Abstract
Purpose
This cross-sectional study was performed to assess the relationship between simple snoring and metabolic syndrome (MetS).
Methods
A total of 5635 participants including 300 healthy volunteers without snoring allegedly were initially included from 2007 to 2016. Polysomnographic variables, anthropometric measurements, and biochemical indicators were collected. The polynomial linear trend test was used to assess the linear trend across snoring intensity for metabolic score, and logistic regression was used to evaluate the odds ratios (ORs) for MetS after controlling for age, sex, obesity, smoking status, and alcohol consumption.
Results
The final study population consisted of 866 participants. Simple snorers showed more severe metabolic disorders and higher prevalence of MetS than nonsnorers. A significant linear trend was observed between snoring intensity and metabolic score. Simple snoring was significantly associated with increased odds for MetS among all participants (OR = 2.328, 95% CI: 1.340–4.045) and female participants (OR = 2.382, 95% CI: 1.136–4.994) after multivariable adjustment. With regard to MetS components, simple snoring was significantly associated with increased odds for hypertension (OR = 1.730, 95% CI: 1.130–2.650), abdominal obesity (OR = 1.810, 95% CI: 1.063–3.083), and hyper-triglycerides (TG) (OR = 1.814, 95% CI: 1.097–2.998) among all participants, with hypertension (OR = 3.493, 95% CI: 1.748–6.979) among males and with abdominal obesity (OR = 2.306, 95% CI: 1.245–4.270) and hyper-TG (OR = 2.803, 95% CI: 1.146–6.856) among females after multivariable adjustment.
Conclusions
After excluding the influence of repeated apnea and hypoxia, simple snoring was still significantly associated with MetS, especially in women. Furthermore, the associations were more obvious for hypertension among males and for abdominal obesity and hyper-TG among females. In addition to OSA, simple snoring also should be valued.
1. Introduction
Snoring is commonly described as a coarse and vibratory sound during sleep resulting from partial obstruction of inspiration in the oropharynx [1]. The prevalence of snoring varies from 2% to 85% [1–3]. Simple snoring may represent the beginning of a sleep-disordered breathing (SDB) continuum, which ranges from partial airway collapse and mildly increased upper airway resistance to complete airway collapse and severe obstructive sleep apnea (OSA) lasting for 60 s or more [2, 4]. There is accumulating evidence that snoring is associated with several health problems, including sleepiness, cardiovascular diseases, metabolic syndrome (MetS), and all-cause mortality [5–7].
MetS, a combination of excess abdominal obesity, dyslipidemia, hypertension, hyperglycemia, and insulin resistance (IR) [8], is related to increased risk of cardiovascular events and mortality [9]. Previous studies have demonstrated a relationship between snoring and MetS, but patients with OSA were not excluded from the study populations [10–12]. Such association between snoring and MetS may be mediated by OSA, as heavy snoring is always accompanied with sleep apnea [3], and both snoring and sleep apnea are related to mechanical obstruction of the upper airway [13]. However, most common snorers do not have OSA [4]. Therefore, further research is required to determine whether simple snoring itself, as a more common disease, is independently related to increased odds for MetS and its components.
To exclude the effects of more severe sleep apnea, we conducted a cross-sectional study among non-OSA participants to examine whether simple snoring itself is associated with increased odds for MetS and metabolic disorders, such as obesity, hypertension, dyslipidemia, and IR.
2. Methods
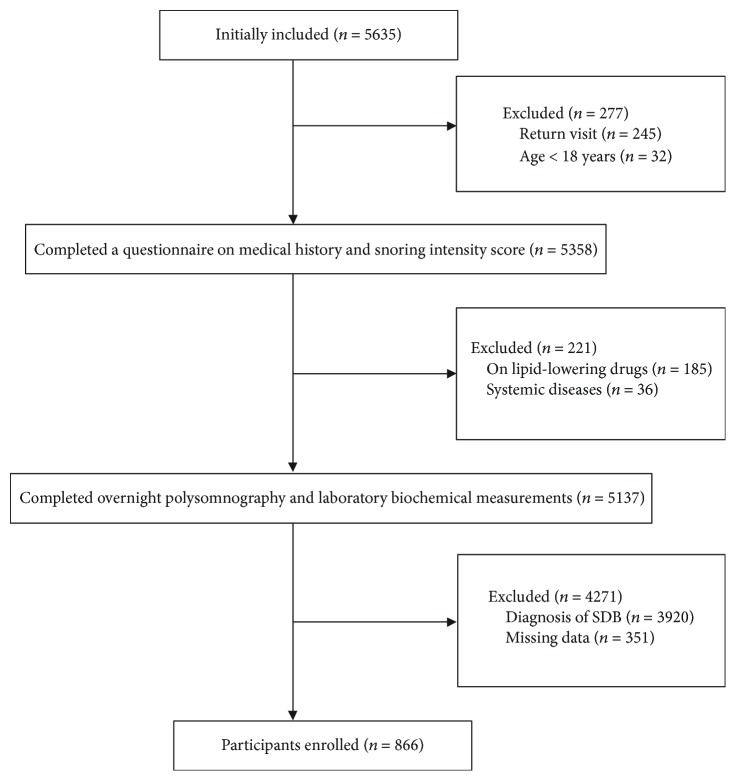
A total of 5635 participants were initially included in the study. Among them, 300 were healthy volunteers specially recruited without snoring allegedly; the other participants were patients referring to the sleep center of Shanghai Jiao Tong University Affiliated Sixth People's Hospital for suspected SDB from 2007 to 2016. They were mainly from cities in southeastern China and all completed surveys regarding smoking habits, alcohol consumption, and medical history. 4769 participants were excluded for the following reasons: return visit; age < 18 years; taking lipid-reducing medications prior to the study, which could affect the serum lipid profiles' levels; various systemic diseases (i.e., malignancy, chronic kidney disease, and unstable cardiopulmonary diseases, such as congestive heart failure or intrinsic pulmonary disease); diagnosis of SDB; and missing data (lacking information of smoking, drinking, lipid-lowering medication taking, etc.). Finally, a total of 866 participants including 187 nonsnorers and 679 simple snorers were enrolled in the analysis (Figure 1). Written informed consent was obtained from each participant according to the guidelines outlined by the National Ethics Regulation Committee. This study was approved by the Internal Review Board of the Institutional Ethics Committee of Shanghai Jiao Tong University Affiliated Sixth People's Hospital and was conducted in accordance with the tenets of the Declaration of Helsinki.
Figure 1.
Flow chart of study population enrollment.
2.1. Anthropometric and Metabolic Measurements
All measurements were performed using the standard methods mentioned in our previous research [14], with the participants dressed in lightweight clothing and with bare feet. Body mass index (BMI) was calculated as body mass in kilograms divided by the square of the patient's height in meters. Neck circumference (NC) was measured at the level of laryngeal prominence, waist circumference (WC) in the middle between the 12th rib and the iliac crest, and hip circumference (HC) at the level of the anterior superior iliac spine at the broadest circumference below the waist using a measuring tape. The waist-to-hip ratio (WHR) was determined as WC (cm)/HC (cm). In accordance with the guidelines of the American Society of Hypertension [15], blood pressure was measured at approximately 08:00 with patients in a seated position using a mercury sphygmomanometer after a 5 min rest. It was recorded as the mean of three measurements taken at 1 min intervals.
A fasting blood sample was taken from the antecubital vein of each patient in the morning after polysomnographic monitoring. Fasting serum glucose and lipid profiles were measured in the hospital laboratory using routine procedures. Serum lipid profiles included total cholesterol (TC), triglycerides (TG), high-density lipoprotein (HDL), low-density lipoprotein (LDL), apolipoprotein A-I (apoA-I), apolipoprotein B (apoB), apolipoprotein E (apoE), and lipoprotein(a) (Lpa) (Hitachi, Tokyo, Japan). An immunoradiological method was used to measure the fasting serum insulin level. Insulin sensitivity was evaluated using the homeostasis model assessment of insulin resistance (HOMA-IR): HOMA-IR = fasting glucose (mmol/L) × fasting insulin(μU/mL)/22.5 [16].
2.2. Polysomnography
Overnight polysomnography (PSG) (Alice 4 or 5, Philips Respironics, Pittsburgh, PA) was performed from 22:00 to 06:00 according to the criteria of the American Academy of Sleep Medicine [17], including electroencephalogram (EEG), left and right electrooculogram (EOG), genioglossus electromyogram, electrocardiogram (ECG), pulse oxygen saturation, nose and mouth airflow, thoracic-abdominal movement, and body position. Apnea was defined as the complete cessation of airflow lasting for at least 10 s. Hypopnea was defined as a ≥50% reduction in airflow for at least 10 s with a decrease in oxyhemoglobin saturation of ≥3% or a ≥30% reduction in airflow for at least 10 s with a decrease in oxyhemoglobin saturation of ≥4%. The apnea hypopnea index (AHI) was defined as the number of apnea and hypopnea events per hour during sleep. The diagnosis of OSA was determined by AHI, and an AHI ≥5 events/h was defined as OSA [17].
2.3. Snoring Assessment
As snoring sounds are commonly described as a nuisance by the bed partner of the affected individual, information on snoring was collected from a bed partner or family member. Snoring intensity was evaluated using a 10 cm visual analogue scale (VAS) from 0 to 10: 0 represents no snoring, 1–3 represents minimally annoying, 4–6 represents moderately annoying, 7–9 represents annoying, and 10 represents extremely annoying [18]. We defined 0 as no snoring, 1–3 as mild snoring, 4–6 as moderate snoring, and 7–9 as severe snoring. Besides, in order to ensure a more even distribution for analysis, we incorporated 10 into the severe snoring group.
2.4. Metabolic Score
MetS was defined according to the NCEP ATP III criteria with the modified WC criteria for Asians [19] as the presence of at least three of the following five clinical features: (1) elevated WC: ≥90 cm in men and ≥80 cm in women; (2) elevated TG: ≥1.70 mmol/L; (3) reduced HDL <1.03 mmol/L in men and <1.30 mmol/L in women; (4) elevated blood pressure: SBP ≥130 mmHg or DBP ≥85 mmHg or on antihypertensive drug treatment in a patient with a history of hypertension; and (5) elevated fasting glucose ≥5.6 mmol/L or on drug treatment for elevated glucose. A metabolic score was established as the total number of positive diagnostic criteria of metabolic syndrome in each participant [14].
2.5. Statistical Analysis
All statistical analyses were performed using SPSS (version 23.0; SPSS Inc., Chicago, IL). All values were examined for normal distribution prior to statistical analysis. Data are presented as the median (interquartile range [IQR]), mean ± standard deviation (SD), or n (%) if they are skewed, normally distributed, or categorical. Normally distributed or skewed variables were analyzed using the independent samples t-test or Mann–Whitney U test, respectively. Categorical variables were analyzed using the chi-square test or Fisher's exact test. The polynomial linear trend test was used to assess the linear trends across snoring intensity for metabolic score. Independent associations between snoring and MetS and its components were analyzed, using multivariable logistic regression after adjusting for relevant covariates. Odds ratios (ORs) are presented with 95% confidence intervals (CIs). In all analyses, P < 0.05 was taken to indicate statistical significance.
3. Results
3.1. Basic Characteristics
The 866 participants were divided according to snoring intensity into the simple snoring group (n = 679) and nonsnoring group (n = 187). The proportion of males was greater in the simple snoring group than in the nonsnoring group (63.0% vs. 41.7%, respectively, P < 0.001). Compared to the nonsnoring group, simple snorers were more obese (evidenced by higher BMI, NC, WC, HC, and WHR, P < 0.001), had higher fasting glucose levels, insulin levels, and HOMA-IR levels (P < 0.001), and showed more severe lipid abnormalities (i.e., hyper-TC, hyper-TG, hyper-LDL, hyper-apoB, and hypo-apoA-I, P < 0.05). Furthermore, simple snorers had a higher prevalence of MetS (18.0% vs. 9.1%, respectively, P = 0.003), and among simple snorers, the percent of participants scored from 0 to 5 was 24.9%, 33.6%, 23.6%, 12.7%, 4.1%, and 1.2%, respectively; among controls, the percent was 31%, 40.6%, 19.3%, 6.4%, 2.7%, and 0, respectively. Simple snorers were more likely to be current smokers and alcohol drinkers (P < 0.001) (Table 1).
Table 1.
Basic characteristics of nonsnorer participants and simple snorers.
| Nonsnorers (n = 187) | Simple snorers (n = 679) | P value | |
|---|---|---|---|
| Age (years) | 42.00 (33.00, 49.00) | 36.00 (30.00, 46.00) | 0.002 |
| Male (%) | 78 (41.7%) | 428 (63.0%) | <0.001 |
| BMI (kg/m2) | 23.04 ± 3.11 | 24.15 ± 3.59 | <0.001 |
| NC (cm) | 34.67 ± 3.29 | 36.80 ± 3.55 | <0.001 |
| WC (cm) | 83.09 ± 10.09 | 86.99 ± 10.41 | <0.001 |
| HC (cm) | 94.37 ± 7.07 | 97.10 ± 7.07 | <0.001 |
| WHR (cm) | 0.88 ± 0.07 | 0.90 ± 0.07 | 0.011 |
| SBP (mmHg) | 119.19 ± 10.71 | 119.83 ± 14.39 | 0.573 |
| DBP (mmHg) | 76.55 ± 8.18 | 76.24 ± 9.72 | 0.687 |
| FPG (mmol/L) | 4.99 (4.62, 5.29) | 5.05 (4.73, 5.37) | 0.036 |
| FINS (μU/mL) | 5.52 (4.08, 8.48) | 7.85 (5.65, 11.30) | <0.001 |
| HOMA-IR | 1.20 (0.88, 1.90) | 1.77 (1.23, 2.57) | <0.001 |
| TC (mmol/L) | 4.22 (3.49, 4.82) | 4.37 (3.80, 4.96) | 0.001 |
| TG (mmol/L) | 0.90 (0.63, 1.32) | 1.18 (0.78, 1.72) | <0.001 |
| HDL (mmol/L) | 1.14 (0.96, 1.34) | 1.09 (0.96, 1.29) | 0.237 |
| LDL (mmol/L) | 2.39 (1.81, 2.93) | 2.39 (1.81, 2.93) | <0.001 |
| apoA-I (g/L) | 1.14 (0.98, 1.29) | 1.09 (0.96, 1.23) | 0.023 |
| apoB (g/L) | 0.68 (0.57, 0.78) | 0.76 (0.65, 0.88) | <0.001 |
| apoE (mg/dL) | 3.92 (3.24, 4.71) | 3.90 (3.20, 4.79) | 0.748 |
| Lpa (mg/dL) | 6.40 (3.75, 14.75) | 8.10 (4.40, 16.80) | 0.051 |
| MetS, n (%) | 17 (9.1) | 122 (18) | 0.003 |
| Smokers, n (%) | 15 (8.0) | 149 (21.9) | <0.001 |
| Alcohol drinkers, n (%) | 16 (8.6) | 198 (29.2) | <0.001 |
BMI: body mass index; NC: neck circumference; WC: waist circumference; HC: hip circumference; WHR: waist-to-hip ratio; SBP: systolic blood pressure; DBP: diastolic blood pressure; FBG: fasting blood glucose; FINS: fasting insulin; HOMA-IR: homeostasis model assessment of insulin resistance; TC: total cholesterol; TG: triglyceride; HDL: high-density lipoprotein cholesterol; LDL: low-density lipoprotein cholesterol; apoA-I: apolipoprotein A-I; apoB: apolipoprotein B; apoE: apolipoprotein E; Lpa: lipoprotein(a); MetS: metabolic syndrome.
Compared to female simple snorers, males snored louder (higher VAS score, P < 0.001), were more obese (evidenced by higher BMI, NC, WC, HC, and WHR, P < 0.05), had higher systolic and diastolic blood pressure (P < 0.001), and had more severe dyslipidemia (i.e., higher TG, LDL, and apoB and lower HDL, apoA-I, and apoE, P < 0.05). In addition, males were more likely to be current smokers and alcohol drinkers than females (P < 0.001) (Table 2).
Table 2.
Basic characteristics of participants according to gender.
| Nonsnorers | Simple snorers | |||||
|---|---|---|---|---|---|---|
| Male | Female | P value | Male | Female | P value | |
| (n = 78) | (n = 109) | (n = 428) | (n = 251) | |||
| Age (years) | 38.31 ± 12.64 | 42.16 ± 10.43 | 0.029 | 36.84 ± 11.42 | 40.60 ± 12.39 | <0.001 |
| BMI (kg/m2) | 23.14 ± 3.08 | 22.96 ± 3.15 | 0.074 | 24.44 ± 3.22 | 23.66 ± 4.11 | 0.010 |
| NC (cm) | 37.14 ± 2.98 | 32.89 ± 2.18 | <0.001 | 38.42 ± 2.73 | 34.04 ± 3.07 | <0.001 |
| WC (cm) | 86.08 ± 11.37 | 80.95 ± 8.49 | 0.001 | 89.22 ± 9.27 | 83.18 ± 11.15 | <0.001 |
| HC (cm) | 95.25 ± 7.25 | 93.74 ± 6.90 | 0.154 | 97.80 ± 6.68 | 95.89 ± 7.55 | 0.001 |
| WHR (cm) | 0.90 ± 0.08 | 0.86 ± 0.06 | 0.001 | 0.91 ± 0.06 | 0.87 ± 0.07 | <0.001 |
| SBP (mmHg) | 118.15 ± 8.27 | 119.93 ± 12.14 | 0.237 | 121.78 ± 13.74 | 116.50 ± 14.89 | <0.001 |
| DBP (mmHg) | 76.19 ± 8.23 | 76.81 ± 8.17 | 0.614 | 77.38 ± 9.57 | 74.28 ± 9.69 | <0.001 |
| FPG (mmol/L) | 5.06 (4.55, 5.30) | 4.97 (4.66, 5.26) | 0.969 | 5.23 ± 0.88 | 5.12 ± 0.85 | 0.094 |
| FINS (μU/mL) | 5.07 (3.42, 7.74) | 5.87 (4.47, 8.59) | 0.085 | 9.25 ± 5.75 | 10.28 ± 16.57 | 0.341 |
| HOMA-IR | 1.13 (0.76, 1.63) | 1.29 (0.94, 2.03) | 0.173 | 2.24 ± 1.89 | 2.43 ± 4.30 | 0.517 |
| TC (mmol/L) | 4.08 (3.39, 4.71) | 4.25 (3.63, 4.85) | 0.266 | 4.45 ± 0.84 | 4.40 ± 0.96 | 0.450 |
| TG (mmol/L) | 0.94 (0.67, 1.56) | 0.87 (0.62, 1.19) | 0.013 | 1.57 ± 1.26 | 1.23 ± 0.94 | <0.001 |
| HDL (mmol/L) | 1.05 (0.88, 1.21) | 1.21 (1.03, 1.41) | <0.001 | 1.08 ± 0.26 | 1.24 ± 0.28 | <0.001 |
| LDL (mmol/L) | 2.41 (1.77, 2.99) | 2.37 (1.90, 2.86) | 0.934 | 2.79 ± 0.73 | 2.64 ± 0.83 | 0.016 |
| apoA-I (g/L) | 1.06 (0.92, 1.20) | 1.22 (1.03, 1.34) | <0.001 | 1.07 ± 0.19 | 1.19 ± 0.23 | <0.001 |
| apoB (g/L) | 0.68 (0.56, 0.78) | 0.67 (0.58, 0.78) | 0.833 | 0.78 ± 0.16 | 0.73 ± 0.18 | <0.001 |
| apoE (mg/dL) | 3.62 (3.02, 4.25) | 4.07 (3.45, 4.75) | 0.160 | 4.04 ± 1.33 | 4.32 ± 1.40 | 0.013 |
| Lpa (mg/dL) | 6.10 (3.85, 11.55) | 7.40 (3.70, 18.10) | 0.051 | 13.76 ± 15.54 | 14.54 ± 18.06 | 0.578 |
| MetS, n (%) | 7 (9%) | 10 (9.2%) | 0.963 | 74 (17.3%) | 48 (19.1%) | 0.548 |
| Smoking, n (%) | 15 (19.2%) | 0 | <0.001 | 143 (33.4%) | 6 (2.4%) | <0.001 |
| Alcohol drinkers, n (%) | 15 (19.2%) | 1 (0.9%) | <0.001 | 177 (41.4%) | 21 (8.4%) | <0.001 |
BMI: body mass index; NC: neck circumference; WC: waist circumference; HC: hip circumference; WHR: waist-to-hip ratio; SBP: systolic blood pressure; DBP: diastolic blood pressure; FBG: fasting blood glucose; FINS: fasting insulin; HOMA-IR: homeostasis model assessment of insulin resistance; TC: total cholesterol; TG: triglyceride; HDL: high-density lipoprotein cholesterol; LDL: low-density lipoprotein cholesterol; apoA-I: apolipoprotein A-I; apoB: apolipoprotein B; apoE: apolipoprotein E; Lpa: lipoprotein(a); MetS: metabolic syndrome.
3.2. Prevalence of MetS
The prevalence rates of MetS and its components in our study are shown in Table 2. The prevalence rate of MetS was higher in simple snorers (18.0% vs. 9.1%, respectively, P = 0.003). Among its components, the prevalence rates of hypertension (29.3% vs. 18.7%, respectively, P = 0.004) and hyper-TG (25.9% vs. 12.8%, respectively, P < 0.001) were significantly higher in simple snorers than in nonsnorers.
Subgroup analysis showed that there were differences between simple snorers and the nonsnoring group in both male and female participants. Among male participants, simple snorers only had a higher prevalence rate of hypertension compared with nonsnorers (33.2% vs. 14.1%, P = 0.001). Among female participants, simple snorers had a higher prevalence rate of MetS (19.1% vs. 9.2%, P = 0.018) and higher prevalence rates of abdominal obesity (30.7% vs. 22.0%, P = 0.005) and hyper-TG (16.3% vs. 6.4%, P = 0.011) compared with nonsnorers (Table 3).
Table 3.
Prevalence of MetS in nonsnorers and simple snorers.
| Nonsnorers | Simple snorers | P value | ||
|---|---|---|---|---|
| Total | MetS | 17 (9.1%) | 122 (18.0%) | 0.003 |
| Hypertension | 35 (18.7%) | 199 (29.3%) | 0.004 | |
| Abdominal obesity | 23 (12.3%) | 113 (16.6%) | 0.148 | |
| Hyper-TG | 24 (12.8%) | 176 (25.9%) | <0.001 | |
| Hypo-HDL | 101 (54.0%) | 362 (53.3%) | 0.866 | |
| Hyperglycemia | 21 (11.2%) | 108 (15.9%) | 0.112 | |
|
| ||||
| Males | MetS | 7 (9.0%) | 74 (17.3%) | 0.065 |
| Hypertension | 11 (14.1%) | 142 (33.2%) | 0.001 | |
| Abdominal obesity | 5 (6.4%) | 36 (8.4%) | 0.551 | |
| Hyper-TG | 17 (21.8%) | 135 (31.5%) | 0.084 | |
| Hypo-HDL | 37 (47.4%) | 207 (48.4%) | 0.880 | |
| Hyperglycemia | 8 (10.3%) | 72 (16.8%) | 0.144 | |
|
| ||||
| Females | MetS | 10 (9.2%) | 48 (19.1%) | 0.018 |
| Hypertension | 15 (19.0%) | 57 (22.7%) | 0.885 | |
| Abdominal obesity | 24 (22.0%) | 77 (30.7%) | 0.005 | |
| Hyper-TG | 7 (6.4%) | 41 (16.3%) | 0.011 | |
| Hypo-HDL | 64 (58.7%) | 155 (61.8%) | 0.587 | |
| Hyperglycemia | 13 (11.9%) | 36 (14.3%) | 0.539 | |
MetS: metabolic syndrome; TG: triglyceride; HDL: high-density lipoprotein cholesterol.
3.3. Association between Snoring and MetS
A significant linear trend was observed between snoring intensity and metabolic score (P for trend = 0.008) (Figure 2). Simple snoring was associated with MetS, even after adjusting for age, sex, smoking status, and alcohol consumption (OR = 2.328, 95% CI: 1.340–4.045). Gender stratification analysis showed that such association was only significant among female participants (OR = 2.382, 95% CI: 1.136–4.994) (Table 4).
Figure 2.
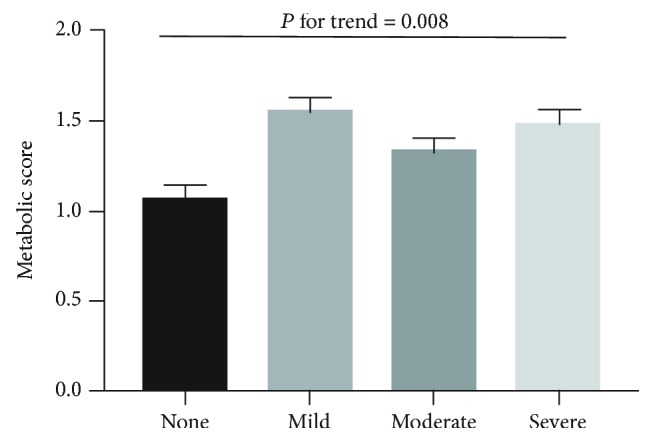
Adjusted mean metabolic scores across snoring severity. The data were adjusted for age, sex, smoking status, and alcohol consumption.
Table 4.
Odds ratios for MetS.
| Total | Males | Females | ||||
|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | |
| Unadjusted | 2.190 | (1.282–3.742) | 2.120 | (0.938–4.794) | 2.341 | (1.137–4.821) |
| Multivariable adjusted∗ | 2.328 | (1.340–4.045) | 1.615 | (0.931–4.936) | 2.382 | (1.136–4.994) |
∗Adjusted for age, sex, smoking status, and alcohol consumption.
Focusing on separate MetS components (Table 5), simple snoring was significantly associated with hypertension (OR = 1.730, 95% CI: 1.130–2.650) among all participants after adjusting for confounding factors, such as age, gender, smoking status, alcohol consumption, and other MetS components. However, the results of gender stratification analysis showed that this relationship was only obvious in men (OR = 3.493, 95% CI: 1.748–6.979).
Table 5.
Odds ratios for MetS components.
| Total | Males | Females | |||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | OR | 95% CI | OR | 95% CI | ||
| Hypertension | Unadjusted | 1.800 | (1.203, 2.694) | 3.024 | (1.550, 5.902) | 1.041 | (0.606, 1.787) |
| Multivariable adjusted∗ | 1.730 | (1.130, 2.650) | 3.493 | (1.748, 6.979) | 0.899 | (0.501, 1.613) | |
|
| |||||||
| Abdominal obesity | Unadjusted | 1.424 | (0.880, 2.303) | 1.341 | (0.509, 3.531) | 2.237 | (1.262, 3.965) |
| Multivariable adjusted∗ | 1.810 | (1.063, 3.083) | 0.943 | (0.335, 2.653) | 2.306 | (1.245, 4.270) | |
|
| |||||||
| Hyper-TG | Unadjusted | 2.376 | (1.498, 3.771) | 1.653 | (0.930, 2.938) | 2.845 | (1.233, 6.562) |
| Multivariable adjusted∗ | 1.814 | (1.097, 2.998) | 1.443 | (0.774, 2.690) | 2.803 | (1.146, 6.856) | |
|
| |||||||
| Hypo-HDL | Unadjusted | 0.972 | (0.703, 1.345) | 1.038 | (0.640, 1.683) | 1.135 | (0.718, 1.796) |
| Multivariable adjusted∗ | 0.876 | (0.615, 1.249) | 0.808 | (0.477, 1.368) | 0.897 | (0.551, 1.460) | |
|
| |||||||
| Hyperglycemia | Unadjusted | 1.495 | (0.908, 2.461) | 1.770 | (0.816, 3.838) | 1.236 | (0.627, 2.437) |
| Multivariable adjusted∗ | 1.217 | (0.709, 2.092) | 1.696 | (0.736, 3.911) | 0.899 | (0.425, 1.899) | |
∗Adjusted for age, sex, smoking status, alcohol consumption, and other MetS components. TG: triglyceride; HDL: high-density lipoprotein cholesterol.
Simple snoring was significantly associated with abdominal obesity (OR = 1.810, 95% CI: 1.063–3.083) after adjusting for confounding factors, and this relationship was only significant in women (OR = 2.306, 95% CI: 1.245–4.270). Simple snoring was also significantly associated with hyper-TG (OR = 1.814, 95% CI: 1.097–2.998), and gender stratification analysis showed that this relationship was only significant in women (OR = 2.803, 95% CI: 1.146–6.856) after multivariable adjustment.
The associations between simple snoring and hypo-HDL/hyperglycemia were not significant.
No significant interaction between sex and snoring on MetS was observed through joint classification analysis (P for interaction = 0.836) (Figure 3).
Figure 3.
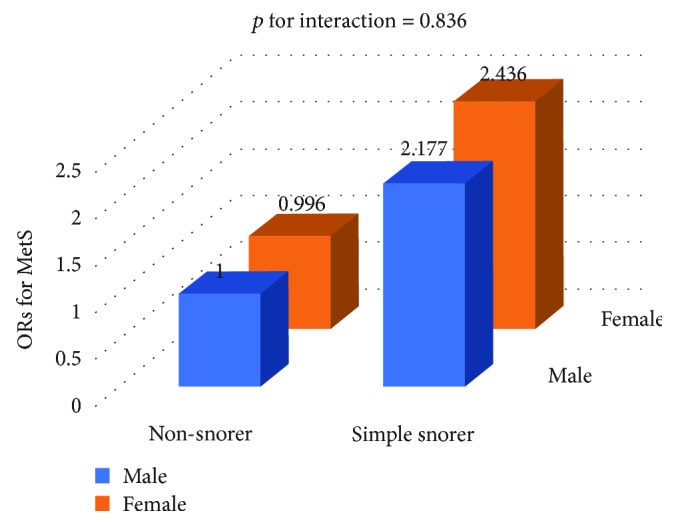
ORs for MetS according to gender and snoring status. ORs according to joint classification were adjusted for age, smoking status, and alcohol consumption.
4. Discussion
The present study indicated that simple snoring was associated with higher prevalence of MetS, and there was a positive linear trend for metabolic score across snoring severity after adjusting for multiple variables. Furthermore, we found that simple snoring was independently associated with MetS and that female snorers were more vulnerable to metabolic disorders. No interaction was observed between sex and simple snoring on MetS.
Previous studies have demonstrated a relationship between snoring and MetS, but did not exclude the impact of repeated apnea and hypoxia [10, 11, 20]. A similar relationship was still found after excluding OSA patients from the whole sample. Furthermore, some studies showed that associations between snoring and MetS/MetS components only existed among females [7, 13, 21–24]. In the present study, although men showed more typical symptoms, such as snoring, than women, the metabolic effects of snoring were even greater in women. Furthermore, simple snoring was significantly associated with MetS in female snorers but not in male snorers. After controlling for confounding factors, the association with abdominal obesity/hyper-TG was more obvious among women. However, no significant interaction was observed between sex and snoring in our study. The mechanism involved in this gender difference may be as follows. Firstly, approximately 7% of premenopausal women have polycystic ovary syndrome, show hyperandrogenism, and have increased vulnerability to sleep disorders and metabolic disorders [7, 21]. Secondly, the levels of sex hormone secretion are reduced in postmenopausal women, with the most pronounced changes seen in estrogen reduction. The hormone levels change from estrogen predominance to androgen predominance, followed by increased incidence of snoring and metabolic disorders. Thirdly, compared with men, women tend to accumulate less visceral fat, but there are fewer α-adrenergic receptors in visceral adipose tissue in men indicating higher rates of lipolysis [25]. Finally, there existed gene-by-sex interaction on hepatic steatosis and females could accumulate more hepatic TG than males genetically [26].
Obesity is considered a predisposing factor for severe snoring, whereas snoring may further promote the development of obesity [27]. Abdominal obesity is not only a component of MetS, but a promoting factor of other components of MetS, such as IR, resulting in more serious metabolic problems [28]. Thus, the relationships among snoring, obesity, and MetS are complex. Elmasry et al. [29] suggested that snoring and obesity lead to increased odds for diabetes, with obesity playing the leading role, whereas snoring further increases the odds for developing diabetes on the basis of obesity. Other studies [11, 29–31] have shown that snoring increases the odds for metabolic disturbances, but the effect of snoring may be significantly attenuated or even eliminated after adjusting for obesity-related indicators, such as BMI or WHR. Thus, obesity may play a leading role in the interaction between snoring and metabolic disorders by activating chronic inflammatory reactions, as well as adipokine disorders [11]. In this study, simple snorers were more obese, and simple snoring was significantly associated with abdominal obesity after adjustment, including for other MetS components.
The relationship between snoring and hypertension remains unclear. Lindberg et al. reported that snoring was an independent risk factor for hypertension in men younger than 50 years old, whereas snoring did not significantly affect hypertension in older adults [32]. Hu et al. reported that snorers had significantly higher systolic and diastolic blood pressure levels, and snoring was significantly associated with hypertension in women [23]. Bixler et al. reported that in both men and women, snoring was independently correlated with hypertension, which was more obvious in young and normal-weight participants [33]. Two related studies in the Korean population indicated that snoring significantly increased the prevalence of hypertension independent of obesity [20]. In contrast, Nieto et al. reported that AHI was significantly associated with hypertension, whereas the relationship between habitual snoring and hypertension was not obvious [34]. In the present study, simple snorers showed significantly higher prevalence rates of hypertension than nonsnorers, and simple snoring was an independent risk factor for hypertension, which was more obvious in men than in women. The main underlying mechanism may lie in estrogen, which could influence artery stiffness, inhibit vascular remodel, and modulate the renin-angiotensin aldosterone system [35].
Previous studies have also drawn inconsistent conclusions regarding the relationship between snoring and IR. Some researchers reported that snoring was associated with diabetes and insulin sensitivity [29, 36], whereas other studies showed that although snoring was more common in men, it significantly increased the odds for diabetes or impaired glucose tolerance only in women [13, 21, 24]. A recent meta-analysis concluded that there was a strong association between snoring and diabetes in women, but not in men [7]. The association between simple snoring and hyperglycemia was not significant in our study, but simple snorers showed higher fasting glucose and insulin levels as well as a tendency for higher IR than nonsnorers.
There have been few previous studies on the relationship between snoring and dyslipidemia. Shin et al. reported that as snoring frequency increased, TG levels increased and HDL levels decreased and that snoring was associated with hyper-TG and hypo-HDL, but the effect of snoring was significantly attenuated after adjusting for other confounding factors. Cho et al. reported that snorers had higher prevalence rates of hyper-TG and hypo-HDL, but snoring did not increase the odds for dyslipidemia after taking various confounding factors into consideration [20]. In our study, simple snorers had higher levels of TC, TG, LDL, and apoB, lower apoA-I levels, and higher prevalence of hyper-TG than nonsnorers. Furthermore, the components of dyslipidemia may differ between genders, e.g., males showed higher TG, LDL, and apoB and lower HDL, apoA-I, and apoE than females. However, after multivariable adjustment, simple snoring was significantly associated with increased odds for hyper-TG only among females.
The mechanisms underlying the relationship between simple snoring and MetS have not been clarified. Through direct mechanical injury to the endothelium and local initiation of proinflammatory response, snoring vibration transmission may accelerate the development of carotid atherosclerotic plaque and contribute to MetS [37–40]. The physiological disturbances caused by snoring increase the number of microarousals during sleep [22], which could disrupt the restorative value of sleep, increase the activity of the sympathetic nervous system, and have a harmful impact on the hypothalamic-pituitary-adrenal axis with consequent elevations in serum cortisol, ultimately contributing to metabolic dysfunction [13, 41–45].
To our knowledge, this is the first study to investigate the associations between MetS/MetS components and simple snoring excluding the impact of OSA. Our study showed that snoring without repeated apnea and hypoxia was significantly associated with increased odds for MetS, which requires more attention regarding hypertension among male snorers and abdominal obesity and hyper-TG among female snorers. Our study also had some limitations. First, although self-reported snoring and snoring reported by roommates were found to be reliable measures in epidemiological studies, as validated by all-night sleep recording [46, 47], these are subjective measures to evaluate snoring. Second, as the duration of snoring was not collected, its impact on MetS could not be assessed.
In conclusion, the present study investigated the relationships between simple snoring and MetS/MetS components and concluded that, after eliminating the effects of repeated apnea and hypoxia, simple snoring was still independently associated with increased odds for MetS, especially in women. Furthermore, it suggested that clinicians should implement interventions, such as suggesting low-fat diet, low-sugar diet, and increased exercise for simple snorers to prevent MetS.
Acknowledgments
We thank all of the participants and survey staffs for their participation. The study was supported by the National Key R&D Program of China (2017YFC0112500), the National Natural Science Foundation of China (81770987, 81700896, 81701306, and 81770988), the Innovation Program of Shanghai Municipal Education Commission (2017-01-07-00-02-E00047), the Multicenter Clinical Research Project from School of Medicine, Shanghai Jiao Tong University (DLY201502), and the Shanghai Shen Kang Hospital Development Center Project (SHDC12015101).
Contributor Information
Jian Guan, Email: guanjian0606@sina.com.
Huaming Zhu, Email: zhmtiger@126.com.
Bin Chen, Email: chen-bin@sjtu.edu.cn.
Data Availability
The clinical data used to support the findings of this study are available from the corresponding authors upon request.
Conflicts of Interest
None of the authors has potential conflict of interests related to the content of the manuscript.
Authors' Contributions
Juanjuan Zou and Yiqun Fu designed the research. Fan Song, Yunyan Xia, and Huajun Xu conducted the research. Juanjuan Zou, Yingjun Qian, and Jianyin Zou analyzed the data. Juanjuan Zou wrote the first draft. Jian Guan, Bin Chen, and Shankai Yin made critical manuscript revisions. All authors read and approved the final manuscript. Juanjuan Zou and Fan Song contributed equally to this work.
References
- 1.Chung J. W., Kim N., Wee J. H., et al. Clinical features of snoring patients during sedative endoscopy. The Korean Journal of Internal Medicine. 2019;34(2):305–314. doi: 10.3904/kjim.2017.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouscoulet L. T., Vázquez-García J. C., Muiño A., et al. Prevalence of sleep related symptoms in four Latin American cities. Journal of Clinical Sleep Medicine. 2008;4(6):579–585. [PMC free article] [PubMed] [Google Scholar]
- 3.Cho J. G., Witting P. K., Verma M., et al. Tissue vibration induces carotid artery endothelial dysfunction: a mechanism linking snoring and carotid atherosclerosis? Sleep. 2011;34(6):751–757. doi: 10.5665/SLEEP.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hu F. B., Willett W. C., Manson J. A. E., et al. Snoring and risk of cardiovascular disease in women. Journal of the American College of Cardiology. 2000;35(2):308–313. doi: 10.1016/S0735-1097(99)00540-9. [DOI] [PubMed] [Google Scholar]
- 5.Rich J., Raviv A., Raviv N., Brietzke S. E. An epidemiologic study of snoring and all-cause mortality. Otolaryngology-Head and Neck Surgery. 2011;145(2):341–346. doi: 10.1177/0194599811402475. [DOI] [PubMed] [Google Scholar]
- 6.Kalchiem-Dekel O., Westreich R., Regev A., Novack V., Goldberg M., Maimon N. Snoring intensity and excessive daytime sleepiness in subjects without obstructive sleep apnea. Laryngoscope. 2016;126(7):1696–1701. doi: 10.1002/lary.25876. [DOI] [PubMed] [Google Scholar]
- 7.Xiong X., Zhong A., Xu H., Wang C. Association between self-reported habitual snoring and diabetes mellitus: a systemic review and meta-analysis. Journal of Diabetes Research. 2016;2016:7. doi: 10.1155/2016/1958981.1958981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coughlin S. R., Mawdsley L., Mugarza J. A., Calverley P. M., Wilding J. P. Obstructive sleep apnoea is independently associated with an increased prevalence of metabolic syndrome. European Heart Journal. 2004;25(9):735–741. doi: 10.1016/j.ehj.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 9.Gami A. S., Witt B. J., Howard D. E., et al. Metabolic syndrome and risk of incident cardiovascular events and death. Journal of the American College of Cardiology. 2007;49(4):403–414. doi: 10.1016/j.jacc.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 10.Thomas G. N., Jiang C. Q., Lao X. Q., et al. Snoring and vascular risk factors and disease in a low-risk Chinese population: the Guangzhou Biobank Cohort Study. Sleep. 2006;29(7):896–900. doi: 10.1093/sleep/29.7.896. [DOI] [PubMed] [Google Scholar]
- 11.Sun L., Pan A., Yu Z., et al. Snoring, inflammatory markers, adipokines and metabolic syndrome in apparently healthy Chinese. PLoS One. 2011;6(11, article e27515) doi: 10.1371/journal.pone.0027515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim C. E., Shin S., Lee H. W., Lim J., Lee J. K., Kang D. Frequency of loud snoring and metabolic syndrome among Korean adults: results from the Health Examinees (HEXA) Study. International Journal of Environmental Research and Public Health. 2017;14(11, article 1294) doi: 10.3390/ijerph14111294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Al-Delaimy W. K., Manson J. E., Willett W. C., Stampfer M. J., Hu F. B. Snoring as a risk factor for type II diabetes mellitus: a prospective study. American Journal of Epidemiology. 2002;155(5):387–393. doi: 10.1093/aje/155.5.387. [DOI] [PubMed] [Google Scholar]
- 14.Fu Y., Xu H., Xia Y., et al. Excessive daytime sleepiness and metabolic syndrome in men with obstructive sleep apnea: a large cross-sectional study. Oncotarget. 2017;8(45):79693–79702. doi: 10.18632/oncotarget.19113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weber M. A., Schiffrin E. L., White W. B., et al. Clinical practice guidelines for the management of hypertension in the community: a statement by the American Society of Hypertension and the International Society of Hypertension. Journal of Hypertension. 2014;32(1):3–15. doi: 10.1097/HJH.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 16.Matthews D. R., Hosker J. P., Rudenski A. S., Naylor B. A., Treacher D. F., Turner R. C. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412–419. doi: 10.1007/bf00280883. [DOI] [PubMed] [Google Scholar]
- 17.Iber C., Ancoli-Israel S., Chesson AL, Quan Stuart, et al. The AASM Manual for the Scoring of Sleep and Associated Events: Rules, Terminology and Technical Specifications. 1st ed. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 18.Rohrmeier C., Fischer R., Merz A. K., Ettl T., Herzog M., Kuehnel T. S. Are subjective assessments of snoring sounds reliable? European Archives of Oto-Rhino-Laryngology. 2015;272(1):233–240. doi: 10.1007/s00405-014-3211-3. [DOI] [PubMed] [Google Scholar]
- 19.Grundy S. M., Cleeman J. I., Daniels S. R., et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112(17):2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- 20.Cho N., Joo S. J., Kim J. K., et al. Relation of habitual snoring with components of metabolic syndrome in Korean adults. Diabetes Research and Clinical Practice. 2006;71(3):256–263. doi: 10.1016/j.diabres.2005.06.011. [DOI] [PubMed] [Google Scholar]
- 21.Valham F., Stegmayr B., Eriksson M., Hägg E., Lindberg E., Franklin K. A. Snoring and witnessed sleep apnea is related to diabetes mellitus in women. Sleep Medicine. 2009;10(1):112–117. doi: 10.1016/j.sleep.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Leineweber C., Kecklund G., Åkerstedt T., Janszky I., Orth-Gomér K. Snoring and the metabolic syndrome in women. Sleep Medicine. 2003;4(6):531–536. doi: 10.1016/S1389-9457(03)00160-6. [DOI] [PubMed] [Google Scholar]
- 23.Hu F. B., Willett W. C., Colditz G. A., et al. Prospective study of snoring and risk of hypertension in women. American Journal of Epidemiology. 1999;150(8):806–816. doi: 10.1093/oxfordjournals.aje.a010085. [DOI] [PubMed] [Google Scholar]
- 24.Lindberg E., Berne C., Franklin K. A., Svensson M., Janson C. Snoring and daytime sleepiness as risk factors for hypertension and diabetes in women—a population-based study. Respiratory Medicine. 2007;101(6):1283–1290. doi: 10.1016/j.rmed.2006.10.015. [DOI] [PubMed] [Google Scholar]
- 25.Williams C. M. Lipid metabolism in women. Proceedings of the Nutrition Society. 2004;63(1):153–160. doi: 10.1079/pns2003314. [DOI] [PubMed] [Google Scholar]
- 26.Norheim F., Hui S. T., Kulahcioglu E., et al. Genetic and hormonal control of hepatic steatosis in female and male mice. Journal of Lipid Research. 2017;58(1):178–187. doi: 10.1194/jlr.M071522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shin M. H., Kweon S. S., Choi B. Y., et al. Self-reported snoring and metabolic syndrome: the Korean Multi-Rural Communities Cohort Study. Sleep and Breathing. 2014;18(2):423–430. doi: 10.1007/s11325-013-0902-8. [DOI] [PubMed] [Google Scholar]
- 28.Perry I. J., Wannamethee S. G., Walker M. K., Thomson A. G., Whincup P. H., Shaper A. G. Prospective study of risk factors for development of non-insulin dependent diabetes in middle aged British men. BMJ. 1995;310(6979):560–564. doi: 10.1136/bmj.310.6979.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elmasry A., Janson C., Lindberg E., Gislason T., Tageldin M. A., Boman G. The role of habitual snoring and obesity in the development of diabetes: a 10-year follow-up study in a male population. Journal Internal Medicine. 2000;248(1):13–20. doi: 10.1046/j.1365-2796.2000.00683.x. [DOI] [PubMed] [Google Scholar]
- 30.Roopa M., Deepa M., Indulekha K., Mohan V. Prevalence of sleep abnormalities and their association with metabolic syndrome among Asian Indians: Chennai Urban Rural Epidemiology Study (CURES-67) Journal of Diabetes Science and Technology. 2010;4(6):1524–1531. doi: 10.1177/193229681000400630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ford E. S., Wheaton A. G., Chapman D. P., Li C., Perry G. S., Croft J. B. Associations between self-reported sleep duration and sleeping disorder with concentrations of fasting and 2-h glucose, insulin, and glycosylated hemoglobin among adults without diagnosed diabetes. Journal of Diabetes. 2014;6(4):338–350. doi: 10.1111/1753-0407.12101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindberg E., Janson C., Gislason T., Svärdsudd K., Hetta J., Boman G. Snoring and hypertension: a 10 year follow-up. European Respiratory Journal. 1998;11(4):884–889. doi: 10.1183/09031936.98.11040884. [DOI] [PubMed] [Google Scholar]
- 33.Bixler E. O., Vgontzas A. N., Lin H. M., et al. Association of hypertension and sleep-disordered breathing. Archives of Internal Medicine. 2000;160(15):2289–2295. doi: 10.1001/archinte.160.15.2289. [DOI] [PubMed] [Google Scholar]
- 34.Nieto F. J., Young T. B., Lind B. K., et al. Association of sleep-disordered breathing, sleep apnea, and hypertension in a large community-based study. JAMA. 2000;283(14):1829–1836. doi: 10.1001/jama.283.14.1829. [DOI] [PubMed] [Google Scholar]
- 35.Di Giosia P., Giorgini P., Stamerra C. A., Petrarca M., Ferri C., Sahebkar A. Gender differences in epidemiology, pathophysiology, and treatment of hypertension. Current Atherosclerosis Reports. 2018;20(3):p. 13. doi: 10.1007/s11883-018-0716-z. [DOI] [PubMed] [Google Scholar]
- 36.Renko A. K., Hiltunen L., Laakso M., Rajala U., Keinänen-Kiukaanniemi S. The relationship of glucose tolerance to sleep disorders and daytime sleepiness. Diabetes Research and Clinical Practice. 2005;67(1):84–91. doi: 10.1016/j.diabres.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 37.Lee S. A., Amis T. C., Byth K., et al. Heavy snoring as a cause of carotid artery atherosclerosis. Sleep. 2008;31(9):1207–1213. [PMC free article] [PubMed] [Google Scholar]
- 38.Amatoury J., Howitt L., Wheatley J. R., Avolio A. P., Amis T. C. Snoring-related energy transmission to the carotid artery in rabbits. Journal of Applied Physiology. 2006;100(5):1547–1553. doi: 10.1152/japplphysiol.01439.2005. [DOI] [PubMed] [Google Scholar]
- 39.Curry B. D., Bain J. L. W., Yan J. G., et al. Vibration injury damages arterial endothelial cells. Muscle & Nerve. 2002;25(4):527–534. doi: 10.1002/mus.10058. [DOI] [PubMed] [Google Scholar]
- 40.Kim J., Pack A. I., Riegel B. J., et al. Objective snoring time and carotid intima-media thickness in non-apneic female snorers. Journal of Sleep Research. 2017;26(2):147–150. doi: 10.1111/jsr.12471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Spiegel K., Leproult R., Van Cauter E. Impact of sleep debt on metabolic and endocrine function. The Lancet. 1999;354(9188):1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 42.Punjabi N. M., Shahar E., Redline S., et al. Sleep-disordered breathing, glucose intolerance, and insulin resistance: the Sleep Heart Health Study. American Journal of Epidemiology. 2004;160(6):521–530. doi: 10.1093/aje/kwh261. [DOI] [PubMed] [Google Scholar]
- 43.Schöbel C., Fietze I., Glos M., et al. Nocturnal snoring decreases daytime baroreceptor sensitivity. Respiratory Medicine. 2014;108(7):1049–1055. doi: 10.1016/j.rmed.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 44.Zou J., Xia Y., Xu H., et al. Independent relationships between cardinal features of obstructive sleep apnea and glycometabolism: a cross-sectional study. Metabolism. 2018;85:340–347. doi: 10.1016/j.metabol.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 45.Stamatakis K. A., Punjabi N. M. Effects of sleep fragmentation on glucose metabolism in normal subjects. Chest. 2010;137(1):95–101. doi: 10.1378/chest.09-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Virkkula P., Bachour A., Hytönen M., Malmberg H., Salmi T., Maasilta P. Patient- and bed partner-reported symptoms, smoking, and nasal resistance in sleep-disordered breathing. Chest. 2005;128(4):2176–2182. doi: 10.1378/chest.128.4.2176. [DOI] [PubMed] [Google Scholar]
- 47.Fischer R., Kuehnel T. S., Merz A. K., Ettl T., Herzog M., Rohrmeier C. Calculating annoyance: an option to proof efficacy in ENT treatment of snoring? European Archives of Oto-Rhino-Laryngology. 2016;273(12):4607–4613. doi: 10.1007/s00405-016-4160-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The clinical data used to support the findings of this study are available from the corresponding authors upon request.