Abstract
Quantifying cortisol concentration in hair is a non-invasive biomarker of long-term hypothalamic-pituitary-adrenal (HPA) activation, and thus can provide important information on laboratory animal health. Marmosets (Callithrix jacchus) and capuchins (Cebus apella) are New World primates increasingly used in biomedical and neuroscience research, yet published hair cortisol concentrations for these species are limited. Review of the existing published hair cortisol values from marmosets reveals highly discrepant values and the use of variable techniques for hair collection, processing, and cortisol extraction. In this investigation we utilized a well-established, standardized protocol to extract and quantify cortisol from marmoset (n = 12) and capuchin (n = 4) hair. Shaved hair samples were collected from the upper thigh during scheduled exams and analyzed via methanol extraction and enzyme immunoassay. In marmosets, hair cortisol concentration ranged from 2710 – 6267 pg/mg and averaged 4070 ± 304 pg/mg. In capuchins, hair cortisol concentration ranged from 621 – 2089 pg/mg and averaged 1092 ± 338 pg/mg. Hair cortisol concentration was significantly different between marmosets and capuchins, with marmosets having higher concentrations than capuchins. The incorporation of hair cortisol analysis into research protocols provides a non-invasive measure of HPA axis activity over time, which offers insight into animal health. Utilization of standard protocols across laboratories is essential to obtaining valid measurements and allowing for valuable future cross-species comparisons.
Keywords: Hypothalamic-pituitary-adrenal axis, glucocorticoids, Callithrix, Cebus
Introduction
Common marmosets (Callithrix jacchus) and tufted capuchin monkeys (Cebus apella) are small New World primates (approximately 300 – 500g and 2.5 – 4kg, respectively) increasingly used in biomedical and neuroscience research (Mansfield, 2003; Phillips et al., 2014). Marmosets display important similarities to humans in physiology, neuroanatomy, reproduction, development, cognition, and social complexity (Mitchell & Leopold, 2015; Tardif et al., 2003). As such, marmosets are valuable models of human disease, studies of aging and neurodegenerative diseases associated with aging, and social behavior (Miller et al., 2016; Tardif, Mansfield, Ratnam, Ross, & Ziegler, 2011). For example, marmoset models for Parkinson’s disease (induced by 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) are used to study pathogenesis and evaluate potential therapies (Yun, Ahn, & Kang, 2015); marmoset models for multiple sclerosis (MS; induced by recombinant human myelin oligodendrocyte glycoprotein, rhMOG) have yielded important discoveries regarding immune mechanisms and how infection can contribute to the pathogenesis of MS (‘t Hart, Kap, Morandi, Laman, & Gran, 2016; ‘t Hart et al., 2000). Capuchins have a propensity to engage in complex manipulative behavior including the use of tools in the wild and the use of precision grips (Costello & Fragaszy, 1988; Fragaszy, Visalberghi, & Fedigan, 2004; Phillips, 1998; Spinozzi, Lagana, & Truppa, 2007). They have multiple premotor areas in the frontal lobe (Dum & Strick, 1991, 2005; Ohbayashi, Picard, & Strick, 2016), a proprioceptive cortical area 2, and a well-developed cortical area 5 which is associated with motor planning, visually guided reaching, grasping, and manipulation (Padberg et al., 2007). Capuchins are thus valuable models for investigating the motor areas of the cerebral cortex and their involvement in motor control and movement generation (Ohbayashi et al., 2016).
Glucocorticoid hormones are commonly used as a biomarker of health as these hormones are involved in numerous physiological processes including the conversion of sugar, fat, and protein stores into useable energy and the inhibition of swelling and inflammation. Chronic elevation of cortisol can lead to deleterious effects such as the destruction of hippocampal neurons and suppression of immune responses (Sharpley, McFarlane, & Slominski, 2011). These hormones are one indicator of the body’s response to stress and are regulated by the hypothalamic-adrenal-pituitary (HPA) axis. Quantifying concentrations of cortisol is a valid assessment of HPA axis activity, and thus chronic stress, if extracted from a type of specimen that reflects HPA activity over time. Acute cortisol concentrations are often measured using blood serum, urine, or saliva. However, these types of specimen are subject to circadian rhythm fluctuations and, in some cases, the stress of sample collection. These confounding factors compromise the ability of serum, urine, or saliva samples to provide valid measures of chronic cortisol levels. Measuring cortisol from hair more accurately reflects long-term HPA axis activity as free circulating glucocorticoids are slowly deposited into the hair shaft over a period of weeks (J. Meyer & Novak, 2012). In humans, hair grows approximately 1 cm per month (LeBeau, Montgomery, & Brewer, 2011); in marmosets and capuchins, hair grows approximately 0.5 cm per month (Phillips, personal observation). Hair cortisol can thus provide a retrospective non-invasive measure of activity of the HPA axis over relatively long periods of time.
Despite the utility of cortisol extraction from hair, only a handful of published research has quantified hair cortisol from common marmosets and to our knowledge only one publication has reported values for capuchins (Clara, Tommasi, & Rogers, 2008; Fourie & Bernstein, 2011; Franke et al., 2016). Review of these existing published hair cortisol values from marmosets reveals highly discrepant values ranging from approximately 2.75 × 103 - 1.27 × 107 pg/mg obtained with variable techniques (see Table 1 for summary). Yamanashi et al. (Yamanashi, 2013; 2016) identified several factors that affect results of cortisol extraction from hair – sampling site, weight of hair sample used, degree of grinding, and extraction time – and emphasized the importance of using consistent and reliable techniques. Additionally, exposure to natural sunlight (UV radiation) has been shown to decrease hair cortisol concentration (Wester, van der Wulp, Koper, de Rijke, & van Rossum, 2016). Here we provide hair cortisol concentrations for common marmosets and tufted capuchins, using a standard protocol (J. Meyer, Novak, Hamel, & Rosenberg, 2014) to establish baseline levels for these species.
Table 1.
Hair cortisol concentrations reported for common marmosets (Callithrix jacchus) and tufted capuchins (Cebus apella).
| Species | Hair Cortisol (pg/mg) | Weight of Sample (mg) | Sampling Site | Technique | Degree of Grinding | Extraction Time | n | Source |
|---|---|---|---|---|---|---|---|---|
| Callithrix jacchus | 62,300 ± 50,480 | 10 | back or shoulder | scissors to mince hair | N/A | 24 hrs | 12 | Fourie & Bernstein, 2011 |
| Callithrix jacchus | 1.81 × 106 – 1.27 × 107 | 8 – 20 | not stated | scissors to mince hair | N/A | 48 hrs | 11 | Clara et al. 2008 |
| Callithrix jacchus | 2751 ± 829 (grp A) 2587 ± 401 (grp B) |
50 | posterior vertex region of neck | bead beater | powder | 24 hrs | 20 | Franke et al. 2016 |
| Cebus apella | 26,040 ± 11,190 | 10 | back or shoulder | scissors to mince hair | N/A | 24 hrs | 4 | Fourie & Bernstein, 2011 |
Notes: For all but the Clara et al. 2008 data, hair cortisol concentrations are provided as mean +/− standard deviation. Clara et al. 2008 did not provide summary data of hair cortisol concentration; we have reported here the estimated low and high concentrations. All concentrations were converted to pg/mg for ease of comparison across studies. Samples that were minced with scissors did not undergo grinding.
Method
Animals and housing
The subjects of this study were adult common marmosets (Callithrix jacchus, n = 12; female n = 8) and adult tufted capuchins (Cebus apella; n = 4; female n = 2), housed at the Southwest National Primate Research Center, Texas Biomedical Research Institute, San Antonio, TX. Marmosets were socially housed in family groups and non-breeding at the time of sample collection, with room temperatures ranging between 76 and 84˚ F (set point of 80˚ F) and a 12h light-dark cycle with lights off at 19:00. Fresh food was available ad libitum; the base diet consisted of a purified diet (Harlan Teklad TD130059 PWD) and Mazuri diet (AVP Callitrichid 5LK6). Marmosets also received small amounts of fresh fruit, seeds, or dairy products daily as enrichment. Dietary, nutritional, and husbandry specifics for these subjects followed those outlined in Layne and Power (2003). Capuchins were socially housed and non-breeding at the time of sample collection in an indoor-outdoor enclosure enriched with perches, swings, and PVC tubes and joints. New World monkey chow and water were available ad libitum; fresh fruit or nuts were provided daily. At the time of hair collection (and for several months prior), the capuchins and marmosets were in breeding protocols and not involved in research projects. This research was approved by the Institutional Animal Care and Use Committee at Texas Biomedical Research Institute, adhered to the American Society of Primatologists Principles for the Ethical Treatment of Non-Human Primates, and abided by all applicable U.S. Federal laws governing research with nonhuman primates.
Sample collection and cortisol assay
Hair sample collection occurred during routine health checks while animals were anesthetized. Between 170 and 800 mg of hair was shaved from the animal’s thigh with an electric shaver, taking care not to nick the skin as blood contamination can falsely elevate cortisol levels (Yamanashi, Morimura, Mori, Hayashi, & Suzuki, 2013). Hair samples were stored at room temperature out of direct light in 15 mL screw-cap polypropylene centrifuge tubes until processed within a week of collection.
The protocol for extracting cortisol from hair followed Meyer et al. (2014) and has been validated by several other laboratories (Davenport, Tiefenbacher, Lutz, Novak, & Meyer, 2006; Dettmer, Novak, Meyer, & Suomi, 2014). Samples were washed three times with isopropanol, allowed to air-dry under a hood for 5–7 days, and then ground to a fine powder using a ball mill (MM400; Retsch, Newtown, PA). To extract cortisol, 50 mg of the finely ground hair was incubated in 1.0 mL of methanol for 24 hours. Samples were then centrifuged for 1 minute at 14,000 rpm to pellet the powdered hair. A 600 μL sample of the supernatant was evaporated for 45 minutes using a nitrogen evaporator. Samples were then reconstituted with 200 μL of assay buffer and diluted 1:40 before being analyzed in duplicate via enzyme immunoassay (EIA) using a commercially available expanded range high sensitivity salivary cortisol EIA kit (#1–3002; Salimetrics; State College, PA). Resulting values (μg/dL) were converted to pg/mg for analysis. Inter- and intra- coefficients of variance were 11% and 4%, respectively. These assays were performed in both the Phillips and Meyer laboratories and showed excellent reproducibility (rs(10) = 0.85, p < 0.01). According to the manufacturer, the antibody has high selectivity for cortisol with a sensitivity < 0.007 μg/dL. The amount of cross-reactivity of the antibody with other steroids is as follows: prednisolone = 0.568%, cortisone = 0.130%, 11-deoxycortisol = 0.156%, 21-deoxycortisol = 0.041%, dexamethasone = 19.2%, triamcinolone = 0.086%, corticosterone = 0.214%, progesterone = 0.015%, and testosterone = 0.006%. Cross-reactivity of the antibody with prednisone, 17α-hydroxyprogesterone, 17β-estradiol, DHEA, transferrin, and aldosterone is not detectable.
To assess whether the Salimetrics kit was accurately measuring cortisol in methanolic extracts from New World monkey hair extracts, we performed parallelism tests of serially diluted extracts from two different marmosets used in the present study. Parallelism was excellent, with correlation coefficients of 0.990 and 0.999 for the two samples. These results support the conclusion that the present procedure afforded a valid measure of hair cortisol concentrations in marmosets and presumably also capuchin monkeys that, like marmosets, have very high circulating cortisol levels compared to Old World primate species.
Results
The range of obtained hair cortisol concentrations for marmosets and capuchins is provided in Table 2. The assumption of normality was tested for the obtained cortisol concentrations for each species. The hair cortisol concentrations for marmosets deviated significantly from normality (Shapiro-Wilk = 0.828, df = 12, p < 0.02). Examination of a boxplot revealed an extreme score in one marmoset (See Figure 1 and Table 2). This animal was healthy; the reason for the extreme value is unknown. This data point was removed from further analysis. Hair cortisol concentrations for capuchins did not deviate significantly from normality.
Table 2.
Obtained hair cortisol concentrations from common marmosets and tufted capuchins.
| Subject | Sex | Age (in yrs) | Hair Cortisol (pg/mg) | Weight of Sample (mg) |
|---|---|---|---|---|
| Marmosets | ||||
| M1 | F | 2.5 | 6267 | 49.6 |
| M2 | F | 3 | 2710 | 49.6 |
| M3 | F | 3 | 3302 | 49.8 |
| M4 | F | 3.5 | 3531 | 49.7 |
| M5* | F | 4 | 8906 | 49.7 |
| M6 | F | 4 | 4780 | 49.4 |
| M7 | M | 3 | 4293 | 49.5 |
| M8 | M | 3 | 2984 | 49.8 |
| M9 | M | 3.5 | 4010 | 49.8 |
| M10 | M | 4 | 3631 | 49.3 |
| M11 | M | 5 | 4751 | 49.8 |
| M12 | M | 6 | 4512 | 49.4 |
| Capuchins | ||||
| C1 | F | 26 | 920 | 49.7 |
| C2 | F | 32 | 739 | 49.4 |
| C3 | M | 8 | 621 | 49.4 |
| C4 | M | 10 | 2089 | 49.4 |
This subject’s value was determined to be an outlier and was removed from analysis.
Figure 1.
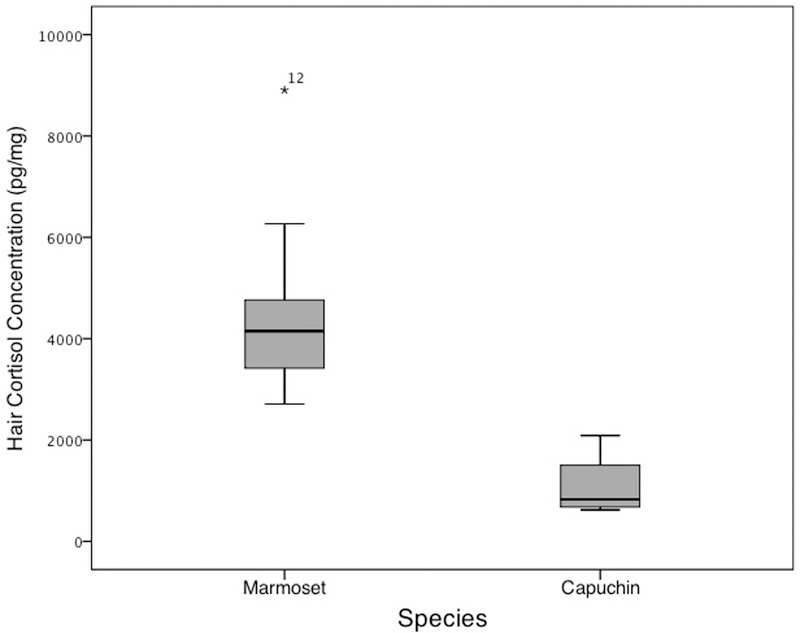
Boxplot of hair cortisol concentrations grouped by species
There were no significant sex differences in hair cortisol concentration for marmosets (female M = 4118 ± 1418 pg/mg; male M = 4030 ± 644 pg/mg; t(9) = 4.19, p = 0.07). A test of significance for sex differences was not conducted for capuchins due to the small sample size in each condition. However, examination of these values indicates considerable overlap in cortisol concentration between male and female capuchins.
Hair cortisol concentrations were significantly higher in marmosets (M = 4070 ± 304 pg/mg) than in capuchins (M = 1092 ± 338 pg/mg), t(13) = 5.42, p < 0.001.
Discussion
We provide values for baseline hair cortisol concentrations for common marmosets and tufted capuchin monkeys. The results for marmosets are similar to those reported by Franke et al. (2016), who followed the same assay protocol. As seen in Table 1, other studies have obtained notably different hair cortisol values in common marmosets. Clara et al. (2008) reported the most dissimilar results, with concentrations over 1000 times greater than the findings of this study. Perhaps it is not surprising considering that Clara et al. (2008) also utilized the most dissimilar protocol.
To our knowledge, only one study, Fourie and Bernstein (2011), has published hair cortisol concentration of tufted capuchins (refer to Table 1 for summary values). Their values are approximately 30 times greater than the results obtained in the present study. Methodological differences between the two studies, as elaborated on below, likely account for these disparate values.
The importance of using a standard protocol – both within and across species – cannot be overstated. In comparing the obtained results with previously published hair cortisol values for marmosets and capuchins, the following should be kept in mind. In the present study, hair was sampled from all animals consistently from the upper thigh. Only Clara et al. (2008) did not explicitly state from which body region hair was sampled; as samples were acquired opportunistically (as animals came to the front of the cage for a food treat), we assume the regions sampled varied across individuals. Yamanashi et al. (2013) found variation in hair cortisol concentrations across different body regions. Degree of grinding also was found to significantly influence obtained hair cortisol concentration (Yamanashi et al., 2016). We used a ball mill to grind the hair into a fine powder, as did Franke et al. (2016). Another method used by Fourie and Bernstein (2011) and Clara et al. (2008) involved mincing the hair into 1–2 mm pieces with scissors. Yamanashi et al. (2013) also concluded higher extraction times resulted in significant increases in hair cortisol concentration. Extraction time of 24 hours appears to be the most widely used (Fourie & Bernstein, 2011; Franke et al., 2016); the Meyer protocol recommends methanol extraction for 24 hours (2014). Clara et al. (2008) performed extraction for 48 hours, which may also contribute to their relatively high values. These discrepancies emphasize the importance of comparing cortisol concentrations utilizing consistent methodologies.
It is unclear whether sex differences in basal cortisol concentrations are typical in primates. We did not detect sex differences in cortisol concentration in marmosets. Laudenslager et al. (2012) detected significant sex differences in hair cortisol concentration in vervet monkeys (Chlorocebus aethiops sabaeus). This effect emerged after puberty, wherein males had lower concentrations than females. A study of hair cortisol concentration in rhesus monkeys (Macaca mulatta) reported a significant sex difference, yet this was found in only one of the two facilities studied (Lutz et al., 2016). Crockett et al. (Crockett, Bowers, Sackett, & Bowden, 1993) examined urinary cortisol responses to potentially stressful laboratory events in long-tailed macaques (Macaca fascicularis). Females tended to have higher cortisol concentration than males in response to these events, which suggests that there are sex differences in reactivity. Whether marmosets display such differences is unknown. We should note that the basic unit for marmoset social groups is a mated pair and their offspring, rather than the large multimale-multifemale social groups seen in vervet monkeys and macaques. Additionally, endocrine physiology may be markedly different between New and Old World primates. Coe et al. (1992) conducted a comparative study of endocrine activity across prosimians, Old World primates, and New World monkeys. Cortisol levels were found to be 2 – 10 times greater in New World monkeys than Old World primates. This difference, which is also reflected in hair cortisol, is due to the phenomenon of glucocorticoid resistance seen in some New World primates that is a result of species variation in glucocorticoid receptor binding affinity and receptor signaling (Charmandari & Kino, 2010). As such, monkeys in the Callitrichidae family, which includes marmosets, have markedly elevated cortisol concentrations (Lipsett, Chrousos, Tomita, Brandon, & Loriaux, 1985; Scammell, 2000). However, members of the Cebidae family, which includes capuchins, have somewhat lower cortisol concentrations which suggests little or only modest glucocorticoid resistance in these Neotropical primates. The reason for these species differences in glucocorticoid receptor binding affinity and cortisol secretion is currently unknown.
Hair cortisol is increasingly used as a biomarker of chronic stress in nonhuman primates (Carlitz, Kirschbaum, Stalder, & van Schaik, 2014; Carlitz et al., 2016; Rakotoniaina et al., 2017). These measures can contribute to captive management by providing quantifiable data about how individuals respond to standard stressors in captive environments such as group formation, social separation, and social rank (J. S. Meyer & Hamel, 2014). Hair cortisol can also be used to assess long-term stress in wild primate populations, such as investigating the impact of anthropogenic disturbances on wild populations. Carlitz et al. (2016) examined whether chimpanzees living in an environment with human interferences of forest fragmentation, ecotourism, and illegal logging had increased long-term stress levels as quantified by hair cortisol concentration. In another study, hair cortisol concentration correlated negatively with survival in a population of wild grey mouse lemurs (Microcebus murinus) (Rakotoniaina et al., 2017).
Thus, hair cortisol analysis provides a retrospective and non-invasive measure of HPA axis activity over time. Utilization of standard protocols across laboratories is essential to obtaining valid measurements and allowing for valuable future cross-species comparisons.
Acknowledgements
We are grateful to the veterinary staff of the SNPRC for assistance in sample collection. This research was supported by the Pilot Research Program at the Southwest National Primate Research Center (NIH grant P51 OD011133) and the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under award number RS15N090296 to KAP. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Declarations of interest: None.
References
- ‘t Hart BA, Kap YS, Morandi E, Laman JD, & Gran B (2016). EBV infection and multiple sclerosis: Lessons from a marmoset model. Trends in Molecular Medicine, 22(12), 1012–1024. doi: 10.1016/j.molmed.2016.10.007 [DOI] [PubMed] [Google Scholar]
- ‘t Hart BA, van Meurs M, Brok HP, Massacesi L, Bauer J, Boon L, … Laman JD (2000). A new primate model for multiple sclerosis in the common marmoset. Immunology Today, 21(6), 290–297. [DOI] [PubMed] [Google Scholar]
- Carlitz EHD, Kirschbaum C, Stalder T, & van Schaik CP (2014). Hair as a long-term retrospective cortisol calendar in orang-utans (Pongo spp.): New perspectives for stress monitoring in captive management and conservation. . General and Comparative Endocrinology, 195, 151–156. [DOI] [PubMed] [Google Scholar]
- Carlitz EHD, Miller R, Kirschbaum C, Gao W, Hänni C, & van Schaik CP (2016). Measuring hair cortisol concentrations to assess the effect of anthropogenic impacts on wild chimpanzees (Pan troglodytes). PLoS ONE, 11(4), e0151870. doi:doi: 10.1371/journal.pone.0151870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charmandari E, & Kino T (2010). Chrousos syndrome: a seminal report, a phylogenetic enigma and the clinical implications of glucocorticoid signaling changes. European Journal of Clinical Investigation, 40(10), 932–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clara E, Tommasi L, & Rogers LJ (2008). Social mobbing calls in common marmosets (Callithrix jacchus): effects of experience and associated cortisol levels. Animal Cognition, 11, 349–358. doi: 10.1007/s10071-007-0125-0 [DOI] [PubMed] [Google Scholar]
- Coe CL, Savage A, & Bromley LJ (1992). Phylogenetic influences on hormone levels across the primate order. American Journal of Primatology, 28, 81–100. [DOI] [PubMed] [Google Scholar]
- Costello M, & Fragaszy DM (1988). Prehension in Cebus and Samiri: I. Grip type and hand preference American Journal of Primatology, 15, 234–245. [DOI] [PubMed] [Google Scholar]
- Crockett CM, Bowers CL, Sackett GP, & Bowden DM (1993). Urinary cortisol responses of longtailed macaques to five cage sizes, tethering, sedation, and room change. American Journal of Primatology, 30, 55–74. [DOI] [PubMed] [Google Scholar]
- Davenport MD, Tiefenbacher S, Lutz CK, Novak MA, & Meyer JS (2006). Analysis of endogenous cortisol concentrations in the hair of rhesus macaques. General and Comparative Endocrinology, 147, 255–261. doi: 10.1016/j.ygcen.2006.01.005 [DOI] [PubMed] [Google Scholar]
- Dettmer AM, Novak MA, Meyer JS, & Suomi SJ (2014). Population density-dependent hair cortisol concentrations in rhesus monkeys (Macaca mulatta). Psychoneuroendocrinology, 42, 59–67. doi: 10.1016/j.psyneuen.2014.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, & Strick PL (1991). The origin of corticospinal projections from the premotor areas in the frontal lobe. Journal of Neuroscience, 11(3), 667–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dum RP, & Strick PL (2005). Frontal lobe inputs to the digit representations of the motor areas on the lateral surface of the hemisphere. Journal of Neuroscience, 25(6), 1375–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fourie NH, & Bernstein RM (2011). Hair cortisol levels track phylogenetic and age related differences in hypothalamic-pituitary-adrenal (HPA) axis activity in non-human primates. General and Comparative Endocrinology, 174, 150–155. doi: 10.1016/j.ygcen.2011.08.013 [DOI] [PubMed] [Google Scholar]
- Fragaszy DM, Visalberghi E, & Fedigan LM (2004). The Complete Capuchin Cambridge: Cambridge University Press. [Google Scholar]
- Franke SK, Van kesteren RE, Hofman S, Wubben JAM, Smit AB, & Philippens, I. H. C. H. M. (2016). Individual and familial susceptibility to MPTP in a common marmoset model for Parkinson’s disease. Neurodegenerative Diseases, 16(5–6), 293–303. doi: 10.1159/000442574 [DOI] [PubMed] [Google Scholar]
- Laudenslager ML, Jorgensen MJ, & Fairbanks LA (2012). Developmental patterns of hair cortisol in male and female nonhuman primates: Lower hair cortisol levels in vervet males emerge at puberty. Psychoneuroendocrinology, 37(10), 1736–1739. doi: 10.1016/j.psyneuen.2012.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layne DG, & Power RA (2003). Husbandry, handling, and nutrition for marmosets. Comparative Medicine, 53(4), 383–392. [PubMed] [Google Scholar]
- LeBeau MA, Montgomery MA, & Brewer JD (2011). The role of variations in growth rate and sample collection on interpreting results of segmental analyses of hair. Forensic Science International, 210, 110–116. doi: 10.1016/j.forsciint.2011.02.015 [DOI] [PubMed] [Google Scholar]
- Lipsett MB, Chrousos GP, Tomita M, Brandon DD, & Loriaux DL (1985). The defective glucocorticoid receptos in man and nonhuman primates. Recent Progress in Hormone Research, 41, 199–247. [DOI] [PubMed] [Google Scholar]
- Lutz CK, Coleman K, Worlein JM, Kroeker R, Menard MT, Rosenberg K, … Novak MA (2016). Factors influencing alopecia and hair cortisol in rhesus macaques (Macaca mulatta) Journal of Medical Primatology, 45(4), 180–188. doi: 10.111/jmp.12220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield K (2003). Marmoset models commonly used in biomedical research. Comparative Medicine, 53, 383–392. [PubMed] [Google Scholar]
- Meyer J, & Novak M (2012). Minireview: Hair cortisol: A novel biomarker of hypothalamic-pituitary-adrenocortical activity. Endocrinology, 153, 4120–4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer J, Novak M, Hamel A, & Rosenberg K (2014). Extraction and analysis of cortisol from human and monkey hair. Journal of Visualized Experiments, 83, e50882. doi: 10.3791/50882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer JS, & Hamel AF (2014). Models of stress in nonhuman primates and their relevance for human psychopathology and endocrine dysfunction. ILAR Journal, 55(2), 347–360. doi: 10.1093/ilar/ilu023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CT, Freiwald WA, Leopold DA, Mitchell JF, Silva AC, & Wang X (2016). Marmosets: A neuroscientific model of human social behavior. Neuron, 90(2), 219–233. doi: 10.1016/j.neuron.2016.03.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell JF, & Leopold DA (2015). The marmoset monkey as amodel for visual neuroscience. Neuroscience Research, 93, 20–46. doi: 10.1016/j.neures.2015.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohbayashi M, Picard N, & Strick PL (2016). Inactivation of the dorsal premotor area disrupts internally generated, but not visually guided, sequential movements. Journal of Neuroscience, 36(6), 1971–1976. doi:10.1523.JNEUROSCI.2356-15.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padberg J, Franca JG, DCooke DF, Soares JGM, Rosa MGP, Fiorani M Jr., … Krubitzer L (2007). Parallel evolution of cortical areas involved in skilled hand use. J Neurosci, 27, 10106–10115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips KA (1998). Tool use in wild capuchin monkeys (Cebus albifrons trinitatis). American Journal of Primatology, 46, 259–261. [DOI] [PubMed] [Google Scholar]
- Phillips KA, Bales KL, Capitanio JP, Conley A, Czoty PW, ‘t Hart BA, … Voytko ML (2014). Why primate models matter. American Journal of Primatology, 76(9), 801–827. doi: 10.1002/ajp.22281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakotoniaina JH, Kappeler PM, Kaesler E, Hamalainen AM, Kirschbaum C, & Kraus C (2017). Hair cortisol concentrations correlate negatively with survival in a wild primate population. BMC Ecology, 17, 30. doi: 10.1186/s12898-017-0140-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scammell JG (2000). Steroid resistance in the squirrel monkey: An old subject revisited. ILAR Journal, 41(1), 19–25. doi: 10.1093/ilar.41.1.19 [DOI] [PubMed] [Google Scholar]
- Sharpley CF, McFarlane JR, & Slominski A (2011). Stress-linked cortisol concentrations in hair: What we know and what we need to know. Reviews in the Neurosciences, 23(1), 111–121. doi: 10.1515/RNS.2011.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinozzi G, Lagana T, & Truppa V (2007). Hand use by tufted capuchins (Cebus apella) to extract a small ood item from a tube: digit movements, hand preference, and performance. American Journal of Primatology, 69(3), 336–352. [DOI] [PubMed] [Google Scholar]
- Tardif SD, Mansfield KG, Ratnam R, Ross CN, & Ziegler TE (2011). The marmoset as a model of aging and age-related diseases. ILAR Journal, 52(1), 54–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tardif SD, Smucny DA, Abbott DH, Mansfield K, Schultz-Darken NJ, & Yamamoto ME (2003). Reproduction in captive common marmosets (Callithrix jacchus). Comparative Medicine, 53, 364–368. [PubMed] [Google Scholar]
- Wester VL, van der Wulp NR, Koper JW, de Rijke YB, & van Rossum EF (2016). Hair cortisol and cortisone and decreased by natural sunlight. Psychoneuroendocrinology, 72, 94–96. doi: 10.1016/j.psyneuen.2016.06.016 [DOI] [PubMed] [Google Scholar]
- Yamanashi Y (2013). Cortisol analysis of hair of camptive chimpanzees (Pan troglodytes). General and Comparative Endocrinology, 194, 55–63. [DOI] [PubMed] [Google Scholar]
- Yamanashi Y, Morimura N, Mori Y, Hayashi M, & Suzuki J (2013). Cortisol analysis of hair of captive chimpanzees (Pan troglodytes) General and Comparative Endocrinology, 194, 55–63. [DOI] [PubMed] [Google Scholar]
- Yamanashi Y, Teramoto M, Morimura N, Hirata S, Suzuki J, Hayashi N, … Idani G (2016). Analysis of hair cortisol levels in captive chimpanzees: Effect of various methods on cortisol stability and variability. MethodsX, 3, 110–117. doi: 10.1016/j.mex.2016.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun J-W, Ahn J-B, & Kang B-C (2015). Modeling Parkinson’s disease in the common marmoset (Callithrix jacchus): overview of models, methods, and animal care. Laboratory Animal Research, 31(4), 155–165. doi: 10.5625/lar.2015.31.4.155 [DOI] [PMC free article] [PubMed] [Google Scholar]


