Abstract
BACKGROUND
Many patients remain without a diagnosis despite extensive medical evaluation. The Undiagnosed Diseases Network (UDN) was established to apply a multidisciplinary model in the evaluation of the most challenging cases and to identify the biologic characteristics of newly discovered diseases. The UDN, which is funded by the National Institutes of Health, was formed in 2014 as a network of seven clinical sites, two sequencing cores, and a coordinating center. Later, a central biorepository, a metabolomics core, and a model organisms screening center were added.
METHODS
We evaluated patients who were referred to the UDN over a period of 20 months. The patients were required to have an undiagnosed condition despite thorough evaluation by a health care provider. We determined the rate of diagnosis among patients who subsequently had a complete evaluation, and we observed the effect of diagnosis on medical care.
RESULTS
A total of 1519 patients (53% female) were referred to the UDN, of whom 601 (40%) were accepted for evaluation. Of the accepted patients, 192 (32%) had previously undergone exome sequencing. Symptoms were neurologic in 40% of the applicants, musculoskeletal in 10%, immunologic in 7%, gastrointestinal in 7%, and rheumatologic in 6%. Of the 382 patients who had a complete evaluation, 132 received a diagnosis, yielding a rate of diagnosis of 35%. A total of 15 diagnoses (11%) were made by clinical review alone, and 98 (74%) were made by exome or genome sequencing. Of the diagnoses, 21% led to recommendations regarding changes in therapy, 37% led to changes in diagnostic testing, and 36% led to variant-specific genetic counseling. We defined 31 new syndromes.
CONCLUSIONS
The UDN established a diagnosis in 132 of the 382 patients who had a complete evaluation, yielding a rate of diagnosis of 35%. (Funded by the National Institutes of Health Common Fund.)
MANY PATIENTS WITH CHRONIC DISEASes remain without a diagnosis despite extensive medical evaluation. In 2008, the Undiagnosed Diseases Program (UDP) was established at the National Institutes of Health (NIH) Clinical Center to meet the needs of patients with undiagnosed diseases and to investigate the biologic characteristics of the diseases.1 During the first 2 years of the UDP, 1191 patient records were reviewed. Of the 160 patients who were admitted for a comprehensive evaluation, 24% received a diagnosis.1 The diagnoses included entirely new syndromes, rare diseases, and unusual presentations of common diseases.
In 2014, the Undiagnosed Diseases Network (UDN), which is funded by the NIH, was established as a network of seven clinical sites, two sequencing cores, and a coordinating center.2 Later, a central biorepository, a metabolomics core, and a model organisms screening center were added. A Web-based portal, the UDN Gateway, was opened to the public on September 16, 2015.3-5 The aim of the UDN is to provide wider access to cross-disciplinary expertise and to leverage specific advantages of the collaborative network, such as deep subspecialty expertise. Diagnostic evaluation is provided at no cost to patients.
Since the publication of the original UDP report in 2012,1 the cost of human genome sequencing has fallen dramatically and algorithms for analyzing the data have improved.6,7 At many academic medical centers in the United States, genome sequencing is now a routine part of the care of patients with genetic or presumed genetic diseases. Summary reports that include rates of molecular diagnoses have been produced at clinical and research laboratories.8-11 However, many patients who undergo genome sequencing remain without a diagnosis. Even among patients with a putative molecular diagnosis, data on clinical confirmation of the molecular diagnosis, the effect of the diagnosis on medical care, and extension of the findings toward the overall understanding of the disease are limited, because laboratories often do not have access to follow-up information. We report data from the first 1519 consecutive applicants to the UDN.
METHODS
PATIENTS
The study was approved by the central institutional review board at the National Human Genome Research Institute (registration number, 00000014).12 A detailed description of UDN processes is provided in a manual of operations.13 In brief, patients refer themselves to the UDN or are referred by a health care provider. Since the number of patients who can be accepted each year is limited, preference is given to applicants in whom a diagnosis is most likely to be established and applicants whose disease, when researched, is most likely to generate new knowledge about the underlying pathogenic mechanism. The criteria that are used in deciding which patients to accept include the presence of an undiagnosed condition despite thorough evaluation by a health care provider, the presence of at least one objective finding, agreement to the storage and sharing of information and biologic materials, the ability to travel to a clinical site for evaluation or availability to participate in a telemedicine-based consultation, and the ability and willingness to engage in additional clinical and research workup. No criteria are based on the presence of specific symptoms or the involvement of specific systems. All applicants are considered.
EVALUATIONS
Patients who meet the criteria and in whom a diagnosis is not made by clinical review alone are eligible for a multidisciplinary evaluation, which includes the use of diagnostic tools such as exome and genome sequencing and metabolomics testing (Table S3 in the Supplementary Appendix, available with the full text of this article at NEJM.org). In-person evaluations include detailed and standardized phenotyping, which is performed by multiple specialists with the use of a customized implementation of PhenoTips, a software tool for the collection of phenotypic information.14 After the evaluation, a report, a letter that describes key findings and follow-up recommendations, and records are sent to the patient or family, the referring provider, and to other providers who are designated by the patient or family.
At the model organisms screening center, which has a drosophila core and a zebrafish core, candidate genes (and their variants) are evaluated for pathogenicity. A central biorepository provides secure storage, tracking, and distribution of collected biologic materials.
DIAGNOSES
Diagnoses are coded at the individual clinical sites and entered into the UDN Gateway. Because criteria for establishing a diagnosis are inconsistent in the literature, we formed a subcommittee to formalize working definitions of diagnosis and the effect of diagnosis on medical care.
Diagnosis and its effect on medical care are decided at the level of the individual patient by the treating clinical team. A tool was designed to help the clinical team reach a consensus that is focused on the following factors: whether a diagnosis has really been made, the level of confidence in the diagnosis, and whether the diagnosis changed the patient’s medical care and, if so, the way in which care changed. For certification of a diagnosis, the clinical team is required to provide information in additional categories, including the method by which the diagnosis was established and the extent to which the diagnosis explains the patient’s presenting symptoms. The additional categories were developed by the subcommittee; the clinical teams are left to interpret the wording and are required to do so through consensus.
A diagnosis that is based on the association of a previously unreported clinical presentation with a new gene (or gene region) is straightforward to classify. A more challenging group of diagnoses arises from variations in existing gene–disease associations. Clinical teams that wish to establish a diagnosis that is based on an existing gene–disease association with a variant phenotype are provided the opportunity to explain their reasoning through annotations to the information provided in the additional categories.
Established diagnostic guidelines are used if they are available. For diseases that do not have clear diagnostic criteria, diagnosis involves a synthesis of the available objective data and the judgment of the treating clinician. If the diagnosis is associated with minimal uncertainty, then it is considered to be a certain diagnosis. If the diagnosis is associated with an element of uncertainty but not enough to dismiss it for the purposes of clinical decision making, then it is considered to be a high-likelihood diagnosis.
Investigators are asked to describe, in freeform text, their recommendations regarding any changes in medical care that should result from the diagnosis. They are also asked to comment on the following factors: whether the diagnosis had no effect on the patient’s care; whether it led to a change in care other than medical therapy, such as a change in the diagnostic strategy; whether it led to a recommendation regarding a change in medical therapy; and whether it led to variant-specific genetic counseling.
To increase the likelihood that a diagnosis is established, the UDN shares deidentified phenotypic and genotypic data in publicly accessible databases, including the Database of Genotypes and Phenotypes (dbGaP),15 PhenomeCentral,16 and Clin-Var.17 Through PhenomeCentral, the information is available to other databases that participate in the Matchmaker Exchange.18 Patients also have the option of making their data available on the public-facing UDN website, in the form of a participant page (Fig. S2 in the Supplementary Appendix).
STATISTICAL ANALYSIS
Data on UDN patients were gathered directly from the operations group at each site. Statistical testing was performed with the use of the R statistical package, version 3.3.3.
RESULTS
PATIENTS
From September 16, 2015, through May 23, 2017, a total of 1519 patients were referred to the UDN; 811 were female, and 615 were younger than 18 years of age. A total of 601 patients were accepted for evaluation; 321 were female, and 350 were younger than 18 years of age (Table 1). The percentage of accepted patients was higher among pediatric applicants than among adult applicants (57% vs. 28%, P<0.001). The mean (±SD) age of accepted pediatric patients was 8±5 years, and the mean age of accepted adults was 39±16 years.
Table 1.
Demographic Characteristics and Primary Symptoms of Applicants to the Undiagnosed Diseases Network.*
| Variable | All Applicants (N = 1519)† |
Pediatric Applicants (N = 615) |
Accepted Pediatric Applicants (N = 350) |
Adult Applicants (N = 904) |
Accepted Adult Applicants (N = 251) |
|
|---|---|---|---|---|---|---|
| Sex — no. (%)‡ | ||||||
| Male | 704 (46) | 318 (52) | 170 (49) | 386 (43) | 109 (43) | |
| Female | 811 (53) | 297 (48) | 180 (51) | 514 (57) | 141 (56) | |
| Other | 4 (<1) | 0 | 0 | 4 (<1) | 1 (<1) | |
| Age — yr | 29±22 | 8±5 | 8±5 | 43±16 | 39±16 | |
| Race or ethnic group — no. (%)‡ | ||||||
| White | 1178 (78) | 452 (73) | 259 (74) | 726 (80) | 197 (78) | |
| Asian | 82 (5) | 41 (7) | 24 (7) | 41 (5) | 14 (6) | |
| Black | 73 (5) | 28 (5) | 18 (5) | 45 (5) | 13 (5) | |
| Multiracial | 65 (4) | 34 (6) | 16 (5) | 31 (3) | 7 (3) | |
| American Indian or Alaska Native | 3 (<1) | 1 (<1) | 0 | 2 (<1) | 0 | |
| Native Hawaiian or other Pacific Islander | 1 (<1) | 1 (<1) | 1 (<1) | 0 | 0 | |
| Other | 117 (8) | 58 (9) | 32 (9) | 59 (7) | 20 (8) | |
| Hispanic or Latino ethnic group — no. (%)‡ | ||||||
| Hispanic or Latino | 156 (10) | 110 (18) | 64 (18) | 46 (5) | 19 (8) | |
| Not Hispanic or Latino | 1133 (75) | 424 (69) | 237 (68) | 709 (78) | 185 (74) | |
| Unknown or not reported | 230 (15) | 81 (13) | 49 (14) | 149 (16) | 47 (19) | |
| Primary symptoms — no. (%) | ||||||
| Neurologic | 607 (40) | 285 (46) | 171 (49) | 322 (36) | 112 (45) | |
| Musculoskeletal or orthopedic | 148 (10) | 63 (10) | 43 (12) | 85 (9) | 25 (10) | |
| Immunologic or allergic | 104 (7) | 28 (5) | 15 (4) | 76 (8) | 17 (7) | |
| Gastrointestinal | 99 (7) | 38 (6) | 15 (4) | 61 (7) | 7 (3) | |
| Rheumatologic | 86 (6) | 12 (2) | 10 (3) | 74 (8) | 18 (7) | |
| Cardiac or vascular | 52 (3) | 21 (3) | 12 (3) | 31 (3) | 13 (5) | |
| Endocrinologic | 43 (3) | 17 (3) | 11 (3) | 26 (3) | 5 (2) | |
| Respiratory | 31 (2) | 11 (2) | 3 (1) | 20 (2) | 6 (2) | |
| Hematologic | 26 (2) | 8 (1) | 3 (1) | 18 (2) | 6 (2) | |
| Infectious | 20 (1) | 1 (<1) | 0 | 19 (2) | 1 (<1) | |
| Dermatologic | 16 (1) | 2 (<1) | 1 (<1) | 14 (2) | 4 (2) | |
| Renal | 15 (1) | 6 (1) | 4 (1) | 9 (1) | 5 (2) | |
| Other | 272 (18) | 123 (20) | 62 (18) | 149 (16) | 32 (13) |
Plus–minus values are means ±SD. P values (calculated with Fisher’s exact test) for the comparison of pediatric applicants with accepted pediatric applicants did not show significant differences (P<0.01) for any variables. P values for the comparison of adult applicants with accepted adult applicants did not show significant differences for any variables except for neurologic symptoms. P values for the comparison of pediatric applicants with adult applicants showed significant differences for the following variables: male sex, female sex, white race, Hispanic or Latino ethnic group, not Hispanic or Latino ethnic group, neurologic symptoms, immunologic or allergic symptoms, rheumatologic symptoms, and infectious symptoms. Percentages may not total 100 because of rounding.
By May 23, 2017, a total of 1203 applications had been reviewed or withdrawn and 316 applications were still under review at the clinical sites.
Data on sex, race, and ethnic group were reported by the patient, a family member, or a representative.
The geographic distribution of the applicants is shown in Figure S1 in the Supplementary Appendix. The majority of the applicants (78%) and accepted patients identified as white. The percentage of patients who identified as Hispanic or Latino was significantly higher among pediatric applicants than among adult applicants (18% vs. 5%, P<0.001), and the percentage of patients who identified as white was significantly lower (74% vs. 80%, P = 0.002).
Although the UDN is open to the possibility of accepting any patient, the primary symptoms of applicants were most commonly neurologic (in 40%), musculoskeletal (10%), immunologic (7%), gastrointestinal (7%), and rheumatologic (6%) (Table 1). Primary neurologic symptoms were more common among accepted adult patients than among all the adult applicants (occurring in 45% vs. 36%, P = 0.01).
STANDARDIZED PHENOTYPING
All accepted patients underwent standardized phenotyping, performed by a cross-disciplinary team of specialists with the use of Human Phenotype Ontology (HPO) terms. As of May 23, 2017, a total of 181 records had been uploaded to Phenome-Central. The mean number of clinical symptoms and physical findings (HPO terms) per record was 26.
EXOME AND GENOME SEQUENCING
Exome and genome sequencing were available to the UDN sites. As of May 23, 2017, a total of 357 patients had undergone sequencing through the UDN; 3 pediatric patients had undergone both exome and genome sequencing. In total, 360 sequencing assays were performed, of which 54% were exome sequencing and 46% were genome sequencing. In addition, 192 patients (32%) had undergone exome sequencing before referral to the UDN. Most of the patients who had previously undergone exome sequencing underwent genome sequencing through the UDN; 63% of the patients who underwent genome sequencing through the UDN had previously undergone exome sequencing.
DIAGNOSIS
As of May 23, 2017, a total of 382 patients had a complete evaluation; 132 of those patients received a diagnosis, yielding a rate of diagnosis of 35%. Of the diagnoses, 11% were made by clinical review alone, 11% by directed clinical testing, 4% by nonsequencing genomewide diagnostic assays (single-nucleotide polymorphism [SNP] array, oligonucleotide array, or karyotyping), and the remainder (74%) by exome or genome sequencing (Table 2). Of the 195 patients who underwent exome sequencing through the UDN, 55 (28%) received a diagnosis; 6 of the 55 patients (11%) had undergone exome sequencing before referral to the UDN. Of the 165 patients who underwent genome sequencing, 32 (19%) received a diagnosis; many of these patients (17 of the 32 patients; 53%) had undergone exome sequencing before referral to the UDN (Table 2, and Table S2 in the Supplementary Appendix). One patient received a diagnosis by both exome and genome sequencing.
Table 2.
Diagnoses According to Method.
| Diagnosis | Patients with Complete Evaluation (N = 382) |
|---|---|
| no./total no. (%) | |
| Diagnosis by any method | 132/382 (35) |
| Clinical review | 15/132 (11) |
| Directed clinical testing | 14/132 (11) |
| Nonsequencing genomewide diagnostic assay | 5/132 (4) |
| Exome or genome sequencing* | 98/132 (74) |
| Reanalysis of previously obtained sequencing data | 11/132 (8) |
| Exome sequencing through the UDN | 55/132 (42) |
| Had previously undergone exome sequencing | 6/132 (5) |
| Genome sequencing through the UDN | 32/132 (24) |
| Had previously undergone exome sequencing | 17/132 (13) |
| No diagnosis | 250/382 (65) |
One diagnosis was established by both exome and genome sequencing.
The diagnoses fell into different categories: 77 (58%) were recognized presentations of a known syndrome, 24 (18%) were unusual presentations of a known syndrome, 16 (12%) were new syndromes associated with a known gene or gene region, and 15 (11%) were new syndromes associated with a new gene or gene region. In effect, 31 new syndromes were described (Table 3, and Table S2 in the Supplementary Appendix).
Table 3.
Examples of New Syndromes Described by the Undiagnosed Diseases Network.
| Syndrome | Gene | Description | Reference |
|---|---|---|---|
| Hypotonia, ataxia, and delayed development syndrome (HADDS) | EBF3 | Syndrome characterized by congenital hypotonia, delayed psychomotor development, variable intellectual disability with speech delay, variable dysmorphic facial features, and ataxia | Chao et al.19 |
| Shashi–Pena syndrome (SHAPNS) | ASXL2 | Neurodevelopmental syndrome characterized by delayed psychomotor development, variable intellectual disability, hypotonia, enlarged head circumference, glabellar nevus flammeus, and deep palmar creases | Shashi et al.20 |
| Neurodevelopmental disorder with epilepsy, cataracts, feeding difficulties, and delayed brain myelination (NECFM) | NACC1 | Syndromic form of severe-to-profound intellectual disability with delayed psychomotor development and seizures in infancy | Schoch et al.21 |
| Mitochondrial complex V (ATP synthase) deficiency, nuclear type 5 (MC5DN5) | ATP5F1D | Metabolic disorder with episodic lethargy, 3-methylglutaconic aciduria, and hyperammonemia | Oláhová et al.22 |
Of the 147 patients who underwent sequencing through the UDN and also had previously undergone sequencing, 48 (33%) received a diagnosis. The diagnoses included new diseases associated with a new gene, as well as new diseases associated with a variant in an area of the genome that had previously been poorly covered. Some of the diagnoses relied on data from case reports that had been published (independent of the UDN) since the patient’s original evaluation had been carried out, and some relied on the consideration of alternative inheritance mechanisms or mosaicism (Table S2 in the Supplementary Appendix). Of the 48 patients, 11 (23%) received a diagnosis after reanalysis of their previously obtained sequencing data and another 30 (63%) underwent repeat sequencing through the UDN. Of the 234 patients who had not previously undergone exome sequencing, 84 (36%) received a diagnosis.
EFFECT OF DIAGNOSIS ON MEDICAL CARE
The diagnoses affected medical care in a variety of ways. In 28 (21%) of the patients who received a diagnosis, the diagnosis led to a recommendation regarding a change in therapy. In 49 (37%), the diagnosis led to a change in care other than therapy, such as the narrowing of diagnostic testing. In 48 (36%), the diagnosis led to variant-specific genetic counseling but did not lead to a change in the diagnostic or therapeutic strategy.
Among the patients who received recommendations regarding a change in therapy, the recommendation was related to a known drug in 22 (79%) of the patients, a vitamin in 7 (25%), a coenzyme in 2 (7%), and a transplant in 1 (4%). Some of the patients received more than one recommendation. There was an observed positive treatment effect for 8 patients. There was an unclear or negative effect for 6 patients. Therapy was not initiated for 4 patients, and the outcome could not be determined for 10 patients.
MODEL ORGANISMS SCREENING
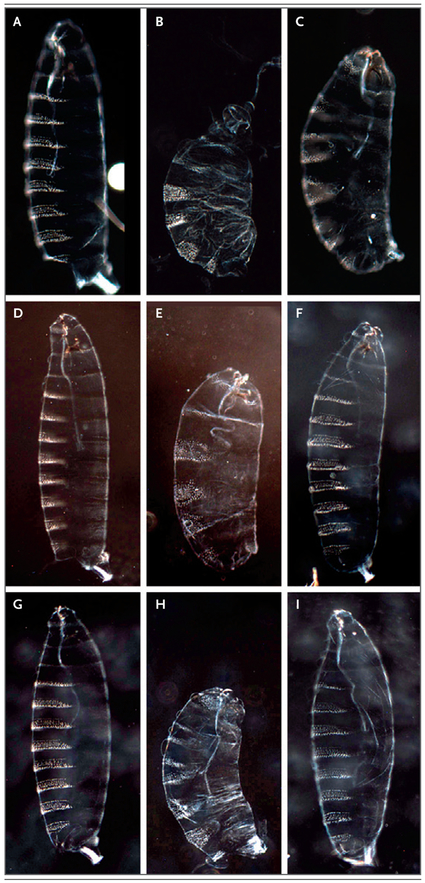
The model organisms screening center was directly involved in the diagnosis of eight patients in this study. Diagnoses were related to the discovery of variants in EBF3 as a cause of neurodevelopmental disorders19 and to the discovery of a broader functional spectrum of CACNA1A alleles in early developmental delay.23 One diagnosis was related to the discovery of a de novo variant in NR5A1 in a patient with a 46,XX genotype and male sex characteristics.24 Functional studies in drosophila validated that the de novo NR5A1 p.R92W variant altered gene function (Fig. 1). Later identification of this variant in additional patients led to the characterization of a new syndrome.
Figure 1. Drosophila Study of NR5A1 p.R92W Variant.
Panels A through I show preparations of cuticles from first-instar drosophila larvae. The first column (Panels A, D, and G) shows cuticles from wild-type controls, in which green fluorescent protein was expressed during egg maturation. Eight prominent abdominal denticle belts and three faint thoracic denticle belts are visible in each panel. The second column shows cuticles from embryos with expression of human NR5A1 at six times the baseline level (Panel B), three times the baseline level (Panel E), and the baseline level (Panel H). Only four or five abdominal denticle belts are present in each panel; overexpression of NR5A1 very severely affects development and causes a phenotype that is very similar to the phenotype observed with the wild-type fly homologue (not shown). The third column shows cuticles from embryos with expression of mutant NR5A1 (NR5A1 p.R92W variant) at six times the baseline level (Panel C), three times the baseline level (Panel F), and the baseline level (Panel I). When mutant NR5A1 is expressed at the baseline level and three times the baseline level, there is no loss of denticle belts, a finding suggestive of a loss-of-function mutation. However, when mutant NR5A1 is expressed at six times the baseline level, it causes loss of some segments, a finding suggestive of a severe loss-of-function mutation but not of a complete null allele. In the first row (Panels A, B, and C), virgin maternal triple driver (MTD) GAL4 females were crossed with UAS males, contributing six doses of GAL4 to the embryo. In the second row (Panels D, E, and F), virgin MTD GAL4/+, UAS/+ females were crossed with male siblings of the same genotype, contributing three doses of GAL4. In the third row (Panels G, H, and I), virgin NGT40 GAL4/+, UAS/+ females were crossed with male siblings of the same genotype, contributing just one dose of GAL4.
METABOLOMICS TESTING
Data that were generated at the metabolomics core were used to support the diagnosis of homozygous missense variants in ATP5F1D (encoding the delta subunit of mitochondrial ATP synthase) in a patient with altered plasma levels of Krebs cycle intermediates22 and were used to confirm the diagnosis of 2,4-dienoyl coenzyme A reductase deficiency due to variants in NADK2, on the basis of elevated levels of lysine in the patient’s plasma and urine. In a patient with multisystem involvement, identification of consistently high levels of urinary organic acids suggested that a deficiency in 3-hydroxy-3-methylglutaryl coenzyme A lyase (encoded by HMGL) could account for many of the symptoms. This prompted a reexamination of the exome sequencing data, which revealed a deletion in exon 1 of HMGL. RNA sequencing revealed a 50% lower level of HMGL expression in fibroblasts from the patient than in fibroblasts from eight unaffected persons.
COST
Although detailed financial information was not available for most of the patients, we analyzed data on all billable medical procedures from a selected sample of 14 patients who had received all their health care before and during the UDN evaluation within the same health care system. Among these patients, the average cost of care before acceptance to the UDN was $198,651, and the average cost of the UDN evaluation was $15,116 (7% of the total cost). Among the patients who received a diagnosis, the average cost of care before acceptance was $305,428, and the average cost of the UDN evaluation was $18,903 (6% of the total cost). These cost estimates suggest that the UDN approach has the potential to cut short an expensive medical diagnostic odyssey, and they are consistent with recent cost-effectiveness analyses for genome sequencing.25-27
DISCUSSION
In this study, we present data on referral and acceptance patterns, diagnosis and its effect on medical care, and follow-up scientific investigations among the 1519 patients who were referred to the UDN during a 20-month period, of whom 601 were accepted for detailed evaluation. We found that, although one third of the accepted patients had previously undergone exome sequencing, the UDN established a clinical diagnosis at a rate of 35%. A specific therapy was recommended for 21% of the patients who received a diagnosis. As a result of these efforts, 31 new syndromes were identified.
By virtue of research funding, the UDN is able to perform testing and coordination in a way that would be challenging in a traditional health care delivery system. However, many features that distinguish the UDN approach could be implemented more broadly in local clinics. The first and most important feature is the wide availability of sequencing. The second is the systematic documentation of phenotype to facilitate case sharing. The third is the close communication among experts and collaborators, including extended data sharing between investigators on a virtual private cloud and more broadly on the Internet, in networks such as Matchmaker Exchange18 and on participant pages on the UDN website.28 We also encourage and help UDN patients to make use of the Internet and social-media platforms to find similar patients. In many situations, finding just one similar case can be critical in proving the causality of a putative mechanism. Finally, we collect data on the psychosocial effect of undiagnosed conditions to improve the patients’ experience and to identify resources in their communities that could facilitate further care.
One hope in demonstrating the usefulness of these approaches is to allow for their broader adoption outside the limited scope of the UDN. A critical aspect of broadening access is the need for qualified clinicians and genetic counselors. Another is the funding mechanism. Although data from this study and from others suggest that money can be saved when the medical odyssey is cut short,25,26 attention should be focused on the aspects of the model that are most likely to be scalable, since infrastructure costs for such networks are not generally supported by health care systems.
We report rates of clinical diagnosis that integrate data from all domains of the UDN. Data on diagnosis and its effect on medical care that are reported in this study reflect consensus-based decisions from clinical teams with detailed knowledge of the individual patient and family. A rate of clinical diagnosis of 35% is in line with previous reports but should be viewed in light of the 32% of referred patients who had undergone clinical exome sequencing before referral to the UDN. Overall, the rate of diagnosis in that group of patients was 33%. Diagnoses in patients who had previously undergone exome sequencing were established by several means. In some cases, the gene region had been poorly covered in previous exome sequencing or a second case came to light, which narrowed the list of potential causative variants. In other cases, the variant had previously been included on the list of variants of unknown significance, but other, newer data, such as data from trio sequencing, allowed it to rise to the top of the list of potential causative variants. A reflex to genome sequencing led to the diagnosis in some cases.
Our approach has limitations. Patients with neurologic conditions made up the majority of study applicants and enrollees. This reflects the stated mission of the UDN to enroll patients who are most likely to benefit from the UDN approach, but in turn, it limits the generalizability of insight from the UDN to patients with presentations that primarily involve other systems. The majority of patients were white and resided near major clinical sites. Active outreach to underrepresented and underserved populations continues.
In summary, the UDN represents an effort to help patients who remain without a diagnosis despite, in most cases, years of medical attention. An important issue is the extent to which this model or lessons from this network can be applied to the broader health care system. One role of the UDN should be to demonstrate or refute the usefulness of new diagnostic approaches so that the findings can be integrated into the health care system. Indeed, some of the diagnoses described in this study could be made in the community with better financial support for testing and counseling. However, other diagnoses were established with the use of additional resources of the UDN. The centralized institutional review board and infrastructure, which provide patients from across the nation with a singular gateway to a coordinated network that draws on the expertise of hundreds of specialists, were critical to many of the diagnoses.
Supplementary Material
Acknowledgments
Supported by awards from the National Institutes of Health (NIH) Common Fund, through the Office of Strategic Coordination and the Office of the NIH Director, to the clinical sites (U01HG007709, to Baylor College of Medicine; U01HG007672, to Duke University; U01HG007690, to Harvard-affiliated teaching hospitals; U01HG007708, to Stanford Medicine; U01HG007703, to the University of California, Los Angeles; and U01HG007674, to Vanderbilt University), the coordinating center (U01HG007530, to Harvard Medical School), the sequencing cores (U01HG007942, to Baylor College of Medicine; and U01HG007943, to HudsonAlpha Institute for Biotechnology), the metabolomics core (U01TR001395, to Pacific Northwest National Laboratory), and the model organisms screening center (U54NS093793, to Baylor College of Medicine) and by an NIH grant (R01GM113230, to Dr. Pick).
Footnotes
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We thank the patients and families who participated in the study, particularly those who remain without a diagnosis at this time.
A complete list of members of the Undiagnosed Diseases Network is provided in the Supplementary Appendix, available at NEJM.org.
Contributor Information
Kimberly Splinter, Harvard Medical School, Boston
David R. Adams, National Institutes of Health Clinical Center, Maryland
Carlos A. Bacino, Baylor College of Medicine, Houston
Hugo J. Bellen, Baylor College of Medicine, Houston
Jonathan A. Bernstein, Stanford University, Stanford
Alys M. Cheatle-Jarvela, University of Maryland, College Park, Maryland
Christine M. Eng, Baylor College of Medicine, Houston
Cecilia Esteves, Harvard Medical School, Boston
William A. Gahl, National Institutes of Health Clinical Center, Maryland
Rizwan Hamid, Vanderbilt University, Nashville
Howard J. Jacob, HudsonAlpha Institute for Biotechnology, Huntsville, AL
Bijal Kikani, University of Maryland, College Park, Maryland
David M. Koeller, Oregon Health and Science University, Portland
Isaac S. Kohane, Harvard Medical School, Boston
Brendan H. Lee, Baylor College of Medicine, Houston
Joseph Loscalzo, Harvard Medical School, Brigham and Women’s Hospital, Boston
Xi Luo, Baylor College of Medicine, Houston
Alexa T. McCray, Harvard Medical School, Boston
Thomas O. Metz, Pacific Northwest National Laboratory, Richland, WA
John J. Mulvihill, National Institutes of Health Clinical Center, Maryland
Stanley F. Nelson, University of California, Los Angeles, Los Angeles, California
Christina G.S. Palmer, University of California, Los Angeles, Los Angeles, California
John A. Phillips, III, Vanderbilt University, Nashville
Leslie Pick, University of Maryland, College Park, Maryland
John H. Postlethwait, University of Oregon, Eugene
Chloe Reuter, Stanford University, Stanford
Vandana Shashi, Duke University, Durham, NC
David A. Sweetser, Harvard Medical School, Boston; Massachusetts General Hospital, Boston
Cynthia J. Tifft, National Institutes of Health Clinical Center, Maryland
Nicole M. Walley, Duke University, Durham, NC
Michael F. Wangler, Baylor College of Medicine, Houston
Monte Westerfield, University of Oregon, Eugene
Matthew T. Wheeler, Stanford University, Stanford
Anastasia L. Wise, National Human Genome Research Institute, Bethesda, Maryland
Elizabeth A. Worthey, HudsonAlpha Institute for Biotechnology, Huntsville, AL
Shinya Yamamoto, Baylor College of Medicine, Houston
Euan A. Ashley, Stanford University, Stanford
REFERENCES
- 1.Gahl WA, Markello TC, Toro C, et al. The National Institutes of Health Undiagnosed Diseases Program: insights into rare diseases. Genet Med 2012;14:51–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gahl WA, Wise AL, Ashley EA. The Undiagnosed Diseases Network of the National Institutes of Health: a national extension. JAMA 2015;314:1797–8. [DOI] [PubMed] [Google Scholar]
- 3.Ramoni RB, Mulvihill JJ, Adams DR, et al. The Undiagnosed Diseases Network: accelerating discovery about health and disease. Am J Hum Genet 2017;100:185–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The Undiagnosed Diseases Network home page (https://gateway.undiagnosed.hms.harvard.edu/static/start.html).
- 5.Reuter CM, Brimble E, DeFilippo C, et al. A new approach to rare diseases of children: the Undiagnosed Diseases Network. J Pediatr 2018;196:291–297.e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Human Genome Research Institute. The cost of sequencing a human genome. July 6, 2016. (http://www.genome.gov/sequencingcosts/).
- 7.Ashley EA. Towards precision medicine. Nat Rev Genet 2016;17:507–22. [DOI] [PubMed] [Google Scholar]
- 8.Yang Y, Muzny DM, Xia F, et al. Molecular findings among patients referred for clinical whole-exome sequencing. JAMA 2014;312:1870–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee H, Deignan JL, Dorrani N, et al. Clinical exome sequencing for genetic identification of rare Mendelian disorders. JAMA 2014;312:1880–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Need AC, Shashi V, Hitomi Y, et al. Clinical application of exome sequencing in undiagnosed genetic conditions. J Med Genet 2012;49:353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bick D, Fraser PC, Gutzeit MF, et al. Successful application of whole genome sequencing in a medical genetics clinic. J Pediatr Genet 2017;6:61–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Splinter K, Hull SC, Holm IA, et al. Implementing the single institutional review board model: lessons from the Undiagnosed Diseases Network. Clin Transl Sci 2018;11:28–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.About the Undiagnosed Diseases Network (https://undiagnosed.hms.harvard.edu/).
- 14.Girdea M, Dumitriu S, Fiume M, et al. PhenoTips: patient phenotyping software for clinical and research use. Hum Mutat 2013;34:1057–65. [DOI] [PubMed] [Google Scholar]
- 15.Tryka KA, Hao L, Sturcke A, et al. NCBI’s Database of Genotypes and Phenotypes: dbGaP. Nucleic Acids Res 2014;42:D975–D979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Buske OJ, Girdea M, Dumitriu S, et al. PhenomeCentral: a portal for phenotypic and genotypic matchmaking of patients with rare genetic diseases. Hum Mutat 2015;36:931–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Landrum MJ, Lee JM, Benson M, et al. ClinVar: public archive of interpretations of clinically relevant variants. Nucleic Acids Res 2016;44(D1):D862–D868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Philippakis AA, Azzariti DR, Beltran S, et al. The Matchmaker Exchange: a platform for rare disease gene discovery. Hum Mutat 2015;36:915–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chao H-T, Davids M, Burke E, et al. A syndromic neurodevelopmental disorder caused by de novo variants in EBF3. Am J Hum Genet 2017;100:128–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shashi V, Pena LDM, Kim K, et al. De novo truncating variants in ASXL2 are associated with a unique and recognizable clinical phenotype. Am J Hum Genet 2017;100:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schoch K, Meng L, Szelinger S, et al. A recurrent de novo variant in NACC1 causes a syndrome characterized by infantile epilepsy, cataracts, and profound developmental delay. Am J Hum Genet 2017;100:343–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oláhová M, Yoon WH, Thompson K, et al. Biallelic mutations in ATP5F1D, which encodes a subunit of ATP synthase, cause a metabolic disorder. Am J Hum Genet 2018;102:494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo X, Rosenfeld JA, Yamamoto S, et al. Clinically severe CACNA1A alleles affect synaptic function and neurodegeneration differentially. PLoS Genet 2017;13(7):e1006905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bashamboo A, Donohoue PA, Vilain E, et al. A recurrent p.Arg92Trp variant in steroidogenic factor-1 (NR5A1) can act as a molecular switch in human sex development. Hum Mol Genet 2016;25:5286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Palmer EE, Schofield D, Shrestha R, et al. Integrating exome sequencing into a diagnostic pathway for epileptic encephalopathy: evidence of clinical utility and cost effectiveness. Mol Genet Genomic Med 2018;6:186–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dragojlovic N, Elliott AM, Adam S, et al. The cost and diagnostic yield of exome sequencing for children with suspected genetic disorders: a benchmarking study. Genet Med 2018. January 4 (Epub ahead of print). [DOI] [PubMed] [Google Scholar]
- 27.Tan TY, Dillon OJ, Stark Z, et al. Diagnostic impact and cost-effectiveness of whole-exome sequencing for ambulant children with suspected monogenic conditions. JAMA Pediatr 2017;171:855–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Undiagnosed Diseases Network. Our participants (https://undiagnosed.hms.harvard.edu/our-participants/).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.