Abstract
“Absence of bile ducts (BD) in >50% of portal tracts” is currently the most widely accepted criterion for the diagnosis of ductopenia. In this study, we describe an alternative method for quantitative assessment of BDs based on the percentage of portal tracts containing unpaired hepatic arteries (HA). Diagnostic criteria for ductopenia were defined as follows: 1- presence of at least one unpaired HA in ≥10% of all portal tracts; 2- at least 2 unpaired HAs present in different portal tracts in a given sample. In liver biopsies from patients with primary biliary cirrhosis and suspected chronic allograft rejection (n=32), BD loss was detected in 59.4% of patients using the unpaired HA method compared to 43.7% (P=0.31), 21.9% (P=0.005), and 12.5% (P=0.001) by the traditional method, depending on specific adequacy criteria utilized (no adequacy criteria, >10 portal tracts, or >5 complete portal tracts per biopsy, respectively). The percentage of portal tracts containing bile duct(s) was significantly affected by the degree of portal inflammation, fibrosis stage, percentage of complete portal tracts, and biopsy width, while none of these factors influenced the prevalence of unpaired arteries. Conclusion: The unpaired HA method showed higher sensitivity for the detection of mild degrees of bile duct loss compared to the traditional method and was not influenced by factors that affected the percentage of portal tracts containing BDs.
Introduction
Decreased numbers of bile ducts (BD) in peripheral portal tracts (ductopenia) is seen in a variety of liver disorders, including primary biliary diseases (primary biliary cirrhosis [PBC], primary sclerosing cholangitis [PSC]), syndromic (Alagille syndrome) and nonsyndromic paucity of bile ducts, α−1 antitrypsin deficiency, chronic ductopenic rejection, idiopathic adulthood ductopenia, among others.1–4,6,13,14,16–18,24,26 In many of these conditions, establishing the presence of ductopenia is a key step in the diagnostic process. An accurate quantitative evaluation of BDs in liver biopsies samples is, therefore, essential. Absence of BDs in greater than 50% of portal tracts in an adequate sample is currently the most widely accepted definition of ductopenia.3,4,13,17,22,25 More recent studies of normal livers, however, have shown that BDs are present in approximately 93% of all portal tracts and in 96% of portal tracts containing a hepatic artery (HA),7 indicating a significant gap between normal samples and those fulfilling current criteria for BD paucity. The current “50% rule” is largely derived from early morphometric studies of autopsy and wedge biopsies of Allagile’s syndrome1,2 and PBC patients.16 In recent years, small needle biopsies have become the most common type of liver sample seen by pathologists, and quantitative assessment of BDs in such samples is accompanied by several practical difficulties, including the presence of a significant proportion of incomplete and/or tangentially sectioned portal tracts showing no BDs, dense inflammatory infiltrates obscuring the visualization of BDs, and problems related to specimen adequacy for the evaluation of ductopenia.
In the present study, we explore the concept of hepatic artery-bile duct parallelism, whereby a close anatomic relationship exists between HA branches and BDs within portal tracts, to further characterize the potential use of HA branches as landmark structures for a more precise quantitative assessment of interlobular BDs. Specifically, we sought to build a large database of morphologic measurements of normal portal tracts, with particular attention to parameters that could be useful in quantitative analyses of BDs in biopsy samples. The goal of this study is to further characterize the normal distribution of portal tracts showing parallelism between HAs and BDs and to provide additional guidelines for the evaluation of ductopenia using HA branches as anatomic landmarks, which may be useful in the interpretation of liver biopsy samples.
Materials and methods
Case selection
This study was carried out in two phases. In phase 1, histologically normal liver resection specimens (autopsies, lobectomy specimens, and allograft donor wedge biopsies) were studied and a database of reference parameters was established, as described below. In phase 2, two groups of liver needle core biopsy samples were studied, including non-ductopenic conditions (group 1) and ductopenic disorders (group 2). The two groups in phase 2 were used to validate the ductopenia criteria established from morphometric data derived from the analysis of normal livers during phase 1.
For phase 1, ten histologically normal liver sections from ten patients were selected from our institutional database. We included five autopsy cases, three liver donor (pre-perfusion) wedge biopsies, and two hepatic right lobectomy specimens. For autopsy cases, inclusion criteria included a grossly normal liver, normal histology (minimal non-specific portal inflammation and minimal [<5%] steatosis was considered acceptable), absence of any history of primary liver or biliary disease, normal levels of bilirubin, alkaline phosphatase, glutamic oxlacetic transaminase (SGOT), and glutamic pyruvic transaminase (SGPT), and absence of significant autolytic changes. For liver donor wedge biopsies, inclusion criteria included no known history of liver disease and normal liver histology (minimal non-specific portal inflammation and minimal [<5%] steatosis was considered acceptable). Biopsies from donors with positive viral hepatitis serology (extended donor criteria) were excluded. We also included sections from four patients undergoing right hepatic lobectomy for small metastatic colon cancer lesions. For these patients, inclusion criteria included presence of a single small tumor (2 cm or less in greatest diameter), no previous chemotherapy or radiotherapy, grossly normal non-neoplastic liver parenchyma, sections taken several centimeters away from metastatic lesion (at a different hepatic segment), normal levels of bilirubin, alkaline phosphatase, glutamic oxlacetic transaminase, and glutamic pyruvic transaminase, and normal histology.
In phase 2, we selected a total of 62 liver needle core biopsy samples from our institutional database in order to further evaluate the applicability of morphometric parameters derived from resection specimens to biopsy samples. For this purpose, we divided our biopsy cases into two groups: group 1 (control)- patients with either no liver disease (normal needle core biopsies from liver transplant donors, n=10) or patients diagnosed with conditions not associated with BD loss (non-ductopenic disorders), including, chronic hepatitis C patients (n=10), steatohepatitis (n=5), and autoimmune hepatitis (n=5); and group 2- ductopenic liver diseases, including PBC cases (n=21), and clinically suspected chronic allograft rejection (n=11). PBC cases were selected based on positive anti-mitochondrial antibody and consistent histopathologic abnormalities. Clinical suspicion for chronic rejection was based primarily on persistent cholestatic pattern of liver enzymes (predominant bilirubin and alkaline phosphatase elevation). Quantitative analysis of bile ducts was not used as a selection criterion for patients in either group. Demographic information for the two groups of patients is summarized in Table 1.
Table 1.
Patient demographic information, biopsy specimens.
| Group |
n |
Age |
Gender |
Portal inflam.* |
Stage* |
Clinical features |
|---|---|---|---|---|---|---|
| Donor | 10 | 45 (28–59) | M=5, F=5 | 0 (0–0) | 0 (0–0) | |
| HCV | 10 | 51 (37–66) | M=6, F=4 | 2 (1–2) | 3 (1–4) | All HCV RNA positive, untreated. |
| SH | 5 | 52 (39–65) | M=5, F=5 | 1 (0–2) | 3 (0–4) | |
| AIH | 5 | 32 (14–42) | M=3, F=7 | 2 (2–3) | 3 (0–4) | ANA positive (n=9). SMA positive (n=1) |
| PBC | 21 | 60 (38–76) | M=0, F=21 | 2 (0–3) | 2 (0–3) | AMA positive, titer > 1:160 (n=21). |
| CR | 11 | 43 (23–58) | M=6, F=5 | 1 (0–2) | 2 (0–4) | Clinically suspected chronic rejection based on persistent alkaline phosphates and/or bilirubin elevation (9/11), posttrasnplantation period (>6 months, n=10/11), and history of multiple previous episodes of acute cellular rejection (n=10/11). |
Abbreviations: n, number of patients; M, male; F, female; HCV, hepatitis C virus; RNA, ribonucleic acid; SH, steatohepatitis; AIH, autoimmune hepatitis; ANA, anti-nuclear antibody; PBC, primary biliary cirrhosis; AMA, anti-mitochondrial antibody; CR, chronic rejection.
Data presented as median (range).
Data collection
For resection specimens and wedge biopsies (phase 1), an area of approximately 1 cm2 was demarcated on each slide and high-resolution digital imaging was obtained of 50 contiguous portal tracts within this area, regardless of size, orientation, or presence of specific portal structures. The three liver donor wedge biopsies combined yielded 50 portal tracts, counted in a similar fashion. Digital images were then obtained at a magnification that ranged from 100X to 400X, depending on the size and orientation of individual portal tracts. Multiple images taken at different magnifications were occasionally necessary for large or longitudinally sectioned portal areas. A total of 500 normal portal tracts were studied in this initial part of the study. The presence and number of portal structures (HAs, BDs, and portal veins) were recorded before digital imaging was obtained. Subsequently, digital imaging was used to assess the following: portal tract size, portal tract orientation, diameter of bile duct(s), diameter of HA(s), HA orientation, and distance from each HA profile to closest interlobular BD. Bile ductules and canals of Hering were not counted. Portal tracts were labeled as subcapsular if located within 2 mm of the Glisson’s capsule. All other portal tracts did not receive any special designation.
For biopsy specimens (phase 2), the following information was collected: length and width of biopsy core(s), total number of portal tracts, percentage of complete and incomplete portal tracts, presence of BD and HA profile(s), and stage (using Ludwig’s system for PBC cases and modified Batts-Ludwig system for all other cases). Degree of portal inflammation was scored 0–3 (grade 0, no significant portal inflammation; grade 1, mild inflammation in any number of portal tracts; grade 2, dense inflammation present in a minority of portal tracts; grade 3, dense inflammation present in the majority of portal tracts). Dense inflammation was defined as an infiltrate that either filled or expanded portal tracts; otherwise, the infiltrate was considered mild. At least 3 hematoxylin and eosin (H&E) levels and a Masson’s trichrome stained slide were reviewed in each case.
Definitions
Hepatic artery: defined by the presence of smooth muscle cells (tunica media), surrounding an endothelium-lined luminal space. In round profiles, arterial size was measured at the point of maximum external diameter from the exterior aspect of the tunica media. In oval, elliptical, or irregular profiles, the arterial size was determined by measuring the maximal transverse, rather than longitudinal, external diameter of the vessel (Figure 1A).
Figure 1.
A - Portal tract (yellow) and portal structures (hepatic artery, red line; bile duct, green line) and hepatic artery-bile duct distance (black line) are measured as illustrated above. Note the presence of ductules located at the periphery of the portal tract (arrows) (hematoxylin and eosin, 400X magnification). B- In incomplete portal tracts, a hepatic artery branch >20 μm can be considered “unpaired” (arrow) if a bile duct is not present within a radius of 10 hepatic artery diameters from the edge of the arterial profile, as illustrated. In the example above, the arterial profile located at the center of the biopsy core can be labeled as “unpaired”, while the artery closer to the biopsy edge cannot. Abbreviations: HA, hepatic artery; PV, portal vein (Trichrome stain, 400X magnification).
Bile duct: defined as tubular structures within the portal tracts, formed by cuboidal or short columnar epithelial cells with an open lumen, continuously surrounded by connective tissue (i.e. not in direct contact with hepatocytes at the limiting plate), generally measuring > 15–20 μm in diameter or roughly equivalent in size to its accompanying HA branch.7,15,19,21 In very small portal tracts, a centrally located ductal structure with an accompanying artery of similar diameter was considered a duct, rather than a ductule, regardless of size.7 In round profiles, duct size was measured at the point of maximum external diameter from the exterior aspect of the epithelium, at the level of the basement membrane. In oval, elliptical, or irregular profiles, duct size was determined by measuring the maximal transverse, rather than longitudinal, external diameter (Figure 1).
Bile ductule: Defined as tubular profiles located at the periphery of the portal tracts, generally measuring < 15–20 μm in diameter and/or in direct contact with hepatocytes at the limiting plate.7,15,19,21
Hepatic artery and bile duct orientation: round or slightly oval arterial or ductal profiles were considered well-oriented (transverse section); elliptical or elongated profiles not cut longitudinally were considered partially oriented (oblique sections); and markedly irregular, longitudinal, or multiple profiles in a linear distribution were classified as poorly oriented (longitudinal sections).
Portal tract: foci within the parenchyma containing connective tissue and at least one embedded luminal structures (BD, artery, or vein), each with a continuous area of surrounding connective tissue. The presence of connective tissue was especially relevant in portal tracts containing a single structure (referred to as “monads”, as described by Crawford et al.7). The precise delineation of portal spaces in advanced fibrosis and cirrhosis was often challenging. In difficult cases, we assessed the number of portal tracts using portal structures (especially hepatic artery branches) as anatomic landmarks. Each hepatic artery profile, or group of tangentially cut profiles within the same fibrous area, usually accompanied by at least one other portal structure and surrounding fibrous tissue, was regarded as one portal tract. The diameter of portal tracts (μm) was measured at the point of maximum caliber in the transverse axis, in order to minimize the effects of oblique or tangential sectioning.
Portal tract orientation: each portal tract profile was classified as well-oriented, partially oriented, or poorly oriented, based on a combination of the following criteria: 1- portal tract contour orientation (longitudinal diameter measures less than twice the length of the transverse diameter); 2 – orientation of BD or HA profile (as defined above). Each portal tract was classified as: a) well oriented, when meeting both criteria; b) partially oriented – when meeting only one but not both criteria; and c) poorly oriented – when neither criteria are met.
Complete portal tract: a portal tract completely surrounded by hepatocytes in a biopsy sample (i.e, the entire circumference of the portal tract is present within the biopsy core).
Incomplete portal tract: a portal tract not completely seen in a biopsy sample (i.e., portal tract connective tissue and/or portal structures present at the edge of biopsy).
Unpaired hepatic artery: a) a HA branch of any size within a complete portal tract not accompanied by a BD; b) in an incomplete portal tract, a HA branch measuring > 20 μm with no bile duct present within a radius of 10 HA diameters (Figure 1B). If multiple hepatic arteries are present, any individual arterial profile within a given portal tract may qualify as “unpaired” provided the above conditions are met. For the purposes of this study, small HA branches (generally <15–20μm) were not considered unpaired if accompanied by any ductal structure of similar size, even if the latter showed some features characteristic of ductules (as defined above).
Hepatic artery-bile duct distance: in order to measure this variable, the largest HA-BD distance in an individual portal tract (in case of multiple HAs and/or BDs) was identified. This distance was then measured (μm), from the external aspect of the HA nearest to the BD to the level of the basement membrane of the bile duct opposite to the HA, using the diameter of the arterial profile located the furthest away from its nearest BD (Figure 1A).
Hepatic artery-bile duct parallelism: presence of at least one BD profile in a portal tract containing a HA, regardless of the distance between these structures or their size.
Statistical analysis
Continuous data were described using mean and standard deviation and compared using the Student’s t-test. Proportions were compared using chi-square test. All probability values were 2-tailed, and a P value <0.05 was considered statistically significant. Statistical analysis was performed using the SPSS for Windows statistical package.
Results
Basic morphometric data and rates of parallelism in histologically normal liver resection and wedge biopsy specimens (phase 1)
Morphologic analysis of 500 portal tracts of normal livers (autopsy, resection, and wedge biopsy specimens) revealed presence of at least one HA profile and at least one BD profile in 89.9% and 93.8% of portal tracts, respectively. An average of 1.49 ± 0.9 HA and 1.46 ± 0.8 BD profiles were present per portal tract. Poorly oriented portal tracts tended to have a larger number of profiles of any portal structure compared to those with better orientation on histologic sectioning. The rate of HA-BD parallelism for portal tracts containing at least one HA profile was 96.6% for all 500 portal tracts (746 arteries). The rate of HA-BD parallelism did not change significantly according to portal tract orientation and showed little variation among the twelve samples taken from ten different patients (94–98%). Morphometric data obtained from histologically normal liver resection samples is summarized in Table 2.
Table 2.
Morphometric data of normal liver samples.
| All portal tracts |
Well-oriented PTs |
Partially oriented PTs |
Poorly oriented PTs |
P value* |
|
|---|---|---|---|---|---|
| Triadsa | 80% (401/500) | 85.1% (160/188) | 81.5% (141/173) | 70.5% (98/139) | P=0.002 |
| Diadsa | 15% (75/500) | 10.1% (19/188) | 13.3% (23/173) | 25.8% (36/139) | P=0.007 |
| Monadsa | 5% (24/500) | 4.7% (9/188) | 5.8% (10/173) | 3.5% (5/139) | P>0.05 |
| % HA-BD parallelism (when HA present)a | 96.6% (483/500) | 97.9% (184/188) | 97.1% (168/173) | 93.5% (130/139) | P>0.05 |
| % HA-BD parallelism per patient | 94–98% | – | – | – | – |
| HA w/o BDa | 3.4% (17/500) | 2.1% (4/188) | 2.9% (5/173) | 5.7% (8/139) | P>0.05 |
| BD w/o HAa | 7.6% (38/500) | 3.2% (6/188) | 8.1% (14/173) | 13.0% (18/139) | P=0.001 |
| Mean # of structuresb | 4.28 ± 2.58 | 3.86 ± 1.4 | 4.28 ± 1.9 | 4.79 ± 2.3 | P<0.0001 |
| Mean # HA profilesb | 1.49 ± 0.9 | 1.47 ± 0.9 | 1.49 ± 1.0 | 1.51 ± 1.0 | P>0.05 |
| Mean # BD profilesb | 1.46 ± 0.8 | 1.26 ± 0.6 | 1.46 ± 0.8 | 1.7 ± 1.0 | P<0.0001 |
| Mean # PV profilesb | 1.32 ± 0.8 | 1.13 ± 0.6 | 1.32 ± 0.7 | 1.56 ± 1.0 | P<0.0001 |
| Mean HA diameterb | 28 ± 18.4 | – | – | – | – |
| Mean BD diameterb | 26 ± 12.5 | – | – | – | – |
| Mean HA-BD dist. (μm) b | 98.8 ± 75.86 | 91.7 ± 55.2 | 101.6 ± 72.4 | 122.7 ± 99.9 | P<0.0004 |
| Mean HA-BD dist. (HADs) b | 4 ± 2.8 | 3.7 ± 2.8 | 4.1 ± 2.4 | 4.9 ± 2.9 | P<0.0002 |
| PT size (um) b | 163 ± 92.1 | 160 ± 82.0 | 158 ± 74.687.2% | 172 ± 120.686.3% | P>0.05 |
| % HA present a | 89.8%(449/500) | 95.2% (179/188) | 87.2% (151/173) | 86.3% (120/139) | P=0.008 |
| % BD present a | 93.8% (469/500) | 95.2% (179/188) | 93.0% (161/173) | 92.8% (129/139) | P>0.05 |
| % PV present a | 91.8% (459/500) | 90.4% (170/188) | 95.3% (165/173) | 89.2% (124/139) | P>0.05 |
| BD/PT ratio | 1.4 | 1.2 | 1.4 | 1.6 | – |
| BD/HA ratio | 0.97 | 0.8 | 0.9 | 1.1 | – |
| BD/PT ratio per patient | 1.34–1.6 | – | – | – | – |
| BD/HA ratio per patient | 0.81–1.23 | – | – | – | – |
Abbreviations: PT, portal tract; HA, hepatic artery; BD, bile duct; HAD, hepatic artery diameter; PV, portal vein.
data expressed in percentage and absolute numbers.
data presented in form of mean ± standard deviation.
P value refers to difference between well oriented and poorly oriented portal tracts.
Normal liver sections obtained from lobectomy specimens performed for single small metastatic colorectal carcinoma lesions did not significantly differ from autopsy or liver donor samples in regard to percentage of portal tracts containing BD, HA-BD parallelism, or any other features addressed in this study. A total of 17 portal tracts containing a HA branch without an accompanying BD were identified among 500 portal tracts in normal livers (3.4%).
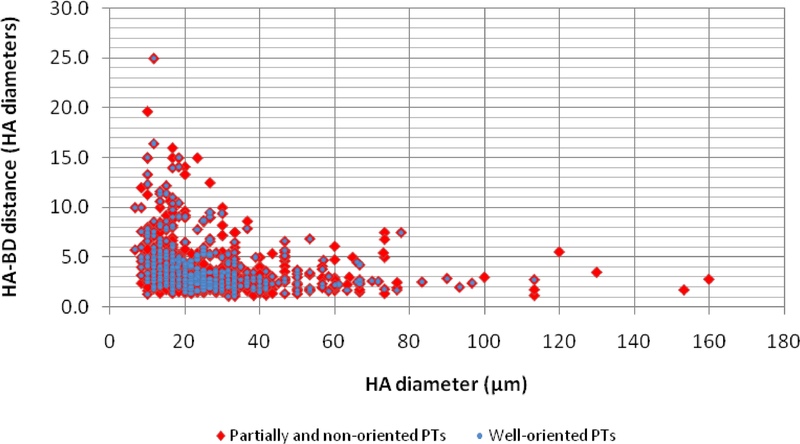
Knowledge about the expected distance between HAs and BDs may be important in the interpretation of incomplete portal tracts in liver biopsy specimens. The mean HA-BD distance for all portal tracts was 4 ± 2.8 HA diameters (or 98.8 ± 75.86 μm). For arterial profiles > 20 μm, the average HA-BD distance was 3.3 ± 2.0 HA diameters. In this group of arteries, when at least one BD was present, the nearest ductal profile was located within 10 HA diameters from any given artery in 99.1% of cases. Although poor portal tract orientation increased the average HA-BD distance, nearly all measurements still lay within 10 HA diameters, as seen in well oriented portal tracts. Significantly greater variation was observed for smaller sized arteries (mean 5.1 ± 3.5 HADs), with nearly 1 out of 10 BDs being located > 10 HA diameters from its nearest artery (Table 2).
Although portal tracts containing HAs unaccompanied by BDs tended to be smaller than other portal tracts, the average arterial diameter was similar. No significant difference in rates of parallelism was seen in subcapsular portal tracts (within 2 mm of the liver capsule) compared to all others. Arterial branches unaccompanied by ductal structures were frequently seen adjacent to the liver capsule, embedded in the subcapsular connective tissue. Since these arteries were not located within the hepatic parenchyma, or within a portal tract, they were not considered unpaired arteries as defined in this study. In one biopsy from a patient with NASH (group 1), a single “arterialized” vessel was present in a centrilobular region, surrounded by prominent fibrosis. The centrilobular location of this vessel was recognized on routine stains and confirmed with glutamine synthetase immunohistochemistry, which highlights centrilobular hepatocytes. This vascular structure likely represents an arterialized central vein and was not counted as an unpaired HA branch.
Morphometric analysis of liver needle biopsy samples (phase 2)
In group 1 (30 needle biopsy cores, 452 portal tracts) the average biopsy length was 16.1 mm (range, 8–38 mm) and the average width was 0.87 mm (range, 0.5–1 mm). The average number of portal tracts per biopsy was 15.1 (range, 8–28). In average, only 33.1% (range 0–64.7%) of portal tracts were complete, representing an average absolute number of 5.1 (range, 0–16) per biopsy. In group 2 (32 needle core biopsies, 337 portal tracts), the average length of biopsies was 15.7 cm (range 7–35 cm) and the average width was 0.87 mm (range 0.5–1 mm). The average number of portal tracts per biopsy was 10.5 (range 4–32). As in group 1, only a minority of portal tracts (average 40 ± 20.7%) were complete, representing an absolute number of 4.2 (range, 0–16) per biopsy. At least one BD profile was present in 82.8 ± 14% of portal tracts in group 1 and in 53.7 ± 27.2% in group 2 (P<0.0001) (Table 5). For groups 1 and 2 combined, the percentage of biopsies containing at least 5, 10, 15, or 20 portal tracts for all biopsies was 98.4%, 61.2%, 33.8%, 16.1% respectively, if all portal tracts are included, and 37%, 11.2%, 3.2%, and 0% if only complete portal tracts are considered.
Table 5.
Proposed diagnostic criteria for ductopenia in liver needle biopsy samples.
| 1) Unpaired hepatic arteries* present in at least 10% of portal tracts. |
| 2) At least 2 unpaired hepatic arteries present (in different portal tracts) in a given sample, regardless of the total number of portal tracts. |
Unpaired hepatic artery: a hepatic artery branch of any size within a complete portal tract not accompanied by a bile duct; or, in an incomplete portal tract, a hepatic artery branch measuring > 20 μm with no bile duct present within a radius of 10 hepatic artery diameters.
In group 1, an average of 0.83% (range 0–5.8%) of portal tracts contained at least one unpaired artery, as defined in this study, or 0.17 (range 0–1) portal tracts containing at least one unpaired artery per biopsy. In contrast, this number was significantly higher (21.5% [range 0–62.5%], or 2.2 [range 0–8] unpaired arteries per biopsy, P<0.0001) in group 2 (Table 3). Although the majority of unpaired arteries were present within complete portal tracts, 28.9 % (22/76) were located within incomplete portal tract profiles. In the control group, the prevalence of unpaired arteries in complete and incomplete portal tracts was not significantly different to that seen in the normal liver resection group (2.1% and 0.4%, respectively, versus 3.4%, P>0.10).
Table 3.
Morphometric data of liver biopsy samples.
| Group 1 (n=30) (mean ± SD) |
Group 2 (n=32) (mean ± SD) |
P value |
|
|---|---|---|---|
| Biopsy length (mm) | 16.1 ± 7.1 | 14.5 ± 7.1 | P>0.05 |
| Biopsy width (mm) | 0.87 ± 0.2 | 0.81 ± 0.1 | P>0.05 |
| # of PTs | 15.1 ± 5.3 | 10.5 ± 5.7 | P>0.05 |
| % complete PTs | 33.1 ± 20.9 | 40 ± 20.8 | P>0.05 |
| # complete PTs | 5.1 ± 4.1 | 4.2 ± 3.3 | P>0.05 |
| PT inflammation | None-mild:50% | None-mild:53.2% | P>0.05 |
| Mod-sev: 50% | Mod-sev: 46.8% | P>0.05 | |
| Stage | 0–1: 40% | 0–1: 40.6% | P>0.05 |
| 2–3: 60% | 2–3: 59.4% | P>0.05 | |
| % compl. PTs w/ BDs | 95.6 ± 9.5 | 51.2 ± 32.9 | P<0.0001 |
| % incompl. PTs w/ BDs | 76.6 ± 19.6 | 53.3 ± 29.6 | P<0.0001 |
| % PTs w/ BDs (all) | 82.8 ± 14 | 53.7 ± 27.2 | P<0.0001 |
| Donor | 91.8 ± 8.2 | – | |
| Hep C | 74 ± 12.2 | – | |
| SH | 88.9 ± 16.4 | – | |
| AIH | 76.5 ± 13 | – | |
| PBC | – | 57.1 ± 24.2 | |
| CR | – | 47.2 ± 32.4 | |
| % complete PTs w/ unpaired artery | 2.1 ± 7.1 | 36.8 ± 34.3 | P<0.0001 |
| % incomplete PTs w/ unpaired artery | 0.4 ± 1.9 | 8.8 ± 13.6 | P<0.0001 |
| % of all PTs w/ unpaired hepatic artery | 0.83 ± 1.9 | 21.5 ± 18.4 | P<0.0001 |
| Donor | 1.6 ± 2.6 | – | |
| Hep C | 0.4 ± 1.4 | – | |
| SH | 0.9 ± 2 | – | |
| AIH | 0 | – | |
| PBC | – | 19.6 ± 15.7 | |
| CR | – | 25.2 ± 23.2 |
Abbreviations: SD, standard deviation; PT, portal tract; BD, bile duct; Mod, moderate; Sev, severe; Hep C, hepatitis C; SH, steatohepatitis; AIH, autoimmune hepatitis; PBC, primary biliary cirrhosis; CR, chronic rejection.
Portal inflammation
The degree of portal inflammation influenced the identification of BDs in the control group. In group 1, the percentage of portal tracts containing at least one BD was 89.8% for biopsies showing no or mild portal inflammation and 74.8% for biopsies with moderate or severe portal inflammation (P<0.0001). A statistically significant difference was also seen between the latter two subgroups if only complete portal tracts were analyzed (99.3% versus 91.3%, respectively, P=0.02) (Table 4). The effect of portal inflammation on identification of BDs did not reach statistical significance in group 2. The percentage of portal tracts containing unpaired HAs was not affected by the degree of portal inflammation in neither group 1 nor group 2.
Table 4.
Histologic features that may influence the % of portal tracts containing bile ducts in biopsy samples.
| % Complete PTs | % PTs w/ BDs | % PTs w/ unpaired hepatic artery |
|---|---|---|
| Portal inflammation | ||
| Group 1: | 82.8 ± 13.9% | 0.83 ± 1.91% |
| None-mild | 89.8 ± 11.8% | 1.0 ± 2.1% |
| Mod-severe | 74.8 ± 11.9% | 0.64 ± 1.6% |
| P=0.001 | P>0.05 | |
| Group 2: | 53.7 ± 27.1% | 21.5 ± 18.4% |
| None-mild | 57 ± 29.1% | 21.3 ± 21% |
| Mod-severe | 50 ± 25.2% | 21.7 ± 15.6% |
| P>0.05 | P>0.05 | |
| Fibrosis stage | ||
| Group 1: | 82.9 ± 14% | 0.8 ± 1.9% |
| 0–2 | 89.7 ± 10.8% | 0.7 ± 1.9% |
| 3–4 | 76 ± 13.6% | 1,0 ± 2.0% |
| P=0.004 | P>0.05 | |
| Group 2: | 53.8 ± 27.1% | 21.5 ± 18.4% |
| 0–2 | 55.2 ± 26.4% | 23 ± 19.4% |
| 3–4 | 45.8 ± 33.2% | 14 ± 10.2% |
| P>0.05 | P>0.05 | |
| Biopsy width | ||
| Group 1: | 82.9 ± 14% | 0.8 ± 1.9% |
| ≥1mm | 88.8 ± 11.2% | 0.7± 1.9% |
| <1mm | 76.9 ± 14.2% | 1,0 ± 2.0% |
| P=0.01 | P>0.05 | |
| Group 2: | 53.8 ± 27.2% | 21.6 ± 18.4% |
| ≥1mm | 62.4 ± 20.4% | 14.4 ± 11.2% |
| <1mm | 49.8 ± 29.3% | 24.8 ± 20.3% |
| P>0.05 | P>0.05 | |
| % Complete portal tracts | ||
| Group 1: | 82.9 ± 14% | 0.8 ± 1.9% |
| >40% | 94.8 ± 8.7% | 1,0 ± 2.1% |
| ≤40% | 76.4 ± 12.3% | 0.8 ± 1.8% |
| P<0.0001 | P>0.05 | |
| Group 2: | 53.7 ± 27.1% | 21.5 ± 18.4% |
| >40% | 52.3 ± 32.1% | 24.9 ± 20.2% |
| ≤40% | 55.1 ± 23.0% | 18.6 ± 16.8% |
| P>0.05 | P>0.05 |
Abbreviations: PT, portal tract; BD, bile duct
Fibrosis stage
In the control group, a significantly lower percentage of portal tracts containing BDs was observed in biopsies with advanced fibrosis (stages 3 and 4) compared to biopsies with no or mild fibrosis (stages 0–2) (76% and 89.7%, respectively, P=0.004), while no significant difference was observed in group 2. The prevalence of unpaired arteries was not affected by the degree of fibrosis in either group (Table 4).
Percentage of complete PTs
Among all biopsy samples analyzed, only 35.3% of portal tracts were complete. In group 1, biopsies with high percentage of complete portal tracts (>40%) had a significantly higher percentage of portal tracts containing BDs compared to biopsies with few complete PTs (≤40%) (94.8 versus 76.4%, respectively, P<0.0001). The prevalence of portal unpaired HA was not influenced by percentage of complete portal tracts (Table 4).
The percentage of complete portal tracts was lower in biopsies with moderate to severe portal inflammation (mean 30 ± 16.1%) compared to biopsies with no to mild portal inflammation (mean 40.4 ± 24.3%) (P=0.07) and biopsies showing advanced fibrosis – stages 3 and 4 (mean 24.9 ± 16.6%) compared to biopsies showing early or no fibrosis – stages 0–2 (mean 42.8 ± 19.9%) (P=0.01), presumably due to expansion of portal tracts secondary to inflammation and fibrosis, respectively. The mean percentage of complete portal tracts in biopsies measuring less than one millimeter was 28.8 ± 17.3% compared to 36.8 ± 24% in those measuring 1mm or more (P=0.3).
Biopsy width
In group 1, a greater percentage of portal tracts contained at least one BD in biopsies measuring 1mm or more in width (88.8%) compared to narrower biopsies (76.9%) (P=0.01), while no statistically significant difference was observed in the prevalence of unpaired arteries. The impact of biopsy width on percentage of portal tracts containing ducts was not significant in group 2 (Table 4).
Proposed diagnostic criteria for ductopenia
Based on data collected from histologically normal liver resection samples, we defined the concept of “unpaired HA”, to be used in the analysis of liver core biopsies. We also hypothesized that knowledge about the expected HA-BD distance may be useful when dealing with incomplete portal tracts in liver needle biopsy samples. The average distance between these structures was 4 ± 2.8 HA diameters (98.8 ± 75.8μm). This distance was shortest in well oriented portal tracts and longest in those with poor orientation (3.7 versus 4.9 HA diameters, respectively, P<0.001). Regardless of portal tract orientation, however, HA-BD distance was consistently below 10 HA diameters for HAs measuring >20μm but varied greatly for smaller sized arteries (Figure 2). Based on these observations, as outlined in methods, the concept of “unpaired HA” was defined.
Figure 2:
Hepatic artery-bile duct (HA-BD) distance, measured in hepatic artery diameters (HADs), according to size of hepatic artery in 500 portal tracts (PTs). For arterial profiles > 20 μm, nearly all HA-BD pairs are located within within 10 HADs from each other, while a greater variation exists for smaller arteries. A slightly more cohesive distribution of HA-BD distances is seen among well-oriented portal tracts (blue) compared to partially oriented and unoriented tracts (red).
Since the highest value for percentage of portal tracts showing at least one unpaired artery in the control biopsy group was 5.8%, a cutoff value of 10% was stipulated as diagnostic of BD paucity in this study. Presence of at least two unpaired HAs in separate portal tracts was also included as a criterion ductopenia. The latter requirement was adopted in order to avoid the theoretical risk that a single unpaired HA might be present in a sample with few portal tracts and, by itself, be diagnostic of ductopenia, although this situation was not encountered in any of the control cases in our study. The diagnostic criteria for ductopenia proposed in this study are summarized in Table 5. The application of the unpaired HA method is exemplified in Figure 3.
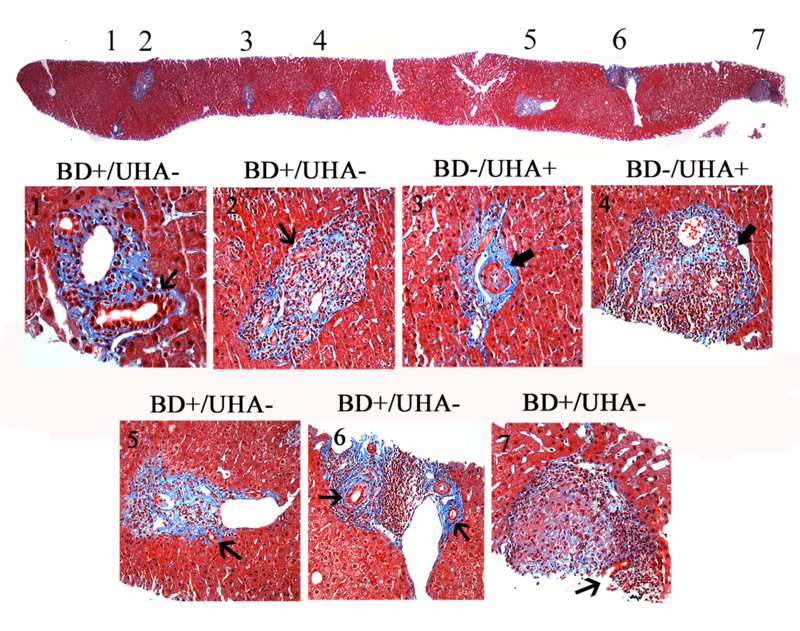
Figure 3:
The application of the unpaired hepatic artery method in the diagnosis of bile duct loss is illustrated above. In this biopsy from a patient with stage 1 primary biliary cirrhosis, 5 of 7 portal tracts contain at least one bile duct (thin arrows), therefore showing no evidence of bile duct loss by the traditional method. However, two of 7 portal tracts (28.5%) contain unpaired hepatic arteries (UHA) (broad arrows). Portal tract #3 is complete (present in its entirety in the biopsy core) and contains UHAs. Portal tract #4 is incomplete (cut at the biopsy edge) and contains one arterial profile (broad arrow) that qualifies as “unpaired” (>20μm, with at least 10 hepatic artery diameters of surrounding tissue). The presence of two or more portal tracts (≥ 10% of all tracts in the sample) containing an unpaired artery is indicative of bile duct loss by the unpaired hepatic artery method. Abbreviations: BD, bile duct; UHA, unpaired hepatic artery. Masson’s trichrome (20X magnification, top image; 400x remaining images).
Subgroup analysis using current diagnostic criteria versus the “unpaired hepatic artery” method
We compared the percentage of patients fulfilling criteria for ductopenia using the current “50% rule” with that obtained by applying our proposed criteria. Since the “50% rule” derives from resection/autopsy samples,1 no specific recommendations exist in regards to how incomplete portal tracts containing no BDs (which represented 23.3% and 46.6% of all portal tracts in groups 1 and 2, respectively) should be interpreted. Likewise, no universally accepted adequacy criteria to be used in this setting are available. For these reasons, the “50% rule” was applied according to different combinations of the most commonly cited recommendations. None of the biopsies in the control group met criteria for ductopenia by either the “50% rule” or by the unpaired artery system. Both systems, therefore, had 100% specificity in our study. In group 2, 59.4% (19/32) of cases met criteria for BD paucity using the unpaired HA system compared to 43.7% (14/32) and 31.2% (10/32) by the traditional method when all portal tracts or only complete portal tracts, respectively, are taken into account. If at least 10 portal tracts or 5 complete portal tracts are required for a sample to be considered adequate, only 21.8% (7/32) and 15.6% (5/32) of biopsies, respectively, would be considered diagnostic by the traditional method (Table 5). Within group 2, biopsies of PBC patients were positive for ductopenia in 57.1% (12/21) of cases using the unpaired artery criteria, compared to 38% (8/21), 19% (4/21), and 9.5% (2/21) by the traditional criterion, using any number of portal tracts, at least 10 portal tracts, or at least 5 complete portal tracts, respectively, as requirement for adequacy. Similar values were seen in the liver transplantation group: 63.6% (7/11) by the new method, compared to 54.5% (6/11), 27.2% (3/11), and 27.2% (3/11) by the traditional method, depending on the adequacy criterion utilized. Unpaired arteries present within incomplete portal tracts were necessary for diagnosis of ductopenia in the majority (10 of 19) of cases diagnosed by our method.
Patient follow-up
Follow-up information was available for 19 of 21 patients with PBC (mean follow-up time of 49.1 months, range: 10–133 months) and for all 11 posttransplantation patients with suspected chronic rejection (mean follow-up time of 11.6 months, range: 1–24 months). Among PBC patients, 21% (4/19) required liver transplantation during follow-up but none of the patients died, while in the posttransplantation group no re-transplantation procedures were performed but a 54.5% mortality rate was observed. Of the four patients with PBC requiring liver transplantation (mean follow-up time of 30.7 months, range: 12–64 months), two were diagnosed with ductopenia by the traditional method and three by the unpaired HA method on their last pre-transplantation biopsy. In the posttranplantation group, all patients who died met criteria for ductopenia using both the traditional method and the unpaired HA method (mean post-biopsy follow-up of 12 months, range: 1–29 months).
Discussion
Alagille and colleagues,1 in 1975, described a peculiar condition affecting young children, characterized by several malformations and developmental abnormalities. Of particular interest was the fact that this disease, later known as Alagille’s syndrome, was associated with a severe cholestatic liver disease and was histologically characterized by “hepatic ductular hypoplasia”, or paucity of BDs. In their original description, the authors noted that the ratio of interlobular BDs to portal areas in these patients was between 0 and 0.4, compared to 0.9 to 1.8 in normal children.1,2,12 Although no details are available regarding the normal population included in this study, these reference values (or slight variations thereof) have since gained wide acceptance in the diagnosis of BD paucity. Presence of BD in less than 50% of portal tracts in a given sample (i.e. “50% rule”), albeit somewhat arbitrary,25 is currently the most widely used criterion for diagnosis of ductopenia in all age groups.3,4,13,17,22,25,26
Nakanuma et al.16 reported the rate of parallelism between HAs and BDs in normal livers to be approximately 70–80%. For the purposes of that study, the two structures had to be located within a distance of three arterial diameters from each other in order to constitute a pair. Using slightly more lenient criteria, Crawford et al.7 defined a HA-BD pair as the coexistence of the two structures in the same portal tract within 100μm from each other. The authors identified a bile duct in 96% of portal tracts containing a HA. In our study, parallelism was defined as the presence of an artery and a duct within a portal tract, regardless of the distance between them. The rate of HA-BD parallelism was 96.6% (483/500 portal tracts), varying from 94%−96% among the twelve normal tissue sections analyzed in the first part of the study. Parallelism rates decreased slightly with lack of orientation of individual portal tracts on histologic sectioning (94.2%, 97.1%, and 97.9% in unoriented, partially oriented, and well-oriented portal tracts) (Table 2). In general, the rate of HA-BD parallelism was high (similar to that reported by Crawford et al.) and no specific characteristic of portal tracts seemed to influence the degree of parallelism to any significant extent. No significant variation in the rate of parallelism was seen among different patients included in this study (age range: 37–84 years) (Table 2).
Unpaired hepatic artery method and its application
Based on the morphometric features of normal livers described above, we defined specific criteria that would potentially identify even mild degrees of BD loss based on greater than expected prevalence of portal unpaired HA in needle biopsies from patients diagnosed with ductopenic disorders. Given the very low numbers of complete portal tracts encountered in average needle biopsy samples, the concept of “unpaired HA”, was expanded to also include a subset of arterial profiles present in incomplete portal tracts. Even when present within incomplete portal tracts, certain arterial branches, when unaccompanied by BDs, can be presumed to represent unpaired arteries, as defined in this study, if sufficient amount of tissue is present around the arterial profile. More specifically, a cutoff value of 10 HA diameters was chosen because it encompasses the expected distance between >99% of all HA-BD pairs and provides a high degree of certainty regarding the absence of a BD within a given portal tract when the latter is not present within this radius from a HA profile (Figure 2). The minimal diameter necessary for an arterial profile to be taken into account in this setting is 20μm, or roughly the diameter of 3 red blood cells, as smaller vessels often accompany bile ducts located >10 HA diameters away and cannot confidently be labeled as “unpaired” when seen in incomplete tracts. In addition to their spatially erratic relationship with interlobular BDs, very small arterial branches often accompany very small ductal structures that may be difficult to differentiate from ductules. Identification of unpaired HAs within incomplete portal tracts represented an essential component of our method, as the latter finding was necessary in the majority (10/19) of cases meeting criteria for ductopenia according to our proposed set of rules. Therefore, careful consideration of arterial vessels in incomplete portal tracts proved useful, provided that arterial size and distance to closest biopsy edge are observed.
A cutoff value of 10% of portal tracts containing at least one unpaired HA was chosen as a diagnostic criterion in light of the fact that, in the control group, an average of only 0.83 ± 1.9% of the HAs were classified as unpaired (mean of 0.17 ± 0.38 unpaired artery per biopsy [range 0–1]). Therefore, a non-ductopenic sample would be extremely unlikely to show a prevalence of unpaired arteries of 10% or greater. The highest percentage of portal tracts containing unpaired arteries of all biopsies in our control group (n=30) was 5.8% (one unpaired artery). The second criterion, requiring the presence of at least 2 unpaired arteries, was included to avoid a diagnosis of ductopenia based on abnormal findings in a single portal tract (i.e. one portal tract containing an unpaired artery representing > 10% of portal tracts), although this event was not observed in any of our control cases (Table 5).
In group 2, 43.7% of biopsies were diagnostic for ductopenia using “absence of BDs in >50% of portal tracts” as the sole criterion, with no further specifications. Application of the criterion above yielded a specificity of 100% in this group, although a large percentage of samples did not fulfill adequacy criteria by current standards, as discussed below. Sensitivity increased at the expense of positive results in the control group even with only slightly higher cutoff values (60% or higher). The traditional method was associated with significantly lower sensitivity (as low as 15.6%) for detection of BD loss if adequacy criteria were observed. Using the unpaired HA method, 59.4% (19/32) of cases in group 2 showed an increased prevalence of unpaired arteries (diagnostic for ductopenia)(Table 5), while none of the cases in group 1 were classified as positive.
The main objective of this study was to compare two different approaches to detect the presence of bile duct loss on biopsy samples. Ideally, our study group would include cases showing various degrees of bile duct loss as well as patients with no detectable bile duct loss by the traditional method. In order for our proposed method to be useful, cases showing obvious ductopenia by the traditional method should also be easily diagnosed by the unpaired HA method. Likewise, most or all mild/early bile duct loss cases diagnosed by the traditional method should also be identified as such by the new method. In addition, we hoped to be able to diagnose bile duct loss in some of those patients in the “at risk for ductopenia” group who had normal bile duct counts by the traditional method, while maintaining excellent specificity (no “overdiagnoses” in the non-ductopenic/control group). For the reasons mentioned above, we have included all patients with PBC and clinically suspected chronic rejection undergoing liver biopsy during a period of several years at our institution, without selecting patients based on either presence or severity of ductopenia. The inclusion of predominantly early-stage PBC cases reflects the typical population of PBC patients who usually undergoes liver biopsy at our institution (i.e, asymptomatic middle aged women with elevated alkaline phosphatase and positive AMA). Since it is in the early stages of PBC (and chronic rejection) that most cases of mild or equivocal bile duct loss are found, we felt that this represented the ideal setting for our method to be tested against the traditional method. For similar reasons we chose to include posttransplantation patients with persistent cholestatic liver test abnormalities (clinically suspected chronic rejection) instead of pathologically defined chronic rejection, as ductopenia, by definition, would have been present in these cases.
Influence of portal inflammation
The degree of portal tract inflammatory infiltrate also seemed to influence the identification of BD profiles, with greater percentage of portal tracts without BDs being observed in biopsies showing higher inflammatory scores. While it would be plausible to speculate that, in some ductopenic disorders, higher grades of portal inflammation might have a direct association with lower BD counts compared to cases showing less prominent infiltrates (i.e. an association with disease activity or severity), the increased percentage of ductless portal tracts in biopsies showing higher inflammation scores in non-ductopenic disorders (group 1) has been attributed to poor visualization of BDs.20,10
Although BD damage is known to occur in certain chronic liver diseases not typically associated ductopenia,5,8,23 previous studies have suggested that a mild degree of ductopenia, not fulfilling traditional criteria, may be present in this setting.23 Goldin e al.9 found a significantly lower BD to portal tract ratio in biopsies from patients with hepatitis C compared to autopsy and normal biopsy controls. Unfortunately, no data regarding the degree of inflammation in each group was presented in this study. Our data indicate that dense inflammatory infiltrates within portal tracts (seen in chronic hepatitis C and autoimmune hepatitis in our study population) correlates with lower percentages of portal tracts containing BD profiles on H&E-stained slides. BDs were identified in 74% (57.8–100%) of portal tracts in our group of hepatitis C and 76.5% (62.5–92.3%) of portal tracts in patients with autoimmune hepatitis. Rubio et al.20 presented strong evidence that portal inflammation, in cases of chronic hepatitis, can significantly affect the visualization of BDs - which are readily identified by cytokeratin 7 immunohistochemistry. An interesting finding in our study, in this regard, is the fact that dense inflammatory infiltrates obscured the visualization of both BDs and HAs. Consequently, the prevalence of unpaired HAs remained relatively constant and was not affected by the degree of portal inflammation in the control group (Table 4). This observation provides further evidence that, unless some degree of concomitant arterial loss existed, no significant BD loss was present in our control chronic hepatitis cases. Therefore, we believe the poor visualization of small HA branches in biopsies showing portal inflammation at least in part corrects for the negative influence of inflammatory infiltrates on the identification of BD profiles on H&E-stained slides. In ductopenic disorders, however, the relatively subtle effect of inflammation on BD counts is often overshadowed by true BD loss (Table 5).
While cytokeratin immunohistochemistry has been shown to improve recognition of BD profiles in densely inflamed portal tracts20,10 and is used in many institutions as an ancillary tool in the evaluation of suspected ductopenic disorders, it is unclear how results should be interpreted in this context, since none of the studies from which current diagnostic criteria for ductopenia are derived have evaluated the role of immunohistochemical techniques. Moreover, as acknowledged in previous studies,10 cytokeratin immunohistochemistry often highlights severely damaged, “dysplastic” BDs, partial BD profiles, as well as small clusters of biliary cells within portal tracts. It is arguable whether such markedly abnormal structures, frequently not discernable on H&E-stained slides, retain any physiologic function or whether they should be counted as BDs.
Influence of fibrosis stage, percentage of complete portal tracts, and biopsy width
The implications of a low percentage of complete portal tracts, advanced fibrosis, and sample width in needle biopsy samples have not been addressed in previous studies in the context of quantitative evaluation of BDs. Our data indicate that a low percentage of complete portal tracts, advanced fibrosis, as well as width <1mm of a given biopsy sample may significantly affect the percentage of portal tracts containing BDs, and these features may represent important variables to be taken into consideration for evaluation of ductopenia in select cases. A combination of the factors above probably accounts for the significantly lower percentage of portal tracts containing BDs in non-ductopenic disorders, particularly in hepatitis C and autoimmune hepatitis. Interestingly, none of the above factors seemed to influence the prevalence of portal unpaired HAs, likely due to the fact that the prevalence of HA profiles within portal tracts are influenced by the same variables that affect BD counts and to a similar degree. Therefore, as for inflammation, the lower percentage of portal tracts containing a HA profile in biopsies showing advanced fibrosis, low percentage of complete portal tracts, and width <1mm at least in part corrects for the negative influence of these factors on the identification of BDs when the unpaired HA method is employed (Table 4). The effect of a combination of the above factors, especially the degree of fibrosis, may also explain why it was not possible to identify more severe bile duct loss in advanced stages of ductopenic disorders (e.g. stage III and IV PBC).
Finally, the minimal number of portal tracts that would qualify a biopsy sample as adequate for a quantitative evaluation of BDs by the traditional method is debatable. Up to 20 portal tracts were initially thought to be necessary for an accurate assessment of a sample for ductopenia.2,26 However, such numbers are only rarely obtained in needle core biopsies. In our study, the latter number of portal tracts was seen in only 16.1% of all biopsies. Different adequacy criteria have been suggested in literature for biopsy specimens being evaluated for ductopenia. Among the most lenient recommendations are: 1- presence of at least 10 portal tracts;17,13,4 or 2- presence of at least 5 complete portal tracts in a biopsy sample.22,11 The traditional method was associated with significantly lower sensitivity (21.8% and 12.5%, respectively) if the above adequacy criteria were applied. Therefore, when the traditional method of evaluating ductopenia is utilized, lack of minimum requirements for specimen adequacy is an exceedingly common problem. Although specimen adequacy was not specifically addressed in this study, we believe the unpaired HA method may offer the advantage of being capable of diagnosing ductopenia in specimens which do not fulfill traditional adequacy requirements. In group 2, 47.3% and 63.1% of patients who met criteria for ductopenia by the unpaired HA criteria had less than 10 portal tracts and less than 5 complete portal tracts, respectively, present in their biopsies. The latter numbers would render these samples inadequate for evaluation by current standards. In spite of the small number of portal structures, however, these “inadequate” samples could safely be considered diagnostic for BD paucity based on our data. While the key finding in the traditional method (i.e. absence of BDs in a portal tract) is a relatively common occurrence in liver biopsy specimens, unpaired HAs are distinctly uncommon regardless of any specific portal tract features and, therefore, represent a much more significant finding, indicative of bile duct loss even when present in low numbers.
Conclusion
Ductopenia has traditionally been defined as absence of BDs in > 50% of portal tracts in a given sample. No specific guidelines exist, however, regarding how to approach some practical problems that arise when applying this criterion to needle core biopsy specimens, such as the interpretation of incomplete portal tracts and the diagnosis of BD loss in suboptimal specimens. In this study, we explored the anatomic relationship between BDs and HA branches and provide histometric data for the evaluation of ductopenia based solely on the well-described concept of HA-BD parallelism. Our data indicate that the presence of two or more portal tracts containing unpaired HAs, representing at least 10% of all portal tracts in the specimen, is indicative of BD loss.
The unpaired HA method described here was able to identify a greater number of cases of ductopenia compared to the traditional method when applied to liver biopsy specimens from a population of patients at risk for BD loss. In addition, the unpaired HA method provides precise recommendations on how to interpret incomplete portal tracts, which represent a large proportion of tracts in needle biopsy samples. Finally, the prevalence of unpaired arteries was not influenced by factors that affected the percentage of portal tracts containing BDs, serving as a reliable indicator of BD loss regardless of associated histopathologic abnormalities.
Table 6:
Comparison between traditional diagnostic criteria with the “unpaired hepatic artery” method for diagnosis of ductopenia
| Criteria |
% Diagnostic biopsies (group 2) |
P value (versus UHA method) |
|---|---|---|
| 1. Unpaired hepatic artery method criteria | 59.4% | |
| Traditional Method: | ||
| 2. <50% of all PTs containing at least one BD (no adequacy criteria observed) | 43.7% | P=0.31 |
| 3. <50% of PTs containing at least one BD (only complete PTs considered) | 31.2% | P=0.04 |
| 5. <50% of all PTs containing at least one BD ( at least 10 PTs present) | 21.8% | P=0.005 |
| 4. <50% of all PTs containing at least one BD (at least 5 complete PTs present) | 15.6% | P<0.001 |
Abbreviations: UHA, unpaired hepatic artery; PT, portal tract; BD, bile duct.
Acknowledgments
Financial support: Core Services performed through Vanderbilt University Medical Center’s Digestive Disease Research Center supported by NIH grant P30DK058404.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Alagille D, Odievre M, Gautier M, Dommergues JP. Hepatic ductular hypoplasia associated with characteristic fascies, vertebral malformations, retarded physical, mental, and sexual development, and cardiac murmur. J Pediatr. 1975;86(1):63–71. [DOI] [PubMed] [Google Scholar]
- 2.Alagille D Intra-hepatic biliary atresia (hepatic ductular hypoplasia) In Berenger SR: Liver Disease in Infancy and Childhood (Baltimore: William & Wilkins, p. 129, 1976). [Google Scholar]
- 3.Balistreri WF. Neonatal cholestasis: lessons from the past, issues for the future. Sem Liver Dis. 1987;7(2):61–66. [PubMed] [Google Scholar]
- 4.Burt AD. Primary biliary cirrhosis and other ductopenic diseases. Clin Liver Dis. 2002;6(2):363–380. [DOI] [PubMed] [Google Scholar]
- 5.Carpenter HA, Czaja AJ. The role of histologic evaluation in the diagnosis and management of autoimmune hepatitis and its variants. Clin Liver dis. 2002;6(3)685–705. [DOI] [PubMed] [Google Scholar]
- 6.Casali AM, Carbone G, Cavalli G. Intrahepatic bile duct loss in primary sclerosing cholangitis: a quantitative study. Histopathology. 1998;32(5):449–453. [DOI] [PubMed] [Google Scholar]
- 7.Crawford AR, Lin XZ, Crawford JM. The normal adult human liver biopsy: a quantitative reference standard. Hepatology. 1998;28(2):323–331. [DOI] [PubMed] [Google Scholar]
- 8.Czaja AJ, Muratori P, Muratori L, Carpenter HA, Bianchi FB. Diagnosis and therapeutic implications of bile duct injury in autoimmune hepatitis. Liver Intl. 2004;24(4):322–329. [DOI] [PubMed] [Google Scholar]
- 9.Goldin RD, Patel NK, Thomas HC. Hepatitis C and bile duct loss. J Clin Pathol. 1996;49(10):836–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harrison RF, Patsiaoura K, Hubscher SG. Cytokeratin immunostaining for detection of biliary epithelium: its use in counting bile ducts in cases of liver allograft rejection. J Clin Pathol. 1994;47(4):303–308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Henriksen NT, Langmark F, Sorland SJ, Fausa O, Landaas S, Aagenaes O. Hereditary cholestasis combined with peripheral pulmonary stenosis and other anomalies. Acta Paediatr Scand. 1977;66:7–15. [DOI] [PubMed] [Google Scholar]
- 12.Kahn E, Markowitz J, Aiges H, Daum F. Human ontogeny of the bile duct to portal tract space ratio. Hepatology. 1989;10(1):21–23. [DOI] [PubMed] [Google Scholar]
- 13.Ludwig J, Wiesner RH, LaRusso NF. Idiopathic adulthood ductopenia: a cause of chronic cholestatic liver disease and biliary cirrhosis. J Hepatol. 1988;7(2):193–199. [DOI] [PubMed] [Google Scholar]
- 14.Marshall MK. Primary biliary cirrhosis. N Engl J Med. 1996;335(21):1570–1580. [DOI] [PubMed] [Google Scholar]
- 15.Nakanuma Y, Hoso M, Sanzen T, Sasaki M. Microstructure and development of the normal and pathologic biliary tract in humans, including blood supply. Microsc Res Tech. 1997;38(6):552–570. [DOI] [PubMed] [Google Scholar]
- 16.Nakanuma Y, Ohta G. Histometric and serial section observations of the intrahepatic bile ducts in primary biliary cirrhosis. Gastroenterol. 1979;76(6):1326–1332. [PubMed] [Google Scholar]
- 17.Nakanuma Y, Tsuneyama K, Harada K. Pathology and pathogenesis of intrahepatic bile duct loss. J Hepatobiliary Pancreat Surg. 2003;8(4):303–315. [DOI] [PubMed] [Google Scholar]
- 18.Riely CA. Familial intrahepatic cholestatic syndromes. Sem Liver Dis. 1987;7(2):119–133. [DOI] [PubMed] [Google Scholar]
- 19.Roskams TA, Theise ND, Balabaud C, et al. Nomenclature of the finer branches of the biliary tree: canals, ductules, and ductular reactions in human livers. Hepatology. 2004;39:1739–1745. [DOI] [PubMed] [Google Scholar]
- 20.Rubio CA. Qualitative and quantitative differences between bile ducts in chronic hepatitis and in primary biliary cirrhosis. J Clin Pathol. 2000;53(10):765–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Saxena R, Theise ND, Crawford JM. Microanatomy of the human liver – exploring the hidden interfaces. Hepatology. 1999;30(6):1339–1346. [DOI] [PubMed] [Google Scholar]
- 22.Sinha J, Magid M, VanHuse C, et al. Bile duct paucity in infancy. Sem Liver Dis. 2007;27(3):319–323. [DOI] [PubMed] [Google Scholar]
- 23.Souza P, Prihoda TJ, Hoyumpa AM, Sharkey FE. Morphologic features resembling transplant rejection in core biopsies of native livers from patients with hepatitis C. Hum Pathol. 2009;40(1):92–97. [DOI] [PubMed] [Google Scholar]
- 24.Talwalkar JA, Lindor KD. Primary biliary cirrhosis. Lancet. 2003;362(9377):53–61. [DOI] [PubMed] [Google Scholar]
- 25.Wiesner RH, Ludwig J, van Hoek B, Krom RAF. Current concept in cell-mediated hepatic allograft rejection leading to ductopenia and liver failure. Hepatology. 1991;14(4):721–729. [DOI] [PubMed] [Google Scholar]
- 26.Witzleben CL. Bile duct paucity (“intrahepatic atresia”). Perspect Pediatr Pathol. 1982;7:185–201. [PubMed] [Google Scholar]