Abstract
Background
Symptom awareness, behavioral factors, and other barriers associated with timely STI healthcare provision in men is not well-studied.
Methods
Men attending an STI clinic answered a questionnaire regarding their symptoms, sexual behavior, and socio-demographic and behavioral characteristics. Characteristics of symptomatic men were compared between those who did and did not delay seeking healthcare services. Delayed care-seeking was defined as clinic attendance >7 days after symptoms while early care-seeking was defined as clinic attendance ≤7 days.
Results
Over one quarter [n=43] (27.7%) of men with urethritis symptoms (urethral discharge and/or dysuria) delayed seeking care for more than 7 days. Compared with men that sought treatment within 7 days, those that delayed care worried for longer periods that their symptoms were STI-related, were more likely to attempt self-treatment of STI symptoms, were more likely to continue engaging in sexual activity, and were less likely to use a condom during their last sexual encounter. Conversely, men that delayed care-seeking were less likely to have urethral discharge on physical exam, to have ≥5 polymorphonuclear leukocytes, and to test positive for Neisseria gonorrhoeae. When compared to men that sought care earlier, men that delayed care-seeking had fewer overall and new partners in the past 30 days.
Conclusions
Our data suggest that over a quarter of men aware of STI symptoms delay seeking health services. Interventions that promote better patient understanding of the importance of symptom recognition and that facilitate timely access to care may provide new opportunities to reduce STI transmission.
Summary
A study in a STD clinic in Birmingham, AL found that over one quarter of men aware of STI symptoms delay seeking healthcare for over 7 days.
Introduction
Sexually transmitted infections (STIs) have a profound impact on sexual and reproductive health, and rank among the top five disease categories for which adults seek health care.1 The consequences of untreated STIs include pelvic inflammatory disease, infertility, adverse pregnancy outcomes, cervical cancer, and an increased risk for acquiring and transmitting HIV.2 The most common manifestation of STIs in men is urethritis which may be caused by a number of pathogens including Neisseria gonorrhoeae (NG), Chlamydia trachomatis (CT), Mycoplasma genitalium, Trichomonas vaginalis, and other pathogens.3,4 Typical symptoms of the urethritis syndrome are genital discharge, dysuria, and urethral and penile irritation.5
Healthcare-seeking culminates from the complex process involving symptom perception, interpretation, appraisal and decision-making linked to the ability and motivation to access healthcare.6 Fortenberry has described the time-period between symptom recognition and actual presentation for evaluation as the “procrastination” interval.6 Depending on the perceived seriousness of symptoms, the procrastination interval might be reduced or lengthy. For symptomatic men, timely and appropriate therapy represents a necessary secondary prevention step.7 Sexual activity during this procrastination period provides opportunities for STI transmission and delayed treatment may lead to development of complications. Few studies have examined sexual behaviors and clinical characteristics in regard to delay in healthcare seeking among men with STI symptoms. The objective of this study was to identify the proportion of men who delayed care-seeking after becoming aware of their urethritis symptoms and to characterize factors associated with that behavior.
Methods
Enrollment took place at the Jefferson County Department of Health (JCDH) STD clinic, which is accessible to Jefferson county residents on weekdays and offers STI screening, evaluation, and treatment at minimal to no cost. Participants were men ≥18 years of age that had not taken antibiotics in the previous 30 days. Evaluation included a detailed non-validated symptoms questionnaire and collection of a urethral swab for Gram stain and urine for STI pathogen testing. Participants in the parent study included men that presented for screening, were referred by a partner or healthcare provider, and/or those that presented and answered symptom specific questions regarding urethral discharge, dysuria, urinary frequency, genital irritation, genital itching, genital lesions, or specified other symptoms. For the current analysis, only men that reported urethral discharge and/or dysuria, the main predictors of urethritis, noted in previous work using this data were included.8 Participants were classified as heterosexual, gay, or bisexual based on the reported sex of their partners. To determine if participants had sexual encounters after the onset of reported symptoms, we asked separate questions about symptom duration and the interval since the participant’s last sex, as well as whether or not condoms were used at that time and then calculated any difference between the number of days a participant had signs or symptom and the number of days since that participant’s last sexual encounter. To assess participants’ awareness of STIs, we also asked those reporting symptoms if and for how long they were concerned that their symptoms were due to a possible STI. Directed physical examinations were performed by trained clinicians for the presence of urethral discharge. The timing of health service seeking was categorized by men that sought care early, ≤ 7 days, versus those that delayed care-seeking for >7 days. All study procedures were reviewed and approved by the University of Alabama at Birmingham institutional review board (IRB) and the JCDH research review committee. Informed content was obtained for all men prior to enrollment.
Gram stains were read in blinded fashion by a single expert microscopist (JRS). A urethritis diagnosis was based upon the presence of urethritis symptoms and either a discharge detected on examination or the presence of ≥5 polymorphonuclear leukocytes per oil immersion field (PMNs/OIF) by microscopy of urethral secretions. Nucleic acid amplification testing (NAAT) on urine was performed to detect CT and NG using the Cepheid GeneXpert® CT/NG assay (Sunnyvale, CA).
Comparisons across groups for categorical variables were performed using Chi-square tests or Fisher’s Exact test where appropriate while comparisons for continuous variables were based on ANOVA or in the case of non-normally distributed data, the Kruskal-Wallis test. All statistics were performed using SAS 9.4 (Cary, NC) and an alpha-level of 0.05 determined statistical significance.
Results
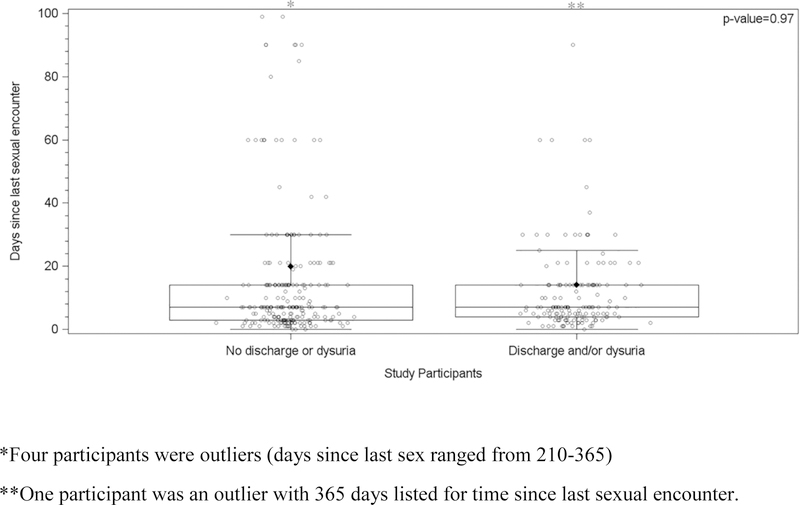
Between March 2015 and January 2017, 385 eligible men were enrolled. The median age was 25 years old (range, 18 to 77) and the majority were black (90.8%), heterosexual (91%), and reported having a prior STI (64%). Participants’ most common reason for clinic attendance was the presence of urethral symptoms (47.5%), followed by STI screening (28.1%) and then healthcare provider referral (24.4%). Symptoms and their distribution for this population have been reported previously and urethral discharge and dysuria were considered indicative of urethritis.8 Both asymptomatic and symptomatic men reported a median time since last sexual encounter of 7 days (range, 0 to 365 days), p-value=0.97. (Figure 1)
Figure 1.
Median days since last sexual encounter for participants with and without discharge and/or dysuria symptoms
The most common reason for seeking healthcare at JCDH among participants in this cohort was the presence of STI symptoms. One hundred fifty-five men (40.3%) with self-reported symptoms of discharge or dysuria were included. Of the men presenting with urethritis symptoms, 112 (72.3%) sought healthcare services within 7 days, while 43 (27.7%) delayed seeking care for longer than 7 days. More than one third of men with symptoms reported they had engaged in sex since they first thought they needed to attend a clinic for health services, and, as anticipated, a large proportion (60%) of those that waited longer than 7 days were more likely to have sex while symptomatic. Men that delayed care reported that they worried for a longer time period that they may have an STI when compared to those that sought care earlier, median days of worry 14 (range, 2–90 days) versus 4 (1–21 days) (p-value<0.001). Additionally, those that delayed care were more likely to attempt home remedies to self-treat STI symptoms (28.6%) when compared to those that sought care earlier (10.7%), p-value=0.01. Men that delayed care more than 7 days had fewer new and overall partners in the past 30 days (p-value≤0.01); were more likely to engage in sexual activity (60.5% vs 27.7%), p-value<0.001; and were less likely to use a condom during their last sexual encounter (18.6% vs. 35.1%), p-value=0.05. Physical exam revealed that men who delayed care were less likely to have urethral discharge (46.5% vs.74.3%), p-value=0.001 and, were less likely to have ≥5PMNs (48.8% vs. 79.5%), p-value<0.001. Those described as not being diagnosed with urethritis had negative NAAT results for CT and NG and had <5PMNs. (Table 1)
Table 1.
Characteristics of men with symptoms of urethritis attending an urban Alabama STI clinic by time to care provision
| Variable | Overall N=155 |
Sought care within 7 days N=112 (72.3%) |
Sought care after 7 days N=43 (27.7%) |
p-value |
|---|---|---|---|---|
| Age | 24.0 (18.0–65.0) | 25.0 (18.0–65.0) | 24.0 (19.0–55.0) | 0.75 |
| Partner Type | 0.65 | |||
| Women | 142 (92.2%) | 103 (92.0%) | 39 (92.9%) | |
| Men | 9 (5.84%) | 6 (5.36%) | 3 (7.14%) | |
| Women and Men | 3 (1.95%) | 3 (2.68%) | 0 (0%) | |
| Days participant worried they may have an STI | 4.00 (1.00–90.0) | 4.00 (1.00–21.0) | 14.0 (2.00–90.0) | <0.001 |
| Attempted remedies to self-treat symptoms | 24 (15.6%) | 12 (10.7%) | 12 (28.6%) | 0.01 |
| Total Lifetime Sexual Partners | 18.0 (3.0–1200) | 20.0 (3.00–1200) | 14.0 (3.00–200) | 0.26 |
| History of STDs | 115 (76.2%) | 87 (79.8%) | 28 (66.7%) | 0.09 |
| Number of partners in the last 30 days | 1 (0–10) | 1 (0–10) | 1 (0–4) | <0.001 |
| Number of new partners in the last 30 days | 0 (0–5) | 1 (0–5) | 0 (0–3) | 0.01 |
| Continued engaging in sexual activity after symptom onset | 57 (36.8%) | 31 (27.7%) | 26 (60.5%) | <0.001 |
| Condom use last sex | 47 (30.5%) | 39 (35.1%) | 8 (18.6%) | 0.05 |
| Circumcised | 138 (90.2%) | 98 (89.1%) | 40 (93.0%) | 0.56 |
| Discharge from penis on physical exam | 101 (66.5%) | 81 (74.3%) | 20 (46.5%) | 0.001 |
| ≥5 PMNs | 110 (71.0%) | 89 (79.5%) | 21 (48.8%) | <0.001 |
| GeneXpert® CT Positive | 51 (32.9%) | 35 (31.3%) | 16 (37.2%) | 0.69 |
| GeneXpert® NG Positive | 55 (35.5%) | 47 (42.0%) | 8 (18.6%) | 0.01 |
| CT and NG Coinfection | 23 (15.1%) | 16 (14.7%) | 7 (16.3%) | 0.81 |
| Urethritis Type | <0.001 | |||
| Gonococcal Urethritis | 55 (35.5%) | 47 (42.0%) | 8 (18.6%) | |
| Chlamydial NGU, ≥5 PMNs | 25 (16.1%) | 16 (14.3%) | 9 (20.9%) | |
| Chlamydial NGU, <5PMNs | 6 (3.87%) | 4 (3.57%) | 2 (4.65%) | |
| NCNGU | 31 (20.0%) | 27 (24.1%) | 4 (9.30%) | |
| Not urethritis | 38 (24.5%) | 18 (16.1%) | 20 (46.5%) |
Note: Values expressed as N (%), Mean ± Standard Deviation, or Median (Range); p-values for Comparisons across Groups for Categorical Variables are Based on Chi-square Test or Fisher’s Exact Tests where appropriate; p-values for Continuous Variables are Based on ANOVA or Kruskal-Wallis Test for Median; CT= Chlamydia trachomatis; NG= Neisseria gonorrhoeae; PMN=polymorphonuclear leukocytes; NGU=non-gonococcal urethritis; NCNGU=non-chlamydial non-gonococcal urethritis
Discussion
Our findings expand on observations from previous studies in observing that a substantial proportion of men waited longer than seven days to seek healthcare after the onset of symptoms. 9–14 Denison and colleagues found in a study conducted in New Zealand that 39% of men with symptoms delayed seeking care for more than one week.15 A study of UK genitourinary medicine clinic patients revealed that over 45% had been symptomatic for more than one week before seeking care.12 One previous US study showed that 38% of men with symptoms delayed care seeking for greater than seven days.16 Data from our study shed light on the fact that while men with symptoms delayed care seeking, they also worried about the possibility of having an STI. This presents an avenue to explore for structural and social interventions that might encourage seeking STI-related services.
Also of concern is the fact that these men continued to have unprotected sex and even expanded their numbers of partners during this period of reported worry about an STI. Our findings in this regard are similar to those reported previously.14,15,17 Of those studies, two described an association of delay behavior with sex while symptomatic.15,17 In contrast, one study found that sexual activity while symptomatic was associated with more expeditious healthcare seeking.14 Among participants in this study that delayed seeking care, less than one fifth reported using a condom during their last sexual encounter. Taken together, these data suggest the need for behavioral interventions that focus on partner safety in addition to personal wellness.
From our data, we cannot infer what factors play a role in influencing healthcare seeking behavior for a potential STI. Men in this study were asked how many days their symptoms of discharge and dysuria were present before contacting health services, but clinical facility delays were not assessed in this study. Barriers to seeking timely testing and treatment need to be identified and reduced as a means of reducing transmission of STI. It is possible that the time to contact and attend health services was affected by factors such as opening hours and distance to services. Moreover, men with symptoms may avoid care because they choose to wait and see if symptoms disappear, try self-treatment, or may avoid care due to denial or stigma.6,18 Other barriers may include apathy or perception of STIs as not serious, fear of invasive testing or treatment, self-consciousness or embarrassment during exams, inconvenience or being too busy, or the financial cost of an STI test. Barriers to service utilization and means of reducing them may vary with factors such as age, race, and education levels and by social and structural factors such as community size, income inequality, stigma, and incarceration.19–21
Publicly funded STD clinics provide an essential resource for providing prevention services to patients at increased risk for STI acquisition. Ideally a range of options for STI services should be available in major urban areas, including categorical STD clinics, private providers, and HMOs. Understanding the variance in behavior and lifestyle of patients is important to pinpoint potential STI risks. Various public health behavioral models (the Health Belief Model, the theory of reasoned action, the protection-motivation theory, the social cognitive theory, and the theory of planned behavior) each provide a theoretical basis for further research exploring care seeking and sexual behaviors following symptom onset.6,14,18,22,23
Several limitations of this study should be recognized and considered. Our assessment of delay in seeking care as well as other STD-related behaviors were not from a validated scale; and due to the observational design, we cannot determine a causal relationship between the associated factors and the outcome of delay. For comparison to other studies in the literature, an arbitrary cut point of 7 days was selected for the healthcare-seeking interval in which men participating in this study were classified as seeking health services early if they attended clinic within 7 days of symptom onset, while men attending clinic after 7 days were described as delayed healthcare seekers. Study participants were recruited from the JCDH STD clinic, the primary care provider for sexual health services in the Birmingham, Alabama metropolitan area; thus, results may not be generalizable to men utilizing other sources of care or to other STD clinics in different urban areas in the United States. Also, patterns of symptom recognition and treatment-seeking behavior may vary in men in other settings and parts of the country. Additionally, although classification of the duration of participant symptoms based on self-report lack objectivity as patients might not have remembered the exact time symptoms started, prior episodes of STIs, or specific details about their sexual history, we would anticipate that errors related to self-report would make our data conservative estimates of these variables.
In conclusion, this study is consistent with others that found both individual and structural factors to be associated with the time to seek care for STIs. A substantial proportion of patients with genitourinary symptoms attending JCDH STD clinic delayed seeking care for a week or more while not reducing the risk of transmission. The study represents the reality of service seeking in the Birmingham metropolitan area and as such provides a meaningful glimpse into care seeking despite the fact that care-seeking may involve overcoming barriers to access. Our findings suggest the use of interventions that improve access to clinics and testing options, encourage symptomatic persons to seek medical care more rapidly, and address concerns about stigma need to be evaluated in the context of care seeking behaviors. Our findings provide a unique profile of men attending an urban public STD clinic in the Southeastern United States, allowing for a contemporary contribution of delay behavior data in a setting of limited resources.
Acknowledgments
Dr. Schwebke is a consultant for Talis Corporation, Hologic, Lupin Pharmaceuticals, Toltec, and StarPharma; Dr. Van Der Pol receives research support, consulting fees and/or honorarium from the following: Abbott Molecular, BD Diagnostics, Binx Health, BioFire Diagnostics, Hologic, Rheonix, Roche and SpeeDx; and Dr. Hook has received honoraria, research support, or consulting fees from Cepheid, BD Diagnostics, Gen-Probe Hologic, and Roche Diagnostics.
The present study was funded by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health Sexually Transmitted Infection Cooperative Research Center grant U19AI113212 (EWH, PI).
The authors are grateful to the nurses at JCDH, Hanne Harbison and Meghan Whitfield, for specimen collection and to Paula Dixon and Austin Culver for assistance with specimen processing/testing.
Footnotes
Reagents and test kits for this study were supplied by Cepheid (Sunnyvale, CA).
References
- 1.Sexually transmitted infections: more than 1 million people acquire a sexually transmitted infection every day: fact sheet World Health Organization; 2014. [Google Scholar]
- 2.Galvin SR, Cohen MS. The role of sexually transmitted diseases in HIV transmission. Nat Rev Microbiol 2004;2(1):33–42. [DOI] [PubMed] [Google Scholar]
- 3.Martin DH. “Urethritis in males”. In: Holmes KK, SP, Stamm WE, et al. , editors. 4th ed; 2008. Sexually Transmitted Diseases New York, NY: McGraw Hill; 1107–1126. [Google Scholar]
- 4.Horner PJ, Martin DH. Mycoplasma genitalium Infection in Men. The Journal of infectious diseases 2017;216(suppl_2):S396–S405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Workowski KA, Bolan GA, Centers for Disease C, Prevention. Sexually transmitted diseases treatment guidelines, 2015. MMWR Recomm Rep 2015;64(RR-03):1–137. [PMC free article] [PubMed] [Google Scholar]
- 6.Fortenberry JD. Health care seeking behaviors related to sexually transmitted diseases among adolescents. Am J Public Health 1997;87(3):417–420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.“Prevention of STDs”. In: Eng TR, Butler WT. Institute of Medicine. 1997. The Hidden Epidemic: Confronting Sexually Transmitted Diseases Washington, DC: The National Academies Press. doi: 10.17226/5284.448. [DOI] [PubMed] [Google Scholar]
- 8.Jordan SJ, Aaron KJ, Schwebke JR, Van Der Pol BJ, Hook EW 3rd. Defining the Urethritis Syndrome in Men Using Patient Reported Symptoms. Sexually transmitted diseases 2018;45(7):e40–e42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hook EW, 3rd, Richey CM, Leone P, et al. Delayed presentation to clinics for sexually transmitted diseases by symptomatic patients. A potential contributor to continuing STD morbidity. Sexually transmitted diseases 1997;24(8):443–448. [DOI] [PubMed] [Google Scholar]
- 10.Kramer MA, Aral SO, Curran JW. Self-reported behavior patterns of patients attending a sexually transmitted disease clinic. Am J Public Health 1980;70(9):997–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Upchurch DM, Brady WE, Reichart CA, Hook EW, 3rd. Behavioral contributions to acquisition of gonorrhea in patients attending an inner city sexually transmitted disease clinic. The Journal of infectious diseases 1990;161(5):938–941. [DOI] [PubMed] [Google Scholar]
- 12.Mercer CH, Sutcliffe L, Johnson AM, et al. How much do delayed healthcare seeking, delayed care provision, and diversion from primary care contribute to the transmission of STIs? Sex Transm Infect 2007;83(5):400–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fonck K, Mwai C, Rakwar J, Kirui P, Ndinya-Achola JO, Temmerman M. Healthcare-seeking behavior and sexual behavior of patients with sexually transmitted diseases in Nairobi, Kenya. Sexually transmitted diseases 2001;28(7):367–371. [DOI] [PubMed] [Google Scholar]
- 14.Irwin DE, Thomas JC, Spitters CE, et al. Self-reported sexual activity and condom use among symptomatic clients attending STD clinics. Sex Transm Dis 1999;26(5):286–290. [DOI] [PubMed] [Google Scholar]
- 15.Denison HJ, Woods L, Bromhead C, et al. Healthcare-seeking behaviour of people with sexually transmitted infection symptoms attending a Sexual Health Clinic in New Zealand. The New Zealand medical journal 2018;131(1481):40–49. [PMC free article] [PubMed] [Google Scholar]
- 16.Malek AM, Chang CC, Clark DB, Cook RL. Delay in Seeking Care for Sexually Transmitted Diseases in Young Men and Women Attending a Public STD Clinic. The open AIDS journal 2013;7:7–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moses S, Ngugi EN, Bradley JE, et al. Health care-seeking behavior related to the transmission of sexually transmitted diseases in Kenya. Am J Public Health 1994;84(12):1947–1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meyer-Weitz A, Reddy P, Van den Borne HW, Kok G, Pietersen J. Health care seeking behaviour of patients with sexually transmitted diseases: determinants of delay behaviour. Patient education and counseling 2000;41(3):263–274. [DOI] [PubMed] [Google Scholar]
- 19.Adimora AA, Schoenbach VJ. Social context, sexual networks, and racial disparities in rates of sexually transmitted infections. The Journal of infectious diseases. 2005;191 Suppl 1:S115–122. [DOI] [PubMed] [Google Scholar]
- 20.Hatzenbuehler ML, Phelan JC, Link BG. Stigma as a fundamental cause of population health inequalities. American journal of public health 2013;103(5):813–821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Buffardi AL, Thomas KK, Holmes KK, Manhart LE. Moving upstream: ecosocial and psychosocial correlates of sexually transmitted infections among young adults in the United States. Am J Public Health 2008;98(6):1128–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Montanaro EA, Bryan AD. Comparing theory-based condom interventions: health belief model versus theory of planned behavior. Health psychology : official journal of the Division of Health Psychology, American Psychological Association 2014;33(10):1251–1260. [DOI] [PubMed] [Google Scholar]
- 23.Crepaz N, Horn AK, Rama SM, et al. The efficacy of behavioral interventions in reducing HIV risk sex behaviors and incident sexually transmitted disease in black and Hispanic sexually transmitted disease clinic patients in the United States: a meta-analytic review. Sexually transmitted diseases 2007;34(6):319–332. [DOI] [PubMed] [Google Scholar]