Abstract
Objective:
Trigeminal nerve stimulation (TNS), a minimal risk, non-invasive neuromodulation method, has showed potential benefits for attention-deficit/hyperactivity disorder (ADHD) in an unblinded open study. This blinded sham-controlled trial was conducted to assess efficacy and safety of TNS for ADHD, as well as potential changes in brain spectral power using resting-state quantitative electroencephalography (qEEG).
Method:
62 children aged 8–12 years, with full-scale IQ ≥ 85 and KSADS-diagnosed ADHD, were randomized to four weeks nightly treatment with active or sham TNS, followed by one-week without intervention. Assessments included weekly clinician-administered ADHD-Rating Scales (ADHD-RS) and Clinical Global Impression (CGI) scales, and qEEG at baseline and week 4.
Results:
ADHD-RS totals showed significant group-by-time interactions (F = 8.12, df = 1/228, p = .005); week 4 Cohen’s d = .5. CGI-Improvement also favored active treatment (Chisq = 8.75, df = 1/168, p = .003); number-needed-to-treat (NNT) = 3. Resting-state qEEG showed increased spectral power in right frontal and frontal midline frequency bands with active TNS. Neither group had clinically meaningful adverse events.
Conclusion:
This study demonstrates TNS efficacy for ADHD in a blinded sham-controlled trial, with estimated treatment effect size similar to non-stimulants. TNS is well-tolerated and minimal risk. Additional research should examine treatment response durability and potential impact on brain development with sustained use.
Clinical trial registration information:
Developmental Pilot Study of External Trigeminal Nerve Stimulation for ADHD; http://clinicaltrials.gov/; NCT02155608.
Keywords: attention-deficit/hyperactivity disorder, clinical trial, neuromodulation, trigeminal nerve stimulation
INTRODUCTION
Although stimulant medications are regarded as the most effective and commonly employed treatment for Attention-Deficit/Hyperactivity Disorder (ADHD),1 side effect concerns, social stigma, and parental preferences for non-medication approaches contribute to a lack of long-term compliance.2, 3 In addition to standard psychosocial interventions such as parent management training and academic accommodations, there has been increasing interest in other non-medication approaches to ADHD, including EEG-based neurofeedback, computer-based working memory training, and noninvasive brain stimulation methods such as transcranial direct stimulation and transcranial magnetic stimulation. However, scientific studies of these modalities have largely failed to demonstrate positive effects.4–8
Trigeminal nerve stimulation (TNS) is a non-invasive, minimal risk neuromodulation method approved in Canada and Europe for adult treatment of medication-resistant major depression,9, 10 and epilepsy.11 Similar to the vagus nerve, the trigeminal conveys sensory inputs from skin, muscles, and skull to extensive connections within the locus coeruleus, reticular activating system, and nucleus tractus solitarius,12 regions involved in selective maintenance of attention.13 Recent data provide increased evidence that TNS exerts its effects via central projections to cortical structures.14 TNS utilizes a small stimulator worn during sleep to emit a low-level current. Thin wires extend from the TNS device to an adhesive electrode worn across the forehead over branch V1 of the trigeminal nerve. Assuming that benefits of vagal stimulation rely in part on the same brain connections, it was hypothesized that TNS similarly improve seizures and mood, but without costs and risks associated with surgical device implantation.
Several TNS depression studies suggested a potential role in ADHD. First, item-analysis of mood rating scales revealed that TNS was associated with selective improvements in concentration and attention (Ian Cook, personal communication). Second, a small positron emission tomography (PET) study showed that acute TNS activated several brain regions implicated in ADHD and executive function, including the anterior cingulate cortex (ACC) and the inferior frontal, medial, and middle frontal gyri, as well as the parietotemporal cortex.15 Finally, TNS is extremely well tolerated in adults and virtually without adverse events, suggesting suitability for pediatric testing.16
A preliminary open trial in ADHD-diagnosed youth suggested TNS was 1) readily accepted by parents and children; 2) associated with substantial reductions in parent and clinician ADHD symptom ratings and significant improvements on multiple indices of parent-reported executive functioning; and, 3) associated with dramatic improvements in laboratory measures of response inhibition.17 Treatment was well tolerated and without meaningful adverse events.
The present study investigated the potential efficacy of TNS for ADHD treatment in a four-week double-blind sham-controlled trial, followed by one blinded week without treatment to assess response persistence. This is the first blinded sham-controlled trial of TNS for ADHD or any pediatric condition. Secondary aims included assessment of cortical activation mechanisms, measured with quantitative electroencephalography (qEEG), as well effects on anxiety, mood, sleep, growth, and safety. The study further assessed time course effects, provided estimates of treatment effect sizes, and measured the success of blinding procedures in anticipation of future clinical trials.
METHOD
Participants
Participants were recruited through community advertisements and internet postings. Children aged 8 to 12 years with DSM-5 ADHD, based on the Kiddie Schedule for Affective Disorders and Schizophrenia (KSADS-PL)18 and clinical interview, minimum total of 24 on the clinician-administered parent ADHD-IV Rating Scale (ADHD-RS),19 baseline Clinical Global Impression-Severity Score (CGI-S) ≥ 4,20 estimated full-scale IQ ≥ 85 based on WASI subtests,21 and able to cooperate with EEG and other study procedures were enrolled. Exclusion criteria were current major depression or autism spectrum disorder, lifetime psychosis, mania, seizure disorder, or head injury with loss of consciousness, or baseline suicidality. Children were medication free for at least one month prior to participation and remained so throughout the trial. Before screening, parents and children received thorough verbal and written descriptions of study requirements and provided written permission/assent. The UCLA Institutional Review Board approved all study procedures.
Study Design
The study was a four-week, double-blind, sham-controlled trial, followed by one blinded week without intervention. Screening included diagnostic and IQ assessment,18, 21 clinician-completed parent ADHD-RS and CGI-S rating, parent-completed Childhood Behavioral Checklist (CBCL),22 and the parent- and child-rated Affective Reactivity Index (ARI).23 Eligible participants returned at baseline for repeated clinician ratings, additional parent- and child-completed behavioral measures, computerized tests of executive function, and EEG. Randomization was 1:1, using random block lengths of four and six, to active or sham TNS, with equal stratification on low (≤ 6) or high parent ARI scores to assess potential effects on irritability. Families were taught proper electrode placement and device operation at baseline. Active or sham TNS was administered nightly during sleep. Participants returned after one week for repeated measurement of behavioral and cognitive outcomes and assessment of blinding integrity (Early Impressions Questionnaire, below). Clinician and parent behavioral ratings were repeated weekly. After week 4, behavioral, cognitive, and EEG measures were repeated and treatment (active or sham) discontinued. Participants and investigators remained blinded for one additional week when final behavioral and cognitive outcomes were repeated to assess potential benefit persistence post discontinuation.
TNS Intervention
TNS procedures were based on previous work in epilepsy,11, 24 adult depression,9, 10 post-traumatic stress disorder10 and ADHD.17 Stimulation was via a CE-mark approved neurostimulator, the Monarch eTNS System™ (NeuroSigma, Inc., Los Angeles CA). The stimulator was worn on the child’s pajamas or t-shirt and attached with thin wires to disposable, silver-gel, self-adhesive patch electrodes. Parents applied patches across their child’s forehead to provide bilateral stimulation of V1 trigeminal branches for approximately 8 hours nightly. Patches were removed each morning. The active condition utilized a 120-Hz repetition frequency, with 250-μs pulse width, and a duty cycle of 30 seconds on/30 seconds off. Stimulator current settings between 2 and 4 milli-amperes (mA) (range: 0–10 mA) were established at baseline by titration, which identified a stimulation level below the participant’s subjective level of discomfort. Power was provided by 9-volt lithium medical-grade batteries (Energizer L522, Eveready Battery Co., St. Louis, MO), which were replaced every day.
Active and sham systems were identical in appearance and operation. Participants were informed via a scripted presentation that “pulses may come so fast or so slowly that the nerves in the forehead might or might not detect a sensation.” Each night parents turned on the device, pressed the “up” button until the stimulation was uncomfortable or until the device reached the maximum current, and then pressed “down” to reduce it by one 0.1mA step. In active devices, current flowed to the patch and was limited to a safe range. Some, but not all, in both active and sham groups reported feeling some sensation, which generally faded with time. With sham, no current flowed, so participants adjusted settings without actually controlling current.
One research assistant who managed study devices had access to group assignments. All other staff, parents, and participants were blinded to randomized group. To assess study blinding effectiveness, parents completed an Early Impressions Questionnaire25 after the initial treatment week to quantify expectations of success with their assigned condition.
Quantitative Electroencephalography
qEEG acquisition followed previously used procedures.26 Participants underwent qEEG recording, including a five minute, eyes-open resting condition. Recordings were carried out using an Electrical Geodesics (EGI; Eugene, Oregon) GES300 system with 128-electrode high-impedance Hydrocel Geodesic Sensor Nets. Data were referenced to Cz, impedance threshold set at 50 kOhms (per manufacturer standard), and sampling rate was 1000 Hertz (Hz). Eye movements were monitored by electrodes placed on the outer canthus of each eye for horizontal movements (REOG, LEOG) and by electrodes above the eyes for vertical eye movements. Key head landmarks (nasion, inion, preauricular notches) and 3-D electrode locations were recorded (Polhemus, Inc.) to allow three-dimensional reconstruction of scalp electrode positions.
Continuous EEG data were imported into the EEGLAB environment for processing.27 The EEG data were: 1) high pass filtered (>1 Hz), 2) re-referenced to the channel average, 3) rejected for excessive noise, and 4) decomposed using independent components analysis (ICA), which separates brain from non-brain (e.g., muscle, eye) artifacts that contribute to scalp recorded signals. Independent components were inspected for spatial, spectral, and temporal properties to identify those with patterns corresponding to non-brain sources of signal such as eye blinks, lateral eye movement, cardiac artifacts, single channel artifacts and high-frequency line noise; these components were excluded from further analyses. Cleaned data were then back-projected into channel space for resting state analyses. Fourier transform was used to estimate spectral power in frequencies from 1–50 Hz for the following channels: F3/4, Fz, C3/4, Cz, P3/4, PZ and averaged across standard frequency bands: delta (1–3 Hz), theta (4–7 Hz), alpha (8–12 Hz), beta 1 (13–16 Hz), beta 2 (17–25 Hz), gamma 1 (30–40 Hz) and gamma 2 (40–50 Hz).
Outcome Measures
The primary efficacy outcome measure was the clinician completed ADHD-RS Total Score,19 based on parental interview and all available clinical information, completed at baseline and over subsequent weeks. Secondary behavioral outcomes included weekly clinician-scored CGI-Improvement (CGI-I) scales,20 weekly parent-completed Behavioral Rating Inventory of Executive Functioning (BRIEF) Scales,28 Conners Global Index,29 Children’s Sleep Habits Questionnaire (CSHQ),30 and teacher-completed Conners Global Index.29 Ratings at baseline and weeks 4 and 5 included the parent and child completed ARI and Multidimensional Anxiety Scale for Children (MASC),31 and clinician-completed Children’s Depression Rating Scale (CDRS-R).32 Secondary cognitive outcomes included the computer-based Spatial Working Memory test33 and Attention Network Task34 at baseline and weeks 1, 4, and 5. qEEG was conducted at baseline and weeks 1 and 4. Cognitive outcomes will be presented in a subsequent publication addressing neurobiological response mechanisms. Safety was assessed by height, weight, and vital sign measurements at each clinic visit, and weekly open-ended adverse event inquiries, parent-completed Side Effects Rating Scales17, and clinician-completed Columbia Suicide Severity Rating Scales (C-SSRS).35
Statistical Analysis
All analyses were conducted in SAS 9.4. To confirm successful randomization, we compared groups on baseline demographic and clinical characteristics using t-tests and chi-square tests as appropriate. Subsequently, data were assessed for normality and sphericity and outcome variables plotted as a function of time to determine forms of treatment trajectories (e.g., linear, quadratic, piecewise linear with change of slope, etc.).
Our primary analytic tool was the general linear mixed model (GLMM) with treatment group (active vs. sham), time (in weeks), and group-by-time interactions to test for differential treatment effects as primary predictors, along with subject level random intercepts. GLMMs properly account for correlations induced by repeated measurements within subjects and automatically handle missing values, allowing maximum use of available data. As such, all participants with baseline data were included in analyses. We fitted a single model for each dimensional outcome from baseline to end of the four-weeks. Separate models were fit for the blinded discontinuation period between weeks 4 and 5.
Categorical outcomes were assessed using chi square (X2). For CGI-I, a binary variable was created wherein scores of “1” or “2” (very much improved or much improved) were deemed “improved” versus those scores > 2 considered “not improved”. CGI-I was determined weekly in reference to baseline. Adverse event frequencies within each group were tallied over the study course based on the Side Effects Rating Scale and spontaneous report. Likert scale values from the Early Impression Questionnaire were assessed via logistic regression as predictive of treatment group to assess validity of blinding procedures. Effect size differences between groups were estimated using Cohen’s d and number-needed-to-treat (NNT). For Cohen’s d, cutoff values for small, medium, and large effects were defined as .2, .5, and .8, respectively.36 For NNT, small, medium, and large effects were defined as 9, 4, and 2, respectively.37
Effects of multiple testing were minimized by identifying the ADHD-RS total score a priori as the single primary outcome. However, for a developmental pilot the identification of sensitive outcomes and protocol parameters carried more importance for future research design than minimizing Type I error. All results are therefore reported using an uncorrected significance level of α=.05.
RESULTS
Demographics and Disposition
Of 79 individuals screened, 62 were eligible and randomized to active (n=32) or sham (n=30) TNS. Of those ineligible, 13 failed inclusion criteria, 2 met exclusion criteria, and 2 failed to return after initial screening. One participant randomized to sham left the trial after week 3. One additional participant in each group withdrew between weeks 4 and 5. qEEG data for 3 participants were excluded due to excessive movement artifact; leaving a total of 56 participants (active n=30; sham n=26) for EEG analyses. Participant characteristics are summarized in Table 1. No significant group differences were found for age, sex, race/ethnicity, height, weight, vital signs, IQ, ADHD subtype, or baseline behavioral ratings.
Table 1.
Participant Characteristics at Baseline by Assigned Treatment Group.a
| Total
Sample (N=62) |
Active
Group (n=32) |
Sham Group (n=30) |
||||
|---|---|---|---|---|---|---|
| Age, y, mean (SD) | 10.4 | (1.4) | 10.3 | (1.4) | 10.5 | (1.4) |
| Sex, n (%) | ||||||
| Male | 40 | (65) | 19 | (60) | 21 | (70) |
| Race/Ethnicity, n (%) | ||||||
| White | 40 | (65) | 20 | (63) | 20 | (67) |
| Black | 4 | (6) | 4 | (13) | 0 | |
| Asian | 10 | (16) | 5 | (16) | 5 | (17) |
| Mixed/Other | 8 | (13) | 3 | (9) | 5 | (17) |
| Hispanic | 10 | (16) | 5 | (16) | 5 | (17) |
| Height, cm, mean (SD) | 142.2 | (9.9) | 142.8 | (10.1) | 141.5 | (9.9) |
| Weight, kg, mean (SD) | 37.1 | (10.5) | 38.8 | (12.3) | 35.4 | (8.1) |
| Systolic BP, Mean (SD) | 107 | (11.8) | 108.5 | (11.53) | 106.2 | (12.2) |
| Diastolic BP, Mean (SD) | 64.3 | (7.9) | 65.0 | (8.2) | 63.6 | (7.6) |
| Pulse, Mean (SD) | 76.7 | (11.6) | 71.7 | (9.2) | 76.6 | (13.1) |
| Full Scale IQ, Mean (SD) | 108.9 | (13.2) | 110.4 | (12.3) | 107.3 | (14.2) |
| ADHD Subtype, n (%) | ||||||
| Combined | 39 | (63) | 22 | (69) | 17 | (57) |
| Inattentive | 21 | (34) | 9 | (28) | 12 | (40) |
| Hyperactive/Impulsive | 2 | (3) | 1 | (3) | 1 | (3) |
| Comorbidity, n (%) | ||||||
| ODD | 20 | (32) | 11 | (34) | 9 | (30) |
| DMDD | 17 | (27) | 10 | (31) | 7 | (23) |
| Social Phobia | 10 | (16) | 7 | (21) | 3 | (10) |
| Separation Anxiety | 2 | (3) | 1 | (3) | 1 | (3) |
| Generalized Anxiety | 10 | (16) | 6 | (19) | 4 | (13) |
| Any Anxiety | 18 | (29) | 11 | (3) | 7 | (23) |
| Enuresis | 6 | (12) | 5 | (16) | 1 | (3) |
| Encopresis | 2 | (3) | 0 | 2 | (7) | |
| Tourette’s Syndrome | 2 | (3) | 2 | (6) | 0 | |
| Motor Tic | 1 | (2) | 0 | 1 | (3) | |
| ADHD-RS-T, mean (SD) | 32.5 | (6.2) | 32.1 | (6.3) | 32.8 | (6.2) |
| ARI-P, mean (SD) | 4.5 | (3.7) | 4.4 | (3.9) | 4.5 | (3.9) |
| MASC-Child, mean (SD) | 60.6 | (25.7) | 59.0 | (26.2) | 62.4 | (25.5) |
| MASC-Parent, mean (SD) | 47.4 | (19.2) | 46.2 | (19.2) | 48.7 | (19.2) |
| CDRS-R, mean (SD) | 9.71 | (6.4) | 10.4 | (6.9) | 9.0 | (5.8) |
| CGI-S, n (%) | ||||||
| 4 | 21 | (34) | 10 | (31) | 11 | (37) |
| 5 | 41 | (66) | 22 | (69) | 19 | (63) |
Note: ADHD = attention-deficit/hyperactivity disorder; ADHD-RS-T = ADHD Rating Scale Total Score; ARI-P = Affective Reactivity Index- Parent Report; CDRS-R = Children’s Depression Rating Scale; CGI-S = Clinical Global Impression Severity Scale; DMDD = Disruptive Mood Dysregulation Disorder; MASC = Manifest Anxiety Scale for Children; ODD = Oppositional Defiant Disorder.
No significant differences between groups (all p > .05).
Efficacy Measures
Initial analyses demonstrated that dependent variables were normally distributed and that assumptions of sphericity were not violated. Plotted ADHD-RS totals over time suggested a non-linear pattern, with decreasing scores in both groups during the first week, followed by ongoing improvement, albeit slower, in the active group vs. a flattening response trajectory with sham (Figure 1). Consequently, dimensional behavioral outcomes were fitted via a mixed effects model with group-by-time interactions to test for treatment effects using a piecewise linear time trend. This was parameterized in the model as a standard linear variable, time (ranging from baseline to 4 weeks) and a second variable, time2, defined as 0 at baseline and time past week 1 for subsequent weeks. The time2 coefficient represents the change in slope after the initial week. Height, weight, and vital signs demonstrated linear patterns and were evaluated using time only, as were measures taken only at baseline and week 4.
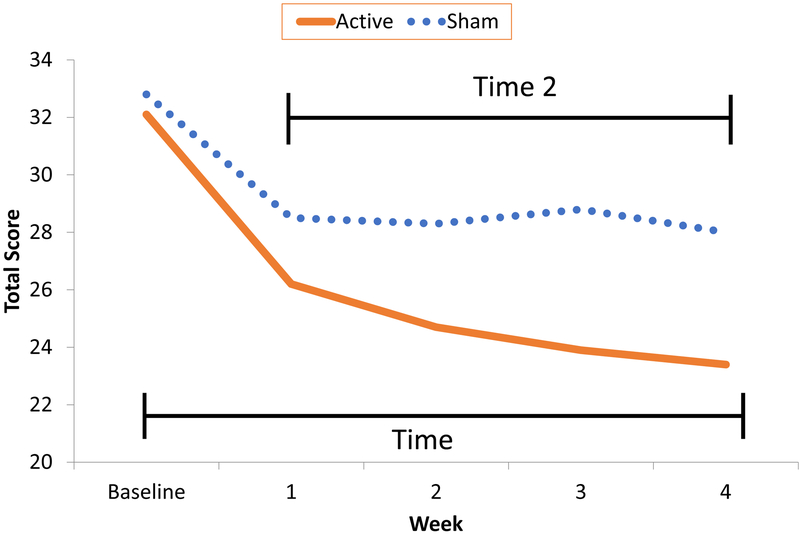
Figure 1. Attention-Deficit/Hyperactivity Disorder-Rating Scale (ADHD-RS) Total Scores Over Four-Week Blinded Trial: Active vs. Sham Trigeminal Nerve Stimulation.
ADHD-RS totals showed significant group-by-time interaction, demonstrating a differential treatment effect (F = 8.12, df = 1/228, p = .005). The significant main effect of time (F = 39.97, df = 1/228, p < .0001) revealed initial improvement in both groups, greater with active TNS. Time2 also demonstrated a significant effect (F = 28.96, df = 1/228, p < .0001), but no group-by-time2 interaction, indicating an equal leveling-off of improvement following week 1. Estimated Cohen’s d at week 4 was 0.50, suggesting a medium-size treatment effect. CGI-I over the 4-week course similarly favored active over sham (X2 = 8.75, df = 1/168, p = .003). Improvement rates for active vs. sham were 25% vs. 13%, 34% vs. 15%, 47% vs. 12%, and 52% vs. 14% based on raw CGI-I at weeks 1, 2, 3, and 4 respectively, with a trend for increasing improvement with active TNS over time (X2 = 5.08, df = 3/168, p = .17). Number-needed-to-treat (NNT) based on CGI-I at week 4 was 3.
Table S1, available online, summarizes other exploratory outcomes with significant effects. The same pattern of time, time2, and group-by-time effects was found with both Inattentive and Hyperactive-Impulsive ADHD-RS subscales as with total scores. A similar piecewise linear trajectory, but no group or interactive effects, was seen with the parent-completed Conners. The MASC-Parent Report showed trends for time (F = 3.58. df = 1/53, p = .06) and group-by-time (F = 2.90, df 1/53, p = .09) effects, with estimated Cohen’s d =.33. The CSHQ revealed significant time and time2 effects, but no group-by-time interactions, for Bedtime Resistance, Sleep Anxiety, and Total Sleep Problems. Other behavioral outcomes, including the MASC Child Report, CDRS-R, BRIEF, remaining CSHQ scales, teacher Conners, and ARI scales were not significant.
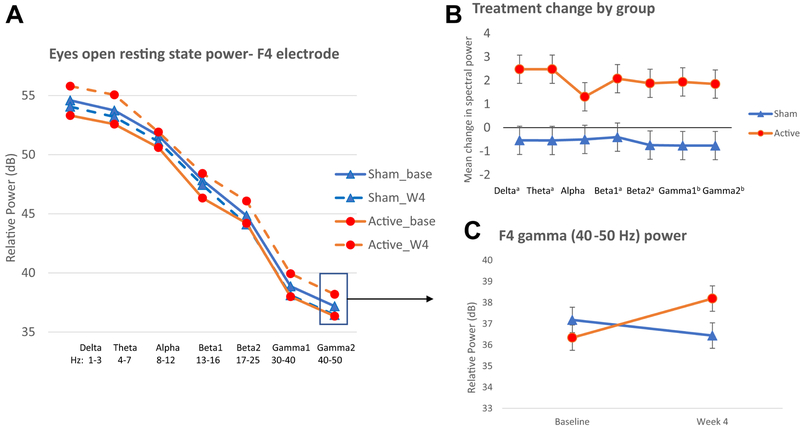
With resting state qEEG, active TNS demonstrated increased broadband power, whereas sham exhibit decreased power in the right frontal region (Figure 2). Treatment groups did not differ at any channel or frequency band at baseline (all p’s > 0.3). EEG spectral power statistics are summarized in Table S2, available online, and reveal significant group-by-time effects for frequency bands in the right frontal (F4 delta, theta, beta, gamma) and frontal midline (Fz gamma) channels, with trend level effects for frequency bands in the mid-frontal region (Fz delta, theta, beta). Left frontal region (F3) effects were generally in the same direction but did not reach significance (all p’s > 0.2). No significant group, time, or group-by-time effects were seen in central or parietal electrodes (all p’s > 0.2).
Figure 2. Treatment Related Change in Electroencephalography (EEG) Spectral Power at F4 Electrode.
Note: During eyes-open resting state, active Trigeminal Nerve Stimulation (TNS) treatment was associated with increased broad band spectral power from baseline to Week 4 (orange solid and dashed line, respectively) compared to sham treatment, which showed no change or slight decrease from baseline to week 4 (blue solid and dashed lines, respectively), particularly in the right frontal region (panel A). Amount of change for each treatment group in the active and sham TNS groups (panel B), suggests increased power in the active group and decreased power in the sham group across multiple frequency bands. Depiction of the significant group by time interaction effect for F4 gamma power (panel C), data for other frequency bands and the Fz electrode show similar patterns. Base=baseline, W4=week4 treatment end, a p<0.05, b p<0.01.
To facilitate functional interpretation of qEEG changes, significant EEG outcomes and ADHD behavioral ratings were evaluated using Pearson partial correlations with age as a covariate. Week 4 changes in right frontal (F4 theta, beta bands) and frontal midline (Fz Gamma 1) regions were significantly associated with changes in ADHD-RS total and hyperactive/impulsive scores (r’s range −.34 to −.41) (see Table S3, available online). Spectral power changes had weaker correlations with inattentive symptoms and none were statistically significant (all p’s > 0.13). These correlations suggest that treatment-related spectral power increases in frontal midline and right frontal regions were associated with lower ADHD-RS scores, particularly hyperactive-impulsive, at trial end.
Discontinuation Outcomes
ADHD-RS totals worsened in both groups between weeks 4 and 5 following treatment discontinuation. Week 4 mean (SD) scores for active vs. sham groups were 23.39 (7.88) and 27.50 (8.08) respectively; with week 5 scores of 25.52 (7.84) and 29.11 (7.79). Time effect was significant (F = 6.23, df = 1/57, p = .02), with a trend for group differences (F = 4.18, df = 1/57, p = .05), but no significant group-by-time interaction (F = .12, df = 1/57, p = .73), suggesting both groups deteriorated at similar rates. Week 5 CGI-I ratings showed 13% improved in active vs.7% improved in sham groups compared to baseline (X2 = .53, df = 1, p = .46). Cohen’s d at Week 5 =.46, suggesting maintenance of a medium-size treatment effect one week after treatment cessation.
Safety and Tolerability
Significant increases in weight and pulse were seen with active TNS compared with sham over four weeks, but there were no group differences in increased height or blood pressure (Table 2). There were no serious adverse events in either group and no participant withdrew for adverse events. C-SSRS showed no responses suggestive of suicidality. Side Effects Rating Scale responses are summarized in Table 3, with notable increases in fatigue, headache, and increased appetite with active TNS, and increased hyperactivity with sham. Table S4, available online, summarizes spontaneously reported adverse events. One initially concerning adverse event, skin whitening/discoloration under the patch site in some darker skinned participants, occurred in active and sham groups and was attributed to patch removal and concomitant loss of superficial skin layers. Skin discoloration resolved with subsequent sun exposure and time.
Table 2.
Vital Sign Changes Over Double Blind: Active vs. Sham Trigeminal Nerve Stimulation
| Measure | Active | Sham | Effect | F | df | P | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Visit 0 | Visit 4 | Visit 0 | Visit 4 | |||||||||
| Mean | (SD) | Mean | (SD) | Mean | (SD) | Mean | (SD) | |||||
| Height cm | 142.8 | (10.1) | 143.1 | (10.0) | 141.5 | (9.9) | 142.3 | (9.8) | Group | <1 | 1/229 | .59 |
| Time | 5.83 | 1/229 | .02 | |||||||||
| Group*Time | 1.03 | 1/229 | .31 | |||||||||
| Weight kg | 38.8 | (12.3) | 39.7 | (10.5) | 35.4 | (8.1) | 35.7 | (10.3) | Group | 1.62 | 1/128 | .21 |
| Time | 5.18 | 1/128 | .02 | |||||||||
| Group*Time | 6.89 | 1/128 | .01 | |||||||||
| Pulse bpm | 71.7 | (9.2) | 81.8 | (12.7) | 76.6 | (13.1) | 75.2 | (12.6) | Group | <1 | 1/128 | .79 |
| Time | 1.10 | 1/128 | .30 | |||||||||
| Group*Time | 4.61 | 1/128 | .03 | |||||||||
| Systolic BP | 108.5 | (11.5) | 111.0 | (12.7) | 106.2 | (12.2) | 107.8 | (12.5) | Group | <1 | 1/128 | .93 |
| Time | <1 | 1/122 | .76 | |||||||||
| Group*Time | <1 | 1/128 | .39 | |||||||||
| Diastolic BP | 65.0 | (8.2) | 65.1 | (9.3) | 63.6 | (7.6) | 61.0 | (9.2) | Group | <1 | 1/128 | .64 |
| Time | 1.75 | 1/128 | .19 | |||||||||
| Group*Time | 1.49 | 1/128 | .22 | |||||||||
Note: Boldface type indicates significance at p < .05.
Table 3.
Percent Participants Endorsing Side Effects on Rating Scale at Some Point Over Four-Week Blinded Trial: Active vs. Sham Trigeminal Nerve Stimulation
| Side Effect (% Reporting) |
Active (N=32) |
Sham (N=30) |
Side
Effect (% Reporting) |
Active (N=32) |
Sham (N=30) |
|---|---|---|---|---|---|
| Trouble sleeping | 19 | 17 | Rapid heartbeat | 3 | 0 |
| Nightmares | 6 | 0 | Out of breath | 3 | 3 |
| Drowsy | 22 | 13 | Nausea | 3 | 0 |
| Hyperactive | 41 | 63 | Stomachache | 6 | 3 |
| Fatigue | 13 | 3 | Constipation | 9 | 7 |
| Feels strange | 0 | 7 | Frequent urination | 6 | 0 |
| Tingling | 3 | 0 | Frequent sweating | 3 | 3 |
| Headache | 13 | 0 | Decreased appetite | 3 | 3 |
| Stuffy nose | 16 | 20 | Increased appetite | 19 | 7 |
| Muscle cramps | 3 | 3 | Skin rash | 6 | 0 |
| Muscle twitch | 0 | 7 | Finding words | 0 | 7 |
| Tremor | 0 | 3 | Apathy | 6 | 7 |
| Slurred speech | 0 | 3 | Clenching teeth | 13 | 7 |
Assessment of Study Blinding
Responses on the Early Impressions Questionnaire showed no differences predictive of group assignment on questions pertaining to belief in having an active or sham device: 1) how successful do you think your current treatment will be in reducing ADHD symptoms (Odds Ratio = .93, 95% CI = .76–1.15, p = .50), or 2) how much do you feel the current treatment will help reduce ADHD symptoms (Odds Ratio = .90, 95% CI = .70–1.14, p = .37).
DISCUSSION
This study demonstrated the efficacy and safety of TNS in ADHD treatment, confirming and expanding previous open-label findings.17 ADHD-RS response patterns suggest that the greatest degree of TNS-related improvement occurs during the first week, with additional improvement accruing with ongoing use. The week 4 medium-sized treatment effect is within the same range typically evidenced with non-stimulant ADHD medications.38 Weekly CGI-I ratings further indicate that response rates increase with sustained treatment, at least over four weeks. Worsening scores over the discontinuation week likely reflect in part an awareness of treatment cessation in both groups. Even with the parallel score declines, however, lower active ADHD-RS scores at week 5 compared with sham suggest some persistent benefit after treatment discontinuation. Together, results support the utility of TNS as a component of clinical ADHD management.
At a mechanistic level, TNS is thought to stimulate the nucleus tractus solitarius, which relays signals to cortical and subcortical structures such as the thalamus, hypothalamus, amygdala, locus coeruleus, reticular activating system, anterior cingulate and insula.12, 14, 17 Treatment-related changes in resting state qEEG measures suggest that middle and right frontal regions show increased activation with active TNS relative to sham. Furthermore, these changes are primarily associated with improvement in hyperactive and impulsive symptom changes. Previous scalp qEEG studies reported increased power in delta, theta, and beta frequency bands at right frontal electrodes with successful stopping within a stop signal task,39, 40 suggesting a significant association between right frontal cortex and inhibitory control. The right inferior frontal cortex, pre-supplemental motor area (SMA), and subthalamic nuclei (STN) are thought to be part of a fronto-basal-ganglia network utilized in suppression of motor behavior.41 Taken together, we hypothesize that the neurophysiological mechanism underlying TNS treatment effects in ADHD is activation of the fronto-basal ganglia network, resulting in increased EEG power in middle and right frontal electrodes and subsequent improvement in hyperactive and impulsive behaviors.
Many studies of nonmedication ADHD treatments are biased towards false positive findings, particularly when blinding is compromised or raters are highly invested in treatment success.42 Results from the Early Impressions Questionnaire showed no differences in outcome expectations between treatment groups after one week using the randomized device, suggesting that our sham procedures successfully accomplished double-blinding of group assignment. Improvements seen in both active and sham groups at week 1 likely reflect some placebo-response secondary to the high level of parental involvement in administering treatment. Nonetheless, further improvement over subsequent weeks with active TNS suggests emergence of true treatment effects, demonstrated in both clinician-rated ADHD-RS and CGI-I scores. In contrast, parent Conners ratings have significant time effects in both groups, but no group-by-treatment differences, likely due to some placebo response among all raters. EEG findings, which demonstrated clear treatment-related-differences in cortical activation, provide independent verification of positive behavioral outcomes unbiased by rater expectations. Small but measurable TNS effects on parent-reported anxiety provides further evidence of positive response.
As with previous reports, results confirm that TNS carries minimal risk and is well tolerated and accepted by ADHD-affected children and their parents.17 Adverse events had minimal clinical significance. While reports of headache and fatigue were associated with active TNS, no one abandoned treatment due to side effects. Increases in weight and reported appetite in the active group are not readily explained and require ongoing investigation in longer studies.
The potential significance of observed increased heart rate with active TNS remains unclear. Prior acute studies of TNS have revealed both increases17 and decreases14 in pulse. As with the vagus nerve, TNS is known to elicit parasympathetic activity, which is expected to result in pulse decreases or bradycardia.43 Pulse increases in this study, while statistically significant, were not within a clinically abnormal range and were not associated with clinical symptoms. ADHD stimulants are also associated with small increases in heart rate that are not viewed as clinically meaningful. Results derived from this small sample might also represent outlier findings not generalizable to larger groups. The issue clearly requires further investigation, but is not inconsistent with the assertion that TNS poses minimal risk.
The study assessed acute response to TNS over four weeks. It does not inform on whether additional improvement would accrue with ongoing treatment or whether benefits persist over time. There might have been some bias toward non-medication approaches to ADHD management by parents of study participants, but this view is common among many parents seeking ADHD treatment for their children. As such, results from this study should be widely generalizable, but support for TNS would be strengthened if replicated in additional patient groups. We did not assess potential utility of TNS as adjunctive therapy to standard ADHD interventions. The study failed to support several hypotheses arising from the open-label trial, particularly positive benefits seen in executive functioning, measured by the BRIEF, and selected sleep measures, measured by the CSHQ. However, since mean ratings on these measures were subclinical, it is unknown whether improvement might be evidenced if limited to those individuals with clinically significant difficulties. These relationships require additional analysis.
TNS is a non-medication minimal risk intervention with proven efficacy in reducing ADHD symptoms. Although the present study finds that only slightly more than half of those receiving therapy have clinically meaningful improvement, the virtual lack of significant side effects should make it a popular treatment choice for many patients with ADHD, particularly for parents who prefer to avoid psychotropic medication. The quality of evidence for TNS exceeds that which is available for many commercially available complementary interventions. TNS is potentially a valuable new addition to the ADHD treatment armamentarium.
Supplementary Material
Acknowledgments
This study was supported by National Institute of Mental Health grant R34 MH101282 (to Drs. McGough and Loo, Co-PIs). Study devices and some materials were provided by NeuroSigma, Inc. in response to an investigator-initiated request.
Disclosure: Dr. McGough has provided expert testimony on behalf of Janssen, Shire, and Tris Pharmaceuticals. Dr. Leuchter has received research support from Neuronetics, Breast Cancer Foundation, CHDI Foundation, and NeuroSigma. He has served as a consultant to Ionis Pharmaceuticals, CHDI Foundation, and NeoSyn Inc. He serves as Chief Scientific Officer for Brain Biomarker Analytics LLC (BBA); has stock options in NeoSync. Inc.; and equity interest in BBA. Dr. Cook has received research support to UCLA from NeoSync. Inc.; has been an advisor to Arctica Health, Cereve, and HeartCloud; has served as part of the management team of NeuroSigma, Inc. (on leave since 6/2016); and has been allocated stock options. His patents are assigned to the University of California. Drs. Sturm, Cowen, Sugar, and Loo, Ms. Tung, and Ms. Salgari report no biomedical financial interests or potential conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This study was presented as an abstract at the American Academy of Child and Adolescent Psychiatry’s 64th Annual Meeting, Washington, DC, October 23–28, 2017.
REFERENCES
- 1.Danielson ML, Visser SN, Chroni-Tuscano A, DuPaul GJ. A national description of treatment among United States children and adolescent with attention-deficit/hyperactivity disorder. J Pediatr. 2018;192:240–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brinkman WB, Simon JO, Epstein JN. Reasons why children and adolescent with attention-deficit/hyperactive disorder stop and restart taking medicine. Acad Pediatr. 2018:18:273–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Coletti DJ, Pappadopulos E, Katsiotas NJ, Berest A, Jensen PS, Kafantaris V. Parent perspectives on the decision to initiate medication treatment of attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol. 2012;22:226–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cortese S, Ferrn M, Brandeis D, et al. Neurofeedback for attention-deficit/hyperactivity disorder: meta-analysis of clinical and neuropsychological outcomes from randomized controlled trials. J Am Acad Child Adolesc Psychiatry. 2016;55:444–455. [DOI] [PubMed] [Google Scholar]
- 5.Cortese S, Ferrn M, Brandeis D, et al. Cognitive training for attention-deficit/hyperactivity disorder: meta-analysis of clinical and neuropsychological outcomes from randomized controlled trials. J Am Acad Child Adolesc Psychiatry. 2015;54:164–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cosmo C, Baptista AF, deAraújo AN, et al. A randomized, double-blind, sham-controlled trial of transcranial direct current stimulation in attention-deficit/hyperactivity disorder. PLoS One. 2015;10:e0135371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubio B, Boes AD, Laganiere S, Rotenberg A, Jeurissen D, Pascual-Leone A. Noninvasive brain stimulation in pediatric ADHD: a review. J Child Neurol. 2016;31:784–796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weaver L, Rostain AL, Mace W, Akhtar U, Moss E, O’Reardon JP. Transcranial magnetic stimulation (TMS) in the treatment of attention-deficit/hyperactivity disorder. J ECT. 2012;28:98–103. [DOI] [PubMed] [Google Scholar]
- 9.Schrader LM, Cook IA, Miller PR, Maremont ER, DiGiorgio CM. Trigeminal nerve stimulation in major depressive disorder: first proof of concept in an open pilot trial. Epilepsy Behav. 2011;22:475–478. [DOI] [PubMed] [Google Scholar]
- 10.Cook IA, Abrams M, Leuchter AF. Trigeminal nerve stimulation for comorbid posttraumatic stress disorder and major depressive disorder. Neuromodulation. 2016;19:299–305. [DOI] [PubMed] [Google Scholar]
- 11.Cook AI, Kealey CP, DeGiorgio CM. The potential use of trigeminal nerve stimulation in the treatment of epilepsy. Ther Deliv. 2015;6:273–275. [DOI] [PubMed] [Google Scholar]
- 12.Nolte J The human brain: an introduction to its functional anatomy. 4th ed St. Louis, MO; 1999. [Google Scholar]
- 13.Peterson SE, Posner MI. The attention system of the human brain: 20 years after. Annu Rev Neurosci. 2012;35:73–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mercante B, Enrico P, Floris G, et al. Trigeminal nerve stimulation induces FOS immunoreactivity in selected brain regions, increased hippocampal cell proliferation and reduces seizure severity in rats. Neuroscience. 2017;361:69–80. [DOI] [PubMed] [Google Scholar]
- 15.Cook IA, Espinoza R, Leuchter AF. Neuromodulation for depression: invasive and noninvasive (deep brain stimulation, transcranial magnetic stimulation, trigeminal nerve stimulation). Neurosurg Clin N Am. 2014;25:103–16. [DOI] [PubMed] [Google Scholar]
- 16.DeGiorgio CM, Fanselow EE, Schrader LM, Cook IA. Trigeminal nerve stimulation: seminal animal and human studies for epilepsy and depression. Neurosurg Clin N Am. 2011;22:449–56. [DOI] [PubMed] [Google Scholar]
- 17.McGough JJ, Loo SK, Sturm A, Cowen J, Leuchter AF, Cook IA. An eight-week, open-trial, pilot feasibility study of trigeminal nerve stimulant in youth with attention-deficit/hyperactivity disorder. Brain Stimul. 2015;8:299–304. [DOI] [PubMed] [Google Scholar]
- 18.Kaufman J, Birmaher B, Brent D, et al. Schedule for affective disorders and schizophrenia for school aged children – present and lifetime version (K-SADS-PL). J Am Acad Child Adolesc Psychiatry. 1997;36:980–988. [DOI] [PubMed] [Google Scholar]
- 19.DuPaul GJ, Power RJ, Anastopoulos AD, Reid R. ADHD Rating Scale-IV Checklist , Norms and Clinical Interpretations. New York: Guilford Press; 1998. [Google Scholar]
- 20.Guy W EDCEU Assessment Manual for Psychopharmacology (Revised). Washington, D.C.: US Dept. Health, Education, and Welfare; 1976. [Google Scholar]
- 21.Wechsler Abbreviated Scale of Intelligence, 3rd Edition Pearson Education Inc.; 1997. [Google Scholar]
- 22.Achenbach TM. Manual for the Child Behavior Checklist/4–18. Burlington, VT: University of Vermont, Department of Psychiatry; 1999. [Google Scholar]
- 23.Stringaris A, Goodman R, Ferdinando S, et al. The Affective Reactivity Index: a concise irritability scale. J Child Psychol Psychiatry. 2012;53:1109–1117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.DeGiorgio CM, Soss J, Cook IA, et al. Randomized controlled trial of trigeminal nerve stimulation for drug-resistant epilepsy. Neurology. 2013;80:786–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Devilly GJ, Borkovec TD. Psychometric properties of the credibility/expectancy questionnaire. J Beh Ther Exp Psychiatry. 2000;31:73–86. [DOI] [PubMed] [Google Scholar]
- 26.Loo SK, Bilder RM, Cho AL, et al. Effects of d-methylphenidate, guanfacine, and their combination on electroencephalogram resting state spectral power in attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2016;55:674–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004;134:9–21. [DOI] [PubMed] [Google Scholar]
- 28.Gioia GA, Isquith PK, Guy SC, Kenworthy L. Test review Behavior Rating Inventory of Executive Functioning. Child Neuropsychology. 2000;6:235–238.11419452 [Google Scholar]
- 29.Conners CK. Conners’ Global Index. Canada: Multi-Health Systems Inc.; 1997. [Google Scholar]
- 30.Owens JA, Spiritio A, McGuinn M. The Children’s Sleep Habits Questionnaire (CSHQ): psychometric properties of a survey instrument for school-aged children. Sleep. 2000;15:1043–1051. [PubMed] [Google Scholar]
- 31.March J Multidimensional Anxiety Scale for Children. Canada: Multi-Health Systems Inc.; 1997. [Google Scholar]
- 32.Poznanski EO, Freeman LN, Mokros HB. Children’s Depression Rating Scale-Revised. Psychopharmacol Bull. 1985;21:979–989. [Google Scholar]
- 33.Glahn DC, Kim J, Cohen MS, et al. Maintenance and manipulation in spatial working memory: dissociations in the prefrontal cortex. Neuroimage. 2002;17:201–213. [DOI] [PubMed] [Google Scholar]
- 34.Fan J, Wu Y, Fossella JA, Posner MI. Assessing the heritability of attentional networks. BMC Neurosci. 2001;2:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Posner K, Brown GK, Stanley B, et al. Columbia-Suicide Severity Rating Scale: initial validity and internal consistency findings from three multisite studies with adolescents and adults. Am J Psychiatry. 2011;168:1266–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cohen J Statistical Power Analysis for the Behavioral Sciences, Second Edition Mahwah, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 37.Kramer HC, Kupfer DJ. Size of treatment effects and their importance to clinical research and practice. Biol Psychiatry. 2006;59:990–996. [DOI] [PubMed] [Google Scholar]
- 38.Faraone SV, Biederman J, Spencer TJ, Aleardi M. Comparing the efficacy of medications for ADHD using meta-analysis. MedGenMed. 2006;8:4. [PMC free article] [PubMed] [Google Scholar]
- 39.Huster RJ, Enriquez-Geppert S, Lavalle CF, Falkenstein M, Herrmann CS. Electroencephalography of response inhibition tasks: functional networks and cognitive contributions. Int J Psychophysiol. 2013;87:217–233. [DOI] [PubMed] [Google Scholar]
- 40.Wagner J, Wessel J, Gharahmeni A, Aron A. Establishing a right frontal beta signature for stopping active in scalp electroencephalography: implications for testing inhibitory control in other task contexts. J Cognitive Neuroscience. 2018;1:107–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wessel JR, Aron AR. On the globality of motor suppression: unexpected events and their influence on behavior and cognition. Neuron. 2017:93:259–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sonuga-Barke EJ, Brandeis D, Cortese S, et al. Nonpharmacological interventions for ADHD: systematic review and meta-analyses of randomized controlled trials of dietary and psychological treatments. Am J Psychiatry. 2013;170:275–289. [DOI] [PubMed] [Google Scholar]
- 43.Kumada M, Dampney RA, Reis DJ. The trigeminal depressor response: a novel vasodepressor response originating from the trigeminal system. Brain Res. 177;119:305–326. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.