Abstract
Our objective was to determine the relationship of T1rho and T2 relaxation mapping to the biochemical and biomechanical properties of articular cartilage through selective digestion of proteoglycans and collagens. Femoral condyles were harvested from porcine knee joints and treated with either chondroitinase ABC (cABC) followed by collagenase, or collagenase followed by cABC. Magnetic resonance (MR) images were acquired and cartilage explants were harvested for biochemical, biomechanical, and histological analyses before and after each digestion. Targeted enzymatic digestion of proteoglycans with cABC resulted in elevated T1rho relaxation times and decreased sulfated glycosaminoglycan (sGAG) content without affecting T2 relaxation times. In contrast, extractable collagen and T2 relaxation times were increased by collagenase digestion; however, neither was altered by cABC digestion. Aggregate modulus decreased with digestion of both components. Overall, we found that targeted digestion of proteoglycans and collagens had varying effects on biochemical, biomechanical, and imaging properties. T2 relaxation times were altered with changes in extractable collagen, but not changes in proteoglycan. However, T1rho relaxation times were altered with proteoglycan loss, which may also coincide with collagen disruption. Since it is unclear which matrix components are disrupted first in osteoarthritis, both markers may be important for tracking disease progression.
Keywords: MRI, validation, osteoarthritis, articular cartilage, collagens, proteoglycan, biomarkers
Introduction
Osteoarthritis (OA) is a common and debilitating disease, affecting approximately 27 million adults in the United States [29]. While there are a number of risk factors for OA, including age, obesity, genetics, and joint injury, studies suggest that biomechanical factors play a critical role in the onset and progression of this disease [2, 50, 51, 64, 66]. OA is characterized primarily by the progressive loss of articular cartilage and remodeling of surrounding joint tissues [38]. Articular cartilage consists primarily of type II collagen fibers, proteoglycans, and water, with a small volume fraction of chondrocytes in the extracellular matrix (ECM). A breakdown in these components is an early indicator of the presence of OA [20]. Specific biochemical changes associated with disease onset and progression include a decrease in both proteoglycan and collagen content and a disruption of the collagen organization within the cartilage matrix, which is attributed in part to the activity of various enzymes such as matrix metalloproteinases (MMPs) and aggrecanases [7, 20, 23, 27, 28].
Changes in the biomechanical properties of articular cartilage that occur with OA include decreased aggregate modulus as well as increased matrix permeability [35, 37, 49]. The loss of proteoglycans in the matrix results in increased hydraulic permeability and decreased fixed charge density, affecting the ability of cartilage to appropriately support mechanical loads [34, 37]. Additionally, disruption of collagen integrity has been shown to decrease the compressive modulus of cartilage and lead to further loss of proteoglycans [14, 16, 19]. However, it remains to be determined whether the in vivo changes associated with OA involve degradation of the collagen or the glycosaminoglycan components first, and whether loss of one of the major constituents renders other tissue components susceptible to further enzymatic degradation [24, 36]. The ability to non-invasively determine the sequence of changes in cartilage composition, as well as their relationship to tissue mechanical properties, could greatly facilitate the early diagnosis and treatment of OA.
Quantitative magnetic resonance (MR) imaging techniques, namely T1rho and T2 relaxation mapping, have been used to study changes in both proteoglycan concentration (T1rho), as well as collagen concentration and organization (T2) within articular cartilage [1, 19, 26, 32, 43, 44, 61, 63, 64]. These quantitative MR imaging techniques allow for a non-invasive assessment of early changes in the cartilage matrix associated with knee OA. However, there is little data quantifying the relationship of both T1rho and T2 imaging biomarkers to the biochemical and biomechanical properties of cartilage following selective and sequential degradation of cartilage components. Therefore, our study utilized a novel repeated measures approach to specifically and sequentially digest cartilage components to elicit changes in imaging biomarkers (T1rho and T2), biochemical properties, and biomechanical properties. We hypothesized that proteoglycan digestion would increase T1rho relaxation times and decrease sulfated glycosaminoglycan (sGAG) content, while collagen digestion would increase T2 relaxation times and the percentage of extractable collagen. Furthermore, these changes would result in decreases in cartilage aggregate modulus.
Materials and Methods
Specimen Preparation
Lateral femoral condyles (n=16) were harvested from skeletally mature, female porcine knee joints obtained from a local abattoir. Knee joints had no gross evidence of OA. Immediately after removal from the femur, the lateral condyles were fixed to the bottom of a plastic container and immersed in a solution of Dulbecco’s modified Eagle’s medium (DMEM) containing 10 units/mL penicillin/streptomycin/fungizone (PSF) and incubated for one hour at 37°C. Following the PSF wash, the antibiotics were removed and replaced with phosphate-buffered saline (PBS) to cover the condyles.
Study Procedure
For MR imaging, all condyles were fixed to the bottom of a plastic container and immersed in PBS (Figure 1). PBS immersed condyles were placed in the same orientation in a 3T scanner (Trio Tim, Siemens) with an 8 channel knee coil. Sagittal GRE T1rho- and T2- weighted images were acquired in single slices perpendicular to the articular surface (Figure 2A). A T1rho- weighted pulse sequence with the following imaging parameters was used: FOV=14 cm, matrix=256×256 pixels, slice thickness=3 mm, TR/TE=3500/13 ms, B1=500 Hz. A series of seven T1rho-weighted images was collected using spin lock times (TSL) of 5, 10, 20, 40, 60, 80, and 100 ms. T2 relaxation times were acquired from the T2 imaging sequence with the following parameters: FOV=16 cm, matrix=384×384 pixels, slice thickness = 3 mm, TR=3500 ms, and the following echo times (TE)=13.8, 27.6, 41.4, 55.2, 69.0, 82.8, 96.6 ms [5, 19]. The resulting signal intensities were fit to a set of exponential equations in order to derive T1rho and T2 relaxation times [5, 19].
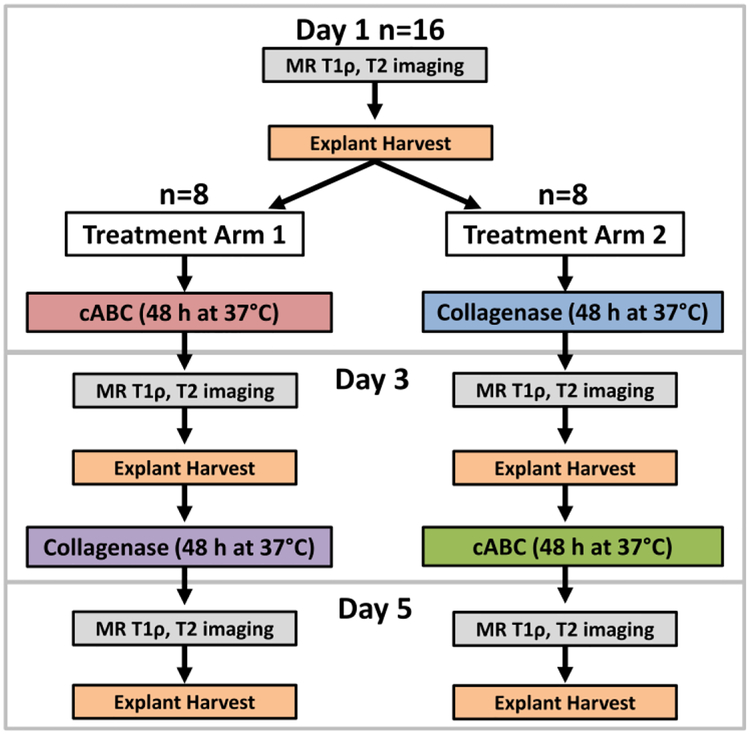
Figure 1.
Study design showing both treatment arms and the order in which MR imaging, explant harvesting, and enzymatic depletion occurred. cABC=chondroitinase ABC
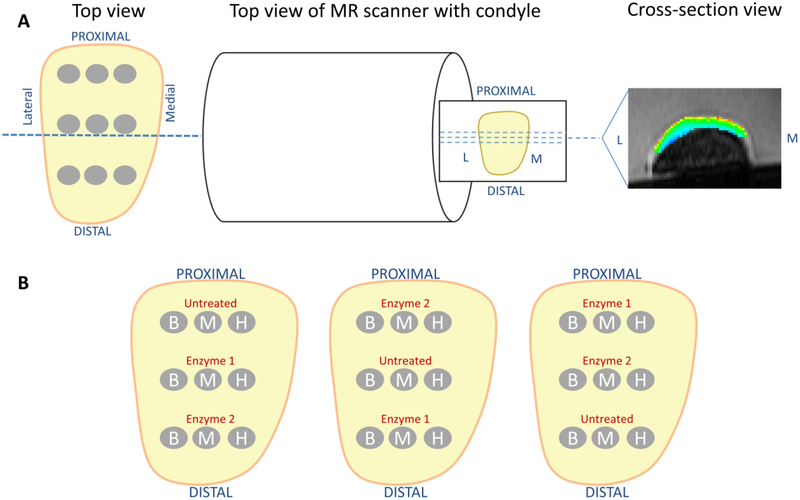
Figure 2.
A) One representative slice showing sample harvest locations relative to the direction of MR scanner orientation and T1rho and T2 slice acquisition. All condyles were positioned with the same orientation within the MR scanner. B) Diagrams indicating the three different variations of locations of all harvested explants. For treatment arm 1, enzyme 1 is cABC and enzyme 2 is collagenase. For treatment arm 2, enzyme 1 is collagenase and enzyme 2 is cABC. B = Biochemical, M = Mechanical, H = Histology.
Following the initial MR imaging, three 5mm cartilage explants were harvested from each condyle in an alternating sequence for biochemical, biomechanical, and histological analyses (untreated group) (Figure 2B). Enzymatic digestions were then performed to selectively and sequentially target proteoglycans and collagens. Chondroitinase ABC (cABC) (Sigma) was used to deplete sGAG via digestion of chondroitin and dermatan sulfates and hyaluronic acid from proteoglycans [47, 64]. Collagenase was chosen to digest collagen fibrils in the ECM [18, 53]. In treatment arm 1, condyles were digested with 0.15 units/mL cABC (Sigma) at 37°C for 48 hours with agitation (Figure 1). In treatment arm 2, condyles were digested with 10 units/mL type II collagenase (Worthington) at 37°C for 48 hours with agitation (Figure 1). Enzymes were applied for 48 hours to allow ample time to diffuse into the cartilage extracellular matrix. Following enzymatic digestion, all condyles were reaffixed to the plastic container and immersed in PBS for a second set of MR images. A second set of three explants was then harvested and stored for biochemical, biomechanical, and histological analyses. The condyles were then digested in either 10 units/mL type II collagenase for 48 hours at 37°C in treatment arm 1 or with 0.15 units/mL cABC for 48 hours at 37oC in treatment arm 2 (Figure 1). Following digestion, condyles were MR imaged for a third and final time and three additional explants were then harvested for analyses.
Histological Analyses
For histological analyses, frozen explants were placed in optimal cutting temperature (OCT) medium (Sakura Finetek, Torrance, CA) and frozen at −20°C. Tissues were cut to 8μm thick slices, placed on slides, and allowed to dry. Sections were formalin fixed for 10 minutes and stained with Harris Hematoxylin with glacial acetic acid (Poly Scientific, Bay Shore, NY), 0.02% aqueous fast green (Sigma-Aldrich), and Safranin-O (Sigma-Aldrich) to visually assess cell nuclei, collagens, and proteoglycans, respectively. Stained sections were viewed on an Axiovert S100 microscope (Zeiss) and photographed using a digital camera (Nikon, Melville, NY).
Biochemical Analyses
The explants for biochemical analyses were cut in half perpendicular to the cartilage surface, wet weights were determined, and samples were then lyophilized for 24 hours and weighed again to attain dry weights. The water content (%) was calculated as the difference between the wet and dry weights divided by the original wet weight and multiplied by 100.
One half of each explant for biochemical analyses was papain digested at 65°C for 24 hours [48]. sGAG content was determined using the dimethylmethylene blue (DMMB) assay with a standard curve of chondroitin-4-sulfate type A from bovine trachea (Sigma) in papain [13]. The second half of each explant for biochemical analyses was digested in α-chymotrypsin (Sigma) at 37°C overnight to solubilize the extractable (cleaved) collagen [22]. After α-chymotrypsin digestion, the supernatant was collected and the remaining tissue was digested in papain at 65°C for 24 hours. Collagen content was measured in the papain digested and chymotrypsin supernatant samples, using the hydroxyproline assay and a standard curve of trans-4-hydroxyproline (Sigma) [10, 67]. Total collagen content was calculated as the sum of the collagen content in both the papain and α-chymotrypsin digested samples. To quantify the percent of extractable collagen in each explant, the collagen content in the α-chymotrypsin fraction was divided by the total collagen content and multiplied by 100.
Confined Compression Testing
Disks (3 mm in diameter) were harvested from the cartilage explants and placed in a previously described confined compression setup [19, 39]. Compressive loads were applied with a rigid porous platen in a confined compression materials testing machine (ELF 3100, Bose, Minnetonka, MN). A 2 gf tare load was used to equilibrate the explants. A step compressive load of 17 gf was then applied and the samples were allowed to equilibrate until the change in displacement was less than 0.001 mm over a period of 100 seconds [19, 39]. The resulting aggregate modulus was determined using a non-linear least-squares regression model [39, 41].
Statistical Analyses
Repeated measures analysis of variance (ANOVA) was used to determine statistically significant differences in outcome measures with a normal distribution (sGAG, % extractable collagen, % water content, T1rho, T2) (p<0.05). Post-hoc testing was performed using the Fisher’s least square difference test. A non-parametric analysis was performed for outcome measures that were not normally distributed (total collagen and aggregate modulus) using the Kruskal-Wallis ANOVA test by ranks (p<0.05).
Results
Histological Analyses
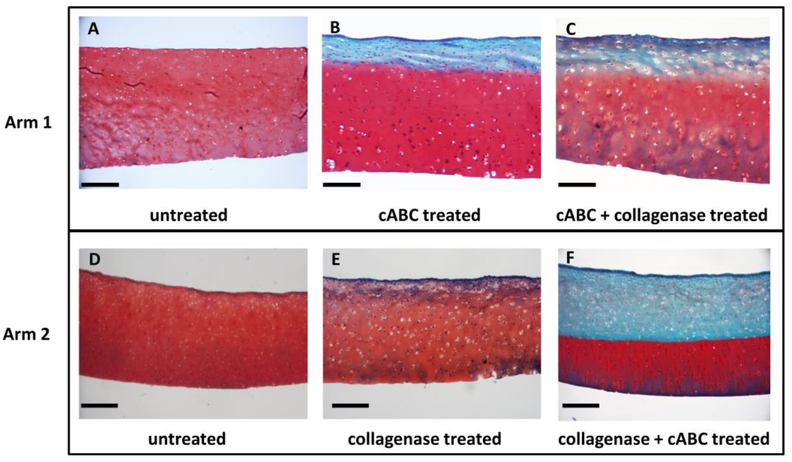
Histological staining of cartilage explants revealed intact cartilage matrices with abundant Safranin-O staining in the untreated group (Figure 3A, D). In treatment arm 1 with cABC treatment, approximately the top quarter of the tissue was depleted of sGAG staining (Figure 3B). Further digestion of the tissue by collagenase revealed no obvious changes in fast green or proteoglycan staining throughout the tissue (Figure 3C). In treatment arm 2, collagenase digestion yielded a slight decrease in Safranin-O staining throughout the tissue and no changes in fast green staining (Figure 3E). However, following subsequent cABC treatment, tissues demonstrated loss of proteoglycan staining throughout the top half of the tissue and no obvious changes in collagen content (Figure 3F).
Figure 3.
Cartilage explants were stained with safranin-O (red), fast green (blue), and hematoxylin (black) to indicate the presence of proteoglycans, collagen, and cell nuclei, respectively. Images are representative samples from each group. cABC = chondroitinase ABC. Scale bar is 100 μm.
Biochemical Composition
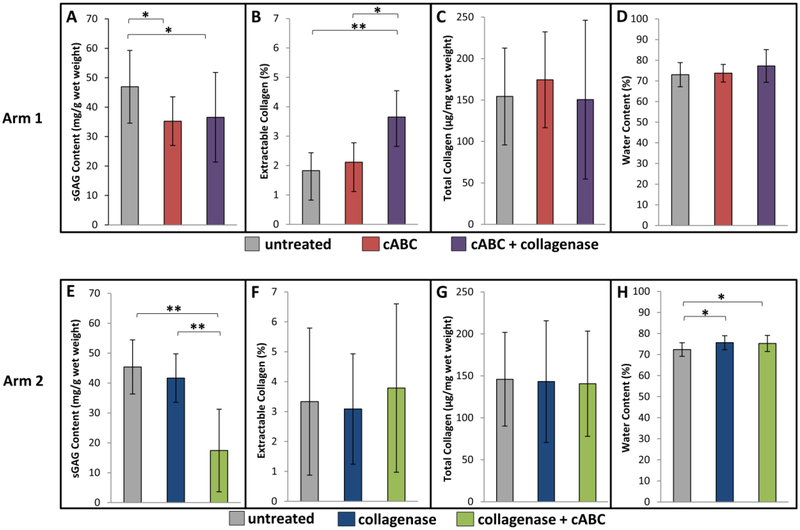
In treatment arm 1, sGAG content decreased when condyles were digested with cABC alone (Figure 4A, p<0.05), and there was no further decrease in the sGAG content by subsequent collagenase digestion. In treatment arm 2, sGAG content slightly decreased on average with the addition of collagenase, but these differences were not statistically significant; however, sGAG content significantly decreased when condyles were subsequently digested with cABC (Figure 4E, p<0.001).
Figure 4.
Mean (±SD) (A, E) sGAG Content; (B, F) % Extractable Collagen; (C, G) Total Collagen Content; and (D, H) % Water Content. cABC = chondroitinase ABC. * p<0.05, ** p<0.001
In treatment arm 1, the percentage of extractable collagen was not altered with cABC digestion, but did increase significantly with subsequent collagenase treatment (Figure 4B, p<0.05). In treatment arm 2, no significant changes were measured in the percentage of extractable collagen between treatment groups (Figure 4F). Additionally, total collagen content did not significantly change between treatment groups in either treatment arm (Figure 4C, 4G).
The percent water content did not change significantly between treatment groups in treatment arm 1 (Figure 4D). However, in treatment arm 2, water content increased significantly when condyles were first treated with collagenase, and when collagenase was followed by cABC, as compared to the untreated group (Figure 4H, p<0.05).
Biomechanical Properties
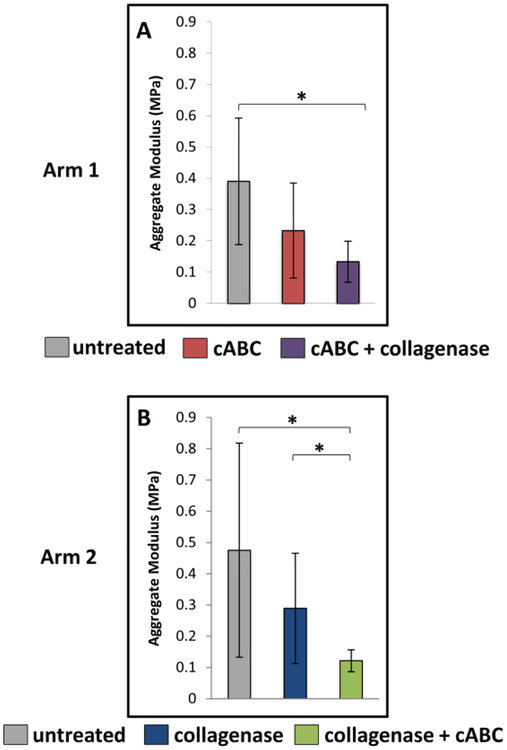
In treatment arm 1, aggregate modulus decreased on average with cABC digestion, but this difference was not significant (Figure 5A); however, with subsequent collagenase digestion the aggregate modulus significantly decreased compared to the untreated group (p<0.05). In treatment arm 2, there was a significant decrease in aggregate modulus between the collagenase and collagenase + cABC groups (p<0.05) and between the untreated and collagenase + cABC groups (Figure 5B, p<0.05).
Figure 5.
Mean (±SD) Aggregate Modulus of cartilage explants for treatment arm 1 (A) and treatment arm 2 (B). cABC = chondroitinase ABC. * p<0.05
MR Imaging Analyses
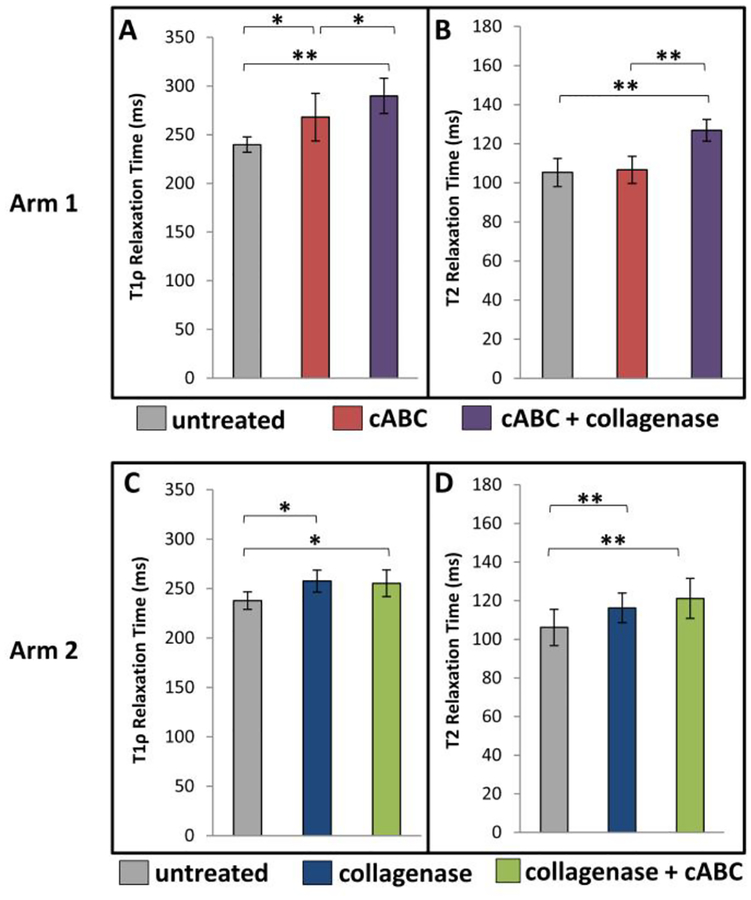
With targeted digestion using cABC in treatment arm 1, T1rho relaxation times significantly increased (Figures 6A, 7, p<0.05), but no change in T2 relaxation times was observed (Figures 6B, 7). Subsequent digestion using collagenase resulted in a further increase in T1rho relaxation times (Figures 6A, 7, p<0.05) and a significant increase in T2 relaxation times (Figures 6B, 7, p<0.001).
Figure 6.
Mean (±SD) (A, C) T1rho relaxation time and (B, D) T2 relaxation time of cartilage from treatment arm 1 (A, B) and treatment arm 2 (C, D). * p<0.05, ** p<0.001
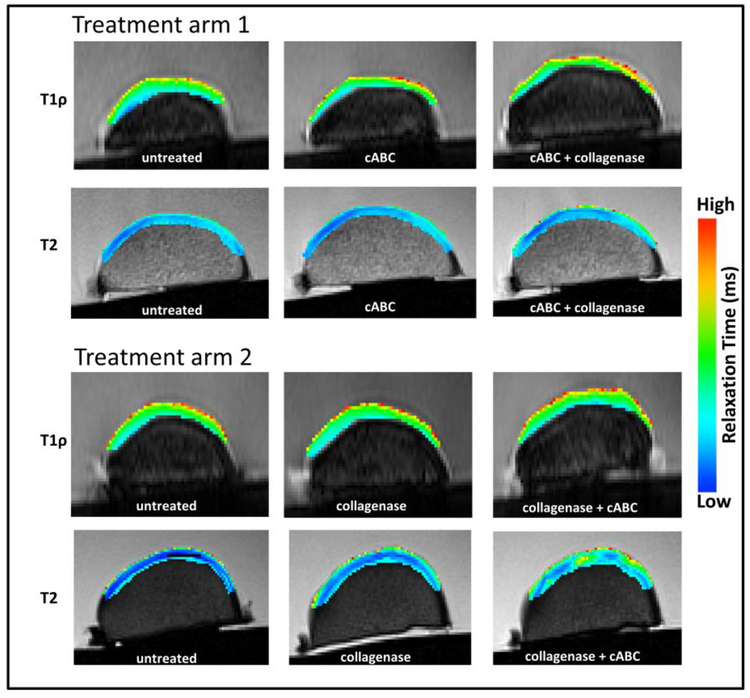
Figure 7.
Representative sagittal T1rho and T2 color maps of porcine articular cartilage from untreated and enzymatically depleted samples.
When condyles were treated with collagenase alone in treatment arm 2, T1rho relaxation times significantly increased (Figures 6C, 7, p<0.05), but did not increase further following cABC digestion. Additionally, when condyles were treated with collagenase alone in treatment arm 2, T2 relaxation times significantly increased (Figures 6D, 7, p<0.001) with no further increase in T2 relaxation times when condyles were subsequently digested with cABC.
Discussion
Early detection of articular cartilage degeneration is important for the diagnosis of OA as well as evaluating disease progression and potential therapeutic interventions. Non-invasive quantitative MR imaging techniques can assess early signs of OA, such as the loss of ECM components and disruption of ECM organization [19, 30, 31, 60, 63]. Specifically, T1rho and T2 relaxation mapping are two MR imaging techniques that allow for the quantification of proteoglycan and collagen organization within articular cartilage, respectively [1, 11, 25, 26, 31, 32, 43, 45, 63]. Several studies have previously examined the relationship of these MR imaging parameters with the mechanical and biochemical properties of bovine, porcine, and human cartilage [26, 43, 44, 61]. However, this study is unique in that T1rho and T2 relaxation mapping were used to assess changes in cartilage composition with corresponding changes in biomechanical and biochemical properties of articular cartilage following sequential enzymatic digestion of cartilage components. We demonstrated that cABC digestion of sGAGs led to elevated T1rho relaxation times, while T2 relaxation times remained unchanged. Additionally, disruption of the collagen organization through administration of collagenase increased the percent of extractable collagen, concomitant with increased T2 relaxation times. Aggregate modulus significantly decreased with the loss of both sGAGs and collagens.
Histological analyses provided visual evidence of selective depletion of cartilage components. In the first treatment arm, upon initial treatment with cABC, explants demonstrated reduced Safranin-O staining while the integrity of the collagen matrix appeared intact, as evidenced by fast green staining in the superficial zone. In the second treatment arm, samples demonstrated continued presence of proteoglycans when treated with collagenase and a large reduction in the intensity of safranin-O staining in explants that were treated with cABC. Supporting these results, our biochemical analyses demonstrated decreased proteoglycans with cABC digestion, but no significant change in the total collagen content following collagenase digestion. We found significant increases in the percentage of extractable collagen in the first treatment arm following collagenase digestion, which corresponded to an increase in T2 relaxation times, a surrogate measure for changes in collagen organization within the matrix [32, 42, 62].
Targeted digestion of cartilage components in this study demonstrates the utility of T1rho and T2 relaxation times as non-invasive measures of cartilage composition. Specifically, selective digestion of sGAGs by cABC resulted in a statistically significant increase in T1rho relaxation times with no change in T2 relaxation times. These results confirm the findings of other studies that have used T1rho relaxation mapping to quantify changes in proteoglycan content within articular cartilage [1, 8, 12, 19, 32, 47, 54, 58, 60, 64]. For example, Li et al. demonstrated higher T1rho relaxation times in the cartilage of OA patients than in healthy subjects and also correlated T1rho relaxation times with OA severity [30, 32]. They also observed no relationship between T1rho and collagen content [32]. Similarly, Duvvuri et al. demonstrated increased T1rho relaxation times in bovine patellar cartilage that had undergone enzymatic depletion of proteoglycans. Furthermore, no significant changes were observed in the T1rho relaxation times of samples treated with collagenase [12]. Additionally, a previous study from our group showed that in porcine knee joints, T1rho relaxation times were increased in OA regions compared to normal regions of the same joint and were negatively correlated with sGAG content in the tissue [19]. Further supporting the relationship of T1rho and proteoglycan content, Akella et al. depleted proteoglycans by enzymatic degradation and found a strong correlation between changes in proteoglycan content and T1rho relaxation times [1]. However, the present study also examined the mechanical properties of cartilage and found significant changes in the aggregate modulus of the tissue following enzymatic digestion, coinciding with changes in proteoglycan and collagen content by imaging and biochemical analyses.
In this study, we reversed the order of digestion in the two arms to isolate the influence of sequence-specific effects. While there may have been a minor loss of extracellular matrix components throughout the experiment, this likely resulted in negligible effects compared to the influence of enzymatic digestion. In this study, we harvested explants from the same condyles throughout the course of the experiment and removed each explant with minimal disruption to the surrounding tissue. However, the removal of the explants likely allowed greater access of the enzymes to the tissue immediately adjacent to the explant harvest sites. Therefore, subsequent imaging and explant harvests occurred in areas away from prior harvest sites (Figure 2A). All samples were treated in the same fashion to maintain consistency among groups. Furthermore, while biochemical and histological explants provided data at a single site, imaging provided measures from a broader region across the condyle. Therefore, there may be some variability between measures acquired using imaging and biochemical, biomechanical, and histological analyses.
An important conclusion from the present study was the finding that T2 relaxation mapping is not sensitive to changes in proteoglycan content within cartilage. Specifically, we found no changes in T2 relaxation times following targeted enzymatic digestion of sGAGs with cABC. However, T1rho relaxation times were increased by both cABC and collagenase digestion. This increase in T1rho relaxation times following the loss of the collagen matrix integrity may be due to loss of proteoglycans that had been restrained by the collagen fibrils, influx of water, or a combination of both. Furthermore, in treatment arm 2, there was a significant increase in T1rho relaxation times following collagen digestion with no further increase after cABC degradation, suggesting that many of the proteoglycans were released from the ECM during the collagenase digestion (Figure 6C). On the other hand, we found that following cABC digestion, the addition of collagenase resulted in statistically significant increases in T2 relaxation times, corresponding to an increase in the percentage of extractable collagen. These results are similar to those of Fleck et al. who showed that sequential treatment of cABC followed by collagenase resulted in increased cartilage degradation compared to cABC or collagenase alone [15]. Therefore, the action of collagenase preceded by cABC may have a greater effect on collagen matrix degradation than collagenase alone [15] or than collagenase prior to cABC.
Our findings suggest that T2 relaxation times are sensitive to disruption in collagen organization. In these experiments, the total amount of collagen was not changed but the amount of extractable collagen increased with collagenase digestion. This is consistent with previous studies in the literature [32, 46, 62]. Specifically, Li et al. found no correlation between T2 relaxation times and total collagen composition [32]. Similarly, Wei et al. found no significant relationship between T2 relaxation times and collagen content; the authors speculated that T2 relaxation times may better reflect alterations in collagen organization rather than a change in collagen content within the cartilage [62]. Interestingly, T2 relaxation times have been found to be higher in cartilage with moderate OA compared to severe OA [9, 19] possibly due to alterations in collagen alignment and organization near the articular surface that occur before the loss of extracellular matrix during OA progression [4, 17, 55]. In the present study, to measure degraded collagen we treated explants with α-chymotrypsin to solubilize the extractable (cleaved) collagen [3, 21, 22]. We have previously shown a significant increase in the percentage of extractable collagen in OA regions of cartilage compared to normal regions of cartilage within the same joint [19]. Disruptions in the collagen organization occurring within the ECM may allow water to enter the tissue, resulting in increases in water content, extractable collagen, and T2 signal. Taken together, these findings are consistent with several previous studies showing that enzymatic digestion of collagen in vitro or in early OA may not alter total collagen content despite observed changes in collagen organization, imaging properties, and tissue mechanical properties [19, 32, 62].
The biochemical properties of cartilage were also affected by selective digestion of proteoglycans and collagens, which serves to validate our digestion protocols and the results of our imaging analyses. The loss of both collagen integrity and proteoglycans resulted in a significant reduction in aggregate modulus; therefore, the compressive stiffness of cartilage is likely sensitive to the combined loss of proteoglycans and disruption in the organization and alignment of collagen fibrils within the matrix. Additionally, in a cytokine-induced model of bovine cartilage degeneration, Wheaton et al. demonstrated progressive changes in biochemical content with increasing duration of interleukin-1 (IL-1) exposure and these changes corresponded to reduced aggregate modulus [64]. Compressive stiffness has also been shown to be reduced by disruptions to collagen fibril alignment at the cartilage surface that occur early in the progression of OA [16, 59]. Changes in the aggregate modulus, or stiffness, of the tissue may be the result of a reduction in the matrix components that contribute to the fixed charge density and, thus, stability of the matrix. The superficial zone may play an important role in the compressive viscoelastic behavior of cartilage and changes in the properties near the surface, namely collagen fibrillation, may preferentially affect the mechanical properties of the tissue [52]. A decrease in cartilage stiffness occurred with depletion of proteoglycans and further with a disruption of the collagen matrix, which is also reflected by an increase in the percentage of extractable collagen and an increase in T2 relaxation times. In treatment arm 2, the collagenase digestion did not significantly affect sGAG content as measured biochemically, despite changes in T1rho relaxation times. This may be due to differences between the local measurements of proteoglycan content assessed using biochemistry versus the more global assessment of T1rho relaxation times across the surface of the condyle, or could indicate that T1rho is a more sensitive measure of changes in proteoglycan content. Importantly, disruption of both collagen and proteoglycans resulted in the largest changes in mechanical properties. Therefore, our results demonstrate the ability to selectively and sequentially deplete cartilage components with corresponding changes in biochemical and biomechanical properties and imaging biomarkers.
In applying these imaging biomarkers clinically, it is important to note that these tools are sensitive to cartilage loading history [19, 40, 56]. For example, in healthy human subjects, we have shown that walking results in decreased T1rho relaxation times, consistent with water exudation and an increase in proteoglycan concentration with joint loading [19]. Future work could potentially use these imaging biomarkers, in combination with novel tools quantifying the mechanical response of cartilage [6, 33, 57, 65], to investigate the relationship between altered mechanical loading and changes in cartilage composition in populations at risk of developing OA.
Our results indicate that selective depletion of proteoglycans and collagens can elicit changes in T1rho and T2 relaxation times, respectively. However, T1rho relaxation time may be a better measure of proteoglycan loss that occurs coincident with collagen degradation and thus, a more sensitive imaging biomarker of ECM disruption. Interestingly, sequential digestion of proteoglycans and collagens did not result in the same T1rho and T2 relaxation times or biochemical properties. As it is unclear whether proteoglycan loss precedes collagen breakdown or vice versa in the pathway to OA, both T1rho and T2 imaging biomarkers are therefore useful for tracking the progression from healthy to degraded cartilage. Early identification of cartilage degeneration, such as that detected using T1rho and T2 imaging, is critical to the design of therapeutic interventions aimed at ultimately halting the initiation and progression of this devastating disease.
Acknowledgements
The authors would like to thank the National Institutes of Health (AR065527, AR066477, AG15768, AG028716, AG46927), the Veteran’s Affairs Rehabilitation Research Service Award, the Orthopaedic Research and Education Foundation, and the AO Foundation for financial support of this work. Additionally, the authors would like to thank the Duke Center for Advanced Magnetic Resonance Development (CAMRD) for their assistance with MR imaging.
Footnotes
The authors have no conflicts of interest.
References
- 1.Akella SV, Regatte RR, Gougoutas AJ, Borthakur A, Shapiro EM, Kneeland JB, Leigh JS, and Reddy R, Proteoglycan-induced changes in T1rho-relaxation of articular cartilage at 4T. Magn Reson Med, 2001. 46(3): p. 419–23. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong CG and Mow VC, Variations in the intrinsic mechanical properties of human articular cartilage with age, degeneration, and water content. J Bone Joint Surg Am, 1982. 64(1): p. 88–94. [PubMed] [Google Scholar]
- 3.Bank R, Krikken M, Beekman B, Stoop R, Maroudas A, Lafeber F, and te Koppele J, A simplified measurement of degraded collagen in tissues: application in healthy, fibrillated and osteoarthritic cartilage. Matrix Biology, 1997. 16(5): p. 233–43. [DOI] [PubMed] [Google Scholar]
- 4.Billinghurst RC, Dahlberg L, Ionescu M, Reiner A, Bourne R, Rorabeck C, Mitchell P, Hambor J, Diekmann O, Tschesche H, Chen J, Van Wart H, and Poole AR, Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J Clin Invest, 1997. 99(7): p. 1534–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borthakur A, Wheaton A, Charagundla SR, Shapiro EM, Regatte RR, Akella SV, Kneeland JB, and Reddy R, Three-dimensional T1rho-weightedMRI at 1.5 Tesla. J Magn Reson Imaging, 2003. 17(6): p. 730–6. [DOI] [PubMed] [Google Scholar]
- 6.Carter TE, Taylor KA, Spritzer CE, Utturkar GM, Taylor DC, Moorman CT 3rd, Garrett WE, Guilak F, McNulty AL, and DeFrate LE, In vivo cartilage strain increases following medial meniscal tear and correlates with synovial fluid matrix metalloproteinase activity. J Biomech, 2015. 48(8): p. 1461–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Catterall JB, Stabler TV, Flannery CR, and Kraus VB, Changes in serum and synovial fluid biomarkers after acute injury (NCT00332254). Arthritis Res Ther, 2010. 12(6): p. R229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi JA and Gold GE, MR imaging of articular cartilage physiology. Magn Reson Imaging Clin N Am, 2011. 19(2): p. 249–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.David-Vaudey E, Ghosh S, Ries M, and Majumdar S, T2 relaxation time measurements in osteoarthritis. Magn Reson Imaging, 2004. 22(5): p. 673–82. [DOI] [PubMed] [Google Scholar]
- 10.Detamore MS and Athanasiou KA, Effects of growth factors on temporomandibular joint disc cells. Arch Oral Biol, 2004. 49(7): p. 577–83. [DOI] [PubMed] [Google Scholar]
- 11.Duvvuri U, Kudchodkar S, Reddy R, and Leigh J, T(1rho) relaxation can assess longitudinal proteoglycan loss from articular cartilage in vitro. Osteoarthritis and Cartilage, 2002. 10(11): p. 838–844. [DOI] [PubMed] [Google Scholar]
- 12.Duvvuri U, Reddy R, Patel SD, Kaufman JH, Kneeland JB, and Leigh JS, T1rho- relaxation in articular cartilage: effects of enzymatic degradation. Magn Reson Med, 1997. 38(6): p. 863–7. [DOI] [PubMed] [Google Scholar]
- 13.Farndale RW, Sayers CA, and Barrett AJ, A direct spectrophotometric microassay for sulfatedglycosaminoglycans in cartilage cultures. Connect Tissue Res, 1982. 9(4): p. 247–8. [DOI] [PubMed] [Google Scholar]
- 14.Fazaeli S, Ghazanfari S, Everts V, Smit TH, and Koolstra JH, The contribution of collagen fibers to the mechanical compressive properties of the temporomandibular joint disc. Osteoarthritis Cartilage, 2016. 24(7): p. 1292–301. [DOI] [PubMed] [Google Scholar]
- 15.Fleck AKM, Kruger U, Carlson K, Waltz C, McCallum SA, Lucas Lu X, and Wan LQ, Zonal variation of MRI-measurable parameters classifies cartilage degradation. J Biomech, 2017. 65: p. 176–184. [DOI] [PubMed] [Google Scholar]
- 16.Grenier S, Bhargava MM, and Torzilli PA, An in vitro model for the pathological degradation of articular cartilage in osteoarthritis. J Biomech, 2014. 47(3): p. 645–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Guilak F, Ratcliffe A, Lane N, Rosenwasser MP, and Mow VC, Mechanical and biochemical changes in the superficial zone of articular cartilage in canine experimental osteoarthritis. J Orthop Res, 1994. 12(4): p. 474–84. [DOI] [PubMed] [Google Scholar]
- 18.Harris ED Jr., Parker HG, Radin EL, and Krane SM, Effects of proteolytic enzymes on structural and mechanical properties of cartilage. Arthritis Rheum, 1972. 15(5): p. 497–503. [DOI] [PubMed] [Google Scholar]
- 19.Hatcher CC, Collins AT, Kim SY, Michel LC, Mostertz WC 3rd, Ziemian SN, Spritzer CE, Guilak F, DeFrate LE, and McNulty AL, Relationship between T1rho magnetic resonance imaging, synovial fluid biomarkers, and the biochemical and biomechanical properties of cartilage. J Biomech, 2017. 55: p. 18–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hollander AP, Pidoux I, Reiner A, Rorabeck C, Bourne R, and Poole AR, Damage to type II collagen in aging and osteoarthritis starts at the articular surface, originates around chondrocytes, and extends into the cartilage with progressive degeneration. J Clin Invest, 1995. 96(6): p. 2859–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hosseininia S, Weis MA, Rai J, Kim L, Funk S, Dahlberg LE, and Eyre DR, Evidence for enhanced collagen type III deposition focally in the territorial matrix of osteoarthritic hip articular cartilage. Osteoarthritis Cartilage, 2016. 24(6): p. 1029–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hosseininia S, Lindberg LR, and Dahlberg LE, Cartilage collagen damage in hip osteoarthritis similar to that seen in knee osteoarthritis; a case-control study of relationship between collagen, glycosaminoglycan and cartilage swelling. BMC Musculoskelet Disord, 2013. 14: p. 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Janusz MJ, Bendele AM, Brown KK, Taiwo YO, Hsieh L, and Heitmeyer SA, Induction of osteoarthritis in the rat by surgical tear of the meniscus: Inhibition ofjoint damage by a matrix metalloproteinase inhibitor. Osteoarthritis Cartilage, 2002. 10(10): p. 785–91. [DOI] [PubMed] [Google Scholar]
- 24.Karsdal MA, Madsen SH, Christiansen C, Henriksen K, Fosang AJ, and Sondergaard C, Cartilage degradation is fully reversible in the presence of aggrecanase but not matrix metalloproteinase activity. Arthritis Res Ther, 2008. 10(3): p. R63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keenan KE, Besier TF, Pauly JM, Han E, Rosenberg J, Smith RL, Delp SL, Beaupre GS, and Gold GE, Prediction of glycosaminoglycan content in human cartilage by age, T1rho and T2MRI. Osteoarthritis Cartilage, 2011. 19(2): p. 171–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kurkijarvi JE, Nissi MJ, Kiviranta I, Jurvelin JS, and Nieminen MT, Delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) and T2 characteristics of human knee articular cartilage: topographical variation and relationships to mechanical properties. Magn Reson Med, 2004. 52(1): p. 41–6. [DOI] [PubMed] [Google Scholar]
- 27.Lark MW, Bayne EK, Flanagan J, Harper CF, Hoerrner LA, Hutchinson NI, Singer II, Donatelli SA, Weidner JR, Williams HR, Mumford RA, and Lohmander LS, Aggrecan degradation in human cartilage. Evidence for both matrix metalloproteinase and aggrecanase activity in normal, osteoarthritic, and rheumatoid joints. J Clin Invest, 1997. 100(1): p. 93–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Larsson S, Lohmander LS, and Struglics A, Synovial fluid level of aggrecan ARGS fragments is a more sensitive marker of joint disease than glycosaminoglycan or aggrecan levels: a cross sectional study. Arthritis Res Ther, 2009. 11(3): p. R92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lawrence R, Felson D, Helmick C, Arnold L, Choi H, Devo R, Gabriel S, Hirsch R, Hochberg M, Hunder M, Jordan J, Katz J, Kremers H, and Wolfe F, Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis & Rheumatism, 2008. 58(1): p. 26–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Li X, Benjamin Ma C, Link TM, Castillo DD, Blumenkrantz G, Lozano J, Carballido-Gamio J, Ries M, and Majumdar S, In vivo T(1rho) and T(2) mapping of articular cartilage in osteoarthritis of the knee using 3 T MRI. Osteoarthritis Cartilage, 2007. 15(7): p. 789–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li X, Pai A, Blumenkrantz G, Carballido-Gamio J, Link T, Ma B, Ries M, and Majumdar S, Spatial distribution and relationship of T1rho and T2 relaxation times in knee cartilage with osteoarthritis. Magn Reson Med, 2009. 61(6): p. 1310–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li X, Cheng J, Lin K, Saadat E, Bolbos RI, Jobke B, Ries MD, Horvai A, Link TM, and Majumdar S, Quantitative MRI using T1rho and T2 in human osteoarthritic cartilage specimens: correlation with biochemical measurements and histology. Magn Reson Imaging, 2011. 29(3): p. 324–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu B, Lad NK, Collins AT, Ganapathy PK, Utturkar GM, McNulty AL, Spritzer E, Moorman CT 3rd, Sutter EG, Garrett WE, and DeFrate LE, In Vivo Tibial Cartilage Strains in Regions of Cartilage-to-Cartilage Contact and Cartilage-to-Meniscus Contact in Response to Walking. Am J Sports Med, 2017: 45(12): p. 2817–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lu XL, Mow VC, and Guo XE, Proteoglycans and mechanical behavior of condylar cartilage. J Dent Res, 2009. 88(3): p. 244–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lyyra T, Arokoski JP, Oksala N, Vihko A, Hyttinen M, Jurvelin JS, and Kiviranta I, Experimental validation of arthroscopic cartilage stiffness measurement using enzymatically degraded cartilage samples. Phys Med Biol, 1999. 44(2): p. 525–35. [DOI] [PubMed] [Google Scholar]
- 36.Madsen SH, Sumer EU, Bay-Jensen AC, Sondergaard BC, Qvist P, and Karsdal MA, Aggrecanase- and matrix metalloproteinase-mediated aggrecan degradation is associated with different molecular characteristics of aggrecan and separated in time ex vivo. Biomarkers, 2010. 15(3): p. 266–76. [DOI] [PubMed] [Google Scholar]
- 37.Maroudas A, Ziv I, Weisman N, and Venn M, Studies of hydration and swelling pressure in normal and osteoarthritic cartilage. Biorheology, 1985. 22(2): p. 159–69. [DOI] [PubMed] [Google Scholar]
- 38.Martin JA and Buckwalter JA, Roles of articular cartilage aging and chondrocyte senescence in the pathogenesis of osteoarthritis. Iowa Orthop J, 2001. 21: p. 1–7. [PMC free article] [PubMed] [Google Scholar]
- 39.McNulty AL, Rothfusz NE, Leddy HA, and Guilak F, Synovial fluid concentrations and relative potency of interleukin-1 alpha and beta in cartilage and meniscus degradation. J Orthop Res, 2013. 31(7): p. 1039–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mosher TJ, Smith HE, Collins C, Liu Y, Hancy J, Dardzinski BJ, and Smith MB, Change in knee cartilage T2 at MR imaging after running: a feasibility study. Radiology, 2005. 234(1): p. 245–9. [DOI] [PubMed] [Google Scholar]
- 41.Mow VC, Kuei SC, Lai WM, and Armstrong CG, Biphasic creep and stress relaxation of articular cartilage in compression? Theory and experiments. J Biomech Eng, 1980. 102(1): p. 73–84. [DOI] [PubMed] [Google Scholar]
- 42.Nieminen MT, Rieppo J, Toyras J, Hakumaki JM, Silvennoinen J, Hyttinen MM, Helminen HJ, and Jurvelin JS, T2 relaxation reveals spatial collagen architecture in articular cartilage: a comparative quantitative MRI and polarized light microscopic study. Magn Reson Med, 2001. 46(3): p. 487–93. [DOI] [PubMed] [Google Scholar]
- 43.Nieminen MT, Toyras J, Rieppo J, Hakumaki JM, Silvennoinen J, Helminen HJ, and Jurvelin JS, Quantitative MR microscopy of enzymatically degraded articular cartilage. Magn Reson Med, 2000. 43(5): p. 676–81. [DOI] [PubMed] [Google Scholar]
- 44.Nieminen MT, Toyras J, Laasanen MS, Silvennoinen J, Helminen HJ, and Jurvelin JS, Prediction of biomechanical properties of articular cartilage with quantitative magnetic resonance imaging. J Biomech, 2004. 37(3): p. 321–8. [DOI] [PubMed] [Google Scholar]
- 45.Nishioka H, Hirose J, Nakamura E, Oniki Y, Takada K, Yamashita Y, and Mizuta H, T1rho and T2 mapping reveal the in vivo extracellular matrix of articular cartilage. J Magn Reson Imaging, 2012. 35(1): p. 147–55. [DOI] [PubMed] [Google Scholar]
- 46.Nissi MJ, Salo EN, Tiitu V, Liimatainen T, Michaeli S, Mangia S, Ellermann J, and Nieminen MT, Multi-parametric MRI characterization of enzymatically degraded articular cartilage. J Orthop Res, 2016. 34(7): p. 1111–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Regatte RR, Akella SV, Borthakur A, Kneeland JB, and Reddy R, Proteoglycan depletion-induced changes in transverse relaxation maps of cartilage: comparison of T2 and T1rho. Acad Radiol, 2002. 9(12): p. 1388–94. [DOI] [PubMed] [Google Scholar]
- 48.Rowland CR, Lennon DP, Caplan AI, and Guilak F, The effects of crosslinking of scaffolds engineered from cartilage ECM on the chondrogenic differentiation of MSCs. Biomaterials, 2013. 34(23): p. 5802–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sah RL, Yang AS, Chen AC, Hant JJ, Halili RB, Yoshioka M, Amiel D, and Coutts RD, Physical properties of rabbit articular cartilage after transection of the anterior cruciate ligament. J Orthop Res, 1997. 15(2): p. 197–203. [DOI] [PubMed] [Google Scholar]
- 50.Sanchez-Adams J, Leddy HA, McNulty AL, O’Conor CJ, and Guilak F, The mechanobiology of articular cartilage: bearing the burden of osteoarthritis. Curr Rheumatol Rep, 2014. 16(10): p. 451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Setton LA, Elliott DM, and Mow VC, Altered mechanics of cartilage with osteoarthritis: human osteoarthritis and an experimental model of joint degeneration. Osteoarthritis Cartilage, 1999. 7(1): p. 2–14. [DOI] [PubMed] [Google Scholar]
- 52.Setton LA, Zhu W, and Mow VC, The biphasicporoviscoelastic behavior of articular cartilage: role of the surface zone in governing the compressive behavior. Journal of Biomechanics, 1993. 26(4–5): p. 581–92. [DOI] [PubMed] [Google Scholar]
- 53.Shingleton WD, Hodges DJ, Brick P, and Cawston TE, Collagenase: a key enzyme in collagen turnover. Biochem Cell Biol, 1996. 74(6): p. 759–75. [DOI] [PubMed] [Google Scholar]
- 54.Souza RB, Kumar D, Calixto N, Singh J, Schooler J, Subburaj K, Li X, Link TM, and Majumdar S, Response of knee cartilage T1rho and T2 relaxation times to in vivo mechanical loading in individuals with and without knee osteoarthritis. Osteoarthritis Cartilage, 2014. 22(10): p. 1367–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stoop R, Buma P, van der Kraan PM, Hollander AP, Billinghurst RC, Meijers TH, Poole AR, and van den Berg WB, Type II collagen degradation in articular cartilage fibrillation after anterior cruciate ligament transection in rats. Osteoarthritis Cartilage, 2001. 9(4): p. 308–15. [DOI] [PubMed] [Google Scholar]
- 56.Subburaj K, Kumar D, Souza R, Alizai H, Li X, Link T, and Majumdar S, The acute effect of running on knee articular cartilage and meniscus magnetic resonance relaxation times in young healthy adults. American Journal of Sports Medicine, 2012. 40(9): p. 2134–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sutter EG, Widmyer MR, Utturkar GM, Spritzer CE, Garrett WE Jr., and DeFrate LE, In vivo measurement of localized tibiofemoral cartilage strains in response to dynamic activity. Am J Sports Med, 2015. 43(2): p. 370–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tang SY, Souza RB, Ries M, Hansma PK, Alliston T, and Li X, Local tissue properties of human osteoarthritic cartilage correlate with magnetic resonance T(1) rho relaxation times. J Orthop Res, 2011. 29(9): p. 1312–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Temple-Wong MM, Bae WC, Chen MQ, Bugbee WD, Amiel D, Coutts RD, Lotz M, and Sah RL, Biomechanical, structural, and biochemical indices of degenerative and osteoarthritic deterioration of adult human articular cartilage of the femoral condyle. Osteoarthritis Cartilage, 2009. 17(11): p. 1469–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsushima H, Okazaki K, Takayama Y, Hatakenaka M, Honda H, Izawa T, Nakashima Y, Yamada H, and Iwamoto Y, Evaluation of cartilage degradation in arthritis using T1rho magnetic resonance imaging mapping. Rheumatol Int, 2012. 32(9): p. 2867–75. [DOI] [PubMed] [Google Scholar]
- 61.Wayne JS, Kraft KA, Shields KJ, Yin C, Owen JR, and Disler DG, MR imaging of normal and matrix-depleted cartilage: correlation with biomechanical function and biochemical composition. Radiology, 2003. 228(2): p. 493–9. [DOI] [PubMed] [Google Scholar]
- 62.Wei B, Du XT, Liu J, Mao FY, Zhang X, Liu S, Xu Y, Zang FC, and Wang LM, Associations between the properties of the cartilage matrix and findings from quantitative MRI in human osteoarthritic cartilage of the knee. International Journal of Clinical and Experimental Pathology, 2015. 8(4): p. 3928–3936. [PMC free article] [PubMed] [Google Scholar]
- 63.Wheaton AJ, Dodge GR, Borthakur A, Kneeland JB, Schumacher HR, and Reddy R, Detection of changes in articular cartilage proteoglycan by T(1rho) magnetic resonance imaging. J Orthop Res, 2004. 23(1): p. 102–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wheaton AJ, Dodge GR, Elliott DM, Nicoll SB, and Reddy R, Quantification of cartilage biomechanical and biochemical properties via T1rho magnetic resonance imaging. Magn Reson Med, 2005. 54(5): p. 1087–93. [DOI] [PubMed] [Google Scholar]
- 65.Widmyer MR, Utturkar GM, Leddy HA, Coleman JL, Spritzer CE, Moorman CT 3rd, Defrate LE, and Guilak F, High body mass index is associated with increased diurnal strains in the articular cartilage of the knee. Arthritis Rheum, 2013. 65(10): p. 2615–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wilusz RE, Zauscher S, and Guilak F, Micromechanical mapping of early osteoarthritic changes in the pericellular matrix of human articular cartilage. Osteoarthritis Cartilage, 2013. 21(12): p. 1895–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Woessner JF Jr., The determination of hydroxyproline in tissue and protein samples containing small proportions of this imino acid. Arch Biochem Biophys, 1961. 93: p. 440–7. [DOI] [PubMed] [Google Scholar]