Abstract
Objectives:
To investigate potential risk factors for perinatal (intrauterine and intrapartum) mother-to-child transmission of HIV (MTCT) in women unexposed to Antiretroviral Therapy (ART) during pregnancy.
Methods:
We compared factors according to perinatal MTCT outcome among 2,275 ART-naïve (until the onset of labor) HIV-infected women in the Breastfeeding, Antiretrovirals and Nutrition study (2004–2010) in Lilongwe, Malawi. Factors included HIV viral load during pregnancy, food security, demographic characteristics, hematologic and blood chemistry measures, medical history and physical factors. Associations with perinatal MTCT and interactions with maternal viral load were assessed using simple and multivariable logistic regression.
Results:
There were 119 (115 intrauterine, 4 intrapartum) cases of perinatal MTCT; only one to a mother with <1,000 HIV copies/mL. Maternal viral loads >10,000 copies/mL were common (63.1%). Lower maternal viral load (<1,000 copies/mL and 1,000.1–10,000 copies/mL) was associated with reduced odds of perinatal MTCT [adjusted odds ratio (aOR), 0.1; 95% confidence interval (CI), 0.01–0.4 and aOR, 0.2; 95% CI, 0.1–0.4, respectively), compared with maternal viral load >10,000 copies/mL. Low CD4+ T cell count (≤ 350 cells/μL) was only associated with perinatal MTCT in unadjusted models. Food shortage (aOR, 1.8; 95% CI; 1.2-2.6), sexually transmitted infection (past year) (aOR, 1.9; 95% CI; 1.0–3.7), histories of herpes zoster (aOR, 3.0; 95% CI; 1.6–5.6) and tuberculosis (aOR, 2.5; 95% CI; 1.1–5.7) were associated with increased odds of perinatal MTCT.
Conclusions:
These findings confirm that lowering maternal HIV viral load is most important in preventing perinatal MTCT, and support efforts to address food shortage, STD and TB prevention, while informing programs to improve ART coverage in pregnancy.
Background
Approximately 150,000 children < 15 years old were newly infected with human immunodeficiency virus (HIV) in 2015 [1], a majority through mother-to-child transmission of HIV (MTCT) [2]. Transmission from the mother can occur in utero, at the time of delivery, or postnatally through breastfeeding [3]. In non-breastfeeding populations, it is possible to achieve rates of perinatal MTCT in infants born to HIV-infected mothers of <2% with antiretroviral therapy (ART) during pregnancy and delivery [4, 5]. However, despite a dramatic expansion in ART coverage of pregnant, HIV-infected women, it is estimated that, globally, more than a quarter of women with known HIV infection did not receive ART during pregnancy in 2014 [6]. Maternal HIV viral load is a strong risk factor for MTCT, but other demographic, behavioral, clinical, virologic and genetic factors have been identified [7]. Because there remains a substantial number of HIV-infected women giving birth who are not on ART, and because, even with optimal ART during pregnancy and delivery the risk of transmission to the infant is not zero, it is important to understand the full spectrum of risk factors for MTCT.
We conducted a retrospective analysis of data from the Breastfeeding, Antiretrovirals and Nutrition (BAN) study to investigate potential risk factors for perinatal MTCT in a population of HIV-infected women who were ART naïve until receiving ART at the onset of labor.
Methods
Recruitment and study procedures
This analysis uses data from the BAN study: a randomized, controlled trial of two ART regimens to prevent MTCT during breastfeeding in Lilongwe, Malawi, that recruited and followed participants from March 2004 to January 2010 (www.ClinicalTrials.gov number NCT00164736) [8–10].
The BAN study screened and consented 3,572 pregnant women who tested HIV positive at antenatal visits at six clinics in Lilongwe, Malawi from March 2004 to February 2009, and randomized 2,369 women at delivery who met the antenatal and postnatal eligibility criteria. Antenatal criteria were age ≥ 14 years, antiretroviral unexposed, no serious pregnancy complications, intentions to breastfeed, CD4+ T cell count ≥250 cells/μL (≥200 cells/μL before July 24, 2006), hemoglobin ≥ 7g/dL and plasma alanine aminotransferase (ALT) ≤ 2.5 times the upper limit of normal. Postnatal criteria included infant birth weight ≥ 2000 g and no conditions precluding study interventions (maternal or infant). All women provided written informed consent. The BAN study protocol was approved by the Malawi National Health Science Research Committee and by institutional review boards at the University of North Carolina (UNC) at Chapel Hill and the US Centers for Disease Control and Prevention (CDC). Participants received perinatal MTCT antiretroviral prophylaxis. Mothers (at onset of labor) and infants received a single dose of oral nevirapine. All mothers received zidovudine (300 mg) plus lamivudine (150 mg), twice-daily, from the onset of labor and for 7 days after birth and all infants received zidovudine (2 mg per kilogram of body weight) and lamivudine (4 mg per kilogram) twice-daily for 7 days.
Tests for hematologic measures and blood chemistry measures were conducted on samples collected at screening. Results of routine syphilis tests were obtained with participant consent. At delivery/randomization, mothers were interviewed to collect medical history, demographic and food security information with updates to maternal medical history solicited by study staff at prenatal visits scheduled at 28, 32 and 36 weeks of gestational age and at delivery; included were questions about tuberculosis (TB) and malaria treatment history, Herpes zoster, oral thrush, sickle cell, hepatitis and sexually transmitted infection (STI) history. Demographic information included age, marital/cohabitation status, highest level of education completed and literacy. HIV viral load testing was conducted on specimens taken prenatally at registration using the NucliSens assay (bioMerieux, Durham, NC). If no viral load test could be completed at registration, testing was conducted on a specimen collected at a subsequent prenatal visit. Maternal physical exams, which included triceps skinfold; mean upper arm circumference; and abnormal genitourinary symptom assessments, were performed at screening, prenatal visits and at delivery/randomization. Women presenting with symptoms of malaria were tested via microscopy.
Outcome assessment
Infants were tested for HIV via DNA PCR at birth and at 1, 2 and 4 week post-partum visits with the Amplicor 1.5 DNA polymerase-chain-reaction (PCR) assay (Roche Molecular Systems). Positive tests were confirmed by testing a specimen obtained at the next visit. For infants lost to follow-up before a confirmatory test was obtained, a second specimen from the same day was tested at the reference laboratory at the University of North Carolina. Of the 2,369 women who met the BAN study eligibility criteria, cases were 119 mothers who transmitted HIV to their infants by the 2-week postpartum visit; controls were 2,156 mothers whose infants were HIV uninfected after the 2-week postpartum visit; 94 mothers whose infants’ last HIV test was negative and were lost to follow up before the 2-week postpartum visit were excluded because intrapartum MTCT could not be ruled out.
Statistical methods
Bivariate associations between exposures and perinatal MTCT of HIV were assessed using chi-square tests and Fisher exact tests. The Mann-Whitney test was used to compare the distribution of viral load during pregnancy according to perinatal MTCT status. Transmission rates were estimated with their binomial exact 95% confidence intervals (CI) according to viral load category. Simple logistic regression was used to evaluate the effect of variables on perinatal MTCT. Model selection for multivariate logistic regression was conducted via backward manual selection, beginning with all variables with p-values ≤ 0.1 in bivariate analysis, along with interaction terms between each covariate and three category maternal viral load (≤1,000 copies/mL, <1,000–10,000 copies/mL, >10,000 copies/mL). Interaction terms were retained if their removal significantly changed the likelihood ratio test results. Remaining covariates were removed sequentially (largest p-value first) via backwards selection using a change-in-estimate approach. Covariates significantly associated with the outcome (p < 0.05) or whose removal caused a change >10% (|ln(ORreduced/ORfull)| > 0.10) in the estimate of any odds ratio were retained in the model. Statistical analyses were conducted using SAS 9.4.
Results
Approximately 30% of the HIV-infected pregnant women in this study had a CD4+ T cell count ≤350 cells/μL during pregnancy, but above the exclusionary threshold of 200 or 250 cells/uL; the percentage with maternal viral loads greater than 10,000 copies/mL during pregnancy was high (63.1%), while less than 10% had maternal viral loads < 1,000 copies/mL (Table 1). Only 34.6% had more than a primary education. Anemia during pregnancy, reporting a current food shortage and physical measures related to poor nutrition were all common (>40%). Low blood albumin, low lymphocyte count and neutropenia were all common (>33%) (Table 1).
Table 1.
Bivariate and multivariate associations between maternal characteristics and perinatal HIV transmission in the BAN Study, Lilongwe, Malawi 2004–2010
| Overall (N=2275) | Cases (N=119) | Controls (N=2156) | OR (95% CI) | Adjusted OR (95% CI)* | |
|---|---|---|---|---|---|
| N (%) | N (%) | N (%) | |||
| Maternal CD4+ T cell count during pregnancy† | |||||
| 200–350 cells/μL | 684 (30.1%) | 43 (36.1%) | 641 (29.7%) | 1.6 (1.02, 2.6) | -- |
| 350.1–500 cells/μL | 757 (33.3%) | 43 (36.1%) | 714 (33.1%) | 1.5 (0.9, 2.3) | -- |
| >500 cells/μL (referent) | 834 (36.7%) | 33 (27.7%) | 801 (37.2%) | -- | -- |
| Viral load during pregnancy†‡ | |||||
| <1,000 copies/mL | 200 (9.4%) | 1 (0.9%) | 199 (9.9%) | 0.1 (0.01, 0.4) | 0.1 (0.01, 0.4) |
| 1,000.1–10,000 copies/mL | 583 (27.5%) | 8 (7.3%) | 575 (28.6%) | 0.2 (0.1, 0.4) | 0.2 (0.1, 0.4) |
| >10,000 copies/mL | 1341 (63.1%) | 101 (91.8%) | 1240 (61.6%) | -- | -- |
| Age at enrollment | |||||
| 15–24 (referent) | 986 (43.3%) | 50 (42.0%) | 936 (43.4%) | -- | -- |
| 25–34 | 1157 (50.9%) | 60 (50.4%) | 1097 (50.9%) | 1.0 (0.7, 1.5) | -- |
| >35 | 132 (5.8%) | 9 (7.6%) | 123 (5.7%) | 1.4 (0.7, 2.9) | -- |
| Married or living with partner | 2110 (92.9%) | 114 (95.8%) | 1996 (92.7%) | 1.8 (0.7, 4.5) | -- |
| >Primary education† | 784 (34.6%) | 30 (25.2%) | 754 (35.1%) | 0.6 (0.4, 0.95) | -- |
| Literate | 1705 (77.0%) | 81 (71.7%) | 1624 (77.3%) | 0.7 (0.5, 1.1) | -- |
| Anemia during pregnancy§ | 1220 (53.6%) | 74 (62.2%) | 1146 (53.2%) | 1.4 (0.99, 2.1) | -- |
| Currently facing a food shortage† | 932 (41.9%) | 61 (51.3%) | 871 (41.4%) | 1.5 (1.03, 2.2) | 1.8 (1.2, 2.6) |
| Low Triceps Skin Fold (<14mm) | 953 (42.2%) | 54 (45.4%) | 899 (42.0%) | 1.1 (0.8, 1.7) | -- |
| Low Mid-Upper Arm Circumference (<26mm) | 1053 (46.7%) | 59 (49.6%) | 994 (46.5%) | 1.1 (0.8, 1.6) | -- |
| Clinically diagnosed malaria during pregnancy | 298 (13.1%) | 14 (11.8%) | 284 (13.2%) | 0.9 (0.5, 1.6) | -- |
| Self-reported past medical history of TB† | 69 (3.1%) | 8 (6.7%) | 61 (2.8%) | 2.5 (1.2, 5.3) | 2.5 (1.1, 5.7) |
| Self-reported past medical history of oral thrush | 144 (6.4%) | 7 (5.9%) | 137 (6.4%) | 0.9 (0.4, 2.0) | -- |
| Self-reported sexually transmitted infection in last 12 months | 155 (6.9%) | 13 (10.9%) | 142 (6.7%) | 1.7 (0.9, 3.1) | 1.9 (1.0, 3.7) |
| Self-reported past medical history of herpes zoster† | 107 (4.7%) | 17 (14.3%) | 90 (4.2%) | 3.8 (2.2, 6.6) | 3.0 (1.6, 5.6) |
| Syphilis Infection during pregnancy | 74 (4.6%) | 4 (5.2%) | 70 (4.4%) | 0.9 (0.3, 2.5) | -- |
| Vaginal sores, discharge or itching present during pregnancy | 405 (17.8%) | 24 (20.2%) | 381 (17.7) | 1.2 (0.7, 1.9) | -- |
| Low Maternal Albumin (<3 g/dL)† | 1434 (64.1%) | 87 (74.4%) | 1347 (63.5%) | 1.7 (1.1, 2.5) | -- |
| Low Maternal Lymphocytes (<1.8 K/μL) | 805 (35%) | 39 (33.1%) | 766 (35.6%) | 0.9 (0.6, 1.3) | -- |
| Maternal Neutropenia (<3.5 K/μL) | 973 (42.9%) | 59 (50.0%) | 914 (42.5%) | 1.4 (0.9, 2.0) | -- |
Significant ORs are bolded
Age, literacy, CD4+ T cell count, education , anemia, and albumin were initially entered into multivariate model, but not retained in final due to lack of association with the outcome after adjustment
Chi-square or Fischer exact test p-value < 0.05
151 Pregnant women missing viral load
Hemoglobin <11 g/dL
Among the 119 perinatally HIV-infected infants in our study, 115 were HIV PCR-positive at birth and 4 were HIV PCR-negative at birth but positive by 2 weeks of life. Transmitting mothers had significantly higher median maternal viral load during pregnancy compared to non-transmitting mothers (61,830 vs 16,529 copies/ml, p<0.001).
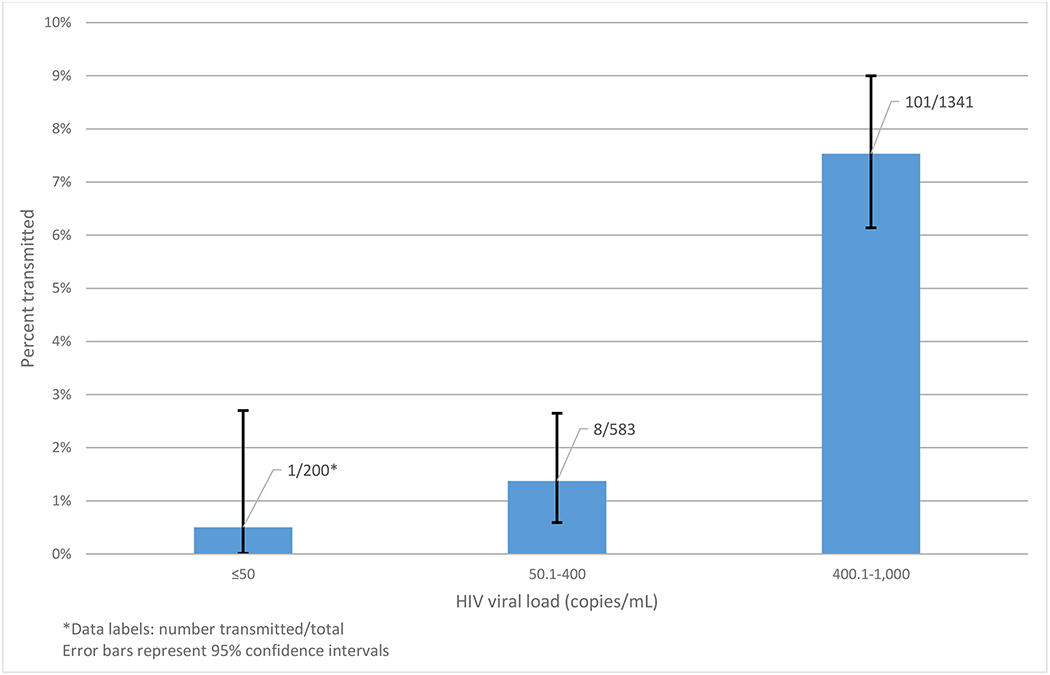
Women who transmitted HIV perinatally to their infants were more likely to have CD4+ T cell counts ≤ 350 cells/μL during pregnancy compared to those who did not transmit (36.1% for transmitters vs. 29.7% for non-transmitters, odds ratio (OR): 1.6, 95% confidence interval (CI): 1.02, 2.6). Viral load measurement took place a median of 12 weeks before delivery. Transmitters (91.8%) were more likely to have viral loads > 10,000 copies/mL during pregnancy than non-transmitters (61.6%). Having viral load during pregnancy <1,000 copies/mL (OR: 0.1, 95% CI: 0.01, 0.4) and between 1,000 and 10,000 copies/mL (OR: 0.2, 95% CI: 0.1, 0.4) were both associated with significantly lower odds of perinatal transmission compared with those with viral loads during pregnancy ≥10,000 copies/mL. Of the 200 HIV-infected pregnant women with viral loads during pregnancy < 1,000 copies/mL, there was only one episode of perinatal MTCT (viral load: 361 copies/mL), a rate of 0.5% (95% CI: 0.0, 2.7) (Figure 1). The correspondingrates for pregnant women with viral loads during pregnancy between 1,000 copies/mL and 10,000 copies/mL and for pregnant women with viral loads during pregnancy ≥10,000, respectively, were 1.4% (95% CI: 0.6, 2.7) and 7.5% (95% CI: 6.1, 9.0).
Figure 1.
Percentage of HIV-infected pregnant women who transmitted HIV perinatally in the Breastfeeding, Antiretrovials and Nutrition (BAN) study in Lilongwe, Malawi (2004–2010), by prenatal maternal viral load category
Transmitters (25.2%) had lower odds of having any formal post-primary education than non-transmitters (35.1%) (OR: 0.6, 95% CI: 0.04, 0.95). Reporting a current food shortage was more common in transmitters (51.3%) than in non-transmitters (41.4%) (OR: 1.5, 95% CI: 1.03, 2.2). Self-reported histories of individual diseases were uncommon, but there were differences between transmitters and non-transmitters. Tuberculosis was more common in transmitters (6.7%) than in non-transmitters (2.8%) (OR: 2.5, 95% CI: 1.2, 5.3), as was self-reported history of herpes zoster (14.3% in transmitters vs. 4.2% in non-transmitters, OR: 3.8, 95% CI: 2.2, 6.6) (Table 1). The association between self-reported STI within the last six months and perinatal MTCT was not significant (OR 1.7, 95% CI: 0.9 – 3.1). Low blood albumin was significantly associated with perinatal MTCT (OR: 1.7, 95% CI: 1.1, 2.5) (Table 1).
The final multivariable logistic regression model included categorized viral load (<1,000 copies/mL, 1,000 – 9,999 copies/mL and ≥10,000 copies/mL) (Table 1), reporting facing a food shortage, self-reported STI in the last 12 months, and past medical histories of herpes zoster and TB (Table 1). Individuals in each of the two lower viral load categories, <1,000 copies/mL and 1,000–9,999 copies/mL, had lower adjusted odds (aOR: 0.1, 95% CI: 0.01, 0.4 and aOR: 0.2 95% CI: 0.1, 0.4, respectively) of perinatal MTCT compared with those with viral loads >10,000 copies/mL. Reporting facing a food shortage (aOR: 1.8, 95% CI: 1.2, 2.6), self-reported STI in the last 12 months (aOR: 1.9, 95% CI: 1.0004, 3.7), and past medical histories of herpes zoster (aOR: 3.0, 95% CI: 1.6, 5.6) and TB (aOR: 2.5, 95% CI: 1.1, 5.7) were all associated with higher adjusted odds of perinatal MTCT.
Discussion
In this analysis of risk factors for perinatal MTCT of HIV among 2,275 HIV-infected pregnant women participating in the BAN study in Lilongwe, Malawi, the strongest risk factor was high prenatal viral load. Women with prenatal viral loads between 1,000 and 9,999 copies/mL had 80% lower odds and women with prenatal viral loads <1,000 copies/mL had 90% lower odds of perinatal MTCT, compared with women who had viral loads ≥10,000 copies/mL. In fact, there was only one case of HIV transmission among women in the lowest viral load category. Other factors associated with perinatal transmission included facing a food shortage, self-reported sexually transmitted infection in the last 12 months, history of herpes zoster and history of TB. Given the intrapartum initiation of ART prophylaxis in our study, we have likely underestimated the rate of perinatal MTCT. The HPTN 040 trial of only postnatal infant prophylaxis found similar rates of in utero MTCT (5.7%) to the rates of MTCT by two weeks of age that we observed in the BAN study (4.4–5.5%) {Chasela, 2010 #612}. Given that most (over 90%) intrapartum transmissions will be detected by 2 weeks of age, {Dunn, 1995 #1208}, it is likely that most peripartum transmissions were prevented in the BAN study.
Maternal viral load is a known risk factor for MTCT [7], and risk of MTCT increases with higher viral loads [11–15]. The risk of perinatal MTCT exists throughout pregnancy, and for women not on ART, this risk is highest during late gestation/labor and delivery [3]. Mandelbrot, et al. showed that ART initiation before conception and viral load suppression (<50 copies/mL at delivery) can virtually eliminate perinatal MTCT [15]. The women in this study did not receive ART during pregnancy until receiving single dose nevirapine at the onset of labor, yet 9.4% had viral loads <1,000 copies/mL. The lone case of perinatal MTCT among these women had history of maternal TB infection, also associated with transmission in this study, but no other unique characteristics. The overall 5.2% perinatal MTCT rate, combined with the very low transmission rate among those with viral loads <1,000 copies/mL, reinforces the importance of early ART, ideally before pregnancy, for optimal prevention of MTCT of HIV, in accordance with current WHO guidelines [16].
The possible role of food insecurity – represented in this study by the question “Are you currently facing a food shortage?” in MTCT is not well characterized in the scientific literature, but it may be a cause of nutritional deficiencies and an indicator of lower socio-economic status [17][18][19][20] However, placebo-controlled randomized trials of nutritional supplements have not shown significant effects on MTCT [21]. In our study, low blood albumin (<3.5K/μL), which is both a potential indicator of malnutrition [22–24] and of HIV disease progression [23, 25], was associated in our unadjusted model with HIV transmission during the intrapartum period. Low blood albumin was also associated with CD4+ T cell count ≤ 350 cells/μL, low MUAC and low TSF in this study (data not shown). However, the odds of transmission of HIV during the intrapartum period was not associated in either the adjusted or unadjusted models with physical measures of poor nutrition. It’s possible that food shortages in this study functioned as an indicator of social determinants of health [26, 27].
Histories of TB and herpes zoster may be proxies for immunosuppression [28–33]. Gupta et al. found that maternal TB was an independent risk factor for MTCT and proposed that this association may be due to unobserved, transitory increases in maternal viral loads, increased maternal immune system activation or an association between TB and an unknown or unmeasured MTCT risk factor [34]. Our results support the finding that TB is associated with MTCT independent of maternal viral load, although it is possible that there were variations in immune status and viral load not captured. The significance in the multivariable model of self-reported herpes zoster history also supports this conclusion.
The estimate for the association between self-reported history of STI within the past year and perinatal MTCT was fairly large (adjusted OR: 1.9), although the lower end of the confidence interval was very close to 1 and the association was not significant in bivariate analysis. There is research showing several lower genital tract infections to be associated with MTCT and with HIV genital shedding [35]. Herpes simplex virus type 2 (HSV-2) is associated with MTCT, due to genital ulcers with high concentrations of HIV RNA and also possibly direct viral interaction and maternal immune activation [35–38]. There is contradictory evidence on the effect of syphilis on MTCT [39, 40][37, 41]. Unadjusted associations between genital warts and MTCT may be confounded by advanced HIV disease and higher maternal viral load [42, 43].
This research is subject to some limitations, including potential unmeasured residual confounding. Our viral load data may not capture transitory changes in HIV disease status potentially related to perinatal transmission. Medical history data are self-reported and may be subject to recall bias, which would likely be non-differential and tend to bias results closer to the null hypothesis. Due to participants receiving nevirapine prophylaxis during labor, our results may not be generalizable HIV-infected pregnant women who give birth without antiretroviral HIV prophylaxis. Individuals missing covariate data and not included in the final logistic regression model (n=186) were less likely to be anemic and more likely to self-report history of herpes zoster infection in the past 12 months, risk factors for MTCT. However, the number of subjects missing is small, and we do not expect this to have a large effect on the results. The trial selection criteria, such as requiring a CD4 count above ≥ 250 cells/mL (≥200 cells/μL before July 24, 2006), may have resulted in a population less likely to experience MTCT, and the risk factors identified here may not be representative of those for women who do not meet the selection criteria. Despite these limitations, this study contains a large sample of ART-naïve HIV-infected pregnant women in a resource-limited setting, a difficult-to-study but still quite prevalent population, and was able to account for a range of factors, including medical, demographic, food security, nutritional status and hematologic factors.
In conclusion, our study confirms that with maternal viral load suppression, most instances of perinatal MTCT, can be avoided. Clearly, every effort must be made to begin ART and reduce viral load in HIV-infected pregnant women. Our data also suggest that food insecurity, history of diseases indicating immunosuppression, and history of other STIs may be risk factors for perinatal MTCT. These findings can help inform decisions to target populations for programs seeking to improve coverage of ART during pregnancy, and may support efforts to recognize and treat maternal TB, STIs and other infections prior to and during pregnancy, and efforts to improve maternal nutritional status antenatally, to achieve worldwide elimination of pediatric HIV infection and improve outcomes for HIV-infected pregnancy women.
Table 2.
Adjusted Odds Ratios for Perinatal HIV Transmission in the BAN Study, Lilongwe, Malawi 2004–2010
| Variable | Adjusted Odds Ratio* | 95% Confidence Interval |
|---|---|---|
| Viral Load | ||
| <1,000 | 0.1 | (0.01, 0.4) |
| 1,000 – 9,999 | 0.2 | (0.1, 0.4) |
| >10,000 | ref | -- |
| Facing a Food Shortage | 1.8 | (1.2, 2.6) |
| Self-reported sexually transmitted infection in last 12 months | 1.9 | (1.00, 3.7) |
| Past Medical History of Herpes Zoster | 3.0 | (1.6, 5.6) |
| Past Medical History of Tuberculosis | 2.5 | (1.1, 5.7) |
Age, literacy, CD4+ T cell count, education, anemia, and albumin were initially entered into multivariate model, but not retained in final due to lack of association with the outcome after adjustment
Note: final model includes 2,089 individuals with complete covariate data. 186 (8.2%) were missing data from the following categories: viral load (n=151, 7.1%), food shortage (n=50, 2.3%), STI history (n=22, 1.0%), tuberculosis history (n=10, 0.5%), herpes zoster history (n=13, 0.6%).
Adjusted for all other variables in multivariable model
Acknowledgments
We are grateful to the BAN Study Team http://links.lww.com/INF/D345 at University of North Carolina Chapel Hill, Centers for Disease Control and Prevention, Atlanta, and UNC Project in Lilongwe.
Footnotes
Publisher's Disclaimer: CDC Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
The authors have no conflicts of interest or funding to disclose
References
- [1].Prevention Gap Report . UN Joint Programme on HIV/AIDS (UNAIDS); 2016. [Google Scholar]
- [2].Global HIV/AIDS response : epidemic update and health sector progress towards universal access: progress report 2011. UNICEF, UNAIDS, World Health Organization; 2011. [Google Scholar]
- [3].Kourtis AP, Lee FK, Abrams EJ, Jamieson DJ, Bulterys M. Mother-to-child transmission of HIV-1: timing and implications for prevention. Lancet Infect Dis. 2006;6:726–32. [DOI] [PubMed] [Google Scholar]
- [4].Cooper ER, Charurat M, Mofenson L, Hanson IC, Pitt J, Diaz C, et al. Combination antiretroviral strategies for the treatment of pregnant HIV-1-infected women and prevention of perinatal HIV-1 transmission. J Acquir Immune Defic Syndr. 2002;29:484–94. [DOI] [PubMed] [Google Scholar]
- [5].Lallemant M, Jourdain G, Le Coeur S, Mary JY, Ngo-Giang-Huong N, Koetsawang S, et al. Single-Dose Perinatal Nevirapine plus Standard Zidovudine to Prevent Mother-to-Child Transmission of HIV-1 in Thailand. N Engl J Med. 2004;351:217–28. [DOI] [PubMed] [Google Scholar]
- [6].progress report on the global plan towards the elimination of new HIV infections among children by 2015 and keeping their mothers alive Joint United Nations Programme on HIV/AIDS (UNAIDS); 2013. [Google Scholar]
- [7].Kourtis AP, Bulterys M. Mother-to-child transmission of HIV: pathogenesis, mechanisms and pathways. Clin Perinatol. 2010;37:721–37, [DOI] [PubMed] [Google Scholar]
- [8].van der Horst C, Chasela C, Ahmed Y, Hoffman I, Hosseinipour M, Knight R, et al. Modifications of a large HIV prevention clinical trial to fit changing realities: a case study of the Breastfeeding, Antiretroviral, and Nutrition (BAN) protocol in Lilongwe, Malawi . Contemp Clin Trials. 2009;30:24–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Chasela CS, Hudgens MG, Jamieson DJ, Kayira D, Hosseinipour MC, Kourtis AP, et al. Maternal or Infant Antiretroviral Drugs to Reduce HIV-1 Transmission. N Engl J Med. 2010;362:2271–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jamieson DJ, Chasela CS, Hudgens MG, King CC, Kourtis AP, Kayira D, et al. Maternal and infant antiretroviral regimens to prevent postnatal HIV-1 transmission: 48-week follow-up of the BAN randomised controlled trial. Lancet. 2012;379:2449–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Mayaux MJ, Dussaix E, Isopet J, Rekacewicz C, Mandelbrot L, Ciraru-Vigneron N, et al. Maternal virus load during pregnancy and mother-to-child transmission of human immunodeficiency virus type 1: the French perinatal cohort studies. SEROGEST Cohort Group. J Infect Dis. 1997;175:172–5. [DOI] [PubMed] [Google Scholar]
- [12].Study TEC. Maternal viral load and vertical transmission of HIV-1: an important factor but not the only one. AIDS. 1999;13:1377–85. [PubMed] [Google Scholar]
- [13].Garcia PM, Kalish LA, Pitt J, Minkoff H, Quinn TC, Burchett SK, et al. Maternal Levels of Plasma Human Immunodeficiency Virus Type 1 RNA and the Risk of Perinatal Transmission. N Engl J Med. 1999;341:394–402. [DOI] [PubMed] [Google Scholar]
- [14].Mofenson LM, Lambert JS, Stiehm ER, Bethel J, Meyer WA, Whitehouse J, et al. Risk Factors for Perinatal Transmission of Human Immunodeficiency Virus Type 1 in Women Treated with Zidovudine. N Engl J Med. 1999;341:385–93. [DOI] [PubMed] [Google Scholar]
- [15].Mandelbrot L, Tubiana R, Le Chenadec J, Dollfus C, Faye A, Pannier E, et al. No Perinatal HIV-1 Transmission From Women With Effective Antiretroviral Therapy Starting Before Conception. Clin Infect Dis. 2015;61:1715–25. [DOI] [PubMed] [Google Scholar]
- [16].World Health Organization . Guideline on when to start antiretroviral therapy and on pre-exposure prophylaxis for HIV: World Health Organization; 2015. [PubMed] [Google Scholar]
- [17].Anema A, Vogenthaler N, Frongillo EA, Kadiyala S, Weiser SD. Food insecurity and HIV/AIDS: current knowledge, gaps, and research priorities. Curr HIV/AIDS Rep. 2009;6:224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Mehta S, Manji KP, Young AM, Brown ER, Chasela C, Taha TE, et al. Nutritional indicators of adverse pregnancy outcomes and mother-to-child transmission of HIV among HIV-infected women. Am J Clin Nutr. 2008;87:1639–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Semba RD, Miotti PG, Chiphangwi JD, Saah AJ, Canner JK, Dallabetta GA, et al. Maternal vitamin A deficiency and mother-to-child transmission of HIV-1. Lancet. 1994;343:1593–7. [DOI] [PubMed] [Google Scholar]
- [20].Zijenah LS, Moulton LH, Iliff P, Nathoo K, Munjoma MW, Mutasa K, et al. Timing of mother-to-child transmission of HIV-1 and infant mortality in the first 6 months of life in Harare, Zimbabwe. AIDS. 2004;18:273–80. [DOI] [PubMed] [Google Scholar]
- [21].Dreyfuss ML, Fawzi WW. Micronutrients and vertical transmission of HIV-1. Am J Clin Nutr. 2002;75:959–70. [DOI] [PubMed] [Google Scholar]
- [22].Forse RA, Shizgal HM. Serum albumin and nutritional status. JPEN J Parenter Enteral Nutr. 1980;4:450–4. [DOI] [PubMed] [Google Scholar]
- [23].Süttmann U, Ockenga J, Selberg O, Hoogestraat L, Deicher H, Müller MJ. Incidence and prognostic value of malnutrition and wasting in human immunodeficiency virus-infected outpatients. JAIDS Journal of Acquired Immune Deficiency Syndromes. 1995;8:239–46. [DOI] [PubMed] [Google Scholar]
- [24].Friedman AN, Fadem SZ. Reassessment of albumin as a nutritional marker in kidney disease. J Am Soc Nephrol. 2010;21:223–30. [DOI] [PubMed] [Google Scholar]
- [25].Feldman JG, Burns DN, Gange SJ, Bacchetti P, Cohen M, Anastos K, et al. Serum albumin as a predictor of survival in HIV-infected women in the Women’s Interagency HIV Study*. AIDS. 2000;14:863–70. [DOI] [PubMed] [Google Scholar]
- [26].Abu-Saad K, Fraser D. Maternal nutrition and birth outcomes. Epidemiol Rev. 2010;32:5–25. [DOI] [PubMed] [Google Scholar]
- [27].Christian P, Mullany LC, Hurley KM, Katz J, Black RE. Nutrition and maternal, neonatal, and child health. Semin Perinatol. 2015;39:361–72. [DOI] [PubMed] [Google Scholar]
- [28].Colebunders R, Mann JM, Francis H, Hila K, Izaley L, Ilwaya M, et al. Herpes Zoster in African Patients: A Clinical Predictor of Human Immunodeficiency Virus Infection. J Infect Dis. 1988;157:314–8. [DOI] [PubMed] [Google Scholar]
- [29].Glesby MJ, Moore RD, Chaisson RE. Herpes zoster in patients with advanced human immunodeficiency virus infection treated with zidovudine. J Infect Dis. 1993;168:1264–8. [DOI] [PubMed] [Google Scholar]
- [30].Buchbinder SP, Katz MH, Hessol NA, Liu JY, O’Malley PM, Underwood R, et al. Herpes zoster and human immunodeficiency virus infection. J Infect Dis. 1992;166:1153–6. [DOI] [PubMed] [Google Scholar]
- [31].Glesby MJ, Hoover DR, Tan T, Shi Q, Gao W, French AL, et al. Herpes zoster in women with and at risk for HIV: data from the Women’s Interagency HIV Study. J Acquir Immune Defic Syndr. 2004;37:1604–9. [DOI] [PubMed] [Google Scholar]
- [32].Lange C, van Leth F, Sester M. Viral load and risk of tuberculosis in HIV infection. JAIDS Journal of Acquired Immune Deficiency Syndromes. 2016;71:e51–e3. [DOI] [PubMed] [Google Scholar]
- [33].Lawn SD, Badri M, Wood R. Tuberculosis among HIV-infected patients receiving HAART: long term incidence and risk factors in a South African cohort. AIDS. 2005;19:2109–16. [DOI] [PubMed] [Google Scholar]
- [34].Gupta A, Bhosale R, Kinikar A, Gupte N, Bharadwaj R, Kagal A, et al. Maternal tuberculosis: A risk factor for mother-to-child transmission of human immunodeficiency virus. J Infect Dis. 2011;203:358–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].King CC, Ellington SR, Kourtis AP. The role of co-infections in mother-to-child transmission of HIV. Current HIV research. 2013;11:10–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Van de Perre P, Segondy M, Foulongne V, Ouedraogo A, Konate I, Huraux j-M, et al. Herpes simplex virus and HIV-1: deciphering viral synergy. The Lancet infectious diseases. 2008;8:490–7. [DOI] [PubMed] [Google Scholar]
- [37].Cowan FM, Humphrey JH, Ntozini R, Mutasa K, Morrow R, Iliff P. Maternal herpes simplex virus type 2 infection, syphilis and risk of intra-partum transmission of HIV-1: results of a case control study. AIDS. 2008;22:193–201. [DOI] [PubMed] [Google Scholar]
- [38].Bollen LJ, Whitehead SJ, Mock PA, Leelawiwat W, Asavapiriyanont S, Chalermchockchareonkit A, et al. Maternal herpes simplex virus type 2 coinfection increases the risk of perinatal HIV transmission: possibility to further decrease transmission? AIDS. 2008;22:1169–76. [DOI] [PubMed] [Google Scholar]
- [39].Mwapasa V, Rogerson SJ, Kwiek JJ, Wilson PE, Milner D, Molyneux ME, et al. Maternal syphilis infection is associated with increased risk of mother-to-child transmission of HIV in Malawi. AIDS. 2006;20:1869–77. [DOI] [PubMed] [Google Scholar]
- [40].Thorne C, Malyuta R, Semenenko I, Pilipenko T, Stelmah A, Posokhova S, et al. Mother-to-child transmission risk is increased among HIV-infected pregnant women in Ukraine with serological test results positive for syphilis. Clin Infect Dis. 2008;47:1114–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Schulte JM, Burkham S, Hamaker D, Louis MES, Paffel JM, Hutcheson D, et al. Syphilis among HIV-infected mothers and their infants in Texas from 1988 to 1994. Sex Transm Dis. 2001;28:315–20. [DOI] [PubMed] [Google Scholar]
- [42].Van Dyke RB, Korber BT, Popek E, Macken C, Widmayer SM, Bardeguez A, et al. The Ariel Project: A prospective cohort study of maternal-child transmission of human immunodeficiency virus type 1 in the era of maternal antiretroviral therapy. J Infect Dis. 1999;179:319–28. [DOI] [PubMed] [Google Scholar]
- [43].Mandelbrot L, Mayaux M-j, Boogain A, Berrebi A. Obstetric factors and mother-to-child transmission of human immunodeficiency virus type 1: The French perinatal cohorts. Am J Obstet Gynecol. 1996;175:661–7. [DOI] [PubMed] [Google Scholar]