Abstract
Objective
The goal of this study was to investigate the role of CD 19+ B cells within the brain and spinal cord during CNS autoimmunity in a peptide-induced, primarily T-cell–mediated experimental autoimmune encephalomyelitis (EAE) model of MS. We hypothesized that CD19+ B cells outside the CNS drive inflammation in EAE.
Methods
We generated CD19.Cre+/− α4-integrinfl/fl mice. EAE was induced by active immunization with myelin oligodendrocyte glycoprotein peptide (MOGp35-55). Multiparameter flow cytometry was used to phenotype leukocyte subsets in primary and secondary lymphoid organs and the CNS. Serum cytokine levels and Ig levels were assessed by bead array. B-cell adoptive transfer was used to determine the compartment-specific pathogenic role of antigen-specific and non–antigen-specific B cells.
Results
A genetic ablation of α4-integrin in CD19+/− B cells significantly reduced the number of CD19+ B cells in the CNS but does not affect EAE disease activity in active MOGp35-55-induced disease. The composition of B-cell subsets in the brain, primary lymphoid organs, and secondary lymphoid organs of CD19.Cre+/− α4-integrinfl/fl mice was unchanged during MOGp35-55-induced EAE. Adoptive transfer of purified CD19+ B cells from CD19.Cre+/− α4-integrinfl/fl mice or C57BL/6 wild-type (WT) control mice immunized with recombinant rMOG1-125 or ovalbumin323-339 into MOGp35-55-immunized CD19.Cre+/− α4-integrinfl/fl mice caused worse clinical EAE than was observed in MOGp35-55-immunized C57BL/6 WT control mice that did not receive adoptively transferred CD19+ B cells.
Conclusions
Observations made in CD19.Cre+/− α4-integrinfl/fl mice in active MOGp35-55-induced EAE suggest a compartment-specific pathogenic role of CD19+ B cells mostly outside of the CNS that is not necessarily antigen specific.
Recent clinical trials with B-cell–depleting anti-CD20 therapeutic monoclonal antibodies illustrated a pathogenic role for B lymphocytes in MS.1–4 Whether B-cell depletion outside of the CNS is sufficient to provide a detectable benefit in MS or whether a reduction in the number of B lymphocytes within the CNS compartment is required to diminish inflammation remains incompletely understood.
In 1992, it was determined that the binding of leukocytes to inflamed CNS venules was inhibited by antibodies against α4-integrin.5 Natalizumab, a humanized recombinant monoclonal antibody, was the first approved α4-integrin antagonist for treatment of relapsing forms of MS.6 Natalizumab is highly effective in decreasing the number of CD19+ B cells in CSF.7
The goal of this study was to investigate the role of α4-integrin ablation in CD19+ B cells in a peptide-induced, primarily T-cell–mediated experimental autoimmune encephalomyelitis (EAE) model and to identify compartment-specific contributions of B cells to disease initiation and perpetuation. A T-cell–mediated EAE model was chosen to reflect the role of α4-integrin in B cells in patients with MS as closely as possible. Genetically, MS is most strongly associated with human leukcoyte antigen-DRB1*15:01,8,9 an association that implies a pathogenic involvement of an antigen-specific CD4+ T cell in MS.
Flow cytometry was used to phenotype leukocyte subsets in lymphoid organs and the CNS. Serum cytokine levels and immunoglobulin (Ig) levels were assessed by ELISA. B-cell adoptive transfer was used to determine the compartment-specific pathogenic role of antigen-specific B cells.
Methods
Generation of CD19.Cre+/− α4-integrin–deficient mice
Because α4-integrin is an absolute requirement for normal organ development, α4-integrin–deficient (α−/−) mice are embryonic lethal.10 Thus, it is not possible to conduct EAE experiments in animals that are completely devoid of α4-integrin. To examine how the deficiency of α4-integrin affects the migration of dendritic cells and B cells into the CNS and T-cell reactivation and retention in the CNS, we used cre-loxP–mediated recombination11 to create B-cell lineage–specific α4-integrin gene knockout mice. Specifically, we crossed female mice that are homozygous for the α4-integrin–floxed allele (α4f/f)12 with commercially available CD19.Cre+ males for the ablation of α4-integrin in B cells. Insertion of cre disrupts the CD19 coding sequence, leading to a CD19 deficiency and a concomitant reduction in germinal centers (GCs) in homozygous animals. Consequently, CD19.Cre+/+ mice behave functionally very similarly to B-cell–deficient mice. CD19.Cre+/+ mice on the C57BL/6 background were used to generate CD19.Cre+/− α4-integrinfl/fl mice that appear developmentally normal and fertile. C57BL/6 mice were purchased from (The Jackson Laboratories, Bar Harbor, MN). α4-integrinfl/fl mice were used as controls. Male and female mice were used for experiments. We observed no differences regarding disease scores, cellular composition, or any of the biochemical and cellular outcomes between the 2 sexes.
Peptides
Mouse myelin oligodendrocyte glycoprotein peptide (MOGp)35-55 (MEVGWYRSPFSRVVHLYRNGK) and ovalbumin (OVA)323-339 (ISQAVHAAHAEINEAGR) were synthesized by solid-phase Fmoc chemistry by QCB, Inc. (Hopkinton, MA) and CS Bio (Menlo Park, CA). Recombinant rMOG1-125 was as donation of Dr. Hans-Christian von Büdingen at the University of California, San Francisco (UCSF).
Experimental autoimmune encephalomyelitis
To induce active EAE, experimental mice were immunized subcutaneously with myelin MOGp35-55 (200 μg/100 μL/mouse), emulsified in an equal volume of complete Freund adjuvant containing 4 mg/mL H37Ra Mycobacterium tuberculosis (Difco, BD, Franklin Lakes, NJ) in each flank as described.13
For B-cell adoptive transfer, spleens of donor mice immunized with MOG1-125 or OVA323-339 were removed at day 12, and single-cell suspensions were prepared as previously described.14 The Miltenyi kit 130-090-862 was used to purify a total of 10 × 106 CD19+ donor B cells (Miltenyi Biotec, San Diego, CA). Briefly, highly pure resting B cells were isolated by magnetic labeling and depleted of CD43-expressing B cells (activated B cells, plasma cells [PCs], and CD5+B-1a cells) and non–B cells. Purified cells were subsequently transferred IV into recipient CD19.cre+/− α4-integrinfl/fl mice that were then immediately immunized with MOGp35-55. For all experiments, individual animals were observed and scored as described.13
Isolation of lymph node cells and splenocytes
Lymph node (LN) cells and splenocytes were isolated by pressing through a 70-μM nylon mesh cell strainer as described.13
Percoll PLUS density gradient
In all experiments in which tissue is referred to as CNS, and not specifically as brains or spinal cords, CNS leukocytes were isolated by Percoll PLUS (GE Healthcare Bio-Sciences, Pittsburgh, PA) gradient as previously described.13
Enzymatic CNS digestions
For some experiments, brains and spinal cords were dissociated enzymatically as described.13
Flow cytometry
To determine the absolute number of B cells and T cells in different compartments during EAE, multiparameter flow cytometry was used. Cells from bone marrows (BMs), spleens, LNs, brains, and spinal cords were brought into single-cell suspension as described.13 Cells were stained for 30 minutes at 4°C with the following antibodies: CD45 PE-Cyannin-7 (30-F11; eBioscience, San Diego, CA) CD3 Alexa Flour 700 (17A2, eBioscience), CD19 PE-Texas Red (6D5, Invitrogen, Waltham, MA), CD19 PE (1D3 BD Bioscience, San Jose, CA), CD49d FITC (PS/2, Santa Cruz Biotechnology, Dallas, TX), CD4 APC (RM4-5, BD Bioscience), CD45R B220-APC (RA3-6B2, BioLegend, San Diego, CA), PE, PE Dazzle 594, PE-Texas Red, AF700, Pac Orange, PerCP, CD138 BV421(281-2, BioLegend), CD11b (APC, APC-Cy-7, PE, PerCP/Cy5.5, V450, B700 (M1/70, BioLegend), CD23 PE-Cy7 (B3B4, BioLegend), CD5 PE-Cy5 (53-7.3, BioLegend), CD1d PE (1B1, BioLegend), GL-7 FITC (GL7, BioLegend), and CD21/CD35 BV510 (7G6, BD Bioscience, San Jose, CA). Isotype-matched mAbs were used to set the gates. Next, 30,000–300,000 gated events were acquired on an FACSAria II flow cytometer (BD Biosciences), equipped with Diva acquisition software (BD Biosciences). FlowJo (BD Biosciences) software was also used for some data analysis.
Intracellular cytokine staining
Phorbol 12-myristate 13-acetate (500 ng), ionomycin (500 ng), and GolgiPlug (1 μL) (BD Biosciences) were added to 1 × 106 cells and incubated for 3 hours at 37°C. Cells were then washed in phosphate-buffered saline and stained following the extracellular staining protocol for CD45-Alexa Fluor 700. After extracellular staining, CD19+ cells were purified as described and fixed using Fixation Buffer (BioLegend) for 15 minutes in the dark at room temperature. Cells were then washed with Permeabilization Buffer (BioLegend) twice and then stained using interferon gamma (IFNγ)-PE-Cy7 (BD Biosciences), interleukin (IL)-17A-FITC (BioLegend), tumor necrosis factorα-PE (eBioscience), and IL-6-APC (BioLegend). Cells were then washed, and acquired, and analyzed by flow cytometry.
ELISA
Serum samples were collected by submandibular bleeding at days 13, 19, and 29 during early active EAE, maximum EAE disease activity, and during chronic EAE. Quantitative ELISA for IL-17, IL-10, IL-4, IL-5, and IFNγ was performed using paired mAb specific for corresponding cytokines as per the manufacturer's recommendations (BD Biosciences or R&D Systems). IgM and IgG serum levels were also determined by ELISA. The results of ELISA assays are expressed as an average of triplicate wells ± SD. The EPOCH (BioTek, Winooski, VT) ELISA plate reader and software were used for data analysis (Molecular Devices Corporation, Sunnyvale, CA).
T-cell proliferation assay
To determine the capability of B cells to serve as APC to CD4+ T cells, flow cytometric proliferation assays using the green fluorescent dye carboxyfluorescein succinimidyl ester or V450 (BD Biosciences) were performed according to published methods.15 On day 15 after active induction of EAE, spleens and LNs were harvested and processed into single-cell suspension. Splenocytes and lymphocytes were stained with carboxyfluorescein succinimidyl ester and plated at 1 × 106 cells per well. The cells were then restimulated with 10 μg/mL of MOGp35-55 and incubated at 37°C for 5 days. Con A at a concentration of 2 mg/mL and media were used as controls. On day 6, the cells were collected, blocked with Fc block antibody, stained with APC CD4 antibody, and acquired and analyzed by flow cytometry.
Histology
Following fixation in 10% buffered formalin, LNs were processed and embedded in paraffin blocks. Four-micrometer sections were cut, mounted on Fisherbrand Superfrost Plus glass slides (Fisher Scientific, Pittsburgh, PA), and stained with hematoxylin & eosin (Fisher Scientific) or anti-CD19.
Statistical analysis
For parametric tests, data were assessed for normality using the Kolmogorov-Smirnov test. Normally distributed values were compared using the unpaired 2-sided Student t test. Correlations between continuous and categorical variables were assessed using the Mann-Whitney U-test. All statistical tests were 2 sided, and p < 0.05 indicated significance. All analyses were performed with Prism 7 (Graphpad, La Jolla, CA).
Standard protocol approvals and registrations
All experimental animals were maintained in a specific pathogen-free facility at the University of Texas (UT) Southwestern Medical Center. All protocols involving mice handling were approved by the UT Southwestern animal care facility.
Data availability
Data not shown will be shared by request.
Results
The percentage of CD49d+ CD19+ B cells is diminished in primary and secondary lymphoid organs in naive CD19.Cre+/− α4-integrinfl/fl mice
To characterize CD19.Cre+/− α4-integrinfl/fl mice, the expression of α4-integrin (CD49d) was determined by multiparameter flow cytometry. The percentage of CD49d+ CD19+ B cells in the BM, LNs, spleens, and Peyer patches is significantly diminished in naive CD19.Cre+/− α4-integrinfl/fl mice compared with C57BL/6 wild-type (WT) control mice (figure 1A). Cre recombination efficiency is incomplete16 and determined mostly by the nucleotide sequence in the spacer region of the lox site. Also, there is a negative correlation between the Cre/lox recombination efficiency and the length of DNA between the 2 lox sites. As stated above, other investigators found a deletion efficiency between 75% and 80% in BM-derived pre–B cells and approximately 90% in splenic B cells.17
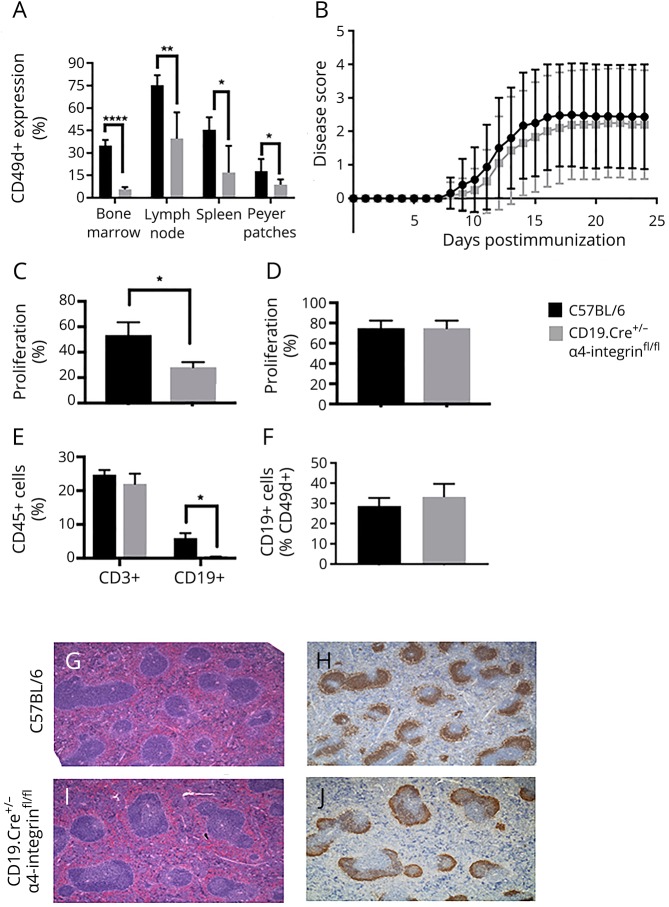
Figure 1. CD19.Cre+/− α4-integrinfl/fl mice are fully susceptible to actively MOGp35-55-induced experimental autoimmune encephalomyelitis.
(A) The percentage of CD49d+ CD19+ B cells in the bone marrow, lymph nodes, spleens, and Peyer patches is significantly diminished in naive CD19.Cre+/− α4-integrinfl/fl mice compared with C57BL/6 control mice. Cells were immunophenotyped by multiparameter flow cytometry. (B) CD19.Cre+/− α4-integrinfl/fl mice behave similar to C57BL/6 wild-type (WT) mice regarding the disease incidence, onset, and severity of active experimental autoimmune encephalomyelitis (EAE). α4-integrinfl/fl mice were used as controls and showed similar disease activity (data not shown). Active EAE was induced in CD19.Cre+/− α4-integrinfl/fl and C57BL/6 age-matched control mice by subcutaneous immunizations with MOGp35-55 in incomplete Freund adjuvant (IFA) containing 4 mg/mL mycobacteria. Mice received intraperitoneal injection of 200 μL of pertussis toxin (Ptx) at 200 ng/mL on days 0 and 2. Mice were observed daily, and EAE severity was scored using a 5-point scale. CD4+ T-cell recall responses to MOGp35-55 in (C) spleens were significantly diminished in CD19.Cre+/− α4-integrinfl/fl mice, but (D) indistinguishable to those in C57BL/6 WT mice in lymph nodes. Cell proliferation was determined by a flow cytometric proliferation assay using the green fluorescent dye CFSE or V450. (E) At maximum disease activity (days 13–15), the percentage of CD3+ T cells in the CNS was similar in CD19.Cre+/− α4-integrinfl/fl and C57BL/6 WT mice. In contrast, the percentage of CD19+ B cells in the CNS was significantly diminished in CD19.Cre+/− α4-integrinfl/fl mice. (F) The percentage of α4-integrin–positive (CD49d+) CD19+ B cells in the CNS during maximum EAE disease activity was comparable between both mouse strains. Lymphocytes were immunophenotyped by multiparameter flow cytometry. The number, appearance, and architecture of germinal centers in lymph nodes of (G and H) C57BL/6 WT control mice were comparable to those in (I and J) CD19.Cre+/− α4-integrinfl/fl mice. Panels G and I were stained with hematoxylin & eosin, and panels H and J were stained with anti-CD19. Magnification for G–J is ×4. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. CFSE = carboxyfluorescein succinimidyl ester.
CD19.Cre+/− α4-integrinfl/fl mice immunized with MOGp35-55 display a regular clinical EAE course
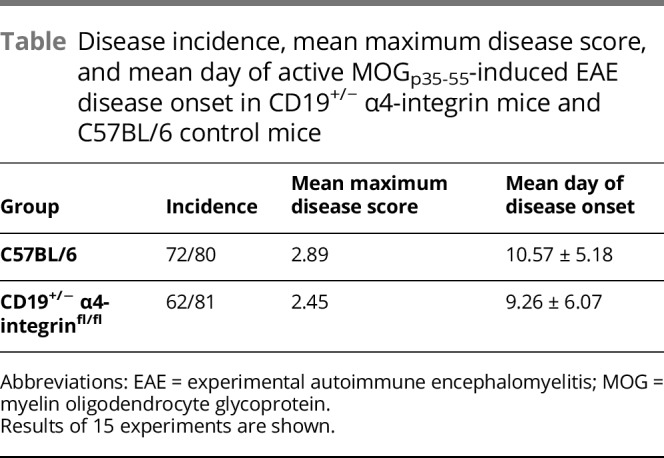
To determine the effect of α4-integrin expression of B cells in a T-cell–mediated active model of EAE, CD19.Cre+/− α4-integrinfl/fl mice and C57BL/6 control mice were immunized with MOGp35-55. No significant differences regarding the EAE disease incidence, severity, or phenotype were observed between the mouse strains (figure 1B and table). α4-integrinfl/fl mice were also used as controls and showed similar disease activity (data not shown).
Table.
Disease incidence, mean maximum disease score, and mean day of active MOGp35-55-induced EAE disease onset in CD19+/− α4-integrin mice and C57BL/6 control mice
α4-integrin expression on splenic B cells affects antigen-specific CD4+ T-cell proliferation
It was previously demonstrated that α4-integrin can participate in costimulation of CD4+ T cells.18,19 Yet other investigators showed that anti-CD49d plus anti-CD3/anti-CD28–coated polystyrene beads induced significantly greater T-cell proliferation than anti-CD3/anti-CD28 polystyrene beads alone.20 To test the effect of α4-integrin ablation on the capability of CD19+ B cells to present antigen to antigen-reactive CD4+ T cells, flow cytometric proliferation assays were performed. CD4+ T-cell recall responses to MOGp35-55 in spleens were significantly diminished in CD19.Cre+/− α4-integrinfl/fl mice (figure 1C). However, in LNs, we did not observe any difference in CD4+ T-cell proliferation between CD19.Cre+/− α4-integrinfl/fl mice and C57BL/6 WT control mice (figure 1D). In both mouse strains, T-cell proliferation was substantial. Our observations appear to differ from those of other investigators, who showed that B cells do not present MOGp35-55 to T cells.21 The Methods section of that article states that “…B cells were magnetically activated cell sorting (MACS; Miltenyi Biotec, Bergisch Gladbach, Germany)-separated from lymph nodes or spleens.” Thus, a differential capability of B cells isolated from either LNs or spleens on MOG peptide presentation may not have been fully tested.
The number of B cells in the CNS during active MOGp35-55-induced EAE is diminished in CD19.Cre+/− α4-integrinfl/fl mice
To examine the effect of α4-integrin deletion in CD19+ B cells on the ability of B lymphocytes to enter the CNS, cells were isolated from brains and spinal cords by Percoll gradient and analyzed by multiparameter flow cytometry. As MOGp35-55-induced EAE is a T-cell–mediated form of EAE, the number of CD3+ T cells was also assessed by the same method. At maximum disease activity (days 13–15), the percentage of CD3+ T cells in the CNS was similar in CD19.Cre+/− α4-integrinfl/fl mice and C57Bl/6 WT mice (figure 1E). In contrast, the percentage of CD19+ B cells in the CNS was significantly reduced in CD19.Cre+/− α4-integrinfl/fl mice (figure 1E). These observations suggest that antigen-specific B cells within the CNS play a minor role in initiating and perpetuating EAE, given that these mice showed the same disease susceptibility and disease course as C57Bl/6 WT mice. Immunohistochemical studies to determine a differential anatomic distribution of B cells in the CNS between CD19.Cre+/− α4-integrinfl/fl mice and C57Bl/6 WT mice were not performed, as the absolute number of B cells in CD19.Cre+/− α4-integrinfl/fl mice was very low.
CD19+ B cells from CD19.Cre+/− α4-integrinfl/fl mice use CD49d to gain access to the CNS during active MOGp35-55-induced EAE
To determine whether CD19+ B cells from CD19.Cre+/− α4-integrinfl/fl mice that gain access to the CNS during active EAE use α4-integrin, the percentage of α4-integrin–positive (CD49d+) CD19+ B cells was determined by multiparameter flow cytometry and found to be comparable between CD19.Cre+/− α4-integrinfl/fl mice and C57Bl/6 WT controls (figure 1F).
The number and architecture of GCs in LNs of CD19.Cre+/− α4-integrinfl/fl mice are normal
CD19.Cre mice express cre under the transcriptional control of the B lineage–restricted CD19 gene.17 Insertion of cre disrupts the CD19 coding sequence, leading to a CD19 deficiency and a concomitant reduction in GCs in CD19.Cre+/+ mice. At day 15 during the acute phase of MOGp35-55-induced EAE, the number and architecture of GCs in LNs of CD19.Cre+/− α4-integrinfl/fl mice and C57BL/6 WT control mice were comparable by hematoxylin & eosin (H&E) staining (figure 1, G and H) and anti-CD19 staining (figure 1, I and J).
B cells of CD19.Cre+/− α4-integrinfl/fl mice have normal cytokine profiles during active MOGp35-55-induced EAE
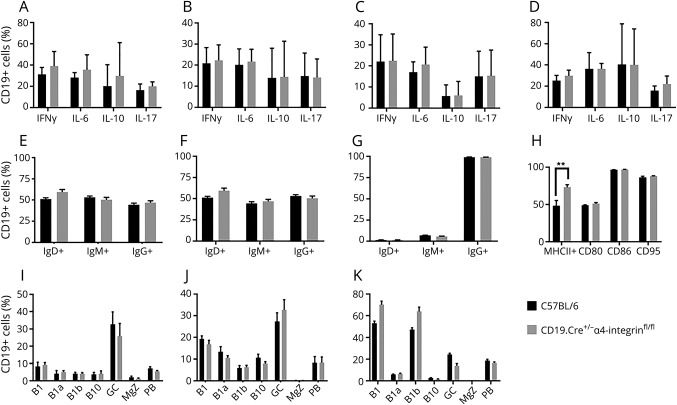
To test whether the absence of α4-integrin in CD19+ B cells affects their differentiation during inflammatory conditions, experimental animals were killed at day 13 during the acute phase of MOGp35-55-induced EAE, and B cells in disease-relevant compartments were immunophenotyped by multiparameter flow cytometry and intracellular cytokine staining. Compared with B cells from C57BL/6 WT control mice, the percentage of CD19+ B cells expressing IFNγ, IL-6, IL-10, or IL-17 in CD19.Cre+/− α4-integrinfl/fl mice was similar in the BM (figure 2A), spleen (figure 2B), LNs (figure 2C), and brain (figure 2D) of CD19.Cre+/− α4-integrinfl/fl mice.
Figure 2. CD19+ B cells in the CNS of CD19.Cre+/− α4-integrinfl/fl mice are phenotypically similar to wild-type (WT) B cells during myelin oligodendrocyte glycoprotein peptide (MOGp35-55)-induced experimental autoimmune encephalomyelitis.
Experimental animals were killed at day 13 during the acute phase of MOGp35-55-induced experimental autoimmune encephalomyelitis (EAE), and B cells in disease-relevant compartments were immunophenotyped by multiparameter flow cytometry and intracellular cytokine staining. Compared with B cells from C57BL/6 WT control mice, the percentage of CD19+ B cells expressing interferon gamma (IFNγ), interleukin (IL)-6, IL-10, or IL-17 in CD19.Cre+/− α4-integrinfl/fl mice was similar in the (A) bone marrow (BM), (B) spleen, (C) lymph node (LNs), and (D) brain of CD19.Cre+/− α4-integrinfl/fl mice. The percentages of CD19+ B220+ B cells expressing IgD, IgM, IgG, and MHC II in (E) LNs and (F) spleen were comparable in both mouse strains. (G) In the CNS, the surface expression of MHC II was similar in CD19.Cre+/− α4-integrinfl/fl mice and controls (H) In the brain, the expression of the costimulator molecules CD80 and CD86 and the first apoptosis signal receptor CD95 was indistinguishable between CD19.Cre+/− α4-integrinfl/fl mice and C57BL/6 WT controls. The percentage of CD11b+CD23B220lo B1 B cells, CD11b+CD23B220lo/−CD5+ B1a B cells, CD11b+CD23B220lo/−CD5− B1b B cells, B220+CD5+CD1dhi B10 B cells, CD19+CD23+CD5− naive follicular B cells (FB), B220+CD138−CD95+GL7+ germinal center (GC) B cells, B220+CD1d+CD5−CD23−CD21+ marginal zone (MGZ) B cells, CD138+B220low/– plasma blast (PB), and B220−CD138+ plasma cells (PC) was similar in both mouse strains at (I) day 13 during early active EAE, (J) day 19 during maximum EAE disease activity, and (K) day 29 during chronic EAE. Lymphocytes were immunophenotyped by multiparameter flow cytometry. A gating strategy used to identify some of the B-cell subsets is shown in Figure 3. **p < 0.01.
Outside the CNS, B cells of CD19.Cre+/− α4-integrinfl/fl mice express normal maturation and activation markers during active MOGp35-55-induced EAE
As stated above, α4-integrin is considered a costimulatory molecule through which CD19+ B cells are capable of interacting with CD4+ T cells. To further elucidate the effect of α4-integrin deficiency on CD19+ B cells on their maturation and activation in secondary lymphoid organs, experimental animals were killed at day 13 during the acute phase of MOGp35-55-induced EAE, and B cells in spleens and LNs were immunophenotyped by multiparameter flow cytometry. The percentages of CD19+ B220+ B cells expressing IgD, IgM, IgG, and major histocompatibility complex (MHC) II in LNs (figure 2E) and spleen (figure 2F) were comparable in CD19.Cre+/− α4-integrinfl/fl mice and C57BL/6 WT control mice.
CNS B cells from CD19.Cre+/− α4-integrinfl/fl mice do not upregulate activation markers during active MOGp35-55-induced EAE
The absolute number of CD19+ B cells is diminished in the CNS of CD19.Cre+/− α4-integrinfl/fl mice compared with C57BL/6 WT control mice during acute MOGp35-55-induced EAE (figure 1E). To evaluate the activation state of these cells, CNS B cells were isolated and assessed by multiparameter flow cytometry during active EAE at day 13 after active induction or EAE with MOGp35-55. The surface expression of MHC II was similar in CNS-derived B cells from CD19.Cre+/− α4-integrinfl/fl and C57BL/6 WT controls (figure 2G). Also, the expression of the costimulator molecules CD80 and CD86 and the first apoptosis signal receptor CD95 was comparable in brain-derived B cells from CD19.Cre+/− α4-integrinfl/fl mice and C57BL/6 WT controls (figure 2H).
The composition of B-cell subsets in the brain of CD19.Cre+/− α4-integrinfl/fl mice is unchanged during MOGp35-55-induced EAE
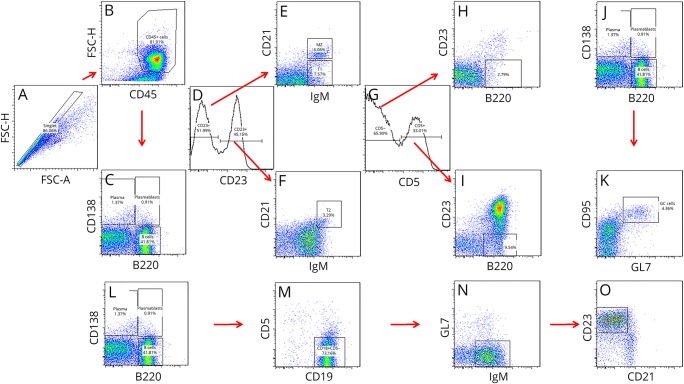
To determine whether the deletion of α4-integrin on CD19+ B cells affects B-cell subsets differentially in their capability to enter the CNS, the percentage of innate and adaptive B-lymphocyte subsets, including CD11b+CD23−B220− B1 B cells, CD11b+CD23−B220lo/−CD5− B1b B cells, CD11b+CD23−B220lo/−CD5+ B1a B cells, CD19+CD5+CD1dhi B10 B cells, CD19+CD23+CD5− naive follicular B cells, B220+CD138−CD95+GL7+ GCs B cells, B220+CD1d+CD5−CD23−CD21+ marginal zone (MGZ) B cells, CD19low/–CD138+B220low/– plasma blast (PB), and CD19−CD138+ PCs, was assessed by multiparameter flow cytometry during acute MOGp35-55-induced EAE at day 13 during early active EAE (figure 2I), day 19 during maximum EAE disease activity (figure 2J), and day 29 during chronic EAE (figure 2K) after active induction or EAE with MOGp35-55 and was found to be similar in CD19.Cre+/− α4-integrinfl/fl mice and C57BL/6 WT controls at all time points. A gating strategy used to identify some of the B-cell subsets is shown in figure 3.
Figure 3. Immunophenotyping of murine B cells.
B-lymphocyte subsets were immunophenotyped by multiparameter flow cytometry. A gating strategy that was used to identify some of the B-cell subpopulations is shown. (A) First, cellular area, height, and width measurements were obtained in a channel with linear scale to gate on singlets and exclude doublets. (B) Next, gates were set to include CD45+ leukocytes and exclude FSClow cell debris out. (C) CD138 and B220 were used to gate on plasmablasts (CD138+B220low/–), plasma cells (CD138+B220–), and B cells (B220+). Panels D–F show the gating for (E) B220+CD1d+CD5−CD23−CD21+IgM+ marginal zone B cells, (E) B220+CD1d+CD5−CD23−CD21−IgM+ transitional 1 (T1) B cells, and (F) B220+CD1d+CD5−CD23+CD21+IgM+ T2 B cells. Panels G–I show gating for B1 B-cell populations, and the identification of (H) CD11b+CD23−B220lo/−CD5− B1b B cells and (I) CD11b+CD23−B220lo/−CD5+ B1a B cells. Panels J and K show the identification of B220+CD138−CD95+GL7+ germinal center B cells. Panels L–O illustrate the gating for B220+CD19+CD5−GL7−CD23+CD21lo/int naive follicular B cells. FSC = forward side scatter.
The composition of B-cell subsets in primary and secondary lymphoid organs of CD19.Cre+/− α4-integrinfl/fl mice is similar to those of WT control mice during MOGp35-55-induced EAE
To assess the effect of α4-integrin ablation on CD19+ B cells on B-cell phenotypes in lymphoid organs, innate and adaptive B-lymphocyte subsets were characterized by flow cytometry.
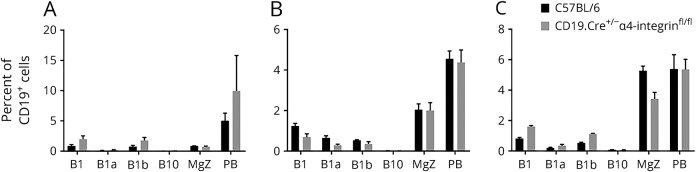
We were unable to detect a change in composition of CD19+CD11b+CD23−B220− B1 B cells, CD19+CD11b+CD23−B220−CD5+ B1a B cells, CD19+CD11b+CD23−B220−CD5− B1b B cells, CD19+CD5+CD1dhi B10 B cells, CD19+B220+CD1d+CD5−CD23−CD21+ MGZ B cells, and CD19low/–CD138+B220low/– PB CD19.Cre+/− α4-integrinfl/fl mice and C57BL/6 WT controls at day 13 during early active EAE in the BM (figure 4A), LNs (Figure 4B), and spleen (figure 4C).
Figure 4. CD19+ B cells in primary and secondary lymphoid organs of CD19.Cre+/− α4-integrinfl/fl mice are phenotypically similar to wild-type B cells during MOGp35-55-induced experimental autoimmune encephalomyelitis.
(A) In the bone marrow, (B) lymph nodes, and (C) spleen, the percentage of CD19+CD11b+CD23−B220− B1 B cells, CD19+CD11b+CD23−B220−CD5+ B1a B cells, CD19+CD11b+CD23−B220−CD5− B1b B cells, CD19+CD5+CD1dhi B10 B cells, CD19+B220+CD1d+CD5−CD23−CD21+ marginal zone (MGZ) B cells, and CD19low/–CD138+B220low/– plasma blast (PB) was similar in CD19.Cre+/− α4-integrinfl/fl mice and C57BL/6 WT controls at day 13 during early active EAE. Lymphocytes were immunophenotyped by multiparameter flow cytometry.
Serum soluble inflammatory markers are unchanged in CD19.Cre+/− α4-integrinfl/fl mice during different stages of MOGp35-55-induced EAE
Deletion of α4-integrin on CD19+ B cells diminishes the ability of CD19+ B cells to enter the CNS during acute MOGp35-55-induced EAE (figure 1E). It is conceivable that inflammatory B cells accumulate in peripheral blood, altering the inflammatory milieu in that compartment. This was observed in patients with MS treated with natalizumab.22 We found that the serum expression of the cytokines IL-17A (figure 5A), IL-10 (Figure 5B), IL-4 (Figure 5C), IL-5 (figure 5D), and IFNγ (figure 5E), as measured by ELISA (ELISA) during acute and chronic MOGp35-55-induced EAE on days 13, 19, and 29, was comparable between CD19.Cre+/− α4-integrinfl/fl mice and C57BL/6 WT control mice.
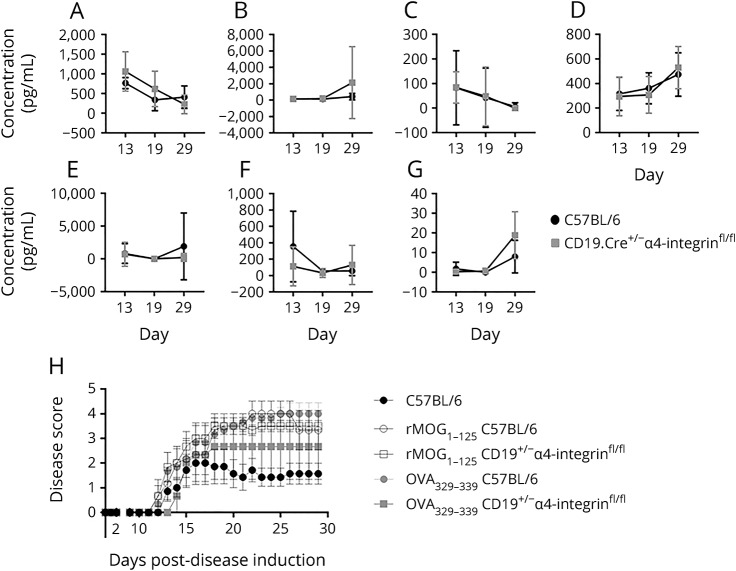
Figure 5. Activated B cells outside of the CNS drive disease activity in myelin oligodendrocyte glycoprotein peptide (MOGp35-55)-induced experimental autoimmune encephalomyelitis (EAE) in an antigen no-specific manner.
The serum expression of the cytokines (A) IL-17A, (B) IL-10, (C) IL-4, (D) IL-5, and (E) IFNγ as measured by ELISA during acute and chronic MOGp35-55-induced EAE on days 13, 19, and 29 was comparable between CD19.Cre+/− α4-integrinfl/fl mice and C57BL/6 wild-type (WT) control mice. Serum levels of (F) IgM and (G) IgG measured by ELSA at the same time points were also similar between mouse strains. (H) Adoptive transfer of purified CD19+ B cells from CD19.Cre+/− α4-integrinfl/fl mice or C57BL/6 WT control mice immunized with recombinant (r)MOG1-125 or ovalbumin (OVA)323-339 into CD19+/− α4-integrinfl/fl mice that were then immediately immunized with MOGp35-55 caused worse clinical EAE than was observed in MOGp35-55-immunized C57BL/6 WT control mice (“C57BL/6”) that did not receive adoptively transferred CD19+ B cells.
Serum Ig levels are unchanged in CD19.Cre+/− α4-integrinfl/fl mice during different stages of MOGp35-55-induced EAE
To further investigate whether the sequestration of CD19+ B cells through deletion of α4-integrin affects their activation, proliferation, and antibody secretion, IgM and IgG levels in serum were determined by ELISA. Serum levels of IgM (figure 5F) and IgG (figure 5G) as measured by ELISA were also similar between CD19.Cre+/− α4-integrinfl/fl mice and C57BL/6 WT control mice on days 13, 19, and 29 of MOGp35-55-induced EAE. Given that the absolute levels of IgM and IgG were similar between the mouse strains, and that other investigators previously showed that B cells from mice on the C57BL/6 background do not readily recognize MOGp35-55,21 we did not test the serum levels of MOGp35-55-specific IgM or IgG.
Activated peripheral B cells contribute to disease severity in MOGp35-55-induced EAE
To determine the compartment-specific pathogenic role of B cells in the induction and perpetuation of the EAE animal model, CD19.Cre+/− α4-integrinfl/fl mice were immunized with MOGp35-55. As demonstrated, B cells in these mice have a diminished capacity to enter the CNS (figure 1E). Adoptive transfer of purified CD19+ B cells from CD19.Cre+/− α4-integrinfl/fl mice and C57BL/6 WT mice immunized with rMOG1-125 or the control antigen OVA323-339 worsened the clinical course of EAE in recipient mice immunized with MOGp35-55 (figure 5H), although these changes were not significant.
Discussion
There is accumulating evidence to suggest that B cells and myelin-specific antibodies play crucial roles in the pathogenesis of MS. Whether B-cell depletion mediates its beneficial effects in patients with MS through B cells in the periphery, in the CNS, or both is currently not completely understood.
Other investigators investigated the role of α4-integrin deficiency in CD19+ B cells in EAE.23 These investigators showed that B-cell very late activation antigen-4–deficient mice developed more severe clinical disease than control mice after active induction of EAE with MOGp35-55 and that that this clinical effect is due to the requirement of regulatory B cells (Bregs) for α4-integrin to enter the CNS. Bregs were defined as CD19+IL-10+ or CD1dhiCD5+ B-cell subsets. Another group of investigators also tested CD19Cre Itga4fl/fl mice in MOGp35-55-induced active EAE.24 CD19Cre Itga4fl/fl mice developed more severe EAE than control mice, and these investigators demonstrated a critical role for α4-integrin on the generation of Bregs in peripheral immune organs.
Our own results diverge from these reports in that we could not detect a difference in active EAE disease severity between CD19.Cre+/− α4-integrinfl/fl mice and C57BL/6 WT control mice. These experiments were conducted 15 times (table), and we are confident in our findings. Differences in EAE disease severity and phenotype can be affected by conditions under which mouse colonies are maintained, as well as other factors that may affect the overall inflammatory milieu of experimental conditions. This may explain the differences in results. It is also conceivable that different mice were used by the other investigators for their experiments. The investigators of the 2 previous studies state that they used CD19.Creα4fl/fl mice23 or that they used CD19Cre Itga4fl/fl mice.25 It is not stated whether mice were heterozygous or homozygous for CD19.cre or a mixture of both. As stated in the Methods section, CD19.Cre+/+ mice behave functionally very similarly to B-cell–deficient mice, which develop more severe EAE when immunized with MOGp35-55.26
We found one potentially meaningful biological difference between CD19.Cre+/− α4-integrinfl/fl mice and C57BL/6 control mice. There was decreased MOGp35-55-specific CD4+ T-cell proliferation in spleens, which is an expected result, given that α4-integrin can participate in costimulation of CD4+ T cells.18,19
The observation that CD19+ B cells of CD19.Cre+/− α4-integrinfl/fl mice did deplete α4-integrin and that they were significantly less capable of entering the CNS after mice were immunized with MOGp35-55 suggests 3 interpretations: (1) Antigen-activated B cells contribute to disease burden in EAE in a seemingly dose-dependent manner, (2) they do not absolutely require recognition of a CNS autoantigen or a mimic thereof to do so, and (3) they do not require full α4-integrin–mediated access to the CNS to exacerbate disease. The first interpretation is supported by work of other investigators who have previously demonstrated that B-cell–deficient C57BL/6 mice are resistant to active rMOG-induced EAE26 and that the administration of a B-cell–depleting monoclonal antibody prevents or reverses established rMOG-induced EAE in C57BL/6 mice.21 Weber and colleagues21 further showed that B-cell depletion results in a reduction of MOGp35-55-specific Th1 and Th17 cells. Our own data suggest that the differentiation of MOGp35-55-specific T cells to become encephalitogenic does not absolutely require the presentation of autoantigen, but is likely driven by other B-cell factors, including soluble inflammatory mediators. The third interpretation of this experiment is supported by our demonstration that B cells from CD19.Cre+/− α4-integrinfl/fl mice are significantly impaired in their ability to enter the CNS (figure 1E). Finally, it should be emphasized that our findings do not conclusively rule out a role of antigen-specific B cells in the CNS on the course of CNS autoimmunity, as threshold effects may be difficult to detect with the EAE experiments that were conducted.
Our data suggest that the beneficial effects of anti-CD20 therapies in patients with MS are mediated through their effects on peripheral B cells. We also conclude that benefits observed in patients with MS during anti-α4-integrin therapy are likely not predominantly due to its effect of suppressing the migration of B cells into the CNS. The interpretation of our findings should be limited to early forms of MS, as the EAE model that was used and the observation period that was used do not provide insight into late or progressive forms of CNS autoimmunity. Magliozzi et al27 were the first investigators to show that some patients with secondary-progressive MS develop lymphoid tissue in the meninges that resemble B-cell follicles in secondary lymphoid organs. B cells in these structures may be susceptible to anti-CD20 therapies with monoclonal antibodies or small molecules, and it is conceivable that their depletion may benefit patients afflicted with that MS phenotype.
Acknowledgment
The authors thank Dr. Thalia Papayannopoulou at the University of Washington for providing them with α4-integrinflox/flox mice. Dr. Stuve was funded by a Merit Review grant (federal award document number [FAIN] I01BX001674) from the United States (U.S.) Department of Veterans Affairs, Biomedical Laboratory Research and Development.
Glossary
- BM
bone marrow
- DC
dendritic cell
- EAE
experimental autoimmune encephalomyelitis
- GC
germinal center
- IFN
interferon gamma
- IL
interleukin
- LN
lymph node
- MGZ
marginal zone
- MOG
myelin oligodendrocyte glycoprotein
- OVA
ovalbumin
- PB
plasma blast
- PC
plasma cell
- WT
wild type
Appendix. Authors
Study funding
No targeted funding reported.
Disclosure
R.Z. Hussain, P.C. Cravens, W.A. Miller-Little, R. Doelger, V. Grandos, and E. Herndon report no disclosures. D.T. Okuda received advisory and consulting fees from Celgene, Genentech, Genzyme, EMD Serono, and Novartis and research support from Biogen; served on the scientific advisory board of Osmotica; and served on the speakers' bureau of Acorda, Genzyme, and Teva. T.N. Eagar serves as section editor of Archives of Pathology and received research support from the NIH. O. Stuve served on the scientific advisory boards of TG Therapeutics and Genentech-Roche; served on the editorial board of Therapeutic Advances in Neurological Disorders; consulted for Celgene, EMD Serono, and Novartis; and received research support from Sanofi and Genzyme. Disclosures available: Neurology.org/NN.
References
- 1.Hauser SL, Waubant E, Arnold DL, et al. B-cell depletion with rituximab in relapsing-remitting multiple sclerosis. N Engl J Med 2008;358:676–688. [DOI] [PubMed] [Google Scholar]
- 2.Hawker K, O'Connor P, Freedman MS, et al. Rituximab in patients with primary progressive multiple sclerosis: results of a randomized double-blind placebo-controlled multicenter trial. Ann Neurol 2009;66:460–471. [DOI] [PubMed] [Google Scholar]
- 3.Hauser SL, Bar-Or A, Comi G, et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N Engl J Med 2017;376:221–234. [DOI] [PubMed] [Google Scholar]
- 4.Montalban X, Hauser SL, Kappos L, et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N Engl J Med 2017;376:209–220. [DOI] [PubMed] [Google Scholar]
- 5.Yednock TA, Cannon C, Fritz LC, Sanchez-Madrid F, Steinman L, Karin N. Prevention of experimental autoimmune encephalomyelitis by antibodies against alpha 4 beta 1 integrin. Nature 1992;356:63–66. [DOI] [PubMed] [Google Scholar]
- 6.Frohman EM, Racke MK, Raine CS. Multiple sclerosis—the plaque and its pathogenesis. N Engl J Med 2006;354:942–955. [DOI] [PubMed] [Google Scholar]
- 7.Stüve O, Marra CM, Jerome KR, et al. Immune surveillance in multiple sclerosis patients treated with natalizumab. Ann Neurol 2006;59:743–747. [DOI] [PubMed] [Google Scholar]
- 8.Haines JL, Ter Minassian M, Bazyk A, et al. A complete genomic screen for multiple sclerosis underscores a role for the major histocompatability complex. The Multiple Sclerosis Genetics Group. Nat Genet 1996;13:469–471. [DOI] [PubMed] [Google Scholar]
- 9.Sawcer S, Jones HB, Feakes R, et al. A genome screen in multiple sclerosis reveals susceptibility loci on chromosome 6p21 and 17q22. Nat Genet 1996;13:464–468. [DOI] [PubMed] [Google Scholar]
- 10.Yang JT, Rayburn H, Hynes RO. Cell adhesion events mediated by alpha 4 integrins are essential in placental and cardiac development. Development 1995;121:549–560. [DOI] [PubMed] [Google Scholar]
- 11.Sternberg N, Hamilton D. Bacteriophage P1 site-specific recombination. I. Recombination between loxP sites. J Mol Biol 1981;150:467–486. [DOI] [PubMed] [Google Scholar]
- 12.Scott LM, Priestley GV, Papayannopoulou T. Deletion of alpha4 integrins from adult hematopoietic cells reveals roles in homeostasis, regeneration, and homing. Mol Cell Biol 2003;23:9349–9360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hussain RZ, Miller-Little WA, Doelger R, et al. Defining standard enzymatic dissociation methods for individual brains and spinal cords in EAE. Neurol Neuroimmunol Neuroinflamm 2018;5:e437 doi: 10.1212/NXI.0000000000000437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cravens PD, Hussain RZ, Zacharias TE, et al. Lymph node-derived donor encephalitogenic CD4+ T cells in C57BL/6 mice adoptive transfer experimental autoimmune encephalomyelitis highly express GM-CSF and T-bet. J Neuroinflammation 2011;8:73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karandikar NJ, Crawford MP, Yan X, et al. Glatiramer acetate (Copaxone) therapy induces CD8(+) T cell responses in patients with multiple sclerosis. J Clin Invest 2002;109:641–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu H, Marth JD, Orban PC, Mossmann H, Rajewsky K. Deletion of a DNA polymerase beta gene segment in T cells using cell type-specific gene targeting. Science 1994;265:103–106. [DOI] [PubMed] [Google Scholar]
- 17.Rickert RC, Roes J, Rajewsky K. B lymphocyte-specific, Cre-mediated mutagenesis in mice. Nucleic Acids Res 1997;25:1317–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Davis LS, Oppenheimer-Marks N, Bednarczyk JL, McIntyre BW, Lipsky PE. Fibronectin promotes proliferation of naive and memory T cells by signaling through both the VLA-4 and VLA-5 integrin molecules. J Immunol 1990;145:785–793. [PubMed] [Google Scholar]
- 19.Shimizu Y, van Seventer GA, Horgan KJ, Shaw S. Costimulation of proliferative responses of resting CD4+ T cells by the interaction of VLA-4 and VLA-5 with fibronectin or VLA-6 with laminin. J Immunol 1990;145:59–67. [PubMed] [Google Scholar]
- 20.Theien BE, Vanderlugt CL, Eagar TN, et al. Discordant effects of anti-VLA-4 treatment before and after onset of relapsing experimental autoimmune encephalomyelitis. J Clin Invest 2001;107:995–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weber MS, Prod'homme T, Patarroyo JC, et al. B-cell activation influences T-cell polarization and outcome of anti-CD20 B-cell depletion in central nervous system autoimmunity. Ann Neurol 2010;68:369–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krumbholz M, Meinl I, Kumpfel T, Hohlfeld R, Meinl E. Natalizumab disproportionately increases circulating pre-B and B cells in multiple sclerosis. Neurology 2008;71:1350–1354. [DOI] [PubMed] [Google Scholar]
- 23.Lehmann-Horn K, Sagan SA, Winger RC, et al. CNS accumulation of regulatory B cells is VLA-4-dependent. Neurol Neuroimmunol Neuroinflamm 2016;3:e212 doi: 10.1212/NXI.0000000000000212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glatigny S, Wagner CA, Bettelli E. Correction: cutting edge: integrin alpha4 is required for regulatory B cell control of experimental autoimmune encephalomyelitis. J Immunol 2017;198:3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Glatigny S, Duhen R, Oukka M, Bettelli E. Cutting edge: loss of alpha4 integrin expression differentially affects the homing of Th1 and Th17 cells. J Immunol 2011;187:6176–6179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lyons JA, San M, Happ MP, Cross AH. B cells are critical to induction of experimental allergic encephalomyelitis by protein but not by a short encephalitogenic peptide. Eur J Immunol 1999;29:3432–3439. [DOI] [PubMed] [Google Scholar]
- 27.Magliozzi R, Howell O, Vora A, et al. Meningeal B-cell follicles in secondary progressive multiple sclerosis associate with early onset of disease and severe cortical pathology. Brain 2007;130:1089–1104. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data not shown will be shared by request.