Abstract
Background
We investigated whether apigenin could mitigate myocardial reperfusion injury in rats, and a possible mechanism was proposed.
Material/Methods
The I-R injury model was established in rats along with a sham group as control, and the expressions of microRNA-15b (miR-15b), JAK2, and p-JAK2 in the myocardia of the 2 groups were detected. Apoptosis and reactive oxygen species (ROS) were also detected. Rats in the I-R injury model were divided into 3 groups in vivo: the 1I-R group, the 2I-R+solvent group, and the 3I-R+apigenin group. Expression of miR-15b, JAK2, p-JAK2, STAT3, and p-STAT3 in the myocardia of the 3 groups were detected. ROS content, apoptosis, MDA content, SOD, and CAT activities were detected. Rat myocardial H9C2 cells were cultured in vitro and divided into 5 treatment groups in vitro; expressions of miR-15b, JAK2, p-JAK2, STAT3, and p-STAT3 in H9C2 cells were detected, and the apoptosis and ROS content were detected by flow cytometry.
Results
We found that the increased miR-15b expression during myocardial I-R injury in rats downregulated the expression of JAK2 and activity of the JAK2-STAT3 pathway, promoted myocardial apoptosis and ROS production, and aggravated myocardial I-R injury. Apigenin treatment can downregulate miR-15b expression, increase the expression of JAK2 and the activity of JAK2-STAT3 pathway, reduce myocardial apoptosis and ROS production, and alleviate myocardial I-R injury.
Conclusions
Api treatment downregulated the expression of miR-15b and upregulated the expression of JAK2 and the activity of the JAK2-STAT3 pathway, thereby alleviating myocardial I-R injury, cardiomyocyte apoptosis, and ROS production in vitro.
MeSH Keywords: Apigenin, Myocardium, Reperfusion Injury
Background
Coronary artery reperfusion after acute myocardial infarction (AMI) is the most effective way to save the ischemic myocardium, protect myocardial function, and prevent patient deaths [1,2]. However, recanalization inevitably causes ischemia-reperfusion (I-R) injury of the infarct myocardium, which leads to more serious myocardial damage [3,4].
Janus kinase (JAK) signal transducers and signal transducer and activator of transcription (STAT) are widely expressed in a variety of tissues and cells, and participate in many biological processes, such as cell survival, proliferation, apoptosis, and migration [5,6]. The STAT protein family consists of 7 members, among which STAT3 is considered to have the most important functions. The expression or abnormal functional activity of STAT3 is associated with I-R injury in a variety of tissues and organs [1–3]. JAK2 is a member of the JAK kinase family. Many studies have shown that JAK2 expression or functional changes are related to I-R injury in various organs such as myocardium [10], brain [11], and kidney [7]. MicroRNA (miRNA) is a class of single-stranded, noncoding RNA molecules made up of 22–25 nucleotides. miRNA induces cleavage or blockage of translation of the target mRNAs by complete or incomplete complementary binding to the 3′ untranslated region (UTR) of mRNAs. The role of abnormal expression and function in myocardial [12], spinal cord [13], and renal [14] I-R injury has attracted increasing attention. Studies have shown that miR-15b plays a role in the I-R injury of cardiomyocytes [15,16] and nerve cells [17].
Apigenin (Api) is 4, 5, 7-trihydroxyflavone, which is a flavonoid compound with a molecular formula of C15H10O5 [18]. In recent years, it has been reported that Api can alleviate myocardial I-R injury [19] and reduce infarct area after AMI [20,21]. However, the specific mechanism of its protective effect against myocardial I-R injury remains unclear. Studies have shown that apigenin is closely related to JAK2-STAT3 pathway activity and ischemia-reperfusion injury [22]. The activity of the JAK2-STAT3 pathway is closely related to injury to the heart [8,10,12], kidneys [7,9], and brain [11]. Luo found that osthole reduced renal reperfusion injury by regulating JAK2-STAT3 pathway activity [7]. Liu found that miR-15b is involved in the myocardial ischemia-reperfusion injury process [16]. However, it is not clear whether the protective effect of apigenin on myocardial ischemia-reperfusion occurs via miR-15b. Therefore, the present study investigated whether apigenin plays a role in regulating miR-15b and whether it affects JAK2-STAT3 pathway activity and myocardial I-R injury.
Material and Methods
Main reagents and materials
Healthy adult male Sprague-Dawley rats (6 weeks, weighing 220–240 g) were purchased from Shanghai Slack Experimental Animal Co. DMEM medium, FBS, type II collagenase, Tyrisin, and Opti-MEM were from Gibco (NY). Rabbit anti-rat JAK2 and p-JAK2 were from Abcam (Cambridge, UK). Rabbit anti-rat STAT3, p-STAT3, and beta-actin were from Boston (MA). Conjugated goat anti-rabbit IgG (H+L) was from Raw Biology (Shanghai, China). Lipofectamine 2000 transfection reagent and pMIR-Report luciferase were from Invitrogen (NY). PrimeScript RT Reagent kit was from Dalian (China). Luciferase activity detection kit, miR-15b inhibitor, antagomir-NC, and antagomir miR-15b were from Guangzhou (China). Lipid peroxidation product malondialdehyde (MDA), superoxide dismutase (SOD), catalase (CAT) activity detection kit, fluorescent dye, caspase-3 activity detection kit, cell apoptosis detection kit, Bradford Protein Assay kit, and RIPA lysate were from Biyuntian Biotechnology Co. (Nanjing, Jiangsu). Apigenin was purchased from Shanghai Pureone Biotechnology Co. (China). Chloral hydrate, PEG400, DMSO, and apigenin were from Sigma (San Francisco, CA). PBST, skimmed milk powder and SDS-polyacrylamide were from Biomart (Beijing, China). Ethanol was from China National Pharmaceutical Group (Shanghai, China). The FC500MCL flow cytometer was from Beckman Coulter (Brea City, FL). The CFX96 Real-Time PCR system and ELX808 microplate spectrophotometer were from Bio-Rad (CA). The electrophoresis apparatus, Fluoroskan Ascent FL, was from Thermo (NY).
I-R injury model in rats
All rats were intraperitoneally anesthetized with 10% chloral hydrate. After opening the chest at the fourth rib, the heart was exposed and the left anterior descending branch of the coronary artery was identified and ligated. If the ECG monitoring showed an elevation of ST segment above 0.1 mV in the Q lead or T waves, and the color of the heart turned pale with weakened heartbeat, the AMI model was considered to be successfully built. After 60 min, the ligature was loosened and the blood supply to the myocardium was restored. Apical reddening was a sign of successful reperfusion, upon which myocardial I-R injury was considered successfully built. A sham operation group (Sham group) receiving thoracotomy but no ligation of the left anterior descending coronary artery was used as a control group. The rats were killed at 12 h or 24 h in the 2 groups after surgery, and myocardial tissues were collected. The expressions of related genes and proteins were detected.
Apigenin treatment of rat I-R injury model
In the apigenin intervention experiment, the rats with induction of I-R injury were randomly divided into 3 groups: the I-R group, in which the rats were intraperitoneally injected with normal saline (4 mg/kg) 10 min before the experiment, and then the I-R model was induced; the I-R+solvent control group (I-R+Solvent) was intraperitoneally injected with 4 mL/Kg containing 10% of PEG400 and 10% ethanol solvent 10 min before the experiment; and the I-R+apigenin treatment group (I-R+apigenin), in which the rats were intraperitoneally injected with apigenin (4 mg/kg) 10 min before the experiment. Myocardial tissues were harvested at 24 h after surgery in the 3 groups. Western blot analysis was used to detect protein expression, caspase-3 activity was detected by spectrophotometry, ROS content was detected by flow cytometry, and MDA content and SOD activity were detected as well.
Detection of caspase-3 activity in rat myocardium
About 5 mg myocardial tissue was shredded and a homogenate was prepared with a tissue homogenizer. We added 100 μL lysis buffer to the homogenate, cracked it on ice for 20 min, and centrifuged it at 4°C for 10–15 min, and the supernatant was then collected into a new 1.5-ml centrifuge tube. The quality of the extracted proteins was determined by using a BCA kit. According to the kit’ instruction manual, the steps were as follow: pNA (10mM) provided by the kit was diluted to 0, 10, 20, 50, 100, and 200 μM, and the standards of different concentrations were prepared. The absorbance value at 405 nm was determined as A405, and the standard curve was made. Into each well of the 96-well plates, we added 65 μL assay buffer, 25 μL lysate supernatant, and 10μL LAc-DEVD-pNA (2 mM) sequentially, then the 96-well plate was capped and incubated at 37°C for 2 h. A405 was measured immediately when the color changes were obvious, and the A405 of the experimental group divided by A405 of the control group ×100% was the relative enzyme active unit.
Detection of MDA content and SOD activity
In accordance with the instructions of the MDA and SOD detection kits, the rat myocardial tissue was cut into pieces. Tissue homogenate was prepared with a tissue homogenizer and then centrifuged at 500 g for 5 min, then the supernatant was taken. The MDA content and SOD activity of the homogenate solution were measured for rats in accordance with the kit instructions.
Cell transfection
We added 40 pmol miR-15b mimic or miR-NC into the 50 μL Opti-MEM with gentle mixing and then stored at it at room temperature for 5 min. After adding 2 μL Lipofectamine 2000 into 50 μL Opti-MEM, the tube was placed at room temperature for 5 min after mixing gently. The 2 solutions were carefully mixed at room temperature for 20 min and then added into the H9C2 cells that were inoculated in the 24-well plate (10% FBS-containing DMEM, 60% confluency). Then, the cells were cultured in the incubator at 37°C and the medium was replaced by fresh 10% FBS-containing DMEM, before further culture for 72 h. Finally, the cells were digested with trypsin and collected.
Grouping and in vitro induction of I-R
I-R induction: H9C2 cells were added to the low-glucose DMEM medium and serum-free medium to simulate an ischemic environment in vitro. In an incubator with 95% N2/5% CO2, a hypoxic environment was simulated. After 12 h, the culture medium was replaced by a conventional serum-containing medium and was cultured in a 5% CO2/95% air incubator for another 12 h. Related indexes were detected.
H9C2 cells in a good growth state and reaching the logarithmic phase were divided into 5 treatment groups: I-R induction group, I-R+solvent+miR-NC group, I-R+solvent+miR-15b mimic group, I-R+miR-NC+Api (40 μM) group, and I-R+Api (40 μM)+miR-15b mimic group. After transfection for 72 h, I-R treatment was carried out according to the above methods, and the conventionally cultured H9C2 cells were used as the control (control). The cells were collected and the gene and protein expressions were detected. The apoptosis and ROS content were detected by flow cytometry, and the MDA content and SOD activity were detected using the kits.
Flow cytometry detection of cell apoptosis in vitro
Cells were collected, washed twice with PBS, and digested with 0.25% pancreatin. Then, the cells were collected and centrifuged at 300 g for 5 min. After washing with PBS twice, 100 μL of Annexin V binding solution was added to the cell suspension, followed by the addition of 5 μL Annexin V-FITC to the cell suspension. After the addition of 5 μL PI solution, the cells were incubated at room temperature away from light for 15 min, then we added with 400 μL 1×Annexin V binding solution. Cell apoptosis was measured by using FC500MCL flow cytometer within 1 h.
Flow cytometry detection of ROS content in vivo and in vitro
Detection of ROS content in myocardial tissues in vivo was performed as follows: rat myocardial tissues were collected at 24 h after surgery, digested by 0.1% type II collagenase for 30 min, and the tissue fragments were removed. The cell suspension was centrifuged at 250 g for 5min, and 500 μL d DCFH-DA diluted in DMEM was added to the cell precipitate (1: 1000 dilution). After incubation at 37°C for 30 min and washing with PBS, the cells were resuspended in 500 μL PBS, and ROS content was measured with a FC500MCL flow cytometer.
In vitro cultured myocytes were detected for ROS content as follows: After washing with PBS twice, the cells were digested with 0.25% trypsin, collected, and centrifuged at 300 g for 5 min. Next, 0.1% DCFH-DA probe diluted in 500 μL DMEM medium was added into the cell precipitate. The cells were incubated at 37°C for 30 min with gentle mixing for 3–5 times to ensure uniform and full contact between the cells and ROS probes. The cells were washed with PBS twice. After resuspension in 500 μL PBS, ROS content was immediately measured with a FC500MCL flow cytometer.
Luciferase reporter gene assay
Into the 24-well plate, 1×105 H9C2 cells were incubated. After cell growth to 50% fusion, Lipofectamine® 2000 was used to transfect pMIR-JAK2-WT (or pMIR-JAK2-MUT), and miR-15b mimic (or miR-NC, miR-15b inhibitor) into the H9C2 cells, and was cultured in serum-free medium, which was replaced with serum-free medium after 6 h. The cells were further cultured for 48 h and we detected dual luciferase activity. The activity of firefly luciferase was measured as follows: 100 μL cell lysate was added to each well of the 24-well plate. After incubation at room temperature for 15 min, 20 μL cell lysate was mixed with 100 μL LAR II, and luciferase activity was determined using fluorescein as a substrate. Then, the Renilla luciferase activity was determined using 100 μL fluorescein reagent. The ratio of firefly luciferase activity to Renilla luciferase activity was calculated.
qRT-PCR detection of miR-15b and JAK2 gene expressions
Trizol reagent was used for RNA extraction, followed by reverse transcription of RNA into cDNA using a PrimeScript cDNA RT reagent kit. Using cDNA as a template, PCR amplification reaction was carried out under the action of TaqDNA polymerase. The 10-μL reverse transcription system contained 2×SYBR Green Mixture 5.0 μL, 5 μm/L primers 0.5 μL each, cDNA, and dilution with ddH2O to 10 μL. The reverse transcription conditions were 50°C 15 min and 85°C for 5 min. PCR reaction conditions were: denaturation at 95°C 5 min, and 40 cycles on a Bio-Rad CFX96 real-time quantitative PCR system at 95°C×15 s and 60°C×1 min. The data were recorded.
Western blot detection of protein expressions
The cells were lysed in RIPA buffer and the proteins were extracted. From each sample, 40 μg of protein was separated on 10% SDS-PAGE gels and transferred to a nitrocellulose membrane (300 mA, 90 min). The membranes were then blocked with 5% non-fat milk for 60 min, incubated with primary polyclonal antibodies against JAK2, p-JAK2, STAT3, p-STAT3, and β-actin (the dilution times were 1: 2000, 1: 1000, 1: 2000, 1: 1000 and 1: 10000, respectively) at 4°C overnight. After removing the unbound antibodies, the membrane was incubated with secondary antibodies at room temperature for 60 min (1: 15 000 diluted HRP-conjugated goat anti-Rabbit IgG (H+L)), and ECL was determined for the membrane. This was followed by exposure to X-rays for 1–2 min, film development, and fixation in the darkroom, and film scanning.
Statistical analysis
Statistical analyses were carried out using SPSS 18 software. All data are expressed as mean ±SD. Statistical significance was determined using the t test for pairwise comparisons. For multiple comparisons, univariate ANOVA was performed with Bonferroni correction. P<0.05 was considered statistically significant.
Results
There was a miRNA-target regulatory relationship between miR-15b and JAK2
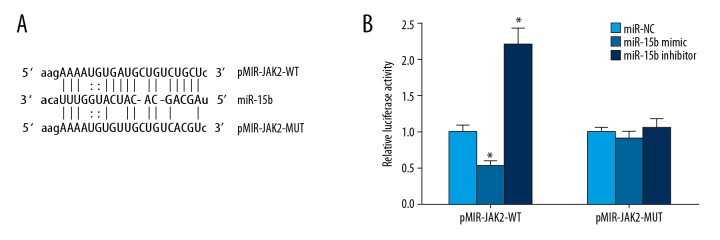
The online prediction results at the microRNA.org website showed that there was a target complementary binding site between miR-15b and JAK2 mRNA’s 3′-UTR (Figure 1A). The double-luciferase reporter gene assay showed that the transfection of miR-15b mimic significantly reduced relative luciferase activity in the H9C2 cells transfected by pMIR-JAK2-WT, and the transfection of miR-15b inhibitor significantly increased the relative luciferase activity in the H9C2 cells transfected by pMIR-JAK2-WT. However, the transfection of both had no significant effect on the relative luciferase activity of the pMIR-JAK2-MUT-transfected H9C2 cells (Figure 1B). This indicated that there was a miRNA-target regulatory relationship between miR-15b and JAK2.
Figure 1.
Relationship between miR-15b and JAK2. (A) A schematic diagram of the interaction site between miR-15b and JAK2 mRNA’s 3′-UTR. (B) Double-luciferase reporter gene assay. * P<0.05 compared with miR-NC.
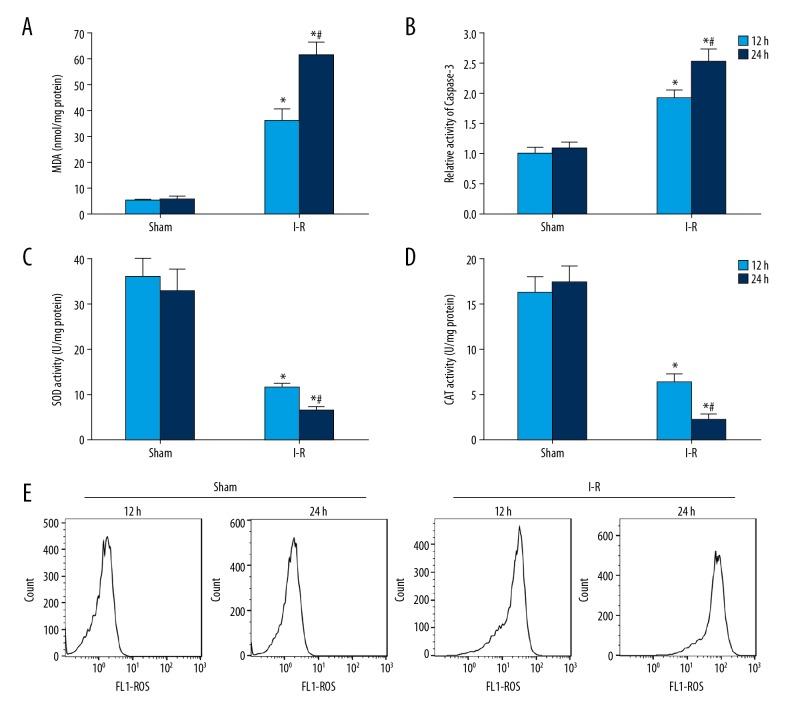
Oxidative stress and myocardial apoptosis were significantly increased in I-R rats
The tests of lipid peroxidation and antioxidant level showed that compared with the sham group, MDA content in the myocardium of I-R model rats increased significantly (Figure 2A), while the activities of SOD (Figure 2C) and CAT (Figure 2D) decreased significantly. The results of spectrophotometry showed that compared with sham rats, caspase-3 activity in myocardium of I-R model rats was significantly enhanced (Figure 2B). The results of flow cytometry showed that, compared with sham rats, the ROS content in the myocardium of I-R rats increased significantly (Figure 2E).
Figure 2.
The oxidative stress and apoptosis of myocardium in I-R rats increased significantly. (A) Detection of MDA content in rat myocardium. (B) Caspase-3 activity in rat myocardium was detected by spectrophotometry. (C) SOD activity in rat myocardium was detected by kits. (D) CAT activity in rat myocardium was detected by kits. (E) Flow cytometry was used to detect ROS content in rat myocardium. * P<0.05 compared with the sham group; # P<0.05 at 24 h compared with 12 h.
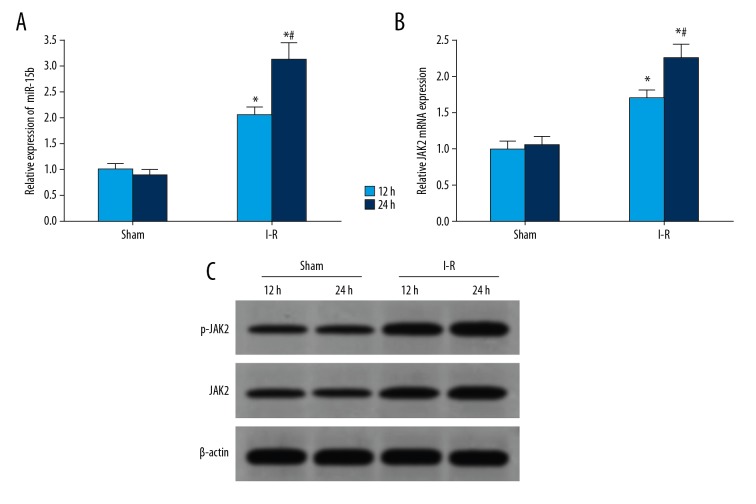
The expressions of miR-15b and JAK2 in the myocardium of I-R rats increased significantly
The results of qRT-PCR showed that the expression of miR-15b in myocardial tissues of rats in the I-R group was significantly higher than that in the sham group, and the expression of miR-15b was increased with the prolonged duration of I-R (Figure 3A). Compared with the sham group, the expression of JAK2 mRNA in the myocardium of the I-R group was increased significantly, and that of JAK2 mRNA was also increased with the prolonged duration of I-R (Figure 3B). Western blot analysis showed that the expressions of JAK2 and p-JAK2 proteins in the myocardium of the I-R group at 12 h and 24 h after surgery were significantly higher than that of the sham rats (Figure 3C).
Figure 3.
The expressions of miR-15b and JAK2 in the myocardium of I-R rats were increased significantly. (A) qRT-PCR detection of the expression of miR-15b in rat myocardium. (B) qRT-PCR detection of the expression of JAK2 mRNA in rat myocardium. (C) Western blot detection of protein expressions in rat myocardium. * P<0.05 versus sham; # P<0.05 at 24 h versus 12 h.
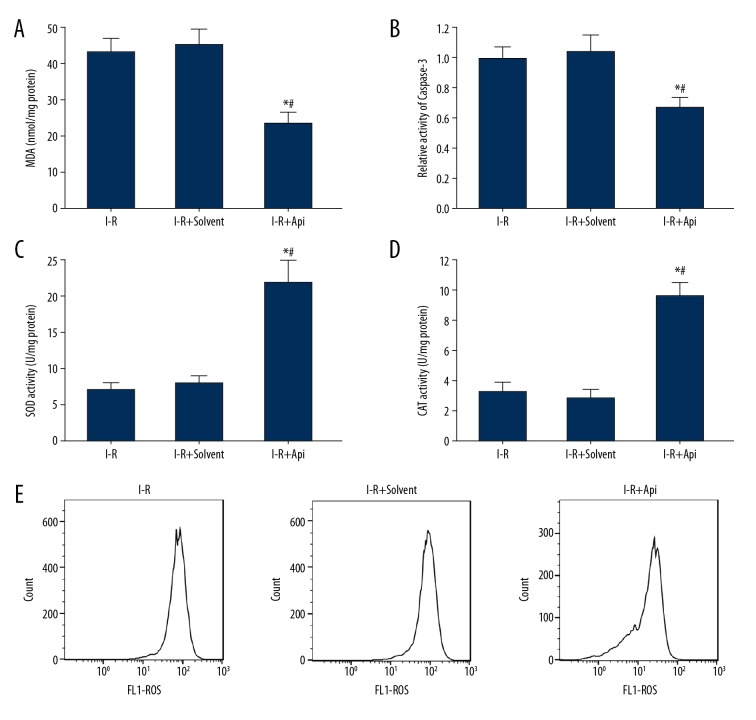
Api reduced the degree of oxidative stress and apoptosis in I-R rats
By detecting the lipid peroxidation and antioxidant levels at 24 h after surgery, we found that, compared with the I-R group and I-R+solvent group, the MDA content in myocardial tissues of the I-R+Api group significantly decreased (Figure 4A), while the activities of SOD (Figure 4B) and CAT (Figure 4C) increased significantly. The results of spectrophotometry showed that, compared with the I-R group and I-R+solvent group, caspase-3 activity in the myocardium of the I-R+Api group was significantly decreased (Figure 4D). Additionally, flow cytometry showed that, compared with the I-R group and I-R+solvent group, the ROS content in the myocardium of the I-R+Api group significantly decreased (Figure 4E). There was no significant difference in the contents of MDA, SOD, CAT, and caspase-3 in the myocardium between the I-R group and I-R+solvent group (P>0.05).
Figure 4.
Api treatment reduced oxidative stress and apoptosis in the myocardium of I-R rats. (A) MDA content in rat myocardium. (B) Caspase-3 activity in rat myocardium. (C) SOD activity in rat myocardium. (D) CAT enzyme activity in rat myocardium. (E) Flow cytometry detection of ROS content in rat myocardium. * P<0.05 versus I-R; # P<0.05 versus I-R+solvent.
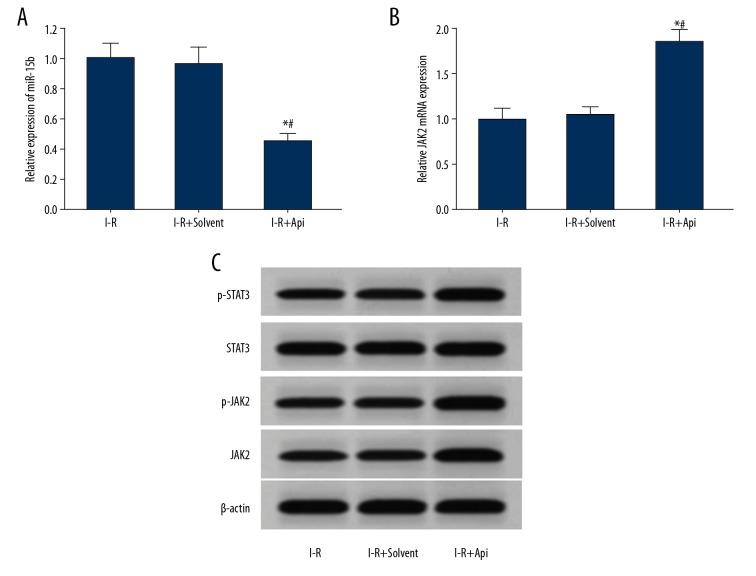
Api reduced the expression of miR-15b and increased the expression of JAK2 in the myocardium of I-R rats
qRT-PCR results showed that, compared with the I-R group and I-R+solvent group, the expression of miR-15b in the myocardium of the I-R+Api group was significantly decreased (Figure 5A), while the expression of JAK2 mRNA was increased significantly (Figure 5B). Western blot analysis showed that the expressions of JAK2, p-JAK2, and p-STAT3 proteins in the myocardium of the I-R+Api group were increased significantly compared with the I-R group and I-R+Solvent group (Figure 5C).
Figure 5.
Api reduced the expressions of miR-15b in the myocardium of I-R rats and increased the expression of JAK2. (A) qRT-PCR detection of miR-15b expression in rat myocardium. (B) qRT-PCR detection of the expression of JAK2 mRNA in rat myocardium. (C) Western blot detection of protein expressions in rat myocardium. * P<0.05 versus I-R; # P<0.05 versus I-R+Solvent.
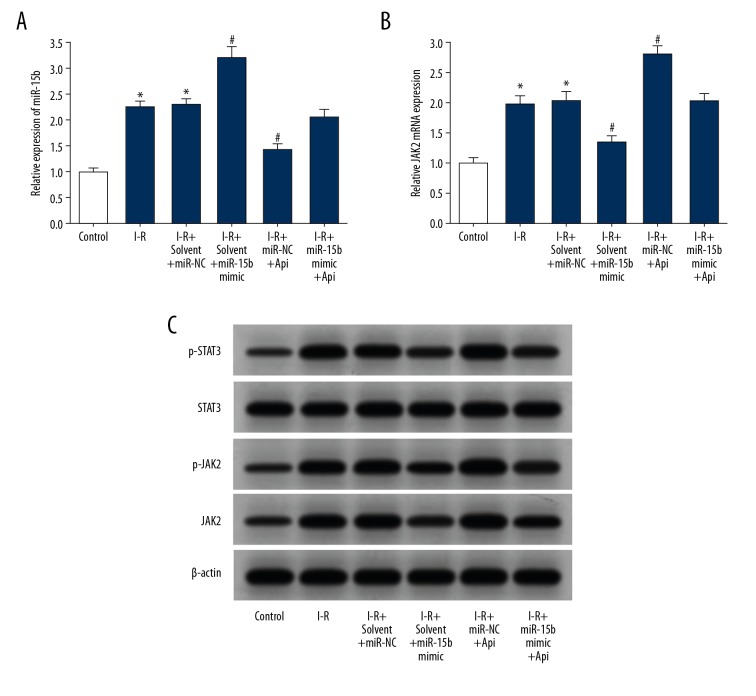
Api decreased the expression of miR-15b in H9C2 cells and increased the expression of JAK2
The results of qRT-PCR detection showed that, compared with the control group, the expression of miR-15b in H9C2 cells of the I-R group was significantly increased (Figure 6A), and that of JAK2 mRNA was increased significantly (Figure 6B). Transfection of miR-15b mimic increased the expression of miR-15b in H9C2 cells under I-R condition, while significantly reducing the expression of JAK2 mRNA. Additionally, Api treatment significantly reduced the expression of miR-15b in H9C2 cells under I-R condition, while significantly increasing the expression of JAK2 mRNA. Transfection of miR-15b mimic after Api treatment attenuated the downregulation of miR-15b expression induced by Api, and attenuated Api-induced upregulation of JAK2 mRNA expression. Western blot analysis showed that the expressions of JAK2, p-JAK2, and p-STAT3 in the I-R group were significantly increased compared with those of the group control, and the transfection with miR-15b mimic reduced the expression of JAK2 in H9C2 cells under I-R condition. The expressions of JAK2, p-JAK2, and p-STAT3 proteins in H9C2 cells were increased significantly under Api treatment following I-R injury. Transfection with miR-15b mimic after Api treatment attenuated the upregulation of JAK2, p-JAK2, and p-STAT3 protein expressions induced by Api (Figure 6C).
Figure 6.
Api reduced the expression of miR-15b in H9C2 cells and increased the expression of JAK2. (A) qRT-PCR was used to detect the expression of miR-15b in H9C2 cells. (B) qRT-PCR was used to detect the expression of JAK2 mRNA in H9C2 cells. (C) Western blot detection of protein expressions in H9C2 cells. * P<0.05 versus control; # P<0.05 versus I-R+solvent+miR-NC.
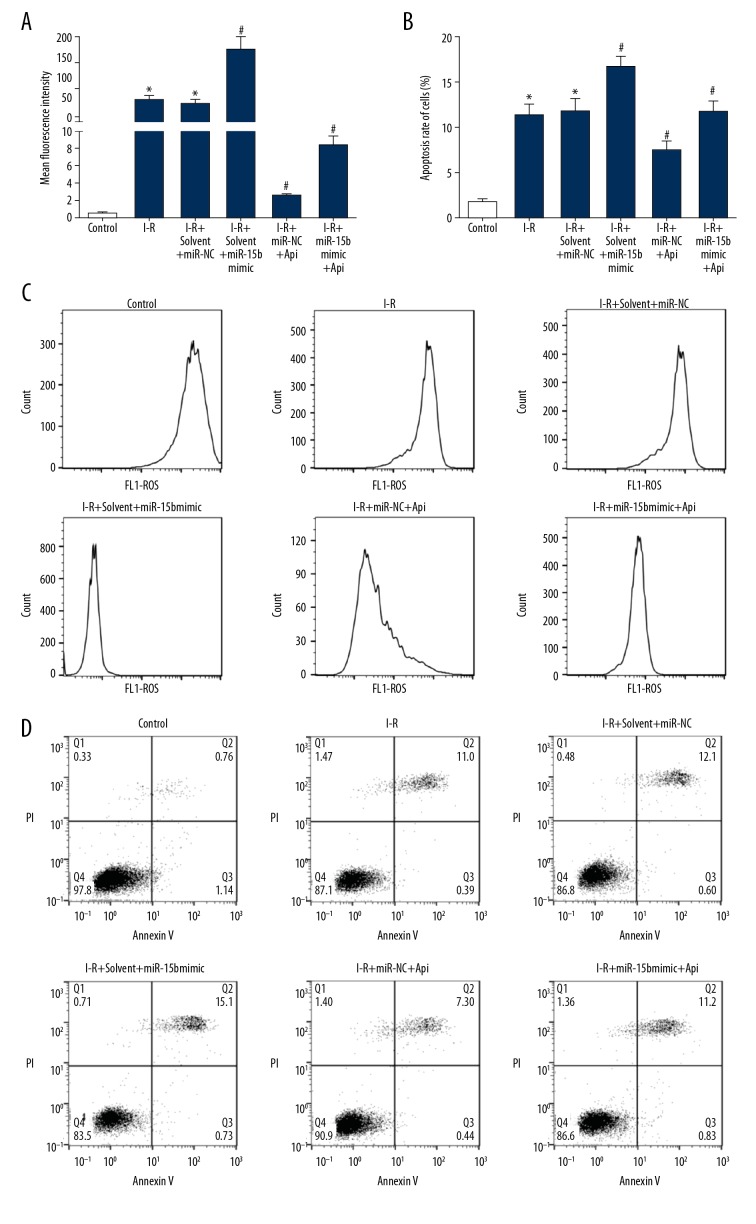
The effects of apigenin on the levels of apoptosis and ROS content in H9C2 cells under I-R condition
According to flow cytometry, the I/R group showed a significant increase in ROS production (Figure 7A, 7C) and apoptosis rate (Figure 7B, 7D), as compared with the control group. Compared with the I-R+solvent+miR-NC group, transfection with miR-15b mimic increased ROS production and apoptosis rate in H9C2 cells under I-R condition. Compared with the I-R+solvent+miR-NC group, Api treatment reduced ROS production and apoptosis rate in H9C2 cells under I-R condition. Transfection with miR-15b mimic after Api treatment increased ROS production and promoted apoptosis, which attenuated the protective effect of Api on H9C2 cells.
Figure 7.
Api significantly decreased H9C2 cell apoptosis and ROS production under I-R condition. (A) ROS content in H9C2 cells of each treatment group. (B) Apoptosis rate of H9C2 cells in each treatment group. (C) Flow cytometry was used to detect ROS content in H9C2 cells. (D) Flow cytometry indicated apoptosis of the H9C2 cells. * P<0.05 versus control; # P<0.05 versus I-R+solvent+miR-NC.
Discussion
When there is a factor that activates the JAK-STAT signaling pathway, the cell membrane receptor may be dimerized. The dimerized receptor can phosphorylate JAK kinase, which further leads to the phosphorylation of the receptor tyrosine kinase. As a result, STAT will fill the STAT site through the SH2 domain to the tyrosine phosphorylation site of the receptor complex. At this time, JAK kinase phosphorylation will activate the STAT protein that is adjacent to its spatial location, and cleave the activated STAT from the receptor complex, forming a dimer and transferring from the cytoplasm to the nucleus. This further promotes transcription and expression of the genes related to cell proliferation, cell cycle, and apoptosis regulation [4,22]. STAT3 is the most important member among the 7 members of the STAT protein family (STAT1, STAT2, STAT3, STAT4, STAT5a, STAT5b, and STAT6), and is the most widely studied. Abnormal expression or functional activity of STAT3 plays a role in mediating I-R injury in various organs such as the heart, brain, and kidneys [1–3,7,8]. The JAK kinase protein family includes JAK1, JAK2, JAK3, and TYK2. JAK2 is a non-receptor tyrosine kinase and is an intensely studied JAK kinase. The JAK2-STAT3 pathway is closely related to the inflammatory and immune response [5,6,11,23], I-R injury [7,8,10,24], oxidative stress [25,26], and other pathophysiological processes. Many studies have shown that changes in the expression or functions of JAK2 are related to I-R injury in various organs such as myocardium [10], brain [11], and kidneys [7]. Many researchers believe that miR-15b plays a role in the I-R injury of cardiomyocytes [15,16], neurons [17], and many other cells. Bioinformatics analysis indicated that there was a complementary binding site between miR-15b and the 3′-UTR of STAT3 mRNA, suggesting the possible miRNA-target regulatory relationship between the two. Api is widely present in celery, apples, oranges, Plantago, rattan, and Agastache rugosus, as a natural flavone with antioxidant and anti-lipid peroxidation activities. Api possesses anti-inflammatory, antiviral, free radical scavenging, and antioxidant functions. The following functions have been reported in Api: dilating blood vessels, regulating blood pressure and blood lipids, improving vasoconstriction, reducing myocardial damage, protecting heart functions, and other cardiovascular pharmacological effects. In I-R injury, Api can reduce the I-R injury of various organs such as myocardium [20], kidneys [27], liver [28], and brain [29], but the specific mechanism for the protective effect against I-R injury is not completely clear. Studies have shown that Api participates in regulation of the JAK2-STAT3 pathway [27], but whether Api regulates I-R injury through miR-15b and JAK2 is unclear.
The results of this study showed that, compared with the sham group, the MDA content, ROS production, and caspase-3 activity in the myocardium of the I-R model rats increased significantly, whereas the activities of SOD and CAT enzymes decreased significantly. The above results indicate that the oxidative stress of rat myocardium, as well as cell apoptosis, was increased significantly in the I-R model. Detections of gene and protein expressions showed that the expression of miR-15b in the myocardium of the I-R model group was significantly higher than that of the sham group; the expression of miR-15b was higher with the prolonged duration of I-R; and the expressions of JAK2 and p-JAK2 proteins were significantly higher at 12 h and 24 h after surgery in model rats than in sham rats. At present, the relationship between JAK2 and I-R injury is not fully understood, and the research findings are inconsistent. Some studies have found that the expression and function of JAK2-STAT3 can be attenuated after I-R injury. For example, Li et al. [10] observed that, compared with the sham group, the functional activity of JAK2-STAT3 pathway in the myocardial tissues of SD rats was significantly lower than that of the sham group. A decrease in JAK2-STAT3 pathway activity is an adverse factor precipitating I-R and is involved in promoting the I-R lesion of the myocardium. To date, most studies have observed that the activity of JAK2-STAT3 pathway is enhanced in I-R injury; however, an increase in JAK2-STAT3 pathway activity is a pathogenic factor in I-R injury, or related to stress protection after I-R. Research results on this topic are conflicting. For example, Hu et al. [30] showed that after I-R injury, enhanced JAK2-STAT3 pathway activity was a promoter of I-R injury. An injection of nicotiflorin to the tail vein reduced apoptosis and I-R injury by inhibiting the JAK2-STAT3 pathway. Lv et al. [31] also observed enhancement of functional activity of the JAK2-STAT3 pathway in I-R injury, and fenofibrate treatment inhibited activity of the JAK2-STAT3 pathway and attenuated renal I-R injury. These finding indicate that enhancement of JAK2-STAT3 pathway activity is related to attenuation of I-R injury.
Zhao et al. [24] showed that preconditioning with berberine (BBR) alleviated oxidative stress and reduced apoptosis rate in the myocardium of I-R model rats. This further attenuated I-R injury and lowered the sensitivity of H9C2 cells to I-R injury in the I-R model. JAK2 inhibitor inhibits JAK2-STAT3, thus attenuating the protective effect of BBR on I-R injury. Wu et al. [32] showed that JAK2-STAT3 pathway activity in rat myocardium was obviously enhanced during I-R injury. Sevoflurane further enhances activity of the JAK2-STAT3 pathway and protects the myocardium against harm, whereas AG490 inhibits JAK2-STAT3 and weakens the protective effect of sevoflurane against myocardial I-R injury. Yang et al. [33] showed that melatonin enhances functional activity of the JAK2-STAT3 pathway in the tissues under I-R condition, which leads to a decrease in oxidative stress, apoptosis, and I-R injury; however, AG490 exerts the opposite effect. Liu et al. [34] showed that, compared with the sham group, the activity of the JAK2-STAT3 pathway in the myocardium of I-R rats was significantly enhanced. The intraperitoneal injection of N (2)-L-alanyl-L-glutamine (NALG) effectively activates the JAK2-STAT3 signal pathway, reduces the apoptosis of myocardial cells, and improves myocardial function, thereby alleviating I-R injury.
The present study showed that the injection of Api to the I-R model in rats significantly increased the JAK2 expression and JAK2-STAT3 pathway activity in the myocardium of rats. Other changes included reducing MDA content, ROS production, and caspase-3 activity, while increasing the activities of CAT and SOD enzymes. This suggests that Api activates JAK2-STAT3 activity by upregulating JAK2 expression, thereby reducing oxidative stress, apoptosis, and I-R injury. Liu et al. [22] showed that Api alleviates I-R injury by activating the JAK2-STAT3 pathway. However, researchers such as Liu did not elucidate the specific mechanism of Api in regulating JAK2-STAT3 activity [22]. Our study found that pretreatment with Api reduced the elevation of miR-15b expression in I-R myocardium and increased the expression of JAK2. The results showed that an increase in miR-15b expression was an adverse factor in I-R injury, while JAK2-STAT3 was a protective factor. The elevated expression of miR-15b inhibits JAK2 expression and effective activation of JAK2-STAT3, while Api preconditioning inhibits miR-15b and indirectly increases JAK2 expression and JAK2-STAT3 activity. That was proposed as the mechanism by which I-R injury of the myocardium was relieved. Furthermore, in vitro studies demonstrated that overexpression of miR-15b attenuates the protective effect of Api against the upregulation of JAK2 and the I-R injury to H9C2 cardiomyocytes. In summary, Api plays a role in reducing I-R injury by inhibiting miR-15b expression.
Many studies have reported that miR-15b is an adverse factor promoting I-R injury. Liu et al. [15] showed that miR-15b expression was increased during I-R injury in rat myocardium; overexpression of miR-15b enhanced the apoptotic induction of myocardial cells by I-R treatment, while the downregulation of miR-15b protected against myocardial I-R injury. Shi et al. [17] showed that the miR-15b expression in brain tissues with I-R was significantly higher after cerebral infarction. Sevoflurane can reduce I-R injury in brain tissues by inhibiting miR-15b expression, which was consistent to the observations of the present study.
Our results demonstrate that an increase in miR-15b expression during I-R injury in rat myocardium was an adverse factor restricting the protective mechanism of JAK2 expression and JAK2-STAT3 activity. In contrast, Api reduced miR-15b expression, while increasing the expression of JAK2 and activating JAK2-STAT3. Api treatment protected against myocardial apoptosis and I-R injury. To the best of our knowledge, this has not been previously reported. miR-15b can downregulate JAK2 expression and reduce JAK2-STAT3 pathway activity, and further studies are required to confirm this.
Conclusions
Api treatment downregulated miR-15b expression, increased JAK2 expression and JAK2-STAT3 pathway activity, and alleviated myocardial I-R injury and cardiomyocyte apoptosis and ROS production in vitro.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Chen S, Hua F, Lu J, et al. Effect of dexmedetomidine on myocardial ischemia-reperfusion injury. Int J Clin Exp Med. 2015;8(11):21166–72. [PMC free article] [PubMed] [Google Scholar]
- 2.Giustino G, Dangas GD. Ischemia-reperfusion injury and ischemic post-conditioning in acute myocardial infarction: Lost in translation. Catheter Cardiovasc Interv. 2017;90(7):1068–69. doi: 10.1002/ccd.27436. [DOI] [PubMed] [Google Scholar]
- 3.Pinelli S, Agrinier N, Bouchahda N, et al. Myocardial reperfusion for acute myocardial infarction under an optimized antithrombotic medication: What can you expect in daily practice? Cardiovasc Revasc Med. 2018;19(5):369–81. doi: 10.1016/j.carrev.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 4.Vogel B, Mehta SR, Mehran R. Reperfusion strategies in acute myocardial infarction and multivessel disease. Nat Rev Cardiol. 2017;14(11):665–78. doi: 10.1038/nrcardio.2017.88. [DOI] [PubMed] [Google Scholar]
- 5.Yang H, Ma P, Cao Y, et al. ECPIRM, a potential therapeutic agent for cutaneous T-cell lymphoma, inhibits cell proliferation and promotes apoptosis via a JAK/STAT pathway the biological effect of ECPIRM in CTCL cell. Anticancer Agents Med Chem. 2017;32(7):1452–63. doi: 10.2174/1871520617666170327115657. [DOI] [PubMed] [Google Scholar]
- 6.Ouyang J, Pan X, Lin H, et al. GKN2 increases apoptosis, reduces the proliferation and invasion ability of gastric cancer cells through down-regulating the JAK/STAT signaling pathway. Am J Transl Res. 2017;9(2):803–11. [PMC free article] [PubMed] [Google Scholar]
- 7.Luo LN, Xie Q, Zhang XG, Jiang R. Osthole decreases renal ischemia-reperfusion injury by suppressing JAK2/STAT3 signaling activation. Exp Ther Med. 2016;12(4):2009–14. doi: 10.3892/etm.2016.3603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Das A, Salloum FN, Durrant D, et al. Rapamycin protects against myocardial ischemia-reperfusion injury through JAK2-STAT3 signaling pathway. J Mol Cell Cardiol. 2012;53(6):858–69. doi: 10.1016/j.yjmcc.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lei C, Deng J, Wang B, et al. Reactive oxygen species scavenger inhibits STAT3 activation after transient focal cerebral ischemia-reperfusion injury in rats. Anesth Analg. 2011;113(1):153–59. doi: 10.1213/ANE.0b013e31821a9fbe. [DOI] [PubMed] [Google Scholar]
- 10.Li D, Lu N, Han J, et al. Eriodictyol attenuates myocardial ischemia-reperfusion injury through the activation of JAK2. Front Pharmacol. 2018;30(9):33. doi: 10.3389/fphar.2018.00033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wu Y, Xu J, Xu J, et al. Study on the mechanism of JAK2/STAT3 signaling pathway-mediated inflammatory reaction after cerebral ischemia. Mol Med Rep. 2018;17(4):5007–12. doi: 10.3892/mmr.2018.8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xie XJ, Fan DM, Xi K, et al. Suppression of microRNA-135b-5p protects against myocardial ischemia/reperfusion injury by activating JAK2/STAT3 signaling pathway in mice during sevoflurane anesthesia. Biosci Rep. 2017;37(3):465–81. doi: 10.1042/BSR20170186. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Liu K, Yan L, Jiang X, et al. Acquired inhibition of microRNA-124 protects against spinal cord ischemia-reperfusion injury partially through a mitophagy-dependent pathway. J Thoracic Cardiovasc Surg. 2017;154(5):1498–508. doi: 10.1016/j.jtcvs.2017.05.046. [DOI] [PubMed] [Google Scholar]
- 14.He Y, Liu JN, Zhang JJ, Fan W. Involvement of microRNA-181a and Bim in a rat model of retinal ischemia-reperfusion injury. Int J Ophthalmol. 2016;9(1):33–40. doi: 10.18240/ijo.2016.01.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu L, Zhang G, Liang Z, et al. MicroRNA-15b enhances hypoxia/reoxygenation-induced apoptosis of cardiomyocytes via a mitochondrial apoptotic pathway. Apoptosis. 2014;19(1):19–29. doi: 10.1007/s10495-013-0899-2. [DOI] [PubMed] [Google Scholar]
- 16.Liu LF, Liang Z, Lv ZR, et al. MicroRNA-15a/b are up-regulated in response to myocardial ischemia/reperfusion injury. J Geriatr Cardiol. 2012;9(1):28–32. doi: 10.3724/SP.J.1263.2012.00028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi H, Sun BL, Zhang J, et al. miR-15b suppression of Bcl-2 contributes to cerebral ischemic injury and is reversed by sevoflurane preconditioning. CNS Neurol Disord Drug Targets. 2013;12(3):381–91. doi: 10.2174/1871527311312030011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Skondras I, Lambropoulou M, Tsaroucha A, et al. The role of Apigenin in testicular damage in experimental ischemia-reperfusion injury in rats. Hippokratia. 2015;19(3):225–30. [PMC free article] [PubMed] [Google Scholar]
- 19.Hu J, Li Z, Xu LT, et al. Protective effect of apigenin on ischemia/reperfusion injury of the isolated rat heart. Cardiovasc Toxicol. 2015;15(3):241–49. doi: 10.1007/s12012-014-9290-y. [DOI] [PubMed] [Google Scholar]
- 20.Chen C, He H, Luo Y, et al. Involvement of Bcl-2 signal pathway in the protective effects of apigenin on anoxia/reoxygenation-induced myocardium injury. J Cardiovasc Pharmacol. 2016;67(2):152–63. doi: 10.1097/FJC.0000000000000331. [DOI] [PubMed] [Google Scholar]
- 21.Du H, Hao J, Liu F, et al. Apigenin attenuates acute myocardial infarction of rats via the inhibitions of matrix metalloprotease-9 and inflammatory reactions. Int J Clin Exp Med. 2015;8(6):8854–59. [PMC free article] [PubMed] [Google Scholar]
- 22.Liu Y, Wang L, Du Y, et al. Effects of apigenin pretreatment against renal ischemia/reperfusion injury via activation of the JAK2/STAT3 pathway. Biomed Pharmacother. 2017;95(11):1799–808. doi: 10.1016/j.biopha.2017.09.091. [DOI] [PubMed] [Google Scholar]
- 23.Abed El-Gaphar OAM, Abo-Youssef AM, Halal GK. Levetiracetam mitigates lipopolysaccharide-induced JAK2/STAT3 and TLR4/MAPK signaling pathways activation in a rat model of adjuvant – induced arthritis. Eur J Pharmacol. 2018;826(5):85–95. doi: 10.1016/j.ejphar.2018.02.041. [DOI] [PubMed] [Google Scholar]
- 24.Zhao GL, Yu LM, Gao WL, et al. Berberine protects rat heart from ischemia/reperfusion injury via activating JAK2/STAT3 signaling and attenuating endoplasmic reticulum stress. Acta Pharmacol Sin. 2016;37(3):354–67. doi: 10.1038/aps.2015.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang G, Huang X, Liu M, et al. Secoisolariciresinol diglucoside prevents the oxidative stress-induced apoptosis of myocardial cells through activation of the JAK2/STAT3 signaling pathway. Int j Mol Med. 2018;41(6):3570–76. doi: 10.3892/ijmm.2018.3560. [DOI] [PubMed] [Google Scholar]
- 26.Duan W, Yang Y, Yi W, et al. New role of JAK2/STAT3 signaling in endothelial cell oxidative stress injury and protective effect of melatonin. PLoS One. 2013;8(3):694–714. doi: 10.1371/journal.pone.0057941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Swierkot J, Nowak B, Czarny A, et al. The activity of JAK/STAT and NF-kappaB in patients with rheumatoid arthritis. Adv Clin Exp Med. 2016;25(4):709–17. doi: 10.17219/acem/61034. [DOI] [PubMed] [Google Scholar]
- 28.Tsaroucha AK, Tsiaousidou A, Ouzounidis N, et al. Intraperitoneal administration of apigenin in liver ischemia/reperfusion injury protective effects. Saudi J Gastroenterol. 2016;22(6):415–22. doi: 10.4103/1319-3767.195556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tu F, Pang Q, Huang T, et al. Apigenin ameliorates post-stroke cognitive deficits in rats through histone acetylation-mediated neurochemical alterations. Med Sci Monit. 2017;23(8):4004–13. doi: 10.12659/MSM.902770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hu GQ, Du X, Li YJ, et al. Inhibition of cerebral ischemia/reperfusion injury-induced apoptosis: Nicotiflorin and JAK2/STAT3 pathway. Neural Regen Res. 2017;12(1):96–102. doi: 10.4103/1673-5374.198992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lv J, Wang X, Liu SY, et al. Protective effect of Fenofibrate in renal ischemia reperfusion injury: Involved in suppressing kinase 2 (JAK2)/transcription 3 (STAT3)/p53 signaling activation. Pathol Biol (Paris) 2015;63(6):236–42. doi: 10.1016/j.patbio.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 32.Wu J, Yu J, Xie P, et al. Sevoflurane postconditioning protects the myocardium against ischemia/reperfusion injury via activation of the JAK2-STAT3 pathway. Peer J. 2017;5(4):e3196. doi: 10.7717/peerj.3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang Y, Duan W, Jin Z, et al. JAK2/STAT3 activation by melatonin attenuates the mitochondrial oxidative damage induced by myocardial ischemia/reperfusion injury. J Pineal Res. 2013;55(3):275–86. doi: 10.1111/jpi.12070. [DOI] [PubMed] [Google Scholar]
- 34.Liu S, Yang Y, Song YQ, et al. Protective effects of N(2)LalanylLglutamine mediated by the JAK2/STAT3 signaling pathway on myocardial ischemia reperfusion. Mol Med Rep. 2018;17(4):5102–8. doi: 10.3892/mmr.2018.8543. [DOI] [PMC free article] [PubMed] [Google Scholar]