Abstract
Inducible bronchus associated lymphoid tissue (iBALT) is a tertiary lymphoid structure (TLOs) that resembles secondary lymphoid organs (SLOs). iBALT is induced in the lung in response to antigen exposure. In some cases, such as infection with Mycobacterium tuberculosis (Mtb), the formation of iBALT structure is indicative of an effective, protective immune response. However, with persistent exposure to antigen during chronic inflammation, allergy, or autoimmune diseases, iBALT may be associated with exacerbation of inflammation. iBALT is characterized by well-organized T and B areas enmeshed with conventional dendritic cells (cDCs), follicular dendritic cells (FDCs) and stromal cells, usually located surrounding airways or blood vessels. Several of the molecular signals and cellular contributors that mediate formation of iBALT structures have been recently identified. This review will outline the recent findings associated with the formation and maintenance of iBALT, and their contributions toward a protective or pathogenic function in pulmonary disease outcome.
iBALT -Structure and Function
SLOs are immune structures distributed throughout the human body that serve as localized reservoirs of immune cells poised to be primed and activated quickly upon infection or inflammatory insult (1). SLOs, such as lymph nodes and Peyer’s patches, are seeded during embryonic development in the absence of infectious or inflammatory stimuli. These structures are formed as part of a highly ordered series of events, guided by lymphoid tissue inducer (LTi) cells that require lymphotoxin alpha (LTα), and occurring early during embryonic development (2–4). On the other hand, iBALT is a TLO specifically induced by antigenic stimuli. These stimuli can be from infectious exposures, environmental insults, self-antigens, or other sources. While several cell types and molecular signals function similarly in SLOs and TLOs, the anatomical feature that makes iBALT unique is it’s localization which is generally near major bronchi, but often during inflammation or infection may also localize within the perivascular or interstitial areas of the lung (5, 6). Additionally, iBALT is also unique in that it is induced after antigenic or inflammatory stimulation, and antigen or pathogen specific factors often skew the characteristics of iBALT. Endothelial, epithelial, and stromal cells are thought to secrete the initial inflammatory signals that trigger the initiation of iBALT and TLO formation (7, 8). DCs and macrophages, which are amongst the first immune cells that seed the nascent iBALT, further propagate the cascade of proinflammatory cytokines and chemokine signals to initiate the recruitment of more DCs, B cells and T cells (9). As iBALT formation progresses, the nascent structure becomes more organized with segregated B and T cell zones forming sustained germinal centers (GC)-like structures (9), a marker of iBALT. LTα and TNF-α are key signals required for the maintenance of SLOs (1, 10), but in the context of iBALT are known to play a role only in some models of infection and chronic inflammation (5, 11, 12). IL-1α is also required for induction of iBALT formation, as IL-1α deficiency is correlated with a decreased frequency of conventional dendritic cells (cDCs) and failure to induce iBALT during viral infection, and iBALT formation could be reversed by exogenous IL-1α administration (11, 13). IL-1α is produced during the early stages of pulmonary influenza infection by stromal cells and is required for control of viral replication and iBALT formation through the induction of CXCL13, which is also driven by LTα (13). Early production of IL-23, IL-17 and IL-22 also constitute critical cytokine signals required for iBALT formation during infections such as Mycobacterium tuberculosis (Mtb) (14–16), by inducing the secretion of chemokines associated with recruitment of T and B cells to the nascent iBALT (17). Of the homeostatic chemokines, CCL19, CCL21 and CXCL12 and CXCL13 and their receptors CCR7, CXCR4, and CXCR5 are associated with the structural organization of iBALT (12, 18, 19). CCL19 and CCL21, constitutively expressed by stromal cells and follicular dendritic cells (FDCs) recruit CCR7-expressing cDCs, as well as naïve, antigen-specific, and memory T cells to the nascent T cell zones of the iBALT structure (5, 18). Accordingly, recruited cDCs and T cells can localize around the tightly packed B cell follicles (5, 20, 21). During infection with bacterial pathogens such as Mtb, absence of CCR7 and CCL19/CCL21 deficiency resulted in delayed antigen presentation and T cell priming, poorly formed B cell follicle formation and increased susceptibility to infection (22, 23), supporting a non-redundant role for the CCR7-CCL19/CCL21 axis in iBALT induction and function. CCL21 along with CXCL13 can maintain iBALT even in absence of LTα (5). CXCL13, produced mainly by FDCs, follicular B cells, and other stromal cells within iBALT (5) can mediate the homing of CXCR5-expressing B and T cells (including a subset of Tfh cells) to the follicular compartment of iBALT (5, 24). Accordingly, CXCL13 or CXCR5 deficiency coincided with defects in T and B cell homing into iBALT structures, thus impacting the control of bacterial infections such as Mtb (24). Migration into nascent iBALT and the specific localization of T and B cells within iBALT driven by the aforementioned chemokines represent the initial steps in the development of bona fide iBALT structures in lung. The unique localization of iBALT near the airways raises the possibility that perhaps iBALT samples antigen effectively and rapidly due to this unique anatomical localization. With time, the developing iBALT becomes more structured with organized, segregated B and T cell zones (9) interspersed with FDC, cDC and stromal cells. FDC and cDC likely provide antigen, survival, and costimulatory signals to activate and sustain iBALT-harbored T and B cells (9, 25). Previously activated T and B cells can infiltrate iBALT, and naïve T and B cells may also be activated by local APC or by direct antigen recognition within iBALT structures (26). Activated T cells, such as Th1, Th2, Th17 or even Tregs, along with DCs within the iBALT can produce effector molecules, including cytokines and chemokines, required to control pathogens and may contribute directly to the outcome of the disease (25, 27). T cells and DCs can also provide help for antibody production by B cells that may act systemically or locally during infections (25, 28). Whether T cells differentiate within iBALT or whether they migrate into iBALT once they are differentiated in SLOs is not fully understood. However, T cells actively participate in the protective immune responses against pathogens in protective iBALT or can drive dysregulated pathological responses associated with persistent inflammation during chronic pulmonary conditions such as chronic obstructive pulmonary disease (COPD), autoimmune diseases or allergy.
The protective and pathogenic roles of iBALT
iBALT structures can maintain a pool of locally-activated, antigen-specific lymphocytes able to induce a rapid and effective immune response (5, 29). As such, the presence of iBALT has important implications in the progression and outcome of pathogen exposures and chronic inflammation. The triggers that induce protective versus pathological outcomes for iBALT are just beginning to be characterized, but likely implicate the type of antigen, the type of T cell response induced, and the chronicity of the stimulation conditions. Understanding the initial seeding events that drive iBALT formation may explain the distinct roles of iBALT during infection and inflammation. Here we explore the key differences between protective and pathogenic iBALT and their effects on health and disease.
Protective iBALT
A proposed function of protective iBALT is to harbor a local supply of B and T cells within the lung resulting in a rapid, localized immune response and sustained and rapid activation of antigen specific lymphocytes in the tissue (29). The initial response to microbial pathogens occurs through the recognition of pathogen associated molecular patterns (PAMPs) by pattern recognition receptors (PRRs) on immune cells. This interaction can lead to the induction of specific, polarizing cytokine and chemokine cascades, thus allowing the immune system to tailor the response to effectively contain and neutralize the pathogen through the recruitment of specialized myeloid populations and T helper subsets. The specific PAMPs and PRRs that are required for the formation of iBALT are not fully defined; though we hypothesize that the type of PAMP and PRR involved will impact subsequent immune response and skew the initial production of chemokines and molecules required for the recruitment of immune cells to allow formation of the iBALT. In line with this hypothesis, Fleige et al. demonstrated that the type of pathogen determines which key factors are required for the formation and maturation of iBALT (19). Specifically, by comparing Modified Vaccinia Virus Ankara (MVA) and Pseudomonas aeruginosa pulmonary infection models, some of the initial interactions with host receptors that can alter the characteristics of iBALT were defined. In the case of P. aeruginosa, TLR signaling through MyD88 was shown to drive the formation of FDC-lacking iBALT and required the production of IL-17. In contrast, MVA-induced iBALT was highly organized with FDCs surrounding densely packed B cell- follicles, and did not require IL-17 signaling (19). Thus, characteristics of iBALT during infection are dictated by early cellular interactions, inflammatory signals, and the type of pathogen. In response to, and in conjunction with cytokines induced by PRRs, early signals from infected myeloid cells, epithelial cells, Th17 helper T cells, type 3 innate lymphoid cells (ILC3s) and γδ T cells likely induce the inflammatory cascade of cytokines including IL-23, IL-22, IL-17, TNF-α and LTα that drive and maintain iBALT formation during pulmonary infections (29, 30). These tissue resident cells likely produce the signals that drive the earliest immune responses, even before the recruitment and activation of monocytes, DCs, B cells, and T helper cells occurs. These early signals, specifically IL-17 and LTα, are known to be upstream drivers of CXCR5 and CXCL13 (15, 17, 31), required for iBALT formation and organization of B cell follicles (5, 10, 14, 22, 24, 32, 33), and play important protective roles in host immune responses to a variety of viral (31, 34), and bacterial infections (35–40) (Table 1). Early IL-17 is required for protective immunity against the highly pathogenic H5N1 influenza virus infection by a mechanism involving induction of CXCL13 and recruitment of CXCR5+ B cells into the lung tissue (31). Accordingly, IL-17−/− mice had increased inflammation and decreased total B cells in the lung (19, 31), and in vitro, IL-17 axis blockade led to defects in chemotaxis of human B cells (41). These findings suggest that localization and organization of B cells in follicles was likely impaired without IL-17 signaling in these models. Beyond their roles in acute infection, IL-17, IL-22 and IL-23 are also required for the long term control of Mtb, and deficiency of these cytokines correlated with reduced B cell follicle formation and a failure to control bacterial replication during later stages of infection (15). These studies are supported by a role for IL-22 in driving the development of TLOs (8, 42) possibly due to their effects on stromal cells which express IL-22R and mediate downstream signaling events for induction of CXCL-13 and CXCL-12 (42). Additionally, LTα−/− mice showed defective B and T cell follicle organization, lower numbers of proliferating B cells, and lower IgG serum titers during Pneumocystis jirovecii infection, although these mice were still able to clear the infection and did have smaller, but persistent iBALT (12). These findings suggest that LTα while not required for iBALT induction, is required for the organization and long term maintenance of iBALT structures. Together, IL-17 and IL-22 modulate the recruitment of B cells, Tfh and other CXCR5+ cells to nascent iBALT, thus providing critical early signals for formation and organization of protective iBALT (Table 1).
Table 1.
Published studies describing the protective and pathogenic roles of iBALT in pulmonary diseases
| Protective function | |||
|---|---|---|---|
| Agent/disease | Cellular drivers | Molecular signature | References |
| Mycobacterium tuberculosis | Tfh, FDCs, GC B-cells, | Th17, CXCL13, CCL19, CCL21, IL-23, IL-17, IL-22 | (14–16) (22, 23) (24) (42) (50) (52) (53) |
| Pneumocystis | Th2, Th17 | CXCL13, IL-13, IL-17, LTα | (12) |
| Viral infections | CD11c+ DCs, lung stromal cells, GC, B-cells | IL-17, CXCL13, CCL19, CCL21, LTα, IL-1α | (5) (13) (25) (26) (27, 31) |
| Pseudomonas aeruginosa | Neutrophils, B and T-cells | CXCL12, CXCR4, IL-17 | (48) (49) |
| Pathological functions | |||
| Rheumatoid Arthritis | Autorreactive T cells, B cells, FDC | CCL19, CCL21, LTβ | (21) |
| Inhalation Allergy/Asthma | Eosinophils, B cells, FDC, Th2, endothelial cells | IL-1α, IgE, CCL19, CCL21 | (11) (58, 69) |
| COPD | DCs, autoimmune B cells | CCL20, CCR7, CXCL13, LTα, IL-1β,IL-18,IFN-γ | (65) (70) (73) (74, 76) (77) |
| Pulmonary Arterial Hypertension | T cells, B cells, DC | CXCL13, CCL20, LTα, | (66) (67) |
Without early signals from IL-17 and IL-22, chemokine signals are dysregulated or abrogated, leading to defective or inappropriate recruitment and organization of myeloid and lymphoid cells required for protection against pathogen exposure. Proper migration and specific localization of B and T cells into iBALT occurs through CXCL13/CXCR5 and CCL19/CCL21/CCR7 signaling (14, 18, 22, 24, 43). Particularly, CCL19 and CCL21 are important for not only pulmonary recruitment of naive CD4+ T cells, but also CD11c+ cDCs, antigen specific IFN-γ producing T cells and development of iBALT within granulomas during Mtb infection (22). Beyond inducing migration of naïve cells or already differentiated T and B cells, early secreted chemokines also induce the differentiation of naïve T cells toward specific T helper subsets. CCL19 by itself can enhance T cell proliferation by inducing maturation of DCs and specifically program DCs to induce Th1 responses (44).
T cells are crucial for iBALT formation as treatment with anti-CD4 antibody resulted in the reduction of lymphoid follicles within iBALT in several infection models (12, 45). Recruited or locally differentiated T helper subsets in iBALT may include Th1, Th2, Th17, Tregs, and Tfh cells, which are poised to exert their effector functions to provide a rapid local recall response (5, 26, 29) or control exacerbated inflammation (46, 47). Induction of particular T helper subsets determines the type of immune response occurring in iBALT and impacts the outcome of the disease. For instance, Th1, Th17, and Tfh-like cells expressing CXCR5 in the lung were associated with iBALT formation, control of Mtb infection, and host survival (17, 24, 39). Similarly, the Th1 immune response induced in iBALT during influenza infection correlated with improved viral clearance, decreased lung pathology, increased survival rates and sustained memory responses, even in absence of SLOs (5, 26, 27). Furthermore, Th2 and Th17 cells were required to control Pneumocystis infection and induction of protective iBALT in a CXCL13-dependent manner (12). Early induction of iBALT correlated with increased recruitment of T and B cells during chronic infection with P. aeruginosa. Interestingly, as infection progressed, cellular infiltration and associated inflammation decreased, suggesting that iBALT may also serve to regulate or dampen the local immune response during chronic pulmonary infections (48, 49), most likely through the recruitment of IL-10 producing Tregs. iBALT may contribute to the induction of long term memory B cell responses by maintaining plasmablasts and long lived plasma cells, which migrate to the bone marrow, suggesting that the presence of long term memory and recall responses during influenza virus infection partially depends on sustained input of B cells migrating from iBALT in the lung (25). As such, the presence of iBALT correlates with an increased recall response after secondary antigen exposure, thus improving the outcome of repeated exposure to the same infection. The presence of pre-existing iBALT can also improve outcome following challenge with other pulmonary infections, regardless of the initial antigen that seeded it (28). This is done by sustaining the activation of a local pool of CD4+ T cells with different antigen specificities, and can be maintained even in the absence of the triggering antigen (9). Furthermore, it was shown that secondary immune responses initiated in iBALT induced less inflammation and were potentially less damaging to the host than the immune response induced systemically (5). Taken together, in the context of secondary infection, the presence of iBALT is generally considered to be protective by inducing an improved recall response. In the non-human primate model of TB, a model that closely mimics human tuberculosis the presence of B cell follicles correlates with protection during tuberculosis (50, 51), as iBALT structures are prominent in lungs of latently infected primates, and negligible in cavitary lung lesions of Mtb-infected primates, and patients with acute pulmonary tuberculosis (52, 53). These findings highlight the importance of iBALT in protective immune responses against Mtb, influenza, and other infections, suggesting iBALT as an important local source of cellular and humoral effectors to mediate local immunity in the lung. It is also possible that iBALT functions as a protective feature by limiting infected cells within the lymphoid follicle and preventing dissemination to other organs (54).
Delineating the protective role of iBALT and identifying the mechanisms of protection will be a major step forward in developing therapies or vaccines for pulmonary infections. The initial seeding, and the early host and pathogen specific factors that induce iBALT are not well understood. Broader knowledge of the PAMPs and damage associated molecular signals (DAMPs) that can trigger iBALT formation will provide new insights into the development of molecule-based therapies or vaccines to induce iBALT. In a recent study performed by Griffiths et al., pulmonary delivery of Mtb antigen primed DCs led to increased and rapid iBALT formation, near-sterilizing immunity and improved disease outcome, suggesting that early antigen acquisition, delivery, and rapid antigen presentation may be key to protection against tuberculosis (45). In the context of vaccination, one could potentially administer an attenuated version of a pathogen to induce protective, long-lasting iBALT as was done by Kaushal et al (50). Pulmonary delivery of an attenuated strain of Mtb lacking functional Sigma H factor or sigH (MtbΔsigH), a master regulator of Mtb oxidative stress responses, induced effective and long-lasting iBALT structures (50). Upon subsequent lethal challenge with aerosolized Mtb, MtbΔsigH vaccinated macaques demonstrated increased iBALT formation, decreased inflammation, improved recruitment of T cells and superior Mtb control when compared to the macaques vaccinated with the currently used vaccine, M. bovis BCG (50). Similar approaches with other attenuated strains or different pathogens could induce the local proliferation of iBALT in the lung, providing long-lasting immunity to specific pathogens. Furthermore, the induction of iBALT using intranasally delivered nanoparticles was shown to accelerate the immune response to influenza and other infections, increasing IgG and IgA production, reducing lung pathology and protecting mice against a spectrum of respiratory viral pathogens (28). Delivery of mucosally administered nano-emulsion based vaccines for TB induced IL-17 responses and iBALT containing lymphoid follicles in the lung and protected mice upon Mtb challenge (55). These findings suggest that targeting specific cytokines such as IL-17 could induce iBALT structures as potential therapeutic strategy to include in combination with current or new vaccines to improve vaccine-mediated protection to a broader variety of pathogens.
Pathogenic iBALT
Although it is believed that iBALT in the context of long term inflammatory responses is detrimental to the host, the mechanisms associated with iBALT induction and maintenance are less understood for pulmonary chronic inflammatory conditions. During pulmonary chronic inflammatory conditions, it is thought that different antigen classes can trigger iBALT, including inhaled particulates, allergens, self-antigens, and DAMPs (11, 56, 57). Whether iBALT contributes to exacerbate inflammation during chronic conditions or is induced as a consequence of an already established inflammatory condition remains unclear. However, the presence of iBALT correlates with worsened pathology rather than resolution of inflammation in chronic inflammatory diseases.
In most cases, regardless of the antigen or triggering signals, a sustained inflammatory response with influx of granulocytes such as neutrophils, eosinophils, and basophils, as well as inappropriately activated T and B cells, yields detrimental consequences for the host (6, 57–59). During chronic inflammatory conditions, IL-17 and LTα likely play a similar early role in driving iBALT induction as during protective iBALT formation. However, prolonged production of these drivers can lead to sustained inflammatory cytokine and chemokine production, thus rendering inflammatory iBALT over time. These events favor dysregulated inflammatory responses and result in maintenance of activated lymphocytic and myeloid cell accumulation, thus leading to tissue damage and the development of autoimmune diseases. For instance, contrasting the protective role of IL-17 in early induction of iBALT during acute influenza, Mtb and Pneumocystis infection (12, 15, 23, 32), heightened Th17 responses can cause inflammation and pathology during respiratory syncytial virus infection and influenza (60, 61) (Table 1). These findings highlight a dichotomous role for this cytokine axis that may be dependent on time and pathogen persistence. The increased pathology over time correlates with an enhanced influx of neutrophils and IL-13 production that promotes the activation of Th2 lymphocytes and excessive mucus production (62). In alum or silica exposure models of airway inflammation, alveolar macrophages underwent cell death and produced IL-1α, which acts as a DAMP that can trigger iBALT, thus inducing further allergic responses associated with IgE antibody production (11). High levels of IL-6 and TNF-α in bronchoalveolar lavage (BAL) fluid were also found following environmental exposure to crystalline silica (cSiO2) in mice. Respiratory exposure to cSiO2 induced a robust inflammatory response associated with eosinophilia, increased production of immunoglobulins, and proinflammatory cytokines and chemokines in BAL fluid corresponding with systemic lupus erythematous (SLE)-type autoimmune response (63). Since SLE is a systemic disease, the persistence of iBALT structures resulted in dysregulated inflammatory responses suggesting that iBALT structure may serve as a platform for triggering systemic autoimmune responses (63).
Due to chronic stimuli, several chronic inflammatory conditions containing iBALT eventually lead to exacerbation and tissue damage by driving the production of autoreactive antibodies by activated B cells present in iBALT. Increased production of molecules associated with B cell follicles such as B cell activating factor of the TNF family (BAFF), inducible costimulator (ICOS)-Ligand, and LTα correlated with increased numbers of iBALT structures in patients with pulmonary complications associated with RA (21), COPD (64), and Sjögren syndrome (21). In patients with COPD, LTα was associated with pathology by driving enhanced expression of CXCL13 and B cell recruitment within iBALT, yielding hallmarks of the disease such as production of autoantibodies and exacerbated inflammation (65). Furthermore, in RA patients, clinical parameters of disease such as the presence of anti-cyclic citrullinated peptide (anti-CCP) antibodies correlated with the presence of iBALT and increased lung tissue damage, supporting a pathogenic role for B cells and iBALT during chronic inflammation (21). In situ production of autoantibody (66) against fibroblasts or epithelial cells in iBALT was reported in patients with pulmonary arterial hypertension (67). Induction of pulmonary hypertension also correlated with increased number and size of iBALT (67) resulting in increased local inflammation and autoantibody production. Therefore, strategies targeting reduction in iBALT formation during these chronic conditions may be effective approaches to prevent or reverse pulmonary hypertension.
As previously mentioned, signals and antigens driving the initial seeding of iBALT may also skew the T helper responses and the mechanisms underlying chronic disease. CCL19 and CCL21 produced by lymphatic endothelial cells (LEC) in the lung may mediate iBALT formation during chronic allergic inflammation (58), possibly due to the recruitment of CCR7+ cDCs and T cells (56). Furthermore IL-5 and IL-7 produced by LECs within iBALT is required for the maintenance of pathogenic Th2 memory T cells, which are present in many chronic inflammatory diseases such as allergy and asthma (58, 68). In chronic allergic inflammation, iBALT sustains enhanced memory Th2 responses with local production of environmental-reactive IgG1, IgA and IgE in the lungs, which further worsens inflammation after antigen exposure (11, 58, 69). Overall, the production of antibodies in this model is thought to be MyD88 and IL-18 independent, although IgE was specifically driven by IL-4 secreted by Tfh cells (11). The pathogenic consequences of enhanced local production of IgE involve induction of eosinophilia, mononuclear cell infiltration and airway hyper-reactivity to antigen exposure, suggesting a detrimental role for IgE producing B cells in iBALT during the allergic response (69) (Table 1).
COPD is associated with a mixture of Th1, Th2, and Th17 responses, which contribute to worsened disease condition and are exacerbated in the presence of iBALT in both humans and mice model (57, 59, 70–72). Particularly, IL-18, a proinflammatory cytokine derived mainly from epithelial cells and myeloid cells, was shown to be associated with worsened lung pathology during COPD by a mechanism involving induction of high levels of IFN-γ producing Th1 cells, which contributed to exacerbated inflammation and disease severity (73). IL-17 producing Th17 cells, along with IL-1β, are thought to be the main drivers of neutrophilic inflammation in COPD (59, 72, 74) by a mechanism involving upregulation of B and T cell attracting chemokine CXCL12. Furthermore, high serum levels of IL-17 correlated with exacerbated disease and increased numbers of circulating neutrophils in COPD patients (74). Although the identification of the triggering antigen in COPD is not clear, lung infections, which are common in these patients (75) may contribute to disease severity (76). More recent evidence suggests that genetic and metabolic factors can also impact iBALT formation and progression. Mutations in serpin family E member 2 (SERPINE2), an extracellular matrix–associated glycoprotein is associated with COPD development (77). SERPINE2 deficiency was associated with enhanced production of molecules associated with iBALT induction and inflammation including IL-17, TNF-α, LTα, CXCL13, IFN-γ, IL-2 and spontaneous mononuclear cell infiltration, thus boosting formation of iBALT in the lungs (77). Furthermore, using a mouse model of COPD, Jia et al. reported that oxysterol metabolism of cholesterol drove iBALT formation and the induction of COPD; similar to what occurs in SLOs. Increased production of a cholesterol-derived metabolite, 7a,25-hydroxy cholesterol, enhanced B cell recruitment into iBALT by interacting with Epstein-Barr virus induced G protein coupled receptor 2 (EBI2), a G protein coupled receptor also expressed in lymphocytes and DCs (78). Pharmacological inhibition of the involved oxysterol pathway was shown to resolve this observed B cell-driven iBALT and abrogated associated emphysema in mice (78), presenting a new possible therapeutic target to treat patients with this clinical condition. Thus, different signals have been described to induce or maintain iBALT structures in the context of chronic inflammatory diseases. The ability to block signals or DAMPs associated with the formation of pathogenic iBALT could lead to less autoreactivity associated with autoimmune diseases, and the ability to resolve pathogenic iBALT and unwanted inflammation which would be a potential step forward in treatment.
Conclusions
Although the signals and cellular components present in protective and pathological iBALT may be somewhat similar, the impact on pathogen exposure, infection, or inflammation can be distinct. For example, some of the requirements for B and T cell associated chemokines CXCL13, CCL19 and CCL21, Tfh and FDC signals, and the signals driving germinal center formation are ubiquitous across both protective and pathological iBALT structures. However, as discussed above, distinct differences in antigen exposure, duration of antigen exposure, early cytokine signals and downstream soluble and cellular inflammatory mediators may drive distinct cellular differences in structure and function of protective versus pathological iBALT structures. For example, while IL-17 is critical for initiation of early chemokine signals to seed iBALT formation, sustained and prolonged IL-17 production might lead to detrimental accumulation of macrophages and neutrophils resulting in chronic inflammation. Thus, it is likely that iBALT formation is a short-term protective solution for pathogen control, but if left unresolved, it may result in long term localized pathological iBALT. Another possible difference in protective versus pathological iBALT is likely the type of Th response induced by the antigen. While early Th1 and Th17 responses appear to be involved in protective iBALT formation and function, prolonged Th17 and Th2 responses are associated with environmental or self-reactive IgE and IgA. As a downstream event, the effect of chronic Th17 and Th2 responses on lung epithelia and sustained presence of granulocytes are also likely major determinants of the function of protective versus pathological iBALT structures. Finding immunological targets that can limit pathological iBALT while promoting protective iBALT structure may provide novel therapeutic strategies to drive immunity during pulmonary infections while alleviating chronic pulmonary symptoms in allergy, asthma, COPD and even autoimmune diseases. Potential targets would likely be cytokines involved in iBALT initiation and formation such as IL-17, IL-22, LTα, and the associated chemokines CXCL13 and CXCL12. However, since the molecules and pathways associated with pathogenic and pathological iBALT formation largely overlap targeting specific molecules to abrogate formation or maintenance needs to be carefully interrogated to avoid potential undesired effects or compromised immunity. Additional studies addressing the mechanisms of persistence of iBALT, antigen presentation and Th differentiation within iBALT and local immunity within iBALT could open up a new field of host directed immuno-therapeutics targeting iBALT formation.
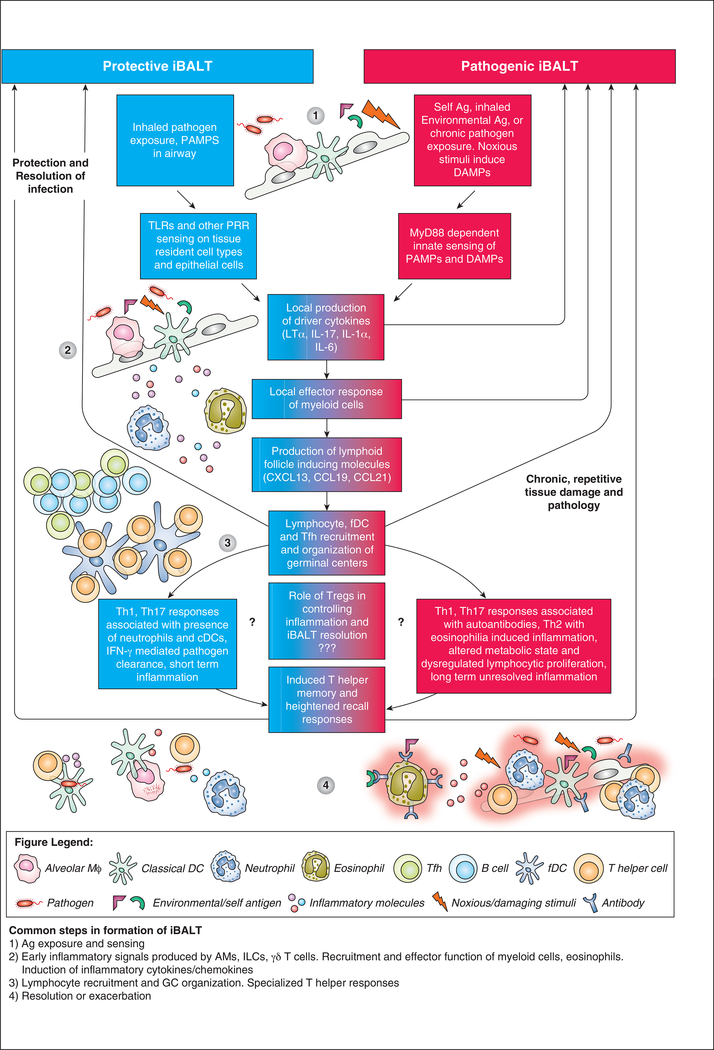
Figure 1: The common and distinct mechanisms of protective and pathogenic iBALT formation.
(1) Protective iBALT is induced in response to PAMPs recognized by PRR expressed on innate immune cells or epithelial cells. Pathogenic iBALT is induced in response to chronic pathogen exposure, self-antigens or environmental antigens which induce DAMPs and inflammation. (2) The sensing of PAMPs and DAMPs results in the production of iBALT-driving cytokines that induce the local response of myeloid cells, production of inflammatory molecules, and recruitment of more myeloid cells, lymphocytes, FDC and Tfh. (3)These signals and associated cellular recruitment drive the formation and organization of germinal centers. (4) In protective iBALT, responses are associated with early recruitment of neutrophils and cDCs, IFN-γ mediated pathogen clearance and short-term inflammation. In pathogenic iBALT, responses are associated with autoantibodies and uncontrolled inflammation, with some diseases specifically highlighted by eosinophilia induced inflammation. Other changes include altered metabolic state and dysregulated lymphocyte proliferation with subsequent long-term unresolved inflammation.
Acknowledgements
We thank Dr. Shyamala Thirunavukkarasu and Ms. Nicole Howard for scientific editing of the manuscript.
This work was supported by Washington University in St Louis, NIH grants R01 AI134236, R01 AI111914, R01 HL105427, R01 AI123780 and NIH/NHLBI T32 AI007172 to MDD.
Footnotes
anti-cyclic citrullinated peptide (anti-CCP), bronchoalveolar lavage (BAL), chronic obstructive pulmonary disease (COPD), conventional dendritic cells (cDCs), damage associated molecular signals (DAMPs), Epstein-Barr virus induced G protein coupled receptor 2 (EBI2), follicular dendritic cells (FDCs), germinal centers (GC), Inducible bronchus associated lymphoid tissue (iBALT), lymphotoxin alpha (LTα), lymphatic endothelial cells (LEC), Modified Vaccinia Virus Ankara (MVA), Mycobacterium tuberculosis (Mtb), pattern recognition receptors (PRRs), pathogen associated molecular patterns (PAMPs), secondary lymphoid organs (SLOs), rheumatoid arthritis (RA), serpin family E member 2 (SERPINE2), systemic lupus erythematous (SLE), tertiary lymphoid organs (TLOs), type 3 innate lymphoid cells (ILC3s).
References
- 1.Ruddle NH, and Akirav EM 2009. Secondary lymphoid organs: responding to genetic and environmental cues in ontogeny and the immune response. J Immunol 183: 2205–2212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Randall TD, Carragher DM, and Rangel-Moreno J 2008. Development of secondary lymphoid organs. Annu Rev Immunol 26: 627–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yadava K, and Marsland BJ 2013. Lymphoid follicles in chronic lung diseases. Thorax 68: 597–598. [DOI] [PubMed] [Google Scholar]
- 4.Drayton DL, Liao S, Mounzer RH, and Ruddle NH 2006. Lymphoid organ development: from ontogeny to neogenesis. Nat Immunol 7: 344–353. [DOI] [PubMed] [Google Scholar]
- 5.Moyron-Quiroz JE, Rangel-Moreno J, Kusser K, Hartson L, Sprague F, Goodrich S, Woodland DL, Lund FE, and Randall TD 2004. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat Med 10: 927–934. [DOI] [PubMed] [Google Scholar]
- 6.Hwang JY, Randall TD, and Silva-Sanchez A 2016. Inducible Bronchus-Associated Lymphoid Tissue: Taming Inflammation in the Lung. Front Immunol 7: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chiavolini D, Rangel-Moreno J, Berg G, Christian K, Oliveira-Nascimento L, Weir S, Alroy J, Randall TD, and Wetzler LM 2010. Bronchus-associated lymphoid tissue (BALT) and survival in a vaccine mouse model of tularemia. PloS one 5: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barone F, Nayar S, Campos J, Cloake T, Withers DR, Toellner KM, Zhang Y, Fouser L, Fisher B, Bowman S, Rangel-Moreno J, Garcia-Hernandez Mde L, Randall TD, Lucchesi D, Bombardieri M, Pitzalis C, Luther SA, and Buckley CD 2015. IL-22 regulates lymphoid chemokine production and assembly of tertiary lymphoid organs. Proceedings of the National Academy of Sciences of the United States of America 112: 11024–11029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Halle S, Dujardin HC, Bakocevic N, Fleige H, Danzer H, Willenzon S, Suezer Y, Hammerling G, Garbi N, Sutter G, Worbs T, and Forster R 2009. Induced bronchus-associated lymphoid tissue serves as a general priming site for T cells and is maintained by dendritic cells. J Exp Med 206: 2593–2601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.De Togni P, Goellner J, Ruddle NH, Streeter PR, Fick A, Mariathasan S, Smith SC, Carlson R, Shornick LP, Strauss-Schoenberger J, and et al. 1994. Abnormal development of peripheral lymphoid organs in mice deficient in lymphotoxin. Science (New York, N.Y.) 264: 703–707. [DOI] [PubMed] [Google Scholar]
- 11.Kuroda E, Ozasa K, Temizoz B, Ohata K, Koo CX, Kanuma T, Kusakabe T, Kobari S, Horie M, Morimoto Y, Nakajima S, Kabashima K, Ziegler SF, Iwakura Y, Ise W, Kurosaki T, Nagatake T, Kunisawa J, Takemura N, Uematsu S, Hayashi M, Aoshi T, Kobiyama K, Coban C, and Ishii KJ 2016. Inhaled Fine Particles Induce Alveolar Macrophage Death and Interleukin-1alpha Release to Promote Inducible Bronchus-Associated Lymphoid Tissue Formation. Immunity 45: 1299–1310. [DOI] [PubMed] [Google Scholar]
- 12.Eddens T, Elsegeiny W, Garcia-Hernadez ML, Castillo P, Trevejo-Nunez G, Serody K, Campfield BT, Khader SA, Chen K, Rangel-Moreno J, and Kolls JK 2017. Pneumocystis-Driven Inducible Bronchus-Associated Lymphoid Tissue Formation Requires Th2 and Th17 Immunity. Cell Rep 18: 3078–3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Neyt K, GeurtsvanKessel CH, Deswarte K, Hammad H, and Lambrecht BN 2016. Early IL-1 Signaling Promotes iBALT Induction after Influenza Virus Infection. Front Immunol 7: 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gopal R, Rangel-Moreno J, Slight S, Lin Y, Nawar HF, Fallert Junecko BA, Reinhart TA, Kolls J, Randall TD, Connell TD, and Khader SA 2013. Interleukin-17-dependent CXCL13 mediates mucosal vaccine-induced immunity against tuberculosis. Mucosal Immunol 6: 972–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khader SA, Guglani L, Rangel-Moreno J, Gopal R, Junecko BA, Fountain JJ, Martino C, Pearl JE, Tighe M, Lin YY, Slight S, Kolls JK, Reinhart TA, Randall TD, and Cooper AM 2011. IL-23 is required for long-term control of Mycobacterium tuberculosis and B cell follicle formation in the infected lung. J Immunol 187: 5402–5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Treerat P, Prince O, Cruz-Lagunas A, Munoz-Torrico M, Salazar-Lezama MA, Selman M, Fallert-Junecko B, Reinhardt TA, Alcorn JF, Kaushal D, Zuniga J, Rangel-Moreno J, Kolls JK, and Khader SA 2017. Novel role for IL-22 in protection during chronic Mycobacterium tuberculosis HN878 infection. Mucosal Immunol: 1069–1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, Locksley RM, Haynes L, Randall TD, and Cooper AM 2007. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat Immunol 8: 369–377. [DOI] [PubMed] [Google Scholar]
- 18.Fleige H, Bosnjak B, Permanyer M, Ristenpart J, Bubke A, Willenzon S, Sutter G, Luther SA, and Forster R 2018. Manifold Roles of CCR7 and Its Ligands in the Induction and Maintenance of Bronchus-Associated Lymphoid Tissue. Cell Rep 23: 783–795. [DOI] [PubMed] [Google Scholar]
- 19.Fleige H, Ravens S, Moschovakis GL, Bolter J, Willenzon S, Sutter G, Haussler S, Kalinke U, Prinz I, and Forster R 2014. IL-17-induced CXCL12 recruits B cells and induces follicle formation in BALT in the absence of differentiated FDCs. J Exp Med 211: 643–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woodland DL, and Randall TD 2004. Anatomical features of anti-viral immunity in the respiratory tract. Semin Immunol 16: 163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rangel-Moreno J, Hartson L, Navarro C, Gaxiola M, Selman M, and Randall TD 2006. Inducible bronchus-associated lymphoid tissue (iBALT) in patients with pulmonary complications of rheumatoid arthritis. J Clin Invest 116: 3183–3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Khader SA, Rangel-Moreno J, Fountain JJ, Martino CA, Reiley WW, Pearl JE, Winslow GM, Woodland DL, Randall TD, and Cooper AM 2009. In a murine tuberculosis model, the absence of homeostatic chemokines delays granuloma formation and protective immunity. J Immunol 183: 8004–8014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kahnert A, Hopken UE, Stein M, Bandermann S, Lipp M, and Kaufmann SH 2007. Mycobacterium tuberculosis triggers formation of lymphoid structure in murine lungs. The Journal of infectious diseases 195: 46–54. [DOI] [PubMed] [Google Scholar]
- 24.Slight SR, Rangel-Moreno J, Gopal R, Lin Y, Fallert Junecko BA, Mehra S, Selman M, Becerril-Villanueva E, Baquera-Heredia J, Pavon L, Kaushal D, Reinhart TA, Randall TD, and Khader SA 2013. CXCR5+ T helper cells mediate protective immunity against tuberculosis. J Clin Invest 123: 712–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.GeurtsvanKessel CH, Willart MA, Bergen IM, van Rijt LS, Muskens F, Elewaut D, Osterhaus AD, Hendriks R, Rimmelzwaan GF, and Lambrecht BN 2009. Dendritic cells are crucial for maintenance of tertiary lymphoid structures in the lung of influenza virus-infected mice. J Exp Med 206: 2339–2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moyron-Quiroz JE, Rangel-Moreno J, Hartson L, Kusser K, Tighe MP, Klonowski KD, Lefrancois L, Cauley LS, Harmsen AG, Lund FE, and Randall TD 2006. Persistence and responsiveness of immunologic memory in the absence of secondary lymphoid organs. Immunity 25: 643–654. [DOI] [PubMed] [Google Scholar]
- 27.Richert LE, Harmsen AL, Rynda-Apple A, Wiley JA, Servid AE, Douglas T, and Harmsen AG 2013. Inducible bronchus-associated lymphoid tissue (iBALT) synergizes with local lymph nodes during antiviral CD4+ T cell responses. Lymphat Res Biol 11: 196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wiley JA, Richert LE, Swain SD, Harmsen A, Barnard DL, Randall TD, Jutila M, Douglas T, Broomell C, and Young M 2009. Inducible Bronchus-associated lymphoid tissue elicited by a protein cage nanoparticle enhances protection in mice against diverse respiratory viruses. PloS one 4: 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones GW, and Jones SA 2016. Ectopic lymphoid follicles: inducible centres for generating antigen-specific immune responses within tissues. Immunology 147: 141–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lockhart E, Green AM, and Flynn JL 2006. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J Immunol 177: 4662–4669. [DOI] [PubMed] [Google Scholar]
- 31.Wang X, Chan CC, Yang M, Deng J, Poon VK, Leung VH, Ko KH, Zhou J, Yuen KY, Zheng BJ, and Lu L 2011. A critical role of IL-17 in modulating the B-cell response during H5N1 influenza virus infection. Cell Mol Immunol 8: 462–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rangel-Moreno J, Carragher DM, de la Luz Garcia-Hernandez M, Hwang JY, Kusser K, Hartson L, Kolls JK, Khader SA, and Randall TD 2011. The development of inducible bronchus-associated lymphoid tissue depends on IL-17. Nat Immunol 12: 639–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grogan JL, and Ouyang W 2012. A role for Th17 cells in the regulation of tertiary lymphoid follicles. Eur J Immunol 42: 2255–2262. [DOI] [PubMed] [Google Scholar]
- 34.Hamada H, Garcia-Hernandez Mde L, Reome JB, Misra SK, Strutt TM, McKinstry KK, Cooper AM, Swain SL, and Dutton RW 2009. Tc17, a unique subset of CD8 T cells that can protect against lethal influenza challenge. J Immunol 182: 3469–3481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsuzaki G, and Umemura M 2007. Interleukin-17 as an Effector Molecule of Innate and Acquired Immunity against Infections. Microbiol Immunol 51: 1139–1147. [DOI] [PubMed] [Google Scholar]
- 36.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shellito JE, Bagby GJ, Nelson S, Charrier K, Peschon JJ, and Kolls JK 2001. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J Exp Med 194: 519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Warfel JM, and Merkel TJ 2013. Bordetella pertussis infection induces a mucosal IL-17 response and long-lived Th17 and Th1 immune memory cells in nonhuman primates. Mucosal Immunol 6: 787–796. [DOI] [PubMed] [Google Scholar]
- 38.Kimizuka Y, Kimura S, Saga T, Ishii M, Hasegawa N, Betsuyaku T, Iwakura Y, Tateda K, and Yamaguchi K 2012. Roles of interleukin-17 in an experimental Legionella pneumophila pneumonia model. Infection and immunity 80: 1121–1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khader SA, and Gopal R 2010. IL-17 in protective immunity to intracellular pathogens. Virulence 1: 423–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rudner XL, Happel KI, Young EA, and Shellito JE 2007. Interleukin-23 (IL-23)-IL-17 cytokine axis in murine Pneumocystis carinii infection. Infection and immunity 75: 3055–3061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Halwani R, Al-Kufaidy R, Vazquez-Tello A, Pureza MA, BaHammam AS, Al-Jahdali H, Alnassar SA, Hamid Q, and Al-Muhsen S 2014. IL-17 Enhances Chemotaxis of Primary Human B Cells during Asthma. PloS one 9: e114604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khader SA, Guglani L, Rangel-Moreno J, Gopal R, Fallert Junecko BA, Fountain JJ, Martino C, Pearl JE, Tighe M, Lin YY, Slight S, Kolls JK, Reinhart TA, Randall TD, and Cooper AM 2011. IL-23 Is Required for Long-Term Control of Mycobacterium tuberculosis and B Cell Follicle Formation in the Infected Lung. J Immunol 187: 5402–5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lo JC, Chin RK, Lee Y, Kang HS, Wang Y, Weinstock JV, Banks T, Ware CF, Franzoso G, and Fu YX 2003. Differential regulation of CCL21 in lymphoid/nonlymphoid tissues for effectively attracting T cells to peripheral tissues. J Clin Invest 112: 1495–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marsland BJ, Battig P, Bauer M, Ruedl C, Lassing U, Beerli RR, Dietmeier K, Ivanova L, Pfister T, Vogt L, Nakano H, Nembrini C, Saudan P, Kopf M, and Bachmann MF 2005. CCL19 and CCL21 induce a potent proinflammatory differentiation program in licensed dendritic cells. Immunity 22: 493–505. [DOI] [PubMed] [Google Scholar]
- 45.Griffiths KL, Ahmed M, Das S, Gopal R, Horne W, Connell TD, Moynihan KD, Kolls JK, Irvine DJ, Artyomov MN, Rangel-Moreno J, and Khader SA 2016. Targeting dendritic cells to accelerate T-cell activation overcomes a bottleneck in tuberculosis vaccine efficacy. Nature communications 7: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Foo SY, and Phipps S 2010. Regulation of inducible BALT formation and contribution to immunity and pathology. Mucosal Immunol 3: 537–544. [DOI] [PubMed] [Google Scholar]
- 47.Foo SY, Zhang V, Lalwani A, Lynch JP, Zhuang A, Lam CE, Foster PS, King C, Steptoe RJ, Mazzone SB, Sly PD, and Phipps S 2015. Regulatory T cells prevent inducible BALT formation by dampening neutrophilic inflammation. J Immunol 194: 4567–4576. [DOI] [PubMed] [Google Scholar]
- 48.Iwata M, and Sato A 1991. Morphological and immunohistochemical studies of the lungs and bronchus-associated lymphoid tissue in a rat model of chronic pulmonary infection with Pseudomonas aeruginosa. Infection and immunity 59: 1514–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kitazawa H, Sato A, and Iwata M 1997. A study of bronchus-associated lymphoid tissue in a rat model of chronic pulmonary infection with Pseudomonas aeruginosa. Kansenshogaku Zasshi 71: 214–221. [DOI] [PubMed] [Google Scholar]
- 50.Kaushal D, Foreman TW, Gautam US, Alvarez X, Adekambi T, Rangel-Moreno J, Golden NA, Johnson AM, Phillips BL, Ahsan MH, Russell-Lodrigue KE, Doyle LA, Roy CJ, Didier PJ, Blanchard JL, Rengarajan J, Lackner AA, Khader SA, and Mehra S 2015. Mucosal vaccination with attenuated Mycobacterium tuberculosis induces strong central memory responses and protects against tuberculosis. Nature communications 6: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaushal D, Mehra S, Didier PJ, and Lackner AA 2012. The non-human primate model of tuberculosis. J Med Primatol 41: 191–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ulrichs T, Kosmiadi GA, Trusov V, Jorg S, Pradl L, Titukhina M, Mishenko V, Gushina N, and Kaufmann SH 2004. Human tuberculous granulomas induce peripheral lymphoid follicle-like structures to orchestrate local host defence in the lung. J Pathol 204: 217–228. [DOI] [PubMed] [Google Scholar]
- 53.Ulrichs T, Kosmiadi GA, Jorg S, Pradl L, Titukhina M, Mishenko V, Gushina N, and Kaufmann SH 2005. Differential organization of the local immune response in patients with active cavitary tuberculosis or with nonprogressive tuberculoma. The Journal of infectious diseases 192: 89–97. [DOI] [PubMed] [Google Scholar]
- 54.Khader SA, Gaffen SL, and Kolls JK 2009. Th17 cells at the crossroads of innate and adaptive immunity against infectious diseases at the mucosa. Mucosal Immunol 2: 403–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ahmed M, Smith DM, Hamouda T, Rangel-Moreno J, Fattom A, and Khader SA 2017. A novel nanoemulsion vaccine induces mucosal Interleukin-17 responses and confers protection upon Mycobacterium tuberculosis challenge in mice. Vaccine 35: 4983–4989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kawakami M, Narumoto O, Matsuo Y, Horiguchi K, Horiguchi S, Yamashita N, Sakaguchi M, Lipp M, Nagase T, and Yamashita N 2012. The role of CCR7 in allergic airway inflammation induced by house dust mite exposure. Cellular immunology 275: 24–32. [DOI] [PubMed] [Google Scholar]
- 57.Roos AB, Sanden C, Mori M, Bjermer L, Stampfli MR, and Erjefalt JS 2015. IL-17A Is Elevated in End-Stage Chronic Obstructive Pulmonary Disease and Contributes to Cigarette Smoke-induced Lymphoid Neogenesis. Am J Respir Crit Care Med 191: 1232–1241. [DOI] [PubMed] [Google Scholar]
- 58.Shinoda K, Hirahara K, Iinuma T, Ichikawa T, Suzuki AS, Sugaya K, Tumes DJ, Yamamoto H, Hara T, Tani-Ichi S, Ikuta K, Okamoto Y, and Nakayama T 2016. Thy1+IL-7+ lymphatic endothelial cells in iBALT provide a survival niche for memory T-helper cells in allergic airway inflammation. Proceedings of the National Academy of Sciences of the United States of America 113: E2842–2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ponce-Gallegos MA, Ramírez-Venegas A, and Falfán-Valencia R 2017. Th17 profile in COPD exacerbations. International Journal of Chronic Obstructive Pulmonary Disease 12: 1857–1865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bystrom J, Al-Adhoubi N, Al-Bogami M, Jawad AS, and Mageed RA 2013. Th17 lymphocytes in respiratory syncytial virus infection. Viruses 5: 777–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gopal R, Rangel-Moreno J, Fallert Junecko BA, Mallon DJ, Chen K, Pociask DA, Connell TD, Reinhart TA, Alcorn JF, Ross TM, Kolls JK, and Khader SA 2014. Mucosal pre-exposure to th17-inducing adjuvants exacerbates pathology after influenza infection. Am J Pathol 184: 55–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Whittaker L, Niu N, Temann UA, Stoddard A, Flavell RA, Ray A, Homer RJ, and Cohn L 2002. Interleukin-13 mediates a fundamental pathway for airway epithelial mucus induced by CD4 T cells and interleukin-9. American journal of respiratory cell and molecular biology 27: 593–602. [DOI] [PubMed] [Google Scholar]
- 63.Bates MA, Brandenberger C, Langohr I, Kumagai K, Harkema JR, Holian A, and Pestka JJ 2015. Silica Triggers Inflammation and Ectopic Lymphoid Neogenesis in the Lungs in Parallel with Accelerated Onset of Systemic Autoimmunity and Glomerulonephritis in the Lupus-Prone NZBWF1 Mouse. PloS one 10: 1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gosman MM, Willemse BW, Jansen DF, Lapperre TS, van Schadewijk A, Hiemstra PS, Postma DS, Timens W, and Kerstjens HA 2006. Increased number of B-cells in bronchial biopsies in COPD. Eur Respir J 27: 60–64. [DOI] [PubMed] [Google Scholar]
- 65.Litsiou E, Semitekolou M, Galani IE, Morianos I, Tsoutsa A, Kara P, Rontogianni D, Bellenis I, Konstantinou M, Potaris K, Andreakos E, Sideras P, Zakynthinos S, and Tsoumakidou M 2013. CXCL13 production in B cells via Toll-like receptor/lymphotoxin receptor signaling is involved in lymphoid neogenesis in chronic obstructive pulmonary disease. Am J Respir Crit Care Med 187: 1194–1202. [DOI] [PubMed] [Google Scholar]
- 66.Colvin KL, Cripe PJ, Ivy DD, Stenmark KR, and Yeager ME 2013. Bronchus-associated lymphoid tissue in pulmonary hypertension produces pathologic autoantibodies. Am J Respir Crit Care Med 188: 1126–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Perros F, Dorfmuller P, Montani D, Hammad H, Waelput W, Girerd B, Raymond N, Mercier O, Mussot S, Cohen-Kaminsky S, Humbert M, and Lambrecht BN 2012. Pulmonary Lymphoid Neogenesis in Idiopathic Pulmonary Arterial Hypertension. Am J Resp Crit Care 185: 311–321. [DOI] [PubMed] [Google Scholar]
- 68.Shinoda K, Hirahara K, and Nakayama T 2017. Maintenance of pathogenic Th2 cells in allergic disorders. Allergol Int 66: 369–376. [DOI] [PubMed] [Google Scholar]
- 69.Chvatchko Y, Kosco-Vilbois MH, Herren S, Lefort J, and Bonnefoy JY 1996. Germinal center formation and local immunoglobulin E (IgE) production in the lung after an airway antigenic challenge. J Exp Med 184: 2353–2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, Cherniack RM, Rogers RM, Sciurba FC, Coxson HO, and Pare PD 2004. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med 350: 2645–2653. [DOI] [PubMed] [Google Scholar]
- 71.Polverino F, Cosio BG, Pons J, Laucho-Contreras M, Tejera P, Iglesias A, Rios A, Jahn A, Sauleda J, Divo M, Pinto-Plata V, Sholl L, Rosas IO, Agusti A, Celli BR, and Owen CA 2015. B Cell-Activating Factor. An Orchestrator of Lymphoid Follicles in Severe Chronic Obstructive Pulmonary Disease. Am J Respir Crit Care Med 192: 695–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yanagisawa H, Hashimoto M, Minagawa S, Takasaka N, Ma R, Moermans C, Ito S, Araya J, Budelsky A, Goodsell A, Baron JL, and Nishimura SL 2017. Role of IL-17A in murine models of COPD airway disease. Am J Physiol Lung Cell Mol Physiol 312: L122–l130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Briend E, Ferguson GJ, Mori M, Damera G, Stephenson K, Karp NA, Sethi S, Ward CK, Sleeman MA, Erjefalt JS, and Finch DK 2017. IL-18 associated with lung lymphoid aggregates drives IFNgamma production in severe COPD. Respir Res 18: 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Zou Y, Chen X, Liu J, Zhou DB, Kuang X, Xiao J, Yu Q, Lu X, Li W, Xie B, and Chen Q 2017. Serum IL-1beta and IL-17 levels in patients with COPD: associations with clinical parameters. Int J Chron Obstruct Pulmon Dis 12: 1247–1254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Patel IS, Seemungal TA, Wilks M, Lloyd-Owen SJ, Donaldson GC, and Wedzicha JA 2002. Relationship between bacterial colonisation and the frequency, character, and severity of COPD exacerbations. Thorax 57: 759–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brusselle GG, Demoor T, Bracke KR, Brandsma CA, and Timens W 2009. Lymphoid follicles in (very) severe COPD: beneficial or harmful? Eur Respir J 34: 219–230. [DOI] [PubMed] [Google Scholar]
- 77.Solleti SK, Srisuma S, Bhattacharya S, Rangel-Moreno J, Bijli KM, Randall TD, Rahman A, and Mariani TJ 2016. Serpine2 deficiency results in lung lymphocyte accumulation and bronchus-associated lymphoid tissue formation. FASEB J 30: 2615–2626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jia J, Conlon TM, Sarker RS, Tasdemir D, Smirnova NF, Srivastava B, Verleden SE, Gunes G, Wu X, Prehn C, Gao J, Heinzelmann K, Lintelmann J, Irmler M, Pfeiffer S, Schloter M, Zimmermann R, Hrabe de Angelis M, Beckers J, Adamski J, Bayram H, Eickelberg O, and Yildirim AO 2018. Cholesterol metabolism promotes B-cell positioning during immune pathogenesis of chronic obstructive pulmonary disease. EMBO Mol Med 10: 1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]