Abstract
Idiopathic pulmonary fibrosis (IPF) is a disease of older adults leading to progressive dyspnea and reduced exercise capacity, typically resulting in death within 3–5 years of diagnosis. Underlying genetic susceptibility combined with environmental insults is proposed to trigger a chronic wound repair response, leading to activation of the fibrotic cascade. Perturbations in several molecular pathways mediate vulnerability of the alveolar epithelium to injurious agents, including the unfolded protein response, autophagy, mitophagy, and cellular senescence. These cellular responses are intricately intertwined and link genetic susceptibility to the progressive fibrotic phenotype. Ongoing studies investigating these pathways in type II alveolar epithelial cells show promise for identifying new targeted interventions that could prevent or halt the progression of IPF.
Keywords: Idiopathic pulmonary fibrosis, endoplasmic reticulum stress, autophagy, mitophagy, senescence
Introduction
Idiopathic pulmonary fibrosis (IPF) is a progressive scarring disease of the lung characterized histologically by significant architectural distortion involving the distal lung parenchyma. Characteristic abnormalities include cystic “honeycomb change”, with bronchiolization of the distal airspaces and areas of alveolar epithelial hyperplasia, especially prominent overlying fibroblastic foci, which are interstitial collections of myofibroblasts embedded within a myxoid stroma. Other interstitial and mesenchymal cell changes include abundant collagen deposition, smooth muscle cell hyperplasia and malformed vasculature.1–3 Areas of scarred lung, first most notable in the subpleural and paraseptal spaces, march progressively inward over time, classically with fibroblastic foci at the leading edge.4 Despite these well characterized histological features, it is unknown which cellular populations are instigators of disease and which are reactive. Several lines of evidence support the model first proposed by Katzenstein and colleagues more than two decades ago hypothesizing that epithelial injury and failure of appropriate healing/regeneration are central to the pathogenesis of IPF.5
Genetic studies provided some of the most compelling clues that defects of the alveolar epithelium underlie disease progression. Healthy pulmonary alveoli are composed of thin type I alveolar epithelial cells (AT1) in close proximity to endothelial cells forming a surface for gas exchange and type II alveolar epithelial cells (AT2) which produce the phospholipid rich surfactant. A subset of AT2 cells also are maintained as stem cells and slowly renew the alveolar epithelium through production of daughter cells that differentiate into AT1 cells.6–8 Over 25 genes and genetic loci have been identified in sporadic and familial cases of IPF that increase the risk of developing disease. The most prominent common genetic variant linked to disease is a promoter polymorphism in the MUC5B gene, whose expression is restricted to lung epithelium. The first studies of Mendelian families with pulmonary fibrosis identified mutations in epithelial-restricted genes, and further work has demonstrated that disease-associated mutations primarily cluster into two categories—surfactant production (SFTPC, SFTPA2, ABCA3) and telomere maintenance (TERT, RTEL, TERC, DKC1, TINF2, PARN).9,10 These genetic insights focused attention on AT2 cells and subsequent studies have suggested these cells have an increased susceptibility to injury. While the possible sources of epithelial injury are numerous, proposed triggers including cigarette smoking, air pollutants and chronic viral or bacterial infection.
Endoplasmic Reticulum Stress
One indicator of cellular injury or stress is activation of the unfolded protein response (UPR). The endoplasmic reticulum (ER) assists in folding of integral membrane and secretory proteins. Accumulation of unfolded proteins within the ER, termed ER stress, triggers the UPR, a signaling cascade directed at reducing the amount of unfolded protein within the cell and resolution of ER stress. Three proteins, PKR-like endoplasmic reticulum kinase (PERK), activating transcription factor 6 (ATF6) and inositol requiring enzyme 1 α (IRE1α), start a downstream cascade that will result in upregulation of chaperone proteins to promote proper protein folding, decreased protein synthesis, increased degradation of unfolded proteins, and if necessary, apoptosis (Figure 1). Balance of these pathways ideally leads to proteostasis and cellular survival.11
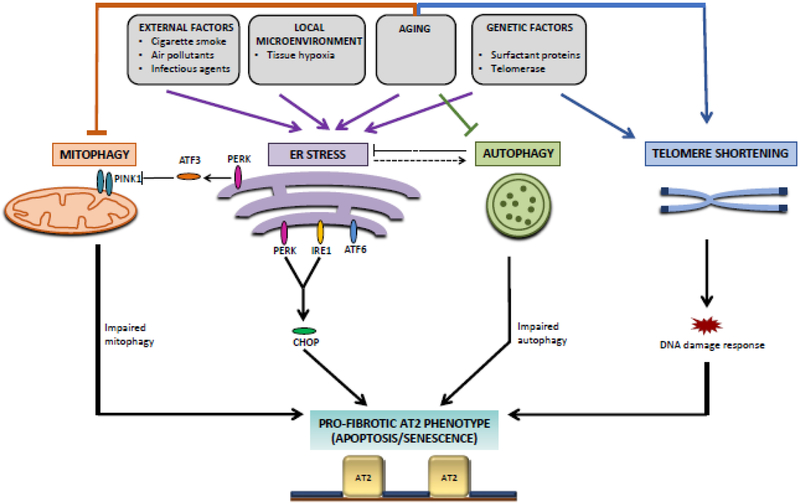
Figure 1. Molecular pathways influencing pro-fibrotic phenotypes of type II alveolar epithelial (AT2) cells in IPF.
Genetic and environmental factors converge to regulate pathways that affect the ability of AT2 cells to respond to injury, including ER stress, mitophagy, autophagy, and DNA damage response. Pathological interactions and imbalances between these pathways result in pro-fibrotic phenotypes in AT2 cells, including senescence and apoptosis, which contribute to IPF pathogenesis.
Many of the SFTPC mutations that have been identified in patients with IPF cluster in the region encoding the BRICHOS domain, a portion of the C-terminus of the pro-peptide essential for protein folding and trafficking. These mutations result in misfolded SP-C that accumulates in the endoplasmic reticulum with resulting ER stress and activation of the unfolded protein response (UPR).12,13 In vivo experiments have suggested that ER stress is upstream of pulmonary fibrosis. A transgenic mouse model expressing the disease-associated SP-CL188Q variant specifically in AT2 cells had an exaggerated fibrotic response to bleomycin induced injury. Similarly, stimulation of ER stress through administration of tunicamycin predisposes the murine lung to a more robust fibrotic response to injury.14 Importantly, in familial and sporadic cases of IPF with and without SFTPC mutations, markers of ER stress and of activation of the UPR (BiP, EDEM, p-eIF2α, XBP-1) are expressed in the hyperplastic AT2 cells overlying fibroblastic foci.13,15 This suggests that ER stress and activation of the UPR may be a generalized feature of IPF. Recent publications have begun to reveal the downstream mechanisms connecting ER stress to pulmonary fibrosis.
If ER stress persists in a cell, apoptosis can be triggered. Downstream of PERK in the UPR is the pro-apoptotic factor, C/EBP homologous protein (CHOP). CHOP is expressed in hyperplastic AT2 cells in IPF lungs. In a mouse model, after single administration of intratracheal bleomycin, minimal induction of CHOP was observed. However, repetitive bleomycin administration resulted in robust activation of CHOP in hyperplastic AT2 cells around areas of fibrosis, similar to what is observed in the IPF lung. In this model, mice deficient in CHOP had reduced AT2 apoptosis and were protected from pulmonary fibrosis.16 These data suggest that failure to resolve ER stress with the eventual progression to AT2 apoptosis and impaired re-epithelialization is essential to the fibrotic cascade.
Other triggers of ER stress identified in the lung include infection (viral and bacterial), reactive oxygen species production, hypoxia, and aging. One proposed mechanism by which the IPF-associated MUC5B risk allele contributes to disease pathogenesis is through induction of ER stress.17 This may occur through the metabolic demands of overexpressing a large glycoprotein similar to the strain induced by mutant SP-C. Another possibility is that impairment in mucociliary clearance leads to retention of microorganisms and the resulting chronic inflammation activates the UPR as has been suggested in cystic fibrosis.18 The prolific nature of the stressors may explain the frequently observed phenotype of ER stress observed in the lungs of IPF patients. Additionally, AT2 cells in the IPF lung have a reduced capacity to resolve ER stress due to impairment in autophagy.
Autophagy
Autophagy is an important homeostatic mechanism and response to cellular stress. During autophagy, portions of the cytoplasm and cellular organelles are encapsulated in double membraned autophagosomes which fuse with lysosomes to degrade the content. ER stress activates autophagy which in turn removes the expanded ER and promotes cell survival.19 In IPF lungs, the overlying hyperplastic epithelial cells and fibroblasts of fibroblastic foci both display markers of impaired autophagy despite activation of ER stress pathways.20 Furthermore, genetic variants in TOLLIP modulate risk of developing IPF providing genetic evidence of a link between autophagy and IPF. TOLLIP is an autophagy receptor downstream of Toll-like receptor signaling important for recognizing ubiquitinated proteins and delivering them for degradation.21
Failure to induce autophagy in response to ER stress promoted pulmonary fibrosis in a mouse model. Knockout of the autophagy gene, Atg4b, lead to a decrease of both basal and induced autophagy. Exposure of Atg4b knockout mice to tunicamycin to induce ER stress generated increased epithelial cell apoptosis and prominent septal thickening and collagen deposition compared to control mice.19 These data demonstrate that impaired autophagy compromises the capacity of the lung to deal with ER stress.
The most common SFTPC variant (I73T) found in both sporadic and familial cases of IPF has also been mechanistically linked to inadequate autophagy in a mouse model.22 In contrast to BRICHOS domain mutations that lead to misfolding and accumulation in the ER, SP-CI73T is abnormally trafficked directly to the plasma membrane. Cells expressing this variant do not develop ER stress. Rather, expression of SP-CI73T leads to a late block in autophagy demonstrated by accumulation of both LC3 and p62.23 A mouse model expressing SP-CI73T in the endogenous SFTPC locus developed spontaneous alveolitis and fibrotic remodeling. AT2 cells accumulated p62 and LC3-II and transmission electron microscopy of lung sections revealed numerous intracytoplasmic double membrane inclusions within hyperplastic AT2 cells consistent with impaired autophagy. The level of SP-CI73T expressed correlated directly with the degree of lung injury.22 Taken together, these studies suggest that AT2s are particularly sensitive to ER stress and are dependent on autophagy both as a response to ER stress and to resolve other types of injury.
Mitophagy
The susceptibility of AT2 cells to ER stress likely correlates with their role as an active secretory cell. Their secretory function necessitates a large number of mitochondria relative to other pulmonary cell types. Failure to maintain healthy mitochondria has also been identified as an underlying mechanism of pulmonary fibrosis and is intimately connected to ER stress pathways and autophagy. Damaged mitochondria are targeted for lysosomal degradation via autophagy in a selective process termed mitophagy. Stressed mitochondria are recognized by the degradative machinery through stabilization of PINK1 on the mitochondrial outer membrane. PINK1 then recruits PARK2, an E3-ubiquitin ligase, to the mitochondrial membrane. The resulting ubiquitinated mitochondrial proteins are recognized by p62 which targets the mitochondria for the autophagosome through interaction with LC3.24
AT2 cells, but not fibroblasts, in fibrotic regions of IPF lungs accumulate enlarged and dysmorphic mitochondria and this correlates with markers of ER stress and impaired autophagy. Indeed, induction of ER stress through tunicamycin administration was sufficient to induce accumulation of enlarged mitochondria in AT2 cells in a mouse model.25 This effect is mediated through the PERK arm of the UPR which leads to induction of ATF3, a transcriptional repressor of PINK1.26 Pink1 −/− mice demonstrated increased numbers of swollen mitochondria and had spontaneous deposition of collagen within the alveolar wall. They also had increased susceptibility to fibrosis following viral infection.25
These three pathways (UPR, autophagy and mitophagy) are intricately intertwined in the normal cellular response to stress and perturbation in any of these pathways in the lung appears to adversely affect AT2 cells. As each pathway has been investigated, links have been formed that reinforce the hypothesis that epithelial cell injury and failure to resolve stress is central to the pathogenesis of IPF.
Cellular Senescence and Telomere Shortening
Cells that are unable to resolve stress eventually progress to apoptosis or undergo cellular senescence, a permanent cell cycle arrest. Increased markers of senescence have been identified in IPF lungs in both epithelial cells and fibroblasts within fibroblastic foci and areas of honeycomb change.27,28 The arrest of the growth cycle does not lead to a dormant cell but rather senescent cells adopt the senescence-associated secretory phenotype (SASP) and produce a variety of chemokines, cytokines, growth factors and matrix metalloproteinases. Conditioned medium from senescent lung fibroblasts and epithelial cells have been demonstrated to induce expression of several pro-fibrotic genes involved in extracellular matrix production in healthy primary human fibroblasts in vitro27 and cultured fibroblasts.28 In an in vivo murine model of pulmonary injury, bleomycin treatment induced a marker of senescence, p16, in both fibroblasts and epithelial cells. Selective induction of apoptosis in p16+ cells resulted in improved physiologic parameters, including lung compliance and exercise capacity, following bleomycin exposure, but did not impact histologic fibrosis.27 These data suggest that both the epithelial and fibroblast SASP is profibrotic in the lung and that epithelial-mesenchymal cross talk mediated by senescent cells may perpetuate the fibrotic phenotype. However, it is unclear which senescent population, epithelial or fibroblast, might be pro-fibrotic in vivo and to what degree cellular senescence is contributing to the pathogenesis of pulmonary fibrosis.
Senescence is a topic of interest due to the significant risk that aging (known to increase cellular senescence) and shortened telomeres confers for the development of disease.9,29 Telomere shortening is a known trigger for cellular senescence and can induce the DNA damage response pathway. As described above, multiple variants in genes involved in telomere maintenance have been identified that increase the risk of developing IPF. However, mouse models deficient in telomere maintenance genes (Tert, Terc) have failed to reliably recapitulate human pulmonary phenotypes despite developing other manifestations of short telomere syndromes. Tert-null mice developed spontaneous failure of rapidly renewing tissue with manifestations including infertility, intestinal villous atrophy and bone marrow failure30, but do not spontaneously develop pulmonary fibrosis and have provided inconsistent and even contradictory phenotypes in response to injury.31–33 Mice lacking Terc are prone to emphysema when exposed to cigarette smoke but do not develop fibrosis.34 It is unknown at this time why the mouse lung is not consistently affected by deficiency of telomere maintenance genes. Notably, patients with IPF have shorter telomeres both in peripheral blood leukocytes and in AT2s even without a known mutation in telomere maintenance genes.35 This suggests that there may be a driver of shortened telomeres in IPF independent of genetic factors.
Several groups have utilized conditional deletion of shelterin complex components as an approach to recapitulate the phenotype of a telomere-DNA damage response. In contrast to telomerase components (Tert, Terc, Dkc1), shelterin components Trf1 and Trf2 are negative regulators of telomere length. Studies using these models have provided evidence that the alveolar stem cell compartment of the lung is negatively impacted by induction of DNA-damage response and cellular senescence. Mice deficient in telomeric repeat-binding factor 2 (Trf2) develop uncapping of telomeres and a robust DNA damage response that occurs within a single generation.29 Conditional knockout of Trf2 specifically in AT2 cells resulted in reduced AT2 cell proliferation and development of spontaneous inflammation in vivo. Bleomycin injury in these mice was 100% fatal which could not be explained histopathologically though AT2 cells again demonstrated impaired proliferation. Importantly, while proliferation was reduced, there was not an increase in apoptosis, suggesting induction of senescence. Trf2-null and late generation Terc-null AT2 cells also had markedly reduced colony forming capacity in vitro demonstrating impairment in stem cell function by DNA damage at telomeres.29 In another report, Trf1-null mice developed severe spontaneous pulmonary fibrosis but did not have shortened telomeres. They did develop a robust DNA damage response in response to the telomere damage and both increased senescence and apoptosis in AT2 cells was observed.32 Notably, Trf-1 deletion specifically in AT2 cells was sufficient to induce fibrosis while deletion selectively in collagen expressing cells was not profibrotic in mice.36 Together, these studies suggest that induction of DNA damage responses through failure to maintain telomeres specifically in the alveolar epithelium is sufficient to induce injury and senescence. Further investigation is needed to define the precise mechanisms and mediators linking epithelial senescence to progressive lung fibrosis.
Future Directions
There is a growing body of evidence suggesting that lung epithelial injury and dysfunction are central factors in the pathogenesis of pulmonary fibrosis. Exciting data have emerged that integrate injury and cellular stress response pathways (UPR, autophagy, mitophagy) together in IPF pathogenesis providing a possible final downstream cascade that can be triggered by diverse injuries (Figure 1). In contrast, the molecular mechanisms tying telomere shortening to pulmonary fibrosis have remained somewhat elusive though links to the DNA damage response and cellular senescence provide a starting point for additional studies and also point to the epithelium as a key regulator of the fibrotic cascade. Determining shared molecular mechanisms that lead to pulmonary fibrosis will be essential in identifying novel treatments that can be applied to a diverse patient population with varied genetic underpinnings and inciting injuries.
Funding:
National Institutes of Health, National Heart, Lung, and Blood Institute T32HL094296 (NIW), K08HL130595 (JAK), and P01HL92870 (TSB), Department of Veterans Affairs (TSB), Francis Family Foundation (JAK), Doris Duke Charitable Foundation (JAK), Department of Defense PR160522 (TSB)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: TSB and JAK have grant funding from Boehringer Ingelheim and TSB has grant funding from Celgene.
References
- 1.Cosgrove GP, Brown KK, Schiemann WP, et al. Pigment epithelium-derived factor in idiopathic pulmonary fibrosis: a role in aberrant angiogenesis. Am J Respir Crit Care Med. 2004;170(3):242–251. [DOI] [PubMed] [Google Scholar]
- 2.Cavazza A, Rossi G, Carbonelli C, et al. The role of histology in idiopathic pulmonary fibrosis: an update. Respir Med. 2010;104 Suppl 1:S11–22. [DOI] [PubMed] [Google Scholar]
- 3.Myers JL, Katzenstein AL. Beyond a consensus classification for idiopathic interstitial pneumonias: progress and controversies. Histopathology. 2009;54(1):90–103. [DOI] [PubMed] [Google Scholar]
- 4.Leslie KO. My approach to interstitial lung disease using clinical, radiological and histopathological patterns. J Clin Pathol. 2009;62(5):387–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.AL K Pathogenesis of “fibrosis” in interstitial pneumonia: an electron microscopic study. Hum Pathol. 1985;16(10):1015–1024. [DOI] [PubMed] [Google Scholar]
- 6.Evans MJ, Cabral LJ, Stephens RJ, et al. Renewal of alveolar epithelium in the rat following exposure to NO2. Am J Pathol. 1973;70(2):175–198. [PMC free article] [PubMed] [Google Scholar]
- 7.Desai TJ, Brownfield DG, Krasnow MA. Alveolar progenitor and stem cells in lung development, renewal and cancer. Nature. 2014;507(7491):190–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nabhan AN, Brownfield DG, Harbury PB, et al. Single-cell Wnt signaling niches maintain stemness of alveolar type 2 cells. Science. 2018;359(6380):1118–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kropski JA, Blackwell TS, Loyd JE. The genetic basis of idiopathic pulmonary fibrosis. Eur Respir J. 2015;45(6):1717–1727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kaur A, Mathai SK, Schwartz DA. Genetics in Idiopathic Pulmonary Fibrosis Pathogenesis, Prognosis, and Treatment. Front Med (Lausanne). 2017;4:154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burman A, Tanjore H, Blackwell TS. Endoplasmic reticulum stress in pulmonary fibrosis. Matrix Biol. 2018;68–69:355–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mulugeta S, Nguyen V, Russo SJ, et al. A surfactant protein C precursor protein BRICHOS domain mutation causes endoplasmic reticulum stress, proteasome dysfunction, and caspase 3 activation. Am J Respir Cell Mol Biol. 2005;32(6):521–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lawson WE, Crossno PF, Polosukhin VV, et al. Endoplasmic reticulum stress in alveolar epithelial cells is prominent in IPF: association with altered surfactant protein processing and herpesvirus infection. Am J Physiol Lung Cell Mol Physiol. 2008;294(6):L1119–1126. [DOI] [PubMed] [Google Scholar]
- 14.Lawson WE, Cheng DS, Degryse AL, et al. Endoplasmic reticulum stress enhances fibrotic remodeling in the lungs. Proc Natl Acad Sci U S A. 2011;108(26):10562–10567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Korfei M, Ruppert C, Mahavadi P, et al. Epithelial endoplasmic reticulum stress and apoptosis in sporadic idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2008;178(8):838–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burman A, Kropski JA, Calvi CL, et al. Localized hypoxia links ER stress to lung fibrosis through induction of C/EBP homologous protein. JCI Insight. 2018;3(16). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz DA. Idiopathic Pulmonary Fibrosis Is a Genetic Disease Involving Mucus and the Peripheral Airways. Ann Am Thorac Soc. 2018;15:S192–S197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ribeiro CM, Paradiso AM, Schwab U, et al. Chronic airway infection/inflammation induces a Ca2+i-dependent hyperinflammatory response in human cystic fibrosis airway epithelia. J Biol Chem. 2005;280(18):17798–17806. [DOI] [PubMed] [Google Scholar]
- 19.Maciel M, Hernandez-Barrientos D, Herrera I, et al. Impaired autophagic activity and ATG4B deficiency are associated with increased endoplasmic reticulum stress-induced lung injury. Aging (Albany NY). 2018;10(8):2098–2112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Araya J, Kojima J, Takasaka N, et al. Insufficient autophagy in idiopathic pulmonary fibrosis. Am J Physiol Lung Cell Mol Physiol. 2013;304(1):L56–69. [DOI] [PubMed] [Google Scholar]
- 21.Noth I, Zhang Y, Ma SF, et al. Genetic variants associated with idiopathic pulmonary fibrosis susceptibility and mortality: a genome-wide association study. Lancet Respir Med. 2013;1(4):309–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nureki SI, Tomer Y, Venosa A, et al. Expression of mutant Sftpc in murine alveolar epithelia drives spontaneous lung fibrosis. J Clin Invest. 2018;128(9):4008–4024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hawkins A, Guttentag SH, Deterding R, et al. A non-BRICHOS SFTPC mutant (SP-CI73T) linked to interstitial lung disease promotes a late block in macroautophagy disrupting cellular proteostasis and mitophagy. Am J Physiol Lung Cell Mol Physiol. 2015;308(1):L33–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsubouchi K, Araya J, Kuwano K. PINK1-PARK2-mediated mitophagy in COPD and IPF pathogeneses. Inflamm Regen. 2018;38:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bueno M, Lai YC, Romero Y, et al. PINK1 deficiency impairs mitochondrial homeostasis and promotes lung fibrosis. J Clin Invest. 2015;125(2):521–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bueno M, Brands J, Voltz L, et al. ATF3 represses PINK1 gene transcription in lung epithelial cells to control mitochondrial homeostasis. Aging Cell. 2018;17(2). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Schafer MJ, White TA, Iijima K, et al. Cellular senescence mediates fibrotic pulmonary disease. Nature Communications. 2017;8:14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minagawa S, Araya J, Numata T, et al. Accelerated epithelial cell senescence in IPF and the inhibitory role of SIRT6 in TGF-beta-induced senescence of human bronchial epithelial cells. Am J Physiol Lung Cell Mol Physiol. 2011;300(3):L391–401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alder JK, Barkauskas CE, Limjunyawong N, et al. Telomere dysfunction causes alveolar stem cell failure. Proc Natl Acad Sci U S A. 2015;112(16):5099–5104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strong MA, Vidal-Cardenas SL, Karim B, et al. Phenotypes in mTERT+/− and mTERT−/− Mice Are Due to Short Telomeres, Not Telomere-Independent Functions of Telomerase Reverse Transcriptase. Molecular and cellular biology. 2011;31(12):2369–2379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Degryse AL, Xu XC, Newman JL, et al. Telomerase deficiency does not alter bleomycin-induced fibrosis in mice. Exp Lung Res. 2012;38(3):124–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Povedano JM, Martinez P, Flores JM, et al. Mice with Pulmonary Fibrosis Driven by Telomere Dysfunction. Cell Rep. 2015;12(2):286–299. [DOI] [PubMed] [Google Scholar]
- 33.Liu T, Chung MJ, Ullenbruch M, et al. Telomerase activity is required for bleomycin-induced pulmonary fibrosis in mice. J Clin Invest. 2007;117(12):3800–3809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alder JK, Guo N, Kembou F, et al. Telomere length is a determinant of emphysema susceptibility. Am J Respir Crit Care Med. 2011;184(8):904–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alder JK, Chen JJ, Lancaster L, et al. Short telomeres are a risk factor for idiopathic pulmonary fibrosis. Proc Natl Acad Sci U S A. 2008;105(35):13051–13056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Naikawadi RP, Disayabutr S, Mallavia B, et al. Telomere dysfunction in alveolar epithelial cells causes lung remodeling and fibrosis. JCI Insight. 2016;1(14):e86704. [DOI] [PMC free article] [PubMed] [Google Scholar]