Abstract
Background
Molluscum contagiosum is a common skin infection that is caused by a pox virus and occurs mainly in children. The infection usually resolves within months in people without immune deficiency, but treatment may be preferred for social and cosmetic reasons or to avoid spreading the infection. A clear evidence base supporting the various treatments is lacking.
This is an update of a Cochrane Review first published in 2006, and updated previously in 2009.
Objectives
To assess the effects of specific treatments and management strategies, including waiting for natural resolution, for cutaneous, non‐genital molluscum contagiosum in people without immune deficiency.
Search methods
We updated our searches of the following databases to July 2016: the Cochrane Skin Group Specialised Register, CENTRAL, MEDLINE, Embase, and LILACS. We searched six trial registers and checked the reference lists of included studies and review articles for further references to relevant randomised controlled trials. We contacted pharmaceutical companies and experts in the field to identify further relevant randomised controlled trials.
Selection criteria
Randomised controlled trials of any treatment of molluscum contagiosum in people without immune deficiency. We excluded trials on sexually transmitted molluscum contagiosum and in people with immune deficiency (including those with HIV infection).
Data collection and analysis
Two review authors independently selected studies, assessed methodological quality, and extracted data from selected studies. We obtained missing data from study authors where possible.
Main results
We found 11 new studies for this update, resulting in 22 included studies with a total of 1650 participants. The studies examined the effects of topical (20 studies) and systemic interventions (2 studies).
Among the new included studies were the full trial reports of three large unpublished studies, brought to our attention by an expert in the field. They all provided moderate‐quality evidence for a lack of effect of 5% imiquimod compared to vehicle (placebo) on short‐term clinical cure (4 studies, 850 participants, 12 weeks after start of treatment, risk ratio (RR) 1.33, 95% confidence interval (CI) 0.92 to 1.93), medium‐term clinical cure (2 studies, 702 participants, 18 weeks after start of treatment, RR 0.88, 95% CI 0.67 to 1.14), and long‐term clinical cure (2 studies, 702 participants, 28 weeks after start of treatment, RR 0.97, 95% CI 0.79 to 1.17). We found similar but more certain results for short‐term improvement (4 studies, 850 participants, 12 weeks after start of treatment, RR 1.14, 95% CI 0.89 to 1.47; high‐quality evidence). For the outcome 'any adverse effect', we found high‐quality evidence for little or no difference between topical 5% imiquimod and vehicle (3 studies, 827 participants, RR 0.97, 95% CI 0.88 to 1.07), but application site reactions were more frequent in the groups treated with imiquimod (moderate‐quality evidence): any application site reaction (3 studies, 827 participants, RR 1.41, 95% CI 1.13 to 1.77, the number needed to treat for an additional harmful outcome (NNTH) was 11); severe application site reaction (3 studies, 827 participants, RR 4.33, 95% CI 1.16 to 16.19, NNTH over 40).
For the following 11 comparisons, there was limited evidence to show which treatment was superior in achieving short‐term clinical cure (low‐quality evidence): 5% imiquimod less effective than cryospray (1 study, 74 participants, RR 0.60, 95% CI 0.46 to 0.78) and 10% potassium hydroxide (2 studies, 67 participants, RR 0.65, 95% CI 0.46 to 0.93); 10% Australian lemon myrtle oil more effective than olive oil (1 study, 31 participants, RR 17.88, 95% CI 1.13 to 282.72); 10% benzoyl peroxide cream more effective than 0.05% tretinoin (1 study, 30 participants, RR 2.20, 95% CI 1.01 to 4.79); 5% sodium nitrite co‐applied with 5% salicylic acid more effective than 5% salicylic acid alone (1 study, 30 participants, RR 3.50, 95% CI 1.23 to 9.92); and iodine plus tea tree oil more effective than tea tree oil (1 study, 37 participants, RR 0.20, 95% CI 0.07 to 0.57) or iodine alone (1 study, 37 participants, RR 0.07, 95% CI 0.01 to 0.50). Although there is some uncertainty, 10% potassium hydroxide appears to be more effective than saline (1 study, 20 participants, RR 3.50, 95% CI 0.95 to 12.90); homeopathic calcarea carbonica appears to be more effective than placebo (1 study, 20 participants, RR 5.57, 95% CI 0.93 to 33.54); 2.5% appears to be less effective than 5% solution of potassium hydroxide (1 study, 25 participants, RR 0.35, 95% CI 0.12 to 1.01); and 10% povidone iodine solution plus 50% salicylic acid plaster appears to be more effective than salicylic acid plaster alone (1 study, 30 participants, RR 1.43, 95% CI 0.95 to 2.16).
We found no statistically significant differences for other comparisons (most of which addressed two different topical treatments). We found no randomised controlled trial evidence for expressing lesions or topical hydrogen peroxide.
Study limitations included no blinding, many dropouts, and no intention‐to‐treat analysis. Except for the severe application site reactions of imiquimod, none of the evaluated treatments described above were associated with serious adverse effects (low‐quality evidence). Among the most common adverse events were pain during application, erythema, and itching. Included studies of the following comparisons did not report adverse effects: calcarea carbonica versus placebo, 10% povidone iodine plus 50% salicylic acid plaster versus salicylic acid plaster, and 10% benzoyl peroxide versus 0.05% tretinoin.
We were unable to judge the risk of bias in most studies due to insufficient information, especially regarding concealment of allocation and possible selective reporting. We considered five studies to be at low risk of bias.
Authors' conclusions
No single intervention has been shown to be convincingly effective in the treatment of molluscum contagiosum. We found moderate‐quality evidence that topical 5% imiquimod was no more effective than vehicle in terms of clinical cure, but led to more application site reactions, and high‐quality evidence that there was no difference between the treatments in terms of short‐term improvement. However, high‐quality evidence showed a similar number of general side effects in both groups. As the evidence found did not favour any one treatment, the natural resolution of molluscum contagiosum remains a strong method for dealing with the condition.
Plain language summary
Treatments for molluscum contagiosum, a common viral skin infection in children
Review question
We reviewed the evidence for the effect of any treatment on the common viral skin infection molluscum contagiosum. We excluded people with a repressed immune system or sexually transmitted molluscum contagiosum.
Background
Molluscum contagiosum in healthy people is a self limiting, relatively harmless viral skin infection. It mainly affects children and adolescents and is rare in adults. It occurs worldwide, but seems much more frequent in geographic areas with warm climates. Molluscum contagiosum usually presents as single or multiple pimples filled with an oily substance. People may seek treatment for social and cosmetic reasons and because of concerns about spreading the disease to others. Treatment is intended to speed up the healing process.
Study characteristics
We searched the literature to July 2016. We included 22 trials (total of 1650 participants). Twenty of the studies evaluated topical treatment, and two studies evaluated treatment taken by mouth (oral). Comparisons included physical therapies, as well as topical and oral treatments. Most studies were set in hospital outpatient or emergency departments, and were performed in North America, the UK, Asia, or South America. Participants were of both sexes and were mainly children or young adults. Follow‐up duration varied from 3 to 28 weeks after randomisation. Only five studies had longer than 3 months' follow‐up.
Five studies reported commercial funding, three studies obtained medication for free from pharmaceutical companies, 12 studies did not mention the source of funding, one study reported charity funding, and one study reported they had had no financial support.
Key results
We found that many common treatments for molluscum, such as physical destruction, have not been adequately evaluated. Some of the included treatments are not part of standard practice.
We found moderate‐quality evidence that topical 5% imiquimod is probably no more effective than vehicle (i.e. the same cream but without imiquimod) in achieving short‐, medium‐, and long‐term clinical cure. High‐quality (and thus more certain) evidence showed that topical 5% imiquimod is no better than placebo at improving molluscum up to three months after the start of treatment.
High‐quality evidence showed that 5% imiquimod differed little or not at all in the number of side effects compared to vehicle. However, moderate‐quality evidence suggests that there are probably more application site reactions when using topical 5% imiquimod compared with vehicle.
Low‐quality evidence, based on one or two mostly small studies, revealed the following results for the outcome short‐term clinical cure: 5% imiquimod less effective than cryospray or 10% potassium hydroxide; 10% Australian lemon myrtle oil more effective than olive oil; 10% benzoyl peroxide cream more effective than 0.05% tretinoin; 5% sodium nitrite co‐applied with 5% salicylic acid more effective than 5% salicylic acid alone; and iodine plus tea tree oil more effective than tea tree oil or iodine alone. We found more uncertain (low‐quality) evidence to suggest that 10% potassium hydroxide is more effective than saline; homeopathic calcarea carbonica is more effective than placebo; 2.5% solution of potassium hydroxide is less effective than 5% solution of potassium hydroxide; and 10% povidone iodine solution and 50% salicylic acid plaster are more effective than salicylic acid plaster alone.
Except for the severe application site reactions of imiquimod, none of these treatments led to serious adverse effects (low‐quality evidence). Pain during treatment application, redness, and itching were among the most reported adverse effects.
We found no differences between the treatments assessed in the other comparisons.
We found no randomised trials for several commonly used treatments, such as expressing lesions with an orange stick or topical hydrogen peroxide. Since most lesions resolve within months, unless better evidence for the superiority of active treatments emerges, molluscum contagiosum can be left to heal naturally.
Quality of the evidence
For topical imiquimod, the quality of the evidence for clinical cure, short‐term improvement, and adverse effects was moderate to high. For all other comparisons, the quality of the evidence for short‐term clinical cure and adverse effects was low. Common limitations of the included studies were that the numbers of participants were small, the investigators were not blinded, and participants who did not complete the study (numerous in some studies) were not included in the analyses.
Summary of findings
Summary of findings for the main comparison. Imiquimod versus vehicle for cutaneous molluscum contagiosum.
| Imiquimod versus vehicle for cutaneous molluscum contagiosum | ||||||
| Patient or population: molluscum contagiosum Setting: dermatology outpatient departments Intervention: topical imiquimod Comparison: topical vehicle | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with topical vehicle | Risk with topical imiquimod | |||||
| Short‐term clinical cure (up to 3 months after start of treatment) (completely cleared short term) Assessed with: observer assessed Follow‐up: mean 12 weeks | Study population | RR 1.33 (0.92 to 1.93) | 850 (4 RCTs) | ⊕⊕⊕○ MODERATE 1 | Analysis 1.1 | |
| 118 per 1000 | 156 per 1000 (108 to 227) | |||||
| Medium‐term clinical cure (after 3 months and up to 6 months after start of treatment) (completely cleared medium term) Assessed with: observer assessed Follow‐up: mean 16 weeks | Study population | RR 0.88 (0.67 to 1.14) | 702 (2 RCTs) | ⊕⊕⊕○ MODERATE 2 | Analysis 1.2 | |
| 272 per 1000 | 239 per 1000 (182 to 310) | |||||
| Long‐term clinical cure (beyond 6 months after start of treatment) (completely cleared long term) Assessed with: observer assessed Follow‐up: mean 28 weeks | Study population | RR 0.97 (0.79 to 1.17) | 702 (2 RCTs) | ⊕⊕⊕○ MODERATE 2 | Analysis 1.3 | |
| 401 per 1000 | 389 per 1000 (317 to 469) | |||||
| Short‐term clinical improvement (up to 3 months after start of treatment) Assessed with: observer assessed Follow‐up: mean 12 weeks | Study population | RR 1.14 (0.89 to 1.47) | 850 (4 RCTs) | ⊕⊕⊕⊕ HIGH 3 | Analysis 1.4 | |
| 487 per 1000 | 555 per 1000 (433 to 716) | |||||
| Any adverse effect | Study population | RR 0.97 (0.88 to 1.07) | 827 (3 RCTs) | ⊕⊕⊕⊕ HIGH 4 | Analysis 1.7 | |
| 688 per 1000 | 667 per 1000 (606 to 736) | |||||
| Application site reactions | Study population | RR 1.41 (1.13 to 1.77) | 827 (3 RCTs) | ⊕⊕⊕○ MODERATE 5 | Analysis 1.8. This outcome was not prespecified in our protocol. | |
| 261 per 1000 | 368 per 1000 (295 to 462) | |||||
| Severe application site reactions | Study population | RR 4.33 (1.16 to 16.19) | 827 (3 RCTs) | ⊕⊕⊕○ MODERATE 5 | Analysis 1.9. This outcome was not prespecified in our protocol. | |
| 7 per 1000 | 29 per 1000 (8 to 110) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
1Downgraded by one level due to imprecision (< 300 events). We decided not to downgrade for risk of bias as out of four studies, the largest three were judged to be at low risk of bias.
2Downgraded by one level due to imprecision (< 300 events). We decided not to downgrade for risk of bias as both studies were judged to be at low risk of bias.
3We decided not to downgrade for risk of bias as out of four studies, the largest three were judged to be at low risk of bias. We also decided not to downgrade for inconsistency as removing one outlier eliminated inconsistency but hardly affected pooled estimate.
4We decided not to downgrade for risk of bias as all three studies were judged to be at low risk of bias.
5Downgraded by one level due to imprecision (< 300 events). We decided not to downgrade for risk of bias as all three studies were judged to be at low risk of bias.
Background
Description of the condition
Molluscum contagiosum is a viral skin infection most frequently encountered in children (Chen 2013). The infection is caused by the molluscum contagiosum virus, which is classified within the family of poxviruses (Poxviridae) (Buller 1991). The virus is assumed to be the only remaining poxvirus that specifically affects human beings (Chen 2013).
Infection follows after contact with infected people or contaminated objects (Chen 2013). Molluscum contagiosum usually presents as single or multiple (usually no more than 20) painless, spherical, shiny, pearly white papules that classically have a central dimple. Their size may vary from tiny 1 mm papules to large nodules over 1 cm in diameter. The lesions may itch (Rogers 1998).
In addition to the common form of benign skin tumours (mostly found in children), there is also a sexually transmitted variant of molluscum contagiosum that occurs on genital, perineal, pubic, and surrounding skin (Czelusta 2000). Molluscum contagiosum lesions may also appear in or around the mouth (Whitaker 1991). Molluscum contagiosum has also been observed with other diseases in people with immune deficiency (Gottlieb 1994; Mansur 2004). People with HIV infection are particularly prone to molluscum contagiosum; prevalence in this population has been reported to range from 5% to 18% (Hira 1988; Husak 1997; Matis 1987). The focus of this review was the common form of molluscum contagiosum only.
Epidemiology
Molluscum contagiosum occurs worldwide. Previous reviews have reported that it as more frequent in geographic areas with warm climates, but this may be due to selective publication of local outbreaks (Olsen 2014). Infection is rare in children under the age of 1 year, typically occurring in the 2‐ to 5‐year‐old age group (Rogers 1998). The age of peak incidence is reported to be between the ages of 2 and 3 years in Fiji (Postlethwaite 1967), and between 1 and 4 years in the Congo (formerly Zaire) (Torfs 1959). In Papua New Guinea the annual incidence rate for children under 10 years of age was 6% (Sturt 1971). Population‐based occurrence rates are scarce for high‐income countries. In a large questionnaire study among parents of children attending kindergartens and elementary schools, the reported prevalence of molluscum contagiosum was 5.6% and 7.4%, respectively (Niizeki 1984). Much higher prevalence rates have been reported during outbreaks in closed communities (Overfield 1966). In 1878 an outbreak in an English school was reported involving 9 children (Liveing 1878). A recent meta‐analysis of five cross‐sectional surveys among children (age range 0 to 16 years) resulted in a pooled prevalence rate of 2.8% (95% confidence interval 0.0 to 5.9) (Olsen 2014).
In the USA, the estimated number of physician visits for molluscum contagiosum from 1990 to 1999 was 280,000 per year (Molino 2004). One out of 6 Dutch children aged 15 years have visited their doctor for molluscum contagiosum at least once (Koning 1994). There is generally no difference in incidence between males and females (Koning 1994; Relyveld 1988; Sturt 1971); however, an unequal sex ratio was found in studies from Japan (Niizeki 1984), Alaska (Overfield 1966), and Fiji (Hawley 1970), where boys were affected more often. This is probably due to habits associated with the spread of the infection, such as swimming (Niizeki 1984; Postlethwaite 1967). Outbreaks may occur among children who bathe or swim together. A history of eczema was found in 62% of children with molluscum contagiosum in Australia (Braue 2005). In the adolescent and adult age groups sexual transmission becomes important.
Natural history
The estimated incubation period varies from 14 days to 6 months (Sterling 1998). Lesions enlarge slowly and may reach a diameter of 5 to 10 mm in 6 to 12 weeks (Sterling 1998). After trauma (e.g. scratching) or spontaneously after several months, inflammatory changes result in the production of white fluid, crusting, and eventual destruction of the lesions. The duration of both the individual lesion and of the entire episode is highly variable. Crops of molluscum may appear to come and go for several months, and although most cases are self limiting and resolve within six to nine months, some may persist for more than three or four years.
A Japanese study described spontaneous resolution on average 6.5 months after infection in 205 out of 217 children (94.5%) affected by molluscum contagiosum (Takemura 1983). One month after the first consultation with the dermatologist, 23% of the children were cured. A recent community cohort study in the UK, Olsen 2015, followed 306 children with molluscum contagiosum aged 4 to 15 years. Only 19% of the children were reported to have received treatment. Mean time to resolution was 13.3 months; 30% had not resolved by 18 months, and 13% had not resolved by 24 months.
Secondary bacterial infection can occur, and when severe can result in scarring. This must be distinguished from the milder inflammatory reactions that molluscum lesions show after a scratching or when they are starting to resolve spontaneously, which may prompt parents to take their child to the general practitioner thinking they have become infected (Highet 1992).
Particularly in atopic people (who are prone to asthma, hay fever, or eczema), there is a tendency for a patch of eczema (which is often particularly itchy) to develop around one or more of the lesions a month or more after their arrival (Beaulieu 2000; De Oreo 1956). Erythema annulare centrifugum (a widespread rash of red inflammatory rings) has also been reported (Vasily 1978). Chronic conjunctivitis and superficial punctate keratitis may likewise complicate lesions on or near the eyelids (Haellmigk 1966; Redmond 2004). The eczema and conjunctivitis diminish naturally when the molluscum lesion is removed.
Molluscum contagiosum behaves differently in HIV‐infected individuals. As immunodeficiency progresses, molluscum contagiosum becomes more common and resistance to therapy increases. Frequently, multiple lesions in atypical areas such as the face and neck can be found (Husak 1997). Only limited data are available on the course of the disease in this group of people.
Description of the intervention
In people without an immune deficiency molluscum contagiosum is a self limiting disease (Chen 2013). Therapy is often not necessary for recovery, and awaiting spontaneous resolution is an important management strategy (Brown 2006; Chen 2013; Jones 2007; Olsen 2015; Takemura 1983). Most lesions resolve within months without scarring in otherwise healthy people (Ordoukhanian 1997). Treatment is intended to accelerate this process. Destruction of the lesions and the production of an inflammatory response are means by which resolution of the lesions could be hastened (Sterling 1998).
Reasons to treat molluscum contagiosum include the following:
alleviating discomfort, including itching;
cosmetic reasons;
social stigma associated with many visible lesions;
limiting its spread to other areas of the body and to other people;
preventing scarring and secondary infection; and
preventing trauma and bleeding of lesions.
There are a large number of treatment options for molluscum contagiosum (see Table 2 for an overview). These treatment options can be divided into three major categories:
1. Treatment modalities and examples of references.
| Treatment class | Treatment modality | Included studies | Other studies |
| 'Doing nothing' | Awaiting natural resolution | — | Olsen 2015; Takemura 1983 |
| Placebo | Antony 2001; Eichenfield 2005; Manchanda 1997b; Paller 2005a; Paller 2005b | — | |
| Surgical treatments | Cryotherapy | Al‐Mutairi 2010 | Barton 2002; Caballero 1996; Salmanpour 2006 |
| Curettage | Hanna 2006; Machado 2010 | de Waard 1990; Simonart 2008 | |
| Curettage with punch | — | Quan 2000 | |
| Electric cauterisation | — | He 2001 | |
| Physical expression (squeezing) | — | Weller 1999 | |
| Pricking | — | Wishart 1903 | |
| Pulsed dye laser | — | Hammes 2001 | |
| Topical treatments | Acidified nitrite | Ormerod 1999 | Gräfe 2000 |
| Adapalene | — | Scheinfeld 2007 | |
| Australian lemon myrtle oil | Burke 2004 | — | |
| Benzoyl peroxide | Saryazdi 2004 | — | |
| Bromogeramine | — | He 2001 | |
| Cantharidin | Coloe Dosal 2014; Hanna 2006 | Funt 1961; Funt 1979; Ross 2004; Silverberg 2000 | |
| Cidofovir | — | Davies 1999; Toro 2000; Zabawski 1999 | |
| Diphencyprone | — | Kang 2005; Kyu 1993 | |
| Griseofulvin | — | Salmanpour 2006 | |
| Honey | — | Holt 2015 | |
| Hydrogen peroxide cream | — | Bigardi 2003; Semkova 2014 | |
| Hyperthermia | — | Gao 2016 | |
| Imiquimod | Al‐Mutairi 2010; Eichenfield 2005; Hanna 2006; Paller 2005a; Paller 2005b; Seo 2010; Theos 2004 | Arican 2006; Barba 2001; Bayerl 2003; Hengge 2003; Lim 2003; Liota 2000; Metkar 2008; Skinner 2000; Skinner 2002; Syed 1998 | |
| Iodine | Markum 2012 | — | |
| Iodine combined with tea tree oil | Markum 2012 | — | |
| Milkweed | — | Behl 1970 | |
| Povidone iodine plus salicylic acid | Markum 2012; Ohkuma 1990 | — | |
| Phenol | Leslie 2005 | Weller 1999 | |
| Podophyllotoxin (HIV patients) | — | Markos 2001; Syed 1994; Teilla‐Hamel 1996 | |
| Potassium hydroxide | Bazza 2007; Machado 2010; Seo 2010; Short 2006; Uçmak 2013 | Metkar 2008; Romiti 1999; Romiti 2000 | |
| Retinoic acid | — | Hund 1975 | |
| Salicylic acid | Hanna 2006; Leslie 2005; Ohkuma 1990 | — | |
| Salicylic acid combined with lactic acid | Machado 2010 | — | |
| Salicylic acid combined with sodium nitrite | Ormerod 1999 | — | |
| Silver nitrate | — | Niizeki 1999 | |
| Tea tree oil | Markum 2012 | — | |
| Tretinoin | Saryazdi 2004 | — | |
| Yellow oxide of mercury | — | Davis 1896 | |
| Systemic treatments | Cimetidine | Antony 2001 | Cunningham 1998; Dohil 1996; Sharma 1998; Yasher 1999 |
| Calcarea carbonica (homeopathy) | Manchanda 1997b | Manchanda 1997a | |
| Griseofulvin | — | Singh 1977 | |
| Combinations of above | Potassium iodide followed by X‐rays | — | Cope 1915 |
physical destruction of the lesions;
topical agents (i.e. those applied directly to the lesions); and
systemic treatment (i.e. those affecting the whole body).
In the past, many authors have recommended physical destruction as the preferred method for treatment of molluscum contagiosum (Smith 2002; Stulberg 2003; Williams 1991). Dermatology textbooks mention removal of the lesion with a sharp curette (curettage) or the application of liquid nitrogen (cryotherapy) as being simple and usually effective treatments (Lowy 1999; Sterling 1998). Gentle squeezing or pricking with a sterile needle are alternatively recommended destructive therapies (Berger 1996). Most of these therapies need to be repeated at three to four weekly intervals. Treatment may be painful and may result in scarring (Friedman 1987). Squeezing of lesions may even lead to the formation of large abscesses due to the disruption of virus into the deeper layer of the skin (dermis) (Brandrup 1989).
Topical preparations such as podophyllotoxin, liquefied phenol, tretinoin, cantharidin, or potassium hydroxide are also used (Hughes 2013; Metkar 2008; Saryazdi 2004; Silverberg 2000; Syed 1994; Weller 1999). In children, prior application of local anaesthetic cream may reduce the pain of treatment involving physical destruction or local inflammation (de Waard 1990; Rosdahl 1988), although severe side effects have been reported in a case of excessive application of lidocaine‐prilocaine (Wieringa 2006). Other proposed topical treatments include immune response modifiers such as imiquimod and cidofovir.
Systemic treatment with cimetidine has been suggested as a possible treatment because of its systemic immunomodulatory effects; it increases lymphocyte proliferation and inhibits suppressor T‐cell function (Orlow 1993; Sterling 1998).
Little data are available with regard to prevailing practice. In a survey among paediatric dermatologists in the USA in 2008, respondents seemed to favour cantharidin, followed by imiquimod, watchful waiting, curettage, and cryotherapy (Coloe 2009). This differs from, for example, general practice in the Netherlands, where waiting for natural resolution is the most popular option (Van der Linden 2005). A more recent survey among physicians from various specialties in the USA showed that treatment preferences differed widely between specialties (Hughes 2013).
How the intervention might work
The working mechanism differs according to the type of treatment (Chen 2013). Curettage aims to remove the lesions entirely. Other techniques like pricking with a needle, cryotherapy, or pulsed‐dye laser aim at damaging the lesion, which may in itself induce an immune response. Topical preparations such as podophyllotoxin, tretinoin, cantharidin, or potassium hydroxide are supposed to evoke a local inflammatory response. Application of phenol or trichloroacetic acid also aims to destroy the lesions. Another topical preparation, imiquimod, supposedly induces an immune response. Cimetidine is a systemic immune modulator. Antiviral agents, especially cidofovir, have been used both systemically and locally (Chen 2013).
Why it is important to do this review
Molluscum contagiosum is a common reason for consultation in family practice and dermatology. Many treatment options are available, some of which are painful and some that may leave scars. A decision may be made in favour of active therapy to prevent further spread, relieve symptoms, prevent scarring, and for cosmetic and social reasons. Indeed, many parents are concerned about the stigma associated with the lesions. Children with molluscum may be excluded from attending nursery and from participating in physical activities such as swimming. However, the scientific basis for treatment is unclear. Consequently, many practitioners find themselves in a dilemma as to whether or not to promote active treatment and, if they do decide on an active treatment strategy, are unclear as to which is the best option. We carried out this systematic review to evaluate treatment options for molluscum contagiosum.
Objectives
To assess the effects of specific treatments and management strategies, including waiting for natural resolution, for cutaneous, non‐genital molluscum contagiosum in people without immune deficiency.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) for the treatment of molluscum contagiosum. We excluded trials on sexually transmitted molluscum contagiosum and in people with an immune deficiency (including those with HIV infection).
Types of participants
People with a diagnosis of molluscum contagiosum, except for those with immune deficiency or sexually transmitted molluscum contagiosum.
In general, treatment was based on a clinical diagnosis only, as molluscum contagiosum is an easy diagnosis to make and confusion is rare among clinicians. We therefore considered additional diagnostic criteria, such as histological examination and laboratory investigations, as unnecessary.
Types of interventions
All treatments aimed at eradicating molluscum contagiosum lesions, including:
physical interventions;
systemic treatments;
topical agents; and
awaiting natural resolution.
We excluded studies on other aspects of the treatment of molluscum contagiosum, for example on reducing pain in the studies that used analgesic EMLA (eutectic mixture of local anaesthetics) cream (de Waard 1990; Juhlin 1980).
Types of outcome measures
Primary outcomes
Short‐term clinical cure (up to three months after start of treatment).
We defined clinical cure as complete disappearance (clearance) of molluscum contagiosum skin lesions, as assessed by a physician.
Secondary outcomes
Medium‐ and long‐term clinical cure (after three months and up to six months after start of treatment, and beyond six months, respectively).
Short‐, medium‐, and long‐term improvement (including cure, intervals as above).
Time to cure.
Recurrences after 3, 6, and 12 months.
Adverse effects of treatment such as pain, blistering, sensitisation, scarring, erosion, and pigmentary changes.
Spread to other people.
Disease‐related quality of life.
Where included studies used the term 'complete clearance' or 'free of lesions' or 'cured or > 90% cleared', we classed these as our primary outcome 'short‐term clinical cure (up to three months after start of treatment)' or our secondary outcome 'medium‐ and long‐term cure (after three months and up to six months, and beyond six months, respectively)', and where they referred to 'partial clearance', we took this to mean our secondary outcome 'improvement'.
We did not initially specify secondary outcomes (2) and (7) in the protocol, but added them afterwards since we considered improvement at the end of the study important, as was disease‐related quality of life. For secondary outcome (2), we would combine 'improvement' and 'cure' (even though cure alone was a seperate outcome) because 'improvement' would be hard to interpret without also including those who were cured. For example: suppose in group A, 30% of participants were cured and another 20% improved. In group B, 40% of participants were cured and 10% improved. Comparing improvement rates between A and B (20% versus 10%) is misleading, whereas combining cure and improvement (50% versus 50%) is not.
Search methods for identification of studies
We aimed to identify all relevant RCTs regardless of language or publication status (published, unpublished, in press, or in progress).
Electronic searches
For this update, we revised all of our search strategies in line with current Cochrane Skin practices. Details of the previous search strategies are available in Van der Wouden 2009.
We searched the following databases up to 21 July 2016:
the Cochrane Skin Group Specialised Register using the search strategy in Appendix 1;
the Cochrane Central Register of Controlled Trials (CENTRAL) 2016, Issue 6, in the Cochrane Library using the strategy in Appendix 2;
MEDLINE via Ovid (from 1946) using the strategy in Appendix 3;
Embase via Ovid (from 1974) using the strategy in Appendix 4; and
LILACS (Latin American and Caribbean Health Service Information database) (from 1982) using the strategy in Appendix 5.
Trials registers
For this update, we searched the following trials registers up to 4 August 2016, using 'molluscum' as the search term:
ISRCTN registry (www.isrctn.com);
ClinicalTrials.gov (www.clinicaltrials.gov);
Australian New Zealand Clinical Trials Registry (www.anzctr.org.au);
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (apps.who.int/trialsearch/);
EU Clinical Trials Register (www.clinicaltrialsregister.eu); and
Netherlands Trial Register (www.trialregister.nl).
We searched Google combining the keyword 'molluscum' with author names of pertinent studies.
Searching other resources
Reference lists
We checked the bibliographies of included studies and review articles for further references to relevant studies.
Correspondence
We obtained further relevant published and unpublished trials via correspondence with selected pharmaceutical companies and authors of recent publications.
Three unpublished studies undertaken by 3M were brought to our attention by Dr Ken Katz, an American dermatologist.
Data collection and analysis
Selection of studies
Two review authors (of SK, RvdS, EK, JCvdW) independently read all abstracts or titles of identified trials. If one of the review authors considered the article to be potentially relevant, we obtained a full‐text copy of the article for further consideration. Two review authors (as before) independently examined all full‐text articles to determine whether or not they met our inclusion criteria. Disagreements were resolved by discussion between the review authors, with referral to a third review author (JCvdW or SK) when necessary.
We excluded trials on sexually transmitted molluscum contagiosum and in people with immune deficiency (including those with HIV infection), in order to increase homogeneity of studies. If the full text of a study was not available, we considered published abstracts for the review.
If an RCT included a variety of skin diseases, of which one was molluscum contagiosum, the number of molluscum participants needed to be at least five in the active treatment and placebo groups. We added this criterion after approval of the protocol when a we found a study that included 10 molluscum participants with a 9:1 distribution over the two treatment groups (Caballero 1996).
If the setting of the study was not explicitly mentioned in the text, we assumed it to be carried out at the affiliation of the first author.
Data extraction and management
Two review authors (of SK, RvdS, EK, JCvdW) independently carried out data extraction using specially developed and piloted data extraction forms. Discrepancies were resolved by a third review author (JCvdW or SK). We obtained missing data from study authors where possible. One review author (JCvdW) entered the data.
As planned in our protocol, the review authors were not blinded to the names of authors, journals, or institutions.
Assessment of risk of bias in included studies
Two review authors (of SK, RvdS, EK, JCvdW) independently assessed the included studies for risk of bias using Cochrane's 'Risk of bias' tool (Higgins 2011). The review authors were not blinded to the names of authors, journals, or institutions. A third review author (JCvdW or SK) acted as arbitrator when necessary. Our assessment included an evaluation of the following components.
Method of generation of the randomisation sequence: it was considered adequate when a computer program or a random number table was used, or a statistician was involved.
Method of allocation concealment: it was considered adequate if the assignment could not be foreseen.
Blinding of participants, clinicians: it was considered adequate when the study was called 'double‐blind' and precautions were taken to mask the nature of the interventions.
Blinding of outcome assessors: it was considered adequate when the study was called 'double‐blind' and it was unlikely that difference in treatment resulted in unmasking (e.g. in the case of staining due to one of the treatments).
Incomplete outcome data addressed (short, medium, and long term): it was scored 'unclear' if not reported and 'high risk' if > 20% of participants lost to follow‐up (short term) or > 30% lost to follow‐up (long term) (Back Review Group 2008).
Free of selective reporting: it was considered adequate if the reported outcomes were similar to those mentioned in the study protocol.
Free of other bias, such as baseline imbalance, compliance, and unit of analysis errors in the case of multiple lesions.
Items (5), (6), and (7) differed from the original protocol or were absent, and were initially adapted for the 2009 update. Items (3) and (4) were combined in one item in the previous versions of this review.
Assessment of quality of the evidence
We used GRADE to assess the overall quality of the evidence for each outcome of each comparison. Starting from 'high quality' (because we only included RCTs), we downgraded the quality for serious study limitations (high risk of bias), indirectness of evidence, serious inconsistency, imprecision of effect estimates (fewer than 300 events), or potential publication bias.
Measures of treatment effect
For dichotomous outcomes, we expressed the results as risk ratios (RR) with 95% confidence intervals (CI) and as a number needed to treat (NNT) only for comparisons with two or more studies and only in case of statistically significant outcomes (the latter in order to avoid confusing confidence interval boundaries). For continuous outcomes, we expressed the results as weighted mean differences (WMD) with 95% CI.
Following the Cochrane Skin Group recommendations, we decided post hoc to re‐analyse results from individual studies with borderline significance and with low numbers of events (fewer than 10 in total) or a total sample size of less than 30, using Fisher’s exact test. The resulting P value was leading in interpreting the results.
Unit of analysis issues
We could expect unit of analysis issues regarding studies potentially using within‐participant comparisons (e.g. split‐body studies) and cross‐over trials. An additional problem of split‐body studies is when a locally applied treatment could induce a systemic response. Our protocol stated that data from these trials were to be analysed using techniques appropriate for paired designs, with the help of a statistician (Van der Wouden 2004).
Dealing with missing data
We attempted to obtain missing data from the trial authors. If this was not possible or not feasible, we used the data as reported.
Assessment of heterogeneity
We planned to explore heterogeneity between the studies using the I² statistic. If substantial heterogeneity (I² statistic > 50%) existed between studies for the primary outcome, we planned to explore the reasons for the heterogeneity, for example by using sensitivity analyses to examine the effects of excluding studies with high risk of bias.
Assessment of reporting biases
We planned to assess reporting bias by comparing the published trial publications with the study protocol.
Data synthesis
For studies with a similar type of intervention, we conducted meta‐analyses to calculate a weighted treatment effect across trials using a random‐effects model (DerSimonian and Laird model) (Higgins 2011).
When the same comparison between two interventions was made in more than one study, and studies appeared to have been executed in similar groups and settings, we used statistical tests for homogeneity between studies. In those studies where the available data were sufficiently homogenous and where a pooled estimate of the treatment effect made sense, we conducted a meta‐analysis.
Where a quantitative synthesis was not possible we provided a narrative synthesis of included trials, presenting the main characteristics of trials and their results.
For studies with a similar type of intervention, we conducted meta‐analyses to calculate a weighted treatment effect across trials using a random‐effects model (DerSimonian and Laird model).
Sensitivity analysis
We planned to use sensitivity analyses to examine the effects of excluding studies with high risk of bias.
Summary of findings
We developed 'Summary of findings' tables subsequent to our protocol. For this update we produced a 'Summary of findings' table for what we believe is the most important comparison of this update: imiquimod versus vehicle.
Results
Description of studies
Results of the search
Our update of the database searches to July 2016 identified 64 records. We identified an additional 15 records from other sources including trial registers, resulting in a total of 79 records to be screened. We excluded 50 records based on titles and abstracts. Of the remaining 29 records, 5 were not available in full text and for 3 others were available in full text but insufficient information was provided to decide on inclusion or exclusion (see Characteristics of studies awaiting classification). We identified one ongoing study (see Characteristics of ongoing studies). We excluded a further nine studies (see Characteristics of excluded studies). We included 11 new studies (see Characteristics of included studies).
We combined these studies with those previously identified for this review, resulting in a total of 22 included studies. A flow diagram summarising the study selection process is shown in Figure 1.
1.
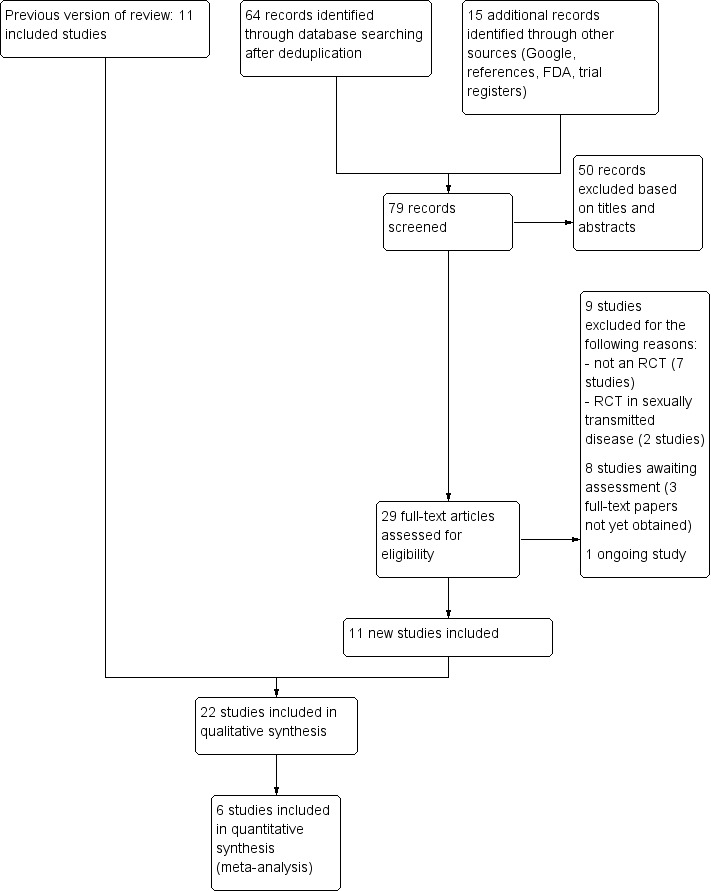
Flow diagram of inclusion for this update.
Included studies
We identified 11 new studies for this update, resulting in a total of 22 included studies. The newly included studies are: Al‐Mutairi 2010; Chathra 2015; Coloe Dosal 2014; Eichenfield 2005; Handjani 2014; Machado 2010; Markum 2012; Paller 2005a; Paller 2005b; Seo 2010; Uçmak 2013.
Setting
Eight trials were performed in North America, five in the UK, eight in Asia, and one in South America (see Characteristics of included studies).
Most included studies were set in hospital outpatient or emergency departments. Only people without immune deficiency (non‐HIV participants) and non‐genital molluscum contagiosum participants were included in the studies. Participants therefore consisted primarily of children and young adults (adolescents).
Participants
The 22 included studies involved a total of 1650 randomised molluscum participants, more than three times as many as in the previous version of this review. The number of participants in each study ranged from 20, in Manchanda 1997b and Short 2006, to 379, in Paller 2005b.
Design
Most of the studies had two trial arms. Four studies had three arms (Leslie 2005; Machado 2010; Markum 2012; Ohkuma 1990), and one study had four arms (Hanna 2006). The number of participants per trial arm ranged from 5, in Ohkuma 1990, to 253, in Paller 2005b. Manchanda 1997b reported on two studies, both making the same comparison but one in a cross‐over design and one in a parallel design. We chose not to include the cross‐over study because fewer than five molluscum participants were assigned to one of the treatment arms. See below for more details on the study designs. Follow‐up duration ranged from 3 to 28 weeks after randomisation. Only five studies had longer than 3 months' follow‐up (Al‐Mutairi 2010; Antony 2001; Eichenfield 2005; Leslie 2005; Paller 2005b).
Publications
We obtained 17 studies as full‐text articles; in five cases these were unpublished manuscripts (Bazza 2007; Eichenfield 2005; Paller 2005a; Paller 2005b; Short 2006). The three unpublished studies by 3M, all addressing the same comparison, imiquimod versus placebo, were brought to our attention by Dr Ken Katz, an American dermatologist. These unpublished studies were mentioned in an FDA summary, Papadopoulos 2007, and subsequently in several journal articles (Katz 2013; Katz 2014; Katz 2015). Upon our request, the director of Meda Pharma BV (Amstelveen, the Netherlands) provided us with the full reports of these three trials.
Two studies were available only as published abstracts (Antony 2001; Saryazdi 2004). We requested and obtained additional information from the authors of several of the included studies (Manchanda 1997b; Ohkuma 1990; Short 2006).
Funding
Five studies reported obtaining commercial funding (Burke 2004; Eichenfield 2005; Markum 2012; Paller 2005a; Paller 2005b); three other studies obtained medication for free from pharmaceutical companies (Hanna 2006; Leslie 2005; Machado 2010); one study reported charity funding (Coloe Dosal 2014); and one study reported receiving no financial support (Chathra 2015). The other 12 studies did not report on funding.
Interventions
Twenty studies evaluated topical therapies for molluscum contagiosum. Two studies included curettage as a treatment arm (Hanna 2006; Machado 2010). Two studies investigated systemic treatments (Antony 2001; Manchanda 1997b). Below we have provided some information regarding interventions, measurements, and loss to follow‐up for each study. For further details, see Characteristics of included studies.
Topical therapy
Three studies by 3M Pharmaceuticals all compared 5% imiquimod with its vehicle in children (Eichenfield 2005; Paller 2005a; Paller 2005b). Paller 2005a used a 1:1 randomisation scheme; the other two studies used a 2:1 scheme. In total, 532 children were randomised to the imiquimod arms and 295 children were randomised to the vehicle arms. Treatment frequency and duration varied from daily for 8 weeks, in Paller 2005a, to 3 times weekly for a maximum of 16 weeks (Eichenfield 2005; Paller 2005b). The total number of evaluable participants was 623, that is 76% of those randomised. Outcomes were lesion clearance, lesion counts, time to complete clearance, and side effects after 4, 8, 12, 16, 18, and 28 weeks after the start of treatment (Eichenfield 2005; Paller 2005b); and lesion clearance, lesion counts, time to complete clearance, and side effects 12 weeks after the start of treatment (Paller 2005a).
Al‐Mutairi 2010 assessed the effect of 5% imiquimod 5 times a week (n = 37) with that of cryospray once a week (n = 37) for a maximum of 16 weeks in children 2 to 12 years of age. No loss to follow‐up was reported. Outcomes were cure at 3, 6, 12, and 16 weeks; cosmetic outcome; and adverse effects.
Bazza 2007 assessed the effect of 5% potassium hydroxide compared to 0.9% saline twice daily. The design was a within‐participant comparison, with treatment randomised to the right or left side of the body. Treatment was continued for a maximum period of three weeks. The study included 30 participants, of whom 20 participants (2 to 12 years of age) completed the study. Outcomes were complete clearance of lesions and side effects after 12 weeks.
Burke 2004 assessed the effect of 10% Australian lemon myrtle tree oil once daily (n = 16). The control group (n = 15) received the vehicle, olive oil. Treatment was continued for 21 days. The mean age of the participants was 4.6 years. Four children were lost to follow‐up. Outcome was complete clearance or greater than 90% reduction in number of lesions after 3 weeks.
Chathra 2015 compared 5% imiquimod (n = 20) to 10% potassium hydroxide (n = 20) 3 times a week for up to 12 weeks. Age range was 1 to 18 years. There was no loss to follow‐up. Outcome was complete clearance after 4, 8, and 12 weeks.
Coloe Dosal 2014 compared 0.7% cantharidin (n = 16) with its vehicle (n = 16) for a maximum of 8 weeks, applied at 5 subsequent visits. Age of the participants was 5 to 10 years. No follow‐up data were available for three children in the 0.7% cantharidin group. Outcomes were complete clearance, lesion counts, and adverse effects at approximately 8 weeks after the start of treatment.
Handjani 2014 compared 10% potassium hydroxide 2 times daily (n = 15) with cryotherapy once weekly (n = 15) for up to 4 weeks. Age of the participants was 1 to 24 years, mean 6.4 years. There was no loss to follow‐up. Outcomes were lesion response and side effects 4 weeks after the start of treatment.
Hanna 2006 compared four treatments: 5% imiquimod (n = 29), 0.7% cantharidin (n = 30), 16.7% salicylic acid/16.7% lactic acid (n = 29), and curettage (physical destruction) (n = 31). Treatment frequency varied and neither treatment duration nor the time point for measuring whether participants were cured was reported. Age of the participants was 1 to 16 years. Loss to follow‐up was unclear. Outcome was the number of visits required. The intervals between study visits were not reported, therefore outcome data were not suitable for analysis.
Leslie 2005 compared the effect of 10% phenol/70% alcohol (n = 41) to 12% salicylic acid (n = 37) and to 70% alcohol (n = 36). Age of the participants was 1 to 15 years. Treatment frequency varied. Participants returned for additional treatment for up to six months. Thirty‐one participants (27%) were lost to follow‐up. Outcome was complete clearance of lesions after six months.
Machado 2010 reported on a study with three arms: 10% potassium hydroxide two times daily (n = 17); 14% salicylic acid plus 14% lactic acid in collodion once daily (n = 16); and curettage (n = 17). The first two groups were treated for 90 days; curettage was performed only once. Age of the participants was 3 to 15 years. No losses to follow‐up were reported. Outcomes were cure and adverse effects 90 days after the start of treatment.
Markum 2012 also reported on a study with three arms: iodine (n = 16); tea tree oil (Melaleuca alternifolia) (n = 18); and tea tree oil combined with iodine (n = 19) twice daily for a maximum of 30 days. The mean age of participants was 6.3 years. A total of five children were lost to follow‐up. Outcomes were cure or reduction in the number of lesions of greater than 90% and adverse effects 30 days after the start of treatment.
Ohkuma 1990 had three intervention arms: 10% povidone iodine solution combined with 50% salicylic acid plaster (n = 20), povidone iodine alone (n = 5), and salicylic plaster alone (n = 10), all once daily. Age of the participants was 2 to 9 years. Outcome was time to cure.
Ormerod 1999 assessed the effect of 5% sodium nitrite co‐applied daily with 5% salicylic acid, under occlusion (n = 16). The control group received an identical cream with 5% salicylic acid but without sodium nitrite (n = 14). Treatment was for three months or until participants were cured or dropped out if sooner. The median age of the participants was 6 years. Outcomes were time to complete resolution and adverse events.
Saryazdi 2004 compared the effect of 10% benzoyl peroxide cream with 0.05% tretinoin cream twice daily for 4 weeks. Participants were children; their age was not specified. The total number of participants was 30; we assumed these were equally divided between the 2 treatments. Outcomes were lesion count, lesion free, and side effects six weeks after the start of treatment.
Seo 2010 compared the effect of once daily 5% imiquimod cream (n = 15) with that of once daily 10% potassium hydroxide solution (n = 15) for a maximum of 12 weeks. Age of the participants was 1 to 36 years. Three participants were lost to follow‐up. Outcomes were cure and adverse effects after 2, 4, 8, and 12 weeks.
Short 2006 assessed the application of a 10% potassium hydroxide solution (n = 10) twice daily. The control group received saline (n = 10). Assessment of the therapeutic response took place up to 90 days after the start of treatment or 1 month after clearance, or both. Age of the participants was 2 to 9 years. One child was lost to follow‐up. Outcomes were time to resolution and adverse events three months after the start of treatment.
Theos 2004 assessed the effect of 5% imiquimod (n = 12) versus vehicle (n = 11) 3 times a week for up to 12 weeks. Participants were assessed every 2 weeks after treatment initiation, for up to 12 weeks. Age of the participants was 1 to 9 years. Two children were lost to follow‐up. Outcomes were complete and partial clearance and adverse events after 4, 8, and 12 weeks.
Uçmak 2013 compared 2.5% potassium hydroxide (n = 14) with 5% potassium hydroxide (n = 15) twice daily for 60 days. Age of the participants was 1 to 18 years. Three participants were lost to follow‐up. Outcomes were cure and adverse effects after 15, 30, 45, and 60 days.
Systemic therapy
Antony 2001 assessed the effect of 35 mg/kg/day of cimetidine given once daily as an oral suspension for three months. Thirty‐eight participants, aged 1 to 16 years, were enrolled in the trial, but assignment details were provided only for the 19 participants who completed the study. Eight of these participants were randomised to the treatment arm of the trial. The 11 participants in the control arm received a matched oral suspension. The follow‐up period was four months from the start of treatment. Outcomes were complete clearance after four months of treatment and reduction of lesions.
Manchanda 1997b evaluated different potencies of the homoeopathic drug calcarea carbonica given daily for 15 days (n = 14). Six participants were randomised to receive plain sugar globules as a placebo. Age of the participants was 0 to 30 years. Follow‐up duration was not reported. Outcome was improvement (not clear after what period).
Excluded studies
The most common reasons why studies were excluded was that they were case series rather than RCTs, or because the participant groups were outside the focus of the review. See the 'Characteristics of excluded studies' tables.
Studies awaiting classification
We have assigned eight studies to awaiting classification. Two studies classified as ongoing in the previous version of this review have likely been completed (NCT01348386; NCT02665260), however we are unaware of papers describing the results of these studies. For three other studies identified in the current search but rather old, we were unsuccessful in obtaining full‐text papers, and abstracts are missing as well (Elzawahry 1964; Tanissa 1951; Unknown Chinese author 1991). For the remaining three studies, full‐text papers were available, but insufficient information was provided to decide on inclusion or exclusion (Köse 2013; Muzaffar 2014; Rajouria 2011). All three studies compared topical treatments: a Turkish study compared a 10% potassium hydroxide solution with a combination of salicylic and lactic acid; a study from Pakistan compared two different potassium hydroxide solutions (5% versus 10%); and a study from Nepal compared 5% potassium hydroxide solution with 0.05% tretinoin cream. We have contacted the authors for additional information, but have been unsuccessful thus far.
Ongoing studies
We found one ongoing study comparing topical treatments: 10% sandalwood cream versus placebo cream. It is being conducted in the USA (NCT02024581).
Risk of bias in included studies
Figure 2 and Figure 3 show the results of the 'Risk of bias' assessment. Reasons for the choices that were made for each individual study can be found in the Characteristics of included studies. Overall, most study reports provided insufficient information to judge risk of bias, especially for allocation concealment and selective reporting. We considered only five studies to be at low risk of bias: Coloe Dosal 2014; Eichenfield 2005; Markum 2012; Paller 2005a; Paller 2005b; all of these studies were identified during the 2016 update.
2.
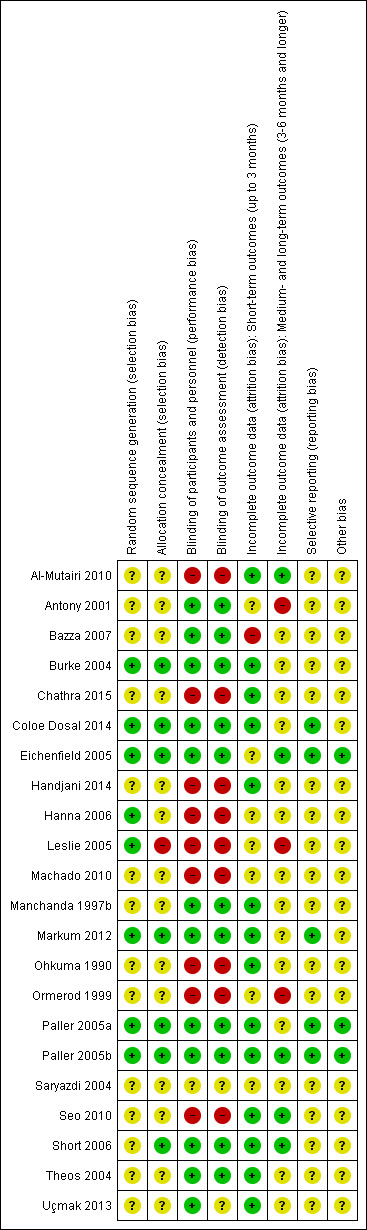
Risk of bias table: review authors' judgements about each methodological quality item for each included study.
3.
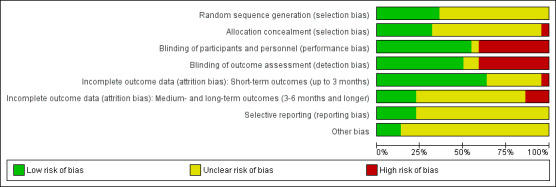
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Twenty studies were described in the text as randomised trials. We obtained additional information from the authors of the other two papers, who confirmed in writing that the participants were randomised into the different treatment groups (Manchanda 1997b; Ohkuma 1990). Eight papers described the way the randomisation sequence was generated (Burke 2004; Coloe Dosal 2014; Eichenfield 2005; Hanna 2006; Leslie 2005; Markum 2012; Paller 2005a; Paller 2005b), which we judged as at low risk of bias (Figure 2). In a personal communication, Manchanda informed us that in his study this was "generated manually" (Manchanda 1997b).
Only seven studies described whether the investigators took any precautions to conceal the allocation schedule from those involved in entering participants into the study (Burke 2004; Coloe Dosal 2014; Eichenfield 2005; Markum 2012; Paller 2005a; Paller 2005b; Short 2006).
Blinding
Eleven of the studies were described as double‐blind. However, only four of these studies provided information about the similarity in appearance and smell of treatments (Burke 2004; Coloe Dosal 2014; Manchanda 1997b; Markum 2012). In four so‐called vehicle‐controlled studies, visual similarity was not explicitly stated but can be assumed (Eichenfield 2005; Paller 2005a; Paller 2005b; Theos 2004). None of the papers provided information on whether blinding was maintained throughout follow‐up. Ormerod reported brown staining on the skin in six participants with active treatment, but none of the controls, which may have unblinded outcome assessment (Ormerod 1999). In some studies blinding was impossible due to the comparison that was made (e.g. topical cream versus cryospray (Al‐Mutairi 2010)). Blinding was unclear in Saryazdi 2004. In Uçmak 2013, participants seemed to be blinded but for the outcome assessors this was unclear. We considered the following studies not to be (fully) blinded: Al‐Mutairi 2010; Chathra 2015; Handjani 2014; Hanna 2006; Leslie 2005; Ohkuma 1990; Ormerod 1999; Seo 2010. Further details are provided in Characteristics of included studies.
Incomplete outcome data
In Figure 2, we have reported this item separately for short‐term outcomes and longer‐term outcomes. For short‐term outcomes, only one study reported a loss to follow‐up of more than 20% (Bazza 2007). Six studies did not report any loss to follow‐up (Al‐Mutairi 2010; Chathra 2015; Handjani 2014; Machado 2010; Ohkuma 1990; Saryazdi 2004). For longer‐term outcomes, three studies reported a loss to follow‐up of over 30%.
Selective reporting
We could not evaluate selective reporting for most of the included studies because neither a study protocol was available, nor a trial register providing information on outcomes. We judged five studies to be free of reporting bias (Coloe Dosal 2014; Eichenfield 2005; Markum 2012; Paller 2005a; Paller 2005b). Several studies reported that efficacy was assessed at several time points but results were provided only at the last time point. We did not qualify this as high risk of bias.
Other potential sources of bias
For most studies, it was unclear whether other sources of bias played a role, because no information was provided on baseline characteristics or adherence to the treatment protocol (Figure 2). One study reported that the treatment adherence differed between study arms (Machado 2010), and other studies reported considerable baseline imbalances for lesion count (Theos 2004), sex (Seo 2010), and skin dryness (Coloe Dosal 2014), but the differences were not statistically significant. We judged three studies to be at low risk of bias for this item because there was no relevant baseline imbalance and information was provided on adherence to the treatment protocol (Eichenfield 2005; Paller 2005a; Paller 2005b). For funding sources of studies, see above under Funding. We did not take the source of funding into account for the 'Risk of bias' assessment.
Effects of interventions
See: Table 1
Our prespecified outcomes were as follows.
Primary outcomes
Short‐term clinical cure (up to three months after the start of treatment).
We defined clinical cure as complete disappearance (clearance) of molluscum contagiosum skin lesions, as assessed by a physician.
Secondary outcomes
Medium‐ and long‐term clinical cure (after three months and up to six months after start of treatment, and beyond six months, respectively).
Short‐, medium‐, and long‐term improvement (including cure, intervals as above).
Time to cure.
Recurrences after 3, 6, and 12 months.
Adverse effects of treatment such as pain, blistering, sensitisation, scarring, erosion, and pigmentary changes.
Spread to other people.
Disease‐related quality of life.
We describe here the prespecified outcomes found for all comparisons. Comparisons are sorted by type of treatment. Only two comparisons were addressed by more than one study and allowed pooling of study data: topical imiquimod versus vehicle cream (four studies), and imiquimod versus potassium hydroxide (two studies). For each comparison we have only reported the outcomes that were addressed in the included studies. If an outcome is not reported on, this is because the outcome was not assessed by the study/studies within that comparison.
Where included studies used the term 'complete clearance' or 'free of lesions' or 'cured or > 90% cleared', we classed these as our primary outcome 'short‐term clinical cure (up to three months after start of treatment)' or our secondary outcome 'medium‐ and long‐term cure (after three months and up to six months, and after six months, respectively)', and where they referred to 'partial clearance', we took this to mean our secondary outcome 'improvement'.
We could not include the results of the study by Hanna and colleagues in the analysis, as the outcome (cure rate) was reported only in number of visits, without stating at what time these visits took place (Hanna 2006). Four studies did not report adverse or side effects (Antony 2001; Leslie 2005; Manchanda 1997b; Ohkuma 1990). One study reported the rates but not the nature of the adverse effects (Hanna 2006).
See Table 1 for our grading of the evidence for the comparison 'imiquimod versus vehicle'.
Topical treatments
Two of our secondary outcomes were not reported in any of the studies: 6. Spread to other people and 7. Disease‐related quality of life. Only five studies reported on our secondary outcome 3. Time to cure (Eichenfield 2005; Paller 2005b; Ohkuma 1990; Ormerod 1999; Short 2006). With regard to our secondary outcome 5. Adverse effects of treatment such as pain, blistering, sensitisation, scarring, erosion, and pigmentary changes, three studies reported separately on: any side effect, application site reactions, and severe application site reactions (Eichenfield 2005; Paller 2005a; Paller 2005b).
Imiquimod versus vehicle
The quality of the evidence for this comparison was moderate to high, depending on the outcome; see Table 1 for details.
Primary outcomes
1. Short‐term clinical cure (up to three months after the start of treatment)
Application of 5% imiquimod cream resulted in complete clearance in 79/544 participants versus 36/306 of the control group, who received vehicle cream (4 studies, 850 participants, risk ratio (RR) 1.33, 95% confidence interval (CI) 0.92 to 1.93, moderate‐quality evidence for little or no difference, Analysis 1.1) (time point: 12 weeks after start of treatment) (Eichenfield 2005; Paller 2005a; Paller 2005b; Theos 2004). The pooled analysis showed no heterogeneity (I² = 0%).
1.1. Analysis.
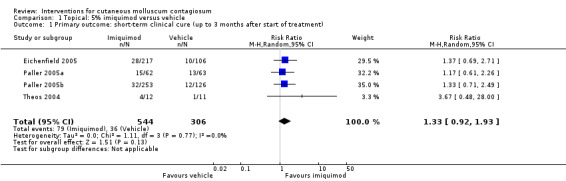
Comparison 1 Topical: 5% imiquimod versus vehicle, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).
Secondary outcomes
1.Medium‐ and long‐term cure (after three months and up to six months, and beyond six months, respectively)
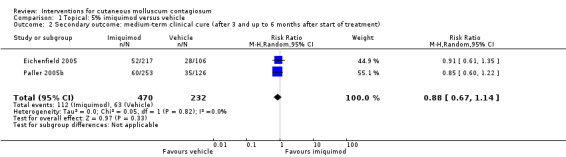
Eighteen weeks after start of treatment, application of 5% imiquimod cream resulted in complete clearance in 112/470 participants versus 63/232 participants in the control (vehicle) group (2 studies, RR 0.88, 95% CI 0.67 to 1.14, I² = 0%, moderate‐quality evidence for no clear difference, Analysis 1.2) (Eichenfield 2005; Paller 2005b).
1.2. Analysis.
Comparison 1 Topical: 5% imiquimod versus vehicle, Outcome 2 Secondary outcome: medium‐term clinical cure (after 3 and up to 6 months after start of treatment).
Twenty‐eight weeks after start of treatment, application of 5% imiquimod cream resulted in complete clearance in 180/470 participants versus 92/232 participants in the control (vehicle) group (2 studies, RR 0.97, 95% CI 0.79 to 1.17, I² = 0%, moderate‐quality evidence for no difference, Analysis 1.3) (Eichenfield 2005; Paller 2005b).
1.3. Analysis.
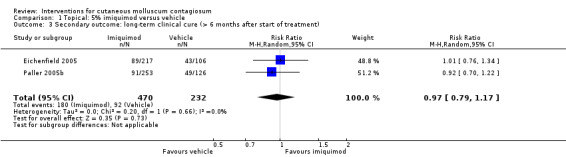
Comparison 1 Topical: 5% imiquimod versus vehicle, Outcome 3 Secondary outcome: long‐term clinical cure (> 6 months after start of treatment).
2. Improvement
Twelve weeks after start of treatment, application of 5% imiquimod cream resulted in partial or complete clearance in 273/544 participants versus 149/306 participants in the control (vehicle) group (4 studies, RR 1.14, 95% CI 0.89 to 1.47, high‐quality evidence for little or no difference, Analysis 1.4) (Eichenfield 2005; Paller 2005a; Paller 2005b; Theos 2004). The I² value was 65%, showing substantial heterogeneity. Exploring heterogeneity, in the forest plot the small study Theos 2004 showed as a clear outlier. Excluding this study reduced I² to 10%, and resulted in a small change of the pooled outcome (RR 1.05, 95% CI 0.91 to 1.21).
1.4. Analysis.
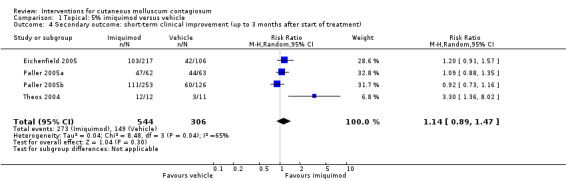
Comparison 1 Topical: 5% imiquimod versus vehicle, Outcome 4 Secondary outcome: short‐term clinical improvement (up to 3 months after start of treatment).
Eighteen weeks after start of treatment, application of 5% imiquimod cream resulted in partial or complete clearance in 341/470 participants versus 174/232 participants in the control (vehicle) group (2 studies, RR 0.97, 95% CI 0.87 to 1.08, I² = 26%, high‐quality evidence for no difference, Analysis 1.5) (Eichenfield 2005; Paller 2005b).
1.5. Analysis.
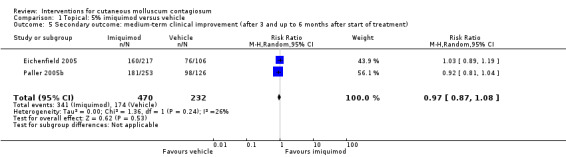
Comparison 1 Topical: 5% imiquimod versus vehicle, Outcome 5 Secondary outcome: medium‐term clinical improvement (after 3 and up to 6 months after start of treatment).
3. Time to cure
In two unpublished studies, the median time to cure was the same in the group treated with 5% imiquimod and the group treated with its vehicle: 16 weeks in Eichenfield 2005 and 18 weeks in Paller 2005b (high‐quality evidence).
4. Recurrence
In two unpublished studies, recurrence was observed in 1/52 and 3/60 in the group treated with 5% imiquimod and 0/28 and 0/35 in the group treated with its vehicle after 28 weeks (Eichenfield 2005 and Paller 2005b, respectively) (RR 2.70, 95% CI 0.31 to 23.23, I² = 0%, moderate‐quality evidence, Analysis 1.6).
1.6. Analysis.
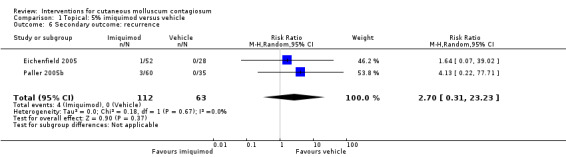
Comparison 1 Topical: 5% imiquimod versus vehicle, Outcome 6 Secondary outcome: recurrence.
5. Adverse effects of treatment such as pain, blistering, sensitisation, scarring, erosion, and pigmentary changes
Three of the four related studies comparing topical 5% imiquimod with vehicle reported extensively on adverse effects (Eichenfield 2005; Paller 2005a; Paller 2005b); we had copies of the full trial reports of the 3M studies as submitted to the US Food and Drug Administration. In the imiquimod group of Theos 2004, pruritis was reported by 6/12 participants versus 5/11 in the vehicle group. Pain/tenderness was reported by one participant in each group. Other reported side effects were not interpretable because of unclear denominators.
In Eichenfield 2005, any side effect was reported in 149/217 versus 78/106 participants in the 5% imiquimod and vehicle group, respectively; application site reactions were reported in 77/217 versus 21/106 participants; 7 versus 1 of these were qualified as severe. In Paller 2005a, any side effect was reported in 42/62 versus 41/63 participants in the 5% imiquimod and vehicle group, respectively; application site reactions were reported in 32/62 versus 25/63 participants; 6 versus 1 of these were qualified as severe. In Paller 2005b, any side effect was reported in 166/253 versus 84/126 participants in the 5% imiquimod and vehicle group, respectively; application site reactions were reported in 80/253 versus 31/126 participants; 3 versus 0 of these were qualified as severe. We pooled the results of the three 3M studies, with 827 evaluable participants. For any adverse effect, the pooled RR was 0.97 (95% CI 0.88 to 1.07, I² = 0%, high‐quality evidence for no difference, Analysis 1.7). For application site reactions, the pooled RR was 1.41 (95% CI 1.13 to 1.77, I² = 0%, moderate‐quality evidence for probably more harm for imiquimod, Analysis 1.8). The proportion of participants experiencing an application site reaction was 189/532 (36%) versus 77/295 (26%), giving a number needed to treat for an additional harmful outcome (NNTH) of 11. For severe application site reactions, the pooled RR was 4.33 (95% CI 1.16 to 16.19, I² = 0%, moderate‐quality evidence for probably more harm for imiquimod, Analysis 1.9). The proportion of participants experiencing a severe application site reaction was 16/532 (3%) versus 2/295 (0.7%), giving a NNTH of over 40.
1.7. Analysis.
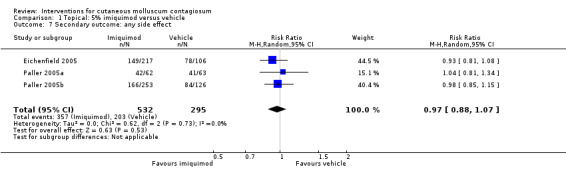
Comparison 1 Topical: 5% imiquimod versus vehicle, Outcome 7 Secondary outcome: any side effect.
1.8. Analysis.
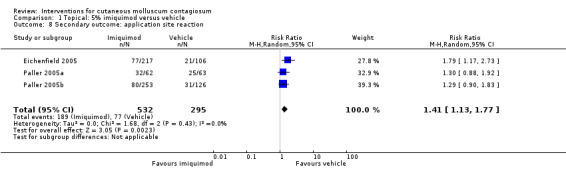
Comparison 1 Topical: 5% imiquimod versus vehicle, Outcome 8 Secondary outcome: application site reaction.
1.9. Analysis.
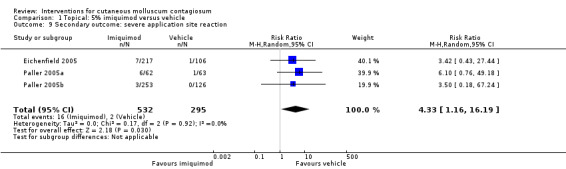
Comparison 1 Topical: 5% imiquimod versus vehicle, Outcome 9 Secondary outcome: severe application site reaction.
Imiquimod versus cryospray
We considered the evidence for all outcomes for this comparison to be of low quality due to small study size and serious risk of bias.
Primary outcomes
1. Short‐term clinical cure (up to three months after the start of treatment)
Application of 5% imiquimod cream resulted in complete clearance in 22/37 participants versus 37/37 participants who received cryospray after 6 weeks (RR 0.60, 95% CI 0.46 to 0.78, Analysis 2.1) (Al‐Mutairi 2010). (We selected the 6‐week time point for this analysis because the 12‐week time point was close to the 16‐week time point ‐ see below).
2.1. Analysis.
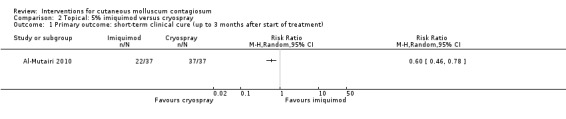
Comparison 2 Topical: 5% imiquimod versus cryospray, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).
Secondary outcomes
1. Medium‐ and long‐term cure (after three months and up to six months, and beyond six months, respectively)
Application of 5% imiquimod cream resulted in complete clearance in 34/37 participants versus 37/37 participants who received cryospray after 16 weeks (RR 0.92, 95% CI 0.83 to 1.20, Analysis 2.2) (Al‐Mutairi 2010).
2.2. Analysis.
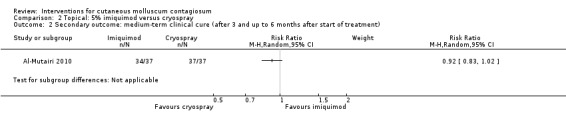
Comparison 2 Topical: 5% imiquimod versus cryospray, Outcome 2 Secondary outcome: medium‐term clinical cure (after 3 and up to 6 months after start of treatment).
4. Recurrence
Al‐Mutairi 2010 reported 0/37 in the imiquimod group and 3/37 in the cryospray group with recurrence after 5 months (RR 0.14, 95% CI 0.01 to 2.67, Analysis 2.3).
2.3. Analysis.
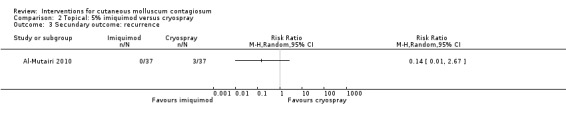
Comparison 2 Topical: 5% imiquimod versus cryospray, Outcome 3 Secundary outcome: recurrence.
5. Adverse effects of treatment such as pain, blistering, sensitisation, scarring, erosion, and pigmentary changes
In the 5% imiquimod group of Al‐Mutairi 2010, 27/37 participants reported pain during application; 28/37, erythema; 9/37, itching; 5/37, a burning sensation; and 2/37, pigmentary changes. In the cryospray group, 22/37 participants reported pain during application; 34/37, a burning sensation; 18/37, erosions; 17/37, erythema; 11/37, itching; 9/37, vesicles/bullae; 15/37, pigmentary changes; and 8/37, scarring/atrophy.
Imiquimod versus potassium hydroxide
We considered the evidence for all outcomes for this comparison to be of low quality due to small study size and serious risk of bias.
Primary outcomes
1. Short‐term clinical cure (up to three months after the start of treatment)
Two small studies compared 5% imiquimod cream with 10% potassium hydroxide (Chathra 2015; Seo 2010), resulting in complete clearance 12 weeks after start of treatment in 18/34 (imiquimod) versus 27/33 (potassium hydroxide) (RR 0.65, 95% CI 0.46 to 0.93), favouring 10% potassium hydroxide (I² = 0%) and resulting in a number needed to treat for an additional beneficial outcome of 3 (Analysis 3.1).
3.1. Analysis.
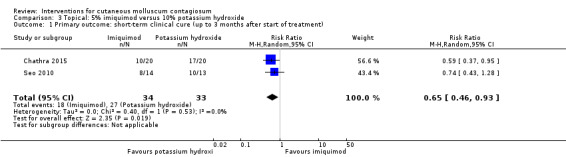
Comparison 3 Topical: 5% imiquimod versus 10% potassium hydroxide, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).
Secondary outcomes
5. Adverse effects of treatment such as pain, blistering, sensitisation, scarring, erosion, and pigmentary changes.
Two small studies compared 5% imiquimod cream with 10% potassium hydroxide (Chathra 2015; Seo 2010). In the 5% imiquimod group 10/33 participants reported adverse effects (erythema, burning, itching, ulceration, scaling, hypo‐ and hyperpigmentation), versus 16/34 in the 10% potassium hydroxide group (RR 0.68, 95% CI 0.25 to 1.81, Analysis 3.2). The I² value was 57%, showing substantial heterogeneity; however, as only two studies were included in the analysis we were unable to investigate heterogeneity.
3.2. Analysis.
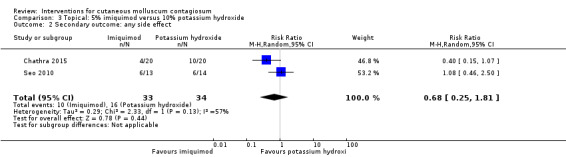
Comparison 3 Topical: 5% imiquimod versus 10% potassium hydroxide, Outcome 2 Secondary outcome: any side effect.
Lemon myrtle oil versus olive oil
We considered the evidence for all outcomes for this comparison to be of low quality due to small study size and serious imprecision.
Primary outcomes
1. Short‐term clinical cure (up to three months after the start of treatment)
Application of 10% lemon myrtle oil resulted in complete disappearance (or reduction of > 90% of lesions) after three weeks in 9/16 participants versus 0/15 of the control group, who received only the vehicle (olive oil) (RR 17.88, 95% CI 1.13 to 282.72, Analysis 4.1) (Burke 2004). The Fisher exact test resulted in a P value of 0.001.
4.1. Analysis.
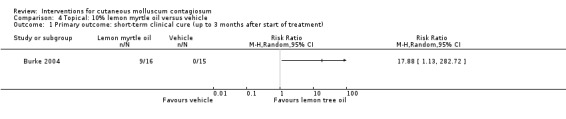
Comparison 4 Topical: 10% lemon myrtle oil versus vehicle, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).
Secondary outcomes
5. Adverse effects of treatment such as pain, blistering, sensitisation, scarring, erosion, and pigmentary changes.
Application of 10% lemon myrtle oil resulted in local redness in 2/16 participants versus 1/15 participants in the control (vehicle) group (Burke 2004).
Benzoyl peroxide versus tretinoin
We considered the evidence for the outcome for this comparison to be of low quality due to small study size, unknown risk of bias, and imprecision.
Primary outcomes
1. Short‐term clinical cure (up to three months after the start of treatment)
Application of 10% benzoyl peroxide resulted in complete disappearance of lesions after six weeks in 11/15 participants compared to 5/15 participants who received 0.05% tretinoin (RR 2.20, 95% CI 1.01 to 4.79, Analysis 5.1) (Saryazdi 2004).
5.1. Analysis.
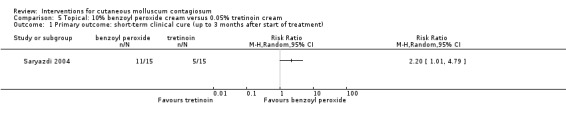
Comparison 5 Topical: 10% benzoyl peroxide cream versus 0.05% tretinoin cream, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).
Potassium hydroxide versus saline
We considered the evidence for all outcomes for this comparison to be of low quality due to small study size, serious risk of bias, and imprecision.
Primary outcomes
1. Short‐term clinical cure (up to three months after the start of treatment)
The Bazza 2007 study randomised treatments to the right or left side of the body, comparing application of 5% potassium hydroxide to 0.9% saline. On both sides of the body, 17/20 participants showed complete clearance. Due to the absence of paired data in the study report, it was impossible to take the split‐body design into account in the analysis, therefore we did not pool the study results with those of Short 2006.
Short 2006 made a similar comparison, showing 10% potassium hydroxide solution to be successful in 7/10 participants (70%) compared with 2/10 (20%) in the saline group, which was not statistically significant (RR 3.50, 95% CI 0.95 to 12.90, Analysis 6.1). The Fisher exact test resulted in a P value of 0.07.
6.1. Analysis.
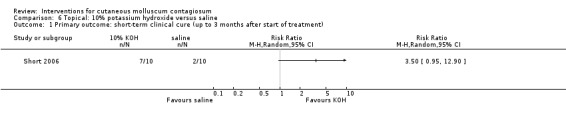
Comparison 6 Topical: 10% potassium hydroxide versus saline, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).
Secondary outcomes
5. Adverse effects of treatment such as pain, blistering, sensitisation, scarring, erosion, and pigmentary changes
In Short 2006 most participants treated with 10% potassium hydroxide reported mild stinging, and two participants reported severe stinging, one of whom withdrew. Also, two participants developed post‐inflammatory pigmentary changes at the treatment site (unclear in which group). No participants in the saline group withdrew due to side effects.
In Bazza 2007 12/20 participants reported mild to moderate stinging; 2/20, severe stinging; and 2/20, hypopigmentation in the group treated with 5% potassium hydroxide. In the group receiving topical 0.9% saline, 8/20 participants reported mild to moderate stinging and 2/20 reported hypopigmentation.
Potassium hydroxide versus potassium hydroxide
We considered the evidence for all outcomes for this comparison to be of low quality due to small study size, serious risk of bias, and imprecision.
Primary outcome
1. Short‐term clinical cure (up to three months after the start of treatment)
Uçmak 2013 compared 2.5% and 5% solutions of potassium hydroxide, and found complete clearance in 3/13 versus 8/12 participants after 60 days (RR 0.35, 95% CI 0.12 to 1.01, Analysis 7.1), in favour of the 5% solution. Fisher's exact test resulted in a P value of 0.047.
7.1. Analysis.
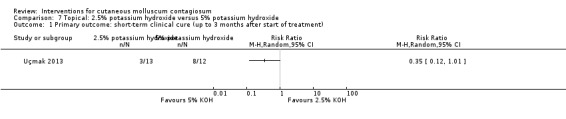
Comparison 7 Topical: 2.5% potassium hydroxide versus 5% potassium hydroxide, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).
Secondary outcomes
2. Improvement
Uçmak 2013 compared 2.5% and 5% solutions of potassium hydroxide, and found clinical cure or improvement in 8/13 versus 10/12 participants after 60 days (RR 0.74, 95% CI 0.45 to 1.22, Analysis 7.2), favouring the 5% solution.
7.2. Analysis.
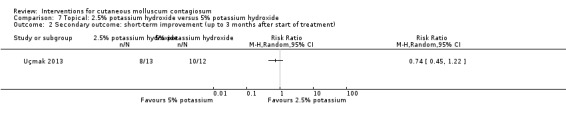
Comparison 7 Topical: 2.5% potassium hydroxide versus 5% potassium hydroxide, Outcome 2 Secondary outcome: short‐term improvement (up to 3 months after start of treatment).
5. Adverse effects of treatment such as pain, blistering, sensitisation, scarring, erosion, and pigmentary changes
Uçmak 2013 compared 2.5% and 5% solutions of potassium hydroxide, and found at least one adverse effect at 15 days in 11/13 versus 11/12 participants; at 60 days: 6/13 versus 5/12, respectively. The most common adverse effect was a burning sensation.
Potassium hydroxide versus salicylic acid plus lactic acid
We considered the evidence for all outcomes for this comparison to be of low quality due to small study size, serious risk of bias, and imprecision.
Primary outcome
1. Short‐term clinical cure (up to three months after the start of treatment)
Machado 2010 compared 10% potassium hydroxide to a combination of 14% salicylic acid plus 14% lactic acid, and found 14/17 versus 15/16 participants were cured after a maximum of 90 days treatment (RR 0.88, 95% CI 0.68 to 1.13, Analysis 8.1).
8.1. Analysis.
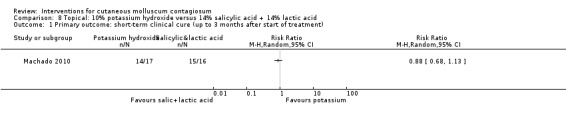
Comparison 8 Topical: 10% potassium hydroxide versus 14% salicylic acid + 14% lactic acid, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).
Secondary outcomes
5. Adverse effects of treatment such as pain, blistering, sensitisation, scarring, erosion, and pigmentary changes.
In the 10% potassium hydroxide group of Machado 2010, 10/17 participants reported moderate pain; in the group treated with 14% salicylic acid plus 14% lactic acid, 8/16 reported mild pain.
Potassium hydroxide versus curettage
We considered the evidence for all outcomes for this comparison to be of low quality due to small study size, serious risk of bias, and imprecision.
Primary outcome
1. Short‐term clinical cure (up to three months after the start of treatment)
Machado 2010 compared 10% potassium hydroxide to curettage, and found 14/17 versus 15/17 participants were cured after a maximum of 90 days treatment (RR 0.93, 95% CI 0.71 to 1.24, Analysis 9.1).
9.1. Analysis.
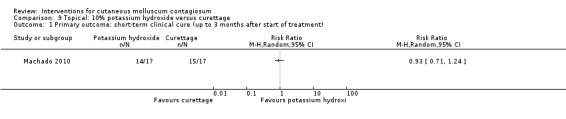
Comparison 9 Topical: 10% potassium hydroxide versus curettage, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).
Secondary outcomes
5. Adverse effects of treatment such as pain, blistering, sensitisation, scarring, erosion, and pigmentary changes
In the 10% potassium hydroxide group of Machado 2010, 10/17 participants reported moderate pain; in the curettage group, 12/17 reported mild pain.
Potassium hydroxide versus cryotherapy
We considered the evidence for all outcomes for this comparison to be of low quality due to small study size and serious risk of bias.
Primary outcome
1. Short‐term clinical cure (up to three months after the start of treatment)
Handjani 2014 compared 10% potassium hydroxide to cryotherapy, and found 13/15 versus 14/15 participants were cured after a maximum of 4 weeks treatment (RR 0.93, 95% CI 0.73 to 1.18, Analysis 10.1).
10.1. Analysis.
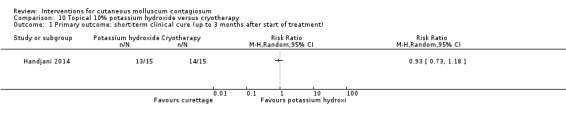
Comparison 10 Topical 10% potassium hydroxide versus cryotherapy, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).
Secondary outcomes
2. Improvement
Handjani 2014 compared 10% potassium hydroxide to cryotherapy, and found 14/15 versus 15/15 participants were cured or improved after a maximum of 4 weeks treatment (RR 0.94, 95% CI 0.78 to 1.12, Analysis 10.2).
10.2. Analysis.
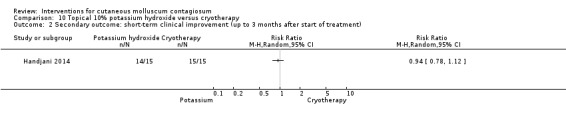
Comparison 10 Topical 10% potassium hydroxide versus cryotherapy, Outcome 2 Secondary outcome: short‐term clinical improvement (up to 3 months after start of treatment).
5. Adverse effects of treatment such as pain, blistering, sensitisation, scarring, erosion, and pigmentary changes
Handjani 2014 found hyperpigmentation in 4/15 participants (10% potassium hydroxide) and 7/15 (cryotherapy), and hypopigmentation in 6/15 (10% potassium hydroxide) and 5/15 (cryotherapy).
Povidone iodine versus salicylic acid plaster
We considered the evidence for all outcomes for this comparison to be of low quality due to small study size, serious risk of bias, and imprecision.
Primary outcome
1. Short‐term clinical cure (up to three months after the start of treatment)
Application of 10% povidone iodine was effective in 3/5 participants (60%) compared to 7/10 (70%) who received salicylic acid plaster alone (RR 0.86, 95% CI 0.38 to 1.95, not statistically significant) (Ohkuma 1990) (Analysis 11.1). (Please note that this study did not clearly state when cure was measured but stated "treatment continued as long as necessary, range was 7‐64 days, mean 26 days".)
11.1. Analysis.
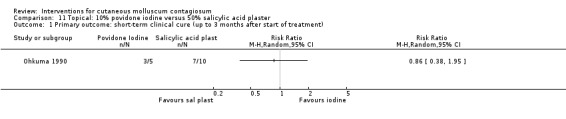
Comparison 11 Topical: 10% povidone iodine versus 50% salicylic acid plaster, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).
Secondary outcomes
3. Time to cure
Application of 10% povidone iodine resulted in a mean time to cure of 86 days versus 47 days for the group receiving salicylic acid plaster.
Povidone iodine versus povidone iodine plus salicylic acid plaster
We considered the evidence for all outcomes for this comparison to be of low quality due to small study size, serious risk of bias, and imprecision.
Primary outcome
1. Short‐term clinical cure (up to three months after the start of treatment)
Application of 10% povidone iodine solution was effective in 3/5 participants (60%) compared with 20/20 (100%) who received 10% povidone iodine plus salicylic acid plaster (RR 0.60, 95% CI 0.30 to 1.18, Analysis 12.1) (Ohkuma 1990). See note above after results of Analysis 11.1.
12.1. Analysis.
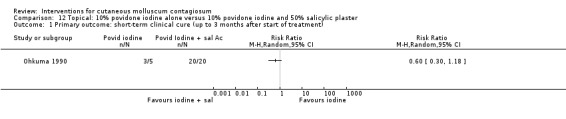
Comparison 12 Topical: 10% povidone iodine alone versus 10% povidone iodine and 50% salicylic plaster, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).
Secondary outcomes
3. Time to cure
Application of 10% povidone iodine resulted in a mean time to cure of 86 days versus 26 days for the group receiving 10% povidone iodine plus salicylic acid plaster.
Povidone iodine plus salicylic acid plaster versus salicylic acid plaster
We considered the evidence for all outcomes for this comparison to be of low quality due to small study size, serious risk of bias, and imprecision.
Primary outcome
1. Short‐term clinical cure (up to three months after the start of treatment)
Application of 10% povidone iodine solution plus 50% salicylic acid plaster was effective in curing 20/20 participants (100%) compared with 7/10 (70%) who received salicylic acid plaster alone (RR 1.43, 95% CI 0.95 to 2.16, Analysis 13.1) (Ohkuma 1990). Fisher's exact test resulted in a P value of 0.03. See note above after results of Analysis 11.1.
13.1. Analysis.
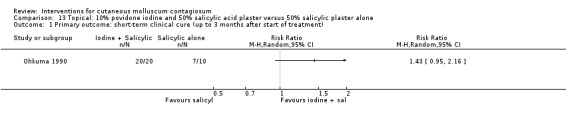
Comparison 13 Topical: 10% povidone iodine and 50% salicylic acid plaster versus 50% salicylic plaster alone, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).
Secondary outcomes
3. Time to cure
Application of 10% povidone iodine plus salicylic acid plaster resulted in a mean time to cure of 26 days versus 47 days for the group receiving salicylic acid plaster (Ohkuma 1990).
Cantharidin versus vehicle
We considered the evidence for all outcomes for this comparison to be of low quality due to small study size and imprecision.
Primary outcome
1. Short‐term clinical cure (up to three months after the start of treatment)
Coloe Dosal 2014 compared 0.7% cantharidin cream to its vehicle, and found 2/13 versus 1/16 participants free of lesions at the end of 5 treatments over 2 months (RR 2.46, 95% CI 0.25 to 24.21, Analysis 14.1).
14.1. Analysis.
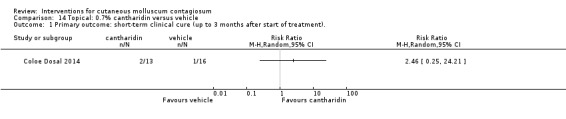
Comparison 14 Topical: 0.7% cantharidin versus vehicle, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment)..
Secondary outcomes
5. Adverse effects of treatment such as pain, blistering, sensitisation, scarring, erosion, and pigmentary changes
In Coloe Dosal 2014, 3/13 participants reported pain; 12/13, blistering; and 6/13, hypo‐ or hyperpigmentation in the group treated with 0.7% cantharidin. In the vehicle group, the same effects were found in 1/16, 8/16, and none of the participants, respectively.
Salicylic acid versus sodium nitrate in salicylic acid
We considered the evidence for all outcomes for this comparison to be of low quality due to small study size, serious risk of bias, and imprecision.
Primary outcome
1. Short‐term clinical cure (up to three months after the start of treatment)
Treatment with 5% sodium nitrite co‐applied daily with 5% salicylic acid under occlusion resulted in significantly more participants with complete resolution of lesions three months after start of treatment: 12/16 (75%) compared with 3/14 (21%) participants in the control group, which was treated with an identical cream but omitting sodium nitrite (RR 3.50, 95% CI 1.23 to 9.92, Analysis 15.1) (Ormerod 1999).
15.1. Analysis.
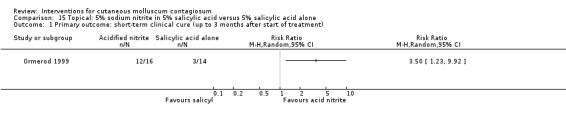
Comparison 15 Topical: 5% sodium nitrite in 5% salicylic acid versus 5% salicylic acid alone, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).
5. Adverse effects of treatment such as pain, blistering, sensitisation, scarring, erosion, and pigmentary changes
In the group treated with 5% sodium nitrite co‐applied with 5% salicylic acid under occlusion, brown staining was reported in 6 of the 16 participants (Ormerod 1999). Four out of 16 participants (25%) in this group stopped the treatment because of irritation and lack of efficacy. Two additional participants, who were cured, complained of significant irritation. In the group treated with 5% salicylic acid only, 4/14 participants complained of irritation.
Salicylic acid versus alcohol
We considered the evidence for all outcomes for this comparison to be of low quality due to small study size, serious risk of bias, and imprecision.
Secondary outcomes
1. Medium‐ and long‐term cure (after three months and up to six months, and beyond six months, respectively)
As part of a three‐arm study, Leslie 2005 compared 12% salicylic acid with 70% alcohol, and assessed cure after a maximum of 6 months. In the 12% salicylic acid group, 21/37 participants were cured versus 16/36 in the 70% alcohol group (RR 1.28, 95% CI 0.81 to 2.02, Analysis 16.1).
16.1. Analysis.
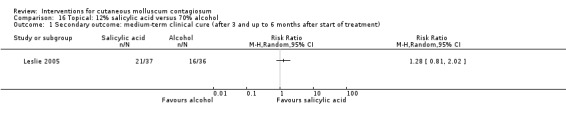
Comparison 16 Topical: 12% salicylic acid versus 70% alcohol, Outcome 1 Secondary outcome: medium‐term clinical cure (after 3 and up to 6 months after start of treatment).
Salicylic acid versus phenol plus alcohol
We considered the evidence for all outcomes for this comparison to be of low quality due to small study size, serious risk of bias, and imprecision.
Secondary outcomes
1. Medium‐ and long‐term cure (after three months and up to six months, and beyond six months, respectively)
As part of a three‐arm study, Leslie 2005 compared 12% salicylic acid with 10% phenol plus 70% alcohol, and assessed cure after a maximum of 6 months. In the 12% salicylic acid group, 21/37 participants were cured versus 17/41 in the 10% phenol plus 70% alcohol group (RR 1.37, 95% CI 0.86 to 2.17, not statistically significant, Analysis 17.1).
17.1. Analysis.
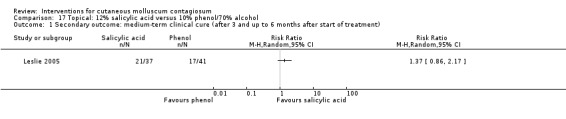
Comparison 17 Topical: 12% salicylic acid versus 10% phenol/70% alcohol, Outcome 1 Secondary outcome: medium‐term clinical cure (after 3 and up to 6 months after start of treatment).
Salicylic acid plus lactic acid versus curettage
We considered the evidence for all outcomes for this comparison to be of low quality due to small study size, serious risk of bias, and imprecision.
Primary outcome
1. Short‐term clinical cure (up to three months after the start of treatment)
The third comparison of Machado 2010 was a combination of 14% salicylic acid plus 14% lactic acid versus curettage, resulting in 14/17 versus 15/17 participants showing complete clearance (RR 1.06, 95% CI 0.86 to 1.32, Analysis 18.1).
18.1. Analysis.
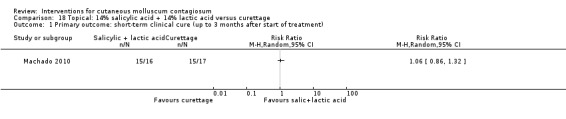
Comparison 18 Topical: 14% salicylic acid + 14% lactic acid versus curettage, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).
Secondary outcomes
5. Adverse effects of treatment such as pain, blistering, sensitisation, scarring, erosion, and pigmentary changes
In the group treated with 14% salicylic acid plus 14% lactic acid, 8/16 participants reported mild pain, whereas in the curettage group 12/17 reported mild pain.
Alcohol versus phenol plus alcohol
We considered the evidence for all outcomes for this comparison to be of low quality due to small study size, serious risk of bias, and imprecision.
Secondary outcomes
1. Medium‐ and long‐term cure (after three months and up to six months, and beyond six months, respectively)
As part of a three‐arm study, Leslie 2005 compared 70% alcohol plus 10% phenol with 70% alcohol, and assessed cure after a maximum of 6 months. In the 70% alcohol group, 16/36 participants were cured versus 17/41 in the 70% alcohol plus 10% phenol group (RR 1.07, 95% CI 0.64 to 1.79, Analysis 19.1).
19.1. Analysis.
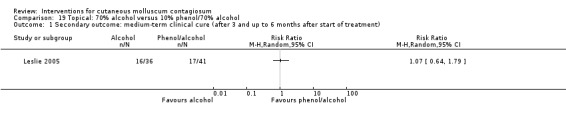
Comparison 19 Topical: 70% alcohol versus 10% phenol/70% alcohol, Outcome 1 Secondary outcome: medium‐term clinical cure (after 3 and up to 6 months after start of treatment).
Iodine versus tea tree oil
We considered the evidence for all outcomes for this comparison to be of low quality due to small study size, serious risk of bias, and imprecision.
Primary outcome
1. Short‐term clinical cure (up to three months after the start of treatment)
In a three‐armed study, Markum 2012 compared iodine, tea tree oil, and the two combined. Application of iodine compared to tea tree oil resulted in clinical cure (> 90% reduction of lesions) in 1/16 versus 3/18 participants (RR 0.38, 95% CI 0.04 to 3.25, Analysis 20.1).
20.1. Analysis.
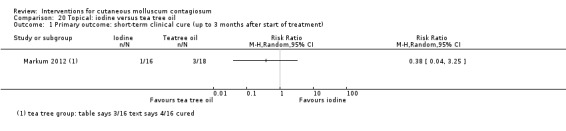
Comparison 20 Topical: iodine versus tea tree oil, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).
Secondary outcomes
2. Improvement
Application of iodine compared to tea tree oil resulted in clinical improvement in 5/16 versus 8/18 participants (RR 0.70, 95% CI 0.29 to 1.71, Analysis 20.2) (Markum 2012).
20.2. Analysis.
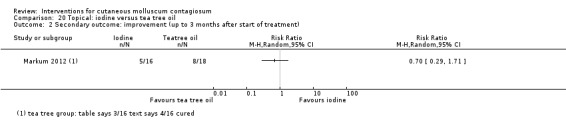
Comparison 20 Topical: iodine versus tea tree oil, Outcome 2 Secondary outcome: improvement (up to 3 months after start of treatment).
5. Adverse effects of treatment such as pain, blistering, sensitisation, scarring, erosion, and pigmentary changes
Redness was reported in 2/16 participants treated with iodine and 1/18 participants treated with tea tree oil. None of the iodine‐treated participants and 4/18 participants treated with tea tree oil noted a sensation of warmth during application (Markum 2012).
Iodine versus iodine plus tea tree oil
We considered the evidence for all outcomes for this comparison to be of low quality due to small study size and imprecision.
Primary outcome
1. Short‐term clinical cure (up to three months after start of treatment)
In a three‐armed study, Markum 2012 compared iodine, tea tree oil, and the two combined. Application of iodine compared to tea tree oil combined with iodine resulted in > 90% reduction of lesions in 1/16 versus 16/19 participants (RR 0.07, 95% CI 0.01 to 0.50, Analysis 21.1).
21.1. Analysis.
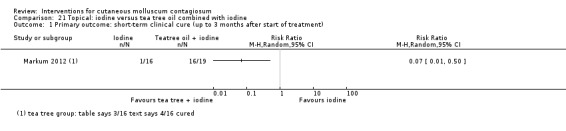
Comparison 21 Topical: iodine versus tea tree oil combined with iodine, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).
Secondary outcomes
2. Improvement
Application of iodine compared to tea tree oil combined with iodine resulted in improvement in 5/16 versus 18/19 participants (RR 0.29, 95% CI 0.14 to 0.62, Analysis 21.2).
21.2. Analysis.
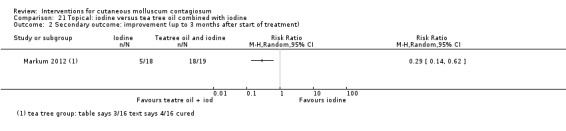
Comparison 21 Topical: iodine versus tea tree oil combined with iodine, Outcome 2 Secondary outcome: improvement (up to 3 months after start of treatment).
5. Adverse effects of treatment such as pain, blistering, sensitisation, scarring, erosion, and pigmentary changes
Redness was reported in 2/16 participants treated with iodine and 1/19 participants treated with iodine combined with tea tree oil. None of the iodine‐treated participants and 3/19 participants treated with the combination noted a sensation of warmth during application (Markum 2012).
Tea tree oil versus iodine plus tea tree oil
We considered the evidence for all outcomes for this comparison to be of low quality due to small study size and imprecision.
Primary outcome
1. Short‐term clinical cure (up to three months after start of treatment)
Application of tea tree oil alone compared to iodine combined with tea tree oil resulted in > 90% reduction of lesions in 3/18 versus 16/19 participants (RR 0.20, 95% CI 0.07 to 0.57, Analysis 22.1) (Markum 2012), favouring the combined treatment.
22.1. Analysis.
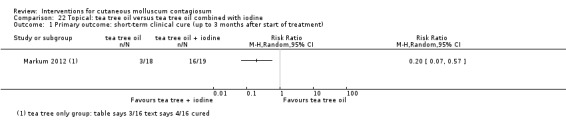
Comparison 22 Topical: tea tree oil versus tea tree oil combined with iodine, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).
Secondary outcomes
2. Improvement
Application of tea tree oil alone compared to iodine combined with tea tree oil resulted in improvement in 8/18 versus 18/19 participants (RR 0.47, 95% CI 0.28 to 0.79, Analysis 22.2) (Markum 2012), favouring the combined treatment.
22.2. Analysis.
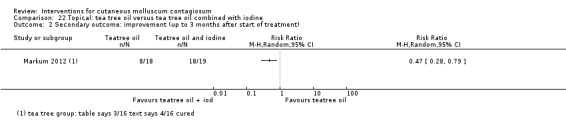
Comparison 22 Topical: tea tree oil versus tea tree oil combined with iodine, Outcome 2 Secondary outcome: improvement (up to 3 months after start of treatment).
5. Adverse effects of treatment such as pain, blistering, sensitisation, scarring, erosion, and pigmentary changes
Redness was reported in 1/18 participants treated with tea tree oil and 1/19 participants treated with the combination. The number of participants noting a sensation of warmth during application was 4/18 and 3/19 treated with tea tree oil or the combination (Markum 2012).
Systemic treatments
Of the two studies addressing systemic treatments, only one study reported on our primary outcome but did not include any of our secondary outcomes (Manchanda 1997b). The other study reported on two of our secondary outcomes (Antony 2001).
Cimetidine versus placebo
We considered the evidence for all outcomes for this comparison to be of low quality due to small study size, serious risk of bias, and imprecision.
Secondary outcomes
1. Medium‐ and long‐term cure (after three months and up to six months, and beyond six months, respectively)
Treatment with systemic cimetidine 35 mg/kg/day cleared lesions completely in 4/8 participants (50%) after 4 months of treatment, compared with 5/11 participants (46%) given placebo in the same period (Analysis 23.1) (Antony 2001). The difference was not statistically significant (RR 1.10, 95% CI 0.43 to 2.84). No data were reported for the 50% (19/38) of participants who withdrew from the study.
23.1. Analysis.
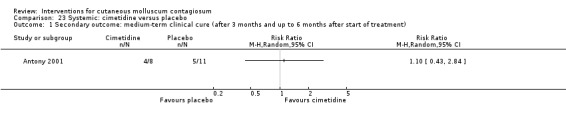
Comparison 23 Systemic: cimetidine versus placebo, Outcome 1 Secondary outcome: medium‐term clinical cure (after 3 months and up to 6 months after start of treatment).
2. Improvement
Treatment with systemic cimetidine 35 mg/kg/day resulted in completely cleared lesions or self reported improvement in 7/8 participants after 4 months of treatment, compared with 9/11 participants given placebo in the same period (Analysis 23.2) (Antony 2001). The difference was not statistically significant (RR 1.07, 95% CI 0.73 to 1.57).
23.2. Analysis.
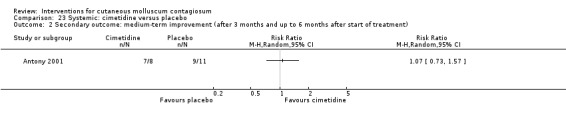
Comparison 23 Systemic: cimetidine versus placebo, Outcome 2 Secondary outcome: medium‐term improvement (after 3 months and up to 6 months after start of treatment).
Calcarea carbonica versus placebo
We considered the evidence for all outcomes for this comparison to be of low quality due to small study size, serious risk of bias, and imprecision.
Primary outcome
1. Short‐term clinical cure (up to three months after start of treatment)
Treatment with calcarea carbonica resulted in 100% improvement in 13/14 participants in the treatment arm and 1/6 in the placebo arm of the trial (RR 5.57, 95% CI 0.93 to 33.54, Analysis 24.1) (Manchanda 1997b). Fisher's exact test resulted in a P value of 0.002. However, study duration, time to resolution, and adverse events were not reported, and the study was not analysed by the intention‐to‐treat principle. The number of dropouts (20/104 for the whole trial, including other skin conditions) was unclear for the molluscum participants.
24.1. Analysis.
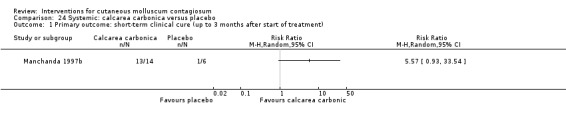
Comparison 24 Systemic: calcarea carbonica versus placebo, Outcome 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment).
Discussion
Summary of main results
Twenty‐two studies, with a total of 1650 participants, examined the effects of topical (20 studies) and systemic (2 studies) interventions. Altogether, we could extract evaluable data for 24 comparisons.
We found limited evidence of low quality from 11 comparisons for the short‐term cure efficacy of the following topical treatments: cryospray when compared to 5% imiquimod; 10% potassium hydroxide compared to 5% imiquimod; 5% sodium nitrite co‐applied with 5% salicylic acid compared to 5% salicylic acid alone; tea tree oil combined with iodine compared to tea tree oil or iodine alone; 10% Australian lemon myrtle oil compared to olive oil; and 10% benzoyl peroxide compared to 0.05% tretinoin. We found some evidence to suggest that 10% potassium hydroxide is more effective than saline; 5% solution of potassium hydroxide is favoured compared to 2.5% solution of potassium hydroxide; 10% povidone iodine solution plus 50% salicylic acid plaster is favoured compared to salicylic acid plaster alone; and homeopathic calcarea carbonica is favoured compared to placebo. We found no statistically significant differences for the other comparisons not including imiquimod.
The addition of three unpublished trial reports, with a total of over 800 participants, resulted in high‐ to moderate‐quality evidence for the comparison of topical 5% imiquimod versus vehicle. We conclude that compared to vehicle, topical 5% imiquimod is probably no more effective in terms of clinical cure, makes little or no difference in terms of short‐term improvement or local side effects, but appears to induce more application site reactions.
Overall, study limitations included lack of blinding, many dropouts, and no intention‐to‐treat analysis. Small study sizes resulted in broad confidence intervals and may have led to important differences being missed. None of the evaluated treatment options were associated with serious adverse effects, except for 5% imiquimod (severe application site reactions).
Although this update of our original review identified 11 new studies for inclusion, the overall conclusions have hardly changed, due to the small size of most of the studies and methodological shortcomings. We found no strong evidence either for or against the most commonly used treatment options for molluscum contagiosum. The evidence identified by this systematic review is therefore insufficient to propose any one intervention for molluscum contagiosum.
Overall completeness and applicability of evidence
Of the 11 trials added during the 2016 update, only three contributed to comparisons for which trials had been found in previous versions of this review. The other eight addressed new comparisons, thus increasing the fragmentation of the evidence. There remains an evidence gap regarding many promoted and used treatment options for molluscum contagiosum, with many interventions assessed by single, low‐quality studies. For example, of the two studies that included a curettage arm (Hanna 2006; Machado 2010), the outcomes of Hanna 2006 were not suitable for inclusion in this review, and Machado 2010 was a very small study, with 50 participants divided over three study arms. We could include only two studies on cryotherapy (Al‐Mutairi 2010; Handjani 2014).
Due to the small sample sizes of the low‐quality, single‐study comparisons that were included and for which no differences were found, it is possible that clinically relevant differences could be found if treatments would be evaluated in larger samples.
About half of the studies did not compare an active treatment to some sort of placebo, but rather compared two active treatments. This implies that for these interventions, the magnitude of benefit compared to placebo or doing nothing is unclear.
Several issues remain unclear due to lack of details in the published papers. For example, it is unclear whether duration of treatment, as used in Ormerod 1999, can be taken as a valid indicator for time to cure given dropouts and other possible reasons for stopping treatment. Although Antony 2001 did not report on adverse events, the 50% loss to follow‐up rate in the trial might have been caused by adverse effects of the treatment. It is unclear which dosing regimen was used in Manchanda 1997b when evaluating calcarea carbonica.
Several of the outcomes important to participants and clinicians were not measured in most of the studies we found, or not at all; these included recurrences, and, especially, spread to other people and quality of life.
We initially chose our primary outcome measure to be clinical cure after one month, calculated from the last day of treatment. However, this may not be the most appropriate outcome measure to cover the variety of treatments for molluscum. For example, when comparing a method of physical destruction (e.g. curettage) with a topical treatment that is applied during several days or weeks, our primary outcome measure might favour the first type of treatment. For this update we have adapted our primary outcome to make it more manageable: short‐term clinical cure (up to three months after start of treatment). A time point beyond one month after start of treatment for assessing cure was also suggested by Mc Cuaig 2011, commenting on the 2009 update of this review. An example of when time to cure since the last day of treatment is not appropriate is when treatment is continued until resolution of all lesions (e.g. Ohkuma 1990). Although no clear‐cut solution seems available, and so far few trials have studied physical destruction (e.g. Hanna 2006; Machado 2010), it is advisable to always consider multiple outcome measures and also to take the burden of treatment into account.
We could perform a meta‐analysis for only two comparisons: 5% imiquimod versus its vehicle and 5% imiquimod versus 10% potassium hydroxide.
We excluded studies on genital molluscum contagiosum and in participants with immune deficiency, so our conclusions do not apply to these participant groups, as the need for treatment is probably higher and may require different treatments.
Quality of the evidence
We included 22 studies with a total of 1650 participants in this update. Most of the included studies had small sample sizes, with a median study size of just over 30 molluscum participants, and only five studies with more than 100 participants. This impacted on our GRADE assessments. The small studies may have limited power, which was reflected in the wide confidence intervals around the risk ratios. In addition, many of the studies had large losses to follow‐up, up to 50% (Antony 2001).
Furthermore, most of the included studies used a control treatment that was not a placebo. Examples of comparator treatments in these studies were olive oil, saline, and alcohol, which may have had potential treatment effects. It was therefore difficult to compare the net effect of interventions due to the absence of a placebo group.
We could assign a low risk of bias for only a small proportion of items in the 'Risk of bias' table (Figure 2). The lack of reported details on several methodological issues and follow‐up periods, together with the small number of participants, gives rise to doubts about the validity of the results of some of the studies. We were not able to assess publication bias in this review, for example by constructing a funnel plot, due to the lack of directly comparable studies.
Notably, we judged five of the nine studies included in the 2016 update to be at low risk of bias (Coloe Dosal 2014; Eichenfield 2005; Markum 2012; Paller 2005a; Paller 2005b), suggesting a trend toward better study design or reporting, or both. However, with the exception of the three 'imiquimod versus vehicle' studies, most of the studies added during this update were small. The imiquimod studies overall had a low risk of bias (Eichenfield 2005; Paller 2005a; Paller 2005b; Theos 2004), therefore we did not downgrade the outcomes in Table 1 for this domain.
As most of the evidence included in this review was based on low‐quality studies, we were unable to reach a robust conclusion regarding the objective of the review.
Potential biases in the review process
Despite conducting a thorough search, we cannot be certain that we did not miss relevant randomised trials. One study is ongoing, and several others are awaiting classification and have not yet been incorporated into the review. Given that 19 of the included studies were performed or published, or both after 2000, we expect more studies comparing treatments for molluscum contagiosum to be published in the coming years.
We made several amendments to the original protocol, some of which were 'data‐driven' in the sense that we encountered situations that we had not expected when we developed the protocol. This may have introduced bias.
Agreements and disagreements with other studies or reviews
At the time of the 2009 update of this review, we had found one other systematic review (Schmitt 2008). This review included six randomised trials, all of which were also included in our review. The conclusions of this review were similar to ours. During the 2016 update, we identified a new partly systematic (they described their databases, search strategy, and search date, but did not select studies or extract data in couples, did not state clear inclusion criteria, and did not assess risk of bias) review (Chen 2013), which addressed not only epidemiology, virology, and immunology of molluscum contagiosum, but also clinical management. They summarised the findings of the previous version of this review (Van der Wouden 2009), and mentioned three new trials (Al‐Mutairi 2010; Köse 2013; Seo 2010), the first two of which have been included in this update, and the last which is awaiting assessment. They concluded that no evidence‐based consensus has been reached on which is the best treatment for molluscum contagiosum in people without immune deficiency.
The cure rates found in the non‐randomised study by Weller 1999 for physical expression and phenol ablation (75% and 77% of lesions, respectively, after one month) compare favourably to the 23% of children found cured in the Japanese cohort study on the natural history of the disease (Takemura 1983).
In the vehicle group of the 3M studies, clearance rates were 12% within 3 months; 27% after 3 to 6 months; and 40% beyond 6 months. Another, more recent cohort study from the UK (Olsen 2015), where only a small proportion of children were reported to have received treatment, found somewhat lower cure rates: around 10% after 6 months; 40% after 12 months; 75% after 18 months; and 80% after 24 months. Comparing these figures, 'vehicle' seems to work better than no treatment at all.
Authors' conclusions
Implications for practice.
We can provide no reliable evidence‐based recommendations for the treatment of molluscum contagiosum at present, except for 5% imiquimod, which based on moderate‐quality evidence from three unpublished studies is probably no more effective in terms of clinical cure than its vehicle but is probably more harmful in terms of applications site reactions, and based on high‐quality evidence is no more effective than its vehicle in terms of short‐term improvement.
We found only a few randomised controlled trials that addressed physical destruction of molluscum lesions; these studies were small and at serious risk of bias. Until robust evidence emerges for effective and safe treatment, expectant management, that is awaiting spontaneous resolution of the molluscum lesions, remains a strong option for treating the condition. The studies in Studies awaiting classification may alter the conclusions of the review once assessed.
Implications for research.
Additional well‐designed, adequately powered, and preferably blinded randomised controlled studies are needed to provide high‐quality clinical trial evidence upon which to base clinical decision‐making. Future studies evaluating treatments for molluscum contagiosum should, as a priority, focus on commonly promoted and commonly used options for treatment (e.g. curettage, cryotherapy, salicylic acid) and preferably include a placebo or vehicle arm as long as no high‐quality evidence supporting a treatment option is available. When comparing different types of treatments, the use of the double‐dummy technique should be considered where possible. Regarding sample sizes, we suggest following GRADE guidance for information size (www.gradeworkinggroup.org).
Limited data on the natural history of molluscum contagiosum is available. Additional studies into the rate of resolution without active interventions are therefore needed, preferably assessing this after various follow‐up times (e.g. 1, 3, 6, and 12 months). This will help guide decisions concerning the use of active treatments.
Outcome measures of future trials should not only include cure, but also recurrence rates, spread of the disease to other people, disease‐related quality of life, and scarring.
A standardised outcome measure (e.g. time to resolution of the lesions or resolution after three months) would make studies easier to compare. However, the difference in the nature of treatments, for example repeated topical application of a chemical substance versus physical destruction of the lesions, will probably hamper the harmonisation of timing of outcome measurement.
Statistical power must be considered in conjunction with outcomes that are meaningful for people with molluscum contagiosum. For example, it is likely that a treatment that results in statistically fewer lesions may not be considered worthwhile because this reduction may not be sufficient to improve appearance or quality of life. People should be able to weigh costs and benefits, taking into account resolution of lesions, adverse effects, and treatment burden.
Molluscum contagiosum is a common disease in people with immune deficiency (e.g. people living with HIV). There is also a sexually transmitted variant that affects sexually active people without immune deficiency, which we excluded from this review. There is a need for reviews of studies of treatments for these important subgroups of people with molluscum contagiosum.
What's new
| Date | Event | Description |
|---|---|---|
| 21 July 2016 | New search has been performed | A new search led to the addition of 11 new included studies, and we updated the review in line with MECIR standards. |
| 21 July 2016 | New citation required and conclusions have changed | New evidence added regarding the use of topical imiquimod |
History
Protocol first published: Issue 2, 2004 Review first published: Issue 2, 2006
| Date | Event | Description |
|---|---|---|
| 7 March 2012 | Amended | The lead author's contact details have been updated. |
| 7 December 2009 | Amended | Unpublished data (Short 2002) has now been published as Short 2006. |
| 22 July 2009 | New search has been performed | New search, 6 new trials added. 'Risk of bias' table added, Discussion rearranged, various minor adaptations. |
| 21 June 2008 | Amended | Converted to new review format |
| 6 December 2005 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The authors thank Marjolein Berger, Chris Butler, Sanjay Gajadin, Jack Menke, and Marjolein Tasche for their involvement in writing previous versions of our review. The authors would also like to thank Adrie Hollestein, Ken Katz, Daan Muris, Kazutomo Ohkuma, Anthony Ormerod, Jane Sterling, and Hywel Williams for drawing our attention to relevant studies.
Drs. Manchanda, Kazutomo Ohkuma, and Anthony Ormerod kindly provided additional information regarding their studies, and Kate Short and Mohammed Bazza generously sent us their full paper before it was submitted for publication. Derya Uçmak kindly sent us their paper. The editorial base provided help in tracing and translating papers. We also thank Himiko Luiken for translating the unique study on the natural history of molluscum contagiosum (Takemura 1983), and Taixiang Wu for interviewing Dr He on details of her study design (He 2001). We thank Alireza Firooz for assessing the paper Salmanpour 2006, and Susheera Chatpoedprai for providing additional information on their study (Chatproedrai 2007). Clemens van Ede (at that time medical director of Meda Pharma BV, Netherlands) kindly provided the trial reports of the three unpublished 3M studies (Eichenfield 2005; Paller 2005a; Paller 2005b).
For the current update of this review, the Cochrane Skin editorial base wishes to thank Bob Boyle, Cochrane Dermatology Editor for this review; Matthew Grainge, Statistical Editor; Ching‐Chi Chi, Methods Editor; the clinical referees, Paul Martin and Hywel Williams; and the consumer referee, Jack Tweed; as well as Lisa Winer, who copy edited the review.
Appendices
Appendix 1. Cochrane Skin Group Specialised Register (CRS) search strategy
"mollusc* contagios*" or "water wart*"
Appendix 2. CENTRAL (Cochrane Library) search strategy
#1 MeSH descriptor: [Molluscum Contagiosum] explode all trees #2 mollusc* contagios*:ti,ab,kw #3 water wart*:ti,ab,kw #4 {or #1‐#3}
Appendix 3. MEDLINE (Ovid) search strategy
1. Molluscum Contagiosum/ 2. mollusc$ contagios$.mp. 3. water wart$.mp. 4. or/1‐3 5. randomized controlled trial.pt. 6. controlled clinical trial.pt. 7. randomized.ab. 8. placebo.ab. 9. clinical trials as topic.sh. 10. randomly.ab. 11. trial.ti. 12. 5 or 6 or 7 or 8 or 9 or 10 or 11 13. exp animals/ not humans.sh. 14. 12 not 13 15. 4 and 14
[Lines 5‐14: Cochrane Highly Sensitive Search Strategy for identifying randomized trials in MEDLINE: sensitivity‐ and precision‐maximizing version (2008 revision)]
Appendix 4. Embase (Ovid) search strategy
1. crossover procedure.sh. 2. double‐blind procedure.sh. 3. single‐blind procedure.sh. 4. (crossover$ or cross over$).tw. 5. placebo$.tw. 6. (doubl$ adj blind$).tw. 7. allocat$.tw. 8. trial.ti. 9. randomized controlled trial.sh. 10. random$.tw. 11. or/1‐10 12. exp animal/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 13. human/ or normal human/ 14. 12 and 13 15. 12 not 14 16. 11 not 15 17. molluscum contagiosum/ 18. mollusc$ contagios$.mp. 19. water wart$.mp. 20. or/17‐19 21. 16 and 20
Appendix 5. LILACS search strategy
(mollusc$ contagios$) or (molusco contagioso)
In LILACS we searched using the Controlled clinical trials topic‐specific query filter and the terms above.
Data and analyses
Comparison 1. Topical: 5% imiquimod versus vehicle.
Comparison 2. Topical: 5% imiquimod versus cryospray.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Secondary outcome: medium‐term clinical cure (after 3 and up to 6 months after start of treatment) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 3 Secundary outcome: recurrence | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 3. Topical: 5% imiquimod versus 10% potassium hydroxide.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) | 2 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.46, 0.93] |
| 2 Secondary outcome: any side effect | 2 | 67 | Risk Ratio (M‐H, Random, 95% CI) | 0.68 [0.25, 1.81] |
Comparison 4. Topical: 10% lemon myrtle oil versus vehicle.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 5. Topical: 10% benzoyl peroxide cream versus 0.05% tretinoin cream.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 6. Topical: 10% potassium hydroxide versus saline.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 7. Topical: 2.5% potassium hydroxide versus 5% potassium hydroxide.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Secondary outcome: short‐term improvement (up to 3 months after start of treatment) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 8. Topical: 10% potassium hydroxide versus 14% salicylic acid + 14% lactic acid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 9. Topical: 10% potassium hydroxide versus curettage.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 10. Topical 10% potassium hydroxide versus cryotherapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Secondary outcome: short‐term clinical improvement (up to 3 months after start of treatment) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 11. Topical: 10% povidone iodine versus 50% salicylic acid plaster.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 12. Topical: 10% povidone iodine alone versus 10% povidone iodine and 50% salicylic plaster.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 13. Topical: 10% povidone iodine and 50% salicylic acid plaster versus 50% salicylic plaster alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 14. Topical: 0.7% cantharidin versus vehicle.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment). | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 15. Topical: 5% sodium nitrite in 5% salicylic acid versus 5% salicylic acid alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 16. Topical: 12% salicylic acid versus 70% alcohol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Secondary outcome: medium‐term clinical cure (after 3 and up to 6 months after start of treatment) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 17. Topical: 12% salicylic acid versus 10% phenol/70% alcohol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Secondary outcome: medium‐term clinical cure (after 3 and up to 6 months after start of treatment) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 18. Topical: 14% salicylic acid + 14% lactic acid versus curettage.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 19. Topical: 70% alcohol versus 10% phenol/70% alcohol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Secondary outcome: medium‐term clinical cure (after 3 and up to 6 months after start of treatment) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 20. Topical: iodine versus tea tree oil.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Secondary outcome: improvement (up to 3 months after start of treatment) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 21. Topical: iodine versus tea tree oil combined with iodine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Secondary outcome: improvement (up to 3 months after start of treatment) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 22. Topical: tea tree oil versus tea tree oil combined with iodine.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Secondary outcome: improvement (up to 3 months after start of treatment) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 23. Systemic: cimetidine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Secondary outcome: medium‐term clinical cure (after 3 months and up to 6 months after start of treatment) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Secondary outcome: medium‐term improvement (after 3 months and up to 6 months after start of treatment) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Comparison 24. Systemic: calcarea carbonica versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Primary outcome: short‐term clinical cure (up to 3 months after start of treatment) | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Al‐Mutairi 2010.
| Methods | Randomised controlled trial | |
| Participants | 74 children 2 to 12 years of age, hospital outpatient clinic in Kuwait | |
| Interventions | Imiquimod 5% for up to 16 weeks versus cryospray for up to 2 weeks | |
| Outcomes | Cure at 3, 6, 12, and 16 weeks; cosmetic outcome; adverse effects | |
| Notes | Funding: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details except 'randomly assigned' |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Probably not blinded: cream versus cryospray |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Probably not blinded: cream versus cryospray |
| Incomplete outcome data (attrition bias) Short‐term outcomes (up to 3 months) | Low risk | All participants had complete follow‐up. |
| Incomplete outcome data (attrition bias) Medium‐ and long‐term outcomes (3‐6 months and longer) | Low risk | All participants had complete follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | Protocol not available |
| Other bias | Unclear risk | No baseline imbalance for gender, MC area, morphology, MC location, or baseline lesion count, but no data on compliance |
Antony 2001.
| Methods | Double‐blind randomised placebo‐controlled trial | |
| Participants | 38 patients (1 to 16 years; M/F: 18/20) were enrolled, Dept of Dermatology, UK. | |
| Interventions | 35 mg/kg/day cimetidine, given once daily as oral suspension versus a matching placebo for 3 months | |
| Outcomes | Complete clearance after 4 months of treatment. Reduction of lesions. Adverse events: not mentioned | |
| Notes | 50% dropout rate. Published abstract only. Funding: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomized". No details in abstract |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment is not described in the abstract. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double‐blind placebo‐controlled"; "The dose of cimetidine was 35 mg/kg‐1/day‐1"; "The placebo group received a manufactured placebo". Probably done, placebo controlled, both suspensions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Double‐blind placebo‐controlled"; "The dose of cimetidine was 35 mg/kg‐1/day‐1"; "The placebo group received a manufactured placebo". Probably done, placebo controlled, both suspensions |
| Incomplete outcome data (attrition bias) Short‐term outcomes (up to 3 months) | Unclear risk | Not reported in the abstract |
| Incomplete outcome data (attrition bias) Medium‐ and long‐term outcomes (3‐6 months and longer) | High risk | 4 months: 19/35 completed the treatment course. Quote: "The number of patients who received placebo or cimetidine was similar in the groups that did not attend or withdrew." > 30% withdrawals |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Quote: "The mean age and sex of the patients and incidence of atopic disease in each treatment group was similar." No compliance data |
Bazza 2007.
| Methods | Randomised controlled trial. Body sides were randomised left‐right. | |
| Participants | 30 children (2 to 12 years of age; M/F: 18/12) were recruited, Dept of Dermatology, UK. | |
| Interventions | Sterile normal 0.9% saline versus 5% potassium hydroxide for 3 weeks | |
| Outcomes | Complete clearance of lesions and side effects after 12 weeks | |
| Notes | Unpublished, year of study unclear. Unpublished paper obtained in 2007. Funding: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Where treatment with 0.9% NS and 5% KOH solution was randomised to right or left side of body". Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Where treatment with 0.9% NS and 5% KOH solution was randomised to right or left side of body". Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "30 patients were recruited in this double‐blind study". "All subjects were given seven bottles clearly labelled R and seven bottles labelled L, for use on the right and left side of the body respectively (patient and investigator did not know which is active site)" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "30 patients were recruited in this double‐blind study". "All subjects were given seven bottles clearly labelled R and seven bottles labelled L, for use on the right and left side of the body respectively (patient and investigator did not know which is active site)" |
| Incomplete outcome data (attrition bias) Short‐term outcomes (up to 3 months) | High risk | 12 weeks: 10/30 did not complete study, 2 withdrew due to severe stinging from KOH, and 8 children were lost to follow‐up. > 30% dropouts |
| Incomplete outcome data (attrition bias) Medium‐ and long‐term outcomes (3‐6 months and longer) | Unclear risk | No medium‐ or long‐term follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | No baseline comparison. No compliance data |
Burke 2004.
| Methods | Randomised controlled trial | |
| Participants | 31 children, mean age 4.6 years. Sex not reported. USA, outpatient clinic | |
| Interventions | 10% lemon myrtle oil or vehicle (olive oil) for 3 weeks | |
| Outcomes | Complete clearance or > 90% reduction in number of lesions after 3 weeks | |
| Notes | Funding: Center for Biomedical Research, a commercial institute involved in drug research and sale. Partner of Naturopathix (ZymaDerm for molluscum) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Children were randomised to active treatment or vehicle (virgin olive oil) by blindly choosing a token numbered from 1 to 100. Odd numbers were assigned to active treatment even numbers to vehicle" |
| Allocation concealment (selection bias) | Low risk | Quote: "Children were randomised to active treatment or vehicle (virgin olive oil) by blindly choosing a token numbered from 1 to 100." "Parents and physicians were blinded to treatment protocol. A treatment key was held by a participating pharmacist (no patient contact) until study completion" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Parents and physicians were blinded to treatment protocol. A treatment key was held by a participating pharmacist (no patient contact) until study completion." "A mild synthetic lemon fragrance not containing citral was added to scent the control olive oil preparation. This fragrance by itself had no therapeutic effect." Vehicle controlled. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Parents and physicians were blinded to treatment protocol. A treatment key was held by a participating pharmacist (no patient contact) until study completion." "A mild synthetic lemon fragrance not containing citral was added to scent the control olive oil preparation. This fragrance by itself had no therapeutic effect." Vehicle controlled. |
| Incomplete outcome data (attrition bias) Short‐term outcomes (up to 3 months) | Low risk | 21 days: 4/31 withdrew: 1/16 in lemon myrtle oil group lost to follow‐up; 3/15 missing in vehicle group, withdrew because of worsening of the molluscum. Withdrawn participants included in analysis as failures. |
| Incomplete outcome data (attrition bias) Medium‐ and long‐term outcomes (3‐6 months and longer) | Unclear risk | The study did not address medium‐ and long‐term outcomes. |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | The mean number of lesions at enrolment did not differ between treatment groups. No sex or age comparison between groups. No compliance data |
Chathra 2015.
| Methods | Randomised trial | |
| Participants | Children 1 to 18 years with a minimum of 3 molluscum lesions, target sample size 40, Karnataka, India | |
| Interventions | Imiquimod 5% cream application alternate nights versus 10% potassium hydroxide solution applied alternate nights for 12 weeks | |
| Outcomes | Complete clearance, time points 4, 8, and 12 weeks | |
| Notes | Funding: this study reported that they had no support. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The lottery method"; no details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Different instructions, therefore not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Different instructions, therefore not blinded |
| Incomplete outcome data (attrition bias) Short‐term outcomes (up to 3 months) | Low risk | No loss to follow‐up |
| Incomplete outcome data (attrition bias) Medium‐ and long‐term outcomes (3‐6 months and longer) | Unclear risk | No medium‐ and long‐term outcomes |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk | No baseline imbalance in terms of age, gender, and number of lesions. No compliance data |
Coloe Dosal 2014.
| Methods | Randomised controlled trial | |
| Participants | 32 children, 5 to 10 years of age, recruited in local paediatricians' offices, university clinics and through mass emails to university students and staff, North Carolina, USA | |
| Interventions | Cantharidin collodion 0.7% versus vehicle collodion for approximately 8 weeks | |
| Outcomes | Complete clearance, lesion counts, adverse effects, approximately 8 weeks after start of treatment | |
| Notes | Funding: Doris Duke Charitable Foundation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization schedule was prepared before first recruitment using permuted blocks of size 2" |
| Allocation concealment (selection bias) | Low risk | After eligibility was assessed, study personnel "assigned the next unique subject identification number and dispensed the appropriate drug" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Patients, parents and investigators were blinded to treatment assignment" and "placebo was identical in texture and smell" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Patients, parents and investigators were blinded to treatment assignment" and "placebo was identical in texture and smell" |
| Incomplete outcome data (attrition bias) Short‐term outcomes (up to 3 months) | Low risk | 2 participants dropped out immediately after randomisation because they did not meet all eligibility criteria (< 20%). |
| Incomplete outcome data (attrition bias) Medium‐ and long‐term outcomes (3‐6 months and longer) | Unclear risk | No long‐term data |
| Selective reporting (reporting bias) | Low risk | Reported outcomes similar to those in trial register resume. |
| Other bias | Unclear risk | Imbalance in dry skin. Allocation bias was due to dropout. |
Eichenfield 2005.
| Methods | Randomised controlled trial | |
| Participants | 323 children, 2 to 12 years of age, with molluscum contagiosum in 19 outpatient clinics in the USA were randomised. | |
| Interventions | Imiquimod cream 5% vs vehicle cream 3 times weekly for 16 weeks | |
| Outcomes | Lesion clearance, lesion counts, time to complete clearance, side effects after 4, 8, 12, 16, 18, and 28 weeks | |
| Notes | Funding by pharmaceutical company (3M), unpublished | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subjects were randomly assigned to a treatment arm in blocks of 6 according to a computer‐generated randomizations schedule. Randomization was 2:1 (active:vehicle) for a planned number of 300 subjects to be randomised into the study" (p.40) |
| Allocation concealment (selection bias) | Low risk | "The treatment assignments were concealed from the subjects, investigators and study staff, and the 3M clinical research team. The clinical packaging group at 3M Pharmaceuticals held the master code for the treatment randomizations schedule, and supplied the investigators with each subject’s treatment assignment as a hidden (tear‐off) panel on the study cream label, which was affixed to the blinded Drug Label page" (p.43) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | See allocation concealment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See allocation concealment. |
| Incomplete outcome data (attrition bias) Short‐term outcomes (up to 3 months) | Unclear risk | Unclear when participants dropped out, so impossible to distinguish short‐term from long‐term. Primary analysis by intention‐to‐treat |
| Incomplete outcome data (attrition bias) Medium‐ and long‐term outcomes (3‐6 months and longer) | Low risk | 53/323 participants dropped out, reasons mentioned (< 30%). |
| Selective reporting (reporting bias) | Low risk | All outcomes seem to have been reported. |
| Other bias | Low risk | No baseline imbalance, compliance data available, primary analysis by intention‐to‐treat |
Handjani 2014.
| Methods | Open randomised trial | |
| Participants | 30 people with molluscum contagiosum in Iran | |
| Interventions | 10% potassium hydroxide solution versus cryotherapy | |
| Outcomes | Lesion response and side effects 4 weeks after start of treatment | |
| Notes | Funding: not mentioned | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "the simple randomization method"; no details |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Different administration, so could not be blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Different administration, so could not be blinded |
| Incomplete outcome data (attrition bias) Short‐term outcomes (up to 3 months) | Low risk | No loss to follow‐up |
| Incomplete outcome data (attrition bias) Medium‐ and long‐term outcomes (3‐6 months and longer) | Unclear risk | Not applicable, no medium‐ and long‐term outcomes |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk | No baseline comparison; no compliance data |
Hanna 2006.
| Methods | Randomised controlled trial | |
| Participants | 124 children, 1 to 16 years of age, dermatology clinic, Montreal, Canada M/F: 57/67 |
|
| Interventions | 4 arms: curettage, topical cantharidin 0.7%, topical salicylic acid 16.7% + lactic acid 16.7%, topical imiquimod cream 5% | |
| Outcomes | Number of visits required. Intervals between study visits not reported, so outcome data not suitable for inclusion. | |
| Notes | Total number of participants unclear. Percentage of group 3 in Table 1 does not correspond to number mentioned in text. Funding: 3 pharmaceutical companies provided treatments for free. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomizations list was generated by specialized computer software (PC‐PLAN, Dalal, 1996)" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The randomizations list was generated by specialized computer software (PC‐PLAN, Dalal, 1996)." Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "This is not a double‐blind study." Physical versus topical treatment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "This is not a double‐blind study." Physical versus topical treatment |
| Incomplete outcome data (attrition bias) Short‐term outcomes (up to 3 months) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) Medium‐ and long‐term outcomes (3‐6 months and longer) | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | No baseline comparison. No compliance data |
Leslie 2005.
| Methods | Randomised controlled trial | |
| Participants | 114 children, 1 to 15 years of age, sex not reported, UK, outpatient departments of teaching hospital and district general hospital | |
| Interventions | Topical salicylic acid 12%, or phenol 10% + 70% alcohol, or 70% alcohol at monthly visits for a maximum of 6 months | |
| Outcomes | Complete clearance of lesions after 6 months | |
| Notes | Funding: pharmaceutical company provided medication | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The participants were randomised according to a random number table" |
| Allocation concealment (selection bias) | High risk | Quote: "The investigators were not blinded to randomizations" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "The patients in the salicylic acid groups were aware of their treatments. The other two groups treated with vehicle or phenol were single‐blinded, as the patients/parents were unaware of which treatment they received." "The vehicle and diluted phenol were prepared by the hospital pharmacy and labelled with a letter." "The investigators were not blinded for the randomization" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "The patients in the salicylic acid groups were aware of their treatments. The other two groups treated with vehicle or phenol were single‐blinded, as the patients/parents were unaware of which treatment they received." "The vehicle and diluted phenol were prepared by the hospital pharmacy and labelled with a letter." "The investigators were not blinded for the randomization" |
| Incomplete outcome data (attrition bias) Short‐term outcomes (up to 3 months) | Unclear risk | No short‐term outcomes reported. |
| Incomplete outcome data (attrition bias) Medium‐ and long‐term outcomes (3‐6 months and longer) | High risk | Up to 6 months: 31/114 lost to follow‐up: 13/37 in salicylic acid arm, 9/41 in dilute phenol arm, 9/36 in alcohol arm. > 30% dropouts |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Quote: "The baseline characteristics of the three groups were similar." See also Table I, Baseline characteristics. No compliance data |
Machado 2010.
| Methods | Randomised controlled trial | |
| Participants | 50 children, 3 to 15 years of age, recruited in a hospital outpatient clinic in Brazil | |
| Interventions | (1) potassium hydroxide 10% in aqueous solution; (2) 14% salicylic acid + 14% lactic acid in collodion; both for 3 months or (3) curettage once | |
| Outcomes | Cure, adverse effects 90 days after start of treatment | |
| Notes | 2 groups applied medication at home; treatment duration was not reported for these groups. These participants were seen every 15 days until day 90 after start of treatment. The third group underwent curettage (once). These participants were seen day 7 and 90 after treatment. Outcomes were reported at 90 days, but as we do not know how long topical treatments were applied and assuming that parents were instructed to stop treatment when lesions had resolved, it is hard to say whether these are short‐ or long‐term outcomes (apart from the curettage group). Funding: 2 pharmaceutical companies provided medication. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Patients were allocated randomly into three study groups”; insufficient information |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Different treatments: topical treatment versus curettage |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Different treatments: topical treatment versus curettage. Also, follow‐up moments differed between treatment groups (topical: every 15 days for 90 days; curettage: days 7 and 90 after the procedure) |
| Incomplete outcome data (attrition bias) Short‐term outcomes (up to 3 months) | Unclear risk | Proportions in table and text show that these were not based on number of participants randomised, so there must have been loss to follow‐up; magnitude unclear. |
| Incomplete outcome data (attrition bias) Medium‐ and long‐term outcomes (3‐6 months and longer) | Unclear risk | No medium‐ or long‐term follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | Unclear risk | No baseline comparison. Imbalance in treatment adherence (not statistically significant) |
Manchanda 1997b.
| Methods | Double‐blind randomised controlled trial, addressing various types of warts (n = 124), including molluscum contagiosum (n = 20) | |
| Participants | 14 molluscum patients (sex distribution unknown) randomised to the treatment arm, 6 patients were randomised to receive plain sugar globules as a placebo (personal communication Dr Manchanda). 10 participants were aged below 10 years; 7 from 10 to 20 years; and 3 from 21 to 30 years (personal communication with Dr Manchanda). India, Homoeopathic Medical College & Hospital, New Delhi | |
| Interventions | Different potencies of homeopathic drug calcarea carbonica daily for 15 days (n = 14) versus sugar globules (placebo). Unclear which participants received what potency | |
| Outcomes | Improvement (not clear after what period) | |
| Notes | Paper reports on (1) cross‐over study and (2) parallel study. We excluded the cross‐over study because 1 arm had fewer than 5 participants. Funding: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "In this research design, each case was initially given a drug code in 30 potency which could be either active drug or placebo." Randomisation not mentioned in paper, "sequence was generated manually" (personal communication) |
| Allocation concealment (selection bias) | Unclear risk | Quote: "In this research design, each case was initially given a drug code in 30 potency which could be either active drug or placebo." "Therefore it was found that after decoding method of concealment is not described" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Two types of placebo controlled double‐blind clinical trials were undertaken." "The subjects were given both drug and placebo." Quote (personal communication): “The identity of the drugs was kept secret in a sealed cover which was opened only at the time un‐blinding the experiment." "The plain sugar globules looks like homoeopathic drug Calcerea carbonica was used as placebo." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Two types of placebo controlled double‐blind clinical trials were undertaken." "The subjects were given both drug and placebo." Quote (personal communication): “The identity of the drugs was kept secret in a sealed cover which was opened only at the time un‐blinding the experiment." "The plain sugar globules looks like homoeopathic drug Calcerea carbonica was used as placebo." |
| Incomplete outcome data (attrition bias) Short‐term outcomes (up to 3 months) | Low risk | 15 days: 20/124 dropouts, unclear what skin disease and group assignment |
| Incomplete outcome data (attrition bias) Medium‐ and long‐term outcomes (3‐6 months and longer) | Unclear risk | No long‐term outcome |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | No baseline comparison. No compliance data |
Markum 2012.
| Methods | Randomised controlled trial | |
| Participants | 53 children (aged 9 months to 15 years according to trial register), recruited in outpatient clinic in Idaho, USA | |
| Interventions | (1) iodine; (2) tea tree oil (Melaleuca alternifolia); (3) tea tree oil + iodine for 30 days | |
| Outcomes | Cure or reduction in the number of lesions > 90%, adverse effects, 30 days after start of treatment | |
| Notes | Funding: Center for Biomedical Research, a commercial institute involved in drug research and sale. Partner of Naturopathix (ZymaDerm for molluscum) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | According to trial register: "Subject or subject's parent blindly chose a numbered token from an opaque container containing tokens numbered 1‐99. Numbers 1‐33 assigned to iodine treatment, numbers 34‐66 assigned to tea tree oil treatment, numbers 67‐99 assigned to tea tree oil + iodine treatment" and "Randomization by sealed container" |
| Allocation concealment (selection bias) | Low risk | According to trial register: "Subject or subject's parent blindly chose a numbered token from an opaque container containing tokens numbered 1‐99. Numbers 1‐33 assigned to iodine treatment, numbers 34‐66 assigned to tea tree oil treatment, numbers 67‐99 assigned to tea tree oil + iodine treatment" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: “Patients and physicians were blinded to treatment protocol.” “A mild synthetic lemon fragrance not containing citral was added to scent the iodine olive oil preparation.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: “Patients and physicians were blinded to treatment protocol.” “A mild synthetic lemon fragrance not containing citral was added to scent the iodine olive oil preparation.” |
| Incomplete outcome data (attrition bias) Short‐term outcomes (up to 3 months) | Low risk | 5/53 participants (less than 20%) were lost to follow‐up (Markum 2012, from Table 1 of publication). |
| Incomplete outcome data (attrition bias) Medium‐ and long‐term outcomes (3‐6 months and longer) | Unclear risk | No long‐term data |
| Selective reporting (reporting bias) | Low risk | Reported outcomes similar to those mentioned in trial register |
| Other bias | Unclear risk | No baseline comparison. No compliance data |
Ohkuma 1990.
| Methods | Randomised controlled trial (written correspondence Dr Ohkuma), the method of generation of randomisation sequence remained unclear, as was the concealment of allocation. It was also unclear if participants were analysed according to the group to which they were randomised (intention‐to‐treat analysis) and how blinding was performed. | |
| Participants | 35 patients with molluscum contagiosum, aged between 2 and 9 years (M/F: 21/14), Japan, Department of Dermatology | |
| Interventions | 3 interventions were compared: 10% povidone iodine solution combined with 50% salicylic acid plaster (n = 20), iodine alone (n = 5), and salicylic plaster alone (n = 10). | |
| Outcomes | Time to cure. Study duration unknown, but paper indicated that treatment continued as long as necessary; range was 7 to 64 days; mean 26 days for iodine + plaster, 86 days for iodine only, and 47 days for plaster only . | |
| Notes | No baseline comparison, no compliance data Funding: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised (personal communication, not in paper). Insufficient information about the sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Iodine versus salicylic plaster: hard to mask |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Iodine versus salicylic plaster: hard to mask |
| Incomplete outcome data (attrition bias) Short‐term outcomes (up to 3 months) | Low risk | No loss reported, all participants in outcome table. Follow‐up period unclear. Duration of treatment ranged from 7 to 64 days. |
| Incomplete outcome data (attrition bias) Medium‐ and long‐term outcomes (3‐6 months and longer) | Unclear risk | No medium‐ or long‐term follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Quote: "In the former, two girls and three boys between the age of 3 and 5 were included and 4 girls and 6 boys between 2 and 9 comprised the latter control group." No imbalance for sex. No compliance data |
Ormerod 1999.
| Methods | Group sequential double‐blind randomised trial | |
| Participants | 30 molluscum patients were enrolled, 16 in the acidified nitrite group and 14 controls, with a median age of 6 years, 22 girls and 8 boys. UK, Department of Dermatology | |
| Interventions | 5% sodium nitrite co‐applied daily with 5% salicylic acid under occlusion versus identical cream with 5% salicylic acid omitting sodium nitrite, for 3 months | |
| Outcomes | Time to complete resolution, adverse events, study duration 3 months | |
| Notes | No baseline imbalance for duration and number of lesions, no compliance data. Funding: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Group sequential design in which subjects were randomised to receive either"; insufficient information about the sequence generation |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Double‐blind, group sequential design in which subjects were randomised to receive either 5% sodium nitrite co‐applied with 5% salicylic acid under occlusion, or identical cream with 5% salicylic acid but omitting sodium nitrite, as a control." Not done, active intervention was associated with brown staining. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Double‐blind, group sequential design in which subjects were randomised to receive either 5% sodium nitrite co‐applied with 5% salicylic acid under occlusion, or identical cream with 5% salicylic acid but omitting sodium nitrite, as a control." Not done, active intervention was associated with brown staining. |
| Incomplete outcome data (attrition bias) Short‐term outcomes (up to 3 months) | Unclear risk | Only long‐term data |
| Incomplete outcome data (attrition bias) Medium‐ and long‐term outcomes (3‐6 months and longer) | High risk | 21/30 dropouts after 3 months (> 30%) |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | No compliance data. Duration and number of lesions were very similar (communication with author). |
Paller 2005a.
| Methods | Randomised controlled trial | |
| Participants | 125 children, 1 to 12 years of age, with molluscum contagiosum in 9 outpatient clinics in the USA and Canada were randomised. | |
| Interventions | Imiquimod cream 5% vs vehicle cream daily for 8 weeks | |
| Outcomes | Lesion clearance, lesion counts, time to complete clearance, side effects, 12 weeks after start of treatment | |
| Notes | Funding by pharmaceutical company (3M), unpublished | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Subjects were randomly assigned to a treatment arm in blocks of 4 according to a computer‐generated randomizations.” (p.32) |
| Allocation concealment (selection bias) | Low risk | “Subjects/legal parental custodian(s) and investigators were unaware of the study assignment” (p.25) "This was a double‐blind, vehicle‐controlled study. 3M held the master code for the treatment randomizations schedule and supplied the investigators with each subject’s randomizations code as a hidden (tear‐off) disclosure panel on the study cream carton label. The randomizations code for an individual subject was to be broken only in case of an emergency, such as a serious adverse event (SAE)." (p.34) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | See allocation concealment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See allocation concealment. |
| Incomplete outcome data (attrition bias) Short‐term outcomes (up to 3 months) | Low risk | Not applicable: primary analysis by intention‐to‐treat |
| Incomplete outcome data (attrition bias) Medium‐ and long‐term outcomes (3‐6 months and longer) | Unclear risk | No long‐term outcomes |
| Selective reporting (reporting bias) | Low risk | All outcomes seem to have been reported. |
| Other bias | Low risk | No baseline imbalance, compliance data available, primary analysis by intention‐to‐treat |
Paller 2005b.
| Methods | Randomised controlled trial | |
| Participants | 379 children, 2 to 12 years of age, with molluscum contagiosum in 19 outpatient clinics in the USA were randomised. | |
| Interventions | Imiquimod cream 5% vs vehicle cream 3 times weekly for 16 weeks | |
| Outcomes | Lesion clearance, lesion counts, time to complete clearance, side effects, 4, 8, 12, 16, 18, and 28 weeks after start of treatment | |
| Notes | Funding by pharmaceutical company (3M), unpublished | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Subjects were randomly assigned to a treatment arm in blocks of 6 according to a computer‐generated randomizations schedule. Randomization was 2:1 (active:vehicle)" (p.40) |
| Allocation concealment (selection bias) | Low risk | "This was a double‐blind, vehicle‐controlled study; accordingly, the treatment assignments were concealed from the subjects, investigators and study staff, and the 3M clinical research team. The clinical packaging group at 3M Pharmaceuticals held the master code for the treatment randomizations schedule, and supplied the investigators with each subject’s treatment assignment as a hidden (tear‐off) panel on the study cream label, which was affixed to the blinded Drug Label page" (p.43) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | See allocation concealment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See allocation concealment. |
| Incomplete outcome data (attrition bias) Short‐term outcomes (up to 3 months) | Low risk | Primary analysis by intention‐to‐treat |
| Incomplete outcome data (attrition bias) Medium‐ and long‐term outcomes (3‐6 months and longer) | Low risk | 48/379 discontinued, reasons were mentioned (< 30%). |
| Selective reporting (reporting bias) | Low risk | All outcomes seem to have been reported. |
| Other bias | Low risk | No baseline imbalances, reported on compliance, primary analysis by intention‐to‐treat |
Saryazdi 2004.
| Methods | Randomised trial | |
| Participants | 30 children, age and sex unknown, Iran, hospital dermatology clinic | |
| Interventions | Topical benzoyl peroxide 10% cream versus tretinoin 0.05% cream, 2 times daily for 4 weeks | |
| Outcomes | Lesion count, lesion free, and side effects, 6 weeks after start of treatment | |
| Notes | Information based on abstract; proportions cured used to estimate absolute numbers. Abstract published in 2004; unclear when study was carried out. Funding: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Investigator masked"; no further details |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Investigator masked"; no further details |
| Incomplete outcome data (attrition bias) Short‐term outcomes (up to 3 months) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) Medium‐ and long‐term outcomes (3‐6 months and longer) | Unclear risk | No medium‐ or long‐term follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Efficacy was assessed at 2, 4, and 6 weeks, but the paper only reports results at week 6. |
| Other bias | Unclear risk | No baseline characteristics or compliance data |
Seo 2010.
| Methods | Randomised controlled trial | |
| Participants | 30 patients, 1 to 36 years of age, setting unclear, Korea | |
| Interventions | Imiquimod cream 5% versus potassium hydroxide solution 10% "for 3 months" (see Notes) | |
| Outcomes | Cure, adverse effects | |
| Notes | Applied medication until all lesions were cleared. Mean duration of treatment > 4 months; this is inconsistent with 'time after treatment'. Funding: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open study, method of application differed between treatment arms. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open study, so investigators were aware of treatment assignment. |
| Incomplete outcome data (attrition bias) Short‐term outcomes (up to 3 months) | Low risk | 3 participants lost to follow‐up (< 20%) |
| Incomplete outcome data (attrition bias) Medium‐ and long‐term outcomes (3‐6 months and longer) | Low risk | 3 participants lost to follow‐up (< 30%) |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk | Imbalance in sex. No compliance data |
Short 2006.
| Methods | Double‐blind, randomised, placebo‐controlled trial | |
| Participants | 20 children from a paediatric dermatology clinic, age range 2 to 12 years, M/F 6/14. UK, Department of Dermatology, London | |
| Interventions | Application of 10% potassium hydroxide solution twice daily applied with a cotton swab, continued until the lesions showed signs of inflammation (n = 10). The control group received saline (n = 10), for a maximum of 3 months. | |
| Outcomes | Time to resolution, adverse events 3 months after start of treatment | |
| Notes | Number of participants who completed the study differs between unpublished paper (18/20) and published paper (19/20). Latter number included in corrected version of 2009 update (December 2009). Funding: not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The children were randomly allocated by the dispensing pharmacist to receive one of two treatments". Insufficient information |
| Allocation concealment (selection bias) | Low risk | Quote: "The children were randomly allocated by the dispensing pharmacist to receive one of two treatments." Central allocation: pharmacy controlled |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Both the patients and the observer were blinded". "Both solutions were dispensed in identical, unlabeled bottles. The sequence was not revealed until the end of the study." Staining and stinging reported in the potassium hydroxide group. Participant, care provider, and outcome assessor probably blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Both the patients and the observer were blinded". "Both solutions were dispensed in identical, unlabeled bottles. The sequence was not revealed until the end of the study." Staining and stinging reported in the potassium hydroxide group. Participant, care provider, and outcome assessor probably blinded. |
| Incomplete outcome data (attrition bias) Short‐term outcomes (up to 3 months) | Low risk | 2 weeks: 1/20 did not complete study. 1/10 in the potassium hydroxide group withdrew after 2 weeks because of discomfort of the skin localised to the application site. |
| Incomplete outcome data (attrition bias) Medium‐ and long‐term outcomes (3‐6 months and longer) | Low risk | Not applicable: no long‐term results |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | No baseline imbalance for sex, lesion site, and numbers. No compliance data |
Theos 2004.
| Methods | Randomised controlled trial | |
| Participants | 23 children, 1 to 9 years of age, M/F 12/11, USA, Alabama, Illinois, New York | |
| Interventions | Imiquimod cream 5% or vehicle 3 times a week for 12 weeks | |
| Outcomes | Complete or partial clearance (> 30% decrease from baseline lesion count), adverse events after 4, 8, and 12 weeks | |
| Notes | Presented as a pilot study. Funding: not mentioned, but 1 of the authors was reported to be a consultant for 3M Pharmaceuticals, and was also the principal investigator of 2 other unpublished studies funded by this company (Paller 2005a; Paller 2005b). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Eligible patients were randomised to either imiquimod or vehicle". Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Eligible patients were randomised to either imiquimod or vehicle". Insufficient information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "In a Double Blind, Randomized Pilot Trial"; "imiquimod vs vehicle". Only participants and physicians involved, so probably at low risk of bias. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "In a Double Blind, Randomized Pilot Trial"; "imiquimod vs vehicle". Only participants and physicians involved, so probably at low risk of bias. |
| Incomplete outcome data (attrition bias) Short‐term outcomes (up to 3 months) | Low risk | 2 weeks: 2/23 did not complete the study (discontinued treatment) |
| Incomplete outcome data (attrition bias) Medium‐ and long‐term outcomes (3‐6 months and longer) | Unclear risk | No medium‐ or long‐term follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Unclear |
| Other bias | Unclear risk | Baseline imbalance for mean lesion count, imiquimod: 27.0 versus vehicle: 19.4 (not statistically significant). No compliance data |
Uçmak 2013.
| Methods | Randomised controlled trial | |
| Participants | 29 children, 15 months to 18 years of age, outpatient clinic, Turkey | |
| Interventions | Potassium hydroxide 2.5% versus potassium hydroxide 5% twice daily for 60 days | |
| Outcomes | Cure, adverse effects after 15, 30, 45, and 60 days | |
| Notes | Funding: not mentioned, but authors report having no conflicts of interest. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details except "randomised study" |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The solution vials were indistinguishable, regardless of content, and were stored at room temperature.” But paper does not use the word 'blinded'. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear whether outcome assessors were aware of treatment assignment |
| Incomplete outcome data (attrition bias) Short‐term outcomes (up to 3 months) | Low risk | 1 participant in potassium hydroxide 2.5% group and 2 participants in potassium hydroxide 5% group "removed from study" due to irregular attendance at follow‐up visits. 1 further participant in potassium hydroxide 5% group quit study due to excessive burning. Total number of loss to follow‐up: 4/29 (< 20%) |
| Incomplete outcome data (attrition bias) Medium‐ and long‐term outcomes (3‐6 months and longer) | Unclear risk | No long‐term outcomes |
| Selective reporting (reporting bias) | Unclear risk | No protocol |
| Other bias | Unclear risk | No baseline comparison, no compliance data |
KOH: potassium hydroxide MC: molluscum contagiosum NS: normal saline
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arican 2006 | Open‐label study, imiquimod 5% cream (n = 12) |
| Barton 2002 | HIV‐infected patients (n = 40) |
| Bayerl 2003 | Open‐label study, imiquimod 5% cream (n = 13) |
| Caballero 1996 | RCT comparing 2 types of cryotherapy for cutaneous skin lesions; 124 participants, of which 10 were molluscum patients, distributed 9:1 over 2 arms |
| Can 2014 | Patient series, 10% potassium hydroxide (n = 40) |
| Cathcart 2009 | Patient series, topical cantharidin (n = 54) |
| Chatproedrai 2007 | Pulsed dye laser (n = 20), not randomised (personal communication) |
| de Waard 1990 | Study on analgesic effect of lidocaine/prilocaine (EMLA) cream before physical therapy. Not a focus of this review (n = 83) |
| He 2001 | Large parallel controlled study (n = 1656), with 4 arms, no randomisation (personal communication with Dr He through Taixiang Wu) |
| Hengge 2000 | Open‐label study, imiquimod 5% cream, no control group, patients with common warts or molluscum contagiosum (n = 65) |
| Holt 2011 | Patient series, topical treatment with Manuka honey (n = 15) |
| Juhlin 1980 | Study on analgesic effect of lidocaine/prilocaine (EMLA) cream before physical therapy. Not a focus of this review (n = 24) |
| Lim 2003 | Patient series of topical 5% imiquimod (n = 4) |
| Manchanda 1997a | Cross‐over study of patients with different types of warts (n = 43), 10 molluscum patients. 1 of the treatment arms (placebo first?) had fewer than 2 participants. |
| Metkar 2008 | Non‐randomised, comparative study of imiquimod 5% cream versus 10% potassium hydroxide (n = 40) |
| Myhre 2008 | Open‐label study, imiquimod 5% cream, no control group (n = 22) |
| Puri 2009 | RCT of people with sexually transmitted disease, not a focus of this review |
| Rosdahl 1988 | Study on analgesic effect of lidocaine/prilocaine (EMLA) cream before physical therapy. Not a focus of this review (n = 55) |
| Sadick 2009 | Randomised split‐face study in 20 patients with disseminated facial molluscum contagiosum and HIV infection, not a focus of this review |
| Salmanpour 2006 | Not randomised but alternate assignment (personal communication, Alireza Firooz) |
| Schalka 2010 | RCT comparing two topical analgesics before curettage (n = 40). Not a focus of this review |
| Simonart 2008 | Not a randomised trial, curettage (n = 73) |
| Skinner 2000 | Case report, 3 children, topical imiquimod 5% |
| Syed 1994 | RCT, n = 150, mainly genital lesions, not a focus of this review |
| Syed 1998 | RCT, n = 100, mainly genital lesions, not a focus of this review |
| Weller 1999 | Controlled trial (n = 16) comparing phenol ablation and physical expression. Lesions were unit of treatment and analysis. No randomisation |
| Yabut‐Catalasan 2003 | Controlled trial (n = 34) of children aged 2 to 12 years. 10% potassium hydroxide versus placebo. Not randomised, but alternate assignment |
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Elzawahry 1964.
| Methods | Unknown, no full‐text paper and no abstract |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Köse 2013.
| Methods | Randomised trial |
| Participants | Patients with molluscum contagiosum in Turkey |
| Interventions | 10% potassium hydroxide solution versus salicylic and lactic acid combination |
| Outcomes | Lesion response and side effects |
| Notes | Full text obtained August 2016. Email contact about unclear randomisations ("Patients were randomised into two treatment groups according to appealing number." "The treatment groups were not matched with the baseline characteristics because of the randomizations by application rank." (p.301) (8 August 2016) |
Muzaffar 2014.
| Methods | Possibly randomised (participants were divided into 2 groups) |
| Participants | Children aged 2 to 14 years with molluscum contagiosum in Pakistan |
| Interventions | 5% or 10% potassium hydroxide solution |
| Outcomes | Complete clearance, partial clearance, adverse effects |
| Notes | Summer 2016: asked author for additional information regarding randomisation (unclear from paper) |
NCT01348386.
| Methods | Double‐blind, randomised clinical trial, in 3 groups |
| Participants | Children aged 2 to 6 years with molluscum contagiosum in Spain. Planned number of participants: 60 |
| Interventions | Application of topical 10% potassium hydroxide in an aqueous solution; 15% potassium hydroxide; placebo |
| Outcomes | Primary outcome: efficacy (disappearance of lesions) after 60 days |
| Notes | Email correspondence in January 2015: results are expected soon |
NCT02665260.
| Methods | From trial register: Allocation: Randomized Endpoint Classification: Safety/Efficacy Study Intervention Model: Crossover Assignment Masking: Double Blind (Subject, Caregiver, Investigator) Primary Purpose: Treatment |
| Participants | n=100 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Cantharidin 0.7% topical, cantharidin 0.7% topical with occlusion, placebo, or placebo with occlusion. Treatments were applied at weeks 0 and 3 (blinded phase). At week 6, all participants were treated with open‐label, topical cantharidin 0.7% without occlusion every 3 weeks until all lesions resolved (open‐label phase). |
| Outcomes | Primary outcome measures: Percentage of participants who achieve complete clearance at 6 weeks and 33 weeks [Time Frame: 33 weeks] [Designated as safety issue: No] Assess percentage of participants who achieve a lesion count of zero at 6 weeks (end of blinded phase) and 33 weeks (end of open‐label phase) Secondary outcome measures: Frequency of adverse events [Time Frame: 33 weeks] [Designated as safety issue: No] Assessed by patient‐reported outcomes questionnaire at each visit |
| Notes | Study completion date: January 2016 |
Rajouria 2011.
| Methods | Randomised (abstract) or non‐randomised (methods) controlled clinical trial |
| Participants | Children with molluscum contagiosum in tertiary care centre in Nepal |
| Interventions | 5% potassium hydroxide solution versus 0.05% tretinoin cream |
| Outcomes | Number of lesions, local and systemic side effects |
| Notes | February 2015: asked author for additional information regarding randomisation (conflicting statements in paper) |
Tanissa 1951.
| Methods | Unknown, no full‐text paper or abstract |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Unknown Chinese author 1991.
| Methods | Unknown, no full‐text paper or abstract |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes |
Characteristics of ongoing studies [ordered by study ID]
NCT02024581.
| Trial name or title | A Dose Range‐Finding Phase 2 Trial of a Botanical Drug for the Treatment of Molluscum Contagiosum in Pediatric Subjects (original title, later changed to: A Single‐center, Double‐blind, Placebo‐controlled, Randomized Safety and Efficacy Trial of a Botanical Drug Product, East Indian Sandalwood Oil (EISO), at One Dose Level for the Treatment of Molluscum Contagiosum in Pediatric Subjects |
| Methods | Double‐blind, randomised controlled trial |
| Participants | Children 2 to 17 years of age with molluscum contagiosum, in Texas USA, planned number of participants: 60 |
| Interventions | 10% East Indian sandalwood oil cream administered twice a day for 90 days versus placebo cream |
| Outcomes | The primary purpose of this study is to determine the safety profile of East Indian sandalwood. Safety will be assessed by evaluating adverse events with respect to severity, duration, and relationship to study drug compared to placebo. Secondary outcomes: change in lesion count; improvement in Global Aesthetic Improvement Scale score; complete resolution of lesions; improvement in Evaluator's Global Severity Score. |
| Starting date | September 2015, estimated study completion date September 2016 |
| Contact information | Dr John C Browning, drbrowning@texasdls.com |
| Notes | See History of Changes in trial register (duration changed from 60 to 90 days; upper age limit changed; 3 strengths changed into 1; dates; title changed). Results of previously announced dose‐finding study unknown, asked by email July 2015. |
Differences between protocol and review
The title of the published protocol was inadvertently left as 'Interventions for molluscum contagiosum in children', although a decision had been made not to restrict the review to children.
Differences between the protocol and the current update
For differences between other published versions, please see the 'Differences between protocol and review' sections within the original publications.
Objectives: In the protocol, we had planned to assess the effects of treatments, but in this and the previous updates we broadened this to include management strategies because waiting for natural resolution is a recognised option for dealing with molluscum contagiosum. We amended the text from that which was in the protocol to make our objectives more clear.
Types of studies: In the protocol, we said that "studies should compare one or more treatments with another, with placebo, or with no treatment (waiting for natural response)"; we removed this sentence in this and previous updates because it refers to comparisons rather than studies.
Types of interventions: We had planned to include randomised trials of all treatments for molluscum contagiosum, but narrowed this to include only treatments aimed at eradicating molluscum contagiosum lesions, and excluded studies on other aspects of the treatment of molluscum contagiosum, for example on reducing pain in the studies that assessed the effect of using an analgesic EMLA (eutectic mixture of local anaesthetics) cream before the actual intervention took place. This was because the analgesic was not used to eradicate the molluscum lesions.
Primary outcomes: We decided that our original choice for 'short‐term clinical cure' of one month was not realistic, and therefore changed it to three months. We have also clarified our primary outcome to make it more manageable: short‐term clinical cure (up to three months after treatment). (Please see Overall completeness and applicability of evidence for a more detailed description of why we felt the original choice was not realistic.) We also deleted the term 'elevated' in the description, as we felt it was unnecessary and could possibly cause confusion, the implication being that there are elevated and non‐elevated forms of the lesion.
Where included studies used the term 'complete clearance' or 'free of lesions' or 'cured or > 90% cleared', we classed these as our primary outcome 'short‐term clinical cure (up to three months after start of treatment)' or our secondary outcome 'medium‐ and long‐term cure (after three months and up to six months, and after six months, respectively)'. Where studies have referred to 'partial clearance', we took this to mean our secondary outcome 'improvement'.
Secondary outcomes: We did not initially specify the outcome 'disease‐related quality of life' in the protocol, but added it afterwards as we considered it to be a relevant additional measure.
We also added 'short‐, medium‐, and long‐term improvement (including cure, intervals as above)' as a secondary outcome as we considered it to be important. For this outcome we combined 'improvement' and 'cure' (even though cure alone was a seperate outcome) because 'improvement' would be hard to interpret without also including those who were cured. For example: suppose in group A, 30% were cured and another 20% improved. In group B, 40% were cured and 10% improved. Comparing improvement rates between A and B (20% versus 10%) is misleading, whereas combining cure and improvement (50% versus 50%) is not.
Electronic searches: We expanded the number of trial registries that we planned to search when we became aware of the existence of these registries and in line with current Cochrane Skin practices. For similar reasons, we added Google as an additional electronic search strategy.
Selection of studies: If a randomised controlled trial included a variety of skin diseases, of which one was molluscum contagiosum, the number of molluscum participants needed to be at least five in the active treatment and placebo groups in order to reduce the role of extremely small studies. We added this criterion after the protocol was approved when we found a study that included 10 molluscum participants with a 9:1 distribution over the two treatment groups (Caballero 1996). The criterion also applied to Manchanda 1997a.
Selection of studies: If the setting of the study was not explicitly mentioned in the text, we assumed it to be carried out at the affiliation of the first author. Also, if the full text of a study was not available, we considered published abstracts for this update, as we have done this for previous versions of the review.
Assessment of risk of bias in included studies: In this update, we assessed each study using Cochrane's 'Risk of bias' tool (Higgins 2011), as this is now required. Also items (5), (6) and (7), which differed from the original protocol or were absent, were added or amended for the 2009 update as recommended. In previous versions of this review, items (3) and (4) were combined. For the 2016 update we further clarified how we decided what constituted an 'adequate' assessment and therefore low risk of bias.
Measures of treatment effect: Following the recent Cochrane Skin Group recommendations, we decided post hoc to re‐analyse results from individual studies with borderline significance and with low numbers of events (fewer than 10 in total) or a total sample size of less than 30, using Fisher’s exact test. The resulting P value was leading in interpreting the results.
Data synthesis: We had planned to express dichotomous results as odds ratios, but changed this to risk ratios and as a number needed to treat where appropriate because these are easier for most readers to understand. We decided to report numbers needed to treat only for comparisons with more than one study and only in the case of statistically significant differences, the latter because numbers needed to treat for differences that are statistically not significant produce large and uncertain confidence intervals.
When the same comparison between two interventions was made in more than one study, and studies appeared to have been executed in similar groups and settings, we planned to use statistical tests for homogeneity between studies. In those studies where the available data were sufficiently homogenous and where a pooled estimate of the treatment effect made sense, we planned to conduct a meta‐analysis. However, we could not implement these plans in most cases due to lack of data.
Assessment of reporting biases: Subsequent to the protocol, we aimed to assess reporting bias by comparing the published trial publications with the study protocol, but no protocols were available.
Unit of analysis issues: In our methods we planned to use special analytic techniques for paired (split‐body) designs; however, we were unable to do this as the paired data were not available to us.
Dealing with missing data: Although this was not specified in the protocol, we considered participants who dropped out or were lost to follow‐up as treatment failures.
Unit of analysis issues/Assessment of heterogeneity/Sensitivity analysis: We had planned analyses not documented in the protocol, including the use of sensitivity analyses to examine the effects of excluding studies with lower reported methodological quality, as well as how to analyse cross‐over trials and within‐participant designed trials. However, we did not undertake these analyses because of the small number of studies for each comparison.
Sensitivity analysis: We planned to use sensitivity analyses to examine the effects of excluding studies with high risk of bias. However, we did not undertake these analyses because of the small number of studies for each comparison.
Summary of findings: We developed 'Summary of findings' tables subsequent to our protocol. We have produced one for this update.
Quality of evidence: We used GRADE to assess the quality of evidence for each primary outcome and key secondary outcomes.
Contributions of authors
JCvdW was the contact person with the editorial base, co‐ordinated contributions from the coauthors, and wrote the final draft of the review. SK, EJK, RvdS, and JCvdW screened papers against the eligibility criteria. JCvdW obtained data on ongoing and unpublished studies. SK, EJK, RvdS, and JCvdW appraised the quality of papers. SK, EJK, RvdS, and JCvdW extracted data for the review and sought additional information about papers. JCvdW and EJK entered data into Review Manager 5. JCvdW and EJK analysed and interpreted data. JCvdW worked on the Methods section. All review authors commented on draft versions of this update. SK drafted the clinical sections of the Background and responded to the clinical comments of the referees. JCvdW responded to the methodology and statistics comments of the referees. AS was the consumer coauthor and checked the review for readability and clarity, as well as ensuring outcomes were relevant to consumers. JCvdW is the guarantor of the update.
Disclaimer
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Skin Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Sources of support
Internal sources
-
Department of General Practice, Erasmus MC, Rotterdam, Netherlands.
in kind support
-
Department of General Practice and Elderly Care Medicine, VUmc University Medical Center, Amsterdam, Netherlands.
in kind support
External sources
-
The National Institute for Health Research (NIHR), UK.
The NIHR, UK, is the largest single funder of the Cochrane Skin Group.
Declarations of interest
Johannes C van der Wouden: My institution has received money from the following non‐profit sources which have no real or potential vested interest in the findings of this Cochrane review: Editorial Board, Huisarts en Wetenschap (monthly journal of Dutch College of GPs); Advisory Board of Achmea Health Database (health insurance company). The funds did not come from sources that produce any of the drugs that might be included in the review or competitors to the drugs in the review.
Renske van der Sande: nothing to declare. Emma J Kruithof: nothing to declare. Annet Sollie: nothing to declare. Lisette WA van Suijlekom‐Smit: I have received a travel grant from Pfizer (American College of Rheumatology Annual Meeting 2014 Boston). Sander Koning: nothing to declare.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Al‐Mutairi 2010 {published data only}
- Al‐Mutairi N, Al‐Doukhi A, Al‐Farag S, Al‐Haddad A. Comparative study on the efficacy, safety, and acceptability of imiquimod 5% cream versus cryotherapy for molluscum contagiosum in children. Pediatric Dermatology 2010;27(4):388‐94. [CENTRAL: CN‐00760650; PUBMED: 19804497] [DOI] [PubMed] [Google Scholar]
Antony 2001 {published data only}
- Antony F, Cliff S, Ahmad A, Holden C. Double‐blind placebo‐controlled study of oral cimetidine treatment for molluscum contagiosum. British Journal of Dermatology 2001;145(Suppl 59):126. [CENTRAL: CN‐00430863] [Google Scholar]
Bazza 2007 {unpublished data only}
- Bazza MA, Ryatt KS. Sterile normal 0.9% saline as a effective 5% potassium hydroxide in treatment of molluscum contagiosum, and safer (title incomplete). Unpublished manuscript 2007:1‐4. [CENTRAL: CN‐00747643]
Burke 2004 {published data only}
- Burke BE, Baillie JE, Olson RD. Essential oil of Australian lemon myrtle (Backhousia citriodora) in the treatment of molluscum contagiosum in children. Biomedecine & Pharmacotherapie [Biomedicine & Pharmacotherapy] 2004;58(4):245‐7. [CENTRAL: CN‐00489728; PUBMED: 15183850] [DOI] [PubMed] [Google Scholar]
Chathra 2015 {published data only}
- Chathra N, Sukumar D, Bhat RM, Kishore BN, Martis J, Kamath G, et al. A comparative study of 10% KOH solution and 5% imiquimod cream for the treatment of molluscum contagiosum in the pediatric age group. Indian Dermatology Online Journal 2015;6(2):75–80. [CTRI/2013/08/003941; PUBMED: 25821725] [DOI] [PMC free article] [PubMed] [Google Scholar]
Coloe Dosal 2014 {published data only}
- Coloe Dosal J, Stewart PW, Lin JA, Williams CS, Morrell DS. Cantharidin for the treatment of molluscum contagiosum: a prospective, double‐blinded, placebo‐controlled trial. Pediatric Dermatology 2014;31(4):440‐9. [CENTRAL: CN‐00999446; PUBMED: 22897595] [DOI] [PMC free article] [PubMed] [Google Scholar]
Eichenfield 2005 {unpublished data only}
- EIchenfield LF. Phase‐III double‐blind vehicle‐controlled study of imiquimod cream 5% for the treatment of molluscum contagiosum in pediatric subjects (3M Pharmaceuticals study number 1494‐IMIQ). Unpublished study report. 3M Pharmaceuticals, St. Paul (MN, USA) 2005.
Handjani 2014 {published data only}
- Handjani F, Behazin E, Sadati MS. Comparison of 10% potassium hydroxide solution versus cryotherapy in the treatment of molluscum contagiosum: an open randomized clinical trial. Journal of Dermatological Treatment 2014;25(3):249‐50. [CENTRAL: CN‐00961439; PUBMED: 23924070] [DOI] [PubMed] [Google Scholar]
Hanna 2006 {published data only}
- Hanna D, Hatami A, Powell J, Marcoux D, Maari C, Savard P, et al. A prospective randomized trial comparing the efficacy and adverse effects of four recognized treatments of molluscum contagiosum in children. Pediatric Dermatology 2006;23(6):574‐9. [CENTRAL: CN‐00609029; PUBMED: 17156002] [DOI] [PubMed] [Google Scholar]
- McCuaig CC, Hatami A, Powell J, Maari C, Marcoux D, Thibeault H. Mollusca contagiosa: what treatment to use when?. Journal of the European Academy of Dermatology and Venereology : JEADV 2005;19(Suppl 2):8. [CENTRAL: CN‐00602169] [Google Scholar]
Leslie 2005 {published data only}
- Leslie KS, Dootson G, Sterling JC. Does treatment of molluscum contagiosum affect clearance?. British Journal of Dermatology 2004;151(Suppl 68):67. [CENTRAL: CN‐00487868] [Google Scholar]
- Leslie KS, Dootson G, Sterling JC. Topical salicylic acid gel as a treatment for molluscum contagiosum in children. Journal of Dermatological Treatment 2005;16(5‐6):336‐40. [CENTRAL: CN‐00554512; PUBMED: 16428156] [DOI] [PubMed] [Google Scholar]
Machado 2010 {published data only}
- Machado RB, Leal TF, Bonfa R, Werlang ME, Blessmann Weber M. Molluscum contagiosum in children: comparative treatments [Molusco contagioso em crianças: tratamentos comparativos]. Surgical & Cosmetical Dermatology 2010;2:272‐5. [Google Scholar]
Manchanda 1997b {published data only}
- Manchanda RK, Mehan N, Bahl R, Atey R. Double blind placebo controlled clinical trials of homeopathic medicines in warts and molluscum contagiosum. Central Council of Research in Homeopathy Quarterly Bulletin 1997;19(3&4):25‐9. [CENTRAL: CN‐00478663] [Google Scholar]
Markum 2012 {published data only}
- Markum E, Baillie J. Combination of essential oil of Melaleuca alternifolia and iodine in the treatment of molluscum contagiosum in children. Journal of Drugs in Dermatology 2012;11(3):349‐54. [CENTRAL: CN‐00880968; PUBMED: 22395586] [PubMed] [Google Scholar]
Ohkuma 1990 {published data only}
- Ohkuma M. Molluscum contagiosum treated with iodine solution and salicylic acid plaster. International Journal of Dermatology 1990;29(6):443‐5. [CENTRAL: CN‐00269315; PUBMED: 2397974] [DOI] [PubMed] [Google Scholar]
Ormerod 1999 {published data only}
- Ormerod AD, White MI, Shah SA, Benjamin N. Molluscum contagiosum effectively treated with a topical acidified nitrite, nitric oxide liberating cream. British Journal of Dermatology 1999;141(6):1051‐3. [CENTRAL: CN‐00274687; PUBMED: 10606851] [DOI] [PMC free article] [PubMed] [Google Scholar]
Paller 2005a {unpublished data only}
- Paller A. Double‐blind vehicle‐controlled study of imiquimod cream 5% in pediatric subjects with molluscum contagiosum to evaluate safety and efficacy of daily dosing during 8 weeks of treatment (3M Pharmaceuticals study number 1490‐IMIQ). Unpublished study report. 3M Pharmaceuticals, St. Paul (MN, USA) July 2005 (amendment April 2006).
Paller 2005b {unpublished data only}
- Paller A. Phase‐III double‐blind vehicle‐controlled study of imiquimod cream 5%, for the treatment of molluscum contagiosum in pediatric subjects (3M Pharmaceuticals study number 1495‐IMIQ). Unpublished study report. 3M Pharmaceuticals, St. Paul (MN, USA) November 2005.
Saryazdi 2004 {published data only}
- Saryazdi S. The comparative efficacy of benzoyl peroxide 10% cream and tretinoin 0.05% cream in the treatment of molluscum contagiosum. Pediatric Dermatology 2004;21(3):399. [CENTRAL: CN‐00520460] [Google Scholar]
Seo 2010 {published data only}
- Seo SH, Chin HW, Jeong DW, Sung HW. An open, randomized, comparative clinical and histological study of imiquimod 5% cream versus 10% potassium hydroxide solution in the treatment of molluscum contagiosum. Annals of Dermatology 2010;22(2):156‐62. [CENTRAL: CN‐01046394] [DOI] [PMC free article] [PubMed] [Google Scholar]
Short 2006 {published and unpublished data}
- Short KA, Fuller LC, Higgins EM. Double‐blind randomized placebo‐controlled trial of the use of topical potassium hydroxide in the treatment of molluscum contagiosum. British Journal of Dermatology 2002;147(Suppl 62):95. [CENTRAL: CN‐00487907; CENTRAL: CN‐00406990] [DOI] [PubMed] [Google Scholar]
- Short KA, Fuller LC, Higgins EM. Double‐blind, randomised, placebo‐controlled trial of the use of topical 10% potassium hydroxide solution of molluscum contagiosum. Unpublished manuscript. [DOI] [PubMed]
- Short KA, Fuller LC, Higgins EM. Double‐blind, randomized, placebo‐controlled trial of the use of topical 10% potassium hydroxide solution in the treatment of molluscum contagiosum. Pediatric Dermatology 2006;23(3):279‐81. [CENTRAL: CN‐00570792; PUBMED: 16780480] [DOI] [PubMed] [Google Scholar]
Theos 2004 {published data only}
- Theos AU, Cummins R, Silverberg NB, Paller AS. Effectiveness of imiquimod cream 5% for treating childhood molluscum contagiosum in a double‐blind, randomized pilot trial. Cutis 2004;74(2):134‐8,141‐2. [CENTRAL: CN‐00491567; PUBMED: 15379366] [PubMed] [Google Scholar]
Uçmak 2013 {published data only}
- Uҫmak D, Akkurt MZ, Kacar SD, Sula B, Arica M. Comparative study of 5% and 2.5% potassium hydroxide solution for molluscum contagiosum in children. Cutaneous and Ocular Toxicology 2014;33(1):54‐9. [CENTRAL: CN‐00982389; PUBMED: 23713782] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Arican 2006 {published data only}
- Arican O. Topical treatment of molluscum contagiosum with imiquimod 5% cream in Turkish children. Pediatrics International 2006;48(4):403‐5. [PUBMED: 16911087] [DOI] [PubMed] [Google Scholar]
Barton 2002 {published data only}
- Barton SE, Chard S. Facial molluscum: treatment with cryotherapy and podophyllotoxin. International Journal of STD & AIDS 2002;13(4):277‐8. [PUBMED: 11963912] [DOI] [PubMed] [Google Scholar]
Bayerl 2003 {published data only}
- Bayerl C, Feller G, Goerdt S. Experience in treating molluscum contagiosum in children with imiquimod 5% cream. British Journal of Dermatolology 2003;149(Suppl 66):25‐9. [PUBMED: 14616342] [DOI] [PubMed] [Google Scholar]
Caballero 1996 {published data only}
- Caballero Martinez F, Plaza Nohales C, Perez Canal C, Lucena Martin MJ, Holgado Catalán M, Olivera Cañadas G. Cutaneous cryosurgery in family medicine: dimethyl ether‐propane spray versus liquid nitrogen. Atencion Primaria 1996;18(5):211‐6. [PUBMED: 8963007] [PubMed] [Google Scholar]
Can 2014 {published data only}
- Can B, Topaloğlu F, Kavala M, Turkoglu Z, Zindancı I, Sudogan S. Treatment of pediatric molluscum contagiosum with 10% potassium hydroxide solution. Journal of Dermatological Treatment 2014;25(3):246‐8. [PUBMED: 22639976] [DOI] [PubMed] [Google Scholar]
Cathcart 2009 {published data only}
- Cathcart S, Coloe J, Morrell DS. Parental satisfaction, efficacy, and adverse events in 54 patients treated with cantharidin for molluscum contagiosum infection. Clinical Pediatrics 2009;48(2):161‐5. [PUBMED: 18936288] [DOI] [PubMed] [Google Scholar]
Chatproedrai 2007 {published data only}
- Chatproedrai S, Suwannakarn K, Wananukul S, Theamboonlers A, Poovorawan Y. Efficacy of pulsed dye laser (585 nm) in the treatment of molluscum contagiosum subtype 1. Southeast Asian Journal of Tropical Medicine & Public Health 2007;38(5):849‐54. [PUBMED: 18041301] [PubMed] [Google Scholar]
de Waard 1990 {published data only}
- Waard‐van der Spek FB, Oranje AP, Lillieborg S, Hop WC, Stolz E. Treatment of molluscum contagiosum using a lidocaine/prilocaine cream (EMLA) for analgesia. Journal of the American Academy of Dermatology 1990;23(4 Pt 1):685‐8. [PUBMED: 2229496] [DOI] [PubMed] [Google Scholar]
He 2001 {published data only}
- He H, Lu JY, Fang J, et al. Observation on effect of four kinds of therapy for molluscum contagiosum. Chinese Journal of Dermatovenereology 2001;15(5):308‐9. [CENTRAL: CN‐00454307] [Google Scholar]
Hengge 2000 {published data only}
- Hengge UR, Esser S, Schultewolter T, Behrendt C, Meyer T, Stockfleth E, et al. Self‐administered topical 5% imiquimod for the treatment of common warts and molluscum contagiosum. British Journal of Dermatology 2000;143(5):1026‐31. [PUBMED: 11069514] [DOI] [PubMed] [Google Scholar]
Holt 2011 {published data only}
- Holt S, Webster‐Longin M, Perrin K, Baker T, Beasley R. The use of honey in the treatment of molluscum contagiosum in children. Presented at World Dermatology Congress, Seoul, Korea. May 2011.
Juhlin 1980 {published data only}
- Juhlin L, Evers H, Broberg F. A lidocaine‐prilocaine cream for superficial skin surgery and painful lesions. Acta Dermato‐Venereologica 1980;60(6):544‐6. [PUBMED: 6162348] [DOI] [PubMed] [Google Scholar]
Lim 2003 {published data only}
- Lim DS, Egan CA. Insights into a novel treatment for molluscum. Acta Paediatrica 2003;92(2):265‐6. [PUBMED: 12710660] [DOI] [PubMed] [Google Scholar]
Manchanda 1997a {published data only}
- Manchanda RK, Mehan N, Bahl R, Atey R. Double blind placebo controlled clinical trials of homeopathic medicines in warts and molluscum contagiosum. Central Council for Research in Homeopathy Quarterly Bulletin 1997;19(3&4):25‐30. [CENTRAL: CN‐00478663] [Google Scholar]
Metkar 2008 {published data only}
- Metkar A, Pande S, Khopkar U. An open, nonrandomized, comparative study of imiquimod 5% cream versus 10% potassium hydroxide solution in the treatment of molluscum contagiosum. Indian Journal of Dermatology, Venereology and Leprology 2008;74(6):614‐8. [PUBMED: 19171985] [DOI] [PubMed] [Google Scholar]
Myhre 2008 {published data only}
- Myhre PE, Levy ML, Eichenfield LF, Kolb VB, Fielder SL, Meng TC. Pharmacokinetics and safety of imiquimod 5% cream in the treatment of molluscum contagiosum in children. Pediatric Dermatology 2008;25(1):88‐95. [PUBMED: 18304162] [DOI] [PubMed] [Google Scholar]
Puri 2009 {published data only}
- Puri N. A study on the use of imiquimod for the treatment of genital molluscum contagiosum and genital warts in female patients. Indian Journal of Sexually Transmitted Diseases 2009;30(2):84‐8. [PUBMED: 21938126] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rosdahl 1988 {published data only}
- Rosdahl I, Edmar B, Gisslén H, Nordin P, Lillieborg S. Curettage of molluscum contagiosum in children: analgesia by topical application of lidocaine/prilocaine cream (EMLA). Acta Dermato‐Venereologica 1988;68(2):149‐53. [PUBMED: 2453995] [PubMed] [Google Scholar]
Sadick 2009 {published data only}
- Sadick N, Sorhaindo L. A comparative split‐face study of cryosurgery and trichloroacetic acid 100% peels in the treatment of HIV‐associated disseminated facial molluscum contagiosum. Cutis 2009;83(6):299‐302. [PUBMED: 19681340] [PubMed] [Google Scholar]
Salmanpour 2006 {published data only}
- Salmanpour R, Razmavar MR, Seyf I. Treatment of molluscum contagiosum with griseofulvin or cryotherapy. Iranian Journal of Dermatology 2006;9(1):5. [CENTRAL: CN‐00615975] [Google Scholar]
Schalka 2010 {published data only}
- Schalka S, Addor FAS, Silva VDM, Pinto PC, Milan ALK, Pannuti EMB. A randomized, open‐label, comparative clinical study for the evaluation of safety, tolerability and analgesic efficacy of two different presentations of topical medication in the treatment of dermatological curettage of molluscum contagiosum in pediatric patients [Estudo clinico randomizado, aberto e comparativo para avaliacao de seguranca, tolerabilidade e eficacia analgesica de duas diferentes apresentacoes de medicamento topico na realizacao de curetagem dermatologica de molusco contagioso em pacientes pediatricos]. Revista Brasileira de Medicina 2010;67(6):201‐7. [CENTRAL: CN‐00803780] [Google Scholar]
Simonart 2008 {published data only}
- Simonart T, Maertelaer V. Curettage treatment for molluscum contagiosum: a follow‐up survey study. British Journal of Dermatology 2008;159(5):1144‐7. [PUBMED: 18795919] [DOI] [PubMed] [Google Scholar]
Skinner 2000 {published data only}
- Skinner RB Jr, Ray S, Talanin NY. Treatment of molluscum contagiosum with topical 5% imiquimod cream. Pediatric Dermatology 2000;17(5):420. [PUBMED: 11085678] [DOI] [PubMed] [Google Scholar]
Syed 1994 {published data only}
- Syed TA, Lundin S, Ahmad M. Topical 0.3% and 0.5% podophyllotoxin cream for self‐treatment of molluscum contagiosum in males. A placebo‐controlled, double‐blind study. Dermatology 1994;189(1):65‐8. [CENTRAL: CN‐00102127; PUBMED: 8003791] [DOI] [PubMed] [Google Scholar]
Syed 1998 {published data only}
- Syed TA, Goswami J, Ahmadpour OA, Ahmad SA. Treatment of molluscum contagiosum in males with an analog of imiquimod 1% in cream: a placebo‐controlled, double‐blind study. Journal of Dermatology 1998;25(5):309‐13. [PUBMED: 9640884] [DOI] [PubMed] [Google Scholar]
Weller 1999 {published data only}
- Weller R, O'Callaghan CJ, MacSween RM, White MI. Scarring in molluscum contagiosum: comparison of physical expression and phenol ablation. BMJ 1999;319(7224):1540. [PUBMED: 10591712] [DOI] [PMC free article] [PubMed] [Google Scholar]
Yabut‐Catalasan 2003 {published data only}
- Yabut‐Catalasan RO, Paliza AC. 10% Potassium Hydroxide as treatment for molluscum contagiosum: a double‐blind, placebo‐controlled study. Journal of the Philippine Dermatological Society 2003;12(1):28‐35. [CENTRAL: CN‐00602272] [Google Scholar]
References to studies awaiting assessment
Elzawahry 1964 {published data only}
- Zawahry M. Virus skin diseases: frequency and trial of antiviral agent. Journal of the Egyptian Medical Association 1963;46:1303‐14. [PUBMED: 14162107] [PubMed] [Google Scholar]
Köse 2013 {published data only}
- Köse O, Özmen I, Arca E. An open, comparative study of 10% potassium hydroxide solution versus salicylic and lactic acid combination in the treatment of molluscum contagiosum in children. Journal of Dermatological Treatment 2013;24(4):300‐4. [PUBMED: 22214282] [DOI] [PubMed] [Google Scholar]
Muzaffar 2014 {published data only}
- Muzaffar F, Faiz F. Comparison of 5% potassium hydroxide with 10% potassium hydroxide solution in treatment of molluscum contagiosum: comparative study. Journal of Pakistan Association of Dermatologists 2014;24(4):337‐41. [www.jpad.org.pk/Oct%20Dec%202014/11.%20Original%20article%20KoH%20in%20molluscum%20contagiosum.pdf] [Google Scholar]
NCT01348386 {published data only}
- NCT01348386. Efficacy and tolerance of potassium hydroxide (10% and 15%) in molluscum contagiosum (EKOH‐MOL 2008) [Efficacy and tolerance of the topical application of potassium hydroxide (10% and 15%) in the treatment of molluscum contagiosum]. clinicaltrials.gov/ct2/show/NCT01348386 Date first received: 4 May 2011.
NCT02665260 {unpublished data only}
- NCT02665260. Safety and efficacy study of topical cantharidin for the treatment of molluscum contagiosum [Randomized pilot study investigating the safety and efficacy of topical cantharidin for the treatment of molluscum contagiosum]. clinicaltrials.gov/ct2/show/NCT02665260 Date first received: 31 December 2016.
Rajouria 2011 {published data only}
- Rajouria EA, Amatya A, Karn D. Comparative study of 5% potassium hydroxide solution versus 0.05% tretinoin cream for Molluscum Contagiosum in children. Kathmandu University Medical Journal 2011;9(36):291‐4. [PUBMED: 22710541] [DOI] [PubMed] [Google Scholar]
Tanissa 1951 {published data only}
- Tanissa A. 62 cases of molluscum contagiosum in a boarding‐school; trial therapy with sulfonamides, podophyllin and aureomycin [Sessenta e dois casos de "molluscum contagiosum" em um internato. Ensaios teraputicos pelas sulfonamidas, podofilina e aureomicina]. Gazeta Médica Portuguesa 1951;4(1):77‐83. [ref 44 of Cochrane Skin Group search 2014; PUBMED: 14823272] [PubMed] [Google Scholar]
Unknown Chinese author 1991 {published data only}
- Unknown Chinese author(s). Different concentrations of iodine tincture in the treatment of molluscum contagiosum. Chinese Journal of Dermatology 1991;24(6):405. [ref 1 of Cochrane Skin Group search 2014] [Google Scholar]
References to ongoing studies
NCT02024581 {published data only}
- NCT02024581. A dose range‐finding phase 2 trial of a botanical drug for the treatment of molluscum contagiosum in pediatric subjects [A single‐center, double‐blind, placebo‐controlled, randomized safety and efficacy trial of a botanical drug product, East Indian Sandalwood Oil (EISO), at one dose level for the treatment of molluscum contagiosum in pediatric subjects]. clinicaltrials.gov/ct2/show/NCT02024581 Date first received: 19 December 2013.
Additional references
Back Review Group 2008
- Cochrane Back Review Group. Sources of risk of bias. www.cochrane.iwh.on.ca/pdfs/RoBassessform_June2008.rtf (accessed 3 February 2009).
Barba 2001
- Barba AR, Kapoor S, Berman B. An open label safety study of topical imiquimod 5% cream in the treatment of molluscum contagiosum in children. Dermatology Online 2001;7(1):20. [PUBMED: 11328641] [PubMed] [Google Scholar]
Beaulieu 2000
- Beaulieu Ph, Pepin E, Aboucaya P, Bennassy I, Blaise F, Blechaye‐Butaye F, et al. Molluscum contagiosum. Epidemiological study of 452 cases in private practice [Molluscum contagiosum. Etude épidémiologique de 452 observations en pratique libérale]. Nouvelle Dermatologique 2000;19:231. [Google Scholar]
Behl 1970
- Behl PN, Bhatia BK. Clinical trial of milkweed (Asclepius curussavica) in the treatment of warts. Indian Journal of Dermatology 1970;15(2):49‐50. [PUBMED: 4914368] [PubMed] [Google Scholar]
Berger 1996
- Berger TG, Tappero JW. Chapter 25 Human immunodeficiency virus infection and the cutaneous complications of immunosuppression. In: Arndt KA, et al. editor(s). Cutaneous medicine and surgery. Vol. 2, Philadelphia: WB Saunders, 1996:1098‐9. [Google Scholar]
Bigardi 2003
- Bigardi A, Milani M. Successful treatment of molluscum contagiosum skin infection with hydrogen peroxide 1% cream. Journal of the European Academy of Dermatology and Venereology 2003;17(Suppl 3):419. [Google Scholar]
Brandrup 1989
- Brandrup F, Asschenfeldt P. Molluscum contagiosum‐induced comedo and secondary abscess formation. Pediatric Dermatology 1989;6(2):118‐21. [PUBMED: 2748472] [DOI] [PubMed] [Google Scholar]
Braue 2005
- Braue A, Ross G, Varigos G, Kelly H. Epidemiology and impact of childhood molluscum contagiosum: a case series and critical review of the literature. Pediatric Dermatology 2005;22(4):287‐94. [PUBMED: 16060861] [DOI] [PubMed] [Google Scholar]
Brown 2006
- Brown J, Janniger CK, Schwartz RA, Silverberg NB. Childhood molluscum contagiosum. Internation Journal of Dermatology 2006;45(2):93‐9. [PUBMED: 16445494] [DOI] [PubMed] [Google Scholar]
Buller 1991
- Buller RM, Palumbo GJ. Poxvirus pathogenesis. Microbiological Reviews 1991;55(1):80‐122. [PUBMED: 1851533] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2013
- Chen X, Anstey AV, Bugert JJ. Molluscum contagiosum virus infection. Lancet Infectious Diseases 2013;13(10):877‐88. [PUBMED: 23972567] [DOI] [PubMed] [Google Scholar]
Coloe 2009
- Coloe J, Morrell DS. Cantharidin use among pediatric dermatologists in the treatment of molluscum contagiosum. Pediatric Dermatology 2009;26(4):405‐8. [PUBMED: 19689514] [DOI] [PubMed] [Google Scholar]
Cope 1915
- Cope LF. A case of molluscum contagiosum cured by X rays. Lancet 1915;185(4788):1179. [DOI: 10.1016/S0140-6736(01)66721-7] [DOI] [Google Scholar]
Cunningham 1998
- Cunningham BB, Paller AS, Garzon M. Inefficacy of oral cimetidine for nonatopic children. Pediatric Dermatology 1998;15(1):71‐2. [PUBMED: 9496815] [DOI] [PubMed] [Google Scholar]
Czelusta 2000
- Czelusta A, Yen‐Moore A, Straten M, Carrasco D, Tyring SK. An overview of sexually transmitted diseases. Part III. Sexually transmitted diseases in HIV‐infected patients. Journal of the American Academy of Dermatology 2000;43(3):409‐32. [PUBMED: 10954653] [DOI] [PubMed] [Google Scholar]
Davies 1999
- Davies EG, Thrasher A, Lacey K, Harper J. Topical cidofovir for severe molluscum contagiosum. Lancet 1999;353(9169):2042. [PUBMED: 10376628] [DOI] [PubMed] [Google Scholar]
Davis 1896
- Davis AE. Report of a case of molluscum contagiosum which got well under the use of yellow oxide of mercury ointment. Annals of Ophthalmology and Otology 1896;5:404. [Google Scholar]
De Oreo 1956
- Binkley GW, Oreo GA, Johnson HH. An eczematous reaction associated with molluscum contagiosum. AMA Archives of Dermatology 1956;74(4):344‐8. [PUBMED: 13361506] [DOI] [PubMed] [Google Scholar]
Dohil 1996
- Dohil M, Prendiville JS. Treatment of molluscum contagiosum with oral cimetidine: clinical experience in 13 patients. Pediatric Dermatology 1996;13(4):310‐2. [PUBMED: 8844752] [DOI] [PubMed] [Google Scholar]
Friedman 1987
- Friedman M, Gal D. Keloid scars as a result of CO2 laser for molluscum contagiosum. Obstetrics and Gynecology 1987;70(3 Pt 1):394‐6. [PUBMED: 3627587] [PubMed] [Google Scholar]
Funt 1961
- Funt TR. Canthadirin treatment of molluscum contagiosum. Archives of Dermatology 1961;83:504‐5. [PUBMED: 13702631] [DOI] [PubMed] [Google Scholar]
Funt 1979
- Funt TR, Mehr KA. Cantharidin: a valuable office treatment of molluscum contagiosum. Southern Medical Journal 1979;72(8):1019. [PUBMED: 472800] [PubMed] [Google Scholar]
Gao 2016
Gottlieb 1994
- Gottlieb SL, Myskowski PL. Molluscum contagiosum. International Journal of Dermatology 1994;33(7):453‐61. [PUBMED: 7928025] [DOI] [PubMed] [Google Scholar]
Gräfe 2000
- Gräfe A, Fischer S, Bohn M, Neumann Ch, Kölmel K. Treatment of warts with NO releasing ointment [Die Behandlung von Dellwarzen mit einer NO‐freisetzenden Creme (5% Natriumnitrit and 5% Zitronensäure in Basiscreme DAC)]. Zeitschrift für Hautkrankheiten 2000;75:492. [Google Scholar]
Haellmigk 1966
- Haellmigk C. Keratoconjunctivitis in molluscum contagiosum of the eyelids [Keratokonjunktivitis bei Molluscum contagiosum der Lider]. Klinische Monatsblatter fur Augenheilkunde 1966;148(1):87‐91. [PUBMED: 5978089] [PubMed] [Google Scholar]
Hammes 2001
- Hammes S, Greve B, Raulin C. Molluscum contagiosum: treatment with pulsed dye laser. Hautarzt 2001;52(1):38‐42. [PUBMED: 11220237] [DOI] [PubMed] [Google Scholar]
Hawley 1970
- Hawley TG. The natural history of molluscum contagiosum in Fijian children. Journal of Hygiene 1970;68(4):631‐2. [PUBMED: 5276334 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hengge 2003
- Hengge UR, Cusini M. Topical immunomodulators for the treatment of external genital warts, cutaneous warts and molluscum contagiosum. British Journal of Dermatology 2003;149(Suppl 66):15‐9. [PUBMED: 14616340] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org. The Cochrane Collaboration.
Highet 1992
- Highet AS. Molluscum contagiosum. Archives of Disease in Childhood 1992;67(10):1248‐9. [PUBMED: 1444521] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hira 1988
- Hira SK, Wadhawan D, Kamanga J, Kavindele D, Macuacua R, Patil PS, et al. Cutaneous manifestations of human immunodeficiency virus in Lusaka, Zambia. Journal of the American Academy of Dermatology 1988;19(3):451‐7. [PUBMED: 2971691] [DOI] [PubMed] [Google Scholar]
Holt 2015
- Holt S, Webster‐Longin M, Perrin K, Baker T, Beasley R. The use of honey in the treatment of molluscum contagiosum in children. Conference abstract, presented at World Dermatology Congress, Seoul, Korea 2011.
Hughes 2013
- Hughes CM, Damon IK, Reynolds MG. Understanding U.S. healthcare providers' practices and experiences with molluscum contagiosum. PLoS One 2013;8(10):e76948. [PUBMED: 24155912] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hund 1975
- Hund G. Vitamin A‐acid therapy of molluscum contagiosa in hemophilia A [Vitamin‐A‐Säure‐Therapie von Mollusca contagiosa bei Haemophilie A]. Zeitschrift fur Hautkrankheiten 1975;50(7):291‐2. [PUBMED: 1226947] [PubMed] [Google Scholar]
Husak 1997
- Husak R, Garbe C, Orfanos CE. Molluscum contagiosum in HIV‐Infection. Clinical manifestation, relation to immune status and prognostic value in 39 patients. Der Hautarzt; Zeitschrift fur Dermatologie, Venerologie, und Verwandte Gebiete 1997;48(2):103‐7. [PUBMED: 9173055] [DOI] [PubMed] [Google Scholar]
Jones 2007
- Jones S, Kress D. Treatment of molluscum contagiosum and herpes simplex virus cutaneous infections. Cutis 2007;79(4 Suppl):11‐7. [PUBMED: 17508491] [PubMed] [Google Scholar]
Kang 2005
- Kang SH, Lee D, Hoon Park J, Cho SH, Lee SS, Park SW. Treatment of molluscum contagiosum with topical diphencyprone therapy. Acta Dermatato‐Venereologica 2005;85(6):529‐30. [PUBMED: 16396805] [DOI] [PubMed] [Google Scholar]
Katz 2013
- Katz KA, Swetman GL. Imiquimod, molluscum, and the need for a better “best pharmaceuticals for children” act. Pediatrics 2013;132(1):1‐3. [PUBMED: 23796740] [DOI] [PubMed] [Google Scholar]
Katz 2014
- Katz KA. Imiquimod is not an effective drug for molluscum contagiosum. Lancet Infectious Diseases 2014;14(5):372‐3. [PUBMED: 24758995] [DOI] [PubMed] [Google Scholar]
Katz 2015
- Katz KA. Dermatologists, imiquimod, and treatment of molluscum contagiosum in children: righting wrongs. JAMA Dermatology 2015;151(2):125‐6. [PUBMED: 25587702] [DOI] [PubMed] [Google Scholar]
Koning 1994
- Koning S, Bruijnzeels MA, Suijlekom‐Smit LW, Wouden JC. Molluscum contagiosum in Dutch general practice. British Journal of General Practice 1994;44(386):417‐9. [PUBMED: 8790656 ] [PMC free article] [PubMed] [Google Scholar]
Kyu 1993
- Kyu Han K, Koo Il S, Jin Ho C, Kyung Chan P, Hee Chul E. The effect of diphenylcyclopropenone immunotherapy on molluscum contagiosum. Annals of Dermatology 1993;5(2):79‐82. [Google Scholar]
Liota 2000
- Liota E, Smith KJ, Buckley R, Menon P, Skelton H. Imiquimod therapy for molluscum contagiosum. Journal of Cutaneous Medical Surgery 2000;4(2):76‐82. [PUBMED: 11179929] [DOI] [PubMed] [Google Scholar]
Liveing 1878
- Liveing R. Molluscum contagiosum. Lancet 1878;112(2875):494. [Google Scholar]
Lowy 1999
- Lowy DR. Molluscum contagiosum. In: Fitzpatrick TB, Freedberg IM editor(s). Fitzpatrick's Dematology in General Medicine. 5th Edition. Vol. 2, New York: McGraw‐Hill, 1999:2478‐81. [Google Scholar]
Mansur 2004
- Mansur AT, Goktay F, Gunduz S, Serdar ZA. Multiple giant molluscum contagiosum in a renal transplant recipient. Transplant Infectious Disease 2004;6(3):120‐3. [PUBMED: 15569228] [DOI] [PubMed] [Google Scholar]
Markos 2001
- Markos AR. The successful treatment of molluscum contagiosum with podophyllotoxin (0.5%) self‐application. International Journal of STD & AIDS 2001;12(12):833. [PUBMED: 11791521] [PubMed] [Google Scholar]
Matis 1987
- Matis WL, Triana A, Shapiro R, Eldred L, Polk BF, Hood AF. Dermatologic findings associated with human immunodeficiency virus infection. Journal of the American Academy of Dermatology 1987;17(5 Pt 1):746‐51. [PUBMED: 3680653] [DOI] [PubMed] [Google Scholar]
Mc Cuaig 2011
- Cuaig C, Silverberg N. Commentaries on 'Interventions for cutaneous molluscum contagiosum'. Evidence‐based Child Health 2011;6:1602‐4. [DOI: 10.1002/ebch.833] [DOI] [Google Scholar]
Molino 2004
- Molino AC, Fleischer AB Jr, Feldman SR. Patient demographics and utilization of health care services for molluscum contagiosum. Pediatric Dermatology 2004;21(6):628‐32. [PUBMED: 15575844] [DOI] [PubMed] [Google Scholar]
Niizeki 1984
- Niizeki K, Kano O, Kondo Y. An epidemic study of molluscum contagiosum. Relationship to swimming. Dermatologica 1984;169(4):197‐8. [PUBMED: 6500123] [DOI] [PubMed] [Google Scholar]
Niizeki 1999
- Niizeki K, Hahimoto K. Treatment of molluscum contagiosum with silver nitrate paste. Pediatric Dermatology 1999;16(5):395‐7. [PUBMED: 10571843] [DOI] [PubMed] [Google Scholar]
Olsen 2014
- Olsen JR, Gallacher J, Piguet V, Francis NA. Epidemiology of molluscum contagiosum in children: a systematic review. Family Practice 2014;31(2):130‐6. [PUBMED: 24297468] [DOI] [PubMed] [Google Scholar]
Olsen 2015
- Olsen JR, Gallacher J, Finlay AY, Piquet V, Francis NA. Time to resolution and effect on quality of life of molluscum contagiosum in children in the UK: a prospective community cohort study. Lancet Infectious Diseases 2015;15(2):190‐5. [PUBMED: 25541478] [DOI] [PubMed] [Google Scholar]
Ordoukhanian 1997
- Ordoukhanian E, Lane AT. Warts and molluscum contagiosum: beware of treatments worse than the disease. Postgraduate Medicine 1997;101(2):223‐32, 235. [PUBMED: 9046937] [DOI] [PubMed] [Google Scholar]
Orlow 1993
- Orlow SJ, Paller A. Cimetidine therapy for multiple viral warts in children. Journal of the American Academy of Dermatology 1993;28(5 Pt 1):794‐6. [PUBMED: 8496433] [DOI] [PubMed] [Google Scholar]
Overfield 1966
- Overfield TM, Brody JA. An epidemiologic study of molluscum contagiosum in Anchorage, Alaska. Journal of Pediatrics 1966;69(4):640‐2. [PUBMED: 5921341] [DOI] [PubMed] [Google Scholar]
Papadopoulos 2007
- Papadopoulos EJ. Clinical executive summary. Application type: NDA, Submission number: 20723; Submission code: SE8‐020. FDA report March 15, 2007 (electronic signature).
Postlethwaite 1967
- Postlethwaite R, Watt JA, Hawley TG, Simpson I, Adam H. Features of molluscum contagiosum in the northeast of Scotland and in Fijian village settlements. Journal of Hygiene 1967;65(3):281‐91. [PUBMED: 5233985] [DOI] [PMC free article] [PubMed] [Google Scholar]
Quan 2000
- Quan LT, Orengo I. Surgical pearl: curetting with a punch. Journal of the American Academy of Dermatology 2000;43(5 Pt 1):854‐5. [PUBMED: 11050593] [DOI] [PubMed] [Google Scholar]
Redmond 2004
- Redmond RM. Molluscum contagiosum is not always benign. BMJ 2004;329(7462):403. [PUBMED: 15310623] [DOI] [PMC free article] [PubMed] [Google Scholar]
Relyveld 1988
- Relyveld J, Bergink AH, Nijhuis HGJ. Epidemiological notes. Leg ulcers, warts and dying circumstances [Epidemiologische notities. Ulcus cruris, wratten en sterfsituatie]. Huisarts en Wetenschap 1988;31:266‐7. [Google Scholar]
Rogers 1998
- Rogers M, Barnetson RSC. Diseases of the skin. In: Campbell AGM, McIntosh N, et al. editor(s). Forfar and Arneil's Textbook of Pediatrics. 5th Edition. New York: Churchill Livingstone, 1998:1633‐5. [Google Scholar]
Romiti 1999
- Romiti R, Ribeiro AP, Grinblat BM, Rivitti EA, Romiti N. Treatment of molluscum contagiosum with potassium hydroxide: a clinical approach in 35 children. Pediatric Dermatology 1999;16(3):228‐31. [PUBMED: 10383783] [DOI] [PubMed] [Google Scholar]
Romiti 2000
- Romiti R, Ribeiro AP, Romiti N. Evaluation of the effectiveness of 5% potassium hydroxide for the treatment of molluscum contagiosum. Pediatric Dermatology 2000;17(6):495. [PUBMED: 11189099] [DOI] [PubMed] [Google Scholar]
Ross 2004
- Ross GL, Orchard DC. Combination topical treatment of molluscum contagiosum with cantharidin and imiquimod 5% in children: a case series of 16 patients. Australasian Journal of Dermatology 2004;45(2):100‐2. [PUBMED: 15068455] [DOI] [PubMed] [Google Scholar]
Scheinfeld 2007
- Scheinfeld N. Treatment of molluscum contagiosum: a brief review and discussion of a case successfully treated with adapelene. Dermatology Online Journal 2007;13(3):15. [PUBMED: 18328209] [PubMed] [Google Scholar]
Schmitt 2008
- Schmitt J, Diepgen TL. Molluscum contagiosum. In: Williams H, Bigby M, Diepgen T, Herxheimer A, Naldi L, Rzany B editor(s). Evidence‐based dermatology. 2nd Edition. Oxford: Blackwell, 2008:Web chapter, accessed 18 February 2009. [Google Scholar]
Semkova 2014
- Semkova K, Palamaras I, Robles W. Hydrogen peroxide 1% cream under occlusion for treatment of molluscum contagiosum in an 8‐month‐old infant: an effective and safe treatment option. Clinical and Experimental Dermatology 2014;39(4):560‐1. [PUBMED: 24825145] [DOI] [PubMed] [Google Scholar]
Sharma 1998
- Sharma AK. Cimetidine therapy for multiple molluscum contagiosum lesions. Dermatology 1998;197(2):194‐5. [PUBMED: 9840981] [PubMed] [Google Scholar]
Silverberg 2000
- Silverberg NB, Sidbury R, Mancini AJ. Childhood molluscum contagiosum: experience with cantharidin therapy in 300 patients. Journal of the American Academy of Dermatology 2000;43(3):503‐7. [PUBMED: 10954663] [DOI] [PubMed] [Google Scholar]
Singh 1977
- Singh OP, Kanwar AJ. Griseofulvin therapy in molluscum contagiosum. Archives of Dermatology 1977;113(11):1615. [PUBMED: 931417] [DOI] [PubMed] [Google Scholar]
Skinner 2002
- Skinner RB. Treatment of molluscum contagiosum with imiquimod 5% cream. Journal of the American Academy of Dermatology 2002;47(Suppl 4):221‐4. [DOI] [PubMed] [Google Scholar]
Smith 2002
- Smith KJ, Skelton H. Molluscum contagiosum: recent advances in pathogenic mechanisms, and new therapies. American Jorunal of Clinical Dermatology 2002;3(8):535‐45. [PUBMED: 12358555] [DOI] [PubMed] [Google Scholar]
Sterling 1998
- Sterling JC, Kurtz JB. Viral infections. In: Champion RH, Burton JL, Ebling FJG editor(s). Textbook of Dermatology. 6th Edition. Oxford: Blackwell, 1998:1005‐8. [Google Scholar]
Stulberg 2003
- Stulberg DL, Hutchinson AG. Molluscum contagiosum and warts. American Family Physician 2003;67(6):1233‐40. [PUBMED: 12674451] [PubMed] [Google Scholar]
Sturt 1971
- Sturt RJ, Muller HK, Francis GD. Molluscum contagiosum in villages of the West Sepik district of New Guinea. Medical Journal of Australia 1971;2(15):751‐4. [PUBMED: 5117269] [DOI] [PubMed] [Google Scholar]
Takemura 1983
- Takemura T, Ohkuma K, Nagai, H, Saito T. The natural history of molluscum contagiosum. Examination and treatment of dermatological diseases 1983;5(7):667‐70. [Google Scholar]
Teilla‐Hamel 1996
- Teilla‐Hamel D, Roux A, Loeb G. Pharmacokinetics and safety profile of topical podophyllotoxin (0.5% solution) on molluscum contagiosum in children. European Journal of Dermatology 1996;6:437‐40. [Google Scholar]
Torfs 1959
- Torfs M, Lambelin G. Considerations on Molluscum Contagiosum in the tropics [Considerations sur le Molluscum Contagiosum en milieu tropical]. Annales de la Societe Belge de Medecine Tropicale 1959;39:703‐9. [PubMed] [Google Scholar]
Toro 2000
- Toro JR, Wood LV, Patel NK, Turner ML. Topical cidofovir: a novel treatment for recalcitrant molluscum contagiosum in children infected with human immunodeficiency virus 1. Archives of Dermatology 2000;136(8):983‐5. [PUBMED: 10926733] [DOI] [PubMed] [Google Scholar]
Van der Linden 2005
- Linden MW, Suijlekom‐Smit LWA, Schellevis FG, Wouden JC. [The child in general practice] Het kind in de huisartspraktijk. Rotterdam/Utrecht: Erasmus MC/NIVEL, 2005. [Google Scholar]
Vasily 1978
- Vasily DB, Bhatia SG. Erythema annulare centrifugum and molluscum contagiosum. Archives of Dermatology 1978;114(12):1853. [PUBMED: 736595] [DOI] [PubMed] [Google Scholar]
Whitaker 1991
- Whitaker SB, Wiegand SE, Budnick SD. Intraoral molluscum contagiosum. Oral Surgery, Oral Medicine, Oral Pathology 1991;72(3):334‐6. [PUBMED: 1923422] [DOI] [PubMed] [Google Scholar]
Wieringa 2006
- Wieringa JW, Ketel AG, Houten MA. Coma in a child after treatment with the 'magic salve' lidocaine‐prilocaine cream [Coma bij een peuter na behandeling met de 'toverzalf' lidocaine‐prilocainecreme]. Nederlands Tijdschrift voor Geneeskunde 2006;150(33):1805‐7. [PUBMED: 16967588] [PubMed] [Google Scholar]
Williams 1991
- Williams LR, Webster G. Warts and molluscum contagiosum. Clinical Dermatology 1991;9(1):87‐93. [PUBMED: 1933729] [DOI] [PubMed] [Google Scholar]
Wishart 1903
- Wishart J. The local treatment of psoriasis and molluscum contagiosum. Lancet 1903;161(4154):1030‐1. [Google Scholar]
Yasher 1999
- Yasher SS, Shamiri B. Oral cimetidine treatment of molluscum contagiosum. Pediatric Dermatology 1999;16(6):493. [PUBMED: 10651572] [PubMed] [Google Scholar]
Zabawski 1999
- Zabawski EJ Jr, Cockerell CJ. Topical cidofovir for molluscum contagiosum in children. Pediatric Dermatology 1999;16(5):414‐5. [PUBMED: 10627225] [PubMed] [Google Scholar]
References to other published versions of this review
Van der Wouden 2004
- Wouden JC, Gajadin S, Berger MY, Butler CC, Koning S, Menke J, et al. Interventions for molluscum contagiosum in children. Cochrane Database of Systematic Reviews 2004, Issue 2. [DOI: 10.1002/14651858.CD004767] [DOI] [PubMed] [Google Scholar]
Van der Wouden 2006
- Wouden JC, Koning S, Suijlekom‐Smit LWA, Berger M, Butler C, Menke J, et al. Interventions for cutaneous molluscum contagiosum. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD004767] [DOI] [PubMed] [Google Scholar]
Van der Wouden 2009
- Wouden JC, Sande R, Suijlekom‐Smit LWA, Berger M, Butler CC, Koning S. Interventions for cutaneous molluscum contagiosum. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD004767] [DOI] [PubMed] [Google Scholar]


