Abstract
Background
Women with a prior caesarean delivery have an increased risk of uterine rupture and for women subsequently requiring induction of labour it is unclear which method is preferable to avoid adverse outcomes. This is an update of a review that was published in 2013.
Objectives
To assess the benefits and harms associated with different methods used to induce labour in women who have had a previous caesarean birth.
Search methods
We searched Cochrane Pregnancy and Childbirth's Trials Register (31 August 2016) and reference lists of retrieved studies.
Selection criteria
Randomised controlled trials (RCTs) comparing any method of third trimester cervical ripening or labour induction, with placebo/no treatment or other methods in women with prior caesarean section requiring labour induction in a subsequent pregnancy.
Data collection and analysis
Two review authors independently assessed studies for inclusion and trial quality, extracted data, and checked them for accuracy.
Main results
Eight studies (data from 707 women and babies) are included in this updated review. Meta‐analysis was not possible because studies compared different methods of labour induction. All included studies had at least one design limitation (i.e. lack of blinding, sample attrition, other bias, or reporting bias). One study stopped prematurely due to safety concerns.
Vaginal PGE2 versus intravenous oxytocin (one trial, 42 women): no clear differences for caesarean section (risk ratio (RR) 0.67, 95% confidence interval (CI) 0.22 to 2.03, evidence graded low), serious neonatal morbidity or perinatal death (RR 3.00, 95% CI 0.13 to 69.70, evidence graded low), serious maternal morbidity or death (RR 3.00, 95% CI 0.13 to 69.70, evidence graded low). Also no clear differences between groups for the reported secondary outcomes. The GRADE outcomes vaginal delivery not achieved within 24 hours, and uterine hyperstimulation with fetal heart rate changes were not reported.
Vaginal misoprostol versus intravenous oxytocin (one trial, 38 women): this trial stopped early because one woman who received misoprostol had a uterine rupture (RR 3.67, 95% CI 0.16 to 84.66) and one had uterine dehiscence. No other outcomes (including GRADE outcomes) were reported.
Foley catheter versus intravenous oxytocin (one trial, subgroup of 53 women): no clear difference between groups for vaginal delivery not achieved within 24 hours (RR 1.47, 95% CI 0.89 to 2.44, evidence graded low), uterine hyperstimulation with fetal heart rate changes (RR 3.11, 95% CI 0.13 to 73.09, evidence graded low), and caesarean section (RR 0.93, 95% CI 0.45 to 1.92, evidence graded low). There were also no clear differences between groups for the reported secondary outcomes. The following GRADE outcomes were not reported: serious neonatal morbidity or perinatal death, and serious maternal morbidity or death.
Double‐balloon catheter versus vaginal PGE2 (one trial, subgroup of 26 women): no clear difference in caesarean section (RR 0.97, 95% CI 0.41 to 2.32, evidence graded very low). Vaginal delivery not achieved within 24 hours, uterine hyperstimulation with fetal heart rate changes, serious neonatal morbidity or perinatal death, and serious maternal morbidity or death were not reported.
Oral mifepristone versus Foley catheter (one trial, 107 women): no primary/GRADE outcomes were reported. Fewer women induced with mifepristone required oxytocin augmentation (RR 0.54, 95% CI 0.38 to 0.76). There were slightly fewer cases of uterine rupture among women who received mifepristone, however this was not a clear difference between groups (RR 0.29, 95% CI 0.08 to 1.02). No other secondary outcomes were reported.
Vaginal isosorbide mononitrate (IMN) versus Foley catheter (one trial, 80 women): fewer women induced with IMN achieved a vaginal delivery within 24 hours (RR 2.62, 95% CI 1.32 to 5.21, evidence graded low). There was no difference between groups in the number of women who had a caesarean section (RR 1.00, 95% CI 0.39 to 2.59, evidence graded very low). More women induced with IMN required oxytocin augmentation (RR 1.65, 95% CI 1.17 to 2.32). There were no clear differences in the other reported secondary outcomes. The following GRADE outcomes were not reported: uterine hyperstimulation with fetal heart rate changes, serious neonatal morbidity or perinatal death, and serious maternal morbidity or death.
80 mL versus 30 mL Foley catheter (one trial, 154 women): no clear difference between groups for the primary outcomes: vaginal delivery not achieved within 24 hours (RR 1.05, 95% CI 0.91 to 1.20, evidence graded moderate) and caesarean section (RR 1.05, 95% CI 0.89 to 1.24, evidence graded moderate). However, more women induced using a 30 mL Foley catheter required oxytocin augmentation (RR 0.81, 95% CI 0.66 to 0.98). There were no clear differences between groups for other secondary outcomes reported. Several GRADE outcomes were not reported: uterine hyperstimulation with fetal heart rate changes, serious neonatal morbidity or perinatal death, and serious maternal morbidity or death.
Vaginal PGE2 pessary versus vaginal PGE2 tablet (one trial, 200 women): no difference between groups for caesarean section (RR 1.09, 95% CI 0.74 to 1.60, evidence graded very low), or any of the reported secondary outcomes. Several GRADE outcomes were not reported: vaginal delivery not achieved within 24 hours, uterine hyperstimulation with fetal heart rate changes, serious neonatal morbidity or perinatal death, and serious maternal morbidity or death.
Authors' conclusions
RCT evidence on methods of induction of labour for women with a prior caesarean section is inadequate, and studies are underpowered to detect clinically relevant differences for many outcomes. Several studies reported few of our prespecified outcomes and reporting of infant outcomes was especially scarce. The GRADE level for quality of evidence was moderate to very low, due to imprecision and study design limitations.
High‐quality, adequately‐powered RCTs would be the best approach to determine the optimal method for induction of labour in women with a prior caesarean birth. However, such trials are unlikely to be undertaken due to the very large numbers needed to investigate the risk of infrequent but serious adverse outcomes (e.g. uterine rupture). Observational studies (cohort studies), including different methods of cervical ripening, may be the best alternative. Studies could compare methods believed to provide effective induction of labour with low risk of serious harm, and report the outcomes listed in this review.
Keywords: Female; Humans; Pregnancy; Vaginal Birth after Cesarean; Dinoprostone; Dinoprostone/administration & dosage; Early Termination of Clinical Trials; Labor, Induced; Labor, Induced/methods; Misoprostol; Misoprostol/administration & dosage; Oxytocics; Oxytocics/administration & dosage; Oxytocin; Oxytocin/administration & dosage; Randomized Controlled Trials as Topic; Uterine Rupture; Uterine Rupture/etiology
Plain language summary
Induction methods for women who have had a prior caesarean birth
What is the issue?
Labour induction is a common procedure, carried out when it is judged to be safer for a baby to be born than to continue a pregnancy. When a woman who has had a caesarean in the past gives birth, current clinical practice supports helping her to have a vaginal birth. However, there is a higher risk of complications from induction for women who have previously had a caesarean section.
Methods for induction include: prostaglandin medication (including oral or vaginal prostaglandins E2 (PGE2) or misoprostol); mifepristone; mechanical methods (including Foley catheters and double‐balloon catheters); nitric oxide donors (such as isosorbide mononitrate); and oxytocin. This review looked at the harms and benefits of different methods for induction of labour in women with a prior caesarean birth, if induction of labour was required in their current pregnancy.
Why is this important?
Lots of women have caesareans: across the world between one in four and one in two babies are born by caesarean section. Many women go on to have another pregnancy, and we want to know how to deliver these babies safely. Women with a prior caesarean birth have an increased risk of uterine scar rupture, particularly when labour is induced. This is a serious complication, often leading to negative outcomes for mother and child, such as hysterectomy, genitourinary tract injury, and postpartum blood transfusions for the mother, and neurological impairment or even death for the child.
What evidence did we find?
We searched for studies on 31 August 2016. Eight small randomised controlled trials are included in this updated review, with data from 707 women and babies. The studies compared different methods of inducing labour, so results could not be combined.
There were design problems in all of the trials: women and health professionals knew which induction method was being used in seven out of eight trials, which may have affected clinical decisions. Women were left out of the analysis in some trials, and trials often did not report important outcomes (vaginal birth not achieved within 24 hours of induction, overstimulation of the uterus with changes to the baby's heart rate, caesarean section, serious illness or death of the baby, serious illness or death of the mother).
The trials were too small to show clear differences. The quality of the evidence was very low, low, or moderate, because the trials were small and had high risk of bias. We cannot be certain about the results, and future research may show something different.
What does this mean?
There is not enough information available from randomised controlled trials to advise on the best methods of labour induction in women with a previous caesarean birth. More high‐quality randomised controlled trials are needed to find out which method is best for mothers and babies. However, such trials are unlikely to be carried out because they would need a very large number of participants in order to study the risk of infrequent but serious outcomes (such as rupture of the woman's uterus). Other types of studies (i.e. non‐randomised controlled trials) might be the best alternative. Future research could focus on those methods of induction that are believed to be effective and have a low risk of serious harm. The outcomes identified as important in this review could be utilised in future studies.
Summary of findings
Summary of findings for the main comparison. Vaginal PGE2 versus intravenous (IV) oxytocin.
| Vaginal PGE2 compared with IV oxytocin for term labour induction for women with a previous caesarean section | ||||||
| Patient or population: women with one previous lower segment caesarean section and requiring labour induction due to prolonged pregnancy or pre‐eclampsia, singleton in cephalic presentation, GA ≥ 37 weeks, BS < 9, no cephalopelvic disproportion anticipated Setting: UK Intervention: vaginal prostaglandin E2 (2.5 mg pessary) Comparison: intravenous oxytocin | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with oxytocin | Risk with prostaglandin E2 | |||||
| Vaginal delivery not achieved within 24 hours | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Uterine hyperstimulation with fetal heart rate changes | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Caesarean section | Study population | RR 0.67 (0.22 to 2.03) | 42 (1 RCT) | ⊕⊕⊝⊝ Low1 | ||
| 286 per 1000 | 191 per 1000 (63 to 580) | |||||
| Serious neonatal morbidity/perinatal death | Study population | RR 3.00 (0.13 to 69.70) | 42 (1 RCT) | ⊕⊕⊝⊝ Low1 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Serious maternal morbidity or death | Study population | RR 3.00 (0.13 to 69.70) | 42 (1 RCT) | ⊕⊕⊝⊝ Low1 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BS: Bishop score; CI: Confidence interval; GA: gestational age; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1Wide CI crossing the line of no effect, small sample size, and few events (imprecision, downgraded 2 levels).
Summary of findings 2. Vaginal misoprostol versus intravenous (IV) oxytocin.
| Vaginal misoprostol compared with IV oxytocin for term labour induction for women with a previous caesarean section | ||||||
| Patient or population: women with a previous caesarean section Setting: USA Intervention: vaginal misoprostol 25 μg every 6 hours (maximum of 4 doses) Comparison: intravenous oxytocin "per a standardised infusion protocol" see Wing 1998 (dose/regime not reported) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with misoprostol | Risk with oxytocin | |||||
| Vaginal delivery not achieved within 24 hours | not reported | |||||
| Uterine hyperstimulation with fetal heart rate changes | not reported | |||||
| Caesarean section | not reported | |||||
| Serious neonatal morbidity or perinatal death | not reported | |||||
| Serious maternal morbidity or death | not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
Summary of findings 3. Foley catheter versus intravenous (IV) oxytocin.
| Foley catheter compared with IV oxytocin for term labour induction for women with a previous caesarean section | ||||||
| Patient or population: pregnant women with a previous low transverse caesarean section, singleton live pregnancy with cephalic presentation, period of gestation > 28 weeks and BS < 5 were included in the study, with unfavourable cervix Setting: Chandigarh, India. July 2004–November 2005 Intervention: Foley catheter balloon inflated with 30 mL of sterile saline Comparison: intravenous oxytocin (low dose IV oxytocin, starting at 1 mU/min and increasing if contractions were not frequent after 1 hour) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with oxytocin | Risk with Foley catheter | |||||
| Vaginal delivery not achieved within 24 hours | Study population | RR 1.47 (0.89 to 2.44) | 53 (1 RCT) | ⊕⊕⊝⊝ Low1 | ||
| 444 per 1000 | 653 per 1000 (396 to 1000) | |||||
| Uterine hyperstimulation with fetal heart rate changes | Study population | RR 3.11 (0.13 to 73.09) | 53 (1 RCT) | ⊕⊕⊝⊝ Low1 | ||
| 0 per 1000 | 0 per 1000 (0 to 0) | |||||
| Caesarean section | Study population | RR 0.93 (0.45 to 1.92) | 53 (1 RCT) | ⊕⊕⊝⊝ Low1 | ||
| 370 per 1000 | 344 per 1000 (167 to 711) | |||||
| Serious neonatal morbidity or perinatal death | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Serious maternal morbidity or death | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BS: Bishop score; CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1Wide confidence interval crossing the line of no effect, small sample size, and few events (imprecision, downgraded 2 levels).
Summary of findings 4. Double‐balloon catheter versus vaginal PGE2.
| Double‐balloon catheter compared with vaginal PGE2 for term labour induction for women with a previous caesarean section | ||||||
|
Patient or population: women with a previous caesarean section (subgroup of all women in the study) with intact fetal membranes, cephalic position and unfavourable cervix, with indications for induction of labour
Setting: 7 labour wards in Denmark, December 2002‐September 2005 Intervention: double‐balloon catheter inserted through the cervical canal with 80 mL of saline installed stepwise in the uterine balloon and 80 mL saline in the cervicovaginal balloon Comparison: vaginal prostaglandin E2 (dinoprostone 3 mg vaginal tablet) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with prostaglandin E2 | Risk with double‐balloon catheter | |||||
| Vaginal delivery not achieved within 24 hours | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Uterine hyperstimulation with fetal heart rate changes | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Caesarean section | Study population | RR 0.97 (0.41 to 2.32) | 16 (1 RCT) | ⊕⊝⊝⊝ Very low1, 2 | ||
| 571 per 1000 | 554 per 1000 (234 to 1000) | |||||
| Serious neonatal morbidity or perinatal death | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Serious maternal morbidity or death | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1One study with design limitations (risk of bias, downgraded 1 level). 2Wide confidence interval crossing the line of no effect, small sample size, and few events (imprecision, downgraded 2 levels).
Summary of findings 5. Oral mifepristone versus Foley catheter.
| Oral mifepristone compared with Foley catheter for term labour induction for women with a previous caesarean section | ||||||
| Patient or population: pregnant women, 40 weeks' gestation, single cephalic presentation, 1 previous low segment caesarean section Setting: India, 2012‐2014 Intervention: oral mifepristone (400 mg) orally at 40 + 5. All women were reassessed 24 hours and 48 hours later. If BS > 6, amniotomy was performed, followed by oxytocin infusion. If after 48 hours, BS was < 6, induction of labour was done with oxytocin infusion Comparison: Foley catheter with 30 mL normal saline inserted at 40 + 5 | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with mifepristone | Risk with Foley catheter | |||||
| Vaginal delivery not achieved within 24 hours | not reported | |||||
| Uterine hyperstimulation with fetal heart rate changes | not reported | |||||
| Caesarean section | not reported | |||||
| Serious neonatal morbidity or perinatal death | not reported | |||||
| Serious maternal morbidity or death | not reported | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BS: Bishop score; CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
Summary of findings 6. Vaginal isosorbide mononitrate versus Foley catheter.
| Vaginal isosorbide mononitrate versus Foley catheter for term labour induction for women with a previous caesarean section | ||||||
| Patient or population: pregnant women with 1 previous lower segment caesarean section at 37 weeks and beyond, with a BS of ≤ 6, intact membranes, reactive non‐stress test, normal umbilical arterial Doppler indices, absence of labour and willingness of women to participate in the study Setting: Egypt Intervention: vaginal isosorbide mononitrate (40 mg) inserted into the posterior fornix of the vagina once Comparison: Foley catheter No. 14‐16 Fr inserted into the endocervical canal, beyond the internal os and inflated with 50‐60 mL of normal saline | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with Foley catheter | Risk with isosorbide mononitrate | |||||
| Vaginal delivery not achieved within 24 hours | Study population | RR 2.63 (1.32 to 5.21) | 80 (1 RCT) | ⊕⊕⊝⊝ Low1, 2 | ||
| 200 per 1000 | 526 per 1000 (264 to 1000) | |||||
| Uterine hyperstimulation with fetal heart rate changes | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Caesarean section | Study population | RR 1.00 (0.39 to 2.59) | 80 (1 RCT) | ⊕⊝⊝⊝ Very low1, 3 | ||
| 175 per 1000 | 175 per 1000 (68 to 453) | |||||
| Serious neonatal morbidity or perinatal death | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Serious maternal morbidity or death | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BS: Bishop score; CI: Confidence interval; Fr: French; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1 One study with design limitations (risk of bias, downgraded 1 level). 2 Small sample size (imprecision, downgraded 1 level). 3 Wide CI crossing the line of no effect, small sample size (imprecision, downgraded 2 levels).
Summary of findings 7. 80 mL versus 30 mL Foley catheter.
| 80 mL Foley catheter versus 30 mL Foley catheter for term labour induction for women with a previous caesarean section | ||||||
| Patient or population: pregnant women who previously had a lower segment CS and now have a singleton cephalic presentation after at least 36 completed weeks, not in labour, with intact membranes and BS of < 6 Setting: a large tertiary centre in South India, which carries out ˜15,000 deliveries every year. October 2011‐December 2013 Intervention: a 16 Fr Foley catheter was introduced into the cervix beyond the internal os and the bulb inflated with 80 mL of sterile water Comparison: a 16 Fr Foley catheter was introduced into the cervix beyond the internal os and the bulb inflated with 30 mL of sterile water | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with 30 mL Foley catheter | Risk with 80 mL Foley catheter | |||||
| Vaginal delivery not achieved within 24 hours | Study population | RR 1.05 (0.91 to 1.20) | 154 (1 RCT) | ⊕⊕⊕⊝ Moderate1 | ||
| 818 per 1000 | 859 per 1000 (745 to 982) | |||||
| Uterine hyperstimulation with fetal heart rate changes | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Caesarean section | Study population | RR 1.05 (0.89 to 1.24) | 154 (1 RCT) | ⊕⊕⊕⊝ Moderate1 | ||
| 766 per 1000 | 805 per 1000 (682 to 950) | |||||
| Serious neonatal morbidity or perinatal death | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Serious maternal morbidity or death | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BS: Bishop score; CI: Confidence interval; Fr: French; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1Small sample size (imprecision, downgraded 1 level).
Summary of findings 8. Vaginal PGE2 pessary versus vaginal PGE2 tablet.
| Vaginal PGE2 pessary versus vaginal PGE2 tablet for term labour induction for women with a previous caesarean section | ||||||
| Patient or population: women with a previous caesarean section, a live singleton fetus (37‐42 weeks of gestation) in cephalic presentation and a reactive non‐stress test, BS of ≤ 7 before onset of labour, no spontaneous contractions (< 4 contractions within 20 minutes) Setting: large Governmental hospital, Saudi Arabia. February 2009‐March 2013 Intervention: vaginal PGE2 pessary (10 mg dinoprostone sustained‐release vaginal pessary) Comparison: vaginal PGE2 tablet (1.5 mg dinoprostone vaginal tablet) | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with dinoprostone tablet | Risk with dinoprostone pessary | |||||
| Vaginal delivery not achieved within 24 hours | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Uterine hyperstimulation with fetal heart rate changes | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Caesarean section | Study population | RR 1.09 (0.74 to 1.60) | 200 (1 RCT) | ⊕⊝⊝⊝ Very low1, 2 | ||
| 330 per 1000 | 360 per 1000 (244 to 528) | |||||
| Serious neonatal morbidity or perinatal death | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| Serious maternal morbidity or death | ‐ | ‐ | ‐ | ‐ | ‐ | Not reported |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). BS: Bishop score; CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | ||||||
1One study with design limitations (risk of bias, downgraded 1 level). 2Wide confidence crossing the line of no effect, small sample size (imprecision, downgraded 2 levels).
Background
This review is an update of a review first published in 2013 (Jozwiak 2013).
Description of the condition
Worldwide, caesarean birth is common. In Australia in 2007, almost 31% of women gave birth by caesarean section (Laws 2009), with similar figures reported from the USA (Martin 2009). While the overall rate of caesarean section is lower in the UK, accounting for approximately 25% of all births (NHS 2009), rates of almost 50% have been reported in some private hospitals in Argentina, Brazil and Chile (Belizan 1999). Women who have had a prior caesarean birth are at increased risk of complications during a subsequent labour, including risk of uterine rupture, presenting unique circumstances related to the mode of birth in a subsequent pregnancy. The particular benefits and harms associated with both elective repeat caesarean section and vaginal birth after caesarean section are discussed in the Cochrane Review 'Planned elective repeat caesarean section versus planned vaginal birth for women with a previous caesarean section' (Dodd 2004). Current clinical practice guidelines support vaginal birth and trial of labour among women who have had a prior caesarean birth (ACOG 2006; RCOG 2008).
Induction of labour is a common obstetric intervention, with between 20% and 30% of births reported to occur following induction of labour (Laws 2009; Martin 2009; Peristat 2008). The percentage of women requiring induction of labour after a previous caesarean birth is thought to be similar to that of other pregnant women (Locatelli 2004). For women who have had a previous caesarean birth and who require induction of labour in a subsequent pregnancy, it is unclear whether labour should be induced, or if birth should occur by repeat elective caesarean section. This question is considered in more detail in the Cochrane Review 'Elective repeat caesarean section versus induction of labour for women with a previous caesarean birth' (Dodd 2006).
An uncommon, but potentially life‐threatening complication for both the woman and her infant associated with vaginal birth, is that of uterine scar rupture (where the previous caesarean scar breaks down). Uterine scar rupture is associated with a significant risk of maternal morbidity, such as hysterectomy, genitourinary tract injury, postpartum blood transfusions, and maternal death (Chuahan 2003; Zwart 2008). Increased infant morbidity and perinatal death have been reported (Chuahan 2003). Although there is variation in findings in different studies, in women who have had previous caesarean birth, uterine rupture is reported to occur in about 8 in 1000 births with spontaneous labour, however, this risk is thought to be almost doubled when labour is induced (NIH consensus 2010).
The focus of this current systematic review is to address the method of induction of labour, should it be required, in women who have had a previous caesarean section. The review draws on the methodology of the Cochrane generic protocol related to methods of induction of labour (Hofmeyr 2009).
Description of the intervention
Induction of labour is carried out when the risks of continuing the pregnancy outweigh the benefits. Common indications for labour induction include post‐term pregnancy, prelabour rupture of membranes, intrauterine growth restriction of the fetus, maternal hypertensive disorders, and other maternal conditions. Many different methods are available for labour induction, including pharmacological methods (mainly prostaglandin analogues and oxytocin), and mechanical methods, such as Foley catheters.
How the intervention might work
Prospective and retrospective cohort studies have shown an increased risk of uterine rupture in women who have had a prior caesarean birth following induction of labour, especially when prostaglandin preparations are used for cervical ripening (Landon 2004; Lydon‐Rochelle 2001; Smith 2004). The risk of uterine rupture following mechanical dilation for ripening of the cervix is reported to be lower than with prostaglandins (Bujold 2004; Landon 2004; Ravasia 2000), approximating the risk after spontaneous onset of labour.
The observed increase in risk of uterine rupture following prostaglandin administration may reflect changes that are induced in the connective tissue of the uterine scar, thereby, weakening it. Equally, it could be reflective of the woman’s cervix being ‘unfavourable’ for labour (Bujold 2004; Kayani 2005), which in turn has been recognised to be associated with adverse maternal and infant outcomes following the trial of labour (Landon 2005).
The use of oxytocin to induce labour in women who have had a prior caesarean birth is also associated with an increased risk of uterine rupture (36/10,000 women without the use of oxytocin compared with 87/10,000 women following oxytocin use) (Landon 2005).
Clinical practice guidelines vary worldwide in relation to induction of labour for women who have had a previous caesarean section. The Society of Obstetricians and Gynaecologists of Canada clinical practice guidelines state that prostaglandins E2 (PGE2) should only be used in exceptional circumstances, and after appropriate counselling on the risk of uterine rupture, recommending that a Foley catheter be used in these women (SOGC 2005). The UK National Institute for Clinical Excellence (NICE) guidelines do not make any explicit recommendations, but do not discourage the use of prostaglandin (RCOG 2008). In contrast, practice guidelines issued by the American College of Obstetricians and Gynaecologists state that the use of prostaglandins for cervical ripening or induction of labour in most women who have had a previous caesarean section should be discouraged (ACOG 2006).
Why it is important to do this review
Cohort studies suggest that for women who have had a previous caesarean birth and require induction of labour in a subsequent pregnancy, there are potential benefits and harms associated with the induction of labour. These benefits and harms may vary considerably with the method used to induce labour.
Objectives
To assess the benefits and harms associated with different methods used to induce labour in women who have had a previous caesarean birth and require induction of labour in a subsequent pregnancy.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (with reported data for women and infants) comparing any method of term cervical ripening or labour induction, with placebo/no treatment or other methods, not including the comparison of induction of labour versus expectant management. Quasi‐randomised controlled trials, cluster‐randomised trials, and those presented only as an abstract were eligible for inclusion. Cross‐over trials are not relevant to this intervention and were not eligible for inclusion.
Types of participants
Pregnant women with a live fetus, who have had a previous caesarean section, requiring induction of labour in the third trimester of pregnancy.
Types of interventions
All methods of cervical ripening or labour induction including: prostaglandin medication (including oral or vaginal PGE2 and misoprostol); mifepristone; mechanical methods (including Foley catheters and double‐balloon catheters); oxytocin, or placebo compared with placebo or any other method were included.
Types of outcome measures
Clinically relevant outcomes for trials of methods of cervical ripening/labour induction have been prespecified and published in the Cochrane generic protocol relating to induction of labour (Hofmeyr 2009).
Primary outcomes
1. Vaginal delivery not achieved within 24 hours (or period specified by trial authors) 2. Uterine hyperstimulation with fetal heart rate (FHR) changes 3. Caesarean section 4. Serious neonatal morbidity or perinatal death (e.g. seizures, birth asphyxia defined by trialists, neonatal encephalopathy, disability in childhood) 5. Serious maternal morbidity or death (e.g. uterine rupture, admission to intensive care unit, septicaemia)
Secondary outcomes
Measures of effectiveness
6. Cervix unfavourable/unchanged after 12 to 24 hours 7. Oxytocin augmentation
Complications
8. Uterine hyperstimulation without FHR changes 9. Uterine rupture 10. Epidural analgesia 11. Instrumental vaginal delivery 12. Meconium‐stained liquor 13. Apgar score less than 7 at five minutes 14. Neonatal intensive care unit admission 15. Neonatal encephalopathy 16. Perinatal death 17. Disability in childhood 18. Neonatal infection 19. Neonatal antibiotics 20. Maternal side‐effects (all) 21. Maternal nausea 22. Maternal vomiting 23. Maternal diarrhoea 24. Other maternal side‐effects 25. Postpartum haemorrhage 26. Chorioamnionitis 27. Endometritis 28. Maternal antibiotics 29. Serious maternal complications (e.g. intensive care unit admission, septicaemia but excluding uterine rupture) 30. Maternal death
Measures of satisfaction
31. Woman not satisfied 32. Caregiver not satisfied
'Uterine rupture' includes all clinically significant ruptures of unscarred or scarred uteri. Trivial scar dehiscence noted incidentally at the time of surgery was excluded. In the reviews, we use the term 'uterine hyperstimulation without FHR changes' to include uterine tachysystole (more than five contractions per 10 minutes for at least 20 minutes) and uterine hypersystole/hypertonus (a contraction lasting at least two minutes) and 'uterine hyperstimulation with FHR changes' to denote uterine hyperstimulation syndrome (tachysystole or hypersystole with FHR changes such as persistent decelerations, tachycardia or decreased short‐term variability).
Outcomes are included in the analysis if data are available according to treatment allocation and reasonable measures were taken to minimise observer bias. While all the above outcomes were sought, only outcomes with available data appear in the analysis tables. Data not pre‐stated were extracted and reported as not pre‐specified.
Search methods for identification of studies
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
We searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (31 August 2016).
The Register is a database containing over 23,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth in the Cochrane Library and select the ‘Specialized Register ’ section from the options on the left side of the screen.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set which has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Ongoing studies).
Searching other resources
We searched the reference lists of retrieved studies.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, see Jozwiak 2013.
For this update, we used the following methods for assessing the 12 reports that were identified as a result of the updated search.
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted the third review author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted the third review author. Data were entered into Review Manager 5 (RevMan 5) software (RevMan 2014) and checked for accuracy.
When information regarding any of the above was unclear, we planned to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). Any disagreement was resolved by discussion or by involving a third assessor.
1. Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random‐number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
2. Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively‐numbered, sealed, opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
3.1. Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
3.2. Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
4. Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses that we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as‐treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
5. Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
6. Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
7. Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of interventions (Higgins 2011a). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses ‐ see Sensitivity analysis.
Assessing the quality of the evidence using GRADE
For this update we have assessed the quality of the evidence using the GRADE approach as outlined in the GRADE handbook in order to assess the quality of the body of evidence relating to the following outcomes for the main comparisons.
Vaginal delivery not achieved within 24 hours (or period specified by trial authors)
Uterine hyperstimulation with fetal heart rate changes
Caesarean section
Serious neonatal morbidity or perinatal death (e.g. seizures, birth asphyxia defined by trialists, neonatal encephalopathy, disability in childhood)
Serious maternal morbidity or death (e.g. uterine rupture, admission to intensive care unit, septicaemia)
We used GRADEpro Guideline Development Tool (GRADEpro GDT) to import data from RevMan 5 (RevMan 2014) in order to create 'Summary of findings' tables. We produced a summary of the intervention effect and a measure of quality for each of the above outcomes using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
We used the mean difference if outcomes were measured in the same way between trials. We used the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
Our searches did not identify any cluster‐randomised trials for inclusion in the analyses. In future updates, if we identify any cluster‐randomised controlled trials we will include them in our analyses along with the individually randomised trials. We will adjust their sample sizes using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b) using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
Cross‐over trials
Cross‐over trials are inappropriate for this intervention.
Multi‐armed trials
We identified one multi‐arm trial, however only two arms were reported in the study publication. We contacted the trial authors to request data on the other arms but did not receive a reply. If we had received these data, we would have combined all relevant experimental intervention groups of the study into a single group and all relevant control intervention groups into a single control group when we analysed the data. If we had considered one of the arms irrelevant, we would have excluded it from analysis.
Dealing with missing data
For included studies, we noted levels of attrition. In future updates, if we include more eligible studies, we will explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect using sensitivity analysis.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, that is, we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We did not combine data from the included studies. In future updates we will assess statistical heterogeneity in each meta‐analysis using the Tau², I² (Higgins 2003) and Chi² statistics (Deeks 2011). We will regard heterogeneity as substantial if I² is greater than 30% and either Tau² is greater than zero, or there is a low P value (less than 0.10) in the Chi² test for heterogeneity. If we identify substantial heterogeneity (above 30%), we will explore it by pre‐specified subgroup analysis.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate it.
Data synthesis
Meta‐analysis was not possible because the studies compared different methods of labour induction. In future updates we will carry out statistical analysis using RevMan 5 software (RevMan 2014). We will use fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: that is, where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar.
If there is clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity is detected, we will use random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials is considered clinically meaningful. The random‐effects summary will be treated as the average range of possible treatment effects and we will discuss the clinical implications of treatment effects differing between trials. If the average treatment effect is not clinically meaningful, we will not combine trials. If we use random‐effects analyses, we will present the results as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
In future updates, if we identify substantial heterogeneity, we will investigate it using subgroup analyses and sensitivity analyses. We will considered whether an overall summary is meaningful, and if it is, we will use a random‐effects analysis to produce it.
We plan to carry out the following subgroup analyses:
previous vaginal birth (yes versus no);
number of previous caesarean births (one versus two versus three or more);
indication for previous caesarean birth(s) (failure to progress versus fetal distress versus other);
indication for labour induction (hypertensive disorders versus post‐term pregnancy versus intrauterine growth restriction versus maternal disease versus other indication);
favourability of the cervix (favourable versus unfavourable);
status of membranes (ruptured versus unruptured);
gestational age (37 to 40 weeks versus 40 to 41 weeks versus more than 41 weeks).
We will restrict planned subgroup analysis to the primary outcomes.
We will assess subgroup differences by interaction tests available within RevMan 5 (RevMan 2014). We will report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
In future updates, we will carry out sensitivity analyses, where appropriate, to explore the effect of trial quality assessed by concealment of allocation, high attrition rates, or both, with poor‐quality studies being excluded from the analyses in order to assess whether this makes any difference to the overall result.
Results
Description of studies
Results of the search
The updated search of Cochrane Pregnancy and Childbirth's Trials Register retrieved 12 reports (see Figure 1). We included six new studies (10 reports) (Hassan 2014; Lokkegaard 2015; Manish 2016; Meetei 2015; Rezk 2014; Sharma 2015). One new study was excluded (Ramya 2015) and one study is ongoing (NCT02196103).
1.
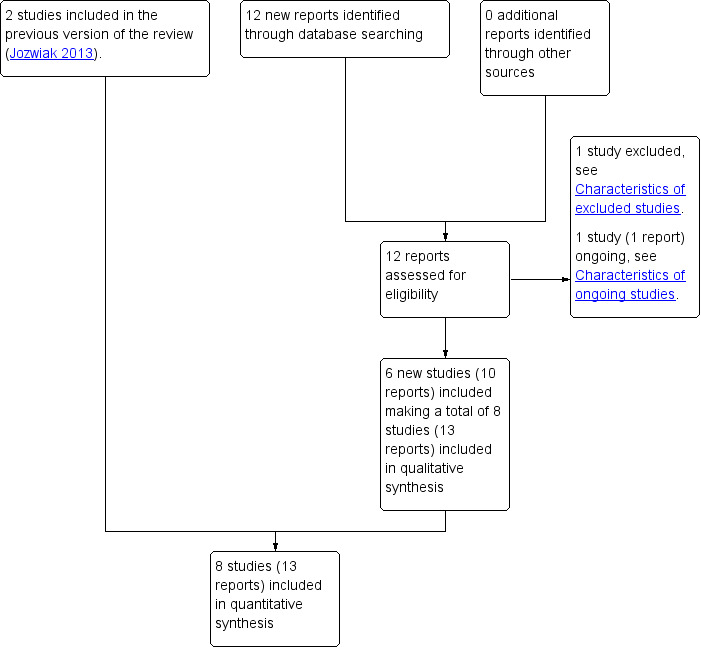
Study flow diagram
Included studies
See Characteristics of included studies.
Altogether we included eight studies in the review (Hassan 2014; Lokkegaard 2015; Manish 2016; Meetei 2015; Rezk 2014; Sharma 2015; Taylor 1993; Wing 1998). A subset of the women who participated in Lokkegaard 2015 had a prior caesarean section, and it was only this subset of results that we included in our review. Only data for women who were 37 weeks' gestation or more in Meetei 2015 were included in this review (unpublished data supplied by the trial author).
Design
Seven of the included studies were two‐arm randomised controlled trials (Hassan 2014; Lokkegaard 2015; Manish 2016; Meetei 2015; Rezk 2014; Taylor 1993; Wing 1998), one was a four‐arm randomised controlled trial however only two arms were reported in the publications (Sharma 2015). Trials compared different methods of labour induction.
Sample sizes
The studies range in size from 26 women (Lokkegaard 2015) to 200 women (Hassan 2014). The total number of women contributing data to the review is 707.
Setting
Three studies took place in India (Manish 2016; Meetei 2015; Sharma 2015), one in Saudi Arabia (Hassan 2014), one in Denmark (Lokkegaard 2015), one in Egypt (Rezk 2014), one in the UK (Taylor 1993), and one in the USA (Wing 1998).
Participants
All participants were women with a prior caesarean section. In Lokkegaard 2015 this was a subgroup within a trial of all women undergoing induction.
All studies looked at induction at term, or approaching term. The gestational age at which women were eligible varied: in Sharma 2015 women were 40 weeks' gestation, in Manish 2016, Rezk 2014 and Taylor 1993 they were at least 37 weeks gestational age, and in Hassan 2014 they were 37 to 42 weeks' gestation. The inclusion criteria in Meetei 2015 is from 28 weeks gestational age. The gestational age of women who took part is not reported, although it states that the majority of women were between 38 and 40 weeks. In personal communication, the trial author reported that 26 out of 30 women in the Foley catheter group, and 27 out of 30 in the oxytocin group were 37 weeks' gestation or more, and provided data for this subgroup of women. Lokkegaard 2015 does not state a specific gestational age among the inclusion criteria. In the whole study the earliest gestational age was 32 + 5, however this information is not given in the published report for the subgroup of women with a prior caesarean. In personal communication, the trial author reported that all women with a prior caesarean were 37 weeks' gestation or more. The gestational age for included women is not stated in Wing 1998.
The indications for induction of labour varied between studies. Taylor 1993 included only women with post‐term pregnancy or pre‐eclampsia. Sharma 2015 included only women with a post‐term pregnancy (defined by the trialists as from 40 weeks five days). Hassan 2014, Lokkegaard 2015, Manish 2016, Meetei 2015, Rezk 2014 and Wing 1998 used broad criteria for induction, including post‐term pregnancy, pre‐eclampsia or hypertension, gestational diabetes mellitus, oligohydramnios and intrauterine growth restriction.
Seven studies specified cephalic presentation in the inclusion criteria (Hassan 2014; Lokkegaard 2015; Manish 2016; Meetei 2015; Sharma 2015; Taylor 1993; Wing 1998). Seven studies included only women with a singleton pregnancy (Hassan 2014; Manish 2016; Meetei 2015; Rezk 2014; Sharma 2015; Taylor 1993; Wing 1998). Lokkegaard 2015 did not exclude multiple pregnancies, however there were none in the subgroup of women with a previous caesarean section. Six studies specified that women could participate if they had had a previous low transverse or lower segment caesarean section (Hassan 2014; Manish 2016; Meetei 2015; Rezk 2014; Sharma 2015; Taylor 1993). Wing 1998 included women with one prior caesarean, but found that verifying the type of incision was "often impossible in our population", and Lokkegaard 2015 did not report on the number or nature of prior caesarean(s).
Hassan 2014, Lokkegaard 2015, Manish 2016 and Rezk 2014 specified in their inclusion criteria that membranes had to be unruptured, as did the previous trial cited by Wing 1998 as having similar inclusion and exclusion criteria to this study (Wing 1996). Meetei 2015 and Sharma 2015 excluded women with premature rupture of membranes from their studies, and Taylor 1993 did not describe the status of membranes for women to be included in the trial.
The inclusion criteria of studies specified Bishop scores of less than or equal to seven (Hassan 2014), less than or equal to six (Rezk 2014), less than six (Lokkegaard 2015; Manish 2016), less than five (Meetei 2015), less than or equal to four (Wing 1998), and modified Bishop score less than nine (Taylor 1993). Sharma 2015 did not specify.
No information was reported on the indication for the previous caesarean section, or whether women had had a previous vaginal birth, in Lokkegaard 2015, Manish 2016, Meetei 2015, Rezk 2014 and Sharma 2015. In Taylor 1993, women whose only previous pregnancy was delivered by caesarean section were included, so no women had had a prior vaginal birth. The indications for previous caesarean sections are listed in a table in the report. Wing 1998 required that women had not had a vaginal birth since their caesarean section. Some women in Manish 2016 had had more than one previous pregnancy (seven out of 70 in the 30 mL group, four out of 70 in the 80 mL group), however it does not report whether these previous births were caesarean or vaginal deliveries. Hassan 2014 reports that 56 out of 100 women in the tablet group and 62 out of 100 women in the pessary group had had a prior vaginal delivery, in addition to their previous caesarean section.
Interventions
Three studies made a comparison with oxytocin: vaginal PGE2 (Taylor 1993), vaginal misoprostol (Wing 1998), and Foley catheter (Meetei 2015). Two additional studies made a comparison with Foley catheter: oral mifepristone (Sharma 2015), and vaginal isosorbide mononitrate (Rezk 2014). One study compared double‐balloon catheter with vaginal PGE2 (Lokkegaard 2015), one compared 30 mL Foley catheter with 80 mL Foley catheter (Manish 2016), and one compared vaginal PGE2 tablet with vaginal PGE2 pessary (Hassan 2014).
The dose of intravenous oxytocin was started at 1 mU/minute and increased if contractions were not frequent after one hour in Meetei 2015, "per a standardized infusion protocol" in Wing 1998, and in Taylor 1993, the dose was not reported. Amniotomy was done at the start of oxytocin administration Taylor 1993 and Wing 1998, but is not reported in Meetei 2015. PGE2 was administered as a 2.5 mg vaginal pessary (Taylor 1993), or 3 mg vaginal tablet (Lokkegaard 2015). The dose of misoprostol was 25 μg intravaginally every six hours to a maximum of four doses (Wing 1998). The Foley catheter balloon was inserted to the endocervix and inflated with 30 mL of sterile saline (Meetei 2015; Sharma 2015) or inserted into the cervix beyond the internal os and inflated with 80 mL or 30 mL of sterile water (Manish 2016) or 50 mL to 60 mL of normal saline (Rezk 2014). The double‐balloon catheter was inflated with 80 mL of saline in the uterine balloon and 80 mL of saline in the cervicovaginal balloon (Lokkegaard 2015). Women received a dose of 400 mg mifepristone orally (Sharma 2015). Women received either 1.5 mg PGE2 (dinoprostone) vaginal tablet into the posterior vaginal fornix for a maximum of three doses with six‐hourly intervals, or a single dose of PGE2 (dinoprostone) 10 mg sustained‐release vaginal pessary into the posterior vaginal fornix (Hassan 2014).
Outcomes
Three studies reported very few of our prespecified outcomes or did not report them in a form that could be included in the review (Lokkegaard 2015; Sharma 2015; Wing 1998). Perinatal outcomes were especially scarce. Wing 1998 reported only uterine rupture, and Sharma 2015 reported oxytocin augmentation and uterine rupture. Caesarean section and neonatal unit admission were the only prespecified outcomes reported for the subgroup of women with a previous caesarean in Lokkegaard 2015.
Five studies reported more of our prespecified outcomes (Hassan 2014; Manish 2016; Meetei 2015; Rezk 2014; Taylor 1993), including the primary outcomes: any delivery not achieved within 24 hours (Manish 2016; Meetei 2015; Rezk 2014), uterine hyperstimulation with FHR changes (Meetei 2015), caesarean section (Hassan 2014; Manish 2016; Meetei 2015; Rezk 2014; Taylor 1993), serious neonatal morbidity or perinatal death (Taylor 1993), and serious maternal morbidity or death (Taylor 1993). In several studies (e.g. Hassan 2014; Manish 2016; Rezk 2014) the composite outcomes were not reported, but individual elements of them were, for example, perinatal death, uterine rupture, and maternal admission to intensive care unit.
Excluded studies
See Characteristics of excluded studies.
We excluded nine studies (Arraztoa 1994; Ben‐Aroya 2001; Hamdan 2009; Lelaidier 1994; Morales 1986; Ramya 2015; Rayburn 1999; Sciscione 2001; Spallicci 2007).
The studies by Arraztoa 1994, Morales 1986 and Rayburn 1999 compared a pharmacological method of induction of labour with ongoing expectant management of the pregnancy. The Ben‐Aroya 2001 study did not involve a randomised comparison, while Hamdan 2009 and Ramya 2015 compared weekly membrane sweeping with weekly vaginal examination, in women who did not require induction of labour.
The Lelaidier 1994 study compared mifepristone with placebo as a pre‐induction agent, followed by vaginal prostaglandin induction in all women after an observation period of four days. Spallicci 2007 compared hyaluronidase with placebo in women with a prior caesarean birth at term, who did not require induction of labour. Sciscione 2001 compared transcervical Foley catheter with misoprostol to induce labour in women with a prior caesarean birth. However, the trial inclusion criteria were modified to exclude women with a prior caesarean birth, following the occurrence of a uterine rupture in the misoprostol group.
Risk of bias in included studies
Assessment of the methodological quality of the included studies was based on risk of bias in relation to selection bias (method of randomisation and allocation concealment), performance bias, detection bias, attrition bias (loss of participants from the analyses) and reporting bias. Summaries of 'Risk of bias' assessments for each study, and for included trials overall, are set out in Figure 2 and Figure 3.
2.
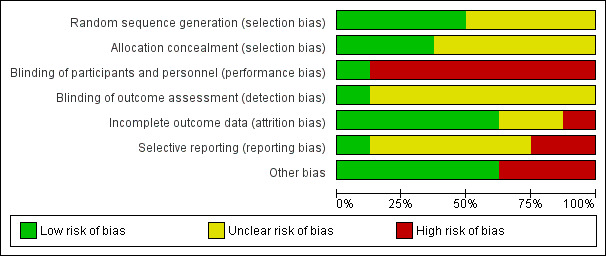
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.
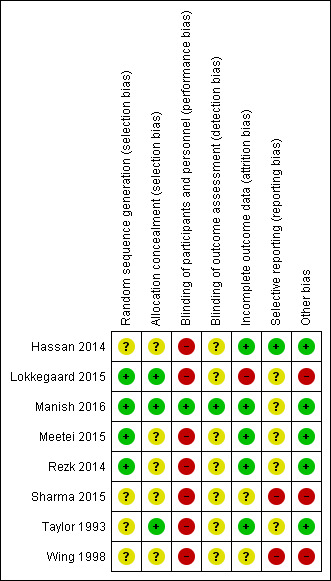
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation (selection bias)
Generation of the randomisation sequence
Three studies reported using a computer‐generated random sequence (Lokkegaard 2015; Manish 2016; Rezk 2014) and one used Tippets random number table (Meetei 2015), which we judged were at low risk of bias. The method of generating the randomisation sequence was not described in two studies (Hassan 2014; Wing 1998), which we judged to be unclear risk of bias. Sharma 2015 reported an intention to use computer‐generated randomisation in the study protocol, but this was not reported in the study report. We contacted the study author to clarify the method, but no response has been received, so their method was judged to be unclear risk of bias. Taylor 1993 described using "predetermined code envelope" which we also judged to be at unclear risk of bias.
Allocation concealment
In three of the studies, the method for concealing group allocation at the point of randomisation was at low risk of bias: randomised by a central telephone automatic voice‐response system (Lokkegaard 2015), and sealed, opaque envelopes (Manish 2016; Taylor 1993). The method for concealing group allocation was not described in Hassan 2014, Meetei 2015, Rezk 2014 or Wing 1998, which we judged to be at unclear risk of bias. Sharma 2015 reported an intention to use "sequentially numbered, sealed, opaque envelopes" in the study protocol, but this was not described in the study report, and enquiries to clarify the method were not answered, so we decided it was at unclear risk of bias.
Blinding (performance bias and detection bias)
Manish 2016 is the only study that reported any attempt to blind women or health professionals to group allocation. In this study, the decision to perform caesarean section was left to the discretion of an obstetrician who was unaware of group allocation. Women were unlikely to be aware of how much sterile water was in the Foley catheter, despite the personnel responsible for inserting and removing the catheter knowing. Therefore blinding was not perfect, but the bias was minimised and we judged it to be low risk of bias.
No other studies were blinded (Hassan 2014; Lokkegaard 2015; Meetei 2015; Rezk 2014; Sharma 2015; Taylor 1993; Wing 1998). It was not feasible to blind women or health professionals to most of these comparisons. This may have had an effect on other treatment decisions. All included studies have consequently been assessed as high risk of bias due to lack of blinding. It might have been possible to blind outcome assessors, but this was not described in any studies, so they were all judged to be at unclear risk of bias.
Incomplete outcome data (attrition bias)
Five studies were judged to be at low risk of attrition bias. All women appear to be accounted for and there is no mention of women dropping out of the study in Hassan 2014, Manish 2016, Meetei 2015, Rezk 2014 and Taylor 1993.
Two studies had unclear risk of attrition bias. In Sharma 2015, all women recruited appear to be accounted for in the results. However, the omission of two arms of the study may suggest bias. There is insufficient information in Wing 1998 to judge whether all women were accounted for.
One study was at high risk of bias. Women in Lokkegaard 2015 who went into spontaneous labour before induction, who were not in labour after 48 hours, or who had been coded as "VBAC" (vaginal birth after caesarean section) in error were classed as ‘failure’ and excluded from the results. There were 13 women randomised to each group, however results for only 10 are reported in the publication, and in correspondence from the study author additional 'failures' were identified. In the Minprostin group, two women began labour before induction, three were not in labour after 48 hours, and one was wrongly coded as VBAC. In the balloon group, three began labour before induction, one was not VBAC and 0 were not in labour after 48 hours. Unfortunately, despite additional information from the study authors, there were still missing data on outcomes, and we were unable to add the excluded women in an intention‐to‐treat analysis.
Selective reporting (reporting bias)
Two studies were judged to be at high risk of reporting bias. The protocol for Sharma 2015 describes a four‐arm study, but only two arms were reported in the publication. The abstracts from which it was assessed do not report any prespecified neonatal outcomes, and report only two arms of the four comparison groups set out in the protocol. No response was received from the authors when additional details were requested. In Wing 1998, uterine rupture is the only outcome reported. It is likely that other outcomes were prespecified and these are not reported.
Four studies were at unclear risk of reporting bias: Meetei 2015, Rezk 2014 and Taylor 1993 were assessed from published reports, without protocols available. It is unclear whether all prespecified outcomes were reported in these studies. Several secondary outcomes prespecified in the protocol for Manish 2016 were not reported in the published study report. In Lokkegaard 2015 the subset of participants with a previous VBAC were not the primary focus of this study, so few outcomes are reported for these women.
One study was at low risk of reporting bias: Hassan 2014 was assessed from a published report with no protocol available, however outcomes were comprehensively reported.
Other potential sources of bias
Five studies were at low risk: the groups were comparable at baseline, and no other potential sources of bias were identified (Hassan 2014; Manish 2016; Meetei 2015; Rezk 2014; Taylor 1993).
Three studies were considered to be at high risk of other bias: the inconsistencies between the study protocol and published report for Sharma 2015 suggest that this study is at high risk of other bias. In Lokkegaard 2015 the primary endpoints of the full study are reported by both intention‐to‐treat and per‐protocol analyses, indicating that a large proportion of women did not receive the allocated treatment. Wing 1998 was stopped prematurely due to safety concerns.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6; Table 7; Table 8
We did not combine data from the six included studies because they used different methods of induction of labour, so we did not consider meta‐analysis appropriate.
1. Vaginal PGE2 inserts versus intravenous oxytocin
A single study involving 42 women compared vaginal PGE2 with intravenous oxytocin for induction of labour (Taylor 1993). See Table 1.
Primary outcomes
There were no differences identified between the two treatment groups for caesarean section (risk ratio (RR) 0.67, 95% confidence interval (CI) 0.22 to 2.03, one study, 42 women, evidence graded low, Analysis 1.1). There was only one event for serious neonatal morbidity or perinatal death and serious maternal morbidity or death, so the analysis of differences between groups was not meaningful (one study, 42 women, evidence graded low, Analysis 1.2; one study, 42 women, evidence graded low,Analysis 1.3).
1.1. Analysis.
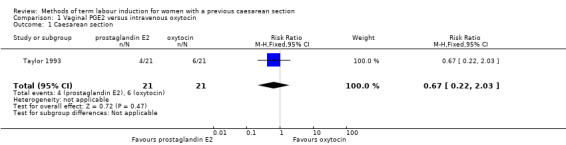
Comparison 1 Vaginal PGE2 versus intravenous oxytocin, Outcome 1 Caesarean section.
1.2. Analysis.
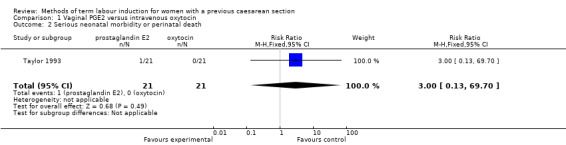
Comparison 1 Vaginal PGE2 versus intravenous oxytocin, Outcome 2 Serious neonatal morbidity or perinatal death.
1.3. Analysis.
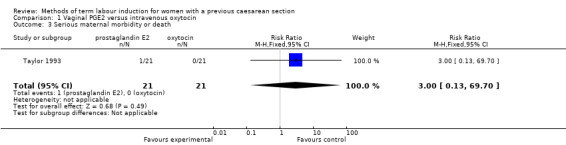
Comparison 1 Vaginal PGE2 versus intravenous oxytocin, Outcome 3 Serious maternal morbidity or death.
The study did not report the following primary/GRADE outcomes: vaginal delivery not achieved within 24 hours, anduterine hyperstimulation with fetal heart rate (FHR) changes.
Secondary outcomes
One woman was identified as having a uterine rupture following prostaglandin administration, so the analysis is not meaningful (one study, 42 women, Analysis 1.4). There were no differences identified in the secondary maternal or infant outcomes, including use of epidural analgesia (RR 1.42, 95% CI 0.93 to 2.17, one study, 42 women, Analysis 1.5), instrumental vaginal delivery (RR 1.25, 95% CI 0.39 to 4.02, one study, 42 women, Analysis 1.6), or Apgar score of less than seven at five minutes (no events, one study, 42 infants, Analysis 1.7).
1.4. Analysis.
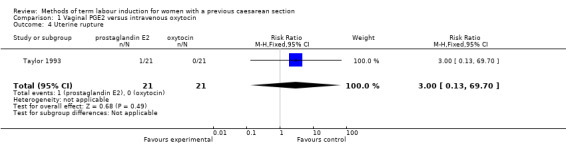
Comparison 1 Vaginal PGE2 versus intravenous oxytocin, Outcome 4 Uterine rupture.
1.5. Analysis.
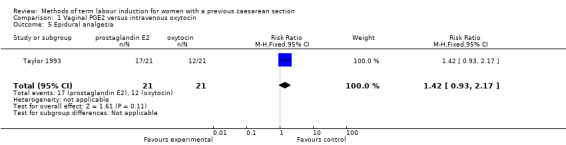
Comparison 1 Vaginal PGE2 versus intravenous oxytocin, Outcome 5 Epidural analgesia.
1.6. Analysis.
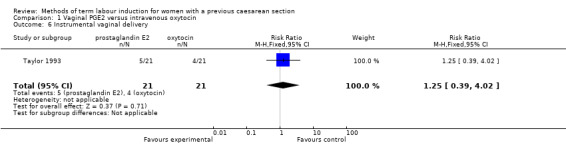
Comparison 1 Vaginal PGE2 versus intravenous oxytocin, Outcome 6 Instrumental vaginal delivery.
1.7. Analysis.
Comparison 1 Vaginal PGE2 versus intravenous oxytocin, Outcome 7 Apgar score < 7 at 5 minutes.
The study did not report the following secondary outcomes: cervix unfavourable/unchanged after 12 to 24 hours, oxytocin augmentation, uterine hyperstimulation without FHR changes, meconium‐stained liquor, neonatal intensive care unit admission, neonatal encephalopathy, perinatal death, disability in childhood, neonatal infection, neonatal antibiotics, maternal side‐effects (all), maternal nausea, maternal vomiting, maternal diarrhoea, other maternal side‐effects, postpartum haemorrhage, chorioamnionitis, endometritis, maternal antibiotics, serious maternal complications (e.g. intensive care unit admission, septicaemia but excluding uterine rupture), maternal death, woman not satisfied, and caregiver not satisfied.
2. Vaginal misoprostol versus intravenous oxytocin
There was one study comparing vaginal misoprostol and intravenous oxytocin included in the review (Wing 1998). No GRADE outcomes were reported. See Table 2.
Primary outcomes
This trial was stopped following recruitment and randomisation of 38 women (17 women misoprostol group; 21 women oxytocin group) and no primary outcomes or GRADE outcomes were reported (vaginal delivery not achieved within 24 hours, uterine hyperstimulation with FHR changes, caesarean section, serious neonatal morbidity or perinatal death, and serious maternal morbidity or death).
Secondary outcomes
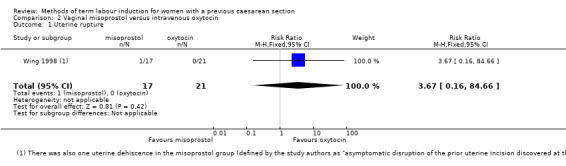
The only outcome reported was uterine rupture, which occurred in one woman in the misoprostol group (RR 3.67, 95% CI 0.16 to 84.66, one study, 38 women, Analysis 2.1). One woman in the misoprostol group also experienced uterine dehiscence.
2.1. Analysis.
Comparison 2 Vaginal misoprostol versus intravenous oxytocin, Outcome 1 Uterine rupture.
The study did not report the following secondary outcomes: cervix unfavourable/unchanged after 12 to 24 hours, oxytocin augmentation, uterine hyperstimulation without FHR changes, epidural analgesia, instrumental vaginal delivery, meconium‐stained liquor, Apgar score less than seven at five minutes, neonatal intensive care unit admission, neonatal encephalopathy, perinatal death, disability in childhood, neonatal infection, neonatal antibiotics, maternal side‐effects (all), maternal nausea, maternal vomiting, maternal diarrhoea, other maternal side‐effects, postpartum haemorrhage, chorioamnionitis, endometritis, maternal antibiotics, serious maternal complications (e.g. intensive care unit admission, septicaemia but excluding uterine rupture), maternal death, woman not satisfied, and caregiver not satisfied.
3. Foley catheter versus intravenous oxytocin
One study comparing Foley catheter with intravenous oxytocin was included (Meetei 2015). The study author supplied unpublished data for the women who were 37 weeks' gestation or more (53 of the 60 women who participated). See Table 3.
Primary outcomes
There was no difference between oxytocin and Foley catheter in the number of women who delivered within 24 hours (RR 1.47, 95% CI 0.89 to 2.44, one study, 53 women, evidence graded low, Analysis 3.1), uterine hyperstimulation with FHR changes (RR 3.11, 95% CI 0.13 to 73.09, one study, 53 women, evidence graded low, Analysis 3.2), or the number of women requiring a caesarean section (RR 0.93, 95% CI 0.45 to 1.92, one study, 53 women, evidence graded low,Analysis 3.3).
3.1. Analysis.
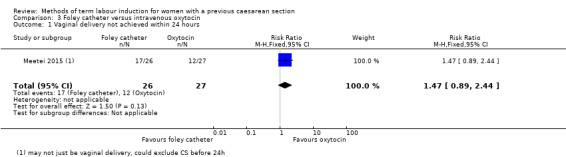
Comparison 3 Foley catheter versus intravenous oxytocin, Outcome 1 Vaginal delivery not achieved within 24 hours.
3.2. Analysis.
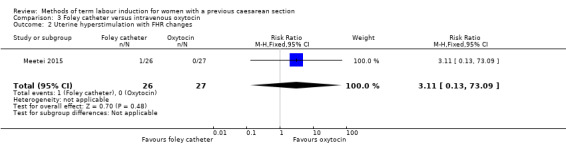
Comparison 3 Foley catheter versus intravenous oxytocin, Outcome 2 Uterine hyperstimulation with FHR changes.
3.3. Analysis.
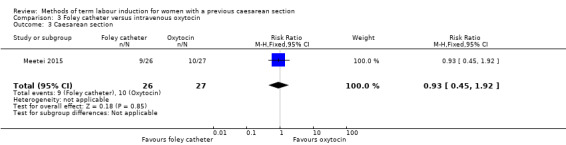
Comparison 3 Foley catheter versus intravenous oxytocin, Outcome 3 Caesarean section.
The study did not report the following primary/GRADE composite outcomes: serious neonatal morbidity or perinatal death, and serious maternal morbidity or death.
Secondary outcomes
There was no difference between the groups in the number of women requiring oxytocin augmentation (RR 1.04, 95% CI 0.81 to 1.32, one study, 53 women, Analysis 3.4), uterine rupture (no events, one study, 53 women, Analysis 3.5), instrumental vaginal delivery (RR 7.26, 95% CI 0.39 to 134.01, one study, 53 women, Analysis 3.6), postpartum haemorrhage (RR 3.11, 95% CI 0.13 to 73.09, one study, 53 women, Analysis 3.7) and chorioamnionitis (not estimable, one study, 53 women, Analysis 3.8). However the number of events for each of these outcomes was very low and the study did not include enough women to show differences between the groups. Two women in the oxytocin group had scar dehiscence, while none in the Foley catheter group did.
3.4. Analysis.
Comparison 3 Foley catheter versus intravenous oxytocin, Outcome 4 Oxytocin augmentation.
3.5. Analysis.
Comparison 3 Foley catheter versus intravenous oxytocin, Outcome 5 Uterine rupture.
3.6. Analysis.
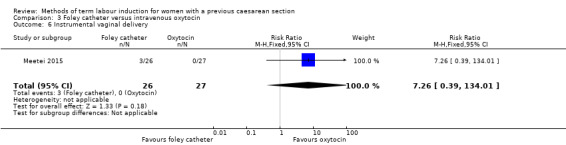
Comparison 3 Foley catheter versus intravenous oxytocin, Outcome 6 Instrumental vaginal delivery.
3.7. Analysis.
Comparison 3 Foley catheter versus intravenous oxytocin, Outcome 7 Postpartum haemorrhage.
3.8. Analysis.
Comparison 3 Foley catheter versus intravenous oxytocin, Outcome 8 Chorioamnionitis.
The study did not report the following secondary outcomes: cervix unfavourable/unchanged after 12 to 24 hours, uterine hyperstimulation without FHR changes, epidural analgesia, meconium‐stained liquor, Apgar score less than seven at five minutes, neonatal intensive care unit admission, neonatal encephalopathy, perinatal death, disability in childhood, neonatal infection, neonatal antibiotics, maternal side‐effects (all), maternal nausea, maternal vomiting, maternal diarrhoea, other maternal side‐effects, endometritis, maternal antibiotics, serious maternal complications (e.g. intensive care unit admission, septicaemia but excluding uterine rupture), maternal death, woman not satisfied, and caregiver not satisfied.
4. Double‐balloon catheter versus vaginal PGE2
One study compared double‐balloon catheter with vaginal PGE2 (Lokkegaard 2015). Data from the subgroup of women who had had a previous caesarean section were included in this review. See Table 4.
Primary outcomes
There was no difference between the groups for caesarean section (RR 0.97, 95% CI 0.41 to 2.32, one study, 16 women, evidence graded very low,Analysis 4.1).
4.1. Analysis.
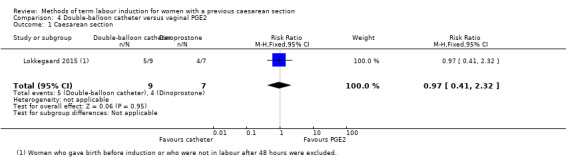
Comparison 4 Double‐balloon catheter versus vaginal PGE2, Outcome 1 Caesarean section.
The study did not report the following primary/GRADE outcomes: vaginal delivery not achieved within 24 hours, uterine hyperstimulation with FHR changes, serious neonatal morbidity or perinatal death, and serious maternal morbidity or death.
Failed induction was reported (three out of 12 women in the double‐balloon catheter group, and five out of 12 women in the dinoprostone group), however we did not included this outcome in this review as it included women who started labour before induction began and women who had not delivered 48 hours after induction began.
Secondary outcomes
No babies in this subgroup of the study were admitted to neonatal unit (not estimable, one study, 20 infants, Analysis 4.2).
4.2. Analysis.
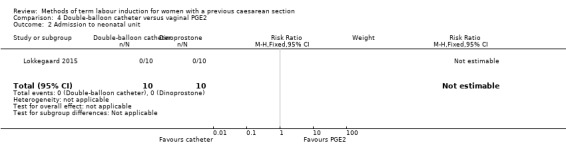
Comparison 4 Double‐balloon catheter versus vaginal PGE2, Outcome 2 Admission to neonatal unit.
The study did not report the following secondary outcomes: cervix unfavourable/unchanged after 12 to 24 hours, oxytocin augmentation, uterine hyperstimulation without FHR changes, uterine rupture, epidural analgesia, instrumental vaginal delivery, meconium‐stained liquor, Apgar score less than seven at five minutes, neonatal encephalopathy, perinatal death, disability in childhood, neonatal infection, neonatal antibiotics, maternal side‐effects (all), maternal nausea, maternal vomiting, maternal diarrhoea, other maternal side‐effects, postpartum haemorrhage, chorioamnionitis, endometritis, maternal antibiotics, serious maternal complications (e.g. intensive care unit admission, septicaemia but excluding uterine rupture), maternal death, woman not satisfied, and caregiver not satisfied.
5. Oral mifepristone versus Foley catheter
One study compared oral mifepristone versus Foley catheter (Sharma 2015). No GRADE outcomes were reported. See Table 5.
Primary outcomes
The study did not report any of the primary/GRADE outcomes: vaginal delivery not achieved within 24 hours, uterine hyperstimulation with FHR changes, caesarean section, serious neonatal morbidity or perinatal death, and serious maternal morbidity or death.
Secondary outcomes
More women who were induced with Foley catheter than mifepristone required further oxytocin augmentation (RR 0.54, 95% CI 0.38 to 0.76, one study, 107 women, Analysis 5.1). The number of women who had a uterine rupture was slightly lower with mifepristone (three out of 57, compared with nine out of 50), however this does not show a clear difference between groups (RR 0.29, 95% CI 0.08 to 1.02, one study, 107 women, Analysis 5.2).
5.1. Analysis.
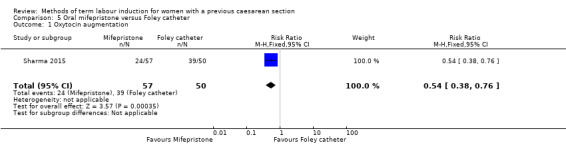
Comparison 5 Oral mifepristone versus Foley catheter, Outcome 1 Oxytocin augmentation.
5.2. Analysis.
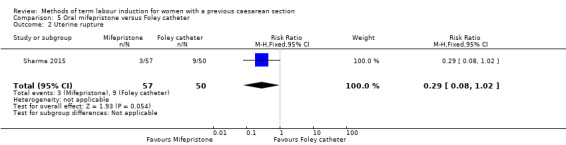
Comparison 5 Oral mifepristone versus Foley catheter, Outcome 2 Uterine rupture.
The study did not report the following secondary outcomes: cervix unfavourable/unchanged after 12 to 24 hours, uterine hyperstimulation without FHR changes, epidural analgesia, instrumental vaginal delivery, meconium‐stained liquor, Apgar score less than seven at five minutes, neonatal intensive care unit admission, neonatal encephalopathy, perinatal death, disability in childhood, neonatal infection, neonatal antibiotics, maternal side‐effects (all), maternal nausea, maternal vomiting, maternal diarrhoea, other maternal side‐effects, postpartum haemorrhage, chorioamnionitis, endometritis, maternal antibiotics, serious maternal complications (e.g. intensive care unit admission, septicaemia but excluding uterine rupture), maternal death, woman not satisfied, and caregiver not satisfied.
6. Vaginal isosorbide mononitrate versus Foley catheter
One study compared vaginal isosorbide mononitrate (IMN) versus Foley catheter (Rezk 2014). See Table 6.
Primary outcomes
More women who were induced using IMN rather than Foley catheter had not achieved a vaginal delivery within 24 hours (RR 2.62, 95% CI 1.32 to 5.21, one study, 80 women, evidence graded low,Analysis 6.1). There was no difference in the number of women who had a caesarean section (RR 1.00, 95% CI 0.39 to 2.59, one study, 80 women, evidence graded very low,Analysis 6.2).
6.1. Analysis.
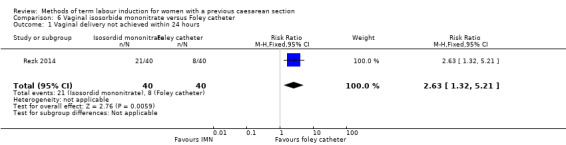
Comparison 6 Vaginal isosorbide mononitrate versus Foley catheter, Outcome 1 Vaginal delivery not achieved within 24 hours.
6.2. Analysis.
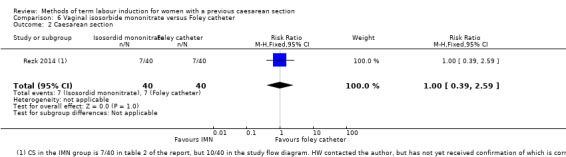
Comparison 6 Vaginal isosorbide mononitrate versus Foley catheter, Outcome 2 Caesarean section.
The study did not report the following primary/GRADE outcomes: uterine hyperstimulation with FHR changes, serious neonatal morbidity or perinatal death, and serious maternal morbidity or death.
Secondary outcomes
More women who in the IMN group compared with the Foley catheter group received oxytocin augmentation (RR 1.65, 95% CI 1.17 to 2.32, one study, 80 women, Analysis 6.3).
6.3. Analysis.
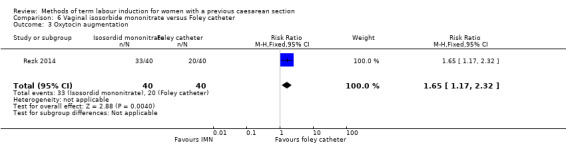
Comparison 6 Vaginal isosorbide mononitrate versus Foley catheter, Outcome 3 Oxytocin augmentation.
The number of women may have been too small to show clear differences for some outcomes: slightly more infants in the IMN group had an Apgar score less than seven at five minutes (IMN: 20 out of 40, Foley catheter: 12 out of 40; RR 1.67, 95% CI 0.95 to 2.93, Analysis 6.8); slightly more women experience puerperal pyrexia with Foley catheter (IMN: five out of 40, Foley catheter: 12 out of 40; RR 0.42, 95% CI 0.16 to 1.07, Analysis 6.11); and slightly more women experienced headaches with IMN (IMN: 10 out of 40, Foley catheter: three out of 40; RR 3.33, 95% CI 0.99 to 11.22, Analysis 6.13).
6.8. Analysis.
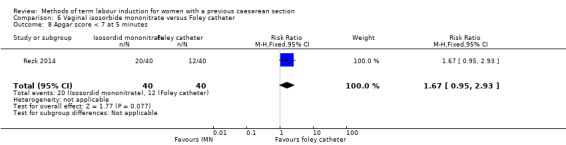
Comparison 6 Vaginal isosorbide mononitrate versus Foley catheter, Outcome 8 Apgar score < 7 at 5 minutes.
6.11. Analysis.
Comparison 6 Vaginal isosorbide mononitrate versus Foley catheter, Outcome 11 Puerperal pyrexia (other maternal side‐effects).
6.13. Analysis.
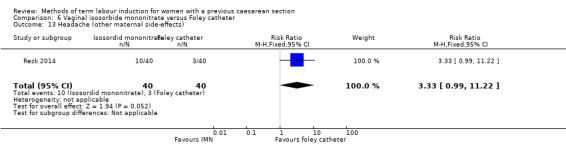
Comparison 6 Vaginal isosorbide mononitrate versus Foley catheter, Outcome 13 Headache (other maternal side‐effects).
In this study of 80 women and infants, there was no clear difference between groups for: uterine rupture (no events, Analysis 6.4); epidural analgesia (RR 1.00, 95% CI 0.39 to 2.59, Analysis 6.5); instrumental vaginal delivery (RR 0.80, 95% CI 0.23 to 2.76, Analysis 6.6); meconium‐stained liquor (RR 2.00, 95% CI 0.19 to 21.18, Analysis 6.7); neonatal intensive care unit admission (RR 2.50, 95% CI 0.51 to 12.14, Analysis 6.9); maternal nausea and vomiting (RR 3.00, 95% CI 0.33 to 27.63, Analysis 6.10); palpitation (RR 2.50, 95% CI 0.51 to 12.14, Analysis 6.12); postpartum haemorrhage (RR 2.00, 95% CI 0.90 to 4.43, Analysis 6.14); and woman not satisfied (RR 1.75, 95% CI 0.56 to 5.51, Analysis 6.15).
6.4. Analysis.
Comparison 6 Vaginal isosorbide mononitrate versus Foley catheter, Outcome 4 Uterine rupture.
6.5. Analysis.
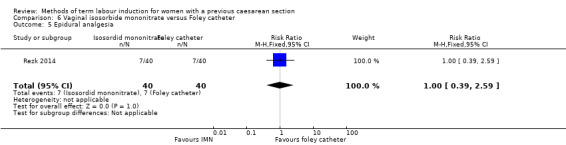
Comparison 6 Vaginal isosorbide mononitrate versus Foley catheter, Outcome 5 Epidural analgesia.
6.6. Analysis.
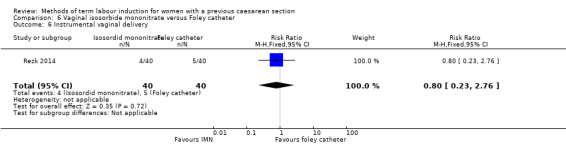
Comparison 6 Vaginal isosorbide mononitrate versus Foley catheter, Outcome 6 Instrumental vaginal delivery.
6.7. Analysis.
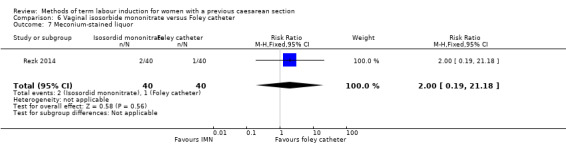
Comparison 6 Vaginal isosorbide mononitrate versus Foley catheter, Outcome 7 Meconium‐stained liquor.
6.9. Analysis.
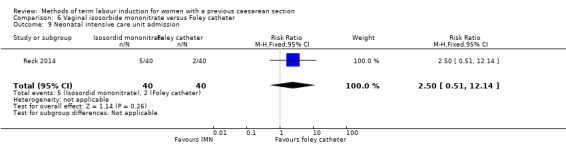
Comparison 6 Vaginal isosorbide mononitrate versus Foley catheter, Outcome 9 Neonatal intensive care unit admission.
6.10. Analysis.
Comparison 6 Vaginal isosorbide mononitrate versus Foley catheter, Outcome 10 Maternal nausea and vomiting.
6.12. Analysis.
Comparison 6 Vaginal isosorbide mononitrate versus Foley catheter, Outcome 12 Palpitation (other maternal side‐effects).
6.14. Analysis.
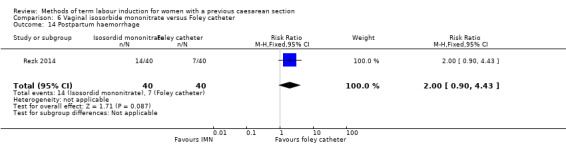
Comparison 6 Vaginal isosorbide mononitrate versus Foley catheter, Outcome 14 Postpartum haemorrhage.
6.15. Analysis.
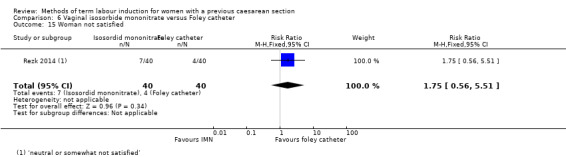
Comparison 6 Vaginal isosorbide mononitrate versus Foley catheter, Outcome 15 Woman not satisfied.
The study did not report the following secondary outcomes: cervix unfavourable/unchanged after 12 to 24 hours, uterine hyperstimulation without FHR changes, neonatal encephalopathy, perinatal death, disability in childhood, neonatal infection, neonatal antibiotics, maternal side‐effects (all), maternal diarrhoea, chorioamnionitis, endometritis, maternal antibiotics, serious maternal complications (e.g. intensive care unit admission, septicaemia but excluding uterine rupture), maternal death, and caregiver not satisfied.
7. Foley catheter (80 mL) versus Foley catheter (30 mL)
One study compared 80 mL Foley catheter versus 30 mL Foley catheter (Manish 2016). See Table 7.
Primary outcomes
There was no clear difference between groups for vaginal delivery not achieved within 24 hours (RR 1.05, 95% CI 0.91 to 1.20, one study, 154 women, evidence graded moderate,Analysis 7.1) and caesarean section (RR 1.05, 95% CI 0.89 to 1.24, one study, 154 women, evidence graded moderate,Analysis 7.2).
7.1. Analysis.
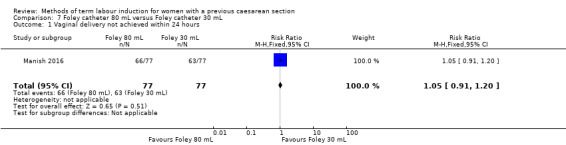
Comparison 7 Foley catheter 80 mL versus Foley catheter 30 mL, Outcome 1 Vaginal delivery not achieved within 24 hours.
7.2. Analysis.
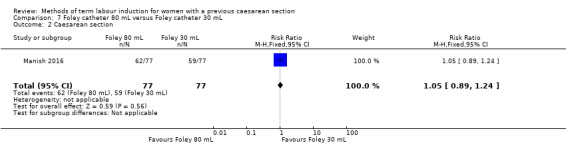
Comparison 7 Foley catheter 80 mL versus Foley catheter 30 mL, Outcome 2 Caesarean section.
The study did not report the following primary/GRADE outcomes: uterine hyperstimulation with FHR changes, serious neonatal morbidity or perinatal death, and serious maternal morbidity or death.
Secondary outcomes
More women who were induced using a 30 mL Foley catheter required oxytocin augmentation (RR 0.81, 95% CI 0.66 to 0.98, one study, 154 women, Analysis 7.3).
7.3. Analysis.
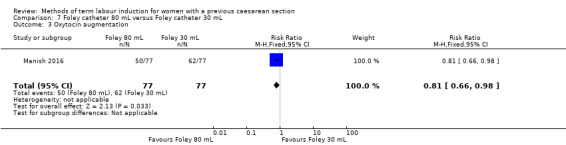
Comparison 7 Foley catheter 80 mL versus Foley catheter 30 mL, Outcome 3 Oxytocin augmentation.
In this study of 154 women and infants, there was no clear difference between groups for: uterine rupture (RR 1.00, 95% CI 0.06 to 15.70, Analysis 7.4); epidural analgesia (no events, Analysis 7.5); instrumental vaginal delivery (RR 0.92, 95% CI 0.43 to 1.95, Analysis 7.6); Apgar score less than seven at five minutes (RR 1.00, 95% CI 0.06 to 15.70, Analysis 7.7); neonatal intensive care unit admission (RR 2.00, 95% CI 0.19 to 21.60, Analysis 7.8); neonatal encephalopathy (RR 3.00, 95% CI 0.12 to 72.52, Analysis 7.9); perinatal death (RR 3.00, 95% CI 0.12 to 72.52, Analysis 7.10); neonatal infection (no events, Analysis 7.11); cord prolapse (other maternal side‐effects) (no events, Analysis 7.12); postpartum haemorrhage (RR 0.33, 95% CI 0.01 to 8.06, Analysis 7.13); and chorioamnionitis (RR 0.33, 95% CI 0.04 to 3.13, Analysis 7.14).
7.4. Analysis.
Comparison 7 Foley catheter 80 mL versus Foley catheter 30 mL, Outcome 4 Uterine rupture.
7.5. Analysis.
Comparison 7 Foley catheter 80 mL versus Foley catheter 30 mL, Outcome 5 Epidural analgesia.
7.6. Analysis.
Comparison 7 Foley catheter 80 mL versus Foley catheter 30 mL, Outcome 6 Instrumental vaginal delivery.
7.7. Analysis.
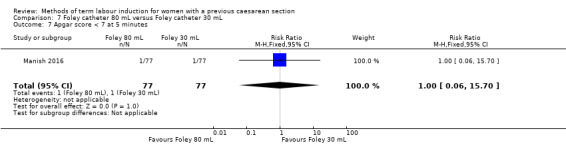
Comparison 7 Foley catheter 80 mL versus Foley catheter 30 mL, Outcome 7 Apgar score < 7 at 5 minutes.
7.8. Analysis.
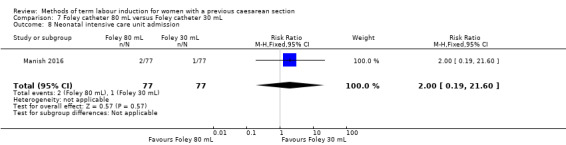
Comparison 7 Foley catheter 80 mL versus Foley catheter 30 mL, Outcome 8 Neonatal intensive care unit admission.
7.9. Analysis.
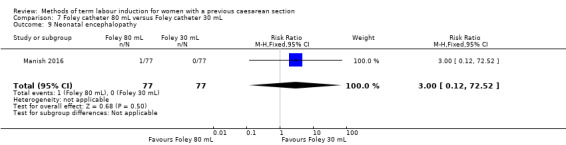
Comparison 7 Foley catheter 80 mL versus Foley catheter 30 mL, Outcome 9 Neonatal encephalopathy.
7.10. Analysis.
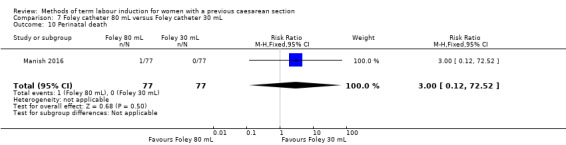
Comparison 7 Foley catheter 80 mL versus Foley catheter 30 mL, Outcome 10 Perinatal death.
7.11. Analysis.
Comparison 7 Foley catheter 80 mL versus Foley catheter 30 mL, Outcome 11 Neonatal infection.
7.12. Analysis.
Comparison 7 Foley catheter 80 mL versus Foley catheter 30 mL, Outcome 12 Cord prolapse (other maternal side‐effects).
7.13. Analysis.
Comparison 7 Foley catheter 80 mL versus Foley catheter 30 mL, Outcome 13 Postpartum haemorrhage.
7.14. Analysis.
Comparison 7 Foley catheter 80 mL versus Foley catheter 30 mL, Outcome 14 Chorioamnionitis.
The study did not report the following secondary outcomes: cervix unfavourable/unchanged after 12 to 24 hours, uterine hyperstimulation without FHR changes, meconium‐stained liquor, disability in childhood, neonatal antibiotics, maternal side‐effects (all), maternal nausea, maternal vomiting, maternal diarrhoea, endometritis, maternal antibiotics, serious maternal complications (e.g. intensive care unit admission, septicaemia but excluding uterine rupture), maternal death, woman not satisfied, and caregiver not satisfied.
8. Vaginal PGE2 tablet versus vaginal PGE2 pessary
One study compared PGE2 (dinoprostone) vaginal tablet versus vaginal pessary (Hassan 2014). See Table 8.
Primary outcomes
There was no clear difference between groups in the number of women requiring a caesarean section (RR 1.09, 95% CI 0.74 to 1.60, one study, 200 women, evidence graded very low, Analysis 8.1).
8.1. Analysis.
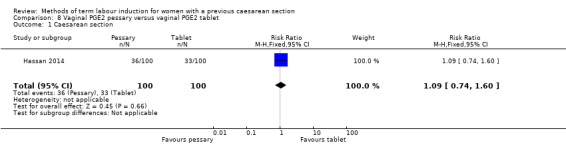
Comparison 8 Vaginal PGE2 pessary versus vaginal PGE2 tablet, Outcome 1 Caesarean section.
The study did not report the following primary/GRADE outcomes: vaginal delivery not achieved within 24 hours, uterine hyperstimulation with FHR changes, serious neonatal morbidity or perinatal death, and serious maternal morbidity or death.
Secondary outcomes
In this study of 200 women and infants, there was no clear difference between groups for: oxytocin augmentation (RR 1.50, 95% CI 0.81 to 2.78, Analysis 8.2); uterine hyperstimulation (FHR change not mentioned) (RR 0.50, 95% CI 0.05 to 5.43, Analysis 8.3); uterine rupture (RR 0.33, 95% CI 0.01 to 8.09, Analysis 8.4); Apgar score less than seven at five minutes (RR 0.80, 95% CI 0.22 to 2.89, Analysis 8.5); neonatal intensive care unit admission (RR 0.75, 95% CI 0.17 to 3.27, Analysis 8.6); neonatal infection (RR 1.00, 95% CI 0.06 to 15.77, Analysis 8.7); postpartum haemorrhage (RR 1.00, 95% CI 0.14 to 6.96, Analysis 8.8); chorioamnionitis (RR 1.00, 95% CI 0.06 to 15.77, Analysis 8.9); endometritis (RR 1.50, 95% CI 0.26 to 8.79, Analysis 8.10); and maternal intensive care unit admission (no events, Analysis 8.11).
8.2. Analysis.
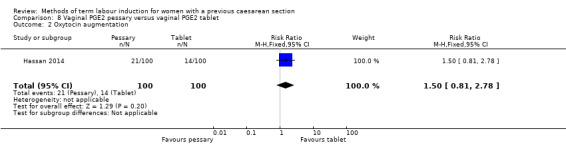
Comparison 8 Vaginal PGE2 pessary versus vaginal PGE2 tablet, Outcome 2 Oxytocin augmentation.
8.3. Analysis.
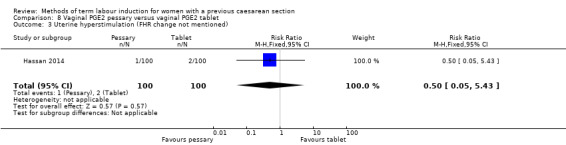
Comparison 8 Vaginal PGE2 pessary versus vaginal PGE2 tablet, Outcome 3 Uterine hyperstimulation (FHR change not mentioned).
8.4. Analysis.
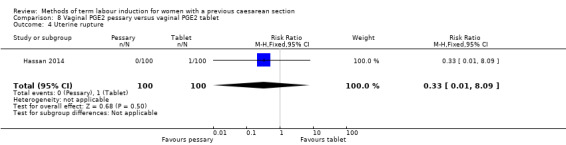
Comparison 8 Vaginal PGE2 pessary versus vaginal PGE2 tablet, Outcome 4 Uterine rupture.
8.5. Analysis.
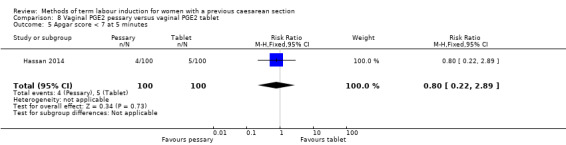
Comparison 8 Vaginal PGE2 pessary versus vaginal PGE2 tablet, Outcome 5 Apgar score < 7 at 5 minutes.
8.6. Analysis.
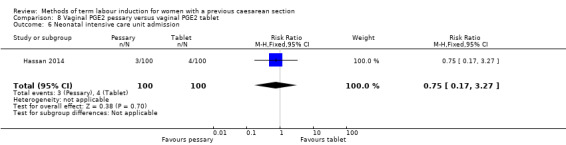
Comparison 8 Vaginal PGE2 pessary versus vaginal PGE2 tablet, Outcome 6 Neonatal intensive care unit admission.
8.7. Analysis.
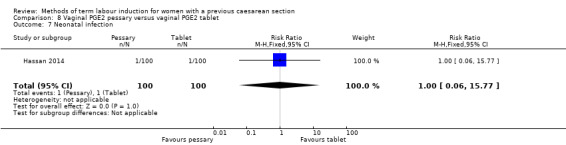
Comparison 8 Vaginal PGE2 pessary versus vaginal PGE2 tablet, Outcome 7 Neonatal infection.
8.8. Analysis.
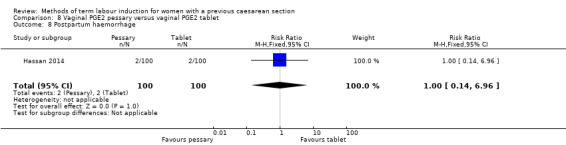
Comparison 8 Vaginal PGE2 pessary versus vaginal PGE2 tablet, Outcome 8 Postpartum haemorrhage.
8.9. Analysis.
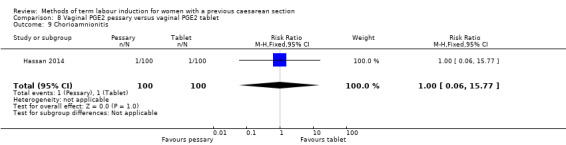
Comparison 8 Vaginal PGE2 pessary versus vaginal PGE2 tablet, Outcome 9 Chorioamnionitis.
8.10. Analysis.
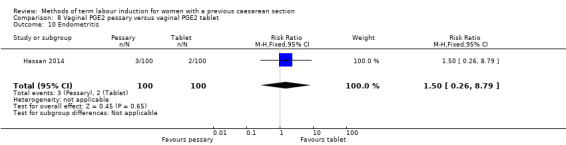
Comparison 8 Vaginal PGE2 pessary versus vaginal PGE2 tablet, Outcome 10 Endometritis.
8.11. Analysis.
Comparison 8 Vaginal PGE2 pessary versus vaginal PGE2 tablet, Outcome 11 Maternal intensive care unit admission (serious maternal complications).
The study did not report the following secondary outcomes: cervix unfavourable/unchanged after 12 to 24 hours, epidural analgesia, instrumental vaginal delivery, meconium‐stained liquor, neonatal encephalopathy, perinatal death, disability in childhood, neonatal antibiotics, maternal side‐effects (all), maternal nausea, maternal vomiting, maternal diarrhoea, other maternal side‐effects, maternal antibiotics, maternal death, woman not satisfied, and caregiver not satisfied.
Discussion
Summary of main results
Eight studies were included in this updated review (Hassan 2014; Lokkegaard 2015; Manish 2016; Meetei 2015; Rezk 2014; Sharma 2015; Taylor 1993; Wing 1998), with a total of 707 women participating in these studies, or the eligible subgroups within them. Three studies compared an intervention with intravenous oxytocin: vaginal PGE2 (Taylor 1993), vaginal misoprostol (Wing 1998), and Foley catheter (Meetei 2015). One study compared double‐balloon catheter with vaginal PGE2 (Lokkegaard 2015), one compared oral mifepristone with Foley catheter (Sharma 2015), and one trial compared vaginal isosorbide mononitrate (a nitric oxide donor) with Foley catheter (Rezk 2014). One study compared 80 mL Foley catheter with 30 mL Foley catheter (Manish 2016), and one study compared vaginal PGE2 pessary with vaginal PGE2 tablet (Hassan 2014).
The available evidence from randomised controlled trials relating to methods of induction of labour for women with a prior caesarean section is inadequate. The available studies are underpowered to detect clinically relevant differences in the primary and secondary outcome measures, and many important outcomes were not reported. As the studies compared different methods of labour induction, no meta‐analysis was possible.
No clear differences were found between vaginal PGE2 and intravenous oxytocin for the outcomes reported by Taylor 1993 (42 women): caesarean section, serious neonatal morbidity or perinatal death, serious maternal morbidity or death, uterine rupture, epidural analgesia, instrumental vaginal delivery, and Apgar score less than seven at five minutes.
One woman who received vaginal misoprostol rather than intravenous oxytocin had a uterine rupture and one had a uterine dehiscence, prompting Wing 1998 to prematurely end the trial. Despite this, there was no clear difference between groups, possibly due to the small number of participants. None of our other prespecified primary or secondary outcomes were reported in this study of 38 women.
One study comparing Foley catheter with intravenous oxytocin in a study of 53 women (Meetei 2015) found no difference between groups for all reported primary and secondary outcomes: vaginal delivery within 24 hours, uterine stimulation with fetal heart rate changes, caesarean section, oxytocin augmentation, uterine rupture, instrumental vaginal delivery, postpartum haemorrhage, and chorioamnionitis.
There was no clear difference between women who were induced using a double‐balloon catheter versus vaginal PGE2 for caesarean section and admission to the neonatal unit in Lokkegaard 2015 (26 women) for the outcomes reported: caesarean section, and admission to neonatal unit. 'Failed induction' was reported, however this combined women who had begun labour between randomisation and induction, as well as women who had not delivered 48 hours after induction, so it was not considered meaningful to include this. No other primary or secondary outcomes were reported.
None of the primary outcomes were reported by Sharma 2015 (107 women). Women induced with oral mifepristone received less oxytocin augmentation than those induced with Foley catheter. There were slightly fewer cases of uterine rupture in the mifepristone group, however this was not a clear difference between groups.
One study comparing induction with vaginal isosorbide mononitrate (IMN) versus Foley catheter (80 women, Rezk 2014) found that fewer women induced with isosorbide mononitrate achieved a vaginal delivery within 24 hours. More women induced with IMN required oxytocin augmentation. There were no clear differences for the other reported outcomes: caesarean section, uterine rupture, epidural analgesia, instrumental vaginal delivery, meconium‐stained liquor, Apgar score less than seven at five minutes, neonatal intensive care unit admission, maternal nausea and vomiting, puerperal pyrexia, palpitation, headache, postpartum haemorrhage, and woman not satisfied. There were slightly more infants with an Apgar score less than seven at five minutes in the IMN group, slightly more women experienced puerperal pyrexia in the Foley catheter group, and slightly more women experienced headaches with IMN, however the low number of participants meant that these were not clear differences between the groups of women.
Manish 2016 compared 80 mL Foley catheter with 30 mL Foley catheter (154 women). There was no clear difference between groups for the primary outcomes: vaginal delivery not achieved within 24 hours and caesarean section. More women who were induced using a 30 mL Foley catheter required oxytocin augmentation. There were no clear differences in the other reported secondary outcomes: uterine rupture, epidural analgesia, instrumental vaginal delivery, Apgar score less than seven at five minutes, neonatal intensive care unit admission, neonatal encephalopathy, perinatal death, neonatal infection, cord prolapse, postpartum haemorrhage, and chorioamnionitis.
One study of 200 women (Hassan 2014) showed no difference between induction with vaginal PGE2 pessary and vaginal PGE2 tablet for any of the reported outcomes: caesarean section, oxytocin augmentation, uterine hyperstimulation (FHR change not mentioned), uterine rupture, Apgar score less than seven at five minutes, neonatal intensive care unit admission, neonatal infection, postpartum haemorrhage, chorioamnionitis, endometritis, and maternal intensive care unit admission.
Overall completeness and applicability of evidence
There is insufficient information available from randomised trials to inform the optimal method of induction of labour in women with a prior caesarean birth. Several of the studies were at high risk of bias, and did not report important outcomes.
Quality of the evidence
All of the trials included in this review had design limitations and some had serious design limitations. It would be difficult to blind women or health professionals to these interventions, and only one study described blinding outcome assessors and the health professionals making clinical decisions (Manish 2016). One study had high risk of attrition bias due to excluding a high proportion of women from the analysis (Lokkegaard 2015). The risk of reporting bias was high in two studies, one that reported few of the prespecified outcomes from two arms of a four‐arm study (Sharma 2015), and one that reported only uterine rupture and was stopped prematurely due to safety concerns (Wing 1998).
Several studies reported very few of our prespecified outcomes (Lokkegaard 2015; Sharma 2015; Wing 1998), or did not report them in a form that could be included in the review. Infant outcomes were especially scarce, with none of our prespecified infant outcomes reported by Meetei 2015, Sharma 2015 and Wing 1998.
Studies reported none (Sharma 2015; Wing 1998), one (Hassan 2014; Lokkegaard 2015), two (Manish 2016; Rezk 2014), or three (Meetei 2015; Taylor 1993) of our five primary/GRADE outcomes. Caesarean section was reported by six studies (Hassan 2014; Lokkegaard 2015; Manish 2016; Meetei 2015; Rezk 2014; Taylor 1993), vaginal delivery not achieved within 24 hours was reported by three studies (Manish 2016; Meetei 2015; Rezk 2014), uterine hyperstimulation with fetal heart rate changes was reported by one study (Meetei 2015), and the composite outcomes serious neonatal morbidity or perinatal death, and serious maternal morbidity or death were reported by one study (Taylor 1993).
The GRADE level of evidence for outcomes were: moderate (vaginal delivery not achieved within 24 hours, and caesarean section in Manish 2016), low (vaginal delivery not achieved within 24 hours, uterine hyperstimulation with fetal heart rate changes, and caesarean section in Meetei 2015; vaginal delivery not achieved within 24 hours in Rezk 2014; caesarean section, serious neonatal morbidity or perinatal death, and serious maternal morbidity or death in Taylor 1993), and very low (caesarean section in Lokkegaard 2015; caesarean section in Rezk 2014; caesarean section in Hassan 2014).
Decisions to downgrade the evidence were based on small sample sizes in every comparison, and high risk of bias in some studies (Hassan 2014; Lokkegaard 2015; Meetei 2015; Rezk 2014; Taylor 1993). GRADE could not be assessed for misoprostol versus oxytocin (Wing 1998) or mifepristone versus Foley catheter (Sharma 2015), because none of the prespecified GRADE outcomes were reported.
Potential biases in the review process
The assessment of risk of bias involves subjective judgements. This potential limitation is minimised by following the procedures in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a) with review authors independently assessing studies and resolving any disagreement through discussion, and if required involving a third assessor in the decision.
Agreements and disagreements with other studies or reviews
While there is limited information available from randomised trials, lower‐quality evidence is available from observational studies.
In a large retrospective population‐based study, Lydon‐Rochelle 2001 evaluated the risk of uterine rupture among women with a prior caesarean birth, comparing the risks following the spontaneous onset of labour, as well as following induction of labour (using both prostaglandin and other methods to induce labour). Where labour occurred spontaneously, the risk of uterine rupture was reported to be 5.2 per 1000 women (56 of 10,789 women), increasing to 7.7 per 1000 women (15 of 1960 women) where labour was induced with “non‐prostaglandin” methods, and further increasing to 24.5 per 1000 women (nine of 366 women) where labour was induced with prostaglandin preparations. When expressed as a risk ratio (RR) comparing the chance of uterine rupture among women who had a repeat elective caesarean section, spontaneous labour increased the chance of rupture by three‐fold (RR 3.3, 95% confidence interval (CI) 1.8 to 6.0), induction with non‐prostaglandin methods by almost five‐fold (RR 4.9, 95% CI 2.4 to 9.7), and induction with prostaglandin preparations by over 15.5‐fold (RR 15.6, 95% CI 8.1 to 30.0). Specific information was not presented for different prostaglandin preparations (for example, PGE2, or misoprostol).
The US‐based NICHD group conducted a prospective evaluation of women with a prior caesarean birth (Landon 2004). In this study, induction of labour following the use of prostaglandin medication was associated with a non‐significant increase in risk of uterine rupture when compared with mechanical methods of induction, for example, the Foley catheter (risk of uterine rupture 140 per 10,000 inductions following PGE2 compared with 89 per 10,000 inductions following mechanical dilation of the cervix with a Foley catheter) (Landon 2004). In contrast, Scottish data from more than 36,000 women with a prior caesarean birth, of whom 4600 women had labour induced with prostaglandins, demonstrated an increased risk of uterine rupture and subsequent perinatal death following prostaglandin induction (risk of uterine rupture 4.5 per 10,000 non‐induced labours versus 11 per 10,000 labours induced with prostaglandins in women with a prior caesarean) (Smith 2004). Raviasia et al conducted a retrospective cohort study reviewing all births between 1992 and 1998 in a Canadian hospital (Ravasia 2000). In this series, of the 172 women who underwent induction with prostaglandins, five suffered a uterine rupture (2.9%), compared with one of 129 in women who were induced with a Foley catheter (0.78%), and two of 274 women who did not require cervical ripening (0.73%). In a similar study evaluating mechanical cervical dilation in women with a prior caesarean section, Bujold 2004 demonstrated a similar risk of uterine rupture between Foley catheter induction and spontaneous onset of labour (1.78% versus 1.2%).
The risk associated with uterine scar rupture following the use of misoprostol is less well documented. Misoprostol is an oral prostaglandin E1 analogue, licensed for use in the treatment of gastric ulcer disease. There is increasing recognition of its use as a prostaglandin agent to induce labour following oral, vaginal and buccal administration (Alfirevic 2006; Hofmeyr 2010; Muzonzini 2004). However, its use to induce labour in women with a previous caesarean has been questioned, with several case reports indicating an increased risk of uterine rupture (Bennett 1997; Choy‐Hee 2001; Cunha 1999; Phillips 1996; Plaut 1999).
While there are documented potential risks associated with induction of labour among women with a previous caesarean section, induction of labour is considered by many to be preferable to a repeat elective caesarean section. In an Australian survey of practice relating to care of women with a prior caesarean section in a subsequent birth, two‐thirds of obstetrician respondents indicated that induction of labour was preferable to a repeat elective caesarean (Dodd 2003). While manufacturers of both oxytocin and PGE2 specifically list previous caesarean as a contraindication to use in their product information brochures, almost two‐thirds of Australian obstetricians (Dodd 2003) and 25% of Canadian obstetricians (Brill 2003) use vaginal PGE2 in this setting. Additionally, 80% of Australian obstetricians use oxytocin in women with a previous caesarean birth (Dodd 2003). These figures are similar to those reported in England, where 76% of obstetricians would consider use of prostaglandin analogues, and 86% of consultants would use oxytocin to induce labour in women with a previous caesarean birth (Gupta 2011).
Authors' conclusions
Implications for practice.
There is insufficient information available from randomised controlled trials to inform clinical decisions regarding the optimal method of induction of labour in women with a prior caesarean birth. For women with an unfavourable cervix who require induction of labour, the risks and benefits of mechanical and pharmacologic options of cervical ripening, as well as labour induction and augmentation need to be considered. Whilst the data in this review are insufficient to inform practice in terms of the best method of induction for women with a prior caesarean, it is important to highlight that one study, which used misoprostol, was stopped early due to serious complications associated with its use.
Implications for research.
Appropriately designed and conducted randomised trials are required to evaluate methods of induction of labour for women who have had a prior caesarean birth, including evaluation of mechanical methods of induction, with adequate reporting of clinically relevant maternal and infant outcomes. As these are not likely to be undertaken due to results from previous reports, and if undertaken are likely to be underpowered to evaluate the risk of infrequent but serious adverse outcomes, we suggest adequately powered prospective cohort studies. These studies could compare methods believed to provide effective induction of labour with low risk of serious harm, and report the outcomes identified as important in this review.
What's new
| Date | Event | Description |
|---|---|---|
| 31 August 2016 | New search has been performed | Search updated and 12 trial new reports were identified. For this update we have included a further six studies (from 10 reports), and excluded one study. One study is ongoing. We have updated the methods in line with the standard methods used by Cochrane Pregnancy and Childbirth and we have used GRADE to assess the quality of the body of evidence. |
| 31 August 2016 | New citation required but conclusions have not changed | No change to conclusions. |
Acknowledgements
Helen West is supported by the National Institute for Health Research (NIHR) Cochrane Programme Grant Project: 13/89/05 – Pregnancy and childbirth systematic reviews to support clinical guidelines.
Anna Cuthbert for data extraction; Denise Atherton for administrative assistance; Lynn Hampson for the literature search.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure and Cochrane Programme Grant funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Data and analyses
Comparison 1. Vaginal PGE2 versus intravenous oxytocin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.22, 2.03] |
| 2 Serious neonatal morbidity or perinatal death | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 69.70] |
| 3 Serious maternal morbidity or death | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 69.70] |
| 4 Uterine rupture | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 69.70] |
| 5 Epidural analgesia | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.93, 2.17] |
| 6 Instrumental vaginal delivery | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.39, 4.02] |
| 7 Apgar score < 7 at 5 minutes | 1 | 42 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Vaginal misoprostol versus intravenous oxytocin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Uterine rupture | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.67 [0.16, 84.66] |
Comparison 3. Foley catheter versus intravenous oxytocin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.89, 2.44] |
| 2 Uterine hyperstimulation with FHR changes | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.11 [0.13, 73.09] |
| 3 Caesarean section | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.45, 1.92] |
| 4 Oxytocin augmentation | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.81, 1.32] |
| 5 Uterine rupture | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Instrumental vaginal delivery | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.26 [0.39, 134.01] |
| 7 Postpartum haemorrhage | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.11 [0.13, 73.09] |
| 8 Chorioamnionitis | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Double‐balloon catheter versus vaginal PGE2.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 1 | 16 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.41, 2.32] |
| 2 Admission to neonatal unit | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 5. Oral mifepristone versus Foley catheter.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Oxytocin augmentation | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.38, 0.76] |
| 2 Uterine rupture | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.29 [0.08, 1.02] |
Comparison 6. Vaginal isosorbide mononitrate versus Foley catheter.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.63 [1.32, 5.21] |
| 2 Caesarean section | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.39, 2.59] |
| 3 Oxytocin augmentation | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.65 [1.17, 2.32] |
| 4 Uterine rupture | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Epidural analgesia | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.39, 2.59] |
| 6 Instrumental vaginal delivery | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.23, 2.76] |
| 7 Meconium‐stained liquor | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.18] |
| 8 Apgar score < 7 at 5 minutes | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.95, 2.93] |
| 9 Neonatal intensive care unit admission | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.51, 12.14] |
| 10 Maternal nausea and vomiting | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 27.63] |
| 11 Puerperal pyrexia (other maternal side‐effects) | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.42 [0.16, 1.07] |
| 12 Palpitation (other maternal side‐effects) | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.5 [0.51, 12.14] |
| 13 Headache (other maternal side‐effects) | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.33 [0.99, 11.22] |
| 14 Postpartum haemorrhage | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.90, 4.43] |
| 15 Woman not satisfied | 1 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.56, 5.51] |
Comparison 7. Foley catheter 80 mL versus Foley catheter 30 mL.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.91, 1.20] |
| 2 Caesarean section | 1 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.89, 1.24] |
| 3 Oxytocin augmentation | 1 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.66, 0.98] |
| 4 Uterine rupture | 1 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.70] |
| 5 Epidural analgesia | 1 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Instrumental vaginal delivery | 1 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.43, 1.95] |
| 7 Apgar score < 7 at 5 minutes | 1 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.70] |
| 8 Neonatal intensive care unit admission | 1 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.19, 21.60] |
| 9 Neonatal encephalopathy | 1 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 72.52] |
| 10 Perinatal death | 1 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.12, 72.52] |
| 11 Neonatal infection | 1 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Cord prolapse (other maternal side‐effects) | 1 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Postpartum haemorrhage | 1 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.06] |
| 14 Chorioamnionitis | 1 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.04, 3.13] |
Comparison 8. Vaginal PGE2 pessary versus vaginal PGE2 tablet.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Caesarean section | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.74, 1.60] |
| 2 Oxytocin augmentation | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.81, 2.78] |
| 3 Uterine hyperstimulation (FHR change not mentioned) | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.05, 5.43] |
| 4 Uterine rupture | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.09] |
| 5 Apgar score < 7 at 5 minutes | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.8 [0.22, 2.89] |
| 6 Neonatal intensive care unit admission | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.17, 3.27] |
| 7 Neonatal infection | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.77] |
| 8 Postpartum haemorrhage | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.14, 6.96] |
| 9 Chorioamnionitis | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.77] |
| 10 Endometritis | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.26, 8.79] |
| 11 Maternal intensive care unit admission (serious maternal complications) | 1 | 200 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Hassan 2014.
| Methods | 2‐arm RCT | |
| Participants | Setting: large Governmental hospital, Saudi Arabia. February 2009‐March 2013 Inclusion criteria: women with a previous CS, a live singleton fetus (37‐42 weeks of gestation) in cephalic presentation and a reactive non‐stress test, BS of ≤ 7 before onset of labour, no spontaneous contractions (< 4 contractions within 20 min) Exclusion criteria: women in active labour or with uterine surgery other than lower segment CS, ruptured membranes, chorioamnionitis, antepartum haemorrhage, contraindication to prostaglandins use (e.g. bronchial asthma or glaucoma), contraindication to vaginal delivery, nonvertex presentation, multiple pregnancy, major fetal anomalies or demise |
|
| Interventions | Experimental intervention: single dose of dinoprostone 10 mg sustained‐release vaginal pessary (Propess; controlled Therapeutics (Scotlantd) Ltd., East Kilbride, UK) into the posterior vaginal fornix. The dinoprostone pessary releases at a steady rate (0.3 mg/h). It remained in the vagina for up to 24 h, as recommended by the manufacturer. It was removed if it was still present 24 h after placement, if a worrisome fetal heart rate pattern persisted, or if the woman had efficient uterine contractions (3‐4 contractions in 10 min). Total number randomised: n = 100 Comparison intervention: 1.5 mg dinoprostone vaginal tablet (Prostin E2; Parmacia & Upjohn, Puurs, Belgium) into the posterior vaginal fornix for a maximum of 3 doses with 6‐hourly intervals between each dose. Before application of each dose, vaginal examination to ascertain the BS and CTG was performed to assess fetal well‐being and frequency of uterine contractions. Total number randomised: n = 100 |
|
| Outcomes | Primary outcome: vaginal delivery rate. Secondary outcomes: induction to delivery time, maternal satisfaction score for the birth process obtained within 24 h of delivery (a VAS of 0‐10, with a greater score denoting better satisfaction), maternal and neonatal complications | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Women included in this study were randomly allocated into two equal groups”, no description of random sequence generation |
| Allocation concealment (selection bias) | Unclear risk | No allocation concealment described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | “The study was open labelled; thus, women and clinicians were aware of the treatment allocation scheme.” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Open‐label study, no mention of blinding outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women appear to be accounted for |
| Selective reporting (reporting bias) | Low risk | Assessed from published report with no protocol available, however outcomes were comprehensively reported |
| Other bias | Low risk | Analysis was done by ITT. Baseline characteristics, including indications for labour induction, were similar between groups |
Lokkegaard 2015.
| Methods | 2‐arm multicentre RCT, with adequate randomisation | |
| Participants | Setting: 7 labour wards in Denmark, December 2002‐September 2005 Inclusion criteria: pregnant women with intact fetal membranes, cephalic position and unfavourable cervix, with indications for induction of labour (e.g. prolonged pregnancy (> 42 weeks’ gestation), pre‐eclampsia/hypertension, placental insufficiency, gestational diabetes mellitus and twins). Women with previous CSs were included (and data were presented as a subgroup of all women) Exclusion criteria: spontaneous labour, rupture of membranes, placenta previa, acute fetal distress, specific vaginal/cervical infections (e.g. group‐B Streptococcus, Condyloma and acute herpes), asthma, glaucoma and latex allergy |
|
| Interventions | Experimental intervention: double‐balloon catheter inserted through the cervical canal with 80 mL of saline installed stepwise in the uterine balloon and 80 mL saline in the cervicovaginal balloon. Removed after 12 h, followed by amniotomy. Stimulation with oxytocin 2‐3 h after amniotomy was allowed (10 IU) oxytocin/500 mL saline administered intravenously at 20 mL/h, increasing up to 180 mL/h) Failed induction = if the BS had increased since the randomisation (BS > 6), if the catheter insertion was not achieved, if amniotomy could not be performed within 4 h after removal of the catheter. Total number randomised: n = 13 with previous CS (4 later excluded: 1 not VBAC, 3 "failed induction") (n = 412 in total) Control/comparison intervention: vaginal PGE2 (dinoprostone) 3 mg vaginal tablet Amniotomy or second dinoprostone tablet after 4‐5 h Stimulation with oxytocin 2‐3 h after amniotomy was allowed (dose/regime as in experimental group) Failed induction = if amniotomy could not be achieved and if labour was not established within 48 h after the first dinoprostone administration Total number randomised: n = 13 with previous CS (6 later excluded: 1 not VBAC, 5 "failed induction") (n = 413 in total) |
|
| Outcomes | Rate of failed inductions, median induction delivery time (hours), CS frequency, admission to neonatal unit (other outcomes may be available from the study authors, e.g. Apgar score, assisted delivery) | |
| Notes | HW emailed Dr Lokkegaard requesting additional data on outcomes: vaginal delivery, assisted vaginal delivery, and Apgar score < 7 at 5 min. These were reported for all women but not the subset who had a previous CS, by ITT. We received a response, with incomplete additional data, inconsistent with the published report. In correspondence, Dr Lokkegaard reported that all women in the subset of women with a prior CS were 37 weeks of gestation or more. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated, stratified for parity and department |
| Allocation concealment (selection bias) | Low risk | Randomised by a telephone automatic voice‐response randomisation system administered from the Perinatal Epidemiology Research Unit, Aarhus University Hospital, Denmark |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not possible for these interventions. Knowledge of the intervention may have influenced clinical decision‐making |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Women who went into spontaneous labour before induction, who were not in labour after 48 h, or who had been coded as VBAC in error were classed as ‘failure’ and excluded from the results. There were 13 women randomised to each group, however results for only 10 are reported in the publication, and in correspondence from the author additional 'failures' were identified. In the PGE2 group, 2 women began labour before induction, 3 were not in labour after 48 h, and 1 was wrongly coded as VBAC. In the balloon group, 3 began labour before induction, 1 was not VBAC and 0 were not in labour after 48 h. Unfortunately, despite additional information from the study authors, there were still missing data on outcomes, and we were unable to add the excluded women in an ITT analysis |
| Selective reporting (reporting bias) | Unclear risk | The subset of participants with a previous VBAC were not the primary focus of this study, so few outcomes are reported for these women |
| Other bias | High risk | Groups were similar at baseline. However more women in the catheter group went into spontaneous labour between recruitment and induction, potentially introducing bias. The primary endpoints of the full study are reported by both ITT and per‐protocol analyses, showing that a large number of women did not receive the allocated treatment. |
Manish 2016.
| Methods | 2‐arm RCT, with adequate randomisation | |
| Participants | Describe setting: a large tertiary centre in South India, which carries out ˜15,000 deliveries every year. October 2011‐December 2013 Inclusion criteria: pregnant women who previously had a lower segment CS and now have a singleton cephalic presentation after at least 36 completed weeks, not in labour, with intact membranes and BS of < 6 Exclusion criteria: women who had endometritis in a previous pregnancy, inter‐delivery interval of < 18 months, extension of the uterine incision onto upper segment at the previous CS and an estimates fetal weight of ≥ 4 kg and women with a previous preterm CS |
|
| Interventions | Experimental intervention: a 16 Fr Foley catheter was introduced into the cervix beyond the internal os and the bulb inflated with 80 mL of sterile water Total number randomised: n = 77 Comparison intervention: a 16 Fr Foley catheter was introduced into the cervix beyond the internal os and the bulb inflated with 30 mL of sterile water Total number randomised: n = 77 The Foley catheter was folded and left in the vagina for 12 h, after which it was removed. Assessment of the cervix and artificial rupture of membranes was done at the time of catheter removal or earlier if the catheter expelled spontaneously. All women were monitored continuously with an electronic fetal monitor. Oxytocin for augmentation or induction was considered if women did not have regular uterine contractions lasting for 30 seconds, every 3 min |
|
| Outcomes | Primary: percentage of women achieving vaginal delivery within 24 h of induction Secondary: effectiveness: number of women delivering vaginally in 12 h, BS at amniotomy, duration from induction to delivery, delivery by CS, need for augmentation with oxytocin, number of units of oxytocin used. Maternal complications: uterine rupture, scare dehiscence, postpartum haemorrhage, abruption placentae, chorioamnionitis. Neonatal complications: Apgar < 7 at 5 min, cord pH < 7.1, neonatal intensive care unit admission, neonatal encephalopathy, neonatal sepsis |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Generated with permuted block randomisation of sizes two, four and six using SAS 9.3.1.” |
| Allocation concealment (selection bias) | Low risk | “Serially numbered, opaque, sealed envelopes containing the allocated group were opened in a central research office after confirming that the participant was eligible for the study. The treating doctor had not access to the envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | It is not described as blinded: the personnel responsible for inserting and removing the Foley catheter would presumably be aware of the group allocation. However, women are likely to have been unaware of which group they were in. However, it does say that, “The decision to perform CS was left to the discretion of the obstetrician managing the labour ward who was unaware of the group allocation”, so the impact of imperfect blinding due to the procedure was minimised. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “Data on baseline characteristics and outcomes were collected by research officers who were unaware of the allocated group.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women appear to be accounted for. 1 woman in each group inadvertently received the wrong allocation, and it’s unclear which group these women were analysed with: the ITT intervention, or the intervention they received |
| Selective reporting (reporting bias) | Unclear risk | Protocol available: some prespecified outcomes were not reported: uterine hyperstimulation with RHR change, meconium‐stained liquor, and satisfaction of caregiver and mother |
| Other bias | Low risk | Similar baseline characteristics, e.g. BS at induction, and indication for induction |
Meetei 2015.
| Methods | 2‐arm RCT, with adequate randomisation | |
| Participants | Setting: Chandigarh, India. July 2004–November 2005 Inclusion criteria: pregnant women with a previous low transverse CS, singleton live pregnancy with cephalic presentation, period of gestation > 28 weeks and BS < 5 were included in the study, with unfavourable cervix admitted for induction of labour. Exclusion criteria: previous classical or T‐shaped incision, unknown scar, transfundal uterine surgery, medical or obstetric complications that preclude vaginal delivery, placenta previa, low lying placenta, undiagnosed vaginal bleeding, maternal heart disease, premature/preterm premature rupture of membranes, interval between previous CS and present pregnancy/conception < 6 months, cervicovaginal infection, history of unclean vaginal examination and history of infection in previous CS |
|
| Interventions | Experimental intervention: cervical ripening with Foley catheter balloon inflated with 30 mL of sterile saline. Catheter inserted and inflated, observed for 12 h, then BS was rechecked or earlier if Foley expelled before 12 h. Oxytocin started after 12 h, if the woman was not yet in active labour (starting at 1 mU/min and increasing up to a maximum of 32 mU/min). Total number randomised: n = 30 (26 out of 30 were 37 weeks' gestation or more) Control/comparison intervention: cervical ripening with low dose IV oxytocin (starting at 1 mU/min and increasing if contractions were not frequent after 1 hour). After 12 h BS was rechecked, and oxytocin for induction or augmentation was increased as in the Foley group up to a maximum of 32 mU/min. Total number randomised: n = 30 (27 out of 30 were 37 weeks' gestation or more) |
|
| Outcomes | BS before and after 12 h of ripening, percentage and time interval of women entering spontaneous labour, method of delivery, induction‐delivery interval, complications, neonatal outcome | |
| Notes | The inclusion criteria is from 28 weeks GA, and the published report states that the majority of women were between 38 and 40 weeks. HW contacted the study author to request data for women from 37 weeks GA onwards. In personal correspondence, Dr Meetei reported that 26 out of 30 women in the Foley‐catheter group, and 27 out of 30 in the oxytocin group were 37 weeks' gestation or more, and provided data for this subgroup of women | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Used Tippets random number table |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described. Unlikely, given the characteristics of the different methods of induction being compared. This may have affected clinical decisions and therefore introduced bias |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women appear to be accounted for. There is no mention of women dropping out of the study |
| Selective reporting (reporting bias) | Unclear risk | Assessed from published report, without protocol. It is unclear whether all prespecified outcomes were reported |
| Other bias | Low risk | Groups were comparable at baseline. No power calculation is reported |
Rezk 2014.
| Methods | 2‐arm RCT | |
| Participants | Describe setting: Egypt Inclusion criteria: the study was conducted on 80 healthy pregnant women with previous 1 lower segment CS at 37 weeks and beyond, with a BS of ≤ 6, intact membranes, reactive non‐stress test, normal umbilical arterial Doppler indices, absence of labour and willingness of women to participate in the study. The indications for the induction of labour were pregnancy‐induced hypertension, oligohydramnios, intrauterine growth restrictions and controlled diabetes mellitus Exclusion criteria: women with intrauterine fetal death, twins pregnancy, polyhydramnios, placenta previa, severe anaemia, severe hypertension, uncontrolled diabetes, coagulopathy and any contraindication for the labour induction were excluded from the study |
|
| Interventions | Experimental intervention: intracervical Foley catheter was inserted, inflated and placed on traction. Under aseptic conditions, with the women lying in the lithotomy position, the cervix was assessed and Foley catheter No. 14‐16 Fr was inserted into the endocervical canal, beyond the internal os and the balloon was inflated with 50‐60 mL of normal saline. The catheter was strapped to the thigh with gentle traction. The catheter was checked for its position and the traction at 3‐6 h intervals. The catheter was either removed at 12 h or expelled spontaneously and it was checked whether the BS had improved or whether a spontaneous rupture of the membranes had occurred. Total number randomised: n = 40 Comparison intervention: women received moistened 1 tablet of isosorbide mononitrate 40 mg (Monomak, October Pharma, Egypt) inserted into the posterior fornix of the vagina once. Total number randomised: n = 40 Women were examined regularly at 3 h, 6 h, 9 h, 12 h and 24 h after starting the method of induction to evaluate the change in BS. Vital signs were monitored every 30 min. AROM was performed for all women when their cervical dilatation reached 3‐4 cm and IV oxytocin infusion was started if there was no efficient uterine contractions. An oxytocin infusion was started at 2 mU/min and increased in increments of 1‐2 mU/min at 15‐30 min intervals as needed to achieve adequate uterine contraction pattern (≥ 200 MVU). Opiate and epidural analgesia was given on the woman’s request and at the discretion of the obstetrician. Continuous CTG was done during delivery and the modified WHO partograph was followed up for the labour management) |
|
| Outcomes | Primary outcome measures included changes in BS, time from initiation till the onset of labour, the induction to delivery interval, the mode of delivery and the length of the second and third stages of labour. Maternal adverse effects, acceptability and neonatal outcome (Apgar score at 5 min, neonatal weight and admission to neonatal intensive care unit) were recorded as secondary outcomes. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation in 1:1 ratio was carried out using computer‐generated simple random tables |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not possible to blind the study participants from knowledge of which intervention a participant received because methods were clearly different |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram, all women accounted for |
| Selective reporting (reporting bias) | Unclear risk | This study was assessed from a published report without access to the protocol, so we do not know if all prespecified outcomes were reported |
| Other bias | Low risk | Groups appear to be comparable at baseline |
Sharma 2015.
| Methods | 4‐arm RCT, with adequate randomisation (only 2 arms are reported in the poster abstract giving results of the study) | |
| Participants | Setting: India, 2012‐2014 Inclusion criteria: pregnant women, 40 weeks' gestation, single cephalic presentation, 1 previous low segment CS. Postdates (gestation 40 weeks 5 days) Exclusion criteria: described in the study protocol as: interconceptional period less than 18 months, estimated fetal birthweight > 4 kg, poor modified bio‐physical profile (amniotic fluid index), poor dating (not sure of dates, no ultrasonography in first trimester or 2 serial ultrasounds 4 weeks apart in second trimester), premature rupture of membranes/chorioamnionitis, evidence of fetal distress at admission, intrauterine fetal death, any maternal disease, i.e. hypertension, diabetes, renal disease, liver disease, cardiac disease, epilepsy or any chronic medication, any contraindication for vaginal delivery (cephalo‐pelvic disproportion ruled out), type of CS not known (classical or low segment) |
|
| Interventions | In the study protocol, the groups are listed as: Comparator: cervical sweeping and stretching at 40 weeks + 5 days Intervention 1: single dose mifepristone (200 mg) orally at 40 weeks + 5 days Intervention 2: single dose mifepristone (400 mg) orally at 40 + 5 Intervention 3: transcervical Foley catheter with 30 mL normal saline inserted at 40 + 5 However, the poster abstract reports intervention 2 versus intervention 3, and does not mention the other 2 arms of the study: Experimental intervention: single dose mifepristone (400 mg) orally at 40 + 5. All women were reassessed 24 h and 48 h later. If BS > 6, amniotomy was done, followed by oxytocin infusion. If after 48 h, BS was < 6, induction of labour was done with oxytocin infusion. Total number randomised: n = 57 Control/comparison intervention: transcervical Foley catheter with 30 mL normal saline inserted at 40 + 5. Total number randomised: n = 50 |
|
| Outcomes | Spontaneous onset of labour, duration of labour, need and amount of oxytocin required, scar dehiscence, incidence of CS, neonatal outcomes (pH of cord, hypoglycaemia, intensive care unit admission) | |
| Notes | HW contacted the study authors, requesting additional information on the methodology (how blinding was achieved), and results of the 2 arms described in the protocol but not in the published reports. No response was received | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as “computer generated randomisation” in the study protocol, not described in the study report |
| Allocation concealment (selection bias) | Unclear risk | Described as “sequentially numbered, sealed, opaque envelopes” in the study protocol, not described in the study report |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Described as “double blind double dummy” in the study protocol, but the description does not fit with having a fake Foley catheter. Not described in the study report |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Described as “double blind double dummy” in the study protocol. Not described in the study report |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All women recruited appear to be accounted for in the results. However, the omission of 2 arms of the study may suggest bias |
| Selective reporting (reporting bias) | High risk | The protocol described a 4‐arm study, but only 2 arms were reported in the publication. The brief poster abstract does not report any prespecified neonatal outcomes, and reports only 2‐arms of the 4 comparison groups set out in the protocol |
| Other bias | High risk | The inconsistencies between the study protocol and published report (such as the omission of 2 arms of the study, information on the methodology, and prespecified outcomes) suggest that this study is at high risk of bias |
Taylor 1993.
| Methods | Prospective randomised trial; sealed, numbered envelopes; no sample size calculation | |
| Participants | Setting: UK Women requiring labour induction due to prolonged pregnancy or pre‐eclampsia, 1 previous pregnancy delivered by lower segment CS, singleton in cephalic presentation, GA ≥ 37 weeks, BS < 9, no cephalopelvic disproportion anticipated |
|
| Interventions | Amniotomy and IV oxytocin (n = 21) versus 2.5 mg vaginal PGE2 pessary, followed by amniotomy 3 h later + oxytocin (if necessary) 6 h later (n = 21) | |
| Outcomes | Induction to delivery time, analgesia, mode of delivery, uterine rupture | |
| Notes | Only half of the women included had an unfavourable cervix (BS < 6) 1 uterine rupture in PGE 2 group (after oxytocin) reported in abstract Sellers 1988 (see Taylor 1993) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "predetermined code envelope." |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding was not described in the report |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data of all women included were reported, no ITT analysis done |
| Selective reporting (reporting bias) | Unclear risk | Published report includes expected outcomes, but no outcome measures were prespecified in the methods section |
| Other bias | Low risk | The baseline characteristics were comparable between the groups |
Wing 1998.
| Methods | Prematurely terminated RCT, due to safety concerns | |
| Participants | Setting: USA Women with a singleton pregnancy in cephalic presentation with 1 prior CS were eligible for inclusion |
|
| Interventions | 25 μg vaginal misoprostol every 6 h (maximum of 4 doses) (n = 17) versus IV oxytocin "per a standardised infusion protocol" (dose/regime not reported) (n = 21) | |
| Outcomes | Not described in detail, included uterine tachysystole, hypertonus, hyperstimulation syndrome, uterine dehiscence (defined at laparotomy or digital examination), uterine rupture (that required emergency laparotomy) | |
| Notes | 2 uterine ruptures occurred in the misoprostol group and the trial was ended prematurely due to safety concerns | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Method not described in the report |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding was not feasible due to the nature of the interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding was not described in the report |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There is insufficient information to judge whether all women are accounted for |
| Selective reporting (reporting bias) | High risk | This report only describes the cases of uterine rupture in detail. The study was assessed from a published report, without protocol. It is likely that other outcomes were prespecified and these are not reported |
| Other bias | High risk | The study was terminated prematurely due to safety concerns |
AROM: artificial rupture of membranes BS: Bishop score CS: caesarean section CTG: cardiotocograph Fr: French catheter scale GA: gestational age ITT: intention‐to‐treat IU: international unit IV: intravenous MVU: Montevideo Units PGE2: prostaglandin E2 RCT: randomised controlled trial RHR: resting heart rate VAS: visual analogue scale VBAC: vaginal birth after caesarean section WHO: World Health Organization
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arraztoa 1994 | Compared, in women with a prior caesarean birth in spontaneous labour, a pharmacologic approach (oxytocin + epidural) to expectant management (spontaneous evolution). Did not compare methods of induction for women in whom labour was induced |
| Ben‐Aroya 2001 | Not a RCT, but rather a cohort study |
| Hamdan 2009 | Compared weekly membrane sweeping to weekly vaginal examination, did not compare 2 different methods of cervical ripening or induction. Women did not require induction of labour |
| Lelaidier 1994 | Mifepristone was used as a pre‐induction agent, only after the women were randomised |
| Morales 1986 | 2‐arm quasi‐randomised trial comparing induction using oxytocin versus expectant management for women with a previous caesarean section |
| Ramya 2015 | Compared weekly membrane sweeps from 39 weeks GA to no intervention/expectant management. The aim of the intervention was reducing post‐term pregnancies, not induction of labour |
| Rayburn 1999 | Compared weekly administration of cervical PGE2 gel to expectant management in women with a prior caesarean birth, not 2 different methods of cervical ripening or induction. Women with indications for induction of labour were excluded |
| Sciscione 2001 | Initially all women were included, subsequently women with a prior caesarean were excluded |
| Spallicci 2007 | Women did not require induction of labour |
GA: gestational age RCT: randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
NCT02196103.
| Trial name or title | Management of labour in patients with previous cesarean section |
| Methods | 2‐arm randomised controlled trial, randomisation method unclear, open label |
| Participants | Setting: Israel Inclusion criteria: pregnant women, prelabour rupture of the membranes at > 34 weeks (ruptured membranes in the 24 h prior to inclusion in the study), unripe cervix. Singleton in cephalic position. 1 previous CS. No contractions Exclusion criteria: any contraindication for the vaginal delivery (i.e. placenta previa, non vertex presentation), regular uterine contractions (3‐5/10 min), diagnosis of uterine rupture was made over 24 h prior to study inclusion, evidence of chorioamnionitis (T 37.6 oC with uterine tenderness and maternal or fetal tachycardia or purulent discharge or WBC ≥ 20,000), suspected placental abruption or presence of a significant haemorrhage, non‐reassuring fetal status (as determined by fetal heart rate monitoring and/or bio‐physical profile) necessitating immediate intervention |
| Interventions | Experimental intervention: double‐balloon cervical catheter Control/Comparison intervention: expectant management |
| Outcomes | Vaginal delivery rate, safety (fetal heart rate, uterine haemorrhage, maternal haemodynamic changes, uterine atony), satisfaction (maternal experience and satisfaction) |
| Starting date | This study is not yet open for participant recruitment |
| Contact information | Asnat Walfisch MD, Hillel Yaffe Medical Center |
| Notes | NCT02196103 |
CS: caesarean section WBC: white blood count
Differences between protocol and review
We have updated our methods to include the use of GRADE to assess the quality of the body of evidence and we have included 'Summary of findings' tables.
Trials using a cluster‐RCT design are now eligible for inclusion in this review (and we include methods for dealing with them) but none were identified for this update. We also include methods for dealing with trials that have multiple‐arms.
Contributions of authors
For the review update, Helen West and Marta Jozwiak assessed study eligibility, methodological quality, and performed data extraction. Helen West entered the data, conducted the GRADE assessment, produced the 'Summary of findings' tables, and drafted the review update. Jodie Dodd checked the data and commented on the review.
Sources of support
Internal sources
(HW) Cochrane Pregnancy and Childbirth Group, Department of Women's and Children's Health, The University of Liverpool, Liverpool, UK.
External sources
The University of Adelaide, Discipline of Obstetrics and Gynaecology, Australia.
The Australian National Health and Medical Research Council Practitioner Fellowship, Australia.
(HW) NIHR Cochrane Programme Grant Project: 13/89/05 – Pregnancy and childbirth systematic reviews to support clinical guidelines, UK.
Declarations of interest
Jodie Dodd: none known.
Marta Jozwiak was involved in two RCTs on the topic of induction of labour but these are not eligible for inclusion in this review (the participants had not had a previous caesarean section). She was also involved in an observational study looking at induction of labour in women with a caesarean section (PROBAAT‐S study) – this study has not yet been published but would not be eligible for inclusion in this review as it is not a randomised controlled trial.
Helen West's contribution to this project was supported by the National Institute for Health Research, via Cochrane Programme Grant funding to Cochrane Pregnancy and Childbirth. NIHR has no influence on the content or conclusions of this review.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Hassan 2014 {published data only}
- Hassan MF, El‐Tohamy O. Comparative study for success rate of vaginal birth after cesarean section following labor induction by two forms of vaginal dinoprostone: a pilot study. Open Journal of Obstetrics and Gynecology 2014;4:33‐41. [Google Scholar]
Lokkegaard 2015 {published data only}
- Lokkegaard E, Lundstrom M, Kjaer MM, Christensen IJ, Pedersen HB, Nyholm H. Prospective multi‐centre randomised trial comparing induction of labour with a double‐balloon catheter versus dinoprostone. Journal of Obstetrics and Gynaecology 2015;35(8):797‐802. [DOI] [PubMed] [Google Scholar]
- NCT01255839. A prospective multi‐centre randomised comparison on induction of labour with double‐balloon installation device versus prostaglandin e2 minprostin. clinicaltrials.gov/ct2/show/NCT01255839 Date first received: 6 December 2010.
Manish 2016 {published data only}
- CTRI/2014/02/004412. Randomised trial comparing intrauterine balloon catheter with 30 ml fluid with intrauterine balloon catheter with 80 ml of fluid to start labor in women with one previous caesarean section. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=4199 Date first received: 17 February 2014.
- Manish P, Rathore S, Benjamin SJ, Abraham A, Jeyaseelan V, Mathews JE. A randomised controlled trial comparing 30 ml and 80 ml in Foley catheter for induction of labour after previous caesarean section. Tropical Doctor 2016;46(6):205‐11. [DOI] [PubMed] [Google Scholar]
Meetei 2015 {published data only}
- Meetei LT, Suri V, Aggarwal N. Induction of labor in patients with previous cesarean section with unfavorable cervix. JMS ‐ Journal of Medical Society 2015;28(1):29‐33. [Google Scholar]
Rezk 2014 {published data only}
- Rezk M, Sanad Z, Dawood R, Masood A, Emarh M, Halaby AA. Intracervical Foley catheter versus vaginal isosorbid mononitrate for induction of labor in women with previous one cesarean section. Journal of Clinical Gynecology and Obstetrics 2014;3(2):55‐61. [Google Scholar]
Sharma 2015 {published data only}
- CTRI/2012/06/002733. Induction of labor in women with previous one cesarean section: prospective double blind randomized control trial comparing the effect of mifepristone with sweeping stretching and trans‐cervical Folley’s catheterization. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=4745 Date first received: 19 June 2012.
- Sharma C, Soni A, Thakur S, Verma S. Induction of labor in women with previous cesarean section; mifepristone versus trans‐cervical catheter. A randomized controlled trial. International Journal of Gynecology and Obstetrics 2015;131(Suppl 5):E216. [Google Scholar]
- Sharma C, Soni A, Thakur S, Verma S. Induction of labour in women with previous one caesarean section; mifepristone versus transcervical Foley's catheter. A randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2015;122(Suppl S1):303. [Google Scholar]
Taylor 1993 {published data only}
- Sellers SM, Ah‐Moye M, MacKenzie IZ. Comparison of vaginal prostaglandin E2 and intravenous oxytocin for induction of labour in women previously delivered by caesarean section. 1st European Congress on Prostaglandins in Reproduction; 1988 July 6‐9; Vienna, Austria. 1988:128.
- Taylor AVG, Sellers S, Ah‐Moye M, MacKenzie IZ. A prospective random allocation trial to compare vaginal prostaglandin E2 with intravenous oxytocin for labour induction in women previously delivered by caesarean section. Journal of Obstetrics and Gynaecology 1993;13(5):333‐6. [Google Scholar]
Wing 1998 {published data only}
- Wing DA, Lovett K, Paul RH. Disruption of prior uterine incision following misoprostol for labor induction in women with previous cesarean delivery. Obstetrics & Gynecology 1998;91(5 Pt 2):828‐30. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Arraztoa 1994 {published data only}
- Arraztoa JA, Jensen L, Clavero M, Castillo H. Delivery conduction in patients with cicatrix of a prior Cesarean section. Pilot study. Revista Chilena de Obstetricia y Ginecologia 1994;59(2):95‐100; discussion 100‐1. [PubMed] [Google Scholar]
Ben‐Aroya 2001 {published data only}
- Ben‐Aroya Z, Hallak M, Segal D, Friger M, Katz M, Mazor M. Ripening of the uterine cervix in post cesarean parturient: PGE2 vs intracervical Foley catheter. American Journal of Obstetrics and Gynecology 2001;184(1):S117. [Google Scholar]
Hamdan 2009 {published data only}
- Hamdan M, Sidhu K, Sabir N, Omar SZ, Tan PC. Serial membrane sweeping at term in planned vaginal birth after cesarean: a randomized controlled trial. Obstetrics & Gynecology 2009;114(4):745‐51. [DOI] [PubMed] [Google Scholar]
Lelaidier 1994 {published data only}
- Lelaidier C, Baton C, Benifla JL, Fernandez H, Bourget P, Frydman R. Mifepristone for labour induction after previous caesarean section. British Journal of Obstetrics and Gynaecology 1994;101:501‐3. [DOI] [PubMed] [Google Scholar]
Morales 1986 {published data only}
- Morales WJ, Lazar AJ. Expectant management of rupture of membranes at term. Southern Medical Journal 1986;79:955‐8. [DOI] [PubMed] [Google Scholar]
Ramya 2015 {published data only}
- Ramya V, Ghose S, Pallavee P. Membrane sweeping for vaginal birth after caesarean section and its outcome ‐a comparative study. Journal of Clinical and Diagnostic Research 2015;9(8):QC01‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rayburn 1999 {published data only}
- Gittens L, Schenkel C, Strassberg S, Apuzzio J. Vaginal birth after cesarean section: comparison of outpatient use of prostaglandin gel to expectant management. American Journal of Obstetrics and Gynecology 1996;174(1 Pt 2):354. [Google Scholar]
- Rayburn W, Lucas M, Gittens L, Goodwin TM, Baxi L, Gall S, et al. Attempted vaginal birth after cesarean section: a multicenter comparison of outpatient prostaglandin E2 gel with expectant management. Primary Care Update for Ob/Gyns 1998;5(4):182‐3. [DOI] [PubMed] [Google Scholar]
- Rayburn WF, Gittens LN, Lucas MJ, Gall SA, Martin ME. Weekly administration of prostaglandin e2 gel compared with expectant management in women with previous cesareans Prepidil gel study group. Obstetrics & Gynecology 1999;94(2):250‐4. [DOI] [PubMed] [Google Scholar]
Sciscione 2001 {published data only}
- Manley J, Nguyen L, Shlossman P, Colmorgen G, Sciscione A. A randomized prospective comparison of the intracervical Foley bulb to intravaginal misoprostol (cytotec) for preinduction cervical ripening. American Journal of Obstetrics and Gynecology 1999;180(1 Pt 2):S76. [DOI] [PubMed] [Google Scholar]
- Sciscione AC, Nguyen L, Manley J, Pollock M, Maas B, Colmorgen G. A randomized comparison of transcervical Foley catheter to intravaginal misoprostol for preinduction cervical ripening. Obstetrics & Gynecology 2001;97(4):603‐7. [DOI] [PubMed] [Google Scholar]
Spallicci 2007 {published data only}
- Spallicci MD, Chiea MA, Singer JM, Albuquerque PB, Bittar RE, Zugaib M. Use of hyaluronidase for cervical ripening: a randomized trial. European Journal of Obstetrics & Gynecology and Reproductive Biology 2007;130(1):46‐50. [DOI] [PubMed] [Google Scholar]
- Spallicci MDB, Bittar RE. Randomized double blind study of ripening the cervix with hyaluronidase in term gestations [Estudo clinico aleatorizado com grupo controle e mascaramento duplo da maturacao do colo uterino pela hialuronidase em gestacoes a termo]. Revista Brasileira de Ginecologia e Obstetricia 2003;25(1):67. [Google Scholar]
References to ongoing studies
NCT02196103 {published data only}
- NCT02196103. Management of labor in patients with previous cesarian section and premature rupture of membranes who desire TOLAC: comparison between the use of standard expectant management and the double‐balloon catheter device. A prospective randomized study. clinicaltrials.gov/ct2/show/NCT02196103 Date first received: 15 July 2014.
Additional references
ACOG 2006
- ACOG. Committee Opinion No 342: induction of labor for vaginal birth after cesarean delivery. Obstetrics & Gynecology 2006;108(2):465‐8. [DOI] [PubMed] [Google Scholar]
Alfirevic 2006
- Alfirevic Z, Weeks A. Oral misoprostol for induction of labour. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD001338.pub2] [DOI] [PubMed] [Google Scholar]
Belizan 1999
- Belizan JM, Althabe F, Barros FC, Alexander S. Rates and implications of caesarean sections in Latin America: ecological study. BMJ 1999;319(7222):1397‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bennett 1997
- Bennett BB. Uterine rupture during induction of labor at term with intravaginal misoprostol. Obstetrics & Gynecology 1997;89(5 Pt 2):832‐3. [DOI] [PubMed] [Google Scholar]
Brill 2003
- Brill Y, Kingdom J, Thomas J, Fraser W, Milne JK, Thomas M, et al. The management of VBAC at term: a survey of Canadian obstetricians. Journal of Obstetrics and Gynaecology Canada: JOGC 2003;25(4):300‐10. [DOI] [PubMed] [Google Scholar]
Bujold 2004
- Bujold E, Blackwell SC, Gauthier RJ. Cervical ripening with transcervical Foley catheter and the risk of uterine rupture. Obstetrics & Gynecology 2004;103(1):18‐23. [DOI] [PubMed] [Google Scholar]
Choy‐Hee 2001
- Choy‐Hee L, Raynor BD. Misoprostol induction of labor among women with a history of cesarean delivery. American Journal of Obstetrics and Gynecology 2001;184(6):1115‐7. [DOI] [PubMed] [Google Scholar]
Chuahan 2003
- Chuahan SP, Martin JN, Henrichs CE, Morrison JC, Magann EF. Maternal and perinatal complications with uterine rupture in 147,075 patients who attempted vaginal birth after cesarean delivery: a review of the literature. American Journal of Obstetrics and Gynecology 2003;189:408‐17. [DOI] [PubMed] [Google Scholar]
Cunha 1999
- Cunha M, Bugalho A, Bique C, Bergstrom S. Induction of labor by vaginal misoprostol in patients with previous cesarean delivery. Acta Obstetricia et Gynecologica Scandinavica 1999;78(7):653‐4. [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Dodd 2003
- Dodd J, Crowther CA. Vaginal birth after caesarean section: a survey of practice in Australia and New Zealand. Australian and New Zealand Journal of Obstetrics and Gynaecology 2003;43(3):226‐31. [DOI] [PubMed] [Google Scholar]
Dodd 2004
- Dodd JM, Crowther CA, Huertas E, Guise JM, Horey D. Planned elective caesarean section versus planned vaginal birth for women with a previous caesarean birth. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD004224.pub2] [DOI] [PubMed] [Google Scholar]
Dodd 2006
- Dodd JM, Crowther CA. Elective repeat caesarean section versus induction of labour for women with a previous caesarean birth. Cochrane Database of Systematic Reviews 2012, Issue 5. [DOI: 10.1002/14651858.CD004906.pub3] [DOI] [PubMed] [Google Scholar]
Gupta 2011
- Gupta P, Elmardi A, Bathula U, Chandru S, Charlesworth D. Induction of labour in women with one previous caesarean section. Journal of Obstetrics and Gynaecology 2011;21(2):131‐3. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hofmeyr 2009
- Hofmeyr GJ, Alfirevic Z, Kelly AJ, Kavanagh J, Thomas J, Neilson JP, et al. Methods for cervical ripening and labour induction in late pregnancy: generic protocol. Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD002074.pub2] [DOI] [Google Scholar]
Hofmeyr 2010
- Hofmeyr GJ, Gülmezoglu AM, Pileggi C. Vaginal misoprostol for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2010, Issue 10. [DOI: 10.1002/14651858.CD000941.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kayani 2005
- Kayani SI, Alfirevic Z. Uterine rupture after induction of labour in women with previous caesarean section. BJOG: an international journal of obstetrics and gynaecology 2005;112(4):451‐5. [DOI] [PubMed] [Google Scholar]
Landon 2004
- Landon MB, Hauth JC, Leveno KJ, Spong CY, Leindecker S, Varner MW, et al. Maternal and perinatal outcomes associated with a trial of labor after prior cesarean delivery. New England Journal of Medicine 2004;351(25):2581‐9. [DOI] [PubMed] [Google Scholar]
Landon 2005
- Landon MB, Leindecker S, Spong CY, Hauth JC, Bloom S, Varner MW, et al. The MFMU Cesarean Registry: factors affecting the success of trial of labor after previous cesarean delivery. American Journal of Obstetrics and Gynecology 2005;193(3 Pt 2):1016‐23. [DOI] [PubMed] [Google Scholar]
Laws 2009
- Laws P, Sullivan EA. Australia's Mothers and Babies 2007. Cat. no. PER 48. Canberra: AIHW, 2009. [Google Scholar]
Locatelli 2004
- Locatelli A, Regalia AL, Ghidini A, Ciriello E, Biffi A, Pezzullo JC. Risks of induction of labour in women with a uterine scar from previous low transverse caesarean section. BJOG: an international journal of obstetrics and gynaecology 2004;111(12):1394‐9. [DOI] [PubMed] [Google Scholar]
Lydon‐Rochelle 2001
- Lydon‐Rochelle M, Holt VL, Easterling TR, Martin DP. Risk of uterine rupture during labor among women with a prior cesarean delivery. New England Journal of Medicine 2001;345(1):3‐8. [DOI] [PubMed] [Google Scholar]
Martin 2009
- Martin JA, Sutton PD, Ventura SJ, Menacker F, Kirmeyer S, Mathews TJ. National Vital Statistics Reports. Births: Final Data for 2006. www.cdc.gov/nchs/data/nvsr/nvsr57/nvsr57_07.pdf (accessed July 2011).
Muzonzini 2004
- Muzonzini G, Hofmeyr GJ. Buccal or sublingual misoprostol for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD004221.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
NHS 2009
- NHS. NHS Maternity Statistics 2008‐09. The Health and Social Care Information Centre, 2009. [Google Scholar]
NIH consensus 2010
Peristat 2008
- Euro‐PERISTAT Project. The European Perinatal Health Report. www.europeristat.com 2008.
Phillips 1996
- Phillips K, Berry C, Mathers AM. Uterine rupture during second trimester termination of pregnancy using mifepristone and a prostaglandin. European Journal of Obstetrics, Gynecology, and Reproductive Biology 1996;65(2):175‐6. [DOI] [PubMed] [Google Scholar]
Plaut 1999
- Plaut MM, Schwartz ML, Lubarsky SL. Uterine rupture associated with the use of misoprostol in the gravid patient with a previous cesarean section. American Journal of Obstetrics and Gynecology 1999;180(6 Pt 1):1535‐42. [DOI] [PubMed] [Google Scholar]
Ravasia 2000
- Ravasia DJ, Wood SL, Pollard JK. Uterine rupture during induced trial of labor among women with previous cesarean delivery. American Journal of Obstetrics and Gynecology 2000;183(5):1176‐9. [DOI] [PubMed] [Google Scholar]
RCOG 2008
- National Collaborating Centre for Women’s and Children’s Health. Induction of Labour. Clinical Guideline no 70. London: RCOG press, 2008. [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Smith 2004
- Smith GC, Pell JP, Pasupathy D, Dobbie R. Factors predisposing to perinatal death related to uterine rupture during attempted vaginal birth after caesarean section: retrospective cohort study. BMJ 2004;329(7462):375. [DOI] [PMC free article] [PubMed] [Google Scholar]
SOGC 2005
- Society of Obstetricians and Gynaecologists of Canada. SOGC clinical practice guidelines. Guidelines for vaginal birth after previous caesarean birth. Number 155 (Replaces guideline Number 147), February 2005. International Journal of Gynaecology & Obstetrics 2005;89(3):319‐31. [DOI] [PubMed] [Google Scholar]
Wing 1996
- Wing DA, Paul RH. A comparison of differing dosing regimens of vaginally administered misoprostol for cervical ripening and labor induction. American Journal of Obstetrics and Gynecology 1996;175:158‐64. [DOI] [PubMed] [Google Scholar]
Zwart 2008
- Zwart JJ, Richters JM, Ory F, Vries JI, Bloemenkamp KW, Roosmalen J. Severe maternal morbidity during pregnancy, delivery and puerperium in the Netherlands: a nationwide population‐based study of 371 000 pregnancies. BJOG: an international journal of obstetrics and gynaecology 2008;115:842‐50. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Jozwiak 2013
- Jozwiak M, Dodd JM. Methods of term labour induction for women with a previous caesarean section. Cochrane Database of Systematic Reviews 2013, Issue 3. [DOI: 10.1002/14651858.CD009792.pub2] [DOI] [PubMed] [Google Scholar]
Jozwiak 2012
- Jozwiak M, Dodd JM. Methods of term labour induction for women with a previous caesarean section. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD009792] [DOI] [PubMed] [Google Scholar]


