Abstract
Background
Chest physiotherapy is widely prescribed to assist the clearance of airway secretions in people with cystic fibrosis. Oscillating devices generate intra‐ or extra‐thoracic oscillations orally or external to the chest wall. Internally they create variable resistances within the airways, generating controlled oscillating positive pressure which mobilises mucus. Extra‐thoracic oscillations are generated by forces outside the respiratory system, e.g. high frequency chest wall oscillation. This is an update of a previously published review.
Objectives
To identify whether oscillatory devices, oral or chest wall, are effective for mucociliary clearance and whether they are equivalent or superior to other forms of airway clearance in the successful management of secretions in people with cystic fibrosis.
Search methods
We searched the Cochrane Cystic Fibrosis and Genetic Disorders Group Trials Register comprising references identified from comprehensive electronic database searches and hand searches of relevant journals and abstract books of conference proceedings. Latest search of the Cystic Fibrosis Trials Register: 27 April 2017.
In addition we searched the trials databases ClinicalTrials.gov and the WHO International Clinical Trials Registry Platform. Latest search of trials databases: 26 April 2017.
Selection criteria
Randomised controlled studies and controlled clinical studies of oscillating devices compared with any other form of physiotherapy in people with cystic fibrosis. Single‐treatment interventions (therapy technique used only once in the comparison) were excluded.
Data collection and analysis
Two authors independently applied the inclusion criteria to publications and assessed the quality of the included studies.
Main results
The searches identified 76 studies (302 references); 35 studies (total of 1138 participants) met the inclusion criteria. Studies varied in duration from up to one week to one year; 20 of the studies were cross‐over in design. The studies also varied in type of intervention and the outcomes measured, data were not published in sufficient detail in most of these studies, so meta‐analysis was limited. Few studies were considered to have a low risk of bias in any domain. It is not possible to blind participants and clinicians to physiotherapy interventions, but 11 studies did blind the outcome assessors.
Forced expiratory volume in one second was the most frequently measured outcome and while many of the studies reported an improvement in those people using a vibrating device compared to before the study, there were few differences when comparing the different devices to each other or to other airway clearance techniques. One study identified an increase in frequency of exacerbations requiring antibiotics whilst using high frequency chest wall oscillation when compared to positive expiratory pressure. There were some small but significant changes in secondary outcome variables such as sputum volume or weight, but not wholly in favour of oscillating devices. Participant satisfaction was reported in 15 studies but this was not specifically in favour of an oscillating device, as some participants preferred breathing techniques or techniques used prior to the study interventions. The results for the remaining outcome measures were not examined or reported in sufficient detail to provide any high level evidence.
Authors' conclusions
There was no clear evidence that oscillation was a more or less effective intervention overall than other forms of physiotherapy; furthermore there was no evidence that one device is superior to another. The findings from one study showing an increase in frequency of exacerbations requiring antibiotics whilst using an oscillating device compared to positive expiratory pressure may have significant resource implications. More adequately‐powered long‐term randomised controlled trials are necessary and outcomes measured should include frequency of exacerbations, individual preference, adherence to therapy and general satisfaction with treatment. Increased adherence to therapy may then lead to improvements in other parameters, such as exercise tolerance and respiratory function. Additional evidence is needed to evaluate whether oscillating devices combined with other forms of airway clearance is efficacious in people with cystic fibrosis.There may also be a requirement to consider the cost implication of devices over other forms of equally advantageous airway clearance techniques. Using the GRADE method to assess the quality of the evidence, we judged this to be low or very low quality, which suggests that further research is very likely to have an impact on confidence in any estimate of effect generated by future interventions.
Keywords: Adolescent; Adult; Child; Humans; Breathing Exercises; Chest Wall Oscillation; Chest Wall Oscillation/instrumentation; Cystic Fibrosis; Cystic Fibrosis/complications; Cystic Fibrosis/physiopathology; Forced Expiratory Volume; Lung Diseases, Obstructive; Lung Diseases, Obstructive/etiology; Lung Diseases, Obstructive/therapy; Mucus; Mucus/secretion; Randomized Controlled Trials as Topic; Vibration; Vibration/therapeutic use
The use of vibrating devices to help people with cystic fibrosis clear their airways of mucus
Review question
We reviewed the evidence about the effect of vibrating devices (e.g. Flutter, acapella, cornet, Quake®, intrapulmonary percussive ventilation, high frequency chest wall oscillators (e.g. Vest®), VibraLung® and MetaNeb®) to help people with cystic fibrosis clear their airways of mucus. This is an update of a previously published review.
Background
People with cystic fibrosis have too much sticky mucus in their lungs which can lead to constant infection and inflammation. This damages their airways and worsens lung function over time. People with cystic fibrosis use chest physiotherapy to clear the mucus from their lungs. They can use different methods alone or in combination with others ‐ manual techniques, breathing techniques and mechanical devices. Vibrating devices (also sometimes known as oscillators) use pressure generated either inside or outside of the body to clear the mucus.
Search date
Evidence is current to 26 April 2017.
Study characteristics
The review included 35 studies with 1138 people with cystic fibrosis aged between 4 and 63 years of age. Studies compared different physiotherapy treatments and people were selected for one treatment or the other randomly. Not many studies looked at the same types of physiotherapy over the same period of time; studies ranged in duration from two days to 13 months.
Key results
Given the differences in study design, it was difficult to combine the results from these studies in a useful way.
We did not find any clear evidence that vibrating devices were better than any other form of physiotherapy which they were compared to in these studies, or that one device was better than another. One study found that people using an vibrating device needed additional antibiotics for a chest infection more often than those using positive expiratory pressure. When recommending the most suitable method of airway clearance, physiotherapists should consider the needs of the people they are treating.
For the future, larger and longer trials are needed to measure the frequency of lung infections, preference, adherence to and general satisfaction with treatment, financial constraints should also be taken into consideration. We think adherence is important, because if people with cystic fibrosis are willing to stick to their physiotherapy regimen, there may be improvements in other outcomes such as exercise tolerance, respiratory function and mortality.
Quality of the evidence
Overall, we thought most studies had some design problems which might affect our confidence in some of the results. In about a quarter of studies there were concerns that not all the results were reported clearly and in about a third of the studies the reasons for people withdrawing from a trial were not clearly explained. In comparisons of different types of physiotherapy, a person and their physiotherapist will always know which treatment they are receiving and this might affect their answers to some questions, such as which treatment makes them feel better, but we only thought this was a problem in a few studies. We used a scoring system called GRADE to assess the quality of the evidence, we then judged it to be either low or very low quality, which suggests that further research is very likely to affect our confidence in the results in this review for of any of the interventions analysed.
Summary of findings
Summary of findings for the main comparison.
Oscillating devices compared with positive expiratory pressure (PEP) for cystic fibrosis
| Oscillating devices compared with positive expiratory pressure (PEP) for cystic fibrosis | ||||||
|
Patient or population: adults and children with cystic fibrosis Settings: outpatients and hospitalised patients Intervention: oscillating devices Comparison: positive expiratory pressure (PEP) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| PEP | Oscillating devices1 | |||||
|
FEV₁: % predicted Follow‐up: less than 1 week to 1 year |
There were no statistically significant differences between oscillating devices and PEP in terms of FEV₁ % predicted post‐intervention or change from baseline at any time point. | NA | 510 (15 studies) | ⊕⊝⊝⊝ very low3,4 | ||
|
FEF25‐75 : % predicted Follow‐up: less than 1 week to 1 year |
There were no statistically significant differences between oscillating devices and PEP in terms of FEF25‐75 % predicted post‐intervention or change from baseline at any time point. | NA | 355 (9 studies) | ⊕⊝⊝⊝ very low3,4 | ||
|
FVC Follow‐up: less than 1 week to 1 year |
There were no statistically significant differences between oscillating devices and PEP in terms of FVC post‐intervention or change from baseline at any time point. | NA | 362 (9 studies) | ⊕⊝⊝⊝ very low3,4 | ||
|
Sputum: volume (mL) Follow‐up: up to 1 week |
The mean sputum volume in the PEP group was 8.5 mL. | The mean sputum volume in the oscillating device group was 1.8 mL lower (6.6 mL lower to 3.0 mL higher). | NA | 23 (1 study) | ⊕⊕⊝⊝ low4,5 | A second study recruiting 30 participants reported that there was an increase in sputum volume when HFCWO was compared to participants' usual ACT; however, it was not clear exactly what interventions were included in the usual ACT treatment arm. |
|
Sputum: weight (dry or wet) (g) Follow‐up: up to 2 weeks |
3 out of 4 studies reported no statistically significant difference between oscillating devices and PEP in terms of sputum weight (g). 1 study reported that a significantly greater weight of sputum was yielded using PEP compared to HFCWO. |
NA | 104 (4 studies) | ⊕⊕⊝⊝ low4,6 | ||
|
Frequency of exacerbations2 Follow‐up: up to one year |
2 out of 4 studies reported no statistically significant difference between oscillating devices and PEP. 2 out of 4 studies reported that significantly more hospitalizations or participants requiring antibiotics in the oscillating devices groups compared to the PEP groups. |
NA | 219 (4 studies) | ⊕⊕⊝⊝ low4,6 | ||
|
Participant‐reported satisfaction with treatment intervention Follow‐up: less than 1 week to 1 year |
Some differences were reported between treatment groups in single domains of satisfaction questionnaires or measurement scales (in favour of or against oscillating devices). Overall across the 7 studies, no consistent differences were reported in terms of satisfaction of any treatment intervention. |
NA | 242 (7 studies) |
⊕⊝⊝⊝ very low3,4,7 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ACT: airway clearance technique; CI: confidence interval; FEF25‐75 : mid‐expiratory flow; FEV₁: forced expiratory volume at one second;FVC: forced vital capacity; HFCWO: high frequency chest wall oscillation;IPV: intrapulmonary percussive ventilation; NA: not applicable; PEP: positive expiratory pressure. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1. The oscillating devices included in the trials under this comparison were HFCWO, flutter, IPV, acapella and cornet.
2. Frequency of exacerbations were measured as defined by Rosenfeld as a consequence of the treatment intervention (Rosenfeld 2001).
3. Downgraded twice due to serious risk of bias; many judgements of high risk of bias across the included studies due to reasons such as inadequate allocation concealment, lack of blinding of participants, clinicians and outcome assessors, incomplete outcome data and selective reporting (see Risk of bias in included studies for further information).
4. Downgraded once due to imprecision: many included studies had very small sample sizes, short treatment durations and employed cross‐over designs. As results were not presented from paired analyses for these studies, we treated the cross‐over trials as if they were parallel trials which is a conservative approach as it does not take into account within‐patient correlation. Sensitivity analyses indicates that results were robust to this approach.
5. Downgraded once due to unclear risk of bias; the study was published as an abstract only and very limited information was available regarding the study design.
6. Downgraded once due to risk of bias; judgements of high risk of bias across some of the included studies due to reasons such as inadequate allocation concealment, lack of blinding of participants clinicians and outcome assessors, incomplete outcome data and selective reporting (see Risk of bias in included studies for further information).
7. Downgraded once due to applicability; three of the studies reported anecdotal findings in terms of participant satisfaction or preference for a treatment arm without numerical results to support these findings.
Summary of findings 2.
Oscillating devices compared with breathing techniques for cystic fibrosis
| Oscillating devices compared with breathing techniques for cystic fibrosis | ||||||
|
Patient or population: adults and children with cystic fibrosis Settings: outpatients and hospitalised patients Intervention: oscillating devices Comparison: breathing techniques | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Breathing techniques | Oscillating devices1 | |||||
|
FEV₁: % predicted or L Follow‐up: less than 1 week to 1 year |
6 out of 7 studies reported no statistically significant differences between oscillating devices and breathing techniques in terms of FEV₁ (% predicted or L). 1 study reported a significant advantage for active cycle of breathing techniques compared to HFWCO in terms of FEV₁ (L). |
NA | 184 (7 studies) |
⊕⊕⊝⊝ low3,4 | ||
|
FEF25‐75 Follow‐up: 5 days |
There were no statistically significant differences between oscillating devices and breathing techniques in terms of FEF25‐75. | NA | 7 (1 study) | ⊕⊝⊝⊝ very low5,6 | ||
|
FVC Follow‐up: less than 1 week to 1 year |
4 out of 5 studies reported no statistically significant differences between oscillating devices and breathing techniques in terms of FVC. 1 study reported a significant advantage for active cycle of breathing techniques compared to HFWCO in terms of FVC % predicted. |
NA | 154 (6 studies) | ⊕⊕⊝⊝ low3,4 | ||
|
Sputum: volume (g) Follow‐up: up to 1 month |
The mean sputum volume in the breathing technique group was 3.6 g. | The mean sputum volume in the oscillating device group was 0.9 g higher (1.72 g lower to 3.52 g higher). | NA | 14 (1 study) | ⊕⊕⊝⊝ low5,7 | |
|
Sputum: weight (dry or wet) (g) Follow‐up: up to 2 weeks |
3 out of 5 studies reported no statistically significant difference between oscillating devices and breathing technique in terms of sputum weight (g). 2 out of 5 studies reported that a significantly greater weight of sputum was yielded using breathing techniques compared to oscillating devices. |
NA | 92 (5 studies) | ⊕⊕⊝⊝ low3,4 | ||
|
Frequency of exacerbations2 Follow‐up: NA |
Outcome not reported in any study. | NA | NA | NA | ||
|
Participant‐reported satisfaction with treatment intervention Follow‐up: up to 2 weeks |
Some differences were reported between treatment groups in single domains of satisfaction questionnaires or measurement scales (in favour of or against oscillating devices). Overall across the 5 studies, no consistent differences were reported in terms of satisfaction of any treatment intervention. |
NA | 92 (5 studies) | ⊕⊕⊝⊝ low3,4 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; FEF25‐75 : mid‐expiratory flow; FEV₁: forced expiratory volume at one second;FVC: forced vital capacity; HFCWO: high frequency chest wall oscillation;L: litres; MD: mean difference; NA: not applicable. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1. The oscillating devices included in the trials under this comparison were HFCWO, flutter and cornet.
2. Frequency of exacerbations were measured as defined by Rosenfeld as a consequence of the treatment intervention (Rosenfeld 2001).
3. Downgraded once due to risk of bias; judgements of high risk of bias across some of the included studies due to reasons such as lack of blinding of participants clinicians and outcome assessors, incomplete outcome data and selective reporting (see Risk of bias in included studies for further information)
4. Downgraded once due to imprecision: many included studies had very small sample sizes, short treatment durations and employed cross‐over designs. As results were not presented from paired analyses for these studies, we treated the cross‐over trials as if they were parallel trials which is a conservative approach as it does not take into account within‐patient correlation. Sensitivity analyses indicates that results were robust to this approach.
5. Downgraded once due to risk of bias: the single included study was at high risk of bias due to lack of blinding and reported limited information regarding other aspects of the methodological design
6. Downgraded once due to serious imprecision: a single cross‐over study recruiting only seven participants over a 5‐day period contributed to the outcome and no numerical data were available.
7. Downgraded once due to imprecision: a single cross‐over study recruiting only 14 participants contributed to the outcome.
Summary of findings 3.
Oscillating devices compared with conventional physiotherapy for cystic fibrosis
| Oscillating devices compared with conventional physiotherapy for cystic fibrosis | ||||||
|
Patient or population: adults and children with cystic fibrosis Settings: outpatients and hospitalised patients Intervention: oscillating devices Comparison: conventional physiotherapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Conventional physiotherapy | Oscillating devices1 | |||||
|
FEV₁: % predicted Follow‐up: less than 1 week up to 3 years |
There were no statistically significant differences between oscillating devices and conventional physiotherapy in terms of FEV₁ % predicted post‐intervention or change from baseline at any time point. | NA | 363 (10 studies) | ⊕⊝⊝⊝ very low3,4 | ||
|
FEF25‐75: % predicted Follow‐up: less than 1ne week up to 3 years |
There were no statistically significant differences between oscillating devices and conventional physiotherapy in terms of FEF25‐75 % predicted post‐intervention or change from baseline at any time point. | NA | 319 (8 studies) | ⊕⊝⊝⊝ very low3,4 | ||
|
FVC Follow‐up: less than 1 week up to 3 years |
There were no statistically significant differences between oscillating devices and conventional physiotherapy in terms of FVC post‐intervention or change from baseline at any time point. | NA | 268 (7 studies) |
⊕⊝⊝⊝ very low3,4 | ||
|
Sputum: volume Follow‐up: up to 1 week |
Both studies found a statistically significant advantage for the oscillating device compared to the conventional physiotherapy in terms of volume of sputum. | NA | 17 (2 studies) | ⊕⊕⊝⊝ low4,5 | ||
| Sputum: weight (dry or wet) | 6 out of 8 studies reported no statistically significant difference between oscillating devices and conventional physiotherapy in terms of sputum weight (g). 1 study reported that a significantly greater weight of sputum was yielded using conventional physiotherapy compared to HFCWO. 1 study reported that a significantly greater weight of sputum was yielded using HFCWO compared to conventional physiotherapy. |
NA | 188 (8 studies) | ⊕⊝⊝⊝ very low3,4 | ||
|
Frequency of exacerbations2 Follow‐up: less than 1 week up to 3 years |
There were no significant differences between oscillating devices and conventional physiotherapy in terms of days of hospitalisation or time to next pulmonary exacerbation. | NA | 262 (4 studies) | ⊕⊝⊝⊝ very low3,4 | ||
|
Participant‐reported satisfaction with treatment intervention Follow‐up: less than 1 week up to 3 years |
Some differences were reported between treatment groups in single domains of satisfaction questionnaires or measurement scales (in favour of or against oscillating devices). Overall across the 9 studies, no consistent differences were reported in terms of satisfaction of any treatment intervention. |
NA | 345 (9 studies) | ⊕⊝⊝⊝ very low3,4,6 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; FEF25‐75 : mid‐expiratory flow; FEV₁: forced expiratory volume at one second;FVC: forced vital capacity; HFCWO: high frequency chest wall oscillation; IPV: intrapulmonary percussive ventilation; NA: Not applicable. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1. The oscillating devices included in the trials under this comparison were HFCWO, flutter and IPV.
2. Frequency of exacerbations were measured as defined by Rosenfeld as a consequence of the treatment intervention (Rosenfeld 2001).
3. Downgraded twice due to serious risk of bias; many judgements of high risk of bias across the included studies due to reasons such as inadequate allocation concealment, lack of blinding of participants, clinicians and outcome assessors, incomplete outcome data and selective reporting (see Risk of bias in included studies for further information).
4. Downgraded once due to imprecision: many included studies had very small sample sizes, short treatment durations and employed cross‐over designs. As results were not presented from paired analyses for these studies, we treated the cross‐over trials as if they were parallel trials which is a conservative approach as it does not take into account within‐patient correlation. Sensitivity analyses indicates that results were robust to this approach.
5. Downgraded once due to unclear risk of bias; limited information was available regarding the methodological designs of the 2 studies.
6. Downgraded once due to applicability; 4 of the studies reported anecdotal findings in terms of participant satisfaction or preference for a treatment arm without numerical results to support these findings.
Summary of findings 4.
Oscillating devices compared with different oscillating devices for cystic fibrosis
| Oscillating devices compared with different oscillating devices for cystic fibrosis | ||||||
|
Patient or population: adults and children with cystic fibrosis Settings: outpatients and hospitalised patients Intervention: oscillating devices Comparison: a different oscillating device | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Oscillating Devices1 | Oscillating devices1 | |||||
|
FEV₁ Follow‐up: less than 1e week up to 3 years |
There were no statistically significant differences between oscillating devices in terms of FEV₁ at any time point. | NA | 316 (5 studies) |
⊕⊝⊝⊝ very low3,4 | ||
|
FEF25‐75 Follow‐up: less than 1 week up to 3 years |
There were no statistically significant differences between oscillating devices in terms of FEF25‐75 at any time point. | NA | 211 (3 studies) | ⊕⊝⊝⊝ very low3,4 | ||
|
FVC Follow‐up: less than 1 week up to 3 years |
There were no statistically significant differences between oscillating devices in terms of FVC at any time point. | NA | 286 (4 studies) | ⊕⊝⊝⊝ very low3,4 | ||
|
Sputum: volume Follow‐up: NA |
Outcome not reported. | NA | NA | NA | ||
|
Sputum: weight (dry or wet) Folllow‐up: 6 days |
The results of the study showed that wet and dry sputum weight in the IPV group was significantly greater than in the HFCWO group. | NA | 24 (1 study) |
⊕⊕⊝⊝ low4,5 | ||
|
Frequency of exacerbations2 Follow‐up: 24 weeks |
There were no statistically significant differences between oscillating devices in terms of frequency of hospitalisations or need for home intravenous therapies. | NA | 16 (1 study) | ⊕⊝⊝⊝ very low6,7 | ||
|
Participant‐reported satisfaction with treatment intervention Follow‐up: less than 1 week up to 3 years |
Some differences were reported between treatment groups in single domains of satisfaction questionnaires or measurement scales (in favour of or against oscillating devices). Overall across the 5 studies, no consistent differences were reported in terms of satisfaction of any treatment intervention. |
NA | 265 (5 studies) | ⊕⊝⊝⊝ very low3,4 | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; FEF25‐75 : mid‐expiratory flow; FEV₁: forced expiratory volume at one second;FVC: forced vital capacity; HFCWO: high frequency chest wall oscillation; IPV: intrapulmonary percussive ventilation; NA: Not applicable. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1. The oscillating devices included in the trials under this comparison were HFCWO, flutter, IPV and cornet.
2. Frequency of exacerbations were measured as defined by Rosenfeld as a consequence of the treatment intervention (Rosenfeld 2001).
3. Downgraded twice due to serious risk of bias; many judgements of high risk of bias across the included studies due to reasons such as lack of blinding of participants, clinicians and outcome assessors, incomplete outcome data and selective reporting (see Risk of bias in included studies for further information).
4. Downgraded once due to imprecision: many included studies had very small sample sizes, short treatment durations and employed cross‐over designs. As results were not presented from paired analyses for these studies, we treated the cross‐over trials as if they were parallel trials which is a conservative approach as it does not take into account within‐patient correlation. Sensitivity analyses indicates that results were robust to this approach.
5. Downgraded once due to unclear risk of bias; the study was potentially as risk of bias due to the administration of the interventions and limited information was available regarding the study design.
6. Downgraded once due to serious risk of bias; the study was at risk of attrition bias and selective reporting bias.
7. Downgraded once due to imprecision: the study recruited only 16 participants and numerical data were not available for the outcome.
Background
Description of the condition
Cystic fibrosis (CF) is a common inherited life‐limiting genetic disorder. The genetic defect causes mucus hypersecretion within the airways leading to airway obstruction and mucus plugging (Zach 1990). Airway damage and progressive loss of respiratory function is a consequence of persistent infection and inflammation within the lungs (Cantin 1995; Konstan 1997).
Description of the intervention
Chest physiotherapy is currently implemented at initial diagnosis. It is recommended that it should be carried out for the maintenance of a clear chest with an additional recognition for altered or more aggressive therapies during times of respiratory exacerbation. Dependent on the age of the individual, chest physiotherapy will traditionally take the form of manual therapies. Conventional manual therapies would require the assistance of another person to perform the techniques of percussion and vibrations, with the addition of postural drainage when this was felt to add to the technique. With the advent of a more modern approach to physiotherapy, self‐administered techniques are more frequently used. These self‐administered techniques do not necessitate postural drainage or indeed the assistance of another person. They can be done in a sitting position (if preferred) and use different methods of breathing or different devices to assist mucus clearance. Oscillatory devices are designed to interrupt the expiratory airflow. These devices are either intra‐ or extra‐thoracic. Intra‐thoracic oscillatory devices are placed in the mouth and provide resistance during exhalation which results in the airways vibrating thus loosening the mucus. Extra‐thoracic oscillatory devices, such as an inflatable vest attached to a machine, vibrate at variable frequencies and intensities as set by the operator to ensure the individual's comfort and associated concordance. Fuller descriptions of all the interventions to be compared in the review can be found below in Types of interventions.
In this review we have considered the use of oscillation and oscillatory devices as a means of airway clearance and the consequent impact this type of intervention has on the individual with CF and in particular when compared with other recognised forms of airway clearance.
How the intervention might work
Respiratory infections are the primary cause of morbidity and mortality in CF and therefore chest physiotherapy is considered to be an important treatment for the assistance and clearance of the sticky mucus found within the airways of people with CF.
Oscillations, or interruptions in expiratory airflow have been postulated to mechanically reduce the viscoelasticity of sputum and enhance mucociliary clearance (Newbold 2005). Oscillations, both internally and externally, have also been considered to improve airway patency by preventing spontaneous compression through the introduction of alternating positive pressure where the consequent vibration loosens mucus allowing ease of expectoration (Oermann 2001; Pryor 1994).
Why it is important to do this review
Other Cochrane Reviews have considered the benefits of different forms of chest physiotherapy in people with CF (Main 2005;; McIlwaine 2015; McKoy 2016). They compare oscillatory devices with another recognised single therapy; conventional chest physiotherapy (Main 2005), positive expiratory pressure (PEP) (McIlwaine 2015) and active cycle of breathing techniques (ACBT) (McKoy 2016). It is the intention of this review to complement the information previously provided. This review will examine the effect and acceptability of oscillatory devices when compared to all other techniques including comparing types of oscillatory device currently used for airway clearance.
This is an updated version of previous reviews (Morrison 2007; Morrison 2009; Morrison 2014).
Objectives
To identify whether oscillatory devices, oral or chest wall, are effective for mucociliary clearance and whether they are equivalent or superior to other forms of airway clearance in the successful management of secretions in people with CF.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) and quasi ‐RCT's.
Types of participants
Children (aged up to 16 years) and adults (16 years and above) with any degree of disease severity, with defined CF, diagnosed clinically and by sweat or genetic testing. Trials with participants enrolled during a period of stability or during a pulmonary exacerbation were both considered.
Types of interventions
Oscillatory devices, both oral and chest wall, for airway clearance compared with another recognised airway clearance technique either as a single technique (e.g. oscillation versus active cycle of breathing technique (ACBT)) or in conjunction with another recognised airway clearance technique (e.g. oscillation and ACBT versus ACBT alone).
Interventions of variable duration would be considered and separated according to term of intervention. Single treatment interventions (where the therapy technique was used only once in the comparison) were not considered.
Specific techniques considered for comparison are likely to fall in to one of the following categories:
1. Oscillatory devices
Devices which have an oscillatory component which consider intra‐ or extra‐thoracic oscillations.
Intra‐thoracic oscillations are generated orally and created using variable resistances within the airways generating controlled oscillating positive pressure which mobilises respiratory secretions. When the oscillation frequency approximates the resonance frequency of the pulmonary system, endobronchial pressure oscillations are amplified and result in vibrations of the airways. These vibrations loosen mucus from the airway walls. The intermittent increases in endobronchial pressure reduce the collapsibility of the airways during exhalation, increasing the likelihood of clearing mucus from the tracheobronchial tract. The airflow accelerations increase the velocity of the air being exhaled, facilitating the movement of mucus up the airways (Konstan 1994). Exhalation through these devices generates both oscillations of positive pressure in the airways and repeated accelerations of expiratory airflow that have been shown to result in improved sputum clearance (Rogers 2005).
The devices frequently employed for this purpose are:
a. Flutter
A small plastic device containing a large ball bearing which repeatedly interrupts the outward flow of air (Konstan 1994; Pryor 1999).
b. Acapella
A flow operated oscillatory PEP device, which uses a counterweighted plug and magnet to generate the oscillatory resistance (Volsko 2003).
c. Cornet
A horn‐shaped tube which houses a rubber inner tube. The degree of rotation of this inner tube reflects the resistance generated. As the individual exhales through the horn the inner tube unfurls generating a rhythmic bending and unbending of the inner tube within the horn throughout the expiration phase (Pryor 1999).
d. Quake® (Thayer Medical, Tucson, Arizona, USA)
This device oscillates a column of air in both inspiratory and expiratory phases of respiration. It does not rely on an oscillating valve like the Flutter and the acapella, as it uses a manually turned cylinder that fits within another cylinder. Airflow occurs only when slots within the two cylinders line up. Therefore, the airflow is interrupted at regular intervals as the user turns the crank. The rate at which the device is cranked will determine the frequency of the flow interruption. Since the resulting vibration is not determined by the patients rate of flow, the Quake® theoretically may be more helpful for patients with severe obstructive lung disease who are unable to generate high peak expiratory flow rates.
e. Intrapulmonary percussive ventilation (IPV)
This provides continuous oscillation to the airways via the mouth (Homnick 1995).
f. Extra‐ thoracic oscillations (HFCWO)
Extra‐thoracic oscillations are generated by forces external to the respiratory system, e.g. high frequency chest wall oscillation (HFCWO) (Warwick 1991). External chest wall oscillations are applied using an inflatable vest attached to a machine which vibrates at a variable frequencies and intensities as set by the operator to ensure the individual's comfort and associated concordance. This type of device can also be called the Vest® or Hayek Oscillator.
g. The VibraLung®
The VibraLung® is an acoustic percussor, where sound waves are applied directly to the tracheobronchial tract at frequencies that cover the range of resonant frequencies of the human tracheobronchial tract (5 to 1,200 Hz). This causes a vibration within the airways and mucus directly, instead of indirectly through the chest wall. Additionally, the VibraLung® incorporates positive expiratory pressure (PEP) through its mouthpiece design with the inclusion of two tiny holes to provide resistance to exhalation (Wheatley 2013).
h. Metaneb®
The MetaNeb® System is a pneumatic compressor system which delivers continuous high frequency oscillation (CHFO) and continuous positive expiratory pressure (CPEP) to facilitate the clearance of mucus from the lungs, provide aerosol delivery and lung expansion therapy. Flow, pressure and percussive rate are all adjustable (Patel 2013).
2. Positive expiratory pressure (PEP)
Positive expiratory pressure is another well‐recognised and well‐utilised clearance method. Devices can be used to open up and recruit obstructed lung, allowing air to move behind secretions and assist in mobilising them. Breathing out against a slight resistance (10 to 20 cm H₂O) prevents the smaller bronchial tubes from collapsing down and thus permits the continuing upward movement of any secretions (McIlwaine 2015). Masks, mouthpieces or a novel Bubble PEP system offer more choice when considering this approach.
Hi‐PEP is a modification of PEP which involves the full forced expiration against a fixed mechanical resistance usually between 80 to 140 cm H₂O (Prasad 1993) .
3. Breathing techniques
When the individual is considered to be moving toward independence and chooses not to use a device, the techniques frequently adopted are autogenic drainage (AD) and the active cycle of breathing technique (ACBT).
a. Autogenic drainage
This term describes a series of breathing exercises devised by the Belgian physiotherapist Jean Chevaillier. The aim is to dislodge and collect mucus from the lungs and then clear these secretions by breathing at various lung volumes (Chevaillier 1984; Schöni 1989). There are three phases ‐ the Unstick, Collect and Evacuate when breathing at low, mid and high lung volumes to mobilise, collect and expectorate secretions respectively.
b. Active cycle of breathing technique
(Pryor 1999; Webber 1986; Webber 1990) This consists of three breathing techniques: breathing control is used between other techniques to allow relaxation; thoracic (chest) expansion exercises with the emphasis on inspiration, expiration being quiet and relaxed; and the forced expiration technique or huff is used to mobilise and clear secretions. One or two forced expirations are combined with a period of breathing control. A huff from high lung volume (when a breath has been taken in) will clear secretions from the upper airways and a huff from mid to low lung volume will clear secretions from the lower more peripheral airways.
4. Conventional chest physiotherapy
Conventional therapy techniques typically consisting of techniques such as modified postural drainage, percussion and manual vibrations or shakings are likely to have been introduced in infancy, or if the initial diagnosis was made in childhood (Prasad 1993). They may also include huffing and directed cough (Main 2005). If the diagnosis of CF was made during adolescence or indeed adulthood, many people prefer to use techniques which enable independence from an operator and which can easily be fitted around an active lifestyle.
As a consequence of many different descriptions of therapy techniques it was considered by the authors that certain manual therapies could be combined and considered as one 'type' of therapy. For this reason we have grouped the techniques of postural drainage and percussion (PD&P), postural drainage and clapping (PD& C) and postural drainage percussion and vibration (PDPV) under the term conventional physiotherapy (CPT), for unless otherwise stated we have assumed that CPT is a derivative of, or comparable to, the other terms used in the grouping.
5. Exercise
Where an individual with CF has few respiratory symptoms, exercise can often be the treatment of choice as a means of airway clearance or as an adjunct to other techniques. It has been recognised as contributing to enhanced quality of life (QoL) and improvements in functional exercise tolerance in people with chronic respiratory diseases such as CF. In addition exercise has been shown to increase respiratory muscle endurance, increase sputum expectoration and preserve respiratory function in some individuals with CF, where a higher level of aerobic fitness also correlated with a decreased risk of mortality (Radtke 2015; Webb 1995).
Types of outcome measures
Primary outcomes
-
Respiratory function
forced expiratory volume at one second (FEV₁)
mid‐expiratory flow (FEF25‐75 )
forced vital capacity (FVC)
expiratory reserve volume (ERV) or reserve volume (RV)
Secondary outcomes
-
Sputum
volume
weight (dry or wet)
Exercise tolerance (as measured by recognised standard exercise tests e.g. walk tests, step tests or cycle ergometry)
Quality of life (QoL) indices, e.g. CF QOL questionnaire
Level of oxygen saturation in response to treatment
Frequency of exacerbations (as defined by Rosenfeld (Rosenfeld 2001)) as a consequence of the treatment intervention
Participant reported satisfaction with treatment intervention
Lung clearance index
Search methods for identification of studies
There are no restrictions regarding language or publication status.
Electronic searches
We identified relevant trials from the Group's Cystic Fibrosis Trials Register using the term: 'oscillating devices'. The Cystic Fibrosis Trials Register is compiled from electronic searches of the Cochrane Central Register of Controlled Trials (CENTRAL) (updated each new issue of the Cochrane Library), weekly searches of MEDLINE, a search of Embase to 1995 and the prospective handsearching of two journals ‐ Pediatric Pulmonology and the Journal of Cystic Fibrosis. Unpublished work is identified by searching the abstract books of three major cystic fibrosis conferences: the International Cystic Fibrosis Conference; the European Cystic Fibrosis Conference and the North American Cystic Fibrosis Conference. For full details of all searching activities for the register, please see the relevant sections of the Group's website.
Date of last search of the Cystic Fibrosis Trials Register: 27 April 2017.
We also searched the relevant clinical trials databases clinicaltrials.gov/ and WHO ICTRP using the terms 'cystic fibrosis' AND 'oscillation". Date of the latest search: 26 April 2017.
Data collection and analysis
Selection of studies
Two authors (LM and JA) independently reviewed all citations and abstracts identified by the search to determine which papers assessed should be included. If disagreement had occurred, the authors planned to seek resolution by consensus.
Data extraction and management
Both authors (LM and JA) independently performed data extraction and recorded data on forms developed for this purpose. If disagreement had occurred, the authors planned to seek resolution by consensus.
We planned to group outcome data those measured at one, three, six, 12 months and annually thereafter. If outcome data were recorded at other time periods, then we planned to consider examining these as well. We have subsequently considered these time points and felt that to combine data measured at two weeks with data measured at four weeks was inappropriate. Therefore, we have split the original proposed time point of one month and reported data at up to two weeks (Arens 1994; Braggion 1995; Darbee 2005; Davies 2012; Gondor 1999; Grzincich 2008; Hare 2002; Kluft 1996; Milne 2004; Osman 2010; Phillips 2004; Pike 1999; Varekojis 2003a; Warwick 1990; West 2010) and at over two weeks and up to one month (Homnick 1998; Padman 1999a).
We have considered trials identifying interventions of varying duration separately; we considered those of one to 12 weeks as short term; those over 12 to 24 weeks medium term; and those over 24 weeks as long term. We did not consider single‐treatment interventions, as it is unlikely that an individual can be instructed in the most appropriate usage of such devices or treatment techniques in a single session. We have identified three multiple‐arm trials which consider more than one oscillatory device when compared with conventional physiotherapy. In order to achieve a comparison we have set up 'dummy' study ID's which allow the data from the study to be entered more than once on the same graph (Modi 2006b; Padman 1999b; Varekojis 2003b). In addition, there are other trials which consider two or more therapies. Due to the limitations of the analysis we are able to carry out in RevMan 5, the participants in the control groups will appear in more than one comparison, i.e. be counted twice, and we would caution the reader to consider this when interpreting the graphs.
Assessment of risk of bias in included studies
Cochrane Reviews incorporate a recommended approach for assessing risk of bias in included studies. It is a two‐part tool, addressing the six specific domains (namely sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting and ‘other issues’). Each domain includes one or more specific entries in a ‘Risk of bias’ table. Within each entry, the first part of the tool involves describing what was reported to have happened in the study. The second part of the tool involves assigning a judgement relating to the risk of bias for that entry. This is achieved by answering a pre‐specified question about the adequacy of the study in relation to the entry, such that a judgement of ‘Yes’ indicates low risk of bias, ‘No’ indicates high risk of bias, and ‘Unclear’ indicates unclear or unknown risk of bias.
In order to establish the risk of bias in the included studies, the two authors independently assessed the methodological rigour and quality of selected studies and reported on the six domains as recommended by theCochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and incorporated the criteria on quality assessment described by Jüni (Jüni 2001).
Generation of allocation sequence
We considered this as adequate, and a low risk of bias, if a computer algorithm or a similar process based on chance was used to randomise participants to treatment groups. We identified this as inadequate, and a high risk of bias, if sequences which could be attributed to prognosis, degree of disease severity, age etc were employed. We considered this unclear, and thus the risk of bias was also be unclear, where the generation of allocation sequence has not been identified.
Concealment of allocation
We considered concealment of allocation adequate where it was not possible for the investigators to foresee the allocation of participants to a particular treatment group, e.g. centralised or pharmacy‐controlled randomisation, pre‐numbered or coded identical containers administered serially to participants, on‐site locked computer system, or sequentially numbered, sealed , opaque envelopes. This means the study has a low risk of bias. We considered the concealment of allocation inadequate if the investigator was able to predict the allocation, e.g., alternation; the use of case record numbers, dates of birth or day of the week; thus the study has a high risk of bias. We graded this (and the risk of bias) as unclear if the concealment of allocation has not been described.
Blinding
We reported on the degree of blinding employed in each study. Given the treatment interventions and the specific devices for chest clearance which we have considered in this review, blinding of the investigator and participants was not possible: however, blinding of the person analysing the data was possible. The risk of bias is likely to be lower when these aspects of the trial are blinded, but frequently when the study compares the use of a device this is not practically possible.
Incomplete outcome data and intention‐to‐treat analysis
We described the completeness of outcome data for each main outcome and commented on attrition and exclusions from the study. If there was a discrepancy between total numbers randomised and numbers in each intervention group we reported on these and any reasons given for this occurrence.
We reported on whether the original investigators employed an intention‐to‐treat analysis (analysis based on the initial treatment allocation, not on the treatment eventually administered). We assessed whether the numbers and reasons for dropouts and withdrawals in all intervention groups were described or whether it was specified that there were no dropouts or withdrawals. If information is lacking on missing data, the risk of bias will increase.
Selective reporting
We considered the possibility that selective reporting influences the number of published articles and for this reason have also included abstracts and articles of non‐English language in our review. Following translation, we have included these articles in the review and entered data into the meta‐analysis where available.
We considered there to be selective reporting, if statistically non‐significant results were selectively withheld from publication. The most common reasons for non‐publication of results are ‘lack of clinical importance’ or lack of statistical significance. Therefore, meta‐analyses excluding unpublished outcomes are likely to overestimate intervention effects. We have tried where possible to include all identified outcomes within the meta‐analysis whether they demonstrated statistical significance or not and consequently reducing the likelihood of selective reporting within this review.
Within this review we have highlighted those references where selective reporting may have occurred. Examples of this include where we were unable to compare the original protocol of the study with the final paper and so we were unable to determine whether exclusions had occurred. Additionally, there were articles which identified particular variables in their outcome measures; however, these were not fully reported in their results. In these instances we have highlighted the discrepancies in the tables Characteristics of included studies and reported within the text of the review.
Other potential sources of bias
We considered that external bias could also influence the number of published articles and again this can be noted from the tables Characteristics of included studies. It is apparent that funding may have been sought, or indeed researchers identified, to consider mechanical or other devices, and their benefits, when compared to other techniques that do not necessitate potentially expensive equipment. This fact may limit the frequency of studies that include expensive equipment as research may be limited due to lack of funding streams.
Measures of treatment effect
For binary outcome measures, we planned to seek data on the number of participants with each outcome event, by allocated treated group, irrespective of compliance and whether or not the individual was later thought to be ineligible or otherwise excluded from treatment or follow up. We aimed to calculate a pooled estimate of the treatment effect for each outcome across studies using relative risk where appropriate.
For continuous outcomes, we recorded either mean relative change from baseline for each group or mean post‐treatment or intervention values and their standard deviations (these will be presented separately). If standard errors were reported, we planned to calculate the standard deviations if possible. We calculated a pooled estimate of treatment effect by calculating the mean difference.
Unit of analysis issues
When conducting a meta‐analysis combining results from cross‐over trials we planned to use the methods recommended by Elbourne (Elbourne 2002). However, only limited data were available and we entered the first‐arm data only from one trial (Oermann 2001); for the remainder we treated the cross‐over trials as if they were parallel trials. Elbourne states that this approach will produce conservative results as it does not take into account within‐patient correlation (Elbourne 2002). Also each participant appears in both the treatment and control group, so the two groups are not independent.
This review comprises data from both parallel and cross‐over studies, in the analysis of the data we have combined the results from both types of trial. In order to minimise the carry‐over effect from one arm to another, we have included only the data from the first arm of the cross‐over trial where possible as suggested by Curtin (Curtin 2002a); although taking data from the first arm of the trial reduces carry‐over it may offer a less efficient treatment estimate consequently leading to selection bias.
There were several studies which examined multiple treatment arms where more than one device was compared to conventional chest physiotherapy (Modi 2006a; Padman 1999a; Varekojis 2003a). We created duplicate references for each of these studies to enable data from both types of oscillatory device to be entered into the analysis. There was one paper where oscillatory devices were compared with the "usual" airway clearance technique (which encompassed a number of alternative therapies); however, we did not consider it meaningful to extract these data for inclusion in the analysis (Osman 2010). One further paper compared five different devices and again we were not able to extract the data in a clinically relevant way to be included in the analysis (Pryor 2010).
A further consideration noted in the study by Varekojis was that the data collected referred to the number of sputum samples rather than number of participants included in the study (Varekojis 2003a). This does accurately reflect how the comparison influenced sputum expectorated by participants, particularly as the number of samples compared were not equal. There were 24 participants in this study with six sets of sputum data anticipated for each treatment option; however, some of the sputum cups were contaminated by hemetemesis, one dried prior to wet weight being measured and one sputum cup was lost prior to weighing, leading to a discrepancy in terms of sputum samples across the intervention groups i.e. 142 compared to 143.
Dealing with missing data
If data were missing from the original trial reports, we planned to seek clarification from the authors. In the instance of a discrepancy between data in abstracts and the published article we sought clarification from the author and acted appropriately when considering data analysis.
Assessment of heterogeneity
The greater the consistency between the primary studies in a meta‐analysis, the more generalisable are the results. Heterogeneity refers to the genuine differences between studies rather than those that occur by chance. We planned to test for heterogeneity using the I² statistic (Higgins 2003). The values of I² lie between 0% to 100%, and we planned to use a simplified categorization of heterogeneity where we judge heterogeneity as low if the I² value is up to 25%, moderate up to 50% and high up to 75% (Higgins 2003). If this value were to be greater than 75% we would consider heterogeneity as extremely high.
Assessment of reporting biases
Many of the papers measured the outcome variables routinely and often during clinic visits. In the analysis of the papers, where possible, we have included the appropriate time points; however, it was often the case that measurements were recorded on completion of the study and it is these data that have been included in the tables of analyses. We examined the papers to assess when outcome variables were measured and which time points were reported. We recorded the data for each time point reported and noted if data were not presented for any of the outcomes. We looked for sponsorship of the trials by companies and whether this had been acknowledged in the papers. Furthermore, we noted if adverse events which could be a direct consequence of the use of the oscillating devices were reported in these papers.
Data synthesis
We analysed data using a fixed‐effect model, but if we had included sufficient studies for each outcome (at least four) and we had identified significant heterogeneity (where heterogeneity was 50% or greater), we planned to use a random‐effects model in the final analysis of the data.
Subgroup analysis and investigation of heterogeneity
There were insufficient combined data in the meta‐analysis for each comparison and outcome to allow for any of the planned subgroup analysis. If we had included a sufficient number of studies in the review and had identified moderate or high degrees of heterogeneity between studies in the meta‐analyses, we planned to investigate this by performing subgroup analysis of the following:
children (up to 16 years) compared to adults;
different treatment regimens (frequency per day and duration of treatment sessions) and concomitant medications (e.g. the use of bronchodilators or hypertonic saline);
participants with acute exacerbations compared to those with stable disease.
Sensitivity analysis
We also planned to perform the following sensitivity analyses to assess how robust the results of our meta‐analysis are:
study quality i.e. RCT compared to quasi‐RCT;
differing baseline characteristics of studies (specifically disease severity as measured by FEV₁ and defined as severely (FEV₁ < 45% predicted), moderately (FEV₁ > 46% to < 65% predicted) and minimally affected (FEV₁ > 65% predicted)).
The studies we included in the review were a mixture of cross‐over and parallel designed studies. In a post hoc change we decided to perform a sensitivity analysis including and excluding the studies with a cross‐over design to assess whether the study design had an effect on the results.
Summary of findings tables
In a post hoc change in line with current Cochrane guidance, at the 2017 update we added a summary of findings table for each comparison presented in the review. We selected the following seven outcomes to report (chosen based on relevance to clinicians and consumers):
FEV₁
FEF25‐75
FVC
Sputum volume
Sputum weight (dry or wet)
Frequency of exacerbations (as defined by Rosenfeld (Rosenfeld 2001)) as a consequence of the treatment intervention
Participant reported satisfaction with treatment intervention
We determined the quality of the evidence using the GRADE approach; and downgraded evidence in the presence of a high risk of bias in at least one study, indirectness of the evidence, unexplained heterogeneity or inconsistency, imprecision of results, high probability of publication bias. We downgraded evidence by one level if they considered the limitation to be serious and by two levels if very serious.
Results
Description of studies
Results of the search
A total of 302 references were identified from searches of the Cystic Fibrosis and Genetic Disorders Group's Cystic Fibrosis Trials Register combined with studies identified through attendance at international conferences. Searches of the international trials databases did not provide any further relevant studies for inclusion in this review. After initial consideration, those studies obviously not relevant to the review question or duplicated were discounted, leaving 119 references to 76 studies requiring closer inspection. Following further examination, 38 studies (50 references) were excluded, details of which can be found in the tables (Characteristics of excluded studies). There are 35 studies (65 references) included in the review, details of which are provided in the tables and and summarised in the text below (Characteristics of included studies). Three studies (four references), each as yet presented only as abstracts (Herrero 2016; Patel 2013; Wheatley 2013), have been listed under 'Studies awaiting classification' pending publication of the full study reports (Characteristics of studies awaiting classification). This is summarised in a study flow diagram (Figure 1).
Figure 1.
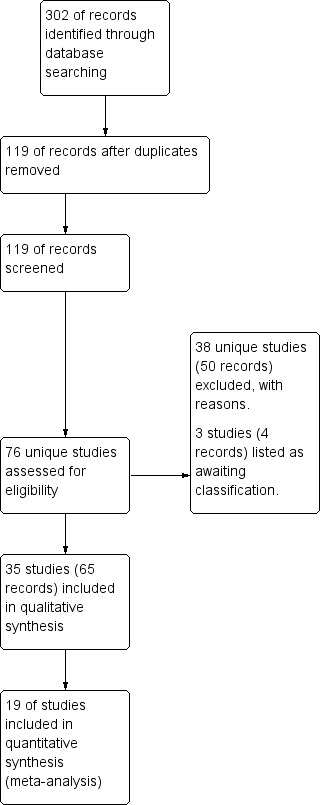
Study flow diagram.
Included studies
Of the included studies, 11 were published as abstracts only (Davies 2012; Giles 1996; Gotz 1995; Grzincich 2008; Hare 2002; Lyons 1992; Marks 2001; Modi 2006a; Pike 1999; Prasad 2005; Warwick 1990), with only two of these providing data that could be included in the meta‐analyses (Giles 1996; Grzincich 2008).
Trial design
A cross‐over design was used in 20 studies, and the remaining 15 studies used a parallel design. A total of 16 studies were generated by research carried out in the USA and 15 of these were single centre. The UK generated eight single‐centre studies, and the remaining 11 studies came from Europe, Canada and Australia. In addition there were four multi‐centre studies, two from the USA and one from Canada and one from Germany. Study duration varied widely; ranging from two days (Braggion 1995) up to 2.8 years (Modi 2006a) and duration was unspecified in three studies analysed.
Participants
The 35 included studies involved a total of 1138 participants and the numbers in each trial varied from five (Hansen 1990) to 166 (Modi 2006a). Participant age (when identified) varied from four years to 63 years of age; on closer inspection 19 of the studies included children younger than 16 years of age. Eight studies did not describe their gender split, and in the 27 studies which did, 22 of them had a greater number of male participants. There were 16 studies which did not identify whether participants were in a stable condition or experiencing an exacerbation. In the 19 studies that did report this factor, seven studies included participants who were deemed stable at the time of study initiation and 12 studies included participants who were admitted to hospital for the management of clinical exacerbations.
Interventions
As a consequence of many different descriptions of therapy techniques, it was considered by the authors that certain manual therapies could be combined and considered as one 'type' of therapy. For this reason we have grouped the techniques of postural drainage and percussion (PD&P), postural drainage and clapping (PD&C) and postural drainage percussion and vibration (PDPV) under the term conventional physiotherapy (CPT). Unless otherwise stated we have assumed that CPT is a derivative of, or comparable to, the other terms used in the grouping.
There were 10 studies which failed to identify the frequency of interventions performed on a daily basis. Where this was reported, the most common frequency of treatment interventions was twice daily with a range of one to four times daily. Where there were treatment comparisons, these were done at the same time of day and the same frequency of interventions occurred.
Outcomes measured
Once again the diversity of outcomes measured was great. However, the most frequently used clinical outcome measure was respiratory function (28 studies included respiratory function parameters in their outcome data), followed by sputum weight (14 studies) and individual satisfaction (11 studies).
Excluded studies
A total of 38 studies were excluded.
The authors consider it unlikely that an individual can be instructed in the appropriate usage of therapy devices or treatment techniques in a single session and consequently 21 studies were excluded using this criteria (Borka 2012; Dosman 2003; Dunn 2013; Dwyer 2017; Elkins 2004; Elkins 2005; Fainardi 2011; Grosse‐Onnebrink 2017; Hartsell 1978; Kempainen 2007; Konstan 1994; Lagerkvist 2006; Lindemann 1992; Marks 1998; Marks 2004; McCarren 2006; Natale 1994; Newhouse 1998; Scherer 1998; Stites 2006; Van Ginderdeuren 2008). Despite best efforts in specifying appropriate search terms 10 studies had to be excluded on the grounds that they did not concern either the population under review (Cegla 1993) or indeed the types of devices we were comparing on this occasion (Cantin 2005; Jarad 2010; Kraemer 1996; Kirkpatrick 1995; Liedtke 1996; Morris 1982; Salh 1989; Skopnik 1986; Webber 1984). One study was excluded as a consequence of incomplete data being reported in the abstract and the authors not being available for a response to requests for the missing data (Roos 1987). A further study was excluded as it did not contain any of the outcome measures we had identified as useful to this review (Majaesic 1996). One study of hypertonic saline in conjunction with acapella was excluded as the only difference between treatment groups was the timing of hypertonic saline administration (O'Neil 2017) The remaining four studies were excluded as (following translation of the full papers) were not RCTs (Amelina 2014; Orlik 2000a; Orlik 2000b; Orlik 2001).
Studies awaiting classification
Three studies, which have only been presented as abstracts, are awaiting further assessment pending further publications to clarify eligibility criteria (Herrero 2016; Patel 2013; Wheatley 2013) and details are given in the tables (Characteristics of studies awaiting classification).
The Herrero study is a cross‐over RCT comparing a combined therapy (nebulised hypertonic saline plus oscillatory PEP (Acapella®) to nebulised hypertonic saline alone. Each treatment arm lasted five days (running consecutively) with a one‐week washout period in between arms. The study was conducted across seven CF centres in Spain and recruited 19 participants with stable CF. Outcomes measured included sputum volume, pulmonary function, cough and personal preference (Herrero 2016).
The Patel study compared HFCWO to the Metaneb® during a 14‐day study period. The primary outcome measure was the time to cessation of sputum expectorated, with data for respiratory function and participant satisfaction also collected (Patel 2013).
The Wheatley study had two phases, but only the second phase will likely be eligible for inclusion in the review. This part of the study includes 12 participants in a hospital setting and compares the VibraLung® to the Vest® over a five‐day period of two sessions per day (Wheatley 2013).
We will further assess these studies for inclusion in the review when the full papers have been published and more data are available.
Risk of bias in included studies
Further details can be found in the risk of bias sections of the Characteristics of included studies tables.
Allocation
Considering the risk of bias graphs (Figure 2; Figure 3), we can determine that approximately 10% of the published studies had a high risk of allocation bias. This was apparent where there was no clear evidence that the allocation sequence could not be compromised by those entering participants into the studies. Methods of allocation were frequently omitted or described as alternate, and means of randomisation, concealment and sequence generation were not clearly identified. Approximately a further 85% of published studies had an unclear risk of bias, leaving only 5% of studies with a low risk of allocation bias.
Figure 2.
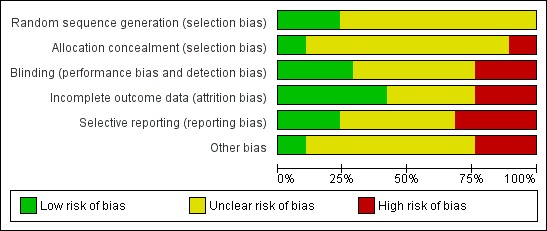
Risk of bias: review authors' judgments about each risk of bias item presented as percentages across all included studies.
Figure 3.
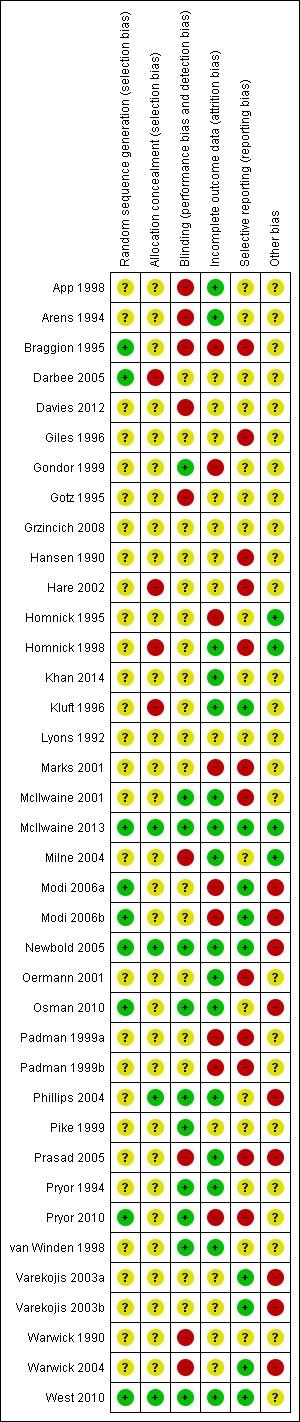
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Generation of sequence
Six studies were judged to have a low risk of bias (Braggion 1995; Darbee 2005; McIlwaine 2013; Newbold 2005; Osman 2010; West 2010). Randomisation according to Latin square design described by Williams (Williams 1949) was used in only one study (Braggion 1995). Darbee used a coin toss to decide which group the first participant was allocated to (Darbee 2005). A random numbers table and block randomisation were used in only one study which used sealed envelopes to conceal allocation; the envelopes were opened in sequence, which may itself be a form of allocation bias (Newbold 2005). A computer‐generated randomisation table was used in two studies (McIlwaine 2013; Osman 2010). In one study, the allocation sequence was generated by placing 36 pieces of paper (18 PEP mask and 18 acapella) in double‐sealed envelopes one of which was opened for each enrolled participant by a research assistant not otherwise involved with the study and then the envelope was discarded (West 2010).
The remaining studies (n = 29) had an unclear risk of bias.
Concealment of allocation
We judged four studies to have a low risk of bias (McIlwaine 2013; Newbold 2005; Phillips 2004; West 2010). The McIlwaine study used a process of central allocation by an independent statistician, following which the allocation sequence was sent to the study coordinator, thus reducing the risk of bias significantly (McIlwaine 2013). The three remaining studies used sealed envelopes to conceal allocation (Newbold 2005; Phillips 2004; West 2010). Three studies were judged to have a high risk of bias (Darbee 2005; Hare 2002; Homnick 1998). Darbee used a coin toss to decide which group the first participant was allocated to, thereafter allocation was by alternation i.e. could be foretold; therefore we judged there to be a high risk of bias from this method (Darbee 2005). The other two studies with a high risk of bias also used alternate allocation (Hare 2002; Homnick 1998). In the remaining studies (n = 28), allocation concealment was not discussed and we judged these to have an unclear risk of bias.
Blinding
As the therapies being compared require participant participation and on occasion the inclusion of a device, it is not possible to blind participants and the clinicians who are implementing the treatments to the treatment group. However, it is possible to blind those individuals collecting data and assessing outcomes. Of the 35 studies available for analysis, only 11 studies identified that blinding of some or all of the outcome assessors or investigators had taken place and hence were judged to have a low risk of bias (Gondor 1999; McIlwaine 2001; McIlwaine 2013; Newbold 2005; Osman 2010; Phillips 2004; Pike 1999; Pryor 1994; Pryor 2010; van Winden 1998; West 2010). Of the remaining 24 studies, 16 were characterised as unclear on the subject of blinding, principally as this had not been discussed throughout the paper (Darbee 2005;Davies 2012; Giles 1996; Grzincich 2008; Hansen 1990; Hare 2002; Homnick 1995; Homnick 1998; Khan 2014; Kluft 1996; Lyons 1992; Marks 2001; Modi 2006a; Oermann 2001; Padman 1999a; Varekojis 2003a); eight studies had not used any recognisable means of blinding assessors and were judged to have a high risk of bias (App 1998; Arens 1994; Braggion 1995; Gotz 1995; Milne 2004; Prasad 2005; Warwick 1990; Warwick 2004).
Incomplete outcome data
Incomplete data were essentially due to participant dropout. Reasons for withdrawal were given in 13 studies, which we judged to have a low risk of bias (App 1998; Arens 1994; Homnick 1998; Kluft 1996; McIlwaine 2001; McIlwaine 2013; Milne 2004; Newbold 2005; Oermann 2001; Osman 2010; Phillips 2004; van Winden 1998; West 2010). Principally this was reported as being: due to chest infections leading to withdrawal by the investigators (App 1998; McIlwaine 2001; McIlwaine 2013; Oermann 2001); early discharge and consequent incomplete data collection (Osman 2010; West 2010); and failure to comply with the treatment regimen (Arens 1994; Kluft 1996; McIlwaine 2001; McIlwaine 2013; Newbold 2005). In the remaining 22 studies, reasons for any withdrawals that occurred were not given, leading to a potentially higher risk of bias.
Selective reporting
Many of the papers measured the outcome variables routinely and often during clinic visits. In the analysis of the papers, where possible, we have included the appropriate time points. However, it was often the case that measurements were recorded on completion of the study and it is these data that have been included in the tables of analyses. The authors found occasionally that some parameters, e.g, blood oxygen measurements, were taken but were not commented upon in the published paper. Four studies were thought to have the potential for selective reporting where information had been collected but no further comments were made; e.g., days lost from work or school although identified as being an outcome variable had not been reported in the results (App 1998; Braggion 1995; Marks 2001; Modi 2006a).
Other potential sources of bias
The possibility of bias due to order of the treatments was unlikely as in all of the studies where a cross‐over occurred, the order of treatment interventions were randomised or alternated. Study fatigue is always a consideration when using small populations such as those with CF; and indeed one study by Newbold identified this as a reason why some participants declined inclusion into the study (Newbold 2005). However, as the majority of the studies included in this review are short term, one might surmise that there was little opportunity for study fatigue to impact upon adherence and this was not an outcome we chose to measure.
The possibility of publication bias is an important point to consider. The effect of this is that published studies may not be truly representative of all valid studies undertaken, and this bias may distort meta‐analyses and systematic reviews of large numbers of studies. The problem may be particularly significant when the research is sponsored by entities that may have a financial interest in achieving favourable results.
When specific devices were used, there was some evidence of sponsorship by way of provision of equipment by the manufacturers and this may be considered as a source of bias. However, this did not necessarily favour the device over other modalities. The original authors in the seven studies where this occurred, acknowledged the manufacturers for their sponsorship (Darbee 2005; Gondor 1999; Hare 2002; McIlwaine 2013; Modi 2006a; Osman 2010; Padman 1999a). There were few reported incidences of adverse reactions to the therapy regimens implemented, and in the unlikely event of an adverse event occurring, it was not found to have occurred as a consequence of the device under scrutiny. This may be due to the safety of these devices or indeed may reflect reporting bias; however, we are unable to reach a firm conclusion about this.
One paper also reports that a natural competition between two different therapists was created leading to the potential that the data could be skewed depending on how competitive or enthusiastic the therapists were (Warwick 2004).
Three papers had a discrepancy between number of participants and a greater number of data sets collected (Homnick 1998; Pryor 2010; Varekojis 2003a). Whilst the papers acknowledged this fact, it may have led to duplication of data and a consequent skewing of results.
One study reported that the measured levels of oxygen saturation were higher at baseline in the HFCWO group than in the group using their 'usual' airway clearance techniques, which could be a potential source of bias as groups were not balanced at the beginning of the intervention (Osman 2010).
In the West study, there were clear differences at baseline for age, FEV₁, and exercise performance; participants allocated to the PEP arm of the study were older, had a greater FEV₁ and exercise ability than those in the acapella arm (West 2010). These discrepancies were attributed to the smaller than proposed sample size.
In addition, where the studies included children under 16 years of age, it is a possibility that parental influence may have occurred as there appeared to be no measures in place to eliminate or reduce this possibility.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Oscillating devices versus PEP
A total of 15 studies reported on this comparison (Braggion 1995; Darbee 2005; Davies 2012; Gotz 1995; Grzincich 2008; McIlwaine 2001; McIlwaine 2013; Newbold 2005; Oermann 2001; Osman 2010; Padman 1999a; Prasad 2005; Pryor 2010; van Winden 1998; West 2010). When comparing oscillatory devices with PEP, there were studies which used PEP alone as the control and other studies where oscillatory treatments were compared to treatment regimens which may also have included PEP, e.g. two studies compared two different oscillating devices (HFCWO, Flutter or Cornet) to PEP (Osman 2010; Pryor 2010). A number of different oscillating devices were used in this comparison: five used HFCWO (Braggion 1995; Darbee 2005; Grzincich 2008; McIlwaine 2013; Osman 2010;); five used flutter (McIlwaine 2001; Newbold 2005; Osman 2010; Pryor 2010; van Winden 1998); one used IPV (Gotz 1995); one used acapella (West 2010) and two used cornet (Prasad 2005; Pryor 2010). The Padman study was comprised of three arms comparing flutter to PEP and CPT, but in this section we present only the data from the comparison of flutter and PEP (Padman 1999a).
The Pryor study compared flutter, cornet, PEP, ACBT and AD; however, due to the multiple comparators we have not included the data in our meta‐analysis. Khan, Davies and Osman compared an oscillating device to 'usual airway clearance technique' which included several comparator interventions combined and we found it difficult to breakdown the data meaningfully and therefore have not included it in our meta‐analysis (Davies 2012; Khan 2014; Osman 2010).
Darbee measured outcomes pre‐ and post‐treatment at admission and at discharge (mean number of days in hospital was nine) (Darbee 2005). We have reported the post‐treatment values at discharge and entered these under the 'over one week and up to two week' time point.
Primary outcomes
1. Respiratory function
a. FEV₁
A total of 15 studies reported on this outcome; however, only nine had data that could be entered into the meta‐analysis (Braggion 1995; Darbee 2005; Grzincich 2008; McIlwaine 2001; McIlwaine 2013; Newbold 2005; Padman 1999a; van Winden 1998; West 2010) and six reported information we were only able to include narratively (Davies 2012; Gotz 1995; Khan 2014; Osman 2010; Prasad 2005; Pryor 2010).
Four studies reported post‐intervention data for FEV₁ per cent (%) predicted (Braggion 1995; Darbee 2005; Grzincich 2008; van Winden 1998). Grzincich recorded FEV₁ pre and 30 minutes post each treatment intervention during a three‐day study period (Grzincich 2008). Braggion evaluated the time points of up to one week in their analysis for HFCWO compared with PEP (Braggion 1995). Darbee also compared HFCWO and PEP and reported data at hospital discharge (average duration nine days) (Darbee 2005). Another study evaluated FEV₁ % predicted when comparing flutter and PEP at over two weeks and up to one month (van Winden 1998). None of these were statistically significant (Analysis 1.1).
Analysis 1.1.
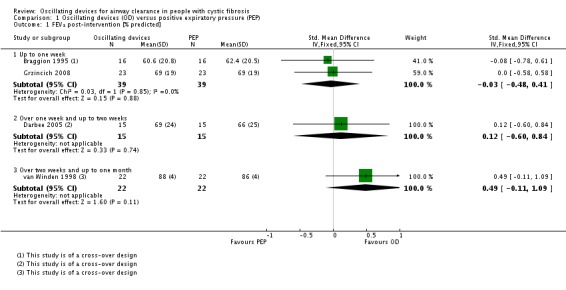
Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 1 FEV₁ post‐intervention [% predicted].
Five studies reported FEV₁ % predicted as the change from baseline (McIlwaine 2001; McIlwaine 2013; Newbold 2005; Padman 1999a; West 2010). One study reported data collected at up to two weeks (West 2010). One study reported data at over two weeks and up to one month (Padman 1999a). Only three studies published data which could be combined and which evaluated FEV₁ % predicted as change from baseline at one year (McIlwaine 2001; McIlwaine 2013; Newbold 2005). There were no statistically significant changes identified for the change from baseline in FEV₁ % predicted at any of the time points evaluated in the meta‐analysis (Analysis 1.2).
Analysis 1.2.
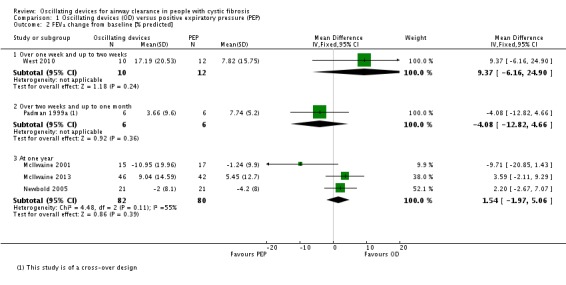
Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 2 FEV₁ change from baseline [% predicted].
There were six studies which did not have data available for inclusion in our meta‐analysis and these reported FEV₁ narratively (Davies 2012; Gotz 1995; Khan 2014; Osman 2010; Prasad 2005; Pryor 2010). The paper by Gotz considered the comparison of IPV with PEP for a period of two months; the study investigators felt IPV was not superior to PEP when FEV₁ was evaluated using a repeated measures analysis of variance, but there were no data given to support this finding (Gotz 1995). Prasad compared the cornet to PEP and the primary outcome measured was FEV₁ (Prasad 2005). However, there were no statistically significant differences identified despite a small increase in FEV₁ % predicted from baseline in both treatment arms (PEP 2.2% and cornet 4.3%) over the 12‐month study period. In concurrence with this finding, the Pryor study (n = 75) compared five different therapy techniques over a 12‐month period and found no statistically significant differences among any of the treatment modalities in the primary outcome of the change from baseline in FEV₁ % predicted (P = 0.35); the authors also noted that there was significant decline across the entire study population over the 12‐month period (P = 0.02) (Pryor 2010). Three studies comparing HFCWO to 'usual ACT' reported on FEV₁ % predicted (Davies 2012; Khan 2014; Osman 2010). Davies and Osman both reported that there was no significant change in FEV₁ between groups using HFCWO or their normal ACT (Davies 2012; Osman 2010). Khan (paper written in Russian with data translated) suggested that there was a change in FEV₁ using HFCWO as compared to control but this was not identified as significant (Khan 2014).
b. FEF25‐75
Nine studies reported on this outcome (Braggion 1995; Darbee 2005; Grzincich 2008; McIlwaine 2001; McIlwaine 2013; Newbold 2005; Padman 1999a; Pryor 2010; van Winden 1998); we were able to enter data from eight of these into the tables, but one study did not report data we could enter into our analysis (Pryor 2010).
Two studies evaluated the time points of up to one week in their analysis and considered FEF25‐75 % predicted for HFCWO compared with PEP, but there were no statistically significant results (Braggion 1995; Grzincich 2008). One further study also measured FEF25‐75 % predicted, with no statistical differences between flutter and PEP after one week and up to two weeks of treatment (Darbee 2005); the same was true of a fourth study which reported at two weeks of treatment (van Winden 1998) (Analysis 1.3).
Analysis 1.3.
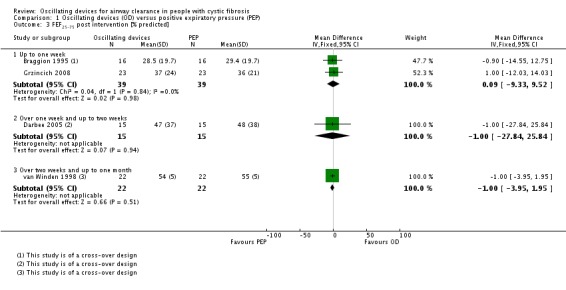
Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 3 FEF25‐75 post intervention [% predicted].
Five studies reported on the change from baseline in FEF25‐75 % predicted ; none of these results were statistically significant (Analysis 1.4). One study reported no statistically significant differences between PEP and acapella after 10 days of treatment when evaluating FEF25‐75 % predicted (West 2010), When analysed in this review at the 'up to two weeks' time point. One of these showed no statistical differences between PEP and flutter at over two weeks and up to one month (Padman 1999a). In three studies no significant difference in the mean change or annual rate of change was found when FEF25‐75 was measured at one year and compared between PEP and flutter (McIlwaine 2001; McIlwaine 2013; Newbold 2005). The main publication for the later McIlwaine study reported that FEF25‐75 % predicted was trending downwards in the HFCWO group, but increased again between visit 5 (at 9 months) and visit 6 (at 12 months). The researchers found 30 out of 46 participants in the HFCWO group required antibiotics for a pulmonary exacerbation during this time, which they suggested could be a treatment effect and may be the reason for the (statistically non‐significant) increase in FEF25‐75 % predicted (McIlwaine 2013).
Analysis 1.4.
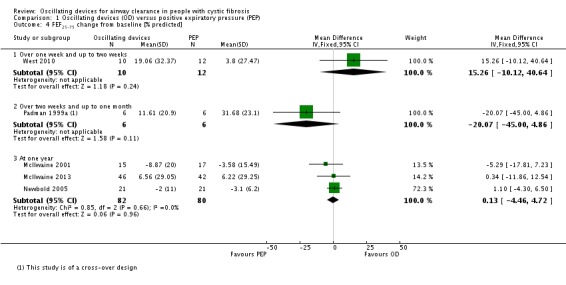
Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 4 FEF25‐75 change from baseline [% predicted].
One study reported no statistically significant differences between the treatment modalities of PEP and oscillatory devices of either flutter or cornet when evaluating FEF25‐75 (Pryor 2010).
c. FVC
Nine studies reported on this outcome (Braggion 1995; Darbee 2005; Grzincich 2008; McIlwaine 2001; McIlwaine 2013; Newbold 2005; Pryor 2010; van Winden 1998; West 2010); eight studies were included in our meta‐analysis, but again there were no data from the Pryor paper available to enter into our analysis (Pryor 2010).
Two studies evaluated the time points of up to one week in their analysis and considered FVC % predicted for HFCWO compared with PEP; there were no statistically significant changes (Braggion 1995; Grzincich 2008). Further studies showed no significant differences in FVC % predicted between flutter and PEP after over one week and up to two weeks of treatment (Darbee 2005) or after two weeks and up to one month (van Winden 1998) (Analysis 1.5).
Analysis 1.5.
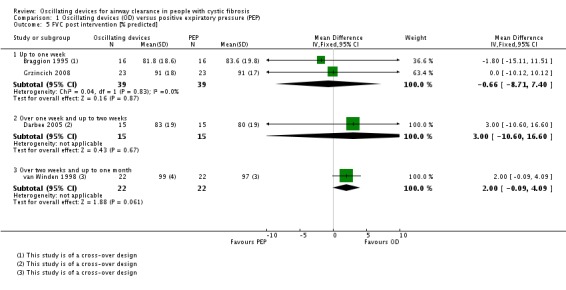
Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 5 FVC post intervention [% predicted].
The remaining four studies in our meta‐analysis reported on the change from baseline in FVC % predicted (McIlwaine 2001; McIlwaine 2013; Newbold 2005; West 2010). West compared PEP and acapella and reported data at up to two weeks (West 2010). The remaining three studies compared PEP and flutter and we found no significant difference in the mean change or annual rate of change (McIlwaine 2001; McIlwaine 2013; Newbold 2005) (Analysis 1.6). We initially analysed these data using a fixed‐effects analysis, but identified a high degree of heterogeneity (I² = 71%). As stated in our methods, we re‐analysed the data using a random‐effects analysis, but in both cases the result was not statistically significant. As for FEV₁, close contact and phone calls from study coordinators may also have contributed to increased adherence identified in the later McIlwaine study and this high adherence may explain the increase in FVC % predicted in both groups from their baseline measurements (McIlwaine 2013).
Analysis 1.6.
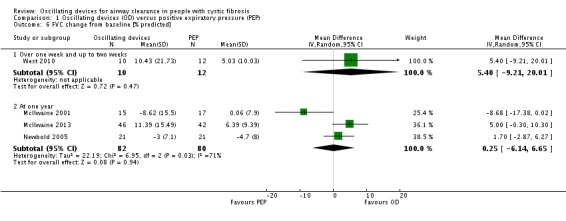
Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 6 FVC change from baseline [% predicted].
Finally, Pryor reported no statistically significant difference between any of the treatment modalities for FVC (Pryor 2010).
d. expiratory reserve volume (ERV) or reserve volume (RV)
One study reported on this outcome, but did not provide data we were able to enter into the tables (Pryor 2010).
The investigators found no statistically significant difference between the treatment modalities in the parameter of RV as a percentage of total lung capacity (Pryor 2010).
Secondary outcomes
1. Sputum
a. volume
Two studies reported on this outcome (Grzincich 2008; Khan 2014). Only one study provided data for analysis (Grzincich 2008); sputum was collected following each treatment session when HFCWO was compared with PEP but results were not statistically significant (Analysis 1.7). The second study reported that there was an increase in sputum volume when HFCWO was compared to participants' usual ACT; however there were no data included and we are unaware of what interventions were included in the usual ACT treatment arm (Khan 2014).
Analysis 1.7.
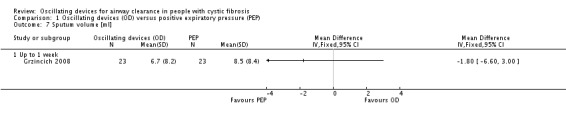
Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 7 Sputum volume [ml].
b. weight (dry or wet)
Four studies reported on this outcome; only one had data for analysis (West 2010), the remaining three had no data to enter (Braggion 1995; Davies 2012; Osman 2010). When West compared PEP to acapella this was found to clear more sputum; however, this was not statistically significant (Analysis 1.8). These data, as with any wet sputum data, may not be clinically relevant as frequently wet sputum is mixed with salivary secretions and consequently may be misinterpreted as a greater volume and or weight of sputum collected. This is a point which is true for all wet weight sputum collected and not specific to this particular paper. Two studies of the studies which did not present data reported no statistical difference in the wet or dry weight of sputum expectorated when HFCWO was compared with PEP (Braggion 1995) or 'usual' airway clearance (Davies 2012). The remaining study was of a short duration (up to one week) and the investigators reported that a significantly greater weight of sputum was yielded when using usual ACT (which included PEP) (P < 0.001) compared to HFCWO (Osman 2010).
Analysis 1.8.
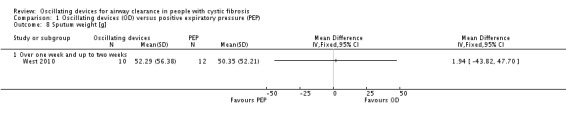
Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 8 Sputum weight [g].
2. Exercise tolerance
Two studies reported on this outcome (Pryor 2010; West 2010). West assessed exercise performance by measuring the % change in distance achieved in the modified 10 m shuttle test (West 2010). Data showed no difference between treatments at up to two weeks (Analysis 1.12); however, the authors reported that 13 out of 22 participants had an improvement in exercise performance of greater than 10%, which they considered to be clinically significant. We were unable to enter data in the meta‐analysis for the second study (Pryor 2010). The study compared both cornet and flutter as oscillatory devices to PEP (and breathing techniques of AD and ACBT which will be discussed later in this review) and there were no statistical differences reported for exercise tolerance using the modified shuttle walk test (Pryor 2010).
Analysis 1.12.
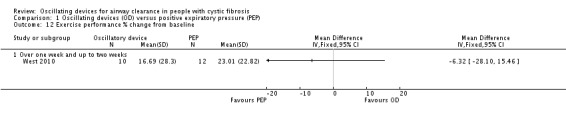
Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 12 Exercise performance % change from baseline.
3. QoL
Four studies reported on QoL indices (McIlwaine 2013; Newbold 2005; Prasad 2005; Pryor 2010). We able to enter data from the McIlwaine and Newbold studies into our analysis (McIlwaine 2013; Newbold 2005).
The McIlwaine study did not demonstrate a statistically significant difference in any domain evaluated (McIlwaine 2013). The Newbold study demonstrated that there were no significant differences between flutter and PEP in either of the two QoL scores utilised, which were the Quality of Well‐being Scale (QWBS) and the Chronic Respiratory Disease Index Questionnaire (CRQ) (Newbold 2005) (Analysis 1.9). One study comparing the cornet against PEP reported that there was a significant correlation between changes over 12 months in all parameters in the QWBS where current health was assessed, but no correlation when retrospective health over the previous 12 months was evaluated (Prasad 2005). The authors suggested the use of a more sensitive measure when evaluating perceived changes, particularly when children are considered (Prasad 2005). In the final study the investigators found no statistically significant differences among the treatment modalities in QoL indices (Pryor 2010).
Analysis 1.9.
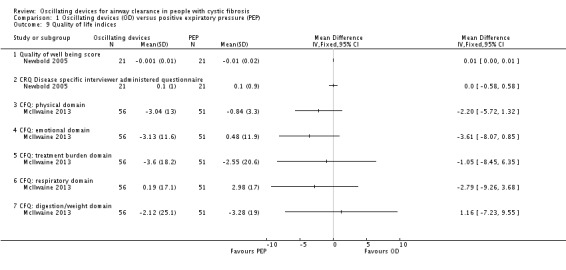
Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 9 Quality of life indices.
4. Level of oxygen saturation in response to treatment
Interpretation of oxygen saturation is done using a finger probe and is most frequently written as SaO₂; however, some studies have recorded this as SpO₂, for ease of clarity we will be consistent in our reporting and use the abbreviation of SaO₂ for oxygen saturation values. Five studies reported on this outcome, but none of these had data available for analysis (Darbee 2005; Gotz 1995; Osman 2010; Padman 1999a; van Winden 1998).
The Darbee paper reported % SaO₂ but only within group differences and not between group data, thus we were not able to enter this into our analysis (Darbee 2005). The paper stated that PEP breathing was associated with increases in SaO₂ during treatment (P < 0.00004), but HFCWO therapy resulted in decreases in SaO₂ (P < 0.00004) (Darbee 2005). One study evaluated the value of IPV compared with PEP and found there to be no significant difference between the two techniques when considering the change in SaO₂ (Gotz 1995). Padman reported that the level of oxygen saturation remained over 95% in all participants; those in the PEP group had a tendency towards a further increase, although neither result was identified as statistically significant (Padman 1999a). The Osman paper measured the change in SaO₂ where measured data were higher in the HFCWO arm at baseline, during treatment and 30 minutes following treatment (however these were not significant) and as a consequence of their study grouping multiple comparators (breathing exercises, flutter, PEP and CPT) when compared with HFCWO, we felt it was difficult to breakdown the data meaningfully and so have not included the data in the meta analysis (Osman 2010). The fifth study found no significant difference in transcutaneous SaO₂ either before, immediately after or 30 minutes after completion of the physiotherapy treatments of PEP or flutter (van Winden 1998). There was some evidence of desaturation to under 92% identified in one participant using the PEP and six participants using the flutter; in all but one of these participants this episode of desaturation lasted less than two minutes.
5. Frequency of exacerbations
Four studies reported on the frequency of exacerbations (McIlwaine 2001; McIlwaine 2013; Newbold 2005; Prasad 2005); two of which had data which could be entered into our analysis (McIlwaine 2013; Newbold 2005).
The Newbold study found no significant difference in the mean number of hospitalizations owing to pulmonary exacerbations when flutter was compared with PEP (P = 0.2) (Newbold 2005) (Analysis 1.10). In the later study by McIlwaine, the number of pulmonary exacerbations per participant was reported as 1.14 in the PEP group as compared to 2.0 per participant in the HFCWO group (P = 0.007) (McIlwaine 2013). Additionally in this study, whilst the overall incidence was low, the number of pulmonary exacerbations requiring IV antibiotics in the HFCWO group was three times more than the PEP group (19 as compared to 6). After contact with the study investigators, we have received data that can be entered into our analysis for the number of participants experiencing an exacerbation requiring antibiotics. Significantly fewer participants in the PEP group than in the HFCWO group required antibiotics for a pulmonary exacerbation, OR 4.10 (95% CI 1.42 to 11.84); however, this result was no longer significant when considering just IV antibiotics (Analysis 1.11).
Analysis 1.10.
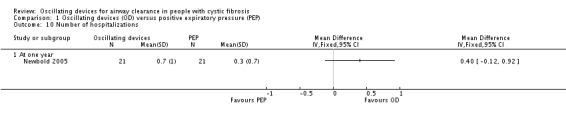
Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 10 Number of hospitalizations.
Analysis 1.11.
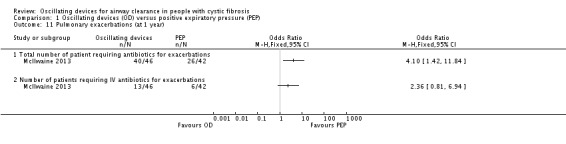
Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 11 Pulmonary exacerbations (at 1 year).
In an earlier study comparing flutter and PEP, McIlwaine suggested a statistically significant difference between the two groups in hospitalizations for a decline in pulmonary function; there were five hospitalizations in the PEP group and 18 in the flutter group (P = 0.03) (McIlwaine 2001). We were not able to enter these data in the meta‐analysis as it is not clear how many individuals experienced these hospitalizations. It is important to state that these hospitalizations did not occur until at least the sixth month of this year‐long study and there was a disproportionate number of participants using the flutter being admitted. Despite this being the case, there were no further withdrawals from the study because of significant clinical deterioration (McIlwaine 2001). The fourth study found no difference in pulmonary exacerbations requiring antibiotics when the cornet was compared with PEP over a 12‐month study period (Prasad 2005).
6. Participant‐reported satisfaction with treatment intervention
Seven studies reported on this outcome (Braggion 1995; McIlwaine 2013; Osman 2010; Padman 1999a; Prasad 2005; van Winden 1998; West 2010). Only one had data available for our analysis (West 2010).
West reported on user satisfaction using a five‐point scale to rate their satisfaction with aspects of efficacy, convenience, comfort, and overall satisfaction (West 2010). There was no significant difference between acapella and PEP (Analysis 1.13).
Analysis 1.13.
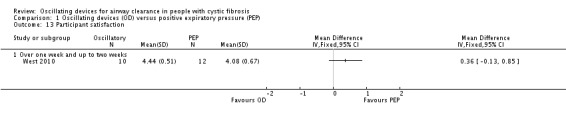
Comparison 1 Oscillating devices (OD) versus positive expiratory pressure (PEP), Outcome 13 Participant satisfaction.
Osman reported on participant satisfaction based on comfort, efficacy, preference and frequency of urinary leakage; as a consequence of their study grouping multiple comparators (breathing exercises, flutter, PEP and CPT) when compared with HFCWO. We felt it was difficult to breakdown the data meaningfully and so have not included the data in the meta analysis (Osman 2010). However, in this paper 17 participants (55%) who completed the study, expressed a preference for their usual ACT over HFCWO (Osman 2010).
Tolerance to the treatments of HFCWO and PEP were discussed in the paper by Braggion, who referred to the results as good, but without statistical or other evidence to support this finding (Braggion 1995). Similarly in the paper by McIlwaine, participant satisfaction based on comfort, independence and flexibility showed no difference in the comfort and independence parameters, but PEP scored more highly when considering flexibility (P > 0.001) (McIlwaine 2013). When flutter was compared to PEP in the study by Padman, all participants reported they felt better and ease of expectoration was cited as having improved, although there were no data provided to support this finding (Padman 1999a). Prasad compared the cornet to PEP, with no significant changes in parameters over the year‐long study or indeed between treatment groups (Prasad 2005). One child in each group withdrew, with the reasons given being the device was either too difficult to clean or they preferred their original device. There was no correlation in the decision to continue with the device at the end of the study. The remaining study reported finding no statistical differences in satisfaction between the techniques of flutter and PEP (van Winden 1998).
7. Lung clearance index (LCI)
Only one study reported on this outcome (Prasad 2005). Despite no statistical evidence of effect of the treatment, the authors felt that LCI was a more sensitive measure of abnormal lung function and further studies should be directed at the clinical relevance of LCI as an outcome measure when conventional outcomes appear normal (Prasad 2005).
Oscillating devices versus breathing techniques
Seven studies reported on this comparison (App 1998; Milne 2004; Osman 2010; Phillips 2004; Pike 1999; Pryor 1994; Pryor 2010). Again, a variety of oscillating devices were employed: three studies compared flutter to breathing techniques (App 1998; Milne 2004; Pike 1999) and two studies compared HFWCO to breathing techniques (Osman 2010; Phillips 2004). The earlier Pryor study compared flutter combined with ACBT to ACBT alone (Pryor 1994) and the later Pryor study compared flutter, cornet, PEP, ACBT and AD and reported no statistically significant differences between the techniques for any outcome (Pryor 2010). In the Osman paper, as a consequence of their study grouping multiple comparators (breathing exercises, flutter, PEP and CPT) when compared with HFCWO, we felt it was difficult to breakdown the data meaningfully and so have not included the data in the meta‐analysis (Osman 2010).
Primary outcomes
1. Respiratory function
a. FEV₁
Seven studies reported on this outcome (App 1998; Milne 2004; Osman 2010; Phillips 2004; Pike 1999; Pryor 1994; Pryor 2010). We were able to enter data from the App study for FEV₁ % predicted at the time point 'over two weeks and up to one month' into our analysis (App 1998), and these showed no significant results when comparing flutter with AD (Analysis 2.1).
Analysis 2.1.
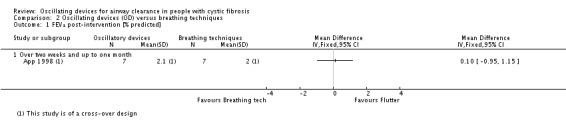
Comparison 2 Oscillating devices (OD) versus breathing techniques, Outcome 1 FEV₁ post‐intervention [% predicted].
Five of the studies were of short duration and reported results up to one week, but no data were available for analysis. The Milne study found no statistical difference between flutter and ACBT (Milne 2004). Osman reported that there was no significant change in FEV₁ between groups using HFCWO or their normal ACT (Osman 2010). In the study comparing HFCWO to ACBT, Phillips reported statistically significant results for FEV₁ (L) (P = 0.03) in favour of ACBT (Phillips 2004). The study by Pike did not report any significant differences between treatments for pulmonary function (Pike 1999). The earlier Pryor study showed no statistical differences between ACBT and flutter and ACBT combined (Pryor 1994).
The later study by Pryor, which was 12 months in duration, found no statistical differences between treatment techniques of ACBT, AD, cornet, flutter and PEP when considering FEV₁ (P = 0.35) during the study period (Pryor 2010).
b. FEF25‐75
One study reported on this outcome and the investigators found no statistical difference between flutter and ACBT (Milne 2004).
c. FVC
Six studies reported on this outcome (App 1998; Milne 2004; Phillips 2004; Pike 1999; Pryor 1994; Pryor 2010)); but we were only able to analyse data from one of these (App 1998).
App found no significant changes throughout the study period of one month when comparing flutter and AD in a cross‐over study (Analysis 2.2). The investigators did, however, identify a tendency towards improvement of up to 6.5% from baseline in both groups, but attributed this to non‐specific improvement or the possibility of a training effect (App 1998).
Analysis 2.2.
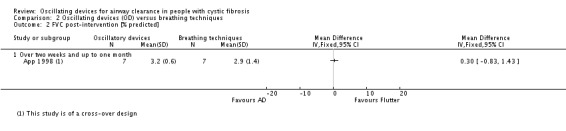
Comparison 2 Oscillating devices (OD) versus breathing techniques, Outcome 2 FVC post‐intervention [% predicted].
Four studies were of short duration and reported results up to one week, but no data were available for analysis. Phillips compared HFCWO with the breathing techniques of ACBT and reported statistically significant results for FVC in favour of ACBT (Phillips 2004). The remaining three short‐duration studies demonstrated no significant differences between treatments for pulmonary function (Milne 2004; Pike 1999; Pryor 1994).
The later 12‐month study by Pryor found no statistical differences between treatment techniques of ACBT, AD, cornet, flutter and PEP (Pryor 2010).
d. ERV or RV
No studies reported on this outcome.
Secondary outcomes
1. Sputum
a. volume
One study reported on this outcome and provided data to enter into the analysis (App 1998).
App considered the use of flutter and AD, but there was no statistically significant difference between the two techniques, despite acknowledging a tendency for expectorated sputum volume to be greater following treatment with the flutter regardless of therapeutic order (Analysis 2.3).
Analysis 2.3.
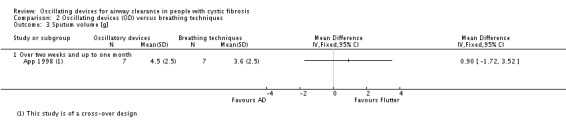
Comparison 2 Oscillating devices (OD) versus breathing techniques, Outcome 3 Sputum volume [g].
b. weight (dry or wet)
Five studies reported on this outcome (Milne 2004; Osman 2010; Phillips 2004; Pike 1999; Pryor 1994); but data were only available for the analysis from one study (Milne 2004).
The Milne data showed no significant difference in sputum weight in the short‐term study when flutter was compared to ACBT (Analysis 2.4).
Analysis 2.4.
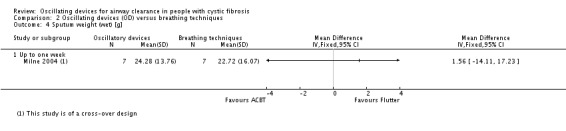
Comparison 2 Oscillating devices (OD) versus breathing techniques, Outcome 4 Sputum weight (wet) [g].
In the study by Phillips, it was stated that the weight of expectorated sputum was greater with sessions of ACBT that with HFCC, but this was not significant at the 24‐hour time point (Phillips 2004).
Pike also considered the outcome of wet sputum weight (Pike 1999). Using the cross‐over paired t‐test and McNemar's Chi² test for statistical analysis, they found no significant differences between treatments of flutter and ACBT when measuring wet sputum weight. As with the Pryor study, one of the monitored sessions was in supine (Pike 1999; Pryor 1994). It is not clear whether the participants had previously carried out their flutter therapy prior to adopting this position. It is not possible to use flutter in a postural drainage position other than sitting unless adaptations were made, and the implementation of adaptation was not apparent from the study methodology. Pryor considers the variables of ACBT alone versus ACBT with flutter (Pryor 1994). They report that there was a significant increase in the weight of sputum expectorated (P < 0.001) when ACBT alone was used.The remaining study was of a short duration (up to one week) and the investigators demonstrated that a significantly greater weight of sputum was yielded when using usual airway clearance techniques (of which included breathing techniques) (P < 0.001) compared to HFCWO (Osman 2010).
2. Exercise tolerance
One study reported on this outcome and found no statistical differences between treatment techniques of ACBT, AD, cornet, flutter and PEP when considering modified shuttle walk scores (Pryor 2010).
3. QoL
One study reported on this outcome and found no statistical differences between treatment techniques of ACBT, AD, Cornet, Flutter and PEP when considering the QoL score of CRQ and Short Form‐36 (Pryor 2010).
4. Level of oxygen saturation in response to treatment
Two studies reported on this outcome, but neither had any data available for analysis (Osman 2010; Pike 1999). In the cross‐over trial with two treatments per day, Pike reported that there was no statistically significant difference in SaO₂ between treatments (flutter or ACBT) (Pike 1999). The Osman paper measured the change in SaO₂ where measured data were higher in the HFCWO arm at baseline, during treatment and 30 minutes following treatment; however, these were not significant. As previously stated, due to the study design we felt it was difficult to breakdown the data meaningfully and so have not included the data in the analysis (Osman 2010).
5. Frequency of exacerbations as a consequence of the treatment intervention
There were no studies reporting on this outcome for this comparison.
6. Participant reported satisfaction with treatment intervention
FIve studies of short duration (up to two weeks) reported on this outcome, but none had any data which we could enter into our analysis (Milne 2004; Osman 2010; Phillips 2004; Pike 1999; Pryor 1994).
Milne considered satisfaction and whether participants were likely to change their preferred therapy following the study period; there were no statistical differences in satisfaction between the techniques and approximately 45% chose to continue with flutter either independently or in conjunction with ACBT (Milne 2004). Osman considered participant satisfaction in terms of comfort, efficacy and urinary leakage; however, these data are from combined interventions and we were unable to breakdown the data meaningfully in order to include it in our meta‐analysis (Osman 2010). The investigators in the Osman study identified that 55% of their study population preferred their normal ACT compared to HFCWO; in this study the normal ACT was either ACBT or AD 83% of control participants (Osman 2010). Phillips found that all participants in their cohort of 10 found the technique of ACBT to be comfortable (Phillips 2004). In the same study, 40% of participants found the HFCWO to be comfortable, but difficult to clear secretions (80%); however, all participants felt ACBT made it easier to clear secretions (Phillips 2004). Participant preference was reported in the Pike study, with 100% recommending ACBT and 55% flutter (P < 0.008) (Pike 1999). Comfort and convenience were considered in the Pryor study, with the addition of effect on breathlessness and if the participants were likely to change their regimen based on preference (Pryor 1994). Here participants found both treatments easy to use but most preferred ACBT due to helpfulness at clearing secretions. The three participants who did indicate a preference for flutter had discontinued within the month following completion of the study (Pryor 1994).
7. Lung clearance index
No studies reported on this outcome.
Oscillating devices versus conventional physiotherapy
A total of 16 studies reported on this comparison (Arens 1994; Braggion 1995; Giles 1996; Gondor 1999; Hansen 1990; Hare 2002; Homnick 1995; Homnick 1998; Kluft 1996; Lyons 1992; Modi 2006a; Padman 1999b; Osman 2010; Varekojis 2003a; Warwick 1990; Warwick 2004). There were three studies considering multiple treatment arms where more than one oscillatory device was compared to conventional chest physiotherapy (Modi 2006a; Padman 1999a; Varekojis 2003a). For each of these studies a duplicate reference was created to enable data from both types of oscillatory device to be entered into the analysis.
Six studies compared HFCWO to CPT (Arens 1994; Braggion 1995; Hansen 1990; Kluft 1996; Warwick 1990; Warwick 2004); three studies compared flutter to CPT (Giles 1996; Gondor 1999; Homnick 1998); and two studies compared IPV to CPT (Hare 2002; Homnick 1995). Four further studies compared multiple oscillating devices and CPT (Modi 2006a; Osman 2010; Padman 1999b; Varekojis 2003b). One study compared flutter, HFCWO and PD&P (Modi 2006a; Modi 2006b); another study compared flutter, PEP and CPT (Padman 1999a; Padman 1999b); the third study compared IPV, HFCWO and PD&P (Varekojis 2003a; Varekojis 2003b). These three studies presented data for different oscillating devices compared to a single arm of conventional physiotherapy and these data will be presented separately in this review as follows: flutter versus PD&P (Modi 2006a), HFCWO versus PD&P (Modi 2006b), flutter versus PEP (Padman 1999a), flutter versus CPT (Padman 1999b), IPV versus PD&P (Varekojis 2003a) and HFCWO versus PD&P (Varekojis 2003b). As a consequence of the Osman study grouping multiple interventions (breathing exercises, flutter, PEP and CPT) when compared with HFCWO, we have not included the results in the meta‐analysis (Osman 2010).
Primary outcomes
1. Respiratory function
a. FEV₁
A total of 10 studies reported 11 data sets on this outcome (Arens 1994; Braggion 1995; Giles 1996;Gondor 1999; Hare 2002; Homnick 1995; Homnick 1998; Modi 2006a; Modi 2006b; Osman 2010; Padman 1999b).
Four studies reported on absolute post treatment values for FEV₁ % predicted which we entered into our analysis (Braggion 1995; Giles 1996; Gondor 1999; Homnick 1995). These were reported at different time points and we were only able to combine data for the time point 'up to one week' (Analysis 3.1). Braggion compared FEV₁ % predicted for HFCWO compared with CPT and reported data at the time point of 'up to one week' but found no statistically significant differences between groups (Braggion 1995). The Gondor paper presents absolute data at day 7 and day 14 for this outcome, neither of which showed a significant difference between groups in our analysis (Analysis 3.1). However, the Gondor paper reports that there was a significant improvement in FEV₁ in both treatment groups over the two‐week treatment period, with the flutter group having a significantly higher increase from baseline by day 7 than the CPT group; further increases in FEV₁ from day 7 to the end of treatment were not significant and the investigators did not find any statistical difference between the treatment groups (Gondor 1999). Giles also reported this outcome and demonstrated no significant difference between flutter and CPT during or after the treatment periods (Giles 1996). The Homnick data also showed no significant differences in FEV₁ % predicted between IPV and CPT at the end of the six‐month study period (Homnick 1995).
Analysis 3.1.
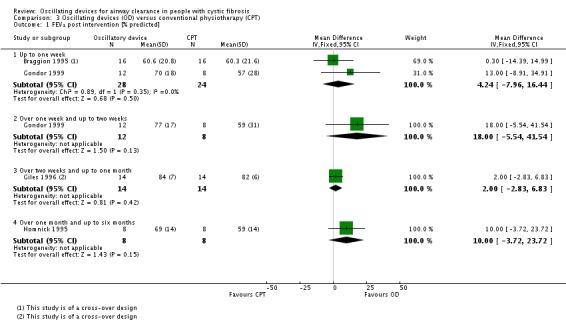
Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 1 FEV₁ post intervention [% predicted].
Hare noted significant improvements from admission to discharge in the IPV group for FEV₁, but data were not provided to support this claim (Hare 2002). In the later Homnick study, investigators found no significant differences between flutter and CPT in % predicted FEV₁ (Homnick 1998).
Two studies reported data on the change in FEV₁ % predicted from baseline which we were able to enter into the analysis (Arens 1994; Padman 1999b). Arens reported data for the change from baseline in FEV₁ % predicted at time points of 'up to one week' and 'over one week and up to two weeks' for the comparison between HFCWO and CPT (Arens 1994); Padman reported data for this outcome at 'over two weeks and up to one month' (Padman 1999b). Analysis showed no significant differences between treatment groups at any time point (Analysis 3.2).
Analysis 3.2.

Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 2 FEV₁ change from baseline [% predicted].
Osman reported that there was no significant change in FEV₁ between groups using HFCWO or their normal ACT (Osman 2010). As already stated, these data are from several comparator interventions combined and not included it in our meta‐analysis.
In the Modi study, no differences were identified between the three therapies (PD&P, FD, HFCWO) in FEV₁ % predicted (Modi 2006a; Modi 2006b). The data presented in the paper can not be analysed here as they report longitudinal decline in respiratory function as % predicted from baseline adjusted for BMI rather than a post intervention or change from baseline measure of lung function.
b. FEF25‐75
Eight studies reported nine data sets on this outcome (Arens 1994; Braggion 1995; Gondor 1999; Hare 2002; Homnick 1995; Homnick 1998; Modi 2006a; Modi 2006b; Padman 1999a). Only five had data suitable for analysis; of these, four presented data for absolute values of FEF25‐75 % predicted (Braggion 1995; Gondor 1999; Homnick 1995) (Analysis 3.3) and two presented change data (Arens 1994; Padman 1999a) (Analysis 3.4).
Analysis 3.3.
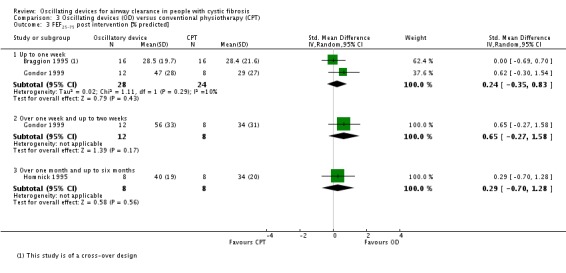
Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 3 FEF25‐75 post intervention [% predicted].
Analysis 3.4.
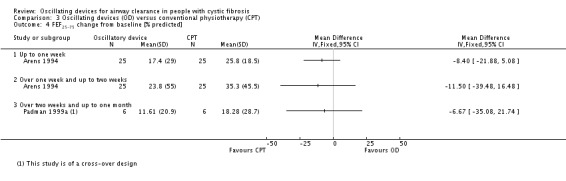
Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 4 FEF25‐75 change from baseline [% predicted].
Two studies evaluated FEF25‐75 % predicted at the time point of up to one week (Braggion 1995; Gondor 1999). There were no statistically significant differences when comparing HFCWO with CPT (Braggion 1995), or when comparing flutter with CPT (Gondor 1999). The combined results also showed no significant difference (Analysis 3.3). In the earlier Homnick study, no significant differences were noted between IPV and CPT at the end of the six‐month study period (Homnick 1995).
Arens reported on the change from baseline in FEF25‐75 % predicted; the analysis did not show any statistically significant differences between HFCWO and CPT at either up to one week or over one week and up to two weeks (Arens 1994). Padman presented data for the time point over two weeks and up to one month which were similarly non‐significant (Analysis 3.4).
Two studies did not present data which could be entered into the meta‐analysis (Hare 2002; Homnick 1998). Significant improvements were described in the Hare study from hospital admission to discharge in the IPV group for FEF 25‐75, but data were not provided to support this claim when IPV was compared with CPT (Hare 2002). The later Homnick study reported no significant difference in FEF25‐75 % predicted at hospital discharge after admission for an exacerbation (Homnick 1998).
In the Modi study no statistically significant differences were identified between PD&P and FD or PD&P and HFCWO for FEF25–75% predicted however with FD and HFCWO there was considered to be a significant difference P=0.035 (Modi 2006a; Modi 2006b). The data presented in the paper can not be analysed here as they report longitudinal decline in respiratory function as % predicted from baseline adjusted for BMI rather than a post intervention or change from baseline measure of lung function.
c. FVC
Seven studies (eight data sets) reported on this outcome (Braggion 1995; Giles 1996; Gondor 1999; Hare 2002; Homnick 1995; Homnick 1998; Modi 2006a; Modi 2006b). Four of which had data suitable for analysis (Braggion 1995; Giles 1996; Gondor 1999; Homnick 1995).
Two studies evaluated FVC % predicted at up to one week in a comparison of HFCWO and CPT (Braggion 1995) and flutter and CPT (Gondor 1999). When entered into our meta‐analysis the data show no significant differences between treatment groups (Analysis 3.5). In the study by Giles, there was no significant difference between flutter and CPT during or after the treatment period of over two weeks and up to one month (Giles 1996). In the earlier Homnick study, no significant differences were noted between IPV and CPT at the end of the six‐month study period (Homnick 1995).
Analysis 3.5.
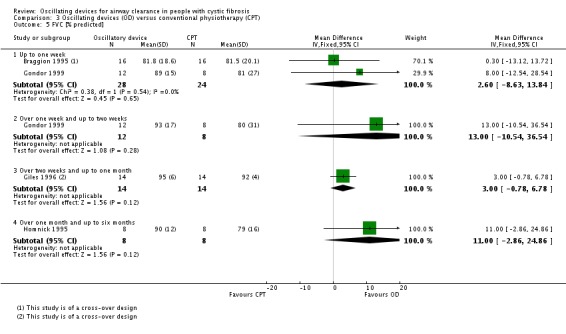
Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 5 FVC [% predicted].
The later Homnick study compared flutter to CPT and reported no significant difference in FVC % predicted at hospital discharge after admission for an exacerbation (Homnick 1998). Hare compared IPV to CPT and also noted no significant difference between treatment groups at the end of the treatment period (Hare 2002).
In the Modi study, no differences were identified between the three therapies (PD&P, FD, HFCWO) in FVC % predicted (Modi 2006a; Modi 2006b).The data presented in the paper can not be analysed here as they report longitudinal decline in respiratory function as % predicted from baseline adjusted for BMI rather than a post intervention or change from baseline measure of lung function.
d. ERV or RV
Three studies reported on this outcome (Arens 1994; Hare 2002; Homnick 1998). Data were only available from the Arens study for the change from baseline at time points of 'up to one week' and 'over one week and up to two weeks' (Arens 1994). There was no significant difference for RV when comparing HFCWO and CPT at either time point (Analysis 3.6).
Analysis 3.6.
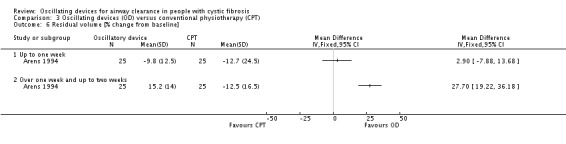
Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 6 Residual volume [% change from baseline].
The remaining two studies reported on time points of 'up to two weeks' (Hare 2002; Homnick 1998). In the Hare study, significant improvements were noted from admission to discharge in the IPV group for RV, but again no data were supplied to support this finding (Hare 2002). In the study by Homnick, no significant differences were found between flutter and CPT, although RV improved significantly in each group from baseline to discharge (Homnick 1998).
Secondary outcomes
1. Sputum
a. volume
Two studies of short duration with time points of 'up to one week' reported on this outcome, but data were not suitable to enter into our analysis (Hansen 1990; Lyons 1992).
Hansen compared the HFCWO with CPT and reported a statistical difference in the volume of mucus cleared in favour of HFCWO (P < 0.001) (Hansen 1990). In the Lyons paper, the authors report the only statistically significant variable was sputum volume, where less sputum was produced on the "flutter only" day (P = 0.0015) (Lyons 1992). The suggestion therefore made by the authors is that flutter cannot be substituted for CPT.
b. weight (dry or wet)
Eight studies reported nine sets of information on this outcome (Arens 1994; Braggion 1995; Giles 1996; Kluft 1996; Osman 2010; Varekojis 2003a; Varekojis 2003b; Warwick 1990; Warwick 2004). There are two sets of data from the Varekojis study, one for IPV compared to PD&P (Varekojis 2003a) and one for HFCWO compared to PD&P (Varekojis 2003b). Six data sets were available to enter into the analysis from five studies (Arens 1994; Giles 1996; Kluft 1996; Varekojis 2003b; Warwick 2004).
FIve studies presented six sets of data for up to one week for both dry (Analysis 3.7) and wet sputum weight (Analysis 3.8). There were no overall significant differences between oscillating devices and CPT for either outcome at this time point (Analysis 3.7; Analysis 3.8). Although not significant in our analysis, Kluft reported a significant result in favour of HFCWO in the published paper for dry weight (P < 0.01, using a Wilcoxon signed rank test) (Kluft 1996). The results from the Kluft study for wet sputum weight significantly favoured oscillating devices compared to CPT when entered into our analysis, MD 3.90 g (95 % CI 0.08 g to 7.72 g) (Kluft 1996). When the duration of sampling was further analysed it would appear that the sputum was collected over a six‐day period (Kluft 1996), yielding a greater sampling period than the other studies in the comparison which were collected over a 1‐hour to a 24‐hour period. One study reported sputum weights at over one week and up to two weeks (Warwick 2004); and only one further study reported sputum weights over two weeks (Giles 1996).
Analysis 3.7.
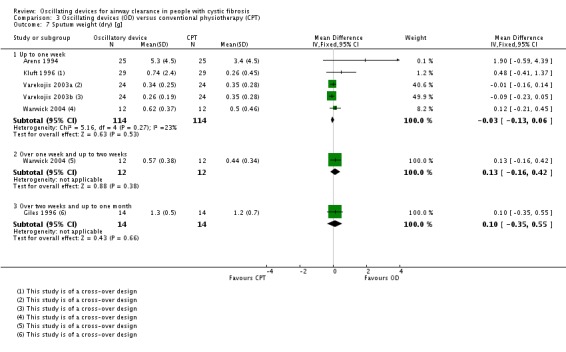
Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 7 Sputum weight (dry) [g].
Analysis 3.8.
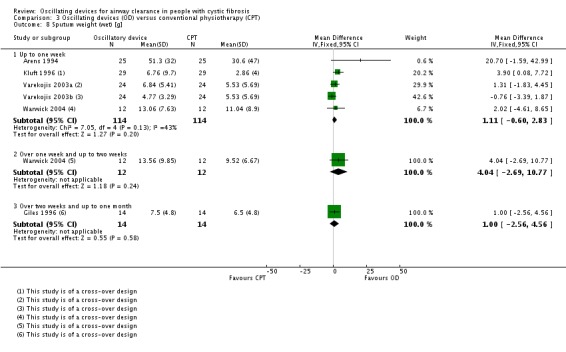
Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 8 Sputum weight (wet) [g].
The remaining three studies do not have data available to enter into the meta‐analysis. The first of these studies, no data were provided, but the investigators noted that there was no significant difference in sputum weight (either wet or dry) between the HFCWO group or the CPT group when measured at the end of the treatment period (Braggion 1995). The second study was of a short duration (up to one week) and the investigators demonstrated that a significantly greater weight of sputum was yielded when using breathing techniques (P < 0.001) compared to HFCWO (Osman 2010). However, as a consequence of this study using multiple comparators we found it difficult to break down the data meaningfully and have not included the data in the meta‐analysis. In the remaining study, Warwick measured both wet and dry sputum weight following treatments of either CPT and HFCWO in his 1990 abstract. He found that there was no statistical difference (P = 0.221) for the wet weights but a significant difference for dry weights (P = 0.046)favouring the HFCWO in the 13 pairs of samples analysed (Warwick 1990).
2. Exercise tolerance
One study reported on this outcome using the six‐minute walk test (Gondor 1999). The analysis showed no significant differences between treatment groups when walk distance was evaluated after two weeks of treatment (Analysis 3.9).
Analysis 3.9.
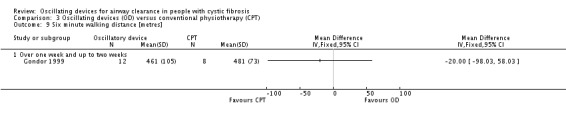
Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 9 Six minute walking distance [metres].
3. QoL
The Modi study reports health‐related QoL for 12 domains in the CFQ (Modi 2006a; Modi 2006b). The ITT analysis of data from the final questionnaire revealed a mean (SD) difference between treatment groups only in the domain of 'Body Image' (PD&P = 87.9 (3.1), flutter = 82.6 (3.4), HFCWO = 78.2 (3.1), P = 0.03). The change in each CFQ domain from baseline to the first or fifth assessment after randomization, and the change from baseline to the final CFQ assessment obtained (ITT analysis) only showed a difference in the 'Social' domain at the final assessment. However, after correcting for multiple comparisons in the CFQ analyses, these results were non‐significant. The CFQ 'Respiratory Domain' score was positively correlated with the overall satisfaction score in the Treatment Satisfaction Survey (TSS) at the final assessment point (R = 0.23, P < 0.006).
4. Level of oxygen saturation in response to treatment
Four studies reported on this outcome (Arens 1994; Gondor 1999; Osman 2010; Padman 1999b), but only one provided data which could be entered into our analysis (Arens 1994).Data from both time points, up to one week and over one week and up to two weeks, did not favour either treatment (Analysis 3.10). However, the clinical relevance of this result is questionable as frequently activities such as ACTs correspond with a transient decrease in SaO₂ and there is no suggestion that it did not return to normal pre‐treatment values within a reasonable time period (e.g. 20 minutes).
Analysis 3.10.
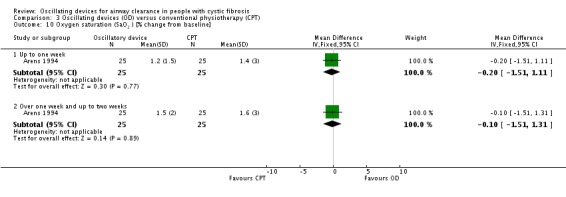
Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 10 Oxygen saturation (SaO2 ) [% change from baseline].
Gondor monitored SaO₂ during the study period and reported no significant differences between treatment with either flutter or CPT (Gondor 1999). Padman reported that SaO₂ was higher than 95% in all participants, with no statistical difference between treatments (Padman 1999b). The Osman paper measured the change in SaO₂ during treatment and 30 minutes following treatment; SaO₂ levels were higher in the HFCWO arm at baseline, however, the differences between groups were not significant (Osman 2010). As already stated, the study grouped multiple comparators (breathing exercises, flutter, PEP and CPT) to compare with HFCWO and so we have not included the data in the meta‐analysis.
5. Frequency of exacerbations as a consequence of the treatment intervention
Four studies reported on this outcome, with three providing data for analysis on the number of days of hospitalisation (Arens 1994; Gondor 1999; Homnick 1995; Modi 2006a; Modi 2006b). Our analysis showed no significant difference between oscillating devices or CPT at either 'up to one week' or 'over one week and up to two weeks' (Analysis 3.11). The Modi study reported no significant difference in the time to next pulmonary exacerbation across the comparators (Modi 2006a; Modi 2006b).
Analysis 3.11.
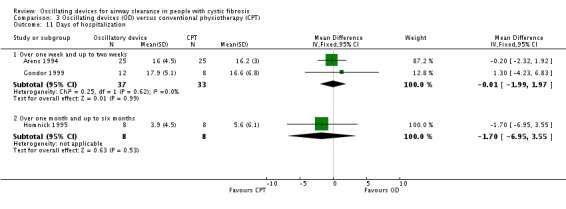
Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 11 Days of hospitalization.
6. Participant reported satisfaction with treatment intervention
Nine studies with eleven separate comparisons reported on this outcome (Arens 1994; Braggion 1995; Giles 1996; Hare 2002; Homnick 1995; Modi 2006a; Modi 2006b; Osman 2010; Padman 1999b; Varekojis 2003a; Varekojis 2003b). We were only able to enter data from the Varekojis study and the Modi study in our analysis for this outcome (Modi 2006a; Modi 2006b; Varekojis 2003a; Varekojis 2003b).
In the short term, Varekojis looked at the comparisons of IPV and PD&P (Varekojis 2003a) and HFCWO and PD&P (Varekojis 2003b). There was no statistical difference reported by the investigators in either treatment arm compared to PD&P when participant satisfaction was measured by Friedmans test. Similarly, our analysis also showed no significant difference between groups (Analysis 3.12). Conversely, when Modi reported on the long‐term treatment satisfaction (up to three years) looking at subsets of comfort, convenience and efficacy as well as overall satisfaction, significant differences were found for all measures favouring both flutter and HFCWO over PD&P (Analysis 3.13). When the investigators carried out sub‐analysis of the data according to age, they found that oscillating devices gave adolescent participants a degree of independence from their care givers which may have impacted on their improved preference over PD&P. It should be noted that the last reported TSS scores were associated with participant withdrawal from the study, indicating individuals with lower TSS were more likely to withdraw. (Modi 2006a; Modi 2006b).
Analysis 3.12.
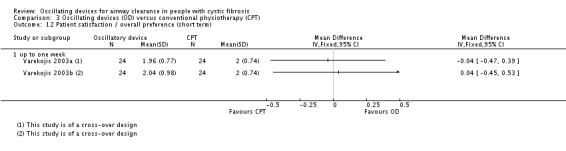
Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 12 Patient satisfaction / overall preference (short term).
Analysis 3.13.
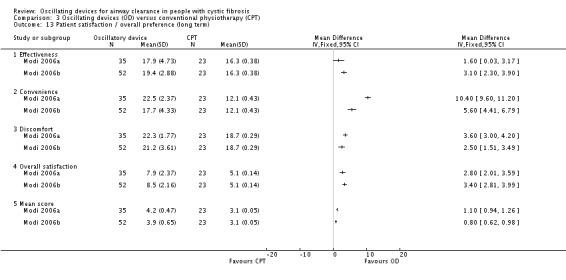
Comparison 3 Oscillating devices (OD) versus conventional physiotherapy (CPT), Outcome 13 Patient satisfaction / overall preference (long term).
Arens reported participant satisfaction from the HFCWO arm of the trial only; 88% (22 participants) in this treatment group expressed satisfaction with this technique and requested this therapy in the management of further exacerbations (Arens 1994). Braggion discussed tolerance to the treatments of HFCWO and PD&P and referred to the results as "good", but without statistical or other evidence to support this finding (Braggion 1995). Giles reported that flutter was preferred by participants based on comfort and convenience (Giles 1996). In the paper by Hare, participants in the IPV group were reported to be generally satisfied with the device (Hare 2002). Homnick evaluated participant satisfaction in the IPV group (the questionnaire was not given to the CPT group) and reported that all eight respondents would continue to use the IPV device if given the opportunity (Homnick 1995).In the Padman study, it was reported that participants felt they were in control of all their therapies, felt physically better and mucus was more easily expectorated with no preference given to any modality (Padman 1999b). The Osman study considered participant satisfaction in terms of comfort, efficacy and urinary leakage; however, these data are from combined interventions and we felt we were unable to breakdown the data meaningfully therefore have not included this data in the meta‐analysis. Investigators reported that 55% of the study population preferred their normal ACT compared to HFCWO (Osman 2010).
7. Lung clearance index
No studies reported on this outcome.
Different oscillating devices compared
Six studies (eight data sets) compared different oscillating devices (Marks 2001; Modi 2006a; Modi 2006b; Oermann 2001; Osman 2010; Pryor 2010; Varekojis 2003a; Varekojis 2003b). One study compared flutter and IPV (Marks 2001); three data sets compared flutter to HFCWO (Modi 2006a; Modi 2006b; Oermann 2001; Osman 2010); one study compared flutter to cornet (Pryor 2010); and the final study compared IPV and HFCWO (Varekojis 2003a; Varekojis 2003b). In order to avoid making this review more complicated, we have only listed below the outcomes for which we have any information. As a consequence of the investigators of the Osman study grouping several interventions (breathing exercises, flutter, PEP and CPT) when compared with HFCWO, we were unable to breakdown the data meaningfully and therefore have not included the results in the meta‐analysis; however, we would suggest that the reader consider the previous sections for general statements on relevant results within this paper (Osman 2010).
Flutter compared to IPV
Primary outcomes
1. Respiratory function
a. FEV₁
Marks reported no significant difference (P = 0.208) at the end of the 24‐week treatment period (Marks 2001).
b. FEF25‐75
Marks reported no significant difference (P = 0.126) at the end of the 24‐week treatment period (Marks 2001).
c. FVC
Marks reported no significant difference (P = 0.292) at the end of the 24‐week treatment period (Marks 2001).
Secondary outcomes
5. Frequency of exacerbations as a consequence of the treatment intervention
No difference was reported between groups when frequency of hospitalisations or need for home intravenous therapies was considered (Marks 2001).
6. Participant‐reported satisfaction with treatment intervention
Marks reported that IPV was well‐tolerated with 67% of participants wanting to continue using it instead of other ACTs (Marks 2001).
Flutter compared to HFCWO
Primary outcomes
1. Respiratory function
a. FEV₁
Three reported on this outcome (Modi 2006a; Oermann 2001; Osman 2010). Modi reported non significant change over the 12 months period between the different comparators (Modi 2006a). Oermann was a cross‐over trial and we have presented data from the first arm of the trial only (at one month) (Oermann 2001). Oermann reports absolute values for FEV₁ % predicted and the analysis shows these were not statistically significant, although tending to favour flutter (Analysis 4.1). The Osman study reported that no statistically significant change in FEV₁ % predicted was observed within or between either regimen of HFCWO or usual ACTs when compared with baseline (data not able to be meaningfully analysed in this review) (Osman 2010).
Analysis 4.1.
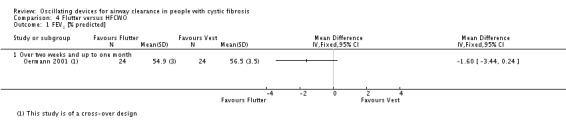
Comparison 4 Flutter versus HFCWO, Outcome 1 FEV1 [% predicted].
b. FEF25‐75
Oermann also reported on FEF25‐75 % predicted and again we have presented first‐arm data only (Oermann 2001). Absolute values for FEF25‐75 % predicted were not statistically significant, although again tending to favour flutter (Analysis 4.2).The Modi study reported that there was considered to be a significant difference in FEF 25‐75 between FD and HFCWO P=0.035 (Modi 2006a).
Analysis 4.2.
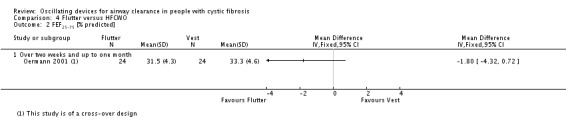
Comparison 4 Flutter versus HFCWO, Outcome 2 FEF25‐75 [% predicted].
c. FVC
Oermann reported on this outcome with data for FVC % predicted and we have presented only data from the first arm of the trial (Oermann 2001). Results for FVC % predicted were not statistically significant, but tended to favour flutter (Analysis 4.3). Modi reported a non significant change over the 12 months period between the different comparators (Modi 2006a).
Analysis 4.3.
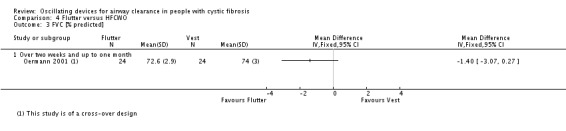
Comparison 4 Flutter versus HFCWO, Outcome 3 FVC [% predicted].
Secondary outcomes
4. Level of oxygen saturation in response to treatment
One study reported on this outcome but, as stated above, we have not been able to include any data in the meta‐analysis (Osman 2010).
6. Participant‐reported satisfaction with treatment intervention
Three studies reported on this outcome (Modi 2006a; Modi 2006b; Oermann 2001; Osman 2010), but we were only able to enter data into the analysis for one of these (Modi 2006a; Modi 2006b). Modi reported on differences between flutter and HFCWO regarding treatment satisfaction (Modi 2006a; Modi 2006b) with results clearly favouring flutter for convenience, whilst all other scores showed no difference, but the strong result for convenience means that the overall score is just significant in favour of flutter. Oermann also conducted a participant satisfaction survey considering efficacy, convenience and comfort. Whilst no significant difference was found between therapies for comfort, flutter was found to score significantly more for convenience (P < 0.02), as was seen in the Modi study, and HFCWO scored highest for efficacy (P < 0.02) (Oermann 2001). However, the investigators also reported that 13% of participants preferred their pre‐study therapy regimen of PD&P because of familiarity with the technique (Oermann 2001). Osman considered participant satisfaction in terms of comfort, efficacy and urinary leakage; the study identified that 55% of their study population preferred their normal ACT compared to HFCWO (Osman 2010).
Flutter compared to Cornet
Primary outcomes
1. Respiratory function
a. FEV₁
Pryor reported no statistical differences between treatment techniques of flutter and cornet when considering FEV₁ (P = 0.35) (Pryor 2010).
c. FVC
Pryor found no statistical differences between flutter and cornet for FVC (Pryor 2010).
Secondary outcomes
2. Exercise tolerance
Pryor used the modified shuttle walk score and found no statistical differences between flutter and cornet (Pryor 2010).
3. QoL
Pryor used the CRQ to assess QoL and found no statistical differences between flutter and cornet (Pryor 2010).
IPV and HFCWO
Secondary outcomes
1. Sputum
b. weight (dry or wet)
In the Varekojis study, the investigators collected 142 sputum samples from the IPV group and 143 samples from the HFCWO group. The paper states that the wet sputum weight in the IPV group was significantly greater than in the HFCWO group (P < 0.05, by Tukey's honest significant difference test) (Varekojis 2003a; Varekojis 2003b). However, on inspection of the data it became apparent that this evaluation was based on the number of samples in the analysis rather that the number of participants and their relevant corresponding sputum samples.The number of samples compared were not equal; 24 participants were included in the study with six sets of sputum data anticipated for each treatment option. However, some of the sputum cups were contaminated by hemetemesis (vomiting of blood), one dried prior to wet weight being measured and one sputum cup was lost prior to weighing, which accounts for the discrepancy in terms of sputum samples across the intervention groups.
6. Participant‐reported satisfaction with treatment intervention
Using a Friedmans test comparing the HFCWO and IPV, Varekojis reported no significant difference in preference between the techniques (Varekojis 2003b).
Discussion
Summary of main results
The initial aim of this review was to determine whether oscillatory devices as used in cystic fibrosis (CF) were effective for airway clearance and, if so, were they equivalent to, or superior to other recognised airway clearance techniques or devices. Outcomes included pulmonary function, sputum weight and volume, individual preference, quality of life (QoL) measures and number of hospitalisations per study period. Frequency of exacerbations was identified as an outcome and analysed as "days of hospitalizations" throughout the literature reviewed. Single‐treatment studies were excluded. There were no studies identified through the search process that compared exercise with any form of oscillatory device, or indeed where the acapella or Quake® were included as a comparison with any other form of airway clearance. We have also not yet been able to include data from studies of the newer devices (Metaneb® and VibraLung®). Therefore, these have not been included in the analysis or any further comments made. None of the intended subgroup analyses were possible due to either the small numbers of studies or to insufficient detail allowing the separating of subgroup data within any study.
There were no significant differences between participants on enrolment to studies when considering demographics, spirometry, anthropometrics and clinical scores. Most studies identified improvements in outcome measures from the beginning to the end of the study periods, although between‐group differences were most frequently not significant. Where there have been small but significant changes in secondary outcome variables, such as sputum volume or weight, this has not been wholly in favour of oscillatory devices. Sputum weight and volume may be considered to be somewhat misleading as an outcome variable as some individuals have difficulty expectorating and have a tendency to swallow their secretions. This can therefore significantly alter the results obtained.
It is the authors' opinion that oscillatory devices can be effective in clearing secretions, but despite evidence showing improvement in sputum volume, there is no statistically significant evidence to suggest that the use of these devices is superior to other physiotherapy techniques when respiratory function is the primary outcome in the short term. A study by Newbold compared flutter with positive expiratory pressure (PEP) in a 13‐month intervention and found there to be no statistical differences in respiratory function or health‐related QoL over the study period (Newbold 2005). In the most recent study looking at high frequency chest wall oscillation (HFCWO) versus PEP over a 12‐month period, results significantly favour the use of PEP over HFCWO when considering the number of exacerbations requiring antibiotics (but not those specifically requiring intravenous antibiotics) occurring in each group (McIlwaine 2013). When comparing the different types of oscillatory devices, there have been no statistical differences noted between any of the primary or secondary outcomes evaluated. On occasion, there were reported preferences for flutter over HFCWO; however, these did not reach statistical significance. It would appear therefore that oscillatory devices are a recognised therapy, but they are not superior to any other form of airway clearance or that one device is superior to another. It should also be acknowledged that longer‐term studies are essential when considering the differences between alternative airway clearance therapies.
Overall completeness and applicability of evidence
The literature appears to be representative of the airway clearance techniques available to participants with CF. Apart from the lack of evidence with regard to either acapella, Quake® or exercise, all therapy techniques are included, with recognition that alternative devices are in development (e.g., the Metaneb® and VibraLung®) and we await further information before being able to fully present these options. The literature also includes representation from both children and adults with mild to severe disease. Oscillatory devices have been compared with each other and all other recognised airway clearance techniques.
A total of 20 studies involved flutter as a comparison, 15 studies included HFCWO, five included IPV, two included cornet and one included acapella. There were no studies comparing, Quake®, Metaneb® or VibraLung® with any other treatment. These are more recently developed devices and that may account for the limited, or lack of, literature evaluating the efficacy of these devices. These devices may be included in future comparative studies as the variety of treatment options become more readily available for all people with CF.
Most studies have been short term and the literature recognises that short‐term studies demonstrating improved sputum clearance have not demonstrated preservation of respiratory function,decreased morbidity or shown improved QoL over the long term (Varekojis 2003a; Varekojis 2003b). Modi was unable to identify differences in clinical effectiveness as measured by FEV₁ decline over the duration of this three‐year study; however, perceived effectiveness as suggested by treatment satisfaction and convenience may lead to better adherence and result in long‐term improvements (Modi 2006a; Modi 2006b). In 2004, Milne also reported that in short‐term studies, it would be unlikely that changes, if they existed, would be apparent in single‐treatment days (Milne 2004). Whilst we did not include studies where one treatment session was compared with another, we did include those studies where a single day of treatment was included, if the therapy was conducted more than once during that day.
The greater the consistency between the primary studies in a meta‐analysis, the more generalisable are the results. Heterogeneity refers to substantial differences between studies rather than those that occur by chance. We planned to test for heterogeneity using the I² statistic (Higgins 2003); however, due to the limited data available for meta‐analysis, testing for heterogeneity was not always applicable.
Three instances of moderate to high heterogeneity were identified in our analysis. In the test for subgroup differences in the analysis, an I² value of 59% was calculated for the comparison oscillating devices versus PEP for the change from baseline in mid‐peak expiratory flow (FEF25‐75) (% predicted) between one study lasting one month (Padman 1999a) and three studies with a study period of one year (McIlwaine 2001; McIlwaine 2013; Newbold 2005); we believe this is due to the difference in study duration (Analysis 1.4). Between the same three studies, a value for I² of 71% was calculated for the change from baseline in FVC (% predicted) when we analysed the data using a fixed‐effect model (McIlwaine 2001; McIlwaine 2013; Newbold 2005). We re‐analysed these data using a random‐effects model, but the result remained statistically not significant (Analysis 1.6). These studies seem to be very diverse; however, due to the low number of studies, we were unable to investigate the causes of these instances of heterogeneity further.
Of the studies reviewed, 26 were less than three months in duration; and of these, 13 were considered to be of less than one‐week duration.
It is recognised that three of the studies have multiple treatment arms and in the analysis we have considered each treatment separately (Modi 2006a; Modi 2006b; Padman 1999a; Padman 1999b; Varekojis 2003a; Varekojis 2003b). It is potentially possible to conduct a multiple treatment analysis where all the treatments are assessed simultaneously across studies; however, it was not within the capabilities of the authors to conduct such an analysis, but this should be considered in future studies to ensure clarity of the meta‐analysis.Two of the studies included in the 2017 update have compared oscillatory devices with 'usual' airway clearance techniques, but the data for each individual technique were not identifiable and the authors were not able to extract data for analysis.
Quality of the evidence
The following limitations all highlight the need for further good quality RCTs.
Methodological quality of included studies
For the purposes of this review we have included only randomised controlled trials (RCTs) and quasi‐RCTs, which are the highest quality of research studies available. This has led to the inclusion of 35 individual studies, one third of which were published only as abstracts, thus limiting the amount of salient information we were able to retrieve from them with respect to methodology and data available for analysis.
Few studies were considered to be of relatively high methodological quality, and therefore at a low risk of bias, e.g. where there was definite evidence of allocation concealment and blinding of some of the researchers (Newbold 2005; Phillips 2004; McIlwaine 2013; West 2010). Only four studies reported blinded allocation to treatment, it is not possible to blind the participants to the physiotherapy interventions included in this review; however, in nine studies there was evidence that those researchers who were collecting lung function or sputum samples or performing other relevant testing were blinded to the treatment intervention. The potential for a high risk of bias due to lack of blinding in all these studies cannot be excluded.
A sensitivity analysis for those outcomes where data from parallel studies were compared with data from cross‐over studies was performed (Analysis 3.2; Analysis 3.3; Analysis 3.4; Analysis 3.5; Analysis 3.7; Analysis 3.8). The results however remained non‐significant and we conclude that the results are therefore robust.
Small numbers
There were relatively small numbers of participants enrolled in the included studies (range 5 to 166) and 50% of those studies included children. We appreciate the hypotheses by Newbold that adults have “fixed damage” and therefore less potential improvement as a consequence of chest physiotherapy (CPT) and that also the mean rate of decline in adults tends to be slower (Newbold 2005). This may have an impact on the accuracy of evidence when measuring the effect of a device on lung function irrespective of the duration of the study.
Inconsistency in study design
The included studies employed different interventions and outcome measures and therefore many could not be combined for inclusion into the meta‐analysis. As CPT is delivered by a therapist it must be considered that differences in technique delivery may have an impact on results achieved. This factor is most apparent in the Warwick study where competition between therapists was encouraged (Warwick 2004).
Applicability to present day practice
Ten of the included studies were conducted over 20 years ago. Applicability of the results to present day practice may be compromised due to changes in population characteristics and interventions, including more aggressive management of lung infection and improvements in antibiotics.
Quality of evidence
The quality of the evidence was assessed using the GRADE methodology and four Summary of Findings tables were generated (one for each comparison presented) (Table 1; Table 2; Table 3; Table 4). All of the included studies were graded as having low or very low quality evidence. Downgrading of evidence occurred for a number of reasons including small sample size, short duration of treatment and the use of a cross‐over design rather than a parallel design. There were also inadequacies noted in allocation concealment, a lack of blinding and incomplete data which suggested a high risk of bias. Anecdotal evidence and limited information on some of the intervention methodology further downgraded the evidence. This low or very low quality evidence suggests that if further research was conducted and resolution of these inadequacies existed then results would significantly influence the confidence in the estimate of effect of the interventions.
Potential biases in the review process
Several of the studies reported that they had received funding from the manufacturers of specific devices. In particular Hill‐Rom (manufacturers of "the Vest®") sponsored three studies (Darbee 2005; Modi 2006a; Osman 2010). Two of the studies evaluating flutter were provided with the devices by Scandipharm (Gondor 1999; Padman 1999a); and one intrapulmonary percussive ventilation (IPV) study was provided with the equipment by Vortran Ltd (Hare 2002). One study comparing HFCWO and PEP were loaned or provided with the devices respectively (McIlwaine 2013).
The risk of bias due to the carry‐over effect is also a major problem of combined design meta‐analysis on the assumption that the first period is devoid of bias, but this may lead to a biased subset of studies in a meta‐analysis and it is the price of a less efficient treatment estimate (Curtin 2002b). There is a possibility that this type of bias may have occurred in our meta‐analyses since the pooled data includes 11 studies of a cross‐over design and six of a parallel design. In addition, whilst the studies have considered treatment of participants when they are in a "stable" state and generally excluded participants who have had an increase in symptomology (either before inclusion or during the study) it is possible that participants have had different disease severity or levels of anxiety associated to their disease. As stated by Curtin, "such differences in study populations could be a source of heterogeneity in the treatment effect which is not caused by the design but confounded by it" (Curtin 2002a). As there were insufficient studies with data to allow sub‐group analysis it is not possible to further investigate the issue of heterogeneity.
Two studies reported flutter therapy in the supine position, but no supportive evidence was given to suggest how this was achieved (Pike 1999; Pryor 1994). Under normal circumstances, the manufacturers' guidelines are to use the flutter in a sitting position.
In his 1998 study, Homnick also suggested that the sample size of each comparison group should be 219 participants to achieve 80% power when considering FEV₁ as a quality primary outcome variable (Homnick 1998). With this factor in mind, all of the studies included in this review would be under‐powered and consequently any evidence of no improvement should be regarded with some caution, as the issue of power is more important when the findings are of no difference between interventions.
One particular study reported that when some of the eligible participants were approached regarding entry to the study, they felt that they were happy with their current therapy regimen and on occasion they felt they had been over‐studied (Newbold 2005).
Adherence to therapies has a major impact upon outcome measures; however, few of the studies considered this as a factor when evaluating the treatment intervention. Only those participants who adhered to twice‐daily treatment (based on diary records) at a level of 85% were included in the McIlwaine study (McIlwaine 2001). Although we did not specifically address adherence as an outcome measure, we did consider other subjective parameters such as QoL indices, tolerance and participant‐reported satisfaction, all of which ultimately impact upon a person's adherence to the therapy in question. Interest in participating in a study due to perceived effectiveness of a treatment, may have led to improved adherence and treatment satisfaction (McIlwaine 2013). Close contact and phone calls from study coordinators may also have contributed to increased adherence identified in the McIlwaine study; and the high adherence may explain the significant increase in percent predicted FEV₁ in both groups from their baseline measurements, which was reported in the paper (no data provided) (McIlwaine 2013).
One further point is the pooling of data between adults and children. Monitoring of the flutter technique is more challenging in children than adults, where the child may be less sensitive to the requirement for adjusting the gradient of the flutter to enable optimum oscillation. This could therefore reflect a difference in outcome if, in children, the treatment is not being optimised because of lack of understanding of the technique. However, if in practice children and adults are treated the same, a random‐effects analysis will be appropriate to give the average treatment effect across all adults and children.
Agreements and disagreements with other studies or reviews
The literature recognises that there is little, if any, evidence to support the use of one airway clearance technique or device over another. The findings of this review agree with the previous Cochrane Reviews which looked at PEP physiotherapy and CPT in people with CF (McIlwaine 2015; Main 2005).
Authors' conclusions
Individual preference continues to be a factor when introducing a airway clearance technique or therapy adjunct, such as an oscillatory device. It is also important to consider the impact the device may have on the individual at particular stages of their disease. It would appear no single treatment technique is suitable for everyone and the therapist delivering airway clearance should be well‐educated in all aspects of airway clearance and associated therapy techniques. This would enable the appropriate selection and inclusion of airway clearance techniques or devices into the management of the individual. As there is no appreciable difference between the devices or therapies used in airway clearance, the healthcare provider should consider a cost‐benefit analysis for their individual patients based on financial burdens and possible insurance cover where appropriate. In particular, where the frequency of exacerbations was shown to be increased whilst using the Vest® when compared to the PEP in the recent McIlwaine study (McIlwaine 2013), this may have significant resource implications for the individual and the healthcare provider. Individual preference and acknowledgement of personal health beliefs are also important, as is age‐appropriateness of the therapy techniques, which may have a considerable impact on concordance with therapies suggested or offered.
Many of the studies included QoL scales and satisfaction questionnaires; however, few incorporated measures of adherence. When there is no marker of superiority between airway clearance techniques, it may be prudent to include time to next exacerbation, frequency of exacerbations, individual preference, adherence to therapy and general satisfaction with treatment as potential outcome measures in further studies of these techniques. As a consequence of adherence to therapy, we may then see improvements in other parameters such as exercise tolerance and respiratory function.
Most of the studies reviewed were of short duration i.e. less than three months (n = 26), and of these 13 were of less than one week duration. Only 12 studies were of longer duration, which in this review extended to 2.8 years (Modi 2006a). This would suggest for the future researcher that longer‐term studies would add more weight to the perceived benefit of airway clearance techniques or therapy devices or both. It has been suggested in adherence literature that the introduction of new novel therapies increases the individual adherence for up to a three‐month "honeymoon" period after which time the individual tends to resort to previous levels of adherence. This should be considered by the designers of future studies when deciding on the duration of their study. More adequately‐powered long‐term RCTs (parallel or cross‐over in design) need to be included in this review before clinically valuable information can be gained with regard to treatment efficacy and safety.
Acknowledgements
The review authors would like to acknowledge the previous contribution of Jennifer Agnew who was part of the original review team.
The review authors would like to acknowledge the patient assistance of our Managing Editors, Mrs Nikki Jahnke (without whose invaluable support this review would not have reached completion), Miss Tracey Remmington, and our editor, Professor Alan Smyth.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Data and analyses
Comparison 1.
Oscillating devices (OD) versus positive expiratory pressure (PEP)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV₁ post‐intervention [% predicted] | 4 | Std. Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Up to one week | 2 | 78 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.48, 0.41] |
| 1.2 Over one week and up to two weeks | 1 | 30 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.12 [‐0.60, 0.84] |
| 1.3 Over two weeks and up to one month | 1 | 44 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.49 [‐0.11, 1.09] |
| 2 FEV₁ change from baseline [% predicted] | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Over one week and up to two weeks | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 9.37 [‐6.16, 24.90] |
| 2.2 Over two weeks and up to one month | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | ‐4.08 [‐12.82, 4.66] |
| 2.3 At one year | 3 | 162 | Mean Difference (IV, Fixed, 95% CI) | 1.54 [‐1.97, 5.06] |
| 3 FEF25‐75 post intervention [% predicted] | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Up to one week | 2 | 78 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐9.33, 9.52] |
| 3.2 Over one week and up to two weeks | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐27.84, 25.84] |
| 3.3 Over two weeks and up to one month | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐3.95, 1.95] |
| 4 FEF25‐75 change from baseline [% predicted] | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Over one week and up to two weeks | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 15.26 [‐10.12, 40.64] |
| 4.2 Over two weeks and up to one month | 1 | 12 | Mean Difference (IV, Fixed, 95% CI) | ‐20.07 [‐43.00, 4.86] |
| 4.3 At one year | 3 | 162 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐4.46, 4.72] |
| 5 FVC post intervention [% predicted] | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Up to one week | 2 | 78 | Mean Difference (IV, Fixed, 95% CI) | ‐0.66 [‐8.71, 7.40] |
| 5.2 Over one week and up to two weeks | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 3.0 [‐10.60, 16.60] |
| 5.3 Over two weeks and up to one month | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐0.09, 4.09] |
| 6 FVC change from baseline [% predicted] | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 Over one week and up to two weeks | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 5.40 [‐9.21, 20.01] |
| 6.2 At one year | 3 | 162 | Mean Difference (IV, Random, 95% CI) | 0.25 [‐6.14, 6.65] |
| 7 Sputum volume [ml] | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Up to 1 week | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Sputum weight [g] | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8.1 Over one week and up to two weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Quality of life indices | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Quality of well being score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 CRQ Disease specific interviewer administered questionnaire | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.3 CFQ: physical domain | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.4 CFQ: emotional domain | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.5 CFQ: treatment burden domain | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.6 CFQ: respiratory domain | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.7 CFQ: digestion/weight domain | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Number of hospitalizations | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 10.1 At one year | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Pulmonary exacerbations (at 1 year) | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 11.1 Total number of patient requiring antibiotics for exacerbations | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11.2 Number of patients requiring IV antibiotics for exacerbations | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 12 Exercise performance % change from baseline | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1 Over one week and up to two weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Participant satisfaction | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1 Over one week and up to two weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2.
Oscillating devices (OD) versus breathing techniques
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV₁ post‐intervention [% predicted] | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Over two weeks and up to one month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 FVC post‐intervention [% predicted] | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Over two weeks and up to one month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Sputum volume [g] | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Over two weeks and up to one month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Sputum weight (wet) [g] | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Up to one week | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3.
Oscillating devices (OD) versus conventional physiotherapy (CPT)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV₁ post intervention [% predicted] | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Up to one week | 2 | 52 | Mean Difference (IV, Fixed, 95% CI) | 4.24 [‐7.96, 16.44] |
| 1.2 Over one week and up to two weeks | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 18.0 [‐5.54, 41.54] |
| 1.3 Over two weeks and up to one month | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [‐2.83, 6.83] |
| 1.4 Over one month and up to six months | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | 10.0 [‐3.72, 23.72] |
| 2 FEV₁ change from baseline [% predicted] | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Up to one week | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 Over one week and up to two weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 Over two weeks and up to one month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 FEF25‐75 post intervention [% predicted] | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 3.1 Up to one week | 2 | 52 | Std. Mean Difference (IV, Random, 95% CI) | 0.24 [‐0.35, 0.83] |
| 3.2 Over one week and up to two weeks | 1 | 20 | Std. Mean Difference (IV, Random, 95% CI) | 0.65 [‐0.27, 1.58] |
| 3.3 Over one month and up to six months | 1 | 16 | Std. Mean Difference (IV, Random, 95% CI) | 0.29 [‐0.70, 1.28] |
| 4 FEF25‐75 change from baseline [% predicted] | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Up to one week | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Over one week and up to two weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Over two weeks and up to one month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 5 FVC [% predicted] | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Up to one week | 2 | 52 | Mean Difference (IV, Fixed, 95% CI) | 2.60 [‐8.63, 13.84] |
| 5.2 Over one week and up to two weeks | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 13.0 [‐10.54, 36.54] |
| 5.3 Over two weeks and up to one month | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 3.0 [‐0.78, 6.78] |
| 5.4 Over one month and up to six months | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | 11.0 [‐2.86, 24.86] |
| 6 Residual volume [% change from baseline] | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Up to one week | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Over one week and up to two weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Sputum weight (dry) [g] | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Up to one week | 5 | 228 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.13, 0.06] |
| 7.2 Over one week and up to two weeks | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [‐0.16, 0.42] |
| 7.3 Over two weeks and up to one month | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.35, 0.55] |
| 8 Sputum weight (wet) [g] | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Up to one week | 5 | 228 | Mean Difference (IV, Fixed, 95% CI) | 1.11 [‐0.60, 2.83] |
| 8.2 Over one week and up to two weeks | 1 | 24 | Mean Difference (IV, Fixed, 95% CI) | 4.04 [‐2.69, 10.77] |
| 8.3 Over two weeks and up to one month | 1 | 28 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐2.56, 4.56] |
| 9 Six minute walking distance [metres] | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Over one week and up to two weeks | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Oxygen saturation (SaO2 ) [% change from baseline] | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 10.1 Up to one week | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐1.51, 1.11] |
| 10.2 Over one week and up to two weeks | 1 | 50 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐1.51, 1.31] |
| 11 Days of hospitalization | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 11.1 Over one week and up to two weeks | 2 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐1.99, 1.97] |
| 11.2 Over one month and up to six months | 1 | 16 | Mean Difference (IV, Fixed, 95% CI) | ‐1.70 [‐6.95, 3.55] |
| 12 Patient satisfaction / overall preference (short term) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 12.1 up to one week | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13 Patient satisfaction / overall preference (long term) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 13.1 Effectiveness | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.2 Convenience | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.3 Discomfort | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.4 Overall satisfaction | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 13.5 Mean score | 2 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4.
Flutter versus HFCWO
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 [% predicted] | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 Over two weeks and up to one month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 FEF25‐75 [% predicted] | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 2.1 Over two weeks and up to one month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 FVC [% predicted] | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3.1 Over two weeks and up to one month | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Treatment satisfaction (long term) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4.1 Effectiveness | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.2 Convenience | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.3 Discomfort | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.4 Overall satisfaction | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4.5 Mean score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Analysis 4.4.
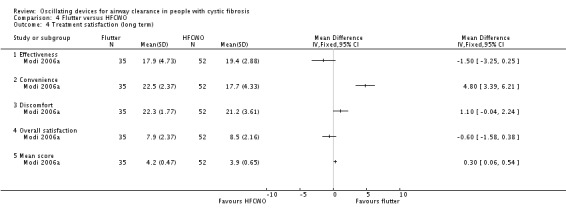
Comparison 4 Flutter versus HFCWO, Outcome 4 Treatment satisfaction (long term).
What's new
Last assessed as up‐to‐date: 12 June 2017.
| Date | Event | Description |
|---|---|---|
| 29 June 2017 | Amended | Contact details updated. |
History
Protocol first published: Issue 4, 2007 Review first published: Issue 1, 2009
| Date | Event | Description |
|---|---|---|
| 12 June 2017 | Amended | Error in Abstract corrected ‐ a sentence referring to a previously included study, which was excluded at the 2017 update (Orlik 2001), had been left in the Results section of the Abstract; this sentence has now been removed. |
| 26 April 2017 | New search has been performed | A search of the Cystic Fibrosis and Genetic Disorders Review Group's Cystic Fibrosis Trials Register identified 15 references to 10 studies for possible inclusion in this update of the review. A total of three new studies have been included at this update. Two were newly identified studies (one reference each) (Khan 2014; West 2010) and one further reference was an additional reference to a study previously listed as 'Awaiting classification' which is now also included (Davies 2012). One study was only available in abstract form (two references) and has been listed as 'Awaiting classification' until further details are available to allow a judgement on inclusion or exclusion (Herrero 2016). 10 references to six studies were excluded (Amelina 2014; Dwyer 2017; Fainardi 2011; Grosse‐Onnebrink 2017; Kempainen 2007; O'Neil 2017). Following communication with the authors of the previously included Orlik studies, we established that there were no random elements in the allocation procedure of these studies. These therefore do not meet the review's inclusion criteria and have been excluded at this 2017 update (Orlik 2000a; Orlik 2000b; Orlik 2001). |
| 26 April 2017 | New citation required but conclusions have not changed | Despite the inclusion of new studies, our conclusions remain the same. Jennifer Agnew has stepped down from the review team and a new author, Stephanie Innes, has joined. |
| 11 August 2014 | Amended | An error in the text of the plain language summary has been corrected. |
| 17 July 2014 | New search has been performed | A search of the Group's Cystic Fibrosis Trials Register identified 14 new references. A further study was identified through further hand searching (Borka 2012). Two of the new references were additional references to already included studies (Lyons 1992; Modi 2006a). One new study (five references) has been included (McIlwaine 2013). Five studies,with single references to each, were excluded (Borka 2012; Dosman 2003; Dunn 2013; Jarad 2010; Van Ginderdeuren 2008). Three new studies have been listed as 'Awaiting classification' (Davies 2012a; Patel 2013; Wheatley 2013). A reference previously listed under 'Studies awaiting classification' was an additional reference to an already included study (Modi 2006a). In the 'Types of interventions' we have included two new types of devices that have recently come onto the market, both of which have an oscillatory component to their function. The VibraLung (VL) is an acoustic percussor and incorporates positive expiratory pressure. The MetaNeb is a pneumatic compressor system delivering continuous high frequency oscillation and positive expiratory pressure. |
| 17 July 2014 | New citation required but conclusions have not changed | Despite the inclusion of a new study with 92 participants, the conclusions of our review remain the same (McIlwaine 2013). |
| 3 December 2010 | New search has been performed | A search of the Group's Cystic Fibrosis Trials Register identified three new references to two new studies (Grzincich 2008; Kraemer 1996). We included the Grzincich study as this compared HFCWO with PEP (Grzincich 2008). We excluded the two references to the Kraemer study as they did not compare oscillation with another form of physiotherapy treatment (Kraemer 1996). We identified the full paper to a previously identified abstract and this now supercedes all previous references and has been included in this review (Osman 2010). We also identified the full paper to another previously included study (Modi 2006a) and this is listed as 'Awaiting classification' until the next update when we will include any new data. Three studies which were listed as 'Awaiting classification' have now been translated and assessed for eligibility.These studies were included in this updated review (Orlik 2000c; Orlik 2000c; Orlik 2001a). |
| 10 November 2008 | Amended | Converted to new review format. |
Differences between protocol and review
Update 2017
We originally planned to group outcome data those measured at one, three, six, 12 months and annually thereafter. We subsequently considered these time points and felt that to combine data measured at two weeks with data measured at four weeks was inappropriate. Therefore we have split the original proposed time point of one month and separately reported data at up to two weeks and at over two weeks and up to one month.
We have also amended the wording of our eligibility criteria so it is clear we are only considering randomised or quasi‐randomised studies and not all controlled clinical studies (which may not have any random element involved in allocation to treatment groups).
Update 2014
Two further devices (MetaNeb® and VibraLung®) have been added to the list of possible oscillatory devices in 'Types of interventions'. The data for these devices are only included in abstract form and we await full publication or additional information. The references to the studies of these devices are included under 'Characteristics of studies awaiting classification'.
Update 2010 Since the publication of the protocol there has been a new version of the RevMan software released. The full review has been developed using the RevMan 5 programme and consequently there are several sections now included which were previously not available.
A further device (Quake®) has been added to the list of possible oscillatory devices in 'Types of interventions'. There are currently no trial data published for this device.
Original review A new team of review authors have worked on the review and taken on the protocol from the previous review team. They have added a further planned subgroup analysis which they felt was clinically relevant. Furthermore in a second post hoc change, they decided to perform a sensitivity analysis including and excluding the studies with a cross‐over design to assess whether the study design had an effect on the results.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | RCT. Cross‐over design. Duration: 4 weeks. Location: multicentre in Germany. |
|
| Participants | 17 participants initially randomised. 3 drop outs reported (1 for time reasons and the other 2 for acute chest exacerbation), therefore 14 (6 males, 8 females) analysed (7 in each treatment group). Age range 4 ‐ 41 years, mean (SD) 19.6 (10.3) years. Participants had a positive diagnosis of CF by means of sweat test or clinical history or both. | |
| Interventions | Flutter versus AD twice daily for 4 weeks. | |
| Outcomes | Respiratory function (FEV₁, FVC) and sputum volume. | |
| Notes | This paper also considered the implications of the flutter on sputum viscoelasticity but this was not an outcome measured in this review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Abstract states randomised cross‐over design; however the methodology does not report any details of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Abstract does not report any details of allocation concealment. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not possible to blind participants and clinicians. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were 3 dropouts occurring after randomisation; 1 for time reasons and the other 2 for acute chest exacerbation. ITT not discussed. |
| Selective reporting (reporting bias) | Unclear risk | Blood oxygen saturations were taken, but there are no data to support a change in this parameter if it were to have occurred during the study or as a consequence of the intervention. |
| Other bias | Unclear risk | None identified. |
| Methods | RCT. Parallel design. Location: single centre in USA. Duration: 2 weeks, follow up not stated. |
|
| Participants | 50 (32 males, 18 females) participants randomised. Age range 16.9 ‐ 24.9 years. Participants with CF and an acute exacerbation who had been admitted to hospital. | |
| Interventions | HFCWO for 30 min 3x daily in sitting whilst receiving nebuliser. CPT 30 min 3x daily in 6 different PD positions, following 15 min of nebuliser. 25 participants randomised to each treatment group. Treatment 2 weeks in duration. | |
| Outcomes | Respiratory function (VC, FEV₁, FEF and RV), sputum weight in g both wet and dry at 1 hour and 24 hours. Participants reported satisfaction with technique and % change in Sa0₂. Outcome measurements taken at admission, 7 days and 14 days. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported how sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding of assessors or participants. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 dropouts were identified and this was due to failure to comply with the therapy regimen. |
| Selective reporting (reporting bias) | Unclear risk | Not possible to compare original trial protocol with final paper. |
| Other bias | Unclear risk | None identified. |
| Methods | RCT. Cross‐over design (1‐day washout between treatments). Location: single centre in Italy. Duration: 2 days for each of 3 treatments with 1 rest day in between treatment 1 and 2. No follow up reported. |
|
| Participants | 16 (8 males, 8 females) participants. Mean (SD) age 20.3 (4) years, range 15 ‐ 27 years. All participants had FEV₁ >40%, sputum volume >30 ml/day and were accustomed to ACTs. Mean (SD) Schwachmann score 65.1 (11). Mean (SD) Crispin Norman score 18.5 (4.3). | |
| Interventions | 3 interventions: PD (specific PD positions were not identified); PEP; HFCWO. 15 min saline nebulised prior to treatment. 2 treatments per day for 2 days, then rest 1 day; next intervention for 2 days, then 1 rest day; then the final intervention. Each session lasting 50 min (not clear if this included the 15 min of nebulisation). | |
| Outcomes | RFTs (FEV₁) 30 minutes pre‐ and post‐treatment, wet and dry sputum weight collected in 50 min of treatment and 30 min following. Only spontaneous coughs were allowed and the number of cough manoeuvres were counted and documented. Each treatment was scored for efficacy and tolerance by participant and for tolerance by therapist (method of efficacy or tolerance scoring was not defined). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised according to Latin square design described by Williams. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of participants or assessors not performed. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Withdrawals had not been discussed |
| Selective reporting (reporting bias) | High risk | Efficacy and tolerance for the treatments were scored by the participant, and tolerance was also scored by the physiotherapist. These were then referred to as good but with no further evaluation of this score made. |
| Other bias | Unclear risk | None identified. |
| Methods | Quasi‐RCT Cross‐over design. Location: single centre in USA. Duration: average length of hospital stay was 11 days (range 9 ‐ 15 days); no follow‐up reported. |
|
| Participants | 15 participants (8 males, 7 females). Aged at least 7 years, mean (SD) age 17.5 (4.2) years. Participants were admitted to hospital for acute exacerbation. All participants performed HFCWO 1 ‐ 3 times daily as outpatients before admission, but none had performed PEP. | |
| Interventions | PEP versus HFCWO. Both treatments were alternated within 48 hours of hospital admission and then reversed prior to discharge. Treatment lasted 30 minutes. | |
| Outcomes | RFTs and SaO₂ measured before and after every intervention. Each intervention was only done twice i.e. day 1 or 2 following admission then day ‐1 or ‐2 prior to discharge. | |
| Notes | Average length of hospital stay was 11 days (range 9 ‐ 15 days). 3 participants discharged while still receiving intravenous antibiotics, for these participants the final measurement was taken within 48 hours of the final dose of antibiotic. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were assigned to treatment order by numbering them consecutively, 1 through 15, at study entry. On the basis of a coin toss at admission, participant 1 and all odd‐numbered participants were randomly assigned to perform HFCWO on day 1 and PEP breathing on day 2, and even‐numbered participants performed PEP breathing on day 1 and HFCWO on day 2. At discharge, participants received treatment in the order opposite the treatment order at admission. |
| Allocation concealment (selection bias) | High risk | Used alternation. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details given. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No details given. |
| Selective reporting (reporting bias) | Unclear risk | Not possible to compare original trial protocol with final abstract. |
| Other bias | Unclear risk | The authors thanked Hill‐Rom for providing the Vest® device. |
| Methods | RCT. Parallel design. Location: single centre in UK. Duration: median length of stay for controls was 14 days, median length of stay for HFCWO group was 13 days. |
|
| Participants | 36 participants with CF admitted to hospital with an acute infective pulmonary exacerbation. Mean (SD) age: HFCWO group 25.8 (7.3) years; control group 29.8 (1.7) years. Sex: 23 (64%) males. |
|
| Interventions | Intervention: HFCWO (device was the Vest®, Hill Rom Model 205), participants paused to huff and cough as necessary. Control: usual airway clearance techniques (including ACBT, AD, PEP, manual techniques or oscillating PEP), further details not given. Treatment given 4x daily ‐ 2x supervised by a physiotherapist and 2x carried out independently. |
|
| Outcomes | FEV₁, FVC, length of hospital stay and sputum weight. Additional reference to this study also considered FEF25‐75. |
|
| Notes | Abstracts only and entry on clinicaltrials.gov (NCT01057524) available ‐ no full paper. Further breakdown of data has been requested for inclusion in meta‐analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not discussed. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not blinded. Difficult to blind participants to a device trial, but assessors not blinded either and no reasons given for this. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No drop outs mentioned or missing data discussed. |
| Selective reporting (reporting bias) | Unclear risk | All parameters stated as recorded were discussed over the 2 abstracts, but no full paper. |
| Other bias | Unclear risk | Not discussed but there is a possibility of involvement of the manufacturers in provision of the Vest® devices for the 36 participants. |
| Methods | RCT. Cross‐over design (2‐week washout period). Location: single centre in USA. Duration: 4 weeks of treatment followed by 2‐week washout and then 4 weeks of alternative treatment; follow‐up not stated. |
|
| Participants | 14 participants. Age and sex of the participants was unspecified, but as parents were also questioned it would suggest they were concerned with a paediatric population. | |
| Interventions | PD&P versus flutter. 2x daily for 15 min each treatment. | |
| Outcomes | Participant preference, wet and dry sputum weight, FVC and FEV₁ were measured pre‐study baseline and at the end of each treatment period. Sputum collected on the last treatment of each treatment period. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not discussed. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not discussed. |
| Selective reporting (reporting bias) | High risk | Parents were also questioned therefore it may be reasonable to assume that they may have influenced the children's decision as to preference. |
| Other bias | Unclear risk | Abstract only. |
| Methods | RCT. Parallel design. Location: single centre in USA. Duration: length of hospital stay (2 weeks). |
|
| Participants | 23 participants enrolled, 3 participants excluded due to being discharged prior to 14 days of inpatient stay. Data from 20 participants (11 males, 9 females) with CF, enrolled on admission to hospital. Age 5 ‐ 21 years. | |
| Interventions | 2‐week intervention of either flutter (n = 12) or CPT (n = 8). Frequency during the day was not specified. | |
| Outcomes | SaO₂, exercise tolerance (as measured by the 6MWD) and FEF, FVC and FEV₁ were measured at entry, day 7 and day 14. | |
| Notes | 20 participants included but two of them refused to walk so the data are from 18 participants – but the paper does not state which group(s) the two belonged to who dropped out, so "n" is unknown for each group in this outcome. Data have been recorded in the analysis using the numbers originally in each group therefore there may be bias attributed to one or other group as it is not clear which participants would not perform the walk test. SaO₂ was monitored during admission but no other data were reported for this parameter, apart from P < 0.05 by day 14. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not discussed. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Pulmonary function and exercise technicians were blinded as to which treatment interventions the participants were receiving. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 3 participants excluded due to being discharged prior to 14 days of inpatient stay, therefore not all their data were collected. |
| Selective reporting (reporting bias) | Unclear risk | Not possible to compare original trial protocol with final paper. Additionally the lung function parameters are not identified and may not be those frequently observed. SaO₂ was monitored during admission but no other data were reported for this parameter, apart from P < 0.05 by day 14. |
| Other bias | Unclear risk | Scandipharm Pharmaceuticals were thanked by the authors for providing the flutter valves. |
| Methods | RCT. Cross‐over design (2‐week washout between treatment arms). Location: single centre in Austria. Duration: 2x 4‐week treatment periods with 2‐week washout in between. |
|
| Participants | 7 participants. Age and sex of the participants was not identified. | |
| Interventions | 2x daily IPV versus 2x daily PEP. 2x 4‐week periods of treatment, 2‐week washout between where there was no PT. | |
| Outcomes | FEV₁, PO₂. Measured before, 10, & 40 min after first treatment of the day once per week. | |
| Notes | Abstract only, full paper not published as yet. Due to carryover effect the analysis was confined to the first treatment period; therefore this was analysed NOT as a cross‐over but a parallel study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, method not discussed. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding was not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Data not reported, only generalised conclusions made. |
| Selective reporting (reporting bias) | Unclear risk | Not possible to compare trial protocol with the published abstract. No full paper available to exclude selective reporting bias. |
| Other bias | Unclear risk | Abstract only. |
| Methods | RCT. Parallel design Duration: first 3 days of hospitalisation for an exacerbation.HFCWO or PEP |
|
| Participants | 23 participants (12 females). Mean age 25 years. | |
| Interventions | HFCWO at setting of 20 Hz for 30 minutes compared with 30 minutes of PEP for the first 3 days of treatment. | |
| Outcomes | FEV₁, FVC and FEF 25‐75 were assessed pre and 30 minutes post intervention. Sputum volume was collected after each intervention. | |
| Notes | Abstract only, full paper not published as yet. No identification how many participants were randomised to each treatment. There were no statistical data given but reference made to state P < 0.05 was significant but this was not attributed to any result. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, method not discussed. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not discussed; data provided but significance not statistically represented. |
| Selective reporting (reporting bias) | Unclear risk | Not possible to compare original trial protocol with final paper. Additionally a P value was suggested in the abstract but not attributed to any specific outcome measured. |
| Other bias | Unclear risk | Abstract only. Details of methodology are scarce |
| Methods | RCT. Cross‐over design. Location: single centre in USA. Duration: not defined. |
|
| Participants | 5 participants. Age and sex of participants not stated. | |
| Interventions | HFCWO versus CPT. 30 sessions of each therapy lasting same duration, but duration of treatment was not defined. | |
| Outcomes | Sputum weight. Primarily looking at the pressure and frequencies generated by the vest and the mucus collection was an aside. Measured before and after duration of intervention (30 days). | |
| Notes | In addition a gentleman not wanting to be included in the study used the Vest® for 12 months and experienced a significant increase in his RFTs and restoration of ventilation to the upper lobes of his chest on scanning. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but method not discussed. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not discussed. |
| Selective reporting (reporting bias) | High risk | Only reporting of respiratory function was descriptive of the man not included in the study. |
| Other bias | Unclear risk | None identified. |
| Methods | Quasi‐RCT (alternate assignment). Parallel design. Location: single centre in USA. Duration: 2 weeks. |
|
| Participants | 14 participants (10 males, 4 females). Age 8 ‐ 28 years. All participants admitted to hospital for 2‐week course of IV antibiotics, acute. No complications identified, no difference between groups in terms of clinical score, but clinical score not defined. | |
| Interventions | Percussive device (IPV) versus CPT, not stated how many participants were randomised to each treatment group 4 times per day for 2 weeks. | |
| Outcomes | FVC, FEV₁ and FEF25‐75 and RV. Participant‐reported satisfaction was noted. Measurements taken at admission and discharge. | |
| Notes | Abstract only, no full paper. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but no further details given. |
| Allocation concealment (selection bias) | High risk | Alternate assignment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not discussed. |
| Selective reporting (reporting bias) | High risk | Cliinical score was used as an outcome measure but no clear definition of this parameter given. Significant differences were suggested but no data provided to support this. |
| Other bias | Unclear risk | Supported by Vortran Medical Technology 1, Inc., Sacramento, CA. |
| Methods | RCT. Parallel design. Location: single centre in USA. Duration: total study period of 180 days ‐ 30‐day run in (participant kept a daily log of CPT and aerosol treatment administered) followed by 150 days of treatment. |
|
| Participants | 20 participants stratified by Schwachmann score and randomised to standard treatment or IPV. 4 dropped out, 16 participants (8 from each group (5 males, 3 females)) completed trial. IPV group mean (range) age: 12 (5 ‐ 24) years. CPT group mean (range) age 10 (5 ‐ 18) years. Participants were well matched to CF severity index, Schwachmann score. Mild to moderate disease severity. | |
| Interventions | IPV at least 2x per day compared to standard manual CPT at least 2x daily (included manual percussion for 2 min in each of 10 PD positions). Aerosol treatment was saline or N‐cromolyn and an appropriate volume of albuterol via standard aerosolisation. | |
| Outcomes | FVC, FEV₁ and FEF25‐75 measured at baseline, 30 days and at 180 days. Mean days of antibiotic use were documented both for oral and IV antibiotics as needed for hospitalisations. |
|
| Notes | Aerosolisation of saline or N‐cromolyn and an appropriate volume of albuterol was used via standard aerosolisation in the CPT group. This was the same volume of saline and albuterol as was used in the IPV group. IPV is thought to aid secretion removal by introducing simultaneous application of aerosolisation and intrathoracic percussion using mini‐bursts of gases. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | 20 participants stratified by Schwachmann score and randomised to standard treatment or IPV. No further details were reported. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No reasons for drop out of 4 participants following randomisation were discussed. |
| Selective reporting (reporting bias) | Unclear risk | Not possible to compare study protocol with final paper. |
| Other bias | Low risk | Adverse reaction noted and detailed in one participant who experienced minor haemoptysis. |
| Methods | Quasi‐RCT (alternate allocation). Cross‐over design. Location: single centre in USA. Duration: length of hospitalisation. |
|
| Participants | 22 enrolled into study , the data for 33 hospitalisations (20 males, 13 females) presented. Mean (range) age: 12 (7‐ 44) years. CF confirmed by sweat test and/or genetic testing. | |
| Interventions | 4x daily flutter (each treatment was 15 min) versus 4x daily CPT (each treatment was 30 min). | |
| Outcomes | Change from baseline FVC, FEV₁, FEF25‐75, FEV₁/FVC, TLC, RV, RV/TLC. Measured at admission and discharge which was mean (SD) 8.9 (2.5) days of treatment in the flutter arm and 8.8 (2.4) days in the CPT arm. | |
| Notes | Although 22 participants enrolled into the study, data were collected for 33 hospitalisations over the study period therefore baseline demographics may include some duplication of data. Subgroup analyses of 15 participants with only one admission and the initial admission of 7 were done with no change from overall outcome of the total 33 data sets analysed. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Initial participant randomised, but not stated how. Others followed alternating schedule. |
| Allocation concealment (selection bias) | High risk | Alternate assignment. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Open label. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop outs reported. |
| Selective reporting (reporting bias) | High risk | Although 22 participants enrolled into the study the data were collected for 33 hospitalisations over the study period therefore baseline demographics may include some duplication of data. Subgroup analyses of 15 participants with only one admission and the initial admission of 7 were done with no change from overall outcome of the total 33 data sets analysed. |
| Other bias | Low risk | Participants were monitored for side effects including haemoptysis, hypoxia and pneumothorax but none were identified. |
| Methods | RCT. Parallel design. Location: single centre in Russia. Duration: 10 'procedures' (not clear how many procedures per day). |
|
| Participants | 30 children aged 5 ‐ 17 years. | |
| Interventions | HFCWO versus control (control not mentioned so alternative ACT unknown, assumed that 15 participants were randomised to each treatment group). | |
| Outcomes | FEV₁, FVC, exercise tolerance, sputum volume and SpO₂ But as we are unaware of the alternative "control" ACT we cannot include the data in the meta‐analysis. |
|
| Notes | Only abstract in English, therefore translation required but even following translation the paper had limited quality and limited information as to the actual interventions and their frequency. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Stratified randomisation declared, not described. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not mentioned. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop outs. All 30 data sets included in their analysis |
| Selective reporting (reporting bias) | Unclear risk | No objective data on sputum volume, although it was stated there was an improvement following the intervention. |
| Other bias | Unclear risk | Not clear as abstract only in English and no clear evidence of excluded bias in translated paper. |
| Methods | Quasi‐RCT (alternate allocation). Cross‐over design. Location: single centre in USA. Duration: 8 days (treatments alternating daily for 4 days). |
|
| Participants | 29 participants (15 males, 14 females). Age range 7 ‐ 47 years. Diagnosis of CF and clinical evidence of chronic disease. | |
| Interventions | 3x daily 30 min CPT/PD versus 3x daily 30 min HFCWO. Participants continued to receive their standard bronchodilators prior to therapies. | |
| Outcomes | Sputum weight (wet and dry). Each participant provided 3 samples per day for 4 days and all 12 samples were used to calculate the means and standard deviations. | |
| Notes | 1 individual not enrolled due to intolerance of HFCWO, although had met the inclusion criteria ‐ never really entered the study therefore not really a drop out. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Initially randomly assigned, but method not stated then treatment assignments alternating daily. |
| Allocation concealment (selection bias) | High risk | Alternate. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 individual not enrolled due to intolerance of HFCWO, although had met the inclusion criteria ‐ never really entered the trial therefore not really a drop out. |
| Selective reporting (reporting bias) | Low risk | Potential adverse effects were identified but none occurred. |
| Other bias | Unclear risk | Not discussed. |
| Methods | RCT. Cross‐over design (4 treatment arms, no washout). Location: single centre in UK. Duration: 4 successive days (1 day per treatment arm). |
|
| Participants | 12 participants (5 males, 7 females). Mean age 21 years (range 16 ‐ 28). | |
| Interventions | PD&P versus Flutter alone versus Flutter with PD&P versus sham flutter with PD&P. 3x a day for each treatment. | |
| Outcomes | Used sputum volume and peak flows only. Measured after 24‐hour period for 4 days. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but method not discussed. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not discussed. |
| Selective reporting (reporting bias) | Unclear risk | Not discussed. |
| Other bias | Unclear risk | Abstract only. |
| Methods | RCT. Parallel design. Location: single centre in USA. Duration: 24 weeks with 7‐day run in period. |
|
| Participants | 16 participants (9 males, 7 females). Only results from 15 participants (8 flutter and 7 IPV) analysed. 1 participant became pregnant and she was withdrawn from the study. Age not specified, but similar mean age was expressed. | |
| Interventions | 2x daily flutter versus 2x daily IPV; 8 randomised to each treatment group | |
| Outcomes | Frequency of exacerbation, participant reported satisfaction, FEV₁, FVC, FEF25‐75, Schwachmann scores. Spirometry and Schwachmann scores were measured at enrolment and at baseline following the 7‐day run in period, every 4 weeks during the study and at 24 weeks (the end of the study). | |
| Notes | Unsure regarding the need to have all participants do the week run in with flutter, was this to eliminate bias for the flutter or to ensure all had similar experience before randomisation? Had they used either of the devices before? Abstracts only. There does not appear to have been a full paper published yet. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but method not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | One participant was excluded due to pregnancy; we are not made aware of when she was withdrawn and her data were not reported. |
| Selective reporting (reporting bias) | High risk | Days lost from work or school although identified as being an outcome variable have not been reported in the results. |
| Other bias | Unclear risk | Abstract only. |
| Methods | RCT. Parallel design. Location; single centre in Canada. Duration: 12 months. |
|
| Participants | 40 participants (24 males, 16 females) were randomised. Age range 7 ‐ 17 years. Participants had stable CF (judged by clinical evaluation, chest radiograph and pulmonary function) with FEV₁ 47 ‐ 107% and attended British Colombia's Children's Hospital CF Clinic. No participant entered the study within 1 month of hospitalisation for a pulmonary exacerbation. | |
| Interventions | 2x daily flutter versus 2x daily PEP (20 randomised to each treatment group). | |
| Outcomes | Mean annual rate of decline in % predicted of FEV₁, FVC and FEF 25‐75, number of exacerbations (hospitalisations), adherence or compliance with therapy. Measured at beginning of study, at 3‐monthly intervals and at 12 months. | |
| Notes | Most of the hospitalisations did not occur until months 7 ‐ 9 of the study. People who did not adhere to treatment to a level of 85% adherence to 2x daily ACT as depicted in diaries were withdrawn by the researchers; but those who dropped out did so because they felt flutter to be ineffective. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised to either one group or another but generation of sequence not discussed. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Physicians were blinded to the method of physiotherapy received. Pulmonary function technician and radiographer were also blinded as to the airway clearance method. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop outs were reported and subgroup analysis carried out. |
| Selective reporting (reporting bias) | High risk | Less than 85% adherence over 1 month of treatment was considered not adherent to therapies and those participants were withdrawn. |
| Other bias | Unclear risk | There was a discrepancy between those withdrawn for non‐compliance between the final paper which reported 2 from the PEP group and the 1998 abstract which reported 3 withdrawals . The author was contacted and advised that the final paper contained the correct information. |
| Methods | RCT. Parallel design with 2‐month washout period post randomisation and prior to start of trial. Location: multicentre (12 centres) in Canada. Duration 12 months. |
|
| Participants | 107 participants (children and adults aged 6 ‐ 47 years) enrolled in the study and randomised. PEP Group: 51 participants (mean age 13.5 years). 25 female, 26 male. HFCWO Group: 56 participants (mean age 14.3 years). 25 female, 31 male. 19 dropouts within the study ‐ 16 occurred prior to or at the time of randomisation (8 from each group ‐ reasons given). At visit 2 (start of treatment arm) 43 were included in the PEP arm and 48 in the HFCWO arm. The study results were analysed on an ITT premise based on these participant numbers. Between visits 2 and 6 there was 1 further dropout from the PEP group and 2 from the HFCWO group. 88 were analysed following completion of the study. |
|
| Interventions | 1 ‐ 2 sessions/day ‐ participants to remain on individual regimen prescribed prior to study 30 min of HFCWO (6x 5 min cycles) versus PEP (6 cycles of 15 PEP breaths followed by 2 ‐ 3 huffs). |
|
| Outcomes | Time to exacerbation and frequency of exacerbation, health‐related quality of life measurements, change in respiratory function parameters, participant preference. | |
| Notes | Only randomised participants were included in the ITT analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised by an independent statistician using a computer‐generated randomisation table. |
| Allocation concealment (selection bias) | Low risk | Central allocation ‐ computer‐generated by independent statistician. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Although participants could not be blinded to treatment, physicians and respiratory therapists performing the respiratory assessments and lung function tests were unaware of the treatment assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop outs were reported and data sets for all included were complete. ITT identified. At visit 2 when participants were to begin prescribed arm of treatment, there were 8 dropouts in each arm with similar reasons given. By the end of the study, there was 1 further dropout from the PEP group (diagnosed with CFRD) and 2 treatment‐related from the HFCWO group (1 due to reflux and vomiting associated with treatment; 1 did not like HFCWO). |
| Selective reporting (reporting bias) | Low risk | On comparison with the protocol published on the clinical trials register, all outcomes identified are reported within the final paper. However, the data are presented as medians and percentiles which makes analysis problematic. |
| Other bias | Low risk | Both types of device (HFCWO and PEP) were loaned by their respective companies. It is considered therefore that this would not constitute bias as both groups were potentially equally influenced. The study was limited by the fact that the majority of participants were on PEP prior to the study, although attempts were made to limit any potential bias from this by having a washout period. |
| Methods | RCT (pilot study). Cross‐over design (1 day washout between 2 treatment arms). Location; single‐centre in South Africa. Duration: 5 days (2 days per treatment with 1 day washout in between). |
|
| Participants | 7 participants with CF; mean age 28 years (range 16 ‐ 42 years). | |
| Interventions | Flutter versus ACBT. Group A: flutter, then washout, then ACBT. Group B: ACBT, then washout, then flutter. | |
| Outcomes | Daily 24‐hour sputum samples and lung function tests (FEV₁, FVC, PEF, FEF25, FEF50, FEF75), questionnaire at end of study. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised to either one group or another but generation of sequence not discussed. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Additional person was used to perform the lung function tests and is included in the acknowledgements it is not clear if this person was blinded to the treatments. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data sets were complete for all those participants included in the study. |
| Selective reporting (reporting bias) | Unclear risk | No major side effects were experienced with either technique. |
| Other bias | Low risk | Possible limitations of the study were discussed and the author suggested various means to improve the findings of the study. |
| Methods | RCT. Parallel design (3 arms). Location: multicentre (20 centres) in USA. Duration: 3 years, but terminated early, duration of study participation ranged from 1.3 to 2.8 years. |
|
| Participants | 166 participants with CF enrolled initially. 15 dropped out (11 from the CPT group and 4 from the flutter group) in the initial 60 days of the study with a further 41 withdrawing due to lost to follow up; lack of time; treatment preference and decrease in health. Data missing from 5 participants. Randomised 58 (31%) to PD&P, 51 (30%) to flutter and 57 (39%) to HFCWO. Gender split: 54% male. Mean (range) age: 14.2 (7 ‐ 44) years. Participants split into 86 children (7 ‐ 12 years), 44 adolescents (13 ‐ 17 years) and 36 adults (over 18 years). Mean FEV₁ 88.2%. | |
| Interventions | Flutter versus HFCWO versus PD&P. Flutter: self‐administered in 3 stages ‐ (1) loosening and mobilisation breaths (2) mucus mobilisation and (3) expectoration. PD&P: treatment administered by caregiver using a wedge and consisted of positioning, percussion (vibration ) and forced expiratory technique with coughing between 6 positions; after each position participants instructed to do 3 forced expiratory technique and cough. Each treatment was 2x daily |
|
| Outcomes | Rate of decline in FEV₁, time to need for antibiotics for pulmonary exacerbations, use of other pulmonary therapies, participant satisfaction, adherence, quality of life. Measurements of satisfaction were recorded before and after study and every 4 months with phone diary, but no identification of type of activities outlined in this abstract. ‐ details in online supplement | |
| Notes | This study ID refers to the flutter versus PD&P section of the study. 166 participants enrolled and a total of 56 withdrew (15 before Day 60, 41 after Day 60). 110 left in at early study termination and those who withdrew after Day 60 included in ITT analysis (n = 151). Funded by Hill‐Rom. Sample size calculation undertaken (60 participants per group) to detect a difference between annual rates of decline in FEV₁ of 2% predicted. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Electronic randomisation stratified by age |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Drop outs apparent over the 3 abstracts but reasons not discussed. 15 dropped out and data missing from 5 participants. 130 provided adherence data in the 2006 abstract, but other abstracts and main papers describe166 participants. Uneven drop outs across treatment arms and age groups led to early termination. |
| Selective reporting (reporting bias) | Low risk | Measurements of satisfaction were recorded before and after study and every 4 months with phone diary, these were identified as effectiveness, convenience, comfort, and overall satisfaction. Satisfaction with the therapy was an independent predictor of withdrawing. |
| Other bias | High risk | Study supported by Hill‐Rom, Inc and the CF Foundation. |
| Methods | RCT. Parallel design (3 arms). Location: multicentre (20 centres) in USA. Duration: 3 years, but terminated early, duration of study participation ranged from 1.3 to 2.8 years. |
|
| Participants | 166 participants with CF enrolled initially. 15 dropped out (11 from the CPT group and 4 from the flutter group) in the initial 60 days of the study with a further 41 withdrawing due to lost to follow up; lack of time; treatment preference and decrease in health. Data missing from 5 participants. Randomised 58 (31%) to PD&P, 51 (30%) to flutter and 57 (39%) to HFCWO. Gender split: 54% male. Mean (range) age: 14.2 (7 ‐ 44) years. Participants split into 86 children (7 ‐ 12 years), 44 adolescents (13 ‐ 17 years) and 36 adults (over 18 years). Mean FEV₁ 88.2%. | |
| Interventions | Flutter versus HFCWO versus PD&P. Each treatment was 2x daily HFCWO: self‐administered using the Vest® using HFCWO, deep breathing and forced expiratory technique with coughing between each frequency. Each frequency to be done for 5 minutes with deep breathing to total lung capacity every 2 minutes and each cycle followed by 3 forced expiratory techniques. PD&P: treatment administered by caregiver using a wedge and consisted of positioning, percussion (vibration) and forced expiratory technique with coughing between 6 positions; after each position participants instructed to do 3 forced expiratory technique and cough. |
|
| Outcomes | Rate of decline in FEV₁, time to need for antibiotics for pulmonary exacerbations, use of other pulmonary therapies, participant satisfaction, adherence, quality of life. Measurements of satisfaction were recorded before and after study and every 4 months with phone diary, but no identification of type of activities outlined in this abstract. ‐ details in online supplement | |
| Notes | THIS DUPLICATE REFERENCE HAS BEEN CREATED TO ALLOW DATA FOR BOTH TYPES OF OSCILLATING DEVICE TO BE ENTERED IN THE ANALYSIS. This study ID refers to the HFCWO versus PD&P section of the study. 166 participants enrolled and a total of 56 withdrew (15 before Day 60, 41 after Day 60). 110 left in at early study termination and those who withdrew after Day 60 included in ITT analysis (n = 151). Funded by Hill‐Rom. Sample size calculation undertaken (60 participants per group) to detect a difference between annual rates of decline in FEV₁ of 2% predicted. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Electronic randomisation stratified by age. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Drop outs apparent over the 3 abstracts but reasons not discussed. 15 dropped out and data missing from 5 participants. 130 provided adherence data in the 2006 abstract, but other abstracts and main papers describe 166 participants. Uneven drop outs across treatment arms and age groups led to early termination. |
| Selective reporting (reporting bias) | Low risk | Measurements of satisfaction were recorded before and after study and every 4 months with phone diary, these were identified as effectiveness, convenience, comfort, and overall satisfaction. Satisfaction with the therapy was an independent predictor of withdrawing. |
| Other bias | High risk | Study supported by Hill‐Rom, Inc and the CF Foundation. |
| Methods | RCT. Parallel design. Location: single centre in Canada. Duration: 13 months. |
|
| Participants | 43 adults (25 males) with CF. FEV₁ > 40% predicted. No hospitalisations within 1 month of study entry, no change in medications within 1 month of study entry and willingness to attend 5 follow‐up appointments. Exclusion criteria ‐ absence of daily cough or daily production of sputum. Flutter group: mean (SD) age 31 (8.5) years. PEP group: mean (SD) age 28 (8.1) years. |
|
| Interventions | Flutter versus PEP mask (21 randomised to each treatment group out of 42 participants included in analysis) 5 ‐ 10 exhalations through the flutter with the degree of tilt adjusted to optimise the vibrations. Cycle is repeated until the individual felt "clear" or for approximately 20 minutes. 10 ‐ 15 breaths through the PEP followed by a huff or cough, followed by period of relaxed breathing. Cycle repeated 5 ‐ 6 times taking approximately 20 minutes to complete. Participants were advised to perform their therapy 2x per day following any bronchodilator therapy. They were instructed to only use their Ffutter or PEP mask for the duration of the study. Followed every 3 months for 13 months. First month was the "training" month. |
|
| Outcomes | Lung function tests (FEV₁, FVC, FEF25‐75%), Quality of Well‐being Scale, Chronic Respiratory Disease Index Questionnaire, daily diary record. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers table and block randomisation. |
| Allocation concealment (selection bias) | Low risk | Sealed opaque envelope. Envelopes opened in sequence, and this may itself be a risk of allocation bias. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Lung function assessor was blinded to the device used by the participant and also to what stage they were at in the study period. It was not possible to blind the physiotherapist teaching the participant how to use the device nor indeed the participant themselves. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One drop out due to not attending at clinic appointments. Paper states that although not all participants attended every follow up assessment, baseline and final measures were obtained for all 42. All but 3 (1 flutter; 2 PEP) attended at least 4 follow‐up visits in the 13‐month period. |
| Selective reporting (reporting bias) | Low risk | Information available for all outcome variables measured. |
| Other bias | High risk | Flutter group and PEP group had different mean pulmonary function values at recruitment (flutter group higher). This led to divergence between groups in mean pulmonary function values at 1st and 2nd follow‐up visits. Study fatigue is always a consideration when using small populations such as those with CF; and this study this as a reason why some participants declined inclusion into the study |
| Methods | RCT. Cross‐over design (2‐week washout period). Location: multicentre (3 centres) in USA. Duration: 12 weeks (2‐week run in period followed by 4‐week treatment and 2‐week washout with alternative 4‐week treatment). |
|
| Participants | 29 participants enrolled (14 males). Aged 6 years or greater. Mean (range) age ‐ 23 (9 to 39) years. Diagnosis of CF confirmed by sweat test. Required ability to reliably perform spirometry and lung volume measurements, to have baseline FVC of 50 ‐ 80 % predicted and be clinically stable for 1 month prior to enrolment. Excluded if in concurrent study or history of massive haemoptysis within 1 month or pneumothorax within 6 months of entrance. 5 participants withdrew (4 exited due to illness and 1 due to non‐compliance with clinic visits). | |
| Interventions | HFCWO versus oscillating PEP (flutter). As prescribed previous to study ‐ no mention whether this was 2x daily etc. 4 weeks in each arm, 2‐week lead‐in and wash out periods during which time they resumed their normal routine therapies which were not outlined. | |
| Outcomes | FEV₁, FVC, FEF25‐75%, participant satisfaction scores in domains of efficacy, convenience and comfort. Participant preference was measured as baseline and pre/post each intervention (5 data points). | |
| Notes | 5 withdrawals, ITT was identified. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Prospective randomisation, further details not given on method. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5 participants withdrew (4 exited due to illness and 1 due to non‐compliance with clinic visits). ITT identified. |
| Selective reporting (reporting bias) | High risk | Only participants who completed both therapies were included in the final analysis. As we do not know what their normal therapy was perhaps they had already done a comparison? |
| Other bias | Unclear risk | None identified. |
| Methods | RCT. Cross‐over design (no washout). Location: single centre in UK. Duration: 4 days. |
|
| Participants | 30 participants recruited (22 males). Mean age: 29.7 years. Mean FEV₁: 37.7 % |
|
| Interventions | HFCWO versus "usual" ACT (83% of "usual" therapy was described as ACBT, AD, flutter or PEP). Participants received either HFCWO on days 1 and 3 and the "usual" ACT on days 2 and 4 or vice versa. Sessions were 2x daily for 30 min. |
|
| Outcomes | Wet weight of expectorated sputum, respiratory function, oxygen saturation monitoring, perceived efficacy and preference were measured. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Allocation to HFCWO or usual ACT on Day 1 was determined using a computer‐generated randomisation table. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Not possible to blind participants or clinicians, but paper states: "An independent observer, blind to the daily method of airway clearance used, performed the spirometry, weighed the sputum samples and collected the 10 cm VAS throughout the study." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 sputum samples were removed from total of 116 collected as they were incomplete. |
| Selective reporting (reporting bias) | Unclear risk | Powered to detect a 4 g difference in expectorated sputum. |
| Other bias | High risk | Supported by Robery Luff Foundation and Hill‐Rom Ltd. Levels of oxygen saturation measured were higher at baseline in the HFCWO group which potentially could influence outcome as groups were not balanced at the beginning of the intervention. |
| Methods | RCT. Cross‐over design (used CPT between therapies as a washout period length of which was not defined). Location: single centre in USA. Duration: each therapy lasted 1 month. |
|
| Participants | 15 participants aged 5 ‐ 17 years with CF. Not stated how many were males and how many females. Participants were clinically stable and able to perform RFT's, no hospitalisations in the month prior to study. 5 excluded due to hospital admission for acute exacerbation, 4 withdrew (no reason given). 6 participants completed the study. | |
| Interventions | Flutter versus PEP versus CPT/PD. Each therapy was performed for 15 min 3x daily for 1 month. No changes in established medication regimen. |
|
| Outcomes | RFTs (FEV₁, FEF25‐75) performed at beginning and end of each new therapy, SaO₂, participant satisfaction. | |
| Notes | This study ID refers to the flutter versus PEP section of the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Each participant was arbitrarily assigned to 1 of 3 groups of randomly sequenced therapies, no further details of method. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 9 withdrawals after randomisation took place, 5 excluded due to hospital admission for acute exacerbation, 4 withdrew (no reason given). |
| Selective reporting (reporting bias) | High risk | Gender split was not stated. Participants stated they felt better but there were no criteria given from which to establish this. |
| Other bias | Unclear risk | Scandipharm provided the flutter devices for the trial. |
| Methods | RCT. Cross‐over design (used CPT between therapies as a washout period length of which was not defined). Location: single centre in USA. Duration: each therapy lasted 1 month. |
|
| Participants | 15 participants aged 5 ‐ 17 years with CF. Not stated how many were males and how many females. Participants were clinically stable and able to perform RFT's, no hospitalisations in the month prior to study. 5 excluded due to hospital admission for acute exacerbation, 4 withdrew (no reason given). 6 participants completed the study. | |
| Interventions | Flutter versus PEP versus CPT/PD. Each therapy was performed for 15 min 3x daily for 1 month. No changes in established medication regimen. |
|
| Outcomes | RFTs (FEV₁, FEF25‐75) performed at beginning and end of each new therapy, SaO₂, participant satisfaction. | |
| Notes | THIS DUPLICATE REFERENCE HAS BEEN CREATED TO ALLOW DATA FOR BOTH TYPES OF OSCILLATING DEVICE TO BE ENTERED IN THE ANALYSIS. This study ID refers to the Flutter versus CPT section of the study. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Each participant was arbitrarily assigned to 1 of 3 groups of randomly sequenced therapies, no further details of method. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 9 withdrawals after randomisation took place, 5 excluded due to hospital admission for acute exacerbation, 4 withdrew (no reason given). |
| Selective reporting (reporting bias) | High risk | Gender split was not stated. Participants stated they felt better but there were no criteria given from which to establish this. |
| Other bias | Unclear risk | Scandipharm provided the flutter devices for the study. |
| Methods | RCT. Cross‐over design (no washout period). Location: single centre in UK. Duration: 2 days. |
|
| Participants | 10 participants (7 males, 3 females). Median (range) age: 14 (9 ‐ 16) years. CF diagnosed via sweat chloride testing or genetic testing. Participants admitted to the Brompton Hospital with an acute exacerbation as defined by conventional criteria and were adept at self‐treatment of ACBT. | |
| Interventions | ABCT versus HFCWO. 2 supervised treatments of either ACBT or HFCWO on 2 successive dates for 20 min. | |
| Outcomes | FVC, FEV₁ (measured immediately before, immediately after and 10 min after each treatment), wet sputum weight (measured over 24‐hour period, during treatment and 15 minutes after treatment), participant preference (measured at the end of the study). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but generation of sequence not identified. |
| Allocation concealment (selection bias) | Low risk | Via sealed envelope. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Individual who collected sputum weight was blinded to therapy type. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data set complete, no drop outs identified. |
| Selective reporting (reporting bias) | Unclear risk | Not possible to compare study protocol with final paper. |
| Other bias | High risk | Paper identifies potential weakness of the study in that is short term and concludes that potentially a longer term study may have demonstrated improved adherence. |
| Methods | RCT. Cross‐over design (no washout period). Location: single centre in UK. Duration: 2 days of treatment and measurements taken at end. |
|
| Participants | 21 participants (12 males, 9 females). Median age 26 years. | |
| Interventions | Flutter and forced expiration versus ACBT. First treatment was performed 2x on Day 1 and then the other treatment 2x the following day. |
|
| Outcomes | RFTs, sputum weight, oxygen saturations and participant satisfaction were the outcome measures. | |
| Notes | Abstract only, no full paper as yet published. Cross‐over paired T‐test and McNemars Chi² tests were used for statistical analysis. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but method not discussed. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Independent observer measured pulmonary function and oxygen saturations. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No dropouts identified or discussed. |
| Selective reporting (reporting bias) | Unclear risk | Not discussed. |
| Other bias | Unclear risk | Abstract only. |
| Methods | RCT. Parallel design. Location: single centre in UK. Duration: 12 months. |
|
| Participants | 30 participants (20 girls, 10 boys matched). Age range 6 ‐ 15 years; mean age 11.5 years. 15 to each treatment arm. BMI, LCI and FEV₁ were well matched. One from each group withdrew because either they preferred their previous device, or they found it too fiddly to clean. | |
| Interventions | PEP versus cornet. The treatment was used as their main ACT for 12 months. | |
| Outcomes | FEV₁; LCI; pulmonary exacerbations; health perception; quality of life. FEV₁ and LCI were measured at start 6 months and 12 months. Quality of Well‐Being Scale, health perception and frequency of exacerbations measured at beginning and end. | |
| Notes | Abstracts only, no full paper published as yet. Blinding not possible. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was stratified for age, sex and FEV₁, further details not given. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not possible. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One child from each group dropped out after randomisation, reason given. |
| Selective reporting (reporting bias) | High risk | Ongoing study which spanned 3 abstracts, but no full paper as yet identified. |
| Other bias | High risk | The authors themselves questioned whether quality of life measures were reliable in children as they may be unable to accurately compare current health to that experienced the previous year. |
| Methods | RCT. Cross‐over design (no washout period). Location: single centre in UK. Duration: 2 days. |
|
| Participants | 24 participants (14 males, 10 females) with positive sweat test for CF were randomised, but only 20 included in the study. 4 participants withdrew (3 males, 1 female); 2 had to have drug regimens changed; 2 withdrew due to technical problems with oximeter and sputum collection. Age range 16 ‐ 36 years; mean age 24.4 years. Stable as according to no clinical findings. | |
| Interventions | ACBT versus flutter and ACBT. 2 supervised treatments per day then alternate treatment on following day. In addition 2 different postural drainage positions were used, but no statistical difference noted between treatments. |
|
| Outcomes | RFTs, wet sputum weight and participant satisfaction. | |
| Notes | No statistical data presented on RFTs apart from there being no statistical significance in the results. Most found both regimens easy to use, with majority finding ACBT easier to clear secretions. 17 out of 20 felt they would continue with ACBT. On follow‐up the 3 participants who said they would continue with the flutter at home had discontinued it within the month and resumed ACBT. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequentially admitted into the study, randomised to treatment regimens, but method not stated. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Independent observer used to measure lung function, sputum weight and oxygen saturations. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4 withdrawals after randomisation, (reasons given) analysis only on 20 remaining. |
| Selective reporting (reporting bias) | Unclear risk | Not discussed. |
| Other bias | Unclear risk | Not discussed. |
| Methods | RCT. Parallel design. Location: single centre in UK. Duration: 12 months. |
|
| Participants | 75 participants (47 males) enrolled. Aged over 16 years with positive diagnosis of CF. Median (SD) age: ACBT 31.1 (9.7) years, AD 25.9 (6.5) years, cornet 25.3 (8.3) years, flutter 32.1 (7.5) years, PEP 29.3 (12) years. Sex: ACBT ‐ 11/15 male, AD 10/15 male, cornet 8/15 male, flutter 10/15 male, PEP 8/15 male. FEV₁ >25% predicted. Exclusion criteria: respiratory exacerbation, recent acquisition of Burkholderia cepacia, previous history of pneumothorax, pregnancy, currently on transplantation waiting list and current haemoptysis. |
|
| Interventions | ACBT versus cornet versus AD versus flutter versus PEP (15 to each treatment group). Duration and frequency of treatments were individualised for each participant. |
|
| Outcomes | FEV₁, FVC , MEF, RV%/TLC, BMI, modified shuttle walk test, chronic respiratory disease questionnaire, Short form‐36 and number of IV antibiotics required. Participants were observed for 1 year with outcomes measured every month. | |
| Notes | Lung function data available on 65 participants only, as 10 lost to follow‐up. Blinding of assessor but unclear as to whether person responsible for care was blinded to the randomisation. Used ITT. 53 completed study on technique to which they had been randomised. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was computerised and stratified according to FEV₁ % predicted and sputum expectorated. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of assessor but unclear as to whether person responsible for care was blinded to the randomisation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 75 participants were randomised but data only available for 65 participants due to loss to follow‐up. Used ITT. Withdrawals due to pleurodesis, listing for transplantation, one participant moved away, 3 withdrew with no reasons given, 1 did not want any more testing. |
| Selective reporting (reporting bias) | High risk | All outcomes were reported, although not all data provided. |
| Other bias | Unclear risk | None reported and no evidence of any other likely bias. |
| Methods | RCT. Cross‐over design (1‐week washout period). Location: single centre in the Netherlands. Duration: 6 weeks (each treatment 2 weeks and 1 week wash in/wash out period). |
|
| Participants | 22 participants with CF confirmed by sweat test or DNA mutation analysis. Mean age 12 years; range 7 ‐ 17 years. Sex: 12 males, 10 females. Clinically stable for 2 weeks before study. |
|
| Interventions | Flutter versus PEP mask. 2x daily, 2 weeks in each arm, 1 week wash‐in and wash‐out period. | |
| Outcomes | FVC, FEV₁, RV/TLC, FEF25‐75% predicted, participant satisfaction. Outcomes were all measured before and after each treatment intervention. | |
| Notes | Outcome assessor blinded. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but method not discussed. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants and clinicians could not be blinded, but outcome assessor was blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study and their data were included. |
| Selective reporting (reporting bias) | Unclear risk | Not clear what happened in the run‐in or wash out period between cross‐over. |
| Other bias | Unclear risk | None identified. |
| Methods | RCT. Cross‐over design (no washout). Location: single centre in USA. Duration: 2 days in each arm which were consecutive so 6 days in total. |
|
| Participants | 28 participants recruited, 24 (10 females, 14 males) analysed, reasons for withdrawals not reported. Mean age 24 years, range 14 ‐ 34 years. | |
| Interventions | PD&P versus IPV versus HFCWO. 3 treatments per day each lasting 30 min (24 min of therapy followed by 6 min of directed coughing). PD&P was delivered by pulmonary nurses; IPV and HFCWO delivered by respiratory therapists. This suggests inconsistency of personnel when delivering treatment modalities. |
|
| Outcomes | Wet and dry sputum weight collected over the 60‐minute period, participant satisfaction questionnaire. | |
| Notes | This study ID refers to the IPV versus PD&P section of the study. It is not clear whether sputum was collected for each of the 6 treatment days or for first or last 60 min per treatment technique. Study reports that 4 participants received each of the 6 possible treatment sequences ‐ this suggests that they had more than 1 admission during the study time which may lead to duplication of data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but method not discussed. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 4 withdrawals following randomisation, but reasons for withdrawals not reported. |
| Selective reporting (reporting bias) | Low risk | Limitations of the study were outlined. |
| Other bias | High risk | Study reports that 4 participants received each of the 6 possible treatment sequences ‐ this suggests that they had more than 1 admission during the study time which may lead to duplication of data. Pulmonary nurses were used to perform physiotherapy techniques which may have had an impact on the accuracy and efficacy of treatments delivered. |
| Methods | RCT. Cross‐over design (no washout). Location: single centre in USA. Duration: 2 days in each arm which were consecutive so 6 days in total. |
|
| Participants | 28 participants recruited, 24 (10 females, 14 males) analysed, reasons for withdrawals not reported. Mean age 24 years, range 14 ‐ 34 years. | |
| Interventions | PD&P versus IPV versus HFCWO. 3 treatments per day each lasting 30 min (24 min of therapy followed by 6 min of directed coughing). PD&P was delivered by pulmonary nurses; IPV and HFCWO delivered by respiratory therapists. This suggests inconsistency of personnel when delivering treatment modalities. |
|
| Outcomes | Wet and dry sputum weight, participant satisfaction questionnaire. | |
| Notes | THIS DUPLICATE REFERENCE HAS BEEN CREATED TO ALLOW DATA FOR BOTH TYPES OF OSCILLATING DEVICE TO BE ENTERED IN THE ANALYSIS. This study ID refers to the HFCWO vs PD&P section of the study. It is not clear whether sputum was collected for each of the 6 treatment days or for first or last 60 min per treatment technique. Study reports that 4 participants received each of the 6 possible treatment sequences ‐ this suggests that they had more than 1 admission during the study time which may lead to duplication of data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised, but method not discussed. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not discussed. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 4 withdrawals following randomisation, but reasons for withdrawals not reported. |
| Selective reporting (reporting bias) | Low risk | Limitations of the study were outlined. |
| Other bias | High risk | Study reports that 4 participants received each of the 6 possible treatment sequences ‐ this suggests that they had more than 1 admission during the study time which may lead to duplication of data. Pulmonary nurses were used to perform physiotherapy techniques which may have had an impact on the accuracy and efficacy of treatments delivered. |
| Methods | RCT. Cross‐over design. Location: single centre in USA. Duration: not clear. |
|
| Participants | Reported 13 pairs of samples but number of participants was not specified, therefore we can only assume there were 13 adolescents or adults. Age and sex not specified. | |
| Interventions | HFCWO versus CPT. Participants were randomised to 2 groups each with 4 sessions. 1st group: CPT, HFCWO, HFCWO, CPT. 2nd group: HFCWO, CPT, CPT, HFCWO. | |
| Outcomes | Wet and dry sputum weight. | |
| Notes | Interventions looks like 2 sessions just one the reverse of the other. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but method not discussed. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not possible to blind participants or clinicians. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reported 13 pairs of samples but number of participants was not specified, therefore we can only assume there were 13 adolescents or adults. |
| Selective reporting (reporting bias) | Unclear risk | Abstract only. |
| Other bias | Unclear risk | Age and sex of participants not stated. |
| Methods | RCT. Cross‐over design Location: single centre in USA. Duration: 2 weeks (2 study days in each week). |
|
| Participants | 12 participants (all males) with CF. Mean (range) age 29.2 (19 ‐ 50) years. Consistent sputum producers; all volunteers with no illness within 6 weeks of study. | |
| Interventions | HFCWO versus CPT. HFCWO: 5 minutes at 6 frequencies, followed by 3 huffs and directed coughs at the end of each cycle; treatment time 36 ‐ 40 min. CPT: 10 hand positions, 3 huffs and directed cough after each position treatment lasting about 45 ‐ 50 min. All treatments preceded by nebulisers. |
|
| Outcomes | Wet and dry sputum weight measured at end of each session, data reported at end of week 1 and end of week 2. | |
| Notes | As this study also appeared to compare the efficacy of 2 different therapists therefore we cannot be absolutely clear that the HFCWO was solely responsible for any and all improvements in sputum weight. In addition the hand positions used by the therapist were not defined and commonly we would use a variety of 13 postural drainage positions if this was the technique being evaluated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Described as randomised but method not discussed. |
| Allocation concealment (selection bias) | Unclear risk | Not discussed. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not possible to blind participants or clinicians,but paper states "all the subjects were analysed as soon as possible by a single scientist (LGH) with no knowledge of subject source or therapy given" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Complete data sets for all participants. |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting. All parameters measured were discussed. |
| Other bias | High risk | Paper also reports that a natural competition between two different therapists was created. In addition the hand positions used by the therapist were not defined. |
| Methods | RCT. Parallel design. Location: single centre in Australia. Duration: at least 10 days. |
|
| Participants | 23 children and adolescents with CF admitted to hospital for IV antibiotics for a respiratory exacerbation (as defined by Wood 2002). Needed previous experience at home with any PEP device. 1 from acapella group was discharged early on Day 6, so only 10 analysed in that group. Age mean (SD) range: PEP 13.5 (3.3) 7 ‐ 18 years; acapella 10.4 (2.2) 7 ‐ 13 years. Sex: PEP 9 females, 3 males; acapella 8 females, 2 males, the gender of the one participant from the acapella group who was discharged early was not identified in the paper. FEV₁ % predicted mean (SD) range: PEP 74.67 (19.8)%, 56% ‐ 114%; acapella 58.9 (23)%, 29% ‐ 95%. Exercise performance (m) mean (SD) range; PEP 798.3 (233.6), 390 ‐ 1100 m; acapella 576 (293.7), 290 ‐ 1200 m. |
|
| Interventions | PEP mask (n = 12) versus acapella (n = 11). 2 supervised treatment sessions each day for a 10‐day period. Treatment was standardised to consist of 10 sets with the allocated device in a sitting position. Each set consisted of 10 breaths through the device followed by one or two huffs and cough. The pressure settings for the device were standardised for the study to provide between 15 and 20 cm H₂O of positive pressure. All participants received concurrent IV antibiotics; any other treatment was in accordance with direction from a respiratory physician who was not aware of the treatment allocation of participants. |
|
| Outcomes | Lung function, exercise performance (modified shuttle walk test), wet weight of sputum and satisfaction questionnaire. Outcomes measured prior to randomisation and after 10 days. |
|
| Notes | Sample size calculation undertaken (18 participants per treatment arm needed to detect a 10% change in FEV₁). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 36 pieces of paper (18 PEP and 18 acapella) were put in double‐sealed envelopes and a research assistant (who was not involved with recruitment, assessment, or treatment) withdrew 1 envelope, determined group allocation, and then discarded the envelope. |
| Allocation concealment (selection bias) | Low risk | 36 pieces of paper (18 PEP mask and 18 acapella) were placed in double‐sealed envelopes and for each participant a research assistant (who was not involved with recruitment, assessment, or treatment) withdrew one envelope to determine group allocation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Outcome assessors were blinded for lung function and modified 10‐metre shuttle test. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 22 out of 23 participants completed the study; 1 participant was discharged home on day 6 for home IV treatment and was not available to complete the 10 days of treatment. |
| Selective reporting (reporting bias) | Low risk | Limitations of the study were identified ‐ specifically smaller than planned sample size due to changes in clinical practice. This impacts the power of the study to detect an effect if one exists and may have contributed to lack of statistical differences between the intervention groups. |
| Other bias | Unclear risk | There appeared to be differences at baseline for age, FEV₁, and exercise performance. The PEP mask group was older, had a higher FEV₁, and could cover more distance in the 10‐metre shuttle test. It was noted that parents were allowed to assist their child in completing the satisfaction questionnaire. |
6MWD: six minute walk distance ACBT: active cycle of breathing ACT: airway clearance technique AD: autogenic drainage BMI: body mass index CF: cystic fibrosis CFRD: cystic fibrosis‐related diabetes CPT: chest physiotherapy FEF: forced expiratory flow FEV₁: forced expiratory volume at one second FVC: forced vital capacity HFCC: high frequency chest compression HFCWO: high force chest wall oscillation IPV: intrapulmonary percussive ventilator ITT: intention to treat IV: intravenous LCI: lung clearance index MEF: mid‐expiratory flow PD: postural drainage PD&C : postural drainage and clapping PD&P: postural drainage and percussion PEF: peak expiratory flow PEP: positive expiratory pressure PO₂: partial pressure of oxygen RCT: randomised controlled trial RFT: respiratory function test RV: residual volume SaO₂: pulse oximetry SD: standard deviation SpO₂: peripheral capillary oxygen saturation, an estimate of the amount of oxygen in the blood TLC: total lung capacity VC: vital capacity
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Amelina 2014 | No randomisation. Despite what is inferred by abstract, after translation of the full paper there is no evidence or comment made as regards randomisation. |
| Borka 2012 | Study considers comparing treatment sequence, when broken down into individual treatment options it is a single intervention study. |
| Cantin 2005 | Use of the frequencer which is not a therapy modality for comparison. |
| Cegla 1993 | Only one participant with CF involved in the study, therefore no comparable participants relevant to this review. |
| Dosman 2003 | Single‐intervention study. |
| Dunn 2013 | Single‐intervention study. |
| Dwyer 2017 | Single‐intervention study. |
| Elkins 2004 | Single‐intervention study. In addition uses non‐invasive ventilation which was not in our inclusion characteristics as a therapy modality for comparison. |
| Elkins 2005 | Single‐dose comparison study. |
| Fainardi 2011 | Single‐intervention study. |
| Grosse‐Onnebrink 2017 | Single‐intervention study. |
| Hartsell 1978 | Single‐dose comparison study. Uses a mechanical percussor which we have not formally included. Some of the study participants did not have CF. |
| Jarad 2010 | HAT is not a recognised airway clearance technique and therefore not in our inclusion criteria. In particular we stated that external oscillation applied to the chest wall should have an effect on expiratory airflow. This is not the case with HAT and consequently should not be judged as an airway clearance adjunct. |
| Kempainen 2007 | Single intervention and comparison made with HFCWO and different pressures and variable frequencies. |
| Kirkpatrick 1995 | Use of acoustic percussion, not a therapy we have chosen to compare as not inclusive of oscillation therapy as a comparator. |
| Konstan 1994 | After careful consideration of the methodology of the paper it was considered to be comparing single interventions only. |
| Kraemer 1996 | Evaluating a bronchodilator in sequence with flutter. This did not evaluate an oscillatory device with another form of ACT. |
| Lagerkvist 2006 | Single‐dose comparative study. Also principally looking at blood gases, tensions and RFTs were an aside. |
| Liedtke 1996 | Efficacy of beta2‐inhalation therapy in combination with respiratory physiotherapy. Not an oscillatory comparison with another ACT. |
| Lindemann 1992 | Single‐dose comparison study. |
| Majaesic 1996 | Outcome measure is sputum viscosity, which is not one of our outcome measures. |
| Marks 1998 | Single‐dose comparison study. |
| Marks 2004 | Single‐dose comparison study. |
| McCarren 2006 | Single‐dose comparison study. Physiological effects of vibration is not an outcome measure of the review. |
| Morris 1982 | Use of mechanical percussor, which is not in the inclusion criteria for therapies to be compared. |
| Natale 1994 | Comparison of 3 treatment techniques, but only single doses of each. |
| Newhouse 1998 | Single‐dose study. |
| O'Neil 2017 | Both groups received same oscillating device regimen, difference between groups was the timing of administration of hypertonic saline. |
| Orlik 2000a | CCT not RCT or quasi‐RCT. |
| Orlik 2000b | CCT not RCT or quasi‐RCT. |
| Orlik 2001 | CCT not RCT or quasi‐RCT. |
| Roos 1987 | Study was not completed when abstract was published. Authors were contacted but they were unable to provide us with any data to support this or any subsequently related study. |
| Salh 1989 | Assessing exercise for sputum clearance but not compared with oscillatory therapies. |
| Scherer 1998 | Single‐dose study. |
| Skopnik 1986 | Use of 'Knock and Vibration' therapy, which is not part of our review inclusion criteria. |
| Stites 2006 | Single‐dose comparison and outcome measure of drug deposition whilst using the device rather than evaluating it as an airway clearance system. |
| Van Ginderdeuren 2008 | Single‐dose study. |
| Webber 1984 | Self percussor, not in criteria for comparison as not oscillatory. |
ACT: airway clearance technique CF: cystic fibrosis HAT: hydro‐acoustic therapy RFT: respiratory function test
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Randomised cross‐over trial. Duration: each treatment arm lasted 5 consecutive days with 1 week washout period in between. Multicentre: 7 centres in Spain. |
| Participants | 19 CF stable participants, mean age (SD) 24.2 yrs (7.6) and FEV₁ 70.8% predicted (24.3). |
| Interventions | Intervention A: combined therapy (nebulised hypertonic saline plus oscillatory PEP (Acapella®)). Intervention B: classic nebulised hypertonic saline. |
| Outcomes | Sputum volume (during nebulisation, the subsequent physiotherapy and 24 h post‐physiotherapy). Pulmonary function, Leicester Cough Questionnaire (LCQ) and Cough and Sputum Assessment Questionnaire (CASA‐Q) (evaluated before and after each intervention). Participant preference (assessed using a Likert test (range 6–30). |
| Notes |
| Methods | Randomised parallel study. |
| Participants | 18 participants randomised to Metaneb®, 14 participants randomised to HFCWO. All admitted to hospital for management of a severe pulmonary exacerbation. Age (median (range)): 29 (19 ‐ 48) years. Mean BMI: 22.3 kg/m2. Mean FEV₁ % predicted: 41.4%. |
| Interventions | Metaneb® compared to HFCWO over a 14‐day period of hospitalisation. Frequency and duration of each treatment not identified. |
| Outcomes | Participant satisfaction, sputum expectorated, spirometry and CFQ‐R. |
| Notes | Await publication of full paper and further data requested for inclusion in analysis. |
| Methods |
Phase I: cross‐over RCT. Phase II: parallel RCT. |
| Participants |
Phase I 10 participants with mild to moderate disease Mean (SD) age: 30 (7) years. Mean (SD) height: 168 (10) cm. Mean (SD) weight: 67 (14) kg. Mean (SD) BMI: 24 (4) kg/m2. Mean (SD) BSA: 1.7 (0.2) m2. Mean (SD) FEV₁ % predicted: 70 (24) %. Mean (SD) FVC % predicted: 85 (20) %. Phase II 12 hospitalised participants (VibraLung® group n = 3; Vest® group n = 9). Mean (SD) age: 23 (6) years. Mean (SD) height: 165 (6) cm. Mean (SD) weight: 60 (10) kg. Mean (SD) BMI: 22 (3) kg/m2. Mean (SD) BSA: 1.7 (0.2) m2. Mean (SD) FEV₁ % predicted: 60 (20) %. Mean (SD) FVC % predicted: 76 (18) %. |
| Interventions |
Phase I: single intervention where VibraLung® used with sound or without sound for 20 minutes; on 2nd visit crossed over to alternative treatment. Phase II: 5 days of in‐hospital therapy for 2 sessions/day with either VibraLung® or the Vest®. |
| Outcomes |
Phase I: pulmonary function; lung diffusion for carbon monoxide and nitric oxide; lung clearance index; symptoms; oxygen saturation. Measurements at baseline, 1‐hour and 4‐hours post‐treatment. Phase II: sputum collected for 20 minutes post‐treatment. |
| Notes | Only Phase II likely eligible for inclusion; await full publication of results. |
BMI: body mass index BSA: body surface area CFQ‐R: cystic fibrosis questionnaire ‐ revised FEV₁: forced expiratory volume at one second FVC: forced vital capacity HAT: hydro acoustic therapy HFCWO: high frequency chest wall oscillation PEP: positive expiratory pressure RCT: randomised controlled trial SD: standard deviation
Contributions of authors
LM conceived and drafted the protocol and the review. JA commented on the protocol and the review. Both authors independently selected studies for inclusion in the review and extracted data.
From the 2017 update, JA stepped down from the review team and SI took on the role of co‐author.
LM acts as guarantor for this review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
This systematic review was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Cystic Fibrosis and Genetic Disorders Group.
Declarations of interest
Lisa Morrison declares that she has no interest in any of the papers or references within this document and has received no funding in whole or in part for any of this work.
Stephanie Innes: none known.
Edited (no change to conclusions)
References
References to studies included in this review
- App EM, Danzl G, Schweiger K, Kieselmann R, Reinhardt D, Lindemann H, et al. Sputum rheology changes in cystic fibrosis lung disease following two different types of physiotherapy ‐ VRP1 (flutter) versus autogenic drainage [abstract]. American Journal of Respiratory and Critical Care Medicine. 1995; Vol. 151, issue 4 Suppl:A737. [Google Scholar]; App EM, Kieselmann R, Reinhardt D, Lindemann H, Dasgupta B, King M, et al. Sputum rheology changes in cystic fibrosis lung disease following two different types of physiotherapy: flutter vs autogenic drainage. Chest 1998;114(1):171‐7. [DOI] [PubMed] [Google Scholar]
- Arens R, Gozal D, Omlin KJ, Vega J, Boyd KP, Keens TG, et al. Comparison of high frequency chest compression and conventional chest physiotherapy in hospitalized patients with cystic fibrosis. American Journal of Respiratory and Critical Care Medicine 1994;150(4):1154‐7. [DOI] [PubMed] [Google Scholar]; Arens R, Gozal D, Omlin KJ, Vega J, Boyd KP, Woo MS, et al. Comparative efficacy of high frequency chest compression and conventional chest physiotherapy in hospitalized patients with cystic fibrosis. Pediatric Pulmonology. 1993; Vol. Suppl 9:239. [DOI] [PubMed] [Google Scholar]
- Braggion C, Cappelletti LM, Cornacchia M, Zanolla L, Mastella G. Short‐term effects of three chest physiotherapy regimens in patients hospitalized for pulmonary exacerbations of cystic fibrosis: a cross‐over randomized study. Pediatric Pulmonology 1995;19(1):16‐22. [DOI] [PubMed] [Google Scholar]; Cappelletti LM, Cornacchia M, Braggion C, Zanolla L, Mastella G. Short‐term effects of 3 physiotherapy (CPT) regimens in cystic fibrosis (CF) patients hospitalized for a pulmonary exacerbation: a cross‐over randomized trial. In: Proceedings of 18th European Cystic Fibrosis Conference; 1993 May 21‐26; Madrid, Spain. 1993:W9.3. [Google Scholar]
- Darbee JC, Kanga JF, Ohtake PJ. Physiologic Eeidence for high‐frequency chest wall oscillation and positive expiratory pressure breathing in hospitalized subjects with cystic fibrosis. Physical Therapy 2005;85(12):1278‐89. [PubMed] [Google Scholar]; Darbee JC, Kanga JF, Ohtake PJ. Physiologic evidence for high frequency chest wall oscillation and positive expiratory pressure breathing in hospitalized patients with cystic fibrosis. Pediatric Pulmonology 2005;40(Suppl 28):322. [PubMed] [Google Scholar]
- Banks A, Davies G, Agent P, Osman L, Bilton D, Hodson M. The use of high frequency chest wall oscillation during an acute infective pulmonary exacerbation of cystic fibrosis. European Respiratory Journal 2012;40. [Abstract no: 3483; CENTRAL: 1100493; CFGD Register: PE197b; CRS: 5500050000000359; EMBASE: 71925445] [Google Scholar]; Davies GA, Banks AE, Agent P, Osman LP, Bilton D, Hodson ME. The use of high frequency chest wall oscillation during an acute infective pulmonary exacerbation of cystic fibrosis. Pediatric Pulmonology 2012;47(S35):366. [Abstract no: 396; CFGD Register: PE197a; ] [Google Scholar]
- Giles D, Sontag M, Wagener J, Accurso F. Effect of one month of treatment with flutter valve or postural drainage and clapping on pulmonary function and sputum recovery in cystic fibrosis. Pediatric Pulmonology. 1996; Vol. Suppl 13:307. [Google Scholar]
- Gondor M, Nixon PA, Mutich R, Rebovich P, Orenstein DM. Comparison of flutter device and chest physical therapy in the treatment of cystic fibrosis pulmonary exacerbation. Pediatric Pulmonology 1999;28(4):255‐60. [DOI] [PubMed] [Google Scholar]; Gondor M, Nixon PA, Rebovich PJ, Orenstein DM. A comparison of the flutter device and chest physical therapy in the treatment of cystic fibrosis pulmonary exacerbation. Pediatric Pulmonology 1996;Suppl 13:307. [DOI] [PubMed] [Google Scholar]
- Gotz M, Wolkerstorfer A. Physiotherapy in cystic fibrosis: Intrapulmonary percussive ventilation (IPV) versus positive expiratory pressure (PEP). Pediatric Pulmonology 1995;20(Suppl 12):267. [Google Scholar]
- Grzincich GL, Longon F, Faverzani S, Chetta A, Spaggiari C, Pisi G. Short‐term effects of high‐frequency chest compression (HFCC) and positive expiratory pressure (PEP) in adults with cystic fibrosis. Proceedings of European Respiratory Society Annual Congress; 2008 Oct 4‐8; Berlin, Germany. 2008:502s. [Google Scholar]
- Hansen LG, Warwick WJ. High‐frequency chest compression system to aid in clearance of mucus from the lung. Biomedical Instrumentation & Technology 1990;24(4):289‐94. [PubMed] [Google Scholar]
- Hare KL, Homnick DN, Cucos D, Marks JH. The PercussiveTech HF device compared to standard chest physiotherapy in hospitalized patients with cystic fibrosis. Pediatric Pulmonology 2002;Suppl 24:316. [Google Scholar]
- Homnick D, Spillers C, White F. The intrapulmonary percussive ventilator compared to standard aerosol therapy and chest physiotherapy in the treatment of patients with cystic fibrosis. Pediatric Pulmonology 1994;Suppl 10:266. [Google Scholar]; Homnick DN, White F, Castro C. Comparison of effects of an intrapulmonary percussive ventilator to standard aerosol and chest physiotherapy in treatment of cystic fibrosis. Pediatric Pulmonology 1995;20(1):50‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Homnick DN, Anderson K, Marks JH. Comparison of the flutter device to standard chest physiotherapy in hospitalized patients with cystic fibrosis: a pilot study. Chest 1998;114(4):993‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]; Homnick DN, Marks JH. Comparison of the flutter device to standard chest physiotherapy in hospitalized patients with cystic fibrosis. Pediatric Pulmonology 1996;Suppl 13:308. [DOI] [PubMed] [Google Scholar]
- Khan MA, Lian NA, Mikitchenko NA. The use of high‐frequency chest wall oscillation for the combined treatment of the children presenting with mucoviscidosis. Voprosy Kurortologii, Fizioterapii, i Lechebnoi Fizicheskoi Kultury 2014, (3):22‐6. [CFGD Register: PE227; CRS: 5500135000001513; PUBMED: 25087417] [PubMed] [Google Scholar]
- Kluft J, Beker L, Castagnino M, Gaiser J, Chaney H, Fink RJ. A comparison of bronchial drainage treatments in cystic fibrosis. Pediatric Pulmonology 1996;22(4):271‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lyons E, Chatham K, Campbell IA, Prescott RJ. Evaluation of the flutter VRP1 device in young adults with cystic fibrosis. 11th International Cystic Fibrosis Congress; 1992; Dublin, Ireland. 1992:AHP30. [Google Scholar]; Lyons E, Chatham K, Campbell IA, Prescott RJ. Evaluation of the flutter VRP1 device in young adults with cystic fibrosis. Thorax 1992;47(3):237P. [CFGD Register: PE60b] [Google Scholar]
- Marks JH, Hare K, Homnick DN. The PercussiveTech HF compared to the flutter in cystic fibrosis patients: a six month pilot study. Proceedings of 24th European Cystic Fibrosis Conference; 2001 June 6‐9; Vienna, Austria. 2001:P101. [Google Scholar]; Marks JH, Homnick DN, Hare K, Cucos D. The PercussiveTech HF compared to the flutter device in cystic fibrosis patients: a six month pilot study. Pediatric Pulmonology 2001;32(Suppl 22):309. [Google Scholar]
- Davidson AGF, McIlwaine PM, Wong LTK, Peacock D. "Flutter versus PEP": A long‐term comparative trial of positive expiratory pressure (PEP) versus oscillating positive expiratory pressure (Flutter) physiotherapy techniques. In: Proceedings of 22nd European Cystic Fibrosis Conference; 1998 June 13‐19; Berlin, Germany. 1998:71. [Google Scholar]; McIlwaine PM, Wong LT, Peacock D, Davidson AG. Long‐term comparative trial of positive expiratory pressure versus oscillating positive expiratory pressure (flutter) physiotherapy in the treatment of cystic fibrosis. Journal of Pediatrics 2001;138(6):845‐50. [DOI] [PubMed] [Google Scholar]; McIlwaine PM, Wong LTK, Peacock D, Davidson AGF. "Flutter versus PEP": A long‐term comparative trial of positive expiratory pressure (PEP) versus oscillating positive expiratory pressure (FLUTTER) physiotherapy. Pediatric Pulmonology 1997;23(Suppl 14):299. [Google Scholar]
- Alarie N, Agnew JL, McIlwaine M, Ratjen F, Davidson G, Lands LC. Canadian National Airway Clearance Study: how physically active are CF patients?. Pediatric Pulmonology 2012;47(S35):367. [Abstract no: 398; CFGD Register: PE187e; ]22102598 [Google Scholar]; Alarie N, Agnew LL, McIlwaine MP, Ratjen F, Davidson GF, Milner R, et al. Evaluation of physical activity using the habitual activity estimation scale (HAES) questionnaire in a multicenter study. Journal of Cystic Fibrosis 2013;12 Suppl 1:S28. [Abstract no: WS14.1; CFGD Register: PE187f; ] [Google Scholar]; McIlwaine M, Agnew J, Alarie N, Ratjen F, Lands L, Milner R, et al. Canadian national airway clearance study: patient satisfaction with positive expiratory pressure versus high frequency chest wall oscillation. Pediatric Pulmonology 2012;47(S35)(S35):367. [Abstract no: 397; CFGD Register: PE187b; ]22102598 [Google Scholar]; McIlwaine M, Agnew JL, Alarie N, Lands L, Ratjen F, Milner R, et al. Canadian national airway clearance study: positive expiratory pressure mask versus high frequency chest wall oscillation. Journal of Cystic Fibrosis 2012;11 Supplement 1:S23. [Abstract no: WS10.6; CFGD Register: PE187a] [Google Scholar]; McIlwaine MP, Alarie N, Davidson GF, Lands LC, Ratjen F, Milner R, et al. Long‐term multicentre randomised controlled study of high frequency chest wall oscillation versus positive expiratory pressure mask in cystic fibrosis. Thorax 2013;68(8):746‐51. [CFGD Register: PE187c; ] [DOI] [PubMed] [Google Scholar]; McIlwaine MP, Alarie N, Davidson GF, Lands LC, Ratjen F, Milner R, et al. Online Supplement to "Long‐term multicentre randomised controlled study of high frequency chest wall oscillation versus positive expiratory pressure mask in cystic fibrosis" [online]. Thorax 2013;68(8):746‐51 Online. [CFGD Register: PE187d; ] [DOI] [PubMed] [Google Scholar]
- Milne SM, Eales CJ. A pilot study comparing two physiotherapy techniques in patients with cystic fibrosis. South African Journal of Physiotherapy 2004;60(2):3‐6. [Google Scholar]
- Accurso FJ, Sontag MK, Koenig JM, Quittner AL. Multi‐center airway secretion clearance study in cystic fibrosis. Pediatric Pulmonology 2004;38(Suppl 27):314. 15334509 [Google Scholar]; Modi AC, Cassedy AE, Quittner AL, Accurso F, Sontag M, Koenig JM, et al. Trajectories of adherence to airway clearance therapy for patients with cystic fibrosis. Journal of Pediatric Psychology 2010;35(9):1028‐37. [CFGD Register: PE152e] [DOI] [PubMed] [Google Scholar]; Modi AC, Sontag MK, Koenig JM, Accurso FJ, Quittner AL, Investigators and Coordinators of the Airway Secretion Clearance Study. Adherence to airway clearance therapies in patients with cystic fibrosis. Journal of Cystic Fibrosis 2006;5 Suppl:S97. [Google Scholar]; Quittner AL, Modi AC, Accurso FJ, Koenig JM, Sontag MK, Oermann C, et al. Treatment satisfaction, health‐related quality of life and airway clearance therapies in patients with cystic fibrosis. Pediatric Pulmonology 2004;38(Suppl 27):314. 15334509 [Google Scholar]; Sontag MK, Quittner AL, Modi AC, Koenig JM, Giles D, Oermann CM, et al. Lessons learned from a randomized trial of airway secretion clearance techniques in cystic fibrosis. Pediatric Pulmonology 2010;45(3):291‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Accurso FJ, Sontag MK, Koenig JM, Quittner AL. Multi‐center airway secretion clearance study in cystic fibrosis. Pediatric Pulmonology 2004;38(Suppl 27):314. 15334509 [Google Scholar]; Modi AC, Cassedy AE, Quittner AL, Accurso F, Sontag M, Koenig JM, et al. Trajectories of adherence to airway clearance therapy for patients with cystic fibrosis. Journal of Pediatric Psychology2010; Vol. 35, issue 9:1028‐37. [CFGD Register: PE152e] [DOI] [PubMed]; Modi AC, Sontag MK, Koenig JM, Accurso FJ, Quittner AL, Investigators and Coordinators of the Airway Secretion Clearance Study. Adherence to airway clearance therapies in patients with cystic fibrosis. Journal of Cystic Fibrosis 2006;5 Suppl:S97. [Google Scholar]; Quittner AL, Modi AC, Accurso FJ, Koenig JM, Sontag MK, Oermann C, et al. Treatment satisfaction, health‐related quality of life and airway clearance therapies in patients with cystic fibrosis. Pediatric Pulmonology 2004;38(Suppl 27):314. 15334509 [Google Scholar]; Sontag MK, Quittner AL, Modi AC, Koenig JM, Giles D, Oermann CM, et al. Lessons learned from a randomized trial of airway secretion clearance techniques in cystic fibrosis. Pediatric Pulmonology 2010;45(3):291‐300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newbold ME, Brooks D, Tullis DE, Ross BG. Effectiveness of the flutter device versus the PEP mask in the treatment of adult cystic fibrosis. Pediatric Pulmonology 2000;30(Suppl 20):304. [Google Scholar]; Newbold ME, Tullis E, Corey, M, Ross B, Brooks D. The flutter device versus the PEP mask in the treatment of adults with cystic fibrosis. Physiotherapy Canada 2005;57(3):199‐207. [Google Scholar]
- Oermann CM, Sockrider MM, Giles D, Sontag MK, Accurso FJ, Castile RG. Comparison of high‐frequency chest wall oscillation and oscillating positive expiratory pressure in the home management of cystic fibrosis: a pilot study. Pediatric Pulmonology 2001;32(5):372‐7. [DOI] [PubMed] [Google Scholar]
- Osman LP, Roughton M, Hodson ME, Pryor JA. High frequency chest wall oscillation in cystic fibrosis. Journal of Cystic Fibrosis 2008;7(Suppl 2):295. [CFGD Register: PE171a]18234567 [Google Scholar]; Osman LP, Roughton M, Hodson ME, Pryor JA. Short‐term comparative study of high frequency chest wall oscillation and European airway clearance techniques in patients with cystic fibrosis. Thorax 2010;65(3):196‐200. [CFGD Register: PE171b; ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padman R, Geouque DM, Engelhardt MT. Effects of the flutter device on pulmonary function studies among pediatric cystic fibrosis patients. Delaware Medical Journal 1999;71(1):13‐8. [PubMed] [Google Scholar]
- Padman R, Geouque DM, Engelhardt MT. Effects of the flutter device on pulmonary function studies among pediatric cystic fibrosis patients. Delaware Medical Journal 1999;71(1):13‐8. [PubMed] [Google Scholar]
- Phillips GE, Pike S, Jaffe A, Bush A. Comparison of the active cycle of breathing techniques and external high frequency oscillation jacket for clearance of secretions in children with cystic fibrosis. Thorax 1998;53(Suppl 4):A61. [Google Scholar]; Phillips GE, Pike SE, Jaffe A, Bush A. Comparison of active cycle of breathing and high‐frequency oscillation jacket in children with cystic fibrosis. Pediatric Pulmonology 2004;37(1):71‐5. [DOI] [PubMed] [Google Scholar]
- Pike SE, Machin AC, Dix KJ, Pryor JA, Hodson ME. Comparison of flutter VRPI and forced expirations (FE) with active cycle of breathing techniques (ACBT) in subjects with cystic fibrosis (CF). The Netherlands Journal of Medicine 1999;54(Suppl):S55‐6. [Google Scholar]
- Main E, Tannenbaum E, Stanojevic S, Scrase E, Prasad A. The effects of positive expiratory pressure (PEP) or oscillatory positive pressure (RC Cornet®) on FEV1 and lung clearance index over a twelve month period in children with CF. Pediatric Pulmonology 2006;41(Suppl 29):351. [Google Scholar]; Prasad A, Tannenbaum E, Bryon M, Main E. One year trial of two airway clearance techniques in children with cystic fibrosis: limitations of the quality of well‐being scale. Pediatric Pulmonology 2005;40(Suppl 28):323. [Google Scholar]; Tannenbaum E, Prasad SA, Main E, Scrase E. Long‐term effects of positive expiratory pressure (PEP) or oscillatory positive pressure (RC Cornet®) on FEV1 and perceived health in children with CF. Proceedings of 28th European Cystic Fibrosis Conference; 2005 June 22‐25; Crete, Greece. 2005:S100. [Google Scholar]
- Pryor JA, Webber BA, Hodson ME, Warner JO. The Flutter VRP1 as an adjunct to chest physiotherapy in cystic fibrosis. 11th International Cystic Fibrosis Congress; 1992; Dublin, Ireland. 1992:WP 102. [DOI] [PubMed] [Google Scholar]; Pryor JA, Webber BA, Hodson ME, Warner JO. The Flutter VRP1 as an adjunct to chest physiotherapy in cystic fibrosis. Respiratory Medicine 1994;88(9):677‐81. [DOI] [PubMed] [Google Scholar]
- Pryor JA, Tannenbaum E, Cramer D, Scott SF, Burgess J, Gyi K, et al. A comparison of five airway clearance techniques in the treatment of people with Cystic Fibrosis [abstract]. Journal of Cystic Fibrosis 2006;5(Suppl):S76. [DOI] [PubMed] [Google Scholar]; Pryor JA, Tannenbaum E, Scott SF, Burgess J, Cramer D, Gyi K, et al. Beyond postural drainage and percussion: Airway clearance in people with cystic fibrosis. Journal of Cystic Fibrosis 2010;9(3):187‐92. [DOI] [PubMed] [Google Scholar]
- Winden CMQ, Visser A, Hop W, Sterk PJ, Beckers S, Jongste JC. Effects of Flutter and PEP‐MASK on expectoration and lung function in cystic fibrosis. Israel Journal of Medical Sciences 1996;32(Suppl):S275. [Google Scholar]; Winden CMQ, Visser A, Hop W, Sterk PJ, Beckers S, Jongste JC. Effects of flutter and PEP mask physiotherapy on symptoms and lung function in children with cystic fibrosis. European Respiratory Journal 1998;12(1):143‐7. [DOI] [PubMed] [Google Scholar]
- Castile R, Tice J, Flucke R, Filbrun D, Varekojis S, McCoy K. Comparison of three sputum clearance methods in in‐patients with cystic fibrosis. Pediatric Pulmonology 1998;26(Suppl 17):329. [MEDLINE: ] [Google Scholar]; Varekojis SM, Douce FH, Flucke RL, Filbrun DA, Tice JS, McCoy KS, et al. A comparison of the therapeutic effectiveness of and preference for postural drainage and percussion, intrapulmonary percussive ventilation, and high‐frequency chest wall compression in hospitalized cystic fibrosis patients. Respiratory Care 2003;48(1):24‐8. [PubMed] [Google Scholar]
- Castile R, Tice J, Flucke R, Filbrun D, Varekojis S, McCoy K. Comparison of three sputum clearance methods in in‐patients with cystic fibrosis. Pediatric Pulmonology 1998;26(Suppl 17):329. [MEDLINE: ] [Google Scholar]; Varekojis SM, Douce FH, Flucke RL, Filbrun DA, Tice JS, McCoy KS, et al. A comparison of the therapeutic effectiveness of and preference for postural drainage and percussion, intrapulmonary percussive ventilation, and high‐frequency chest wall compression in hospitalized cystic fibrosis patients. Respiratory Care 2003;48(1):24‐8. [PubMed] [Google Scholar]
- Warwick WJ, Wielnski CI. Matched pair comparison of manual chest physical therapy (CPT) and the thairapy bronchial drainage vest (ThBVD) system. Pediatric Pulmonology 1990;9 Suppl 5:177. 2277738 [Google Scholar]
- Warwick WJ, Wielinski CL, Hansen LG. Comparison of expectorated sputum after manual chest physical therapy and high‐frequency chest compression. Biomedical Instrumentation Technology 2004;38(6):470‐5. [DOI] [PubMed] [Google Scholar]
- West K, Wallen M, Follett J. Acapella vs. PEP mask therapy: a randomised trial in children with cystic fibrosis during respiratory exacerbation. Physiotherapy Theory and Practice 2010;26(3):143‐9. [CENTRAL: 753270; CFGD Register: PE213; CRS: 5500050000000049; PUBMED: 20331370] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Amelina EL, Krasovskii SA, Usacheva MV, Krylova NA. [Use of high‐frequency chest wall oscillation in an exacerbation of chronic pyo‐obstructive bronchitis in adult patients with cystic fibrosis]. [Russian]. Terapevticheskii Arkhiv 2014;86(12):33‐6. [CFGD Register: PE226b; CRS: 5500135000000122; PUBMED: 25804037] [DOI] [PubMed] [Google Scholar]; Krasovskij S, Amelina E, Usacheva M, Samoylenko V, Krilova N. High frequency chest wall oscillation (HFCWO) in the treatment of acute pulmonary exacerbation in adult cystic fibrosis (CF) patients. European Respiratory Society Annual Congress; 2013 Sept 7‐11; Barcelona, Spain 2013;42:741s. [Abstract no: P3599; CENTRAL: 990024; CFGD Register: PE226a; CRS: 5500050000000365] [Google Scholar]
- Borka P, Gyurkovits K, Bódis J. Comparative study of PEP mask and Flutter on expectoration in cystic fibrosis patients. Acta Physiologica Hungarica 2012;99(3):324‐331. [DOI] [PubMed] [Google Scholar]
- Cantin AM, Berthiaume Y. Clearance of airway secretions with the Frequencer in patients with cystic fibrosis. Pediatric Pulmonology 2004;38(Suppl 27):322. [Google Scholar]
- Cegla UH, Retzow A. Physical therapy with VRP1 in chronic obstructive respiratory tract diseases‐‐results of a multicenter comparative study [Physiotherapie mit dem VRP1 bei chronisch obstruktiven Atemwegserkrankungen‐‐Ergebnisse einer multizentrischen Vergleichsstudie]. Pneumologie 1993;47(11):636‐9. [PubMed] [Google Scholar]
- Dosman CF, Zuberbuhler PC, Tabak JI, Jones RL. Effects of positive end‐expiratory pressure on oscillated volume during high frequency chest compression in children with cystic fibrosis. Canadian Respiratory Journal 2003;10(2):94‐8. [CFGD Register: PE144; ] [DOI] [PubMed] [Google Scholar]
- Dunn C, Davies Z, Everson C, Zirbes J, Kim L, Milla C. Study of acute effects on pulmonary function and sputum production with high frequency chest oscillation (HFCWO) and postural drainage aided by handheld percussion (P‐HP). Pediatric Pulmonology 2013;48 Suppl 36:359. [Abstract no: 421; CFGD Register: PE205b; ] [Google Scholar]; Dunn C, Davies Z, Kim L, Zirbes J, Everson C, Milla C. Comparison of acute effects of conventional high frequency chest oscillation (HFCWO) and hand held percussor (Electro‐Flo 5000) for airway clearance in cystic fibrosis patients. Journal of Cystic Fibrosis 2013;12 Suppl 1:S103. [Abstract no: 215; CFGD Register: PE205a; ] [Google Scholar]
- Dwyer TJ, Zainuldin R, Daviskas E, Bye PT, Alison JA. Effects of treadmill exercise versus Flutter(R) on respiratory flow and sputum properties in adults with cystic fibrosis: a randomised, controlled, cross‐over trial. BMC Pulmonary Medicine 2017;17(1):14. [CFGD Register: PE239 ; CRS: 5500135000001880; PUBMED: 28077104] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkins MR, Eberl S, Alison J, Bye P. The effect of bi‐level non‐invasive ventilation on mucociliary clearance in subjects with cystic fibrosis. Pediatric Pulmonology 2004;38(Suppl 27):315. [Google Scholar]
- Elkins MR, Eberl S, Constable C, White J, Robinson M, Daviskas E, et al. The effect of manual chest physiotherapy, positive expiratory pressure (PEP), and oscillating PEP on mucociliary clearance in subjects with cystic fibrosis. Pediatric Pulmonology 2005;40(Suppl 28):321. [Google Scholar]
- Fainardi V, Longo F, Faverzani S, Tripodi MC, Chetta A, Pisi G. Short‐term effects of high‐frequency chest compression and positive expiratory pressure in patients with cystic fibrosis. Journal of Clinical Medicine Research 2011;3(6):279‐84. [CENTRAL: 983057; CFGD Register: PE211; CRS: 5500125000000625; PUBMED: 22393338] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosse‐Onnebrink J, Mellies U, Olivier M, Werner C, Stehling F. Chest physiotherapy can affect the lung clearance index in cystic fibrosis patients. Pediatric Pulmonology 2017;52(5):625‐31. [CENTRAL: 1343708; CFGD Register: PE238; CRS: 5500135000001898; PUBMED: 28125158] [DOI] [PubMed] [Google Scholar]
- Hartsell M, Traver G, Taussig LM. Comparison of manual percussion and vibration (P &V) vs. mechanical vibration (MV) alone on maximal expiratory flows. 19th Cystic Fibrosis Club Abstracts. 1978:49. [Google Scholar]
- Jarad NA, Powell T, Smith CE, Cartwright P, Nedwell J. The efficacy, preference and safety of a novel method of sputum clearance, hydro acoustic therapy, on adult patients with cystic fibrosis. Thorax 2006;61(Suppl 2):ii120; P194. [CFGD Register: PE172a] [Google Scholar]; Jarad NA, Powell T, Smith E. Evaluation of a novel sputum clearance technique‐‐hydro‐acoustic therapy (HAT) in adult patients with cystic fibrosis: a feasibility study. Chronic Respiratory Diseases 2010;7(4):217‐27. [CFGD Register: PE172b] [DOI] [PubMed] [Google Scholar]
- Kempainen R, Hazelwood A, Williams C, Dunitz J, Billings J, Milla C. Comparison of airway clearance efficacy of sine and triangular wave high frequency chest wall oscillation in patients with cystic fibrosis. Pediatric Pulmonology 2006;41 Suppl 29:351. [CFGD Register: PE166a] [Google Scholar]; Kempainen RR, Milla C, Dunitz J, Savik K, Hazelwood A, Williams C, et al. Comparison of settings used for high‐frequency chest‐wall compression in cystic fibrosis. Respiratory Care 2010;55(6):695‐701. [CENTRAL: 761016; CFGD Register: PE166c; CRS: 5500050000000193; PUBMED: 20507651] [PubMed] [Google Scholar]; Kempainen RR, Williams CB, Hazelwood A, Rubin BK, Milla CE. Comparison of high‐frequency chest wall oscillation with differing waveforms for airway clearance in cystic fibrosis. Chest 2007;132(4):1227‐32. [CFGD Register: PE166b] [DOI] [PubMed] [Google Scholar]
- Kirkpatrick KR, Howard D, Ter‐Pogossian M, Kollef MH. A direct comparison of manual chest percussion with acoustic percussion, an experimental treatment for cystic fibrosis [abstract]. American Journal of Respiratory and Critical Care Medicine 1995;151(4 Suppl):A738. [Google Scholar]
- Konstan MW, Stern RC, Doershuk CF. Efficacy of the Flutter device for airway mucus clearance in patients with cystic fibrosis. Journal of Pediatrics 1994;124(5 (Pt 1)):689‐93. [DOI] [PubMed] [Google Scholar]
- Kraemer D, Liedtke C, Casaulta Aebischer C. Bronchodilator inhalation (BD) treatment in sequence with flutter VRP1 chest physiotherapy (CPT) in patients with cystic fibrosis. Israel Journal of Medical Sciences 1996;32(Suppl):S192. [Google Scholar]
- Lagerkvist AL, Sten G, Lindblad A, Redfors S. Chest physiotherapy with positive expiratory pressure (PEP) and oscillating positive expiratory pressure (flutter) in patients with cystic fibrosis‐a comparative study. Proceedings of 21st European Cystic Fibrosis Conference; 1997 June 1‐6; Davos, Switzerland. 1997:132. [Google Scholar]; Lagerkvist AL, Sten GM, Redfors SB, Lindblad AG, Hjalmarson O. Immediate changes in blood‐gas tensions during chest physiotherapy with positive expiratory pressure and oscillating positive expiratory pressure in patients with cystic fibrosis. Respiratory Care 2006;51(10):1154‐61. [PubMed] [Google Scholar]
- Liedtke D, Casaulta Aebischer C, Martin N, Schibler A, Kraemer R. Mucociliar clearance (MCC) in patients with cystic fibrosis (CF) ‐ Efficacy of beta2‐inhalation therapy (beta2) in combination with respiratory physiotherapy [abstract] [Mukoziliare Clearance bei (MCC) bei Patienten mit cystischer Fibrose (CF) ‐ Effizienz der beta2‐Inhalationstherapie (beta2) in Kombination mit Atemphysiotherapie (APT)]. Schweizerische Medizinische Wochenschrift 1996;126(Suppl 78):29S. [Google Scholar]
- Lindemann H. The value of physical therapy with VRP 1‐Desitin ("Flutter") [Zum Stellenwert der Physiotherapie mit dem VRP 1‐Desitin ("Flutter")]. Pneumologie 1992;46(12):626‐30. [PubMed] [Google Scholar]
- Majaesic CM, Montgomery M, Jones R, King M. Reduction in sputum viscosity using high frequency chest compressions (HFCC) compared to conventional chest physiotherapy (CCP). Pediatric Pulmonology 1996;Suppl 13:308. [Google Scholar]
- Marks JH, Fooy C, Anderson K, Homnick DN. Nebulized albuterol delivered with positive expiratory pressure (PEP) and the flutter device in patients with cystic fibrosis: an assessment of bronchodilator response compared to standard nebulizer therapy. American Journal of Respiratory and Critical Care Medicine. 1998; Vol. 157, issue 3 Suppl:A130. [Google Scholar]
- Marks J, Hare K, Homnick D. The PercussiveTech HF device compared to standard chest physiotherapy in patients with cystic fibrosis. Proceedings of 13th International Cystic Fibrosis Congress; 2000 June 4‐8; Stockholm, Sweden. 2000:151. [Google Scholar]; Marks JH, Hare KL, Homnick DN. Pulmonary function and sputum production in patients with cystic fibrosis: a pilot study comparing the percussive tech HF device and standard chest physiotherapy. Pediatric Pulmonology 1999;28(Suppl 19):290. [DOI] [PubMed] [Google Scholar]; Marks JH, Hare KL, Saunders RA, Homnick DN. Pulmonary function and sputum production in patients with cystic fibrosis: a pilot study comparing the PercussiveTech HF device and standard chest physiotherapy. Chest 2004;125(4):1507‐11. [DOI] [PubMed] [Google Scholar]
- McCarren B, Alison JA. Comparison of vibration to other physiotherapy interventions for secretion clearance. European Respiratory Journal 2005;26(Suppl 49):497s. [Google Scholar]; McCarren B, Alison JA. Physiological effects of vibration in subjects with cystic fibrosis. European Respiratory Journal 2006;27(6):1204‐9. [DOI] [PubMed] [Google Scholar]
- Morris D, Barbero G, Konig P, Woodruff C, Kline J, Martinez R. The effect of mechanical and manual percussion on pulmonary function in cystic fibrosis patients. 23rd Annual Meeting Cystic Fibrosis Club Abstracts; 1982 May 14; Washington D.C. 1982:135. [Google Scholar]
- Natale JE, Pfeifle J, Homnick DN. Comparison of intrapulmonary percussive ventilation and chest physiotherapy. A pilot study in patients with cystic fibrosis. Chest 1994;105(6):1789‐93. [DOI] [PubMed] [Google Scholar]
- Newhouse P, White F, Marks J, Homnick D. Pulmonary function testing and sputum production in patients with cystic fibrosis: A pilot study comparing the flutter device, intrapulmonary percussive ventilator, and standard chest physiotherapy. Pediatric Pulmonology 1995;Suppl 12:269. [Google Scholar]; Newhouse PA, White F, Marks JH, Homnick DN. The intrapulmonary percussive ventilator and flutter device compared to standard chest physiotherapy in patients with cystic fibrosis. Clinical Pediatrics 1998;37(7):427‐32. [DOI] [PubMed] [Google Scholar]
- O'Neil K, Moran F, Bradbury I, Downey DG, Rendall J, Tunney MM, et al. Exploring the timing of hypertonic saline (HTS) and airways clearance techniques (ACT) in cystic fibrosis (CF): a crossover study. Thorax 2016;71 Suppl 3:A134. [Abstract no: P95; CENTRAL: 1253123; CFGD Register: BD231a; CRS: 5500135000001677] [Google Scholar]; O'Neill K, Moran F, Tunney MM, Elborn JS, Bradbury I, Downey DG, et al. Timing of hypertonic saline and airway clearance techniques in adults with cystic fibrosis during pulmonary exacerbation: Pilot data from a randomised crossover study. BMJ Open Respiratory Research 2017;4(1). [CENTRAL: 1297377; CFGD Register: BD231b; CRS: 5500135000001896; CTG: NCT01753869; EMBASE: 614069626] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlik T. Evaluation of the efficiency of selected thoracic physiotherapy methods used in the treatment of patients with cystic fibrosis [Ocena efektywnosci wybranych metod fizjoterapii klatki piersiowej stosowanej w leczeniu chorych na mukowiscydoze]. Medycyna Wieku Rozwojowego 2000;4(3):233‐46. [PubMed] [Google Scholar]
- Orlik T. Estimation of autodrainage methods in a selected group of cystic fibrosis patients with home environment factors taken into consideration [Ocena metod autodren azu w wybranej grupie chorych na mukowiscydoze z uwzglednieniem czynnika strodowiskowego]. Medycyna Wieku Rozwojowego 2000;4(3):247‐59. [PubMed] [Google Scholar]
- Orlik T, Sands D. Long‐term evaluation of effectiveness for selected chest physiotherapy methods used in the treatment of cystic fibrosis [Dlugofalowa ocena skutecznosci wybranych metod fizjoterapii klatki piersiowej stoswanych w leczeniu chorych na mukowiscydoze]. Medycyna Wieku Rozwojowego 2001;5(3):245‐57. [PubMed] [Google Scholar]; Orlik T, Sands D. Long‐term study of efficiencies of select physiotherapy methods used in the treatment of cystic fibrosis. Proceedings of 24th European Cystic Fibrosis Conference; 2001 June 6‐9; Vienna, Austria. 2001:P113. [Google Scholar]
- Roos S, Birrer P, Rüdeberg A, Kraemer R. First experience with intrapulmonary percussive ventilation (IPV) in the treatment of patients with cystic fibrosis. Proceedings of 15th Annual Meeting of the European Working Group for Cystic Fibrosis; 1987; Oslo, Norway. 1987. [Google Scholar]
- Salh W, Bilton D, Dodd M, Webb AK. Effect of exercise and physiotherapy in aiding sputum expectoration in adults with cystic fibrosis. Thorax 1989;44(12):1006‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer TA, Barandun J, Martinez E, Wanner A, Rubin EM. Effect of high‐frequency oral airway and chest wall oscillation and conventional chest physical therapy on expectoration in patients with stable cystic fibrosis. Chest 1998;113(4):1019‐27. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Skopnik H, Waters W, Horowitz AE, Roth B, Benz‐Bohm G. Evaluation of Knock and Vibrattion Therapy and Autogenic drainage in Cystic Fibrosis Using a Ventilation Zintigraphical method [Beurteilung der Klopf und Vibrationstherapie und autogenic drainage bei cystischer fibrose mittels eines ventilationsszintigrafischen Verfahrens]. Monatsscurift fur Kinderheilkunde. 1986; Vol. 134:583. [Google Scholar]
- Stites SW, Perry GV, Peddicord T, Cox G, McMillan C, Becker B. Effect of high‐frequency chest wall oscillation on the central and peripheral distribution of aerosolized diethylene triamine penta‐acetic acid as compared to standard chest physiotherapy in cystic fibrosis. Chest 2006;129(3):712‐7. [DOI] [PubMed] [Google Scholar]
- Ginderdeuren F, Verbanck S, Cauwelaert K, Vanlaethem S, Schuermans D, Vincken W, et al. Chest physiotherapy in cystic fibrosis: short‐term effects of autogenic drainage preceded by wet inhalation of saline versus autogenic drainage preceded by intrapulmonary percussive ventilation with saline. Respiration 2008;76(2):175‐80. [CFGD Register: BD186] [DOI] [PubMed] [Google Scholar]
- Webber BA, Parker RA, Hofmeyr JL, Hodson ME. Evaluation of self‐percussion during postural drainage using the forced expiration technique (FET). Proceedings of 9th International Cystic Fibrosis Congress; 1984 June 9‐15; Brighton, England. 1984:2.12. [Google Scholar]
References to studies awaiting assessment
- Herrero Cortina B, San Miguel Pagola M, Cebria i Ranzo MA, Gomez Romero M, Diaz Gutierrez F, Reychler G. Short‐term effects of hypertonic saline nebulization combined with oscillatory positive expiratory pressure in cystic fibrosis: randomised crossover trial. Journal of Cystic Fibrosis 2016;15 Suppl 1:S33. [Abstract no: WS21.3; CENTRAL: 1155402; CFGD Register: BD229a; CRS: 5500135000001528] [Google Scholar]; San Miguel Pagola M, Herrero Cortina B, Cebria i Iranzo MA, Gomez Romero M, Diaz Gutierrez F, Reychler G. Hypertonic saline nebulization combined with oscillatory positive expiratory pressure accelerate sputum clearance in cystic fibrosis: A randomised crossover trial. European Respiratory Journal 2016;48(Suppl 60):PA1369. [CENTRAL: 1343707; CFGD Register: BD229b; CRS: 5500135000001897] [Google Scholar]
- Patel P, Fukushima L, Balekian A, Chou W, Lu A, Gali V, et al. Is Metaneb comparable to high frequency chest compression in the setting of a severe pulmonary exacerbation in adults with cystic fibrosis. Pediatric Pulmonology 2013;48 Suppl 36:359. [Abstract no: 420; CFGD Register: PE209; ] [Google Scholar]
- Wheatley CM, Baker SE, Daines C, Phan H, Morgan WJ, Snyder EM. Influence of the vibralung device on pulmonary function and sputum expectoration in patients with cystic fibrosis. Pediatric Pulmonology 2013;48 Suppl 36:357. [Abstract no: 416; CFGD Register: PE208; ] [Google Scholar]
Additional references
- Cantin A. Cystic fibrosis lung inflammation: early, sustained, and severe. American Journal of Respiratory and Critical Care Medicine 1995;151(4):939‐41. [DOI] [PubMed] [Google Scholar]
- Chevaillier J. Autogenic drainage. In: Lawson D editor(s). Cystic Fibrosis: Horizons. London: Churchill Livingstone, 1984:65‐78. [Google Scholar]
- Curtin F, Altman DG, Elbourne E. Meta‐analysis combining parallel and cross‐over clinical trials. I: Continuous outcomes. Statistics in Medicine 2002;21:2131–44. [DOI] [PubMed] [Google Scholar]
- Curtin F, Altman DG, Elbourne E. Meta‐analysis combining parallel and cross‐over clinical trials. III: The issue of carryover. Statistics in Medicine 2002;21:2161‐73. [DOI] [PubMed] [Google Scholar]
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140‐9. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Altman DG, Sterne JAC on behalf of the CSMG and the CBMG, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from cochrane.handbook.org.
- Jüni P, Altman DG, Egger M. Systematic reviews in health care: Assessing the quality of controlled clinical trials. BMJ 2001;323(7303):42‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konstan MW, Berger M. Current understanding of the inflammatory process in cystic fibrosis: onset and etiology. Pediatric Pulomonolgy 1997;24(2):137‐42. [DOI] [PubMed] [Google Scholar]
- Main E, Prasad A, Schans C. Conventional chest physiotherapy compared to other airway clearance techniques for cystic fibrosis. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD002011.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlwaine M, Button B, Dwan K. Positive expiratory pressure physiotherapy for airway clearance in people with cystic fibrosis. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD003147.pub4] [DOI] [PubMed] [Google Scholar]
- Mckoy NA, Wilson LM, Saldanha IJ, Odelola OA, Robinson KA. Active cycle of breathing technique for cystic fibrosis. Cochrane Database of Systematic Reviews 2016, Issue 7. [DOI: 10.1002/14651858.CD007862.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasad SA. Current concepts in physiotherapy. Journal of the Royal Society of Medicine 1993;86(Suppl 20):23‐9. [PMC free article] [PubMed] [Google Scholar]
- Pryor J. Physiotherapy for airway clearance in adults. European Respiratory Journal 1999;14(6):1418‐24. [DOI] [PubMed] [Google Scholar]
- Radtke T, Nolan SJ, Hebestreit H, Kriemler S. Physical exercise training for cystic fibrosis. Cochrane Database of Systematic Reviews 2015, Issue 6. [DOI: 10.1002/14651858.CD002768.pub3] [DOI] [PubMed] [Google Scholar]
- Rogers D, Doull IJM. Physiological principles of airway clearance techniques used in the physiotherapy management of cystic fibrosis. Current Paediatrics 2005;15(3):233‐8. [Google Scholar]
- Rosenfeld M, Emerson J, Williams‐Warren J, Pepe M, Smith A, Montgomery AB, Ramsey B. Defining a pulmonary exacerbation in cystic fibrosis. Journal of Pediatrics 2001;139(3):359‐65. [DOI] [PubMed] [Google Scholar]
- Schöni MH. Autogenic drainage: a modern approach to physiotherapy in cystic fibrosis. Journal of Royal Society of Medicine 1989;82(Suppl 16):32‐7. [PMC free article] [PubMed] [Google Scholar]
- Volsko TA, DiFiore JM, Chatburn RL. Performance comparison of two oscillatory positive pressure devices: Acapella versus Flutter. Respiratory Care 2003;48(2):124‐30. [PubMed] [Google Scholar]
- Warwick WJ, Hansen LG. The long‐term effect of high frequency chest compression therapy on pulmonary complications of cystic fibrosis. Pediatric Pulmonology 1991;11(3):265‐71. [DOI] [PubMed] [Google Scholar]
- Webb AK, Dodd ME, Moorcroft J. Exercise and cystic fibrosis. Journal of the Royal Society of Medicine 1995;88(Suppl 25):30‐6. [PMC free article] [PubMed] [Google Scholar]
- Webber BA, Hofmeyer JL, Moran MDL, Hodson ME. Effects of postural drainage, incorporating forced expiration technique, on pulmonary function in cystic fibrosis. British Journal of Diseases of the Chest 1986;80(4):353‐9. [DOI] [PubMed] [Google Scholar]
- Webber BA. The active cycle of breathing exercises. Cystic Fibrosis News 1990;Aug/Sept:10‐1. [Google Scholar]
- Williams, EJ. Experimental designs balanced for the estimation of residual effects of treatments. Australian Journal of Scientific Research 1949;Series A, Volume 2:149‐68. [Google Scholar]
- Zach MS. Lung disease in cystic fibrosis ‐ an updated concept. Pediatric Pulmonology 1990;8(3):188‐202. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- Morrison L, Agnew J. Oscillating devices for airway clearance in people with cystic fibrosis. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD006842] [DOI] [Google Scholar]
- Morrison L, Agnew J. Oscillating devices for airway clearance in people with cystic fibrosis. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD006842.pub2] [DOI] [PubMed] [Google Scholar]
- Morrison L, Agnew J. Oscillating devices for airway clearance in people with cystic fibrosis. Cochrane Database of Systematic Reviews 2014, Issue 7. [DOI: 10.1002/14651858.CD006842.pub3] [DOI] [PubMed] [Google Scholar]


