Abstract
Background
Multi‐vessel coronary disease in people with ST elevation myocardial infarction (STEMI) is common and is associated with worse prognosis after STEMI. Based on limited evidence, international guidelines recommend intervention on only the culprit vessel during STEMI. This, in turn, leaves other significantly stenosed coronary arteries for medical therapy or revascularisation based on inducible ischaemia on provocative testing. Newer data suggest that intervention on both the culprit and non‐culprit stenotic coronary arteries (complete intervention) may yield better results compared with culprit‐only intervention.
Objectives
To assess the effects of early complete revascularisation compared with culprit vessel only intervention strategy in people with STEMI and multi‐vessel coronary disease.
Search methods
We searched the Cochrane Central Register of Controlled Trials, MEDLINE, Embase, World Health Organization International Clinical Trials Registry Platform Search Portal, and ClinicalTrials.gov. The date of the last search was 4 January 2017. We applied no language restrictions. We handsearched conference proceedings to December 2016, and contacted authors and companies related to the field.
Selection criteria
We included only randomised controlled trials (RCTs), wherein complete revascularisation strategy was compared with a culprit‐only percutaneous coronary intervention (PCI) for the treatment of people with STEMI and multi‐vessel coronary disease.
Data collection and analysis
We assessed the methodological quality of each trial using the Cochrane 'Risk of bias' tool. We resolved the disagreements by discussion among review authors. We followed standard methodological approaches recommended by Cochrane. The primary outcomes were long‐term (one year or greater after the index intervention) all‐cause mortality, long‐term cardiovascular mortality, long‐term non‐fatal myocardial infarction, and adverse events. The secondary outcomes were short‐term (within the first 30 days after the index intervention) all‐cause mortality, short‐term cardiovascular mortality, short‐term non‐fatal myocardial infarction, revascularisation, health‐related quality of life, and cost. We analysed data using fixed‐effect models, and expressed results as risk ratios (RR) with 95% confidence intervals (CI). We used GRADE criteria to assess the quality of evidence and we conducted Trial Sequential Analysis (TSA) to control risks of random errors.
Main results
We included nine RCTs, that involved 2633 people with STEMI and multi‐vessel coronary disease randomly assigned to either a complete (n = 1381) versus culprit‐only (n = 1252) revascularisation strategy. The complete and the culprit‐only revascularisation strategies did not differ for long‐term all‐cause mortality (65/1274 (5.1%) in complete group versus 72/1143 (6.3%) in culprit‐only group; RR 0.80, 95% CI 0.58 to 1.11; participants = 2417; studies = 8; I2 = 0%; very low quality evidence). Compared with culprit‐only intervention, the complete revascularisation strategy was associated with a lower proportion of long‐term cardiovascular mortality (28/1143 (2.4%) in complete group versus 51/1086 (4.7%) in culprit‐only group; RR 0.50, 95% CI 0.32 to 0.79; participants = 2229; studies = 6; I2 = 0%; very low quality evidence) and long‐term non‐fatal myocardial infarction (47/1095 (4.3%) in complete group versus 70/1004 (7.0%) in culprit‐only group; RR 0.62, 95% CI 0.44 to 0.89; participants = 2099; studies = 6; I2 = 0%; very low quality evidence). The complete and the culprit‐only revascularisation strategies did not differ in combined adverse events (51/2096 (2.4%) in complete group versus 57/1990 (2.9%) in culprit‐only group; RR 0.84, 95% CI 0.58 to 1.21; participants = 4086; I2 = 0%; very low quality evidence). Complete revascularisation was associated with lower proportion of long‐term revascularisation (145/1374 (10.6%) in complete group versus 258/1242 (20.8%) in culprit‐only group; RR 0.47, 95% CI 0.39 to 0.57; participants = 2616; studies = 9; I2 = 31%; very low quality evidence). TSA of long‐term all‐cause mortality, long‐term cardiovascular mortality, and long‐term non‐fatal myocardial infarction showed that more RCTs are needed to reach more conclusive results on these outcomes. Regarding long‐term repeat revascularisation more RCTs may not change our present result. The quality of the evidence was judged to be very low for all primary and the majority of the secondary outcomes mainly due to risk of bias, imprecision, and indirectness.
Authors' conclusions
Compared with culprit‐only intervention, the complete revascularisation strategy may be superior due to lower proportions of long‐term cardiovascular mortality, long‐term revascularisation, and long‐term non‐fatal myocardial infarction, but these findings are based on evidence of very low quality. TSA also supports the need for more RCTs in order to draw stronger conclusions regarding the effects of complete revascularisation on long‐term all‐cause mortality, long‐term cardiovascular mortality, and long‐term non‐fatal myocardial infarction.
Keywords: Female, Humans, Male, Cause of Death, Coronary Stenosis, Coronary Stenosis/complications, Coronary Stenosis/mortality, Coronary Stenosis/surgery, Myocardial Revascularization, Myocardial Revascularization/adverse effects, Myocardial Revascularization/methods, Myocardial Revascularization/mortality, Randomized Controlled Trials as Topic, ST Elevation Myocardial Infarction, ST Elevation Myocardial Infarction/etiology, ST Elevation Myocardial Infarction/mortality, ST Elevation Myocardial Infarction/surgery
Plain language summary
Complete versus culprit‐only revascularisation in ST elevation heart attack with multi‐vessel disease
Review question
In people with narrowing of multiple coronary arteries (blood vessels that surround and supply the heart with blood), along with one completely occluded (blocked) that is causing a heart attack, whether it is better to open all arteries or only the one that is causing the heart attack.
Background
The co‐existence of multiple significantly narrowed coronary vessels (called multi‐vessel disease) with a completely occluded coronary artery that is causing the heart attack, is commonly seen among people having a heart attack. Current treatment of these narrowed or completely obstructed coronary arteries involves an intravascular (within a blood vessel) procedure known as percutaneous coronary intervention, which uses a balloon that is positioned and inflated at the site of the blockage thereby opening the artery and restoring normal blood flow. This is usually followed by placement of a stent (small mesh tube) to avoid the previously narrowed arteries closing again. In addition to the blocked artery, there may be other narrowed coronary arteries, but several cardiology societies recommend intervening only on the vessel(s) causing the heart attack thereby leaving the other narrowed arteries untreated unless the person continues to have symptoms.
Study characteristics
We searched for clinical trials in adults who had percutaneous coronary intervention for the management of heart attack and multi‐vessel disease. The evidence is current to 4 January 2017. Only four trials reported funding from government organisations or charitable institutions. The other trials did not mention the source of funding and no private companies were mentioned as sources of finance. In the included trials, both the participants and researchers were aware of what treatment the participants received which may have biased the results. One trial ended enrolment earlier than planned because the difference between treatment was significant. This may have overestimated the difference between intervention groups. For most trials, the number of participants that were included was not enough to see a potential difference between treatments.
Key results
We included nine clinical trials with 2633 people with heart attack and multi‐vessel disease. Compared with participants who underwent opening of only the coronary artery that caused heart attack, people who underwent treatment on all narrowed vessels had fewer deaths from diseases of the heart and blood supply (called cardiovascular disease), required fewer treatments to open the problematic coronary arteries, and had fewer heart attacks at the end of one year or later since the treatment. Based on our analyses, although the treatment on all narrowed vessels appears to be a better treatment strategy, there still exists a need for more well‐designed clinical trials to confirm that this approach is associated with fewer deaths from cardiovascular diseases or heart attack, or both.
Quality of the evidence
The evidence is of very low quality. For instance, the number of participants in the included studies was insufficient, the medical team was aware of the study group that the participants were allocated to and that may have affected our conclusions. There is a need for well‐designed clinical trials with more participants to determine which treatment strategy is superior.
Summary of findings
Summary of findings for the main comparison. Complete revascularisation compared to culprit‐only revascularisation in ST elevated myocardial infarction with multi‐vessel disease.
| Complete revascularisation compared to culprit‐only revascularisation in ST elevated myocardial infarction with multi‐vessel disease | ||||||
| Patient or population: people with STEMI and MVD. Intervention: complete revascularisation. Comparison: culprit only. | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with culprit only | Risk with complete revascularisation | |||||
| Long‐term all‐cause mortality (≥ 1 year after the intervention) | Study population | RR 0.80 (0.58 to 1.11) | 2417 (8 RCTs) | ⊕⊝⊝⊝ Very low 1,2,3,4 | PRAMI study terminated early. CvLPRIT and PRAMI concerning for attrition bias. Only CvLPRIT was judged to have low risk for selection bias. | |
| 63 per 1000 | 50 per 1000 (37 to 70) | |||||
| Long‐term cardiovascular mortality (≥ 1 year after the intervention) | Study population | RR 0.50 (0.32 to 0.79) | 2229 (6 RCTs) | ⊕⊝⊝⊝ Very low 1,2,3,4 | PRAMI study terminated early. CvLPRIT and PRAMI concerning for attrition bias. Only CvLPRIT was judged to have low risk for selection bias. | |
| 47 per 1000 | 23 per 1000 (15 to 37) | |||||
| Long‐term myocardial infarction (≥ 1 year after the intervention) | Study population | RR 0.62 (0.44 to 0.89) | 2099 (6 RCTs) | ⊕⊝⊝⊝ Very low 1,2,3,4 | PRAMI study terminated early. CvLPRIT and PRAMI concerning for attrition bias. Only CvLPRIT was judged to have low risk for selection bias. | |
| 70 per 1000 | 43 per 1000 (31 to 62) | |||||
| Overall adverse events (pooled short and long term) | Study population | OR 0.84 (0.58 to 1.21) | 4086 (6 RCTs) | ⊕⊝⊝⊝ Very low 1,2,3,4 | PRAMI study terminated early. CvLPRIT and PRAMI concerning for attrition bias. Only CvLPRIT was judged to have low risk for selection bias. Open label to the operator may affect this outcome. | |
| 29 per 1000 | 24 per 1000 (17 to 35) | |||||
| Short‐term all‐cause mortality (within the first 30 days after the intervention) | Study population | RR 0.65 (0.18 to 2.37) | 696 (2 RCTs) | ⊕⊝⊝⊝ Very low 1,2,3,4 | HELP‐AMI trial did not describe in detail their methodology to analyse for bias. | |
| 15 per 1000 | 10 per 1000 (3 to 36) | |||||
| Long‐term revascularisation (≥ 1 year after the intervention) | Study population | RR 0.47 (0.39 to 0.57) | 2616 (9 RCTs) | ⊕⊝⊝⊝ Very low 1,2,3 | PRAMI study terminated early. CvLPRIT and PRAMI concerning for attrition bias. Only CvLPRIT was judged to have low risk for selection bias. Open label to the operator may affect this outcome. | |
| 208 per 1000 | 98 per 1000 (81 to 118) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MVD: multi‐vessel disease; RCT: randomised controlled trial; RR: risk ratio; STEMI: ST elevated myocardial infarction. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded due to publication (reporting) bias.
2 Downgraded due to study limitations (largely risk of attrition bias and selection bias).
3 Downgraded because of indirectness: black and Hispanic people, as well as women were under‐represented.
4 Downgraded due to imprecision.
Background
Description of the condition
The World Health Organization (WHO) reported that in 2012 there were 17.5 million global deaths due to cardiovascular diseases, accounting for 31% of all deaths (WHO 2014); furthermore, they estimated about 20 million cardiovascular associated deaths in 2015 (WHO 2005). In contemporary practice, among the people who present to the hospital with ST elevation myocardial infarction (STEMI), between 40% and 65% have concurrent multi‐vessel disease (MVD) or a combination of a completely occluded coronary artery and significant but incomplete stenosis of other coronary vessels (Dziewierz 2010; Jo 2011; Park 2014; Sorajja 2007). As the burden of cardiovascular diseases affects hospital systems worldwide, there is growing interest among healthcare providers to examine and improve the various treatment strategies involved in the management of STEMI in people with co‐existing MVD.
Several studies have shown that the co‐existence of culprit and non‐culprit coronary artery stenotic lesions leads to worse outcomes. For instance, people with STEMI and MVD have higher one‐year rates of the composite outcome of death, recurrent myocardial infarction, and need for revascularisation compared with people with single‐vessel disease (Corpus 2004; Halkin 2005; Jaski 1992; Muller 1991; Sorajja 2007). The American College of Cardiology (ACC)/American Heart Association (AHA) (ACCF/AHA 2013) and by the European Society of Cardiology (ESC) (ESC 2012) discouraged the early intervention of the non‐culprit; however, these recommendations were based on limited evidence. At that time, standard of care for people presenting with STEMI and MVD was to undergo sole intervention on the culprit lesion followed by intervention on other significant coronary artery stenoses involving non‐culprit arteries, in a staged fashion; or a multi‐vessel intervention in cases of cardiogenic shock or persistent ischaemia after primary percutaneous coronary intervention (P‐PCI). However, more recent randomised controlled trials (RCT) have demonstrated that simultaneous intervention on both culprit and non‐culprit lesions can be safely performed and that it may improve patient outcomes (CvLPRIT 2015; DANAMI‐3‐PRIMULTI 2015; PRAGUE‐13 2015; PRAMI 2013). This evidence subsequently resulted in an upgrade in the ACC/AHA and ESC/European Association for Cardio‐Thoracic Surgery (EACTS) recommendations on the intervention of non‐infarct‐related arteries (IRA) stenotic lesions from possibly intervention that can produce harm or no benefit (class III recommendation) to an intervention that may be considered in selected people with MVD (class IIb recommendation level of evidence B) per ACC/AHA/Society for Cardiovascular Angiography and Interventions (SCAI) 2015 guidelines (ACC/AHA/SCAI 2015), class IIa for staged intervention or IIb for simultaneous multi‐vessel percutaneous coronary intervention (PCI) at the index procedure with a level of evidence B per ESC 2014 guidelines (ESC 2014).
Description of the intervention
The current standard of care for treatment of people with STEMI is P‐PCI on the completely occluded artery within 90 minutes from first medical contact (ACCF/AHA 2013; ESC 2012; ESC 2014; NICE 2013). Upon presentation to the hospital, a person with STEMI is taken to the catheterisation laboratory where angiography of the coronary arteries is performed in an attempt to identify the culprit lesion, that is, the narrowed atherosclerotic lesion of the coronary vessel responsible for the ischaemic changes seen on the electrocardiogram. The culprit lesion is treated with P‐PCI comprising of balloon angioplasty followed by the insertion of a stent.
In addition to the culprit vessel, it is possible for a person to have other significantly stenosed coronary arteries. These non‐culprit stenoses may not be responsible for the person's STEMI on presentation, but may eventually lead to acute or chronic ischaemic heart disease (Corpus 2004; Halkin 2005; Jaski 1992; Sorajja 2007). In people with non‐culprit artery disease amenable to PCI, three different treatment strategies can be adopted: 1. P‐PCI on the culprit artery along with medical management, with revascularisation of the non‐culprit lesions only in the setting of recurrent symptoms, infarction, or significant inducible ischaemia on provocative testing; 2. complete revascularisation including the culprit and non‐culprit arteries during the same procedure; and 3. staged intervention on the non‐culprit arteries later during the same hospitalisation or shortly after discharge.
How the intervention might work
STEMI is a consequence of a sudden complete occlusion of a coronary artery, leading to infarction or myocardial cell death. P‐PCI is currently the preferred treatment option for a completely occluded coronary artery in the setting of STEMI. This procedure restores the blood supply to a previously ischaemic region, thereby reducing cell death and preserving as much viable myocardium as possible. An early invasive approach for the partially occluded non‐culprit lesion(s), in the setting of MVD, might help in simultaneously restoring and improving blood supply to the remaining myocardium that is at potential risk for future ischaemic events.
There is cumulative evidence that STEMI is a pro‐inflammatory process that might play a role in the instability of the atherosclerotic plaques and subsequent higher risk of cardiac events surrounding the STEMI (Arroyo‐Espliguero 2004; Kubo 2010). Therefore, complete revascularisation could potentially prevent subsequent cardiac events by treating any unstable inflamed atherosclerotic plaques and thus preventing complete obstruction (subsequent STEMI), transient obstruction/embolisation (non‐STEMI), or progression over time to ischaemia and symptoms of angina (unstable angina or refractory angina). Intervention on the non‐culprit lesions might impose a higher risk of vascular complications including periprocedural infarction, renal impairment due to the contrast load, and additional risk of bleeding when performed as a staged procedure. In addition, PCI with stent implantation always carries the risk of later stent thrombosis.
Why it is important to do this review
A timely P‐PCI with revascularisation of the culprit vessel remains the mainstay of treatment for people presenting with STEMI across different continents. However, management of significantly stenotic lesions of the non‐culprit vessel has been an area of constant debate and the recommendations differ among the various published guidelines worldwide. In the US, for instance, the 2013 ACC/AHA guidelines discouraged routine simultaneous P‐PCI of non‐culprit lesions with the culprit vessel in haemodynamically stable people because of concerns of worse outcomes (class III (harm)/level of evidence B) (ACCF/AHA 2013), and ESC encouraged intervention of the culprit vessel only (ESC 2012). With those guidelines, simultaneous intervention of culprit and non‐culprit vessels was recommended only in certain contexts such as in cardiogenic shock or when there was persistence of symptoms of cardiac ischaemia despite intervention on the culprit lesion (class I/level of evidence C ACCF/AHA 2013) (class IIa/level of evidence B ESC 2012). Based on more recent evidence provided by RCTs suggesting better outcomes with complete revascularisation in people with STEMI and MVD (CvLPRIT 2015; DANAMI‐3‐PRIMULTI 2015; PRAGUE‐13 2015; PRAMI 2013), the ACC/AHA and ESC guidelines updated their guidelines. The ACC/AHA/SCAI 2015 and ESC 2014 guidelines now state that simultaneous intervention on significantly stenotic non‐culprit lesions can be considered in selected people with STEMI and MVD, thus upgrading their recommendations to class IIb. The fact that the strength of the recommendation is still IIb suggests that more robust evidence is needed to make stronger recommendations in this regard. In contrast, the National Institute for Health and Care Excellence (NICE) guidelines do not make any recommendations regarding intervention on non‐culprit coronary arteries due to lack of evidence (NICE 2013).
This lack of strong recommendations in regards to intervention on both culprit and non‐culprit arteries during STEMI emphasises the need for good‐quality evidence followed by systematic analysis, to support more specific recommendations. Such information, when available, can be of great help to healthcare providers allowing them to allocate resources more efficiently thereby improving outcomes related to this common condition amidst concerns over healthcare expenditure and varying insurance reimbursements.
Objectives
To assess the effects of early complete revascularisation compared with culprit vessel only intervention strategy in people with STEMI and MVD.
Methods
Criteria for considering studies for this review
Types of studies
We included only RCTs comparing complete revascularisation versus culprit‐only PCI strategy in people with STEMI and MVD. We included studies reported as full‐text, those published as abstract, and unpublished data.
Types of participants
We included adults aged 18 years and above, who underwent P‐PCI for the management of STEMI, with concurrent non‐culprit significant lesions (as defined by the authors of the trial), identified at the time of the index procedure.
Types of interventions
We included studies that looked at a population of people presenting with STEMI and who were initially treated with a P‐PCI and coronary angiography to assess the extent of coronary vessel obstruction in the various branches of the coronary tree. Further, in these people, the culprit and the non‐culprit arteries were identified based on correlation with participants' changes on electrocardiograms. Following initial treatment of the culprit lesion, participants in these RCTs were randomised to receive either complete or culprit vessel only intervention. A comparison was then made between these two strategies, defined as:
Complete revascularisation strategy: involving additional revascularisation of the clinically significant stenotic non‐culprit lesion(s) (at least 50% obstruction but less than 100%) at the index procedure or in a second intervention. This was carried out in all eligible participants, except if contraindicated;
Culprit vessel‐only intervention: involving no additional invasive approach but rather medical management of all eligible participants, even with evidence of non‐culprit artery disease diagnosed at the time of P‐PCI. Exceptions were made if the participant had clinical symptoms that warranted further testing and intervention (e.g. new‐onset chest pain, dynamic changes on electrocardiogram, or a positive stress test).
Types of outcome measures
Primary outcomes
Long‐term all‐cause mortality: defined as death from any cause at one year or greater after the intervention.
Long‐term cardiovascular mortality: defined as death from cardiovascular cause at one year or greater after the intervention.
Long‐term non‐fatal myocardial infarction: spontaneous myocardial infarction measured at one year or greater after the intervention.
Adverse events: acute kidney injury, stroke, and bleeding (defined as GUSTO (Global Utilization of Streptokinase and t‐PA for Occluded Coronary Arteries) severe or moderate and TIMI (Thrombolysis in Myocardial Infarction) major or minor) at 30 days (short‐term) and one year or later (long‐term) after the initial intervention.
Secondary outcomes
Short‐term all‐cause mortality: defined as death from any cause measured within the first 30 days (short‐term) after the index intervention.
Short‐term cardiovascular mortality: defined as death from cardiovascular cause within the first 30 days after the index intervention.
Short‐term non‐fatal myocardial infarction: spontaneous myocardial infarction (excluding periprocedural elevation of cardiac enzymes) and measured within the first 30 days after the index intervention.
Revascularisation: defined as the need for revascularisation with either coronary artery bypass graft surgery (CABG) or PCI. Measured within the first 30 days (short‐term) and at one year or greater (long‐term) after the intervention.
Health‐related quality of life: measured with any validated health‐related quality of life instrument at one year or greater after the intervention.
Cost: measured at one‐year follow‐up or greater.
Search methods for identification of studies
Electronic searches
On 4 January 2017, we searched the following sources from their inception and we imposed no restriction on language of publication:
Cochrane Central Register of Controlled Trials (CENTRAL): 2016, Issue 11 (Wiley);
Database of Abstracts of Reviews of Effects (DARE): Issue 2 of 4, April 2015 (Wiley);
Health Technology Assessment Database (HTA): Issue 4 of 4, October 2016 (Wiley);
Ovid MEDLINE(R) 1946 to December Week 1 2016, Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations 3 January 2017 and Ovid MEDLINE(R) Epub Ahead of Print 3 January 2017;
Embase 1974 to 4 January 2017; Embase Classic 1947 to 1973; MEDLINE 1966 to 4 January 2017 (embase.com);
Conference Proceedings Citation Index‐Science (CPCI‐S) 1990 to 4 January 2017 (Web of Science).
We applied the Cochrane sensitivity‐maximizing RCT filter (Lefebvre 2011) to MEDLINE (Ovid) but did not apply it to the MEDLINE In‐Process or Epub searches. For Embase, we used the multi‐term Embase filter with the best balance of sensitivity and specificity (Wong 2006) translated from Ovid to embase.com syntax.
For details of terms used in search strategies please see Appendix 1.
Searching other resources
In order to identify articles potentially missed through the electronic searches, we:
handsearched reference lists of all included studies and relevant reviews retrieved by electronic searching to identify other potentially eligible trials or ancillary publications;
conducted a search on 8 December 2016 for other systematic reviews and Health Technology Assessment reports in Epistemonikos (www.epistemonikos.org);
handsearched on 8 December 2016, conference proceedings via their websites for 2011 to 2016 (congress365.escardio.org), ACC Annual Scientific Sessions (www.onlinejacc.org/content/meeting‐abstract‐supplements), AHA Annual Scientific Sessions (circ.ahajournals.org), and Transcatheter Cardiovascular Therapeutics Abstracts (www.tctmd.com);
contacted corresponding authors of included studies for any additional published or unpublished data;
attempted to contact the authors of trials when information in the study report was lacking or unclear.
We also searched the following trial registers:
ClinicalTrials.gov (www.clinicaltrials.gov/);
European (EU) Clinical Trials Register (www.clinicaltrialsregister.eu/);
WHO International Clinical Trials Registry Platform (apps.who.int/trialsearch/).
Data collection and analysis
Selection of studies
Two review authors (SH and CB) independently screened titles and abstracts for inclusion of all the potential studies that the search identified and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. If there were any disagreements, a third review author arbitrated. We retrieved the full‐text study reports/publication and two review authors (SH and CB) independently screened the full‐text and identified studies for inclusion, and identified and recorded reasons for exclusion of the ineligible studies. We resolved any disagreements through discussion or, if required, we consulted a third review author. We identified and excluded duplicate publications and collated multiple reports of the same study so that each study, rather than each report, became a unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Figure 1) and Characteristics of excluded studies table.
1.
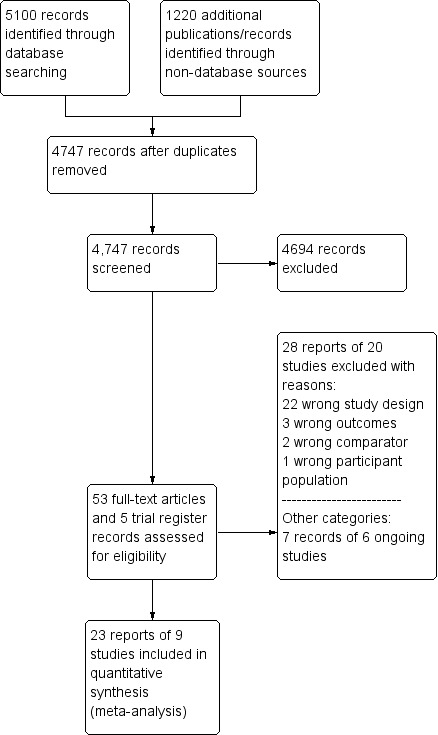
Study flow diagram.
Data extraction and management
We used a data collection form for study characteristics and outcome data that had been piloted on at least one study in the review. One review author (CB) extracted study characteristics from included studies including:
methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and location, study setting, withdrawals, and date of study;
participants: number, mean age, age range, gender, severity of the condition, diagnostic criteria, inclusion criteria, and exclusion criteria;
interventions: intervention, comparison, concomitant medications, and excluded medications;
outcomes: primary and secondary outcomes specified and collected, and time points reported;
notes: funding for trial and notable conflicts of interest of trial authors.
Two review authors (CB and SH) independently extracted outcome data from included studies. We resolved any disagreements by consensus or by involving a third review author. One review author (CB) transferred data into the Review Manager 5 (RevMan 2014). We double‐checked that data were entered correctly by comparing the data presented in the systematic review with those of the study reports. A second review author (SH) spot‐checked study characteristics for accuracy against the trial report.
Trial Sequential Analysis
We performed Trial Sequential Analysis (TSA) for the outcomes long‐term all‐cause mortality, long‐term cardiovascular mortality, long‐term non‐fatal myocardial infarction, and long‐term revascularisation. We applied TSA using free software (www.ctu.dk/tsa). TSA reduces the risk of random errors due to sparse data and repetitive testing of the accumulating data (Wetterslev 2008). We calculated the diversity‐adjusted required information size, that is, number of participants needed to detect or reject a hypothesis. In our meta‐analysis, the required information size for dichotomous outcomes was based on the proportion in the control group; assumption of a plausible relative risk reduction (RRR) of 20% observed in the included trials; a risk of type I error (alpha) of 2%; a risk of type II error (beta) of 10%; and the observed diversity of the meta‐analysis (Jakobsen 2014). The underlying assumption of TSA is that testing for significance may be performed each time a new trial is added to the meta‐analysis. We added the trials according to the year of publication, and if more than one trial was published in a year, we added trials alphabetically according to the last name of the first author or the trial name. On the basis of the required information size, we constructed trial sequential monitoring boundaries (Thorlund 2011; Wetterslev 2008). These boundaries determine the statistical inference one may draw regarding the cumulative meta‐analysis that has not reached the required information size; if the trial sequential monitoring boundary is crossed by the cumulative Z curve before the required information size is reached, firm evidence may perhaps be established and further trials may be superfluous. In contrast, if the boundary benefit or harm is not surpassed, it is most probably necessary to continue doing trials in order to detect or reject a certain intervention effect. That can be determined by assessing if the cumulative Z‐curve crosses the trial sequential boundaries for futility.
Assessment of risk of bias in included studies
Two review authors (SH and CB) independently assessed the risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion or by involving a third review author. We assessed the risk of bias according to the following domains (Wood 2008).
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias (e.g. industry funding).
We graded each potential source of bias as high, low, or unclear and provide a quote from the study report together with a justification for our judgement in the 'Risk of bias' table. We summarised the risk of bias judgements across different studies for each of the domains listed. Wherever the information on the risk of bias related to unpublished data or correspondence with a trialist, we noted the same in the 'Risk of bias' table.
We conducted the review according to the published protocol (Hirji 2015), and reported any deviations from it under the Differences between protocol and review. When considering treatment effects, we took into account the risk of bias for the studies that contributed to such an outcome.
Measures of treatment effect
We analysed dichotomous data as risk ratios (RR) with 95% confidence intervals (CI) and continuous data as mean difference (MD) or standardised mean difference (SMD) with 95% CI. SMD will be used if the outcome is measured in a variety of ways, for instances with different scales and MD will be used if the outcomes is measured with the same method. We entered data presented as a scale with a consistent direction of effect. We described skewed data reported as medians and interquartile ranges narratively.
Unit of analysis issues
All included trials were randomised at the individual participant level.
Dealing with missing data
We contacted investigators to verify key study characteristics and obtain missing numerical outcome data wherever possible (e.g. when a study was published as abstract only). Where this was not feasible, and the missing data were thought to introduce serious bias, we explored the impact of including such studies on the overall assessment of results using a sensitivity analysis.
Assessment of heterogeneity
We examined heterogeneity using the I2 statistic, which quantifies inconsistency across studies to assess the impact of heterogeneity on the meta‐analysis, with an I2 statistic of 50% or more indicative of a considerable level of inconsistency.
If we identified substantial heterogeneity, we reported it and explored possible causes by prespecified subgroup analysis and performed the analysis using a random‐effects model. In the event of substantial clinical, methodological, or statistical heterogeneity, we decided not to report study results as pooled effect estimates.
Assessment of reporting biases
We were unable to assess reporting bias as the number of included studies was insufficient for an informative funnel plot (Higgins 2011).
Data synthesis
If there was evidence for homogeneous effects across studies, we analysed the data using RR and summarised all data using a fixed‐effect model (Riley 2011; Wood 2008). In addition, we performed statistical analyses according to the statistical guidelines contained in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Subgroup analysis and investigation of heterogeneity
We carried out the following subgroup analyses and investigated interaction.
Drug‐eluting stent (DES) compared to bare‐metal stents (BMS).
Sex.
People with diabetes mellitus compared to people without diabetes mellitus.
Non‐culprit and culprit intervention during the same procedure compared to in separate interventions (staged).
Low risk of bias articles compared to high risk of bias articles.
Participants in cardiogenic shock compared to participants not in cardiogenic shock.
We used the following outcomes in subgroup analyses.
Long‐term all‐cause mortality.
Long‐term cardiovascular mortality.
Long‐term non‐fatal myocardial infarction.
We used the formal test for subgroup interactions in Review Manager 5 (RevMan 2014).
Sensitivity analysis
We performed sensitivity analyses to explore the influence of the following factors on effect sizes.
Restricting the analysis to published studies.
Restricting the analysis to trials at low risk of bias, as specified in Assessment of risk of bias in included studies.
Restricting the analysis to trials using the following filters: language of publication, source of funding (industry), and country.
Restricting the analysis to published trials that utilised mostly DES.
Reaching conclusions
Two review authors (CB and CG) independently assessed the quality of the evidence using the GRADE approach and the GRADE profiler (GRADEpro) 3.6 (GRADEpro GDT) to assess the quality of evidence related to each of the key outcomes listed in the Types of outcome measures (Chapter 12.2, Cochrane Handbook for Systematic Reviews of Interventions; Higgins 2011). A summary of the evidence is included in the Table 1.
We based our conclusions only on findings from the quantitative or narrative synthesis of included studies for this review.
We avoided making recommendations for practice and our implications for research were meant to suggest priorities for future research and outline any remaining uncertainties in the area.
Results
Description of studies
For a detailed description of studies, see the Characteristics of included studies, Characteristics of excluded studies, and Characteristics of ongoing studies tables.
Results of the search
Our comprehensive literature search identified 5100 records; of these, we identified 53 full‐text papers and five clinical trial register records for further examination. We excluded the other studies on the basis of their titles or abstracts, which either did not meet the inclusion criteria or were not relevant to the question under trial (see Figure 1 for the PRISMA flow chart). After screening the full‐text of the selected publications, nine trials (23 publications) met the inclusion criteria. All studies were in English, except for one (Zhang 2015). Although we sought additional information from the authors of all studies, two responded to these requests and only one provided additional data (DANAMI‐3‐PRIMULTI 2015).
Included studies
A detailed description of the characteristics of included studies is presented in the Characteristics of included studies table; Table 2; Table 3; and Table 4. The following is a succinct overview.
1. Summary of included studies.
| Study | Dates | Complete revascularisation (staged vs 1 time) | Intervention criteria in non‐culprit vessel | Mean follow‐up (years) | Description multi‐vessel disease | Country | Number of centres |
| CvLPRIT 2015 | May 2011 to May 2013 | At index procedure or before discharge. 65% of participants in invasive group had at index procedure. | > 70% diameter stenosis in 1 plane or > 50% in 2 planes. | 2.5 | Culprit vessel plus ≥ 1 non‐infarct‐related epicardial artery with ≥ 1 lesion deemed angiographically significant (> 70% stenosis in 1 plane or > 50% in 2 planes). | UK | 7 |
| Dambrink and Ghani 2010 | June 2004 to February 2007. | Staged 7.5 days after P‐PCI. | FFR < 0.75 and in stenosis > 90%, PCI was performed without FFR measurement. PCI was with BMS or DES. | 3 | ≥ 1 significant stenosis (> 50% stenosis in ≥ 1 view) in ≥ 2 major epicardial coronary arteries, or the combination of a side branch and a main epicardial vessel provided that they supplied different territories. | The Netherlands | 1 |
| DANAMI‐3‐PRIMULTI 2015 | March 2011 to February 2014 | Staged 2 days after P‐PCI. | FFR < 0.8 and those > 90% stenotic arteries visually. | 2.2 | Significant stenosis (> 50% stenosis visually in arteries > 2 mm diameter) in ≥ 1 of the non‐culprit epicardial coronary arteries or their major side branches in addition to the infarct‐related artery. | Denmark | 2 |
| Estevez Loureiro 2014 | 2010 to 2013 | Staged. | Complete. Criteria not described in study. | 1 | NR. | Spain | NR |
| HELP AMI 2004 | NR | Index procedure. | Not described. | 1 | NR. | Not described | NR |
| Politi 2009 | January 2003 to December 2007 | At index procedure or staged mean 56 days after P‐PCI. 50% participants of complete revascularisation had at intervention of the non‐culprit lesions at index procedure. | > 70% diameter stenosis. | 2.5 | > 70% diameter stenosis of ≥ 2 epicardial coronary arteries or their major branches by visual estimation. | Not described | NR |
| PRAGUE‐13 2015 | September 2008 to December 2014 | Staged between 3 and 40 days after P‐PCI. | > 70% stenosis of non‐culprit coronary artery. | 3 | ≥ 1 vessel, beside the culprit vessel, with significant stenosis (> 70% stenosis). | Czech Republic | 6 |
| PRAMI 2013 | April 2008 to January 2013 | At index procedure. | Stenosis ≥ 50%. | 2 | The presence of stenosis ≥ 50% in ≥ 1 coronary artery other than the culprit vessel. | UK | 5 |
| Zhang 2015 | January 2009 to June 2012 | Staged between 7 and 10 days after P‐PCI. | 75% to 90%. | 2 | Non‐culprit vessel with significant stenosis (75% to 90% stenosis). | China | NR |
BMS: bare‐metal stent; DES: drug‐eluting stent; FFR: fractional flow reserve; NR: not reported in the article; PCI: percutaneous coronary intervention; P‐PCI: primary percutaneous coronary intervention.
2. Baseline information.
| Study | Group | Sample size (n) | Participants (n (%)) | Dropouts (n (%)) | % Male | Mean age (years) | % HTN | % DM | % HLD | % Prior MI | % Anterior STEMI |
| CvLPRIT 2015 | Complete | 150 | 139 (92.7) | 11 (7.3) | 85.3 | 64.6 | 36 | 12.7 | 27.3 | 4.7 | 36 |
| Culprit‐only | 146 | 139 (95.2) | 8 (5.5) | 76.7 | 65.3 | 35 | 13.7 | 23.3 | 3.4 | 35.6 | |
| Dambrink and Ghani 2010 | Complete | 80 | 71 (88.8) | 1 (1.3) | 80 | 62 | 26.3 | 6.3 | 15 | 6.3 | 21.3 |
| Culprit‐only | 41 | 41 (100) | 1 (2.4) | 80.5 | 61 | 42.5 | 5 | 30 | 4.9 | 23.3 | |
| DANAMI‐3‐PRIMULTI 2015 | Complete | 314 | 294 (93.6) | 1 (0.3) | 80 | 64 | 41.4 | 9.2 | NR | 5.4 | 33.4 |
| Culprit‐only | 313 | 313 (100) | 0 | 81.5 | 63 | 46.6 | 13.4 | NR | 8.6 | 35.8 | |
| Estevez Loureiro 2014 | Complete | 100 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Culprit‐only | 99 | NR | NR | NR | NR | NR | NR | NR | NR | NR | |
| HELP AMI 2004 | Complete | 52 | NR | NR | 88.5 | 63.5 | 36.5 | 11.5 | 41.2 | NR | 52 |
| Culprit‐only | 17 | NR | NR | 82.4 | 65.3 | 58.8 | 41.2 | 53 | NR | 59 | |
| Politi 2009 | Complete | 130 | NR | NR | 78.5 | 64 | 57 | 16.2 | NR | NR | 45.4 |
| Culprit‐only | 84 | NR | NR | 76.2 | 66.5 | 60 | 23.8 | NR | NR | 41.7 | |
| PRAGUE‐13 2015 | Complete | 106 | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Culprit‐only | 108 | NR | NR | NR | NR | NR | NR | NR | NR | NR | |
| PRAMI 2013 | Complete | 234 | 223 (95.3) | 10 (4.3) | 75.6 | 62 | 40.2 | 15 | NR | 8.1 | 28.6 |
| Culprit‐only | 231 | 229 (99) | 8 (3.5) | 80.5 | 62 | 40.3 | 20.8 | NR | 7 | 38.5 | |
| Zhang 2015 | Complete | 215 | NR | NR | 61 | 62.3 | 64.2 | 36.7 | 35.3 | NR | 36.7 |
| Culprit‐only | 213 | NR | NR | 67.1 | 62 | 61 | 35.2 | 36.6 | NR | 40 |
DM: diabetes mellitus; HLD: hyperlipidaemia; HTN: hypertension; MI: myocardial infarction; n: number of participants; NR: not reported in the article; STEMI: ST elevated myocardial infarction.
3. Procedure details.
| Study | Group | Symptoms to PCI time (minute) | PCI without stenting (n (%)) | DES (n (%)) | BMS (n (%)) | 2‐Vessel disease (n (%)) | 3‐Vessel disease (n (%)) | Received PCI non‐culprit (n (%)) | DAPT | DAPT duration |
| CvLPRIT 2015 | Complete | 182 | NR | 141 (94) | NR | 119 (79.3) | 31 (20.7) | 139 (92.7) | Yes | NR |
| Culprit‐only | 159 | NR | 127 (87) | NR | 110 (75.3) | 36 (24.7) | 0 | |||
| Dambrink and Ghani 2010 | Complete | NR | 6 (7.5) | 18 (22.5) | 56 (70) | 60 (75) | 20 (25) | 48 (60) | Yes | 1 month |
| Culprit‐only | NR | 7 (17.1) | 7 (7.1) | 27 (66) | 33 (80.5) | 8 (19.5) | 0 | |||
| DANAMI‐3‐PRIMULTI 2015 | Complete | NR | 12 (3.8) | 298 (95) | 0 | NR | 97 (31) | 193 (61.5) | Yes | 1 year |
| Culprit‐only | NR | 18 (5.8) | 290 (92.7) | 0 | NR | 100 (32) | 0 | |||
| Estevez Loureiro 2014 | Complete | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Culprit‐only | NR | NR | NR | NR | NR | NR | NR | |||
| HELP AMI 2004 | Complete | 210 | 0 | 52 (100) | 0 | 36 (69) | 16 (30.8) | NR | Yes | 1 month |
| Culprit‐only | 236 | 0 | 17 (100) | 0 | 9 (53) | 8 (47) | NR | |||
| Politi 2009 | Complete | NR | NR | 11 (8.5) | NR | NR | 48 (37) | NR | NR | NR |
| Culprit‐only | NR | NR | 10 (12) | NR | NR | 21 (25) | NR | |||
| PRAGUE‐13 2015 | Complete | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| Culprit‐only | NR | NR | NR | NR | NR | NR | NR | |||
| PRAMI 2013 | Complete | NR | 1 (< 1) | 147 (63) | 86 (37) | 143 (61.1) | 91 (39) | 223 (95.3) | Yes | 1 month |
| Culprit‐only | NR | 0 | 135 (58) | 96 (42) | 155 (67.1) | 76 (33) | 2 (1) | |||
| Zhang 2015 | Complete | 214 | 0 | 215 (100) | 0 | NR | NR | NR | NR | NR |
| Culprit‐only | 227 | 0 | 213 (100) | 0 | NR | NR | NR |
BMS: bare‐metal stent; DAPT: dual antiplatelet therapy; DES: drug‐eluting stent; n: number of participants; NR: not reported in the article; PCI: percutaneous coronary intervention.
Source of data
All included trials had published data in established journals, except for the PRAGUE‐13 (PRAGUE‐13 2015) and Estevez (Estevez Loureiro 2014) trials, which only had abstracts. We contacted all corresponding authors, two replied and only one (DANAMI‐3‐PRIMULTI 2015) provided additional data (Appendix 2).
Comparisons
All included trials compared culprit‐only versus complete revascularisation in people with acute STEMI and MVD. Six trials performed the intervention on the non‐culprit vessels as a staged intervention (Dambrink and Ghani 2010; DANAMI‐3‐PRIMULTI 2015; Estevez Loureiro 2014; Politi 2009; PRAGUE‐13 2015; Zhang 2015), and three trials performed the intervention of the non‐culprit vessels at the same index procedure (HELP AMI 2004; Politi 2009; PRAMI 2013). In one trial, the investigators encouraged the interventionists to perform the complete revascularisation at the same index procedure (CvLPRIT 2015); however, 35% of the procedures were staged. The Politi 2009 trial had three intervention groups: complete revascularisation during the index procedure; complete revascularisation in a staged procedure; and culprit vessel‐only revascularisation.
Overview of study populations
The nine trials included 2633 participants, 1381 participants were randomised to the complete revascularisation group and 1252 to the culprit‐only intervention group.
Among the four publications that reported dropout, 1449 (96%) participants finished the trial, 727 (93.4%) in the complete revascularisation group and 722 (98.8%) in the culprit‐only intervention group (CvLPRIT 2015; Dambrink and Ghani 2010; DANAMI‐3‐PRIMULTI 2015; PRAMI 2013).
Trial design
The included trials were conducted between 2003 and 2014, and the duration of follow‐up ranged from one to three years, with a mean follow‐up of 2.1 years.
Four trials were multi‐centre (CvLPRIT 2015; DANAMI‐3‐PRIMULTI 2015; PRAGUE‐13 2015; PRAMI 2013), one was single centre (Dambrink and Ghani 2010), and four trials did not mention the number of centres (Estevez Loureiro 2014; HELP AMI 2004; Politi 2009; Zhang 2015). The number of centres per trial ranged between one and seven.
Two trials terminated prematurely, one because of slow enrolment (Dambrink and Ghani 2010) and the other because there was a marked benefit from the complete revascularisation strategy (PRAMI 2013).
Settings
Six of the nine trials were performed in Europe (Spain, Denmark, UK, Czech Republic, and the Netherlands), one in China (Zhang 2015), and two trials did not report where the study was carried out, although based on the authorship, it is likely that they were carried out in Italy (HELP AMI 2004; Politi 2009).
Participants
The majority of participants were men, with male percentage per group between 61% and 89%. The mean age was 63.5 years and the age mean per study ranged between 62 and 65 years. Included studies did not report the ethnicity of the participants.
With the exception of two studies (Estevez Loureiro 2014; PRAGUE‐13 2015), all trials reported comorbidities. The trials that reported comorbidities included people with past medical history of diabetes mellitus, arterial hypertension, hyperlipidaemia, smoking, myocardial infarction, prior CABG, prior PCI, stroke, heart failure, and chronic kidney disease. These trials had between 5% and 41% of participants with diabetes and between 26% and 64% of participants with hypertension, which were therefore the most common comorbidities among the included participants, followed by previous myocardial infarction.
With the exception of two studies (Estevez Loureiro 2014; PRAGUE‐13 2015), all trials reported comedications. In the studies that reported comedications, after the intervention participants received standard medical treatment for the duration of the follow‐up. The most commonly reported drugs were aspirin and clopidogrel in seven out of nine trials; six of the nine trials reported using beta‐blockers, angiotensin‐converting enzyme inhibitor, angiotensin receptor blockers, glycoprotein IIb/IIIa inhibitor, and statins. Five trials reported using dual antiplatelet after PCI, where this combination treatment was provided for at least one month (CvLPRIT 2015; Dambrink and Ghani 2010; DANAMI‐3‐PRIMULTI 2015; HELP AMI 2004; PRAMI 2013).
The major exclusion criteria from the included trials were cardiogenic shock in seven trials (CvLPRIT 2015; DANAMI‐3‐PRIMULTI 2015; HELP AMI 2004; Politi 2009; PRAGUE‐13 2015; PRAMI 2013; Zhang 2015), clear indication for CABG in six trials (Dambrink and Ghani 2010; DANAMI‐3‐PRIMULTI 2015; HELP AMI 2004; Politi 2009; PRAGUE‐13 2015; PRAMI 2013), prior CABG in five trials (CvLPRIT 2015; Dambrink and Ghani 2010; Politi 2009; PRAMI 2013; Zhang 2015), and technically impossible PCI and chronic coronary obstruction in six trials (CvLPRIT 2015; Dambrink and Ghani 2010; DANAMI‐3‐PRIMULTI 2015; Politi 2009; PRAMI 2013; Zhang 2015). Only one trial did not report the exclusion criteria (Estevez Loureiro 2014).
Diagnosis
All trials reported that the participants had acute STEMI and MVD diagnosed during the P‐PCI. Among the studies that described the definition of MVD, they considered as such, those cases where significant stenosis was present in at least one other major epicardial artery besides the culprit vessel. Significant stenosis was defined as greater than 50% stenosis in one or two planes by visual inspection (CvLPRIT 2015; Dambrink and Ghani 2010; DANAMI‐3‐PRIMULTI 2015; PRAMI 2013) or greater than 70% in one plane (CvLPRIT 2015; Politi 2009; PRAGUE‐13 2015). After diagnosing significant stenosis by visual inspection of the coronary angiogram, in two studies the investigators performed a fractional flow reserve (FFR) measurement to decide whether the stenosis was haemodynamically significant and required intervention. The cutoff value for FFR that defined haemodynamically significant stenosis was 0.8 or less (DANAMI‐3‐PRIMULTI 2015) and less than 0.75 (Dambrink and Ghani 2010).
Interventions
All 2633 participants underwent the intervention after randomisation. In two trials, all participants underwent PCI with DES placement (HELP AMI 2004; Zhang 2015), while two other trials did not describe the type of stent utilised (Estevez Loureiro 2014; PRAGUE‐13 2015). For more details please refer to Table 4.
Outcomes
Six trials explicitly stated the primary and secondary outcomes in their publications; only three trials did not specify their secondary outcomes (Estevez Loureiro 2014; Politi 2009; Zhang 2015). The most commonly defined primary outcome in the publications and protocols were non‐fatal myocardial infarction, all‐cause mortality, revascularisation, and cardiac mortality.
Reporting of outcomes
All trials collected a median of 1.2 (range one to three) primary outcomes and a median of 3.8 (range one to eight) secondary outcomes. All included trials assessed non‐fatal myocardial infarction, all except one trial assessed revascularisation (Zhang 2015), all except one trial assessed all‐cause mortality (Estevez Loureiro 2014), and all except two trials assessed cardiac mortality (Dambrink and Ghani 2010; HELP AMI 2004).
Four trials reported bleeding (CvLPRIT 2015; Dambrink and Ghani 2010; DANAMI‐3‐PRIMULTI 2015; PRAMI 2013), and three trials reported acute kidney injury (CvLPRIT 2015; Politi 2009; PRAMI 2013). For a summary of all assessed outcomes in each trial, see the Characteristics of included studies table.
Excluded studies
We excluded twenty‐eight articles after careful evaluation of the full publication (Figure 1). The main reasons for exclusion were: wrong study design and wrong comparator. For further details, see the Characteristics of excluded studies table.
Risk of bias in included studies
For details on risk of bias of included studies see the Characteristics of included studies table. For an overview of review authors' judgements about each risk of bias item for individual trials and across all trials see Figure 2 and Figure 3.
2.
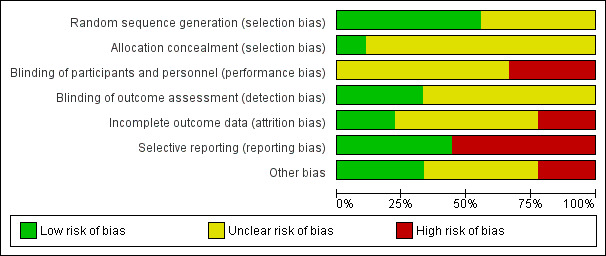
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
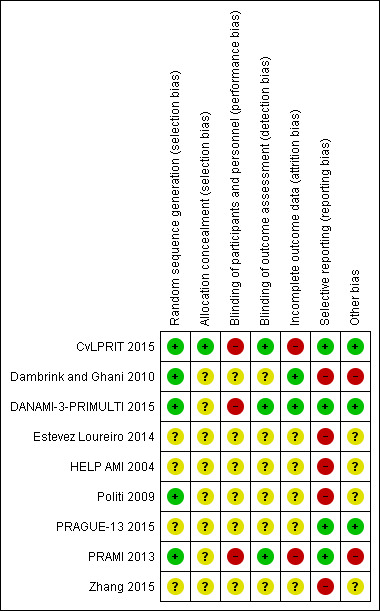
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Five out of the nine included trials utilised electronically generated random numbers to allocate the included participants into one of the intervention groups (CvLPRIT 2015; Dambrink and Ghani 2010; DANAMI‐3‐PRIMULTI 2015; Politi 2009; PRAMI 2013). Four out of the nine trials did not describe their methods of generating the allocation sequence (Estevez Loureiro 2014; HELP‐AMI 2004; PRAGUE‐13 2015; Zhang 2015). Only one trial detailed how the allocation concealment was ensured (CvLPRIT 2015). Therefore, only the CvLPRIT trial was at low risk of selection bias. The remaining eight trials were at high risk of selection bias.
Blinding
Because of the nature of the intervention, all studies were open label for the operators. Several trials described that they were open label for the participants (CvLPRIT 2015; DANAMI‐3‐PRIMULTI 2015; PRAMI 2013), and blinded for investigators and outcome assessors (CvLPRIT 2015;Dambrink and Ghani 2010; DANAMI‐3‐PRIMULTI 2015; PRAMI 2013). One study described that it was open label for investigators and participants (PRAGUE‐13 2015), and two trials did not state if they were blinded to participants (Dambrink and Ghani 2010; Zhang 2015). With the exception of the outcome of revascularisation, there was low risk of performance and detection bias since the majority of the other assessed outcomes were objective.
Incomplete outcome data
Only four trials reported the number of withdrawals (CvLPRIT 2015; Dambrink and Ghani 2010; DANAMI‐3‐PRIMULTI 2015; PRAMI 2013). The number of dropouts per group were similar; however, given the low number of events, there was considerably high risk of attrition bias in two trials (CvLPRIT 2015; PRAMI 2013).
Selective reporting
Among the four studies that were registered in a clinical trial database or had a published protocol, there was a low risk of reporting bias (CvLPRIT 2015; DANAMI‐3‐PRIMULTI 2015; PRAGUE‐13 2015; PRAMI 2013).
Other potential sources of bias
Only four trials reported funding from national institutions or charitable institutions (CvLPRIT 2015; DANAMI‐3‐PRIMULTI 2015; PRAGUE‐13 2015; PRAMI 2013). The other trials did not mention the source of funding and no private companies were mentioned as sources of finance.
Effects of interventions
See: Table 1
Baseline characteristics
For details of baseline characteristics see Table 3.
Complete revascularisation strategy or culprit vessel‐only intervention
Primary outcomes
Long‐term all‐cause mortality
Eight studies found that complete and culprit‐only revascularisation strategies did not differ significantly regarding long‐term all‐cause mortality (65/1274 (5.1%) in complete group versus 72/1143 (6.3%) in culprit‐only group; RR 0.80, 95% CI 0.58 to 1.11; participants = 2417; I2 = 0%; very low quality evidence) (Analysis 1.1) (CvLPRIT 2015; Dambrink and Ghani 2010; DANAMI‐3‐PRIMULTI 2015; HELP AMI 2004; Politi 2009; PRAGUE‐13 2015; PRAMI 2013; Zhang 2015).
1.1. Analysis.
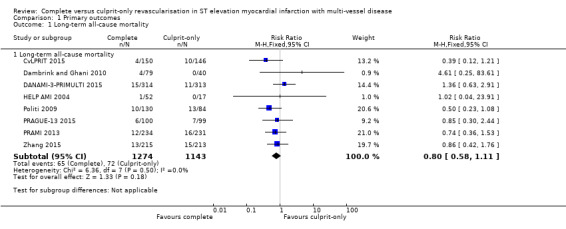
Comparison 1 Primary outcomes, Outcome 1 Long‐term all‐cause mortality.
The TSA showed that more studies are needed to demonstrate that the complete revascularisation strategy is related to an at least 20% long‐term all‐cause mortality RRR compared to the culprit‐only intervention strategy (RR 0.80, TSA‐adjusted CI 0.56 to 1.10) (Figure 4).
4.
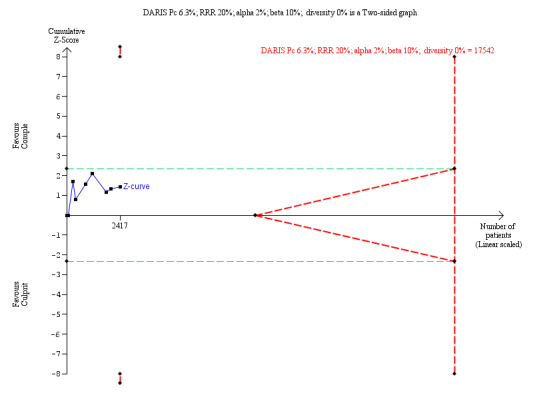
Trial Sequential Analysis for complete versus culprit‐only revascularisation on long‐term all‐cause mortality. The diversity‐adjusted required information size (DARIS) was calculated based on an expected relative risk reduction (RRR) of 20% from proportion event in control (Pc) group of 6.3% with an alpha of 2% and beta of 10%.
Long‐term cardiovascular mortality
Six studies found that, when compared to the culprit‐only intervention strategy, complete revascularisation was associated with a lower cardiovascular mortality in the long‐term or one year after the index procedure (28/1143 (2.4%) in complete group versus 51/1086 (4.7%) in culprit‐only group; RR 0.50, 95% CI 0.32 to 0.79; participants = 2229; I2 = 0%; very low quality evidence) (Analysis 1.2) (CvLPRIT 2015; DANAMI‐3‐PRIMULTI 2015; Politi 2009; PRAGUE‐13 2015; PRAMI 2013; Zhang 2015).
1.2. Analysis.
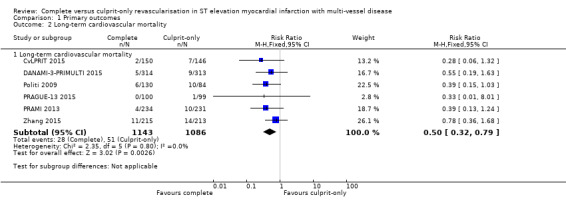
Comparison 1 Primary outcomes, Outcome 2 Long‐term cardiovascular mortality.
The TSA showed that more studies are needed to demonstrate that the complete revascularisation strategy is related to an at least 20% long‐term cardiovascular mortality RRR compared with the culprit‐only intervention strategy (RR 0.51, TSA‐adjusted CI 0.08 to 3.24) (Figure 5).
5.
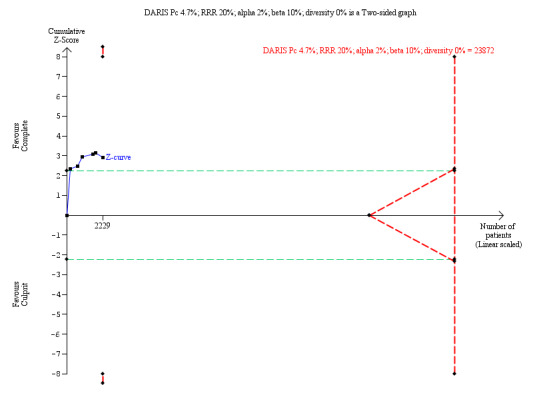
Trial Sequential Analysis for complete versus culprit‐only revascularisation on long‐term cardiovascular mortality. The diversity‐adjusted required information size (DARIS) was calculated based on an expected relative risk reduction (RRR) of 20% from Pc group of 4.7% with an alpha of 2% and beta of 10%.
Long‐term non‐fatal myocardial infarction
Six studies found that complete revascularisation strategy was superior to the culprit‐only intervention in terms of long‐term non‐fatal myocardial infarction (47/1095 (4.3%) in complete group versus 70/1004 (7.0%) in culprit‐only group; RR 0.62, 95% CI 0.44 to 0.89; participants = 2099; I2 = 0%; very low quality evidence) (Analysis 1.3) (CvLPRIT 2015; DANAMI‐3‐PRIMULTI 2015; HELP AMI 2004; Politi 2009; PRAMI 2013; Zhang 2015).
1.3. Analysis.
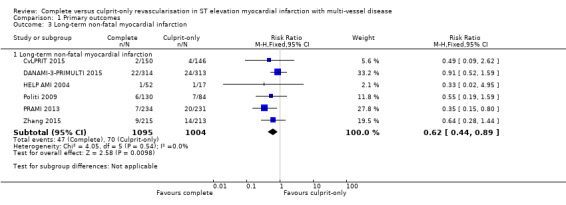
Comparison 1 Primary outcomes, Outcome 3 Long‐term non‐fatal myocardial infarction.
The TSA showed that more studies are needed to demonstrate that the complete revascularisation strategy is related to an at least 20% long‐term non‐fatal myocardial infarction RRR compared to the culprit‐only intervention strategy (RR 0.64, TSA‐adjusted CI 0.14 to 2.82) (Figure 6).
6.
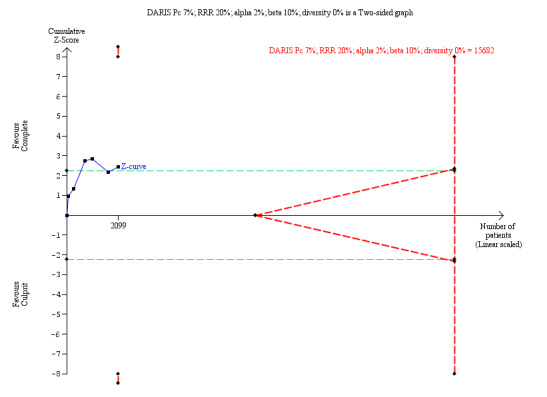
Trial Sequential Analysis for complete versus culprit‐only revascularisation on long‐term non‐fatal myocardial infarction. The diversity‐adjusted required information size (DARIS) was calculated based on an expected relative risk reduction (RRR) of 20% from Pc group of 7.0% with an alpha of 2% and beta of 10%.
Adverse events
Six studies contributed to the pooled analysis of all adverse events comprising acute kidney injury, stroke, and bleeding in the short‐term (within the first 30 days after the index procedure) and long‐term, demonstrated that the frequency of the combined adverse event rate was similar in both groups (51/2096 (2.4%) in complete group versus 57/1990 (2.9%) in culprit‐only group; RR 0.84, 95% CI 0.58 to 1.21; participants = 4086; I2 = 0%; very low quality evidence).
Acute kidney injury
Two studies found no difference in the occurrence of acute kidney injury in the short‐term between complete or culprit‐only revascularisation strategy (4/364 (1.1%) in complete group versus 6/315 (1.9%) in culprit‐only group; RR 0.50, 95% CI 0.14 to 1.81; participants = 679; I2 = 0%; very low quality evidence) (Politi 2009; PRAMI 2013). Similarly, one trial found no difference in the occurrence of acute kidney injury in the long‐term between complete or culprit‐only revascularisation strategy (2/150 (1.3%) in complete group versus 2/146 (1.4%) in culprit‐only group; RR 0.97, 95% CI 0.14 to 6.82; participants = 296) (Analysis 1.4) (CvLPRIT 2015).
1.4. Analysis.
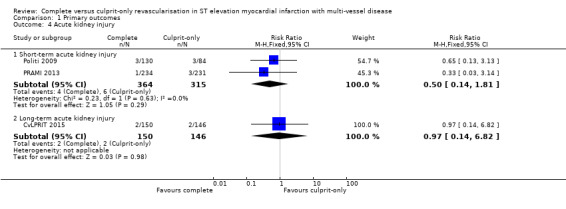
Comparison 1 Primary outcomes, Outcome 4 Acute kidney injury.
Stroke
One trial found no difference in the occurrence of short‐term stroke between complete or culprit‐only revascularisation strategy (2/234 (0.9%) in complete group versus or 0/231 (0%) in culprit‐only group; RR 4.94, 95% CI 0.24 to 102.26; participants = 465; very low quality evidence) (PRAMI 2013). Two trials showed that complete and culprit‐only revascularisation strategies were associated with similar rate of stroke in the long‐term (2/256 (0.8%) in complete group versus 5/254 (2%) in culprit‐only group; RR 0.45, 95% CI 0.10 to 2.01; participants = 510; I2 = 14) (Analysis 1.5) (CvLPRIT 2015; PRAGUE‐13 2015).
1.5. Analysis.
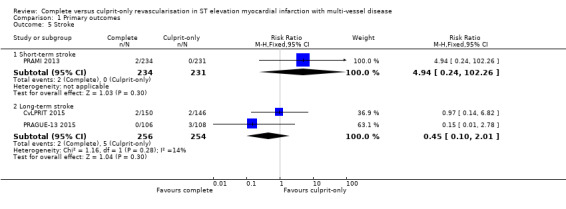
Comparison 1 Primary outcomes, Outcome 5 Stroke.
Bleeding
Three trials showed that there was no difference in major bleeding rate between groups in the short‐term (21/628 (3.3%) in complete group versus 19/585 (3.2%) in culprit‐only group; RR 1.00, 95% CI 0.53 to 1.86; participants = 1213; I2 = 0%; very low quality evidence) (Dambrink and Ghani 2010; DANAMI‐3‐PRIMULTI 2015; PRAMI 2013). Two trials demonstrated that the frequency of bleeding in the long‐term was similar between groups (20/464 (4.3%) in complete group versus 25/459 (5.4%) in culprit‐only group; RR 0.79, 95% CI 0.45 to 1.41; participants = 923; I2 = 0%) (Analysis 1.6) (CvLPRIT 2015; DANAMI‐3‐PRIMULTI 2015).
1.6. Analysis.
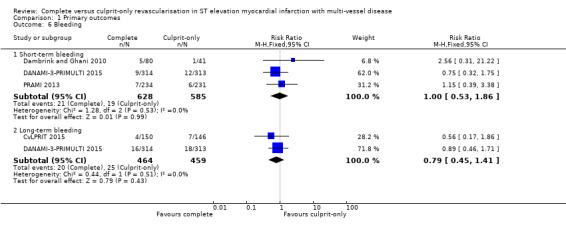
Comparison 1 Primary outcomes, Outcome 6 Bleeding.
Secondary outcomes
Short‐term all‐cause mortality
Two trials showed that the complete and culprit‐only revascularisation strategies did not differ significantly in terms of short‐term all‐cause mortality (4/366 (1.1%) in complete group versus 5/330 (1.5%) in culprit‐only group; RR 0.65, 95% CI 0.18 to 2.37; participants = 696; I2 = 0%; very low quality evidence) (Analysis 2.1) (DANAMI‐3‐PRIMULTI 2015; HELP AMI 2004).
2.1. Analysis.
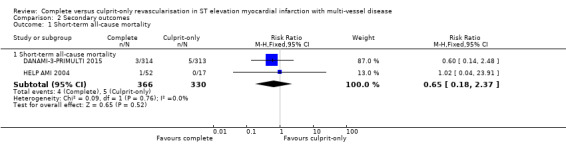
Comparison 2 Secondary outcomes, Outcome 1 Short‐term all‐cause mortality.
Short‐term cardiovascular mortality
One trial showed that the complete and culprit‐only revascularisation strategies did not differ significantly regarding short‐term cardiovascular mortality (1/314 (0.3%) in complete group versus 3/313 (1%) in culprit‐only group; RR 0.33, 95% CI 0.03 to 3.18; participants = 627; I2 = 0%) (Analysis 2.2) (DANAMI‐3‐PRIMULTI 2015).
2.2. Analysis.
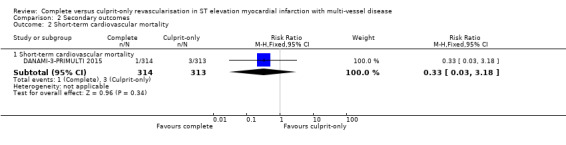
Comparison 2 Secondary outcomes, Outcome 2 Short‐term cardiovascular mortality.
Short‐term non‐fatal myocardial infarction
One trial showed that the complete and culprit‐only revascularisation strategies did not differ significantly regarding short‐term non‐fatal myocardial infarction (7/314 (2.2%) in complete group versus 4/313 (1.3%) in culprit‐only group; RR 1.74, 95% CI 0.52 to 5.90; participants = 627; I2 = 0%) (Analysis 2.3) (DANAMI‐3‐PRIMULTI 2015).
2.3. Analysis.
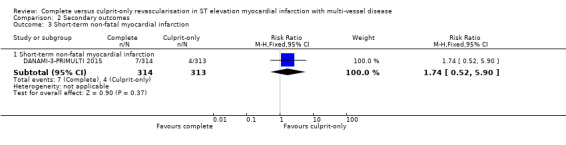
Comparison 2 Secondary outcomes, Outcome 3 Short‐term non‐fatal myocardial infarction.
Short‐term and long‐term revascularisation
Two trials showed that there was no difference in terms of revascularisation between groups in the short‐term (6/366 (1.6%) in complete group versus 10/330 (3%) in culprit‐only group; RR 0.53, 95% CI 0.20 to 1.45; participants = 696; I2 = 0%) (DANAMI‐3‐PRIMULTI 2015; HELP AMI 2004). Nine trials showed that the complete revascularisation strategy was associated with significantly lower rates of revascularisation in the long‐term (145/1374 (10.6%) in complete group versus 258/1242 (20.8%) in culprit‐only group; RR 0.47, 95% CI 0.39 to 0.57; participants = 2616; I2 = 31%; very low quality evidence) (Analysis 2.4) (CvLPRIT 2015; Dambrink and Ghani 2010; DANAMI‐3‐PRIMULTI 2015; Estevez Loureiro 2014; HELP AMI 2004; Politi 2009; PRAGUE‐13 2015; PRAMI 2013; Zhang 2015).
2.4. Analysis.
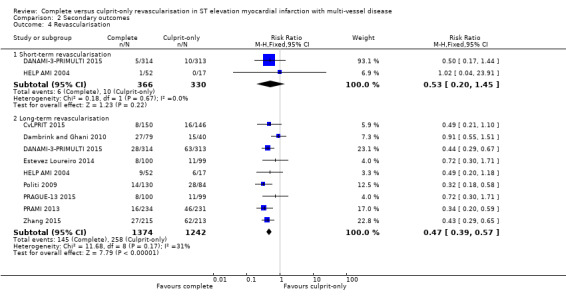
Comparison 2 Secondary outcomes, Outcome 4 Revascularisation.
In the TSA, the cumulative Z‐curve crossed the sequential monitoring boundaries for benefit. This result indicates that the complete revascularisation strategy is associated to an at least 20% long‐term non‐fatal myocardial infarction RRR compared with the culprit‐only strategy (RR 0.49, TSA‐adjusted CI 0.31 to 0.79) and further trials may not change this result (Figure 7).
7.
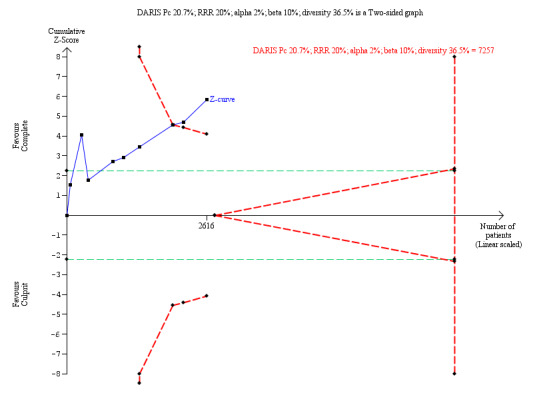
Trial Sequential Analysis for complete versus culprit‐only revascularisation on long‐term revascularisation. The diversity‐adjusted required information size (DARIS) was calculated based on an expected relative risk reduction (RRR) of 20% from Pc group of 20.7% with an alpha of 2% and beta of 10%.
Health‐related quality of life
No studies reported long‐term health‐related quality of life.
Cost
Only one study reported on costs and found no difference in one‐year cost between the groups (MD Euros ‐1948.00, 95% CI ‐9171.85 to 5275.85; participants = 69; I2 = 0%) (Analysis 2.5) (HELP AMI 2004).
2.5. Analysis.
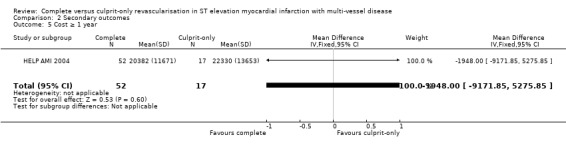
Comparison 2 Secondary outcomes, Outcome 5 Cost ≥ 1 year.
Subgroup analyses
Drug‐eluting stent compared to bare‐metal stents
Two trials utilised DES only in few participants (20% of the included participants in Dambrink and Ghani 2010 and 10.2% in the Politi 2009 trial received DES), while the other studies mostly placed DES in their revascularisation procedures. In those participants who underwent revascularisation with DES, complete revascularisation was favoured over the culprit‐only revascularisation strategy in terms of long‐term cardiovascular mortality (22/913 (2.4%) in complete group versus 40/903 (4.4%) in culprit‐only group; RR 0.54, 95% CI 0.33 to 0.91; participants = 1816; studies = 4; I2 = 0%) and long‐term non‐fatal myocardial infarction (41/965 (4.2%) in complete group versus 63/920 (6.8%) in culprit‐only group; RR 0.63, 95% CI 0.43 to 0.93; participants = 1885; studies = 5; I2 = 0%). In contrast, for participants in whom revascularisation was performed with BMS, neither of the revascularisation strategies was statistically favoured in terms of long‐term cardiovascular mortality (6/130 (4.6%) in complete group versus 10/84 (12%) in culprit‐only group; RR 0.39, 95% CI 0.15 to 1.03; participants = 214; studies = 1) or long‐term non‐fatal myocardial infarction (6/130 (4.6%) in complete group versus 7/84 (8.3%) in culprit‐only group; RR 0.55, 95% CI 0.19 to 1.59; participants = 214; studies = 1).
Sex
Given the poor subgroup reporting in the included trials, we were unable to perform analysis by sex.
People with diabetes mellitus compared to people without diabetes mellitus
Given the poor subgroup reporting in the included trials, we were unable to perform analysis by people with or without diabetes mellitus.
Non‐culprit and culprit intervention during the same procedure compared to in separate interventions (staged)
The complete revascularisation strategy performed at the index procedure was associated with lesser long‐term non‐fatal myocardial infarction (12/501 (2.4%) in complete group versus 32/478 (6.7%) in culprit‐only group; RR 0.37, 95% CI 0.19 to 0.71; participants = 979; studies = 4; I2 = 0%), while when the intervention on the non‐culprit lesions was deferred for a second intervention or staged procedure, the complete and culprit‐only revascularisation strategies had similar long‐term non‐fatal myocardial infarction (35/594 (5.9%) in complete group versus 45/610 (7.4%) in culprit‐only group; RR 0.80, 95% CI 0.52 to 1.23; participants = 1204; studies = 3; I2 = 0%). Complete revascularisation was also associated with lesser long‐term cardiovascular mortality when performed either during the index procedure (10/449 (2.2%) in complete group versus 27/461 (5.9%) in culprit‐only group; RR 0.40, 95% CI 0.20 to 0.82; participants = 910; studies = 3; I2 = 0%) or in a staged manner (18/694 (2.6%) in complete group versus 34/709 (4.8%) in culprit‐only group; RR 0.56, 95% CI 0.32 to 0.98; participants = 1403; studies = 4; I2 = 0%). It is important to note that in the CvLPRIT 2015 trial, which was grouped with the studies that performed complete revascularisation at the index procedure, 35% of the complete revascularisation procedures were performed in a staged manner.
Low risk of bias articles compared to high risk of bias articles
Given the poor subgroup reporting in the included trials, we were unable to perform analysis by low or high risk of bias.
Participants in cardiogenic shock compared to participants not in cardiogenic shock
Given the poor subgroup reporting in the included trials, we were unable to perform analysis by participants in or not in cardiogenic shock.
Sensitivity analyses
We performed sensitivity analyses restricting the analysis to studies that were published, were in English language only, with revascularisation guided by FFR, and the type of stent used. The overall results were unaffected by excluding the studies with those characteristics from the pooled analysis.
Assessment of reporting bias
We were unable to create a funnel plot because we did not pool more than 10 trials for the analysis of any outcome.
Ongoing trials
We identified six ongoing clinical trials that are comparing complete PCI revascularisation versus PCI of culprit‐only revascularisation in people with STEMI (see Characteristics of ongoing studies table).
Discussion
Summary of main results
The meta‐analysis of nine RCTs showed that, compared with the culprit‐only intervention, complete revascularisation in people with STEMI and MVD seems to be associated with a lower long‐term cardiovascular mortality, long‐term revascularisation need, and long‐term non‐fatal myocardial infarction.
Overall completeness and applicability of evidence
Even though this systematic review included nine fairly well‐designed RCTs and there was significant consistency across the studies due to similar inclusion/exclusion criteria, similar participant populations, similar procedures, and criteria to decide about intervening on the non‐culprit vessel, the overall quality of evidence was very low mostly due to serious problem of imprecision, indirectness, and study limitations.
The fact that the included studies in this pooled analysis were conducted recently and were contemporary with regards to the standards of care utilised, including DES and up‐to‐date medical therapy, along with the flexible inclusion and exclusion criteria, ensures high applicability of these results to current‐day clinical practice. Even though the studies were performed in different parts of the world, some continents were under‐represented. For instance, none of the included trials studied participants in North America, South America, or Australia. Women were also under‐represented, and although none of the trials reported the race of the studied participants, it is plausible that black and Hispanic people were not included or were under‐represented. Therefore, these findings have to be applied cautiously in those under‐represented populations and geographic locations.
Quality of the evidence
We utilised the GRADE assessment tool to evaluate the quality of evidence for the most relevant outcomes. In general, we judged the outcomes to have very low quality of evidence. The quality of the evidence was downgraded because of the potential risk of bias, indirectness of findings, and imprecision. Given the number of studies, we were unable to exclude publication bias and we found no evidence of plausible confounding or dose‐response gradient.
One of the main limitations of the included studies was the open‐label design, which potentially may increase the risk of performance or detection bias. To attenuate these biases, the investigators blinded the outcome assessors and we, in our analysis, included mostly objective outcomes such as mortality and non‐fatal myocardial infarction. However, we do expect that the rate of revascularisation and acute kidney injury were possibly influenced by detection and performance bias. Five of the included trials lacked a published protocol (Dambrink and Ghani 2010; Estevez Loureiro 2014; HELP AMI 2004; Politi 2009; Zhang 2015), which was concerning for risk of reporting bias; for those studies that had a published protocol, we found no evidence of reporting bias. The only study that effectively reported the allocation concealment and random sequence generation was the CvLPRIT 2015 trial, which we judged to have low risk of selection bias, while the others had high risk for selection bias.
Although the studies had few dropouts, given the small number events, we considered that the proportion of participants that left the trials might have increased the risk of attrition bias in at least two studies (CvLPRIT 2015; PRAMI 2013). Moreover, the early termination of one study because of significant difference between groups (PRAMI 2013), may also have introduced bias to the results (e.g. overestimate the beneficial results from the complete revascularisation strategy).
Another explanation for the very low quality of evidence comes from the fact that women and presumably minority group such as Hispanic and black people were under‐represented in the included studies, which would affect the external applicability of these findings; therefore, we considered this a serious limitation in indirectness of the evidence. Another limitation that justified downgrading the quality of the evidence for imprecision was that the included studies had a small number of events and several outcomes did not meet the optimal information size, which was confirmed with the TSA.
Potential biases in the review process
In order to ensure applicability of these results to patient care, all‐cause mortality was added as another primary outcome after publication of the protocol. The definition of “type of intervention” was changed to include RCTs that performed revascularisation as a second intervention at another hospitalization, which is a common practice. Additionally an element that should be considered as a potential source of bias during the review process is that the decision on intervening the non‐culprit coronary arteries was slightly different among studies. For instance, trials included in this review used slightly different degrees of stenosis while others utilised FFR measurements. Finally, some studies performed the complete revascularisation at the same index procedure while others performed the complete revascularisation at different times.
Agreements and disagreements with other studies or reviews
Major international guidelines, including those from the ACCF/AHA 2013 and ESC 2012, favour timely intervention on the culprit artery and tend to discourage simultaneous interventions upon non‐culprit lesions in the absence of objective signs or symptoms of persistent cardiac ischaemia, due to lack of good‐quality evidence and even concerns of possible harm. The results of our meta‐analysis suggest that in fact complete revascularization of significantly stenotic (50‐70% stenotic) non‐culprit lesions, regardless of signs or symptoms of ischaemia, may improve important cardiovascular outcomes.
Our findings are clearly in conformity with the newly updated ACC/AHA/SCAI 2015 and ESC 2014 guidelines, which allow for consideration of simultaneous intervention on both culprit and non‐culprit lesions during the same index procedure, thus suggesting possible benefits in otherwise haemodynamically stable people presenting with STEMI and MVD.
There are several meta‐analyses that have previously shown that complete revascularisation and culprit‐only intervention strategies have similar outcomes. For example, Vlaar 2011 showed in their meta‐analysis of four prospective studies that there was no difference in short (odds ratio (OR) 1.98, 95% CI 0.57 to 6.85) or long‐term mortality (OR 1.45, 95% CI 0.61 to 3.46). Similarly Bagai 2013, after analysing three RCTs, showed that the strategies were similar (OR 0.81, 95% CI 0.32 to 2.06).
In contrast, more recently published meta‐analyses have shown that complete revascularisation strategy is superior to the culprit‐only intervention strategy. Sardar 2015 included five RCTs and they found that, compared to a culprit‐vessel‐only intervention strategy, complete revascularisation was associated with a significant reduction in revascularisation (OR 0.32, 95% CI 0.21 to 0.49), cardiovascular mortality (OR 0.44, 95% CI 0.20 to 0.94), and recurrent myocardial infarction (OR 0.35, 95% CI 0.18 to 0.69). In Elgendy 2015, the pooled analysis of six RCTs showed that the complete revascularisation strategy lowered the risk of urgent revascularisation (RR 0.55, 95% CI 0.35 to 0.86), but the strategies did not differ in terms of mortality (RR 0.68, 95% CI 0.4 to 1.14) and myocardial infarction (RR 0.56, 95% CI 0.24 to 1.27). In the editorial comment by Bhatt 2015, the pooled analysis of the PRAMI 2013 and CvLPRIT 2015 trials showed that, compared with the culprit‐only intervention group, the complete revascularisation strategy was associated with a lower cardiovascular mortality, revascularisation, and myocardial infarction. Similarly, Pandit 2014 demonstrated that the complete revascularisation strategy was associated with reduced cardiovascular deaths (OR 0.39, 95% CI 0.18 to 0.83), revascularisation (OR 0.28, 95% CI 0.18 to 0.44), and non‐fatal myocardial infarction (OR 0.38, 95% CI 0.20 to 0.75). In Bangalore 2015, the complete revascularisation strategy was associated with lower mortality (RR 0.60, 95% CI 0.38 to 0.97), cardiovascular mortality (RR 0.38, 95% CI 0.20 to 0.73), and revascularisation (RR 0.42, 95% CI 0.31 to 0.57). In Bainey 2014, it was shown that complete revascularisation strategy lowered in‐hospital mortality (OR 0.24, 95% CI 0.06 to 0.91) and need for revascularisation (OR 0.31, 95% CI 0.17 to 0.57). In terms of long‐term mortality, the strategies appeared to be similar (OR 0.61, 95% CI 0.28 to 1.33).
Although our present systematic review is consistent with the previously published reviews and meta‐analyses, this is the largest, most comprehensive, and only systematic review that included only RCTs, showing that complete revascularisation strategy is associated with a long‐term reduction in cardiovascular mortality, non‐fatal myocardial infarction, and revascularisation. Furthermore, this is the only systematic review in this area that has utilised GRADE to evaluate the quality of the evidence, and to include a TSA in order to control the type I and II errors and to predict if the total number of participants included in the meta‐analysis was enough to draw conclusions regarding the effects of complete revascularisation.
Authors' conclusions
Implications for practice.
Given the increasing prevalence of classic cardiovascular risk factors, the prevalence of ST elevation myocardial infarction (STEMI) continues to rise globally, leading to significant mortality, morbidity, and healthcare costs. The updated version of American College of Cardiology (ACC)/American Heart Association (AHA)/Society for Cardiovascular Angiography and Interventions (SCAI) and European Society of Cardiology (ESC) guidelines now consider it reasonable to intervene on the non‐culprit vessel, at the time of the index procedure, in people with STEMI. The evidence generated by this meta‐analysis shows that the complete revascularisation may be superior but we have very little confidence in the effect estimate since the quality of evidence is very low and there is still need of further research to support or disprove that potential difference.
Implications for research.
STEMI and multi‐vessel disease (MVD) frequently coexist, and this combination is associated with worse outcomes. In order to improve the care of this subgroup of people, it is crucial to understand fully the pathophysiology of the coronary artery disease progression after STEMI and the correct treatment options for the non‐culprit stenotic vessels. This systematic review supports the view that complete revascularisation may be a better treatment option than the culprit‐only intervention strategy; however, based on the low quality of the evidence and TSA, more studies are needed in order to draw firm conclusions regarding long‐term all‐cause mortality, cardiovascular mortality, and non‐fatal myocardial infarction. The additional important question as to whether it is better to intervene on the non‐culprit vessel at the index procedure or staged needs to be further studied in a randomised controlled trial designed for this purpose. Such trials ought to be designed according to the SPIRIT Statement (Standard Protocol Items: Recommendations for Interventional Trials) and reported according to the CONSORT Statement.
Unfortunately, we could not access data that would allow us to study specific subgroups of people, such as people with diabetes, women, or certain races, which may benefit in particular from one strategy over another. Finally, even though the findings of this systematic review suggest that the complete revascularisation strategy seems better than the current standard of care, further research is needed to confirm the benefit obtained by complete revascularisation, and to determine the optimal mode, timing, and extent of revascularisation of the non‐culprit arteries in people with STEMI and MVD.
What's new
| Date | Event | Description |
|---|---|---|
| 8 May 2017 | Amended | Minor correction to author name, addition of Published Note for ACUTE COMPARE trial. |
Notes
It was noted that at the time of submission of this meta‐analysis, the ACUTE COMPARE trial was published. In an updated version we will include that trial.
Acknowledgements
We acknowledge the important role of the institutions that foster our career development, the Cochrane Heart Group, and the reviewers that provided interesting and useful criticisms.
We also thank the contribution of Dr Wenliang Song and Lanye He in the translation of the article Zhang 2015; and Ronald E Pachon for his participation at early stages of this manuscript.
Appendices
Appendix 1. Search strategies
|
CENTRAL, DARE, and HTA (Wiley) 1 MeSH descriptor: [Myocardial Infarction] explode all trees 2 ((myocard* or heart) near/3 infarct*):ab,ti,kw 3 (heart next/1 attack*):ab,ti,kw 4 ((stun* or hibernat*) near/3 myocard*):ab,ti,kw 5 'cardiogenic shock':ab,ti,kw 6 (st near/2 elevat* near/4 ('myocardial infarction' or 'myocardial infarctions' or mi)):ab,ti,kw 7 stemi:ab,ti,kw 8 {or #1‐#7} 9 MeSH descriptor: [Percutaneous Coronary Intervention] explode all trees 10 pci:ab,ti,kw or ppci:ab,ti,kw 11 ('percutaneous coronary' near/6 (intervention* or revascularization*)):ab,ti,kw 12 ((transluminal or 'trans luminal') near/6 coronary):ab,ti,kw 13 angioplast*:ab,ti,kw 14 atherectom*:ab,ti,kw 15 (balloon near/2 (coronary or dilat*)):ab,ti,kw 16 MeSH descriptor: [Stents] explode all trees 17 stent*:ab,ti,kw 18 {or #9‐#17} 19 (multi* near/4 vessel):ab,ti,kw 20 (multivessel or 'multi‐vessel'):ab,ti,kw 21 ('infarct related' or IRA or 'non infarct related' or 'non‐IRA'):ab,ti,kw 22 (culprit or 'culprit‐only' or 'non‐culprit' or nonculprit or bystander):ab,ti,kw 23 {or #19‐#22} 24 #8 and #18 and #23 Ovid MEDLINE(R) 1946 to December Week 1 2016, Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations January 03, 2017 and Ovid MEDLINE(R) Epub Ahead of Print January 03, 2017 1. exp Myocardial Infarction/ 2. ((myocard* or heart) adj3 infarct*).tw. 3. heart attack*.tw. 4. ((stun* or hibernat*) adj3 myocard*).tw. 5. cardiogenic shock.tw. 6. (ST adj2 elevat* adj4 (myocardial infarction* or MI)).tw. 7. stemi.tw. 8. or/1‐7 9. exp Percutaneous Coronary Intervention/ 10. (PCI or PPCI).tw. 11. (percutaneous coronary adj6 (intervention* or revascularization*)).tw. 12. ((transluminal or trans‐luminal) adj6 coronary).tw. 13. angioplast*.tw. 14. atherectom*.tw. 15. (balloon adj2 (coronary or dilat*)).tw. 16. exp Stents/ 17. stent*.tw. 18. or/9‐17 19. (multi* adj4 vessel).tw. 20. (multivessel or multi‐vessel).tw. 21. (infarct related or IRA or non infarct related or non‐IRA).tw. 22. (culprit or culprit‐only or non‐culprit or nonculprit or bystander).tw. 23. or/19‐22 24. randomized controlled trial.pt. 25. controlled clinical trial.pt. 26. randomized.ab. 27. placebo.ab. 28. drug therapy.fs. 29. randomly.ab. 30. trial.ab. 31. groups.ab. 32. 24 or 25 or 26 or 27 or 28 or 29 or 30 or 31 33. exp animals/ not humans.sh. 34. 32 not 33 35. 8 and 18 and 23 36. 34 and 35 EMBASE (embase.com) #28 #26 AND #27 #27 #8 AND #18 AND #23 #26 #24 NOT #25 1122006 #25 'animal'/exp OR 'nonhuman'/exp NOT 'human'/exp #24 random*:ab,ti OR placebo* OR (double NEXT/1 blind*):ab,ti #23 #19 OR #20 OR #21 OR #22 #22 culprit:ab,ti OR 'culprit‐only':ab,ti OR 'non‐culprit':ab,ti OR nonculprit:ab,ti OR bystander:ab,ti #21 'infarct related':ab,ti OR ira:ab,ti OR 'non infarct related':ab,ti OR 'non‐ira':ab,ti #20 multivessel:ab,ti OR 'multi‐vessel':ab,ti #19 (multi* NEAR/4 vessel):ab,ti #18 #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 #17 stent*:ab,ti #16 'stent'/exp #15 (balloon NEAR/2 (coronary OR dilat*)):ab,ti #14 atherectom*:ab,ti #13 angioplast*:ab,ti #12 ((transluminal OR 'trans luminal') NEAR/6 coronary):ab,ti #11 ('percutaneous coronary' NEAR/6 (intervention* OR revascularization*)):ab,ti #10 pci:ab,ti OR ppci:ab,ti #9 'interventional cardiovascular procedure'/exp #8 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 #7 stemi:ab,ti #6 (st NEAR/2 elevat* NEAR/4 ('myocardial infarction' OR 'myocardial infarctions' OR mi)):ab,ti #5 'cardiogenic shock':ab,ti #4 ((stun* OR hibernat*) NEAR/3 myocard*):ab,ti #3 (heart NEXT/1 attack*):ab,ti #2 ((myocard* OR heart) NEAR/3 infarct*):ab,ti #1 'heart infarction'/exp Conference Proceedings Citation Index‐ Science (CPCI‐S)‐‐1990‐present (Web of Science) # 21 #20 AND #15 AND #7 # 20 #19 OR #18 OR #17 OR #16 # 19 TS=(culprit or "culprit‐only" or "non‐culprit" or nonculprit or bystander) # 18 TS=("infarct related" or IRA or "non infarct related" or "non‐IRA") # 17 TS=(multivessel or "multi‐vessel") # 16 TS=(multi* near/4 vessel) # 15 #14 OR #13 OR #12 OR #11 OR #10 OR #9 OR #8 # 14 TS=(stent*) # 13 TS=(balloon near/2 (coronary or dilat*)) # 12 TS=(atherectom*) # 11 TS=(angioplast*) # 10 TS=((transluminal or "trans luminal") near/6 coronary) # 9 TS=(PCI or PPCI) # 8 TS=("percutaneous coronary" near/6 (intervention* or revascularization*)) # 7 #6 OR #5 OR #4 OR #3 OR #2 OR #1 # 6 TS=(stemi) # 5 TS=(st near/2 elevat* near/4 ("myocardial infarction" or "myocardial infarctions" or mi)) # 4 TS=("cardiogenic shock") # 3 TS=((stun* or hibernat*) near/3 myocard*) # 2 TS=("heart attack" OR "heart attacks") # 1 TS=((myocard* or heart) near/3 infarct*) ClinicalTrials.gov (Expert search) multivessel OR "multi vessel" OR "infarct related" OR "non infarct related" OR culprit OR "non culprit" World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) Search Portal (Standard search) multivessel OR multi vessel OR infarct related OR non infarct related OR culprit OR non culprit European (EU) Clinical Trials Register multivessel OR "multi vessel" OR "infarct related" OR "non infarct related" OR culprit OR "non culprit" Epistemonikos (http://www.epistemonikos.org) (Advance search) multivessel OR "multi vessel" OR "infarct related" OR "non infarct related" OR culprit OR "non culprit" |
Appendix 2. Survey of authors providing information on included trials
| Characteristic | Date trial author asked for additional information | Date trial author replied | Trial author provided data |
| CvLPRIT 2015 | 26 May 2016 | No reply | |
| Dambrink and Ghani 2010 | 26 May 2016 | No reply | |
| DANAMI‐3‐PRIMULTI 2015 | 26 May 2016 | 27 May 2016 | 1 July 2016 |
| Estevez Loureiro 2014 | 26 May 2016 | No reply | |
| HELP AMI 2004 | 26 May 2016 | No reply | |
| Politi 2009 | 26 May 2016 | No reply | |
| PRAGUE‐13 2015 | 26 May 2016 | No reply | |
| PRAMI 2013 | 26 May 2016 | 31 May 2016 | Did not provide additional data |
| Zhang 2015 | 26 May 2016 | No reply |
Data and analyses
Comparison 1. Primary outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Long‐term all‐cause mortality | 8 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Long‐term all‐cause mortality | 8 | 2417 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.58, 1.11] |
| 2 Long‐term cardiovascular mortality | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Long‐term cardiovascular mortality | 6 | 2229 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.32, 0.79] |
| 3 Long‐term non‐fatal myocardial infarction | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Long‐term non‐fatal myocardial infarction | 6 | 2099 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.44, 0.89] |
| 4 Acute kidney injury | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Short‐term acute kidney injury | 2 | 679 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.50 [0.14, 1.81] |
| 4.2 Long‐term acute kidney injury | 1 | 296 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.14, 6.82] |
| 5 Stroke | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Short‐term stroke | 1 | 465 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.94 [0.24, 102.26] |
| 5.2 Long‐term stroke | 2 | 510 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.10, 2.01] |
| 6 Bleeding | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Short‐term bleeding | 3 | 1213 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.53, 1.86] |
| 6.2 Long‐term bleeding | 2 | 923 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.45, 1.41] |
Comparison 2. Secondary outcomes.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Short‐term all‐cause mortality | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Short‐term all‐cause mortality | 2 | 696 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.18, 2.37] |
| 2 Short‐term cardiovascular mortality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Short‐term cardiovascular mortality | 1 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.03, 3.18] |
| 3 Short‐term non‐fatal myocardial infarction | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Short‐term non‐fatal myocardial infarction | 1 | 627 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.74 [0.52, 5.90] |
| 4 Revascularisation | 9 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Short‐term revascularisation | 2 | 696 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.20, 1.45] |
| 4.2 Long‐term revascularisation | 9 | 2616 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.39, 0.57] |
| 5 Cost ≥ 1 year | 1 | 69 | Mean Difference (IV, Fixed, 95% CI) | ‐1948.0 [‐9171.85, 5275.85] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
CvLPRIT 2015.
| Methods | RCT multi‐centre. Randomisation ratio: 1:1. Number of study centres: 7 centres in the UK. |
|
| Participants |
Inclusion criteria: suspected or confirmed acute MI, significant ST elevation or LBBB on ECG (in cases of LBBB, angiographic confirmation of culprit coronary occlusion was required), < 12 hours of symptom onset, scheduled for P‐PCI for clinical reasons, provision of verbal assent followed by written informed consent, MVD, the non‐culprit vessel had to be a major (> 2 mm) epicardial coronary artery or branch (> 2 mm) and be suitable for stent implantation. Exclusion criteria: any exclusion criteria for P‐PCI; aged < 18 years; clear indication for, or contraindication to, multi‐vessel P‐PCI according to operator judgement; previous Q‐wave MI; people with prior CABG, cardiogenic shock, ventricular septal defect, or moderate/severe mitral regurgitation; chronic kidney disease (Cr > 200 μmol/L or eGFR < 30 mL/minute/1.73 m2); suspected or confirmed thrombosis of a previously stented artery; where the only significant non‐IRA lesion is a chronic total occlusion. Diagnostic criteria MVD: culprit vessel plus at least 1 non‐culprit coronary artery with at least 1 lesion deemed angiographically significant. Significant stenosis: > 70% diameter stenosis in 1e plane or > 50% in 2 planes. Sample size: complete revascularisation n = 150 and culprit‐only revascularisation n = 146. |
|
| Interventions |
Complete revascularisation: complete revascularisation at the same procedure, unless operator decided, for clinical reasons, that the procedure needed to be staged, in the cases of staged intervention, it was mandated that the non‐culprit lesions be treated during the index admission. Culprit‐only revascularisation: intervention on the culprit artery unless participant needed revascularisation based on ischaemic symptoms or significant ischaemia evidenced in imaging tests. |
|
| Outcomes |
Primary: composite of all‐cause mortality, recurrent MI, heart failure, and ischaemia‐driven revascularisation within 12 months after index procedure. Secondary: cardiovascular death, individual components of the primary endpoint, stroke, major bleeding, and contrast‐induced nephropathy. |
|
| Notes | Protocol ID: ISRCTN70913605. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | An interactive voice‐response program was utilised to randomise participants. |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed immediately after the angiography and before the intervention of the culprit artery via a centralised 24/7 telephone randomisation service. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study was open label for the participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes adjudicator clinicians were blinded to the group allocation. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Similar dropout rate in both groups, 7.3% culprit‐only vs 5.5% complete revascularisation group; however, given the small number of events, the result may be affected by attrition bias. |
| Selective reporting (reporting bias) | Low risk | Study reported the primary outcomes indicated in the published protocol in www.isrctn.com ISRCTN70913605. |
| Other bias | Low risk | Study was funded by the national funding institution British Heart Foundation and Medical Research Council (MRC)/National Institutes of Health Research (NIHR) (UK) and the funding institution was not involved in the study other than economically. |
Dambrink and Ghani 2010.
| Methods | RCT. Randomisation ratio: 2:1. Number of study centres: 1 centre in the Netherlands. |
|
| Participants |
Inclusion criteria: MVD with successful P‐PCI for STEMI. Exclusion criteria: urgent indication for additional revascularisation, aged > 80 years, chronic occlusion of 1 of the non‐culprit artery(ies), prior CABG, left main stenosis of ≥ 50%, restenotic lesions in non‐culprit artery(ies), chronic atrial fibrillation, limited life‐expectancy, or other factors that made complete follow‐up unlikely. Diagnostic criteria MVD: ≥ 1 significant stenosis in at least 2 major epicardial coronary arteries or the combination of a side branch and a main epicardial vessel provided that they supplied different territories. Significant stenosis: diameter ≥ 50% in luminal diameter in at least 1 view. FFR < 0.75 defined ischaemic stenosis and those were intervened only, and > 90% stenosis were intervened without FFR measurement. Sample size: complete revascularisation n = 80 and culprit‐only revascularisation n = 41. |
|
| Interventions |
Complete revascularisation: staged intervention on significant stenotic non‐culprit lesions compatible with ischaemia (FFR < 0.75) with plain angioplasty, BMS, or DES. Culprit‐only revascularisation: medical management after P‐PCI of culprit artery only unless ischaemic symptoms were elicited with exercise testing, dobutamine stress echocardiography, or myocardial scintigraphy, in those cases ischaemia‐guided revascularisation was performed. |
|
| Outcomes |
Primary: EF at 6 months. Secondary: change in EF, wall motion score, left ventricle end‐systolic and end‐diastolic volume, and MACE. |
|
| Notes | Early termination because of slow enrolment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed with a computer program. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned how allocation concealment was insured. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned in the article. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Only mentioned in the study that echocardiographic and radionucleotide data were blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In the complete revascularisation group 1.3% dropped out, vs 2.4% in the culprit‐only group. |
| Selective reporting (reporting bias) | High risk | Study did not have a published protocol and was not registered on any clinical trial databases. |
| Other bias | High risk | Study had early termination because of slow enrolment. Unclear source of funding. |
DANAMI‐3‐PRIMULTI 2015.
| Methods | RCT multi‐centre. Randomisation ratio: 1:1. Number of study centres: 2 centres in Denmark. |
|
| Participants |
Inclusion criteria: chest pain < 12 hours' duration and ST elevation > 0.1 mV in at least 2 contiguous leads and with diameter stenosis of > 50% in ≥ non‐culprit artery(ies). Exclusion criteria: intolerance to contrast media, anticoagulant, antithrombotic drugs, unconsciousness or cardiogenic shock, stent thrombosis, indications for CABG, or increased bleeding risk. Diagnostic criteria MVD: significant stenosis in ≥ 1 of the non‐culprit artery(ies) or their major side branches in addition to that in the culprit artery. Significant stenosis: > 50% stenosis visually in arteries > 2 mm diameter and FFR ≤ 0.8 or > 90% stenosis visually regardless FFR measurement. Sample size: complete revascularisation n = 314 and culprit‐only revascularisation n = 313. |
|
| Interventions |
Complete revascularisation: PCI of culprit and in a second intervention 48 hours after P‐PCI and before discharge, FFR‐guided PCI in all non‐culprit significant stenotic lesions and > 90% stenotic despite FFR measurement. Culprit‐only revascularisation: Intervention on the culprit‐only. |
|
| Outcomes |
Primary: composite of all‐cause mortality, non‐fatal MI, and ischaemia‐driven (subjective or objective) revascularisation of lesions in non‐culprit artery(ies) 1 year' follow‐up. Secondary: all‐cause mortality, non‐fatal MI, cardiac death, urgent or non‐urgent PCI of lesions in non‐culprit artery(ies). |
|
| Notes | Protocol ID: NCT01960933. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed electronically via a centralised web‐based system. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned how allocation concealment was insured. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Even though the study was open label there was an independent events committee that adjudicated all events. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In the complete revascularisation group around 0.3% dropped out, while in the culprit‐only group all participants completed the study. |
| Selective reporting (reporting bias) | Low risk | Study reported the primary outcomes indicated in the published protocol in www.ClinicalTrial.gov NCT01960933. |
| Other bias | Low risk | Study was funded by national funding institution (Danish Agency of Science, Technology and Innovation and Danish Council for Strategic Research) and the funding institution was not involved in the study other than economically. |
Estevez Loureiro 2014.
| Methods | RCT. Randomisation ratio: 1:1. Number of study centres: not described in abstract. |
|
| Participants |
Inclusion criteria: people with STEMI and MVD. Exclusion criteria: not described in abstract. Diagnostic criteria MVD: not described in abstract. Significant stenosis: not described in abstract. Sample size: complete revascularisation n = 100 and culprit‐only revascularisation n = 99. |
|
| Interventions |
Invasive: staged complete intervention after P‐PCI. Conservative: intervention of culprit‐only, unless participants had residual ischaemia based on stress echocardiogram, these participants would go for staged intervention. |
|
| Outcomes |
Primary: composite of cardiovascular death, non‐fatal MI, revascularisation of any vessel, or admission due to heart failure. Secondary: not described in abstract. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned in the article. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned in the article. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned in the article. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned in the article. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not mentioned in the article. |
| Selective reporting (reporting bias) | High risk | Study did not have a published protocol and was not registered on any clinical trial databases. |
| Other bias | Unclear risk | Unclear source of funding. |
HELP AMI 2004.
| Methods | RCT multi‐centre. Randomisation ratio: 3:1. Number of study centres: not described in the article. |
|
| Participants |
Inclusion criteria: ischaemic chest pain started < 12 hours before hospital admission with or without ST‐segment elevation of ≥ 1 mm in ≥ 2 contiguous electrocardiographic leads (peripheral leads) or 2 mm in the precordial leads. MVD amenable to angioplasty of at least 2 lesions (culprit artery and ≥ 1 (maximum 3) lesions in a major non‐culprit coronary artery(ies)). Exclusion criteria: presence of significant lesions in vein grafts or arterial conduits or in segments previously treated with angioplasty or stent, recent thrombolysis (< 1 week), cardiogenic shock, defined as hypotension with systolic blood pressure < 90 mmHg and tachycardia > 100 beats/minute, not due to hypovolaemia or requiring inotropic support or balloon counter pulsation. Single‐vessel disease, left main stenosis of ≥ 50%, intention to treat > 1 totally occluded major epicardial vessel, diffuse calcification or severe tortuosity in the culprit and non‐culprit arteries preventing the implantation of the study stents. A sided branch > 2 mm which required being covered by the stent, unless the operator was willing and technically able to maintain patency of this side branch with either further balloon angioplasty or stent placement. Diagnostic criteria MVD: not defined in the article. Significant stenosis: not defined in the article. Sample size: complete revascularisation n = 53 and culprit‐only revascularisation n = 17. |
|
| Interventions |
Complete revascularisation: PCI of all, culprit and non‐culprit coronary artery lesions suitable to intervention with a heparin‐coated stent. Culprit‐only revascularisation: PCI of culprit artery only and intervention on non‐culprit artery(ies) was performed at discretion of the investigator, based on clinical status (persistent or recurrent angina), evidence of ischaemia in non‐invasive tests (perfusion scintigraphy or stress echo), angiographic severity of non‐culprit lesions and clinical relevance of the affected vessels as well as organisation standards of the participating centres. |
|
| Outcomes |
Primary: 12‐month revascularisation. Secondary: in‐hospital revascularisation, reinfarction, and death. Procedural in‐hospital and total hospital cost, 12 months' follow‐up. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned in the article. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned in the article. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned in the article. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned in the article. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not mentioned in the article. |
| Selective reporting (reporting bias) | High risk | Study did not have a published protocol and was not registered on any clinical trial databases. |
| Other bias | Unclear risk | Unclear source of funding. |
Politi 2009.
| Methods | RCT. Number of study centres: not described. |
|
| Participants |
Inclusion criteria: people with prolonged (> 30 minutes) chest pain, started < 12 hours before hospital arrival and ST elevation of ≥ 1 mm in ≥ 2 contiguous limb electrocardiographic leads or 2 mm in precordial leads. Exclusion criteria: cardiogenic shock at presentation (systolic blood pressure ≤ 90 mmHg despite drug therapy), left main coronary disease (≥ 50% diameter stenosis), previous CABG surgery, severe valvular heart disease, and unsuccessful procedures. Diagnostic criteria MVD: stenosis of ≥ 2 epicardial coronary arteries or their major branches by visual estimation. Significant stenosis: > 70% diameter. Sample size: complete revascularisation n = 130 (65 staged and 65 at index procedure complete revascularisation) and culprit‐only revascularisation n = 84. |
|
| Interventions |
Complete revascularisation: revascularisation of all, culprit and non‐culprit significant stenosis at the index procedure or staged. Culprit‐only revascularisation: intervention on the culprit vessel only. |
|
| Outcomes |
Primary: MACE, cardiac or non‐cardiac death, in‐hospital death, re‐infarction, re‐hospitalisation for acute coronary syndrome, and revascularisation. Secondary: not mentioned. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed with a computer program. |
| Allocation concealment (selection bias) | Unclear risk | Lack of information regarding how the allocation was concealed. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned in the article. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned in the article. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts were not reported in the article. |
| Selective reporting (reporting bias) | High risk | Study did not have a published protocol and was not registered on any clinical trial databases. |
| Other bias | Unclear risk | Unclear source of funding. |
PRAGUE‐13 2015.
| Methods | RCT multi‐centre. Randomisation ratio: 1:1. Number of study centres: 6 centres in Czech Republic. |
|
| Participants |
Inclusion criteria: people with STEMI, angiographically successful primary PCI of culprit artery (TIMI flow grades II or III), ≥ 1 other significant stenoses of non‐culprit artery(ies) found by coronary angiography (diameter of artery ≥ 2.5 mm), enrolment ≥ 48 hours following onset of symptoms. Exclusion criteria: stenosis of the left main of left coronary artery ≥ 50%, haemodynamically significant valvular disease, people in cardiogenic shock during STEMI, haemodynamic instability, angina pectoris > grade 2 CCS lasting 1 month prior to STEMI. Diagnostic criteria MVD: ≥ 1 vessel, beside of the culprit vessel, with significant stenosis. Significant stenosis: > 70% stenosis of non‐culprit artery(ies). Sample size: complete revascularisation n = 106 and culprit‐only revascularisation n = 108. |
|
| Interventions |
Complete revascularisation: PCI of the culprit artery and staged intervention for the non‐culprit artery(ies) between days 3 and 40 after the index procedure. Culprit‐only revascularisation: intervention on the culprit artery only. |
|
| Outcomes |
Primary: composite endpoint of death, non‐fatal acute MI, and stroke. Secondary: cardiovascular death, recurrent MI, target vessel failure, progression of studied stenosis of non‐culprit artery, stroke, hospitalisation for heart failure, changes in left ventricular EF, hospitalisation for unstable angina pectoris, outcomes of questionnaire regarding angina pectoris, target vessel revascularisation, non‐culprit target lesion revascularisation. |
|
| Notes | Only abstract published. Protocol ID: NCT01332591. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned in the abstract or in protocol posted on www.ClinicalTrial.gov. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned in the abstract or in protocol posted on www.ClinicalTrial.gov. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Open‐label study. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Open‐label study. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropouts not reported in the abstract. |
| Selective reporting (reporting bias) | Low risk | Abstract reported the primary outcomes indicated in the published protocol in www.ClinicalTrial.gov NCT01332591. |
| Other bias | Low risk | Study was funded by a Research Grant from the Czech Ministry of Health and by The International Clinical Research Center of St. Anne's University Hospital Brno (FNUSA‐ICRC) which is funded by the European Union. |
PRAMI 2013.
| Methods | RCT multi‐centre. Randomisation ratio: 1:1. Number of study centres: 5 centres in the UK. |
|
| Participants |
Inclusion criteria: people of any age with STEMI and MVD detected at the time of angiography. Exclusion criteria: cardiogenic shock, unable to provide consent, previous CABG, non‐infarct artery stenosis of͵≥ 50% in the left main stem or the ostial branch of both the left anterior descending and circumflex arteries (because these are indications for CABG), or if the only non‐infarct stenosis was a chronic total occlusion (because it was felt that PCI in such circumstances was contraindicated owing to a low success rate). Diagnostic criteria MVD: presence of significant stenosis in ≥ 1 coronary artery other than the culprit vessel. Significant stenosis: stenosis ≥ 50%. Sample size: complete revascularisation n = 234 and culprit‐only revascularisation n = 231. |
|
| Interventions |
Complete revascularisation: intervention on all, culprit and non‐culprit arteries with stenosis of ≥ 50%. Culprit‐only revascularisation: PCI of culprit vessel only, except in people with refractory angina with objective evidence of ischaemia which may require staged intervention. |
|
| Outcomes |
Primary: composite of death from cardiac causes, non‐fatal MI, or refractory angina. Secondary: death from non‐cardiac causes and revascularisation procedure. |
|
| Notes | Study was stopped earlier because of a highly significant difference between groups, favouring the complete revascularisation group. The study was funded by Bart's and the London Trust (BLT) Charitable Foundation (UK). Protocol ID: ISRCTN73028481. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was computer‐generated. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned how allocation concealment was insured. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Study was open label for the participant. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Independent cardiologist and cardiac surgeon who were not notified about study‐group assignments examined specified primary and secondary outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Similar dropout rate in both groups, 4.3% in the complete vs 3.5% in the culprit‐only intervention group; however, given the small number of events, the results may be susceptible to attrition bias. |
| Selective reporting (reporting bias) | Low risk | Study reported the primary outcomes indicated in the published protocol in www.isrctn.com ISRCTN73028481. |
| Other bias | High risk | Early termination of the study may overestimate/underestimate certain differences. |
Zhang 2015.
| Methods | RCT. Randomisation ratio: 1:1. Number of study centres: not mentioned in the study. |
|
| Participants |
Inclusion criteria: people with STEMI, non‐culprit artery(ies) with significant stenosis, blood vessel > 2.5 mm and suitable for PCI. Exclusion criteria: cardiogenic shock, CABG, undetermined culprit vessel, person refused PCI, non‐culprit vessel occlusion is chronic, blood vessel diameter < 2.5 mm, lesions non‐suitable for PCI, non‐culprit vessel stenosis > 90%. Diagnostic criteria MVD: non‐culprit vessel significant stenosis. Significant stenosis: between 75% and 90%. Sample size: complete revascularisation n = 215 and culprit‐only revascularisation n = 213. |
|
| Interventions |
Complete revascularisation: PCI of the culprit vessel and staged intervention for the non‐culprit lesions between days 7 and 10 after the index procedure. Culprit‐only revascularisation: PCI of culprit vessel only and intervention on non‐culprit vessels was performed if participant had evidence of ischaemia (symptoms, ECG changes, or nuclear study consistent with ischaemia). |
|
| Outcomes | All‐cause mortality, MACE (MI and cardiac death), hospitalisation due to cardiac reasons (angina, heart failure, re‐hospitalisation for PCI), total hospitalisation time, stent number and hospital cost. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not mentioned in the article the method for randomisation. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned in the article the method for randomisation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned in the article. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned in the article. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not mentioned in the article. |
| Selective reporting (reporting bias) | High risk | Study did not have a published protocol and was not registered on any clinical trial databases. |
| Other bias | Unclear risk | Unclear source of funding. |
BMS: bare‐metal stent; CABG: coronary artery bypass graft; CCS: Canadian Cardiovascular Society; Cr: creatinine; DES: drug‐eluting stent; ECG: electrocardiogram; EF: ejection fraction; eGFR: estimated glomerular filtration rate; FFR: fractional flow reserve; LBBB: left bundle branch block; MACE: major adverse cardiovascular event; MI: myocardial infarction; MVD: multi‐vessel disease; n: number of participants; non‐IRA: non‐infarct related artery; PCI: percutaneous coronary intervention; P‐PCI: primary percutaneous coronary intervention; RCT: randomised controlled trial; STEMI: ST elevated myocardial infarction; TIMI: Thrombolysis in Myocardial Infarction.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| APEX AMI 2010 | Wrong study design. |
| Chen 2005 | Wrong study design. |
| Corpus 2004 | Wrong study design. |
| Hamza 2016 | Wrong outcomes. |
| Han 2008 | Wrong study design. |
| Hong 2001 | Wrong study design. |
| HORIZONS‐AMI 2011 | Wrong study design. |
| Ijsselmuiden 2004 | Wrong participant population. |
| Jin 2008 | Wrong study design. |
| Khattab 2008 | Wrong study design. |
| Liu 2015 | Wrong study design. |
| Maamoun 2011 | Wrong study design. |
| Ochala 2004 | Wrong comparator. |
| PRIMA trial 2013 | Wrong comparator. |
| Qarawani 2008 | Wrong study design. |
| Samson 1990 | Wrong study design. |
| Tajstra 2012 | Wrong study design. |
| Tapsiz 2014 | Wrong study design. |
| Tarasov 2013 | Wrong study design. |
| Valenti 2013 | Wrong study design. |
Characteristics of ongoing studies [ordered by study ID]
ASSIST‐CMR.
| Trial name or title | Revascularisation Strategies for STEMI; The CMR Endpoint Study. |
| Methods | Open‐label RCT. |
| Participants | People with acute STEMI and MVD as evidenced by ≥ 1 significant (≥ 70% by visual assessment or FFR < 0.80 for 50% to 70% stenosis) stenosis in non‐culprit artery(ies). |
| Interventions | 1 time primary PCI of the culprit and non‐culprit lesions vs intervention of the culprit vessel only. |
| Outcomes | Primary: infarct size by CMR. Secondary: MACE rate at 12 months. |
| Starting date | April 2014. |
| Contact information | Shahar Lavi shahar.lavi@lhsc.on.ca. |
| Notes |
COCUA.
| Trial name or title | Complete Lesion Versus Culprit Lesion Revascularisation (COCUA). |
| Methods | Not described. |
| Participants | People with acute STEMI and MVD. |
| Interventions | 1 time primary PCI of the culprit and non‐culprit lesions vs intervention of the culprit vessel only. |
| Outcomes | Not described. |
| Starting date | July 2011. |
| Contact information | Seung Woon Rha swrha617@yahoo.co.kr. |
| Notes | www.ClinicalTrial.gov NCT01180218. |
COMPARE ACUTE.
| Trial name or title | Comparison Between FFR Guided Revascularisation Versus Conventional Strategy in Acute STEMI Patients with MVD (CompareAcute). |
| Methods | Open‐label RCT. |
| Participants | People with acute STEMI and MVD. |
| Interventions | FFR‐guided revascularisation strategy vs culprit vessel only intervention. |
| Outcomes | Primary: composite endpoint of all‐cause mortality, non‐fatal myocardial infarction, any revascularisation, and cerebrovascular events (MACCE) at 12 months between groups. Secondary: composite endpoint of cardiac death, myocardial infarction, revascularisation, stroke and major bleeding, composite of hospitalisation for heart failure and unstable angina pectoris, all‐cause mortality, stent thrombosis, bleeding, treatment costs, and each component of the primary endpoint. |
| Starting date | May 2011. |
| Contact information | Steffen Helqvist. |
| Notes | www.ClinicalTrial.gov NCT01399736. |
COMPLETE.
| Trial name or title | Complete vs. Culprit‐only Revascularisation to Treat Multi‐vessel Disease After Primary PCI for STEMI (COMPLETE). |
| Methods | Open‐label RCT. |
| Participants | People with acute STEMI and MVD. |
| Interventions | Staged complete revascularisation strategy vs culprit vessel only intervention. |
| Outcomes | Primary: composite of cardiovascular death or new myocardial infarction. Secondary: composite of cardiovascular death, new myocardial infarction, ischaemia‐driven revascularisation, or hospitalisation for unstable angina or heart failure. |
| Starting date | December 2012. |
| Contact information | Shamir Mehta smehta@mcmaster.ca. |
| Notes | www.ClinicalTrial.gov NCT01740479. |
CROSS‐AMI.
| Trial name or title | Strategies of Revascularisation in Patients with ST‐segment Elevation Myocardial Infarction (STEMI) and Multivessel Disease. |
| Methods | Open‐label RCT. |
| Participants | People with acute STEMI and MVD. |
| Interventions | Staged complete revascularisation strategy vs culprit vessel only intervention and stress echocardiography and revascularisation if required. |
| Outcomes | Primary: combined event of cardiovascular death/re‐myocardial infarction/revascularisation of any vessel/admission due to heart failure. Secondary: incidence of acute renal failure (contrast‐induced nephropathy), cost analysis of both strategies, death, cardiovascular death, re‐myocardial infarction, revascularisation of any vessel, admission due to heart failure. |
| Starting date | September 2010. |
| Contact information | Rodrigo Estevez‐Loureiro, MD. |
| Notes |
FIT.
| Trial name or title | FIT (Fast Infarction Treatment): Complete Revascularisation During Primary Percutaneous Coronary Intervention (PCI) Can be Achieved Safely With an Improved Clinical Outcome During the Indexed Hospitalisation. |
| Methods | Double‐blind RCT. |
| Participants | People with acute STEMI and MVD. |
| Interventions | Complete revascularisation strategy vs culprit vessel only intervention. |
| Outcomes | Primary: death at 30 days, stent thrombosis, target vessel failure and re‐acute myocardial infarction. Secondary: bleeding, TIMI frame count and vascular site access complications. |
| Starting date | July 2010. |
| Contact information | Azienda Ospedaliera San Camillo Forlanini. |
| Notes |
CMR: cardiac magnetic resonance; FFR: fractional flow reserve; MACE: major adverse cardiovascular event; MVD: multi‐vessel disease; RCT: randomised controlled trial; STEMI: ST elevated myocardial infarction; TIMI: Thrombolysis in Myocardial Infarction.
Differences between protocol and review
We determined that a revision to the review title was needed based on alignment with current terminology, the protocol for this review was titled "Early invasive versus conservative strategy for non‐infarct related artery lesions in ST elevation myocardial infarction with multi‐vessel disease".
We have changed the order of the authors and added the following authors: Deepak L Bhatt, Asishana A Osho, Christian Gluud, Henning Kelbæk, Thomas Engstrøm, Dan Eik Høfsten); and removed Ronald E Pachon.
We did not apply any filters to the Conference Proceedings Index search because the relatively small retrieval set of the base search did not warrant applying a filter.
We did not search Current Controlled Trials MetaRegister (www.controlled‐trials.com/mrct/) as it is no longer active or available on the Internet.
We believe that the important outcome long‐term all‐cause mortality was omitted from the protocol and it was considered that this is a crucial outcome to judge the effectiveness of one intervention over the other. We judged this outcome as relevant or more relevant compared to cardiovascular mortality and adverse events, therefore we added this as another primary outcome. Moreover, all‐cause mortality is likely a less biased outcome. We have added all‐cause mortality as another outcome to be evaluate under the subgroup analysis section. All‐cause mortality is a crucial patient oriented outcome that is important to evaluate for potential subgroup differences.
In the protocol, we planned to include only participants who received revascularisation before discharge in the complete revascularisation group. In the review, we included participants who received revascularisation of the non‐culprit vessel (at least 50% obstruction but less than 100%) at the index procedure or at a second intervention including after discharge.
We removed in‐stent thrombosis and PCI‐related myocardial infarction measured at 30 days and one year after the intervention from the primary outcome adverse events because it overlapped with the outcome short‐ and long‐term myocardial infarction.
We considered the outcomes short‐term cardiovascular and all‐cause mortality, non‐fatal myocardial infarction, and revascularisation as those that occurred within the first 30 days after the index procedure.
We added the sensitivity analysis "Restricting the analysis to published trials that utilised mostly DES" because we thought it was reasonable to attribute heterogeneity or a certain effect size because of certain specific type of stent.
In addition to the cumulative meta‐analysis we conducted Trial Sequential Analysis of the ones that we judged to be the most relevant outcomes.
Contributions of authors
CB: protocol writing, trial selection, data extraction, data analysis, data interpretation, review writing, and future review updates.
SH: protocol writing, trial selection, data extraction, data analysis, data interpretation, review writing, and future review updates.
DB: data interpretation and review writing.
RK: data interpretation and review writing.
DF: data analysis, data interpretation, and review writing.
EO: data analysis, data interpretation, and review writing.
KA: data analysis, data interpretation, and review writing.
AS: data analysis, data interpretation, and review writing.
MS: data analysis, data interpretation, and review writing.
SZ: data analysis, data interpretation, and review writing.
AO: data analysis, data interpretation, and review writing.
CG: TSA expert, data analysis, data interpretation, and review writing.
HK: data interpretation and review writing.
TE: data interpretation and review writing.
DH: data interpretation and review writing.
JB: data analysis, data interpretation, and review writing.
The first and second review authors contributed equally to this review.
Sources of support
Internal sources
Self supported, Other.
External sources
Self supported, Other.
Declarations of interest
CB: none known.
SH: none known.
DB: Advisory Board: Cardax, Elsevier Practice Update Cardiology, Medscape Cardiology, Regado Biosciences; Board of Directors: Boston VA Research Institute, Society of Cardiovascular Patient Care; Chair: American Heart Association Quality Oversight Committee; Data Monitoring Committees: Duke Clinical Research Institute, Harvard Clinical Research Institute, Mayo Clinic, Population Health Research Institute (including for his role on the DSMB of COMPLETE); Honoraria: American College of Cardiology (Senior Associate Editor, Clinical Trials and News, ACC.org), Belvoir Publications (Editor in Chief, Harvard Heart Letter), Duke Clinical Research Institute (clinical trial steering committees), Harvard Clinical Research Institute (clinical trial steering committee), HMP Communications (Editor in Chief, Journal of Invasive Cardiology), Journal of the American College of Cardiology (Guest Editor; Associate Editor), Population Health Research Institute (clinical trial steering committee), Slack Publications (Chief Medical Editor, Cardiology Today’s Intervention), Society of Cardiovascular Patient Care (Secretary/Treasurer), WebMD (CME steering committees); Other: Clinical Cardiology (Deputy Editor), NCDR‐ACTION Registry Steering Committee (Chair), VA CART Research and Publications Committee (Chair); Research Funding: Amarin, Amgen, AstraZeneca, Bristol‐Myers Squibb, Eisai, Ethicon, Forest Laboratories, Ischemix, Lilly, Medtronic, Pfizer, Roche, Sanofi Aventis, The Medicines Company; Royalties: Elsevier (Editor, Cardiovascular Intervention: A Companion to Braunwald’s Heart Disease); Site Co‐Investigator: Biotronik, Boston Scientific, St. Jude Medical; Trustee: American College of Cardiology; Unfunded Research: FlowCo, PLx Pharma, Takeda.
RK: none known.
DF: has received compensation for travel expenses related to his membership on the board of the Alliance for a Healthier Generation and the American Heart Association. Dr Faxon has also received compensation for consulting as a member of a Data Safety Monitoring Board from Medtronic, Boston Scientific, and Biotronik. Dr Faxon has received stock options from RIVA Medical as well as honoraria from the American Heart Association for his service as an editor of Circulation. All compensation received is unrelated to this review.
EO: has received compensation for consulting from Abiomed, Astra Zeneca, Biotie, Boehringer Ingelheim, Bristol Meyers Squibb, Daiichi Sankyo, Eli Lilly & Company, Faculty Connection, Gilead Sciences, Ikaria, Ivivi, Janssen Pharmaceuticals, LipoScience, Merck, Pozen, Roche, Sanofi Aventis, Stealth Peptides, The Medicines Company, and Web MD. Dr Ohman has received institutional grants for clinical trials from Daiichi Sankyo, Eli Lilly & Company, Gilead Sciences, and Janssen Pharmaceuticals. Dr Ohman has received payment for lectures from Gilead Sciences, Janssen Pharmaceuticals, and LipoScience. All compensation received is unrelated to this review.
KA: none known.
AS: none known.
MS: none known.
SZ: has received compensation for lectures from GSK and Arbor Pharmaceuticals for topics unrelated to this review.
AO: none known.
CG: none known.
HK: none known.
TE: fees from Boston Scientific, St. Jude Medical, Astra Zeneca, and Bayer.
DH: none known.
JB: none known.
Edited (no change to conclusions)
References
References to studies included in this review
CvLPRIT 2015 {published data only}
- Gershlick AH, Khan JN, Kelly DJ, Greenwood JP, Sasikaran T, Curzen N, et al. Randomized trial of complete versus lesion‐only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. Journal of the American College of Cardiology 2015;65(10):963‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelion AD, Pakkal M, Chowdhury F, Niagara N, Birchall J, Dixon K, et al. Extent and prognostic significance of scar and inducible ischaemia following primary PCI for STEMI with multi vessel disease: Insights from the CvLPRIT Nuclear Sub study. European Heart Journal. 2015; Vol. 16:i36‐7.
- Kelly DJ, McCann GP, Blackman D, Curzen NP, Dalby M, Greenwood JP, et al. Complete Versus culprit‐Lesion only PRimary PCI Trial (CVLPRIT): a multicentre trial testing management strategies when multivessel disease is detected at the time of primary PCI: rationale and design. EuroIntervention 2013;8(10):1190‐8. [DOI] [PubMed] [Google Scholar]
- Khan JN, Greenwood JP, Nazir SA, Dalby M, Curzen N, Hetherington S. The complete versus lesion only primary PCI trial‐cardiovascular MRI substudy (CvLPRIT‐CMR). Journal of the American College of Cardiology 2015;65(10):A17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dambrink and Ghani 2010 {published data only}
- Dambrink JH, Debrauwere JP, van't Hof AWJ, Ottervanger JP, Gosselink ATM, Hoorntje JCA, et al. Non‐culprit lesions detected during primary PCI: treat invasively or follow the guidelines?. EuroIntervention 2010;5(8):968‐75. [PubMed] [Google Scholar]
- Ghani A, Dambrink JHE, van't Hof AWJ, Ottervanger JP, Gosselink ATM, Hoorntje JCA. Treatment of non‐culprit lesions detected during primary PCI: long‐term follow‐up of a randomised clinical trial. Netherlands Heart Journal 2012;20(9):347‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
DANAMI‐3‐PRIMULTI 2015 {published data only}
- Engstrøm T, Kelbæk H, Hedqvist S, Høfsten DE, Kløvgaard L, Holmvang L, et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST‐segment elevation myocardial infarction and multivessel disease (DANAMI‐3‐PRIMULTI): an open‐label, randomised controlled trial. Lancet 2015;386(9994):665‐71. [DOI] [PubMed] [Google Scholar]
- Høfsten DE, Kelbæk H, Hedqvist S, Kløvgaard L, Engstrøm T, Holmvang L, et al. The Third DANish Study of Optimal Acute Treatment of Patients with ST‐segment Elevation Myocardial Infarction: ischemic postconditioning or deferred stent implantation versus conventional primary angioplasty and complete revascularization versus treatment of culprit lesion only: Rationale and design of the DANAMI 3 trial program. American Heart Journal 2015;169(5):613‐21. [DOI] [PubMed] [Google Scholar]
- Sadjadieh G, Engstrøm T, Hedqvist S, Høfsten DE, Koeber L, Pedersen F. Bleeding episodes in "complete, staged" versus "culprit only" revascularization in patients with multivessel disease and ST‐segment elevation myocardial infarction ‐ a DANAMI‐3‐Primulti substudy. European Heart Journal 2015;36:681. [DOI] [PubMed] [Google Scholar]
- Sadjadieh G, Engstrøm T, Helqvist S, Høfsten DE, Køber L, Pedersen F, et al. Bleeding episodes in "complete, staged" versus "culprit only" revascularisation in patients with multivessel disease and ST‐segment elevation myocardial infarction: a DANAMI‐3‐PRIMULTI substudy. EuroIntervention 2016;12(10):1231‐8. [DOI] [PubMed] [Google Scholar]
Estevez Loureiro 2014 {unpublished data only}
- Estevez Loureiro R, Calvino‐Santos R, Peteiro J, Bouzas‐Mosquera A, Salgado‐Fernandez J, Soler‐Martin MR, et al. Preventive revascularization does not offer clinical advantage over a selective invasive strategy in patients with ST‐segment elevation myocardial infarction and multivessel disease. European Heart Journal 2014;35:477. [Google Scholar]
HELP AMI 2004 {published data only}
- Mario C, Mara S, Flavio A, Imad S, Antonio M, Anna P, et al. Single vs multivessel treatment during primary angioplasty: results of the multicentre randomized HEpacoat™ for cuLPrit or multivessel stenting for Acute Myocardial Infarction (HELP AMI) Study. International Journal of Cardiovascular Interventions 2004;6(3‐4):128‐33. [DOI] [PubMed] [Google Scholar]
Politi 2009 {published data only}
- Politi L, Rossi R, Sgura FA, Monopoli DE, Girolamo A, Guerri E, et al. Multivessel coronary disease in patients with ST‐elevation myocardial infarction undergoing primary angioplasty: different strategies of treatment and long‐term outcomes. Journal of the American College of Cardiology 2009;53(10):A397. [Google Scholar]
- Politi L, Sgura F, Rossi R, Monopoli D, Guerri E, Lezzi C, et al. A randomised trial of target‐vessel versus multi‐vessel revascularisation in ST‐elevation myocardial infarction: major adverse cardiac events during long‐term follow‐up. Heart (British Cardiac Society) 2010;96(9):662‐7. [DOI] [PubMed] [Google Scholar]
- Politi L, Sgura F, Rossi R, Monopoli D, Guerri E, Lezzi C, et al. A randomised trial of target‐vessel versus multi‐vessel revascularisation in ST‐elevation myocardial infarction: major adverse cardiac events during long‐term follow‐up. [Erratum]. Heart (British Cardiac Society) 2014;100(5):350. [DOI] [PubMed] [Google Scholar]
PRAGUE‐13 2015 {unpublished data only}
- Hlinomaz O, Grouch L, Polokova L, Lehar F, Vekov T, Griva M, et al. Multivessel coronary disease diagnosed at the time of primary PCI for STEMI: complete revascularisation versus conservative strategy. European Heart Journal 2015;36:825. [Google Scholar]
- Hlinomaz O, Grouch L, Polokova L, Lehar F, Vekov T, Griva M, et al. Multivessel coronary disease diagnosed at the time of primary PCI for STEMI: complete revascularisation versus conservative strategy. Prague‐13 trial. Kardiologicka Revue 2015;17(3):214‐220. [Google Scholar]
PRAMI 2013 {published data only}
- Mansion K, Carrick D, Payne AR, McClure J, Mason M, Petrie M, et al. Infarct burden following multivessel PCI vs. infarct‐only PCI in patients with acute STEMI: the Glasgow PRAMI CMR sub‐study. Journal of Cardiovascular Magnetic Resonance 2015;17:9. [Google Scholar]
- Mansion K, Carrick D, Payne AR, McClure J, Mason M, Petrie M, et al. Left ventricular outcomes following multivessel PCI versus infarct‐only PCI in patients with acute STEMI: the Glasgow PRAMI CMR sub‐study. Journal of the American College of Cardiology 2015;65(10):A1937. [Google Scholar]
- Mansion K, Carrick D, Payne AR, McClure J, Mason M, Petrie M, et al. Left ventricular outcomes following multivessel PCI vs. infarct artery‐only PCI in patients with acute STEMI: the Glasgow PRAMI CMR sub‐study. Journal of Cardiovascular Magnetic Resonance 2015;17:P104. [Google Scholar]
- Mansion K, Carrick D, Payne AR, McClure J, Mason M, Petrie M, et al. Left ventricular outcomes following multivessel PCI vs. infarct‐only PCI in patients with acute STEMI: the Glasgow PRAMI CMR sub‐study. Heart (British Cardiac Society) 2015;101:A62. [Google Scholar]
- Wald DS, Morris JK, Wald NJ, Chase AJ, Edwards RJ, Hughes LO. Randomized trial of preventive angioplasty in myocardial infarction. New England Journal of Medicine 2013;369(12):1115‐23. [DOI] [PubMed] [Google Scholar]
Zhang 2015 {published data only}
- Zhang J, Wang Q, Yang H, Ma L, Fu X, Hou W, et al. Evaluation of different revascularization strategies for patients with acute myocardial infarction with lesions of multiple coronary arteries after primary percutaneous coronary intervention and its economic evaluation. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2015;27:169‐74. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
APEX AMI 2010 {published data only}
- Toma M, Buller CE, Westerhout CM, Fu Y, O'Neill WW, Holmes DR Jr. Non‐culprit coronary artery percutaneous coronary intervention during acute ST‐segment elevation myocardial infarction: insights from the APEX‐AMI trial. European Heart Journal 2010;31(14):1701‐7. [DOI] [PubMed] [Google Scholar]
Chen 2005 {published data only}
- Chen LY, Lennon RJ, Grantham JA, Berger PB, Mathew V, Singh M, et al. In‐hospital and long‐term outcomes of multivessel percutaneous coronary revascularization after acute myocardial infarction. American Journal of Cardiology 2005;95(3):349‐54. [DOI] [PubMed] [Google Scholar]
Corpus 2004 {published data only}
- Corpus RA, House JA, Marso SP, Grantham JA, Huber KC Jr, Laster SB, et al. Multivessel percutaneous coronary intervention in patients with multivessel disease and acute myocardial infarction. American Heart Journal 2004;148(3):493‐500. [DOI] [PubMed] [Google Scholar]
Hamza 2016 {published and unpublished data}
- Hamza M, Elgendy I. Erratum to: a randomized trial of complete versus culprit‐only revascularization during primary percutaneous coronary intervention in diabetic patients with acute ST elevation myocardial infarction and multi vessel disease. Journal of Interventional Cardiology 2016;29(4):441. [DOI] [PubMed] [Google Scholar]
- Hamza M, Elgendy I. TCT‐139 A randomized trial of complete versus culprit‐only revascularization during primary percutaneous coronary intervention in diabetic patients with acute ST elevation myocardial infarction and multi vessel disease. Journal of the American College of Cardiology 2016;68(18S):B56‐7. [DOI] [PubMed] [Google Scholar]
- Hamza M, Elgendy IY. A randomized trial of complete versus culprit‐only revascularization during primary percutaneous coronary intervention in diabetic patients with acute ST elevation myocardial infarction and multi vessel disease. Journal of Interventional Cardiology 2016;29(3):241‐7. [DOI] [PubMed] [Google Scholar]
Han 2008 {published data only}
- Han YL, Wang B, Wang XZ, Li Y, Wang SL, Jing QM, et al. Comparative effects of percutaneous coronary intervention for infarct‐related artery only or for both infarct‐ and non‐infarct‐related arteries in patients with ST‐elevation myocardial infarction and multi‐vessel disease. Chinese Medical Journal 2008;121(23):2384‐7. [PubMed] [Google Scholar]
Hong 2001 {published data only}
- Hong MK, Park SW, Lee CW, Rhee KS, Song JM, Kang DH, et al. Six‐month angiographic follow‐up after intravascular ultrasound‐guided stenting of infarct‐related artery: comparison with non‐infarct‐related artery. American Heart Journal 2001;141(5):832‐6. [DOI] [PubMed] [Google Scholar]
HORIZONS‐AMI 2011 {published data only}
- Claessen BEPM, Dangas GD, Weisz G, Witzenbichler B, Guagliumi G, Mockel M, et al. Prognostic impact of a chronic total occlusion in a non‐infarct related artery in patients with ST‐elevation myocardial infarction: three‐year results from the HORIZONS‐AMI trial. Journal of the American College of Cardiology 2011;58(20):B75. [Google Scholar]
- Claessen BEPM, Dangas GD, Weisz G, Witzenbichler B, Guagliumi G, Mockel M, et al. Prognostic impact of a chronic total occlusion in a non‐infarct‐related artery in patients with ST‐segment elevation myocardial infarction: 3‐year results from the HORIZONS‐AMI trial. European Heart Journal 2012;33(6):768‐75. [DOI] [PubMed] [Google Scholar]
- Claessen BEPM, Weisz G, Dangas GD, Colombo A, Park SJ, Moses JW, et al. Impact of TIMI 0/1 flow in non‐infarct related arteries in patients with ST‐elevation myocardial infarction on myocardial blush and long‐term mortality: three‐year results from the HORIZONS‐AMI trial. European Heart Journal. 2011; Vol. 32:413.
- Kornowski R, Gersh BJ, Dangas GD, Wong SC, Witzenbichler B, Guagliumi G, et al. Prognostic impact of staged vs. "one‐time" intervention for multivessel disease during primary PCI in STEMI: Insights from the HORIZONS‐AMI trial. American Journal of Cardiology 2009;104(6):8D. [Google Scholar]
- Kornowski R, Mehran R, Dangas G, Assali A, Nikolsky E, Gersh BJ. Prognostic impact of staged versus "one‐time" multivessel PCI in acute ST‐segment elevation myocardial infarction: long‐term analysis from the HORIZONS‐AMI trial. Journal of the American College of Cardiology 2011;57(14):E1653. [DOI] [PubMed] [Google Scholar]
- Kornowski R, Mehran R, Dangas G, Nikolsky E, Gersh BJ, Assali A, et al. Prognostic impact of staged versus "one‐time" multivessel percutaneous intervention in acute myocardial infarction: Analysis from the HORIZONS‐AMI (Harmonizing Outcomes with Revascularization and Stents in Acute Myocardial Infarction) trial. Journal of the American College of Cardiology 2011;58(7):704‐11. [DOI] [PubMed] [Google Scholar]
Ijsselmuiden 2004 {published data only}
- Ijsselmuiden AJ, Ezechiels J, Westendorp IC, Tijssen JG, Kiemeneij F, Slagboom T. Complete versus culprit vessel percutaneous coronary intervention in multivessel disease: a randomized comparison. American Heart Journal 2004;148(3):467‐74. [DOI] [PubMed] [Google Scholar]
Jin 2008 {published data only}
- Jin Z, Rha SW, Chen KY, Minami Y, Na JO, Suh SY, et al. Culprit‐lesion Revascularization versus complete revascularization in patients with acute myocardial infarction undergoing primary percutaneous coronary intervention with drug‐eluting stents. American Journal of Cardiology 2008;101(8B):23C. [Google Scholar]
Khattab 2008 {published data only}
- Khattab AA, Abdel‐Wahab M, Rother C, Lisa B, Toelg R, Kassen G, et al. Multi‐vessel stenting during primary percutaneous coronary intervention for acute myocardial infarction. A single‐center experience. Clinical Research in Cardiology 2008;97(1):32‐8. [DOI] [PubMed] [Google Scholar]
Liu 2015 {published data only}
- Liu W. Earlier complete revascularization improves the long term outcome of primary coronary intervention for patients with ST elevation myocardial infarction and multi‐vessel disease. Journal of the American College of Cardiology 2015;66(15):B106‐7. [Google Scholar]
Maamoun 2011 {published data only}
- Maamoun W, Elkhart N, Elarasy R. Safety and feasibility of complete simultaneous revascularization during primary PCI in patients with STEMI and multi‐vessel disease. Egyptian Heart Journal 2011;63(1):39‐43. [Google Scholar]
Ochala 2004 {published data only}
- Ochala A, Smolka GA, Wojakowski W, Dudek D, Dziewierz A, Krolikowski Z. The function of the left ventricle after complete multivessel one‐stage percutaneous coronary intervention in patients with acute myocardial infarction. Journal of Invasive Cardiology 2004;16(12):699‐702. [PubMed] [Google Scholar]
PRIMA trial 2013 {published data only}
- Ochala A, Smolka G, Wojakowski W, Krol M, Skowerski M, Gasior Z, et al. Multivessel, 1‐stage percutaneous coronary intervention in patients with acute myocardial infarction ‐ PRIMA trial: safety, efficacy, and costs in 12‐month follow‐up. American Journal of Cardiology 2013;92(6A):3L‐3L. [Google Scholar]
Qarawani 2008 {published data only}
- Qarawani D, Nahir M, Abboud M, Hasanov Y, Hasin Y. Culprit only versus complete coronary revascularization during primary PCI. International Journal of Cardiology 2008;123(3):288‐92. [DOI] [PubMed] [Google Scholar]
Samson 1990 {published data only}
- Samson M, Meester HJ, Feyter PJ, Strauss B, Serruys PW. Successful multiple segment coronary angioplasty: effect of completeness of revascularization in single‐vessel multilesions and multivessels. American Heart Journal 1990;120(1):1‐12. [DOI] [PubMed] [Google Scholar]
Tajstra 2012 {published data only}
- Tajstra M, Gasior M, Gierlotka M, Pres D, Hawranek M, Trzeciak P, et al. Comparison of five‐year outcomes of patients with and without chronic total occlusion of noninfarct coronary artery after primary coronary intervention for ST‐segment elevation acute myocardial infarction. American Journal of Cardiology 2012;109(2):208‐13. [DOI] [PubMed] [Google Scholar]
Tapsiz 2014 {published data only}
- Tapsiz M, Seker T, Ucar H, Sahin DY, Sen O, Baykan OA, et al. The optimal timing of second intervention to non‐infarct related critical lesions in STEMI patients with multi‐vessel disease undergoing primary percutaneous coronary intervention. Journal of the American College of Cardiology 2014;64(11):B17. [Google Scholar]
Tarasov 2013 {published data only}
- Tarasov RS, Ganiukov VI, Popov VA, Shushpannikov PA, Barbarash OL, Barbarash LS. Effect of the terms of complete revascularization on the outcomes of treatment of patients with ST segment elevation myocardial infarction and multivessel coronary artery disease. Angiologiia i Sosudistaia Khirurgiia = Angiology and Vascular Surgery 2013;19(4):14‐20. [PubMed] [Google Scholar]
- Tarasov RS, Ganiukov VI, Shushpannikov PA, Barbarash OL, Barbarash LS. Optimal timing of the second stage of revascularization in the treatment of patients with ST‐elevation myocardial infarction and multivascular involvement. Kardiologiia 2013;53(7):9‐12. [PubMed] [Google Scholar]
Valenti 2013 {published data only}
- Valenti R, Marrani M, Cantini G, Comito V, Migliorini A, Carrabba N, et al. Prognostic impact of non‐infarct‐related‐artery chronic total occlusion revascularization in patients with acute myocardial infarction treated by primary angioplasty. Journal of the American College of Cardiology 2013;62(18):B114. [Google Scholar]
References to ongoing studies
ASSIST‐CMR {unpublished data only}
- Revascularisation Strategies for STEMI; The CMR Endpoint Study.. Ongoing study April 2014..
COCUA {unpublished data only}
- Complete Lesion Versus Culprit Lesion Revascularisation (COCUA).. Ongoing study July 2011..
COMPARE ACUTE {published data only}
- Smits P, Lunde K, Omerovic E, Schotborgh C, Richardt G, Abdel‐Wahab M, et al. FFR guidance during primary PCI in multivessel STEMI patients: insights from the ongoing COMPARE‐ACUTE trial. EuroIntervention. 2015.
- Smits PC, Vlachojannis GJ, Lunde K, Omerovic E, Schotborgh CE, Richardt G, et al. FFR‐guided complete revascularization during primary PCI: preliminary data from the COMPARE ACUTE trial. Journal of the American College of Cardiology 2014;64(11):B95. [Google Scholar]
COMPLETE {unpublished data only}
- Complete vs. Culprit‐only Revascularisation to Treat Multi‐vessel Disease After Primary PCI for STEMI (COMPLETE).. Ongoing study December 2012..
CROSS‐AMI {unpublished data only}
- Strategies of Revascularisation in Patients with ST‐segment Elevation Myocardial Infarction (STEMI) and Multivessel Disease.. Ongoing study September 2010..
FIT {unpublished data only}
- FIT (Fast Infarction Treatment): Complete Revascularisation During Primary Percutaneous Coronary Intervention (PCI) Can be Achieved Safely With an Improved Clinical Outcome During the Indexed Hospitalisation.. Ongoing study July 2010..
Additional references
ACC/AHA/SCAI 2015
- Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2015 ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST‐elevation myocardial infarction: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention and the 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. Circulation 2016;133(11):1135‐47. [DOI] [PubMed] [Google Scholar]
ACCF/AHA 2013
- O'Gara PT, Kushner FG, Ascheim DD, Casey DE Jr, Chung MK, Lemos JA, et al. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology 2013;61:485‐510. [DOI] [PubMed] [Google Scholar]
Arroyo‐Espliguero 2004
- Arroyo‐Espliguero R, Avanzas P, Cosin‐Sales J, Aldama G, Pizzi C, Kaski JC. C‐reactive protein elevation and disease activity in patients with coronary artery disease. European Heart Journal 2004;25:401‐8. [DOI] [PubMed] [Google Scholar]
Bagai 2013
- Bagai A, Thavendiranathan P, Sharif W, Al Lawati HA, Cheema AN. Non‐infarct‐related artery revascularization during primary percutaneous coronary intervention for ST‐segment elevation myocardial infarction: a systematic review and meta‐analysis. American Heart Journal 2013;166(4):684‐93 e1. [DOI] [PubMed] [Google Scholar]
Bainey 2014
- Bainey KR, Mehta SR, Lai T, Welsh RC. Complete vs culprit‐only revascularization for patients with multivessel disease undergoing primary percutaneous coronary intervention for ST‐segment elevation myocardial infarction: a systematic review and meta‐analysis. American Heart Journal 2014;167(1):1‐14.e2. [DOI] [PubMed] [Google Scholar]
Bangalore 2015
- Bangalore S, Toklu B, Wetterslev J. Complete versus culprit‐only revascularization for ST‐segment‐elevation myocardial infarction and multivessel disease: a meta‐analysis and Trial Sequential Analysis of randomized trials. Circulation. Cardiovascular Interventions 2015;8(4):e002142. [DOI] [PubMed] [Google Scholar]
Bhatt 2015
- Bhatt DL. Do we really know the CvLPRIT in myocardial infarction? or just stent all lesions?. Journal of the American College of Cardiology 2015;65(10):973‐5. [DOI] [PubMed] [Google Scholar]
Dziewierz 2010
- Dziewierz A, Siudak Z, Rakowski T, Zasada W, Dubiel JS, Dudek D. Impact of multivessel coronary artery disease and noninfarct‐related artery revascularization on outcome of patients with ST‐elevation myocardial infarction transferred for primary percutaneous coronary intervention (from the EUROTRANSFER Registry). American Journal of Cardiology 2010;106:342‐7. [DOI] [PubMed] [Google Scholar]
Elgendy 2015
- Elgendy IY, Huo T, Mahmoud A, Bavry AA. Complete versus culprit‐only revascularization in patients with multi‐vessel disease undergoing primary percutaneous coronary intervention: a meta‐analysis of randomized trials. International Journal of Cardiology 2015;186:98‐103. [DOI] [PubMed] [Google Scholar]
ESC 2012
- Steg PG, James SK, Atar D, Badano LP, Blomstrom‐Lundqvist C, Borger MA, et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation. European Heart Journal 2012;33(20):2569‐619. [DOI] [PubMed] [Google Scholar]
ESC 2014
- Authors/Task Force members, Windecker S, Kolh P, Alfonso F, Collet JP, Cremer J, et al. 2014 ESC/EACTS guidelines on myocardial revascularization: the Task Force on Myocardial Revascularization of the European Society of Cardiology (ESC) and the European Association for Cardio‐Thoracic Surgery (EACTS) developed with the special contribution of the European Association of Percutaneous Cardiovascular Interventions (EAPCI). European Heart Journal 2014;35(37):2541‐619. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version 3.6. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Halkin 2005
- Halkin A, Singh M, Nikolsky E, Grines CL, Tcheng JE, Garcia E, et al. Prediction of mortality after primary percutaneous coronary intervention for acute myocardial infarction: the CADILLAC risk score. Journal of the American College of Cardiology 2005;45(9):1397‐405. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jakobsen 2014
- Jakobsen JC, Wetterslev J, Winkel P, Lange T, Gluud C. Thresholds for statistical and clinical significance in systematic reviews with meta‐analytic methods. BMC Medical Research Methodology 2014;14:120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jaski 1992
- Jaski BE, Cohen JD, Trausch J, Marsh DG, Bail GR, Overlie PA, et al. Outcome of urgent percutaneous transluminal coronary angioplasty in acute myocardial infarction: comparison of single‐vessel versus multivessel coronary artery disease. American Heart Journal 1992;124(6):1427‐33. [DOI] [PubMed] [Google Scholar]
Jo 2011
- Jo HS, Park JS, Sohn JW, Yoon JC, Sohn CW, Lee SH, et al. Culprit‐lesion‐only versus multivessel revascularization using drug‐eluting stents in patients with ST‐segment elevation myocardial infarction: a Korean acute myocardial infarction registry‐based analysis. Korean Circulation Journal 2011;41:718‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kubo 2010
- Kubo T, Imanishi T, Kashiwagi M, Ikejima H, Tsujioka H, Kuroi A, et al. Multiple coronary lesion instability in patients with acute myocardial infarction as determined by optical coherence tomography. American Journal of Cardiology 2010;105:318‐22. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Muller 1991
- Muller DW, Topol EJ, Ellis SG, Sigmon KN, Lee K, Califf RM. Multivessel coronary artery disease: a key predictor of short‐term prognosis after reperfusion therapy for acute myocardial infarction. Thrombolysis and Angioplasty in Myocardial Infarction (TAMI) Study Group. American Heart Journal 1991;121(4 Pt 1):1042‐9. [DOI] [PubMed] [Google Scholar]
NICE 2013
- National Institute for Health and Care Excellence. Myocardial infarction with ST‐segment elevation: the acute management of myocardial infarction with ST‐segment elevation, 2013. www.nice.org.uk/guidance/cg167 (accessed 26 November 2015).
Pandit 2014
- Pandit A, Aryal MR, Aryal Pandit A, Hakim FA, Giri S, Mainali NR. Preventive PCI versus culprit lesion stenting during primary PCI in acute STEMI: a systematic review and meta‐analysis. BMJ (Clinical Research Ed.) 2014;1(1):e000012. [DOI] [PMC free article] [PubMed] [Google Scholar]
Park 2014
- Park DW, Clare RM, Schulte PJ, Pieper KS, Shaw LK, Califf RM, et al. Extent, location, and clinical significance of non‐infarct‐related coronary artery disease among patients with ST‐elevation myocardial infarction. JAMA 2014;312(19):2019‐27. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riley 2011
- Riley RD, Higgins JP, Deeks JJ. Interpretation of random effects meta‐analyses. BMJ 2011;342:d549. [DOI] [PubMed] [Google Scholar]
Sardar 2015
- Sardar P, Chatterjee S, Giri J, Alfonso F, Helmy T, Ledley GS, et al. Intervention strategies for multi‐vessel disease in patients with ST‐segment elevation myocardial infarction: a meta‐analysis of randomized trials. International Journal of Cardiology 2015;179:225‐7. [DOI] [PubMed] [Google Scholar]
Sorajja 2007
- Sorajja P, Gersh BJ, Cox DA, McLaughlin MG, Zimetbaum P, Costantini C, et al. Impact of multivessel disease on reperfusion success and clinical outcomes in patients undergoing primary percutaneous coronary intervention for acute myocardial infarction. European Heart Journal 2007;28(14):1709‐16. [DOI] [PubMed] [Google Scholar]
Thorlund 2011 [Computer program]
- Thorlund K, Engstrøm J, Wetterslev J, Brok J, Imberger G, Gluud C. User manual for Trial Sequential Analysis (TSA). Copenhagen, Denmark: Copenhagen Trial Unit, Centre for Clinical Intervention Research, 2011.
Vlaar 2011
- Vlaar PJ, Mahmoud KD, Holmes DR Jr, Valkenhoef G, Hillege HL, Horst IC, et al. Culprit vessel only versus multivessel and staged percutaneous coronary intervention for multivessel disease in patients presenting with ST‐segment elevation myocardial infarction: a pairwise and network meta‐analysis. Journal of the American College of Cardiology 2011;58(7):692‐703. [DOI] [PubMed] [Google Scholar]
Wetterslev 2008
- Wetterslev J, Thorlund K, Brok J, Gluud C. Trial sequential analysis may establish when firm evidence is reached in cumulative meta‐analysis. Journal of Clinical Epidemiology 2008;61:64‐75. [DOI] [PubMed] [Google Scholar]
WHO 2005
- World Health Organization. Preventing chronic diseases: a vital investment. WHO global report, 2005. www.who.int/chp/chronic_disease_report/full_report.pdf (accessed 26 November 2015).
WHO 2014
- World Health Organization. Global status report on noncommunicable diseases 2014. http://apps.who.int/iris/bitstream/10665/148114/1/9789241564854_eng.pdf?ua=1 (accessed 28 February 2017).
Wong 2006
- Wong SS, Wilczynski NL, Haynes RB. Developing optimal search strategies for detecting clinically sound treatment studies in EMBASE. Journal of the Medical Library Association 2006;94(1):41‐7. [PMC free article] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Hirji 2015
- Hirji SA, Bravo CA, Pachon RE, Faxon DP, Ohman EM, Anderson KL, et al. Early invasive versus conservative strategy for non‐infarct related artery lesions in ST elevation myocardial infarction with multi‐vessel disease. Cochrane Database of Systematic Reviews 2015, Issue 12. [DOI: 10.1002/14651858.CD011986] [DOI] [Google Scholar]


