Abstract
Background
The pattern of infections among neutropenic patients with cancer has shifted in the last decades to a predominance of gram‐positive infections. Some of these gram‐positive bacteria are increasingly resistant to beta‐lactams and necessitate specific antibiotic treatment.
Objectives
To assess the effectiveness of empirical anti‐gram‐positive (antiGP) antibiotic treatment for febrile neutropenic patients with cancer in terms of mortality and treatment failure. To assess the rate of resistance development, further infections and adverse events associated with additional antiGP treatment.
Search methods
For the review update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (2017, Issue 2), MEDLINE (May 2012 to 2017), Embase (May 2012 to 2017), LILACS (2012 to 2017), conference proceedings, ClinicalTrials.gov trial registry, and the references of the included studies. We contacted the first authors of all included and potentially relevant trials.
Selection criteria
Randomised controlled trials (RCTs) comparing one antibiotic regimen versus the same regimen with the addition of an antiGP antibiotic for the treatment of febrile neutropenic patients with cancer.
Data collection and analysis
Two review authors independently assessed trial eligibility and risk of bias, and extracted all data. Risk ratios (RR) with 95% confidence intervals (CIs) were calculated. A random‐effects model was used for all comparisons showing substantial heterogeneity (I2 > 50%). Outcomes were extracted by intention‐to‐treat and the analysis was patient‐based whenever possible.
Main results
Fourteen trials and 2782 patients or episodes were included. Empirical antiGP antibiotics were tested at the onset of treatment in 12 studies, and for persistent fever in two studies. The antiGP treatment was a glycopeptide in nine trials. Eight studies were assessed in the overall mortality comparison and no significant difference was seen between the comparator arms, RR of 0.90 (95% CI 0.64 to 1.25; 8 studies, 1242 patients; moderate‐quality data). Eleven trials assessed failure, including modifications as failures, while seven assessed overall failure disregarding treatment modifications. Failure with modifications was reduced, RR of 0.72 (95% CI 0.65 to 0.79; 11 studies, 2169 patients; very low‐quality data), while overall failure was the same, RR of 1.00 (95% CI 0.79 to 1.27; 7 studies, 943 patients; low‐quality data). Sensitivity analysis for allocation concealment and incomplete outcome data did not change the results. Failure among patients with gram‐positive infections was reduced with antiGP treatment, RR of 0.56 (95% CI 0.38 to 0.84, 5 studies, 175 patients), although, mortality among these patients was not changed.
Data regarding other patient subgroups likely to benefit from antiGP treatment were not available. Glycopeptides did not increase fungal superinfection rates and were associated with a reduction in documented gram‐positive superinfections. Resistant colonisation was not documented in the studies.
Authors' conclusions
Based on very low‐ or low‐quality evidence using the GRADE approach and overall low risk of bias, the current evidence shows that the empirical routine addition of antiGP treatment, namely glycopeptides, does not improve the outcomes of febrile neutropenic patients with cancer.
Keywords: Humans, Anti‐Bacterial Agents, Anti‐Bacterial Agents/adverse effects, Anti‐Bacterial Agents/therapeutic use, Febrile Neutropenia, Febrile Neutropenia/drug therapy, Febrile Neutropenia/mortality, Glycopeptides, Glycopeptides/adverse effects, Glycopeptides/therapeutic use, Gram‐Positive Bacterial Infections, Gram‐Positive Bacterial Infections/drug therapy, Gram‐Positive Bacterial Infections/mortality, Neoplasms, Neoplasms/complications, Randomized Controlled Trials as Topic, Treatment Failure
Plain language summary
Spectrum of the initial antibiotic treatment for cancer patients with fever and low leucocytes counts
Background: cancer patients develop neutropenia, a decrease in the subset of leucocytes responsible for protection against bacteria, as a result of chemotherapy or cancer. Neutropenia predisposes the patients to severe bacterial infections. Standard antibiotic regimens for cancer patients with neutropenia and fever are directed at most of the bacteria that can cause infections. However, a subset of resistant bacteria belonging to the gram‐positive group (Staphylococcus aureus and Streptococci) remain untreated unless specific antibiotics are added to the treatment.
Review question: we assessed whether the addition of specific anti gram‐positive antibiotics prior to identification of a causative bacteria improves survival and cure among cancer patients with fever and neutropenia.
Search dates: the evidence is current to February 2017.
Study characteristics: we included randomised controlled trials that compared a standard antibiotic regimen versus the same regimen with an antibiotic directed at gram‐positive bacteria. Overall, 14 randomised controlled trials were included with 2782 patients or episodes of infection. The antibiotics were given to cancer patients with neutropenia and fever as first‐line treatment (12 trials) or for recurrent fever (two trials).
Study funding sources: In 9/14 of the trials the trial received funding from the industry.
Key results: mortality did not differ between patients groups. Antibiotic treatment was more frequently modified among patients who did not initially receive specific antibiotics against gram‐positive bacteria, but overall treatment failures were not different. We attempted to examine the durations of fever and hospital stay, but these were not consistently reported. The addition of specific antibiotics against gram‐positive bacteria resulted in more adverse events, mainly rash. We conclude that antibiotic treatment directed against resistant gram‐positive bacteria can await identification of specific bacteria and need not be given routinely prior to bacterial identification.
Quality of the evidence: overall, the quality of the evidence was low to very low but was based on randomised controlled trials, most of which were at low risk of bias. A limitation of the results for mortality was that all‐cause mortality was not reported and could not be obtained in 6/14 of the studies. The trials did not examine specific circumstances that might mandate empirical use of antibiotics against gram‐positive bacteria and thus the evidence is relevant to cancer patients with fever, without low blood pressure, or a focus of infection that might be caused by gram‐positive bacteria.
Summary of findings
Summary of findings for the main comparison. Mortality with anti‐gram‐positive antibiotics compared to placebo for the treatment of febrile neutropenic patients with cancer.
| Mortality with anti‐gram‐positive antibiotics compared to placebo for the treatment of febrile neutropenic patients with cancer | ||||||
| Patient or population: febrile neutropenic patients with cancer Setting: in‐hospital Intervention: anti‐gram‐positive antibiotics Comparison: placebo or added anti‐gram‐positive antibiotics | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with anti gram‐positive antibiotics | |||||
| Overall mortality | Study population | RR 0.90 (0.64 to 1.25) | 1242 (8 RCTs) | ⊕⊕⊕⊝ MODERATE 1 2 | ||
| 104 per 1,000 | 94 per 1,000 (67 to 130) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Lack of blinding should not affect the objective outcome of mortality
2 Wide CI ranging from a large benefit of anti‐gram‐positive antibiotics to possible harm
Summary of findings 2. Treatment failure with anti‐gram‐positive antibiotics compared to placebo for the treatment of febrile neutropenic patients with cancer.
| Treatment failure with anti gram‐positive antibiotics compared to placebo for the treatment of febrile neutropenic patients with cancer | ||||||
| Patient or population: febrile neutropenic patients with cancer Setting: in‐hospital Intervention: anti‐gram‐positive antibiotics Comparison: placebo or added anti‐gram‐positive antibiotics | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with treatment failure | |||||
| Overall failure (disregarding modifications) | Study population | RR 1.00 (0.79 to 1.27) | 943 (7 RCTs) | ⊕⊕⊝⊝ LOW 1 | ||
| 187 per 1,000 | 187 per 1,000 (148 to 238) | |||||
| Failure, modifications included | Study population | RR 0.72 (0.65 to 0.79) | 2169 (11 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 | ||
| 463 per 1,000 | 333 per 1,000 (301 to 366) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Lack of blinding in most studies, subjective outcome
2 Indirectness: outcome driven by treatment modifications, an outcome not relevant to this patient population
Summary of findings 3. Adverse events with anti‐gram‐positive antibiotics compared to placebo for the treatment of febrile neutropenic patients with cancer.
| Adverse events with anti gram‐positive antibiotics compared to placebo for the treatment of febrile neutropenic patients with cancer | ||||||
| Patient or population: febrile neutropenic patients with cancer Setting: in‐hospital Intervention: anti‐gram‐positive antibiotics Comparison: placebo or added anti‐gram‐positive antibiotics | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with Adverse events | |||||
| Any adverse events | Study population | RR 1.74 (1.50 to 2.01) | 1936 (9 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 | ||
| 192 per 1,000 | 335 per 1,000 (289 to 387) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Lack of blinding
2 Adverse events were not described in all studies and are interpreted differently in each study
Background
Advances in therapy for cancer patients are associated with an increased risk of infection. Newer chemotherapeutic regimens, indwelling intravenous catheters, and bone marrow transplantation for both haematological and solid tumour cancer patients constitute major risk factors for infection. These cause bone marrow suppression with resulting neutropenia and damage to the physiological barriers of infection such as skin and mucous membranes. Infections are the most common cause of death among cancer patients and they are a common rate‐limiting factor for continuing cancer therapy (Nesher 2014).
Gram‐negative bacteria were the most common cause for bacteriologically‐documented infections when empirical treatment for neutropenic cancer patients was proposed. Eventually, gram‐positive bacteria have replaced the gram‐negative bacteria as the most commonly documented infection. These include mainly Staphylococci, Streptococcus species, Enterococci and Corynebacterium species. Between 1973 to 1994, the European Organisation for Research and Treatment of Cancer International Antimicrobial Therapy Cooperative Group (EORTC‐IATCG) has conducted several multi‐centre randomised controlled trials (RCTs) of empirical therapy in cancer patients with fever and neutropenia (EORTC 1978; EORTC 1983; EORTC 1986; EORTC 1987; EORTC 1991; EORTC 1993; EORTC 1995; EORTC 1996). In these trials, the frequency of gram‐positive isolates increased steadily from 29% to 69% of single‐organism bacteraemias, while the rate of single‐agent gram‐negative bacteraemias dropped from 71% to 31%. In addition, the overall mortality associated with treated infection has decreased from around 25% to 6% in trials conducted during recent years (Del Favero 2001; EORTC 1996; Gurwith 1978). Of late, epidemiology might be reverting to a predominance of gram‐negative bacteria, at least in some locations (Montassier 2013; Nesher 2014; Yan 2016; Yapici 2016.
Several explanations may underlie the changes in the epidemiology of febrile neutropenia. The increase in infections due to gram‐positive bacteria is probably due mainly to the widespread use of centrally placed venous catheters, which have the propensity to be colonised by gram‐positive bacteria (Press 1984). Mucositis induced by intensive chemotherapy is similarly associated with gram‐positive bacteria. Quinolone prophylaxis decreases both the incidence of gram‐negative and gram‐positive infections, but infections occurring despite prophylaxis are more likely to be gram‐positive (Bucaneve 2005; Gafter‐Gvili 2005). The recent resurgence of gram‐negative infections might be related to discontinuation of quinolone prophylaxis as a consequence to rising resistance of gram‐negative bacteria to quinolones.
Gram‐positive bacteria among cancer patients are frequently resistant to the beta‐lactams, which are currently recommended for the empirical treatment of febrile neutropenic cancer patients. Methicillin (an antistaphylococcal penicillin)‐resistant Staphylococcus aureus (MRSA) is common in the healthcare setting, with methicillin‐resistance rates reaching up to 50% of all S. aureus isolates in high‐endemicity locations in Europe (EARS‐NET). However, the observed prevalence of MRSA has stabilised or declined in the last decade worldwide (Akova 2016). This reduction has been assumed to result from improved infection control. Coagulase‐negative staphylococci are commonly responsible for bloodstream infections in cancer patients and resistance rates of 90% for methicillin, 68.4% for ciprofloxacin, and 48.5% for clindamycin have been reported in the USA (May 2014).
Current guidelines for the use of antimicrobial agents in febrile neutropenic patients advise against routine empirical treatment with glycopeptides, prior to identification of the causative pathogen or its susceptibilities (Averbuch 2013; Cometta 2007; Freifeld 2011; Penack 2011). Exceptions are defined in patients with hypotension, severe sepsis and septic shock, and those with severe mucositis. Pre‐emptive treatment is advised for patients with suspected catheter‐related infections (with clinical signs of catheter infection). European guidelines recommend the consideration of empirical glycopeptides in centres where resistant gram‐positive bacteria (that is methicillin‐resistant S. aureus or penicillin‐resistant streptococci) are predominant (Cometta 2007). Targeted treatments are recommended for patients with documented infections caused by beta‐lactam resistant gram‐positive bacteria, and for those with bacteraemia caused by gram‐positive bacteria, prior to final identification of the pathogen and susceptibility testing (Averbuch 2013; Freifeld 2011).
Withholding broad‐spectrum anti‐gram‐positive (antiGP) treatment is not necessarily detrimental and may even be advantageous. Early empirical antibiotic treatment for febrile neutropenic patients was suggested when gram‐negative organisms dominated. Such early treatment reduced mortality since gram‐negative infections are notoriously rapidly fatal. Infections due to gram‐positive bacteria, especially those caused by coagulase‐negative staphylococci, may be less rapidly fatal permitting initiation of specific antibiotic treatment when an infection is documented (Rosa 2014). Administration of glycopeptides may be associated with adverse effects, especially when combined with aminoglycosides or other nephrotoxic agents (Finch 2005). Moreover, use of glycopeptides has been associated with emergence of glycopeptide‐resistant enterococci and S. aureus resistant to glycopeptides (Montecalvo 1994; Sievert 2002; Tenover 2001).
Considering an overall mortality rate among patients with febrile neutropenia of around 6%, the sample size needed to assess the effect of antiGP treatment is large (Other published versions of this review). We therefore conducted a meta‐analysis of trials comparing the treatment for febrile neutropenia with or without specific antiGP coverage. We looked for specific patient subgroups for whom antiGP treatment may be specifically indicated.
Objectives
To assess whether the addition of empirical anti‐gram‐positive (antiGP) antibiotic treatment in febrile neutropenic cancer patients reduces mortality and treatment failure.
To assess the rate of resistance development, further infections and adverse events caused by additional antiGP treatment.
Methods
Criteria for considering studies for this review
Types of studies
Any randomised controlled trial (RCT) or quasi‐RCT. Studies with a dropout rate above 30% were excluded, unless an intention‐to‐treat (ITT) analysis was possible for any of the review‐defined outcomes.
Types of participants
Febrile neutropenic patients with cancer with suspected or documented infections.
Types of interventions
We included studies assessing first‐line treatment (that is treatment instituted before final identification of causative pathogen(s) and their susceptibilities) for all patients or those with risk factors for gram‐positive infections (e.g. suspected catheter‐related infections, hypotension, mucositis) or pre‐emptive therapy (for patients with identification of gram‐positive cocci in blood before final identification), both at onset of treatment (empirical treatment) and for fever persisting beyond 48 to 72 hours after treatment initiation (first modification). Only studies comparing one antibiotic regimen with or without a placebo versus the same antibiotic regimen with the addition of an antiGP antibiotic (as defined) were included. Studies comparing different antibiotic regimens, including an antiGP antibiotic in one arm, were excluded.
The following antiGP antibiotics were included.
-
Glycopeptides:
vancomycin;
teicoplanin.
-
Beta‐lactams:
penicillinase‐resistant penicillins, oxacillin, cloxacillin, dicloxacillin, flucloxacillin, or nafcillin;
first‐generation cephalosporins, cefazolin;
advanced‐generation cephalosporins: cefepime*, ceftaroline*, cetobiprole*.
-
Lincosamines:
clindamycin.
-
Streptogramins:
quinupristin‐dalfopristin.
-
Oxazolidinones:
linezolid;
tedizolid.
-
Sulphonamides:
trimethoprim‐sulphamethoxazole*.
-
Lipopeptides:
daptomycin;
dalbavancin;
oritavancin.
-
Glycylcyclines:
tigecycline*.
Antibiotics marked with * are active also against gram‐negative bacteria (see investigation of heterogeneity and subgroup analyses).
Types of outcome measures
Primary outcomes
Overall mortality at end of study follow‐up and up to 30 days following end of treatment. We extracted 30‐day mortality. If not reported, we used overall mortality data at the latest point of study follow‐up when the follow‐up did not exceed 30 days.
Secondary outcomes
Treatment failure, as defined in the study, once including any modification of the empirical antibiotic regimen in the definition of failure (modifications included), and once disregarding treatment modifications (overall failure) (Consensus 1990)
Duration of fever and hospital stay among survivors
Removal of central catheter
Addition of amphotericin (antifungal antibiotic)
Superinfection: new, persistent, or worsening symptoms or signs of infection associated with the isolation of a new pathogen (different pathogen, or same pathogen with different susceptibilities), or the development of a new site of infection
Colonisation by resistant bacteria: the isolation of bacteria during or following antibiotic therapy, without signs or symptoms of infection
Development of resistance: change in susceptibility of pathogens isolated at initiation of antibiotic therapy
Adverse events
The adverse events were described as:
any serious adverse events that were fatal, life‐threatening, or requiring inpatient hospitalisation or prolongation of existing hospitalisation (death due to adverse event, anaphylaxis, nephrotoxicity requiring renal replacement therapy, pseudomembranous colitis); serious adverse events were not independent of the primary outcome, overall mortality;
any adverse events that resulted in significant disability or incapacity (e.g. nephrotoxicity, ototoxicity, bleeding severe skin reactions);
any important medical events that might not be immediately life‐threatening or result in death or hospitalisation but might jeopardise the patient or require intervention to prevent one of the above outcomes;
any adverse events that required discontinuation of medication;
any adverse event.
Search methods for identification of studies
Electronic searches
A comprehensive search strategy was formulated in an attempt to identify all relevant studies regardless of language or publication status, in combination with the search strategy for clinical trials developed by Cochrane and detailed in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). The following databases were searched using the tailored search strategies detailed in Appendix 1, Appendix 2, and Appendix 3.
For this review update the searches were re‐run on 08 March 2017.
Cochrane Central Register of Controlled Trials (CENTRAL 2017, Issue 2). MEDLINE (May 2012 to February Week 4 2017). Embase (May 2012 to 2017 week 10). LILACS (2012 to March 2017).
Searching other resources
The bibliographies of all included studies and pertinent reviews were scanned for additional references. We contacted the first or corresponding author of each included study, and the researchers active in the field for information regarding unpublished trials or complementary information on their own trials. We searched the following conference proceedings for unpublished trials: Interscience Conference on Antimicrobial Agents and Chemotherapy (ICAAC) (1995 to 2017); European Congress of Clinical Microbiology and Infectious Diseases (ECCMID) (2003 to 2017). We searched for ongoing and unpublished trials in the National Institutes of Health database (https://clinicaltrials.gov/).
Data collection and analysis
Selection of studies
One review author (OBK for the 2017 update) inspected the abstract of each reference identified in the search and applied the inclusion criteria. Where potentially relevant articles were identified, the full‐text article was obtained and inspected independently by two review authors (MP, OBK).
Data extraction and management
Two review authors extracted the data from the included trials independently into a data extraction sheet. Differences in the data that were extracted were resolved by discussion with a third review author (LL). Justification for excluding studies from the review was documented. We contacted authors of all included trials, and trials in the assessment for inclusion for clarifications and further information. Data regarding all‐cause mortality and randomisation methods were primarily requested.
For the mortality comparison, we extracted results by ITT, including all individuals randomised in the outcome assessment. Where this was impossible, we extracted the data by available‐case analysis. We compared the main analysis, including both types of studies, to the ITT analysis. All other outcome data were extracted preferentially by ITT and combined with the available‐case analysis. For sensitivity analysis, we imputed failure for all dropouts and presented an ITT analysis including all randomised individuals in the denominator. We could not include all studies in this comparison as some trials did not report the number of dropouts per study arm, prohibiting imputation for dropouts.
The following data were extracted, checked, and recorded.
Trial characteristics
Year (defined as recruitment initiation year) and country of study
Trial sponsor
Publication status: published in journal; abstract or proceeding; unpublished
ITT analysis: performed; possible to extract; efficacy analysis
Randomisation methods: allocation generation and concealment
Blinding
Failure definition: including time of failure assessment
Study follow‐up duration
Performance of surveillance cultures
Patient characteristics
Number of patients with clinically documented infections
Number of patients with bacteriologically documented infections
Number of patients with documented infections due to gram‐positive bacteria: any gram‐positive, Staphlococcus epidermidis, Staphylococcus aureus; Streptococci
Number of patients with bacteraemia
Number of patients with gram‐positive bacteraemia: any gram‐positive, Staphylococcus epidermidis, Staphylococcus aureus; Streptococci
Number of patients with gram‐negative bacteraemia
Number of patients with infections caused by bacteria resistant to the administered antibiotic regimen: methicillin‐resistant staphylococci; other
Infection characteristics
Number of patients with clinically documented infections
Number of patients with bacteriologically documented infections
Number of patients with documented gram‐positive infections: any gram‐positive, Staphlococcus epidermidis, Staphylococcus aureus; Streptococci
Number of patients with bacteraemia
Number of patients with gram‐positive bacteraemia: any gram‐positive, Staphylococcus epidermidis, Staphylococcus aureus; Streptococci
Number of patients with gram‐negative bacteraemia
Number of patients with infections caused by bacteria resistant to the administered antibiotic regimen: methicillin‐resistant staphylococci; other
Intervention characteristics
Antibiotics type and dose
Treatment duration
Treatment modifications
Previous antibiotic regimen for the first modification trials
Measures of outcome
Measures of outcome as defined under Types of outcome measures, extracted as number of patients per group
Assessment of risk of bias in included studies
The risk of bias of the included trials was assessed for allocation sequence generation, allocation concealment, blinding of participants, personnel and outcome assessors and incomplete outcome data using the Cochrane 'Risk of bias' tool (Higgins 2011b). Other risk of bias was considered when patients were randomised more than once into the trial (see Unit of analysis issues). 'Risk of bias' assessment was performed independently by two review authors (MP, OBK). 'Risk of bias' assessment was based on the evidence of a strong association between poor allocation concealment and overestimation of effect, and was defined low risk of bias; adequate allocation concealment, moderate risk of bias; unclear allocation concealment, and high risk of bias; inadequate allocation concealment) (Schulz 1995).
Measures of treatment effect
Dichotomous data were analysed by calculating the risk ratio (RR) for each trial, with the uncertainty of each result being expressed using the 95% confidence interval (CI). We planned to extract time‐to‐event data for hospitalisation, fever and treatment durations according to the method described by Parmar (Parmar 1998).
Unit of analysis issues
Some trials allowed the inclusion of several episodes for each patient; these outcomes for different episodes in the same patient are not independent. Ideally, such trials should be analysed allowing for clustering of episodes within patients, but this clustering is often ignored giving rise to spuriously narrow confidence intervals on the estimated effects of treatment. To minimise such problems, we extracted the number of patients and episodes per trial. Where data were available, we used the number of patients with the outcome and number of patients randomised, rather than basing the analysis on episodes.
Assessment of heterogeneity
Heterogeneity in the results of the trials was assessed using a Chi2 test of heterogeneity (P less than 0.1) and the I2 statistic. We planned to explore heterogeneity by performing subgroup analyses and meta‐regression (see Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
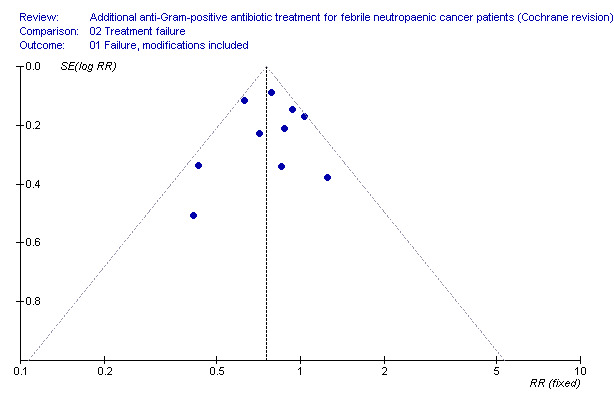
A funnel plot of log odds ratio (OR) for efficacy against the sample size was examined in order to assess potential selection bias (publication and language). In addition, the standard normal deviate (SND), defined as the OR divided by its standard error, was regressed against the estimate's precision (regression equation: SND = a + b x precision) in order to summarise any potential selection bias (Egger 1997). In this equation, the SND reflects the degree of funnel plot asymmetry as measured by the intercept from regression of standard normal deviates against precision.
Data synthesis
Meta‐analysis was performed using the fixed‐effect model for comparisons showing no substantial heterogeneity (I2 less than 50%) and the random‐effects model for other comparisons. The effect of risk of bias on results was examined using sensitivity analysis restricting the analysis to trials at low risk of bias for allocation concealment and reporting results by ITT.
Univeraite meta‐regression was performed using Comprehensive Meta Analysis V3.
Subgroup analysis and investigation of heterogeneity
We planned to compare the effects of empirical treatment with and without additional antiGP antibiotics in the following patient subgroups:
patients diagnosed eventually with gram‐positive infections;
patients with central venous catheters;
patients having received quinolone prophylaxis.
Meta‐regression using Comprehensive Meta‐analysis Version 2 was performed to assess the relationship between the rate of gram‐positive infections in the studies and their estimated treatment effects, in order to assess the hypothesis that antiGP treatment would appear more effective with increasing prevalence of gram‐positive infections.
In this 2017 update, we added a subgroup analysis of trials in which the antiGP antibiotic provides coverage also against gram‐negative bacteria. Thus, this analysis is restricted to antiGPs whose spectrum of coverage includes not only gram‐positive bacteria.
Sensitivity analysis
The effect of risk of bias on results was examined using sensitivity analysis restricting the analysis to trials at low risk of bias for allocation concealment and reporting results by ITT.
Results
Description of studies
Results of the search
The original search strategy resulted in 331 references. After reviewing all abstracts, we retrieved 42 studies for full‐text inspection. We updated the search in 2013 and 3515 new references were screened, but no new trials were identified for inclusion. The current 2017 update revealed 2723 new references with one study identified for inclusion (Bucaneve 2014). See Figure 1.
1.
Study flow diagram.
Included studies
We included 22 publications representing 14 individual randomised controlled trials (RCTs) corresponding to our inclusion criteria. The trials were conducted between 1979 and 2014.
Glycopeptides were tested in nine trials (vancomycin five, teicoplanin four) (see Characteristics of included studies). These trials were performed between 1984 and 2000. Other anti‐gram‐positive (antiGP) drugs were tested in five trials: cephalothin in two (Lawson 1979; Verhagen 1987), flucloxacillin and trimethoprim‐sulphamethoxazole in one trial each (de Pauw 1985; Menichetti 1986, respectively), and tigecycline in the last trial identified in the 2017 update (Bucaneve 2014). The last two antiGP antibiotics cover also gram‐negative bacteria (Bucaneve 2014; Menichetti 1986). The basic antibiotic regimens are specified in the table 'Characteristics of included studies'. Ceftazidime, the most commonly used beta‐lactam used as a basic regimen, was used alone in five trials and with amikacin in two trials.
The antiGP antibiotic was tested at the onset of antibiotic treatment as the first line (empirical) regimen in all trials but two, which assessed its addition for persistently febrile patients (first modification) after 72 to 96 hours of imipenem monotherapy (Erjavec 2000), or after 48 to 60 hours of piperacillin‐tazobactam monotherapy (Cometta 2003). We did not identify studies assessing pre‐emptive antiGP treatment.
Nine trials randomised 1993 patients. Five studies allowed patient re‐entry for separate neutropenic febrile episodes, thus randomising episodes instead of patients. Three trials included 352 episodes representing 292 patients (Del Favero 1987; Erjavec 2000; Menichetti 1986), and two trials included 437 episodes without specifying the number of patients (Lawson 1979; Marie 1991). Overall, 2782 febrile episodes were included and 2549 were evaluated.
All trials included patients with haematological malignancies, except for one trial that was restricted to patients with solid tumours (Molina 1993). One trial included only patients with haematological malignancies (Bucaneve 2014). Two trials did not specify patients' age. In the remaining trials, children less than 16 years were included in six trials, and the mean age ranged between 38 to 48 years. With regard to exclusion of patients at risk for infections due to gram‐positive bacteria, the two first modification trials excluded patients with documented or suspected catheter‐related infections (Cometta 2003; Erjavec 2000). Two empirical studies excluded patients with a documented focus of infection (Marie 1991; Novakova 1991), of which one also excluded patients in septic shock (Marie 1991). Otherwise, no restrictions related to the criteria suggested for empirical antiGP treatment (see Background) were imposed on patient inclusion.
The rate of single‐agent gram‐positive bacteraemia varied between 6% and 28% (Table 4) and did not correlate with the study year.
1. Study year and % gram positive (GP) out of single‐agent bacteraemias.
| Study ID | Study year | GP bacteraemia |
| Empirical | ||
| Menichetti 1986 | 1983 | 13% |
| Del Favero 1987 | 1984 | 19% |
| Karp 1986 | 1984 | 28% |
| Novakova 1991 | 1987 | 25% |
| Ramphal 1992 | 1988 | 25% |
| EORTC 1991 | 1988 | 18% |
| Molina 1993 | 1992 | 6% |
| Bucaneve 2014 | 2010 | 25% |
| Semi‐empirical | ||
| Cometta 2003 | 2000 | 11% |
Excluded studies
Twenty studies were excluded. Two studies were excluded on account of a high percentage of dropouts. An EORTC trial randomised 841 patients and evaluated 419 patients (EORTC 1983). Martino and colleagues reported outcomes for a 10‐month period and 158 patients of a trial which was conducted for 15 months and included 232 patients (Martino 1992). The reasons for exclusion of the remaining studies are listed in the Characteristics of excluded studies table.
Risk of bias in included studies
Results are summarised in Figure 2 and Figure 3 and detailed per study in Characteristics of included studies. Generation of the randomisation sequence was described as low risk in 12 of the 14 trials. Allocation concealment was described as low risk in nine trials, and four additional trials used sealed envelopes that were not described as opaque (classified as unclear). The two first modification trials were double‐blinded (Cometta 2003; Erjavec 2000), as was a single empirical trial (Karp 1986). All remaining trials were open‐label.
2.
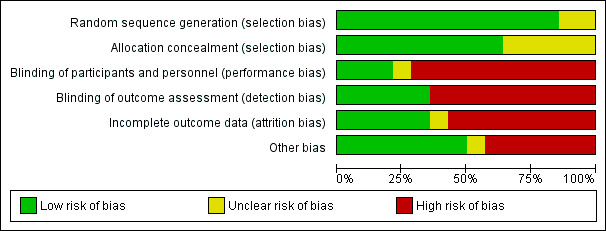
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
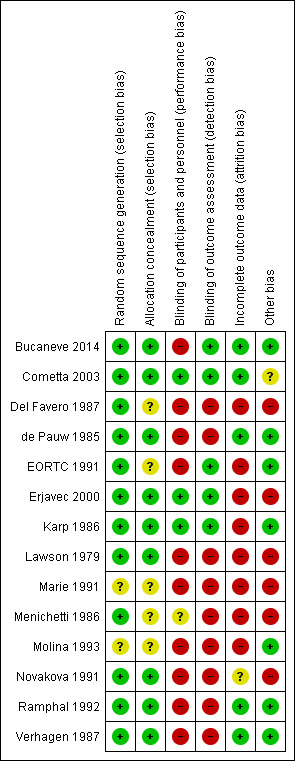
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Full intention‐to‐treat (ITT) analysis for failure and mortality was reported in four trials (Bucaneve 2014; Cometta 2003; de Pauw 1985; Verhagen 1987) and for mortality alone in two (Menichetti 1986; Novakova 1991). Four additional trials provided the number of patients excluded from each study arm, allowing an ITT analysis by imputing failure for dropouts (Del Favero 1987; Erjavec 2000; Karp 1986; Novakova 1991).
Five trials permitted patient re‐inclusion, referring to episodes or infections instead of individual patients, as stated above ('Other bias'). Results per patient were unavailable from the publication even when the number of included patients was known. Results from these trials were analysed together with the remaining trials.
Patients' consent was reported in eight trials and approval of the ethics committee in four, all of which required patient consent. Eight trials reported funding by industry, while no external sources of funding were stated in the other trials.
Effects of interventions
See: Table 1; Table 2; Table 3
Mortality
Eight studies, including 1242 participants, reported overall mortality. The adjusted mean mortality rate in these studies was 9.0%. The risk ratio (RR) for death was 0.9 (95% confidence interval (CI) 0.64 to 1.25; 8 studies, 1242 participants; Analysis 1.1), values lower than 1 favouring the antiGP arm. The quality of this evidence was rated as moderate, downgraded for imprecision (Table 1). Two trials used a glycopeptide empirically, two used a glycopeptide semi‐empirically, and four used another antiGP antibiotic empirically. No difference in mortality was seen in each of these groups. Considering only studies with low‐risk allocation concealment, or those reporting mortality by ITT results, the results were similar, below 1 (Analysis 1.2; Analysis 1.3). Overall, no heterogeneity was seen with this comparison, which was performed using the fixed‐effect model (I2 = 0%).
1.1. Analysis.
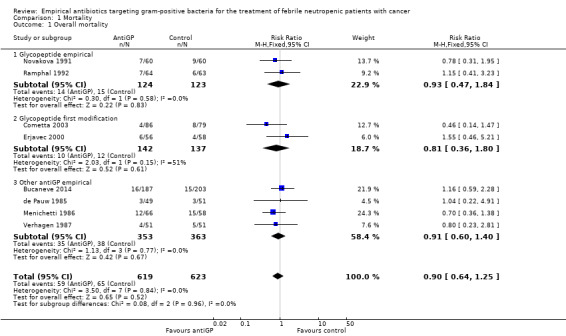
Comparison 1 Mortality, Outcome 1 Overall mortality.
1.2. Analysis.
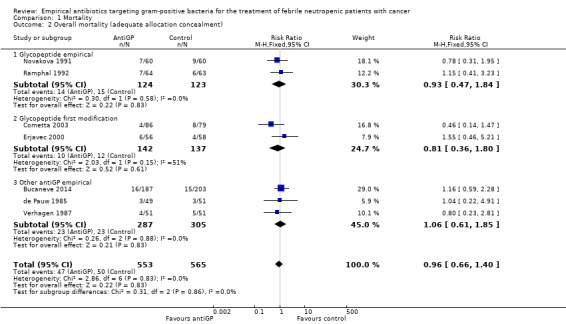
Comparison 1 Mortality, Outcome 2 Overall mortality (adequate allocation concealment).
1.3. Analysis.
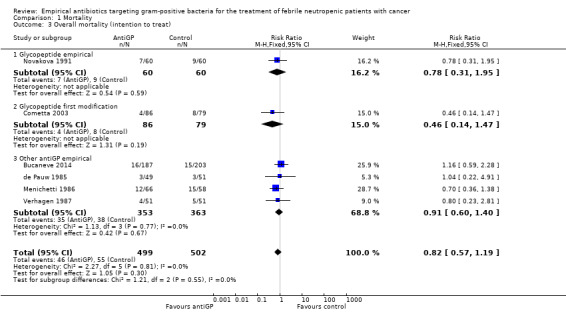
Comparison 1 Mortality, Outcome 3 Overall mortality (intention to treat).
Data regarding mortality among patient subgroups were scarce. Only five studies were included in the comparison for patients in whom a gram‐positive infection was documented (Analysis 1.4). Only 13 deaths were recorded; hence although overall mortality among patients receiving antiGP treatment was almost twice that in the control group, this was not interpreted into a difference. The rate of single‐agent gram‐positive bacteraemia (Table 4) was reported only for five of the studies in the mortality analysis and no association was observed between this rate and the RRs for mortality, but the analysis was limited by the paucity of data with extreme 95% CI for the ratio of odds ratios (ORs). Excluding two studies in which the antiGP was active also against gram‐negative bacteria did not change results (Analysis 1.5). Data for the other pre‐defined subgroup analyses were not available. Inspecting the funnel plot did not reveal a small‐study effect (Figure 4).
1.4. Analysis.
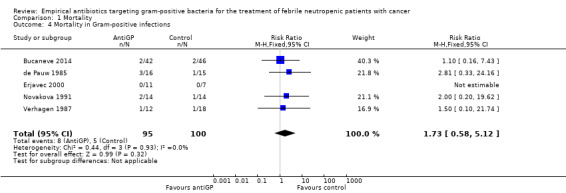
Comparison 1 Mortality, Outcome 4 Mortality in Gram‐positive infections.
1.5. Analysis.
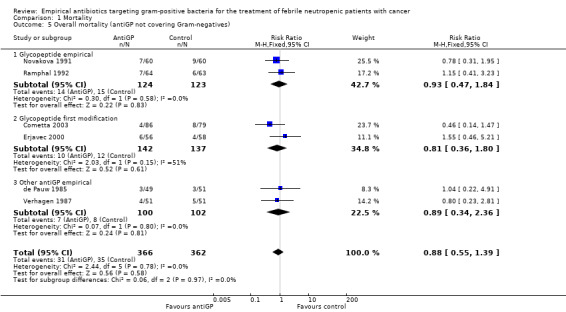
Comparison 1 Mortality, Outcome 5 Overall mortality (antiGP not covering Gram‐negatives).
4.
Failure.
Eight trials compared infection‐related fatality, two of which did not report overall mortality. The RR was 1.15 (95% CI 0.76 to 1.75; 8 studies, 1810 participants; Analysis 1.6).
1.6. Analysis.
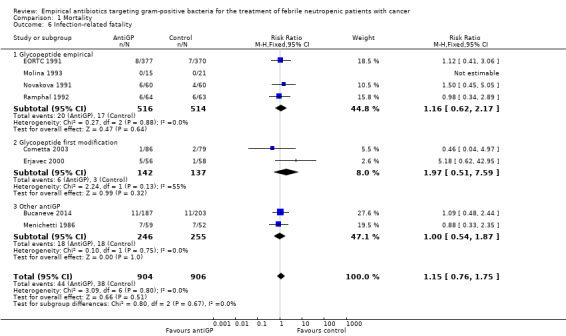
Comparison 1 Mortality, Outcome 6 Infection‐related fatality.
Treatment failure
Overall failure, disregarding treatment modifications, was assessed in seven studies including 943 participants and was similar in both study arms (RR 1.00, 95% CI 0.79 to 1.27; 7 studies, 943 participants; Analysis 2.1, low‐quality evidence). When modifications were counted as causes for treatment failure, an advantage in favour of antiGP treatment was evident (RR 0.72, 95% CI 0.65 to 0.79, 11 studies, 2169 participants; Analysis 2.2, very low‐quality evidence). The quality of the evidence for failure was downgraded for lack of blinding for both outcomes and indirectness of the outcome for treatment failure with modifications (Table 2). The advantage originated from studies that assessed the initial empirical administration of antiGP antibiotics and was demonstrated both for empirical glycopeptides and other antiGP agents. No benefit was observed for the addition of glycopeptides for persistent fever (first modification), in two double‐blind studies (Cometta 2003; Erjavec 2000; Analysis 2.2). Results were not affected by randomisation methods, with similar direction of effects when the analysis was limited to studies with adequate allocation concealment (Analysis 2.3) or those permitting analysis by ITT(Analysis 2.4). Excluding the double‐blind trials decreased the risk ratio (RR 0.67, 95% CI 0.60 to 0.75), demonstrating the effect of the open design on treatment modifications. The funnel plot for failure was centred approximately symmetrically around the effect estimate (Figure 5).
2.1. Analysis.
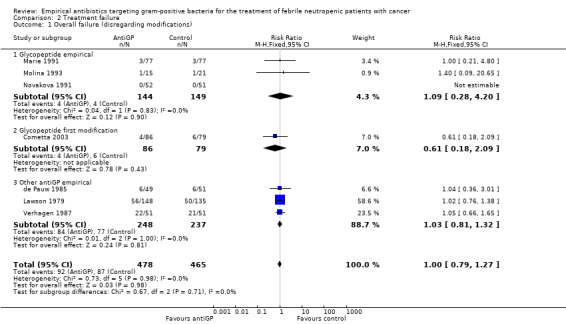
Comparison 2 Treatment failure, Outcome 1 Overall failure (disregarding modifications).
2.2. Analysis.
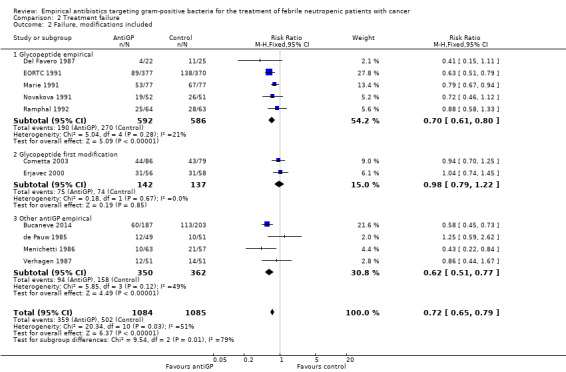
Comparison 2 Treatment failure, Outcome 2 Failure, modifications included.
2.3. Analysis.
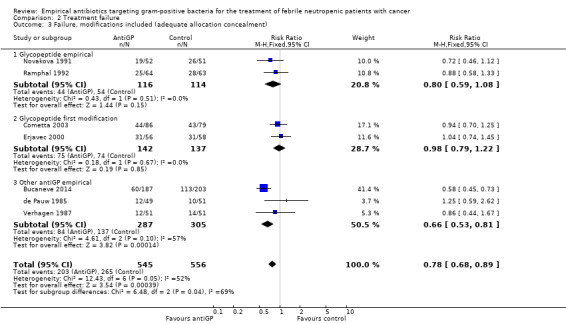
Comparison 2 Treatment failure, Outcome 3 Failure, modifications included (adequate allocation concealment).
2.4. Analysis.
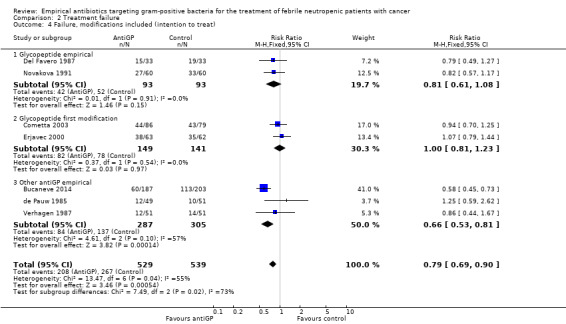
Comparison 2 Treatment failure, Outcome 4 Failure, modifications included (intention to treat).
5.
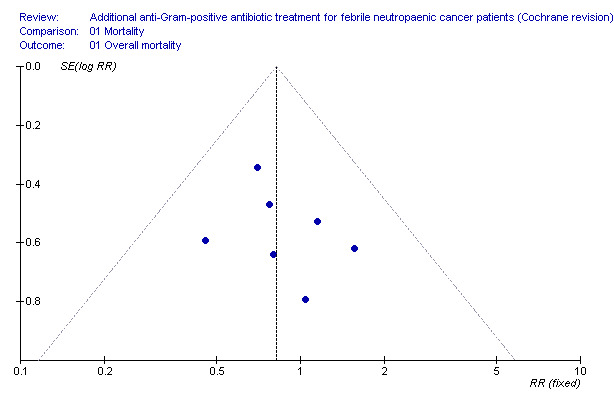
Mortality.
In subgroup analyses of failure with modifications, when excluding antiGP antibiotics covering gram‐negative bacteria, there was no longer an advantage to non‐glycopeptide empirical antiGP antibiotics (Analysis 2.5). An analysis restricted to patients who ultimately had a gram‐positive infection, was composed of studies assessing empirical antiGP treatment and demonstrated a large advantage to this intervention, (RR 0.56, 95% CI 0.38 to 0.84; 5 studies, 175 patients; Analysis 2.6). Meta‐regression demonstrated no association between the rate of single gram‐positive bacteraemia in the studies and their risk ratio for failure with antiGP treatment. No data were available for the subgroup of patients with central catheters or those having received quinolone prophylaxis.
2.5. Analysis.
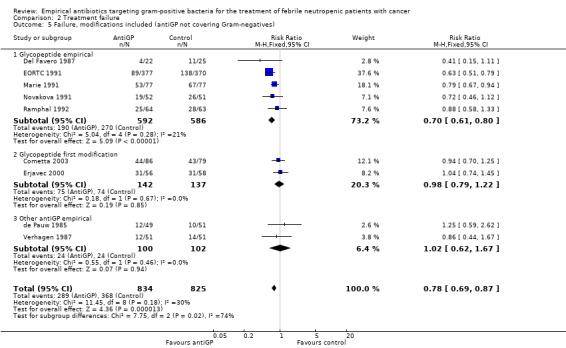
Comparison 2 Treatment failure, Outcome 5 Failure, modifications included (antiGP not covering Gram‐negatives).
2.6. Analysis.
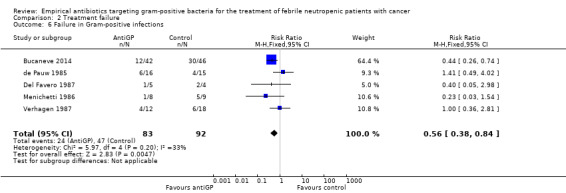
Comparison 2 Treatment failure, Outcome 6 Failure in Gram‐positive infections.
Duration of fever was not reported comparatively, but three studies compared the number of persistently febrile patients at 72 hours after the initiation of empirical antibiotic treatment (Analysis 2.7). An advantage to the antiGP arm was seen, but the number of patients evaluated was small (312 patients). Amphotericin was added more frequently to the control arm (RR 1.23, 95% CI 0.84 to 1.80, 5 studies, 1201 participants, Analysis 2.8). Substantial heterogeneity was seen in the comparisons of persistent fever and addition of amphotericin, which were analysed using the random‐effects model.
2.7. Analysis.
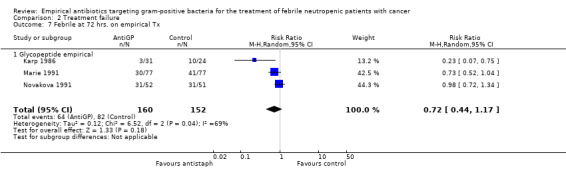
Comparison 2 Treatment failure, Outcome 7 Febrile at 72 hrs. on empirical Tx.
2.8. Analysis.
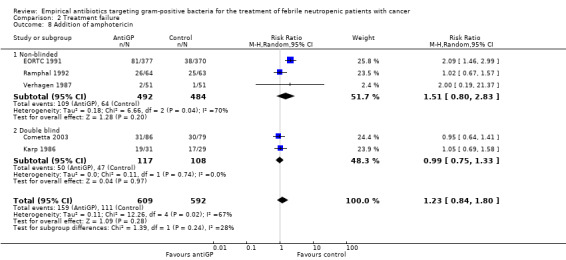
Comparison 2 Treatment failure, Outcome 8 Addition of amphotericin.
Superinfections and adverse events
AntiGP treatment did not increase superinfection rates (Analysis 3.1). Focusing on bacterial superinfections, we observed a decrease with antiGPs (RR 0.41, 95% CI 0.27 to 0.6, Analysis 3.2); specifically gram‐positive superinfections (RR 0.24, 95% CI 0.14 to 0.4, Analysis 3.3). The rate of fungal superinfections was similar in both arms (RR 1.04, 95% CI 0.60 to 1.82, Analysis 3.4). No study assessed the effect of additional antiGP treatment on the rate of colonisation with resistant microorganisms or development of resistance.
3.1. Analysis.
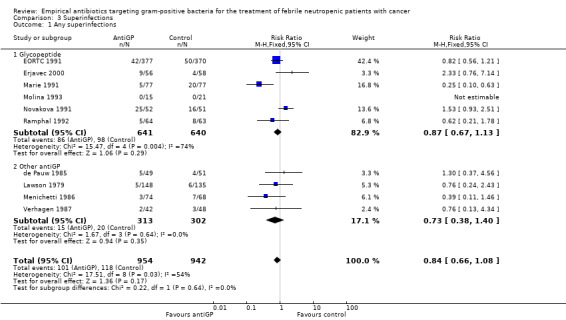
Comparison 3 Superinfections, Outcome 1 Any superinfections.
3.2. Analysis.
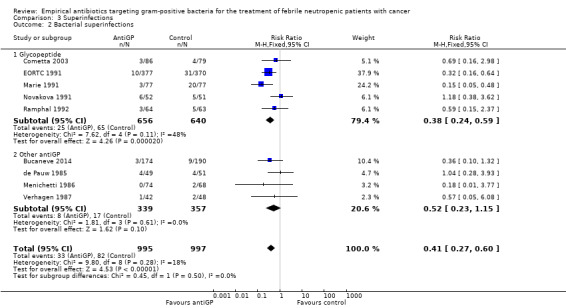
Comparison 3 Superinfections, Outcome 2 Bacterial superinfections.
3.3. Analysis.
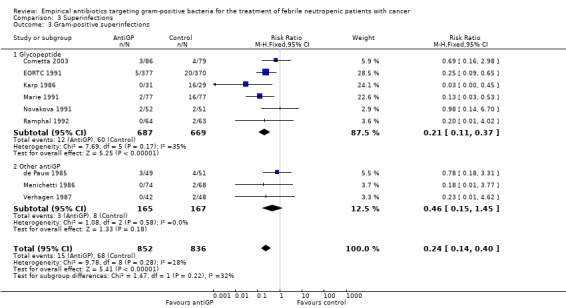
Comparison 3 Superinfections, Outcome 3 Gram‐positive superinfections.
3.4. Analysis.
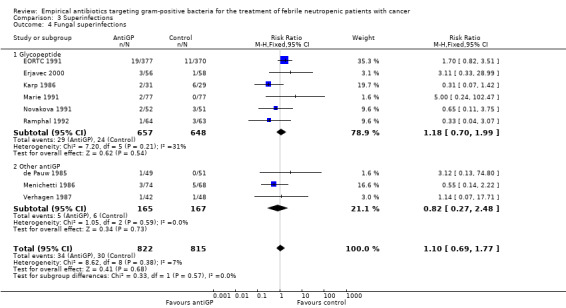
Comparison 3 Superinfections, Outcome 4 Fungal superinfections.
Adverse events were more frequent in the antiGP arm (Analysis 4.1) (Table 3) with very low quality of evidence. However, this originated mainly from a difference in skin reactions (Analysis 4.2) rather than in adverse events incurring significant morbidity. Nephrotoxicity did not differ between the study groups (Analysis 4.3).
4.1. Analysis.
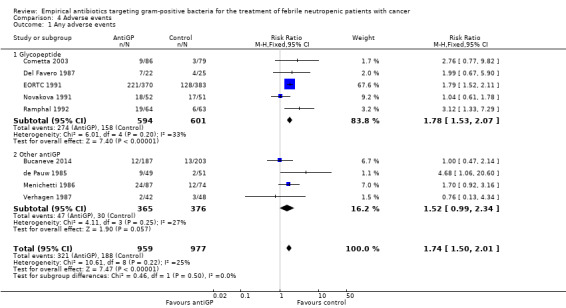
Comparison 4 Adverse events, Outcome 1 Any adverse events.
4.2. Analysis.
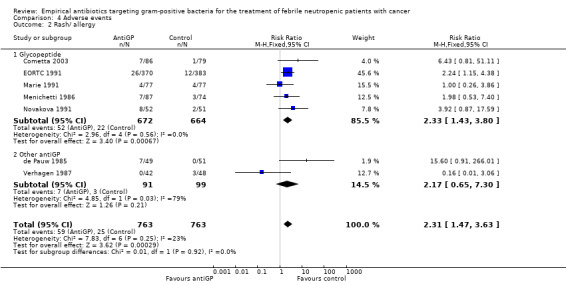
Comparison 4 Adverse events, Outcome 2 Rash/ allergy.
4.3. Analysis.
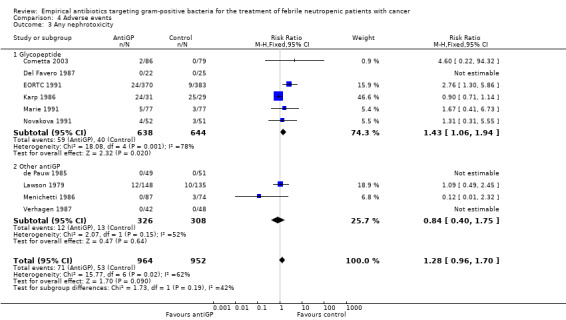
Comparison 4 Adverse events, Outcome 3 Any nephrotoxicity.
Other outcomes
Duration of hospital stay was inconsistently reported and summarised heterogeneously, as means or medians without appropriate CIs, in the included trials. Thus results could not be combined. Duration of fever and removal of central catheters were not reported in the studies.
Discussion
We show that the current evidence does not point to a reduction in the risk of death with the empirical addition of anti‐gram‐positive (antiGP) antibiotics. Twelve studies assessed their addition to the initial antibiotic regimen among non‐selected febrile neutropenic patients. Two studies assessed their addition after 48 to 72 hours of persistent fever. Both combined and separately, these studies show that there is no difference in overall 30‐day patient mortality (RR 0.90, 95% CI 0.64 to 1.25) (Table 1). Failure of the empirical antibiotic regimen, denoting mainly the need to add or change antibiotic therapy, was more common in the control arm in studies assessing the initial, empirical, addition of antiGP treatment. No such advantage was demonstrated for the addition of glycopeptides for persistent fever. Most studies included in the review were conducted at the time when practice guidelines suggested the addition of empirical antiGP treatment on day three to five for persistent fever (Hughes 1990). Similarly, amphotericin was added more frequently to the control arm. Current guidelines advise addition of antifungal therapy on day five to seven for persistent fever with neutropenia (Freifeld 2011). Thus, treatment modifications and the addition of amphotericin may represent persistence of fever regardless of the incidence of uncontrolled infection or fungal infections. Overall failure, whether or not antibiotic treatment was modified, was equal in both study arms (Table 2). Results were similar when analysing the subgroup of patients ultimately diagnosed with gram‐positive infections and, similarly, there was no association between the percentage of gram‐positive bacteria among bacteraemic patients in each trial and the RR for mortality or treatment failure. The rate of antiGP infections did not correlate with study year as expected, possibly due to the differing locations and inclusion criteria of the studies included in the review. The quality of the evidence ranged from moderate for mortality to very low for failure comprising treatment modifications.
In this update, one trial examining the effects of empirical tigecycline added to a backbone of piperacillin‐tazobactam was included to the review and did not change the overall conclusions (Bucaneve 2014). The addition of this study led to a new subgroup analysis of antiGP whose spectrum comprises and might enhance coverage against gram‐negative bacteria. Exclusion of two such trials ( Bucaneve 2014, Menichetti 1986) from the mortality analysis cancelled the advantage of empirical non‐glycopeptide antiGP treatment.
Adverse events were more common in the antiGP arm as expected, but the difference was in minor adverse events (Table 3). The most feared adverse outcome of adding an antibiotic, especially a glycopeptide, is the induction or selection of resistance. Studies conducted in other settings have shown that excessive use of glycopeptides is associated with increased rates of vancomycin‐resistant enterococci and, vancomycin‐resistant staphylococci (Gardete 2014). Studies included in this review assessed superinfection rates and these did not increase in the antiGP arm. Rather, gram‐positive bacterial superinfections were reduced in the treatment arm, possibly reflecting reduced detection of these infections in the presence of antiGP antibiotic treatment. However, there are no data on colonisation from these studies. Therefore, we do not know whether patients treated with glycopeptides were more likely to carry resistant gram‐positive bacteria, an important factor when considering future infections and the environment. The assessment of resistance induction may require a longer timescale than possible in randomised trials.
Several limitations of our analysis should be noted. Firstly, all‐cause mortality was reported only in eight of 14 included studies. We contacted the authors of the six studies with missing mortality data, of which four replied that the data could no longer be retrieved. Secondly, the definition for treatment failure varied between studies, such that we could not combine all studies to assess treatment failure, with or without treatment modifications. We have encountered methodological issues in included studies, which we could not correct for in the meta‐analysis. Randomising patients more than once creates episode clusters in which individual outcomes are not independent. Since data could not be extracted only for the first episode of each included patient, we could not enter the data correctly for the analysis. ITT analysis was frequently missing and could not be reproduced since the number of patients excluded from each study arm was not consistently reported. While the handling of loss to follow‐up with regard to measurable outcomes must entail some assumptions (carry‐over, imputations, etc.), all randomised patients can be included in the all‐cause mortality comparison. Adequate randomisation should ensure that deaths unrelated to infection are equally distributed between trial arms. Finally, our results pertain to patients with uncomplicated low‐ or high‐risk febrile neutropenia, that is patients presenting without specific risk factors such as catheter‐related infection, skin or soft‐tissue infection, pneumonia, or haemodynamic instability, who were excluded from all existing trials. Current guidelines recommend empirical glycopeptide treatment for these patients (Freifeld 2011).
Authors' conclusions
Implications for practice.
Our conclusions are in accordance with current practice guidelines (Freifeld 2011). Non‐selective empirical use of glycopeptides, initially or for persistent fever, is discouraged. Data from existing trials cannot aid in the selection of patient subgroups for whom an advantage does exist.
Implications for research.
Further trials assessing empirical glycopeptide or other novel anti‐gram‐positive (antiGP) antibiotics may be justified only if the prevalence of resistant gram‐positive infections increases. Two trials (Cometta 2003; Erjavec 2000) tested the addition of a glycopeptide for fever persisting more than 48 hours showing no benefit to this intervention, in line with the clinical practice of a longer time period of persisting fever. Future trials should perhaps assess the value of empirical antiGPs for fever persisting for a longer duration (e.g. five to seven days).
Further research should focus on risk factors defining specific patient groups who will benefit from the addition of glycopeptides prior to microbiological documentation of these infections.
Our analysis highlights the pitfalls of assessing treatment failure in these and similar studies. Results are dependent on the definition of failure. In most studies, failure was defined as a change in the empirical antibiotic regimen, an outcome that is not necessarily associated with patient morbidity. Survival is the ultimate goal of chemotherapy in cancer patients. Usually not chosen as a primary outcome due to the sample size calculation considerations, all‐cause mortality should be reported in all trials assessing the management of febrile neutropenic patients. Other patient‐relevant outcomes include number of febrile days, hospital days for patients surviving the infectious episode and adherence to chemotherapy regimen.
We showed that the use of glycopeptides was associated with fewer gram‐positive superinfections. However, we do not rule out the possibility of resistance induced by their use by this as the trials did not assess the rates of colonisation with resistant microorganisms. Future studies must incorporate methods for surveillance of colonisation to correctly represent the effects of glycopeptide use on future infections and the environment.
All future studies should adhere to better methodological standards (Consort statement). Specifically, patients should be included in the study only once, data regarding overall mortality should be reported by ITT, and the number of exclusions after randomisation for all other outcomes should be reported per study arm.
What's new
| Date | Event | Description |
|---|---|---|
| 8 March 2017 | New citation required but conclusions have not changed | One new randomised controlled trial added (Bucaneve 2014); conclusion unchanged. |
| 8 March 2017 | New search has been performed | Searches updated March 2017 |
History
Protocol first published: Issue 4, 2002 Review first published: Issue 3, 2005
| Date | Event | Description |
|---|---|---|
| 2 December 2013 | Amended | Title amended as a result of recent feedback. |
| 16 August 2013 | New search has been performed | Text updated and new search dates added. Two review authors removed (Abigail Fraser and Michal Cohen) and one new author (Yaakov Dickstein) added. |
| 10 August 2013 | New citation required but conclusions have not changed | Search updated, no new studies identified for inclusion. |
| 15 August 2007 | New search has been performed | New studies sought but none found. We updated the search in August 2007 and no new studies were found. We added new anti‐Gram positive antibiotics to included interventions. |
Acknowledgements
We thank the authors who have responded to our request for further data (Ben E De Pauw, Judith E Karp, Gerald P Bodey). We thank the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancers Group for their support throughout the review and update process.
We would like to thank Abigail Fraser and Michal Cohen who contributed to the first version of the review. We would like to express our appreciation to Heather Dickinson for her thoughtful and educative review of our original work.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Gynaecological, Neuro‐oncology and Orphan Cancer Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor Neoplasms explode all trees #2 (neoplasm* or tumor* or tumour* or cancer* or malignan* or carcinoma* or adenocarcinoma* or lymphoma* or leukem* or luekaem*) #3 (#1 OR #2) #4 MeSH descriptor Neutropenia, this term only #5 neutropeni* #6 (granulop?en* or granulocytop?en*) #7 (immunosuppress* or immuno‐suppress*) #8 (#4 OR #5 OR #6 OR #7) #9 MeSH descriptor Gram‐Positive Bacterial Infections explode all trees with qualifier: DT #10 MeSH descriptor Anti‐Bacterial Agents explode all trees #11 (antibiotic* or anti‐bacterial or antibacterial) #12 (vancomycin or teicoplanin or oxacillin or cloxacillin or dicloxacillin or flucloxacillin or nafcillin or cefazolin or clindamycin or quinupristin‐dalfopristin or linezolid or trimethoprim‐sulphamethoxazole or daptomycin or tigecycline or ceftaroline or cetobiprole or tedizolid or dalbavancin or oritavancin) #13 (#9 OR #10 OR #11 OR #12) #14 (#3 AND #8 AND #13)
Appendix 2. MEDLINE search strategy
1 exp Neoplasms/ 2 (neoplasm* or tumor* or tumour* or cancer* or malignan* or carcinoma* or adenocarcinoma* or lymphoma* or leukem* or luekaem*).mp. 3 1 or 2 4 Neutropenia/ 5 neutropeni*.mp. 6 (granulop?en* or granulocytop?en*).mp. 7 (immunosuppress* or immuno‐suppress*).mp. 8 4 or 5 or 6 or 7 9 exp Gram‐Positive Bacterial Infections/dt [Drug Therapy] 10 exp Anti‐Bacterial Agents/ 11 (antibiotic* or anti‐bacterial or antibacterial).mp. 12 (vancomycin or teicoplanin or oxacillin or cloxacillin or dicloxacillin or flucloxacillin or nafcillin or cefazolin or clindamycin or quinupristin‐dalfopristin or linezolid or trimethoprim‐sulphamethoxazole or daptomycin or tigecycline or ceftaroline or cetobiprole or tedizolid or dalbavancin or oritavancin).mp. 13 9 or 10 or 11 or 12 14 3 and 8 and 13 15 randomized controlled trial.pt. 16 controlled clinical trial.pt. 17 randomized.ab. 18 placebo.ab. 19 clinical trials as topic.sh. 20 randomly.ab. 21 trial.ti. 22 15 or 16 or 17 or 18 or 19 or 20 or 21 23 14 and 22
key: mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier pt=publication type ab=abstract sh=subject heading ti=title
Appendix 3. EMBASE search strategy
1 exp neoplasm/ 2 (neoplasm* or tumor* or tumour* or cancer* or malignan* or carcinoma* or adenocarcinoma* or lymphoma* or leukem* or luekaem*).mp. 3 1 or 2 4 exp neutropenia/ 5 neutropeni*.mp. 6 (granulop?en* or granulocytop?en*).mp. 7 (immunosuppress* or immuno‐suppress*).mp. 8 4 or 5 or 6 or 7 9 Gram positive infection/dt [Drug Therapy] 10 exp antibiotic agent/ 11 (antibiotic* or anti‐bacterial or antibacterial).mp. 12 (vancomycin or teicoplanin or oxacillin or cloxacillin or dicloxacillin or flucloxacillin or nafcillin or cefazolin or clindamycin or quinupristin‐dalfopristin or linezolid or trimethoprim‐sulphamethoxazole or daptomycin or tigecycline or ceftaroline or cetobiprole or tedizolid or dalbavancin or oritavancin).mp. 13 9 or 10 or 11 or 12 14 3 and 8 and 13 15 crossover procedure/ 16 double‐blind procedure/ 17 randomized controlled trial/ 18 single‐blind procedure/ 19 random*.mp. 20 factorial*.mp. 21 (crossover* or cross over* or cross‐over*).mp. 22 placebo*.mp. 23 (double* adj blind*).mp. 24 (singl* adj blind*).mp. 25 assign*.mp. 26 allocat*.mp. 27 volunteer*.mp. 28 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27 29 14 and 28
key mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword
Data and analyses
Comparison 1. Mortality.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall mortality | 8 | 1242 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.64, 1.25] |
| 1.1 Glycopeptide empirical | 2 | 247 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.47, 1.84] |
| 1.2 Glycopeptide first modification | 2 | 279 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.36, 1.80] |
| 1.3 Other antiGP empirical | 4 | 716 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.60, 1.40] |
| 2 Overall mortality (adequate allocation concealment) | 7 | 1118 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.66, 1.40] |
| 2.1 Glycopeptide empirical | 2 | 247 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.47, 1.84] |
| 2.2 Glycopeptide first modification | 2 | 279 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.36, 1.80] |
| 2.3 Other antiGP empirical | 3 | 592 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.61, 1.85] |
| 3 Overall mortality (intention to treat) | 6 | 1001 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.57, 1.19] |
| 3.1 Glycopeptide empirical | 1 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.31, 1.95] |
| 3.2 Glycopeptide first modification | 1 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.14, 1.47] |
| 3.3 Other antiGP empirical | 4 | 716 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.60, 1.40] |
| 4 Mortality in Gram‐positive infections | 5 | 195 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.58, 5.12] |
| 5 Overall mortality (antiGP not covering Gram‐negatives) | 6 | 728 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.55, 1.39] |
| 5.1 Glycopeptide empirical | 2 | 247 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.47, 1.84] |
| 5.2 Glycopeptide first modification | 2 | 279 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.36, 1.80] |
| 5.3 Other antiGP empirical | 2 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.34, 2.36] |
| 6 Infection‐related fatality | 8 | 1810 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.76, 1.75] |
| 6.1 Glycopeptide empirical | 4 | 1030 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.62, 2.17] |
| 6.2 Glycopeptide first modification | 2 | 279 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [0.51, 7.59] |
| 6.3 Other antiGP | 2 | 501 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.54, 1.87] |
Comparison 2. Treatment failure.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Overall failure (disregarding modifications) | 7 | 943 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.79, 1.27] |
| 1.1 Glycopeptide empirical | 3 | 293 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.28, 4.20] |
| 1.2 Glycopeptide first modification | 1 | 165 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.18, 2.09] |
| 1.3 Other antiGP empirical | 3 | 485 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.81, 1.32] |
| 2 Failure, modifications included | 11 | 2169 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.65, 0.79] |
| 2.1 Glycopeptide empirical | 5 | 1178 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.61, 0.80] |
| 2.2 Glycopeptide first modification | 2 | 279 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.79, 1.22] |
| 2.3 Other antiGP empirical | 4 | 712 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.51, 0.77] |
| 3 Failure, modifications included (adequate allocation concealment) | 7 | 1101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.68, 0.89] |
| 3.1 Glycopeptide empirical | 2 | 230 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.59, 1.08] |
| 3.2 Glycopeptide first modification | 2 | 279 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.79, 1.22] |
| 3.3 Other antiGP empirical | 3 | 592 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.53, 0.81] |
| 4 Failure, modifications included (intention to treat) | 7 | 1068 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.69, 0.90] |
| 4.1 Glycopeptide empirical | 2 | 186 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.61, 1.08] |
| 4.2 Glycopeptide first modification | 2 | 290 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.81, 1.23] |
| 4.3 Other antiGP empirical | 3 | 592 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.53, 0.81] |
| 5 Failure, modifications included (antiGP not covering Gram‐negatives) | 9 | 1659 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.69, 0.87] |
| 5.1 Glycopeptide empirical | 5 | 1178 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.61, 0.80] |
| 5.2 Glycopeptide first modification | 2 | 279 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.79, 1.22] |
| 5.3 Other antiGP empirical | 2 | 202 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.62, 1.67] |
| 6 Failure in Gram‐positive infections | 5 | 175 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.38, 0.84] |
| 7 Febrile at 72 hrs. on empirical Tx | 3 | 312 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.44, 1.17] |
| 7.1 Glycopeptide empirical | 3 | 312 | Risk Ratio (M‐H, Random, 95% CI) | 0.72 [0.44, 1.17] |
| 8 Addition of amphotericin | 5 | 1201 | Risk Ratio (M‐H, Random, 95% CI) | 1.23 [0.84, 1.80] |
| 8.1 Non‐blinded | 3 | 976 | Risk Ratio (M‐H, Random, 95% CI) | 1.51 [0.80, 2.83] |
| 8.2 Double blind | 2 | 225 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.75, 1.33] |
Comparison 3. Superinfections.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any superinfections | 10 | 1896 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.66, 1.08] |
| 1.1 Glycopeptide | 6 | 1281 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.67, 1.13] |
| 1.2 Other antiGP | 4 | 615 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.38, 1.40] |
| 2 Bacterial superinfections | 9 | 1992 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.27, 0.60] |
| 2.1 Glycopeptide | 5 | 1296 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.24, 0.59] |
| 2.2 Other antiGP | 4 | 696 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.23, 1.15] |
| 3 Gram‐positive superinfections | 9 | 1688 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.14, 0.40] |
| 3.1 Glycopeptide | 6 | 1356 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.11, 0.37] |
| 3.2 Other antiGP | 3 | 332 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.15, 1.45] |
| 4 Fungal superinfections | 9 | 1637 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.69, 1.77] |
| 4.1 Glycopeptide | 6 | 1305 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.70, 1.99] |
| 4.2 Other antiGP | 3 | 332 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.27, 2.48] |
Comparison 4. Adverse events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any adverse events | 9 | 1936 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.74 [1.50, 2.01] |
| 1.1 Glycopeptide | 5 | 1195 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.78 [1.53, 2.07] |
| 1.2 Other antiGP | 4 | 741 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.99, 2.34] |
| 2 Rash/ allergy | 7 | 1526 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.31 [1.47, 3.63] |
| 2.1 Glycopeptide | 5 | 1336 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.33 [1.43, 3.80] |
| 2.2 Other antiGP | 2 | 190 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.17 [0.65, 7.30] |
| 3 Any nephrotoxicity | 10 | 1916 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.96, 1.70] |
| 3.1 Glycopeptide | 6 | 1282 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.43 [1.06, 1.94] |
| 3.2 Other antiGP | 4 | 634 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.40, 1.75] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bucaneve 2014.
| Methods | Randomised controlled, open‐label, superiority trial | |
| Participants | Patients with neutropenia <1000/mL3 expected to decline to <500/mL3 and fever>>= 38.5°C on one occasion or 38°C on two or more occasions within 12 hours. Mean age 54 years (18‐76), with haematologic malignancies receiving intensive chemotherapy or conditioning regimens for autologous haematopoietic stem‐cell transplantation | |
| Interventions | Tigecycline 50 mg x 2 (loading 100 mg) added to one arm Non‐intervention antibiotics:piperacillin‐tazobactam 4.5 g x 3. |
|
| Outcomes | Failure Mortality (all‐cause and infection‐related) Superinfections (breakthrough bacteraemia) Adverse events |
|
| Notes | Empirical design Multicentre ‐ Italy |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation list was created by using a computer random generator program (Epistat, version 2) and was stratified by centre and underlying disease with a 1:1 allocation by using a block size of eight |
| Allocation concealment (selection bias) | Low risk | Central randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Data entered into the computer system and outcomes were adjudicated by a blinded central committee |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis including all randomised patients reported |
| Other bias | Low risk | Patients randomised only once |
Cometta 2003.
| Methods | Randomised controlled trial, double‐blinded | |
| Participants | Patients with neutropenia < 1000/mm3 anticipated to fall to 500/mm3 and fever ≥ 38.5 or > 38 in two measurements Mean age 42(4‐78) with all types of cancer | |
| Interventions | Vancomycin 15 mg/kg x 2 versus placebo for persistent fever at 48‐60 hrs Non‐intervention antibiotics: piperacillin‐tazobactam | |
| Outcomes | Failure Mortality (all‐cause and infection‐related) Superinfections Adverse events | |
| Notes | First modification design Multicentre ‐ Europe, Middle East, North America | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation. Randomisation was dynamically performed after the application of a randomisation algorithm, which used the minimisation technique of a global imbalances function between the 2 treatment arms, with the following 3 stratification variables: name and location of study centre, infection documentation at randomisation, and underlying disease |
| Allocation concealment (selection bias) | Low risk | Central randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients included in mortality and failure analyses |
| Other bias | Unclear risk | Patients randomised only once |
de Pauw 1985.
| Methods | Randomised controlled trial, open‐label | |
| Participants | Patients with neutropenia <1000/mm3 and fever > 38.5 in two measurements, associated with chills. Mean age 34 (16‐75), with any type of cancer | |
| Interventions | Flucloxacillin 2 g x 4 added to one arm Non‐intervention antibiotics: ceftazidime | |
| Outcomes | Failure Superinfections Mortality (all‐cause) Adverse events | |
| Notes | Empirical design Single centre, open‐label, the Netherlands. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Consecutive opaque and sealed envelopes were opened |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients included in mortality and failure analyses |
| Other bias | Low risk | Patients randomised only once |
Del Favero 1987.
| Methods | Open‐label. | |
| Participants | Patients with neutropenia < 1000/mm3 and fever > 38. Mean age 39 (8‐71), with acute leukaemia | |
| Interventions | Teicoplanin 5 mg/kg x 1 added to one arm Non‐intervention antibiotics: ceftazidime + amikacin | |
| Outcomes | Failure Adverse events | |
| Notes | Empirical design Single centre, Italy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random permuted blocks |
| Allocation concealment (selection bias) | Unclear risk | Consecutive, sealed envelopes (opacity not mentioned) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label |
| Incomplete outcome data (attrition bias) All outcomes | High risk | |
| Other bias | High risk | Patients included for different episodes and analysis by episode |
EORTC 1991.
| Methods | Randomised controlled trial, open‐label | |
| Participants | Patients with neutropenia < 1000/mm3 and fever > 38. Mean age 38 (1‐88), with any type of cancer | |
| Interventions | Vancomycin 500 mg x 4 added to one arm Non‐intervention antibiotics: ceftazidime + amikacin | |
| Outcomes | Failure Mortality (infection‐related only) Superinfections Adverse events | |
| Notes | Empirical design Multicentre, Canada | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised sequence stratified by groups of six patients |
| Allocation concealment (selection bias) | Unclear risk | Consecutive sealed envelopes (opacity not mentioned) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | |
| Other bias | Low risk | Patients included only once |
Erjavec 2000.
| Methods | Randomised controlled trial, double‐blind | |
| Participants | Patients with neutropenia < 500/mm3 or < 1000 and decreasing. Fever ≥ 38 Mean age 48.2 yrs with any type of cancer | |
| Interventions | Teicoplanin 400 mg x 1 versus placebo for persistent fever at 72‐96 hrs Non‐intervention antibiotics: imipenem | |
| Outcomes | Failure Mortality (all‐cause and infection‐related) Superinfections | |
| Notes | First modification design Single centre, the Netherlands | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐assisted randomisation |
| Allocation concealment (selection bias) | Low risk | Randomisation performed by the hospital pharmacy |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | High risk | |
| Other bias | High risk | Patients included for different episodes and analysis by episode |
Karp 1986.
| Methods | Randomised controlled trial, double‐blind | |
| Participants | Patients with neutropenia < 500/mm3 and fever > 38.3. Mean age 40 (19‐63) with acute leukaemia or post autologous bone marrow rescue transplantation | |
| Interventions | Vancomycin 500 mg x 4 added to one arm Non‐intervention antibiotics: ticarcillin + gentamicin | |
| Outcomes | Failure Superinfections Adverse events | |
| Notes | Empirical design Single centre, USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by table of random numbers |
| Allocation concealment (selection bias) | Low risk | Randomisation concealed centrally within the oncology pharmacy |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | High risk | |
| Other bias | Low risk | Patients included only once |
Lawson 1979.
| Methods | Randomised controlled trial, open‐label | |
| Participants | Patients with neutropenia < 1000/mm3 and fever > 38.3 Patients with all types of cancer | |
| Interventions | Cephalothin 3 g x 4 added to one arm Non‐intervention antibiotics: ticarcillin + tobramycin | |
| Outcomes | Failure Superinfections Adverse events | |
| Notes | Empirical design Single centre, USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Randomisation kept in the pharmacy |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | High risk | |
| Other bias | High risk | Patients included for different episodes and analysis by episode |
Marie 1991.
| Methods | Randomised controlled trial, open‐label | |
| Participants | Patients with neutropenia < 500/mm3 and fever > 38.5 for 3 hrs or > 38 for 6 hrs. Mean age 46 yrs, with any type of cancer | |
| Interventions | Vancomycin added to one arm Non‐intervention antibiotics: ceftazidime | |
| Outcomes | Failure Superinfections Adverse events | |
| Notes | Empirical design Multicentre, France Only data from protocol one of three consecutive study protocols were extracted | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes (opacity not mentioned) |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Number of randomised patients not stated |
| Other bias | High risk | Patients included for different episodes and analysis by episode |
Menichetti 1986.
| Methods | Randomised controlled trial, single‐blind | |
| Participants | Patients with neutropenia < 1000/mm3, fever > 38 Mean age 45 (9‐82) yrs, with all types of cancer | |
| Interventions | Trimethoprim/sulphamethoxazole 2.5 mg/kg x 4 (max 640 mg per day) added to one arm Non‐intervention antibiotics: piperacillin + amikacin | |
| Outcomes | Failure Mortality (all‐cause and infection‐related) Superinfections Adverse events | |
| Notes | Empirical design Single centre, Italy | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes (opacity not mentioned) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Single‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | High risk | |
| Other bias | High risk | Patients included for different episodes and analysis by episode |
Molina 1993.
| Methods | Randomised controlled trial, open‐label | |
| Participants | Patients with neutropenia <1000/mm3, fever > 38 Solid cancer | |
| Interventions | Teicoplanin 6 mg/kg/day added to one arm Non‐intervention antibiotics: piperacillin+amikacin | |
| Outcomes | Failure Mortality (infection‐related only) Superinfections | |
| Notes | Empirical design Single centre, Spain | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not stated |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Number of randomised patients not stated |
| Other bias | Low risk | Patients included only once |
Novakova 1991.
| Methods | Randomised controlled trial, open‐label | |
| Participants | Patients with neutropenia < 500/mm3, fever > 38.3 or > 38 in two measurements, without a focus of infection on admission. Mean age 40 (16‐69) yrs with all types of cancer | |
| Interventions | Teicoplanin 800 mg x 2 then 400 mg x 1 added to GP arm Non‐intervention antibiotics: ceftazidime | |
| Outcomes | Failure Mortality (all‐cause and infection‐related) Superinfections Adverse events | |
| Notes | Empirical design Single centre, the Netherlands | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Consecutive opaque and sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Low risk for mortality, high risk for failure |
| Other bias | High risk | Patients included for different episodes and analysis by episode |
Ramphal 1992.
| Methods | Randomised controlled trial, open‐label | |
| Participants | Patients with neutropenia < 500/mm3 of < 1000/mm3 and falling, fever > 38.5 or > 38 in two measurements. Mean age 41(18‐83) yrs, with all types of cancer | |
| Interventions | Vancomycin 1 g x 2 added to one arm Non‐intervention antibiotics: ceftazidime | |
| Outcomes | Failure Mortality (all cause and infection‐related) Superinfections Adverse events | |
| Notes | Empirical design Two centres, USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by a computer random number generator |
| Allocation concealment (selection bias) | Low risk | |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Other bias | Low risk | Patients included only once |
Verhagen 1987.
| Methods | Randomised controlled trial, open‐label | |
| Participants | Patients with neutropenia < 1000/mm3 and fever > 38.5 Mean age 41(14‐78) yrs, with all types of cancer | |
| Interventions | Cephalothin 2 g x 4 added to one arm Non‐intervention antibiotics: ceftazidime | |
| Outcomes | Failure Mortality (all‐cause and infection‐related) Superinfections Adverse events | |
| Notes | Empirical design Single centre, the Netherlands | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Low risk | Consecutive opaque and sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Other bias | Low risk | Patients included only once |
GP: gram‐positive
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Berger 2002 | Prospective surveillance study |
| De Pauw 1997 | Provides a summary based on the analysis of several trials, results of which appears in other included studies |
| Dompeling 1996 | Study includes patients presenting initially with a skin or soft tissue infection, who were assigned non‐randomly to an empirical antibiotic regimen which included vancomycin |
| Elting 1996 | Study provides a retrospective cohort of 415 neutropenic patients with gram‐positive bacteraemia formed from 10 consecutive randomised clinical trials |
| Elting 1997 | The study analyses data from 10 consecutive, randomised clinical trials of antibiotic therapy for febrile episodes in neutropenic patients, some of which are included in the review |
| EORTC 1983 | Dropout rate of 50%. Study randomised 841 patients, of which 149 were excluded due to protocol violations and 273 were not evaluated because of a doubtful or non‐bacterial infection |
| Fauser 1991 | Non‐comparative study: patients were treated with a cephalosporin, an aminoglycoside and teicoplanin |
| Granowetter 1988 | Incompatible comparator antibiotics: ceftazidime versus cephalothin + carbenicillin + gentamicin. Vancomycin was added to ceftazidime treatment arm in the second year of the study as a result of an increase in ceftazidime‐resistant gram‐positive infections |
| Jones 1986 | Incompatible comparator antibiotics: aztreonam + vancomycin versus aztreonam + vancomycin + amikacin versus moxalactam + ticarcillin |
| Kramer 1986 | Vancomycin added to ceftazidime regimen at study entry 49 after revealing a preponderance of gram‐positive superinfections. Study continued with a 2:1 randomised comparison of ceftazidime + vancomycin versus cephalothin + gentamicin + carbenicillin |
| Libanore 1991 | Open prospective study of immunocompromised patients treated with teicoplanin for gram‐positive infections |
| Lim 1990 | Patients randomised to ceftazidime versus ciprofloxacin, with the addition of teicoplanin to both study arms in cases with clinical suspicion of catheter‐associated infection |
| Link 1994 | Antibiotic regimens apart from the antiGP antibiotic differed: acylamino penicillin + third generation cephalosporin + vancomycin versus acylamino penicillin + third generation cephalosporin + aminoglycoside |
| Liu 2000 | Reference identified in CENTRAL (3rd quarter 2007 search) ‐ full text and abstract not available |
| Martino 1992 | Full outcomes are reported for a 10‐month period and 158 episodes, of a trial which was conducted for 15 months and included 232 patients and 265 episodes. The original report including all 232 patients describes outcomes for a subgroup of patients with gram‐positive bacteraemia |
| Moroni 1987 | Antibiotic regimens apart from the antiGP antibiotic differed: ceftazidime + amikacin versus ceftazidime + vancomycin |
| Novakova 1990 | Part of study ID Novakova 1991 which is an included study. The patients treated empirically with teicoplanin were automatically excluded from the present report |
| Pizzo 1982 | Incompatible comparator antibiotics: cephalothin, gentamicin and carbenicillin. In the second phase, patients were randomised to either continue receiving the same treatment with or without additional amphotericin B or discontinue all treatment |
| Pizzo 1986 | Incompatible comparator antibiotics: ceftazidime versus cephalothin + gentamicin + carbenicillin |
| Rubin 1988 | The study is a retrospective review of a randomised, prospective study excluded from this review |
antiGP: anti‐gram‐positive
Differences between protocol and review
In the 2017 update, we reconstructed the characteristics of the review between the different categories. The description of the studies included in the analysis has been moved to the section Types of interventions. The subgroup analysis has been now added to Subgroup analysis and investigation of heterogeneity.
The inclusion criteria and the search strategy has been updated to include new antiGP antibiotics. A subgroup analysis has been added of antiGP antibiotics whose spectrum of coverage comprises gram‐negative bacteria and those whose spectrum of coverage is restricted to gram‐positive bacteria. This was deemed necessary since the new antiGP antibiotics have a combined spectrum of coverage. Furthermore, studies with more than 30% dropouts were excluded from analysis. In addition, 'Summary of findings' tables were provided to point out the major conclusions.
Contributions of authors
Mical Paul (contact reviewer) ‐ reference search, article retrieval, study inclusion and exclusion, data extraction, analysis, and writing. Ofrat Beyar Katz‐ reference search, study inclusion and exclusion, data extraction, analysis, and writing. Yaakov Dickstein ‐ 2013 review update, writing. Sara Borok ‐ reference search, article retrieval, study inclusion and exclusion, data extraction, analysis, and review. Liat Vidal ‐ analysis and review. Leonard Leibovici ‐ reference search, study inclusion and exclusion, data extraction, analysis, writing and review.
Sources of support
Internal sources
Rabin Medical Center, Beilinson Campus, Israel.
Tel‐Aviv University, Sackler Faculty of Medicine, Israel.
External sources
EU 5th Framework ‐ TREAT project (grant number: 1999‐11459), Other.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bucaneve 2014 {published data only}
- Bucaneve G, Micozzi A, Picardi M, Ballanti S, Cascavilla N, Salutari P, et al. Results of a multicenter, controlled, randomized clinical trial evaluating the combination of piperacillin/tazobactam and tigecycline in high‐risk hematologic patients with cancer with febrile neutropenia. Journal of Clinical Oncology 2014;32(14):1463‐71. [DOI] [PubMed] [Google Scholar]
Cometta 2003 {published data only}
- Cometta A, Kern WV, Bock R, Paesmans M, Vandenbergh M, Crokaert F, et al. Vancomycin versus placebo for treating persistent fever in patients with neutropenic cancer receiving piperacillin‐tazobactam monotherapy. Clinical Infectious Diseases 2003;37(3):382‐9. [DOI] [PubMed] [Google Scholar]
- Cometta A, Kern WV, Debock R, Paesmans M, Vandenbergh M, Crokaert F, et al. An EORTC‐IATG double‐blind trial of vancomycin (Van) versus placebo (Pla) for persistent fever in neutropenic cancer patients (NCP) given piperacillin/tazobactam (PT) monotherapy. 41st Interscience Conference on Antimicrobial Agents and Chemotherapy. 2001:445.
- Viscoli C, Cometta A, Kern WV, Bock R, Paesmans M, Crokaert F, et al. Piperacillin‐tazobactam monotherapy in high‐risk febrile and neutropenic cancer patients. Clinical Microbiology and Infection 2006;12:212‐6. [DOI] [PubMed] [Google Scholar]
- Wade JC, Glasmacher A. Vancomycin does not benefit persistently febrile neutropenic people with cancer. Cancer Treatment Reviews 2004;30(1):119‐26. [DOI] [PubMed] [Google Scholar]
Del Favero 1987 {published data only}
- Favero A, Menichetti F, Guerciolini R, Bucaneve G, Baldelli F, Aversa F, et al. Prospective randomized clinical trial of teicoplanin for empiric combined antibiotic therapy in febrile, granulocytopenic acute leukemia patients. Antimicrobial Agents and Chemotherapy 1987;31(7):1126‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
de Pauw 1985 {published and unpublished data}
- Pauw B, Williams K, Neeff J, Bothof T, Witte T, Holdrinet R, et al. A randomized prospective study of ceftazidime versus ceftazidime plus flucloxacillin in the empiric treatment of febrile episodes in severely neutropenic patients. Antimicrobial Agents and Chemotherapy 1985;28(6):824‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
EORTC 1991 {published data only}
- EORTC‐1991. Vancomycin added to empirical combination antibiotic therapy for fever in granulocytopenic cancer patients. European Organization for Research and Treatment of Cancer (EORTC) International Antimicrobial Therapy Cooperative Group and the National Cancer Institute of Canada‐Clinical Trials Group. Journal of Infectious Diseases 1991;163(5):951‐8. [PubMed] [Google Scholar]
Erjavec 2000 {published data only}
- Erjavec Z, Vries‐Hospers HG, Laseur M, Halie RM, Daenen S. A prospective, randomized, double‐blinded, placebo‐controlled trial of empirical teicoplanin in febrile neutropenia with persistent fever after imipenem monotherapy. Journal of Antimicrobial Chemotherapy 2000;45(6):843‐9. [DOI] [PubMed] [Google Scholar]
Karp 1986 {published and unpublished data}
- Karp JE, Dick JD, Angelopulos C, Charache P, Green L, Burke PJ, et al. Empiric use of vancomycin during prolonged treatment‐induced granulocytopenia. Randomized, double‐blind, placebo‐controlled clinical trial in patients with acute leukemia. American Journal of Medicine 1986;81(2):237‐42. [DOI] [PubMed] [Google Scholar]
Lawson 1979 {published and unpublished data}
- Lawson RD, Gentry LO, Bodey GP, Keating MJ, Smith TL. A randomized study of tobramycin plus ticarcillin, tobramycin plus cephalothin and ticarcillin, or tobramycin plus mezlocillin in the treatment of infection in neutropenic patients with malignancies. American Journal of Medical Sciences 1984;287(1):16‐23. [DOI] [PubMed] [Google Scholar]
Marie 1991 {published data only}
- Marie JP, Pico J, Lapierre V, Maulard C, Pappo M, Chiche D, et al. Empirical treatment of febrile episodes in cancer patients with prolonged neutropenia: comparative study of ceftazidime alone, ceftazidime+amikacin, and ceftazidime+vancomycin [Traitement empirique des episodes febriles survenant chez les patients cancereux presentant une neutropenie prolongee: essai comparatif ceftazidime seule, ceftazidime+amikacine et ceftazidime+vancomycine]. Medicine et Maladies Infectieuses (Medicine and Infectious Diseases) 1991;21:386‐8. [Google Scholar]
- Marie JP, Pico JL, Chiche D, Fitoussi F, Delmer A, Baume D, et al. Antibiotic therapy protocol using ceftazidime 3g/day alone or in combination with vancomycin or amikacin. In febrile episodes in neutropenic patients. Presse Medicale (Medical Press) 1988;17(37):1968‐70. [PubMed] [Google Scholar]
- Marie JP, Vekhoff A, Pico JL, Guy H, Andremont A, Richet H. Neutropenic infections: a review of the French Febrile Aplasia Study Group trials in 608 febrile neutropenic patients. Journal of Antimicrobial Chemotherapy 1998;41 Suppl D:57‐64. [DOI] [PubMed] [Google Scholar]
- Pico JL, Marie JP, Chiche D, Guiguet M, Andremont A, Lapierre V, et al. Should vancomycin be used empirically in febrile patients with prolonged and profound neutropenia? Results of a randomized trial. European Journal of Medicine 1993;2(5):275‐80. [PubMed] [Google Scholar]
Menichetti 1986 {published data only}
- Menichetti F, Favero A, Guerciolini R, Tonato M, Aversa F, Roila F, et al. Empiric antimicrobial therapy in febrile granulocytopenic patients. Randomized prospective comparison of amikacin plus piperacillin with or without parenteral trimethoprim/sulphamethoxazole. Infection 1986;14(6):261‐7. [DOI] [PubMed] [Google Scholar]
Molina 1993 {published data only}
- Molina F, Pedro L, Rosell R, Barnadas A, Font A, Maurel J, et al. Randomized open and prospective study of two antibiotic schedules (with and without teicoplanin) for post‐chemotherapy episodes of neutropenic fever. Oncologia: IV Congresso Nacional de la SEOM. 1993; Vol. 16:247.
Novakova 1991 {published and unpublished data}
- Pauw BE, Novakova IR, Donnelly JP. Options and limitations of teicoplanin in febrile granulocytopenic patients. British Journal of Haematology 1990;76(Suppl 2):1‐5. [DOI] [PubMed] [Google Scholar]
- Novakova I, Donnelly JP, Pauw B. Ceftazidime as monotherapy or combined with teicoplanin for initial empiric treatment of presumed bacteremia in febrile granulocytopenic patients. Antimicrobial Agents and Chemotherapy 1991;35(4):672‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novakova I, Donnelly JP, Muytjens HL, Pauw B. Ceftazidime as monotherapy or combined with teicoplanin for initial empiric treatment of presumed bacteremia in febrile granulocytopenic patients. 28th Interscience Conference Antimicrobials Agents and Chemotherapy. American Society of Microbiology, 1988:16. [DOI] [PMC free article] [PubMed]
Ramphal 1992 {published data only}
- Ramphal R, Bolger M, Oblon DJ, Sherertz RJ, Malone JD, Rand KH, et al. Vancomycin is not an essential component of the initial empiric treatment regimen for febrile neutropenic patients receiving ceftazidime: a randomized prospective study. Antimicrobial Agents and Chemotherapy 1992;36(5):1062‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Verhagen 1987 {published and unpublished data}
- Verhagen CS, Pauw B, Witte T, Janssen J, Williams K, Mulder P, et al. Randomized prospective study of ceftazidime versus ceftazidime plus cephalothin in empiric treatment of febrile episodes in severely neutropenic patients. Antimicrobial Agents and Chemotherapy 1987;31(2):191‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Berger 2002 {published data only}
- Berger C, Kindli K, Niggli D, Nadal D. Meropenem therapy in febrile neutropenic children with cancer seldom affords addition of a glycopeptide. 42nd Interscience Conference on Antimicrobial Agents and Chemotherapy. American Society for Microbiology, 2002:251.
De Pauw 1997 {published data only}
- Pauw BE, Raemaekers JMM, Schattenberg T, Donnelly JP. Empirical and subsequent use of antibacterial agents in the febrile neutropenic patient. Journal of Internal Medicine. Supplement 1997;242(740):69‐77. [PubMed] [Google Scholar]
Dompeling 1996 {published data only}
- Dompeling EC, Donnelly JP, Deresinski SC, Feld R, Lane‐Allman EF, Pauw BE. Early identification of neutropenic patients at risk of grampositive bacteraemia and the impact of empirical administration of vancomycin. European Journal of Cancer 1996;32A(8):1332‐9. [DOI] [PubMed] [Google Scholar]
Elting 1996 {published data only}
- Elting LS, Rubenstein EB, Rolston KV, Manzullo E, Bodey GP. Impact of vancomycin (vanc) use on duration of fever and mortality in neutropenic patients with gram positive bacteremia (GPB). Proceedings of the Annual Meeting of the American Society of Clinial Oncology. 1996.
Elting 1997 {published data only}
- Elting LS, Rubenstein EB, Rolston KV, Bodey GP. Outcomes of bacteremia in patients with cancer and neutropenia: observations from two decades of epidemiological and clinical trials. Clinical Infectious Diseases 1997;25:247‐59. [DOI] [PubMed] [Google Scholar]
EORTC 1983 {published data only}
- EORTC‐1983. Combination of amikacin and carbenicillin with or without cefazolin as empirical treatment of febrile neutropenic patients. The International Antimicrobial Therapy Project Group of the European Organization for Research and Treatment of Cancer. Journal of Clinical Oncology 1983;1(10):597‐603. [DOI] [PubMed] [Google Scholar]
Fauser 1991 {published data only}
- Fauser AA, Lang E, Dolken G, Bross KJ, Schmid J, Sorgel F. Treatment of severe sepsis in bone marrow transplant recipients with teicoplanin in combination with beta‐lactams and aminoglycosides. Infection 1991;19:195‐200. [DOI] [PubMed] [Google Scholar]
Granowetter 1988 {published data only}
- Granowetter L, Wells H, Lange BJ. Ceftazidime with or without vancomycin vs. cephalothin, carbenicillin and gentamicin as the initial therapy of the febrile neutropenic pediatric cancer patient. Pediatric Infectious Diseases Journal 1988;7:165‐70. [DOI] [PubMed] [Google Scholar]
Jones 1986 {published data only}
- Jones PG, Rolston KV, Fainstein V, Elting L, Walters RS, Bodey GP. Aztreonam therapy in neutropenic patients with cancer. American Journal of Medicine 1986;81:243‐8. [DOI] [PubMed] [Google Scholar]
Kramer 1986 {published data only}
- Kramer BS, Ramphal R, Rand KH. Randomized comparison between two ceftazidime‐containing regimens and cephalothin‐gentamicin‐carbenicillin in febrile granulocytopenic cancer patients. Antimicrobial Agents and Chemotherapy 1986;30(1):64‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Libanore 1991 {published data only}
- Libanore M, Bicocchi R, Pantaleoni M, Montanari P, Singhinolfi L, Ghinelli F. Teicoplanin in the therapy of Gram‐positive infections in immunocomprised patients [La Teicoplanina nella Terapia di Infezioni da Gram‐positivi nell'ospite immunocompomesso]. Giornale Italiano di Chemioterapia (Italian Journal of Chemotherapy) 1991;38:167‐9. [PubMed] [Google Scholar]
Lim 1990 {published data only}
- Lim S, Smith MP, Goldstone AH, Machin SJ. A randomized prospective study of ceftazidime and ciprofloxacin with or without teicoplanin as an empiric antibiotic regimen for febrile neutropenic patients. British Journal of Haematology 1990;76 Suppl 2:41‐4. [DOI] [PubMed] [Google Scholar]
Link 1994 {published data only}
- Link H, Maschmeyer G, Meyer P, Hiddenmann W, Stille W, Helmerking M, et al. Interventional antimicrobial therapy in febrile neutropenic patients. Annals of Hematology 1994;69:231‐43. [DOI] [PubMed] [Google Scholar]
- Maschmeyer G, Link H, Hiddenmann W, Meyer P, Helmerking M, Adam D. Interventional antimicrobial strategy in febrile neutropenic patients. Results of a multicenter study in 1260 patients with hematological malignancies. The Interventional Antimicrobial Strategy Study Group, Paul Erlich Society for Chemotherapy. Onkologie (Oncology) 1990;13:38‐42. [DOI] [PubMed] [Google Scholar]
Liu 2000 {published data only}
- Liu AJ, Chen SL. Analyze the curative effect of norvancomycin using at 44 patients with agranulocyte after chemotherapy. Contemporary Medicine 2000;6(5):63‐4. [Google Scholar]
Martino 1992 {published data only}
- Martino P, Micozzi A, Gentile G, Raccah R, Girmenia C, Mandelli F. Piperacillin plus amikacin vs. piperacillin plus amikacin plus teicoplanin for empirical treatment of febrile episodes in neutropenic patients receiving quinolone prophylaxis. Clinical Infectious Diseases 1992;15(2):290‐4. [DOI] [PubMed] [Google Scholar]
- Micozzi A, Venditti M, Amadori S, Pulsoni A, Tirindelli C, Martino P. Teicoplanin in the treatment of gram‐positive bacteraemia in neutropenic patients. British Journal of Haematology 1990;76 Suppl 2:19‐23. [DOI] [PubMed] [Google Scholar]
Moroni 1987 {published data only}
- Moroni C, Viscoli C, Boni L, Garaventa A, Dini G, Haupt R, et al. A randomized study of the empirical antibiotic therapy of the infections in neutropenic patients, affected by neoplastic diseases in paediatric age and under a fever attack. Giornale di Malattie Infettive e Parassitarie (Journal of Infectious and Parasitic Dieases) 1987;39:1194‐8. [Google Scholar]
Novakova 1990 {published data only}
- Novakova IR, Donnelly JP, Verhagen CS, Pauw BE. Teicoplanin as modification of initial empirical therapy in febrile granulocytopenic patients. Journal of Antimicrobial Chemotherapy 1990;25(6):985‐93. [DOI] [PubMed] [Google Scholar]
Pizzo 1982 {published data only}
- Pizzo AP, Robichaud KJ, Fred AG, Witebsky FG. Empiric antibiotic and antifungal therapy for cancer patients with prolonged fever and granulocytopenia. American Journal of Medicine 1982;72:101‐11. [DOI] [PubMed] [Google Scholar]
Pizzo 1986 {published data only}
- Pizzo P, Hathorn JW, Hiemenz J, Browne M, Commers J, Cotton D, et al. A randomized trial comparing ceftazidime alone with combination antibiotic therapy in cancer patients with fever and neutropenia. New England Journal of Medicine 1986;315:552‐8. [DOI] [PubMed] [Google Scholar]
Rubin 1988 {published data only}
- Rubin M, Hathorn JW, Marshall D, Gress J, Steinberg SM, Pizzo PA. Gram‐positive infections and the use of vancomycin in 550 episodes of fever and neutropenia. Annals of Internal Medicine 1988;108(1):30‐5. [DOI] [PubMed] [Google Scholar]
Additional references
Akova 2016
- Akova M. Epidemiology of antimicrobial resistance in bloodstream infections. Virulence 2016;7(3):252‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Averbuch 2013
- Averbuch D, Orasch C, Cordonnier C, Livermore DM, Mikulska M, Viscoli C. European guidelines for empirical antibacterial therapy for febrile neutropenic patients in the era of growing resistance: summary of the 2011 4th European Conference on Infections in Leukemia. Haematologica 2013;98(12):1826‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bucaneve 2005
- Bucaneve G, Micozzi A, Menichetti F, Martino P, Dionisi MS, Martinelli G, et al. Levofloxacin to prevent bacterial infection in patients with cancer and neutropenia. New England Journal of Medicine 2005;353:977‐87. [DOI] [PubMed] [Google Scholar]
Cometta 2007
- Cometta A, Marchetti O, Calandra T. Empirical use of anti‐Gram‐positive antibiotics in febrile neutropaenic cancer patients with acute leukaemia. European Journal of Cancer 2007;5 Suppl:23‐31. [Google Scholar]
Consensus 1990
- From the Immunocompromised Host Society. The design, analysis, and reporting of clinical trials on the empirical antibiotic management of the neutropenic patient. Report of a consensus panel. Journal of Infectious Diseases 1990;161(3):397‐401. [DOI] [PubMed] [Google Scholar]
Consort statement
- Consort statement. http://www.consort‐statement.org/.
Del Favero 2001
- Favero A, Menichetti F, Martino P, Bucaneve G, Micozzi A, Gentile G, et al. A multicenter, double‐blind, placebo‐controlled trial comparing piperacillin‐tazobactam with and without amikacin as empiric therapy for febrile neutropenia. Clinical Infectious Diseases 2001;33(8):1295‐301. [DOI] [PubMed] [Google Scholar]
EARS‐NET
- Antibiotic resistance and antibiotic consumption. http://ecdc.europa.eu/EN/HEALTHTOPICS/ANTIMICROBIAL‐RESISTANCE‐AND‐CONSUMPTION/Pages/antimicrobial‐resistance‐and‐anitmicrobial‐consumption.aspx.
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
EORTC 1978
- Schimpff SC, Gaya H, Klastersky J, Tattersall MH, Zinner SH. Three antibiotic regimens in the treatment of infection in febrile granulocytopenic patients with cancer. The EORTC international antimicrobial therapy project group. Journal of Infectious Diseases 1978;137(1):14‐29. [PubMed] [Google Scholar]
EORTC 1986
- Klastersky J, Glauser MP, Schimpff SC, Zinner SH, Gaya H. Prospective randomized comparison of three antibiotic regimens for empirical therapy of suspected bacteremic infection in febrile granulocytopenic patients. Antimicrobial Agents and Chemotherapy 1986;29(2):263‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
EORTC 1987
- The EORTC International Antimicrobial Therapy Cooperative Group. Ceftazidime combined with a short or long course of amikacin for empirical therapy of gram‐negative bacteremia in cancer patients with granulocytopenia. New England Journal of Medicine 1987;317(27):1692‐8. [DOI] [PubMed] [Google Scholar]
EORTC 1993
- The International Antimicrobial Therapy Cooperative Group of the European Organization for Research and Treatment of Cancer. Efficacy and toxicity of single daily doses of amikacin and ceftriaxone versus multiple daily doses of amikacin and ceftazidime for infection in patients with cancer and granulocytopenia. Annals of Internal Medicine 1993;119(7 Pt 1):584‐93. [DOI] [PubMed] [Google Scholar]
EORTC 1995
- Cometta A, Zinner S, Bock R, Calandra T, Gaya H, Klastersky J, et al. Piperacillin‐tazobactam plus amikacin versus ceftazidime plus amikacin as empiric therapy for fever in granulocytopenic patients with cancer. The International Antimicrobial Therapy Cooperative Group of the European Organization for Research and Treatment of Cancer. Antimicrobial Agents and Chemotherapy 1995;39(2):445‐52. [DOI] [PMC free article] [PubMed] [Google Scholar]
EORTC 1996
- Cometta A, Calandra T, Gaya H, Zinner SH, Bock R, Favero A, et al. Monotherapy with meropenem versus combination therapy with ceftazidime plus amikacin as empiric therapy for fever in granulocytopenic patients with cancer. The International Antimicrobial Therapy Cooperative Group of the European Organization for Research and Treatment of Cancer and the Gruppo Italiano Malattie Ematologiche Maligne dell'Adulto Infection Program. Antimicrobial Agents and Chemotherapy 1996;40(5):1108‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Finch 2005
- Finch RG, Eliopoulos GM. Safety and efficacy of glycopeptide antibiotics. Journal of Antimicrobial Chemotherapy 2005;55:5‐13. [DOI] [PubMed] [Google Scholar]
Freifeld 2011
- Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, et al. Infectious Diseases Society of America. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the infectious diseases society of america. Clinical Infectious Diseases 2011;52(5):e56‐93. [DOI] [PubMed] [Google Scholar]
Gafter‐Gvili 2005
- Gafter‐Gvili A, Fraser A, Paul M, Leibovici L. Meta‐analysis: antibiotic prophylaxis reduces mortality in neutropenic patients. Annals of Internal Medicine 2005;142:979‐95. [DOI] [PubMed] [Google Scholar]
Gardete 2014
- Gardete S, Tomasz A. Mechanisms of vancomycin resistance in Staphylococcus aureus. Journal of Clinical Investigation 2014;124(7):2836–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gurwith 1978
- Gurwith M, Brunton JL, Lank B, Ronald AR, Harding GK, McCullough DW. Granulocytopenia in hospitalized patients. II. A prospective comparison of two antibiotic regimens in the empiric therapy of febrile patients. American Journal of Medicine 1978;64(1):127‐32. [DOI] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org. Chichester, UK: John Wiley & Sons, Ltd.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Hughes 1990
- Hughes WT, Armstrong D, Bodey GP, Feld R, Mandell GL, Meyers JD, et al. From the Infectious Diseases Society of America. Guidelines for the use of antimicrobial agents in neutropenic patients with unexplained fever. Journal of Infectious Diseases 1990;161(3):381‐96. [DOI] [PubMed] [Google Scholar]
May 2014
- May L, Klein EY, Rothman RE, Laxminarayan R. Trends in antibiotic resistance in coagulase‐negative staphylococci in the United States, 1999 to 2012. Antimicrobial Agents and Chemotherapy 2014;58(3):1404‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Montassier 2013
- Montassier E, Batard E, Gastinne T, Potel G, Cochetière MF. Recent changes in bacteremia in patients with cancer: a systematic review of epidemiology and antibiotic resistance. European Journal of Clinical Microbiology and Infectious Diseases 2013;32(7):841‐50. [DOI] [PubMed] [Google Scholar]
Montecalvo 1994
- Montecalvo MA, Horowitz H, Gedris C, Carbonaro C, Tenover FC, Issah A, et al. Outbreak of vancomycin‐, ampicillin‐, and aminoglycoside‐resistant Enterococcus faecium bacteremia in an adult oncology unit. Antimicrobial Agents and Chemotherapy 1994;38(6):1363‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nesher 2014
- Nesher L, Rolston KV. The current spectrum of infection in cancer patients with chemotherapy related neutropenia. Infection 2014;42(1):5‐13. [DOI] [PubMed] [Google Scholar]
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(24):2815‐34. [DOI] [PubMed] [Google Scholar]
Penack 2011
- Penack O, Buchheidt D, Christopeit M, Lilienfeld‐Toal M, Massenkeil G, Hentrich M, et al. German Society of Hematology and Oncology. Management of sepsis in neutropenic patients: guidelines from the infectious diseases working party of the German Society of Hematology and Oncology. Annals of Oncology 2011;22(5):1019‐29. [DOI] [PubMed] [Google Scholar]
Press 1984
- Press OW, Ramsey PG, Larson EB, Fefer A, Hickman RO. Hickman catheter infections in patients with malignancies. Medicine (Baltimore) 1984;63(4):189‐200. [DOI] [PubMed] [Google Scholar]
Rosa 2014
- Rosa RG, Dos Santos RP, Goldani LZ. Mortality related to coagulase‐negative staphylococcal bacteremia in febrile neutropenia: A cohort study. Canadian Journl of Infectious Diseases and Medical Microbiology 2014;25(1):e14‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [DOI] [PubMed] [Google Scholar]
Sievert 2002
- Sievert DM, Boulton ML, Stoltman G, Jonhnson D, Stobierski MG, Downes FP, et al. Staphylococcus aureus resistant to vancomycin ‐ United States, 2002. Morbidity and Mortality Weekly Report 2002;51(26):565‐7. [PubMed] [Google Scholar]
Tenover 2001
- Tenover FC, Biddle JW, Lancaster MV. Increasing resistance to vancomycin and other glycopeptides in Staphylococcus aureus. Emerging Infectious Diseases 2001;7(2):327‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yan 2016
- Yan CH, Xu T, Zheng XY, Sun J, Duan XL, Gu JL, et al. Epidemiology of febrile neutropenia in patients with hematological disease‐a prospective multicentre survey in China. Zhonghua Xue Ye Xue Za Zhi 2016;37(3):177‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yapici 2016
- Yapici O, Gunseren F, Yapici H, Merdin A, Yaylali ÜÜ, Merdin FA. Evaluation of febrile neutropenic episodes in adult patients with solid tumors. Molecular and Clinical Oncology 2016;4(3):379‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Paul 2002
- Paul M, Vidal L, Cohen M, Clark O, Soares‐Weiser K, Leibovici L. Additional anti‐Gram‐positive antibiotic treatment for febrile neutropaenic cancer patients. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD003914] [DOI] [PubMed] [Google Scholar]
Paul 2005a
- Paul M, Borok S, Fraser A, Vidal L, Cohen M, Leibovici L. Additional anti‐Gram‐positive antibiotic treatment for febrile neutropenic cancer patients. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD003914.pub2] [DOI] [PubMed] [Google Scholar]
Paul 2005b
- Paul M, Borok S, Fraser A, Vidal L, Leibovici L. Empirical antibiotics against Gram‐positive infections for febrile neutropenia: systematic review and meta‐analysis of randomized controlled trials. Journal of Antimicrobial Chemotherapy 2005;55(4):436‐44. [DOI] [PubMed] [Google Scholar]
Paul 2014
- Paul M, Dickstein Y, Borok S, Vidal L, Leibovici L. Empirical antibiotics targeting Gram‐positive bacteria for the treatment of febrile neutropenic patients with cancer. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD003914.pub3] [DOI] [PubMed] [Google Scholar]


