Abstract
Background
The common cold is a spontaneously remitting infection of the upper respiratory tract, characterised by a runny nose, nasal congestion, sneezing, cough, malaise, sore throat, and fever (usually < 37.8º C). The widespread morbidity caused by the common cold worldwide is related to its ubiquitousness rather than its severity. The development of vaccines for the common cold has been difficult because of antigenic variability of the common cold virus and the indistinguishable multiple other viruses and even bacteria acting as infective agents. There is uncertainty regarding the efficacy and safety of interventions for preventing the common cold in healthy people. This is an update of a Cochrane review first published in 2011 and previously updated in 2013.
Objectives
To assess the clinical effectiveness and safety of vaccines for preventing the common cold in healthy people.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (September 2016), MEDLINE (1948 to September 2016), Embase (1974 to September 2016), CINAHL (1981 to September 2016), and LILACS (1982 to September 2016). We also searched three trials registers for ongoing studies and four websites for additional trials (February 2017). We included no language or date restrictions.
Selection criteria
Randomised controlled trials (RCTs) of any virus vaccines compared with placebo to prevent the common cold in healthy people.
Data collection and analysis
Two review authors independently evaluated methodological quality and extracted trial data. We resolved disagreements by discussion or by consulting a third review author.
Main results
We found no additional RCTs for inclusion in this update. This review includes one RCT dating from the 1960s with an overall high risk of bias. The RCT included 2307 healthy participants, all of whom were included in analyses. This trial compared the effect of an adenovirus vaccine against placebo. No statistically significant difference in common cold incidence was found: there were 13 (1.14%) events in 1139 participants in the vaccines group and 14 (1.19%) events in 1168 participants in the placebo group (risk ratio 0.95, 95% confidence interval 0.45 to 2.02; P = 0.90). No adverse events related to the live vaccine were reported. The quality of the evidence was low due to limitations in methodological quality and a wide 95% confidence interval.
Authors' conclusions
This Cochrane Review was based on one study with low‐quality evidence. We found no conclusive results to support the use of vaccines for preventing the common cold in healthy people compared with placebo. We identified a need for well‐designed, adequately powered RCTs to investigate vaccines for the common cold in healthy people. Any future trials on medical treatments for preventing the common cold should assess a variety of virus vaccines for this condition. Outcome measures should include common cold incidence, vaccine safety, and mortality related to the vaccine.
Plain language summary
Vaccines for preventing the common cold
Review question
We looked at whether vaccines can help to prevent the common cold.
Background
The common cold is caused by viral infection of the upper respiratory tract, and people usually get better when the virus dies. People with common cold feel unwell, have runny noses, nasal congestion, sneezing, and cough with or without sore throat, and slightly elevated temperatures. Treatments are aimed at relieving symptoms.
Globally, the common cold causes widespread illness. It has been difficult to produce vaccines to prevent the common cold due to the many viruses involved. The effect of vaccines on preventing the common cold in healthy people is still unknown.
Search date
For this update we searched the literature up to 2 September 2016.
Study characteristics
We found no new studies in this update. This review includes one previously identified randomised controlled trial performed in 1965. This study involved 2307 healthy people at a training facility for the United States Navy and evaluated the effect of a live weakened (attenuated) adenovirus vaccine compared to a fake vaccine (placebo).
Study funding sources
This study was funded by a government institution.
Key results
There were no differences in the frequency of occurrence of the common cold between those who received the vaccine compared to those who received a fake vaccine. There were no adverse events related to the vaccine. However, due to the low numbers of people included in the study and numbers of colds, as well as flaws in the study design, our confidence in the results is low. Further research may be able to clarify if vaccines can prevent common cold, since the current evidence does not support the use of adenovirus vaccine to prevent common cold in healthy people.
Quality of the evidence
We assessed the quality of the evidence as low due to high risk of bias and low numbers of people included in the study and numbers of colds, which resulted in imprecision.
Summary of findings
Summary of findings for the main comparison. Virus vaccines compared to placebo for preventing the common cold in healthy people.
| Virus vaccines compared to placebo for preventing the common cold in healthy people | ||||||
| Patient or population: healthy people Settings: outpatients at Great Lakes Naval Training Center Intervention: virus vaccines for preventing the common cold¹ Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Virus vaccines for preventing the common cold | |||||
| Incidence of the common cold Number of participants with common cold by group Follow‐up: mean 9 weeks | Study population | RR 0.95 (0.45 to 2.02) | 2307 (1 study)² | ⊕⊕⊝⊝ low³ ⁴ | ||
| 12 per 1000 | 11 per 1000 (5 to 24) | |||||
| Vaccine safety | The study stated that there were no adverse events related to the vaccine. | 2307 (1 study)² | ⊕⊕⊝⊝ low³ ⁵ | |||
| Mortality related to the vaccine ‐ not reported | See comments | See comments | See comments | See comments | See comments | The included study did not report this outcome. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence. High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Adenovirus vaccine used for preventing the common cold. 2Griffin 1970. 3Downgraded one level due to high risk of bias for this outcome. 4Downgraded one level due to imprecision: few events (N = 27) and wide 95% confidence interval. 5Downgraded one level due to imprecision: zero events reported in a narrative fashion.
Background
Description of the condition
There are no standardised definitions for a common cold (see Appendix 1). The common cold is a spontaneously remitting infection of the upper respiratory tract, characterised by a runny nose, nasal congestion, and sneezing, and sometimes cough, malaise, sore throat, and fever (usually < 100º F). A temperature of 100º F (37.8º C) or higher for three to four days is typically associated with influenza and other respiratory diseases (Appendix 2) (DDCP 2010; Heikkinen 2003). While benign in nature, the common cold is the most frequent illness experienced in humans. Children experience six to 11 upper respiratory tract infections per year (Evans 1997; Leder 2003; Nelson 2000), and adults experience two to four episodes per year (Evans 1997; Grüber 2008; Harrison 2008). Because it causes frequent absences from school and work, the common cold has become a significant economic burden (Glezen 2000; Hall 2001; Henrickson 1994; Henrickson 2003); the cost in the United States is estimated at more than USD 60 billion each year (Poland 2009). Furthermore, bacterial complications can lead to morbidity and mortality (Thompson 2003; Wat 2004).
The aetiology of the common cold is diverse (Appendix 3) (Heikkinen 2003). Children, the elderly, and other age groups with comorbidities such as prematurity, chronic lung diseases (chronic obstructive pulmonary disease), congenital heart disease, and asthma are more prone to viral infections that cause the common cold, such as respiratory syncytial virus (RSV), rhinovirus, parainfluenza, coronavirus, and adenovirus (non‐polio) (Edlmayr 2009; Jackson 2008; Krasinski 1985; Peltola 2008). Human rhinovirus (HRV) is responsible for 50% to 80% of common colds and is an important cause of morbidity, reduced productivity, and inappropriate use of antibiotics and over‐the‐counter medications. In humans, the coronavirus (HCoV 229E) causes the common cold by infecting the upper respiratory tract. This is mainly encountered in children, and re‐infection occurs in adults (Eriksson 2006). The primary factors that contribute to the spread of this disease are poor hand hygiene, overcrowding, and captive populations (schools and daycare centres) (Harrison 2008).
Description of the intervention
Treatment of the common cold is symptomatic. Studies have shown that simple preventive measures are important but may be difficult to enforce practically (Jefferson 2011). Another method of prevention could be vaccination.
The development of vaccines for the common cold has been challenging because of multiple aetiologies, Poland 2009, and antigenic variability of the common cold viruses (Bembridge 1998; Hussell 1998). In the case of rhinovirus, there are over 167 different rhinoviral serotypes (Ren 2017). For this reason, it is difficult to create a vaccine that can give total protection. However, the future for vaccines for the common cold looks promising, considering the current knowledge of the full genomes of HRV serotypes (Palmemberg 2009). Immune responses are triggered whenever a person is infected with the same virus but with different antigenic molecules (Tobin 2008).
One of the most common causes of respiratory diseases are rhinoviruses. A recombinant vaccine has been reported, produced with rhinovirus‐derived VP1, a surface protein that is critically involved in the infection of respiratory cells, and a non‐allergenic peptide of the major grass pollen type allergen Ph1 p1 (Edlmayr 2009).
Adenovirus is a commonly recognised pathogen of the upper respiratory tract and has been particularly common in captive populations (Binn 2007). Adenovirus serotype 4 (Ad4) and serotype 7 vaccines were used during immunisation programmes starting in 1971. Unfortunately, their interruption triggered the re‐emergence of adenovirus‐produced diseases in crowded locations. An example of this reappearance was documented in United States military training sites in 1999, where Ad4 accounted for 98% of all diagnoses (Russell 2006).
Epidemiological and clinical studies have revealed important changes with regard to clinical adenovirus infection, including alterations in its antigenic presentation, geographical distribution, and virulence (Gray 2007). Adenoviral vaccines delivered orally have been used for decades to prevent respiratory illnesses. New studies have concluded that these vaccines are safe and have brought about a good immune response in the studied populations.
Respiratory syncytial virus causes approximately 5% of common colds in adults (Heikkinen 2003). The vaccine development for RSV has had some problems due to antigenic variability, especially in proteins F and G. People who were vaccinated with the formalin‐inactivated vaccine displayed X‐ray evidence of severe pneumonia and bronchiolitis due to pulmonary Arthus reaction and a process of immunopotentiation. This process was induced by a T helper (Th)‐2 and Th17 T cell responses with the enrolment of T cells, neutrophils, and eosinophils causing inflammation and tissue damage (Rey‐Jurado 2017). Where an effective vaccine can be offered, it should be administered to children younger than six months of age, when immune systems are still immature. For this reason another approach is the development of a vaccine for maternal immunisation as it has been demonstrated that RSV‐neutralising antibodies are transferred efficiently through the placenta from the pregnant woman to the newborn (Munoz 2003). Furthermore, a phase II clinical trial has found that a recombinant F nanoparticle vaccine formulation reduces the incidence of RSV infection when compared to placebo (the incidence was 11% versus 21%, respectively) in healthy women of childbearing age (Glenn 2016).
Vaccines for parainfluenza (HPIV3 cp45) are safe and immunogenic in seronegative children aged between six and 18 months (Belshe 2004a). The vaccine has also demonstrated less risk of transmission than others (wt HPIV3), making it possible to develop more randomised trials. Bovine parainfluenza virus vaccines are also being developed, which have been well‐tolerated, effective, and immunogenic in infants (Belshe 2004b).
How the intervention might work
Almost all vaccines work by inducing antibodies in the serum to interfere with microbial invasion of the bloodstream, or in the mucosa, and to block adherence of pathogens to epithelial cells (Pichichero 2009). To protect the body, antibodies must be efficient, neutralising agents or have opsonisation and phagocytosis properties. Correlates of protection after vaccination are sometimes absolute quantities, but are often relative. Most infections are prevented at a particular response level, but some could occur above that level because of a large challenge dose or deficient host factors. There may be more than one correlate of protection for a disease; authors refer to these as "co‐correlates". Either the effector or central memory may co‐correlate with protection. Cell‐mediated immunity may also operate as a correlate or co‐correlate of protection against disease, rather than against infection (Plotkin 2008). Some studies suggest that vaccines that mimic natural infection and take into account the structure of pathogens seem to be effective in inducing long‐term protective immunity (Kang 2009).
Why it is important to do this review
Common cold vaccines would reduce the prevalence of this disease in more than 25 million people with upper respiratory tract infections each year (Gonzales 2001).
The common cold results in an important economic burden with over 189 million missed school days, Roxas 2007, and 8 million to 20 million days of restricted activity (Adams 1999).
If randomised controlled trials demonstrate that there is a vaccine providing efficacy and safety to prevent the common cold, scientists could continue research in this area.
Objectives
To assess the clinical effectiveness and safety of vaccines for preventing the common cold in healthy people.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs). We did not apply limits with respect to follow‐up periods.
Types of participants
Healthy people aged between 6 months and 90 years.
Types of interventions
Any vaccine that prevents the common cold, which protects against RSV, rhinovirus, parainfluenza, or adenovirus (non‐polio), irrespective of dose, schedule, or administration route, versus placebo. We excluded trials on the prevention of influenza A and B because influenza and the common cold are two different diseases (Jefferson 2012). See Appendix 3 for details.
Types of outcome measures
Primary outcomes
Incidence of the common cold after vaccination, regardless of the causal agent determined by laboratory or clinical examination.
Vaccine safety, i.e. adverse events ("any untoward medical occurrence that may present during treatment with a pharmaceutical product but which does not necessarily have a causal relationship with this treatment") (Nebeker 2004); and adverse drug reactions ("a response to a drug which is noxious, uninitiated and which occurs at doses normally used in men for prophylaxis, diagnosis, or therapy of disease, or for the modification of physiologic functions") (Nebeker 2004).
Mortality related to the vaccine.
Secondary outcomes
We did not consider secondary outcomes.
Search methods for identification of studies
Electronic searches
We searched the following databases up to 2 September 2016:
CENTRAL (the Cochrane Central Register of Controlled Trials), which contains the Cochrane Acute Respiratory Infections Specialised Register (CENTRAL; August 2016, Issue 8), in the Cochrane Library (searched 2 September); using the strategy in Appendix 4;
MEDLINE via Ovid (from 1948 to 2 September 2016) using the strategy in Appendix 5;
Embase via Elsevier (from 1974 to 2 September 2016) using the strategy in Appendix 6;
CINAHL via EBSCO (from 1981 to 2 September 2016) using the strategy in Appendix 7; and
LILACS via BIREME (from 1982 to 2 September 2016) using the strategy in Appendix 8.
We used the Cochrane Highly Sensitive Search Strategy to identify randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision); Ovid format (Lefebvre 2011). We adapted the search strategy to search Embase, CINAHL, and LILACS.
We searched the following trial registries on 2 February 2017:
ISRCTN registry (www.isrctn.com);
ClinicalTrials.gov (clinicaltrials.gov/); and
World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (www.who.int/ictrp/search/en/).
We did not restrict the results by language, dates, or publication status (published, unpublished, in press, or in progress).
Searching other resources
We checked the reference lists of all relevant trials and identified reviews. We searched the following websites for trials on 2 February 2017:
US Food and Drug Administration (www.fda.gov);
European Medicines Agency (www.emea.europa.eu);
Medicines & Healthcare Products Regulatory Agency (www.mhra.gov.uk/index.htm);
Evidence in Health and Social Care (www.evidence.nhs.uk/).
Data collection and analysis
Selection of studies
Two review authors (MJMZ, JVAF) independently screened the titles and abstracts of studies identified as a result of the search for possible inclusion in the review. We retrieved full‐text reports of potentially relevant studies. Two review authors (MJMZ, JVAF) independently screened the full texts to identify studies for inclusion and identified and recorded reasons for exclusion of the ineligible studies. Any disagreements were resolved through discussion or by consulting a third review author (DSR) when needed. We identified and excluded duplicates and collated multiple reports of the same study so that each study, rather than each report, was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram and Characteristics of included studies table (Moher 2009). We imposed no language restrictions.
Data extraction and management
We used a data collection form for study characteristics and outcome data that we had piloted on at least one study in the review. Two review authors (DSR, CVG) extracted study characteristics from the included studies. We extracted the following study characteristics.
Methods: study design, total duration of study, details of any 'run in' period, number of study centres and location, study setting, withdrawals, and date of study.
Participants: N, mean age, age range, gender, severity of condition, diagnostic criteria, baseline lung function, smoking history, inclusion criteria, and exclusion criteria.
Interventions: intervention, comparison, concomitant medications, and excluded medications.
Outcomes: primary and secondary outcomes specified and collected, and time points reported.
Notes: funding for trial, and notable conflicts of interest of trial authors.
Two review authors (DSR, CVG) independently extracted outcome data from the included studies. We had planned to note in the Characteristics of included studies table if outcome data were not reported in a usable way. We had planned to resolve disagreements by consensus or by involving a third review author (RH). One review author (DSR) transferred data into the Review Manager 5 file (RevMan 2014). We double‐checked that the data were entered correctly by comparing the data presented in the systematic review with the study report. A second review author (MJMZ) spot‐checked study characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
Three review authors (DSR, CVG, RH) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreements by discussion or by involving another review author (MJMZ). We assessed the risk of bias according to the following domains.
Random sequence generation.
Allocation concealment.
Blinding of participants and personnel.
Blinding of outcome assessment.
Incomplete outcome data.
Selective outcome reporting.
Other bias.
We graded each potential source of bias as high, low, or unclear and provided quotes from the study report together with a justification for our judgement in the 'Risk of bias' table. Where necessary, we considered blinding separately for different key outcomes.
Measures of treatment effect
We calculated the risk ratio (RR) with 95% confidence intervals (CIs) for incidence of the common cold.
We entered outcome data for the study into a data table in Review Manager 5 to calculate the treatment effects (RevMan 2014). We used RR for dichotomous outcomes.
Unit of analysis issues
The unit of analysis was the participant. We collected and analysed a single measurement for each outcome from each participant.
Dealing with missing data
We had planned to contact investigators or study sponsors to verify key study characteristics and to obtain missing numerical outcome data where possible (e.g. when a study was identified only as an abstract). Where this was not possible, and the missing data were thought to introduce serious bias, we planned to explore the impact of including such studies in the overall assessment of results by a sensitivity analysis.
If numerical outcome data were missing, such as standard deviations or correlation coefficients, and could not be obtained from the authors, we planned to calculate them from other available statistics such as P values, according to the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
However, we did not apply these approaches because this update included only one study.
Assessment of heterogeneity
We had planned use the I² statistic to measure heterogeneity among the trials in each analysis; however, this update did not include meta‐analysis. If in future updates we identify substantial heterogeneity, we will report this and explore possible causes by prespecified subgroup analysis.
Assessment of reporting biases
We did not assess publication bias using a funnel plot because we included only one trial. For future updates, we will attempt to assess whether the review is subject to publication bias by using a funnel plot if 10 or more trials are included.
Data synthesis
We carried out statistical analysis using Review Manager 5 software (RevMan 2014). For future updates, we will summarise findings using a fixed‐effect model according the Cochrane Handbook of Systematic Reviews for Interventions (Higgins 2011).
GRADE and 'Summary of findings' tables
We created a 'Summary of findings' table using the following outcomes: incidence of the common cold, vaccine safety, and mortality. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of the evidence as it relates to the study that contributed data (Atkins 2004). We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), using GRADEpro GDT software (GRADEpro GDT 2014). We justified all decisions to down‐ or upgrade study quality using footnotes, and made comments to aid the reader's understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
In subsequent updates of this review, when sufficient data are available, we plan to carry out the following subgroup analyses:
children and adults;
country of study; and
different responses in relation to different viral agents.
We will explore sources of heterogeneity in the assessment of the primary outcome measure by subgroup analyses and meta‐regression analyses. The meta‐regression analyses will assess the effect of methodological quality (high versus low), type of virus vaccines, and participant characteristics. We will only conduct meta‐regression if 10 or more RCTs are included.
Sensitivity analysis
For future updates, we plan to conduct sensitivity analyses comparing the results using all trials as follows.
Trials with high methodological quality (studies classified as having a 'low risk of bias' versus those identified as having a 'high risk of bias') (Higgins 2011).
Trials that performed intention‐to‐treat versus per‐protocol analyses.
We will also evaluate the risk of attrition bias, as estimated by the percentage of participants lost. We will exclude trials with a total attrition of more than 30% or where differences between the groups exceeded 10%, or both, from meta‐analysis, but will include them in the review.
Results
Description of studies
Results of the search
For this 2016 update we assessed 221 results from our electronic searches (Figure 1). We excluded 218 records based on assessment of title and abstract. We obtained three full‐text reports, which we excluded after full‐text assessment. Consequently, we did not include any new studies for this update. Only one study was included (Griffin 1970).
1.
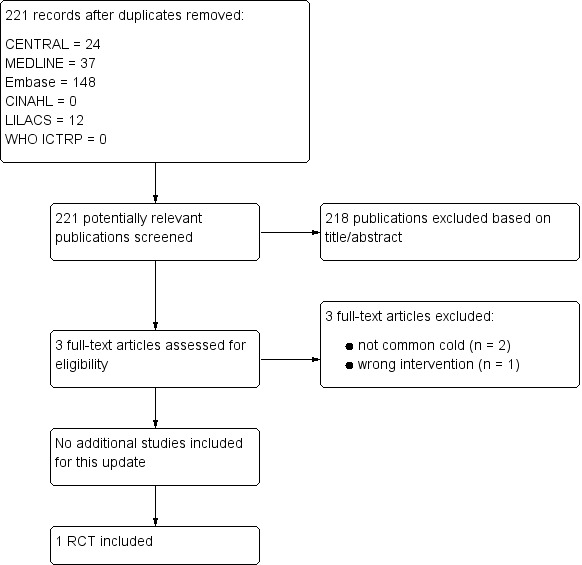
Study flow diagram
Included studies
This review included one RCT involving 2307 healthy people (Griffin 1970). See the Characteristics of included studies table.
Excluded studies
In the previous review we excluded 41 studies (Belshe 1982; Belshe 1992; Belshe 2004a; Belshe 2004b; Clements 1991; DeVincenzo 2010; Doggett 1963; Dudding 1972; Falsey 1996; Falsey 2008; Fulginiti 1969; Gomez 2009; Gonzalez 2000; Greenberg 2005; Hamory 1975; Karron 1995a; Karron 1995b; Karron 1997; Karron 2003; Karron 2005; Langley 2009; Lee 2001; Lee 2004; Lin 2007; Lyons 2008; Madhi 2006; Munoz 2003; Murphy 1994; Paradiso 1994; Piedra 1995; Pierce 1968; Power 2001; Ritchie 1958; Simoes 2001; Tang 2008; Top 1971; Tristram 1993; Watt 1990; Welliver 1994; Wilson 1960; Wright 1976). In this update, we excluded three new studies: two did not evaluate the common cold as an outcome (Glenn 2016; Karron 2015), and the third study involved an intervention that was not relevant to this review (probiotics) (Kumpu 2015).
See the Characteristics of excluded studies tables.
Risk of bias in included studies
Griffin 1970 had overall low methodological quality. See Figure 2 and Figure 3.
2.
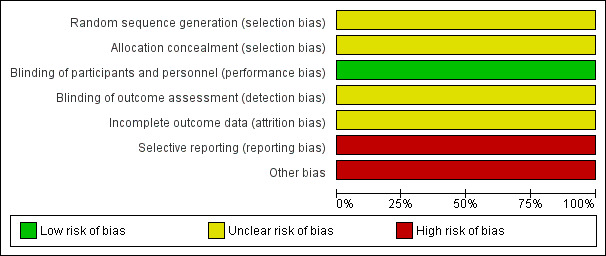
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages
3.
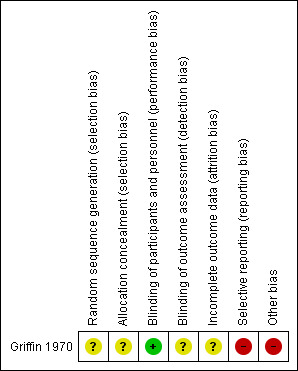
'Risk of bias' summary: review authors' judgements about each risk of bias item for the included study
Allocation
We assessed Griffin 1970 as at unclear risk of bias for random sequence generation and allocation concealment since the information provided was inadequate for judgement of this domain.
Blinding
We assessed Griffin 1970 as at low risk of bias for blinding of participants and personnel, but unclear for outcome assessor. Although a placebo was used, there could be a risk of detection bias because common cold does not require hospitalisation.
Incomplete outcome data
We assessed Griffin 1970 as at unclear risk of attrition bias because the information provided was insufficient to enable assessment of this domain.
Selective reporting
Griffin 1970 was at high risk of bias for selective reporting. The study protocol was not available, but it was clear that the published reports include all expected outcomes. However, some of the outcomes were described in a narrative fashion and did not specify incidence for each group.
Other potential sources of bias
Griffin 1970 had a high risk for other sources of bias. Participants' base characteristics were not described, and because there was no detailed information relating to assessment of selection bias, information was insufficient to evaluate if both groups were comparable.
Effects of interventions
See: Table 1
Results were based on one RCT (Griffin 1970, N = 2307 healthy people), which we assessed as providing low‐quality evidence. See Table 1.
Primary outcomes
1. Incidence of the common cold
Griffin 1970 (2307 participants, 27 events) showed that adenovirus vaccine was associated with a non‐statistically significant reduction in the incidence of the common cold compared with placebo (RR 0.95, 95% CI 0.45 to 2.02; P = 0.90; Analysis 1.1). We downgraded the quality of the evidence to low due to high risk of bias and imprecision; there were few events in each group, resulting in imprecision represented by a wide 95% confidence interval.
1.1. Analysis.
Comparison 1 Adenovirus vaccines versus placebo, Outcome 1 Incidence of the common cold.
2. Vaccine safety
Griffin 1970 reported that there were no adverse events related to the live vaccine preparation. We downgraded the quality of the evidence to low due to high risk of bias and imprecision (0 events).
3. Mortality related to vaccine
Griffin 1970 did not assess this outcome.
Discussion
Summary of main results
We included one RCT that met our inclusion criteria. Critical appraisal of Griffin 1970 did not support the use of any virus vaccines for preventing the common cold in healthy people. We did not find significant differences in the incidence of the common cold in people treated with adenovirus vaccines compared with placebo. Griffin 1970 did not evaluate main clinical outcomes such as mortality related to the vaccine. This RCT reported that there were no adverse events related to the vaccine. The relative effect of any of the vaccines for viruses that cause the common cold remains unclear.
Overall completeness and applicability of evidence
The included trial did not detect statistically significant differences between the treatment groups (Griffin 1970).
When dealing with such neutral results, we need to keep in mind that 'absence of evidence' is not 'evidence of absence' (Altman 1995; Fermi Paradox 2012). The fact that this review did not detect any differences between the intervention groups does not imply that placebo and adenovirus vaccine have the same effect on preventing the common cold. The first possible explanation is failure to determine an appropriate sample size (Green 2002; Schulz 1995), in this case resulting in small differences in the incidence of the common cold and few events in the comparison groups. In a remarkable paper from 28 years ago, Freiman 1978 suggested that "many of the therapies labelled as 'no different from control' in trials using inadequate samples, have not received a fair test" and that "concern for the probability of missing an important therapeutic improvement because of small sample sizes deserves more attention in the planning of clinical trials". Moher 1998 emphasised that "most trials with negative results did not have large enough sample sizes to detect a 25% or a 50% relative difference". Moreover, it has been suggested that the most important therapies adopted in clinical practice have shown more modest benefits (Kirby 2002).
Quality of the evidence
The results for the primary outcomes 'incidence of the common cold' and 'vaccine safety' were based on low‐quality evidence due to imprecision (low number of events and wide confidence intervals) and methodological limitations. The random sequence generation, allocation, sample size, and base characteristics of participants were not reported. Furthermore, the study may be at high risk of detection bias, since the common cold syndrome rarely requires hospitalisation, and the effect of the intervention could not be adequately evaluated. The report of adverse events was not individualised for each group.
Potential biases in the review process
In the process of performing a systematic review, there is a group of biases known as 'significance‐chasing' (Ioannidis 2010). This group includes publication bias, selective outcome reporting bias, selective analysis reporting bias, and fabrication bias (Ioannidis 2010). Publication bias represents a major threat to the validity of systematic reviews, particularly in reviews that include small trials. However, we did an exhaustive search that included many RCTs that did not evaluate common cold outcomes.
Agreements and disagreements with other studies or reviews
We found no other reviews or studies that investigated vaccines for the common cold. We excluded 11 non‐RCTs that evaluated vaccines for upper respiratory tract infections (Belshe 1982; Clements 1991; Doggett 1963; Dudding 1972; Fulginiti 1969; Hamory 1975; Karron 1997; Ritchie 1958; Watt 1990; Wilson 1960; Wright 1976). However, only one study evaluated the incidence of common cold (Ritchie 1958), while the others focused on immunologic outcomes. Ritchie 1958 prepared an "autologous vaccine" developed from the nasal secretions of 125 healthy volunteers, who were then inoculated with this product, while 75 served as a control. The results showed a lower incidence of common cold in the vaccine group than in the control group.
Authors' conclusions
Implications for practice.
This Cochrane Review update found very limited evidence on the effects of vaccines for the common cold in healthy people. We included only one randomised controlled trial, which did not report differences between comparison groups. Review findings were based on only one trial assessed as providing low‐quality evidence at high risk of bias and with imprecise estimates. Griffin 1970 involved 2307 participants and assessed adenovirus vaccine compared with placebo.
We found insufficient evidence to support the use of vaccines for the common cold. Prescription of virus vaccines for preventing the common cold in healthy people can neither be supported nor rejected, unless new evidence from large, high‐quality trials alters this conclusion. This Cochrane Review does not provide evidence about other virus vaccines for preventing the common cold in healthy people.
Implications for research.
This Cochrane Review update highlights the need for well‐designed, high‐quality randomised trials to assess the effectiveness and safety of virus vaccines to prevent the common cold in healthy people. Future trials should include outcomes such as common cold incidence, vaccine safety, mortality related to the vaccine, and adverse events related to vaccine administration. Future trials should be conducted by independent researchers and reported according to CONSORT guidelines (Ioannidis 2004; Moher 2010), and using the Foundation of Patient‐Centered Outcomes Research recommendations (Anonymous 2012; Gabriel 2012).
What's new
| Date | Event | Description |
|---|---|---|
| 2 September 2016 | New citation required but conclusions have not changed | We recruited three new authors to update this review. |
| 2 September 2016 | New search has been performed | We updated our searches and excluded three new trials (Glenn 2016; Karron 2015; Kumpu 2015). |
History
Protocol first published: Issue 3, 2000 Review first published: Issue 6, 2013
| Date | Event | Description |
|---|---|---|
| 22 January 2015 | New search has been performed | Searches conducted |
| 16 March 2011 | New citation required and major changes | Protocol taken over by a new team of review authors |
| 26 February 2009 | Amended | Protocol withdrawn Issue 3, 2009 |
Acknowledgements
The authors wish to thank Tom Jefferson and David Tyrell, who coauthored the first published version of this review; Anne Lyddiatt, Gulam Khandaker, Lisa Jackson, Mark Griffin, and Meenu Singh for commenting on the draft protocol; and Theresa Wrangham, John Jordan, Viviana Rodriguez, and Meenu Singh for commenting on the first draft review. The authors wish to express their thanks to Liz Dooley, Managing Editor of the Cochrane Acute Respiratory Infections Group, for her comments, which improved the quality of this review.
Daniel Simancas‐Racines is a PhD candidate at the Department of Pediatrics, Gynecology and Obstetrics, and Preventive Medicine, Universitat Autònoma de Barcelona, Spain.
Appendices
Appendix 1. Glossary
| Term | Definition | Reference |
| Common cold | The common cold is a self limiting acute upper respiratory tract infection, characterised by rhinorrhoea, nasal congestion, sneezing, cough, sore throat, fever, and malaise. | Heikkinen 2003 |
| Vaccination | Inoculation with a vaccine, i.e. a preparation of microbial antigen often combined with adjuvants administered to an individual in order to induce protective immunity against microbial infections. The antigen may be in the form of live, avirulent micro‐organisms or purified macromolecular components of micro‐organisms. | Abbas 2001 |
| Immune system | The collection of cells, tissues, and molecules that mediate resistance to infections | Abbas 2001 |
| Cell‐mediated immunity | The arm of the adaptative immune response whose role is to combat infections by intracellular microbes. This type of immunity is mediated by T lymphocytes. | Abbas 2001 |
| Antigenical variability | Microbes have evolved mechanisms to evade immunity. Many bacteria and viruses mutate their antigenic surface molecules and can no longer be recognised by antibodies produced in response to previous infection. | Abbas 2001 |
| Serotypes | An antigenically distinct subset of a species of an infectious organism that is distinguished from other subsets by serologic (i.e. serum antibody) tests. Humoral immune response to one serotype of microbes, e.g. influenza virus, may not be protective against another serotypes. | Abbas 2001 |
| Immune responses | Once a foreign organism has been recognised, the immune system enlists the participation of a variety of cells and molecules to mount an appropriate response in order to eliminate or neutralise the organism. | Goldsby 2000 |
| Antigenic molecules | Any molecule capable of being recognised by an antibody or T‐cell receptor. Any substance that elicits an immune response. | Goldsby 2000; Roitt 2004 |
| Allergens | An antigen that elicits an immediate hypersensitivity (allergic) reaction. Allergens are proteins, or chemicals bound to proteins, that induce immunoglobulin E antibody production in atopic individuals. | Abbas 2001 |
| Immunopotentiation | Non‐specific immunostimulation given by various agents that can stimulate the immune response. It is believed that the mechanism of action is through some modification of local cytokines or growth of innate immune mechanisms. An increase in the functional capacity of the immune response |
Gorczynski 2007 |
| Opsonisation | The process by which particulate antigens are rendered more susceptible to phagocytosis The process of attaching opsonins, such as immunoglobulin G or complement fragments, to microbial surfaces to target microbes for phagocytosis |
Abbas 2001; Goldsby 2000 |
| Phagocytosis | Macrophages are capable of ingesting and digesting exogenous antigens, such as whole micro‐organisms and insoluble particles, and endogenous matter, such as injured or dead host cells, cellular debris, and activated clotting factors. The process by which certain cells of the innate immune system, including macrophages and neutrophils, engulf large particles (> 0.5 µm diameter), such as intact microbes. The cell surrounds the particle by a cytoskeleton‐dependent process, leading to formation of an intracellular vesicle called a phagosome, which contains the ingested particle. |
Abbas 2001; Goldsby 2000 |
Appendix 2. Differences between clinical characteristics of the common cold and influenza
| Feature | Common cold | Influenza | References |
| Aetiological agent | > 100 viral strains; rhinovirus most common | 3 strains of influenza virus: influenza A, B, C |
DDCP 2010; Gwaltney 1967; Gwaltney 2000; Heikkinen 2003; Roxas 2007; Thompson 2003 |
| Site of infection | Upper respiratory tract | Entire respiratory system | |
| Symptom onset | Gradual: 1 to 3 days | Sudden: within a few hours | |
| Fever, chills | Occasional, low grade (< 100º F) | Fever is usually present with the flu, in up to 80% of all flu cases. A temperature of 100º F or higher for 3 to 4 days is typically associated with the flu. | |
| Headache | Frequent, usually mild | Characteristic, more severe | |
| General aches, pains | Mild, if any | Characteristic, often severe and affecting the entire body | |
| Cough, chest congestion | Mild to moderate, with hacking cough | Common, may become severe | |
| Sore throat | Common, usually mild | Sometimes present | |
| Runny, stuffy nose | Very common, accompanied by bouts of sneezing | Sometimes present | |
| Fatigue, weakness | Mild, if any | Usual, may be severe and last 2 to 3 weeks | |
| Extreme exhaustion | Never | Frequent, usually in early stages of illness | |
| Season | Year around, peaks in winter months | Most cases between November and February | |
| Antibiotics helpful | No, unless secondary bacterial infection develops | No, unless secondary bacterial infection develops |
Appendix 3. Viral causes of the common cold
| Virus | Estimated annual proportion of cases | References |
| Rhinoviruses | 30% to 50%; during autumn 80%. Once considered to be limited to the upper airway, now recognised as an important cause of lower respiratory infections | Arruda 1997; Gwaltney 1985; Heikkinen 2003; Lemanske 2005; Monto 1993; Mäkelä 1998; Regamey 2008 |
| Coronaviruses | 7% to 18% in adults with upper respiratory infections. Responsible for 2.1% of hospital admissions for acute respiratory tract infections in all age groups | Larson 1980; Lau 2006; Mäkelä 1998; Nicholson 1997 |
| Influenza viruses | 5% to 15% | Heikkinen 2003 |
| Respiratory syncytial virus (RSV) | In low‐income countries, 15% to 20% In hospital the proportion of children aged between birth and 5 months with RSV acute lower respiratory tract infections ranged between 9% and 87%. Among children up to at least 5 years of age reported with RSV, on average 39% (range 20% to 62%) were < 6 months old; on average 24% of cases (range 14% to 38%) were children aged 6 to 11 months. An average of 63% of children were thus under 1 year of age. On average 20% (range 13% to 29%) of the children were between 1 and 2 years of age. Respiratory syncytial virus accounts for approximately 10,000 deaths annually in people over the age of 65 years in the USA. Respiratory syncytial virus in adults, 5% infection annually |
Berman 1991; Falsey 2005; Thompson 2003 |
| Parainfluenza viruses | Acute respiratory infections cause 3% to 18% of all admissions to paediatric hospitals; 9% to 30% of these patients depending on the time of year. Parainfluenza viruses account for 17% of hospitalised illness‐associated virus isolation. In low‐income countries 7% to 10% This virus causes 50% to 74.2% of croup cases. |
Berman 1991; Denny 1983; Henrickson 2003 |
| Adenoviruses | In low‐income countries can be summarised as 2% to 4% | Berman 1991 |
| Metapneumovirus | 10% short epidemic | Esper 2003; Kahn 2003; Nissen 2002; Risnes 2005 |
| Unknown | 20% to 30% | Monto 1993; Mäkelä 1998 |
Appendix 4. CENTRAL search strategy
#1 [mh "Common Cold"] #2 "common cold*":ti,ab #3 "coryza":ti,ab #4 (acute near/5 ("upper respiratory infection*" or "upper respiratory tract infection*" or urti or uri)):ti,ab #5 [mh "Picornaviridae Infections"] #6 [mh Rhinovirus] #7 "rhinovir*":ti,ab #8 "hrv":ti,ab #9 [mh "Paramyxoviridae Infections"] #10 [mh "parainfluenza virus 1, human"] or [mh "parainfluenza virus 3, human"] #11 [mh "parainfluenza virus 2, human"] or [mh "parainfluenza virus 4, human"] #12 "parainfluenza*":ti,ab #13 [mh coronavirus] or [mh "coronavirus 229e, human"] or [mh "coronavirus oc43, human"] #14 [mh "Coronavirus Infections"] #15 "coronavir*":ti,ab #16 [mh adenoviridae] or [mh "adenoviruses, human"] #17 [mh "Adenovirus Infections, Human"] #18 "adenovir*":ti,ab #19 [mh "respiratory syncytial viruses"] or [mh "respiratory syncytial virus, human"] #20 [mh "Respiratory Syncytial Virus Infections"] #21 ("respiratory syncytial virus*" or rsv):ti,ab #22 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 #23 [mh Vaccines] #24 [mh Vaccination] #25 (vaccin* or inocul* or immuni*):ti,ab #26 #23 or #24 or #25 #27 #22 and #26
Appendix 5. MEDLINE (Ovid) search strategy
1 Common Cold/ 2 common cold*.tw. 3 coryza.tw. 4 (acute adj5 (upper respiratory infection* or upper respiratory tract infection* or urti or uri)).tw. 5 Picornaviridae Infections/ 6 Rhinovirus/ 7 rhinovir*.tw. 8 hrv.tw. 9 Paramyxoviridae Infections/ 10 parainfluenza virus 1, human/ or parainfluenza virus 3, human/ 11 parainfluenza virus 2, human/ or parainfluenza virus 4, human/ 12 parainfluenza*.tw. 13 coronavirus/ or coronavirus 229e, human/ or coronavirus oc43, human/ 14 Coronavirus Infections/ 15 coronavir*.tw. 16 exp adenoviridae/ or adenoviruses, human/ 17 Adenovirus Infections, Human/ 18 adenovir*.tw. 19 respiratory syncytial viruses/ or respiratory syncytial virus, human/ 20 Respiratory Syncytial Virus Infections/ 21 (respiratory syncytial virus* or rsv).tw. 22 or/1‐21 23 exp Vaccines/ 24 exp Vaccination/ 25 (vaccin* or inocul* or immuni*).tw. 26 or/23‐25 27 22 and 26
Appendix 6. Embase (Elsevier) search strategy
#27. #23 AND #26 #26. #24 OR #25 #25. random*:ab,ti OR placebo*:ab,ti OR factorial*:ab,ti OR crossover*:ab,ti OR 'cross over':ab,ti OR 'cross‐over':ab,ti OR volunteer*:ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR ((singl* OR doubl*) NEAR/1 blind*):ab,ti #24. 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp #23. #18 AND #22 #22. #19 OR #20 OR #21 #21. 'vaccination'/de #20. vaccin*:ab,ti OR immuni*:ab,ti OR inocul*:ab,ti #19. 'vaccine'/exp #18. #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 #17. 'respiratory syncytial virus':ab,ti OR 'respiratory syncytial viruses':ab,ti OR rsv:ab,ti #16. 'respiratory syncytial pneumovirus'/de OR 'respiratory syncytial virus infection'/de #15. adenovir*:ab,ti #14. 'adenovirus'/exp OR 'human adenovirus infection'/de #13. coronavir*:ab,ti #12. 'coronavirus'/de OR 'coronavirus infection'/de #11. parainfluenza*:ab,ti #10. 'parainfluenza virus 1'/de OR 'parainfluenza virus 2'/de OR 'parainfluenza virus 3'/de OR 'parainfluenza virus 4'/exp #9. 'parainfluenza virus'/exp #8. 'paramyxovirus infection'/de #7. rhinovir*:ab,ti OR hrv:ab,ti #6. 'rhinovirus infection'/de OR 'human rhinovirus'/de #5. coryza:ab,ti #4. 'acute upper respiratory infection':ab,ti OR 'acute upper respiratory infections':ab,ti OR 'acute upper respiratory tract infection':ab,ti OR 'acute upper respiratory tract infections':ab,ti OR (acute NEAR/5 (urti OR uri)):ab,ti #3. 'viral upper respiratory tract infection'/de OR 'upper respiratory tract infection'/de #2. 'common cold':ab,ti OR 'common colds':ab,ti #1. 'common cold'/de OR 'common cold symptom'/de
Appendix 7. CINAHL (EBSCO) search strategy
S34 S23 and S33 S33 S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 S32 (MH "Quantitative Studies") S31 TI placebo* or AB placebo* S30 (MH "Placebos") S29 TI random* or AB random* S28 TI (singl* mask* or doubl* mask* or tripl* mask* or trebl* mask*) or AB (singl* mask* or doubl* mask* or tripl* mask* or trebl* mask*) S27 TI (singl* blind* or doubl* blind* or trebl* blind* or tripl* blind*) or AB (singl* blind* or doubl* blind* or trebl* blind* or tripl* blind*) S26 TI clinic* w1 trial* or AB clinic* w1 trial* S25 PT clinical trial S24 (MH "Clinical Trials+") S23 S18 and S22 S22 S19 or S20 or S21 S21 TI (vaccin* or immuni* or inocula*) or AB (vaccin* or immuni* or inocula*) S20 (MH "Immunization") S19 (MH "Vaccines+") S18 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 S17 TI (respiratory syncytial virus* or rsv ) or AB (respiratory syncytial virus* or rsv) S16 (MH "Respiratory Syncytial Virus Infections") S15 (MH "Respiratory Syncytial Viruses") S14 TI adenovir* or AB adenovir* S13 TI coronavir* or AB coronavir* S12 (MH "Coronavirus+") S11 (MH "Coronavirus Infections") S10 TI parainfluenza* or AB parainfluenza* S9 (MH "Paramyxovirus Infections") S8 (MH "Paramyxoviruses") S7 TI hrv or AB hrv S6 TI rhinovir* or AB rhinovir* S5 (MH "Picornavirus Infections") S4 TI (upper respiratory tract infection* or upper respiratory infection*) or AB (upper respiratory tract infection* or upper respiratory infection*) S3 TI coryza or AB coryza S2 TI common cold* or AB common cold* S1 (MH "Common Cold")
Appendix 8. LILACS (BIREME) search strategy
(mh:"Common Cold" OR "common cold" OR "common colds" OR coryza OR "Resfriado Común" OR "Resfriado Comum" OR "Coriza Aguda" OR "Upper Respiratory Tract Infections" OR "upper respiratory tract infection" OR "Infecciones del Tracto Respiratorio Superior" OR "Infecciones de las Vías Respiratorias Superiores" OR "Infecções do Trato Respiratório Superior" OR "Infecções das Vias Respiratórias Superiores" OR "Infecções das Vias Aéreas Superiores" OR "Infecções do Sistema Respiratório Superior" OR mh:"Picornaviridae Infections" OR "Infecciones por Picornaviridae" OR "Infecções por Picornaviridae" OR "Picornavirus Infections" OR mh:rhinovirus OR rhinovir* OR "Virus de la Coriza" OR "Virus del Resfriado Común" OR "Vírus da Coriza" OR "Vírus do Resfriado Comum" OR hrv OR mh:"Paramyxoviridae Infections" OR parainfluenza* OR mh:"Parainfluenza Virus 1, Human" OR mh:"Parainfluenza Virus 2, Human" OR mh:"Parainfluenza Virus 3, Human" OR mh:"Parainfluenza Virus 4, Human" OR mh:"Coronavirus Infections" OR coronavir* OR mh:coronavirus OR mh:"Coronavirus 229E, Human" OR mh:"Coronavirus OC43, Human" OR mh:"Coronavirus NL63, Human" OR mh:adenoviridae OR mh:"Adenoviruses, Human" OR mh:"Adenovirus Infections, Human" OR adenovir* OR mh:"Respiratory Syncytial Viruses" OR "Virus Sincitiales Respiratorios" OR "Vírus Sinciciais Respiratórios" OR "Virus Sincitial Respiratorio" OR "Vírus Sincicial Respiratório" OR mh:"Respiratory Syncytial Virus, Human" OR "respiratory syncytial virus" OR "Virus Humano Respiratorio Sincitial" OR mh:"Respiratory Syncytial Virus Infections" OR "Infecciones por Virus Sincitial Respiratorio" OR "Infecções por Vírus Respiratório Sincicial" OR rsv) AND (mh:vaccines OR vaccin* OR vacunas OR vacinas OR mh:d20.215.894* OR mh:vaccination OR vacunación OR vacinação OR mh:"Mass Vaccination" OR mh:immunization OR inmunización OR imunização OR mh:e02.095.465.425.400* OR mh:e05.478.550* OR mh:n02.421.726.758.310* OR mh:n06.850.780.200.425* OR mh:n06.850.780.680.310* OR mh:sp2.026.182.113* OR mh:sp8.946.819.838* OR immuni* OR inmuni* OR imuni*) AND db:("LILACS") AND type_of_study:("clinical_trials")
Data and analyses
Comparison 1. Adenovirus vaccines versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Incidence of the common cold | 1 | 2307 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.45, 2.02] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Griffin 1970.
| Methods |
Design: double‐blind, RCT (2 arms) Country: USA (1 site) Clinical setting: Great Lakes Naval Training Center Follow‐up: 9 weeks' basic‐training period Intention‐to‐treat: yes Randomisation unit: participant Analysis unit: participant |
|
| Participants | Great Lakes Naval Training Center, new recruits Randomised: 2307 participants Vaccines group: 1139 (49.3%) Placebo group: 1168 (50.7%) Participants receiving intervention: 1139 Vaccines group: 1139 (49.3%) Placebo group: 1168 (50.7%) Lost post‐randomisation: 0% Analysed participants: Vaccines group: 1139 (49.3%) Placebo group: 1168 (50.7%) Age median (mean (SD)): did not report Gender (number of men): did not report Inclusion criteria:
Exclusion criteria: not reported |
|
| Interventions | Experimental group: the vaccines used were composed of orally administered live adenovirus 4, parenterally administered inactivated adenovirus 4, and parenterally administered inactivated adenovirus 4 and 7 preparations Control group: placebo Co‐interventions
|
|
| Outcomes | This RCT did not specify primary or secondary outcomes. Incidence of admissions of participants with respiratory illness (not only hospitalised participants)
Toxic effects |
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Epidemiologic design of this study consisted of the random assignment of one half of the recruits ..." (p. 982). Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Double‐blind procedure was followed with paramedical personnel administering the appropriate vaccine or placebo to recruits on their third day after arrival at Great Lakes, just prior to initiation of basic training" (p. 982) Quote: "Placebo for the parenterally administered vaccines consisted of an injection of physiological saline, and that for the orally administered vaccine consisted of an identical appearing inert gelatin capsule" (p. 982) Comment: Blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "Since recruits with the common cold syndrome rarely require hospitalizations, the effect of the adenovirus vaccines on this clinical entity can not be adequately evaluated." Comment: Although placebo was used, there could be a risk of detection bias because common cold does not require hospitalization |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Comment: The study protocol is not available, but it is clear that the published reports include all expected outcomes. However, some are described in a narrative fashion and not per group. Quote: "... there was no observable toxic reaction to this new live vaccine preparation within the study design." (p. 985) |
| Other bias | High risk | The sample size was not reported. There is no table with basal characteristics of the participants |
RCT: randomised controlled trial SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Belshe 1982 | Not RCT |
| Belshe 1992 | RCT. Did not evaluate common cold |
| Belshe 2004a | RCT. Did not evaluate common cold |
| Belshe 2004b | RCT. Did not evaluate common cold |
| Clements 1991 | Not RCT |
| DeVincenzo 2010 | RCT. Did not evaluate common cold |
| Doggett 1963 | Not RCT |
| Dudding 1972 | Not RCT |
| Falsey 1996 | RCT. Did not evaluate common cold |
| Falsey 2008 | RCT. Did not include healthy people |
| Fulginiti 1969 | Not RCT |
| Glenn 2016 | RCT. Did not evaluate common cold |
| Gomez 2009 | RCT. Did not evaluate common cold |
| Gonzalez 2000 | RCT. Did not evaluate common cold |
| Greenberg 2005 | RCT. Included participants aged < 6 months |
| Hamory 1975 | Not RCT |
| Karron 1995a | RCT. Did not evaluate common cold |
| Karron 1995b | RCT. Did not evaluate common cold |
| Karron 1997 | Not RCT |
| Karron 2003 | RCT. Did not evaluate common cold |
| Karron 2005 | RCT. Did not evaluate common cold |
| Karron 2015 | RCT. Did not evaluate common cold |
| Kumpu 2015 | RCT. Did not evaluate a vaccine (evaluated a probiotic) |
| Langley 2009 | RCT. Did not evaluate common cold |
| Lee 2001 | RCT. Included participants aged < 6 months |
| Lee 2004 | Non‐vaccine interventions |
| Lin 2007 | RCT. Did not evaluate common cold |
| Lyons 2008 | RCT. Did not evaluate common cold |
| Madhi 2006 | RCT. Included participants aged < 6 months |
| Munoz 2003 | RCT. Included pregnant women |
| Murphy 1994 | Update on vaccines topic |
| Paradiso 1994 | RCT. Did not evaluate common cold |
| Piedra 1995 | RCT. Did not evaluate common cold |
| Pierce 1968 | RCT. Did not evaluate common cold |
| Power 2001 | RCT. Did not evaluate common cold |
| Ritchie 1958 | Not RCT |
| Simoes 2001 | Meta‐analysis |
| Tang 2008 | RCT. Did not evaluate common cold |
| Top 1971 | RCT. Did not evaluate common cold |
| Tristram 1993 | RCT. Did not evaluate common cold |
| Watt 1990 | Not RCT |
| Welliver 1994 | RCT. Did not evaluate common cold |
| Wilson 1960 | Not RCT |
| Wright 1976 | Not RCT |
RCT: randomised controlled trial
Differences between protocol and review
Three new authors contributed to this update: Juan VA Franco, Maria L Felix, and Maria José Martinez‐Zapata.
We considered risk of bias as unclear for blinding in the previous version of this review. For this update, we reassessed this as low because the study used a placebo.
We added two additional primary outcomes, vaccine safety and mortality related to the vaccine, to Table 1.
We did not search Scirus for this update since this service became unavailable in 2014.
Contributions of authors
Conceiving the review: DSR
Designing the review: DSR, CVG, MLF, RH
Co‐ordinating the review: DSR
Data collection for the review: DSR
Screening search results: JVAF, MJMZ
Appraising quality of papers: DSR, CVG, RH, JVAF, MJMZ
Extracting data from papers: DSR
Writing to authors of papers for additional information: DSR
Obtaining and screening data on unpublished studies: JVAF, MJMZ
Data management for the review: DSR
Entering data into Review Manager 5: DSR, JVAF, MJMZ
Interpretation of data: All authors
Providing a methodological perspective: MJMZ
Providing a clinical perspective: CVG, MLF, RH
Writing the review: DSR, JVAF, MJMZ
All authors contributed to the improvement of this updated review and approved the final version of the review.
Sources of support
Internal sources
-
Universidad Tecnológica Equinoccial, Ecuador.
Methodological
-
Centro de Investigación en Salud Pública y Epidemiología Clínica (CISPEC). Facultad de Ciencias de la Salud Eugenio Espejo, Ecuador.
Methodological
-
Instituto Universitario Hospital Italiano, Argentina.
Methodological
External sources
-
Instituto de Salud Carlos III, Spain.
Mª José Martinez Zapata is funded by a Miguel Servet research contract from the Instituto de Salud Carlos III (CP15/00116).
Declarations of interest
Daniel Simancas‐Racines: None known. Juan VA Franco: None known. Claudia V Guerra: None known. Maria L Felix: None known. Ricardo Hidalgo: None known. Maria José Martinez‐Zapata: None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Griffin 1970 {published data only}
- Griffin JP, Greenberg BH. Live and inactivated adenovirus vaccines. Clinical evaluation of efficacy in prevention of acute respiratory disease. Archives of Internal Medicine 1970;125(6):981‐6. [PUBMED: 4378136] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Belshe 1982 {published data only}
- Belshe RB, Voris LP, Mufson MA. Parenteral administration of live respiratory syncytial virus vaccine: results of a field trial. Journal of Infectious Diseases 1982;145(3):311‐9. [PUBMED: 7037983] [DOI] [PubMed] [Google Scholar]
Belshe 1992 {published data only}
- Belshe RB, Karron RA, Newman FK, Anderson EL, Nugent SL, Steinhoff M, et al. Evaluation of a live attenuated, cold‐adapted parainfluenza virus type 3 vaccine in children. Journal of Clinical Microbiology 1992;30(8):2064‐70. [PUBMED: 1323576] [DOI] [PMC free article] [PubMed] [Google Scholar]
Belshe 2004a {published data only}
- Belshe RB, Newman FK, Anderson EL, Wright PF, Karron RA, Tollefson S, et al. Evaluation of combined live, attenuated respiratory syncytial virus and parainfluenza 3 virus vaccines in infants and young children. Journal of Infectious Diseases 2004;190(12):2096‐103. [PUBMED: 15551207] [DOI] [PubMed] [Google Scholar]
Belshe 2004b {published data only}
- Belshe RB, Newman FK, Tsai TF, Karron RA, Reisinger K, Roberton D, et al. Phase 2 evaluation of parainfluenza type 3 cold passage mutant 45 live attenuated vaccine in healthy children 6‐18 months old. Journal of Infectious Diseases 2004;189(3):462‐70. [PUBMED: 14745704] [DOI] [PubMed] [Google Scholar]
Clements 1991 {published data only}
- Clements ML, Belshe RB, King J, Newman F, Westblom TU, Tierney EL, et al. Evaluation of bovine, cold‐adapted human, and wild‐type human parainfluenza type 3 viruses in adult volunteers and in chimpanzees. Journal of Clinical Microbiology 1991;29(6):1175‐82. [PUBMED: 1650789] [DOI] [PMC free article] [PubMed] [Google Scholar]
DeVincenzo 2010 {published data only}
- DeVincenzo J, Lambkin‐Williams R, Wilkinson T, Cehelsky J, Nochur S, Walsh E, et al. A randomized, double‐blind, placebo‐controlled study of an RNAi‐based therapy directed against respiratory syncytial virus. Proceedings of the National Academy of Sciences of the United States of America 2010;107(19):8800‐5. [PUBMED: 20421463] [DOI] [PMC free article] [PubMed] [Google Scholar]
Doggett 1963 {published data only}
- Doggett JE, Bynoe ML, Tyrrell DA. Some attempts to produce an experimental vaccine with rhinoviruses. British Medical Journal 1963;1(5322):34‐6. [PUBMED: 14028369] [DOI] [PMC free article] [PubMed] [Google Scholar]
Dudding 1972 {published data only}
- Dudding BA, Bartelloni PJ, Scott RM, Top FH Jr, Russell PK, Buescher EL. Enteric immunization with live adenovirus type 21 vaccine. I. Tests for safety, infectivity, immunogenicity, and potency in volunteers. Infection and Immunity 1972;5(3):295‐9. [PUBMED: 4564559] [DOI] [PMC free article] [PubMed] [Google Scholar]
Falsey 1996 {published data only}
- Falsey AR, Walsh EE. Safety and immunogenicity of a respiratory syncytial virus subunit vaccine (PFP‐2) in ambulatory adults over age 60. Vaccine 1996;14(13):1214‐8. [PUBMED: 8961507] [DOI] [PubMed] [Google Scholar]
Falsey 2008 {published data only}
- Falsey AR, Walsh EE, Capellan J, Gravenstein S, Zambon M, Yau E, et al. Comparison of the safety and immunogenicity of 2 respiratory syncytial virus (rsv) vaccines ‐ nonadjuvanted vaccine or vaccine adjuvanted with alum ‐ given concomitantly with influenza vaccine to high‐risk elderly individuals. Journal of Infectious Diseases 2008;198(9):1317‐26. [PUBMED: 18855558] [DOI] [PubMed] [Google Scholar]
Fulginiti 1969 {published data only}
- Fulginiti VA, Eller JJ, Sieber OF, Joyner JW, Minamitani M, Meiklejohn G. Respiratory virus immunization. I. A field trial of two inactivated respiratory virus vaccines; an aqueous trivalent parainfluenza virus vaccine and an alum‐precipitated respiratory syncytial virus vaccine. American Journal of Epidemiology 1969;89(4):435‐48. [PUBMED: 4305199] [DOI] [PubMed] [Google Scholar]
Glenn 2016 {published data only}
- Glenn GM, Fries LF, Thomas DN, Smith G, Kpamegan E, Lu H, et al. A randomized, blinded, controlled, dose‐ranging study of a respiratory syncytial virus recombinant fusion (F) nanoparticle vaccine in healthy women of childbearing age. Journal of Infectious Diseases 2016; Vol. 213, issue 3:411‐22. [DOI] [PubMed]
Gomez 2009 {published data only}
- Gomez M, Mufson MA, Dubovsky F, Knightly C, Zeng W, Losonsky G. Phase‐I study MEDI‐534, of a live, attenuated intranasal vaccine against respiratory syncytial virus and parainfluenza‐3 virus in seropositive children. Pediatric Infectious Disease Journal 2009;28(7):655‐8. [PUBMED: 19483659] [DOI] [PubMed] [Google Scholar]
Gonzalez 2000 {published data only}
- Gonzalez IM, Karron RA, Eichelberger M, Walsh EE, Delagarza VW, Bennett R, et al. Evaluation of the live attenuated cpts 248/404 RSV vaccine in combination with a subunit RSV vaccine (PFP‐2) in healthy young and older adults. Vaccine 2000;18(17):1763‐72. [PUBMED: 10699324] [DOI] [PubMed] [Google Scholar]
Greenberg 2005 {published data only}
- Greenberg DP, Walker RE, Lee MS, Reisinger KS, Ward JI, Yogev R, et al. A bovine parainfluenza virus type 3 vaccine is safe and immunogenic in early infancy. Journal of Infectious Diseases 2005;191(7):1116‐22. [PUBMED: 15747247] [DOI] [PubMed] [Google Scholar]
Hamory 1975 {published data only}
- Hamory BH, Hamparian VV, Conant RM, Gwaltney JM Jr. Human responses to two decavalent rhinovirus vaccines. Journal of Infectious Diseases 1975;132(6):623‐9. [PUBMED: 172561] [DOI] [PubMed] [Google Scholar]
Karron 1995a {published data only}
- Karron RA, Wright PF, Hall SL, Makhene M, Thompson J, Burns BA, et al. A live attenuated bovine parainfluenza virus type 3 vaccine is safe, infectious, immunogenic, and phenotypically stable in infants and children. Journal of Infectious Diseases 1995;171(5):1107‐14. [PUBMED: 7751684] [DOI] [PubMed] [Google Scholar]
Karron 1995b {published data only}
- Karron RA, Wright PF, Newman FK, Makhene M, Thompson J, Samorodin R, et al. A live human parainfluenza type 3 virus vaccine is attenuated and immunogenic in healthy infants and children. Journal of Infectious Diseases 1995;172(6):1445‐50. [PUBMED: 7594701] [DOI] [PubMed] [Google Scholar]
Karron 1997 {published data only}
- Karron RA, Wright PF, Crowe JE Jr, Clements‐Mann ML, Thompson J, Makhene M, et al. Evaluation of two live, cold‐passaged, temperature‐sensitive respiratory syncytial virus vaccines in chimpanzees and in human adults, infants, and children. Journal of Infectious Diseases 1997;176(6):1428‐36. [PUBMED: 9395351] [DOI] [PubMed] [Google Scholar]
Karron 2003 {published data only}
- Karron RA, Belshe RB, Wright PF, Thumar B, Burns B, Newman F, et al. A live human parainfluenza type 3 virus vaccine is attenuated and immunogenic in young infants. Pediatric Infectious Disease Journal 2003;22(5):394‐405. [PUBMED: 12792378] [DOI] [PubMed] [Google Scholar]
Karron 2005 {published data only}
- Karron RA, Wright PF, Belshe RB, Thumar B, Casey R, Newman F, et al. Identification of a recombinant live attenuated respiratory syncytial virus vaccine candidate that is highly attenuated in infants. Journal of Infectious Diseases 2005;191(7):1093‐104. [PUBMED: 15747245] [DOI] [PubMed] [Google Scholar]
Karron 2015 {published data only}
- Karron RA, Mateo JS, Thumar B, Schaap‐Nutt A, Buchholz UJ, Schmidt AC, et al. Evaluation of a live‐attenuated human parainfluenza type 1 vaccine in adults and children. Journal of Pediatric Infectious Diseases Society 2015; Vol. 4, issue 4:e143‐6. [DOI] [PMC free article] [PubMed]
Kumpu 2015 {published data only}
- Kumpu M, Kekkonen RA, Korpela R, Tynkkynen S, Järvenpää S, Kautiainen H, et al. Effect of live and inactivated Lactobacillus rhamnosus GG on experimentally induced rhinovirus colds: randomised, double blind, placebo‐controlled pilot trial. Beneficial Microbes 2015; Vol. 6, issue 5:631‐9. [DOI] [PubMed]
Langley 2009 {published data only}
- Langley JM, Sales V, McGeer A, Guasparini R, Predy G, Meekison W, et al. A dose‐ranging study of a subunit Respiratory Syncytial Virus subtype A vaccine with and without aluminum phosphate adjuvantation in adults > or = 65 years of age. Vaccine 2009;27(42):5913‐9. [PUBMED: 19651171] [DOI] [PubMed] [Google Scholar]
Lee 2001 {published data only}
- Lee MS, Greenberg DP, Yeh SH, Yogev R, Reisinger KS, Ward JI, et al. Antibody responses to bovine parainfluenza virus type 3 (PIV3) vaccination and human PIV3 infection in young infants. Journal of Infectious Diseases 2001;184(7):909‐13. [PUBMED: 11509996] [DOI] [PubMed] [Google Scholar]
Lee 2004 {published data only}
- Lee FE, Walsh EE, Falsey AR, Betts RF, Treanor JJ. Experimental infection of humans with A2 respiratory syncytial virus. Antiviral Research 2004;63(3):191‐6. [PUBMED: 15451187] [DOI] [PubMed] [Google Scholar]
Lin 2007 {published data only}
- Lin JT, Zhang JS, Su N, Xu JG, Wang N, Chen JT, et al. Safety and immunogenicity from a phase I trial of inactivated severe acute respiratory syndrome coronavirus vaccine. Antiviral Therapy 2007;12(7):1107‐13. [PUBMED: 18018769] [PubMed] [Google Scholar]
Lyons 2008 {published data only}
- Lyons A, Longfield J, Kuschner R, Straight T, Binn L, Seriwatana J, et al. A double‐blind, placebo‐controlled study of the safety and immunogenicity of live, oral type 4 and type 7 adenovirus vaccines in adults. Vaccine 2008;26(23):2890‐8. [PUBMED: 18448211] [DOI] [PubMed] [Google Scholar]
Madhi 2006 {published data only}
- Madhi SA, Cutland C, Zhu Y, Hackell JG, Newman F, Blackburn N, et al. Transmissibility, infectivity and immunogenicity of a live human parainfluenza type 3 virus vaccine (HPIV3cp45) among susceptible infants and toddlers. Vaccine 2006;24(13):2432‐9. [PUBMED: 16406170] [DOI] [PubMed] [Google Scholar]
Munoz 2003 {published data only}
- Munoz FM, Piedra PA, Glezen WP. Safety and immunogenicity of respiratory syncytial virus purified fusion protein‐2 vaccine in pregnant women. Vaccine 2003;21(24):3465‐7. [PUBMED: 12850361] [DOI] [PubMed] [Google Scholar]
Murphy 1994 {published data only}
- Murphy BR, Hall SL, Kulkarni AB, Crowe JE Jr, Collins PL, Connors M, et al. An update on approaches to the development of respiratory syncytial virus (RSV) and parainfluenza virus type 3 (PIV3) vaccines. Virus Research 1994;32(1):13‐36. [PUBMED: 8030364] [DOI] [PubMed] [Google Scholar]
Paradiso 1994 {published data only}
- Paradiso PR, Hildreth SW, Hogerman DA, Speelman DJ, Lewin EB, Oren J, et al. Safety and immunogenicity of a subunit respiratory syncytial virus vaccine in children 24 to 48 months old. Pediatric Infectious Disease Journal 1994;13(9):792‐8. [PUBMED: 7808848] [DOI] [PubMed] [Google Scholar]
Piedra 1995 {published data only}
- Piedra PA, Glezen WP, Kasel JA, Welliver RC, Jewel AM, Rayford Y, et al. Safety and immunogenicity of the PFP vaccine against respiratory syncytial virus (RSV): the western blot assay aids in distinguishing immune responses of the PFP vaccine from RSV infection. Vaccine 1995;13(12):1095‐101. [PUBMED: 7491817] [DOI] [PubMed] [Google Scholar]
Pierce 1968 {published data only}
- Pierce WE, Rosenbaum MJ, Edwards EA, Peckinpaugh RO, Jackson GG. Live and inactivated adenovirus vaccines for the prevention of acute respiratory illness in naval recruits. American Journal of Epidemiology 1968;87(1):237‐46. [PUBMED: 4295428] [DOI] [PubMed] [Google Scholar]
Power 2001 {published data only}
- Power UF, Nguyen TN, Rietveld E, Swart RL, Groen J, Osterhaus AD, et al. Safety and immunogenicity of a novel recombinant subunit respiratory syncytial virus vaccine (BBG2Na) in healthy young adults. Journal of Infectious Diseases 2001;184(11):1456‐60. [PUBMED: 11709789] [DOI] [PubMed] [Google Scholar]
Ritchie 1958 {published data only}
- Ritchie JM. Autogenous vaccine in prophylaxis of the common cold. Lancet 1958;1(7021):615‐8. [PUBMED: 13515297] [DOI] [PubMed] [Google Scholar]
Simoes 2001 {published data only}
- Simoes EA, Tan DH, Ohlsson A, Sales V, Wang EE. Respiratory syncytial virus vaccine: a systematic overview with emphasis on respiratory syncytial virus subunit vaccines. Vaccine 2001;20(5‐6):954‐60. [PUBMED: 11738763] [DOI] [PubMed] [Google Scholar]
Tang 2008 {published data only}
- Tang RS, Spaete RR, Thompson MW, MacPhail M, Guzzetta JM, Ryan PC, et al. Development of a PIV‐vectored RSV vaccine: preclinical evaluation of safety, toxicity, and enhanced disease and initial clinical testing in healthy adults. Vaccine 2008;26(50):6373‐82. [PUBMED: 18822334] [DOI] [PubMed] [Google Scholar]
Top 1971 {published data only}
- Top FH Jr, Buescher EL, Bancroft WH, Russell PK. Immunization with live types 7 and 4 adenovirus vaccines. II. Antibody response and protective effect against acute respiratory disease due to adenovirus type 7. Journal of Infectious Diseases 1971;124(2):155‐60. [PUBMED: 4330998] [DOI] [PubMed] [Google Scholar]
Tristram 1993 {published data only}
- Tristram DA, Welliver RC, Mohar CK, Hogerman DA, Hildreth SW, Paradiso P. Immunogenicity and safety of respiratory syncytial virus subunit vaccine in seropositive children 18‐36 months old. Journal of Infectious Diseases 1993;167(1):191‐5. [PUBMED: 8418166] [DOI] [PubMed] [Google Scholar]
Watt 1990 {published data only}
- Watt PJ, Robinson BS, Pringle CR, Tyrrell DA. Determinants of susceptibility to challenge and the antibody response of adult volunteers given experimental respiratory syncytial virus vaccines. Vaccine 1990;8(3):231‐6. [PUBMED: 2363300] [DOI] [PubMed] [Google Scholar]
Welliver 1994 {published data only}
- Welliver RC, Tristram DA, Batt K, Sun M, Hogerman D, Hildreth S. Respiratory syncytial virus‐specific cell‐mediated immune responses after vaccination with a purified fusion protein subunit vaccine. Journal of Infectious Diseases 1994;170(2):425‐8. [PUBMED: 8035030] [DOI] [PubMed] [Google Scholar]
Wilson 1960 {published data only}
- Wilson JS, Grant PJ, Miller DL, Taylor CE, McDonald JC. Trial of adenovirus vaccine in Royal Air Force recruits. British Medical Journal 1960;1(5179):1081‐3. [PUBMED: 13845090] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wright 1976 {published data only}
- Wright PF, Shinozaki T, Fleet W, Sell SH, Thompson J, Karzon DT. Evaluation of a live, attenuated respiratory syncytial virus vaccine in infants. Journal of Pediatrics 1976;88(6):931‐6. [PUBMED: 178852] [DOI] [PubMed] [Google Scholar]
Additional references
Abbas 2001
- Abbas A, Lichtman A. Glossary. Basic Immunology. Vol. 1, Philadelphia, PA: WB Saunders Company, 2001. [Google Scholar]
Adams 1999
- Adams PF, Hendershot GE, Marano MA. Current estimates from the National Health Interview Survey 1996. Vital Health Statistics 1999;10(200):1‐203. [MEDLINE: ] [PubMed] [Google Scholar]
Altman 1995
- Altman DG, Bland JM. Absence of evidence is not evidence of absence. BMJ 1995;311(7003):485. [PUBMED: 7647644] [DOI] [PMC free article] [PubMed] [Google Scholar]
Anonymous 2012
- Anonymous. Methodological standards and patient‐centeredness in comparative effectiveness research: the PCORI perspective. JAMA 2012;307(15):1636‐40. [PUBMED: 22511692] [DOI] [PubMed] [Google Scholar]
Arruda 1997
- Arruda E, Pitkäranta A, Witek TJ Jr, Doyle CA, Hayden FG. Frequency and natural history of rhinovirus infections in adults during autumn. Journal of Clinical Microbiology 1997;35(11):2864‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, Flottorp S, et al. GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328(7454):1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bembridge 1998
- Bembridge G, Garcia‐Beato R, Lòpez J, Melero J, Taylor G. Subcellular site of expression and route of vaccination influence pulmonary eosinophilia following respiratory syncytial virus challenge in BALB/c mice sensitized to the attachment G protein. Journal of Immunology 1998;161(5):2473‐80. [MEDLINE: ] [PubMed] [Google Scholar]
Berman 1991
- Berman S. Epidemiology of acute respiratory infections in children of developing countries. Reviews of Infectious Diseases 1991;13(Suppl 6):454‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Binn 2007
- Binn LN, Sanchez JL, Gaydos JC. Emergence of adenovirus type 14 in US military recruits ‐ a new challenge. Journal of Infectious Diseases 2007;196(10):1436‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
DDCP 2010
- Division of Disease Control and Prevention, Utah Department of Health. Difference between cold and flu symptoms. health.utah.gov/epi/diseases/flu/ColdvsFlu.pdf 2010 (accessed prior to 12 May 2017).
Denny 1983
- Denny FW, Murphy TF, Clyde WA, Collier AM, Henderson FW. Croup: an 11 year study in a pediatric practice. Pediatrics 1983;71(6):871‐6. [MEDLINE: ] [PubMed] [Google Scholar]
Edlmayr 2009
- Edlmayr J, Niespodziana K, Linhart B, Focke‐Tejk lM, Westrtschnig K, Scheiblhober S, et al. A combination vaccine for allergy and rhinovirus infections based on rhinovirus‐derived surface protein VP1 and a nonallergenic peptide of the major timothy grass pollen allergen PHl p1. Journal of Immunology 2009;182(10):6298‐306. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Eriksson 2006
- Eriksson KK, Makia D, Maier R, Ludewig B, Thiel V. Towards a coronavirus‐based HIV multigene vaccine. Clinical and Developmental Immunology 2006;13(2‐4):353‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Esper 2003
- Esper F, Boucher D, Weibel C, Martinello RA, Kahn JS. Human metapneumovirus infection in the United States: clinical manifestations associated with a newly emerging respiratory infection in children. Pediatrics 2003;111(6 pt 1):1407‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Evans 1997
- Evans AS, Kaslow RA (editors). Viral Infection of Humans: Epidemiology and Control. 4th Edition. New York: Plenum Press, 1997. [Google Scholar]
Falsey 2005
- Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory syncytial virus infection in elderly and high‐risk adults. New England Journal of Medicine 2005;352(17):1749‐59. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Fermi Paradox 2012
- Fermi Paradox. www.crystalinks.com/fermiparadox.html 2012 (accessed 12 July 2012).
Freiman 1978
- Freiman JA, Chalmers TC, Smith H Jr, Kuebler RR. The importance of beta, the type II error and sample size in the design and interpretation of the randomized control trial. Survey of 71 "negative" trials. New England Journal of Medicine 1978;299(13):690‐4. [PUBMED: 355881] [DOI] [PubMed] [Google Scholar]
Gabriel 2012
- Gabriel SE, Normand SL. Getting the methods right ‐ the Foundation of Patient‐Centered Outcomes Research. New England Journal of Medicine 2012;367(9):787‐90. [DOI: 10.1056/NEJMp1207437] [DOI] [PubMed] [Google Scholar]
Glezen 2000
- Glezen WP, Greenberg SB, Atmar RL, Piedra PA, Couch RB. Impact of respiratory infection on persons with chronic underlying conditions. JAMA 2000;283(4):499‐505. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Goldsby 2000
- Goldsby R, Kingt T, Osborne B. Overview of the immune system. Kuby Immunology. 4th Edition. Vol. 3, New York, NY: WH Freeman Co, 2000. [Google Scholar]
Gonzales 2001
- Gonzales R, Malone DC, Maselli JH, Sande MA. Excessive antibiotic use for acute respiratory infections in the United States. Clinical Infectious Diseases 2001;33(6):757‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Gorczynski 2007
- Gorczynski R, Stanley J. Problem‐based immunology [Inmunología basaqda en la resolución de problemas]. Inmunología Basada en Problemas. Vol. 138, Madrid: Elsevier Saunders, 2007. [Google Scholar]
GRADEpro GDT 2014 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version Accessed: October 15th, 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Gray 2007
- Gray GC, McCarthy T, Lebeck MG, Schnurr OP, Russell KL, Kajon AE, et al. Genotype prevalence and risk factors for severe clinical adenovirus infection, United States 2004‐2006. Clinical Infectious Diseases 2007;45(9):1120‐31. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Green 2002
- Green SB. Design of randomized trials. Epidemiologic Reviews 2002;24(1):4‐11. [PUBMED: 12119855] [DOI] [PubMed] [Google Scholar]
Grüber 2008
- Grüber C, Keil T, Kulig M, Roll S, Wahn U, Wahn V, MAS‐90 Study Group. History of respiratory infections in the first 12 yr among children from a birth cohort. Pediatric Allergy and Immunology 2008;9(6):505‐12. [PUBMED: 18167154] [DOI] [PubMed] [Google Scholar]
Gwaltney 1967
- Gwaltney JM, Hendley J, Simon G, Jordan WSJ. Rhinovirus infections in an industrial population II. Characteristics of illness and antibody response. JAMA 1967;202(6):494‐500. [PUBMED: 4293015] [PubMed] [Google Scholar]
Gwaltney 1985
- Gwaltney JM. Virology and immunology of the common cold. Rhinology 1985;23(4):265‐71. [MEDLINE: ] [PubMed] [Google Scholar]
Gwaltney 2000
- Gwaltney JM. The common cold. In: Mandell GL, Bennett JE, Dolin R editor(s). Principles and Practices of Infectious Diseases. 5th Edition. New York, NY: Churchill Livingstone, 2000:651‐6. [Google Scholar]
Hall 2001
- Hall CV. Respiratory syncytial virus and parainfluenza virus. New England Journal of Medicine 2001;344(25):1917‐28. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Harrison 2008
- Fauci AS, Brownwald E, Kasper D, Hauser S, Longo D, Hameson L, et al. Harrison Principios de Medicina Interna. 17th Edition. McGraw Hill, 2008. [Google Scholar]
Heikkinen 2003
- Heikkinen T, Jarvinen A. The common cold. Lancet 2003;361(9351):51‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Henrickson 1994
- Henrickson KJ, Kuhn SM, Savatski LL. Epidemiology and cost of infection with human parainfluenza viruses types 1 and 2 in young children. Clinical Infectious Diseases 1994;18(5):770‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Henrickson 2003
- Henrickson KJ. Parainfluenza viruses. Clinical Microbiology Reviews 2003;16(2):242‐64. [PUBMED: 12692097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hussell 1998
- Hussell T, Georgiou A, Sparer T, Matthews S, Pala P, Openshaw P. Host genetic determinants of vaccine‐induced eosinophilia during RSV infection. Journal of Immunology 1998;161(11):6215‐22. [PUBMED: 9834108] [PubMed] [Google Scholar]
Ioannidis 2004
- Ioannidis JP, Evans SJ, Gotzsche PC, O'Neill RT, Altman DG, Schulz K, et al. Better reporting of harms in randomized trials: an extension of the CONSORT statement. Annals of Internal Medicine 2004;141(10):781‐8. [PUBMED: 15545678] [DOI] [PubMed] [Google Scholar]
Ioannidis 2010
- Ioannidis JP. Meta‐research: the art of getting it wrong. Research Synthesis Methods 2010;1(3‐4):169‐84. [PUBMED: 26061464] [DOI] [PubMed] [Google Scholar]
Jackson 2008
- Jackson DJ, Gangnon RE, Evans MD, Roberg KA, Anderson EL, Pappas TE, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high‐risk children. American Journal of Respiratory and Critical Care Medicine 2008;178(7):667‐72. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jefferson 2011
- Jefferson T, Mar CB, Dooley L, Ferroni E, Al‐Ansary LA, Bawazeer GA, et al. Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database of Systematic Reviews 2011, Issue 7. [DOI: 10.1002/14651858.CD006207.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jefferson 2012
- Jefferson T, Rivetti A, Harnden A, Pietrantonj C, Demicheli V. Vaccines for preventing influenza in healthy children. Cochrane Database of Systematic Reviews 2012, Issue 8. [DOI: 10.1002/14651858.CD004879.pub4] [DOI] [PubMed] [Google Scholar]
Kahn 2003
- Kahn JS. Human metapneumovirus: a newly emerging respiratory pathogen. Current Opinion in Infection Diseases 2003;16(3):255‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Kang 2009
- Kang SM, Compans RW. Host responses from innate to adaptive immunity after vaccination: molecular and cellular events. Molecular Cell 2009;27(1):5‐14. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kirby 2002
- Kirby A, Gebski V, Keech AC. Determining the sample size in a clinical trial. Medical Journal of Australia 2002;177(5):256‐7. [PUBMED: 12197821] [DOI] [PubMed] [Google Scholar]
Krasinski 1985
- Krasinski K. Severe respiratory syncytial virus infection: clinical features, nosocomial acquisition and outcome. Pediatric Infectious Disease 1985;4(3):250‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Larson 1980
- Larson HE, Reed SE, Tyrrell DA. Isolation of rhinoviruses and coronaviruses from 38 colds in adults. Journal of Medical Virology 1980;5(3):221‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lau 2006
- Lau S, Woo P, Yip L, Tse H, Tesoi H, Ching V, et al. Coronavirus HKU1 and other coronavirus infection in Hong Kong. Journal of Clinical Microbiology 2006;44(6):2063‐71. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Leder 2003
- Leder K, Sinclair MI, Mitakakis TZ, Hellard ME, Forbes A. A community‐based study of respiratory episodes in Melbourne, Australia. Australian and New Zealand Journal of Public Health 2003;27(4):399–404. [PUBMED: 14705301] [DOI] [PubMed] [Google Scholar]
Lemanske 2005
- Lemanske RF Jr, Jackson DJ, Gangnon RE, Evans MD, Li Z, Shult PA, et al. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. Journal of Allergy and Clinical Immunology 2005;116(3):571‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moher 1998
- Moher D, Pham B, Jones A, Cook DJ, Jadad AR, Moher M, et al. Does quality of reports of randomised trials affect estimates of intervention efficacy reported in meta‐analyses?. Lancet 1998;352(9128):609‐13. [PUBMED: 9746022] [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA Statement. BMJ 2009;339:2535. [PMC free article] [PubMed] [Google Scholar]
Moher 2010
- Moher D, Hopewell S, Schulz KF, Montori V, Gotzsche PC, Devereaux PJ, et al. CONSORT 2010 explanation and elaboration: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c869. [PUBMED: 20332511] [DOI] [PMC free article] [PubMed] [Google Scholar]
Monto 1993
- Monto AS, Sullivan KM. Acute respiratory illness in the community. Frequency of illness and the agents involved. Epidemiology and Infection 1993;110(1):145‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mäkelä 1998
- Mäkelä MJ, Puhakka T, Ruuskanen O, Leinonen M, Saikku P, Kimpimäki M, et al. Viruses and bacteria in the etiology of the common cold. Journal of Clinical Microbiology 1998;36(2):539‐42. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nebeker 2004
- Nebeker JR, Barach P, Samore MH. Clarifying adverse drug events: a clinician's guide to terminology, documentation, and reporting. Annals of Internal Medicine 2004;140(10):795‐801. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nelson 2000
- Kliegman R, Behrman RE, Jenson HB. Nelson Tratado de Pediatría. 16th Edition. McGraw Hill, 2000. [Google Scholar]
Nicholson 1997
- Nicholson KG, Kent J, Hammersley V, Cancio E. Acute viral infections of upper respiratory tract in elderly people living in the community: comparative, prospective population based study of disease burden. BMJ 1997;315(7115):1060‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nissen 2002
- Nissen MD, Siebert DJ, Mackay IM, Sloots TP, Withers SJ. Evidence of human metapneumovirus in Australian children. Medical Journal of Australia 2002;176(4):188. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Palmemberg 2009
- Palmemberg AC, Spiro D, Kuzmickas R, Wang S, Djikeng A, Rathe JA, et al. Sequencing and analyses of all known human rhinovirus genomes reveal structure and evolution. Science 2009;324(5923):55‐9. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Peltola 2008
- Peltola V, Waris M, Osterback R, Susi P, Hyypiä T, Ruuskanen O. Clinical effects of rhinovirus infections. Journal of Clinical Virology 2008;43(4):411‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pichichero 2009
- Pichichero ME. Booster vaccinations: can immunologic memory outpace disease pathogenesis?. Pediatrics 2009;124(6):1633‐41. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Plotkin 2008
- Plotkin SA. Vaccines: correlates of vaccine‐induced immunity. Clinical Infectious Diseases 2008;47(3):401‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Poland 2009
- Poland G, Barry MA. Common cold, uncommon variation. New England Journal of Medicine 2009;360(21):2245‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Regamey 2008
- Regamey N, Kaiser L. Rhinovirus infections in infants: is respiratory syncytial virus ready for the challenge?. European Respiratory Journal 2008;32(2):249‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Ren 2017
- Ren L, Yang D, Ren X, Li M, Mu X, Wang Q, et al. Genotyping of human rhinovirus in adult patients with acute respiratory infections identified predominant infections of genotype A21. Scientific Reports 2017;7:41601. [PUBMED: 28128353] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rey‐Jurado 2017
- Rey‐Jurado E, Kalergis AM. Immunological features of respiratory syncytial virus‐caused pneumonia ‐ implications for vaccine design. International Journal of Molecular Sciences 2017;18(3):556. [DOI: 10.3390/ijms18030556] [DOI] [PMC free article] [PubMed] [Google Scholar]
Risnes 2005
- Risnes KR, Radtke A, Nordbø SA, Grammeltvedt AT, Døllner H. Human metapneumovirus ‐ occurrence and clinical significance. Tidsskrift for den Norske Lægeforening 2005;20:2769‐72. [MEDLINE: ] [PubMed] [Google Scholar]
Roitt 2004
- Roitt I, Delves P. Glossary. Essential Immunology. 10th Edition. Vol. 466, Blackwell, 2004. [Google Scholar]
Roxas 2007
- Roxas M, Jurenka J. Colds and influenza: a review of diagnosis and conventional, botanical and nutritional considerations. Alternative Medicine Review 2007;12(1):25‐48. [MEDLINE: ] [PubMed] [Google Scholar]
Russell 2006
- Russell KL, Hawksworth AW, Ryan MA, Strickler J, Irvine M, Hansen CJ, et al. Vaccine‐preventable adenoviral respiratory illness in US military recruits, 1999‐2004. Vaccine 2006;24(15):2835‐42. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Hayes RJ, Altman DG. Empirical evidence of bias. Dimensions of methodological quality associated with estimates of treatment effects in controlled trials. JAMA 1995;273(5):408‐12. [PUBMED: 7823387] [DOI] [PubMed] [Google Scholar]
Thompson 2003
- Thompson WW. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003;289(2):179‐86. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tobin 2008
- Tobin G, Trujillo JD, Bushnell R, Lin G, Chaudhuri RA, Largo J, et al. Deceptive imprinting and immune refocusing in vaccine design. Vaccine 2008;26(49):6189‐99. [DOI] [PubMed] [Google Scholar]
Wat 2004
- Wat D. The common cold: a review of the literature. European Journal of Internal Medicine 2004;15(2):79‐88. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Felix 2011
- Felix ML, Guerra CV, Hinojosa MA, Cabezas CI, Hidalgo R, Samaniego DH, et al. Vaccines for the common cold. Cochrane Database of Systematic Reviews 2011, Issue 4. [DOI: 10.1002/14651858.CD002190.pub3] [DOI] [Google Scholar]
Simancas‐Racines 2013
- Simancas‐Racines D, Guerra CV, Hidalgo R. Vaccines for the common cold. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD002190.pub4] [DOI] [PubMed] [Google Scholar]


