Abstract
Background
Abnormal blood flow patterns in fetal circulation detected by Doppler ultrasound may indicate poor fetal prognosis. It is also possible that false positive Doppler ultrasound findings could lead to adverse outcomes from unnecessary interventions, including preterm delivery.
Objectives
The objective of this review was to assess the effects of Doppler ultrasound used to assess fetal well‐being in high‐risk pregnancies on obstetric care and fetal outcomes.
Search methods
We updated the search of Cochrane Pregnancy and Childbirth's Trials Register on 31 March 2017 and checked reference lists of retrieved studies.
Selection criteria
Randomised and quasi‐randomised controlled trials of Doppler ultrasound for the investigation of umbilical and fetal vessels waveforms in high‐risk pregnancies compared with no Doppler ultrasound. Cluster‐randomised trials were eligible for inclusion but none were identified.
Data collection and analysis
Two review authors independently assessed the studies for inclusion, assessed risk of bias and carried out data extraction. Data entry was checked. We assessed the quality of evidence using the GRADE approach.
Main results
Nineteen trials involving 10,667 women were included. Risk of bias in trials was difficult to assess accurately due to incomplete reporting. None of the evidence relating to our main outcomes was graded as high quality. The quality of evidence was downgraded due to missing information on trial methods, imprecision in risk estimates and heterogeneity. Eighteen of these studies compared the use of Doppler ultrasound of the umbilical artery of the unborn baby with no Doppler or with cardiotocography (CTG). One more recent trial compared Doppler examination of other fetal blood vessels (ductus venosus) with computerised CTG.
The use of Doppler ultrasound of the umbilical artery in high‐risk pregnancy was associated with fewer perinatal deaths (risk ratio (RR) 0.71, 95% confidence interval (CI) 0.52 to 0.98, 16 studies, 10,225 babies, 1.2% versus 1.7 %, number needed to treat (NNT) = 203; 95% CI 103 to 4352, evidence graded moderate). The results for stillbirths were consistent with the overall rate of perinatal deaths, although there was no clear difference between groups for this outcome (RR 0.65, 95% CI 0.41 to 1.04; 15 studies, 9560 babies, evidence graded low). Where Doppler ultrasound was used, there were fewer inductions of labour (average RR 0.89, 95% CI 0.80 to 0.99, 10 studies, 5633 women, random‐effects, evidence graded moderate) and fewer caesarean sections (RR 0.90, 95% CI 0.84 to 0.97, 14 studies, 7918 women, evidence graded moderate). There was no comparative long‐term follow‐up of babies exposed to Doppler ultrasound in pregnancy in women at increased risk of complications.
No difference was found in operative vaginal births (RR 0.95, 95% CI 0.80 to 1.14, four studies, 2813 women), nor in Apgar scores less than seven at five minutes (RR 0.92, 95% CI 0.69 to 1.24, seven studies, 6321 babies, evidence graded low). Data for serious neonatal morbidity were not pooled due to high heterogeneity between the three studies that reported it (1098 babies) (evidence graded very low).
The use of Doppler to evaluate early and late changes in ductus venosus in early fetal growth restriction was not associated with significant differences in any perinatal death after randomisation. However, there was an improvement in long‐term neurological outcome in the cohort of babies in whom the trigger for delivery was either late changes in ductus venosus or abnormalities seen on computerised CTG.
Authors' conclusions
Current evidence suggests that the use of Doppler ultrasound on the umbilical artery in high‐risk pregnancies reduces the risk of perinatal deaths and may result in fewer obstetric interventions. The results should be interpreted with caution, as the evidence is not of high quality. Serial monitoring of Doppler changes in ductus venosus may be beneficial, but more studies of high quality with follow‐up including neurological development are needed for evidence to be conclusive.
Plain language summary
Doppler ultrasound of fetal vessels in pregnancies at increased risk of complications
What is the issue?
Most babies in high‐income countries grow well in the womb. However, when the mother has a medical problem such as diabetes, high blood pressure, heart or kidney problems, or the placenta does not develop properly, this may affect the growth of the baby. Also, sometimes babies do not grow well for reasons we do not fully understand. Babies with poor growth are more likely to have complications, resulting in babies being ill or dying. Doppler ultrasound detects changes in the pattern of blood flow through the baby's circulation. These changes may identify babies who have problems.
Why is this important?
If babies with growth problems are identified, interventions such as early delivery might help to prevent serious illness and death. However, using Doppler ultrasound could increase interventions such as caesarean section.
What evidence did we find?
We searched for evidence in March 2017. We found 19 trials involving over 10,000 women. Eighteen of these studies compared the use of Doppler ultrasound of the umbilical artery of the unborn baby with no Doppler or with cardiotocography (CTG, sometimes called electronic fetal monitoring). One more recent trial compared Doppler examination of other fetal blood vessels (ductus venosus) with computerised CTG (short‐term variation).
Evidence from included studies was assessed as moderate to very low‐quality due to incomplete reporting of methods and uncertainty of findings; when the strength of the evidence is low or very low, this means future research may change the results and we cannot be certain about them.
Results showed that Doppler ultrasound of the umbilical artery may decrease the number of babies who die, and may lead to fewer caesarean sections and inductions of labour. There was no clear difference in the number of stillbirths, births using forceps or ventouse, or babies with a low Apgar score five minutes after birth. Findings for serious problems in the neonate were not consistent in different studies. In babies with growth restriction, when the decision to deliver was based on late ductus venosus changes or abnormalities on computerised CTG, this appeared to improve long‐term (two‐year) developmental outcome.
What does this mean?
Doppler ultrasound in high‐risk pregnancies appears to reduce the number of babies who die, and may also lead to fewer obstetric interventions. However, the evidence was of moderate to very low‐quality. Further studies of high‐quality with long‐term follow‐up would help us to be more certain.
Summary of findings
Summary of findings for the main comparison. Umbilical artery Doppler ultrasound compared to no Doppler ultrasound in high‐risk pregnancies.
| Umbilical artery Doppler ultrasound compared to no Doppler ultrasound in high‐risk pregnancies | ||||||
| Patient or population: pregnant women at increased risk of fetal complications Setting: antenatal clinics or inpatient wards in hospitals in Australia (3) UK (6) US (2) Sweden (1) South Africa (2) Ireland (1) The Netherlands (1) France (1) Canada (1) Intervention: umbilical artery Doppler ultrasound Comparison: no Doppler ultrasound | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no Doppler ultrasound | Risk with umbilical artery Doppler ultrasound | |||||
| Any perinatal death after randomisation | Study population | RR 0.71 (0.52 to 0.98) | 10225 (16 RCTs) | ⊕⊕⊕⊝ MODERATE 1 2 | ||
| 17 per 1000 | 12 per 1000 (9 to 17) | |||||
| Serious neonatal morbidity | Study population | 1098 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 3 4 | We did not pool the data for this outcome due to high heterogeneity (the direction of effect in the 2 studies contributing data were not consistent). | ||
| Stillbirth | Study population | RR 0.65 (0.41 to 1.04) | 9560 (15 RCTs) | ⊕⊕⊝⊝ LOW 1 2 5 | ||
| 9 per 1000 | 6 per 1000 (4 to 9) | |||||
| Apgar < 7 at 5 minutes | Study population | RR 0.92 (0.69 to 1.24) | 6321 (7 RCTs) | ⊕⊕⊝⊝ LOW 1 5 | ||
| 29 per 1000 | 26 per 1000 (20 to 36) | |||||
| Caesarean section (elective and emergency) | Study population | RR 0.90 (0.84 to 0.97) | 7918 (14 RCTs) | ⊕⊕⊕⊝ MODERATE 1 2 | ||
| 263 per 1000 | 237 per 1000 (221 to 255) | |||||
| Induction of labour | Study population | RR 0.89 (0.80 to 0.99) | 5633 (10 RCTs) | ⊕⊕⊕⊝ MODERATE 1 2 | ||
| 334 per 1000 | 298 per 1000 (268 to 331) | |||||
| Long‐term infant neurodevelopmental outcome (impairment at 2 years) | Study population | ‐ | (0 studies) | ‐ | There has been no comparative long‐term follow‐up of babies exposed to Doppler ultrasound in pregnancy in women at increased risk of complications. | |
| see comment | see comment | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 All studies assessed as having design limitations due to lack of information.
2 Although there was some evidence of funnel plot asymmetry suggesting small‐study effect (with studies with smaller sample sizes appearing to have a more pronounced effect), we did not downgrade for publication bias because, for our selected outcomes, individual studies did not reach statistical significance and there was low heterogeneity across all studies for this outcome.
3 High heterogeneity (I² statistic 76%) with direction of effect different in the 2 studies contributing data.
4 95% CI crossing the line of no effect. Low event rate.
5 Wide 95% CI crossing the line of no effect.
Background
The previous version of this review (Neilson 1996) was split into two separate reviews, for which new protocols were prepared. This present review covers Doppler ultrasound of fetal vessels including umbilical arteries in women at high risk of fetal compromise. The other review covers Doppler ultrasound of utero‐placental circulation (Utero‐placental Doppler ultrasound for improving pregnancy outcome;Stampalija 2010). In addition, we will update the review of 'routine' use of Doppler ultrasound in low‐risk pregnant women (Fetal and umbilical Doppler ultrasound in normal pregnancy;Alfirevic 2015).
Description of the condition
When it comes to the provision of antenatal care or research, pregnant women tend to be divided into low‐ and high‐risk populations; however, the boundaries between the groups are often blurred. For most researchers, ‘high‐risk status’ includes maternal conditions associated with increased perinatal mortality and morbidity such as diabetes, hypertensive disorders (chronic hypertension and pre‐eclampsia), cardiac, renal, and autoimmune disorders (Fisk 2001; Graves 2007; Westergaard 2001). More recently, thrombophilias (congenital and acquired) have been added to this list (Alfirevic 2002; Greer 1999).
Of the conditions specific to pregnancy, fetal growth restriction, antepartum haemorrhage, multiple pregnancy, and prolonged pregnancy tend to be regarded as ‘high risk’ (Bernstein 2000; Westergaard 2001).
It is important to stress that fetal growth restriction is often confused with the concept of being small‐for‐gestational age. Some fetuses are constitutionally small and they do not have increased perinatal morbidity and mortality. Our inability to distinguish easily between small, but healthy fetuses and those who are failing to reach their growth potential has hampered attempts to find appropriate treatment for growth restriction. Growth‐restricted fetuses, who may or may not be small‐for‐dates are at increased risk of mortality and serious morbidity (intraventricular haemorrhage, bronchopulmonary dysplasia, necrotising enterocolitis, infection, pulmonary haemorrhage, hypothermia and hypoglycaemia) (Fisk 2001). Early antenatal detection, treatment where appropriate, and timely delivery could minimise the risks significantly.
In multiple pregnancies, most of the excess morbidity and mortality can be attributed to preterm birth and to pathology associated with twin‐to‐twin transfusion syndrome (TTTS) in monochorionic pregnancies. However, growth discordance or selective intrauterine growth restriction (IUGR) are more common that TTTS (Ortibus 2009). The pathophysiological nature of the TTTS differs from other placental pathology with specific impact on the fetal haemodynamics. Different monitoring and treatment strategies are needed for this condition and for this reason we planned to exclude this subgroup of multiple pregnancies from this review if such information was available.
The most commonly used methods for the assessment of fetal well‐being in high‐risk pregnancies include fetal cardiotocography (CTG) (Grivell 2015), biophysical profile (Lalor 2008) and Doppler studies of the fetal circulation. This review focuses on the role of fetal and umbilical Doppler ultrasound as a test of fetal well‐being in high‐risk pregnancies.
Description of the intervention
The use of Doppler ultrasound to investigate the pattern of waveforms in the umbilical artery during pregnancy was first reported in 1977 from Dublin (Fitzgerald 1977). The waveforms were derived from the changes in the ultrasound frequency of the Doppler signal, which targeted circulating fetal blood within the umbilical artery. Such flow velocity waveforms (FVW) from the feto‐placental circulation are dependent on the fetal cardiac contraction force, density of the blood, the vessel wall elasticity and peripheral or downstream resistance (Giles 1985; Owen 2001). It was suggested that the FVWs should be obtained with the mother in a semirecumbent position during a period of fetal inactivity, as the impedance indices are moderated by fetal breathing and elevated fetal heart rates (Mires 2000).
Different types of measurements have been described in an attempt to quantify the Doppler signals accurately and reproducibly (Chen 1996; Mari 2009; Owen 2001). The indices are calculated as ratios between peak systolic velocity (A), end‐diastolic peak velocity (B) and mean velocity. The most common in clinical practice are pulsatility index (PI = (A ‐ B)/mean)) and resistant index (RI = (A ‐ B)/A) (Burns 1993). Ideally, the measurements have to be done on several consecutive identical wave forms with the angle of the insonation as close to zero as possible (Burns 1993).
Observational studies have demonstrated that, in the presence of normal placental function, the umbilical artery waveform has a pattern compatible with a low‐resistance system, displaying forward blood flow throughout the cardiac cycle (Neilson 1987).
Initial studies have focused on umbilical arteries and veins, but better equipment has allowed studies of carotid and intracranial arteries, aorta, coronary circulation (Baschat 2002), mesenteric artery and the venous circulation (ductus venosus, inferior vena cava and vena Galena) (Cheema 2004; Owen 2001). The assessment of utero‐placental arteries has also been investigated (Trudinger 1985a; Trudinger 1985b) and has been reviewed in a separate Cochrane review (Utero‐placental Doppler ultrasound for improving pregnancy outcome;Stampalija 2010).
When inadequate vascularisation of the placenta occurs (placental insufficiency), the haemodynamic changes in the feto‐placental circulation develop, often in a progressive fashion. Doppler indices from the umbilical artery start to increase when approximately 60% to 70% of the placental vascular tree is not functioning (Thompson 1990). This tends to be followed by a decrease in the impedance to blood flow in the middle cerebral artery as a consequence of 'brain sparing effect' (Hecher 2001), while the resistance increases in aortic blood flow (Ferrazzi 2002; Hecher 2001). This redistribution of the blood flow allows preferential oxygenation of fetal vital organs such as brain and heart. Late Doppler changes include absent or reverse end diastolic flow in the umbilical artery (Al‐Ghazali 1990; Nicholaides 1988) and increase in the resistance of venous blood flow (ductus venosus and inferior vena cava) (Baschat 2001; Ferrazzi 2002). Higher resistance in venous circulation reflects the elevation of right heart afterload and increase of the intraventricular pressure caused by hypoxaemia of the myocardium. Those changes correlate well with fetal acidosis (Bilardo 1990; Weiner 1990).
How the intervention might work
The time scale over which placental insufficiency and fetal compensatory changes develop varies and depends on underlying maternal and fetal pathology and gestational age. It is, therefore, difficult to apply the same management protocol to all women with abnormal Doppler findings. Normal Doppler findings do provide some reassurance and may, in some circumstances, reduce the need for hospitalisation and additional fetal monitoring, but this is not always the case. There is also some suggestion that normal umbilical artery Doppler ultrasound cannot be assumed to mean low risk where the fetus is small (Figueras 2008). An abnormal Doppler finding tends to trigger management protocols that vary significantly, not only between low‐ and high‐income countries, but also from unit to unit in the same country. The most important factors that determine subsequent management are gestation, availability of additional monitoring methods (computerised CTG, biophysical profile, Doppler), and neonatal intensive care availability.
The Growth Restriction Intervention Trial (GRIT) study showed that although the delay in delivery (around four days) may lead to more stillbirths, the overall number of perinatal deaths is not reduced by an immediate delivery (GRIT 2003). Importantly, the study showed that at two years follow‐up, the immediate delivery group showed a trend towards more neurological disability (GRIT 2004).
Recently, considerable interest has been generated by observations that ductus venous flow may be a good predictor of perinatal outcome (Baschat 2001; Bilardo 2004; Ferrazzi 2002). The TRUFFLE study was designed to compare reduced short‐term variation on computerized CTG, early ductus venosus changes or late ductus venosus changes as a trigger for delivery of the growth‐restricted babies between 26+0‐31+6 gestational weeks and results from that trial have now been published and are included in the review (Lees 2005; Lees 2015).
Ultimately, the goal of any Doppler‐triggered management protocol is to improve perinatal mortality and morbidity. An unnecessary early intervention may result in excess morbidity from prematurity, whilst a delay may result in a stillbirth or severely compromised newborn (GRIT 2003).
Why it is important to do this review
The first meta‐analysis of umbilical artery Doppler in high‐risk pregnancies was published in 1995 (Alfirevic 1995; Neilson 1995), demonstrating improvement with Doppler in a number of clinical outcomes and possible reduction in perinatal deaths. Since then, ultrasound technology has developed further and much more complex assessment of fetal circulation has become standard clinical practice in fetal medicine units worldwide. However, the potential for benefit from the knowledge generated by these new methods has to be balanced with the potential for harm. Any suggestion of fetal compromise in high‐risk women is likely to lead to considerable anxiety in families and clinicians, further diagnostic testing, and early (possibly very preterm) birth often by caesarean section.
Another Cochrane review analysed the role of Doppler ultrasound in routine practice (Bricker 2007), with doubts expressed about its benefit as a screening tool in all pregnancies (Alfirevic 2015). The use of utero‐placental Doppler ultrasound is the subject of another Cochrane review (Utero‐placental Doppler ultrasound for improving pregnancy outcome;Stampalija 2010). However, when both fetal and utero‐placental Doppler assessments are used in high‐risk pregnancies, the study will be included here because clinical judgements tend to rest on the fetal assessment.
Objectives
The objective of this review was to assess the effects of Doppler ultrasound used to assess fetal well‐being in high‐risk pregnancies on obstetric care and fetal outcomes.
Methods
Criteria for considering studies for this review
Types of studies
All randomised trials and quasi‐randomised studies comparing Doppler ultrasound (fetal and umbilical circulations) in pregnancies considered to be at high risk of fetal compromise. Cluster‐randomised trials were eligible for inclusion, as were abstracts if enough information was available for assessment and data extraction. Cross‐over trials were not eligible for inclusion.
Types of participants
Women with pregnancies considered to be at 'high risk' for fetal compromise, e.g. intrauterine growth restriction, post‐term pregnancies, previous pregnancy loss, women with hypertension, women with diabetes, or other maternal pathology (e.g. thrombophilia). We planned to include twin pregnancies, separating monochorionic and dichorionic pregnancies, where possible.
Types of interventions
Doppler ultrasound of the fetal and umbilical vessels for fetal assessment in pregnancies in high‐risk populations. We excluded utero‐placental Doppler studies (as these are assessed in a separate review). However, where umbilical artery or fetal Doppler was combined with utero‐placental Doppler, the study has been included in this review.
Comparisons
Doppler ultrasound of fetal vessels versus no Doppler ultrasound of fetal vessels (including comparisons of Doppler ultrasound of fetal vessels revealed versus Doppler ultrasound of fetal vessels concealed).
Doppler ultrasound of fetal vessels versus other forms of monitoring, e.g. cardiotocography, biophysical profile.
Comparison of different forms of Doppler ultrasound of fetal vessels versus other types of Doppler ultrasound of fetal vessels.
Combination of umbilical artery or fetal Doppler with utero‐placental Doppler (uterine artery Doppler) versus either no other monitoring or additional monitoring.
Early ductus venosus Doppler ultrasound versus computerized CTG.
Late ductus venosus Doppler ultrasound versus computerized CTG.
Early versus late ductus venosus Doppler ultrasound.
Types of outcome measures
We selected outcome measures with the help of a proposed core data set of outcome measures (Devane 2007).
Main outcomes
Any perinatal death after randomisation.
Serious neonatal morbidity ‐ composite outcome including hypoxic ischaemic encephalopathy, intraventricular haemorrhage (IVH), bronchopulmonary dysplasia (BPD), necrotising enterocolitis (NEC).
Additional outcomes of interest
Stillbirth.
Neonatal death.
Any potentially preventable perinatal death*.
Fetal acidosis.
Apgar score less than seven at five minutes.
Caesarean section (both elective and emergency).
Spontaneous vaginal birth.
Operative vaginal birth.
Induction of labour.
Oxytocin augmentation.
Neonatal resuscitation required.
Infant requiring intubation/ventilation.
Neonatal fitting/seizures.
Preterm labour (onset of labour before 37 completed weeks of pregnancy).
Gestational age at birth.
Infant respiratory distress syndrome.
Meconium aspiration.
Neonatal admission to special care or intensive care unit, or both.
Hypoxic ischaemic encephalopathy (a condition of injury to the brain).
Intraventricular haemorrhage (IVH).
Bronchopulmonary dysplasia (BPD).
Necrotising enterocolitis (NEC).
Infant birthweight.
Length of infant hospital stay.
Long‐term infant/child neurodevelopmental outcome.
Women's views of their care.
* Perinatal death excluding chromosomal abnormalities, termination of pregnancies, birth before fetal viability (as defined by trialists) and fetal death before use of the intervention.
Non‐prespecified outcomes were also reported if we considered them to be important.
Search methods for identification of studies
The following methods section of this review was based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
We searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (31 March 2017).
The Register is a database containing over 22,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate Pregnancy and Childbirth’s Trials Register (including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL), the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth in the Cochrane Library and select the ‘Specialised Register ’ section from the options on the left side of the screen.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set which has been fully accounted for in the relevant review sections (Included studies; Excluded studies).
Searching other resources
We also planned to look for additional studies in the reference lists of the studies identified.
We did not apply any language or date restrictions.
Data collection and analysis
For methods used in the previous version of this review, seeAlfirevic 2013.
For this update, the following methods were used for assessing the reports that were identified as a result of the updated search.
The following methods section of this review was based on a standard template used by Cochrane Pregnancy and Childbirth.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. We resolved any disagreement through discussion or, if required, we consulted the third review author.
Data extraction and management
We designed a form to extract data. For eligible studies, two review authors extracted the data using the agreed form. We resolved discrepancies through discussion or, if required, we consulted the third review author. Data were entered into Review Manager software (RevMan 2014) and checked for accuracy.
When information regarding any of the above was unclear, we planned to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any nonrandom process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or nonopaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding was unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high, or unclear risk of bias for participants;
low, high, or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high, or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to reinclude missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses (see Sensitivity analysis).
Assessment of the quality of the evidence using the GRADE approach
For this update, the quality of the evidence was assessed using the GRADE approach, as outlined in the GRADE handbook in order to assess the quality of the body of evidence relating to the following outcomes for the main comparisons.
Any perinatal death after randomisation.
Serious neonatal morbidity.
Stillbirth.
Caesarean section (elective and emergency).
Induction of labour.
Apgar less than seven at five minutes.
Long‐term infant neurodevelopmental outcome.
GRADEpro Guideline Development Tool was used to import data from Review Manager 5 (RevMan 2014) in order to create ’Summary of findings’ tables. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates, or potential publication bias.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratios with 95% confidence intervals.
Continuous data
We used the mean difference if outcomes were measured in the same way between trials. If appropriate, we would have used the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
We planned to include cluster‐randomised trials in the analyses along with individually‐randomised trials. We planned to adjust their sample sizes using the methods described in the Cochrane Handbook for Systematic Reviews of Interventions using an estimate of the intracluster correlation coefficient (ICC) derived from the trial (if possible), from a similar trial, or from a study of a similar population. If we had used ICCs from other sources, we planned to report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we had identified both cluster‐randomised trials and individually‐randomised trials, we planned to synthesise the relevant information. We considered it reasonable to combine the results from both if there was little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit was considered to be unlikely.
We also planned to acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
Cross‐over trials were not considered eligible for inclusion.
Multiple pregnancies
Trials of multiple pregnancies were eligible for inclusion. We planned to adjust for clustering to take into account the non‐independence of babies from the same pregnancy (Gates 2004), however, we were unable to do this because of the lack of reported intercorrelation coefficients (ICC). Treating babies from multiple pregnancies as if they were independent, when they are more likely to have similar outcomes than babies from different pregnancies, would overestimate the sample size and give confidence intervals that were too narrow. Each woman can be considered a cluster in multiple pregnancy, with the number of individuals in the cluster being equal to the number of fetuses in her pregnancy. Analysis using cluster trial methods allows calculation of relative risk and adjustment of confidence intervals. Usually, this will mean that the confidence intervals get wider. Although this may make little difference to the conclusion of a trial, it avoids misleading results in those trials where the difference may be substantial.
In future updates, if information on ICCs are reported, we will adjust for clustering in the analyses, wherever possible, and use the inverse variance method for adjusted analyses, as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Studies with multiple treatment groups
Trials with multiple treatment groups were eligible for inclusion. In trials with multiple intervention groups, we planned to select one pair of interventions and exclude the others and to include two or more independent comparisons, as described in section 16.5.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). One of the included trials, (Lees 2013), included three relevant intervention groups and all were included in three separate independent comparisons: early ductus venosus Doppler ultrasound versus CTG; late ductus venosus Doppler ultrasound versus CTG; and early ductus venosus Doppler ultrasound versus late.
Dealing with missing data
For included studies, levels of attrition were noted. In future updates, if more eligible studies are included, the impact of including studies with high levels of missing data in the overall assessment of treatment effect will be explored by using sensitivity analysis.
For all outcomes, analyses were carried out, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², and the I² and Chi² statistics. We regarded heterogeneity as substantial if I² was greater than 30% and either Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. If we identified substantial heterogeneity (above 30%), we planned to explore it by prespecified subgroup analysis.
Assessment of reporting biases
If there were 10 or more studies in the meta‐analysis, we investigated reporting biases (such as publication bias) using funnel plots. We assessed funnel plot asymmetry visually. If asymmetry was suggested by a visual assessment, we planned to perform exploratory analyses to investigate it (Harbord 2006).
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar.
If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary, if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If we did not consider that the average treatment effect was clinically meaningful, we did not combine trials. If we used random‐effects analyses, the results were presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and the I² statistic.
Subgroup analysis and investigation of heterogeneity
Where we identified substantial heterogeneity, we investigated it using subgroup analyses and sensitivity analyses. We considered whether an overall summary was meaningful, and if it was, we used random‐effects analysis to produce it.
We planned the following a priori subgroup analyses for all outcomes, rather than undertaking separate reviews on singleton and multiple pregnancies:
singleton pregnancies versus multiple pregnancies;
monochorionic twins versus dichorionic twins.
We presented separate data for singleton versus multiple pregnancies, but there was insufficient information in the trial reports to carry out planned subgroup analysis for monochorionic versus dichorionic twins.
We carried out the following additional a priori subgroup analyses for the primary outcomes:
where the fetus was suspected small‐for‐gestational age;
where the woman had hypertension or pre‐eclampsia;
where the woman had diabetes;
prolonged pregnancy;
where there had been previous pregnancy loss.
We assessed subgroup differences by interaction tests available within RevMan 5 (RevMan 2014). We reported the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
We planned sensitivity analyses to explore the effect of trial quality assessed by adequate labelled sequence generation and adequate allocation concealment, with poor‐quality studies (unclear or high risk of bias) being excluded from the analyses in order to assess whether this made any difference to the overall result.
Results
Description of studies
Results of the search
In the previous version of the review, the search identified 29 studies, of which 18 were included, and one study was ongoing; results for this trial have now been published and were included in this updated version of the review (Lees 2013; Lees 2015) (search date 31 March 2017, see: Figure 1). Findings were therefore based on 19 trials involving 10,667 women. In the previous version of the review, 10 trials were excluded and no further trials have been excluded in this update. For further details of trial characteristics, please refer to the tables of Characteristics of included studies and Characteristics of excluded studies.
1.
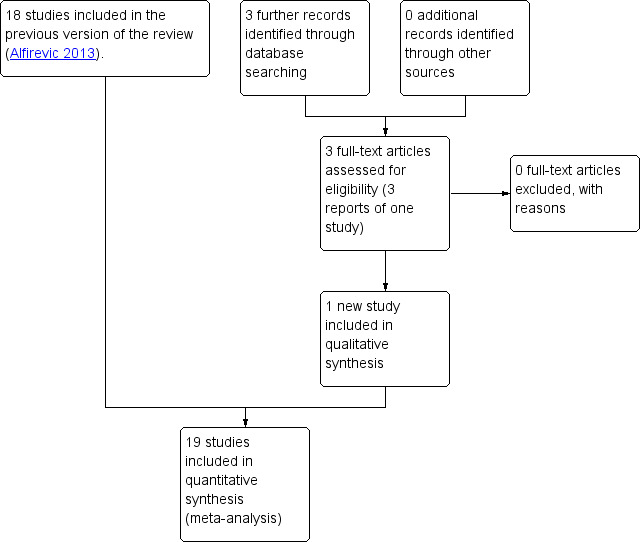
Study flow diagram.
Included studies
Most studies included Doppler assessments of umbilical artery in both experimental and control groups, with the Doppler results being revealed to clinicians only in the 'Doppler group' (Biljan 1992; Burke 1992; De Rochambeau 1992; Giles 2003; Johnstone 1993; Lees 2013; Lees 2015; Neales 1994 [pers comm]; Newnham 1991; Nienhuis 1997; Nimrod 1992; Norman 1992; Ott 1998; Pattinson 1994; Trudinger 1987; Tyrrell 1990). Doppler ultrasound of the umbilical artery was used as an addition to the standard fetal monitoring (e.g. cardiotocography (CTG), biophysical profile, fetal biometry).
Eight of these studies involved singleton pregnancies only (Biljan 1992; De Rochambeau 1992; Lees 2013; Neales 1994 [pers comm]; Nienhuis 1997; Ott 1998; Trudinger 1987; Tyrrell 1990) and one study of 539 women involved twin pregnancies only (Giles 2003). Two studies assessed a mixture of singleton and multiple pregnancies with 40/2289 (1.7%) being twin pregnancies in Johnstone 1993 and 40/505 (7.9%) being twin pregnancies in Newnham 1991. Four studies did not state whether they included just singleton pregnancies or not (Burke 1992; Nimrod 1992; Norman 1992; Pattinson 1994).
Four studies compared Doppler ultrasound alone versus CTG alone in women whose pregnancies were considered at increased risk of problems (Almstrom 1992; Haley 1997; Hofmeyr 1991; Williams 2003). Of these, three involved singleton pregnancies only (Almstrom 1992; Haley 1997; Williams 2003) and one study did not specify (Hofmeyr 1991).
Gestational age for inclusion in studies was not reported in six studies, and the remainder of the studies varied in the gestational ages they included, from 24 weeks' gestation to those studies looking at the value of Doppler ultrasound when women had gone beyond 40 weeks (Characteristics of included studies).
One study compared three different monitoring strategies to trigger delivery in mothers with early fetal growth restriction: early changes in ductus venosus (pulsatility index > 95th percentile) versus late changes in ductus venosus (absent or negative A‐wave) versus short term variation from computerised CTG (cCTG) (Lees 2013). However, all women were monitored by cCTG and safety net criteria for delivery based on cCTG applied to all women, irrespective of randomised group.
Excluded studies
Ten of the 29 potentially eligible studies were excluded. In five studies, the participants were described as 'unselected populations' (Davies 1992; Newnham 1993; Omtzigt 1994; Schneider 1992; Whittle 1994); in one study, the participants were women considered at low risk of complications (Mason 1993); one study was not a randomised study (McCowan 1996); in one study, the full report was not available and there were no data in the conference abstract (Gonsoulin 1991), and in two studies the information was considered unreliable (McParland 1988; Pearce 1992).
Risk of bias in included studies
The quality of the 19 completed included studies was difficult to assess due to lack of information, particularly in terms of randomisation and concealment of allocation (Figure 2). For this reason, we did not carry out planned sensitivity analysis excluding studies at high risk of bias.
2.
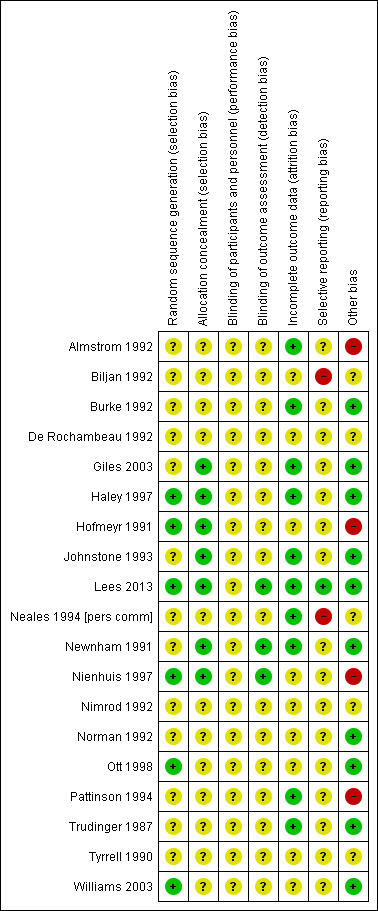
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Only four studies had adequate sequence generation and allocation concealment (Haley 1997; Hofmeyr 1991; Lees 2013; Nienhuis 1997). Two studies had adequate sequence generation but allocation concealment was unclear (Ott 1998; Williams 2003) and in two studies allocation concealment was adequate, but sequence generation was unclear (Giles 2003; Newnham 1991). In three studies, concealment allocation was judged as adequate, but sequence generation was unclear (Giles 2003; Johnstone 1993; Newnham 1991). The remaining 10 studies had both unclear sequence generation and unclear concealment allocation (Almstrom 1992; Biljan 1992; Burke 1992; De Rochambeau 1992; Neales 1994 [pers comm]; Nimrod 1992; Norman 1992; Pattinson 1994; Trudinger 1987; Tyrrell 1990).
Blinding
Blinding women and/or staff in these trials was not generally feasible. Even in the studies where Doppler ultrasound was either revealed or concealed, some outcomes, such as induction of labour and caesarean section were clearly going to be influenced by the knowledge of Doppler results, but it might have been possible to avoid bias in neonatal assessment. Unfortunately, the information on the attempts to protect against biased assessment was often not available. In three studies (Lees 2013; Newnham 1991; Nienhuis 1997), assessors of neonatal outcomes were indeed blind to Doppler results.
Incomplete outcome data
Incomplete outcome data were addressed adequately in 10 studies (Almstrom 1992; Burke 1992; Giles 2003; Haley 1997; Johnstone 1993; Lees 2013; Neales 1994 [pers comm]; Newnham 1991; Pattinson 1994; Trudinger 1987) and unclear in nine studies (Biljan 1992; De Rochambeau 1992; Hofmeyr 1991; Nienhuis 1997; Nimrod 1992; Norman 1992; Ott 1998; Tyrrell 1990; Williams 2003). Only a few studies provided full information on the number of women approached to take part in the studies, the numbers eligible for inclusion, and the overall refusal rate. While not sources of bias as such, high exclusion and refusal rates might affect the generalisability of the findings and the interpretation of the results.
Selective reporting
Almost all the studies, except three, were assessed as at unclear risk of selective reporting bias because we did not assess the trial protocols. Two studies were considered to have some degree of selective reporting bias (Biljan 1992; Neales 1994 [pers comm]). In one multiple‐intervention study, the protocol was available, there was no evidence of reporting bias, and each group to which participants were randomised was presented (Lees 2013).
Other potential sources of bias
Ten studies were judged to be free of other sources of bias (Burke 1992; Giles 2003; Haley 1997; Johnstone 1993; Lees 2013; Newnham 1991; Norman 1992; Ott 1998; Trudinger 1987; Williams 2003); five studies were unclear (Biljan 1992; De Rochambeau 1992; Neales 1994 [pers comm]; Nimrod 1992; Tyrrell 1990); and four studies were considered to have some other source of bias, mainly baseline imbalances (Almstrom 1992; Hofmeyr 1991; Nienhuis 1997; Pattinson 1994).
Sensitivity analyses
For sensitivity analyses by quality of studies, we used both adequately labelled sequence generation and adequate allocation concealment as essential criteria for high quality. Only three of the 18 studies in the main comparison for umbilical artery met these criteria (Haley 1997; Hofmeyr 1991; Nienhuis 1997), see Figure 2.
Effects of interventions
See: Table 1
This review included 19 studies involving 10,667 women.
1) Umbilical artery Doppler ultrasound versus no Doppler ultrasound (18 studies, 10,156 women)
We included all completed studies examining umbilical artery Doppler ultrasound, including those that compared Doppler ultrasound alone versus CTG alone, as we wished to get an overall assessment of whether using Doppler ultrasound was beneficial. Findings for important outcomes for this overall assessment are set out in Table 1.
A separate comparison of studies where Doppler was used as an alternative to CTG was also undertaken, and these findings are reported below under 3) 'Umbilical Doppler ultrasound alone versus CTG alone'.
As mentioned above, the quality of the studies included in this comparison was often unclear due to lack of information, particularly in terms of randomisation and concealment allocation.
Main outcomes
It is important to emphasise that this review still remains underpowered to detect clinically important differences in serious neonatal morbidity.
Any perinatal mortality after randomisation (16 studies, 10,225 babies)
There was a clear difference in perinatal mortality between the two groups (risk ratio (RR) 0.71, 95% confidence interval (CI) 0.52 to 0.98, 16 studies, 10,225 babies, 1.2% versus 1.7%, number needed to treat (NNT) 203, 95% CI 103 to 4352, Analysis 1.1, evidence graded moderate). A sensitivity analysis including only the three studies of high quality (low risk of bias for sequence generation and concealment allocation) (Haley 1997; Hofmeyr 1991; Nienhuis 1997) showed no clear difference, though the numbers were small and this analysis lacked the power of the overall analysis (RR 0.61, 95% CI 0.24 to 1.53, three studies, 1197 babies) (data not shown).
1.1. Analysis.
Comparison 1 Umbilical artery Doppler ultrasound versus no Doppler ultrasound, Outcome 1 Any perinatal death after randomisation.
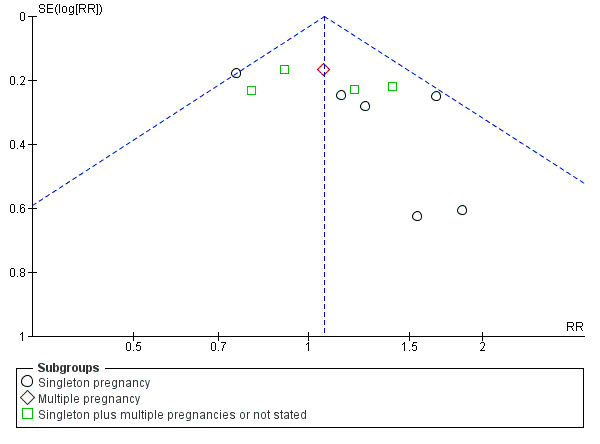
There was no evidence that the treatment effect varied between subgroups as the CIs overlapped (as indicated by the subgroup interaction test (test for subgroup differences: Chi² = 0.80, df = 2 (P = 0.67), I² = 0%; Analysis 1.1)), although the RR for the singleton subgroup was somewhat lower compared with the others (RR 0.59 compared with 0.88, 0.78 and 0.71). There was evidence of funnel plot asymmetry ('small‐study effects', P = 0.057, using Harbord 2006) which might indicate publication bias. We noted that the results of individual studies all crossed the line of no effect and there was overall low heterogeneity for this outcome, therefore, we did not downgrade the evidence (Figure 3). However, possible publication bias was a concern given that the result of the pooled meta‐analysis was borderline.
3.
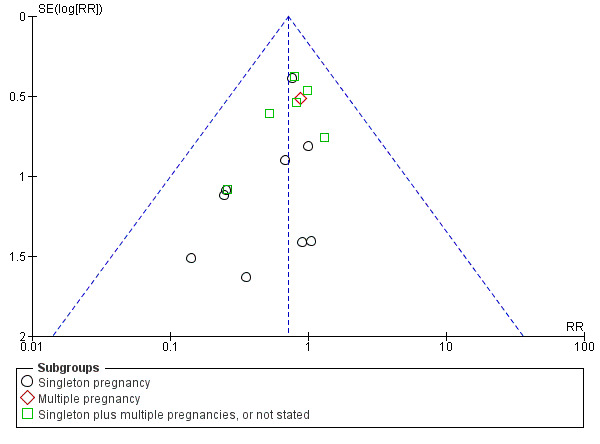
Funnel plot of comparison: 1 Doppler ultrasound versus no Doppler ultrasound, outcome: 1.1 Any perinatal death after randomisation.
It is also important to note that we did not adjust for the nonindependence of twins because of the lack of reported intercorrelation coefficients (ICC).
Serious neonatal morbidity (three studies, 1098 babies)
Only three studies reported relevant neonatal morbidity data (Newnham 1991; Norman 1992; Tyrrell 1990); one study reported no events and the two studies which contributed data showed no clear differences in serious perinatal morbidity between women having Doppler ultrasound and those monitored by standard methods (Analysis 1.2, evidence graded very low). The heterogeneity was high (Tau² = 3.84, Chi²: P = 0.04, I² = 76%) and the numbers of babies with serious morbidity were too small to be able to say anything with any degree of certainty. Thus, we decided, on the advice of our statistician, not to pool the data for this outcome. No studies reported serious neonatal morbidity in multiple pregnancies.
1.2. Analysis.
Comparison 1 Umbilical artery Doppler ultrasound versus no Doppler ultrasound, Outcome 2 Serious neonatal morbidity.
Additional outcomes
The data for stillbirths (RR 0.65, 95% CI 0.41 to 1.04, 9560 babies, 15 studies, Analysis 1.3, evidence graded low), neonatal deaths (RR 0.81, 95% CI 0.53 to 1.24, 8167 babies, 13 studies, Analysis 1.4) and low Apgar score (RR 0.92, 95% CI 0.69 to 1.24; 6321 babies, 7 studies, I² = 30%, Analysis 1.6, evidence graded low) were consistent with the overall picture showing fewer adverse outcomes in the Doppler group, but the CIs crossed the line of no effect.
1.3. Analysis.
Comparison 1 Umbilical artery Doppler ultrasound versus no Doppler ultrasound, Outcome 3 Stillbirth.
1.4. Analysis.
Comparison 1 Umbilical artery Doppler ultrasound versus no Doppler ultrasound, Outcome 4 Neonatal death.
1.6. Analysis.
Comparison 1 Umbilical artery Doppler ultrasound versus no Doppler ultrasound, Outcome 6 Apgar < 7 at 5 minutes.
The clear difference favouring the Doppler group in perinatal deaths, seen in Analysis 1.1, was also present when the analysis focused just on potentially preventable perinatal deaths (RR 0.67, 95% CI 0.46 to 0.98, 16 studies, 10,225 babies, Analysis 1.5).
1.5. Analysis.
Comparison 1 Umbilical artery Doppler ultrasound versus no Doppler ultrasound, Outcome 5 Any potentially preventable perinatal death*.
The reduction in elective and emergency caesarean sections with the use of Doppler ultrasound was clear (RR 0.90, 95% CI 0.84 to 0.97, 14 studies, 7918 women, Analysis 1.7, evidence graded moderate), though the upper limit of the CI was close to one. When caesarean sections were reported as either elective or emergency, the reduction in caesareans appeared to be confined to the emergency procedures (elective only: RR 1.07, 95% CI 0.93 to 1.22; 6627 women; 11 studies; Analysis 1.8; emergency only: average RR 0.81, 95% CI 0.67 to 0.98, 6175 women, 10 studies, Tau² = 0.04; Chi² = 16.21, P = 0.06, I² = 44%, Analysis 1.9). This is something that will be explored in a meta‐regression in future updates if more data become available.
1.7. Analysis.
Comparison 1 Umbilical artery Doppler ultrasound versus no Doppler ultrasound, Outcome 7 Caesarean section (elective and emergency).
1.8. Analysis.
Comparison 1 Umbilical artery Doppler ultrasound versus no Doppler ultrasound, Outcome 8 Caesarean section ‐ elective.
1.9. Analysis.
Comparison 1 Umbilical artery Doppler ultrasound versus no Doppler ultrasound, Outcome 9 Caesarean section ‐ emergency.
There was also some evidence of possible publication bias in the funnel plots (Figure 4; Figure 5; Figure 6). The Harbord test (Harbord 2006) for all caesarean sections did not suggest evidence of asymmetry (P = 0.12) but there did appear to be asymmetry by visual inspection indicating that there might have been some small studies missing, although none of the individual published studies showed clear differences between the groups. Possible publication bias is of concern because the pooled meta‐analysis CI was close to the line of no effect . With elective caesarean sections, there was evidence of asymmetry (P = 0.1) and the visual assessment indicating the 'missing' studies were those below a relative risk of one, so the pooled result is likely to be even closer to the null. For emergency caesarean sections, there was evidence of asymmetry (P = 0.09), again this being a small‐study effect. Heterogeneity can sometimes contribute to funnel plot asymmetry, so overall we should be cautious about the significance of the pooled result.
4.
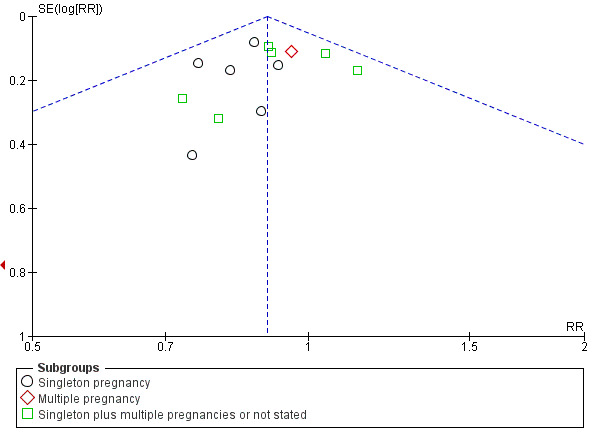
Funnel plot of comparison: 1 Doppler ultrasound versus no Doppler ultrasound, outcome: 1.8 Cesarean section (elective and emergency).
5.
Funnel plot of comparison: 1 Doppler ultrasound versus no Doppler ultrasound, outcome: 1.9 Cesarean section ‐ elective.
6.
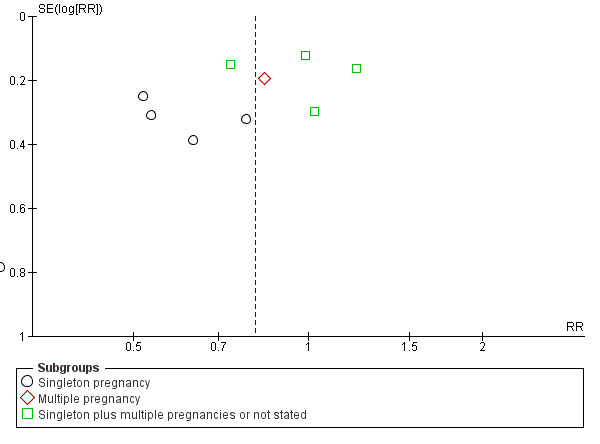
Funnel plot of comparison: 1 Doppler ultrasound versus no Doppler ultrasound, outcome: 1.10 Cesarean section ‐ emergency.
Caesarean section results for subgroups based on the populations (singletons, multiples, not specified) were consistent with the overall effect in terms of the direction and size. However, the heterogeneity in the subgroup of emergency caesarean section was high and, therefore, a random‐effects model was used for pooling (average RR 0.81, 95% CI 0.67 to 0.98; test for subgroup differences: Chi² = 7.47, df = 2 (P = 0.02), I² = 73.2%; Analysis 1.9). This analysis provided evidence that the average RR across studies was clearly less than one, indicating a reduction in emergency caesarean section. However, we also calculated the 95% prediction interval (PI) for the underlying effect in any future studies (PI = 0.49 to 1.35); this indicated that the underlying RR may be greater than one in an individual study, due to the between‐study heterogeneity.
Overall, there were no clear differences identified in spontaneous vaginal births (RR 1.04, 95% CI 0.98 to 1.10; 2504 women; 5 studies, Analysis 1.10) and operative vaginal births (RR 0.95, 95% CI 0.80 to 1.14; 2813 women; 4 studies; Analysis 1.11) for women having the umbilical artery Doppler ultrasound compared with women not having the Doppler ultrasound.
1.10. Analysis.
Comparison 1 Umbilical artery Doppler ultrasound versus no Doppler ultrasound, Outcome 10 Spontaneous vaginal birth.
1.11. Analysis.
Comparison 1 Umbilical artery Doppler ultrasound versus no Doppler ultrasound, Outcome 11 Operative vaginal birth.
There was, however, an average reduction in induction of labour for women with the umbilical artery Doppler intervention (average RR 0.89, 95% CI 0.80 to 0.99, 10 studies, 5633 women, random‐effects (Tau² = 0.01, Chi²: P = 0.08, I² = 41%), PI 0.68 to 1.16, Analysis 1.12, evidence graded moderate). Although the average effect across studies was evident, the prediction interval suggested that, due to the between‐study heterogeneity, we could not rule out the possibility that the underlying effect in a future study might actually increase induction of labour. There might be some clinical heterogeneity around the assessment of induction of labour due to the varying methods and timings of this intervention.
1.12. Analysis.
Comparison 1 Umbilical artery Doppler ultrasound versus no Doppler ultrasound, Outcome 12 Induction of labour.
There was no difference identified overall in intubation or ventilation (average RR 1.42, 95% CI 0.87 to 2.30, six studies, 3136 babies, Analysis 1.13). Again, random‐effects were used because of high heterogeneity (Tau² = 0.14, Chi²: P = 0.09, I² = 47%) and a wide prediction interval was estimated due to the large heterogeneity and small number of studies in the meta‐analysis (PI 0.41 to 4.94, Analysis 1.13).
1.13. Analysis.
Comparison 1 Umbilical artery Doppler ultrasound versus no Doppler ultrasound, Outcome 13 Infant requiring intubation/ventilation.
There was evidence of a difference between subgroups (interaction test for inverse variance analysis: Chi² = 8.67, df = 2 (P = 0.01)) suggesting that there might be an effect in singletons, but not in multiple pregnancies. The data were limited because there is only one trial in multiples and one with singleton and multiples combined. Further studies are needed to confirm if there is a difference here or not.
There was no clear difference identified in neonatal fitting/seizures (RR 0.35, 95% CI 0.01 to 8.49, 150 babies, 1 study, Analysis 1.14), or preterm labour (RR 1.12, 95% CI 0.72 to 1.75; 626 women, 2 studies Analysis 1.15), though sample sizes were small for both outcomes.
1.14. Analysis.
Comparison 1 Umbilical artery Doppler ultrasound versus no Doppler ultrasound, Outcome 14 Neonatal fitting/seizures.
1.15. Analysis.
Comparison 1 Umbilical artery Doppler ultrasound versus no Doppler ultrasound, Outcome 15 Preterm labour.
Overall, there was a small increase in gestational age (weeks) for babies exposed to umbilical artery Doppler ultrasound (average mean difference (MD) 0.21, 95% CI ‐0.02 to 0.43, eight studies, 4066 babies, random‐effects (Tau²= 0.04, Chi²: P = 0.11, I² = 40%, Analysis 1.16). However, the prediction interval suggested that, due to between‐study heterogeneity, we cannot rule out that a future study might show a decrease in gestational age. This finding should, therefore, be interpreted with caution.
1.16. Analysis.
Comparison 1 Umbilical artery Doppler ultrasound versus no Doppler ultrasound, Outcome 16 Gestational age at birth (weeks).
There were no clear differences found in risk of infant respiratory distress syndrome (RDS) in singleton pregnancies (no study reported multiples) (RR 1.06, 95% CI 0.07 to 16.48, 107 babies; 1 study; Analysis 1.17), neonatal admission to special care baby unit (SCBU) and/or neonatal intensive care unit (NICU) (RR 0.95, 95% CI 0.89 to 1.03, 9334 babies, 12 studies, Analysis 1.18) , hypoxic ischaemic encephalopathy (average RR 0.65, 95% CI 0.01 to 33.07, 1045 babies, 2 studies, I² = 72%, Analysis 1.19), intraventricular haemorrhage (RR 1.42, 95% CI 0.47 to 4.30, 2008 babies, 4 studies, Analysis 1.20), or birthweight (MD 31.33, 95% CI ‐8.70 to 71.37; 3887 babies; 7 studies; Analysis 1.21).
1.17. Analysis.
Comparison 1 Umbilical artery Doppler ultrasound versus no Doppler ultrasound, Outcome 17 Infant respiratory distress syndrome (RDS).
1.18. Analysis.
Comparison 1 Umbilical artery Doppler ultrasound versus no Doppler ultrasound, Outcome 18 Neonatal admission to SCBU and/or NICU.
1.19. Analysis.
Comparison 1 Umbilical artery Doppler ultrasound versus no Doppler ultrasound, Outcome 19 Hypoxic ischaemic encephalopathy.
1.20. Analysis.
Comparison 1 Umbilical artery Doppler ultrasound versus no Doppler ultrasound, Outcome 20 Intraventricular haemorrhage.
1.21. Analysis.
Comparison 1 Umbilical artery Doppler ultrasound versus no Doppler ultrasound, Outcome 21 Birthweight (grams).
There was a reduction in the length of infant hospital stay (days) in singleton pregnancies that had umbilical artery Doppler intervention, (standardised MD (SMD) ‐0.28, 95% CI ‐0.40 to ‐0.16, three studies, 1076 babies, Analysis 1.22).
1.22. Analysis.
Comparison 1 Umbilical artery Doppler ultrasound versus no Doppler ultrasound, Outcome 22 Length of infant hospital stay (days).
We also included reported data for all other prespecified secondary outcomes when available, none of which conclusively showed clinically important differences between groups.
Non‐prespecified outcomes
For completeness, we also included the graphs for eight clinically relevant outcomes that were not prespecified in our protocol. There were fewer antenatal admissions in the Doppler group (RR 0.72, 95% CI 0.60 to 0.88, 893 women, 2 studies, Analysis 1.24) but all other outcomes showed no clear difference between the groups.
1.24. Analysis.
Comparison 1 Umbilical artery Doppler ultrasound versus no Doppler ultrasound, Outcome 24 Antenatal admissions (not prespecified).
Birth less than 34 weeks (RR 2.04, 95% CI 0.62 to 6.69, 976 women, 2 studies, I² = 52%, Analysis 1.23);
Phototherapy for neonatal jaundice (RR 0.15, 95% CI 0.01 to 2.87, 150 babies, 1 study, Analysis 1.25);
Abnormal neurological development at 9 months (RR 0.61, 95% CI 0.26 to 1.45, 137 babies, 1 study, Analysis 1.26);
Hospitalisation for IUGR neonatal (RR 1.03, 95% CI 0.75 to 1.41, 142 babies, 1 study, Analysis 1.27);
Fetal distress in labour (RR 0.35, 95% CI 0.10 to 1.22, 289 women, 1 study, Analysis 1.28);
Birthweight < 5 percentile (RR 1.16, 95% CI 0.51 to 2.64; 289 babies, 1 study, Analysis 1.29);
Periventricular leucomalacia (RR 0.33, 95% CI 0.01 to 8.00, 545 babies, 1 study, Analysis 1.30);
Antenatal hospital stay (days) (MD ‐0.60, 95% CI ‐2.39 to 1.19, 426 women, 1 study, Analysis 1.31).
1.23. Analysis.
Comparison 1 Umbilical artery Doppler ultrasound versus no Doppler ultrasound, Outcome 23 Birth < 34 weeks (not prespecified).
1.25. Analysis.
Comparison 1 Umbilical artery Doppler ultrasound versus no Doppler ultrasound, Outcome 25 Phototherapy for neonatal jaundice (not prespecified).
1.26. Analysis.
Comparison 1 Umbilical artery Doppler ultrasound versus no Doppler ultrasound, Outcome 26 Abnormal neurological development at 9 months (not prespecified).
1.27. Analysis.
Comparison 1 Umbilical artery Doppler ultrasound versus no Doppler ultrasound, Outcome 27 Hospitalisation for IUGR neonatal (not prespecified).
1.28. Analysis.
Comparison 1 Umbilical artery Doppler ultrasound versus no Doppler ultrasound, Outcome 28 Fetal distress in labour (not prespecified).
1.29. Analysis.
Comparison 1 Umbilical artery Doppler ultrasound versus no Doppler ultrasound, Outcome 29 Birthweight < 5 percentile (not prespecified).
1.30. Analysis.
Comparison 1 Umbilical artery Doppler ultrasound versus no Doppler ultrasound, Outcome 30 Periventricular leucomalacia (not prespecified).
1.31. Analysis.
Comparison 1 Umbilical artery Doppler ultrasound versus no Doppler ultrasound, Outcome 31 Antenatal hospital stay (days) (not prespecified).
Oxytocin augmentation, requirement for neonatal resuscitation, preterm labour (onset of labour before 37 completed weeks of pregnancy), meconium aspiration, bronchopulmonary dysplasia (BPD), necrotising enterocolitis (NEC), long‐term infant/child neurodevelopmental outcome, and women's views of their care were not reported in any trial under this comparison.
2) Umbilical artery Doppler ultrasound versus no Doppler ultrasound (all subgroups)
Six studies reported main outcomes by subgroups.
Any perinatal mortality after randomisation
Five studies assessed women with suspected small‐for‐gestational age (SGA)/IUGR (Almstrom 1992; Haley 1997; Neales 1994 [pers comm]; Nienhuis 1997; Pattinson 1994) (RR 0.72, 95% CI 0.38 to 1.35; 1292 women; 5 studies), one study assessed women with hypertension/pre‐eclampsia (Pattinson 1994) (RR 3.57, 95% CI 0.42 to 30.73; 89 women; 1 study) and one study assessed women with a previous pregnancy loss (Norman 1992) (RR 0.26, 95% CI 0.03 to 2.17; 53 women; 1 study). Findings are reported in Analysis 2.1. No clear differences were found in any of the subgroups. As only one study assessed women with hypertension/pre‐eclampsia, and women with a previous pregnancy loss, there were not enough data to perform a meaningful subgroup analysis and therefore data were not pooled for this analysis.
2.1. Analysis.
Comparison 2 Umbilical artery Doppler ultrasound versus no Doppler ultrasound (all subgroups), Outcome 1 Any perinatal death after randomisation.
One small study (Norman 1992) assessed serious neonatal morbidity in women with a previous pregnancy loss but did not report any morbidity in either group (Analysis 2.2). We were unable to carry out planned subgroup analysis examining monochorionic twins versus dichorionic twins due to lack of data.
2.2. Analysis.
Comparison 2 Umbilical artery Doppler ultrasound versus no Doppler ultrasound (all subgroups), Outcome 2 Serious neonatal morbidity.
No additional outcomes were reported under this comparison.
3) Umbilical artery Doppler ultrasound as an alternative to CTG monitoring (four studies, 2834 women)
Four trials were included in this comparison (Almstrom 1992; Haley 1997; Hofmeyr 1991; Williams 2003). Unfortunately, this analysis had much less power for assessing main clinical outcomes than the main comparison (which included 12 studies where additional methods of fetal monitoring were used in both groups).
In terms of quality, two of the four studies were judged to be at low risk of bias (Haley 1997; Hofmeyr 1991) whilst the rest were classified as 'unclear' because of the lack of information on randomisation and the allocation process.
Main outcomes
Any perinatal mortality after randomisation
Overall, there was no clear difference identified in perinatal mortality (RR 0.45, 95% CI 0.17 to 1.15, four studies, 2813 babies, Analysis 3.1). Only two studies were judged to have adequate sequence generation and allocation concealment (Haley 1997; Hofmeyr 1991) and using only these in a sensitivity analysis similarly showed no clear difference identified in perinatal mortality (RR 0.58, 95% CI 0.20 to 1.73, two studies, 1047 babies, data not shown).
3.1. Analysis.
Comparison 3 Umbilical artery Doppler ultrasound alone versus CTG alone, Outcome 1 Any perinatal death after randomisation.
There was no evidence that the treatment effect varied between subgroups as the CIs overlapped.
None of the studies provided data on serious perinatal morbidity.
Additional outcomes
There were no clear differences between groups for stillbirths (RR 0.48, 95% CI 0.14 to 1.71, four studies, 2813 babies, Analysis 3.2), neonatal death (RR 0.52, 95% CI 0.16 to 1.72, three studies, 1473 babies, Analysis 3.3), potentially preventable deaths (RR 0.38, 95% CI 0.12 to 1.18, four studies, 2813 babies, Analysis 3.4), and Apgar score < 7 at five minutes (RR 0.86, 95% CI 0.54 to 1.37; 2663 babies; three studies; Analysis 3.5). The same was true for all other additional outcomes, with the exception of caesarean section rate and length of hospital stay for neonates.
3.2. Analysis.
Comparison 3 Umbilical artery Doppler ultrasound alone versus CTG alone, Outcome 2 Stillbirth.
3.3. Analysis.
Comparison 3 Umbilical artery Doppler ultrasound alone versus CTG alone, Outcome 3 Neonatal death.
3.4. Analysis.
Comparison 3 Umbilical artery Doppler ultrasound alone versus CTG alone, Outcome 4 Any potentially preventable perinatal death*.
3.5. Analysis.
Comparison 3 Umbilical artery Doppler ultrasound alone versus CTG alone, Outcome 5 Apgar < 7 at 5 minutes.
Overall rates of caesarean section, when both elective and emergency caesareans were combined, showed fewer caesareans in the umbilical artery Doppler group (RR 0.89, 95% CI 0.79 to 1.01, four studies, 2813 babies, Analysis 3.6). Interestingly, the results from three studies that reported emergency and elective caesareans separately showed fewer emergency caesareans (RR 0.66, 95% CI 0.52 to 0.84, three studies, 1473 women, Analysis 3.8) and more elective caesareans (RR 1.53, 95% CI 1.12 to 2.09, three studies, 1473 women, Analysis 3.7) in the umbilical artery Doppler group. There were too few studies to explore this differential effect in a formal meta‐regression, but lack of heterogeneity for these outcomes suggested that the effect of the umbilical artery Doppler studies on the type of caesareans was real.
3.6. Analysis.
Comparison 3 Umbilical artery Doppler ultrasound alone versus CTG alone, Outcome 6 Caesarean section (elective and emergency).
3.8. Analysis.
Comparison 3 Umbilical artery Doppler ultrasound alone versus CTG alone, Outcome 8 Caesarean section ‐ emergency.
3.7. Analysis.
Comparison 3 Umbilical artery Doppler ultrasound alone versus CTG alone, Outcome 7 Caesarean section ‐ elective.
There were no clear differences between the groups for spontaneous vaginal birth (RR 1.06, 95% CI 0.97 to 1.15, 1323 women, 2 studies, Analysis 3.9), operative vaginal birth (RR 0.98, 95% CI 0.81 to 1.17, 2663 women, 3 studies, Analysis 3.10), induction of labour (RR 0.67, 95% CI 0.32 to 1.40, 576 women, 2 studies, I² = 74%, Analysis 3.11), infant requiring intubation/ventilation (RR 1.54, 95% CI 0.26 to 9.08, 576 babies, 2 studies, Analysis 3.12), neonatal fitting/seizures (RR 0.35, 95% CI 0.01 to 8.49, 150 babies, 1 study, Analysis 3.13), gestational age at birth (MD 0.23, 95% CI ‐0.00 to 0.47; 1473 babies, 3 studies, Analysis 3.14), neonatal admission to SCBU and/or NICU (RR 0.87, 95% CI 0.73 to 1.03, 2813 babies, 4 studies, Analysis 3.15), and infant birthweight (MD 38.41, 95% CI ‐6.14 to 82.97, 2813 babies, 4 studies, Analysis 3.16).
3.9. Analysis.
Comparison 3 Umbilical artery Doppler ultrasound alone versus CTG alone, Outcome 9 Spontaneous vaginal birth.
3.10. Analysis.
Comparison 3 Umbilical artery Doppler ultrasound alone versus CTG alone, Outcome 10 Operative vaginal birth.
3.11. Analysis.
Comparison 3 Umbilical artery Doppler ultrasound alone versus CTG alone, Outcome 11 Induction of labour.
3.12. Analysis.
Comparison 3 Umbilical artery Doppler ultrasound alone versus CTG alone, Outcome 12 Infant requiring intubation/ventilation.
3.13. Analysis.
Comparison 3 Umbilical artery Doppler ultrasound alone versus CTG alone, Outcome 13 Neonatal fitting/seizures.
3.14. Analysis.
Comparison 3 Umbilical artery Doppler ultrasound alone versus CTG alone, Outcome 14 Gestational age at birth.
3.15. Analysis.
Comparison 3 Umbilical artery Doppler ultrasound alone versus CTG alone, Outcome 15 Neonatal admission to SCBU and/or NICU.
3.16. Analysis.
Comparison 3 Umbilical artery Doppler ultrasound alone versus CTG alone, Outcome 16 Infant birthweight (grams).
There was a reduction in the length of infant hospital stay with umbilical artery Doppler ultrasound compared with CTG (SMD ‐0.25, 95% CI ‐0.41 to ‐0.08, two studies, 576 babies, Analysis 3.17). The two studies that reported this outcome included just singleton pregnancies. However, the number of babies involved was too small to be able to say anything with any degree of certainty.
3.17. Analysis.
Comparison 3 Umbilical artery Doppler ultrasound alone versus CTG alone, Outcome 17 Length of infant hospital stay (days).
Fetal acidosis, oxytocin augmentation, requirement for neonatal resuscitation, preterm labour (onset of labour before 37 completed weeks of pregnancy), infant respiratory distress syndrome, meconium aspiration, hypoxic ischaemic encephalopathy (a condition of injury to the brain), intraventricular haemorrhage (IVH), bronchopulmonary dysplasia (BPD), necrotising enterocolitis (NEC), long‐term infant/child neurodevelopmental outcome, and women's views of their care were not reported in any trial under this outcome.
Non‐prespecified outcomes
For completeness, we also included the graphs for three clinically relevant outcomes that were not prespecified in our protocol. There were fewer antenatal admissions in the Doppler group (RR 0.70, 95% CI 0.55 to 0.90, 426 women, 1 study, Analysis 3.18), but no clear difference between groups in phototherapy rates for neonatal jaundice (RR 0.15, 95% CI 0.01 to 2.87, 150 babies, 1 study, Analysis 3.19), or antenatal hospital stay (days) (MD ‐0.60, 95% CI ‐2.39 to 1.19, 426 women, 1 study, Analysis 3.20).
3.18. Analysis.
Comparison 3 Umbilical artery Doppler ultrasound alone versus CTG alone, Outcome 18 Antenatal admissions (not prespecified).
3.19. Analysis.
Comparison 3 Umbilical artery Doppler ultrasound alone versus CTG alone, Outcome 19 Phototherapy for neonatal jaundice (not prespecified).
3.20. Analysis.
Comparison 3 Umbilical artery Doppler ultrasound alone versus CTG alone, Outcome 20 Antenatal hospital stay (days) (not prespecified).
4) Umbilical artery Doppler ultrasound as an alternative to CTG monitoring (all subgroups)
Three studies reported primary outcomes by subgroups. Two studies assessed women with suspected SGA/IUGR (Almstrom 1992; Haley 1997) and one study assessed women with hypertension/pre‐eclampsia (Pattinson 1994). There was no clear difference in perinatal mortality between groups for women with suspected SGA/IUGR (RR 0.33, 95% CI 0.05 to 2.09; 572 women; 2 studies) or women with hypertension/pre‐eclampsia (RR 3.57, 95% CI 0.42 to 30.73, 89 women,1 study). Findings were reported in Analysis 4.1. Studies assessed only perinatal mortality and none assessed serious neonatal morbidity. It was not possible to carry out any meaningful subgroup analysis due to a lack of data.
4.1. Analysis.
Comparison 4 Umbilical artery Doppler ultrasound alone versus CTG alone (all subgroups), Outcome 1 Any perinatal death after randomisation.
No additional outcomes were reported in any trials under this comparison.
5) Early ductus venosus Doppler ultrasound versus computerised CTG (one study, 333 women)
Two arms of a three‐arm trial recruiting women with singleton pregnancies compared these interventions (Lees 2013). This study was of high quality (low risk of bias for sequence generation and concealment allocation).
Main outcomes
There was no clear difference in any perinatal death after randomisation (RR 0.84, 95% CI 0.39 to 1.82; 333 infants, Analysis 5.1). Serious neonatal morbidity was reported separately as death or survival following severe morbidity; for the infants surviving following severe morbidity, there was no clear evidence of a difference between groups (RR 1.10, 95% CI 0.75 to 1.61; 333 women, Analysis 5.2).
5.1. Analysis.
Comparison 5 Early ductus venosus Doppler ultrasound versus CTG, Outcome 1 Any perinatal death after randomisation.
5.2. Analysis.
Comparison 5 Early ductus venosus Doppler ultrasound versus CTG, Outcome 2 Survival following severe neonatal morbidity.
Additional outcomes
There were insufficient data to show clear differences between early ductus venosus Doppler ultrasound versus CTG for stillbirth (RR 1.99, 95% CI 0.37 to 10.71, 333 babies, 1 study, Analysis 5.3), neonatal death (RR 0.60, 95% CI 0.22 to 1.60, 333 babies, 1 study, Analysis 5.4), any potentially preventable perinatal death (RR 0.83, 95% CI 0.37 to 1.86, 333 babies, 1 study, Analysis 5.5), fetal acidosis (RR 0.25, 95% CI 0.03 to 2.20, 333 babies, 1 study, Analysis 5.6), Apgar less than seven at five minutes (RR 0.87, 95% CI 0.44 to 1.72, 333 babies, 1 study, Analysis 5.7), infant requiring intubation/ventilation (RR 0.87, 95% CI 0.67 to 1.13, 333 babies, 1 study, Analysis 5.8), intraventricular haemorrhage (RR 8.95, 95% CI 0.49 to 164.87, 333 babies, 1 study, Analysis 5.9), bronchopulmonary dysplasia (RR 0.87, 95% CI 0.55 to 1.38, 333 babies, 1 study, Analysis 5.10), necrotising enterocolitis (RR 0.33, 95% CI 0.03 to 3.15; 333 babies, 1 study, Analysis 5.11), infant birthweight (grams) (MD 38.00, 95% CI ‐31.53 to 107.53, 333 babies, 1 study, Analysis 5.12), long‐term infant neurodevelopmental outcome (impairment at two years) (RR 0.60, 95% CI 0.30 to 1.18; 333 infants, 1 study, Analysis 5.13), long‐term infant neurodevelopmental outcome (cerebral palsy at two years) (RR 0.20, 95% CI 0.02 to 1.68, 333 infants, 1 study, Analysis 5.14), infant survival at two years without neurodevelopmental impairment (RR 1.07, 95% CI 0.92 to 1.23, 333 infants, 1 study, Analysis 5.15), and sepsis (proven) (RR 0.93, 95% CI 0.60 to 1.45, 333 babies, 1 study, Analysis 5.16).
5.3. Analysis.
Comparison 5 Early ductus venosus Doppler ultrasound versus CTG, Outcome 3 Stillbirth.
5.4. Analysis.
Comparison 5 Early ductus venosus Doppler ultrasound versus CTG, Outcome 4 Neonatal death.
5.5. Analysis.
Comparison 5 Early ductus venosus Doppler ultrasound versus CTG, Outcome 5 Any potentially preventable perinatal death*.
5.6. Analysis.
Comparison 5 Early ductus venosus Doppler ultrasound versus CTG, Outcome 6 Fetal acidosis.
5.7. Analysis.
Comparison 5 Early ductus venosus Doppler ultrasound versus CTG, Outcome 7 Apgar < 7 at 5 minutes.
5.8. Analysis.
Comparison 5 Early ductus venosus Doppler ultrasound versus CTG, Outcome 8 Infant requiring intubation/ventilation.
5.9. Analysis.
Comparison 5 Early ductus venosus Doppler ultrasound versus CTG, Outcome 9 Intraventricular haemorrhage.
5.10. Analysis.
Comparison 5 Early ductus venosus Doppler ultrasound versus CTG, Outcome 10 Bronchopulmonary dysplasia.
5.11. Analysis.
Comparison 5 Early ductus venosus Doppler ultrasound versus CTG, Outcome 11 Necrotising enterocolitis.
5.12. Analysis.
Comparison 5 Early ductus venosus Doppler ultrasound versus CTG, Outcome 12 Infant birthweight (grams).
5.13. Analysis.
Comparison 5 Early ductus venosus Doppler ultrasound versus CTG, Outcome 13 Long‐term infant neurodevelopmental outcome (impairment at 2 years).
5.14. Analysis.
Comparison 5 Early ductus venosus Doppler ultrasound versus CTG, Outcome 14 Long‐term infant neurodevelopmental outcome (cerebral palsy at 2 years).
5.15. Analysis.
Comparison 5 Early ductus venosus Doppler ultrasound versus CTG, Outcome 15 Infant survival at 2 years without neurodevelopmental impairment (not prespecified).
5.16. Analysis.
Comparison 5 Early ductus venosus Doppler ultrasound versus CTG, Outcome 16 Sepsis (proven) (not prespecified).
Caesarean section (both elective and emergency), spontaneous vaginal birth, operative vaginal birth, induction of labour, oxytocin augmentation, requirement for neonatal resuscitation, neonatal fitting/seizures, preterm labour (onset of labour before 37 completed weeks of pregnancy), gestational age at birth, infant respiratory distress syndrome, meconium aspiration, neonatal admission to special care or intensive care unit, or both, hypoxic ischaemic encephalopathy (a condition of injury to the brain), length of infant hospital stay, and women's views of their care were not reported in this trial.
6) Late ductus venosus Doppler ultrasound versus computerised CTG (one study, 336 women)
Two arms of a three‐arm trial compared these interventions (Lees 2013). This trial recruited women with singleton pregnancies only. The study was of high quality (low risk of bias for sequence generation and concealment allocation).
Main outcomes
There was no clear difference in any perinatal death after randomisation (RR 1.28, 95% CI 0.64 to 2.55, 336 infants, 1 study Analysis 6.1). For the infants surviving following severe morbidity, there was no clear evidence of difference between groups (RR 0.98, 95% CI 0.66 to 1.45; 336 infants, 1 study, Analysis 6.2).
6.1. Analysis.
Comparison 6 Late ductus venosus Doppler ultrasound versus CTG, Outcome 1 Any perinatal death after randomisation.
6.2. Analysis.
Comparison 6 Late ductus venosus Doppler ultrasound versus CTG, Outcome 2 Survival following severe neonatal morbidity.
Additional outcomes
Fewer infants whose birth was triggered by late ductus venosus Doppler ultrasound had long‐term infant neurodevelopmental impairment at two years (RR 0.34, 95% CI 0.15 to 0.79; 336 infants, 1 study, Analysis 6.13).
6.13. Analysis.
Comparison 6 Late ductus venosus Doppler ultrasound versus CTG, Outcome 13 Long‐term infant neurodevelopmental outcome (impairment at 2 years).
There were insufficient data to show clear differences between late ductus venosus Doppler ultrasound versus CTG for stillbirth (RR 2.93, 95% CI 0.60 to 14.31, 336 babies; 1 study, Analysis 6.3), neonatal death (RR 1.07, 95% CI 0.47 to 2.46, 336 babies, 1 study, Analysis 6.4), any potentially preventable perinatal death (RR 1.22, 95% CI 0.59 to 2.53, 336 babies, 1 study, Analysis 6.5), fetal acidosis (RR 0.11, 95% CI 0.01 to 2.00; 336 babies; 1 study; Analysis 6.6), Apgar less than seven at five minutes (RR 1.28, 95% CI 0.69 to 2.37, 336 babies, 1 study, Analysis 6.7), infant requiring intubation/ventilation (RR 0.94, 95% CI 0.73 to 1.20, 336 babies, 1 study, Analysis 6.8), intraventricular haemorrhage (RR 16.60, 95% CI 0.97 to 285.35, 336 babies, 1 study, Analysis 6.9), bronchopulmonary dysplasia (RR 0.95, 95% CI 0.61 to 1.48; 336 babies, 1 study, Analysis 6.10), necrotising enterocolitis (RR 0.98, 95% CI 0.20 to 4.77; 336 babies, 1 study, Analysis 6.11), infant birthweight (grams) (MD 25.00, 95% CI ‐40.06 to 90.06; 336 babies, 1 study, Analysis 6.12), long‐term infant neurodevelopmental outcome (cerebral palsy at two years) (RR 0.09, 95% CI 0.00 to 1.59, 336 infants, 1 study, Analysis 6.14), infant survival at two years without neurodevelopmental impairment (RR 1.17, 95% CI 1.02 to 1.34, 336 infants, 1 study, Analysis 6.15), and sepsis (proven) (RR 0.68, 95% CI 0.42 to 1.11, 336 babies, 1 study, Analysis 6.16).
6.3. Analysis.
Comparison 6 Late ductus venosus Doppler ultrasound versus CTG, Outcome 3 Stillbirth.
6.4. Analysis.
Comparison 6 Late ductus venosus Doppler ultrasound versus CTG, Outcome 4 Neonatal death.
6.5. Analysis.
Comparison 6 Late ductus venosus Doppler ultrasound versus CTG, Outcome 5 Any potentially preventable perinatal death*.
6.6. Analysis.
Comparison 6 Late ductus venosus Doppler ultrasound versus CTG, Outcome 6 Fetal acidosis.
6.7. Analysis.
Comparison 6 Late ductus venosus Doppler ultrasound versus CTG, Outcome 7 Apgar < 7 at 5 minutes.
6.8. Analysis.
Comparison 6 Late ductus venosus Doppler ultrasound versus CTG, Outcome 8 Infant requiring intubation/ventilation.
6.9. Analysis.
Comparison 6 Late ductus venosus Doppler ultrasound versus CTG, Outcome 9 Intraventricular haemorrhage.
6.10. Analysis.
Comparison 6 Late ductus venosus Doppler ultrasound versus CTG, Outcome 10 Bronchopulmonary dysplasia.
6.11. Analysis.
Comparison 6 Late ductus venosus Doppler ultrasound versus CTG, Outcome 11 Necrotising enterocolitis.
6.12. Analysis.
Comparison 6 Late ductus venosus Doppler ultrasound versus CTG, Outcome 12 Infant birthweight (grams).
6.14. Analysis.
Comparison 6 Late ductus venosus Doppler ultrasound versus CTG, Outcome 14 Long‐term infant neurodevelopmental outcome (cerebral palsy at 2 years).
6.15. Analysis.
Comparison 6 Late ductus venosus Doppler ultrasound versus CTG, Outcome 15 Infant survival at 2 years without neurodevelopmental impairment (not prespecified).
6.16. Analysis.
Comparison 6 Late ductus venosus Doppler ultrasound versus CTG, Outcome 16 Sepsis (proven) (not prespecified).
Caesarean section (both elective and emergency), spontaneous vaginal birth, operative vaginal birth, induction of labour, oxytocin augmentation, requirement for neonatal resuscitation, neonatal fitting/seizures, preterm labour (onset of labour before 37 completed weeks of pregnancy), gestational age at birth, infant respiratory distress syndrome, meconium aspiration, neonatal admission to special care or intensive care unit, or both, hypoxic ischaemic encephalopathy (a condition of injury to the brain), length of infant hospital stay, and women's views of their care were not reported in this trial.
7) Early ductus venosus Doppler ultrasound versus late ductus venosus Doppler ultrasound (one study, 337 women)
The three‐arm trial by Lees 2013, including women with singleton pregnancies, allowed comparison of early versus late ductus venosus Doppler ultrasound. The study was of high quality (low risk of bias for sequence generation and concealment allocation).
Main outcomes
There was no clear difference in any perinatal death after randomisation (RR 0.66, 95% CI 0.32 to 1.36; one study, 337 infants, Analysis 7.1). For the infants surviving following severe morbidity, there was no clear evidence of any difference between groups (RR 1.13, 95% CI 0.77 to 1.65, 337 infants, Analysis 7.2).
7.1. Analysis.
Comparison 7 Early ductus venosus Doppler ultrasound versus late, Outcome 1 Any perinatal death after randomisation.
7.2. Analysis.
Comparison 7 Early ductus venosus Doppler ultrasound versus late, Outcome 2 Survival following severe neonatal morbidity.
Additional outcomes
There were insufficient data to show clear differences between early ductus venosus Doppler ultrasound changes versus late changes for stillbirth (RR 0.68, 95% CI 0.20 to 2.36; 337 babies; 1 study, Analysis 7.3), neonatal death (RR 0.56, 95% CI 0.21 to 1.47, 337 babies, 1 study, Analysis 7.4), any potentially preventable perinatal death (RR 0.68, 95% CI 0.31 to 1.47, 337 babies, 1 study, Analysis 7.5), fetal acidosis (RR 3.05, 95% CI 0.13 to 74.43; 337 babies; 1 study, Analysis 7.6), Apgar less than seven at five minutes (RR 0.68, 95% CI 0.36 to 1.29, 337 babies, 1 study, Analysis 7.7), infant requiring intubation/ventilation (RR 0.93, 95% CI 0.71 to 1.21; 337 babies, 1 study, Analysis 7.8), intraventricular haemorrhage (RR 0.51, 95% CI 0.16 to 1.66; 337 babies, 1 study, Analysis 7.9), bronchopulmonary dysplasia (RR 0.92, 95% CI 0.58 to 1.46; 337 babies, 1 study, Analysis 7.10), necrotising enterocolitis (RR 0.34, 95% CI 0.04 to 3.23, 337 babies, 1 study, Analysis 7.11), infant birthweight (grams) (MD 13.00, 95% CI ‐59.31 to 85.31, 337 babies, 1 study, Analysis 7.12), long‐term infant neurodevelopmental outcome (any impairment at two years) (RR 1.75, 95% CI 0.70 to 4.32, 337 infants, 1 study, Analysis 7.13), cerebral palsy at two years (RR 3.05, 95% CI 0.13 to 74.43, 337 babies, 1 study, Analysis 7.14)), infant survival at two years without neurodevelopmental impairment (RR 0.91, 95% CI 0.80 to 1.03, 337 infants, 1 study, Analysis 7.15), and sepsis (proven) (RR 1.37, 95% CI 0.84 to 2.25, 337 babies, 1 study, Analysis 7.16).
7.3. Analysis.
Comparison 7 Early ductus venosus Doppler ultrasound versus late, Outcome 3 Stillbirth.
7.4. Analysis.
Comparison 7 Early ductus venosus Doppler ultrasound versus late, Outcome 4 Neonatal death.
7.5. Analysis.
Comparison 7 Early ductus venosus Doppler ultrasound versus late, Outcome 5 Any potentially preventable perinatal death*.
7.6. Analysis.
Comparison 7 Early ductus venosus Doppler ultrasound versus late, Outcome 6 Fetal acidosis.
7.7. Analysis.
Comparison 7 Early ductus venosus Doppler ultrasound versus late, Outcome 7 Apgar < 7 at 5 minutes.
7.8. Analysis.
Comparison 7 Early ductus venosus Doppler ultrasound versus late, Outcome 8 Infant requiring intubation/ventilation.
7.9. Analysis.
Comparison 7 Early ductus venosus Doppler ultrasound versus late, Outcome 9 Intraventricular haemorrhage.
7.10. Analysis.
Comparison 7 Early ductus venosus Doppler ultrasound versus late, Outcome 10 Bronchopulmonary dysplasia.
7.11. Analysis.
Comparison 7 Early ductus venosus Doppler ultrasound versus late, Outcome 11 Necrotising enterocolitis.
7.12. Analysis.
Comparison 7 Early ductus venosus Doppler ultrasound versus late, Outcome 12 Infant birthweight (grams).
7.13. Analysis.
Comparison 7 Early ductus venosus Doppler ultrasound versus late, Outcome 13 Long‐term infant neurodevelopmental outcome (impairment at 2 years).
7.14. Analysis.
Comparison 7 Early ductus venosus Doppler ultrasound versus late, Outcome 14 Long‐term infant neurodevelopmental outcome (cerebral palsy at 2 years).
7.15. Analysis.
Comparison 7 Early ductus venosus Doppler ultrasound versus late, Outcome 15 Infant survival at 2 years without neurodevelopmental impairment (not prespecified).
7.16. Analysis.
Comparison 7 Early ductus venosus Doppler ultrasound versus late, Outcome 16 Sepsis (proven) (not prespecified).
Caesarean section (both elective and emergency), spontaneous vaginal birth, operative vaginal birth, induction of labour, oxytocin augmentation, requirement for neonatal resuscitation, neonatal fitting/seizures, preterm labour (onset of labour before 37 completed weeks of pregnancy), gestational age at birth, infant respiratory distress syndrome, meconium aspiration, neonatal admission to special care or intensive care unit, or both, hypoxic ischaemic encephalopathy (a condition of injury to the brain), length of infant hospital stay, and women's views of their care were not reported in this trial.
Subgroup analysis
A single study examined early or late ductus venosus Doppler ultrasound changes compared with CTG and no data were available to examine outcomes in clinical subgroups.
Discussion
Summary of main results
Nineteen trials involving 10,667 women were included in this update of the review.
Overall, the use of Doppler ultrasound versus no Doppler ultrasound in high‐risk pregnancy was associated with a reduction in perinatal deaths. There were also fewer inductions of labour and fewer caesarean sections. No clear difference was found in stillbirth, operative vaginal births, nor in Apgar score less than seven at five minutes. Serious neonatal morbidity was not pooled due to high heterogeneity between the three studies that reported it.
Four of the trials included in the main comparison compared the use of umbilical artery Doppler ultrasound with CTG. In these studies there was insufficient evidence to detect a clear difference in perinatal mortality. There were no clear differences between groups for other primary or secondary outcomes, apart from length of hospital stay which appeared to be reduced in the umbilical artery Doppler ultrasound group although the number of babies involved was too small to be able to say anything with any degree of certainty.
This update included one new three‐arm trial (Lees 2013) examining early and late ductus venosus Doppler changes, which was not incorporated into the main meta‐analyses. This study was at low risk of bias and included follow‐up to age two, however, it was underpowered to detect clinically important differences in the main outcomes of this review. The observed improvement in long‐term neurological outcomes in the cohort of babies in whom triggers for delivery were late changes in ductus venosus are of considerable interest. Ideally, this observation should be replicated in adequately powered studies. It is important to stress that all randomised women in Lees 2013 were also monitored with computerised cardiotocography and there were clearly defined safety net criteria. In effect, the beneficial effect in this high‐risk group of fetuses, if present, came from a comprehensive and serial assessment of fetal well‐being that included combination of Doppler ultrasound and computerised cardiotocography.
Overall completeness and applicability of evidence
The first meta‐analysis showing that Doppler studies of the umbilical artery, when used in singleton high‐risk pregnancies, resulted in the reduction in perinatal deaths without an increase in obstetric interventions was published in 1995 (Alfirevic 1995). This Cochrane review update confirms these results, although formal quality assessment of the included studies revealed very few studies of high quality by today's standards. An international agreement on how best to report clinical trials is relatively recent (CONSORT 2001) and most studies simply did not report information on random sequence generation and allocation blinding that is nowadays considered essential for quality assessment. This makes formal quality assessment of older studies very imprecise, resulting in most them being labelled as 'of unclear quality'.
The other criticism of the current evidence is lack of a hitherto agreed intervention(s) that should follow an abnormal Doppler finding. Doppler ultrasound can be regarded as a screening or diagnostic test and as such cannot, by itself, influence clinically important outcomes. It is the clinical decisions influenced by Doppler findings that may or may not change the outcome. The evidence from this review suggested that better timing of caesarean sections may be the 'cause' of reduced perinatal mortality. An overall decrease in caesarean sections appeared to be confined to emergency procedures which led us to believe that clinicians with no access to Doppler studies are more often faced with a seriously compromised baby in labour.
It is difficult to say to what extent this review constitutes the 'definitive' evidence of benefit (and absence of harm) for Doppler ultrasound. Some may argue that this meta‐analysis is an ideal example of the epidemiological evidence that should trigger a definitive, high‐quality large multi‐centre clinical trial with an agreed treatment protocol that follows an abnormal Doppler finding in the umbilical artery. Most clinicians feel that a window of opportunity for such a trial is long gone, at least in singleton pregnancies with suspected 'placental insufficiency'. However, it is quite possible that for some 'high‐risk' groups, Doppler of the umbilical artery does not offer any protection (e.g. post‐term pregnancy, uncomplicated dichorionic pregnancy). Large enough clinical trials of umbilical artery Doppler in these groups of women are unlikely to be funded as clinical attention focuses on more sophisticated use of Doppler ultrasound. It is hoped that more clinical trials evaluating such techniques (e.g. Doppler studies of the fetal ductus venosus and cerebroplacental ratio) will be of high quality, with adequate power to detect important differences in neonatal morbidity.
Quality of the evidence
The trials were generally at unclear risk of bias due to incomplete reporting of methods (see Figure 2), and there was evidence of possible publication bias, shown by asymmetric funnel plots for some analyses (see Figure 3; Figure 4; Figure 5; Figure 6).
GRADE assessments of the evidence were moderate for three outcomes: perinatal death, caesarean section, and induction of labour, low for stillbirth and Apgar score less than seven at five minutes, and very low for serious neonatal mortality for singletons. No trials reported serious neonatal morbidity for multiples. Overall, the evidence was downgraded due to missing information on trial methods (all outcomes), heterogeneity (neonatal morbidity) and imprecision (neonatal morbidity, stillbirth, Apgar score less than seven at five minutes), and we also suspected possible publication bias for several outcomes, although we did not downgrade for this reason (perinatal death, caesarean section, induction of labour, and Apgar score less than seven at five minutes) (seeTable 1).
Only three studies in the main comparison (Haley 1997; Hofmeyr 1991; Nienhuis 1997) and one study in an additional comparison (Lees 2013) had adequate sequence generation and allocation concealment. Blinding women and/or staff in these trials was not generally feasible, and may have biased treatment decisions. In just three studies (Lees 2013; Newnham 1991; Nienhuis 1997), assessors of neonatal outcomes were blind to Doppler results. Full information on the number of women approached to take part in the studies, the numbers eligible for inclusion, and the overall refusal rate were not provided in most studies. While not sources of bias as such, high exclusion and refusal rates may affect the generalisability of the findings and the interpretation of the results.
These limitations in the current evidence mean that the results should be interpreted with some caution. Future research may change the results and our certainty about them.
To try to avoid bias associated with uneven post‐randomisation exclusions, we used the number of randomised women as our denominators. Where there is loss to follow‐up or missing data, using the number randomised as the denominator results in a more conservative effect estimate. If trial investigators used other denominators (e.g. the numbers included at different stages of follow‐up), it would mean that results in this review and those in published trial reports might differ slightly.
Potential biases in the review process
The assessment of risk of bias involves subjective judgements. This potential limitation is minimised by following the procedures in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), with two or more review authors independently assessing studies and resolving any disagreement through discussion, and, if required, involving a third assessor in the decision. We undertook a comprehensive, systematic search of databases to reduce the potential for publication bias, without language or publication status restrictions.
Agreements and disagreements with other studies or reviews
Systematic reviews and meta‐analysis
Imdad 2011 reviewed published literature on the effectiveness of fetal movement monitoring and Doppler velocimetry for the detection and surveillance of high risk pregnancies, and their effect in the prevention of stillbirths. Pooled results from sixteen studies showed that Doppler velocimetry of umbilical and fetal arteries in high risk pregnancies leads to a reduction of 29% in perinatal mortality compared with no Doppler velocimetry (RR 0.71, 95% CI 0.52 to 0.98). The pooled results for impact of Doppler ultrasound versus no ultrasound on stillbirths showed a reduction of 35% (RR 0.65, 95% CI 0.41 to 1.04), although the result did not reach statistical significance. These results are in agreement with our findings.
In a critical appraisal of the use of umbilical artery Doppler ultrasound in high risk pregnancies, Westergaard 2001 aimed to determine which high‐risk pregnancies benefit from the use of Doppler velocimetry. Thirteen randomised controlled trials were divided into a "well‐defined studies", meaning studies that included pregnancies with strictly defined IUGR and/or hypertensive disease of pregnancy (six studies), and "general risk studies", meaning studies that included a variety of high‐risk pregnancies.
The Odds Ratio (OR) for perinatal mortality (singleton pregnancies and not‐malformed fetuses) was 0.66 (95% CI 0.36 to 1.22) in "well‐defined studies", and 0.68 (95% CI 0.43 to 1.08) in "general risk studies", respectively. (The same paper reported an audit of perinatal deaths by 32 international experts which concluded that more perinatal deaths were potentially avoidable by use of Doppler velocimetry in "well‐defined studies" than in "general risk studies".)
In the meta‐analysis for the "well‐defined studies" there was a significant reduction in antenatal admission (OR 0.56; 95% CI 0.43 to 0.72), inductions of labor and elective caesarean sections (OR 0.73; 95% CI 0.61 to 0.88), and overall caesarean sections (OR 0.78; 95% CI 0.65 to 0.94) respectively. Thus, the authors concluded that only in pregnancies with suspected IUGR and/or hypertensive disease of pregnancy would the use of umbilical artery Doppler velocimetry reduce the number of perinatal deaths and unnecessary obstetric interventions.
In the meta‐analysis in this review subgroup analysis for primary outcomes only was defined a priori in the protocol. We considered separately pregnancies with small for gestational age fetuses from those with hypertensive disease of pregnancy (Analysis 2.1). We did not include data from the study by Johnstone (Johnstone 1993) in the subgroup analysis, as this trial included pregnancies as being at risk by referral, although there was a subset of women with hypertension or suspected IUGR (754/2289).
Authors' conclusions
Implications for practice.
Doppler studies of the umbilical artery improves perinatal outcomes in high‐risk pregnancies thought to be at risk of placental insufficiency. The clear definition of suspected placental insufficiency, frequency of Doppler studies and timing of delivery in the presence of abnormal umbilical artery Doppler studies remains elusive. Women with hypertensive disorders and small‐for‐date fetuses are obvious candidates, whilst the role of umbilical artery Doppler in other risk groups like post‐term, diabetes and uncomplicated dichorionic twin pregnancy is still debatable.
Implications for research.
As discussed, a case could be made for a larger trial of umbilical artery Doppler ultrasound than has been mounted hitherto, particularly in risk groups where the risk of fetal growth restriction caused by impaired placental blood flow is relatively low. Observational studies suggest that fetal vessels other than the umbilical artery may be better markers of fetal well‐being, fetal ductus venosus and middle cerebral artery, in particular. It is hoped that future clinical studies evaluating the possible added benefit of these tests will comply with the most recent CONSORT statement (www.consort‐statement.org) and use clinical outcomes from this Cochrane review as the minimum data set.
Further studies of management protocols based on fetal monitoring of ductus venosus and middle cerebral artery with or without computerised cardiotocography should be encouraged. It is critically important that such studies collect all clinically important information including long‐term neurological follow‐up data.
What's new
| Date | Event | Description |
|---|---|---|
| 31 March 2017 | New citation required but conclusions have not changed | One study previously in ongoing section was included in this update (Lees 2013). The conclusions remain the same. |
| 31 March 2017 | New search has been performed | Search updated and no new studies identified. The quality of the evidence was assessed using the GRADE approach and a 'Summary of findings' table was incorporated. |
History
Protocol first published: Issue 1, 2009 Review first published: Issue 1, 2010
| Date | Event | Description |
|---|---|---|
| 30 September 2013 | New citation required but conclusions have not changed | No new trials identified. |
| 30 September 2013 | New search has been performed | Search updated. Methods updated. |
Acknowledgements
In the previous version of this review, Alfirevic 2010, Gill Gyte was supported by the NIHR NHS Cochrane Collaboration Programme grant scheme award for NHS‐prioritised centrally‐managed, pregnancy and childbirth systematic reviews: CPGS02. We thank Gill for her contributions to previous versions of this review.
This project was supported by the National Institute for Health Research, via Cochrane programme grant funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Therese Dowswell is supported by the NIHR Cochrane Programme Grant Project: 13/89/05 – Pregnancy and childbirth systematic reviews to support clinical guidelines.
Data and analyses
Comparison 1. Umbilical artery Doppler ultrasound versus no Doppler ultrasound.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any perinatal death after randomisation | 16 | 10225 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.52, 0.98] |
| 1.1 Singleton pregnancy | 9 | 4661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.35, 1.01] |
| 1.2 Multiple pregnancy | 1 | 1052 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.32, 2.41] |
| 1.3 Singleton plus multiple pregnancies, or not stated | 6 | 4512 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.51, 1.19] |
| 2 Serious neonatal morbidity | 3 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 Singleton pregnancy | 1 | 500 | Risk Ratio (M‐H, Random, 95% CI) | 0.13 [0.02, 0.99] |
| 2.2 Multiple pregnancy | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Singleton plus multiple pregnancies, or not stated | 2 | 598 | Risk Ratio (M‐H, Random, 95% CI) | 2.95 [0.31, 28.14] |
| 3 Stillbirth | 15 | 9560 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.41, 1.04] |
| 3.1 Singleton pregnancy | 8 | 3996 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.31, 1.19] |
| 3.2 Multiple pregnancy | 1 | 1052 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.11, 4.00] |
| 3.3 Singleton plus multiple pregnancy, or not stated | 6 | 4512 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.35, 1.39] |
| 4 Neonatal death | 13 | 8167 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.53, 1.24] |
| 4.1 Singleton pregnancy | 7 | 2656 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.31, 1.53] |
| 4.2 Multiple pregnancy | 1 | 1052 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.29, 3.46] |
| 4.3 Singleton plus multiple pregnancies, or not stated | 5 | 4459 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.48, 1.45] |
| 5 Any potentially preventable perinatal death* | 16 | 10225 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.46, 0.98] |
| 5.1 Singleton pregnancy | 9 | 4661 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.30, 1.13] |
| 5.2 Multiple pregnancy | 1 | 1052 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.32, 2.41] |
| 5.3 Singleton plus multiple pregnancies or not stated | 6 | 4512 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.41, 1.15] |
| 6 Apgar < 7 at 5 minutes | 7 | 6321 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.69, 1.24] |
| 6.1 Singleton pregnancy | 4 | 2555 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.45, 1.09] |
| 6.2 Multiple pregnancy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Singleton plus multiple pregnancies or not stated | 3 | 3766 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.77, 1.73] |
| 7 Caesarean section (elective and emergency) | 14 | 7918 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.84, 0.97] |
| 7.1 Singleton pregnancy | 7 | 2929 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.75, 0.95] |
| 7.2 Multiple pregnancy | 1 | 526 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.77, 1.19] |
| 7.3 Singleton plus multiple pregnancies or not stated | 6 | 4463 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.84, 1.05] |
| 8 Caesarean section ‐ elective | 11 | 6627 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.93, 1.22] |
| 8.1 Singleton pregnancy | 6 | 1934 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.90, 1.38] |
| 8.2 Multiple pregnancy | 1 | 526 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.77, 1.47] |
| 8.3 Singleton plus multiple pregnancies or not stated | 4 | 4167 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.84, 1.26] |
| 9 Caesarean section ‐ emergency | 10 | 6175 | Risk Ratio (M‐H, Random, 95% CI) | 0.81 [0.67, 0.98] |
| 9.1 Singleton pregnancy | 5 | 1482 | Risk Ratio (M‐H, Random, 95% CI) | 0.58 [0.43, 0.78] |
| 9.2 Multiple pregnancy | 1 | 526 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.57, 1.23] |
| 9.3 Singleton plus multiple pregnancies or not stated | 4 | 4167 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.77, 1.20] |
| 10 Spontaneous vaginal birth | 5 | 2504 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.98, 1.10] |
| 10.1 Singleton pregnancy | 2 | 576 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.96, 1.18] |
| 10.2 Multiple pregnancy | 1 | 526 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.90, 1.19] |
| 10.3 Singleton plus multiple pregnancies or not stated | 2 | 1402 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.95, 1.12] |
| 11 Operative vaginal birth | 4 | 2813 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.80, 1.14] |
| 11.1 Singleton pregnancy | 3 | 1916 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.78, 1.22] |
| 11.2 Multiple pregnancy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.3 Singleton plus multiple pregnancies or not stated | 1 | 897 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.68, 1.25] |
| 12 Induction of labour | 10 | 5633 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.80, 0.99] |
| 12.1 Singleton pregnancy | 5 | 1784 | Risk Ratio (M‐H, Random, 95% CI) | 0.79 [0.64, 0.97] |
| 12.2 Multiple pregnancy | 1 | 526 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.80, 1.50] |
| 12.3 Singleton plus multiple pregnancies or not stated | 4 | 3323 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.86, 1.04] |
| 13 Infant requiring intubation/ventilation | 6 | 3136 | Risk Ratio (M‐H, Random, 95% CI) | 1.42 [0.87, 2.30] |
| 13.1 Singleton pregnancy | 4 | 1539 | Risk Ratio (M‐H, Random, 95% CI) | 2.89 [1.40, 5.96] |
| 13.2 Multiple pregnancy | 1 | 1052 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.59, 1.25] |
| 13.3 Singleton plus multiple pregnancies or not stated | 1 | 545 | Risk Ratio (M‐H, Random, 95% CI) | 1.25 [0.79, 1.98] |
| 14 Neonatal fitting/seizures | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.49] |
| 14.1 Singleton pregnancy | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.49] |
| 14.2 Multiple pregnancy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.3 Singleton plus multiple pregnancies or not stated | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Preterm labour | 2 | 626 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.72, 1.75] |
| 15.1 Singleton pregnancy | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.51, 2.07] |
| 15.2 Multiple pregnancy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.3 Singleton plus multiple pregnancy or not stated | 1 | 476 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.18 [0.66, 2.11] |
| 16 Gestational age at birth (weeks) | 8 | 4066 | Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.02, 0.43] |
| 16.1 Singleton pregnancy | 3 | 1043 | Mean Difference (IV, Random, 95% CI) | 0.54 [‐0.00, 1.09] |
| 16.2 Multiple pregnancy | 1 | 1052 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.24, 0.44] |
| 16.3 Singleton plus multiple pregnancies or not stated | 4 | 1971 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.19, 0.31] |
| 17 Infant respiratory distress syndrome (RDS) | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.07, 16.48] |
| 17.1 Singleton pregnancy | 1 | 107 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.07, 16.48] |
| 17.2 Multiple pregnancy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.3 Singleton plus multiple pregnancies or not stated | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Neonatal admission to SCBU and/or NICU | 12 | 9334 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.89, 1.03] |
| 18.1 Singleton pregnancy | 8 | 4511 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.80, 1.06] |
| 18.2 Multiple pregnancy | 1 | 1052 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.88, 1.05] |
| 18.3 Singleton plus multiple pregnancies or not stated | 3 | 3771 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.85, 1.14] |
| 19 Hypoxic ischaemic encephalopathy | 2 | 1045 | Risk Ratio (M‐H, Random, 95% CI) | 0.65 [0.01, 33.07] |
| 19.1 Singleton pregnancy | 1 | 500 | Risk Ratio (M‐H, Random, 95% CI) | 0.09 [0.01, 1.64] |
| 19.2 Multiple pregnancy | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 19.3 Singleton plus multiple pregnancies or not stated | 1 | 545 | Risk Ratio (M‐H, Random, 95% CI) | 4.91 [0.24, 101.79] |
| 20 Intraventricular haemorrhage | 4 | 2008 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.42 [0.47, 4.30] |
| 20.1 Singleton pregnancy | 3 | 1463 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.38, 4.16] |
| 20.2 Multiple pregnancy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.3 Singleton plus multiple pregnancies or not stated | 1 | 545 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.95 [0.12, 71.99] |
| 21 Birthweight (grams) | 7 | 3887 | Mean Difference (IV, Fixed, 95% CI) | 31.33 [‐8.70, 71.37] |
| 21.1 Singleton pregnancy | 3 | 1916 | Mean Difference (IV, Fixed, 95% CI) | 49.34 [‐0.62, 99.31] |
| 21.2 Multiple pregnancy | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 21.3 Singleton plus multiple pregnancies or not stated | 4 | 1971 | Mean Difference (IV, Fixed, 95% CI) | ‐0.95 [‐67.84, 65.95] |
| 22 Length of infant hospital stay (days) | 3 | 1076 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.28 [‐0.40, ‐0.16] |
| 22.1 Singleton pregnancy | 3 | 1076 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.28 [‐0.40, ‐0.16] |
| 22.2 Multiple pregnancy | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 22.3 Singleton plus multiple pregnancies or not stated | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 23 Birth < 34 weeks (not prespecified) | 2 | 976 | Risk Ratio (M‐H, Random, 95% CI) | 2.04 [0.62, 6.69] |
| 23.1 Singleton pregnancy | 1 | 500 | Risk Ratio (M‐H, Random, 95% CI) | 1.17 [0.40, 3.42] |
| 23.2 Multiple pregnancy | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 23.3 Singleton plus multiple pregnancies or not stated | 1 | 476 | Risk Ratio (M‐H, Random, 95% CI) | 3.90 [1.11, 13.65] |
| 24 Antenatal admissions (not prespecified) | 2 | 893 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.60, 0.88] |
| 24.1 Singleton pregnancy | 2 | 893 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.60, 0.88] |
| 24.2 Multiple pregnancy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 24.3 Singleton plus multiple pregnancies or not stated | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25 Phototherapy for neonatal jaundice (not prespecified) | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.87] |
| 25.1 Singleton pregnancy | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.87] |
| 25.2 Multiple pregnancy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 25.3 Singleton plus multiple pregnancies or not stated | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26 Abnormal neurological development at 9 months (not prespecified) | 1 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.26, 1.45] |
| 26.1 Singleton pregnancy | 1 | 137 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.26, 1.45] |
| 26.2 Multiple pregnancy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 26.3 Singleton plus multiple pregnancies or not stated | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 27 Hospitalisation for IUGR neonatal (not prespecified) | 1 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.75, 1.41] |
| 27.1 Singleton pregnancy | 1 | 142 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.75, 1.41] |
| 27.2 Multiple pregnancy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 27.3 Singleton plus multiple pregnancies or not stated | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 28 Fetal distress in labour (not prespecified) | 1 | 289 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.10, 1.22] |
| 28.1 Singleton pregnancy | 1 | 289 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.10, 1.22] |
| 28.2 Multiple pregnancy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 28.3 Singleton plus multiple pregnancies or not stated | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 29 Birthweight < 5 percentile (not prespecified) | 1 | 289 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.51, 2.64] |
| 29.1 Singleton pregnancy | 1 | 289 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.51, 2.64] |
| 29.2 Multiple pregnancy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 29.3 Singleton plus multiple pregnancies or not stated | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 30 Periventricular leucomalacia (not prespecified) | 1 | 545 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.00] |
| 30.1 Singleton pregnancy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 30.2 Multiple pregnancy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 30.3 Singleton plus multiple pregnancies or not stated | 1 | 545 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.00] |
| 31 Antenatal hospital stay (days) (not prespecified) | 1 | 426 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐2.39, 1.19] |
| 31.1 Singleton pregnancy | 1 | 426 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐2.39, 1.19] |
| 31.2 Multiple pregnancy | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 31.3 Singleton plus multiple pregnancies or not stated | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Umbilical artery Doppler ultrasound versus no Doppler ultrasound (all subgroups).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any perinatal death after randomisation | 6 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 SGA/IUGR | 5 | 1292 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.38, 1.35] |
| 1.2 Hypertension/pre‐eclampsia | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.57 [0.42, 30.73] |
| 1.3 Diabetes | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Prolonged pregnancy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.5 Previous pregnancy loss | 1 | 53 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.26 [0.03, 2.17] |
| 2 Serious neonatal morbidity | 1 | 53 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.1 SGA/IUGR | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Hypertension/pre‐eclampsia | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Diabetes | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Prolonged pregnancy | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.5 Previous pregnancy loss | 1 | 53 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 3. Umbilical artery Doppler ultrasound alone versus CTG alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any perinatal death after randomisation | 4 | 2813 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.45 [0.17, 1.15] |
| 1.1 Singleton pregnancy | 3 | 1916 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.07, 1.68] |
| 1.2 Multiple pregnancy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Singleton plus multiple pregnancies or not stated | 1 | 897 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.16, 1.73] |
| 2 Stillbirth | 4 | 2813 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.48 [0.14, 1.71] |
| 2.1 Singleton pregnancy | 3 | 1916 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.28 [0.05, 1.70] |
| 2.2 Multiple pregnancy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Singleton plus multiple pregnancies or not stated | 1 | 897 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.15, 7.41] |
| 3 Neonatal death | 3 | 1473 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.16, 1.72] |
| 3.1 Singleton pregnancy | 2 | 576 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.15, 7.10] |
| 3.2 Multiple pregnancy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Singleton plus multiple pregnancies or not stated | 1 | 897 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.07, 1.72] |
| 4 Any potentially preventable perinatal death* | 4 | 2813 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.38 [0.12, 1.18] |
| 4.1 Singleton pregnancy | 3 | 1916 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.08, 2.11] |
| 4.2 Multiple pregnancy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Singleton plus multiple pregnancies or not stated | 1 | 897 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.07, 1.72] |
| 5 Apgar < 7 at 5 minutes | 3 | 2663 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.54, 1.37] |
| 5.1 Singleton pregnancy | 2 | 1766 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.49, 1.43] |
| 5.2 Multiple pregnancy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.3 Singleton plus multiple pregnancies or not stated | 1 | 897 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.36, 2.39] |
| 6 Caesarean section (elective and emergency) | 4 | 2813 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.79, 1.01] |
| 6.1 Singleton pregnancy | 3 | 1916 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.77, 1.02] |
| 6.2 Multiple pregnancy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Singleton plus multiple pregnancies or not stated | 1 | 897 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.73, 1.14] |
| 7 Caesarean section ‐ elective | 3 | 1473 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [1.12, 2.09] |
| 7.1 Singleton pregnancy | 2 | 576 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.69 [1.07, 2.67] |
| 7.2 Multiple pregnancy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7.3 Singleton plus multiple pregnancies or not stated | 1 | 897 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.40 [0.91, 2.15] |
| 8 Caesarean section ‐ emergency | 3 | 1473 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.52, 0.84] |
| 8.1 Singleton pregnancy | 2 | 576 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.55 [0.36, 0.83] |
| 8.2 Multiple pregnancy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 8.3 Singleton plus multiple pregnancies or not stated | 1 | 897 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.55, 0.98] |
| 9 Spontaneous vaginal birth | 2 | 1323 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.97, 1.15] |
| 9.1 Singleton pregnancy | 1 | 426 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.91, 1.19] |
| 9.2 Multiple pregnancy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.3 Singleton plus multiple pregnancies or not stated | 1 | 897 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.95, 1.19] |
| 10 Operative vaginal birth | 3 | 2663 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.81, 1.17] |
| 10.1 Singleton pregnancy | 2 | 1766 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.80, 1.27] |
| 10.2 Multiple pregnancy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10.3 Singleton plus multiple pregnancies or not stated | 1 | 897 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.68, 1.25] |
| 11 Induction of labour | 2 | 576 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.32, 1.40] |
| 11.1 Singleton pregnancy | 2 | 576 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.32, 1.40] |
| 11.2 Multiple pregnancy | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 11.3 Singleton plus multiple pregnancies or not stated | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Infant requiring intubation/ventilation | 2 | 576 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.26, 9.08] |
| 12.1 Singleton pregnancy | 2 | 576 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.26, 9.08] |
| 12.2 Multiple pregnancy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 Singleton plus multiple pregnancies or not stated | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Neonatal fitting/seizures | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.49] |
| 13.1 Singleton pregnancy | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.35 [0.01, 8.49] |
| 13.2 Multiple pregnancy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.3 Singleton plus multiple pregnancies or not stated | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Gestational age at birth | 3 | 1473 | Mean Difference (IV, Fixed, 95% CI) | 0.23 [‐0.00, 0.47] |
| 14.1 Singleton pregnancy | 2 | 576 | Mean Difference (IV, Fixed, 95% CI) | 0.26 [‐0.06, 0.59] |
| 14.2 Multiple pregnancy | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.3 Singleton plus multiple pregnancies or not stated | 1 | 897 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [‐0.15, 0.55] |
| 15 Neonatal admission to SCBU and/or NICU | 4 | 2813 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.73, 1.03] |
| 15.1 Singleton pregnancy | 3 | 1916 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.64, 0.99] |
| 15.2 Multiple pregnancy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.3 Singleton plus multiple pregnancies or not stated | 1 | 897 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.73, 1.37] |
| 16 Infant birthweight (grams) | 4 | 2813 | Mean Difference (IV, Fixed, 95% CI) | 38.41 [‐6.14, 82.97] |
| 16.1 Singleton pregnancy | 3 | 1916 | Mean Difference (IV, Fixed, 95% CI) | 49.34 [‐0.62, 99.31] |
| 16.2 Multiple pregnancy | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16.3 Singleton plus multiple pregnancies or not stated | 1 | 897 | Mean Difference (IV, Fixed, 95% CI) | ‐4.0 [‐102.42, 94.42] |
| 17 Length of infant hospital stay (days) | 2 | 576 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.41, ‐0.08] |
| 17.1 Singleton pregnancy | 2 | 576 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.25 [‐0.41, ‐0.08] |
| 17.2 Multiple pregnancy | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17.3 Singleton plus multiple pregnancies or not stated | 0 | 0 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Antenatal admissions (not prespecified) | 1 | 426 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.55, 0.90] |
| 18.1 Singleton pregnancy | 1 | 426 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.70 [0.55, 0.90] |
| 18.2 Multiple pregnancy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18.3 Singleton plus multiple pregnancies or not stated | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Phototherapy for neonatal jaundice (not prespecified) | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.87] |
| 19.1 Singleton pregnancy | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.87] |
| 19.2 Multiple pregnancy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19.3 Singleton plus multiple pregnancies or not stated | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20 Antenatal hospital stay (days) (not prespecified) | 1 | 426 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐2.39, 1.19] |
| 20.1 Singleton pregnancy | 1 | 426 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐2.39, 1.19] |
| 20.2 Multiple pregnancy | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 20.3 Singleton plus multiple pregnancies or not stated | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Umbilical artery Doppler ultrasound alone versus CTG alone (all subgroups).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any perinatal death after randomisation | 3 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 SGA/IUGR | 2 | 572 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.05, 2.09] |
| 1.2 Hypertension/pre‐eclampsia | 1 | 89 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.57 [0.42, 30.73] |
| 1.3 Diabetes | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Prolonged pregnancy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.5 Previous pregnancy loss | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 5. Early ductus venosus Doppler ultrasound versus CTG.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any perinatal death after randomisation | 1 | 333 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.39, 1.82] |
| 1.1 Singleton pregnancy | 1 | 333 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.39, 1.82] |
| 2 Survival following severe neonatal morbidity | 1 | 333 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.75, 1.61] |
| 3 Stillbirth | 1 | 333 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [0.37, 10.71] |
| 3.1 Singleton pregnancy | 1 | 333 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.99 [0.37, 10.71] |
| 4 Neonatal death | 1 | 333 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.22, 1.60] |
| 4.1 Singleton pregnancy | 1 | 333 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.22, 1.60] |
| 5 Any potentially preventable perinatal death* | 1 | 333 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.37, 1.86] |
| 5.1 Singleton pregnancy | 1 | 333 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.37, 1.86] |
| 6 Fetal acidosis | 1 | 333 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.20] |
| 6.1 Singleton pregnancy | 1 | 333 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.25 [0.03, 2.20] |
| 7 Apgar < 7 at 5 minutes | 1 | 333 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.44, 1.72] |
| 7.1 Singleton pregnancy | 1 | 333 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.44, 1.72] |
| 8 Infant requiring intubation/ventilation | 1 | 333 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.67, 1.13] |
| 8.1 Singleton pregnancy | 1 | 333 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.67, 1.13] |
| 9 Intraventricular haemorrhage | 1 | 333 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.95 [0.49, 164.87] |
| 9.1 Singleton pregnancy | 1 | 333 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.95 [0.49, 164.87] |
| 10 Bronchopulmonary dysplasia | 1 | 333 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.55, 1.38] |
| 10.1 Singleton pregnancy | 1 | 333 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.55, 1.38] |
| 11 Necrotising enterocolitis | 1 | 333 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.03, 3.15] |
| 11.1 Singleton pregnancy | 1 | 333 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.03, 3.15] |
| 12 Infant birthweight (grams) | 1 | 333 | Mean Difference (IV, Fixed, 95% CI) | 38.0 [‐31.53, 107.53] |
| 12.1 Singleton pregnancy | 1 | 333 | Mean Difference (IV, Fixed, 95% CI) | 38.0 [‐31.53, 107.53] |
| 13 Long‐term infant neurodevelopmental outcome (impairment at 2 years) | 1 | 333 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.30, 1.18] |
| 13.1 Singleton pregnancy | 1 | 333 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.60 [0.30, 1.18] |
| 14 Long‐term infant neurodevelopmental outcome (cerebral palsy at 2 years) | 1 | 333 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.02, 1.68] |
| 14.1 Singleton pregnancy | 1 | 333 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.02, 1.68] |
| 15 Infant survival at 2 years without neurodevelopmental impairment (not prespecified) | 1 | 333 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.92, 1.23] |
| 15.1 Singleton pregnancy | 1 | 333 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.92, 1.23] |
| 16 Sepsis (proven) (not prespecified) | 1 | 333 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.60, 1.45] |
| 16.1 Singleton pregnancy | 1 | 333 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.60, 1.45] |
Comparison 6. Late ductus venosus Doppler ultrasound versus CTG.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any perinatal death after randomisation | 1 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.64, 2.55] |
| 1.1 Singleton pregnancy | 1 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.64, 2.55] |
| 2 Survival following severe neonatal morbidity | 1 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.66, 1.45] |
| 3 Stillbirth | 1 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.93 [0.60, 14.31] |
| 3.1 Singleton pregnancy | 1 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.93 [0.60, 14.31] |
| 4 Neonatal death | 1 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.47, 2.46] |
| 4.1 Singleton pregnancy | 1 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.47, 2.46] |
| 5 Any potentially preventable perinatal death* | 1 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.59, 2.53] |
| 5.1 Singleton pregnancy | 1 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [0.59, 2.53] |
| 6 Fetal acidosis | 1 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.00] |
| 6.1 Singleton pregnancy | 1 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.11 [0.01, 2.00] |
| 7 Apgar < 7 at 5 minutes | 1 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.69, 2.37] |
| 7.1 Singleton pregnancy | 1 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.69, 2.37] |
| 8 Infant requiring intubation/ventilation | 1 | 336 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.73, 1.20] |
| 8.1 Singleton pregnancy | 1 | 336 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.73, 1.20] |
| 9 Intraventricular haemorrhage | 1 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 16.60 [0.97, 285.35] |
| 9.1 Singleton pregnancy | 1 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 16.60 [0.97, 285.35] |
| 10 Bronchopulmonary dysplasia | 1 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.61, 1.48] |
| 10.1 Singleton pregnancy | 1 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.61, 1.48] |
| 11 Necrotising enterocolitis | 1 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.20, 4.77] |
| 11.1 Singleton pregnancy | 1 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.20, 4.77] |
| 12 Infant birthweight (grams) | 1 | 336 | Mean Difference (IV, Fixed, 95% CI) | 25.0 [‐40.06, 90.06] |
| 12.1 Singleton pregnancy | 1 | 336 | Mean Difference (IV, Fixed, 95% CI) | 25.0 [‐40.06, 90.06] |
| 13 Long‐term infant neurodevelopmental outcome (impairment at 2 years) | 1 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.15, 0.79] |
| 13.1 Singleton pregnancy | 1 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.15, 0.79] |
| 14 Long‐term infant neurodevelopmental outcome (cerebral palsy at 2 years) | 1 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.00, 1.59] |
| 14.1 Singleton pregnancy | 1 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.00, 1.59] |
| 15 Infant survival at 2 years without neurodevelopmental impairment (not prespecified) | 1 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [1.02, 1.34] |
| 15.1 Singleton pregnancy | 1 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [1.02, 1.34] |
| 16 Sepsis (proven) (not prespecified) | 1 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.42, 1.11] |
| 16.1 Singleton pregnancy | 1 | 336 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.42, 1.11] |
Comparison 7. Early ductus venosus Doppler ultrasound versus late.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Any perinatal death after randomisation | 1 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.32, 1.36] |
| 1.1 Singleton pregnancy | 1 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.32, 1.36] |
| 2 Survival following severe neonatal morbidity | 1 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.77, 1.65] |
| 3 Stillbirth | 1 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.20, 2.36] |
| 3.1 Singleton pregnancy | 1 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.20, 2.36] |
| 4 Neonatal death | 1 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.21, 1.47] |
| 4.1 Singleton pregnancy | 1 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.56 [0.21, 1.47] |
| 5 Any potentially preventable perinatal death* | 1 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.31, 1.47] |
| 5.1 Singleton pregnancy | 1 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.31, 1.47] |
| 6 Fetal acidosis | 1 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.05 [0.13, 74.43] |
| 6.1 Singleton pregnancy | 1 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.05 [0.13, 74.43] |
| 7 Apgar < 7 at 5 minutes | 1 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.36, 1.29] |
| 7.1 Singleton pregnancy | 1 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.36, 1.29] |
| 8 Infant requiring intubation/ventilation | 1 | 337 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.71, 1.21] |
| 8.1 Singleton pregnancy | 1 | 337 | Risk Ratio (M‐H, Random, 95% CI) | 0.93 [0.71, 1.21] |
| 9 Intraventricular haemorrhage | 1 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.16, 1.66] |
| 9.1 Singleton pregnancy | 1 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.51 [0.16, 1.66] |
| 10 Bronchopulmonary dysplasia | 1 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.58, 1.46] |
| 10.1 Singleton pregnancy | 1 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.58, 1.46] |
| 11 Necrotising enterocolitis | 1 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.04, 3.23] |
| 11.1 Singleton pregnancy | 1 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.34 [0.04, 3.23] |
| 12 Infant birthweight (grams) | 1 | 337 | Mean Difference (IV, Fixed, 95% CI) | 13.0 [‐59.31, 85.31] |
| 12.1 Singleton pregnancy | 1 | 337 | Mean Difference (IV, Fixed, 95% CI) | 13.0 [‐59.31, 85.31] |
| 13 Long‐term infant neurodevelopmental outcome (impairment at 2 years) | 1 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.70, 4.32] |
| 13.1 Singleton pregnancy | 1 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.75 [0.70, 4.32] |
| 14 Long‐term infant neurodevelopmental outcome (cerebral palsy at 2 years) | 1 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.05 [0.13, 74.43] |
| 14.1 Singleton pregnancy | 1 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.05 [0.13, 74.43] |
| 15 Infant survival at 2 years without neurodevelopmental impairment (not prespecified) | 1 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.80, 1.03] |
| 15.1 Singleton pregnancy | 1 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.80, 1.03] |
| 16 Sepsis (proven) (not prespecified) | 1 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.84, 2.25] |
| 16.1 Singleton pregnancy | 1 | 337 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.84, 2.25] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Almstrom 1992.
| Methods | 2‐arm prospective RCT; randomised block design; individual women. | |
| Participants | Singleton pregnancies with suspected IUGR at 31 completed weeks of pregnancy. IUGR if fetal weight < 2 SD below the mean at 31 weeks. N = 427 women. |
|
| Interventions | Intervention: Doppler of umbilical artery only every 2 weeks till birth unless:
Comparison: CTG (NST). |
|
| Outcomes | Primary: GA at delivery, frequency of CS, frequency of operative delivery for fetal distress, CS, vacuum, forceps, length of stay at NICU. Secondary: number of fetal monitoring occasions, duration of antenatal hospital stay, frequency of labour induction, birthweight, frequency of small‐for‐dates infants, Apgar score at 1 min and 5 min, need for respiratory support. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised block design. No information about how the randomisation was performed. |
| Allocation concealment (selection bias) | Unclear risk | Sealed numbered envelopes according to a randomisation block design. This may mean separate randomisation schedules for the 4 different hospitals. No mention of whether the envelopes were opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding women and/or staff in these trials was not generally feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding women and/or staff in these trials was not generally feasible. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No women were lost to follow‐up. 3 women declined to take part in the trial. 1 woman in the CTG group had to be excluded from data analysis since all her records were mislaid before evaluation. All women seemed to get their allocated Doppler or CTG, so this was an ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes were described in results section, but we did not assess the trial protocol. |
| Other bias | High risk | The study was not stopped earlier. Baseline imbalance:
Differential diagnosis: Almstrom 1995 concluded that obstetricians may have been influenced by the knowledge of a normal umbilical Doppler examination when assessing the CTG in labour. This might have contributed bias to the finding of fewer emergency CS for fetal distress in the Doppler group than in the CTG group. |
Biljan 1992.
| Methods | Randomised controlled study. | |
| Participants | Women with high‐risk singleton pregnancies. N = 674 women randomised. |
|
| Interventions | Intervention: Doppler of umbilical artery revealed. N = 338. Comparison: no Doppler. N = 336. |
|
| Outcomes | Elective births; GA at birth; birthweight; Apgar scores, admissions to NICU, length of time in NICU, number of babies ventilated, length of ventilation, perinatal mortality. | |
| Notes | The information came only from the 2 conference abstracts and personal communication (ZA). Sadly, Dr Biljan has died, so further detailed information on the study is not available. The information on the number of women randomised to each group was obtained from previous published version of this systematic review (Alfirevic 1995), and data on 'potentially preventable perineal deaths' was calculated from data in a previous version of this Cochrane review (Neilson 1996). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "...were randomised..." |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding women and/or staff in these trials was not generally feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding women and/or staff in these trials was not generally feasible. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information provided in the conference abstract to assess this. |
| Selective reporting (reporting bias) | High risk | Only gave data for the significant findings and reported the nonsignificant findings just as lower but not statistically significant. |
| Other bias | Unclear risk | Insufficient information provided in the conference abstract to assess this. |
Burke 1992.
| Methods | Prospective RCT, individual women, 2 trial arms. | |
| Participants | Women with high‐risk pregnancies (suspected IUGR, hypertensive disorders, previous baby < 2.5 kg, antepartum haemorrhage, previous perinatal death, diminished fetal movements, post maturity, diabetes, and others). N = 476 women. |
|
| Interventions | Intervention: Doppler of umbilical artery and fetal biometry and BPP scoring. Comparison: fetal biometry and BPP scoring. |
|
| Outcomes | Primary outcomes: induction of labour, elective and emergency CS, preterm delivery, and perinatal loss. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation by a random number sequence but it was unclear whether this was made by a third independent person. |
| Allocation concealment (selection bias) | Unclear risk | Sealed numbered envelopes but there was no information whether the envelopes were opaque and whether there was an ordered numbered sequence. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding women and/or staff in these trials was not generally feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding women and/or staff in these trials was not generally feasible. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No exclusions after randomisation. Reported as ITT. |
| Selective reporting (reporting bias) | Unclear risk | All outcomes were described in the results section, but we did not assess the trial protocol. |
| Other bias | Low risk | The study was not stopped early. Baseline imbalance: "Doppler examinations were not carried out in the control group unless specifically requested by the consultant in charge of patients" ‐ 2 women in the control group had a Doppler and were not excluded. |
De Rochambeau 1992.
| Methods | 2‐arm RCT of individual women. | |
| Participants | Women with singleton post‐term pregnancies (40 + 3 weeks to 42 + 3 weeks). N = 107 women. |
|
| Interventions | Intervention: Doppler US of umbilical artery. Comparison: no Doppler US, and standard care (FHR). |
|
| Outcomes | CS, RDS and post maturity. | |
| Notes | Paper in French with English abstract, paper was translated. Most of the data were missing. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Women "...were randomly divided...". No information on how the random sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding women and/or staff in these trials was not generally feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding women and/or staff in these trials was not generally feasible. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Describe any loss of participants to follow‐up at each data collection point:
Describe any exclusion of participants after randomisation:
Was the analysis ITT? If not, have the data been able to be reincluded?
|
| Selective reporting (reporting bias) | Unclear risk | There was no list of prespecified outcomes as far as we could ascertain and we did not assess the trial protocol. |
| Other bias | Unclear risk | If the study was stopped early, explain the reasons:
Describe any baseline imbalance:
Describe any differential diagnosis:
|
Giles 2003.
| Methods | Multi‐centred RCT; block randomisation, block of 20. Individual women, 2‐arm trial. |
|
| Participants | Women with twin pregnancies (monochorionic and dichorionic) at 25 weeks. 2 viable apparently normally formed fetuses seen on US scan. Exclusions: fetal anomalies; polyhydramnios/oligohydramnios; demise of 1 twin before 25 weeks. Significance of chorionicity not realised at time randomisation began so no attempt was made to assess chorionicity. N = 539 women. |
|
| Interventions | Intervention: Doppler and biometry US.
Comparison: biometry US.
|
|
| Outcomes | Maternal: antenatal admission, presence of hypertension, gestation at delivery, indication for delivery and mode of delivery. Fetal: US biometry measurements, umbilical artery doppler systolic diastolic ratios and the occurrence of fetal death and causative factors. Neonatal: birthweight, Apgar scores, admission to NICU, admission to special care nursery, requirements for ventilation and occurrence of neonatal death (up to 28 days of life). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “....opaque sealed envelopes containing the randomisation code the envelope being opened by an observer remote from patient care”. |
| Allocation concealment (selection bias) | Low risk | “....opaque sealed envelopes containing the randomisation code the envelope being opened by an observer remote from patient care". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding women and/or staff in these trials was not generally feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding women and/or staff in these trials was not generally feasible. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Describe any loss of participants to follow‐up at each data collection point:
Describe any exclusion of participants after randomisation: Was the analysis ITT? If not, have the data been able to be reincluded?
|
| Selective reporting (reporting bias) | Unclear risk | There seemed to be no evidence of selective reporting bias, but we did not assess the trial protocol. |
| Other bias | Low risk | If the study was stopped early, explain the reasons:
Describe any baseline imbalance:
|
Haley 1997.
| Methods | 2‐arm RCT with stratified block randomisation producing 4 groups: Caucasian primiparous and multiparous women, and Asian primiparous and multiparous women. Randomised in blocks of 8 using table of random numbers. However, the results are not reported by any of these subgroups ‐ only Doppler vs CTG overall. Randomisation was of individual women. |
|
| Participants | Women with singleton fetuses with US examination showing the abdominal circumference < 2 SD of the mean for the GA FHR on charts recommended by British Medical Ultrasound Society. There was no GA constraint although all women were > 26 weeks' gestation. N = 150 women. |
|
| Interventions | Intervention: Doppler of umbilical artery and no CTG. Comparison: CTG. |
|
| Outcomes | Primary: duration of hospital antenatal admission, induction of labour rates. Secondary: number of investigations (CTG or Doppler), number of outpatient visits to hospital, emergency CS rate, length of stay on the NICU, birthweight, and 1 min and 5 min Apgar score. All women were sent a questionnaire asking their views on the process of their care. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocks of 8 using a table of random numbers. |
| Allocation concealment (selection bias) | Low risk | "...randomisation only possible by telephone .... sequentially numbered sealed opaque envelopes..." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding women and/or staff in these trials was not generally feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding women and/or staff in these trials was not generally feasible. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss of participants at follow‐up. No exclusion after the randomisation. ITT analysis. |
| Selective reporting (reporting bias) | Unclear risk | We did not assess the trial protocol. Also, despite the stratified randomisation to look at ethnicity and parity, the results are not reported by any of these subgroups, only Doppler vs CTG overall. |
| Other bias | Low risk | Study went to completion. Baseline imbalance: more women had no live‐in support at home in the CTG group. Differential diagnosis: "...there was not a rigid protocol except that clinicians usually felt that a CTG record gave reassurance for 48 to 72 hours and a Doppler examination for a week or more ...". |
Hofmeyr 1991.
| Methods | 2‐arm RCT, but with additional evaluation by the nonallocated technique. Randomisation was of individual women. |
|
| Participants | Women undergoing evaluation of fetal well‐being in the high‐risk obstetric unit. 867 women randomised. N = 897 women. |
|
| Interventions | Intervention: Doppler US of umbilical artery. Comparison: computerised CTG. |
|
| Outcomes | Number and duration of tests; perinatal outcomes. “Our objective was to determine whether the experimental policy of Doppler study followed when necessary by FHR testing would take less time than routine FHR testing alone". |
|
| Notes | We contacted the authors to ask for clarification of the phrase, "computer generated algorithm based on the hospital number". They kindly responded with an explanation: "allocation was done automatically by a computer programme. Although the algorithm made use of the woman's hospital number, it was impossible for the midwife performing the fetal assessment to predict to which group the women would be allocated. The 'algorithm' was simply a mathematical sequence which was applied to the woman's hospital number to generate an allocation". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...computer generated algorithm based on the hospital number...". We sought clarification from the authors who kindly responded: "allocation was done automatically by a computer programme". |
| Allocation concealment (selection bias) | Low risk | Not described in the paper but we wrote for clarification from the authors who kindly responded: "although the algorithm made use of the woman's hospital number, it was impossible for the midwife performing the fetal assessment to predict to which group the women would be allocated. The 'algorithm' was simply a mathematical sequence which was applied to the woman's hospital number to generate an allocation". |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding women and/or staff in these trials was not generally feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding women and/or staff in these trials was not generally feasible. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Describe any loss of participants to follow‐up at each data collection point:
Describe any exclusion of participants after randomisation:
Was the analysis ITT? If not, have the data been able to be reincluded?
|
| Selective reporting (reporting bias) | Unclear risk | There was no list of prespecified outcomes from the protocol, and we did not assess the trial protocol. |
| Other bias | High risk | If the study was stopped early, explain the reasons:
Describe any baseline imbalance:
|
Johnstone 1993.
| Methods | 2‐arm RCT. Randomisation by Zelen method ‐ only those randomised to Doppler were invited to participate in the trial. Those allocated to CTG were being given normal care so their permission was regarded as not required. Randomisation was of individual women. |
|
| Participants | Women with pregnancies identified clinically as being at increased risk (N = 2289 out of the 8018 women giving birth at the hospital during the time of the study). Doppler or CTG or BPP was given to pregnant women where there was concern by medical staff about antenatal fetal well‐being by random allocation. Women were admitted to the trial if there was a wish for Doppler studies or a referral for AN fetal monitoring (CTG or BPP). So, all women meeting these criteria were randomised regardless of risk. N = 2289 women. |
|
| Interventions | Intervention: Doppler US of umbilical artery (and other monitoring). Comparison: no Doppler ‐ but other monitoring used (CTG/BPP). |
|
| Outcomes | Fetal mortality and morbidity; obstetric interventions; use of other tests of fetal monitoring; impact on obstetric decision making; health and personal costs; women’s satisfaction (to be presented in a separate report). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Just described as randomised. |
| Allocation concealment (selection bias) | Low risk | “Sequentially numbered opaque sealed envelopes were attached by stapling to the case notes of all women attending this hospital. Randomisation was carried out by opening the envelope for every woman who met the criteria described above.” |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding women and/or staff in these trials was not generally feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding women and/or staff in these trials was not generally feasible. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Describe any loss of participants to follow‐up at each data collection point:
Describe any exclusion of participants after randomisation:
Was the analysis ITT? If not, have the data been able to be reincluded?
|
| Selective reporting (reporting bias) | Unclear risk | They seemed to report on their prespecified outcomes but we did not assess the trial protocol. |
| Other bias | Low risk | If the study was stopped early, explain the reasons:
Describe any baseline imbalance:
Describe any differential diagnosis:
Receiving the other intervention:
|
Lees 2013.
| Methods | 3‐arm prospective randomised controlled study of individual women. | |
| Participants | Study in 20 tertiary care hospitals in 5 European countries (Austria, Germay, Italy, The Netherlands, UK). Women over 18 years capable of giving consent. Singleton pregnancy at 26 + 0 to 31 + 6 weeks’ gestation with FGR (defined as abdominal circumference below the 10th percentile based on local standards and abnormal umbilical artery Doppler pulsatility index (PI) above the 95th percentile based on local standards irrespective of the presence or absence of reversed end‐diastolic flow). In all cases, estimated fetal weight was > 500 g. Short‐term variation after 1 hour of CTG tracing had to be > 3.5 ms at 26 to 28 weeks and > 4 at 29 to 31 weeks with ductus venosus PI < 95th percentile. (GA determined by US at 14 and between 14 to 21 + 6 weeks). Women with known or planned impending delivery, major structural abnormality or fetal karyotype abnormality were excluded. N = 511 randomised (8 subsequently excluded). |
|
| Interventions | Randomisation groups: 1. Cardiotocograph short term variation (CTG STV) and timing of delivery was assessed with a criterion for reduced STV. Umbilical artery Doppler measurements were taken but no waveform measurements of the ductus venosus were recorded. (166 allocated, 21 lost to follow‐up, 1 missing neonatal data, 144 in primary analysis). 2. Early abnormality of ductus venosus prompted delivery (early changes pulsatility index > 95th percentile) (n = 167, 25 lost to follow‐up, 142 in primary analysis). 3. Late ductus venosus changes (a wave indicated no or reversed flow) (n = 170, 13 lost to follow‐up, 157 in primary analysis). All measurements were confirmed by a second measurement at least 24 hours later. Monitoring in all groups included umbilical artery Doppler and CTG was recommended at least once a week but could be more frequent depending on local protocol. Irrespective of randomised group, there was a cutoff rescue value for STV based on CTG at 26 to 28.9 weeks that prompted delivery. At 32 weeks, deliveries were according to local protocol. In all groups, delivery could be undertaken based on a maternal indication such as severe pre‐eclampsia or clear CTG abnormalities such as recurrent late decelerations. |
|
| Outcomes | Primary outcome: survival without cerebral palsy or neurosensory impairment, or a Bayley III developmental score of less than 85 at 2 years of age. Secondary outcomes: composite of adverse neonatal outcome defined as fetal or postnatal death (between trial entry in‐utero and discharge home from neonatal services) or 1 or more of the following severe morbidities: BPD (defined as supplemental oxygen to maintain SATs > 90% at 36 weeks), severe cerebral haemorrhage (IVH grade III or IV) cystic periventricular leukomalacia, proven neonatal sepsis (blood culture and requiring antibiotics) or NEC (presence of pneumatosis or perforation on X‐ray or disease present on laparotomy). |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Through central randomisation website. Random block design, stratified by gestation (< 29 vs > 29 weeks) and centre. |
| Allocation concealment (selection bias) | Low risk | Through central randomisation website. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | It was not feasible to blind clinicians to intervention group. Women may have been aware of randomisation group. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Neonatal outcome data entered directly from records and entered into database. Not possible to blind outcome assessment for all outcomes, however, the assessor of the primary outcome was blinded. “Concealment of the allocated monitoring regime was not possible, and clinicians responsible for the care of the women entered in the study and women themselves were aware of the treatment allocation. However, the paediatrician doing the follow‐up examination was masked to follow‐up assessment and data entry allocation”. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Of 511 randomised, missing data for 8 women and babies for the primary outcome. There was some attrition at 2‐year follow‐up (59 lost to follow‐up). ITT analysis. |
| Selective reporting (reporting bias) | Low risk | Protocol available and no evidence of outcome reporting bias. |
| Other bias | Low risk | Demographic data given for whole sample and those with poor composite outcome. Groups appeared similar at baseline. |
Neales 1994 [pers comm].
| Methods | 2‐arm randomised controlled study of individual women. | |
| Participants | Women of 24 weeks or greater gestation with a singleton pregnancy, and ultrasonic evidence of IUGR (abdominal circumference on or below 5th centile for GA). N = 467 women. |
|
| Interventions | Intervention: Doppler US of umbilical artery revealed, weekly or more often if indicated. Documented in notes. Discussed with registrar. Comparison: Doppler US weekly but recorded in separate file and not disclosed to clinicians. |
|
| Outcomes | Obstetric management: gestation at birth, time from enrolment to birth, mode of birth/onset of labour, fetal distress in labour. Neonatal outcome: perinatal mortality, birthweight, admission to NICU, neonatal outcome. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information other than 'randomised'. |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes but there was no information as to whether the envelopes were opaque and whether they were distributed in a sequential order. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding women and/or staff in these trials was not generally feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding women and/or staff in these trials was not generally feasible. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Describe any loss of participants to follow‐up at each data collection point:
Describe any exclusion of participants after randomisation: no exclusion:
Was the analysis ITT? If not, have the data been able to be reincluded?
|
| Selective reporting (reporting bias) | High risk | Not all outcomes available and we did not assess the trial protocol. |
| Other bias | Unclear risk | If the study was stopped early, explain the reasons:
|
Newnham 1991.
| Methods | 2‐arm RCT, stratified for twin pregnancies. Randomisation was of individual women. |
|
| Participants | Women with high‐risk pregnancies, singletons and twins. Defined as those disorders of pregnancy in which an increased risk of retarded fetal growth or impaired fetal well‐being were considered likely. N = 505 women. |
|
| Interventions | Intervention: Doppler of umbilical and utero‐placental (within the placental bed) artery.
Comparison: no Doppler.
|
|
| Outcomes | Primary: duration of neonatal stay in hospital. Secondary: number and type of fetal heart monitoring studies, obstetric interventions, frequency of fetal distress, birthweight, Apgar score, and need for NICU. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Just described as random. |
| Allocation concealment (selection bias) | Low risk | Numbered opaque sealed envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding women and/or staff in these trials was not generally feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors of neonatal outcomes were blind to Doppler results. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Describe any loss of participants to follow‐up at each data collection point:
Describe any exclusion of participants after randomisation:
Was the analysis ITT? If not, have the data been able to be reincluded?
|
| Selective reporting (reporting bias) | Unclear risk | Reported outcomes were the same as those prespecified but we did not assess the trial protocol. |
| Other bias | Low risk | If the study was stopped early, explain the reasons:
Describe any baseline imbalance:
Describe any differential diagnosis:
|
Nienhuis 1997.
| Methods | Randomised controlled study ‐ stratified randomisation and block randomisation. Stratification by GA (< 32 weeks and > 32 weeks) and smoking (regardless of number of cigarettes smoked). Randomisation by individual women, 2‐arm trial. |
|
| Participants | Women with clinically suspected IUGR of > 2 weeks diagnosed by fundal height measurements at the outpatient clinic. Singleton pregnancies. Exclusions: multiple pregnancies, uncertain GA, nonCaucasian origin, maternal or fetal conditional requiring immediate hospitalisation or intervention. N = 161 women. |
|
| Interventions | Intervention: Doppler US of umbilical artery revealed:
Comparison: Doppler US of umbilical artery concealed:
|
|
| Outcomes | Effect on costs in terms of hospitalisation, perinatal outcome, neurological development and postnatal catchup growth, onset and mode of birth, birthweight, and GA at birth. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk |
|
| Allocation concealment (selection bias) | Low risk |
|
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding women and/or staff in these trials was not generally feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors of neonatal outcomes were blind to Doppler results. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Describe any loss of participants to follow‐up at each data collection point:
Describe any exclusion of participants after randomisation:
Was the analysis ITT? If not, have the data been able to be reincluded?
|
| Selective reporting (reporting bias) | Unclear risk | All the outcomes were reported but we did not assess the trial protocol. |
| Other bias | High risk | If the study was stopped early, explain the reasons:
Describe any baseline imbalance:
|
Nimrod 1992.
| Methods | RCT; 2‐arm trial randomising individual women. | |
| Participants | Pregnant women seen at the 'Fetal Assessment Unit' over 40 weeks' gestation. | |
| Interventions | Intervention: pulsed Doppler revealed. Fetal aorta and umbilical artery assessed. BPP and NST also undertaken. Comparison: pulsed Doppler concealed. BPP and NST were reported. |
|
| Outcomes | CS; gestation at birth; meconium in amniotic fluid; need for phototherapy. | |
| Notes | Conference abstract available, but no full publication. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information available. |
| Allocation concealment (selection bias) | Unclear risk | No information available. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding women and/or staff in these trials was not generally feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding women and/or staff in these trials was not generally feasible. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information available. |
| Selective reporting (reporting bias) | Unclear risk | Very limited data in the conference abstract. We did not assess the trial protocol. |
| Other bias | Unclear risk | No information available on which to judge this aspect. |
Norman 1992.
| Methods | RCT. Individual women randomised in 2 arms. | |
| Participants | Women with high‐risk pregnancies with recurrent pregnancy loss (2 or more mid trimester or early third trimester losses which resulted in IUFD, stillbirth or neonatal death) at least 24 weeks' pregnant. 54 women randomised. N = 54 women. |
|
| Interventions | Intervention: Doppler velocimetry of umbilical artery revealed. Comparison: Doppler velocimetry of umbilical artery concealed. |
|
| Outcomes | Maternal intervention, hospital stay, induction of labour, CS, perinatal mortality and morbidity. | |
| Notes | A conference poster (incomplete data). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Women were randomly allocated. |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelope, but no mention of how they were distributed nor whether they were opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding women and/or staff in these trials was not generally feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding women and/or staff in these trials was not generally feasible. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Describe any loss of participants to follow‐up at each data collection point:
Describe any exclusion of participants after randomisation:
Was the analysis ITT? If not, have the data been able to be reincluded?
|
| Selective reporting (reporting bias) | Unclear risk | No information in the poster to enable this to be assessed. Also we did not assess the trial protocol. |
| Other bias | Low risk | If the study was stopped early, explain the reasons:
Describe any baseline imbalance:
Describe any differential diagnosis:
|
Ott 1998.
| Methods | 2‐arm RCT of individual women. | |
| Participants | Women referred to the perinatal laboratory so high‐risk pregnancies (risk of UPI; fetal risk; postdates; maternal diabetes; PROM/PTL; fluid abnormalities). N = 715 women. |
|
| Interventions | Intervention: fetal and umbilical Doppler + modified BPP. Comparison: no Doppler but modified BPP. |
|
| Outcomes | Primary outcome: neonatal morbidity rate (admission to NICU, length of stay in NICU, significant neonatal morbidity). Secondary outcome: GA at delivery, neonatal weight, CS for fetal distress. |
|
| Notes | The outcome of 'significant neonatal morbidity' assessed in this study included central nervous system complications, sepsis, acidosis/asphyxia, cardiomyopathy, anaemia, metabolic outcomes but excluded RDS. Anaemia and metabolic outcomes were not defined. We considered this outcome to be sufficiently different from the review's primary outcome of 'serious neonatal morbidity (composite outcome including hypoxic ischaemic encephalopathy, IVH, BPD, NEC)' that we did not include these data in the meta‐analysis. This study found no significant difference in 'significant neonatal complications' between the Doppler group (8%) and the no Doppler group (6.6%). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Computer‐generated random number allocation system.” |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding women and/or staff in these trials was not generally feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding women and/or staff in these trials was not generally feasible. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Describe any loss of participants to follow‐up at each data collection point:
Describe any exclusion of participants after randomisation:
Was the analysis ITT? If not, have the data been able to be reincluded?
|
| Selective reporting (reporting bias) | Unclear risk | Although the prespecified outcomes in the paper were reported. we were not able to assess the protocol, so are not sure whether there was outcome reporting bias. The authors reported only on CS for fetal distress, and not on all CS. |
| Other bias | Low risk | If the study was stopped early, explain the reasons:
Describe any baseline imbalance:
Describe any differential diagnosis:
|
Pattinson 1994.
| Methods | RCT; block randomisation of individual women:
|
|
| Participants | Women > 28 weeks' pregnant with hypertension and/or suspected SGA fetuses were referred for Doppler US. 212 women with singleton pregnancies. N = 212 women. |
|
| Interventions | Intervention: Doppler velocimetry of umbilical artery revealed:
Comparison: Doppler velocimetry of umbilical artery concealed:
|
|
| Outcomes | Perinatal mortality and morbidity, antenatal hospitalisation, maternal intervention, admission to the NICU, and hospitalisation until discharge from the neonatal wards. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “...randomisation was performed by the person doing the Doppler velocity .......” |
| Allocation concealment (selection bias) | Unclear risk | “......opaque sealed envelopes......”, but no mention of numbered and sequentially ordered envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding women and/or staff in these trials was not generally feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding women and/or staff in these trials was not generally feasible. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Describe any loss of participants to follow‐up at each data collection point:
Describe any exclusion of participants after randomisation:
Was the analysis ITT? If not, have the data been able to be reincluded?
|
| Selective reporting (reporting bias) | Unclear risk | All the outcomes were reported but we did not assess the trial protocol. |
| Other bias | High risk | If the study was stopped early, explain the reasons:
Describe any differential diagnosis:
|
Trudinger 1987.
| Methods | 2‐arm RCT of Individual women. | |
| Participants | Women with high fetal risk (singletons). More than 28 weeks' gestation. N = 300 women. |
|
| Interventions | Intervention: Doppler of umbilical artery revealed:
Comparison: Doppler of umbilical artery concealed:
|
|
| Outcomes | Perinatal mortality, CS, induction of labour, etc. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random number though no information on how they were generated and by whom. |
| Allocation concealment (selection bias) | Unclear risk | “Each patient was asked to draw an envelope containing a random number and those with even numbers were allocated to the Doppler report available group”. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding women and/or staff in these trials was not generally feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding women and/or staff in these trials was not generally feasible. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Describe any loss of participants to follow‐up at each data collection point:
Was the analysis ITT? If not, have the data been able to be reincluded?
|
| Selective reporting (reporting bias) | Unclear risk | No outcome listed in methods section and we did not assess the trial protocol. |
| Other bias | Low risk | If the study was stopped early, explain the reasons:
Describe any baseline imbalance:
Describe any differential diagnosis:
|
Tyrrell 1990.
| Methods | RCT; pragmatic 2‐arm trial. | |
| Participants | Women with high‐risk singleton pregnancies. Specifically, 500 pregnant women at high risk of growth retardation or stillbirth. IUGR clinically suspected or by US scan, previous SGA baby, previous antepartum haemorrhage, hypertension. Exclusions: women with diabetes, twin pregnancies. N = 500 women. |
|
| Interventions | Intervention: routine use of Doppler and BPP testing + other tests:
Comparison: no Doppler and no biophysical assessment but other tests only:
|
|
| Outcomes | Total number of days of antenatal admission, rate of induction of labour (by any method), mode of birth (elective CS and emergency CS), 1 and 5 min Apgar, birthweight, admission to NICU. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk |
|
| Allocation concealment (selection bias) | Unclear risk |
|
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding women and/or staff in these trials was not generally feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding women and/or staff in these trials was not generally feasible. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Describe any loss of participants to follow‐up at each data collection point:
Describe any exclusion of participants after randomisation:
Was the analysis ITT? If not, have the data been able to be reincluded?
|
| Selective reporting (reporting bias) | Unclear risk | Not all outcomes were reported, emergency CS just reported in the text. We did not assess the trial protocol. |
| Other bias | Unclear risk | If the study was stopped early, explain the reasons:
Describe any baseline imbalance:
Describe any differential diagnosis:
|
Williams 2003.
| Methods | Randomised controlled study; block randomisation (block of 4 and 6). Individual women. |
|
| Participants | Women with high‐risk pregnancies: singletons (IUGR 7%, hypertension 10%, diabetes 11%, prolonged pregnancy 43%, decreased fetal movements 22%). GA > 32 weeks. N = 1360 women. |
|
| Interventions | Intervention: umbilical artery Doppler:
Comparison: electronic FHR with NST:
|
|
| Outcomes |
Primary outcome: incidence of CS for fetal distress in labour (nonreassuring FHR). Secondary outcome: total CS, Apgar score 1 and 5 min, the incidence of stillbirth, the presence of meconium, and the incidence of transfer to the NICU with severe neonatal morbidity. |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table with a variable block size of 4 and 6. |
| Allocation concealment (selection bias) | Unclear risk | Sequentially numbered opaque envelopes although no information as to whether they were sealed. “...envelopes were kept in a locked drawer that was accessible only to the unit clerk. The envelops was opened by the nurse/sonographer in the presence of the patient”. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Blinding women and/or staff in these trials was not generally feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding women and/or staff in these trials was not generally feasible. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Describe any loss of participants to follow‐up at each data collection point:
Describe any exclusion of participants after randomisation:
Was the analysis ITT?
|
| Selective reporting (reporting bias) | Unclear risk | All the outcomes were reported but we did not assess the trial protocol. |
| Other bias | Low risk | If the study was stopped early, explain the reasons:
Describe any baseline imbalance:
Describe any differential diagnosis:
|
AEDF: absent end diastolic flow AEDV: absent end diastolic velocity AN: antenatal BPD: bronchopulmonary dysplasia BPP: biophysical profile CS: caesarean section CTG: cardiotocography
D: EDV: end diastolic velocities FHR: fetal heart rate GA: gestational age
HT: ITT: intention‐to‐treat IUFD: intrauterine fetal death IUGR: intrauterine growth retardation IVH: intraventricular haemorrhage min: minute NEC: necrotising enterocolitis NICU: neonatal intensive care unit NS: not significant NST: nonstress test PNM:
PTL: preterm labour PROM: preterm rupture of membranes RCT: randomised controlled trial RDS: respiratory distress syndrome
S:
SAT: SD: standard deviation SGA: small‐for‐gestational age
STV:
UPI: US: ultrasound vs: versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Davies 1992 | Participants were an "unselected population". |
| Gonsoulin 1991 | Full report not available. |
| Mason 1993 | Participants were "low‐risk primigravid women". |
| McCowan 1996 | Conference abstract only but outcomes were comparing women with normal and abnormal Doppler ultrasound readings, so not a randomised comparison. |
| McParland 1988 | This study was never reported in full although it has been partly reported in a review article (McParland 1988) and a full manuscript was given to the review authors by Dr Pearce, who has been accused of publishing reports of trials whose veracity cannot be confirmed (BJOG 1995). Consequently, the Doppler trial data are not now thought by the review authors to be sufficiently reliable to be retained within this review. |
| Newnham 1993 | Participants were an "unselected population". |
| Omtzigt 1994 | Participants were a "non‐selected University Hospital population". |
| Pearce 1992 | Dr Pearce has been accused of publishing reports of trials whose veracity cannot be confirmed (BJOG 1995). Consequently, the Doppler trial data are not now thought by the reviewers to be sufficiently reliable to be retained within this review. |
| Schneider 1992 | Participants were an "unselected pregnant population". |
| Whittle 1994 | Participants were an "unselected population". |
Differences between protocol and review
The secondary outcome of 'any perinatal death after randomisation excluding malformations' was changed to 'any potentially preventable perinatal death', which was defined as 'perinatal death excluding chromosomal abnormalities, termination of pregnancies, birth before fetal viability (less than 500 g) and fetal death before use of the intervention'.
The methods have been updated to the current Cochrane Pregnancy and Childbirth Group standard text, and a 'summary of findings' table has been added to the updated review.
We included the following clinically relevant outcomes that were not prespecified in our protocol.
Antenatal admissions.
Birth less than 34 weeks.
Phototherapy for neonatal jaundice.
Abnormal neurological development at nine months.
Hospitalisation for IUGR neonatal.
Fetal distress in labour.
Birthweight < 5 percentile.
Periventricular leucomalacia.
Antenatal hospital stay (days).
Infant survival at two years.
Sepsis (proven).
Contributions of authors
In an earlier version of this review, T Stampalija (TS) drafted the background section, with Z Alfirevic (ZA) providing comments and suggestions. In this update, T Dowswell (TD) assisted with assessing new studies, grading the evidence and producing the 'Summary of findings' table. All authors commented on drafts.
Sources of support
Internal sources
The University of Liverpool, UK.
External sources
-
National Institute for Health Research (NIHR), UK.
NIHR Cochrane Programme Grant Project: 13/89/05 – Pregnancy and childbirth systematic reviews to support clinical guidelines
Declarations of interest
Zarko Alfirevic: none known.
Tamara Stampalija: none known.
Therese Dowswell: I am paid via my institution by the UK NHS (NIHR programme grant) to work on a range of Cochrane Reviews. In the last 36 months, I have received funding from the WHO to work on other Cochrane reviews. The funders have no influence on the content or conclusions of the reviews I work on.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Almstrom 1992 {published data only}
- Almstrom H, Axelsson O, Cnattingius S, Ekman G, Maesel A, Ulmsten U, et al. Comparison of umbilical‐artery velocimetry and cardiotocography for surveillance of small‐for‐gestational‐age fetuses. Lancet 1992;340:936‐40. [DOI] [PubMed] [Google Scholar]
- Almstrom H, Axelsson O, Ekman G, Ingemarsson I, Maesel A, Arstrom K, et al. Umbilical artery velocimetry may influence clinical interpretation of intrapartum cardiotocograms. Acta Obstetricia et Gynecologica Scandinavica 1995;74:526‐9. [DOI] [PubMed] [Google Scholar]
- Marsal K, Almstrom H, Axelsson O, Cnattingius S, Ekman G, Maesel A, et al. Umbilical artery velocimetry is more effective than cardiotocography for surveillance of growth retarded fetuses. Journal of Perinatal Medicine 1991;19(Suppl 2):84. [Google Scholar]
Biljan 1992 {published and unpublished data}
- Biljan M, Haddad N, McVey K, Williams J. Efficiency of continuous‐wave Doppler in screening high risk pregnancies in a district general hospital (a prospective randomized study on 674 singleton pregnancies). Proceedings of 26th British Congress of Obstetrics and Gynaecology; 1992 July 7‐10; Manchester, UK. 1992:6.
- Biljan MM, McVey KP, Haddad NG. The value of continuous wave doppler assessment of fetal umbilical artery in management of "at risk" pregnancies. Proceedings of 2nd European Congress on Prostaglandins in Reproduction; 1991 April 30 ‐ May 3; The Hague, Netherlands. 1991:189.
Burke 1992 {published data only}
- Burke G, Stuart B, Crowley P, Ni Scanaill S, Drumm J. Does Doppler ultrasound alter the management of high‐risk pregnancy? Care concern and cure in perinatal medicine. 13th European Congress of Perinatal Medicine; 1992 May; Amsterdam, The Netherlands. Parthenon, 1992:597‐604.
- Burke G, Stuart B, Crowley P, Ni Scanaill S, Drumm J. Does Doppler ultrasound alter the management of high‐risk pregnancy?. Journal of Perinatal Medicine 1992;20(Suppl 1):266. [Google Scholar]
De Rochambeau 1992 {published data only}
- Rochambeau B, Jabbour N, Mellier G. Umbilical doppler velocimetry in prolonged pregnancies [La velocimetrie Doppler ombilicale dans les grossesses prolongees]. Revue Francaise de Gynecologie et d Obstetrique 1992;87(5):289‐94. [PubMed] [Google Scholar]
Giles 2003 {published data only}
- Giles W, Bisits A, O'Callaghan S. The doppler assessment in multiple pregnancy study (damp) and metaanalyses of doppler and twins. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S17. [Google Scholar]
- Giles W, Bisits A, O'Callaghan S, Gill A. The doppler assessment in multiple pregnancy randomised controlled trial of ultrasound biometry versus umbilical artery doppler ultrasound and biometry in twin pregnancy. BJOG: an international journal of obstetrics and gynaecology 2003;110(6):593‐7. [PubMed] [Google Scholar]
Haley 1997 {published data only}
- Haley J, Tuffnell DJ, Johnson N. Randomised controlled trial of cardiotocography versus umbilical artery Doppler in the management of small for gestational age fetuses. British Journal of Obstetrics and Gynaecology 1997;104(4):431‐5. [DOI] [PubMed] [Google Scholar]
Hofmeyr 1991 {published data only}
- Hofmeyr GJ, Pattinson R, Buckley D, Jennings J, Redman CWG. Umbilical artery resistance index as a screening test for fetal well‐being. II. Randomized feasibility study. Obstetrics & Gynecology 1991;78:359‐62. [PubMed] [Google Scholar]
Johnstone 1993 {published data only}
- Johnstone FD, Prescott R, Hoskins P, Greer IA, McGlew T, Compton M. The effect of introduction of umbilical Doppler recordings to obstetric practice. British Journal of Obstetrics and Gynaecology 1993;100:733‐41. [DOI] [PubMed] [Google Scholar]
Lees 2013 {published data only}
- Cambridge Consortium ongoing. Trial of umbilical and fetal flow in Europe (TRUFFLE). National Research Register (www.nrr.nhs.uk) (accessed 6 July 2006).
- Lees C, Baumgartner H. The TRUFFLE study ‐ a collaborative publicly funded project from concept to reality: how to negotiate an ethical, administrative and funding obstacle course in the European Union. Ultrasound in Obstetrics and Gynecology 2005;25:105‐7. [DOI] [PubMed] [Google Scholar]
- Lees C, Marlow N, Arabin B, Bilardo CM, Brezinka C, Derks JB, et al. Perinatal morbidity and mortality in early‐onset fetal growth restriction: cohort outcomes of the trial of randomized umbilical and fetal flow in Europe (truffle). Ultrasound in Obstetrics & Gynecology 2013;42(4):400‐8. [DOI] [PubMed] [Google Scholar]
- Lees CC, Marlow N, Wassenaer‐Leemhuis A, Arabin B, Bilardo CM, Brezinka C, et al. 2 year neurodevelopmental and intermediate perinatal outcomes in infants with very preterm fetal growth restriction (TRUFFLE): a randomised trial. Lancet 2015;385(9983):2162‐72. [DOI] [PubMed] [Google Scholar]
- Wassenaer‐Leemhuis AG, Marlow N, Lees C, Wolf H. Can severe neonatal morbidity predict neurodevelopmental impairment (NDI) at two years in growth restricted infants? Secondary analyses from TRUFFLE (Trial of Randomized Umbilical and Fetal Flow in Europe). Pediatric Academic Socieities Annual Meeting; 2015 April 25‐28; San Diego, California, USA. 2015.
Neales 1994 [pers comm] {published data only}
- Neales K. (St Mary's Hospital, Manchester, UK). [personal communication]. Letter to: Z Alfirevic (Department of Women's and Children's Health, The University of Liverpool, Liverpool, UK) 24 January 1994; Vol. Located at: Cochrane Office, Department of Women's and Children's Health, The University of Liverpool, Liverpool, UK.
Newnham 1991 {published data only}
- Newnham JP, O'Dea MRA, Reid KP, Diepeveen DA. Doppler flow velocity waveform analysis in high risk pregnancies: a randomized controlled trial. British Journal of Obstetrics and Gynaecology 1991;98:956‐63. [DOI] [PubMed] [Google Scholar]
Nienhuis 1997 {published data only}
- Nienhuis SJ. Costs and effects of Doppler ultrasound measurements in suspected intrauterine growth retardation. A randomised clinical trial [thesis]. Maastricht: Universitaire Pers Maastricht, 1995. [Google Scholar]
- Nienhuis SJ, Ruissen CJ, Hoogland HJ, Gerver JW, Vles J, Haan J. Cost‐effectiveness of a doppler policy in suspected intrauterine growth retardation ‐ a randomized controlled trial. Proceedings of 13th World Congress of Gynaecology and Obstetrics (FIGO); 1991 Sept 15‐20; Singapore. 1991:32.
- Nienhuis SJ, Vles JS, Gerver WJ, Hoogland HJ. Doppler ultrasonography in suspected intrauterine growth retardation: a randomized clinical trial. Ultrasound in Obstetrics & Gynecology 1997;9(1):6‐13. [DOI] [PubMed] [Google Scholar]
- Ruissen CJ, Nienhuis SJ, Hoogland HJ, Vles JFH, Gerver WJ, Haan J. Cost‐effectiveness of a Doppler based policy of suspected intrauterine growth retardation ‐ a randomised controlled trial. Journal of Maternal Fetal Investigation 1992;1:126. [Google Scholar]
Nimrod 1992 {published data only}
- Nimrod C, Yee J, Hopkins C, Pierce P, Lange I, Fick G, et al. The utility of pulsed Doppler studies in the evaluation of postdate pregnancies. Journal of Maternal Fetal Investigation 1992;1:127. [Google Scholar]
Norman 1992 {published data only}
- Norman K, Pattinson RC, Carstens E. Doppler velocimetry in recurrent pregnancy loss: is there a role? Proceedings of 11th Conference on Priorities in Perinatal Care in South Africa; 1992 March; Caledon, South Africa. 1992:71‐4.
Ott 1998 {published data only}
- Ott WJ, Mora G, Arias F, Sunderji S, Sheldon G. Comparison of the modified biophysical profile to a new biophysical profile incorporating the middle cerebral artery to umbilical artery velocity flow systolic/diastolic ratio. American Journal of Obstetrics and Gynecology 1998;178(6):1346‐53. [DOI] [PubMed] [Google Scholar]
Pattinson 1994 {published data only}
- Pattinson R, Norman K, Odendaal HJ. Management of fetuses suspected of IUGR but with EDVs of the umbilical artery: a randomised controlled trial. Proceedings of 26th British Congress of Obstetrics and Gynaecology; 1992 July 7‐10; Manchester, UK. 1992:5.
- Pattinson RC, Norman K, Odendaal HJ. The role of doppler velocimetry in the management of pregnancies: a randomized controlled trial. Proceedings of 11th Conference on Priorities in Perinatal Care in South Africa; 1992 March; Caledon, South Africa. 1992:59‐63.
- Pattinson RC, Norman K, Odendaal HJ. The role of Doppler velocimetry in the management of high risk pregnancies. British Journal of Obstetrics and Gynaecology 1994;101:114‐20. [DOI] [PubMed] [Google Scholar]
Trudinger 1987 {published data only}
- Trudinger BJ, Cook CM, Giles WB, Connelly AJ, Thompson RS. Umbilical artery flow velocity waveforms in high‐risk pregnancy: randomised controlled trial. Lancet 1987;1:188‐90. [DOI] [PubMed] [Google Scholar]
Tyrrell 1990 {published data only}
- Lilford RJ. (St James' University Hospital, Leeds, UK). [personal communication]. Letter to: The Oxford Database of Perinatal Trials (copy) 24 February 1989; Vol. Located at: Cochrane Office, Department of Women's and Children's Health, The University of Liverpool, Liverpool, UK.
- Tyrrell SN, Lilford RJ, MacDonald HN, Nelson EJ, Porter J, Gupta JK. Randomized comparison of routine vs highly selective use of Doppler ultrasound and biophysical scoring to investigate high risk pregnancies. British Journal of Obstetrics and Gynaecology 1990;97:909‐16. [DOI] [PubMed] [Google Scholar]
Williams 2003 {published data only}
- Williams K, Farquharson D, Bebbington M, Dansereau J, Galerneau F, Wilson RD, et al. A randomized controlled clinical trial comparing non stress testing versus doppler velocimetry as a screening test in a high risk population. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S109. [DOI] [PubMed] [Google Scholar]
- Williams KP, Farquharson DF, Bebbington M, Dansereau J, Galerneau F, Wilson RD, et al. Screening for fetal well‐being in a high‐risk pregnant population comparing the nonstress test with umbilical artery doppler velocimetry: a randomized controlled clinical trial. American Journal of Obstetrics and Gynecology 2003;188(5):1366‐71. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Davies 1992 {published data only}
- Davies JA, Gallivan S, Spencer JAD. Randomised controlled trial of doppler ultrasound screening of placental perfusion during pregnancy. Lancet 1992;340:1299‐303. [PubMed] [Google Scholar]
Gonsoulin 1991 {published data only}
- Gonsoulin W. Umbilical artery Doppler waveform analysis: a randomized study on effect on outcome. American Journal of Obstetrics and Gynecology 1991;164:370. [Google Scholar]
Mason 1993 {published data only}
- Mason GC, Lilford RJ, Porter J, Nelson E, Tyrell S. Randomised comparison of routine vs highly selective use of Doppler ultrasound in low risk pregnancies. British Journal of Obstetrics and Gynaecology 1993;100:130‐3. [DOI] [PubMed] [Google Scholar]
McCowan 1996 {published data only}
- McCowan LME, Harding J, Roberts AB, Barker S, Townend K. Perinatal morbidity in small for gestational age fetuses in relation to umbilical doppler. Proceedings of the 14th Annual Congress of the Australian Perinatal Society in conjunction with the New Zealand Perinatal Society; 1996 March 24‐27; Adelaide, Australia. 1996:10.
McParland 1988 {published data only}
- McParland P, Pearce JM. Doppler blood flow in pregnancy. Placenta 1988;9:427‐50. [DOI] [PubMed] [Google Scholar]
Newnham 1993 {published data only}
- Newnham JP, Evans SF, Michael CA, Stanley FJ, Landau LI. Effects of frequent ultrasound during pregnancy: a randomised controlled trial. Lancet 1993;342:887‐91. [DOI] [PubMed] [Google Scholar]
Omtzigt 1994 {published data only}
- Omtzigt AWJ. Clinical value of umbilical doppler velocimetry [thesis]. Utrecht: University of Utrecht, 1990. [Google Scholar]
- Omtzigt AWJ, Bruinse HW, Reuwer PJHM. A randomized controlled trial on the clinical value of umbilical Doppler velocimetry. I. Obstetrical management. Proceedings of 12th European Congress of Perinatal Medicine; 1990 Sept 11‐14; Lyon, France. 1990:210.
- Omtzigt AWJ, Bruinse HW, Reuwer PJHM. A randomized controlled trial on the clinical value of umbilical Doppler velocimetry: neonatal outcome. Proceedings of 12th European Congress of Perinatal Medicine; 1990 Sept 11‐14; Lyon, France. 1990:189.
- Omtzigt AWJ, Reuwer PJHM, Bruinse HW. A randomized controlled trial on the clinical value of umbilical Doppler velocimetry in antenatal care. American Journal of Obstetrics and Gynecology 1994;170:625‐34. [DOI] [PubMed] [Google Scholar]
Pearce 1992 {published data only}
- Pearce JM. The application of uteroplacental waveforms to complicated pregnancies. Doppler Ultrasound in Perinatal Medicine. Oxford: Oxford University Press, 1992:159‐77. [Google Scholar]
Schneider 1992 {published data only}
- Schneider KTM, Renz S, Furstenau U, Amberg‐Wendland D, Prochaska D, Graeff H. Doppler flow measurements as a screening method during pregnancy: is it worth the effort?. Journal of Maternal Fetal Investigation 1992;1:125. [Google Scholar]
Whittle 1994 {published data only}
- Whittle MJ, Hanretty KP, Primrose MH, Neilson JP. Screening for the compromised fetus: a randomized trial of umbilical artery velocimetry in unselected pregnancies. American Journal of Obstetrics and Gynecology 1994;170:555‐9. [DOI] [PubMed] [Google Scholar]
Additional references
Al‐Ghazali 1990
- Al‐Ghazali WH, Chapman MG, Rissik JM, Allan LD. The significance of absent end‐diastolic flow in the umbilical artery combined with reduced fetal cardiac output estimation in pregnancies at high risk for placental insufficiency. Journal of Obstetrics and Gynaecology 1990;10:271‐5. [Google Scholar]
Alfirevic 1995
- Alfirevic Z, Neilson JP. Doppler ultrasonography in high‐risk pregnancies: systematic review with meta‐analysis. American Journal of Obstetrics and Gynecology 1995;172:1379‐87. [DOI] [PubMed] [Google Scholar]
Alfirevic 2002
- Alfirevic Z, Roberts D, Martlew V. How strong is the association between maternal thrombophilia and adverse pregnancy outcome? A systematic review. European Journal Obstetetrics & Gynecology and Reproductive Biology 2002;101(1):6‐14. [DOI] [PubMed] [Google Scholar]
Alfirevic 2015
- Alfirevic Z, Stampalija T, Medley N. Fetal and umbilical Doppler ultrasound in normal pregnancy. Cochrane Database of Systematic Reviews 2015, Issue 4. [DOI: 10.1002/14651858.CD001450.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Almstrom 1995
- Almstrom H, Axelsson O, Ekman G, Ingemarsson I, Maesel A, Arstrom K, et al. Umbilical artery velocimetry may influence clinical interpretation of intrapartum cardiotocograms. Acta Obstetricia et Gynecologica Scandinavica 1995;74:526‐9. [DOI] [PubMed] [Google Scholar]
Baschat 2001
- Baschat AA, Gembruch U, Harman CR. The sequence of changes in Doppler and biophysical parameters as severe fetal growth restriction worsens. Ultrasound in Obstetrics & Gynecology 2001;18:571‐7. [DOI] [PubMed] [Google Scholar]
Baschat 2002
- Baschat AA, Gembruch U. Evaluation of the fetal coronary circulation. Ultrasound in Obstetrics & Gynecology 2002;20:405‐12. [DOI] [PubMed] [Google Scholar]
Bernstein 2000
- Bernstein IM, Horbar JD, Badger GJ, Ohlsson A, Golan A. Morbidity and mortality among very‐low‐birth‐weight neonates with intrauterine growth restriction. The Vermont Oxford Network. American Journal of Obstetrics and Gynecology 2000;182:198‐206. [DOI] [PubMed] [Google Scholar]
Bilardo 1990
- Bilardo CM, Nicolaides KH, Campbell S. Doppler measurements of fetal and uteroplacental circulations: relationship with umbilical venous blood gases measured at cordocentesis. American Journal of Obstetrics and Gynecology 1990;162:115‐20. [DOI] [PubMed] [Google Scholar]
Bilardo 2004
- Bilardo CM, Wolf H, Stigter RH, Ville Y, Baez E, Visser GHA, et al. Relationship between monitoring parameters and outcome in severe, early intrauterine growth restriction. Ultrasound in Obstetrics & Gynecology 2004;23:119‐25. [DOI] [PubMed] [Google Scholar]
BJOG 1995
- Anonymous. Retraction of articles. British Journal of Obstetrics and Gynaecology 1995;102(11):853. [DOI] [PubMed] [Google Scholar]
Bricker 2007
- Bricker L, Neilson JP. Routine Doppler ultrasound in pregnancy. Cochrane Database of Systematic Reviews 2007, Issue 2. [DOI: 10.1002/14651858.CD001450.pub2] [DOI] [PubMed] [Google Scholar]
Burns 1993
- Burns PN. Principles of Doppler and color flow. Radiology in Medicine 1993;85:3‐16. [PubMed] [Google Scholar]
Cheema 2004
- Cheema R, Dubiel M, Breborowicz G, Gudmundsson S. Fetal cerebral venous Doppler velocimetry in normal and high‐risk pregnancy. Ultrasound in Obstetrics & Gynecology 2004;24:147‐53. [DOI] [PubMed] [Google Scholar]
Chen 1996
- Chen JF, Fowlkes JB, Carson PL, Rubin JM, Adler RS. Autocorrelation of integrated power Doppler signals and its application. Ultrasound in Medicine and Biology 1996;22:1053‐7. [DOI] [PubMed] [Google Scholar]
CONSORT 2001
- Moher D, Schulz KF, Altman DG. The CONSORT statement: revised recommendations for improving the quality of reports of parallel‐group randomised trials. Lancet 2001;357(9263):1191‐4. [PubMed] [Google Scholar]
Devane 2007
- Devane D, Begley CM, Clarke M, Horey D, Oboyle C. Evaluating maternity care: a core set of outcome measures. Birth 2007;34(2):164‐72. [DOI] [PubMed] [Google Scholar]
Ferrazzi 2002
- Ferrazzi E, Bozzo M, Rigano S, Bellotti M, Morabito A, Pardi G, et al. Temporal sequence of abnormal Doppler changes in the peripheral and central circulatory systems of the severely growth‐restricted fetus. Ultrasound in Obstetrics & Gynecology 2002;19:140‐6. [DOI] [PubMed] [Google Scholar]
Figueras 2008
- Figueras F, Eixarch E, Gratacos E, Gardosi J. Predictiveness of antenatal umbilical artery Doppler for adverse pregnancy outcome in small‐for‐gestational‐age babies according to customised birthweight centiles: population‐based study. BJOG: an international journal of obstetrics and gynaecology 2008;115:590‐4. [DOI] [PubMed] [Google Scholar]
Fisk 2001
- Fisk NM, Smith RP. Fetal growth restriction; small for gestational age. In: Chamberlain G, Steer P editor(s). Turnbull's Obstetrics. 3rd Edition. Edinburgh: Churchill Livingstone, 2001:197‐209. [Google Scholar]
Fitzgerald 1977
- Fitzgerald DE, Drumm JE. Non‐invasive measurement of the human circulation using ultrasound: a new method. British Medical Journal 1977;2:1450‐1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gates 2004
- Gates S, Brocklehurst P. How should randomised trial including multiple pregnancies be analysed?. BJOG: an international journal of obstetrics and gynaecology 2004;111:213‐9. [DOI] [PubMed] [Google Scholar]
Giles 1985
- Giles WB, Trudinger BJ, Baird PJ. Fetal umbilical artery flow velocity waveforms and placental resistance: pathological correlation. British Journal of Obstetrics and Gynaecology 1985;92:31‐8. [DOI] [PubMed] [Google Scholar]
Graves 2007
- Graves CR. Antepartum fetal surveillance and timing of delivery in the pregnancy complicated by diabetes mellitus. Clinical Obstetrics and Gynecology 2007;50(4):1007‐13. [DOI] [PubMed] [Google Scholar]
Greer 1999
- Greer IA. Thrombosis in pregnancy: maternal and fetal issues. Lancet 1999;353(9160):1258‐65. [DOI] [PubMed] [Google Scholar]
GRIT 2003
- GRIT Study Group. A randomised trial of timed delivery for the compromised preterm fetus: short term outcomes and Bayesian interpretation. BJOG: an international journal of obstetrics and gynaecology 2002;110:27‐32. [DOI] [PubMed] [Google Scholar]
GRIT 2004
- GRIT study group. Infant wellbeing at 2 years of age in the Growth Restriction Intervention Trial (GRIT): multicentred randomised controlled trial. Lancet 2004;364:513‐20. [DOI] [PubMed] [Google Scholar]
Grivell 2015
- Grivell RM, Alfirevic Z, Gyte Gillian ML, Devane D. Antenatal cardiotocography for fetal assessment. Cochrane Database of Systematic Reviews 2015, Issue 9. [DOI: 10.1002/14651858.CD007863.pub4] [DOI] [Google Scholar]
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25:3443‐57. [DOI] [PubMed] [Google Scholar]
Hecher 2001
- Hecher K, Bilardo CM, Stigter RH, Ville Y, Hackeloer BJ, Kok HJ, et al. Monitoring of fetuses with intrauterine growth restriction: a longitudinal study. Ultrasound in Obstetrics & Gynecology 2001;18:564‐70. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Imdad 2011
- Imdad A, Yakoob MY, Siddiqui S, Bhutta ZA. Screening and triage of intrauterine growth restriction (IUGR) in general population and high risk pregnancies: a systematic review with a focus on reduct ion of IUGR related stillbirths . BMC Public Health 2011 ; 11 ( Suppl 3 ):S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lalor 2008
- Lalor JG, Fawole B, Alfirevic Z, Devane D. Biophysical profile for fetal assessment in high risk pregnancies. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD000038.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lees 2005
- Lees C, Baumgartner H. The TRUFFLE study ‐ a collaborative publicly funded project from concept to reality: how to negotiate an ethical, administrative and funding obstacle course in the European Union. Ultrasound in Obstetrics and Gynecology 2005;25:105‐7. [DOI] [PubMed] [Google Scholar]
Lees 2015
- Lees CC, Marlow N, Wassenaer‐Leemhuis A, Arabin B, Bilardo CM, Brezinka C, et al. 2 year neurodevelopmental and intermediate perinatal outcomes in infants with very preterm fetal growth restriction (TRUFFLE): a randomised trial. Lancet 2015;385(9983):2162‐72. [DOI] [PubMed] [Google Scholar]
Mari 2009
- Mari G. Doppler ultrasonography in obstetrics: from the diagnosis of fetal anemia to the treatment of intrauterine growth‐restricted fetuses. American Journal of Obstetrics and Gynecology 2009;200(6):613.e1‐613.e9. [DOI] [PubMed] [Google Scholar]
Mires 2000
- Mires GJ, Patel NB, Dempster J. Review: The value of fetal umbilical artery flow velocity waveforms in the prediction of adverse fetal outcome in high risk pregnancies. Journal of Obstetrics and Gynaecology 2000;10:261‐70. [Google Scholar]
Neilson 1987
- Neilson JP. Doppler ultrasound. British Journal of Obstetrics and Gynaecology 1987;94:929‐34. [DOI] [PubMed] [Google Scholar]
Nicholaides 1988
- Nicholaides KH, Bilardo CM, Soothill PW, Campbell S. Absence of end diastolic frequencies in umbilical artery: a sign of fetal hypoxia and acidosis. BMJ 1988;297(6655):1026‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nienhuis 1995
- Nienhuis SJ. Costs and effects of Doppler ultrasound measurements in suspected intrauterine growth retardation. A randomised clinical trial [thesis]. Maastricht: Universitaire Pers Maastricht, 1995. [Google Scholar]
Ortibus 2009
- Ortibus E, Lopriore E, Deprest J, Vandenbussche FP, Walther FJ, Diemert A, et al. The pregnancy and long‐term neurodevelopmental outcome of monochorionic diamniotic twin gestations: a multicenter prospective cohort study from the first trimester onward. American Journal of Obstetrics and Gynecology 2009;200(5):494.e1‐494.e8. [DOI] [PubMed] [Google Scholar]
Owen 2001
- Owen P. Fetal assessment in the third trimester: fetal growth and biophysical methods. In: Chamberlain G, Steer P editor(s). Turnbull's Obstetrics. 3rd Edition. Edinburgh: Churchill Livingstone, 2001. [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Stampalija 2010
- Stampalija T, Gyte GML, Alfirevic Z. Utero‐placental Doppler ultrasound for improving pregnancy outcome. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD008363.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thompson 1990
- Thompson RS, Trudinger BJ. Doppler waveform pulsatility index and resistance, pressure and flow in the umbilical placental circulation: an investigation using a mathematical model. Ultrasound in Medicine and Biology 1990;16:449. [DOI] [PubMed] [Google Scholar]
Trudinger 1985a
- Trudinger BJ, Giles WB, Cook CM, Bombardieri J, Collins L. Fetal umbilical artery flow velocity waveforms and placental resistance: clinical significance. British Journal of Obstetrics and Gynaecology 1985;92:23‐30. [DOI] [PubMed] [Google Scholar]
Trudinger 1985b
- Trudinger BJ, Giles WB, Cook CM. Uteroplacental blood flow velocity‐time waveform in normal and complicated pregnancy. British Journal of Obstetrics and Gynaecology 1985;92:39‐45. [DOI] [PubMed] [Google Scholar]
Weiner 1990
- Weiner CP. The relationship between the umbilical artery systolic/diastolic ratio and umbilical blood gas measurements in specimens obtained by cordocentesis. American Journal of Obstetrics and Gynecology 1990;162:1198‐202. [DOI] [PubMed] [Google Scholar]
Westergaard 2001
- Westergaard HB, Langhoff‐Roos J, Lingman G, Marsal K, Kreiner S. A critical appraisal of the use of umbilical artery Doppler ultrasound in high‐risk pregnancies: use of meta‐analyses in evidence‐based obstetrics. Ultrasound in Obstetrics & Gynecology 2001;17:466‐76. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Alfirevic 2010
- Alfirevic Z, Stampalija T, Gyte GML. Fetal and umbilical Doppler ultrasound in high‐risk pregnancies. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD007529.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Alfirevic 2013
- Alfirevic Z, Stampalija T, Gyte GML. Fetal and umbilical Doppler ultrasound in high‐risk pregnancies. Cochrane Database of Systematic Reviews 2013, Issue 11. [DOI: 10.1002/14651858.CD007529.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Neilson 1995
- Neilson JP, Alfirevic Z. Doppler ultrasound for fetal assessment in high risk pregnancies. Cochrane Database of Systematic Reviews 1995, Issue 1. [DOI: 10.1002/14651858.CD000073] [DOI] [PubMed] [Google Scholar]
Neilson 1996
- Neilson JP, Alfirevic Z. Doppler ultrasound for fetal assessment in high risk pregnancies. Cochrane Database of Systematic Reviews 1996, Issue 4. [DOI: 10.1002/14651858.CD000073.pub2] [DOI] [PubMed] [Google Scholar]


