Abstract
Background
Fibromyalgia (FM) is a clinically well‐defined chronic condition of unknown aetiology characterized by chronic widespread pain that often co‐exists with sleep disturbances, cognitive dysfunction and fatigue. Patients often report high disability levels and negative mood. Psychotherapies focus on reducing key symptoms, improving daily functioning, mood and sense of personal control over pain.
Objectives
To assess the benefits and harms of cognitive behavioural therapies (CBTs) for treating FM at end of treatment and at long‐term (at least six months) follow‐up.
Search methods
We searched the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2013, Issue 8), MEDLINE (1966 to 28 August 2013), PsycINFO (1966 to 28 August 2013) and SCOPUS (1980 to 28 August 2013). We searched http://www.clinicaltrials.gov (web site of the US National Institutes of Health) and the World Health Organization Clinical Trials Registry Platform (ICTRP) (http://www.who.int/ictrp/en/) for ongoing trials (last search 28 August,2013), and the reference lists of reviewed articles.
Selection criteria
We selected randomised controlled trials of CBTs with children, adolescents and adults diagnosed with FM.
Data collection and analysis
The data of all included studies were extracted and the risks of bias of the studies were assessed independently by two review authors. Discrepancies were resolved by discussion.
Main results
Twenty‐three studies with 24 study arms with CBTs were included. A total of 2031 patients were included; 1073 patients in CBT groups and 958 patients in control groups. Only two studies were without any risk of bias. The GRADE quality of evidence of the studies was low. CBTs were superior to controls in reducing pain at end of treatment by 0.5 points on a scale of 0 to 10 (standardised mean difference (SMD) ‐ 0.29; 95% confidence interval (CI) ‐0.49 to ‐0.17) and by 0.6 points at long‐term follow‐up (median 6 months) (SMD ‐0.40; 95% CI ‐0.62 to ‐0.17); in reducing negative mood at end of treatment by 0.7 points on a scale of 0 to 10 (SMD ‐ 0.33; 95% CI ‐0.49 to ‐0.17) and by 1.3 points at long‐term follow‐up (median 6 months) (SMD ‐0.43; 95% CI ‐0.75 to ‐0.11); and in reducing disability at end of treatment by 0.7 points on a scale of 0 to 10 (SMD ‐ 0.30; 95% CI ‐0.51 to ‐0.08) and at long‐term follow‐up (median 6 months) by 1.2 points (SMD ‐0.52; 95% CI ‐0.86 to ‐0.18). There was no statistically significant difference in dropout rates for any reasons between CBTs and controls (risk ratio (RR) 0.94; 95% CI 0.65 to 1.35).
Authors' conclusions
CBTs provided a small incremental benefit over control interventions in reducing pain, negative mood and disability at the end of treatment and at long‐term follow‐up. The dropout rates due to any reason did not differ between CBTs and controls.
Plain language summary
Cognitive behavioural therapies for fibromyalgia syndrome
Researchers in The Cochrane Collaboration conducted a review of research about the effects of cognitive‐behavioural therapies (CBTs) on fibromyalgia (FM). After searching for all relevant studies, they found 23 studies with up to 2031 people. Their findings are summarised below.
After about 12 weeks, children, adolescents and adults with FMS, who used CBTs compared to controls, were likely to report that CBT
‐ may reduce slightly pain, negative mood and disability at the end of the treatment;
‐ may reduce slightly pain, negative mood and disability six months after the end of treatment.
There was no difference between CBTs and controls in the number of people who withdrew from treatment.
We do not have precise information about side effects and complications of CBTs. Rare complications may include worsening of co‐existing mental disorders.
What is fibromyalgia and what are cognitive behavioural therapies?
People with FM suffer from chronic widespread pain, sleep problems and fatigue. There is no cure for FM at present, so treatments aim to relieve symptoms and to improve daily functioning.
Cognitive behavioural therapies (CBTs) are widely used psychological treatments for a wide range of health problems, including chronic pain. CBTs are effective in enhancing patients’ beliefs in their own abilities and developing ways to deal with health problems. The primary goals of CBTs are to change negative thoughts and feelings that individuals may have of their physical and mental problems and to change their behaviour accordingly. Patients learn skills (for example, relaxation, activity pacing) to help them to manage their pain better or develop different attitudes towards pain (for example, more acceptance), or both.
Best estimates of what happens to people with FMS when they use CBTs
Pain (higher scores mean worse or more severe pain):
‐ People who used CBTs rated their pain to be 0.5 points lower at the end of treatment (6.3% absolute improvement) and to be 0.6 points lower six months after the end of treatment on a scale of 0 to 10 (4.2% absolute improvement).
‐ People who used CBTs rates their pain to be 6.9 points on a scale of 0‐10.
‐ People who used a control treatment rated their pain to be 7.4 points on a scale of 0 to 10.
Negative mood (higher scores mean worse or more severe negative mood):
‐ People who used CBTs rated their depressed mood to be 0.7 points lower at the end of treatment (10.2% absolute improvement) and to be 1.3 points lower six months after the end of treatment on a scale of 0 to 10 (2.7% absolute improvement).
‐ People who used CBTs rated their negative mood to be 6.1 points on a scale of 0 to 10.
‐ People who had a control treatment rated their negative mood to be 6.8 points on a scale of 0 to 10.
Disability (higher scores mean more disability):
‐ People who used CBTs rated their disability to be 0.7 points lower at the end of treatment (7.2% absolute improvement) and to be 1.2 points lower six months after the end of treatment on a scale of 0 to 10 (11.7% absolute improvement).
‐ Peope who used CBTs rated their disability to be 2.0 points on a scale of 0 to 10.
‐ People who used a control treatment rated their disability to be 2.8 points on a scale of 0 to 10.
Withdrawing from treatment:
‐ The number of people who withdrew from CBTs compared to control interventions due to any reason was equal.
‐ 15 people out of 100 who used CBTs withdrew from treatment due to any reason;
‐ 15 people out of 100 who used control interventions withdrew from treatment due to any reason.
Summary of findings
for the main comparison.
| Cognitive behavioural therapies compared to controls for fibromyalgia | ||||||
|
Patient or population: Patients with fibromyalgia Settings: In‐ and outpatient Intervention: Cognitive behavioural therapies Comparison: Controls (attention control, treatment as usual, waiting list, other active therapy) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| controls | Cognitive behavioural therapies | |||||
|
Pain end of treatment (0‐10 scale) Higher scores indicate higher pain levels |
Mean pain baseline 7.37 (SD 2.10) 3 |
The mean pain in the intervention groups was 0.29 standard deviations lower (0.49 to 0.11 lower) | 1382 (20) | ⊕⊕⊝⊝ low1,2 | SMD ‐0.29 (95% CI ‐0.47 to ‐0.11) 8.5% (95% CI 3.1% to 14.0%) relative improvement 6.3 % (95% 2.3% to 10.3%) CI) fewer points on the scale (absolute change) NNTB 7 (95% CI 5 to19) |
|
|
Pain
follow‐up median 6 months (0‐10 scale) Higher scores indicate higher pain levels |
Mean pain baseline 64.72 (SD 10.44) 4 |
The mean pain in the intervention groups was 0.40 standard deviations lower (0.64 to 0.16 lower) | 822 (14) | ⊕⊕⊝⊝ low1,2 | SMD ‐0.40 (95% CI ‐0.62 to ‐0.17) 6.4% (95% CI 2.7% to 9.9%) relative improvement 4.2% (95% CI 1.8% to 6.5%) fewer points on the scale (absolute change) NNTB 10 (95% CI 6 to 24) |
|
|
Negative mood end of treatment (0‐10 scale) Higher scores indicate higher negative mood levels |
Mean depression baseline 6.82 (SD 3.11) 5 |
The mean negative mood in the intervention groups was 0.33 standard deviations lower (0.49 to 0.17 lower) | 1578 (18) | ⊕⊕⊝⊝ low1,2 | SMD ‐0.33 (95% CI ‐0.49 to ‐0.17) 15.0% (95% CI 7.7% to 22.3%) relative improvement 10.2% (95% CI 5.2% to 15.2%) fewer points on the scale (absolute change) NNTB 6 (95% CI 4 to12) |
|
|
Negative mood
follow‐up median 6 months (0‐50 scale) Higher scores indicate higher negative mood levels |
Mean depression baseline 14.94 (SD 3.11) 6 |
The mean negative mood in the intervention groups was 0.43 standard deviations lower (0.75 to 0.11 lower) | 721 (11) | ⊕⊕⊝⊝ low1,2 | SMD ‐0.43 (95% CI ‐0.75 to ‐0.11) 8.9% (95% CI 2.3% to 15.8%) relative improvement 2.7% (95% CI 0.1% to 4.7%) fewer points on the scale (absolute change) NNTB 11 (95% CI 6 to 43) |
|
|
Disability end of treatment (0‐10 scale) Higher scores indicate disability levels |
Mean physical impairment baseline 2.80 (SD 2.40) 7 | The mean disability in the intervention groups was 0.30 standard deviations lower (0.51 to 0.08 lower) | 1163 (15) | ⊕⊕⊝⊝ low1,2 | SMD ‐0.30 (95% CI ‐0.51 to ‐0.08) 25.8 % (95% CI 6.9% to 43.7% relative improvement 7.2% (95% CI 1.9% to 12.2%) fewer points on the scale (absolute change) NNTB 7 (95% CI 4 to 26) |
|
|
Disability
follow‐up median 6 months (0‐10 scale) Higher scores indicate disability levels |
Mean physical impairment baseline 3.24 (SD 2.26) 8 | The mean disability in the intervention groups was 0.52 standard deviations lower (0.86 to 0.18 lower) | 664 (9) | ⊕⊕⊝⊝ low1,2 | SMD ‐0.52 (95% CI ‐0.86 to ‐0.18) 36.4% (95% CI 1.3% to 60.2%) relative improvement 11.7% (95% CI 4.1% to 19.4%) fewer points on the scale (absolute change) NNTB 4 (95% CI 3 to12) |
|
|
Acceptability end of treatment (dropouts from study due to any reasons) |
136 (94 to 195) per 1000 | 127 (88 to 182) | RR 0.94 (0.65 to 1.35) | 1914 (21) | ⊕⊕⊝⊝ low1 | Absolute risk difference
0% (95% CI ‐1 to 0) Relative per cent change 6% (95% CI 15% improvement to 35% worsening) Not statistically significant |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Some studies with lack of reported allocation concealment, without intention‐to‐treat analysis and with selective outcome reporting
2 High heterogeneity of treatment effect
3 Luciano 2011: N=216 patients; Pain VAS 0‐10 scale
4 Alda 2011: N=113 patients; Pain VAS 0‐100 scale
5 Luciano 2011: N=216 patients; Depression VAS 0‐10 scale
6 Alda 2011; N=113 patients; Hamilton Rating Scale for Depression (0‐50)
7 Luciano 2011: N=216 patients; Physical impairment VAS 0‐10 scale
8 Alda 2011; N=113 patients; Physical impairment VAS 0‐10 scale
Summary of findings 2. Cognitive behavioural therapies versus controls for fibromyalgia.
| Cognitive behavioural therapies versus controls for fibromyalgia | ||||||
|
Patient or population: Patients with fibromyalgia Settings: In‐ and outpatients Intervention: Cognitive behavioural therapies Comparison: Controls (attention control, treatment as usual, waiting list, other active therapy) | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Cognitive behavioural therapies versus controls final treatment | |||||
|
Fatigue end of treatment (0‐10 scale) Higher scores indicate higher fatigue levels |
Mean fatigue score 8.13 (SD 1.89) 3 | The mean fatigue in the intervention groups was 0.25 standard deviations lower (0.49 to 0.02 lower) | 910 (11 studies) | ⊕⊕⊝⊝ low1 | SMD ‐0.25 (95% CI ‐0.49 to ‐0.02) 5.8% (95% CI 0.05% to 11.3%) relative improvement 4.7% (95% CI 0.4% to 9.3%) fewer points on the scale (absolute change) NNTB 9 (95% CI 5 to109) |
|
|
Sleep problems end of treatment (0‐50 scale) Higher scores indicate more sleep problems |
Mean sleep problems score 27.9 (SD 8.8) 4 | The mean sleep problems in the intervention groups was 0.4 standard deviations lower (0.85 lower to 0.05 higher) | 422 (8 studies) | ⊕⊕⊝⊝ low2 | SMD ‐0.40 (95% CI ‐0.85 to 0.05) 0.3% (95% CI ‐0.03% to 1.7%) relative improvement 7.0% (95% CI ‐0.90% to 15.0%) fewer points on the scale (absolute change) NNTB 5 (95% CI ‐45 to 3) |
|
|
Health‐related quality of life end of treatment (0‐80 scale) Higher scores indicate lower health‐related quality of life |
Mean health‐related quality of life score 55.97 (SD 15.95) 5 | The mean health‐related quality of life in the intervention groups was 0.23 standard deviations lower (0.38 to 0.08 lower) | 1238 (13 studies) | ⊕⊕⊝⊝ low1,2 | SMD ‐0.23 (95% CI ‐0.38 to ‐0.08) 0.08% (95% CI 0.03% to 0.13%) relative improvement 4.6% (95% CI 1.6% to 7.6%) fewer points on the scale (absolute change) NNTB 9 (95% CI 6 to 27) |
|
|
Fatigue Follow‐up median 6 months (0‐10 scale) Higher scores indicate higher fatigue levels |
Mean fatigue score Mean 8.32 (SD 2.17)6 |
The mean fatigue in the intervention groups was 0.46 standard deviations lower (0.77 to 0.15 lower) | 429 (6 studies) | ⊕⊕⊝⊝ low1,2 | SMD ‐0.46 (95% CI ‐0.77 to ‐0.15) 1.2% (95% CI 0.4% to 2.0%) relative improvement 10.0% (95% CI 3.2% to 16.7%) fewer points on the scale (absolute change) NNTB 5 (95% CI 3 to 14) |
|
|
Sleep problems
Follow‐up median 6 months (0‐50 scale) Higher scores indicate more sleep problems |
Mean sleep problems score 27.9 (SD 8.8) 4 | The mean sleep problems in the intervention groups was 0.64 standard deviations lower (1.31 lower to 0.03 higher) | 378 (7 studies) | ⊕⊕⊝⊝ low1,2 | SMD ‐0.64 (95% CI ‐1.31 to 0.03) 0.4% (95% CI ‐0.02% to 0.8%) relative improvement 11.2% (95% CI ‐0.53% to 23.1%) fewer points on the scale (absolute change) NNTB 4 (95% CI ‐74 to 2) |
|
|
Health‐related quality of life Follow‐up median 6 months (0‐80 scale) Higher scores indicate lower health‐related quality of life |
Mean health‐related quality if life score 64.48 (SD 10.50) 7 |
The mean health‐related quality of life in the intervention groups was 0.19 standard deviations lower (0.58 lower to 0.21 higher) | 425 (6 studies) | ⊕⊕⊝⊝ low1 | SMD ‐0.19 (95% CI ‐0.58 to 0.21) 0.03% (95% CI ‐0.03% to 0.15%) relative improvement 2.0% (95% CI ‐2.2% to 6.1%) fewer points on the scale (absolute change) NNTB 12 (95% CI ‐17 to 6) |
|
| Acceptability Follow‐up: median 6 months | See comment | See comment | Not estimable | ‐ | See comment | Not assessed |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Some studies with lack of reported allocation concealment, without intention‐to‐treat analysis and with selective reporting 2 High heterogeneity of treatment effect
3 Luciano 2011: N=216 patients; VAS 0‐10 scale
4 Castel 2012; N=60 patients; NRS 0‐50 scale
5 Luciano 2011: N=216 patients; VAS 0‐80 scale
6 Alda 2010; N= 113; VAS 0‐10 scale
7 Alda 2010; N= 113; VAS 0‐100 scale
Background
Description of the condition
The key symptoms of fibromyalgia (FM) are chronic widespread pain associated with cognitive dysfunction, physical fatigue and sleep disturbances (Häuser 2008; Wolfe 2010). Patients often report high disability levels and poor quality of life along with extensive use of medical care (Winkelmann 2011; Wolfe 1997). Lacking a specific laboratory test, diagnosis is established by a history of the key symptoms and the exclusion of somatic diseases sufficiently explaining the key symptoms (Häuser 2008; Wolfe 2010). For a clinical diagnosis the 1990 and 2010 American College of Rheumatology (ACR) criteria (Wolfe 1990; Wolfe 2010) and the Association of the Medical Scientific Societies in Germany (AWMF) diagnostic criteria (Häuser 2010) can be used. In the past other standardised criteria have been used to diagnose FM (Smythe 1981; Yunus 1981).
FM is estimated to affect 1% to 2% of people in the United States (Lawrence 2008) and 2.1% to 2.9% in Europe (Branco 2010; Wolfe 2013).
The definite aetiology (causes) of this syndrome remains unknown. A model of interacting biological and psychosocial variables in the predisposition, triggering and development of the chronicity of FM has been suggested (Sommer 2012a). Depression (Forseth 1999), genes (e.g. 5‐hydroxytryptamine 2A receptor 102T/C polymorphism) (Lee 2012), obesity combined with physical inactivity (Mork 2010), physical and sexual abuse in childhood (Häuser 2011), sleep problems (Mork 2012) and smoking (Choi 2010) predict future development of FM. Depression and post‐traumatic stress disorder worsen FM symptoms (Dell' Osso 2012; Lange 2010).
Several factors are associated with the pathophysiology (functional changes associated with or resulting from disease) of FM, but the relationship is unclear. The functional changes include alteration of pain processing in the brain, reduced reactivity of the hypothalamus‐pituitary‐adrenal axis to stress, increased pro‐inflammatory and reduced anti‐inflammatory cytokine profiles (produced by cells involved in inflammation), and disturbances in neurotransmitters such as dopamine and serotonin (Sommer 2012a). Prolonged exposure to stress, as outlined above, may contribute to these functional changes (Bradley 2009).
Current treatments for FM are not curative. Drugs (Häuser 2013; Moore 2011; Tort 2012) and exercise therapies (Busch 2007) aim to relieve symptoms and improve quality of life and functional abilities.
Description of the intervention
Behavioural and cognitive behavioural psychological therapies are the dominant contemporary psychological treatments for a wide range of health problems, including chronic pain (Morley 2011). Behavioural and cognitive behavioural psychological therapies are used to manage chronic pain by attempting to change negative thoughts about pain, and introduce behaviour modification, including self‐management techniques, to improve function and cope with pain. However, there is no universally accepted definition of which techniques constitute behavioural and cognitive behavioural psychological therapies (Morley 2011). Due to the broad variety of behavioural and cognitive behavioural psychological therapy techniques, we use in the following context the term 'cognitive behavioural therapies' (CBTs). For the purposes of this review we will consider the following techniques (Jensen 2011).
Operant therapy, which requires techniques to increase activity, the inclusion of significant others to reduce reinforcement of pain behaviours, and the reduction of pain‐contingent medication (Fordyce 1976; Thieme 2003).
Traditional cognitive behavioural therapy (CBT), which requires monitoring of one's own thoughts, feelings and behaviours with respect to the target symptom (e.g. by a symptom diary) and the promotion of alternative ways of coping with the target symptom (also labelled as problem‐solving techniques, self management, coping skills), through methods such as activity participation and skill‐building or practice opportunities (Bennett 2006).
Self management education programmes, which require information on the clinical picture of FMS, cognitive and behavioural skills mastery to manage pain and limitations of daily activities, and modelling as supplied by the facilitators to target cognitive, behavioral and emotional change (Burckhardt 2005b; Warsi 2003).
Acceptance‐based CBTs, which include acceptance and commitment therapy, or contextual CBT or mindfulness‐based cognitive therapy. All these therapies use acceptance techniques (e.g. mindfulness meditation training) to facilitate a separation between 'self' and one’s thoughts, feelings and pain experience, and encourage patients to base their actions on their most important values as opposed to their immediate feelings, thoughts and pain (Veehof 2011).
How the intervention might work
CBTs include interventions that are based on the premise that chronic pain and other symptoms of FM are maintained and influenced by emotional and cognitive (conscious intellectual activities such as thinking, reasoning or remembering) as well as behavioural factors. A typical treatment protocol for traditional CBT will involve methods aimed directly at assessing the thoughts associated with pain, the extent of avoidance of unpleasant thoughts and of painful experiences, and the consequences of these. A common focus is on strongly held beliefs about pain and their relationship with behaviour, which typically worsens the situation in the shorter or longer term. Behavioural methods focus on the identification of behaviour that is contingent on pain, or upon events which provide pain relief or comfort, and the development of behaviour that is contingent instead on goal achievement related to the values of the individual with pain (Bennett 2006; Williams 2012). Most CBTs include education (information on the etiology of the disease including importance of psychological factors; treatment options; working mechanisms of psychological and drug therapies).
Why it is important to do this review
The significance of CBTs in the management of FM still needs to be determined. Systematic narrative and quantitative reviews on CBTs in FM have had divergent results. Koulil (Koulil 2007) concluded from six randomized controlled trials (RCTs) that the effects on pain, disability and mood were limited, and that it was mostly CBTs within a multi‐component approach that yielded improvements. Bennett concluded from six RCTs that CBT as a single treatment modality did not offer any distinct advantage over well‐planned group programmes of education or exercise, or both (Bennett 2006). Thieme and coworkers concluded from 14 studies that CBTs were superior to controls in most key domains of FMS post‐treatment and at follow‐up (Thieme 2009). A recent Cochrane review on the efficacy of psychological therapies in chronic pain syndromes included only six studies with FM patients and did not present a subgroup analysis of FM patients (Williams 2012). Another recent review on psychological therapies in FM concluded that CBTs were effective in relieving FM symptoms and superior to other psychological therapies. However, this review included a combination of CBTs with aerobic exercise (multi‐component therapies) and did not compare the results of CBTs with control groups (Glombiewski 2010). A meta‐analysis on CBTs in FM found that CBTs were superior to controls in reducing depressed mood post‐treatment but not superior in reducing pain, fatigue, sleep and limitations in quality of life post‐treatment and at follow‐up. This systematic review included trials with mindfulness‐based stress reduction (MBSR) and excluded trials with self management approaches (Bernardy 2010).
Evidence‐based guidelines on the management of FM have given different grades of recommendation for CBTs. The American Pain Society (Burckhardt 2005a) gave the highest grade of recommendation to CBTs based on a qualitative systematic review. The European League Against Rheumatism only gave a weak (expert opinion) recommendation for CBTs based on a quantitative systematic review (Carville 2008). The Association of the Scientific Medical Societies in Germany gave an open recommendation based on a quantitative systematic review (Köllner 2012). The Canadian Pain Society gave a strong recommendation of CBTs based on a quantitative systematic review (Fitzcharles 2012).
Objectives
To assess the short and long‐term benefits and harms of CBTs compared to control groups in the treatment of FM patients of any age.
Methods
Criteria for considering studies for this review
Types of studies
We selected randomized controlled trials (RCTs) of CBTs of any duration of treatment in FM. According to the eligibility criteria of The Cochrane Collaboration (Higgins 2011), a trial was eligible if, on the basis of the best available information, it was judged that the individuals were definitely or possibly assigned prospectively to one of two (or more) alternative forms of health care using random allocation or some quasi‐random method of allocation (such as alternation, date of birth, or case record number). We also considered cluster‐randomized trials to be eligible. We accepted an attention control, waiting list control, treatment as usual, no therapy and any other active therapy as controls. We included RCTs if they:
were available as a full publication or a report of the RCT in a peer‐reviewed journal or in a database (detailed below);
had a design that placed a CBT as an active treatment of primary interest;
had a credible CBT content (see Types of interventions);
had 10 or more participants in each treatment arm at the end of the assessment (Eccleston 2009).
Types of participants
We included patients of any age with a clinical diagnosis of FM by any published, recognised and standardised criteria (Häuser 2010; Smythe 1981; Wolfe 1990; Wolfe 2010; Yunus 1981). We included studies in which FM patients were mixed with patients having other chronic pain syndromes if the outcomes of FM patients were reported separately or could be provided on request.
Types of interventions
We included RCTs comparing a credible CBT treatment with controls. We judged a psychological treatment to be credible if it was based on an extant CBT model or framework (see Background) and its delivery was from, or supervised by, a healthcare professional trained in an extant CBT model or framework. In addition, the delivery by a lay leader was accepted in the case of self management education programs. The continuation of previous therapies as usual care was allowed.
We included trials comparing face‐to‐face, telephone‐based or internet‐based CBTs as an active treatment of primary interest with controls.
We excluded the following types of psychological therapies from this review.
Biofeedback, hypnosis, mindfulness‐based stress reduction and relaxation training as single therapies. These psychological therapies are also attributed to complementary and alternative medicine (CAM) (National Institutes of Health 2011) and are included in another Cochrane review in preparation on mind‐body therapies in FMS (Theadom 2009). Furthermore we excluded studies that included hypnosis and mindfulness‐based stress reduction as part of a complex CBTs intervention because it would not be possible to separate the effects of CBTs from these therapies.
Studies with education only: any combination of information on the symptoms and management of FM, discussion or emotional support without skills mastery and modelling as supplied by the facilitators.
Studies in which CBTs were combined with any other defined active therapy (physical exercise, physical therapy or drug therapy with defined extent and intensity (so‐called multicomponent therapy), because it is not possible to separate the effects of CBTs from these other active therapies.
Types of outcome measures
We based the selection of outcome measures on the key domains of FM developed through consensus among experts and FM patients (Mease 2009), the goals of CBTs (Bennett 2006; Eccleston 2009), the suggestions of the Initiative of Methods, Measurement and Pain Assessment in Clinical trials (IMMPACT) (Dworkin 2008; Dworkin 2009) and best practice in the reporting of systematic reviews in chronic pain (Moore 2010a). We selected outcome measures for short‐term (at final treatment) and long‐term (follow‐up of at least six months) efficacy.
The primary data type was measurement using continuous scales (Bernardy 2010; Eccleston 2009). We did not meta‐analyse dichotomous outcome data based on clinical improvement (responder analysis). These data have been rarely reported in psychological trials of FMS (Bernardy 2010; Eccleston 2009). We present in Characteristics of included studies which studies reported responder analysis for pain and disability and, if reported, reasons for dropping out. Although standard trial reporting guidance promotes the definition of major outcomes (Dworkin 2008) most psychological trials in chronic pain do not define an a priori major outcome. From each trial we selected the measure considered most appropriate for each of the outcomes. When there was more than one measure for an outcome we gave preference to the measure that was most frequently used (Eccleston 2009). Also, when there was a choice between single‐item and multi‐item self report tools, we chose multi‐item tools on the basis of inferred increased reliability (Eccleston 2009).
We analysed outcome measures at final treatment (end of therapy) and at long‐term (at least six months) follow‐up. Follow‐ups < six months were not considered for the analysis of long‐term follow‐up. If more than one follow‐up after six months had been conducted, the results of the final follow‐up visit were extracted for analysis.
Major outcomes
Self reported pain at end of treatment and at long‐term (at least six months) follow‐up
Self reported negative mood at end of treatment and at long‐term (at least six months) follow‐up
Self reported disability at end of treatment and at long‐term (at least six months) follow‐up
Acceptability: total dropout rate (patients who terminated the trial early for any reason during the treatment period (Cipriani 2009)). We analysed reasons for dropout if reported.
Minor outcomes
Self reported pain self efficacy (beliefs in one's capabilities to manage one's own pain) at end of treatment and at long‐term (at least six months) follow‐up
Self reported fatigue at end of treatment and at long‐term (at least six months) follow‐up
Self reported sleep problems at end of treatment and at long‐term (at least six months) follow‐up
Self reported disease‐specific health‐related quality of life (HRQOL) measured by the Fibromyalgia Impact Questionnaire (FIQ) at end of treatment and at long‐term (at least six months) follow‐up
Search methods for identification of studies
Electronic searches
We ran an electronic search in the Cochrane Central Register of Controlled Trials (CENTRAL (The Cochrane Library 2013, Issue 1), MEDLINE accessed through PubMed (1966 to 15 February 2013), PsycINFO (1966 to 15 February 2013) and SCOPUS (1980 to 15 February 2013). We searched http://www.clinicaltrials.gov (website of the US National Institutes of Health) and the World Health Organization Clinical Trials Registry Platform (ICTRP) (http://www.who.int/ictrp/en/) for ongoing trials.
We used the search terms fibromyalgia, CBTs and their variations (see Appendix 1).
Searching other resources
We searched bibliographies from retrieved relevant articles. We contacted content experts for further possible studies. Our search included all languages.
Data collection and analysis
Selection of studies
Two review authors (KB, PK) independently scrutinised all the titles and abstracts and selected studies based on inclusion and exclusion criteria. A third review author verified the result (WH).
Data extraction and management
Two authors extracted data on the studies (including the methods, participants, interventions, outcomes, and results) independently using a specially designed data extraction form (KB, WH). The types of treatment and reported treatment quality were rated independently by two authors (KB, WH). We resolved disagreements by discussion, if necessary a third review author (AB) was consulted. One author (WH) entered data into Review Manager (RevMan) 5 (RevMan 2011) and a second author (KB) validated the entries.
Assessment of risk of bias in included studies
Two review authors (KB, WH) independently assessed the risk of bias of each included trial. Disagreements were resolved by discussion and consensus, otherwise a third review author (AB) acted as arbiter.
For each included study, we assessed risk of bias against key criteria: random sequence generation; allocation concealment; blinding of outcome assessment; incomplete outcome data; and selective outcome reporting, in accordance with methods recommended by The Cochrane Collaboration (Higgins 2011). We excluded the option of 'blinding participants and personnel' because neither therapists nor patients can be blinded to whether they deliver or receive treatment (Williams 2012).
We explicitly judged each of these criteria as: low risk of bias, high risk of bias, or unclear (either a lack of information or uncertainty over the potential for bias) risk of bias. We present the 'Risk of bias' assessment results in the 'Risk of bias' graph and 'risk of bias' summary figures.
Assessment of quality of the treatment
We assessed the quality of the treatment using five criteria (treatment content and setting, treatment duration, manualisation of the treatment, adherence of the therapist to the manual, therapist training and client engagement) on a quality rating scale designed specifically for application to psychological treatment studies in pain. The total score ranges from 0 to 9 (Yates 2005). We considered scores 0 to 2 to indicate poor quality, scores 3 to 5 average, and scores 6 to 9 excellent treatment quality (Bernardy 2010).
Assessment of study samples
We extracted demographic data (percentage of women, mean age) and history of disease (mean durations of chronic widespread pain or FMS symptoms) from study samples. We evaluated external validity of the study samples by checking if patients with depressive or anxiety disorders were included to assess the representativeness of study samples for FMS patients in clinical practice (Bernardy 2010). We explicitly judged this criterion using the terms high risk of limited external validity (patients with depressive or anxiety disorders were excluded), no risk of limited external validity (patients with depressive or anxiety disorders were included) and unclear risk of limited external validity (insufficient details were given).
Measures of treatment effect
The effect measures of choice were standardised mean difference (SMD) (when different scales are used to measure outcomes) for continuous data and risk ratio (RR) for dichotomous data of CBTs groups and control groups at end of treatment and at final follow‐up. We used a random‐effects model. We expressed precision with 95% confidence intervals (CIs). We used Cohen’s categories to evaluate the magnitude of the effect size, calculated by SMD, with Hedges' g > 0.2 to 0.5 = small effect size, g > 0.5 to 0.8 = medium effect size, and g > 0.8 = large effect size (Cohen 1988). We labelled g < 0.2 to be a 'not substantial' effect size. We converted SMD to relative and absolute change by multiplying by the baseline standard deviation from the control group of a 'representative trial, and relative per cent change by dividing the absolute change by the baseline mean of the control group from the same representative trial for some results in the summary of findings table (Bliddal 2009).
Unit of analysis issues
In the case of unit of analysis issues we followed the suggestions in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). In the case of cross‐over design we used the methods of analysis for cross‐over trials: we analysed paired data if available or provided by request. If no paired data were available we used first‐period data. In the case of repeated observations on participants we selected the longest follow‐up from each study. In the case of different types of CBTs we analysed the different types of CBTs separately. If different types of CBTs were compared with only one control group we adjusted the number of patients in the control group according to the number of patients in the CBT arms. In the case of different types of control groups we used the following preference for comparison with CBTs: attention control, waiting list control, treatment as usual, no therapy, and any other active therapy. We did not combine different types of control groups.
Dealing with missing data
Where means or standard deviations (SDs) were missing, we attempted to obtain these data through contacting the trial authors. Where SDs were not available from trial authors, we calculated them from t values, confidence intervals or standard errors, where reported in articles (Higgins 2011). If these data were not available, we substituted the missing SD by a validated imputation method (Furukawa 2006).
Assessment of heterogeneity
We extracted demographic (average age, percentages of women) and clinical characteristics of the patients (duration of FMS symptoms) as well as study characteristics (country and setting of study, type and duration of CBTs) as potential sources of clinical heterogeneity. We used the I2 statistic to describe the percentage variability of effect estimates that is due to heterogeneity. We combined results in a meta‐analysis using a random‐effects model. I2 values above 50% indicate high heterogeneity, between 25% and 50% moderate heterogeneity, and below 25% low heterogeneity.
Assessment of reporting biases
We avoided language publication bias by including studies irrespective of the language of publication.
We addressed publication bias by visual inspection of funnel plots and tests for funnel plot asymmetry (Begg 1994; Egger 1997) when there were at least 10 studies included in the meta‐analysis (Higgins 2011).
We addressed outcome reporting bias by checking if the means and SDs of all primary and secondary outcomes, as outlined in the methods section of the published studies, had been reported or had been provided on request.
Data synthesis
We examined the combined results using a random‐effects model (inverse variance method) because this model is more conservative than the fixed‐effect model and incorporates both within‐study and between‐study variance and because we expected clinical and statistical heterogeneity. We used the GRADE approach to grade the quality of evidence (Brozek 2009; Higgins 2011). We used the software GradePro (Guyatt 2006). We presented a 'Summary of findings' table with the major outcomes (pain, disability, mood, and acceptability).
The numbers needed to treat for an additional outcome of benefit (NNTB) for continuous variables (pain, fatigue, sleep problems, negative mood, disability, disease‐specific quality of life, self reported pain self efficacy) were calculated using the Wells calculator software available at the Cochrane Musculoskeletal Group editorial office, which estimates the proportion of patients who will benefit from treatment from SMDs. The estimation of responders is nearly independent from the minimally important difference (MID) (Norman 2001). We used a minimal clinically important difference of 15% for the calculation of NNTB from SMDs for all continuous outcomes.
Subgroup analysis and investigation of heterogeneity
We analysed the effects of all types of CBTs pooled together compared to all types of control groups pooled together for major and minor outcomes. We performed subgroup analyses of the efficacy of the different types of CBTs (operant (behavioural) therapies, traditional cognitive behavioural therapies, self management approaches, acceptance‐based cognitive behaviour therapies); face‐to‐face versus other (telephone, internet‐based) CBTs; CBTs in adults (persons ≥18 years) versus children and adolescents (persons < 18 years); and different types of control groups (attention controls, active controls and other types of controls [treatment as usual, waiting list control]) compared to all types of CBTs pooled together. At least two studies should be available for subgroup analysis. We tested for subgroup differences using the test of interaction (Altman 2003).
We performed a subgroup analysis of ultra‐short (< 5 sessions), short‐term (5 to 25 sessions) and long‐term CBTs studies (> 25 sessions) with the primary outcome measures of pain, negative mood and disability to test for dosage effects. We performed a subgroup analysis of studies with low, moderate and high reported treatment quality to test for effects of treatment quality on outcomes. We expected better treatment outcomes in studies with high treatment quality compared to studies with low treatment quality.
Sensitivity analysis
We performed sensitivity analysis of all types of CBTs pooled together compared to all types of control groups pooled together for the three primary outcomes of pain, mood and disability: a) based on the need to substitute means, SDs, or both, by excluding studies with substituted values or values visually extracted from figures; b) based on the risk of bias by excluding studies with high and unclear selection bias, studies with high and unclear attrition bias, studies with high and unclear reporting bias, and studies with high and unclear risk of limited external validity; c) (post hoc decision) excluding studies with < 20 participants per treatment arm in accordance with the Cochrane reviews on psychological therapies for the management of chronic pain in children and adolescents (Eccleston 2012) and in adults (Williams 2012).
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of studies awaiting classification
Results of the search
We identified 1519 studies. We excluded 1477 references as they did not fulfil inclusion criteria related to the interventions evaluated in this review. We identified 42 studies potentially related to CBTs, and the full text was obtained for each of them. Of these 42 studies, 15 did not meet the inclusion criteria and were excluded. Four studies which were identified by a second and third search were included in Characteristics of studies awaiting classification for a total of 23 studies with 24 pairs of study arms to be included in the analysis (Figure 1).
1.
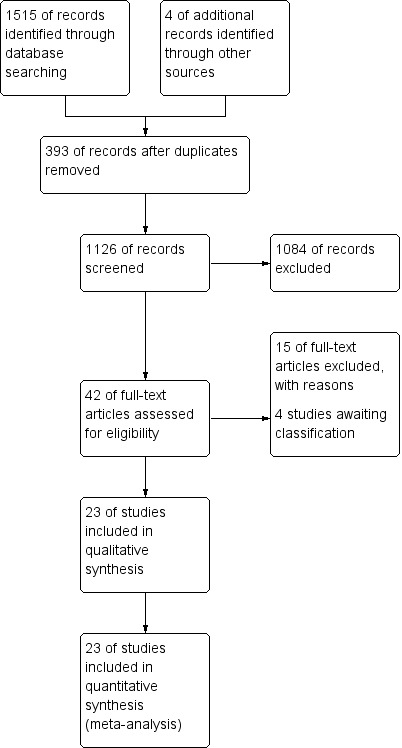
Study flow diagram.
The following studies are awaiting classification and will be included in the update of this review: Three studies with cognitive behavioural therapy (Jensen 2012; Sanchez 2012; Martinez 2013) and one study with acceptance and commitment therapy (Wicksell 2012) which were found in the second search of 28 February 2012 and in the third search of 28 August 2013.
Included studies
The main characteristics of the studies are summarized in Table 3.
1. Main characteristics of included studies.
| Author | Country | Type of CBT | Type of control group | Duration CBT (weeks) | Number of CBT sessions Total treatment time CBT (hours) |
Number of patients in CBT group % women |
Number of patients in control group % women |
| Alda 2011 * | Spain | CBT | TAU | 12 | 6 15 |
57 95 |
56 96 |
| Ang 2010 * | USA | CBT | TAU | 12 | 6 3 |
17 100 |
15 100 |
| Burckhardt 1994 * | Sweden | CBT | Delayed treatment | 6 | 6 9 |
28 100 |
30 100 |
| Castel 2009 * | Spain | CBT | TAU | 11 | 12 18 |
18 94 |
12 86 |
| Castel 2012 * | Spain | CBT | TAU | 14 | 14 28 |
34 94 |
30 100 |
| Edinger 2005 * | USA | CBT | TAU | 6 | 6 6 |
16 94 |
12 100 |
| Falcao 2008 * | Brazil | CBT | TAU | 10 | 20 30 |
30 100 |
30 100 |
| Kashikar‐Zuck 2005 ** | USA | CBT | Active control | 8 | 8 12 |
14 100 |
14 100 |
| Kashikar‐Zuck 2012 ** | USA | CBT | Active control | 8 | 8 6 |
57 95 |
57 90 |
| King 2002 * | USA | CBT | Delayed treatment | 12 | 12 18 |
48 100 |
39 100 |
| Luciano 2011 * | Spain | CBT | TAU | 8 | 8 16 |
108 95 |
108 98 |
| Miro 2011 * | Spain | CBT | Active control | 6 | 6 9 |
20 100 |
20 100 |
| Nicassio 1997 * | USA | CBT | Active control | 10 | 10 15 |
36 89 |
35 89 |
| Oliver 2002 * | USA | Self‐management | TAU | 52 | 10 20 |
207 96 |
193 94 |
| Redondo 2004 * | Spain | CBT | Active control | 8 | 8 20 |
21 100 |
19 100 |
| Rooks 2007 * | USA | Self‐management | Active control | 16 | 8 16 |
51 100 |
50 100 |
| Soares 2002 * | Sweden | CBT | Attention control | 10 | 10 120 |
18 100 |
18 100 |
| Thieme 2003 * | Germany | Operant therapy | Active control | 5 | 25 75 |
42 100 |
21 100 |
| Thieme 2006a * | Germany | Operant therapy | Attention control | 15 | 15 30 |
42 100 |
20 100 |
| Thieme 2006b * | Germany | CBT | Attention control | 15 | 15 30 |
43 100 |
20 100 |
| Vlayen 1996 * | Netherlands | CBT | Active control | 6 | 12 18 |
49 93 |
43 82 |
| Wigers 1996 * | Norway | CBT | TAU | 14 | 15 30 |
20 90 |
20 95 |
| Williams 2010 * | USA | Self‐management | TAU | 26 | NR | 59 95 |
59 95 |
| Woolfolk 2012 * | USA | CBT | TAU | NR | NR | 38 89 |
38 87 |
* Studies included only adults
** Studies included only children and adolescents
NR = Not reported and not provided on request
TAU = Treatment as usual
Settings
Twenty‐three studies with 24 pairs of study arms were analysed. Twelve studies were conducted in Europe (Alda 2011; Burckhardt 1994; Castel 2009; Castel 2012; Luciano 2011; Miro 2011; Redondo 2004; Soares 2002; Thieme 2003; Thieme 2006; Vlayen 1996; Wigers 1996), 10 in North America (Ang 2010; Edinger 2005; Kashikar‐Zuck 2005; Kashikar‐Zuck 2012; King 2002; Nicassio 1997; Oliver 2001; Rooks 2007; Williams 2010; Woolfolk 2012) and one in South America (Falcao 2008). Four studies had been conducted before 2000 (Burckhardt 1994; Nicassio 1997; Vlayen 1996; Wigers 1996), the remaining studies were published after 2000. All studies but one (Thieme 2003) were outpatient‐based. Three studies were conducted in primary care (Alda 2011; Luciano 2011; Rooks 2007), two studies in secondary care (Kashikar‐Zuck 2005; Thieme 2003) and the remaining studies in tertiary care (university centres). All studies except five (Alda 2011; Kashikar‐Zuck 2012; Luciano 2011; Oliver 2001; Rooks 2007) were single‐centre studies.
Types of therapies
Nineteen studies provided traditional CBT (Alda 2011; Ang 2010; Burckhardt 1994; Castel 2009; Castel 2012; Edinger 2005; Falcao 2008; Kashikar‐Zuck 2005; Kashikar‐Zuck 2012; King 2002; Luciano 2011; Miro 2011; Nicassio 1997; Redondo 2004; Soares 2002; Thieme 2006; Vlayen 1996; Wigers 1996; Woolfolk 2012), three studies provided self management education (Oliver 2001; Rooks 2007; Williams 2010) and two studies provided operant therapy (Thieme 2003; Thieme 2006). All studies were conducted by live face‐to‐face contact except one study which was provided by the internet (Williams 2010) and one which was delivered by telephone (Ang 2010). The median duration of all CBTs was 10 (5 to 54) weeks. The median number of sessions was 10 (6 to 60) and the median total hours was 18 (3 to 102) hours.The median of follow‐ups which were performed by 17 of 23 studies was 6 (3 to 48) months. Fourteen studies performed follow‐ups at equal to or greater than six months (Alda 2011; Burckhardt 1994; Castel 2012; Edinger 2005; Kashikar‐Zuck 2012; Nicassio 1997; Redondo 2004; Rooks 2007; Soares 2002; Thieme 2003; Thieme 2006; Vlayen 1996; Wigers 1996; Woolfolk 2012).
Controls
Two studies used waiting list controls (Burckhardt 1994; King 2002), two studies used attention controls (Soares 2002; Thieme 2006), eight studies used active controls (Kashikar‐Zuck 2005; Kashikar‐Zuck 2012; Miro 2011; Nicassio 1997; Redondo 2004; Rooks 2007; Thieme 2003; Vlayen 1996), the remaining studies compared CBTs to treatment as usual.
Patients
A total of 1073 patients in treatment groups and 958 patients in control groups were included in the analysis. The median number of patients in CBTs groups was 36 (14 to 207), in controls 30 (11 to 193). Participants were referred and recruited from a wide range of healthcare settings and other sources (self‐help groups, media advertisements). The median percentage of women in CBTs groups was 96% (89% to 100%). Ten studies included only women (Ang 2010; Burckhardt 1994; Falcao 2008; Kashikar‐Zuck 2005; King 2002; Miro 2011; Redondo 2004; Soares 2002; Thieme 2003; Thieme 2006). The median of the mean age was 47.5 (15.2 to 55.4) years. Two studies included only children and adolescents (Kashikar‐Zuck 2005; Kashikar‐Zuck 2012); the other studies included only adults. The median percentage of Caucasians was 93% (79% to 100%). The percentage of Caucasians in the whole sample was probably high because most European studies did not report ethnicity of the patients included. The studies used different criteria of disease duration. Therefore, we did not calculate median values. Overall, the studies included patients with a long disease duration (more than five years) except in three studies (Falcao 2008; Miro 2011; Soares 2002) which reported a disease duration of less than five years. Disease duration in children and adolescents was reported to be three years in one study (Kashikar‐Zuck 2012).
Diagnosis of FM
FM was diagnosed in the two studies with children and adolescents (Kashikar‐Zuck 2005; Kashikar‐Zuck 2012) according to the Yunus criteria (Yunus 1981). Of the studies with adults, one study (Wigers 1996) used the Smythe criteria (Smythe 1981). The remaining studies used the American College of Rheumatology (ACR) 1990 classification criteria (Wolfe 1990) for diagnosis.
Exclusion of anxiety or depressive disorder
Twelve studies included patients with depressive or anxiety disorders, or both (Alda 2011; Ang 2010; Burckhardt 1994; Castel 2009; Castel 2012; King 2002; Nicassio 1997; Luciano 2011; Thieme 2006; Wigers 1996).
Reported treatment quality
Three studies had a low (Castel 2009; Nicassio 1997; Vlayen 1996), 12 studies had a moderate (Burckhardt 1994; Edinger 2005; Falcao 2008; King 2002; Luciano 2011; Oliver 2001; Redondo 2004; Rooks 2007; Soares 2002; Thieme 2003; Wigers 1996; Woolfolk 2012) and nine studies had a high treatment quality (Alda 2011; Ang 2010; Castel 2012; Kashikar‐Zuck 2005; Kashikar‐Zuck 2012; Miro 2011; Thieme 2006; Williams 2010) (see Table 4).
2. Reported treatment quality.
| Treatment content and setting | Treatment duration | Manualisation | Adherence to manual | Therapist training | Client engagement | Sum | |
| Alda 2011 | 2 | 1 | 2 | 1 | 1 | 0 | 7 |
| Ang 2010 | 2 | 1 | 2 | 1 | 2 | 0 | 8 |
| Burckhardt 1994 | 1 | 1 | 0 | 0 | 1 | 0 | 3 |
| Castel 2009 | 1 | 1 | 0 | 0 | 0 | 0 | 2 |
| Castel 2012 | 1 | 1 | 2 | 1 | 0 | 1 | 6 |
| Edinger 2005 | 1 | 1 | 2 | 0 | 1 | 0 | 5 |
| Falcao 2008 | 1 | 1 | 0 | 0 | 1 |
1 | 4 |
| Kashikar‐Zuck 2005 | 2 | 1 | 2 | 1 | 2 | 1 | 9 |
| Kashikar‐Zuck 2012 | 2 | 1 | 2 | 1 | 2 | 1 | 9 |
| King 2002 | 1 | 1 | 0 | 0 | 0 | 1 | 3 |
| Luciano 2011 | 2 | 1 | 1 | 0 | 1 | 0 | 5 |
| Miro 2011 | 2 | 1 | 2 | 0 | 1 | 0 | 6 |
| Nicassio 1997 | 1 | 1 | 0 | 0 | 0 | 0 | 2 |
| Oliver 2002 | 2 | 1 | 0 | 0 | 0 | 0 | 3 |
| Redondo 2004 | 2 | 1 | 0 | 0 | 0 | 0 | 3 |
| Rooks 2007 | 2 | 1 | 0 | 0 | 0 | 0 | 3 |
| Soares 2002 | 1 | 1 | 0 | 0 | 1 | 0 | 3 |
| Thieme 2003 | 2 | 1 | 2 | 0 | 0 | 0 | 5 |
| Thieme 2006 | 2 | 1 | 1 | 0 | 2 | 0 | 6 |
| Vlayen 1996 | 1 | 1 | 0 | 0 | 0 | 0 | 2 |
| Wigers 1996 | 1 | 1 | 0 | 0 | 1 | 1 | 4 |
| Williams 2010 | 2 | 1 | 1 | 0 | 1 | 1 | 6 |
| Woolfolk 2012 | 1 | 1 | 2 | 0 | 0 | 0 | 4 |
Items and scores of treatment quality scale (Yates 2005)
1. Treatment content and setting: 2 ‐ Adequate: a clear rationale for the treatment has been reported along with an adequate description of its content; 1 ‐ Partial: either a clear rationale or a description of the content of the treatment is reported; 0 ‐ Inadequate:neither the rationale for treatment or the treatment content are adequately reported.
2. Treatment duration: 1 – Reported; 0 ‐ Unknown.
3. Manualistion of treatment: 2 ‐ Adequate: there is reference to use of a manual that describes the active components of the treatment of study. If more than one treatment arm, manuals were used for all the appropriate treatments; 1 ‐ Partial:in trials with more than one treatment arm, the use of a manual is described but not for all the treatments that would be expected to be manualised; 0 ‐ Inadequate: no evidence that a manual has been used, but reference is made to various principles.
4. Adherence to the manual: 1 ‐ Adequate: there is evidence that the investigators have checked adherence to the manual during the period of study via direct observations, tape recording or supervisory processes that explicitly state adherence to the manual; 0 ‐ Inadequate: no evidence of adherence checks reported.
5. Therapist training: 2 ‐ Adequate: there is documentation of explicit training for the treatment of the trial; 1 ‐ Partial: the general level of therapist training is reported and is adequate (professionally qualified) but there is no mention of explicit training for the trial; 0 ‐ Inadequate: there is no convincing evidence that the therapists have an adequate level of training (e.g. graduate level) or explicit training for the trial.
6. Client Engagement: 1 ‐ Adequate: documented that evidence of engagement was sought e.g. checks on homework were made, skills practice in sessions; 0 – inadequate: no evidence that checks were made on level of engagement.
Major outcomes
Pain was assessed by a visual analogue scale (VAS) in 12 studies (Alda 2011; Ang 2010; Burckhardt 1994; Falcao 2008; Kashikar‐Zuck 2005; Kashikar‐Zuck 2012; Luciano 2011; Miro 2011; Redondo 2004; Rooks 2007; Wigers 1996; Woolfolk 2012), by a numeric rating scale in five studies (Castel 2009;Castel 2012; Thieme 2003; Thieme 2006; Williams 2010), by the McGill Pain Questionnaire in three studies (Edinger 2005; Soares 2002; Vlayen 1996) and by a scale with a composed score by one study (Nicassio 1997).
Negative mood was assessed by the Beck Depression Inventory in five studies (Falcao 2008; Redondo 2004; Rooks 2007; Vlayen 1996; Woolfolk 2012), by a single item visual analogue scale (VAS scale) in four studies (Burckhardt 1994; Castel 2009; Luciano 2011; Wigers 1996), by the Center for Epidemiological Studies Depression Scale in three studies (Nicassio 1997; Oliver 2001; Williams 2010), by the Hospital Anxiety and Depression Scale in two studies (Castel 2012; Miro 2011), by the Children Depression Inventory in two studies (Kashikar‐Zuck 2005; Kashikar‐Zuck 2012), and in one study each by the Patient Health Questionnaire 8 (Ang 2010), the Profile of Mood States (Edinger 2005), the depression subscale of the Multidimensional Pain Inventory (MPI) (Thieme 2003) and the Hamilton Rating Scale Depression (Alda 2011).
Disability was assessed by a single item VAS scale in seven studies (Alda 2011; Ang 2010; Burckhardt 1994; Castel 2009; Castel 2012; Falcao 2008; Luciano 2011), by the Short Form Health Survey (SF)‐36 subscale physical functioning in three studies (Rooks 2007; Williams 2010; Woolfolk 2012), in two studies each by the MPI disability subscale (Thieme 2003; Thieme 2006) and by the Functional Disability Index (Kashikar‐Zuck 2005; Kashikar‐Zuck 2012), and in one study by the quality of well‐being index (Nicassio 1997).
Dropout rates suitable for analysis with reasons for discontinuation were reported by 17 studies (Alda 2011; Ang 2010; Burckhardt 1994; Falcao 2008; Kashikar‐Zuck 2005; Kashikar‐Zuck 2012; Luciano 2011; Miro 2011; Oliver 2001; Redondo 2004; Rooks 2007; Soares 2002; Thieme 2003; Thieme 2006; Vlayen 1996; Wigers 1996; Williams 2010).
Minor outcomes
Self reported pain self efficacy was assessed by the Self Efficacy Pain Scale in four studies (Burckhardt 1994; King 2002; Oliver 2001; Rooks 2007), in two studies by the Chronic Pain Self Efficacy Scale (Redondo 2004; Woolfolk 2012), and in one study each by the Coping Strategies Questionnaire (Vlayen 1996), the Arthritis Self Efficacy Scale (Soares 2002), the Pain Castastrophizing Scale (Alda 2011), the Pain Coping Questionnaire (Kashikar‐Zuck 2005), the Pain Management Inventory (Nicassio 1997), the MPI Pain Coping Scale (Thieme 2003) and the Pain‐related Self‐statements Scale (Thieme 2006).
Fatigue was assessed by a single item VAS scale in 10 studies (Alda 2011; Ang 2010; Burckhardt 1994; Castel 2009; Castel 2012; Falcao 2008; Luciano 2011; Redondo 2004; Rooks 2007; Wigers 1996) and in one study by the Multidimensional Fatigue Inventory (Williams 2010).
Sleep problems were assessed by single item VAS scale in three studies (Kashikar‐Zuck 2012; Redondo 2004; Wigers 1996) and in one study each by the Karolinska Sleep Questionnaire (Soares 2002), the Insomnia Symptom Questionnaire (Edinger 2005), the Pittsburg Sleep Quality Index (Miro 2011) and the SF‐36 Sleep Scale (Williams 2010).
Disease‐specific health‐related quality of life was assessed by the FIbromyalgia Impact Questionnaire in 12 studies (Alda 2011; Burckhardt 1994; Castel 2009; Castel 2012; Falcao 2008; Luciano 2011; Miro 2011; Oliver 2001; Redondo 2004; Rooks 2007; Soares 2002; Thieme 2006).
Excluded studies
Fourteen studies were excluded: four studies because they were not randomised studies (Anderson 2007; De Voogd 2003; Goldenberg 1994; Lommel 2011), two studies because the predefined criteria of CBTs were not met (Carleton 2011; Goeppinger 2009), two studies because no separate data from FM patients were reported and were not provided on request (Haugli 2008; Solomon 2002), two studies because of < 10 patients per treatment arm (Garcia 2006; Martinez‐Valero 2008), one study because of the combination of CBT with psychodynamic therapy (Langford 2010), and one study each because the authors did not report the continuous outcomes assessed and did not provide these outcomes on request (Williams 2002), because no details of FM diagnosis were reported (Stuifbergen 2010) and because FM diagnosis was not established according to the inclusion criteria of the study (Lorig 2008).
Risk of bias in included studies
Risk of bias could not be properly assessed in some studies due to poor method reporting. Most of the studies reviewed were published prior to the standardisation of RCT reporting, established by the CONSORT statement (Schulz 2010). In general, the risk of bias of included studies was high for incomplete outcome data and selective reporting (Figure 2, Figure 3 for risk of bias summary and graph). Only two studies were without any risk of bias (Alda 2011; Williams 2010). Detailed information regarding risk of bias assessments of every study are given in the Characteristics of included studies table. Moreover, individual treatment groups were relatively small in size, which makes them susceptible to random chance and small sample bias.
2.
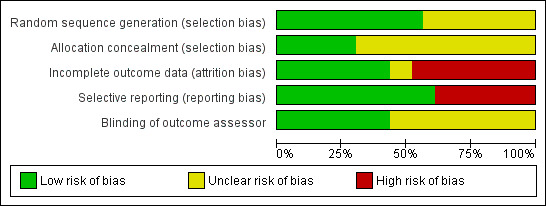
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
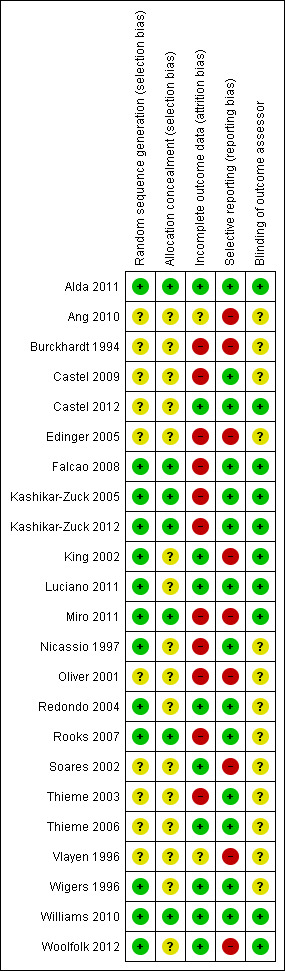
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Reported random sequence generation was adequate in 13 studies (Alda 2011; Falcao 2008; Kashikar‐Zuck 2005; Kashikar‐Zuck 2012; King 2002; Luciano 2011; Miro 2011; Nicassio 1997; Redondo 2004; Rooks 2007; Wigers 1996; Williams 2010; Woolfolk 2012). Reported allocation concealment was adequate in seven studies (Alda 2011; Falcao 2008; Kashikar‐Zuck 2005; Kashikar‐Zuck 2012; Miro 2011; Rooks 2007; Williams 2010).
Blinding
Ten studies reported blinding of the outcome assessor (Alda 2011; Castel 2012; Falcao 2008; Kashikar‐Zuck 2005; Kashikar‐Zuck 2012; King 2002; Luciano 2011; Miro 2011; Williams 2010; Woolfolk 2012).
Incomplete outcome data
Only 11 studies performed an intention‐to‐treat (ITT) analysis (Alda 2011; Castel 2012; Kashikar‐Zuck 2012; King 2002; Luciano 2011; Redondo 2004; Soares 2002; Thieme 2006; Wigers 1996; Williams 2010; Woolfolk 2012).
Selective reporting
Visual inspection of funnel plots was not indicative of publication bias. In the Egger’s test the intercept of the effect size on pain at end of treatment was ‐0.96 (95% CI ‐3.49 to 1.57) with t = 0.79 (two‐tailed P = 0.46). In the Begg’s test Kendall’s tau without continuity correction was ‐0.08 and z = 0.56 (two‐tailed P = 0.43). Both tests were not indicative of publication bias.
Fourteen studies reported all study outcomes or provided these data on request (Alda 2011; Castel 2009; Castel 2012; Falcao 2008; Kashikar‐Zuck 2005; Kashikar‐Zuck 2012; Luciano 2011; Nicassio 1997; Redondo 2004; Rooks 2007; Thieme 2003; Thieme 2006; Wigers 1996; Williams 2010). Nine authors (Ang 2010; Burckhardt 1994; Edinger 2005; King 2002; Miro 2011; Oliver 2001; Soares 2002; Vlayen 1996; Woolfolk 2012) did not provide missing outcomes on request.
Effects of interventions
Of the 15 analyses which compared CBTs with controls, 14 had high heterogeneity and one had moderate heterogeneity. In the subgroup analyses, the heterogeneity of the effect size of operant therapy on pain and disability at end of treatment was > 90%, which is indicative of highly inconsistent findings of the two operant therapy studies (Thieme 2003; Thieme 2006). Subgroup analyses revealed that the high heterogeneity was due to studies with active controls and that heterogeneity in studies with other types of controls was low to moderate (Appendix 2).
Some missing SDs of one study (Burckhardt 1994) had to be calculated by a validated imputation method. Means from one study (Woolfolk 2012) had to be extracted from figures.
CBTs versus controls at end of treatment
Major outcomes
Twenty‐one studies with 1453 participants were entered into an analysis of the effects of CBTs on pain. CBTs had a small effect size on pain of ‐0.29 (95% CI ‐0.47 to ‐0.11) (Figure 4). Nineteen studies with 1649 participants were entered into an analysis of the effects of CBTs on negative mood. CBTs had a small effect size on mood of ‐0.33 (95% CI ‐0.49 to ‐0.17) (Figure 5). Sixteen studies of 1234 participants were entered into an analysis of the effects of CBTs on disability. CBTs had a small effect size on disability of ‐0.30 (95% CI ‐0.51 to ‐0.08) (Figure 6). Twenty‐one studies with 1914 participants were entered into an analysis of the acceptability of CBTs. The risk ratio of dropping out for any reason did not differ between CBTs (15.4%) and controls (14.5%) (RR 0.94; 95% CI 0.65 to 1.35) (see Table 1).
4.
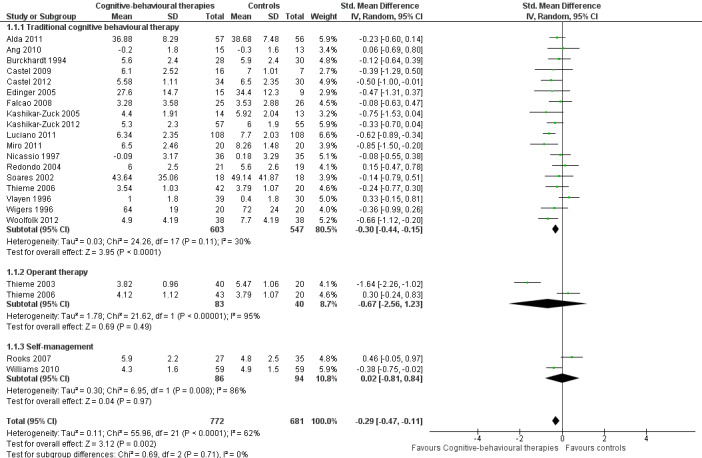
Forest plot of comparison: 1 Cognitive behavioural therapies versus controls at end of treatment, outcome: 1.1 Pain.
5.
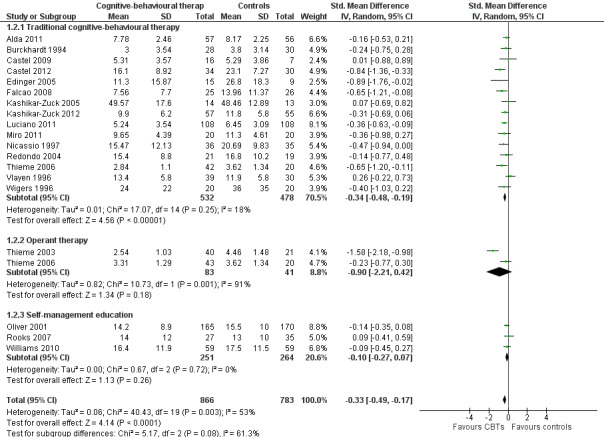
Forest plot of comparison: 1 Cognitive behavioural therapies versus controls at end of treatment, outcome: 1.2 Negative mood.
6.
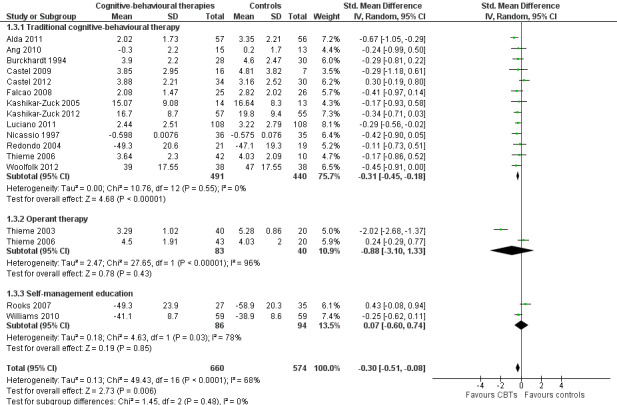
Forest plot of comparison: 1 Cognitive behavioural therapies versus controls at end of treatment, outcome: 1.3 Disability.
Minor outcomes
Eleven studies with 1047 participants were entered into an analysis of the effects of CBTs on pain self efficacy. CBTs had a small effect size on pain self efficacy of ‐0.49 (95% CI ‐0.80 to ‐0.17). Eleven studies with 910 participants were entered into a study of the effects of CBTs on fatigue. CBTs had a small effect size on fatigue of ‐0.25 (95% CI ‐0.49 to ‐0.02). Eight studies of 422 participants were entered into a study of the effects of CBTs on sleep problems. The overall effect on CBTs on sleep problems was ‐0.40 (95% CI ‐ 0.85 to 0.05), which was not statistically significant (P = 0.06). Thirteen studies of 1238 participants were entered into a study of the effects of CBTs on disease‐specific quality of life. CBTs had a small effect size on disease‐specific quality of life of ‐0.23 (95% CI ‐0.38 to ‐0.08) (see Table 2).
CBTs versus controls at long‐term follow‐up
Major outcomes
Fifteen studies with 893 participants were entered into an analysis of the effects of CBTs on pain. CBTs had a small effect size on pain of ‐0.40 (95% CI ‐0.62 to ‐0.17). Twelve studies with 792 participants were entered into an analysis of the effects of CBTs on negative mood. CBTs had a small effect size on negative mood of ‐0.43 (95% CI ‐0.75 to ‐0.11). Ten studies of 735 participants were entered into an analysis of the effects of CBTs on disability. CBTs had a moderate effect size on disability of ‐0.52 (95% CI ‐0.86 to ‐0.18) (see Table 1).
Minor outcomes
Nine studies of 617 participants were entered into an analysis of the effects of CBTs on pain self efficacy. CBTs had a moderate effect size on pain self efficacy of ‐0.75 (95% CI ‐1.27 to ‐0.24). Six studies of 429 participants were entered into a study of the effects of CBTs on fatigue. CBTs had a small effect size on fatigue of ‐0.46 (95% CI ‐0.77 to ‐0.15). Six studies of 425 participants were entered into a study of the effects of CBTs on sleep problems. The overall effect of CBTs on sleep problems of ‐0.64 (95% CI ‐1.31 to 0.03) was not statistically significant. Six studies of 425 participants were entered into a study of the effects of CBTs on disease‐specific quality of life. The overall effect of CBTs on disease‐specific quality of life of ‐0.19 (95% CI ‐0.58 to 0.21) was not statistically significant (see Table 2).
Subgroup analyses
Different types of CBTs
a. End of treatment
There were no statistically significant subgroup differences for the outcomes pain (Chi2 = 0.69, P = 0.71) (Analysis 1.1), negative mood (Chi2 = 5.17, P = 0.08) (Analysis 1.2), disability (Chi2 = 1.45, P = 0.48) (Analysis 1.3) and pain self efficacy (Chi2 = 2.11, P = 0.35) (Analysis 1.4). The effect sizes of operant therapy and self management education on these outcomes were not statistically significant. The effect size of operant therapy on pain was ‐0.67 (95% CI ‐2.56 to 1.23; P = 0.49) and of self management education was 0.02 (95% CI ‐0.81 to 0.84; P = 0.97). Operant therapy had a small effect size on disability of ‐0.30 (95% CI ‐0.44 to ‐0.17; P < 0.0001) (Analysis 1.3). The effect size of operant therapy on negative mood was ‐0.90 (95% CI ‐2.12 to 0.42; P = 0.18) and of self management education was ‐0.10 (95% CI ‐0.27 to 0.07; P = 0.26) (Analysis 1.2). The effect size of operant therapy on disability was ‐0.88 (95% CI ‐3.10 to 1.33); P = 0.43) and of self management education was 0.07 (95% CI ‐0.60 to 0.74; P = 0.06) (Analysis 1.3). The effect size of operant therapy on pain self efficacy was ‐1.18 (95% CI ‐3.01 to 0.64; P = 0.20) and of self management education was ‐0.18 (95% CI ‐0.39 to 0.04; P = 0.11) (Analysis 1.4).
1.1. Analysis.
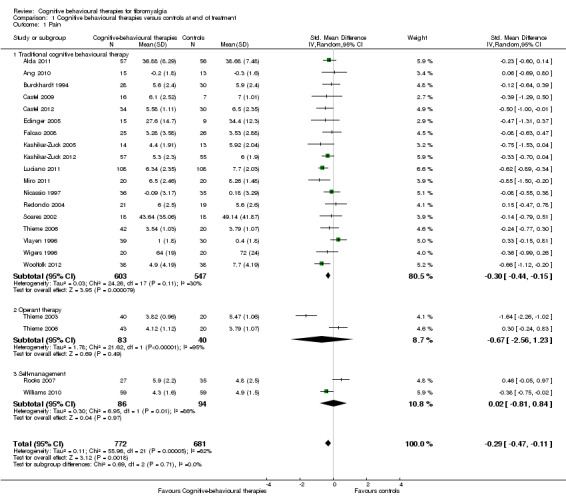
Comparison 1 Cognitive behavioural therapies versus controls at end of treatment, Outcome 1 Pain.
1.2. Analysis.
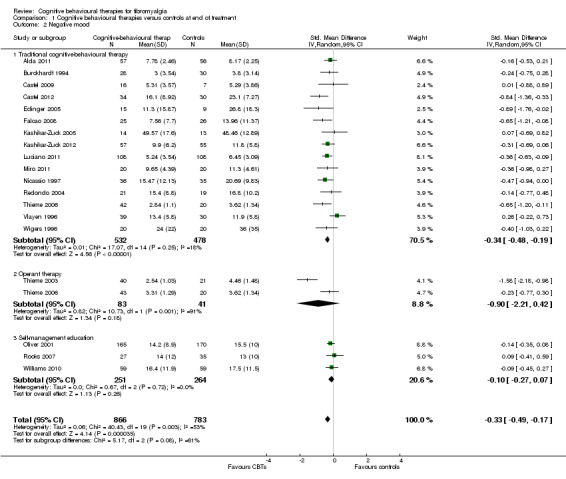
Comparison 1 Cognitive behavioural therapies versus controls at end of treatment, Outcome 2 Negative mood.
1.3. Analysis.
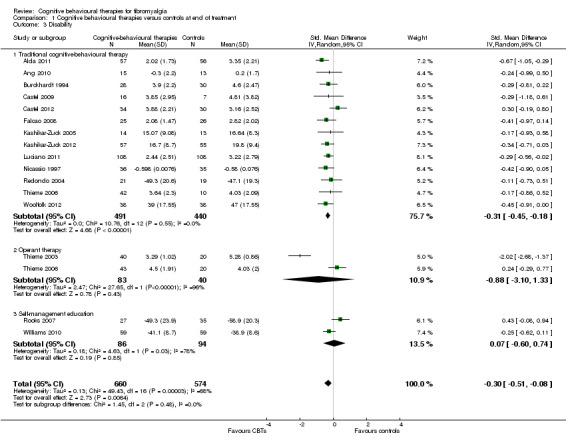
Comparison 1 Cognitive behavioural therapies versus controls at end of treatment, Outcome 3 Disability.
1.4. Analysis.
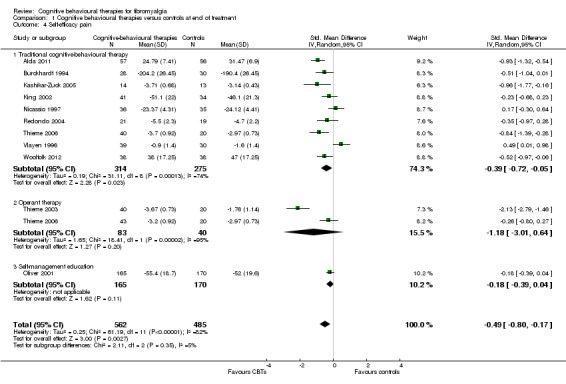
Comparison 1 Cognitive behavioural therapies versus controls at end of treatment, Outcome 4 Self‐efficacy pain.
b. Long‐term follow‐up
There was no statistically significant group difference between traditional CBT and operant therapy on pain (Chi2 = 3.46, P = 0.06) (Analysis 2.1) but there was on negative mood (Chi2 = 6.9, P = 0.0009) (Analysis 2.2), disability (Chi2 = 12.34, P = 0.0004) (Analysis 1.3), pain self efficacy (Chi2 = 3.71, P = 0.05) (Analysis 1.4), fatigue (Chi2 = 3.89, P = 0.05) (Analysis 2.5) and sleep problems (Chi2 = 7.03, P = 0.008) (Analysis 2.6) favouring operant therapy; and in HRQOL (Chi2 = 3.92, P = 0.05) Analysis 2.7) favouring traditional CBT. Of note, the effect sizes of operant therapy on pain (‐1.27; 95% CI ‐2.30 to ‐0.24; P = 0.02), negative mood (‐1.28; 95% CI ‐1.97 to ‐0.49; P = 0.003), disability (‐1.68; 95% CI ‐2.40 to ‐0.96; P < 0.0001) and pain self efficacy (‐1.02; 95% CI ‐1.59 to ‐0.46; P = 0.02) were large and statistically significant. The effect size of traditional CBT on pain of ‐0.28 (95% CI ‐0.43 to ‐0.14; P = 0.001) was small and on negative mood of (‐0.28; 95% CI ‐0.58 to 0.02; P = 0.07) was not statistically significant; on disability (‐0.32; 95% CI ‐0.55 to ‐0.09; P = 0.007) the effect size was small and statistically significant. No study with self management education performed long‐term follow‐up.
2.1. Analysis.
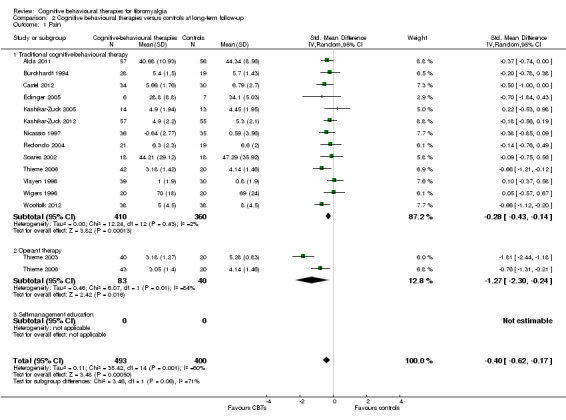
Comparison 2 Cognitive behavioural therapies versus controls at long‐term follow‐up, Outcome 1 Pain.
2.2. Analysis.
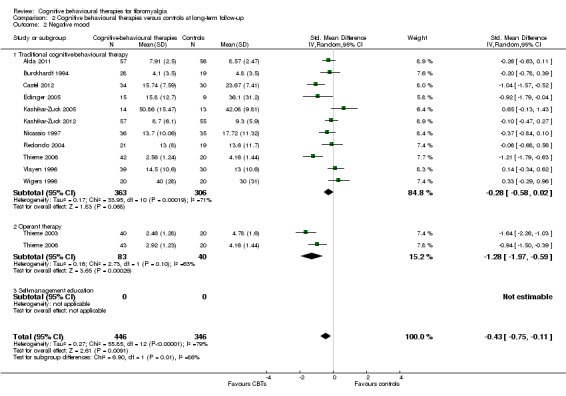
Comparison 2 Cognitive behavioural therapies versus controls at long‐term follow‐up, Outcome 2 Negative mood.
2.5. Analysis.
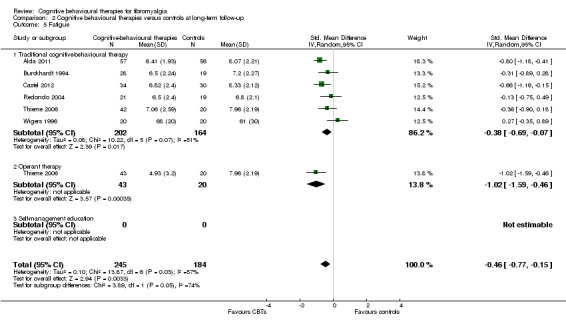
Comparison 2 Cognitive behavioural therapies versus controls at long‐term follow‐up, Outcome 5 Fatigue.
2.6. Analysis.
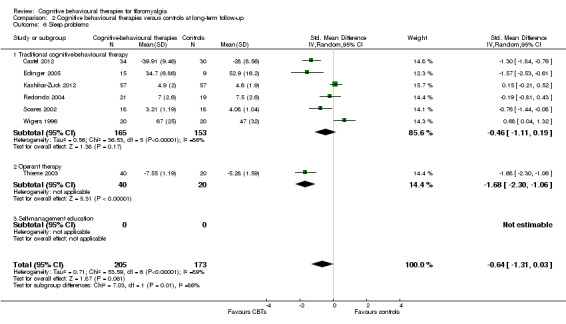
Comparison 2 Cognitive behavioural therapies versus controls at long‐term follow‐up, Outcome 6 Sleep problems.
2.7. Analysis.
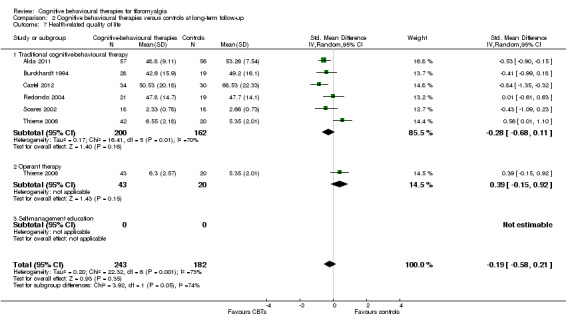
Comparison 2 Cognitive behavioural therapies versus controls at long‐term follow‐up, Outcome 7 Health‐related quality of life.
Types of controls
The effect sizes of CBTs on pain, negative mood and disability at end of treatment and at long‐term follow‐up compared to active controls were not statistically significant. The effect sizes of CBTs on pain, negative mood and disability at end of treatment compared to attention controls were only statistically significant for negative mood, but not for pain and disability at end of therapy. The effects sizes of CBTs on pain, disability and negative mood compared to treatment as usual and waiting list control at end of treatment were small and statistically significant. The effect sizes of CBTs on pain and on disability at long‐term follow‐up compared treatment as usual or waiting list control were small and statistically significant, the one on negative mood was not statistically significant (Appendix 2).
Type of delivery of treatment
The effect sizes of internet or telephone therapy CBTs on pain, negative mood and disability at end of treatment were not statistically significant. The effect sizes of face‐to‐face CBTs on pain, negative mood and disability at end of treatment were small and statistically significant (Appendix 3).
Age of study participants
The effect size of traditional CBT on pain at end of treatment in children and adolescents was small and statistically significant. The effect sizes on negative mood and disability at end of treatment were not statistically significant. The effect sizes of traditional CBT on pain, negative mood and disability in adults at end of treatment were small and statistically significant (Appendix 4).
Treatment duration
Two studies which did not report treatment duration (Williams 2010; Woolfolk 2012) were excluded from this analysis. One study (Ang 2010) with < five hours was labelled 'ultra‐short term' CBTs. This study had no statistically significant effects on pain and disability at end of treatment. Studies with > 25 hours (Castel 2012; Falcao 2008; Thieme 2006; Wigers 1996) and > 50 hours (Soares 2002; Thieme 2003) were included in a group labelled 'long‐term' CBTs. At end of treatment, the effect sizes of long‐term CBTs on pain and disability were not statistically significant, the effect size on negative mood was moderate and statistically significant. The remaining studies with a study intensity of 5 to 25 hours were included in a group labelled 'short‐term' CBTs. At end of treatment, the effect sizes of short‐term CBTs on negative mood and disability were small and statistically significant, the effect size on pain was not statistically significant (Appendix 5).
Reported treatment quality
The effect sizes of CBTs with low reported treatment quality on pain, negative mood and disability at end of treatment were not statistically significant. The effect sizes of CBTs with moderate reported treatment quality on pain and negative mood at end of treatment were small and statistically significant, the effect size on disability was not statistically significant. The effect sizes of CBTs with high reported treatment quality on pain and negative mood at end of treatment were small and statistically significant, the effect size on disability was not statistically significant (Appendix 6).
Sensitivity analyses
Removing studies with data substituted or extracted from figures, with selection bias, with attrition bias, with reporting bias, without ITT, and with exclusion of patients with depressive or anxiety disorders did not change the magnitude and significance of the effect sizes of CBTs on pain, negative mood and disability at end of treatment; except that the effect size of CBTs on disability at end of treatment was not statistically significant after excluding studies with data extracted from figures or substituted values. Moreover, the effect size of CBTs on pain and disability was not statistically significant in studies after excluding studies which did not include patients with depressive or anxiety disorders, or both. Removing studies with < 20 participants per treatment arm did not change the magnitude and significance of the effect sizes of CBTs on pain, negative mood and disability at end of treatment (Appendix 7).
Discussion
Summary of main results
Low quality evidence from 20 trials (1382 patients with FM) indicates that CBTs provided a small decrease in pain at the end of about 12 weeks treatment, and from 14 trials (822 patients with FM) that CBTs provided a small decrease in pain at the end of about six months follow‐up. Low quality evidence from 18 trials (1578 patients with FM) indicates that CBTs provided a small decrease in negative mood at the end of about 12 weeks treatment, and from 11 trials (721 patients with FM) that CBTs provided a small decrease of negative mood at the end of about six months follow‐up. Low quality evidence from 15 trials (1163 patients with FM) indicates that CBTs provided a small decrease in disability at the end of about 12 weeks treatment, and from nine trials (664 patients with FM) that CBTs provided a moderate decrease in disability at the end of about six months follow‐up (Table 1). Subgroup analyses demonstrated that positive effects of CBTs were only detectable for CBT at end of treatment and at long‐term follow‐up and for operant therapy at follow‐up, but not for operant therapy at end of treatment and not for self management education programs at end of treatment. Positive effects were only verifiable for face‐to‐face CBTs but not for internet‐based and telephone‐based CBTs at end of treatment. Positive effects were only traceable in the comparison of CBTs with treatment as usual and waiting lists controls but not with other active treatments (for example aerobic exercise) or with attention control (except negative mood) at end of treatment. Studies which included patients with anxiety and depression disorders exerted only a reduction of negative mood but not of pain and disability at end of treatment.
Overall completeness and applicability of evidence
The tests conducted were not indicative of a publication bias. Nevertheless, we cannot rule out the possibility that negative study results with CBTs had not been published or had been missed by our search strategy.
The applicability (external validity) of evidence is strong for the following reasons. 1. The studies were not only performed in university centres but also in primary and secondary care. 2. Patients with anxiety or depressive disorder, or both, which are frequently associated with FM were included in some studies. 3. Subgroup analysis demonstrated the efficacy of CBTs in children and adolescents and in adults. However, the majority of the patients were middle‐aged Caucasian women, making it difficult to apply the results to the total FM population, especially to male and non‐Caucasian patients. No study performed a subgroup analysis for male and non‐Caucasian patients. In summary, the external validity of CBTs studies in FM is much stronger than in the phase III clinical trials for drugs approved for FM treatment by the Food and Drug Administration (duloxetine, milnacipran and pregabalin). These studies were conducted only in research centres and excluded patients with anxiety and depressive disorder, except the duloxetine studies (Sommer 2012b).
Quality of the evidence
The quality of evidence of this review was based on the data presented in peer reviewed journals and some additional details which were provided on request by the study authors. The overall quality of evidence for the primary outcomes in the majority of studies was low (Table 1) due to studies without or with unclear randomisation, allocation concealment, ITT analysis and selective reporting. However, sensitivity analyses demonstrated that the study results were robust against these risks of bias.
Potential biases in the review process
We cannot be certain that other studies that have not been published (with positive or negative results) were not identified.
We might have underestimated the methodological and treatment quality of some studies which might not have reported some details required for the treatment quality score used. We relied on the reported data for quality assessment and did not ask authors for further details because we did not want to introduce a 'response' bias. We have experienced in previous reviews on psychological therapies that it was impossible to get any details, on request, of studies conducted before 2005 (Bernardy 2010; Bernardy 2011). Even some authors of studies conducted after 2010 did not respond to our requests for outcomes not reported for this review.
Efficacy outcomes were analysed by some studies using last observation carried forward to impute for missing data. This procedure may lead to an overestimation of efficacy. However, the recommended baseline observation carried forward method (Moore 2010b) has been used by some other studies.
We had to calculate missing values by established imputation methods and to extract data from figures, for one study each. Excluding these studies from analyses did not change the significance and magnitude of the effect sizes.
The results of subgroup analyses are not robust due to the small number of studies in the operant and self‐management group and in the internet/telephone‐based groups.
Agreements and disagreements with other studies or reviews
The results of this review were more favourable for CBTs than previous reviews on CBTs in FM which searched the literature to June 2009 (Bernardy 2010) and December 2010 (Köllner 2012). By including new studies with large sample sizes and positive results into this review, positive effects of CBTs on pain, fatigue and disability could be demonstrated that were not detectable in our previous reviews (Bernardy 2010; Köllner 2012). Positive effects of CBTs on depression and self efficacy pain in FM patients were demonstrated in the previous reviews (Bernardy 2010; Köllner 2012)..
A Cochrane review on psychological therapies in chronic pain syndromes, except headache, in adults searched the literature until September 2011 (Williams 2012). The authors found that CBTs had small positive effects on disability, but not on pain or mood, when compared with active controls. CBTs had small to moderate effects on pain, disability and mood at end of treatment when compared with treatment as usual or waiting list. All except a small effect on mood disappeared at follow‐up. Subgroup analyses stratified according to the type of pain syndrome were not conducted. Our results are mainly in line with this review (Williams 2012): in FM, CBTs were only superior with small effect sizes in reducing pain, negative mood and disability at the end of treatment when compared to treatment as usual and waiting list but not when compared to attention controls (except for negative mood) and to other active therapies such as exercise.
A Cochrane review on psychological therapies in chronic pain syndromes in children and adolescents searched the literature until August 2008 (Eccleston 2012). The authors included one study in FM (Kashikar‐Zuck 2005). They found a significant and large effect of CBTs compared to controls in non‐headache treatments on pain at end of treatment and at long‐term follow‐up (Eccleston 2012). The effects of the two studies of CBTs in FM children and adolescents compared to controls were small and statistically significant on pain at end of treatment but were not statistically significant on negative mood and disability.
Of note, the effects of operant therapy compared to active controls in FM were not statistically significant at end of treatment, but they were large and statistically significant at long‐term follow‐up in this review. This time‐dependent efficacy of operant therapy in FM has not been sufficiently outlined by previous reviews on CBTs in FM (Bernardy 2010)).
Authors' conclusions
Implications for practice.
Traditional cognitive behavioural therapy and operant therapy reduce the key symptoms of FM after six months. These types of therapies should be offered face‐to face. The limited data available on internet‐ or telephone‐based CBTs do not allow a recommendation to use this type of delivery. Treatment intensity should be between five and 25 hours. Evidence was not found for benefit of self management programs as single therapy.
Even if the assessment of adverse events in most CBTs studies was insufficient and two studies reported a dropout rate of up to 5% of patients in CBTs groups due to worsening of co‐morbid mental disorders, the risk‐benefit ratio of CBTs is more favourable than that of drugs which have been approved by the US Food and Drug Administration (FDA) for FM treatment. Adverse events and dropouts due to adverse events were higher in drug than in CBTs trials (see Häuser 2010; Sommer 2012b). In addition, the positive effects of CBTs, especially of operant therapy, increase within six months after the end of therapy, whereas the positive effects of drugs disappear within two weeks after the cessation of therapy (Saxe 2012).
In some countries (for example USA), CBTs might not be easily available for patients and might be expensive and not covered by insurance (Rheumatologist Los Angeles 2012). In contrast, aerobic exercise (for example walking) is available everywhere with very low costs because it can be carried out by the patient himself without professional guidance, or after a short education. We found that CBTs were not superior to other active treatments such as aerobic exercise in the reduction of FM symptoms. However, aerobic exercise of low to moderate intensity is effective in reducing FM key symptoms (pain, fatigue, negative mood) at end of treatment and long‐term follow‐up (Busch 2007; Winkelmann 2012), to the same amount as CBTs (Nüesch 2013). Therefore, aerobic exercise and not CBTs are the first non‐pharmacologic treatment option of choice for FM (Eich 2012). This conclusion is not based on the results of head‐to‐head comparisons of CBTs versus aerobic exercise but on the higher availability and lower costs of aerobic exercise compared to CBTs.
Implications for research.
The following research priorities would help to understand the effects of CBTs in FM according to EPICOT (Brown 2006), and to definitively convince patients, clinicians, researchers and payers of the benefits of CBTs in FM.
Evidence
No further small sample size studies with low to moderate methodology and treatment quality comparing CBTs with treatment as usual are needed. Large and high quality studies should compare CBTs with an adequate psychological placebo or best available drug therapy or aerobic exercise. Any new RCT needs to be designed and reported taking explicit account of the challenges identified and discussed in this review and in the ones of psychological therapies in chronic pain in children and adolescents (Eccleston 2012) and in adults (Williams 2012).
Population (external validity)
Studies are needed in all age groups. Future studies should include more adolescents, men and seniors. Clinical studies in FM should be conducted with patient samples representative of clinical practice, including patients with co‐morbid anxiety and depressive disorders and inflammatory rheumatic diseases. Subgroup analyses should be performed for these subpopulations. Moreover, studies should include patients with different disease durations and different severity levels all over the world.
Intervention (treatment quality)
Standardised empirical protocols should be used. Therapists providing interventions in trials should have been trained in the use of the protocol. Therapist adherence to the protocol (treatment integrity) and patient adherence to behavioural skill training (treatment uptake) should be monitored (Kashikar‐Zuck 2010). Attendance rates should be reported. Future intervention studies should be designed to assess dose–response curves for improvement of FM symptoms and evaluate follow‐up periods following completion of the active intervention period. These studies should include ongoing, intermittent assessment of CBTs interventions and FM outcome measures to understand the stability of responses and program characteristics (intensity, duration, frequency) needed to maintain gains.
Comparisons
An attention control with the same amount of time spent with the patients by therapists with the same experience in psychotherapy as in the treatment groups is the gold standard to control for unspecific effects of psychological therapies (Kashikar‐Zuck 2010; Wampold 2005). Of note, the superiority of CBTs over controls which we found in this review is based on the comparison of CBTs with treatment as usual and waiting list control, which were inadequate according to the gold standard. A recent large trial with mindfulness‐based stress reduction (MBSR) failed to demonstrate a superiority of MBSR over an adequate 'psychological placebo' (Schmidt 2011). Whether CBTs add a relevant benefit over an adequate psychological placebo still needs to be determined. The significance of CBTs compared to other established FM therapies such as aerobic exercise and drugs recommended for FM (amitriptyline, duloxetine, milnacipran, pregabalin) still needs to be determined.
Outcomes
A core set of outcome measures for psychological trials in FM is needed. The set should include the key domains of FM developed through consensus among experts and FM patients (Mease 2009) and the suggestions of the Initiative of Methods, Measurement and Pain Assessment in Clinical trials (IMMPACT) (Dworkin 2008; Dworkin 2009). Key domains include pain, sleep problems and fatigue. Responder outcomes (for example number of patients with ≥ 50% pain reduction, number of patients with ≥ 14% in the FIQ‐total score) should be reported. There is increasing recognition that psychological treatments have the potential for negative outcomes for some patients (Kashikar‐Zuck 2010). Standards, in which potential negative outcomes should be routinely monitored, by which methods, still have to be defined by the research community. In addition, a discussion of what we think is feasible as the outcome of any type of therapy in FM is appropriate. Average effect sizes across trials are relatively small, as they are across pharmacological and physical treatments in FM (Nüesch 2013; Winkelmann 2012). This raises the issue of whether FM trials should still focus on mere symptom reduction. FM trials on psychological therapies, especially acceptance‐based CBTs, could provide a better answer on how to live more satisfactorily with FM symptoms (Williams 2012).
Disease‐relevant timelines
Follow‐up of all study participants (including members of the control group) should be for at least one year (Brachaniec 2009).
Study type and methodology (methodology quality)
The following requirements should be met for the 'green' criterion of the suggested Cochrane Collaboration traffic light system (Moore 2010a). Randomization should be performed by an adequate method. Treatment allocation should be undertaken independently. Investigators and assessment staff should be blinded to prevent inadvertent bias. Analysis should be performed by an ITT analysis based on baseline observation carried forward method. Patient‐associated barriers to participate in CBTs should be investigated.
Major tasks of future research are: a) the definition of subgroups (e.g. FM patients with and without major depression or post‐traumatic stress disorder, pain persistence and pain avoidance behaviour) with development of more tailored therapies to these subgroups (Lange 2010; Loevinger 2012; Lumley 2011; van Koulil 2011), b) the identification of common (e.g. therapeutic relationship) and specific (e.g. exposure) treatment mechanisms by more frequent measures than pre‐post assessment allowing examination of lagged relationships over time (Thurns 2011) c) the development of cost‐effective telephone or internet‐assisted therapies to overcome the costs of CBTs and limited availability of providers with specialized training (Kashikar‐Zuck 2010; McBeth 2012) and d) the application of new study designs beyond the "gold standard" of RCTs, e.g. clinical effectiveness trials where clinical effectiveness is "the product of efficacy, tolerability, utility, cost, and speed" (Moore 2010c).
What's new
| Date | Event | Description |
|---|---|---|
| 1 June 2017 | Review declared as stable | See Published notes. |
Notes
We performed a full search in February 2016 intending to complete a full update, but we did not identify any potentially relevant studies likely to change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. If appropriate, we will update the review if new evidence likely to change the conclusions is published. Patrick Welsch joined the author team and worked on the potential update, and we acknowledge his contribution.
Acknowledgements
We thank all reviewers for their helpful comments.
Appendices
Appendix 1. Search strategies and hits retrieved
MEDLINE (via PubMed)
| #1 | Search "behaviour therapy"[All Fields] OR "behavior therapy"[MeSH Terms] OR ("behavior"[All Fields] AND "therapy"[All Fields]) OR "behavior therapy"[All Fields] | 121430 |
| #2 | Search "cognitive therapy"[MeSH Terms] OR ("cognitive"[All Fields] AND "therapy"[All Fields]) OR "cognitive therapy"[All Fields] | 51940 |
| #3 | Search "cognitive behaviour therapy"[All Fields] OR ("cognitive"[All Fields] AND "behavior"[All Fields] AND "therapy"[All Fields]) OR "cognitive behavior therapy"[All Fields] | 11060 |
| #4 | Search acceptance‐based[All Fields] AND ("cognitive behaviour therapy"[All Fields] OR "cognitive therapy"[MeSH Terms] OR ("cognitive"[All Fields] AND "therapy"[All Fields]) OR "cognitive therapy"[All Fields] OR ("cognitive"[All Fields] AND "behavior"[All Fields] AND "therapy"[All Fields]) OR "cognitive behavior therapy"[All Fields]) | 51 |
| #5 | Search ("exposure"[All Fields] AND "therapy"[All Fields]) OR "exposure therapy"[All Fields] | 66828 |
| #6 | Search "implosive therapy"[MeSH Terms] OR ("implosive"[All Fields] AND "therapy"[All Fields]) OR "implosive therapy"[All Fields] | 544 |
| #7 | Search "aversive therapy"[MeSH Terms] OR ("aversive"[All Fields] AND "therapy"[All Fields]) OR "aversive therapy"[All Fields] | 1505 |
| #8 | Search imaginal exposure therapy[all] | 8 |
| #9 | Search "acceptance" [All Fields] AND "commitment" [All Fields] AND ("therapy"[Subheading] OR "contextual" [All Fields] AND "cognitive‐behavioral [All Fields]" AND ("therapy"[Subheading] OR "mindfulness‐based" [All fields] AND "cognitive" [All fields] AND ("therapy"[Subheading] OR "therapeutics"[MeSH Terms] OR "therapeutics"[All Fields]) | 190 |
| #10 | Search "desensitization, psychologic"[MeSH Terms] OR ("desensitization"[All Fields] AND "psychologic"[All Fields]) OR "psychologic desensitization"[All Fields] OR ("desensitization"[All Fields] AND "psychologic"[All Fields]) OR "desensitization, psychologic"[All Fields] | 2043 |
| #11 | Search cbt[all] | 4748 |
| #12 | Search "self care"[MeSH Terms] OR ("self"[All Fields] AND "care"[All Fields]) OR "self care"[All Fields] | 110710 |
| #13 | Search self [All fields] OR "management" [All fields"] OR "self management" [All Fields] | 56496 |
| #14 | Search "education"[Subheading] OR "education"[All Fields] OR "educational status"[MeSH Terms] OR ("educational"[All Fields] AND "status"[All Fields]) OR "educational status"[All Fields] OR "education"[All Fields] OR "education"[MeSH Terms] | 900276 |
| #15 | Search psychoeducation[all Fields] | 1203 |
| #16 | Search #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR 11 OR #12 OR #13 OR #14 OR #15 | 1173401 |
| #17 | Search "fibromyalgia"[MeSH Terms] OR "fibromyalgia"[All Fields] OR "fibrositis"[All Fields] OR FMS[all] | 12147 |
| #18 | Search randomised controlled trial[pt] OR controlled clinical trial[pt] OR randomised[tiab] OR placebo[tiab] OR drug therapy[sh] OR randomly[tiab] OR trial[tiab] OR groups[tiab] | 3213345 |
| #19 | Search animals[mh] NOT humans[mh] | 3810178 |
| #20 | Search #18 NOT #19 | 2757341 |
| #21 | Search #16 AND #17 AND #20 | 602 |
CENTRAL (The Cochrane Library 2013, Issue 1)
#1 (behavior therapy) or (cognitive therapy) or (cognitive behavior therapy) or (cognitive behaviour therapy) or (behaviour therapy) in Trials 26033
#2 (acceptance based) or (exposure therapy) or (implosive therapy) or (aversive therapy) or (imaginal exposure therapy) in Trials 9230
#3 (desensitization) or (psychologic desensitization) or (cbt) or (self care ) or (self management) in Trials 18267
#4 (education) or (psychoeducation) in Trials 31332
#5 (acceptance ) and (commitment) in Trials 143
#6 MeSH descriptor Behavior Therapy explode all trees 340
#7 (#1 OR #2 OR #3 OR #4 OR #5 OR #6) 67827
#8 (fibromyalgia) or (fibrositis) or (FMS) in Trials 1147
#9 MeSH descriptor Fibromyalgia explode all trees 30
#10 (#8 OR #9) 1147
#11(randomised controlled trial):pt or (controlled trial ):pt or (randomised controlled trial) or (random allocation) or (double blind method) in Trials 507367
#12 (single blind method) or (clinical trial):pt or (placebo$) or (random$) or (clinical trial) in Trials 484316
#13 (#11 OR #12) 569365
#14 (animal):ti,ab,kw in Trials 12506
#15 (human):ti,ab,kw in Trials 460556
#16 (#14 AND NOT #15) 353
#17 (#13 AND NOT #16) 569098
#18 (#7 AND #10 AND #17) 334
of these:
122 in Cochrane Reviews
42 in Other Reviews
1 in Methods Studies
1 in Technology Assessments
4 in Economic Evaluations
3 in Cochrane Groups
161 in Cochrane Central Register of Controlled Trials
SCOPUS Recherche via SciVerse/Elsevier
#1 (TITLE‐ABS‐KEY(behavior therapy) OR TITLE‐ABS‐KEY(behaviour therapy) OR TITLE‐ABS‐KEY(cognitive therapy)) AND DOCTYPE(ar OR re)
159,847 1
#2 (TITLE‐ABS‐KEY("behavior therapy") OR TITLE‐ABS‐KEY("behaviour therapy") OR TITLE‐ABS‐KEY("cognitive therapy")) AND DOCTYPE(ar OR re)
57,531
#3 (TITLE‐ABS‐KEY("cognitive behavior therapy") OR TITLE‐ABS‐KEY("cognitive behaviour therapy")) AND DOCTYPE(ar OR re) 3,388
#4 (TITLE‐ABS‐KEY("acceptance based") OR TITLE‐ABS‐KEY("exposure therapy") OR TITLE‐ABS‐KEY("implosive therapy") OR TITLE‐ABS‐KEY("aversive therapy") OR TITLE‐ABS‐KEY("imaginal exposure therapy")) AND DOCTYPE(ar OR re) 2,668
#5 (TITLE‐ABS‐KEY("acceptance and commitment") OR TITLE‐ABS‐KEY("desensitization") OR TITLE‐ABS‐KEY("psychologic desensitization"))
AND DOCTYPE(ar OR re) 29,207
#6 (TITLE‐ABS‐KEY("self care") OR TITLE‐ABS‐KEY("self management")) AND DOCTYPE(ar OR re) 35,570
#7 (TITLE‐ABS‐KEY(education) OR TITLE‐ABS‐KEY(psychoeducation)) AND DOCTYPE(ar OR re) 885,411
#8 ((TITLE‐ABS‐KEY(behavior therapy) OR TITLE‐ABS‐KEY(behaviour therapy) OR TITLE‐ABS‐KEY(cognitive therapy)) AND DOCTYPE(ar OR re)) OR ((TITLE‐ABS‐KEY("behavior therapy") OR TITLE‐ABS‐KEY("behaviour therapy") OR TITLE‐ABS‐KEY("cognitive therapy")) AND DOCTYPE(ar OR re)) OR ((TITLE‐ABS‐KEY("cognitive behavior therapy") OR TITLE‐ABS‐KEY("cognitive behaviour therapy")) AND DOCTYPE(ar OR re)) OR ((TITLE‐ABS‐KEY("acceptance based") OR TITLE‐ABS‐KEY("exposure therapy") OR TITLE‐ABS‐KEY("implosive therapy") OR TITLE‐ABS‐KEY("aversive therapy") OR TITLE‐ABS‐KEY("imaginal exposure therapy")) AND DOCTYPE(ar OR re)) OR ((TITLE‐ABS‐KEY("acceptance and commitment") OR TITLE‐ABS‐KEY("desensitization") OR TITLE‐ABS‐KEY("psychologic desensitization")) AND DOCTYPE(ar OR re)) OR ((TITLE‐ABS‐KEY("self care") OR TITLE‐ABS‐KEY("self management")) AND DOCTYPE(ar OR re)) OR ((TITLE‐ABS‐KEY(education) OR TITLE‐ABS‐KEY(psychoeducation)) AND DOCTYPE(ar OR re)) 1,073,177
#9 (TITLE‐ABS‐KEY(fibromyalgia) OR TITLE‐ABS‐KEY(fibrositis) OR TITLE‐ABS‐KEY(fms)) AND DOCTYPE(ar OR re) 16,778
#10 (TITLE‐ABS‐KEY("randomised controlled trial") OR TITLE‐ABS‐KEY("controlled trial") OR TITLE‐ABS‐KEY(placebo) OR TITLE‐ABS‐KEY("single blind") OR TITLE‐ABS‐KEY("double blind")) AND DOCTYPE(ar OR re) 560,247
#11 (((TITLE‐ABS‐KEY(behavior therapy) OR TITLE‐ABS‐KEY(behaviour therapy) OR TITLE‐ABS‐KEY(cognitive therapy)) AND DOCTYPE(ar OR re)) OR ((TITLE‐ABS‐KEY("behavior therapy") OR TITLE‐ABS‐KEY("behaviour therapy") OR TITLE‐ABS‐KEY("cognitive therapy")) AND DOCTYPE(ar OR re)) OR ((TITLE‐ABS‐KEY("cognitive behavior therapy") OR TITLE‐ABS‐KEY("cognitive behaviour therapy")) AND DOCTYPE(ar OR re)) OR ((TITLE‐ABS‐KEY("acceptance based") OR TITLE‐ABS‐KEY("exposure therapy") OR TITLE‐ABS‐KEY("implosive therapy") OR TITLE‐ABS‐KEY("aversive therapy") OR TITLE‐ABS‐KEY("imaginal exposure therapy")) AND DOCTYPE(ar OR re)) OR ((TITLE‐ABS‐KEY("acceptance and commitment") OR TITLE‐ABS‐KEY("desensitization") OR TITLE‐ABS‐KEY("psychologic desensitization")) AND DOCTYPE(ar OR re)) OR ((TITLE‐ABS‐KEY("self care") OR TITLE‐ABS‐KEY("self management")) AND DOCTYPE(ar OR re)) OR ((TITLE‐ABS‐KEY(education) OR TITLE‐ABS‐KEY(psychoeducation)) AND DOCTYPE(ar OR re))) AND ((TITLE‐ABS‐KEY(fibromyalgia) OR TITLE‐ABS‐KEY(fibrositis) OR TITLE‐ABS‐KEY(fms)) AND DOCTYPE(ar OR re)) AND ((TITLE‐ABS‐KEY("randomised controlled trial") OR TITLE‐ABS‐KEY("controlled trial") OR TITLE‐ABS‐KEY(placebo) OR TITLE‐ABS‐KEY("single blind") OR TITLE‐ABS‐KEY("double blind")) AND DOCTYPE(ar OR re)) 375
PsycINFO via Ovid
| Searches | Results | |
| 1 | (behavior therapy or behaviour therapy).ab. or behavior therapy.sh. or behaviour therapy.sh. or behavior therapy.ti. or behaviour therapy.ti. or cognitive therapy.sh. or cognitive therapy.ab. or cognitive therapy.ti. or cognitive therapy.id. or behavior therapy.id. or behaviour therapy.id. | 29335 |
| 2 | (cognitive behavior therapy or cognitive behaviour therapy).ab. or cognitive behavior therapy.ti. or cognitive behaviour therapy.ti. or cognitive behavior therapy.id. or cognitive behaviour therapy.id. | 5289 |
| 3 | acceptance based.ab. or acceptance based.ti. or acceptance based.id. or exposure therapy.ti. or exposure therapy.ab. or exposure therapy.id. or implosive therapy.ab. or implosive therapy.ti. or implosive therapy.id. or aversive therapy.ab. or aversive therapy.ti. or aversive therapy.id. or imaginal exposure therapy.ab. or imaginal exposure therapy.ti. or imaginal exposure therapy.id. | 1792 |
| 4 | desensitization.ab. or desensitization.ti. or desensitization.id. or psychologic desensitization.ti. or psychologic desensitization.ab. or psychologic desensitization.id. | 5125 |
| 5 | (acceptance and commitment).ab. or (acceptance and commitment).ti. or (acceptance and commitment).id. or cbt.ti. or cbt.ab. or cbt.id. | 8048 |
| 6 | self care.ab. or self care.ti. or self care.id. or self management.ti. or self management.ab. or self management.id. | 9926 |
| 7 | education.ab. or education.ti. or education.id. or psychoeducation.ti. or psychoeducation.ab. or psychoeducation.id. | 234026 |
| 8 | 1 or 2 or 3 or 4 or 5 or 6 or 7 | 279039 |
| 9 | fibromyalgia.ab. or fibromyalgia.ti. or fibromyalgia.id. or fibrositis.ti. or fibrositis.ab. or fibrositis.id. or fms.ab. or fms.ti. or fms.id. or fibromyalgia.sh. | 2364 |
| 10 | randomised controlled trial.af. or randomised controlled trial.pt. or controlled clinical trial.af. or controlled clinical trial.pt. or double blind.af. or single blind.af. or placebo.af. or random$.af. | 330948 |
| 11 | 8 and 9 and 10 | 134 |
National Institutes of Health (NIH)
fibromyalgia and cognitive behavioral therapy and randomised controlled trial 5
fibromyalgia and operant therapy and randomised controlled trial 1
fibromyalgia and behavioral therapy and randomised controlled trial 17
fibromyalgia and self‐management and randomised controlled trial 2
fibromyalgia and acceptance therapy and randomised controlled trial 1
fibromyalgia and commitment therapy and randomised controlled trial 1
Total 27
Clinical Trials Registry Platform (ICTRP)
fibromyalgia and cognitive behavioral therapy and randomised controlled trial 23
fibromyalgia and operant therapy or behavioral therapy and randomised controlled trial 2
fibromyalgia and self‐management and randomised controlled trial 5
fibromyalgia and (acceptance OR commitment) therapy and randomised controlled trial 23
Total 53
Appendix 2. Subgroup analysis according to type of controls
|
Outcome title (End of treatment) |
Number of study arms /patients | SMD (95% CI); P value |
Hetero‐ geneity I2 [%] |
| Attention controls | |||
| Pain Negative mood Disability |
3/161 2/125 2/115 |
‐0.01 (‐0.35 to 0.32); 0.94 ‐0.44 (‐0.85 to ‐0.03); 0.04 0.09 (‐0.33 to 0.51); 0.69 |
5 13 0 |
| Active controls | |||
| Pain Negative mood Disability |
8/481 8/482 6/372 |
‐0.31 (‐0.76 to 0.14); 0.18 ‐0.30 (‐0.67 to 0.06); 0.11 ‐0.43 (‐1.01 to 0.16); 0.15 |
82 74 86 |
| Treatment as usual or waiting list | |||
| Pain Negative mood Disability |
11/811 10/1042 9/747 |
‐0.40(‐0.54 to ‐0.26); < 0.0001 ‐0.30(‐0.46 to ‐0.15); 0.0001 ‐0.31(‐0.48 to ‐0.14); 0.0003 |
0 26 19 |
|
Outcome title (Long term follow‐up) |
Number of study arms /patients | SMD (95% CI); P value |
Hetero‐ geneity I2 [%] |
| Attention control : Only one study available | |||
| Active controls | |||
| Pain Negative mood Disability |
6/379 6/379 5/313 |
‐0.36 (‐0.86 to 0.15); 0.16 ‐0.24 (‐0.76 to 0.28); 0.36 ‐0.23 (‐0.79 to 0.34); 0.43 |
82 82 82 |
| Treatment as usual or waiting list | |||
| Pain Negative mood Disability |
6/353 5/288 4/300 |
‐0.39 (‐0.61 to ‐0.18); 0.0003 ‐0.40 (‐0.68 to 0.06); 0.09 ‐0.49 (‐0.72 to ‐0.26); < 0.001 |
0 70 0 |
Appendix 3. Subgroup analysis according to type of delivery of treatment
|
Outcome title (End of treatment) |
Number of study arms /patients | SMD (95% CI); P value |
Hetero‐ geneity I2 [%] |
| Internet/telephone | |||
| Pain Negative mood Disability |
2/146 1/118 2/146 |
‐0.29 (‐0.65 to 0.07); 0.12 ‐0.09 (‐0.45 to 0.27) ‐0.25 (‐0.58 to 0.07); 0.13 |
8 0 |
| Face‐to face | |||
| Pain Negative mood Disability |
19/1307 18/1531 15/1116 |
‐0.29(‐0.49 to ‐0.10); 0.004 ‐0.35(‐0.51 to ‐0.18); < 0.0001 ‐0.30(‐0.55 to ‐0.06); 0.01 |
65 54 2 |
Abbreviations: CI= Confidence Interval; SMD: standardised mean difference
Appendix 4. Subgroup analysis according to age of participants
|
Outcome title (End of treatment) |
Number of study arms /patients | SMD (95% CI); P value |
Hetero‐ geneity I2 [%] |
|
| Children/adolescents | ||||
| Pain Negative mood Disability |
2/139 2/139 2/139 |
‐0.41 (‐0.74 to ‐0.07); 0.02 ‐0.24 (‐0.57 to 0.20); 0.16 ‐0.31 (‐0.64 to 0.03); 0.07 |
0 0 0 |
|
| Adults | ||||
| Pain Negative mood Disability |
20/1314 18/1515 14/1095 |
‐0.27(‐0.47 to ‐0.07); 0.008 ‐0.35(‐0.52 to ‐0.168; < 0.001 ‐0.30(‐0.54 to ‐0.06); 0.02 |
65 57 72 |
|
Appendix 5. Subgroup analysis according to treatment duration
|
Outcome title (End of treatment) |
Number of study arms /patients | SMD (95% CI); P value |
Hetero‐ geneity I2 [%] |
| < 5 sessions | |||
| Pain Negative mood Disability |
1/28 no data 1/28 |
0.06 (‐0.69 to 0.80) no data ‐0.24 (‐0.99 to 0.50) |
|
| 5‐25 sessions | |||
| Pain Negative mood Disability |
12/855 13/1190 9/722 |
‐0.22(‐0.45 to 0.01); 0.07 ‐0.20(‐0.32 to ‐0.09); 0.0006 ‐0.26(‐0.45 to ‐0.07); 0.008 |
60 0 33 |
| > 25 sessions | |||
| Pain Negative mood Disability |
7/376 6/341 5/290 |
‐0.37 (‐0.80 to 0.06); 0.09 ‐0.72 (‐1.08 to ‐0.36); < 0.0001 ‐0.40 (‐1.18 to 0.38); 0.32 |
75 59 89 |
Appendix 6. Subgroup analysis according to reported treatment quality
|
Outcome title (End of treatment) |
Number of study arms /patients | SMD (95% CI); P value | ||
| Low reported treatment quality | ||||
| Pain Negative mood Disability |
3/163 3/163 2/94 |
0.04 (‐0.32 to 0.41); 0.82 ‐0.08 (‐0.59 to 0.42); 0.74 ‐0.30 (‐0.71 to 0.02); 0.16 |
||
| Moderate reported treatment quality | ||||
| Pain Negative mood Disability |
11/725 9/887 7/563 |
‐0.34(‐0.64 to ‐0.03); 0.03 ‐0.43(‐0.70 to ‐0.15); 0.002 ‐0.43(‐0.87 to 0.02); 0.06 |
||
| High reported treatment quality | ||||
| Pain Negative mood Disability |
9/627 7/599 8/577 |
‐0.31 (‐0.50 to‐0.11); 0.002 ‐0.31 (‐0.50 to ‐0.12); 0.001 ‐0.19 (‐0.43 to 0.05); 0.12 |
||
Appendix 7. Sensitvity analyses
|
Outcome title (End of treatment) |
Number of studies /patients | SMD (95% CI); P value |
Hetero‐ geneity I2 [%] |
| Studies without data extracted from figures and or substituted | |||
| Pain Negative mood Disability |
19/1319 18/1591 14/1162 |
‐0.27 (‐0.47 to ‐0.08); 0.006 ‐0.34 (‐0.50 to ‐0.17); < 0.0001 ‐0.20 (‐0.65 to 0.05); 0.12 |
64 55 76 |
| Studies without selection bias | |||
| Pain Negative mood Disability |
12/966 10/839 10/886 |
‐0.31(‐0.51 to ‐0.11); 0.002 ‐0.24(‐0.38 to ‐0.11); 0.0005 ‐0.30 (‐0.47 to ‐0.13); 0.004 |
53 0 31 |
| Studies without attrition bias | |||
| Pain Negative mood Disability |
11/940 9/828 9/854 |
‐0.33 (‐0.49 to‐0.16); 0.0001 ‐0.32 (‐0.47 to ‐0.18); <0.0001 ‐0.24 (‐0.43 to ‐0.04); 0.02 |
33 2 43 |
| Studies without reporting bias | |||
| Pain Negative mood Disability |
13/1122 15/1123 14/1072 |
‐0.30 (‐0.52 to ‐0.08); 0.007 ‐0.38 (‐0.57 to ‐0.19); < 0.0001 ‐0.28 (‐0.53 to ‐0.03); 0.03 |
67 54 73 |
| Studies with patients with depressive and/or anxiety disorders included | |||
| Pain Negative mood Disability |
11/836 9/772 9/750 |
‐0.18 (‐0.38 to 0.03); 0.09 ‐0.34 (‐0.49 to ‐0.18); < 0.0001 ‐0.14 (‐0.37 to 0.09); 0.23 |
48 9 53 |
| Studies with ≥ 20 patients per treatment arm | |||
| Pain Negative mood Disability |
17/1299 17/1559 13/1116 |
‐0.31 (‐0.52 to ‐0.10); 0.04 ‐0.36 (‐0.53 to ‐0.19); < 0.0001 ‐0.32 (‐0.57 to ‐0.07); 0.01 |
69 59 75 |
Data and analyses
Comparison 1. Cognitive behavioural therapies versus controls at end of treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 21 | 1453 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.29 [‐0.47, ‐0.11] |
| 1.1 Traditional cognitive behavioural therapy | 18 | 1150 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.44, ‐0.15] |
| 1.2 Operant therapy | 2 | 123 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.67 [‐2.56, 1.23] |
| 1.3 Self‐management | 2 | 180 | Std. Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.81, 0.84] |
| 2 Negative mood | 19 | 1649 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.49, ‐0.17] |
| 2.1 Traditional cognitive‐behavioural therapy | 15 | 1010 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.48, ‐0.19] |
| 2.2 Operant therapy | 2 | 124 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐2.21, 0.42] |
| 2.3 Self‐management education | 3 | 515 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.10 [‐0.27, 0.07] |
| 3 Disability | 16 | 1234 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.30 [‐0.51, ‐0.08] |
| 3.1 Traditional cognitive‐behavioural therapy | 13 | 931 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.31 [‐0.45, ‐0.18] |
| 3.2 Operant therapy | 2 | 123 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.88 [‐3.10, 1.33] |
| 3.3 Self‐management education | 2 | 180 | Std. Mean Difference (IV, Random, 95% CI) | 0.07 [‐0.60, 0.74] |
| 4 Self‐efficacy pain | 11 | 1047 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐0.80, ‐0.17] |
| 4.1 Traditional cognitive‐behavioural therapy | 9 | 589 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.72, ‐0.05] |
| 4.2 Operant therapy | 2 | 123 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.18 [‐3.01, 0.64] |
| 4.3 Self‐management education | 1 | 335 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.39, 0.04] |
| 5 Acceptability | 21 | 1914 | Risk Ratio (M‐H, Random, 95% CI) | 0.94 [0.65, 1.35] |
| 5.1 Traditional cognitive‐behavioural therapy | 17 | 1169 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.65, 1.46] |
| 5.2 Operant therapy | 2 | 126 | Risk Ratio (M‐H, Random, 95% CI) | 0.43 [0.03, 7.34] |
| 5.3 Self‐management education | 3 | 619 | Risk Ratio (M‐H, Random, 95% CI) | 1.33 [0.81, 2.19] |
| 6 Fatigue | 11 | 910 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.49, ‐0.02] |
| 6.1 Traditional cognitive‐behavioural therapy | 9 | 667 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.65, ‐0.10] |
| 6.2 Operant therapy | 1 | 63 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.44, 0.62] |
| 6.3 Self‐management education | 2 | 180 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.33, 0.40] |
| 7 Sleep problems | 8 | 422 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.85, 0.05] |
| 7.1 Traditional cognitive‐behavioural therapy | 6 | 244 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐1.11, 0.11] |
| 7.2 Operant therapy | 1 | 60 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.91, 0.17] |
| 7.3 Self‐management education | 1 | 118 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.27, 0.45] |
| 8 Health‐related quality of life | 13 | 1238 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.38, ‐0.08] |
| 8.1 Traditional cognitive‐behavioural therapy | 11 | 778 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [‐0.49, ‐0.18] |
| 8.2 Operant therapy | 1 | 63 | Std. Mean Difference (IV, Random, 95% CI) | 0.19 [‐0.34, 0.72] |
| 8.3 Self‐management education | 2 | 397 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.36, 0.33] |
1.5. Analysis.
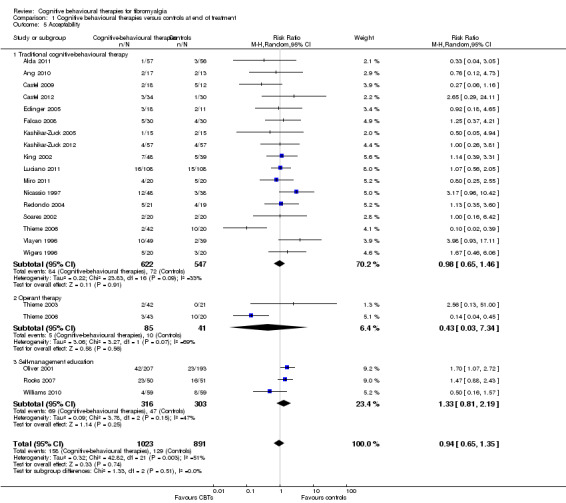
Comparison 1 Cognitive behavioural therapies versus controls at end of treatment, Outcome 5 Acceptability.
1.6. Analysis.
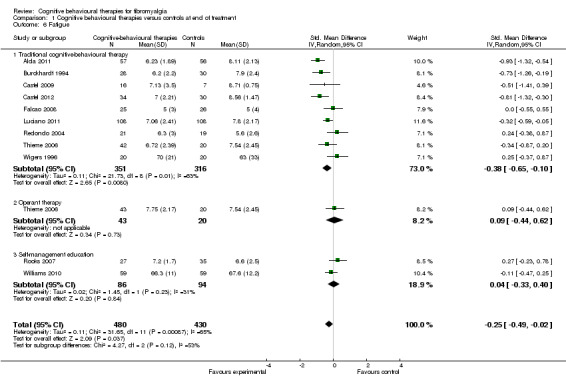
Comparison 1 Cognitive behavioural therapies versus controls at end of treatment, Outcome 6 Fatigue.
1.7. Analysis.
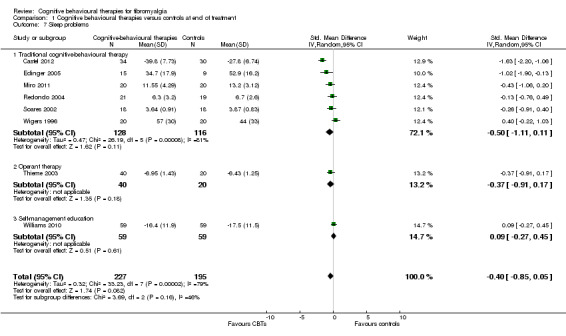
Comparison 1 Cognitive behavioural therapies versus controls at end of treatment, Outcome 7 Sleep problems.
1.8. Analysis.
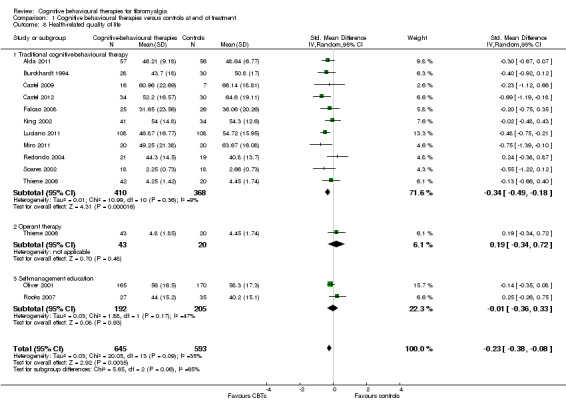
Comparison 1 Cognitive behavioural therapies versus controls at end of treatment, Outcome 8 Health‐related quality of life.
Comparison 2. Cognitive behavioural therapies versus controls at long‐term follow‐up.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 14 | 893 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.62, ‐0.17] |
| 1.1 Traditional cognitive‐behavioural therapy | 13 | 770 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.43, ‐0.14] |
| 1.2 Operant therapy | 2 | 123 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.27 [‐2.30, ‐0.24] |
| 1.3 Self‐management education | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Negative mood | 12 | 792 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.43 [‐0.75, ‐0.11] |
| 2.1 Traditional cognitive‐behavioural therapy | 11 | 669 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.58, 0.02] |
| 2.2 Operant therapy | 2 | 123 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.28 [‐1.97, ‐0.59] |
| 2.3 Self‐management education | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Disability | 10 | 735 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐0.86, ‐0.18] |
| 3.1 Traditional cognitive‐behavioural therapy | 9 | 612 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.55, ‐0.09] |
| 3.2 Operant therapy | 2 | 123 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.68 [‐2.40, ‐0.96] |
| 3.3 Self‐management education | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Self‐efficacy pain | 9 | 617 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.27, ‐0.24] |
| 4.1 Traditional cognitive‐behavioural therapy | 8 | 494 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.52 [‐1.04, ‐0.00] |
| 4.2 Operant therapy | 2 | 123 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.69 [‐2.76, ‐0.62] |
| 4.3 Self‐management education | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Fatigue | 6 | 429 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.77, ‐0.15] |
| 5.1 Traditional cognitive‐behavioural therapy | 6 | 366 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.69, ‐0.07] |
| 5.2 Operant therapy | 1 | 63 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.02 [‐1.59, ‐0.46] |
| 5.3 Self‐management education | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Sleep problems | 7 | 378 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐1.31, 0.03] |
| 6.1 Traditional cognitive‐behavioural therapy | 6 | 318 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐1.11, 0.19] |
| 6.2 Operant therapy | 1 | 60 | Std. Mean Difference (IV, Random, 95% CI) | ‐1.68 [‐2.30, ‐1.06] |
| 6.3 Self‐management education | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Health‐related quality of life | 6 | 425 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.58, 0.21] |
| 7.1 Traditional cognitive‐behavioural therapy | 6 | 362 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.68, 0.11] |
| 7.2 Operant therapy | 1 | 63 | Std. Mean Difference (IV, Random, 95% CI) | 0.39 [‐0.15, 0.92] |
| 7.3 Self‐management education | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
2.3. Analysis.
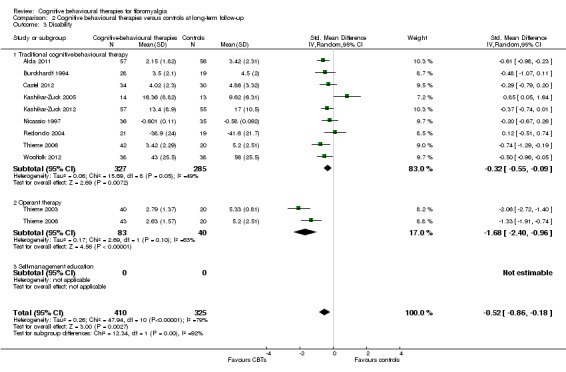
Comparison 2 Cognitive behavioural therapies versus controls at long‐term follow‐up, Outcome 3 Disability.
2.4. Analysis.
Comparison 2 Cognitive behavioural therapies versus controls at long‐term follow‐up, Outcome 4 Self‐efficacy pain.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Alda 2011.
| Methods |
Study setting: Europe (Spain). Multicentre, primary healthcare centres, outpatient based. Patients were recruited from any of the 41 primary healthcare centres in the city of Zaragoza Study design: Parallel Duration therapy: 10‐12 weeks Follow‐up: 6 months Analysis: ANOVA for comparisons between groups (baseline), intention‐to‐treat approach, ANCOVA (covariate: baseline scores) to examine differences among outcomes of the groups post‐treatment and follow‐up, effect sizes (Cohen’s d) |
|
| Participants |
Patients: Treatment Group: 57 patients, 95% female, 100% European, mean age 46 years: mean years since diagnosis:12.9 (7.1) Control Group: 56 patients, 96% female, 100% European, mean age 47 years; mean years since diagnosis: 11.7 (4.0) Inclusion: ACR 1990 criteria for FM, age 18 to 65 years, able to understand and read Spanish, no psychological treatment during the preceding two years, no pharmacological treatment at that time or willing to discontinue it for two weeks before start of the study Exclusion: Axis I psychiatric disorders (dementia, schizophrenia, paranoid disorder, alcohol and/or drug abuse), axis II psychiatric disorders or other medical disorders that prevented patient from following the treatment protocol, women that were pregnant or nursing |
|
| Interventions |
Treatment Group: CBT group: Cognitive restructuring, cognitive and behavioural coping strategies (1.5h/week), total: 15 h Control Group: Treatment as usual (TAU): General practitioners received a guide for the treatment of fibromyalgia in primary care and selected a pharmacological treatment as well as the frequency of patient visits that they considered adequate Co‐medication allowed: Occasionally minor analgesics, no pregabalin, gabapentin, opioids, antidepressants permitted Other co‐therapies: None |
|
| Outcomes |
Primary Outcomes Self reported pain: Visual Analogue Scale pain (VAS pain) 0‐100 Self reported negative mood: Hamilton Rating Scale for Depression (HAM‐D) 0‐50 Self reported disability: FIQ physical disability 0‐10; data provided on request Acceptability: Total dropout rate Secondary Outcomes Self reported self efficacy pain: Pain Catastrophizing Scale (PCS) Helplessness 0‐24 Self reported fatigue: FIQ (VAS) 0‐10; data provided on request Self reported sleep problems: Not assessed Self reported disease‐specific health‐related quality of life: Fibromyalgia Impact Questionnaire (FIQ) total 0‐100 |
|
| Notes | 1. Study arm recommended pharmacological therapy (pregabalin 300‐600 mg/d or duloxetine 60‐120 mg/d) not used for comparison 2. Reasons for dropout: ‐ Treatment Group: 1x lack of efficacy, 4x patient decision, 3x lost to follow‐up ‐ Control group: 1x did not receive allocated intervention because person moved to another city, 2x adverse events, 3x lack of efficacy, 2x patient decision, 2 loss to follow‐up 3. Attendance rates: Not reported 4: Responder analysis: None 5. Funding sources and declaration of interest of the primary researchers: The study was funded by a grant from the Carlos III Health Institute of the Spanish Ministry of Health and Consumption (ETES PI07/90959).The authors declared that they have no competing interests |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random sequence |
| Allocation concealment (selection bias) | Low risk | Allocation sequence generated by a member of research group not involved in study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis by last observation carried forward method |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Blinding of outcome assessor | Low risk | Study personnel who conducted psychological assessments were blinded to participants' treatment conditions |
Ang 2010.
| Methods |
Study setting: North America (USA). Details not reported, Outpatient based Study design: Parallel Duration therapy: 6 weeks Follow‐up: 12 weeks Analysis: T‐test, Chi2 tests, Fisher’s exact test to compare baseline characteristics and FIQ scores at week 12, mixed‐effects linear model approach to compare treatment groups (with random subject effect and fixed effects for treatment group, visit (week 6 or 12), treatment group‐visit interaction, baseline NFR threshold, study entry medications |
|
| Participants |
Patients: Treatment Group: 17 patients 100% female, 81% white, mean age 50.5 years; Years of FMS 11.8 (4.6) Control Group: 15 patients, 100% female, 80% white, mean age 47 years; Years of FMS 12.3 (7.9) Inclusion: ACR 1990 criteria for FM, moderately symptomatic with respect to pain intensity (FIQ pain score >3, FIQ physical impairment score ≥2), female Exclusion: Peripheral neuropathy, diabetes mellitus, demyelinating disorders, inflammatory rheumatic diseases |
|
| Interventions |
Treatment Group: CBT: telephone sessions: time‐contingent activity pacing, pleasant activity scheduling, relaxation, automatic thoughts and pain, cognitive restructuring, stress management + workbook (0,5h/week), total: 3 hours Control Group: TAU: customary care received from subject’s treating physician Co‐medication allowed: Stable doses and regimen of medication throughout the study, 48‐hour washout of NSAIDs prior to nociceptive flexion reflex threshold test Other Co‐therapies: Not reported |
|
| Outcomes |
Primary Outcomes Self reported pain: FIQ pain (VAS) 0‐10 Self reported negative mood: Patient Health Questionnaire 8‐item depression scale (PHQ‐8) 0‐24. Post‐treatment data not reported and not provided on request. Self reported disability: FIQ Physical Impairment 0‐10 Acceptability: Total drop out rate Secondary Outcomes Self reported self efficacy pain: Not assessed Self reported fatigue: FIQ fatigue 0‐10; data provided on request Self reported sleep problems: Not assessed Self reported disease‐specific health‐related quality of life: FIQ total 0‐100 |
|
| Notes |
1. Reasons for dropout (post‐treatment and follow‐up): Treatment Group: 1x refused further follow‐up, 1x nociceptive flexion reflex threshold test too painful; Control Group: 1x refused further follow‐up, 1x nociceptive flexion reflex threshold test too painful 2. Attendance rates: Not reported 3. Responder analysis: 33% of the patients in the CBT group and 15% of the patients in the TAU group reported a ≥ 14% reduction of the FIQ total score 4. Funding sources and declaration of interest of the primary researchers: No details of funding reported. Dr. Ang has received consulting fees (less than $10,000) from Eli Lilly |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detailed information |
| Allocation concealment (selection bias) | Unclear risk | No detailed information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No Intention‐to‐treat analysis |
| Selective reporting (reporting bias) | High risk | Not all outcomes reported and not provided on request |
| Blinding of outcome assessor | Unclear risk | No detailed information |
Burckhardt 1994.
| Methods |
Study setting: Europe (Sweden). Single‐centre, recruitment in occupational and primary health clinics, outpatient based Study design: Parallel Duration therapy: 6 weeks Follow‐up: 3 and 6 months Analysis: ANOVA (FIQ), MANOVA (QOLS‐S, FAI, SELF pain, SELF function, SELF other, 6‐min walk, chair test, myalgic score), if omnibus F significant ANOVA on individual outcomes, independent group t‐tests, paired different t‐tests for within group change |
|
| Participants |
Patients: Treatment group: 28 patients; Control group: 30 patients; Total sample:100% female, 99% white, mean age 46.5 years; Duration of symptoms not reported Inclusion: ACR 1990 criteria for FM, normal results in laboratory tests (haemoglobin, free thyroxine, erythrocyte sedimentation rate, antinuclear antibodies, rheumatoid factor, creatine phosphokinase), understand Swedish Exclusion: Other rheumatic diseases |
|
| Interventions |
Treatment Group: CBT, group: education, relaxation, assertiveness training, coping strategies, problem solving techniques (1x1.5h/week), total: 9h Control Group: Delayed treatment control Co‐medication allowed: Yes, not controlled for Other Co‐therapies: Not reported |
|
| Outcomes |
Primary Outcomes Self reported pain: FIQ pain 0‐10; SD not reported and not provided on request; SDs calculated by imputation method Self reported negative mood: FIQ depression 0‐10; SD not reported and not provided on request; SDs calculated by imputation method Self reported disability: FIQ physical impairment 0‐10; SD not reported and not provided on request; SDs calculated by imputation method Acceptability: Total dropout rate Secondary Outcomes Self reported self efficacy pain: Self‐Efficacy (SES) pain scale 500‐50; SD not reported and calculated by reported P‐value Self reported fatigue: FIQ fatigue 0‐10; SD not reported and not provided on request; SDs calculated by imputation method Self reported sleep problems: Not assessed Self reported disease‐specific health‐related quality of life: FIQ total 0‐80; SD not reported and not provided on request; SDs calculated by imputation method |
|
| Notes | 1. Study arm CBT plus physical therapy not used for comparison 2. Reasons for drop‐out ‐Total sample: 6x did not return for retesting (no separate data reported ‐Experimental groups: 7x did only attend 1 or 2 classes 3. Attendance rates: Not reported 4. Responder analysis: None 5. Funding sources and declaration of interest of the primary researchers: No details reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detailed information |
| Allocation concealment (selection bias) | Unclear risk | No detailed information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No Intention‐to‐treat analysis |
| Selective reporting (reporting bias) | High risk | Except FIQ‐subscale scores all other outcomes not reported in detail at follow‐up and not provided on request |
| Blinding of outcome assessor | Unclear risk | No detailed information |
Castel 2009.
| Methods |
Study setting: Europe (Spain). SIngle centre, recruitment in university pain clinic, outpatient based Study design: Parallel Duration therapy: 12 weeks Follow‐up: None Analysis: T‐tests and Chi2 analysis to compare treatment conditions in demographic and pre‐treatment outcome variables, paired t‐tests to evaluate changes in each condition treatment, univariate analyses to compare between treatments, percentage of patients that experienced a significant change in pain intensity was evaluated, cut‐off of 30% accepted as indicator of clinically important improvement, evaluation of Pearson correlation between hypnotisability and pre‐post‐treatment differences |
|
| Participants |
Patients: Treatment Group: 18 patients, 94% female, race not reported, mean age 43.0 years; pain duration 10.2 (10.7) years Control Group: 12 patients, 86% female, race not reported, mean age 49.6 years; pain duration 7.1 (5.6) years Inclusion: ACR 1990 criteria for FM, age between 18 years and less than 60 years, having a minimum of 6 months history of chronic pain, having at least 6 years of education Exclusion: One or more additional severe chronic medical pain conditions, significant suicidal ideation, severe psychopathology (e. g. psychosis), moderate to severe cognitive impairment, presence of pending litigation |
|
| Interventions |
Treatment Group: CBT, group: didactic presentation of information about FMS and theory of pain perception, relaxation training, cognitive restructuring, assertiveness training, behavioral goal setting, problem solving, training in outcome generalization, maintenance of gains, audio CD of a relaxation exercise to listen to at home (1.5h/week), total: 18h Control Group: TAU: Pharmacological treatment including analgesics, antidepressants, sedatives, myorelaxants Comedication allowed: Standard medication management Other Cotherapies: Not reported |
|
| Outcomes |
Primary Outcomes Self reported pain: Numeric Pain Rating Scale (NPRS) 0‐10 NR Self reported negative mood: FIQ depression 0‐10; Data provided on request Self reported disability: FIQ physical impairment 0‐10; Data provided on request Acceptability: Total dropout rate Secondary Outcomes Self reported self efficacy pain: Not assessed Self reported fatigue: FIQ fatigue 0‐10; data provided on request Self reported sleep problems: Not assessed Self reported disease‐specific health‐related quality of life: FIQ total 0‐100 |
|
| Notes | 1. Study arm CBT plus hypnosis (not used for comparison) 2.Reasons for dropout: Not reported 3. Attendance rates: Not reported 4. Responder analysis: None 5. Funding sources and declaration of interest of the primary researchers: No details reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detailed information |
| Allocation concealment (selection bias) | Unclear risk | No detailed information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No Intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes reported or provided on request |
| Blinding of outcome assessor | Unclear risk | No detailed information |
Castel 2012.
| Methods |
Study setting: Europe (Spain). Single centre, recruitment in university pain clinic, outpatient based Study design: Parallel Duration therapy: 14 weeks Follow‐up: 3 and 6 months Analysis: T‐tests with Bonferroni correction. Linear mixed model analysis (interaction between time and group) for outcome measures |
|
| Participants |
Patients: Treatment Group: 34 patients, 94% female, race not reported, mean age 50 years; pain duration 13.6 (9.2) years Control Group: 30 patients, 100% female, race not reported, mean age 48.7 years; pain duration 11.6 (6.9) years Inclusion: ACR 1990 criteria for FM, age between 18 years and less than 65 years Exclusion: One or more additional severe chronic medical pain conditions, significant suicidal ideation, severe psychopathology (e.g. psychosis), moderate to severe cognitive impairment |
|
| Interventions |
Treatment Group: CBT, group (except session 2):Education on FMS and pain perception theory (Session 1, autogenic training (session 2), cognitive restructuring training (sessions 3‐5), cognitive behavioural training for primary insomnia (sessions 6‐8), assertiveness training (sessions 9‐10), activity pacing and pleasant activity scheduling training (sessions 11‐12), behavioral goal setting (session 13), life values and relapse training (session 14); audio CD of autogenic training to practice at home;1 session/week for 4 weeks (2h/week), total:28h Control Group: TAU: Pharmacological treatment including analgesics, antidepressants, anticonvulsants and myorelaxants, as appropriate Co‐medication allowed: Standard medication management Other Co‐therapies: Not reported |
|
| Outcomes |
Primary Outcomes Self reported pain: Numeric rating scale 0‐10 Self reported negative mood: Hospital Anxiety and Depression Scale (HADS) total score 0‐42 Self reported disability: FIQ physical impairment 0‐10: Data provided on request Acceptability: Total dropout rate Secondary Outcomes Self reported self efficacy pain: Not assessed Self reported fatigue: FIQ Fatigue 0‐10; Data provided on request Self reported sleep problems: SF‐36 sleep problems index 50‐0 Self reported disease‐specific health‐related quality of life: FIQ total score 0‐100 |
|
| Notes | 1. Treatment arm CBT plus hypnosis not used for comparison 2. Reasons for dropout: Not reported 3. Attendance rates: Patients in CBT groups attended a mean of 12.3 (SD 1.7) sessions 4. Responder analysis: 8.8% of the patients in the CBT group reported a ≥30% pain reduction at final treatment and 17.6% at six months follow‐up. 16.7 % of the patients in the TAU group reported a ≥30% pain reduction at final treatment and 13.3% at six months follow‐up. 55.9% of the patients in the CBT group reported a ≥14% reduction of the FIQ total score at final treatment and 58.8% at six months follow‐up. 23.3% of the patients in the TAU group reported a ≥14% reduction in the FIQ total score at final treatment and 20% at six months follow‐up. 5. Funding sources and declaration of interest of the primary researchers: No details reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details reported |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis by last observation carried forward method |
| Selective reporting (reporting bias) | Low risk | All outcomes reported or provided on request |
| Blinding of outcome assessor | Low risk | Psychologist blinded to participants' group assignment |
Edinger 2005.
| Methods |
Study setting: North America (USA). Newspaper recruitment, University centre, outpatient based Study design: Parallel Duration therapy: 6 weeks Follow‐up: 6 months Analysis: 3 (group) x 2 (post‐treatment versus follow‐up) ANCOVA adjusted for baseline values, pair‐wise tests |
|
| Participants |
Patients: Treatment Group: 16 patients, 94% female, 94% white, mean age 50 years; duration of symptoms not reported Control Group: 11 patients, 100% female, 92% white, mean age 48.3 years; duration of symptoms not reported Inclusion: ACR 1990 criteria for FM, aged 21 to 65 yrs,meet structured interview criteria for insomnia, at least 60 minutes of total nocturnal wake time on average over 1 week of sleep log monitoring Exclusion: Pregnancy, breastfeeding, not practicing contraception, co‐morbid sleep‐disruptive medical condition, Axis I depressive (other than dysthymia), anxiety or substance abuse disorder, severe hypnotic dependence, symptoms of sleep apnoea, restless legs syndrome, circadian rhythm disorder, apnoea‐hypopnoea index or periodic limb movement‐related arousal index of 15 or more per hour |
|
| Interventions |
Treatment Group: CBT, group: Insomnia therapy with education and stimulus control (1x1h/wk), total: 6h Control Group: TAU Co‐medication allowed: Yes Other Co‐therapies: Not reported |
|
| Outcomes |
Primary Outcomes Self reported pain: McGill Pain Questionnaire total score (MPQ) 0‐78 Self reported negative mood: Profile of mood states (POMS), range 0‐ 260 Self reported disability: SF‐36 physical functioning 50‐0 not reported and not provided on request Acceptability: Total dropout rate Secondary Outcomes Self reported self efficacy pain: Not assessed Self reported fatigue: Not assessed Self reported sleep problems: Insomnia Symptom Questionnaire (ISQ) 0‐57 Self reported disease‐specific health‐related quality of life: Not assessed |
|
| Notes | 1. Treatment arm sleep hygiene not used for comparison 2. Reasons for dropout: Not reported 3: Attendance rates: 16/18 in the CBT group attended ≥1 treatment session 4: Responder analysis: None 5. Funding sources and declaration of interest of the primary researchers: The study was supported by grant R21‐ AR052368 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases (Dr Edinger). Dr Edinger received honoraria from Fisson Communications, Sepracor, and Axis Healthcare; and Dr Rice has provided expert testimony and medical record review as a defence expert in FM for several attorneys (he is willing to provide further information about the financial details of the testimony on appropriate request) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detailed information |
| Allocation concealment (selection bias) | Unclear risk | No detailed information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No intention‐to‐treat analysis |
| Selective reporting (reporting bias) | High risk | Not all outcomes reported and not provided on request |
| Blinding of outcome assessor | Unclear risk | No detailed information |
Falcao 2008.
| Methods |
Study setting: South America (Brazil). Single centre, recruitment in rheumatology university clinic, outpatient based Study design: Parallel Duration therapy: 10 weeks Follow‐up: 3 months Analysis: Chi2 or student t‐test analyses between groups, analyses of variance (repeated measures) between groups at different time points |
|
| Participants |
Patients: Treatment Group: 30 patients, 100% female, 80% Caucasian, mean age 45 years; disease duration 3.5 (2.4) years Control Group: 30 patients, 100% female, 77% Caucasian, mean age 46 years; disease duration 3.7 (4.8) years Inclusion: ACR 1990 criteria for FM, age 18 to 65 years, female, at least 4 years of formal education (elementary school), patients had not received any kind of treatment for their disease Exclusion: Other rheumatic diseases, known hypersensibility to amitriptyline, cyclobenzaprine or paracetamol, use of psychotropic drugs, psychiatric diseases, work‐related litigation |
|
| Interventions |
Treatment Group: CBT,group: progressive muscle relaxation training, cognitive restructuring, stress management + routine medical visits (3h/week), total: 30h Control Group: TAU: Medication and routine medical visits Co‐medication allowed: Patients in both groups: amitriptyline 12.5mg/day during first week, then increase to 25mg/day, those with intolerance or side effects were given cyclobenzaprine 5mg/day, use of paracetamol was allowed 750mg if patients had pain (max. dose of 2250mg/day) Other Co‐therapies: None |
|
| Outcomes |
Primary Outcomes Self reported pain: VAS pain 0‐10 Self reported negative mood: Beck Depression Inventory (BDI) 0‐54 Self reported disability: FIQ 0‐10; data provided on request Acceptability: Total dropout rate Secondary Outcomes Self reported self efficacy pain: Not assessed Self reported fatigue: FIQ fatigue 0‐10; data provided on request Self reported sleep problems: Not assessed Self reported disease‐specific health‐related quality of life: FIQ total 0‐100 |
|
| Notes | 1. Reasons for dropout: ‐ Experimental group:2x move to another city, 3x gave up after 1st, 5th or 6th CBT session ‐ Control group: 1x use of psychotropic drugs, 3x gave up study at different times 2: Attendance rates: All completers in the CBT group attended more than 80% of the sessions. 3. Responder analysis: None 4. Funding sources and declaration of interest of the primary researchers: No details reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomised by drawing lots with concealed allocation |
| Allocation concealment (selection bias) | Low risk | Yes (see above) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes reported or provided on request |
| Blinding of outcome assessor | Low risk | Evaluation performed by a physiotherapist who was blind to treatment allocation |
Kashikar‐Zuck 2005.
| Methods |
Study setting: North America (USA).Single centre, paediatric rheumatology non‐university clinic, outpatient based Study design: Cross‐over Duration therapy: 8 weeks Follow‐up: None Analysis: T‐ tests for pre‐treatment differences, repeated measures ANOVA 2 (baseline versus time 2) x 2 (CBT versus control), within‐subjects effects, difference between 2 conditions based upon sequence of treatment delivery by analysis with proc mixed model, effect sizes (time 1 to time 2, time 2 to time 3, time 1 to time 3) |
|
| Participants |
Patients: Total group: 14 patients each in both groups; 100% female, 93% white, mean age 15.8 years; duration of symptoms not reported Inclusion: Age between 13 and 17 years, Juvenile Primary Fibromyalgia criteria, stabilization on medication for at least 4 weeks prior to enrolment, VAS pain 0‐10 score at least 3 an a 10 cm VAS, functional disability score greater than 7 (mild disability) Exclusion: Co‐morbid rheumatic disease, developmental delay or impairment, major depressive disorder |
|
| Interventions |
Treatment Group: CBT, single, group and with parents: Relaxation, distraction, activity pacing, cognitive and problem‐solving techniques (1x1.5h/week), total: 12h Control Group: Active control, single: Self‐monitoring with diary Co‐medication allowed: Yes (standard medical care) Other Co‐therapies: Not reported |
|
| Outcomes |
Primary Outcomes Self reported pain: VAS pain 0‐10 Self reported negative mood: Children’s Depression Inventory (CDI) T score Self reported disability: Functional Disability Inventory (FDI) 0‐60 Acceptability: Total dropout rate Secondary Outcomes Self reported self efficacy pain: Pain Coping Questionnaire (PCQ) 1‐5 Self reported fatigue: Not assessed Self reported sleep problems: Not assessed Self reported disease‐specific health‐related quality of life: Not assessed |
|
| Notes | 1. Reasons for dropout: ‐ Treatment Group: 1x due to distance ‐ Control Group:1x no contact, 1x family issues 2. Attendance rates: 90% of all participants attended all sessions 3.Responder analysis: None 4. Funding sources and declaration of interest of the primary researchers: Supported by grants of the Cincinnati Children's Hospital Reserach Foundation and National Institutes Helath Grant 1P60AR47784‐01) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated pseudo‐random number list |
| Allocation concealment (selection bias) | Low risk | 1:1 allocation ratio for the subjects as a single block was used. An assistant who was blinded enrolled the subject and opened a sealed envelope with the subject's study number |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Blinding of outcome assessor | Low risk | An assistant and a rheumatologist who were blinded |
Kashikar‐Zuck 2012.
| Methods |
Study setting: North America (USA). Multicentre paediatric university rheumatology centres, outpatient based Study design: Parallel Duration therapy: 8 weeks Follow‐up: 6 months Analysis: Mixed modelling for repeated measures with fixed factors being group, time and group‐by‐time interaction, intention‐to‐treat |
|
| Participants |
Patients: Treatment Group: 57 patients; 95% female, 84% white, mean age 15.2 years; disease duration 3.3 (3.1) years Control Group: 57 patients; 90% female, 97% white, mean age 14.9 years; disease duration 2.5 (1.8) years Inclusion: Juvenile FM classification criteria determined by a paediatric rheumatologist, age 11 to 18, stable medication for 8 weeks, willing to continue receiving stable medication for the duration of the study, average pain severity ≥4 on VAS 0‐10 based on 1 week of daily pain diaries, score of >7 on FDI Exclusion: Other rheumatic disease (juvenile arthritis, lupus), documented developmental delay, current panic disorder or major depression or lifetime bipolar disorder or psychosis, use of opioids |
|
| Interventions |
Treatment Group: CBT, individual: Education about rationale for behavioural pain management, training in relaxation, distraction, activity pacing, problem solving, using calming statements, relapse prevention strategies; parents included in 3 out of 8 sessions: training in behavioural management techniques (1x45min/week), total: 6h Control Group: Active control: FMS education, individual: education and discussion about FM, pain medications, general lifestyle issues (diet, sleep, exercise), impact of juvenile’s FM on patient’s lifestyle; parents attended 3 out of 8 sessions (1x45min/week), total: 6h Co‐medication allowed: Yes, 9 patients were prescribed new antidepressant medication Other Co‐therapies: Not reported |
|
| Outcomes |
Primary Outcomes Self reported pain: VAS pain 0‐10 Self reported negative mood: CDI T score Self reported disability: Functional Disability Index (FDI) 0‐60 Acceptability: Total dropout rate Secondary Outcomes Self reported self efficacy pain: Not assessed Self reported fatigue: Not assessed Self reported sleep problems: VAS sleep 0‐10 Self reported disease‐specific health‐related quality of life: Not assessed |
|
| Notes | 1. Reasons for dropout: ‐ Treatment group: 1x time constraints, 1x family reasons, 1x loss to follow‐up, 1x psychiatric hospitalisation for non‐study related reasons, 6 months follow‐up 3x lost to follow‐up; ‐Control group: 2x dropped out before attending any sessions, other reasons for drop‐out not reported, 6‐months follow‐up: 3x lost to follow‐up 2: Attendance rates: Not reported 3. Responder analysis: 14.0% in the CBT‐group and 8.6% in the control group reported a ≥30% pain reduction 4. Funding sources and declaration of interest of the primary researchers:Supported by the National Institute of Arthtitis and Musculoskeletal and Skin Diseases grant R01‐AR‐0500208 to Dr. Kashikar‐Zuck. Dr Passo received consulting fees, speaking fees and/or honoraria from Pfizer (less than 10 000$) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization based upon a computer generated randomisation list, stratified by site |
| Allocation concealment (selection bias) | Low risk | Treatment allocation by biostatistician |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Intention‐to‐treat‐analysis reported, but no imputation methods for dropouts used |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Blinding of outcome assessor | Low risk | Study uses a single‐blind design, assessment staff were all blinded to the patients' treatment condition |
King 2002.
| Methods |
Study setting: North America (Canada). Single university centre, self referral or referral by rheumatologists, outpatient based Study design: Parallel Duration therapy: 12 weeks Follow‐up: 3 months Analysis: ANOVA with repeated measures (group versus time), significant results were examined using Tukey multiple comparisons, independent t‐tests and Chi2 tests to compare demographic and baseline variables |
|
| Participants |
Patients: Treatment group; 48 patients; 100% female, race not reported, mean age 44.9 years; duration of symptoms 10.9 (10.7) years Control Group: 39 patients; 100% female, race not reported, mean age 47.3 years; duration of symptoms 9.6 (7.9) years Inclusion: ACR 1990 criteria for FM, age between 18‐65, diagnosis confirmed by rheumatologist, female Exclusion: Any conditions that precluded ability to exercise (severe cardiac arrhythmia, dizziness, severe shortness of breath), inflammatory arthritis, systemic lupus erythematosus, rheumatoid arthritis |
|
| Interventions |
Treatment Group: CBT, group: Self management strategies, information about cause of FMS, goal setting, maximizing energy for household chores or personal activities, pain or fatigue coping strategies, benefits of exercise, evaluating alternative therapies, barriers to behavior change (1.5h‐2h/week), total: 18h‐24h Control Group: Delayed treatment control Co‐medication allowed: Instruction not to change medication, but if participants documented changes in their usual treatment and it was not major they remained in the study Other Co‐therapies: Not reported |
|
| Outcomes |
Primary Outcomes Self reported pain: FIQ pain 0‐10 not reported Self reported negative mood: FIQ depression 0‐10 not reported and not provided on request Self reported disability: FIQ physical impairment 0‐10 not reported and not provided on request Acceptability: Total dropout rate Secondary Outcomes Self reported self efficacy pain: Chronic Pain Self Efficacy Scale (CPSS) pain: 10‐0 Self reported fatigue: FIQ fatigue 0‐10 not reported and not provided on request Self reported sleep problems: Not assessed Self reported disease‐specific health‐related quality of life: FIQ 0‐80 |
|
| Notes | 1. Study arms exercise and exercise plus education not used for comparison) 2. Reasons for dropout: ‐ Treatment and control group: End of treatment and follow up: Not reported 3. Attendance rate: 83.3% (SD 10.8) of the sessions in CBT group were attended 4. Responder analysis: None 5. Funding sources and declaration of interest of the primary researchers: Funded by grants from the Medical Services Incorporated Foundation and from the Health Services Research and Innovation Fund, Alberta Health |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random assignment was done in blocks of 4 to 16 subjects |
| Allocation concealment (selection bias) | Unclear risk | No detailed information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis by baseline carried forward method |
| Selective reporting (reporting bias) | High risk | Not all outcomes reported and not provided on request. |
| Blinding of outcome assessor | Low risk | Both assessors were blinded to the subjects' group randomisation |
Luciano 2011.
| Methods |
Study setting: Europe (Spain). Multicentre, general practitioners, outpatient based Study design: Parallel Duration therapy: 8 weeks Follow‐up: None Analysis: T‐tests and Chi2 to examine baseline differences, repeated measures analysis of variance (factor 1: intervention and control, factor 2: pretreatment and post‐treatment), comparison of baseline differences between responders and non‐responders |
|
| Participants |
Patients: Treatment Group: 108 patients; 97% female, race not reported, mean age 55.2 years; years since diagnosis 15.2 (11.7) Control Group: 108 patients; 98% female, race not reported, mean age 55.4 years; years since diagnosis 14.3 (10.6) Inclusion: ACR 1990 criteria for FMS, age 18 to 75 years Exclusion: Diagnosis of FM based on ACR 1990 criteria, cognitive impairment, presence of physical/psychiatric limitations that impeded participation in the study assessments, life expectancy of less than 12 months, absence of schooling |
|
| Interventions |
Treatment Group: CBT: information about symptoms, usual course, comorbid medical conditions, potential causes of illness, influence of psychosocial factors on pain, current pharmacological and non‐pharmacological treatments, benefits of regular exercise, autogenic training (2h/week), total: 16h Control Group: TAU: pharmacological treatment and counselling about aerobic exercise Co‐medication allowed: Yes Other Co‐therapies: Not reported |
|
| Outcomes |
Primary Outcomes Self reported pain: FIQ pain 0‐10 Self reported negative mood: FIQ depression 0‐10 Self reported disability: FIQ physical impairment 0‐10 Acceptability: Total dropout rate Secondary Outcomes Self reported self efficacy pain: Not assessed Self reported fatigue: FIQ general fatigue 0‐10 Self reported sleep problems: Not assessed Self reported disease‐specific health‐related quality of life: FIQ total 0‐80 |
|
| Notes | 1. Reasons for dropout (both groups): 16x not interested in the study, 2x family burden, 2x not able to comply with the treatment schedule, 1x relocation 2. Attendance rates: not reported 3:Responder analysis: 35% of the patients in the CBT groups and 17% of the patients in the TAU group reported a ≥20% reduction of the FIQ total score at final treatment 4. Funding sources and declaration of interest of the primary researchers:The research project and Nuria Martınez’s pre‐doctoral contract were funded by a grant from the “Agencia d’Avaluacio´ de Tecnologia i Recerca Mediques” (AATRM 077/25/06). Juan V. Luciano received a postdoctoral contract from the “Instituto de Salud Carlos III” (Red RD06/0018/0017) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomisation list |
| Allocation concealment (selection bias) | Unclear risk | No details reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis by baseline carried forward method |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Blinding of outcome assessor | Low risk | Research assistant was not involved in the treatment and was blind to group allocation |
Miro 2011.
| Methods |
Study setting: Europe (Spain). Single centre, university rheumatology and pain department, outpatient based Study design: Randomized, parallel Duration therapy: 6 weeks Follow‐up: No Analysis: T‐test, Mann‐Whitney U, Chi² to compare baseline measures, ANCOVA 2 (alerting signal) x 3 (orienting cue) x 2 (congruency) x 2 (time) with age as covariate, repeated measures ANOVA (CBT vs SH x time, pre vs post‐treatment), calculation of effect sizes (Cohen’s d), paired comparisons of sign. effects with student’s t test, Reliable Change Index for clinical variables that changed over time as a result of therapy and Pearson’s analysis |
|
| Participants |
Patients: Treatment Group: 20 patients, 100% female, race not reported, mean age 44.0 years; duration FMS 4.2 (3.4) years Control Group: 20 patients, 100% female, race not reported, mean age 50.2 years; duration FMS 4.7 (4.3) years Inclusion: ACR 1990 criteria for FM, criteria for insomnia Exclusion: 25 to 60 years, insomnia/cognitive dysfunction were better explained by being pregnant, medical history of significant head injury, neurological disorder, major concomitant medical condition, major depressive disorder with suicide ideation, other major axis I diagnosis, symptoms of sleep disruptive co‐morbidities with insomnia, apnoea‐hypopnoea index or periodic limb movement‐related arousal index of 15 or more per hour of sleep, severe hypnotic dependence (use of hypnotic in a higher than recommended dosage or repeated episodes of rebound insomnia on withdrawal), being treated with another psychological or physical therapy at the moment of the study |
|
| Interventions |
Treatment Group: CBT, group: Education, sleep restriction, stimulus control instructions, relaxation training, cognitive therapy for dysfunctional beliefs related to insomnia (1.5h/week), total: 9h Control Group: Active control: Sleep hygiene, group: education sleep hygiene rules (1,5h/wk), total: 9h Co‐medication allowed: Stable doses of medication Other Co‐therapies: None |
|
| Outcomes |
Primary Outcomes Self reported pain: MPQ VAS pain 1‐10 Self reported negative mood: Hospital Anxiety and Depression Scale (HADS) subscale depression 0‐21 Self reported disability: FIQ impairment 0‐10 not reported and not provided on request Acceptability: Total dropout rate Secondary Outcomes Self reported self efficacy pain: Not assessed Self reported fatigue: FIQ fatigue 0‐10 not reported and not provided on request Self reported sleep problems: Pittsburgh Sleep Quality Index (PSQI) 0‐21 Self reported disease‐specific health‐related quality of life: FIQ 0‐100 |
|
| Notes | 1. Reasons for dropout: ‐Treatment Group: 1x did not receive CBT due to changes in work time, 1x did not attend assessment at end of treatment ‐ Control Group: 2x did not attend assessment at end of treatment 2: Attendance rates: Not reported 3. Responder analysis: 60% of patients in the CBT‐group and 30% of the patients in the control group clinically significant (% not reported) reduction of the FIQ daily functioning score at end of treatment 4. Funding sources and declaration of interest of the primary researchers: The study was financially supported by the Spanish Ministry of Science and Innovation (research projects SEJ2006‐07513, PSI2008‐03595PSIC and PSI2009‐1365PSIC) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Simple randomisation (1:1) was implemented by a computerised number generator designed by a researcher with no clinical involvement in the trial |
| Allocation concealment (selection bias) | Low risk | Yes (see above) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No intention‐to‐treat analysis |
| Selective reporting (reporting bias) | High risk | Not all outcomes reported and not provided on request |
| Blinding of outcome assessor | Low risk | The assessment of the outcome measures was performed by an examiner who was blinded to group assignment |
Nicassio 1997.
| Methods |
Study setting: North America (USA). Single centre university psychiatry department, outpatient based, referral by community, private or university rheumatology clinics, FMS support groups Study design: Parallel Duration therapy: 10 weeks Follow‐up: 6 months Analysis: MANOVA between groups at pre‐treatment on clinical criteria and intervening variables, MANOVA between treatment conditions and across time (pre‐, post‐treatment, follow‐up periods) on clinical criteria and intervening variables (helplessness, active coping, passive coping, quality of social support), change score correlations to see whether helplessness and passive coping mediate changes, regression analysis (predictors: helplessness, passive coping) |
|
| Participants |
Patients: Total group: 89% female, 86% white, mean age 53.1 years; CBT group 36 patients, control group 35 patients; duration of symptoms not reported Inclusion: Diagnosis of FM confirmed by rheumatologist according to ACR 1990 criteria, stabilization on medication for at least 2 months prior to the study, support person who would be willing to participate in the study Exclusion: Concomitant rheumatologic conditions, cardiovascular disease, central nervous system disorders, psychosis, bipolar illness |
|
| Interventions |
Treatment Group: CBT, group: education, relaxation, activity pacing, pain coping, involvement of support person reinforcing adherence to protocol (1x1.5h/week), total: 15h Control Group: Attention control, group: lectures, group discussion, support (1.5h/week), total: 15h Co‐medication allowed: Yes, not controlled for Other Co‐therapies: Not reported |
|
| Outcomes |
Primary Outcomes Self reported pain: Pain index composed by (a) pain scale of the FIQ, b) number of discrete body areas endorsed as painful form a human figure drawing, c) pain rating index of MPQ, d) flare index: frequency times the squared average intensity of pain flares over previous month; no minimum and maximum scores available Self reported negative mood: Center for Epidemiological Studies‐Depression Scale (CES‐D) 0‐60 Self reported disability: Quality of well‐being 1‐0 Acceptability: Total dropout rate Secondary Outcomes Self reported self efficacy pain: Pain Management Inventory Subscale active coping Self reported fatigue: Not assessed Self reported sleep problems: Not assessed Self reported disease‐specific health‐related quality of life: Not assessed |
|
| Notes | 1. Reasons for dropout: not reported 2. Attendance rates: Not reported 3: Responder analysis: None 4. Funding sources and declaration of interest of the primary researchers: Multipurpose Arthritis and Musculoskeletal Diseases Center Grant AR40770 and grant from the General Clinical Research Centers M01RR00827 of the MCRR from the US National Institute of Health |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Subjects were randomly assigned with the aid of a random number table from within blocks to the interventions |
| Allocation concealment (selection bias) | Unclear risk | No detailed information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Blinding of outcome assessor | Unclear risk | No detailed information |
Oliver 2001.
| Methods |
Study setting: North America (USA). Multicentre, recruitment via health maintenance organisation, university psychology department, outpatient based Study design: Parallel Duration therapy: 12 months and 2 weeks Follow‐up: None Analysis: Chi2 test and ANOVA to examine pre‐existing differences on demographic characteristics, ANCOVA to examine 3 (group) x 2 (time of assessment) interactions |
|
| Participants |
Patients: Treatment Group: 207 patients; 96% female, 85% Caucasian, mean age 55.1 years; duration of FMS symptoms 14.4 (14.2) years Control Group: 193 patients; 94% female, 100% European, mean age 52.9 years; duration of FMS‐symptoms 11.7 (12.1) years Inclusion: ACR 1990 criteria for FM, diagnosis by a physician Exclusion: Not reported |
|
| Interventions |
Treatment Group: Self management education program: Social support and education, group: tasks aimed at promoting empathy and sharing coping techniques, health education in lecture format (2h/session), total: 20h Control Group: Non‐treatment control, details not reported (TAU?) Co‐medication allowed: NR Other Co‐therapies: NR |
|
| Outcomes |
Primary Outcomes Self reported pain: FIQ pain 0‐10 not reported and not provided on request Self reported negative mood: CES‐D 0‐60 Self reported disability: Not assessed Acceptability: Total dropout rate Secondary Outcomes Self reported self efficacy pain: SES pain 100‐0 Self reported fatigue: FIQ sleep 0‐10 not reported and not provided on request Self reported sleep problems: Not assessed Self reported disease‐specific health‐related quality of life: FIQ 0‐100 |
|
| Notes | 1. Study arm social support not used for comparison 2. Reasons for dropout: ‐ Control group: 42x no reason, 8x not interested, 5x moved, 9x inconvenience, 4x other, 4x surgery/illness Control Group: 6x no reason, 8x not interested, 4x moved, 4x other, 1x surgery/illness ‐ Treatment Group: 207/165 (80%), reasons for dropout: 42x no reason, 8x not interested, 5x moved, 9x inconvenience, 4x other, 4x surgery/illness; Control Group: 193/170 (88%), reasons for dropout: 6x no reason, 8x not interested, 4x moved, 4x other, 1x surgery/illness 3: Attendance rates: Patients in the CBT group attended an average total of 8.4 (SD 6.2) of the 20 meetings 4: Responder analysis: None 5. Funding sources and declaration of interest of the primary researchers: NIH grant AR‐440020 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detailed information |
| Allocation concealment (selection bias) | Unclear risk | No detailed information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No intention‐to‐treat analysis |
| Selective reporting (reporting bias) | High risk | Not all outcomes reported and not provided on request |
| Blinding of outcome assessor | Unclear risk | No detailed information |
Redondo 2004.
| Methods |
Study setting: Europe (Spain). Single centre, referral by general practitioners, rheumatology university, outpatient based Study design: Parallel Duration therapy: 8 weeks Follow‐up: 12 months Analysis: Chi2 and Fisher exact tests to compare categorical variables between groups, t‐test to compare means for independent variables, paired t‐tests to compare paired variables, intention‐to‐treat |
|
| Participants |
Patients: Total group: 100% female, race not reported, mean age not reported; 21 patients in CBT and 19 patients in control group; duration of symptoms not reported Inclusion: ACR 1990 criteria for FM Exclusion: Serious concomitant disease |
|
| Interventions |
Treatment Group: CBT, group: Education, relaxation, coping with pain and daily activities, problem solving, prevention of relapses (1x2.5h/week), total: 20h Control Group: Active control: pool and cycle ergometer aerobic training (5x45 min/week), total: 30h Co‐medication allowed: Flexible medication with NSAID, amitriptyline and acetaminophen allowed Other Co‐therapies: Not reported |
|
| Outcomes |
Primary Outcomes Self reported pain: VAS pain 0‐10 Self reported negative mood: BDI 0‐54 Self reported disability: SF 36 Physical functioning 50‐0 Acceptability: Total dropout rate Secondary Outcomes Self reported self efficacy pain: Chronic Pain Self Efficacy Scale (CPSS) 10‐0 Self reported fatigue: VAS sleep 0‐10 Self reported sleep problems: VAS fatigue 0‐10 Self reported disease‐specific health‐related quality of life: FIQ 0‐100 |
|
| Notes | 1. Reasons for dropout: ‐Treatment Group: 2x no subjective improvement with proposed treatment, 1x move, 2x did not complete entire evaluation ‐ Control Group: 2x concomitant illnesses, 2x did not complete entire evaluation 2. Attendance rates: 72.1% (SD 24.2) of the CBT sessions were attended 3.Responder analysis: None 4. Funding sources and declaration of interest of the primary researchers: No details reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization by means of a random numbers table |
| Allocation concealment (selection bias) | Unclear risk | No detailed information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis by baseline carried forward method |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Blinding of outcome assessor | Unclear risk | No detailed information |
Rooks 2007.
| Methods |
Study setting: North America (USA). Multicentre (2 community fitness facilities, one hospital wellness centre), outpatient based, recruitment via general practitioners, letters Study design: Parallel Duration therapy: 16 weeks Follow‐up: 6 months Analysis: Paired t‐test or Kruskal‐Wallis for within‐group changes, analyses of variance for multiple comparisons to compare mean changes scores across the groups, 2‐sample t‐test, Wilcoxon rank sum test and Fisher exact test to compare baseline values and demographic variables between completers and non‐completers |
|
| Participants |
Patients: Treatment group: 51 patients; 100% female, 93% White, mean age 51 years; years since diagnosis 6 (5) Control Group: 50 patients; 100% female, 83% White, mean age 48 years; years since diagnosis 5 (4) Inclusion: ACR 1990 criteria for FM, diagnosis confirmed by primary care physician, age 18‐75 years Exclusion: Medical conditions that limited a person’s ability to perform the exercise protocol or for whom moderate‐level exercise was contraindicated |
|
| Interventions |
Treatment Group: Self management education program, group: basic self‐management techniques to accomplish daily activities, manage symptoms, suggested ways to incorporate wellness activities (2h/session): 16h Control Group: Active control: Aerobic and flexibility exercise (1h/session), total: 32h Co‐medication allowed: Not reported Other Co‐therapies: Not reported |
|
| Outcomes |
Primary Outcomes Self reported pain: VAS pain 0‐10 Self reported negative mood: BDI 0‐54 Self reported disability: SF 36 physical function 50‐0 Acceptability: Total dropout rate Secondary Outcomes Self reported self efficacy pain: SES pain 100‐0 Self reported fatigue: FIQ fatigue 0‐10 Self reported sleep problems: Not assessed Self reported disease‐specific health‐related quality of life: FIQ 0‐100 |
|
| Notes | 1. Study arms strength training, and combination a of strength training, aerobic, flexibility exercise not used for comparison 2. Reasons for dropout: ‐ Treatment Group: 7x dissatisfied with randomisation, 7x schedule conflicts, 6x lost to follow‐up, 1x other health problems, 1x travel issues, 1x FMS pain ‐ Control Group: 5x lost to follow‐up, 4x other health problems, 4x schedule conflicts, 1x travel issues, 1x in a randomisation group, 1x FMS pain 3: Attendance rates: Mean attendance rate 77% in CBT and 73% in aerobic exercise group 4. Responder analysis: 20% of the patients in the CBT group and 25% of the patients in the combined group (CBT plus exercise) reported ≥20% reduction of the FIQ‐total score at final treatment 5. Funding sources and declaration of interest of the primary researchers: The study was supported by an Arthritis Foundation Investigator Award (Dr Rooks) and National Institutes of Health grants K23 AR48305 (Dr Rooks), RO3 AR047398 (Dr Rooks), K24 AR02123 (Dr Katz), P60 AR47782 (Dr Iversen and Katz), and RR01032 (Dr Gautan). Financial disclosure: None reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated single‐page listings of random group assignment |
| Allocation concealment (selection bias) | Low risk | Individual pages were placed in an opaque envelopes, sealed, numbered sequentially and stored in a locked cabinet |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Intention‐to‐treat analyses (BOCF) reported, but only outcomes of completers reported |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Blinding of outcome assessor | Unclear risk | No detailed information |
Soares 2002.
| Methods |
Study setting: Europe (Sweden). Single centre, psychology university department, outpatient based; referral by general practitioners Study design: Parallel Duration therapy: 10 weeks Follow‐up: 6 months Analysis: 3x2 repeated measures ANOVA (group: treatment, control, waiting list control group x test occasion: pre, post), 2x3 ANOVA (group: treatment, control x test occasion: pre, post, follow‐up), one‐tailed t‐tests, Chi² tests |
|
| Participants |
Patients: Total group: 100% female, race not reported, mean age 45 years; 18 patients each in CBT and control group; duration pain 3.4 (3.2) years in CBT and 4.1 (3.8) in control group Inclusion: Diagnosis of FM in the past 2 years according to ACR 1990 criteria by rheumatologist, female, age between 18 and 64 years Exclusion: Other serious illnesses (e.g. other rheumatic diseases), ongoing alcohol or drug abuse, receiving other therapies |
|
| Interventions |
Treatment Group: CBT, single and group: education, problem solving, pain and self management (5x1h individual, 15x2h group), total: 120h Control Group: Attention control, group: education, discussion (2x2 h individual, 15x2h group), total: 102h Co‐medication allowed: No Other Co‐therapies: No |
|
| Outcomes |
Primary Outcomes Self reported pain: MPQ total 0‐78 Self reported negative mood: FIQ depressed mood 0‐10 not reported and not provided on request Self reported disability: FIQ disability 0‐10 not reported and not provided on request Acceptability: Total dropout rate Secondary Outcomes Self reported self efficacy pain: ASES pain: 100‐10 Self reported fatigue: FIQ Fatigue 0‐10 not reported and not provided on request Self reported sleep problems: Karolinska Sleep Questionnaire (KSQ) sleep quality 0‐75 Self reported disease‐specific health‐related quality of life: FIQ 0‐80 |
|
| Notes | 1. Waiting list control not used for comparison because of lack of follow‐up assessment 2.Reasons for dropout (only for total sample reported): declined participation after randomisation 3. Attendance rates: Not reported 4. Responder analysis: None 5. Funding sources and declaration of interest of the primary researchers: No details reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details reported |
| Allocation concealment (selection bias) | Unclear risk | No detailed information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis by baseline carried forward method |
| Selective reporting (reporting bias) | High risk | Follow‐up data not reported in total |
| Blinding of outcome assessor | Unclear risk | No detailed information |
Thieme 2003.
| Methods |
Study setting: Europe (Germany). Single rheumatology centre, inpatient based, recruitment from the regular patients of a hospital for rheumatic diseases Study design: Parallel Duration therapy: 5 weeks Follow‐up: 6 and 15 months Analysis: Repeated measures analysis of variances, t‐tests, effect‐sizes, reliable change index |
|
| Participants |
Patients: Treatment Group: 42 patients, 100% female, race not reported, mean age 47 years; duration pain 17.1 (7,1) years Control Group: 21 patients, 100% female, race not reported, mean age 49 years; duration pain 15.6 (6.3) years Inclusion: ACR 1990 criteria for FM Exclusion: Inflammatory cause of the pain, neurologic complications, duration pain < 4 months, pregnancy, severe somatic diseases, major psychiatric disorder, problems with German language |
|
| Interventions |
Treatment Group: Operant therapy: Education; structured time‐contingent exercises, reduction of medication, increase of bodily activity, reduction of interference of pain with daily activities; reduction of health care utilisation; time contingent exercises and intake and reduction of medication, assertiveness training, 5 weeks: 75 h Control Group: Active control: Education, antidepressants, passive physical therapy exercises: 5 weeks: 75 h Co‐medication allowed: Yes, intake management part of therapy Other Co‐therapies: Yes |
|
| Outcomes |
Primary Outcomes Self reported pain: Multidimensional Pain Inventory (MPI), pain intensity 0‐6 Self reported negative mood: MPi affective distress 0‐6 Self reported disability: MPI Interference 0‐6 Acceptability: Total dropout rate Secondary Outcomes Self reported self efficacy pain: MPI self‐efficacy 0‐6 Self reported fatigue: Not assessed Self reported sleep problems: Sleep behaviour (hours) Self reported disease‐specific health‐related quality of life: Not assessed |
|
| Notes | 1. Reasons for dropout: Treatment group: 1 x severe depressive episode, 1 x bipolar disorder Control Group: no dropouts 2: Attendance rate: Not reported 3. Responder analysis: 65% of patients in the OBT group and 0% of the patients in the control group reported a clinically relevant reduction (based on the reliability of change index) of the MPI pain interference score at final treatment 4. Funding sources and declaration of interest of the primary researchers: No details reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detailed information |
| Allocation concealment (selection bias) | Unclear risk | No detailed information |
| Incomplete outcome data (attrition bias) All outcomes | High risk | No intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Blinding of outcome assessor | Unclear risk | No detailed information |
Thieme 2006.
| Methods |
Study setting: Europe (Germany). Single university psychology centre, outpatient based; referral by rheumatologists Study design: Parallel Duration therapy: 15 weeks Follow‐up: 6 and 12 months Analysis: MANOVA, ANOVA, t‐tests, effect sizes |
|
| Participants |
Patients: CBT Group: 42 patients,100% female, race not reported, mean age 49 years; duration pain 9.1 (8.5) years Operant therapy Group: 43 patients, 100% female, race not reported, mean age 43 years; duration pain 9 (10.1) years Control Group: 40 patients, 100% female, race not reported, mean age 48 years; duration pain 8.7 (8.8) years Inclusion: ACR 1990 criteria for FM, pain for a period of at least 6 months, married, willingness of the spouse to participate, ability to complete the questionnaires and understand the treatment components Exclusion: Inflammatory rheumatic diseases and any concurrent major somatic disease (e.g. cancer, diabetes) |
|
| Interventions |
Treatment Group: CBT: Problem‐Solving, stress and pain coping strategies, relaxation, education, homework. 15 weekly 2‐hour sessions: 30 hours Operant therapy: Changing observable pain behaviours, punishment, video feedback of expressions of pain, contingent positive reinforcement of pain‐incompatible behaviours, time contingent exercises and intake and reduction of medication, increase of bodily activity, role‐plays,15 weekly 2‐hour sessions: 30 hours Control Group: Attention control: general discussions in groups around medical and psychosocial problems of FM,15 weekly 2‐hour sessions: 30 hours Co‐medication allowed: Yes, intake management part of therapy Other Co‐therapies: Yes |
|
| Outcomes |
Primary Outcomes Self reported pain: MPI, pain intensity 0‐6 Self reported negative mood: MPI Affective Distress 0‐6 Self reported disability: FIQ physical impairment 0‐10 Acceptability: Total dropout rate Secondary Outcomes Self reported self efficacy pain: Pain‐related self statements scale (PRSS) active coping scale range; data provided on request Self reported fatigue: FIQ fatigue 0‐10. Data provided on request Self reported sleep problems: Not assessed Self reported disease‐specific health‐related quality of life: FIQ‐Total 0‐8, data provided on request |
|
| Notes | 1. Reasons for dropout: CBT Treatment Group: 2x depression, OBT Treatment Group: 2x Major depression, 1x lack of motivation Control Group: 20xdetoriation of symptoms 2: Attendance rate: 95.7% of the sessions were attended and 94.5% of the homework was completed in the OBT group. 86.8% of the sessions were attended and 94.3% of the homework was completed in the CBT group. 3. Responder analysis: 53.5% of patients in the OBT group, 45.2% of the patients in the CBT group and 5.0% of the patients in the control group reported a ≥ 50% pain reduction at 12 months follow‐up. 56.1% of patients in the OBT group, 36.1% of the patients in the CBT group and 7.5% of the patients in the control group reported a ≥ 50% reduction of the FIQ physical impairment score at 12 months follow‐up (Thieme 2007) 4. Funding sources and declaration of interest of the primary researchers: This study was supported by grants from the Deutsche Forschungsgemeinschaft to KT (Th 899‐1/2 and 899‐2/2) and HF (FL 156/26, Clinical Research Unit 107 'Learning, plasticity and pain'), the Max‐Planck Award for International Cooperation to HF, and the National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases to DCT (AR44724 and AR 47298) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detailed information |
| Allocation concealment (selection bias) | Unclear risk | No detailed information |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis by last observation carried forward method |
| Selective reporting (reporting bias) | Low risk | All outcomes reported or provided on request |
| Blinding of outcome assessor | Unclear risk | No detailed information |
Vlayen 1996.
| Methods |
Study setting: Europe (Netherlands). Single university psychology centre, outpatient based; referral by rheumatology department of general hospital Study design: Parallel Duration therapy: 6 weeks Follow‐up: 6 and 12 months follow‐up Analysis: Comparisons between groups (baseline): Univariate analysis of variance, chi‐square. Group differences: ANCOVA, MANCOVA. Reliability of change index |
|
| Participants |
Patients: Treatment Group: 49 patients, 93% female, race not reported, mean age 45 years; duration pain 10.4 (7.7) years Control Group: 43 patients, 82% female, race not reported, mean age 43 years; duration pain 10.2 (8.8) years Inclusion: ACR 1990 criteria for FM, age 18‐65 years Exclusion: Illiteracy, pregnancy, substance abuse, involvement in any litigation concerning disability income, medical disorders and acute diseases, use of supportive equipment for ambulation, severe psychopathology |
|
| Interventions |
Treatment Group: CBT: Education and Information, reconceptualization, skills acquisition and generalization phase. Relaxation, Homework.12 sessions a 90 min: 18 hours Control Group: Active control: Education and information on chronic pain; physical exercise, intensity and duration not reported, at the end of the session; 12 sessions a 90 min: 18 hours Co‐medication allowed: Not reported Other Co‐therapies: Not reported |
|
| Outcomes |
Primary Outcomes Self reported pain: McGill Pain Questionnaire (MPQ), present rating intensity 0‐100 Self reported negative mood: Beck Depression Inventory (BDI) 0‐ 63 Self reported disability: Not assessed Acceptability: Total dropout rate Secondary Outcomes Self reported self efficacy pain: Coping strategies questionnaire (CSQ) 7‐1 Self reported fatigue: Not assessed Self reported sleep problems: Not assessed Self reported disease‐specific health‐related quality of life: Not assessed |
|
| Notes | 1. Waiting list control not used for comparison, because outcomes not reported at follow‐up 2. Reasons for dropout: ‐ CBT group: 6x by treatment staff because of group cohesion difficulties: 3x absence during 3 consecutive sessions: 1x worsening of health condition ‐ WLC: 1x refusal to comply with assessments 3: Attendance rates: Not reported 4. Responder analysis: 23.9% of the patients in the CBT group and 35.9% of the patients in the control group reported a clinically‐relevant change (based on the reliability of change index) in the composite main outcome measure (pain coping and control, relaxation, tension, headache) at final treatment. 5. Funding sources and declaration of interest of the primary researchers: Supported by grant 28‐2055 from the Dutch Prevention Fund |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No detailed information |
| Allocation concealment (selection bias) | Unclear risk | No detailed information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear information |
| Selective reporting (reporting bias) | High risk | Not all CSQ subscales reported |
| Blinding of outcome assessor | Unclear risk | No detailed information |
Wigers 1996.
| Methods |
Study setting: Europe (Norway). Single centre, Physical medicine and psychiatry centre, outpatient based; recruitment from local patient association and physical outpatient clinics Study design: Parallel Duration therapy: 14 weeks Follow‐up: 4 years Analysis: Comparisons between groups (baseline): t‐tests, ANOVA. Group differences: regression analysis. Kruskal‐Wallis, Mann‐Whitney. Responder analysis |
|
| Participants |
Patients: Treatment Group: 20 patients 90% female, race not reported, mean age 44 years; symptom duration 11 (10) years Control Group: 20 patients, 95% female, race not reported, mean age 46 years; symptom duration 11 (9) years Inclusion: Diagnostic criteria of Smythe and Yunus Exclusion: None |
|
| Interventions |
Treatment Group: CBT: Stress Mangement Treatment (SMT): cognitive behavioural stress management package including relaxation and coping strategies. Total hours: 30 Control Group: TAU Co‐medication allowed: Allowed, but no details reported Other Co‐therapies: Not reported |
|
| Outcomes |
Primary Outcomes Self reported pain: Visual Analogue Scale pain (VAS pain) 0‐ 10 Self reported negative mood: Visual Analogue Scale Depression (VAS) 0‐10 Self reported disability: Not assessed Acceptability: Total dropout rate Secondary Outcomes Self reported self efficacy pain: Not assessed Self reported fatigue: Visual Analogue Scale (VAS) 0‐10 Self reported sleep problems: Visual Analogue Scale (VAS) 0‐10 Self reported disease‐specific health‐related quality of life: Not assessed |
|
| Notes | 1. Treatment arm aerobic exercise not used for comparison 2. Reasons for dropout: ‐ Treatment Group: 2 x Moved, 1x acquired Cancer, 1x transport problems, 2x initiated additional treatments (2). Follow‐up: 1 x Moved, 1x not wishing to participate (1) Control Group: 3x initiated additional treatments follow‐up: 4x moved 3. Attendance rates: 68% in the CBT group 4. Responder analysis: 20% of the patients in the CBT group and 5% of the patients in the TAU group reported a ≥30% pain reduction at final treatment. 5. Funding sources and declaration of interest of the primary researchers: The study was supported by the Research Counsil of Norway (101417/320) and the Norwegian Fibromyalgia Association |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization by drawing lots |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Blinding of outcome assessor | Unclear risk | No details reported |
Williams 2010.
| Methods |
Study setting: North America (USA). Single university research centre, outpatient based, referral by primary or specialist care physician Study design: Parallel Duration therapy: 6 months Follow‐up: None Analysis: Comparisons between groups (baseline): t‐tests and Chi‐quadrat, intention‐to‐treat approach, ANCOVA, responder analysis to anyone who reported 30% improvement in pain, an 0.50 SD in physical function via Fisher's exact test |
|
| Participants |
Patients: Treatment Group: 59 patients, 95% female, 98% white, mean age 50 years; duration FM 9.5 (6.9) years Control Group: 59 patients, 95% female, 97% white, mean age 51 years; duration FM 9.3 (6.1) years Inclusion: ACR 1990 criteria for FM, age > 18 years, under standard medical care of a physician the last 3 months prior to assessment, possess basic computer literacy and access Exclusion: Severe physical impairment, co‐morbid medical illnesses capable of causing a worsening of physical functional status independent of FM, any present psychiatric disorder involving psychosis, suicide attempts or current risk, substance abuse, prior CBT for pain management, pending status associated with disability compensation or receipt of disability compensation in the last 2 years |
|
| Interventions |
Treatment Group: Self management education program: Web‐enhanced Behavioral Self‐Management: website included 13 modules segregated into: educational lectures, education, behavioral and cognitive skills to help with symptom management, behavioral and cognitive skills to facilitate adaptive life style changes for managing FM. Each module featured a video lecture. Total hours: NA, but mean number of skills used every month and in total Control Group: TAU Co‐medication allowed: Yes Other Co‐therapies: Not reported |
|
| Outcomes |
Primary Outcomes Self reported pain: Pain Severity scale of the Brief Pain Inventory (BPI) 0‐10 Self reported negative mood: CED‐S 0‐60 Self reported disability: SF‐36 Physical Functioning Scale 50‐0 Acceptability: Total dropout rate Secondary Outcomes Self reported self efficacy pain: Not assessed Self reported fatigue: Multidimensional Fatigue Inventory (MFI) 4‐20 Self reported sleep problems: MOS Sleep Scale 50‐0 Self reported disease‐specific health‐related quality of life: Not assessed |
|
| Notes | 1. Reasons for dropout: ‐ Treatment Group: 1xmedical complications, 3xpersonal choice ‐ Control Group: 1x medical complications, 6x personal choice 1x relocation 2. Attendance rate: 89‐94% of the patients in the self management education group used at least 1 skill by month 3. Responder analysis: 29% of the patients in the self management education group and 8% of the patients in the TAU group reported a ≥30% pain reduction at final treatment. 31% of the patients in the self management education group and 6% of the patients in the TAU group reported a ≥0.5 SD improvement of the subscale physical functioning of the SF‐36 at end of treatment 4. Funding sources and declaration of interest of the primary researchers: Supported in part by Grant numbers R01‐AR050044 (NIAMS/ NIH), and DAMD 17‐00‐2‐0018 (Department of Defense). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation program |
| Allocation concealment (selection bias) | Low risk | Allocation sequence was done by computerised randomisation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | BOCF was used in ITT analysis for missing endpoint values |
| Selective reporting (reporting bias) | Low risk | All outcomes reported |
| Blinding of outcome assessor | Low risk | Study personnel were blinded |
Woolfolk 2012.
| Methods |
Study setting: North America (USA). Single university psychology centre, outpatient based; referral by rheumatologists Study design: Parallel Duration therapy: 10 weeks Follow‐up: 9 months Analysis: Comparisons between groups (baseline): t‐tests and Chi‐quadrat, intention‐to‐treat approach in all analysis, one‐way analysis of co‐variances with one fixed‐effect was conducted on the primary outcome measure (VAS) at post‐treatment, Hedges g. Data were also examined using the perspective of clinical significance (30% improvement) |
|
| Participants |
Patients: Treatment Group: 38 patients, 89% female, 79% white, mean age 48 years Control Group: 38 patients, 87% female, 74% white, mean age 50 years Average widespread pain 11.5 years for both groups Inclusion: ACR 1990 criteria for FM, age 18 to 70 years Exclusion: pain from traumatic injury or structural or regional rheumatic disease, rheumatoid arthritis, inflammatory arthritis, autoimmune disease, unstable medical or psychiatric illness, active suicidal ideation, a history of psychosis, current psychoactive substance dependence, medication regimen not stable last 2 months. Pregnancy. Participation in psychotherapy |
|
| Interventions |
Treatment Group: CBT: Individually administered CBT, group: relaxation, activity regulation, facilitation of emotional awareness, cognitive restructuring, interpersonal communication training. Total hours: Not reported Control Group: Unaugmented TAU Co‐medication allowed: Not reported Other Co‐therapies: Not reported |
|
| Outcomes |
Primary Outcomes Self reported pain: VAS pain 0‐10 Self reported negative mood: Beck Depression Inventory (BDI) 0‐42; Outcomes incompletely reported (non means, only F‐ and P‐values) and not provided on request Self reported disability: Medical Outcomes Study (MOS) SF‐36 Physical Functioning Scale 50‐0; Mean extracted from figure; SD not provided on request and calculated by reported P value Acceptability: Total dropout rate Secondary Outcomes Self reported self efficacy pain: Pain‐Management Subscale of the Chronic Pain Self Efficacy Scale (CPSE): 10‐0 Mean extracted from figure; Self reported fatigue: Not assessed Self reported sleep problems: Not assessed Self reported disease‐specific health‐related quality of life: Not assessed |
|
| Notes | 1. Reasons for dropout: Not reported for both groups 2. Attendance rate: Not reported 3. Responder analysis: 65.% of the patients in the CBT group and 5.2% of the patients in the TAU group reported a ≥30% pain reduction at end of treatment 4. Funding sources and declaration of interest of the primary researchers: No details reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated random number sequence |
| Allocation concealment (selection bias) | Unclear risk | Not reported in detail |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention to‐treat analysis. Missing data were imputed by last observation carried forward method |
| Selective reporting (reporting bias) | High risk | Outcome depression not reported |
| Blinding of outcome assessor | Low risk | Study personnel masked to participants' treatment condition |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Anderson 2007 | No randomisation |
| Carleton 2011 | Attention modification training in a laboratory; delivery was not from, or supervised by, a healthcare professional qualified in psychology, psychiatry or psychosomatic medicine |
| De Voogd 2003 | No randomisation |
| Garcia 2006 | Less than 10 patients per treatment arm |
| Goeppinger 2009 | Did not meet the criteria of self management. No modelling as supplied by the facilitators |
| Goldenberg 1994 | No randomisation |
| Haugli 2008 | No separate data of FM‐patients presented; not provided on request |
| Langford 2010 | Combination of cognitive behavioural therapy with interpersonal (psychodynamic) therapy |
| Lommel 2011 | No randomisation |
| Lorig 2008 | Details of FM diagnosis not reported |
| Martinez‐Valero 2008 | < 10 patients per treatment arm |
| Solomon 2002 | No separate data of FM patients presented; not provided on request |
| Stuifbergen 2010 | Details of FM diagnosis not reported and not provided on request |
| Van Oosterwijck 2013 | Pain physiology education without self‐management education |
| Williams 2002 | Outcomes reported not suited for meta‐analysis: 25% of the patients in CBT group and 12% of the patients in the TAU group reported improvement of ≥ 6.5 points on the physical component score of the SF‐36. Outcomes suited for meta‐analysis not provided on request |
Characteristics of studies awaiting assessment [ordered by study ID]
Jensen 2012.
| Methods | Not yet assessed |
| Participants | 43 FM patients |
| Interventions | Cognitive behavioural therapy compared to waiting list control |
| Outcomes | Not yet assessed |
| Notes | New study |
Martinez 2013.
| Methods | Not yet assessed |
| Participants | 64 FM patients with insomnia |
| Interventions | Cognitive behavioural therapy for insomnia compared to sleep hygiene group |
| Outcomes | Not yet assessed |
| Notes | New study |
Sanchez 2012.
| Methods | Not yet assessed |
| Participants | 26 FM patients |
| Interventions | Cognitive behavioural therapy for insomnia compared to sleep hygiene group |
| Outcomes | Not yet assessed |
| Notes | New study |
Wicksell 2012.
| Methods | Not yet assessed |
| Participants | 40 FM patients |
| Interventions | Acceptance and commitment therapy |
| Outcomes | Not yet assessed |
| Notes | New study |
Differences between protocol and review
To establish consistency between the SOF table and major outcomes, pain self efficacy was changed from a major to minor outcome. We restricted the subgroup and sensitivity analyses to the major outcomes pain, negative mood and disability at end of treatment, except the subgroup comparison of the different types of CBTs which also included these outcomes at long‐term follow‐up. We did not meta‐regress duration of therapy with the major outcome measures pain, mood and disability but performed a subgroup analysis of short‐term (5 to 25 sessions) and long‐term CBTs studies (> 25 sessions). We did not meta‐regress the incremental year of study publication with the major outcome measures pain, mood and disability. We did not perform a sensitivity analysis by excluding studies with low treatment quality. Based on the comments of the reviewers, we changed the classification of control groups for subgroup analysis and we performed an analysis of all types of CBTs pooled together compared to all types of control groups pooled together for the three major outcomes of pain, mood and disability excluding studies with < 20 participants per treatment arm in accordance with the Cochrane reviews on psychological therapies for the management of chronic pain in children and adolescents (Eccleston 2012) and in adults (Williams 2012).
Contributions of authors
Kathrin Bernardy a. Protocol review and revision b. Systematic review study selection, methodology, data extraction, analysis and interpretation, and co‐author of review Petra Klose a. Protocol review proof b. Search of literature c. Systematic review study selection, data extraction, revision and review final proof Angela J Busch a. Topic conception and methodological aspects b. Protocol review proof c. Systematic review dispute resolution for methodology, review of final interpretations of findings, and review final proof Ernest HS Choy a. Protocol review proof b. Review of final interpretations of findings and of final proof
Winfried Häuser a. Topic conception and methodological aspects b. Protocol development and revision c. Systematic review study selection, data analysis, systematic review dispute resolution for methodological quality and data extraction, interpretation of findings, and author of review
Sources of support
Internal sources
None, Other.
External sources
None, Other.
Declarations of interest
WH received honoraria for educational lectures from Abott, Eli‐Lilly, Janssen‐Cilag, Mundipharma and Pfizer. WH serves on the advisory board panel of Daiichi Sankyo. KB received a travel grant from Pfizer and WH from Eli‐Lilly. EC has served on advisory panels of Daiichi Sankyo, Pierre Fabre Medicament, Jazz Pharmaceutical, Allergan and Pfizer. EC has also lectured in meetings organised by Pierre Fabre Medicament, Eli Lilly and Pfizer. The Rheumatology Department of EC received a research grant from Pierre Fabre Medicament. The funding was not used for the production of the review. AB and PK have no conflicts of interest to declare.
None of the authors has done or plans to do a study in this field.
None of the authors received any funding for this review.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Alda 2011 {published and unpublished data}
- Alda M, Luciano JV, Andrés E, Serrano‐Blanco A, Rodero B, Hoyo YL, et al. Effectiveness of cognitive behaviour therapy for the treatment of catastrophisation in patients with fibromyalgia: a randomised controlled trial. Arthritis Research Therapy 2011;13:R173. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ang 2010 {published data only}
- Ang DC, Chakr R, Mazzuca S, France CR, Steiner J, Stump T. Cognitive‐behavioral therapy attenuates nociceptive responding in patients with fibromyalgia: a pilot study. Arthritis Care Research (Hoboken) 2010;62:618‐23. [DOI] [PubMed] [Google Scholar]
Burckhardt 1994 {published data only}
- Burckhardt CS, Mannerkopi K, Hedenberg L, Bjelle A. A randomized, controlled clinical trial of education and physical training for women with fibromyalgia. Journal of Rheumatology 1994;21:714‐20. [PubMed] [Google Scholar]
Castel 2009 {published and unpublished data}
- Castel A, Salvat M, Sala J, Rull M. Cognitive‐behavioural group treatment with hypnosis: A randomized pilot trial in fibromyalgia. Contemporary Hypnosis 2009;26:48‐59. [Google Scholar]
Castel 2012 {published and unpublished data}
- Castel A, Cascón R, Padrol A, Sala J, Rull M. Multicomponent cognitive‐behavioral group therapy with hypnosis for the treatment of fibromyalgia: long‐term outcome. Journal of Pain 2012;13:255‐65. [DOI] [PubMed] [Google Scholar]
Edinger 2005 {published data only}
- Edinger JD, Wohlgemuth WK, Krystal AD, Rice JR. Behavioral insomnia therapy for fibromyalgia patients: a randomized clinical trial. Archives of Internal Medicine 2005;165:2527‐35. [DOI] [PubMed] [Google Scholar]
Falcao 2008 {published and unpublished data}
- Falcao DM, Sales L, Leite JR, Feldman D, Valim V, Natour J. Cognitive‐behavioral therapy for the treatment of fibromyalgia syndrome: A randomized controlled trial. Journal of Musculoskeletal Pain 2008;16:133‐40. [Google Scholar]
Kashikar‐Zuck 2005 {published data only}
- Kashikar‐Zuck S, Swain NF, Jones BA, Graham TB. Efficacy of cognitive‐behavioral intervention for juvenile primary fibromyalgia syndrome. Journal of Rheumatology 2005;32:1594‐602. [PubMed] [Google Scholar]
Kashikar‐Zuck 2012 {published data only}
- Kashikar‐Zuck S, Ting TV, Arnold LM, Bean J, Powers SW, Graham TB, et al. Cognitive behavioral therapy for the treatment of juvenile fibromyalgia: a multi‐site, single‐blind, randomized, controlled clinical trial. Arthritis Rheumatism 2012;64:297‐305. [DOI] [PMC free article] [PubMed] [Google Scholar]
King 2002 {published data only}
- King SJ, Wessel J, Bhambhani Y, Sholter D, Maksymowych W. The effects of exercise and education, individually or combined, in women with fibromyalgia. Journal of Rheumatology 2002;29:2620‐7. [PubMed] [Google Scholar]
Luciano 2011 {published data only}
- Luciano JV, Martínez N, Peñarrubia‐María MT, Fernández‐Vergel R, García‐Campayo J, Verduras C, et al. FibroQoL Study Group. Effectiveness of a psychoeducational treatment program implemented in general practice for fibromyalgia patients: a randomized controlled trial. Clinical Journal of Pain 2011;27:383‐91. [DOI] [PubMed] [Google Scholar]
Miro 2011 {published data only}
- Miró E, Lupiáñez J, Martínez MP, Sánchez AI, Díaz‐Piedra C, Guzmán MA, Buela‐Casal G. Cognitive‐behavioral therapy for insomnia improves attentional function in fibromyalgia syndrome: a pilot, randomized controlled trial. Journal of Health Psychology 2011;16:770‐82. [DOI] [PubMed] [Google Scholar]
Nicassio 1997 {published data only}
- Nicassio PM, Radojevic V, Weisman MH, Schuman C, Kim J, Schoenfeld‐Smith K, Krall T. A comparison of behavioral and educational interventions for fibromyalgia. Journal of Rheumatology 1997;24:2000‐7. [PubMed] [Google Scholar]
Oliver 2001 {published data only}
- Oliver K, Cronan TA, Walen HR, Tomita M. Effects of social support and education on health care costs for patients with fibromyalgia. Journal of Rheumatology 2001;28:2711‐9. [PubMed] [Google Scholar]
Redondo 2004 {published data only}
- Redondo JR, Justo CM, Moraleda FV, Velayos YG, Puche JJ, Zubero JR, et al. Long‐term efficacy of therapy in patients with fibromyalgia: a physical exercise‐based program and a cognitive‐behavioral approach. Arthritis Rheumatism 2004;51:184‐92. [DOI] [PubMed] [Google Scholar]
Rooks 2007 {published data only}
- Rooks DS, Gautam S, Romeling M, Cross ML, Stratigakis D, Evans B, et al. Group exercise, education, and combination self‐management in women with fibromyalgia: a randomized trial. Archives of Internal Medicine 2007;12:2192‐200. [DOI] [PubMed] [Google Scholar]
Soares 2002 {published data only}
- Soares JJF, Grossi G. A randomised, controlled comparison of educational and behavioral interventions for woman with fibromyalgia. Scandinavian Journal of Occupational Therapy 2002;9:35‐45. [Google Scholar]
Thieme 2003 {published data only}
- Thieme K, Gromnica‐Ihle E, Flor H. Operant behavioral treatment of fibromyalgia: a controlled study. Arthritis & Rheumatism 2003;43:314‐20. [DOI] [PubMed] [Google Scholar]
Thieme 2006 {published and unpublished data}
- Thieme K, Flor H, Turk DC. Psychological pain treatment in fibromyalgia syndrome: Efficacy of operant behavioral and cognitive behavioral treatments. Arthritis Research & Therapy 2006;8:R121. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vlayen 1996 {published data only}
- Vlaeyen JW, Teeken‐Gruben NJ, Goossens ME, Mölken MP, Pett RA, Eek H, Heuts PH. Cognitive‐educational treatment of fibromyalgia: A randomized clinical trial. I. Clinical effects. Journal of Rheumatology 1996;23:1237‐45. [PubMed] [Google Scholar]
Wigers 1996 {published data only}
- Wigers SH, Stiles TC, Vogel PA. Effects of aerobic exercise versus stress management treatment in fibromyalgia. Scandinavian Journal of Rheumatology 1996;25:77‐86. [DOI] [PubMed] [Google Scholar]
Williams 2010 {published data only}
- Williams DA, Kuper D, Segar M, Mohan N, Sheth M, Clauw DJ. Internet‐enhanced management of fibromyalgia: a randomized controlled trial. Pain 2010;151:694‐702. [DOI] [PMC free article] [PubMed] [Google Scholar]
Woolfolk 2012 {published data only}
- Woolfolk RL, Allen LA, Apter JT. Affective‐cognitive behavioral therapy for fibromyalgia: a randomized controlled trial. Pain Research Treatment 2012;2012:937873. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Anderson 2007 {published data only}
- Anderson FJ, Winkler AE. An integrated model of group psychotherapy for patients with fibromyalgia. International Journal of Group Psychotherapy 2007;57:451‐74. [DOI] [PubMed] [Google Scholar]
Carleton 2011 {published data only}
- Carleton RN, Richter AA, Asmundson GJ. Attention modification in persons with fibromyalgia: a double blind, randomized clinical trial. Cognitive Behaviour Therapy 2011;40:279‐90. [DOI] [PubMed] [Google Scholar]
De Voogd 2003 {published data only}
- Voogd JN, Knipping AA, Blécourt ACE, Rijswijk MH. Treatment of fibromyalgia syndrome with psychomotor therapy and marital counselling. Journal of Musculoskeletal Pain 2003;1:273‐80. [Google Scholar]
Garcia 2006 {published data only}
- Garcia J, Simon MA, Duran M, Canceller J, Aneiros FJ. Differential efficacy of a cognitive‐behavioral intervention versus pharmacological treatment in the management of fibromyalgic syndrome. Psychology, Health & Medicine 2006;11:498‐50. [DOI] [PubMed] [Google Scholar]
Goeppinger 2009 {published data only}
- Goeppinger J, Lorig KR, Ritter PL, Mutatkar S, Villa F, Gizlice Z. Mail‐delivered arthritis self‐management tool kit: A randomized trial and longitudinal followup. Arthritis & Rheumatism 2009;61:867‐75. [DOI] [PubMed] [Google Scholar]
Goldenberg 1994 {published data only}
- Goldenberg DL, Kaplan KH, Nadeau MG, Brodeur C, Smith S, Schmid CH. A controlled study of stress‐reduction, cognitive‐behavioral treatment program in fibromyalgia. Journal of Musculoskeletal Pain 1994;2:53‐66. [Google Scholar]
Haugli 2008 {published data only}
- Haugli L, Steen E, Laerum E, Nygard R, Finset A. Pychological distress and employment status. Effects of a group learning programme for patients with chronic musculoskeletal pain. Psychology, Health & Medicine 2003;8:135‐48. [Google Scholar]
Langford 2010 {published data only}
- Langford. The efficacy of a combined cognitive‐behavioural and interpersonal therapy approach to the treatment of fibromyalgia syndrome: A randomized controlled trial. Dissertation Abstracts International: Section B: The Sciences and Engineering 2010;71:2052. [Google Scholar]
Lommel 2011 {published data only}
- Lommel K, Bandyopadhyay A, Martin C, Kapoor S, Crofford L. A pilot study of a combined intervention for management of juvenile primary fibromyalgia symptoms in adolescents in an inpatient psychiatric unit. International Journal of Adolescent Medicine and Health 2011;23:193‐7. [DOI] [PubMed] [Google Scholar]
Lorig 2008 {published data only}
- Lorig KR, Ritter PL, Laurent DD, Plant K. The internet‐based arthritis self‐management program: a one‐year randomized trial for patients with arthritis or fibromyalgia. Arthritis & Rheumatism 2008;15:1009‐17. [DOI] [PubMed] [Google Scholar]
Martinez‐Valero 2008 {published data only}
- Martínez‐Valero C, Castel A, Capafons A, Sala J, Espejo B, Cardeña E. Hypnotic treatment synergizes the psychological treatment of fibromyalgia: a pilot study. The American Journal of Clinical Hypnosis 2008;50:311‐21. [DOI] [PubMed] [Google Scholar]
Solomon 2002 {published data only}
- Solomon DH, Warsi A, Brown‐Stevenson T, Farrell M, Gauthier S, Mikels D, Lee TH. Does self‐management education benefit all populations with arthritis? A randomized controlled trial in a primary care physician network. Journal of Rheumatology 2002;29:362‐8. [PubMed] [Google Scholar]
Stuifbergen 2010 {published data only}
- Stuifbergen AK, Blozis SA, Becker H, Phillips L, Timmerman G, Kullberg V, et al. A randomized controlled trial of a wellness intervention for women with fibromyalgia syndrome. Clinical Rehabilitation 2010;24:305‐18. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van Oosterwijck 2013 {published data only}
- Oosterwijck J, Meeus M, Paul L, Schryver M, Pascal A, Lambrecht L, Nijs J. Pain Physiology Education Improves Health Status and Endogenous Pain Inhibition in Fibromyalgia: A Double‐Blind Randomized Controlled Trial. Clin J Pain 2013;Jan 30:epub ahead of print. [DOI] [PubMed] [Google Scholar]
Williams 2002 {published data only}
- Williams DA, Cary MA, Groner KH, Chaplin W, Glazer LJ, Rodriguez AM, Clauw DJ. Improving physical functional status in patients with fibromyalgia: a brief cognitive behavioral intervention. Journal of Rheumatology 2002;29:1280‐6. [PubMed] [Google Scholar]
References to studies awaiting assessment
Jensen 2012 {published data only}
- Jensen KB, Kosek E, Wicksell R, Kemani M, Olsson G, Merle JV, et al. Cognitive Behavioral Therapy increases pain‐evoked activation of the prefrontal cortex in patients with fibromyalgia. Pain 2012;153:1495‐503. [DOI] [PubMed] [Google Scholar]
Martinez 2013 {published data only}
- Martínez MP, Miró E, Sánchez AI, Díaz‐Piedra C, Cáliz R, Vlaeyen JW, Buela‐Casal G. Cognitive‐behavioral therapy for insomnia and sleep hygiene in fibromyalgia: a randomized controlled trial. J Behav Med 2013;Jun 7:epub ahead of print. [DOI] [PubMed] [Google Scholar]
Sanchez 2012 {published data only}
- Sanchez A, Diaz‐Piedra C, Miro E, Martinez MP, Galvez R, Buela‐Casal G. Effects of cognitive‐behavioral therapy for insomnia on polysomnographic parameters in fibromyalgia patients. International Journal of Clinical and Health Psychology 2012;12:39‐53. [Google Scholar]
Wicksell 2012 {published data only}
- Wicksell RK, Kemani M, Jensen K, Kosek E, Kadetoff D, Sorjonen K, et al. Acceptance and commitment therapy for fibromyalgia: A randomized controlled trial. European Journal of Pain 2013;17(4):599‐611. [DOI] [PubMed] [Google Scholar]
Additional references
Altman 2003
- Altman DG, Bland JM. Interaction revisited: the difference between two estimates. BMJ 2003;326(7382):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
Begg 1994
- Begg CB, Mazumdar M. Operating characteristics of a rank correlation test for publication bias. Biometrics 1994;50(4):1088‐101. [PubMed] [Google Scholar]
Bennett 2006
- Bennett R, Nelson D. Cognitive behavioral therapy for fibromyalgia. Nature Clinical Practice. Rheumatology 2006;2:416‐24. [DOI] [PubMed] [Google Scholar]
Bernardy 2010
- Bernardy K, Füber N, Köllner V, Häuser W. Efficacy of cognitive‐behavioral therapies in fibromyalgia syndrome ‐ a systematic review and meta‐analysis of randomized controlled trials. Journal of Rheumatology 2010;37(10):1991‐2005. [DOI] [PubMed] [Google Scholar]
Bernardy 2011
- Bernardy K, Füber N, Klose P, Häuser W. Efficacy of hypnosis/guided imagery in fibromyalgia syndrome‐‐a systematic review and meta‐analysis of controlled trials. BMC Musculoskeletal Disorders 2011;15:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bliddal 2009
- Bliddal H, Christensen R. The treatment and prevention of knee osteoarthritis: a tool for clinical decision‐making. Expert Opinion on Pharmacotherapy 2009;10:1793‐804. [DOI] [PubMed] [Google Scholar]
Brachaniec 2009
- Brachaniec M, Depaul V, Elliott M, Moore L, Sherwin P. Partnership in action: an innovative knowledge translation approach to improve outcomes for persons with fibromyalgia. Physiotherapy Canada 2009;61:123‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bradley 2009
- Bradley LA. Pathophysiology of fibromyalgia. American Journal of Medicine 2009;122(12 Suppl):S22‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Branco 2010
- Branco JC, Bannwarth B, Failde I, Abello Carbonell J, Blotman F, Spaeth M, et al. Prevalence of fibromyalgia: a survey in five European countries. Seminars in Arthritis and Rheumatism 2010;39(6):448‐53. [DOI] [PubMed] [Google Scholar]
Brown 2006
- Brown P, Brunnhuber K, Chalkidou K, Chalmers I, Clarke M, Fenton M, et al. How to formulate research recommendations. BMJ 2006;333:804‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brozek 2009
- Brozek JL, Akl EA, Alonso‐Coello P, Lang D, Jaeschke R, Williams JW, et al. Grading quality of evidence and strength of recommendations in clinical practice guidelines. Part 1 of 3. An overview of the GRADE approach and grading quality of evidence about interventions. Allergy 2009;64(5):669‐77. [DOI] [PubMed] [Google Scholar]
Burckhardt 2005a
- Burckhardt CS, Goldenberg D, Crofford L, Gerwin R, Gowans S, Jackson K, et al. Guideline for the management of fibromyalgia syndrome. Pain in adults and children. APS Clinical Practice Guideline Series No. 4. Glenview, IL: American Pain Society, 2005. [Google Scholar]
Burckhardt 2005b
- Burckhardt CS. Educating patients: self‐management approaches. Disability and Rehabilitation 2005;27(12):703‐9. [DOI] [PubMed] [Google Scholar]
Busch 2007
- Busch AJ, Barber KA, Overend TJ, Peloso PM, Schachter CL. Exercise for treating fibromyalgia syndrome.. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD003786.pub2] [DOI] [PubMed] [Google Scholar]
Carville 2008
- Carville SF, Arendt‐Nielsen S, Bliddal H, Blotman F, Branco JC, Buskila D, et al. EULAR evidence based recommendations for the management of fibromyalgia syndrome. Annals of the Rheumatic Diseases 2008;67(4):536‐41. [DOI] [PubMed] [Google Scholar]
Choi 2010
- Choi CJ, Knutsen R, Oda K, Fraser GE, Knutsen SF. The association between incident self‐reported fibromyalgia and nonpsychiatric factors: 25‐years follow‐up of the Adventist Health Study. Journal of Pain 2010;11(10):994‐1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cipriani 2009
- Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JP, Churchill R. Comparative efficacy and acceptability of 12 new‐generation antidepressants: a multiple‐treatments meta‐analysis. Lancet 2009;373(9665):746‐58. [DOI] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical power analysis for the behavioral sciences. Hillsdale: Lawrence Erlbaum Associates, 1988. [Google Scholar]
Dell' Osso 2012
- Dell'Osso L, Carmassi C, Consoli G, Conversano C, Ramacciotti CE, Musetti L, et al. Lifetime post‐traumatic stress symptoms are related to the health‐related quality of life and severity of pain/fatigue in patients with fibromyalgia. Clinical and Experimental Rheumatology 2012;29:S73‐8. [PubMed] [Google Scholar]
Dworkin 2008
- Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. Journal of Pain 2008;9(2):105‐21. [DOI] [PubMed] [Google Scholar]
Dworkin 2009
- Dworkin RH, Turk DC, McDermott MP, Peirce‐Sandner S, Burke LB, Cowan P, et al. Interpreting the clinical importance of group differences in chronic pain clinical trials: IMMPACT recommendations. Pain 2009;146(3):238‐44. [DOI] [PubMed] [Google Scholar]
Eccleston 2009
- Eccleston C, Williams ACDC, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database of Systematic Reviews 2009, Issue 2. [DOI: 10.1002/14651858.CD007407.pub2] [DOI] [PubMed] [Google Scholar]
Eccleston 2012
- Eccleston C, Palermo TM, Williams AC, Lewandowski A, Morley S, Fisher E, Law E. Psychological therapies for the management of chronic and recurrent pain in children and adolescents. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD003968] [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Smith GD, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315:629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Eich 2012
- Eich W, Häuser W, Arnold B, Bernardy K, Brückle W, Eidmann U, et al. Fibromyalgia syndrome: General principles and coordination of clinical care and patient education. Schmerz 2012;26:268‐75. [DOI] [PubMed] [Google Scholar]
Fitzcharles 2012
- Fitzcharles MA, Ste‐Marie PA, Goldenberg DL, Pereira JX, Abbey S, Choinière M, et al. 2012 Canadian Guidelines for the diagnosis and management of fibromyalgia syndrome. www.canadianpainsociety.ca/pdf/Fibromyalgia_Guidelines_2012.pdf 2012. [DOI] [PMC free article] [PubMed]
Fordyce 1976
- Fordyce WE. Behavioral Methods in Chronic Pain and Illness. St. Louis: Mosby, 1976. [Google Scholar]
Forseth 1999
- Forseth KO, Husby G, Gran JT, Førre O. Prognostic factors for the development of fibromyalgia in women with self‐reported musculoskeletal pain. A prospective study. Journal of Rheumatology 1999;26(11):2458‐67. [PubMed] [Google Scholar]
Furukawa 2006
- Furukawa TA, Barbui C, Cipriani A, Brambilla P, Watanabe N. Imputing missing standard deviations in meta‐analyses can provide accurate results. Journal of Clinical Epidemiology 2006;59:7‐10. [DOI] [PubMed] [Google Scholar]
Glombiewski 2010
- Glombiewski JA, Sawyer AT, Gutermann J, Koenig K, Rief W, Hofmann SG. Psychological treatments for fibromyalgia: a meta‐analysis. Pain 2010;151(2):280‐95. [DOI] [PubMed] [Google Scholar]
Guyatt 2006
- Guyatt G, Gutterman D, Baumann M, Adrizzio‐Harris D, Hylek E, Phillips B, et al. Grading strength of recommendations and quality of evidence in clinical guidelines ‐ Report from an ACCP working group. Chest 2006;129:182‐7. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from http://www.cochrane‐handbook.org.
Häuser 2008
- Häuser W, Zimmer C, Felde E, Köllner V. What are the key symptoms of fibromyalgia? Results of a survey of the German Fibromyalgia Association [Was sind die Kernsymptome des Fibromyalgiesyndroms? Umfrageergebnisse der Deutschen Fibromyalgievereinigung]. Schmerz 2008;22(2):176‐83. [DOI] [PubMed] [Google Scholar]
Häuser 2010
- Häuser W, Hayo S, Biewer W, Gesmann M, Kühn‐Becker H, Petzke F, et al. Diagnosis of fibromyalgia syndrome ‐ a comparison of Association of the Medical Scientific Societies in Germany, survey, and American College of Rheumatology criteria. Clinical Journal of Pain 2010;26(6):505‐11. [DOI] [PubMed] [Google Scholar]
Häuser 2011
- Häuser W, Kosseva M, Uceyler N, Klose P, Sommer C. Emotional, physical and sexual abuse in fibromyalgia syndrome ‐ a systematic review with meta‐analysis. Arthritis Care and Research 2011;63(6):808‐20. [DOI] [PubMed] [Google Scholar]
Häuser 2013
- Häuser W, Urrútia G, Tort S, Üçeyler N, Walitt B. Serotonin and noradrenaline reuptake inhibitors (SNRIs) for fibromyalgia syndrome. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD010292] [DOI] [PubMed] [Google Scholar]
Jensen 2011
- Jensen MP. Psychosocial approaches to pain management: an organizational framework. Pain 2011;152(4):717‐25. [DOI] [PubMed] [Google Scholar]
Kashikar‐Zuck 2010
- Kashikar‐Zuck SM. Psychological interventions for pediatric chronic pain ‐ the good news and the challenges ahead. Pain 2010;148:361‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koulil 2007
- Koulil SV, Effting M, Kraaimaat FW, Lankveld WV, Helmond TV, Cats H, et al. A review of cognitive behaviour therapies and exercise programmes for fibromyalgia patients: state of the art and future directions. Annals of the Rheumatic Diseases 66;5:571‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Köllner 2012
- Köllner V, Häuser W, Klimczyk K, Kühn‐Becker H, Settan M, Weigl M, Bernardy K. Psychotherapy for patients with fibromyalgia syndrome: Systematic review, meta‐analysis and guideline. Schmerz 2012;26:291‐6. [DOI] [PubMed] [Google Scholar]
Lange 2010
- Lange M, Petermann F. Influence of depression on fibromyalgia: a systematic review. Schmerz 2010;24(4):26‐33. [DOI] [PubMed] [Google Scholar]
Lawrence 2008
- Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Arthritis and Rheumatism 2008;58(1):26‐35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2012
- Lee YH, Choi SJ, Ji JD, Song GG. Candidate gene studies of fibromyalgia: a systematic review and meta‐analysis. Rheumatology International 2012;32(2):417‐26. [DOI] [PubMed] [Google Scholar]
Loevinger 2012
- Loevinger BL, Shirtcliff EA, Muller D, Alonso C, Coe CL. Delineating psychological and biomedical profiles in a heterogeneous fibromyalgia population using cluster analysis. Clinical Rheumatology 2012;31:677‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lumley 2011
- Lumley MA. Beyond cognitive‐behavioral therapy for fibromyalgia: addressing stress by emotional exposure, processing, and resolution. Arthritis Research & Therapy 2011;13:136. [DOI] [PMC free article] [PubMed] [Google Scholar]
McBeth 2012
- McBeth J, Prescott G, Scotland G, Lovell K, Keeley P, Hannaford P, et al. Cognitive behavior therapy, exercise, or both for treating chronic widespread pain. Archives of Internal Medicine 2012;172:48‐57. [DOI] [PubMed] [Google Scholar]
Mease 2009
- Mease P, Arnold LM, Choy EH, Clauw DJ, Crofford LJ, Glass JM, et al. OMERACT Fibromyalgia Working Group. Fibromyalgia syndrome module at OMERACT 9: domain construct. Journal of Rheumatology 2009;36(10):2318‐29. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2010a
- Moore AR, Eccleston C, Derry S, Wiffen P, Bell RF, Straube S, et al. "Evidence" in chronic pain‐establishing best practice in the reporting of systematic reviews. Pain 2010;150(3):386‐9. [DOI] [PubMed] [Google Scholar]
Moore 2010b
- Moore RA, Smugar SS, Wang H, Peloso PM, Gammaitoni A. Numbers‐needed‐to‐treat analyses‐‐do timing, dropouts, and outcome matter? Pooled analysis of two randomized, placebo‐controlled chronic low back pain trials. Pain 2010;151:592‐7. [DOI] [PubMed] [Google Scholar]
Moore 2010c
- Moore RA, Derry S, McQuay HJ, Straube S, Aldington D, Wiffen P. Clinical effectiveness: an approach to clinical trial design more relevant to clinical practice, acknowledging the importance of individual differences. Pain 2010;149:173‐6. [DOI] [PubMed] [Google Scholar]
Moore 2011
- Moore RA, Wiffen PJ, Derry S, McQuay HJ. Gabapentin for chronic neuropathic pain and fibromyalgia in adults. Cochrane Database of Systematic Reviews 3, Issue 2011. [DOI: 10.1002/14651858.CD007938] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mork 2010
- Mork PJ, Vasseljen O, Nilsen TI. Association between physical exercise, body mass index, and risk of fibromyalgia: longitudinal data from the Norwegian Nord‐Trøndelag Health Study. Arthritis Care and Research 2010;62(5):611‐7. [DOI] [PubMed] [Google Scholar]
Mork 2012
- Mork PJ, Nilsen TI. Sleep problems and risk of fibromyalgia: longitudinal data from the Norwegian HUNT‐study. Arthritis and Rheumatism 2012 Jan;64(1):281‐4. [DOI: 10.1002/art.33346] [DOI] [PubMed] [Google Scholar]
Morley 2011
- Morley S. Efficacy and effectiveness of cognitive behaviour therapy for chronic pain: progress and some challenges. Pain 2011;152 Suppl 3:S99‐106. [DOI] [PubMed] [Google Scholar]
National Institutes of Health 2011
- National Institutes of Health. National Centre for Alternative and Complementary Medicine. What Is complementary and alternative medicine?. http://nccam.nih.gov/health/whatiscam (accessed 15 May 2011).
Norman 2001
- Norman GR, Sridhar FG, Guyatt GH, Walter SD. Relation of distribution‐ and anchor‐based approaches in interpretation of changes in health‐related quality of life. Medical Care 2001;39(10):1039‐47. [DOI] [PubMed] [Google Scholar]
Nüesch 2013
- Nüesch E, Häuser W, Bernardy K, Barth J, Jüni P. Comparative efficacy of pharmacological and non‐pharmacological interventions in fibromyalgia syndrome: network meta‐analysis. Annals of Rheumatic Diseases 2013;72:955‐62. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rheumatologist Los Angeles 2012
- Rheumatologist Los Angeles. What are the psychological therapies available for fibromyalgia?. http://arthritisconsult.com/fibromyalgia‐doctor/psychological‐treatments‐for‐fibromyalgia/ Accessed July 27,2012.
Saxe 2012
- Saxe PA, Arnold LM, Palmer RH, Gendreau RM, Chen W. Short‐term (2‐week) effects of discontinuing milnacipran in patients with fibromyalgia. Current Medical Research and Opinion 2012;28:815‐21. [DOI] [PubMed] [Google Scholar]
Schmidt 2011
- Schmidt S, Grossman P, Schwarzer B, Jena S, Naumann J, Walach H. Treating fibromyalgia with mindfulness‐based stress reduction: results from a 3‐armed randomized controlled trial. Pain 2011;152:361‐9. [DOI] [PubMed] [Google Scholar]
Schulz 2010
- Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;40:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smythe 1981
- Smythe HA. Fibrositis and other diffuse musculoskeletal syndromes. In: Kelley WN, Harris Jr ED, Ruddy S, Sledge CB editor(s). Textbook of Rheumatology. First Edition. Philadelphia: WB Saunders, 1981. [Google Scholar]
Sommer 2012a
- Sommer C, Häuser W, Burgmer M, Engelhardt R, Gerhold K, Petzke F, et al. Etiology and pathophysiology of fibromyalgia syndrome. Schmerz 2012;26(3):259‐67. [DOI] [PubMed] [Google Scholar]
Sommer 2012b
- Sommer C, Häuser W, Alten R, Petzke F, Späth M, Tölle T, et al. Drug therapy of fibromyalgia syndrome: Systematic review, meta‐analysis and guideline. Schmerz 2012;26:297‐310. [DOI] [PubMed] [Google Scholar]
Theadom 2009
- Theadom A, Cropley M, Hankins M, Smith HE. Mind and body therapy for fibromyalgia. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD001980.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Thieme 2007
- Thieme K, Turk DC, Flor H. Responder criteria for operant and cognitive‐behavioral treatment of fibromyalgia syndrome. Arthritis and Rheumatism 2007;57:830‐6. [DOI] [PubMed] [Google Scholar]
Thieme 2009
- Thieme K, Gracely RH. Are psychological treatments effective for fibromyalgia pain?. Current Rheumatology Reports 2009;11(6):443‐50. [DOI] [PubMed] [Google Scholar]
Thurns 2011
- Thorn BE, Burns JW. Common and specific treatment mechanisms in psychosocial pain interventions: the need for a new research agenda. Pain 2011;4:705‐6. [DOI] [PubMed] [Google Scholar]
Tort 2012
- Tort S, Urrútia G, Nishishinya MB, Walitt B. Monoamine oxidase inhibitors (MAOIs) for fibromyalgia syndrome. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD009807] [DOI] [PMC free article] [PubMed] [Google Scholar]
van Koulil 2011
- Koulil S, Kraaimaat FW, Lankveld W, Helmond T, Vedder A, Hoorn H, et al. Cognitive‐behavioral mechanisms in a pain‐avoidance and a pain‐persistence treatment for high‐risk fibromyalgia patients. Arthritis Care and Research (Hoboken) 2010;62:1377‐85. [DOI] [PubMed] [Google Scholar]
Veehof 2011
- Veehof MM, Oskam MJ, Schreurs KM, Bohlmeijer ET. Acceptance‐based interventions for the treatment of chronic pain: a systematic review and meta‐analysis. Pain 2011;152(3):533‐42. [DOI] [PubMed] [Google Scholar]
Wampold 2005
- Wampold BE, Minami T, Tierney SC, Baskin TW, Bhati KS. The placebo is powerful: estimating placebo effects in medicine and psychotherapy from randomized clinical trials. Journal of Clinical Psychology 2005;61:835‐54. [DOI] [PubMed] [Google Scholar]
Warsi 2003
- Warsi A, LaValley MP, Wang PS, Avorn J, Solomon DH. Arthritis self‐management education programs: a meta‐analysis of the effect on pain and disability. Arthritis and Rheumatism 2003;48(8):2207‐13. [DOI] [PubMed] [Google Scholar]
Williams 2012
- Williams AC, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database of Systematic Reviews 2012, Issue 11. [DOI: 10.1002/14651858.CD007407] [DOI] [PMC free article] [PubMed] [Google Scholar]
Winkelmann 2011
- Winkelmann A, Perrot S, Schaefer C, Ryan K, Chandran A, Sadosky A, et al. Impact of fibromyalgia severity on health economic costs: results from a European cross‐sectional study. Applied Health Economics and Health Policy 2011;9(2):125‐36. [DOI] [PubMed] [Google Scholar]
Winkelmann 2012
- Winkelmann A, Häuser W, Friedel E, Moog‐Egan M, Seeger D, Settan M, et al. Physiotherapy and physical therapies for fibromyalgia syndrome: Systematic review, meta‐analysis and guideline. Schmerz 2012;26:276‐86. [DOI] [PubMed] [Google Scholar]
Wolfe 1990
- Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 Criteria for the Classification of Fibromyalgia. Report of the Multicenter Criteria Committee. Arthritis and Rheumatism 1990;33(2):160‐72. [DOI] [PubMed] [Google Scholar]
Wolfe 1997
- Wolfe F, Anderson J, Harkness D, Bennett RM, Caro XJ, Goldenberg DL, et al. A prospective, longitudinal, multicenter study of service utilization and costs in fibromyalgia. Arthritis and Rheumatism 1997;40(9):1553‐5. [DOI] [PubMed] [Google Scholar]
Wolfe 2010
- Wolfe F, Clauw DJ, Fitzcharles MA, Goldenberg DL, Katz RS, Mease P, et al. The American College of Rheumatology preliminary diagnostic criteria for fibromyalgia and measurement of symptom severity. Arthritis and Rheumatism 2010;62(5):600‐10. [DOI] [PubMed] [Google Scholar]
Wolfe 2013
- Wolfe F, Brähler E, Hinz A, Häuser W. Fibromyalgia prevalence, somatic symptom reporting, and the dimensionality of polysymptomatic distress: results from a survey of the general population. Arthritis Care and Research (Hoboken) 2013;65:777‐85. [DOI] [PubMed] [Google Scholar]
Yates 2005
- Yates SL, Morley S, Eccleston E, Williams A. A scale for rating the quality of psychological trials for pain. Pain 2005;117:314‐25. [DOI] [PubMed] [Google Scholar]
Yunus 1981
- Yunus M, Masi AT, Calabro JJ, Miller KA, Feigenbaum SL. Primary fibromyalgia (fibrositis): clinical study of 50 patients with matched normal controls. Seminars in Arthritis and Rheumatism 1981;11(1):151‐71. [DOI] [PubMed] [Google Scholar]


