Abstract
Background
Reducing high blood cholesterol, a risk factor for cardiovascular disease (CVD) events in people with and without a past history of CVD is an important goal of pharmacotherapy. Statins are the first‐choice agents. Previous reviews of the effects of statins have highlighted their benefits in people with CVD. The case for primary prevention was uncertain when the last version of this review was published (2011) and in light of new data an update of this review is required.
Objectives
To assess the effects, both harms and benefits, of statins in people with no history of CVD.
Search methods
To avoid duplication of effort, we checked reference lists of previous systematic reviews. The searches conducted in 2007 were updated in January 2012. We searched the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library (2022, Issue 4), MEDLINE OVID (1950 to December Week 4 2011) and EMBASE OVID (1980 to 2012 Week 1).There were no language restrictions.
Selection criteria
We included randomised controlled trials of statins versus placebo or usual care control with minimum treatment duration of one year and follow‐up of six months, in adults with no restrictions on total, low density lipoprotein (LDL) or high density lipoprotein (HDL) cholesterol levels, and where 10% or less had a history of CVD.
Data collection and analysis
Two review authors independently selected studies for inclusion and extracted data. Outcomes included all‐cause mortality, fatal and non‐fatal CHD, CVD and stroke events, combined endpoints (fatal and non‐fatal CHD, CVD and stroke events), revascularisation, change in total and LDL cholesterol concentrations, adverse events, quality of life and costs. Odds ratios (OR) and risk ratios (RR) were calculated for dichotomous data, and for continuous data, pooled mean differences (MD) (with 95% confidence intervals (CI)) were calculated. We contacted trial authors to obtain missing data.
Main results
The latest search found four new trials and updated follow‐up data on three trials included in the original review. Eighteen randomised control trials (19 trial arms; 56,934 participants) were included. Fourteen trials recruited patients with specific conditions (raised lipids, diabetes, hypertension, microalbuminuria). All‐cause mortality was reduced by statins (OR 0.86, 95% CI 0.79 to 0.94); as was combined fatal and non‐fatal CVD RR 0.75 (95% CI 0.70 to 0.81), combined fatal and non‐fatal CHD events RR 0.73 (95% CI 0.67 to 0.80) and combined fatal and non‐fatal stroke (RR 0.78, 95% CI 0.68 to 0.89). Reduction of revascularisation rates (RR 0.62, 95% CI 0.54 to 0.72) was also seen. Total cholesterol and LDL cholesterol were reduced in all trials but there was evidence of heterogeneity of effects. There was no evidence of any serious harm caused by statin prescription. Evidence available to date showed that primary prevention with statins is likely to be cost‐effective and may improve patient quality of life. Recent findings from the Cholesterol Treatment Trialists study using individual patient data meta‐analysis indicate that these benefits are similar in people at lower (< 1% per year) risk of a major cardiovascular event.
Authors' conclusions
Reductions in all‐cause mortality, major vascular events and revascularisations were found with no excess of adverse events among people without evidence of CVD treated with statins.
Plain language summary
Statins for the primary prevention of cardiovascular disease
Cardiovascular disease (CVD), which comprises heart attacks (myocardial infarction), angina and strokes, is ranked as the number one cause of mortality and is a major cause of morbidity world wide. High blood cholesterol is linked to CVD events and is an important risk factor. Reducing high blood cholesterol, is thus an important way to reduce the chances of suffering a CVD event. Statins ‐ cholesterol lowering drugs ‐ (e.g. simvastatin, pravastatin, atorvastatin) are the first‐choice treatments. Since the early statin randomised controlled trials were reported in the 1990s, several reviews of the effects of statins have been published highlighting their benefits particularly in people with a past history of CVD. Benefits include a reduction in CVD events. Statins have also been shown to reduce the risk of a first event in otherwise healthy individuals at high risk of CVD (primary prevention) but information on possible hazards has not been reported fully. The aim of this updated systematic review is to assess the effects, both in terms of benefits and harms of statins, for the primary prevention of CVD. We searched the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE and EMBASE until 2011. We found 18 randomised controlled trials with 19 trial arms (56,934 patients) dating from 1994 to 2008. All were randomised control trials comparing statins with usual care or placebo. The mean age of the participants was 57 years (range 28 ‐ 97 years), 60.3% were men, and of the eight trials that reported on ethnicity, 85.9 % were Caucasian. Duration of treatment was a minimum one year and with follow‐up of a minimum of six months. All‐cause mortality and fatal and non‐fatal CVD events were reduced with the use of statins as was the need for revascularisation (the restoration of an adequate blood supply to the heart) by means of surgery (coronary artery bypass graft ) or by angioplasty (PTCA). Of 1000 people treated with a statin for five years, 18 would avoid a major CVD event which compares well with other treatments used for preventing cardiovascular disease. Taking statins did not increase the risk of serious adverse effects such as cancer. Statins are likely to be cost‐effective in primary prevention.
Background
Burden of cardiovascular disease
Cardiovascular disease (CVD) encompasses a wide range of disease including coronary heart disease (e.g. heart attack, angina), cerebrovascular disease (ischaemic and haemorrhagic stroke), raised blood pressure, hypertension, rheumatic heart disease and heart failure. In the context of this review the major causes of CVD are unhealthy diets, tobacco use and physical inactivity (WHO 2008).
CVD is ranked as the number one cause of mortality and is a major cause of morbidity world wide accounting for 17 million deaths, 30% of total deaths. Of these, 7.6 million are due to heart attacks and 5.7 million due to stroke (WHO 2008). Over 80% of CVD deaths occur in low‐ and middle‐income countries (WHO 2008). In developing countries, it causes twice a many deaths as HIV, malaria and tuberculosis combined (Gaziano 2007). It has been estimated that between 1990 and 2020, the increase in ischaemic heart disease alone will increase by 29% in men and 48% in women in developed countries and by 120% in women and 127% in men in developing countries (Yusuf 2001). CVD imposes high social costs, including impaired quality of life and reduced economic activity and accounts for a large share of health service resources (Gaziano 2007).
CVD is multi‐factorial in its causation and lifestyle changes are the basis of any treatment strategy, with patients often requiring behavioural counselling. Those unable to achieve or maintain adequate risk reduction through lifestyle changes alone or those at high risk may benefit from pharmacotherapy. High blood cholesterol (hypercholesterolaemia) is a risk factor for both fatal and non‐fatal CVD events in people with and without a past CVD (Prospective Studies Collaboration 2007), and lowering cholesterol, in particular low density lipoprotein (LDL) cholesterol, is an important target for pharmacotherapy. Statins are the first‐choice agents for LDL cholesterol reduction. Since the relation between blood cholesterol and cardiovascular risk is continuous (Chen 1991), there is no definite threshold to initiate treatment. If a threshold for 'high' cholesterol is set at over 3.8 mmol/L, (146.9 mg/dL) this would contribute 4.4 million deaths worldwide and 40.4 million disability‐adjusted life years (DALYs) (Ezzati 2002). Furthermore, the average level of blood cholesterol within a population is an important determinant of the CVD risk of the population. Differences in average levels of blood cholesterol between populations are largely determined by differences in diet, and countries with higher dietary saturated fat intake and a lower ratio of polyunsaturated to saturated fatty acids have higher than average cholesterol levels (Davey Smith 1992).
Trial evidence for use of statins
Since the early statin trials were reported in the early 1990s, several reviews of the effects of statins have been published highlighting the benefits of their use (Baigent 2005; Bartlett 2003; Blauw 1997; Briel 2004; Cheung 2004; Ebrahim 1999; Katerndahl 1999; LaRosa 1994; LaRosa 1999; Law 2003; Pignone 2000; Silva 2006; Thavendiranathan2006; Ward 2007; Wilt 2004). The Cholesterol Treatment Trialists (CTT) Collaboration has used individual patient data in their meta‐analyses to show consistency of treatment benefits across a wide range of patient subgroups (Baigent 2005). More recent evidence from the CCT Collaboration has demonstrated that statins are beneficial in reducing the risk of CVD events in people without prior evidence of CVD (CTT Collaboration 2010). A 2012 CTT Collaboration report further demonstrated a consistent 20% relative risk reduction in major vascular events with statins per 1mmol/L reduction in LDL cholesterol, regardless of baseline risk (CTT Collaboration 2012a). Men and women, old and young, and people with and without CVD all appear to benefit. These findings confirm the efficacy of statins for primary prevention, resolving concerns about possible serious adverse effects and potential sources of bias in the randomised trials highlighted in an earlier version of this Cochrane review.
Adverse effects of statins
There has been some concern, primarily from observational studies, that low levels of blood cholesterol increase the risk of mortality from causes other than coronary heart disease (CHD), including cancer, respiratory disease, liver disease and accidental/violent death. Several studies have now demonstrated that this is mostly, or entirely, due to the fact that people with low cholesterol levels include a disproportionate number whose cholesterol has been reduced by illness ‐ early cancer, respiratory disease, gastrointestinal disease and alcoholism, among others (Iribarren 1997; Jacobs 1997). Thus it appears to be the pre‐existing disease which causes both the low cholesterol and raised mortality (Davey Smith 1992).
The potential adverse effects of statins among people at low risk of CVD were poorly reported and unclear in earlier trials (Jackson 2001), but among those with and without pre‐existing CVD the evidence now suggests that any possible hazards are far outweighed by the benefits of treatment. Two reviews of 18 and 35 trials respectively found that there were similar rates of serious adverse events with statins as compared to placebo (Kashani 2006; Silva 2006). Individual patient data meta‐analyses conducted by the CTT Collaboration have demonstrated unequivocally that there is no excess risk of cancers (CTT Collaboration 2012b), confirming the findings of an earlier review and has reported reductions in all‐cause mortality and no excess of non‐vascular mortality (Dale 2006). Rhabdomyolysis ‐ break down of muscles ‐ which can be serious if not detected and treated early (Beers 2003) may be caused by statins, but this is very rare. In a systematic review of randomised trials of statins with about 35,000 people and 158,000 person years of observation in both treated and placebo groups, rhabdomyolysis was diagnosed in eight treated and five placebo patients, none with serious illness or death (Law 2003).
An increased risk of incident type 2 diabetes associated with statin therapy compared with usual care or placebo has been reported (Mills 2011; Sattar 2010) and with high dose versus usual dose statins (Preiss 2011). The mechanism by which statins may increase diabetes risk is not known. Haemorrhagic stroke appears to be increased by statin treatment, although estimates are imprecise, with an annual risk of 0.5 per 1000 patients treated for five years which is small compared with the benefits seen on the overall risk of stroke (CTT Collaboration 2012a). Two recent meta‐analyses of large‐scale placebo or standard care controlled trials observed a 9% increased risk for incident diabetes associated with statin therapy, with little heterogeneity between studies. In Mills 2011(Mills 2011), 17 randomised controlled trials (RCTs) reported an increased risk of the development of diabetes.
Other possible adverse events derived from small trials have been investigated. In a recent RCT of 1016 adults, statin treatment for six months was associated with increased self‐reporting of reduced energy and fatigue on exertion (Golomb 2012). An earlier RCT of 621 adults found that statins did not adversely affect self‐reported quality of life, mood, hostility, psychological well being or anger expression (Wardle 1996). Small decrements in scores on tests of psychomotor speed and attention were found by Muldoon et al in an RCT of 209 adults, but Muldoon concluded that more research is needed to fully evaluate this (Muldoon 2000). In addition, a systematic review of five statin trials (N = 30,817) found no evidence that statins increased the risk of death from non‐illness mortality (accidents, violence or suicide) (Muldoon 2001).
Guidelines for use of statins
The evidence on the beneficial effects of statins has led expert committees to promote their use on a global scale particularly in the developed world. (Genest 2009; Manuel 2006; NICE 2006; Reiner 2011) Statin prescribing and expenditure have risen rapidly as a result. For example, the European statin prescription average (weighted by population of each country) increased from 11.12 defined daily doses/1000 in 1997 to 41.80/1000 in 2002, an average 31% increase a year (Walley 2004). The expenditure on statin drugs in England was over £20 million in 1993, over £113 million in 1997 (Ebrahim 1998) and has risen to more than £500 million in 2006 (NICE 2006).
Why it is important to do this review
A major limitation of the evidence summaries to date is combining trials of statins in secondary and primary prevention of CVD without reporting benefits and adverse effects separately. A number of systematic reviews have focused on statins in primary prevention, but they differ in their interpretation of the evidence (Brugts 2009; Ebrahim 1999; NICE 2006; Thavendiranathan2006; Vrecer, 2003; Ward 2007). This is largely due to the differing inclusion criteria of the reviews and differences in reporting of outcomes. The most recent systematic review, using individual participant data from the majority of statin trials, provides strong evidence that benefits from statins outweigh any possible serious adverse effects, even at very low levels of CVD event rates (CTT Collaboration 2012a). These new findings counter earlier opinion that the evidence is insufficient to support use of statins in primary prevention for women or in older men (Abramson 2007). Previous reviews, in addition, have not reported other relevant outcomes such as costs, patient quality of life nor have they focused their attention on detailed reporting of adverse side effects.
The aim of this systematic review is to update and include further trials that have been published since the last search (to 2007) and contextualise our findings with those recently published by the CTT Collaboration.
Objectives
To update this review to assess the effects, both harms and benefits, of statins in people with no history of CVD events.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) comparing treatment with statins for at least 12 months with placebo or usual care. Length of follow‐up of outcomes had to be at least six months.
Types of participants
Men and women (aged 18 or more) with no restrictions on total, low or high density lipoprotein cholesterol levels. We limited our inclusion of study population to have less than or equal to 10% of a previous history of CVD (this would include previous angina, myocardial infarction and/or stroke). Trials in which statins were used to treat or control chronic conditions (e.g. Alzheimer’s disease, rheumatoid arthritis, renal disease, macular degeneration, aortic stenosis) were excluded.
Types of interventions
Statins (HMG CoA reductase inhibitors) versus placebo or usual care.
Concommitant interventions
Drug treatments and other interventions were accepted provided they were given to both arms of the intervention groups. Adjuvant treatments with one additional drug where a patient developed excessively high lipids during the trial were accepted.
Types of outcome measures
The following outcomes were collected:
death from all causes;
fatal and non‐fatal CHD, CVD and stroke events;
combined endpoint (fatal and non‐fatal CHD, CHD and stroke events);
change in blood total and low density lipoprotein (LDL) cholesterol concentration;
revascularisation;
adverse events;
quality of life;
costs.
Search methods for identification of studies
As previous comprehensive reviews (Bartlett 2005; Ebrahim 1999; Ward 2007) have been undertaken, we built on this work. The searches conducted in 2007 (Appendix 1) were updated on 10th January 2012 (Appendix 2). We searched the Cochrane Central Register of Controlled Trials (CENTRAL) on The Cochrane Library (2011, Issue 4), MEDLINE OVID (1950 to December Week 4 2011) and EMBASE OVID (1980 to 2012 Week 1). The standard RCT filters used for MEDLINE and EMBASE (Lefebvre 1996) in 2007 were updated in 2012. The Cochrane sensitivity‐ and precision‐maximising RCT filter has been applied to the MEDLINE search (Lefebvre 2011) and the BMJ 2011 has been applied to the EMBASE search. No language restrictions were applied to either searching or trial inclusion. Reference lists of identified review articles and of all included RCTs were searched to find other potentially eligible studies.
Data collection and analysis
Trial selection
Two review authors independently read the results from searches on electronic databases to identify those articles relevant to this systematic review based on title or title and abstract (FT and KW for the original review, FT and AM for the update). Full articles were retrieved for further assessment. The articles were read independently by two review authors (FT and KW for the original review, FT and AM for the update) and a form was designed to describe the characteristics of studies to be included or excluded as set out in the recommendations in the Cochrane Handbook for Systematic Reviews of Interventions 5.0.2 (Higgins 2011).
Assessment of risk of bias
We used criteria described in the Cochrane Handbook of Systematic Reviews 5.0.2 (Higgins 2011) to describe the quality of trials we found. Two review authors independently assessed risk of bias of selected studies (FT and KW for the original review, FT and AM for the update). Any differences of opinion were resolved by discussion and consensus and finally by discussion with a third author (SE). To assess any risk of bias we focused on the following dimensions as recommended in the Cochrane Handbook for Systematic Reviews of Interventions.
Adequate sequence generation (such as computer‐generated random numbers and random number tables, whilst inadequate approaches included the use of alternation, case record numbers, birth dates or days of the week).
Adequate measures to conceal allocation. Concealment was deemed adequate where randomisation was centralised or pharmacy‐controlled, or where the following were used: serially numbered containers, on‐site computer‐based systems where assignment is unreadable until after allocation, other methods with robust methods to prevent foreknowledge of the allocation sequence to clinicians and patients
Blinding was deemed adequate if blinding was applied (whether the participant, care provider or outcome assessors)
Completeness of outcome data was deemed adequate if intention‐to‐treat analysis was performed for each outcome and not what patient numbers the analysis was confined to.
Free of selective reporting was deemed adequate if all stated outcomes were reported on and presented. We highlighted any selective outcome reporting.
A 'Risk of bias' graph for each trial was made available to assess quality.
Data extraction
We designed a data extraction form and included data on our outcomes measures in addition to:
study ID;
quality;
population characteristics terms of CVD risk;
intervention dosage and duration.
To assess baseline risk of CVD the following median/mean values were also extracted:
age;
gender ratio;
proportion of current smokers;
total cholesterol and LDL cholesterol.
Data were independently extracted by two review authors (FT, KW). Any differences of opinion were resolved by discussion and consensus and finally by discussion with a third review author (SE).
Contacting trialists
For unpublished studies or where data were incomplete in published papers, we contacted trial authors to obtain further details.
Data analysis
Risk ratios (RR), odds ratio (OR) and 95% confidence intervals (CI) were calculated for dichotomous data. Quantitative analyses of outcomes was based on 'intention‐to‐treat' (ITT). For continuous data (such as change in blood total cholesterol), we calculated pooled mean differences (MD) (with 95% CI).
We did not add the number of fatal and non‐fatal clinical events together from any of the studies that we included in this review as it was not possible to ascertain whether an individual who had a non‐fatal clinical event followed by a fatal clinical event was counted as a clinical event under both categories. As a result, we have only included the composite of fatal and non‐fatal clinical events if this was reported in the papers. For example, number of stroke events: 10 trials reported this as a composite outcome, but three reported on fatal and five on non‐fatal stroke events. We did not add the fatal and non‐fatal strokes together to ascertain a composite number.
Heterogeneity
Because trials found may not have been carried out according to a common protocol there will usually be variations in patient groups, clinical settings, concomitant care etc. We, therefore, assessed heterogeneity between trial results. Trial data were considered to be heterogeneous where the I2 statistic was > 50%. For analysis, we used the fixed‐effect method unless data were heterogenous in which case we used the random‐effects model. Where significant heterogeneity was present, we attempted to explain the differences based on the patient clinical characteristics and interventions of the included studies.
Publication or other bias
A funnel plot was used to test for asymmetry, which represents the presence of publication bias based on the data for the primary outcome of all‐cause mortality (Sterne 2001). Analyses for potential effect modifiers was initially considered but abandoned due to lack of adequate reporting. We planned to include:
gender;
extent of hyperlipidaemia;
age greater than and less than 65 years.
Sensitivity analysis
Sensitivity analysis was used to explore the influence of the following on effect size:
repeating analysis taking account of study quality;
repeating analysis excluding any large studies to see how they influence the results;
post‐hoc analysis (requested by a peer‐reviewer) excluding those trials with any participants with clinical evidence of CVD.
Results
Description of studies
For the original review 4227 references were identified after removal of duplicates. From reading titles and abstracts 4128 were eliminated as being not relevant to the review. Full papers were obtained for 99 references. From these 99 papers, 72 papers reporting on 48 studies were excluded (see Characteristics of excluded studies). A total of 27 papers reporting on 14 trials were included (see Characteristics of included studies).
For this update, 6442 references were identified after removal of duplicates and off these, 131 full papers were retrieved. From these, 92 papers were relevant; 35 papers related to seven studies included in the original review and 57 papers to five new trials. For one of these (a conference abstract), we were unable to obtain further data (Babes 2010). This study is listed the Table: Characteristics of studies awaiting classification. We excluded 39 papers: 37 related to 36 excluded studies and two related to the previously excluded ASCOT‐LLA trial (Figure 1). Reasons for exclusion remained unchanged and mainly included; treatment length not at least being one year, more than 10% of the population having existing CVD and no relevant outcomes (to this review) being reported (see Characteristics of excluded studies).
1.
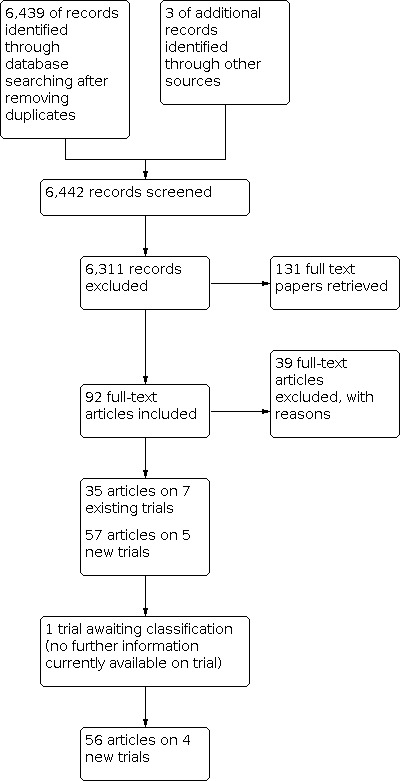
Study flow diagram for the update
Our update identified four new trials with 19,662 additional participants (Bone 2007; CERDIA 2004; METEOR 2010; JUPITER 2008) bringing a total of 18 trials. Of the 18, trials, one tested two different interventions and for the purpose for meta analysis, this trial was counted as two trials (in total 19 trial arms) (CELL A 1996; CELL B 1996). In addition, our updated search identified reporting of new follow‐up data of the three of the 14 trials in the original review. (Adult Japanese MEGA Study; CARDS 2008; WOSCOPS).
The trials dated from 1994 to 2008 and were conducted worldwide, mainly in industrially developed countries (Japan, USA, Europe and JUPITER which included sites in South America, Israel, South Africa and Russia). Fourteen trials recruited patients with specific conditions: nine recruited participants with raised lipids, four with diabetes, two with hypertension and one with microalbuminuria.
All tested the effectiveness of statins compared with placebo; nine tested pravastatin 10 mg to 40 mg per day; two atorvastatin 10 mg to 80 mg per day; two fluvastatin 40 mg to 80 mg per day; two lovastatin 20 mg to 40 mg per day; two rosuvastatin 20 mg to 40 mg per day; and the remaining two simvastatin 20 mg to 40 mg per day (one of these had started patients on cerivastatin 0.4 mg per day which was replaced with simvastatin in August 2001). Five trials also included advice, counselling or information on health‐behaviour modification such as diet, smoking cessation, or exercise.
In total, the 18 trials (with 19 trial arms) recruited 56,934 participants and observed outcomes ranging from one to 5.3 years. The size of the population recruited ranged from 47to 17,802. The mean age of the participants was 57 years (range 28‐97 years), 60.3% included male participants, and of the eight trials that reported on ethnicity, 85.9 % were Caucasian.
Three trials (AFCAPS/TexCAPS 1998; CARDS 2008; JUPITER 2008) were stopped prematurely because significant reductions in primary composite outcomes between the intervention and placebo had been observed. Overall, these trials had recruited 47% of the total study population and were stopped 1.4 to 3.0 years before the pre‐specified end date.
Data on all‐cause mortality were provided in 11 trials. Excluding the four trials whose primary outcome was change in size of carotid artery and one whose primary endpoint was change in bone density, nine of the remaining trials chose a composite primary outcome. Ten trials provided data on fatal and 11 on non‐fatal CHD events, and five trials provided data on fatal and two on non‐fatal CVD events. Ten trials reported on combined stroke events, five provided data on non‐fatal and three on fatal stroke events. Fourteen trials provided data on cholesterol and 12 on adverse events. Four trials provided economic costings, (CARDS 2008; JUPITER 2008; MRC/BHF Heart Protection; WOSCOPS) and one (CELL A 1996; CELL B 1996) provided data on patient perceived quality of life.
Excluding the four trials that solely recruited participants with diabetes, 1% to 20% of the participants had diabetes. Excluding the two trials that recruited participants with hypertension, the remaining studies recruited 15% to 67% with hypertension. The proportion of participants smoking ranged from 10% to 45% in the 17 trials that provided these data. We were unable to ascertain baseline lipid levels for three trials. Baseline total cholesterol levels ranged from 4.81 to 6.97 mmol/L (median 6.17 mmol/lL, and LDL cholesterol from 2.8 to 4.95 mm/L (median 4.1mm/L).
Risk of bias in included studies
In general, there was low risk of bias (Figure 2; Figure 3) though all trials were either fully or partially funded by pharmaceutical companies (five by Bristol Myers and Squibb, three by Pfizer, four by Astra‐Zeneca, two by Merck and one by Bayer, one by Bayer and Merk, one by Pfizer, and the remaining by Sankyo Co Ltd). Three (AFCAPS/TexCAPS 1998; ASPEN 2006; HYRIM 2007) of the 19 trial arms did not provide adequate information on the methods used for randomisation, two of which had recruited more than 2,000 participants. Eighteen trials used blinding to reduce bias, 15 of which used double‐blinding methods. Thirteen used intention‐to‐treat analysis. The drop‐out rates ranged from 2% to 30% for the 12 trials that reported on this. We judged 15 of the trials to be free from selection bias. The MRC/BHF Heart Protection Study (MRC/BHF Heart Protection) only provided data on total CVD events for patients with diabetes in the primary prevention group, and HYRIM reported outcomes on cholesterol on a subset of the population (46%) with no explanation as to how the subset had been derived (HYRIM 2007).
2.

Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
3.

Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
The funnel plot for all‐cause mortality showed no sign of asymmetry (Figure 4).
4.

Funnel plot of comparison: 2 Mortality and Morbidity, outcome: 2.1 Total Mortality.
Effects of interventions
All‐cause mortality
Thirteen trials with 48,060 participants recruited reported on total mortality. During observation, 1077/24,408 (4.4%) died in the statin group compared with 1223/23,652 (5.1%) in the placebo group; yielding an unadjusted NNT for 5 years of 132 (95% confidence interval (CI) 121 to 144). Adjustment to account for total person‐years of follow‐up across all studies by dividing the number of events by total person‐years of follow‐up in the statin and placebo group from the included trials, yields an NNT for 5 years of 96 (95% CI 64 to 244). Only the JUPITER trial showed strong evidence of a reduction in total mortality. When the data were pooled using a fixed‐effect model, a reduction that favoured statin treatment by 14% was observed: (odds ratio (OR) 0.86, 95% CI 0.79 to 0.94). No heterogeneity was observed (Analysis 1.1).
1.1. Analysis.

Comparison 1: Mortality and Morbidity, Outcome 1: Total Mortality
Fatal and non‐fatal CHD events
Fourteen trials with 48,049 participants reported on combined fatal and non‐fatal CHD events. Four trials showed evidence of a reduction in this combined outcome, which was maintained in the pooled analysis using a fixed‐effect model: 820/24,217 (3.4%) in the statin group versus 1114/23,832 (4.6%) in the placebo group; yielding an unadjusted NNT for 5 years of 78 (95% confidence interval (CI) 72 to 85). Adjustment to account for total person‐years of follow‐up across all studies in the same manner as outlined above, yields an NNT for 5 years of 56 (95% CI 46 to 75); risk ratio (RR) 0.73 (95% CI 0.67 to 0.80) (Analysis 1.2).
1.2. Analysis.

Comparison 1: Mortality and Morbidity, Outcome 2: Total Number of CHD Events
Observations on fatal or non‐fatal CHD events are based on 10 and 11 trials respectively. When pooled, a risk reduction in fatal CHD events was observed; 251/23,019 (1.1%) statin group versus 306/23,075 (1.3%) placebo group; RR 0.82 (95% CI 0.70 to 0.96) (Analysis 1.3). Evidence for a reduction in non‐fatal CHD events was also found: 398/20,668 (1.9%) statin group versus 583/20,309 (2.8%); RR 0.67 (95% CI 0.59 to 0.76). No significant heterogeneity was observed using a fixed‐effect model for both analyses(Analysis 1.4).
1.3. Analysis.

Comparison 1: Mortality and Morbidity, Outcome 3: Number of Fatal CHD Events
1.4. Analysis.

Comparison 1: Mortality and Morbidity, Outcome 4: Number of Non‐fatal CHD Events
Fatal and non‐fatal CVD events
Nine trials with 23,805 participants, representing 41.8% of the total population, reported on combined fatal and non‐fatal CVD events. Four of the larger trials with 21,205 participants demonstrated strong evidence of a reduction in this combined outcome. In the pooled analysis using a fixed‐effect model: 1103/11,892 (9.3%) in the statin group versus 1455/11,913 (12.2%) in the placebo group; RR 0.75 (95% CI 0.70 to 0.81). There was no evidence of heterogeneity (Analysis 1.5).
1.5. Analysis.

Comparison 1: Mortality and Morbidity, Outcome 5: Total Number of CVD Events
Five trials reported on fatal CVD events and two reported on non‐fatal CVD events. Reductions in risk were observed in both these endpoints; fatal CVD events; 295/16,962 (1.7%) in the statin group versus 355/17,050 (2.1%) in the placebo group; RR 0.83 (95% CI 0.72 to 0.96) (Analysis 1.6); non‐fatal CVD events 123/4,299 (3%) in the stain group versus 175/4,398 (4%) in the placebo group, RR 0.77 (95% CI 0.62 to 0.96) (Analysis 1.7). No significant heterogeneity was observed using a fixed‐effect model for both analyses.
1.6. Analysis.

Comparison 1: Mortality and Morbidity, Outcome 6: Number of Fatal CVD Events
1.7. Analysis.

Comparison 1: Mortality and Morbidity, Outcome 7: Number of Non‐fatal CVD Events
Fatal and non‐fatal stroke events
Ten trials with 40,295 participants reported on combined fatal and non‐fatal stroke events. Two trials that had been stopped prematurely demonstrated a significant reduction in this combined outcome with the use of statins. This reduction was observed in the pooled analysis using a fixed‐effect model: 345/20,302 (1.7%) in the statin group versus 442/19,993 (2.2%) in the placebo group; RR 0.78 (95% CI 0.68 to 0.89] (Analysis 1.8).
1.8. Analysis.

Comparison 1: Mortality and Morbidity, Outcome 8: Total Number of Stroke Events
Three trials with 27,238 participants reported on fatal stroke events, and five trials with 28,097 participants reported on non‐fatal stroke events. There was no observed difference in fatal stroke. We applied a random‐effects model due to significant heterogeneity (I2= 68%). Two of three trials had been prematurely stopped, and the remaining trial (WOSCOPS) demonstrated a 43% increase in risk of fatal stroke, but this was not significant (Analysis 1.9). Using a fixed‐effect model, a significant risk reduction was seen for non‐fatal stroke events 193/14,243 (1.3%) in the statin group versus 276/13,852 (2%) in the placebo group; RR 0.69 (95% CI 0.58 to 0.83) Analysis 1.10.
1.9. Analysis.

Comparison 1: Mortality and Morbidity, Outcome 9: Number of Fatal Stroke Events
1.10. Analysis.

Comparison 1: Mortality and Morbidity, Outcome 10: Number of Non‐fatal Stroke Events
Combined fatal and non‐fatal CHD, CVD and stroke events
Only four trials with 35,254 participants reported a composite of fatal and non‐fatal events for CHD, CVD and stroke. All the trials showed a significant reduction in this composite outcome with the treatment of statins, which was maintained in the pooled analysis and used a fixed model: 438/17,591 (2.4%%) events versus 678/17,663 (3.8%); RR 0.65 (95% CI 0.58 to 0.73) (Analysis 1.11).
1.11. Analysis.

Comparison 1: Mortality and Morbidity, Outcome 11: Total Number of Fatal and Non‐fatal CHD, CVD and Stroke Events
Revascularisation
Seven trials with 42,403 participants reported on the need for revascularisation procedures during follow‐up: 286/21,166 (1.4%) in the statin group versus 461/21237 (2.2%) in the placebo group underwent either percutaneous transluminal coronary angioplasty (PTCA) or coronary artery bypass graft (CABG). Three of the larger trials were able to demonstrate fewer revascularisation events in the intervention groups compared with the control groups with the use of statins. This was maintained in the pooled analysis using a fixed‐effect model: RR 0.62 (0.54 to 0.72) (Analysis 1.12).
1.12. Analysis.

Comparison 1: Mortality and Morbidity, Outcome 12: Number of Study Participants who underwent Revascularisation
Cholesterol
Fourteen trials provided data on total cholesterol, and 16 trials provided data on LDL cholesterol. For both endpoints, all trials were able to demonstrate significant reductions. For total cholesterol, a net difference of ‐1.05 mmol/L (95% CI ‐1.35 to ‐0.76 mmol/L) was observed (Analysis 2.1), and for LDL cholesterol a net difference of ‐1.00 (95% CI ‐1.16 to ‐0.85 mmol/L) was observed (Analysis 2.2).There was marked heterogeneity of effects in both analysis (I2= 100% and 99%, respectively). It is likely that the heterogeneity is due to differences in the type of statin and dosage used.
2.1. Analysis.

Comparison 2: Lipids (mmol/L), Outcome 1: Total Cholesterol (mmol/L)
2.2. Analysis.

Comparison 2: Lipids (mmol/L), Outcome 2: LDL Cholesterol (mmol/L)
Adverse events
Twelve trials provided data on adverse events. In total 10,838/56,934 (19%) participants experienced an adverse event with adverse event rates ranging from 0% to 97%. Pooling the events rates indicated no difference between the intervention and control groups with the use of statin using a fixed‐effect model: RR 1.00 (95% CI 0.97 to 1.03) (Analysis 3.1). No differences were observed between statin and control with the number of participants stopping statin treatment due to adverse events and those admitted to hospital for an adverse event, though heterogeneity was observed (Analysis 3.2; Analysis 3.3).
3.1. Analysis.

Comparison 3: Adverse Events, Outcome 1: Number of study participants who had adverse events
3.2. Analysis.

Comparison 3: Adverse Events, Outcome 2: Number of study participants who stopped treatment due to adverse events
3.3. Analysis.

Comparison 3: Adverse Events, Outcome 3: Number of study participants who were admitted to hospital
Cancer: 2255/38,739 (5.8%) participants in 11 trials developed cancer (Analysis 3.4). There was no evidence of any excess risk of cancers with a pooled estimate of RR 1.01 (95% CI 0.93 to 1.10) and no heterogeneity.
3.4. Analysis.

Comparison 3: Adverse Events, Outcome 4: Number of study participants who developed cancer
Myalgia and rhabdomyolysis: 3551/37,939 participants in nine trials developed myalgia, but there was no evidence of excess risk with a pooled estimate of 1.03 (95% CI 0.97 to 1.09] with some heterogeneity (I2 = 41%) (Analysis 3.5). Rhabdomyolysis was very rare, affecting three of 19,410 participants on statins in six trials reporting this outcome but with no evidence of any excess risk on statins: RR 1.00 (95% CI 0.23 to 4.38) (Analysis 3.6).The Heart Protection Study outcomes for rhabdomyolysis were five cases in those on statins and three cases among controls, but these findings were not broken down by primary and secondary prevention. Adding these additional events to the estimate above gives RR 1.31 (95% CI 0.47 to 3.62).
3.5. Analysis.

Comparison 3: Adverse Events, Outcome 5: Number of study participants who developed myalgia or muscle pain
3.6. Analysis.

Comparison 3: Adverse Events, Outcome 6: Number of study participants who developed rhabdomyolysis
Type 2 diabetes: reporting of new occurrences of type 2 diabetes was confined to only two trials, AFCAPS/TexCAPS 1998 and JUPITER 2008. Overall, 342/12,205 (2.8%) participants on statins developed diabetes compared with 290/12202 (2.4%) participants on control or placebo, with a relative risk of developing diabetes of 1.18 (95% CI 1.01 to 1.39). This excess risk of diabetes was driven by the JUPITER trial, which used higher statin doses than the AFACPS/TexCAPS trial, which showed no effect on diabetes incidence (Analysis 3.7).
3.7. Analysis.

Comparison 3: Adverse Events, Outcome 7: Number of study participants who developed diabetes
Haemorrhagic stroke: only two trials reported haemorrhagic stroke outcomes which occurred in 45/25634 (0.2%) participants with a RR of 0.97 (95% CI 0.54 to 1.75) (Analysis 3.8).
3.8. Analysis.

Comparison 3: Adverse Events, Outcome 8: Number of study participants who developed haemorrhagic stroke
Other adverse events: weak evidence was found for an increased risk of liver enzyme elevations (10 studies) RR 1.16 (95% CI 0.87 to 1.54) (Analysis 3.9), renal dysfunction (four studies) RR 1.11 (95% CI 0.99 to 1.26) (Analysis 3.10), and arthritis (two studies) RR 1.20 (95% CI 0.82 to 1.75) (Analysis 3.11).
3.9. Analysis.

Comparison 3: Adverse Events, Outcome 9: Number of study participants who had elevated liver enzymes
3.10. Analysis.

Comparison 3: Adverse Events, Outcome 10: Number of study participants who developed renal disorder
3.11. Analysis.

Comparison 3: Adverse Events, Outcome 11: Number of study participants who developed arthritis
Treatment compliance
Of the eight trials that reported treatment compliance there was no difference between the two groups (Analysis 4.1). In the statin group 77% participants and 70% in the placebo group complied with treatment; RR 1.08 (0.98 to 1.18).
4.1. Analysis.

Comparison 4: Treatment Compliance, Outcome 1: Treatment Compliance
Costs
Four trials reported on costs. WOSCOPS found that the use of statin yielded substantial health benefits at a cost which was not prohibitive: an undiscounted gain of 2460 years of life at a cost of £8,121 per life year gained (WOSCOPS). In the JUPITER trial, the authors estimated that rosuvastatin therapy was cost‐effective, using a willingness‐to‐pay threshold of £31,882/QALY, statin therapy had a cost‐effectiveness of £25,796/QALY for CHD and stroke prevention. (JUPITER 2008‐Ohsfeldt 2010) The authors of CARDS estimated the cost of managing CVD events would be lower after five years for patients treated with atorvastatin compared with those on placebo. The cost‐effectiveness of atorvastatin 10 mg/day would be £87,525/QALY at five years, with an incremental cost of £2,320/QALY at 10 years. (CARDS 2008‐Ramsay 2008)
Patient quality of life
There were no reliable data on patient quality of life reported by trials. CELL A+B provided limited data on quality of life, suggesting that the intervention of lifestyle advise plus pravastatin reduced stress and sleeping problems.
Sensitivity analysis
We were unable to locate any unpublished studies. As the study quality was overall rated as good, for the update we confined our sensitivity analysis to comparing studies that were stopped early and followed a protocol and to comparing large and small studies for total mortality and total CHD events. These analyses indicated no change in the overall results in early stopping of trials and for study size for either outcome (Analysis 5.1; Analysis 5.2Analysis 5.3; Analysis 5.4).
5.1. Analysis.

Comparison 5: Sensitivity Analysis, Outcome 1: Early stopping of trials and total mortality
5.2. Analysis.

Comparison 5: Sensitivity Analysis, Outcome 2: Early stopping of trials and total CHD events
5.3. Analysis.

Comparison 5: Sensitivity Analysis, Outcome 3: Study Size for total Mortality
5.4. Analysis.

Comparison 5: Sensitivity Analysis, Outcome 4: Study Size for total CHD events
Excluding the five trials that included up to 10% participants with clinical evidence of CVD (none of the trials published the subgroup without any evidence of CVD) demonstrates very similar findings: total mortality RR 0.80 (95% CI 0.70 to 0.91) versus RR 0.86 (0.79 to 0.94) in all trials; total CHD events RR 0.68 (0.59 to 0.77) versus 0.73 (0.67 to 0.80) in all trials; adverse events RR 0.99 (0.96 to 1.02) versus 1.00 (0.97 to 1.03) in all trials.
Discussion
The trials included in this systematic review showed reductions in all‐cause mortality, composite cardiovascular disease (CVD) endpoints, fatal and non‐fatal CVD events considered separately, total and low density lipoprotein (LDL) cholesterol, and revascularisations. These findings were associated with falls in total and LDL cholesterol in all trials reporting these outcomes. No excess of combined adverse events, cancers, myopathy, rhabdomyolysis, haemorrhagic stroke, liver enzyme elevation, renal dysfunction and arthritis were found, although not all trials reported fully on adverse events. An increased risk of incident diabetes was found in the two trials reporting this outcome. There was limited evidence to suggest that the use of statins for primary prevention may be cost‐effective. However, in light of new evidence derived from the CTT Collaboration on primary prevention, there is a need to up‐date existing cost‐effective analysis. Patient perceived quality of life was reported in only one trial, which showed limited benefit. Sensitivity analysis suggested that early stopping of trials and size of trial did not influence the overall results.
Although the trials intended to recruit only people without evidence of CVD, some trials did include some participants with CVD. Rather than exclude such trials, we set an arbitrary threshold of 10% to avoid any major influence of effects of treatment on those with existing CVD. A sensitivity analysis, excluding the five trials that had up to 10% participants with clinical evidence of CVD at baseline, showed very little difference between effect sizes compared with all the trials included in this review. Our findings concur with previous systematic reviews (Brugts 2009; Ebrahim 1999; NICE 2006). However, previous systematic reviews have included trials where more than 10% of participants had a previous history of CVD which is reflected in their higher baseline all‐cause mortality event rates which were 1.4 per 100 person years at risk (NICE 2006) and 1.7 per 100 person years (Brugts 2009) compared with 1.0 per 100 person years in this review.
The CTT Collaboration has published analyses focusing on the comparison between high and low doses of statins which demonstrate that more intensive treatment lowers LDL cholesterol more, resulting in greater benefits (CTT Collaboration 2010) with no excess risk of non‐vascular mortality. However, a increase in the risk of myopathy and rhabdomyolysis in people treated with statins is confirmed, particularly among those treated with higher rather than lower doses statins (Armitage 2007). Strong evidence of the absence of any adverse effects on cancer risk is also confirmed by a further CTT Collaboration report (CTT Collaboration 2012b).
Our estimates of effects on CVD outcomes and on all‐cause mortality on statins are in line with the recent CTT Collaboration report (CTT Collaboration 2012a). The major finding of this new report is the benefits from statins at low levels of CVD risk: six and 15 major vascular events would be avoided per 1000 people treated for five years in the two lowest baseline risk categories (< 5% five‐year risk, RR 0·57 (0·36 to 0·89) and 5% to 10% five‐year risk RR 0·61 (0·50 to 0·74)) respectively (Figure 1, CTT report), giving NNT values of 167 and 67 respectively. These NNTs are well within the range considered worthwhile in primary prevention (e.g. for treatment of hypertension).
The individual patient data analyses conducted by the CTT Collaboration counter concerns about the interpretation of the evidence of statins for primary prevention. First, the use of composite endpoints derived from different CVD outcomes is overcome since there is sufficient power to demonstrate benefits for individual CVD outcomes. Second, additional data on outcomes are available for most trials, which reduces any effect of selective reporting of outcomes. Third, similar benefits of statins were seen in trials that stopped early and in those running their planned course. Fourth, concerns about effects in low‐risk groups, particularly women, are now demonstrated to be similar to those in other trial participants. Fifth, the benefits of statins outweigh any risks of serious adverse effects since no excess of cancers was found and all‐cause mortality was lower in those on statins. Thus, earlier claims that statins provide no overall benefit in primary prevention in terms of all‐cause mortality (Therapeutics Letter 2003; Therapeutics Letter 2010; Ray 2010) can no longer be substantiated.
Haemorrhagic stroke may be increased by use of statins with an annual excess risk of 0.5 per 1000 people treated over five years per 1·0 mmol/L LDL cholesterol reduction reported by the CTT Collaboration. However, overall stroke events were reduced, indicating a net benefit. This might not be the case in Asian populations where haemorrhagic stroke is more common than ischaemic stroke, and where evidence of association between low blood cholesterol and haemorrhagic stroke has been reported (Ebrahim 2006).
Our review, with sparse data, found an increased risk of type 2 diabetes in those treated with statins: RR 1.18 (95% CI 1.01 to 1.39), which is greater than the that found in a more comprehensive meta‐analysis using both published and unpublished data from 13 trials (both primary and secondary prevention) which reported a relative risk of 1·09; 95% CI 1·02 to 1·17, with a number needed to harm of 255 people treated for four years to result in one case of diabetes (Preiss 2011; Sattar 2010). This increased risk of diabetes appears to be related to baseline fasting glucose levels and metabolic syndrome among participants randomised to statins (Waters 2011). It can be argued that the overall small proportion of people who develop diabetes when treated with a statin is outweighed by the benefits of statins (CTT Collaboration 2012a). However, in the context of primary prevention, patients may expect not to be harmed in any way by 'preventive' treatments. Patient view points of such trade‐offs remain to be assessed and will be important in determining wider use of statins (Smeeth 2012).
All but one of the trials had some form of pharmaceutical industry sponsorship. It is now established that published pharmaceutical industry‐sponsored trials are more likely than non‐industry‐sponsored trials to report results and conclusions that favour drug over placebo due to biased reporting and/or interpretation of trial results (Als‐Nielsen 2003). The reporting of adverse events in these trials is generally poor, with failure to provide details of severity and type of adverse events or to report on health‐related quality of life. However, it seems unlikely that any major life‐threatening hazards associated with statin use exist. Potential non‐fatal but serious hazards of long‐term statin use have not been assessed in trials (e.g. possible cognitive impairments suggested by one small trial: Muldoon 2000). We have focused on adverse events arising in randomised trial populations but these cannot adequately assess rare hazards, such as rhabdomyolysis. Large observational databases are useful for detecting rare hazards associated with use of statins but a causal attribution is more difficult to establish (Hippsley‐Cox 2010; Smeeth 2008).
Our previous conclusion urging caution in the use of statins in people at low risk of cardiovascular events is no longer tenable in light of the CTT Collaboration findings. Several issues remain to be considered before widespread use of statins could be recommended in people at low risk (Ebrahim 2012; Smeeth 2012). These include: i) the feasibility and desirability of having to treat the majority of people over the age of 50 with a statin; ii) the cost‐effectiveness of such a strategy using a conventional healthcare delivery system; iii) diversion of attention from achieving coverage in people at high risk of events; iv) use of alternative public health strategies to lower blood cholesterol; v) the views of patients on life‐long drug therapy; and vi) limited evidence on less serious but nonetheless potentially important adverse effects and quality of life.
The National Institute for Health & Clinical Excellence UK (NICE) has provided some cost‐effectiveness estimates based on data to 2005 and conclude that an annual risk of a CHD event ranging from 3% to 0.5%, the ranges of cost per quality adjusted life year gained (QALY) gained were £10,000 to £31,000 at age 45 years, £13,000 to £40,000 at age 55 years using older generic statins (NICE 2006). Their guidance is to use statins "... as part of the management strategy for the primary prevention of CVD for adults who have a 20% or greater 10‐year risk of developing CVD." Evidence supporting the use of statins as part of an overall strategy of identification of people at high risk of CVD events and lowering blood pressure and blood cholesterol has been produced for low‐ and middle‐income countries (Lim 2007) and is now part of World Health Organization's policy for CVD prevention (WHO 2008b).
Low cost generic statins are now widely available and recent cost‐effectiveness studies show that statins are cost‐saving in the USA even in people at low levels of predicted CHD risk. To gain maximal impact from using statins, 64 million people in the USA (just under half of the over 35 year old population) would need to be put on treatment at a cost of US$2,800 per QALY gained (Lazar 2011). These cost‐effectiveness estimates are likely to be better for more potent statins and in lower cost health services.
Authors' conclusions
Implications for practice.
The totality of evidence now supports the benefits of statins for primary prevention. The individual patient data meta‐analyses now provide strong evidence to support their use in people at low risk of cardiovascular disease. Further cost‐effectiveness analyses are now needed to guide widening their use to these low risk groups.
Implications for research.
In addition to the cost‐effectiveness analyses referred to above, it will be useful to study the effects of public health interventions that attempt to alter diet and physical activity patterns and compare their effects with statins in robust randomised trials given recent evidence of large independent survival benefits of physical fitness in those taking statins in a large prospective cohort study (Kokkinos 2012). Relevant interventions might include nutrition education, exercise prescription, physical education curriculums that may be effective in changing lifestyle behaviours. (Jepson 2000) Studies of patient experiences and views on long‐term use of statins are also needed to improve adherence to treatment. It is likely that further trials will be conducted in younger adults with adverse risk factor profiles which are associated with higher lifetime CVD risk (Berry 2012) and also in children (de Ferranti 2008). It is important that these trials examine comprehensively potential adverse effects of statins and quality of life, reporting on them in an unbiased way.
Feedback
Failure to cite CTT paper and dangerously misleading press release, February 2011
Summary
Clinical Trials Services Unit and Epidemiogical Studies Unit
The Discussion of your paper erroneously stated that the CTT collaborators had not published information about the proportional and absolute benefits of statin therapy among people with no prior history of vascular disease, although these were published in The Lancet in November 2010 (Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive LDL‐lowering therapy: meta‐analysis of individual data from 170,000 participants in 26 randomised trials of statin therapy. Lancet 2010; 376: 1670‐81). It also stated that the CTT collaborators had been “unable to provide the relevant analysis for inclusion in our review”, but we are not aware of having been asked by you (or anyone in your team) to provide such analyses, and wonder whether correspondence may have gone astray.
We are concerned that these mis‐statements in the Cochrane Collaboration paper (and some over‐statements in the related press release, such as the claim that “Given that low cholesterol has been shown to increase [our emphasis] the risk of death from other causes, statins may do more harm than good in some patients”) are dangerously misleading for the public —as well as not meeting the Cochrane Collaboration’s key principle of ‘keeping up to date’. Might it be possible for this Cochrane report to be corrected as a matter of urgency? Professor Colin Baigent, Professor of Epidemiology, MRC Scientist, Hon. Consultant in Public Health Professor Rory Collins, BHF Professor of Medicine and Epidemiology
Reply
The recent CTT Lancet November 2010 paper was not available to our team at the time the review was completed and submitted for publication to the Cochrane Database of Systematic Reviews. We agree that a data point in Figure 3 gives the proportional and absolute effects on major vascular events of a 1mmol/l reduction in LDL cholesterol in trial participants without prior cardiovascular disease. Our estimate of this effect and its precision is similar to the CTT estimate. I am surprised that CTT did not provide more information on other outcomes among participants taking statins for primary prevention. In particular, others have raised the issue of all‐cause mortality in primary prevention trials (Ray et al, Arch Intern Med. 2010;170:1024‐1031) and have expressed concerns about an increased risk of diabetes in those taking statins (Sattar et al, Lancet 2010;375:735‐42). We will, of course, include reference to the CTT paper and will remove the text stating that CTT was “unable to provide the relevant analysis for inclusion in our review”. It should be feasible to make these changes in the next issue. Work is underway to conduct a comprehensive update of this review as soon as possible.
Following discussions with David Tovey and Rory Collins, the press release was withdrawn and a correction issued on 8 March 2011 from by David Tovey, Editor in Chief's office on the homepage of the Cochrane Library (http://www.thecochranelibrary.com/details/editorial/1029211/Correction-by-David-Tovey.html). An email was sent to all recipients of that press release, and correction was attempted of any existing versions of the press release that were still in circulation.
Shah Ebrahim, lead author of Statins for the Primary Prevention of Cardiovascular Disease and Coordinating Editor of the Cochrane Heart Group
Contributors
Colin Baigant & Rory Collins, Shah Ebrahim
Further correspondence with CTT collaboration, April 2011
Summary
22 February 2011
Taylor F et al. Statins for the primary prevention of cardiovascular disease.
Cochrane Database of Systematic Reviews 2011, Issue 1
The Discussion of your paper erroneously stated that the CTT collaborators had not published information about the proportional and absolute benefits of statin therapy among people with no prior history of vascular disease, although these were published in The Lancet in November 2010 (Cholesterol Treatment Trialists’ (CTT) Collaboration. Efficacy and safety of more intensive LDL‐lowering therapy: meta‐analysis of individual data from 170,000 participants in 26 randomised trials of statin therapy. Lancet 2010; 376: 1670‐81). It also stated that the CTT collaborators had been “unable to provide the relevant analysis for inclusion in our review”, but we are not aware of having been asked by you (or anyone in your team) to provide such analyses, and wonder whether correspondence may have gone astray.
We are concerned that these mis‐statements in the Cochrane Collaboration paper (and some over‐statements in the related press release, such as the claim that “Given that low cholesterol has been shown to increase [our emphasis] the risk of death from other causes, statins may do more harm than good in some patients”) are dangerously misleading for the public —as well as not meeting the Cochrane Collaboration’s key principle of ‘keeping up to date’. Might it be possible for this Cochrane report to be corrected as a matter of urgency?
Colin Baigent & Rory Collins
Reply 2 March 2011
Re: Statins for the primary prevention of cardiovascular disease, Cochrane Database of Systematic Reviews 2011, Issue 1.
Thanks for your letter of 22 February 2011. The recent CTT Lancet November 2010 paper was not available to our team at the time the review was completed and submitted for publication to the Cochrane Database of Systematic Reviews. We agree that a data point in Figure 3 gives the proportional and absolute effects on major vascular events of a 1mmol/l reduction in LDL cholesterol in trial participants without prior cardiovascular disease. Our estimate of this effect and its precision is similar to the CTT estimate. I am surprised that CTT did not provide more information on other outcomes among participants taking statins for primary prevention. In particular, others have raised the issue of all‐cause mortality in primary prevention trials (Ray et al, Arch Intern Med. 2010;170:1024‐1031) and have expressed concerns about an increased risk of diabetes in those taking statins (Sattar et al, Lancet 2010;375:735‐42). We will, of course, include reference to the CTT paper and will remove the text stating that CTT was “unable to provide the relevant analysis for inclusion in our review”. It should be feasible to make these changes in the next issue.
The press release was referring to the association of low blood cholesterol (not cholesterol lowering by statins) with haemorrhagic stroke which has been shown by several observational cohorts, including a large Korean civil servants cohort (n=3900 haemorrhagic strokes), but these associations may be confounded. It would obviously be of great value to have a more reliable estimate of this effect by randomization to statins than that reported in the recent CTT paper (RR 1.12 (95% CI: 0.93, 1.35) per 1 mmol/L reduction in LDL cholesterol, webfigure 8) which might be achieved if more trials provided this outcome. More robust estimates would be particularly helpful for low and middle income countries where underlying rates of haemorrhagic stroke remain high and statins, as part of a “polypill” strategy, are being promoted for primary prevention.
We are already working on a full update of the review and have 7,000 citations to work through inclusion/exclusion criteria. In addition to the changes for the next issue, if you want I can arrange to have your letter and my response entered in the correspondence section linked to the review. This would enable your concerns to be immediately linked to the review and be readily available to readers of the review. Let me know your preference.
Shah Ebrahim
4 March 2011 Dear Shah Thank you for your response. One quick point of clarification, the press release actually says "low cholesterol has beenshown to increase [my emphasis] the risk of death from other causes" which is clearly quite different from what you have written in the second paragraph of your letter and is dangerously irresponsible. I wondered, therefore, if — before considering publication — you would like to make this error clear in your letter and ensure that the statement in the press release is formally retracted.
Rory Collins
04 March 2011 Dear Rory I agree the wording is quite wrong. The press statement has not been published, nor is it available to readers of the review itself. I will add a sentence saying that a press release about the review contained a seriously misleading statement that "low cholesterol has been shown to increase the risk of death from other causes". Shah Ebrahim
4 March 2011
Thank you for your proposal to modify your letter which is fine as far as it goes. The statement in this press release (which engendered wide publicity) is, however, so dangerously wrong that I think the Cochrane Collaboration is obliged to issue a public retraction. Please could you forward my correspondence to whoever is responsible for dealing with such serious misrepresentations within the Collaboration? Rory Collins
4 March 2011
In the first instance, if we have published something that is misleading or incorrect in the press release I would suggest that we issue a correction in the release accompanying the next issue. I would like to explore with the writer of the release how this happened, as this is the first time that we have had such a complaint in relation to a press release, to the best of my knowledge. Having said that I am responsible for the sign off of press releases so that any error is entirely my responsibility. I am making some enquiries as a matter of urgency and will let you all know when we have a proposed course of action. David Tovey
4 March 2011
Shah Ebrahim has confirmed that the statement is wrong (see below) and, in public health terms, it is potentially a far more serious misrepresentation than that of the risks of MMR by Wakefield and The Lancet. As a consequence, I think it requires an urgent and specific response by the Cochrane Collaboration and should not just be "buried" in a routine press release. Rory Collins
8 March 2011
This is to update you in relation to our current plans in relation to correction of the press release. Firstly, we are will contact via email in the next 48 hours, all individuals and agencies that received the original press release for Issue 1 and explain the need for a correction of the offending sentence. Secondly, we will publish a correction on The Cochrane Library homepage explaining the error. I anticipate that this will happen later today. Thirdly we will do our utmost to ensure that anywhere where the press release is still “live”, it is modified to a more satisfactory form of words. The Cochrane Collaboration sets a high value on quality, scientific rigour and transparency. In this instance we are grateful to you for pointing out an error in the press release that had evaded our editorial system. Please be assured that we regarded this as a serious matter, and have sought to implement visible and appropriate measures to correct the error. We have also learned lessons from the episode that once implemented will reduce the chance of a similar event in the future. David Tovey
10 March 2011
Thank you for taking some steps towards dealing with this problem as the errors of fact in both the press release, as well as those in the related paper (see our original letter to Shah Ebrahim and his reply: attached), have had a damaging effect on public health (as well as on the credibility of the Cochrane Collaboration). It is very much to your credit that you wish to take final responsibility (as editor) for these errors, but should not the authors also take some of the responsibility (rather than just passing the buck) since they presumably approved the press release which quotes them? I have now had an opportunity to read your Correction on the Cochrane Library website and, though welcome, it seems to me that it is incomplete (given the errors in the original paper) and, indeed, is misleadingly half‐hearted. For example, Shah Ebrahim accepts in his letter to us that, by contrast with what he had claimed in his paper, results for the highly statistical benefits in patients with no prior cardiovascular disease (risk ratio for major vascular events: 0.75; 95% CI 0.69 ‐ 0.82) had been published nearly 3 months beforehand. Your Correction would have been an opportunity to put that straight, rather than to assert that such errors do "not impact in any way on the validity of the accompanying Cochrane Review". Similarly, please could you explain why the claim in the press release that "low cholesterol has been shown to increase the risk of death from other causes, statins may do more harm than good" is, according to the assertion in your correction, "irrelevant to the underlying question being evaluated"? This does not seem to be correct. I'm sorry not to have replied to your letter sooner, but I was waiting to see the Correction before doing so and was looking for it on the Cochrane Collaboration website, where it does not appear. As well as having it on the Cochrane Library website, would it not be appropriate to put this Correction (or, preferably, a more accurate one) on the Cochrane Collaboration website (and any other Cochrane websites), especially since the statin paper is one of its featured reviews? I do hope that you will reconsider the partial (in more than one sense) attempt that you've made so far to redress the serious harm that has been caused to public health by the Cochrane Collaboration and its misinterpretation of the available evidence (which does not seem to be at all consistent with your key principles). Rory Collins
10 March 2011 I suspect we have reached an impasse. I really don’t accept that the response was half‐hearted. To repeat, we have placed a highly visible correction on the homepage of the product that was the subject of the press release, we have sent an email to all recipients of that press release, and we have sought to correct any existing versions of the press release that are still in circulation. I, not the Co‐ordinating Editor, sign off the press release, so this was my error alone. It was, as you pointed out, a seriously incorrect message – implying that the very act of reducing your serum cholesterol might cause early death – and could, if acted upon have caused public harm. For that reason I recognised the need to act decisively and swiftly to correct any wrong impression. I made the point in the correction that the press release mistake was based on a misunderstanding of the Cochrane Review, which had explicitly explained that any possible association was highly unlikely to be based on cause and effect. Therefore I believe it was correct to be clear that the press release was distinct from the review. I recognise that you have also raised questions in relation to the content of the review. As Shah describes in his response, he has taken on board your comments, explained why the Lancet paper was not considered in the original published version, and has sought to amend the review appropriately at the earliest opportunity. For technical /publication reasons there will be an inevitable but short delay before the changes are published. I am aware that you are unlikely to agree, but I am confident that our response to the questions you have raised in relation to the press release and the review has been appropriate, open and positive. David Tovey
11 March 2011
I'm extremely grateful both for your careful response to my email and for what you've been able to do to rectify this problem. I did have a couple of questions in my previous email which I'd be grateful if you'd consider. First, might it be possible to put the Correction on the Cochrane Collaboration website as well, since that would be an obvious place where people alerted by the original press release would go? Second, why do you say in the Correction that the claim in the press release that "low cholesterol has been shown to increase the risk of death from other causes, statins may do more harm than good" is "irrelevant to the underlying question being evaluated" by this meta‐analysis of whether statins do more harm than good? I had thought that this Correction would have provided an opportunity to indicate that errors in the original paper would also be corrected at the earliest possible opportunity. Again, thanks for taking the issue so seriously and for going as far as you have towards repairing the damage caused. Rory Collins
Reply
See above
Contributors
Colin Baigent & Rory Collins, Shah Ebrahim
22nd Febuary 2012,
Summary
Feedback: Dear respected authors and editors of the Cochrane Heart Group, First, in the abstract of the systematic review, statistically significant relative risk reductions were reported for all‐cause mortality, non‐fatal CVD events, and revascularisations. However, I had trouble applying this knowledge in practice because there was no mention of the absolute risk reduction or number needed to treat associated with statin use in this clinical setting. I thought these two values would be of clinical relevance since the review defined primary prevention as treating people without evidence of existing cardiovascular disease, who would be expected to have low baseline risks. Furthermore, despite the statistically significant relative risk reductions of key outcomes without an increase in adverse events found by this review, the authors advised caution before prescribing statins for primary prevention. This was a point of confusion for me, as I could not understand how the authors came to that conclusion. I think that because many people in the world have only access to the Cochrane abstracts and plain language summary, the rationale for the conclusions should be explicit to the reader. Lastly, on the abstract page this review was assessed as up‐to‐date on September 7, 2007, even though it was published on January 19, 2011. I was not sure if that was a mistake.
With Kind Regards, Qiming Roger Wu BSc, BSc(Pharm), RPh, MD Candidate
Submitter agrees with default conflict of interest statement: I certify that I have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback.
Reply
Dear Qiming Roger Wu, Thank you for your feedback on this review of statins for primary prevention of cardiovascular disease. You would like to see absolute risk differences and numbers needed to treat. We have not provided these as they are often misleading in primary prevention. The absolute levels of CVD risk used will depend on a) what is included in 'CVD' (e.g. new angina cases, revasularisations), b) the population in which you practice (CVD incidence varies markedly between countries), c) age group and sex of the population considered. The relative risk reduction figure is stable across outcomes, populations, age and sex groups. The NNT is not and presenting several NNTs is confusing for the reader.
You are concerned about why we recommend caution in using statins for primary prevention despite the statistically significant relative risk reduction. This is because the quality of the trials is variable (early stopping, selective reporting of outcomes), many do not report any adverse events (which is unlikely to be true), and guidelines in UK, USA and Europe do not recommend their use at levels below 20% 10‐year risk of CVD. This is discussed in detail in the main text but not in the abstract for reasons of space. In the abstract we say: 'Other potential adverse events were not reported and some trials included people with cardiovascular disease. Only limited evidence showed that primary prevention with statins may be cost‐effective and improve patient quality of life.' This gives some, but not all, of the rationale for use with caution. You question whether the review is as out of date as it appears. I am afraid it is. This is because doing a Cochrane review on statins requires searching for relevant papers ‐ in this case we had to sift through thousands of abstracts of papers, retrieve hundreds of full papers, assess them in duplicate to reach our final 14 trials for consideration. This takes time and in this case was made worse as the key authors were relocated to work in India on another project that took priority over this review. We have conducted an update and hope that this will be published before the end of 2012. Thank you for your interest in our work.
Best wishes, Shah Ebrahim
Contributors
Qiming Yu, Shah Ebrahim
Response to Taylor et al (2013) Statins for primary preventionof cardiovascular disease,
Summary
Dear Colleagues, I am writing to express concerns about the Cochrane Review update of statins for primary prevention (Taylor et al 2013.) I have three principle concerns which involve Taylor et al’s treatment of 1) potential commercial bias, 2) data transparency, and 3)patient safety. Recent research indicates that commercial bias may be an important factor in evaluating the validity of published trial data and may be more important than a checklist of technical factors in determining bias. Bero, Lexchin, Lundh and other colleagues, for example, have pointed out a variety of issues in this regard (eg Bero et al 2007; Roseman et al 2012; Lundh et al 2012). Most recently, describing the research of Lundh et al’s ( 2012) Cochrane Review on industry sponsorship and research outcomes, Bero (2013; JAMA Intern Med) has stated that standard assessment tools do not adequately grapple with risk of bias. She noted that Lundh et al (2012) reviewed nine studies with seemingly low risk of bias but the relationship between sponsorship and outcomes was in fact stronger. The Cochrane authors, citing Als‐Nielsen (2003) note the possibility of commercial bias briefly but fail to explain why they do not consider it more fully in their analysis. Will the authors clarify why they did not fully address commercial sponsorship as a risk of bias? Taylor et al (2013) rely heavily on industry‐sponsored trials and the meta‐analysis by the CTT indicating that the CTT’s commercial sponsorship isanalysis rests in their use of patient‐level data. However, it is known that many scholars have asked the CTT for data without success (interalia Criqui and Golomb 2004, Walsh and Pignone 2004, Petretta 2008.) As I understand the Review by Taylor and colleagues they seem not to have reviewed that data but are relying on the CTT’s interpretations. Currently many scholars are asking for transparency and the release of primary source data like Clinical Study Reports and patient‐level data (eg Doshi and Jefferson) so that the larger scientific community can be more fully informed. This broader perspective is important in understanding both benefit and harm through a lens other than that provided by industry. Will the authors clarify why they did not ask for full transparency but seemingly relied on CTT analysis? In terms of issues related to harm, the Review is both troubling and confusing. It is known that harms are under‐reported in commercial trials. The Cochrane authors state that 12 trials provided data on Adverse Events, indicating that one third did not provide data on AEs. They previously stated (page 3) that the trial authors were contacted to obtain further details. It is, thus, not clear if the AE data was withheld from the Cochrane reviewers or were not collected in the original trials. The authors state (page 14) that reporting of adverse events in the statin trials is relatively poor, since there is a failure to provide important details of type and severity of AEs. But they then state with (no citations) that it “seems unlikely that major life‐threatening hazards associated with statin use exist.” And they go on to state that potential non‐fatal but serious adverse events have not been assessed in statin trials. They briefly touch on the recent work by Hippsley‐Cox (20120) but seem to discount the value of database analysis for detecting statin harm. It is, thus, not clear why Taylor and colleagues have chosen to deem statins safe for primary prevention for men and women on the basis of clinical trial data with serious limitations in safety information. Nor is it clear why they did not consider information about harm from a wider variety of sources including advisories from countries like New Zealand, UK and the USA. Many sources indicate that clinical trials are not geared towards real‐world experiences of harm. For example, Fernandez and colleagues at the Cleveland Clinic (2011) state that statin myopathy is a common experience that is not reflected in clinical trials and that muscle symptoms occurred in up to 20% of patients on statins. They point out that real‐world conditions should be considered, and note the relatively low level of myalgia (1%‐5%) described in clinical trials. Other institutions have, like the Mayo Clinic, have created Statin Intolerance Clinics acknowledging people’s every day experiences with statin harms. Oskarsson (2011; Neurology) states that there is particular concern about necrotizing statin myopathy wherein recovery does not occur with cessation of statin‐exposure. (See also research by rheumatologists like Mammen and colleagues on treatments for statin‐associated autoimmune necrotizing myopathies.) Pharmacovigilance can play an important role in detecting signals of harm, which the Cochrane authors do not fully consider. Sakaeda et al (2011) analyzed data on muscular and renal events in FDA data base and found evidence of signals of harm warranting clinical trial research. There is also an important social context in adverse event reporting. Research indicates adverse event reporting to be low, possibly because of failure to recognize statin associated symptoms (Dirks and Jones, 2006) or physician reluctance to report (Golomb et al., 2007.) Taylor and colleagues have also not dealt with the issue that statins are considered a category X drug in the USA and are associated with miscarriage and birth defects (see for example Edison and Muenke, 2004 and Prescrire 2005.) Taylor and coseem to suggest that women of child‐bearing capacity (over age 35; page 15) would benefit from statins without considering this important harm. Taylor and colleagues indicate that in using CTT patient level data they have examined the “totality of evidence” for primary prevention statin use. This does not seem possible. I am concerned that the voices of patients are not heard in this review. I do not see evidence of pro‐active research strategies addressing patient safety. The importance of interrogating the quality of safety reporting in clinical trials is seen as valuable within the research community but it is raised in a confusing way in this review; both acknowledging problems and then discounting their relevance. Other sources of data are not adequately considered.
Will the authors explain what they mean by “the totality of evidence” and why they have not attempted to proactively secure safety data from trialists nor consider other forms of independent information about statin‐related harms? Harriet Rosenberg, York University, Health and Society Program (retired)
Reply
Thank you for your interest in our review. We respond to your questions and hope you find them helpful in understanding our approach, both its strengths and its limitations.
1. Will the authors clarify why they did not fully address commercial sponsorship as a risk of bias?
The potential bias associated with commercial sponsorship of trials is well‐recognised. We collected and reported information on the funding source of all trials included in our review. We were unable to evaluate this form of potential bias through comparison of effect sizes between commercially funded trials and non‐commercially funded trials, since every trial was commercially funded. We have addressed commercial sponsorship to the extent possible in this circumstance. We would be pleased to learn of alternative approaches in these circumstances.
2. Will the authors clarify why they did not ask for full transparency but seemingly relied on Cholesterol Treatment Trialists (CTT) analysis?
Our analysis did not rely on the CTT analyses. Our meta‐analyses are based on published data from trials on statins. CTT is based on individual patient data and covers an overlapping set of statin trials in both primary and secondary prevention. We are aware that attempts to obtain individual patient data from CTT does not appear to be feasible, and we did not ask the CTT investigators for data as we did not require their data to carry out our systematic review. It is important to recognise that in any systematic review using Cochrane methods a thorough search is made for all relevant trials, independent of source of funding. We then conducted our analysis using published data and made requests to the individual trial authors, where necessary, for data on specific outcomes. As the CTT had published highly relevant analyses since our 2011 publication, it was imperative for us to update our review in light of their findings. Our findings obtained from the published literature and findings from the CTT are remarkably similar, so we do not believe that there are any issues with the integrity of their analyses.
3. Will the authors explain what they mean by “the totality of evidence” and why they have not attempted to proactively secure safety data from trialists nor consider other forms of independent information about statin‐related harms?
We considered the randomized evidence in this Cochrane review and on that basis believe we have the totality of this evidence. We have attempted to get additional information from trialists but did not set out to duplicate successful efforts that have others have made to get additional data. CTT have been particularly successful in this regard (as have others with respect to type 2 diabetes). Individual patient level data are needed to do this well and is a long term goal for our group. We have also considered other non‐randomized data in our discussion.
We concluded that it “seems unlikely that major life‐threatening hazards associated with statin use exist.” This is based on the all‐cause mortality relative risk of 0.86 (95% CI 0.79 to 0.94). This means that, overall, any unintended effect of statins that was severe enough to cause death would be highly unlikely as we do not see any increased overall death rate on statins. We accept that trials generally do not report unintended effects of treatments well, and statin trials are no exception.
The analyses from the CTT have explored the possibility of hazards due to cancers and found no evidence of any increased risk of any cancer. We believe these data are reliable and are consistent with our own analyses from the trial reports. We also considered myalgia/rhabdomyolosis, type 2 diabetes, haemorrhagic stroke, liver dysfunction, renal dysfunction and arthritis.
We also examined adherence with treatment rates and found 77% participants and 70% in the placebo group complied with treatment; RR 1.08 (0.98 to 1.18), indicating that while 23‐30% of patients stopped treatment, this was more common in those taking placebo than in those taking statins.
We have not made any specific reference to use of statins in women of child‐bearing age, in whom it would be most unlikely for statins to be indicated. We do report that women gain similar benefit to men, which was not clear in previous reviews.
With regard to other potentially serious, but non‐fatal unintended effects of statins, we do not discount Hippsley‐Cox’s report (or Smeeth’s report) as large scale observational data are often the only way of determining possible unexpected harms. However, they are prone to confounding and do not provide the robust evidence that randomized trials do.
There are many observational reports of harms associated with statins, and this literature frequently reports contradictory findings. We are currently conducting a separate systematic review of these studies and are also conducting a further large scale primary care database analysis. These studies are complex to perform, have taken separate funding to conduct, yet should be ready for submission for publication later this year.
Contributors
Shah Ebrahim, George Davey Smith, Tess Moore, Fiona Taylor and Mark Huffman
Query regarding outcomes and mortality figures, December 2013
Summary
1. Why did you decided to use the 10 year follow‐up ('post‐trial period') and not the 'trial period' outcomes for the WOSCOPS study (Tables 1 and 2, NEJM 2007 357;15,1477‐1486) for the quoted outcomes? This increases the weighting of WOSOCPS greatly and affects the event rate and subsequently ARR or NNT based on control rates. 2. Where did you source the PREVEND‐IT total mortality figures of 10/433 in the statin group and 8/431 from? This is not clear to me from reading the source paper and other meta‐analyses list the figures as 13/433 and 12/431 resppectively (Tonelli M et al. Efficacy of statins for primary prevention in people at low cardiovascular risk: a meta‐analysis. CMAJ 2011. DOI:10.1503/cmaj.101280 and Ray K et al Statins and All‐Cause Mortality in High‐Risk Primary Prevention: A Meta‐analysis of 11 Randomized Controlled Trials Involving 65 229 Participants. Arch Intern Med. 2010;170(12):1024‐1031).
Dr Rupert Major, University of Leicester.
Reply
1. If there are longer follow ups available it does make some sense to use them to increase the overall events, particularly if they are sparse which is the case in trials of primary prevention. However, it is generally better to carry out two analyses: trial end outcomes and longest outcomes to explore how publication of longer follow ups may be biasing the overall effect size. There is a tendency for trialists who have found continued benefits to publish such data but not to publish if these findings if they are null. We will carry out this analysis in the next update of this review. NNTs are highly susceptible to the underlying rates used to estimated them and, in my opinion are potentially quite spurious when derived from trial control groups (which tend to be healthier than the populations in which drugs are used, even in primary prevention). Sensitivity analyses are always worth doing using trial control group rates, general population rates (often available from large GP databases), and separated by gender and age groups. 2. With regard to the data abstracted for PREVEND‐IT, I am not able to confirm the source of the data used as I will not be in London where the data abstraction sheets are stored ‐ and won't be until end of February. It is quite possible that these are errors given your cross‐checking with the original paper and other meta‐analyses. So thank you for drawing my attention to this and it will be checked, and if in error, corrected in the next update.
Contributors
Shah Ebrahim and Rupert Major.
Calculation of NNT, January 2017
Summary
At our department we teach EBM to doctors in training for general practice. One of our students have studied statins in primary prevention and used your review thoroughly.
In the calculations of NNT she (ABH) had difficulties following your calculations and two senior researchers (AM+OO) could not help her out.
In relation to total mortality you state:
”All‐cause mortality. Thirteen trials with 48,060 participants recruited reported on total mortality. During observation, 1077/24,408 (4.4%) died in the statin group compared with 1223/23,652 (5.1%) in the placebo group; number needed to treat for five years: 96 (95% confidence interval (CI) 64 to 244).”
However, we calculate ARR: 5.1%‐4.4% = 0.7%, and if we use the traditional formula NNT = 1/ ARR, we get NNT= 143, i.e. larger than your NNT = 96.
Similarly, in relation to fatal and non‐fatal CHD events you state:
“Fourteen trials with 48,049 participants reported on combined fatal and non‐fatal CHD events. Four trials showed evidence of a reduction in this combined outcome, which was maintained in the pooled analysis using a fixed‐effect model: 820/24,217 (3.4%) in the statin group versus 1114/23,832 (4.6%) in the placebo group. Overall NNT for five years: 56 (95% CI 46 to 75);”
But we get ARR = 4.6%‐3.4% = 1.2%, and thus NNT = 83, i.e. again larger than your NNT = 56.
We cannot see in your methods section that you have used any other formula than the usual.
Could you please explain how your NNTs have been calculated?
Best regards,
Anne Boe‐Hansen Dall MD, Anne Møller, MD, PhD, Ole Olsen, M.Sc.
Reply
The discussions of numbers needed to treat (NNT) for total mortality and for fatal and non‐fatal CHD events have been revised to reflect both unadjusted NNTs (calculated directly from the summary data for the trial outcomes) and NNTs adjusted to account for total person‐years of follow‐up across all studies in each outcome (in order to account for the differences in study duration across trials) (1)
1) Smeeth L, Haines A, Ebrahim S. Numbers needed to treat derived from meta‐analyses‐‐sometimes informative, usually misleading. BMJ. 1999 Jun 5;318(7197):1548‐51.
Contributors
Mark Huffman, review author
William Cayley, Cochrane Heart feedback editor
What's new
| Date | Event | Description |
|---|---|---|
| 21 September 2021 | Review declared as stable | The research area is no longer active. |
History
Protocol first published: Issue 2, 2004 Review first published: Issue 1, 2011
| Date | Event | Description |
|---|---|---|
| 21 April 2017 | Amended | Text edited for all‐cause mortality and fatal and non‐fatal CHD events in response to feedback. |
| 21 April 2017 | Feedback has been incorporated | Comment on calculation of NNT and reply added. |
| 18 August 2016 | Amended | Minor corrections on decimal points for outcomes fatal and non‐fatal CVD events and fatal and non‐fatal stroke events) |
| 2 October 2014 | Amended | Contact person changed from Fiona Taylor to Mark Huffman. Acknowledgements amended, adding thanks to translators. |
| 20 January 2014 | Feedback has been incorporated | Query regarding the timing of outcomes and the mortality figures of the PREVEND‐IT trial |
| 11 April 2013 | Feedback has been incorporated | New feedback was added |
| 4 December 2012 | New citation required and conclusions have changed | This update includes 4 new studies and updated date from 3 studies and our conclusions have changed |
| 18 June 2012 | New search has been performed | This review has been updated incorporating findings from the Cholesterol Treatment Trialists Collaboration individual patient data meta‐analyses that extend the findings on benefits of statins to people at lower risk of major cardiovascular events than previously established. Conclusions have been changed. |
| 10 April 2012 | Feedback has been incorporated | Feed back incorporated. |
| 10 April 2012 | New citation required and conclusions have changed | New search to January 2012 found four new trials and published follow‐up data on three existing trials. Results and conclusion have changed in light of the new evidence. |
| 4 July 2011 | Amended | Rectified minor error in reporting of all‐cause mortality data in main text. |
| 7 April 2011 | Amended | Converted to new review format |
| 7 April 2011 | Feedback has been incorporated | Correspondence with CTT collaboration added |
| 8 March 2011 | Feedback has been incorporated | Removed text indicating CTT collaboration had not provided relevant data. Included citation to recent CTT collaboration paper which gives additional confirmation of benefits of statins in primary prevention. Added in response to CTT collaboration correspondence (see Feeback). |
Acknowledgements
For the primary phase of this work we would like thank Gauri Verma who contributed to selecting studies on statin treatment for both primary and secondary prevention of cardiovascular disease undertaken in 2002. For the update of this review many thanks go to Mrityunjay Kumar for helping to abstract data, and David GriersonTaylor for diligently collecting data on share prices of the pharmaceutical companies that supported the included studies. We would also like to thank Kensuke Takaoka, Annie Tremp, Marina Karanikolos, Sandy Gulyurtlu, Taixiang Wu, for their help in translating papers.
Appendices
Appendix 1. Search Strategies 2007
CENTRAL on The Cochrane Library
#1MeSH descriptor Hydroxymethylglutaryl‐CoA Reductase Inhibitors explode all trees #2 statin or statins #3 atorvastatin #4 cerivastatin #5 fluvastatin #6 lovastatin #7 pravastatin #8 simvastatin #9 lipitor #10 baycol #11 lescol #12 mevacor #13 altocor #14 pravachol #15 lipostat #16 zocor #17 rosuvastatin #18 (hydroxymethylglutaryl next coenzyme next reductase next inhibitor) #19 (#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9) #20 (#10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18) #21 (#19 or #20)
MEDLINE on Ovid
1 exp Hydroxymethylglutaryl‐CoA Reductase Inhibitors/ 2 (statin or statins).tw. 3 atorvastatin.tw. 4 cerivastatin.tw. 5 fluvastatin.tw. 6 lovastatin.tw. 7 pravastatin.tw. 8 simvastatin.tw. 9 lipitor.tw. 10 baycol.tw. 11 lescol.tw. 12 mevacor.tw. 13 altocor.tw. 14 pravachol.tw. 15 lipostat.tw. 16 zocor.tw. 17 mevinolin.tw. 18 compactin.tw. 19 fluindostatin.tw. 20 rosuvastatin.tw. 21 or/1‐20 22 exp Cardiovascular Diseases/ 23 cardiovascular.tw. 24 heart disease$.tw. 25 coronary disease$.tw. 26 angina.tw. 27 heart failure.tw. 28 cardiac failure.tw. 29 exp Hyperlipidemia/ 30 hyperlipid$.tw. 31 hypercholesterol$.tw. 32 exp Cholesterol/ 33 cholesterol$.tw. 34 randomized controlled trial.pt. 35 controlled clinical trial.pt. 36 Randomized controlled trials/ 37 random allocation.sh. 38 double blind method.sh. 39 single‐blind method.sh. 40 or/34‐39 41 exp animal/ not human/ 42 40 not 41 43 clinical trial.pt. 44 exp Clinical trials/ 45 (clin$ adj25 trial$).ti,ab. 46 ((singl$ or doubl$ or trebl$ or tripl$) adj (blind$ or mask$)).ti,ab. 47 placebos.sh. 48 placebo$.ti,ab. 49 random$.ti,ab. 50 research design.sh. 51 or/43‐50 52 51 not 41 53 42 or 52 54 or/22‐33 55 21 and 54 and 53
EMBASE on Ovid
1 exp Hydroxymethylglutaryl Coenzyme a Reductase Inhibitor/ 2 (statin or statins).tw. 3 atorvastatin.tw. 4 cerivastatin.tw. 5 fluvastatin.tw. 6 lovastatin.tw. 7 pravastatin.tw. 8 simvastatin.tw. 9 lipitor.tw. 10 baycol.tw. 11 lescol.tw. 12 mevacor.tw. 13 altocor.tw. 14 pravachol.tw. 15 lipostat.tw. 16 zocor.tw. 17 mevinolin.tw. 18 compactin.tw. 19 fluindostatin.tw. 20 rosuvastatin.tw. 21 or/1‐20 22 exp Cardiovascular Disease/ 23 cardiovascular.tw. 24 heart disease$.tw. 25 coronary disease$.tw. 26 angina.tw. 27 heart failure.tw. 28 cardiac failure.tw. 29 exp Hyperlipidemia/ 30 hyperlipid$.tw. 31 hypercholesterol$.tw. 32 exp Cholesterol/ 33 cholesterol$.tw. 34 exp lipid blood level/ 35 or/22‐34 36 21 and 35 37 random$.ti,ab. 38 factorial$.ti,ab. 39 (crossover$ or cross over$ or cross‐over$).ti,ab. 40 placebo$.ti,ab. 41 (double$ adj blind$).ti,ab. 42 (singl$ adj blind$).ti,ab. 43 assign$.ti,ab. 44 allocat$.ti,ab. 45 volunteer$.ti,ab. 46 Crossover Procedure/ 47 Double Blind Procedure/ 48 Randomized Controlled Trial/ 49 Single Blind Procedure/ 50 or/37‐49 51 exp animal/ 52 nonhuman/ 53 exp animal experiment/ 54 or/51‐53 55 exp human/ 56 54 not 55 57 50 not 56 58 36 and 57
Appendix 2. 2 Search Strategies 2012
CENTRAL
#1MeSH descriptor Hydroxymethylglutaryl‐CoA Reductase Inhibitors explode all trees #2 hydroxymethylglutaryl* #3 HMG‐CoA* #4 statin or statins #5 atorvastatin #6 cerivastatin #7 fluvastatin #8 lovastatin #9 pravastatin #10 simvastatin #11 lipitor #12 baycol #13 lescol #14 mevacor #15 altocor #16 pravachol #17 lipostat #18 zocor #19 mevinolin #20 compactin #21 fluindostatin #22 rosuvastatin #23 dalvastatin #24 cranoc #25 canef #26 locol #27 lochol #28 leucol #29 lescol #30 monacolin #31 medostatin #32 mevinacor #33 livalo #34 pitava #35 pitavastatin #36 pravasin #37 mevalotin #38 gerosim #39 lipex #40 zenas #41 crestor #42 meglutol #43 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14) #44 (#15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 OR #29) #45 (#30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 OR #38 OR #39 OR #40 OR #41 OR #42) #46 (#43 OR #44 OR #45)
MEDLINE
1. exp Hydroxymethylglutaryl‐CoA Reductase Inhibitors/ 2. hydroxymethylglutaryl*.tw. 3. HMG‐CoA*.tw. 4. (statin or statins).tw. 5. atorvastatin.tw. 6. cerivastatin.tw. 7. fluvastatin.tw. 8. lovastatin.tw. 9. pravastatin.tw. 10. simvastatin.tw. 11. lipitor.tw. 12. baycol.tw. 13. lescol.tw. 14. mevacor.tw. 15. altocor.tw. 16. pravachol.tw. 17. lipostat.tw. 18. zocor.tw. 19. mevinolin.tw. 20. compactin.tw. 21. fluindostatin.tw. 22. rosuvastatin.tw. 23. dalvastatin.tw. 24. cranoc.tw. 25. canef.tw. 26. locol.tw. 27. lochol.tw. 28. leucol.tw. 29. lescol.tw. 30. monacolin.tw. 31. medostatin.tw. 32. mevinacor.tw. 33. livalo.tw. 34. pitava.tw. 35. pitavastatin.tw. 36. pravasin.tw. 37. mevalotin.tw. 38. gerosim.tw. 39. lipex.tw. 40. zenas.tw. 41. crestor.tw. 42. meglutol.tw. 43. or/1‐42 44. exp Hyperlipidemias/ 45. exp Cholesterol/ 46. exp Cardiovascular Diseases/ 47. cardio*.tw. 48. cardia*.tw. 49. heart*.tw. 50. coronary*.tw. 51. angina*.tw. 52. hyperlipid*.tw. 53. hypercholesterol*.tw. 54. cholesterol*.tw. 55. hypercholester?emia*.tw. 56. hyperlip?emia*.tw. 57. triglycerid*.tw. 58. hypertriglycerid?emia*.tw. 59. hyperlipoprotein?emia*.tw. 60. LDL.tw. 61. HDL.tw. 62. or/44‐61 63. 43 and 62 64. randomized controlled trial.pt. 65. controlled clinical trial.pt. 66. randomized.ab. 67. placebo.ab. 68. clinical trials as topic.sh. 69. randomly.ab. 70. trial.ti. 71. 64 or 65 or 66 or 67 or 68 or 69 or 70 72. exp animals/ not humans.sh. 73. 71 not 72 74. 63 and 73 75. (2007031* or 2007032* or 2007033* or 200704* or 200705* or 200706* or 200707* or 200708* or 200709* or 20011* or 2008* or 2009* or 2010* or 2011*).ed. 76. 74 and 75
EMBASE
1. exp hydroxymethylglutaryl coenzyme A reductase inhibitor/ 2. hydroxymethylglutaryl*.tw. 3. HMG‐CoA*.tw. 4. (statin or statins).tw. 5. atorvastatin.tw. 6. cerivastatin.tw. 7. fluvastatin.tw. 8. lovastatin.tw. 9. pravastatin.tw. 10. simvastatin.tw. 11. lipitor.tw. 12. baycol.tw. 13. lescol.tw. 14. mevacor.tw. 15. altocor.tw. 16. pravachol.tw. 17. lipostat.tw. 18. zocor.tw. 19. mevinolin.tw. 20. compactin.tw. 21. fluindostatin.tw. 22. rosuvastatin.tw. 23. dalvastatin.tw. 24. cranoc.tw. 25. canef.tw. 26. locol.tw. 27. lochol.tw. 28. leucol.tw. 29. lescol.tw. 30. monacolin.tw. 31. medostatin.tw. 32. mevinacor.tw. 33. livalo.tw. 34. pitava.tw. 35. pitavastatin.tw. 36. pravasin.tw. 37. mevalotin.tw. 38. gerosim.tw. 39. lipex.tw. 40. zenas.tw. 41. crestor.tw. 42. meglutol.tw. 43. or/1‐42 44. exp cardiovascular disease/ 45. cardio*.tw. 46. cardia*.tw. 47. heart*.tw. 48. coronary*.tw. 49. angina*.tw. 50. hyperlipidemia/ 51. exp cholesterol/ 52. exp lipid blood level/ 53. hyperlipid*.tw. 54. hypercholesterol*.tw. 55. cholesterol*.tw. 56. hypercholester?emia*.tw. 57. hyperlip?emia*.tw. 58. triglycerid*.tw. 59. hypertriglycerid?emia*.tw. 60. hyperlipoprotein?emia*.tw. 61. LDL.tw. 62. HDL.tw. 63. or/44‐62 64. 43 and 63 65. ((2007* or 2008* or 2009* or 2010* or 2010* or 2011*) not ("200701" or "200702" or "200703" or "200704" or "200705" or "200706" or "200707" or "200708" or "200709" or "200710")).em. 66. (random$ or placebo$ or single blind$ or double blind$ or triple blind$).ti,ab. 67. retracted article/ 68. 66 or 67 69. (animal$ not human$).sh,hw. 70. (book or conference paper or editorial or letter or review).pt. not exp randomized controlled trial/ 71. (random sampl$ or random digit$ or random effect$ or random survey or random regression).ti,ab. not exp randomized controlled trial/ 72. 69 or 70 or 71 73. 68 not 72 74. 64 and 65 and 73 75. limit 74 to embase
Data and analyses
Comparison 1. Mortality and Morbidity.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1.1 Total Mortality | 13 | 48060 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.79, 0.94] |
| 1.2 Total Number of CHD Events | 14 | 48049 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.67, 0.80] |
| 1.3 Number of Fatal CHD Events | 10 | 46094 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.70, 0.96] |
| 1.4 Number of Non‐fatal CHD Events | 11 | 40977 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.59, 0.76] |
| 1.5 Total Number of CVD Events | 9 | 23805 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.70, 0.81] |
| 1.6 Number of Fatal CVD Events | 5 | 34012 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.72, 0.96] |
| 1.7 Number of Non‐fatal CVD Events | 2 | 8696 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.62, 0.96] |
| 1.8 Total Number of Stroke Events | 10 | 40295 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.68, 0.89] |
| 1.9 Number of Fatal Stroke Events | 3 | 27238 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.18, 2.23] |
| 1.10 Number of Non‐fatal Stroke Events | 5 | 28097 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.58, 0.83] |
| 1.11 Total Number of Fatal and Non‐fatal CHD, CVD and Stroke Events | 4 | 35254 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.58, 0.73] |
| 1.12 Number of Study Participants who underwent Revascularisation | 7 | 42403 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.54, 0.72] |
Comparison 2. Lipids (mmol/L).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2.1 Total Cholesterol (mmol/L) | 14 | 34122 | Mean Difference (IV, Random, 95% CI) | ‐1.05 [‐1.35, ‐0.76] |
| 2.2 LDL Cholesterol (mmol/L) | 16 | 41380 | Mean Difference (IV, Random, 95% CI) | ‐1.00 [‐1.16, ‐0.85] |
Comparison 3. Adverse Events.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3.1 Number of study participants who had adverse events | 12 | 40716 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.97, 1.03] |
| 3.2 Number of study participants who stopped treatment due to adverse events | 9 | 21642 | Odds Ratio (M‐H, Random, 95% CI) | 0.86 [0.65, 1.12] |
| 3.3 Number of study participants who were admitted to hospital | 2 | 19707 | Risk Ratio (M‐H, Random, 95% CI) | 0.74 [0.38, 1.41] |
| 3.4 Number of study participants who developed cancer | 11 | 38739 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.93, 1.10] |
| 3.5 Number of study participants who developed myalgia or muscle pain | 9 | 37938 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.97, 1.09] |
| 3.6 Number of study participants who developed rhabdomyolysis | 6 | 38468 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.23, 4.38] |
| 3.7 Number of study participants who developed diabetes | 2 | 24407 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.18 [1.01, 1.39] |
| 3.8 Number of study participants who developed haemorrhagic stroke | 2 | 25634 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.54, 1.75] |
| 3.9 Number of study participants who had elevated liver enzymes | 10 | 40094 | Risk Ratio (M‐H, Random, 95% CI) | 1.16 [0.87, 1.54] |
| 3.10 Number of study participants who developed renal disorder | 4 | 27804 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.99, 1.26] |
| 3.11 Number of study participants who developed arthritis | 2 | 7586 | Odds Ratio (M‐H, Random, 95% CI) | 1.20 [0.82, 1.75] |
Comparison 4. Treatment Compliance.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 4.1 Treatment Compliance | 8 | 41712 | Risk Ratio (M‐H, Random, 95% CI) | 1.08 [0.98, 1.18] |
Comparison 5. Sensitivity Analysis.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 5.1 Early stopping of trials and total mortality | 11 | 46811 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.74, 0.92] |
| 5.1.1 trials stopped early | 3 | 27245 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.72, 0.96] |
| 5.1.2 trials with no early stop | 8 | 19566 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.69, 0.98] |
| 5.2 Early stopping of trials and total CHD events | 14 | 48066 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.67, 0.80] |
| 5.2.1 trials stopped early | 3 | 27265 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.58, 0.79] |
| 5.2.2 trials with no early stop | 11 | 20801 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.69, 0.84] |
| 5.3 Study Size for total Mortality | 11 | 46811 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.74, 0.92] |
| 5.3.1 Over 1000 participants | 6 | 43754 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.74, 0.92] |
| 5.3.2 Under 1000 participants | 5 | 3057 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.50, 1.82] |
| 5.4 Study Size for total CHD events | 14 | 48066 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.73 [0.67, 0.80] |
| 5.4.1 Over 1000 participants | 6 | 43597 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.67, 0.80] |
| 5.4.2 Under 1000 participants | 8 | 4469 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.59 [0.39, 0.90] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
ACAPS 1994.
| Study characteristics | ||
| Methods | Randomised trial 4 x 4 factorial. | |
| Participants | 919 participants based in the USA aged 40 ‐ 79 (mean age of 62); 52% men. None with any clinical evidence of CVD. | |
| Interventions | 20 mg lovastatin + 1 mg warfarin versus placebo followed up for 34 months. | |
| Outcomes | Carotid atherosclerosis, cholesterol, fatal + non‐fatal CHD events, stroke. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocked randomisation stratified by centre |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Carers and patients were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT, no drop‐outs reported |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Funded by pharmaceutical industry |
Adult Japanese MEGA Study.
| Study characteristics | ||
| Methods | Randomised trial. | |
| Participants | 7832 participants with hypercholesterolaemia based in Japan aged 40‐70 (mean age 59); 32% men. None with any clinical evidence of CVD. | |
| Interventions | 10‐20 mg pravastatin versus placebo; all participants got advice on diet; follow‐up 5 years. | |
| Outcomes | Primary: composite of major CVD events, sudden cardiac death, angina and revascularisation. Single outcomes included: all‐cause mortality, total CVD events, fatal and non‐fatal MI, stroke and TIA events, sudden cardiac death, angina and revascularisation, cholesterol, adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation by permuted block method. |
| Allocation concealment (selection bias) | Low risk | Central laboratory. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Single blinded, endpoint committee was blinded only because investigators stated that placebo‐controlled trials are regarded with suspicion by Japanese participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT used 2% dropped out. |
| Selective reporting (reporting bias) | High risk | Not all adverse events reported. We wrote to the authors asking for clarity regarding data on serious events. The authors responded saying they were unable to send the data. |
| Other bias | Low risk | Groups were comparable at baseline and includes all the major prognostic factors. Funded by pharmaceutical industry. |
AFCAPS/TexCAPS 1998.
| Study characteristics | ||
| Methods | Randomised trial. | |
| Participants | 6606 participants in Texas, USA; mean age 58; 57.5% men; 89% Caucasian. None with any clinical evidence of CVD. | |
| Interventions | 20‐40 mg lovastatin compared with placebo; follow‐up for 5.2 years; all participants received advice on diet. | |
| Outcomes | Primary: composite of fatal and non‐fatal MI and fatal CHD events. Single outcomes included: all‐cause mortality, fatal and non‐fatal CVD + stroke events, heart failure and adverse events. | |
| Notes | Trial was stopped prematurely. To be terminated when 320 participants had experienced primary outcome event. Stopped when 267 had done at 5.2 years. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind: participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT used no drop‐outs reported |
| Selective reporting (reporting bias) | Low risk | Other than results for cholesterol |
| Other bias | Unclear risk | Funded by pharmaceutical industry |
ASPEN 2006.
| Study characteristics | ||
| Methods | Randomised trial. | |
| Participants | 2410 participants with type 2 diabetes based in 16 developed countries with mean age 60; 62.5% men; 84% Caucasian. < 10% with clinical evidence of CVD. | |
| Interventions | 10 mg atorvastatin versus placebo; follow‐up of 2.4 years (for primary prevention participants). | |
| Outcomes | Primary: composite of fatal MI, stroke, sudden cardiac death, heart failure, CVD death. Single outcomes included: non‐fatal or silent MI + stroke, revascularisation, resuscitated cardiac arrest, TIA, unstable angina, peripheral arterial disease, Ischaemic heart failure and adverse events. | |
| Notes | Primary prevention participants recruited 2‐3 years into the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind: participants and outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT used 22% drop‐outs reported |
| Selective reporting (reporting bias) | Low risk | Other than not providing results on adverse events for primary prevention group |
| Other bias | Unclear risk | Funded by pharmaceutical industry |
Bone 2007.
| Study characteristics | ||
| Methods | Randomised control trial. | |
| Participants | 626 Post‐menopausal women aged 40‐75 years with dyslipidaemia and no history of CHD or diabetes. None with any clinical evidence of CVD. | |
| Interventions | Atorvastatin (10/20/40/80 mg/day) with matching placebo. All patients were instructed to be on NCEP ATP III diet. | |
| Outcomes | Primary: Percentage change in lumbar spine bone marrow density Seconday: Percentage change in femoral neck etc BMD by DXA. other; adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated pseudo random code |
| Allocation concealment (selection bias) | Low risk | Random permuted blocks |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | States double blind but only reported that participants were blinded to interventions |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT used, 5% dropped out. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Unclear risk | Funded by pharmaceutical industry |
CAIUS 1996.
| Study characteristics | ||
| Methods | Randomised trial. | |
| Participants | 305 participants with hypercholesterolaemia based in Italy with mean age 55; 53% men. None with any clinical evidence of CVD. | |
| Interventions | 40 mg pravastatin versus placebo; follow‐up of three years. | |
| Outcomes | Slope of carotid artery, fatal and non‐fatal MI, angina, revascularisations, cholesterol and adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Independent co‐ordinating centre controlled allocation |
| Allocation concealment (selection bias) | Low risk | Independent co‐ordinating centre controlled allocation |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Double‐blind: participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT used, 13% dropped out |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Funded by pharmaceutical industry |
CARDS 2008.
| Study characteristics | ||
| Methods | Randomised control trial. | |
| Participants | 2838 participants with diabetes based in UK and Ireland aged 40‐75 years (mean 61.7); 68% men; 94.5% Caucasian. None with any clinical evidence of CVD. | |
| Interventions | 10 mg atorvastatin, all patients were given counselling on cessation of smoking; follow up of 3.9‐4 years. | |
| Outcomes | Primary: composite of fatal and non‐fatal MI, acute CHD death, resuscitated cardiac arrest. Single outcomes included: all‐cause mortality, fatal and non‐fatal or silent MI + stroke, revascularisation, resuscitated cardiac arrest, total CVD events, adverse events and cholesterol. | |
| Notes | Trial stopped prematurely due to large beneficial treatment effect. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation code |
| Allocation concealment (selection bias) | Low risk | Staff and patients unaware of computer‐generated randomisation code |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Triple‐blind: participants, personnel and outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT used, no drop‐outs reported |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Funded by pharmaceutical industry |
CELL A 1996.
| Study characteristics | ||
| Methods | Randomised trial; 2 x 3 factorial design. | |
| Participants | 228 participants with hyperlipidaemia based in Sweden with a mean age of 49; 85% men, <10% had clinical evidence of CVD. | |
| Interventions | 10‐40 mg pravastatin plus intensive dietary advice versus placebo; follow‐up for 18 months. | |
| Outcomes | Fatal MI, cholesterol, quality of life. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Not described |
| Allocation concealment (selection bias) | Low risk | Randomisation performed separately for each centre with numbers allocated to intervention and control groups |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind: participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT used, 14.5% dropped out |
| Selective reporting (reporting bias) | High risk | Adverse events rates not provided for each group |
| Other bias | Unclear risk | Funded by pharmaceutical industry |
CELL B 1996.
| Study characteristics | ||
| Methods | Randomised trial; 2 x 3 factorial design. | |
| Participants | 227 participants with hyperlipidaemia based in Sweden with a mean age of 49; 85% men, <10% had clinical evidence of CVD. | |
| Interventions | 10‐40 mg pravastatin plus dietary advice versus placebo; follow‐up for 18 months. | |
| Outcomes | Fatal MI, cholesterol, quality of life. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Not described |
| Allocation concealment (selection bias) | Low risk | Randomisation performed separately for each centre with numbers allocated to intervention and control groups |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind: participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT used, 6% dropped out |
| Selective reporting (reporting bias) | Unclear risk | CVD and adverse events rates not provided for each group |
| Other bias | Unclear risk | Funded by pharmaceutical industry |
CERDIA 2004.
| Study characteristics | ||
| Methods | Parallel group randomised control trial. | |
| Participants | 250 patients with type 2 Diabetes aged 30‐80 years. None with any clinical evidence of CVD. | |
| Interventions | 0.4 mg of Cerivastatin until 08/2001 then Simvastain 20 mg. | |
| Outcomes | Primary outcome: Change in mean common carotid intima media thickness (IMT) after 24 months of intervention. Secondary outcomes: Changes in Mean + maximum IMT at 24 months, CVD events, amputation due to atherosclerotic disease, serum levels of LDL and total cholesterol. | |
| Notes | In August 2001, Cerivstatin was withdrawn from market. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Predetermined computer‐generated randomisation sequence in block of 10. |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | States double blind but only reported that participants were blinded to interventions |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT not used, 27% dropped out. |
| Selective reporting (reporting bias) | Low risk | States double blind but unclear who was blinded. |
| Other bias | Unclear risk | Comparable at baseline, including all major prognostic group however its unclear if it was valid and reliable method to determine outcomes. Funded by pharmaceutical industry. |
Derosa 2003.
| Study characteristics | ||
| Methods | Randomised trial. | |
| Participants | 47 participants with hypercholesterolaemia based in Italy with a mean age of 51; 46% men. None with any clinical evidence of CVD. | |
| Interventions | 80 mg fluvastatin versus placebo; all participants were given advice on diet and exercise ; follow‐up for one year. | |
| Outcomes | Adverse events, cholesterol. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Envelopes containing randomisation codes prepared by statistician |
| Allocation concealment (selection bias) | Low risk | Allocation code could only be identified by statistician and person responsible for statistical analysis |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Single blind: participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT used, no drop‐outs reported |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Funded by pharmaceutical industry |
HYRIM 2007.
| Study characteristics | ||
| Methods | Randomised trial 2 x 2 factorial design. | |
| Participants | 287 men with hypertension based in Norway aged 40‐75 years (mean age 57). None with any clinical evidence of CVD. | |
| Interventions | 40 mg fluvastatin; follow‐up four years. | |
| Outcomes | Primary: composite of fatal and non‐fatal MI, + stroke, angina, sudden CHD death, TIA and heart failure. MACE: composite of cardiac death, fatal and non‐fatal MI and revascularisation. Single outcomes included: adverse events, cholesterol. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind: participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described and no drop‐outs reported |
| Selective reporting (reporting bias) | Low risk | Mostly but not for adverse events and cholesterol level at baseline and at 4‐year follow‐up not provided |
| Other bias | Unclear risk | Groups comparable at baseline, including all major prognostic factors, structured interview for outcomes and side effects confirmed by independent expert committee. Funded by pharmaceutical industry. |
JUPITER 2008.
| Study characteristics | ||
| Methods | Randomised control trial. | |
| Participants | 17,802 participants (intervention:8901, control 8901) > 50 years. None with any clinical evidence of CVD. | |
| Interventions | Rosuvastatin 20 mg daily. | |
| Outcomes | First occurrence of major cardiovascular event, revascularisation, hospital admission for angina, MI, stroke, all‐cause death, CVD death and adverse events. | |
| Notes | Stopped early with a follow‐up of 1.9 years. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation done in block of 4, use of Interactive voice response system‐generated allocation sequence. |
| Allocation concealment (selection bias) | Low risk | Stratified according to the centre |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind, participants and outcomes assessed by end point committee |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT used, no drop‐outs reported |
| Selective reporting (reporting bias) | Low risk | There was limited data on LDL and TC at the end of trial |
| Other bias | High risk | Groups comparable at baseline, including all major prognostic factors, structured interview assessing outcomes and adverse effects confirmed by independent expert committee. Trial was stopped early with a follow‐up of 1.9 years. Funded by pharmaceutical industry. |
KAPS 1995.
| Study characteristics | ||
| Methods | Randomised trial. | |
| Participants | 447 men based in Finland aged 44‐65 years (mean 57). < 10% with clinical evidence of CVD. | |
| Interventions | 40 mg pravastatin versus placebo; follow‐up of 3 years. | |
| Outcomes | Carotid atherosclerotic progression, total mortality, fatal and non‐fatal MI events, stroke, adverse events, cholesterol, other cardiac death, revascularisations, non cardiac death and heart failure. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Biostatistician prepared randomisation scheme |
| Allocation concealment (selection bias) | Low risk | Tablets were masked by pharmaceutical company |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind: participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT was not used, 17% patients dropped out |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Funded by pharmaceutical industry |
METEOR 2010.
| Study characteristics | ||
| Methods | Randomised control trial. | |
| Participants | 984 asymptomatic individuals with a mean age of 57 years. None with any clinical evidence of CVD. | |
| Interventions | Rosuvastatin 40 mg/ day. | |
| Outcomes | Primary: Mean of 12 Carotid Intima media (CIMT) thickness measurements. Secondary: CIMT measurements of left and right common carotid artery. Other relevant outcomes: adverse events, cholesterol levels. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation in block of 7 (5 to intervention and 5 to control) |
| Allocation concealment (selection bias) | Low risk | Blinded study medication |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding both to participants and personnel. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT used 25‐6% dropped out. |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Funded by pharmaceutical industry |
MRC/BHF Heart Protection.
| Study characteristics | ||
| Methods | Randomised trial (2 x 2 factorial design). | |
| Participants | 3982 patients with no prior CHD with diabetes mellitus as a subset of 20,536 UK adults aged 40‐80 years. | |
| Interventions | 40 mg simvastatin compared with placebo, follow‐up 5.3 years for all participants. | |
| Outcomes | Composite of coronary and vascular events, stroke, revascularisations. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Not described |
| Allocation concealment (selection bias) | Low risk | Central telephone system used |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind: participants and outcome assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not described |
| Selective reporting (reporting bias) | High risk | Only CVD event results provided for this subgroup |
| Other bias | Unclear risk | Funded by pharmaceutical industry. |
PHYLLIS 2004.
| Study characteristics | ||
| Methods | Randomised trial 4 x 4 factorial. | |
| Participants | 253 men and women aged 45‐70 (mean age 58) with hypertension, hypercholesterolaemia and asymptomatic carotid atherosclerosis based in Italy. None with any clinical evidence of CVD. | |
| Interventions | 25 mg hydrochlorothiazide + 40 mg pravastatin followed up for 2.6 years. | |
| Outcomes | Primary outcomes: carotid atherosclerosis. Secondary outcomes: non‐fatal MI, CVD death, stroke, cholesterol and cancer. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was computer‐generated in blocks of 4 |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT used, 20% drop‐outs reported |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Funded by pharmaceutical industry |
PREVEND IT 2004.
| Study characteristics | ||
| Methods | Randomised trial 2 x 2 factorial design. | |
| Participants | 864 participants with microalbuminuria based in Holland aged 28‐75 years (mean age 51); 64.5% men; 96% Caucasian. < 10% with clinical evidence of CVD. | |
| Interventions | 40 mg pravastatin versus placebo; follow‐up 3.8 years. | |
| Outcomes | Primary outcome: composite of fatal and non‐fatal CVD events. Single outcomes included fatal CVD events, stroke, heart failure, non‐fatal MI and cholesterol. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was computer‐generated. |
| Allocation concealment (selection bias) | Low risk | Participants randomised were allocated to a treatment number. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | ITT used but confined to CVD events, 6% dropped out |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Funded by pharmaceutical industry |
WOSCOPS.
| Study characteristics | ||
| Methods | Randomised trial. | |
| Participants | 6595 men with hypercholesterolaemia based in Scotland aged 45‐64 (mean age 55). < 10% with clinical evidence of CVD. | |
| Interventions | 40 mg pravastatin versus placebo; follow‐up 4.9 years. | |
| Outcomes | Primary outcome: composite of non‐fatal MI and CHD death. Single outcomes included total mortality, fatal CVD events, cholesterol, revascularisations, non‐fatal MI and CHD death and adverse events. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Blocks of random numbers and treatment assigned randomly |
| Allocation concealment (selection bias) | Low risk | All trial personnel remained unaware of the participant's treatment assignment throughout the study. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind: participants and personnel |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT used, 30% drop‐outs reported |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Unclear risk | Funded by pharmaceutical industry |
BMD: bone mineral density CHD: coronary heart disease CIMT: carotid intima media thickness CVD: cardiovascular disease DXA: Dual‐energy X‐ray absorptiometry ITT: intention‐to‐treat LDL: low density lipoprotein MI: myocardial infarction TC: total cholesterol TIA: transient ischaemic attack
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agewall 2006 | Treatment length was less than a year |
| ALLHAT‐LLT 2002 | 15% patients had history of CVD |
| Ames 2011 | No relevant outcomes reported |
| Anderson 1993 | No placebo ‐ statin + antioxidant versus statin + antioxidant |
| ASCOT‐LLA 2003 | 18% patients had history of CVD |
| ASTRONOMER 2010 | Study is not a primary prevention |
| Bak 1998 | Treatment length was less than a year |
| BCAPS 2001 | 11% patients had history of CVD |
| Boccuzzi 1991 | Not a RCT ‐ all participants were given Simvastatin |
| Branchi 1995 | Control Group was not randomised |
| Byington 1993 | Secondary prevention |
| CASHMERE 2007 | Treatment length was less than a year |
| Cassader 1993 | Treatment length was less than a year |
| CHALLENGER | Patients with CHD were included in study |
| Chan 1996 | Treatment length was less than a year |
| Chuengsamarn 2010 | Study is not a RCT |
| CLIP 2002 | Not a RCT ‐ All participants were given Pravastatin |
| Cowan 2010 | Follow‐up duration was inadequate |
| Coylewright 2008 | Secondary prevention |
| CRISP 1994 | Treatment length was less than a year |
| CURVES 1998 | No placebo ‐ statin versus statin |
| Dangas 1999 | Treatment length was less than a year |
| Davidson 1997 | No placebo ‐ statin versus statin |
| Duffy 2001 | Treatment length was less than a year |
| Egashira 1994 | Not a RCT ‐ All participants were given Pravastatin |
| Eriksson 1998 | No control group ‐ Pravastatin vs. Cholestyramine |
| EXCEL 1990 | Treatment length was less than a year |
| Faergeman 2009 | Comparison of two statins/doses |
| FAST 2002 | Over 40% had CVD and over 14% had CHD |
| Ferrari 1993 | Treatment length was less than a year |
| Gentile 2000 | Treatment length was only 24 weeks |
| Glasser 1996 | Length of treatment was only 12 weeks |
| Gomez‐Garcia 2007 | Follow‐up period was less than a year |
| Guisasola 2009 | Study is not a RCT |
| Hokuriku NK‐104 Study 02 | Not a RCT ‐ All participants were given intravasating |
| Hongo 2010 | No placebo control group |
| Hufnagel 2000 | Treatment length was less than a year |
| Italian Family Physician | Not a RCT ‐ open‐labelled |
| Jardine 2006 | Outcomes provided were aggregated. Unable to ascertain actual numbers for cardiac death and myocardial infarction. |
| JART 2011 | Comparison was between two types of statin |
| JELIS 2009 | No placebo/control group and study was a secondary prevention |
| J‐LIT 2007 | Study is not a RCT |
| Jones 1991 | Treatment length was less than a year |
| Kappelle 2009 | Treatment length was less than a year |
| KLIS 2000 | Not randomised |
| Kojima 2010 | Secondary prevention |
| Lemaitre 2002 | Cohort study |
| Lin 2010 | No relevant outcomes reported. |
| LIPID 2010 | Secondary prevention |
| Mareev 2008 | Previous history of cardiovascular disease in most patients |
| McDermott 2003 | Participants were not randomised to statins or no statins |
| Mizuguchi 2008 | Study outcomes are not relevant to current review. |
| Mohler 2003 | Patients recruited had peripheral arterial disease |
| Mok 2009 | Secondary prevention |
| Muldoon 1997 | Treatment length is only six months |
| Nephrotic Syndrome Study | Treatment length was less than a year |
| Ohta 2000 | Treatment length was less than a year |
| Oi 1997 | No placebo or control group |
| Olzowy 2007 | Outcomes of the study are not relevant to review. |
| Ormiston 2003 | Not a RCT ‐ all participants were given statins |
| Pavia 2000 | Intervention included Amlodipine |
| Pitt 1999 | No placebo ‐ statins versus angioplasty |
| POSCH 1990 | Statins were not used |
| Pravastatin Multi 1993 | Treatment length was less than a year |
| PROSPER 2002 | More than 10% of the participants had CVD |
| Safaei 2007 | Study is not a RCT |
| SANDS 2008 | Comparison of two different treatment algorithms which included statins |
| Schmermund 2006 | Comparision of 10 mg vs 80 mg of statin. |
| Sen 2000 | Treatment length was less than a year |
| Sprecher 1994 | Treatment length was less than a year |
| Stein 1997 | Treatment length was less than a year |
| Su 2000 | Treatment length was less than a year |
| Tanaka 2001 | Treatment length was less than a year |
| Tarin 2010 | Secondary prevention |
| Teixeira 2011 | No relevant outcomes |
| Tekin 2008 | Not randomised |
| Thomas 1993 | Treatment length was less than a year |
| Thrombosis Prevention | Statins were not used |
| Togha 2009 | Patients had a chronic disease. |
| Tran 2007 | The data are based on inadequate length of treatment |
| Wallace 2003 | Treatment length was less than a year |
| Wu 2007 | No comparison group |
| Yu‐An 1998 | Treatment length was less than one year |
| Zachoval 2000 | Comparison of two statins |
CHD: coronary heart disease CVD: cardiovascular disease RCT: randomised controlled trial
Characteristics of studies awaiting classification [ordered by study ID]
Babes 2010.
| Methods | RCT |
| Participants | Young adults |
| Interventions | Statin |
| Outcomes | Endothelial dysfunction |
| Notes | We wrote on three separate occasions to the authors for further information on this study and did not receive a response. |
RCT: randomised controlled trial
Contributions of authors
Professor Shah Ebrahim and Professor George Davey Smith: Origination of idea, preparation of review on which this review is based, control of content.
Fiona Taylor: Assessed relevance and quality of papers, extracted data, analysed data and prepared the manuscript.
Mark Huffman: Helped screen abstracts for the update, contributed to the analysis and interpretation of data for the update.
Ana Macedo: Abstracted data for the update, helped assess adverse events and wrote to authors.
Kirsten Ward: Obtained papers, assessed relevance and quality of papers, extracted data, organised and analysed data.
Theresa Moore: Contributed to the early work on this review in addition to screening of abstracts for the update
Margaret Burke: Developed search strategy, ran searches and assessed relevance of papers.
Sources of support
Internal sources
Department of Social Medicine, University of Bristol, UK
External sources
Department of Health Funding for the Cochrane Heart Group, UK
Declarations of interest
None known.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
ACAPS 1994 {published data only}
- Cui Y, Watson DJ, Girman CJ, Shapiro DR, Gotto AM, Hiserote P, et al. Effects of increasing high-density lipoprotein cholesterol and decreasing low-density lipoprotein cholesterol on the incidence of first acute coronary events (from the Air Force/Texas Coronary Atherosclerosis Prevention Study). American Journal of Cardiology 2009;104(6):829-34. [DOI] [PubMed] [Google Scholar]
- Furberg C, Adams HP Jr, Appelgate WB, Byington RP, Espeland MA, Hartwell T, et al. Effect of lovastatin on early carotid atherosclerosis and cardiovascular events. Asymptomatic Carotid Artery Progression Study (ACAPS) Research Group. Circulation 1994;90(4):1679-87. [DOI] [PubMed] [Google Scholar]
- Kendrick J, Shlipak MG, Targher G, Cook T, Lindenfeld J, Chonchol M. Effect of Lovastatin on primary prevention of cardiovascular events in mild CKD and kidney function loss: A post hoc analysis of the Air Force/Texas Coronary Atherosclerosis Prevention Study. American Journal of Kidney Diseases 2009;55(1):42-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Splichal JE, Ornstein DL, Hong-Dice YG, Downs JR, Fischer JR. Lovastatin for the prevention of Melanoma: Analysis of AFCAPS/TexCaps. In: Proceedings of the American Society of Clinical Oncology. Vol. 20 (abstr 1399). 2001.
Adult Japanese MEGA Study {published data only}
- Brookes L, Nakamura H. MEGA: Management of elevated cholesterol in the primary prevention group of adult Japanese. American Heart Association 2005 Scientific Sessions 2008.
- Kushioro T, Mizuno K, Nakaya N, Ohashi Y, Tajima N, Teramoto T, et al for the Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese Study Group. Pravastatin for cardiovascular event primary prevention in patients with mild-to-moderate hypertension in the management of elevated cholesterol in the primary prevention group of adult Japanese (MEGA) study. Hypertension 2009;53:135-41. [DOI] [PubMed] [Google Scholar]
- Management of Elevated Cholesterol in the Primary Prevention Group of Adult Japanese (MEGA) Study Group. Design and baseline characteristics of a study of primary prevention of coronary events with pravastatin among Japanese with mildly elevated cholesterol levels. Circulation Journal 2004:860-7. [DOI] [PubMed]
- Nakagami, TNishimura, RSone, H. The role of cardiovascular risk factors in postmenopausal hypercholesterolemic women with abnormal fasting glucose: A post hoc analysis of the MEGA Study. In: Diabetologia. Vol. Conference abstract. 2010.
- Nakamura H, Arakawa K, Itakura H, Kitabatake A, Goto Y, Toyota T, et al, MEGA Study Group. Primary prevention of cardiovascular disease with pravastatin in Japan (MEGA Study): a prospective randomised controlled trial. Lancet 2006;368(9542):1155-63. [DOI] [PubMed] [Google Scholar]
- Nakamura H. Primary prevention of cardiovascular diseases among hypercholesterolemic Japanese with a low dose of pravastatin. Atherosclerosis Supplements 2007;8:13-7. [DOI] [PubMed] [Google Scholar]
- Nakamura H. Primary prevention trial by lowering hyperlipidemia on the cardiovascular disease (MEGA Study). [Japanese]. Nippon Ronen Igakkai Zasshi - Japanese Journal of Geriatrics 2009;46(1):18-21. [DOI] [PubMed] [Google Scholar]
- Nakamura H. The design and background characteristics of the study on the primary prevention of coronary events with pravastatin among Japanese with mildly elevated cholesterol levels (Japanese Mega Study). Atherosclerosis 2000;151(1):136. [Google Scholar]
- TeramotoT, Nakaya N, Yokoyama S, Ohashi Y, Mizuno K. Association between lowering low-density lipoprotein cholesterol with pravastatin and primary prevention of cardiovascular disease in mild to moderate hypercholesterolemic Japanese. Journal of Atherosclerosis & Thrombosis 2010;17:879-87. [DOI] [PubMed] [Google Scholar]
- Uchiyama S, Nakaya N, Mizuno K, Ohashi Y, Tajima N, Kushiro T, et al, MEGA Study Group. Risk factors for stroke and lipid-lowering effect of Pravastatin on the risk of stroke in Japanese patients with hypercholesterolemia: Analysis of data from the MEGA Study, a large randomized controlled trial. Journal of the Neurological Sciences 2009;284(1-2):72-6. [DOI] [PubMed] [Google Scholar]
AFCAPS/TexCAPS 1998 {published data only}
- Downs JR, Clearfield M, Weis S, Whitney E, Shapiro DR, Beere PA, et al. Primary prevention of acute coronary events with lovastatin in men and women with average cholesterol levels: results of AFCAPS/TexCAPS. Air Force/Texas Coronary Atherosclerosis Prevention Study. JAMA 1998;279(20):1615-22. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Rifai N, Clearfield M, Downs JR, Weis SE, Miles JS, et al. Measurement of C-reactive protein for the targeting of statin therapy in the primary prevention of acute coronary events. New England Journal of Medicine. 2001;344(26):1959-65. [DOI] [PubMed] [Google Scholar]
ASPEN 2006 {published data only}
- Knopp RH, D'Emden M, Smilde JG, Pocock SJ, on behalf of the ASPEN Study Group. Efficacy and safety of atorvastatin in the prevention of cardiovascular end points in subjects with type 2 diabetes: the Atorvastatin Study for Prevention of Coronary Heart Disease Endpoints in non-insulin-dependent diabetes mellitus (ASPEN). Diabetes Care 2006;29(7):1478-85. [DOI] [PubMed] [Google Scholar]
Bone 2007 {published data only}
- Bone HG, Kiel DP, Lindsay RS, Lewiecki EM, Bolognese MA, Leary ET, et al. Effects of atorvastatin on bone in postmenopausal women with dyslipidemia: A double-blind, placebo-controlled, dose-ranging trial. Journal of Clinical Endocrinology and Metabolism 2007;92(12):4671-77. [DOI] [PubMed] [Google Scholar]
CAIUS 1996 {published data only}
- Baldassarre D, Veglia F, Gobbi C, Gallus G, Ventura A, Crepaldi G, et al. Intima-media thickness after pravastatin stabilizes also in patients with moderate to no reduction in LDL-cholesterol levels: the carotid atherosclerosis Italian ultrasound study. Atherosclerosis 2000;151:575-83. [DOI] [PubMed] [Google Scholar]
- Mercuri M, Bond G, Sirtori CR, Veglia F, Crepaldi G, Feruglio S, et al. Pravastatin reduces carotid intima-media thickness progression in an asymptomatic hypercholesterolemic Mediterranean population: the Carotid Atherosclerosis Italian Ultrasound Study. American Journal of Medicine 1996;101:627-34. [DOI] [PubMed] [Google Scholar]
- Sirtori CR, Bianchi G, Bond MG, D'Alo G, Gallus G, Liberatore S, et al. Pravastatin intervention trial on carotid artery atherosclerosis in patients with mild hypercholesterolemia: the CAIUS Study. International Journal of Cardiac Imaging 1995;11(Suppl 2):119-24. [Google Scholar]
CARDS 2008 {published data only}
- Armani A, Toth P. The CARDS Trial: Diabetic patients dealt a winning hand. Current Atherosclerosis Reports 2006;8:429-32. [DOI] [PubMed] [Google Scholar]
- Charlton-Menys V, Betteridge DJ, Colhoun H, Fuller J, France M, Hitman GA, et al. Apolipoproteins, cardiovascular risk and statin response in type 2 diabetes: the Collaborative Atorvastatin Diabetes Study (CARDS). Diabetologia 2009;52(2):218-25. [DOI] [PubMed] [Google Scholar]
- Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, Neil HAW, Livingstone SJ, et al. Primary prevention of cardiovascular disease with atorvastatin in type 2 diabetes in the Collaborative Atorvastatin Diabetes Study (CARDS): multicentre randomised placebo-controlled trial. Lancet 2004;364:685-96. [DOI] [PubMed] [Google Scholar]
- Colhoun HM, Betteridge J, Durrington PN, Hitman GA, Neil HA, Livingstone SJ, et al, CARDS Investigators. Effects of atorvastatin on kidney outcomes and cardiovascular disease in patients with diabetes: an analysis from the Collaborative Atorvastatin Diabetes Study (CARDS). American Journal of Kidney Diseases 2009;54(5):810-9. [DOI] [PubMed] [Google Scholar]
- Colhoun HM, Thomason MJ, Mackness MI, Maton SM, Betteridge DJ, Durrington PN, et al. Design of the Collaborative Atorvastatin Diabetes Study (CARDS) in patients with Type 2 Diabetes. Diabetic Medicine 2002;19:201-11. [DOI] [PubMed] [Google Scholar]
- Fernandez De Bobadilla J, Lopez De Sa E, Troncoso IA, Moreno Gómez R, Rubio-Terrés C, Soto Alvarez J. Cost-effectiveness analysis of the use of atorvastatin in patients with type 2 diabetes mellitus: A pharmacoeconomic model of the CARDS study [Analisis coste-efectividad del uso de atorvastatina en pacientes diabeticos de tipo 2: Modelo farmacoeconomico del estudio CARDS]. Anales de Medicina Interna 2006;23(5):213-9. [DOI] [PubMed] [Google Scholar]
- Hitman GA, Colhoun H, Newman C, Szarek M, Betteridge DJ, Durrington PN, et al, CARDS Investigators. Stroke prediction and stroke prevention with Atorvastatin in the Collaborative Atorvastatin Diabetes Study (CARDS). Diabetic Medicine 2007;24(12):1313-21. [DOI] [PubMed] [Google Scholar]
- Jinnouchi Y, Yamagishi S, Takeuchi M, Ishida S, Jinnouchi Y, Jinnouchi J, et al. Atorvastatin decreases serum levels of advanced glycation end products (AGEs) in patients with type 2 diabetes. Clinical and Experimental Medicine 2006;6(4):191-3. [DOI] [PubMed] [Google Scholar]
- Neil HA, Demicco DA, Luo D, Betteridge DJ, Colhoun HM, Durrington PN, et al, CARDS Study Investigators. Analysis of efficacy and safety in patients aged 65-75 years at randomization: Collaborative Atorvastatin Diabetes Study (CARDS). Diabetes Care 2006;29(11):2378-84. [DOI] [PubMed] [Google Scholar]
- Newman CB, Szarek M, Colhoun HM, Betteridge DJ, Durrington PN, Hitman GA, et al. The safety and tolerability of atorvastatin 10 mg in the collaborative atorvastatin diabetes study (CARDS). Diabetes and Vascular Disease Research 2008;5(3):177-83. [DOI] [PubMed] [Google Scholar]
- Ramsey SD, Clarke LD, Roberts CS, Sullivan SD, Johnson SJ, Liu LZ. An economic evaluation of atorvastatin for primary prevention of cardiovascular events in type 2 diabetes. PharmacoEconomics 2008;26(4):329-39. [DOI] [PubMed] [Google Scholar]
CELL A 1996 {published data only}
- Lindholm LH, Ekbom T, Dash C, Eriksson M, Tibblin G, Schersten B, et al. The impact of health care advice given in primary care on cardiovascular risk. BMJ 1995;310(6987):1105-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm LH, Ekbom T, Dash C, Isacsson A, Schersten B, for the CELL Study Group. Changes in cardiovascular risk factors by combined pharmacological strategies: the main results of the CELL Study. Journal of Internal Medicine 1996;240:13-22. [DOI] [PubMed] [Google Scholar]
CELL B 1996 {published data only}
- Lindholm LH, Ekbom T, Dash C, Eriksson M, Tibblin G, Schersten B, et al. The impact of health care advice given in primary care on cardiovascular risk. BMJ 1995;310(6987):1105-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindholm LH, Ekbom T, Dash C, Isacsson A, Schersten B, for the CELL Study Group. Changes in cardiovascular risk factors by combined pharmacological and nonpharmacological strategies: the main results of the CELL Study. Journal of Internal Medicine 1996;240:13-22. [DOI] [PubMed] [Google Scholar]
CERDIA 2004 {published data only}
- Beishuizen ED, Jukema JW, Tamsma JT, de Ree MA, Vijver JC, Putter H, et al. No effect of statin therapy on silent myocardial ischemia in patients with Type 2 diabetes without manifest cardiovascular disease. Diabetes Care 2005;28(7):1675-9. [DOI] [PubMed] [Google Scholar]
- Beishuizen ED, Tamsma JT, Jukema JW, de Ree MA, Vijver JC, Meinders AE, et al. The effect of statin therapy on endothelial function in type 2 diabetes without manifest cardiovascular disease. Diabetes Care 2005;28(7):1668-74. [DOI] [PubMed] [Google Scholar]
- Beishuizen ED, Van De Ree MA, Jukema JW, Tamsma JT, Vijver JC, Meinders AE, et al. Two year statin therapy does not alter the progression of intima medica thickness in patients with type 2 diabetes without manifest cardiovascular disease. Diabetes Care 2004;27(12):2887-91. [DOI] [PubMed] [Google Scholar]
Derosa 2003 {published data only}
- Derosa G, Mugellini A, Ciccarelli L, Fogari R. Randomized, double-blind, placebo-controlled comparison of the action of orlistat, fluvastatin, or both on anthropometric measurements, blood pressure, and lipid profile in obese patients with hypercholesterolemia prescribed a standardized diet. Clinical Therapeutics 2003;25(4):1107-22. [DOI] [PubMed] [Google Scholar]
HYRIM 2007 {published data only}
- Hjelstuen A, Anderssen SA, Holme I, Seljeflot I, Klemsdal TO. Effect of lifestyle and/or statin treatment on soluble markers of atherosclerosis in hypertensives. Scandanavian Cardiovascular Journal 2007;41(5):313-20. [DOI] [PubMed] [Google Scholar]
- Sigmund A, Hjelstuen AK, Hjermann I, Bjerkan K, Holme I. Fluvastatin and lifestyle modification for reduction of carotid intima-media thickness and left ventricular mass progression in drug-treated hypertensives. Atherosclerosis 2004;178:387-97. [DOI] [PubMed] [Google Scholar]
JUPITER 2008 {published data only}
- Albert MA, Glynn RJ, Fonseca FA, Lorenzatti AJ, Ferdinand KC, MacFadyen JG, et al. Race, ethnicity, and the efficacy of rosuvastatin in primary prevention: the Justification for the Use of statins in Prevention: An interventionTrial Evaluating Rosuvastatin (JUPITER) trial. American Heart Journal 2011;162(1):106-14. [DOI] [PubMed] [Google Scholar]
- Bloom JM. Rosuvastatin, C-reactive protein, LDL cholesterol, and the JUPITER trial. Lancet 2009;374. [DOI] [PubMed]
- Danchin N. Rosuvastatin, C-reactive protein, LDL cholesterol, and the JUPITER trial. Lancet 2009;374. [DOI] [PubMed]
- De Tena, JG. Rosuvastatin, C-reactive protein, LDL cholesterol, and the JUPITER trial. Lancet 2009;374. [DOI] [PubMed]
- Everett BM, Glynn RJ, MacFadyen JG, Ridker PM. Rosuvastatin in the prevention of stroke among men and women with elevated levels of C-reactive protein: Justification for the use of statins in prevention: An intervention trial evaluating rosuvastatin (JUPITER). Circulation 2009;121:143-50. [DOI] [PubMed] [Google Scholar]
- Feeman Jr WE. Rosuvastatin, C-reactive protein, LDL cholesterol, and the JUPITER trial. Lancet 2009;374. [DOI] [PubMed]
- Fonseca FAH, Izar MC. Primary prevention of vascular events in patients with high levels of C-reactive protein: the JUPITER study. Expert Review of Cardiovascular Therapy 2009;7(9):1041-56. [DOI] [PubMed] [Google Scholar]
- Glynn RJ, Danielson E, Fonseca FAH, Genest J, Gotto AM Jr, Kastelein JJP, et al. A randomized trial of Rosuvastatin in the prevention of venous thromboembolism. New England Journal of Medicine 2009;360(18):1851-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn RJ, MacFadyen JG, Ridker PM. Tracking of high senstivity C-reactive protein after an initially elevated concentration: The JUPITER study. Clinical Chemistry 2008;55(2):305-12. [DOI] [PubMed] [Google Scholar]
- Glynn RJ, Wolfgang K, Nordestgaard BG, Shepherd J, Ridker PM. Rosuvastatin for primary prevention in older persons with elevated C-reactive protein and low to average low-density lipoprotein cholesterol levels: Exploratory analysis of a randomised trial. Annals of Internal Medicine 2010;152:488-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha AK, Hwang U, Keyhani S, et al (Eds). Statins lower the risk of first cardiovascular event in patients with high C-reactive protein and normal LDL cholesterol (outcomes research in review) [Rosuvastatin to prevent vascular event in men and women with elevated C-reactive protein; Ridker PM, Danielson E, Fonseca FAH et al. New England Journal of Medicine, 2008;359:2195-207]. Journal of Clinical Outcomes Management 2009;16(1):09, 12-13. [DOI] [PubMed]
- Kappagoda CT, Amsterdam EA. Another look at the results of JUPITER trial. American Journal of Cardiology 2009;104:1603-05. [DOI] [PubMed] [Google Scholar]
- Kerst AJFA. A randomised study of efficacy of statins in the prevention of venous thromboembolism [Een gerandomiseerd onderzoek naar de werkzaamheid van een statine bij de preventie van veneuze trombo-embolieen]. Geneesmiddelenbulletin 2009;43:104-5. [Google Scholar]
- Koenig W, Ridker PM. Rosuvastatin for primary prevention in patients with European systematic coronary risk evaluation risk >=5 or Framingham risk >20%: Post hoc analyses of the JUPITER trial requested by European health authorities. European Heart Journal 2011;32(1):75-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kones R. The JUPITER study, CRP screening and agressive statin therapy-implications for the primary prevention of cardiovascular disease. Therapeutic Advances in Cardiovascular Disease 2009;3(4):309-15. [DOI] [PubMed] [Google Scholar]
- Mora S, Glynn RJ, Hsia J, MacFadyen JG, Genest J, Ridker PM. Statins for the primary prevention of cardiovascular events in women with elevated high-sensitivity C-reactive protein or dyslipidemia: Results from the justification for the use of statins in prevention: An intervention trial evaluating rosuvastatin (JUPITER) and meta-analysis of women from primary prevention trials. Circulation 2010;121(9):1069-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissen SE. The Jupiter trial; Key findings, controversies and implications. Current Cardiology Reports 2009;11(2):81-2. [DOI] [PubMed]
- O'Keefe JH, Carter MD, Lavie CJ, Bell DS. The gravity of JUPITER ( Justification for the use of statins in primary prevention: An intervention trial evaluating Rosuvastatin). Postgraduate Medicine 2009;121(3):113-8. [DOI] [PubMed] [Google Scholar]
- Ohsfeldt RL, Gandhi SK, Smolen LJ, Jensen MM, Fox KM, Gold A, et al. Cost-effectiveness of rosuvastatin in patients at risk of cardiovascular disease based on findings from the JUPITER trial. Journal of Medical Economics 2010;13(3):428-37. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Danielson E, Fonseca FA, Genest J, Gotto AM Jr, Kastelein JJ, et al, JUPITER Trial Study Group. Reduction in C-reactive protein and LDL cholesterol and cardiovascular event rates after initiation of Rosuvastatin: A prospective study of JUPITER trial. Lancet 2009;373(9670):1175-82. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Danielson E, Fonseca FAH, Genest J, Gotto AM Jr, Kastelein JJ, et al, JUPITER Study Group. Rosuvastatin to prevent vascular events in men and women with elevated C-Reactive protein. New England Journal of Medicine 2008;359(21):2195-207. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Fonseca FA, Genest J, Gotto AM, Kastelein JJ, Khurmi NS, et al, JUPITER Trial Study Group. Baseline characteristics of participants in the JUPITER trial, a randomized placebo-controlled primary prevention trial of statin therapy among individuals with low low-density lipoprotein cholesterol and elevated high-sensitivity C-reactive protein. American Journal of Cardiology 2007;100(11):1659-64. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Genest J, Boekholdt SM, Libby P, Gotto AM, Nordestgaard BG, et al, JUPITER Trial Study Group. HDL cholesterol and residual risk of first cardiovascular events after treatment with potent statin therapy: An analyis from JUPITER trial. Lancet 2010;376(9738):333-9. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Glynn RJ. The JUPITER trial: Responding to the critics. American Journal of Cardiology 2010;106(9):1351-6. [DOI] [PubMed] [Google Scholar]
- Ridker PM, MacFadyen J, Cressman M, Glynn RJ. Efficacy of Rosuvastatin among men and women with moderate chronic kidney disease and elevated high sensitvity C-reactive protein. Journal of American College of Cardiology 2010;55(12):1266-73. [DOI] [PubMed] [Google Scholar]
- Ridker PM, MacFadyen J, Libby P, Glynn RJ. Relation of baseline high-sensitivity C-reactive protein level to cardiovascular outcomes with rosuvastatin in the justification for use of statins in prevention: An intervention trial evaluating Rosuvastatin (JUPITER). American Journal of Cardiology 2010;106:204-9. [DOI] [PubMed] [Google Scholar]
- Ridker PM, MacFadyen JG, Nordestgraard BG, Koenig W, Kastelein JJ, Genest J, et al. Rosuvastatin for primary prevention among individuals with elevated high-sensitivity C-reactive protein and 5% to 10% and 10% to 20% 10-year risk: Implications of the Justification for use of statins in prevention: An intervention trial evaluating Rosuvastatin (JUPITER) trial for "intermediate risk". Circulation:Cardiovascular Quality and Outcomes 2010;3(5):447-52. [DOI] [PubMed] [Google Scholar]
- Ridker PM. Rosuvastatin in the primary prevention of cardiovascular disease among patients with low levels of low-density lipoprotein cholesterol and elevated high sensitivity C-reactive protein; Rationale and design of the JUPITER trial. Circulation 2003;108:2292-7. [DOI] [PubMed] [Google Scholar]
- Ridker PM. The JUPITER trial: results, controversies, and implications for prevention. Circulation Cardiovascular Quality & Outcomes 2009;2(3):279-85. [DOI] [PubMed] [Google Scholar]
- Rosenstein R, Parra D. Rosuvastatin, C-reactive protein, LDL cholesterol, and the JUPITER trial. Lancet 2009;374. [DOI] [PubMed]
- Segura J, Ruilope. Rosuvastatin, C-reactive protein, LDL cholesterol, and the JUPITER trial. Lancet 2009;374. [DOI] [PubMed]
- Sgueglia GA, Crea F. The risks of new hypothesis: why did JUPITER patients have twice the predicted event rates of reduction. Journal of Cardiovascular Medicine 2011;12:66-70. [DOI] [PubMed] [Google Scholar]
- Slejko JF, Page RL, Sullivan PW. Statin therapy is cost-effective for vascular event prevention in adults with elevated C-reactive protein: Implications of Jupiter. In: Journal of the American College of Cardiology. Vol. Conference. 2010. [DOI] [PubMed]
- Sniderman AD. Rosuvastatin, C-reactive protein, LDL cholesterol, and the JUPITER trial. Lancet 2009;374. [DOI] [PubMed]
- Watson KE. The JUPITER trial: How will it change clinical practice. Reviews in Cardiovascular Medicine 2009;10(2):91-6. [PubMed] [Google Scholar]
KAPS 1995 {published data only}
- Salonen R, Nyyssonen K, Porkkala E, Rummukainen J, Belder R, Park J-S, et al. Kuopio Atherosclerosis Prevention Study (KAPS). A population-based primary preventive trial of the effect of LDL lowering on atherosclerotic progression in carotid and femoral arteries. Circulation 1995;92:1758-64. [DOI] [PubMed] [Google Scholar]
METEOR 2010 {published data only}
- Bots M, Palmer M, Grobbee D, Crouse J, O'Leary D, Evans G et al. C-Reactive protein lowering with rosuvastatin in the METEOR study. In: Atherosclerosis Supplement. Vol. 10, Issue 2. 2009. [DOI] [PubMed]
- Bots ML, Palmer MK, Dogan S, et al. Intensive lipid lowering may reduce progression of carotid atherosclerosis within 12 months of treatment: the METEOR study. Journal of Internal Medicine 2009;265:698-707. [DOI] [PubMed] [Google Scholar]
- Crouse J. Effects of statin therapy on the carotid artery. In: Atherosclerosis Supplement. Vol. 10, Issue 2. 2009.
- Crouse JR 3rd, Grobbee DE, O'Leary DH, Bots ML, Evans GW, Palmer MK, et al, METEOR Study Group. Carotid intima-media thickness in low-risk individuals with asymptomatic atherosclerosis: Baseline data from the METEOR study. Current Medical Research and Opinion 2007;23(3):641-8. [DOI] [PubMed] [Google Scholar]
- Crouse JR 3rd, Raichlen JS, Riley WA, Evans GW, Palmer MK, O'Leary DH, et al, METEOR Study Group. Effect of rosuvastatin on progression of carotid intima-media thickness in low-risk individuals with subclinical atherosclerosis: The METEOR Trial. JAMA 2007;297(12):1344-53. [DOI] [PubMed] [Google Scholar]
- Lauer MS. Primary prevention of atherosclerotic cardiovascular disease. The high public burden of low individual risk. JAMA 2007;297(12):1376-8. [DOI] [PubMed] [Google Scholar]
- Peters SA, Palmer MK, Grobbee DE, Crouse JR, O'Leary DH, Raichlen JS, et al. C-reactive protein lowering with rosuvastatin in the METEOR study. Journal of Internal Medicine 2010;268(2):155-61. [DOI] [PubMed] [Google Scholar]
- Sanne Peters S, Den Ruiters H. Completeness of carotid intima-media thickness measurements. Analaysis of the METEOR study. In: European Journal of Cardiovascular Prevention and Rehabilitation. Vol. 18. 2011.
MRC/BHF Heart Protection {published data only}
- Heart Protection Study Collaborative Group. C-reactive protein concentration and the vasculr benefits of statin theray: an analysis of 20,536 patients of the Heart Protection Study. Lancet 2011;377:469-76. [Google Scholar]
- Heart Protection Study Collaborative Group. Effects of 11 year mortality and morbidiity of lowering LDL cholesterol with simvastatin for about 5 years in 20,536 high-risk individuals: a randomised controlled trial. Lancet 2011;378:2013-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of Choleterol lowering with simvastatin in 20 536 high-risk individuals: a randomised placebo-controlled trial. Lancet 2002;360:7-22. [Google Scholar]
- Heart Protection Study Collaborative Group. Randomised trial of the effects of cholesterol-lowering with simvastatin on peripheral vascular and other major vascular outcomes. Journal of Vascular Surgery 2007;45:645-54. [Google Scholar]
- Heart Protection Study Collaborative Group. Statin cost-effectiveness in the United States for people at different vascular risk levels. Circulation-Cardiovascular Quality and Outcomes 2009;2:65-72. [DOI] [PubMed] [Google Scholar]
- MRC/BHF Heart Protection Study Collaborative Group. Effects of simvastatin 40 mg daily on muscle and liver adverse effects in a 5-year randomized placebo-controlled trial in 20,536 high-risk people. BMC Clinical Pharmacology 2009;9:1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
PHYLLIS 2004 {published data only}
- Mancia G, Parati G, Revera M, Bilo G, Giuliano A, Veglia F, et al. Statins, antihypertensive treatment, and blood pressure control in clinic and over 24 hours: evidence from PHYLLIS randomised double blind trial. BMJ 2010;340:c1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanchetti A, Crepaldi G, Bond M, Gallus G, Veglia F, Mancia G, et al, PHYLLIS Investigators. Different effects of anti-hypertensive regimens based on fosinopril or hydrochlorothiazide with or without lipid lowering pravastatin on progression of asymptomatic carotid atherosclerosis: principal results of PHYLLIS- a randomised double blind trial. Stroke 2004;35(12):2807-12. [DOI] [PubMed] [Google Scholar]
PREVEND IT 2004 {published data only}
- Asselbergs FW, Diercks GFH, Hillege HL, Boven AJ, Janssen WMT, Voors AA, et al. Effects of fosinopril and pravastatin on cardiovascular events in subjects with microalbinuria. Circulation 2004;110:2809-16. [DOI] [PubMed] [Google Scholar]
- Asselbergs FW, Hillege HL, Van Gilst WH. Framingham score and microalbuminuria: combined future targets for primary prevention? Kidney international.Supplement 2004;66(supplement 92):S111-4. [DOI] [PubMed] [Google Scholar]
- Asselbergs FW, Harst P, Roon AM, Hillege HL, Jong PE, Gans RO, et al. Long-term effects of pravastatin and fosinopril on peripheral endothelial function in albuminuric subjects. Atherosclerosis 2008;196(1):349-55. [DOI] [PubMed] [Google Scholar]
WOSCOPS {published data only}
- Caro J, Klittich W, McGuire A, Ford I, Norrie J, Pettit D, et al. The West of Scotland Coronary Prevention Study: economic benefit analysis of primary prevention with pravastatin. BMJ 1997;315:1577-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford I, Murray H, Packard CJ, Shepherd J, Macfarlane PW, Cobbe SM, Scotland Coronary Prevention Study Group. Long-term follow-up of the West of Scotland coronary prevention study. New England Journal of Medicine 2007;357(15):1477-86. [DOI] [PubMed] [Google Scholar]
- Macfarlane PW, Norrie J. The value of the electrocardiogram in risk assessment in primary prevention: Experience from the West Scotland Coronary Prevention Study. Journal of Electrocardiology 2007;40:101-9. [DOI] [PubMed] [Google Scholar]
- Sheperd J, Park JS. Prevention of heart disease: is LDL reduction the outcome of choice? No, there is more. Value In Health 1998;1(2):120-4. [DOI] [PubMed] [Google Scholar]
- Shepherd J, . Cholesterol lowering with statins: How WOSCOPS confounded the skeptics. Atherosclerosis Supplements 2007;s8:9-12. [DOI] [PubMed] [Google Scholar]
- Shepherd J, Cobbe SM, Ford I, Isles CG, Latimer AR, MacFarlane PW, et al. Prevention of coronary heart disease with pravastatin in men with hypercholesterolemia. New England Journal of Medicine 1995;33(20):1301-7. [DOI] [PubMed] [Google Scholar]
- The West of Scotland Coronary Prevention Study Group. A coronary primary prevention study of Scottish men aged 45-64 years: trial design. Journal of Clinical Epidemiology 1992;45(8):849-60. [DOI] [PubMed] [Google Scholar]
- The West of Scotland Coronary Prevention Study Group. Baseline risk factors and their associations with outcome in the West of Scotland Coronary Prevention Study. American Journal of Cardiology 1997;79:756-62. [DOI] [PubMed] [Google Scholar]
- The West of Scotland Coronary Prevention Study Group. Compliance and adverse event withdrawal: their impact on the West of Scotland Coronary Prevention Study. European Heart Journal 1997;18:1718-24. [DOI] [PubMed] [Google Scholar]
- West of Scotland Coronary Prevention Group. West of Scotland Coronary Prevention Study: identification of high-risk groups and comparison with other cardiovascular intervention trials. Lancet 1996;348:1339-42. [PubMed] [Google Scholar]
- West of Scotland Coronary Prevention Study Group. Influence of pravastatin and plasma lipids on clinical events in the West of Scotland Coronary Prevention Study (WOSCOPS). Circulation 1998;97:1440-5. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Agewall 2006 {published data only}
- Agewall S, Hernberg A. Atorvastatin normalizes endothelial function in healthy smokers. Clinical Science 2006;111(1):87-91. [DOI] [PubMed] [Google Scholar]
ALLHAT‐LLT 2002 {published data only}
- The Anti-hypertensive and Lipid Lowering Treatment to Prevent Heart Attack Trial (ALLHAT-LLT). Major outcomes in moderately hypercholesterolemic, hypertensive patients randomised to pravastatin versus usual care. JAMA 2002;288:2998-3007. [DOI] [PubMed] [Google Scholar]
Ames 2011 {published data only}
- Ames P, Sokoll K, Maviver H, Batuca J, Lopez L, Emery P. Effect of atorvastatin on inflammatory markers, nitric oxide meatbolites and carotid intima media thickness in systemic lupus erythematosus. In: Journal of Thrombosis and Haemostasis. Vol. 9. 2011:57th Annual SSC meeting.
Anderson 1993 {published data only}
- Anderson TJ, Meredith IT, Yeung AC, Frei B, Selwyn AP, Ganz P. The effect of cholesterol-lowering and anti-oxidant therapy on endothelium-dependent coronary vasomotion. New England Journal of Medicine 1993;332:488-93. [DOI] [PubMed] [Google Scholar]
ASCOT‐LLA 2003 {published data only}
- Chapman N, Chang CL, Caulfiend M, Dahloef B, Feder G, Sever PS, et al. Ethnic variation in lipid lowering in response to a statin (Evirest): a substudy of the Anglo-Scandinavian Cardiac Outcomes Trial (ASCOT). Ethnicity and Disease 2011;21(2):150-7. [PubMed] [Google Scholar]
- Collier D, Poulter N, Dahloef B, Secer P, Wedel H, Buch J, et al, ASCOT Investigators. Impact of atorvastatin among older and younger patients in the Anglo-Scandinavian Cardiac Outcomes Trial Lipid- Lowering arm.. Journal of Hypertension 2011;29(3):592-9. [DOI] [PubMed] [Google Scholar]
- O'Brien E. Anglo-Scandinavian Cardiac Outcomes Trial ASCOT. Main protocol summary & sub-study protocols. Journal of Human Hypertension 2001;15(Suppl 1):S1-S96. [DOI] [PubMed] [Google Scholar]
- Sever PS, Dahlof B, Poulter NR, Wedel H, Beevers G, Caulfield M, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cardiac Outcomes Trial - Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Lancet 2003;361:1149-58. [DOI] [PubMed] [Google Scholar]
- Sever PS, Dahlof B, Poulter NR, Wedel H, Beevers G, Caulfield M, et al. Prevention of coronary and stroke events with atorvastatin in hypertensive patients who have average or lower-than-average cholesterol concentrations, in the Anglo-Scandinavian Cariac Outcomes Trial - Lipid Lowering Arm (ASCOT-LLA): a multicentre randomised controlled trial. Zeitschrift für Kardiologie 2003;92:613. [DOI] [PubMed] [Google Scholar]
- Sever PS, Dahlof B, Poulter NR, Wedel H, Beevers G, Caulfield M, et al. Rationale, design, methods and baseline demography of participants of the Anglo-Scandinavian Cardiac Outcomes Trial. Journal of Hypertension 2001;19:1139-47. [DOI] [PubMed] [Google Scholar]
- Svilaas A, Kjeldsen S, Midtbo K, Westheim A, Syvertsen JO. Statin therapy for hypertensive patients [Statinbehandling av blodtrykkspasienter]. Tidsskrift for Den Norske Laegeforening 2004;124:165-6. [PubMed] [Google Scholar]
ASTRONOMER 2010 {published data only}
- Chan KL, Ni A, Tam J, Sochowski R, Dumesnil JG, Giannocaro JP, et al. Interaction between rosuvastatin and high sensitivity c-reactive protein on progression of aortic stenosis. In: Journal of the American College of Cardiology. Vol. Volume 55, issue 10A. Conference, 2010.
- Chan KL, Teo K, Dumesnil JG, Ni A, Tam J, ASTRONOMER Investigators. Effect of lipid lowering with rosuvastatin on progression of aortic stenosis: Results of the aortic stenosis progression observation: Measuring effects of rosuvastatin (Astronomer) trial. Circulation 2010;121(2):306-14. [DOI] [PubMed] [Google Scholar]
Bak 1998 {published data only}
- Bak AA, Huizer J, Leijten PA, Rila H, Grobbee DE. Diet and pravastatin in moderate hypercholesterolaemia: a randomized trial in 215 middle-aged men free from cardiovascular disease. Journal of Internal Medicine. 1998;244(5):371-8. [DOI] [PubMed] [Google Scholar]
BCAPS 2001 {published data only}
- Hedblad B, Wikstand J, Janzon L, WedelH, Berglund G. Low dose metaprolol CR/XL and fluvastatin slow progression of carotid intima-media thickness: main results from the Beta-Blocker Cholesterol-Lowering Asymptomatic Plaque Study. Circulation 2001;103:1721-6. [DOI] [PubMed] [Google Scholar]
Boccuzzi 1991 {published data only}
- Boccuzzi SJ, Bocanegra TS, Walker JF, Shapiro DR, Keegan ME. Long-term safety and efficacy profile of simvastatin. American Journal of Cardiology 1991;68(11):1127-31. [DOI] [PubMed] [Google Scholar]
Branchi 1995 {published data only}
- Branchi A, Rovellini A, Fiorenza AM, Sommariva D. Effects of bezafibrate and of 2 HMG-CoA reductase inhibitors on lipoprotein (a) level in hypercholesterolemic patients. International Journal of Clinical Pharmacology & Therapeutics. 1995;33(6):345-50. [PubMed] [Google Scholar]
Byington 1993 {published data only}
- Byington RP, Furberg CD, Crouse JR, Bond M, Espeland M. PLAC 2: effects of pravastatin on progression of carotid atherosclerosis and clinical events. European Heart Journal 1993;14(s):20. [Google Scholar]
CASHMERE 2007 {published data only}
- Pfizer. Carotid Atorvastatin Study in Hyperlipidemic Post-MEnopausal Women: a Randomised Evaluation of atorvastatin versus placebo (CASHMERE). Protocol A2581051. PhRMA Web Synopsis 2007.
Cassader 1993 {published data only}
- Cassader M, Ruiu G, Gambino R, Alemanno N, Veglia F, Pagano G. Hypercholesterolemia in non-insulin-dependent diabetes mellitus: different effect of simvastatin on VLDL and LDL cholesterol levels. Atherosclerosis 1993;99:47-53. [DOI] [PubMed] [Google Scholar]
CHALLENGER {published data only}
- Miyauchi K, Takaya N, Hirose T, Ikeda F, Kawamori R, Ohishi H, et al. Rationale and design of the carotid plaque in human for all evaluations with aggressive rosuvastatin therapy (CHALLENGER trial): evaluation by magnetic resonance imaging. Circulation Journal 2009;73(1):111-5. [DOI] [PubMed] [Google Scholar]
Chan 1996 {published data only}
- Chan P, Tomlinson B, Lee CB, Pan WH, Lee YS. Beneficial effects of pravastatin on fasting hyperinsulinemia in elderly hypertensive hypercholesterolemic subjects. Hypertension. 1996;28(4):647-51. [DOI] [PubMed] [Google Scholar]
Chuengsamarn 2010 {published data only}
- Chuengsamarn S, Rattanamongkoulgul S, Suwanwalaikorn S, Wattanasirichaigoon S, Kaufman L. Effects of statins vs. non-statin lipid-lowering therapy on bone formation and bone mineral density biomarkers in patients with hyperlipidemia. Bone 2010;46(4):1011-5. [DOI] [PubMed] [Google Scholar]
CLIP 2002 {published data only}
- Saito Y, Shirai K, Sasaki N, Shinomiya M, Yoshida S, for the Committee of the Chiba Lipid Intervention Program Study. Prognosis of hypercholesterolemic patients taking pravastatin for five years: the Chiba Lipid Intervention Program (CLIP) study. Journal of Athersclerosis and Thrombosis 2002;9(2):99-108. [DOI] [PubMed] [Google Scholar]
Cowan 2010 {published data only}
- Cowan DC, Cowan JO, Palmay R, Williamson A, Taylor R. Simvastatin in the treatment of asthma: lack of steroid-sparing effect. Thorax 2010;65:891-6. [DOI] [PubMed] [Google Scholar]
Coylewright 2008 {published data only}
- Coylewright M, Blumenthal RS, Post W. Placing COURAGE in context: review of the recent literature on managing stable coronary artery disease. Mayo Clinic Proceedings 2008;83(7):799-805. [DOI] [PubMed] [Google Scholar]
CRISP 1994 {published data only}
- LaRosa JC, Applegate W, Crouse JR III, Hunninghake DB, Grimm R, Knopp R, et al. Cholesterol lowering in the elderly. Results of the Cholesterol Reduction in Seniors Program (CRISP) pilot study. Archives of Internal Medicine. 1994;154(5):529-39. [DOI] [PubMed] [Google Scholar]
- Santanello NC, Barber BL, Applegate WB, Elam J, Curtis C, Hunninghake DB, et al. Effect of pharmacologic lipid lowering on health-related quality of life in older persons: Results from the Cholesterol Reduction in Seniors Program (CRISP) Pilot Study. Journal of the American Geriatric Society 1997;45(1):8-14. [DOI] [PubMed] [Google Scholar]
CURVES 1998 {published data only}
- Jones PH, Kafonek S, Laurora I, Hunninghake DB. Comparative dose efficacy study of atorvastatin with that of lovastatin, pravastatin, simvastatin and fluvastatin in patients With hypercholesterolemia (the CURVES Study). American Journal of Cardiology 1998;81(5):582-7. [DOI] [PubMed] [Google Scholar]
Dangas 1999 {published data only}
- Dangas G, Badimon JJ, Smith DA, Unger AH, Levine D, Shao JH, et al. Pravastatin therapy in hyperlipidemia: effects on thrombus formation and the systemic hemostatic profile. Journal of the American College of Cardiology. 1999;33(5):1294-304. [DOI] [PubMed] [Google Scholar]
Davidson 1997 {published data only}
- Davidson M, McKenney J, Stein E, Schrott H, Bakker-Arkema R, Fayyad R, et al for the Atorvastatin Study Group. Comparison of one-year efficacy and safety of atorvastatin versus lovastatin in primary hypercholesterolemia. American Journal of Cardiology 1997;79:1475-81. [DOI] [PubMed] [Google Scholar]
Duffy 2001 {published data only}
- Duffy SJ, O'Brien RC, New G, Harper RW, Meredith IT. Effect of anti-oxidant treatment and cholesterol lowering on resting arterial tone, metabolic vasodilation and endothelial function in the human forearm: a randomized, placebo-controlled study. Clinical & Experimental Pharmacology & Physiology. 2001;28(5-6):409-18. [DOI] [PubMed] [Google Scholar]
Egashira 1994 {published data only}
- Egashira K, Hirooka Y, Kai H, Sugimachi M, Suzuki S, Inou T, er al. Reduction in serum cholesterol with pravastatin improves endothelium-dependent coronary vasomotion in patients with hypercholesterolemia. Circulation 1994;89:2519-24. [DOI] [PubMed] [Google Scholar]
Eriksson 1998 {published data only}
- Eriksson M, Hadell K, Holme I, Walldius G, Kjellstrom T. Compliance with and efficacy of treatment with pravastatin and cholestyramine: a randomized study on lipid-lowering in primary care. Journal of Internal Medicine. 1998;243(5):373-80. [DOI] [PubMed] [Google Scholar]
EXCEL 1990 {published data only}
- Bradford RH, Shear CL, Chremos AN, Dujovne C, Downton M, Franklin FA, et al. Expanded Clinical Evaluation of Lovastatin (EXCEL) study results. I. Efficacy in modifying plasma lipoproteins and adverse event profile in 8245 patients with moderate hypercholesterolemia. Archives of Internal Medicine 1991;151(1):43-9. [DOI] [PubMed] [Google Scholar]
- Bradford RH, Shear CL, Chremos AN, Dujovne C, Franklin FA, Hesney M, et al. Expanded clinical evaluation of lovastatin (EXCEL) study: design and patient characteristics of a double-blind, placebo-controlled study in patients with moderate hypercholesterolemia. American Journal of Cardiology. 1990;66(8):44B-55B. [DOI] [PubMed] [Google Scholar]
- Bradford RH, Shear CL, Chremos AN, Dujovne CA, Franklin FA, Grillo RB, et al. Expanded Clinical Evaluation of Lovastatin (EXCEL) study results: two-year efficacy and safety follow-up. American Journal of Cardiology 1994;74(7):667-73. [DOI] [PubMed] [Google Scholar]
- Bradford RH, Shear CL, Chremos AN, Franklin FA, Nash DT, Hurley DP, et al. Expanded clinical evaluation of lovastatin (EXCEL) study results: III. Efficacy in modifying lipoproteins and implications for managing patients with moderate hypercholesterolemia. American Journal of Medicine 1991;91(1B):18S-24S. [DOI] [PubMed] [Google Scholar]
Faergeman 2009 {published data only}
- Faergeman O, Holme I, Fayyad R, Bhatia S, Grundy SM, Kastelein JJ, et al, Steering Committees of IDEAL and TNT Trials. Plasma triglycerides and cardiovascular events in the Treating to New Targets and Incremental Decrease in End-Points through Aggressive Lipid Lowering trials of statins in patients with coronary artery disease. American Journal of Cardiology 2009;104(4):459-63. [DOI] [PubMed] [Google Scholar]
FAST 2002 {published data only}
- Sawayama Y, Shimizu C, Maeda N, Tatsukawa M, Kinukawa N, Koyanagi S, et al. Effects of probucal and pravastatin on common carotid atherosclerosis in patients with asymptomatic hypercholesterolemia. Fukuoka atherosclerosis trial (FAST). Journal of the American College of Cardiology 2002;39(4):610-6. [DOI] [PubMed] [Google Scholar]
Ferrari 1993 {published data only}
- Ferrari P, Weidmann P, Riesen WF, Martius F, Luban S, Pasotti E, et al. Pravastatin in the treatment of primary hypercholesterolemia: a Swiss multicenter study [Pravastatin zur Behandlung der primaren Hypercholesterinamie: Schweizer Multizenter-Studie]. Schweizerische Medizinische Wochenschrift [Journal Suisse de Medecine] 1993;123(37):1736-41. [PubMed] [Google Scholar]
Gentile 2000 {published data only}
- Gentile S, Turco S, Guarino G, Sasso CF, Amodio M, Magliano P, et al. Comparative efficacy study of atorvastatin vs. simvastatin, pravastatin, lovastatin and placebo in type 2 diabetic patients with hypercholesterolaemia. Diabetes, Obesity and Metabolism 2000;2(6):355-62. [DOI] [PubMed] [Google Scholar]
Glasser 1996 {published data only}
- Glasser SP, DiBianco R, Effron BA, Faas F, Germino FW, Shane LE, et al. The efficacy and safety of pravastatin in patients aged 60 to 85 years with low-density lipoprotein cholesterol > 160 mg/dl. The American Journal Of Cardiology 1996;77:83-5. [DOI] [PubMed] [Google Scholar]
Gomez‐Garcia 2007 {published data only}
- Gómez-García A, Martínez Torres G, Ortega-Pierres LE, Rodríguez-Ayala E, Alvarez-Aguilar C. Rosuvastatin and metformin decrease inflammation and oxidative stress in patients with hypertension and dyslipidemia [Rosuvastatina y metformina reducen la inflamacion y el estres oxidativo en pacientes con hipertension y dislipemia]. Revista Espanola de Cardiologia 2007;60(12):1242-9. [DOI] [PubMed] [Google Scholar]
Guisasola 2009 {published data only}
- Guisasola MC, Dulin E, Almendral J, Garcia-Barreno P. Reduction of heat shock protein antibody levels by statin therapy. Lipids 2009;44:317-24. [DOI] [PubMed] [Google Scholar]
Hokuriku NK‐104 Study 02 {published data only}
- Noji Y, Higashikata T, Inazu A, Nohara A, Ueda K, Miyamoto S, et al. Long-term treatment with pitavastatin (NK-104), a new HMG-CoA reducatase inhibitor, of patients with heterozygous familial hypercholesterolemia. Atherosclerosis 2002;163:157-64. [DOI] [PubMed] [Google Scholar]
Hongo 2010 {published data only}
- Hongo M, Kumazaki S, Izawa A, Kasai H, Tanita T, Yazaki Y. Rosuvastatin improves arterial stiffness in hypertensive patients with dyslipidemia. In: European Heart Journal. Conference.
Hufnagel 2000 {published data only}
- Hufnagel G, Vrtovsnik CMF, Queffeulou G, Kossari N, Mignon F. Effects of atorvastatin on dyslipidemia in uraemic patients on peritoneal dialysis. Nephrology Dialysis Transplantation 2000;15:684-8. [DOI] [PubMed] [Google Scholar]
Italian Family Physician {published data only}
- Cattin L, Da Col PG, Bordin P, Battello C, Petrucco A, Fonda M on behalf of the Italian Postmarketing Surveillance Simvastatin Study Group. Efficacy and safety of simvastatin in current clinical practice: The Italian Family Physician Simvastatin Study. Current Therapeutic Research 1996;57(6):418-29. [Google Scholar]
Jardine 2006 {published data only}
- Jardine A Holdaas H, Fellstrom B, Cole E, Nyberg G, Grönhagen-Riska C, et al, ALERT Study Investigators. Fluvastatin prevents cardiac death and myocardial infarction in renal transplant recipients: post hoc analysis of the ALERT study. American Journal of Transplantation 2006;4(6):988-95. [DOI] [PubMed] [Google Scholar]
JART 2011 {published data only}
- Hohara R, Daida H, Hata M, Kaku K, Kawamori R. Effect of intensive lipid lowering therapy with rosuvastatin of carotid intima-media thickness in Japanese patients: Justification of Atherosclorosis Regression Treatment (JART) study. Circulation 2012 (Epub 2011);76:221-9. [DOI] [PubMed] [Google Scholar]
JELIS 2009 {published data only}
- Oikawa S, Yokoyama M, Origasa H, Matsuzaki M, Matsuzawa Y, Saito Y, et al, JELIS Investigators, Japan. Suppressive effect of EPA on the incidence of coronary events in hypercholesterolemia with impaired glucose metabolism: Sub-analysis of the Japan EPA Lipid Intervention Study (JELIS). Atherosclerosis 2009;206(2):535-9. [DOI] [PubMed] [Google Scholar]
J‐LIT 2007 {published data only}
- ShimamotoK, Kita T, Mabuchi H, Matsuzaki M, Matsuzawa Y, Nakaya N, et al, J-LIT Study Group. Effects of hypertension and type 2 diabetes mellitus on the risk of total cardiovascular events in Japanese patients with hypercholesterolemia: implications from the Japan Lipid Intervention Trial (J-LIT). Hypertension Research - Clinical & Experimental 2007;30(2):119-23. [DOI] [PubMed] [Google Scholar]
Jones 1991 {published data only}
- Jones PH, Farmer JA, Cressman MD, McKenney JM, Wright JT, Proctor JD, et al. Once-daily pravastatin in patients with primary hypercholesterolemia: a dose-response study. Clinical Cardiology 1991;14:146-51. [DOI] [PubMed] [Google Scholar]
Kappelle 2009 {published data only}
- Kappelle, PJWH, Zwang L, Huisman MV, Banga JD, Sluiter WJ, Dallinga-Thie GM, et al. Atorvastatin affects low density lipoprotein and non-high density lipoprotein cholesterol relations with apolipoprotein B in type 2 diabetes mellitus: Modification by triglycerides and cholesteryl ester transfer protein. Expert Opinion on Therapeutic Targets 2009;13(7):743-51. [DOI] [PubMed] [Google Scholar]
KLIS 2000 {published data only}
- Sasaki J, Arakawa K, KLIS group. Abstract at XIIth International Symposium on Atherosclerosis, Stockholm, Sweden, June 25-29 2000. Pravastatin use and risk of coronary events and cerebral infarction in Japanese men with hypercholesterolemia: The Kyushu Lipid Intervention Study (KLIS). Atherosclerosis 2000;151(1):37. [Google Scholar]
- The Kyushu Lipid Intervention Study Group. A coronary primary intervention study of Japanese men: study design, implementation and baseline data. Journal of Atherosclerosis & Thrombosis 1996;3(2):95-104. [DOI] [PubMed] [Google Scholar]
Kojima 2010 {published data only}
- Kojima S, Sakamoto T, Ogawa H, Kitagawa A, Matsui K, Shimomura H, et al, Multicenter Study for Aggressive Lipid-lowering Strategy by HMG-CoA Reductase Inhibitors Investigators. Standard-dose statin therapy provides incremental clinical benefits in normocholesterolemic diabetic patients. Circulation Journal 2010;74(4):779-85. [DOI] [PubMed] [Google Scholar]
Lemaitre 2002 {published data only}
- Lemaitre RN, Psaty BM, Heckbert SR, Kronmal RA, Newman AB, Burke GL. Therapy with hydroxymethylglutaryl coenzyme a reductase inhibitors (statins) and associated risk of incident cardiovascular events in older adults: evidence from the Cardiovascular Health Study. Archives of Internal Medicine 2002;162(12):1395-400. [DOI] [PubMed] [Google Scholar]
Lin 2010 {published data only}
- Lin Z, Zhang Z, Zhang R, Shu P, Wu S. Effects of rosuvasatin on left ventricular cardiac function, arteriosclerotic plaque and high sensitive C-reactive protein in hyepertensive patienrs with mild LdL-C elevation. Journal of Southern Medical University (China) 2010;30:588-90. [PubMed] [Google Scholar]
LIPID 2010 {published data only}
- Cui J, Forbes A, Kirby A, Marshner I, Simes J, Hunt D, et al. Semi-parametric risk prediction models for recurrent cardiovascular events in the LIPID study. BMC Medical Research Methodology 2010;10:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mareev 2008 {published data only}
- Mareev VI, Belenkov IuN, Oganov RG, Barbir-Zhagor B, Mareev Vn, Belenkov Iv. Atorvastatin in treatment of patients with coronary heart disease and dislipidemiya and high general risk: efficiency and safety estimation. Design and main results of ATLANTIKA [Russian]. Kardiologiia 2008;48:4-13. [PubMed] [Google Scholar]
McDermott 2003 {published data only}
- McDermott MM, Guralnik JM, Greenland P, Pearce WH, Criqui MH, Liu K, et al. Statin use and leg functioning in patients with and without lower-extremity peripheral arterial disease. Circulation 2003;107:757-61. [DOI] [PubMed] [Google Scholar]
Mizuguchi 2008 {published data only}
- Mizuguchi Y, Oishi Y, Miyoshi H, Iuchi A, Nagase N, Oki T. Impact of statin therapy on left ventricular function and carotid arterial stiffness in patients with hypercholesterolemia. Circulation Journal 2008;72(4):538-44. [DOI] [PubMed] [Google Scholar]
Mohler 2003 {published data only}
- Mohler E, Hiatt W, Creager M. Cholesterol reduction with atorvastatin improves walking distance in patients with peripheral arterial disease. Circulation 2003;108:1481-6. [DOI] [PubMed] [Google Scholar]
Mok 2009 {published data only}
- Mok VCT, Lam WWM, Chen XY, Wong A, Ng PW, Tsoi TH. Statins for asymptomatic middle cerebral artery stenosis: The Regression of Cerebral Artery Stenosis study. Cerebrovascular Diseases 2009;28(1):18-25. [DOI] [PubMed] [Google Scholar]
Muldoon 1997 {published data only}
- Muldoon MF, Barger SD, Ryan CM, Flory JD, Lehoczky JP, Matthews KA, et al. Effects of lovastatin on cognitive function and psychological well-being. American Journal of Medicine 2000;108(7):538-46. [DOI] [PubMed] [Google Scholar]
- Muldoon MF, Flory JD, Marsland A, Manuck SB, Whiteside TL, Rabin B. Effects of lovastatin on the immune system. American Journal of Cardiology. 1997;80(10):1391-4. [DOI] [PubMed] [Google Scholar]
Nephrotic Syndrome Study {published data only}
- Olbricht CJ, Wanner C, Thiery J, Basten A, for the Simvastatin in Nephrotic Syndrome Study Group. Simvastatin in nephrotic syndrome. Kidney International 1999;56(Suppl. 71):S-113-6. [DOI] [PubMed] [Google Scholar]
Ohta 2000 {published data only}
- Ohta H, Masuda A, Fuyuki T, Sugimoto I, Suda Y, Makita K, et al. Usefulness of HMG-CoA reductase inhibitor in Japanese hyperlipidemic women within seven years of menopause. Hormone Research. 2000;53(3):120-4. [DOI] [PubMed] [Google Scholar]
Oi 1997 {published data only}
- Oi K, Komori H. Abstract of the 11th Internationial Symposium on Atherosclerosis Paris, 5-9 October 1997. 2.P.51 Escape phenomenon during the long-term administration of pravastatin for hyperlipidemia associated with diabetes. Atherosclerosis 1997;134(1-2):127. [Google Scholar]
Olzowy 2007 {published data only}
- Olzowy B, Canis M, Hempel JM. Effect of atorvastatin on progression of sensorineural hearing loss and tinnitus in the elderly: Results of a prospective, randomized, double-blind clinical trial. Otology and Neurotology 2007;28:455-8. [DOI] [PubMed] [Google Scholar]
Ormiston 2003 {published data only}
- Ormiston T, Wolkowitz OM, Reus VI, Manfredi F. Behavioral implications of lowering cholesterol levels: a double-blind pilot study. Psychosomatics 2003;44(5):412-4. [DOI] [PubMed] [Google Scholar]
Pavia 2000 {published data only}
- Pavia Lopez A, Zamorano J, Kim JH, Erdine S, Al Khadra A, Westergaard M, et al. Treatment strategies for cardiovascular risk factor management in patients with hypertension and additional risk factors-experiences from the usual care arm of the Crucial trial. In: Journal of Hypertension. Vol. Conference. 2010:p e276–e277.
Pitt 1999 {published data only}
- Pitt B, Waters D, Brown WV, Boven AJ, Schwartz L, Title LM, et al. Aggressive lipid-lowering therapy compared with angioplasty in stable coronary artery disease. New England Journal of Medicine 1999;341(2):70-6. [DOI] [PubMed] [Google Scholar]
POSCH 1990 {published data only}
- Buchwald H, Campos CT, Boen JR, Nguyen PA, Williams SE, for the POSCH Group. Disease-free intervals after partial ileal bypass in patients with coronary heart disease and hypercholesterolemia: Report from the Program on the Surgical Control of the Hyperlipidemias (POSCH). Journal of American College Cardiology 1995;26:351-7. [DOI] [PubMed] [Google Scholar]
- Buchwald H, Campos CT, Matts JP, Fitch LL, Long JM, Varco RL, et al. Women in the POSCH trial. Effects of aggressive cholesterol modification in women with coronary heart disease. Annals of Surgery 1992;216(4):389-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchwald H, Matts JP, Fitch LL, Campos CT, Sanmarco ME, Amplatz K, et al. Changes in sequential coronary arteriograms and subsequent coronary events. JAMA 1992;268(11):1429-33. [PubMed] [Google Scholar]
- Buchwald H, Varco RL, Matts JP, Long JM, Fitch LL, Campbell GS, et al. Effect of partial ileal bypass surgery on mortality and morbidity from coronary heart disease in patients with hypercholesterolemia. New England Journal of Medicine 1990;323:946-55. [DOI] [PubMed] [Google Scholar]
Pravastatin Multi 1993 {published data only}
- The Pravastatin Multinational Study Group for Cardiac Risk Patients. Effects of pravastatin in patients with serum total cholesterol levels from 5.2 to 7.8 mmol/liter (200 to 300 mg/dl) plus two additional atherosclerotic risk factors. American Journal of Cardiology. 1993;72(14):1031-7. [DOI] [PubMed] [Google Scholar]
PROSPER 2002 {published data only}
- Avorn J, Benner J, Ford I, Ganz DA, Gaw A, Glynn RJ, et al. Measuring the cost-effectiveness of lipid-lowering drugs in the elderly: the outcomes research and economic analysis components of the PROSPER trial. Controlled Clinical Trials 2002;23(6):757-73. [DOI] [PubMed] [Google Scholar]
- Baztan JJ, Hornillos M, Rodriguez-Manas L. More on PROSPER. Lancet 2003;361(9363):1135. [DOI] [PubMed] [Google Scholar]
- Blauw GJ, Shepherd J, Murphy MB, PROSPER study group. Dementia and statins. PROSPER study group. Lancet 2001;357(9259):881. [DOI] [PubMed] [Google Scholar]
- Collins R, Armitage J. High-risk elderly patients PROSPER from cholesterol-lowering therapy. Lancet 2002;360(9346):1618-9. [DOI] [PubMed] [Google Scholar]
- Fiorenza AM, Sommariva D, Branchi A. The PROSPER trial. Lancet 2003;361(9355):428. [DOI] [PubMed] [Google Scholar]
- Ford, I, Blauw, GJ, Murphy, MB, Shepherd J, Cobbe SM, Bollen EL, et al, and the PROSPER Study Group. A Prospective Study of Pravastatin in the Elderly at Risk (PROSPER): screening experience and baseline characteristics. Current Controlled Trials in Cardiovascular Medicine 2002;3(1):8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houx PJ, Shepherd J, Blauw GJ, Murphy MB, Ford I, Bollen EL, et al. Testing cognitive function in elderly populations: the PROSPER study. PROspective Study of Pravastatin in the Elderly at Risk. Journal of Neurology, Neurosurgery & Psychiatry. 2002;73(4):385-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kulbertus H, Scheen AJ. The PROSPER Study (PROspective study of pravastatin in the elderly at risk) [L'etude clinique du mois. L'etude PROSPER (PROspective study of pravastatin in the elderly at risk)]. Revue Medicale de Liege 2002;57(12):809-13. [PubMed] [Google Scholar]
- PROSPER-no authors listed. Pravastatin benefits elderly patients: results of PROSPER study. Cardiovascular Journal of Southern Africa 2003;14(1):48. [PubMed] [Google Scholar]
- Shepherd J, Blauw GJ, Murphy MB, Bollen EL, Buckley BM, Cobbe SM, et al. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet. 2002;360(9346):1623-30. [DOI] [PubMed] [Google Scholar]
- Shepherd J, Blauw GJ, Murphy MB, Cobbe SM, Bollen EL, Buckley BM, et al. The design of a prospective study of Pravastatin in the Elderly at Risk (PROSPER). PROSPER Study Group. PROspective Study of Pravastatin in the Elderly at Risk. American Journal of Cardiology. 1999;84(10):1192-7. [DOI] [PubMed] [Google Scholar]
- Shepherd J. Preventing the next event in the elderly: the PROSPER perspective. Atherosclerosis Supplements 2003;4:17-22. [DOI] [PubMed] [Google Scholar]
Safaei 2007 {published data only}
- Safaei H, Janghorbani M, Aminorroaya A, Amini M. Lovastatin effects on bone mineral density in postmenopausal women with type 2 diabetes mellitus. Acta Diabetologica 2007;44(2):76-82. [DOI] [PubMed] [Google Scholar]
SANDS 2008 {published data only}
- Howard BV, Roman MJ, Devereux RB, Fleg JL, Galloway JM, Henderson JA, et al. Effect of lower targets for blood pressure and LDL cholesterol on atherosclerosis in diabetes: The SANDS randomized trial. JAMA 2008;299(14):1678-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schmermund 2006 {published data only}
- Schmermund A, Achenbach S, BuddeT, Buziashvili Y, Förster A, Friedrich G, et al. Effect of intensive versus standard lipid-lowering treatment with atorvastatin on the progression of calcified coronary atherosclerosis over 12 months: A multicenter, randomized, double-blind trial. Circulation 2006;113(3):427-37. [DOI] [PubMed] [Google Scholar]
Sen 2000 {published data only}
- Sen K, Misra A, Kumar A. Double blind randomized trial of efficacy of simvastatin on retinopathy in hyperlipidemic diabetic patients. In: Journal of the Association of Physicians of India. 2000.
Sprecher 1994 {published data only}
- Sprecher DL, Abrams J, Allen JW, Keane WF, Chrysant SG, Ginsberg H, et al. Low-dose combined therapy with fluvastatin and cholestyramine in hyperlipidemic patients. Annals of Internal Medicine 1994;120:537-43. [DOI] [PubMed] [Google Scholar]
Stein 1997 {published data only}
- Stein E, Sprecher D, Allenby KS, Tosielle RL, Whalen E, Ripa SR, the Cerivastatin Study Group. Cerivastatin, a new potent synthetic HMG Co-A reductase inhibitor: effect of 0.2mg daily in subjects with primary hypercholesterolemia. Journal of Cardiovascular Pharmacology and Therapeutics 1997;2(1):7-16. [DOI] [PubMed] [Google Scholar]
Su 2000 {published data only}
- Su SF, Hsiao CL, Chu CW, Lee BC, Lee TM. Effects of pravastatin on left ventricular mass in patients with hyperlipidemia and essential hypertension. American Journal of Cardiology. 2000;86(5):514-8. [DOI] [PubMed] [Google Scholar]
Tanaka 2001 {published data only}
- Akira T, Nobuhiro Y, Saito Y, Kawakami M, Ohashi Y, Akanuma Y. A double-blind trial on the effects of atorvastatin on glycemic control in Japanese diabetic patients with hypercholesterolemia. Clinica Chimica Acta 2001;312:41-47. [DOI] [PubMed] [Google Scholar]
Tarin 2010 {published data only}
- Tarin N, Tunan-Fernandez JT, Sanz P, Espana E, Turbi C, Mischke J et al. Statins reduce the incidence of cardiovascular events in postmenopausal women in secondary but not in primary prevention. A retrospective analysis of the Ruth study. In: European Heart Journal. 2010.
Teixeira 2011 {published data only}
- Teixeira A, Buffani A, Tavaraes A, Ribeiro A, Zanella M, Kohlmann O Jr, et al. Effects of fluvastatin on insulin resistance and cardiac morpholgy in hypertensive patients. Journal of Human Hypertension 2011;25(8):492-9. [DOI] [PubMed] [Google Scholar]
Tekin 2008 {published data only}
- Tekin A, Tekin G, Sezgin AT, Muderrisoglu H. Short- and long-term effect of simvastatin therapy on the heterogeneity of cardiac repolarization in diabetic patients. Pharmacological Research 2008;57:393-7. [DOI] [PubMed] [Google Scholar]
Thomas 1993 {published data only}
- Thomas ME, Harris KP, Ramaswamy C, Hattersley JM, Wheeler DC, Varghese Z, et al. Simvastatin therapy for hypercholesterolemic patients with nephrotic syndrome or significant proteinuria. Kidney International. 1993;44(5):1124-9. [DOI] [PubMed] [Google Scholar]
Thrombosis Prevention {published data only}
- The Medical Research Council's General Practice Research Framework. Thrombosis Prevention Trial: randomised trial of low-intensity oral anticoagulation with warfarin and low-dose aspirin in the primary prevention of ischaemic heart disease in men at increased risk. Lancet 1998;351:233-41. [PubMed] [Google Scholar]
Togha 2009 {published data only}
- Togha M, Karvigh SA, Nabavi M, Moghadam NB, Harirchian MH, Sahraian MA, et al. Simvastatin treatment in patients with relapsing-remitting multiple sclerosis receiving interferon beta 1a: a double-blind randomized controlled trial. Multiple Sclerosis 2010;16(7):848-54. [DOI] [PubMed] [Google Scholar]
Tran 2007 {published data only}
- Tran YB, Frial T, Miller PS. Statin's cost-effectiveness: a Canadian analysis of commonly prescribed generic and brand name statins. Canadian Journal of Clinical Pharmacology 2007;14(2):e205-14. [PubMed] [Google Scholar]
Wallace 2003 {published data only}
- Wallace A, Chinn D, Rubin G. Taking Simvastatin in the morning compared with in the evening: randomised controlled trial. BMJ 2003;327:788. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wu 2007 {published data only}
- Wu J, Wu G, Wang MP, Liu DL, Hu ZJ, Xu GY. Effect of atorvastatin on carotid intima-medial of thickness of primary hypertension patients of Han nationality. National Medical Journal of China 2007;87(31):2215-18. [PubMed] [Google Scholar]
Yu‐An 1998 {published data only}
- Yu-An DP, Huey-Herng SW, An HC, Pei D. Efficacy and safety of fluvastatin in patients with non-insulin-dependent diabetes mellitus and hypercholesterolemia. Atherosclerosis 1998;136:S42. [Google Scholar]
Zachoval 2000 {published data only}
- Zachoval R, Parhofer KG, Schwandt P, Gerbes AL. Cerivastatin and Pravastatin in the hyperlipoproteinaemia therapy in patients after liver transplantation - Results of a randomised, cross-over study. Zeitschrift fur Gastroenterologie. 2000;38:531. [Google Scholar]
References to studies awaiting assessment
Babes 2010 {published and unpublished data}
- Babes E, Babes V, Popescu M, Rus M, Bustea C. Simvastatin therapy and endothelial function in young adults with subclinical atheroslerosis. In: Cardiovascular Research. Vol. 87 (Suppl 1). 16-19th July 2010:S94.
Additional references
Abramson 2007
- Abramson J, Wright JM. Are lipid-lowering guidelines evidence-based? Lancet 2007;369:168-9. [DOI] [PubMed] [Google Scholar]
Als‐Nielsen 2003
- Als-Nielsen B, Chen W, Gluud C, Kjaergard LL. Association of funding and conclusions in randomized drug trials: a reflection of treatment effect or adverse events? JAMA 2003;290(7):921-8. [DOI] [PubMed] [Google Scholar]
Armitage 2007
- Armitage J. The safety of statins in clinical practice. Lancet 2007;370:1781-90. [DOI] [PubMed] [Google Scholar]
Baigent 2005
- Baigent C, Keech A, Kearney PM, Blackwell L, Buck G, Pollicino C, et al. Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005;366(9493):1267-78. [DOI] [PubMed] [Google Scholar]
Bartlett 2003
- Bartlett C, Davey P, Dieppe P, Doyal L, Ebrahim S, Egger M. Women, older persons, and ethnic minorities: factors associated with their inclusion in randomised controlled trials of statins 1990 to 2001. Heart 2003;89:327-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bartlett 2005
- Bartlett C, Doyal L, Ebrahim S, Davey P, Bachmann M, Egger M, et al. The causes and effects of socio-demographic exclusions from clinical trials. Health Technology Assessment 2005;9(38):1-152. [DOI] [PubMed] [Google Scholar]
Beers 2003
- Beers MH, Berkow R (Eds). The Merck Manual of Diagnosis and Therapy. Http://www.merck.com (accessed 20 November 2003).
Berry 2012
- Berry JD, Dyer A, Cai X, Garside DB, Ning H, Thomas A, et al. Lifetime risks of cardiovascular disease. New England Journal of Medicine 2012;366(4):321-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Blauw 1997
- Blauw GJ, Lagaay AM, Smelt AH, Westendorp RG. Stroke, statins, and cholesterol. A meta-analysis of randomized, placebo-controlled, double-blind trials with HMG-CoA reductase inhibitors. Stroke 1997;28:946-50. [DOI] [PubMed] [Google Scholar]
BMJ 2011
- BMJ Clinical Evidence. Default search strategies used for BMJ Clinical Evidence: Embase randomised controlled trial strategy. http://clinicalevidence.bmj.com/ceweb/about/search_filters.jsp (accessed 2 February 2011).
Briel 2004
- Briel M, Studer M, Glass TR, Bucher HC. Effects of statins on stroke prevention in patients with and without coronary heart disease: a meta-analysis of randomized controlled trials. American Journal of Medicine 2004;117(8):596-606. [DOI] [PubMed] [Google Scholar]
Brugts 2009
- Brugts JJ, Yetgin T, Hoeks SE, Gotto AM, Shepherd J, Westendorp RG, et al. The benefits of statins in people without established cardiovascular disease but with cardiovascular risk factors: meta-analysis of randomised controlled trials. BMJ 2009;338:b2376. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 1991
- Chen ZM, Peto R, Collins R. Serum cholesterol concentration and coronary heart disease in a population with low cholesterol concentrations. BMJ 1991;303:276-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheung 2004
- Cheung BM, Lauder IJ, Lau CP, Kumana CR. Meta-analysis of large randomized controlled trials to evaluate the impact of statins on cardiovascular outcomes. British Journal of Clinical Pharmacology 2004;57(5):640-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
CTT Collaboration 2010
- CTT Collaboration. Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomized trials. Lancet 2010;376:1670-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
CTT Collaboration 2012a
- Cholesterol Treatment Trialists (CTT) Collaboration . The effects of lowering LDL cholesterolwith statin therapy in people at low risk of vascular disease: meta-analysis of individualdata from 27 randomised trials. Lancet 2012;378:doi:10.1016/S0140-6736(12)60367-5. [Google Scholar]
CTT Collaboration 2012b
- Cholesterol Treatment Trialists' (CTT) Collaboration. Lack of effect of lowering LDLcholesterol on cancer: meta-analysis of individual data from 175,000 people in 27randomised trials of statin therapy. PLoS One 2012;7 (1):e29849. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dale 2006
- Dale KM, Coleman CI, Henyan NN, Kluger J, White CM. Statins and cancer risk: a meta-analysis. JAMA 2006;295(1):74-80. [DOI] [PubMed] [Google Scholar]
Davey Smith 1992
- Davey Smith G, Shipley MJ, Marmot MGM, Rose G. Plasma cholesterol concentrations and mortality in the Whitehall study. JAMA 1992;267:70-6. [PubMed] [Google Scholar]
de Ferranti 2008
- Ferranti S, Ludwig DS. Storm over statins--the controversy surrounding pharmacologic treatment of children. New England Journal of Medicine 2008;359(13):1309-12. [DOI] [PubMed] [Google Scholar]
Ebrahim 1998
- Ebrahim S, Davey Smith G, McCabe C, Payne N, Pickin M, Sheldon TA, et al. Cholesterol and heart disease: screening and treatment. Quality in Health Care 1998;7:232-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ebrahim 1999
- Ebrahim S, Smith GD, McCabe C, Payne N, Pickin M, Sheldon TA, et al. What role for statins? A review and economic model. Health Technology Assessment 1999;3(19):1-91. [MEDLINE: ] [PubMed] [Google Scholar]
Ebrahim 2006
- Ebrahim S, Sung J, Song YM, Ferrer RL, Lawlor DA, Davey Smith G. Serum cholesterol, haemorrhagic stroke, ischaemic stroke, and myocardial infarction: Korean national health system prospective cohort study. BMJ 2006;333(7557):22. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ebrahim 2012
- Ebrahim S, Casas JP. Statins for all by the age of 50? Lancet 2012;380(9841):545-7. [DOI: 10.1016/S0140-6736(12)60694-1] [DOI] [PubMed] [Google Scholar]
Ezzati 2002
- Ezzati M, Lopez AD, Rodgers A, Vander Hoorn S, Murray CJL, and the Comparative Risk Assessment Collaborating Group. Selected major risk factors and global and regional burden of disease. Lancet 2002;360:1347-60. [DOI] [PubMed] [Google Scholar]
Gaziano 2007
- Gaziano T. Reducing the growing burden of cardiovascular disease in the developing world. Health Affairs 2007;26(1):13-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Genest 2009
- Genest J, McPherson R, Frohlich J, Anderson T, Campbell N, Carpentier A, et al. 2009 Canadian Cardiovascular Society/Canadian guidelines for the diagnosis and treatment of dyslipidemia and prevention of cardiovascular disease in the adult – 2009 recommendations. Canadian Journal of Cardiology 2009;25(10):567-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
Golomb 2012
- Golomb B Evans M, Dimsdale J, White H. Effects of statin on energy and fatigue and exertion: results from a randomised controlled trial. Archives of Internal Medicine 2012;172(15):1180-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Hippsley‐Cox 2010
- Hippisley-Cox J. Unintended effects of statins in men and women in England and wales: population based cohort study using the QResearch database.. BMJ 2010;340:c2197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Iribarren 1997
- Iribarren C, Jacobs DR, Sidney S, Claxton AJ, Gross MD, Sadler M, et al. Serum total cholesterol and risk of hospitalisation, and death from respiratory disease. International Journal of Epidemiology 1997;26:1191-202. [DOI] [PubMed] [Google Scholar]
Jackson 2001
- Jackson PR, Wallis EJ, Haq IU, Ramsay E. Statins for primary prevention: at what coronary risk is safety assured? Journal of Clinical Pharmacology 2001;52:439-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jacobs 1997
- Jacobs DR, Herbert B, Schreiner PJ, Sidney S, Iribarren C, Hulley S. Reduced cholesterol is associated with recent minor illness. The CARDIA study. American Journal of Epidemiology 1997;146:558-64. [DOI] [PubMed] [Google Scholar]
Jepson 2000
- Jepson R. The effectiveness of Interventions to change health-related behaviours: a review of reviews. Medical Research Council Social & Public Health Sciences Unit. MRC Social & Public Health Sciences Unit Occasional Paper No 3 May 2000.
Kashani 2006
- Kashani A, Phillips CO, Foody JM, Wang Y, Mangalmurti S, Ko DT, et al. Risks associated with statin therapy: A systematic overview of randomized clinical trials. Circulation 2006;114(25):2788-97. [DOI] [PubMed] [Google Scholar]
Katerndahl 1999
- Katerndahl DA, Lawler WR. Variability in meta-analytic results concerning the value of cholesterol reduction in coronary heart disease: A meta-meta-analysis. American Journal of Epidemiology 1999;149(5):429-41. [DOI] [PubMed] [Google Scholar]
Kokkinos 2012
- Kokkinos PF, Faselis C, Myers J, Panagiotakos D, Daumas M. Interactive effects of fitness and statin treatment on mortality risk in veterans with dyslipidaemia: a cohort study. Lancet 2012;Online publication:28 November 2012. [DOI: 10.1016/S0140-6736(12)61426-3] [DOI] [PubMed] [Google Scholar]
LaRosa 1994
- LaRosa JC, Applegate W, Crouse JR 3rd, Hunninghake DB, Grimm R, Knopp R, et al. Cholesterol lowering in the elderly. Results of the Cholesterol Reduction in Seniors Program (CRISP) pilot study. Archives of Internal Medicine 1994;154(5):529-39. [DOI] [PubMed] [Google Scholar]
LaRosa 1999
- LaRosa JC, He J, Vupputuri S. Effect of statins on risk of coronary disease. A meta-analysis of randomized controlled trials. JAMA 1999;282:2340-6. [DOI] [PubMed] [Google Scholar]
Law 2003
- Law MR, Wald NJ, Rudnicka AR. Quantifying effect of statins on low density lipoprotein cholesterol, ischaemic heart disease, and stroke: systematic review and meta-analysis. BMJ 2003;326:1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lazar 2011
- Lazar LD, Pletcher MJ, Coxson PG, Bibbins-Domingo K, Goldman L. Cost-effectiveness of statin therapy for primary prevention in a low-cost statin era. Circulation 2011;124:146-53. [DOI] [PubMed] [Google Scholar]
Lefebvre 1996
- Lefebvre C, Mcdonald S. Development of a sensitive search strategy for reports of randomized controlled trials in EMBASE. In: Paper presented at the Fourth International Cochrane Colloquium 20-24 Oct 1996. 1996.
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies: In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011] The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org 2011.
Lim 2007
- Lim SS, Gaziano TA, Gakidou E, Reddy KS, Farzadfar F, Lozano R, et al. Prevention of cardiovascular disease in high-risk individuals in low-income and middle-income countries: health effects and costs. Lancet 2007;370(9604):2054-62. [DOI] [PubMed] [Google Scholar]
Manuel 2006
- Manuel D, Kwong K, Tanuseputro P, Lim J, Mustard C, Anderson GM, et al. Effectiveness and efficiency of different guidelines on statin treatment for preventing deaths from coronary heart disease: modeling study. BMJ 2006;332(7555):1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mills 2011
- Mills EJ, Wu P, Chong G, Ghement I, Singh S, Akl EA, et al. Efficacy and safety of statin treatment for cardiovascular disease: a network meta-analysis of 170,255 patients from 76 randomized trials. QJM 2011;104(2):109-24. [DOI] [PubMed] [Google Scholar]
Muldoon 2000
- Muldoon MF, Barger SD, Ryan CM, Flory JD, Lehoczky JP, Matthews KA, et al. Effects of lovastatin on cognitive function and psychological wellbeing. American Journal of Medicine 2000;108:538-47. [DOI] [PubMed] [Google Scholar]
Muldoon 2001
- Muldoon MF, Manuck SB, Mendelsohn AB, Kaplan JR, Belle SH. Cholesterol reduction and nonillness mortality: metaanalysis of randomised clinical trials. BMJ 2001;322:11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
NICE 2006
- National Institute for Health and Clinical Excellence. Technology Appraisal TA94: Statins for the prevention of cardiovascular events in patients at increased risk of developing cardiovascular disease or those with established cardiovascular disease. 2006. http://www.nice.org.uk/TA094 [accessed 10.10.2010] (accessed 10 October 2010).
Pignone 2000
- Pignone M, Phillips C, Mulrow C. Use of lipid lowering drugs for primary prevention of coronary heart disease: metaanalysis of randomised trials. BMJ 2000;321:1-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Preiss 2011
- Preiss D, Seshasai SR, Welsh P, Murphy SA, Ho JE, Waters DD, et al. Risk of incident diabetes with intensive-dose compared with moderate-dose statin therapy: a meta-analysis. JAMA 2011;305:2556-64. [DOI] [PubMed] [Google Scholar]
Prospective Studies Collaboration 2007
- Prospective Studies Collaboration. Collaborative meta-analysis of 61 studies of vascular risk factors (blood cholesterol, blood pressure, body mass index, diabetes) and cause-specific mortality. Lancet 2007;370:1829-39. [DOI] [PubMed] [Google Scholar]
Ray 2010
- Ray KK, Sreenivasha RKS, Erqou S, Sever P, Jukema JW, Ford I, et al. Statins and all-cause mortality in high-risk primary prevention. A meta-analysis of 11 randomized controlled trials involving 65,229 participants. Archives of Internal Medicine 2010;170:1024-31. [DOI] [PubMed] [Google Scholar]
Reiner 2011
- Reiner Z, Catapano A, Backer G. ESC/EAS guidelines for the management of dyslipidaemias. The Task Force on the management of dyslipidaemias of the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS). European Heart Journal 2011;32:1345-61. [DOI] [PubMed] [Google Scholar]
Sattar 2010
- Sattar N, Preiss D, Murray HM, Welsh P, Buckley BM, Craen AJ, et al. Statins and risk of incident diabetes: a collaborative meta-analysis of randomised statin trials. Lancet 2010;375(9716):735-42. [DOI] [PubMed] [Google Scholar]
Silva 2006
- Silva MA, Swanson AC, Gandhi PJ, Tataronis GR. Statin-related adverse events: a meta-analysis. Clinical Therapeutics 2006;28(1):26-35. [DOI] [PubMed] [Google Scholar]
Smeeth 2008
- Smeeth L, Douglas I, Hall AJ, Hubbard R, Evans S. Effect of statins on a wide range of health outcomes: a cohort study validated by comparison with randomized trials.. British Joutnal of Clinical Pharmacology 2008;67(1):99–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smeeth 2012
- Smeeth L, Hemingway H. Improving vascular health: are pills the answer? BMJ 2012;344:doi: 10.1136/bmj.e3802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sterne 2001
- Sterne J, Egger M, Davey Smith G. Investigating and dealing with publication and other biases. In: Egger M, Davey Smith G, Altman D, editors(s). Systematic reviews in health care. Meta-analysis in context. 2 edition. London: British Medical Journal Books, 2001:189-208. [Google Scholar]
Thavendiranathan2006
- Thavendiranathan P, Bagai A, Brookhart MA, Choudhry NK. Primary prevention of cardiovascular diseases with statin therapy: a meta-analysis of randomized controlled trials. Archives of Internal Medicine 2006;166(21):2307-13. [DOI] [PubMed] [Google Scholar]
Therapeutics Letter 2003
- Jauca C, Wright JM. Do statins have a role in primary prevention? Therapeutics Letter 2003;48.
Therapeutics Letter 2010
- Wright JM. Do statins have a role in primary prevention? An update. Therapeutics Letter 2010;77.
Vrecer, 2003
- Vrecer M, Turk S, Drinovec J, Mrhar A. Use of statins in primary and secondary prevention of coronary heart disease and ischemic stroke. Meta-analysis of randomized trials. International Journal of Clinical Pharmacology Therapeutics 2003;41(12):567-77. [DOI] [PubMed] [Google Scholar]
Walley 2004
- Walley T, Folino-Gallo P, Schwabe U, Van Ganse E. Variations and increase in use of statins across Europe. BMJ 2004;328:385-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ward 2007
- Ward S, Lloyd Jones M, Pandor A, Holmes M, Ara R, Ryan A, et al. A systematic review and economic evaluation of statins for the prevention of coronary events. Health Technology Assessment 2007;11(14):1-160. [DOI] [PubMed] [Google Scholar]
Wardle 1996
- Wardle J, Armitage J, Collins R, Wallendszus K, Keech A, Lawson A. Randomised placebo controlled trial of effect on mood of lowering cholesterol concentration. BMJ 1996;313:75-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Waters 2011
- Waters DD, Ho JE, DeMicco DA, Breazna A, Arsenault BJ, Wun CC, et al. Predictors of new-onset diabetes in patients treated with atorvastatin: results from 3 large randomized clinical trials. Journal of the American College of Cardiology 2011;57(14):1535-45. [DOI] [PubMed] [Google Scholar]
WHO 2008
- WHO. The World Health report. Accessed online at www.who.int/cardiovascular_diseases/en/2008 September 30th. Geneva: WHO, 2008. [Google Scholar]
WHO 2008b
- World Health Organization. 2008-2013 Action Plan for the global strategy for the prevention and control of noncommunicable diseases. Geneva: WHO, 2008. [Google Scholar]
Wilt 2004
- Wilt TJ, Bloomfield HE, MacDonald R, Nelson D, Rutks I, Ho M, et al. Effectiveness of statin therapy in adults with coronary heart disease. Archives of Internal Medicine 2004;164(13):1427-36. [DOI] [PubMed] [Google Scholar]
Yusuf 2001
- Yusuf S, Reddy S, Ounpuu S, Anand S. Global burden of cardiovascular diseases. Part 1: General considerations, the epidemic transition, risk factors, and impact of urbanisation. Circulation 2001;104:2746-53. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Taylor 2009
- Taylor F, Ward K, Moore THM, Burke M, Davey Smith G, Ebrahim S. Statins for the primary prevention of cardiovascular disease. Cochrane Database of Systematic Reviews 2009, Issue 1. Art. No: CD004816. [DOI: 10.1002/14651858.CD004816.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor 2011
- Taylor F, Ward K, Moore THM, Burke M, Davey Smith G, Casas JP, et al. Statins for the primary prevention of cardiovascular disease. Cochrane Database of Systematic Reviews 2011, Issue 1. Art. No: CD004816. [DOI: 10.1002/14651858.CD004816.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]


