Abstract
In this study, we explore the relationship between orbit anatomy and different ecological factors in carnivorous mammals from a phylogenetic perspective. We calculated the frontation (α), convergence (β), and orbitotemporal (Ω) angles of the orbit from 3D coordinates of anatomical landmarks in a wide sample of carnivores with different kinds of visual strategy (i.e. photopic, scotopic, and mesopic), habitat (i.e. open, mixed, and closed), and substrate use (i.e. arboreal, terrestrial, and aquatic). We used Bloomberg's K and Pagel's λ to assess phylogenetic signal in frontation, convergence, and orbitotemporal angles. The association of orbit orientation with skull length and ecology was explored using phylogenetic generalized least squares and phylogenetic manova, respectively. Moreover, we also computed phylomorphospaces from orbit orientation. Our results indicate that there is not a clear association between orbit orientation and the ecology of living carnivorans. We hypothesize that the evolution of the orbit in mammalian carnivores represents a new case of an ecological bottleneck specific to carnivorans. New directions for future research are discussed in light of this new evidence.
Keywords: habitat use, mammalian carnivores, orbit anatomy, phylogenetic signal, visual strategy
Introduction
Orbit (eye socket; Fig. 1A) orientation is an important ecomorphological indicator in vertebrates due to its visual implications (e.g. Cartmill, 1970, 1974, 2017). Animals with more lateral orbits exhibit high panoramic vision (or monocular vision) with little or no overlap among visual fields. Monocular vision has been studied across different prey species, such as artiodactyls, equids, and lagomorphs, and a strong relationship has been established between orbit lateralization and fast detection of predators (e.g. Walls, 1942; Hughes, 1977). In contrast, species with forward‐facing orbits exhibit low panoramic vision with substantial overlap among visual fields (or binocular vision). Binocular vision has the advantages of more light capture, better depth perception, and better contrast, which provide several visual advantages for active predation (e.g. Pirenne, 1943; Campbell & Green, 1965; Hughes, 1977; Garamszegi et al. 2002; Heesy, 2004, 2008). Additionally, binocular vision provides the ability to detect an object when something is obstructing the view by the overlap of the same visual information extracted from similar images presented to each eye (e.g. Allman, 1977; Cartmill, 2017). Therefore, binocular vision is typical of predatory behaviour because it allows stereoscopic depth perception (e.g. Heesy, 2008). For this reason, orbit anatomy has been used to decipher visual abilities in a plethora of extinct taxa including early tetrapods (MacIver et al. 2017), non‐mammalian synapsids (Angielczyk & Schmitz, 2014), dinosaurs (Schmitz & Motani, 2011), and ichthyosaurs (Motani et al. 1999).
Figure 1.
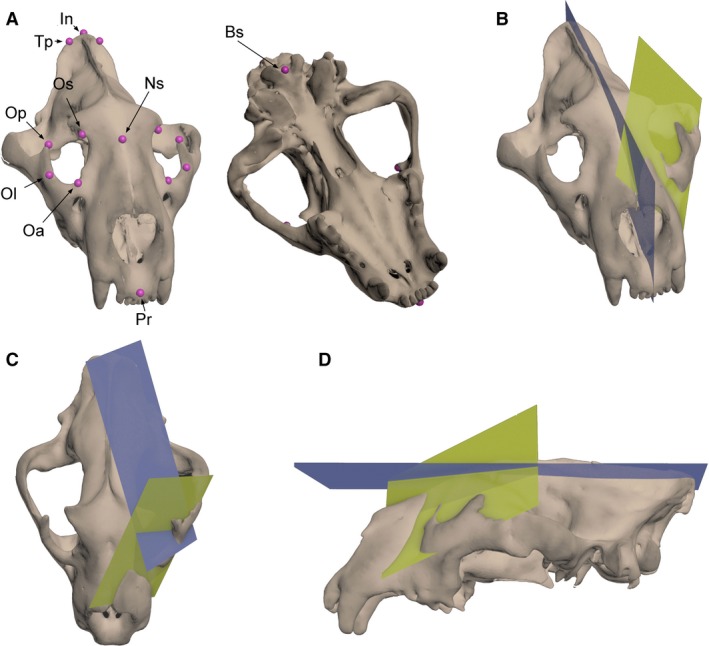
Calculation of orbit orientation in carnivores. (A) Anatomical landmarks in three dimensions used to define the planes to analyse the orientation of the orbit: Op (orbitale posterius), most posterior point of the orbit; Oa (orbitale anterius), most anterior point of the orbit; Ol (orbitale lateralis), most lateral point of the orbit; Os (orbitale superius), highest point of the orbit; Tp, most posterior point of the temporal fossa; Pr, prostion; Ns, nasion; In, inion; Bs, basion. (B) Convergence angle (β) measured as the dihedral angle between the sagittal plane (blue) and the orbital plane (green). (C) Orbitotemporal angle (Ω) measured as the dihedral angle between the temporal plane (blue) and the orbital plane (green); (D) Frontation angle (α) measured as the dihedral angle between the dorsal plane (blue) and the orbital plane (green).
Previous researchers have demonstrated that specific angles of the orbit in mammals are associated with substrate use (e.g. Cartmill, 1974) or with circadian activity pattern (e.g. Crompton, 1995). Despite the fact that locomotion in a complex arboreal environment does not require binocular vision (Cartmill, 1974, 1992, 2017), arboreal taxa show the highest level of orbit convergence among mammals (Heesy, 2008). Moreover, as more convergent orbits have larger zones of binocular visual field overlap, and scales similarly across mammalian orders (Heesy, 2004), orbit orientation is a good proxy for binocular visual field overlap.
Previous studies have demonstrated that 45% of orbit orientation variance in mammals is explained by ecological factors such as activity pattern, substrate preference, and the degree of faunivory (Heesy, 2008). The same analyses performed solely in non‐primate eutherian mammals demonstrated that 44% of orbit orientation variance is explained by ecological factors. Other authors have also demonstrated a significant correlation between orbit orientation in mammals and the degree of canopy cover in the inhabited ecosystem (Changizi & Shimojo, 2008). All these studies have been performed using broad taxonomic samples with the main goal of finding general ecological patterns shaping orbit orientation and size across mammals. However, detailed studies focused on specific taxonomic groups with more refined ecological categories are less common.
In this article, we perform a detailed study on orbit orientation in the mammalian order Carnivora as a proxy for visual field overlap to explore the relationship between visual strategy and different ecological factors. Specifically, we characterize orbit orientation based on the frontation (α), convergence (β), and orbitotemporal (Ω) angles (e.g. Cartmill, 1970; Ravosa et al. 2000; Heesy, 2005), and we test the association of these angles with the type of vision (i.e. photopic, scotopic or mesopic), the vegetation structure of the inhabited ecosystem (i.e. closed, mixed or open habitats), and substrate preference (i.e. terrestrial, arboreal or aquatic) under a phylogenetic framework.
Our specific goals are (1) to explore whether orbit orientation is associated with the ecology of carnivores; (2) to test for the effect of skull length, i.e. allometric effects, on orbit orientation; (3) to investigate the influence of phylogeny on orbit orientation by mapping frontation, convergence, and orbitotemporal angles onto a carnivoran phylogenetic tree; and (4) to depict a phylomorphospace for orbit orientation that allows the phylogenetic occupation of this morphospace to be reconstructed. Our hypothesis is that orbit orientation is correlated with the specific ecology of carnivorans, as previously demonstrated in other studies with a broader taxonomic range (e.g. Heesy, 2008).
Materials and methods
Taxa sampled
We collected 192 skulls of 107 species belonging to 13 families of living mammalian carnivores: Ailuridae (1), Canidae (20), Felidae (23), Hyaenidae (3), Ursidae (7), Mustelidae (13), Herpestidae (5), Viverridae (4), Eupleridae (3), Procyonidae (6), Phocidae (12), Otariidae (6), and Odobenidae (1) (see Table 1 and Fig. 2.
Table 1.
Species of the order Carnivora included in the study with average values of frontation (Ω), convergence (β), and orbitotemporal (Ω) angles, as well as skull length (Sk. Length). The ecological classifications were established following the literature (see text for details)
| Family | Specie | Activity | Habitat | Substrate | α (°) | β (°) | Ω (°) | Sk. Length (mm) |
|---|---|---|---|---|---|---|---|---|
| Ailuridae | Ailurus fulgens | Nocturnal | Closed | Arboreal | 54.72 | 59.18 | 46.30 | 97.79 |
| Canidae | Canis aureus | Nocturnal | Mixted | Terrestrial | 58.55 | 44.46 | 37.46 | 146.78 |
| Canidae | Canis latrans | Crepuscular | Mixted | Terrestrial | 60.93 | 46.35 | 41.12 | 174.56 |
| Canidae | Canis lupus | Crepuscular | Mixted | Terrestrial | 60.51 | 46.04 | 41.80 | 213.53 |
| Canidae | Canis mesomelas | Nocturnal | Opened | Terrestrial | 57.58 | 45.85 | 39.02 | 143.40 |
| Canidae | Cerdocyon thous | Nocturnal | Closed | Terrestrial | 57.78 | 45.56 | 34.22 | 132.64 |
| Canidae | Chrysocyon brachyurus | Nocturnal | Mixted | Terrestrial | 65.97 | 46.06 | 39.51 | 194.33 |
| Canidae | Cuon alpinus | Crepuscular | Mixted | Terrestrial | 58.81 | 44.39 | 35.66 | 164.04 |
| Canidae | Lycalopex culpaeus | Nocturnal | Opened | Terrestrial | 52.72 | 50.48 | 37.54 | 144.56 |
| Canidae | Lycalopex gymnocercus | Nocturnal | Opened | Terrestrial | 51.76 | 50.53 | 32.75 | 118.58 |
| Canidae | Lycalopex vetulus | Nocturnal | Mixted | Terrestrial | 55.34 | 48.44 | 35.91 | 101.52 |
| Canidae | Lycaon pictus | Crepuscular | Opened | Terrestrial | 54.26 | 50.24 | 41.06 | 181.34 |
| Canidae | Nyctereutes procyonoides | Nocturnal | Closed | Arboreal | 56.51 | 49.16 | 37.49 | 107.82 |
| Canidae | Otocyon megalotis | Crepuscular | Opened | Terrestrial | 59.01 | 42.95 | 34.50 | 102.53 |
| Canidae | Speothos venaticus | Diurnal | Closed | Terrestrial | 53.66 | 50.01 | 34.24 | 123.53 |
| Canidae | Urocyon cinereoargenteus | Nocturnal | Closed | Arboreal | 66.15 | 41.93 | 40.86 | 106.80 |
| Canidae | Vulpes chama | Nocturnal | Opened | Terrestrial | 59.99 | 40.95 | 44.14 | 90.62 |
| Canidae | Vulpes lagopus | Nocturnal | Opened | Terrestrial | 54.89 | 45.37 | 34.24 | 123.10 |
| Canidae | Vulpes macrotis | Nocturnal | Opened | Terrestrial | 54.14 | 47.56 | 34.10 | 108.44 |
| Canidae | Vulpes velox | Nocturnal | Opened | Terrestrial | 55.05 | 45.58 | 34.93 | 103.34 |
| Canidae | Vulpes vulpes | Nocturnalr | Mixted | Terrestrial | 51.66 | 49.20 | 34.08 | 140.85 |
| Canidae | Vulpes zerda | Nocturnal | Opened | Terrestrial | 56.32 | 45.84 | 35.38 | 87.38 |
| Eupleridae | Cryptoprocta ferox | Nocturnal | Closed | Arboreal | 54.13 | 46.40 | 30.54 | 103.63 |
| Eupleridae | Eupleres goudotii | Crepuscular | Closed | Terrestrial | 53.20 | 42.84 | 22.12 | 79.09 |
| Eupleridae | Fossa fossana | Nocturnal | Closed | Terrestrial | 50.14 | 46.66 | 40.53 | 84.80 |
| Felidae | Acinonyx jubatus | Diurnal | Opened | Terrestrial | 72.38 | 41.65 | 52.77 | 155.01 |
| Felidae | Caracal caracal | Nocturnal | Mixted | Terrestrial | 64.27 | 48.95 | 48.15 | 108.82 |
| Felidae | Homotherium serum † | – | – | – | 66.93 | 37.24 | 37.36 | 264.14 |
| Felidae | Leopardus colocolo | Diurnal | Opened | Terrestrial | 67.27 | 47.61 | 47.84 | 83.86 |
| Felidae | Leopardus geoffroyi | Nocturnal | Mixted | Terrestrial | 65.75 | 45.05 | 41.53 | 94.19 |
| Felidae | Leopardus guigna | Nocturnal | Closed | Terrestrial | 67.90 | 43.11 | 42.17 | 79.89 |
| Felidae | Leopardus pardalis | Nocturnal | Closed | Terrestrial | 66.53 | 51.38 | 49.70 | 120.06 |
| Felidae | Leopardus wiedii | Nocturnal | Closed | Arboreal | 67.61 | 49.91 | 51.60 | 83.00 |
| Felidae | Leptailurus serval | Nocturnal | Opened | Terrestrial | 65.80 | 48.11 | 50.01 | 105.69 |
| Felidae | Lynx canadensis | Nocturnal | Closed | Terrestrial | 66.01 | 43.93 | 44.72 | 103.00 |
| Felidae | Lynx lynx | Nocturnal | Closed | Terrestrial | 65.67 | 44.97 | 48.53 | 129.72 |
| Felidae | Lynx pardinus | Nocturnal | Closed | Terrestrial | 70.03 | 45.10 | 49.39 | 111.71 |
| Felidae | Lynx rufus | Nocturnal | Mixted | Terrestrial | 72.52 | 43.81 | 51.64 | 101.77 |
| Felidae | Panthera leo | Nocturnal | Abierta | Terrestrial | 53.41 | 53.25 | 39.95 | 278.68 |
| Felidae | Panthera onca | Nocturnal | Closed | Terrestrial | 57.42 | 57.28 | 45.44 | 207.86 |
| Felidae | Panthera pardus | Nocturnal | Mixted | Terrestrial | 59.19 | 55.33 | 46.96 | 165.04 |
| Felidae | Panthera tigris | Nocturnal | Closed | Terrestrial | 58.24 | 54.97 | 45.94 | 288.63 |
| Felidae | Pardofelis marmorata | Diurnal | Closed | Terrestrial | 69.35 | 51.69 | 56.01 | 79.42 |
| Felidae | Prionailurus bengalensis | Nocturnal | Closed | Terrestrial | 60.13 | 53.24 | 48.12 | 89.56 |
| Felidae | Prionailurus viverrinus | Nocturnal | Mixted | Terrestrial | 63.07 | 50.74 | 43.43 | 111.24 |
| Felidae | Prionailurus rubiginosus | Nocturnal | Closed | Arboreal | 62.20 | 49.99 | 45.29 | 61.20 |
| Felidae | Puma concolor | Nocturnal | Mixted | Terrestrial | 69.12 | 47.80 | 46.61 | 169.57 |
| Felidae | Puma yagouarundi | Diurnal | Mixted | Arboreal | 67.99 | 45.55 | 42.96 | 80.46 |
| Felidae | Smilodon fatalis † | – | – | – | 70.33 | 38.19 | 41.08 | 281.28 |
| Herpestidae | Herpestes edwardsi | Diurnal | Closed | Terrestrial | 67.57 | 34.77 | 27.76 | 64.24 |
| Herpestidae | Herpestes ichneumon | Diurnal | Mixted | Terrestrial | 68.62 | 35.36 | 29.55 | 69.80 |
| Herpestidae | Herpestes javanicus | Diurnal | Closed | Terrestrial | 72.73 | 32.04 | 30.31 | 69.80 |
| Herpestidae | Mungos mungo | Diurnal | Mixted | Terrestrial | 66.29 | 37.76 | 26.89 | 63.50 |
| Herpestidae | Suricata suricatta | Diurnal | Opened | Terrestrial | 71.15 | 41.79 | 44.01 | 51.57 |
| Hyaenidae | Crocuta crocuta | Nocturnal | Mixted | Terrestrial | 63.20 | 38.20 | 32.92 | 105.97 |
| Hyaenidae | Hyaena hyaena | Nocturnal | Opened | Terrestrial | 59.26 | 42.96 | 43.18 | 249.23 |
| Hyaenidae | Proteles cristata | Nocturnal | Opened | Terrestrial | 68.58 | 41.69 | 42.77 | 115.53 |
| Mustelidae | Aonyx capensis | Diurnal | Closed | Aquatic | 55.63 | 49.08 | 38.69 | 113.76 |
| Mustelidae | Eira barbara | Diurnal | Closed | Terrestrial | 64.97 | 46.62 | 41.35 | 103.14 |
| Mustelidae | Enhydra lutris | Diurnal | Opened | Aquatic | 57.63 | 41.75 | 19.94 | 123.24 |
| Mustelidae | Galictis vittata | Diurnal | Closed | Terrestrial | 60.84 | 43.51 | 30.44 | 80.24 |
| Mustelidae | Ichneuma striatus | Nocturnal | Mixted | Terrestrial | 57.91 | 46.65 | 30.24 | 61.95 |
| Mustelidae | Ichneumia albicauda | Nocturnal | Mixted | Terrestrial | 68.75 | 37.14 | 33.95 | 87.85 |
| Mustelidae | Lutra lutra | Nocturnal | Mixted | Aquatic | 48.01 | 42.13 | 45.57 | 218.39 |
| Mustelidae | Martes americana | Nocturnal | Closed | Terrestrial | 56.31 | 42.49 | 24.28 | 66.55 |
| Mustelidae | Martes foina | Nocturnal | Mixted | Terrestrial | 60.62 | 45.27 | 36.94 | 74.83 |
| Mustelidae | Martes pennanti | Crepuscular | Closed | Terrestrial | 55.88 | 48.50 | 32.75 | 101.08 |
| Mustelidae | Meles meles | Nocturnal | Mixted | Terrestrial | 56.70 | 46.63 | 33.05 | 119.47 |
| Mustelidae | Mephitis mephitis | Nocturnal | Mixted | Terrestrial | 62.35 | 39.11 | 24.57 | 61.57 |
| Mustelidae | Mustela nigripes | Nocturnal | Opened | Terrestrial | 49.21 | 49.18 | 26.41 | 63.41 |
| Mustelidae | Pteronura brasiliensis | Diurnal | Mixted | Aquatic | 38.96 | 55.40 | 15.61 | 142.31 |
| Mustelidae | Taxidea taxus | Nocturnal | Mixted | Terrestrial | 50.72 | 51.32 | 23.59 | 115.57 |
| Odobenidae | Odobenus rosmarus | Diurnal | Opened | Aquatic | 65.67 | 25.86 | 12.15 | 332.06 |
| Otariidae | Arctocephalus australis | Diurnal | Opened | Aquatic | 50.90 | 43.94 | 10.65 | 194.88 |
| Otariidae | Callorhinus ursinus | Diurnal | Opened | Aquatic | 43.58 | 57.89 | 9.13 | 159.70 |
| Otariidae | Eumetopias jubatus | Diurnal | Opened | Aquatic | 66.03 | 30.09 | 20.25 | 342.61 |
| Otariidae | Otaria flavescens | Diurnal | Opened | Aquatic | 61.87 | 33.29 | 17.58 | 269.34 |
| Otariidae | Zalophus californianus | Diurnal | Opened | Aquatic | 56.12 | 38.26 | 11.31 | 205.09 |
| Phocidae | Cystophora cristata | Diurnal | Opened | Aquatic | 36.81 | 61.32 | 18.28 | 225.32 |
| Phocidae | Erignathus barbatus | Diurnal | Opened | Aquatic | 35.75 | 60.26 | 14.97 | 109.43 |
| Phocidae | Histriophoca fasciata | Diurnal | Opened | Aquatic | 38.08 | 55.15 | 9.62 | 179.86 |
| Phocidae | Hydrurga leptonyx | Diurnal | Opened | Aquatic | 40.88 | 52.88 | 11.45 | 347.21 |
| Phocidae | Leptonychotes weddellii | Diurnal | Opened | Aquatic | 36.20 | 57.04 | 19.64 | 281.83 |
| Phocidae | Lobodon carcinophaga | Diurnal | Opened | Aquatic | 49.62 | 44.46 | 6.03 | 233.94 |
| Phocidae | Mirounga angustirostris | Diurnal | Opened | Aquatic | 31.44 | 61.98 | 11.37 | 378.88 |
| Phocidae | Mirounga leonina | Diurnal | Opened | Aquatic | 34.03 | 61.46 | 13.77 | 355.16 |
| Phocidae | Monachus monachus | Diurnal | Opened | Aquatic | 40.93 | 58.13 | 17.73 | 242.13 |
| Phocidae | Monachus tropicalis | Diurnal | Opened | Aquatic | 38.00 | 61.46 | 20.65 | 258.85 |
| Phocidae | Pagophilus groenlandicus | Diurnal | Opened | Aquatic | 33.48 | 59.65 | 5.55 | 197.77 |
| Phocidae | Phoca vitulina | Diurnal | Opened | Aquatic | 36.25 | 59.39 | 8.10 | 156.24 |
| Phocidae | Pusa hispida | Diurnal | Opened | Aquatic | 30.01 | 66.10 | 9.89 | 149.38 |
| Procyonidae | Bassaricyon alleni | Crepuscular | Closed | Arboreal | 65.85 | 48.31 | 44.92 | 71.42 |
| Procyonidae | Bassaricyon gabbii | Nocturnal | Closed | Arboreal | 68.43 | 47.94 | 50.80 | 73.31 |
| Procyonidae | Nasua narica | Diurnal | Closed | Arboreal | 64.12 | 40.24 | 29.63 | 61.83 |
| Procionidae | Nasua nasua | Diurnal | Closed | Arboreal | 54.10 | 46.80 | 26.49 | 101.10 |
| Procyonidae | Potos flavus | Nocturnal | Closed | Arboreal | 70.87 | 48.89 | 46.09 | 68.99 |
| Procyonidae | Procyon lotor | Nocturnal | Mixted | Terrestrial | 56.87 | 55.98 | 38.76 | 101.04 |
| Ursidae | Ailuropoda melanoleuca | Diurnal | Closed | Terrestrial | 53.09 | 48.76 | 30.51 | 128.00 |
| Ursidae | Helarctos malayanus | Nocturnal | Closed | Terrestrial | 71.59 | 48.63 | 52.05 | 238.64 |
| Ursidae | Melursus ursinus | Nocturnal | Mixted | Terrestrial | 63.89 | 48.54 | 44.48 | 194.04 |
| Ursidae | Tremarctos ornatus | Diurnal | Mixted | Terrestrial | 66.26 | 50.31 | 49.09 | 201.90 |
| Ursidae | Ursus americanus | Diurnal | Closed | Terrestrial | 70.64 | 42.36 | 43.31 | 235.83 |
| Ursidae | Ursus arctos | Crepuscular | Mixted | Terrestrial | 69.55 | 48.76 | 50.75 | 286.83 |
| Ursidae | Ursus maritimus | Diurnal | Opened | Aquatic | 68.68 | 43.94 | 43.86 | 335.63 |
| Ursidae | Ursus thibetanus | Diurnal | Mixted | Terrestrial | 65.44 | 50.36 | 44.88 | 232.76 |
| Viverridae | Arctictis binturong | Nocturnal | Closed | Arboreal | 61.80 | 46.52 | 38.49 | 138.36 |
| Viverridae | Cynogale bennettii | Nocturnal | Closed | Aquatic | 58.60 | 46.61 | 33.67 | 109.50 |
| Viverridae | Paguma larvata | Nocturnal | Closed | Terrestrial | 48.70 | 57.54 | 41.49 | 116.89 |
| Viverridae | Paradoxurus hermaphroditus | Nocturnal | Closed | Terrestrial | 52.61 | 59.04 | 34.98 | 102.88 |
| Viverridae | Viverra tangalunga | Nocturnal | Closed | Terrestrial | 52.40 | 45.92 | 30.61 | 112.80 |
†denote extinct taxa.
Figure 2.
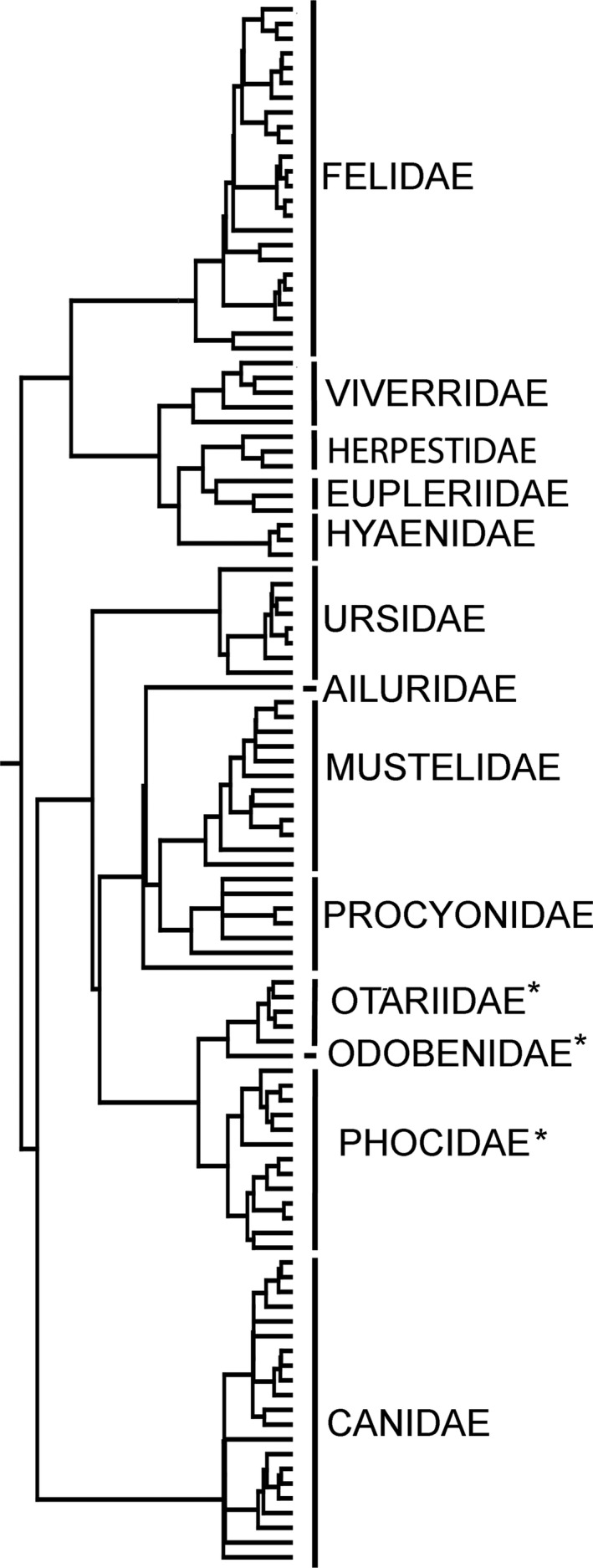
Phylogenetic tree for the species sampled in this paper. The supertree of Nyakatura & Bininda‐Emonds (2012) has been pruned to include only the species sampled in our study, and we have included the extinct sabre‐tooths using the topology and branch lengths of Piras et al. (2013). Asterisks denote pinnipeds.
The skulls are housed in the collections of the Department of Anatomy at the University of Valladolid (Spain), Smithsonian National Museum of Natural History (USNM, Washington, DC, USA), American Museum of Natural History (AMNH, USA), National Museum of Scotland (NMS, UK) and the Museum für Naturkunde Berlin (MFN, Germany).
We collected only adult individuals, as indicated by closed basilar synchondroses and complete tooth eruption. We also included two sabre‐tooths, Smilodon fatalis and Homotherium serum (collected from DigiMorph, http://digimorph.org/), as fossil species, without living analogues, adapted to deploy a specialized killing bite (Slater & Van Valkenburgh, 2008; Figueirido et al. 2010, 2018).
Orbit orientation
All the data were collected by A.P.‐R. with a MicroScribe G2X by digitizing 14 three‐dimensional landmarks (Fig. 1A) to define the orbital, sagittal, and temporal planes (Fig. 1B–D). Their 3D coordinates (x, y, z) were imported into excel using the Immersion software package (Immersion, Inc., San Jose, CA, USA). We also constructed an additional plane, i.e. the dorsal plane (Fig. 1D), perpendicular to the sagittal plane. The sagittal plane was defined with the points Pr (Prostion), Nn (Nasion), In (Inion), and Bs (Basion; Fig. 1A,B). The orbital plane is defined as the plane that contains the following points: the orbitale superius (Os), the orbitale anterius (Oa) and the orbitale posterius (Op). The temporal plane is defined as the plane that contains the orbitale superius (Os), the orbitale lateralis (Ol) and the most posterior point of the temporal fossa (Tp) (Fig. 1C).
As the carnivores included in our sample lack the postorbital bar, we used the Op instead of the most inferior point of the orbit, the orbitale inferior (Oi, in Heesy, 2008) to define the orbital plane. We used Op because it is a better landmark for defining the plane of the orbit than Oi, as the latter seems more influenced by the shape of the tooth row than is the former in carnivores (C. Casares‐Hidalgo, pers. obs.). Moreover, the distance between Oa and Op is longer than between Oa and Oi, better capturing the variation in convergence (Fig. 1C). However, as in the Heesy (2008) dataset several taxa have a postorbital bar, his selection of Oi as a landmark to define the orbit plane was an adequate one.
The orbit orientation was defined by the frontation (α), convergence (β), and orbitotemporal (Ω) angles (e.g. Cartmill, 1970; Heesy, 2008). The frontation angle (α) is measured as the dihedral angle between the dorsal plane and the orbital plane (Fig. 1D). The convergence angle (β) is defined as the dihedral angle between the orbit and sagittal planes (Fig. 1B). The dihedral angle between the orbital and temporal planes (Fig. 1C) is defined here as the orbitotemporal angle (Ω). This angle was taken specifically for this study to characterize the relationship between the orbit and the disposition of the neurocranium and temporal muscles (Cartmill, 1974, 2017; Heesy, 2004, 2005, 2008; Finarelli & Goswami, 2009). We wrote a routine in wolfram mathematica v. 11.1 (available from the authors upon request) to calculate the dihedral angles between planes.
The orientation angles were measured on the left and right sides, and they were averaged per specimen to avoid possible undesirable effects due to fluctuating asymmetry. Afterwards, all the specimens per species were averaged to avoid possible effects of static allometry.
Ecological variables
We classified all living species sampled in this study according to (1) their visual strategy (i.e. photopic, scotopic or mesopic); (2) the type of habitat they inhabit (i.e. open, mixed or closed); and (3) their substrate preference (i.e. arboreal, terrestrial or aquatic). We classified photopic carnivores as those species that are active (i.e. hunting or breeding behaviour) in the presence of bright light/photopic conditions, scotopic carnivores as those being active in low‐light conditions, and mesopic carnivores as those being active during twilight and/or sunrise (crepuscular) or indifferently during day and night (cathemeral). We classified the type of habitat (open, mixed or closed) according to the value of vegetation cover (Table 1). Carnivores that forage in trees (i.e. hunting or breeding behaviour) have been classified as arboreal. In contrast, those species that forage in aquatic environments (at least partially) and show adaptations for swimming or diving have been classified as aquatic. The rest of the species that usually forage on the ground have been classified as terrestrial.
The information about these ecological variables was taken from the IUCN database (http://www.iucnredlist.org), the PanTHERIA online database (http://esapubs.org/Archive/ecol/E090/184/default.htm) and, when the information was insufficient or doubtful, from Mammalian Species (http://www.science.smith.edu/msi/), Nowak (2005), Sillero‐Zubiri et al. (2004), and Wilson & Mittermeier (2009).
Quantifying the influence of phylogeny
To assess the presence of phylogenetic signal in orbit orientation, we assembled a phylogenetic tree (Fig. 2) using mesquite (Maddison & Maddison, 2016). We pruned the molecular supertree of Nyakatura & Bininda‐Emonds (2012) to exclude those carnivorous species do not sampled in this study. The extinct sabre‐tooths were incorporated using the topology and branch length published by Piras et al. (2013).
We calculated the K statistic for continuous traits (Blomberg et al. 2003) for the frontation (α), convergence (β), and orbitotemporal (Ω) angles using the function phylosig in the phytools r package (Revell, 2012). The index assumes a Brownian motion (BM) evolutionary model [i.e. purely neutral (random) evolution of a trait with variance directly proportional to the branch lengths] and varies from 0 to ≫ 1, where 0 indicates no phylogenetic signal (the trait has evolved independently of phylogeny, and close relatives are not more similar than distant relatives) and values > 1 reflect that close relatives are more similar than expected under BM. Interestingly, the K statistic has the advantage of allowing direct comparison of phylogenetic signal strengths not only across traits but also across different phylogenetic trees (Molina‐Venegas & Rodríguez, 2017). The statistical significance of K was assessed based on a comparison of the observed phylogenetically independent contrasts and the expected contrast under 999 randomizations (Blomberg et al. 2003).
Additionally, we calculated Pagel's λ (Pagel, 1993) to assess the degree of phylogenetic signal in the frontation, convergence, and orbitotemporal angles using the function phylosig in the phytools R package (Revell, 2012). Pagel's λ is a parameter for the correlations between species relative to the correlation expected under Brownian evolution. It scales between zero (indicating an absence of correlation between species) and 1.0 (indicating a correlation between species equal to the Brownian expectation).
To reconstruct the past phenotypes of extinct ancestral species from the trait values of their extant descendants, we mapped the frontation, convergence, and orbitotemporal angles on the phylogeny of Fig. 2. Using the R package phytools (Revell, 2012), we estimated the ancestral characters at internal nodes assuming a Brownian mode of evolution. However, it is worth mentioning that these analyses should be used with caution, as potential inaccuracy in reconstructing ancestral traits of the internal nodes of the phylogeny in the absence of fossil taxa has been demonstrated (Finarelli & Flynn, 2006).
The influence of skull length on orbit orientation and the relationships between orbit angles
We explored the relationship between orbit orientation and body size to control for allometric effects, as Changizi & Shimojo (2008) demonstrated that orbit convergence increases with body size for species in forests but not in other leafy and semi‐leafy environments. Therefore, as body size could be a determinant of orbit orientation, we decided to test for allometry in orbit angles as a preliminary test to discard possible allometric effects on the orbit of mammalian carnivores. Accordingly, we regressed α, β, and Ω with skull length as a proxy for carnivore body size (Van Valkenburgh, 1990). This was tested with ordinary least squares (OLS) regression analysis of log‐transformed variables. We also explored the relationships among the angles (α–β, α–Ω, and β‐Ω) using OLS regression analysis of log‐transformed variables with PAST (Hammer & Harper, 2004).
However, species cannot be treated as statistically independent data points because they are phylogenetically related (Felsenstein, 1985). This violates the assumption of independent sampling and hence inflates the classic type I error (e.g. Harvey & Pagel, 1991). To avoid this, we performed phylogenetic generalized least square (PGLS) analysis (Martins & Hansen, 1997) using the geiger package of R (Harmon et al. 2007) with 10 000 permutations and using a Brownian motion model of evolution.
Testing the influence of ecology on orbit orientation
To assess the relationship of orbit orientation with the ecological variables, we used phylogenetic manova (Garland et al. 1993) with α, β, and Ω as a multivariate response variable and visual strategy, substrate preference, and type of habitat as predictor variables, all while taking phylogeny into account. We used the aov.phylo function in the geiger package of R (Harmon et al. 2007), assuming Brownian motion. For obvious reasons, both of the sabre‐tooths were excluded from the analysis. We used a Brownian model of evolution and 1000 replicates to test for statistical significance. To test for specific relationships among pairs of predictor and response variables while taking phylogeny into account, we also used phylogenetic anova (Garland et al. 1993).
Depicting the orbit orientation phylomorphospace
To investigate the distribution of carnivorans in phenotypic space, we computed a principal components analysis (PCA) from the correlation matrix of the log‐transformed frontation, convergence, and orbitotemporal angles and skull length in the 104 species analysed. Another PCA was performed excluding pinnipeds from the same log‐transformed variables.
The reconstructed ancestral values were plotted into the shape spaces obtained in the PCAs. Subsequently, the branches of the tree were connected (Klingenberg & Ekau, 1996; Rohlf, 2002; Polly, 2008; Astúa, 2009; Gidaszewski et al. 2009; Figueirido et al. 2010, 2013, 2016; Klingenberg & Gidaszewski, 2010) to construct orbit phylomorphospaces. We used the function phylomorphospace in the phytools R package (Revell, 2012).
Results and Discussion
Inter‐family differences in orbit orientation
The values of frontation (α), convergence (β), and orbitotemporal (Ω) angles across all the carnivoran families are shown as box plots in Fig. 3. The families in these box‐plots are ordered according to the increasing value in each orbit angle.
Figure 3.
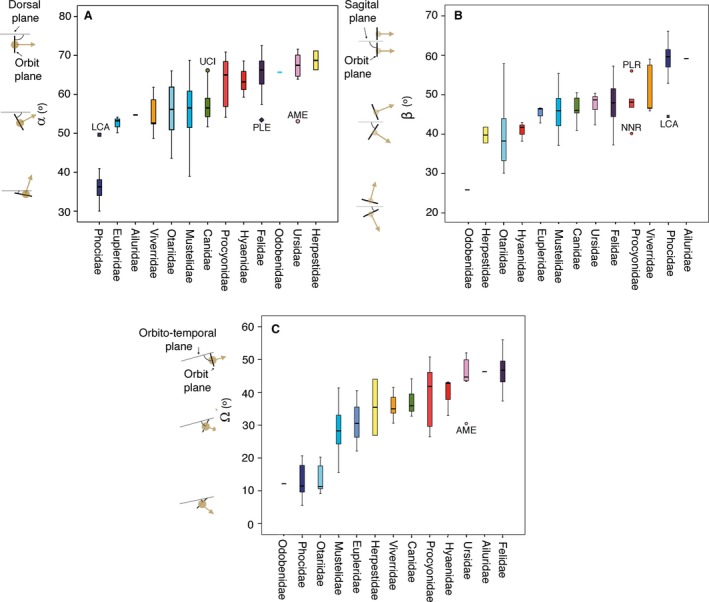
Box plots showing the range of frontation (α), convergence (β), and orbitotemporal (Ω) angles across all carnivoran families analysed here. The vertical line inside each box is the median. Box length is the interquartile range (IQR) and shows the difference between the 75th and 25th percentiles. Horizontal bars enclose values of 5–95%. UCI, Urocyon cinereoargenteus; PLE, Panthera leo; AME, Ailuropoda melanoleuca; PLR, Procyon lotor; NNR, Nasua narica; LCA, Lobodon carcinophaga.
The family Phocidae shows the lowest values of frontation among the sample (Fig. 3A), which a priori could be interpreted as an adaptation to have the orbits facing upwards as a consequence of diving in the mesopelagic zone (e.g. Levenson & Schusterman, 1999). However, otariids have similar values of frontation to other fissipeds, but contrary to phocids, they are adapted to forage in the epipelagic zone. Moreover, the giant otter (Pteronura brasiliensis), which is the mustelid with the lowest frontation angle in our sample and overlapping with the values of phocids, does not dive in deep zones (Carter & Rosas, 1997), and the deepest‐diving marine otter (Enhydra lutris) has a value of frontation similar to other mustelids. The walrus (Odobenus rosmarus) has the lowest value of convergence (Fig. 3B).
Strikingly, pinnipeds have the lowest values of orbitotemporal angles and are well differentiated from fissipeds (Fig. 3C). Among fissipeds, the families with the largest orbitotemporal angles are felids, the red panda and ursids (Fig. 3C), and those with low orbitotemporal angles are mustelids and euplerids (Fig. 3C).
Skull length does not influence orbit orientation
The linear regression analysis of frontation and orbitotemporal angles against skull length indicated that both angles are associated with size (Table 2; Fig. 4A,C). However, both significant associations disappeared when taking the phylogenetic relationships of species into account (Table 2; Fig. 4A,C). In contrast, the linear regression analysis of orbit convergence against skull length was not significant (Table 2; Fig. 5B).
Table 2.
Summary statistics for bivariate regressions computed to test the influence of skull length on orbit orientation as well as the relationships between the angles
| Regressions | Model | r 2 | F | P‐value |
|---|---|---|---|---|
| α‐Skl | OLS | 0.0829 | 0.0500 | < 0.01 |
| PGLS | 0.0021 | 0.2187 | 0.691 | |
| β‐Skl | OLS | 0.0005 | 0.0500 | > 0.05 |
| PGLS | 0.0089 | 0.9331 | 0.405 | |
| Ω‐Skl | OLS | 0.1253 | 14.8660 | < 0.01 |
| PGLS | 0.0065 | 0.6800 | 0.490 | |
| α‐β | OLS | 0.4020 | 69.8950 | < 0.01 |
| PGLS | 0.4507 | 85.3290 | < 0.01 | |
| α‐Ω | OLS | 0.5715 | 139.1380 | < 0.01 |
| PGLS | 0.0788 | 8.8904 | 0.008 | |
| β‐Ω | OLS | 0.0202 | 2.1530 | > 0.05 |
| PGLS | 0.0042 | 0.4358 | 0.589 |
Figure 4.
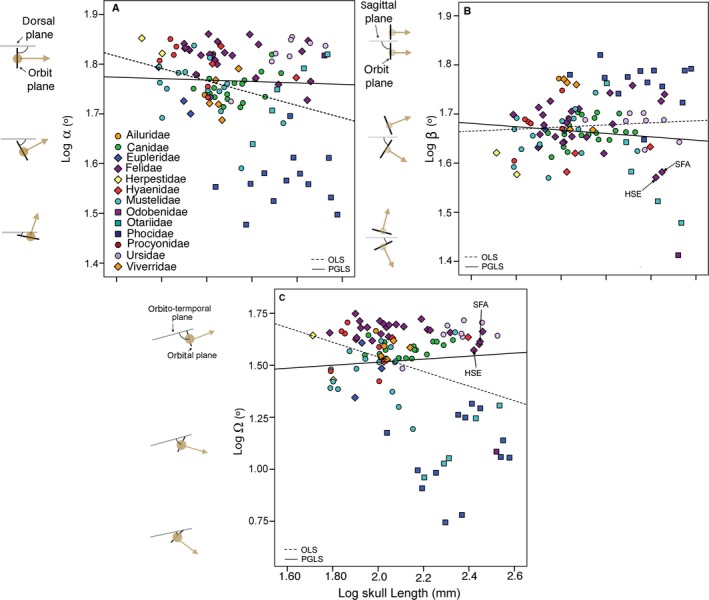
Bivariate plots of orbit angles against skull length. (A) Skull length against frontation angle (α). (B) Skull length against convergence angle (β). (C) Skull length against orbitotemporal angle (Ω).All variables are log‐transformed. HSE, Homotherium serum; SFA, Smilodon fatalis. Dotted line, regression line using ordinary least squares (OLS); Black line, regression line using phylogenetic generalized least squares (PGLS). All variables are log‐transformed.
Figure 5.
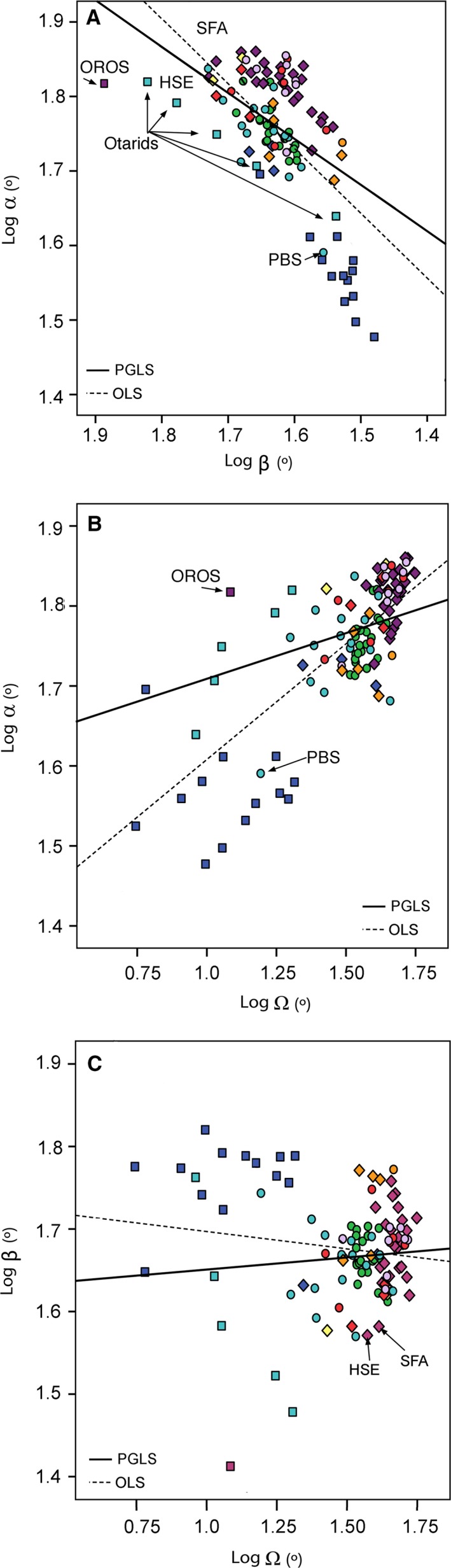
Bivariate plots of the association among orbit angles. (A) Frontation (α) against convergence (β). (B) Orbitotemporal (Ω) against convergence (β). (C) Convergence (β) against orbitotemporal (Ω). All variables are log‐transformed. Black line, regression line using ordinary least squares (OLS); dotted line, regression line using phylogenetic generalized least squares (PGLS). HSE, Homotherium serum; SFA, Smilodon fatalis; PBS, Pteronura brasiliensis; OROS, Odobenus rosmarus.
Therefore, our data suggest that orbit orientation in carnivores is not influenced by skull length, which means that longer or shorter skulls are not characterized by specific orbit orientations. However, although we included a sample with a wide range of body masses, according to Cartmill (2017) orbit orientation is not ever expected to vary with size across large size ranges.
The relationships between angles
The linear regression analysis of frontation against convergence was highly significant, even when the phylogenetic relationships were taken into account (Table 2; Fig. 5A). It is striking that, at least in mammalian carnivores, an increase in frontation entails a decrease in convergence and vice versa, which indicates that not all combinations of frontation/convergence are possible.
Similarly, the linear regression analysis of frontation compared with the orbitotemporal angle was highly significant, even when phylogeny was taken into account (Table 2; Fig. 5B). On the other hand, the linear regression analysis between convergence and the orbitotemporal angle was not significant (Table 2; Fig. 5C).
A strong influence of phylogeny on orbit orientation
Orbit orientation in carnivores exhibited a significant phylogenetic signal, as indicated by both Bloomberg's K and Pagel's lambda (α: K = 0.7308, P‐value = 0.001; λ = 0.994, P‐value < 0.001; β: K = 0.338; P‐value < 0.001; λ = 0.984; P‐value < 0.001; Ω: K = 0.806; P‐value < 0.001; λ = 0.872).
Mapping the values of orbit angles on the phylogenetic tree of Fig. 2, it is possible to trace the history of these traits through the evolution of the clade. Strikingly, different groups have acquired larger frontation values than the one reconstructed for the common ancestor of all carnivoran families (e.g. ursids, canids, procyonids). This indicates that larger frontation angles seem to have evolved independently in multiple groups of carnivorans (α; Fig. 6A). In contrast, the evolution of convergence (β; Fig. 6B) seem to be more conservative, although multiple groups also experience the independent acquisition of larger convergence values (e.g. pantherines or phocids) and few cases experience a decrease in convergence values (i.e. Odobenus rosmarus and Eumetopias jubatus). It is also important to note that the distribution of values of the orbitotemporal angle is similar to frontation (Ω; Fig. 5C).
Figure 6.
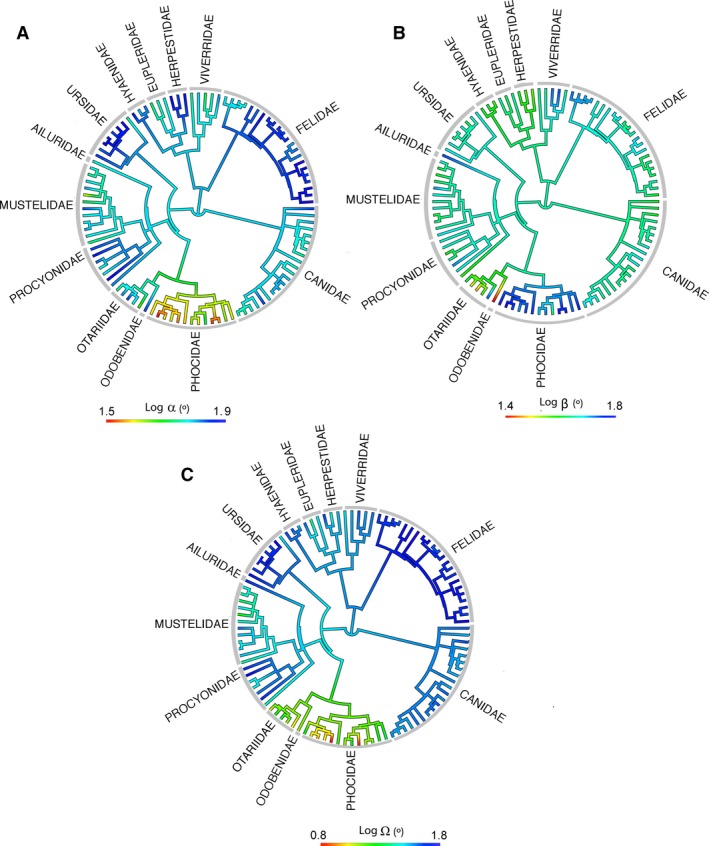
Values of (A) frontation (α), (B) convergence (β), and (C) orbitotemporal angles (Ω) plotted on the phylogenetic tree for the species sampled in this paper.
Orbit orientation is not related to ecology
Non‐phylogenetic manova revealed that orbit orientation was statistically associated with visual strategy, habitat, and substrate preference (Table 3). Taking the phylogenetic relationships of the species into account, orbit orientation was statistically associated with neither visual strategy nor habitat use (Table 3). However, the significant association of orbit orientation with substrate preference (Table 3) was probably due to the ecological homogeneity among pinnipeds. Pinnipeds [Phocidae (earless or true seals), Otariidae (sea lions and fur seals), and Odobenidae (one extant species of walrus)] have particularly long skulls and a unique combination of frontation, convergence, and orbitotemporal angles. Moreover, they are aquatic, diurnal, and inhabit the same areas. As a matter of fact, when we repeated the analyses excluding pinnipeds, the association between orbit orientation and substrate preference was not significant (Table 3).
Table 3.
Results obtained from phylogenetic manova using Brownian model of evolution and 1000 simulations
| df | Wilks’ lambda | Approx‐F | num‐df | den‐df | P | Pphy | |
|---|---|---|---|---|---|---|---|
| Sample including pinnipeds | |||||||
| Activity | 2 | 0.643 | 8.142 | 6 | 198 | 0.000 | 0.120 |
| Habitat | 2 | 0.684 | 6.907 | 6 | 198 | 0.000 | 0.054 |
| Substrate | 2 | 0.331 | 24.332 | 6 | 198 | 0.000 | 0.005 |
| Sample excluding pinnipeds | |||||||
| Activity | 2 | 0.836 | 2.504 | 6 | 160 | 0.024 | 0.289 |
| Habitat | 2 | 0.750 | 4.122 | 6 | 160 | 0.001 | 0.071 |
| Substrate | 2 | 0.829 | 2.628 | 6 | 160 | 0.019 | 0.112 |
P, standard (non‐phylogenetic) P‐value; Pphy, phylogenetic P‐values; df, degrees of freedom.
To detect which predictor (independent) variable specifically influences a given response (dependent) variable, we performed a set of phylogenetic anova to test for specific associations between pairs of variables. Accordingly, frontation was directly associated with habitat and substrate preference, even when taking the phylogenetic relationships of the species into account (Table 4). On the other hand, the orbitotemporal angle was statistically associated with habitat vision and substrate preference, even when taking phylogeny into account (Table 4). However, all these significant associations disappeared when we re‐analysed the data excluding pinnipeds, with the exception of the association between the orbitotemporal angle and substrate preference (Table 4). However, we urge readers to interpret these results with caution, as this significant association may simply be a statistical artefact, given that randomization could inflate the degrees of freedom, and hence giving a ‘false‐positive’ result in statistical tests. In any case, as the orbitotemporal angle reflects to a great extent the disposition of masticatory muscles – mainly the temporalis and masseter muscles (Cartmill, 1970; Heesy, 2005), the observed association between the orbitotemporal angle and the use of substrate could be indirectly reflecting a feeding diet requiring special cranial adaptations (e.g. Figueirido et al. 2010).
Table 4.
Results obtained from phylogenetic anova using Brownian model of evolution and 1000 simulations
| df | Sum‐Sq | Mean‐Sq | F‐value | P | Pphy | ||
|---|---|---|---|---|---|---|---|
| Sample including pinnipeds | |||||||
| Alpha | Vision | 2.000 | 0.109 | 0.055 | 8.053 | 0.001 | 0.252 |
| Habitat | 2.000 | 0.163 | 0.082 | 13.034 | 0.000 | 0.032 | |
| Substrate | 2.000 | 0.328 | 0.164 | 35.435 | 0.000 | 0.028 | |
| Beta | Vision | 2.000 | 0.002 | 0.001 | 0.220 | 0.803 | 0.957 |
| Habitat | 2.000 | 0.004 | 0.002 | 0.458 | 0.634 | 0.843 | |
| Substrate | 2.000 | 0.011 | 0.005 | 1.301 | 0.277 | 0.829 | |
| Omega | Vision | 2.000 | 1.760 | 0.880 | 25.446 | 0.000 | 0.009 |
| Habitat | 2.000 | 1.413 | 0.706 | 18.584 | 0.000 | 0.012 | |
| Substrate | 2.000 | 3.437 | 1.719 | 95.640 | 0.000 | 0.001 | |
| Sample excluding pinnipeds | |||||||
| Alpha | Vision | 2.000 | 0.004 | 0.002 | 0.747 | 0.477 | 0.795 |
| Habitat | 2.000 | 0.002 | 0.001 | 0.354 | 0.703 | 0.824 | |
| Substrate | 2.000 | 0.013 | 0.007 | 2.647 | 0.077 | 0.361 | |
| Beta | Vision | 2.000 | 0.003 | 0.002 | 0.927 | 0.400 | 0.743 |
| Habitat | 2.000 | 0.007 | 0.003 | 1.879 | 0.159 | 0.348 | |
| Substrate | 2.000 | 0.003 | 0.001 | 0.723 | 0.488 | 0.760 | |
| Omega | Vision | 2.000 | 0.023 | 0.012 | 1.040 | 0.358 | 0.713 |
| Habitat | 2.000 | 0.009 | 0.004 | 0.376 | 0.688 | 0.792 | |
| Substrate | 2.000 | 0.163 | 0.081 | 8.516 | 0.000 | 0.032 | |
P, standard (non‐phylogenetic) test; Pphy, phylogenetic P‐values; df, degrees of freedom.
Orbit orientation phylomorphospace
The phylomorphospace depicted from the superimposition of the phylogeny shown in Fig. 2 onto the bivariate plot, depicted from the first two PCs obtained from PCA of orbit orientation, is shown in Fig. 7. Figure 7A depicts the phylomorphospace from the sample of fissipeds plus pinnipeds. Whereas PCI explains 55.45% of the original variance, PCII explain 25.30%, which jointly explains more than 80% of the original variance. The scores of the species on this phylomorphospace reveal a clear separation between fissipeds and pinnipeds. Species with high scores on PCI have very high values of frontation and orbitotemporal angles, and very low values of skull length and convergence. Species with low scores on PCI are characterized by having high values of convergence and skull length. On the other hand, species with high scores on PCII are characterized by very high convergence values and moderate orbitotemporal ones. Those species scoring negatively on this eigenvector are characterized by high values of skull length and frontation angle.
Figure 7.
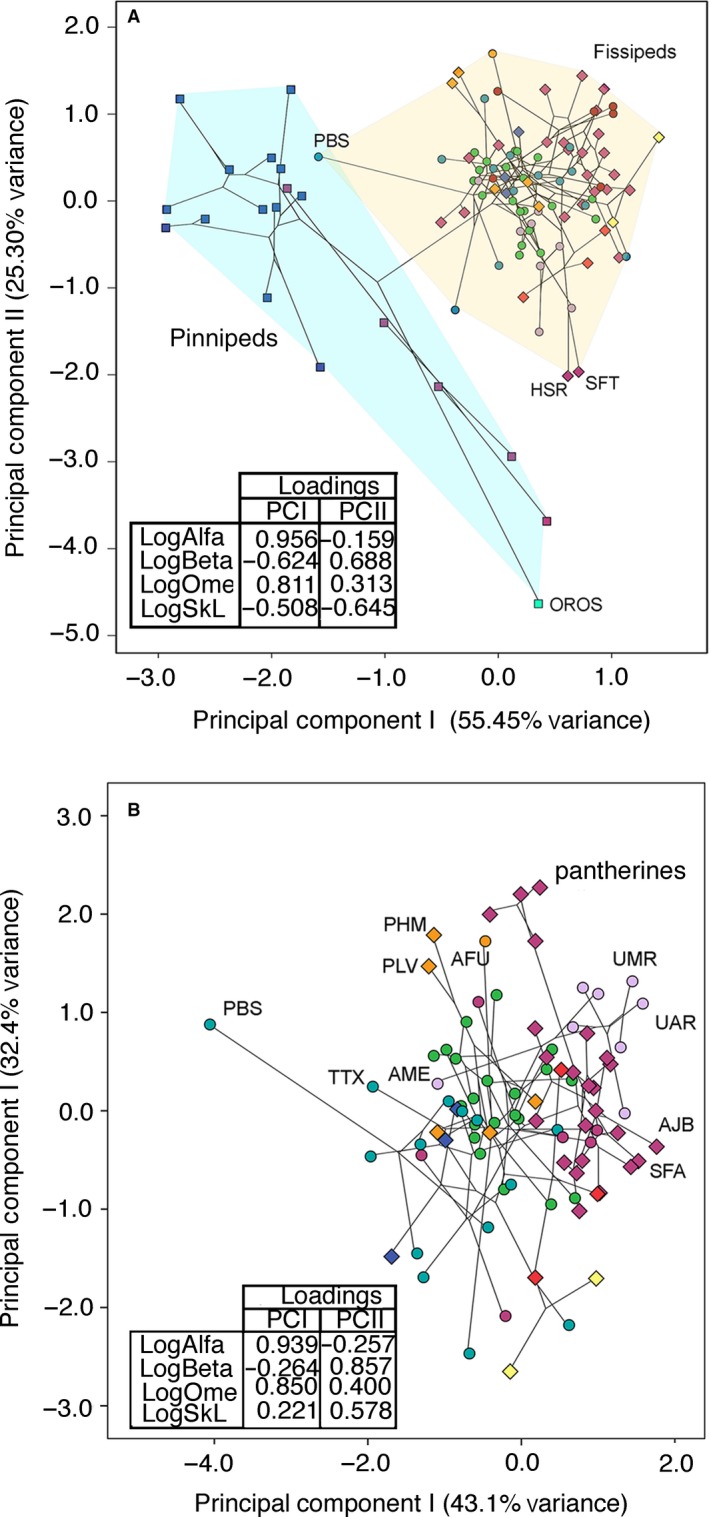
Phylomorphospaces of orbit orientation and skull length in carnivores. (A) Phylomorphospace depicted from the first two PCs obtained from the PCA computed from the sample of pinnipeds plus fissipeds. (B) Phylomorphospace depicted from the first two PCs obtained from the PCA computed from the sample excluding pinnipeds. AME, Ailuropoda melanoleuca; AFU, Ailurus fulgens; HSR, Homotherium serum; SFT, Smilodon fatalis; PBS, Pteronura brasiliensis; OROS, Odobenus rosmarus; TTX, Taxidea taxus; PLV, Paguma larvata; PHM, Paradoxurus hermaphroditus; UMR, Ursus maritimus; UAR, Ursus arctos; AJB, Acinonyx jubatus. Symbols as in Fig. 4.
The combination of both PCs separates pinnipeds from fissipeds in this morphospace (Fig. 7A). While phocids are separated from fissipeds plus otariids and odobenids on PCI, otariids and odobendis are separated from the rest of the sample on PCII. Strikingly, despite the variety of ecologies exhibited by fissipeds in comparison with pinnipeds, they occupy a much lower portion of this morphospace than pinnipeds.
We also performed a second phylomorphospace from PCA computed with a sample restricted to fissipeds (Fig. 7B). The first PC explains 43.10% of the original variance, and the second PC explains 32.36%, which jointly explain more than 75% of the total variation. Whereas on the first PC all variables score positively, excepting the convergence angle, on PCII all variables score positively, excepting frontation. Therefore, ursids, felids, and hyaenids score on the positive end of PCI and the rest of the sample score negatively.
Conclusions
Our results indicate that variation in orbit orientation is not influenced by variation in skull length and therefore allometry has a negligible effect on orbit orientation in mammalian carnivores (Fig. 5; Table 2). The significance of this relationship found in some studies (e.g. Changizi & Shimojo, 2008) could be due to the taxa included in the sample. This does not contradict the ‘X‐ray hypothesis’, but it encourages testing their asseverations from another point of view, such as using a visual axis instead of an orbit axis.
The frontation and convergence angles are negatively correlated, indicating that carnivores with more forward‐facing orbits (more convergence) also have more upward‐facing orbits (less frontation). In contrast, the frontation and orbitotemporal angles are positively correlated, indicating that carnivores with more downward‐facing orbits (more frontation) also have less parallel temporal and orbit planes. This significant association indicates a strong influence of the shape and size of the temporal fossa in shaping frontation. Although further exploration is necessary to infer how modularity and integration in the carnivoran skull can affect orbit orientation, Finarelli & Goswami (2009) demonstrated that encephalization is related to orbit orientation, at least in felids and canids.
Our results also demonstrate that orbit orientation is only statistically associated with substrate preference and not with preferred habitat or visual strategy, even when taking phylogeny into account (Table 3). However, when we re‐analysed the sample excluding pinnipeds, the association between orbit orientation and substrate preference was not significant (Table 3). Similarly, the analyses performed between specific pairs of orbit angles and ecological variables also revealed significant associations between frontation and the orbitotemporal angles when the complete sample of species was analysed (Table 4). Again, when we re‐analysed the data excluding pinnipeds, none of the associations was significant, the sole exception being that between orbitotemporal angle and substrate preference. The reason for the absence of significant correlation between orbit orientation and the ecology of the taxa when pinnipeds are excluded is that they have particularly long skulls and a unique combination of frontation, convergence, and orbitotemporal angles. Moreover, they are all aquatic, diurnal, and inhabit very open areas. Therefore, their presence in the analysis significantly biases the results towards having significant associations.
The significant correlation found between the orbitotemporal angle and substrate preference is probably related to feeding behavior, as the orbitotemporal angle mainly reflects the disposition of masseter and temporalis muscles involved in chewing, gape angle, and struggling with prey (e.g. Figueirido et al. 2010, 2012, 2013) in the case of predators (e.g. pantherines).
In any case, the lack of association between orbit orientation and the visual strategy or the type of habitat of carnivores was totally unexpected. Previous studies have found significant associations between them (e.g. Walls, 1942; Hughes, 1977; Noble et al. 2000; Heesy, 2008), but including more mammalian orders, such as primates, rodents or ungulates. For example, Heesy (2008) showed in a large sample of non‐primate eutherian mammals that approximately 40% of the variance in orbit orientation was highly influenced by ecological factors such as activity pattern, substrate preference, and the degree of faunivory.
One explanation for the lack of association between orbit anatomy and visual strategy in carnivores found in our study could be the nocturnal bottleneck hypothesis (see Heesy & Hall, 2010 for a review). The ancestral condition of nocturnality in mammals provided useful adaptations in the eye tissues for the entire range of vision. In fact, several researchers have found evidence for genetic and physiological adaptations related to the nocturnal bottleneck that have not been reflected in the orbit anatomy (e.g. Hut et al. 2000; Hall et al. 2012; Gerkema et al. 2013).
The lack of a significant association between the evolutionary increases or decreases in orbit angles (Fig. 6) among different carnivoran families and the habitat preference could be explained because the ancestral stem carnivoramorphans (early miacoids) acquired a range of orbit angles that were useful for the entire range of ecologies exhibited by derived monophyletic groups of the crown‐clade Carnivora. Therefore, the adaptation of different orbit orientations to specific ecological niches has simply been unnecessary. In fact, early Tertiary families of carnivoramorphans, the paraphyletic ‘Miacidae’ and monophyletic Viverravidae (Wesley‐Hunt & Flynn, 2005), which are traditionally joined as Miacoidea (Flynn & Galiano, 1982; Hunt & Tedford, 1993), were ecologically diverse, with species adapted to arboreal, scansorial, and cursorial locomotion, and ranging in size from weasel‐sized to coyote‐sized forms (Flynn et al. 1998).
If this hypothesis holds, then the orbit anatomy of mammalian carnivorans represents a case of an ‘ecological bottleneck’ probably acquired in early Cenozoic representatives of stem taxa. However, differences between orbit axis and eye axis (the direction in which the orbits are directed and the direction in which the eyes are watching, respectively) could also explain the lack of correlation in fissipeds between orbit orientation and habitat preference. Cartmill (2017) observed that neither visual nor orbit orientation could be directly inferred from the orientation of the orbits in smaller mammals. In fact, most mammals have conjugate eye movements, i.e. both eyes gaze in the same direction with visual axes directed at the same point in space (Walls, 1942). In other words, orbit orientation does not necessarily reflect visual orientation (Simons, 2009).
In summary, this study demonstrates a lack of association between orbit anatomy and ecology in carnivoran mammals and shows that extrapolating visual strategies and the type of habitat for extinct carnivores from orbit anatomy should be done with caution. Our results also highlight the need to develop more accurate methods to characterize eye axis instead of orbit axis.
Conflict of interest
None of the authors has any conflict of interest to declare.
Author contributions
A.P.R., C.C.H. collected the data. C.C.H., B.F. performed the analyses. B.F., C.C.H., F.J.P. designed the study. M.F.G. wrote the script in mathematica to calculate the angles. B.F., C.C.H., M.F.G. wrote the paper.
Acknowledgements
The authors are especially grateful to Christopher Heesy for his comments and suggestions during the elaboration of the study. The authors are also grateful to Ramón Muñoz‐Chapuli, Amelia Victoria de Andrés and Paul Palmqvist for their comments. Alberto Martín‐Serra and Francisco Serrano also made significant comments and improvements on an earlier version of this MS. The authors thank all the staff from the museums visited for kindly providing access to the specimens under their care. Three anonymous reviewers and Lars Schmitz provided insightful comments that significantly improved the rigour of the manuscript. This paper has been funded by the Spanish Ministry of Economy and Competitiveness (MINECO) grant to B.F. (CGL2015‐68300‐P).
References
- Allman J (1977) Evolution of the visual system in the early primates. Prog Psychobiol Physiol Psychol 7, 53. [Google Scholar]
- Angielczyk KD, Schmitz L (2014) Nocturnality in synapsids predates the origin of mammals by over 100 million years. Proc R Soc B 281, 20141642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Astúa D (2009) Evolution of scapula size and shape in didelphid marsupials (Didelphimorphia: Didelphidae). Evolution 63, 2438–2456. [DOI] [PubMed] [Google Scholar]
- Blomberg SP, Garland T Jr, Ives AR, et al. (2003) Testing for phylogenetic signal in comparative data: behavioral traits are more labile. Evolution 57, 717–745. [DOI] [PubMed] [Google Scholar]
- Campbell FW, Green DG (1965) Optical and retinal factors affecting visual resolution. J Physiol 181, 576–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter SK, Rosas FC (1997) Biology and conservation of the giant otter Pteronura brasiliensis . Mamm Rev 27, 1–26. [Google Scholar]
- Cartmill M (1970). The orbits of arboreal mammals: a reassessment of the arboreal theory of primate evolution. Doctoral Dissertation, University of Chicago, Department of Anthropology.
- Cartmill M (1974) Rethinking primate origins. Science 184, 436–443. [DOI] [PubMed] [Google Scholar]
- Cartmill M (1992) New views on primate origins. Evol Anthropol Issues News Rev 1, 105–111. [Google Scholar]
- Cartmill M (2017) Arboreal adaptations and the origin of the order Primates In: The Functional and Evolutionary Biology of Primates (ed. Tutle R.), pp. 97‐122. New York: Routledge. [Google Scholar]
- Changizi MA, Shimojo S (2008) “X‐ray vision” and the evolution of forward‐facing eyes. J Theor Biol 254, 756–767. [DOI] [PubMed] [Google Scholar]
- Crompton RH (1995) Visual predation, habitat structure, and the ancestral primate niche In: Creatures of the Dark. The Nocturnal Prosimians. (eds Alterman L, Doyle GA, Kay Izard M.), pp. 11–30. Boston, MA: Springer. [Google Scholar]
- Felsenstein J (1985) Phylogenies and the comparative method. Am Nat 125, 1–15. [Google Scholar]
- Figueirido B, Serrano‐Alarcón FJ, Slater GJ, et al. (2010) Shape at the cross‐roads: homoplasy and history in the evolution of the carnivoran skull towards herbivory. J Evol Biol 23, 2579–2594. [DOI] [PubMed] [Google Scholar]
- Figueirido B, Serrano‐Alarcón FJ, Palmqvist P (2012) Geometric morphometrics shows differences and similarities in skull shape between the red and giant pandas. Journal of Zoology 286, 293–302. [Google Scholar]
- Figueirido B, Tseng ZJ, Martín‐Serra A (2013) Skull shape evolution in durophagous carnivorans. Evolution 67, 1975–1993. [DOI] [PubMed] [Google Scholar]
- Figueirido B, Martín‐Serra A, Janis CM (2016) Ecomorphological determinations in the absence of living analogues: the predatory behavior of the marsupial lion (Thylacoleo carnifex) as revealed by elbow joint morphology. Paleobiology 42, 508–531. [Google Scholar]
- Figueirido B, Lautenschlager S, Pérez‐Ramos A, et al. (2018) Distinct predatory behaviors in Scimitar‐ and Dirk‐toothed sabertooth cats. Curr Biol 28, 3260–3266. [DOI] [PubMed] [Google Scholar]
- Finarelli JA, Flynn JJ (2006) Ancestral state reconstruction of body size in the Caniformia (Carnivora, Mammalia): the effects of incorporating data from the fossil record. Syst Biol 55, 301–313. [DOI] [PubMed] [Google Scholar]
- Finarelli JA, Goswami A (2009) The evolution of orbit orientation and encephalization in the Carnivora (Mammalia). J Anat 214, 671–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn JJ, Galiano H (1982) Phylogeny of early Tertiary Carnivora: with a description of a new species of Protictis from the Middle Eocene of northwestern Wyoming. Am Mus Novit no. 2725.
- Flynn JJ, Janis CM, Scott KM, et al. (1998) Early Cenozoic Carnivora (‘Miacoidea’). Evol Tertiary Mamm North America 1, 110–123. [Google Scholar]
- Garamszegi LZ, Møller AP, Erritzøe J (2002) Coevolving avian eye size and brain size in relation to prey capture and nocturnality. Proc R Soc Lond B Biol Sci 269, 961–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland T Jr, Dickerman AW, Janis CM, et al. (1993) Phylogenetic analysis of covariance by computer simulation. Syst Biol 42, 265–292. [Google Scholar]
- Gerkema MP, Davies WI, Foster RG, et al. (2013) The nocturnal bottleneck and the evolution of activity patterns in mammals. Proc R Soc Lond B Biol Sci 280, 20130508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidaszewski NA, Baylac M, Klingenberg CP (2009) Evolution of sexual dimorphism of wing shape in the Drosophila melanogaster subgroup. BMC Evol Biol 9, 110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall MI, Kamilar JM, Kirk EC (2012) Eye shape and the nocturnal bottleneck of mammals. Proc R Soc Lond B Biol Sci 279, 4962–4968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer O, Harper DAT (2004) PAST version 1.3. Available from https://folk.uio.no/ohammer/past/
- Harmon LJ, Weir JT, Brock CD, et al. (2007) GEIGER: investigating evolutionary radiations. Bioinformatics 24, 129–131. [DOI] [PubMed] [Google Scholar]
- Harvey PH, Pagel MD (1991) The Comparative Method in Evolutionary Biology, Vol. 239. Oxford: Oxford University Press. [Google Scholar]
- Heesy CP (2004) On the relationship between orbit orientation and binocular visual field overlap in mammals. Anat Rec 281, 1104–1110. [DOI] [PubMed] [Google Scholar]
- Heesy CP (2005) Function of the mammalian postorbital bar. J Morphol 264, 363–380. [DOI] [PubMed] [Google Scholar]
- Heesy CP (2008) Ecomorphology of orbit orientation and the adaptive significance of binocular vision in primates and other mammals. Brain Behav Evol 71, 54–67. [DOI] [PubMed] [Google Scholar]
- Heesy CP, Hall MI (2010) The nocturnal bottleneck and the evolution of mammalian vision. Brain Behav Evol 75, 195–203. [DOI] [PubMed] [Google Scholar]
- Hughes A (1977) The topography of vision in mammals of contrasting life style: comparative optics and retinal organisation In: The Visual System in Vertebrates. (ed Crescitelli F.), pp. 613–756. Berlin: Springer‐Verlag. [Google Scholar]
- Hunt RM Jr, Tedford RH (1993) Phylogenetic relationships within the aeluroid Carnivora and implications of their temporal and geographic distribution In: Mammal Phylogeny (eds Novacek MJ, McKenna M, Szalay FS.), pp. 53–73. New York: Springer Verlag. [Google Scholar]
- Hut RA, Scheper A, Daan S (2000) Can the circadian system of a diurnal and a nocturnal rodent entrain to ultraviolet light? J Comp Physiol A 186, 707–715. [DOI] [PubMed] [Google Scholar]
- Klingenberg CP, Ekau W (1996) A combined morphometric and phylogenetic analysis of an ecomorphological trend: pelagization in Antarctic fishes (Perciformes: Nototheniidae). Biol J Linn Soc 59, 143–177. [Google Scholar]
- Klingenberg CP, Gidaszewski NA (2010) Testing and quantifying phylogenetic signals and homoplasy in morphometric data. Syst Biol 59, 245–261. [DOI] [PubMed] [Google Scholar]
- Levenson DH, Schusterman RJ (1999) Dark adaptation and visual sensitivity in shallow and deep‐diving pinnipeds. Mar Mamm Sci 15, 1303–1313. [Google Scholar]
- MacIver MA, Schmitz L, Ugurcan M, et al. (2017) Massive increase in visual range preceded the origin of terrestrial vertebrates. Proc Natl Acad Sci U S A 114, E2375–E2384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddison WP, Maddison DR (2016) Mesquite: a modular system for evolutionary analysis. Vers. 3.10.
- Martins EP, Hansen TF (1997) Phylogenies and the comparative method: a general approach to incorporating phylogenetic information into the analysis of interspecific data. Am Nat 149, 646–667. [Google Scholar]
- Molina‐Venegas R, Rodríguez MÁ (2017) Revisiting phylogenetic signal; strong or negligible impacts of polytomies and branch length information? BMC Evol Biol 17, 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motani R, Rothschild BM, Wahl JR (1999) Large eyeballs in diving ichthyosaurs. Nature 402, 747. [DOI] [PubMed] [Google Scholar]
- Noble VE, Kowalski EM, Ravosa MJ (2000) Orbit orientation and the function of the mammalian postorbital bar. J Zool 250, 405–418. [Google Scholar]
- Nowak RM (2005) Walker's Carnivores of the World. Baltimore: JHU Press. [Google Scholar]
- Nyakatura K, Bininda‐Emonds OR (2012) Updating the evolutionary history of Carnivora (Mammalia): a new species‐level supertree complete with divergence time estimates. BMC Biol 10, 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagel M (1993) Seeking the evolutionary regression coefficient: an analysis of what comparative methods measure. J Theor Biol 164, 191–205. [DOI] [PubMed] [Google Scholar]
- Piras P, Maiorino L, Teresi L, et al. (2013) Bite of the cats: relationships between functional integration and mechanical performance as revealed by mandible geometry. Syst Biol 62, 878–900. [DOI] [PubMed] [Google Scholar]
- Pirenne MH (1943) Binocular and uniocular threshold of vision. Nature 152, 698–699. [Google Scholar]
- Polly PD (2008) Adaptive zones and the pinniped ankle: a 3D quantitative analysis of carnivoran tarsal evolution In: Mammalian Evolutionary Morphology: A Tribute to Frederick S. Szalay. (eds Sargis E, Dagosto M.), pp. 165–194. Dordrecht: Springer. [Google Scholar]
- Ravosa MJ, Noble VE, Hylander WL, et al. (2000) Masticatory stress, orbital orientation and the evolution of the primate postorbital bar. J Hum Evol 38, 667–693. [DOI] [PubMed] [Google Scholar]
- Revell LJ (2012) phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol Evol 3, 217–223. [Google Scholar]
- Rohlf J (2002) Geometric morphometrics and phylogeny In: Morphology, Shape, and Phylogeny. (eds MacLeod N, Forey PL.), pp. 175–193. London: Taylor & Francis. [Google Scholar]
- Schmitz L, Motani R (2011) Nocturnality in dinosaurs inferred from scleral ring and orbit morphology. Science 332, 705–708. [DOI] [PubMed] [Google Scholar]
- Sillero‐Zubiri C, Hoffmann M, Macdonald DW (eds) (2004) Canids: Foxes, Wolves, Jackals, and Dogs: Status Survey and Conservation Action Plan, pp. x + ‐430. Gland: IUCN. [Google Scholar]
- Simons EL (2009) Convergence and frontation in fayum anthropoid orbits In: Primate Craniofacial Function and Biology. (eds Vinyard C, Ravosa MJ, Wall C.), Chapter 18, pp. 407–429. Boston: Springer. [Google Scholar]
- Slater GJ, Van Valkenburgh B (2008) Long in the tooth: evolution of sabertooth cat cranial shape. Paleobiology 34, 403–419. [Google Scholar]
- Van Valkenburgh B (1990) Skeletal and dental predictors of body mass in carnivores In: Body Size in Mammalian Paleobiology: Estimation and Biological Implications. (eds Damuth J, McFadden B.), pp. 1–205. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Walls GL (1942) The vertebrate eye and its adaptive radiation. Cranbrook Inst Sci Bull 9, 1–785. [Google Scholar]
- Wesley‐Hunt GD, Flynn JJ (2005) Phylogeny of the Carnivora: basal relationships among the carnivoramorphans, and assessment of the position of ‘Miacoidea’ relative to Carnivora. J Syst Paleontol 3, 1–28. [Google Scholar]
- Wilson DE, Mittermeier RA (2009) Handbook of the Mammals of the World (No. C/599.012 H3).


