Abstract
The aim of this study was to determine the main stages of submandibular salivary gland development during the embryonic period in humans. In addition, we studied submandibular salivary gland development in rats on embryonic days 14–16 and expression in the submandibular salivary gland region with the monoclonal antibody HNK‐1. Serial sections from 25 human embryos with a greatest length ranging from 10 to 31 mm (Carnegie stages 16–23; weeks 5.5–8 of development) and Wistar rats of embryonic days (E) 14–16 were analysed with light microscopy. Five stages of submandibular salivary gland development were identified. The prospective stage (1), between weeks 5.5 and early week 6, is characterized by a thickening of the epithelium of the medial paralingual groove in the floor of the mouth corresponding to the primordium of the submandibular salivary gland parenchyma. At this stage, the primordium of the parasympathetic ganglion lies below the lingual nerve. The primordium of the submandibular salivary gland parenchyma is observed in rats on E14 in the medial paralingual groove with mesenchymal cells, underlying the lingual nerve. These cells are HNK‐1‐positive, corresponding to the primordium of the parasympathetic ganglion. The bud stage (2), at the end of week 6 in humans and on E15 in rats, is characterized by the proliferation and invagination of the epithelial condensation, surrounded by an important condensation of the mesenchyme. The pseudoglandular stage (3) at week 6.5 is characterized by the beginning of the formation of lobes in the condensed mesenchyme. The canalicular stage (4), between week 7 and 7.5, is characterized by the appearance of a lumen in the proximal part of the submandibular duct. The innervation stage (5) occurs during week 8, with the innervation of the submandibular and interlobular ducts. Nervous branches arriving from the parasympathetic ganglion innervate the glandular parenchyma. Numerous blood vessels are observed nearby. Our results suggest that submandibular salivary gland development requires interactions among epithelium, mesenchyme, parasympathetic ganglion and blood vessels.
Keywords: development, embryology, embryonic stage, submandibular salivary gland
Introduction
The development of the submandibular salivary gland (SMG) has attracted many investigators, with attention mainly focused on rodents. As previously described, SMG development is generally classified into the prebud, initial bud, pseudoglandular, canalicular, and terminal bud stages (Jaskoll & Melnick, 1999; Tucker, 2007). In mice, the first sign of the SMG is an epithelial thickening observed close to the developing tongue on embryonic day (E) 11.5 (prebud stage). This thickening invaginates in the mesenchyme derived from the neural crest (Jaskoll et al. 2002; Teshima et al. 2016a) at E12.5 (bud stage). On E14, the SMG shows a multilobular aspect (pseudoglandular stage). On E14.5, the solid epithelial ducts initiate lumen formation via the apoptosis of internal cells (canalicular stage) (Tucker, 2007; Teshima et al. 2016b). The gland continues to develop until puberty (Tucker, 2007). Similar events are observed in rats with a 2‐day delay (Denny et al. 1997).
Several descriptive studies have provided a sound foundation for knowledge on SMG development in humans (Moral, 1913; Thoma, 1919; Dozin, 1966; Johns, 1977; Gibson, 1983; El‐Mohandes et al. 1987; García‐García et al. 1991; Mérida‐Velasco et al. 1993; Chi, 1996; Guizetti & Radlanski, 1996; Sperber, 2001).
Submandibular salivary gland morphogenesis requires complex coordinated events. SMG development depends on epithelial growth and proliferation, together with interactions with the surrounding mesenchyme, blood vessels and nerves (Yamamoto et al. 2008; Knosp et al. 2015; Kwon et al. 2017).
It has been suggested that the medial paralingual groove appears in the floor of the mouth during week 5 as a consequence of tongue development. The lateral paralingual groove would appear soon after due to gum growth (Dozin, 1966; García‐García et al. 1991; Mérida‐Velasco et al. 1993; Guizetti & Radlanski, 1996).
The SMG itself initiates development at week 6 as a solid epithelial bud in the medial paralingual groove (Sperber, 2001). Some authors have reported that this epithelial bud invaginates within the surrounding mesenchyme distal to the parasympathetic submandibular ganglion (PSG), which lies next to the lingual nerve (Moral, 1913; Thoma, 1919; Dozin, 1966; García‐García et al. 1991; Mérida‐Velasco et al. 1993). However, other investigators have suggested that this development starts where the future papillae are located (Johns, 1977; Sperber et al. 2010).
The aim of this work was to analyse the development of the SMG in human embryos with a greatest length (GL) ranging from 10 to 31 mm [Carnegie stages (CS) 16–23] and the development of the SMG in E14–E16 rat embryos. In addition, we immunolabelled rat embryos of these stages at the SMG region with the monoclonal antibody HNK‐1, which recognizes neural crest cells (Lipinski et al. 1983; Mérida‐Velasco et al. 2012). Our results suggest the existence of five stages of SMG development, correlated with either defined morphological events or the relationship with the surrounding mesenchyme, nerves and blood vessels.
Material and methods
Study on human embryos
The submandibular gland region was investigated bilaterally in 25 human embryos that belong to the large collection kept at the Embryology Institute of the University Complutense of Madrid (UCM). All specimens were products of ectopic pregnancies or spontaneous abortions managed at the Department of Obstetrics of UCM, and none of the material indicated any possible malformation. The study was approved by the Ethics Committee of UCM. The GL of the embryos ranged from 10 to 31 mm. The number and CS of the embryos were as follows: three belonged to stage 16, four to stage 17, three to stage 18, three to stage 19, five to stage 20, one to stage 21, three to stage 22, and four to stage 23. The parameters used to determine the postconceptional age were the GL and external and internal criteria (O'Rahilly & Müller, 2001, 2010). All specimens were fixed in 10% formalin and embedded in paraffin following standard procedures. Each specimen was sliced in toto on a single plane. Sections 7–10 μm in thickness were obtained and stained with haematoxylin‐eosin (HE), azan, Bielschowsky's stain or Masson's trichrome dye depending on specimen size (MacManus & Mowry, 1968). Digital photomicrographs were taken with a Nikon® DXM 1200 microscope (Nikon Corp., Tokyo, Japan), and transferred and edited on a PC Pentium IV using Act One and Adobe photoshop® CS6 software. Although we observed the left or right submandibular gland region in each of the 25 human embryos (see above), all figures were prepared using the same orientation, always showing the right submandibular gland region. The nomenclature used corresponds to the English version of the Terminologia Anatomica (2011) of the Federative International Programme for Anatomical Terminology.
Study in rats
The experimental protocol used for rats was approved by the Ethics Committee for Animal Experimentation of University Complutense of Madrid (Spain). Wistar rat embryos from stages E14 to E16 (considering the fertilization day as E0, based on the vaginal plug) were used for the study. The specimens were extracted from anaesthetized and dissected mothers and fixed overnight in 4% paraformaldehyde in 0.1 m phosphate‐buffered saline (PBS, pH 7.4) at 4 °C. Five specimens at each stage were used. The embryos were dehydrated and embedded in paraffin. Serial sections were cut to a 6–8‐μm thickness and mounted on poly‐l‐lysine precoated slides. For orientation, adjacent sections were stained with HE.
Immunohistochemical procedure
Sections were pre‐treated with 0.3% hydrogen peroxide in PBS for 4 h at room temperature before washing in PBS (3 × 10 min). They were then incubated overnight at 4 °C in 10% normal goat serum (Sigma‐Aldrich®) and 0.2% Triton X‐100 (Merck, Germany) diluted in PBS. The sections were incubated overnight with the mouse monoclonal antibody HNK‐1 (Becton Dickinson, CA, USA) in a 1/20 dilution at 4 °C and then washed with PBS (3 × 10 min). Next, the sections were again incubated at 4 °C for 12 h with a horseradish peroxidase‐conjugated secondary antibody (Chemicon) diluted 1/2500 and then rinsed in PBS (3 × 10 min). The preparations were treated with 3,3’ diaminobenzidine (Sigma‐Aldrich®). After a final rinse in PBS (3 × 10 min), the preparations were dehydrated in a series of alcohols, cleared in xylene and mounted in Eukitt (O. Kindler GmbH and Co., Germany). The controls were treated as described above with the exception that the incubation in the primary antibody solution was omitted to demonstrate that the secondary antibody only reacted with the corresponding primary antibody.
Results
Submandibular gland development in humans
Carnegie stages 16–17 (CS16–17)
The medial paralingual groove appeared for the first time at CS16 (11‐mm‐GL human embryo) as a condensation of epithelial cells observed in the floor of the mouth, separated from the surrounding mesenchyme by a clearly defined basal membrane. This epithelial condensation constituted the prospective area of the SMG parenchyma.
In the same region, the lingual nerve described a curve opened cranially on its way to the anlage of the tongue. Some basophilic cells surrounded by blood vessels were observed in relation to the lingual nerve at the level of its curve, constituting the primordium of the PSG (Fig. 1a,b).
Figure 1.
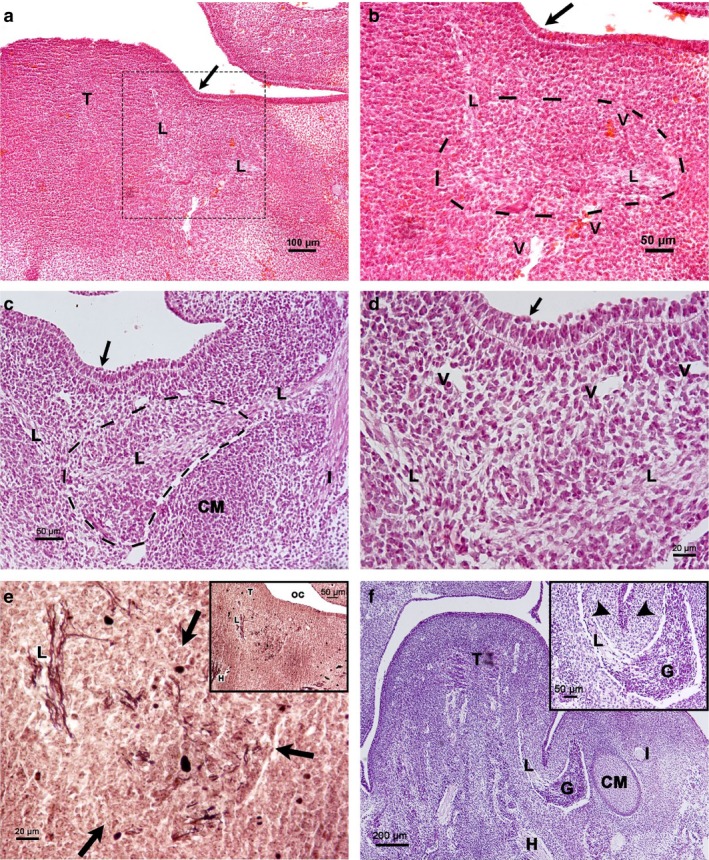
Carnegie stages 16–17. Frontal sections of the floor of the mouth. (a) Human embryo 11 mm GL. Haematoxylin‐eosin staining. The medial paralingual groove (arrow). L, lingual nerve; T, tongue. (b) Magnification of the squared area of (a). Primordium of the PSG (framed by a dashed line) in relationship to the lingual nerve (L); arrow, medial paralingual groove; V, vessel. (c). Human embryo 12 mm GL. Azan staining. The cells of the prospective area of the SMG show apical polarization (arrow). Primordium of Meckel's cartilage (CM) between the alveolar inferior nerve (I) and lingual nerve (L). Primordium of the PSG in relation to the lingual nerve (framed by a dashed line). (d). Magnification of (c). Prospective area of the SMG parenchyma (arrow). L, lingual nerve; V, vessel. (e) Human embryo 12 mm GL. Bielschowsky's staining. Primordium of the PSG (arrows) in relation to the lingual nerve (L). (e) Inset corresponding to a panoramic view. H, hypoglossal nerve; L, lingual nerve; T. tongue; OC, oral cavity. (f) Human embryo 13 mm GL. Azan staining. (f) Inset corresponding to invagination of the prospective area of the SMG parenchyma (arrowheads). CM, Meckel's cartilage; G, parasympathetic submandibular ganglion; H, hypoglossal nerve; I, inferior alveolar nerve; L, lingual nerve; T. tongue.
The lateral paralingual groove appeared at CS17 (12‐mm‐GL human embryo). The prospective area of the SMG parenchyma shows a prismatic pseudostratified epithelium, well delimited from the underlying mesenchyme by a clear basal membrane. The nuclei of most epithelial cells are elongated, with their greatest edge perpendicular to the plane of the basal membrane. They are situated at different heights, although they do not reach the most basal regions. At the surface, nuclei are round and less compact. Some blood vessels were observed between the condensed mesenchyme and the PSG primordium. The inferior alveolar and lingual nerves are divisions of the mandibular nerve, and the primordium of the Meckel's cartilage was observed between them. The primordium of the PSG developed next to the lingual nerve (Fig. 1c,d). A Bielschowsky‐stained 12‐mm‐GL human embryo showed the distribution of this nerve among the PSG primordium cells, and the epithelial condensation coincided cranially with this primordium (Fig. 1e).
The parenchyma of the SMG proliferated as a solid cord by the end of CS17 (13‐mm‐GL embryo), being continuous with the epithelial sulcus. This cord invaginated the surrounding condensed mesenchyme next to the lingual nerve and the PSG (Fig. 1f).
Carnegie stages 18–19 (CS18–19)
At these stages, the primordium of the SMG parenchyma was surrounded by a clearly condensed mesenchyme, which was the anlage of the glandular stroma (Fig. 2a,b). The observation of sagittal sections allowed the demonstration of the presence of the primordium of parenchyma dorsal to the PSG, initiating a lobulation process. The primordium of the glandular stroma preceded the glandular parenchyma. The PSG emitted branches to the anlage of the glandular stroma (Fig. 2c,d).
Figure 2.
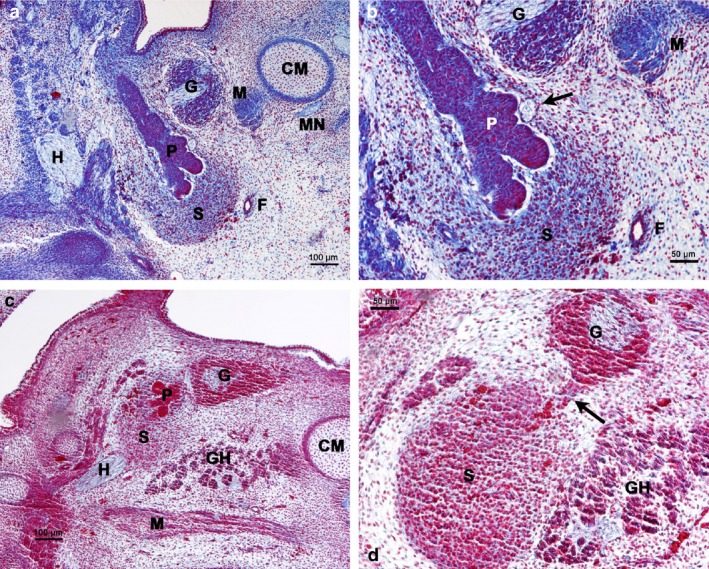
Carnegie stages 18–19. (a) Human embryo 16 mm GL. Frontal section of the submandibular gland region. Azan staining. Primordium of the glandular stroma (S) and glandular parenchyma (P). CM, Meckel's cartilage; F, facial artery; G, parasympathetic submandibular gland; H, hypoglossal nerve; M, mylohyoid muscle; MN, mylohyoid nerve. (b) Magnification of (a). Arrow indicates nerve. F, facial artery; G, parasympathetic submandibular ganglion; M, mylohyoid muscle; P, primordium of the glandular parenchyma; S, primordium of the glandular stroma. (c) Human embryo 15 mm GL. Sagittal section of the submandibular gland region. Haematoxylin‐eosin staining. The primordium of the parenchyma (P) and stroma (S) of the SMG is located dorsal to parasympathetic submandibular ganglion (G). CM, Meckel's cartilage; GH, geniohyoid muscle; H, hypoglossal nerve; M, mylohyoid muscle. (d) Magnification of (c). Nervous branch (arrow) from the parasympathetic submandibular ganglion (G) that reaches the primordium of the glandular stroma (S). GH, geniohyoid muscle.
Carnegie stages 20–21 (CS20‐E21)
The sublingual papillae were located at the anterior part of the mouth floor, flanking the tongue in the mandibular symphysis region (Fig. 3a).
Figure 3.
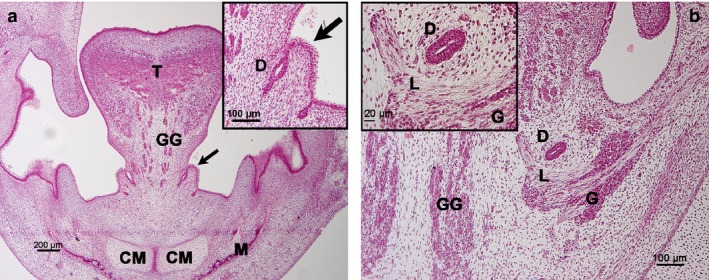
Carnegie stages 20–21. Frontal section of the symphyseal region. Haematoxylin‐eosin staining. (a) Human embryo 20 mm GL. Sublingual papilla (arrow). CM, Meckel's cartilage; GG, genioglossus muscle; M, mandible; T, tongue. (a) Inset corresponds to sublingual papilla (arrow). D, Submandibular duct. (b) Human embryo 20 mm GL. (b) Inset corresponds to submandibular duct (D). G, parasympathetic submandibular ganglion; GG, genioglossus muscle; L, lingual nerve.
The submandibular duct was formed by a bistratified cuboidal epithelium and showed a central lumen. It was located cranial to the lingual nerve and the PSG (Fig. 3b).
Carnegie stages 22‐23 (CS22–23)
Observation of a 26‐mm‐GL (CS22) human embryo detected with Bielschowsky's stain showed the parasympathetic innervation reaching the primordium of the glandular parenchyma and the submandibular duct (Fig. 4a,b). The interlobar excretory ducts were formed by a bistratified cuboidal epithelium and contained a lumen. The primordium of the glandular stroma was formed by a vascularized mesenchyme (Fig. 4c).
Figure 4.
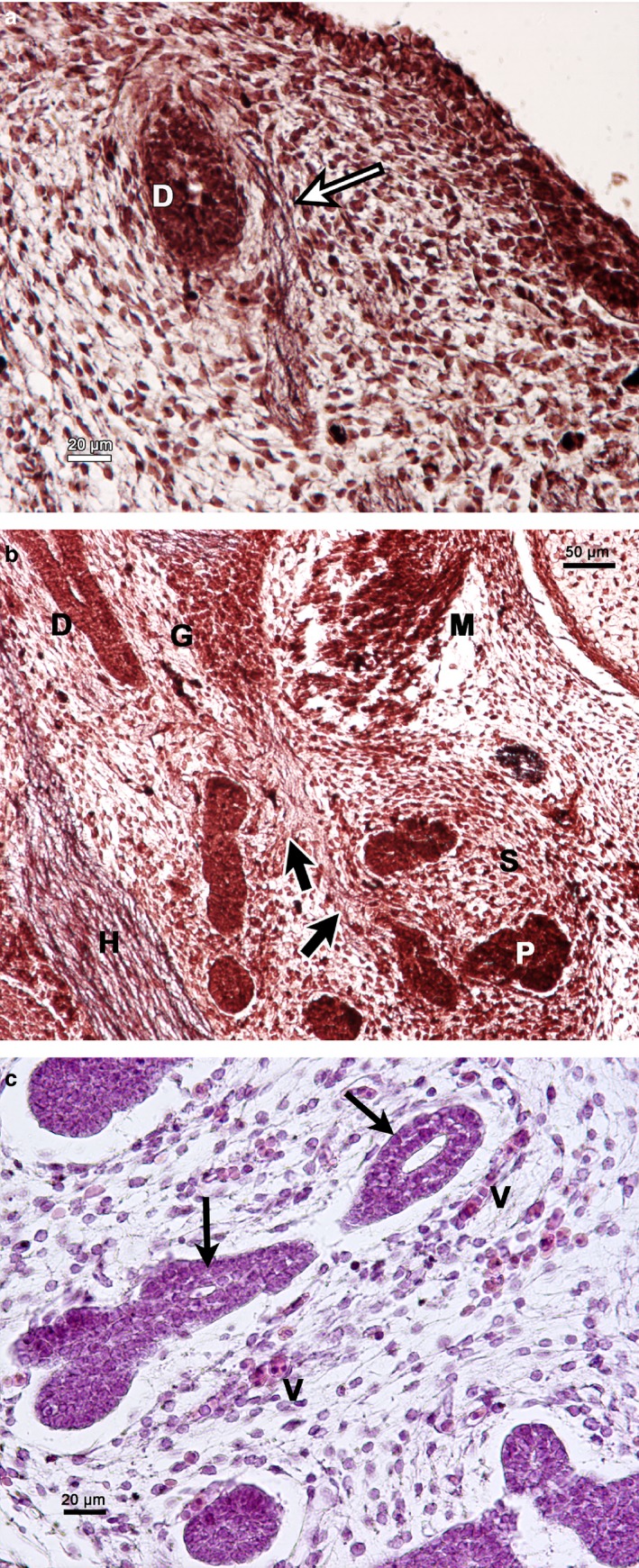
Carnegie stage 22–23. (a) Human embryo 26 mm GL. Bielschowsky's staining. Frontal section of the innervation (arrow) of the submandibular duct (D). (b) Human embryo 26 mm GL. Bielschowsky's staining. Frontal section of the innervation (arrows) of the glandular parenchyma (P). D, submandibular duct; G, parasympathetic submandibular ganglion; H, hypoglossal nerve; M, mylohyoid muscle; S, primordium of the glandular stroma. (c) Human embryo 31 mm GL. Azan staining. Interlobular duct with lumen (arrows). V, vessels.
Submandibular gland development in rats
On E14, the medial paralingual groove showed an epithelial condensation in the floor of the mouth. This condensation corresponded to the prospective area of the SMG parenchyma. The inferior alveolar and lingual nerves were observed below and were HNK‐1‐positive. Some mesenchymal cells in relation to the lingual nerve were also HNK‐1‐positive and corresponded to the primordium of the PSG (Fig. 5a‐c).
Figure 5.
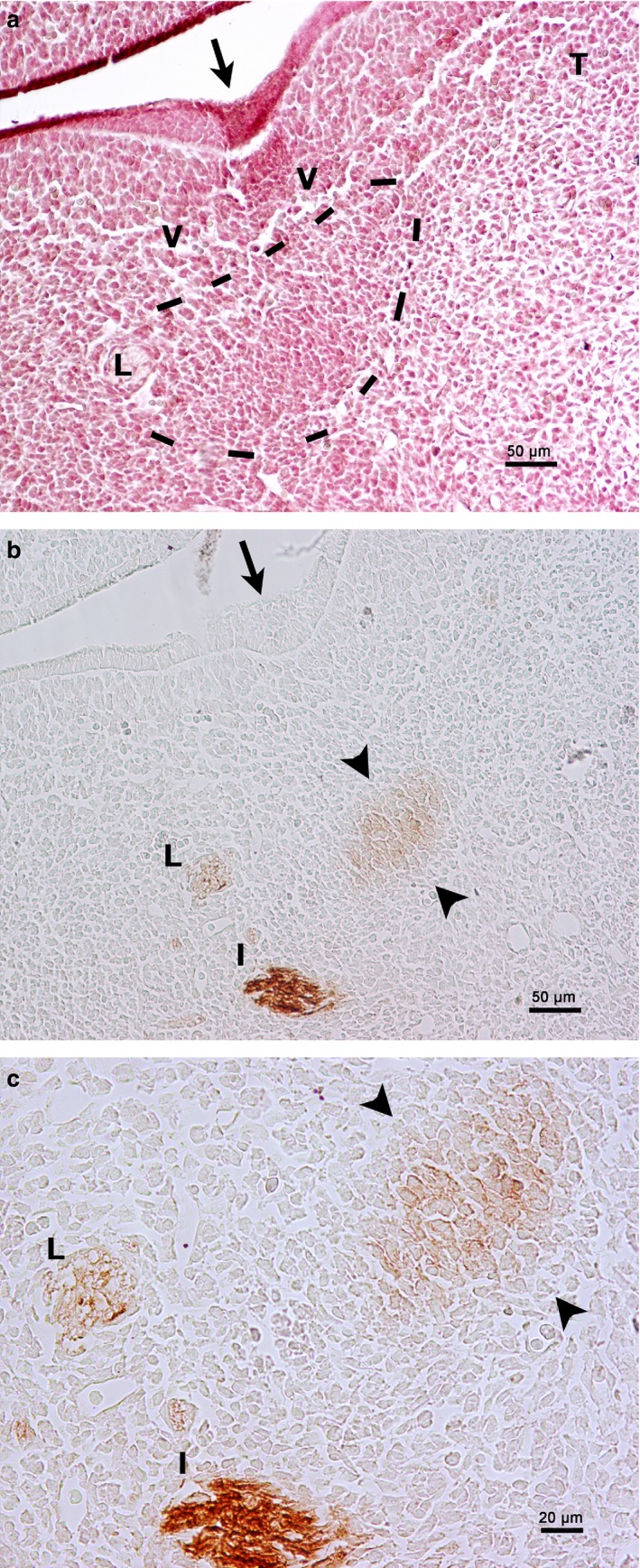
Rat embryo E14. Frontal section of the floor of the mouth. (a) Haematoxylin‐eosin staining. The medial paralingual groove is beginning to form (arrow). Primordium of the PSG (framed by a dashed line) in relationship to the lingual nerve (L). T, tongue; V, vessel. (b) HNK‐1 immunoperoxidase labelling. HNK‐1 is positive to the lingual nerve (L), the inferior alveolar nerve (I) and the mesenchyme related to the lingual nerve (arrowheads). Arrow, medial paralingual groove. (c) Magnification of (a). HNK‐1 is positive to the lingual nerve (L), the inferior alveolar nerve (I) and the mesenchyme related to the lingual nerve, which corresponds to the primordium of the parasympathetic submandibular ganglion (arrowheads).
On E15, both the medial and lateral paralingual grooves showed proliferating cells that started invaginating in the surrounding mesenchyme, constituting the primordia of the submandibular and sublingual glands, respectively. Both structures were closely placed to the HNK‐1‐positive lingual nerve and PSG (Fig. 6a,b).
Figure 6.
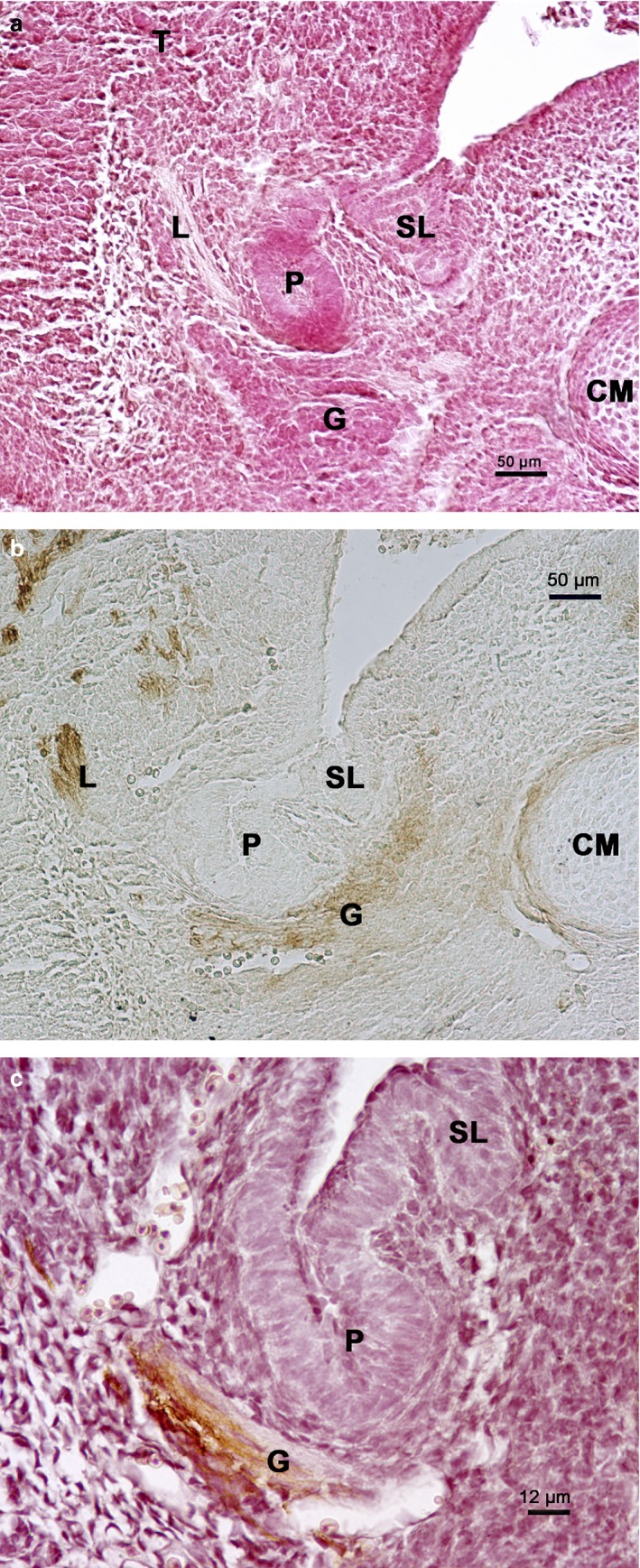
Rat embryo E15. Frontal section of the submandibular gland region. (a) Haematoxylin‐eosin staining. Primordium of the submandibular gland parenchyma (P) and sublingual gland parenchyma (SL). CM, Meckel's cartilage; G, parasympathetic submandibular ganglion; L, lingual nerve; T, tongue. (b) HNK‐1 immunoperoxidase labelling. Primordium of the submandibular gland parenchyma (P) and sublingual gland parenchyma (SL). CM, Meckel's cartilage; G, parasympathetic submandibular ganglion; L, lingual nerve. (c) HNK‐1 immunoperoxidase labelling contrasted with haematoxylin‐eosin. Primordium of the submandibular gland parenchyma (P) and sublingual gland parenchyma (SL). G, parasympathetic submandibular ganglion.
On E16, nervous and vascular structures were observed in the mesenchyme surrounding the glandular parenchyma, which was the primordium of the glandular stroma. The primordium of the glandular parenchyma started the lobulation process (Fig. 7).
Figure 7.
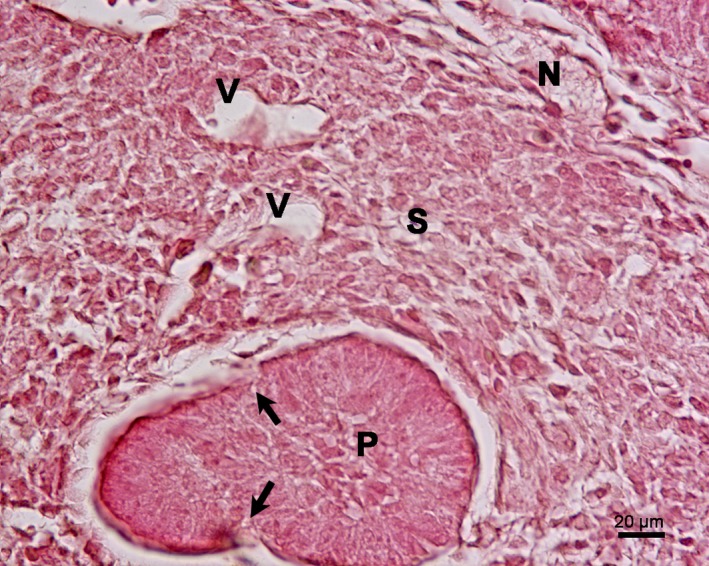
(a) Rat embryo E16. Frontal section of the primordium of the submandibular gland. Haematoxylin‐eosin staining. Lobulation begins in the primordium of the glandular parenchyma (P) (arrows). In the primordium of the glandular stroma (S), there are vessels (V) and nerves (N).
Discussion
Although numerous descriptive studies provided a sound foundation for SMG development in humans, analysis of the embryonic period is scarce.
Our results established five stages in the development of the SMG in humans from weeks 5.5 to 8 of development:
Prospective stage, corresponding to the period between week 5.5 and the beginning of week 6 (CS16 and early CS17).
Bud stage, corresponding to the end of week 6 (late CS17).
Pseudoglandular stage, matching week 6.5 (CS18–19).
Canalicular stage, corresponding to the period between week 7 and 7.5 (CS20–21).
Innervation of the parenchyma stage, matching week 8 (CS22–23).
According to other investigators, the medial paralingual groove, which forms as a consequence of the growth of the tongue, is the first structure to appear in the floor of the mouth (Dozin, 1966; García‐García et al. 1991; Mérida‐Velasco et al. 1993; Guizetti & Radlanski, 1996). Our results indicate the initial presence of the medial paralingual groove at CS16 in humans and at E14 in rats.
At this time point in humans (CS16, 11‐mm‐GL human embryo), an epithelial condensation is observed in the medial paralingual groove. This condensation is found over the route of the lingual nerve on its way to the tongue blastema, and its presence defines the prospective stage. Dozin (1966) reported a similar observation in an embryo with a crown–rump length of 12 mm. According to Dozin (1966), the epithelial condensation is thought to be the primordium of the SMG parenchyma and coincides with the primordium of the PSG. The PSG is formed by basophilic cells, with abundant surrounding blood vessels in relation to the lingual nerve. This is in contrast to the observations made by García‐García et al. (1991) and Mérida‐Velasco et al. (1993), who reported epithelial condensation appearing dorsal to the PSG anlage.
In rats, the epithelial condensation primordium of the SMG appears on E14 in the medial paralingual groove and in relation to the lingual nerve and to HNK‐1‐positive cells, which corresponds to the primordium of the PSG. Indeed, in rats, the cranial ganglia and nerves are HNK‐1‐positive (Mérida‐Velasco et al. 2012). This stage corresponds to the prebud stage of the mouse (E11.5: Jaskoll & Melnick, 1999; Tucker, 2007). In rats, the SMG develops with an approximate difference of 2 days (Denny et al. 1997).
Furthermore, the PSG forms from neural crest cells (Hauser & Hoffman, 2015; Adameyko & Fried, 2016; Dupin et al. 2018). It has been recently demonstrated that the parasympathetic ganglia derive from peripheral Schwann cell precursors (Dyachuk et al. 2014; Espinosa‐Medina et al. 2014). Schwann cell precursors are multipotent embryonic progenitors covering all developing peripheral nerves. Within specific developing tissues, these cells detach from nerves and generate neuroendocrine cells, autonomic neurons, mature Schwann cells, melanocytes and other cell types. These properties of Schwann cell precursors bear resemblances to their parental population, neural crest cells (Petersen & Adameyko, 2017; Furlan & Adameyko, 2018).
We suggest that the development of the primordium of the SMG parenchyma and that of the PSG occur in parallel. Knosp et al. (2015) reported that the Wnt signals from the epithelium are necessary for the formation of the PSG. Moreover, in CS17‐stage human embryos (prospective stage), a manifest change in the epithelium is observed. At this stage these cells have the appearance of pseudostratified prismatic epithelium. Most nuclei are elongated and have their greatest edge perpendicular to the basal membrane without reaching it. Nuclei in the superficial layer are round and less compact. We suggest that the changes in the epithelium occurring at the prospective stage determine the location at which the sublingual papillae develop.
During the bud stage at the end of week 6, late CS17, the epithelial condensation proliferates and invaginates as a solid cord contiguous with the floor of the mouth in the surrounding condensed mesenchyme in relation to the lingual nerve and the primordium of the PSG. This finding advances those reported by García‐García et al. (1991) and Mérida‐Velasco et al. (1993), who considered that the invagination period started at week 7 (CS20). The epithelial invagination occurs in mice on E12.5 (Tucker, 2007). In rats, epithelial invagination is established during E15 and is related to the lingual nerve and the primordium of the PSG, both positively marked with HNK1.
In humans, during the pseudoglandular stage (CS18–19; week 6.5), the primordium of the glandular parenchyma proliferates dorsal to the PSG. The formation of lobes begins. The lobes are surrounded by a condensed mesenchyme, which constitutes the primordium of the glandular stroma. The PSG appears to produce nervous branches reaching the mesenchyme, which was also reported by Dozin (1966). This process is observed in rats on E16. Recent reports indicate that parasympathetic innervation regulates epithelial duct tubulogenesis (Nedvetsky et al. 2014; Hauser & Hoffman, 2015).
In humans, the lumen of the submandibular duct appears during the canalicular stage (CS20–21; week 7.5). This lumen reaches the interlobar ducts on CS22–23. This result agrees with that presented by García‐García et al. (1991), who reported that the ductal lumen starts at the sublingual papilla location and extends towards the glandular parenchyma. In mice, this process is caused by programmed cell death (Tucker, 2007) and is accompanied by changes in cell polarity (Teshima et al. 2016b).
Innervation of the primordium of the SMG parenchyma and submandibular duct occurs at week 8 (innervation of the parenchyma stage). This coincides with the appearance of a lumen in the interlobar ducts, although nerves coming from the PSG reach the primordia of the glandular stroma earlier. Nedvetsky et al. (2014) reported that the parasympathetic nerve produced vasoactive intestinal peptide (VIP), which promotes both ductal growth and lumen formation. In addition, the presence of blood vessels is evident in the mesenchyme surrounding the glandular parenchyma of E16 rats. A recent report indicated the importance of endothelial factors to glandular development in mice (Kwon et al. 2017).
During the embryogenesis of mammals, epithelial–mesenchymal interactions play a decisive role in the design and development of tissues (Grobstein, 1953; De la Cuadra‐Blanco et al. 2003). The development of the SMG is an example of such an epithelial–mesenchymal interaction (Denny et al. 1997). The mesenchyme constituting the anlage of the SMG stroma surrounds and precedes the anlage of the SMG parenchyma and condenses in CS17 human embryos. Other researchers (Dozin, 1966; García‐García et al. 1991; Mérida‐Velasco et al. 1993) reported this phenomenon in only older embryos. Jaskoll et al. (2002), using Wnt1‐Cre linage tracing, reported that this mesenchyme derives from the neural crest. The migratory ability of neural crest‐derived mesenchymal cells is known (Erickson & Perris, 1993; Dupin et al. 2018). We suggest that the sublingual papilla moves anteriorly due to the growth of oral structures such as the tongue (Guizetti & Radlanski, 1996) and the Meckel cartilage in the mandibular symphysis (Rodríguez‐Vázquez et al. 1997), and that the SMG migrates dorsally to its final position under the influence of the mesenchyme and PSG.
In summary, in both humans and rats, submandibular salivary gland embryonic development requires coordinated interactions among the epithelium, mesenchyme, PSG and blood vessels.
Conflict of interest
The authors have no conflict of interest to declare.
Author contributions
Design: L.Q.T., J.V.S.C., J.R.M.V. Acquisition of data: L.A.A.A., J.A.M.G., C.C.B. Data analysis/interpretation: L.Q.T., L.A.A.A., J.A.M.G., C.C.B., J.R.M.V. Drafting of the manuscript: L.Q.T., L.A.A.A., J.A.M.G., C.C.B., J.R.M.V. Critical revision of the manuscript: M.C.M.A., J.V.S.C. Approval of the article: all authors.
Acknowledgements
The authors wish to thank the Wiley Editing Services for the English editing of this manuscript, and all individuals who donate their bodies and tissues for the advancement of education and research.
References
- Adameyko I, Fried K (2016) The nervous system orchestrates and integrates craniofacial development: a review. Front Physiol 7, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anatomica Terminologia (2011) Federative Committee on Anatomical Terminology. 2nd edn Stuttgart: Georg Thieme Verlag. [Google Scholar]
- Chi JG (1996) Prenatal development of human major salivary glands. Histological and immunohistochemical characteristics with reference to adult and neoplastic salivary glands. J Korean Med Sci 11, 203–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De la Cuadra‐Blanco C, Peces‐Peña MD, Mérida‐Velasco JR (2003) Morphogenesis of the human lacrimal gland. J Anat 203, 531–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denny PC, Ball WD, Redman RS (1997) Salivary glands: a paradigm for diversity of gland development. Crit Rev Oral Biol Med 8, 51–75. [DOI] [PubMed] [Google Scholar]
- Dozin A (1966) Introduction à l’étude de la morphogenèse des glandes sous‐maxillaires et sublinguales chez l'embryon humain. Arch Biol (Liege) 77, 459–610. [PubMed] [Google Scholar]
- Dupin E, Calloni GW, Coelho‐Aguiar JM, et al. (2018) The issue of the multipotency of the neural crest cells. Dev Biol. 10.1016/j.ydbio.2018.03.024. [DOI] [PubMed] [Google Scholar]
- Dyachuk V, Furlan A, Shahidi MK, et al. (2014) Parasympathetic neurons originate from nerve‐associated peripheral glial progenitors. Science 345, 82–87. [DOI] [PubMed] [Google Scholar]
- El‐Mohandes EA, Botros KG, Bondok AA (1987) Prenatal development of the human submandibular gland. Acta Anat (Basel) 130, 213–218. [DOI] [PubMed] [Google Scholar]
- Erickson CA, Perris R (1993) The role of cell‐cell and cell‐matrix interactions in the morphogenesis of the neural crest. Dev Biol 159, 60–74. [DOI] [PubMed] [Google Scholar]
- Espinosa‐Medina I, Outin E, Picard CA, et al. (2014) Neurodevelopment. Parasympathetic ganglia derive from Schwann cell precursors. Science 345, 87–90. [DOI] [PubMed] [Google Scholar]
- Furlan A, Adameyko I (2018) Schwann cell precursor: a neural crest cell in disguise? Dev Biol. 10.1016/j.ydbio.2018.02.008 [DOI] [PubMed] [Google Scholar]
- García‐García JD, Mérida‐Velasco JA, Barranco‐Zafra RJ, et al. (1991) Development of ductus submandibularis in the human submandibular gland. Eur Arch Biol 102, 1–7. [Google Scholar]
- Gibson MH (1983) The prenatal human submandibular gland: a histological, histochemical and ultrastructural study. Anat Anz 153, 91–105. [PubMed] [Google Scholar]
- Grobstein C (1953) Inductive epitheliomesenchymal interaction in cultured organ rudiments of the mouse. Science 118, 52–55. [DOI] [PubMed] [Google Scholar]
- Guizetti B, Radlanski RJ (1996) Development of the submandibular gland and its closer neighboring structures in human embryos and fetuses of 19–67 mm CRL. Ann Anat 178, 509–514. [DOI] [PubMed] [Google Scholar]
- Hauser BR, Hoffman MP (2015) Regulatory mechanisms driving salivary gland organogenesis. Curr Top Dev Biol 115, 111–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaskoll T, Melnick M (1999) Submandibular gland morphogenesis: stage‐specific expression of TGF‐alpha/EGF, IGF, TGF‐beta, TNF, and IL‐6 signal transduction in normal embryonic mice and the phenotypic effects of TGF‐beta2, TGF‐beta3, and EGF‐r null mutations. Anat Rec 256, 252–268. [DOI] [PubMed] [Google Scholar]
- Jaskoll T, Zhou YM, Chai Y, et al. (2002) Embryonic submandibular gland morphogenesis: stage‐specific protein localization of FGFs, BMPs, Pax6 and Pax9 in normal mice and abnormal SMG phenotypes in FgfR2‐IIIc+/Δ, BMP7−/− and Pax6−/− mice. Cells Tissues Organs 170, 83–98. [DOI] [PubMed] [Google Scholar]
- Johns ME (1977) The salivary glands: anatomy and embryology. Otolaryngol Clin North Am 10, 261–271. [PubMed] [Google Scholar]
- Knosp WM, Knox SM, Lombaert IM, et al. (2015) Submandibular parasympathetic gangliogenesis requires sprouty‐dependent Wnt signals from epithelial progenitors. Dev Cell 32, 667–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HR, Nelson DA, DeSantis KA, et al. (2017) Endothelial cell regulation of salivary gland epithelial patterning. Development 144, 211–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipinski M, Braham K, Caillaud JM, et al. (1983) HNK‐1 antibody detects an antigen expressed on neuroectodermal cells. J Exp Med 158, 1775–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacManus J, Mowry RW (1968) Técnica Histológica. Madrid: Atika S.A. [Google Scholar]
- Mérida‐Velasco JA, Sánchez‐Montesinos I, Espín‐Ferra J, et al. (1993) Development of the human submandibular salivary gland. J Dent Res 72, 1227–1232. [DOI] [PubMed] [Google Scholar]
- Mérida‐Velasco JR, De La Cuadra Blanco C, Mérida‐Velasco JA (2012) Development of the juxta‐oral organ in rat embryo. Anat Rec (Hoboken) 295, 769–775. [DOI] [PubMed] [Google Scholar]
- Moral H (1913) Über die ersten Entwickelungsstadien der Glandula submaxillaris. Anat Hefte 47, 277–382. [Google Scholar]
- Nedvetsky PI, Emmerson E, Finley JK, et al. (2014) Parasympathetic innervation regulates tubulogenesis in the developing salivary gland. Dev Cell 30, 449–462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Rahilly R, Müller F (2001) Human Embryology & Teratology. New York: Wiley‐Liss. [Google Scholar]
- O'Rahilly R, Müller F (2010) Developmental stages in human embryos: revised and new measurements. Cells Tissues Organs 192, 73–84. [DOI] [PubMed] [Google Scholar]
- Petersen J, Adameyko I (2017) Nerve‐associated neural crest: peripheral glial cells generate multiple fates in the body. Curr Opin Genet Dev 45, 10–14. [DOI] [PubMed] [Google Scholar]
- Rodríguez‐Vázquez JF, Mérida‐Velasco JR, Mérida‐Velasco JA, et al. (1997) Development of Meckel's cartilage in the symphyseal region in man. Anat Rec 249, 249–254. [DOI] [PubMed] [Google Scholar]
- Sperber G (2001) Craniofacial Development, pp. 167–170. London: BC Decker Inc. [Google Scholar]
- Sperber GH, Sperber SM, Guttmann GD (2010) Craniofacial Embryogenetics and Development, pp. 187–190. Shelton: PMPH‐USA. [Google Scholar]
- Teshima TH, Lourenco SV, Tucker AS (2016a) Multiple cranial organ defects after conditionally knocking out. Front Physiol 7, 488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teshima TH, Wells KL, Lourenço SV, et al. (2016b) Apoptosis in early salivary gland duct morphogenesis and lumen formation. J Dent Res 95, 277–283. [DOI] [PubMed] [Google Scholar]
- Thoma KH (1919) A contribution to the knowledge of the development of the submaxillary and sublingual salivary glands in human embryos. J Dent Res 1, 95–143. [Google Scholar]
- Tucker AS (2007) Salivary gland development. Semin Cell Dev Biol 18, 237–244. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Fukumoto E, Yoshizaki K, et al. (2008) Platelet‐derived growth factor receptor regulates salivary gland morphogenesis via fibroblast growth factor expression. J Biol Chem 283, 23139–23149. [DOI] [PubMed] [Google Scholar]


