Abstract
Background
Approximately 20% of stroke patients experience clinically significant levels of anxiety at some point after stroke. Physicians can treat these patients with antidepressants or other anxiety‐reducing drugs, or both, or they can provide psychological therapy. This review looks at available evidence for these interventions. This is an update of the review first published in October 2011.
Objectives
The primary objective was to assess the effectiveness of pharmaceutical, psychological, complementary, or alternative therapeutic interventions in treating stroke patients with anxiety disorders or symptoms. The secondary objective was to identify whether any of these interventions for anxiety had an effect on quality of life, disability, depression, social participation, caregiver burden, or risk of death.
Search methods
We searched the trials register of the Cochrane Stroke Group (January 2017). We also searched the Cochrane Central Register of Controlled Trials (CENTRAL; the Cochrane Library; 2017, Issue 1: searched January 2017); MEDLINE (1966 to January 2017) in Ovid; Embase (1980 to January 2017) in Ovid; the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1937 to January 2017) in EBSCO; and PsycINFO (1800 to January 2017) in Ovid. We conducted backward citation searches of reviews identified through database searches and forward citation searches of included studies. We contacted researchers known to be involved in related trials, and we searched clinical trials registers for ongoing studies.
Selection criteria
We included randomised trials including participants with a diagnosis of both stroke and anxiety for which treatment was intended to reduce anxiety. Two review authors independently screened and selected titles and abstracts for inclusion.
Data collection and analysis
Two review authors independently extracted data and assessed risk of bias. We performed a narrative review. We planned to do a meta‐analysis but were unable to do so as included studies were not sufficiently comparable.
Main results
We included three trials (four interventions) involving 196 participants with stroke and co‐morbid anxiety. One trial (described as a 'pilot study') randomised 21 community‐dwelling stroke survivors to four‐week use of a relaxation CD or to wait list control. This trial assessed anxiety using the Hospital Anxiety and Depression Scale and reported a reduction in anxiety at three months among participants who had used the relaxation CD (mean (standard deviation (SD) 6.9 (± 4.9) and 11.0 (± 3.9)), Cohen's d = 0.926, P value = 0.001; 19 participants analysed).
The second trial randomised 81 participants with co‐morbid anxiety and depression to paroxetine, paroxetine plus psychotherapy, or standard care. Mean levels of anxiety severity scores based on the Hamilton Anxiety Scale (HAM‐A) at follow‐up were 5.4 (SD ± 1.7), 3.8 (SD ± 1.8), and 12.8 (SD ± 1.9), respectively (P value < 0.01).
The third trial randomised 94 stroke patients, also with co‐morbid anxiety and depression, to receive buspirone hydrochloride or standard care. At follow‐up, the mean levels of anxiety based on the HAM‐A were 6.5 (SD ± 3.1) and 12.6 (SD ± 3.4) in the two groups, respectively, which represents a significant difference (P value < 0.01). Half of the participants receiving paroxetine experienced adverse events that included nausea, vomiting, or dizziness; however, only 14% of those receiving buspirone experienced nausea or palpitations. Trial authors provided no information about the duration of symptoms associated with adverse events. The trial of relaxation therapy reported no adverse events.
The quality of the evidence was very low. Each study included a small number of participants, particularly the study of relaxation therapy. Studies of pharmacological agents presented details too limited to allow judgement of selection, performance, and detection bias and lack of placebo treatment in control groups. Although the study of relaxation therapy had allocated participants to treatment using an adequate method of randomisation, study recruitment methods might have introduced bias, and drop‐outs in the intervention group may have influenced results.
Authors' conclusions
Evidence is insufficient to guide the treatment of anxiety after stroke. Further well‐conducted randomised controlled trials (using placebo or attention controls) are required to assess pharmacological agents and psychological therapies.
Plain language summary
Interventions for treating anxiety after stroke
Review question
To determine whether any treatments might reduce the symptoms of anxiety, and subsequently improve quality of life, for people who have had a stroke.
Background
Anxiety after stroke occurs frequently and can be treated with antidepressants or other anxiety‐reducing drugs, or both, or with psychological therapy.
Study characteristics
Evidence is current to January 2017. We found three studies with 196 stroke survivors who had received a diagnosis of anxiety. One study assessed the effect of a relaxation CD used five times a week for one month for participants with a diagnosis of anxiety. Two studies assessed the use of antidepressants in participants who had both anxiety and depression.
Key results
One study found that participants were less anxious three months after using a relaxation CD when compared with those who were given no therapy. One study reported that participants were less anxious when treated with an antidepressant medicine (paroxetine), or with paroxetine and psychotherapy, than with standard care. This study reported that half of the participants receiving paroxetine experienced side effects that included nausea, vomiting, or dizziness. The third study also reported that participants were less anxious when treated with an antidepressant (buspirone hydrochloride) than with standard care, and only 14% of those receiving buspirone hydrocholoride reported nausea or palpitations.
Quality of the evidence
We judged that the quality of this evidence was very low. Studies were few and each included a small number of participants. Studies assessing antidepressants did not include comparison with a placebo drug, and information in both study reports was insufficient to permit assessment of whether other biases had been introduced. The study of relaxation therapy was very small, with loss of two participants who used the CD, and the study recruitment process may have attracted participants who had a positive bias towards psychological therapies.
Conclusion
Current evidence is insufficient to guide the treatment of anxiety after stroke. Additional well‐conducted randomised trials are needed.
Summary of findings
Summary of findings for the main comparison. Interventions for treating anxiety after stroke.
| Interventions for treating anxiety after stroke | ||||||
| Patient or population: stroke survivors with anxiety Settings: out of hospital Intervention: pharmacological or psychological treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No. of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Interventions | |||||
| Proportion of stroke patients without clinical diagnosis of an anxiety disorder | See comment | See comment | Not estimable | 19 (1 study) | ⊕⊝⊝⊝ very lowa,b | Clinical anxiety at 3 months: 4/9 in intervention group no longer had anxiety, 1/10 in control group no longer had anxiety |
| Proportion of stroke patients scoring outside anxiety range; or changes from baseline on an anxiety rating scale | See comment | See comment | Not estimable | 196 (3 studies) | ⊕⊝⊝⊝ very lowc,d | Statistically significant difference in anxiety scores on HADS‐A scale at 3 months, with reduction in anxiety for those using therapeutic CD (P value = 0.001); statistically significant differences in HAM‐A scores at 6 weeks and 4 weeks with reduced anxiety for those taking paroxetine and paroxetine with psychological therapy and those taking buspirone, respectively (P value < 0.01) |
| Co‐morbid depression | See comment | See comment | Not estimable | 175 (2 studies) | See comment | Reduction in depression symptoms according to HAM‐D at 6 weeks and at 4 weeks for those taking paroxetine and paroxetine with psychological therapy and those taking buspirone, respectively |
| Quality of life ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Outcome not reported in any study |
| Social activities ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Outcome not reported in any study |
| Activities of daily living | See comment | See comment | Not estimable | 81 (1 study) | ⊕⊝⊝⊝ very lowb,e | Improvement in activities of daily living in all groups, but greatest improvement in those taking paroxetine with psychological therapy |
| Principal caregiver burden ‐ not reported | See comment | See comment | Not estimable | ‐ | See comment | Outcome not reported in any study |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
aStudy was unblinded, with further risks of recruitment bias and drop‐outs. Downgraded one level for risk of bias bOnly one study with small number of participants, downgraded two levels for imprecision cLimited detail in Wang 2005 and Zhang 2005 for effective assessment of bias; lack of blinding, risks of recruitment bias, and drop‐outs in Golding 2016. Downgraded two levels for risk of bias dOnly three studies with few participants, all with different interventions that are not comparable. Downgraded one level for indirectness and one level for imprecision eLimited detail in studies, unable to effectively assess risk of bias; downgraded one level
Background
Description of the condition
Stroke and anxiety disorders are major public health problems. Although stroke is the leading cause of adult disability (Department of Health 2007; Intercollegiate Stroke Working Party 2012), anxiety is the most common mental health disorder (Lepine 2002). Prevalence of anxiety after stroke ranges from 20% to 25% (Campbell Burton 2013), and it remains a common problem several years after the stroke event (Ayerbe 2013; Langhorne 2000). Anxiety is more common among younger or female people after stroke, those unable to work after stroke, and those from lower income backgrounds (Ayerbe 2013; Broomfield 2015; Menlove 2015).
Several distinct types of anxiety disorders are known, such as general anxiety disorder (GAD), panic disorder, social phobia, obsessive‐compulsive disorder (OCD), and post‐traumatic stress disorder (PTSD). Although categorically different, these disorders share similar hallmark characteristics of excessive and irrational fear, subjective apprehension, and difficulty and distress in managing daily tasks (Gelder 2006). Furthermore, although an anxiety disorder is diagnosed, many individuals experience significant levels of physical (e.g. heart palpitations, shortness of breath), cognitive (e.g. feeling of losing control), or behavioural (e.g. avoidance of certain stimuli) symptoms of anxiety that can affect their daily lives. All types of anxiety disorders have been observed in stroke patients (House 1991; Max 2002), and have been shown to have a negative impact on quality of life (Ahlsio 1984). Co‐morbidity with depression is also very high (Castillo 1993). Studies have found that depression is more severe and longer lasting in those with co‐morbid anxiety (Shimoda 1998), and stroke patients with co‐morbid anxiety and depression have higher levels of impairment in activities of daily living, greater cognitive impairment, and fewer social ties than those with depression alone (Shimoda 1998).
Differentiating between normal worries and emergence of pathological anxiety disorders, or clinically significant levels of anxiety symptoms, is difficult for several reasons. Advanced age and limited verbal ability, both of which are common within the stroke population, increase the difficulty involved in identifying persons with anxiety (Van Rijswijk 2009). Other practical problems, such as difficulty accessing specialist mental health services, not presenting for treatment, and lack of clinical guidelines specific to stroke patients with anxiety problems, mean that individuals may go untreated (Fernandez 2007).
Description of the intervention
We were interested in pharmaceutical, psychological, or any alternative therapy whose primary purpose was to treat anxiety disorders or significant levels of anxiety symptoms in stroke patients. Given the potential diversity of anxiety states, we did not limit our criteria to an a priori list of therapies. However, we did expect to find studies that treated anxiety according to evidence‐based guidelines, such as those recommended by the National Institute for Health and Clinical Excellence (NICE 2011), which outline pharmaceutical and psychological interventions that can be used to treat members of the general population with specific anxiety disorders. To our knowledge, no specific guidelines have been developed for the treatment stroke patients with anxiety.
Pharmaceutical therapies
Several classes of drugs can be used to treat anxiety disorders. These drugs vary according to the neurotransmitters that they are purported to affect.
Selective serotonin reuptake inhibitors (SSRIs) are a class of antidepressant drugs used to treat anxiety. Serotonin is a neurotransmitter involved in regulating mood. SSRIs, such as fluoxetine, sertraline, escitalopram, paroxetine, and citalopram, are commonly prescribed for panic disorder, OCD, PTSD, and social phobia (NIMH 2009). Pharmacologically, SSRIs inhibit post‐release reuptake of serotonin by presynaptic nerve terminals, hence increasing the level of available serotonin in the brain (Craig 2003).
Tricyclic antidepressants (TCAs) (e.g. imipramine) are an older generation of antidepressant drugs developed in the 1950s; they have been replaced for the most part by SSRIs. However, TCAs are still recommended in clinical guidelines for treating GAD and panic disorder (NICE 2011). TCAs act as serotonin and norepinephrine reuptake inhibitors, which results in increased extracellular concentrations of these neurotransmitters and hence enhanced neurotransmission.
Benzodiazepines (e.g. diazepam, alprazolam) are anxiolytics used to treat GAD and social phobia (Baldwin 2005), and in some instances specific phobia (NICE 2011; NICE 2014). These drugs enhance the effect of the gamma‐aminobutyric acid (GABA) neurotransmitter, thereby reducing the somatic symptoms associated with anxiety, such as muscle tension and insomnia, but they are recommended only for short‐term use.
Zopiclone, zolpidem, and zaleplon (Z‐drugs) are hypnotics that can be prescribed to help patients with the sleep disturbance associated with GAD and PTSD (NICE 2005). These drugs behave in a similar way to benzodiazepines, except they have a shorter half‐life.
Psychological therapies
Various forms of psychological therapies are available for treating anxiety. They are particularly suited to certain forms of anxiety, such as social anxiety (NICE 2013), and may be welcomed by individuals (especially older people) who may prefer not to use psychotropic drugs. This preference is based on concern about dependence, prior negative experiences, and the fact that many individuals do not view their psychological symptoms as a medical illness. Several forms of psychological therapies are described below.
Behaviour therapy is based on learning theory and consists of approaches for developing adaptive ways of behaving. The aim of behaviour therapy is to treat anxiety through techniques designed to reinforce desired behaviours while eliminating undesired behaviours.
Cognitive therapy is based on the cognitive model, which hypothesises that a person’s emotions and behaviours are influenced by their perception of events. Hence it is not the situation itself that determines how people feel but rather the way they construe the situation (Beck 1979).
Cognitive‐behavioural therapy (CBT) incorporates elements from both cognitive and behavioural therapies with the goal of changing a person’s thoughts, beliefs, attitudes, and expectations, and how a person acts (similar to behaviour therapy). It is 'present‐centred' and directs individuals to identify the current issues that are causing them distress, with the support of a trained psychological practitioner. Individuals talk with their therapist about specific problems in a structured manner and may be given homework consisting of activities to be completed before their next session. CBT is characterised as structured, goal‐oriented, and time‐limited (Beck 1997).
Complementary or alternative therapies
Although we cannot provide an exhaustive description of all interventions that can be used to treat anxiety, patients may choose from a mix of alternative therapies. For example, self‐help manuals may assist patients in gaining understanding or insight into their emotional problems and can be used to treat anxiety disorders or severe symptoms with limited therapist involvement (Van Boeijen 2005). Other therapies such as exercise training, which may act as a buffer for stress or trigger the release of monoamine neurotransmitters, and relaxation therapy, which teaches individuals to recognise symptoms of anxiety and how to respond to them via a technique that reduces arousal, have also been used to treat anxiety (Jorm 2004).
How the intervention might work
Pharamaceutical interventions work by altering the level of certain neurotransmitters in the brain, and psychological interventions aim to alter maladaptive behaviour and cognition to improve emotional functioning. Treatments in the complementary and alternative category work through multiple mechanisms. Additionally, patients receiving standard care, or those waiting to receive an intervention, may experience a reduction in anxiety symptoms through a placebo effect that is not directly related to the action of the intervention or treatment.
Why it is important to do this review
Anxiety after stroke has received substantially less attention than other psychological outcomes by both clinicians and researchers. Systematic reviews have been carried out to assess the effectiveness of interventions used to treat depression and emotionalism when they occur after stroke (Hackett 2008; Hackett 2010). The previous version of this Cochrane Review included only two small trials, thus highlighting a gap in the literature and knowledge base (Campbell Burton 2011). Studies in stroke (Shimoda 1998) and non‐stroke populations (Wittchen 2003) have shown that anxiety increases the risk and severity of depression. Hence, early treatment of anxiety may reduce the risk of subsequent depression and its associated adverse consequences. Clinical guidelines for treating anxiety have been established, but their effectiveness in stroke populations remains unknown. We chose to evaluate any intervention whose primary aim was to treat anxiety after stroke, as evidence suggests diversity among patient preferences (Hyde 2005; Riedel‐Heller 2005). We did expect that most of the trials retrieved would provide pharmaceutical or psychologically based interventions.
Objectives
The primary objective was to assess the effectiveness of pharmaceutical, psychological, complementary, or alternative therapeutic interventions in treating stroke patients with anxiety disorders or symptoms. The secondary aim was to identify whether any of these interventions for anxiety had an effect on quality of life, disability, depression, social participation, caregiver burden, or risk of death.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) in which the primary aim of the intervention was to treat anxiety in people with a clinical diagnosis of stroke (Hatano 1976) were eligible for inclusion in this review. Review authors applied no restrictions on language or study location. We expected eligible trials to compare the effect of an intervention plus usual care against placebo, a different intervention, or different doses or frequency of interventions. Trials had to include a placebo or standard care control arm; otherwise they were not eligible for inclusion.
Types of participants
All stroke patients enrolled into an RCT must have received a clinical diagnosis of an anxiety disorder according to the Diagnostic and Statistical Manual of Mental Disorders (DSM‐III (APA 1980), DSM‐III‐R (APA 1987), DSM‐IV (APA 1994), DSM‐IV‐TR (APA 2000)) or had to meet similar diagnostic criteria. Stroke patients in RCTs deemed to have significant levels of anxiety symptoms as established by a predetermined defined cut‐off score on an anxiety screening tool were also eligible. Review authors applied no restrictions on age distribution or gender. Studies with mixed populations of ischaemic or haemorrhagic stroke were eligible, but we excluded studies assessing treatment effect in an exclusively subarachnoid haemorrhage patient population, as the characteristics, treatment, and management of these patients can be substantially different from those of other stroke patients. Studies treating stroke patients for other conditions such as depression, cognitive impairment, or physical disability were also ineligible, unless we could determine that all patients had co‐morbid anxiety upon enrolment into the trial and that treatment for anxiety was one of the main objectives of the trial.
Types of interventions
We evaluated RCTs comparing pharmaceutical interventions administered to stroke patients versus placebo or standard care. The drug had to be administered for the purpose of treating anxiety. We excluded trials in which drugs were administered for other purposes, such as neuroprotection. We also evaluated RCTs that compared psychological interventions versus placebo or standard care for the purpose of treating anxiety. We expected that these types of interventions would have a clearly defined psychological component; would be structured, delivered, and supervised by trained staff; and would be time‐limited. We excluded interventions whose purpose was to prevent anxiety or simply to provide information or educate patients. We did not include trials of interventions such as occupational therapy or co‐ordinator visitation for stroke support unless they had a definitive psychological component aimed at treating anxiety.
Types of outcome measures
Primary outcomes
Proportion of stroke patients without a clinical diagnosis of an anxiety disorder according to the DSM (APA 1994) or another standard diagnostic classification at the end of scheduled follow‐up
Proportion of stroke patients scoring outside the anxiety symptom range (as defined by study authors); or with changed scores from baseline on an anxiety rating scale or via self‐report at the end of scheduled follow‐up
Secondary outcomes
Co‐morbid depression, as diagnosed by DSM or determined by a depression rating scale such as the Beck Depression Inventory (BDI) (Beck 1961), the Hamilton Depression Scale (HAM‐D) (Hamilton 1960), or the Montgomery‐Asberg Depression Rating Scale (Montgomery 1979)
Quality of life as measured on scales such as the 36‐item Short Form Questionnaire (SF‐36) (Ware 1993)
Social activities as measured on scales such as the Frenchay Activities Index (Wade 1985)
Activities of daily living as measured on scales such as the Barthel Index (Mahoney 1965)
Principal caregiver burden as measured by scales such as the Zarit Caregiver Burden Interview (Zarit 1980)
Any adverse consequence resulting from treatment for anxiety such as drug tolerance, co‐dependence on the counsellor, or death. We also recorded rates of loss to follow‐up in different arms of trials as a possible indicator of treatment acceptability
Search methods for identification of studies
See the 'Specialized Register' section of the Cochrane Stroke Group module. We attempted to identify all relevant trials regardless of language or publication status, and we arranged translation of relevant papers when necessary.
Electronic searches
We searched the trials register of the Cochrane Stroke Group (January 2017), the Cochrane Central Register of Controlled Trials (CENTRAL; the Cochrane Library; 2017, Issue 1: searched January 2017) (Appendix 1); MEDLINE (1966 to January 2017) in Ovid (Appendix 2); Embase (1980 to January 2017) in Ovid (Appendix 3); the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1937 to January 2017) in EBSCO (Appendix 4); and PsycINFO (1800 to January 2017) in Ovid (Appendix 5).
We developed the MEDLINE search strategy with the help of the Cochrane Stroke Group Information Specialist and adapted it for use with the other databases. We searched the following trial registers to identify additional published, unpublished, and ongoing clinical trials.
ClinicalTrials.gov (http://clinicaltrials.gov) (January 2016).
World Health Organization (WHO) International Clinical Trials Registry Portal (http://apps.who.int/trialsearch/) (September 2016).
ISRCTN Registry (http://www.isrctn.com/) (January 2016).
Searching other resources
We identified reviews from the results of database searches and conducted backward citation searches for potentially eligible trials. We used Google Scholar (scholar.google.co.uk) to conduct forward citation searching of included studies. We contacted known researchers to ask for information on completed and ongoing clinical trials.
Data collection and analysis
Selection of studies
Two review authors (SRL, H‐YYC) independently screened all reports yielded by the searches of electronic databases, and excluded citations that were clearly irrelevant based on title and abstract. We retrieved the full texts of remaining articles and reviewed them for inclusion on the basis of eligibility criteria for the review. If consensus could not be reached, we consulted a third review author (PK) for adjudication.
Data extraction and management
Two review authors (SRL and H‐YYC) independently extracted data and recorded them on a paper extraction form designed to capture key information. The two review authors reconciled the data extraction and entered the data into Review Manager 5 (RevMan 2014). We recorded core data elements such as study details, methods, information about participants, and outcomes for analysis.
Assessment of risk of bias in included studies
We assessed study bias in accordance with the Cochrane tool for assessing risk of bias (Higgins 2011). This instrument includes six domains whereby different types of potential bias can be evaluated, including sequence generation, allocation concealment, blinding (of participants, personnel, and outcome assessors), incomplete outcome data, selective outcome reporting, and other unspecified types of bias (e.g. conflict of interest). We identified respective biases from each study and displayed them in a tabular format. We summarised risks qualitatively and attempted to describe their impact on research findings.
Measures of treatment effect
We prepared a narrative description of all studies. Included trials measured anxiety using the Hamilton Anxiety Scale (HAM‐A) (Hamilton 1959) and the Hospital Anxiety and Depression Scale (HADS). The HAM‐A, a rating scale that was developed to quantify the severity of anxiety symptoms, is often used in psychotropic drug evaluation. It consists of 14 items, each defined by a series of symptoms. Each item is rated on a five‐point scale, ranging from 0 (not present) to 4 (severe). Total scores on the HAM‐A range from 0 to 56. A score of 14 or higher has been suggested to indicate clinically significant anxiety (Maier 1988). The HADS is commonly used to assess levels of patient anxiety and depression. The HADS evaluates 14 items (seven for anxiety and seven for depression) and uses a scale of 0 to 3 for each item, with a total score of 21 possible for each subscale (Zigmond 1983). Scores of 8 or above on either HADS subscale are commonly taken to indicate clinical significance (Bjelland 2002).
Unit of analysis issues
In the event that outcomes were repeatedly observed in participants (e.g. follow‐up at four and six weeks), we reported the measurement taken at the longest time point post intervention from each study.
Dealing with missing data
We planned to contact study authors to obtain information about missing data and, if we could not obtain this, we planned to conduct a 'what if' sensitivity analysis to explore the impact that missing data could have on the final outcome.
Assessment of heterogeneity
The intent was to measure heterogeneity by using the I2 statistic. If higher than 50% (a level considered moderate to substantial), we would have calculated the treatment effect by using the random‐effects method, which assumes that different studies are estimating different but related intervention effects and so provides a more conservative intervention effect estimate and wider confidence intervals (DerSimonian 1986).
Assessment of reporting biases
We planned to construct a funnel plot estimate to assess the potential influence of reporting bias if we had included more than 10 studies in the systematic review.
Data synthesis
Two review authors (SRL and H‐YYC) independently extracted data from the included studies. One review author (SRL) entered data into RevMan (RevMan 2014) and the other (H‐YYC) cross‐checked the data entered. Review authors resolved disagreements by referring to the original study report.
Subgroup analysis and investigation of heterogeneity
Several factors could impact study heterogeneity and effect size. We initially planned to undertake subgroup analyses on certain clinically relevant factors, such as specific type of anxiety disorder (e.g. GAD, social phobia), length of time treatment was administered, or length of time since stroke at entry into the trial.
Sensitivity analysis
To test robustness of findings and examine the degree to which findings influenced effect size, we planned to analyse data and include studies that executed allocation concealment, double blinding, and fidelity to administered intervention to the highest standard.
'Summary of findings' table
We used the principles of GRADE (Grades of Recommendation, Assessment, Development and Evaluation Working Group; Guyatt 2008) to assess the quality of the body of evidence associated with the following specific outcomes in our review.
Proportion of patients without a clinical diagnosis of anxiety.
Proportion of patients scoring outside the anxiety symptom range; or with change scores from baseline on an anxiety rating scale.
Co‐morbid depression.
Quality of life.
Social activities.
Activities of daily living.
Principal caregiver burden.
We constructed a 'Summary of findings' table by using GRADE software (gradepro.org). The GRADE approach appraises the quality of the body of evidence according to the extent to which one can be confident that an estimate of effect or association reflects the item assessed. The quality of a body of evidence is based on within‐study risk of bias (methodological quality), directness of the evidence, heterogeneity of the data, precision of effect estimates, and risk of publication bias.
Results
Description of studies
We identified no trials that compared any intervention with a placebo control. See Characteristics of included studies and Characteristics of excluded studies.
Results of the search
We identified 4223 records for the 2017 update through electronic database searches. We contacted three researchers known to be working in the field, who provided information on their current work to enable us to assess study eligibility. We identified 20 additional records from the Cochrane Stroke Group trials register. We removed duplicates and sifted 4069 titles and abstracts. We identified nine reviews for backward citation searching and evaluated potential studies from these reviews, alongside 39 potentially relevant full‐text articles; we identified one study that met the inclusion criteria for this review (Golding 2016), in addition to the two studies in the previous review update (Wang 2005; Zhang 2005). See Figure 1.
1.
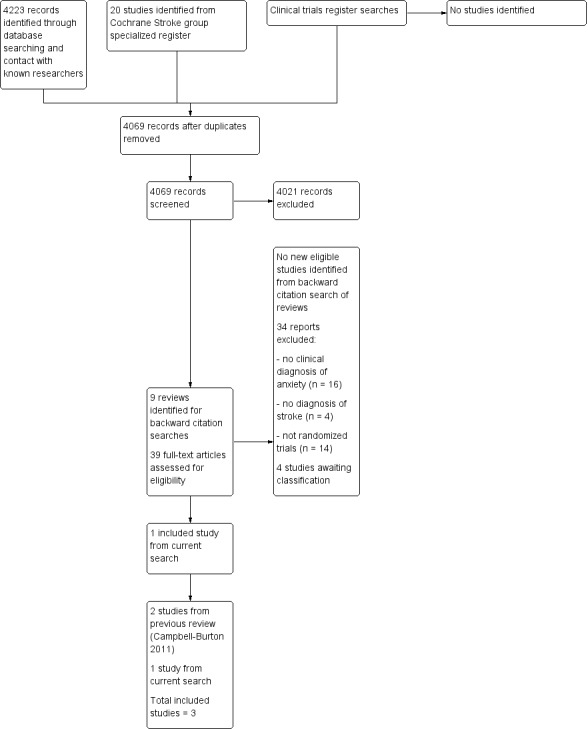
Search flow diagram (from searches conducted for the review update, October 2010 to January 2017).
Included studies
Three trials with a total of 196 randomised participants met our inclusion criteria (Golding 2016; Wang 2005; Zhang 2005).
Golding 2016 conducted what is described as a 'pilot study' to assess the effectiveness of a self‐help relaxation therapy. Participants were 21 stroke survivors who had anxiety and were living in the community. After a telephone interview and assessment, intervention group members (11 participants) were sent a self‐help autogenic relaxation CD; they were asked to follow the instructions on it five times per week for one month; and they were asked to complete a diary sheet. The control group (10 participants) did not receive the CD until the end of the study period at three months. Individuals were excluded if they were unable to complete rating scales via telephone, had a Telephone Interview Cognitive Score (TICS) ≤ 20, had significant difficulties with language or were non‐English speaking, had a co‐morbid psychiatric disorder other than an affective disorder, or were currently receiving other psychological interventions. Study investigators determined anxiety at baseline and at one month, two months, and three months using the HADS anxiety subscale (HADS‐A). They did not measure any additional outcomes. The mean age of participants was 67.8 years in the intervention group, and 62.4 years in the control group.
Wang 2005 evaluated the effectiveness of the SSRI paroxetine and of combination paroxetine and psychotherapy. Eighty‐one first‐ever stroke patients who met Chinese Classification and Diagnostic Criteria of Mental Disorders (CCMD‐3) criteria were randomised to one of the three groups. The first group (27 participants) received 20 mg of paroxetine per day, and the second group (27 participants) received the same amount of paroxetine per day along with psychiatrist‐administered supportive psychotherapy for 30 to 60 minutes once per week. A parallel control group with 27 participants received routine treatment only. Study authors did not specify the length of time since stroke at the time of participant recruitment. Patients who were in a coma or aphasic, had severe cognitive dysfunction or other serious disease, or who had been prescribed depression or antipsychotic medications in the three months before the start of the trial were excluded. Investigators provided interventions for six weeks and used HAM‐A and HAM‐D scales to assess the severity of anxiety and depression symptoms at baseline and at two, four, and six weeks during treatment. They assessed scores on the Barthel Index measuring activities of daily living at all time points. The mean age of participants was 62.4 years in the drug only group, 64.0 years in the drug plus psychotherapy group, and 63.2 years in the standard care group.
Zhang 2005 examined the effect of the anxiolytic drug buspirone hydrochloride against standard care. Researchers recruited 94 stroke patients with co‐morbid anxiety and depression according to the CCMD‐3. They deemed that individuals in an unstable condition were ineligible but provided no description of the unstable conditions. Investigators administered buspirone for four weeks to those in the intervention arm of the study at 20 to 30 mg per dose during the first week and at 40 to 60 mg per dose during the second week. They provided no information about the amount administered during the third or fourth week. Researchers measured anxiety and depression using HAM‐A and HAM‐D scales at baseline, and at two and four weeks during the intervention. The mean age of participants was 57.8 years for the intervention group and 59.2 years for the control group. Study authors reported no other secondary outcomes of interest.
Excluded studies
We excluded 40 studies after assessing the full text of the article during the most recent search. We excluded 24 of these studies as they used the wrong study design, did not include participants who had a diagnosis of stroke, or did not include a treatment aimed at reducing anxiety. Sixteen studies measured anxiety, often alongside depression, and aimed to relieve psychological symptoms exclusively or in addition to physical symptoms. None of these studies required participants to have a clinical diagnosis of anxiety for study participation (Aidar 2012; Aidar 2013; Akerlund 2013; Chaiyawat 2012; Chan 2012; Hoffmann 2015; Ihle‐Hansen 2014; Immink 2014; Jouzi 2010; Karaiskos 2012; Kongkasuwan 2014; Kulishova 2014; Mikami 2014; Peng 2015; Wu 2012; Xue 2013). We excluded these key studies and listed them under Characteristics of excluded studies. In addition, we excluded eight key trials from the original review (Kimura 2003; Li 2005; Liu 2004; Mok 2004; Morrison 1998; Rorsman 2006; Wu 2008; Ye 2006) and reported reasons for exclusion under Characteristics of excluded studies.
Studies awaiting classification
Four studies are awaiting classification (Doogan 2012; Guilan 2013; Kerr 2014; Yates 2015). These studies were published as abstracts only, without author contact details. All studies included participants who were stroke survivors but provided insufficient detail to establish whether included participants were required to have a diagnosis of anxiety.
Ongoing studies
We identified no eligible studies in clinical trials registers.
Risk of bias in included studies
We have provided a summary of 'Risk of bias' assessments in Figure 2.
2.
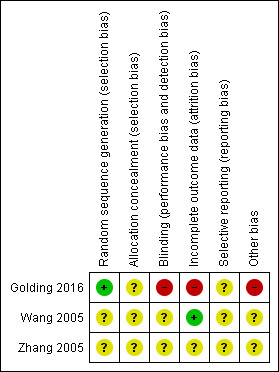
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Golding 2016 used a random number generator that we judged to be adequate with low risk of bias. However, details in the paper were insufficient to show whether allocation of participants was adequately concealed. Wang 2005 stated that investigators used simple random sampling, and Zhang 2005 indicated researchers used a random number list for participants who met the inclusion criteria. However, neither study described the randomisation process, hence the integrity of sequence generation and allocation concealment was unclear.
Blinding
It was not possible to blind participants to the study intervention in Golding 2016, which presented an inevitably high risk of performance and detection bias. Neither Wang 2005 nor Zhang 2005 provided information about blinding. As these studies included no placebo control group, blinding would likely be possible only for independent outcome assessors.
Incomplete outcome data
Golding 2016 was a small study with few but potentially significant losses and no intention‐to‐treat analysis; therefore, we judged this trial to have high risk of attrition bias. Wang 2005 reported no loss to follow‐up and did not describe adherence to the treatment protocol. Zhang 2005 reported outcomes for participants who remained until study completion. Hence, this study is classified as an 'available case analysis'.
Selective reporting
We found no evidence of selective outcome reporting in any of the included trials. Investigators reported all outcomes as described in the methods section of the full study report. However, we did not obtain the research protocols, and study authors did not report clinical trial registration, so we do not know if other outcomes were measured but not reported.
Other bias
Methods of recruitment in Golding 2016 had increased risk of bias, as interested participants responded to advertisements in publications intended for stroke survivors. It is possible that participants who contacted the research team had a positive bias towards psychological therapies for treatment of anxiety, although the study did not assess this potential bias.
Effects of interventions
See: Table 1
It was not appropriate to combine trial data on pharmacological therapies with data on relaxation therapy; therefore we did not perform a meta‐analysis.
In summary, Golding 2016 found preliminary evidence in this pilot study that an autogenic relaxation CD may reduce anxiety among stroke survivors living in the community. Wang 2005 found that both paroxetine and paroxetine plus psychotherapy reduced the severity of anxiety symptoms as measured by the HAM‐A when compared with standard care. Zhang 2005 found that buspirone hydrocholoride was effective in reducing anxiety symptoms when compared with standard care.
We have described the effectiveness of interventions compared with standard care in the prespecified outcomes below and have reported results of the GRADE assessment, with explanations of decisions for each outcome, in Table 1.
Primary outcomes
Proportion of stroke patients without a clinical diagnosis of an anxiety disorder
In Golding 2016, four members of the intervention group were no longer considered to have clinical levels of anxiety at three months, compared with one participant in the control group. Investigators reported loss of two participants after randomisation in the intervention group with no intention‐to‐treat analysis. Therefore, results include nine participants in the intervention group and 10 participants in the control group. We judged this evidence as having very low quality.
Wang 2005 and Zhang 2005 did not report this outcome.
Proportion of stroke patients scoring outside the anxiety symptom range; or with changed scores from baseline on an anxiety rating scale
Golding 2016 measured levels of anxiety at one, two, and three months post stroke, but, as per the review protocol, we have considered analysis only at the final time point. At three months post stroke, mean scores on the HADS‐A scale were 6.9 (± standard deviation (SD) 4.9) in the intervention group, and 11.0 (SD ± 3.9) in the control group, showing a statistically significant difference (P value = 0.001). Again these results include nine participants in the intervention group and 10 participants in the control group.
In Wang 2005, mean HAM‐A anxiety scores at baseline in the drug only, drug plus psychotherapy, and standard care groups were 14.0 (SD ± 2.8), 13.9 (SD ± 2.9), and 13.8 (SD ± 2.8), respectively. At six weeks, mean anxiety scores were significantly lower in the two intervention groups relative to the controls at 5.4 (SD ± 1.7) and 3.8 (SD ± 1.8) in the drug only and drug plus psychotherapy groups, but the mean anxiety score was 12.8 (SD ± 1.9) in the control group. Relative to the standard care group, this represents 58% and 71% lower mean anxiety scores in the paroxetine and paroxetine plus psychotherapy groups, respectively. Cohen's d was 4.10 for the paroxetine only group versus the control group, and 4.86 for the paroxetine plus psychotherapy group versus the control group. Both of these differences were statistically significant (P value < 0.01).
In Zhang 2005, four weeks after trial initiation, the mean anxiety score on the HAM‐A decreased from 22.7 (SD ± 5.2) to 6.5 (SD ± 3.1) in the intervention group. This decrease was significantly larger than that seen in the standard care group (P value < 0.01), for which the mean anxiety score decreased from 22.5 (SD ± 4.3) to 12.6 (SD ± 3.4) after four weeks. The mean anxiety score in the intervention group was 50% lower than that in the standard care group (Cohen's d effect size = 1.87).
HAM‐A scores range from zero to 56; a score greater than 14 indicates mild to moderate anxiety symptoms. Study authors in Golding 2016 used a lower cut‐off of ≥ 6, which they recommended as the most sensitive for a stroke population. On this basis, the reduction in anxiety scores among intervention groups in each trial appears to be clinically meaningful. However, by using GRADE, we judged that all evidence for this outcome was of very low quality.
Secondary outcomes
Co‐morbid depression
The possible range on the HAM‐D is zero to 54, with higher scores indicating more severe symptoms. In Wang 2005, mean depression severity scores were 18.2 (SD ± 1.4), 18.8 (SD ± 3.1), and 18.0 (SD ± 1.3) at baseline in the paroxetine, paroxetine plus psychotherapy, and standard care groups, respectively. Although results showed no change in the control group after six weeks (mean 17.5, SD ± 1.1), both the drug only group and the drug plus psychotherapy group had significantly fewer depression symptoms (mean 10.1, SD ± 1.1; mean 8.9, SD ± 1.2), respectively.
In Zhang 2005, buspirone was effective in significantly reducing depression symptoms as measured on the HAM‐D in the intervention group compared with the control group. The mean depression score decreased from 24.6 (SD ± 4.7) to 8.3 (SD ± 2.8) in the intervention group, and from 23.4 (SD ± 5.3) to 13.4 (SD ± 2.7) in the standard care group.
We judged that the evidence for this outcome was of very low quality.
Quality of life
Studies did not report this outcome.
Social activities
Studies did not report this outcome.
Activities of daily living
Only one trial reported changes in functional status as measured by the Barthel Index of activities of daily living (ADLs) (Wang 2005). Investigators found that ADLs improved significantly in all three groups of participants, with the greatest improvement noted in the drug plus psychotherapy group (which increased from 62.0 (SD ± 23.1) to 90.2 (SD ± 7.3)), followed by the drug‐only group (which increased from 60.9 (SD ± 23.9) to 84.3 (SD ± 8.4)), with standard care controls showing the least improvement (the increase was from 61.5 (SD ± 24.3) to 78.3 (SD ± 15.0)). We judged the evidence for this outcome to be of very low quality.
Principal caregiver burden
Studies did not report this outcome.
Adverse consequences
Wang 2005 reported 26 adverse events, all in participants given paroxetine or paroxetine with psychotherapy; nine participants given paroxetine reported nausea and vomiting, and five reported dizziness, 10 participants given paroxetine with psychotherapy reported nausea and vomiting, and two reported dizziness.
In Zhang 2005, three participants reported dizziness and two reported palpitations. Again, all adverse events occurred in the intervention group.
Combining data from Wang 2005 and Zhang 2005 revealed that intervention agents increased the risk of dizziness (risk ratio, Mantel‐Haenszel, random‐effects 7.32 (95% confidence interval 0.96 to 55.95)). See Analysis 1.1.
1.1. Analysis.
Comparison 1 Pharmacological agents versus control, Outcome 1 Dizziness.
In Golding 2016, one participant reported that the training made his "eyes feel funny", and participants did not describe any other adverse consequences of therapy.
Loss to follow‐up and intervention fidelity
No participants were lost to follow‐up in Wang 2005. However, in both intervention and control groups, 23% of participants were lost in Zhang 2005. Reasons given for drop‐out in the intervention group were unsatisfactory treatment effect, drug side effects, and subsequent prescription of benzodiazepines. Recurrent stroke, prescribing of benzodiazepines, and withdrawal were reasons given for loss to follow‐up in the control group. Wang 2005 and Zhang 2005 did not report data on intervention fidelity (other than loss to follow‐up). Loss of two participants in the intervention group in Golding 2016 was due to personal reasons and to a change in health circumstances; two of the participants in the intervention group used the CD less than once a week, rather than five times per week as directed.
Discussion
Summary of main results
We found three published trials and were unable to identify any ongoing trials. Among the three published trials, anxiety symptom severity as measured by the Hamilton Anxiety Scale (HAM‐A) or the Hospital Anxiety and Depression Scale (HADS) was the outcome of interest. None of these studies evaluated clinical anxiety disorders or included a placebo control group. Study results suggest that both paroxetine and buspirone are effective pharmacological therapies for treatment of anxiety after stroke. However, in the absence of a placebo control arm, the true level of effectiveness is unknown. Combining paroxetine and psychotherapy did not confer significant additional benefit for stroke patients. Paroxetine appeared to be well tolerated, as no drop‐outs occurred among participants, but a large proportion experienced symptoms of nausea or dizziness. Buspirone was also effective in reducing anxiety, but investigators reported substantial loss to follow‐up and some adverse events. Loss to follow‐up in the buspirone trial is unusual as results show an equally high level of drop‐out in the control group. The addition of Golding 2016 to the most recent update provides limited evidence that relaxation therapy may reduce anxiety among stroke survivors.
Overall completeness and applicability of evidence
This review was intentionally broad because we suspected that the literature on interventions used to treat anxiety after stroke was not as established as for some of the other post‐stroke psychological conditions. We attempted to collate comprehensive evidence relevant to the review question by conducting a thorough evidence search.
In the original review, the two included studies provided very little information about the populations from which participants were selected, and we could not ascertain whether the results were generalisable to the stroke population (Campbell Burton 2011). We also noted that the inclusion criteria for the two trials included in the original review required participants to have both anxiety and depression according to the Chinese Classification and Diagnostic Criteria of Mental Disorders (CCMD‐3), and therefore, the evidence could not be attributable to stroke survivors with anxiety alone. The inclusion of Golding 2016 in this most recent update provided evidence for a more specific community‐based population of stroke survivors with anxiety; however, study authors used a much lower threshold for clinical anxiety with the HAM‐A scale than in previous studies. Indeed, it should be noted that although the HAM‐A is widely used in pharmaceutical studies of anxiety, it is not appropriate for use as a diagnostic or screening instrument. The HAM‐A focuses primarily on the phobic and autonomic arousal symptoms of anxiety, and gives little weight to the psychic symptoms. Given the physical consequences of stroke, it would be misleading to attribute all physical symptoms solely to anxiety after stroke. Therefore, the evidence presented in this review is limited by the measurement scales used to assess anxiety in this population.
Quality of the evidence
Two studies assessing pharmacological agents provided limited methodological details for adequate judgement of risk of bias across all domains. The third study, which assessed relaxation therapy, inevitably had high risk of bias due to the inability to blind participants to treatment allocation. Clinical trial registration was lacking in all studies and study sample sizes were small, including one study with just 21 participants, which reported two drop‐outs and had high risk of recruitment bias. The pharmacological studies inadequately described comparison groups. In using GRADE to assess the quality of the evidence, we were particularly concerned about risk of bias in these studies, as well as the limited number of studies including few participants. We downgraded the evidence by two levels for risk of bias and by one or two levels for imprecision; we therefore rated the evidence for each reported outcome in this review as very low.
Potential biases in the review process
To the extent possible, we worked to ensure minimal bias in the review process. We undertook an extensive literature search guided by the Cochrane Stroke Group, and we contacted key researchers in the field to obtain information about studies with a focus on post‐stroke anxiety. Additionally, we did not limit findings to English language papers. Two review authors independently decided whether studies should be included and independently extracted data.
Agreements and disagreements with other studies or reviews
To our knowledge, no other systematic reviews have examined interventions used to treat anxiety after stroke.
Authors' conclusions
Implications for practice.
Currently, evidence is insufficient to guide practice in treatment of anxiety after stroke. The pharmaceutical therapies evaluated indicate that, when compared with standard care, medication may be an effective approach for reducing anxiety symptoms in stroke patients with co‐morbid anxiety and depression. The clinical significance of this decrease is unclear, as study authors did not provide any information about the proportion of study participants no longer meeting the anxiety criteria. Research indicates that a reduction of more than 50% on the Hamilton Anxiety Scale is indicative of tangible improvement in the level of anxiety (Ye 2006). However, the quality of the evidence for pharmaceutical therapies in this review is very low. The relaxation therapy evaluated in this review was examined in a small pilot study, and although study authors reported a statistically significant reduction in anxiety at three months after use of an autogenic relaxation CD for one month, risk of bias inherent in the study design was high, and not all participants used the CD as often as directed.
Implications for research.
Given the high prevalence of anxiety after stroke, placebo‐controlled or attention‐control trials are needed to identify effective treatments for this condition, as it can have a negative impact on other aspects of life. Future research evaluating interventions to treat post‐stroke anxiety should assess outcomes such as quality of life and caregiver burden, as the trials in this review provided no information on the impact of treatment on any of these outcomes. It will also be useful for trials to further investigate the effectiveness of psychological interventions, and for studies to recruit participants with anxiety only, as well as those with co‐morbid anxiety and depression. Research into the uptake and acceptability of psychological interventions for anxiety after stroke would also be valuable.
What's new
| Date | Event | Description |
|---|---|---|
| 7 September 2016 | New search has been performed | We updated all searches. We included in the review 1 new study with 21 participants. The review now includes 3 studies with 196 participants. We added a 'Summary of findings' table and updated the results to reflect outcome measures |
| 7 September 2016 | New citation required but conclusions have not changed | We made no change to the conclusions of the review. We added new review authors (Sharon R Lewis and Ho‐Yan Y Chun) to the review |
Acknowledgements
We are very grateful to Hazel Fraser, who facilitated the entire review process; Brenda Thomas and Josh Chyene of the Cochrane Stroke Group, who helped develop the search strategies and searched the Cochrane Stroke Group Trials Register and the Cochrane Central Register of Controlled Trials; and Mark Clowes of the University of Leeds, who assisted with the various database searches. Additionally, we are indebted to Professor Mei‐Chuin Tseng, whose translation of several articles made the review possible. We thank Professor Reg Morris, Dr Niall Broomfield, and Professor Ian Kneebone for providing additional information regarding eligibility of unpublished research.
This review update was supported in part by the UK National Institute for Health Research (NIHR), which funded Sharon Lewis' work through a research grant awarded for the 'Back to Normal' project. Dr Yvonne Chun was in receipt of a Chief Scientist Office (CSO) Clinical Academic Fellowship during completion of work on this update. Many thanks to the peer reviewers for their helpful comments on the first published version of the review (Professor Peter Langhorne, Dr Maree Hackett, Professor Gillian Mead, Ms Ashma Krishan, and Mrs Brenda Thomas) and this review update (Josh Cheyne, Dr Valentina Assi, Dr Tammy Hoffman, Dr Maree Hackett, Dr Terry Quinn, and two consumer reviewers: Heather Goodare and Julie Gildie).
Appendices
Appendix 1. CENTRAL search strategy
1. stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc* or cva* or apoplexy* or SAH
2. (brain* or cerebr* or cerebell* or intracran* or intracerebral) near/5 (isch?emi* or infarct* or thrombo* or emboli* or occlus*)
3. (brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid) near/5 (haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed*)
4. hemipleg* or hemipar* or paresis or paretic
5. brain injur*
6. #1 or #2 or #3 or #4 or #5
7. anxiety or anxieties or anxious or agoraphobi* or phobi* or panic disorder* or panic attack* or (obsess* near/3 compuls*) or post?traumatic stress* or PTSD
8. feel* near/5 (apprehens* or dread or disaster* or fear* or worry or worried or terror)
9. "beck anxiety inventory" or "hamilton anxiety scale" or "hospital anxiety and depression scale" or "self‐rating anxiety scale" or "state trait anxiety inventory"
10. #7 or #8 or #9
Appendix 2. MEDLINE search strategy
1. cerebrovascular disorders/ or exp basal ganglia cerebrovascular disease/ or exp brain ischemia/ or exp carotid artery diseases/ or exp intracranial arterial diseases/ or exp "intracranial embolism and thrombosis"/ or exp intracranial hemorrhages/ or stroke/ or exp brain infarction/ or vasospasm, intracranial/ or vertebral artery dissection/
2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw.
3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw.
4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw.
5. hemiplegia/ or exp paresis/
6. (hemipleg$ or hemipar$ or paresis or paretic).tw.
7. brain injuries/ or brain injury, chronic/
8. or/1‐7
9. anxiety/
10. anxiety disorders/ or agoraphobia/ or obsessive‐compulsive disorder/ or panic disorder/ or phobic disorders/ or exp stress disorders, traumatic/
11. exp Anti‐Anxiety Agents/
12. (anxiety or anxieties or anxious or agoraphobi$ or phobi$ or panic disorder$ or panic attack$ or (obsess$ adj3 compuls$) or post?traumatic stress$ or PTSD).tw.
13. (feel$ adj5 (apprehens$ or dread or disaster$ or fear$ or worry or worried)).tw.
14. manifest anxiety scale/
15. or/9‐14
16. Randomized Controlled Trials as Topic/
17. random allocation/
18. Controlled Clinical Trials as Topic/
19. control groups/
20. clinical trials as topic/ or clinical trials, phase i as topic/ or clinical trials, phase ii as topic/ or clinical trials, phase iii as topic/ or clinical trials, phase iv as topic/
21. double‐blind method/
22. single‐blind method/
23. Placebos/
24. placebo effect/
25. cross‐over studies/
26. randomized controlled trial.pt.
27. controlled clinical trial.pt.
28. (clinical trial or clinical trial phase i or clinical trial phase ii or clinical trial phase iii or clinical trial phase iv).pt.
29. (random$ or RCT or RCTs).tw.
30. (controlled adj5 (trial$ or stud$)).tw.
31. (clinical$ adj5 trial$).tw.
32. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
33. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw.
34. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw.
35. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
36. (cross‐over or cross over or crossover).tw.
37. (placebo$ or sham).tw.
38. trial.ti.
39. (assign$ or allocat$).tw.
40. controls.tw.
41. or/16‐40
42. exp animals/ not humans.sh.
43. 8 and 15 and 41
44. limit 43 to yr="2010‐Current"
Appendix 3. Embase search strategy
1. cerebrovascular disease/ or basal ganglion hemorrhage/ or exp brain hematoma/ or exp brain hemorrhage/ or exp brain infarction/ or exp brain ischemia/ or exp carotid artery disease/ or cerebral artery disease/ or cerebrovascular accident/ or exp intracranial aneurysm/ or exp occlusive cerebrovascular disease/ or stroke/
2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw.
3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw.
4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw.
5. paralysis/ or hemiparesis/ or hemiplegia/ or paresis/
6. (hemipleg$ or hemipar$ or paresis or paretic).tw.
7. brain injury/
8. or/1‐7
9. anxiety/
10. exp anxiety disorder/
11. exp anxiolytic agent/
12. (anxiety or anxieties or anxious or agoraphobi$ or phobi$ or panic disorder$ or panic attack$ or (obsess$ adj3 compuls$) or post?traumatic stress$ or PTSD).tw.
13. (feel$ adj5 (apprehens$ or dread or disaster$ or fear$ or worry or worried or terror)).tw.
14. beck anxiety inventory/ or hamilton anxiety scale/ or "hospital anxiety and depression scale"/ or self‐rating anxiety scale/ or state trait anxiety inventory/
15. or/9‐14
16. Randomized Controlled Trial/ or "randomized controlled trial (topic)"/
17. Randomization/
18. Controlled clinical trial/ or "controlled clinical trial (topic)"/
19. control group/ or controlled study/
20. clinical trial/ or "clinical trial (topic)"/ or phase 1 clinical trial/ or phase 2 clinical trial/ or phase 3 clinical trial/ or phase 4 clinical trial/
21. Crossover Procedure/
22. Double Blind Procedure/
23. Single Blind Procedure/ or triple blind procedure/
24. placebo/ or placebo effect/
25. (random$ or RCT or RCTs).tw.
26. (controlled adj5 (trial$ or stud$)).tw.
27. (clinical$ adj5 trial$).tw.
28. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw.
29. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw.
30. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw.
31. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
32. (cross‐over or cross over or crossover).tw.
33. (placebo$ or sham).tw.
34. trial.ti.
35. (assign$ or allocat$).tw.
36. controls.tw.
37. or/16‐36
38. (exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/) not (human/ or normal human/ or human cell/)
39. 8 and 15 and 37
40. limit 39 to yr="2010 ‐Current"
Appendix 4. CINAHL search strategy
1.(MH "Cerebrovascular Disorders") OR (MH "Basal Ganglia Cerebrovascular Disease+") OR (MH "Carotid Artery Diseases+") OR (MH "Cerebral Ischemia+") OR (MH "Cerebral Vasospasm") OR (MH "Intracranial Arterial Diseases+") OR (MH "Intracranial Embolism and Thrombosis") OR (MH "Intracranial Hemorrhage+") OR (MH "Stroke") OR (MH "Vertebral Artery Dissections")
2.TI ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH ) or AB ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH )
3.TI ( brain* or cerebr* or cerebell* or intracran* or intracerebral ) or AB ( brain* or cerebr* or cerebell* or intracran* or intracerebral )
4.TI ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* ) or AB ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* )
5.S3 and S4
6.TI ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid ) or AB ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid )
7.TI ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) or AB ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* )
8.S6 AND S7
9.(MH "Hemiplegia")
10.TI ( hemipleg* or hemipar* or paresis or paretic ) or AB ( hemipleg* or hemipar* or paresis or paretic )
11.(MH "Brain Injuries") OR (MH "Head Injuries")
12.TI (brain or head or intracran* or cerebr* or cerebell*) N5 (injur* or contusion* or hypoxi* or damage* or inflamm* or concussion or trauma$ or fractur* or neoplasm* or lesion* or tumor* or tumour* or cancer* or infection*)
13.S1 OR S2 OR S5 OR S8 OR S9 OR S10 OR S11 OR S12
14.(MH "Anxiety") OR (MH "Anxiety Disorders") OR (MH "Fear")
15.TX (taylor manifest anxiety scale) or TX "state trait anxiety inventory"
16.TX (anxiet* or anxious or agoraphobi* or phobi* or panic disorder* or panic attack* or (obsess* N3 compuls*) or post?traumatic stress* or PTSD)
17.TX (feel* N5 (apprehens* or dread or disaster* or fear* or worr* or terror))
18.S14 OR S15 OR S16 OR S17
19.(MH "Randomized Controlled Trials") or (MH "Random Assignment") or (MH "Random Sample+")
20.(MH "Clinical Trials") or (MH "Intervention Trials") or (MH "Therapeutic Trials")
21.(MH "Double‐Blind Studies") or (MH "Single‐Blind Studies") or (MH "Triple‐Blind Studies")
22.(MH "Control (Research)") or (MH "Control Group") or (MH "Placebos") or (MH "Placebo Effect")
23.(MH "Crossover Design") OR (MH "Quasi‐Experimental Studies")
24.PT (clinical trial or randomized controlled trial)
25.TI (random* or RCT or RCTs) or AB (random* or RCT or RCTs)
26. TI (controlled N5 (trial* or stud*)) or AB (controlled N5 (trial* or stud*))
27.TI (clinical* N5 trial*) or AB (clinical* N5 trial*)
28.TI ((control or treatment or experiment* or intervention) N5 (group* or subject* or patient*)) or AB ((control or treatment or experiment* or intervention) N5 (group* or subject* or patient*))
29.TI ((control or experiment* or conservative) N5 (treatment or therapy or procedure or manage*)) or AB ((control or experiment* or conservative) N5 (treatment or therapy or procedure or manage*))
30.TI ((singl* or doubl* or tripl* or trebl*) N5 (blind* or mask*)) or AB ((singl* or doubl* or tripl* or trebl*) N5 (blind* or mask*))
31.TI (cross‐over or cross over or crossover) or AB (cross‐over or cross over or crossover)
32.TI (placebo* or sham) or AB (placebo* or sham)
33.TI trial
34.TI (assign* or allocat*) or AB (assign* or allocat*)
35.TI controls or AB controls
36.TI (quasi‐random* or quasi random* or pseudo‐random* or pseudo random*) or AB (quasi‐random* or quasi random* or pseudo‐random* or pseudo random*)
37.S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36
38.S13 AND S18 AND S37
Appendix 5. PsycINFO search strategy
1. cerebrovascular disorders/ or cerebral hemorrhage/ or exp cerebral ischemia/ or cerebral small vessel disease/ or cerebrovascular accidents/ or subarachnoid hemorrhage/ 2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw. 3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw. 4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw. 5. hemiparesis/ or hemiplegia/ 6. (hemipleg$ or hemipar$ or paresis or paretic).tw. 7. brain injur$.tw. 8. or/1‐7 9. exp anxiety/ 10. exp anxiety disorders/ or panic/ or panic attack/ or fear/ 11. anxiety management/ 12. state trait anxiety inventory/ or taylor manifest anxiety scale/ 13. (anxiety or anxieties or anxious or agoraphobi$ or phobi$ or panic disorder$ or panic attack$ or (obsess$ adj3 compuls$) or post?traumatic stress$ or PTSD).tw. 14. (feel$ adj5 (apprehens$ or dread or disaster$ or fear$ or worry or worried or terror)).tw. 15. or/9‐14 16. 8 and 15 17. random sampling/ 18. experiment controls/ 19. placebo/ 20. (empirical study or treatment outcome clinical trial).md. 21. clinical trials/ or Treatment Effectiveness Evaluation/ 22. random$.tw. 23. (controlled adj5 (trial$ or stud$)).tw. 24. (clinical$ adj5 trial$).tw. 25. ((control or treatment or experiment$ or intervention) adj5 (group$ or subject$ or patient$)).tw. 26. (quasi‐random$ or quasi random$ or pseudo‐random$ or pseudo random$).tw. 27. ((multicenter or multicentre or therapeutic) adj5 (trial$ or stud$)).tw. 28. ((control or experiment$ or conservative) adj5 (treatment or therapy or procedure or manage$)).tw. 29. ((singl$ or doubl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).tw. 30. (coin adj5 (flip or flipped or toss$)).tw. 31. (cross‐over or cross over or crossover).tw. 32. placebo$.tw. 33. sham.tw. 34. (assign$ or alternate or allocat$ or counterbalance$ or multiple baseline).tw. 35. controls.tw. 36. (treatment$ adj6 order).tw. 37. or/17‐36 38. 16 and 37
Data and analyses
Comparison 1. Pharmacological agents versus control.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Dizziness | 2 | 153 | Risk Ratio (M‐H, Random, 95% CI) | 7.32 [0.96, 55.95] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Golding 2016.
| Methods | RCT, feasibility study | |
| Participants | Location: UK, community setting 21 stroke survivors experiencing anxiety and living in the community, HADS‐A ≥ 6 Group 1: 60% male, mean age 67.8 years (SD 7.5) Group 2: 50% male, mean age 62.4 years (SD 8.4) |
|
| Interventions | Intervention group 1: 10 participants, self‐help autogenic relaxation CD, asked to follow instructions 5 times per week for 1 month, asked to complete a diary sheet Intervention group 2: 10 participants, received CD at end of follow‐up Duration: 3 months Study dates: not stated |
|
| Outcomes | HADS‐A at months 1, 2, and 3 Loss to follow‐up: 1 withdrew at 1 week for personal reasons (group 1), not included in analysis; 1 withdrew after 1 month owing to additional health concerns (group1), included in analysis |
|
| Notes | Exclusions: inability to complete rating scales via telephone; TICS ≤ 20; significant difficulties with language or non‐English speaking; co‐morbid psychiatric disorder other than an affective disorder; currently receiving other psychological intervention Funding sources/declarations of interest: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of a random number generator. Researcher unaware of group assignment at this stage |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) Anxiety | High risk | Not possible. Participants may have had a positive bias towards the intervention |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Evaluated by participant who was not blinded |
| Selective reporting (reporting bias) | Unclear risk | Clinical trial registration not reported; therefore not possible to assess risk of bias |
| Other bias | High risk | Recruitment methods (advertisement circulated at 97 stroke survivor groups and placed in a national stroke survivor publication) may have led to a participant population biased towards the intervention and proactively seeking support for anxiety |
Wang 2005.
| Methods | RCT | |
| Participants | Location: China 81 CT/MRI confirmed first ever stroke according to CCMD‐3 criteria with co‐morbid anxiety and depression Group 1: 52% male, mean age 62.4 years (SD 6.1) Group 2: 52% male, mean age 64.0 years (SD 5.3) Group 3: 52% male, mean age 63.2 years (SD 5.7) |
|
| Interventions | Intervention group 1: 27 participants, paroxetine 20 mg daily + routine treatment Intervention group 2: 27 participants, paroxetine 20 mg daily + routine treatment + psychiatrist‐administered individual supportive psychotherapy (30 to 60 minutes per week) Group 3: 27 participants, control group routine treatment only Duration: 6 weeks Study dates: March 2002 to September 2009 |
|
| Outcomes | Anxiety (HAM‐A), depression (HAM‐D), BI at 2, 4, and 6 weeks Loss to follow‐up: none Adverse events: 26
Other outcomes: neurological impairment (SSS), activities of daily living (BI) |
|
| Notes | Exclusions: coma, aphasia, severe cognitive dysfunction, other serious diseases, depression or antipsychotic medications within 3 months, allergic to paroxetine, or bipolar disorder Funding sources/declarations of interest: no details |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random number list (details not provided) |
| Allocation concealment (selection bias) | Unclear risk | Unknown |
| Blinding (performance bias and detection bias) Anxiety | Unclear risk | Unknown |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Not applicable; data available from all participants recruited to the study |
| Selective reporting (reporting bias) | Unclear risk | All outcomes measured at start of the trial reported at all time points |
| Other bias | Unclear risk | Unknown |
Zhang 2005.
| Methods | RCT | |
| Participants | Location: China 94 participants (47 each in control and intervention groups) with clinical diagnosis of stroke according to CCMD‐3 criteria and affective disorders (72 included in final analysis) Intervention group: 64% male, 57.8 years (SD 6.4) Control group: 61% male, 59.2 years (SD 5.8) |
|
| Interventions | Intervention group: 36 participants, buspirone hydrochloride 20 to 30 mg daily in first week, 40 to 60 mg in second week + routine care Control group: 36 participants, routine care (no description of routine care) Duration: 4 weeks Study dates: May 2001 to June 2002 |
|
| Outcomes | Anxiety (HAM‐A) and depression (HAM‐D) at 2 and 4 weeks Loss to follow‐up: 22 (11 in each group)
Adverse effects: 5
Other outcomes: American Heart Stroke Outcome Classification |
|
| Notes | Exclusion: patients with unstable conditions Funding sources/declarations of interest: no details |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) Anxiety | Unclear risk | No information provided |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | High number of losses but balanced between groups |
| Selective reporting (reporting bias) | Unclear risk | No information provided |
| Other bias | Unclear risk | No information provided |
BI: Barthel Index CCMD‐3: Chinese Classification of Mental Disorders Version 3 CT: computed tomography HADS‐A: Hospital Anxiety and Depression Scale ‐ anxiety subscale HAM‐A: Hamilton Anxiety Scale HAM‐D: Hamilton Depression Rating Scale MRI: magnetic resonance imaging RCT: randomised controlled trial SD: standard deviation SSS: Scandinavian Stroke Scale TICS: Telephone Interview of Cognitive Status
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aidar 2012 | RCT, assessing exercise programme on levels of depression and anxiety among stroke survivors. Excluded from review as participants were not required to have a clinical diagnosis of anxiety to be eligible |
| Aidar 2013 | RCT, assessing exercise programme on levels of depression and anxiety among stroke survivors. Excluded from review as participants were not required to have a clinical diagnosis of anxiety to be eligible |
| Akerlund 2013 | RCT, assessing rehabilitation programme for stroke survivors to include working memory training. Included secondary outcome assessment of anxiety but excluded from review as participants were not required to have a clinical diagnosis of anxiety to be eligible |
| Chaiyawat 2012 | RCT, assessing rehabilitation programme for stroke survivors. Assessment of HADS, although did not report anxiety scores separately. Excluded from review as participants were not required to have a clinical diagnosis of anxiety to be eligible |
| Chan 2012 | RCT, assessing yoga for stroke survivors. Includes assessment of anxiety and depression, but excluded from review as participants were not required to have a clinical diagnosis of anxiety to be eligible |
| Hoffmann 2015 | RCT, assessing self‐management intervention programme specifically designed to target anxiety and depression among stroke survivors. Excluded from review as participants were not required to have a clinical diagnosis of anxiety to be eligible |
| Ihle‐Hansen 2014 | RCT, assessing multi‐disciplinary interventions to target and reduce risks of further vascular incident among stroke survivors. Included secondary outcome assessment of HADS. Excluded from review as participants were not required to have a clinical diagnosis of anxiety to be eligible |
| Immink 2014 | RCT, assessing yoga for stroke survivors. Included assessment of anxiety, but excluded from review as participants were not required to have a clinical diagnosis of anxiety to be eligible |
| Jouzi 2010 | RCT, assessing massage therapy given to stroke survivors. Included assessment of anxiety, but excluded from review as participants were not required to have a clinical diagnosis of anxiety to be eligible |
| Karaiskos 2012 | RCT, assessing pharmacological agents administered to stroke survivors. Included assessment of anxiety, but excluded from review as participants were not required to have a clinical diagnosis of anxiety to be eligible |
| Kimura 2003 | Assessing pharmacological agent for stroke survivors with clinical diagnosis of moderate to severe depression but excluded participants with GAD. Cohort study design; therefore excluded from review |
| Kongkasuwan 2014 | RCT, assessing creative therapy combined with conventional rehabilitation programmes for stroke survivors. Included assessment of anxiety, but excluded from review as participants were not required to have a clinical diagnosis of anxiety to be eligible |
| Kulishova 2014 | Assessing transcranial magnetic stimulation for stroke survivors. Information taken from English abstract only. Does not appear to be an RCT. Assessment of anxiety is a secondary outcome and we have assumed that participants were unlikely to be required to have a clinical diagnosis of anxiety; therefore we have excluded this study |
| Li 2005 | Assessing early functional training that included component of supportive treatment without antianxiety or antidepressant prescriptions. Not an RCT, and intervention not compared against placebo or standard care; therefore excluded from review |
| Liu 2004 | RCT, assessing the use of pharmacological agents among stroke survivors with anxiety. However, agents were not compared against placebo or standard care; therefore excluded from review |
| Mikami 2014 | RCT, assessing effectiveness of an antidepressant on GAD, but not all participants were required to have GAD for study inclusion; therefore we excluded this study |
| Mok 2004 | RCT, assessing slow stroke back massage for stroke survivors. Included assessment of anxiety using Chinese State Trait Anxiety Inventory, but participants were not required to meet a cut‐off criterion for anxiety; therefore we excluded this study |
| Morrison 1998 | Assessing use of a self‐help workbook aimed at enhancing non‐avoidant coping and increasing personal control over recovery. Participants were stroke survivors and anxiety was assessed, but participants were not required to have anxiety for eligibility. Study used a quasi‐experimental cohort design; therefore we excluded this study |
| Peng 2015 | RCT, assessing a neuro‐linguistic programme (NLP) intervention for stroke survivors. Included assessment of anxiety and depression, but we excluded it from review as participants were not required to have a clinical diagnosis of anxiety to be eligible |
| Rorsman 2006 | RCT, assessing electroacupuncture and transcutaneous electrical nerve stimulation among stroke survivors. Included assessment of anxiety, but excluded from review as participants were not required to have a clinical diagnosis of anxiety to be eligible |
| Wu 2008 | RCT, comparing alprazolam with acupuncture for stroke survivors with neurosis. Does not include comparison against placebo or standard care; therefore excluded from this review |
| Wu 2012 | RCT, assessing early psychological and physical rehabilitation programme. Included assessment of anxiety, but excluded from review as participants were not required to have a clinical diagnosis of anxiety to be eligible |
| Xue 2013 | RCT, assessing use of mecobalamine among stroke survivors. Currently published only as an abstract. Included assessment of anxiety but not as a primary outcome. We have assumed therefore that participants were not required to have a clinical diagnosis of anxiety to be eligible and have excluded this study |
| Ye 2006 | RCT, comparison of pharmacological agents and rehabilitative training for stroke survivors with anxiety and depression. Did not include comparison against placebo or standard care; therefore excluded from this review |
GAD: generalised anxiety disorder HADS: Hospital Anxiety and Depression Scale RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Doogan 2012.
| Methods | Unclear if study is an RCT |
| Participants | Stroke survivors |
| Interventions | Inpatient rehabilitation programme to include psycho‐education and relaxation |
| Outcomes | Distress, before and after intervention |
| Notes | Study is currently published only as an abstract. Information in abstract is insufficient to establish review eligibility. No study author contact details in abstract. Attempted to source study author contact details through the university, but no response to email enquiries |
Guilan 2013.
| Methods | RCT |
| Participants | Stroke survivors |
| Interventions | Enhanced rehabilitation programme |
| Outcomes | Self‐rating of anxiety and depression as primary outcomes |
| Notes | Study is currently published only as an abstract. No study author contact details in abstract and unable to source any possible contacts for this study author. Information in abstract is insufficient to establish whether participants were required to have a diagnosis of anxiety; therefore unable to assess if study meets review eligibility |
Kerr 2014.
| Methods | RCT, pilot study |
| Participants | Stroke survivors |
| Interventions | In‐hospital intervention of early motivational interviewing |
| Outcomes | HADS, Quality of Life Index |
| Notes | Study is currently published only as an abstract. Information in abstract is insufficient to establish whether participants were required to have a diagnosis of anxiety; therefore unable to assess if study meets review eligibility. No study author contact details in abstract. Sourced possible email address through other publication, but no response to email enquiries |
Yates 2015.
| Methods | RCT |
| Participants | Stroke survivors |
| Interventions | Guided computerised cognitive‐behavioural therapy |
| Outcomes | Beck Anxiety and Depression Inventories |
| Notes | Study is currently published only as an abstract. Information in abstract is insufficient to establish whether participants were required to have a diagnosis of anxiety; therefore unable to assess if study meets review eligibility |
HADS: Hospital Anxiety and Depression Scale RCT: randomised controlled trial
Differences between protocol and review
For this update, we did not search the Allied and Complementary Medicine Database (AMED) or the Proquest Digital Dissertations database. We did not search conference proceedings other than those searched for the Cochrane Stroke Group trials register, nor PscyBITE (Psychological database for Brain Impairment Treatment Efficacy), and we did not contact the Association of the British Pharmaceutical Industry.
We produced a 'Summary of findings' table for this update, for the first seven primary and secondary outcomes. We edited the 'Results' section to add narrative data under subheadings for each outcome.
Contributions of authors
Peter Knapp is a senior lecturer and an applied health researcher. C Alexia Campbell Burton is a healthcare value consultant, John Holmes is a senior lecturer in old age liaison psychiatry, Jenni Murray is a senior research fellow in complex health services research, David Gillespie is a practising clinical psychologist, C. Elizabeth Lightbody is a reader in health services research, Caroline L Watkins is a nursing professor of stroke and care of older people, Ho‐Yan Y Chun is a clinical academic fellow, and Sharon R Lewis is a research associate for systematic reviews of health interventions.
For the first published version of the review (Campbell Burton 2011): Campbell Burton co‐ordinated and led the review. All review authors contributed to drafting of the protocol. Campbell Burton and Knapp carried out independent screening of papers and formed consensus on studies for inclusion in the review. Murray and Campbell Burton independently extracted data. Campbell Burton conducted data entry and analysis and wrote the first draft of the review. All review authors contributed to the final draft.
For the updated review: Knapp led the review, Lewis co‐ordinated the review, and Chun and Lewis carried out independent screening of papers and, with Knapp, formed consensus on studies for inclusion in the review. Chun and Lewis independently extracted data. Lewis conducted data entry and analysis and wrote the first draft of the review. All review authors contributed to the final draft.
Sources of support
Internal sources
University of Leeds, UK.
External sources
-
NIHR Cochrane Programme Grant, UK.
13/89/16. 'Back to normal': speed and quality of recovery after surgery, major injury and critical care. This funding supported the work of Sharon Lewis
CSO Clinical Academic Fellowship (CAF/15/07) awarded to Dr Yvonne Chun during the period of the review update, UK.
Declarations of interest
Peter Knapp: none known. C. Alexia Campbell Burton: none known. John Holmes: none known. Jenni Murray: none known. David Gillespie: none known. C. Elizabeth Lightbody: none known. Caroline L Watkins: none known. Ho‐Yan Y Chun: none known. Sharon R Lewis: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Golding 2016 {published data only}
- Golding K, Kneebone I, Fife‐Schaw C. Self‐help relaxation for post‐stroke anxiety: a randomised, controlled pilot study. Clinical Rehabilitation 2016;30(2):174‐80. [25780259] [DOI] [PubMed] [Google Scholar]
Wang 2005 {published data only}
- Wang X, Yan H, Xiao CL. A clinical trial of paroxetine and psychotherapy in patients with poststroke depression and anxiety. Chinese Mental Health Journal 2005;19(8):564‐6. [Google Scholar]
Zhang 2005 {published data only}
- Zhang YX, Zhang HL, Wang H. Effects of buspirone hydrochloride on post‐stroke affective disorder and neural function. Chinese Journal of Clinical Rehabilitation 2005;9(12):8‐9. [Google Scholar]
References to studies excluded from this review
Aidar 2012 {published data only}
- Aidar FJ, Oliveira RJ, Silva AJ, Matos DG, Mazini Filho ML, Hickner RC, et al. The influence of resistance exercise training on the levels of anxiety in ischemic stroke. Stroke Research and Treatment 2012, issue 298375. [2012744313] [DOI] [PMC free article] [PubMed]
Aidar 2013 {published data only}
- Aidar FJ, Garrido ND, Silva AJ, Reis VM, Marinho DA, Oliveira RJ. Effects of aquatic exercise on depression and anxiety in ischemic stroke subjects. Health (1949‐4998). lrvine, California: Scientific Research Publishing, 2013; Vol. 5, issue 2:222‐228 7p. [104182342.: Language: English. Entry Date: 20130625. Revision Date: 20150711. Publication Type: Journal Article]
Akerlund 2013 {published data only}
- Akerlund E, Esbjornsson E, Sunnerhagen KS, Bjorkdahl A. Can computerized working memory training improve impaired working memory, cognition and psychological health?. Brain Injury 2013;27(13‐14):1649‐57. [24087909] [DOI] [PubMed] [Google Scholar]
Chaiyawat 2012 {published data only}
- Chaiyawat P, Kulkantrakorn K. Randomized controlled trial of home rehabilitation for patients with ischemic stroke: impact upon disability and elderly depression. Psychogeriatrics 2012;12(3):193‐9. [22994618] [DOI] [PubMed] [Google Scholar]
Chan 2012 {published data only}
- Chan W, Immink MA, Hillier S. Yoga and exercise for symptoms of depression and anxiety in people with poststroke disability: a randomized, controlled pilot trial. Alternative Therapies in Health & Medicine 2012;18(3):34‐43. [22875560] [PubMed] [Google Scholar]
Hoffmann 2015 {published data only}
- Hoffmann T, Ownsworth T, Eames S, Shum D. Evaluation of brief interventions for managing depression and anxiety symptoms during early discharge period after stroke: a pilot randomized controlled trial. Topics in Stroke Rehabilitation 2015;22(2):116‐26. [25936543] [DOI] [PubMed] [Google Scholar]
Ihle‐Hansen 2014 {published data only}
- Ihle‐Hansen H, Thommessen B, Fagerland MW, Oksengard AR, Wyller TB, Engedal K, et al. Effect on anxiety and depression of a multifactorial risk factor intervention program after stroke and TIA: a randomized controlled trial. Aging & Mental Health 2014;18(5):540‐6. [23957255] [DOI] [PubMed] [Google Scholar]
Immink 2014 {published data only}
- Immink MA, Hillier S, Petkov J. Randomized controlled trial of yoga for chronic poststroke hemiparesis: motor function, mental health, and quality of life outcomes. Topics in Stroke Rehabilitation 2014;21(3):256‐71. [24985393] [DOI] [PubMed] [Google Scholar]
Jouzi 2010 {published data only}
- Jouzi M. Assessment of the effect of massage therapy on stroke patients. Medical Sciences Journal of Islamic Azad University Tehran Medical Branch 2010;19(4):8. [105150738.: Language: Persian. Entry Date: 20100430. Revision Date: 20150711. Publication Type: Journal Article] [Google Scholar]
Karaiskos 2012 {published data only}
- Karaiskos D, Tzavellas E, Spengos K, Vassilopoulou S, Paparrigopoulos T. Duloxetine versus citalopram and sertraline in the treatment of poststroke depression, anxiety, and fatigue. Journal of Neuropsychiatry & Clinical Neurosciences 2012;24(3):349‐53. [23037649] [DOI] [PubMed] [Google Scholar]
Kimura 2003 {published data only}
- Kimura M, Tateno A, Robinson RG. Treatment of poststroke generalized anxiety disorder comorbid with poststroke depression. American Journal of Geriatric Psychiatry 2003;11(3):320‐7. [PubMed] [Google Scholar]
Kongkasuwan 2014 {published data only}
- Kongkasuwan R. Creative therapy: the holistic approach in life enhancing for Thai stroke rehabilitation. Physical Medicine and Rehabilitation 2014;10:S333. [71643912] [Google Scholar]
Kulishova 2014 {published data only}
- Kulishova TV, Shinkorenko OV. [The effectiveness of early rehabilitation of the patients presenting with ischemic stroke]. Voprosy Kurortologii, Fizioterapii i Lechebnoi Fizicheskoi Kultury 2014;6:9‐12. [25730927] [PubMed] [Google Scholar]
Li 2005 {published data only}
- Li Y. Effect of early functional training on psychological rehabilitation in stroke patients. Chinese Journal of Clinical Rehabilitation 2005;9(1):176‐7. [Google Scholar]
Liu 2004 {published data only}
- Liu L, Liu YH, Liang JH. Recent effect of drug intervention on post‐stroke anxiety. Chinese Journal of Clinical Rehabilitation 2004;8(30):6600‐1. [Google Scholar]
Mikami 2014 {published data only}
- Mikami K, Jorge RE, Moser DJ, Arndt S, Jang M, Solodkin A, et al. Prevention of post‐stroke generalized anxiety disorder, using escitalopram or problem‐solving therapy. Journal of Neuropsychiatry and Clinical Neurosciences 2014;26(4):323‐8. [2015672817] [DOI] [PubMed] [Google Scholar]
Mok 2004 {published data only}
- Mok E, Pang Woo C. The effects of slow stroke back massage on anxiety and shoulder pain in elderly stroke patients. Complementary Therapies in Nursing and Midwifery 2004;10(4):209‐16. [DOI] [PubMed] [Google Scholar]
Morrison 1998 {published data only}
- Morrison VL, Johnston M, MacWalter RS. Pollard BS. Improving emotional outcomes following acute stroke: a preliminary evaluation of a work‐book based intervention. Scottish Medical Journal 1998;43(2):52‐3. [DOI] [PubMed] [Google Scholar]
Peng 2015 {published data only}
- Peng Y, Lu Y, Wei W, Yu J, Wang D, Xiao Y, et al. The effect of a brief intervention for patients with ischemic stroke: a randomized controlled trial. Journal of Stroke and Cerebrovascular Diseases 2015;24(8):1793‐802. [2015153225] [DOI] [PubMed] [Google Scholar]
Rorsman 2006 {published data only}
- Rorsman I, Johansson B. Can electroacupuncture or transcutaneous nerve stimulation influence cognitive and emotional outcome after stroke?. Journal of Rehabilitation Medicine 2006;38(1):13‐9. [DOI] [PubMed] [Google Scholar]
Wu 2008 {published data only}
- Wu P, Liu S. Clinical observation on post‐stroke anxiety neurosis treated by acupuncture. Journal of Traditional Chinese Medicine 2008;28(3):186‐8. [DOI] [PubMed] [Google Scholar]
Wu 2012 {published data only}
- Wu DY, Guo M, Gao YS, Kang YH, Guo JC, Jiang XL, et al. Clinical effects of comprehensive therapy of early psychological intervention and rehabilitation training on neurological rehabilitation of patients with acute stroke. Asian Pacific Journal of Tropical Medicine 2012;5(11):914‐6. [2012666894] [DOI] [PubMed] [Google Scholar]
Xue 2013 {published data only}
- Xue YD, Shi N, Gao XR. Mecobalamine in the treatment of acute stroke and post‐stroke depression. Journal of the American Geriatrics Society 2013;61:S333. [71370251] [Google Scholar]
Ye 2006 {published data only}
- Ye LX, Wang YD, Wang H, Liang DS. Effect of paxil and berhomine on poststroke anxiety‐depression and neurological recovery. Chinese Journal of Clinical Rehabilitation 2006;10(6):153‐5. [Google Scholar]
References to studies awaiting assessment
Doogan 2012 {published data only}
- Doogan CE, Carter SN. Me, My Stroke and I. An evaluation of an inpatient psychoeducational and relaxation group to promote awareness and reduce anxiety. International Journal of Stroke 2012;7:55‐6. [70951797] [Google Scholar]
Guilan 2013 {published data only}
- Guilan C, Jing D, Sumei Z. Influence of '5E' rehabilitation model on anxiety and depression in hemiplegic stroke patients. Chinese Nursing Research 2013;27(5A):1205. [107962960.: Language: Chinese. Entry Date: 20131115. Revision Date: 20150819. Publication Type: Journal Article] [Google Scholar]
Kerr 2014 {published data only}
- Kerr D, Mackey E, Wijeratne T, McCann T. Early motivational interviewing on post‐stroke depressive symptoms: pilot randomised controlled trial of the good mood intervention program. International Journal of Stroke 2014;9:39‐40. [71775556] [DOI] [PubMed] [Google Scholar]
Yates 2015 {published data only}
- Yates M, Simblett SK, Wagner AP, Gracey F, Ring H, Bateman A. 'Beating the blues' after a stroke: a pilot randomised controlled trial of guided computerised cognitive behavioural therapy for treating symptoms of depression and anxiety after a stroke. Clinical Rehabilitation 2015;4:406‐7. [CN‐01101630] [Google Scholar]
Additional references
Ahlsio 1984
- Ahlsiö B, Britton M, Murray V, Theorell T. Disablement and quality of life after stroke. Stroke 1984;15:886‐90. [PUBMED: 6236588] [DOI] [PubMed] [Google Scholar]
APA 1980
- American Psychiatric Association. Diagnostic and Statistical Manual for Mental Disorders (DSM‐III). 3rd Edition. Washington DC: American Psychiatric Press, 1980. [Google Scholar]
APA 1987
- American Psychiatric Association. Diagnostic and Statiscal Manual of Mental Disorders (DSM‐III‐R). 3rd Edition. Washington DC: American Psychiatric Press, 1987. [Google Scholar]
APA 1994
- American Psychiatric Association. Diagnostic and Statisical Manual of Mental Disorders. 4th Edition. Washington DC: American Psychiatric Press, 1994. [Google Scholar]
APA 2000
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders. 4th Edition. Washington DC: American Psychiatric Press, 2000. [Google Scholar]
Ayerbe 2013
- Ayerbe L, Ayis SA, Crichton S, Wolfe CDA, Rudd AG. Natural history, predictors and associated outcomes of anxiety up to 10 years after stroke: the South London Stroke Register. Age and Ageing 2013;43:542‐7. [PUBMED: 24375225] [DOI] [PubMed] [Google Scholar]
Baldwin 2005
- Baldwin DS, Anderson IM, Nutt DJ, Bandelow B, Bond A, Davidson JRT, et al. Evidence‐based guidelines for the pharmacological treatment of anxiety disorders: recommendations from the British Association for Psychopharmacology. Journal of Psychopharmacology 2005;19:567‐96. [PUBMED: 16272179] [DOI] [PubMed] [Google Scholar]
Beck 1961
- Beck AT, Ward C, Mendelson M. An inventory for measuring depression. Archives of General Psychiatry 1961;4:561‐71. [PUBMED: 13688369] [DOI] [PubMed] [Google Scholar]
Beck 1979
- Beck AT, Rush AJ, Shaw BF. Cognitive Therapy of Depression. New York: The Guildford Press, 1979. [Google Scholar]
Beck 1997
- Beck AT. The past and future of cognitive therapy. Journal of Psychotherapy Practice 1997;6:276‐84. [PMC free article] [PubMed] [Google Scholar]
Bjelland 2002
- Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the Hospital Anxiety and Depression Scale. An updated literature review. Journal of Psychosomatic Research 2002;52(2):69‐77. [PUBMED: 11832252] [DOI] [PubMed] [Google Scholar]
Broomfield 2015
- Broomfield NM, Scoular A, Welsh P, Walters M, Evans JJ. Poststroke anxiety is prevalent at the population level, especially among socially deprived and younger age community stroke survivors. International Journal of Stroke 2015;10:897‐902. [PUBMED: 24206439] [DOI] [PubMed] [Google Scholar]
Campbell Burton 2013
- Campbell Burton CA, Murray J, Holmes J, Astin F, Greenwood D, Knapp P. Frequency of anxiety after stroke: a systematic review and meta‐analysis of observational studies. International Journal of Stroke 2013;8(7):545‐59. [PUBMED: 23013268] [DOI] [PubMed] [Google Scholar]
Castillo 1993
- Castillo CS, Starkstein SE, Fedoroff JP, Price TR, Robinson RG. Generalized anxiety disorder after stroke. Journal of Nervous and Mental Disease 1993;181:100‐6. [DOI] [PubMed] [Google Scholar]
Craig 2003
- Craig CR, Stitzel RE. Modern Pharmacology With Clinical Applications. 6th Edition. Baltimore, MD: Lippincott Williams & Wilkins, 2003. [Google Scholar]
Department of Health 2007
- Department of Health/Vascular Program/Stroke. National Stroke Strategy. www.gov.uk/stroke 2007.
DerSimonian 1986
- DerSimonian R, Laird N. Meta‐analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
Fernandez 2007
- Fernandez A, Haro JM, Martinez‐Alonso M, Demyttenaere K, Brugha TS, Autonell J, et al. Treatment adequacy for anxiety and depressive disorders in six European countries. The British Journal of Psychiatry : The Journal of Mental Science 2007;190:172‐3. [PUBMED: 17267936] [DOI] [PubMed] [Google Scholar]
Gelder 2006
- Gelder M, Harrison P, Cowen P. Shorter Oxford Textbook of Psychiatry. 5th Edition. New York: Oxford University Press, 2006. [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians?. BMJ (Clinical research ed.) 2008;336(7651):995‐8. [PUBMED: 18456631] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hackett 2008
- Hackett ML, Anderson CS, House A, Xia J. Interventions for treating depression after stroke. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD003437] [DOI] [PubMed] [Google Scholar]
Hackett 2010
- Hackett ML, Yang M, Anderson CS. Pharmaceutical interventions for emotionalism after stroke. Cochrane Database of Systematic Reviews 2010, Issue 2. [DOI: 10.1002/14651858.CD003690.pub2] [DOI] [PubMed] [Google Scholar]
Hamilton 1959
- Hamilton M. The assessment of anxiety states by rating. British Journal of Medical Psychology 1959;32:50‐5. [DOI] [PubMed] [Google Scholar]
Hamilton 1960
- Hamilton M. Rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry 1960;23:56‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hatano 1976
- Hatano S. Experience from a multicentre stroke register: a preliminary report. Bulletin of the World Health Organization 1976;54:541‐53. [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (eds). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org.
House 1991
- House AM, Dennis M, Mogridge L, Warlow C, Hawton K, Jones L. Mood disorders in the year after first stroke. British Journal of Psychiatry 1991;158:83‐90. [DOI] [PubMed] [Google Scholar]
Hyde 2005
- Hyde J, Calnan M, Prior L, Lewis G, Kessler D, Sharp D. A qualitative study exploring how GPs decide to prescribe antidepressants. British Journal of General Practice 2005;55:755‐62. [PMC free article] [PubMed] [Google Scholar]
Intercollegiate Stroke Working Party 2012
- Intercollegiate Stroke Working Party. National Clinical Guideline for Stroke (4th edition). London: Royal College of Physicians, 2012. [Google Scholar]
Jorm 2004
- Jorm AF, Christensen H, Griffiths KM, Parslow RA, Rodgers B, Blewitt KA. Effectiveness of complementary and self‐help treatments for anxiety disorders. Medical Journal of Australia 2004;181 Suppl:S29‐S46. [DOI] [PubMed] [Google Scholar]
Langhorne 2000
- Langhorne P, Stott DJ, Robertson L, MacDonald J, Jones L, McAlpine C, et al. Medical complications after stroke ‐ multicenter study. Stroke 2000;31:1223‐9. [DOI] [PubMed] [Google Scholar]
Lepine 2002
- Lepine JP. The epidemiology of anxiety disorders: prevalence and societal costs. Journal of Clinical Psychiatry 2002;63 Suppl 14:4‐8. [PubMed] [Google Scholar]
Mahoney 1965
- Mahoney FI, Barthel DW. Functional evaluation: the Barthel Index. Maryland State Medical Journal 1965;14:61‐5. [PubMed] [Google Scholar]
Maier 1988
- Maier W, Buller R, Philipp M, Heuser I. The Hamilton Anxiety Scale: reliability, validity and sensitivity to change in anxiety and depressive disorders. Journal of Affective Disorders 1988;14(1):61‐8. [PUBMED: 2963053] [DOI] [PubMed] [Google Scholar]
Max 2002
- Max JE, Mathews K, Lansing AE, Robertson BA, Fox PT, Lancaster JL, et al. Psychiatric disorders after childhood stroke. Journal of the American Academiy of Child and Adolescent Psychiatry 2002;41:555‐62. [DOI] [PubMed] [Google Scholar]
Menlove 2015
- Menlove L, Crayton E, Kneebone I, Allen‐Crooks R, Otto E, Harder H. Predictors of anxiety after stroke: a systematic review of observational studies. Journal of Stroke and Cerebrovascular Diseases 2015;24:1107‐17. [PUBMED: 25816724] [DOI] [PubMed] [Google Scholar]
Montgomery 1979
- Montgomery SA, Asberg M. A new depression scale designed to be sensitive to change. British Journal of Psychiatry 1979;134:382‐9. [DOI] [PubMed] [Google Scholar]
NICE 2005
- National Institute for Health and Care Excellence. Post‐traumatic Stress Disorder. The Management of PTSD in Adults and Children in Primary and Secondary Care. London: The Royal College of Psychiatrists and the British Psychological Society, 2005. [Google Scholar]
NICE 2011
- National Institute for Health and Care Excellence. Generalised anxiety disorder and panic disorder (with or without agoraphobia) in adults. Management in Primary, Secondary and Community Care, National Institute for Health and Care Excellence, London 2011.
NICE 2013
- National Institute for Health and Care Excellence. Social anxiety disorders: recognition, assessment and treatment (CG159). https://www.nice.org.uk/guidance/cg159?unlid=3733086092016228153435 (accessed 8 July 2016). [PubMed]
NICE 2014
- National Institute for Health and Care Excellence. Quality standard (Q553): anxiety disorders. https://www.nice.org.uk/Guidance/QS53 (accessed 8 July 2016).
NIMH 2009
- Anxiety disorders: National Institute of Mental Health. Bethesda, MD: US Department of Health and Human Services, 2009.
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riedel‐Heller 2005
- Riedel‐Heller SG, Matschinger H, Angermeyer MC. Mental disorders ‐ who and what might help? Help‐seeking and treatment preferences of the lay public. Social Psychiatry and Psychiatric Epidemiology 2005;40:167‐74. [DOI] [PubMed] [Google Scholar]
Shimoda 1998
- Shimoda K, Robinson RG. Effect of anxiety disorder on impairment and recovery from stroke. Journal of Neuropsychiatry and Clinical Neurosciences 1998;10:34‐40. [DOI] [PubMed] [Google Scholar]
Van Boeijen 2005
- Boeijen CA, Balkom AJLM, Oppen P, Blankenstein N, Cherpanath A, Dyck R. Efficacy of self help manuals for anxiety disorders in primary care: a systematic review. Family Practice 2005;22(2):192‐6. [DOI] [PubMed] [Google Scholar]
Van Rijswijk 2009
- Rijswijk E, Hout H, Lisdonk E, Zitman F, Weel C. Barriers in recognising, diagnosing and managing depressive and anxiety disorders as experienced by family physicians; a focus group study. BMC Family Practice 2009;10(52):1‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wade 1985
- Wade DT, Legh‐Smith J, Hewer RL. Social activities after stroke: measurement and natural history using the Frenchay Activities Index. International Rehabilitation Medicine 1985;7:176‐81. [DOI] [PubMed] [Google Scholar]
Ware 1993
- Ware JE, Snow KK, Kosinski M, Gandek B. SF‐36 Health Survey: Manual and Interpretation Guide. Boston: New England Medical Center Health Institute, 1993. [Google Scholar]
Wittchen 2003
- Wittchen HU, Beesdo K, Bittner A, Goodwin RD. Depressive episodes ‐ evidence for a causal role of primary anxiety disorders?. European Psychiatry 2003;18:384‐93. [DOI] [PubMed] [Google Scholar]
Zarit 1980
- Zarit SH, Rever KE, Bach‐Peterson J. Relatives of the impaired elderly: correlates of feelings of burden. Gerontologist 1980;20:649‐55. [DOI] [PubMed] [Google Scholar]
Zigmond 1983
- Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatrica Scandinavica 1983;67(6):361‐70. [PUBMED: 6880820] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Campbell Burton 2010
- Campbell Burton CA, Knapp P, Holmes J, Murray J, Gillespie D, Lightbody CE, et al. Interventions for treating anxiety after stroke (Protocol). Cochrane Database of Systematic Reviews 2010, Issue 12. [DOI: 10.1002/14651858.CD008860] [DOI] [PubMed] [Google Scholar]
Campbell Burton 2011
- Campbell Burton CA, Holmes J, Murray J, Gillespie D, Lightbody CE, Watkins CL, et al. Interventions for treating anxiety after stroke. Cochrane Database of Systematic Reviews 2011, Issue 12. [DOI: 10.1002/14651858.CD008860] [DOI] [PubMed] [Google Scholar]


