Abstract
This cross-sectional analysis characterizes venture capital investments in otolaryngologic devices by therapeutic area using data from a private capital market data provider from 2008 through 2017.
Venture capital (ie, private, early-stage, and high-risk) funding of start-up or small medical device manufacturers is critical for translating scientific understanding of disease into novel therapies.1 However, venture capital investment in device firms has recently declined amid concerns about reduced reimbursement.1,2,3 Such uncertainties may additionally induce investors to concentrate resources on potentially lucrative technologies of lesser benefit to public health (eg, cosmetic treatments).2 Identifying gaps in venture capital funding may therefore help policy makers implement legislation promoting the development of therapeutics for unmet medical needs.1 The nature and extent of venture capital investment in otolaryngologic devices is unknown. We therefore sought to characterize these investments over time and by therapeutic area.
Methods
We conducted a retrospective cross-sectional analysis of PitchBook, a private capital market data provider,4,5 to identify venture capital investment from January 1, 2008, through December 31, 2017, in device firms developing therapeutics indicated for the treatment of otolaryngologic disease; diagnostic and surgical (eg, endoscopic) device firms were excluded. We included device firms based on company descriptions within PitchBook and product pipeline information on manufacturer websites. Institutional review board approval was not needed for this study without human or animal data.
Data were analyzed from November 19 through December 10, 2018. For each firm, we categorized the therapeutic area (facial plastics; head and neck oncology; laryngology; otology and neurotology; pediatrics; rhinology; and sleep) and extracted the year and amount of all investments. We used descriptive statistics to characterize trends over time and by therapeutic area; investments of undisclosed amount were excluded from analysis.
Results
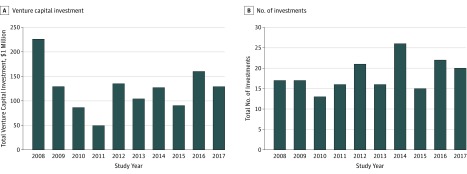
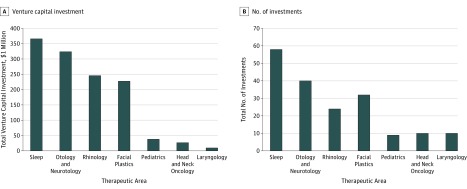
From January 1, 2008, through December 31, 2017, venture capital providers made 210 investments in 126 therapeutic otolaryngologic device firms. After excluding investments of undisclosed amount (27 of 210 [12.8%]), venture capital investment totaled approximately $1.2 billion during this period. The annual number of investments (mean, 18; range, 13-26) and investment amount (mean, $123.7 million; range, $49.5-$226.0 million) remained stable, with a few exceptions (Figure 1). Investment varied widely by therapeutic area (Figure 2). Most investment targeted firms developing devices indicated for the treatment of sleep disorders ($365.9 million [29.6%]; eg, obstructive sleep apnea) or otologic and neurotologic conditions ($323.7 million [26.2%]; eg, hearing loss). All rhinologic device firms ($245.7 million [19.9%]) receiving investment were developing treatments for chronic rhinosinusitis. Investment in facial plastics firms ($227.4 million [18.4%]) was primarily for devices indicated for aesthetic enhancement ($127.5 million [56.1%]) and nasal valve dysfunction ($99.4 million [43.7%]). Pediatric otolaryngology, head and neck oncology, and laryngology accounted for a small proportion ($74.3 million [6.0%]) of total investment.
Figure 1. Annual Venture Capital Investment in Therapeutic Otolaryngologic Device Firms.
Data were acquired for the period from January 1, 2008, through December 31, 2017. Excludes 27 investments of undisclosed amount. Adapted with permission from PitchBook.5
Figure 2. Total Venture Capital Investment in Therapeutic Otolaryngologic Device Firms.
Data were acquired for the period from January 1, 2008, through December 31, 2017, and stratified by therapeutic area. Excludes 27 investments of undisclosed amount. Adapted with permission from PitchBook.5
Discussion
Although venture capital investment in medical technology has declined overall,1,2,3 our findings demonstrate that funding of device firms developing treatments for otolaryngologic diseases remained stable during the past decade and totaled approximately $1.2 billion. However, the distribution of funding varied widely by therapeutic area. Most investment was allocated toward devices indicated for the treatment of sleep disorders and otologic and neurotologic conditions, whereas funding of therapeutics for children and patients with laryngeal disorders was limited.
Although device firms focused on prevalent conditions—such as obstructive sleep apnea, hearing loss, and chronic rhinosinusitis—may often serve as attractive investment opportunities, venture capital providers may be less inclined to fund firms focused on other therapeutic areas within otolaryngology owing to concerns about market size (eg, for palliative tracheal stents) or regulatory challenges (eg, pediatric research protections).6 To incentivize further investment and promote technological innovation in underfunded therapeutic areas, policy makers should consider legislation that would lower financial barriers for device manufacturers, such as exemption from the medical device excise tax, or federal grants or tax credits to partially offset clinical research expenses, which are presently available to pharmaceutical companies developing drugs indicated for the treatment of rare diseases.
This study has limitations. By excluding investments of undisclosed amounts from analysis, we underestimate the level of investment in therapeutic otolaryngologic devices. Inherent uncertainties in the characterization of nonpublic venture capital funding may additionally affect estimation of investment levels.4
References
- 1.Fleming JJ. The decline of venture capital investment in early-stage life sciences poses a challenge to continued innovation. Health Aff (Millwood). 2015;34(2):271-276. doi: 10.1377/hlthaff.2014.1051 [DOI] [PubMed] [Google Scholar]
- 2.Ackerly DC, Valverde AM, Diener LW, Dossary KL, Schulman KA. Fueling innovation in medical devices (and beyond): venture capital in health care. Health Aff (Millwood). 2009;28(1):w68-w75. doi: 10.1377/hlthaff.28.1.w68 [DOI] [PubMed] [Google Scholar]
- 3.Makower J, Aabed M, Denend L FDA impact on US medical technology innovation: a survey of over 200 medical technology companies. Stanford University. https://www.medtecheurope.org/wp-content/uploads/2015/07/01112010_FDA-impact-on-US-medical-technology-innovation_Backgrounder.pdf November 2010. Accessed December 14, 2018.
- 4.Kaplan SN, Lerner J Venture capital data: opportunities and challenges. Harvard Business School. https://www.hbs.edu/faculty/Publication%20Files/17-012_10de1f93-30e4-4a98-858c-4137556ec037.pdf. 2016. Accessed November 25, 2018.
- 5.PitchBook https://pitchbook.com/. Accessed December 10, 2018.
- 6.US Food and Drug Administration FDA awards five grants to advance the development of pediatric medical devices. https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm620288.htm. Published September 12, 2018. Accessed December 16, 2018.