Key Points
Question
How do the safety and efficacy of minimally interrupted dabigatran therapy compare with uninterrupted warfarin therapy in candidates for atrial fibrillation catheter ablation?
Findings
In this randomized clinical trial of 442 participants undergoing ablation for atrial fibrillation, 2 thromboembolic events occurred in the warfarin group before ablation, but none in the dabigatran group. Major bleeding and thromboembolic event rates were 1.4% and 0, respectively, in the dabigatran group (n = 220) and 5.0% and 0.5%, respectively, in the warfarin group (n = 222) from the start of the ablation procedure until 3 months after ablation.
Meaning
In this patient population, minimally interrupted dabigatran therapy did not increase thromboembolic events and was associated with fewer bleeding complications than uninterrupted warfarin therapy.
This randomized clinical trial compares the safety and efficacy of minimally interrupted dabigatran vs uninterrupted warfarin therapy in patients undergoing catheter ablation for atrial fibrillation.
Abstract
Importance
Uninterrupted dabigatran therapy reduces stroke risk in patients with nonvalvular atrial fibrillation (NVAF) undergoing ablation and is associated with a lower bleeding risk than uninterrupted warfarin therapy. Minimally interrupted direct oral anticoagulant therapy is widely used, but data from controlled studies are insufficient.
Objective
To compare the safety and efficacy of minimally interrupted dabigatran vs uninterrupted warfarin therapy in patients undergoing catheter ablation for NVAF.
Design, Setting, and Participants
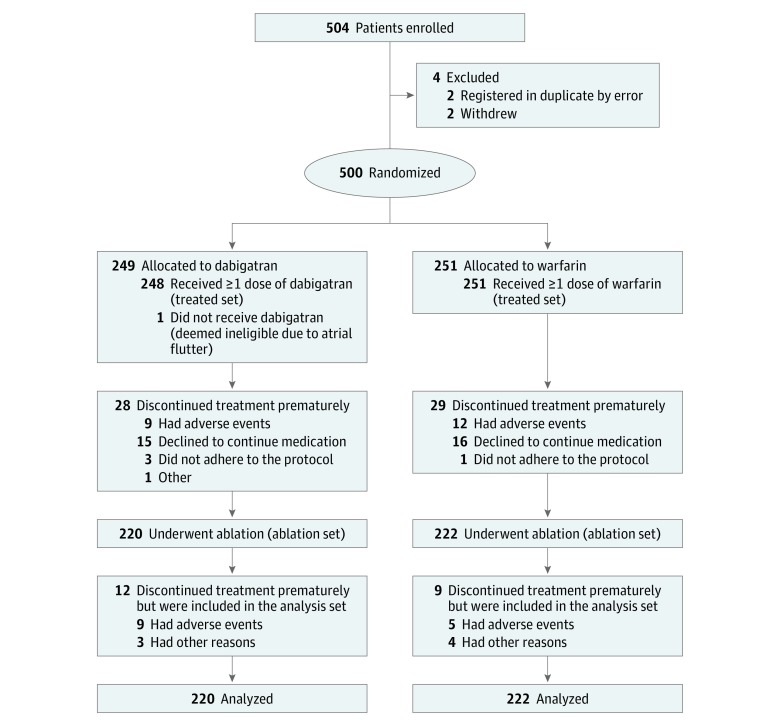
The ABRIDGE-J (ABlation peRIoperative DabiGatran in use Envisioning in Japan) trial is a open-label, randomized clinical trial performed in 28 Japanese treatment centers. A total of 504 patients scheduled for NVAF ablation were enrolled; 500 were randomized to the study treatments; 499 received at least 1 dose of dabigatran etexilate (n = 248) or warfarin potassium (n = 251); and 442 underwent ablation (220 in the dabigatran group and 222 in the warfarin group). Data were collected from May 1, 2014, through September 14, 2015, and analyzed from March 7, 2017, through January 28, 2019.
Interventions
Appropriate dose anticoagulation was administered 4 weeks before and at least 3 months after ablation in all patients. Dabigatran therapy was interrupted before catheter ablation (holding of 1-2 doses) and resumed after ablation.
Main Outcomes and Measures
Primary end points were the incidence of embolism during the perioperative period and atrial thrombus just before the ablation. The main secondary end point was the incidence of major bleeding events until 3 months after ablation.
Results
Of the 442 patients who underwent ablation, 74.9% were men and the median age was 66 years (interquartile range, 59-71 years). Before ablation, 1 cerebral infarction and 1 thrombus in the left atrium occurred in the warfarin group, but no events occurred in the interrupted dabigatran group. After ablation, the mean (SD) incidence of major bleeding events was significantly lower with dabigatran (3 patients [1.4% {0.8%}; 95% CI, 0.4%-4.2%]) vs warfarin (11 patients [5.0% {1.5%}; 95% CI, 2.8%-8.8%]; P = .03). No thromboembolic events occurred after ablation in the dabigatran group; 1 (0.5%) occurred in the warfarin group.
Conclusions and Relevance
In patients undergoing ablation for NVAF, anticoagulation with minimally interrupted dabigatran therapy did not increase thromboembolic events and was associated with fewer bleeding complications than uninterrupted warfarin therapy.
Trial Registration
umin.ac.jp Identifier: UMIN000013129
Introduction
Perioperative anticoagulation is required to minimize the risk of embolic events, including stroke and transient ischemic attack, after catheter ablation for atrial fibrillation (AF).1,2,3,4,5 Recent randomized clinical studies6,7,8 and meta-analyses9,10,11 showed that uninterrupted therapy with direct oral anticoagulants (DOACs) reduced the risk of stroke in patients undergoing ablation for nonvalvular AF (NVAF). In particular, these studies showed that DOACs are associated with lower6 or noninferior7,8,9,10,11,12,13 risks of thromboembolic or bleeding events compared with uninterrupted warfarin.
The 2017 consensus statement on AF ablation1 recommends performing the ablation procedure without anticoagulant interruption with warfarin or dabigatran (class I); however, it also stated that holding 1 to 2 doses of DOAC before AF ablation is reasonable (class IIa). In the American College of Cardiology expert consensus decision pathway for periprocedural management of anticoagulation,14 AF ablation is classified as having a low or intermediate bleeding risk, and interruption of DOAC is recommended for a variable duration based on the patient’s estimated creatinine clearance rate.
At present, minimally interrupted DOACs are widely used,15 and previous cohort studies16,17,18 and propensity score matching or meta-analyses studies11,19,20 found that interrupted DOACs had a noninferior risk of major bleeding and thromboembolism compared with warfarin. Recently, a randomized clinical study12 reported no stroke and similarly low rates of major bleeding between uninterrupted and minimally interrupted apixaban. However, the data from clinical studies of other DOACs are insufficient. The aim of the ABRIDGE-J (ABlation peRIoperative DabiGatran in use Envisioning in Japan) study was to evaluate the safety and efficacy of minimally interrupted dabigatran vs uninterrupted warfarin therapy during the perioperative period of catheter ablation.
Methods
Trial Design
This prospective, randomized, open-label, multicenter, clinical interventional superiority trial included patients undergoing catheter ablation for NVAF across 28 sites in Japan. Patients were enrolled from May 1, 2014, through September 14, 2015 (Figure 1). All end points were adjudicated by a panel of experts in a blinded manner. The trial was conducted in accordance with the Declaration of Helsinki21 as well as International Conference on Harmonisation Good Clinical Practice guidelines.22 The trial protocol (Supplement 1) was approved by the institutional review board or the independent ethics committee at each participating center (eAppendix 1 in Supplement 2). Written informed consent was obtained from all patients before trial entry. This report follows the Consolidated Standards of Reporting Trials Extension (CONSORT Extension) reporting guidelines.
Figure 1. CONSORT Flow Diagram.
Participants
Patients aged 20 to 85 years when informed consent was obtained were eligible for ABRIDGE-J if they had paroxysmal or persistent NVAF with AF ablation planned, had documented AF, and were eligible for treatment with dabigatran or warfarin according to the prescribing guidelines in Japan. Key exclusion criteria were valvular AF, defined as the presence of a prosthetic heart valve (annuloplasty with or without a prosthetic ring, commissurotomy, and/or valvuloplasty were permitted), hemodynamically significant mitral valve stenosis, or rheumatic heart disease.
Interventions
After qualifying for the trial, patients were randomly allocated to dabigatran etexilate (Prazaxa) or warfarin potassium (1:1 ratio) treatment using a registration system. The random allocation sequence, participant enrollment, and assignment of participants to the interventions were conducted by Mebix Inc. Dabigatran was administered at a dosage of 150 or 110 mg twice daily. The 110-mg twice-daily dosage of dabigatran was administered in patients with moderate renal disorders (creatinine clearance rate of 30-50 mL/min, calculated using the Cockcroft–Gault formula), those concomitantly receiving p-glycoprotein antagonists, or those with a high risk of bleeding (age ≥70 years, with a history of gastrointestinal tract hemorrhage), according to the dose reduction criteria in the package insert.23 Dabigatran was administered for at least 4 weeks. Based on the Japanese guideline,24 the target prothrombin time–international normalized ratio (PT-INR) in the warfarin group was set at 2.0 to 3.0 for patients younger than 70 years and 1.6 to 2.6 for patients 70 years or older. Warfarin was administered for at least 4 weeks after the target PT-INR was reached. The percentage of time that each patient’s INR was within the target therapeutic range was determined using the Rosendaal method.25 If PT-INR on the day of ablation was less than the target value, ablation was performed depending on the result of transesophageal echocardiography.
In the dabigatran group, dabigatran therapy was interrupted before catheter ablation (1-2 doses were put on hold before ablation). The timing of discontinuation was based on the scheduled time of ablation and the treating physician’s discretion. Use of heparin bridging was based on the Japanese recommendations and guidelines.24 Heparin bridging was recommended if dabigatran therapy was discontinued at least 24 hours before the ablation procedure. In the warfarin group, warfarin therapy was continued without interruption.
Ablation of AF was performed according to clinical recommendations.26 Unfractionated heparin was administered during the ablation procedure, after placing the femoral sheaths, but before or immediately after transseptal puncture. The interval between the final dose of dabigatran and the transseptal puncture was defined as the dabigatran to ablation (D-A) interval. An activated clotting time of 300 to 400 seconds was to be achieved and maintained, where possible, during the ablation procedure. After the ablation procedure, protamine was administered to reverse the effects of heparin if the activated clotting time after the procedure was at least 300 seconds, and hemostasis was confirmed at the puncture site. After ablation, dabigatran therapy was resumed. Anticoagulation was continued in both groups for longer than 3 months after the procedure (eFigure 1 in Supplement 2). Any decision to continue anticoagulation with nontrial medication after the follow-up period was based on recommendations, guidelines, and the treating physician’s discretion. All patients were followed up for 12 months after ablation.
End Points
The primary end points, which were assessed from the time of randomization, were the incidence of embolism during the perioperative period and the existence or nonexistence of an atrial thrombus just before ablation, detected by transesophageal echocardiography or intracardiac echocardiography. All potential end point events were reviewed by an independent events committee.
Secondary end points were assessed from the start of the ablation procedure (ie, first femoral puncture) until 3 months after ablation. The main secondary end point was the incidence of adjudicated major bleeding events, which were defined according to the International Society on Thrombosis and Haemostasis (ISTH) criteria.27 Other secondary safety and efficacy end points consisted of a composite incidence of major bleeding events, thromboembolic events (stroke, systemic embolism, and transient ischemic attack), all-cause death, and a composite incidence of all bleeding events, including minor bleeding, thromboembolic events, and all-cause death, during and within 3 months after ablation. Bleeding events that did not satisfy the ISTH criteria for major bleeding were considered minor bleeding events. The net clinical benefit28 is a composite of thromboembolic events, all-cause death, and major bleeding until 3 months after ablation. The net clinical benefit was calculated as A − (1.5 × B), where A was the difference in the incidence of thromboembolism or total deaths between groups (warfarin − dabigatran) and B was the difference in the incidence of major bleeding events between groups (dabigatran − warfarin).
All adverse events that occurred during the trial were to be recorded and analyzed and were subsequently classified as serious or nonserious. Safety and efficacy end points were reported only as adverse events.
Statistical Analysis
Data were analyzed from May 7, 2017, to January 28, 2019. Based on the subanalysis of the RE-LY (Randomized Evaluation of Long-term Anticoagulation Therapy) trial,29 and considering the potential number of dropouts, we set the target number of participants at 450 (225 per group). A more detailed description of the target number estimate and analyses sets in this study are described in eAppendix 2 in Supplement 2. The statistical analysis plan can be found in Supplement 1.
Patients in both groups were matched by risk factors of ischemic stroke included in the CHA2DS2-VASc score (congestive heart failure [or left ventricular systolic dysfunction; 1 point], hypertension [blood pressure consistently >140/90 mm Hg or medication-treated hypertension; 1 point], age ≥75 years [2 points], diabetes mellitus [1 point], prior stroke or transient ischemic attack or thromboembolism [2 points], vascular disease [eg, peripheral artery disease, myocardial infarction, or aortic plaque; 1 point], age 65-74 years [1 point], and sex category (ie, female sex; 1 point]), type of AF, and number of antiplatelet drugs used. Tests for independent samples were selected throughout because other unmatched variables that might influence treatment efficacy could remain.
In each medical institution, the numbers of patients in the warfarin and the dabigatran groups were allocated as evenly as possible. Both groups were compared using the χ2 test without continuity correction. Continuous variables were expressed as mean and SD or median and interquartile range (IQR; quartiles 1-3). Categorical variables were expressed as numbers and percentages. Primary and secondary end points were compared between groups. For each end point, the incidence of the event for each treatment group and its odds ratios and 95% CIs were calculated. The χ2 test (≥5 events) and Fisher exact test (<5 events) were performed for the intergroup comparison of the incidence. Time-to-event analysis was performed with Kaplan-Meier estimates, and the log-rank test was used to determine between-group differences. We considered 2-sided P < .05 statistically significant. Missing data were not imputed. All analyses were performed using SAS software (version 9.4 for Windows; SAS Institute, Inc).
Results
Participants
The trial enrolled 504 patients, of whom 500 were randomized and 499 received at least 1 dose of the treatment drug (dabigatran, n = 248; warfarin, n = 251). The ablation set included 442 patients (331 men [74.9%] and 111 women [25.1%]; median age, 66 years [interquartile range, 59-71 years]), of whom 220 were randomized to the dabigatran group and 222 to the warfarin group (Figure 1). Demographic and clinical characteristics were well balanced between groups (Table 1). Most patients (dabigatran, 95.0%; warfarin, 95.5%) received trial medication for 3 months after ablation. The mean (SD) time in therapeutic range in the warfarin group was 60.1% (34.1%). In the dabigatran group, a dose of 150 mg twice daily was administered in 97 patients (44.1%), and a dose of 110 mg twice daily was administered in 123 patients (55.9%). eTable 1 in Supplement 2 shows the details of INR data in warfarin-treated patients by timing of measurement and activated partial thromboplastin time (APTT) data in dabigatran-treated patients.
Table 1. Baseline Demographic and Clinical Characteristics (Ablation Set).
| Characteristic | Study Group | |
|---|---|---|
| Dabigatran (n = 220) | Warfarin (n = 222) | |
| Age, median (IQR), y | 65.0 (59.0-71.0) | 66.0 (59.0-71.0) |
| Male, No. (%) | 171 (77.7) | 160 (72.1) |
| Body weight, median (IQR), kg | 66.9 (59.7-75.0) | 66.2 (59.7-73.0) |
| Body mass index, median (IQR)a | 24.2 (22.1-26.4) | 23.9 (22.0-26.4) |
| CHA2DS2-VAScb score, median (IQR)c | 2 (1-3) | 2 (1-3) |
| HAS-BLED score, median (IQR)d | 1 (0-2) | 1 (1-2) |
| Atrial fibrillation, No. (%) | ||
| Paroxysmal | 138 (62.7) | 138 (62.2) |
| Persistent | 52 (23.6) | 55 (24.8) |
| Long-standing persistent | 30 (13.6) | 29 (13.1) |
| Time from the first atrial fibrillation, median (IQR), y | 1.0 (0.3-4.5) | 1.5 (0.3-4.3) |
| Medical history, No. (%) | ||
| Congestive heart failure | 8 (3.6) | 14 (6.3) |
| Previous stroke | 15 (6.8) | 12 (5.4) |
| Coronary artery disease | 7 (3.2) | 14 (6.3) |
| Previous myocardial infarction | 2 (0.9) | 6 (2.7) |
| Previous gastrointestinal tract bleeding | 0 | 3 (1.4) |
| Previous gastric ulcer | 5 (2.3) | 9 (4.1) |
| Renal dysfunction | 15 (6.8) | 16 (7.2) |
| Liver dysfunction | 6 (2.7) | 12 (5.4) |
| Alcohol abuse | 2 (0.9) | 4 (1.8) |
| Diabetes | 36 (16.4) | 34 (15.3) |
| Hypertension | 123 (55.9) | 126 (56.8) |
| Cardiomyopathy | 5 (2.3) | 5 (2.3) |
| Respiratory disease | 9 (4.1) | 8 (3.6) |
| Medication use, No. (%) | ||
| Warfarin | 67 (30.5) | 68 (30.6) |
| Dabigatran | 39 (17.7) | 33 (14.9) |
| Rivaroxaban | 34 (15.5) | 35 (15.8) |
| Apixaban | 26 (11.8) | 24 (10.8) |
| Aspirin | 13 (5.9) | 14 (6.3) |
| Clopidogrel bisulfate | 3 (1.4) | 3 (1.4) |
| Ticlopidine hydrochloride | 0 | 0 |
| Cilostazol | 0 | 1 (0.5) |
| Eicosapentaenoic acid | 3 (1.4) | 2 (0.9) |
| NSAID | 1 (0.5) | 7 (3.2) |
| Labile INR, No. (%) | 20 (9.1) | 14 (6.3) |
| LVEF, median (IQR), % | 65.9 (60.1-70.5) | 65.0 (60.0-70.0) |
| Serum creatinine level, median (IQR), mg/dL | 0.86 (0.74-0.96) | 0.84 (0.71-0.94) |
| Creatinine clearance, median (IQR), mL/min | 80.1 (65.4-94.1) | 77.8 (64.6-97.2) |
| eGFR, median (IQR), mL/min/1.73 m2 | 67.1 (59.1-75.8) | 67.8 (60.4-75.5) |
| Systolic blood pressure, median (IQR), mm Hg | 128 (120-142) | 133 (120-142) |
| Diastolic blood pressure, median (IQR), mm Hg | 78 (70-86) | 78 (70-87) |
| Heart rate, median (IQR), bpm | 70 (61-82) | 67 (59-81) |
| TEE and ICE, No. (%) | ||
| TEE within 48 h before ablation | 163 (74.1) | 154 (69.4) |
| ICE during the procedure | 52 (23.6) | 58 (26.1) |
| TEE and/or ICE | 188 (85.5) | 187 (84.2) |
| Type of ablation, No. (%) | ||
| Prior atrial fibrillation ablation | 0 | 0 |
| Radiofrequency catheter ablation PVI | 176 (80.0) | 171 (77.0) |
| Cryoballoon PVI | 34 (15.5) | 44 (19.8) |
| SVCI | 40 (18.2) | 30 (13.5) |
| Radiofrequency catheter ablation CTI | 124 (56.4) | 123 (55.4) |
| Cryoablation CTI | 25 (11.4) | 25 (11.3) |
| Linear ablation | 48 (21.8) | 47 (21.2) |
Abbreviations: CTI, cavotricuspid isthmus; eGFR, estimated glomerular filtration rate; HAS-BLED, hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile international normalized ratio, elderly, drugs/alcohol concomitantly; ICE, intracardiac echocardiography; INR, international normalized ratio; IQR, interquartile range; LVEF, left ventricular ejection fraction; NSAID, nonsteroidal anti-inflammatory drug; PVI, pulmonary vein isolation; SVCI, superior vena cava isolation; TEE, transesophageal echocardiography.
SI conversion factor: To convert serum creatinine to μmol/L, multiply by 88.4; creatinine clearance to mL/s, multiply by 0.0167.
Calculated as weight in kilograms divided by height in meters squared.
CHA2DS2-VASc indicates the following: C, congestive heart failure (or left ventricular systolic dysfunction; 1 point); H, hypertension (blood pressure consistently above 140/90 mm Hg [or treated hypertension on medication]; 1 point); A2, age 75 years or older (2 points); D, diabetes mellitus (1 point); S2, prior stroke or transient ischemic attack or thromboembolism (2 points); V, vascular disease (eg, peripheral artery disease, myocardial infarction, aortic plaque; 1 point); A, age 65 to 74 years (1 point); Sc, Sex category (ie, female sex; 1 point).
A score of at least 2 indicates a high risk of stroke.
A score of at least 3 indicates a high risk of major bleeding.
Interruption of Dabigatran Therapy
One or 2 doses of dabigatran were withheld before ablation. The median D-A time was 19.5 hours (IQR, 16.3-26.2 hours). In 137 patients, the D-A interval was less than 24 hours, meaning that 1 morning dose on the day of ablation was withheld. Of these patients, heparin bridging was performed in 20 patients (14.6%). In the remaining 83 patients, the D-A interval was at least 24 hours, meaning that the evening dose on the day before ablation and the morning dose on the day of ablation were withheld. Of these patients, heparin bridging was performed in 58 patients (69.9%). No significant difference was found in demographic and clinical characteristics between dabigatran subgroups with D-A interval of less than 24 hours and at least 24 hours except transesophageal echocardiography performance within 48 hours before ablation (91 of 137 [66.4%] vs 72 of 83 [86.7%]; P < .001) (eTable 2 in Supplement 2). Dabigatran therapy was restarted at a median time of 8.3 hours (IQR, 4.4-18.7 hours) after ablation.
End Points
Primary End Points
The incidence of embolism during the perioperative period from the time of randomization was 1 patient (cerebral infarction) in the warfarin group and none in the dabigatran group. Another patient in the warfarin group had a left atrial appendage thrombus detected by intracardiac echocardiography just before ablation. These 2 patients did not undergo an ablation procedure. Clinical characteristics of the patients were shown in eTable 3 in Supplement 2. Both patients presented with AF at the event, and the PT-INRs were not sufficiently prolonged.
Secondary End Points
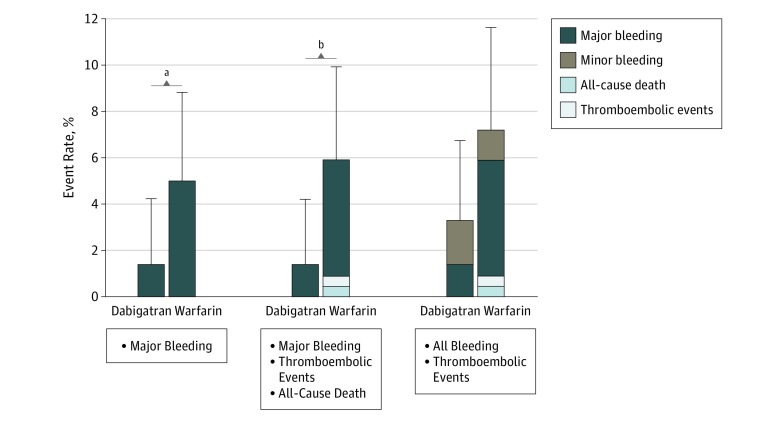
In terms of the main secondary end point, 14 patients had adjudicated major bleeding events during the 3-month postablation period. Sites of major bleeding are shown in Table 2. The mean (SD) event rate of major bleeding was significantly lower in the dabigatran group (3 patients [1.4% {0.8%}; 95% CI, 0.4%-4.2%]) compared with the warfarin group (11 patients [5.0% {1.5%}; 95% CI, 2.8%-8.8%]; absolute risk difference, −3.6% [95% CI, −6.8 to −0.4]) (Figure 2). The relative risk reduction compared with warfarin was 72.5% (risk ratio, 0.273 for dabigatran vs warfarin [95% CI, 0.076-0.980]; P = .03) (eFigure 2 in Supplement 2). In subgroup analyses, a significant reduction in the major bleeding risk in the dabigatran group vs the warfarin group was consistently observed across the following subgroups: those aged 65 years or older and younger than 75 years (−4.81% [95% CI, −8.92% to −0.70%]), male (−5.08% [95% CI, −9.16% to −1.00%]), those with a CHA2DS2-VASc score of 1 (−11.32% [95% CI, −19.85% to −2.79%]), and those undergoing radiofrequency ablation for pulmonary vein isolation (−4.21% [95% CI, −7.95% to −0.48%]) (eFigure 3 in Supplement 2).
Table 2. Major Bleeding Events, Thromboembolic Events, and All-Cause Death for 3 Months in the Ablation Set.
| Event or Death | No. (%) of Participants | Time From Ablation, d |
|---|---|---|
| Major bleeding event | ||
| Dabigatran group (n = 220) | ||
| Pericardial effusion | 1 (0.5) | 1 |
| Femoral arteriovenous fistula | 1 (0.5) | 2 |
| Intraperitoneal bleeding | 1 (0.5) | 1 |
| Total | 3 (1.4) | NA |
| Warfarin group (n = 222) | ||
| Pericardial hemorrhage | 2 (0.9) | 1 |
| 1 | ||
| Groin bleeding/hematoma | 3 (1.4) | 1 |
| 1 | ||
| 71 | ||
| Femoral arteriovenous fistula | 1 (0.5) | 1 |
| Femoral pseudoaneurysm | 1 (0.5) | 3 |
| Retroperitoneal bleeding | 1 (0.5) | 3 |
| Subcutaneous bleeding | 1 (0.5) | 2 |
| Compartment syndrome | 1 (0.5) | 2 |
| Cardiac tamponade | 1 (0.5) | 1 |
| Total | 11 (5.0) | NA |
| Thromboembolic event | ||
| Warfarin group (n = 222) | ||
| Cerebral infarction | 1 (0.5) | 86 |
| Total | 1 (0.5) | NA |
| All-cause death | ||
| Warfarin group (n = 222) | ||
| Cardiac arrest | 1 (0.5) | 14 |
| Total | 1 (0.5) | NA |
Abbreviation: NA, not applicable.
Figure 2. Event Rates of Major Bleeding and Composite Outcomes.
Error bars denote upper bound of 95% CIs.
aP = .03.
bP = .01.
Although no ischemic events of stroke, systemic embolism, transient ischemic attack, or all-cause death occurred in the dabigatran group, 1 cerebral infarction (PT-INR of 1.56 at event) and 1 death (cardiac arrest with unknown cause) occurred in the warfarin group within 3 months after ablation (Table 2). The composite incidence of major bleeding, thromboembolic events, and all-cause death until 3 months was lower in the dabigatran group vs the warfarin group (3 patients [mean {SD}, 1.4% {0.8%}; 95% CI, 0.4%-4.2%] vs 13 patients [mean {SD}, 5.9% {1.6%}; 95% CI, 3.5%-9.9%]; P = .01) (Figure 2). The composite incidence of all bleeding, thromboembolic events, and all-cause death until 3 months was 7 patients (3.2% [1.2%]; 95% CI, 1.5%-6.6%) in the dabigatran group and 16 patients (7.2% [1.7%]; 95% CI, 4.5%-11.6%) in the warfarin group, with no significant difference between groups (Figure 2). The net clinical benefit of dabigatran, measured by a composite end point consisting of thromboembolic events, all-cause death, and major bleeding up to 3 months after ablation, was 6.3. No thromboembolic events occurred after ablation in the dabigatran group; 1 (0.5%) occurred in the warfarin group.
Adverse Events
Serious adverse events until 3 months after ablation were reported in 21 patients (9.5%) in the dabigatran group and 28 (12.6%) in the warfarin group (eTable 4 in Supplement 2). Differences between groups were not significant.
Comparison of Patients With and Without Major Bleeding
No significant differences were found in baseline characteristics between patients with and without major bleeding events in the dabigatran group, including CHA2DS2-VASc and HAS-BLED (hypertension, abnormal renal/liver function, stroke, bleeding history or predisposition, labile INR, elderly, drugs/alcohol concomitantly) scores (eTable 5 in Supplement 2). In the warfarin group, patients with major bleeding events had a higher rate of alcohol abuse vs patients without major bleeding events (2 of 11 [18.2%] vs 2 of 211 [0.9%]; P = .01). No difference occurred in anticoagulation management before and during the ablation procedure between patients with and without major bleeding events in the dabigatran group.
Management of anticoagulation during the ablation procedure is shown in eTable 6 in Supplement 2. The dabigatran group had a significantly longer time to reach the activated clotting time target (median, 0.68 [IQR, 0.42-1.03] vs 0.47 [IQR, 0.28-0.68] hours), higher total heparin dose to reach the activated clotting time target (median, 10 000 [IQR, 8000-12 000] vs 6000 [IQR, 5000-8000] IU), higher heparin dose per hour (median, 6243.0 [IQR, 4615.4-8063.4] vs 4587.8 [IQR, 3428.6-6621.0] IU/h), longer total heparinization time (median, 2.25 [IQR, 1.63-3.15] vs 2.08 [IQR, 1.33-2.92] hours), and total heparin dose during the procedure (median, 14 000 [IQR, 11 000-18 000] vs 9000 [IQR, 6760-12 000] IU) compared with the warfarin group (all P ≤ .01).
Comparison Between Dabigatran Subgroups With the Holding of 1 vs 2 Doses
Detailed results of patients in the dabigatran subgroups with D-A intervals of less than 24 hours and at least 24 hours are described in eAppendix 3 and eFigure 4 in Supplement 2. All 3 patients with major bleeding events in the dabigatran group had the D-A interval of at least 24 hours.
The APTT and D-dimer levels at various times in dabigatran subgroups were compared (eTable 7 in Supplement 2). In the subgroup with a D-A interval of at least 24 hours and without heparin bridging, the APTT on the day of ablation (right before ablation) was significantly shorter (median, 32.2 [IQR, 29.8, 36.9] seconds) than that in subgroups with a D-A interval of less than 24 hours without heparin bridging (median, 36.7 [IQR, 33.6-42.5] seconds), with a D-A interval of less than 24 hours with heparin bridging (median, 42.3 [IQR, 37.7-49.5] seconds), and with a D-A interval of at least 24 hours with heparin bridging (median, 41.9 [IQR, 32.8-48.6] seconds) (all P < .01).
Discussion
Main Findings
To our knowledge, ABRIDGE-J is the first randomized clinical trial of minimally interrupted DOAC vs uninterrupted warfarin therapy in patients with AF undergoing catheter ablation. During the perioperative period, no participants in the minimally interrupted dabigatran group had a thromboembolism compared with 1 case each of cerebral infarction and intracardiac thrombus in the warfarin group. The incidence of major bleeding events at 3 months after ablation was significantly lower with dabigatran vs warfarin (1.4% vs 5.0%; P = .03). In the warfarin group of 222 patients, 3 (1.4%) developed tamponade or pericardial hemorrhage and 5 (2.3%) developed groin-site complications. Our findings support the recommendation of minimally interrupted dabigatran therapy with a higher level of evidence than that of the 2017 consensus statement on AF ablation.1
In this study, no participants in the dabigatran group had an embolism. In the RE-CIRCUIT (Uninterrupted Dabigatran Etexilate in Comparison to Uninterrupted Warfarin in Pulmonary Vein Ablation) trial,6 only 1 patient had embolism in the warfarin group during the 8-week postablation period, but no intracardiac thrombus events occurred. In terms of the secondary end points used in the present study, the rates of major bleeding to 8 weeks were 1.6% in the dabigatran group and 6.9% in the warfarin group in the RE-CIRCUIT trial.6 In the warfarin group, which consisted of 318 patients, bleeding events included tamponade in 6 patients (1.9%), groin hematomas in 8 (2.5%), and groin bleeding in 2 (0.6%). The complication rates in the warfarin group in the RE-CIRCUIT trial6 and in the present study were higher than those observed in other ablation trials, such as COMPARE (Role of Coumadin in Preventing Thromboembolism in Atrial Fibrillation [AF] Patients Undergoing Catheter Ablation),5 VENTURE-AF (Study Exploring Two Treatment Strategies in Patients With Atrial Fibrillation Who Undergo Catheter Ablation Therapy),7 and AEIOU (Apixaban Evaluation of Interrupted or Uninterrupted Anticoagulation for Ablation of Atrial Fibrillation).12 Several possible reasons explain this increase. Bleeding events in the RE-CIRCUIT trial6 and in the present study were defined using ISTH criteria, which have a lower threshold for hemoglobin loss than the definition used in the COMPARE study. In the AXAFA-AFNET5 (Anticoagulation Using the Direct Factor Xa Inhibitor Apixaban During Atrial Fibrillation Catheter Ablation: Comparison to Vitamin K Antagonist Therapy Atrial Fibrillation Network) trial,13 the investigators described major bleeding rates using the TIMI (Thrombolysis in Myocardial Infarction) and ISTH criteria. Bleeding rates in the warfarin group were 0.3% using the TIMI criteria and 3.1% using the ISTH criteria. Another possible reason is procedural or operator-related biases in each trial. The present study included many low-volume institutions and might include less experienced operators. The incidence of all bleeding events in our study was higher than in others, with no difference between the dabigatran and warfarin groups. Because the minimally interrupted dabigatran group had significantly lower major bleeding rates, dabigatran might prevent the worsening of mild bleeding complications to severe bleeding events. Our subgroup analyses (eFigure 3 in Supplement 2) revealed that dabigatran reduced the major bleeding risk in the radiofrequency ablation subgroup, but not in the cryoballoon ablation subgroup. In the FIRE and ICE study,30 the radiofrequency ablation group had a higher frequency of serious bleeding events compared with the cryoballoon ablation group. This finding explains why no difference in major bleeding events occurred between groups within the cryoballoon ablation subgroup in the present study.
Interval of Dabigatran Interruption
In this study, dabigatran therapy was interrupted by holding 1 or 2 doses before ablation. All 3 major bleeding events in the dabigatran group occurred in the subgroup with D-A interval of at least 24 hours (the evening dose on the day before ablation and the morning dose on the day of ablation were withheld). The composite incidence of major bleeding events, thromboembolic events, and all-cause death was significantly lower in the dabigatran subgroup with a D-A interval of less than 24 hours (holding of 1 dose) vs the warfarin group and vs dabigatran subgroup with at least 24 hours (holding of 2 doses) (eFigure 4 in Supplement 2).
Regarding heparin bridging, the American College of Cardiology expert consensus decision pathway for periprocedural management of anticoagulation14 and the American Heart Association scientific statement on non–vitamin K antagonist oral anticoagulants in the acute care and periprocedural setting31 recommended heparin bridging only for patients with a high thromboembolic risk, such as those with a history of systemic embolus in the last 6 weeks. In patients with low or intermediate risks of thrombosis, heparin bridging does not prevent thromboembolic events and increases bleeding events.32
In this study, a low rate of major bleeding events was observed in patients with a D-A interval of less than 24 hours (eFigure 4 in Supplement 2). Furthermore, on the day of ablation, the median APTT in patients with a D-A interval of at least 24 hours without heparin bridging was significantly lower (32.2 seconds) than that of other subgroups (all P < .001) (eTable 7 in Supplement 2).
Prior Studies
Previous nonrandomized studies have compared interrupted dabigatran and uninterrupted warfarin therapy for NVAF ablation.33,34 In their case-control study, Kim et al33 reported the results of 763 consecutive patients who underwent AF ablation using interrupted dabigatran (n = 191) or uninterrupted warfarin (n = 572) for periprocedural anticoagulation. In that study,33 2 doses (the evening dose on the day before the procedure and the morning dose) were skipped, and they described the prevalence of major (2.1%) and minor (2.6%) bleeding complications in the dabigatran group as similar to those in the warfarin group (2.1% and 3.3%, respectively). The authors also found that clopidogrel bisulfate use and higher CHAsDS2-VASc score were independent risk factors for bleeding complications in the warfarin group, but not the dabigatran group. However, their study was also underpowered for the assessment of efficacy in the prevention of thromboembolic events because no cases of thromboembolism developed in either group.
Lakkireddy et al34 reported an observational study from a registry for AF ablation. A total of 145 patients receiving dabigatran with the dose held on the morning of the procedure were matched with an equal number of patients undergoing AF ablation with uninterrupted warfarin therapy. Three thromboembolic complications (2.1%) occurred in the dabigatran group vs none in the warfarin group (P = .25). The dabigatran group had a significantly higher major bleeding rate (6% vs 1%; P = .02), total bleeding rate (14% vs 6%; P = .03), and composite of bleeding and thromboembolic complications (16% vs 6%; P = .009) compared with the warfarin group. The reasons for the higher thromboembolic and bleeding rates in the dabigatran group are unknown; however, that observational study might be limited by institution or operator bias. Although other clinical characteristics were similar, the significant difference in medication use between groups might be due to bias of the institution or operator.
Limitations
Our study has some notable limitations. First, the sample size was relatively small, and the study was underpowered to determine differences in thromboembolic rates between groups. The benefit of minimally interrupted dabigatran in terms of prevention of thromboembolism could not be analyzed. Subgroup analyses were post hoc, and no adjustment was made for this. Furthermore, the subgroup analysis between dabigatran with holding of 1 vs 2 doses was not randomized. In addition, one-third of patients received heparin bridging, which likely affected both safety and efficacy. This study was conducted in Japanese patients; thus, the findings cannot be generalized to other ethnic populations. Future studies should compare uninterrupted DOAC and minimally interrupted DOAC without heparin bridging.
Conclusions
In patients undergoing NVAF catheter ablation, anticoagulation with minimally interrupted dabigatran did not increase perioperative thromboembolic events. Minimally interrupted dabigatran was associated with fewer bleeding complications than uninterrupted warfarin.
Trial Protocol
eAppendix 1. List of Centers
eAppendix 2. Sample Size Estimate Calculation and Statistical Analysis Set
eAppendix 3. Comparison Between Dabigatran Subgroups With the Holding of 1 vs 2 Doses
eTable 1. APTT and PT-INR Data During Treatment
eTable 2. Comparison Between Dabigatran Subgroups
eTable 3. Clinical Characteristics of Patients With a Primary End Point
eTable 4. Serious Adverse Events After Ablation
eTable 5. Characteristics of Patients With and Without Major Bleeding
eTable 6. Management of Anticoagulation During the Ablation Procedure
eTable 7. APTT and D-dimer Data in Dabigatran Subgroups
eFigure 1. Study Design
eFigure 2. Kaplan-Meier Plot of Time to First Adjudicated Major Bleeding Event (Ablation Set)
eFigure 3. Subgroup Analysis of the Incidence of Adjudicated Major Bleeding Events Using Univariable Associations
eFigure 4. Event Rates of Major Bleeding and Composite Outcomes in the Warfarin and Dabigatran Subgroups With D-A Interval <24 Hours and ≥24 Hours
Data Sharing Statement
References
- 1.Calkins H, Hindricks G, Cappato R, et al. 2017 HRS/EHRA/ECAS/APHRS/SOLAECE expert consensus statement on catheter and surgical ablation of atrial fibrillation: executive summary. J Arrhythm. 2017;33(5):-. doi: 10.1016/j.joa.2017.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Santangeli P, Di Biase L, Horton R, et al. Ablation of atrial fibrillation under therapeutic warfarin reduces periprocedural complications: evidence from a meta-analysis. Circ Arrhythm Electrophysiol. 2012;5(2):302-311. doi: 10.1161/CIRCEP.111.964916 [DOI] [PubMed] [Google Scholar]
- 3.Di Biase L, Burkhardt JD, Mohanty P, et al. Periprocedural stroke and management of major bleeding complications in patients undergoing catheter ablation of atrial fibrillation: the impact of periprocedural therapeutic international normalized ratio. Circulation. 2010;121(23):2550-2556. doi: 10.1161/CIRCULATIONAHA.109.921320 [DOI] [PubMed] [Google Scholar]
- 4.Wazni OM, Beheiry S, Fahmy T, et al. Atrial fibrillation ablation in patients with therapeutic international normalized ratio: comparison of strategies of anticoagulation management in the periprocedural period. Circulation. 2007;116(22):2531-2534. doi: 10.1161/CIRCULATIONAHA.107.727784 [DOI] [PubMed] [Google Scholar]
- 5.Di Biase L, Burkhardt JD, Santangeli P, et al. Periprocedural stroke and bleeding complications in patients undergoing catheter ablation of atrial fibrillation with different anticoagulation management: results from the Role of Coumadin in Preventing Thromboembolism in Atrial Fibrillation (AF) Patients Undergoing Catheter Ablation (COMPARE) randomized trial. Circulation. 2014;129(25):2638-2644. doi: 10.1161/CIRCULATIONAHA.113.006426 [DOI] [PubMed] [Google Scholar]
- 6.Calkins H, Willems S, Gerstenfeld EP, et al. ; RE-CIRCUIT Investigators . Uninterrupted dabigatran versus warfarin for ablation in atrial fibrillation. N Engl J Med. 2017;376(17):1627-1636. doi: 10.1056/NEJMoa1701005 [DOI] [PubMed] [Google Scholar]
- 7.Cappato R, Marchlinski FE, Hohnloser SH, et al. ; VENTURE-AF Investigators . Uninterrupted rivaroxaban vs uninterrupted vitamin K antagonists for catheter ablation in non-valvular atrial fibrillation. Eur Heart J. 2015;36(28):1805-1811. doi: 10.1093/eurheartj/ehv177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kuwahara T, Abe M, Yamaki M, et al. Apixaban versus warfarin for the prevention of periprocedural cerebral thromboembolism in atrial fibrillation ablation: multicenter prospective randomized study. J Cardiovasc Electrophysiol. 2016;27(5):549-554. doi: 10.1111/jce.12928 [DOI] [PubMed] [Google Scholar]
- 9.Garg J, Chaudhary R, Krishnamoorthy P, Shah N, Bozorgnia B, Natale A. Safety and efficacy of uninterrupted periprocedural apixaban in patients undergoing atrial fibrillation catheter ablation: a meta-analysis of 1,057 patients. J Atr Fibrillation. 2016;8(6):1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Garg J, Chaudhary R, Krishnamoorthy P, Shah N, Natale A, Bozorgnia B. Safety and efficacy of uninterrupted periprocedural rivaroxaban in patients undergoing atrial fibrillation catheter ablation: a metaanalysis of 1,362 patients. Int J Cardiol. 2016;203:906-908. doi: 10.1016/j.ijcard.2015.11.085 [DOI] [PubMed] [Google Scholar]
- 11.Hohnloser SH, Camm AJ. Safety and efficacy of dabigatran etexilate during catheter ablation of atrial fibrillation: a meta-analysis of the literature. Europace. 2013;15(10):1407-1411. doi: 10.1093/europace/eut241 [DOI] [PubMed] [Google Scholar]
- 12.Reynolds MRJ, Allison JS, Natale A, et al. A prospective randomized trial of apixaban dosing during atrial fibrillation ablation: the AEIOU Trial. JACC Clin Electrophysiol. 2018;4(5):580-588. doi: 10.1016/j.jacep.2017.11.005 [DOI] [PubMed] [Google Scholar]
- 13.Kirchhof P, Haeusler KG, Blank B, et al. Apixaban in patients at risk of stroke undergoing atrial fibrillation ablation. Eur Heart J. 2018;39(32):2942-2955. doi: 10.1093/eurheartj/ehy176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Doherty JU, Gluckman TJ, Hucker WJ, et al. 2017 ACC expert consensus decision pathway for periprocedural management of anticoagulation in patients with nonvalvular atrial fibrillation: a report of the American College of Cardiology Clinical Expert Consensus Document Task Force. J Am Coll Cardiol. 2017;69(7):871-898. doi: 10.1016/j.jacc.2016.11.024 [DOI] [PubMed] [Google Scholar]
- 15.Potpara TS, Larsen TB, Deharo JC, et al. ; Scientific Initiatives Committee of European Heart Rhythm Association (EHRA) . Oral anticoagulant therapy for stroke prevention in patients with atrial fibrillation undergoing ablation: results from the First European Snapshot Survey on Procedural Routines for Atrial Fibrillation Ablation (ESS-PRAFA). Europace. 2015;17(6):986-993. doi: 10.1093/europace/euv132 [DOI] [PubMed] [Google Scholar]
- 16.Providência R, Marijon E, Albenque JP, et al. Rivaroxaban and dabigatran in patients undergoing catheter ablation of atrial fibrillation. Europace. 2014;16(8):1137-1144. doi: 10.1093/europace/euu007 [DOI] [PubMed] [Google Scholar]
- 17.Winkle RA, Mead RH, Engel G, Kong MH, Patrawala RA. Peri-procedural interrupted oral anticoagulation for atrial fibrillation ablation: comparison of aspirin, warfarin, dabigatran, and rivaroxaban. Europace. 2014;16(10):1443-1449. doi: 10.1093/europace/euu196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Steffel J, Ruff CT, Hamershock RA, et al. First experience with edoxaban and atrial fibrillation ablation: insights from the ENGAGE AF-TIMI 48 trial. Int J Cardiol. 2017;244:192-195. doi: 10.1016/j.ijcard.2017.05.098 [DOI] [PubMed] [Google Scholar]
- 19.Bassiouny M, Saliba W, Rickard J, et al. Use of dabigatran for periprocedural anticoagulation in patients undergoing catheter ablation for atrial fibrillation. Circ Arrhythm Electrophysiol. 2013;6(3):460-466. Erratum in: Circ Arrhythm Electrophysiol. 2013;6:e79. doi: 10.1161/CIRCEP.113.000320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bin Abdulhak AA, Khan AR, Tleyjeh IM, et al. Safety and efficacy of interrupted dabigatran for peri-procedural anticoagulation in catheter ablation of atrial fibrillation: a systematic review and meta-analysis. Europace. 2013;15(10):1412-1420. doi: 10.1093/europace/eut239 [DOI] [PubMed] [Google Scholar]
- 21.World Medical Association World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310(20):2191-2194. doi: 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 22.International Conference on Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (2016). Integrated Addendum to ICH E6(R1): Guideline for Good Clinical Practice E6(R2). https://www.ich.org/fileadmin/Public_Web_Site/ICH_Products/Guidelines/Efficacy/E6/E6_R2__Step_4_2016_1109.pdf. Published November 9, 2016. Accessed March 6, 2019.
- 23.Dabigatran package insert. http://www.bij-kusuri.jp/leaflet/attach/pdf/pxa_cap75_pi.pdf. Version 10, September 2017. Accessed August 9, 2018.
- 24.Guidelines for indications and procedural techniques of catheter ablation (JCS 2012). http://www.j-circ.or.jp/guideline/pdf/JCS2012_okumura_h.pdf. Published 2012. Accessed August 9, 2018.
- 25.Rosendaal FR, Cannegieter SC, van der Meer FJ, Briët E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69(3):236-239. doi: 10.1055/s-0038-1651587 [DOI] [PubMed] [Google Scholar]
- 26.Calkins H, Kuck KH, Cappato R, et al. 2012 HRS/EHRA/ECAS expert consensus statement on catheter and surgical ablation of atrial fibrillation: recommendations for patient selection, procedural techniques, patient management and follow-up, definitions, endpoints, and research trial design. Europace. 2012;14(4):528-606. doi: 10.1093/europace/eus027 [DOI] [PubMed] [Google Scholar]
- 27.Schulman S, Kearon C; Subcommittee on Control of Anticoagulation of the Scientific and Standardization Committee of the International Society on Thrombosis and Haemostasis. Definition of major bleeding in clinical investigations of antihemostatic medicinal products in nonsurgical patients. J Thromb Haemost. 2005;3(4):692-694. doi: 10.1111/j.1538-7836.2005.01204.x [DOI] [PubMed] [Google Scholar]
- 28.Singer DE, Chang Y, Fang MC, et al. The net clinical benefit of warfarin anticoagulation in atrial fibrillation. Ann Intern Med. 2009;151(5):297-305. doi: 10.7326/0003-4819-151-5-200909010-00003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hori M, Connolly SJ, Ezekowitz MD, Reilly PA, Yusuf S, Wallentin L; RE-LY Investigators . Efficacy and safety of dabigatran vs. warfarin in patients with atrial fibrillation: sub-analysis in Japanese population in RE-LY trial. Circ J. 2011;75(4):800-805. doi: 10.1253/circj.CJ-11-0191 [DOI] [PubMed] [Google Scholar]
- 30.Kuck KH, Brugada J, Fürnkranz A, et al. ; FIRE AND ICE Investigators . Cryoballoon or radiofrequency ablation for paroxysmal atrial fibrillation. N Engl J Med. 2016;374(23):2235-2245. doi: 10.1056/NEJMoa1602014 [DOI] [PubMed] [Google Scholar]
- 31.Raval AN, Cigarroa JE, Chung MK, et al. ; American Heart Association Clinical Pharmacology Subcommittee of the Acute Cardiac Care and General Cardiology Committee of the Council on Clinical Cardiology; Council on Cardiovascular Disease in the Young; and Council on Quality of Care and Outcomes Research . Management of patients on non–vitamin K antagonist oral anticoagulants in the acute care and periprocedural setting: a scientific statement from the American Heart Association. Circulation. 2017;135(10):e604-e633. doi: 10.1161/CIR.0000000000000477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Douketis JD, Spyropoulos AC, Kaatz S, et al. ; BRIDGE Investigators . Perioperative bridging anticoagulation in patients with atrial fibrillation. N Engl J Med. 2015;373(9):823-833. doi: 10.1056/NEJMoa1501035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kim JS, She F, Jongnarangsin K, et al. Dabigatran vs warfarin for radiofrequency catheter ablation of atrial fibrillation. Heart Rhythm. 2013;10(4):483-489. doi: 10.1016/j.hrthm.2012.12.011 [DOI] [PubMed] [Google Scholar]
- 34.Lakkireddy D, Reddy YM, Di Biase L, et al. Feasibility and safety of dabigatran versus warfarin for periprocedural anticoagulation in patients undergoing radiofrequency ablation for atrial fibrillation: results from a multicenter prospective registry. J Am Coll Cardiol. 2012;59(13):1168-1174. doi: 10.1016/j.jacc.2011.12.014 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
eAppendix 1. List of Centers
eAppendix 2. Sample Size Estimate Calculation and Statistical Analysis Set
eAppendix 3. Comparison Between Dabigatran Subgroups With the Holding of 1 vs 2 Doses
eTable 1. APTT and PT-INR Data During Treatment
eTable 2. Comparison Between Dabigatran Subgroups
eTable 3. Clinical Characteristics of Patients With a Primary End Point
eTable 4. Serious Adverse Events After Ablation
eTable 5. Characteristics of Patients With and Without Major Bleeding
eTable 6. Management of Anticoagulation During the Ablation Procedure
eTable 7. APTT and D-dimer Data in Dabigatran Subgroups
eFigure 1. Study Design
eFigure 2. Kaplan-Meier Plot of Time to First Adjudicated Major Bleeding Event (Ablation Set)
eFigure 3. Subgroup Analysis of the Incidence of Adjudicated Major Bleeding Events Using Univariable Associations
eFigure 4. Event Rates of Major Bleeding and Composite Outcomes in the Warfarin and Dabigatran Subgroups With D-A Interval <24 Hours and ≥24 Hours
Data Sharing Statement