Abstract
Background
Diabetic macular oedema (DMO) is a common complication of diabetic retinopathy. Antiangiogenic therapy with anti‐vascular endothelial growth factor (anti‐VEGF) modalities can reduce oedema and thereby improve vision and prevent further visual loss. These drugs have replaced laser photocoagulation as the standard of care for people with DMO.
Objectives
The 2014 update of this review found high‐quality evidence of benefit with antiangiogenic therapy with anti‐VEGF modalities, compared to laser photocoagulation, for the treatment of DMO.The objective of this updated review is to compare the effectiveness and safety of the different anti‐VEGF drugs in preserving and improving vision and quality of life using network meta‐analysis methods.
Search methods
We searched various electronic databases on 26 April 2017.
Selection criteria
We included randomised controlled trials (RCTs) that compared any anti‐angiogenic drug with an anti‐VEGF mechanism of action versus another anti‐VEGF drug, another treatment, sham or no treatment in people with DMO.
Data collection and analysis
We used standard Cochrane methods for pair‐wise meta‐analysis and we augmented this evidence using network meta‐analysis methods. We focused on the relative efficacy and safety of the three most commonly used drugs as interventions of direct interest for practice: aflibercept and ranibizumab, used on‐label; and off‐label bevacizumab.
We collected data on three efficacy outcomes (gain of 15 or more Early Treatment Diabetic Retinopathy Study (ETDRS) letters; mean change in best‐corrected visual acuity (BCVA); mean change in central retinal thickness (CRT)), three safety outcomes (all severe systemic adverse events (SSAEs); all‐cause death; arterial thromboembolic events) and quality of life.
We used Stata 'network' meta‐analysis package for all analyses. We investigated the risk of bias of mixed comparisons based on the variance contribution of each study, having assigned an overall risk of bias to each study.
Main results
Twenty‐four studies included 6007 participants with DMO and moderate vision loss, of which two studies randomised 265 eyes of 230 participants and one was a cross‐over study on 56 participants (62 eyes) that was treated as a parallel‐arm trial. Data were collected on drugs of direct interest from three studies on aflibercept (975 eyes), eight studies on bevacizumab (515 eyes), and 14 studies on ranibizumab (1518 eyes). As treatments of indirect interest or legacy treatment we included three studies on pegaptanib (541 eyes), five studies on ranibizumab plus prompt laser (557 eyes), one study on ranibizumab plus deferred laser (188 eyes), 13 studies on laser photocoagulation (936 eyes) and six studies on sham treatment (793 eyes).
Aflibercept, bevacizumab and ranibizumab were all more effective than laser for improving vision by 3 or more lines after one year (high‐certainty evidence). Approximately one in 10 people improve vision with laser, and about three in 10 people improve with anti‐VEGF treatment: risk ratio (RR) versus laser 3.66 (95% confidence interval (CI) 2.79 to 4.79) for aflibercept; RR 2.47 (95% CI 1.81 to 3.37) for bevacizumab; RR 2.76 (95% CI 2.12 to 3.59) for ranibizumab. On average there was no change in visual acuity (VA) with laser after one year, compared with a gain of 1 or 2 lines with anti‐VEGF treatment: laser versus aflibercept mean difference (MD) −0.20 (95% CI −0.22 to −0.17) logMAR; versus bevacizumab MD −0.12 (95% CI −0.15 to −0.09) logMAR; versus ranibizumab MD −0.12 (95% CI −0.14 to −0.10) logMAR. The certainty of the evidence was high for the comparison of aflibercept and ranibizumab with laser and moderate for bevacizumab comparison with laser due to inconsistency between the indirect and direct evidence.
People receiving ranibizumab were less likely to gain 3 or more lines of VA at one year compared with aflibercept: RR 0.75 (95% CI 0.60 to 0.94), moderate‐certainty evidence. For every 1000 people treated with aflibercept, 92 fewer would gain 3 or more lines of VA at one year if treated with ranibizumab (22 to 148 fewer). On average people receiving ranibizumab had worse VA at one year (MD 0.08 logMAR units, 95% CI 0.05 to 0.11), moderate‐certainty evidence; and higher CRT (MD 39 µm, 95% CI 2 µm to 76 µm; low‐certainty evidence). Ranibizumab and bevacizumab were comparable with respect to aflibercept and did not differ in terms of VA: RR of gain of 3 or more lines of VA at one year 1.11 (95% CI 0.87 to 1.43), moderate‐certainty evidence, and difference in change in VA was 0.00 (95% CI −0.02 to 0.03) logMAR, moderate‐certainty evidence. CRT reduction favoured ranibizumab by −29 µm (95% CI −58 µm to −1 µm, low‐certainty evidence). There was no evidence of overall statistical inconsistency in our analyses.
The previous version of this review found moderate‐certainty evidence of good safety of antiangiogenic drugs versus control. This update used data at the longest available follow‐up (one or two years) and found that aflibercept, ranibizumab and bevacizumab do not differ regarding systemic serious adverse events (SSAEs) (moderate‐ or high‐certainty evidence). However, risk of bias was variable, loop inconsistency could be found and estimates were not precise enough on relative safety regarding less frequent events such as arterial thromboembolic events or death (low‐ or very low‐certainty evidence).
Two‐year data were available and reported in only four RCTs in this review. Most industry‐sponsored studies were open‐label after one year. One large publicly‐funded study compared the three drugs at two years and found no difference.
Authors' conclusions
Anti‐VEGF drugs are effective at improving vision in people with DMO with three to four in every 10 people likely to experience an improvement of 3 or more lines VA at one year. There is moderate‐certainty evidence that aflibercept confers some advantage over ranibizumab and bevacizumab in people with DMO at one year in visual and anatomic terms. Relative effects among anti‐VEGF drugs at two years are less well known, since most studies were short term. Evidence from RCTs may not apply to real‐world practice, where people in need of antiangiogenic treatment are often under‐treated and under‐monitored.
We found no signals of differences in overall safety between the three antiangiogenic drugs that are currently available to treat DMO, but our estimates are imprecise for cardiovascular events and death.
Keywords: Humans; Angiogenesis Inhibitors; Angiogenesis Inhibitors/therapeutic use; Antibodies, Monoclonal; Antibodies, Monoclonal/therapeutic use; Antibodies, Monoclonal, Humanized; Antibodies, Monoclonal, Humanized/therapeutic use; Aptamers, Nucleotide; Aptamers, Nucleotide/therapeutic use; Bevacizumab; Diabetic Retinopathy; Diabetic Retinopathy/complications; Laser Coagulation; Laser Coagulation/methods; Macular Edema; Macular Edema/drug therapy; Macular Edema/surgery; Randomized Controlled Trials as Topic; Ranibizumab; Receptors, Vascular Endothelial Growth Factor; Receptors, Vascular Endothelial Growth Factor/therapeutic use; Recombinant Fusion Proteins; Recombinant Fusion Proteins/therapeutic use; Triamcinolone; Triamcinolone/therapeutic use; Vascular Endothelial Growth Factor A; Vascular Endothelial Growth Factor A/antagonists & inhibitors
Anti‐vascular endothelial growth factor (anti‐VEGF) drugs for diabetic macular oedema
What is the aim of this review? The aim of this Cochrane Review was to find out which is the best type of anti‐VEGF drug for diabetic macular oedema (DMO). Cochrane researchers collected and analysed all relevant studies to answer this question and found 24 studies.
Key messages Anti‐VEGF drugs given by injection into the eye improve vision in people with diabetic macular oedema as compared to no average improvement with laser photocoagulation. One of these drugs, aflibercept, probably works slightly better after one year. There did not appear to be important harms from any of these drugs.
What was studied in the review? The light‐sensitive tissue at the back of the eye is known as the retina. The central area of the retina is called the macula. People with diabetes can develop problems in the retina, known as retinopathy. Some people with diabetic retinopathy can also develop oedema (swelling or thickening) at the macula. DMO is a common complication of diabetic retinopathy and can lead to visual loss.
One type of treatment for DMO is anti‐VEGF. This drug is given by means of an injection into the eye. It can reduce the swelling at the back of the eye and prevent visual loss. There are three main types of anti‐VEGF drugs in use: aflibercept (EyeleaTM), bevacizumab (Avastin) and ranibizumab (LucentisTM). Only aflibercept and ranibizumab have received marketing authorisation for the treatment of DMO. All three drugs are used to prevent visual loss and improve vision. They do this by slowing down the growth of new blood vessels and thereby reducing the swelling at the back of the eye. They may have adverse effects, particularly related to effects on blood vessels in the rest of the body. These effects may include stroke and heart attack.
What are the main results of the review? Cochrane researchers found 24 relevant studies. Fourteen of these studies were industry‐sponsored studies from USA, Europe or Asia. Ten studies were independent of industry funding and were from USA, Europe, Middle East and South America.
These studies investigated ranibizumab, bevacizumab and aflibercept. These anti‐VEGF drugs were compared with no treatment, placebo treatment, laser treatment, or each other. The drugs were given every month, every two months, as needed or 'treat and extend', which means that the time period between treatments is extended if the condition has stabilised. Decisions about re‐treating were based on visual acuity or by looking at the back of the eye.
The review reveals the following results.
• All three anti‐VEGF drugs prevent visual loss and improve vision in people with DMO (high‐certainty evidence).
• People receiving ranibizumab were probably slightly less likely to improve vision compared with aflibercept at one year after the start of treatment (moderate‐certainty evidence). Approximately three in 10 people improve vision by 3 or more lines with ranibizumab and one in 10 additional people can achieve this with aflibercept.
• People receiving ranibizumab and bevacizumab probably have a similar visual outcome at one year after the start of treatment (moderate‐certainty evidence).
• Aflibercept, ranibizumab and bevacizumab are similar for common and serious systemic harms (such as any disease leading to hospitalisation, disability or death) (moderate‐ or high‐certainty evidence) but is less certain for arterial thromboembolic events (mainly stroke, myocardial infarction and vascular death) and death of any cause (very low‐certainty evidence).
How up to date is this review? Cochrane researchers searched for studies that had been published up to 26 April 2017.
Summary of findings
Summary of findings for the main comparison.
Antiangiogenic therapy versus control
| Antiangiogenic therapy versus control | |||||
| Patient or population: people with diabetic macular oedema Settings: ophthalmology clinics Interventions: laser photocoagulation, aflibercept, bevacizumab, ranibizumab | |||||
| Outcomes | Assumed risk* | Corresponding risk and relative risk** (95% CI) , mixed evidence | Certainty of evidence and reason for downgrading | ||
| Laser photocoagulation | Aflibercept | Bevacizumab | Ranibizumab | ||
| Gain 3+ lines of visual acuity at 1 year | 100 per 1000 |
366 per 1000 (279 to 479) RR: 3.66 (2.79 to 4.79) |
247 per 1000 (181 to 337) RR: 2.47 (1.81 to 3.37) |
276 per 1000 (212 to 359) RR: 2.76 (2.12 to 3.59) |
⊕⊕⊕⊕ high |
|
Visual acuity change at 1 year Measured on the logMAR scale, range −0.3 to 1.3. Higher values represent worse visual acuity. |
On average visual acuity improved by −0.01 logMAR units in the laser group between the start of treatment and 1 year (effectively no change) | Average change in visual acuity was −0.20 (−0.22 to −0.17) logMAR units better with aflibercept compared with laser photocoagulation | Average change in visual acuity was −0.12 (−0.15 to −0.09) logMAR units better with bevacizumab compared with laser photocoagulation | Average change in visual acuity was −0.12 (−0.14 to −0.10) logMAR units better with ranibizumab compared with laser photocoagulation | ⊕⊕⊕⊕ high for aflibercept and ranibizumab ⊕⊕⊕ moderate for bevacizumab (−1 for inconsistency of indirect versus direct evidence) |
|
Central retinal thickness μm (CRT) change at 1 year The aim of treatment is to reduce central retinal thickness so thinner is better. |
On average CRT changed by −64 μm in the laser group between the start of treatment and 1 year (became thinner) | Average change in CRT was −114 (−147 to −81) μm more (thinner) with aflibercept compared with laser photocoagulation | Average change in CRT was −46 (−78 to −14) μm more (thinner)with bevacizumab compared with laser photocoagulation | Average change in CRT was −75 (−100 to −50) μm more (thinner) with ranibizumab compared with laser photocoagulation | ⊕⊕⊕⊕ high |
|
Quality of life: NEI‐VFQ composite score at 6 to 12 months An improvement by 5 units is clinically significant. |
On average the composite score improved by +2 units in the laser group between the start of treatment and 6 to 12 months | Average change in composite score was 5.14 (2.96 to 7.32) with ranibizumab compared with laser photocoagulation | ⊕⊕⊕ moderate (−1 for risk of bias) | ||
| All serious systemic adverse events at 1 to 2 years | 200 per 1000 |
196 per 1000 (166 to 232) RR: 0.98 (0.83 to 1.16) |
186 per 1000 (146 to 238) RR: 0.93 (0.73 to 1.19) |
194 per 1000 (160 to 234) RR: 0.97 (0.80 to 1.17) |
⊕⊕⊕⊕ high |
| Arterial thromboembolic events at 1 to 2 years | 45 per 1000 |
38 per 1000 (16 to 94) RR: 0.88 (0.37 to 2.13) |
41 per 1000 (15 to 117) RR: 0.94 (0.33 to 2.66) |
48 per 1000 (23 to 101) RR: 1.09 (0.52 to 2.29) |
⊕⊕ low (−2 for imprecise estimates) |
| Death at 1 to 2 years | 20 per 1000 |
20 per 1000 (7 to 61) RR: 1.01 (0.34 to 3.03) a |
32 per 1000 (9 to 114) RR: 1.61 (0.45 to 5.69) |
18 per 1000 (8 to 40) RR: 0.90 (0.40 to 2.01) |
⊕⊕ low for bevacizumab and aflibercept (−2 for imprecise estimates) ⊕ very low for aflibercept (additional −1 direct evidence inconsistent, higher risk) |
| The assumed risk in the laser group was estimated as the row sum of the events divided by the row sum of the participants (eyes) for dichotomous variables, and as the (unweighted) median change of visual acuity or central retina thickness. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ** The risk ratio was estimated from mixed (direct and indirect) comparisons. CI: Confidence interval; RR: Risk ratio. | |||||
| GRADE Working Group grades of evidence High‐certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate‐certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low‐certainty: we are very uncertain about the estimate. | |||||
Summary of findings 2.
Ranibizumab versus aflibercept for diabetic macular oedema
| Ranibizumab versus aflibercept for diabetic macular oedema | ||||||
| Patient or population: people with diabetic macular oedema Settings: ophthalmology clinics Interventions: aflibercept, ranibizumab | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI), mixed evidence** | Certainty of the evidence (GRADE) | Reason for downgrading certainty of evidence | ||
| Assumed risk | Corresponding risk | |||||
| Aflibercept | Ranibizumab | |||||
| Gain 3+ lines of visual acuity at 1 year | 370 per 1000 | 278 per 1000 (222 to 348) | RR: 0.75 (0.60 to 0.94) | ⊕⊕⊕ moderate | −1 for imprecision as confidence intervals include both clinically important and clinically unimportant effects | |
|
Visual acuity change at 1 year Measured on the logMAR scale, range −1.3 to 1.3. Higher values represent worse visual acuity. |
On average visual acuity improved by−0.23 logMAR units in the aflibercept group between the start of treatment and 1 year | Average change in visual acuity was 0.08 (0.05 to 0.11) logMAR units worse with ranibizumab compared with aflibercept |
⊕⊕⊕ moderate | −1 for imprecision as confidence intervals include both clinically important and clinically unimportant effects | ||
|
Central retinal thickness μm (CRT) change at 1 year The aim of treatment is to reduce central macular thickness so thinner is better. |
On average CRT changed by −181 μm in the aflibercept group between the start of treatment and 1 year (became thinner) | Average change in CRT was 39 (2 to 76) μm more (thicker) with ranibizumab compared with aflibercept |
⊕⊕ low | −1 for high heterogeneity in two direct comparisons and large predictive intervals −1 for imprecision |
||
| Quality of life at 1 year | No data available. | |||||
| All serious systemic adverse events at 1 to 2 years | 345 per 1000 |
338 per 1000 (283 to 411) |
RR 0.98 (0.82 to 1.19) |
⊕⊕⊕⊕ high | ||
| Arterial thromboembolic events at 1 to 2 years | 60 per 1000 |
74 per 1000 (29 to 191) |
RR 1.24 (0.48 to 3.19) |
⊕ very low | Inconsistency between direct and indirect evidence (−1), and imprecise estimates (−2) | |
| Death at 1 to 2 years | 30 per 1000 |
35 per 1000 (11 to 108) |
RR 1.16 (0.38 to 3.58) |
⊕ very low | Inconsistency between direct and indirect evidence (−1), and imprecise estimates (−2) | |
| The assumed risk in the aflibercept group was estimated as the row sum of the events divided by the row sum of the participants (eyes) for dichotomous variables, and as the (unweighted) median change of visual acuity or central retina thickness. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ** The risk ratio was estimated from mixed (direct and indirect) comparisons. CI: Confidence interval; RR: Risk ratio. |
||||||
| GRADE Working Group grades of evidence High‐certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate‐certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low‐certainty: we are very uncertain about the estimate. | ||||||
Summary of findings 3.
Ranibizumab versus bevacizumab for diabetic macular oedema
| Ranibizumab versus bevacizumab for diabetic macular oedema | |||||
| Patient or population: people with diabetic macular oedema Settings: ophthalmology clinics Interventions: bevacizumab, ranibizumab | |||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI), mixed evidence** | Certainty of the evidence (GRADE) | Reason for downgrading certainty of evidence | |
| Assumed risk | Corresponding risk | ||||
| Bevacizumab | Ranibizumab | ||||
| Gain 3+ lines of visual acuity at 1 year | 300 per 1000 | 333 per 1000 (261 to 429) | RR 1.11 (0.87 to 1.43) | ⊕⊕⊕ moderate | Imprecise estimate (−1) |
|
Visual acuity change at 1 year Measured on the logMAR scale, range −1.3 to 1.3. Higher values represent worse visual acuity. |
On average visual acuity improved by −0.19 logMAR units in the bevacizumab group between the start of treatment and 1 year | Average change in visual acuity was0.00 (−0.02 to 0.03) logMAR units (same) with ranibizumab compared with bevacizumab | ⊕⊕⊕ moderate | Unclear risk of bias (−1) | |
|
Central retinal thickness (CRT) change at 1 year The aim of treatment is to reduce central macular thickness so thinner is better. |
On average CRT changed by −98 μm in the bevacizumab group between the start of treatment and 1 year (became thinner) | Average change in CRT was −29 (−58 to −1) μm more (thinner) with ranibizumab compared with bevacizumab | ⊕⊕ low | Unclear risk of bias (−1) Imprecise estimate (−1) | |
| Quality of life at 1 year | No data available | ||||
| All serious systemic adverse events at 1 to 2 years | 240 per 1000 |
250 per 1000 (202 to 307) |
RR 1.04 (0.84 to 1.28) |
⊕⊕⊕ moderate | Unclear risk of bias (−1) |
| Arterial thromboembolic events at 1 to 2 years | 60 per 1000 |
70 per 1000 (26 to 189) |
RR 1.17 (0.43 to 3.13) |
⊕ very low | Unclear risk of bias (−1) Imprecise estimate (−2) |
| Death at 1 to 2 years | 40 per 1000 |
29 per 1000 (9 to 95) |
RR 0.73 (0.22 to 2.37) |
⊕ very low | High risk of bias (−2) Imprecise estimate (−2) |
| The assumed risk in the bevacizumab group was estimated as the row sum of the events divided by the row sum of the participants (eyes) for dichotomous variables, and as the (unweighted) median change of visual acuity or central retina thickness. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ** The risk ratio was estimated from mixed (direct and indirect) comparisons. CI: Confidence interval; RR: Risk ratio. | |||||
| GRADE Working Group grades of evidence High‐certainty: further research is very unlikely to change our confidence in the estimate of effect. Moderate‐certainty: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐certainty: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low‐certainty: we are very uncertain about the estimate. | |||||
Background
Description of the condition
Diabetic retinopathy (DR) is the most frequent and severe ocular complication of diabetes mellitus (DM) and the leading cause of blindness in the working age population in developed countries (Frank 2004; Klein 1984; Tranos 2004).
Diabetic macular oedema (DMO) is the swelling of the retina resulting from the exudation and accumulation of extracellular fluid and proteins in the macula (Ciulla 2003), due to the breakdown of the blood‐retina barrier with an increase in vascular permeability (Antcliff 1999). About a third of people with diabetes have DR and one in 10 is affected by DMO (Yau 2012). The prevalence of DMO increases with diabetes duration, haemoglobin A1c, and blood pressure levels and is higher in people with type 1 compared with type 2 diabetes (Yau 2012).
Intraretinal fluid accumulation results in significant reduction in visual acuity that may be reversible in the short term, but prolonged oedema can cause irreversible damage resulting in permanent visual loss. Blurred vision represents the most common clinical symptom of DMO. Other symptoms can include metamorphopsia (distortion of visual image), floaters, changes in contrast sensitivity, photophobia (visual intolerance to light), changes in colour vision and scotomas (a localised defect of the visual field).
During the last decades, the clinical gold standard to detect macular oedema has been fundus examination with contact lens, but non‐contact lenses can also be used for this purpose with good sensitivity. Optical coherence tomography (OCT) has progressively been used as an objective and reproducible tool to measure retinal thickness and has been suggested to be the new gold standard for diagnosing DMO (Olson 2013; Ontario HTA 2009). The most severe form of DMO is CSMO, which was defined by the Early Treatment Diabetic Retinopathy Study (ETDRS) as: retinal oedema within 500 µm of the centre of the fovea; hard exudates within 500 µm of the centre of the fovea, if associated with adjacent retinal thickening (which may be outside the 500 µm limit); and one disc area of retinal oedema (1500 µm) or larger, any part of which is within one disc diameter of the centre of the fovea (ETDRS 1985). Since its introduction, OCT was found to be in good agreement with the clinical gold standard (slit‐lamp examination with a contact lens) for detecting the presence of macular oedema and was found to be potentially more sensitive in cases of mild foveal thickening (Brown 2004). A simple OCT‐based classification of DMO is often used as centre‐involving or non‐centre‐involving DMO (Browning 2008).
Description of the intervention
Antiangiogenic therapy has been believed a standard of care for treatment of DMO and has largely replaced laser photocoagulation (Jampol 2014), than which it was proven to be more effective (Virgili 2014). Anti‐vascular endothelial growth factor (anti‐VEGF) treatments inhibit VEGF angiogenic activity, binding to VEGF protein and thus preventing its receptor activation or interaction. These drugs were originally hypothesised as an alternative adjunctive treatment for DMO (Cunningham 2005), following evidence that VEGF‐A plays a key role in the occurrence of increased vascular permeability in ocular diseases such as DMO (Aiello 2005).
Grid or focal laser photocoagulation could not be used in all patients with DMO; thus, either laser or sham procedures were current practice comparators in initial studies on the efficacy of antiangiogenic drugs for DMO (Macugen 2005; RESOLVE 2010; RESTORE 2011; Soheilian 2007), and only recently have directly comparative RCTs been conducted (DRCRnet 2015).
Safety of intravitreal antiangiogenic therapy is acceptable; endophthalmitis, the major adverse event (< 1/1000 injections) is related to the surgical injection procedure, rather than the drug itself. These drugs were shown not to increase systemic adverse events such as arterial thromboembolic events, but differences between drugs are not well known (Virgili 2014).
Another therapeutic option for DMO treatment is represented by steroids, administered as intravitreal injections or sustained release implants in order to obtain high local concentrations, maximising their anti‐inflammatory, angiostatic and anti‐permeability effects while minimising systemic toxicity (Ciulla 2004; Haller 2010; Kuppermann 2010). However, intravitreal steroids may cause cataract and ocular hypertension and the visual outcome is dependent on the lens status or the need for cataract surgery after about one year (Haller 2010; Campochiaro 2010). Currently, some investigators think intravitreal steroids are preferred in patients with anti‐VEGF resistant and chronic DMO (Hussain 2015), as an alternative to switching between anti‐VEGF drugs. This is also consistent with the EU label of the only approved dexamethasone intravitreal implant in Europe: "Ozurdex is indicated for the treatment of adult patients with visual impairment due to diabetic macular oedema (DME) who are pseudophakic or who are considered insufficiently responsive to, or unsuitable for non‐corticosteroid therapy" (accessed on EMA on 4 December 2016).
For ranibizumab, the EU label prescribes a 0.5 mg dosage, and that "treatment is initiated with one injection per month until maximum visual acuity is achieved and/or there are no signs of disease activity i.e. no change in visual acuity and in other signs and symptoms of the disease under continued treatment. In patients with wet AMD, DME and RVO, initially, three or more consecutive, monthly injections may be needed. Thereafter, monitoring and treatment intervals should be determined by the physician and should be based on disease activity, as assessed by visual acuity and/or anatomical parameters" (accessed on EMA on 4 December 2016). In the USA, ranibizumab "0.3 mg is recommended to be administered by intravitreal injection once a month (approximately 28 days)" (accessed on FDA on 4 December 2016).
Aflibercept has been approved in the USA, as accessed on FDA on 4 December 2016, and "the recommended dose for EYLEA is 2 mg (0.05 mL) administered by intravitreal injection every 4 weeks (monthly) for the first 5 injections followed by 2 mg (0.05 mL) via intravitreal injection once every 8 weeks (2 months)". The EU label is similar (accessed on EMA on 4 December 2016).
Bevacizumab is widely used off‐label although its use has been questioned based on regulatory or safety issues (Banfi 2013), but is still key for treating chorioretinal vascular disease in low‐ and middle‐income countries thanks to its low cost (Stewart 2016).
How the intervention might work
VEGF plays a key role in the occurrence of increased vascular permeability in ocular diseases such as DMO (Aiello 2005). Anti‐VEGF agents inhibit VEGF angiogenic activity, binding to VEGF protein thus preventing its receptor activation and interaction.
Why it is important to do this review
DMO results in a significant burden of low vision and blindness, thus the extent of the existing evidence base for the effectiveness and safety of these agents needs to be assessed and updated. There is a continuing clinical need to establish evidence‐based recommendations regarding anti‐VEGF agents.
Objectives
The 2014 update of this review found high‐quality evidence of benefit with antiangiogenic therapy with anti‐VEGF modalities, compared to laser photocoagulation, for the treatment of DMO. As was concluded in the previous version (Virgili 2014), the objective of this updated review is to compare the effectiveness and safety of the different anti‐VEGF drugs in preserving and improving vision and quality of life using network meta‐analysis methods.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs).
Types of participants
People with DMO for whom anti‐VEGF treatment is indicated. We expected to include most of the studies also included in Virgili 2014.
Types of interventions
Any antiangiogenic drug with anti‐VEGF modalities compared with another drug with anti‐VEGF modalities, laser treatment, sham treatment or no treatment. The reasons for selecting treatments of direct and indirect treatment have been discussed in the Description of the intervention section. As explained above, we remark that steroids may be compared with anti‐VEGF drugs but this needs a different approach, specifically patient subgroups and timing, and their inclusion could lead to violation of similarity in a review aiming to compare different anti‐VEGF drugs such as this.
Regarding drug dose and monitoring/retreatment regimen, in efficacy analyses we included schemes that are either on‐label or commonly used in clinical practice, such as the PRN regimen, as presented in the Description of the intervention section. Particularly, both 0.3 mg and 0.5 mg ranibizumab dose are included as available in studies. These two ranibizumab doses were merged into one group in our NMA since studies suggest no difference between them when used monthly (Heier 2016). Regarding aflibercept, we selected the bi‐monthly retreatment regimen since this is the approved label in the USA. We used all available data regardless of safety and dose for safety analyses as previously done in Moja 2014.
Types of outcome measures
Primary outcomes
Best‐corrected visual acuity (BCVA) expressed as the proportion of participants with at least 15 ETDRS letters (3 ETDRS lines or 0.3 logMAR) of improvement in BCVA from baseline to 12 months.
Secondary outcomes
Mean change in BCVA from baseline to 12 months, measured using ETDRS charts.
Mean change in central retinal thickness (CRT), from baseline to 12 months, measured using optical coherence tomography (OCT).
Mean change in quality of life from baseline to 12 months, measured using a validated instrument.
Measurements at varying lengths of follow‐up were pooled at annual intervals, plus or minus six months, the primary analysis being that at 12 months. The time point closer to 12 months, or the latest time point in the window frame in the case of symmetry, was chosen where multiple time points were available.
Adverse events
The following adverse events were considered.
All‐cause mortality.
Arterial thromboembolic events (ATC 1994).
Systemic serious adverse events (SSAEs).
Adverse events were analysed at the longest available follow‐up time (Moja 2014).
Search methods for identification of studies
Electronic searches
The Cochrane Eyes and Vision Information Specialist conducted systematic searches in the following databases for randomised controlled trials and controlled clinical trials. There were no language or publication year restrictions. The date of the search was 26 April 2017.
Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 3) (which contains the Cochrane Eyes and Vision Trials Register) in the Cochrane Library (searched 26 April 2017) (Appendix 1);
MEDLINE Ovid (1946 to 26 April 2017) (Appendix 2);
Embase Ovid (1980 to 26 April 2017) (Appendix 3);
LILACS (Latin American and Caribbean Health Science Information database (1982 to 26 April 2017) (Appendix 4);
ISRCTN registry (www.isrctn.com/editAdvancedSearch; searched 26 April 2017) (Appendix 5);
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (www.clinicaltrials.gov; searched 26 April 2017) (Appendix 6);
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (www.who.int/ictrp; searched 26 April 2017) (Appendix 7).
Searching other resources
We handsearched the reference lists of the included trials for other possible trials. We accessed the Novartis Clinical Trials database (www.novctrd.com/ctrdWebApp/clinicaltrialrepository/public/main.jsp) on 28 May 2014 and checked all trials indexed under the headings: Ophthalmic Disorders and ranibizumab.
Data collection and analysis
Selection of studies
Two review authors independently selected the studies for inclusion. The titles and abstracts of all reports identified by the electronic searches and handsearching were examined by the review authors. We classified the abstracts as (a) definitely include, (b) unsure and (c) definitely exclude. We obtained and re‐assessed full‐text copies of those classified as either (a) definitely include or (b) unsure. Having reviewed the full‐text copies, we classified the studies as (1) included, (2) awaiting assessment and (3) excluded. Studies identified by both review authors as 'excluded' were excluded and documented in the review. Studies identified as 'included' were included and assessed for methodological quality. The review authors were unmasked to the report authors, institutions and trial results during this assessment. Disagreements between the two review authors were resolved by a third review author.
Data extraction and management
Two review authors independently extracted the data for the primary and secondary outcomes onto paper data extraction forms developed by the Cochrane Eyes and Vision Group. A pilot test of this form was carried out using a small number of studies. We resolved discrepancies by discussion. One review author entered all data into Review Manager 5 (Review Manager 5 2014). The entered data were checked by a second author. In case standard deviations were not available in the publication, and could not be obtained from the authors, these were imputed from standard deviations of other studies with the same comparison.
Assessment of risk of bias in included studies
Two review authors independently assessed the included trials for bias according to the methods described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011b). The following parameters were assessed: sequence generation; allocation concealment; masking (blinding) of participants, personnel and outcome assessors; incomplete outcome data; selective outcome reporting. We evaluated these parameters for each outcome measure or class of outcome measure. We classified each parameter as low risk of bias, high risk of bias or unclear.
If the information available in the published trial reports was inadequate to assess methodological quality, we contacted the trial authors for clarification. We had planned that if they did not respond within six months we would assess the trial based on the available information. However, in the latest update of this review we assessed the trial had the authors not responded within one month.
We followed Salanti 2014 to assess the risk of bias of mixed evidence (mixed evidence not defined previously).
Summary risk of bias for each trial: we considered all domains but gave more importance to allocation concealment and masking of outcome assessor.
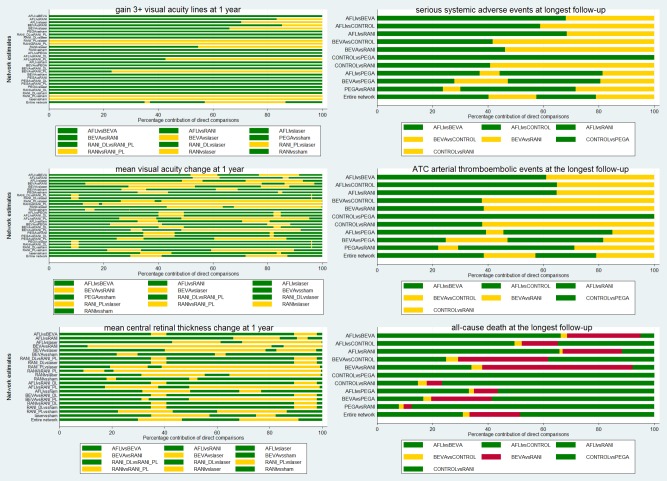
Summary risk of bias for the mixed evidence: based on the percentage contribution of each direct comparison to each network estimate using the contribution plot (Chaimani 2013).
We finally integrated the risk of bias of a given comparison with the assessment of transitivity, or similarity of the characteristics of the studies. We expected the transitivity assumption would hold as long as treatment comparisons were not related to:
acute versus chronic DMO, defined using the cut‐off of three or more years of duration;
average severity of DMO using OCT CRT of 400 micrometres as a cut‐off;
treatment regimen, such as monthly versus less than monthly and number of injections in the first year;
drug dose for ranibizumab, since this is commercially available in two doses (0.3 mg in the USA, 0.5 mg otherwise);
whether the trial was industry sponsored.
Measures of treatment effect
Data analysis followed the guidelines set out in Chapter 9 of the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). For dichotomous outcomes, we calculated a summary risk ratio (RR). For continuous outcome, we calculated the mean difference (MD). We planned to calculate a standardised mean difference (SMD) had different scales been used to measure the same continuous outcome.
We did not use ranking measures in this review, since our main interest was to compare only three drugs: aflibercept, bevacizumab and ranibizumab.
Unit of analysis issues
The unit of randomisation was the eye of individual participants. We included one cross‐over study comparing ranibizumab and bevacizumab and treated this as a parallel arm study (Wiley 2016), which equals to assume a moderate (0.5) correlation within‐person. However, relative drug safety is impossible to assess with a paired design.
We accepted studies presenting systemic adverse events as the unit of analyses, i.e. when an individual suffers from more than one severe adverse event in the study.
Dealing with missing data
Where data were missing due to dropping out of participants, we conducted a primary analysis based on participants with complete data (available case analysis). Following the guidance available in Chapter 16 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a), we considered that missing outcome data are missing at random if the reasons for loss to follow‐up are documented and judged to be unrelated to outcome in both study arms.
Assessment of reporting biases
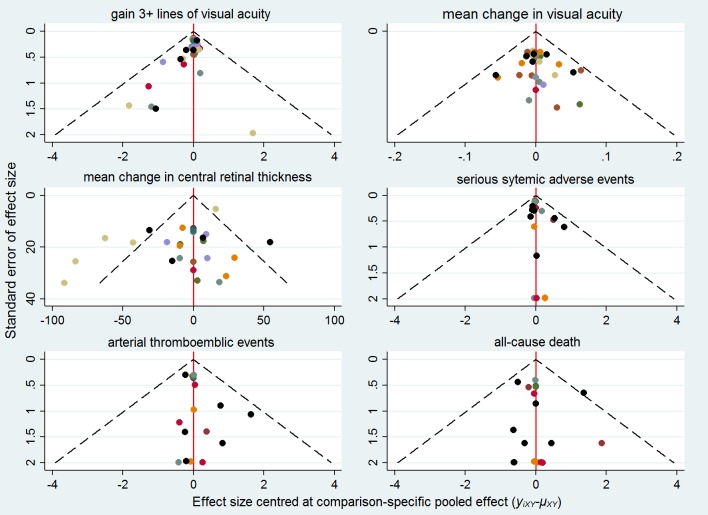
To investigate small‐study bias at the network level we employed the comparison‐adjusted funnel plot, which is an adaptation of the funnel plot. We subtracted from each study‐specific effect size the mean of meta‐analysis of the study‐specific comparison and plotted it against the study’s standard error (Chaimani 2013).
Data synthesis
Methods for direct treatment comparisons
If there was no substantial statistical heterogeneity, and if there was no clinical heterogeneity between the trials, we combined the results in a meta‐analysis using a random‐effects model. A fixed‐effect model was used if the number of trials was three or less. In the case of substantial statistical heterogeneity (that is I² value more than 50%) or clinical heterogeneity, we combined the results in a meta‐analysis using a random‐effects model if the individual trial results were all consistent in the direction of the effect (that is the RR or MD and confidence intervals largely fall on one side of the null line); when the individual trial results were inconsistent in the direction of the effect, we did not combine study results but presented a narrative or tabulated summary of each study.
Methods for indirect and mixed comparisons
We performed network meta‐analysis using the methodology of the multivariate meta‐analysis model where different treatment comparisons are treated as different outcomes (Salanti 2012). For this analysis, we used the 'network' suite of commands available in STATA (StataCorp, 2011; Stata Statistical Software: Release 14. College Station, TX) (White 2015).
We presented mixed effects as RRs or MDs against laser photocoagulation as a single comparison. We prepared league tables presenting mixed comparisons in the inferior‐left part and direct comparisons in the superior‐right part of the table in order to allow for the inspection of both types of evidence. The same information was presented graphically. We also presented the contribution of direct and indirect evidence to mixed evidence using contribution plots (Chaimani 2013).
Assessment of statistical heterogeneity
In standard pairwise meta‐analyses, we estimated heterogeneity variances for each direct comparison. We assessed statistically the presence of heterogeneity within each pairwise comparison using the I² statistic (Higgins 2011b). The I² statistic measures the percentage of variability that cannot be attributed to random error. In network meta‐analysis, we assumed a common estimate for the heterogeneity variance across the different comparisons. The assessment of statistical heterogeneity in the entire network was based on the magnitude of the heterogeneity variance parameter (τ²) estimated from the network meta‐analysis models.
Assessment of statistical inconsistency
Local approaches for evaluating inconsistency
To evaluate the presence of inconsistency locally, we used the node‐splitting approach (Dias 2010). We assumed a common heterogeneity estimate within each loop.
Global approaches for evaluating inconsistency
To check the assumption of consistency in the entire network, we used the 'design‐by‐treatment' model using the 'network' command in STATA (White 2015). This method accounts for different sources of inconsistency that can occur when studies with different designs (two‐arm trials versus three‐arm trials) give different results as well as disagreement between direct and indirect evidence. Using this approach, we judged the presence of inconsistency from any source in the entire network based on a Chi² test.
'Summary of findings' table and GRADE assessment
We prepared one 'Summary of findings' table for each relevant comparison, including all seven outcomes in a table (GRADEpro 2014). As originally intended, the primary analysis was conducted at 12 months. Relevant comparisons were identified to answer the question of which antiangiogenic drug is most effective among on‐label (aflibercept, ranibizumab) and off‐label (bevacizumab) drugs that are currently available. Because most of the available evidence is around ranibizumab, we reported on the comparison of aflibercept and bevacizumab with ranibizumab. Analyses conducted at 24 months were presented textually because a network meta‐analysis was not feasible.
We graded the certainty of the evidence for mixed estimates as explained above. We started from the premise that RCTs provide high‐certainty evidence and downgraded for each GRADE parameter to get an overall certainty for each outcome as high, moderate, low or very low (Higgins 2014; Salanti 2014; Schünemann 2011). We estimated the absolute risk in the control group from the data in the included studies as the raw proportion with event for dichotomous outcomes and the median value for continuous outcomes.
Sensitivity and subgroup analyses
We had not planned sensitivity analyses but we decided post‐hoc to conduct one excluding studies which were assessed as being at overall high or unclear risk of bias. Moreover, we acknowledge that DRCRnet 2015 emphasised that the differences in absolute benefit between aflibercept, bevacizumab and ranibizumab at one year were dependent on baseline visual acuity, but when we considered this post hoc subgroup analysis we did not find enough study data to conduct such meta‐regression analyses.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies and Characteristics of studies awaiting classification.
The previous version of this review included 18 trials. Update searches run in April 2017 yielded a further 1166 records (Figure 1). After 397 duplicates were removed, the Cochrane Information Specialist screened the remaining 769 records and removed 577 references that were not relevant to the scope of the review. We screened the remaining 192 references and obtained 19 full‐text reports for further assessment. We identified eight reports of six new trials for inclusion in the review (DRCRnet 2015, Ishibashi 2014, Lopez‐Galvez 2014, REVEAL 2015, Turkoglu 2015, Wiley 2016). A further four trials were deemed eligible but did not provide sufficient data for analysis (Chen 2016; Huang 2016; Jovanovic 2015;Fouda 2017. We have contacted these authors to ask for further information and will assess these studies if we receive additional data. We excluded one study (NCT02985619 (BEVATAAC)) and have identified six new ongoing studies and will assess these for inclusion in the review when data becomes available (NCT02194634;NCT02259088; NCT02348918; NCT02645734; NCT02699450; NCT02712008).
Figure 1.
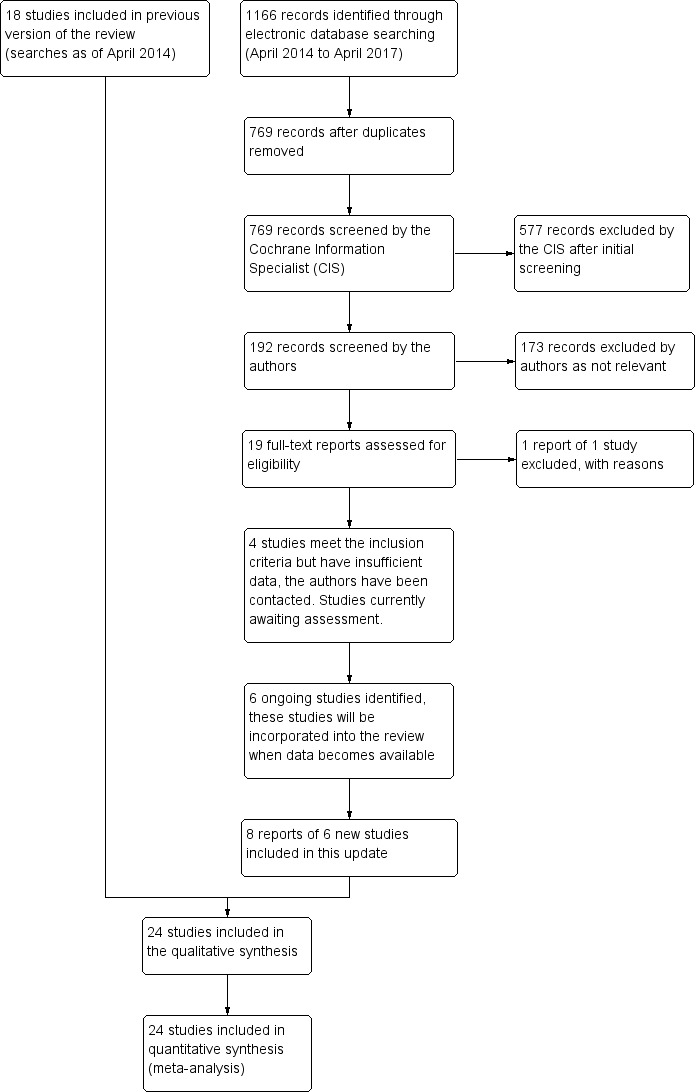
Study flow diagram.
Included studies
We included a total of 24 studies in this updated systematic review and network meta‐analysis. BOLT 2010, DA VINCI 2011, Ishibashi 2014, Korobelnik 2014, Macugen 2005, Macugen 2011, READ2 2009, RELATION 2012, RESOLVE 2010, RESPOND 2013, RESTORE 2011, and RISE‐RIDE were industry‐sponsored, multicentre RCTs conducted in the USA or Europe, whereas REVEAL 2015 was industry‐sponsored but conducted in Asia. Ahmadieh 2008, Azad 2012, Ekinci 2014, LUCIDATE 2014, Nepomuceno 2013, Soheilian 2007, and Turkoglu 2015 were independent studies conducted in Brazil, India, Iran, Turkey, and the UK, five of which included bevacizumab. DRCRnet 2010, DRCRnet 2015, Wiley 2016 were publicly‐sponsored studies, mainly by the US National Eye Institute, and conducted in the USA or UK. DRCRnet 2015 was the only large parallel‐arm study that compared all commercially available drugs (aflibercept, bevacizumab, ranibizumab) and was a large publicly‐funded trial comparing aflibercept, bevacizumab and ranibizumab with monthly monitoring and treatment as needed (PRN). Wiley 2016 was a cross‐over trial comparing the same three drugs. Lopez‐Galvez 2014 was an open‐label trial comparing ranibizumab with laser; it was conducted in Spain and results were available only in abstract form.
Only six trials maintained the randomisation scheme at two years' follow‐up (BOLT 2010; DRCRnet 2010; DRCRnet 2015; Macugen 2011; READ2 2009; RISE‐RIDE). Two industry‐sponsored trials used randomisation up to two years (Macugen 2011; RISE‐RIDE), while three others obtained follow‐up data but allowed anti‐VEGF treatment in the control arm after one year (Korobelnik 2014; RESOLVE 2010; RESTORE 2011).
We did not extract data on comparisons of antiangiogenic therapy with triamcinolone and other intravitreal steroids, which were study arms in Ahmadieh 2008, Azad 2012, DRCRnet 2010 and Soheilian 2007, for reasons presented above and also because this comparison is the subject of another Cochrane Review (Grover 2008). Standard deviations of change in CRT were imputed from other studies in REVEAL 2015.
Types of participants
Trials included participants with DMO diagnosed clinically, and often these trials used OCT for confirming macular centre involvement. Baseline visual acuity of participants was generally between 20/200 and 20/40. Most trials required a three‐ to six‐month interval from previous central or peripheral laser, and a few small studies required that participants had not received previous antiangiogenic treatment.
Types of interventions
Eleven studies assessed ranibizumab (DRCRnet 2010; Lopez‐Galvez 2014; LUCIDATE 2014; READ2 2009; RELATION 2012; RESOLVE 2010; RESPOND 2013; RESTORE 2011; REVEAL 2015; RISE‐RIDE; Turkoglu 2015), six investigated bevacizumab (Ahmadieh 2008; Azad 2012; BOLT 2010; Ekinci 2014; Nepomuceno 2013; Soheilian 2007), two pegaptanib (Macugen 2005; Macugen 2011), and three aflibercept (DA VINCI 2011; and two studies conducted in the USA and Europe using the same protocol, which we will refer to as a single study (Korobelnik 2014). DRCRnet 2015 and Wiley 2016 were the only studies comparing ranibizumab, bevacizumab or aflibercept directly. The drug dose was the same in most studies (0.5 mg ranibizumab, 1.25 mg bevacizumab, 0.3 mg pegaptanib, 2 mg aflibercept) except for RESOLVE 2010 where dose adjustment was allowed for ranibizumab, and also RISE‐RIDE, DRCRnet 2015 and Wiley 2016 where 0.3 mg ranibizumab was also delivered.
Anti‐VEGF treatment regimens were monthly in RISE‐RIDE, in one arm of Korobelnik 2014 and in Wiley 2016. Monthly, bimonthly and 'as needed' or pro re nata (PRN) regimens were adopted in four arms of DA VINCI 2011, and we selected PRN for efficacy data extraction because this is current practice with other anti‐VEGF drugs. Ahmadieh 2008 was a short‐term study which delivered only the first three injections. Most other studies adopted three initial injections followed by various maintenance regimens. Two studies on aflibercept, reported in Korobelnik 2014 (VISTA and VIVID), compared laser photocoagulation with both monthly injections (2q4) and a regimen of five initial monthly injections followed by bimonthly injections (2q8) followed by a 'treat‐and‐extend' regimen in year two.
PRN retreatment criteria were based on: visual acuity only in Nepomuceno 2013 and REVEAL 2015; OCT only in BOLT 2010, Macugen 2011 and READ2 2009; OCT and visual acuity in Azad 2012, DRCRnet 2010, DRCRnet 2015, Ekinci 2014, RESOLVE 2010 and in the PRN arm of DA VINCI 2011; inclusion of clinical examination or at the examiners' discretion in Macugen 2005, RESTORE 2011 and Soheilian 2007. They were unclear in Lopez‐Galvez 2014, RELATION 2012 and RESPOND 2013.
Types of outcomes
The data structure of our efficacy and safety outcomes can be seen in Table 5 where the sum of cases for each outcome is shown.
Table 1.
Reporting of all outcomes across studies
| Study |
Gain 3+ VA lines |
Mean VA change |
Mean CMT change |
QOL | SSAE | ATC | Death | |||||||
| Follow‐up year | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 | 1 | 2 |
| Ahmadieh 2008 | 78 | 78 | ||||||||||||
| Azad 2012 | 40 | 40 | 40 | 40 | ||||||||||
| BOLT 2010 | 80 | 65 | 80 | 65 | 80 | 65 | 80 | 80 | 80 | |||||
| DA VINCI 2011 | 89 | 89 | 87 | 89 | 89 | 89 | ||||||||
| DRCRnet 2010 | 668 | 486 | 668 | 486 | 617 | 483 | 240 | 505 | 505 | |||||
| DRCRnet 2015 | 620 | 577 | 620 | 577 | 620 | 577 | 660 | 660 | 660 | |||||
| Ekinci 2014 | 100 | 100 | 100 | 100 | 100 | |||||||||
| Ishibashi 2014 | 233 | 233 | 233 | 233 | 233 | 233 | ||||||||
| Korobelnik 2014 | 573 | 573 | 573 | 865 | 865 | 865 | ||||||||
| LUCIDATE 2014 | 33 | 33 | 33 | 33 | 33 | 33 | ||||||||
| Macugen 2005 | 86 | 86 | 86 | 86 | 86 | |||||||||
| Macugen 2011 | 260 | 207 | 260 | 236 | 286 | 286 | 286 | |||||||
| Nepomuceno 2013 | 60 | 60 | 60 | 60 | ||||||||||
| READ2 2009 | 115 | 115 | 117 | 117 | ||||||||||
| RELATION 2012 | 128 | 128 | 128 | 128 | ||||||||||
| RESOLVE 2010 | 151 | 151 | 151 | 151 | 151 | 151 | ||||||||
| RESPOND 2013 | 203 | 203 | 202 | 237 | 237 | |||||||||
| RESTORE 2011 | 343 | 343 | 343 | 299 | 345 | 345 | 345 | |||||||
| REVEAL 2015 | 390 | 390 | 268 | |||||||||||
| RISE‐RIDE | 509 | 504 | 500 | 500 | ||||||||||
| Soheilian 2007 | 87 | 85 | 85 | 96 | ||||||||||
| Turkoglu 2015 | 70 | 70 | 70 | |||||||||||
| Wiley 2016 | 124 | 124 | ||||||||||||
| Total studies | 17 | 5 | 21 | 3 | 16 | 3 | 4 | 1 | 12 | 6 | 10 | 5 | 12 | 5 |
| Total participants | 4031 | 1844 | 4489 | 1128 | 3491 | 1125 | 838 | 504 | 1598 | 2631 | 1322 | 2396 | 1639 | 2816 |
Numbers in the table are the total number of eyes for each study,as available by follow‐up year (1 or 2) and outcome measure.
Studies awaiting assessment
Several trials were included as ongoing in the previous version of this review. We checked the completion status on the study trial register and tried to contact the authors, but were not able to obtain additional information (NCT00387582; NCT00997191 (IBeTA); NCT01445899 (MATISSE); NCT01565148 (IDEAL)).
Two Chinese trials (Chen 2016, 72 participants; Huang 2016, 78 participants) compared ranibizumab plus laser or, respectively, ranibizumab to grid laser. These trials provided baseline and final CRT data at six months as well as the proportion with visual improvement, but the improvement cut‐off was unclear, as was the measurement tool.
Jovanovic 2015 included 72 participants (120 eyes) randomised to either bevacizumab (one or more injections with or without macular laser photocoagulation depending on results after four to six weeks) or macular laser alone to treat DMO. However, results were not provided at desired fixed follow‐up times by each randomisation group.
Fouda 2017 included 42 participants (70 eyes) randomised to aflibercept or ranibizumab and treated with three initial injections and then PRN. The authors did not find any significant difference between the two drugs in terms of BCVA, but used decimal rather than logMAR visual acuity and we could not use these data in analyses (authors contacted). The authors reported no difference regarding CRT and a smaller but statistically significant number of injections with aflibercept versus ranibizumab.
Excluded studies
See 'Characteristics of excluded studies' table for the list of exclusions with reasons.
Risk of bias in included studies
See 'Risk of bias in included studies'; Figure 2.
Figure 2.
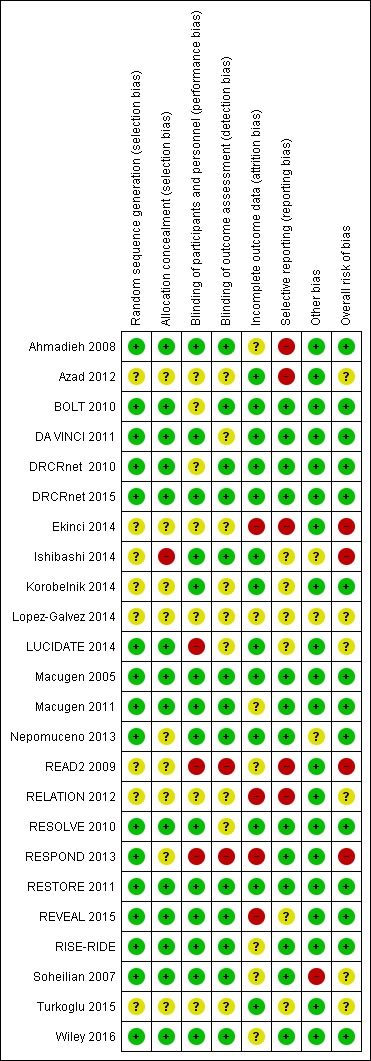
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Sequence generation was judged at low risk of bias in 12 studies and was unclear in nine (Azad 2012; Ekinci 2014; Lopez‐Galvez 2014; Ishibashi 2014; Korobelnik 2014; READ2 2009; RELATION 2012; RESPOND 2013; Turkoglu 2015). Method for allocation concealment was also unclear in these studies, as they were in Nepomuceno 2013. Allocation concealment was judged at high risk of bias in Ishibashi 2014.
Blinding
Masking of participants and outcome assessors was obtained in 14 and 12 trials respectively, and was unclear in seven and nine trials respectively. LUCIDATE 2014, READ2 2009 and RESPOND 2013 were unmasked.
Incomplete outcome data
Eleven trials were judged at low risk of attrition bias (Azad 2012; BOLT 2010; DA VINCI 2011; DRCRnet 2010; DRCRnet 2015; Korobelnik 2014; LUCIDATE 2014; Macugen 2005; Nepomuceno 2013; RESOLVE 2010; RESTORE 2011); and eight trials were judged at unclear risk of bias in which some participants were missing but reasons for missingness were not fully reported (Ahmadieh 2008; Ishibashi 2014; Macugen 2011; READ2 2009; RISE‐RIDE; Soheilian 2007; Turkoglu 2015; Wiley 2016). Five trials were judged at high risk of attrition bias: Ekinci 2014 excluded 15 participants after randomisation due to ocular and systemic complications; Lopez‐Galvez 2014 lost about 20% of participants in each arm and did not report the reasons; RELATION 2012, RESPOND 2013 and REVEAL 2015 lost many more participants in the laser arm than in the ranibizumab arms.
Selective reporting
Table 5 shows the reporting of all outcomes across 24 trials. Reporting was almost complete for mean VA change at one year (21 studies, 4489 complete cases). Mean CRT change was available in 16 studies (3491 cases). Gain of 3 or more VA lines was reported at one year in 17 studies (4031 cases). SSAEs at one or two years were reported from 18 studies (4229 cases). ATC thromboembolic events were reported in 15 (3718 cases) and death in 17 (4455 cases).
Other potential sources of bias
The baseline visual acuity was not balanced in Soheilian 2007; the visual acuity was around 20/100 in the bevacizumab and bevacizumab‐triamcinolone arms and 20/70 in the laser arm, suggesting that milder CSMO was included in the laser arm. The trial investigators adjusted for baseline values in the analyses, which also took into account the within‐participant correlation (150 eyes of 129 participants, 16% of participants with both eyes in the analyses). However, we could not take within‐participant correlation into account when analysing dichotomous visual acuity.
Three studies included both eyes of some participants in analyses: Ahmadieh 2008 14 out of 101 participants; Nepomuceno 2013 15 out of 48 participants; Wiley 2016 6 out of 56 participants.
RELATION 2012 was terminated early when ranibizumab was approved for DMO in Germany. Early termination was unlikely to be associated with treatment effect.
Effects of interventions
See: Table 1; Table 2; Table 3
Antiangiogenic drugs versus laser photocoagulation or control: efficacy and safety
Table 1 presents the evidence on the comparison of each drug with laser photocoagulation (efficacy at one year) or control (laser photocoagulation or sham at the longest available follow‐up of one or two years).
Efficacy at one year
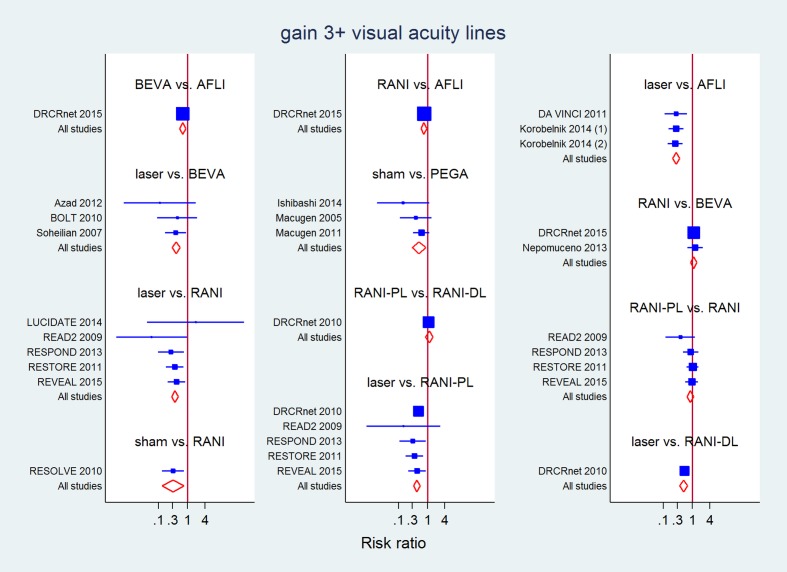
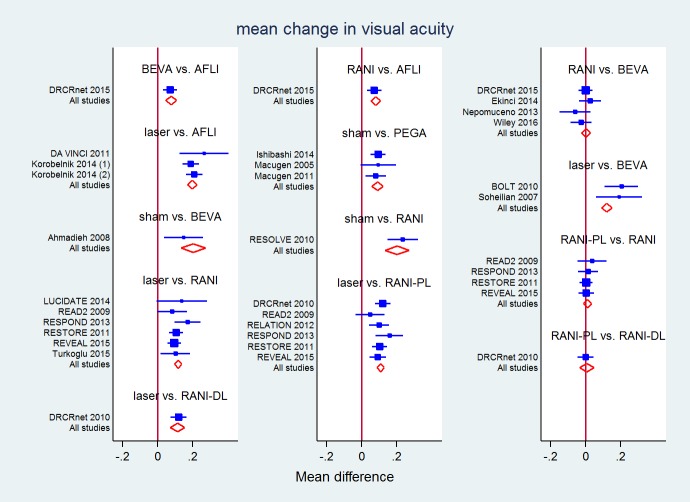
As found in the previous version of this review based on direct meta‐analyses (Virgili 2014), there was high‐certainty of evidence of benefit for aflibercept, bevacizumab and ranibizumab compared to laser photocoagulation at one year. Specifically, aflibercept, bevacizumab and ranibizumab were all more effective than laser for improving vision by 3 or more lines after one year, since about one in 10 people improve vision with laser, and about three in 10 people improve with anti‐VEGF treatment: risk ratio (RR) versus laser was 3.66 (95% CI 2.79 to 4.79) for aflibercept; 2.47 (95% CI 1.81 to 3.37) for bevacizumab; and 2.76 (95% CI 2.12 to 3.59) for ranibizumab. Regarding change of mean BCVA, on average there was no change with laser after one year, compared with a gain of 1 or 2 lines with anti‐VEGF treatment: laser versus aflibercept mean difference (MD) −0.20 (95% CI −0.22 to −0.17) logMAR; versus bevacizumab −0.12 (95% CI −0.15 to −0.09) logMAR; versus ranibizumab −0.12 (95% CI −0.14 to −0.10) logMAR (negative logMAR in favour of anti‐VEGF group).
The certainty of evidence was moderate for bevacizumab versus laser regarding mean BCVA change due to inconsistency of direct and indirect evidence.
Safety at the longest available follow‐up
This network meta‐analysis confirms that aflibercept, bevacizumab and ranibizumab do not increase the risk of all SSAEs compared to laser photocoagulation or sham at one year. We considered this evidence of high‐certainty. (Table 1), Of notice, SSAEs are a generic indicator of harm, mostly including hospitalisation or death for any cause and unrelated to antiangiogenic effect.
Regarding 'Antiplatelet Trialists Collaboration arterial thromboembolic events' and all‐cause death, no statistically significant difference was found between any anti‐VEGF drug and control, but the certainty of the evidence was generally low due to imprecision (large 95% CIs).
Quality of life
Only RESTORE 2011, RESPOND 2013 and Turkoglu 2015 presented quality of life data for ranibizumab versus laser photocoagulation at six to 12 months (3 studies, 412 participants). Ranibizumab improved NEI‐VFQ composite score by 5.14 units (95% CI 2.96 to 7.32) compared to laser (Table 1). The certainty of the evidence was moderate due to risk of bias issues (RESPOND 2013 was unmasked and Turkoglu 2015 was unclear for most items).
Macugen 2011 obtained QOL data at two years and we did not include these data since pegaptanib was not of direct interest and sham, rather than laser, was the comparator. RISE‐RIDE obtained QOL data at two years and was not included since sham, rather than laser, was the control group.
Ranibizumab versus aflibercept and bevacizumab
Efficacy at one year
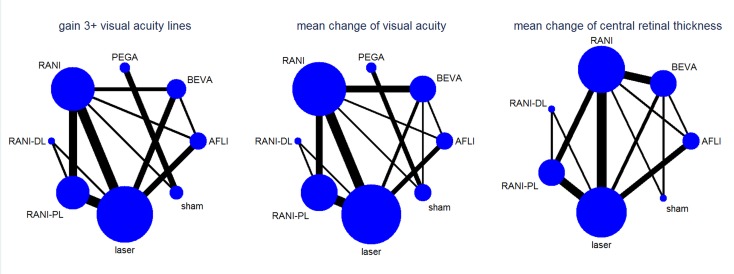
Table 6 presents the number of studies (participants/eyes) in all treatment arms of the network for the efficacy outcomes at one year. Figure 3 presents the corresponding networks' structure. As seen, more data was available for ranibizumab, alone or combined with laser, with respect to aflibercept and bevacizumab. Figure 4, Figure 5 and Figure 6 present forest plots with effects for each study, estimates from direct pairwise meta‐analysis and mixed estimate from the network meta‐analysis. Table 2 and Table 3 present comparisons of ranibizumab versus aflibercept and bevacizumab.
Table 2.
Network structure: efficacy at 12 months
| Laser | Aflibercept | Bevacizumab | Pegaptanib | Ranibizumab | Ranibizumab deferredlaser | Ranibizumab prompt laser | Sham | Overall | |
| Gain 3+ lines | 12 | 4 | 5 | 3 | 8 | 1 | 5 | 3 | 17 |
| 1074 | 539 | 344 | 410 | 713 | 188 | 545 | 218 | 4031 | |
| Mean VA change | 13 | 4 | 7 | 3 | 11 | 1 | 6 | 4 | 21 |
| 1131 | 539 | 476 | 410 | 861 | 188 | 629 | 255 | 4489 | |
| Mean CRT change | 11 | 4 | 7 | 10 | 1 | 4 | 2 | 16 | |
| 986 | 538 | 476 | 779 | 175 | 451 | 86 | 3491 | ||
| QOL | 4 | 4 | |||||||
| 838 | 838 |
For each efficacy outcome, numbers in the table are the total number of studies (upper line for each outcome) and the total number of eyes (lower line for each outcome), as available by treatment and measured at one year.
Figure 3.
Network structure for efficacy outcomes at 1 year
Figure 4.
All direct and mixed comparisons: gain of 3 or more lines of visual acuity at 1 year
Figure 5.
All direct and mixed comparisons: mean change in visual acuity at 1 year
Figure 6.
All direct and mixed comparisons: mean change in central retinal thickness at 1 year (micron)
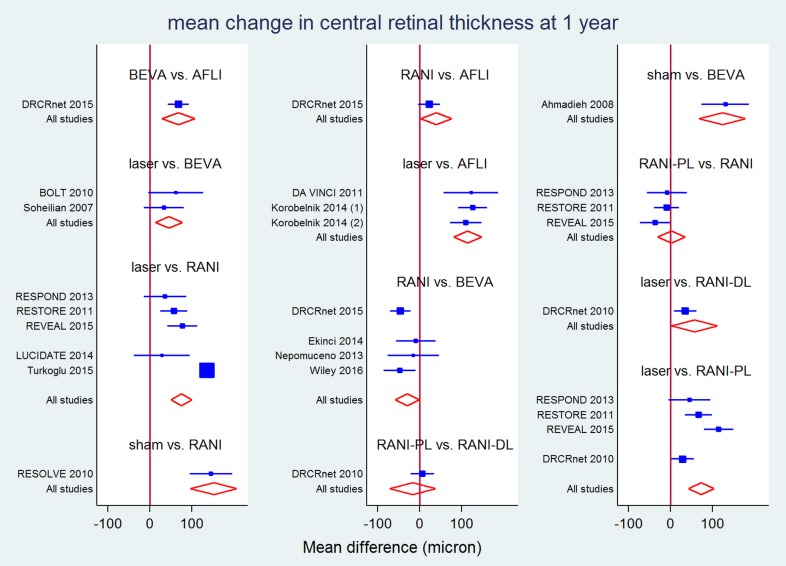
Comparing the available drugs as monotherapy, all efficacy outcomes significantly favoured aflibercept over ranibizumab and bevacizumab (Table 7; Table 8; Table 9). Compared with ranibizumab and bevacizumab, aflibercept increased the chances of gaining 3 or more lines (17 studies, 4031 eyes) by about 30%, since the RR for gain was 0.75 (95% CI 0.60 to 0.94) and 0.68 (95% CI 0.53 to 0.86) versus ranibizumab and bevacizumab, respectively. The corresponding figures for mean BCVA change (21 studies, 2689 eyes) were a difference of 0.08 (95% CI 0.05 to 0.11) logMAR and 0.08 (95% CI 0.05 to 0.11) logMAR and were 38.90 (95% CI 2.27 to 75.52) micron and 68.32 (95% CI 28.69 to 107.96) micron for CRT change (16 studies, 3491 eyes), all favouring aflibercept.
Table 3.
Gain of 3 or more lines of visual acuity at 12 months: direct (upper‐right triangle) and mixed (lower‐left triangle) estimates
| LASER | 3.82 (2.61 to 5.58) | 2.74 (1.34 to 5.61) | 2.82 (1.82 to 4.38) | 1.88 (1.31 to 2.70) | 2.30 (1.74 to 3.03) | ||
| 3.66 (2.79 to 4.79) | AFLI | 0.68 (0.52 to 0.90) | 0.77 (0.59 to 0.99) | ||||
| 2.47 (1.81 to 3.37) | 0.68 (0.53 to 0.86) | BEVA | 1.14 (0.88 to 1.48) | ||||
| 1.70 (0.58 to 4.94) | 0.46 (0.16 to 1.34) | 0.69 (0.24 to 1.89) | PEGA | 0.51 (0.30 to 0.89) | |||
| 2.76 (2.12 to 3.59) | 0.75 (0.60 to 0.94) | 1.11 (0.87 to 1.43) | 1.62 (0.58 to 4.57) | RANI | 0.90 (0.67 to 1.21) | 0.31 (0.13 to 0.76) | |
| 2.02 (1.46 to 2.81) | 0.55 (0.37 to 0.82) | 0.82 (0.54 to 1.24) | 1.19 (0.40 to 3.58) | 0.73 (0.51 to 1.06) | RANI‐DL | 1.10 (0.80 to 1.51) | |
| 2.33 (1.81 to 3.00) | 0.64 (0.47 to 0.86) | 0.94 (0.68 to 1.31) | 1.37 (0.47 to 3.99) | 0.85 (0.65 to 1.09) | 1.15 (0.85 to 1.56) | RANI‐PL | |
| 0.87 (0.35 to 2.17) | 0.24 (0.10 to 0.59) | 0.35 (0.14 to 0.87) | 0.51 (0.30 to 0.89) | 0.32 (0.13 to 0.76) | 0.43 (0.17 to 1.11) | 0.37 (0.15 to 0.93) | SHAM |
P value for overall inconsistency = 0.883 in the network meta‐analysis.
Values in the table are risk ratios and 95% confidence intervals. Values in bold are ones where the 95% confidence intervals does not include 1 (null effect).
Table 4.
Mean visual acuity change at 12 months: direct (upper‐right triangle) and mixed (lower‐left triangle) estimates
| LASER | −0.20 (−0.24 to −0.17) | −0.20 (−0.28 to −0.12)a | −0.11 (−0.13 to −0.08) | −0.12 (−0.16 to −0.075) | −0.10 (−0.13 to −0.08) | ||
| −0.20 (−0.22 to −0.17) | AFLI | 0.07 (0.03 to 0.11) | 0.04 (0.00 to 0.08) | ||||
| −0.12 (−0.15 to −0.09)a | 0.08 (0.05 to 0.11) | BEVA | −0.02 (−0.05 to 0.01) | ||||
| 0.01 (−0.09 to 0.07) | 0.19 (0.11 to 0.27) | 0.11 (0.04 to 0.19) | PEGA | 0.08 (0.03 to 0.13) | |||
| −0.12 (−0.14 to −0.10) | 0.08 (0.05 to 0.11) | 0.00 (−0.02 to 0.03) | −0.11 (−0.19 to −0.04) | RANI | 0.01 (−0.02 to 0.03) | 0.23 (0.15 to 0.32) | |
| −0.11 (−0.13 to −0.09) | 0.08 (0.04 to 0.13) | 0.01 (−0.04 to 0.06) | −0.11 (−0.19 to −0.02) | 0.00 (−0.04 to 0.05) | RANI‐DL | 0.00 (−0.05 to 0.05) | |
| −0.11 (−0.14 to −0.08) | 0.09 (0.06 to 0.12) | 0.01 (−0.02 to 0.05) | −0.10 (−0.18 to −0.02) | 0.01 (−0.01 to 0.03) | 0.01 (−0.04 to 0.05) | RANI‐PL | |
| 0.08 (0.01 to 0.15) | 0.28 (0.21 to 0.35) | 0.20 (0.13 to 0.27) | 0.09 (0.06 to 0.12) | 0.20 (0.13 to 0.27) | 0.20 (0.11 to 0.28) | 0.19 (0.12 to 0.26) | SHAM |
a P value for differences between direct and indirect estimates = 0.031 in the network meta‐analysis.
P value for overall inconsistency = 0.665.
Table 5.
Mean central retinal thickness change at 12 months: direct (upper‐right triangle) and mixed (lower‐left triangle) estimates
| LASER | −119 (−143 to −95) | −44 (−82 to −5) | −71 (−120 to −22)^ | −35 (−62 to −8) | −64 (−103 to −25)b* | |
| −114 (−147 to −81) | AFLI | 68 (43 to 94) | 22 (−4 to 48) | |||
| −46 (−78 to −14) | 68 (29 to 108) | BEVA | −38 (−56 to −20) | 132 (72 to 187) | ||
| −75 (−100 to −50) | 39 (2 to 76) | −29 (−58 to −1) | RANI | −19 (−39 to 2)a | 1470 (95 to 196) | |
| −57 (−111 to −2) | 57 (−6 to 120) | −11 (−73 to 51) | 18 (−40 to 76) | RANI‐DL | 6 (−22 to 34) | |
| −72.90 (−103 to −42)b | 41 (−2 to 84) | −27 (−68 to 13) | 2 (−31 to 35)a | −16 (−71 to 38) | RANI‐PL | |
| 77 (18 to 137) | 191 (127 to 256) | 123 (67 to 179) | 153 (97 to 208) | 134 (55 to 213) | 150 (87 to 214) | SHAM |
a P value for differences between direct and indirect estimates = 0.003.
b P value for differences between direct and indirect estimates = 0.044.
* P value for heterogeneity = 0.002; I² = 80% in the direct meta‐analysis.
^ P value for heterogeneity = 0.000; I² = 91% in the direct meta‐analysis.
P value for overall inconsistency = 0.209 in the network meta‐analysis.
Ranibizumab and bevacizumab did not differ in term of functional outcomes: RR of gain 1.11 (95% CI 0.87 to 1.43) and difference in mean VA change 0.00 (95% CI −0.02 to 0.03) logMAR. However, CRT reduction favoured ranibizumab by −29.4 (95% CI −58.2 to −0.70) micron.
There was no evidence of overall statistical inconsistency in our efficacy analyses (Table 7; Table 8; Table 9). We found evidence of statistical inconsistency in one comparison (bevacizumab versus laser) for mean BCVA change and in the loop connecting ranibizumab, ranibizumab plus prompt laser and laser for mean CRT change, where two direct meta‐analyses also showed high heterogeneity in the same loop.
Mean risk of bias was low for mixed and direct comparisons among aflibercept, bevacizumab and ranibizumab for all efficacy outcomes, except for the comparison between bevacizumab and ranibizumab regarding mean BCVA change and mean CRT change, which were judged at unclear risk of bias (Figure 7).
Figure 7.
Contribution plot of mean overall study risk of bias to pairwise network estimates. Legends show the risk of bias of each direct comparison.
We had not pre‐planned any subgroup analyses and were unable to obtain data to carry out post hoc subgroup analyses by baseline BCVA. DRCRnet 2015, the only large study comparing the three drugs, found that aflibercept was superior to bevacizumab and ranibizumab for participants with lower vision (69 ETDRS letter or less or approximately 20/50 or 0.4 logMAR or worse), whereas differences between the three drugs were unimportant for participants with better vision.
Efficacy at two years
Three publicly funded studies (BOLT 2010; DRCRnet 2010; DRCRnet 2015) and two industry‐sponsored studies (Macugen 2011; RISE‐RIDE) provided data at two years. There was only one study for each comparison, making data unsuitable for a network meta‐analysis.
Only DRCRnet 2015 (complete cases: aflibercept n = 201, bevacizumab n = 185, ranibizumab n = 191) compared different antiangiogenic drugs, and found no VA differences between ranibizumab 0.3 mg and aflibercept (gain 3+ VA lines, RR 0.94, 95% CI 0.73 to 1.22; difference in mean VA change 0.01, 95% CI −0.04 to 0.06). Ranibizumab and bevacizumab did not differ in terms of gain of 3 or more VA lines (RR 0.94, 95% CI 0.72 to 1.24) but the difference in mean VA change favoured ranibizumab (mean difference −0.05, 95% CI −0.09 to 0.00), although it was not precisely estimated. Although effects on CRT favoured aflibercept over ranibizumab and ranibizumab over bevacizumab, none was statistically significant: mean difference −22 micron (95% CI −50 to 6 micron) and −23 micron (95% CI −52 to 6 micron) respectively.
We were unable to obtain data allowing subgroup analyses by baseline BCVA. DRCRnet 2015 found that such subgroup differences were attenuated at two years.
Safety at the longest available follow‐up
Table 10 presents the number of studies (participants/eyes) in the network for safety outcomes at the longest available follow‐up, and Figure 8 presents the corresponding network structure. Figure 9, Figure 10 and Figure 11 present forest plots for each study as well as their direct meta‐analysis and mixed estimates from the network meta‐analysis.
Table 6.
Network structure: safety at the longest available follow‐up
| Laser | Aflibercept | Bevacizumab | Pegaptanib | Ranibizumab | Sham | Overall | |
| SSAE | 9 | 3 | 6 | 2 | 10 | 5 | 18 |
| 1013 | 556 | 410 | 186 | 1303 | 528 | 4229 | |
| ATC* | 10 | 3 | 4 | 2 | 8 | 2 | 15 |
| 824 | 846 | 330 | 188 | 1113 | 184 | 3718 | |
| Death | 11 | 3 | 4 | 2 | 10 | 3 | 17 |
| 903 | 846 | 333 | 188 | 1521 | 434 | 4455 |
For each safety outcome, numbers in the table are the total number of studies (upper line for each outcome) and the total number of eyes (lower line for each outcome), as available by treatment and measured at the longest available follow‐up.
(*) combined incidence of (1) cardiovascular, hemorrhagic, and unknown death; (2) nonfatal MI; and (3) nonfatal stroke.
Figure 8.
Network structure for safety outcomes at 1 year
Figure 9.
All direct and mixed comparisons: serious systemic adverse events at the longest available follow‐up (1 or 2 years)
Figure 10.
All direct and mixed comparisons: arterial thromboembolic events at the longest available follow‐up (1 or 2 years)
Figure 11.
All direct and mixed comparisons: all‐cause death at the longest available follow‐up (1 or 2 years)
Two‐year data were available and reported in only four RCTs in this review. Most industry‐sponsored studies were open‐label after one year. Differently from efficacy analyses at one year, our safety analyses included data from RISE‐RIDE on ranibizumab with monthly treatments up to two years, as well as data from the monthly treatment arm (2q4) of Korobelnik 2014, which became PRN in the second year.
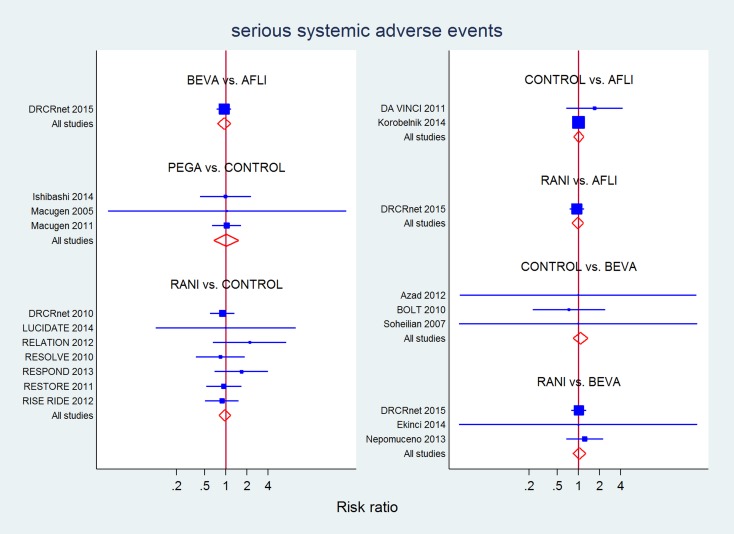
Though no analysis suggested a difference among drugs for any safety outcome, only estimates for SSAEs (18 studies, 4229 eyes) reached sufficient precision to exclude very large differences among drugs. Overall, no difference was detected in mixed evidence estimates for any drug compared to laser or sham. Moreover, RR 95% CI width excluded differences of 20% to 30% or more between aflibercept, bevacizumab and ranibizumab, while estimates for pegaptanib were less precise (Table 11). No overall (P = 0.86) or loop‐specific inconsistency was detected.
Table 7.
All serious systemic adverse events (longest available follow‐up)
| CONTROL | 0.95 (0.75 to 1.20) | 1.29 (0.43 to 3.84) | 1.02 (0.67 to 1.53) | 0.98 (0.76 to 1.25) |
| 0.98 (0.83 to 1.16) | AFLI | 0.95 (0.75 to 1.20) | 1.04 (0.83 to 1.32) | |
| 0.93 (0.73 to 1.19) | 0.95 (0.76 to 1.18) | BEVA | 0.96 (0.77 to 1.20) | |
| 1.02 (0.64 to 1.64) | 1.04 (0.63 to 1.72) | 1.09 (0.64 to 1.86) | PEGA | |
| 0.97 (0.80 to 1.17) | 0.98 (0.82 to 1.19) | 1.04 (0.84 to 1.28) | 0.95 (0.57 to 1.58) | RANI |
P value for overall inconsistency = 0.859.
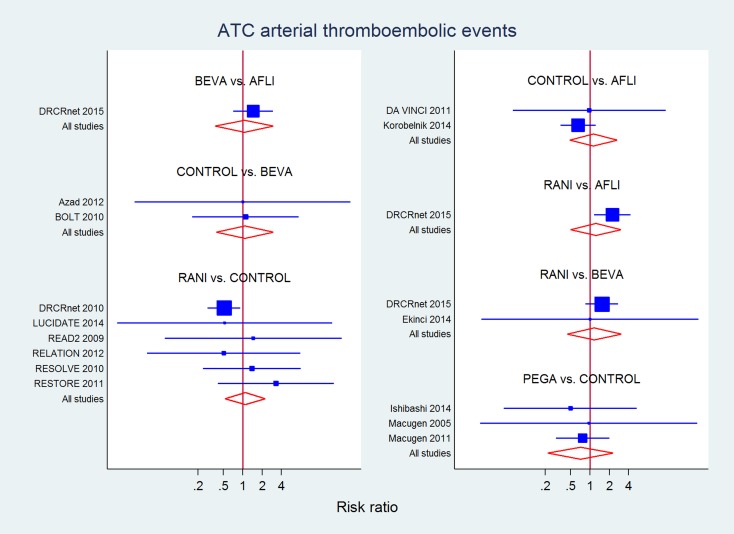
Fifteen studies (3718 eyes) contributed to this analysis on 'Antiplatelet Trialists Collaboration arterial thromboembolic events' (Table 12). No difference was detected in mixed evidence estimates for any drug compared to laser or sham or between drugs, but estimates were very imprecise. No overall inconsistency was detected (P = 0.19), but direct evidence from DRCRnet 2015 showed increased risk for ranibizumab compared to aflibercept (RR 2.26, 95% CI 1.15 to 4.23) which was larger and inconsistent with indirect evidence (P = 0.002), resulting in mixed evidence showing no difference (RR 1.25, 95% CI 0.50 to 3.05).
Table 8.
Antiplatelet Trialists Collaboration arterial thromboembolic events at the longest available follow‐up
| CONTROL | 1.50 (0.81 to 2.79) | 0.92 (0.17 to 5.12) | 0.78 (0.31 to 1.97) | 0.64 (0.38 to 1.07) |
| 0.88 (0.37 to 2.13) | AFLI | 1.46 (0.71 to 2.98) | 2.26 (1.15 to 4.23)a | |
| 0.94 (0.33 to 2.66) | 1.06 (0.36 to 3.11) | BEVA | 1.51 (0.85 to 2.69) | |
| 0.79 (0.20 to 3.02) | 0.89 (0.18 to 4.43) | 0.83 (0.15 to 4.61) | PEGA | |
| 1.09 (0.52 to 2.29) | 1.24 (0.48 to 3.19)a | 1.17 (0.43 to 3.13) | 1.17 (0.43 to 3.16) | RANI |
a P value for differences between direct and indirect estimates = 0.002.
P value for overall inconsistency = 0.274 in the network meta‐analysis.
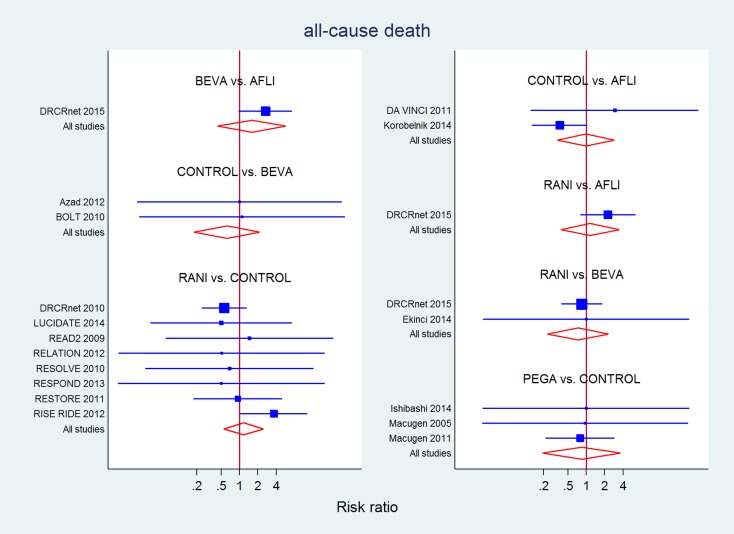
Seventeen studies (4455 eyes) contributed to the analysis of 'all‐cause mortality' (Table 13). No difference was detected for direct, indirect and mixed evidence estimates for any drug compared to laser or sham or between drugs, but estimates were imprecise.
Table 9.
All‐cause mortality at the longest available follow‐up
| CONTROL | 1.69 (0.30 to 9.42)a | 0.95 (0.06 to 14.85) | 0.82 (0.25 to 2.65) | 0.64 (0.32 to 1.25)d |
| 1.01 (0.34 to 3.03)a | AFLI | 2.67 (0.97 to 7.37)b | 2.26 (0.80 to 6.40)c | |
| 1.61 (0.45 to 5.69) | 1.59 (0.43 to 5.94)b | BEVA | 0.85 (0.40 to 1.83) | |
| 0.81 (0.16 to 4.03) | 0.81 (0.12 to 5.62) | 0.51(0.07 to 3.90) | PEGA | |
| 0.90 (0.40 to 2.01) | 1.16 (0.38 to 3.58)c | 0.73 (0.22 to 2.37) | 1.44 (0.24 to 8.48) | RANI |
a P value for differences between direct and indirect estimates = 0.011.
b P value for differences between direct and indirect estimates = 0.030.
c P value for differences between direct and indirect estimates = 0.015.
d P value for differences between direct and indirect estimates = 0.022.
P value for overall inconsistency = 0.087 in the network meta‐analysis.
Mean risk of bias was low for mixed and direct comparisons between aflibercept and ranibizumab and unclear for bevacizumab versus ranibizumab for SSAEs. Regarding ATC arterial thromboembolic events and all‐cause death, risk of bias was low for aflibercept versus ranibizumab but it was unclear or high for bevacizumab versus ranibizumab (Figure 7).
Quality of the evidence
See above for the discussion of risk of bias of mixed evidence in pairwise comparisons of interest.
Statistical heterogeneity between studies
When a direct meta‐analysis was possible, no heterogeneity of effects was found for the following outcomes: gain of 3 or more BCVA lines, mean BCVA change, SSAEs, ATC arterial thrombembolic events, death. As reported above, there was high heterogeneity in the meta‐analysis of change in CRT for the comparisons of ranibizumab with laser (I² = 91%) and ranibizumab plus deferred laser versus laser (I² = 80%), but not in other two meta‐analyses in this network.
Estimates of between‐study standard deviation τ in the network meta‐analyses suggested little heterogeneity for dichotomous outcomes, except for ATC arterial thrombembolic events when it was moderate (τ = 0.51) . Values for BCVA change and CRT change were 8‐10 logMAR and 27 micron, respectively. These values mean that heterogeneity was negligible for VA change, but was compatible with a predictive intervals width increased by at least 100 micron for the CRT change.
Similarity between studies
Table 14 shows baseline characteristics (BCVA, CRT) and the number of injections across study and treatment arms. Overall, most studies included participants with mean BCVA about 20/60 and CRT between 400 and 500 micron, which we believe sufficiently homogeneous. The number of injections was high compared with current practice (seven to 10 in year one), except for few small studies delivering a low number of injections. No heterogeneity was suspected between studies using 0.3 versus 0.5 mg ranibizumab. In the safety analyses, we included two studies with monthly injections — one arm of Korobelnik 2014 for aflibercept and RISE‐RIDE for ranibizumab — and, again, no heterogeneity seemed to arise from lower intensity regimens in other studies. Regarding sponsorship, there were fewer industry‐sponsored studies on bevacizumab, but these studies were also smaller than other studies, and the impact of such differences cannot be assessed. Finally, we did not consider the OCT model used in each study as a source of heterogeneity in CRT change since this was balanced between the arms of each study.
Table 10.
Similarity among studies. baseline values and number of injections
| Study | Participants | Interventions |
Mean n. injections |
Visual acuity (logMAR) |
Retinal thickness (µm) |
Study sponsor |
| Ahmadieh 2008 | 78 | Bevacizumab Sham |
None reported | |||
| BOLT 2010 | 80 | Bevacizumab Laser |
9* 3* |
0.59 0.61 |
507 482 |
Public |
| DA VINCI 2011 | 89 | Aflibercept Laser |
3.6 to 5.5 1.7 |
0.55 | 441 | Industry |
| DRCRnet 2010 | 668 | Laser Ranibizumab‐DL Ranibizumab‐PL |
3* 9* 8* |
0.38 0.39 0.38 |
Public | |
| DRCRnet 2015 | 620 | Aflibercept Bevacizumab Ranibizumab |
9.2 9.4 9.7 |
0.40 | 412 414 407 |
Public |
| Ekinci 2014 | 100 | Bevacizumab Ranibizumab |
5.1 6.5 |
0.22 0.24 |
484 490 |
Public |
| Ishibashi 2014 | 233 | Pegaptanib Sham |
4 | 0.56 | Industry | |
| Korobelnik 2014 | 268 | Aflibercept Laser |
8.5 2.4 |
0.50 | Industry | |
| Lopez‐Galvez 2014 | 83 | Ranibizumab Laser |
5.3 2.1 |
Industry | ||
| LUCIDATE 2014 | 33 | Laser Ranibizumab |
2.6 9 |
0.42 0.30 |
489 455 |
No details |
| Macugen 2005 | 86 | Pegaptanib Sham |
5 4.5 |
0.56 0.58 |
476 423 |
Industry |
| Macugen 2011 | 260 | Pegaptanib Sham |
8.3 8.4 |
0.56 0.58 |
442 465 |
Industry |
| RELATION 2012 | 128 | Laser Ranibizumab‐PL |
Industry | |||
| Nepomuceno 2013 | 60 | Bevacizumab Ranibizumab |
9.8 7.7 |
0.60 0.63 |
451 421 |
Public |
| READ2 2009 | 115 | Laser Ranibizumab Ranibizumab‐PL |
4.4 5.3 2.9 |
0.60 0.54 0.60 |
228 190 263 |
Industry |
| RESOLVE 2010 | 151 | Ranibizumab Sham |
10.2 8.9 |
0.50 0.48 |
455 449 |
Industry |
| RESPOND 2013 | 203 | Laser Ranibizumab Ranibizumab‐PL |
9.2 8.8 |
0.46 0.44 0.40 |
458 448 422 |
Industry |
| RESTORE 2011 | 343 | Laser Ranibizumab Ranibizumab‐PL |
7.3 7 6.8 |
0.46 0.40 0.42 |
412 427 416 |
Industry |
| REVEAL 2015 | 390 | Laser Ranibizumab Ranibizumab‐PL |
1.9 7.8 7 |
0.54 0.52 0.52 |
395 419 430 |
Industry |
| Soheilian 2007 | 85 | Bevacizumab Laser |
0.71 0.55 |
352 319 |
Public | |
| Turkoglu 2015 | 70 | Laser Ranibizumab |
0.84 0.80 |
460 488 |
None reported | |
| Wiley 2016 | 124 | Bevacizumab Ranibizumab |
3 3 |
0.42 | 477 | Public |
DL: plus deferred laser PL: plus prompt laser
(*): median, not mean, available and reported
Selective reporting
Comparison adjusted funnel plots showed some asymmetry for the outcome 'gain of 3 or more VA lines', but no specific direct comparison seemed to be affected (Figure 12). Instead, asymmetric observations to the left of the non‐significance area were seen for the outcome 'mean CRT change at one year' regarding the comparison 'ranibizumab versus laser', which also suffered high heterogeneity.
Figure 12.
Comparison‐adjusted funnel plot for all outcome measures
Overall quality of evidence for the main comparisons between drugs
Table 2 and Table 3 show summary data as well as the overall quality of evidence for the comparisons of interest in this review, based on the data reported above.
Sensitivity analyses on studies at low risk of bias
We conducted analyses of efficacy outcomes after excluding 10 studies at unclear or high risk of bias. These analyses confirmed the findings with all studies.
Gain of 3 or more BCVA lines: ranibizumab versus aflibercept RR 0.74 (95% CI 0.59 to 0.93); bevacizumab versus ranibizumab RR 0.89 (95% CI 0.69 to 1.15); no overall or comparison‐specific inconsistency.
Mean change in BCVA: ranibizumab versus aflibercept 0.08 (95% CI 0.05 to 0.12) logMAR; bevacizumab versus ranibizumab 0.0 (95% CI 0.2 to −0.03); no overall inconsistency (P = 0.130), some inconsistency for the comparisons of bevacizumab versus laser (P = 0.046) and ranibizumab versus laser (P = 0.043), same direction of effects.
Mean change in CRT: ranibizumab versus aflibercept 84 (95% CI 43 to 125) micron; bevacizumab versus ranibizumab 16 (95% CI −35 to 67) micron; overall inconsistency was detected (P = 0.012), which was due to the loop ranibizumab plus prompt laser versus ranibizumab plus deferred laser versus laser (P < 0.001 for all comparisons).
Therefore, sensitivity analyses confirmed the results of main analyses.
Discussion
Summary of main results
This review and network meta‐analysis confirms the findings of the previous version which found high‐certainty evidence that antiangiogenic therapy provides benefit over laser treatment in people with DMO at one year and concluded that further studies should compare different drugs. This update found four studies with direct comparisons between drugs and augmented this evidence with indirect comparisons in a network meta‐analysis based on 24 included studies.
At one year, all efficacy outcomes significantly favoured aflibercept over ranibizumab and bevacizumab. Aflibercept increased the chances of gaining 3 or more BCVA lines by about 30%, and conferred an advantage of between half and 1 BCVA line over the other drugs (moderate‐certainty of evidence). Ranibizumab and bevacizumab did not differ in terms of functional outcomes (moderate‐certainty of evidence), but ranibizumab was more effective in terms of CRT reduction (low‐certainty of evidence). There was no evidence of statistical inconsistency in our analyses, except for the comparison between ranibizumab and bevacizumab for mean BCVA change, but the differences between direct and indirect evidence were clinically irrelevant.
The BCVA difference between aflibercept versus ranibizumab or bevacizumab was largely below the threshold of 1 ETDRS line (five letters or 0.1 logMAR) that was used for non‐inferiority in trials on DMO (OZDRY 2015, PLACID 2013) and AMD (CATT 2011), suggesting this difference was not clinically relevant.
The previous version of this review found moderate‐certainty evidence of good safety of antiangiogenic drugs, including aflibercept, bevacizumab, ranibizumab and pegaptanib, versus control. This update found high‐certainty evidence that aflibercept, ranibizumab and bevacizumab do not differ regarding SAEs, excluding RR differences between drugs by more than 25%. However, estimates were imprecise on well‐defined hard events such as death or arterial thromboembolic events (very low‐certainty evidence).
Two‐year data on direct comparisons were available only for one large multicentre publicly funded study showing smaller differences between aflibercept, bevacizumab and ranibizumab as compared to one year (DRCRnet 2015).
Overall completeness and applicability of evidence
This review confirms and enhances the findings of DRCRnet 2015: that aflibercept confers some advantage over bevacizumab and ranibizumab at one year. Two‐year data were available and reported in only four RCTs in this review. Most industry sponsored studies were open‐label after one year. Thus, long‐term outcomes have to be inferred from observational trials.
We could not investigate subgroup effects in this review due to lack of subgroup data. DRCRnet 2015 found the relative benefit with aflibercept versus ranibizumab and bevacizumab is larger when visual acuity is lower than about 20/50, and modest above this level. Moreover, we could not investigate the potential effect of differences in dose and regimen. Nonetheless, our review includes studies with a broad range of characteristics, but without major differences in populations. Particularly, 0.3 mg ranibizumab was used in the direct comparison with aflibercept and bevacizumab in DRCRnet 2015; yet indirect evidence, mostly based on 0.5 mg ranibizumab, was consistent. Regarding treatment frequency: the only study on monthly 0.3 mg ranibizumab was RISE‐RIDE for indirect evidence, but this could not be included since one‐year data were not available. We excluded the monthly aflibercept treatment arms of Korobelnik 2014 for efficacy outcomes at one year since this is not the registered label.
We would like to remark that this evidence is obtained in clinical trials with high treatment and monitoring standards. A pragmatic RCT would be needed to assess the real‐world effectiveness of anti‐VEGF treatment for DMO, since it could be dependent on the adequacy of monitoring treatment response, which is also sensitive to resource constraints, as found for AMD (Pagliarini 2014). Moreover, evidence on safety from non‐randomised, real‐world data was not included in our review. As found for AMD, real‐world studies suggest that people with DMO may differ from those in RCTs (Ziemssen 2017). However there is more compelling real‐world evidence that patients are under‐treated and have less favourable outcomes than in RCTs for AMD (Chong 2016) compared to DMO (Jiang 2015; Patrao 2016).
Quality of the evidence
The quality of evidence has been presented above with reasons for downgrading shown in Table 2 and Table 3. Overall, inconsistency was not an issue in our network meta‐analyses. We also think that transitivity and generalisablilty, or indirectness according to GRADE (Schünemann 2011), were not a problem since studies included a broad range of people with DMO that resembles those in clinical practice. Minor funnel plot asymmetry was detected only for CRT change and did not involve the treatments of direct interest in this review.
The tight monitoring of participants in RCTs differs from clinical practice, where a lower number of intravitreal injections and under‐treatment are common. As for age‐related macular degeneration, this may overestimate benefit with anti‐VEGF treatment. However, effect differences among drugs may be less biased if similar regimens are compared in RCTs, although this remains presumptive.
Potential biases in the review process
Bevacizumab is an off‐label drug for treating DMO in most countries. Because small RCTs using bevacizumab may have been conducted but not published because no difference was found, we could have missed small unpublished studies.
Agreements and disagreements with other studies or reviews
Although we did not systematically search for other reviews on anti‐VEGF treatments for DMO, the previous version of this review, which focused on anti‐VEGF drugs effects compared to control, reported on other network meta‐analyses which were inconclusive (Ford 2012; MEDCAC 2012; Regnier 2014); and included a much smaller set of studies, as did Korobelnik 2015 more recently. Zhang 2016 conducted a network meta‐analysis of 21 studies of anti‐VEGF drugs versus any control, including studies on intravitreal steroids, such as dexamethasone and triamcinolone, and one retrospective comparative, non‐randomised study (Arevalo 2013). The authors concluded that aflibercept was superior to other drugs at 12 months: they found a difference of about 0.04 logMAR (2 ETDRS letters) between aflibercept and ranibizumab as well as between ranibizumab and bevacizumab, but these did not reach statistical significance, as did differences in retinal thickness. Differently from Zhang 2016, we included a larger number of studies on anti‐VEGF drugs, but not intravitreal steroids since their benefit profile, as well as their local and systemic harm profile, only partly overlap with that of anti‐VEGF drugs and similarity of studies regarding study design and target population would be less likely achieved. Currently, some investigators think intravitreal steroids are preferred in people with anti‐VEGF resistant and chronic DMO (Hussain 2015), as an alternative to switching between anti‐VEGF drugs. We suggest that a network meta‐analysis including both anti‐VEGF drugs and steroids is of interest, but a different approach should be used, specifically regarding heterogeneity of effects by time horizon and participants' subgroups.
The European Society of Retina Specialists have recently published guidelines on the management of DMO, which cover a broad spectrum of clinical questions ranging from imaging interpretation to diabetes management (EURETINA 2017). These guidelines rely on individual study results, particularly those of DRCRnet 2015 which compared aflibercept, bevacizumab and ranibizumab directly regarding anti‐VEGF drug choice. They concluded that "aflibercept is the drug of choice in DME eyes with baseline BCVA below 69 letters, as it shows superiority to bevacizumab over 2 years and over ranibizumab in the first year of treatment" and that "all three medications are equivalent in improving vision in eyes with a baseline BCVA letter score of 69 or more". Our review does not provide additional evidence regarding the effect of baseline vision on visual outcome, since few studies maintained randomisation beyond one year and subgroup data were not available to conduct a network meta‐analysis. Regarding the difference between aflibercept and ranibizumab at one year, EURETINA 2017 stated that "it remains unclear to which extent the slower effect of ranibizumab seen in Protocol T [DRCRnet 2015] compared to aflibercept can be accounted to the lower dose (0.3 mg) of ranibizumab used in this study". Our review found that the difference between aflibercept and ranibizumab was consistent with indirect evidence based on studies that mostly used ranibizumab 0.5 mg, suggesting no dose effect as previously suggested (Heier 2016).
Authors' conclusions
There is moderate‐certainty evidence that aflibercept confers some advantage in improving visual function over ranibizumab and bevacizumab in people with DMO at one year. An anatomic benefit was found with ranibizumab over bevacizumab (low‐certainty evidence), but there was little difference on functional outcomes (low‐ and moderate‐certainty evidence). Relative effects among anti‐VEGF drugs at two years are less well known, since most studies did not maintain randomisation after one year or were short term. A single large publicly‐funded trial found no differences in visual outcomes among these drugs at two years. Evidence from RCTs may not apply to real‐world practice, where people in need of antiangiogenic treatment are often under‐treated and under‐monitored.
We found no signals of differences in safety between the three antiangiogenic drugs that are currently available to treat DMO, particularly for a summary outcome measure such as the sum of all SSAEs (high‐ or moderate‐certainty evidence). However, our estimates were imprecise regarding arterial thromboembolic events and all‐cause death (very low‐certainty evidence).
Further studies should be directed to effectiveness in real‐world use and focus on monitoring and treatment regimens. A network meta‐analysis including steroids for DMO is needed, which should take into account different harms as well as account for differences in populations (e.g. regarding pseudophakic patients and chronic DMO). A network meta‐analysis of the relative safety of different antiangiogenic drugs could be conducted including people with different diseases.
Feedback
Feedback, 25 June 2013
Summary
Comments:1. In the electronic searches,did you not find the article: Lim JW, Lee HK, Shin MC. Comparison of intravitreal bevacizumab alone or combined with triamcinolone versus triamcinolone in diabetic macular edema: A randomized clinical trial. Ophthalmologica. 2012;227(2):100‐6. The article was published online: October 12, 2011, so it should have been found in the last electronic search, June 2012. I understand this article would have been excluded because of the triamcinolone comparison (it compares bevacizumab 1.25 mg versus bevacizumab 1.25 mg plus triamcinolone 2 mg versus triamcinolone 2 mg) but maybe It should appear in the 'Characteristics of excluded studies' section? 2. About the outcome results for 'Quality of life': Quality of life results should be included from the RESTORE 2011 trial. In the RESTORE 2011 trial (RESTORE 2011) data on quality of life have been reported using EQ‐5D and NEI VFQ‐25. It reported 12 months results, so it could also have been included. Mitchell P, Bandello F, Schmidt‐Erfurth U, Lang G, Massin P, Schlingemann R, et al. The RESTORE 2011 Study ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118(4):615–25. 3. In the section Effects of interventions/Anti‐VEGF versus sham treatment/ Quality of the evidence: "READ2 2009 provided visual gain, but not visual loss data". This section evaluates anti‐VEGF versus sham treatment and the READ trial is about ranibizumab versus laser. 4. For the included study: DRCRnet 2010 {published data only} Diabetic Retinopathy Clinical Research Network, Elman MJ, Aiello LP, Beck RW, Bressler NM, Bressler SB, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 2010;117(6):1064‐77. It seems that you have also considered results from this trial, from the 2011 publication for 2 years results (Analysis 3.7‐3.11): Elman MJ, Bressler NM, Qin H, Beck RW, Ferris FL 3rd, Friedman SM, et al. Expanded 2‐year follow‐up of ranibizumab plus prompt laser or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2011;118(4):609‐614. The values of “N”, total population evaluated belong to 2011 publication; the numbers are higher than those belonging to the 2010 publication. So this reference should also be cited. 5. For the included study: READ2 2009 {published data only} Nguyen QD, Shah SM, Khwaja AA, Channa R, Hatef E, Do DV, et al. Two‐year outcomes of the ranibizumab for edema of the mAcula in diabetes (READ‐2) study. Ophthalmology 2010;117(11):2146–51. The results that are considered in the review belong to the article by Nguyen 2009 (results and follow up at 6 months). Nguyen QD, Shah SM, Heier JS, Do DV, Lim J, Boyer D, et al. Primary end point (six months) results of the Ranibizumab for Edema of the mAcula in diabetes. Ophthalmology. 2009;116 (11):2175‐81. All the analyses have been done with the 6 months follow up. Because after six months all patients could be treated with ranibizumab, data were not collected beyond six months. So this reference should also be cited. 6. In the 'Characteristics of included studies' table for RISE‐RIDE, the 'outcomes' section should be completed.
7. In Tables 2, 5, 6, 7, 8 and 9 'bevacacizumab' should be corrected to 'bevacizumab'.
Reply
We thank Ruth Ubago Pérez for her comments submitted through the Feedback system in The Cochrane Library.
1. In the 'Characteristics of excluded studies' table, we have added that not only Paccola 2008, but also Lim 2012 were excluded because another Cochrane review focuses on the use of intravitreal steroids in people with diabetic macular oedema.
2. We will include quality of life data in the next review update.
3. We have removed this sentence.
4 and 5. We have added these references.
6. We have completed the 'Outcomes' section.
7. We have corrected these typos.
Contributors
Comment from Ruth Ubago Pérez, Pharmacist Technician, Andalusian Agency for Health Technology Assessment, Spain Reply from Gianni Virgili (lead author of review)
Acknowledgements
Cochrane Eyes and Vision (CEV) created and executed the search strategies. We thank Maria Diener‐West, Catey Bunce, Tianjing Li and Paolo Lanzetta for their comments on the review and Anupa Shah for her assistance throughout the review process.
Wen Xing and Catey Bunce provided visual acuity data on the BOLT 2010 study.
Dr Oliver Zeitz and Dr Christopher James (Bayer HealthCare) provided additional data regarding DA VINCI 2011.
Meagan Huggins provided clarification regarding the randomisation process in DRCRnet 2010.
Dr Oliver Comyn provided data on LUCIDATE 2014.
Dr Kana Inoue and Dr Norio Watanabe extracted data from Ishibashi 2014.
Appendices
Appendix 1. CENTRAL search strategy
#1 MeSH descriptor: [Macular Edema] explode all trees #2 macula* near/3 oedema #3 macula* near/3 edema #4 maculopath* #5 CME or CSME or CMO or CSMO #6 DMO or DME #7 #1 or #2 or #3 or #4 or #5 or #6 #8 MeSH descriptor: [Diabetes Mellitus] explode all trees #9 MeSH descriptor: [Diabetic Retinopathy] this term only #10 MeSH descriptor: [Diabetes Complications] this term only #11 diabet* #12 retinopath* #13 #8 or #9 or #10 or #11 or #12 #14 MeSH descriptor: [Angiogenesis Inhibitors] explode all trees #15 MeSH descriptor: [Angiogenesis Inducing Agents] explode all trees #16 MeSH descriptor: [Endothelial Growth Factors] explode all trees #17 macugen* or pegaptanib* or lucentis* or rhufab* or ranibizumab* or bevacizumab* or avastin* or or aflibercept* #18 anti adj2 VEGF* #19 #14 or #15 or #16 or #17 or #18 #20 #7 and #13 and #19
Appendix 2. MEDLINE Ovid search strategy
1. randomized controlled trial.pt. 2. (randomized or randomised).ab,ti. 3. placebo.ab,ti. 4. dt.fs. 5. randomly.ab,ti. 6. trial.ab,ti. 7. groups.ab,ti. 8. or/1‐7 9. exp animals/ 10. exp humans/ 11. 9 not (9 and 10) 12. 8 not 11 13. exp macular edema/ 14. (macula$ adj3 oedema).tw. 15. (macula$ adj3 edema).tw. 16. maculopath$.tw. 17. (CME or CSME or CMO or CSMO).tw. 18. (DMO or DME).tw. 19. or/13‐18 20. exp diabetes mellitus/ 21. diabetic retinopathy/ 22. diabetes complications/ 23. diabet$.tw. 24. retinopath$.tw. 25. or/20‐24 26. exp angiogenesis inhibitors/ 27. angiogenesis inducing agents/ 28. endothelial growth factors/ 29. exp vascular endothelial growth factors/ 30. (macugen$ or pegaptanib$ or lucentis$ or rhufab$ or ranibizumab$ or bevacizumab$ or avastin$ or aflibercept$).tw. 31. (anti adj2 VEGF$).tw. 32. or/26‐31 33. 19 and 25 and 32 34. 12 and 33
The search filter for trials at the beginning of the MEDLINE strategy is from the published paper by Glanville 2006.
Appendix 3. Embase Ovid search strategy
1. exp randomized controlled trial/ 2. exp randomization/ 3. exp double blind procedure/ 4. exp single blind procedure/ 5. random$.tw. 6. or/1‐5 7. (animal or animal experiment).sh. 8. human.sh. 9. 7 and 8 10. 7 not 9 11. 6 not 10 12. exp clinical trial/ 13. (clin$ adj3 trial$).tw. 14. ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. 15. exp placebo/ 16. placebo$.tw. 17. random$.tw. 18. exp experimental design/ 19. exp crossover procedure/ 20. exp control group/ 21. exp latin square design/ 22. or/12‐21 23. 22 not 10 24. 23 not 11 25. exp comparative study/ 26. exp evaluation/ 27. exp prospective study/ 28. (control$ or prospectiv$ or volunteer$).tw. 29. or/25‐28 30. 29 not 10 31. 30 not (11 or 23) 32. 11 or 24 or 31 33. exp retina macula edema/ 34. (macula$ adj3 oedema).tw. 35. (macula$ adj3 edema).tw. 36. maculopath$.tw. 37. (CME or CSME or CMO or CSMO).tw. 38. (DMO or DME).tw. 39. or/33‐38 40. exp diabetes mellitus/ 41. diabetic retinopathy/ 42. diabet$.tw. 43. retinopath$.tw. 44. or/40‐43 45. angiogenesis/ 46. exp angiogenesis inhibitors/ 47. angiogenic factor/ 48. endothelial cell growth factor/ 49. exp vasculotropin/ 50. (macugen$ or pegaptanib$ or lucentis$ or rhufab$ or ranibizumab$ or bevacizumab$ or avastin or aflibercept$).tw. 51. (anti adj2 VEGF$).tw. 52. (endothelial adj2 growth adj2 factor$).tw. 53. or/45‐52 54. 39 and 44 and 53 55. 32 and 54
Appendix 4. LILACS search strategy
macula$ edema or macula$ oedema or DMO or DME or CMO or CME or CSMO and angiogenesis or endothelial growth factor or macugen$ or pegaptanib$ or lucentis$ or rhufab$ or ranibizumab$ or bevacizumab$ or avastin or aflibercept$
Appendix 5. ISRCTN search strategy
(diabetic macular edema) AND (macugen OR pegaptanib OR lucentis OR rhufab OR ranibizumab OR bevacizumab OR avastin OR aflibercept)
Appendix 6. ClinicalTrials.gov search strategy
Diabetic Macular Edema AND (Macugen OR Pegaptanib OR Lucentis OR Rhufab OR Ranibizumab OR Bevacizumab OR Avastin OR aflibercept)
Appendix 7. WHO ICTRP search strategy
diabetic macular edema = Condition AND macugen OR pegaptanib OR lucentis OR rhufab OR ranibizumab OR bevacizumab OR avastin OR aflibercept = Intervention
Appendix 8. Dataset used in network meta‐analyses
This dataset should be pasted into an excel spreadsheet and imported using STATA 'import excel' command
| study | outcome | year | arm | events | total | mean | sd | riskofbias | comments |
| Ahmadieh 2008 | cmtchange | 1 | BEVA | 41 | ‐95,7 | 172,5 | low | ||
| Ahmadieh 2008 | cmtchange | 1 | sham | 37 | 34,9 | 63,9 | low | ||
| Ahmadieh 2008 | vachange | 1 | BEVA | 41 | ‐0,18 | 0,26 | low | ||
| Ahmadieh 2008 | vachange | 1 | sham | 37 | ‐0,03 | 0,24 | low | ||
| Azad 2012 | ATC | 1 | BEVA | 0 | 20 | unclear | |||
| Azad 2012 | ATC | 1 | laser | 0 | 20 | unclear | |||
| Azad 2012 | death | 1 | BEVA | 0 | 20 | unclear | |||
| Azad 2012 | death | 1 | laser | 0 | 20 | unclear | |||
| Azad 2012 | gain3+ | 1 | BEVA | 4 | 20 | unclear | |||
| Azad 2012 | gain3+ | 1 | laser | 0 | 20 | unclear | |||
| Azad 2012 | SSAE | 1 | BEVA | 0 | 20 | unclear | |||
| Azad 2012 | SSAE | 1 | laser | 0 | 20 | unclear | |||
| BOLT 2010 | ATC | 2 | BEVA | 2 | 42 | low | |||
| BOLT 2010 | ATC | 2 | laser | 2 | 38 | low | |||
| BOLT 2010 | cmtchange | 1 | BEVA | 42 | ‐130 | 122 | low | ||
| BOLT 2010 | cmtchange | 2 | BEVA | 37 | 355 | 174 | low | ||
| BOLT 2010 | cmtchange | 1 | laser | 38 | ‐68 | 171 | low | ||
| BOLT 2010 | cmtchange | 2 | laser | 28 | 360 | 125 | low | ||
| BOLT 2010 | death | 1 | BEVA | 0 | 42 | low | |||
| BOLT 2010 | death | 1 | laser | 0 | 38 | low | |||
| BOLT 2010 | gain3+ | 1 | BEVA | 5 | 42 | low | |||
| BOLT 2010 | gain3+ | 2 | BEVA | 12 | 37 | low | |||
| BOLT 2010 | gain3+ | 1 | laser | 2 | 38 | low | |||
| BOLT 2010 | gain3+ | 2 | laser | 1 | 28 | low | |||
| BOLT 2010 | SSAE | 2 | BEVA | 6 | 42 | low | |||
| BOLT 2010 | SSAE | 2 | laser | 4 | 38 | low | |||
| BOLT 2010 | vachange | 1 | BEVA | 42 | ‐0,113 | 0,153 | low | ||
| BOLT 2010 | vachange | 2 | BEVA | 37 | 0,412 | 0,266 | low | ||
| BOLT 2010 | vachange | 1 | laser | 38 | 0,092 | 0,263 | low | ||
| BOLT 2010 | vachange | 2 | laser | 28 | 0,604 | 0,252 | low | ||
| Chen 2016 | |||||||||
| DA VINCI 2011 | ATC | 1 | AFLI | 1 | 44 | low | |||
| DA VINCI 2011 | ATC | 1 | laser | 1 | 45 | low | |||
| DA VINCI 2011 | cmtchange | 1 | AFLI | 44 | ‐180,3 | 124,4 | low | ||
| DA VINCI 2011 | cmtchange | 1 | laser | 43 | ‐58,4 | 177,6 | low | ||
| DA VINCI 2011 | death | 1 | AFLI | 0 | 44 | low | |||
| DA VINCI 2011 | death | 1 | laser | 1 | 45 | low | |||
| DA VINCI 2011 | gain3+ | 1 | AFLI | 19 | 45 | low | |||
| DA VINCI 2011 | gain3+ | 1 | laser | 5 | 44 | low | |||
| DA VINCI 2011 | SSAE | 1 | AFLI | 6 | 45 | low | |||
| DA VINCI 2011 | SSAE | 1 | laser | 10 | 44 | low | |||
| DA VINCI 2011 | vachange | 1 | AFLI | 45 | ‐0,24 | 0,222 | low | ||
| DA VINCI 2011 | vachange | 1 | laser | 44 | 0,026 | 0,414 | low | ||
| DRCRnet 2010 | ATC | 2 | laser | 17 | 130 | low | |||
| DRCRnet 2010 | ATC | 2 | RANI | 25 | 375 | low | |||
| DRCRnet 2010 | cmtchange | 1 | laser | 271 | ‐102 | 151 | low | ||
| DRCRnet 2010 | cmtchange | 2 | laser | 211 | ‐138 | 149 | low | ||
| DRCRnet 2010 | cmtchange | 1 | RANI‐DL | 175 | ‐137 | 136 | low | ||
| DRCRnet 2010 | cmtchange | 2 | RANI‐DL | 136 | ‐150 | 143 | low | ||
| DRCRnet 2010 | cmtchange | 1 | RANI‐PL | 171 | ‐131 | 129 | low | ||
| DRCRnet 2010 | cmtchange | 2 | RANI‐PL | 136 | ‐141 | 155 | low | ||
| DRCRnet 2010 | death | 2 | laser | 8 | 130 | low | |||
| DRCRnet 2010 | death | 2 | RANI | 13 | 375 | low | |||
| DRCRnet 2010 | gain3+ | 1 | laser | 43 | 293 | low | |||
| DRCRnet 2010 | gain3+ | 2 | RANI | 37 | 211 | low | |||
| DRCRnet 2010 | gain3+ | 1 | RANI‐DL | 52 | 188 | low | |||
| DRCRnet 2010 | gain3+ | 2 | RANI‐DL | 39 | 139 | low | |||
| DRCRnet 2010 | gain3+ | 1 | RANI‐PL | 57 | 187 | low | |||
| DRCRnet 2010 | gain3+ | 2 | RANI‐PL | 39 | 136 | low | |||
| DRCRnet 2010 | SSAE | 2 | laser | 24 | 74 | low | |||
| DRCRnet 2010 | SSAE | 2 | RANI | 48 | 166 | low | |||
| DRCRnet 2010 | vachange | 1 | laser | 293 | ‐0,06 | 0,26 | low | ||
| DRCRnet 2010 | vachange | 2 | laser | 211 | ‐0,06 | 0,3 | low | ||
| DRCRnet 2010 | vachange | 1 | RANI‐DL | 188 | ‐0,18 | 0,24 | low | ||
| DRCRnet 2010 | vachange | 2 | RANI‐DL | 139 | ‐0,18 | 0,28 | low | ||
| DRCRnet 2010 | vachange | 1 | RANI‐PL | 187 | ‐0,18 | 0,22 | low | ||
| DRCRnet 2010 | vachange | 2 | RANI‐PL | 136 | ‐0,14 | 0,26 | low | ||
| DRCRnet 2015 | ATC | 2 | AFLI | 12 | 224 | low | |||
| DRCRnet 2015 | ATC | 2 | BEVA | 17 | 218 | low | |||
| DRCRnet 2015 | ATC | 2 | RANI | 26 | 218 | low | |||
| DRCRnet 2015 | cmtchange | 1 | AFLI | 208 | ‐169 | 138 | low | ||
| DRCRnet 2015 | cmtchange | 2 | AFLI | 201 | ‐171 | 141 | low | ||
| DRCRnet 2015 | cmtchange | 1 | BEVA | 206 | ‐101 | 121 | low | ||
| DRCRnet 2015 | cmtchange | 2 | BEVA | 185 | ‐126 | 143 | low | ||
| DRCRnet 2015 | cmtchange | 1 | RANI | 206 | ‐147 | 134 | low | ||
| DRCRnet 2015 | cmtchange | 2 | RANI | 191 | ‐149 | 141 | low | ||
| DRCRnet 2015 | death | 2 | AFLI | 5 | 224 | low | |||
| DRCRnet 2015 | death | 2 | BEVA | 13 | 218 | low | |||
| DRCRnet 2015 | death | 2 | RANI | 11 | 218 | low | |||
| DRCRnet 2015 | gain3+ | 1 | AFLI | 87 | 208 | low | |||
| DRCRnet 2015 | gain3+ | 2 | AFLI | 78 | 201 | low | |||
| DRCRnet 2015 | gain3+ | 1 | BEVA | 59 | 206 | low | |||
| DRCRnet 2015 | gain3+ | 2 | BEVA | 64 | 185 | low | |||
| DRCRnet 2015 | gain3+ | 1 | RANI | 66 | 206 | low | |||
| DRCRnet 2015 | gain3+ | 2 | RANI | 70 | 191 | low | |||
| DRCRnet 2015 | SSAE | 2 | AFLI | 88 | 224 | low | |||
| DRCRnet 2015 | SSAE | 2 | BEVA | 81 | 218 | low | |||
| DRCRnet 2015 | SSAE | 2 | RANI | 82 | 218 | low | |||
| DRCRnet 2015 | vachange | 1 | AFLI | 208 | ‐0,266 | 0,222 | low | ||
| DRCRnet 2015 | vachange | 2 | AFLI | 201 | ‐0,256 | 0,248 | low | ||
| DRCRnet 2015 | vachange | 1 | BEVA | 206 | ‐0,194 | 0,202 | low | ||
| DRCRnet 2015 | vachange | 2 | BEVA | 185 | ‐0,2 | 0,236 | low | ||
| DRCRnet 2015 | vachange | 1 | RANI | 206 | ‐0,194 | 0,202 | low | ||
| DRCRnet 2015 | vachange | 2 | RANI | 191 | ‐0,246 | 0,21 | low | ||
| Ekinci 2014 | ATC | 1 | BEVA | 0 | 50 | high | |||
| Ekinci 2014 | ATC | 1 | RANI | 0 | 50 | high | |||
| Ekinci 2014 | cmtchange | 1 | BEVA | 50 | ‐141,5 | 121 | high | SD imputed | |
| Ekinci 2014 | cmtchange | 1 | RANI | 50 | ‐150,5 | 121 | high | SD imputed | |
| Ekinci 2014 | death | 1 | BEVA | 0 | 50 | high | |||
| Ekinci 2014 | death | 1 | RANI | 0 | 50 | high | |||
| Ekinci 2014 | SSAE | 1 | BEVA | 0 | 50 | high | |||
| Ekinci 2014 | SSAE | 1 | RANI | 0 | 50 | high | |||
| Ekinci 2014 | vachange | 1 | BEVA | 50 | ‐0,237 | 0,16 | high | SD imputed | |
| Ekinci 2014 | vachange | 1 | RANI | 50 | ‐0,211 | 0,16 | high | SD imputed | |
| Korobelnik 2014 | ATC | 2 | AFLI | 37 | 578 | low | |||
| Korobelnik 2014 | ATC | 2 | laser | 12 | 287 | low | |||
| Korobelnik 2014 | death | 2 | AFLI | 22 | 578 | low | |||
| Korobelnik 2014 | death | 2 | laser | 4 | 287 | low | |||
| Korobelnik 2014 | SSAE | 2 | AFLI | 97 | 287 | low | |||
| Korobelnik 2014 | SSAE | 2 | laser | 197 | 578 | low | |||
| Korobelnik 2014 (1) | cmtchange | 1 | AFLI | 135 | ‐192,4 | 149,9 | low | ||
| Korobelnik 2014 (1) | cmtchange | 1 | laser | 133 | ‐66,2 | 139 | low | ||
| Korobelnik 2014 (1) | gain3+ | 1 | AFLI | 45 | 135 | low | |||
| Korobelnik 2014 (1) | gain3+ | 1 | laser | 12 | 133 | low | |||
| Korobelnik 2014 (1) | vachange | 1 | AFLI | 135 | ‐0,214 | 0,186 | low | ||
| Korobelnik 2014 (1) | vachange | 1 | laser | 133 | ‐0,024 | 0,212 | low | ||
| Korobelnik 2014 (2) | cmtchange | 1 | AFLI | 151 | ‐183,1 | 153,5 | low | ||
| Korobelnik 2014 (2) | cmtchange | 1 | laser | 154 | ‐73,3 | 176,7 | low | ||
| Korobelnik 2014 (2) | gain3+ | 1 | AFLI | 47 | 151 | low | |||
| Korobelnik 2014 (2) | gain3+ | 1 | laser | 12 | 154 | low | |||
| Korobelnik 2014 (2) | vachange | 1 | AFLI | 151 | ‐0,214 | 0,164 | low | ||
| Korobelnik 2014 (2) | vachange | 1 | laser | 154 | ‐0,004 | 0,25 | low | ||
| LUCIDATE 2014 | ATC | 1 | laser | 0 | 11 | unclear | |||
| LUCIDATE 2014 | ATC | 1 | RANI | 0 | 22 | unclear | |||
| LUCIDATE 2014 | cmtchange | 1 | laser | 11 | ‐102,9 | 88,4 | unclear | ||
| LUCIDATE 2014 | cmtchange | 1 | RANI | 22 | ‐131,5 | 98 | unclear | ||
| LUCIDATE 2014 | death | 1 | laser | 1 | 11 | unclear | |||
| LUCIDATE 2014 | death | 1 | RANI | 1 | 22 | unclear | |||
| LUCIDATE 2014 | gain3+ | 1 | laser | 0 | 11 | unclear | |||
| LUCIDATE 2014 | gain3+ | 1 | RANI | 0 | 22 | unclear | |||
| LUCIDATE 2014 | SSAE | 1 | laser | 2 | 22 | unclear | |||
| LUCIDATE 2014 | SSAE | 1 | RANI | 1 | 11 | unclear | |||
| LUCIDATE 2014 | vachange | 1 | laser | 11 | 0,018 | 0,212 | unclear | ||
| LUCIDATE 2014 | vachange | 1 | RANI | 22 | ‐0,12 | 0,17 | unclear | ||
| Macugen 2005 | ATC | 1 | PEGA | 0 | 44 | low | |||
| Macugen 2005 | ATC | 1 | sham | 0 | 42 | low | |||
| Macugen 2005 | death | 1 | PEGA | 0 | 44 | low | |||
| Macugen 2005 | death | 1 | sham | 0 | 42 | low | |||
| Macugen 2005 | gain3+ | 1 | PEGA | 8 | 44 | low | |||
| Macugen 2005 | gain3+ | 1 | sham | 3 | 42 | low | |||
| Macugen 2005 | SSAE | 1 | PEGA | 0 | 42 | low | |||
| Macugen 2005 | SSAE | 1 | sham | 0 | 44 | low | |||
| Macugen 2005 | vachange | 1 | PEGA | 44 | ‐0,094 | 0,182 | low | ||
| Macugen 2005 | vachange | 1 | sham | 42 | ###### | 0,284 | low | ||
| Macugen 2011 | ATC | 2 | PEGA | 7 | 144 | low | |||
| Macugen 2011 | ATC | 2 | sham | 9 | 142 | low | |||
| Macugen 2011 | death | 2 | PEGA | 4 | 144 | low | |||
| Macugen 2011 | death | 2 | sham | 5 | 142 | low | |||
| Macugen 2011 | gain3+ | 1 | PEGA | 22 | 133 | low | |||
| Macugen 2011 | gain3+ | 2 | PEGA | 25 | 107 | low | |||
| Macugen 2011 | gain3+ | 1 | sham | 13 | 127 | low | |||
| Macugen 2011 | gain3+ | 2 | sham | 15 | 100 | low | |||
| Macugen 2011 | QOL | 1 | PEGA | 121 | 4,5 | 12,52 | low | ||
| Macugen 2011 | QOL | 1 | sham | 115 | 1,3 | 12,52 | low | ||
| Macugen 2011 | SSAE | 2 | PEGA | 28 | 144 | low | |||
| Macugen 2011 | SSAE | 2 | sham | 27 | 142 | low | |||
| Macugen 2011 | vachange | 1 | PEGA | 133 | ‐0,104 | 0,182 | low | ||
| Macugen 2011 | vachange | 1 | sham | 127 | ‐0,024 | 0,284 | low | ||
| Nepomuceno 2013 | cmtchange | 1 | BEVA | 32 | ‐126 | 121 | low | SD imputed | |
| Nepomuceno 2013 | cmtchange | 1 | RANI | 28 | ‐141 | 121 | low | SD imputed | |
| Nepomuceno 2013 | gain3+ | 1 | BEVA | 12 | 32 | low | |||
| Nepomuceno 2013 | gain3+ | 1 | RANI | 13 | 28 | low | |||
| Nepomuceno 2013 | SSAE | 1 | BEVA | 12 | 32 | low | |||
| Nepomuceno 2013 | SSAE | 1 | RANI | 13 | 28 | low | |||
| Nepomuceno 2013 | vachange | 1 | BEVA | 32 | ‐0,23 | 0,113 | low | ||
| Nepomuceno 2013 | vachange | 1 | RANI | 28 | ‐0,29 | 0,212 | low | composite NEIVFQ‐25 score | |
| READ2 2009 | ATC | 1 | laser | 0 | 38 | high | |||
| READ2 2009 | ATC | 1 | RANI | 1 | 79 | high | |||
| READ2 2009 | death | 1 | laser | 0 | 38 | high | |||
| READ2 2009 | death | 1 | RANI | 1 | 79 | high | |||
| READ2 2009 | gain3+ | 1 | laser | 0 | 38 | high | |||
| READ2 2009 | gain3+ | 1 | RANI | 8 | 37 | high | |||
| READ2 2009 | gain3+ | 1 | RANI‐PL | 3 | 40 | high | |||
| READ2 2009 | vachange | 1 | laser | 38 | ‐0,048 | 0,185 | high | composite NEIVFQ‐25 score | |
| READ2 2009 | vachange | 1 | RANI | 37 | ‐0,132 | 0,182 | high | ||
| READ2 2009 | vachange | 1 | RANI‐PL | 40 | ‐0,096 | 0,189 | high | ||
| RELATION 2012 | ATC | 1 | laser | 1 | 43 | unclear | |||
| RELATION 2012 | ATC | 1 | RANI | 1 | 85 | unclear | |||
| RELATION 2012 | death | 1 | laser | 0 | 43 | unclear | |||
| RELATION 2012 | death | 1 | RANI | 0 | 85 | unclear | |||
| RELATION 2012 | SSAE | 1 | RANI | 13 | 85 | unclear | |||
| RELATION 2012 | SSAE | 1 | sham | 3 | 43 | unclear | |||
| RELATION 2012 | vachange | 1 | laser | 43 | ‐0,03 | 0,146 | unclear | ||
| RELATION 2012 | vachange | 1 | RANI‐PL | 85 | ‐0,13 | 0,172 | unclear | ||
| RESOLVE 2010 | ATC | 1 | laser | 3 | 102 | low | |||
| RESOLVE 2010 | ATC | 1 | RANI | 2 | 49 | low | |||
| RESOLVE 2010 | cmtchange | 1 | RANI | 102 | ‐194,2 | 135,1 | low | ||
| RESOLVE 2010 | cmtchange | 1 | sham | 49 | ‐48,4 | 153,4 | low | ||
| RESOLVE 2010 | death | 1 | laser | 1 | 102 | low | |||
| RESOLVE 2010 | death | 1 | RANI | 0 | 49 | low | |||
| RESOLVE 2010 | gain3+ | 1 | RANI | 33 | 102 | low | |||
| RESOLVE 2010 | gain3+ | 1 | sham | 5 | 49 | low | |||
| RESOLVE 2010 | SSAE | 1 | RANI | 14 | 102 | low | |||
| RESOLVE 2010 | SSAE | 1 | sham | 8 | 49 | low | |||
| RESOLVE 2010 | vachange | 1 | RANI | 102 | ‐0,206 | 0,182 | low | ||
| RESOLVE 2010 | vachange | 1 | sham | 49 | 0,028 | 0,284 | low | ||
| RESPOND 2013 | cmtchange | 1 | laser | 61 | ‐107,1 | 146,8 | high | ||
| RESPOND 2013 | cmtchange | 1 | RANI | 71 | ‐143,5 | 146,4 | high | ||
| RESPOND 2013 | cmtchange | 1 | RANI‐PL | 70 | ‐152,2 | 141,9 | high | ||
| RESPOND 2013 | death | 1 | laser | 0 | 79 | high | |||
| RESPOND 2013 | death | 1 | RANI | 0 | 158 | high | |||
| RESPOND 2013 | gain3+ | 1 | laser | 4 | 62 | high | |||
| RESPOND 2013 | gain3+ | 1 | RANI | 17 | 70 | high | |||
| RESPOND 2013 | gain3+ | 1 | RANI‐PL | 15 | 71 | high | |||
| RESPOND 2013 | SSAE | 1 | laser | 6 | 79 | high | |||
| RESPOND 2013 | SSAE | 1 | RANI | 20 | 158 | high | |||
| RESPOND 2013 | vachange | 1 | laser | 62 | ‐0,006 | 0,257 | high | ||
| RESPOND 2013 | vachange | 1 | RANI | 71 | ‐0,178 | 0,159 | high | ||
| RESPOND 2013 | vachange | 1 | RANI‐PL | 70 | ‐0,164 | 0,188 | high | ||
| RESTORE 2011 | ATC | 1 | laser | 1 | 110 | low | |||
| RESTORE 2011 | ATC | 1 | RANI | 7 | 235 | low | |||
| RESTORE 2011 | cmtchange | 1 | laser | 110 | ‐61,3 | 132,3 | low | ||
| RESTORE 2011 | cmtchange | 1 | RANI | 115 | ‐118,7 | 115,1 | low | ||
| RESTORE 2011 | cmtchange | 1 | RANI‐PL | 118 | ‐128,3 | 114,3 | low | ||
| RESTORE 2011 | death | 1 | laser | 2 | 110 | low | |||
| RESTORE 2011 | death | 1 | RANI | 4 | 235 | low | |||
| RESTORE 2011 | gain3+ | 1 | laser | 9 | 110 | low | |||
| RESTORE 2011 | gain3+ | 1 | RANI | 26 | 115 | low | |||
| RESTORE 2011 | gain3+ | 1 | RANI‐PL | 27 | 118 | low | |||
| RESTORE 2011 | QOL | 1 | laser | 96 | 0,6 | 10,84 | low | ||
| RESTORE 2011 | QOL | 1 | RANI | 99 | 5 | 11,01 | low | ||
| RESTORE 2011 | QOL | 1 | RANI‐PL | 104 | 5,4 | 10 | low | ||
| RESTORE 2011 | SSAE | 1 | laser | 15 | 110 | low | |||
| RESTORE 2011 | SSAE | 1 | RANI | 30 | 235 | low | |||
| RESTORE 2011 | vachange | 1 | laser | 110 | ‐0,016 | 0,171 | low | ||
| RESTORE 2011 | vachange | 1 | RANI | 115 | ‐0,122 | 0,129 | low | ||
| RESTORE 2011 | vachange | 1 | RANI‐PL | 118 | ‐0,118 | 0,158 | low | ||
| REVEAL 2015 | cmtchange | 1 | laser | 88 | ‐57,2 | 121 | low | SD imputed | |
| REVEAL 2015 | cmtchange | 1 | RANI | 88 | ‐134,6 | 121 | low | SD imputed | |
| REVEAL 2015 | cmtchange | 1 | RANI‐PL | 92 | ‐171,8 | 121 | low | ||
| REVEAL 2015 | gain3+ | 1 | laser | 10 | 128 | low | |||
| REVEAL 2015 | gain3+ | 1 | RANI | 25 | 133 | low | |||
| REVEAL 2015 | gain3+ | 1 | RANI‐PL | 23 | 129 | low | |||
| REVEAL 2015 | vachange | 1 | laser | 128 | ‐0,036 | 0,165 | low | ||
| REVEAL 2015 | vachange | 1 | RANI | 133 | ‐0,132 | 0,154 | low | ||
| REVEAL 2015 | vachange | 1 | RANI‐PL | 129 | ‐0,128 | 0,213 | low | ||
| RISE RIDE 2012 | death | 2 | RANI | 11 | 250 | low | |||
| RISE RIDE 2012 | death | 2 | sham | 3 | 250 | low | |||
| RISE RIDE 2012 | gain3+ | 2 | RANI | 107 | 252 | low | |||
| RISE RIDE 2012 | gain3+ | 2 | sham | 39 | 257 | low | |||
| RISE RIDE 2012 | QOL | 2 | RANI | 251 | 7,7 | 12,8 | low | ||
| RISE RIDE 2012 | QOL | 2 | sham | 253 | 2,3 | 14,1 | low | ||
| RISE RIDE 2012 | SSAE | 2 | RANI | 22 | 250 | low | |||
| RISE RIDE 2012 | SSAE | 2 | sham | 25 | 250 | low | |||
| Soheilian 2007 | cmtchange | 1 | BEVA | 43 | ‐40 | 133 | unclear | ||
| Soheilian 2007 | cmtchange | 2 | BEVA | 39 | ‐24 | 137 | unclear | ||
| Soheilian 2007 | cmtchange | 1 | laser | 42 | ‐6 | 86 | unclear | ||
| Soheilian 2007 | cmtchange | 2 | laser | 38 | 2 | 108 | unclear | ||
| Soheilian 2007 | gain3+ | 1 | BEVA | 16 | 44 | unclear | |||
| Soheilian 2007 | gain3+ | 1 | laser | 6 | 43 | unclear | |||
| Soheilian 2007 | SSAE | 1 | BEVA | 0 | 48 | unclear | |||
| Soheilian 2007 | SSAE | 1 | laser | 0 | 48 | unclear | |||
| Soheilian 2007 | vachange | 1 | BEVA | 43 | ‐0,21 | 0,27 | unclear | ||
| Soheilian 2007 | vachange | 1 | laser | 42 | ‐0,02 | 0,34 | unclear | ||
| Turkoglu 2015 | cmtchange | 1 | laser | 35 | ‐6 | 24,2 | unclear | ||
| Turkoglu 2015 | cmtchange | 1 | RANI | 35 | ‐142 | 20,2 | unclear | ||
| Turkoglu 2015 | QOL | 1 | laser | 35 | 8,24 | 12,1 | unclear | ||
| Turkoglu 2015 | QOL | 1 | RANI | 35 | 12,4 | 18,2 | unclear | ||
| Turkoglu 2015 | vachange | 1 | laser | 35 | ‐0,028 | 0,188 | unclear | ||
| Turkoglu 2015 | vachange | 1 | RANI | 35 | ‐0,13 | 0,17 | unclear | ||
| Wiley 2016 | cmtchange | 1 | BEVA | 62 | ‐89 | 108,5 | low | ||
| Wiley 2016 | cmtchange | 1 | RANI | 62 | ‐137 | 108,5 | low | ||
| Wiley 2016 | vachange | 1 | BEVA | 62 | ‐0,106 | 0,169 | low | ||
| Wiley 2016 | vachange | 1 | RANI | 62 | ‐0,132 | 0,173 | low | ||
| Ishibashi 2014 | gain3+ | 1 | PEGA | 7 | 117 | 24 week follow‐up | |||
| Ishibashi 2014 | gain3+ | 1 | sham | 1 | 116 | 24 week follow‐up | |||
| Ishibashi 2014 | SSAE | 1 | PEGA | 10 | 117 | 24 week follow‐up | |||
| Ishibashi 2014 | SSAE | 1 | sham | 10 | 116 | 24 week follow‐up | |||
| Ishibashi 2014 | vachange | 1 | PEGA | 117 | ‐0,07 | 0,15 | 24 week follow‐up | ||
| Ishibashi 2014 | vachange | 1 | sham | 116 | 0,024 | 0,176 | 24 week follow‐up | ||
| Ishibashi 2014 | QOL | 1 | PEGA | 117 | 0,5 | 10,37 | composite NEIVFQ‐25 score | ||
| Ishibashi 2014 | QOL | 1 | sham | 116 | ‐1,1 | 10,85 | composite NEIVFQ‐25 score | ||
| Ishibashi 2014 | ATC | 1 | PEGA | 1 | 117 | 24 week follow‐up | |||
| Ishibashi 2014 | ATC | 1 | sham | 2 | 116 | 24 week follow‐up | |||
| Ishibashi 2014 | death | 1 | PEGA | 0 | 117 | 24 week follow‐up | |||
| Ishibashi 2014 | death | 1 | sham | 0 | 116 | 24 week follow‐up |
Appendix 9. Stata instructions to run all analyses
These instructions should be copied and pasted into a STATA command file (.do)
**DATA HAVE TO BE IMPORTED FROM EXCEL FOR EACH OUTCOME MEASURE AS INDICATED
**GAIN 3+ VISUAL ACUITY LINES AT 1 YEAR
*IMPORT DATA
set more off
keep if outcome=="gain3+"
keep if year==1
gen d=events
gen n=total
*NETWORK SETUP AND PRESENTATION
network setup d n, stud(study) trtvar(arm) numcodes armvars(drop) ref("laser") rr
network table
network map, circle title("gain 3+ visual acuity lines", size(medsmall))
graph save net1, replace
*CONSISTENCY AND INCONSISTENCY MODEL ARE FIT
network meta consistency
estimate store cons
network meta inconsistency
network sidesplit all
*THE CONSISTENCY MODEL IS ADEQUATE AND WILL BE USED
estimate restore cons
intervalplot, eform
network forest , xli(1) eform diamond xlab(.1 .3 1 4) ///
ti("gain 3+ visual acuity lines", size(medsmall))
graph save forest_1, replace
*RANKS AND SUCRA
network rank max, all line meanrank gen(prob)
sucra prob*, rankog
*ALL PAIRWISE META‐ANALYSES
gen nonD1=n1‐d1
gen nonD2=n2‐d2
gen nonD3=n3‐d3
gen nonD4=n4‐d4
gen nonD5=n5‐d5
gen nonD6=n6‐d6
gen nonD8=n8‐d8
gen nonD7=n7‐d7
metan d1 nonD1 d7 nonD7 if (d1>=0.5 & d7>=0.5), rr random nograph nointeger label(namevar=study)
metan d2 nonD2 d7 nonD7 if (d2>=0.5 & d7>=0.5), rr random nograph nointeger label(namevar=study)
metan d4 nonD4 d7 nonD7 if (d4>=0.5 & d7>=0.5), rr random nograph nointeger label(namevar=study)
metan d5 nonD5 d7 nonD7 if (d5>=0.5 & d7>=0.5), rr random nograph nointeger label(namevar=study)
metan d6 nonD6 d7 nonD7 if (d6>=0.5 & d7>=0.5), rr random nograph nointeger label(namevar=study)
metan d2 nonD2 d1 nonD1 if (d2>=0.5 & d1>=0.5), rr random nograph nointeger label(namevar=study)
metan d4 nonD4 d1 nonD1 if (d4>=0.5 & d1>=0.5), rr random nograph nointeger label(namevar=study)
metan d4 nonD4 d2 nonD2 if (d4>=0.5 & d2>=0.5), rr random nograph nointeger label(namevar=study)
metan d8 nonD8 d3 nonD3 if (d8>=0.5 & d3>=0.5), rr random nograph nointeger label(namevar=study)
metan d6 nonD6 d4 nonD4 if (d6>=0.5 & d4>=0.5), rr random nograph nointeger label(namevar=study)
metan d8 nonD8 d4 nonD4 if (d8>=0.5 & d4>=0.5), rr random nograph nointeger label(namevar=study)
metan d6 nonD6 d5 nonD5 if (d6>=0.5 & d5>=0.5), rr random nograph nointeger label(namevar=study)
* CONTRIBUTION PLOT AND RISK OF BIAS: RE‐LOAD DATA WITH TREATMENT NAMES (NOCODES OPTION)
*IMPORT DATA
set more off
keep if outcome=="gain3+"
keep if year==1
gen d=events
gen n=total
*NETWORK SETUP AND PRESENTATION
network setup d n, stud(study) trtvar(arm) nocodes armvars(drop) ref("laser") rr
network convert pairs
netweight _y _std _t1 _t2
netweight _y _std _t1 _t2 , bar(by risk mean) aspect(.5)
graph save bias1, replace
*FUNNEL PLOT
netfunnel _y _std _t1 _t2, bycomparison
graph save fun1, replace
**CHANGE IN MEAN VA AT 1 YEAR
*IMPORT DATA
set more off
keep if outcome=="vachange"
keep if year==1
gen n=total
*NETWORK SETUP AND PRESENTATION
network setup mean sd n, stud(study) trtvar(arm) nocodes armvars(drop) ref("laser") md
network table
network map, circle ti("mean change of visual acuity", size(medsmall))
graph save net2, replace
*CONSISTENCY AND INCONSISTENCY MODEL ARE FIT AND ESTIMATES ARE PRESENTED
network meta consistency
estimate store cons
network meta inconsistency
network sidesplit all
*THE CONSISTENCY MODEL IS ADEQUATE AND WILL BE USED
estimate restore cons
intervalplot, null(0) separate labels(laser AFLI BEVA PEGA RANI RANI‐DL RANI‐PL sham) ///
xlab(‐.1 0 .1 .2)
network forest , xli(0) diamond xlab(‐.2 0 .2) ///
ti("mean change in visual acuity", size(medsmall))
graph save forest_2, replace
*RANKS AND SUCRA
network rank min, zero all gen(prob)
sucra prob*, rankog
*ALL PAIRWISE META‐ANALYSES
metan nAFLI meanAFLI sdAFLI nlaser meanlaser sdlaser if (nAFLI>=0.5 & nlaser>=0.5), random nograph nointeger nostandard label(namevar=study)
metan nBEVA meanBEVA sdBEVA nlaser meanlaser sdlaser if (nBEVA>=0.5 & nlaser>=0.5), random nograph nointeger nostandard label(namevar=study)
metan nRANI meanRANI sdRANI nlaser meanlaser sdlaser if (nRANI>=0.5 & nlaser>=0.5), random nograph nointeger nostandard label(namevar=study)
metan nRANI_DL meanRANI_DL sdRANI_DL nlaser meanlaser sdlaser if (nRANI_DL>=0.5 & nlaser>=0.5), random nograph nointeger nostandard label(namevar=study)
metan nRANI_PL meanRANI_PL sdRANI_PL nlaser meanlaser sdlaser if (nRANI_PL>=0.5 & nlaser>=0.5), random nograph nointeger nostandard label(namevar=study)
metan nBEVA meanBEVA sdBEVA nAFLI meanAFLI sdAFLI if (nBEVA>=0.5 & nAFLI>=0.5), random nograph nointeger nostandard label(namevar=study)
metan nRANI meanRANI sdRANI nAFLI meanAFLI sdAFLI if (nRANI>=0.5 & nAFLI>=0.5), random nograph nointeger nostandard label(namevar=study)
metan nRANI meanRANI sdRANI nBEVA meanBEVA sdBEVA if (nRANI>=0.5 & nBEVA>=0.5), random nograph nointeger nostandard label(namevar=study)
metan nsham meansham sdsham nPEGA meanPEGA sdPEGA if (nsham>=0.5 & nPEGA>=0.5), random nograph nointeger nostandard label(namevar=study)
metan nRANI_PL meanRANI_PL sdRANI_PL nRANI meanRANI sdRANI if (nRANI_PL>=0.5 & nRANI>=0.5), random nograph nointeger nostandard label(namevar=study)
metan nsham meansham sdsham nRANI meanRANI sdRANI if (nsham>=0.5 & nRANI>=0.5), random nograph nointeger nostandard label(namevar=study)
metan nRANI_PL meanRANI_PL sdRANI_PL nRANI_DL meanRANI_DL sdRANI_DL if (nRANI_PL>=0.5 & nRANI>=0.5), random nograph nointeger nostandard label(namevar=study)
* CONTRIBUTION PLOT AND RISK OF BIAS
network convert pairs
netweight _y _std _t1 _t2
netweight _y _std _t1 _t2 , bar(by risk mean)
graph save bias2, replace
*FUNNEL PLOT
netfunnel _y _std _t1 _t2, bycomparison
graph save fun2
**MEAN CHANGE IN CENTRAL RETINAL THICKNESS(CRT)
import excel "D:\Dropbox\DTA@CEVG‐IT\DMO\analisi LELLA\dmo_nma_data_long_18nov2016.xlsx", firstrow case(lower) clear
import excel "C:\Users\user\Dropbox\DTA@CEVG‐IT\DMO\analisi LELLA\dmo_nma_data_long_18nov2016.xlsx", firstrow case(lower) clear
set more off
keep if outcome=="cmtchange"
keep if year==1
gen n=total
*NETWORK SETUP AND PRESENTATION
network setup mean sd n, stud(study) trtvar(arm) nocodes armvars(drop) ref("laser") md
network table
network map, circle ti("mean change of central retinal thickness", size(medsmall))
graph save net3, replace
*CONSISTENCY AND INCONSISTENCY MODEL ARE FIT AND ESTIMATES ARE PRESENTED
network meta consistency
estimate store cons
network meta inconsistency
network sidesplit all
*THE CONSISTENCY MODEL IS ADEQUATE AND WILL BE USED
estimate restore cons
intervalplot, null(0) separate labels(laser AFLI BEVA RANI RANI‐DL RANI‐PL sham) ///
xlab(‐.1 0 .1 .2) predict
network forest , xli(0) diamond xlab(‐100 0 100) ///
ti("mean change in central retinal thickness at 1 year", size(medsmall))
graph save forest_3, replace
*RANKS AND SUCRA
network rank min, zero all gen(prob)
sucra prob*, rankog
*ALL PAIRWISE META‐ANALYSES
metan nAFLI meanAFLI sdAFLI nlaser meanlaser sdlaser if (nAFLI>=0.5 & nlaser>=0.5), random nograph nointeger nostandard label(namevar=study)
metan nBEVA meanBEVA sdBEVA nlaser meanlaser sdlaser if (nBEVA>=0.5 & nlaser>=0.5), random nograph nointeger nostandard label(namevar=study)
metan nRANI meanRANI sdRANI nlaser meanlaser sdlaser if (nRANI>=0.5 & nlaser>=0.5), random nograph nointeger nostandard label(namevar=study)
metan nRANI_DL meanRANI_DL sdRANI_DL nlaser meanlaser sdlaser if (nRANI_DL>=0.5 & nlaser>=0.5), random nograph nointeger nostandard label(namevar=study)
metan nRANI_PL meanRANI_PL sdRANI_PL nlaser meanlaser sdlaser if (nRANI_PL>=0.5 & nlaser>=0.5), random nograph nointeger nostandard label(namevar=study)
metan nBEVA meanBEVA sdBEVA nAFLI meanAFLI sdAFLI if (nBEVA>=0.5 & nAFLI>=0.5), random nograph nointeger nostandard label(namevar=study)
metan nRANI meanRANI sdRANI nAFLI meanAFLI sdAFLI if (nRANI>=0.5 & nAFLI>=0.5), random nograph nointeger nostandard label(namevar=study)
metan nRANI meanRANI sdRANI nBEVA meanBEVA sdBEVA if (nRANI>=0.5 & nBEVA>=0.5), random nograph nointeger nostandard label(namevar=study)
metan nRANI_PL meanRANI_PL sdRANI_PL nRANI meanRANI sdRANI if (nRANI_PL>=0.5 & nRANI>=0.5), random nograph nointeger nostandard label(namevar=study)
metan nsham meansham sdsham nRANI meanRANI sdRANI if (nsham>=0.5 & nRANI>=0.5), random nograph nointeger nostandard label(namevar=study)
metan nRANI_PL meanRANI_PL sdRANI_PL nRANI_DL meanRANI_DL sdRANI_DL if (nRANI_PL>=0.5 & nRANI>=0.5), random nograph nointeger nostandard label(namevar=study)
* CONTRIBUTION PLOT AND RISK OF BIAS
network convert pairs
netweight _y _std _t1 _t2
netweight _y _std _t1 _t2 , bar(by riskofbias mean)
graph save bias3, replace
*FUNNEL PLOT
netfunnel _y _std _t1 _t2, bycomparison
graph save fun3, replace
** SERIOUS SYSTEMIC ADVERSE EVENTS (SSAE) AT THE LONGEST AVAILABLE FOLLOW‐UP
*IMPORT DATA
set more off
keep if outcome=="SSAE"
gen d=events
gen n=total
replace arm= "CONTROL" if arm=="laser"
replace arm= "CONTROL" if arm=="sham"
*NETWORK SETUP AND PRESENTATION
network setup d n, stud(study) trtvar(arm) numcodes armvars(drop) ref("CONTROL") rr
network table
network map, circle title("serious systemic adverse events", size(medsmall))
graph save net4, replace
*CONSISTENCY AND INCONSISTENCY MODEL ARE FIT AND ESTIMATES ARE PRESENTED
network meta consistency
estimate store cons
network meta inconsistency
network sidesplit all
*THE CONSISTENCY MODEL IS ADEQUATE AND WILL BE USED
estimate restore cons
intervalplot, eform null(1) separate labels(CONTROL AFLI BEVA PEGA RANI ) ///
xlab(.2 1 5)
network forest , eform xlab( .2 .5 1 2 4) xli(1) diamond ///
ti("serious systemic adverse events", size(medsmall))
graph save forest_4
*RANKS AND SUCRA
network rank min, zero all gen(prob)
sucra prob*, rankog
*ALL PAIRWISE META‐ANALYSES
gen nonD1=n1‐d1
gen nonD2=n2‐d2
gen nonD3=n3‐d3
gen nonD4=n4‐d4
gen nonD5=n5‐d5
metan d1 nonD1 d5 nonD5 if (d1>=0.5 & d5>=0.5), rr random nograph nointeger label(namevar=study)
metan d2 nonD2 d5 nonD5 if (d2>=0.5 & d5>=0.5), rr random nograph nointeger label(namevar=study)
metan d4 nonD4 d5 nonD5 if (d4>=0.5 & d5>=0.5), rr random nograph nointeger label(namevar=study)
metan d2 nonD2 d1 nonD1 if (d2>=0.5 & d1>=0.5), rr random nograph nointeger label(namevar=study)
metan d4 nonD4 d1 nonD1 if (d4>=0.5 & d1>=0.5), rr random nograph nointeger label(namevar=study)
metan d4 nonD4 d2 nonD2 if (d4>=0.5 & d2>=0.5), rr random nograph nointeger label(namevar=study)
metan d4 nonD4 d3 nonD3 if (d2>=0.5 & d1>=0.5), rr random nograph nointeger label(namevar=study)
metan d5 nonD5 d3 nonD3 if (d5>=0.5 & d3>=0.5), rr random nograph nointeger label(namevar=study)
metan d5 nonD5 d4 nonD4 if (d5>=0.5 & d4>=0.5), rr random nograph nointeger label(namevar=study)
* CONTRIBUTION PLOT AND RISK OF BIAS: RE‐LOAD DATA WITH TREATMENT NAMES (NOCODES OPTION)
*IMPORT DATA
set more off
keep if outcome=="SSAE"
gen d=events
gen n=total
replace arm= "CONTROL" if arm=="laser"
replace arm= "CONTROL" if arm=="sham"
network setup d n, stud(study) trtvar(arm) nocodes armvars(drop) ref("CONTROL") rr
network convert pairs
netweight _y _std _t1 _t2
netweight _y _std _t1 _t2 , bar(by risk mean)
graph save bias4, replace
*FUNNEL PLOT
netfunnel _y _std _t1 _t2, bycomparison
graph save fun4
** ATC ARTERIAL THROM BOEMBOLIC EVENTS
*IMPORT DATA
set more off
keep if outcome=="ATC"
gen d=events
gen n=total
replace arm= "CONTROL" if arm=="laser"
replace arm= "CONTROL" if arm=="sham"
*NETWORK SETUP AND PRESENTATION
network setup d n, stud(study) trtvar(arm) nocodes armvars(drop) ref("CONTROL") rr
network table
network map, circle title("ATC arterial thromboembolic events", size(medsmall))
graph save net5, replace
*CONSISTENCY AND INCONSISTENCY MODEL ARE FIT AND ESTIMATES ARE PRESENTED
network meta consistency
estimate store cons
network meta inconsistency
network sidesplit all
*THE CONSISTENCY MODEL IS ADEQUATE AND WILL BE USED
estimate restore cons
intervalplot, eform null(1)
network forest , eform xlab( .2 .5 1 2 4) xli(1) diamond ///
ti("ATC arterial thromboembolic events", size(medsmall))
graph save forest_5, replace
*ALL PAIRWISE META‐ANALYSES
gen nonD1=n1‐d1
gen nonD2=n2‐d2
gen nonD3=n3‐d3
gen nonD4=n4‐d4
gen nonD5=n5‐d5
metan d5 nonD5 d1 nonD1 if (d1>=0.5 & d5>=0.5), rr random nograph nointeger label(namevar=study)
metan d5 nonD5 d2 nonD2 if (d2>=0.5 & d5>=0.5), rr random nograph nointeger label(namevar=study)
metan d5 nonD5 d3 nonD3 if (d3>=0.5 & d5>=0.5), rr random nograph nointeger label(namevar=study)
metan d2 nonD2 d1 nonD1 if (d2>=0.5 & d1>=0.5), rr random nograph nointeger label(namevar=study)
metan d1 nonD1 d3 nonD3 if (d3>=0.5 & d1>=0.5), rr random nograph nointeger label(namevar=study)
metan d2 nonD2 d3 nonD3 if (d3>=0.5 & d2>=0.5), rr random nograph nointeger label(namevar=study)
metan d4 nonD4 d2 nonD2 if (d4>=0.5 & d2>=0.5), rr random nograph nointeger label(namevar=study)
metan d4 nonD4 d3 nonD3 if (d4>=0.5 & d3>=0.5), rr random nograph nointeger label(namevar=study)
metan d5 nonD5 d3 nonD3 if (d5>=0.5 & d3>=0.5), rr random nograph nointeger label(namevar=study)
metan d5 nonD5 d4 nonD4 if (d5>=0.5 & d4>=0.5), rr random nograph nointeger label(namevar=study)
* CONTRIBUTION PLOT AND RISK OF BIAS: RE‐LOAD DATA WITH TREATMENT NAMES (NOCODES OPTION)
*IMPORT DATA
set more off
keep if outcome=="ATC"
gen d=events
gen n=total
replace arm= "CONTROL" if arm=="laser"
replace arm= "CONTROL" if arm=="sham"
network setup d n, stud(study) trtvar(arm) numcodes armvars(drop) ref("CONTROL") rr
network convert pairs
netweight _y _std _t1 _t2
netweight _y _std _t1 _t2 , bar(by risk mean)
graph save bias5, replace
*FUNNEL PLOT
netfunnel _y _std _t1 _t2, bycomparison
graph save fun5
**DEATH AT THE LONGEST AVAILABLE FOLLOW‐UP
*IMPORT DATA
set more off
keep if outcome=="death"
gen d=events
gen n=total
replace arm= "CONTROL" if arm=="laser"
replace arm= "CONTROL" if arm=="sham"
*NETWORK SETUP AND PRESENTATION
network setup d n, stud(study) trtvar(arm) numcodes armvars(drop) ref("CONTROL") rr
network table
network map, circle title("all‐cause death", size(medsmall))
graph save net6, replace
*CONSISTENCY AND INCONSISTENCY MODEL ARE FIT AND ESTIMATES ARE PRESENTED
network meta consistency
estimate store cons
network meta inconsistency
network sidesplit all
*THE CONSISTENCY MODEL IS ADEQUATE AND WILL BE USED
estimate restore cons
intervalplot, eform null(1)
network forest , eform xlab( .2 .5 1 2 4) xli(1) diamond ///
ti("all‐cause death", size(medsmall))
graph save forest_6, replace
*RANKS AND SUCRA
network rank min, zero all gen(prob)
sucra prob*, rankog
*ALL PAIRWISE META‐ANALYSES
gen nonD1=n1‐d1
gen nonD2=n2‐d2
gen nonD3=n3‐d3
gen nonD4=n4‐d4
gen nonD5=n5‐d5
metan d5 nonD5 d1 nonD1 if (d1>=0.5 & d5>=0.5), rr random nograph nointeger label(namevar=study)
metan d5 nonD5 d2 nonD2 if (d2>=0.5 & d5>=0.5), rr random nograph nointeger label(namevar=study)
metan d5 nonD5 d3 nonD3 if (d3>=0.5 & d5>=0.5), rr random nograph nointeger label(namevar=study)
metan d2 nonD2 d1 nonD1 if (d2>=0.5 & d1>=0.5), rr random nograph nointeger label(namevar=study)
metan d1 nonD1 d3 nonD3 if (d3>=0.5 & d1>=0.5), rr random nograph nointeger label(namevar=study)
metan d2 nonD2 d3 nonD3 if (d3>=0.5 & d2>=0.5), rr random nograph nointeger label(namevar=study)
metan d4 nonD4 d2 nonD2 if (d4>=0.5 & d2>=0.5), rr random nograph nointeger label(namevar=study)
metan d4 nonD4 d3 nonD3 if (d4>=0.5 & d3>=0.5), rr random nograph nointeger label(namevar=study)
metan d5 nonD5 d3 nonD3 if (d5>=0.5 & d3>=0.5), rr random nograph nointeger label(namevar=study)
metan d5 nonD5 d4 nonD4 if (d5>=0.5 & d4>=0.5), rr random nograph nointeger label(namevar=study)
* CONTRIBUTION PLOT AND RISK OF BIAS: RE‐LOAD DATA WITH TREATMENT NAMES (NOCODES OPTION)
*IMPORT DATA
set more off
keep if outcome=="death"
gen d=events
gen n=total
replace arm= "CONTROL" if arm=="laser"
replace arm= "CONTROL" if arm=="sham"
network setup d n, stud(study) trtvar(arm) nocodes armvars(drop) ref("CONTROL") rr
network convert pairs
netweight _y _std _t1 _t2
netweight _y _std _t1 _t2 , bar(by risk)
graph save bias6, replace
*FUNNEL PLOT
netfunnel _y _std _t1 _t2, bycomparison
graph save fun6
**COMPOSITE OF EFFICACY OUTCOMES AT 1 YEAR
graph combine net1.gph net2.gph net3.gph , row(1) iscale(.6) imargin(zero)
graph save fig_efficacy
**COMPOSITE OF SAFETY OUTCOMES AT 1‐2 YEARS (LONGEST FU)
graph combine net4.gph net5.gph net6.gph , row(1) iscale(.8) imargin(zero)
graph save fig_safety
**COMPOSITE OF FUNNEL PLOTS
graph combine fun1.gph fun2.gph fun3.gph fun4.gph fun5.gph fun6.gph , col(2)
**COMPOSITE OF CONTRIBUTION PLOTS WITH MEAN BIAS FOR EACH PAIRWISE COMPARISON
graph combine bias1.gph bias4.gph bias2.gph bias5.gph bias3.gph bias6.gph ///
, iscale(.3) col(2)
graph save riskofbias, replace
***QUALITY OF LIFE AT 1 YEAR
*IMPORT DATA
set more off
keep if outcome=="QOL"
keep if year==1
gen n=total
*NETWORK SETUP AND PRESENTATION
network setup mean sd n, stud(study) trtvar(arm) nocodes armvars(drop) ref("laser") md
network table
**ANALYSES AT 2 YEARS
**GAIN 3+ AT 2 YEARS
*IMPORT DATA
set more off
keep if outcome=="gain3+"
keep if year==2
gen d=events
gen n=total
*NETWORK SETUP AND PRESENTATION
network setup d n, stud(study) trtvar(arm) numcodes armvars(drop) ref("laser") rr
network table
network map, circle
*CONSISTENCY FIXED EFFECT MODEL: THIS IS EXPLORATORY
**AND WILL NOT BE USED IN THIS REVIEW
network meta consistency, fixed
network forest , xli(1) eform diamond xlab(.1 .3 1 4) ///
ti("gain 3+ visual acuity lines", size(medsmall))
*PAIRWISE ANALYSES
gen nonD1=n1‐d1
gen nonD2=n2‐d2
gen nonD4=n4‐d4
metan d4 nonD4 d1 nonD1 if (d2>=0.5 & d1>=0.5), rr random nograph nointeger label(namevar=study)
metan d2 nonD2 d4 nonD4 if (d4>=0.5 & d1>=0.5), rr random nograph nointeger label(namevar=study)
**CHANGE IN MEAN VA AT 2 YEARS
*IMPORT DATA
set more off
keep if outcome=="vachange"
keep if year==2
gen n=total
*NETWORK SETUP AND PRESENTATION
network setup mean sd n, stud(study) trtvar(arm) nocodes armvars(drop) ref("laser") md
network table
network map, circle
* PAIRWISE META‐ANALYSES
metan nRANI meanRANI sdRANI nAFLI meanAFLI sdAFLI if (nRANI>=0.5 & nAFLI>=0.5), random nograph nointeger nostandard label(namevar=study)
metan nRANI meanRANI sdRANI nBEVA meanBEVA sdBEVA if (nRANI>=0.5 & nBEVA>=0.5), random nograph nointeger nostandard label(namevar=study)
**CHANGE IN MEAN CRT AT 2 YEARS
*IMPORT DATA
case(lower) clear
set more off
keep if outcome=="cmtchange"
keep if year==2
gen n=total
*NETWORK SETUP AND PRESENTATION
network setup mean sd n, stud(study) trtvar(arm) nocodes armvars(drop) ref("laser") md
network table
network map, circle
* PAIRWISE META‐ANALYSES
metan nRANI meanRANI sdRANI nAFLI meanAFLI sdAFLI if (nRANI>=0.5 & nAFLI>=0.5), random nograph nointeger nostandard label(namevar=study)
metan nRANI meanRANI sdRANI nBEVA meanBEVA sdBEVA if (nRANI>=0.5 & nBEVA>=0.5), random nograph nointeger nostandard label(namevar=study)
*SENSITIVITY ANALYSIS WITH ONLY STUDIES AT LOW RISK OF BIAS
**GAIN 3+ VISUAL ACUITY LINES AT 1 YEAR
*IMPORT DATA
case(lower) clear
set more off
keep if outcome=="gain3+"
keep if year==1 & riskof=="low"
gen d=events
gen n=total
*NETWORK SETUP AND PRESENTATION
network setup d n, stud(study) trtvar(arm) nocodes armvars(drop) ref("laser") rr
network table
network map, circle title("gain 3+ visual acuity lines", size(medsmall))
*CONSISTENCY AND INCONSISTENCY MODEL ARE FIT
network meta consistency
estimate store cons
network meta inconsistency
network sidesplit all
*THE CONSISTENCY MODEL IS ADEQUATE AND WILL BE USED
estimate restore cons
intervalplot, eform
network forest , xli(1) eform diamond xlab(.1 .3 1 4) ///
ti("gain 3+ visual acuity lines", size(medsmall))
*MEAN VA CHANGE AT 1 YEAR
*IMPORT DATA
set more off
keep if outcome=="vachange"
keep if year==1 & riskof=="low"
gen n=total
*NETWORK SETUP AND PRESENTATION
network setup mean sd n, stud(study) trtvar(arm) nocodes armvars(drop) ref("laser") md
network table
network map, circle ti("mean change of visual acuity", size(medsmall))
*CONSISTENCY AND INCONSISTENCY MODEL ARE FIT AND ESTIMATES ARE PRESENTED
network meta consistency
estimate store cons
network meta inconsistency
network sidesplit all
*THE CONSISTENCY MODEL IS ADEQUATE AND WILL BE USED
estimate restore cons
intervalplot, null(0) separate labels(laser AFLI BEVA PEGA RANI RANI‐DL RANI‐PL sham) ///
xlab(‐.1 0 .1 .2)
network forest , xli(0) diamond xlab(‐.2 0 .2) ///
ti("mean change in visual acuity", size(medsmall))
**MEAN CHANGE IN CENTRAL RETINAL THICKNESS
*IMPORT DATA
set more off
keep if outcome=="cmtchange"
keep if year==1 & riskof=="low"
gen n=total
*NETWORK SETUP AND PRESENTATION
network setup mean sd n, stud(study) trtvar(arm) nocodes armvars(drop) ref("laser") md
network table
network map, circle ti("mean change of central retinal thickness", size(medsmall))
*CONSISTENCY AND INCONSISTENCY MODEL ARE FIT AND ESTIMATES ARE PRESENTED
network meta consistency
estimate store cons
network meta inconsistency
network sidesplit all
*THE CONSISTENCY MODEL IS NOT ADEQUATE
intervalplot, null(0) separate labels(laser AFLI BEVA PEGA RANI RANI‐DL RANI‐PL sham) ///
xlab(‐.1 0 .1 .2) predict
Data and analyses
Comparison 1.
Ranibizumab versus laser photocoagulation at 6 to 12 months
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Quality of life: NEI‐VFQ composite score at 6 to 12 months | 3 | 412 | Mean Difference (Fixed, 95% CI) | 5.14 [2.96, 7.32] |
Analysis 1.1.
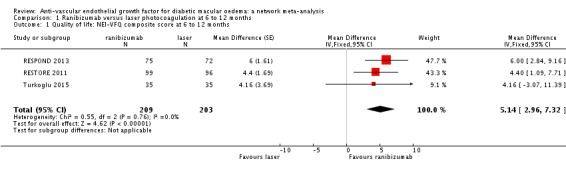
Comparison 1 Ranibizumab versus laser photocoagulation at 6 to 12 months, Outcome 1 Quality of life: NEI‐VFQ composite score at 6 to 12 months.
What's new
Last assessed as up‐to‐date: 26 April 2017.
| Date | Event | Description |
|---|---|---|
| 2 May 2017 | New search has been performed | Issue 6, 2017: Updated protocol: objectives revised as comparing different antiangiogenic drugs using network meta‐analysis technique |
| 2 May 2017 | New citation required and conclusions have changed | Issue 6, 2017: Searches updated and six new studies added (DRCRnet 2015, Ishibashi 2014, Lopez‐Galvez 2014, REVEAL 2015, Turkoglu 2015, Wiley 2016) and conclusions changed |
History
Protocol first published: Issue 4, 2008 Review first published: Issue 4, 2009
| Date | Event | Description |
|---|---|---|
| 4 November 2014 | Amended | Plain language summary title has been amended |
| 17 October 2014 | New citation required but conclusions have not changed | Issue 10, 2014: Five new studies (Azad 2012; Ekinci 2014; Nepomuceno 2013; RELATION 2012; RESPOND 2013) have been included in the update. |
| 17 October 2014 | New search has been performed | Issue 10, 2014: Electronic searches updated. |
| 4 November 2013 | Feedback has been incorporated | The authors have made some edits to the review in response to feedback received. See 'Feedback 1' for further details. |
| 14 March 2013 | Amended | The abstract has been amended to focus on the comparison with laser and presenting absolute effects. |
| 11 November 2012 | New search has been performed | Updated searches yielded seven new trials for inclusion. One trial that had previously been included was excluded. An economic section has been added. One new author Massimo Brunetti has been added to the review team. |
| 11 November 2012 | New citation required and conclusions have changed | Inclusion of seven new studies has changed the conclusions to this review from the previous version. |
Differences between protocol and review
Differences between protocol and review in the first published version of this review
We have added LILACS to the list of databases which have been searched for this review. We have used a sensitivity analysis for the robustness of results in comparisons including only one trial according to a statistical technique derived from a recent publication (Borm 2009).
Changes in update, 2012 compared to the protocol of the previous version
We have specified that studies comparing different anti‐VEGF drugs will also be included in this review, but intravitreal steroids will be excluded as they are the subject of another Cochrane Review. Moreover, we decided not to consider the comparison of bevacizumab with bevacizumab plus triamcinolone, which included two studies; in fact this comparison investigates the additional effect of triamcinolone rather than the benefit of anti‐VEGF drugs.
We have computed indirect comparison odds ratios (OR) of a gain of 3+ and 2+ lines for bevacizumab and pegaptanib versus ranibizumab as the reference drug using random‐effects model logistic regression.
Changes in update, 2014 compared to the protocol of the previous version
We have included five more studies but the conclusions did not change.
We no longer consider economic evidence since antiangiogenic therapy is widely approved and reimbursed.
We eliminated the table on retinal detachment as an ocular adverse event since it proved to be extremely rare in all studies.
Units of analysis issue: in the update of this review we no longer performed a sensitivity analysis regarding the primary outcome to determine the impact of excluding studies with eyes, rather than participants, as the unit of analysis. In fact, a significant amount of evidence from studies with individuals as unit of analysis was achieved for the main comparisons.
Single trial issue: in the 2012 and 2014 updates of the review we did not use the sensitivity analysis on the robustness of single trial results recommended by Borm 2009, as was originally planned. Instead, we calculated the 'Optimal Information Size' to rate the quality of evidence regarding imprecision as recommended by the GRADE study group in Guyatt 2011.
Changes in update, 2016 compared to the protocol of the previous version
The objective was now to compare different anti‐VEGF drugs and a new protocol was developed.
We used network meta‐analysis technique to augment direct evidence with indirect evidence.
We restricted the number of outcomes to three efficacy outcomes, three safety outcomes and quality of life.
We have included six more studies and conclusions are changed.
The sensitivity analysis restricted to low risk of bias studies was added to the protocol.
We included a cross‐over study and treated it as a parallel arm study in efficacy analyses.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Parallel group RCT People were randomly allocated to treatment but in bilateral cases eyes were randomly allocated to treatment |
|
| Participants | Country: Iran Number of people randomised: 101 (115 eyes) Average age: 60 years (range 39 to 74) Sex: 51% women Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Intervention:
Comparator:
"Three consecutive injections were performed at 6‐week intervals. Injections were done under sterile conditions with topical anesthesia and insertion of a lid speculum. For the IVB group, 1.25 mg (0.05 cc) bevacizumab (Avastin, made for F. Hoffmann‐La Roche Ltd Basel, Switzerland by Genentech Inc., San Francisco, CA, USA) was injected intravitreally with a 30‐gauge needle through the superotemporal quadrant." Page 485 "In the control group, a needleless syringe was pressed against the conjunctiva and sclera in each session." Page 485 There was another intervention arm that combined bevacizumab with triamcinolone acetonide, but this is not included in this review (n = 37 eyes) |
|
| Outcomes | Primary outcome:
"Central macular thickness was defined by the average thickness of a central macularregion 1,000 ìm in diameter centered on the patient’s foveola." Page 485 Secondary outcomes:
Follow‐up: 18 and 24 weeks |
|
| Notes | Date study conducted: November 2005 to September 2006 Funding: not reported Conflict of interest: "The authors have no proprietary interest in this study." Trial registration: NCT00370422 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed using a random block permutation method according to a computer‐generated randomization list. The block lengths varied randomly. A random allocation sequence was performed by a biostatistician. Details of the series were unknown to the investigators." Page 485 |
| Allocation concealment (selection bias) | Low risk | "Randomization was performed using a random block permutation method according to a computer‐generated randomization list. The block lengths varied randomly. A random allocation sequence was performed by a biostatistician. Details of the series were unknown to the investigators." Page 485 |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Subjects were masked to the treatment modality. Visual acuity assessment and OCT were performed by optometrists who were masked to the groups." Page 485 |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No incomplete outcome data were reported, but number of participants at 24 weeks' follow‐up was not specified |
| Selective reporting (reporting bias) | High risk | The study protocol is mentioned. However, dichotomous VA outcomes are not provided |
| Other bias | Low risk | 28 eyes of 14 participants (14%) with bilateral CSMO were included in the analysis |
| Overall risk of bias | Low risk | Low risk of bias for most items |
| Methods | Parallel group RCT One eye per person, unclear how eye selected |
|
| Participants | Country: India Number of people randomised: 40 (40 eyes) Average age: 54 years Sex: 42% women Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Intervention:
Comparator:
"IVB [...] injected via pars plana route in the doses mentioned above by a single experienced investigator using full aseptic precautions. Postinjection, all patients were prescribed topical moxifloxacin 0.5% qid for 5 days. Macular grid laser augmentation was performed by a single experienced examiner according to the modified ETDRS protocol with a spot size of 100 μ, pulse duration of 100 ms, and a power of 50–100 mW titrated to produce mild intensity burns in areas showing diffuse leakage on the FFA in a ‘C’ shaped zone between 500 and 3000μ from the foveal center sparing the papilla‐macular bundle." Page 167 Another intervention arm evaluated triamcinolone acetonide, but is not included in this review (n = 20 eyes) |
|
| Outcomes | Outcomes:
Primary outcome: not specified Follow‐up: 1, 3 and 6 months |
|
| Notes | Date study conducted: not reported Funding: not reported Conflict of interest: not reported Trial registration: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up reported |
| Selective reporting (reporting bias) | High risk | VA data and other outcomes incompletely reported |
| Other bias | Low risk | No other bias identified |
| Overall risk of bias | Unclear risk | Unclear risk of bias for most items |
| Methods | Parallel group RCT One eye per person; if both eyes were eligible eye with worse VA was selected |
|
| Participants | Country: UK Number of people randomised: 80 (80 eyes) Average age: 64 years (range 40 to 86) Sex: 31% women Inclusion criteria:
Exclusion criteria: (for study eye)
|
|
| Interventions | Intervention:
Comparator:
"Bevacizumab (1.25 mg in 0.05 ml) (Avastin; Roche Registration Limited, UK) was prepared by Moorfields Pharmaceuticals (London, UK) as a prefilled syringe containing 0.13 ml. In a designated intravitreal treatment room, under sterile conditions, using topical anesthesia and povidone‐iodine 5% into the conjunctival sac and onto the lid margins, and following application of a drape and insertion of a lid speculum, injections were undertaken with a 30‐gauge needle through the supra‐ or infratemporal quadrant, with a drop of ofloxacin placed in the fornix at the end of the procedure. Patency of the central retinal artery was determined by indirect ophthalmoscopy and VA of hand movements or better. The IOP was checked 30 minutes after the injection, and if the pressure was increased (30 mmHg) appropriate treatment was commenced. After the injection, topical ofloxacin was instilled 4 times per day for 4 days". Page 1080 "After baseline IVB, patients received 2 further IVB injections (6‐ and 12‐week time points). Subsequent IVBinjections were guided by an OCT‐based retreatment protocol. In brief, if the thinnest recorded central retinal thickness was less than 270 m at 18 weeks, then treatment was continued only if macular thickness was not “stable.” If central retinal thickness was greater than 270 m at 18 weeks and subsequent visits, then IVB injections were administered until a “stable” macular thickness was attained. “Stable macular thickness” was defined as 3 consecutive visits with the central retinal thickness within 20 m of the patient’s thinnest recorded central retinal thickness. Patients could thereby receive a minimum of 3 injections and a maximum of 9 injections in the first 12 months." Page 1080 "Modified ETDRS MLT comprised 50 m argon laser spot size, laser applied only greater than 500 m from the edge of the FAZ, with focal treatment aiming to cause mild blanching of the retinal pigment epithelium and not darkening/whitening of microaneurysms. Areas of diffuse leakage or nonperfusion were similarly treated in a grid pattern." Page 1080 |
|
| Outcomes | Primary outcome:
Secondary outcomes:
Follow‐up: 12 and 24 months |
|
| Notes | Date study conducted: May 2007 to August 2009 Funding:"Supported by grants from Moorfields Special Trustees and the National Institute for Health Research UK to the Biomedical Research Center for Ophthalmology based at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology." Conflict of interest: "The author(s) have no proprietary or commercial interest in any materials discussed in this article" Trial registration: eudract.ema.europa.eu Identifier: 2007‐000847‐89 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomised into 2 groups by means of an in‐house computerized randomization program. The research investigator was not involved in the randomization process. Patients were stratified for BCVA, with the aim being that both groups would have comparable mean baseline BCVAs." Page 1080 |
| Allocation concealment (selection bias) | Low risk | The doctor had to phone the Clinical Trial Unit in order to obtain a randomisation from the statistician [personal communication from investigators] |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Although the patient and the study physician were not masked to the therapeutic modality, the study optometrist, OCT technician, photographer, graders performing assessment of the FAZ and ETDRS retinopathy grading, and study statistician were all masked to the patient randomization." Page 1080 |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Two patients in the laser group did not complete 12 months of follow‐up (1 patient moved away, and 1 patient could not be contacted). They were last reviewed at the 32‐week time point,with these data being carried forward and an intention‐to‐treat analysis undertaken. All 42 patients in the IVBgroup completed the study." Page 1082 |
| Selective reporting (reporting bias) | Low risk | We could not find a protocol but primary outcomes were stated in the methods and were those routinely used in the field |
| Other bias | Low risk | No other bias identified |
| Overall risk of bias | Low risk | Low risk for most items; we considered masking of outcome assessors, though not of participants and physicians, sufficient to ensure unbiased outcome measurement |
| Methods | Parallel group RCT One eye per person, unclear how eye selected |
|
| Participants | Country: USA, Canada and Austria Number of people randomised: 221 (221 eyes) Average age: 64 years (range 40 to 86) Sex: 31% women Inclusion criteria:
Exclusion criteria: (for study eye)
(in either eye)
(systemic)
|
|
| Interventions | Intervention:
Comparator:
"Patients were randomly assigned in a 1:1:1:1:1 ratio to 1 of 5 treatment regimens in 1 eye only: 0.5 mg VEGF Trap‐Eye every 4 weeks (0.5q4); 2 mg VEGF Trap‐Eye every 4 weeks (2q4); 2 mg VEGF Trap‐Eye for 3 initial monthly doses and then every 8 weeks, (2q8); 2 mg VEGF Trap‐Eye for 3 initial monthly doses and then on an as‐needed (PRN) basis (2 PRN); or macular laser treatment by the modified ETDRS protocol" Page 1820 |
|
| Outcomes | Primary outcome:
Secondary outcomes:
Follow‐up: 24 and 52 weeks |
|
| Notes | Date study conducted: December 2008 to June 2009 Funding: "Sponsored by Regeneron Pharmaceuticals, Inc., Tarrytown, New York." Conflict of interest: "The author(s) have made the following disclosure (s): Diana V. Do: Genentech (financial support), Regeneron Pharmaceuticals (financial support). Ursula Schmidt‐Erfuth: Alcon Labs (consultant, lecturer), Bayer Healthcare (consultant, lecturer), Novartis (consultant, lecturer), Regeneron Pharmaceuticals (lecturer), Pfizer (lecturer). Victor H. Gonzalez: Pfizer (consultant, lecturer), Genentech (lecturer), Eyetech (consultant, lecturer), Regeneron (lecturer). Carmelina M. Gordon: Allergan (consultant), Regeneron Pharmaceuticals (lecturer), Novartis (consultant, lecturer). Michael Tolentino: Genentech (consultant, lecturer), Eyetech (consultant, lecturer), Regeneron Pharmaceuticals (consultant, lecturer). Alyson J Berliner: Regeneron Pharmaceuticals (employee, equity owner). Robert Vitti: Regeneron Pharmaceuticals (employee, equity owner). Rene Rückert: Bayer Schering Pharma (employee). Rupert Sandbrink: Bayer Schering Pharma (employee). David Stein: Regeneron Pharmaceuticals (employee,equity owner). Ke Yang: Regeneron Pharmaceuticals (employee, equity owner). Karola Beckmann: Bayer Schering Pharma (employee). Jeff S.Heier: Genentech (consultant, lecturer), Regeneron Pharmaceuticals (consultant,lecturer), Fovea (consultant). Trial registration:NCT00789477 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization was handled by an IVRS vendor. The study statistician at REGENERON provided the randomization plan and reviewed and approved the dummy rand table. Study Data Management at REGENERON tested the randomization function extensively along with the Clinical team." |
| Allocation concealment (selection bias) | Low risk | "Sites called into IVRS to randomize patients and received the randomization number and drug kit assignment at the completion of the call. The site also received a confirmation email. Neither of these contained the actual randomization assignment. The randomization assignments were kept by the IVRS vendor in a secure, access‐controlled database and were delivered to REGENERON by the IVRS vendor at the primary endpoint database lock." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "To maintain participant masking, sham injections were performed on visits when an active dose was not given, and a sham laser was given to the VEGF Trap‐Eye groups at week 1. Study drug and sham injections and laser and sham laser treatments were performed by an unmasked physician who had no other role in the study except to assess adverse events (AEs) immediately posttreatment. Sham injections followed the active treatment protocol with the exception that no needle was attached to the syringe, and the syringe hub was gently applied to the sclera to mimic an injection. Sham laser consisted of placing a contact lens on the study eye and positioning the patient in front of the laser machine for the approximate duration of a laser treatment." Page 1820‐1 |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | A separate masked physician was assigned to assess adverse events (AEs) and retreatment and rescue criteria and to supervise the masked assessment of efficacy. Every effort was made to ensure that all other study site personnel remained masked to treatment assignment to facilitate an unbiased assessment of efficacy and safety." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
"Two randomised patients did not receive treatment and 19 patients discontinued the study after receiving at least 1 treatment for the following reasons: lost to follow‐up (6 patients), withdrew consent (6 patients), death (3 patients), treatment failures (2 patients), AE (1 patient), and protocol deviation (1 patient). Discontinuations were evenly distributed among the 5 treatment groups." Page 1821 Comment: LOCF used |
| Selective reporting (reporting bias) | Low risk | Primary outcome declared and consistent with our review |
| Other bias | Low risk | No other bias identified |
| Overall risk of bias | Low risk | Low risk of bias for most items |
| Methods | Parallel group and within‐person RCT One or two study eyes per person. If both eyes eligible, right eye randomised first and then left eye assigned to "sham plus prompt laser group". If right eye already assigned to this group then left eye assigned randomly to 1 of the other 3 groups |
|
| Participants | Country: USA Number of people randomised: 691 (854 eyes) Average age: 63 years Sex: 44% women Inclusion criteria:
(in study eye)
Exclusion criteria:
(participant)
|
|
| Interventions | Intervention:
Comparator:
Ranibizumab group was also randomly allocated to prompt laser photocoagulation (187 eyes) which occurred within 3 to 10 days of the injection and deferred laser photocoagulation (188 eyes) which happened after 24 weeks. All eyes in comparator group were treated within 3 to 10 days of the sham injection Complex retreatment algorithm using web‐based, real‐time data‐entry system (page 1066) There was another intervention arm that combined triamcinolone with prompt laser photocoagulation, but this was not included in this review. n = ? (186 eyes) |
|
| Outcomes | Primary outcome: BCVA and safety at 12 months Secondary outcomes: CRT Follow‐up: every 4 weeks for 12 months. After 12 months, the trial was unmasked and follow‐up continued to 3 years |
|
| Notes | Dates participants enrolled: March 2007 to December 2008 Funding: "Supported through a cooperative agreement from the National Eye Institute and the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Department of Health and Human Services EY14231, EY14229, and EY018817. The funding organization (National Institutes of Health) participated in oversight of the conduct of the study and review of the manuscript but not directly in the design or conduct of the study; the collection, management, analysis, or interpretation of the data; or the preparation of the manuscript. Genentech provided the ranibizumab for the study, and Allergan, Inc., provided the triamcinolone for the study. In addition, Genentech and Allergan, Inc., provided funds to the DRCR.net to defray the study’s clinical site costs. As described in the DRCR.net Industry Collaboration Guidelines (available at www.drcr.net), the DRCR.net had complete control over the design of the protocol, the ownership of the data, and all editorial content of presentations and publications related to the protocol." Conflict of interest: "A complete list of all DRCR.net investigator financial disclosures can be found at www.drcr.net" Trial registration: NCT00445003 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation sequence was computer‐generated by the DRCR.net co‐ordinating centre "...study participants with 1 study eye were assigned randomly on the DRCR.net study website (using a permuted blocks design stratified by study eye visual acuity)" Page 1065 |
| Allocation concealment (selection bias) | Low risk | Randomisation assignments were obtained through the DRCR.net study website, therefore no study personnel had access to the list or to the next assignment before it was assigned "study participants with 1 study eye were assigned randomly on the DRCR.net study website (using a permuted blocks design stratified by study eye visual acuity)" Page 1065 |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Study participants in the 3 groups receiving laser were masked to treatment assignment through the primary outcome visit, whereas the ranibizumab deferred laser group was not masked." Page 1065‐6 |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Visual acuity examiners and OCT technicians were masked to treatment group assignment before and at the 1‐year primary outcome visit." Page 1066 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants randomised in each group were: 293 laser, 187 ranibizumab + prompt laser, 188 ranibizumab + deferred laser and 186 IVTA + laser. At 1 year complete participants were 274, 171, 178, 176 respectively (91% to 95%) At 2 years complete participants were 211, 136, 139, 142 respectively (72% to 76%) Causes of missing data were balanced across groups |
| Selective reporting (reporting bias) | Low risk | We could not find a protocol but primary outcomes were stated in the methods and were those routinely used in the field |
| Other bias | Low risk | No other source of bias identified |
| Overall risk of bias | Low risk | Low risk of bias for most items |
| Methods | Parallel group study "One eye of each participant was randomly assigned in a 1:1:1 ratio to be injected with aflibercept (at a dose of 2.0 mg), bevacizumab (1.25 mg), or ranibizumab (0.3 mg). Randomization was performed at the DRCR.net study website, in permuted blocks and with stratification according to study site and visual acuity in the study eye." |
|
| Participants | Country: USA Number of people (eyes) randomised: 660 Average age: 61 years Sex: not reported Inclusion criteria:
(in study eye)
Exclusion criteria:
(participant)
|
|
| Interventions | Interventions:
Randomisation was stratified by site and visual acuity: ≥ 66 letter score/ ≤ 65 letter score. retreatment algorithm: "In general, an eye will continue to receive an injection if the eye is improving or worsening on OCT or visual acuity. The first time an eye has not improved or worsened, the eye will receive an injection. If the eye has not improved or worsened for at least 2 consecutive 4‐week injections and OCT central subfield thickness is <250μ and visual acuity is 20/20 or better, the injection will be deferred." "In general, focal/grid laser will be initiated at or after the 24 week visit if 1) the OCT central subfield thickness is ≥250μ or there is edema that is threatening the fovea and 2) the eye has not improved on OCT or visual acuity from the last two consecutive injections." |
|
| Outcomes | Primary outcome: BCVA and safety at 12 months Secondary outcomes: CRT Follow‐up: after 12 months the trial was unmasked and follow‐up continued to 3 years |
|
| Notes | Dates participants enrolled: March 2007 to December 2008 Funding:"Supported through a cooperative agreement from the National Eye Institute and the National Institute of Diabetes and Digestive and Kidney Diseases, National Institutes of Health, Department of Health and Human Services EY14231, EY14229, and EY018817. The funding organization (National Institutes of Health) participated in oversight of the conduct of the study and review of the manuscript but not directly in the design or conduct of the study; the collection, management, analysis, or interpretation of the data; or the preparation of the manuscript. Genentech provided the ranibizumab for the study, and Allergan, Inc., provided the triamcinolone for the study. In addition, Genentech and Allergan, Inc., provided funds to the DRCR.net to defray the study’s clinical site costs. As described in the DRCR.net Industry Collaboration Guidelines (available at www.drcr.net), the DRCR.net had complete control over the design of the protocol, the ownership of the data, and all editorial content of presentations and publications related to the protocol." Conflict of interest: "A complete list of all DRCR.net investigator financial disclosures can be found at www.drcr.net" Trial registration: NCT00445003 (Protocol T) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The randomisation sequence was computer‐generated by the DRCR.net co‐ordinating centre "Randomization was performed at the DRCR.net study website, in permuted blocks and with stratification according to study site and visual acuity in the study eye." Page 3 |
| Allocation concealment (selection bias) | Low risk | Randomisation assignments were obtained through the DRCR.net study website, therefore no study personnel had access to the list or to the next assignment before it was assigned See above, Page 3 |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Study participants, reading‐center graders, and the medical monitor who reviewed all adverse events were unaware of the treatment group assignments. Visual‐acuity and OCT technicians were unaware of the treatment‐group assignments at the 1‐year visit. Investigators and study coordinators were aware of the treatment group assignments." Page 3 |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Visual‐acuity and OCT technicians were unaware of the treatment‐group assignments at the 1‐year visit." Page 3 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "The 2‐year visit was completed by 90%, 85%, and 88% of the 660 randomised participants (91%, 90%, and 91% excluding deaths) in the aflibercept, bevacizumab, and ranibizumab groups, respectively (Fig S1, available at www.aaojournal.org). There were no substantial differences identified in the baseline characteristics of those who completed and those who did not complete the 2‐year visit (Table S1, available at www.aaojournal.org)." |
| Selective reporting (reporting bias) | Low risk | Outcomes match those in the Study Protocol available at http://publicfiles.jaeb.org/AntiVEGFCompPrtclv5_03_18_14.pdf |
| Other bias | Low risk | No other source of bias identified |
| Overall risk of bias | Low risk | Low risk of bias for most items |
| Methods | Parallel group RCT One eye per person, unclear how eye selected |
|
| Participants | Country: Turkey Number of people randomised unclear: 100 (100 eyes) completed follow‐up Average age: 67 years (range 50 to 89) Sex: 68% women Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Intervention:
Comparator:
"Topical anesthetic drops were instilled, and a drape application and blepharostat attachment were applied. Afterward, fornix lavage was applied using diluted povidone iodine. For Group 1, 1.25 mg (0.05 ml) of bevacizumab was injected into the eye that needed treatment, using a 30 gauge needle; for Group 2, 0.05 mg (0.05 cc) of ranibizumab was injected into the vitreous humor through the lower temporal quadrant, 3.5–4 mm behind the limbus. After the treatment, all patients were treated with topical antibiotics four‐times a day for 1 week." Page 140 Bevacizumab and ranibizumab injections were applied, with an interval of 1 month for the first three doses. Retreatment criteria. “After the third dose of bevacizumab/ranibizumab for patients in Groups 1 and 2, an additional three consecutive bevacizumab/ranibizumab injections were applied if the central macular thickness was greater than 275 µm or if there was an increase in BCVA of at least three letters compared with baseline. After the sixth intravitreal injection, if the central macular thickness was greater than 275 mm or if there was an increase in BCVA of at least two letters, additional intravitreal injections were performed until stable visual acuity was obtained." Page 140 |
|
| Outcomes | Outcomes:
Primary outcome not specified Follow‐up: monthly intervals after treatment to 12 months |
|
| Notes | Dates participants enrolled: 2011 to 2014 Funding: not reported Conflict of interest: "The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties." Page 142 Trial registration: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear if participants, care providers or outcome assessors were masked to treatment method |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear if participants, care providers or outcome assessors were masked to treatment method |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Exclusion after randomisation: 15 participants excluded “Patients with acute ocular infection (endophthalmitis after intravitreal injection, n = 3), stroke, myocardial infarction (n = 2), uncontrolled hypertension (n = 4), pregnancy (n = 1), renal failure (n = 1) and cataract formation during follow‐up period (n = 4) were excluded from the study.” Page 140 |
| Selective reporting (reporting bias) | High risk | We could not find a protocol and our primary outcomes were not reported |
| Other bias | Low risk | No other bias identified |
| Overall risk of bias | High risk | Most items at high or unclear risk of bias |
| Methods | Allocation: randomised endpoint classification; safety/efficacy study intervention model; parallel assignment Masking: double‐masked | |
| Participants | n = 243; country: Japan 43 recruiting hospitals |
|
| Interventions | Drug: pegaptanib sodium (n = 123) Other: sham injection (n = 120) Sex: female 113, male 130 Age: (SD) pegaptanib 65.9 (9.0), sham 66.0 (9.2) years Inclusion criteria:
Exclusion criteria:
|
|
| Outcomes |
|
|
| Notes | Completion Date: August 2012 (results also partly available on ClinicalTrials.gov, accessed on 5 December 2013) Sponsor: Pfizer, two authors (Isogawa N, Esaka E) employee of Pfizer Author contact not found |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information |
| Allocation concealment (selection bias) | High risk | “Pegaptanib sodium or a syringe of sham was enclosed by aluminium bag, then the aluminium bag was put in a box. These boxes were supplied to each hospitals. These box could maintain masking. However, Pfizer Japan Inc, which requested this drug trial, made the mistake of allowing to open the box for some hospitals during transportation, so it was clear that it was possible not to ensure the masking sufficiently.” In 71 cases, there was the evidence of opening the box of study drugs In 172 cases, there was no evidence of opening the box. In 50 cases, there was evidence of not opening the box, so it was clear that masking is sufficient |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Treating physician and his/her assistants were not masked and other staff (physician who did not directly give the drug, orthoptist, clinical research coordinator, nurses, laboratory technician, administrator for study drugs and other staffs) were masked. Participants were blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Lost to follow‐up: 6 pegaptanib, 4 sham |
| Selective reporting (reporting bias) | Unclear risk | No information |
| Other bias | Unclear risk | No information |
| Overall risk of bias | High risk | Unclear or high risk for most items |
| Methods | Parallel group RCT Eyes: 862 eyes from 862 participants. One eye per participant. “For patients who met eligibility criteria in both eyes, the eye with the worst BCVA was selected as the study eye. If a patient had DME with similar BCVA in both eyes, the eye with the clearest media was selected as the study eye. If the ocular media of the both eyes were similar in clarity, the patient’s non‐dominant eye (if identifiable) was selected as the study eye. If neither eye is dominant, the right eye was designated as the study eye.” (Appendix 2) |
|
| Participants | Country: 54 centres in USA (VISTA study, 446 participants) and 73 centres in Europe, Japan, and Australia (VIVID study, 406 participants) Number of people randomised: 852 (852 eyes) Average age: 63 years Sex: 42% women Inclusion criteria:
Exclusion criteria:
See paper for details |
|
| Interventions | Intervention:
Comparator
"Eyes were randomised in a 1:1:1 ratio to receive either 2 mg IAI every 4 weeks (2q4), 2 mg IAI every 8 weeks after 5 initial monthly doses (from baseline to week 16) with sham injections on non‐treatment visits (2q8), or macular laser photocoagulation at baseline and sham injections at every visit (laser control group)" Page 2 |
|
| Outcomes | Primary outcome:
Secondary outcomes:
Follow‐up: 52 weeks |
|
| Notes | Date study conducted: May 2011 to June 2013 Funding: "The VISTA and VIVID studies were funded by Regeneron Pharmaceuticals, Inc., Tarrytown, NY and Bayer HealthCare, Berlin, Germany. The sponsors participated in the design and conduct of the study, analysis of the data, and preparation of the manuscript." Conflict of interest: “Assistance with the study design and conduct and data analysis was provided by Karen Chu, MS, and Xiaoping Zhu, PhD, Regeneron Pharmaceuticals, Inc. (VISTA), and Jana Sachsinger, PhD, and Christiane Norenberg, MS, Bayer HealthCare (VIVID). Editorial and administrative assistance to the authors was provided by Hadi Moini, PhD, and S. Balachandra Dass, PhD, Regeneron Pharmaceuticals, Inc.” Other conflicts of interest reported in the paper. Trial registration: VISTA NCT01363440, VIVID NCT01331681 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No details available |
| Allocation concealment (selection bias) | Unclear risk | No details available |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "A masked investigator assessed safety and efficacy and decided on the need for laser re‐treatment and additional treatment." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Masked graders at independent central reading centers evaluated OCT images for central retinal thickness (center subfield))" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | About 93% participants completed 52‐week follow‐up in each arm and causes of loss to follow‐up were balanced across arms. Slightly higher loss to follow‐up in laser group in VIVID – approx 15% compared to 8% and 11% in aflibercept groups |
| Selective reporting (reporting bias) | Unclear risk | Some differences between trial registration and final reports |
| Other bias | Low risk | No other bias identified |
| Overall risk of bias | Low risk | Though some items could not be fully assessed, we believe randomisation and allocation concealment should be adequate in this multicentre trial aiming at drug registration, as per regulatory requirement |
| Methods | Multicentre, randomised, and open‐label controlled trial | |
| Participants | Country: Spain Number of people randomised: 83 participants (40 ranibizumab, 43 grid laser) Average age: 63.5 (9.4) years. Sex: 59.8% M Inclusion criteria:
|
|
| Interventions | Participants were randomised to intravitreal injection of ranibizumab (0.5 mg) with 3 loading doses and then PRN treatment or to LP (ratio 1:1). | |
| Outcomes | Primary outcome:
Secondary outcomes:
|
|
| Notes | Sponsor: Novartis Trial Registration: NCT00901186 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information (abstract only; authors contacted but no response yet) |
| Allocation concealment (selection bias) | Unclear risk | No information (abstract only; authors contacted but no response yet) |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No information (abstract only; authors contacted but no response yet) |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information (abstract only; authors contacted but no response yet) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 9 and 11 participants lost to follow‐up for ranibizumab and laser respectively |
| Selective reporting (reporting bias) | Unclear risk | No information (abstract only; authors contacted but no response yet) |
| Other bias | Unclear risk | Insufficient information (abstract only; authors contacted but no response yet) |
| Overall risk of bias | Unclear risk | Most items at unclear risk |
| Methods | Parallel group RCT One eye per person, unclear how eye selected "One eye per participant was included to avoid exposure of both eyes to the study drug. If both eyes were eligible, the eye with worse visual acuity became the study eye. Subjects were randomised with 2:1 probability to receive the intervention or standard care (ETDRS macular laser). The randomization list was created using permuted blocks of varying sizes, held by the trial statistician and concealed from the researcher who enrolled, assessed, and allocated treatment to participants." (Page 961) |
|
| Participants | Country: UK Number of people randomised: 37 (37 eyes) Average age: 66 years Sex: 36% women Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Intervention:
Comparator:
"Subjects were randomised with 2:1 probability to receive the intervention or standard care (ETDRS macular laser)." Page 961. "Intravitreal injections of ranibizumab (Lucentis, 0.5 mg in 0.05 mL solution for injection; Novartis Pharmaceuticals UK Ltd., Frimley, United Kingdom) at baseline, 4 weeks, and 8 weeks then every 4 weeks as required according to predefined retreatment criteria to a maximum of 12 injections. Retreatment occurred if BCVA was reduced by 5 letters or more from maximum acuity or if OCT central subfield thickness was more than 300 mm. Subjects in the laser arm received ETDRS macular laser at baseline guided by fluorescein angiography, OCT, and clinical examination. Laser retreatment occurred at 12, 24, and 36 weeks if clinically significant macular edema was still present, in accordance with standard clinical practice at the time; this was guided by the most recent fluorescein angiogram, OCT, and clinical examination results" Page 961 |
|
| Outcomes | Outcomes:
Follow‐up: 48 weeks |
|
| Notes | Date study conducted: November 2010 to July 2011 Sponsor: Moorfields Eye Hospital NHS Foundation Trust Conflict of interest: "Dr Comyn receives travel support from Novartis. Dr Sivaprasad is a consult for and receives payment for lectures or speaker bureaus and travel support from Novartis, Allergan, and Bayer, and receives payment for development of educational materials from Allergan. Dr Holder is a consultant to Servier. Dr Patel receives grant support from Allergan, Heidelberg United Kingdom, and Topcon United Kingdom and is a consultant to Bayer, Novartis, and Thrombogenics. Dr Hykin is a consultant to and receives grant support from Novartis, Allergan, and Bayer. Drs Comyn, Sivaprasad, Peto, Patel, Egan, Bainbridge, and Hykin have received a proportion of their funding from the Department of Health’s National Institute for Health Research Biomedical Research Centre for Ophthalmology at Moorfields Eye Hospital and University College London, Institute of Ophthalmology. Dr Bainbridge is supported by a National Institute for Health Research Professorship. Supported by an unrestricted research grant from Novartis and the National Institute for Health Research Biomedical Research Centre based at Moorfields Eye Hospital National Health Service Foundation Trust and University College London Institute of Ophthalmology." Page 970 Trial registration: NCT01223612 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The randomization list was created using permuted blocks of varying sizes, held by the trial statistician and concealed from the researcher who enrolled, assessed, and allocated treatment to participants." Page 96 |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No sham procedure |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "The microperimetry and electrophysiologic assessors were masked to the patient treatment arm. Evaluation of OCT scans, fundus photographs and fluorescein angiograms was performed by masked Reading Centre graders. The protocol states that the visual acuity assessors were also masked to the patient treatment arm but due to a protocol deviation they had access to the source notes and were potentially unmasked." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 22/25 (88%) of anti‐VEGF group compared to 11/12 (92%) laser group followed up |
| Selective reporting (reporting bias) | Unclear risk | Unclear risk |
| Other bias | Low risk | No other source of bias identified |
| Overall risk of bias | Unclear risk | High or unclear risk of bias for nearly half the items |
| Methods | Parallel group RCT One eye per person, chosen by participant and physician. In 81% of cases the eye with the worse VA was chosen |
|
| Participants | Country: USA Number of people randomised: 172 (172 eyes) Average age: 62 years (range 27 to 89) Sex: 49% women Inclusion criteria:
(study eyes)
(participants)
Exclusion criteria:
|
|
| Interventions | Intervention:
Comparator:
"Intravitreous pegaptanib or sham injections were administered at entry, week 6, and week 12, for a minimum of 3 injections. Thereafter, additional injections were administered every 6 weeks at the discretion of investigators if judged indicated, to a maximum of 6 injections up to week 30. [...] Pegaptanib was formulated for intravitreous injection at 0.3 mg/90 µl, 1 mg/90 µl, and 3 mg/90 µl concentrations in preservative‐free phosphate‐buffered saline (pH 5–7). Pegaptanib was packaged in sterile, single‐use, United States Pharmacopeia type 1 graduated glass 1‐ml syringes with preattached 27‐gauge needles" Page 1748 |
|
| Outcomes | Outcomes:
Follow‐up: 36 weeks |
|
| Notes | Dates participants enrolled: not reported, study published 2005 Funding:"The study was sponsored by Eyetech Pharmaceuticals, Inc., New York, New York, and Pfizer Inc., New York, New York." Page 1747 Conflict of interest: not reported Trial registration: NCT00040313 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were allocated [...] by a dynamic minimization procedure using a stochastic treatment allocation algorithm based on the variance method. Randomization was stratified by study site, size of the thickened retina area [...] and baseline VA [...]". Page 1748 |
| Allocation concealment (selection bias) | Low risk | "An independent fundus photograph and angiogram reading center confirmed eligibility and appropriate retinal thickness classification both for study entry and for randomization and stratification using baseline fluorescein angiography and OCT." Page 1748 |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Study subjects receiving sham or study medication were treated identically in all regards, including ocular antisepsis procedures and subconjunctival anesthetic, except that subjects receiving active treatment had pegaptanib injected into the vitreous, whereas those receiving sham had a needleless syringe pressed against the conjunctiva and sclera. The injection procedure prevented subjects from seeing the syringe and needle, to minimize the risk of unmasking. In all but 3 subjects, injection was administered by a staff member other than the study ophthalmologist responsible for all other aspects of the protocol, to maintain investigator masking." Page 1748 |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk |
"Study subjects receiving sham or study medication were treated identically in all regards, including ocular antisepsis procedures and subconjunctival anesthetic, except that subjects receiving active treatment had pegaptanib injected into the vitreous, whereas those receiving sham had a needleless syringe pressed against the conjunctiva and sclera. The injection procedure prevented subjects from seeing the syringe and needle, to minimize the risk of unmasking. In all but 3 subjects, injection was administered by a staff member other than the study ophthalmologist responsible for all other aspects of the protocol, to maintain investigator masking. Visual acuity was determined by a separate VA examiner masked to treatment." Page 1748 "At baseline and at each study visit thereafter, refraction and VA were determined and OCT was performed by certified examiners masked both to randomization and to findings of the previous measurement." Page 1749 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Nine participants were discontinued from the study before week 36. None in pegaptanib groups 0.3 mg and 1 mg, 3 in pegaptanib 3 mg group (3 mg subgroup: 2 participants by request at weeks 12 and 16 and 1 by other reason at week 1), 6 in sham group (5 participants by request at weeks 6, 11, 18, 30, and 33 and 1 due to death at week 8) |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all (primary and secondary) outcomes that are of interest in the study have been reported in the pre‐specified way |
| Other bias | Low risk | No other source of bias identified |
| Overall risk of bias | Low risk | Low risk of bias for all items |
| Methods | Parallel group RCT One eye per person, unclear how eye selected |
|
| Participants | Country: Australia, Europe, India, North America, and South America Number of people randomised: 288 (288 eyes) Average age: 62 years (20 to 83) Sex: 43% women Inclusion criteria:
(study eye)
Exclusion criteria:
|
|
| Interventions | Intervention:
Comparator:
Participants received pegaptanib 0.3 mg or sham injections every 6 weeks in year 1 (total 9 injections) and could receive focal/grid photocoagulation beginning at week 18. During year 2, participants received injections as often as every 6 weeks according to pre‐specified criteria |
|
| Outcomes | Primary outcome:
Secondary outcomes: (at 12 and 24 months unless otherwise specified)
Follow‐up: 12 and 24 months |
|
| Notes | Dates participants enrolled: September 2005 to July 2009 Funding:"Sponsored by Pfizer Inc, New York, New York. The sponsor participated in the design of the study, in the management, analysis, and interpretation of the data, and in the preparation and review of the manuscript." Page 12 Conflict of interest: The authors were employees of Pfizer, the sponsor Trial registration: NCT00605280 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "[...] subjects were centrally allocated to receive either pegaptanib 0.3 mg or sham injections (1:1) using a dynamic minimization procedure stratified by the site, hemoglobin A1c (<7.6% vs >=7.6%), systolic blood pressure (<140 vs >=140 mmHg), diastolic blood pressure (80 vs 80 mmHg), and baseline BCVA (<54 vs >=54 letters); the dynamic minimization used a stochastic treatment allocation algorithm based on the variance method." Page 3 |
| Allocation concealment (selection bias) | Low risk | "...subjects were centrally allocated..." Page 3 |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "To maintain masking, the intravitreal procedure was identical between the sham and comparator arms, with the difference lying only in the application of an empty barrel of a needleless syringe in the sham procedure designed to mimic the intravitreal injection." Page 3 |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Throughout the study, BCVA was measured at 4 m by the study refractionist/ophthalmologist, who was masked to the subject’s treatment and to the subject’s previous visual acuity (VA) assessments". Page 3 |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | At 1 year 116/144 (81%) pegaptanib‐treated participants and 114/142 (80%) controls completed the 54‐week visit. Adverse events led to discontinuation of 5 treated and 7 control participants At 2 years 66 participants in each group completed the 102 week visit ITT analysis with LOCF was used leading to the analysis of 133 treated and 127 control participants |
| Selective reporting (reporting bias) | Low risk | All primary outcomes reported |
| Other bias | Low risk | No other biases identified |
| Overall risk of bias | Low risk | Low risk of bias for most items |
| Methods | Parallel group RCT and within‐person study People randomised to treatment but two eyes sometimes included. If two eyes included then fellow eye randomised to other treatment |
|
| Participants | Country: Brazil Number of people randomised: 48 (63 eyes) Average age 64 years Sex: 55% women (based on eyes included in analyses) Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Intervention:
Comparator:
“Retreatment with the originally assigned treatment was performed monthly if central subfield thickness was greater than 275 mm.” “If, after 3 consecutive injections, there was not a reduction in central subfield thickness of at least 10% or an increase in BCVA of at least 5 letters compared with baseline, the patient could, at the discretion of the treating ophthalmologist, receive focal/grid laser photocoagulation or continue to receive the same intravitreal medication for an additional 3 consecutive visits.” Page 503 |
|
| Outcomes | Outcomes reported in publication (primary outcome not specified):
On ClinicalTrials.gov following outcomes listed:
|
|
| Notes | Dates participants enrolled: July 2010 to August 2011 Funding:"Fundacao de Amparo a` Pesquisa do Estado de Sao Paulo (FAPESP), grant number 2010/013368; and Fundacao Apoioao Ensino, Pesquisa e Assisteˆncia (FAEPA) do Hospital das Clınicas da Faculdade de Medicina de Ribeirao Preto da Universidade de Sao Paulo." Conflict of interest: Rodrigo Jorge received travel support from Novartis to attend the 2012 American Society of Retina Specialists (ASRS) meeting Trial registration: NCT01487629 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “.... received the randomised treatment according to a computer‐generated sequence” Page 503 |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “Examiners (E.T., F.P.P.A., R.P.) were masked regarding which treatment drug was used for each patient. Throughout the study, a single masked, certified examiner performed BCVA measurements prior to any other study procedure. Patients, OCT technicians, and fundus photographers were also masked to treatment group”. Page 504 |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “Examiners (E.T., F.P.P.A., R.P.) were masked regarding which treatment drug was used for each patient. Throughout the study, a single masked, certified examiner performed BCVA measurements prior to any other study procedure. Patients, OCT technicians, and fundus photographers were also masked to treatment group”. Page 504 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “The 3 patients excluded from the outcomes analyses consisted of 1 patient in the IV ranibizumab group who developed Staphylococcus aureus endophthalmitis after the first injection (this patient chose to exit the study and he did not complete any further study visits); 1 patient in the IV bevacizumab group who developed advanced posterior subcapsular cataract, which precluded adequate SDOCT images, after the ninth follow‐up visit; and 1 patient from the IV bevacizumab group who missed 3 consecutive follow‐up visits.” Page 504 |
| Selective reporting (reporting bias) | Low risk | Both outcomes listed on trial registration reported |
| Other bias | Unclear risk | Fifteen out of 48 participants with both eyes in analyses |
| Overall risk of bias | Low risk | Low risk of bias for most items |
| Methods | Parallel group RCT One eye per person; if both eyes were eligible, the eye with the greater centre subfield thickness was entered |
|
| Participants | Country: USA Number of people randomised: 126 (126 eyes) Average age: 62 years Sex: 59% women Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Intervention:
Comparator:
Participants were randomised 1:1:1 to receive 0.5 mg ranibizumab at baseline and months 1, 3, and 5 (group 1), focal or grid laser photocoagulation at baseline and month 3 if needed (group 2), or a combination of 0.5 mg ranibizumab and focal or grid laser at baseline and month 3 (group 3). Starting at month 6, if retreatment criteria were met, all participants could be treated with ranibizumab Duration: primary outcome at 6 months, extension to 24 and 36 months |
|
| Outcomes | As reported in publications: Primary outcome:
Secondary outcomes:
On ClinicalTrials.gov "Primary Outcome Measures: Improvement in vision of 15 or more letters, or achieve a final vision of 50 letters (20/25) or better if baseline VA was 40 letters (20/40) [Time Frame: 6 mos, 12 mos and 24 mos. Study Extended to 36 mos.] [Designated as safety issue: Yes] Secondary Outcome Measures: Several outcomes related to OCT measurements and fluorescein angiography. [Time Frame: 6 mos, 12 mos and 24 mos, study extended to 36 mos.] [Designated as safety issue: Yes]" Follow‐up: 6 months and 24 months. |
|
| Notes | Dates participants enrolled: not reported Funding: "Sponsored by the Juvenile Diabetes Research Foundation and Genentech, Inc." Conflict of interest: "QDN and PAC have served as members of Expert Panels for Genentech, Inc. without receiving an honorarium during the time of this study, but JHU has recently negotiated a contract through which JHU receives compensation. QDN is a consultant for Bausch and Lomb and has research support from Genentech, Inc., and Regeneron, Inc. PAC serves on the data and safety monitoring committee for a phase III trial sponsored by Regeneron, Inc., and has research support from Genentech, Alimera, and CoMentis for diabetic macular edema trials. Diana Do receives research support from Genentech. These activities are being managed by the Conflict of Interest Committee of the Johns Hopkins University School of Medicine. JSH is a consultant for Genentech, Alcon, Allergan, Bausch and Lomb, Eyemaginations, Fovea, Genzyme, Heidelburg, IScience, ISTA, Jerini, LPath, NeoVista, Nodal Vision, Novagali, Novartis, Optherion, Oxigene, Paloma, Pfizer, Regeneron, Resolvyx, Schering Plough, Scyfix, and VisionCare and has received honoraria from Genentech, Heidelberg, Jerini, NeoVista, Optimedica, and Regeneron. JL has received honoraria from Genentech. DB is a consultant and has received honoraria from Genentech, Novartis, Alcon, Allergan, and Pfizer. PA is a consultant for Genentech" Page 2181 Trial registration: NCT00407381 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Unclear method of sequence generation and information could not be obtained from the authors |
| Allocation concealment (selection bias) | Unclear risk | Unclear method of allocation concealment and information could not be obtained from the authors |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unclear if masked and who was masked and information could not be obtained from the authors |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unclear if masked and who was masked and information could not be obtained from the authors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Participants randomised to each group: 33 ranibizumab, 34 ranibizumab + laser, 34 laser Completed participants at 1 year: 29, 29, 30 (85% to 88%) Completed participants at 2 years: 24, 26, 24 (71% to 76%) Causes of missing data were balanced across groups |
| Selective reporting (reporting bias) | High risk | The primary outcome differed in the protocol and the final report |
| Other bias | Low risk | No other source of bias identified |
| Overall risk of bias | High risk | High risk of bias for nearly half the items and unclear risk for the others |
| Methods | Parallel group RCT One eye per person, eye with worse VA selected |
|
| Participants | Country: Germany Number of people randomised: 128 (128 eyes) Average age: 64 years (range 31 to 79) Sex: 37% women Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Intervention:
Ranibizumab was applied at baseline, 30, 60, 90 days and reapplied at intervals no shorter than 28 days and laser was applied at baseline and re‐applied if needed at intervals no shorter than 3 months |
|
| Outcomes | Primary outcome:
Secondary outcomes:
|
|
| Notes | Dates participants enrolled: July 2010 to May 2011, terminated early Funding: Novartis Conflict of interest: Novartis Trial registration: NCT01131585 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Reported as double‐masked, but no details given |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Reported as double‐masked, but no details given |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing data: combined laser and ranibizumab: 7/85 (7%); laser 11/43 (26%) |
| Selective reporting (reporting bias) | High risk | Only mean change of VA and harms reported |
| Other bias | Low risk | Study terminated early due to European Medicine Agency approval of ranibizumab for DMO but this is independent of effect estimates |
| Overall risk of bias | Unclear risk | Unclear risk of bias for most items |
| Methods | Parallel group RCT One eye per person, eye with worse VA selected |
|
| Participants | Country: unclear exactly where conducted. Investigators from Australia, Denmark, Austria, France, Germany, Italy, Korea, Portugal, Spain, Switzerland, UK Number of people randomised: 151 (151 eyes) Average age: 64 years (range 32 to 85) Sex: 46% women Inclusion criteria:
(study eye)
Exclusion criteria:
|
|
| Interventions | Intervention:
Comparator:
|
|
| Outcomes | Primary outcome:
Secondary outcomes:
|
|
| Notes | Dates participants enrolled: not reported Funding: Novartis Conflict of interest: authors served on advisory boards for Novartis and received honoraria and travel and accommodation payments; Novartis employees assisted with the analysis, interpretation and writing Trial registration:NCT00284050 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Eligible patients were randomised 1:1:1 to either ranibizumab (0.3 mg or 0.5 mg) or sham treatment according to a computer‐generated randomised allocation schedule" Online appendix page 1 |
| Allocation concealment (selection bias) | Low risk | "...allocation schedule (kept at a secure site and accessible only to the injecting physician" Online appendix page 1 "Based on the patient strata the injecting physician would take the treatment allocation card and tear‐off the cover and follow instructions to choose vial from the box as indicated (3 boxes, randomisation block size 3). The randomisation data were kept strictly confidential until database lock; not accessible to anyone involved in the study with the exception of injecting physician(s) and drug accountability monitor." Online appendix page 1 |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Sham injection for masking participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Masking was maintained through appointment of a minimum of 2 investigators at each study site; unmasked injecting physician and a masked evaluating physician (roles could not be switched)." Online appendix page 1 |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants who completed the trial at 1 year: 92/102 ranibizumab and 40/49 sham. Causes of missingness were balanced ITT analysis with LOCF was used |
| Selective reporting (reporting bias) | Low risk | We could not find a protocol, but primary outcomes were stated in the methods and were those routinely used in the field |
| Other bias | Low risk | No other source of bias identified |
| Overall risk of bias | Low risk | Low risk of bias for most items |
| Methods | Parallel group RCT One eye per person, unclear how eye selected |
|
| Participants | Country: Canada Number of people randomised: 239 (239 eyes) Average age: 62 years (range 26 to 87) Sex: 40% women Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Intervention:
Comparator:
For combination and monotherapy, ranibizumab was administered as 3 monthly injections, then 10 months PRN injections given/withheld based on DME stability criteria. Laser was administered according to ETDRS guidelines at intervals of > 3 months |
|
| Outcomes | On ClinicalTrials.gov Primary Outcome Measures: Measure: mean change from baseline in Best Correct Visual Acuity (BCVA) [Time Frame: 12 months] [Designated as safety issue: No] Secondary Outcome Measures: Measure: number of patients with visual acuity above 73 letters [Time Frame: 3, 6, 9 and 12 months] Measure: number of patients with improvement in BCVA [Time Frame: 3, 6, 9 and 12 months] Measure: time course of BCVA changes [Time Frame: 3, 6, 9 and 12 months] Measure: change in central retinal thickness and other anatomical changes [Time Frame: 3, 6, 9 and 12 months] Measure: 15‐letter (3‐line) gain in BCVA [Time Frame: 3, 6, 9 and 12 months] |
|
| Notes | Dates participants enrolled: July 2010 to March 2013 Funding: Novartis Conflict of interest: authors not reported since the study was obtained as a Novartis public report Trial registration: NCT01135914 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was stratified by centre and followed a permutated block size of 6." |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unmasked study (described as open‐label) |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Unmasked study (described as open‐label) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | More missing data in the laser arm (27%), mainly due to lack of efficacy, compared to the 2 ranibizumab arms (5% to 6%) |
| Selective reporting (reporting bias) | Low risk | VA, OCT data and harms adequately reported (only loss of vision not reported) |
| Other bias | Low risk | No other bias identified |
| Overall risk of bias | High risk | High risk of bias for most items |
| Methods | Parallel group RCT One eye per person, eye with worse VA selected unless other eye more suitable for treatment |
|
| Participants | Country: 10 European countries, Australia, Canada, Turkey Number of people randomised: 345 (345 eyes) Average age: 63 years Sex: 42% women Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Intervention:
Comparator
|
|
| Outcomes | Primary outcome:
Secondary outcomes:
Follow‐up: 12 months |
|
| Notes | Dates participants enrolled: not reported Funding: Novartis Conflict of interest: authors reported financial support of Novartis or were Novartis employees Trial registration: NCT00906464 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A randomization list was produced by, or under the responsibility of, Novartis Drug Supply Management using a validated system that automated the random assignment of treatment arms to randomization numbers in the specified ratio." Appendix 1 |
| Allocation concealment (selection bias) | Low risk | Central randomisation using an electronic Case Report Form after each participant was included by study investigators |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The masked BCVA assessor evaluated the visual acuity of the patient and provided the results to the evaluating investigator who also was masked to the treatment assignment. The evaluating investigator was responsible for all other aspects of the study, excluding the injection procedures. Based on all the performed clinical assessments and the visual acuity (VA) results received from the BCVA assessor, the evaluating investigator had to decide on the treatment requirements for the patient each month and communicated this decision to the treating investigator. The treating investigator was unmasked to the treatment assignment and performed all injections or laser treatment as well as the corresponding sham treatments. He/she was required not be involved in any other aspect of the study and not to divulge the patient’s treatment assignment to anyone. Once the designated roles were determined, the roles could not be switched at any time during the conduct of the study. Every effort was made to limit the number of unmasked study personnel to ensure the integrity of this masked study. An independent review and standardized grading of fundus photography, fluorescein angiography, and optical coherence tomography (OCT) images for the patients screened and enrolled in the study was performed at a central reading center that did not have access to any other data of the patients."Appendix 1 |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Participants randomised in each group were: 116 ranibizumab, 118 ranibizumab + laser, 111 laser At 1 year complete participants were 87.9%, 87.3% and 88.3% respectively There were 2 deaths in each of the 3 treatment arms Used ITT analysis with LOCF |
| Selective reporting (reporting bias) | Low risk | We could not find a protocol, but primary outcomes were stated in the methods and were those routinely used in the field |
| Other bias | Low risk | No other source of bias identified |
| Overall risk of bias | Low risk | Low risk of bias for most items |
| Methods | Parallel group RCT One eye per person, eye with worse VA selected unless other eye more suitable for treatment |
|
| Participants | Country: Asian population from 52 centres across 6 countries, or regions: China, Hong Kong, Japan, South Korea, Singapore, and Taiwan. Number of people randomised: 396 (396 eyes) Average age: 61 years Sex: 44% women Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | "Patients were randomised in a 1:1:1 ratio to 1 of 3 treatment arms: intravitreal ranibizumab 0.5 mg injection + sham laser, intravitreal ranibizumab 0.5 mg injection + active laser, or active laser + sham injections for 12 months" Page 1404 Number in each group: ranibizumab + sham laser (n = 133), ranibizumab + active laser (n =132), or sham injection + active laser (n = 131). |
|
| Outcomes | Primary outcome:
Secondary outcomes:
Follow‐up: 12 months |
|
| Notes | Dates participants enrolled: not reported Funding: Novartis Conflict of interest: authors reported financial support of Novartis or were Novartis employees Trial registration: NCT00989989 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "At Visit 2, all patients who fulfilled all the inclusion/exclusion criteria were given the lowest available number on the randomization list. This number assigned them to one of the treatment arms. The investigator entered the randomization number on the electronic case report form. A randomization list was produced by, or under the responsibility of Novartis Drug Supply Management using a validated system that automated the random assignment of treatment arms to randomization numbers in the specified ratio" |
| Allocation concealment (selection bias) | Low risk | See above |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "To ensure successful masking in this double‐masked study, at the start of the study and at each study site, the following site personnel were required to demonstrate their role: BCVA assessor and evaluating investigator (masked to the treatment assignment) and treating investigator (unmasked to the treatment assignment" Page 1404 |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Higher proportion of loss to follow‐up in the laser group; this can decrease the benefit with anti‐VEGF (see below). "Overall, 345 (87.1%) patients completed the study. The proportion of patients who discontinued the study was 7.5% in the ranibizumab arm, 13.6% in the ranibizumab þ active laser treatment arm, and 17.6% in the laser treatment arm (Fig 2, available at www.aaojournal.org). Adverse events (range, 3.0%‐6.8%) were the most common reason for discontinuation across all treatment arms (Fig 2, available at www.aaojournal.org). Unsatisfactory therapeutic effect (n= 7 [5.3%]) was reported only in the laser arm." Page 1405 |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | No other bias identified |
| Overall risk of bias | Low risk | Low risk for most items |
| Methods | Parallel group RCT One eye per person, unclear how eye selected |
|
| Participants | Country: USA and South America Number of people randomised: 759 (759 eyes) Average age: 62 years Sex: 43% women Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Intervention:
Comparator:
"The median number of ranibizumab injections was 24. The mean number of macular laser treatments over 24 months was 1.8 and 1.6 in the sham groups and 0.3 to 0.8 in the ranibizumab groups. Substantially more sham‐treated patients received macular laser under the protocol‐specified criteria or underwent panretinal photocoagulation for proliferative diabetic retinopathy." Page 5 |
|
| Outcomes | Primary outcome:
Secondary outcomes: (at 24 months)
Follow‐up: 24 months |
|
| Notes | Dates participants enrolled: June 2007 to January 2009 Funding: "This study was supported by Genentech Inc. Support for third‐party writing assistance by Ivo Stoilov, MD, CMPP, of Envision Scientific Solutions was provided by Genentech Inc." "The sponsor participated in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation and review of the manuscript." Page 1121 Conflict of interest: "Dr Ip is a consultant/advisor for Eye Technology Ltd, Genentech Inc, NicOx, Notal Vision, QLT Phototherapeutics Inc, Regeneron, and Sirion and has received grant support from Allergan Inc. Drs Hopkins and Ehrlich and Ms Wong are employees of Genentech Inc, a member of the Roche Group. Drs Hopkins and Ehrlich hold equity and/or options in Roche." Page 1121 Trial registration: RIDE NCT00473382 RISE NCT00473330 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was stratified by study eye BCVA (55 vs 55 ETDRS letters), baseline HbA1c (<=8% vs >8%), prior DME therapy in the study eye (yes vs no), and study site. Dynamic randomization was used to obtain approximately a 1:1:1 ratio among groups (Fig 1). Randomization was done via interactive phone system. The sponsor developed the specifications for the randomization, and a third party programmed and held the randomization algorithm." Page 3, Nguyen et al |
| Allocation concealment (selection bias) | Low risk | "Randomization was stratified by study eye BCVA (55 vs 55 ETDRS letters), baseline HbA1c (<=8% vs >8%), prior DME therapy in the study eye (yes vs no), and study site. Dynamic randomization was used to obtain approximately a 1:1:1 ratio among groups (Fig 1). Randomization was done via interactive phone system. The sponsor developed the specifications for the randomization, and a third party programmed and held the randomization algorithm." Page 3, Nguyen et al |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Ocular assessments, including the need for macular laser, were made by evaluating ophthalmologists masked to patients’ treatment assignments. Study treatments were administered by treating ophthalmologists unmasked to treatment assignments but masked to ranibizumab dose. To improve patient masking, all patients received subconjunctival anesthesia before sham or active injections (performed as previously described).22 Study site personnel (except treating physicians and assistants), central reading center personnel, and the sponsor and its agents (except drug accountability monitors) were masked to treatment assignment. Treating physicians were masked to the assigned dose of ranibizumab." Page 3, Nguyen et al |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The 2‐year study period was completed by 83.3% of participants in RISE and by 84.6% in RIDE; causes of missingness not reported |
| Selective reporting (reporting bias) | Low risk | All VA cut‐offs and secondary outcomes available at 2 years, although not at 1 year, as pre‐planned |
| Other bias | Low risk | No other bias identified |
| Overall risk of bias | Low risk | Low risk of bias for most items |
| Methods | Parallel group RCT One or two eyes per person, in bilateral cases unclear how the second eye allocated |
|
| Participants | Country: Iran Number of people randomised: 129 (150 eyes) Average age: 61 years Sex: 49% women Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Intervention:
Comparator:
Re‐treatment was performed at 12‐week intervals whenever indicated There was another intervention arm which combined bevacizumab with triamcinolone, but this is not included in this review (n = 50 eyes) |
|
| Outcomes | Primary outcome:
Secondary outcomes:
|
|
| Notes | Dates participants enrolled: September 2005 to May 2007 Funding: "Supported by the Ophthalmic Research Center of Shahid Beheshti University (MC) Tehran, Iran." Page 1150 Conflict of interest: "The author(s) have no proprietary or commercial interest in any materials discussed in this article" Page 1150 Trial registration: NCT00370669 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization was performed using random block permutation method according to a computer‐generated randomization list. The block length varied randomly (6, 12). Random allocation sequence was performed by a biostatistician. The detail of series was unknown by the study investigators." Page 2 Soheilian 2009 |
| Allocation concealment (selection bias) | Low risk | "Randomization was performed using random block permutation method according to a computer‐generated randomization list. The block length varied randomly (6, 12). Random allocation sequence was performed by a biostatistician. The detail of series was unknown by the study investigators." Page 2 Soheilian 2009 |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "A sham laser procedure (20 seconds) was performed by aiming the laser beam on the macula for the eyes in the IVB and IVB/IVT groups. In the MPC group, a sham injection was done by a needleless syringe pressed against the conjunctiva. To keep the masking process, patients were prevented from seeing the syringes. All procedures were run by staff members other than the study investigators to preserve investigator masking. Best‐corrected VA measurement and OCT were performed by certified examiners masked both to the randomization and to the findings of previous measurements." Page 2‐3 Soheilian 2009 |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | See above |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | There were 6 missing eyes out of 50 at 36 weeks in the IVB group and 12 out of 50 in the photocoagulation group and causes were not clearly unrelated to VA outcome, except for 2 deaths. In a subsequent publication in 2012 the authors reported 39 (78%) and 38 (76%) eyes in the two arms; 8 participants (12 eyes) missing were dead for causes unrelated to treatment, but other causes of death were not reported |
| Selective reporting (reporting bias) | Low risk | The primary outcomes are continuous measures and no arbitrary cut‐points were used |
| Other bias | High risk | There was an imbalance of baseline VA in the IVB and photocoagulation groups: 0.71 logMAR versus 0.55 logMAR. Although there was a potential unit of analysis issue (150 eyes of 129 patients, 16% of participants had both eyes included), comparisons were made in a marginal regression model (based on generalised estimating equation methods) adjusted for the baseline values and to eliminate any possible correlation effects between the 2 eyes of participants in bilateral enrolled cases. However, we could not take correlation into account when analysing dichotomous VA definitions |
| Overall risk of bias | Unclear risk | High or unclear risk of bias for two items to a degree which we believe may influence effect estimates |
| Methods | Prospective study, treatment in the better‐seeing eye | |
| Participants | Country: Turkey Number of people randomised: 70 participants (35 ranibizumab, 35 grid laser) Average age: 64.6 ± 8.2 years ranibizumab; 63.8 ± 7.4 years laser Sex: 21% male ranibizumab, 18% male laser Inclusion criteria:
Exclusion criteria:
|
|
| Interventions | Focal or grid laser photocoagulation treatment was performed in 35 participants and laser settings, including power, spot size, duration and number of burns, were recorded 35 participants received initial injection of ranibizumab 0.5 mg/0.05 mL. All participants of both groups received treatment in their better‐seeing eye. After the induction phase, the intravitreal injections were administered if any of the following changes were observed: presence of visual acuity loss; persistent or recurrent subretinal or intraretinal fluid. |
|
| Outcomes | Group comparisons of absolute scores and mean changes from baseline scores at 6‐month visit were performed using analysis of the Turkish version of VFQ‐25; it has modifications to adjust for Turkish culture and lifestyle. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No data available |
| Allocation concealment (selection bias) | Unclear risk | No data available |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | No data available |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No data available |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up reported |
| Selective reporting (reporting bias) | Unclear risk | No specific statement nor protocol available |
| Other bias | Low risk | No other bias identified |
| Overall risk of bias | Unclear risk | Most items not reported |
| Methods | Randomised, double‐masked, 3‐period, 2‐treatment crossover design with 4 treatment sequence patterns Each of 3 12‐week periods consisted of 3 intravitreous injections of ranibizumab (0.3 mg) or bevacizumab (1.25 mg), given every 4 weeks, with evaluation of the treatment period 4 weeks after the third dose (i.e. weeks 12, 24, and 36) |
|
| Participants | Country: USA and UK Number of people randomised: 56 participants (62 eyes, 6 pz both eyes) Average age: 62 years Sex: 38.7% women Inclusion criteria: Eligible participants had type 1 or type 2 diabetes mellitus, were at least 18 years of age, and could enter one or both eligible eyes in the study. Principal eligibility criteria for a study eye included: (1) presence of DME involving the centre of the macula, (2) ETDRS BCVA letter score of 78 to 24 (Snellen equivalent, 20/32‐20/400), and (3) CSMT of 330 mm or more on Cirrus (Carl Zeiss Meditec, Inc, Dublin, CA) OCT. Exclusion criteria: Eye included presence of factors or other conditions judged to impact the course of oedema or to preclude possible improvement in vision with treatment; PRP, focal or grid laser photocoagulation, or depot corticosteroid injection within the previous 3 months; ocular injection with an anti‐VEGF agent within the previous 2 months; more than 4 injections with an anti‐VEGF agent within the previous year; or prior vitrectomy. Potential participants were excluded for history of renal failure (requiring haemodialysis or renal transplantation) and for a measured systolic BP of more than 180 mmHg or a diastolic BP of more than 110 mmHg. |
|
| Interventions | Each of 3 12‐week periods consisted of 3 intravitreous injections of ranibizumab (0.3 mg) or bevacizumab (1.25 mg), given every 4 weeks, with evaluation of the treatment period 4 weeks after the third dose (i.e. weeks 12, 24, and 36). Each study eye received 9 monthly injections over the course of the trial, according to a pattern of treatments determined by 1 of 4 randomly assigned sequences: R‐R‐B (n = 17), R‐B‐B (n = 15), B‐B‐R (n = 16), or B‐R‐R (n = 14), where R indicates a series of 3 consecutive ranibizumab injections, and B represents a series of 3 consecutive bevacizumab injections. Study eyes meeting predefined criteria for significant worsening of DME at week 12 or later could receive focal or grid laser photocoagulation. Fellow eyes in participants only enrolling 1 eye could receive any necessary ocular treatment. |
|
| Outcomes | The primary outcome: mean change in BCVA from baseline, estimated for a 3‐month dosing period in a linear mixed‐effects model. The main prespecified secondary outcome was the change in CRT, measured as OCT CSMT, estimated for a 3‐month dosing period using the linear mixed‐effects model. |
|
| Notes | June 2012 and January 2014 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Participants were assigned to 1 of the 4 treatment sequences using a randomization list generated by the Data and Statistical Coordinating Center before study initiation, with balance after every 12 enrollments. The list was provided to unmasked pharmacists at each site, who confirmed a valid participant identification code before dispensing study treatment. Both clinical sites used the same randomised list, but selected treatment assignments from opposite ends. For participants entering both eyes in the trial, the right eye was assigned randomly as above to 1 of the 4 treatment sequences, and the left eye was assigned automatically to the sequencewith the inverse schedule (for example, B‐R‐R in the right eye and R‐B‐B in the left eye). Page 2 |
| Allocation concealment (selection bias) | Low risk | Participants were assigned to 1 of the 4 treatment sequences using a randomization list generated by the Data and Statistical Coordinating Center before study initiation, with balance after every 12 enrollments. The list was provided to unmasked pharmacists at each site, who confirmed a valid participant identification code before dispensing study treatment. Both clinical sites used the same randomised list, but selected treatment assignments from opposite ends. For participants entering both eyes in the trial, the right eye was assigned randomly as above to 1 of the 4 treatment sequences, and the left eye was assigned automatically to the sequencewith the inverse schedule (for example, B‐R‐R in the right eye and R‐B‐B in the left eye). Page 2 |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | “Participants and investigators were masked to treatment. Site staff collecting study data, including research coordinators, technicians, and photographers, were also masked.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “Site staff collecting study data, including research coordinators, technicians, and photographers, were also masked.” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | “One participant with a single eye assigned to the R‐B‐B group withdrew after the week 4 visit after a hemorrhagic stroke. All remaining participants completed the study, including the week 12, 24, and 36 visits, and were included in this analysis” |
| Selective reporting (reporting bias) | Low risk | VA (no SD), OCT (no SD) data and harms adequately reported |
| Other bias | Low risk | Six out of 56 participants had both eyes included in analyses |
| Overall risk of bias | Low risk | Low risk of bias for most items |
Abbreviations BCVA: best‐corrected visual acuity BP: blood pressure CRT: central retinal thickness CSFT: central subfield thickness CSMO: clinically significant macular oedema CSMT: central subfield mean thickness DMO: diabetic macular oedema (DME: US spelling edema) ECG: electrocardiogram EQ‐5D: EuroQol 5D ETDRS: Early Treatment Diabetic Retinopathy Study FAZ: foveal avascular zone FFA: fundus fluorescein angiography GLD: greatest linear dimension HbA1c: glycated haemoglobin IOP: intraocular pressure ITT: intention‐to‐treat iv: intravenous IV: intravitreal injection IVB: intravitreal bevacizumab IVT: intravitreal triamcinolone LOCF: last observation carried forward logMAR: log of the Minimum Angle of Resolution NEI VFQ‐25: National Eye Institute Visual Function Questionnaire‐25 OCT: optical coherence tomography PDR: proliferative diabetic retinopathy PFCL: perifoveal capillary loss PRP: panretinal photocoagulation RCT: randomised controlled trial SD: standard deviation SD‐OCT: spectral‐domain optical coherence tomography TD‐OCT: time‐domain optical coherence tomography VA: visual acuity VEGF: vascular endothelial growth factor
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ahmadieh 2013 | Not an RCT |
| CRFB002DFR08 (LUDIC) | Single‐arm study |
| CRFB002DGB14 (RELIGHT) | Single‐arm study |
| CRFB002DNO02 (PTIMAL) | Single‐arm study |
| DRCRnet 2007 | Follow‐up at 12 weeks only |
| DRCRnet 2011 | Follow‐up at 14 weeks only. RCT comparing ranibizumab (2 injections), triamcinolone (1 injection) to sham in participants with DMO undergoing grid and panretinal laser photocoagulation |
| DRCRnet 2012 | Follow‐up of DRCRnet 2010 comparing prompt to deferred laser in participants treated for ranibizumab for DMO: does not report on comparison of ranibizumab with laser |
| Faghihi 2008 | Follow‐up at 16 weeks only |
| NCT02985619 (BEVATAAC) | Triamcinolone as comparator, unpublished study |
| Paccola 2008 | Single injection of intravitreal triamcinolone acetonide (4 mg/0.1 mL) compared to single injection of intravitreal bevacizumab (1.5 mg/0.06 mL). Duration: 24 weeks |
| Solaiman 2010 | Single intravitreal injection of bevacizumab (inadequate dose); follow‐up 6 months |
| Zehetner 2013 | Physiological study of anti‐VEGF levels only |
Abbreviations DMO: diabetic macular oedema RCT: randomised controlled trial VEGF: vascular endothelial growth factor
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Allocation: randomised Endpoint classification: efficacy study Intervention model: parallel assignment Masking: unclear Primary purpose: treatment |
| Participants | 42 (72 eyes); country: China Male 55%, average age 60 years |
| Interventions | Experimental: ranibizumab 0.5 mg; one week after ranibizumab, the participants received grid photocoagulation intervention: macular area C‐shaped manner, spot size 100 μm to 200 μm, 1 spot width apart, level I to II power and exposure time 0.1 second Active comparator: laser only |
| Outcomes | Primary outcome (time frame: 3 and 6 months):
Secondary outcome:
|
| Notes | Articles in Chinese Authors contacted to obtain additional data |
| Methods | Allocation: randomised study but no other information reported Endpoint classification: efficacy study Intervention model: parallel assignment Masking: unclear Primary purpose: treatment |
| Participants | 70 eyes randomised (42 participants); country: Egypt Male % not reported, average age 55 years |
| Interventions | Experimental: ranibizumab 0.5 mg; aflibercept 2 mg |
| Outcomes | Primary outcome (time frame: 12 months): VA and CRT (cut‐off and instrument not specified) Decimal VA used: no significant difference between ranibizumab and aflibercept Reduction in retinal thickening based on OCT: no significant difference between ranibizumab and aflibercept Number of re‐injections after the loading dose: 2.62 (SD 0.68) aflibercept, 3.03 (SD 0.95) ranibizumab (P = 0.02) |
| Notes | Authors contacted to obtain additional data |
| Methods | Allocation: quasi‐randomised, participants with even visit numbers were allocated to intervention group, whereas participants with odd visit numbers were allocated to control group Endpoint classification: efficacy study Intervention model: parallel assignment Masking: unclear Primary purpose: treatment |
| Participants | 78; country: China Male 55%, average age 51 to 54 years |
| Interventions | Experimental: ranibizumab 0.5 mg Active comparator: argon laser 532μm, green, 50 μm to 100 μm spots, intensity 100 mW to 200 mW and exposure time 0.1 second |
| Outcomes | Primary outcome (time frame: 3 and 6 months):
Secondary outcome:
|
| Notes | Article in Chinese Authors contacted to obtain additional data |
| Methods | Allocation: randomised Endpoint classification: efficacy study Intervention model: parallel assignment Masking: unclear Primary purpose: treatment |
| Participants | 72 (120 eyes); country: Poland Average age: 60 years Inclusion criteria: severe DMO that affects the fovea, reduction in VA and/or metamorphopsia, diffuse oedema with or without cystic oedema (confirmed by fluorescein angiography and by OCT), CRT ≥ 300 μm, the absence of hard lipid exudates in the form of plaque in the subfoveal region, no prior laser treatment, no prior VEGF inhibitor treatment, and no previous intravitreal or subtenonian corticosteroid administration. The exclusion criteria were: high risk and advanced proliferative DR (PDR), the presence of other eye diseases that could affect VA, prior eye surgeries |
| Interventions | Experimental: bevacizumab 1.25 mg, one or more injections, with or without laser photocoagulation after 4 to 6 weeks depending on clinical response Active comparator: laser only |
| Outcomes | Primary outcome: not specified Outcommes reported: VA (logMAR), CRT based on OCT Quantitative results are available, but not at specified follow‐up times and by number of injection and combination rather than by assigned subgroup |
| Notes | Sponsor: none reported Authors contacted to obtain additional information |
| Methods | Allocation: randomised Endpoint classification: efficacy study Intervention model: parallel assignment Masking: open‐label Primary purpose: treatment |
| Participants | 49, country: USA |
| Interventions | Experimental: I
Active comparator: II
|
| Outcomes | Primary outcome (time frame: 6 and 12 months):
Secondary outcome:
|
| Notes | Completion Date: February 2009 (No Results) Author contact not found Sponsor: Rocky Mountain Retina Consultants Collaborator: Genentech, Inc. |
| Methods | Allocation: randomised Endpoint classification: safety/efficacy study Intervention model: parallel assignment Masking: open‐label Primary purpose: treatment |
| Participants | 12; country: Brazil |
| Interventions | Procedure: laser photocoagulation Drug: intravitreal triamcinolone Drug: intravitreal bevacizumab |
| Outcomes | Primary outcome (time frame: 1 year):
Secondary outcomes:
|
| Notes | Completion date: November 2011 (no results) Author could not be contacted Sponsor: University of Sao Paulo Collaborator: Fundacao de Amparo a Pesquisa do Estado de Sao Paolo |
| Methods | Allocation: randomised Endpoint classification: safety/efficacy study Intervention model: parallel assignment Masking: double‐masked (participant, caregiver, investigator) |
| Participants | 264; countries: USA, Israel |
| Interventions | Drug: PF‐04523655 (Stratum I) Drug: PF‐04523655 and ranibizumab Drug: ranibizumab Drug: PF‐04523655 (Stratum II) |
| Outcomes | Primary outcomes: Safety and dose‐limiting toxicities (Stratum I): to determine the safety and dose‐limiting toxicities of a single intravitreal (IVT) injection of PF‐04523655 in people with low vision Pharmacokinetics (Stratum I): to determine the pharmacokinetics (PK) of a single IVT injection of PF‐04523655 in people with low vision Safety and tolerability (Stratum II): to evaluate the safety and tolerability of PF‐04523655 alone and in combination with ranibizumab in participants with DMO Efficacy (Stratum II): to evaluate the ability of PF‐04523655 alone and in combination with ranibizumab to improve VA compared to ranibizumab alone in people with DMO |
| Notes | Completion date: November 2013 (no results) Author contact not found Sponsor: Quark Pharmaceuticals Consider putting in excluded studies |
| Methods | Allocation: randomised Endpoint classification: safety/efficacy study Intervention model: factorial assignment Masking: open‐label |
| Participants | 208; country: USA |
| Interventions | Experimental Group 1: drug: iCo‐007 350 µg as an intravitreal injection at baseline followed by another iCo‐007 dose (350 μg) at month 4 Experimental Group 2: drug: iCo‐007 700 µg as an intravitreal injection at baseline followed by another iCo‐007 dose (700 μg) at month 4 Experimental Group 3: drug: iCo‐007 350 µg as an intravitreal injection at baseline followed 7 days later by laser photocoagulation. At month 4, intravitreal injection of iCo‐007 (350 μg) will be given as mandatory treatment. If the eye also meets retreatment criteria, it will also receive the second laser photocoagulation Experimental Group 4: drug: ranibizumab 0.5 mg intravitreal injection at baseline followed by iCo‐007 350 μg intravitreal injection 2 weeks later; re‐treatment with ranibizumab 0.5 mg mandatory at month 4 followed by iCo‐007 350 μg 2 weeks later |
| Outcomes | Primary outcome:
Secondary outcomes:
|
| Notes | Study has passed its completion date and status has not been verified in more than 2 years on ClinicalTrials.gov Author contacted Sponsor: Quan Dong Nguyen Collaborators: Juvenile Diabetes Research Foundation, iCo Therapeutics Inc |
Abbreviations BCVA: best‐corrected visual acuity CFST: central subfield macular thickness CRT: central retinal thickness DMO: diabetic macular oedema EQ‐5D: EuroQol 5D ETDRS: Early Treatment Diabetic Retinopathy Study OCT: optical coherence tomography PRN: pro re nata SD: standard deviation TE: treat and extend VA: visual acuity VEGF: vascular endothelial growth factor VFQ‐25: Visual Function Questionnaire 25‐item
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | A randomised controlled trial to compare the efficacy and safety of 1) macular laser vs 2) repeated intravitreal bevacizumab vs 3) combined repeated intravitreal bevacizumab with macular laser for diabetic macular edema |
| Methods | Parallel group RCT |
| Participants | People with type 2 diabetes and DMO |
| Interventions | Group 1 (Control): macular laser photocoagulation performed every 4 months unless the deferral criteria are met. Group 2: intravitreal bevacizumab injections (1.25 mg each) given at 0, 1, 2 months and repeated en bloc every 4 months unless the deferral criteria are met. Group 3: Intravitreal bevacizumab injections (1.25 mg each) given at 0, 1, 2 months, followed by macular laser photocoagulation at month 3; and repeated en bloc every 4 months unless the deferral criteria are met |
| Outcomes | BCVA at 2 years |
| Starting date | Unknown; trial registered 13 August 2013 |
| Contact information | Joyce Kung (joycekung@cuhk.edu.hk); Carmen Chan (kmcc2001@hotmail.com) |
| Notes | Status checked on Chictr.Org.Cn on 1 December 2016. Author contacted |
| Trial name or title | Comparing the effectiveness and costs of bevacizumab to ranibizumab in participants with diabetic macular edema (BRDME) |
| Methods | Parallel group RCT |
| Participants | 246 people with DMO |
| Interventions | Ranibizumab compared to bevacizumab |
| Outcomes | From clinical trials record: Primary outcome:
Secondary outcome measures:
http://clinicaltrials.gov/show/NCT01635790 |
| Starting date | Study start date: June 2012 Estimated primary completion date: June 2016 (Final data collection date for primary outcome measure). |
| Contact information | Reinier O Schlingemann (r.schlingemann@amc.uva.nl); Monique Wezel (m.wazel@amc.uva.nl) |
| Notes | Recruiting (status checked on ClinicalTrials.gov on 1 December 2016) |
| Trial name or title | Safety and Efficacy Study of Conbercept in Diabetic Macular Edema (DME) (Sailing) |
| Methods | Parallel group RCT |
| Participants | type 1 or type 2 diabetes mellitus with DMO |
| Interventions | Experimental: Conbercept treatment group Conbercept injection and sham laser treatment at day 0 for 1st time, the investigators will decide whether the participants need to get repeated treatment according to monthly assessment. Active Comparator: Laser treatment group Laser treatment and sham injection at day 0 for 1st time, the investigators will decide whether the repeated laser treatment is needed according to monthly results during the visit after 3 months. |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | July 2014 Estimated Study Completion Date: September 2017 Estimated Primary Completion Date: December 2016 (Final data collection date for primary outcome measure) |
| Contact information | Chengdu Kanghong Biotech Co.,Ltd. |
| Notes | This study is recruiting participants. (Status checked on ClinicalTrials.Gov on 5 December 2016) |
| Trial name or title | A 12‐month, Randomized, Efficacy and Safety Study of 0.5 mg Ranibizumab vs Laser in Chinese DME Patients |
| Methods | Parallel group RCT |
| Participants | Participants with DMO with BCVA score between 78 and 39 |
| Interventions | Ranibizumab PRN versus laser |
| Outcomes | Primary outcome measures:
Secondary Outcome Measures:
|
| Starting date | November 2014 Estimated study completion date: January 2017 Estimated primary completion date: January 2017 (final data collection date for primary outcome measure) |
| Contact information | Novartis (Novartis Pharmaceuticals) |
| Notes | This study is ongoing, but not recruiting participants. (Status checked on ClinicalTrials.gov on 5 December 2016) |
| Trial name or title | A Phase 2 Randomized, Controlled, Double‐Masked, Multicenter Clinical Trial Designed to Evaluate the Safety and Exploratory Efficacy of Luminate® (ALG‐1001) as Compared to Avastin® and Focal Laser Photocoagulation in the Treatment of Diabetic Macular Edema |
| Methods | Parallel group RCT |
| Participants | DMO participants |
| Interventions | Experimental: Luminate 1.0 mg group
Experimental: Luminate 2.0 mg group
Experimental: Luminate 3.0 mg group
Active Comparator: Avastin® group
Active Comparator: focal laser photocoagulation group
|
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | October 2014 Estimated study completion date: March 2016 Estimated primary completion date: December 2015 (final data collection date for primary outcome measure) |
| Contact information | Allegro Ophthalmics, LLC |
| Notes | This study is recruiting participants; (status checked on ClinicalTrials.gov on 5 Dec. 2016) |
| Trial name or title | The Effect of Bevacizumab and Ziv‐aflibercept in Diabetic Macular Edema |
| Methods | Parallel group RCT |
| Participants | DMO participants |
| Interventions | Active comparator: injection intravitreous bevacizumab Active comparator: injection ziv‐aflibercept at dose of 1.25 mg Active comparator: injection ziv‐aflibercept at dose of 2.5 mg |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | Study first received: 2 January 2016 Last updated: 4 January 2016 Estimated primary completion date: February 2016 (final data collection date for primary outcome measure) |
| Contact information | Zahra Rabbani Khah, Shahid Beheshti Medical University |
| Notes | This study is recruiting participants; (status checked on ClinicalTrials.gov on 5 December 2016) |
| Trial name or title | A Phase 2 Study of RO6867461 in Participants With Center‐Involving Diabetic Macular Edema (CI‐DME) (BOULEVARD) |
| Methods | Parallel group RCT |
| Participants | DMO |
| Interventions | Experimental: RO6867461 1.5 mg
Experimental: RO6867461 6 mg
Active Comparator: Ranibizumab 0.3 mg
|
| Outcomes | Primary outcome measures:
Secondary Outcome Measures: Percentage of participants gaining ≥ 15 letters from baseline BCVA at Week 24 (time frame: baseline, and Week 24) Percentage of participants with BCVA ≥ 69 letters (20/40 or better) at Week 24 (time frame: Week 24) Percentage of participants with BCVA ≥ 84 letters (20/20 or better) at Week 24 (time frame: Week 24) Mean change from baseline in BCVA at Week 28 (time frame: baseline, and Week 28) Mean change from baseline in foveal centre point thickness at Week 24 and 28, as measures by spectral domain OCT (SD‐OCT) (time frame: baseline, Weeks 24 and 28) Mean change from baseline in mean CSFT at Week 24 and 28, as measures by SD‐OCT (time frame: baseline, Weeks 24 and 28) Percentage of participants with resolution of subretinal and intraretinal fluid at Week 24 and 28, as measures by SD‐OCT (time frame: Weeks 24 and 28) Percentage of participants with resolution of leakage at the macula at Week 24, as measures by FFA (time frame: Week 24) Change from baseline in the size of the foveal avascular zone at Week 24, as measures by FFA (time frame: baseline and Week 24) Change from baseline in plasma levels of vascular endothelial growth factor (VEGF) (time frame: baseline, Weeks 1, 4, 12, 24, 26, and 28 or early termination) Change from baseline in plasma levels of angiopoietin‐2 (Ang‐2) (time frame: baseline, Weeks 1, 4, 12, 24, 26, and 28 or early termination) (designated as safety issue: no) Maximum observed plasma concentration (Cmax) of RO6867461 (time frame: pre‐dose on Days 1, 28, 84, 140, and 168; post‐dose on Days 7, 182, and 196 or early termination) Area under the plasma concentration‐time curve from time zero to extrapolated infinite time [AUC (0‐inf)] of RO6867461 (time frame: pre‐dose on Days 1, 28, 84, 140, and 168; post‐dose on Days 7, 182, and 196 or early termination) Area under the plasma concentration‐time curve from time zero to end of dosing interval [AUC (0‐tau)] of RO6867461 (time frame: pre‐dose on Days 1, 28, 84, 140, and 168; post‐dose on Days 7, 182, and 196 or early termination) Time to reach maximum observed plasma concentration (Tmax) of RO6867461 (time frame: pre‐dose on Days 1, 28, 84, 140, and 168; post‐dose on Days 7, 182, and 196 or early termination) Plasma decay half‐life (t1/2) of RO6867461 (time frame: pre‐dose on Days 1, 28, 84, 140, and 168; post‐dose on Days 7, 182, and 196 or early termination) Number of participants with adverse events (time frame: baseline up to Week 28 or early termination) Number of participants with anti‐RO6867461 antibodies (time frame: baseline, Weeks 1, 4, 12, 20, 24, 26, and 28 or early termination) |
| Starting date | March 2016 Estimated Study Completion Date: October 2017 Estimated Primary Completion Date: October 2017 (final data collection date for primary outcome measure) |
| Contact information | Hoffmann‐La Roche |
| Notes | This study is recruiting participants; (status checked on ClinicalTrials.gov on 5 December 2016) |
| Trial name or title | Anti‐vasculaR Endothelial Growth Factor plUs Anti‐angiopoietin 2 in Fixed comBination therapY: Evaluation for the Treatment of Diabetic Macular Edema (RUBY) |
| Methods | Parallel group RCT |
| Participants | participants with DMO |
| Interventions | Experimental: Group 1 Participants in Group 1 will receive REGN910‐3 dosing regimen 1 Experimental: Group 2 Participants in Group 2 will receive REGN910‐3 dosing regimen 2 Active Comparator: Group 3 Participants in Group 3 will receive IAI |
| Outcomes | Primary outcome measures:
Secondary outcome measures:
|
| Starting date | March 2016 Estimated study completion date: October 2017 Estimated primary completion date: April 2017 (final data collection date for primary outcome measure) |
| Contact information | Regeneron Pharmaceuticals |
| Notes | This study is on ongoing, but not recruiting participants; (status checked on ClinicalTrials.gov on 5 December 2016) |
Abbreviations BCVA: best‐corrected visual acuity CSFT: central subfield thickness CRT: central retinal thickness CSMO: clinically significant macular oedema DMO: diabetic macular oedema (DME: US spelling edema) ETDRS: Early Treatment Diabetic Retinopathy Study FFA: fundus fluorescein angiography IOP: intraocular pressure NA: not available OCT: optical coherence tomography PDR: proliferative diabetic retinopathy PRN: pro re nata (as required in the circumstances) PRP: panretinal photocoagulation RCT: randomised controlled trial VA: visual acuity
Contributions of authors
Conceiving the review: GV, MP
Designing the review: GV, MP, EL
Co‐ordinating the review: GV
Data collection for the review.
Designing search strategies: IG
Undertaking searches: IG
Screening search results: GV, MP
Organising retrieval of papers: IG
Screening retrieved papers against inclusion criteria: GV, Cochrane Eyes and Vision
Appraising quality of papers: GV, MP
Extracting data from papers: GV, MP, EL
Writing to authors of papers for additional information: GV, MP
Obtaining and screening data on unpublished studies: GV, Cochrane Eyes and Vision
Data management for the review.
Entering data into Review Manager 5: GV, MP, EL
Analysis of data: GV, EL
Interpretation of data.
Providing a methodological perspective: GV, EL
Providing a clinical perspective: GV, MP
Providing a policy perspective: GV, MP, EL
Providing a consumer perspective: none
Writing the review: GV, MP, EL
Sources of support
Internal sources
Azienda Ospedaliero‐Universitaria Careggi & University of Florence, based on funding by the Tuscany Region, Italy.
External sources
-
National Institute for Health Research (NIHR), UK.
- Richard Wormald, Co‐ordinating Editor for Cochrane Eyes and Vision (CEV) acknowledges financial support for his CEV research sessions from the Department of Health through the award made by the NIHR to Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology for a Specialist Biomedical Research Centre for Ophthalmology.
- This review was supported by the NIHR, via Cochrane Infrastructure funding to the CEV UK editorial base.
- The Cochrane Review Incentive Scheme provided funding for Jennifer Evans to assist with the 2014 update of this review.
The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Declarations of interest
Gianni Virgili: none known Mariacristina Parravano received payment for participating on the Advisory Board for Allergan, Bayer and Novartis. Jennifer Evans: none known Iris Gordon: none known Ersilia Lucenteforte: none known
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
- Ahmadieh H, Ramezani A, Shoeibi N, Bijanzadeh B, Tabatabaei A, Azarmina M, et al. Intravitreal bevacizumab with or without triamcinolone for refractory diabetic macular edema; a placebo‐controlled, randomized clinical trial. Graefe's Archive for Clinical and Experimental Ophthalmology 2008;246(4):483‐9. [DOI] [PubMed] [Google Scholar]
- Azad R, Sain S, Sharma YR, Mahajan D. Comparison of intravitreal bevacizumab, intravitreal triamcinolone acetonide, and macular grid augmentation in refractory diffuse diabetic macular edema: A prospective, randomized study. Oman Journal of Ophthalmology2012; Vol. 5, issue 3:166‐70. [DOI] [PMC free article] [PubMed]
- Michaelides M, Kaines A, Hamilton RD, Fraser‐Bell S, Rajendram R, Quhill F, et al. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12‐month data: report 2. Ophthalmology 2010;117(6):1078‐86. [DOI] [PubMed] [Google Scholar]; Rajendram R, Fraser‐Bell S, Kaines A, Michaelides M, Hamilton RD, Esposti SD, et al. A 2‐year prospective randomized controlled trial of intravitreal bevacizumab or laser therapy (BOLT) in the management of diabetic macular edema: 24‐month data: report 3. Archives of Ophthalmology 2012;130(8):972‐9. [DOI] [PubMed] [Google Scholar]; Sivaprasad S, Crosby‐Nwaobi R, Esposti S, Peto T, Rajendram R, Michaelides M, et al. Structural and functional measures of efficacy in response to bevacizumab monotherapy in diabetic macular oedema: exploratory analyses of the BOLT Study (Report 4). PloS ONE 2013;8(8):e72755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do DV, Nguyen QD, Boyer D, Schmidt‐Erfurth U, Brown DM, Vitti R, et al. One‐year outcomes of the DA VINCI Study of VEGF Trap‐Eye in eyes with diabetic macular edema. Ophthalmology 2012;119(8):1658‐65. [DOI] [PubMed] [Google Scholar]; Do DV, Schmidt‐Erfurth U, Gonzalez VH, Gordon CM, Tolentino M, Berliner AJ, et al. The DAVINCI Study: phase 2 primary results of VEGF Trap‐Eye in patients with diabetic macular edema. Ophthalmology 2011;118(9):1819‐26. [DOI] [PubMed] [Google Scholar]
- Anonymous. Erratum: Patient‐reported visual function outcomes improve after ranibizumab treatment in patients with vision impairment due to diabetic macular edema: Randomized clinical trial (JAMA Ophthalmology (2013) 131:10 (1339‐1347) DOI: 10.1001/jamaophthalmol.2013.4592). JAMA Ophthalmology 2013;131(12):1652. [DOI] [PubMed] [Google Scholar]; Bressler SB, Qin H, Beck RW, Chalam KV, Kim JE, Melia M, et al. Factors associated with changes in visual acuity and central subfield thickness at 1 year after treatment for diabetic macular edema with ranibizumab. Archives of Ophthalmology 2012;130(9):1153‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]; Bressler SB, Qin H, Melia M, Bressler NM, Beck RW, Chan CK, et al. Exploratory analysis of the effect of intravitreal ranibizumab or triamcinolone on worsening of diabetic retinopathy in a randomized clinical trial. JAMA Ophthalmology 2013;131(8):1033‐40. [DOI] [PMC free article] [PubMed] [Google Scholar]; Dewan V, Lambert D, Edler J, Kymes S, Apte RS. Cost‐effectiveness analysis of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 2012;119(8):1679‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]; Diabetic Retinopathy Clinical Research Network, Elman MJ, Aiello LP, Beck RW, Bressler NM, Bressler SB, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 2010;117(6):1064–77. [DOI] [PMC free article] [PubMed] [Google Scholar]; Elman MJ, Bressler NM, Qin H, Beck RW, Ferris FL 3rd, Friedman SM, et al. Expanded 2‐year follow‐up of ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology 2011;118(4):609‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]; Elman MJ, Qin H, Aiello LP, Beck RW, Bressler NM, Ferris FL, et al. Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: three‐year randomized trial results. Ophthalmology 2012;119(11):2312‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetic Retinopathy Clinical Research Network, Wells JA, Glassman AR, Ayala AR, Jampol LM, Aiello LP, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. New England Journal of Medicine 2015;372(13):1193‐203. [DOI] [PMC free article] [PubMed] [Google Scholar]; Wells JA, Glassman AR, Ayala AR, Jampol LM, Bressler NM, et al. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema: two‐year results from a comparative effectiveness randomized clinical trial. Ophthalmology 2016;123(6):1351‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekinci M, Ceylan E, Cakici O, Tanyildiz B, Olcaysu O, Cagatay HH. Treatment of macular edema in diabetic retinopathy: Comparison of the efficacy of intravitreal bevacizumab and ranibizumab injections. Expert Review of Ophthalmology 2014;9(2):139‐43. [Google Scholar]
- Ishibashi T, Yuzawa M, Yoshimura N, Ohji M, Ishida S, Isogawa N, et al. Japan phase 3 study of pegaptanib sodium in patients with diabetic macular edema. Nippon Ganka Gakkai Zasshi 2014;118(9):773‐82. [PubMed] [Google Scholar]; NCT01100307. A phase 3 study to compare the efficacy and safety of 0.3 mg pegaptanib sodium to sham Injections In subjects with diabetic macular edema [A phase 3, randomized, controlled, double‐masked, multi‐center, comparative, in parallel roups (for 24 weeks), to compare the efficacy and safety of 0.3 mg pegaptanib sodium, with sham injections, and open study (for 30 weeks) to confirm the safety of 0.3 mg pegaptanib sodium in subjects with diabetic macular edema (DME)]. clinicaltrials.gov/show/NCT01100307 (first received 7 April 2010).
- Korobelnik JF, Do DV, Schmidt‐Erfurth U, Boyer DS, Holz FG, Heier JS, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology 2014;121(11):2247‐54. [DOI] [PubMed] [Google Scholar]
- Lopez‐Galvez MI, Arias L, Roura M. Efficacy and safety profile of ranibizumab versus laser photocoagulation in patients with diabetic macular edema. Re‐Des Study. Ophthalmologica 2014;232:115. [Google Scholar]
- Comyn O, Sivaprasad S, Peto T, Neveu MM, Holder GE, Xing W, et al. A randomized trial to assess functional and structural effects of ranibizumab versus laser in diabetic macular edema (the LUCIDATE study). American Journal of Ophthalmology2014; Vol. 157, issue 5:960‐70. [DOI] [PubMed]
- Cunningham ET Jr, Adamis AP, Altaweel M, Aiello LP, Bressler NM, D'Amico DJ, et al. A phase II randomized double‐masked trial of pegaptanib, an anti‐vascular endothelial growth factor aptamer, for diabetic macular edema. Ophthalmology 2005;112(10):1747‐57. [DOI] [PubMed] [Google Scholar]
- Loftus JV, Sultan MB, Pleil AM, Macugen 1013 Study Group. Changes in vision and health‐related quality of life in patients with diabetic macular edema treated with pegaptanib sodium or sham. Investigative Ophthalmology and Visual Science 2011;52(10):7498‐505. [DOI] [PubMed] [Google Scholar]; Sultan MB, Zhou D, Loftus J, Dombi T, Ice KS, Macugen 1013 Study Group. A phase 2/3, multicenter, randomized, double‐masked, 2‐year trial of pegaptanib sodium for the treatment of diabetic macular edema. Ophthalmology 2011;118(6):1107‐18. [DOI] [PubMed] [Google Scholar]
- Nepomuceno AB, Takaki E, Paes de Almeida FP, Peroni R, Cardillo JA, Siqueira RC, et al. A prospective randomized trial of intravitreal bevacizumab versus ranibizumab for the management of diabetic macular edema. American Journal of Ophthalmology 2013;156(3):502‐10. [DOI] [PubMed] [Google Scholar]
- Do DV, Nguyen QD, Khwaja AA, Channa R, Sepah YJ, Sophie R, et al. Ranibizumab for edema of the macula in diabetes study: 3‐year outcomes and the need for prolonged frequent treatment. JAMA Ophthalmology 2013;131(2):139‐45. [DOI] [PubMed] [Google Scholar]; Nguyen QD, Shah SM, Heier JS, Do DV, Lim J, Boyer D, et al. Primary end point (six months) results of the ranibizumab for edema of the mAcula in diabetes (READ‐2) study. Ophthalmology 2009;116(11):2175‐81. [DOI] [PubMed] [Google Scholar]; Nguyen QD, Shah SM, Khwaja AA, Channa R, Hatef E, Do DV, et al. Two‐year outcomes of the ranibizumab for edema of the mAcula in diabetes (READ‐2) study. Ophthalmology 2010;117(11):2146‐51. [DOI] [PubMed] [Google Scholar]
- CRFB002DD13. A 12‐month, two‐armed, randomized, double‐masked, multicenter, phase IIIb study assessing the efficacy and safety of laser photocoagulation as adjunctive to ranibizumab intravitreal injections vs. laser photocoagulation monotherapy in patients with visual impairment due to diabetic macular edema followed by a 12 month follow up period. Novartis clinical trial results database www.novctrd.com/ctrdWebApp/clinicaltrialrepository/public/login.jsp (accessed 2 June 2014). ; NCT01131585. A 12‐month, two‐armed, randomized, double‐masked, multicenter, phase IIIb study assessing the efficacy and safety of laser photocoagulation as adjunctive to ranibizumab intravitreal injections vs. laser photocoagulation monotherapy in patients with visual impairment due to diabetic macular edema followed by a 12 month follow up period. clinicaltrials.gov/show/NCT01131585 (first received 25 May 2010).
- CRFB002D2201. A randomized, double‐masked, multicenter, phase II study assessing the safety and efficacy of two concentrations of ranibizumab (intravitreal injections) compared with non‐treatment control for the treatment of diabetic macular edema with center involvement. Novartis clinical trial results database www.novctrd.com/ctrdWebApp/clinicaltrialrepository/public/login.jsp (accessed 2 June 2014). ; Massin P, Bandello F, Garweg JG, Hansen LL, Harding SP, Larsen M, et al. Safety and efficacy of ranibizumab in diabetic macular edema (RESOLVE Study): a 12‐month, randomized, controlled, double‐masked, multicenter phase II study. Diabetes Care 2010;33(11):2399‐405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger A, Sheidow T, Li R, Rehel B, Takacsy F, Courseau AS. A Canadian 12‐month, phase IIIb study of ranibizumab combination or monotherapy in visual impairment due to diabetic macular edema: Preliminary analysis ("RESPOND"). 16th Annual Canadian Diabetes Association/Canadian Society of Endocrinology and Metabolism Professional Conference and Annual Meetings; 2013 Oct 17‐19; Montreal. 2013. [Google Scholar]; CRFB002DCA05. A Canadian 12‐month, prospective, randomized, open‐label, multicenter, phase IIIb study assessing the efficacy, safety and cost of ranibizumab as combination and monotherapy in patients with visual impairment due to diabetic macular edema. Novartis clinical trial results database www.novctrd.com/ctrdWebApp/clinicaltrialrepository/public/login.jsp (accessed 2 June 2014).
- Anonymous. Erratum: Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: Three year randomized trial results (Ophthalmology 2012;119:2312‐8). Ophthalmology 2014;121(3):805. [DOI] [PMC free article] [PubMed] [Google Scholar]; Lang GE, Berta A, Eldem BM, Simader C, Sharp D, Holz FG, et al. Two‐year safety and efficacy of ranibizumab 0.5 mg in diabetic macular edema: interim analysis of the RESTORE extension study. Ophthalmology2013; Vol. 120, issue 10:2004‐12. [DOI] [PubMed]; Mitchell P, Annemans L, Gallagher M, Hasan R, Thomas S, Gairy K, et al. Cost‐effectiveness of ranibizumab in treatment of diabetic macular oedema (DME) causing visual impairment: evidence from the RESTORE trial. British Journal of Opthalmology2012; Vol. 96, issue 5:688‐93. [DOI] [PMC free article] [PubMed]; Mitchell P, Bandello F, Schmidt‐Erfurth U, Lang GE, Massin P, Schlingemann RO, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology 2011;118(4):615–62. [DOI] [PubMed] [Google Scholar]; Mitchell P, Bressler N, Tolley K, Gallagher M, Petrillo J, Ferreira A, et al. Patient‐reported visual function outcomes improve after ranibizumab treatment in patients with vision impairment due to diabetic macular edema: randomized clinical trial. JAMA Ophthalmology 2013;131(10):1339‐47. [DOI] [PubMed] [Google Scholar]; Schmidt‐Erfurth U, Lang GE, Holz FG, Schlingemann RO, Lanzetta P, Massin P, et al. Three‐year outcomes of individualized ranibizumab treatment in patients with diabetic macular edema: the RESTORE extension study. Ophthalmology 2014;121(5):1045‐53. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Li X, Koh A, Lai TY, Lee FL, Lee WK, et al. The REVEAL Study: ranibizumab monotherapy or combined with laser versus laser monotherapy in asian patients with diabetic macular edema. Ophthalmology 2015;122(7):1402‐15. [DOI] [PubMed] [Google Scholar]
- Bressler NM, Varma R, Suñer IJ, Dolan CM, Ward J, Ehrlich JS, et al. Vision‐related function after ranibizumab treatment for diabetic macular edema: results from RIDE and RISE. Ophthalmology 2014;121(12):2461‐72. [DOI] [PubMed] [Google Scholar]; Brown DM, Nguyen QD, Marcus DM, Boyer DS, Patel S, Feiner L, et al. Long‐term outcomes of ranibizumab therapy for diabetic macular edema: the 36‐month results from two phase III trials: RISE and RIDE. Ophthalmology 2013;120(10):2013‐22. [DOI] [PubMed] [Google Scholar]; Ip MS, Domalpally A, Hopkins JJ, Wong P, Ehrlich JS. Long‐term effects of ranibizumab on diabetic retinopathy severity and progression. Archives of Ophthalmology 2012;130(9):1145‐52. [DOI] [PubMed] [Google Scholar]; Mieler WF, Kim JE, Yau L, Ehrlich JS. Earlier treatment is important in diabetic macular edema: Outcomes from phase III trials of intravitreal ranibizumab. 73rd Scientific sessions of the American Diabetes Association; 2013 Jun 21‐25; Chicago. 2013. [Google Scholar]; Nguyen QD, Brown DM, Marcus DM, Boyer DS, Patel S, Feiner L, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology 2012;119(4):789‐801. [DOI] [PubMed] [Google Scholar]
- Soheilian M, Garfami KH, Ramezani A, Yaseri M, Peyman GA. Two‐year results of a randomized trial of intravitreal bevacizumab alone or combined with triamcinolone versus laser in diabetic macular edema. Retina 2012;32(2):314‐21. [DOI] [PubMed] [Google Scholar]; Soheilian M, Ramezani A, Bijanzadeh B, Yaseri M, Ahmadieh H, Dehghan MH, et al. Intravitreal bevacizumab (avastin) injection alone or combined with triamcinolone versus macular photocoagulation as primary treatment of diabetic macular edema. Retina 2007;27(9):1187‐95. [DOI] [PubMed] [Google Scholar]; Soheilian M, Ramezani A, Obudi A, Bijanzadeh B, Salehipour M, Yaseri M, et al. Randomized trial of intravitreal bevacizumab alone or combined with triamcinolone versus macular photocoagulation in diabetic macular edema. Ophthalmology 2009;116(6):1142‐50. [DOI] [PubMed] [Google Scholar]
- Turkoglu EB, Celık E, Aksoy N, Bursalı O, Ucak T, Alagoz G. Changes in vision related quality of life in patients with diabetic macular edema: ranibizumab or laser treatment?. Journal of Diabetes and its Complications 2015;29(4):540‐3. [DOI] [PubMed] [Google Scholar]
- Wiley HE, Thompson DJ, Bailey C, Chew EY, Cukras CA, Jaffe GJ, et al. A crossover design for comparative efficacy: a 36‐week randomized trial of bevacizumab and ranibizumab for diabetic macular edema. Ophthalmology 2016;123(4):841‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
- Ahmadieh H, Nourinia R, Hafezi‐Moghadam A. Intravitreal fasudil combined with bevacizumab for persistent diabetic macular edema. JAMA Ophthalmology 2013;131(7):923‐4. [DOI] [PubMed] [Google Scholar]
- CRFB002DFR08. Open‐label, multicenter, study of the efficacy and safety of Lucentis® (ranibizumab 0.5 mg) in diabetic patients presenting a visual impairment due to diabetic macular edema in current medical practice (LUDIC). Novartis clinical trial results database. www.novctrd.com/CtrdWeb/displaypdf.nov?trialresultid=9803 (accessed 28 May 2014).
- CRFB002DGB14. RELIGHT ‐ Ranibizumab treatment of diabetic macular oEdema with bimonthLy monItorinG after a pHase of initial Treatment. A UK, 18‐month, prospective, open‐label, multicentre, single‐arm Phase IIIb study, with 12‐month primary endpoint, assessing the efficacy and safety of Ranibizumab in patients with visual impairment. Novartis clinical trial results database www.novctrd.com/ctrdWebApp/clinicaltrialrepository/public/products.jsp?divisionId=2&diseaseAreaID=12 (accessed 28 May 2014).
- CRFB002DNO02. An open‐label, prospective, multicenter, uncontrolled, Proof of concept study assessing the efficacy of Lucentis (ranibizumab) administered by an individualized “treat and extend” dosing regimen in patients with visual impairment due to dIabetic macular edema. Novartis clinical trial results database. www.novctrd.com/CtrdWeb/displaypdf.nov?trialresultid=10423 (accessed 28 May 2014).
- Diabetic Retinopathy Clinical Research Network, Scott IU, Edwards AR, Beck RW, Bressler NM, Chan CK, et al. A phase II randomized clinical trial of intravitreal bevacizumab for diabetic macular edema. Ophthalmology 2007;114(10):1860‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetic Retinopathy Clinical Research Network, Googe J, Brucker AJ, Bressler NM, Qin H, Aiello LP, et al. Randomized trial evaluating short‐term effects of intravitreal ranibizumab or triamcinolone acetonide on macular edema after focal/grid laser for diabetic macular edema in eyes also receiving panretinal photocoagulation. Retina 2011;31(6):1009‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diabetic Retinopathy Clinical Research Network, Elman MJ, Qin H, Aiello LP, Beck RW, Bressler NM, et al. Intravitreal ranibizumab for diabetic macular edema with prompt versus deferred laser treatment: three‐year randomized trial results. Ophthalmology2012; Vol. 119, issue 11:2312‐8. [DOI] [PMC free article] [PubMed]
- Faghihi H, Roohipoor R, Mohammadi SF, Hojat‐Jalali K, Mirshahi A, Lashay A, et al. Intravitreal bevacizumab versus combined bevacizumab‐triamcinolone versus macular laser photocoagulation in diabetic macular edema. European Journal of Ophthalmology 2008;18(6):941‐8. [DOI] [PubMed] [Google Scholar]
- NCT02985619. Bevacizumabe or triamcinolone for persistent diabetic macular edema (BEVATAAC) [Randomized trial evaluating bevacizumabe or triamcinolone for persistent diabetic macular edema]. clinicaltrials.gov/ct2/show/NCT02985619 (first received 1 December 2016).
- Paccola L, Costa RA, Folgosa MS, Barbosa JC, Scott IU, Jorge R. Intravitreal triamcinolone versus bevacizumab for treatment of refractory diabetic macular oedema (IBEME study). British Journal of Ophthalmology 2008;92(1):76‐80. [DOI] [PubMed] [Google Scholar]
- Solaiman KA, Diab MM, Abo‐Elenin M. Intravitreal bevacizumab and/or macular photocoagulation as a primary treatment for diffuse diabetic macular edema. Retina 2010;30(10):1638‐45. [DOI] [PubMed] [Google Scholar]
- Zehetner C, Kirchmair R, Huber S, Kralinger MT, Kieselbach GF. Plasma levels of vascular endothelial growth factor before and after intravitreal injection of bevacizumab, ranibizumab and pegaptanib in patients with age‐related macular degeneration, and in patients with diabetic macular oedema. British Journal of Ophthalmology 2013;97(4):454‐9. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
- Chen ZX, Fu JS, Song W, Wang CX, Zhang YL. Effect analysis of ranibizumab with laser photocoagulation therapy for diabetic macular edema. International Eye Science 2016;16(4):706‐8. [Google Scholar]
- Fouda SM, Bahgat AM. Intravitreal aflibercept versus intravitreal ranibizumab for the treatment of diabetic macular edema. Clinical Ophthalmology 2017;23:567‐71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JD, Song ZY. Clinical study of grid pattern laser photocoagulation with ranibizumab for diabetic macular edema. International Eye Science 2016;16(3):493‐5. [Google Scholar]
- Jovanović S, Čanadanović V, Sabo A, Grgić Z, Mitrović M, Rakić D. Intravitreal bevacizumab injection alone or combined with macular photocoagulation compared to macular photocoagulation as primary treatment of diabetic macular edema. Vojnosanitetski Pregled 2015;72(10):876‐82. [DOI] [PubMed] [Google Scholar]
- NCT00387582. Efficacy study of lucentis in the treatment of diabetic macular edema ‐ a phase II, single center, randomized study to evaluate the efficacy of ranibizumab versus focal laser treatment in subjects with diabetic macular edema [Lucentis in the treatment of macular edema ‐ a phase II, single center, randomized study to evaluate the efficacy of ranibizumab versus focal laser treatment in subjects with diabetic macular edema]. clinicaltrials.gov/show/NCT00387582 (first received 11 October 2006).
- NCT00997191. Intravitreal bevacizumab and intravitreal triamcinolone associated to laser photocoagulation for diabetic macular edema (IBeTA). clinicaltrials.gov/show/NCT00997191 (first received 15 October 2009).
- NCT01445899. PF‐04523655 dose escalation study, and evaluation of PF‐04523655 with/without ranibizumab in diabetic macular edema (DME) (MATISSE) [An open‐label dose escalation study of PF‐04523655 (Stratum I) combined with a prospective, randomized, double‐masked, multi‐center, controlled study (Stratum II) evaluating the efficacy and safety of PF‐04523655 alone and in combination with ranibizumab versus ranibizumab alone in diabetic macular edema (MATISSE STUDY)]. clinicaltrials.gov/ct2/show/study/NCT01445899 (first received 30 September 2011).
- NCT01565148. A randomized, multi‐center, phase II study of the safety, tolerability, and bioactivity of repeated intravitreal injections of iCo‐007 as monotherapy or in combination with ranibizumab or laser photocoagulation in the treatment of diabetic macular edema (the iDEAL Study) (iDEAL) [A randomized, multi‐center, phase II study of the safety, tolerability, and bioactivity of repeated intravitreal injections of iCo‐007 as monotherapy or in combination with ranibizumab or laser photocoagulation in the treatment of diabetic macular edema with involvement of the foveAL center (the iDEAL Study)]. clinicaltrials.gov/ct2/show/study/NCT01565148 (first received 15 March 2012).
References to ongoing studies
- ChiCTR‐TRC‐12002417. A randomized controlled trial to compare the efficacy and safety of 1) macular laser vs. 2) repeated intravitreal bevacizumab vs. 3) combined repeated intravitreal bevacizumab with macular laser for diabetic macular edema. www.chictr.org.cn/hvshowproject.aspx?id=3319 (accessed 17 September 2014).
- NCT01635790. Comparing the effectiveness and costs of bevacizumab to ranibizumab in patients with diabetic macular edema (BRDME) (BRDME). clinicaltrials.gov/ct2/show/NCT01635790 (first received 28 June 2012). [DOI] [PMC free article] [PubMed]
- NCT02194634. Safety and efficacy study of conbercept in diabetic macular edema (DME) (Sailing). clinicaltrials.gov/ct2/show/NCT02194634 (first received 14 July 2014).
- NCT02259088. A 12‐month, randomized, efficacy and safety study of 0.5 mg ranibizumab vs laser in Chinese DME patients [A 12‐month, randomized, double‐masked, multicenter, laser‐controlled phase III study assessing the efficacy and safety of 0.5 mg ranibizumab dosed PRN in subjects with visual impairment due to diabetic macular edema in Chinese patients]. clinicaltrials.gov/ct2/show/NCT02259088 (first received 3 October 2014).
- NCT02348918. A phase 2 randomized, controlled, double‐masked, multicenter clinical trial designed to evaluate the safety and exploratory efficacy of Luminate® (ALG‐1001) as compared to Avastin® and focal laser photocoagulation in the treatment of diabetic macular edema. clinicaltrials.gov/ct2/show/NCT02348918 (first received 12 January 2015).
- NCT02645734. The effect of bevacizumab and ziv‐aflibercept in diabetic macular edema [The comparison between the therapeutic effect of intravitreal bevacizumab and ziv‐aflibercept in diabetic macular edema]. clinicaltrials.gov/ct2/show/NCT02645734 (first received 2 January 2016).
- NCT02699450. A phase 2 study of RO6867461 in participants with center‐involving diabetic macular edema (CI‐DME) (BOULEVARD). clinicaltrials.gov/ct2/show/NCT02699450 (first received 1 March 2016).
- NCT02712008. Anti‐vasculaR endothelial growth factor plUs anti‐angiopoietin 2 in fixed comBination therapY: evaluation for the treatment of diabetic macular edema [A randomized, double‐masked, active‐controlled, phase 2 study of the efficacy, safety, and tolerability of repeated doses of intravitreal REGN910‐3 in patients with diabetic macular edema]. clinicaltrials.gov/ct2/show/NCT02712008 (first received 14 March 2016).
Additional references
- Aiello LP. Angiogenic pathways in diabetic retinopathy. New England Journal of Medicine 2005;353(8):839‐41. [DOI] [PubMed] [Google Scholar]
- Antcliff RJ, Marshall J. The pathogenesis of edema in diabetic maculopathy. Seminars in Ophthalmology 1999;14(4):223‐32. [DOI] [PubMed] [Google Scholar]
- Arevalo JF, Lasave AF, Wu L, Diaz‐Llopis M, Gallego‐Pinazo R, Alezzandrini AA, et al. Intravitreal bevacizumab plus grid laser photocoagulation or intravitreal bevacizumab or grid laser photocoagulation for diffuse diabetic macular edema: results of the Pan‐american Collaborative Retina Study Group at 24 months. Retina 2013;33(2):403‐13. [DOI] [PubMed] [Google Scholar]
- Anonymous. Collaborative overview of randomised trials of antiplatelet therapy‐I: Prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Antiplatelet Trialists' Collaboration. BMJ 1994;308(6921):81‐106. [PMC free article] [PubMed] [Google Scholar]
- Banfi R, Attanasio F, Palazzi N, Colombini S, Falai T, Cecchi M, et al. Bevacizumab versus ranibizumab: why are we not playing the joker?. International Journal of Clinical Pharmacy 2013;35(4):507‐9. [DOI] [PubMed] [Google Scholar]
- Borm GF, Lemmers O, Fransen J, Donders R. The evidence provided by a single trial is less reliable than its statistical analysis suggests. Journal of Clinical Epidemiology 2009;62(7):711‐5. [DOI] [PubMed] [Google Scholar]
- Brown JC, Solomon SD, Bressler SB, Schachat AP, DiBernardo C, Bressler NM. Detection of diabetic foveal edema: contact lens biomicroscopy compared with optical coherence tomography. Archives of Ophthalmology 2004;122(3):330‐5. [DOI] [PubMed] [Google Scholar]
- Browning DJ, Altaweel MM, Bressler NM, Bressler SB, Scott IU, Diabetic Retinopathy Clinical Research Network. Diabetic macular edema: what is focal and what is diffuse?. American Journal of Ophthalmology 2008;146(5):649‐55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campochiaro PA, Heier JS, Feiner L, Gray S, Saroj N, Rundle AC, et al. Ranibizumab for macular edema following branch retinal vein occlusion: six‐month primary end point results of a phase III study. Ophthalmology 2010;117(6):1102‐12. [DOI] [PubMed] [Google Scholar]
- CATT Research Group. Martin DF, Maguire MG, Ying GS, Grunwald JE, Fine SL, Jaffe GJ. Ranibizumab and bevacizumab for neovascular age‐related macular degeneration. New England Journal of Medicine 2011;364(20):1897‐908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaimani A, Higgins JP, Mavridis D, Spyridonos P, Salanti G. Graphical tools for network meta‐analysis in STATA. PLoS One 2013;8(10):e76654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chong V. Ranibizumab for the treatment of wet AMD: a summary of real‐world studies. Eye 2016;30(11):270‐86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciulla TA, Amador AG, Zinman B. Diabetic retinopathy and diabetic macular edema: pathophysiology, screening, and novel therapies. Diabetes Care 2003;26(9):2653‐64. [DOI] [PubMed] [Google Scholar]
- Ciulla TA, Walker JD, Fong DS, Criswell MH. Corticosteroids in posterior segment disease: an update on new delivery systems and new indications. Current Opinions in Ophthalmology 2004;15(3):211‐20. [DOI] [PubMed] [Google Scholar]
- Cunningham ET Jr, Adamis AP, Altaweel M, Aiello LP, Bressler NM, D'Amico DJ, et al. A phase II randomized double‐masked trial of pegaptanib, an anti‐vascular endothelial growth factor aptamer, for diabetic macular edema. Ophthalmology 2005;112(10):1747‐57. [DOI] [PubMed] [Google Scholar]
- Deeks JJ, Higgins JP, Altman DG, editor(s). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration 2011. Available from handbook.cochrane.org.
- Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta‐analysis. Statistics in Medicine 2010;29:932‐944. [DOI] [PubMed] [Google Scholar]
- Early Treatment Diabetic Retinopathy Study Research Group. Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Archives of Ophthalmology 1985;103(12):1796‐806. [PubMed] [Google Scholar]
- Schmidt‐Erfurth U, Garcia‐Arumi J, Bandello F, Berg K, Chakravarthy U, Gerendas BS, et al. Guidelines for the management of diabetic macular edema by the European Society of Retina Specialists (EURETINA). Ophthalmologica 2017 Apr 20 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Ford JA, Elders A, Shyangdan D, Royle P, Waugh N. The relative clinical effectiveness of ranibizumab and bevacizumab in diabetic macular oedema: an indirect comparison in a systematic review. BMJ 2012;345:e5182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank RN. Diabetic retinopathy. New England Journal of Medicine 2004;350(1):48‐58. [DOI] [PubMed] [Google Scholar]
- Glanville JM, Lefebvre C, Miles JN, Camosso‐Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. Journal of the Medical Library Association 2006;94(2):130‐6. [PMC free article] [PubMed] [Google Scholar]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 6 April 2017. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
- Grover D, Li TJ, Chong CC. Intravitreal steroids for macular edema in diabetes. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD005656.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence‐imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [DOI] [PubMed] [Google Scholar]
- Haller JA, Kuppermann BD, Blumenkranz MS, Williams GA, Weinberg DV, Chou C, et al. Randomized controlled trial of an intravitreous dexamethasone drug delivery system in patients with diabetic macular edema. Archives of Ophthalmology 2010;128(3):289‐96. [DOI] [PubMed] [Google Scholar]
- Heier JS, Bressler NM, Avery RL, Bakri SJ, Boyer DS, Brown DM, et al. Comparison of aflibercept, bevacizumab, and ranibizumab for treatment of diabetic macular edema: extrapolation of data to clinical practice. JAMA Ophthalmology 2016;134(1):95‐9. [DOI] [PubMed] [Google Scholar]
- Higgins JP, Deeks JJ, Altman DG, editor(s). Chapter 16: Special topics in statistics. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- Higgins JP, Altman DG, Sterne JAC, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- Higgins JP, Giovane C, Chaimani A, Caldwell DM, Salanti G. Evaluating the quality of evidence from a network meta‐analysis. Value Health 2014;17:A324. [DOI] [PubMed] [Google Scholar]
- Hussain RM, Ciulla TA. Treatment strategies for refractory diabetic macular edema: switching anti‐VEGF treatments, adopting corticosteroid‐based treatments, and combination therapy. Expert Opinion on Biological Therapy 2016;16(3):365‐74. [DOI] [PubMed] [Google Scholar]
- Jampol LM, Bressler NM, Glassman AR. Revolution to a new standard treatment of diabetic macular edema. JAMA 2014;311(22):2269‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S, Barner JC, Park C, Ling YL. Treatment patterns of anti‐vascular endothelial growth factor and laser therapy among patients with diabetic macular edema. Journal of Managed Care and Specialty Pharmacy 2015;21(9):735‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein R, Klein BE, Moss SE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. IV. Diabetic macular edema. Ophthalmology 1984;91(12):1464‐74. [DOI] [PubMed] [Google Scholar]
- Korobelnik JF, Kleijnen J, Lang SH, Birnie R, Leadley RM, Misso K, et al. Systematic review and mixed treatment comparison of intravitreal aflibercept with other therapies for diabetic macular edema (DME). BMC Ophthalmology 2015;15:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuppermann BD, Chou C, Weinberg DV, Whitcup SM, Haller JA, Blumenkranz MS. Intravitreous dexamethasone effects on different patterns of diabetic macular edema. Archives of Ophthalmology 2010;128(5):642‐3. [DOI] [PubMed] [Google Scholar]
- Anti‐vascular endothelial growth factor treatment for diabetic macular edema. www.cms.gov/medicare‐coverage‐database/details/technology‐assessments‐details.aspx?TAId=85&bc=AAAQAAAAAAAA& (accessed 24 Sept 2012).
- Moja L, Lucenteforte E, Kwag KH, Bertele V, Campomori A, Chakravarthy U, et al. Systemic safety of bevacizumab versus ranibizumab for neovascular age‐related macular degeneration. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD011230.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson J, Sharp P, Goatman K, Prescott G, Scotland G, Fleming A, et al. Improving the economic value of photographic screening for optical coherence tomography‐detectable macular oedema: a prospective, multicentre, UK study. Health Technology Assessment 2013;17(51):1‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medical Advisory Secretariat. Optical coherence tomography for age‐related macular degeneration and diabetic macular edema: an evidence‐based analysis. Ontario Health Technology Assessment Series 2009;9(13):1‐22. [PMC free article] [PubMed] [Google Scholar]
- Ramu J, Yang Y, Menon G, Bailey C, Narendran N, Bunce C, et al. A randomized clinical trial comparing fixed vs pro‐re‐nata dosing of Ozurdex in refractory diabetic macular oedema (OZDRY study). Eye 2015;29(12):1603‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliarini S, Beatty S, Lipkova B, Perez‐Salvador Garcia E, Reynders S, et al. A 2‐year, phase IV, multicentre, observational study of ranibizumab 0.5 mg in patients with neovascular age‐related macular degeneration in routine clinical practice: The EPICOHORT study. Journal of Ophthalmology 2014;2014:Article ID 857148. [DOI: 10.1155/2014/857148] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrao NV, Antao S, Egan C, Omar A, Hamilton R, Hykin PG, et al. Real‐world outcomes of ranibizumab treatment for diabetic macular edema in a United Kingdom national health service setting. American Journal of Ophthalmology 2016;172:51‐7. [DOI] [PubMed] [Google Scholar]
- Callanan DG, Gupta S, Boyer DS, Ciulla TA, Singer MA, Kuppermann BD, et al. Dexamethasone intravitreal implant in combination with laser photocoagulation for the treatment of diffuse diabetic macular edema. Ophthalmology 2013;120(9):1843‐51. [DOI] [PubMed] [Google Scholar]
- Regnier S, Malcolm W, Allen F, Wright J, Bezlyak V. Efficacy of anti‐VEGF and laser photocoagulation in the treatment of visual impairment due to diabetic macular edema: a systematic review and network meta‐analysis. PloS ONE 2014;9(7):e102309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- Salanti G. Indirect and mixed‐treatment comparison, network, or multiple‐treatments meta‐analysis: many names, many benefits, many concerns for the next generation evidence synthesis tool. Research Synthesis Methods 2012;3:80‐97. [DOI] [PubMed] [Google Scholar]
- Salanti G, Giovane C, Chaimani A, Caldwell DM, Higgins JP. Evaluating the quality of evidence from a network meta‐analysis. PLoS One 2014;9:e99682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schünemann HJ, Oxman AD, Vist GE, Higgins JP, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions.In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- Stewart MW, Narayanan R, Gupta V, Rosenfeld PJ, Martin DF, Chakravarthy U. Counterfeit Avastin in India: Punish the criminals, not the patients. American Journal of Ophthalmology 2016;170:228‐31. [DOI] [PubMed] [Google Scholar]
- Tranos PG, Wickremasinghe SS, Stangos NT, Topouzis F, Tsinopoulos I, Pavesio CE. Macular edema. Survey of Ophthalmology 2004;49(5):470‐90. [DOI] [PubMed] [Google Scholar]
- White IR. Network meta‐analysis. Stata Journal 2015;15:951‐85. [Google Scholar]
- Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, et al. Global prevalence and major risk factors of diabetic retinopathy. Diabetes Care 2012;35(3):556‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Wang W, Gao Y, Lan J, Xie L. The efficacy and safety of current treatments in diabetic macular edema: a systematic review and network meta‐analysis. PLoS One 2016;11:e0159553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziemssen F, Feltgen N, Holz FG, Guthoff R, Ringwald A, Bertelmann T, et al. Demographics of patients receiving Intravitrealanti‐VEGF treatment in real‐world practice: healthcare research data versus randomized controlled trials. BMC Ophthalmology 2017;17:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
- Parravano M, Menchini F. Antiangiogenic therapy with anti‐vascular endothelial growth factor modalities for diabetic macular oedema. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD007419] [DOI] [PubMed] [Google Scholar]
- Parravano M, Menchini F, Virgili G. Antiangiogenic therapy with anti‐vascular endothelial growth factor modalities for diabetic macular oedema. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD007419.pub2] [DOI] [PubMed] [Google Scholar]
- Virgili G, Parravano M, Menchini F, Brunetti M. Antiangiogenic therapy with anti‐vascular endothelial growth factor modalities for diabetic macular oedema. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD007419.pub3] [DOI] [PubMed] [Google Scholar]
- Virgili G, Parravano M, Menchini F, Evans JR. Anti‐vascular endothelial growth factor for diabetic macular oedema. Cochrane Database of Systematic Reviews 2014, Issue 11. [DOI: 10.1002/14651858.CD007419.pub4] [DOI] [PubMed] [Google Scholar]