Abstract
Background
Cardiovascular disease is the most common cause of death globally. Traditionally, centre‐based cardiac rehabilitation programmes are offered to individuals after cardiac events to aid recovery and prevent further cardiac illness. Home‐based cardiac rehabilitation programmes have been introduced in an attempt to widen access and participation. This is an update of a review previously published in 2009 and 2015.
Objectives
To compare the effect of home‐based and supervised centre‐based cardiac rehabilitation on mortality and morbidity, exercise‐capacity, health‐related quality of life, and modifiable cardiac risk factors in patients with heart disease.
Search methods
We updated searches from the previous Cochrane Review by searching the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE (Ovid), Embase (Ovid), PsycINFO (Ovid) and CINAHL (EBSCO) on 21 September 2016. We also searched two clinical trials registers as well as previous systematic reviews and reference lists of included studies. No language restrictions were applied.
Selection criteria
We included randomised controlled trials, including parallel group, cross‐over or quasi‐randomised designs) that compared centre‐based cardiac rehabilitation (e.g. hospital, gymnasium, sports centre) with home‐based programmes in adults with myocardial infarction, angina, heart failure or who had undergone revascularisation.
Data collection and analysis
Two review authors independently screened all identified references for inclusion based on pre‐defined inclusion criteria. Disagreements were resolved through discussion or by involving a third review author. Two authors independently extracted outcome data and study characteristics and assessed risk of bias. Quality of evidence was assessed using GRADE principles and a Summary of findings table was created.
Main results
We included six new studies (624 participants) for this update, which now includes a total of 23 trials that randomised a total of 2890 participants undergoing cardiac rehabilitation. Participants had an acute myocardial infarction, revascularisation or heart failure. A number of studies provided insufficient detail to enable assessment of potential risk of bias, in particular, details of generation and concealment of random allocation sequencing and blinding of outcome assessment were poorly reported.
No evidence of a difference was seen between home‐ and centre‐based cardiac rehabilitation in clinical primary outcomes up to 12 months of follow up: total mortality (relative risk (RR) = 1.19, 95% CI 0.65 to 2.16; participants = 1505; studies = 11/comparisons = 13; very low quality evidence), exercise capacity (standardised mean difference (SMD) = ‐0.13, 95% CI ‐0.28 to 0.02; participants = 2255; studies = 22/comparisons = 26; low quality evidence), or health‐related quality of life up to 24 months (not estimable). Trials were generally of short duration, with only three studies reporting outcomes beyond 12 months (exercise capacity: SMD 0.11, 95% CI ‐0.01 to 0.23; participants = 1074; studies = 3; moderate quality evidence). However, there was evidence of marginally higher levels of programme completion (RR 1.04, 95% CI 1.00 to 1.08; participants = 2615; studies = 22/comparisons = 26; low quality evidence) by home‐based participants.
Authors' conclusions
This update supports previous conclusions that home‐ and centre‐based forms of cardiac rehabilitation seem to be similarly effective in improving clinical and health‐related quality of life outcomes in patients after myocardial infarction or revascularisation, or with heart failure. This finding supports the continued expansion of evidence‐based, home‐based cardiac rehabilitation programmes. The choice of participating in a more traditional and supervised centre‐based programme or a home‐based programme may reflect local availability and consider the preference of the individual patient. Further data are needed to determine whether the effects of home‐ and centre‐based cardiac rehabilitation reported in the included short‐term trials can be confirmed in the longer term and need to consider adequately powered non‐inferiority or equivalence study designs.
Plain language summary
Home‐based versus supervised centre‐based cardiac rehabilitation
Review question
We compared home‐based cardiac rehabilitation programmes with supervised centre‐based cardiac rehabilitation for adults with myocardial infarction (blood flow to the heart has stopped), angina (chest pain), heart failure or who had undergone revascularisation.
Background
Cardiac rehabilitation aims to restore people with heart disease to health, through a combination of exercise, education and psychological support. Traditionally, centre‐based cardiac rehabilitation programmes (e.g. based at a hospital, gymnasium or in sport centre) are offered to people after cardiac events. Home‐based cardiac rehabilitation programmes have been introduced to increase access and participation.
Search date
We searched up to September 2016.
Study characteristics
We searched for randomised controlled trials (trials that randomly allocate participants to one of two or more treatment groups) looking at the effectiveness of home‐based versus supervised centre‐based cardiac rehabilitation programmes, in adults with heart disease.
We included 23 trials (2890 participants). Most trials were relatively small (median 104 participants, range: 20 to 525). The average age of trial participants ranged from 51.6 to 69 years. Women accounted for only 19% of recruited participants; four trials did not include women.
The mix of people recruited to the trials varied; 10 studies included a mixed population of people with coronary heart disease, five studies included people who had had a heart attack, and four studies each recruited people following revascularisation or who had heart failure.
Study funding sources
Sixteen studies reported sources of funding; seven did not. No study reported funding from an agency with commercial interest in the results.
Key results
We found that home‐ and centre‐based cardiac rehabilitation programmes are similar in benefits measured in terms of numbers of deaths, exercise capacity and health‐related quality of life. Further data are needed to confirm if these short‐term effects of home‐ and centre‐based cardiac rehabilitation can be sustained over time.
Quality of the evidence
Poor reporting made it difficult to assess methodological quality of the included studies and their risk of bias. Evidence quality ranged from very low (total mortality), to moderate (exercise capacity over 12 months and health‐related quality of life). The main reasons for the low assessment of quality was poor reporting in the included studies.
Summary of findings
Summary of findings for the main comparison. Home‐based versus supervised centre‐based cardiac rehabilitation for heart disease.
| Home‐based versus supervised centre‐based cardiac rehabilitation for heart disease | ||||||
| Patient or population: Patients with heart disease Settings: Home and rehabilitation centres Intervention: Home‐based cardiac rehabilitation Comparison: Centre‐based cardiac rehabilitation | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with centre‐based | Risk with home‐base | |||||
| Total mortality Number of deaths Follow‐up: up to 12 months | Study population | RR 1.19 (0.65 to 2.16) | 1505 (11 studies/ 13 comparisons) | ⊕⊝⊝⊝ VERY LOW 1 2 | ||
| 22 per 1,000 | 26 per 1,000 (14 to 47) | |||||
| Exercise capacity ≤ 12 months Validated outcome measure (e.g. VO₂ peak, 6 minute walk test) Follow‐up: 2 to 12 months | The mean exercise capacity ≤ 12 months ranged from ‐2 to 3,509.33 | SMD 0.13 lower (0.28 lower to 0.02 higher) | ‐ | 2255 (22 studies /26 comparisons) | ⊕⊕⊝⊝ LOW 1 3 | Higher score indicates improved activity. A rule of thumb for interpreting SMD is that 0.2 represents a small effect, 0.5 a moderate effect and 0.8 a large effect (Cohen 1988) |
| Withdrawal from the intervention group Number of completers (participants with data at follow‐up) Follow‐up: 2 to 72 months | Study population | RR 1.04 (1.00 to 1.08) | 2615 (22 studies/ 26 comparisons) | ⊕⊕⊝⊝ LOW 1 3 | ||
| 816 per 1,000 | 848 per 1,000 (816 to 881) | |||||
| HRQoL Validated measures of HRQoL (e.g. Short Form Health Survey (SF‐36), Sickness Impact Profile, Nottingham Health Profile) Follow‐up: 2 to 24 months | HRQoL in home‐based cardiac rehabilitation = HRQoL in centre‐based cardiac rehabilitation, in 61/67 domains | Not estimable | 2079 (14 studies/ 15 comparison) | ⊕⊕⊕⊝ MODERATE1 | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Random sequence generation, allocation concealment or blinding of outcome assessors were poorly described in over 50% of included studies; bias likely, therefore quality of evidence downgraded by one level.
2 The 95% CIs includes both no effect, appreciate benefit and appreciable harm (i.e. CI < 0.75 and > 1.25), therefore quality of evidence downgraded by two levels.
3 I² > 50%; heterogeneity may be important and therefore quality of evidence downgraded by one level
Background
Description of the condition
Cardiovascular disease (CVD) is the leading cause of death globally: in 2015 an estimated 17.7 million people died from CVD, representing 31% of all global deaths (WHO 2016). Of these deaths, an estimated 7.4 million were due to coronary heart disease (CHD) and 6.7 million were due to stroke (WHO 2016). Over three quarters of CVD deaths occur in low‐ and middle‐income countries (WHO 2016).
Coronary heart disease is caused by the build‐up of plaque inside the coronary arteries (atherosclerosis), causing arterial narrowing and reducing the flow of oxygen‐rich blood to the heart. The main manifestations of CHD are angina pectoris (chest pain), myocardial infarction (MI), and heart failure. Myocardial infarction occurs when blood flow to the heart muscle is abruptly cut off as the result of a blockage in one or more of the coronary arteries, causing tissue damage. Over time, CHD can weaken the heart muscle and lead to arrhythmias or heart failure. Coronary heart disease causes significant morbidity and mortality, and as a long term condition it contributes greatly to disability in developed countries, accounting for 19% of total disability adjusted life years lost in European countries (European Cardiovascular Disease Statistics 2017). Coronary heart disease can result in difficulties in functionality and performing everyday activities, and impairs sexual function (Racca 2010), all contributing to a reduction in health‐related quality of life (HRQoL) (Gravely‐Witte 2007).
In the United Kingdom (UK), an estimated 2.3 million people live with CHD and the condition accounts for one in five deaths in men and one in 10 deaths in women (Nicholls 2012; Townsend 2012). However, with more people surviving MI (WHO 2008) and heart failure (Kostis 1997), an increasing number of people are now living with CHD and may need support to manage their symptoms and improve their prognosis.
Description of the intervention
Although there are many definitions of cardiac rehabilitation, the following describes their combined key elements: “The coordinated sum of activities required to influence favourably the underlying cause of cardiovascular disease, as well as to provide the best possible physical, mental, and social conditions, so that the patients may, by their own efforts, preserve or resume optimal functioning in their community and through improved health behaviour, slow or reverse progression of disease” (BACPR 2012; Buckley 2013). A central component of cardiac rehabilitation is exercise training (Piepoli 1998; Piepoli 2010). However, in addition to exercise, it is recommended that programmes provide lifestyle education on CHD risk factor management plus counselling and psychological support ‐ so‐called ‘comprehensive cardiac rehabilitation’ (Corrà 2005).
Cardiac rehabilitation is a complex intervention that involves a variety of therapies, including exercise, risk factor education, behaviour change, psychological support, and strategies that are aimed at targeting traditional risk factors for cardiovascular disease. Cardiac rehabilitation should be considered an essential part of the contemporary treatment of heart disease and is considered a priority in countries with a high prevalence of CHD. Cardiac rehabilitation has been shown to improve health‐related quality of life and reduce future morbidity (Anderson 2016; Taylor 2014; Davies 2014). Based on evidence from previous meta‐analyses and systematic reviews, exercise‐based cardiac rehabilitation following a cardiac event, or for patients with heart failure, is a Class I recommendation from the American College of Cardiology/American Heart Association (Balady 2011; Kulik 2015; Smith 2011; Yancy 2013) and the European Society of Cardiology, (McMurray 2012; Roffi 2015; Steg 2012) and is recommended by the National Institute for Health and Care Excellence (NICE 2010; NICE 2013). Service provision, though predominantly centre‐based, varies markedly, and referral, enrolment and completion are sub‐optimal, especially among women and older people (Beswick 2004; Clark 2012). Home‐based cardiac rehabilitation programmes have been increasingly introduced to widen access and participation (Taylor 2009), and interventions aimed at improving patient uptake and adherence to cardiac rehabilitation programmes have been adopted (Karmali 2014).
How the intervention might work
There are a number of mechanisms by which exercise training benefits patients dependent on the cause of their heart disease. For people with CHD, approximately half of the 28% reduction in cardiac mortality achieved with exercise‐based cardiac rehabilitation has been attributed to reductions in major risk factors (e.g. lipids, smoking) (Taylor 2006). For patients with ischaemic causes of heart failure, exercise training appears to improve myocardial perfusion by alleviating endothelial dysfunction thereby dilating coronary vessels, and by stimulating new vessel formation by way of intermittent ischaemia (ExTraMatch 2004). Indeed, Haykowsky 2007 demonstrated that aerobic training in people with heart failure patients improves myocardial contractility and diastolic filling. In their meta‐analysis Haykowsky 2007 demonstrated the benefits of exercise training in people with heart failure in terms of cardiac remodelling as measured by ejection fraction, end‐diastolic volume, and end‐systolic volume. Skeletal muscle dysfunction and wasting may also respond to exercise training (Haykowsky 2007). Regular physical activity by people with heart failure also stimulates vasodilation in the skeletal muscle vasculature and improves oxidative capacity (Hambrecht 1998).The inclusion of psycho‐educational interventions may improve patients' knowledge and risk factor behaviour (Brown 2013; Dickens 2013) and psychological well‐being, including levels of depression and anxiety.
Why it is important to do this review
Although the beneficial effects of cardiac rehabilitation have been shown, participation remains sub‐optimal (Dalal 2012), particularly so by heart failure patients (Dalal 2012; Piepoli 2015). Two of the main reasons people give for not accepting the invitation to attend cardiac rehabilitation are difficulty with regularly attending sessions at their local hospital and reluctance to take part in group‐based classes (Beswick 2004). Home‐based cardiac rehabilitation programmes have therefore been introduced in an attempt to improve rates of participation. In the UK, home‐based cardiac rehabilitation with a self‐help manual ‐ the Heart Manual ‐ supported by a nurse facilitator is a programme of rehabilitation that has been available for over two decades (Lewin 1992). Home‐based cardiac rehabilitation programmes can include supervised and unsupervised elements and increasingly use technology or "telehealth" interventions to support or encourage exercise or behaviour change (Artinian 2007; Neubeck 2009) or to overcome barriers of time and distance (Huang 2015). Figures from the National Audit for Cardiac Rehabilitation (NACR) indicate that approximately 5% of UK sites are currently providing the Heart Manual (NACR 2013), with some 14,000 copies given to patients in UK and abroad each year (Heart Manual 2016). The Heart Manual has also been used in many countries across the world, including Singapore, Italy, Canada, China, Ireland and Cayman (Heart Manual 2016), yet facilitated home‐based options such as the Heart Manual have not increased their share of cardiac rehabilitation provision in the UK in recent years (NACR 2016).
In the previous version of this Cochrane Review, the authors identified five new head‐to‐head randomised controlled trials (345 participants) of home‐ versus centre‐based cardiac rehabilitation (Taylor 2015). Unlike most studies in the original version of the review (Dalal 2010; Taylor 2009), these new studies included patients with heart failure. The authors found the two methods of delivery to be equally effective for improving the clinical and health‐related quality of life outcomes in low risk patients after MI or revascularisation, or with heart failure (Buckingham 2016; Taylor 2015). On the basis of this evidence, together with the absence of evidence of important differences in healthcare costs between the two approaches, the authors concluded that the expansion of home‐based cardiac rehabilitation programmes should continue and that the choice of participating in a more traditional and supervised centre‐based programme or a home‐based programme should reflect the preference of the individual patient (Taylor 2015). More recently, a systematic review was conducted to assess the effectiveness of home‐based cardiac rehabilitation for heart failure compared to either usual medical care (i.e. no cardiac rehabilitation) or centre‐based cardiac rehabilitation on mortality, morbidity, exercise capacity, health‐related quality of life, drop out, adherence rates, and costs (Zwisler 2016). This review found that home‐based cardiac rehabilitation led to short‐term improvements in exercise capacity and health‐related quality of life of heart failure patients compared to usual care, and the magnitude of outcome improvements were similar to those achieved with centre‐based cardiac rehabilitation (Zwisler 2016).
Objectives
To compare the effect of home‐based and supervised centre‐based cardiac rehabilitation on mortality and morbidity, exercise‐capacity, health‐related quality of life, and modifiable cardiac risk factors in patients with heart disease.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs; individual or cluster level), including parallel group, cross‐over or quasi‐randomised designs, were eligible for inclusion. Systematic reviews and meta‐analyses were identified as a means to identify additional RCTs.
Types of participants
The study population included adults (≥18 years) who were post myocardial infarction (MI), have angina, or had undergone revascularisation (coronary artery bypass grafting (CABG), percutaneous transluminal coronary angioplasty or coronary artery stent) or who have had heart failure, who have taken part, or been invited to take part, in cardiac rehabilitation.
Studies were excluded if they included participants with heart transplants, those implanted with either cardiac resynchronisation therapy or implantable defibrillators, or those who had previously received cardiac rehabilitation.
Types of interventions
Home‐based cardiac rehabilitation is defined as a structured programme (that includes exercise training) with clear objectives for the participants, including monitoring, follow up visits, letters or telephone calls from staff or at least self‐monitoring diaries (Jolly 2006). The comparison group was centre‐based cardiac rehabilitation based in a variety of settings (e.g. hospital physiotherapy department, university gymnasium, community sports centre). We included cardiac rehabilitation programmes whether they were based solely on exercise or included other intervention elements (comprehensive cardiac rehabilitation).
Types of outcome measures
Primary outcomes
Total mortality.
-
Cardiac events:
Re‐infarction;
Total revascularisations (including CABG and percutaneous coronary intervention (PCI)); and
Cardiac associated hospitalisation.
Exercise capacity assessed by validated outcome measure (e.g. VO₂ peak, 6 minute walk test).
Validated measures of health‐related quality of life (HRQoL) (e.g. Short Form Health Survey (SF‐36), Sickness Impact Profile, Nottingham Health Profile).
Withdrawal from the exercise programme.
Secondary outcomes
Modifiable coronary risk factors (i.e. blood lipid levels, blood pressure, smoking behaviour).
Adherence to cardiac rehabilitation.
Costs and health service use (e.g. use of medication, primary care contacts).
Reporting of outcomes was not an inclusion or exclusion criterion for this update.
Search methods for identification of studies
Electronic searches
The search from the previously published Cochrane review (Taylor 2015) was updated by searching the following bibliographic databases on 21 September 2016:
CENTRAL Issue 8, 2016 in the Cochrane Library.
Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, MEDLINE Daily and MEDLINE (Ovid, 1946 to 21 September 2016).
Embase (Ovid, 1980 to 2016 Week 38).
PsycINFO (Ovid, 1806 to July Week 4 2016).
CINAHL Plus (EBSCO, 1937 to 21 September 2016).
The searches were run twice for this update; once in August 2016 using the search strategies from the last update and again in September 2016 with additional terms added to the strategies. Date limits were applied to the old terms to only retrieve results added since the last search, but not to the newly added terms.
The search strategies were designed with reference to those of the previous version of this review (Taylor 2015). We searched the databases using a strategy combining selected MeSH terms and free text terms relating to patient education and coronary heart disease (CHD), with filters applied to limit to RCTs. We used the Cochrane sensitivity‐maximising RCT filter for MEDLINE, and for Embase, terms recommended in the Cochrane Handbook for Systematic Reviews of Interventions were applied (Lefebvre 2011). Adaptations of this filter were applied to CINAHL and PsycINFO. We translated the MEDLINE search strategy into the other databases using the appropriate controlled vocabulary as applicable. We imposed no language or other limitations and gave consideration to variations in terms used and spellings of terms in different countries so that studies would not be missed by the search strategy because of such variations. See Appendix 1 for details of the search strategies used.
The reporting of search results was conducted in accordance with PRISMA (Moher 2009). Information about the number of studies identified, included and excluded, and the reasons for exclusion is summarised using a flow diagram (Figure 1).
1.
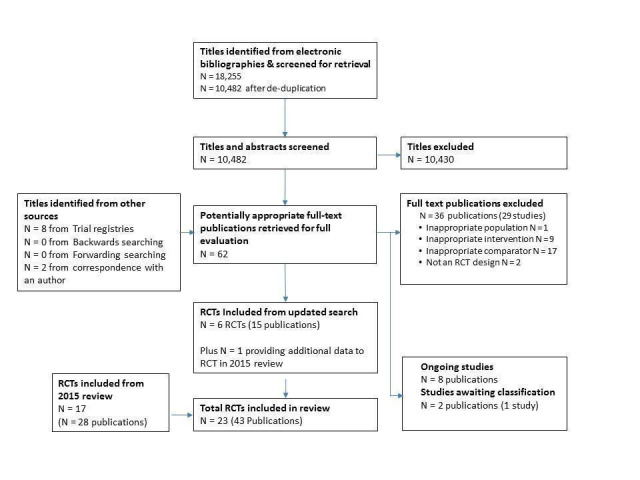
PRISMA Flow Diagram
Searching other resources
We handsearched reference lists of retrieved articles and systematic reviews for any studies not identified by the electronic searches. We also searched clinical trial registers on 7 November 2016; World Health Organization International Clinical Trials Registry Platform (WHO ICTRP; http://www.who.int/ictrp/en) and ClinicalTrials.gov (https://clinicaltrials.gov)) for ongoing clinical trials and sought expert advice. Attempts were made to contact all study authors to obtain relevant information not available in the published manuscript.
Data collection and analysis
Selection of studies
We screened (LA and GAS) the titles and abstracts of identified studies, and discarded clearly irrelevant ones. Two review authors (LA and GAS) then obtained and independently assessed the full‐text reports of all potentially relevant randomised trials for eligibility, based on the defined inclusion criteria. Any disagreement was resolved by discussion and where uncertainty remained, the opinion of a further author (RST) was taken. Excluded studies and reasons for exclusion are detailed in Characteristics of excluded studies. Where necessary, authors of included studies were contacted for missing information.
Data extraction and management
Two independent review authors (LA and GAS) extracted study characteristics of included RCTs using a standardised data collection form which had been piloted on two RCTs included in the review. Data on participant characteristics (e.g. age, sex, CHD diagnosis) details of the intervention (including duration, frequency and delivery), description of usual care and length of follow‐up were extracted. Two independent review authors (LA and GAS) extracted outcome data onto a standardised collection form. If data were presented numerically (in tables or text) and graphically (in figures), the numeric data were used because of possible measurement error when estimating from graphs. Any discrepancies were resolved by arbitration. One review author (LA) transferred extracted data into Review Manager 5.3 (RevMan 2014), and checked data for accuracy against the data collection forms.
If there were multiple reports of the same study, we assessed the duplicate publications for additional data. We extracted outcome results at all follow‐up points post‐randomisation. We contacted study authors where necessary to provide additional information.
Assessment of risk of bias in included studies
Factors considered included the reporting of random sequence generation and allocation concealment, the description of drop‐outs and withdrawals (high risk if >20% loss), consideration of blinding of outcome assessors, and degree of selective outcome reporting. In addition, evidence was sought that the groups were balanced at baseline and whether co‐interventions were delivered equally across the groups. The risk of bias in eligible trials was assessed by two reviewers independently (LA and GAS).
Measures of treatment effect
We extracted outcome results at follow‐up and the focus of this review was the between‐group difference in home‐ versus centre‐based groups. Primary outcomes relating to clinical event data were extracted as dichotomous outcomes for each study. Event data were expressed as risk ratios (RR) with associated 95% confidence intervals (CI), and study sample sizes were based on the number randomised to treatment conditions. For continuous variables, mean differences (MD) and 95% CI were calculated for each outcome, with sample sizes based on number completing assessments at each time‐point. When the results at follow‐up and differences between groups of the individual trials were not reported in the original publication, we calculated P values for the differences using the reported mean and standard deviation with the t‐test command in STATA (StataCorp 2013).
Given the variety of exercise capacity measures reported, results for this outcome were expressed as a standardised mean difference (SMD). Where a trial reported more than one exercise capacity endpoint we used the first one reported in the publication. Other continuous outcomes were pooled as weighted mean differences (WMD).
Unit of analysis issues
In accordance with Section 9.3.1 of the Cochrane Handbook for Systematic Reviews of Intervention (Higgins 2011), we ensured that the analysis was appropriate to the level at which randomisation occurred. All studies included in this review were simple parallel group RCTs, and so there were no issues relating to unit of analysis.
Dealing with missing data
We contacted investigators or study sponsors to verify key study characteristics and obtain missing numerical outcome data where possible (for example when a study was identified as abstract only). For this update, we contacted Grace to request absolute values for adherence data which were presented graphically in the publication (Grace 2016 Mixed). We also contacted Varnfield to obtain six month follow‐up data which were presented graphically (Varnfield 2014). Finally, we contacted Hadadzadeh for further details on study which had been identified as an abstract. This communication also led to the identification of a second study by the same authors which also met our inclusion criteria, but was not yet published (Hadadzadeh 2013; Hadadzadeh 2015).
Assessment of heterogeneity
Heterogeneity amongst included studies was explored qualitatively (by comparing the characteristics of included studies) and quantitatively (using the Chi² test of homogeneity and I² statistic). Where appropriate, the results from included studies were combined for each outcome to give an overall estimate of treatment effect. A fixed‐effect meta‐analysis was used except where statistical heterogeneity was indicated by a I² of ≥ 50%, in which case a random‐effects model was used.
Assessment of reporting biases
The funnel plot and the Egger test (Egger 1997) were used to examine small study bias for outcomes where there were 10 or more studies contributing data to the analysis.
Data synthesis
We processed data in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Where appropriate and possible, results from included studies were combined for each outcome to give an overall estimate of treatment effect, using either a fixed‐effect or random‐effects model.
Summary of findings table
Two independent review authors (LA and GS) employed the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach to interpret result findings and used GRADEpro GDT 2015 to import data from Review Manager to create a 'Summary of findings table'. We created a 'Summary of findings' table using the following outcomes: total mortality, exercise capacity, withdrawal and HRQoL. We used the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness, and publication bias) to assess the quality of a body of evidence as it relates to the studies that contribute data to the meta‐analyses for the prespecified outcomes. We used methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook for Systematic Reviews of Interventions using GRADEpro software (Higgins 2011). We have justified all decisions to downgrade the quality of studies using footnotes, and have made comments to aid readers' understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
We undertook subgroup analysis using meta‐regression to examine potential treatment effect modifiers. We tested the following a priori hypotheses that there may be differences in the effect of home‐ and centre‐based cardiac rehabilitation programmes on total mortality, exercise capacity ≤ 12 months, withdrawal, total cholesterol and blood pressure, across the following subgroups:
case mix (% MI);
type of cardiac rehabilitation (exercise‐only cardiac rehabilitation versus comprehensive cardiac rehabilitation);
'dose' of exercise intervention (dose = number of weeks of exercise training x average number of sessions/week x average duration of session in minutes) (dose ≥ 1000 units versus dose < 1000 units);
follow‐up period;
year of publication;
sample size;
risk of bias (low risk in ≥ 4 items versus < 4 items); and
study location (continent).
Given the relatively small ratio of trials to covariates, multivariable meta‐regression was not appropriate, and instead, limited to a univariate analysis (Deeks 2011). The permute option in STATA was used to allow for multiple testing in meta‐regression (StataCorp 2013).
Results
Description of studies
No cluster RCTs were identified in our searches and therefore only individual RCTs were included in this review.
Results of the search
The original 2009 version of this Cochrane Review contributed 12 trials to this latest analysis (Arthur 2002; Bell 1998; Carlson 2000; Dalal 2007; Daskapan 2005; Gordon 2002 Community; Gordon 2002 Supervised; Jolly 2007; Kassaian 2000; Marchionni 2003; Miller 1984 Brief; Miller 1984 Expanded; Sparks 1993; Wu 2006). The 2015 update identified one previously included trial with longer follow up (Arthur 2002) and five new trials (Cowie 2012; Karapolat 2009; Moholdt 2012; Oerkild 2011; Piotrowicz 2010) and included a total of 17 trials (28 reports).
For this update, 18,255 records were identified through database searches and 10,482 records were screened following de‐duplication. An additional 10 records were identified from other sources. We assessed a total of 62 full text records. We identified one previously included trial with further health‐related quality of life (HRQoL) data (Piotrowicz 2010) and six new trials (Aamot 2014 Treadmill; Grace 2016 Mixed; Hadadzadeh 2013; Hadadzadeh 2015; Kraal 2014; Varnfield 2014). Two of these trials compared a home‐based programme with two supervised centre‐based exercise programmes (Aamot 2014 Treadmill; Grace 2016 Mixed) and this update therefore includes eight additional home‐ versus centre‐based cardiac rehabilitation comparisons.
Two of the studies identified in this update have not yet been published in peer‐reviewed journals (Hadadzadeh 2013; Hadadzadeh 2015). Study and outcome data have been provided by the author of these trials, but in the absence of full study details, it was not possible to assess methodological quality using all domains of the Cochrane risk of bias tool, for these studies.
The study selection process is summarised in the PRISMA flow diagram (Figure 1).
Included studies
The 23 trials (27 home‐ versus centre‐based comparisons) included a total of 2890 participants and all used an individual patient randomisation method (there were no quasi‐randomised studies). Most trials were relatively small in sample size (median 104 participants, range: 20 to 525). The average age of patients in the trials ranged from 51.6 to 69.0 years. With the exception of four trials (Kassaian 2000; Miller 1984 Brief; Sparks 1993; Wu 2006), all included women. However, women accounted for only 19% of all participants who were recruited in the included studies. The mix of participants recruited to included trials varied, with 10 studies including a mixed population of people with coronary heart disease (CHD) (Aamot 2014 Treadmill; Carlson 2000; Gordon 2002 Community; Grace 2016 Mixed; Hadadzadeh 2015; Jolly 2007; Kassaian 2000; Kraal 2014; Oerkild 2011; Piotrowicz 2010), five studies included patients post‐myocardial infarction (MI) (Bell 1998; Dalal 2007; Marchionni 2003; Miller 1984 Brief; Varnfield 2014), four recruited patients following revascularisation (Arthur 2002; Hadadzadeh 2013; Moholdt 2012; Wu 2006), and four studies included participants with heart failure (Cowie 2012; Daskapan 2005; Karapolat 2009; Piotrowicz 2010).
All trials used an individual patient level method for randomisation. Four studies were UK‐based (Bell 1998; Cowie 2012; Dalal 2007; Jolly 2007); four were based in the USA (Carlson 2000; Gordon 2002 Community; Miller 1984 Brief; Sparks 1993); two studies each were from Turkey (Daskapan 2005; Karapolat 2009), Norway (Aamot 2014 Treadmill; Moholdt 2012) and Canada (Arthur 2002; Grace 2016 Mixed); and one each from Denmark (Oerkild 2011), Italy (Marchionni 2003), Netherlands (Kraal 2014); Poland (Piotrowicz 2010), China (Wu 2006), Iran (Kassaian 2000), India (Hadadzadeh 2013), Australia (Varnfield 2014), India and Iran (Hadadzadeh 2015). Most studies reported outcomes up to six months post‐randomisation. Only three studies reported longer‐term follow‐up at 14 months (Marchionni 2003), 18 months (Arthur 2002) and 24 months (Jolly 2007). Sixteen studies compared comprehensive programmes (i.e. exercise plus education and/or psychological management) and the remainder reported only an exercise intervention (Aamot 2014 Treadmill; Daskapan 2005; Karapolat 2009; Kassaian 2000; Miller 1984 Brief; Wu 2006). Three studies compared a comprehensive home‐based programme with an exercise‐only centre‐based programme (Hadadzadeh 2013; Hadadzadeh 2015; Kraal 2014). The cardiac rehabilitation programmes differed considerably in duration (range: 1 to 6 months), frequency (1 to 5 sessions per week) and session length (20 minutes to 60 minutes per session). Most programmes used individually tailored exercise prescription which makes it difficult to precisely quantify the amount of exercise undertaken. Centre‐based programmes typically provided supervised cycle and treadmill exercise, while virtually all home programmes were based on walking, with some level of intermittent nurse or exercise specialist telephone support. Two studies used web‐based or smart phone applications to upload recorded exercise data (Kraal 2014) or to monitor health and exercise, and deliver motivational and educational materials (Varnfield 2014). Most studies recruited lower‐risk patients following an acute MI or revascularisation, and excluded those with significant arrhythmias, ischaemia, or heart failure. Four studies included individuals (315 participants) with New York Heart Association (NYHA) class II or III heart failure (Cowie 2012; Daskapan 2005; Karapolat 2009; Piotrowicz 2010).
Most studies reported sources of trial funding; seven did not (Bell 1998; Carlson 2000; Daskapan 2005; Gordon 2002 Community; Kassaian 2000; Sparks 1993; Wu 2006); and two studies are yet to be published (Hadadzadeh 2013; Hadadzadeh 2015). None of the studies reported that they were funded by an agency with a commercial interest in the results of the study.
Marchionni 2003 reported outcomes for home‐ versus centre‐based care according to three patient age subgroups (i.e. 45 to 65, 66 to 75, > 75 years). Given the data reporting, we pooled these data to obtain single overall outcome results for home‐ and centre‐based groups.
For three studies that report more than one comparator, we reported outcome results separately for each comparison. Gordon et al compared two home‐based exercise groups: a physician‐supervised nurse‐case‐managed programme and a community‐based programme (Gordon 2002 Supervised; Gordon 2002 Community, respectively), versus a centre‐based cardiac rehabilitation programme. The study by Miller et al compared home‐ versus centre‐based cardiac rehabilitation programmes that were either 11 weeks long or 26 weeks long (Miller 1984 Brief; Miller 1984 Expanded, respectively). Grace et al compared a home‐based programme with a supervised mixed‐sex and a supervised women‐only programme (Grace 2016 Mixed), and Aamot et al compared a home‐based programme with a supervised group exercise programme and a treadmill exercise programme (Aamot 2014 Treadmill). We used the method for splitting sample size of shared comparator studies in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (chapter 16.5; Higgins 2011).
Details of included studies are listed in Characteristics of included studies.
Excluded studies
We excluded 36 reports (29 studies): 17 studies included a comparator group which did not receive exercise‐based cardiac rehabilitation or did not compare home‐ versus centre‐based cardiac rehabilitation; nine studies included an intervention which was not exercise‐based; two studies were not RCTs and one study included an inappropriate population. Details of excluded studies are listed in Characteristics of excluded studies.
Risk of bias in included studies
A number of study reports did not contain sufficient detail to assess their potential risk of bias (Figure 2; Figure 3).
2.
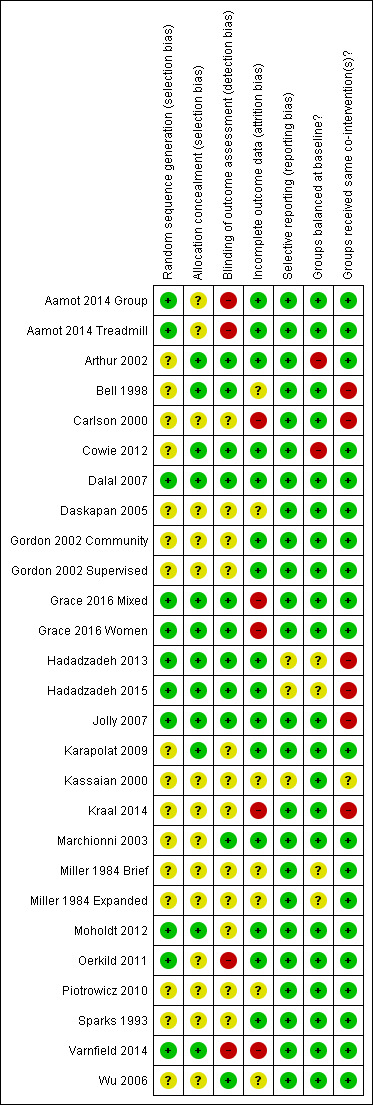
Methodological quality summary: review authors' judgements about each methodological quality item for each included study
3.
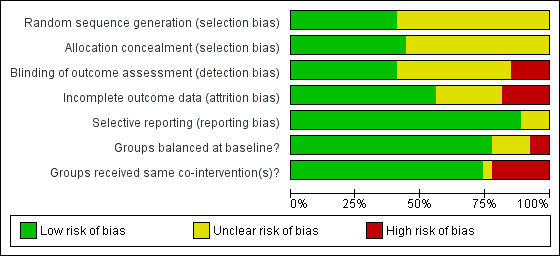
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies
Allocation
Details of generation and concealment of random allocation sequence were particularly poorly reported, with only nine studies adequately describing random sequence generation (Aamot 2014 Treadmill; Aamot 2014 Group; Dalal 2007; Grace 2016 Mixed; Grace 2016 Women; Hadadzadeh 2013; Hadadzadeh 2015; Jolly 2007; Moholdt 2012; Oerkild 2011; Varnfield 2014) and 11 studies adequately reporting random sequence concealment (Arthur 2002; Bell 1998; Cowie 2012; Dalal 2007; Grace 2016 Mixed; Grace 2016 Women; Hadadzadeh 2013; Hadadzadeh 2015; Jolly 2007; Karapolat 2009; Moholdt 2012; Oerkild 2011).
Blinding
Given the nature of these trials, it is not possible to blind participants or carers to group allocation; in such situations, blinding outcome assessors to knowledge of allocation is probably of greater importance. However, only 10 studies stated that they took measures to blind outcome assessment (Arthur 2002; Bell 1998; Cowie 2012; Dalal 2007; Grace 2016 Mixed; Grace 2016 Women; Hadadzadeh 2013; Hadadzadeh 2015; Jolly 2007; Marchionni 2003; Wu 2006).
Incomplete outcome data
Loss to follow‐up varied considerably among studies and was often asymmetric across home‐ and centre‐based cardiac rehabilitation groups. Only a few trials examined the impact of losses to follow‐up or drop out. Five studies were judged to have an unclear risk of attrition bias (Bell 1998; Daskapan 2005; Kassaian 2000; Miller 1984 Brief; Miller 1984 Expanded; Piotrowicz 2010); a further four studies were judged as having a high risk of attrition bias (Carlson 2000; Grace 2016 Mixed; Grace 2016 Women; Kraal 2014; Varnfield 2014).
Selective reporting
We compared the reported outcomes in the results sections to the outcomes described in the methods of the published papers, Most of the included studies fully reported on all the specified outcomes listed in their methods sections; three studies were judged as having an unclear risk of reporting bias (Hadadzadeh 2013; Hadadzadeh 2015; Kassaian 2000). However, the two studies by Hadadzadeh et al have not yet been published and we do not have access to a published protocol or description of the methods, which made reporting bias impossible to assess.
Groups balanced at baseline?
There was generally good evidence of balance in baseline characteristics between groups. However, in two cases there was objective evidence of imbalances in baseline characteristics (Arthur 2002; Cowie 2012), in one study the baseline characteristics were not reported (Miller 1984 Brief) and two additional studies were judged as having unclear risk of bias because they have not yet been published in full and we did not have access to baseline data (Hadadzadeh 2013; Hadadzadeh 2015).
Groups received same co‐interventions?
Most trials were judged to be low risk of bias in terms of whether groups received the same co‐interventions. Because the rehabilitation intervention was usually tailored to the individual participant, it is difficult to quantify the precise level of intervention; however, the intensity of the rehabilitation programme often seemed to differ substantively between home‐ and centre‐based arms. For example, the studies by Bell 1998, Carlson 2000 and Jolly 2007 included hospital cardiac rehabilitation programmes which were fixed in terms of frequency and content over the period of the study. In contrast, the home‐based intervention in these studies consisted of use of the Heart Manual 2016 where the participants could self‐regulate the frequency and nature of rehabilitation sessions they undertook. Kraal 2014 was also judged as having high risk of bias in this domain, as while telephone coaching was offered to the home‐based cohort in this study, no coaching was offered to patients receiving centre‐based cardiac rehabilitation. The study by Kassaian 2000 was judged as having unclear risk of bias because the home‐based programme was not adequately reported, and the two studies by Hadadzadeh et al were judged as unclear risk of bias because the full text was not available (Hadadzadeh 2013; Hadadzadeh 2015).
Effects of interventions
See: Table 1
Primary outcomes
Total mortality
Eleven trials (13 comparisons) reported total mortality up to one year following the intervention (Aamot 2014 Treadmill; Aamot 2014 Group; Bell 1998; Dalal 2007; Daskapan 2005; Hadadzadeh 2013; Jolly 2007; Kraal 2014; Miller 1984 Brief; Miller 1984 Expanded; Moholdt 2012; Oerkild 2011; Piotrowicz 2010). A pooled analysis found no evidence of a significant difference in mortality at three to 12 months of follow‐up between home‐ and centre‐based cardiac rehabilitation (RR 1.19, 95% CI 0.65 to 2.16; participants = 1505; studies = 11 (13 comparisons); I² = 0%; fixed‐effect; very low quality evidence; Analysis 1.1). Jolly 2007 reported there to be no between‐group difference in mortality at 24 months follow‐up (home group: 6/263; centre group: 3/262, P = 0.32).
1.1. Analysis.
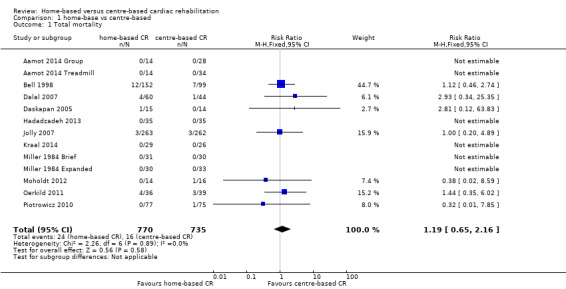
Comparison 1 home‐base vs centre‐based, Outcome 1 Total mortality.
Subgroup analyses
Predictors of treatment effect on total mortality were examined across the longest follow‐up period of each individual study, using univariate meta‐regression. We found no evidence that mortality risk is associated with case mix, type of cardiac rehabilitation, duration of follow‐up, year of publication, study location, study location (continent) or sample size (Table 2). Due to lack of data, we were unable to assess the impact of exercise dose.
1. Results of univariate meta‐regression analysis for total mortality.
| Explanatory variable (n trials) | Exp(slope)* | 95% CI univariate P value | Proportion of variation explained | Interpretation |
| Case mix (% MI patients) (n = 6) | RR = 0.997 | 0.970 to 1.024 P = 0.743 |
Not calculable² | No evidence that RR is associated with case mix |
| Dose of exercise (number of weeks of exercise training x average number of sessions/week x average duration of session in min) (n = ) |
Not calculable¹ | Not calculable¹ | Not calculable¹ | No evidence that RR is associated with increased dose of exercise |
| Type of cardiac rehabilitation (exercise only versus comprehensive cardiac rehabilitation) (n = 7) | RR = 2.464 | 0.038 to 160.487 P = 0.603 |
Not calculable² | No evidence that RR is associated with type of cardiac rehabilitation |
| Duration of follow‐up (months) (n = 7) | RR = 1.022 | 0.872 to 1.198 P = 0.737 |
Not calculable² | No evidence that RR is associated with duration of follow‐up |
| Year of publication (n = 7) | RR = 0.988 | 0.851 to 1.147 P = 0.842 |
Not calculable² | No evidence that RR is associated with year of publication |
| Risk of bias (low risk in ≥ 4 items versus < 4 items) (n = 7) | RR = 0.902 | 0.197 to 4.127 P = 0.868 |
Not calculable² | No evidence that RR is associated with risk of bias |
| Study location (n = 7) | RR = 0.846 | 0.398 to 1.822 P = 0.613 |
Not calculable² | No evidence that RR is associated with study location |
| Sample size (n = 7) | RR = 1.001 | 0.995 to 1.006 P = 0.726 |
Not calculable² | No evidence that RR is associated with sample size |
¹ Not calculable due to insufficient observations ² Not calculable; Tau² of all studies = 0 Abbreviations: MI, myocardial infarction; RR, risk ratio
Small study bias
There was no evidence of funnel plot asymmetry for total mortality (Egger test P = 0.304; Figure 4).
4.
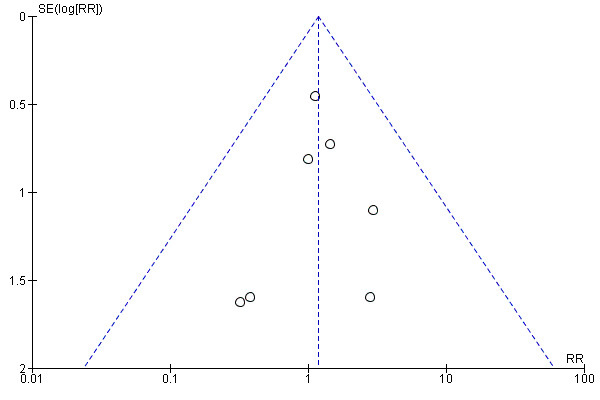
Funnel plot of comparison: 1 home‐base vs centre‐based, outcome: 1.1 Total mortality.
Cardiac events
Only five studies (Arthur 2002; Dalal 2007; Jolly 2007; Oerkild 2011; Piotrowicz 2010) reported cardiac events, including re‐infarction, revascularisation (coronary artery bypass grafting (CABG) and percutaneous coronary intervention (PCI)) or cardiac‐associated hospitalisation. While one study identified in this latest update (Aamot 2014 Treadmill; Aamot 2014 Group) reported that there were "no severe adverse events, defined as cardiac arrests or acute MI", none of the other new studies reported the occurrence of cardiac events. Given the differing nature of the events reported it was not possible to pool the data.
Dalal 2007 and Jolly 2007 reported no difference in revascularisation or recurrent myocardial infarction (MI) events between home‐ and centre‐based cardiac rehabilitation. Piotrowicz 2010 reported no heart failure‐related admissions in either group. Oerkild 2011 stated that “the number and length of acute and non‐acute admissions and adverse events (admission for MI, progressive angina, decompensated congestive heart failure, severe bleeding, new malignant disease and performance of (percutaneous coronary intervention)) to be equally distributed (across groups at 12 months follow‐up)” but did not report numbers of events. The six‐year follow‐up report of the Arthur 2002 study described that a total of 46/79 (62%) centre‐based cardiac rehabilitation patients experienced a hospitalisation compared to 35/70 (50%) in the home‐based group (P = 0.31). However, the total number of hospitalisations in centre‐based patients was greater than that in home‐based participants (79 versus 42, P < 0.0001).
Subgroup analyses
Due to the small number of studies reporting cardiac events, it was not possible to examine the effects of potential treatment effect modifiers on these outcomes.
Small study bias
Due to the small number of studies reporting cardiac events, it was not possible to examine small study bias.
Exercise capacity
With the exception of Hadadzadeh 2013, all included studies reported on exercise or functional capacity in the short‐term (8 weeks to 12 months follow‐up); three (Arthur 2002; Jolly 2007; Marchionni 2003) presented longer‐term data (> 12 months follow‐up) and one reported outcomes at six‐year follow‐up (Arthur 2002). All studies reported absolute exercise capacity at follow‐up, except two trials (3 comparisons; Gordon 2002 Supervised; Gordon 2002 Community; Oerkild 2011) which reported change in exercise capacity at follow‐up compared to baseline. Studies reported exercise capacity using a variety of metrics that included direct measures of oxygen uptake, walking distance and workload on a static cycle.
The pooled analysis showed no evidence of a difference in short‐term exercise capacity between home‐based and centre‐based cardiac rehabilitation (SMD ‐0.13, 95% CI ‐0.28 to 0.02; participants = 2255; studies = 22 (26 comparisons); I² = 63%; random‐effects; low quality evidence; Analysis 1.2).
1.2. Analysis.
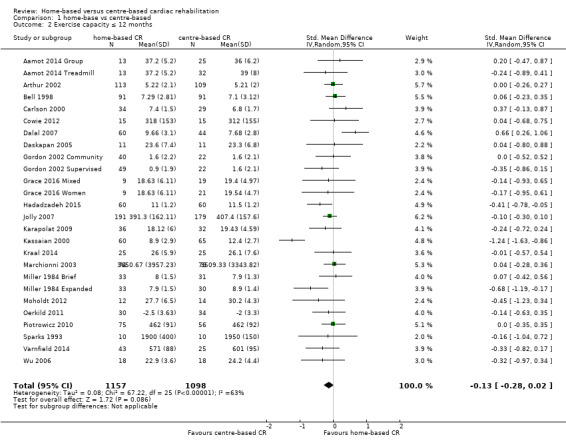
Comparison 1 home‐base vs centre‐based, Outcome 2 Exercise capacity ≤ 12 months.
In a pooled analysis of three studies reporting longer‐term data (> 12 months; Arthur 2002; Jolly 2007; Marchionni 2003), there was no evidence of a difference in exercise capacity following home‐based cardiac rehabilitation compared with centre‐based cardiac rehabilitation (SMD 0.11, 95% CI ‐0.01 to 0.23; participants = 1074; studies = 3; I² = 0%; fixed effect; moderate quality evidence; Analysis 1.3). Arthur 2002 reported that mean peak oxygen consumption (VO₂) at six‐year follow‐up was higher in the 96 participants who had undergone home‐based cardiac rehabilitation (1543 mL/min (SD 444)) compared to the 74 participants who had received centre‐based cardiac rehabilitation (1412 mL/min (SD 356); P = 0.01).
1.3. Analysis.
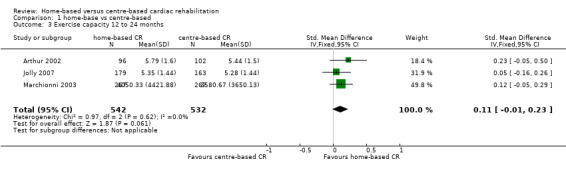
Comparison 1 home‐base vs centre‐based, Outcome 3 Exercise capacity 12 to 24 months.
Subgroup analyses
Predictors of treatment effect on exercise capacity were examined across the longest follow‐up of each individual study, using univariate meta‐regression. We found no evidence that exercise capacity is associated with case mix, dose of exercise, type of cardiac rehabilitation, duration of follow‐up, year of publication, study location, study location (continent) or sample size (Table 3).
2. Results of univariate meta‐regression analysis for exercise capacity.
| Explanatory variable (n trials) | Exp(slope)* |
95% CI Univariate P value |
Proportion of variation explained | Interpretation |
| Case mix (% MI patients) (n = 23) | RR = 0.003 | ‐0.001 to 0.008 P = 0.119 |
11.69% | No evidence that RR is associated with case mix |
| Dose of exercise (number of weeks of exercise training x average number of sessions/week x average duration of session in min) (n = 10) | RR = ‐0.001 | ‐0.003 to 0.001 P = 0.245 |
Not calculable¹ | No evidence that RR is associated with increased dose of exercise |
| Type of cardiac rehabilitation (exercise only versus comprehensive cardiac rehabilitation) (n = 26) | RR = 0.210 | ‐0.026 to 0.447 P = 0.079 |
18.73% | No evidence that RR is associated with type of cardiac rehabilitation |
| Duration of follow‐up (months) (n = 25) | RR = 0.003 | ‐0.007 to 0.013 P = 0.544 |
‐5.17% | No evidence that RR is associated with duration of follow‐up |
| Year of publication (n = 25) | RR = ‐0.002 | ‐0.024 to 0.020 P = 0.841 |
‐5.52% | No evidence that RR is associated with year of publication |
| Risk of bias (low risk in ≥ 4 items versus < 4 items) (n = 25) | RR = 0.097 | ‐0.118 to 0.311 P = 0.360 |
2.94% | No evidence that RR is associated with risk of bias |
| Study location (n = 26) | RR = 0.195 | ‐0.033 to 0.423 P = 0.090 |
15.80% | No evidence that risk ratio is associated with study location |
| Sample size (n = 25) | RR = 0.000 | ‐0.001 to 0.002 P = 0.837 |
‐7.78% | No evidence that RR is associated with sample size |
¹ Not calculable; Tau² of all studies = 0 Abbreviations: MI, myocardial infarction; RR, risk ratio
Small study bias
There was no evidence of funnel plot asymmetry for exercise capacity (Egger test P = 0.661; Figure 5).
5.
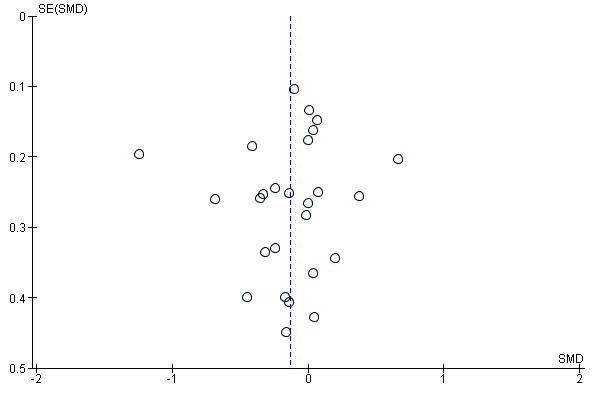
Funnel plot of comparison: 1 home‐base vs centre‐based, outcome: 1.2 Exercise capacity ≤ 12 months.
Health‐related quality of life (HRQoL)
Fourteen of the trials reported validated measures of HRQoL (Table 4). These included four generic HRQoL instruments: EQ‐5D (EuroQoL 1990), Nottingham Health Profile (Hunt 1980), Short‐Form 36 (SF‐36; McHorney 1993), Sickness Impact Profile (Bergner 1976) and two disease‐specific instruments (MacNew; Höfer 2004) and the Minnesota Living With Heart Failure Questionnaire (MLWHF; Rector 1993). This wide variation in HRQoL outcomes meant that pooling across studies was inappropriate.
3. Summary of health‐related quality of life (HRQoL) at follow up for home and centre‐based cardiac rehabilitation.
| Study ID | Follow up | HRQoL measure |
Outcome values at follow up Mean (SD or range) Home‐ versus centre‐based, between group P value |
Between‐group difference |
|
Aamot 2014 Treadmill Home versus treadmill group |
12 weeks | MacNew Emotional domain Social domain Physical domain Global |
6.1 (3.9–6.7) versus 6.0 (4.8–6.5) ns 6.8 (4.9–7.0) versus 6.7 (5.6–6.9) ns 6.4 (4.9–6.9) versus 6.6 (5.4–6.9) ns 6.4 (4.7–6.8) versus 6.3 (5.2–6.7) ns |
Home = Centre Home = Centre Home = Centre Home = Centre |
|
Aamot 2014 Treadmill Home versus group exercise |
12 weeks | MacNew Emotional domain Social domain Physical domain Global |
6.1 (3.9–6.7) versus 6.2 (3.6–6.9) ns 6.8 (4.9–7.0) versus 6.5 (5.0–7.0) ns 6.4 (4.9–6.9) versus 6.4 (5.2–7.0) ns 6.4 (4.7–6.8) versus 6.3 (4.5–6.7) ns |
Home = Centre Home = Centre Home = Centre Home = Centre |
|
Arthur 2002 /Smith 2004 |
6 months 18 months |
SF‐36 PCS MCS SF‐36 PCS MCS |
51.2 (6.4) versus 48.6 (7.1) P = 0.003* 53.5 (6.4 ) versus 52.0 (8.1) P = 0.13* 48.3 (11.7) versus 47.6 (11.7) P = 0.67* 53.0 (10.9) versus 50.2 (10.9) P = 0.07* |
Home > Centre Home = Centre Home = Centre Home = Centre |
| Bell 1998 | 10.5 months | Nottingham Health Profile Energy Pain Emotional reactions Sleep Social isolation Physical mobility |
18.6 (28.4) versus 17.3 (30.7) P = 0.78* 6.6 (15.3) versus 7.4 (15.5) P = 0.74* 6.6 (15.3) versus 7.4 (15.5) P = 0.74* 6.6 (15.3) versus 16.9 (22.8) P = 0.0007* 3.7 (13.6) versus 6.7 (15.0) P = 0.18* 6.9 (13.5) versus 9.1 (15.9) P =0.33* |
Home = Centre Home = Centre Home = Centre Home < Centre Home = Centre Home = Centre |
| Cowie 2012 | 3 months | SF‐36 PCS MCS MLWHF total Physical Emotional |
34.01 (11.04) versus 31.33 (7.97) P = 0.82 44.44 (12.23) versus 48.25 (11.21) P = 0.04 37 (NR) vs 32 (NR) P = 0.18 21 (NR) vs 19 (NR) P = 0.31 7 (NR) vs 7 (NR) P = 0.13 |
Home = Centre Home < Centre Home = Centre Home = Centre Home = Centre |
| Marchionni 2003 | 2 months 8 months 14 months |
Sickness Impact Profile | 2.83 (14.5) versus 4.71 (11.1) P = 0.09* 2.83 (14.5) versus 3.40 (11.1) P = 0.61* 2.00 (8.3) versus 3.70 (11.8) P = 0.06* |
Home = Centre Home = Centre Home = Centre |
| Dalal 2007/Taylor 2007 | 9 months | MacNew Global score EQ‐5D |
5.61 (1.14) versus 5.54 (1.10) P = 0.71 0.74 (0.04) versus 0.78 (0.04) P = 0.57 |
Home = Centre Home = Centre |
| Hadadzadeh 2015 | 12 week | SF 36 Physical Composite Score Mental Composite Score |
51.6 (4.7) versus 52.2 (4.7) P = 0.94 46.4 (4.9) versus 47.6 (6.4) P = 0.10 |
Home = Centre Home = Centre |
| Jolly 2007 | 6 months 12 months 24 months |
EQ‐5D SF‐12 PCS MCS EQ‐5D |
0.74 (0.26) versus 0.76 (0.23) P = 0.37 42.28 (10.9) 42.56 (10.8) P = 0.8 49.19 (10.1) 50.33 (9.6) P = 0.3 0.74 (0.27) versus 0.76 (0.23) P = 0.52* 0.73 (0.29) versus 0.75 (0.26) P = 0.39* |
Home = Centre Home = Centre Home = Centre Home = Centre Home = Centre |
| Karapolat 2009 | 8 weeks | SF‐36 Physical function Physical role Bodily pain General health Vitality Social function Emotional role Mental health |
59.39 (25.35) versus 69.57 (20.94),P = 0.08* 39.81 (41.75) versus 48.21 (45.10) P = 0.43* 62.42 (30.45) versus 74.23 (19.66) P = 0.07* 47.25 (23.42) versus 53.98 (25.00) P =0.33* 66.67 (19.82) versus 69.81 (17.41) P = 0.49* 65.33 (25.60) versus 69.33 (25.14) P = 0.52* 44.74 (39.77) versus 37.16 (39.24) P =0.44* 64.67 (19.04) versus 70.52 (20.37) P = 0.22* |
Home = Centre Home = Centre Home = Centre Home = Centre Home = Centre Home = Centre Home = Centre Home = Centre |
| Kraal 2014 | 12 weeks | MacNew (Dutch translation) Physical scale Emotional scale Social scale Total score |
6.1 (0.6) versus 5.7 (0.8) P = 0.16 5.9 (0.8) versus 5.6 (0.9) P = 0.88 6.4 (0.6) versus 6.1 (0.7) P = 0.26 6.1 (0.5) versus 5.8 (0.7) P = 0.50 |
Home = Centre Home = Centre Home = Centre Home = Centre |
| Moholdt 2012 | 6 months | MacNew Emotional domain Physical domain Social domain |
1.2 (0.2) versus 1.4 (0.2) P > 0.05 1.4 (0.7) versus 1.6 (1.1) P > 0.05 4.3 (0.7) versus 4.3 (1.0) P > 0.05 |
Home = Centre Home = Centre Home = Centre |
| Oerkild 2011 | 3 months 6 months |
SF‐36 PCS SF‐36 MCS SF‐36 PCS SF‐36 MCS |
1.4 (‐1.5 to 4.3) versus 0.5 (‐2.4 to 3.4) P > 0.05 0.8 (‐2.6 to 4.3) versus ‐0.2 (‐3.6 to 3.4) P > 0.05 1.0 (‐1.6 to 3.6) versus 1.2 (‐1.4 to 3.8) P > 0.05 2.3 (‐1.1 to 5.7) versus 2.6 (‐0.9 to ‐6.0) P > 0.05 |
Home = Centre Home = Centre Home = Centre Home = Centre |
|
Piotrowicz 2010/ Piotrowicz 2014 |
8 weeks | SF‐36 Physical function Role limitation caused by physical problems Bodily pain General health Physical component summary Social function Mental health Role limitation caused by physical problems Vitality Mental component summary Total quality of life index |
21.60 (9.65) versus 23.20 (10.71) ns 12.74 (7.17) versus 11.39 (8.43) ns 2.66 (2.22) versus 2.00 (2.07) ns 13.14 (3.80) versus 14.59 (4.03) P < 0.05 50.27 (17.06) versus 51.37 (19.60) ns 2.64 (2.84) versus 1.63 (1.54) P < 0.05 7.15 (4.00) versus 5.89 (3.58) ns 4.93 (6.15) versus 4.35 (6.07) ns 7.25 (3.78) versus 6.76 (3.17) ns 21.68 (12.46) versus 18.56 (9.18) ns 70.50 (25.40) versus 69.20 (26.40) ns |
Home = Centre Home = Centre Home = Centre Home < Centre Home = Centre Home > Centre Home = Centre Home = Centre Home = Centre Home = Centre Home = Centre |
| Varnfield 2014 | 6 weeks 6 months |
EQ5D‐Index | 0.92 (0.9–1.0) versus 0.82 (0.7–0.9) "The HRQoL (EQ5D‐Index) improved significantly in CAP‐CR participants compared with TCR." 0.85 (0.1) versus 0.86 (0.2) "Between‐group difference for changes in EQ5D‐Index was not significant at 6 months" |
Home > Centre Home = Centre |
*P value calculated by authors of this report based on independent 2‐group t‐test Home = Centre: no statistically significant difference (P > 0.05) in HRQoL between home and centre‐based groups at follow up Home > Centre: statistically significant (P ≤ 0.05) higher HRQoL in home versus centre‐based groups at follow up Home < Centre: statistically significant (P ≤ 0.05) lower HRQoL in home versus centre‐based groups at follow up Abbreviations: HRQoL = health related quality of life; MCS: mental component score; MLWHF: Minnesota Living With Heart Failure; PCS: physical component score; SF‐12: 12‐Item Short Form Health Survey; SF‐36: Short Form (36) Health Survey
Taking individual findings of all studies into account, there was no strong evidence of a difference in overall HRQoL outcomes or domain scores at follow‐up between home‐ and centre‐based cardiac rehabilitation.
Individual studies reported consistent improvements in HRQoL at follow‐up with both home‐ and centre‐based cardiac rehabilitation compared to baseline. The notable exception was in two of the three studies which used the EQ‐5D and failed to identify significant improvements with home‐ or centre‐based cardiac rehabilitation (Dalal 2007; Jolly 2007). The third study which used the EQ‐5D reported a significant improvement at six weeks follow‐up for home‐based cardiac rehabilitation, but not for centre‐based cardiac rehabilitation, and reported no improvements in HRQoL at six months follow‐up (Varnfield 2014).
Withdrawal from the intervention programme
Withdrawal from the intervention was inconsistently reported and the reasons were often unclear. Using the number of completers i.e. the number of participants with outcome data at follow‐up, we found some limited evidence of a small increase in the level of completion with home‐based compared with centre‐based programmes (RR 1.04, 95% CI 1.00 to 1.08; participants = 2615; studies = 22 (26 comparisons); I² = 53%; random‐effects; low quality evidence; Analysis 1.4).
1.4. Analysis.
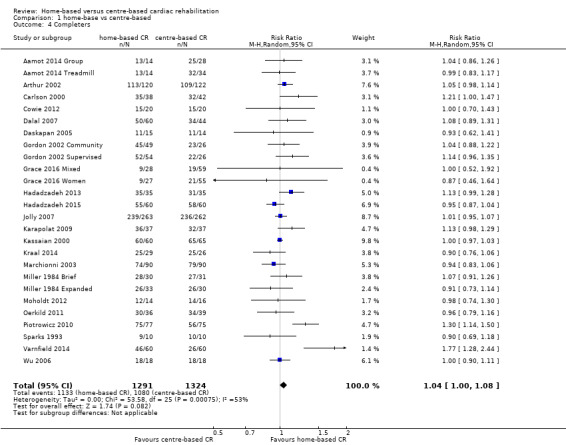
Comparison 1 home‐base vs centre‐based, Outcome 4 Completers.
Subgroup analyses
Predictors of withdrawal were examined across the longest follow‐up period of each individual study using univariate meta‐regression. We found no evidence that withdrawal risk is associated with case mix, dose of exercise, type of cardiac rehabilitation, duration of follow‐up, year of publication, study location, study location (continent), or sample size (Table 5).
4. Results of univariate meta‐regression analysis for withdrawal (no of completers).
| Explanatory variable (n trials) | Exp(slope)* | 95% CI univariate P value | Proportion of variation explained | Interpretation |
| Case mix (% MI patients) (n = 21) | RR = 1.000 | 0.999 to 1.002 P = 0.949 |
‐15.22% | No evidence that RR is associated with case mix |
| Dose of exercise (number of weeks of exercise training x average number of sessions/week x average duration of session in min) (n = 10) |
RR = 0.999 | 0.998 to 1.000 P = 0.217 |
16.94% | No evidence that RR is associated with increased dose of exercise |
| Type of cardiac rehabilitation (exercise only versus comprehensive cardiac rehabilitation) (n = 24) |
RR = 1.041 | 0.975 to 1.111 P = 0.219 |
‐1.56% | No evidence that RR is associated with type of cardiac rehabilitation |
| Duration of follow‐up (months) (n = 23) | RR = 1.000 | 0.997 to 1.003 P = 0.940 |
‐21.09% | No evidence that RR is associated with duration of follow‐up |
| Year of publication (n = 23) | RR = 1.000 | 0.992 to 1.007 P = 0.930 |
‐12.08% | No evidence that RR is associated with year of publication |
| Risk of bias (low risk in ≥ 4 items versus < 4 items) (n = 23) | RR = 0.949 | 0.880 to 1.023 P =0.160 |
32.50% | No evidence that RR is associated with risk of bias |
| Study location (n = 24) | RR = 0.988 | 0.912 to 1.069 P = 0.747 |
‐21.54% | No evidence that RR is associated with study location |
| Sample size (n = 23) | RR = 1.000 | 1.000 to 1.000 P = 0.880 |
‐20.04% | No evidence that RR is associated with sample size |
Abbreviations: MI: myocardial infarction; RR: risk ratio
Small study bias
There was no evidence of funnel plot asymmetry for withdrawal (Egger test P = 0.440; Figure 6).
6.
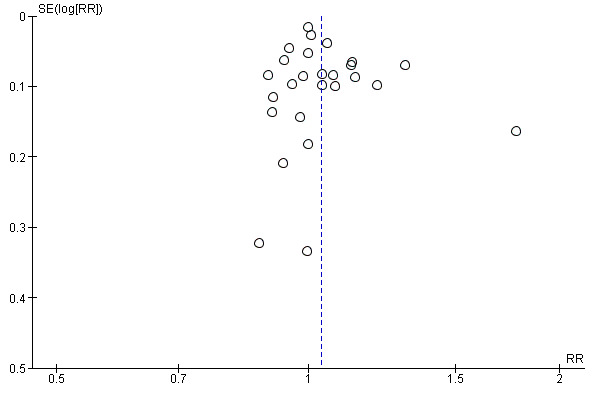
Funnel plot of comparison: 1 home‐base vs centre‐based, outcome: 1.4 Completers.
Secondary outcomes
Modifiable coronary risk factors
Blood lipids
Nine of the included trials (10 comparisons) reported data on blood lipids (Bell 1998; Carlson 2000; Dalal 2007; Gordon 2002 Community; Gordon 2002 Supervised; Jolly 2007; Kassaian 2000; Moholdt 2012; Oerkild 2011; Varnfield 2014). All reported total cholesterol values, seven studies (8 comparisons) reported high density lipoprotein concentrations (Carlson 2000; Gordon 2002 Community; Gordon 2002 Supervised; Jolly 2007; Kassaian 2000; Moholdt 2012; Oerkild 2011; Varnfield 2014), and five studies (6 comparisons) reported low density lipoprotein and triglyceride concentrations (Carlson 2000; Gordon 2002 Community; Gordon 2002 Supervised; Kassaian 2000; Oerkild 2011; Varnfield 2014). All reported absolute follow‐up data except two studies (3 comparisons) where data were reported as the change at follow up from baseline (Gordon 2002 Community; Gordon 2002 Supervised; Oerkild 2011). Study results were expressed as millimols per litre (mmol/L; Bell 1998; Dalal 2007; Jolly 2007) or milligrams per decilitre (mg/dL; Carlson 2000; Gordon 2002 Community; Gordon 2002 Supervised; Kassaian 2000); in the latter case we converted values into mmol/L before pooling for meta‐analysis.
Total cholesterol
The pooled analysis of data at three to 12 months of follow‐up revealed no evidence of a difference in the total cholesterol between home‐ and centre‐based groups (MD 0.06, 95% CI ‐0.10 to 0.23; participants = 1151; studies = 9, comparisons = 10; I² = 57%; random‐effects; Analysis 1.5).
1.5. Analysis.
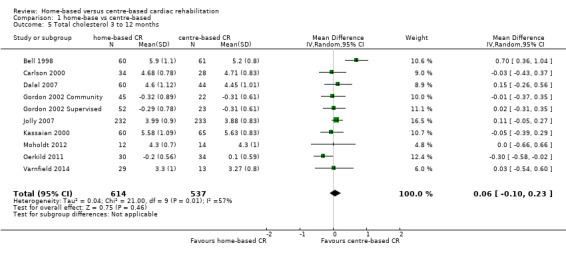
Comparison 1 home‐base vs centre‐based, Outcome 5 Total cholesterol 3 to 12 months.
Jolly 2007 reported no significant difference between home‐ and centre‐based cardiac rehabilitation groups in total cholesterol concentration at 24 months follow up (MD = ‐0.11 mmol/L, 95% CI 0.06 to ‐0.28).
Subgroup analyses
Predictors of total cholesterol were examined across the longest follow‐up period of each individual study using univariate meta‐regression. We found no evidence that the cardiac rehabilitation effect on cholesterol is associated with type of cardiac rehabilitation, duration of follow‐up, year of publication, study location, study location (continent) or sample size (Table 6). However, we found evidence of a relationship between case mix and total cholesterol, with a greater reduction in total cholesterol reported in studies with a higher proportion of participants with MI (Table 6). Due to lack of data, we were unable to assess the impact of exercise dose.
5. Results of univariate meta‐regression analysis for total cholesterol.
| Explanatory variable (n trials) | Exp(slope)* | 95% CI univariate P value | Proportion of variation explained | Interpretation |
| Case mix (% MI patients) (n = 10) | RR = ‐0.007 | ‐0.011 to ‐0.002 P = 0.014 |
88.71% | Evidence that RR is associated with case mix |
| Dose of exercise (number of weeks of exercise training x average number of sessions/week x average duration of session in min) (n = ) |
Not calculable¹ | Not calculable¹ | Not calculable¹ | No evidence that RR is associated with increased dose of exercise |
| Type of cardiac rehabilitation (exercise only vs comprehensive cardiac rehabilitation) (n = 10) |
RR = ‐0.127 | ‐0.822 to 0.567 P = 0.684 |
‐17.11% | No evidence that RR is associated with type of cardiac rehabilitation |
| Duration of follow‐up (months) (n = 10) | RR = ‐0.007 | ‐0.038 to 0.024 P = 0.594 |
‐21.27% | No evidence that RR is associated with duration of follow‐up |
| Year of publication (n = 10) | RR = 0.027 | ‐0.012 to 0.066 P = 0.154 |
31.00% | No evidence that RR is associated with year of publication |
| Risk of bias (low risk in ≥ 4 items versus < 4 items) (n = 10) | RR = ‐0.077 | ‐0.404 to 0.249 P = 0.600 |
‐14.59% | No evidence that RR is associated with risk of bias |
| Study location (n = 10) | RR =0.015 | ‐0.304 to 0.333 P = 0.919 |
‐18.83% | No evidence that RR is associated with study location |
| Sample size (n = 10) | RR = ‐0.001 | ‐0.002 to 0.001 P = 0.347 |
‐7.77% | No evidence that RR is associated with sample size |
¹Not calculable due to insufficient observations Abbreviations: MI: myocardial infarction; RR: risk ratio
Small study bias
There was no evidence of funnel plot asymmetry for total cholesterol (Egger test P = 0.913; Figure 7).
7.
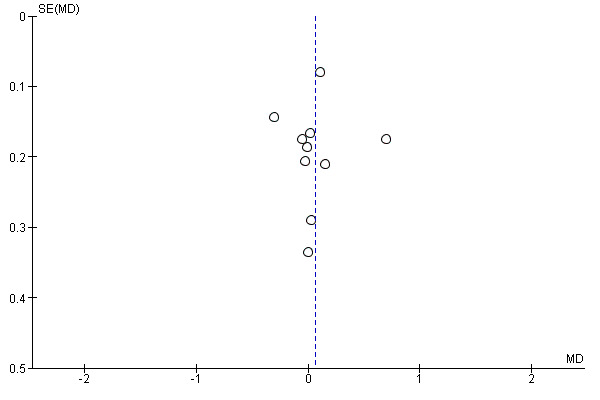
Funnel plot of comparison: 1 home‐base vs centre‐based, outcome: 1.5 Total cholesterol 3 to 12 months.
High density lipoprotein (HDL) cholesterol
The pooled analysis of data at 3 to 12 months of follow up revealed some evidence of a lower high density lipoprotein concentration after centre‐ compared to home‐based cardiac rehabilitation (MD ‐0.07, 95% CI ‐0.11 to ‐0.03; participants = 925; studies = 7; comparisons = 8; I² = 35%; fixed‐effects; Analysis 1.6).
1.6. Analysis.
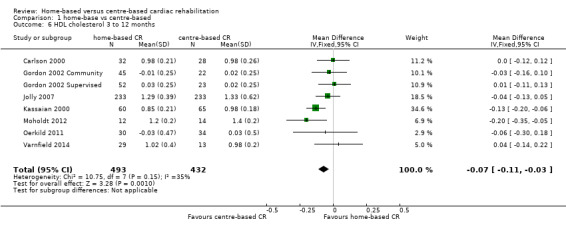
Comparison 1 home‐base vs centre‐based, Outcome 6 HDL cholesterol 3 to 12 months.
Jolly 2007 reported no significant difference between home‐ and centre‐based cardiac rehabilitation groups in high density lipoprotein level at 24 months follow‐up (MD = 0.03 mmol/L, 95% CI ‐0.10 to 0.04).
Low density lipoprotein (LDL) cholesterol
In the pooled analysis of data at 3 to 12 months of follow up there was no evidence of a difference in low density lipoprotein concentration between home‐ and centre‐based cardiac rehabilitation (MD 0.04, 95% CI ‐0.14 to 0.22; participants = 430; studies = 5 comparisons = 6; I² = 54%; random‐effects; Analysis 1.7).
1.7. Analysis.
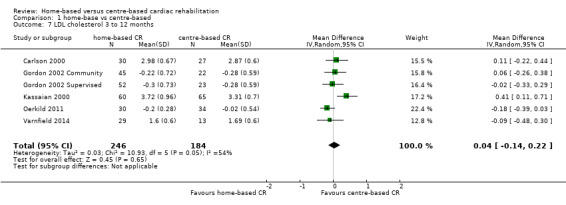
Comparison 1 home‐base vs centre‐based, Outcome 7 LDL cholesterol 3 to 12 months.
Triglycerides
In the pooled analysis of data at 3 to 12 months of follow up there was evidence of slightly lower triglyceride levels in centre‐based cardiac rehabilitation participants (MD 0.15, 95% CI 0.00 to 0.29; participants = 396; studies = 5, comparisons = 6 ; I² = 39%; fixed‐effect; Analysis 1.8).
1.8. Analysis.
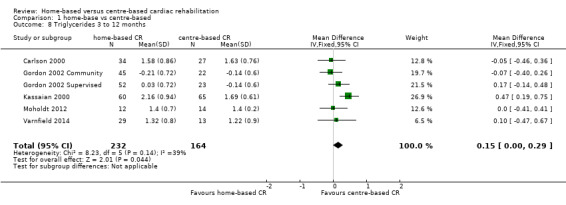
Comparison 1 home‐base vs centre‐based, Outcome 8 Triglycerides 3 to 12 months.
Subgroup analyses
Due to the small number of studies reporting blood lipid levels, it was not possible to examine the effects of potential treatment effect modifiers on these outcomes.
Small study bias
Due to the small number of studies reporting blood lipid levels, it was not possible to examine small study bias in these outcomes.
Blood pressure
Ten and nine of the included trials (12 and 11 comparisons) reported on systolic and diastolic blood pressure respectively (Aamot 2014 Treadmill; Aamot 2014 Group; Carlson 2000; Dalal 2007; Daskapan 2005; Gordon 2002 Community; Gordon 2002 Supervised; Jolly 2007; Kassaian 2000; Oerkild 2011; Varnfield 2014) or systolic blood pressure alone (Bell 1998). Absolute values at follow‐up were reported in all but two studies (3 comparisons; Gordon 2002 Supervised; Gordon 2002 Community; Oerkild 2011) where the change from baseline was reported. We obtained unpublished data for the study by Dalal et al (Dalal 2007).
No evidence of a difference was found at follow‐up between groups in either pooled systolic blood pressure (MD ‐0.27, 95% CI ‐3.13 to 2.60; participants = 1292; studies = 10, comparisons = 12; I² = 55%; random‐effects; Analysis 1.9) or diastolic blood pressure (MD 0.74, 95% CI ‐1.04 to 2.53; participants = 1146; studies = 9, comparisons = 11; I² = 60%; random‐effects; Analysis 1.10) following home‐ or centre‐based cardiac rehabilitation. At 24 months follow up, Jolly 2007 reported no significant difference between home‐ and centre‐based cardiac rehabilitation groups in systolic blood pressure (MD = ‐0.85 mm Hg; 95% CI 2.48 to ‐4.18) or diastolic blood pressure (MD = ‐0.76 mm Hg, 95% CI 1.12 to ‐2.64).
1.9. Analysis.
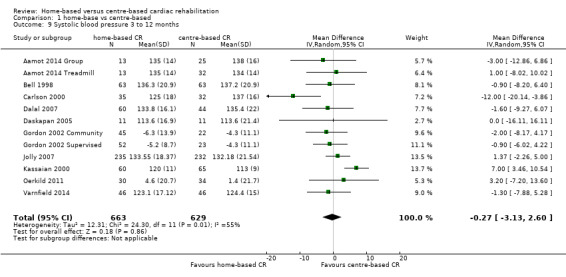
Comparison 1 home‐base vs centre‐based, Outcome 9 Systolic blood pressure 3 to 12 months.
1.10. Analysis.
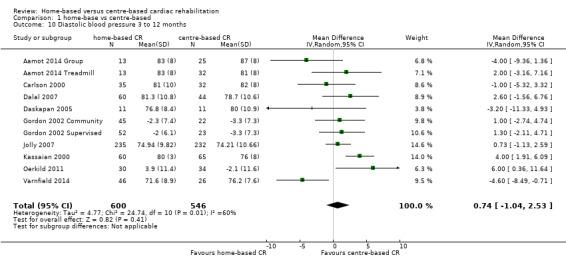
Comparison 1 home‐base vs centre‐based, Outcome 10 Diastolic blood pressure 3 to 12 months.
Subgroup analyses
Predictors of blood pressure were examined across the longest follow‐up period of each individual study using univariate meta‐regression. No statistically significant associations were seen in any of the analyses for systolic blood pressure with the exception of study location (Table 7). No statistically significant associations were seen in any of the analyses for diastolic blood pressure (Table 8).
6. Results of univariate meta‐regression analysis for systolic BP.
| Explanatory variable (n trials) | Exp(slope)* | 95% CI univariate P value | Proportion of variation explained | Interpretation |
| Case mix (% MI patients) (n = 11) | RR = 0.026 | ‐0.095 to 0.146 P = 0.642 |
‐8.81% | No evidence that RR is associated with case mix |
| Dose of exercise (number of weeks of exercise training x average number of sessions/week x average duration of session in min) (n = 4) |
RR = 0.001 | ‐0.110 to 0.112 P = 0.971 |
Not calculable¹ | No evidence that RR is associated with increased dose of exercise |
| Type of cardiac rehabilitation (exercise only versus comprehensive cardiac rehabilitation) (n = 12) |
RR = 5.021 | ‐0.929 to 10.971 P =0.089 |
51.60% | No evidence that RR is associated with type of cardiac rehabilitation |
| Duration of follow‐up (months) (n = 12) | RR = ‐0.053 | ‐0.540 to 0.435 P = 0.815 |
‐22.77% | No evidence that RR is associated with duration of follow‐up |
| Year of publication (n = 12) | RR = ‐0.008 | ‐0.607 to 0.591 P =0.976 |
‐15.85% | No evidence that RR is associated with year of publication |
| Risk of bias (low risk in ≥ 4 items versus < 4 items) (n = 12) | RR = 2.325 | ‐1.376 to 6.026 P =0.192 |
37.06% | No evidence that RR is associated with risk of bias |
| Study location (n = 12) | RR = 4.053 | 0.696 to 7.410 P = 0.023 |
71.21% | Evidence that RR is associated with study location |
| Sample size (n = 12) | RR = ‐0.005 | ‐0.029 to 0.018 P = 0.623 |
‐18.75% | No evidence that RR is associated with sample size |
¹Not calculable; Tau² of all studies = 0 Abbreviations: MI: myocardial infarction; RR: risk ratio
7. Results of univariate meta‐regression analysis for diastolic blood pressure.
| Explanatory variable (n trials) | Exp(slope)* |
95% CI univariate P value |
Proportion of variation explained | Interpretation |
| Case mix (% MI patients) (n = 10) | RR = 0.025 | ‐0.069 to 0.119 P = 0.561 |
‐11.53% | No evidence that risk RR is associated with case mix |
| Dose of exercise (number of weeks of exercise training x average number of sessions/week x average duration of session in min) (n = 4) |
RR = ‐0.017 | ‐0.085 to 0.051 P = 0.391 |
Not calculable¹ | No evidence that RR is associated with increased dose of exercise |
| Type of cardiac rehabilitation (exercise only versus comprehensive cardiac rehabilitation) (n = 11) |
RR = 0.125 | ‐4.719 to 4.970 P = 0.955 |
‐20.57% | No evidence that RR is associated with type of cardiac rehabilitation |
| Duration of follow‐up (months) (n = 11) | RR = ‐0.051 | ‐0.377 to 0.276 P = 0.734 |
‐32.23% | No evidence that RR is associated with duration of follow‐up |
| Year of publication (n = 11) | RR = 0.234 | ‐0.144 to 0.613 P = 0.195 |
40.22% | No evidence that RR is associated with year of publication |
| Risk of bias (low risk in ≥ 4 items versus < 4 items) (n = 11) | RR = 0.761 | ‐2.082 to 3.605 P = 0.560 |
0.88% | No evidence that RR is associated with risk of bias |
| Study location (n = 11) | RR = ‐0.034 | ‐3.196 to 3.128 P = 0.981 |
‐25.38% | No evidence that RR is associated with study location |
| Sample size (n = 11) | RR = ‐0.001 | ‐0.017 to 0.015 P = 0.907 |
‐30.17% | No evidence that risk ratio is associated with sample size |
¹Not calculable; Tau² of all studies = 0 Abbreviations: MI: myocardial infarction; RR: risk ratio
Small study bias
There was no evidence of funnel plot asymmetry for systolic blood pressure (Egger test P = 0.066; Figure 8) or diastolic blood pressure (Egger test P = 0.318; Figure 8).
8.
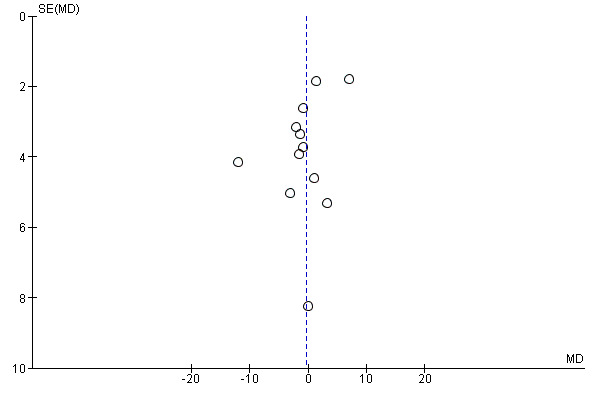
Funnel plot of comparison: 1 home‐base vs centre‐based, outcome: 1.9 Systolic blood pressure 3 to 12 months.
Smoking behaviour
Five studies (6 comparisons) reported on participants' self‐reported smoking behaviour at three to 12 months of follow up (Bell 1998; Dalal 2007; Gordon 2002 Community; Gordon 2002 Supervised; Jolly 2007; Oerkild 2011). There was no evidence indicating a difference in the proportion of smokers at follow up between home‐ and centre‐based cardiac rehabilitation (RR 0.1.02, 95% CI 0.83 to 1.27; participants = 986; studies = 5, comparisons = 6; I² = 0%; fixed‐effect; Analysis 1.11). Jolly 2007 reported no difference in smoking between home‐ and centre‐based arms at 24 months (RR = 1.16, 95% CI 0.58 to 33.3).
1.11. Analysis.
Comparison 1 home‐base vs centre‐based, Outcome 11 Smoking 3 to 12 months.
There was evidence of a consistent reduction in self‐reported smoking behaviour following both home‐ and centre‐based cardiac rehabilitation. This finding was confirmed in the one study that used cotinine‐validated assessments of smoking (Jolly 2007).
Subgroup analyses
Due to the small number of studies reporting smoking, it was not possible to examine the effects of potential treatment effect modifiers on these outcomes.
Small study bias
Due to the small number of studies reporting smoking behaviour, it was not possible to examine small study bias.
Adherence
All but six studies (Bell 1998; Daskapan 2005; Hadadzadeh 2013; Hadadzadeh 2015; Kassaian 2000; Wu 2006) reported adherence to cardiac rehabilitation over the duration of the study (Table 9). There was substantial variation in the way in which adherence was defined and measured, and some studies reported more than one measure of adherence. Pooling across studies was therefore deemed to be inappropriate. Eight studies (11 comparisons: Carlson 2000; Cowie 2012; Dalal 2007; Gordon 2002 Community; Gordon 2002 Supervised; Grace 2016 Mixed; Grace 2016 Women; Jolly 2007; Karapolat 2009; Miller 1984 Brief; Miller 1984 Expanded) found no evidence of a significant difference in the level of adherence between groups, although there was evidence of superior adherence in home‐based cardiac rehabilitation in five studies (Arthur 2002; Kraal 2014; Marchionni 2003; Piotrowicz 2010; Varnfield 2014) and evidence of superior adherence in centre‐based cardiac rehabilitation in one study (Aamot 2014 Treadmill). Three other studies reported adherence (Daskapan 2005; Moholdt 2012; Sparks 1993) but it was not possible to assess if there was a statistically significant difference between home‐ and centre‐based cardiac rehabilitation.
8. Summary of adherence at follow up in home and centre‐based cardiac rehabilitation.
| Trial | Follow‐up | Method/definition of adherence assessment | Findings | Between‐group difference |
|
Aamot 2014 Treadmill Home versus treadmill group |
12 weeks | Completion of 70% of the exercise sessions (considered to be training per protocol). Median (range) number of exercise sessions completed |
Home: 24/28 (86%) versus centre: 34/34 (100%) P = 0.04 Home: 24 (10–24) versus centre: 24 (7–24) |
Home < Centre |
|
Aamot 2014 Treadmill Home versus group exercise |
12 weeks | Completion of 70% of the exercise sessions (considered to be training per protocol). Median (range) number of exercise sessions completed |
Home: 24/28 (86%) versus centre: 28/28 (100%) P = 0.04 Home: 24 (10–24) versus centre: 23 (17–24) |
Home < Centre |
|
Table 7
Arthur 2002 /Smith 2004 |
6 months 18 months |
Number of exercise session reported/week Percentage of patients seeking dietician consultation Percentage of patients seeking psychologist consultation Level of physical activity – Physical Activity Scale for the Elderly |
Home: mean 6.5 (SD 4.6) Centre: mean 3.7 (SD 2.6) P < 0.0001† Home 50% (mean 3.5, SD 2.5 visits) Centre: 53% (mean 3.6, SD 2.3 visits) Home: 42% (mean 2.6, SD 2.4 visits) Centre: 51% (mean 2.5, SD 2.2 visits) Home: mean 232.6 (SD 99.4) Centre: mean 170.0 (SD 89.2) P < 0.0001† |
Home > Centre ? Home = Centre** Home > Centre |
| Carlson 2000 | 6 months | Attendance at all 3 nutrition/risk factor classes Total exercise over follow up – number of sessions ≥ 30 min |
Home: 27/38 (71%) Centre: 33/42 (79%) P = 0.438* Home: mean 111.8 (SD 29.1) Centre: mean 98.1 (SD 33.4) P = 0.06† |
Home = Centre Home = Centre |
| Cowie 2012 | 3 months | Percentage completion of 16 exercise sessions | Home: 77% Centre: 86% P = 0.32 |
Home = Centre |
| Dalal 2007 | 9 months | Number who participated in intervention | Home: 40/60 (67%) Centre: 32/44 (72%) P = 0.51* |
Home = Centre |
| Daskapan 2005 | 3 months | Percentage of sessions attended | Home: 97% Centre: 81% P value not calculable |
? |
| Gordon 2002 Community | 3 months | Percentage of completed scheduled appointments (exercise sessions, office/on site visits, “telephone visits” in accordance with intervention protocol) | Home (MD supervised): 83% Home (community‐based): 86% Centre: 81% |
Home = Centre** |
| Grace 2016 Mixed Home versus mixed sex training | 6 months | Percentage of cardiac rehabilitation sessions attended | Home: 58.12% (SD 34.68) Centre: 51.33% (SD 35.75) P = 0.63 |
Home = Centre |
| Grace 2016 Mixed Home versus women only training | 6 months | Percentage of cardiac rehabilitation sessions attended | Home: 58.12% (SD 34.68) Centre: 54.4% (SD 34.72) P = 0.63 |
Home = Centre |
| Jolly 2007 | 3 months 6 months 12 months 24 months |
Hours of self‐reported activity weighted for intensity | Home: mean 23.2 (SD 22.1) Centre: mean 18.7 (SD 19.3) P = 0.06† Home: mean 16.4 (SD 17.0) Centre: mean 18.1 (SD 25.4) P = 0.4† Home: mean 19.2 (SD 20.8) Centre: mean 15.9 (SD 16.7) P = 0.06† Home: mean 18.9 (SD 18.4) Centre: mean 16.6 (SD 16.4) P = 0.16† |
Home = Centre Home = Centre Home = Centre Home = Centre |
| Karapolat 2009 | 8 weeks | Attendance at exercise sessions | Home: (32/37) 87.5% Centre: (33/37) 90% P = 0.72* |
Home = Centre |
| Kraal 2014 | 12 weeks | Number of sessions attended | Home: Mean = 24 (100 %; SD 7.2; range: 13 to 41) Centre: Mean = 20.5 (86%; SD 4.5 range: 6 to 25) P = 0.049 |
Home > Centre |
| Marchionni 2003 | 4 months | Number of exercise sessions completed | Home: 37.3 (SD 3.4) Centre: 34.3 (SD 4.4) P < 0.0001† |
Home > Centre |
|
Miller 1984 Brief/ DeBusk 1985/ Taylor 1986 |
6 months | Ratio of exercise sessions completed versus prescribed | Home: 50/70 (72%) Centre: 28/40 (71%) P value not calculable |
Home = Centre** |
| Moholdt 2012 | 6 months | Training diaries (only reported for home group) | Home: 7/10 patients (with complete diary data) reported ≥2 weekly interval sessions over 6 months follow up | ? |
| Piotrowicz 2010 | 8 weeks | Percentage of patients who carried out the prescribed exercise training (home group: daily telephone contacts with monitoring centre; centre group: attendance at supervised sessions) | Home: 77/77 (100%) Centre: 59/75 (79%) P < 0.0001† |
Home > Centre |
| Sparks 1993 | 3 months | Percentage of cardiac rehabilitation sessions attended | Home: 93% Centre: 88% P value not calculable |
? |
| Varnfield 2014 | 6 weeks | "Attended baseline assessment and at least 4 weeks (8 of 12 sessions) of centre‐based gym sessions/uploaded exercise data to web portal for a minimum of 4 weeks" | Home: 45/48 (94%) Centre: 25/37 (68%) P < 0.005 |
Home > Centre |
*calculated by authors of this report based on Chi² test †calculated by authors of this report based on independent t‐test Home = Centre: no statistically significant difference (P > 0.05) in health‐related quality of life (HRQoL) between home‐ and centre‐based groups at follow up Home > Centre: statistically significant (P ≤ 0.05) higher HRQoL in home‐ versus centre‐based groups at follow up Home < Centre: statistically significant (P ≤ 0.05) lower HRQoL in home‐ versus centre‐based groups at follow up **Home‐ and centre‐based groups at follow up appear to be similar but P value not reported or calculable ? Home‐ and centre‐based groups at follow up appear different but P value not reported or calculable
Costs and health service use
Six studies reported costs (Carlson 2000; Cowie 2012; Dalal 2007; Jolly 2007; Marchionni 2003; Varnfield 2014; Table 10). Differences in currencies and timing of studies meant that it was not possible compare the costs directly across studies. In four of the five studies, healthcare costs associated with cardiac rehabilitation were lower for the home‐based than centre‐based programmes (Carlson 2000; Dalal 2007; Marchionni 2003; Varnfield 2014), although cost was significantly lower in only one study (Dalal 2007). Jolly 2007 found that home‐based cardiac rehabilitation was more expensive than centre‐based cardiac rehabilitation, although the costs of the two would have been the same if participant costs were included. One study (Cowie 2012) included the costs of a no cardiac rehabilitation control and showed that cardiac rehabilitation costs were offset by a reduction in hospital admissions over five years resulting in a substantive cost saving when compared with control, i.e. GBP ‐3304 per participant for home‐based cardiac rehabilitation and GBP ‐3784 per participant for hospital‐based cardiac rehabilitation. Eight studies reported different aspects of consumption of healthcare resources, including re‐admissions to hospital, primary care consultations and use of secondary care medication (Table 11; Table 12). No significant between‐group differences were seen.
9. Summary of costs in home‐ and centre‐based settings.
| Study | CurrencyYear of costsFollow up | Cardiac rehabilitation programme cost(per patient) | Programme costs considered | Total healthcare cost(per patient) | Additional healthcare costs considered | Comments |
| Carlson 2000 | USD Not reported 6 months |
Home: mean USD 1519 Centre: mean USD 2349 |
Staff, ECG monitoring |
Not reported | ||
| Cowie 2012 | GBP 2013 to 2014 60 months |
Home: GBP mean 197 Centre: GBP mean 221 |
Staff, HR monitors, DVD | Home: mean: GBP 7932 Centre: mean: GBP 7452 |
Hospitalisations, emergency admissions |
|
| Marchionni 2003 | USD 2000 14 months |
Home: mean USD 1650 Centre: mean USD 8841 |
Not reported | Home: USD 13,246 Centre: USD 21,298 |
Not reported | |
| Dalal 2007 | GBP 2002 to 2003 9 months |
Home: mean GBP 170 (SD 8) Centre: mean GBP 200 (SD 3) Difference: mean GBP 30 (95% CI ‐45 to ‐12) P < 0.0001 |
Staff, exercise, equipment, staff travel |
Home: mean GBP 3279 (SD 374) Centre: mean GBP 3201 (SD 443) Difference: mean GBP 78(95% CI ‐1103 to 1191) P = 0.894 |
Rehospitalisations, revascularisations, secondary preventive medication, investigations, primary care consultations |
|
| Jolly 2007 | GBP 2003 24 months |
Home: mean GBP 198 (95% CI 189 to 209) Centre: mean GBP 157 (95% CI 139 to 175) P < 0.05 |
Staff, telephone, consultations, staff travel | Not reported | With inclusion of patient costs (travel and time), the societal costs of home‐ and centre‐bas cardiac rehabilitation were not significantly different |
|
| Varnfield 2014/ Whittaker 2014 | AUD Not reported Based on a 6 week programme |
Home: AUD 1633 Centre: AUD 1845 |
Education, assessment, coaching and mentoring, gymnasium, communication, facility, technology, administration | Patient travel: Home: AUD 80 Centre: AUD 400 |
Re‐admissions ‐ Estimated AUD 39,670 per re‐admission (Collins 2001) |
Based on evidence suggesting that completing a formal rehabilitation programme significantly reduces the risk of a secondary event and readmission, the net‐present value was calculated at AUD 4008 per patient, equating to a saving in health care costs of AUD 2375 per patient |
Abbreviation: ECG = electrocardiogram
10. Summary of healthcare utilisation in home‐ and centre‐based settings.
| Study | Dalal 2007 | Gordon 2002 Community | Bell 1998 | Carlson 2000 | Marchionni 2003 | Jolly 2007 | ||
| Follow up | 9 months | 3 months | 0 to 6 months | 6 to 12 months | 6 months | 14 months | 12 month | 24 month |
|
Rehospitalisations N patient (%) Mean (SD) |
Home 9/60 (15%) Centre 6/44 (14%) P = 0.845 Home 2.2 (0.9)† Centre 1.2 (0.6) P = 0.383 |
Home 21/90 (23%) Centre 19/88 (22%) P = 0.78# |
13/89 (15%) 12/84 (14%) P = 0.95# |
Home 0.46 (SE 0.1) Centre 0.33 (SE 0.1) P = 0.49 |
Home 0.08 (0.34) Centre 0.12 (0.41) P = 0.3 |
Home 0.20 (0.45) Centre 0.26 (0.57) P = 0.3 |
||
|
Primary care consultations Mean (SD) |
Home 6.3 (0.6) Centre 7.0 (0.9) P = 0.514 |
Home 6.6 (3.6)* Centre 6.6 (4.1) P = 1.00# |
5.4 (4.1) 4.6 (3.7) P = 0.19# |
Home 0.65 (1.14) Centre 0.72 (1.54) P = 0.8 |
Home 0.53 (1.14) Centre 0.66 (1.42) P = 0.7 |
|||
|
Secondary prevention medication N patients (%) beta‐blockers ACE inhibitors Statins Antiplatelets |
Home 31/49 (63%) Centre 24/34 (71%) P = 0.49 Home 30/49 (61%) Centre 24/33 (73%) P = 0.28 Home 48/49 (98%)* Centre 30/35 (88%)* P = 0.18 Home 46/49 (94%) Centre 30/35 (86%) P = 0.21 |
Home 36/97 (37%) Centre 17/45 (38%) NS Home 25/97 (26%) Centre 8/45 (18%) NS Home 73/97 (75%) Centre 33/45 (73%) NS Home 94/97 (97%)* Centre 45/45 (100%)* NS |
Home 19/38 Centre 18/42 P = 0.52# Home 4/38 Centre 4/42 P = 0.88# Home 5/38 Centre 8/42 P = 0.47# Home 15/38 Centre 20/42 P = 0.54# |
Home 169 (72.2%) Centre 171 (73.4%) P = 0.8 Home 176 (75.2%)* Centre 161 (69.1%)* P = 0.1 Home 216 (92.3%)** Centre 221 (94.8%)** P = 0.3 Home 227 (97.0%)† Centre 226 (97.0%)† P = 1.0 |
Home 161 (71.6%) Centre 164 (72.2%) P = 0.9 Home 177 (78.7%)* Centre 156 (68.7%)* P = 0.02 Home 195 (86.7%)** Centre 206 (90.7%)** P = 0.2 Home 214 (95.1%)+ Centre 220 (96.9%)+ P = 0.3 |
|||
|
Comments |
†number of nights *lipid lowering drugs |
*antiplatelets & anticoagulants | *GP consultations | *ACEi or Angiotensin II receptor antagonist **cholesterol‐lowering drugs †Aspirin or antiplatelet drugs |
||||
#P value calculated by authors of the present report NS: not statistically significant SE: standard error
11. Summary of healthcare in hospital‐ and centre‐based settings, continued.
| Study | Moholdt 2012 | Oerkild 2011 |
| Follow up | 6 months | 12 months |
|
Rehospitalisations N patient (%) Number Mean (SD) |
Not reported | Number and length of admissions same between groups |
|
Primary care Consultations Mean (SD) |
Not reported | Not reported |
|
Secondary prevention medication N patients (%) beta‐blockers ACE inhibitors Antihypertensives Statins Antiplatelets |
Home: 8/14 (57%) Centre: 15/16 (94%) P = 0.02* Home: 1/14 (7%) Centre: 0/16 (0%) P = 0.28* Home: 6/14 (43%) Centre: 2/16 (13%) P = 0.07* Home: 14/14 (100%) Centre: 14/16 (100%) P = 0.18* |
Not reported |
| Comments |
*P value calculated by review authors ACE: angiotensin‐converting‐enzyme
Discussion
Summary of main results
The mainstay approach to cardiac rehabilitation delivery in many countries is an inpatient and outpatient hospital‐based provision, which often takes place in a supervised university, hospital or community setting. The availability of home‐based programmes may provide an opportunity to widen access and increase participation in cardiac rehabilitation and, may therefore, improve uptake and adherence. Figures from the UK suggest that the dominant mode of delivery in the UK is group‐based, with just 10% cardiac rehabilitation programmes currently offering home‐based cardiac rehabilitation provision (NACR 2016).
This updated review included 23 trials which randomised 2890 participants following an acute myocardial infarction (MI) or revascularisation, or with heart failure, to either home‐ or centre‐based cardiac rehabilitation. The model of home‐based provision in the largest three included trials was the Heart Manual 2016 (Bell 1998; Dalal 2007; Jolly 2007), a cardiac rehabilitation programme that consists of a self‐help manual supported by a nurse facilitator (Lewin 1992). We found no evidence supporting important differences in outcomes for patients receiving home‐based or centre‐based cardiac rehabilitation either in the short‐term (3 to 12 months) or longer‐term (up to 24 months) for mortality, cardiac events, exercise capacity, modifiable risk factors (total cholesterol; low density lipoprotein cholesterol; systolic blood pressure; diastolic blood pressure; proportion of smokers at follow up) or health‐related quality of life. Small outcome differences in favour of centre‐based participants were seen in high density lipoprotein cholesterol and triglycerides. In contrast, in home‐based participants, there was evidence of marginally higher levels of programme completion and adherence to the programme. Healthcare costs seem to depend on the healthcare economy in which cardiac rehabilitation provision is made. However, this review found no consistent evidence to support an important difference in the cost of providing home‐ versus centre‐based programmes. Home‐based programmes often require support from healthcare staff which can be the major cost driver.
Overall completeness and applicability of evidence
The inclusion criteria for this review are broad, in order to reflect current practice where an increasingly diverse patient population is accessing cardiac rehabilitation services (NACR 2016). While the original version of this review was primarily limited to trials in participants with stable coronary heart disease (CHD) either following an acute myocardial infarction (MI) or revascularisation (Taylor 2009), the 2015 update included an additional five trials, which included 345 participants with heart failure (Taylor 2015). However, while this latest update added a further six trials in mixed populations of participants with CHD, none included people with heart failure. Similarly, only 19% of all participants included in this review were women and most participants were from studies that took place in high‐income nations (Europe and North America). It is therefore not clear whether or not our findings generalise to women, or to the wider population in general. However, while ethnicity was poorly reported by most studies, this review included several studies with a substantive proportion of ethnic groups, and in studies that reported ethnicity, fewer than 50% of participants were described as Caucasian. The applicability of our findings to low‐ and middle‐income countries is uncertain.
Interventions varied substantially in content, mode of delivery, level of support or supervision and dose. It could be argued that a benefit of this heterogeneity is that the results are more likely to be applicable to the wider population of people with CHD and clinical practice. However, we must also acknowledge this heterogeneity when interpreting the effect of these interventions on outcomes. This review also included studies which followed participants for as little as eight weeks post‐randomisation, which limits the clinical relevance of the findings. Similarly, fidelity (whether the intervention was delivered as intended) and dose (the quantity of intervention implemented) are important aspects of the delivery of a complex intervention, such as cardiac rehabilitation, and were generally poorly reported by studies included in this review.
Quality of the evidence
The general lack of reporting of methods in the included randomised controlled trial (RCT) reports made it difficult to assess their methodological quality and thereby judge their risk of bias, although there was some evidence of an improvement in the quality of reporting in more recent trials. It was also not possible to consistently judge whether the rehabilitation programmes included in the studies fulfilled recommended quality criteria for delivery of cardiac rehabilitation programmes, such as the BACPR guidelines (BACPR 2012).
Due to this poor reporting, the quality of the evidence for outcomes was assessed as moderate at best. Other reasons for downgrading the quality of evidence included inconsistency (exercise capacity ≤ 12 months and withdrawal) and imprecision (mortality).
Potential biases in the review process
Our review has limitations. Given the inconsistent reporting of outcomes, we were unable to judge the degree of publication bias for all outcomes, although there was no evidence of funnel plot asymmetry or statistically significant Egger tests for any outcome where this was tested (total mortality, exercise capacity, withdrawal, total cholesterol or blood pressure).
Although most participants represented in this review who received home‐based cardiac rehabilitation were exposed to the Heart Manual model, there was evidence of considerable statistical heterogeneity across a number of outcomes among trials. This heterogeneity may well reflect the variety of centre‐based cardiac rehabilitation interventions. Most studies were of relatively short duration, with only three trials reporting outcomes beyond 12 months of follow‐up (Arthur 2002; Jolly 2007; Marchionni 2003). The number of deaths and cardiac events reported by most trials was therefore correspondingly small. Details of interventions were often poorly reported and it was therefore difficult to assess whether the cardiac rehabilitation programmes used would meet current recommendations of good practice (BACPR 2012; Piepoli 2010).
It has been hypothesised that patient preference may have an impact on uptake and adherence to home‐based cardiac rehabilitation and there is evidence that white patients who work full‐ or part‐time and who perceive time constraints as a barrier to adherence are more likely to have a preference for home‐based provision (Grace 2005). However, such a hypothesis is difficult to test in a traditional RCT and therefore our finding of similar adherence between home‐ and centre‐based cardiac rehabilitation needs to be interpreted with caution. Dalal 2007 employed a comprehensive cohort design in addition to the randomised element of home‐ and centre‐based allocation in which there was also a patient preference element (participants could choose between home‐ and hospital‐based cardiac rehabilitation). The study authors reported that outcome differences between the home and hospital arms in the preference (non‐randomised) sample were very similar to those in the randomised comparison. Adherence to home‐based cardiac rehabilitation was also comparable between the randomised (75%) and preference arms (73%). This finding does not support the hypothesis that patients who can choose a programme to suit their lifestyle and preferences will have a higher adherence rate and improved outcomes. However, as with the randomised comparison, the number of participants in the preference arms was small (N = 126).
Agreements and disagreements with other studies or reviews
The findings of this update are consistent with the previous versions of this Cochrane Review (Taylor 2009; Taylor 2015) and another systematic review which reported that home‐based cardiac rehabilitation programmes are as effective as centre‐based programmes in terms of mortality, morbidity, short‐term exercise capacity, blood pressure, smoking cessation and health‐related quality of life (HRQoL) (Crawford‐Faucher 2010).
Our findings are also consistent with a recent systematic review which compared the effectiveness of "telehealth intervention‐delivered cardiac rehabilitation" with centre‐based supervised cardiac rehabilitation (Huang 2015) in nine trials, eight of which are included in this current review. The authors of the review concluded that telehealth intervention‐delivered cardiac rehabilitation does not have significantly inferior outcomes compared to centre‐based supervised programmes in low‐to‐moderate risk patients with CHD (Huang 2015). Similarly, another review which narratively synthesised 11 studies comparing "telerehabilitation" with other delivery models of cardiac rehabilitation in patients with cardiopulmonary diseases (Hwang 2015) found no differences between telerehabilitation and other delivery models, in terms of exercise capacity, quality of life or adverse events, while higher adherence rates were found for patients participating in the telerehabilitation programmes compared with centre‐based exercise.
Finally, our results also concur with a recent systematic review which assessed the effectiveness of home‐based cardiac rehabilitation for heart failure compared to either usual medical care (i.e. no cardiac rehabilitation) or centre‐based cardiac rehabilitation on mortality, morbidity, exercise capacity, HRQoL, adherence and costs (Zwisler 2016). This review found that outcomes and costs were similar between home‐ and centre‐based cardiac rehabilitation with the exception of higher levels of trial completion in the home‐based group.
Authors' conclusions
Implications for practice.
Home‐based and hospital‐ or centre‐based cardiac rehabilitation seem to be of similar effectiveness in improving clinical and health‐related quality of life (HRQoL) outcomes in patients after acute myocardial infarction (MI), revascularisation or with heart failure. This finding, together with a lack of evidence of differences in healthcare costs between the approaches, supports that the choice of participating in a more traditional supervised centre‐ or home‐based programme should reflect local availability and consider the preference of the individual patient.
Implications for research.
Data are needed to determine whether the effects of home‐ and centre‐based cardiac rehabilitation reported in short‐term trials can be confirmed in the longer term. Further comparative trials are needed to assess the relative impact of supervised centre‐ versus home‐based cardiac rehabilitation in patients with heart failure and angina pectoris and need to consider adequately powered non‐inferiority or equivalence study designs. Such studies need to consider economic factors, better methods of assessing and reporting adherence and patient‐related outcomes including costs to the healthcare system and HRQoL.
What's new
| Date | Event | Description |
|---|---|---|
| 20 October 2017 | Amended | correction of mistake in Table 9 |
History
Protocol first published: Issue 2, 2008 Review first published: Issue 1, 2010
| Date | Event | Description |
|---|---|---|
| 20 January 2017 | New citation required but conclusions have not changed | Six new studies included. Conclusions not changed. |
| 14 November 2016 | New search has been performed | The review was updated following a new search in September 2016. |
| 14 October 2014 | New search has been performed | The review has been updated following a new search in October 2014. |
| 9 October 2014 | New citation required but conclusions have not changed | Five new studies were found for inclusion but did not change the conclusions of this review. |
| 19 April 2010 | Amended | Minor changes to the Background section. |
| 10 February 2010 | Amended | Forest plots of 'Mortality' and 'Completers' have been updated as home and hospital group headings were inadvertently reversed in the original review. Added citation in 'Other published versions of this review'. |
Acknowledgements
We are grateful to all authors of the original and previous versions of this Cochrane Review (Dalal 2010; Taylor 2015) for their contributions. We would also like to thank all the study authors who provided additional information about their trials.
Appendices
Appendix 1. Search strategies
September 2016 Strategies
CENTRAL
#1 MeSH descriptor: [Myocardial Ischemia] explode all trees
#2 (myocard* near isch*mi*)
#3 isch*mi* near heart
#4 MeSH descriptor: [Coronary Artery Bypass] explode all trees
#5 coronary
#6 MeSH descriptor: [Coronary Disease] explode all trees
#7 MeSH descriptor: [Myocardial Revascularization] explode all trees
#8 MeSH descriptor: [Myocardial Infarction] explode all trees
#9 myocard* near infarct*
#10 heart near infarct*
#11 MeSH descriptor: [Angina Pectoris] explode all trees
#12 angina
#13 MeSH descriptor: [Heart Failure] explode all trees
#14 heart and (failure or attack)
#15 MeSH descriptor: [Heart Diseases] explode all trees
#16 heart near disease*
#17 myocard*
#18 cardiac*
#19 CABG
#20 PTCA
#21 stent* near (heart or cardiac*)
#22 MeSH descriptor: [Heart Bypass, Left] explode all trees
#23 MeSH descriptor: [Heart Bypass, Right] explode all trees
#24 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23
#25 MeSH descriptor: [Percutaneous Coronary Intervention] explode all trees
#26 (percutaneous coronary near/2 (interven* or revascular*))
#27 MeSH descriptor: [Angioplasty] explode all trees
#28 angioplast*
#29 ((coronary or arterial) near/4 dilat*)
#30 endoluminal repair*
#31 MeSH descriptor: [Stents] explode all trees
#32 stent*
#33 (pci or ptca)
#34 MeSH descriptor: [Atherectomy] explode all trees
#35 atherectom*
#36 acute coronary syndrom*
#37 #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36
#38 MeSH descriptor: [Rehabilitation Centers] explode all trees
#39 MeSH descriptor: [Exercise Therapy] explode all trees
#40 MeSH descriptor: [Sports] this term only
#41 MeSH descriptor: [Physical Exertion] explode all trees
#42 rehabilitat*
#43 (physical* near (fit* or train* or therap* or activit*))
#44 MeSH descriptor: [Exercise] explode all trees
#45 (train*) near (strength* or aerobic or exercise*)
#46 ((exercise* or fitness) near/3 (treatment or intervent* or program*))
#47 MeSH descriptor: [Rehabilitation] explode all trees
#48 MeSH descriptor: [Patient Education as Topic] explode all trees
#49 (patient* near/3 educat*)
#50 ((lifestyle or life‐style) near/3 (intervent* or program* or treatment*))
#51 MeSH descriptor: [Self Care] explode all trees
#52 MeSH descriptor: [Ambulatory Care] explode all trees
#53 MeSH descriptor: [Psychotherapy] explode all trees
#54 psychotherap*
#55 psycholog* near intervent*
#56 relax*
#57 MeSH descriptor: [Relaxation Therapy] explode all trees
#58 MeSH descriptor: [Counseling] explode all trees
#59 counsel*ing
#60 MeSH descriptor: [Cognitive Therapy] explode all trees
#61 MeSH descriptor: [Behavior Therapy] explode all trees
#62 (behavio*r*) near/4 (modif* or therap* or rehab* or change)
#63 MeSH descriptor: [Stress, Psychological] explode all trees
#64 stress near manage*
#65 cognitive* near therap*
#66 MeSH descriptor: [Meditation] explode all trees
#67 meditat*
#68 MeSH descriptor: [Anxiety] this term only
#69 (manage*) near (anxiety or depres*)
#70 CBT
#71 hypnotherap*
#72 goal near/3 setting
#73 (psycho‐educat*) or (psychoeducat*)
#74 motivat* near interv*
#75 MeSH descriptor: [Psychopathology] explode all trees
#76 psychopathol*
#77 MeSH descriptor: [Autogenic Training] explode all trees
#78 autogenic*
#79 self near (manage* or care or motivat*)
#80 distress*
#81 psychosocial* or psycho‐social
#82 MeSH descriptor: [Health Education] explode all trees
#83 ((nutrition or diet or health) near education)
#84 heart manual
#85 home‐based
#86 #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 or #48 or #49 or #50 or #51 or #52 or #53 or #54 or #55 or #56 or #57 or #58 or #59 or #60 or #61 or #62 or #63 or #64 or #65 or #66 or #67 or #68 or #69 or #70 or #71 or #72 or #73 or #74 or #75 or #76 or #77 or #78 or #79 or #80 or #81 or #82 or #83 or #84 or #85
#87 #37 and #86
#88 #24 and #86 Publication Year from 2016 to 2016
#89 #87 or #88
MEDLINE
1. exp Myocardial Ischemia/
2. (myocard* adj3 isch?mi*).tw.
3. (isch?mi* adj3 heart).tw.
4. exp Coronary Artery Bypass/
5. coronary.tw.
6. exp Coronary Disease/
7. exp Myocardial Revascularization/
8. exp Myocardial Infarction/
9. (myocard* adj3 infarct*).tw.
10. (heart adj3 infarct*).tw.
11. exp Angina Pectoris/
12. angina.tw.
13. exp Heart Failure/
14. (heart adj3 (failure or attack)).tw.
15. exp Heart Diseases/
16. (heart adj3 disease*).tw.
17. myocard*.tw.
18. cardiac*.tw.
19. CABG.tw.
20. PTCA.tw.
21. (stent* adj3 (heart or cardiac*)).tw.
22. Heart Bypass, Left/
23. exp Heart Bypass, Right/
24. or/1‐23
25. exp Percutaneous Coronary Intervention/
26. (percutaneous coronary adj2 (interven* or revascular*)).tw.
27. exp Angioplasty/
28. angioplast*.tw.
29. ((coronary or arterial) adj4 dilat*).tw.
30. endoluminal repair*.tw.
31. exp Stents/
32. stent*.tw.
33. (pci or ptca).tw.
34. exp Atherectomy/
35. atherectom*.tw.
36. acute coronary syndrom*.tw.
37. or/25‐36
38. Rehabilitation Centers/
39. exp Exercise Therapy/
40. Sports/
41. Physical Exertion/
42. rehabilitat*.tw.
43. (physical* adj3 (fit* or train* or therap* or activit*)).tw.
44. exp Exercise/
45. (train* adj3 (strength* or aerobic or exercise*)).tw.
46. ((exercise* or fitness) adj3 (treatment or intervent* or program*)).tw.
47. exp Rehabilitation/
48. Patient Education as Topic/
49. (patient* adj3 educat*).tw.
50. ((lifestyle or life‐style) adj3 (intervent* or program* or treatment*)).tw.
51. exp Self Care/
52. exp Ambulatory Care/
53. exp Psychotherapy/
54. psychotherap*.tw.
55. (psycholog* adj3 intervent*).tw.
56. relax*.tw.
57. Relaxation Therapy/
58. exp Counseling/
59. counsel?ing.tw.
60. exp Cognitive Therapy/
61. exp Behavior Therapy/
62. (behavio?r* adj4 (modif* or therap* or rehab* or change)).tw.
63. exp Stress, Psychological/
64. (stress adj3 manage*).tw.
65. (cognitive* adj3 therap*).tw.
66. exp Meditation/
67. meditat*.tw.
68. Anxiety/
69. (manage* adj3 (anxiety or depres*)).tw.
70. CBT.tw.
71. hypnotherap*.tw.
72. (goal adj3 setting).tw.
73. (psycho‐educat* or psychoeducat*).tw.
74. (motivat* adj3 interv*).tw.
75. exp Psychopathology/
76. psychopathol*.tw.
77. exp Autogenic Training/
78. autogenic*.tw.
79. (self adj3 (manage* or care or motivat*)).tw.
80. distress*.tw.
81. (psychosocial* or psycho‐social*).tw.
82. exp Health Education/
83. ((nutrition or diet or health) adj3 education).tw.
84. heart manual.tw.
85. home based.tw.
86. or/38‐85
87. randomized controlled trial.pt.
88. controlled clinical trial.pt.
89. randomized.ab.
90. placebo.ab.
91. drug therapy.fs.
92. randomly.ab.
93. trial.ab.
94. groups.ab.
95. 87 or 88 or 89 or 90 or 91 or 92 or 93 or 94
96. exp animals/ not humans.sh.
97. 95 not 96
98. 2016*.ed.
99. 37 and 86 and 97
100. 24 and 86 and 97 and 98
101. 99 or 100
Embase
1. exp Myocardial Ischemia/
2. (myocard* adj3 isch?mi*).tw.
3. (isch?mi* adj3 heart).tw.
4. exp Coronary Artery Bypass/
5. coronary.tw.
6. exp Coronary Disease/
7. exp Myocardial Revascularization/
8. exp Myocardial Infarction/
9. (myocard* adj3 infarct*).tw.
10. (heart adj3 infarct*).tw.
11. exp Angina Pectoris/
12. angina.tw.
13. exp Heart Failure/
14. (heart adj3 (failure or attack)).tw.
15. exp Heart Diseases/
16. (heart adj3 disease*).tw.
17. myocard*.tw.
18. cardiac*.tw.
19. CABG.tw.
20. PTCA.tw.
21. (stent* adj3 (heart or cardiac*)).tw.
22. Heart Bypass, Left/
23. exp Heart Bypass, Right/
24. or/1‐23
25. exp percutaneous coronary intervention/
26. (percutaneous coronary adj2 (interven* or revascular*)).tw.
27. exp angioplasty/
28. angioplast*.tw.
29. ((coronary or arterial) adj4 dilat*).tw.
30. endoluminal repair*.tw.
31. exp stent/
32. stent*.tw.
33. (pci or ptca).tw.
34. exp atherectomy/
35. atherectom*.tw.
36. acute coronary syndrom*.tw.
37. or/25‐36
38. Rehabilitation Centers/
39. exp Exercise Therapy/
40. Sports/
41. Physical Exertion/
42. rehabilitat*.tw.
43. (physical* adj3 (fit* or train* or therap* or activit*)).tw.
44. exp Exercise/
45. (train* adj3 (strength* or aerobic or exercise*)).tw.
46. ((exercise* or fitness) adj3 (treatment or intervent* or program*)).tw.
47. exp Rehabilitation/
48. Patient Education as Topic/
49. (patient* adj3 educat*).tw.
50. ((lifestyle or life‐style) adj3 (intervent* or program* or treatment*)).tw.
51. exp Self Care/
52. exp Ambulatory Care/
53. exp Psychotherapy/
54. psychotherap*.tw.
55. (psycholog* adj3 intervent*).tw.
56. relax*.tw.
57. Relaxation Therapy/
58. exp Counseling/
59. counsel?ing.tw.
60. exp Cognitive Therapy/
61. exp Behavior Therapy/
62. (behavio?r* adj4 (modif* or therap* or rehab* or change)).tw.
63. exp Stress, Psychological/
64. (stress adj3 manage*).tw.
65. (cognitive* adj3 therap*).tw.
66. exp Meditation/
67. meditat*.tw.
68. Anxiety/
69. (manage* adj3 (anxiety or depres*)).tw.
70. CBT.tw.
71. hypnotherap*.tw.
72. (goal adj3 setting).tw.
73. (psycho‐educat* or psychoeducat*).tw.
74. (motivat* adj3 interv*).tw.
75. exp Psychopathology/
76. psychopathol*.tw.
77. exp Autogenic Training/
78. autogenic*.tw.
79. (self adj3 (manage* or care or motivat*)).tw.
80. distress*.tw.
81. (psychosocial* or psycho‐social*).tw.
82. exp Health Education/
83. ((nutrition or diet or health) adj3 education).tw.
84. heart manual.tw.
85. home based.tw.
86. or/38‐85
87. random$.tw.
88. factorial$.tw.
89. crossover$.tw.
90. cross over$.tw.
91. cross‐over$.tw.
92. placebo$.tw.
93. (doubl$ adj blind$).tw.
94. (singl$ adj blind$).tw.
95. assign$.tw.
96. allocat$.tw.
97. volunteer$.tw.
98. crossover procedure/
99. double blind procedure/
100. randomized controlled trial/
101. single blind procedure/
102. 87 or 88 or 89 or 90 or 91 or 92 or 93 or 94 or 95 or 96 or 97 or 98 or 99 or 100 or 101
103. (animal/ or nonhuman/) not human/
104. 102 not 103
105. 2016*.em.
106. 37 and 86 and 104
107. 24 and 86 and 104 and 105
108. 106 or 107
PsycINFO
1. (myocard* adj3 isch?mi*).tw.
2. (isch?mi* adj3 heart).tw.
3. coronary.tw.
4. exp Myocardial Infarction/
5. (myocard* adj3 infarct*).tw.
6. (heart adj3 infarct*).tw.
7. exp Angina Pectoris/
8. angina.tw.
9. (heart adj3 (failure or attack)).tw.
10. (heart adj3 disease*).tw.
11. myocard*.tw.
12. cardiac*.tw.
13. CABG.tw.
14. PTCA.tw.
15. (stent* adj3 (heart or cardiac*)).tw.
16. or/1‐15
17. exp percutaneous coronary intervention/
18. (percutaneous coronary adj2 (interven* or revascular*)).tw.
19. exp angioplasty/
20. angioplast*.tw.
21. ((coronary or arterial) adj4 dilat*).tw.
22. endoluminal repair*.tw.
23. exp stent/
24. stent*.tw.
25. (pci or ptca).tw.
26. exp atherectomy/
27. atherectom*.tw.
28. acute coronary syndrom*.tw.
29. or/17‐28
30. Rehabilitation Centers/
31. exp Exercise Therapy/
32. Sports/
33. rehabilitat*.tw.
34. (physical* adj3 (fit* or train* or therap* or activit*)).tw.
35. exp Exercise/
36. (train* adj3 (strength* or aerobic or exercise*)).tw.
37. ((exercise* or fitness) adj3 (treatment or intervent* or program*)).tw.
38. exp Rehabilitation/
39. (patient* adj3 educat*).tw.
40. ((lifestyle or life‐style) adj3 (intervent* or program* or treatment*)).tw.
41. exp Self Care/
42. exp Ambulatory Care/
43. exp Psychotherapy/
44. psychotherap*.tw.
45. (psycholog* adj3 intervent*).tw.
46. relax*.tw.
47. Relaxation Therapy/
48. exp Counseling/
49. counsel?ing.tw.
50. exp Cognitive Therapy/
51. exp Behavior Therapy/
52. (behavio?r* adj4 (modif* or therap* or rehab* or change)).tw.
53. (stress adj3 manage*).tw.
54. (cognitive* adj3 therap*).tw.
55. exp Meditation/
56. meditat*.tw.
57. Anxiety/
58. (manage* adj3 (anxiety or depres*)).tw.
59. CBT.tw.
60. hypnotherap*.tw.
61. (goal adj3 setting).tw.
62. (psycho‐educat* or psychoeducat*).tw.
63. (motivat* adj3 interv*).tw.
64. exp Psychopathology/
65. psychopathol*.tw.
66. exp Autogenic Training/
67. autogenic*.tw.
68. (self adj3 (manage* or care or motivat*)).tw.
69. distress*.tw.
70. (psychosocial* or psycho‐social*).tw.
71. exp Health Education/
72. ((nutrition or diet or health) adj3 education).tw.
73. heart manual.tw.
74. home based.tw.
75. or/30‐74
76. random$.tw.
77. factorial$.tw.
78. crossover$.tw.
79. cross‐over$.tw.
80. placebo$.tw.
81. (doubl$ adj blind$).tw.
82. (singl$ adj blind$).tw.
83. assign$.tw.
84. allocat$.tw.
85. volunteer$.tw.
86. control*.tw.
87. "2000".md.
88. or/76‐87
89. 2016*.up.
90. 29 and 75 and 88
91. 16 and 75 and 88 and 89
92. 90 or 91
CINAHL
S88 S86 OR S87
S87 S23 AND S82 AND S85 Limiters ‐ Publication Year: 2016‐2016
S86 S36 AND S82 AND S85
S85 S83 OR S84
S84 (MH "Clinical Trials+")
S83 random* or blind* or allocat* or assign* or trial* or placebo* or crossover* or cross‐over*
S82 S37 OR S38 OR S39 OR S40 OR S41 OR S42 OR S43 OR S44 OR S45 OR S46 OR S47 OR S48 OR S49 OR S50 OR S51 OR S52 OR S53 OR S54 OR S55 OR S56 OR S57 OR S58 OR S59 OR S60 OR S61 OR S62 OR S63 OR S64 OR S65 OR S66 OR S67 OR S68 OR S69 OR S70 OR S71 OR S72 OR S73 OR S74 OR S75 OR S76 OR S77 OR S78 OR S79 OR S80 OR S81
S81 (heart manual) OR (home based)
S80 ((nutrition or diet or health) N3 education)
S79 (MH "Health Education+")
S78 (psychosocial* or psycho‐social)
S77 (distress*)
S76 (self N3 (manage* or care or motivat*))
S75 (autogenic*)
S74 (psychopathol*)
S73 (MH "Psychopathology")
S72 (motivat* N3 interv*)
S71 (psycho‐educat*) or (psychoeducat*)
S70 (goal N3 setting)
S69 (hypnotherap*)
S68 (CBT)
S67 (manage*) N3 (anxiety or depres*)
S66 (MH "Anxiety")
S65 (meditat*)
S64 (MH "Meditation")
S63 (cognitive* N3 therap*)
S62 (stress N3 manage*)
S61 (MH "Stress, Psychological+")
S60 (behavio?r*) N4 (modif* or therap* or rehab* or change)
S59 (MH "Behavior Therapy+")
S58 (MH "Cognitive Therapy")
S57 (counsel?ing)
S56 (MH "Counseling+")
S55 (relax*)
S54 (psycholog* N3 intervent*)
S53 (psychotherap*)
S52 (MH "Psychotherapy+")
S51 (MH "Ambulatory Care")
S50 (MH "Self Care+")
S49 ((lifestyle or life‐style) N3 (intervent* or program* or treatment*))
S48 (patient* N3 educat*)
S47 (MH "Patient Education+")
S46 (MH "Rehabilitation+")
S45 ((exercise* or fitness) N3 (treatment or intervent* or program*))
S44 (train*) N3 (strength* or aerobic or exercise*)
S43 (MH "Exercise")
S42 (physical* N3 (fit* or train* or therap* or activit*))
S41 (rehabilitat*)
S40 (MH "Exertion+")
S39 (MH "Sports")
S38 (MH "Therapeutic Exercise+")
S37 (MH "Rehabilitation Centers+")
S36 S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35
S35 acute coronary syndrom*
S34 atherectom*
S33 (MH "Atherectomy+")
S32 (pci or ptca)
S31 stent*
S30 (MH "Stents+")
S29 endoluminal repair*
S28 ((coronary or arterial) n4 dilat*)
S27 angioplast*
S26 (MH "Angioplasty+")
S25 (percutaneous coronary n2 (interven* or revascular*))
S24 (MH "Angioplasty, Transluminal, Percutaneous Coronary")
S23 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22
S22 (MH "Cardiopulmonary Bypass")
S21 (stent* N3 (heart or cardiac*))
S20 (PTCA)
S19 (CABG)
S18 (cardiac*)
S17 (myocard*)
S16 (heart N3 disease*)
S15 (MH "Heart Diseases+")
S14 (heart N3 (failure or attack))
S13 (MH "Heart Failure+")
S12 (angina)
S11 (MH "Angina Pectoris+")
S10 (heart N3 infarct*)
S9 (myocard* N3 infarct*)
S8 (MH "Myocardial Infarction+")
S7 (MH "Myocardial Revascularization+")
S6 (MH "Coronary Disease+")
S5 (coronary)
S4 (MH "Coronary Artery Bypass+")
S3 (isch?mi* N3 heart)
S2 (myocard* N3 isch?mi*)
S1 (MH "Myocardial Ischemia+")
August 2016 Strategies
CENTRAL
#1 MeSH descriptor: [Myocardial Ischemia] explode all trees
#2 (myocard* near isch*mi*)
#3 isch*mi* near heart
#4 MeSH descriptor: [Coronary Artery Bypass] explode all trees
#5 coronary
#6 MeSH descriptor: [Coronary Disease] explode all trees
#7 MeSH descriptor: [Myocardial Revascularization] explode all trees
#8 MeSH descriptor: [Myocardial Infarction] explode all trees
#9 myocard* near infarct*
#10 heart near infarct*
#11 MeSH descriptor: [Angina Pectoris] explode all trees
#12 angina
#13 MeSH descriptor: [Heart Failure] explode all trees
#14 heart and (failure or attack)
#15 MeSH descriptor: [Heart Diseases] explode all trees
#16 heart near disease*
#17 myocard*
#18 cardiac*
#19 CABG
#20 PTCA
#21 stent* near (heart or cardiac*)
#22 MeSH descriptor: [Heart Bypass, Left] explode all trees
#23 MeSH descriptor: [Heart Bypass, Right] explode all trees
#24 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23
#25 MeSH descriptor: [Rehabilitation Centers] explode all trees
#26 MeSH descriptor: [Exercise Therapy] explode all trees
#27 MeSH descriptor: [Sports] this term only
#28 MeSH descriptor: [Physical Exertion] explode all trees
#29 rehabilitat*
#30 (physical* near (fit* or train* or therap* or activit*))
#31 MeSH descriptor: [Exercise] explode all trees
#32 (train*) near (strength* or aerobic or exercise*)
#33 ((exercise* or fitness) near/3 (treatment or intervent* or program*))
#34 MeSH descriptor: [Rehabilitation] explode all trees
#35 MeSH descriptor: [Patient Education as Topic] explode all trees
#36 (patient* near/3 educat*)
#37 ((lifestyle or life‐style) near/3 (intervent* or program* or treatment*))
#38 MeSH descriptor: [Self Care] explode all trees
#39 MeSH descriptor: [Ambulatory Care] explode all trees
#40 MeSH descriptor: [Psychotherapy] explode all trees
#41 psychotherap*
#42 psycholog* near intervent*
#43 relax*
#44 MeSH descriptor: [Relaxation Therapy] explode all trees
#45 MeSH descriptor: [Counseling] explode all trees
#46 counsel*ing
#47 MeSH descriptor: [Cognitive Therapy] explode all trees
#48 MeSH descriptor: [Behavior Therapy] explode all trees
#49 (behavio*r*) near/4 (modif* or therap* or rehab* or change)
#50 MeSH descriptor: [Stress, Psychological] explode all trees
#51 stress near manage*
#52 cognitive* near therap*
#53 MeSH descriptor: [Meditation] explode all trees
#54 meditat*
#55 MeSH descriptor: [Anxiety] this term only
#56 (manage*) near (anxiety or depres*)
#57 CBT
#58 hypnotherap*
#59 goal near/3 setting
#60 (psycho‐educat*) or (psychoeducat*)
#61 motivat* near interv*
#62 MeSH descriptor: [Psychopathology] explode all trees
#63 psychopathol*
#64 MeSH descriptor: [Autogenic Training] explode all trees
#65 autogenic*
#66 self near (manage* or care or motivat*)
#67 distress*
#68 psychosocial* or psycho‐social
#69 MeSH descriptor: [Health Education] explode all trees
#70 ((nutrition or diet or health) near education)
#71 heart manual
#72 home‐based
#73 #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 or #48 or #49 or #50 or #51 or #52 or #53 or #54 or #55 or #56 or #57 or #58 or #59 or #60 or #61 or #62 or #63 or #64 or #65 or #66 or #67 or #68 or #69 or #70 or #71 or #72
#74 #24 and #73 from 2014 to 2016
MEDLINE
1. exp Myocardial Ischemia/
2. (myocard* adj3 isch?mi*).tw.
3. (isch?mi* adj3 heart).tw.
4. exp Coronary Artery Bypass/
5. coronary.tw.
6. exp Coronary Disease/
7. exp Myocardial Revascularization/
8. exp Myocardial Infarction/
9. (myocard* adj3 infarct*).tw.
10. (heart adj3 infarct*).tw.
11. exp Angina Pectoris/
12. angina.tw.
13. exp Heart Failure/
14. (heart adj3 (failure or attack)).tw.
15. exp Heart Diseases/
16. (heart adj3 disease*).tw.
17. myocard*.tw.
18. cardiac*.tw.
19. CABG.tw.
20. PTCA.tw.
21. (stent* adj3 (heart or cardiac*)).tw.
22. Heart Bypass, Left/
23. exp Heart Bypass, Right/
24. or/1‐23
25. Rehabilitation Centers/
26. exp Exercise Therapy/
27. Sports/
28. Physical Exertion/
29. rehabilitat*.tw.
30. (physical* adj3 (fit* or train* or therap* or activit*)).tw.
31. exp Exercise/
32. (train* adj3 (strength* or aerobic or exercise*)).tw.
33. ((exercise* or fitness) adj3 (treatment or intervent* or program*)).tw.
34. exp Rehabilitation/
35. Patient Education as Topic/
36. (patient* adj3 educat*).tw.
37. ((lifestyle or life‐style) adj3 (intervent* or program* or treatment*)).tw.
38. exp Self Care/
39. exp Ambulatory Care/
40. exp Psychotherapy/
41. psychotherap*.tw.
42. (psycholog* adj3 intervent*).tw.
43. relax*.tw.
44. Relaxation Therapy/
45. exp Counseling/
46. counsel?ing.tw.
47. exp Cognitive Therapy/
48. exp Behavior Therapy/
49. (behavio?r* adj4 (modif* or therap* or rehab* or change)).tw.
50. exp Stress, Psychological/
51. (stress adj3 manage*).tw.
52. (cognitive* adj3 therap*).tw.
53. exp Meditation/
54. meditat*.tw.
55. Anxiety/
56. (manage* adj3 (anxiety or depres*)).tw.
57. CBT.tw.
58. hypnotherap*.tw.
59. (goal adj3 setting).tw.
60. (psycho‐educat* or psychoeducat*).tw.
61. (motivat* adj3 interv*).tw.
62. exp Psychopathology/
63. psychopathol*.tw.
64. exp Autogenic Training/
65. autogenic*.tw.
66. (self adj3 (manage* or care or motivat*)).tw.
67. distress*.tw.
68. (psychosocial* or psycho‐social*).tw.
69. exp Health Education/
70. ((nutrition or diet or health) adj3 education).tw.
71. heart manual.tw.
72. home based.tw.
73. or/25‐72
74. randomized controlled trial.pt.
75. controlled clinical trial.pt.
76. randomized.ab.
77. placebo.ab.
78. drug therapy.fs.
79. randomly.ab.
80. trial.ab.
81. groups.ab.
82. 74 or 75 or 76 or 77 or 78 or 79 or 80 or 81
83. exp animals/ not humans.sh.
84. 82 not 83
85. 24 and 73 and 84
86. (2014* or 2015* or 2016*).ed.
87. 85 and 86
Embase
1. exp Myocardial Ischemia/
2. (myocard* adj3 isch?mi*).tw.
3. (isch?mi* adj3 heart).tw.
4. exp Coronary Artery Bypass/
5. coronary.tw.
6. exp Coronary Disease/
7. exp Myocardial Revascularization/
8. exp Myocardial Infarction/
9. (myocard* adj3 infarct*).tw.
10. (heart adj3 infarct*).tw.
11. exp Angina Pectoris/
12. angina.tw.
13. exp Heart Failure/
14. (heart adj3 (failure or attack)).tw.
15. exp Heart Diseases/
16. (heart adj3 disease*).tw.
17. myocard*.tw.
18. cardiac*.tw.
19. CABG.tw.
20. PTCA.tw.
21. (stent* adj3 (heart or cardiac*)).tw.
22. Heart Bypass, Left/
23. exp Heart Bypass, Right/
24. or/1‐23
25. Rehabilitation Centers/
26. exp Exercise Therapy/
27. Sports/
28. Physical Exertion/
29. rehabilitat*.tw.
30. (physical* adj3 (fit* or train* or therap* or activit*)).tw.
31. exp Exercise/
32. (train* adj3 (strength* or aerobic or exercise*)).tw.
33. ((exercise* or fitness) adj3 (treatment or intervent* or program*)).tw.
34. exp Rehabilitation/
35. Patient Education as Topic/
36. (patient* adj3 educat*).tw.
37. ((lifestyle or life‐style) adj3 (intervent* or program* or treatment*)).tw.
38. exp Self Care/
39. exp Ambulatory Care/
40. exp Psychotherapy/
41. psychotherap*.tw.
42. (psycholog* adj3 intervent*).tw.
43. relax*.tw.
44. Relaxation Therapy/
45. exp Counseling/
46. counsel?ing.tw.
47. exp Cognitive Therapy/
48. exp Behavior Therapy/
49. (behavio?r* adj4 (modif* or therap* or rehab* or change)).tw.
50. exp Stress, Psychological/
51. (stress adj3 manage*).tw.
52. (cognitive* adj3 therap*).tw.
53. exp Meditation/
54. meditat*.tw.
55. Anxiety/
56. (manage* adj3 (anxiety or depres*)).tw.
57. CBT.tw.
58. hypnotherap*.tw.
59. (goal adj3 setting).tw.
60. (psycho‐educat* or psychoeducat*).tw.
61. (motivat* adj3 interv*).tw.
62. exp Psychopathology/
63. psychopathol*.tw.
64. exp Autogenic Training/
65. autogenic*.tw.
66. (self adj3 (manage* or care or motivat*)).tw.
67. distress*.tw.
68. (psychosocial* or psycho‐social*).tw.
69. exp Health Education/
70. ((nutrition or diet or health) adj3 education).tw.
71. heart manual.tw.
72. home based.tw.
73. or/25‐72
74. random$.tw.
75. factorial$.tw.
76. crossover$.tw.
77. cross over$.tw.
78. cross‐over$.tw.
79. placebo$.tw.
80. (doubl$ adj blind$).tw.
81. (singl$ adj blind$).tw.
82. assign$.tw.
83. allocat$.tw.
84. volunteer$.tw.
85. crossover procedure/
86. double blind procedure/
87. randomized controlled trial/
88. single blind procedure/
89. 74 or 75 or 76 or 77 or 78 or 79 or 80 or 81 or 82 or 83 or 84 or 85 or 86 or 87 or 88
90. (animal/ or nonhuman/) not human/
91. 89 not 90
92. 24 and 73 and 91
93. (2014* or 2015* or 2016*).em.
94. 92 and 93
95. limit 94 to embase
PsycINFO
1. (myocard* adj3 isch?mi*).tw.
2. (isch?mi* adj3 heart).tw.
3. coronary.tw.
4. exp Myocardial Infarction/
5. (myocard* adj3 infarct*).tw.
6. (heart adj3 infarct*).tw.
7. exp Angina Pectoris/
8. angina.tw.
9. (heart adj3 (failure or attack)).tw.
10. (heart adj3 disease*).tw.
11. myocard*.tw.
12. cardiac*.tw.
13. CABG.tw.
14. PTCA.tw.
15. (stent* adj3 (heart or cardiac*)).tw.
16. Rehabilitation Centers/
17. exp Exercise Therapy/
18. Sports/
19. rehabilitat*.tw.
20. (physical* adj3 (fit* or train* or therap* or activit*)).tw.
21. exp Exercise/
22. (train* adj3 (strength* or aerobic or exercise*)).tw.
23. ((exercise* or fitness) adj3 (treatment or intervent* or program*)).tw.
24. exp Rehabilitation/
25. (patient* adj3 educat*).tw.
26. ((lifestyle or life‐style) adj3 (intervent* or program* or treatment*)).tw.
27. exp Self Care/
28. exp Ambulatory Care/
29. exp Psychotherapy/
30. psychotherap*.tw.
31. (psycholog* adj3 intervent*).tw.
32. relax*.tw.
33. Relaxation Therapy/
34. exp Counseling/
35. counsel?ing.tw.
36. exp Cognitive Therapy/
37. exp Behavior Therapy/
38. (behavio?r* adj4 (modif* or therap* or rehab* or change)).tw.
39. (stress adj3 manage*).tw.
40. (cognitive* adj3 therap*).tw.
41. exp Meditation/
42. meditat*.tw.
43. Anxiety/
44. (manage* adj3 (anxiety or depres*)).tw.
45. CBT.tw.
46. hypnotherap*.tw.
47. (goal adj3 setting).tw.
48. (psycho‐educat* or psychoeducat*).tw.
49. (motivat* adj3 interv*).tw.
50. exp Psychopathology/
51. psychopathol*.tw.
52. exp Autogenic Training/
53. autogenic*.tw.
54. (self adj3 (manage* or care or motivat*)).tw.
55. distress*.tw.
56. (psychosocial* or psycho‐social*).tw.
57. exp Health Education/
58. ((nutrition or diet or health) adj3 education).tw.
59. heart manual.tw.
60. home based.tw.
61. or/1‐15
62. or/16‐60
63. 61 and 62
64. random$.tw.
65. factorial$.tw.
66. crossover$.tw.
67. cross‐over$.tw.
68. placebo$.tw.
69. (doubl$ adj blind$).tw.
70. (singl$ adj blind$).tw.
71. assign$.tw.
72. allocat$.tw.
73. volunteer$.tw.
74. control*.tw.
75. "2000".md.
76. or/64‐75
77. 63 and 76
78. (2014* or 2015* or 2016*).up.
79. 77 and 78
CINAHL
S76 S74 and S75
S75 EM 20141013‐20160803
S74 S70 and S73
S73 S71 or S72
S72 (MH "Clinical Trials+")
S71 random* or blind* or allocat* or assign* or trial* or placebo* or crossover* or cross‐over*
S70 S23 and S69
S69 S24 or S25 or S26 or S27 or S28 or S29 or S30 or S31 or S32 or S33 or S34 or S35 or S36 or S37 or S38 or S39 or S40 or S41 or S42 or S43 or S44 or S45 or S46 or S47 or S48 or S49 or S50 or S51 or S52 or S53 or S54 or S55 or S56 or S57 or S58 or S59 or S60 or S61 or S62 or S63 or S64 or S65 or S66 or S67 or S68
S68 (heart manual) OR (home based)
S67 ((nutrition or diet or health) N3 education)
S66 (MH "Health Education+")
S65 (psychosocial* or psycho‐social)
S64 (distress*)
S63 (self N3 (manage* or care or motivat*))
S62 (autogenic*)
S61 (psychopathol*)
S60 (MH "Psychopathology")
S59 (motivat* N3 interv*)
S58 (psycho‐educat*) or (psychoeducat*)
S57 (goal N3 setting)
S56 (hypnotherap*)
S55 (CBT)
S54 (manage*) N3 (anxiety or depres*)
S53 (MH "Anxiety")
S52 (meditat*)
S51 (MH "Meditation")
S50 (cognitive* N3 therap*)
S49 (stress N3 manage*)
S48 (MH "Stress, Psychological+")
S47 (behavio?r*) N4 (modif* or therap* or rehab* or change)
S46 (MH "Behavior Therapy+")
S45 (MH "Cognitive Therapy")
S44 (counsel?ing)
S43 (MH "Counseling+")
S42 (relax*)
S41 (psycholog* N3 intervent*)
S40 (psychotherap*)
S39 (MH "Psychotherapy+")
S38 (MH "Ambulatory Care")
S37 (MH "Self Care+")
S36 ((lifestyle or life‐style) N3 (intervent* or program* or treatment*))
S35 (patient* N3 educat*)
S34 (MH "Patient Education+")
S33 (MH "Rehabilitation+")
S32 ((exercise* or fitness) N3 (treatment or intervent* or program*))
S31 (train*) N3 (strength* or aerobic or exercise*)
S30 (MH "Exercise")
S29 (physical* N3 (fit* or train* or therap* or activit*))
S28 (rehabilitat*)
S27 (MH "Exertion+")
S26 (MH "Sports")
S25 (MH "Therapeutic Exercise+")
S24 (MH "Rehabilitation Centers+")
S23 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22
S22 (MH "Cardiopulmonary Bypass")
S21 (stent* N3 (heart or cardiac*))
S20 (PTCA)
S19 (CABG)
S18 (cardiac*)
S17 (myocard*)
S16 (heart N3 disease*)
S15 (MH "Heart Diseases+")
S14 (heart N3 (failure or attack))
S13 (MH "Heart Failure+")
S12 (angina)
S11 (MH "Angina Pectoris+")
S10 (heart N3 infarct*)
S9 (myocard* N3 infarct*)
S8 (MH "Myocardial Infarction+")
S7 (MH "Myocardial Revascularization+")
S6 (MH "Coronary Disease+")
S5 (coronary)
S4 (MH "Coronary Artery Bypass+")
S3 (isch?mi* N3 heart)
S2 (myocard* N3 isch?mi*)
S1 (MH "Myocardial Ischemia+")
UK Clinical Trials Gateway (www.ukctg.nihr.ac.uk/)
"cardiac rehabilitation" AND "home"
WHO ICTRP
"cardiac rehabilitation" AND "home"
Clinicaltrials.gov
"cardiac rehabilitation" AND "home"
Data and analyses
Comparison 1. home‐base vs centre‐based.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Total mortality | 13 | 1505 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.65, 2.16] |
| 2 Exercise capacity ≤ 12 months | 26 | 2255 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.28, 0.02] |
| 3 Exercise capacity 12 to 24 months | 3 | 1074 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.01, 0.23] |
| 4 Completers | 26 | 2615 | Risk Ratio (M‐H, Random, 95% CI) | 1.04 [1.00, 1.08] |
| 5 Total cholesterol 3 to 12 months | 10 | 1151 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.10, 0.23] |
| 6 HDL cholesterol 3 to 12 months | 8 | 925 | Mean Difference (IV, Fixed, 95% CI) | ‐0.07 [‐0.11, ‐0.03] |
| 7 LDL cholesterol 3 to 12 months | 6 | 430 | Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.14, 0.22] |
| 8 Triglycerides 3 to 12 months | 6 | 396 | Mean Difference (IV, Fixed, 95% CI) | 0.15 [0.00, 0.29] |
| 9 Systolic blood pressure 3 to 12 months | 12 | 1292 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐3.13, 2.60] |
| 10 Diastolic blood pressure 3 to 12 months | 11 | 1146 | Mean Difference (IV, Random, 95% CI) | 0.74 [‐1.04, 2.53] |
| 11 Smoking 3 to 12 months | 6 | 986 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.83, 1.27] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aamot 2014 Group.
| Methods |
Study design: Multicentre RCT with 3 parallel groups Number of centres: 2 Country: Norway Dates patients recruited: October 2009 to April 2011 When randomised: After the baseline tests Maximum follow up: 12 weeks |
|
| Participants |
Inclusion criteria: Aged over 18 years, diagnosed MI, CABG surgery, or acute coronary syndrome (ACS), and able to perform a maximal treadmill test. Exclusion criteria: Heart failure, severe arrhythmias, drug abuse, or a medical condition contraindicative to high‐intensity training. N randomised: total: 90; home‐based cardiac rehabilitation: 28; centre‐based cardiac rehabilitation (treadmill exercise): 34; centre‐based cardiac rehabilitation (group exercise): 28 Method of assessment: NR Diagnosis (% of pts): Previous AMI: home‐based cardiac rehabilitation: 71.4%; treadmill exercise: 67.6% group exercise: 64.3% Previous CABG: home‐based cardiac rehabilitation: 21.4%; treadmill exercise: 26.5%; group exercise: 25.0% ACS: home‐based cardiac rehabilitation: 7.2%; treadmill exercise: 5.9% group exercise: 10.7% Age (mean ± SD): total: NR; home‐based cardiac rehabilitation: 58 ± 8 years; treadmill exercise: 56 ± 9 years; group exercise: 58 ± 8 years Percentage male: total: 88.9%; home‐based cardiac rehabilitation: 96.4%; treadmill exercise: 82.4%; group exercise: 89.3% Ethnicity: NR |
|
| Interventions | All participants in all groups performed HIT twice a week for 12 weeks. Every session started with a 10 minute warm up at low‐to‐moderate intensity (50% to 70% of peak heart rate, HR) and continued with four intervals lasting 4 minutes each, at an exercise intensity of 85% to 95% of peak HR. Each interval was separated by 4 minutes of active breaks at an intensity of 70% of peak HR. After the last interval, a cool down period of 3 to 5 minutes was performed at 50% of peak HR. All participants were individually instructed in use of the HR monitor, and how to reach target HR. As aerobic capacity increased, the participants increased work load to maintain relative exercise intensity. Completion of 70% of the exercise sessions was considered to be training per protocol. Description of intervention (home‐based cardiac rehabilitation): The home‐based exercise started with two initial sessions with personal instruction of a physiotherapist where they learned how to perform HIT and to use the HR monitors. These sessions were performed as up‐hill walking or jogging. After the introduction, HIT was performed in preferred exercise mode in their home environment; up‐hill walking, cross country skiing, bicycling, running, or using indoor equipment such as treadmills or cross trainers. All participants varied their exercise mode, but they kept to the exercise design and relative exercise intensity. A Holter electrocardiogram was recorded during the first exercise session to ensure that no arrhythmia occurred during or immediately after exercise. Time of start after event: NR Components: Exercise only Aerobic exercise: Modality: HIT was performed in preferred exercise mode e.g. up‐hill walking, cross country skiing, bicycling, running, or using indoor equipment such as treadmills or cross trainers Dose: Length of session: 45 mins Frequency/no of sessions: twice a week Intensity: 50% to 95% of peak HR Resistance training included? No Total duration: 12 weeks Intermittent nurse or exercise specialist telephone support? NR Co‐interventions: None described Description of comparator (centre‐based cardiac rehabilitation): Treadmill exercise: The treadmills were used at the hospitals, in smaller groups consisting of 3–7 patients. Work load was adjusted individually, either by fast walking with inclination or running with less inclination. A physiotherapist was present to provide monitors and to assist if necessary. Time of start after event: NR Components: Exercise only Aerobic exercise: Modality: Treadmills Dose: Length of session: 45 mins Frequency/no of sessions: twice a week Intensity: 50% to 95% of peak HR Resistance training included? No Total duration: 12 weeks Intermittent nurse or exercise specialist telephone support? NR Co‐interventions: None described Group exercise: The group exercise sessions were held at the hospitals in groups of 10 to 15 people, instructed by a physiotherapist. After a warm up consisting of aerobics, the HIT was organised as circuit training and the intervals performed with a variety of exercises, from running to cycling, squats, and steps. Active breaks could consist of strength exercises (push ups, sit ups) or walking. Time of start after event: NR Components: Exercise only Aerobic exercise: Modality: Circuit training Dose: Length of session: 45 mins Frequency/no of sessions: twice a week Intensity: 50% to 95% of peak HR Resistance training included? No Total duration: 12 weeks Intermittent nurse or exercise specialist telephone support? NR Co‐interventions: None described |
|
| Outcomes | Peak VO₂, HRQoL | |
| Follow up | 12 weeks | |
| Source of funding | This work was supported by the Liaison Committee between the Central Norway Regional Health Authority and the Norwegian University of Science and Technology (NTNU) | |
| Conflicts of interest | The authors declare that there is no conflict of interest | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomization was performed after the baseline tests, by a web‐based randomization system.” |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | “The test personnel were not blinded for allocation.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Home‐based cardiac rehabilitation: 2/28 (7.1 %) lost to follow‐up Treadmill: 2/34 (5.9 %) lost to follow‐up Group exercise: 3/28 (10.7 %) lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods are reported in results section |
| Groups balanced at baseline? | Low risk | “Group differences were not significant” |
| Groups received same co‐intervention(s)? | Low risk | No co‐interventions were received by any group |
Aamot 2014 Treadmill.
| Methods |
Study design: Multicentre RCT with 3 parallel groups No of centres: 2 Country: Norway Dates patients recruited: October 2009 to April 2011 When randomised: After the baseline tests Maximum follow up: 12 weeks |
|
| Participants |
Inclusion criteria: Aged over 18 years, diagnosed MI, CABG surgery, or acute coronary syndrome (ACS), and able to perform a maximal treadmill test. Exclusion criteria: Heart failure, severe arrhythmias, drug abuse, or a medical condition contraindicative to high‐intensity training. N randomised: total: 90; home‐based cardiac rehabilitation: 28; centre‐based cardiac rehabilitation (treadmill exercise): 34; centre‐based cardiac rehabilitation (group exercise): 28 Method of assessment: NR Diagnosis (% of pts): Previous AMI: home‐based cardiac rehabilitation: 71.4%; treadmill exercise: 67.6% group exercise: 64.3% Previous CABG: home‐based cardiac rehabilitation: 21.4%; treadmill exercise: 26.5%; group exercise: 25.0% ACS: home‐based cardiac rehabilitation: 7.2%; treadmill exercise: 5.9% group exercise: 10.7% Age (mean ± SD): total: NR; home‐based cardiac rehabilitation: 58 ± 8 years; treadmill exercise: 56 ± 9 years; group exercise: 58 ± 8 years Percentage male: total: 88.9%; home‐based cardiac rehabilitation: 96.4%; treadmill exercise: 82.4%; group exercise: 89.3% Ethnicity: NR |
|
| Interventions | All participants in all groups performed HIT twice a week for 12 weeks. Every session started with a 10‐minute warm up at low‐to‐moderate intensity (50% to 70% of peak heart rate, HR) and continued with four intervals lasting 4 minutes each, at an exercise intensity of 85% to 95% of peak HR. Each interval was separated by 4 minutes of active breaks at an intensity of 70% of peak HR. After the last interval, a cool down period of 3‐5 minutes was performed at 50% of peak HR. All participants were individually instructed in use of the HR monitor, and how to reach target HR. As aerobic capacity increased, the participants increased work load to maintain relative exercise intensity. Completion of 70% of the exercise sessions was considered to be training per protocol. Description of intervention (home‐based cardiac rehabilitation): The home‐based exercise started with two initial sessions with personal instruction of a physiotherapist where they learned how to perform HIT and to use the HR monitors. These sessions were performed as up‐hill walking or jogging. After the introduction, HIT was performed in preferred exercise mode in their home environment; up‐hill walking, cross country skiing, bicycling, running, or using indoor equipment such as treadmills or cross trainers. All participants varied their exercise mode, but they kept to the exercise design and relative exercise intensity. A Holter electrocardiogram was recorded during the first exercise session to ensure that no arrhythmia occurred during or immediately after exercise. Time of start after event: NR Components: Exercise only Aerobic exercise: Modality: HIT was performed in preferred exercise mode e.g. up‐hill walking, cross country skiing, bicycling, running, or using indoor equipment such as treadmills or cross trainers Dose: Length of session: 45 mins Frequency/no of sessions: twice a week Intensity: 50% to 95% of peak HR Resistance training included? No Total duration: 12 weeks Intermittent nurse or exercise specialist telephone support? NR Co‐interventions: None described Description of comparator (centre‐based cardiac rehabilitation): Treadmill exercise: The treadmills were used at the hospitals, in smaller groups consisting of 3 to 7 patients. Work load was adjusted individually, either by fast walking with inclination or running with less inclination. A physiotherapist was present to provide monitors and to assist if necessary. Time of start after event: NR Components: Exercise only Aerobic exercise: Modality: Treadmills Dose: Length of session: 45 mins Frequency/no of sessions: twice a week Intensity: 50% to 95% of peak HR Resistance training included? No Total duration: 12 weeks Intermittent nurse or exercise specialist telephone support? NR Co‐interventions: None described Group exercise: The group exercise sessions were held at the hospitals in groups of 10 to 15 people, instructed by a physiotherapist. After a warm up consisting of aerobics, the HIT was organised as circuit training and the intervals performed with a variety of exercises, from running to cycling, squats, and steps. Active breaks could consist of strength exercises (push ups, sit ups) or walking. Time of start after event: NR Components: Exercise only Aerobic exercise: Modality: Circuit training Dose: Length of session: 45 mins Frequency/no of sessions: twice a week Intensity: 50% to 95% of peak HR Resistance training included? No Total duration: 12 weeks Intermittent nurse or exercise specialist telephone support? NR Co‐interventions: None described |
|
| Outcomes | Peak VO₂, HRQoL | |
| Follow up | 12 weeks | |
| Source of funding | This work was supported by the Liaison Committee between the Central Norway Regional Health Authority and the Norwegian University of Science and Technology (NTNU) | |
| Conflicts of interest | The authors declare that there is no conflict of interest | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomization was performed after the baseline tests, by a web‐based randomization system.” |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | “The test personnel were not blinded for allocation.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Home‐based cardiac rehabilitation: 2/28 (7.1 %) lost to follow‐up Treadmill: 2/34 (5.9 %) lost to follow‐up Group exercise: 3/28 (10.7 %) lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods are reported in results section |
| Groups balanced at baseline? | Low risk | “Group differences were not significant” |
| Groups received same co‐intervention(s)? | Low risk | No co‐interventions were received by any group |
Arthur 2002.
| Methods |
Study design: Single centre RCT No of centres: 1 Country: Canada Dates patients recruited: July 1997 to October 1998 When randomised: 35 to 49 day post‐CABG surgery, after baseline assessment Maximum follow up: 6 years |
|
| Participants |
Inclusion criteria: 35 to 49 days post‐CABG, able to achieve 40 to 80% of age/sex‐predicted METs on cycle ergometry, read/write English Exclusion criteria: Recurrent angina, positive graded exercise test, unable to attend rehabilitation 3 times weekly, physical limitations, previously participant of out‐patient cardiac rehabilitation N randomised: total: 242; home‐based cardiac rehabilitation: 120; centre‐based cardiac rehabilitation: 122 Method of assessment: NR Diagnosis (% of pts): Previous CABG: 100% Age (mean ± SD): total: 63.3 ± 13 years Percentage male: total: 81% Ethnicity: NR |
|
| Interventions |
Description of home‐based cardiac rehabilitation: Patients also attended 1 hour exercise consultation with exercise specialist at baseline and after 3 months training, completed exercises log reviewed every 2 months, and telephone support call every 2 weeks Time of start after event: 35 to 49 day post‐CABG surgery Components: Exercise, education. psychosocial Aerobic exercise: Modality: walking Dose: Length of session: 40 min/session Frequency/no of sessions: 5 sessions weekly Intensity: 60% to 70% VO₂max Total duration: 6 months Intermittent nurse or exercise specialist telephone support? Home patients were telephoned every 2 weeks by the exercise specialist to monitor progress, assess and document adherence, revise the exercise prescription if necessary, and provide support and education. Exercise logs were reviewed monthly. Co‐interventions: Dietary advice and psychological support Description of centre‐based cardiac rehabilitation: Supervised by exercise specialist and completed exercises log reviewed every month Time of start after event: 35 to 49 day post‐CABG surgery Components:Exercise. education. psychosocial Aerobic exercise: Modality: cycle ergometer, treadmill, track walking, and stair climbing Dose: Length of session: 40 min/session Frequency/no of sessions: 3 sessions weekly Intensity: 60% to 70% VO₂max Total duration: 6 months Co‐interventions: Dietary advice and psychological support |
|
| Outcomes | Primary: exercise capacity (METs) Secondary: HRQoL (SF‐36); cardiac morbidity, mortality |
|
| Follow up | 6 and 18 months and 6 years post randomisation | |
| Source of funding | Heart and Stroke Foundation of Ontario (grant no. T 4004) | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Low risk | "...the data analyst, who had no role in this project, prepared the randomization schedule using a blocked format"; "...the resulting group assignments were than sealed in opaque envelopes that were opened in sequence after consent" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "...the physicians who evaluated the primary variables were blind to the patients assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | CONSORT flow diagram shows loss to follow up 20/242 (8%) at 6 months follow up and 24/242 (10%) at 18 months follow up. No imputation of missing data undertaken |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods section are reported in the results |
| Groups balanced at baseline? | High risk | “There were statistically significant differences at baseline between the two groups in weight, resting heart rate, and social support.” |
| Groups received same co‐intervention(s)? | Low risk | “Similar numbers of patients in the [hospital and home] groups chose to consult with either clinic dietician or psychologist.” |
Bell 1998.
| Methods |
Study design: Multicentre RCT No of centres: 5 district hospitals Country: UK Dates patients recruited: NR When randomised: NR Maximum follow up: 52 weeks |
|
| Participants |
Inclusion criteria: Acute MI (2 of: elevated serum creatinine kinase or oxaloacetic transaminase, prolonged chest pain consistent with AMI, new Q waves or evolutionary ST changes in ECG) Exclusion criteria: Physical infirmity, unable to speak or read English, dementia or psychosis, aged > 75 years, living > 20 miles from CCU, serious persisting medical complications, any other excluding conditions (consultants opinion), for some hospitals ‐ participation in the previous rehabilitation programme N randomised: total: 252; home‐based cardiac rehabilitation: 152; centre‐based cardiac rehabilitation: 100 Method of assessment: NR Diagnosis (% of pts): AMI: 100% Age (mean ± SD): total: 59 ± 8.9 years Percentage male: total: 77% Ethnicity: NR |
|
| Interventions |
Description of home‐based cardiac rehabilitation: Heart Manual Time of start after event: NR Components: Exercise, education and psychological Aerobic exercise: Modality: Walking Dose: Length of session: NR Frequency/no of sessions: NR Intensity: NR Total duration: 6 weeks Intermittent nurse or exercise specialist telephone support? 4 phone calls by facilitator, health education, stress management Co‐interventions: NR Description of centre‐based cardiac rehabilitation: Time of start after event: NR Components: Exercise, education and psychological Aerobic exercise: Modality: Walking Dose: Length of session: ≥ 20 min Frequency/no of sessions: 1 session/week or 4 weeks of 2 sessions/week Intensity: 3 to 4 on Borg RPE scale Total duration: 12 weeks Co‐interventions: Education sessions ‐ CHD causes, medication, risk factor modification, stress management, and exercise |
|
| Outcomes | Primary: exercise capacity (METs) Secondary: total cholesterol; systolic blood pressure; HRQoL (Nottingham Health Profile); smoking; mortality; readmission rate; use of primary care services |
|
| Follow up | 16 and 48 weeks post randomisation (20 and 52 weeks post MI) | |
| Source of funding | NR | |
| Conflicts of interest | NR | |
| Notes | Published as PhD thesis only | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Low risk | “Series of sealed envelopes containing cards evenly distributed between conditions …envelopes were taken sequentially …opened envelopes were retained and returned to trial coordinator” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All measurements were performed 'blind' by members of the medical staff and technicians" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Follow up data on all randomised patients is not reported, no CONSORT flow diagram is reported and it is difficult to determine from the report those who were lost to follow up or who dropped out |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods section are reported in the results |
| Groups balanced at baseline? | Low risk | There were no statistically significant differences in population demographics between the two groups |
| Groups received same co‐intervention(s)? | High risk | Although the intervention for both groups consisted of exercise, education, and stress management, the nature and amount of intervention was quite different |
Carlson 2000.
| Methods |
Study design: Single centre RCT No of centres: 1 Country: USA, single hospital centre Dates patients recruited: NR When randomised: within 2 weeks of entering cardiac rehabilitation Maximum follow up: 6 months |
|
| Participants |
Inclusion criteria: Men and women aged 35 to 75 years referred for the first time to outpatient cardiac rehabilitation, living ≤ 30 miles from the rehabilitation facility, of low‐to‐moderate cardiac risk Exclusion criteria: NR N Randomised: total: 80; home‐based cardiac rehabilitation: 38; centre‐based cardiac rehabilitation: 42 Method of assessment: NR Diagnosis (% of pts): MI: home‐based cardiac rehabilitation: 47%; centre‐based cardiac rehabilitation: 26% Angioplasty: home‐based cardiac rehabilitation: 55%; centre‐based cardiac rehabilitation: 40% CABG: home‐based cardiac rehabilitation: 32%; centre‐based cardiac rehabilitation: 40% Age (mean ± SD): total: NR; home‐based cardiac rehabilitation: 59 ± 10 years; centre‐based: 59 ± 9 years Percentage male: total: NR; home‐based cardiac rehabilitation: 82%; centre‐based cardiac rehabilitation: 83% Ethnicity: NR |
|
| Interventions |
Description of home‐based cardiac rehabilitation: First 4 weeks ‐ 3 hospital based exercise sessions/week with ECG monitoring, progressively reducing frequency of centre‐based sessions Time of start after event: NR Components: Exercise, education, psychosocial Aerobic exercise: Modality: NR Dose: Length of session: 30 to 40 min/session Frequency/no of sessions: 2 to 5 sessions/week Intensity: 60 to 85% aerobic capacity Total duration: 25 weeks Co‐interventions: Weekly educational and counselling meetings that included sessions on exercise, diet, risk factors, drugs, and overcoming barriers to behaviour change. Based on Bandura’s self‐efficacy theory Description of centre‐based cardiac rehabilitation: Centre‐based cardiac rehabilitation(control): Exercise: modality: aerobic exercise Time of start after event: NR Components: e.g. exercise only, exercise and education, exercise and psychosocial Aerobic exercise: Modality: NR Dose: Length of session: 30 to 45 min/session Frequency/no of sessions: 2 to 3 sessions/week Intensity: 60 to 85% aerobic capacity Resistance training included? Total duration: 25 weeks Co‐interventions: Three sessions of education and counselling that included sessions on exercise, diet, risk factors, and drugs |
|
| Outcomes | Primary: peak functional capacity (METs), LDL cholesterol Secondary: total cholesterol, HDL cholesterol, triglycerides, blood pressure, cardiovascular medications, costs, adherence (exercise sessions attended) |
|
| Follow up | 6 months post randomisation | |
| Source of funding | NR | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "...it was not possible to blind the clinicians to the protocol patients were assigned". Outcome blinding not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "...significantly more [centre‐based CR] participants dropped out", "Because more [centre‐based CR] participants dropped out and failed to return for their 6‐month [exercise test] evaluation, this evaluation is a representation of more compliant patients” |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods section are reported in the results |
| Groups balanced at baseline? | Low risk | “…only significant difference between groups was a higher resting systolic blood pressure in [centre‐based CR] …selected demographic and psychological measures including socioeconomic status and social support were comparable between the 2 groups at baseline” |
| Groups received same co‐intervention(s)? | High risk | “The primary differences in the [home‐based CR] compared with the [centre‐based CR] included: …(2) an ongoing weekly education/support group, and (3) education and counselling that emphasized overcoming barriers associated with developing independent exercise and nutrition behaviours”. Although both groups received exercise training, education, and counselling, the amount and nature of this intervention was different between groups |
Cowie 2012.
| Methods |
Study design: Single centre RCT No of centres: 1 Country: UK Dates patients recruited: May 2007 and August 2008 When randomised: After baseline tests Maximum follow up: 8 weeks |
|
| Participants |
Inclusion criteria: (1) left ventricular systolic dysfunction on echocardiography, (2) clinically stable for at least one month, and (3) on optimised medication dosages. Exclusion criteria: (1) significant ischaemic symptoms at low workloads, (2) uncontrollable diabetes, (3) acute systematic illness or fever, (4) recent embolism, (5) acute pericarditis, (6) moderate to severe aortic stenosis, (7) regurgitant valvular heart disease requiring surgery, (8) myocardial infarction within the past three weeks, (9) new onset of atrial fibrillation, (10) signs and symptoms of decompensation, (11) other comorbidities (life‐threatening, uncontrolled, infectious, or exacerbated by exercise). N randomised: total: 60; home‐based cardiac rehabilitation: 20; centre‐based cardiac rehabilitation: 20; control: 20 (usual care – no cardiac rehabilitation ‐ not considered in this review) Method of assessment: Echocardiography Diagnosis (% of pts): NYHA class II/III post‐H: F100% Age (range): total: 66 (35‐85) years; home‐based cardiac rehabilitation: 65.5 (35 to 82) years; centre‐based cardiac rehabilitation: 71.2 (59 to 85) years; control: 61.4 (39 to 79) years Percentage male: total: 85%; home‐based cardiac rehabilitation: 90%; centre‐based cardiac rehabilitation: 80%; control: 85% Ethnicity: NR |
|
| Interventions |
Description of home‐based cardiac rehabilitation: Exercise: 1‐hour aerobic‐based exercise session (DVD and booklet), started with a 15‐minute warm‐up, and ended with a 15‐minute cool‐down. Aerobic overload: 2 x 15 minute circuits (10 simple, functional aerobic exercises e.g. knee lifts, side steps); interspersed with low‐paced ‘active recovery’ (toe tapping or slow walking; 90 seconds for each exercise). Gradually increasing the proportion of time spent on aerobic overload in relation to active recovery provided interval training, which was individually tailored and progressed. Time of start after event: NR Components: Exercise and education Aerobic exercise: Modality: Functional aerobic exercises e.g. knee lifts, side steps interspersed with low‐paced ‘active recovery’ (toe tapping or slow walking) Dose: Length of session: 1 hour Frequency/no of sessions: twice a week Intensity: NR Total duration: eight weeks Intermittent nurse or exercise specialist telephone support? Physiotherapist telephoned every two weeks to modify exercise prescriptions where appropriate. Co‐interventions: Educated on symptoms of unstable heart failure. Use of heart rate monitors to guide training intensity. Encouraged to work at 12 to 13 on the Borg RPE. Advised to adhere to usual heart failure nursing care and daily routines. Description of centre‐based cardiac rehabilitation: As above i.e. 1‐hour aerobic‐based exercise session (physiotherapist‐led) started with a 15‐minute warm‐up, and ended with 15‐minute cool‐down. Aerobic overload: 2 x 15 minute circuits (10 simple, functional aerobic exercises e.g. knee lifts, side steps); interspersed with low‐paced ‘active recovery’ (toe tapping or slow walking; 90 seconds for each exercise). Gradually increasing the proportion of time spent on aerobic overload in relation to active recovery provided interval training, which was individually tailored and progressed. Components: Exercise and education Aerobic exercise: Modality: Functional aerobic exercises e.g. knee lifts, side steps interspersed with low‐paced ‘active recovery’ (toe tapping or slow walking) Dose: Length of session: 1 hour Frequency/no of sessions: twice a week Intensity: NR Total duration: eight weeks Co‐interventions: Educated on symptoms of unstable heart failure. Use of heart rate monitors to guide training intensity. Encouraged to work at 12 to 13 on the Borg RPE. Advised to adhere to usual heart failure nursing care and daily routines |
|
| Outcomes | Exercise capacity (shuttle walk test), health‐related quality of life (SF‐36 and Minnesota Living With Heart Failure) | |
| Follow up | 8 weeks | |
| Source of funding | This work was supported by NHS Ayrshire and Arran’s coronary heart disease Managed Clinical Network | |
| Conflicts of interest | Professor Malcolm Granat is a co‐inventor of the activPALTM and a director of PAL Technologies Ltd., Glasgow, UK. Professor Granat had no involvement in data collection, or analysis of results. No other conflicts of interest declared | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Low risk | “...participants were randomised (using concealed envelopes) to one of three groups” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “...measurements obtained by researcher blind to participants" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 5/20 (25%) centre‐based and 5/20 (25%) dropped out |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods section are reported in the results |
| Groups balanced at baseline? | High risk | "...the mean age of the hospital group was 10 years older than the control group (P = 0.001)" |
| Groups received same co‐intervention(s)? | Low risk | “[both groups were] ...advised to adhere to usual heart failure nursing care and daily routines” |
Dalal 2007.
| Methods |
Study design: Single centre RCT No of centres: 1 Country: UK Dates patients recruited: December 2000 to September 2003 When randomised: Following consent Maximum follow up: 9 months |
|
| Participants |
Inclusion criteria: Confirmed acute myocardial infarction (WHO criteria), ability to read English, registered with family doctor in one of two primary care trusts. Exclusion criteria: Severe heart failure, unstable angina, uncontrolled arrhythmia, history of major psychiatric illness, other significant comorbidity precluding the ability to exercise on the treadmill, patients re‐admitted with acute myocardial infarction who had already received an intervention earlier in the study N randomised: total: 104; home‐based cardiac rehabilitation: 60; centre‐based cardiac rehabilitation: 44 Method of assessment: Confirmed acute myocardial infarction (WHO criteria) Diagnosis (% of pts): Post MI: 100% Age (mean ± SD): total: 62 ± 15 years; home‐based cardiac rehabilitation: 60.6 ± 10.1 years; centre‐based cardiac rehabilitation: 64.3 ± 11.2 years Percentage male: total: 81%; home‐based cardiac rehabilitation: 82%; centre‐based cardiac rehabilitation: 80% Ethnicity: NR |
|
| Interventions |
Description of home‐based cardiac rehabilitation: Heart Manual Time of start after event: Components: Exercise, education and psychosocial Aerobic exercise: Modality: walking Dose: Length of session: NR Frequency/no of sessions: NR Intensity: NR Total duration: 6 weeks Intermittent nurse or exercise specialist telephone support? Home visit in first week after discharge by cardiac rehabilitation nurse followed up by up to 4 telephone calls at 2, 3, 4, and 6 weeks Co‐interventions: NR Description of centre‐based cardiac rehabilitation: Components: Exercise, education and psychosocial Aerobic exercise: Modality: NR Dose: Length of session: NR Frequency/no of sessions: 1 to 5 sessions/week Intensity: NR Total duration: 8 to 10 weeks Co‐interventions: Input from dietician, psychologist, occupational therapist, and pharmacist |
|
| Outcomes | Primary: quality of life (MacNew questionnaire), total cholesterol Secondary: exercise capacity (METs), self‐reported smoking, cardiovascular morbidity, mortality, secondary prevention medication use |
|
| Follow up | 9 months post randomisation | |
| Source of funding | NHS Executive South West (Research and Development) Project Grant D/02/10.99 | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "...computerised random number trial allocation sequence was determined before the study" |
| Allocation concealment (selection bias) | Low risk | "...allocation was transferred to sequentially numbered, opaque, sealed envelopes and concealed from the research nurse, who carried out baseline assessment" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “...the person assessing the primary outcome questionnaires was blinded to allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “...the last known observation carried forward to replace missing values at 9 months for the primary outcome measures.” |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods section are reported in the results |
| Groups balanced at baseline? | Low risk | "The randomized groups were well balanced, apart from a higher proportion of patients in employment in the home based group (51% versus 26%, p=0.013)” |
| Groups received same co‐intervention(s)? | Low risk | Both groups received similar advice regarding exercise, stress management, and education |
Daskapan 2005.
| Methods |
Study design: Single centre RCT No of centres: 1 Country: Turkey Dates patients recruited: 2000 to 2001 When randomised: NR Maximum follow up: 12 weeks |
|
| Participants |
Inclusion criteria: Heart failure > 3 month duration Exclusion criteria: Valvular heart disease, exercise‐induced cardiac arrhythmias, symptomatic myocardial ischaemia within 3 months, taking beta‐blockers N randomised: total: 29; home‐based cardiac rehabilitation: 15; centre‐based cardiac rehabilitation: 14 Method of assessment: Patients fulfilled criteria of the New York Heart Association; class II or III CHF Diagnosis (% of pts): Class II or III NYHA with ischaemic or idiopathic dilated cardiomyopathy: 100% Age (mean ± SD): total: NR; home‐based cardiac rehabilitation: 49 ± 11 years; centre‐based cardiac rehabilitation: 52 ± 8 years Percentage male: total: 73%; home‐based cardiac rehabilitation: 73%; centre‐based cardiac rehabilitation: 73% Ethnicity: NR |
|
| Interventions |
Description of home‐based cardiac rehabilitation: The home‐based exercise training group (HETG) performed 12 weeks of physical training by themselves. Follow up logs completed daily/returned bi‐weekly. Components: Exercise only Aerobic exercise: Modality: Walking Dose: Length of session: 45 min/session (including warm‐up, cool‐down, recovery) Frequency/no of sessions: 3 sessions/week Intensity: up to 60% peak heart rate (RPE 12 to 16) Total duration: 12 weeks Intermittent nurse or exercise specialist telephone support? Weekly phone calls from staff monitoring adherence and progress, monthly phone calls from patients for control purposes Co‐interventions: NR Description of centre‐based cardiac rehabilitation: The supervised exercise training group (SETG) performed 12 weeks of physical training on treadmill at the laboratory Components: Exercise only Aerobic exercise: Modality: Walking on a treadmill Dose: Length of session: 45 min/session (including warm‐up, cool‐down, recovery) Frequency/no of sessions: 3 sessions/week Intensity: up to 60% peak heart rate (RPE 12 to 16) Total duration: 12 weeks Co‐interventions: NR |
|
| Outcomes | (Primary and secondary outcomes not distinguished) exercise capacity (mL/kg/min), resting BP, systolic and diastolic BP, adherence, dropouts, mortality | |
| Follow up | 12 weeks post randomisation | |
| Source of funding | NR | |
| Conflicts of interest | NR | |
| Notes | Data on mortality obtained by personal contact | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of assessors not described |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 3/11 (27%) centre‐based patients and 4/11 (36%) home‐based patients dropped out |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods section are reported in the results |
| Groups balanced at baseline? | Low risk | “Among patients who completed the study, no differences in demographic characteristics were seen between the 2 study groups after randomization (p>0.05).” |
| Groups received same co‐intervention(s)? | Low risk | “We chose lower intensity …training prescriptions in the HETG to avoid any adverse occurrences and also in the SETG to provide comparable training intensity levels between 2 groups.” |
Gordon 2002 Community.
| Methods |
Study design: Single centre RCT No of centres: 1 Country: USA Dates patients recruited: NR When randomised: Following baseline testing Maximum follow up: 12 weeks |
|
| Participants |
Inclusion criteria: Diagnosed CAD; low‐to‐moderate risk of cardiac events (1. no cardiac arrest within 1 year, 2. no complex ventricular dysrhythmia, 3. ejection fraction < 40%. 4. no complicated MI or cardiac surgery, 5. no increasing systolic BP response to exercise testing, 6. no angina pectoris < 5.0 METs); ≥ 4 weeks post‐hospitalisation; aged 21 to 75 years; no life‐threatening illness and/or psychological abnormality; speak/write English; ability to complete exercise treadmill test; ability to attend 36 cardiac rehabilitation sessions Exclusion criteria: NR N randomised: total: 155; physician‐supervised home‐based cardiac rehabilitation: 54; community home‐based cardiac rehabilitation: 49; centre‐based cardiac rehabilitation: 52 Method of assessment: NR Diagnosis (% of pts): History of prior MI: physician‐supervised home‐based cardiac rehabilitation: 29%; community home‐based cardiac rehabilitation: 16%; centre‐based cardiac rehabilitation: 6% History of prior CABG: physician‐supervised home‐based cardiac rehabilitation: 37%; community home‐based cardiac rehabilitation: 40%; centre‐based cardiac rehabilitation: 38% History of prior PTCA: physician‐supervised home‐based cardiac rehabilitation: 42%; community home‐based cardiac rehabilitation: 47%; centre‐based cardiac rehabilitation: 53% Age (mean ± SD): total: NR; physician‐supervised home‐based cardiac rehabilitation: 61 ± 10 years; community home‐based cardiac rehabilitation: 60 ± 9 years; centre‐based cardiac rehabilitation: 60 ± 9 years Percentage male: total: NR; physician‐supervised home‐based cardiac rehabilitation: 73%; community home‐based cardiac rehabilitation: 78%; centre‐based cardiac rehabilitation: 76% Ethnicity: NR |
|
| Interventions |
Description of physician‐supervised home‐based cardiac rehabilitation: Components: Exercise and education Aerobic exercise: Modality: NR Dose: Length of session: individually prescribed (30 to 60 min of aerobic exercise) Frequency/no of sessions: individually prescribed Intensity: 60% to 85% peak HR Total duration: 12 weeks Intermittent nurse or exercise specialist telephone support? appointments: 2 office visits, 4 phone calls Co‐interventions: Written materials, audiotapes, nutrition, weight and stress management, smoking cessation programme, individual CAD risk factors management Description of community home‐based cardiac rehabilitation: Components: Exercise and education Aerobic exercise: Modality: NR Dose: Length of session: individually prescribed (30 to 60 min of aerobic exercise) Frequency/no of sessions: individually prescribed Intensity: 60 to 85% peak HR Total duration: 12 weeks Intermittent nurse or exercise specialist telephone support? 12 on site visits or telephone calls (patient choice) Co‐interventions: Written materials, audiotapes, nutrition, weight and stress management, smoking cessation programme, individual CAD risk factors management Description of centre‐based cardiac rehabilitation: Components: e.g. exercise only, exercise and education, exercise and psychosocial Aerobic exercise: Modality: e.g. running, cycling, skipping. Dose: Length of session: Individually prescribed (30 to 60 min of aerobic exercise) Frequency/no of sessions: 3 sessions/week (total of 36 sessions = appointments) Intensity: 60 to 85% peak HR Total duration: 12 weeks Co‐interventions: Written materials, audiotapes, education on CAD risk factors and lifestyle modification |
|
| Outcomes | (Primary and secondary risk factors not distinguished) maximal oxygen uptake, blood pressure, fasting serum lipids, self‐reported smoking status, rehospitalisation, adherence (completion of appointments) | |
| Follow up | 12 weeks post randomisation | |
| Source of funding | NR | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of assessors not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data for 142 pts who completed exercise testing at baseline and at follow up (not all 155 pts randomised) reported only; numbers of dropouts reported and reasons described |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in methods are reported in results |
| Groups balanced at baseline? | Low risk | “Randomization did not result in statistical significant differences among patients assigned to the 3 interventions” |
| Groups received same co‐intervention(s)? | Low risk | All groups received similar written materials and advice |
Gordon 2002 Supervised.
| Methods |
Study design: Single centre RCT No of centres: 1 Country: USA Dates patients recruited: NR When randomised: Following baseline testing Maximum follow up: 12 weeks |
|
| Participants |
Inclusion criteria: Diagnosed CAD; low‐to‐moderate risk of cardiac events (1. no cardiac arrest within 1 year, 2. no complex ventricular dysrhythmia, 3. ejection fraction < 40%. 4. no complicated MI or cardiac surgery, 5. no increasing systolic BP response to exercise testing, 6. no angina pectoris < 5.0 METs); ≥ 4 weeks post‐hospitalisation; aged 21 to 75 years; no life‐threatening illness and/or psychological abnormality; speak/write English; ability to complete exercise treadmill test; ability to attend 36 cardiac rehabilitation sessions Exclusion criteria: NR N randomised: total: 155; physician‐supervised home‐based cardiac rehabilitation: 54; community home‐based cardiac rehabilitation: 49; centre‐based cardiac rehabilitation: 52 Method of assessment: NR Diagnosis (% of pts): History of prior MI: physician‐supervised home‐based cardiac rehabilitation: 29%; community home‐based cardiac rehabilitation: 16%; centre‐based cardiac rehabilitation: 6% History of prior CABG: physician‐supervised home‐based cardiac rehabilitation: 37%; community home‐based cardiac rehabilitation: 40%; centre‐based cardiac rehabilitation: 38% History of prior PTCA: physician‐supervised home‐based cardiac rehabilitation: 42%; community home‐based cardiac rehabilitation: 47%; centre‐based cardiac rehabilitation: 53% Age (mean ± SD): total: NR; physician‐supervised home‐based cardiac rehabilitation: 61 ± 10 years; community home‐based cardiac rehabilitation: 60 ± 9 years; centre‐based cardiac rehabilitation: 60 ± 9 years Percentage male: total: NR; physician‐supervised home‐based cardiac rehabilitation: 73%; community home‐based cardiac rehabilitation: 78%; centre‐based cardiac rehabilitation: 76% Ethnicity: NR |
|
| Interventions |
Description of physician‐supervised home‐based cardiac rehabilitation: Components: Exercise and education Aerobic exercise: Modality: NR Dose: Length of session: individually prescribed (30 to 60 min of aerobic exercise) Frequency/no of sessions: individually prescribed Intensity: 60% to 85% peak HR Total duration: 12 weeks Intermittent nurse or exercise specialist telephone support? appointments: 2 office visits, 4 phone calls Co‐interventions: Written materials, audiotapes, nutrition, weight and stress management, smoking cessation programme, individual CAD risk factors management Description of community home‐based cardiac rehabilitation: Components: Exercise and education Aerobic exercise: Modality: NR Dose: Length of session: individually prescribed (30 to 60 min of aerobic exercise) Frequency/no of sessions: individually prescribed Total duration: 12 weeks Intermittent nurse or exercise specialist telephone support? 12 on site visits or telephone calls (patient choice) Co‐interventions: Written materials, audiotapes, nutrition, weight and stress management, smoking cessation programme, individual CAD risk factors management Description of centre‐based cardiac rehabilitation: Components: e.g. exercise only, exercise and education, exercise and psychosocial Aerobic exercise: Modality: e.g. running, cycling, skipping. Dose: Length of session: Individually prescribed (30 to 60 min of aerobic exercise) Frequency/no of sessions: 3 sessions/week (total of 36 sessions = appointments) Intensity: 60 to 85% peak HR Total duration: 12 weeks Co‐interventions: Written materials, audiotapes, education on CAD risk factors and lifestyle modification |
|
| Outcomes | (Primary and secondary risk factors not distinguished) maximal oxygen uptake, blood pressure, fasting serum lipids, self‐reported smoking status, rehospitalisation, adherence (completion of appointments) | |
| Follow up | 12 weeks post randomisation | |
| Source of funding | NR | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of assessors not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Data for 142 pts (8%) who completed exercise testing at baseline and at follow up (not all 155 pts randomised) reported only; numbers of dropouts reported and reasons described |
| Selective reporting (reporting bias) | Low risk | All outcomes mentioned in methods are reported in results |
| Groups balanced at baseline? | Low risk | “Randomization did not result in statistical significant differences among patients assigned to the 3 interventions” |
| Groups received same co‐intervention(s)? | Low risk | All groups received similar written materials and advice |
Grace 2016 Mixed.
| Methods |
Study design: Single‐blind, 3 parallel‐arm multicentre RCT No of centres: 6 Country: Canada Dates patients recruited: 1 November 2009 to 31 July 2013 When randomised: After intake assessment Maximum follow up: Six months |
|
| Participants |
Inclusion criteria: Residency in the city where the cardiac rehabilitation programs were offered, proficiency in English, approval to participate in cardiac rehabilitation program by cardiac specialist or general practitioner, and eligibility for home‐based cardiac rehabilitation (i.e. low to moderate risk of an adverse event during exercise as demonstrated by lack of complex ventricular dysrhythmia, New York Heart Association class 1‐2 classification, and left ventricular ejection fraction of > 40%, or Canadian Cardiovascular Society class 1‐2 classification). Exclusion criteria: Musculoskeletal, neuromuscular, visual, cognitive, or serious mental illness, or any serious illness that would preclude cardiac rehabilitation eligibility; deemed not suitable for cardiac rehabilitation by physician; plans to leave area; discharged to a long‐term care facility; and participation in another RCT with behavioural interventions. N randomised: total: 169; home‐based cardiac rehabilitation: 55; comparator 1 (mixed sex): 59 comparator 2 (women only): 55 Method of assessment: Clinical charts were reviewed for inclusion/exclusion criteria Diagnosis (% of pts): PCI: total: 49.1%; home‐based cardiac rehabilitation: 50.0%; mixed sex: 50.0%; women only: 47.3% Angina/ACS/CAD: total: 36.2%; home‐based cardiac rehabilitation: 35.8%; mixed sex: 36.4%; women only: 36.4% MI: total: 35.8%; home‐based cardiac rehabilitation: 34.0%; mixed sex: 38.6%; women only: 34.5% CABG: total: 25.5%; home‐based cardiac rehabilitation: 25.9%; mixed sex: 21.4%; women only: 29.1% Valve: total: 19.4%; home‐based cardiac rehabilitation: 20.4%; mixed sex: 19.3%; women only: 18.5% Age (mean ± SD): total: 63.64 ± 10.42 years; home‐based cardiac rehabilitation: 63.13 ± 10.94 years; mixed sex: 61.56 ± 9.73 years; women only: 66.22 ± 10.21 years Percentage male: total: NR Ethnicity (%white): total: 62.5%; home‐based cardiac rehabilitation: 65.3%; mixed sex: 62.7%; women only: 59.1% |
|
| Interventions | Female patients were randomised to 1 of 3 models: (1)supervised mixed‐sex, (2) supervised women only, or (3) home‐based cardiac rehabilitation. There were 3 cardiac rehabilitation sites involved in the trial, each offering all 3 models of cardiac rehabilitation. The programs lasted 4 to 6 months. At each site, a graded exercise stress test was performed pre‐program and post‐program. Results were used to develop individualised exercise prescriptions and participants were encouraged to accumulate at least 150 minutes of exercise per week at their target heart rate, preferably exercising most days of the week via stationary bicycle/treadmill/walking. Description of intervention (home‐based cardiac rehabilitation): Home‐based cardiac rehabilitation participants had at least 3 onsite visits and then exercised at home. Time of start after event: NR Components: Exercise only Aerobic exercise: Modality: stationary bicycle/treadmill/walking Dose: Participants were encouraged to accumulate at least 150 minutes of exercise per week Length of session: NR Frequency/no of sessions: NR Intensity: Participants exercised according to an individualised exercise prescription which included a target heart rate Resistance training included? No Total duration: 4 to 6 months Intermittent nurse or exercise specialist telephone support? Patients were phoned weekly or biweekly, depending on program protocols and based on patient need. Co‐interventions: Patients were provided the same education materials as patients attending the supervised models at their initial visit, which was reviewed on the phone with program staff. Description of comparator (centre‐based cardiac rehabilitation): Comparator 1: supervised mixed‐sex Comparator 2: supervised women only Time of start after event: NR Components: Exercise only Aerobic exercise: Modality: stationary bicycle/treadmill/walking Dose: Length of session: up to 1 hour Frequency/no of sessions: 1 to 2 times/week Intensity: Individualised target heart rate Resistance training included? Yes Total duration: 4 to 6 months Co‐interventions: Education materials provided |
|
| Outcomes | Adherence to cardiac rehabilitation, exercise capacity | |
| Follow up | 6 months | |
| Source of funding | Heart and Stroke Foundation of Ontario (Grant in Aid no. NA 6682) | |
| Conflicts of interest | None declared | |
| Notes | SD values for adherence data were provided by the author on request | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The randomization sequence was computer generated, in blocks of 6, and stratified by condition…through randomize.net.” |
| Allocation concealment (selection bias) | Low risk | “Recruiters went online to ascertain random allocation and informed patients and CR sites.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “The CR program staff members were not aware of study objectives or which participants were involved in the trial. As a manipulation check, a masked research assistant checked CR charts to confirm the program model attended at the expected CR discharge date. Post‐test CR data extraction, including stress test results, and program adherence were also undertaken by the masked research assistant.” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Home‐based cardiac rehabilitation: 35/55 (64 %) lost to follow‐up Mixed sex centre‐based cardiac rehabilitation: 38/59 (64 %) lost to follow‐up Women only centre‐based cardiac rehabilitation: 34/55 (62 %) lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods were reported in the results section |
| Groups balanced at baseline? | Low risk | There were no significant differences between patients randomized to each of the 3 models (all P>.05) |
| Groups received same co‐intervention(s)? | Low risk | “Patients were provided the same education materials as patients attending the supervised models at their initial visit, which was reviewed on the phone with program staff.” |
Grace 2016 Women.
| Methods |
Study design: Single‐blind, 3 parallel‐arm multicentre RCT No of centres: 6 Country: Canada Dates patients recruited: 1 November 2009 to 31 July 2013 When randomised: After intake assessment Maximum follow up: Six months |
|
| Participants |
Inclusion criteria: Residency in the city where the cardiac rehabilitation programs were offered, proficiency in English, approval to participate in cardiac rehabilitation program by cardiac specialist or general practitioner, and eligibility for home‐based cardiac rehabilitation (i.e. low to moderate risk of an adverse event during exercise as demonstrated by lack of complex ventricular dysrhythmia, New York Heart Association class 1 to 2 classification, and left ventricular ejection fraction of > 40%, or Canadian Cardiovascular Society class 1 to 2 classification). Exclusion criteria: Musculoskeletal, neuromuscular, visual, cognitive, or serious mental illness, or any serious illness that would preclude cardiac rehabilitation eligibility; deemed not suitable for cardiac rehabilitation by physician; plans to leave area; discharged to a long‐term care facility; and participation in another RCT with behavioural interventions. N randomised: total: 169; home‐based cardiac rehabilitation: 55; comparator 1 (mixed sex): 59 comparator 2 (women only): 55 Method of assessment: Clinical charts were reviewed for inclusion/exclusion criteria Diagnosis (% of pts): PCI: total: 49.1%; home‐based cardiac rehabilitation: 50.0%; mixed sex: 50.0%; women only: 47.3% Angina/ACS/CAD: total: 36.2%; home‐based cardiac rehabilitation: 35.8%; mixed sex: 36.4%; women only: 36.4% MI: total: 35.8%; home‐based cardiac rehabilitation: 34.0%; mixed sex: 38.6%; women only: 34.5% CABG: total: 25.5%; home‐based cardiac rehabilitation: 25.9%; mixed sex: 21.4%; women only: 29.1% Valve: total: 19.4%; home‐based cardiac rehabilitation: 20.4%; mixed sex: 19.3%; women only: 18.5% Age (mean ± SD): total: 63.64 ± 10.42 years; home‐based cardiac rehabilitation: 63.13 ± 10.94 years; mixed sex: 61.56 ± 9.73 years; women only: 66.22 ± 10.21 years Percentage male: total: NR Ethnicity (%white): total: 62.5%; home‐based cardiac rehabilitation: 65.3%; mixed sex: 62.7%; women only: 59.1% |
|
| Interventions | Female patients were randomised to 1 of 3 models: (1)supervised mixed sex, (2) supervised women only, or (3) home‐based cardiac rehabilitation There were 3 cardiac rehabilitation sites involved in the trial, each offering all 3 models of cardiac rehabilitation. The programs lasted 4 to 6 months. At each site, a graded exercise stress test was performed pre‐program and post‐program. Results were used to develop individualised exercise prescriptions and participants were encouraged to accumulate at least 150 minutes of exercise per week at their target heart rate, preferably exercising most days of the week via stationary bicycle/treadmill/walking. Description of intervention (home‐based cardiac rehabilitation): Home‐based cardiac rehabilitation participants had at least 3 onsite visits and then exercised at home. Time of start after event: NR Components: Exercise only Aerobic exercise: Modality: stationary bicycle/treadmill/walking Dose: Participants were encouraged to accumulate at least 150 minutes of exercise per week Length of session: NR Frequency/no of sessions: NR Intensity: Participants exercised according to an individualised exercise prescription which included a target heart rate Resistance training included? No Total duration: 4 to 6 months Intermittent nurse or exercise specialist telephone support? Patients were phoned weekly or biweekly, depending on program protocols and based on patient need. Co‐interventions: Patients were provided the same education materials as patients attending the supervised models at their initial visit, which was reviewed on the phone with program staff. Description of comparator (centre‐based cardiac rehabilitation): Comparator 1: supervised mixed sex Comparator 2: supervised women only Time of start after event: NR Components: Exercise only Aerobic exercise: Modality: stationary bicycle/treadmill/walking Dose: Length of session: up to 1 hour Frequency/no of sessions: 1 to 2 times/week Intensity: Individualised target heart rate Resistance training included? Yes Total duration: 4 to 6 months Co‐interventions: Education materials provided |
|
| Outcomes | Adherence to cardiac rehabilitation, exercise capacity | |
| Follow up | 6 months | |
| Source of funding | Heart and Stroke Foundation of Ontario (Grant in Aid no. NA 6682) | |
| Conflicts of interest | None declared | |
| Notes | SD values for adherence data were provided by the author on request | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The randomization sequence was computer generated, in blocks of 6, and stratified by condition…through randomize.net.” |
| Allocation concealment (selection bias) | Low risk | “Recruiters went online to ascertain random allocation and informed patients and CR sites.” |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “The CR program staff members were not aware of study objectives or which participants were involved in the trial. As a manipulation check, a masked research assistant checked CR charts to confirm the program model attended at the expected CR discharge date. Post‐test CR data extraction, including stress test results, and program adherence were also undertaken by the masked research assistant.” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Home‐based cardiac rehabilitation: 35/55 (64 %) lost to follow‐up Mixed sex centre‐based cardiac rehabilitation: 38/59 (64 %) lost to follow‐up Women only centre‐based cardiac rehabilitation: 34/55 (62 %) lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods were reported in the results section |
| Groups balanced at baseline? | Low risk | There were no significant differences between patients randomized to each of the 3 models (all P > 0.05) |
| Groups received same co‐intervention(s)? | Low risk | “Patients were provided the same education materials as patients attending the supervised models at their initial visit, which was reviewed on the phone with program staff.” |
Hadadzadeh 2013.
| Methods | Study design: Single centre RCT No of centres: 1 Country: India Dates patients recruited: 2007 to 2008 When randomised: As recruitment proceeded Maximum follow up: 1 year | |
| Participants |
Inclusion criteria: Low and moderate risk post‐PTCA patients, aged 35 to 75 years Exclusion criteria: High risk post‐PTCA patients; any musculoskeletal; neuromuscular, or any other medical conditions with exercise contraindications; not willing to give consent N randomised: total: 105; home‐based cardiac rehabilitation (HmCR): 35; centre‐based cardiac rehabilitation (HsCR): 35; control (no cardiac rehabilitation ‐ usual standard care in the centre at the time of study): 35 Diagnosis (% of pts): Post‐PTCA patients; 100% Age (mean ± SD): total: 56.1 ± 9.1; home‐based cardiac rehabilitation (HmCR): NR; centre‐based cardiac rehabilitation: NR Percentage male: total: 71.4%; home‐based cardiac rehabilitation (HmCR): NR; centre‐based cardiac rehabilitation: NR Ethnicity: Asian Indian 100% |
|
| Interventions |
Description of intervention (home‐based cardiac rehabilitation): Time of start after event: within 2 weeks post‐PTCA Components: Exercise and education Aerobic exercise: Modality: Brisk walking DOSE: Moderate Length of session: 20 to 60 min, progressively increased in 3 month duration of treatment including 5 to 10 min warm up and cool down Frequency/no of sessions: 3 times/week Intensity: 40% to 70% of HRR, progressively increased in 12 weeks. HRmax was obtained from symptom‐limited Bruce protocol exercise test at baseline. Resistance training included? No Total duration: 3 months (12 weeks) Intermittent nurse or exercise specialist telephone support? Every 2 weeks to increase intensity based on HR, other times as per needed on the telephone Co‐interventions: NR Description of comparator (centre‐based cardiac rehabilitation): Time of start after event: within 2 weeks post‐PTCA Components: Exercise (supervised by trained physical therapist at centre) Aerobic exercise: Yes Modality: Brisk walking on treadmill DOSE: Moderate Length of session: 20 to 60 min, progressively increased in 3 month duration of treatment including 5 to 10 min warm up and cool down Frequency/no of sessions: 3 days/week Intensity: 40% to 70% HRR, progressively increased in 12 weeks Resistance training included? No Total duration: 3 months (12 weeks) Co‐interventions: NR |
|
| Outcomes | Mortality | |
| Follow up | 3 months, 1 year | |
| Source of funding | Manipal University, India | |
| Conflicts of interest | “None” | |
| Notes | This study has not yet been published and we do not have access to the full manuscript. All study information and outcome data were provided by the study author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation through concealed envelope method |
| Allocation concealment (selection bias) | Low risk | Block Randomisation through concealed envelope method |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Home‐based cardiac rehabilitation: 0/35 (0%) lost to follow‐up Centre‐based cardiac rehabilitation: 4/35 (11.4 %) lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | This study has not yet been published and we did not have access to a published protocol or description of the methods |
| Groups balanced at baseline? | Unclear risk | This study has not yet been published and we did not have access to the baseline data |
| Groups received same co‐intervention(s)? | High risk | The home‐based group received education; the centre‐based group did not |
Hadadzadeh 2015.
| Methods | Study design: Multicentre RCT No of centres: 3 Country: India, and Iran Dates patients recruited: 2007 to 2009 When randomised: As recruitment proceeded Maximum follow up: 1 year | |
| Participants |
Inclusion criteria: Low and moderate risk post‐event CAD patients (post‐MI on conservative Rx, CABG, PTCA), aged 35 to 75 years Exclusion criteria: High risk post‐event CAD patients, Any musculoskeletal, neuromuscular, or any other medical conditions with exercise contra‐indications, not willing to give consent. N randomised: total: 180; hospital‐based cardiac rehabilitation: 60; centre‐based cardiac rehabilitation; 60; control (no exercise): 60 Diagnosis (% of pts): Post‐event CAD patients treated conservatively, CABG or PTCA Age (mean ± SD): total: 57 ± 9.3 years; intervention: NR; comparator: NR Percentage male: total: 81.1%; intervention: NR; comparator: NR Ethnicity: Asian Indian 70%, Middle Eastern (white) 30% |
|
| Interventions |
Description of intervention (home‐based cardiac rehabilitation): Time of start after event: within 2 weeks post‐event Components: Exercise and education Aerobic exercise: Yes Modality: Brisk walking DOSE: Moderate Length of session: 20 to 60 min, progressively increased in 3 month duration of treatment including 5 to 10 min warm up and cool down Frequency/no of sessions: 3 times/week Intensity: 40% to 70% HRR, progressively increased over 12 weeks Resistance training included? No Total duration: 3 months (12 weeks) Intermittent nurse or exercise specialist telephone support? Every 2 weeks to increase intensity based on HR, other times as per needed on the phone Co‐interventions: None Description of comparator (centre‐based cardiac rehabilitation): Time of start after event: within 2 weeks post‐event Components: e.g. exercise only Aerobic exercise: Modality: Brisk walking on treadmill DOSE: Moderate Length of session: 20 to 60 min, progressively increased in 3 month duration of treatment including 5 to 10 min warm up and cool down Frequency/no of sessions: 3 days/week Intensity: 40% to 70% HRR, progressively increased over 12 weeks Resistance training included? No Total duration: 3 months (12 weeks) Co‐interventions: None |
|
| Outcomes | Quality of life measured by SF‐36v2, Functional Capacity measured by achieved MET level on a symptom limited Bruce protocol treadmill test | |
| Follow up | 3 months | |
| Source of funding | Manipal University, India; MOE Iran | |
| Conflicts of interest | None | |
| Notes | This study has not been published yet and we do not have access to the full manuscript. All study information and outcome data has been provided by the author of the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation |
| Allocation concealment (selection bias) | Low risk | Concealed envelope method |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Assessors were blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Home‐based cardiac rehabilitation: 5/60 (8.0%) lost to follow‐up Centre‐based cardiac rehabilitation: 2/60 (3.3 %) lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | This study has not yet been published and we did not have access to a published protocol or description of the methods |
| Groups balanced at baseline? | Unclear risk | This study has not yet been published and we did not have access to baseline data |
| Groups received same co‐intervention(s)? | High risk | The home‐based group received education; the centre‐based group did not |
Jolly 2007.
| Methods |
Study design: Multicentre RCT
No of centres: 4
Country: UK
Dates patients recruited: February 2002 to January 2004 When randomised: Following baseline assessment Maximum follow up: 24 months |
|
| Participants |
Inclusion criteria: Acute MI, coronary angioplasty (± stenting) or CABG Exclusion criteria: Inability to speak either English or Punjabi, dementia, severe hearing impairment, sight defects of sufficient severity to prevent reading the Heart Manual, and serious persisting complications N randomised: total: 525; home‐based cardiac rehabilitation: 263; centre‐based cardiac rehabilitation: 262 Method of assessment: Killip Class Diagnosis (% of pts): MI: home‐based cardiac rehabilitation: 49.0%; centre‐based cardiac rehabilitation: 49.2% PTCA: home‐based cardiac rehabilitation: 38.4; centre‐based cardiac rehabilitation: 42.0% CABG: home‐based CR: 12.5; centre‐based cardiac rehabilitation: 8.8% Age (mean ± SD): home‐based cardiac rehabilitation: 60.3 ± 10.5 years; centre‐based cardiac rehabilitation: 61.8 ± 11.0 years Percentage male: home‐based cardiac rehabilitation: 77.2%; centre‐based cardiac rehabilitation: 76.0% Ethnicity: home‐based cardiac rehabilitation: 80.2%; centre‐based cardiac rehabilitation: 79.3% |
|
| Interventions |
Description of home‐based cardiac rehabilitation: The home‐based programme consisted of a manual, three home visits (at 10 days, 6 weeks and 12 weeks) and telephone contact at 3 weeks. Patients who had had an MI were discharged home with the Heart Manual. Additional visits were made as deemed necessary by the rehabilitation nurse. The manual encourages patients to build up their exercise gradually to achieve a minimum of 15 minutes of moderately intense activity daily. Components: Exercise, education and psychosocial Aerobic exercise: Modality: walking Dose: Length of session: minimum of 15 mins Frequency/no of sessions: up to daily Intensity: NR Total duration: 6 weeks Heart Manual programme and 12 weeks nurse support Intermittent nurse or exercise specialist telephone support? Three home visits (at 10 days, 6 weeks and 12 weeks) and telephone contact at 3 weeks Co‐interventions: Education on risk factors, lifestyle changes, medications and stress management (relaxation tapes) Description of centre‐based cardiac rehabilitation: The four centre‐based programmes varied in length, including nine sessions at weekly intervals, 12 sessions over 8 weeks and 24 individualised sessions over 12 weeks. Programmes commenced between 4 weeks and 8 weeks following the cardiac event. Patients exercised to 65% to 75% of their predicted maximal heart rate and the exercise element of the sessions lasted from 25 minutes to 40 minutes plus warm‐up and cool‐down elements. Components: Exercise, education and psychosocial Aerobic exercise: Modality: circuit training, cycle ergometer Dose: Length of session: 25 to 30 min/session Frequency/no of sessions: 1 or 2 sessions/week Intensity: 65% to 75% HRmax Resistance training included? Total duration: 6 to 12 weeks Co‐interventions: Education and stress management (relaxation) |
|
| Outcomes | Primary: serum cholesterol, total cholesterol, HDL cholesterol, blood pressure, exercise capacity (ISWT), smoking (cotinine‐validated) Secondary: quality of life (EQ‐5D), health service utilisation (hospital readmissions, primary care visits, medication), mortality, cardiovascular events, costs |
|
| Follow up | 6, 12, 24 months | |
| Source of funding | Funded by the UK Department of Health through its Health Technology Assessment Programme. National Heart Research funded the development of the Heart Manual for patients following a revascularisation procedure | |
| Conflicts of interest | "None" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients who consented to randomisation were randomised on an individual basis with minimisation by (1) original diagnosis (MI/revascularisation), (2) age (<50/50‐74/75+ years), (3) sex, (4) ethnicity (Caucasian/Asian/other) and (5) hospital of recruitment." |
| Allocation concealment (selection bias) | Low risk | "Allocation was undertaken by the Birmingham Cancer Clinical Trials Unit, a group that was independent from the trial team …When a patient agreed to be randomised…the research nurse telephoned the Clinical Trials Unit…and was given an allocation group." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Assessments were blinded, with follow‐up undertaken by a research nurse who had neither recruited the patient nor provided home cardiac rehabilitation support." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | “A sensitivity analysis was undertaken on the 12‐month data to assess the potential impact of the missing values for the ISWT, [systolic] BP, [diastolic] BP, [total cholesterol] and the Hospital Anxiety and Depression Scale scores.” |
| Selective reporting (reporting bias) | Low risk | all outcomes described in the methods section are reported in the results |
| Groups balanced at baseline? | Low risk | “Demographic characteristics, diagnosis, past medical history and cardiac risk factors were well matched between the two arms at baseline.” |
| Groups received same co‐intervention(s)? | High risk | Although both groups received exercise, education and stress management, the nature and amount of intervention between groups was different |
Karapolat 2009.
| Methods | RCT parallel groups Study design: Single centre RCT No of centres: 1 Country: Turkey Dates patients recruited: 2007 to 2008 When randomised: NR Maximum follow up: 8 weeks |
|
| Participants |
Inclusion criteria: HF as a result of ischaemic and dilated cardiomyopathy, clinical stability for at least 3 months, left ventricular ejection fraction ≤ 40%, NYHA functional class II‐III, optimal and standard pharmacological treatment, the ability to speak and understand Turkish, absence of psychiatric disease, the ability to remain stable during exercise tests, and willingness to volunteer to participate in this study. Exclusion criteria: Neurological orthopaedic, peripheral vascularisation, or severe pulmonary disease; NYHA class IV patients; unstable angina pectoris; poorly controlled or exercise‐induced cardiac arrhythmias; recent acute coronary syndrome or revascularisation (≤ 3 months); significant valvular disease; atrial fibrillation; uncontrolled arterial hypertension; and performing exercise training at regular intervals during the previous 6 weeks. Method of assessment: Standard echocardiography and Tissue Doppler Imaging echocardiography (TDI) N randomised: total: 74; home‐based cardiac rehabilitation: 37; centre‐based cardiac rehabilitation: 37 Diagnosis (% of pts): Heart failure: 100% Age (mean ± SD): home‐based cardiac rehabilitation: 44.05 ± 11.49 years; centre‐based cardiac rehabilitation: 45.16 ± 13.58 years Percentage male: home‐based cardiac rehabilitation: 62%; centre‐based cardiac rehabilitation: 66% Ethnicity: NR |
|
| Interventions |
Description of home‐based cardiac rehabilitation: All sessions were performed at home, supervised by a physician. A specific program was designed for each patient based on individual muscle strength, joint flexibility, and aerobic endurance. Exercise sessions included flexibility exercises, aerobic exercises, and breathing exercises. The flexibility exercises focused on range of motion and included exercises designed to stretch the cervical and lumbar spine and the upper and lower extremities. Training HR measured by monitor. Components: Exercise only Aerobic exercise: Modality: walking Dose: Length of session: NR Frequency/no of sessions: NR Intensity: NR Total duration: 8 weeks Intermittent nurse or exercise specialist telephone support? NR Co‐interventions: NR Description of centre‐based cardiac rehabilitation: Centre‐based cardiac rehabilitation(control): Exercise: All rehabilitation sessions were supervised by a physician. A specific program was designed for each patient based on individual muscle strength, joint flexibility, and aerobic endurance. Exercise sessions included flexibility exercises, aerobic exercises, and breathing exercises. The flexibility exercises focused on range of motion and included exercises designed to stretch the cervical and lumbar spine and the upper and lower extremities. Training HR measured by monitor. Components: e.g. exercise only, exercise and education, exercise and psychosocial Aerobic exercise: Modality: Treadmill Dose: Length of session: 45 to 60 min (including 5 min warm‐up, 30 min aerobic exercise and 5 min cool‐down) Frequency/no of sessions: 3 sessions/week Intensity: 60% to 70% heart rate reserve, level 13 to 15 on the Borg scale Total duration: 8 weeks Co‐interventions: NR |
|
| Outcomes | Exercise capacity, quality of life (SF‐36) | |
| Follow up | 8 weeks | |
| Source of funding | "We have no support for this study" | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Low risk | “...randomized (using concealed envelopes)” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Flow diagram shows loss to follow up 5/37 (14%) hospital‐based, 1/37 (3%) home‐based group; no imputation of missing data undertaken |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods section are reported in the results |
| Groups balanced at baseline? | Low risk | Good balance in patient demographics |
| Groups received same co‐intervention(s)? | Low risk | Only difference between groups is whether exercise training performed in hospital or home |
Kassaian 2000.
| Methods |
Study design: Single centre RCT No of centres: 1 Country: Iran Dates patients recruited: NR When randomised: Immediately after baseline tests (one to two months after acute Q wave MI or CABG) Maximum follow up: 12 weeks |
|
| Participants |
Inclusion criteria: AMI or CABG in last 1 to 2 months, NYHA class < IV, ejection fraction ≥ 30%, able to exercise on a treadmill and participate in exercise programme Exclusion criteria: High‐risk stress test, decompensated CHF (NYHA IV), unstable angina, uncontrolled atrial fibrillation, high‐grade atrioventricular block (grade 2 or 3), active pericarditis or myocarditis, recent pulmonary thromboembolism, exercise‐induced asthma, claudication, fixed‐rate permanent pacemaker, severe medical problem N randomised: total: 125; home‐based cardiac rehabilitation: 60; centre‐based cardiac rehabilitation: 65 Diagnosis (% of pts): MI: total: 23.2%; home‐based cardiac rehabilitation: 13.3%; centre‐based cardiac rehabilitation: 32.3% CABG: total:76.8%; home‐based cardiac rehabilitation: 86.7%; centre‐based cardiac rehabilitation: 67.7% Age (mean ± SD): 55 ± 9.5 years Percentage male: total: 100% Ethnicity: NR |
|
| Interventions |
Description of home‐based cardiac rehabilitation: Patients were taught to count their pulse rate TIme of start after even: One to two months after acute Q wave MI or CABG Components: Exercise only Aerobic exercise: Modality: NR Dose: Length of session: NR Frequency/no of sessions: NR Intensity: “based on exercise test results” Total duration: 12 weeks Intermittent nurse or exercise specialist telephone support? NR Co‐interventions: NR Description of centre‐based cardiac rehabilitation: Components: Exercise only Aerobic exercise: Modality: treadmill Dose: Length of session: 20 to 30 min + 10 min warm‐up + 10 min cool‐down/session Frequency/no of sessions: 3 sessions week Intensity: 60% to 85% (not reported if relative to HRmax) Total duration: 12 weeks Co‐interventions: NR |
|
| Outcomes | (Primary and secondary outcomes not distinguished) systolic BP, diastolic BP, heart rate (all resting and sub‐maximal), functional capacity (METs), BMI, cholesterol: total, LDL, HDL, triglyceride | |
| Follow up | 12 weeks post randomisation | |
| Source of funding | NR | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on loss to follow up or missing data management |
| Selective reporting (reporting bias) | Unclear risk | Not all outcomes reported mentioned in methods section |
| Groups balanced at baseline? | Low risk | “Among patients who completed the study no differences in demographic characteristics were seen between the two study groups after randomisation.” |
| Groups received same co‐intervention(s)? | Unclear risk | Details of home‐based intervention not reported |
Kraal 2014.
| Methods |
Study design: Single centre RCT No of centres: 1 Country: Netherlands Dates patients recruited: March 2013 to March 2014 When randomised: After written consent, one week after cardiac rehabilitation intake Maximum follow up: 12 weeks |
|
| Participants |
Inclusion criteria: Patients entering cardiac rehabilitation after hospitalisation for MI, unstable angina, or a revascularisation procedure (PCI or CABG). Only patients with a low‐to‐moderate risk of future cardiac events according to the Dutch cardiac rehabilitation guidelines were included. Patients were required to have Internet access and a computer at home. Exclusion criteria: None described N randomised: total: 55;intervention: 26; comparator: 26 Method of assessment: NR Diagnosis (% of pts): ACS with PCI: home‐based cardiac rehabilitation: 56%; centre‐based cardiac rehabilitation: 40% ACS without PCI: home‐based cardiac rehabilitation: 16%; centre‐based cardiac rehabilitation: 20% Angina pectoris with PCI: home‐based cardiac rehabilitation: 8%; centre‐based cardiac rehabilitation: 16% Angina pectoris without PCI: home‐based cardiac rehabilitation: 8%; centre‐based cardiac rehabilitation: 0% CABG: home‐based cardiac rehabilitation: 12%; centre‐based cardiac rehabilitation: 24% Age (mean ± SD) (N = 25): total: NR; home‐based cardiac rehabilitation: 60.6 ± 7.5 years; centre‐based cardiac rehabilitation: 56.1 ± 8.7 years Percentage male (N = 25): total: NR; home‐based cardiac rehabilitation: 88%; centre‐based cardiac rehabilitation: 84% Ethnicity: NR |
|
| Interventions |
Description of home‐based cardiac rehabilitation: Patients in the HT group received three initial supervised training sessions. During these sessions, patients received instructions on how to use a wearable heart rate monitor (Garmin Forerunner 70) and how to upload the recorded exercise data to a web application (Garmin Connect) through the Internet. The web application was used to review the training data by the patient, the physical therapist and the exercise specialist. During the first sessions, the patients were also familiarised with the training programme (duration, intensity) and their preferred training modality in the home environment was discussed. After three supervised training sessions, patients in the HT group started training in their home environment. Time of start after event: NR Components: Exercise plus behavioural change Aerobic exercise: Modality: Patient’s preferred training modality Dose: Length of session: 45 to 60 min Frequency/no of sessions: at least two training sessions per week Intensity: 70% to 85% of maximal heart rate Resistance training included? No Total duration: 12 weeks Intermittent nurse or exercise specialist telephone support? Patients received feedback on training frequency, duration and intensity from the physical therapist once a week via telephone. After 12 weeks, the telephonic feedback was terminated and the patients were advised to continue their training with the heart rate monitor. Co‐interventions: Patients in the home‐based training group received coaching from their therapist through weekly telephone calls. During this phone call the therapist gave feedback on training parameters that were measured during the preceding week, and discussed progress with respect to the personal training goals. In addition, based on the principles of motivational interviewing, they discussed barriers and facilitative factors in adhering to the exercise training protocol. Description of centre‐based cardiac rehabilitation: Time of start after event: NR Components: Exercise only Aerobic exercise: Modality: Group‐based training sessions on a treadmill or cycle ergometer, supervised by physical therapists and exercise specialists. Dose: Length of session: 45 to 60 min Frequency/no of sessions: at least two training sessions per week Intensity: 70% to 85% of their maximal heart rate Resistance training included? No Total duration: 12 weeks Co‐interventions: None described |
|
| Outcomes | Exercise capacity; HRQoL; adherence to cardiac rehabilitation | |
| Follow up | 12 weeks | |
| Source of funding | ZonMw, the Dutch Organisation for Health Research and Development (project number 837001003). | |
| Conflicts of interest | The FIT@Home study is executed in collaboration with Philips Research; the heart rate monitors used during home‐based training were provided by Philips Research | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “...patients were randomly allocated to homebased training (HT) or centre‐based training (CT)”. Method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Home‐based cardiac rehabilitation: 4/29 (13.8%) lost to follow‐up Centre‐based cardiac rehabilitation: 1/26 (3.8%) lost to follow‐up Loss to follow‐up was disproportionately higher in the intervention group. "Data were analysed per protocol" |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods section are reported in the results section |
| Groups balanced at baseline? | Low risk | No P values were given, but baseline characteristics appear to be similar in both groups |
| Groups received same co‐intervention(s)? | High risk | “…patients in the HT group started training at home and received coaching from their therapist through weekly telephone calls...” No coaching was given to the centre‐based cardiac rehabilitation group |
Marchionni 2003.
| Methods |
Study design: Single centre RCT No of centres: 1 Country: Italy Dates patients recruited: NR When randomised: NR Maximum follow up: 14 months |
|
| Participants |
Inclusion criteria: Aged > 45 years, MI Exclusion criteria: Severe cognitive impairment; physical disability; left ventricular ejection fraction < 35%; contraindications to vigorous exercise; eligibility for myocardial revascularisation, living too far from cardiac rehabilitation unit N randomised: total: 180; home‐based cardiac rehabilitation: 90; centre‐based cardiac rehabilitation: 90 Method of assessment: NR Diagnosis (% of pts): MI: 100% Age (mean ± SD): total: 69 ± 1.6 years; home‐based cardiac rehabilitation: NR; centre‐based cardiac rehabilitation: NR Percentage male: total: 71%; home‐based cardiac rehabilitation: NR%; centre‐based cardiac rehabilitation: NR Ethnicity: NR |
|
| Interventions |
Description of home‐based cardiac rehabilitation: Components: Exercise only Aerobic exercise: Modality: cycle ergometer Dose: Length of session: NR Frequency/no of sessions: 3 days/week Intensity: 70% to 85% peak HR Total duration: 8 weeks Intermittent nurse or exercise specialist telephone support? Physical therapist home visits every other week Co‐interventions: Monthly family‐oriented support groups Description of centre‐based cardiac rehabilitation: Components: Exercise only Aerobic exercise: cycle ergometer Modality: e.g. running, cycling, skipping. Dose: Length of session: NR Frequency/no of sessions: 3 days/week Intensity: 70% to 85% peak HR Total duration: 12 weeks Co‐interventions: Risk factor management counselling; support group meetings |
|
| Outcomes | Primary: total work capacity Secondary: HRQoL (Sickness Impact Profile), mortality, morbidity (cardiovascular events), healthcare utilisation (medical visits, rehospitalisations), costs, and adherence (number of completed training sessions) |
|
| Follow up | 2, 8, 14 months post randomisation | |
| Source of funding | National Research Council (CNR), the University of Florence, and the Regional Government of Tuscany, Italy | |
| Conflicts of interest | NR | |
| Notes | Subgroup analysis in age groups (middle‐aged: 45 to 65 years, old: 65 to 75 years, very old: >75 years). Data presented separately for 3 age groups. Follow up data on charts only; authors contacted for numerical data at follow up and these have been supplied for total work capacity and Sickness Impact Profile separately for 3 groups; we pooled data across age groups |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Testing personnel were blinded to patient assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "...we performed a sensitivity analysis comparing results obtained with and without replacement of missing data with data obtained with the expectation‐maximization imputation method. Because the 2 analyses provided similar results, which were also similar with missing data substituted with data estimated in a worst‐case scenario, only the data from patients who completed the study are presented" |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods section are reported in the results |
| Groups balanced at baseline? | Low risk | “...baseline sociodemographic and clinical characteristics were similar across the 3 arms of the trial” Baseline characteristics by home and hospital group allocation not reported in tabular format |
| Groups received same co‐intervention(s)? | Low risk | "Patients received an exercise prescription similar to that of the Hosp‐CR group.... A physical therapist made home visits every other week to adjust if necessary the exercise prescription, to enhance adherence with intervention" |
Miller 1984 Brief.
| Methods |
Study design: Single centre RCT No of centres: 1 Country: USA Dates patients recruited: NR When randomised: NR Maximum follow up: 23 weeks |
|
| Participants |
Inclusion criteria: Uncomplicated AMI (elevated serum creatinine kinase or oxaloacetic transaminase, prolonged chest pain consistent with AMI, new Q waves or evolutionary ST changes in ECG) Exclusion criteria: Unable to undertake exercise test, congestive heart failure, unstable angina pectoris, valvular heart disease, atrial fibrillation, bundle branch block, history of bypass, stroke, orthopaedic abnormalities, peripheral vascular disease, chronic pulmonary obstructive disease, obesity N randomised: total: 127; home‐based cardiac rehabilitation: 66 (33 in brief exercise programme subgroup and 33 in extended subgroup); centre‐based cardiac rehabilitation: 61 (31 in brief subgroup and 30 in extended subgroup) Method of assessment: MI was documented by the combination of characteristic elevation of serum creatine kinase or oxaloacetic transaminase, a history of prolonged chest pain consistent with MI, and the appearance of new Q waves or evolutionary ST segment changes. Diagnosis (% of pts): Uncomplicated acute MI: 100% Age (mean ± SD): total: 52 ± 9 years; home‐based cardiac rehabilitation: NR; centre‐based cardiac rehabilitation: NR Percentage male: total: 100% Ethnicity: NR |
|
| Interventions |
Description of home‐based cardiac rehabilitation: Aerobic exercise: Modality: stationary cycling. Portable heart rate monitors and teletransmissions of ECG Dose: Length of session: 30 min/session Frequency/no of sessions: 5 sessions/week Intensity: 70% to 85% HRmax Resistance training included? NR Total duration: 8 weeks (brief) or 23 weeks (extended) Intermittent nurse or exercise specialist telephone support? 2 phone calls/week by staff to verify training intensity, clinical status and medication Co‐interventions: NR Description of centre‐based cardiac rehabilitation: Time of start after event: 3 weeks after infarction Components: Exercise only Aerobic exercise: Modality: walking/jogging; Group based and supervised Dose: Length of session: 60 mins/session Frequency/no of sessions: 5 sessions/week Intensity: 70% to 85% HRmax Resistance training included? NR Total duration: 8 weeks (brief) or 23 weeks (extended) Co‐interventions: NR |
|
| Outcomes | Exercise capacity; mortality and cardiovascular morbidity | |
| Follow up | 23 weeks post randomisation | |
| Source of funding | Grant HL18907 from the NHLBI, Bethesda, and by a grant from the PepsiCo Foundation, Purchase, NY | |
| Conflicts of interest | NR | |
| Notes | Results reported according to the two subgroups, i.e. brief versus extended exercise training and included into analysis separately | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Drop out reported; no imputation of missing data discussed |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods section are reported in the results |
| Groups balanced at baseline? | Unclear risk | Baseline characteristics not reported |
| Groups received same co‐intervention(s)? | Low risk | Both home and centre groups were very closely balanced in terms of the exercise training received |
Miller 1984 Expanded.
| Methods |
Study design: Single centre RCT No of centres: 1 Country: USA Dates patients recruited: NR When randomised: NR Maximum follow up: 23 weeks |
|
| Participants |
Inclusion criteria: Uncomplicated AMI (elevated serum creatinine kinase or oxaloacetic transaminase, prolonged chest pain consistent with AMI, new Q waves or evolutionary ST changes in ECG) Exclusion criteria: Unable to undertake exercise test, congestive heart failure, unstable angina pectoris, valvular heart disease, atrial fibrillation, bundle branch block, history of bypass, stroke, orthopaedic abnormalities, peripheral vascular disease, chronic pulmonary obstructive disease, obesity N randomised: total: 127; home‐based cardiac rehabilitation: 66 (33 in brief exercise programme subgroup and 33 in extended subgroup); centre‐based cardiac rehabilitation: 61 (31 in brief subgroup and 30 in extended subgroup) Method of assessment: MI was documented by the combination of characteristic elevation of serum creatine kinase or oxaloacetic transaminase, a history of prolonged chest pain consistent with MI, and the appearance of new Q waves or evolutionary ST segment changes. Diagnosis (% of pts): Uncomplicated acute MI: 100% Age (mean ± SD): total: 52 ± 9 years; home‐based cardiac rehabilitation: NR; centre‐based cardiac rehabilitation: NR Percentage male: total: 100% Ethnicity: NR |
|
| Interventions |
Description of home‐based cardiac rehabilitation: Aerobic exercise: Modality: stationary cycling. Portable heart rate monitors and teletransmissions of ECG Dose: Length of session: 30 min/session Frequency/no of sessions: 5 sessions/week Intensity: 70% to 85% HRmax Resistance training included? NR Total duration: 8 weeks (brief) or 23 weeks (extended) Intermittent nurse or exercise specialist telephone support? 2 phone calls/week by staff to verify training intensity, clinical status and medication Co‐interventions: NR Description of centre‐based cardiac rehabilitation: Time of start after event: 3 weeks after infarction Components: Exercise only Aerobic exercise: Modality: walking/jogging; Group based and supervised Dose: Length of session: 60 mins/session Frequency/no of sessions: 5 sessions/week Intensity: 70% to 85% HRmax Resistance training included? NR Total duration: 8 weeks (brief) or 23 weeks (extended) Co‐interventions: NR |
|
| Outcomes | Exercise capacity; mortality and cardiovascular morbidity | |
| Follow up | 23 weeks post randomisation | |
| Source of funding | Grant HL18907 from the NHLBI, Bethesda, and by a grant from the PepsiCo Foundation, Purchase, NY | |
| Conflicts of interest | NR | |
| Notes | Results reported according to the two subgroups, i.e. brief versus extended exercise training and included into analysis separately | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Drop out reported; no imputation of missing data discussed |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods section are reported in the results |
| Groups balanced at baseline? | Unclear risk | Baseline characteristics not reported |
| Groups received same co‐intervention(s)? | Low risk | Both home and centre groups were very closely balanced in terms of the exercise training received |
Moholdt 2012.
| Methods |
Study design: Single centre RCT No of centres: 1 Country: Norway Dates patients recruited: NR When randomised: 4 to 8 weeks after CABG surgery Maximum follow up: 6 months |
|
| Participants |
Inclusion criteria: Had coronary artery bypass surgery 4 to 8 weeks before enrolment and clinically stable (defined as the absence of unstable angina pectoris, symptoms of heart failure, pleural liquid limiting respiration, lung disease limiting respiration, ongoing infections, and atrial fibrillation limiting circulation) Exclusion criteria: Left ventricular ejection fraction < 30%, contraindications to vigorous physical activity (unstable angina, uncontrolled abnormal heart rhythms, severe aortic stenosis, suspected or known dissecting aneurysm, infection in the heart or any other systemic infection), pulmonary disease clearly limiting exercise capacity, pregnancy, or drug abuse N randomised: total: 30; home‐based cardiac rehabilitation: 14; centre‐based cardiac rehabilitation: 16 Diagnosis (% of pts): CABG: 100% Age (mean ± SD): total: 63 ± 77 years; home‐based cardiac rehabilitation: 61.7 ± 8.0 years; centre‐based cardiac rehabilitation: 63.6 ± 7.3 years Percentage male: total: 80%; home‐based cardiac rehabilitation: 78.6%; centre‐based cardiac rehabilitation: 81.3% Ethnicity: NR |
|
| Interventions |
Description of home‐based cardiac rehabilitation: Time of start after event: 4 to 8 weeks after CABG surgery Components: Exercise and education Aerobic exercise: Modality: walking, jogging, swimming or cycling (patient choice) Dose: Length of session: 38 min (10 min warm up, 4 x 4 min intervals of high intensity exercise, 4 x 3 min intervals of moderate intensity Frequency/no of sessions: 3 sessions/week Intensity: 70% HRmax (moderate intensity) to 85% to 95% HRmax (high intensity) Resistance training included? Total duration: 6 months Intermittent nurse or exercise specialist telephone support? Co‐interventions: Diet counselling, a smoking cessation program, lectures about healthy lifestyle in general. After discharge from the rehabilitation centre, the patients were advised to keep on exercising at home, and were invited back for follow up testing after 6 months. Description of centre‐based cardiac rehabilitation(residential rehabilitation): Time of start after event: 4 to 8 weeks after CABG surgery Components: Exercise and education Aerobic exercise: Modality: Outdoor walking, cross‐country skiing in winter time, indoor cycling, hall games. Dose: Length of session: NR Frequency/no of sessions: 30 exercise sessions with low intensity, 16 with moderate intensity, and 10 with high intensity Intensity: Up to 11 on the Borg scale (light intensity); 12 to 14 on the Borg scale (moderate intensity); and 15 to 17 on the Borg scale (high intensity) Resistance training included? strength training Total duration: 4 weeks Co‐interventions: Diet counselling, a smoking cessation program, lectures about healthy lifestyle in general. After discharge from the rehabilitation centre, the patients were advised to keep on exercising at home, and were invited back for follow up testing after 6 months. They did not receive a training diary or concrete advice about how to exercise on discharge. |
|
| Outcomes | Primary: peak oxygen consumption Secondary: HRQoL total, HDL cholesterol and triglycerides |
|
| Follow up | 6 months post randomisation | |
| Source of funding | EXTRA funds from the Norwegian Foundation for Health and Rehabilitation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript | |
| Conflicts of interest | The authors declared that no competing interests exist | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Allocation was done by a computer using block randomisation. The first, the smallest and the largest block, were defined by the technicians at the unit of Applied Clinical Research at the university" |
| Allocation concealment (selection bias) | Low risk | "The person including the patients got the allocation results on screen and by e‐mail by logging on to a website." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | CONSORT flow diagram shows loss to follow 4/30 (13%) at 6 months |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods section are reported in the results |
| Groups balanced at baseline? | Low risk | Although no statement of similarity of baseline characteristics, the provided characteristic of both groups appeared similar |
| Groups received same co‐intervention(s)? | Low risk | Co‐interventions received by both groups |
Oerkild 2011.
| Methods |
Study design: Single centre RCT No of centres: 1 Country: Denmark Dates patients recruited: January 2007 to July 2008 When randomised: NR Maximum follow up: 12 months |
|
| Participants | N = 36 pts home‐based intervention; N = 39 pts centre‐based intervention, 100% coronary heart disease, mean age home 74.4 (5.8), mean age centre 74.7 (5.9), 19 males: 17 females home, 26 males: 13 females centre Inclusion criteria: ≥ 65 years old with a ‘new’ event of coronary heart disease defined as AMI, percutaneous transluminal coronary intervention or CABG Exclusion criteria: mental disorders (dementia), social disorders (severe alcoholism and drug abuse), living at nursing home, language barriers and the use of wheelchair N randomised: total: 75; home‐based cardiac rehabilitation: 36; centre‐based cardiac rehabilitation: 39 Method of assessment: NR Medical history (% of pts): Previous MI: home‐based cardiac rehabilitation: 27.8%; centre‐based cardiac rehabilitation: 30.8% Previous PCI: home‐based cardiac rehabilitation: 19.4%; centre‐based cardiac rehabilitation: 18.0% Previous CABG: home‐based cardiac rehabilitation: 16.7%; centre‐based cardiac rehabilitation: 5.4% Heart failure LVEF ≤45%: home‐based cardiac rehabilitation: 38.9%; centre‐based cardiac rehabilitation: 30.8% Age (mean ± SD): total: NR; home‐based cardiac rehabilitation: 74.4 ± 5.8 years; centre‐based cardiac rehabilitation: 74.7 ± 5.9 years Percentage male: total: 60.0%; home‐based cardiac rehabilitation: 52.8%; centre‐based cardiac rehabilitation: 66.7% Ethnicity: NR |
|
| Interventions |
Description of home‐based cardiac rehabilitation: The exercise programmes were individualised but followed international recommendations. A physiotherapist individually tailored the exercise programmes. At 3 months when the intervention ceased, participants were encouraged to continue to exercise 30 min 6 days/week at an 11 to 13 on the Borg scale Time of start after event: NR ("new event") Components: Exercise and education Aerobic exercise: Modality: Self‐passed brisk walking and stationary cycling Dose: Length of session: 30 min Frequency/no of sessions: 6 days/week Intensity: 11 to 13 on a Borg scale Resistance training included? NR Total duration: 6 weeks Intermittent nurse or exercise specialist telephone support? A cardiologist counselled the patients at baseline and after 3, 6 and 12 months. At 4 and 5 months, a telephone call was made to answer any questions, regarding risk factor intervention and medical adjustment. Co‐interventions: Patients were offered six education lectures, two dietary counselling sessions, three practical cooking and (if needed) smoking cessation counselling sessions. Description of centre‐based cardiac rehabilitation: This consisted of a six week intensive programme where patients were offered group‐based supervised exercise training 60 min twice a week and were encouraged to exercise at home to comply with the international recommendations. As for the home programme, a physiotherapist individually tailored the exercise programmes. At 3 months when the intervention ceased, participants were encouraged to continue to exercise 30 min 6 days/week at 11 to 13 on the Borg scale Other: Time of start after event: NR Components: Individually tailored Aerobic exercise: Modality: e.g. running, cycling, skipping. Dose: Length of session: 60 min Frequency/no of sessions: 2 sessions/week Intensity: NR Resistance training included? NR Total duration: 6 weeks Co‐interventions: Patients were offered dietary counselling and (if needed) smoking cessation. A cardiologist counselled the patients at baseline and after 3, 6 and 12 months. At 4 and 5 months, a telephone call was made to answer any questions |
|
| Outcomes | Primary: exercise capacity (VO₂ and 6MWT) Secondary: systolic and diastolic blood pressure; cholesterol (total, HDL, LDL), smoking, HRQoL (SF‐12) |
|
| Follow up | 3 and 12 months | |
| Source of funding | The Velux Foundation | |
| Conflicts of interest | There were no conflicts of interest to declare | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Patients were randomised in alternate block sizes of four to six using computer‐generated randomly permuted blocks” |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Because of the nature of CR, the result of the randomisation could not be blinded and was therefore open to the investigator, involved health personnel and patients” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/75 (5%) drop out |
| Selective reporting (reporting bias) | Low risk | All outcomes outlined in the methods are reported in results |
| Groups balanced at baseline? | Low risk | "Baseline characteristics according to intervention...show no significant difference between the two groups. In addition, no significant differences were found in the use of medication and in socio‐demographic data” |
| Groups received same co‐intervention(s)? | Low risk | “The pharmacological treatment followed international guidelines and were thus identical in the two groups” “Regarding risk factor intervention and medical adjustment, a cardiologist counselled the patients both at home and in the centre intervention at baseline and after 3, 6 and 12 months.” |
Piotrowicz 2010.
| Methods |
Study design: Single centre RCT No of centres: 1 Country: Poland Dates patients recruited: NR When randomised: Following baseline measurements Maximum follow up: 8 weeks |
|
| Participants |
Inclusion criteria: (i) patients of either sex with any aetiology of left ventricular systolic HF (as defined in the European Society of Cardiology (ESC) guidelines) diagnosed for > 3 months; (ii) with a left ventricular ejection fraction ≤ 40% on echocardiography; (iii) in NYHA class II or III; (iv) who were clinically stable and receiving an optimal and stable medication regimen for at least 4 weeks before enrolment; and (v) who were able to exercise using the new model of home‐based exercise. Exclusion criteria: (i) NYHA class I or IV; (ii) unstable angina; (iii) a history of an acute coronary syndrome within the last month, coronary artery bypass grafting within the last 2 months, or initiation of cardiac resynchronisation therapy (CRT) within the last year; (iv) symptomatic and/or exercise‐induced cardiac arrhythmia or conduction disturbances; (v) valvular or congenital heart disease requiring surgical treatment; (vi) hypertrophic cardiomyopathy; (vii) severe pulmonary hypertension or other severe pulmonary disease; (viii) uncontrolled hypertension; (ix) anaemia (haemoglobin,10.0 g/dL); (x) acute and/or decompensated non‐cardiac disease; (xi) physical disability related to severe or neurological problems; (xii) acute or chronic inflammatory disease; (xiii) cancer; (xiv) severe psychiatric disorder; and (xv) patient refusal to participate. N randomised: total: 152; home‐based cardiac rehabilitation (tele‐monitored cardiac rehabilitation): 77; centre‐based cardiac rehabilitation (outpatient‐based standard cardiac rehabilitation): 75 Method of assessment: Two‐dimensional echocardiography Diagnosis (% of pts): Heart failure: 100% Ischaemic: home‐based cardiac rehabilitation: 73.3%; centre‐based cardiac rehabilitation: 85.7% Non‐ischaemic: home‐based cardiac rehabilitation: 26.7%; centre‐based cardiac rehabilitation: 14.3% MI: home‐based cardiac rehabilitation: 64.0%; centre‐based cardiac rehabilitation: 78.6% Age (mean ± SD): total: 58.1 ± 10.2 years; home‐based cardiac rehabilitation: 56.4 ± 10.9 years; centre‐based cardiac rehabilitation: 60.5 ± 8.8 years Percentage male: total: NR; home‐based cardiac rehabilitation: 85%; centre‐based cardiac rehabilitation: 95% Ethnicity: NR |
|
| Interventions |
Description of home‐based cardiac rehabilitation: To make the ET safe for HF patients, the following recommendations were taken into account: (i) special attention was paid to appropriate patient risk stratification before cardiac rehabilitation; (ii) contraindications to ET were never overlooked; (iii) in patients with an implantable cardioverter defibrillator (ICD), maximal training HR was set at 20 bpm lower than the defibrillator discharge threshold; and (iv) in patients with a pacemaker, the rate‐response function was switched on, enabling HR adjustment to the physical effort which facilitates reaching the desired training HR. Exercise training was planned individually for each patient during hospitalisation. The chosen workload reflected individual effort tolerance with regard to: (i) perceived exertion according to the Borg scale and (ii) the training HR range established individually for each patient. In line with the standards, the assumption was that patients should not exceed perceived moderate exertion during the ET (i.e. a score of 11 on the Borg scale). Components: Exercise, education and psychological Aerobic exercise: Modality: Continuous walking training on level ground Length of session: 20 to 45 min (i) warm‐up: 5 to 10mins (breathing and light exercises, callisthenics), (ii) basic aerobic endurance training for 10 to 30 mins (walking), and (iii) a 5 min cooling down (a period when patients could calm down and relax). Frequency/no of sessions: 3 sessions/week Intensity: Individually tailored Resistance training included? NR Total duration: 8 weeks Intermittent nurse or exercise specialist telephone support? NR Co‐interventions: All patients and partners participated in an education programme: how to measure HR, BP, and body weight; evaluate signs and symptoms; level perceived exertion and how to perform exercise training. Each patient received psychological support. Description of centre‐based cardiac rehabilitation: Components: Exercise, education and psychological Aerobic exercise: Modality: Cycle ergometer Dose: Length of session: 20 to 45 min (i) warm‐up: 5 to 10 min (breathing and light exercises, callisthenics), (ii) basic aerobic endurance training for 10 to 30 min (walking), and (iii) a 5 min cooling down (a period when patients could calm down and relax). Frequency/no of sessions: 3 sessions/week Intensity: Individually tailored Resistance training included? NR Total duration: 8 weeks Co‐interventions: All patients and partners participated in an education programme: how to measure HR, BP, and body weight; evaluate signs and symptoms; level perceived exertion and how to perform exercise training. Each patient received psychological support |
|
| Outcomes | Exercise capacity (6‐MWT), quality of life (SF‐36), mortality, hospitalisation | |
| Follow up | 8 weeks | |
| Source of funding | National Institute of Cardiology, Warsaw, Poland (study number 2.9/I/06) | |
| Conflicts of interest | "none declared" | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | CONSORT flow diagram shows 19/75 (25%) of centre‐based group and 2/77 (3%) of home‐based group failed to provide 8 week data; no imputation of missing data undertaken |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods section are reported in the results |
| Groups balanced at baseline? | Low risk | “At baseline there were no significant intergroup differences in terms of demographic and clinical parameters, NYHA functional class, echocardiographic parameters, 6‐MWT distance, functional capacity in [cardiopulmonary exercise testing], medical therapy, or the SF‐36 questionnaire score”. |
| Groups received same co‐intervention(s)? | Low risk | Both groups received some education and psychological support co‐intervention |
Sparks 1993.
| Methods |
Study design: Single centre RCT No of centres: 1 Country: USA Dates patients recruited: NR When randomised: NR Maximum follow up: 12 weeks |
|
| Participants |
Inclusion criteria: Male cardiac patient Exclusion criteria: Not capable of exercising on a bicycle ergometer, serious arrhythmias, symptoms of frequent chest pain, shortness of breath, hypertension N randomised: total: NR; home‐based cardiac rehabilitation 10; centre‐based cardiac rehabilitation: 10 Method of assessment: NR Diagnosis (% of pts): MI, CABG, PTCA Age (mean ± SD): total: 51.6 ± 12 years Percentage male: total: 100% Ethnicity: NR |
|
| Interventions |
Description of home‐based cardiac rehabilitation: Components: Exercise and education Aerobic exercise: Modality: cycle ergometer with trans‐telephonic ECG monitoring Dose: Length of session: 1 hour Frequency/no of sessions: 3 days/week Intensity: 60% to 75% peak HR Resistance training included? NR Total duration: 12 weeks Intermittent nurse or exercise specialist telephone support? trans‐telephonic ECG monitoring Co‐interventions: Education materials on diet, medications, risks and benefits of the exercise Description of centre‐based cardiac rehabilitation: Modality: cycle ergometer Dose: Length of session: 1 hour Frequency/no of sessions: 3 days/week Intensity: 60% to 75% peak HR Resistance training included? NR Total duration: 12 weeks Co‐interventions: Education materials on diet, medications, risks and benefits of the exercise |
|
| Outcomes | Exercise capacity (peak VO₂max); adherence (compliance with exercise); safety (drop out) | |
| Follow up | 12 weeks post randomisation | |
| Source of funding | NR | |
| Conflicts of interest | NR | |
| Notes | Data read from graphs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Blinding of assessors was not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1/20 (5%) drop out reported |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods section were reported in the results |
| Groups balanced at baseline? | Low risk | Although no statement of similarity of baseline characteristics, the characteristics presented appeared similar between groups |
| Groups received same co‐intervention(s)? | Low risk | Education materials on diet, medications, risks and benefits of the exercise given to both groups |
Varnfield 2014.
| Methods |
Study design: Multicentre RCT No of centres: 4 Country: Australia Dates patients recruited: 2009 to 2011 When randomised: Prior to baseline assessment Maximum follow up: 6 months |
|
| Participants |
Inclusion criteria: Post‐MI patients referred to cardiac rehabilitation Exclusion criteria: Unable to participate in self‐management programmes due to medical care needs, operate smart phone for purposes of trial (e.g. vision, hearing, cognitive or dexterity impairment) or attend TCR, or involved in another trial or had no experience with mobile/smart phones. N randomised: total: 120; intervention: 60; comparator: 60 Method of assessment: NR Diagnosis (% of pts): STEMI: home‐based cardiac rehabilitation: 49%; centre‐based cardiac rehabilitation: 56% NSTEMI: home‐based cardiac rehabilitation: 49%; centre‐based cardiac rehabilitation: 44% Angina: home‐based cardiac rehabilitation: 6%; centre‐based cardiac rehabilitation: 5% Heart failure: home‐based cardiac rehabilitation: 4%; centre‐based cardiac rehabilitation: 2% Bypass surgery: home‐based cardiac rehabilitation: 11%; centre‐based cardiac rehabilitation: 5% Angioplasty/stent: home‐based cardiac rehabilitation: 66%; centre‐based cardiac rehabilitation: 80% Age (mean ± SD): total: NR; home‐based cardiac rehabilitation: 54.9 ± 9.6 years; centre‐based cardiac rehabilitation: 56.2 ± 10.1 years Percentage male: total: NR; home‐based cardiac rehabilitation: 91%; centre‐based cardiac rehabilitation: 83% Ethnicity: NR |
|
| Interventions |
Description of home‐based cardiac rehabilitation: The Care Assessment Platform of Cardiac Rehabilitation (CAP‐CR) platform used a smart phone for health and exercise monitoring, and delivery of motivational and educational materials to participants via text messages and pre‐installed audio and video files (including understanding cardiovascular disease symptoms and management). The platform included a web portal with participant data for mentors to provide weekly consultations. Each participant was equipped with a smart phone pre‐installed with health diary and activity monitoring applications; blood pressure monitor; and weight scale. Activity monitoring (step number, duration and intensity) was automatic through the phone’s in‐built accelerometer. Participants were advised to make daily health diary entries: weight, BP, sleep duration and quality, exercise other than automatically monitored steps, stress, meals and, if relevant, alcohol consumption and smoking. Mentors reviewed updated data prior to weekly consultations. Time of start after event: Average = 54 days Components: Exercise and education Aerobic exercise: Modality: walking Dose: Length of session: Target = at least 30 min Frequency/no of sessions: Target = most days of the week Intensity: Borg’s scale 11 to 13 Resistance training included? No Total duration: 6 weeks Intermittent nurse or exercise specialist telephone support? Weekly consultations via the web portal to provide informed, personalised feedback according to goals set. Co‐interventions: Educational materials Description of centre‐based cardiac rehabilitation: The traditional, centre‐based programme (TCR) programme comprised of two supervised exercise and 1 h educational sessions on a weekly basis for 6 weeks at one of four Health Service District community centres. Participants started education sessions once enrolled to cardiac rehabilitation and twice‐weekly exercise sessions commenced once centre appointments became available. Time of start after event: Average = 68 days Components: Exercise and education Aerobic exercise: Modality: Circuit‐based exercise e.g. treadmill, rower, squats and modified push‐ups Dose: Length of session: NR Frequency/no of sessions: twice a week Intensity: Borg’s scale 6 to 10 (light) to 11 to 13 (moderate) Resistance training included? Resistance bands, weights Total duration: 6 weeks Co‐interventions: 1 h educational sessions on a weekly basis for 6 weeks |
|
| Outcomes | Adherence, risk factors (BP, heart rate, weight, BMI, waist circumference (WC), lipid profile), functional capacity and HRQoL Costs are reported separately by Whittaker and Wade 2014 |
|
| Follow up | 6 week and 6 month | |
| Source of funding | A joint venture between Australian eHealth Research Centre and Queensland Health | |
| Conflicts of interest | “None” | |
| Notes | 6 month outcome data provided by the author on request | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Permuted‐block randomisation, by computer‐generated random numbers…” |
| Allocation concealment (selection bias) | Low risk | “…using sequentially numbered opaque, sealed envelopes, was conducted” |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | “We conducted an unblinded RCT in four CR centres”. Blinding of assessors not described |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Home‐based cardiac rehabilitation: 14/60 (23.3 %) lost to follow‐up Centre‐based cardiac rehabilitation: 34/60 (56.7 %) lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes described in the methods were reported in the results section |
| Groups balanced at baseline? | Low risk | “There were no significant differences in baseline demographic and clinical characteristics of participants who commenced CR”. |
| Groups received same co‐intervention(s)? | Low risk | Both groups received educational materials or sessions |
Wu 2006.
| Methods |
Study design: Single centre RCT No of centres: 1 Country: Taiwan (China) Dates patients recruited: NR When randomised: NR Maximum follow up: 12 weeks |
|
| Participants |
Inclusion criteria: No previous CABG, no neurologic impairment like stroke/brain injury, no severe musculoskeletal disease, no complications during hospitalisations like infection, shock, arrhythmia, prolonged ventilation Exclusion criteria: uncontrolled dysrhythmia or continuous ventricular tachycardia during exercise testing, no possibility of completing test at discharge or 12 weeks later N randomised: total: 36; intervention: 18; comparator: 18 Diagnosis (% of pts): Post CABG: 100% Age (mean ± SD): total: 61.9 ± 7.3 years Percentage male: total: 100% Ethnicity: NR |
|
| Interventions |
Description of home‐based cardiac rehabilitation: Exercise documented in record book. Prescription of exercise individually given and updated every 2 weeks by rehabilitation nurse Components: Exercise only Aerobic exercise: Modality: fast walking or jogging Dose: Length of session: 30 to 60 min + 10 min warm‐up + 10 min cool‐down/session Frequency/no of sessions: ≥ 3 sessions/week Intensity: 60% to 85% HRmax Resistance training included? NR Total duration: 12 weeks Intermittent nurse or exercise specialist telephone support? NR Co‐interventions: NR Description of centre‐based cardiac rehabilitation: Exercise supervised by cardiopulmonary physical therapist Components: Exercise only Aerobic exercise: Modality: cycle ergometer, treadmill Dose: Length of session: 30 to 60 min + 10 min warm‐up + 10 min cool‐down/session Frequency/no of sessions: 3 sessions/week (total 36 sessions) Intensity: 60% to 85% HRmax Resistance training included? NR Total duration: 12 weeks Co‐interventions: NR |
|
| Outcomes | (Primary and secondary outcomes not distinguished) exercise capacity (METs) | |
| Follow up | 12 weeks post randomisation | |
| Source of funding | NR | |
| Conflicts of interest | NR | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Subjects were randomly assigned by drawing lots” |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not described |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | “The evaluators of the exercise stress test were also masked to the group assignments." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods section were reported in results |
| Groups balanced at baseline? | Low risk | “Randomization did not result in statistical significances among subjects assigned to the three groups.” |
| Groups received same co‐intervention(s)? | Low risk | Neither group received any co‐interventions |
6MWT = six minute walk test AMI = acute myocardial infarction BP = blood pressure BMI ‐ body mass index CABG = coronary artery bypass graft CAD = coronary artery disease CCU = coronary care unit CHD = coronary heart disease CHF = congestive heart failure ECG = electrocardiogram HF = heart failure HDL = high‐density lipoprotein HR = heart rate HRmax = maximum heart rate HRQoL = health related quality of life ISWT = incremental shuttle walking test ITT = intention to treat LDL = low‐density lipoprotein METs = metabolic equivalents MI = myocardial infarction min = minutes NR = not reported NYHA = New York Heart Association PTCA = percutaneous transluminal coronary angioplasty pts = participants RCT = randomised controlled trial RPE = rating of perceived exertion SF‐36 = Short Form (36) Health Survey SD = standard deviation VO₂max = maximal oxygen consumption
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ades 2000 | Not RCT |
| Austin 2005 | Not home‐ versus centre‐based cardiac rehabilitation comparison |
| Babu 2016 | Comparator group did not receive exercise‐based cardiac rehabilitation |
| Belardinelli 1999 | Not home‐ versus centre‐based cardiac rehabilitation comparison |
| Bubnova 2014 | Comparator group did not receive exercise‐based cardiac rehabilitation |
| Byrnes 2015 | Comparator group did not receive exercise‐based cardiac rehabilitation |
| Chan 2012 | Comparator group did not receive exercise‐based cardiac rehabilitation |
| Chen 2016 | Comparator group did not receive exercise‐based cardiac rehabilitation |
| Chien 2011 | Comparator group did not receive exercise‐based cardiac rehabilitation |
| Chow 2015 | Intervention not exercise‐based cardiac rehabilitation |
| Cinar 2015 | All patients had a Left ventricular Assist Device |
| Corvera‐Tindel 2004 | Not home‐ versus centre‐based cardiac rehabilitation comparison |
| Dracup 2007 | Not home‐ versus centre‐based cardiac rehabilitation comparison |
| Haddadzadeh 2011 | Comparator group did not receive exercise‐based cardiac rehabilitation |
| HF ACTION 2009 | Not home‐ versus centre‐based cardiac rehabilitation comparison |
| Higgins 2001 | Comparator group did not receive exercise‐based cardiac rehabilitation |
| Hovland‐Tanneryd 2016 | Intervention not exercise‐based cardiac rehabilitation |
| Jolly 2009 | Not home‐ versus centre‐based cardiac rehabilitation comparison |
| Khalife‐Zadeh 2015 | Intervention includes home‐ and centre‐based cardiac rehabilitation |
| Kim 2011 | Not a RCT |
| Lear 2014 | Comparator group did not receive exercise‐based cardiac rehabilitation |
| Lee 2013 | Comparator group did not receive exercise‐based cardiac rehabilitation |
| Maddison 2015 | Comparator group did not receive formal exercise‐based cardiac rehabilitation |
| Maru 2015 | Intervention not exercise‐based cardiac rehabilitation |
| McKelvie 2002 | Not home‐ versus centre‐based cardiac rehabilitation comparison |
| Moosavi‐Sohroforouzani 2015 | Not a RCT |
| Mutwalli 2012 | Not home‐ versus centre‐based cardiac rehabilitation comparison |
| Oka 2000 | Relevant outcomes not reported |
| Olson 2015 | Comparator group did not receive exercise‐based cardiac rehabilitation |
| Pfaeffli 2015 | Intervention not exercise‐based cardiac rehabilitation |
| Piotrowicz 2015 | Comparator group did not receive exercise‐based cardiac rehabilitation |
| Salavati 2016 | Comparator group did not receive exercise‐based cardiac rehabilitation |
| Senuzun 2006 | Trial experimental arm received home‐based cardiac rehabilitation; the programme issued in control arm was not described |
| Siabani 2016 | Intervention not exercise‐based cardiac rehabilitation |
| Sinclair 2005 | Trial experimental arm received home‐based cardiac rehabilitation, while the control group did not receive centre based cardiac rehabilitation (only 6% (N = 12) of the participants in the control group were referred to cardiac rehabilitation and only 3% (N = 8) were known to have attended) |
| Takase 2015 | Comparator group did not receive exercise‐based cardiac rehabilitation |
| Tygesen 2001 | Both trial arms received home‐based cardiac rehabilitation |
| Vahedian‐Azimi 2016 | Intervention not exercise‐based cardiac rehabilitation |
| Vibulchai 2016 | Intervention not exercise‐based cardiac rehabilitation |
| Wolkanin‐Bartnik 2011 | Intervention not exercise‐based cardiac rehabilitation |
| Xueyu 2015 | Comparator group did not receive exercise‐based cardiac rehabilitation |
RCT = randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Doletsky 2013.
| Methods | RCT |
| Participants | 70 patients during 3 to 14 days after planned PCI were randomized into three groups |
| Interventions | 1. Ambulatory training in the hospital 3 times per week with ECG control for 8 weeks. 2. One hospital based ECG‐controlled training session followed within the first week with 2 home‐based training sessions on stationary bike under simultaneous tele‐ECG and video‐control with use of Skype via Internet. This was followed by home‐based sessions with patient's weekly phone reports and training diaries. 3. Uncontrolled home‐based training. |
| Outcomes | Peak VO₂ |
| Notes | We were unable to trace authors or find full publication of this conference abstract |
Gelati 2013.
| Methods | RCT |
| Participants | 46 patients, aged 60 years (range 38 ‐75), with Left Ventricle Dysfunction, stable with optimal treatment, with EF< 45%. The patients were in sinusal rhythm. |
| Interventions | Subjects were randomized and stratified to 3 groups: Group 1: 16 patients at high intensity aerobic exercise training, warmed up for 10 minutes at 60‐70% of PHR of exercise test, before walking 4 minute intervals at 90‐95% of PHR of exercise test. Each interval was separated by 3 minutes active pauses, walking at 70% of PHR. Total exercise time was 38 minutes. Group 2: 14 patients at moderate continuous training, walked at 70‐75% of PHR; Group 3: 16 patients at home standard training (control group). The rehabilitation protocol was, 3 times per week for 24 sessions (groups 1 e 2). All patients at the end of the cardiac rehabilitation or after two months (group 3), repeated the baseline tests. |
| Outcomes | Adverse effects (arrhythmias, myocardial ischaemic events and/or symptoms); Nt‐pro BNP; 6MWT |
| Notes | We were unable to trace authors or find full publication of this conference abstract |
Pomeshkina 2012.
| Methods | RCT |
| Participants | 112 patients (mean age 56.8 ± 5.5 years) with coronary artery disease (CAD), 1 month post‐CABG |
| Interventions | Group 1 with supervised cycling training (SCT) (N = 35), Group 2 ‐ home‐based walking training (HBWT) (N = 36) Group 3 ‐ comparison group (N = 41). Subjects did 3 trainings per week for 3 months |
| Outcomes | Adherence to medication |
| Notes | We were unable to find full publication of this conference abstract. Authors were emailed, but no reply was received. |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12616000426482.
| Trial name or title | SMARTphone‐based, early cardiac REHABilitation in patients with acute coronary syndromes: A Randomized Controlled Trial Protocol [SMART‐REHAB Trial] |
| Methods | Randomised, parallel assignment, single blinded |
| Participants |
Inclusion criteria:
Exclusion criteria:
|
| Interventions | The smart phone‐based secondary prevention program will be delivered over 8 weeks starting at time of discharge from hospital through a smart phone application (app). This is a multi‐faceted intervention with particular emphasis on early mobilisation. The app provides a platform to deliver a comprehensive secondary prevention program. The different components of the program include an Exercise Prescription Control group is assigned to usual post‐discharge acute coronary syndrome care which includes traditional cardiac rehabilitation |
| Outcomes | Exercise capacity; risk factors HRQoL |
| Starting date | 04/04/2016 |
| Contact information | Dr Matia Yudi matias.yudi@austin.org.au |
| Notes |
Maddison 2014.
| Trial name or title | The remote exercise monitoring trial for exercise‐based cardiac rehabilitation (REMOTE‐CR): a randomised controlled trial protocol |
| Methods | A two‐arm, parallel, non‐inferiority, randomised controlled trial will be conducted at two sites in New Zealand |
| Participants | 162 adults aged 18 years or more,with a diagnosis of CHD (angina, myocardial infarction, percutaneous coronary intervention or coronary revascularisation) within the previous six months. Participants are current outpatients who have been clinically stable for at least six weeks, are able to perform exercise, and can understand and write English |
| Interventions | 12‐week program of technology‐assisted, home‐based, remote monitored exercise‐based cardiac rehabilitation (intervention) versus 8 to 12 week program of standard supervised exercise‐based cardiac rehabilitation (control) |
| Outcomes | V̇O₂max; cardiovascular risk factors; HRQoL; costs |
| Starting date | Registered 7 August 2014 |
| Contact information | r.maddison@auckland.ac.nz |
| Notes | Study ID number: ACTRN12614000843651 |
NCT01567189.
| Trial name or title | Cost‐effectiveness of Outpatient Versus Hospital Cardiac Rehabilitation (CERC1) |
| Methods | Randomised, parallel assignment, open label |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | Hospital‐based cardiac rehabilitation versus home‐based cardiac rehabilitation |
| Outcomes | Morbidity, re‐admissions, percutaneous or surgical revascularisation, costs |
| Starting date | April 2012 |
| Contact information | Fernando Aros Borau, LUISFDO.AROSBORAU@osakidetza.net |
| Notes |
NCT02047942.
| Trial name or title | Telerehabilitation in Coronary Heart Disease (TRiCH) |
| Methods | Randomised, parallel assignment, single blind |
| Participants | 105 participants Inclusion criteria:
Exclusion criteria:
|
| Interventions | Centre‐based cardiac rehabilitation versus Home‐based training with telemonitoring guidance |
| Outcomes | Exercise tolerance; comparison of evolution of exercise tolerance from baseline to 12 weeks and one year |
| Starting date | February 2014 |
| Contact information | Luc Vanhees, PhD, luc.vanhees@faber.kuleuven.be |
| Notes |
NCT02711631.
| Trial name or title | Feasibility and Effectiveness of Remote Virtual Reality‐Based Cardiac Rehabilitation |
| Methods | Randomised, parallel assignment, single blind |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | MedBIKE ‐ exercise cardiac rehabilitation system that allows participants to perform clinical cardiac rehabilitation at home versus standard cardiac rehabilitation |
| Outcomes | Fitness; compliance |
| Starting date | May 2016 |
| Contact information | Contact: Peter W Wood, MSc; pwwood@ualberta.ca |
| Notes |
NCT02791685.
| Trial name or title | Smartphone Delivered In‐home Cardiopulmonary Rehabilitation |
| Methods | Randomised, parallel assignment, open label |
| Participants | Inclusion criteria: Meet eligibility for cardiac rehabilitation program as defined by Centres for Medicare and Medicaid Services (CMS)
|
| Interventions | MULTIFIT CR program delivered by the Movn smart phone application versus centre‐based CR |
| Outcomes | Six minute walk test; HRQoL |
| Starting date | 2 June 2016 |
| Contact information | Abarmard Zafari, MD, azafari@emory.edu |
| Notes |
NCT02796404.
| Trial name or title | Homebased Monitoring Cardiac Rehabilitation Program (NUUBO) |
| Methods | Randomised, parallel assignment, open label |
| Participants | Inclusion criteria: All of the following:
and at least one of the following:
|
| Interventions | Home‐based cardiac rehabilitation program versus traditional cardiac rehabilitation program |
| Outcomes | Cardiovascular risk factors; functional capacity; adherence; safety |
| Starting date | Aug 2015 |
| Contact information | Raquel Bravo, MD, rbravoescobar@yahoo.es |
| Notes |
NTR5156.
| Trial name or title | Effects of cardiac telerehabilitation in patients with coronary artery disease using a personalized patient‐centred ICT platform: the SmartCare‐CAD study. |
| Methods | Randomised, parallel assignment |
| Participants | Inclusion criteria:
Exclusion criteria:
|
| Interventions | The core component of the study intervention is a secured and personalized patient‐centred web‐based ICT platform. This platform enables patients to register, evaluate and adjust rehabilitation goals, training goals and medication and to upload and inspect exercise training and daily physical activity data (as measured by a heart rate monitor and accelerometer). After three supervised in‐hospital training sessions, patients are given the opportunity to continue exercise training at home, based on prescriptions from their physical therapist. Comparator: Centre based cardiac rehabilitation, consisting of one or more of the following treatments: exercise training, an information program, a relaxation program, psycho‐educative prevention program and/or individual treatment by a psychologist or dietician. Exercise training sessions are performed under direct supervision of a physical therapist specialised in cardiac rehabilitation |
| Outcomes | HRQoL, cost effectiveness |
| Starting date | 01/06/2015 |
| Contact information | Hareld MC Kemps, Máxima Medical Centre, Department of Sport Medicine, P.O. Box 7777 5500 MB Veldhoven The Netherlands; H.Kemps@wxs.nl |
| Notes |
Differences between protocol and review
To reflect current practice and terminology, “percutaneous transluminal coronary angioplasty” (PTCA) was replaced by “percutaneous coronary intervention” (PCI), a term which encompasses the use of balloons, stents and atherectomy.
The order of primary and secondary outcomes has been updated, for clarity.
Due to the increase in the number of studies included in this review, we undertook meta‐regression analysis to examine potential treatment effect modifiers and the text has been updated to reflect this change.
Finally, we created a 'Summary of findings' table using the following outcomes: total mortality, exercise capacity, withdrawal and health‐related quality of life.
Contributions of authors
LA undertook the study selection, data extraction and risk of bias assessment, and led the writing of the updated review.
HD, KJ, AZ, SGD and RJN contributed to a previous version of the review and contributed to the editing of this updated review.
GAS undertook data extraction and risk of bias assessment and contributed to the editing of this updated review.
RST contributed to the original and previous versions of the review, led the analysis of this review and contributed to the editing of the updated review.
The final manuscript was approved by all authors.
Sources of support
Internal sources
No sources of support supplied
External sources
NIHR Cochrane Heart Programme grant, UK.
-
Transparency of the National Health System Drug Reimbursement Decisions, Poland.
co‐financed by EU
Declarations of interest
LA is an author on number of other Cochrane cardiac rehabilitation reviews.
RST, HD, KJ and AC are investigators on randomised controlled trials included in this review. RST, HD and KJ are chief investigators/co‐applicants on an ongoing National Institute for Health Research (NIHR) Programme Grants for Applied Research funded study ‐ Rehabilitation Enablement in Chronic Heart Failure (REACH‐HF) ‐ to develop and evaluate the costs and outcomes of a home‐based self help heart failure rehabilitation manual (RP‐PG‐1210‐12004) http://medicine.exeter.ac.uk/research/healthserv/primarycare/projects/reach‐hf/.
SJD's position at the University of Exeter Medical School is partially supported by the National Institute for Health Research (NIHR) Collaboration for Leadership in Applied Health Research and Care (CLAHRC) for the South West Peninsula. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health in England. The textbook 'Interprofessional Rehabilitation: a person‐centred approach' has a section on adherence in rehabilitation, drawing upon earlier work than this Cochrane Review.
KJ is part funded by NIHR CLAHRC‐WM.
RJN, AZ and GAS declare that they have no conflicts of interest.
Edited (no change to conclusions)
References
References to studies included in this review
Aamot 2014 Group {published data only}
- Aamot IL, Forbord SH, Gustad K, Løckra V, Stensen A, Berg AT, et al. Home‐based versus hospital‐based high‐intensity interval training in cardiac rehabilitation: a randomized study. European Journal of Preventative Cardiology 2014;21(9):1070‐8. [DOI] [PubMed] [Google Scholar]
- Aamot IL, Forbord SH, Løckra V, Gustad K, Stensen A, Berg AT, et al. Implementation of aerobic interval training in cardiac rehabilitation: a randomized clinical study. European Journal of Preventive Cardiology 2012;1:S60. [DOI] [PubMed] [Google Scholar]
Aamot 2014 Treadmill {published data only}
- Aamot IL, Forbord SH, Gustad K, Løckra V, Stensen A, Berg AT, et al. Home‐based versus hospital‐based high‐intensity interval training in cardiac rehabilitation: a randomized study. European Journal of Preventative Cardiology 2014;21(9):1070‐8. [DOI] [PubMed] [Google Scholar]
- Aamot IL, Forbord SH, Løckra V, Gustad K, Stensen A, Berg AT, et al. Implementation of aerobic interval training in cardiac rehabilitation: a randomized clinical study. European Journal of Preventive Cardiology 2012;1:S60. [DOI] [PubMed] [Google Scholar]
Arthur 2002 {published data only}
- Arthur HM, Smith KM, Kodis J, McKelvie R. A controlled trial of hospital versus home‐based exercise in cardiac patients. Medicine and Science in Sports and Exercise 2002;34(10):1544‐50. [DOI] [PubMed] [Google Scholar]
- Smith KM, Arthur HM, McKelvie RS, Kodis J. Differences in sustainability of exercise and health‐related quality of life outcomes following home or hospital‐based cardiac rehabilitation. European Journal of Cardiovascular Prevention and Rehabilitation 2004;11(4):313‐9. [DOI] [PubMed] [Google Scholar]
- Smith KM, McKelvie RS, Thorpe KE, Arthur HM. Six‐year follow‐up of a randomised controlled trial examining hospital versus home‐based exercise training after coronary artery bypass graft surgery. Heart 2011;97(14):1169‐74. [DOI] [PubMed] [Google Scholar]
Bell 1998 {published data only}
- Bell JM. A comparison of a multi‐disciplinary home based cardiac rehabilitation programme with comprehensive conventional rehabilitation in post‐myocardial infarction patients [PhD thesis]. London (UK): University of London, 1998. [Google Scholar]
Carlson 2000 {published data only}
- Carlson J. A comparison of traditional and modified cardiac rehabilitation protocols on compliance to exercise, patient self‐efficacy, cardiovascular outcomes, and program cost [PhD thesis]. East Lansing (MI, USA): Michigan State University, 1999. [Google Scholar]
- Carlson JJ, Johnson JA, Franklin BA, VanderLaan RL. Program participation, exercise adherence, cardiovascular outcomes, and program cost of traditional versus modified cardiac rehabilitation. American Journal of Cardiology 2000;86(1):17‐23. [DOI] [PubMed] [Google Scholar]
- Carlson JJ, Norman GJ, Feltz DL, Franklin BA, Johnson JA, Locke S. Self‐efficacy, psychosocial factors, and exercise behavior in traditional versus modified cardiac rehabilitation. Journal of Cardiopulmonary Rehabilitation 2001;21(6):363‐73. [DOI] [PubMed] [Google Scholar]
Cowie 2012 {published data only}
- Cowie A, Moseley O. Home‐ versus hospital‐based exercise training in heart failure: an economic analysis. British Journal of Cardiology 2014;21:76. [Google Scholar]
- Cowie A, Thow MK, Granat MH, Mitchell SL. A comparison of home and hospital‐based exercise training in heart failure: immediate and long‐term effects upon physical activity level. European Journal of Cardiovascular Prevention and Rehabilitation 2011;18(2):158‐66. [DOI] [PubMed] [Google Scholar]
- Cowie A, Thow MK, Granat MH, Mitchell SL. Effects of home versus hospital‐based exercise training in chronic heart failure. International Journal of Cardiology 2012;158(2):296‐8. [DOI] [PubMed] [Google Scholar]
Dalal 2007 {published and unpublished data}
- Dalal HM, Evans PH, Campbell JL, Taylor RS, Watt A, Read KL, et al. Home‐based versus hospital‐based rehabilitation after myocardial infarction: a randomized trial with preference arms ‐ Cornwall Heart Attack Rehabilitation Management Study (CHARMS). International Journal of Cardiology 2007; Vol. 119, issue 2:202‐11. [DOI] [PubMed]
- Taylor RS, Watt A, Dalal HM, Evans PH, Campbell JL, Read KL, et al. Home‐based cardiac rehabilitation versus hospital‐based rehabilitation: a cost effectiveness analysis. International Journal of Cardiology 2007;119(2):196‐201. [DOI] [PubMed] [Google Scholar]
Daskapan 2005 {published data only}
- Daskapan A, Arikan H, Caglar N, Tunali N, Ataman S. Comparison of supervised exercise training and home‐based exercise training in chronic heart failure. Saudi Medical Journal 2005;26(5):842‐7. [PubMed] [Google Scholar]
- Daskapan A, Arikan H, Caglar N, Turkman N. The effects of two different exercise programs on pulmonary function tests and quality of life in chronic heart failure: a pilot study [Kronik kalp yetmezliginde iki farkli egzersiz programinm solunum fonksiyon testleri ve yasam kalitesi uzerine etkileri: pilot calisma]. Turk Fizyoterapi Rehabilitasyon Gergisi [Turkish Journal of Physiotherapy and Rehabilitation] 2005;16(2):74‐81. [Google Scholar]
Gordon 2002 Community {published data only}
- Gordon NF, English CD, Contractor AS, Salmon RD, Leighton RF, Franklin BA, et al. Effectiveness of three models for comprehensive cardiovascular disease risk reduction. American Journal Cardiology 2002;89(11):1263‐8. [DOI] [PubMed] [Google Scholar]
Gordon 2002 Supervised {published data only}
- Gordon NF, English CD, Contractor AS, Salmon RD, Leighton RF, Franklin BA, et al. Effectiveness of three models for comprehensive cardiovascular disease risk reduction. American Journal of Cardiology 2002;89(11):1263‐8. [DOI] [PubMed] [Google Scholar]
Grace 2016 Mixed {published data only}
- Grace SL, Midence L, Oh P, Brister S, Chessex C, Stewart DE, et al. Cardiac rehabilitation program adherence and functional capacity among women: a randomized controlled trial. Mayo Clinic Proceedings 2016;91(2):140‐8. [DOI] [PubMed] [Google Scholar]
- Midence L, Arthur HM, Oh P, Stewart DE, Grace SL. Women's health behaviours and psychosocial well‐being by cardiac rehabilitation program model: a randomized controlled trial. Canadian Journal of Cardiology 2016;32(8):956‐62. [DOI] [PubMed] [Google Scholar]
- Samayoa L, Arthur HM, Oh P, Brister S, Chessex C, Grace SL. Cardiac rehabilitation program adherence among women following referral to different program models: a randomized controlled trial. Circulation 2014;130(Suppl 2):A19230‐A19230. [Google Scholar]
Grace 2016 Women {published data only}
- Grace SL, Midence L, Oh P, Brister S, Chessex C, Stewart DE, et al. Cardiac rehabilitation program adherence and functional capacity among women: a randomized controlled trial. Mayo Clinic Proceedings 2016;91(2):140‐8. [DOI] [PubMed] [Google Scholar]
- Midence L, Arthur HM, Oh P, Stewart DE, Grace SL. Women's health behaviours and psychosocial well‐being by cardiac rehabilitation program model: a randomized controlled trial. Canadian Journal of Cardiology 2016;32(8):956‐62. [DOI] [PubMed] [Google Scholar]
- Samayoa L, Arthur HM, Oh P, Brister S, Chessex C, Grace SL. Cardiac rehabilitation program adherence among women following referral to different program models: A randomized controlled trial. Circulation. Conference: American Heart Association. 2014; Vol. 130.
Hadadzadeh 2013 {unpublished data only}
- Haddadzadeh MH, Maiya AG, Padmakumar R, et al. Effectiveness of hospital versus home‐based cardiac rehabilitation on left ventricular ejection fraction in post‐PTCA patients: a randomized controlled trial. Journal of Cardiopulmonary Rehabilitation and Prevention 2013;33(5):328‐39. [Google Scholar]
Hadadzadeh 2015 {published data only}
- Hadadzadeh MH, Maiya AG, Shad B, Mirbolouk F, Kumar RP, Borkar SS, et al. Home versus hospital‐based exercise training and associated improvements in functional capacity and quality of life in post‐event coronary artery disease patients: an indo‐Iranian multi‐center randomized controlled trial. Circulation 2015;131:AP280.. [Google Scholar]
Jolly 2007 {published and unpublished data}
- Jolly K, Lip GY, Sandercock J, Greenfield SM, Raftery JP, Mant J, et al. Home‐based versus hospital‐based cardiac rehabilitation after myocardial infarction or revascularisation: design and rationale of the Birmingham Rehabilitation Uptake Maximisation Study (BRUM): a randomised controlled trial [ISRCTN72884263]. BMC Cardiovascular Disorders 2003;3:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jolly K, Lip GY, Taylor RS, Raftery JP, Mant JW, Lane D, et al. The Birmingham Rehabilitation Uptake Maximisation study (BRUM): a randomised controlled trial comparing home‐based with centre‐based cardiac rehabilitation. Heart 2009;95(1):36‐42. [DOI] [PubMed] [Google Scholar]
- Jolly K, Taylor R, Lip GY, Greenfield S, Raftery J, Mant J, et al. The Birmingham Rehabilitation Uptake Maximisation Study (BRUM). Home‐based compared with hospital‐based cardiac rehabilitation in a multi‐ethnic population: cost‐effectiveness and patient adherence. Health Technology Assessment 2007; Vol. 11, issue 35:1‐118. [DOI] [PubMed]
Karapolat 2009 {published data only}
- Karapolat H, Demir E, Bozkaya YT, Eyigor S, Nalbantgil S, Durmaz B, et al. Comparison of hospital‐based versus home‐based exercise training in patients with heart failure: effects on functional capacity, quality of life, psychological symptoms, and hemodynamic parameters. Clinical Research in Cardiology 2009;98(10):635‐42. [DOI] [PubMed] [Google Scholar]
Kassaian 2000 {published data only}
- Kassaian M, Maleki M, Noohi F, Eftekharzadeh M, Arya A, Roshanali F, et al. Comparing effects of supervised vs. home‐based cardiac rehabilitation. Iranian Heart Journal 2000;1(2):95‐102. [Google Scholar]
Kraal 2014 {published data only}
- Kraal JJ, Peek N, Akker‐Van Marle ME, Kemps HMC. Effects of home‐based training with telemonitoring guidance in low to moderate risk patients entering cardiac rehabilitation. European Heart Journal 2015;36:633. [Google Scholar]
- Kraal JJ, Peek N, Akker‐Van Marle ME, Kemps H. Effects and costs of home‐based training with telemonitoring guidance in low to moderate risk patients entering cardiac rehabilitation: The FIT@Home study. BMC Cardiovascular Disorders 2013;13(1):82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraal JJ, Peek N, Akker‐Van Marle ME, Kemps HM. Effects of home‐based training with telemonitoring guidance in low to moderate risk patients entering cardiac rehabilitation: short‐term results of the FIT@Home study. European Journal of Preventive Cardiology 2014;21(2 Suppl):26‐31. [DOI] [PubMed] [Google Scholar]
Marchionni 2003 {published and unpublished data}
- Marchionni N, Fattirolli F, Fumagalli S, Oldridge N, Lungo F, Morosi L, et al. Improved exercise tolerance and quality of life with cardiac rehabilitation of older patients after myocardial infarction: results of a randomized, controlled trial. Circulation 2003; Vol. 107, issue 17:2201‐6. [DOI] [PubMed]
Miller 1984 Brief {published data only}
- DeBusk RF, Haskell WL, Miller NH, Berra K, Taylor CB, Berger WE III, et al. Medically directed at‐home rehabilitation soon after clinically uncomplicated acute myocardial infarction: a new model for patient care. American Journal of Cardiology 1985;55(4):251‐7. [DOI] [PubMed] [Google Scholar]
- Miller NH, Haskell WL, Berra K, DeBusk RF. Home versus group exercise training for increasing functional capacity after myocardial infarction. Circulation 1984;70(4):645‐9. [DOI] [PubMed] [Google Scholar]
- Taylor CB, Houston‐Miller N, Ahn DK, Haskell W, DeBusk RF. The effects of exercise training programs on psychosocial improvement in uncomplicated postmyocardial infarction patients. Journal of Psychosomatic Research 1986;30(5):581‐7. [DOI] [PubMed] [Google Scholar]
Miller 1984 Expanded {published data only}
- DeBusk RF, Haskell WL, Miller NH, Berra K, Taylor CB, Berger WE III, et al. Medically directed at‐home rehabilitation soon after clinically uncomplicated acute myocardial infarction: a new model for patient care. The American Journal of Cardiology 1985;55(4):251‐7. [DOI] [PubMed] [Google Scholar]
- Miller NH, Haskell WL, Berra K, DeBusk RF. Home versus group exercise training for increasing functional capacity after myocardial infarction. Circulation 1984;70(4):645‐9. [DOI] [PubMed] [Google Scholar]
- Taylor CB, Houston‐Miller N, Ahn DK, Haskell W, DeBusk RF. The effects of exercise training programs on psychosocial improvement in uncomplicated postmyocardial infarction patients. Journal of Psychosomatic Research 1986;30(5):581‐7. [DOI] [PubMed] [Google Scholar]
Moholdt 2012 {published data only}
- Moholdt T, Bekken Vold M, Grimsmo J, Slørdahl SA, Wisløff U. Home‐based aerobic interval training improves peak oxygen uptake equal to residential cardiac rehabilitation: a randomized, controlled trial. PLoS One 2012;7:e41199. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oerkild 2011 {published data only}
- Oerkild B, Frederiksen M, Hansen JF, Simonsen L, Skovgaard LT, Prescott E. Home‐based cardiac rehabilitation is as effective as centre‐based cardiac rehabilitation among elderly with coronary heart disease: results from a randomised clinical trial. Age and Ageing 2011;40(1):78‐85. [DOI] [PubMed] [Google Scholar]
Piotrowicz 2010 {published data only}
- Piotrowicz E, Baranowski R, Bilinska M, Stepnowska M, Piotrowska M, Wójcik A, et al. A new model of home‐based telemonitored cardiac rehabilitation in patients with heart failure: effectiveness, quality of life, and adherence. European Journal of Heart Failure 2010;12(2):164‐71. [DOI] [PubMed] [Google Scholar]
- Piotrowicz E, Stepnowska M, Leszczynska‐Iwanicka K, Piotrowska D, Kowalska M, Tylka J, et al. Quality of life in heart failure patients undergoing home‐based telerehabilitation versus outpatient rehabilitation ‐ a randomized controlled study. European Journal of Cardiovascular Nursing 2015;14(3):256‐63. [DOI] [PubMed] [Google Scholar]
Sparks 1993 {published data only}
- Sparks KE, Shaw DK, Eddy D, Hanigosky P, Vantrese J. Alternatives for cardiac rehabilitation patients unable to return to a hospital‐based program. Heart & Lung 1993;22(4):298‐303. [PubMed] [Google Scholar]
Varnfield 2014 {published data only}
- Varnfield M, Karunanithi M, Lee CK, Honeyman E, Arnold D, Ding H, et al. Smartphone‐based home care model improved use of cardiac rehabilitation in postmyocardial infarction patients: results from a randomised controlled trial. Heart 2014;100(22):1770‐9. [DOI] [PubMed] [Google Scholar]
- Walters D, Varnfield M, Karunanithi M, Ding H, Honeyman E, Arnold D, et al. Technology based home‐care model improves outcomes of uptake, adherence and health in cardiac rehabilitation. Heart, Lung and Circulation 2012;21:S315. [Google Scholar]
- Whittaker F, Wade V. The costs and benefits of technology‐enabled, home‐based cardiac rehabilitation measured in a randomised controlled trial. Journal of Telemedicine and Telecare 2014;20(7):419‐22. [DOI] [PubMed] [Google Scholar]
Wu 2006 {published data only}
- Wu SK, Lin YW, Chen CL, Tsai SW. Cardiac rehabilitation vs. home exercise after coronary artery bypass graft surgery: a comparison of heart rate recovery. American Journal of Physical Medicine and Rehabilitation 2006;85(9):711‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Ades 2000 {published data only}
- Ades PA, Pashkow FJ, Fletcher G, Pina IL, Zohman LR, Nestor JR. A controlled trial of cardiac rehabilitation in the home setting using electrocardiographic and voice transtelephonic monitoring. American Heart Journal 2000;139(3):543‐8. [DOI] [PubMed] [Google Scholar]
Austin 2005 {published data only}
- Austin J, Williams R, Ross L, Moseley L, Hutchison S. Randomised controlled trial of cardiac rehabilitation in elderly patients with heart failure. European Journal of Heart Failure 2005;7(3):411‐7. [DOI] [PubMed] [Google Scholar]
- Austin J, Williams WR, Hutchison S. Multidisciplinary management of elderly patients with chronic heart failure: five year outcome measures in death and survivor groups. European Journal of Cardiovascular Nursing 2009;8(1):34‐9. [DOI] [PubMed] [Google Scholar]
- Austin J, Williams WR, Ross L, Hutchison S. Five‐year follow‐up findings from a randomized controlled trial of cardiac rehabilitation for heart failure. European Journal of Cardiovascular Prevention and Rehabilitation 2008;15(2):162‐7. [DOI] [PubMed] [Google Scholar]
Babu 2016 {published data only}
- Babu AS, Desai CV, Maiya AG, Guddattu V, Padmakumar R. Changes in derived measures from six‐minute walk distance following home‐based exercise training in congestive heart failure: a preliminary report. Indian Heart Journal 2016; Vol. 68, issue 4:527‐8. [DOI] [PMC free article] [PubMed]
Belardinelli 1999 {published data only}
- Belardinelli R, Georgiou D, Cianci G, Purcaro A. Randomized, controlled trial of long‐term moderate exercise training in chronic heart failure: effects on functional capacity, quality of life, and clinical outcome. Circulation 1999;99(9):1173‐82. [DOI] [PubMed] [Google Scholar]
- Georgiou D, Chen Y, Appadoo S, Belardinelli R, Greene R, Parides MK, et al. Cost‐effectiveness analysis of long‐term moderate exercise training in chronic heart failure. American Journal of Cardiology 2001;87(8):984‐8. [DOI] [PubMed] [Google Scholar]
Bubnova 2014 {published data only}
- Bubnova MG, Aronov DM, Krasnitskii VB, Ioseliani DG, Novikova NK, Rodzinskaia EM. A home exercise training program after acute coronary syndrome and/or endovascular coronary intervention: efficiency and a patient motivation problem. Terapevticheskii Arkhiv 2014;86(1):23‐32. [PubMed] [Google Scholar]
Byrnes 2015 {published data only}
- Byrnes J, Carrington M, Chan YK, Pollicino C, Dubrowin N, Stewart S, et al. Cost‐effectiveness of a home based intervention for secondary prevention of readmission with chronic heart disease. PLoS One 2015;10(12):e0144545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart S, Carrington MJ, Chan YK, Calderone A, Goldstein S, Scuffham P. Long‐term impact of a nurse‐led, home‐based, prevention program for hospitalised cardiac patients on risk of secondary events: The multicentre young @ heart randomised controlled trial. European Heart Journal 2012;33:443‐4. [Google Scholar]
Chan 2012 {published data only}
- Chan Y, Stewart S, Calderone A, Scuffham P, Goldstein S, Carrington M. Optimising secondary cardiovascular prevention in privately insured cardiac patients: The young @ heart multicentre randomised controlled trial. Heart, Lung and Circulation 2012;21:S311‐2. [Google Scholar]
Chen 2016 {published data only}
- Chen JT, Lin TH, Voon WC, Lai WT, Huang MH, Sheu SH, et al. Beneficial effects of home‐based cardiac rehabilitation on metabolic profiles in coronary heart‐disease patients. Kaohsiung Journal of Medical Sciences 2016;32(5):267‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chien 2011 {published data only}
Chow 2015 {published data only}
- Chow CK, Redfern J, Hillis GS, Thakkar J, Santo K, Hackett ML, et al. Effect of lifestyle‐focused text messaging on risk factor modification in patients with coronary heart disease: a randomized clinical trial. Journal of the American Medical Association 2015;314(12):1255‐63. [DOI] [PubMed] [Google Scholar]
Cinar 2015 {unpublished data only}
- Cinar E, Karapolat H, Capaci K, Engin C, Yagdi T, Ozbaran M, et al. The effect of cardiac rehabilitation on functional capacity, psychological symptoms and quality of life in patients with a left ventricular assist device. Journal of Heart and Lung Transplantation. 2016; Vol. 4 Suppl:S395.
Corvera‐Tindel 2004 {published data only}
- Corvera‐Tindel T, Doering LV, Woo MA, Khan S, Dracup K. Effects of a home walking exercise program on functional status and symptoms in heart failure. American Heart Journal 2004;147(2):339‐46. [DOI] [PubMed] [Google Scholar]
Dracup 2007 {published data only}
- Dracup K, Evangelista LS, Hamilton MA, Erickson V, Hage A, Moriguchi J, et al. Effects of a home‐based exercise program on clinical outcomes in heart failure. American Heart Journal 2007;154(5):877‐83. [DOI] [PubMed] [Google Scholar]
- Evangelista LS, Doering LV, Lennie T, Moser DK, Hamilton MA, Fonarow GC, et al. Usefulness of a home‐based exercise program for overweight and obese patients with advanced heart failure. American Journal of Cardiology 2006;97(6):886‐90. [DOI] [PubMed] [Google Scholar]
- Evangelista LS, Hamilton MA, Fonarow GC, Dracup K. Is exercise adherence associated with clinical outcomes in patients with advanced heart failure?. Physician and Sportsmedicine 2010;38(1):28‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Haddadzadeh 2011 {published data only}
- Haddadzadeh MH, Maiya AG, Padmakumar R, Shad B, Mirbolouk F. Effect of exercise‐based cardiac rehabilitation on ejection fraction in coronary artery disease patients: a randomized controlled trial. Heart Views 2011;12(2):51‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddadzadeh MH, Maiya AG, Shad B, Mirbolouk F, Padma Kumar R. Effectiveness of cardiac rehabilitation on left ventricular ejection fraction in post‐coronary event patients: A successful model for developing countries. Canadian Journal of Cardiology 2011;1(5 Suppl):S242. [Google Scholar]
HF ACTION 2009 {published data only}
- Flynn KE, Piña IL, Whellan DJ, Lin L, Blumenthal JA, Ellis SJ, et al. Effects of exercise training on health status in patients with chronic heart failure: HF‐ACTION randomized controlled trial. JAMA 2009;301(14):1451‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keteyian SJ, Leifer ES, Houston‐Miller N, Kraus WE, Brawner CA, O'Connor CM, et al. Relation between volume of exercise and clinical outcomes in patients with heart failure. Journal of the American College of Cardiology 2012;60(19):1899‐905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor CM, Whellan DJ, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF‐ACTION randomized controlled trial. JAMA 2009;301(14):1439‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed SD, Li Y, Dunlap ME, Kraus WE, Samsa GP, Schulman KA, et al. In‐hospital resource use and medical costs in the last year of life by mode of death (from the HF‐ACTION randomized controlled trial). American Journal of Cardiology 2012;110(8):1150‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed SD, Whellan DJ, Li Y, Friedman JY, Ellis SJ, Pina IL, et al. Economic evaluation of the HF‐ACTION (Heart failure: A controlled trial investigating outcomes of exercise training) randomized controlled trial: An exercise training study of patients with chronic heart failure. Circulation: Cardiovascular Quality and Outcomes 2010;3:374‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whellan DJ, O'Connor CM, Lee KL, Keteyian SJ, Cooper LS, Ellis SJ, et al. Heart failure and a controlled trial investigating outcomes of exercise training (HF‐ACTION): design and rationale. American Heart Journal 2007;153(2):201‐11. [DOI] [PubMed] [Google Scholar]
Higgins 2001 {published data only}
- Higgins HC, Hayes RL, McKenna KT. Rehabilitation outcomes following percutaneous coronary interventions (PCI). Patient Education and Counseling 2001;43(3):219‐30. [DOI] [PubMed] [Google Scholar]
Hovland‐Tanneryd 2016 {published data only}
- Hovland‐Tanneryd AHT, Hagglund EH, Ullman BU, Persson HP, Hagerman IH, Michael MM. PACEMAN‐HF‐pooled analysis from two randomized controlled trials. European Journal of Heart Failure 2016;18:55. [Google Scholar]
Jolly 2009 {published data only}
- Jolly K, Taylor RS, Lip GY, Davies M, Davis R, Mant J, et al. A randomized trial of the addition of home‐based exercise to specialist heart failure nurse care: the Birmingham Rehabilitation Uptake Maximisation study for patients with Congestive Heart Failure (BRUM‐CHF) study. European Journal of Heart Failure 2009;11(2):205‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Khalife‐Zadeh 2015 {published data only}
- Khalife‐Zadeh A, Dorri S, Shafiee S. The effect of cardiac rehabilitation on quality of life in patients with acute coronary syndrome. Iranian Journal of Nursing and Midwifery Research 2015;20(5):588‐93. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2011 {published data only}
- Kim C, Youn JE, Choi HE. The effect of a self exercise program in cardiac rehabilitation for patients with coronary artery disease. Annals of Rehabilitation Medicine 2011;35(3):381‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lear 2014 {published data only}
- Lear SA, Singer J, Banner‐Lukaris D, Horvat D, Park JE, Bates J, et al. Randomized trial of a virtual cardiac rehabilitation program delivered at a distance via the internet. Circulation. Cardiovascular Quality and Outcomes 2014; Vol. 7, issue 6:952‐9. [DOI] [PubMed]
- Lear SA, Singer J, Banner‐Lukaris D, Horvat D, Park JE, Bates J, et al. Randomized trial of a virtual cardiac rehabilitation program delivered at a distance via the internet. Studies in Health Technology and Informatics 2014; Vol. 7, issue 6:952‐9. [DOI] [PubMed]
Lee 2013 {published data only}
- Lee Y, Lee J, Seo H, Kim K, Min D, Lee J, et al. Effects of home‐based exercise training with wireless monitoring on the left ventricular function of acute coronary syndrome patients. Journal of Physical Therapy Science 2013;25(5):631‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Maddison 2015 {published data only}
- Maddison R, Pfaeffli L, Whittaker R, Stewart R, Kerr A, Jiang Y, et al. A mobile phone intervention increases physical activity in people with cardiovascular disease: Results from the HEART randomized controlled trial. European Journal of Preventive Cardiology 2015;22(6):701‐9. [DOI] [PubMed] [Google Scholar]
- Pfaeffli DL, Whittaker R, Dixon R, Stewart R, Jiang Y, Carter K, et al. Acceptability of a mobile health exercise‐based cardiac rehabilitation intervention: a randomized trial. Journal of Cardiopulmonary Rehabilitation and Prevention 2015;35(5):312‐9. [DOI] [PubMed] [Google Scholar]
Maru 2015 {published data only}
- Maru S, Byrnes J, Carrington MJ, Chan YK, Thompson DR, Stewart S, et al. Cost‐effectiveness of home versus clinic‐based management of chronic heart failure: Extended follow‐up of a pragmatic, multicentre randomized trial cohort ‐ The WHICH? study (Which Heart Failure Intervention Is Most Cost‐Effective & Consumer Friendly in Reducing Hospital Care). International Journal of Cardiology 2015;201:368‐75. [DOI] [PubMed] [Google Scholar]
- Newton P, Davidson P, Macdonald P, Stewart S. Why chronic heart failure management programs are so important: Results of the which intervention is most cost‐effective and consumer friendly in reducing hospital care? (WHICH?) trial. Heart, Lung and Circulation 2012;21:S7. [DOI] [PubMed] [Google Scholar]
- Stewart S, Carrington M, Marwick T, Davidson P, Macdonald P, Horowitz J, et al. Which heart failure intervention is most cost‐effective and consumer friendly in reducing hospital care (which?): A multicentre randomised controlled trial. Heart, Lung and Circulation 2012;21:S98‐9. [DOI] [PubMed] [Google Scholar]
McKelvie 2002 {published data only}
- McKelvie RS, Teo KK, Roberts R, McCartney N, Humen D, Montague T, et al. Effects of exercise training in patients with heart failure: the Exercise Rehabilitation Trial (EXERT). American Heart Journal 2002;144(1):23‐30. [DOI] [PubMed] [Google Scholar]
Moosavi‐Sohroforouzani 2015 {published data only}
- IRCT2014092319272N1. Comparison of home‐based exercise rehabilitation with centre‐based cardiac rehabilitation on lipid parameters, hematology and some behavioral indicators with coronary artery disease. www.irct.ir/searchresult.php?keyword=IRCT2014092319272N1&id=19272&number=1&field=a&prt=1&total=1&m=1 (first received 24 March 2015).
- Moosavi‐Sohroforouzani A, Esfarjani F, Sadeghi M, Heidari H. Comparing the effects of home‐based exercise rehabilitation and center‐based cardiac rehabilitation on lipid profiles of the patients with coronary artery disease. Feyz 2015;19(2):135‐43. [Google Scholar]
- Moosavi‐Sohroforouzani A, Esfarjani F, Sadeghi M, Rohafza HR. Assessing psychological factors after home‐based versus centre‐based cardiac rehabilitation in patients with coronary artery disease. Journal of Urmia Nursing and Midwifery Faculty 2015;13(9):‐. [Google Scholar]
Mutwalli 2012 {published data only}
- Mutwalli HA, Fallows SJ, Arnous AA, Zamzami MS. Randomized controlled evaluation shows the effectiveness of a home‐based cardiac rehabilitation program. Saudi Medical Journal 2012;33(2):152‐9. [PubMed] [Google Scholar]
Oka 2000 {published data only}
- Oka RK, Marco T, Haskell WL, Botvinick E, Dae MW, Bolen K, et al. Impact of a home‐based walking and resistance training program on quality of life in patients with heart failure. American Journal of Cardiology 2000;85(3):365‐9. [DOI] [PubMed] [Google Scholar]
Olson 2015 {published data only}
- Olson L, Kapadia S, Lexvold N, Somers V, Friedman P, Schenck L, et al. Remote wireless telemonitoring combined with health coaching (Tele‐HC) to lower readmission rates for patients with acute decompensated heart failure. Journal of Cardiac Failure 2015;1:S47. [Google Scholar]
Pfaeffli 2015 {published data only}
- Pfaeffli DL, Whittaker R, Jiang Y, Stewart R, Rolleston A, Maddison R. Text message and internet support for coronary heart disease self‐management: results From the Text4Heart randomized controlled trial. Journal of Medical Internet Research 2015;17(10):e237. [DOI] [PMC free article] [PubMed] [Google Scholar]
Piotrowicz 2015 {published data only}
- Piotrowicz E, Zielilski T, Bodalski R, Rywik T, Dobraszkiewicz‐Wasilewska B, Sobieszczalska‐Malek M, et al. Home‐based telemonitored Nordic walking training is well accepted, safe, effective and has high adherence among heart failure patients, including those with cardiovascular implantable electronic devices: A randomised controlled study. European Journal of Preventive Cardiology 2015;22(11):1368‐77. [DOI] [PubMed] [Google Scholar]
Salavati 2016 {published data only}
- Salavati M, Fallahinia G, Vardanjani AE, Rafiei H, Mousavi S, Torkamani M. Comparison between effects of home based cardiac rehabilitation programs versus usual care on the patients' health related quality of life after coronary artery bypass graft. Global Journal of Health Science 2016;8(4):196‐202. [DOI] [PMC free article] [PubMed] [Google Scholar]
Senuzun 2006 {published data only}
- Senuzun F, Fadiloglu C, Burke L, Payzin S. Effects of home‐based cardiac exercise program on the exercise tolerance, serum lipid values and self‐efficacy of coronary patients. European Journal of Cardiovascular Prevention and Rehabilitation 2006;13(4):640‐5. [DOI] [PubMed] [Google Scholar]
Siabani 2016 {published data only}
- Siabani S, Driscoll T, Davidson PM, Leeder SR. Efficacy of a home‐based educational strategy involving community health volunteers in improving self‐care in patients with chronic heart failure in western Iran: A randomized controlled trial. European Journal of Cardiovascular Nursing 2016;15(5):363‐71. [DOI] [PubMed] [Google Scholar]
Sinclair 2005 {published data only}
- Sinclair A, Conroy S, Davies M, Bayer A. Post‐discharge home‐based support for older cardiac patients: a randomised controlled trial. Age and Ageing 2005;34(4):338‐43. [DOI] [PubMed] [Google Scholar]
Takase 2015 {published data only}
- Takase K, Matsuo Y, Yanagisawa Y, Higashine K, Oda M, Manabe M, et al. Efficacy of a home‐based exercise program for recently hospitalized chronic heart failure patients. Physiotherapy 2015;101:eS1477. [Google Scholar]
Tygesen 2001 {published data only}
- Tygesen H, Wettervik C, Wennerblom B. Intensive home‐based exercise training in cardiac rehabilitation increases exercise capacity and heart rate variability. International Journal of Cardiology 2001;79(2‐3):175‐82. [DOI] [PubMed] [Google Scholar]
Vahedian‐Azimi 2016 {published data only}
- Vahedian‐Azimi A, Miller AC, Hajiesmaieli M, Kangasniemi M, Alhani F, Jelvehmoghaddam H, et al. Cardiac rehabilitation using the Family‐Centered Empowerment Model versus home‐based cardiac rehabilitation in patients with myocardial infarction: a randomised controlled trial. Open Heart 2016;3(1):e000349. [DOI] [PMC free article] [PubMed] [Google Scholar]
Vibulchai 2016 {published data only}
Wolkanin‐Bartnik 2011 {published data only}
- Wolkanin‐Bartnik J, Pogorzelska H. The effect of patient education on home‐based rehabilitation on physical fitness in patients over 60 after acute myocardial infarction. European Heart Journal 2010;31:377. [Google Scholar]
- Wolkanin‐Bartnik J, Pogorzelska H, Bartnik A. Patient education and quality of home‐based rehabilitation in patients older than 60 years after acute myocardial infarction. Journal of Cardiopulmonary Rehabilitation and Prevention 2011;31(4):249‐53. [DOI] [PubMed] [Google Scholar]
Xueyu 2015 {published data only}
- Xueyu Li, Shunlin Xu, Lijuan Zhou, Rongbin Li, Jianrong Wang. Home‐based exercise in older adults recently discharged from the Hhospital for cardiovascular disease in China. Nursing Research 2015;64(4):246‐55. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Doletsky 2013 {published data only}
- Doletsky A, Andreev DA, Martynova VV, Svet AV. Skype and Tele‐ECG in control of home‐based exercise training after planned PCI‐a useful tool?. European Journal of Preventive Cardiology 2014;1:42. [Google Scholar]
- Doletsky AA, Andreev DA, Minaeva VA, Dikur ON, Giverts IY, Gurova AV, et al. Outpatient and controlled home‐based physical training early after planned PCI. European Journal of Preventive Cardiology 2013;1:S139. [Google Scholar]
Gelati 2013 {published data only}
- Gelati A, Rossetti A, Maddaluna A, Picelli A, Limongelli G, Adone G, et al. High intensity interval training reduces NT‐proBNP in heart failure patients. European Journal of Preventive Cardiology 2013;1:S28. [Google Scholar]
Pomeshkina 2012 {published data only}
- Pomeshkina S, Loktionova E, Arkhipova N, Barbarash O. Home‐based walking training and adherence to medical therapy in patients undergoing coronary artery bypass grafting. European Heart Journal 2012;35:634. [Google Scholar]
References to ongoing studies
ACTRN12616000426482 {unpublished data only}
- ACTRN12616000426482. SMARTphone‐based, early cardiac REHABilitation in patients with acute coronary syndromes: A Randomized Controlled Trial Protocol [SMART‐REHAB Trial]. www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12616000426482 (first received 30 March 2016).
Maddison 2014 {published data only}
- Maddison R, Rawstorn JC, Rolleston A, Whittaker R, Stewart R, Benatar J, et al. The remote exercise monitoring trial for exercise‐based cardiac rehabilitation (REMOTE‐CR): a randomised controlled trial protocol. BMC Public Health 2014;14:1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT01567189 {unpublished data only}
- NCT01567189. Cost‐effectiveness of outpatient versus hospital cardiac rehabilitation (CERC1) [Outpatient cardiac rehabilitation versus hospital: cost‐effectiveness study]. clinicaltrials.gov/ct2/show/NCT01567189 (first received 22 February 2012).
NCT02047942 {unpublished data only}
- NCT02047942. Telerehabilitation in Coronary Heart Disease (TRiCH) [A Randomized Controlled Trial Comparing Home‐based Training With Telemonitoring Guidance Versus Center‐based Cardiac Rehabilitation in Patients With Coronary Heart Disease: the TRiCH‐study]. clinicaltrials.gov/ct2/show/NCT02047942 (first received 27 January 2014).
NCT02711631 {unpublished data only}
- NCT02711631. Feasibility and effectiveness of remote virtual reality‐based cardiac rehabilitation. clinicaltrials.gov/ct2/show/NCT02711631 (first received 17 April 2015).
NCT02791685 {unpublished data only}
- NCT02791685. Smartphone delivered in‐home cardiopulmonary rehabilitation. clinicaltrials.gov/ct2/show/NCT02791685 (first received 2 June 2016).
NCT02796404 {unpublished data only}
- NCT02796404. Homebased Monitoring Cardiac Rehabilitation Program (NUUBO) [Effectiveness of a Homebased Cardiac Rehabilitation Program of Mixed Surveillance Using NUUBO Monitoring Vest in Patients With Ischemic Heart Disease at Moderate Cardiovascular Risk]. clinicaltrials.gov/ct2/show/NCT02796404 (first received 23 May 2016).
NTR5156 {unpublished data only}
- NTR5156. Effects of cardiac telerehabilitation in patients with coronary artery disease using a personalized patient‐centred ICT platform: the SmartCare‐CAD study. trialregister.nl/trialreg/admin/rctview.asp?TC=5156 (first received 22 April 2015).
Additional references
Anderson 2016
- Anderson L, Thompson DR, Oldridge N, Zwisler AD, Rees K, Martin N, et al. Exercise‐based cardiac rehabilitation for coronary heart disease. Cochrane Database of Systematic Reviews 2016, Issue 1. [DOI: 10.1002/14651858.CD001800.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Artinian 2007
- Artinian N. Telehealth as a tool for enhancing care for patients with cardiovascular disease. Journal of Cardiovascular Nursing 2007;22(1):25–31. [DOI] [PubMed] [Google Scholar]
BACPR 2012
- British Association for Cardiovascular Prevention and Rehabilitation (BACPR). The BACPR Standards and Core Components for Cardiovascular Disease Preventionand Rehabilitation. 2nd Edition. http://www.bacpr.com/resources/46C_BACPR_Standards_and_Core_Components_2012.pdf (accessed 14 March 2015).
Balady 2011
- Balady GJ, Ades PA, Bittner VA, Franklin BA, Gordon NF, Thomas RJ, et al. Referral, enrollment, and delivery of cardiac rehabilitation/secondary prevention programs at clinical centers and beyond: a presidential advisory from the American Heart Association. Circulation 2011;124(25):2951‐60. [DOI] [PubMed] [Google Scholar]
Bergner 1976
- Bergner M, Bobbitt RA, Pollard WE, Martin DP, Gilson BS. The sickness impact profile: validation of a health status measure. Medical Care 1976;14(1):57‐67. [DOI] [PubMed] [Google Scholar]
Beswick 2004
- Beswick AD, Rees K, Griebsch I, Taylor FC, Burke M, West RR, et al. Provision, uptake and cost of cardiac rehabilitation programmes: improving services to under‐represented groups. Health Technology Assessment 2004;8(41):1‐166. [DOI] [PubMed] [Google Scholar]
Brown 2013
- Brown JP, Clark AM, Dalal H, Welch K, Taylor RS. Effect of patient education in the management of coronary heart disease: a systematic review and meta‐analysis of randomised controlled trials. European Journal of Preventive Cardiology 2013;20(4):701‐14. [DOI] [PubMed] [Google Scholar]
Buckley 2013
- Buckley JP, Furze G, Doherty P, Speck L, Connolly S, Hinton S, et al. BACPR scientific statement: British standards and core components for cardiovascular disease prevention and rehabilitation. Heart 2012;99(15):169‐71. [DOI] [PubMed] [Google Scholar]
Clark 2012
- Clark AM, King‐Shier KM, Thompson DR, Spaling MA, Duncan AS, Stone JA, et al. A qualitative systematic review of influences on attendance at cardiac rehabilitation programs after referral. American Heart Journal 2012;164(6):835‐45. [DOI] [PubMed] [Google Scholar]
Collins 2001
- Collins L, Scuffham P, Gargett S. Cost‐analysis of gym‐based versus home‐based cardiac rehabilitation programs. Australian Health Review 2001;24(1):51‐61. [DOI] [PubMed] [Google Scholar]
Corrà 2005
- Corrà U, Giannuzzia P, Adamopoulos S, Bjornstad Bjarnason‐Wehernsd HB, Cohen‐Solale A, Dugmore D, et al. Executive Summary of the Position Paper of the Working Group on Cardiac Rehabilitation and Exercise Physiology of the European Society of Cardiology (ESC): core components of cardiac rehabilitation in chronic heart failure. European Journal of Cardiovascular Prevention and Rehabilitation 2005;12(4):32‐105. [DOI] [PubMed] [Google Scholar]
Crawford‐Faucher 2010
- Crawford‐Faucher A. Home‐ and center‐based cardiac rehabilitation equally effective. American Family Physician 2010;82(8):994‐5. [Google Scholar]
Dalal 2012
- Dalal HM, Wingham J, Palmer J, Taylor RS. Why do so few patients with heart failure participate in cardiac rehabilitation? A cross‐sectional survey from England, Wales and Northern Ireland. BMJ Open 2012;2(2):e000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
Davies 2014
- Davies EJ, Moxham T, Rees K, Singh S, Coats AJ, Ebrahim S, et al. Exercise based rehabilitation for heart failure. Cochrane Database of Systematic Reviews 2010, Issue 4. [DOI: 10.1002/14651858.CD003331.pub4] [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Dickens 2013
- Dickens C, Cherrington A, Adeyemi I, Roughley K, Bower P, Garrett C, et al. Characteristics of psychological interventions that improve depression in people with coronary heart disease: a systematic review and meta‐regression. Psychosomatic Medicine 2103;72(2):211‐21. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneiger M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
European Cardiovascular Disease Statistics 2017
- European Heart Network. European Cardiovascular Disease Statistics 2017. ehnheart.org/cvd‐statistics.html (accessed 30 May 2017).
EuroQoL 1990
- EuroQol Group. EuroQol ‐ a new facility for the measurement of health‐related quality of life. Health Policy 1990;16(3):199‐208. [DOI] [PubMed] [Google Scholar]
ExTraMatch 2004
- Piepoli MF, Davos C, Francis DP, Coats AJ. Exercise training meta‐analysis of trials in patients with chronic heart failure (ExTraMATCH). BMJ 2004;328(7433):189. [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADEpro GDT 2015 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version (accessed prior to 12 June 2017). Hamilton (ON): GRADE Working Group, McMaster University, 2015.
Gravely‐Witte 2007
- Gravely‐Witte S, Gucht V, Heiser W, Grace SL, Elderen T. The impact of angina and cardiac history on health‐related quality of life and depression in coronary heart disease patients. Chronic Illness 2007;3(1):66‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hambrecht 1998
- Hambrecht R, Fiehn E, Weigl C, Gielen S, Hamann C, Kaiser R, et al. Regular physical exercise corrects endothelial dysfunction and improves exercise capacity in patients with chronic heart failure. Circulation 1998;98(24):2709‐15. [DOI] [PubMed] [Google Scholar]
Haykowsky 2007
- Haykowsky MJ, Liang Y, Pechter D, Jones LW, McAlister FA, Clark AM. A meta‐analysis of the effect of exercise training on left ventricular remodeling in heart failure patients: the benefit depends on the type of training performed. Journal of the American College of Cardiology 2007;49(24):2329‐36. [DOI] [PubMed] [Google Scholar]
Heart Manual 2016
- Heart Manual Office. The Heart Manual. theheartmanual.com/Pages/default.aspx. Edinburgh: Heart Manual Office, (accessed 29 November 2016).
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available at www.cochrane.org/training/cochrane‐handbook. [Google Scholar]
Huang 2015
- Huang K, Liu W, He D, Huang B, Xiao D, Peng Y, et al. Telehealth interventions versus center‐based cardiac rehabilitation of coronary artery disease: A systematic review and meta‐analysis. European Journal of Preventive Cardiology 2015;22(8):959‐71. [DOI] [PubMed] [Google Scholar]
Hunt 1980
- Hunt SM, McEwan T. The development of a subjective health indicator. Sociology of Health and Illness 1980;2:231‐246. [DOI] [PubMed] [Google Scholar]
Hwang 2015
- Hwang R, Bruning J, Morris N, Mandrusiak A, Russell T. A systematic review of the effects of telerehabilitation in patients with cardiopulmonary diseases. Journal of Cardiopulmonary Rehabilitation and Prevention 2015;35(6):380‐9. [DOI] [PubMed] [Google Scholar]
Höfer 2004
- Höfer S, Lim L, Guyatt G, Oldridge N. The MacNew Heart Disease health‐related quality of life instrument: a summary. Health Quality of Life Outcomes 2004;2:3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jolly 2006
- Jolly K, Taylor RS, Lip GY, Stevens A. Home‐based cardiac rehabilitation compared with centre‐based rehabilitation and usual care: a systematic review and meta‐analysis. International Journal of Cardiology 2006;111(3):343‐51. [DOI] [PubMed] [Google Scholar]
Karmali 2014
- Karmali KN, Davies P, Taylor F, Beswick A, Martin N, Ebrahim S. Promoting patient uptake and adherence in cardiac rehabilitation. Cochrane Database of Systematic Reviews 2014, Issue 6. [DOI: 10.1002/14651858.CD007131.pub3] [DOI] [PubMed] [Google Scholar]
Kostis 1997
- Kostis JB, Davis BR, Cutler J, Grimm RH Jr, Berge KG, Cohen JD, et al. Prevention of heart failure by antihypertensive drug treatment in older persons with isolated systolic hypertension. SHEP Cooperative Research Group. JAMA 1997;278(3):212‐6. [PubMed] [Google Scholar]
Kulik 2015
- Kulik A, Ruel M, Jneid H, Ferguson TB, Hiratzka LF, Ikonomidis JS, et al. Secondary prevention after coronary artery bypass graft surgery: a scientific statement from the American Heart Association. Circulation 2015;131(10):927‐64. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville JC. Chapter 6: Searching for studies. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). Available from www.cochrane‐handbook.org. The Cochrane Collaboration, 2011. [Google Scholar]
Lewin 1992
- Lewin B, Robertson IH, Cay EL, Irving JB, Campbell M. Effects of self‐help post‐myocardial‐infarction rehabilitation on psychological adjustment and use of health services. Lancet 1992;339(8800):1036‐40. [DOI] [PubMed] [Google Scholar]
McHorney 1993
- McHorney CA, Ware JE Jr, Raczek AE. The MOS 36‐Item Short‐Form Health Survey (SF‐36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Medical Care 1993;31(3):246‐63. [DOI] [PubMed] [Google Scholar]
McMurray 2012
- McMurray JV, Adamopoulos S, Anker SD, Auricchio A, Böhm M, Dickstein K, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012 The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. European Journal of Heart Failure 2012;14(8):803‐69. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta‐Analyses: The PRISMA Statement. PLOS Medicine 2009;6(7):e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
NACR 2013
- National Audit of Cardiac Rehabilitation (NACR). The National Audit of Cardiac Rehabilitation Annual Statistical Report 2013. cardiacrehabilitation.org.uk/docs/2013.pdf (accessed 7 December 2016).
NACR 2016
- National Audit of Cardiac Rehabilitation (NACR). The National Audit of Cardiac Rehabilitation Annual Statistical Report 2016. cardiacrehabilitation.org.uk/nacr/docs/BHF_NACR_Report_2016.pdf (accessed 7 December 2016).
Neubeck 2009
- Neubeck L, Redfern J, Fernandez R, Briffad T, Baumane A, Freedman SB. Telehealth interventions for the secondary prevention of coronary heart disease: a systematic review. European Journal of Cardiovascular Prevention and Rehabilitation 2009;16(3):281‐9. [DOI] [PubMed] [Google Scholar]
NICE 2010
- National Institute for Care and health Excellence (NICE). Chronic heart failure in adults: management. CG108. nice.org.uk/guidance/cg108 (accessed prior to 12 June 2017).
NICE 2013
- National Institute for Health and Care Excellence (NICE). Myocardial infarction: cardiac rehabilitation and prevention of further cardiovascular disease. CG172. nice.org.uk/guidance/cg172 (accessed prior to 12 June 2017). [PubMed]
Nicholls 2012
- Nichols M, Townsend N, Luengo‐Fernandez R, Leal J, Gray A, Scarborough P, et al. European Cardiovascular Disease Statistics. Brussels: European Heart Network, Brussels, European Society of Cardiology, Sophia Antipolis, 2012. [Google Scholar]
Piepoli 1998
- Piepoli M, Maugeri FS, Campana M, Ferrari R, Giordano A, Scalvini S, et al. Experience from controlled trials of physical training in chronic heart failure. Protocol and patient factors in effectiveness in the improvement in exercise tolerance. European Heart Journal 1998;19(3):466‐75. [DOI] [PubMed] [Google Scholar]
Piepoli 2010
- Piepoli MF, Corrà U, Benzer W, Bjarnason‐Wehrens B, Dendale P, Gaita D, et al. Secondary prevention through cardiac rehabilitation: from knowledge to implementation. A position paper from the Cardiac Rehabilitation Section of the European Association of Cardiovascular Prevention and Rehabilitation. European Journal of Cardiovascular Prevention and Rehabilitation 2010;17(1):1‐17. [DOI] [PubMed] [Google Scholar]
Piepoli 2015
- Piepoli MF, Binno S, Corrà U, Seferovic P, Conraads V, Jaarsma T, et al. Committee on Exercise Physiology & Training of theHeart Failure Association of the ESC. ExtraHF survey: the first European survey on implementation of exercise training in heart failure patients. European Journal of Heart Failure 2015;17:631–638. [DOI] [PubMed] [Google Scholar]
Racca 2010
- Racca V, Spezzaferri R, Modica M, Mazzini P, Jonsdottir J, Maria R, et al. Functioning and disability in ischaemic heart disease. Disability and Rehabilitation 2010;32(Suppl 1):S42‐9. [DOI] [PubMed] [Google Scholar]
Rector 1993
- Rector TS, Kubo SH, Cohn JN. Validity of the Minnesota Living with Heart Failure Questionnaire as a measure of therapeutic response to enalapril or placebo. American Journal of Cardiology 1993;71(12):1106–7. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan) Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Roffi 2015
- Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, et al. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST‐segment elevation. Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST‐Segment Elevation of the European Society of Cardiology (ESC). European Heart Journal 2015;37(3):267‐315. [DOI] [PubMed] [Google Scholar]
Smith 2011
- Smith SC, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, et al. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation endorsed by the World Heart Federation and the Preventive Cardiovascular Nurses Association. Journal of the American College of Cardiology 2011;58(23):2432‐46. [DOI] [PubMed] [Google Scholar]
StataCorp 2013 [Computer program]
- StataCorp, College Station, TX: StataCorp LP. Stata Statistical Software: Release 13.. StataCorp, College Station, TX: StataCorp LP, 2013.
Steg 2012
- Steg PG, James SK, Atar D, Badano LP, Lundqvist CB, Borger MA, et al. ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST‐segment elevation. European Heart Journal 2012;33:2569‐619. [DOI] [PubMed] [Google Scholar]
Taylor 2006
- Taylor RS, Unal B, Critchley JA, Capewell S. Mortality reductions in patients receiving exercise‐based cardiac rehabilitation: how much can be attributed to cardiovascular risk factor improvements?. European Journal of Cardiovascular Prevention and Rehabilitation 2006;13(3):369‐74. [DOI] [PubMed] [Google Scholar]
Taylor 2014
- Taylor RS, Sagar VA, Davies EJ, Briscoe S, Coats AJS, Dalal H, et al. Exercise‐based rehabilitation for heart failure. Cochrane Database of Systematic Reviews 2014, Issue 4. [DOI: 10.1002/14651858.CD003331.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Townsend 2012
- Townsend N, Wickramasinghe K, Bhatnagar P, Smolina K, Nichols M, Leal J, et al. Coronary Heart Disease Statistics 2012. British Heart Foundation. www.bhf.org.uk/publications/statistics/coronary‐heart‐disease‐statistics‐2012 (accessed prior to 12 June 2017).
WHO 2008
- World Health Organization. The Global Burden of Disease: 2004 update. www.who.int/healthinfo/global_burden_disease/2004_report_update/en/ (accessed prior to 12 June 2017).
WHO 2016
- World Health Organization. Cardiovascular diseases (CVDs): Fact Sheet. www.who.int/mediacentre/factsheets/fs317/en/ (accessed 3 November 2016).
Yancy 2013
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Drazner MH, et al. 2013 ACCF/AHA Guideline for the Management of Heart Failure. A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Journal of the American College of Cardiology 2013;62(16):e147‐239. [DOI] [PubMed] [Google Scholar]
Zwisler 2016
- Zwisler AD, Norton RJ, Dean SG, Dalal H, Tang LH, Wingham J, et al. Home‐based cardiac rehabilitation for people with heart failure: A systematic review and meta‐analysis. International Journal of Cardiology 2016;221:963‐9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Buckingham 2016
- Buckingham, SA, Taylor RS, Jolly K, Zawada A, Dean SG, Cowie A, et al. Home‐based versus centre‐based cardiac rehabilitation: abridged Cochrane systematic review and meta‐analysis. Open Heart 2016;3(2):e000463. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical power analysis in the behavioural sciences. 2nd Edition. Hillsdale NJ: Lawrence Erlbaum Associates Inc, 1988. [Google Scholar]
Dalal 2010
- Dalal HM, Zawada A, Jolly K, Moxham T, Taylor RS. Home based versus centre based cardiac rehabilitation: Cochrane systematic review and meta‐analysis. BMJ 2010;340:b5631. [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor 2009
- Taylor RS, Dalal H, Jolly K, Moxham T, Zawada A. Home‐based versus centre‐based cardiac rehabilitation. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD007130.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Taylor 2015
- Taylor RS, Dalal H, Jolly K, Zawada A, Dean SG, Cowie A, Norton RJ. Home‐based versus centre‐based cardiac rehabilitation. Cochrane Database of Systematic Reviews 2015, Issue 8. [DOI: 10.1002/14651858.CD007130.pub3] [DOI] [PubMed] [Google Scholar]


