Abstract
Background
Circuit class therapy (CCT) offers a supervised group forum for people after stroke to practise tasks, enabling increased practice time without increasing staffing. This is an update of the original review published in 2010.
Objectives
To examine the effectiveness and safety of CCT on mobility in adults with stroke.
Search methods
We searched the Cochrane Stroke Group Trials Register (last searched January 2017), CENTRAL (the Cochrane Library, Issue 12, 2016), MEDLINE (1950 to January 2017), Embase (1980 to January 2017), CINAHL (1982 to January 2017), and 14 other electronic databases (to January 2017). We also searched proceedings from relevant conferences, reference lists, and unpublished theses; contacted authors of published trials and other experts in the field; and searched relevant clinical trials and research registers.
Selection criteria
Randomised controlled trials (RCTs) including people over 18 years old, diagnosed with stroke of any severity, at any stage, or in any setting, receiving CCT.
Data collection and analysis
Review authors independently selected trials for inclusion, assessed risk of bias in all included studies, and extracted data.
Main results
We included 17 RCTs involving 1297 participants. Participants were stroke survivors living in the community or receiving inpatient rehabilitation. Most could walk 10 metres without assistance. Ten studies (835 participants) measured walking capacity (measuring how far the participant could walk in six minutes) demonstrating that CCT was superior to the comparison intervention (Six‐Minute Walk Test: mean difference (MD), fixed‐effect, 60.86 m, 95% confidence interval (CI) 44.55 to 77.17, GRADE: moderate). Eight studies (744 participants) measured gait speed, again finding in favour of CCT compared with other interventions (MD 0.15 m/s, 95% CI 0.10 to 0.19, GRADE: moderate). Both of these effects are considered clinically meaningful. We were able to pool other measures to demonstrate the superior effects of CCT for aspects of walking and balance (Timed Up and Go: five studies, 488 participants, MD ‐3.62 seconds, 95% CI ‐6.09 to ‐1.16; Activities of Balance Confidence scale: two studies, 103 participants, MD 7.76, 95% CI 0.66 to 14.87). Two other pooled balance measures failed to demonstrate superior effects (Berg Blance Scale and Step Test). Independent mobility, as measured by the Stroke Impact Scale, Functional Ambulation Classification and the Rivermead Mobility Index, also improved more in CCT interventions compared with others. Length of stay showed a non‐significant effect in favour of CCT (two trials, 217 participants, MD ‐16.35, 95% CI ‐37.69 to 4.99). Eight trials (815 participants) measured adverse events (falls during therapy): there was a non‐significant effect of greater risk of falls in the CCT groups (RD 0.03, 95% CI ‐0.02 to 0.08, GRADE: very low). Time after stroke did not make a difference to the positive outcomes, nor did the quality or size of the trials. Heterogeneity was generally low; risk of bias was variable across the studies with poor reporting of study conduct in several of the trials.
Authors' conclusions
There is moderate evidence that CCT is effective in improving mobility for people after stroke ‐ they may be able to walk further, faster, with more independence and confidence in their balance. The effects may be greater later after the stroke, and are of clinical significance. Further high‐quality research is required, investigating quality of life, participation and cost‐benefits, that compares CCT with standard care and that also investigates the influence of factors such as stroke severity and age. The potential risk of increased falls during CCT needs to be monitored.
Keywords: Adult, Humans, Walking Speed, Arm, Arm/physiology, Exercise Therapy, Exercise Therapy/adverse effects, Exercise Therapy/methods, Gait, Gait/physiology, Postural Balance, Postural Balance/physiology, Randomized Controlled Trials as Topic, Recovery of Function, Stroke Rehabilitation, Stroke Rehabilitation/methods, Walk Test
Plain language summary
Circuit class therapy for improving mobility after stroke
Review question
Is circuit class therapy better than conventional physiotherapy for improving people's walking after a stroke?
Background
After stroke, people can have difficulty walking. They may become slower, only manage short distances, and may need assistance. They may lose balance more easily and be more fatigued. This can mean they walk even less, and so walking ability can worsen. Rehabilitation can help improve walking, but it is hard to access, particularly later after stroke. Circuit class therapy involves working in groups (rather than individually with a therapist), and doing specific practice of meaningful tasks, and may offer a solution that is more accessible.
Study characteristics
This is an update of the original review in 2010. We considered studies comparing circuit class therapy with conventional therapy for people with stroke, and included only high‐quality studies with a low risk of being biased. We were interested in studies that compared these two approaches and their effects on the way people walk, how far, how fast, and how independently. We also looked for studies that investigated if the circuit classes were more or less likely to be harmful than conventional approaches. The evidence is current to January 2017.
Main results
We found seventeen studies, involving 1297 participants, that compared circuit class rehabilitation with usual care or sham rehabilitation. Most trials reported the benefits of circuit classes for improving walking ability. More specifically, we combined the results from the studies and found moderate evidence that circuit classes were more effective in improving the person's ability to walk further, more independently, and faster and, in some cases, to balance more easily and confidently when compared with other types of therapy. There was a suggestion that people might fall more often in the circuit classes, and that they may be able to get home from rehabilitation hospital more quickly, but these two aspects were not confirmed using statistics. We also found that the positive effects of the circuit classes were experienced equally by people who had had their stroke more than a year ago compared with people who had had their stroke within the year. This means people can continue to improve longer after their stroke than was previously reported. More research is needed to see if it works for all people with any severity of stroke and if some tasks are better to practise than others.
Quality of the evidence
The quality of the studies overall was acceptable, given it is difficult to keep some aspects tightly controlled in rehabilitation studies. However, we have downgraded the quality rating to 'moderate' to acknowledge that some trials have the potential for bias.
Summary of findings
Summary of findings for the main comparison. Circuit class therapy compared with other intervention for improving mobility.
| Circuit class therapy compared with other intervention for improving mobility | ||||||
|
Patient or population: people with stroke Settings: in hospital or community Intervention: mobility‐related circuit class therapy Comparison: any other intervention | ||||||
| Outcomes | Illustrative comparative effects (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed effect | Absolute effect | |||||
| Other intervention | Mobility‐related circuit class therapy | |||||
|
Walking capacity: 6mWT Continous measure of distance walked in 6 minutes in m |
The mean 6mWT distance ranged across control groups from 106 m to 441 m | The mean 6mWT distance in the intervention groups was
60.86 m further (44.55 to 77.17) |
835 (10) | ⊕⊕⊕⊝ moderate | Applicable: difference greater than minimal clinically important difference (MDC) = 34.4m Eng 2004, and 95% CI of difference does not cross MDC Test for differences between subgroups 'early' versus 'later' (< 1 year vs > 1 year post stroke) were not significant. Some studies have unclear risk of bias (downgraded) |
|
|
Walking speed Continuous measure of walking speed measured over a short distance in m/s |
The mean gait speed ranged across control groups from 0.43 m/s to 1.3 m/s | The mean gait speed in the intervention groups was 0.15 m/s faster (0.10 to 0.19) | 744 (8) | ⊕⊕⊕⊝ moderate | Applicable: difference greater than MDC = 0.06 m/s Perera 2006, and 95% CI of difference does not cross MDC Some studies have unclear risk of bias (downgraded) |
|
|
Balance and mobility Timed up and go test. Standing up, walking, returning to sit down in seconds |
The mean speed ranged across control groups from 15 s to 28.6 s. | The mean speed in the intervention group was 3.62 s faster (‐6.06 to ‐1.16) | 488 (5) | ⊕⊕⊝⊝ low |
Applicable: somewhat as difference is not greater than MDC (8 s or 28%) (downgraded). Some studies have unclear risk of bias (downgraded) |
|
|
Independence in mobility Functional ambulation classification. Indicates need for assistance/not to safely mobilise |
The number of independent participants ranged across the control groups from 2 to 92 | The odds ratio of independent classifications in favour of the intervention group was 1.91 (1.01 to 3.6) | 469 (3) | ⊕⊕⊕⊝ moderate | Applicable: better odds of independence in walking is clinically useful. Some studies have unclear risk of bias (downgraded) |
|
| Physical ability Stroke Impact Scale. A self report of overall physical ability (subscale of total Impact) | The mean score for the control groups ranged from 55.4 to 83.73 points (higher is better) | The mean score for the intervention groups was 2.91 points higher (0.00 to 5.82) | 437 (2) | ⊕⊕⊝⊝ low |
Applicable: only somewhat as the mean change score should be 4.5 points to be regarded as clinically important (downgraded) Only two trials (downgraded) |
|
|
Adverse events (falls) from all available trials Counts of numbers of falls |
High risk population | RD 0.03 (‐0.02 to 0.08) | 815 (8) | ⊕⊝⊝⊝ very low | Applicable: 8 out of 17 studies reported falls; 4 of these studies reported no falls in either group. Only small number of studies reported that falls occurred (low event rate with low reporting), wide CIs Difference not statistically significant (downgraded) Some studies have unclear risk of bias (downgraded) Heterogeneity I2 > 50%, (downgraded) |
|
| 91.4 per 1000 | 134 per 1000 | |||||
| CI: confidence interval; RD: risk difference; MDC: minimal detectable change | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different. Low quality: OUr confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of effect. Very low quality: We have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||||
Background
Description of the condition
Stroke is the second most common cause of death globally, and was the third most common cause of disability‐adjusted life‐years worldwide in 2010 (Feigin 2014). The absolute numbers of people with stroke and the overall global burden of stroke are high and, despite medical advances in high‐income countries, these numbers are increasing steadily (Feigin 2014). Disability from stroke can negatively affect people's relationships (Lynch 2008a), the ability to live in the community, and the ability to participate in work and leisure activities. Stroke rehabilitation has been described as a holistic management plan, which is directed towards "enabling a person with impairment to reach their optimal physical, cognitive, emotional, communicative and/or social functional level" (Dawson 2013, p4). In terms of physical function, there are clear benefits from the provision of physical rehabilitation after stroke (Pollock 2014). With increasing numbers of people having strokes, post‐stroke rehabilitation services are in high demand.
Rehabilitation after stroke can be provided in inpatient settings, in peoples' homes, or in community clinics. The financial costs associated with stroke are substantial: for instance, the average per‐person costs of stroke in 2012 in Australia was AUD 27,709 (Deloitte Access Economics 2013), and the burden of disease costs in the USA has been estimated at USD 34 billion per year (Mozaffarian 2015). While there is evidence that rehabilitation at home may be more cost‐effective than other models of service delivery (Hillier 2010), this is not a feasible service for many people with stroke. Given the high demand for services and high costs associated with delivering post‐stroke care, there is pressure on rehabilitation services to provide evidence‐based therapies that are also cost effective.
Description of the intervention
Group circuit class therapy (CCT) is a model of physical therapy delivery wherein participants are given the opportunity to practice active task‐specific exercises (i.e. functional activities) in an intensive manner. The first trials investigating the feasibility of providing physical therapy to patients in groups rather than the traditional one‐therapist‐to‐one‐patient model were published in the late 1990s (Taskinen 1999; Teixeira‐Salmela 1999). The key components of CCT are that physical therapy is provided in groups (more than two participants per therapist) and there is a focus on repetitive practise of functional tasks and exercises that are continually progressed as the participant's function improves (English 2007; Van de Port 2012; Wevers 2009). CCT may comprise either a series of workstations arranged in a circuit (Van de Port 2012; Wevers 2009) or a series of individualised activities within a group setting (English 2007; English 2015). CCT differs from physiological exercise programmes designed to improve strength or aerobic fitness because, although many CCT exercises may have a strength or fitness component, the primary focus is on specific training of everyday motor tasks.
CCT can be directed towards a range of post‐stroke impairments and has been used to improve the use of hemiparetic upper limbs (Blennerhassett 2004), or to improve both mobility and upper limb impairments within the one circuit class session (English 2007; English 2015). However, the majority of studies have investigated the use of CCT for improving mobility (the ability to stand, walk, or run) so mobility‐tailored CCT is the focus of this review.
How the intervention might work
Physical therapy provided to people with stroke for 30 minutes to 60 minutes per day, five to seven days per week, results in significant improvements in independence and motor function compared with no therapy (Pollock 2014). Accordingly, many national clinical guidelines for stroke recommend that people with stroke spend a minimum of between 30 minutes and three hours per day in therapy during inpatient rehabilitation (Intercollegiate Stroke Working Party 2012; Jauch 2013; Lindsay 2010; National Stroke Foundation 2010; Stroke Foundation of New Zealand 2010). Data modelling work has demonstrated that increased time scheduled for therapy is associated with significant post‐stroke improvements in function (Lohse 2014). Further evidence regarding the benefits of increased time in therapy was provided from a recently updated meta‐analysis of clinical trials of physiotherapy after stroke (Verbeek 2014). The meta‐analysis included 80 trials that investigated the effect of providing increased intensity (hours spent) of physiotherapy, and found that increasing time in therapy after stroke is associated with significant, positive effects on walking speed, balance, and activities of daily living. In order to achieve significant positive effects at the body‐function level as well as the activities and participation level, an increase of 17 hours of therapy provided over 10 weeks is necessary (Verbeek 2014). The group nature of CCT interventions potentially allows a greater amount of therapy to be provided to patients within a finite period of time without increasing staffing requirements.
A recent Cochrane Review on physiotherapy for improving mobility after stroke reported that no approach of physiotherapy is clearly more effective than other approaches (Pollock 2014). The review also found that physiotherapy appears to be most beneficial when a mixture of different approaches are provided that are tailored for each patient. Interventions that have proven effectiveness in improving mobility outcomes for people with stroke include balance training (Verbeek 2014), combined strength and cardiovascular training (Verbeek 2014), and treadmill training for people who are able to walk independently (Mehrholz 2014). CCT can potentially improve mobility outcomes as the aforementioned interventions can be incorporated into CCT, and all activities prescribed within CCT are routinely tailored to each participant.
There may be benefits of CCT related to the peer support and social interaction provided by the group environment. Depression after stroke is common, affecting one third of people in the first year following stroke (Hackett 2008). Several small qualitative studies have found benefits to stroke survivors from participating in group activities with peers in terms of learning new coping mechanisms (Morris 2012), experiencing an increased sense of independence and well‐being (Morris 2012), and reducing post‐stroke depression (Stroke Recovery Canada 2009).
The format of CCT is conducive for optimal motor learning after stroke. Given the group nature of the CCT format, participants will usually be prescribed certain activities to perform semi‐supervised or independently, and other activities to perform with assistance of a therapist. When participants are performing the independent activities, the nature of the task‐specific exercise should ensure their attention is on the overall movement outcome (external focus) rather than the individual body part or joint movements (internal focus). Attention to external foci has been associated with better motor‐learning outcomes (Van Vliet 2006; Wulf 2010). The presence of a therapist at each CCT session allows for extrinsic feedback to be given to participants, which is an important contributor for optimal motor learning (Sigrist 2013; Wulf 2010). Further, CCT allows participants to observe other stroke survivors who are learning new motor tasks, which is another mechanism to facilitate motor learning (Wulf 2010).
Why it is important to do this review
Given the fiscal constraints of healthcare systems, rehabilitation services cannot simply increase the amount of therapy provided to people with stroke by scheduling more frequent or longer one‐to‐one therapy sessions, because this involves significant increases in staffing costs. Instead, it is important that novel cost‐effective models of providing increased intensity of therapy are developed, researched, and implemented. CCT has the potential to be an effective means of providing a greater amount of physical therapy for people with stroke both in hospital and community settings. When the clinical effectiveness of CCT is established, then cost implications of this model of therapy provision can be investigated. This is an update of the original review in 2010 which found that there was evidence to support the use of CCT for improving mobility after stroke.
Objectives
To examine the effectiveness and safety of CCT on mobility in adults with stroke.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) comparing CCT with no therapy, sham therapy, or another therapy modality. The earlier review included quasi‐randomised trials due to the paucity of studies. This was not necessary in this update.
Types of participants
We included studies of adults (18 years and older) with stroke (all types, severity, and stages of stroke/rehabilitation).
Types of interventions
We defined CCT as an intervention that involves participants receiving physical rehabilitation in a group environment, with a staff‐to‐client ratio of no greater than 1:3 (that is, no more than one staff member per three clients). We included studies that provided a minimum of once‐weekly CCT sessions for a minimum of four weeks. We only included studies that reported interventions with a focus on repetitive (within session) practise of functional tasks arranged in a circuit, with the aim of improving mobility. We excluded studies of interventions that included exercises solely aimed at improving impairment (such as strengthening, range of motion, or cardiovascular fitness).
Types of outcome measures
We evaluated outcome measures at post‐intervention and at follow‐up wherever available (e.g. three to six months post‐intervention). We did not consider outcomes taken after a single circuit class.
Primary outcomes
In this update the primary outcome of interest was walking capacity as measured using the Six Minute Walk Test (distance walked in six minutes: 6mWT). This is a clinically‐sensitive measure with demonstrated functional benefit for the person with stroke.
Secondary outcomes
Other measures of walking and standing ability including:
walking speed measured over a short distance (e.g. 5 m or 10 m walk test);
functional mobility measures such as the Timed Up and Go (TUG) or the Rivermead Mobility Index (RMI);
measures of standing balance, including the Step Test, Berg Balance Scale or Functional Reach Test.
Measures of impairment, such as:
lower limb strength; and
range of motion.
Measures of activity limitation, such as:
instrumental activities of daily living; and
personal care.
Measures of participation restriction, such as:
health‐related quality of life.
Other measures, such as:
length of hospital stay;
adverse events (including mortality);
self‐reported satisfaction;
locus of control;
economic indicators.
Summary of inclusion criteria
Human participants diagnosed with stroke (haemorrhage or infarct), of any severity/stage/setting (e.g. early: less than six months; or later: more than six months)
Eighteen years of age or older
Receiving CCT as defined
Outcomes evaluated in domains as defined
RCT
Search methods for identification of studies
See the 'Specialized register' section in the Cochrane Stroke Group module. We included all languages, and did not impose any date limits. To improve sensitivity we did not include a trials filter. We arranged for the translation of articles where necessary.
Electronic searches
We searched the Cochrane Stroke Group Trials Register, which was last searched by the Managing Editor in January 2017. We searched for additional articles published since the previous Cochrane systematic review on this topic from January 2008 onwards. Databases searched include the Cochrane Central Register of Controlled Trials (CENTRAL) (in the Cochrane Library 2016, Issue 12, Appendix 1), MEDLINE (in OVID, 1950 to January 2017, Appendix 2), Embase (1980 to January 2017, Appendix 3), CINAHL (1982 to January 2017, Appendix 4), PsycINFO (last searched January 2017, Appendix 5), AMED (1985 to January 2017, Appendix 6), SPORTDiscus (1949 to January 2017, Appendix 7), AGELINE (1978 to March 2015), Current Contents (last searched January 2017), Australasian Medical Index (AMI, 1968 to June 2016), NLM GATEWAY (gateway.nlm.nih.gov, last searched June 2016 for 2014 update), Latin American & Caribbean Health Sciences Literature (LILACS, 1982 to June 2016), IndMed (1985 to January 2017), Educational Resources Information Center (ERIC, 1967 to June 2016), and the Physiotherapy Evidence Database (PEDro, www.pedro.org.au, last searched January 2017). Unique search strings are included in the Appendices, and where not included are adaptations.
Searching other resources
We used the MEDLINE (Ovid) search developed by the Cochrane Stroke Group Information Specialist and adapted it to search the other databases. We included all languages, and imposed no date limits. As the subject area of this review is quite specific we did not include a trials filter. This increased the sensitivity of the search.
In an effort to identify further published, unpublished and ongoing studies, we:
searched for proceedings from stroke‐related conferences that were peer‐reviewed and published in the above databases until 2016;
searched reference lists (from salient articles, journals and books) and unpublished theses;
contacted authors of published trials and other experts in the field;
-
searched the following clinical trials and research registers:
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (apps.who.int/trialsearch/);
US National Institutes of Health Ongoing Trials Register, ClinicalTrials.gov (www.clinicaltrials.gov/);
Computer Retrieval of Information on Scientific Projects (commons.era.nih.gov/common);
ISRCTN Registry www.isrctn.com/ (formerly the Current Controlled Trials);
National Institute of Neurological Disorders and Stroke (www.ninds.nih.gov/);
National Rehabilitation Information Centre (Naric) (including REHABDATA) (www.naric.com/);
Stroke Trials Directory ‐ the Internet Stroke Center (www.strokecenter.org/trials).
Data collection and analysis
Selection of studies
We retrieved papers from the identified lists on the basis of title/abstract, reviewing them against the established criteria for inclusion. If all criteria were met (that is, answers to the five criteria were 'yes' or 'unsure') we retrieved the study in full and reviewed it for final inclusion and then for methodological quality and data extraction. If we disagreed on any aspect of study inclusion we reached consensus through discussion and had a third review author available for consultation if consensus could not be reached.
Data extraction and management
We independently entered data into the Review Manager software, RevMan 5.3 (RevMan 2014), and included full citation details of the study, objectives, design, length, assessment time points, number and characteristics of participants (inclusion and exclusion criteria), description of the intervention, outcome measures, intention‐to‐treat analysis, withdrawals and loss to follow‐up, and adverse events. If we disagreed on any aspect of data extraction or quality evaluation, we reached consensus through discussion and had a third review author available for consultation if consensus could not be reached.
Assessment of risk of bias in included studies
We independently assessed the quality of the studies to be included. We assessed the methodological quality of the included studies for risk of bias using the criteria recommended in section 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) in six domains: sequence generation, allocation concealment, blinding of participants, personnel and outcome assessors, incomplete outcome data, selective reporting and 'other'. We defined 'other' as adequate sample size, based on supplied power calculations. We gave studies an overall summary of the risk of bias for each important outcome (across domains), as well as within and across studies using three levels: low, unclear, or high risk of bias. We also gave a descriptive report on the overall risk of bias in relation to the findings from the meta‐analyses.
Measures of treatment effect
We extracted and analysed data to calculate risk ratio (RR) or mean difference (MD) and 95% confidence intervals (CI). This required the identification of the number of participants in each group in each trial and the total number (for dichotomous data), and the number of participants plus the mean and standard deviations for each group (for continuous data).
Unit of analysis issues
We considered studies with non‐standard designs, for example, cluster randomised trials, if they were assessed as having a low risk of bias. We only considered randomised cross‐over trials prior to cross over (irrespective of wash‐out periods as the changes are assumed to be permanent) and if the study authors provided an analysis of results for the first phase.
Dealing with missing data
We contacted study authors to request appropriate data for meta‐analyses if these were not adequately reported in the retrieved paper. We considered intention‐to‐treat analysis as part of the risk of bias assessment and recorded loss to follow‐up.
Assessment of heterogeneity
We assessed statistical heterogeneity both visually and using the I2 statistic (Higgins 2003). Where I2 was greater than 50% we used random‐effects rather than a fixed‐effect analysis. We also evaluated clinical heterogeneity (clinical and methodological diversity).
Assessment of reporting biases
We minimised reporting biases by the comprehensive search strategies, which had no date or language limits. However, where appropriate we could also examine this statistically via funnel plots and tests for asymmetry if there were sufficient studies (recommended more than 10; Sterne 2011).
Data synthesis
We conducted a meta‐analysis with appropriate data. We considered the degree of heterogeneity to determine whether to use fixed‐effect or random‐effects analyses.
Subgroup analysis and investigation of heterogeneity
We considered performing subgroup analyses to establish effectiveness relative to gender, chronicity, age or stroke severity (respectively men versus women; early (less than one year post‐stroke) versus late (more than one year post‐stroke); young adults versus older; mild/moderate versus severe stroke, if sufficient data were available.
Sensitivity analysis
We conducted sensitivity analyses to determine if pooling results from large trials (more than 100 participants) led to different results compared with pooling data from small trials (fewer than 100 participants), or if trials with low versus high risk of bias influenced the results, when a sufficient number of trials were available.
GRADE assessment and 'Summary of findings' tables
We presented the main results of the review in Table 1 for the comparison of CCT versus 'other' interventions. We reported the outcome measure of walking capacity (6mWT) as the primary outcome; we also included other secondary outcomes in the table that had a sufficient body of evidence (number of trials/number of participants) in recognition that low numbers in either or both of these inevitably leads to a 'very low' GRADE designation.
A 'Summary of findings' table presents information about the certainty of the evidence, the size of the effect of the intervention examined, and the sum of available data for the main outcomes. The 'Summary of findings' table also includes an overall grading of the evidence related to each of the main outcomes using the GRADE approach (GRADE 2013). This defines the certainty or confidence in a body of evidence that an estimate of effect or association is close to the true quantity of specific interest. This certainty involves consideration of within‐trial risk of bias (methodological quality), applicability of evidence, heterogeneity, precision of effect estimates, and risk of publication bias (Higgins 2011). When making decisions for the risk of bias, we downgraded only when we had classed studies as being at high risk of bias for one or more domains or they were classed as being at unclear risk of bias for both domains that contribute to selection bias, or both (GRADE 2013).
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies
Results of the search
We retrieved 101 potential trials in full from the search, of which we included 17 in this review (Figure 1). Twelve were new studies published between 2010 and 2015. Five studies were included from the previous review (Blennerhassett 2004; Dean 2000; Marigold 2005; Mudge 2009a; Pang 2005). We excluded one study from the previous review in this update as it was a pseudo‐randomised trial (English 2007).
1.
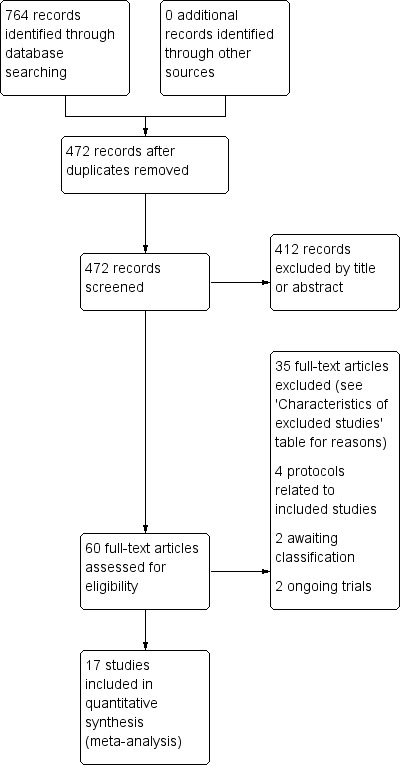
PRISMA flow diagram
Included studies
The 17 included trials were all conducted between 2000 and 2015; four in Australia (Blennerhassett 2004; English 2015; Dean 2012; Marsden 2010), four in Canada (Dean 2000; Marigold 2005; Pang 2005; Tang 2014), two in Korea (Song 2015; Kim 2016a) and the UK (Harrington 2010; Moore 2015) , and one each in Germany (Outermans 2010), India (Verma 2011), , the Netherlands (Van de Port 2012), New Zealand (Mudge 2009a), and Sweden (Holmgren 2010). Four trials were conducted in an inpatient hospital setting (Blennerhassett 2004; English 2015; Song 2015; Verma 2011). The remaining 13 trials were conducted in community settings. A total of 1297 participants were included with sample sizes varying from 12 to 250 participants. Time since stroke onset varied with studies including participants within one month (three trials: Blennerhassett 2004; English 2015; Outermans 2010), three months (three trials: Kim 2016a, Van de Port 2012; Verma 2011), six months (one trial: Holmgren 2010), one year (one trial: Harrington 2010), and more than one year post stroke (eight trials: Dean 2012; Moore 2015; Tang 2014; Dean 2000; Marigold 2005; Marsden 2010; Mudge 2009a; Pang 2005). One trial did not report the exact time since stroke (Song 2015). Only two studies collected objective measures of stroke severity, both of which used the National Institutes of Stroke Scale (Tang 2014; Verma 2011). For the majority of the other studies, stroke severity could be inferred as being mild to moderate, as their inclusion criteria for functional ability was only participants who were able to walk at least 5 m (Tang 2014) or 10 m independently, with or without a walking aid. Two studies included people living at home in the community with no reference to walking ability (Harrington 2010; Marsden 2010), and one included people in the moderate band of stroke severity according to score ranges on the Functional Independence Measure (FIM) (English 2015).
All studies investigated the effects of CCT (workstation‐based, task‐specific practise in a group with a ratio of staff to client of 1:3 or higher) with the aim of improving mobility in people post stroke. Two studies also explicitly aimed to improve cardiorespiratory fitness and included a target heart rate zone within their intervention (Outermans 2010; Tang 2014). Three studies combined CCT with education sessions (Harrington 2010; Holmgren 2010; Marsden 2010) and one combined CCT with mental imagery (Verma 2011). The length of therapy sessions, frequency (sessions per week), and duration of the intervention period varied somewhat between studies but were relatively homogeneous in terms of staffing and content ‐ see Table 2 for a summary of all CCT formats. Five studies reported the percentage of therapy sessions attended and this ranged from 63% (Dean 2012) to 92% (Mudge 2009a), with Harrington 2010 reporting that 61% of participants attended at least 75% of therapy sessions. English 2015 reported the mean total amount of therapy time received per participant (37.3 hours) and Van de Port 2012 reported the total number and average duration of therapy sessions delivered to intervention participants (4461 sessions, mean 72 minutes' duration).
1. Summary of circuit class content in all trials.
| Study ID |
What (CCT content) |
Who |
How (timing, number and duration of sessions) |
Where |
| Blennerhassett 2004 | Mobility CCT in addition to usual care; functional tasks, strengthening exercises | Physiotherapist | 1‐hour sessions 5 days per week for 4 weeks |
Inpatient rehabilitation unit |
| Dean 2000 | Multiple task‐specific training strengthening LL; practice locomotor‐related tasks | Physiotherapists | 1‐hour sessions, 3 days per week for 4 weeks | Community setting |
| Dean 2012 | Progressive balance and strengthening exercises; walking and stair climbing. Home exercise programme and advice to increase walking | Physiotherapist | 45 to 60 minutes per week for 40 weeks over a one‐year period | Community setting |
| English 2015 | Task‐specific, part‐ as well as whole‐practice of tasks; emphasis on repetition and feedback | Physiotherapists, assistants, and physiotherapy students | 90‐minute sessions, 5 times per week for 4 weeks | Inpatient rehabilitation |
| Harrington 2010 | Individual, easily progressed; balance, endurance, strength, flexibility, function and well‐being. Home exercise manuals and encouraged for on‐going exercise | Instructor and physiotherapist with support from volunteers (partners, carers, family members) | 2 sessions per week for 8 weeks. (1 hour exercise plus 1 hour interactive education |
Community setting |
| Holmgren 2010 | Individualised physical activity, functional performance; educational group discussions about fall risk and security | Physiotherapist and occupational therapist | 7 sessions per week divided over 3 days for 5 weeks | Community setting |
| Kim 2016a | Progressive, focused on mobility and gait training as well as physical fitness | Physiotherapist | 90‐minute sessions, 5 days per week for 4 weeks | Inpatient rehabilitation |
| Marigold 2005 | Focused on walking, standing, balance, and sit‐to‐stand tasks | Physical therapist, kinesiologist, and recreation therapist | 1‐hour sessions, 3 times per week for 10 weeks | Community setting |
| Marsden 2010 | Education and exercises for LL function: functional tasks, strength training and balance training | Multidisciplinary team including a physiotherapist, social worker, dietician, clinical nurse consultant, speech pathologist and occupational therapist | 2‐hour sessions (1 hour education + 1 hour exercise) weekly for 7 weeks | Community setting |
| Moore 2015 | Functional movement including stretching, functional strengthening, balance, agility and fitness | Physiotherapist and physical activity instructor | 3 x 45‐ to 60‐minute sessions per week for 19 weeks | Community setting |
| Mudge 2009a | Task‐oriented gait or standing balance activity, strengthening LL | Physiotherapist and 2 physiotherapy students | 50‐ to 60‐minute sessions, 3 times a week for 4 weeks | Community setting |
| Outermans 2010 | Postural control and gait‐related activities: stair climbing, walking and turning | Therapists | 45‐minute sessions, 3 times per week for 4 weeks | Inpatient and outpatient settings |
| Pang 2005 | Fitness and mobility exercise: cardiorespiratory fitness, mobility, leg muscle strength, balance, and hip bone mineral density | Physical therapist, occupational therapist, and exercise instructor | 1‐hour sessions, 3 times per week for 19 weeks | Community setting |
| Song 2015 | Functional training tasks | Physiotherapists | 30‐minute sessions, 3 times per week for 4 weeks | Inpatient rehabilitation |
| Tang 2014 | Brisk level and inclined overground walking, upright and recumbent cycle ergometry, functional movements | 3 instructors | 60‐minute classes, 3 times per week for 6 months | Community setting |
| Van de Port 2012 | Meaningful tasks related to walking competency | Physiotherapist and sports therapists | 90‐minute sessions, 2 times per week for 12 weeks | Community setting |
| Verma 2011 | Meaningful tasks related to walking competency: balance control, stair walking, turning, transfers, and speed walking | Physiotherapist or occupational therapist 1 caretaker to ensure safety |
40‐minute sessions, 7 days per week for 2 weeks | Inpatient and outpatient settings |
CCT: circuit class therapy LL: lower limb
Twelve studies had a comparison group involving alternate 'other interventions', which was matched for length of sessions, frequency, and duration of intervention for eight studies. The description of the comparison 'other interventions' ranged from usual care (English 2015; Kim 2016a; Song 2015; Van de Port 2012; Verma 2011), CCT involving upper limb training only (Blennerhassett 2004; Dean 2000; Dean 2012; Pang 2005), non‐specific exercises such as stretching (Marigold 2005; Moore 2015) or education/social groups (Mudge 2009a). Three studies compared CCT combined with education versus no therapy (Harrington 2010; Holmgren 2010; Marsden 2010). In two studies the comparison group also received mobility‐related CCT but at a lower intensity (without a target heart rate) (Outermans 2010; Tang 2014).
All studies used a composite of measures related to mobility including tests of walking ability (gait speed and capacity), and balance (TUG, Berg Balance Scale (BBS), Step test). Some studies used measures of quality of life, upper limb function, balance self‐efficacy, tests for impairment (strength, VO2max, kinematic data), free‐living walking ability (steps per day using an activity monitor), numbers of adverse events (falls during therapy), satisfaction, and length of stay. Only one study included measures of economic indicators (Harrington 2010). A total of 62 different outcome measures were reported in the included studies.
Excluded studies
We excluded the remaining studies for a variety of reasons including inappropriate methods, or interventions that were either not task‐specific (that is to say the interventions addressed impairments not functional tasks) or not in a group (staff‐to‐client ratio was less than 1:3). See Characteristics of excluded studies for individual reasons for exclusions.
Risk of bias in included studies
Figure 2 summarises the trials together with risk of bias in the six domains, with the most likely risk in the area of selective reporting of outcome data, which was frequently rated as unclear because the majority of included studies did not publish a trial protocol or register their trials. Figure 3 shows the trials individually across the six domains. Three of the 17 trials demonstrated adequate control of risk across all six domains (Dean 2012; English 2015; Mudge 2009a).
2.
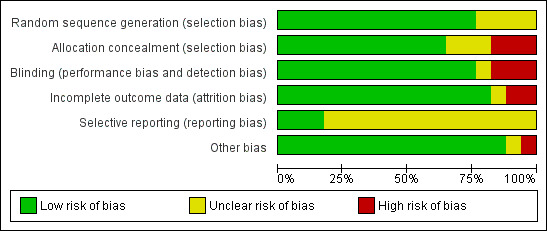
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
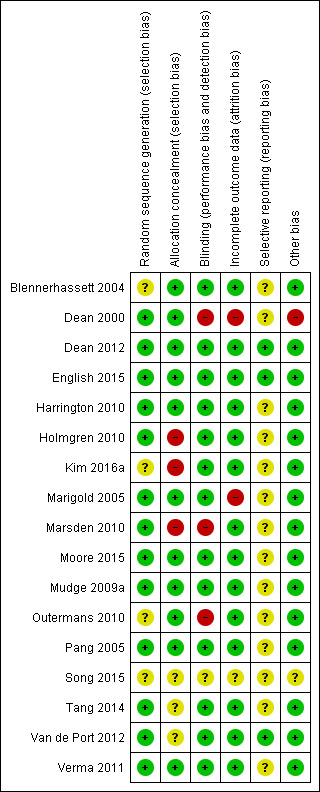
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Allocation
Thirteen studies stated the allocation method for randomising, with the remaining four studies stating that random allocation occurred but not how (Blennerhassett 2004; Kim 2016a; Outermans 2010; Song 2015). Six studies either did not conceal or did not state whether or how allocation was concealed to the administrator of the randomisation process (Holmgren 2010; Kim 2016a; Marsden 2010; Song 2015; Tang 2014; Van de Port 2012).
Blinding
Four studies did not report blinding of assessors involved in the trial (Dean 2000; Marsden 2010; Outermans 2010; Song 2015).
Incomplete outcome data
Three studies did not adequately report and/or account for attrition across the trial groups (Dean 2000; Marigold 2005; Song 2015).
Selective reporting
Only three studies provided a reference to the trial registration or study protocol with all three studies demonstrating complete reporting (Dean 2012; English 2015; Van de Port 2012).
Other potential sources of bias
We noted other potential sources of bias, such as small numbers (Dean 2000), and cursory reporting across all aspects of trial conduct (Song 2015).
Effects of interventions
See: Table 1
CCT versus 'other interventions'
Sufficient clinical homogeneity allowed us to pool study data, comparing CCT for mobility versus 'other intervention(s)'.
Primary outcome
Ten studies (835 participants, 64% of total sample) measured walking capacity using the 6mWT (Blennerhassett 2004; Dean 2000; Dean 2012; English 2015; Kim 2016a; Moore 2015; Mudge 2009a; Pang 2005; Van de Port 2012; Verma 2011). Meta‐analysis demonstrated that overall CCT was superior to the comparison intervention (MD 60.86, 95% CI 44.55 to 77.17; I2 = 27%, Analysis 1.1). Subgroup analysis between trials with participants who were early versus late after stroke onset showed a greater mean difference (improvement) for the later group but failed to reach a significant difference between these subgroups (P = 0.14).
1.1. Analysis.
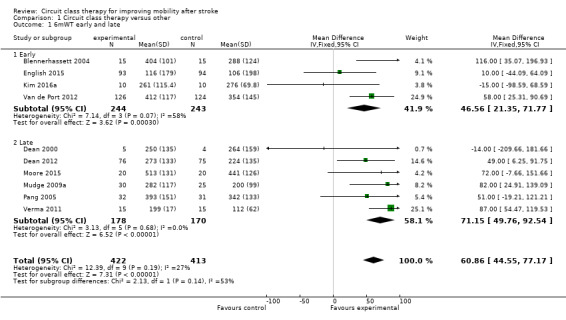
Comparison 1 Circuit class therapy versus other, Outcome 1 6mWT early and late.
Using the GRADE criteria based on the number of participants, the significant effect and relatively narrow CIs, we applied an overall rating of 'moderate', however we downgraded due to uncertain risk of bias across several studies.
Secondary outcomes
Eight studies (744 participants, 57% of total sample) measured gait speed (Dean 2000; Dean 2012; English 2015; Moore 2015; Mudge 2009a; Song 2015; Van de Port 2012; Verma 2011), with meta‐analysis showing a difference between the two groups that reached significance in favour of CCT (MD 0.15, 95% CI 0.10 to 0.19; I2 = 14%, Analysis 1.2). Using the GRADE criteria based on the number of participants, the significant effect and relatively narrow CIs, we applied an overall rating of 'moderate', however we downgraded due to uncertain risk of bias across several studies.
1.2. Analysis.
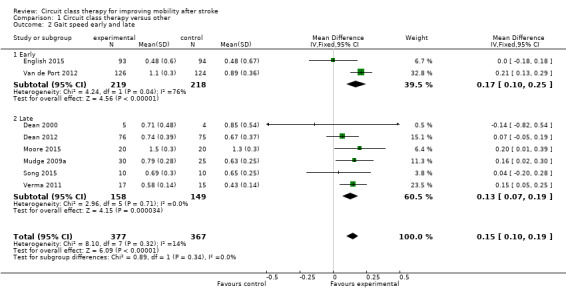
Comparison 1 Circuit class therapy versus other, Outcome 2 Gait speed early and late.
Two studies (50 participants) measured cadence in steps per minute and found a significant effect in favour of CCT (Song 2015; Verma 2011: MD 13.57, 95% CI 7.52 to 19.62; I2 = 0%, Analysis 1.3).
1.3. Analysis.
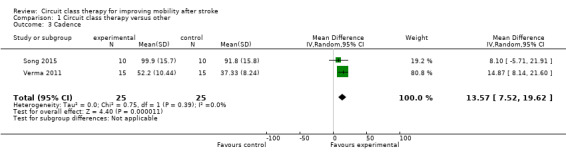
Comparison 1 Circuit class therapy versus other, Outcome 3 Cadence.
Five studies (488 participants) used the TUG test to measure the ability to stand up, walk, and turn around, and meta‐analysis showed a difference between the two groups that reached significance in favour of CCT ((Blennerhassett 2004; Dean 2000; Dean 2012; Marigold 2005; Van de Port 2012: MD ‐3.62, 95% CI ‐6.09 to ‐1.16; I2 = 0%, Analysis 1.4). Two studies (296 participants) measured mobility using the Rivermead Mobility Index (Mudge 2009a; Van de Port 2012). The meta‐analysis showed a significant effect in favour of CCT (MD 0.56, 95% CI 0.17 to 0.95; I2 = 7%, Analysis 1.5). Three studies (469 participants) measured independence in walking using the Functional Ambulation Classification (English 2015; Van de Port 2012; Verma 2011) and found a significant effect in favour of CCT (OR 1.91, 95% CI 1.01 to 3.62; I2 = 34%, Analysis 1.6).
1.4. Analysis.
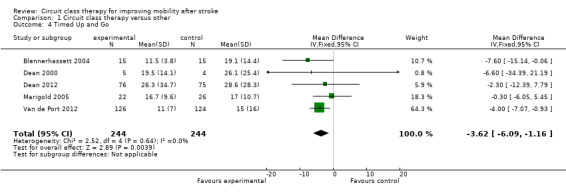
Comparison 1 Circuit class therapy versus other, Outcome 4 Timed Up and Go.
1.5. Analysis.
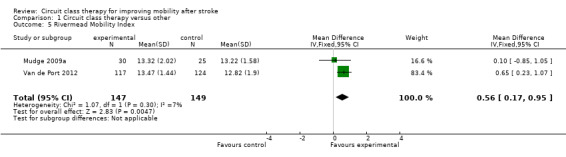
Comparison 1 Circuit class therapy versus other, Outcome 5 Rivermead Mobility Index.
1.6. Analysis.
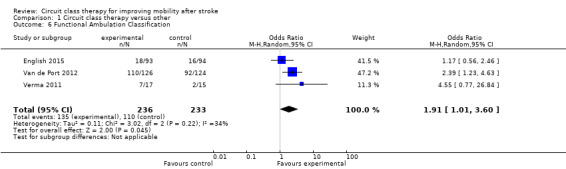
Comparison 1 Circuit class therapy versus other, Outcome 6 Functional Ambulation Classification.
Four studies (171 participants) applied the Berg Balance Scale with meta‐analysis showing no significant between‐group differences (Kim 2016a; Moore 2015; Marigold 2005; Pang 2005: MD 1.21, 95% CI ‐0.62 to 3.04; I2 = 30%, Analysis 1.7). Three studies (190 participants) used the Step Test to measure balance with no significant between‐group differences (Blennerhassett 2004; Dean 2000; Dean 2012: MD 0.98, 95% CI ‐0.40 to 2.37; I2 = 21%, Analysis 1.8). Two studies (103 participants) measured balance self‐efficacy using the Activities‐specific Balance Confidence Scale (ABC) with meta‐analysis showing a significant effect in favour of CCT ((Marigold 2005; Mudge 2009a: MD 7.76, 95% CI 0.66 to 14.87; I2 = 0%, Analysis 1.9).
1.7. Analysis.
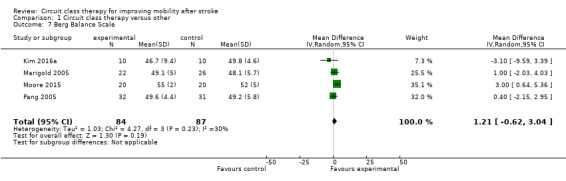
Comparison 1 Circuit class therapy versus other, Outcome 7 Berg Balance Scale.
1.8. Analysis.
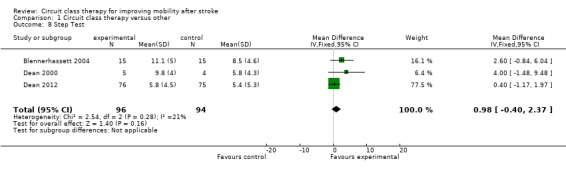
Comparison 1 Circuit class therapy versus other, Outcome 8 Step Test.
1.9. Analysis.
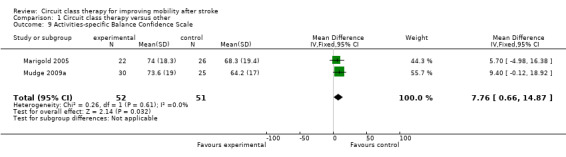
Comparison 1 Circuit class therapy versus other, Outcome 9 Activities‐specific Balance Confidence Scale.
Two studies (437 participants) used the Stroke Impact Scale ‐ physical sub‐scale to measure self‐reported physical ability (English 2015; Van de Port 2012). The meta‐analysis demonstrated a favourable effect for CCT that just met significance (MD 2.91, 95% CI 0.00 to 5.82; I2 = 0%, Analysis 1.10).
1.10. Analysis.
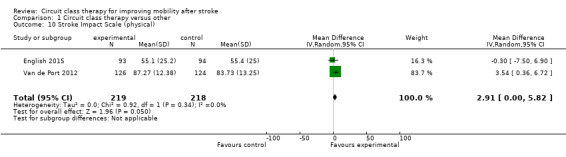
Comparison 1 Circuit class therapy versus other, Outcome 10 Stroke Impact Scale (physical).
Two studies measured fitness using VO2 peak (Moore 2015; Pang 2005, 103 participants). A significant favourable effect was found for CCT participants (MD 2.81, 95% CI 0.90 to 4.72; I2 = 0%, Analysis 1.11). Two studies (206 participants) included measures of average daily step counts and found significant effect in favour of CCT (Mudge 2009a; Dean 2012: MD 1325.66, 95% CI 411.09 to 2240.22; I2 = 29%, Analysis 1.12).
1.11. Analysis.
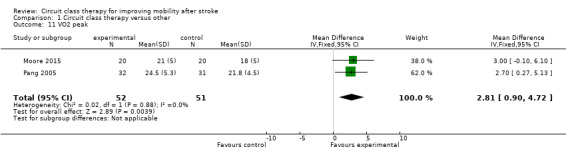
Comparison 1 Circuit class therapy versus other, Outcome 11 VO2 peak.
1.12. Analysis.
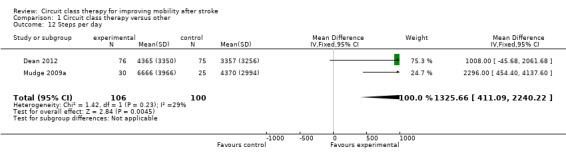
Comparison 1 Circuit class therapy versus other, Outcome 12 Steps per day.
Two trials (217 participants) measured length of stay (Blennerhassett 2004; English 2015). A shorter length of stay was reported for participants receiving CCT (MD ‐16.35, 95% CI ‐37.69 to 4.99; I2 = 51% ), but this was not significant when random effects were applied (given the high heterogeneity) (Analysis 1.13).
1.13. Analysis.
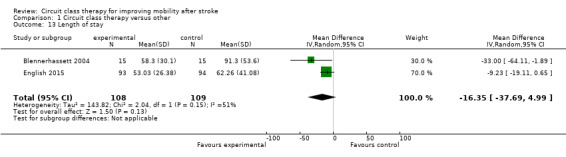
Comparison 1 Circuit class therapy versus other, Outcome 13 Length of stay.
CCT + education versus no intervention
Sufficient clinical homogeneity allowed us to pool study data, comparing CCT + education versus no intervention. Two studies measured balance using the TUG (269 participants) with no significant between group differences found (Harrington 2010; Marsden 2010: MD 0.90, 95% CI ‐0.94 to 2.75; I2 = 0%, Analysis 2.1). The same two studies measured carer burden using the Carer Strain Index (Harrington 2010; Marsden 2010, 174 participants). The meta‐analysis showed a negative effect of the intervention with higher Carer Strain Index (worse functioning) reported by carers of participants in the CCT + education group (MD 1.06, 95% CI 0.39 to 1.73; I2 = 0%, Analysis 2.2).
2.1. Analysis.
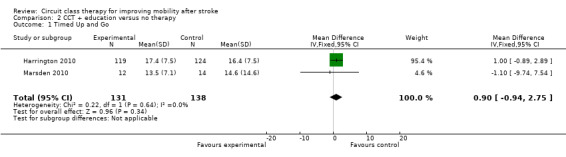
Comparison 2 CCT + education versus no therapy, Outcome 1 Timed Up and Go.
2.2. Analysis.
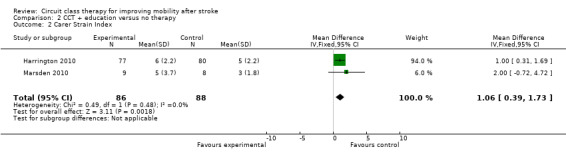
Comparison 2 CCT + education versus no therapy, Outcome 2 Carer Strain Index.
CCT versus a different CCT
Two studies compared mobility‐related CCT provided at high intensity (with target heart rate zones) versus the same exercises at low intensity (Outermans 2010; Tang 2014). The 6mWT was the only outcome in common between these trials, but due to the differences in the duration of the intervention there was insufficient clinical homogeneity to pool data (six months in Tang 2014 versus four weeks in Outermans 2010).
All comparisons
Eight studies (836 participants) reported monitoring adverse events including falls. Of these, four studies reported that there were no falls, and the other four reported between six falls (Pang 2005) and 55 falls (Van de Port 2012). There was a higher risk of falls in the CCT groups (risk difference 0.03, 95% CI ‐0.02, 0.08; I2 = 60%) but this did not reach significance (Analysis 3.1).
3.1. Analysis.
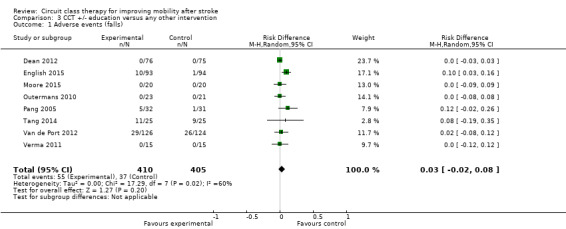
Comparison 3 CCT +/‐ education versus any other intervention, Outcome 1 Adverse events (falls).
Sensitivity analyses: primary outcome
We conducted a sensitivity analysis based on the size of the trial, considering large trials to be those with 100 or more participants (Dean 2012; English 2015; Harrington 2010; Van de Port 2012) and small trials to be those with fewer than 100 participants (Blennerhassett 2004; Dean 2000; Holmgren 2010; Kim 2016a; Marigold 2005; Marsden 2010; Moore 2015; Mudge 2009a; Outermans 2010; Pang 2005; Song 2015; Tang 2014; Verma 2011). The size of effect for the 6mWT was smaller but still significant when pooling only data from large trials (MD 46.31, 95% CI 22.90 to 69.72; participants = 588; studies = 3; I2 = 11%) compared with small trials (MD 74.59, 95% CI 51.85 to 97.32; participants = 247; studies = 7; I2 = 17%).
We also conducted a sensitivity analysis based on risk of bias where the three studies with no/low assessed risk of bias in the six domains confirmed a positive effect in favour of CCT for the 6mWT (Dean 2012; English 2015; Mudge 2009a: MD 46.32, 95% CI 17.40 to 75.24; I2 = 38%) (Analysis 1.14).
1.14. Analysis.
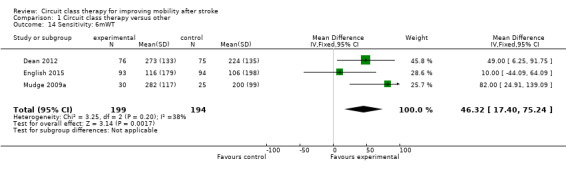
Comparison 1 Circuit class therapy versus other, Outcome 14 Sensitivity: 6mWT.
Discussion
Summary of main results
The primary aim of this review was to investigate the effectiveness of group CCT for improving mobility after stroke. For our primary outcome measure of gait capacity, we found CCT to be superior to other interventions for improving the distance walked on the 6mWT. The minimal clinically‐meaningful improvement on the 6mWT has been estimated at 34.4 m for people later after stroke (Eng 2004) and 61 m for people early after stroke (Perera 2006). Thus, we can be confident that the mean improvement found in the meta‐analysis of 60.86 m represents a real and applicable clinical change. The positive finding for the 6mWT is of functional relevance as it has been shown to be a stronger predictor of the community walking ability than measures of walking speed (Fulk 2010; Mudge 2009b; Rand 2009), which may overestimate community ambulatory ability (Taylor 2006). Furthermore, the 6mWT has been shown to correlate significantly with quality of life after stroke (Muren 2008). We also confirmed that the positive effects were present for people both early and late after stroke suggesting potential for improvement does not necessarily decline. A further positive feature of the primary outcome analyses was that heterogeneity was low. However, we downgraded the GRADE designation to 'moderate' because of the potential for risk of bias in some included studies.
We also found a small favourable effect of CCT in regards to improvements in walking speed, with the magnitude of the between‐group difference (0.15 m per second) being greater than the estimated smallest worthwhile effect of 0.06 m per second (Perera 2006). Perera 2006 suggest that a difference of greater than 0.14 m per second represents a substantial meaningful change for people after stroke. Thus, we can be confident that our results represent real clinical change. Our results suggest that CCT as an intervention has a positive effect on improving independence in walking, with the odds of being fully independent in walking (Functional Ambulation Scale score of 5) after the intervention being significantly greater for intervention participants compared with people allocated to the control intervention.
The evidence for the effectiveness of CCT in improving walking ability after stroke can be considered robust as it is consistently positive across a range of clinic‐based walking measures (6mWT, walking speed, Functional Ambulation Classification) and self‐reported physical function (Stroke Impact Scale ‐ Physical, Rivermead Mobiity Index). The intervention across all studies included a strong emphasis on continuous walking practice. Therefore, the positive results are in line with evidence for intensity and task‐specificity of training, that is to say 'what is trained is what is gained' (Verbeek 2014).
There is some evidence that improvements in walking capacity and ability gained through CCT may also translate into behaviour change. In this updated review, two studies included measures of daily step counts, measured using either a pedometer (Dean 2012) or an ankle‐worn accelerometer (Mudge 2009a). Both trials found that participants who received CCT increased their daily step count to a significantly greater degree than control participants. This is important, as lack of adequate physical activity is linked to increased all‐cause morbidity and mortality (Lollgen 2009) and cardiovascular disease‐specific morbidity and mortality (Thompson 2003), as well as increased risk of stroke (Feigin 2014; McDonnell 2013).
Importantly, CCT may also be an effective method of training for improving cardiorespiratory fitness for people later after stroke. Many studies (Marsden 2013; Marsden 2016; Smith 2012) have reported fitness levels of stroke survivors at less than the minimum requirement for activities of daily living in older adults, that is, 15 mL/kg/min to 18 mL/kg/min (Shephard 2009). An improvement in fitness in the order of magnitude found in our meta‐analysis (2.8 mL/kg/min) is similar to that conferred by exercise interventions with an aerobic component (Marsden 2013). This amount is clinically important as it can improve the exercise reserve of stroke survivors (Ivey 2006) and has the potential to reduce the risk of death (Kodama 2009).
The effectiveness of CCT for improving postural control is less clear. We found significant between‐group differences in favour of CCT for the Activities‐specific Balance Confidence scale and the TUG that exceeded the minimal detectable difference on these measures (Flansbjer 2005; Salbach 2006). However, between‐group differences were non‐significant for the step test, and too small to be clinically worthwhile on the Berg Balance Scale: MD of 1.36 points compared with minimal detectable change of 6.9 points early after stroke (Stevenson 2001), and 4.13 points later after stroke (Flansbjer 2012).
There is some suggestion that providing CCT to people receiving in‐hospital rehabilitation after stroke may reduce length of hospital stay with a mean between‐group difference of 16.35 days. However, the heterogeneity in the study results was higher (I2 = 51%) and the difference just failed to reach significance using random effects in the analysis. There are many factors that influence length of hospital stay. A recent individual patient meta‐analysis was conducted where data were pooled from two large multicentre trials investigating the effect of additional weekend therapy for people with stroke. The meta‐analysis identified a range of factors that significantly contributed to length of rehabilitation hospital stay, including age and degree of disability at admission (English 2016). Interestingly, this paper also reported considerable variability in length of stay between individual hospital sites, highlighting the complexity of factors that influence how long people with stroke spend in inpatient rehabilitation. However, a secondary analysis of data from the CIRCIT trial (English 2015) found that when controlling for other influencing factors, receiving CCT as the sole method of physiotherapy service delivery (as compared to usual care physiotherapy) was an independent predictor of a shorter length of stay, in the order of ‐11.6 days (95% CI ‐21.3 to ‐1.9, P = 0.019) (Abstracts Asian Pacific Stroke Congress p6). Reducing length of stay has the potential for significant savings to the healthcare system, but we currently lack high‐quality economic data to establish the cost effectiveness of such an approach.
With regards to adverse events, there were more falls (albeit not statistically different) reported among participants receiving CCT compared with other interventions. Any intervention aimed at improving mobility and balance after stroke carries an inherent risk of causing falls because it is necessary for participants to undertake activities at the limits of their abilities for the interventions to be effective. The greater falls rate in the intervention group is perhaps not surprising considering that the control group was either undertaking interventions that did not expose the participants to an increased risk of falls; for example, seated upper extremity exercise programmes (Blennerhassett 2004; Dean 2000; Pang 2005), stretching (Marigold 2005; Moore 2015), education (Holmgren 2010; Mudge 2009a), or had significantly less risk exposure because they spent significantly less time engaged in physical therapy sessions (English 2015). Nevertheless, it would be pertinent for future studies to more closely examine the link between CCT and falls in therapy.
Carer burden was reported as increased in two studies comparing CCT plus education against no intervention (Harrington 2010; Marsden 2010). It is unknown how the burden was generated and whether it was simply because of receipt of an intervention per se ‐ this requires clarification in future studies.
Based on the results of the two available trials, there is currently no evidence for superior effectiveness of CCT when combined with education. Similarly, there is insufficient evidence for the relative effectiveness of CCT delivered at higher versus lower intensity (based on heart rate targets).
Overall completeness and applicability of evidence
The content of the intervention provided was similar across all studies with many of the same exercises and activities included (see Characteristics of included studies and Table 2). The majority (11) of the trials were conducted with participants later after stroke (more than one year), compared with earlier after stroke (less than one year, six trials) and whilst our subgroup analyses failed to show a significant difference in effect between the two time frames, there was a larger improvement noted in the later group for several measures. The influence of time alone on recovery after stroke remains largely unknown, although it has been estimated to account for between 16% and 42% of improvements in function in the first six to 10 weeks after stroke (Kwakkel 2004). This may mask any potential benefits of CCT over and above usual care in studies conducted with people earlier after stroke.
There were insufficient data available to examine the impact of CCT on sensorimotor impairment after stroke. No studies included measures of movement kinematics or stroke recovery biomarkers such as imaging. Therefore, we cannot determine the degree to which improvements in mobility measures are related to recovery of motor function, specifically 'true neurological recovery' (Levin 2009) versus compensation and overcoming deconditioning.
This updated review included four trials with sample sizes greater than 100 participants. When we pooled data from only these trials, the magnitude of effect for CCT was smaller, but remained statistically significant for the 6mWT. Smaller trials tend to over‐estimate treatment effects (Pereira 2012). The implications of population heterogeneity across large and small trials need to be considered. Furthermore, our 6mWT results were upheld after a sensitivity analysis for trials with low versus unclear/high risk of bias.
The ability to pool data across trials was somewhat limited by the diversity in outcome measures used. Across the 17 included trials, a total of 62 different outcome measures were used. Lack of commonality in outcome measures is a major issue hampering the progress of stroke rehabilitation and recovery research. An analysis of 38 trials in the Virtual International Stroke Archives in 2012 found at least 44 reported outcome measures, with age being the only common metric across trials (Ali 2013). A group of international experts is currently working on addressing this issue with consensus statements being produced as a result (Bernhardt 2016).
Quality of the evidence
The trials were of varying levels of assessed risk of bias. Most commonly, failure to report one or two domains led to a greater overall risk and it remains to be seen if standards of trial conduct and reporting improve in the future. We cannot differentiate between failure to report versus failure to control the risk and this is a potential source of bias in the review process. Hence we downgraded all GRADE determinations as a result of this uncertainty. Three studies achieved 'low' risk ratings in all six domains, confirming that stroke rehabilitation studies can be conducted and reported in an acceptable manner.
Potential biases in the review process
Potential biases in the review process need to be considered in that the three review authors are stroke rehabilitation trialists and take a pragmatic stand on trial design. For example, we did not assess trials as having a risk of bias where the therapist or the participants were not blinded, as we did not consider this possible in these kinds of clinical trials (other than to maintain the participant naive as to which arm of the trial was of interest to the researchers). The definition of CCT was relatively prescriptive and it may be that studies using an alternate circuit format were not included. For example, Kim 2016b compared group CCT with individualised CCT ‐ however their definition of individualised CCT met the criteria for this review's group CCT, thus not offering a useful comparator. It is important that studies such as this are considered in future, as they may help ascertain which aspects of CCT are effective.
Agreements and disagreements with other studies or reviews
This updated review strengthens the findings of our previous review and the systematic review of Wevers 2009, that CCT is an effective intervention for improving walking ability in people after stroke. The updated findings highlight that the benefit of CCT is reported regardless of time after stroke. This update also provides new evidence that CCT may be an effective method of improving cardiorespiratory fitness and increased daily physical activity (step counts).
Authors' conclusions
Implications for practice.
Based on the existing moderate evidence, circuit class therapy (CCT) is effective in improving walking ability in people after stroke, and this effect was found when delivered in early and late periods after stroke. There is insufficient evidence to determine whether providing physiotherapy using the CCT format for people receiving inpatient rehabilitation may reduce length of hospital stay. Relative to other interventions, there is insufficient evidence to determine whether CCT was associated with an increased risk of falls Therapists should use strategies to reduce the risk of falls while trying to maintain the integrity of the intervention.
Implications for research.
The evidence is becoming clearer and more consistent for the effectiveness of CCT for improving mobility in people after stroke who are able to walk independently. It will be important in future trials to include different subgroups of people with stroke, as well as measurement of changes at the impairment level to help to determine the effect of CCT on true neurological recovery versus compensation. Other aspects of the mechanism of effect are also not clear and likely to be a combination of increased motivation, amount and intensity of practice, as well as the specificity of the practice. Mechanism pathways need further investigation. Further investigation is also needed into the mitigation of risk of falls and the potential strain on carers.
What's new
| Date | Event | Description |
|---|---|---|
| 23 June 2017 | Amended | Correction to forest plot axis label (Analysis 1.1) |
History
Protocol first published: Issue 1, 2009 Review first published: Issue 7, 2010
| Date | Event | Description |
|---|---|---|
| 28 May 2017 | New citation required but conclusions have not changed | Greater number of studies supporting the main conclusion that circuit class therapy is effective at improving mobility for people after stroke. |
| 28 January 2017 | New search has been performed | Searches updated and 12 new trials involving 1005 new participants included. This review now includes 17 trials and 1297 participants. |
| 9 July 2010 | Amended | Minor correction made to the participant characteristics in the Results section of the Abstract and under Included studies in the main Results section of the review. |
Acknowledgements
Anthea Worley and Debbie Booth for assisting with searches. Ashlee Dunn and Gary Crowfoot for assistance with data extraction and editing. Staff of the Cochrane Stroke Group.
Appendices
Appendix 1. CENTRAL search strategy
1. [mh ^"cerebrovascular disorders"] or [mh "basal ganglia cerebrovascular disease"] or [mh "brain ischemia"] or [mh "carotid artery diseases"] or [mh "intracranial arterial diseases"] or [mh "intracranial arteriovenous malformations"] or [mh "intracranial embolism and thrombosis"] or [mh "intracranial hemorrhages"] or [mh ^stroke] or [mh "brain infarction"] or [mh ^"stroke, lacunar"] or [mh ^"vasospasm, intracranial"] or [mh ^"vertebral artery dissection"] 2. (stroke or poststroke or post‐stroke or cerebrovasc* or brain next vasc* or cerebral next vasc* or cva* or apoplex* or SAH):ti,ab,kw (Word variations have been searched) 3. ((brain* or cerebr* or cerebell* or intracran* or intracerebral) near/5 (isch*emi* or infarct* or thrombo* or emboli* or occlus*)):ti,ab,kw (Word variations have been searched) 4. ((brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid) near/5 (haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed*)):ti,ab,kw (Word variations have been searched) 5. [mh ^hemiplegia] or [mh paresis] 6. (hempar* or hemipleg* or brain next injur*):ti,ab,kw (Word variations have been searched) 7. [mh "gait disorders, neurologic"] 8. {or #1‐#6} 9. [mh ^"exercise movement techniques"] or [mh ^"exercise therapy"] or [mh ^"muscle stretching exercises"] or [mh ^"plyometric exercise"] or [mh ^"resistance training"] or [mh ^walking] 10. [mh ^"physical fitness"] or [mh ^"physical exertion"] or [mh ^"physical endurance"] or [mh locomotion] 11. [mh ^sports] or [mh ^bicycling] or [mh ^gymnastics] or [mh ^"weight lifting"] or [mh ^running] 12. [mh ^"task performance and analysis"] or [mh ^"athletic performance"] or [mh ^"mobility limitation"] 13. [mh ^"physical therapy modalities"] or [mh ^"physical therapy specialty"] 14. (physical near/3 (exercise* or therap* or conditioning or activit* or fitness or endurance)):ti,ab,kw (Word variations have been searched) 15. (exercise near/3 (train* or intervention* or protocol* or program* or therap* or activit* or regim*)):ti,ab,kw (Word variations have been searched) 16. (fitness near/3 (train* or intervention* or protocol* or program* or therap* or activit* or regim*)):ti,ab,kw (Word variations have been searched) 17 ((training or conditioning) near/3 (intervention* or protocol* or program* or activit* or regim*)):ti,ab,kw (Word variations have been searched) 18. (sport* or cycl* or bicycl* or treadmill* or run* or walk*):ti,ab,kw (Word variations have been searched) 19. muscle strengthening:ti,ab,kw (Word variations have been searched) 20. ((weight or strength or resistance) near (train* or lift* or exercise*)):ti,ab,kw (Word variations have been searched) 21. {or #9‐#20} 22. [mh ^"fitness centers"] or [mh ^"sports equipment"] 23. (circuit near/3 (class or classes or therapy or training or program* or exercise* or arranged or arrangement)):ti,ab,kw (Word variations have been searched) 24. (sport* equipment or station or work station):ti,ab,kw (Word variations have been searched) 25. (fitness near/3 (center* or centre* or group* or class or classes or training or program*)):ti,ab,kw (Word variations have been searched) 26. (exercise* near/3 (routine* or group* or class or classes)):ti,ab,kw (Word variations have been searched) 27. ((task‐related or sequential) near/3 exercise):ti,ab,kw (Word variations have been searched) 28. group environment:ti,ab,kw (Word variations have been searched) 29. (repetitive pract* or functional task*):ti,ab,kw (Word variations have been searched) 30. {or #22‐#29} 31. [mh ^"cerebrovascular disorders"/RH] or [mh "basal ganglia cerebrovascular disease"/RH] or [mh "brain ischemia"/RH] or [mh "carotid artery diseases"/RH] or [mh "intracranial arterial diseases"/RH] or [mh "intracranial arteriovenous malformations"/RH] or [mh "intracranial embolism and thrombosis"/RH] or [mh "intracranial hemorrhages"/RH] or [mh ^stroke/RH] or [mh "brain infarction"/RH] or [mh ^"stroke, lacunar"/RH] or [mh ^"vasospasm, intracranial"/RH] or [mh ^"vertebral artery dissection"/RH] 32. #8 and #21 33. #31 or #32 34. #30 and #33
Appendix 2. MEDLINE search strategy
We used the following search strategy for MEDLINE (Ovid) and adapted it to search the other databases. As the subject area of this review is quite specific we did not include a trials filter. This increased the sensitivity of the search.
MEDLINE (Ovid)
1. cerebrovascular disorders/ or exp basal ganglia cerebrovascular disease/ or exp brain ischemia/ or exp carotid artery diseases/ or exp intracranial arterial diseases/ or exp "intracranial embolism and thrombosis"/ or exp intracranial hemorrhages/ or stroke/ or exp brain infarction/ or vasospasm, intracranial/ or vertebral artery dissection/ 2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw. 3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw. 4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw. 5. hemiplegia/ or exp paresis/ 6. (hemipleg$ or hemipar$ or paresis or paretic).tw. 7. exp gait disorders, neurologic/ 8. or/1‐7 9. exercise movement techniques/ or exercise therapy/ or muscle stretching exercises/ or plyometric exercise/ or resistance training/ or walking/ 10. physical fitness/ or physical exertion/ or physical endurance/ or exp locomotion/ 11. sports/ or bicycling/ or gymnastics/ or weight lifting/ or running/ 12. "task performance and analysis"/ or athletic performance/ or mobility limitation/ 13. physical therapy modalities/ or physical therapy specialty/ 14. (physical adj3 (exercise$ or therap$ or conditioning or activit$ or fitness or endurance)).tw. 15. (exercise adj3 (train$ or intervention$ or protocol$ or program$ or therap$ or activit$ or regim$)).tw. 16. (fitness adj3 (train$ or intervention$ or protocol$ or program$ or therap$ or activit$ or regim$)).tw. 17. ((training or conditioning) adj3 (intervention$ or protocol$ or program$ or activit$ or regim$)).tw. 18. (sport$ or cycl$ or bicycl$ or treadmill$ or run$ or walk$).tw. 19. muscle strengthening.tw. 20. ((weight or strength or resistance) adj (train$ or lift$ or exercise$)).tw. 21. 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 22. fitness centers/ or sports equipment/ 23. (circuit adj3 (class or classes or therapy or training or program$ or exercise$ or arranged or arrangement)).tw. 24. (sport$ equipment or station or work station).tw. 25. (fitness adj3 (center$ or centre$ or group$ or class or classes or training or program$)).tw. 26. (exercise$ adj3 (routine$ or group$ or class or classes)).tw. 27. ((task‐related or sequential) adj3 exercise$).tw. 28. group environment.tw. 29. (repetitive pract$ or functional task$).tw. 30. 22 or 23 or 24 or 25 or 26 or 27 or 28 or 29 31. cerebrovascular disorders/rh or exp basal ganglia cerebrovascular disease/rh or exp brain ischemia/rh or exp carotid artery diseases/rh or exp intracranial arterial diseases/rh or exp "intracranial embolism and thrombosis"/rh or exp intracranial hemorrhages/rh or stroke/rh or exp brain infarction/rh or vasospasm, intracranial/rh or vertebral artery dissection/rh 32. 8 and 21 33. 31 or 32 34. 30 and 33 35. limit 34 to human
Appendix 3. Embase search strategy
1. cerebrovascular disease/ or brain disease/ or exp basal ganglion hemorrhage/ or exp brain hemangioma/ or exp brain hematoma/ or exp brain hemorrhage/ or exp brain infarction/ or exp brain ischemia/ or exp carotid artery disease/ or exp cerebral artery disease/ or exp cerebrovascular accident/ or exp cerebrovascular malformation/ or exp intracranial aneurysm/ or exp occlusive cerebrovascular disease/ or exp vertebrobasilar insufficiency/ 2. (stroke$ or poststroke or apoplex$ or cerebral vasc$ or brain vasc$ or cerebrovasc$ or cva$ or SAH).tw. 3. ((brain or cerebr$ or cerebell$ or vertebrobasil$ or hemispher$ or intracran$ or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA$ or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$ or hypoxi$)).tw. 4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracran$ or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli$ or putaminal or putamen or posterior fossa or hemispher$ or subarachnoid) adj5 (h?emorrhag$ or h?ematoma$ or bleed$)).tw. 5. hemiparesis/ or hemiplegia/ or paresis/ 6. (hemipleg$ or hemipar$ or paresis or paretic).tw. 7. 1 or 2 or 3 or 4 or 5 or 6 8. exp kinesiotherapy/ or stretching exercise/ or muscle stretching/ or muscle exercise/ or plyometrics/ or resistance training/ or walking/ or exercise/ or circuit training/ or endurance training/ 9. fitness/ or exercise intensity/ or endurance/ or exp locomotion/ 10. physical activity/ or sport/ or body building/ or cycling/ or endurance sport/ or jogging/ or running/ or weight lifting/ 11. task performance/ or physical performance/ or athletic performance/ or walking difficulty/ 12. physiotherapy/ 13. (physical adj3 (exercise$ or therap$ or conditioning or activit$ or fitness or endurance)).tw. 14. (exercise adj3 (train$ or intervention$ or protocol$ or program$ or therap$ or activit$ or regim$)).tw. 15. (fitness adj3 (train$ or intervention$ or protocol$ or program$ or therap$ or activit$ or regim$)).tw. 16. ((training or conditioning) adj3 (intervention$ or protocol$ or program$ or activit$ or regim$)).tw. 17. (sport$ or cycl$ or bicycl$ or treadmill$ or run$ or walk$).tw. 18. muscle strengthening.tw. 19. ((weight or strength or resistance) adj (train$ or lift$ or exercise$)).tw. 20. 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 or 19 21. health center/ or exp sports equipment/ 22. (circuit adj3 (class or classes or therapy or training or program$ or exercise$ or arranged or arrangement)).tw. 23. (sports equipment or station or work station).tw. 24. (fitness adj3 (center$ or centre$ or group$ or class or classes or training or program$)).tw. 25. (exercise$ adj3 (routine$ or group$ or class or classes)).tw. 26. ((task‐related or sequential) adj3 exercise$).tw. 27. group environment.tw. 28. (repetitive pract$ or functional task$).tw. 29. 21 or 22 or 23 or 24 or 25 or 26 or 27 or 28 30. cerebrovascular disease/rh or brain disease/rh or exp basal ganglion hemorrhage/rh or exp brain hemangioma/rh or exp brain hematoma/rh or exp brain hemorrhage/rh or exp brain infarction/rh or exp brain ischemia/rh or exp carotid artery disease/rh or exp cerebral artery disease/rh or exp cerebrovascular accident/rh or exp cerebrovascular malformation/rh or exp intracranial aneurysm/rh or exp occlusive cerebrovascular disease/rh or exp vertebrobasilar insufficiency/rh 31. 7 and 20 32. 30 or 31 33. 29 and 32
Appendix 4. CINAHL search strategy
S1 (MH "Cerebrovascular Disorders") OR (MH "Basal Ganglia Cerebrovascular Disease+") OR (MH "Carotid Artery Diseases+") OR (MH "Cerebral Ischemia+") OR (MH "Cerebral Vasospasm") OR (MH "Intracranial Arterial Diseases+") OR (MH "Intracranial Embolism and Thrombosis") OR (MH "Intracranial Hemorrhage+") OR (MH "Stroke") OR (MH "Vertebral Artery Dissections") S2 (MH "Stroke Patients") OR (MH "Stroke Units") S3 TI ( stroke* or poststroke or apoplex* or cerebral vasc* or brain vasc* or cerebrovasc* or cva* or SAH ) or AB ( stroke* or poststroke or apoplex* or cerebral vasc* or brain vasc* or cerebrovasc* or cva* or SAH ) S4 TI ( brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA* or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying ) or AB ( brain or cerebr* or cerebell* or vertebrobasil* or hemispher* or intracran* or intracerebral or infratentorial or supratentorial or middle cerebral artery or MCA* or anterior circulation or posterior circulation or basilar artery or vertebral artery or space‐occupying ) S5 TI ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* or hypoxi* ) or AB ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* or hypox* ) S6 S4 and S5 S7 TI ( brain* or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli* or putaminal or putamen or posterior fossa or hemispher* or subarachnoid ) or AB ( brain* or cerebr* or cerebell* or intracerebral or intracran* or parenchymal or intraparenchymal or intraventricular or infratentorial or supratentorial or basal gangli* or putaminal or putamen or posterior fossa or hemispher* or subarachnoid ) S8 TI ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) or AB ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) S9 S7 and S8 S10 (MH "Hemiplegia") S11 TI ( hemipleg* or hemipar* or paresis or paretic ) or AB ( hemipleg* or hemipar* or paresis or paretic ) S12 S1 OR S2 OR S3 OR S6 OR S9 OR S10 OR S11 S13 (MH "Exercise") OR (MH "Therapeutic Exercise") OR (MH "Muscle Strengthening") OR (MH "Stretching") OR (MH "Plyometrics") OR (MH "Group Exercise") OR (MH "Muscle Strengthening") OR (MH "Resistance Training") S14 (MH "Physical Fitness") OR (MH "Physical Performance") OR (MH "Physical Activity") OR (MH "Physical Endurance+") OR (MH "Muscle Strength") OR (MH "Locomotion+") S15 (MH "Sports") OR (MH "Cycling") OR (MH "Gymnastics") OR (MH "Weight Lifting") OR (MH "Running") OR (MH "Jogging") S16 (MH "Task Performance and Analysis") OR (MH "Athletic Performance") OR (MH "Physical Mobility") S17 (MH "Physical Therapy") S18 ( TI physical AND TI ( (exercise* or therap* or conditioning or activit* or fitness or endurance) ) ) OR ( AB physical AND AB ( (exercise* or therap* or conditioning or activit* or fitness or endurance) ) ) S19 ( TI exercise AND TI ( (train* or intervention* or protocol* or program* or therap* or activit* or regim*) ) ) OR ( AB exercise AND AB ( (train* or intervention* or protocol* or program* or therap* or activit* or regim*) ) ) S20 ( TI fitness AND TI ( (train* or intervention* or protocol* or program* or therap* or activit* or regim*) ) ) OR ( AB fitness AND AB ( (train* or intervention* or protocol* or program* or therap* or activit* or regim*) ) ) S21 ( TI ( (training or conditioning) ) AND TI ( (intervention* or protocol* or program* or activit* or regim*) ) ) OR ( AB ( (training or conditioning) ) AND AB ( (intervention* or protocol* or program* or activit* or regim*) ) ) S22 TI ( (sport* or cycl* or bicycl* or treadmill* or run* or walk*) ) OR AB ( (sport* or cycl* or bicycl* or treadmill* or run* or walk*) ) S23 TI muscle strengthening OR AB muscle strengthening S24 ( TI ( (weight or strength or resistance) ) AND TI ( (train* or lift* or exercise*) ) ) OR ( AB ( (weight or strength or resistance) ) AND AB ( (train* or lift* or exercise*) ) ) S25 S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 S26 (MH "Fitness Centers") OR ( (MH "Sports Equipment and Supplies") ) S27 ( TI circuit AND TI ( (class or classes or therapy or training or program* or exercise* or arranged or arrangement) ) ) OR ( AB circuit AND AB ( (class or classes or therapy or training or program* or exercise* or arranged or arrangement) ) ) S28 TI ( (sports equipment or station or work station) ) OR AB ( (sports equipment or station or work station) ) S29 ( TI fitness AND TI ( (center* or centre* or group* or class or classes or training or program*) ) ) OR ( AB fitness AND AB ( (center* or centre* or group* or class or classes or training or program*) ) ) S30 ( TI exercise* AND TI ( (routine* or group* or class or classes) ) ) OR ( AB exercise* AND AB ( (routine* or group* or class or classes) ) ) S31 ( TI ( (task‐related or sequential) ) AND TI exercise* ) OR ( AB ( (task‐related or sequential) ) AND AB exercise* ) S32 TI group environment OR AB group environment S33 TI ( (repetitive pract* or functional task*) ) OR AB ( (repetitive pract* or functional task*) ) S34 S26 OR S27 OR S28 OR S29 OR S30 OR S31 OR S32 OR S33 S35 (MH "Cerebrovascular Disorders/RH") OR (MH "Basal Ganglia Cerebrovascular Disease+/RH") OR (MH "Carotid Artery Diseases+/RH") OR (MH "Cerebral Ischemia+/RH") OR (MH "Cerebral Vasospasm/RH") OR (MH "Intracranial Arterial Diseases+/RH") OR (MH "Intracranial Embolism and Thrombosis/RH") OR (MH "Intracranial Hemorrhage+/RH") OR (MH "Stroke/RH") OR (MH "Vertebral Artery Dissections/RH") S36 S9 AND S25 S37 S35 OR S36 S38 S34 AND S37
Appendix 5. PsycINFO search strategy
1. cerebrovascular disorders/ or cerebral hemorrhage/ or cerebral ischemia/ or cerebrovascular accidents/ 2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw. 3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw. 4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw. 5. paralysis/ or hemiplegia/ 6. (hemipleg$ or hemipar$ or paresis or paretic).tw. 7. 1 or 2 or 3 or 4 or 5 or 6 8. aerobic exercise/ or physical fitness/ or exercise/ or movement therapy/ or walking/ or locomotion/ 9. physical activity/ or physical mobility/ or physical agility/ or physical dexterity/ or physical therapy/ 10. athletic training/ or athletic performance/ or sports medicine/ or sports/ or weightlifting/ 11. (physical adj3 (exercise$ or therap$ or conditioning or activit$ or fitness or endurance)).tw. 12. (exercise adj3 (train$ or intervention$ or protocol$ or program$ or therap$ or activit$ or regim$)).tw. 13. (fitness adj3 (train$ or intervention$ or protocol$ or program$ or therap$ or activit$ or regim$)).tw. 14. ((training or conditioning) adj3 (intervention$ or protocol$ or program$ or activit$ or regim$)).tw. 15. (sport$ or cycl$ or bicycl$ or treadmill$ or run$ or walk$).tw. 16. muscle strengthening.tw. 17. ((weight or strength or resistance) adj (train$ or lift$ or exercise$)).tw. 18. or/8‐17 19. apparatus/ 20. (circuit adj3 (class or classes or therapy or training or program$ or exercise$ or arranged or arrangement)).tw. 21. (sport$ equipment or station or work station).tw. 22. (fitness adj3 (center$ or centre$ or group$ or class or classes or training or program$)).tw. 23. (exercise$ adj3 (routine$ or group$ or class or classes)).tw. 24. ((task‐related or sequential) adj3 exercise$).tw. 25. group environment.tw. 26. (repetitive pract$ or functional task$).tw. 27. or/19‐26 28. 27 and 18 and 7
Appendix 6. AMED search strategy
1. cerebrovascular disorders/ or cerebral hemorrhage/ or cerebral infarction/ or cerebral ischemia/ or cerebrovascular accident/ or stroke/ 2. (stroke or poststroke or post‐stroke or cerebrovasc$ or brain vasc$ or cerebral vasc$ or cva$ or apoplex$ or SAH).tw. 3. ((brain$ or cerebr$ or cerebell$ or intracran$ or intracerebral) adj5 (isch?emi$ or infarct$ or thrombo$ or emboli$ or occlus$)).tw. 4. ((brain$ or cerebr$ or cerebell$ or intracerebral or intracranial or subarachnoid) adj5 (haemorrhage$ or hemorrhage$ or haematoma$ or hematoma$ or bleed$)).tw. 5. hemiplegia/ 6. (hemipleg$ or hemipar$ or paresis or paretic).tw. 7. 1 or 2 or 3 or 4 or 5 or 6 8. exp exercise/ or physical fitness/ or exertion/ or lifting/ or exp physical endurance/ or immobility/ or resistance training/ 9. sports/ or bicycling/ or gymnastics/ or exp locomotion/ 10. physical therapy modalities/ or physical therapy speciality/ 11. (physical adj3 (exercise$ or therap$ or conditioning or activit$ or fitness or endurance)).tw. 12. (exercise adj3 (train$ or intervention$ or protocol$ or program$ or therap$ or activit$ or regim$)).tw. 13. (fitness adj3 (train$ or intervention$ or protocol$ or program$ or therap$ or activit$ or regim$)).tw. 14. ((training or conditioning) adj3 (intervention$ or protocol$ or program$ or activit$ or regim$)).tw. 15. (sport$ or cycl$ or bicycl$ or treadmill$ or run$ or walk$).tw. 16. muscle strengthening.tw. 17. ((weight or strength or resistance) adj (train$ or lift$ or exercise$)).tw. 18. 8 or 9 or 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 19. (circuit adj3 (class or classes or therapy or training or program$ or exercise$ or arranged or arrangement)).tw. 20. (sports equipment or station or work station).tw. 21. (fitness adj3 (center$ or centre$ or group$ or class or classes or training or program$)).tw. 22. (exercise$ adj3 (routine$ or group$ or class or classes)).tw. 23. ((task‐related or sequential) adj3 exercise$).tw. 24. group environment.tw. 25. (repetitive pract$ or functional task$).tw. 26. 19 or 20 or 21 or 22 or 23 or 24 or 25 27. 7 and 18 and 26
Appendix 7. SPORTDiscus
S1 DE "CEREBROVASCULAR disease" OR DE "BRAIN ‐‐ Hemorrhage" OR DE "CEREBRAL embolism & thrombosis" OR DE "STROKE" OR DE "BRAIN ‐‐ Wounds & injuries" OR DE "BRAIN damage" OR DE "CEREBROVASCULAR disease ‐‐ Patients" S2 TI ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH ) or AB ( stroke or poststroke or post‐stroke or cerebrovasc* or brain vasc* or cerebral vasc or cva or apoplex or SAH ) S3 ( TI ( brain* or cerebr* or cerebell* or intracran* or intracerebral ) or AB ( brain* or cerebr* or cerebell* or intracran* or intracerebral ) ) and ( TI ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* ) or AB ( ischemi* or ischaemi* or infarct* or thrombo* or emboli* or occlus* ) ) S4 ( TI ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid ) or AB ( brain* or cerebr* or cerebell* or intracerebral or intracranial or subarachnoid ) ) and ( TI ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) or AB ( haemorrhage* or hemorrhage* or haematoma* or hematoma* or bleed* ) ) S5 DE "HEMIPLEGIA" OR DE "HEMIPLEGICS" OR DE "GAIT disorders" S6 TI ( hemipleg* or hemipar* or paresis or paretic ) or AB ( hemipleg* or hemipar* or paresis or paretic ) S7 S1 OR S2 OR S3 OR S4 OR S5 OR S6 S8 DE "EXERCISE" OR DE "EXERCISE ‐‐ Equipment & supplies" OR DE "EXERCISE intensity" OR DE "EXERCISE physiology" OR DE "EXERCISE therapy" S9 (((DE "MUSCLE strength" OR DE "MUSCLE weakness") OR (DE "PLYOMETRICS")) OR (DE "RESISTANCE training (Physical training & conditioning)")) AND (DE "WALKING" OR DE "WALKING (Sports)" OR DE "WALKING (Sports) ‐‐ Training") S10 ((DE "PHYSICAL fitness") OR (DE "ENDURANCE sports" OR DE "ULTRAENDURANCE sports")) OR (DE "LOCOMOTION") S11 (DE "WEIGHT lifting" OR DE "BENCH press" OR DE "DEAD lift (Weight lifting)" OR DE "POWERLIFTING" OR DE "SQUAT (Weight lifting)" OR DE "WEIGHT lifting competitions") OR (DE "RUNNING") S12 (DE "PHYSICAL therapy" OR DE "SPORTS physical therapy" OR DE "RECOVERY training") OR (DE "CIRCUIT training") S13 ( TI physical AND TI ( (exercise* or therap* or conditioning or activit* or fitness or endurance) ) ) OR ( AB physical AND AB ( (exercise* or therap* or conditioning or activit* or fitness or endurance) ) ) S14 ( TI exercise AND TI ( (train* or intervention* or protocol* or program* or therap* or activit* or regim*) ) ) OR ( AB exercise AND AB ( (train* or intervention* or protocol* or program* or therap* or activit* or regim*) ) ) S15 ( TI fitness AND TI ( (train* or intervention* or protocol* or program* or therap* or activit* or regim*) ) ) OR ( AB fitness AND AB ( (train* or intervention* or protocol* or program* or therap* or activit* or regim*) ) ) S16 ( TI ( (training or conditioning) ) AND TI ( (intervention* or protocol* or program* or activit* or regim*) ) ) OR ( AB ( (training or conditioning) ) AND AB ( (intervention* or protocol* or program* or activit* or regim*) ) ) S17 TI ( (sport* or cycl* or bicycl* or treadmill* or run* or walk*) ) OR AB ( (sport* or cycl* or bicycl* or treadmill* or run* or walk*) ) S18 TI muscle strengthening OR AB muscle strengthening S19 ( TI ( (weight or strength or resistance) ) AND TI ( (train* or lift* or exercise*) ) ) OR ( AB ( (weight or strength or resistance) ) AND AB ( (train* or lift* or exercise*) ) ) S20 S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 S21 DE "PHYSICAL fitness centers" OR DE "WEIGHT training facilities" OR DE "GYMNASIUMS" OR DE "HEALTH facilities" OR DE "EXERCISE ‐‐ Equipment & supplies" S22 ( TI circuit AND TI ( (class or classes or therapy or training or program* or exercise* or arranged or arrangement) ) ) OR ( AB circuit AND AB ( (class or classes or therapy or training or program* or exercise* or arranged or arrangement) ) ) S23 TI ( (sports equipment or station or work station) ) OR AB ( (sports equipment or station or work station) ) S24 ( TI fitness AND TI ( (center* or centre* or group* or class or classes or training or program*) ) ) OR ( AB fitness AND AB ( (center* or centre* or group* or class or classes or training or program*) ) ) S25 ( TI exercise* AND TI ( (routine* or group* or class or classes) ) ) OR ( AB exercise* AND AB ( (routine* or group* or class or classes) ) ) S26 ( TI ( (task‐related or sequential) ) AND TI exercise* ) OR ( AB ( (task‐related or sequential) ) AND AB exercise* ) S27 TI group environment OR AB group environment S28 TI ( (repetitive pract* or functional task*) ) OR AB ( (repetitive pract* or functional task*) ) S29 S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 S30 S7 AND S20 AND S29
Data and analyses
Comparison 1. Circuit class therapy versus other.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 6mWT early and late | 10 | 835 | Mean Difference (IV, Fixed, 95% CI) | 60.86 [44.55, 77.17] |
| 1.1 Early | 4 | 487 | Mean Difference (IV, Fixed, 95% CI) | 46.56 [21.35, 71.77] |
| 1.2 Late | 6 | 348 | Mean Difference (IV, Fixed, 95% CI) | 71.15 [49.76, 92.54] |
| 2 Gait speed early and late | 8 | 744 | Mean Difference (IV, Fixed, 95% CI) | 0.15 [0.10, 0.19] |
| 2.1 Early | 2 | 437 | Mean Difference (IV, Fixed, 95% CI) | 0.17 [0.10, 0.25] |
| 2.2 Late | 6 | 307 | Mean Difference (IV, Fixed, 95% CI) | 0.13 [0.07, 0.19] |
| 3 Cadence | 2 | 50 | Mean Difference (IV, Random, 95% CI) | 13.57 [7.52, 19.62] |
| 4 Timed Up and Go | 5 | 488 | Mean Difference (IV, Fixed, 95% CI) | ‐3.62 [‐6.09, ‐1.16] |
| 5 Rivermead Mobility Index | 2 | 296 | Mean Difference (IV, Fixed, 95% CI) | 0.56 [0.17, 0.95] |
| 6 Functional Ambulation Classification | 3 | 469 | Odds Ratio (M‐H, Random, 95% CI) | 1.91 [1.01, 3.60] |
| 7 Berg Balance Scale | 4 | 171 | Mean Difference (IV, Random, 95% CI) | 1.21 [‐0.62, 3.04] |
| 8 Step Test | 3 | 190 | Mean Difference (IV, Fixed, 95% CI) | 0.98 [‐0.40, 2.37] |
| 9 Activities‐specific Balance Confidence Scale | 2 | 103 | Mean Difference (IV, Fixed, 95% CI) | 7.76 [0.66, 14.87] |
| 10 Stroke Impact Scale (physical) | 2 | 437 | Mean Difference (IV, Random, 95% CI) | 2.91 [0.00, 5.82] |
| 11 VO2 peak | 2 | 103 | Mean Difference (IV, Fixed, 95% CI) | 2.81 [0.90, 4.72] |
| 12 Steps per day | 2 | 206 | Mean Difference (IV, Fixed, 95% CI) | 1325.66 [411.09, 2240.22] |
| 13 Length of stay | 2 | 217 | Mean Difference (IV, Random, 95% CI) | ‐16.35 [‐37.69, 4.99] |
| 14 Sensitivity: 6mWT | 3 | 393 | Mean Difference (IV, Fixed, 95% CI) | 46.32 [17.40, 75.24] |
Comparison 2. CCT + education versus no therapy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Timed Up and Go | 2 | 269 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐0.94, 2.75] |
| 2 Carer Strain Index | 2 | 174 | Mean Difference (IV, Fixed, 95% CI) | 1.06 [0.39, 1.73] |
Comparison 3. CCT +/‐ education versus any other intervention.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Adverse events (falls) | 8 | 815 | Risk Difference (M‐H, Random, 95% CI) | 0.03 [‐0.02, 0.08] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Blennerhassett 2004.
| Methods | RCT Mobility CCT versus upper limb CCT | |
| Participants | 30 participants (15 each group) receiving inpatient rehabilitation (mean of 43 days post‐stroke), mean age 55.1 years, able to walk 10 m with close supervision with or without gait aids | |
| Interventions | Intervention: mobility‐related CCT, 10 5‐minute workstations consisting of functional tasks including sit to stand, step ups, obstacle course walking, standing balance, stretching and strengthening exercises); 1 h/day, 5 days/week for 4 weeks Comparison: upper limb‐related CCT, 10 5‐minute workstations consisting of functional tasks to improve reach to grasp, hand eye co‐ordination, stretching and strengthening exercises; 1 h/day, 5 days/week for 4 weeks Staff:participant ratio: 1:4 Both groups received additional CCT therapy in addition to usual care |
|
| Outcomes | 6mWT, Step Test, TUG, LOS, MAS upper arm and hand items, JTHFT | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Card draw: unclear how cards were constructed |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes, independent person |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Assessor blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 100% data at 4 weeks |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol |
| Other bias | Low risk | Adequate sample size |
Dean 2000.
| Methods | RCT Mobility CCT versus upper limb CCT | |
| Participants | 9 participants (intervention = 5, comparison = 4), mean 1.3 years post‐stroke, mean age 62.3 years, able to walk 10 m independently with or without gait aid | |
| Interventions | Intervention: mobility‐related CCT, 10 workstations functional tasks including seated reaching beyond arms' reach, sit to stand, stepping activities, heel lifts, standing balance, strengthening exercises, walking activities; 1 h, 3 times/week for 4 weeks
Comparison: upper limb‐related CCT, workstations consisting of upper limb tasks; 1 h, 3 times/week for 4 weeks Staff:participant ratio: 1:6 |
|
| Outcomes | 6mWT, Step Test, TUG, gait speed, peak vertical ground reaction force through affected lower limb during sit‐to‐stand, laboratory measures of gait kinematics and kinetics | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by lottery: "drawing two cards, one with subject's name and one with group allocation from two separate boxes" |
| Allocation concealment (selection bias) | Low risk | Cards drawn by a person independent of the study |
| Blinding (performance bias and detection bias) All outcomes | High risk | Clinical assessments, with exception of 6mWT, conducted by independent rater; however, this blinding may have been unmasked as the result of this observer inadvertently observing 1 training session |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing data balanced across groups (1 in experimental and 2 in control) for transport or unrelated illness reasons, but no intention‐to‐treat analysis undertaken |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol |
| Other bias | High risk | Very small sample size |
Dean 2012.
| Methods | RCT Mobility CCT + home exercise programme versus upper limb CCT + home exercise programme |
|
| Participants | 151 participants (intervention = 76, comparison = 75), mean 6.0 years post‐stroke, mean age 67.1 years, able to walk 10 m independently with or without gait aid | |
| Interventions | Intervention: mobility‐related CCT, task‐related training with progressive balance, strengthening, standing, walking and stair climbing exercises, home programme and advice to increase walking Comparison: upper‐limb related CCT, task‐related strength and co‐ordination training, cognitive training, home programme and advice to increase use of upper limb and engage in more cognitive tasks Staff:participant ratio: not reported |
|
| Outcomes | 6mWT, gait speed, TUG, 5 x sit‐to‐stand test, step test, timed single leg stance, co‐ordinated stability test, maximal balance range, choice stepping reaction time, number falls in 12 months, falls risk score, knee extensor strength, Short Form‐12, Adelaide Activity Profile, daily step counts | |
| Notes | Adverse events and attendance at classes also reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was computer‐generated prior to commencement of study |
| Allocation concealment (selection bias) | Low risk | Sealed, opaque envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The participants and therapists delivering the intervention could not be blinded to intervention group allocation." Apart from self‐reported falls, "All other outcome measures were collected by an assessor who was blinded to group allocation." Participants asked not to reveal details of the programme to the assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Missing data for individual variables were imputed using regression, where possible." "Overall, missing data amounted to less than 10%." "Attendance records kept by the therapists indicated that only 6 participants (experimental = 1; control = 5) did not attend a single class." All reasons for loss to follow‐up were reported |
| Selective reporting (reporting bias) | Low risk | Protocol available, and all the pre‐specified outcomes were reported |
| Other bias | Low risk | No other sources of bias are evident |
English 2015.
| Methods | RCT (3 arms) CCT (mobility and upper‐limb) versus usual care (one‐to‐one therapy, 5 days/week) versus 7‐day/week therapy (one‐to‐one). Only CCT and usual care arms included in this review |
|
| Participants | 283 participants in whole trial (intervention = 93, comparison = 94), mean 29.8 days post‐stroke, mean age 69.1 years, moderate disability (FIM total score of 40‐80 OR motor score of 38‐62) | |
| Interventions | Intervention: physiotherapy service provided in twice daily 90‐min CCT sessions, 5 days/week primarily focused on mobility. Included task‐specific, individually progressed exercises focused on improving walking and standing activities Comparision: physiotherapy services based on usual care; primarily provided in individual, one‐to‐one therapy sessions 5 days/week Staff:participant ratio: between 1:3 and 1:6 |
|
| Outcomes | 6mWT, gait speed, functional ambulation classification, FIM, Stroke Impact Scale, Wolf Motor Function Test, Australian Quality of Life score | |
| Notes | Data on therapy time provided and adverse events also available | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computer‐generated randomization sequence was blocked to ensure equal numbers for each arm in each block of 15." |
| Allocation concealment (selection bias) | Low risk | "Randomisation was concealed by use of a central telephone service administered by staff not involved in the trial." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | No mention of blinding participants or personnel. It would be unlikely that study participants and staff were blinded due to the nature of the trial. Unlikely to influence outcomes: "All outcomes were assessed by a trained assessor who was blinded to group allocation" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Analyses were first conducted with no imputation of missing data (reported)." The study found that when a multiple imputation was applied, it did not significantly influence the results Figure 1 shows a flow of participants including reasons for participants lost to follow‐up. Usual care therapy = 6/94, 7 days/week = 8/96, usual care = 8/93 |
| Selective reporting (reporting bias) | Low risk | Protocol available, and all the pre‐specified outcomes were reported Cost‐effectiveness sub‐study to be reported in a different paper |
| Other bias | Low risk | No other sources of bias are evident |
Harrington 2010.
| Methods | RCT Mobility CCT + education versus standard care and information sheet about support services |
|
| Participants | 243 participants (intervention = 119, comparison = 124), minimum 12 months post, median 10.3 years post‐stroke, mean age 70.5 years, living in the community and able to participate in groups | |
| Interventions | Intervention: CCT with exercises adapted to ability aimed at improving balance, strength and endurance, plus home exercise programme, plus interactive self‐management education sessions; 1 h exercise and 1 h of education twice a week for 8 weeks Comparison: standard care and information sheet with list of local exercise classes Duration and frequency: not reported Staff:participant ratio: 2:9 |
|
| Outcomes | TUG, RMI, Functional Reach, Frenchay Activities Index, Hosptial Anxiety and Depression Scale, Subjective Impact of Physical and Social Outcome, WHO‐QoL, Carer Strain Index | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "We used computer generated numbers in geographical blocks of 18 participants, with the unit of randomization being the patient." |
| Allocation concealment (selection bias) | Low risk | "Randomization was carried out centrally by an independent assistant who took no part in recruitment." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Due to the nature of the intervention it was not possible to blind either the participants or the individuals involved in running the schemes…" "…outcome was assessed by a blinded assessor." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Analysis was undertaken on an intention‐to‐treat basis Figure 1 shows participant flow, with reasons for loss to follow‐up available |
| Selective reporting (reporting bias) | Unclear risk | No study protocol |
| Other bias | Low risk | No other source of bias evident |
Holmgren 2010.
| Methods | RCT Mobility CCT + education versus education only |
|
| Participants | 34 participants (intervention = 15, comparison = 15), mean time since stroke 0.36 years, mean age 78.5 years, able to walk 10 m independently with or without gait aid (excluded if able to walk outdoors independently) | |
| Interventions | Intervention: mobility‐related CCT, focus on physical activity and functional performance and education about falls risk CCT duration not specified, 7 sessions a week for 5 weeks; education 1 h/week for 5 weeks Comparison: education about coping with hidden dysfunctions after stroke 1 h/week for 5 weeks Staff:participant ratio: not reported |
|
| Outcomes | Short‐form 36, Geriatric Depression Scale | |
| Notes | Secondary outcomes reported from original trial. Original trial not published suggesting possible publication bias | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "…was conducted with a minimization software program, MiniM to avoid imbalances at baseline between the two groups." |
| Allocation concealment (selection bias) | High risk | Two main investigators responsible for randomisation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Single‐blinded Participants were instructed not to reveal anything about group allocation. "All participants were blinded as for the content of the two different groups before randomization." No mention of blinding of staff, however unlikely due to nature of trial. Unlikely to influence outcomes. "The nurses and physiotherapists who performed the clinical test assessments were blinded to group allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis Figure 1 shows the participant flow including reasons for loss to follow‐up All but 1 participant completed the 5‐week intervention period 2 participants dropped out at follow‐up due to health reasons |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | No other sources of bias evident |
Kim 2016a.
| Methods | RCT Mobility CCT versus usual care therapy |
|
| Participants | 20 participants (intervention = 10, comparison = 10), mean time since stroke 30.0 days, mean age 65.6, score 3 or 4 on Functional Ambulation Classification (able to walk with no more than 1 person assisting), less than 3 months post‐stroke | |
| Interventions | Intervention: mobility‐related CCT, including trunk exercises, active sitting practice, sit‐to‐stand practice, standing and walking practice, aerobic exercise and strength training; 90 min/per day, 5 days/week for 4 weeks Comparison: usual care physiotherapy provided in 2 x 30‐min sessions, 5 x per week for 4 weeks. Content based on neurodevelopmental approach and provided in one‐to‐one therapy sessions Staff:participant ratio: at least 2 participants to 1 therapist |
|
| Outcomes | 6mWT, BBS, modified Barthel Index (Korean version), lower limb score of the Fugl‐Meyer assessment | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised via sealed envelope technique, random sequence generation not stated |
| Allocation concealment (selection bias) | High risk | No mention of allocation concealment |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Assessors blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing data |
| Selective reporting (reporting bias) | Unclear risk | No trial protocol |
| Other bias | Low risk | No other sources of bias identified |
Marigold 2005.
| Methods | RCT Mobility‐related CCT versus general balance class | |
| Participants | 59 participants (Group 1 = 28, Group 2 = 31), mean 3.7 years post‐stroke, mean age 67.8 years, able to walk 10 m independently with or without gait aid | |
| Interventions | Intervention: mobility‐related CCT including walking, standing tasks focused on balance, sit to stand; 1‐h sessions, 3 times/week for 10 weeks
Group 2: stretching and slow weight shifting exercises; 1‐h sessions, 3 times/week for 10 weeks Staff:participant ratio: not reported |
|
| Outcomes | BBS, TUG, ABC, NHP, standing postural reflexes using force platform, self‐reported prospective falls diary | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated codes |
| Allocation concealment (selection bias) | Low risk | Person independent of the study |
| Blinding (performance bias and detection bias) All outcomes | Low risk | All assessors were blinded to group assignment, study design and purpose |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Total of 11 lost before post‐intervention testing, another 6 lost before follow‐up No intention‐to‐treat analysis or imputation of missing data |
| Selective reporting (reporting bias) | Unclear risk | Protocol not published so unclear regarding whether all outcomes reported |
| Other bias | Low risk | Adequate sample size |
Marsden 2010.
| Methods | RCT cross‐over design Mobility‐related CCT plus education versus wait list control |
|
| Participants | 26 participants (Group 1 = 12, Group 2 = 14), mean 2.5 years post‐stroke, mean age 71.7 years, clinical diagnosis of stroke | |
| Interventions | Intervention: mobility‐related CCT, 10 x 5‐min workstations consisting of sit to stand, reaching, standing balance, walking figure 8, stationary bike; 1 h exercise and 1 h education, once a week for 7 weeks
Comparison: wait list control Staff:participant ratio: 1:3 |
|
| Outcomes | 6mWT, TUG, Short‐form 36 (physical), Carer Strain Index | |
| Notes | Only first comparison (pre‐cross over) included | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Toss of a coin by a team member |
| Allocation concealment (selection bias) | High risk | Team member responsible for allocation |
| Blinding (performance bias and detection bias) All outcomes | High risk | No mention of blinding of staff or participants, however unlikely due to nature of trial. Unlikely to influence outcomes Primary outcomes assessor blinded Secondary outcome assessors not blinded. Only secondary outcomes used in the meta‐analysis |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat. "… but no values were imputed for survivors or carers who did not attend an assessment session." Only 1 loss to follow‐up (hospitalisation) for intervention group. Figure 1 flow diagram |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | No other sources of bias evident |
Moore 2015.
| Methods | RCT Mobility CCT vs home stretching (matched duration) |
|
| Participants | 40 participants (intervention = 20, comparison = 20), mean time since stroke 1.5 years, mean age 69 years, able to complete 6mWT with or without gait aid | |
| Interventions | Intervention: mobility CCT based on FAME programme including warm‐up, stretching, functional strengthening, balance, agility & fitness, cool down; 45‐60 minutes, 3 times/week for 19 weeks Comparison: home stretching programme of matched duration; 45 to 60 minutes 3 times/week for 19 weeks Staff:participant ratio: not reported |
|
| Outcomes | 6mWT, gait speed, BBS, SIS (physical), VO2 peak, peak work rate, Addenbrook's Cognitive Examination (revised, ACE‐r), blood cholesterol, 2‐hour glucose, HOMA index, blood pressure, BMI, fat mass, brain physiology (cerebral blood flow) | |
| Notes | Adverse events reported, not actual therapy time delivered | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A computerized random number generator was used to allocate treatment by an independent administrator after screening." |
| Allocation concealment (selection bias) | Low risk | "… the administrator was telephoned for the next number in the sequence to enable participant randomisation." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Single‐blind RCT No mention of blinding participants or personnel. It would be unlikely that study participants and staff were blinded due to the nature of the trial. Unlikely to influence outcomes Assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | States participants performed "… > 90% of outcome assessments and exercise sessions." Although these were not defined All participants completed the intervention, none lost to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | No other sources of bias evident |
Mudge 2009a.
| Methods | RCT Mobility‐related CCT versus education or social groups | |
| Participants | 58 participants (Group 1 = 31, Group 2 = 27), mean 4.9 years post‐stroke, mean age 69.1 years, able to walk 10 m independently with or without gait aid | |
| Interventions | Intervention: mobility CCT, 15 2‐min workstations including walking, standing balance and strengthening; 50‐60 min 3 times/week for 4 weeks Comparison: 4 social and 4 educational sessions; duration not specified, twice a week for 4 weeks Staff:participant ratio: 3:9 |
|
| Outcomes | Gait speed, 6mWT, RMI, ABC, steps per day using activity monitor, PADS | |
| Notes | Repetitions and details about exercise intensity recorded each station | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Person independent of the study matched the participants to the codes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Unmasking occurred for 3 out of 58 participants (5%) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2 lost before randomisation, 3 withdrew before post‐intervention assessment and a further 5 lost before follow‐up assessment; losses balanced across groups Intention‐to‐treat analysis undertaken with imputation of missing data using carry forward method |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | Adequate sample size |
Outermans 2010.
| Methods | RCT High‐intensity, mobility‐related CCT versus low‐intensity, mobility‐related CCT |
|
| Participants | 44 participants (intervention = 23, comparison = 21), mean time since stroke 0.75 months, mean age 56.6, able to walk 10 m independently | |
| Interventions | Intervention: high‐intensity mobility CCT, workstations based on Dean 2000 with progressive target heart rate; 45‐60 minutes, 3 times/week for 4 weeks in addition to 30 min/day usual care physiotherapy Comparison: low‐intensity mobility CCT, not clear if same exercises were included, no progression of heart rate; 45‐60 minutes, 3 times/week for 4 weeks in addition to 30 min/day usual care physiotherapy Staff:participant ratio: not reported |
|
| Outcomes | 6mWT, gait speed, BBS, functional reach | |
| Notes | Adverse events, duration (number of days) of training | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Reported as "randomly generated" but description of how was not presented |
| Allocation concealment (selection bias) | Low risk | "Allocation was performed by drawing randomly generated lots enclosed in opaque envelopes." |
| Blinding (performance bias and detection bias) All outcomes | High risk | "All clinical assessments were conducted by one assessor, who was not blinded for allocation. To minimize bias the assessor was not present at the group training at any time. Also previous assessments were not available during post‐test assessment and all instructions were standardized." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat. "Missing values were imputed using the assumption of a worst‐case scenario in which the baseline value was carried forward." Reasons for loss to follow‐up are available: 6 lost in intervention group, 7 in control group |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | No other sources of bias evident |
Pang 2005.
| Methods | RCT Mobility CCT versus upper limb CCT | |
| Participants | 63 participants (Group 1 = 32, Group 2 = 31), mean 5.1 years post‐stroke, mean age 65.3 years, able to walk 10 m independently with or without gait aids | |
| Interventions | Intervention: mobility‐related CCT based on FAME programme including warm‐up, stretching, functional strengthening, balance, agility & fitness, cool down including target heart rate; 1‐h session, 3 times/week for 19 weeks
Comparison: upper‐limb‐related CCT including strengthening, range of motion, functional reach and manipulation tasks; 1‐h session, 3 times/week for 19 weeks Staff:participant ratio: not reported |
|
| Outcomes | 6mWT, BBS, VO2max, knee extension strength (dynamometer), PASIPD, proximal femur BMD | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Drawing ballots |
| Allocation concealment (selection bias) | Low risk | Ballots drawn by person not involved with enrolment, screening, or outcome assessments |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Research personnel who performed outcome assessments were blinded to group assignment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar small amount of missing data across groups Missing data imputed from baseline values and intention‐to‐treat analysis used |
| Selective reporting (reporting bias) | Unclear risk | This study was reported in at least 3 separate papers all including different outcome measures |
| Other bias | Low risk | Adequate sample size |
Song 2015.
| Methods | RCT Mobility CCT vs mobility CCT individually provided vs conventional therapy |
|
| Participants | 30 participants (intervention = 11, comparison (individual) = 10, comparison (conventional therapy) = 9, more than 6 months post‐stroke (mean and upper range not given), mean age 56.2, able to walk 10 m without assistance | |
| Interventions | Intervention: mobility CCT, provided in circuit Comparison (individual): mobility exercises, provided one‐to‐one Comparison (conventional therapy): not described 30 min/day, 3 times/week for 4 weeks Inpatient rehabilitation Staff:participant ratio: not specified |
|
| Outcomes | Gait speed, cadence, self‐esteem scale, motivation of rehabilitation, relationship change scale | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficient information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Unclear risk | Study failed to report any of the above points. Only small sample sizes (n = 9, n = 10, n = 11). Participant assignment was unclear: "Twelve patients were excluded due to health problems, so subjects were randomly assigned to …" |
Tang 2014.
| Methods | RCT Mobility CCT (with aerobic exercise component) vs balance and stretching exercises without aerobic stimulus |
|
| Participants | 50 participants (intervention = 25, comparison = 25), mean 4.2 years post‐stroke, mean 66.4 years, able to walk 5 m independently with or without gait aids | |
| Interventions | Intervention: aerobic training with target progressive heart rate using brisk walking, cycling, step ups, sit to stands Comparison: balance and flexibility non‐aerobic, including balance exercise progressed to be challenging 60‐min sessions 3 times/week for 6 months Staff:participant ratio: 3:12 |
|
| Outcomes | 6mWT, VO2 peak, arterial stiffness, cardiac function, cholesterol, triglycerides, fasting glucose | |
| Notes | Adverse events and adherence to class attendance reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "… performed the randomisation using a computer‐generated 1:1 allocation sequence and permuted block sizes of 2 or 4." |
| Allocation concealment (selection bias) | Unclear risk | States "concealed allocation" with no description |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Single‐blinded trial. Unlikely to influence outcomes "Blinded outcome assessors were used." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat analysis Dropouts described, with only 3 from 1 group and none from the other. Reasons unrelated to the programme |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | No other sources of bias evident |
Van de Port 2012.
| Methods | RCT Mobility CCT versus conventional (one‐to‐one) therapy |
|
| Participants | 250 participants (intervention = 126, comparison = 124), mean 3.2 months post‐stroke, mean age 57 years able to walk 10 m independently with or without walking aid, discharged from inpatient therapy | |
| Interventions | Intervention: mobility related CCT, 8 x 3‐min workstations activities designed to improve walking competency Comparison: individual (one‐to‐one) conventional therapy according to Dutch Guidelines 90 min twice a week for 12 weeks Staff:participant ratio: 1:1.8 |
|
| Outcomes | 6mWT, gait speed, Functional Ambulation Classification, modified stairs test, TUG, timed balance test, RMI, Nottingham Extended Activities of Daily Living, Stroke Impact Scale (mobility), Fatigue Severity Scale, Falls Efficacy Scale, Hospital Anxiety and Depression Scale, motricity index (arm and leg) | |
| Notes | Adverse events and actual therapy time delivered (in minutes) reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "….and randomisation took place using an online minimization procedure." |
| Allocation concealment (selection bias) | Unclear risk | The randomisation scheme was developed and held by an offsite company that provided the online randomisation program. When participants were recruited, members of research team would be notified of group allocation by text message |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Single‐blinded trial. Unlikely to influence outcomes "Three trained RAs who were blinded to treatment allocation, measured all outcomes before randomisation …" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Used last values carried forward for intention‐to‐treat analysis "Of the 250 included patients, one patient in the circuit training group and seven in the usual care group were excluded from the analysis." |
| Selective reporting (reporting bias) | Low risk | Protocol available Cumulative Illness Rating Scale not used in trial paper EuroQoL not used in trial paper Cost benefits not analysed in this paper Slightly different data analysis |
| Other bias | Low risk | No other sources of bias evident |
Verma 2011.
| Methods | RCT Mobility CCT plus mental imagery vs conventional therapy (based on Bobath techniques) |
|
| Participants | 30 participants (intervention = 15, comparison = 15), mean 1.5 years post‐stroke (lower range from 3 months), mean age 54.2 years, Functional Ambulation Classification 2 or above (i.e. able to walk with assistance of 1 person) | |
| Interventions | Intervention: mobility CCT, workstations including balance, stair walking, turning, transfers, and speed walking plus mental imagery Comparison: conventional lower limb therapy based on Bobath techniques 40‐min sessions, 7 days/week for 2 weeks Inpatient and outpatient sessions Staff:participant ratio: 1:4 |
|
| Outcomes | 6mWT, gait speed, Functional Ambulation Classification, Rivermead Visual Gait Assessment, cadence, step length asymmetry, Barthel Index | |
| Notes | Adverse events | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | " … using computer generated random numbers." "A resident physician at the study site conducted the random‐number program." Resident was blinded to the protocol and was not involved in the study |
| Allocation concealment (selection bias) | Low risk | "The intervention assignments were enclosed in sealed envelopes, which were opaque and sequentially numbered." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "The subjects were blinded for intervention of interest." Personnel delivering the intervention would likely not be blinded due to the nature of the program, however unlikely to influence outcomes "… study was an assessor‐blinded RCT." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "An intention‐to‐treat analysis was used with the last observation carried forward for the missing data." "Due to a second stroke, one of the subjects in the experimental group was lost for a follow‐up assessment." |
| Selective reporting (reporting bias) | Unclear risk | No protocol available |
| Other bias | Low risk | No other sources of bias evident |
6mWT: 6 Minute Walk Test ABC: Activities‐specific Balance and Confidence Scale BBS: Berg Balance Scale BMD: bone mineral density CCT: circuit class therapy FIM: Functional Independence Measure ILAS: Iowa Level of Assistance Scale JTHFT: Jebsen Taylor Hand Function Test LOS: length of hospital stay MAS: Motor Assessment Scale NHP: Nottingham Health Profile PADS: Physical Activity and Disability Scale PASIPD: Physical Acitivity Scale for Individuals with Physical Disabilities RCT: randomised controlled trial RMI: Rivermead Mobility Index TUG: Timed Up and Go VO2max: maximum oxygen volume
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Altin 2009 | Intervention: not group format |
| Arya 2012 | Intervention: not group format Aim: not to improve mobility |
| Boss 2014 | Intervention: not group format, not repetitive practice Aim: not to improve mobility |
| Bustamante Valles 2016 | Intervention: CCT group used robotic/technology‐assisted stations not task‐specific training |
| Chu 2004 | Intervention: not task‐specific training |
| Dickstein 2014 | Intervention: not group format, not repetitive practice Aim: not to improve mobility |
| English 2007 | Study design: pseudo randomised |
| English 2014 | Aim: not to improve mobility |
| Faulkner 2014 | Study design: not stroke (TIA) |
| Kim 2010 | Intervention: not group format, no repetitive practice |
| Kim 2012 | Intervention: not group format |
| Kim 2014 | Intervention: not group format, no repetitive practice |
| Kim 2016b | Intervention: compared group CCT with individualised CCT. However individualised CCT fits definition of standard CCT. Therefore no useful comparison for this review. |
| Kowalczewski 2007 | Intervention: not group format |
| Langhammer 2008 | Intervention: not group format, not task‐specific, not circuit |
| Lee 2012 | Intervention: not repetitive practice Aim: not to improve mobility |
| Lee 2015 | Aim: not to improve mobility |
| McDonnell 2014 | Intervention: not repetitive practice Aim: not to improve mobility |
| Mead 2007 | Intervention: not task‐specific |
| Olney 2006 | Intervention: not task‐specific |
| Park 2016 | Intervention: not group format |
| Puckree 2014 | Intervention: not group format |
| Pyöriä 2007 | Intervention: not group format |
| Quaney 2009 | Intervention: not group format, not repetitive practice Aim: not to improve mobility |
| Rimmer 2000 | Intervention: not task‐specific |
| Saeys 2012 | Intervention: not group format, not repetitive practice Aim: not to improve mobility |
| Salbach 2004 | Intervention: not group format |
| Scianni 2010 | Intervention: not group format |
| Sherrington 2008 | Intervention: not task‐specific |
| Shin 2011 | Study design: not group format |
| Sullivan 2007 | Intervention: not circuit format |
| Sunnerhagen 2007 | Intervention: not task‐specific |
| Tanne 2008 | Intervention: not task‐specific |
| Teixeira‐Salmela 1999 | Intervention: not task‐specific |
| Yang 2006 | Intervention: not group format |
CCT: circuit class therapy
Characteristics of studies awaiting assessment [ordered by study ID]
Mota 2011.
| Methods | Experimental design |
| Participants | Victims of stroke |
| Interventions | Physiotherapy intervention using aerobic exercises |
| Outcomes | Gait parameters |
| Notes | Not in English |
Scholten 2014.
| Methods | Possibly a systematic review with 22 RCTs |
| Participants | Unknown |
| Interventions | Fitness training |
| Outcomes | Physical fitness |
| Notes | Not in English |
Characteristics of ongoing studies [ordered by study ID]
Floel 2014.
| Trial name or title | PHYS‐STROKE |
| Methods | Phase III RCT |
| Participants | 215 adults with moderate to severe limitations of walking and ADLs 5‐45 days after stroke |
| Interventions | Physical fitness training plus standard rehabilitation; control relaxation sessions plus standard rehabilitation |
| Outcomes | Gait speed, Barthel Index, QoL, sleep, mood, cognition, arm function, cardiovascular factors. |
| Starting date | October 2013 |
| Contact information | Correspondence: agnes.floeel@charite.de |
| Notes |
Lawal 2015.
| Trial name or title | CCT in Nigeria |
| Methods | Four‐arm RCT |
| Participants | 68 stroke survivors, community dwelling |
| Interventions | CCT of three different durations (60 min, 90 min, 120 min) versus usual care |
| Outcomes | Measures of impairment, activity and participation |
| Starting date | 2014 |
| Contact information | Correspondence: isalawal30@yahoo.com |
| Notes |
Differences between protocol and review
The original protocol included quasi‐randomised trials: the updated review excluded these due to sufficient randomised trials being found. The primary outcome has been refined to walking capacity (rather than a general outcome of improved mobility) but is still defined operationally as the 6mWT, which is used the most extensively in stroke trials. We have included a 'Summary of findings' table in the main report, along with the approach to determining this table and the GRADE designations (in methods).
Contributions of authors
Coralie English and Susan Hillier were involved in all stages of the review. Elizabeth Lynch assisted in assessing risk of bias and in drafting the text of the updated review. Coralie English and Elizabeth Lynch have experience in the clinical use of CCT and Susan Hillier and Coralie English have experience as review authors. Coralie English and Susan Hillier are guarantors of the review.
Declarations of interest
Coralie English: has published a trial investigating the use of CCT with people with stroke (English 2015). Susan Hillier: has published a trial investigating the use of CCT with people with stroke (English 2015). Elizabeth Lynch: none known
Edited (no change to conclusions)
References
References to studies included in this review
Blennerhassett 2004 {published data only}
- Blennerhassett J, Dite W. Additional task‐related practice improves mobility and upper limb function early after stroke: a randomised controlled trial. Australian Journal of Physiotherapy 2004;50:219‐24. [DOI] [PubMed] [Google Scholar]
Dean 2000 {published data only}
- Dean C, Richards C, Malouin F. Task‐related circuit training improves performance of locomotor tasks in chronic stroke: a randomised controlled pilot trial. Archives of Physical Medicine and Rehabilitation 2000;81:409‐17. [DOI] [PubMed] [Google Scholar]
Dean 2012 {published data only}
- Dean CM, Rissel C, Sharkey M, Sherrington C, Cumming RG, Barker RN, et al. Exercise intervention to prevent falls and enhance mobility in community dwellers after stroke: a protocol for a randomised controlled trial. BMC Neurology 2009;9:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean CM, Rissel C, Sherrington C, Sharkey M, Cumming RG, Lord SR, et al. Exercise to enhance mobility and prevent falls after stroke: the community stroke club randomized trial. Neurorehabilitation and Neural Repair 2012;26(9):1046‐57. [DOI] [PubMed] [Google Scholar]
English 2015 {published data only}
- English C, Bernhardt J, Crotty M, Esterman A, Segal L, Hillier S. Circuit class therapy or seven‐day week therapy for increasing rehabilitation intensity of therapy after stroke (CIRCIT): a randomized controlled trial. International Journal of Stroke 2015;10(4):594‐602. [DOI] [PubMed] [Google Scholar]
- Hillier S, English C, Crotty M, Segal L, Bernhardt J, Esterman A. Circuit class or seven‐day therapy for increasing intensity of rehabilitation after stroke: protocol of the CIRCIT trial. International Journal of Stroke 2011;6:560‐5. [DOI] [PubMed] [Google Scholar]
Harrington 2010 {published data only}
- Harrington R, Taylor G, Hollinghurst S, Reed M, Kay H, Wood VA. A community‐based exercise and education scheme for stroke survivors: a randomized controlled trial and economic evaluation. Clinical Rehabilitation 2010;24(1):3‐15. [DOI] [PubMed] [Google Scholar]
Holmgren 2010 {published data only}
- Holmgren E, Gosman‐Hedstroem G, Lindstroem B, Wester P. What is the benefit of a high‐intensive exercise program on health‐related quality of life and depression after stroke? A randomised controlled trial. Advances in Physiotherapy 2010;12:125‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kim 2016a {published data only}
- Kim SM, Han EY, Kim BR, Hyun CW. Clinical application of circuit training for subacute stroke patients: a preliminary study. Journal of Physical Therapy Science 2016;28(1):169‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marigold 2005 {published data only}
- Marigold D, Eng J, Dawson A, Inglis J, Harris J, Gylfadóttir S. Exercise leads to faster postural reflexes, improved balance and mobility, and fewer falls in older persons with chronic stroke. Journal of the American Geriatrics Society 2005;53:426‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marsden 2010 {published data only}
- Marsden D, Quinn R, Pond N, Golledge R, Neilson C, White J, et al. A multidisciplinary group programme in rural settings for community‐dwelling chronic stroke survivors and their carers: a pilot randomized controlled trial. Clinical Rehabilitation 2010;24(4):328‐41. [DOI] [PubMed] [Google Scholar]
Moore 2015 {published data only}
- Moore SA, Hallsworth K, Jakovljevic DG, Blamire AM, He J, Ford GA, et al. Effects of community exercise therapy on metabolic, brain, physical, and cognitive function following stroke: a randomized controlled pilot trial. Neurorehabilitation and Neural Repair 2015;29(7):623‐35. [DOI] [PubMed] [Google Scholar]
Mudge 2009a {published data only}
- Mudge S, Stott N, Barber P. Circuit‐based rehabilitation improves gait endurance but not usual walking activity in chronic stroke: a randomised clinical trial. Archives of Physical Medicine and Rehabilitation 2009;90(12):1989‐96. [DOI] [PubMed] [Google Scholar]
Outermans 2010 {published data only}
- Outermans JC, Peppen RP, Wittink H, Takken T, Kwakkel G. Effects of a high‐intensity task‐oriented training on gait performance early after stroke: a pilot study. Clinical Rehabilitation 2010;24(11):979‐87. [DOI] [PubMed] [Google Scholar]
Pang 2005 {published data only}
- Pang M, Eng J, Dawson A, McKay H, Harris J. A community‐based fitness and mobility exercise program for older adults with chronic stroke: a randomised controlled trial. Journal of the American Geriatrics Society 2005;53:1667‐74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang MY, Harris JE, Eng JJ. A community‐based upper‐extremity group exercise program improves motor function and performance of functional activities in chronic stroke: a randomised controlled trial. Archives of Physical Medicine and Rehabilitation 2006;87:1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Song 2015 {published data only}
- Song HS, Kim JY, Park SD. Effect of the class and individual applications of task‐oriented circuit training on gait ability in patients with chronic stroke. Journal of Physical Therapy Science 2015;27(1):187‐9. [0915‐5287: (Print)] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song HS, Kim JY, Park SD. The effect of class‐based task‐oriented circuit training on the self‐satisfaction of patients with chronic stroke. Journal of Physical Therapy Science 2015;27:127‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tang 2014 {published data only}
- Tang A, Eng JJ, Krassioukov AV, Madden KM, Mohammadi A, Tsang MY, et al. Exercise‐induced changes in cardiovascular function after stroke: a randomized controlled trial. International Journal of Stroke 2014;9(7):883‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Van de Port 2012 {published data only}
- Port IG, Wevers L, Roelse H, Kats L, Lindeman E, Kwakkel G. Cost‐effectiveness of a structured progressive task‐oriented circuit class training programme to enhance walking competency after stroke: the protocol of the FIT‐Stroke trial. BMC Neurology 2009;9:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Port IG, Wevers LE, Lindeman E, Kwakkel G. Effects of circuit training as alternative to usual physiotherapy after stroke: randomised controlled trial. BMJ 2012;344:e2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
Verma 2011 {published data only}
- Verma R, Arya KN, Garg RK, Singh T. Task‐oriented circuit class training program with motor imagery for gait rehabilitation in poststroke patients: a randomized controlled trial. Topics in Stroke Rehabilitation 2011;18 Suppl 1:620‐32. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Altin 2009 {published data only}
- Altin Ertekin O, Gelecek N, Yildirim Y, Akdal G. Supervised versus home physiotherapy outcomes in stroke patients with unilateral visual neglect: a randomized controlled follow‐up study. Journal of Neuroscience 2009;26(3):325‐34. [Google Scholar]
Arya 2012 {published data only}
- Arya KN, Verma R, Garg RK, Sharma VP, Agarwal M, Aggarwal GG. Meaningful task‐specific training (MTST) for stroke rehabilitation: a randomized controlled trial. Topics in Stroke Rehabilitation 2012;19(3):193‐211. [DOI] [PubMed] [Google Scholar]
Boss 2014 {published data only}
- Boss HM, Schaik SM, Deijle IA, Melker EC, Berg BT, Scherder EJ, et al. Safety and feasibility of post‐stroke care and exercise after minor ischemic stroke or transient ischemic attack: MotiveS & MoveIT. NeuroRehabilitation 2014;34(3):401‐7. [DOI] [PubMed] [Google Scholar]
Bustamante Valles 2016 {published data only}
- Bustamante Valles K, Montes S, Jesus Madrigal M, Burciaga A, Martinez M, Johnson M. Technology‐assisted stroke rehabilitation in Mexico: a pilot randomised trial comparing tradition therapy to circuit training in robot/technology‐assisted therapy gym. Journal of Neuroengineering and Rehabilitation 2016; Vol. 13:83. [DOI: 10.1186/s12984-016-0190-1] [DOI] [PMC free article] [PubMed]
Chu 2004 {published data only}
- Chu KS, Eng JJ, Dawson AS, Harris JE, Ozkaplan A, Gylfadóttir S. Water‐based exercise for cardiovascular fitness in people with chronic stroke: a randomised controlled trial. Archives of Physical Medicine and Rehabilitation 2004;85:870‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dickstein 2014 {published data only}
- Dickstein R, Levy S, Shefi S, Holtzman S, Peleg S, Vatine JJ. Motor imagery group practice for gait rehabilitation in individuals with post‐stroke hemiparesis: a pilot study. NeuroRehabilitation 2014;34(2):267‐76. [DOI] [PubMed] [Google Scholar]
English 2007 {published data only}
- English C, Hillier S, Stiller K, Warden‐Flood A. Circuit class therapy versus individual physiotherapy sessions during inpatient stroke rehabilitation: a controlled trial. Archives of Physical Medicine and Rehabilitation 2007;88:955‐63. [DOI] [PubMed] [Google Scholar]
English 2014 {published data only}
- English C, Hillier S, Kaur G, Hundertmark L. People with stroke spend more time in active task practice, but similar time in walking practice, when physiotherapy rehabilitation is provided in circuit classes compared to individual therapy sessions: an observational study. Journal of Physiotherapy 2014;60(1):50‐4. [DOI] [PubMed] [Google Scholar]
Faulkner 2014 {published data only}
- Faulkner J, Lambrick D, Woolley B, Stoner L, Wong LK, McGonigal G. The long‐term effect of exercise on vascular risk factors and aerobic fitness in those with transient ischaemic attack: a randomized controlled trial. Journal of Hypertension 2014;32(10):2064‐70. [DOI] [PubMed] [Google Scholar]
Kim 2010 {published data only}
- Kim MC, Ahn CS, Lee HS, Jang SH, You YY. Change in C‐Reactive protein level according to amounts of exercise in chronic hemiparetic patients with cerebral infarct. Journal of Physical Therapy Science 2010;22(3):279‐84. [Google Scholar]
Kim 2012 {published data only}
- Kim BH, Lee SM, Bae YH, Yu JH, Kim TH. The effect of a task‐oriented training on trunk control ability, balance and gait of stroke patients. Journal of Physical Therapy Science 2012;24(6):519‐22. [Google Scholar]
Kim 2014 {published data only}
- Kim M, Cho K, Lee W. Community walking training program improves walking function and social participation in chronic stroke patients. Tohoku Journal of Experimental Medicine 2014;234(4):281‐6. [DOI] [PubMed] [Google Scholar]
Kim 2016b {published data only}
- Kim B, Park Y, Seo Y, Park S, Cho H, Moon H, et al. Effects of individualised versus group task‐oriented circuit training on balance ability and gait endurance in chronic stroke inpatients. Journal of Physical Therapy Science 2016;28:1872‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kowalczewski 2007 {published data only}
- Kowalczewski J, Gritsenko V, Ashworth N, Ellaway P, Prochazka A. Upper‐extremity functional electric stimulation‐assisted exercises on a workstation in the sub‐acute phase of stroke recovery. Archives of Physical Medicine and Rehabilitation 2007;88:833‐9. [DOI] [PubMed] [Google Scholar]
Langhammer 2008 {published data only}
- Langhammer B, Stanghelle JK, Lindmark B. Exercise and health‐related quality of life during the first year following acute stroke. A randomised controlled trial. Brain Injury 2008;22(2):135‐45. [DOI] [PubMed] [Google Scholar]
Lee 2012 {published data only}
- Lee Y, Lee J, Shin S, Lee S. The effect of dual motor task training while sitting on trunk control ability and balance of patients with chronic stroke. Journal of Physical Therapy Science 2012;24(4):345‐9. [Google Scholar]
Lee 2015 {published data only}
- Lee YH, Park SH, Yoon ES, Lee CD, Wee SO, Fernhall B, et al. Effects of combined aerobic and resistance exercise on central arterial stiffness and gait velocity in patients with chronic poststroke hemiparesis. American Journal of Physical Medicine and Rehabilitation 2015;94(9):687‐95. [DOI] [PubMed] [Google Scholar]
McDonnell 2014 {published data only}
- McDonnell MN, Mackintosh SF, Hillier SL, Bryan J. Regular group exercise is associated with improved mood but not quality of life following stroke. PeerJ 2014;2:e331. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mead 2007 {published data only}
- Mead GE, Greig CA, Cunningham I, Lewis SJ, Dinan S, Saunders DH, et al. Stroke: a randomised trial of exercise or relaxation. Journal of the American Geriatrics Society 2007;55:892‐9. [DOI] [PubMed] [Google Scholar]
Olney 2006 {published data only}
- Olney SJ, Nymark J, Brouwer B, Culham E, Day A, Heard J, et al. A randomised controlled trial of supervised versus unsupervised exercise programs for ambulatory stroke survivors. Stroke 2006;37:476‐81. [DOI] [PubMed] [Google Scholar]
Park 2016 {published data only}
- Park KT, Kim HJ. Effect of the a circuit training program using obstacles on the walking and balance abilities of stroke patients. Journal of Physical Therapy Science 2016;28(4):1194‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Puckree 2014 {published data only}
- Puckree T, Naidoo P. Balance and stability‐focused exercise program improves stability and balance in patients after acute stroke in a resource‐poor setting. PM & R : the Journal of Injury, Function, and Rehabilitation 2014;6(12):1081‐7. [DOI] [PubMed] [Google Scholar]
Pyöriä 2007 {published data only}
- Pyöriä O, Talvitie U, Nyrkkö H, Kautiainen H, Pohjolainen T, Kasper V. The effect of two physiotherapy approaches on physical and cognitive functions and independent coping at home in stroke rehabilitation. A preliminary follow‐up study. Disability and Rehabilitation 2007;29(6):503‐11. [DOI] [PubMed] [Google Scholar]
Quaney 2009 {published data only}
- Quaney BM, Boyd LA, McDowd JM, Zahner LH, He J, Mayo MS, et al. Aerobic exercise improves cognition and motor function poststroke. Neurorehabilitation and Neural Repair 2009;23(9):879‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rimmer 2000 {published data only}
- Rimmer J, Riley B, Creviston T, Nicola T. Exercise training in a predominantly African‐American group of stroke survivors. Medicine and Science in Sports and Exercise 2000;32(12):1990‐6. [DOI] [PubMed] [Google Scholar]
Saeys 2012 {published data only}
- Saeys W, Vereeck L, Truijen S, Lafosse C, Wuyts FP, Heyning PV. Randomized controlled trial of truncal exercises early after stroke to improve balance and mobility. Neurorehabilitation and Neural Repair 2012;26(3):231‐8. [DOI] [PubMed] [Google Scholar]
Salbach 2004 {published data only}
- Salbach NM, Mayo NE, Wood‐Dauphinee S, Hanley JA, Richards CL, Côté R. A task‐orientated intervention enhances walking distance and speed in the first year post stroke: a randomised controlled trial. Clinical Rehabilitation 2004;18:509‐15. [DOI] [PubMed] [Google Scholar]
Scianni 2010 {published data only}
- Scianni A, Teixeira‐Salmela LF, Ada L. Effect of strengthening exercise in addition to task‐specific gait training after stroke: a randomised trial. International Journal of Stroke 2010;5(4):329‐35. [DOI] [PubMed] [Google Scholar]
Sherrington 2008 {published data only}
- Sherrington C, Pamphlett PI, Jacka JA, Olivetti LM, Nugent JA, Hall JM, et al. Group exercise can improve participants' mobility in an outpatient rehabilitation setting: a randomised controlled trial. Clinical Rehabilitation 2008;22:493‐502. [DOI] [PubMed] [Google Scholar]
Shin 2011 {published data only}
- Shin WS, Lee SW, Lee YW, Choi SB, Song CH. Effects of combined exercise training on balance of hemiplegic stroke patients. Journal of Physical Therapy Science 2011;23(4):639‐43. [Google Scholar]
Sullivan 2007 {published data only}
- Sullivan KJ, Brown DA, Klassen T, Mulroy S, Ge T, Azen SP, et al. Effects of task‐specific locomotor and strength training in adults who were ambulatory after stroke: results of the STEPS randomised clinical trial. Physical Therapy 2007;87:1580‐602. [DOI] [PubMed] [Google Scholar]
Sunnerhagen 2007 {published data only}
- Sunnerhagen S. Circuit training in community‐living "younger" men after stroke. Journal of Stroke and Cerebrovascular Diseases 2007;16(3):122‐9. [DOI] [PubMed] [Google Scholar]
Tanne 2008 {published data only}
- Tanne D, Tsabari R, Chechik O, Toledano A, Orion D, Schwammenthal Y, et al. Improved exercise capacity in patients after minor ischaemic stroke undergoing a supervised exercise training program. Israeli Medical Association Journal 2008;10:113‐6. [PubMed] [Google Scholar]
Teixeira‐Salmela 1999 {published data only}
- Teixeira‐Salmela LF, Olney SJ, Nadeau S, Brouwer B. Muscle strengthening and physical conditioning to reduce impairment and disability in chronic stroke survivors. Archives of Physical Medicine and Rehabilitation 1999;80:1211‐8. [DOI] [PubMed] [Google Scholar]
Yang 2006 {published data only}
- Yang Y‐U, Wang R‐Y, Lin K‐H, Chu M‐Y, Chan R‐C. Task‐oriented progressive resistance strength training improves muscle strength and functional performance in individuals with stroke. Clinical Rehabilitation 2006;20(20):860‐70. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Mota 2011 {published data only}
- Mota RS, Bitencourt JS, Conceicao TMA, Cardoso FB, Silva IL, Beresford H. Evaluation of the effectiveness of aerobic exercise in hemiparetic gait [Avaliação do efeito do exercício aeróbico na marcha de indivíduos hemiparéticos]. Revista Brasileira de Ciência e Movimento 2011;19(2):108‐18. [Google Scholar]
Scholten 2014 {published data only}
- Scholten RJPM. Fitness training after a stroke is effective [Fitness training ne een beroerte is effectief]. Nederlands Tijdschrift voor Geneeskunde 2014;158(A7429):864. [Google Scholar]
References to ongoing studies
Floel 2014 {published data only}
- Floel A, Werner C, Grittner U, Hesse S, Jöbges M, Knauss J, et al. Physical fitness training in Subacute Stroke (PHYS‐STROKE) ‐ study protocol for a randomised controlled trial. Trials 2014;15:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lawal 2015 {published data only}
- Lawal IU, Hillier SL, Hamzat TK, Rhoda A. Effectiveness of a structured circuit class therapy model in stroke rehabilitation: a protocol for a randomised controlled trial. BMC Neurology 2015;15:88. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Abstracts Asian Pacific Stroke Congress
- Abstracts Asian Pacific Stroke Congress. Australia, July 14‐17, 2016: Abstracts. Cerebrovascular Diseases 2016;42 Suppl 1:1‐157. [DOI] [PubMed] [Google Scholar]
Ali 2013
- Ali M, English C, Bernhardt J, Sunnerhagen KS, Brady M. More outcomes than trials: a call for consistent data collection across stroke rehabilitation trials. International Journal of Stroke 2013;8(1):18‐24. [1747‐4930] [DOI] [PubMed] [Google Scholar]
Bernhardt 2016
- Bernhardt J, Borschmann K, Boyd L, Carmichael ST, Corbett D, Cramer SC, et al. Moving rehabilitation research forward: developing consensus statements for rehabilitation and recovery research. International Journal of Stroke 2016;11(4):454‐8. [1747‐4930] [DOI] [PubMed] [Google Scholar]
Dawson 2013
- Dawson AS, Knox J, McClure A, Foley N, Teasell R: Stroke Rehabilitation Writing Group. Chapter 5: Stroke rehabilitation. Canadian Best Practice Recommendations For Stroke Care. Ottawa: Heart and Stroke Foundation and the Canadian Stroke Network, 2013. [Google Scholar]
Deloitte Access Economics 2013
- Deloitte Access Economics. The economic impact of stroke in Australia. Deloitte Access Economics 13 March 2013; Vol. strokefoundation.com.au/What‐we‐do/Research/Economic‐impact‐of‐stroke‐in‐Australia:1‐20.
Eng 2004
- Eng JJ, Dawson AS, Chu KS. Submaximal exercise in persons with stroke: test‐retest reliability and concurrent validity with maximal oxygen consumption. Archives of Physical Medicine and Rehabilitation 2004;85(1):113‐8. [0003‐9993] [DOI] [PMC free article] [PubMed] [Google Scholar]
English 2016
- English C, Shields N, Brusco NK, Taylor NF, Watts JJ, Peiris C, et al. Additional weekend therapy may reduce length of rehabilitation stay after stroke: a meta‐analysis of individual patient data. Journal of Physiotherapy 2016;62(3):124‐9. [DOI] [PubMed] [Google Scholar]
Feigin 2014
- Feigin VL, Forouzanfar MH, Krishnamurthi R, Mensah GA, Connor M, Bennett DA, Global Burden of Diseases Injuries and Risk Factors Study 2010 Stroke Experts Group. Global and regional burden of stroke during 1990‐2010: findings from the Global Burden of Disease Study 2010. Lancet 2014;383(9913):245‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Flansbjer 2005
- Flansbjer UB, Holmback AM, Downham D, Patten C, Lexell J. Reliability of gait performance in men and women with hemiparesis after stroke. Journal of Rehabilitation Medicine 2005;37:75‐82. [DOI] [PubMed] [Google Scholar]
Flansbjer 2012
- Flansbjer UB, Blom J, Brogardh C. The reproducibility of Berg Balance Scale and the Single‐leg Stance in chronic stroke and the relationship between the two tests. PM & R : the Journal of Injury, Function, and Rehabilitation 2012;4(3):165‐70. [1934‐1482] [DOI] [PubMed] [Google Scholar]
Fulk 2010
- Fulk GD, Reynolds C, Mondal S, Deutsch JE. Predicting home and community walking activity in people with stroke. Archives of Physical Medicine and Rehabilitation 2010;91(10):1582‐6. [0003‐9993] [DOI] [PubMed] [Google Scholar]
GRADE 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s), GRADE working group. GRADE Handbook. gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html (accessed December 2016).
Hackett 2008
- Hackett ML, Anderson CS, House A, Halteh C. Interventions for preventing depression after stroke. Cochrane Database of Systematic Reviews 2008, Issue 3. [DOI: 10.1002/14651858.CD003689.pub3] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hillier 2010
- Hillier S, Inglis‐Jassiem G. Rehabilitation for community‐dwelling people with stroke: home or centre based? A systematic review. International Journal of Stroke 2010;5(3):178‐86. [DOI] [PubMed] [Google Scholar]
Intercollegiate Stroke Working Party 2012
- Intercollegiate Stroke Working Party. National Clinical Guidelines for Stroke. 4th Edition. London: Royal College of Physicians, 2012. [Google Scholar]
Ivey 2006
- Ivey FM, Hafer‐Macko CE, Macko RF. Exercise rehabilitation after stroke. NeuroRx 2006;3(4):439‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
Jauch 2013
- Jauch E, Saver JL, Adams HP Jr, Bruno A, Connors JJ, Demaerschalk BM, the American Heart Association Stroke Council, Council on Cardiovascular Nursing, Council on Peripheral Vascular Disease and Council on Clinical Cardiology. Guidelines for the early management of patients with acute ischaemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013;44:870‐947. [DOI] [PubMed] [Google Scholar]
Kodama 2009
- Kodama S, Saito K, Tanaka S, Maki M, Yachi Y, Asumi M, et al. Cardiorespiratory fitness as a quantitative predictor of all‐cause mortality and cardiovascular events in healthy men and women: a meta‐analysis. JAMA 2009;301(19):2024‐35. [DOI] [PubMed] [Google Scholar]
Kwakkel 2004
- Kwakkel G, Peppen R, Wagenaar R, Wood‐Dauphinee S, Richards C, Ashburn A, et al. Effects of augmented exercise therapy time after stroke. A meta‐analysis. Stroke 2004;35:2529‐36. [DOI] [PubMed] [Google Scholar]
Levin 2009
- Levin MF, Kleim JA, Wolf SL. What do motor "recovery" and "compensation" mean in patients following stroke?. Neurorehabilitation and Neural Repair 2009;23(4):313‐9. [1545‐9683: (Print)] [DOI] [PubMed] [Google Scholar]
Lindsay 2010
- Lindsay MP, Gubitz G, Bayley M, Hill MD, Davies‐Schinkel C, Singh S: Canadian Stroke Strategy Best Practices and Standards Writing Group. Canadian Best Practice Recommendations For Stroke Care. Ottawa: Canadian Stroke Network, 2010. [Google Scholar]
Lohse 2014
- Lohse KR, Lang CE, Boyd LA. Is more better? Using meta‐data to explore dose‐response relationships in stroke rehabilitation. Stroke 2014;45:2053‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lollgen 2009
- Lollgen H, Bockenhoff A, Knapp G. Physical activity and all‐cause mortality: an updated meta‐analysis with different intensity categories. International Journal of Sports Medicine 2009;30(3):213‐24. [0172‐4622] [DOI] [PubMed] [Google Scholar]
Lynch 2008a
- Lynch EB, Butt Z, Heinemann A, Victorson D, Nowinski CJ, Perez L, et al. A qualitative study of quality of life after stroke: the importance of social relationships. Journal of Rehabiltiation Medicine 2008;40(7):518‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Marsden 2013
- Marsden DL, Dunn A, Callister R, Levi CR, Spratt NJ. Characteristics of exercise training interventions to improve cardiorespiratory fitness after stroke: a systematic review with meta‐analysis. Neurorehabilitation and Neural Repair 2013;27(9):775‐88. [1545‐9683] [DOI] [PubMed] [Google Scholar]
Marsden 2016
- Marsden DL, Dunn A, Callister R, McElduff P, Levi CR, Spratt NJ. A home‐ and community‐based physical activity program can improve the cardiorespiratory fitness and walking capacity of stroke survivors. Journal of Stroke and Cerebrovascular Diseases 2016;25(10):2386‐98. [1052‐3057] [DOI] [PubMed] [Google Scholar]
McDonnell 2013
- McDonnell MN, Hillier SL, Hooker SP, Le A, Judd SE, Howard VJ. Physical activity frequency and risk of incident stroke in a national US study of blacks and whites. Stroke 2013;44(9):2519‐24. [0039‐2499] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mehrholz 2014
- Mehrholz J, Pohl M, Elsner B. Treadmill training and body weight support for walking after stroke. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD002840.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Morris 2012
- Morris R, Morris P. Participants/experiences of hospital‐based peer support groups for stroke patients and carers. Disability and Rehabilitation 2012;34:347‐54. [DOI] [PubMed] [Google Scholar]
Mozaffarian 2015
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics ‐ 2015 update: a report from the American Heart Association. Circulation 2015;131:e29‐322. [DOI] [PubMed] [Google Scholar]
Mudge 2009b
- Mudge S, Stott S. Timed walking tests correlate with daily step activity in person with stroke. Archives of Physical Medicine and Rehabilitation 2009;90:296‐301. [DOI] [PubMed] [Google Scholar]
Muren 2008
- Muren MA, Hutler M, Hooper J. Functional capacity and health‐related quality of life in individuals post‐stroke. Topics in Stroke Rehabilitation 2008;15(1):51‐8. [DOI] [PubMed] [Google Scholar]
National Stroke Foundation 2010
- National Stroke Foundation. Clinical Guidelines for Stroke Management. Melbourne: National Stroke Foundation, 2010. [Google Scholar]
Pereira 2012
- Pereira TV, Horwitz RI, Ioannidis JP. Empirical evaluation of very large treatment effects of medical interventions. JAMA 2012;308(16):1676‐84. [0098‐7484] [DOI] [PubMed] [Google Scholar]
Perera 2006
- Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. Journal of the American Geriatric Society 2006;54(5):743‐9. [0002‐8614: (Print)] [DOI] [PubMed] [Google Scholar]
Pollock 2014
- Pollock A, Baer G, Campbell P, Choo P, Forster A, Morris J, et al. Physical rehabilitation approaches for the recovery of function and mobility following stroke. Cochrane Database of Systematic Reviews 2014, Issue 4. [DOI: 10.1002/14651858.CD001920.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Rand 2009
- Rand D, Eng J, Tang P‐F, Jeng J‐S, Hung C. How active are people with stroke? Use of accelerometers to assess physical activity. Stroke 2009;40:163‐8. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Salbach 2006
- Salbach N, Mayo N, Hanley J, Richards C, Wood‐Dauphinee S. Psychometric evaluation of the original and Canadian French version of the activities‐specific balance confidence scale among people with stroke. Archvies of Physical Medicine and Rehabilitation 2006;87(12):1597‐604. [DOI] [PubMed] [Google Scholar]
Shephard 2009
- Shephard RJ. Maximal oxygen intake and independence in old age. British Journal of Sports Medicine 2009;43(5):342‐6. [DOI] [PubMed] [Google Scholar]
Sigrist 2013
- Sigrist R, Rauter G, Riener R, Wolf P. Augmented visual, auditory, haptic, and multimodal feedback in motor learning: a review. Psychonomic Bulletin and Review 2013;20:21‐53. [DOI] [PubMed] [Google Scholar]
Smith 2012
- Smith AC, Saunders DH, Mead G. Cardiorespiratory fitness after stroke: a systematic review. International Journal of Stroke 2012;7(6):499‐510. [1747‐4930] [DOI] [PubMed] [Google Scholar]
Sterne 2011
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Intervention. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Stevenson 2001
- Stevenson TJ. Detecting change in patients with stroke using the Berg Balance Scale. Australian Journal of Physiotherapy 2001;47(1):29‐38. [0004‐9514: (Print)] [DOI] [PubMed] [Google Scholar]
Stroke Foundation of New Zealand 2010
- Stroke Foundation of New Zealand and New Zealand Guidelines Group. Clinical Guidelines for Stroke Management 2010. Wellington: Stroke Foundation of New Zealand, 2010. [Google Scholar]
Stroke Recovery Canada 2009
- Stroke Recovery Canada. Health Recovery Social Networks: Exploring the experiences of participants in stroke recovery peer support groups. Association for Non‐Profit and Social Economy Research Annual Conference. Ottawa, 2009.
Taskinen 1999
- Taskinen P. The development of health enhancing exercise groups adapted for hemiplegic patients. A pilot study. Neurorehabilitation 1999;13:35‐43. [Google Scholar]
Taylor 2006
- Taylor DW, Stretton C, Mudge S, Garrett N. Does clinic measured gait speed differ from gait speed measured in the community in people with stroke. Clinical Rehabilitation 2006;20:438‐44. [DOI] [PubMed] [Google Scholar]
Thompson 2003
- Thompson PD. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease. Arteriosclerosis, Thrombosis, and Vascular Biology 2003;23(8):1319‐21. [1079‐5642] [DOI] [PubMed] [Google Scholar]
Van Vliet 2006
- Vliet PM, Wulf G. Extrinsic feedback for motor learning after stroke: what is the evidence?. Disability and Rehabilitation 2006;28:831‐40. [DOI] [PubMed] [Google Scholar]
Verbeek 2014
- Verbeek JM, Wegen E, Peppen R, Wees PJ, Hendriks E, Rietberg M, et al. What is the evidence for physical therapy poststroke? A systematic review and meta‐analysis. PLOS ONE 2014;9(2):e87987. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wevers 2009
- Wevers L, Port I, Vermue M, Mead G, Kwakkel G. Effects of a task‐oriented circuit class training on walking competency after stroke. Stroke 2009;40:2450‐9. [DOI] [PubMed] [Google Scholar]
Wulf 2010
- Wulf G, Shea C, Lewthwaite R. Motor skill learning and performance: a review of influential factors. Medical Education 2010;44:75‐84. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
English 2009
- English C, Hillier SL. Circuit class therapy for improving mobility after stroke. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD007513] [DOI] [PMC free article] [PubMed] [Google Scholar]
English 2010
- English C, Hillier SL. Circuit class therapy for improving mobility after stroke. Cochrane Database of Systematic Reviews 2010, Issue 7. [DOI: 10.1002/14651858.CD007513.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


