Abstract
Background
Various nerve blocks with local anaesthetic agents have been used to reduce pain after hip fracture and subsequent surgery. This review was published originally in 1999 and was updated in 2001, 2002, 2009 and 2017.
Objectives
This review focuses on the use of peripheral nerves blocks as preoperative analgesia, as postoperative analgesia or as a supplement to general anaesthesia for hip fracture surgery. We undertook the update to look for new studies and to update the methods to reflect Cochrane standards.
Search methods
For the updated review, we searched the following databases: the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 8), MEDLINE (Ovid SP, 1966 to August week 1 2016), Embase (Ovid SP, 1988 to 2016 August week 1) and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCO, 1982 to August week 1 2016), as well as trial registers and reference lists of relevant articles.
Selection criteria
We included randomized controlled trials (RCTs) involving use of nerve blocks as part of the care provided for adults aged 16 years and older with hip fracture.
Data collection and analysis
Two review authors independently assessed new trials for inclusion, determined trial quality using the Cochrane tool and extracted data. When appropriate, we pooled results of outcome measures. We rated the quality of evidence according to the GRADE Working Group approach.
Main results
We included 31 trials (1760 participants; 897 randomized to peripheral nerve blocks and 863 to no regional blockade). Results of eight trials with 373 participants show that peripheral nerve blocks reduced pain on movement within 30 minutes of block placement (standardized mean difference (SMD) ‐1.41, 95% confidence interval (CI) ‐2.14 to ‐0.67; equivalent to ‐3.4 on a scale from 0 to 10; I2 = 90%; high quality of evidence). Effect size was proportionate to the concentration of local anaesthetic used (P < 0.00001). Based on seven trials with 676 participants, we did not find a difference in the risk of acute confusional state (risk ratio (RR) 0.69, 95% CI 0.38 to 1.27; I2 = 48%; very low quality of evidence). Three trials with 131 participants reported decreased risk for pneumonia (RR 0.41, 95% CI 0.19 to 0.89; I2 = 3%; number needed to treat for an additional beneficial outcome (NNTB) 7, 95% CI 5 to 72; moderate quality of evidence). We did not find a difference in risk of myocardial ischaemia or death within six months, but the number of participants included was well below the optimal information size for these two outcomes. Two trials with 155 participants reported that peripheral nerve blocks also reduced time to first mobilization after surgery (mean difference ‐11.25 hours, 95% CI ‐14.34 to ‐8.15 hours; I2 = 52%; moderate quality of evidence). One trial with 75 participants indicated that the cost of analgesic drugs was lower when they were given as a single shot block (SMD ‐3.48, 95% CI ‐4.23 to ‐2.74; moderate quality of evidence).
Authors' conclusions
High‐quality evidence shows that regional blockade reduces pain on movement within 30 minutes after block placement. Moderate‐quality evidence shows reduced risk for pneumonia, decreased time to first mobilization and cost reduction of the analgesic regimen (single shot blocks).
Plain language summary
Local anaesthetic nerve blocks for people with a hip fracture
Background: Peripheral nerve blocks consist of an injection of local anaesthetics close to the nerves to transiently block pain transmission to the brain. This review examined evidence from randomized controlled trials that evaluated the use of peripheral nerve blocks to manage pain for people with a hip fracture.
Search dates: This is an update of a previously published review. We updated the search in August 2016.
Study characteristics: We included 31 trials (1760 adult participants: 897 randomized to peripheral nerve blocks and 863 to no regional blockade) performed in various countries and published between 1980 and 2016.
Study funding sources: Trials were funded by a charitable organization (n = 3), by a governmental organization (n = 1) or by departmental resources (n = 5), or did not specify the source of funding.
Key results: Compared with other modes of analgesia, peripheral nerve blocks used to treat hip fracture pain reduce pain on movement better within 30 minutes (equivalent to a difference of ‐3.4 on a scale from 0 to 10 between the two analgesic regimens). The risk of pneumonia is also reduced when peripheral nerve blocks are used to treat hip fracture pain. For every 7 people with a hip fracture, one less person will suffer from pneumonia. Studies noted no major complications related to peripheral nerve blocks and reported reduced time to first mobilization after hip fracture surgery (approximately 11 hours earlier). We did not identify enough trial participants to determine if regional blockade makes a difference in terms of acute confusion, myocardial ischaemia and death within six months after surgery. Peripheral nerve block given as a single injection led to reduced cost of analgesic drugs.
Quality of evidence: We rated the quality of evidence as high for reduction of pain on movement within 30 minutes, and as moderate for pneumonia, time to first mobilization and costs of analgesic drugs. We would need more information before we could draw final conclusions on effects of peripheral nerve blocks on the risk of acute confusional state, myocardial ischaemia and mortality.
Summary of findings
Summary of findings for the main comparison. Peripheral nerve blocks for hip fracture.
| Peripheral nerve blocks for hip fracture | ||||||
| Patient or population: patients with hip fracture Settings: trials performed in Argentina (n = 1), Austria (n = 1), Chile (n = 1), China (n = 2), Denmark (n = 2), France (n = 2), Geece (n = 3), Germany (n = 1), India (n = 1), Iran (n = 1), Ireland (n = 1), Israel (n = 1), Korea (n = 1), South Africa (n = 1), Spain (n = 2), Sweden (n = 1), Thailand (n = 1), Turkey (n = 2), United Kingdom (n = 5) and United States of America (n = 1) Intervention: peripheral nerve blocks Comparison: systemic analgesia | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Systemic analgesia | Peripheral nerve blocks | |||||
| Pain on movement at 30 minutes after block placement Follow‐up: 20‐30 minutes | Mean pain on movement at 30 minutes after block placement in the intervention groups was 1.41 standard deviations lower (2.14 to 0.67 lower) | 373 (8 studies) | ⊕⊕⊕⊕ higha,b,c,d,e,f,g,h,i | Equivalent to ‐ 3.4 on a scale from 0 to 10 | ||
| Acute confusional state | Study population | RR 0.69 (0.38 to 1.27) | 676 (7 studies) | ⊕⊝⊝⊝ very lowc,f,h,j,k,l,m,n | ||
| 198 per 1000 | 136 per 1000 (75 to 251) | |||||
| Low | ||||||
| 150 per 1000 | 104 per 1000 (57 to 190) | |||||
| High | ||||||
| 250 per 1000 | 172 per 1000 (95 to 317) | |||||
| Myocardial ischaemia | Study population | RR 0.2 (0.03 to 1.42) | 20 (1 study) | ⊕⊝⊝⊝ very lowb,c,j,m,n,o,p,q | ||
| 500 per 1000 | 100 per 1000 (15 to 710) | |||||
| Low | ||||||
| 100 per 1000 | 20 per 1000 (3 to 142) | |||||
| High | ||||||
| 500 per 1000 | 100 per 1000 (15 to 710) | |||||
| Pneumonia | Study population | RR 0.41 (0.19 to 0.89) | 131 (3 studies) | ⊕⊕⊕⊝ moderatec,f,h,k,l,n,r,s,t | ||
| 269 per 1000 | 110 per 1000 (51 to 239) | |||||
| Low | ||||||
| 50 per 1000 | 20 per 1000 (9 to 44) | |||||
| High | ||||||
| 200 per 1000 | 82 per 1000 (38 to 178) | |||||
| Death Follow‐up: 0‐6 months | Study population | RR 0.72 (0.34 to 1.52) | 316 (7 studies) | ⊕⊕⊝⊝ lowc,f,m,n,o,q,s,u | ||
| 98 per 1000 | 70 per 1000 (33 to 149) | |||||
| Low | ||||||
| 25 per 1000 | 18 per 1000 (9 to 38) | |||||
| High | ||||||
| 150 per 1000 | 108 per 1000 (51 to 228) | |||||
| Time to first mobilisation | Mean time to first mobilisation in intervention groups was 11.25 hours lower (14.34 to 8.15 lower) | 155 (2 studies) | ⊕⊕⊕⊝ moderatea,c,d,e,h,k,n,p,s,v,w | |||
| Cost of analgesic regimens for single shot blocks | Mean cost of analgesic regimens for single shot blocks in intervention groups was 3.48 standard deviations lower (4.23 to 2.74 lower) | 75 (1 study) | ⊕⊕⊕⊝ moderatea,c,l,n,p,q,s,v | |||
| The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI) CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
a50% or more of studies were rated as having unclear or high risk for allocation concealment or blinding of outcome assessor bWe did not downgrade the evidence on inconsistency because we found a reasonable explanation for heterogeneity cDirect comparisons in studies performed on the population of interest and the outcome measured is not a surrogate marker dOptimal information size achieved eWide confidence interval around effect size fNo evidence of publication bias, or applying a correction for the possibility of one would not modify the conclusion gLarge effect size (SMD > 0.8) hNo study used ultrasound guidance, which could have increased success rate of blocks iEffect size was proportional to the concentration of local anaesthetic used in lidocaine equivalent j75% of studies or more were judged at unclear or high risk of bias for allocation concealment or blinding of outcome assessor kModerate amount of heterogeneity or clinical heterogeneity lOptimal information size not achieved mNo evidence of a large effect nNo evidence of a dose response oEstimate included both absence of effect and important benefit pCould not be assessed qNo evidence of confounding factors that would justify upgrading rGroups heterogenous for preoperative characteristics sNo heterogeneity or ≤ 25% tWe upgraded the level of evidence by one owing to a large effect size (RR < 0.5) uWe did not downgrade for risk of bias vWe upgraded the level of evidence on the basis of a large effect size (equivalent to SMD > 0.8) wWe upgraded the level of evidence on the basis of a large effect size (equivalent to SMD of ‐1.87)
Background
Description of the condition
Among women aged 55 years and older in the USA, the Nationwide Inpatient Sample (NIS) for 2000 to 2010 reported 4.9 million hospitalizations for osteoporotic fractures (2.6 million for hip fractures), 2.9 million for myocardial infarction, 3.0 million for stroke and 0.7 million for breast cancer (Singer 2015). Osteoporotic fractures accounted for more than 40% of hospitalizations for these four outcomes, with an age‐adjusted rate of 1124 admissions per 100,000 person‐years. The annual total population facility‐related hospital cost was highest for hospitalizations due to osteoporotic fractures (USD 5.1 billion), followed by myocardial infarction (USD 4.3 billion), stroke (USD 3.0 billion) and breast cancer (USD 0.5 billion) (Singer 2015). Costs of care for hip fractures are high and, when both acute care and the care needed for subsequent dependency were included, exceeded GBP 2 billion in 2012 for the UK as a whole. That same year, the overall rate of return home by 30 days was 44.6% in the UK (http://www.nhfd.co.uk/20/hipfractureR.nsf/). In the USA, from 2003 to 2005, 5.3% (95% confidence interval (CI) 5.2% to 5.4%) of patients with hip fracture returned home in 30 days , and 52.8% of patients with hip fracture (95% CI 52.5% to 53.2%) were discharged to a skilled nursing facility (Brauer 2009). Hip fractures reduce life expectancies when they occur in individuals over 50 years of age. Pooled data from cohort studies revealed that the relative hazard for all‐cause mortality during the first three months after hip fracture was 5.75 (95% CI 4.94 to 6.67) in women and 7.95 (95% CI 6.13 to 10.30) in men (Haentjens 2010).
The term 'hip fracture' refers to a fracture of the proximal femur down to about 5 cm below the lower border of the lesser trochanter.
Description of the intervention
Regional blockade refers to injection of local anaesthetics around neural structures to transiently prevent pain transmission to the brain and may also produce motor blockade of the muscle in a specific area, depending on the type and concentration of local anaesthetic used. Local anaesthetics can be used at the spine level (neuraxial block = epidural or spinal) or around the nerves outside the spine (plexus blocks or peripheral nerve blocks). Local anaesthetic may also be infiltrated directly into wound tissues. All of these blocks can be given as single injections or by continuous infusion through a catheter to prolong their beneficial effects. Regional blockade may be used as a replacement for general anaesthesia during surgery, as adjunctive treatment for preoperative and postoperative pain or to decrease the use of intraoperative systemic drugs during general anaesthesia. Use of regional blockade as a replacement for general anaesthesia is treated in another review (Guay 2016).
How the intervention might work
Most hip fractures occur in an elderly population; more than 30% of individuals are 85 years of age or older (Brauer 2009). Opioid‐related respiratory depression may result in severe brain damage or death (Lee 2015). By reducing the quantity of opioids used before, during and after surgery (Guay 2006), regional blockade may improve the mobility of persons with hip fracture (Saunders 2010), potentially facilitating person's participation in rehabilitation.
Why it is important to do this review
Despite their claim advantages, peripheral nerve blocks still are not widely used for people with hip fracture (Haslam 2013). We therefore decided to re‐evaluate the beneficial/harmful effects of peripheral nerve blocks for hip fracture.
This is an update of a previously published review (Parker 2002).
Objectives
This review focuses on the use of peripheral nerve blocks as preoperative analgesia, as postoperative analgesia or as a supplement to general anaesthesia for hip fracture surgery. We undertook the update to look for new studies and to update the methods to reflect Cochrane standards.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomized controlled trials comparing peripheral nerve blocks inserted preoperatively, operatively or postoperatively versus no regional blockade (control group).
We excluded quasi‐randomized trials (e.g. alternation).
Types of participants
We included adults aged 16 years of age and older with a proximal femoral fracture (hip fracture).
Types of interventions
Peripheral nerve blocks of any type versus no regional blockade added to general or neuraxial anaesthesia.
Types of outcome measures
Primary outcomes
Pain (study author's scale) at rest and on movement 30 minutes after block placement and at 6 to 8, 24, 48 and 72 hours after surgery
Acute confusional state (study author's definition and time points)
Myocardial infarction (study author's definition and time points)
Secondary outcomes
Pneumonia (study author's definition and time points)
Mortality (all death from any cause at any time points chosen by study authors)
Time to first mobilization after surgery
Costs of analgesic regimens (at any time points chosen by study authors)
Pressure sores (study author's definition and time points)
Number of participants transfused in hospital
Myocardial ischaemia (study author's definition and time points)
Opioid consumption in hospital up to 48 hours
Wound infection (study author's definition in hospital)
Participant satisfaction (study author's scale)
Complications related to pain treatment in hospital
Search methods for identification of studies
Electronic searches
We searched the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 8), MEDLINE (Ovid SP, 1966 to August week 1 2016), Embase (Ovid SP, 1988 to August week 1 2016) and the Cumulative Index to Nursing and Allied Health Literature (CINAHL) (EBSCO, 1982 to August week 1 2016). We applied no language or publication status restrictions.
For MEDLINE (Ovid SP), we designed a subject‐specific search strategy and used this as a basis for search strategies used in Embase, CENTRAL and CINAHL. When appropriate, we supplemented the search strategy with search terms used to identify randomized controlled trials. All search strategies can be found in Appendix 1.
Searching other resources
We also looked at http://www.clinicaltrials.gov (May 2015), http://isrctn.org (May 2015), http://www.umin.ac.jp/ctr/index.htm (May 2015), http://www.anzctr.org.au (May 2015), http://www.trialregister.nl/ (May 2015) and http://apps.who.int/trialsearch/ (May 2015) to identify trials in progress. We screened the reference lists of all studies retained (during data extraction) and from the recent meta‐analysis and reviews related to the topic (June 2015). We also screened conference proceedings of anaesthesiology societies for 2012, 2013 and 2014, published in three major anaesthesiology journals: British Journal of Anaesthesiology (May 2015), European Journal of Anaesthesiology (May 2015) and Regional Anesthesia and Pain Medicine (May 2015). We looked for abstracts on the website of the American Society of Anesthesiologists for the same years (2012 to 2014; http://www.asaabstracts.com/strands/asaabstracts/search.htm;jsessionid=4A977E1C98F0AE8995CFF248FE862490) (May 2015).
Data collection and analysis
Selection of studies
Two review authors (JG and SK) independently assessed potentially eligible trials for inclusion. We resolved disagreements by discussion.
Data extraction and management
At least two review authors (JG and SK) independently extracted data for the outcomes listed above for all new trials and resolved differences through discussion. For trials included in the previously published version (Parker 2002), one review author (JG) double‐checked all data against the original articles. When we were unable to extract the data in any form, we contacted the study authors for whom we could find an email address.
Assessment of risk of bias in included studies
Two review authors (JG and SK) evaluated all included studies for risk of bias using the Cochrane tool (Higgins 2011) and entered this information into RevMan. We resolved all differences by discussion. When reports did not provide enough information, we judged the item as unclear.
Measures of treatment effect
We presented results as risk ratio (RR) or risk difference (RD) along with the 95% confidence interval (95% CI) for dichotomous data, and as mean difference (MD) and 95% CI for continuous data. If some of the continuous data were given on different scales, or when results were not provided as mean and standard deviation (SD) (therefore extracted as P values), we produced the results as standardized mean difference (SMD) and 95% CI. For SMD, we considered 0.2 a small effect, 0.5 a medium effect and 0.8 a large effect (Pace 2011). When data showed an effect, we calculated the number needed to treat for an additional beneficial outcome (NNTB) or the number needed to treat for an additional harmful outcome (NNTH) using the odds ratio. We provided results for dichotomous data as RR as often as was feasible, as the odds ratio (OR) is not easily understood by clinicians (Deeks 2002; McColl 1998). We used OR for calculation of NNTB and NNTH (http://www.nntonline.net/visualrx/), as this value is less likely to be affected by the side (benefit or harm) on which data are entered (Cates 2002; Deeks 2002). When we noted no effect, we calculated the optimal information size to make sure that enough participants were included in the retained studies to justify a conclusion on the absence of effect (Pogue 1998) (http://www.stat.ubc.ca/˜rollin/stats/ssize/b2.html). We considered a difference of 25% (increase or decrease) as the minimal clinically relevant difference.
Unit of analysis issues
We included only parallel‐group trials. If a trial included more than two groups, we fused two groups (by using the appropriate formula for adding standard deviations when required) when we thought that they were equivalent according to the criteria chosen a priori for heterogeneity exploration; we separated them and split the control group in half if we thought that they were different.
Dealing with missing data
We contacted study authors to ask for apparently missing data. We did not consider medians as equivalent to means. Instead, we used the P value and the number of participants included in each group to calculate the effect size. We did not use imputed results. We entered data as intention‐to‐treat (ITT) as much as was feasible. If this was not possible, we entered the data on a per‐protocol basis.
Assessment of heterogeneity
We considered clinical heterogeneity before pooling results and examined statistical heterogeneity before carrying out any meta‐analysis. We quantified statistical heterogeneity by using the I2 statistic with data entered in the way (benefit or harm) that yielded the lowest amount. We qualified the amount as low (< 25%), moderate (50%) or high (75%), depending on the value obtained for the I2 statistic (Higgins 2003).
Assessment of reporting biases
We examined publication bias by using a funnel plot, then performed Duval and Tweedie’s trim and fill technique for each outcome. When publication bias is present, this technique yields an adjusted point of estimate that takes into account the number of theoretically missing studies.
Data synthesis
We analysed the data using RevMan 5.3 and ComprehensiveMeta‐Analysis Version 2.2.044 (www.Meta‐Analysis.com) with fixed‐effect (I2 ≤ 25%) or random‐effects models (I2 > 25%). We presented study characteristics in relevant tables (Characteristics of included studies; Characteristics of excluded studies; Characteristics of ongoing studies). We presented risk of bias assessments in graphs and results for each comparison as forests plots or as a narrative review (comparisons with fewer than two available trials or with a high level of heterogeneity after heterogeneity exploration).
Subgroup analysis and investigation of heterogeneity
We focused specifically on comparisons with more than a small amount of heterogeneity (I2 > 25%) (Higgins 2003) and explored heterogeneity by using Egger’s regression intercept (to assess the possibility of a small‐study effect; Rucker 2011); by visually inspecting forest plots with trials placed in order according to a specific moderator, by subgrouping (categorical moderator) or by meta‐regression (continuous moderator). We considered the following factors when exploring heterogeneity: type of block (psoas compartment, fascia iliaca, femoral nerve (we considered three‐in‐one and triple nerve blocks as femoral nerve blocks), femoral lateral cutaneous, obturator etc.), single shot versus continuous block (and duration of use), technique of localization (landmark, nerve stimulator or ultrasound), local anaesthetic concentration in lidocaine equivalent (calculated as follows: lidocaine = 1, bupivacaine = 4, chloroprocaine = 1.5, dibucaine = 4, etidocaine = 4, levobupivacaine = 3.9, mepivacaine = 0.8, prilocaine = 0.9, procaine = 0.5, ropivacaine = 3 and tetracaine = 4) (Berde 2009), time when the block was performed in relation to surgery, ages of participants included, American Society of Anesthesiologists (ASA) physical status of participants, year the study was published, delay from fracture (or hospital admission) to surgery, percentage of female participants, percentage of arthroplasty among participants and route of analgesia in the control group.
Sensitivity analysis
We performed a sensitivity analysis that was based on risk of bias of the study or, if a study was a clear outlier as long as a reason differentiating this study from the other studies could be found.
Quality of evidence and summary of findings
We used the principles of the GRADE approach (Guyatt 2008; Guyatt 2011) to assess the quality of the body of evidence associated with all of our primary outcomes (pain on movement 30 minutes after block placement, acute confusional state, myocardial infarction, pneumonia, death, time to first mobilization and cost of analgesic regimen for single shot blocks) and constructed Table 1 using GradePro (http://tech.cochrane.org/revman/gradepro). For risk of bias, we judged the quality of the evidence as presenting low risk of bias when most information came from studies at low risk of bias; we downgraded quality by one level when most information came from studies at high or unclear risk of bias (allocation concealment and blinding of outcome assessors) and by two levels when the proportion of information from studies at high risk of bias was sufficient to affect interpretation of results. For inconsistency, we downgraded the quality of evidence by one when the I2 statistic was 50% or higher without satisfactory explanation, and by two levels when the I2 statistic was 75% or higher without an explanation. We considered clinical heterogeneity as a factor for inconsistency. We did not downgrade the quality of evidence for indirectness, as all outcomes were based on direct comparisons, were performed on the population of interest and were not surrogate markers (Guyatt 2011a). For imprecision (Guyatt 2011b), we downgraded the quality of evidence by one when the CI around the effect size was large or overlapped with absence of effect and failed to exclude an important benefit or harm (when the number of participants was lower than the optimal information size); and we downgraded quality by two levels when the CI was very wide and included both appreciable benefit and harm. For publication bias, we downgraded the quality of evidence by one when correcting for the possibility of publication bias as assessed by Duval and Tweedie’s fill and trim analysis changed the conclusion. We upgraded the quality of evidence by one when the effect size was large (RR ≤ 0.5 or ≥ 2.0), and by two when the effect size was very large (RR ≤ 0.2 or ≥ 5) (Guyatt 2011c). We applied the same rules for OR when the basal risk was less than 20%. For SMD, we used 0.8 as the cutoff point for a large effect (Pace 2011). We also upgraded the quality by one when we found evidence of a dose‐related response. The quality was upgraded by one when a possible effect of confounding factors would reduce a demonstrated effect or suggest a spurious effect if results show no effect. When the quality of the body of evidence is high, further research is very unlikely to change our confidence in the estimate of effect. When the quality is moderate, further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. When the quality is low, further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. When the quality is very low, any estimate of effect is very uncertain (Guyatt 2008).
Results
Description of studies
Results of the search
Details of the search for this update can be found in Figure 1.
1.
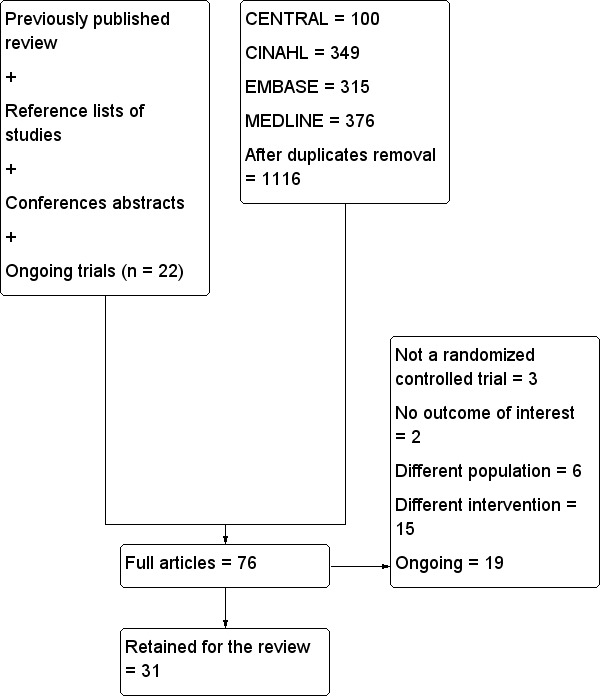
Flow diagram for this update.
n: number.
Included studies
We included 31 trials with 1760 participants: 897 randomized to regional blockade and 863 to no regional blockade. Trials published between 1980 and 2016 were funded by a charitable organization (n = 3; Beaudoin 2013; Cuvillon 2007; Foss 2007), by a governmental organization (n = 1; Nie 2015) or by departmental resources (n = 5; Domac 2015; Gille 2006; Jones 1985; Kullenberg 2004; Luger 2012). Remaining trials did not specify the source of funding. Trials were performed in Argentina (n = 1; Godoy 2010), Austria (n = 1; Luger 2012), Chile (n = 1; Altermatt 2013), China (n = 2; Graham 2008; Nie 2015), Denmark (n = 2; Foss 2007; Spansberg 1996), France (n = 2; Cuvillon 2007; Murgue 2006), Greece (n = 3; Antonopoulou 2006; Diakomi 2014; Mouzopoulos 2009), Germany (n = 1; Gille 2006), India (n = 1; Jadon 2014), Iran (n = 1; Mossafa 2005), Ireland (n = 1; Szucs 2012), Israel (n = 1; Chudinov 1999), Korea (n = 1; Yun 2009), South Africa (n = 1; White 1980), Spain (n = 2; De La Tabla 2010; Segado Jimenez 2009), Sweden (n = 1; Kullenberg 2004), Thailand (n = 1; Iamaroon 2010), Turkey (n = 2; Domac 2015; Tuncer 2003), United Kingdom (n = 5; Coad 1991; Fletcher 2003; Haddad 1995; Hood 1991; Jones 1985) and United States of America (n = 1; Beaudoin 2013). Participants were aged from 59.2 to 88 years (mean or median age of participants included in retained studies) and had an American Society of Anesthesiologists (ASA) physical status between 1.75 and 2.87; the proportion of females included varied between 27% and 95%. The proportion of arthroplasty varied between 0 and 82.5%. Delay from admission to surgery varied between 11 and 283 hours.
Blocks performed included a femoral nerve block (femoral or three‐in‐one block or triple nerve block) (Beaudoin 2013; Coad 1991; Cuvillon 2007; De La Tabla 2010; Fletcher 2003; Gille 2006; Graham 2008; Haddad 1995; Iamaroon 2010; Jadon 2014; Kullenberg 2004; Luger 2012; Murgue 2006; Spansberg 1996; Szucs 2012; Tuncer 2003), a femoral nerve block plus an infiltration above the iliac crest (Hood 1991), a fascia iliaca compartment block (Diakomi 2014; Domac 2015; Foss 2007; Godoy 2010; Mossafa 2005; Mouzopoulos 2009; Nie 2015; Yun 2009), a lateral cutaneous nerve block (Coad 1991; Jones 1985), a lateral cutaneous nerve block plus an obturator nerve block (Segado Jimenez 2009), an obturator nerve block (Segado Jimenez 2009) and a psoas compartment block (Altermatt 2013; Chudinov 1999; White 1980). Blocks were single shot blocks or continuous blocks (infusion or repeated) (Altermatt 2013; Chudinov 1999; Cuvillon 2007; De La Tabla 2010; Gille 2006; Luger 2012; Mouzopoulos 2009; Nie 2015; Spansberg 1996; Szucs 2012; Tuncer 2003) given for a duration ranging from 15 to 92 hours. Techniques of localization used for peripheral nerve blocks included loss of resistance (Chudinov 1999), use of nerve stimulator (Cuvillon 2007; Gille 2006; Graham 2008; Hood 1991; Iamaroon 2010; Jadon 2014; Kullenberg 2004; Spansberg 1996; Szucs 2012; Tuncer 2003), paraesthesia (Haddad 1995), ultrasound (Beaudoin 2013; De La Tabla 2010; Luger 2012) and landmarks (Coad 1991; Diakomi 2014; Domac 2015; Fletcher 2003; Foss 2007; Godoy 2010; Jones 1985; Mossafa 2005; Mouzopoulos 2009; Nie 2015; Segado Jimenez 2009; White 1980). Investigators performed blocks before surgery (Altermatt 2013; Beaudoin 2013; Chudinov 1999; De La Tabla 2010; Diakomi 2014; Domac 2015; Fletcher 2003; Foss 2007; Gille 2006; Godoy 2010; Graham 2008; Haddad 1995; Iamaroon 2010; Jadon 2014; Kullenberg 2004; Luger 2012; Mossafa 2005; Mouzopoulos 2009; Murgue 2006; Szucs 2012; Yun 2009), intraoperatively (Hood 1991; Spansberg 1996; Tuncer 2003; White 1980) or after surgery (Coad 1991; Cuvillon 2007; Jones 1985; Nie 2015; Segado Jimenez 2009). Concentrations of local anaesthetic used in the lidocaine equivalent ranged from 5 to 22.5 mg/mL.
Details of the blocks and of anaesthetic techniques used for the surgery are included in Table 2.
1. Anaesthetic techniques.
| Study | Purpose of blockade | Surgical anaesthesia | Block technique | Comparison |
| Altermatt 2013 | Preoperative and postoperative analgesia | Unspecified | Continuous lumbar plexus with 0.1% bupivacaine in a patient‐controlled analgesia mode | IV morphine |
| Antonopoulou 2006 | Postoperative analgesia | Spinal anaesthesia | Continuous femoral nerve block, nerve stimulator, loaded with 18 mL of 0.25% bupivacaine followed by an infusion of 0.125% levobupivacaine at 3‐4 mL/h | IM pethidine |
| Beaudoin 2013 | Preoperative analgesia | Unspecified | Ultrasound‐guided femoral nerve block performed by an experienced operator with a 7.5 MHz linear probe, participant in Trendelenburg position, cross‐sectional view, 22 G Whitacre needle in‐plane and 25 mL of bupivacaine 0.5% and distal manual pressure for 5 minutes | IM morphine |
| Chudinov 1999 | Preoperative and postoperative analgesia Surgery for some participants |
Treatment group: psoas block alone (3/20) with a sciatic block (5/20), a spinal (11/20) or general anaesthesia (1/20) Control group: neuraxial block (19/20) or general anaesthesia (1/20). |
Psoas, loss of resistance, Chayen's technique 0.8 mL/kg of bupivacaine 0.25%, operated side up (1 epidural spread) | IM meperidine and diclofenac |
| Coad 1991 | Postoperative analgesia | General anaesthesia for all participants with etomidate, nitrous oxide, enflurane, fentanyl and vecuronium | Lateral cutaneous: 15 mL of 0.5% bupivacaine with epinephrine, Eriksson's technique Femoral (3‐in‐1): 15 mL of 0.5% bupivacaine with epinephrine, Winnie's technique |
IM meperidine |
| Cuvillon 2007 | Postoperative analgesia | Spinal anaesthesia for all participants | Continuous femoral nerve block. Nerve stimulator 0.3 to 0.5 mA. Non‐stimulating catheter passed 10‐15 cm past the needle tip loaded with 30 mL of 1.5% lidocaine followed by ropivacaine 0.2% at 10 mL/h for 48 hours | SC morphine |
| De La Tabla 2010 | Preoperative analgesia | Unspecified | Femoral nerve block under dual guidance (ultrasound and nerve stimulator), catheter loaded with 15 mL of ropivacaine 0.2% followed by an infusion of the same solution at 5 mL/h and 10 mL every 30 minutes | IV metamizole and tramadol |
| Diakomi 2014 | Preoperative analgesia (spinal positioning) | Spinal anaesthesia | Fascia iliaca block, Dalen's technique, landmarks, 40 mL of 0.5% ropivacaine | IV fentanyl |
| Domac 2015 | Preoperative analgesia (spinal positioning) plus postoperative analgesia | Spinal anaesthesia | Fascia iliaca block with 15 mL of 0.5% bupivacaine and 15 mL of 2% lidocaine; 2‐3 cm below inguinal ligament at the junction of lateral 1/3 and medial 2/3 of a line from pubis tubercle to anterior iliac spine; 2 pops | IV morphine |
| Fletcher 2003 | Preoperative analgesia | Unspecified | Fermoral (3‐in‐1 nerve block, Wiinie's technique with 20 mL of 0.5% bupivacaine and 5 minutes distal compression) | IV morphine |
| Foss 2007 | Preoperative analgesia | Unspecified | Fascia iliaca block based on Dalen's landmarks with a 24 G blunted needle and 40 mL of 1% mepivacaine with epinephrine | IM morphine |
| Gille 2006 | Preoperative and postoperative analgesia |
Treatment group: spinal anaesthesia for 37/50 and general anaesthesia for 13/50 Control group: spinal anaesthesia for 38/50 and general anaesthesia for 12/50 |
Femoral non‐stimulating catheter: needle 18 G, catheter 20 G (Brown‐Perifix‐Plexus Anaesthesia); 0.5 mA and 0.1 msec. Catheters were advanced about 10 cm past the needle tip and fixed. Loading dose was 40 mL of prilocaine 1% followed 2 hours later by ropivacaine 0.2% 30 mL, repeated every 6 hours. Amount (up to 40 mL; n = 5) and intervals (up to every 4 hours; n = 8) or both (n = 6) adjusted on pain scores | IV metamizole plus oral tilidine and naloxone |
| Godoy 2010 | Preoperative analgesia | Unspecified | Fascia iliaca compartment block, 21G long bevel needle, Dalen's technique with 0.3 mL/kg of 0.25% bupivacaine | IV non‐steroidal anti‐inflammatory drugs |
| Graham 2008 | Preoperative analgesia | Unspecified | Femoral (3‐in‐1) nerve block, Winnie's technique, nerve stimulator and 30 mL of bupivacaine 0.5% (not exceeding 3 mg/kg) | IV morphine |
| Haddad 1995 | Preoperative analgesia | Unspecified | Femoral nerve block with 0.3 mL/kg of bupivacaine 0,25%. Paraesthesia technique with a short bevel needle | IM pethidine, oral co‐dydramol and IM voltarol |
| Hood 1991 | Postoperative analgesia | General anaesthesia for all participants with etomidate, nitrous oxide, isoflurane and alfentanil | Femoral nerve block (triple nerve block) with nerve stimulator < 1.0 mA and 35 mL of prilocaine 0.75% and distal digital pressure plus infiltration above the iliac crest with 8 mL of the same solution | IM papaveratum |
| Iamaroon 2010 | Preoperative analgesia | Spinal anaesthesia | Femoral nerve block with nerve stimulator (0.2 to 0.4 mA) and 20 mL of bupivacaine 0.5% plus 10 mL of saline | IV fentanyl |
| Jadon 2014 | Preoperative analgesia | Spinal anaesthesia | Femoral nerve block with nerve stimulator (0.3 to 0.5 mA) and 15 mL of lidocaine 2% plus 5 mL of distilled water | IV fentanyl |
| Jones 1985 | Postoperative analgesia | General anaesthesia for all participants with thiopental, nitrous oxide, halothane, fentanyl and alcuronium | Lateral cutaneous nerve block with 15 mL of 0.5% bupivacaine, Eriksson's technique | IM pethidine |
| Kullenberg 2004 | Preoperative analgesia | Unspecified | Femoral nerve block with 30 mL of 0.75% ropivacaine. Winnie's approach and nerve stimulator | IM ketobemidon plus tramadol and paracetamol |
| Luger 2012 | Preoperative and postoperative analgesia | Spinal anaesthesia | Ultrasound‐guided femoral (3‐in‐1) nerve block (13‐6 MHz linear probe), catheter inserted ≥ 12‐15 cm past the needle tip) loaded with 30 mL of 0.25% bupivacaine followed by an infusion of 0.125% bupivacaine at 6 mL/h (motor blockade not evaluated) or Lumbar epidural analgesia with 0.125% bupivacaine at 8 mL/h |
IV/SC piritramide or IV paracetamol |
| Mossafa 2005 | Preoperative analgesia | Spinal anaesthesia | Fascia iliaca block with 20 mL of 1.5% lidocaine | IV fentanyl |
| Mouzopoulos 2009 | Preoperative and postoperative analgesia | Epidural anaesthesia | Fascia iliaca block daily, Dalen's technique with 0.3 mL/kg of bupivacaine (0.25%?) | IV and analgesics |
| Murgue 2006 | Preoperative analgesia | Unspecified | Femoral nerve block with nerve stimulator and 20 mL of mepivacaine | IV morphine or IV paracetamol and ketoprofen |
| Nie 2015 | Postoperative analgesia | General anaesthesia with propofol, remifentanil and atracurium | Fascia iliaca block with landmarks (2‐3 cm below the inguinal ligament); catheter inserted at least 10 cm cranially and loaded with 20 to 30 mL (weight basis) of 0.5% ropivacaine followed by 0.25% bupivacaine at 0.1 mL/kg/h for 48 hours | IV patient‐controlled analgesia with fentanyl and tropisetron |
| Segado Jimenez 2009 | Postoperative analgesia | Spinal anaesthesia | Landmarks. Obturator nerve with 15 mL of bupivacaine with a vasoconstrictive agent, proximal to the obturator orifice. Femoral lateral cutaneous (Brown) with 10 mL of the same solution | IV morphine |
| Spansberg 1996 | Postoperative analgesia | Spinal anaesthesia | Femoral nerve block with nerve stimulator, non‐stimulating catheter advanced 8‐15 cm past needle tip. Inserted just before surgery. Loaded with 0.4 L/kg of bupivacaine 0.5%; continuous infusion with 0.14 mL/kg/h of bupivacaine 0.25% for 16 hours after surgery | IM morphine |
| Szucs 2012 | Preoperative and postoperative analgesia | Spinal anaesthesia | Non‐stimulating catheter for femoral nerve block, inserted in the emergency department with a nerve stimulator, 0.4 mA and 0.1 msec, space dilated before catheter insertion with 10 mL of 2% lidocaine, catheter advanced 3 cm past the needle tip and 10 mL of 0.5% bupivacaine through the catheter followed by 0.25% bupivacaine infused at 4 mL per hour for 72 hours | IM morphine |
| Tuncer 2003 | Postoperative analgesia | General anaesthesia for all participants with propofol, nitrous oxide, isoflurane, fentanyl, morphine and atracurium | Femoral (3‐in‐1) nerve block, nerve stimulator 0.1 mA, non‐stimulating catheter advanced 4‐5 cm past the needle tip. Loaded with 30 mL of 2% lidocaine with epinephrine followed by an infusion with bupivacaine 0.125% at 4 mL/h for 48 hours | IV morphine |
| White 1980 | Intraoperative analgesia | General anaesthesia with thiopental, nitrous oxide, halothane and fentanyl or nitrous oxide and alfaxolone/alfadolone | Psoas block with 30 mL of 2% mepivacaine, side to be blocked uppermost, Chayen's technique or Spinal: 0.6 to 0.8 mL of hyperbaric cinchocaine |
Conventional general anaesthesia |
| Yun 2009 | Preoperative analgesia | Spinal anaesthesia | Fascia iliaca block, Dalen's technique with 30 mL of 0.375% ropivacaine | IV alfentanil |
G: gram
h: hour
IM: intramuscular
mA: milliAmpere
mcg/mL: microgram/millilitre
mg/kg: milligram/kilogram
MHz: megahertz
mL: millilitre
msec: millisecond
n: number
SC: subcutaneous
Excluded studies
We excluded 26 studies (Characteristics of excluded studies) because they were not randomized controlled trials (n = 3; Fujihara 2013; Irwin 2012; Luger 2012), included no outcomes of interest for this review (n = 2; Bölükbasi 2013; Hwang 2015), studied a different population (n = 6; Durrani 2013; McRae 2015; Mutty 2007; Schiferer 2007; Segado Jimenez 2010; Sia 2004) or studied a different intervention (n = 15; Bech 2011; Foss 2007; Ghimire 2015; Gorodetskyi 2007; Hussain 2014; Kang 2013; Mannion 2005; Manohara 2015; Marhofer 1998; Matot 2003; Piangatelli 2004; Reavley 2015; Scheinin 2000; Turker 2003; Van Leeuwen 2000).
Studies awaiting classification
We have no studies awaiting classification.
Ongoing studies
We found 19 ongoing trials (ACTRN12609000526279; EUCTR2006‐004001‐26‐GB; EUCTR2008‐004303‐59‐SE; EUCTR2010‐023871‐25‐GB; EUCTR2015‐000078‐36‐DK; ISRCTN07083722; ISRCTN46653818; ISRCTN75659782; ISRCTN92946117; NCT00749489; NCT01052974; NCT01219088; NCT01547468; NCT01593319; NCT01638845; NCT01904071; NCT02381717; NCT02406300; NCT02433548). (See Characteristics of ongoing studies.)
Risk of bias in included studies
We rated randomization as presenting high risk for two of the included studies because investigators provided no details on how randomization was performed and numbers of participants differed highly between groups (Antonopoulou 2006; De La Tabla 2010). We rated eight other studies as having unclear risk for randomization because the report provided no details (Altermatt 2013; Chudinov 1999; Coad 1991; Domac 2015; Mossafa 2005; Segado Jimenez 2009; Tuncer 2003; White 1980) (see Characteristics of included studies; Figure 2; Figure 3).
2.
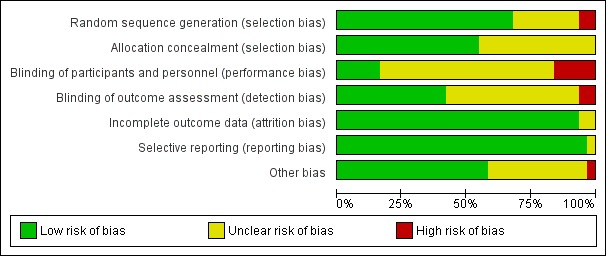
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
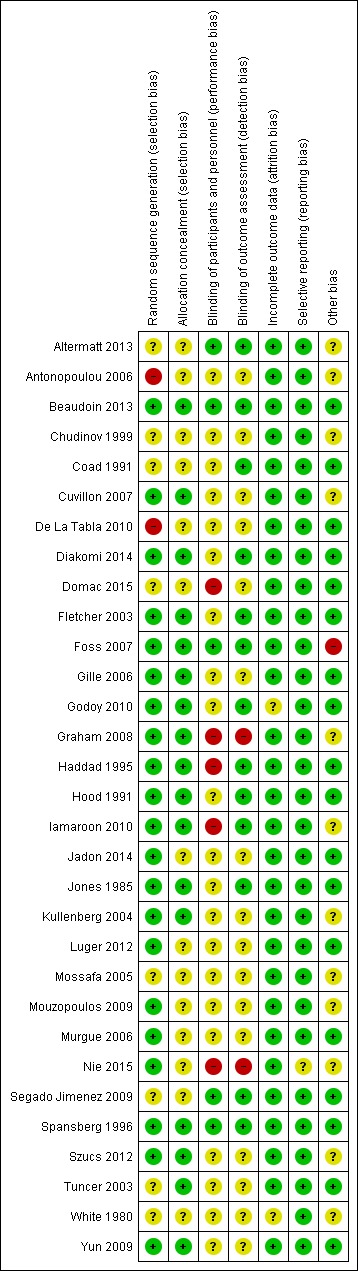
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We rated allocation concealment as introducing unclear or high risk of bias for less than 50% of the studies (see Characteristics of included studies; Figure 2; Figure 3).
Blinding
We judged blinding of outcome assessors as appropriate for less than 50% of the studies (see Characteristics of included studies; Figure 2; Figure 3).
Incomplete outcome data
We rated most studies as having low risk of attrition bias (see Characteristics of included studies; Figure 2; Figure 3).
Selective reporting
We rated no studies as having high risk of bias for this item (see Characteristics of included studies; Figure 2; Figure 3).
Other potential sources of bias
We rated only one study (Foss 2007) as having high risk of bias for other potential sources of bias because participants in the block group had higher pain scores on admission (P = 0.04) (see Characteristics of included studies; Figure 2; Figure 3).
Effects of interventions
See: Table 1
Primary outcomes
1. Pain
1.1 Pain on movement and at rest within 30 minutes after block placement
We did not retain data from two studies for this analysis. Jadon 2014 evaluated pain scores during positioning for spinal anaesthesia five minutes after a femoral nerve block performed with a nerve stimulator and 20 mL of a solution containing 15 mL of lidocaine 2% and 5 mL of distilled water. Parkinson 1989 reported that at five minutes after a femoral nerve block with lidocaine‐HCl and a nerve stimulator, only 6 and 11 participants out of 20 would have a complete or partial femoral nerve block, and 15 minutes would be required for a complete or partial femoral nerve block in all participants. Mossafa 2005 evaluated pain scores during positioning for spinal anaesthesia five minutes after a fascia iliaca block with 20 mL of lidocaine 1.5%. Although some effects on pain scores can be seen at 10 minutes after a fascia iliaca block with lidocaine, maximal effects are more likely to occur after 30 minutes or later (Dochez 2014; Gozlan 2005). We retained eight trials (Diakomi 2014; Domac 2015; Foss 2007; Gille 2006; Iamaroon 2010; Murgue 2006; Szucs 2012; Yun 2009) that included 373 participants evaluating pain on movement within 30 minutes after block placement: at 15 minutes (femoral nerve block with bupivacaine and nerve stimulator, pain during positioning for spinal anaesthesia; Iamaroon 2010), at 20 minutes (fascia iliaca with landmarks and ropivacaine, pain during positioning for spinal anaesthesia, Diakomi 2014; fascia iliaca block with landmarks and ropivacaine, pain during positioning for spinal anaesthesia, Yun 2009; femoral nerve block with a nerve stimulator and mepivacaine, pain during transfer on the radiological table for the X‐ray, Murgue 2006), at 30 minutes (fascia iliaca block with landmarks and mepivacaine, pain during passive elevation of the leg at 15 degrees, Foss 2007; non‐stimulating femoral nerve catheter with prilocaine inserted with a nerve stimulator, pain with passive anteflexion of the hip at 30 degrees, Gille 2006; non‐stimulating femoral nerve catheter with bupivacaine inserted with a nerve stimulator, pain with passive anteflexion of the hip at 30 degrees, Szucs 2012) or after 30 minutes (fascia iliaca with mixture of lidocaine and bupivacaine for pain during positioning for spinal > 30 minutes, Domac 2015). Pain scores were lower with regional blockade (standardized mean difference (SMD) ‐1.41, 95% confidence interval (CI) ‐2.14 to ‐.067; I2 = 90%; Analysis 1.1). On the basis of the standard deviation in the control group of a study at low risk of bias (Diakomi 2014: 2.4), this was equivalent to ‐3.4 on a scale from 0 to 10. Egger's regression intercept showed the possibility of a small‐study effect as a source of heterogeneity (P = 0.02). Duval and Tweedie's trim and fill analysis showed no evidence of publication bias. Investigators in one study may have performed the evaluation before the effect of the local anaesthetic took place in most participants (15 minutes; Iamaroon 2010). When a femoral nerve block using a nerve stimulator is performed with bupivacaine, the median onset time for a complete sensory and motor block would be 30 minutes (5 to 95 percentiles; 15 to 45 minutes; Cuvillon 2009). Excluding this study (Iamaroon 2010) and one study that did not provide the exact concentration of local anaesthetic injected (Murgue 2006) led to an effect size that was correlated with the concentration of local anaesthetic used in lidocaine equivalent (P < 0.00001; Figure 4). We calculated equivalences as mentioned in the methods section (i.e. lidocaine = 1, bupivacaine = 4, chloroprocaine = 1.5, dibucaine = 4, etidocaine = 4, levobupivacaine = 3.9, mepivacaine = 0.8, prilocaine = 0.9, procaine = 0.5, ropivacaine = 3 and tetracaine = 4) (Berde 2009). Therefore, for Diakomi 2014, the concentration in lidocaine equivalent was calculated as 15 mg/mL (ropivacaine 0.5% or ropivacaine 5 mg/mL multiplied by 3 = 15 mg/mL). For Domac 2015, the concentration in lidocaine equivalent was calculated as 20 mg/mL (mixture of 15 mL bupivacaine 0.5% or bupivacaine 5 mg/mL multiplied by 4 = 20 mg/mL and 2% lidocaine or lidocaine 20 mg/mL). For Foss 2007, the equivalence was calculated as 8 mg/mL (mepivacaine 1% or mepivacaine 10 mg/mL multiplied 0.8 = 8 mg/mL). For Gille 2006, the lidocaine equivalent was calculated as 9 mg/mL (1% prilocaine or prilocaine 10 mg/mL multiplied by 0.9 = 9 mg/mL). For Szucs 2012, the equivalence was calculated as 20 mg/mL (10 mL of 2% lidocaine or lidocaine 20 mg/mL and 10 mL of 0.5% bupivacaine or bupivacaine 5 mg/mL multiplied by 4 = 20 mg/mL). For Yun 2009, the equivalence was calculated as 11.25 mg/mL (ropivacaine 0.375% or ropivacaine 3.75 mg/mL multiplied by 3 = 11.25 mg/mL). Results from Diakomi 2014 (mean and SD of the control group 7.5 and 2.4) show that 182 participants (91 per group) would be required in a simple trial to eliminate a difference of 1 on a 0 to 10 scale (alpha 0.05; beta 0.2; two‐sided test) (http://stat.ubc.ca/˜rollin/stats/ssize/n2a.html).
1.1. Analysis.
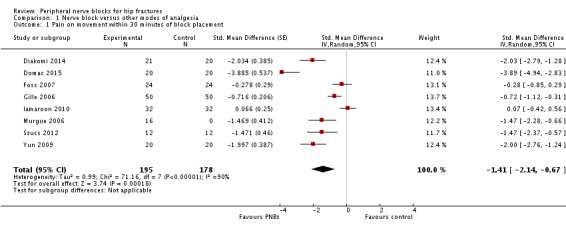
Comparison 1 Nerve block versus other modes of analgesia, Outcome 1 Pain on movement within 30 minutes of block placement.
4.
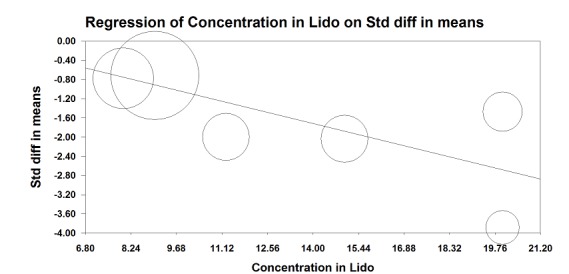
Pain on movement in participants with hip fracture between 20 and 30 minutes after block placement. The effect size is proportionate to the concentration of local anaesthetic (mg/mL) used in lidocaine equivalent (P < 0.00001).
Local anaesthetic concentration in lidocaine equivalent (calculated as follows: lidocaine = 1, bupivacaine = 4, chloroprocaine = 1.5, dibucaine = 4, etidocaine = 4, levobupivacaine = 3.9, mepivacaine = 0.8, prilocaine = 0.9, procaine = 0.5, ropivacaine = 3 and tetracaine = 4).
Quality of evidence for pain on movement at 30 minutes after block placement
We downgraded the level of evidence by one because we rated five of the eight included studies as having unclear risk for blinding of outcome assessment. We did not downgrade the level of evidence on the basis of inconsistency because we found a reasonable explanation for heterogeneity. We used direct comparisons only with studies performed on the population of interest, and this is not a surrogate marker. The optimal information size was achieved, but we downgraded by one level for imprecision owing to a wide confidence interval around the effect size. We found no evidence of publication bias. We upgraded the level of evidence on the basis of a large effect size (SMD > 0.8). We also upgraded the level of evidence by one on the basis of confounding factors. No study used ultrasound guidance, an approach that could have increased block success (Lewis 2015). We upgraded the evidence by one on the basis of a dose‐response relationship (effect size was proportionate to the concentration of local anaesthetic used). We rated the quality of evidence as high.
Nine trials (Beaudoin 2013; Chudinov 1999; Diakomi 2014; Foss 2007; Gille 2006; Godoy 2010; Graham 2008; Iamaroon 2010; Szucs 2012) including 540 participants evaluated pain at rest within 30 minutes after block placement. Of these nine trials, two evaluated a fascia iliaca block with bupivacaine at 15 minutes (Godoy 2010) or a femoral nerve block with bupivacaine with a nerve stimulator, also at 15 minutes (Iamaroon 2010). Because these trials may have evaluated pain scores before the block could be effective (Cuvillon 2009), we excluded them from this analysis. We retained one trial with an evaluation performed at 15 minutes with bupivacaine (Beaudoin 2013) because femoral nerve blocks were performed with ultrasound, and onset of a femoral nerve block may have occurred earlier with ultrasound guidance (mean 16 minutes) compared with use of a nerve stimulator (mean 27 minutes) (Marhofer 1997). We therefore retained seven trials including 322 participants for this analysis. Investigators performed evaluations at 15 minutes (femoral nerve block with bupivacaine with ultrasound guidance, Beaudoin 2013), at 20 minutes (fascia iliaca block with ropivacaine, Diakomi 2014) or at 30 minutes (psoas compartment block with bupivacaine with loss of resistance technique, Chudinov 1999; fascia iliaca block with landmarks and mepivacaine, Foss 2007; non‐stimulating femoral nerve catheter with prilocaine inserted with a nerve stimulator, Gille 2006; femoral nerve block with bupivacaine with a paraesthesia technique or nerve stimulator, Graham 2008; non‐stimulating femoral nerve catheter with bupivacaine inserted with a nerve stimulator, Szucs 2012). Regional blockade decreased pain scores at rest within 30 minutes after block placement (SMD ‐0.80, 95% CI ‐1.25 to ‐0.35; I2 = 72%; Analysis 1.2). When a study at low risk of bias is used (Diakomi 2014), this reduction would be equivalent to 1.7 on a scale from 0 to 10. Egger's regression intercept showed no statistically significant small‐study effect (two‐sided test). Duval and Tweedie's trim and fill analysis showed that two trials might be missing to right of mean for an adjusted point of estimate of ‐0.58 (95% CI ‐1.01 to ‐0.14). Taken individually, only one trial (Foss 2007) did not favour regional blockade and for this study, pain scores before block placement were significantly higher in the regional blockade group. Excluding this trial shows that the effect favouring regional blockade would be SMD ‐0.95 (95% CI ‐1.23 to ‐0.66; I2 = 17%).
1.2. Analysis.
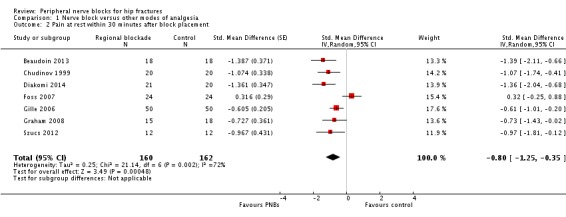
Comparison 1 Nerve block versus other modes of analgesia, Outcome 2 Pain at rest within 30 minutes after block placement.
1.2 Pain on movement and at rest at six to eight hours after surgery
One trial (Domac 2015) gave results for pain on movement at six to eight hours after surgery (SMD 0.00, 95% CI ‐0.62 to 0.62). Results from five trials with 286 participants (Chudinov 1999; Cuvillon 2007; Domac 2015; Nie 2015; Yun 2009) show that peripheral nerve blocks decreased pain scores on a scale from 0 to 10 for pain at rest at 6 to 8 hours after surgery (mean difference (MD) ‐0.38, 95% CI ‐0.70 to 0.06; I2 = 0%; Analysis 1.3). Egger's regression intercept showed no statistically significant small‐study effect (P = 0.05; two‐sided test). Duval and Tweedie's trim and fill analysis calculated that three trials might be missing to right of mean for an adjusted point of estimate of MD ‐0.32 (95% CI ‐0.60 to ‐0.03).
1.3. Analysis.
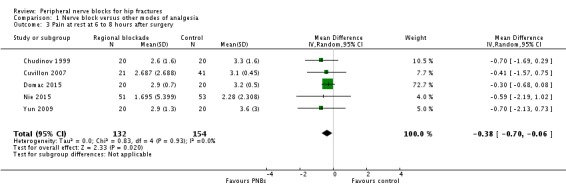
Comparison 1 Nerve block versus other modes of analgesia, Outcome 3 Pain at rest at 6 to 8 hours after surgery.
1.3 Pain on movement and at rest at 24 hours after surgery
Based on four trials with 195 participants (continuous ultrasound‐guided femoral nerve block, De La Tabla 2010; single shot landmark fascia iliaca block, Domac 2015; continuous nerve stimulator‐guided femoral nerve block, Gille 2006; continuous ultrasound‐guided femoral nerve block, Luger 2012) we did not find a difference in pain scores on movement at 24 hours (MD ‐0.39, 95% CI ‐1.08 to 0.30; I2 = 95.6%; Analysis 1.4). Egger's regression intercept showed no statistically significant small‐study effect (two‐sided test). Duval and Tweedie's trim and fill analysis showed that one study might be missing to left of mean for an adjusted point of estimate of SMD ‐0.58 (95% CI ‐1.45 to 0.30; random‐effects model).
1.4. Analysis.
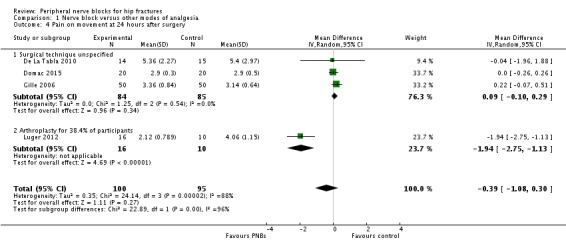
Comparison 1 Nerve block versus other modes of analgesia, Outcome 4 Pain on movement at 24 hours after surgery.
Findings of eight trials (single shot blocks, Domac 2015; Yun 2009; continuous blocks, Chudinov 1999; Cuvillon 2007; De La Tabla 2010; Gille 2006; Luger 2012; Nie 2015) including 435 participants show decreased pain scores at rest at 24 hours (MD ‐0.68, 95% CI ‐1.23 to ‐0.13; I2 = 82%; Analysis 1.5). Egger's regression intercept showed the possibility of a small‐study effect (P = 0.02; two‐sided test). Duval and Tweedie's trim and fill analysis showed no evidence of publication bias. The effect seems as good with a single shot block as with a continuous block: heterogeneity between subgroups was 0% (Analysis 1.5).
1.5. Analysis.
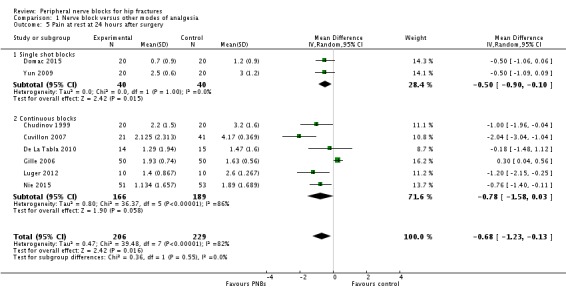
Comparison 1 Nerve block versus other modes of analgesia, Outcome 5 Pain at rest at 24 hours after surgery.
Pain on movement and at rest at 48 hours after surgery
Three trials (De La Tabla 2010; Domac 2015; Gille 2006) gave data for pain on movement at 48 hours, two of which used continuous femoral nerve blocks (De La Tabla 2010; Gille 2006). Results of these two trials (De La Tabla 2010; Gille 2006) including 129 participants show that continuous peripheral nerve blocks do not affect pain on movement at 48 hours after surgery (MD 0.09, 95% CI ‐0.23 to 0.40; I2 = 0%; Analysis 1.6).
1.6. Analysis.
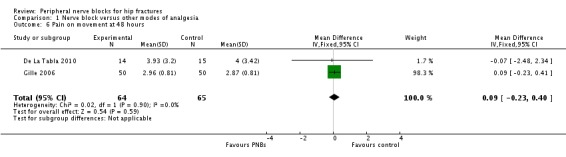
Comparison 1 Nerve block versus other modes of analgesia, Outcome 6 Pain on movement at 48 hours.
Six trials (Chudinov 1999; Cuvillon 2007; De La Tabla 2010; Domac 2015; Gille 2006; Nie 2015) gave results for pain at rest at 48 hours after surgery. Five of these studies used continuous nerve blocks (Chudinov 1999; Cuvillon 2007; De La Tabla 2010; Gille 2006; Nie 2015) and included 335 participants. Peripheral nerve blocks did not affect pain scores at rest at 48 hours (MD ‐0.37, 95% CI ‐0.87 to 0.13; I2 = 72%; Analysis 1.7). The effect may differ with the type of block used: I2 statistic for the difference between subgroups is 85% (P = 0.001). Egger's regression intercept showed no statistically significant small‐study effect (two‐sided test). Duval and Tweedie's trim and fill analysis calculated that one study might be missing to right of mean for an adjusted point estimate of MD ‐0.25 (95% CI ‐0.68 to 0.18; random‐effects model).
1.7. Analysis.
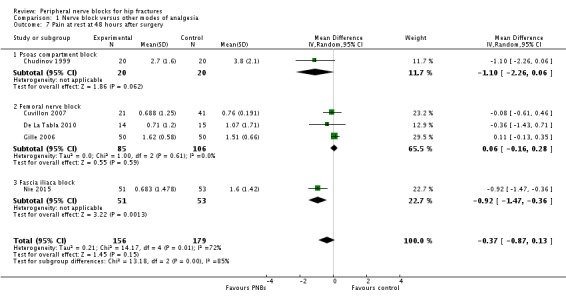
Comparison 1 Nerve block versus other modes of analgesia, Outcome 7 Pain at rest at 48 hours after surgery.
1.5 Pain on movement and at rest at 72 hours after surgery
One trial (Gille 2006) with 100 participants using a continuous femoral nerve block gave results for pain on movement at 72 hours after surgery (MD 0.25, 95% CI ‐0.02 to ‐0.52).
Two trials including 140 participants (psoas compartment block, Chudinov 1999; continuous femoral nerve block, Gille 2006) provided results for pain at rest at 72 hours after surgery for a continuous peripheral nerve block (MD ‐0.48, 95% CI ‐1.83 to 0.87). Data show an effect for a psoas compartment block (MD ‐1.20, 95% CI ‐1.77 to ‐0.63) but not for a femoral nerve block (MD 0.18, 95% CI 0.03 to 0.33). Heterogeneity between subgroups was statistically significant (I2 = 95%; P < 0.00001).
2. Acute confusional state
We have provided definitions used by study authors in Table 3. Based on seven trials (Cuvillon 2007; Godoy 2010; Graham 2008; Kullenberg 2004; Mouzopoulos 2009; Nie 2015; White 1980 with 676 participants, we did not find a difference in the incidence of acute confusional state (RR 0.69, 95% CI 0.38 to 1.27; I2 = 48%). Egger's regression intercept showed no statistically significant small‐study effect (two‐sided test). Duval and Tweedie's trim and fill analysis calculated that one trial might be missing to right of mean for an adjusted point of estimate of RR 0.77 (95% CI 0.40 to 1.45; Analysis 1.9). Given a rate of 19%, the number of participants required to eliminate a 25% decrease would be 1518 (759 per group) (alpha 0.05; beta 0.2; one‐sided test).
2. Outcome definitions for acute confusional state.
| Study | Study authors' definition |
| Cuvillon 2007 | Clinical evaluation "somnolence‐confusion" |
| Godoy 2010 | "episodes of delirium" |
| Graham 2008 | "acute confusional state" |
| Kullenberg 2004 | "transient confusion" |
| Mouzopoulos 2009 | "The primary outcome was perioperative delirium.
Diagnosis of the syndrome was defined using the Diagnostic
and Statistical Manual of Mental Disorders, 4th
edition (DSM‐IV), and Confusion Assessment Method
(CAM) criteria" "Daily patient assessments using the MMSE, DRS‐R‐ 98, and Digit Span test [assessment of attention, range 0 (no attention) to 42 (good attention)] were used to enable the DSM‐IV and CAM diagnoses and assess delirium severity" |
| Nie 2015 | "Presurgery cognitive status was estimated using the mini‐mental state examination before and after surgery. The Confusion Assessment Method was used to diagnose delirium pre‐ and postsurgery" |
| White 1980 | "confused" |
1.9. Analysis.
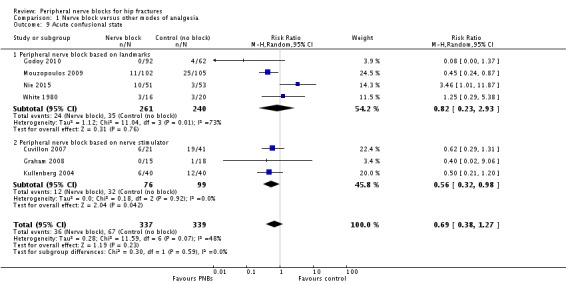
Comparison 1 Nerve block versus other modes of analgesia, Outcome 9 Acute confusional state.
Quality of evidence for acute confusional state
We downgraded the level of evidence by two for risk of bias because we rated 75% or more of the studies as having unclear or high risk of bias for blinding of outcome assessors. We downgraded the level by one for a moderate amount of heterogeneity. We included only direct comparisons performed on the population of interest, and this is not a surrogate marker. We downgraded the level by one for imprecision because the optimal information size was not achieved. We did not downgrade the level of evidence on the basis of the possibility of publication bias because applying a correction for the possibility of one would not modify the conclusion. We found no evidence of a large effect. We downgraded the level of evidence by one for confounding factors because no study used ultrasound guidance, an approach that could have increased block success (Lewis 2015). We rated the quality of evidence as very low.
3. Myocardial infarction/ischaemia
Two trials (Altermatt 2013; Luger 2012) gave results for myocardial ischaemia. Altermatt 2013, with 31 included participants, evaluated effects of a continuous psoas compartment block started preoperatively and maintained until postoperative day 3, and reported the number of ischaemic events (EKG segment analysis) recorded by participants during the observation period as 6 per participant with regional blockade (n = 17) versus 3 per participant with intravenous patient‐controlled analgesia (n = 14) (P = 0.618). Luger 2012 reported that 1 of 10 participants with an ultrasound‐guided continuous femoral nerve block had myocardial ischaemia (serum T troponin levels increased), as did 5 of 10 participants without a peripheral nerve block (RR 0.68, 95% CI ‐2.54 to 0.12). Given an incidence of 30%, 850 participants (425 per group) would be required in a simple trial, to eliminate a 25% reduction in the number of participants experiencing cardiac enzyme elevation (alpha 0.05; beta 0.2; one‐sided test).
Quality of evidence for myocardial ischaemia
We downgraded the level of evidence by two for risk of bias because we judged the included study (Luger 2012) as having unclear risk for allocation concealment and blinding of outcome assessors. We could not assess heterogeneity. The trial performed a direct comparison. We downgraded evidence by two for imprecision owing to inclusion of very few participants/trials in the analysis. We found no evidence of a large effect or confounding factors that would justify upgrading. We found no evidence of a dose‐response effect. We rated the quality of evidence as very low.
Secondary outcomes
1. Pneumonia
Results of three trials (Fletcher 2003; Haddad 1995; White 1980) with 131 participants show that peripheral nerve blocks reduced the risk of pneumonia (RR 0.41, 95% CI 0.19 to 0.89; I2 = 3%; Analysis 1.10). Egger's regression intercept showed no significant evidence of a small‐study effect. Duval and Tweedie's trim and fill analysis revealed no evidence of publication bias. Two trials evaluated a femoral (or three‐in‐one) nerve block (Fletcher 2003; Haddad 1995), and one trial evaluated a psoas compartment block (White 1980). Definitions and time points used included lower respiratory tract infection within six months from hospital notes (Fletcher 2003), short‐term respiratory infection (Haddad 1995) and pneumonia during hospitalization (mean duration 20 days, SD 11.5 days; White 1980). Although all three trials showed a trend towards a reduced incidence of lower respiratory tract infection when a peripheral nerve block was added to the postoperative analgesia regimen, Haddad 1995 reported the largest reduction. The complication rate observed in Haddad 1995 was extremely high compared with the actual rate (Cordero 2016). Given a basal rate of 27%, the NNTB would be 7 (95% CI 5 to 72) and the number of participants required to eliminate a 25% decrease would be 978 (489 per group) (alpha 0.05; beta 0.2; one‐sided test).
1.10. Analysis.
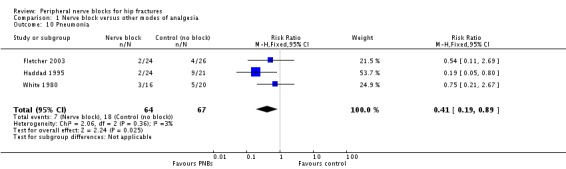
Comparison 1 Nerve block versus other modes of analgesia, Outcome 10 Pneumonia.
Quality of evidence for pneumonia
We downgraded the evidence by one level for risk of bias. Statistical heterogeneity was less than 25% (I2 = 3%). We downgraded evidence by one level for clinical heterogeneity owing to the excessive rate of complications observed in Haddad 1995. We used direct comparisons only with studies performed on the population of interest, and this is not a surrogate marker. The optimal information size was not achieved. We found no evidence of publication bias. We upgraded the level of evidence by one owing to a large effect size (RR 0.41). We upgraded on the basis of confounding factors for technology because no study used ultrasound guidance or a nerve stimulator. We did not upgrade for a dose‐response effect. We rated the quality of evidence as moderate.
2. Mortality
Based on seven trials (Cuvillon 2007; De La Tabla 2010; Fletcher 2003; Haddad 1995; Hood 1991; Jones 1985; White 1980) including 316 participants, we did not find a difference in short‐term (within six months) mortality (RR 0.72, 95% CI 0.34 to 1.52; I2 = 0%; Analysis 1.11). Egger's regression intercept showed no significant evidence of a small‐study effect. Duval and Tweedie's trim and fill analysis showed no evidence of publication bias. Given an incidence of 9.8%, 3228 participants (1614 per group) would have been required to eliminate a 25% reduction (alpha 0.05; beta 0.2; one‐sided test).
1.11. Analysis.
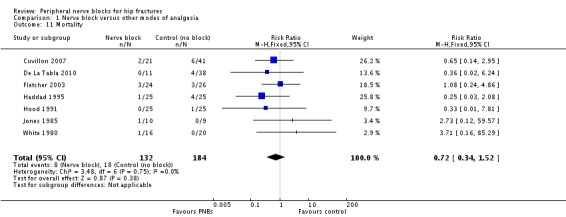
Comparison 1 Nerve block versus other modes of analgesia, Outcome 11 Mortality.
Quality of evidence for mortality within six months
We did not downgrade for risk of bias and we noted no heterogeneity. We used direct comparisons only with studies performed on the population of interest, and this is not a surrogate marker. We downgraded the level of evidence by two for imprecision because the confidence interval included both absence of effect and important benefit. We found no evidence of publication bias nor of large effect or dose‐response effect, and no confounding factors justified upgrading an absence of effect. We rated the quality of evidence as low.
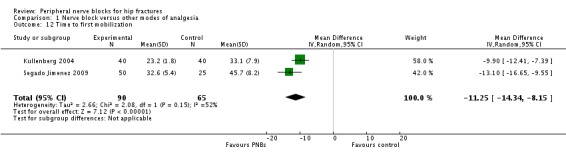
3. Time to first mobilization
Findings of two trials (Kullenberg 2004; Segado Jimenez 2009) with 155 participants show that peripheral nerve blocks reduced time to first mobilization (MD ‐11.25 hours, 95% CI ‐14.34 to ‐8.15 hours; I2 = 52%; Analysis 1.12). On the basis of the findings of Kullenberg 2004 (mean and SD 33.1 and 7.9 hours, respectively), 30 participants (15 per group) would be required to eliminate a 25% difference (alpha 0.05; beta 0.2; two‐sided test) in a simple trial.
1.12. Analysis.
Comparison 1 Nerve block versus other modes of analgesia, Outcome 12 Time to first mobilization.
Quality of evidence for time to first mobilization
We downgraded the level of evidence by one for risk of bias because we rated one study as having unclear risk for allocation concealment and the other as having unclear risk for blinding of outcome assessors. We downgraded quality of evidence by one level for a moderate amount of heterogeneity. We used direct comparisons only with studies performed on the population of interest, and this is not a surrogate marker. The optimal information size was achieved, but we downgraded evidence by one level for imprecision owing to a wide confidence interval around the effect size. We could not assess publication bias. We upgraded the level of evidence on the basis of a large effect size (equivalent to a SMD of ‐1.87). We also upgraded the level of evidence by one on the basis of confounding factors. No study used ultrasound guidance, an approach that could have increased block success (Lewis 2015). We found no evidence of a dose‐response effect. We rated the quality of evidence as moderate.
4. Costs of analgesic regimens
Results of two trials (Cuvillon 2007; Segado Jimenez 2009) with 137 participants show that costs related to analgesia were reduced when regional blockade was used as a single shot block (SMD ‐3.48, 95% CI ‐4.23 to ‐2.74) but were higher when regional blockade was used as a continuous infusion (SMD 0.93, 95% CI 0.37 to 1.48; I2 for heterogeneity between subgroups = 99%).
Quality of evidence for cost of analgesic regimens
We rated the quality of evidence for single shot blocks only. We downgraded the level of evidence by one for risk of bias because we rated the included study as having unclear risk for allocation concealment. The comparison was a direct one. We downgraded the evidence by one level for the small number of trials included. We could not assess publication bias. We upgraded the level of evidence on the basis of a large effect size (SMD > 0.8). We found no confounding factors that would justify upgrading or dose‐response effect. We rated the quality of the evidence as moderate.
5. Pressure sores
Based on three trials (Cuvillon 2007; Haddad 1995; Kullenberg 2004) including 187 participants, we did not find a difference in the incidence of pressure sores (RR 0.47, 95% CI 0.09 to 2.53; I2 = 39.5%; Analysis 1.14). Given an incidence of 6%, 5466 participants (2733 per group) would have been required to eliminate a 25% difference (alpha 0.05; beta 0.2; one‐sided test) in a large trial.
1.14. Analysis.
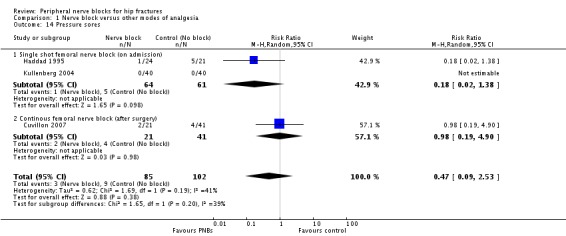
Comparison 1 Nerve block versus other modes of analgesia, Outcome 14 Pressure sores.
6. Number of participants transfused
One trial (Cuvillon 2007) including 62 participants gave results for the number of participants transfused (RR 0.78, 95% CI 0.28 to 2.20). Given an incidence of 22%, 1270 participants (635 per group) would have been required to eliminate a 25% difference (alpha 0.05; beta 0.2; one‐sided test) in a large trial.
7. Opioid consumption
Results from seven trials (Beaudoin 2013; Diakomi 2014; Foss 2007; Kullenberg 2004; Luger 2012; Spansberg 1996; Yun 2009) show that peripheral nerve blocks reduced opioid consumption up to 24 hours after surgery (SMD ‐0.70, 95% CI ‐0.96 to ‐0.44; I2 = 0%; Analysis 1.15). Egger's regression intercept showed no significant evidence of a small‐study effect. Duval and Tweedie's trim and fill analysis calculated that one trial might be missing to left of mean, for an adjusted point of estimate of ‐0.73 (95% CI ‐0.97 to ‐0.49; random‐effects model). We found a reduction in opioid consumption for both single shot and continuous blocks and noted no heterogeneity between subgroups: I2 = 0%.
1.15. Analysis.
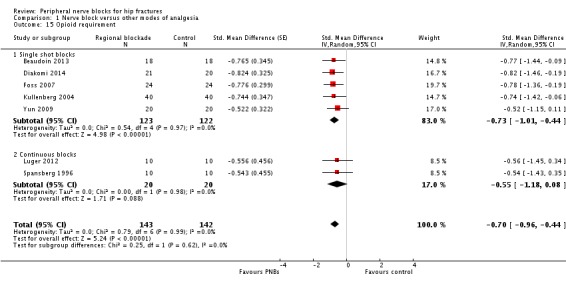
Comparison 1 Nerve block versus other modes of analgesia, Outcome 15 Opioid requirement.
8. Wound infection
One trial (Haddad 1995) including 45 participants gave results for wound infection (RR 1.13, 95% CI 0.24 to 7.12). Given an incidence of 11%, 2736 participants (1368 per group) would have been required to eliminate a 25% difference (alpha 0.05; beta 0.2; one‐sided test) in a large trial.
9. Participant satisfaction
Results of five trials (Domac 2015; Mossafa 2005; Segado Jimenez 2009; Szucs 2012; Tuncer 2003) with 237 participants show that participants were more satisfied with their mode of pain treatment when regional blockade was used (SMD 0.91, 95% CI 0.63 to 1.20; I2 = 0%). Egger's regression intercept showed no significant evidence of a small‐study effect. Duval and Tweedie's trim and fill analysis calculated that two trials might be missing to left of mean, for an adjusted point of estimate of 0.75 (95% CI 0.50 to 1.00; random‐effects model). On the basis of findings from Szucs 2012 (mean and SD of the control group 7.6 and 1.8), the difference would be equivalent to 1 on a scale from 1 to 10.
10. Complications
None of the 31 trials included in this review reported major complications related to regional blockade. A list of complications reported with both modes of pain treatment can be found in Table 4.
3. Complications of blocks and/or analgesic technique.
| Study | Complications related to regional anaesthesia | Complications related to analgesic technique |
| Altermatt 2013 | Not reported | Not reported |
| Antonopoulou 2006 | No complications such as motor block. local
haematoma or infection, inadvertent arterial puncture, direct nerve
damage and cardiovascular or neurological toxicity were observed Five participants had accidental removal or the catheter: 4 during the procedure or while the catheter was secured and 1 while in the ward |
Not reported |
| Beaudoin 2013 | No other adverse events were noted during the study period, and no other adverse events were reported to study investigators | Four‐hour oxygen saturation (%) 96 (93–99) vs (%) 98 (95–99) for regional blockade Adverse events: Hypotension, number (%) 3 (17) vs number (%) 0 (0) for regional blockade Respiratory depression, number (%) 9 (50) vs number (%) 4 (22) for regional blockade Nausea/vomiting, number (%) 5 (28) vs number (%) 5 (28) for regional blockade One participant had an episode of rapid atrial fibrillation requiring diltiazem, but the participant had a history of chronic atrial fibrillation |
| Chudinov 1999 | No major complications were described in group regional blockade. Three participants developed local erythema at the catheter insertion site at the end of the study period No signs of local anaesthetic toxicity were documented One participant developed bilateral blockade (L1‐L3 on the opposite side) |
Not reported |
| Coad 1991 | No complications related to nerve blocks and no case of prolonged motor blockade | Not reported |
| Cuvillon 2007 | Four catheters were prematurely removed: 1 by a confused participant, 2 by nurses (unexplained fever) and 1 by a surgeon (unconfirmed suspicion of local anaesthetic toxicity (ropivacaine blood level < 2 ng/mL)) | More constipation (47% vs 19% for regional blockade) |
| De La Tabla 2010 | Not reported | Not reported |
| Diakomi 2014 | Complications such as local anaesthetic toxicity recorded as well (none reported in results section) Nor did complication rates vary between groups |
Complications such as hypoventilation (breathing rate < 8 breaths/min) were recorded as well Moreover, the 2 groups did not differ in these parameters at any time point until study completion at 24 hours after surgery. Nor did complication rates vary between groups |
| Domac 2015 | Not reported | Not reported |
| Fletcher 2003 | Among study participants, none experienced adverse effects as a result of nerve block administration | No clinically important differences between groups with respect to pulse rate, oxygen saturation or respiratory rate at any time interval. Oxygen saturation 94.87% |
| Foss 2007 | No side effects attributable to femoral nerve block were noted in any participants during their hospital stay | More participants (P = 0.05) were sedated in the morphine group at 180 minutes after block placement No difference was noted between groups in nausea and vomiting, with 3 participants in each group having these side effects Tendency toward lower saturation was noted in the opioid group at 60 and 180 minutes after the block despite oxygen supplementation (P = 0.08) |
| Gille 2006 | One inadvertent arterial puncture and blood aspiration positive for 3 participants Two transient paraesthesias No catheter site infection Ten catheters accidentally removed |
No respiratory depression from systemic analgesia and no allergic reactions All complications were reversible |
| Godoy 2010 | The only complications were local bruises at the site of injection | Two participants with nausea, and 2 with nausea and vomiting |
| Graham 2008 | No immediate complications occurred in either group | No immediate complications were noted in either group |
| Haddad 1995 | No local or systemic complications of femoral nerve blocks were noted | Not reported |
| Hood 1991 | No untoward sequelae were associated with nerve blocks All plasma prilocaine concentrations (maximum 3 pg/mL) were below the suggested threshold for toxicity for prilocaine of 6 pg/mL |
Not reported |
| Iamaroon 2010 | No adverse systemic toxicity of bupivacaine, such as seizure, arrhythmia or cardiovascular collapse was noted in the femoral nerve block group Neither vascular puncture nor paraesthesia occurred No complications, such as haematoma, infection or persistent paraesthesia, were observed within 24 hours after the operation |
No participant in either group had hypoventilation (ventilatory rate < 10/min) or oxygen saturation < 95% |
| Jadon 2014 | Not reported | In participants of fentanyl group, drowsiness was observed that required the presence of more persons for holding the participant during positioning SpO2 was significantly lower in the fentanyl group (P = 0.001). However, no participant in either group had SpO2 < 90% during the procedure Mean arterial blood pressure was significantly lower in the fentanyl group (P = 0.0019) |
| Jones 1985 | No untoward sequelae associated with the nerve block were seen | Not reported |
| Kullenberg 2004 | No complications related to the nerve blockade were noted in this study | Not reported |
| Luger 2012 | Not reported | Not reported |
| Mossafa 2005 | Not reported | Not reported |
| Mouzopoulos 2009 | No complications of femoral nerve block administrations occurred, except 3 local haematomas developed at the injection site, which resolved spontaneously | Not reported |
| Murgue 2006 | Not reported | Not reported |
| Nie 2015 | No adverse effects, such as pain at the insertion site or paraesthesia, were observed No positive cultures were observed with the fascia iliaca block catheter tip, nor were any signs of infection noted in the current study |
Not reported |
| Segado Jimenez 2009 | We did not observe any complications in the realization of regional anaesthetic techniques during or subsequent to the regional anaesthetic techniques | The incidence of side effects (sleepiness, hypotension, constipation, pruritus) was greater in the group with no block than in groups with blocks (P < 0.01) |
| Spansberg 1996 | No haematomas at the site of femoral catheters | Two participants in each group experienced nausea and vomiting |
| Szucs 2012 | For 1 participant, the elastomeric pump failed, resulting in local anaesthetic administered over less than 54 hours instead of 72 hours, and another participant, suffering from acute confusional state, disconnected his pump after 12 hours | The incidence of nausea/vomiting, pruritus or excessive sedation was similar in the 2 groups |
| Tuncer 2003 | Not reported | Side effects (vomiting and pruritus) were observed significantly more frequently with intravenous analgesia |
| White 1980 | No participants showed any evidence of local anaesthetic toxicity | Not reported |
| Yun 2009 | No adverse systemic toxicity of ropivacaine was noted, and neither vascular puncture nor paraesthesia was elicited No complications, such as haematoma or persistent paraesthesia, were observed in participants with a femoral nerve block within 24 hours after the operation |
Hypoventilation (ventilatory rate 6–8/min) or pulse oximetric desaturation (oxygen saturation 88% or 89%) was encountered in 4 participants (20%) in the intravenous analgesia group. This was reverted with assisted manual mask ventilation All participants in the intravenous group experienced mild dizziness, and mild drowsiness was present in 12/20 of them |
%: percentage
L: litre
mg: milligram
min: minute
ng/mL: nanogram/millilitre
pg/mL: picogram/millilitre
Discussion
We found some advantages of peripheral nerve blocks versus systemic analgesia for pain treatment among people with hip fractures. Even at rest, pain after hip fracture is relatively high, particularly among those with subtrochanteric fractures (median 5 out of 10) (Foss 2007). Movement in these individuals immediately after injury is unavoidable: transport from the scene of injury to the hospital, unclothing for medical examination, transport for x‐ray diagnostic confirmation, transfer on the operating room table, positioning for spinal anaesthesia, etc. Movement‐associated median pain ranges from 8 to 10 out of 10, depending on the type of fracture (intracapsular = 8; trochanteric = 9; subtrochanteric = 10) (Foss 2007). As many of these patients are elderly (30% over 85 years of age; Brauer 2009), doses of systemic opioids administered are often limited by the fear of inducing serious adverse events such as respiratory depression in a patient with a full stomach. Compared with systemic analgesia, pain on movement within 30 minutes after block placement will be less by approximately 3.4 out of 10 (Analysis 1.1; Table 1; high quality of evidence). Single shot blocks have been successfully performed by emergency physicians (Beaudoin 2013) or even by trained paramedics at the scene of injury (McRae 2015) without excessive rate of serious complications. Although continuous blocks require greater expertise and are more expensive (Analysis 1.13) than single shot blocks, they may be more suitable than single shot blocks for use in the emergency department and thereafter. Owing to a possibly increased rate of morbidity induced by a longer delay between injury and surgery, authorities usually recommend proceeding to surgical repair of hip fractures as soon as is feasible. However, the delay between hospital arrival and surgery (from 24 to 240 hours in the present review) often exceeds the duration of a single shot block (median between 12 and 22 hours, depending on the drug(s) used; Cuvillon 2009), thus leading to the need for a repeated block (Kullenberg 2004) or reversion to systemic analgesia. Continuous nerve blocks inserted at the emergency department would offer the advantage of covering both preoperative and postoperative analgesia. The exact type of regional block chosen may reflect the preference/training of the practitioner's personnel. However, some study authors have found it particularly difficult to insert epidural analgesia in the emergency department (Luger 2012). Compared with epidural analgesia or psoas compartment blocks, femoral nerve or fascia iliaca blocks may offer several advantages. First, they can be performed with the patient lying in the dorsal decubitus position, and second, as they usually are considered superficial/compressible sites, patients are able to receive any mode of thromboprophylaxis deemed required by their practitioner to suit their medical and surgical condition. When available, ultrasound guidance may be advantageous in terms of decreasing the onset time of the block effect (Marhofer 1997) and increasing the success rate (more blocks have been assessed as sufficient for surgery following sensory or motor testing, and fewer blocks required supplementation or conversion to general anaesthetic, Lewis 2015). The concentration of local anaesthetic used for catheter loading or for performance of a single shot block at the site of injury or in the emergency department should be relatively high. At this phase, a motor block probably poses no clear disadvantage provided that adequate traction/immobilization is ensured, and the effect on pain on movement will be proportionate to the concentration of local anaesthetic used (Figure 4). Owing to the high incidence of acute confusional state seen in these patients, appropriate fixation of these catheters is crucial (Cuvillon 2007), and all connections between the pump and the catheter must be secured (Szucs 2012). If a femoral nerve or a fascia iliaca block is chosen, additional regional blockade will be required for surgery (Chudinov 1999; Johnston 2016). For postoperative analgesia, the difference between peripheral nerve blocks and systemic analgesia was less consistent and may be influenced by the surgical technique (fixation vs arthroplasty; Analysis 1.4) and/or type of block used (Analysis 1.7).
1.13. Analysis.
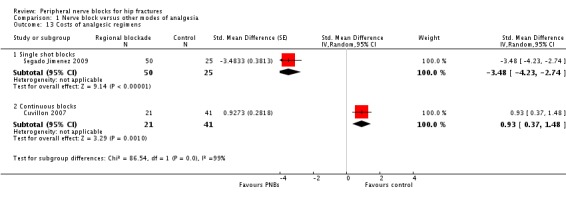
Comparison 1 Nerve block versus other modes of analgesia, Outcome 13 Costs of analgesic regimens.
Use of regional blockade for postoperative analgesia reduces time to first mobilization (Analysis 1.12), hence reducing some complications that may occur secondary to immobilization, such as pneumonia (Analysis 1.10; Table 1; moderate quality of evidence). Use of regional blockade for postoperative analgesia may reduce the risk of pneumonia by half (number needed to treat for an additional beneficial outcome (NNTB) 7, 95% confidence interval (CI) 5 to 72). Our review includes data for time to first mobilization with single shot blocks only. Concerns about risks of inpatient falls have been raised when continuous lower limb peripheral nerve blocks are used for postoperative analgesia. Detailed analysis, however, has revealed that attributable risk for patients who had a continuous peripheral nerve block was not outside the expected probability of postoperative falls after orthopaedic surgery (Johnson 2013). One large retrospective trial found that risk for inpatient falls was higher among older patients, those with a higher comorbidity burden and those with more major complications, but that use of peripheral nerve blocks was not significantly associated with inpatient falls (Memtsoudis 2014). Inpatient falls occur mainly while patients are within their own rooms (while in the bathroom, while going to and from the bathroom or while using a bedside commode) (Johnson 2014). Therefore, with or without peripheral nerve block, fall prevention strategies should continue to include education for all patients (especially elderly patients) and should reinforce practices that monitor patients within their hospital rooms (Johnson 2014).
Acute confusional state is common after hip fracture and may delay rehabilitation and increase hospital length of stay and may impede nursing home placement and even mortality (Pompei 1994). We could not demonstrate a decreased risk of acute confusional state with the use of peripheral nerve blocks, but the number of participants included in the present meta‐analysis is insufficient to eliminate a clinically relevant risk reduction (risk ratio (RR) 0.69, 95% CI 0.38 to 1.27; very low quality of evidence; Analysis 1.9; Table 1). The pathophysiology of acute confusional state in these patients may be multi‐factorial and may include side effects of medications used, hypoxaemia, immobilization, infection and systemic inflammation (Mouzopoulos 2009). Peripheral nerve blocks (or local anaesthetics) may have an influence on any of these factors. Also, peripheral nerve blocks are associated with a clear reduction in opioid consumption (standardized mean difference (SMD) ‐0.70, 95% CI ‐0.96 to ‐0.44; I2 = 0%; Analysis 1.15).
We could not demonstrate a reduction in the incidence of myocardial ischaemia (Table 1; very low quality of evidence), but the number of participants included in our review was clearly insufficient to permit definitive conclusions on this. Likewise, we did not find a reduction in short‐term (up to six months) mortality rate (Analysis 1.11; Table 1; low quality of evidence), but here again, included participants were too few to allow definitive conclusions on this.
Participant satisfaction was also higher when peripheral nerve blocks were used as a modality of pain treatment (SMD 0.91, 95% CI 0.62 to 1.20; I2 = 0%; Analysis 1.16; equivalent to a difference of 1.0 on a scale from 1 to 10).
1.16. Analysis.
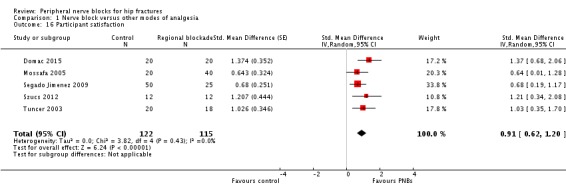
Comparison 1 Nerve block versus other modes of analgesia, Outcome 16 Participant satisfaction.
No trials reported major complications. This is consistent with information derived from large prospective studies indicating that the incidence of nerve injury lasting longer than six months associated with femoral nerve blocks would be relatively low: 0 to 1.2 per 1000 procedures (Auroy 2002; Brull 2007; Sites 2012).
Summary of main results
High‐quality evidence shows that peripheral nerve blocks reduce pain on movement within 30 minutes after block placement. Moderate‐quality evidence shows that peripheral nerve blocks reduce pneumonia, time to first mobilization and cost of analgesic drugs (single shot blocks only).
Overall completeness and applicability of evidence
We are confident that our results reflect the actual available literature. More data are required to evaluate the effects of peripheral nerve blocks on acute confusional state, myocardial ischaemia and death.
Quality of the evidence
We have summarized the quality of evidence in Table 1.
Potential biases in the review process
Our search was extensive. We chose factors for exploration of heterogeneity a priori.
Agreements and disagreements with other studies or reviews
In our previous version of this review (Other published versions of this review), we found that regional blockade reduces pain and opioid consumption, but owing to the limited number of studies available, we did not find a difference between regional blockade and other modes of analgesia in terms of major outcomes. In the present version, we confirm that peripheral nerve blocks reduce pain on movement within 30 minutes after block placement (high quality of evidence) and opioid consumption. We also found a reduction in pneumonia and in time to first mobilization (moderate quality of evidence). We need more data before we can determine if regional blockade influences acute confusional state, myocardial ischaemia and death rates.
Authors' conclusions
Implications for practice.
High‐quality evidence shows that peripheral nerve blocks reduce pain on movement within 30 minutes after block placement, and moderate‐quality evidence shows that they reduce risk of pneumonia, time to first mobilization and cost (single‐injection blocks only). Included trials often excluded patients with dementia (Characteristics of included studies). These patients may be uncooperative and less suitable for awake regional anaesthetic techniques. The American Society of Regional Anesthesia suggests that regional anaesthetic techniques should not be performed routinely in adult patients whose sensorium is compromised by general anaesthesia or deep sedation, but that adult patients with specific conditions (e.g. developmental delay) may be appropriate exceptions to this recommendation after risk versus benefit is considered (Neal 2015).
Implications for research.
The optimal information size was not reached for acute confusional state, myocardial ischaemia and short‐term death (within six months). Therefore, more data are required for these important outcomes.
What's new
| Date | Event | Description |
|---|---|---|
| 16 August 2016 | New search has been performed | We reran the search in August 2016 |
| 16 August 2016 | New citation required and conclusions have changed | Two new authors joined the review We updated the search in June 2015 We updated the review and brought the methods up‐to‐date We found 55 new studies: 20 included, 13 excluded and 22 ongoing. We left no studies awaiting classification |
History
Protocol first published: Issue 3, 1998 Review first published: Issue 2, 1999
| Date | Event | Description |
|---|---|---|
| 6 May 2015 | New search has been performed | This review has been transferred to the Anaesthesia, Critical and Emergency Care Group by the Bone, Joint and Muscle Group |
| 17 February 2009 | New search has been performed | For the second substantive update (Issue 2, 2009), we made the following changes. 1. We included the following newly identified studies: Cuvillon 2007, Fletcher 2003, Foss 2005, Foss 2007, Gille 2006, Kullenberg 2004, Matot 2003, Murgue 2006 and Tuncer 2003. 2. We excluded the following studies: Gorodetskyi 2007, Mannion 2005, Marhofer 1998, Mutty 2007, Schiferer 2007, Turker 2003 and Piangatelli 2004. We made no changes to the conclusions of the review |
| 6 November 2008 | Amended | We converted the review to new review format |
| 21 November 2001 | New citation required and conclusions have changed | In this substantive update (Issue 1, 2002), we included one newly identified study (Scheinin 2000). We made no changes to the conclusions of the review For details on all updates, please see 'Notes' |
Notes
For the first update (Issue 1, 2001), we made the following changes.
Included study of Chudinov 1999 on psoas compartment blocks.
Changed methods score to include item 8.
Changed statistical analysis to relative risks.
Added a synopsis.
In the second update (Parker 2002), we excluded one newly identified study (Van Leeuwen 2000) and included another (Scheinin 2000a). We have not made changes to the conclusions of the review.
We also updated this review in 2009. At that time, Cochrane updates did not earn a new citation unless they included new review authors or made a change to review conclusions.
For the 2016 update, we made the following changes.
Transferred this review to the Anaesthesia, Critical and Emergency Care Group from the Bone, Joint and Muscle Group.
Included two new review authors.
Updated the search in August 2016.
Updated the review and brought the methods up‐to‐date.
Excluded from the review studies evaluating neuraxial blocks (epidural/spinal) and wound infiltration as techniques of regional blockade.
Acknowledgements
The review authors thank Karen Hovhannisyan, who designed the search strategy for this update, as well as the University of Sherbrooke and the University of Quebec in Abitibi‐Temiscamingue for granting access to electronic databases and to medical journals.
We thank Harry Scheinin (Scheinin 2000), Fernando Altermatt (Altermatt 2013), Raquel Ortiz de la Tabla González (De La Tabla 2010), Jochen Gille (Gille 2006), K. Sanem Cakar Turhan (Bölükbasi 2013), Ashok Jadon (Jadon 2014) and Hongling Nie (Nie 2015), who provided additional information for the 2015 update, Karl Sales for traduction of two articles (Segado Jimenez 2009; Segado Jimenez 2010); and Gideon Heinert for translation of Gille 2006.
We would also like to thank Stephan Kettner (Content Editor); Jing Xie (Statistical Editor); Fernanda Fukushima, Pekka Tarkkila, Maya Keplinger, Thomas Fichtner Bendtsen and Thomas Dahl Nielsen (Peer Reviewers); and Patricia Tong (Consumer Referee) for help and editorial advice provided during preparation of this systematic review.
Appendices
Appendix 1. Search strategies
MEDLINE (OVID Web)
1. exp Hip Fractures/ 2. ((hip$ or femur$ or femoral$ or trochant$ or pertrochant$ or intertrochant$ or subtrochant$ or intracapsular$ or extracapsular$) adj4 fracture$).tw. 3. or/1‐2 4. exp Anesthesia/ 5. ((an?esthet$ or an?esthesia) adj4 (regional$ or local$ or general or spinal or epidural)).tw. 6. nerve block.tw. 7. or/4‐6 8. and/3,7
Embase (OVID Web)
1. exp Hip Fracture/ 2. ((hip$ or femur$ or femoral$ or trochant$ or pertrochant$ or subtrochant$ or intracapsular$ or extracapsular$) adj4 fracture$).tw. 3. or/1‐2 4. exp Anesthesia/ 5. exp Analgesia/ 6. Nerve block/ 7. ((an?esthet$ or an?esthesia) adj4 (regional$ or local$ or general or spinal or epidural)).tw. 8. block.tw. 9. or/4‐8 10. and/3,9 11. exp Randomized Controlled trial/ 12. exp Double Blind Procedure/ 13. exp Single Blind Procedure/ 14. exp Crossover Procedure/ 15. Controlled Study/ 16. or/11‐15 17. ((clinical or controlled or comparative or placebo or prospective$ or randomi#ed) adj3 (trial or study)).tw. 18. (random$ adj7 (allocat$ or allot$ or assign$ or basis$ or divid$ or order$)).tw. 19. ((singl$ or doubl$ or trebl$ or tripl$) adj7 (blind$ or mask$)).tw. 20. (cross?over$ or (cross adj1 over$)).tw. 21. ((allocat$ or allot$ or assign$ or divid$) adj3 (condition$ or experiment$ or intervention$ or treatment$ or therap$ or control$ or group$)).tw. 22. or/17‐21 23. or/16,22 24. limit 23 to human 25. 10 and 24
The Cochrane Library (Wiley InterScience)
#1 MeSH descriptor Hip Fractures explode all trees #2 ((hip* or femur* or femoral* or trochant* or pertrochant* or subtrochant* or intracapsular* or extracapsular*) NEAR/4 fracture*):ti,ab,kw #3 (#1 OR #2) #4 MeSH descriptor Anesthesia explode all trees #5 MeSH descriptor Analgesia explode all trees #6 MeSH descriptor Nerve Block explode all trees #7 ((anesthet* or anaesthet* or anesthesia or anaesthesia) NEAR/4 (regional* or local* or general or spinal or epidural)):ti,ab,kw #8 (block):ti,ab,kw #9 (#4 OR #5 OR #6 OR #7 OR #8) #10 (#3 AND #9)
CINAHL (EBSCO host)
S1 (MH "Hip Fractures+") OR TX (((hip$ or femur$ or femoral$ or trochant$ or pertrochant$ or subtrochant$ or intracapsular$ or extracapsular$) N4 fracture$))
S2 ((MH "Anesthesia+") OR (MH "Analgesia+") OR (MH "Nerve Block+") ) OR TX (((an?esthet$ or an?esthesia) N4 (regional$ or local$ or general or spinal or epidural)))
S3 S1 and S2
Data and analyses
Comparison 1. Nerve block versus other modes of analgesia.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain on movement within 30 minutes of block placement | 8 | 373 | Std. Mean Difference (Random, 95% CI) | ‐1.41 [‐2.14, ‐0.67] |
| 2 Pain at rest within 30 minutes after block placement | 7 | 322 | Std. Mean Difference (Random, 95% CI) | ‐0.80 [‐1.25, ‐0.35] |
| 3 Pain at rest at 6 to 8 hours after surgery | 5 | 286 | Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.70, ‐0.06] |
| 4 Pain on movement at 24 hours after surgery | 4 | 195 | Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐1.08, 0.30] |
| 4.1 Surgical technique unspecified | 3 | 169 | Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.10, 0.29] |
| 4.2 Arthroplasty for 38.4% of participants | 1 | 26 | Mean Difference (IV, Random, 95% CI) | ‐1.94 [‐2.75, ‐1.13] |
| 5 Pain at rest at 24 hours after surgery | 8 | 435 | Mean Difference (IV, Random, 95% CI) | ‐0.68 [‐1.23, ‐0.13] |
| 5.1 Single shot blocks | 2 | 80 | Mean Difference (IV, Random, 95% CI) | ‐0.5 [‐0.90, ‐0.10] |
| 5.2 Continuous blocks | 6 | 355 | Mean Difference (IV, Random, 95% CI) | ‐0.78 [‐1.58, 0.03] |
| 6 Pain on movement at 48 hours | 2 | 129 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [‐0.23, 0.40] |
| 7 Pain at rest at 48 hours after surgery | 5 | 335 | Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.87, 0.13] |
| 7.1 Psoas compartment block | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐1.10 [‐2.26, 0.06] |
| 7.2 Femoral nerve block | 3 | 191 | Mean Difference (IV, Random, 95% CI) | 0.06 [‐0.16, 0.28] |
| 7.3 Fascia iliaca block | 1 | 104 | Mean Difference (IV, Random, 95% CI) | ‐0.92 [‐1.47, ‐0.36] |
| 8 Pain at rest at 72 hours after surgery | 2 | 140 | Mean Difference (IV, Random, 95% CI) | ‐0.48 [‐1.83, 0.87] |
| 8.1 Psoas compartment block | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐1.20 [‐1.77, ‐0.63] |
| 8.2 Femoral nerve block | 1 | 100 | Mean Difference (IV, Random, 95% CI) | 0.18 [0.03, 0.33] |
| 9 Acute confusional state | 7 | 676 | Risk Ratio (M‐H, Random, 95% CI) | 0.69 [0.38, 1.27] |
| 9.1 Peripheral nerve block based on landmarks | 4 | 501 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.23, 2.93] |
| 9.2 Peripheral nerve block based on nerve stimulator | 3 | 175 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.32, 0.98] |
| 10 Pneumonia | 3 | 131 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.41 [0.19, 0.89] |
| 11 Mortality | 7 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.34, 1.52] |
| 12 Time to first mobilization | 2 | 155 | Mean Difference (IV, Random, 95% CI) | ‐11.25 [‐14.34, ‐8.15] |
| 13 Costs of analgesic regimens | 2 | Std. Mean Difference (Random, 95% CI) | Subtotals only | |
| 13.1 Single shot blocks | 1 | 75 | Std. Mean Difference (Random, 95% CI) | ‐3.48 [‐4.23, ‐2.74] |
| 13.2 Continuous blocks | 1 | 62 | Std. Mean Difference (Random, 95% CI) | 0.93 [0.37, 1.48] |
| 14 Pressure sores | 3 | 187 | Risk Ratio (M‐H, Random, 95% CI) | 0.47 [0.09, 2.53] |
| 14.1 Single shot femoral nerve block (on admission) | 2 | 125 | Risk Ratio (M‐H, Random, 95% CI) | 0.18 [0.02, 1.38] |
| 14.2 Continous femoral nerve block (after surgery) | 1 | 62 | Risk Ratio (M‐H, Random, 95% CI) | 0.98 [0.19, 4.90] |
| 15 Opioid requirement | 7 | 285 | Std. Mean Difference (Random, 95% CI) | ‐0.70 [‐0.96, ‐0.44] |
| 15.1 Single shot blocks | 5 | 245 | Std. Mean Difference (Random, 95% CI) | ‐0.73 [‐1.01, ‐0.44] |
| 15.2 Continuous blocks | 2 | 40 | Std. Mean Difference (Random, 95% CI) | ‐0.55 [‐1.18, 0.08] |
| 16 Participant satisfaction | 5 | 237 | Std. Mean Difference (Random, 95% CI) | 0.91 [0.62, 1.20] |
1.8. Analysis.
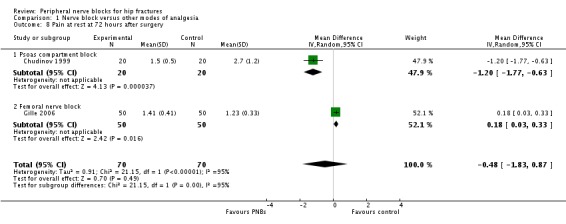
Comparison 1 Nerve block versus other modes of analgesia, Outcome 8 Pain at rest at 72 hours after surgery.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Altermatt 2013.
| Methods | RCT Approved by the ethics committee and informed consents obtained Setting: Chile Funding: unspecified NCT01961895 |
|
| Participants | 31 older than 60 years, ASA II‐III with risk factors for known coronary artery disease (≥ 2 risk factors for coronary heart disease as defined by Wallace 1987) and hip fracture within 48 hours of fracture Exclusion criteria: receiving orthopaedic treatment, coagulopathy (clinic or laboratory), sepsis or infection of the catheter insertion site of the lumbar plexus, neurological diseases evolving Also, disoriented, dementia, chronic renal failure stage IV National Kidney Foundation, glomerular filtration rate between 15 and 29 mL/min/1.73 m2, unable to assess pain, non‐sinus rhythm or conduction abnormalities (right bundle branch block or left atrioventricular block) on admission EKG, with pacemaker, acute coronary syndrome or decompensated cardiovascular disease at entry, allergy to any drugs of the protocol and inability to understand or sign informed consent unaided |
|
| Interventions |
Treatment group: continuous lumbar plexus started preoperatively and continued for 72 hours after surgery (n = 17) Control group: IV PCA with morphine (n = 14) |
|
| Outcomes | Ischaemic events per participant (extracted as P value): continuous EKG monitoring and serial cardiac enzymes | |
| Notes | Conference abstract. Email sent on 25 May 2015. Study authors responded that the manuscript had not been submitted for publication yet and confirmed the registration number. They noted no major cardiac events during the entire period of observation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomized", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double=blind (participant, caregiver, investigator) |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Double‐blind (participant, caregiver, investigator) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Unclear risk | No details on participants enrolled |
Antonopoulou 2006.
| Methods | RCT Setting: Greece Funding; unspecified |
|
| Participants | 84 participants (63 female and 21 male) with hip fracture | |
| Interventions |
Treatment group: continuous femoral nerve block with 0.125% levobupivacaine at 3‐4 mL/h, started after surgery (n = 49) Control group: IM pethidine (n = 35) Spinal anaesthesia and paracetamol after surgery for all participants |
|
| Outcomes | Pain scores during 24 hours (not included in analysis) | |
| Notes | No complications such as motor blockade, local haematoma or infection, inadvertent arterial puncture, direct nerve damage and cardiovascular or neurological toxicity Conference abstract Email sent on 25 May 2015: no reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "randomized", no details. Unequal groups: 48 and 35 |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None lost to follow‐up No failed block Five accidental catheter dislodgements ‐ 4 during procedure of securing the catheter, 1 on the ward |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Unclear risk | No details for each group separately |
Beaudoin 2013.
| Methods | RCT Approved by the ethics committee and written informed consents obtained Setting: United States of America Funding: charity Registered at ClinicalTrials.gov (Identifier NCT01701414) |
|
| Participants | 36 participants Eligible: aged ≥ 55 years, radiographically proven femoral neck or intertrochanteric fracture, normal lower extremity neurovascular examination, able to consent and actively participate in the study, moderate to severe pain (numerical pain rating score 5) at time of enrolment Excluded: known international normalized ratio > 3.0, prior femoral artery vascular surgery on the same side as the fracture, other significant trauma, hypoxia (pulse oximetry < 92%), hypotension (systolic blood pressure < 100 mm Hg), known hypersensitivity to local anaesthetics or morphine |
|
| Interventions |
Treatment group: ultrasound‐guided femoral nerve block plus subcutaneous morphine (n = 18) Control group: sham‐injection (3 mL of saline under ultrasound probe over 5 minutes) plus subcutaneous morphine (n = 18) |
|
| Outcomes | Pain scores at 15 minutes after the block Opioids during 4 hours after the block |
|
| Notes | One participant in the SC group had an episode of rapid atrial fibrillation requiring diltiazem, but this participant had a history of chronic atrial fibrillation. No other adverse events (respiratory depression, hypotension, nausea or vomiting) were noted during the study period, and no other adverse events were reported to study investigators | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | After consent, participants were randomized by sequentially numbered cards in sealed envelopes Internet‐based programme with a 1:1 allocation ratio performed by the research department co‐ordinator, who was not involved in enrolment or data collection |
| Allocation concealment (selection bias) | Low risk | After consent, participants were randomized by sequentially numbered cards in sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Blinded" with sham injection |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Blinded" with sham injection |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant withdrawn from each group (38 randomized and 36 analysed) Two patients enrolled (1 in each arm) dropped out after randomization but before the study procedure. No reason provided |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Low risk | Groups well balanced |
Chudinov 1999.
| Methods | RCT Approved by the ethics committee Setting: Israel Funding: unspecified |
|
| Participants | 40 participants (30 female and 10 male) with a hip fracture undergoing surgery Excluded: patients with severe cardiac, pulmonary, renal or liver dysfunction; systemic infection; decubitus ulcer; dementia; aspirin or anticoagulant treatment; allergy to local anaesthetics Mean age: 80 years (range 67‐96) Percentage female: 75% Lost to follow‐up: none No details on surgical technique provided |
|
| Interventions |
Treatment group: psoas compartment block. Chayen's technique with loss of resistance to air (operated side up), using 2 mg/kg/body weight of 0.25% bupivacaine with adrenaline (0.8 mL/kg) and supplementary doses as required via a catheter inserted 2‐3 cm cephalad past the tip of the needle. Blocks were performed before surgery (16‐48 hours), within 6 hours of admission (n = 20). According to assessment, a sciatic nerve block (n = 5), general anaesthesia (n = 1) or spinal anaesthesia (n = 11) was added for surgery. Catheters were kept for 72 hours after surgery Control group: IM meperidine and diclofenac as required for pain relief (n = 20). Neuraxial block (spinal or epidural n = 19) or general anaesthesia (n =1) for surgery |
|
| Outcomes | Pain relief as assessed by VAS at 30 minutes after block placement and at 8, 24, 48 and 72 hours after surgery Complications of the block: 3 participants showed inflammation at the site of insertion; 1 had epidural spread. Local anaesthetic toxicity: none. No major complications associated with the block |
|
| Notes | Length of follow‐up: 72 hours after surgery | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized trial: method not stated |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No failed block. None lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Unclear risk | Groups well balanced. No details on surgical technique provided |
Coad 1991.
| Methods | RCT Approved by the ethics committee and consents obtained Setting: United Kingdom Funding: unspecified |
|
| Participants | 50 participants with a hip fracture undergoing surgery with a pin and plate or a sliding hip screw
Mean age: 77 years (range 64‐89)
Percentage female: 84%
Lost to follow‐up: none Excluded: receiving analgesic drugs, diagnosis of dementia, regional anaesthesia considered contraindicated |
|
| Interventions |
Treatment group 1: lateral cutaneous nerve of thigh block with 15 mL 0.5% bupivacaine; Eriksson's technique (n = 17) Treatment group 2: femoral (3‐in‐1) nerve block with 15 mL 0.5% bupivacaine; Winnie's technique (n = 17) Control group : IM meperidine (n = 16) All participants had general anaesthesia consisting of fentanyl, etomidate, vecuronium, nitrous oxide and enflurane Blocks performed at completion of the operation |
|
| Outcomes | No complications related to the blocks | |
| Notes | Length of follow‐up: 24 hours | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized trial: method not stated |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Unclear for participants; nurses administering supplemental analgesia were blinded to the treatment group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Nurses administering supplemental analgesia were blinded to treatment |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Low risk | Groups well balanced |
Cuvillon 2007.
| Methods | RCT Approved by the ethics committee and written informed consents obtained Setting: France Funding: charity |
|
| Participants | 62 participants with a hip fracture undergoing surgery.
Mean age: 82 years (range not stated).
Percentage female: 86%. Arthroplasty: 58% Lost to follow‐up: not stated Excluded: more than 72 between fracture and surgery, weight < 40 kg, ASA physical status > IV, neurological disease (alcoholic or diabetic), allergy or contraindication to regional anaesthesia, severe hepatic or renal dysfunction, Mini Mental score < 15/30 |
|
| Interventions |
Treatment group: continuous femoral nerve block. Nerve stimulator, 0.3 to 0.5 mA, catheter introduced 10 to 15 cm past needle tip and loaded with 30 mL of lidocaine 1.5% plus epinephrine. Infusion of 0.2% ropivacaine at 10 mL/h for 48 hours (n = 21) Control groups: intravenous propacetamol 2 G 6‐hourly (n = 21) or subcutaneous morphine 0.05 mg/kg 4‐hourly (n = 20) Propacetamol and morphine before surgery, spinal anaesthesia for surgery for all participants. Treatment group and propacetamol group participants could also receive morphine after surgery if needed |
|
| Outcomes | Pain scores at 8, 24 and 48 hours after surgery Number of participants who required additional opioids during first 48 hours after surgery Confusion/somnolence Transfused Pressure sores Mortality at 6 months Cost of analgesic regimens |
|
| Notes | Length of follow‐up: 6 months Study authors contacted 22 May 2015; no reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized trial: use of numbered envelopes |
| Allocation concealment (selection bias) | Low risk | Randomized trial: use of numbered envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None lost to follow‐up Failed blocked excluded, but no immediate failed block. Four catheter dislodgements; these participants were kept in the analysis (intention‐to‐treat) |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Unclear risk | Groups well balanced except for delay between admission and surgery (median 40 hours in femoral catheter group and 21 and 23,5 hours in the 2 control groups) |
De La Tabla 2010.
| Methods | RCT Setting: Spain Funding: unspecified |
|
| Participants | 49 participants older than 65 years with a neck fracture scheduled for surgical treatment | |
| Interventions |
Treatment group: double guidance (ultrasound and nerve stimulator) femoral nerve block with 15 mL of 0.2% ropivacaine followed by infusion of 0.2% ropivacaine at 5 mL/h plus 10 mL every 30 minutes (n = 11) Control group: intravenous metamizole 2 G every 6 hours (n = 38) Rescue analgesia with 100 mg tramadol and ondansetron 4 mg |
|
| Outcomes | Pain scores at rest and on movement at 24 and 48 hours after surgery Death at 6 months |
|
| Notes | Conference abstract Additional information on pain scores received from study authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | No details and very unequal groups |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow‐up mentioned |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Low risk | Groups well balanced |
Diakomi 2014.
| Methods | RCT Approved by the ethics committee and informed consents obtained Setting: Greece Funding: unspecified NCT02037633 |
|
| Participants | 41 ASA I‐III participants, aged 38 to 94 years, scheduled for hip fracture repair Excluded: contraindications for central nervous blockade, impaired cognition or dementia, multiple fractures, any previous analgesic administration in last 12 hours before surgery |
|
| Interventions |
Treatment group: fascia iliaca block, modified Dalen's technique with 40 mL ropivacaine 0.5% injected while caudal pressure maintained, then turned lateral (fracture side up) for spinal 20 minutes later (n = 21) Control group: IV fentanyl 1.5 mcg/kg, then turned lateral (fracture side up) for spinal 5 minutes later (n = 20) Spinal anaesthesia for surgery. IV PCA with morphine after surgery |
|
| Outcomes | Pain at rest and on movement at 20 minutes after the block (or 5 minutes after fentanyl administration; movement = positioning for spinal anaesthesia) Opioid requirement during first 24 hours after surgery |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly assigned, using a sealed envelope method" |
| Allocation concealment (selection bias) | Low risk | "Patients were randomly assigned, using a sealed envelope method" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Pain scores were assessed by a blind observer who entered the operating room only after the analgesic intervention (IV fentanyl administration or fascia iliaca block performance) had taken place. Landmarks were drawn on all participants, and gauze was applied to the “puncture” site for all participants. Each participant was aware of his/her group allocation because we considered a placebo injection in the inguinal area not acceptable. The observer who recorded participant satisfaction was unaware of group allocation and was not involved in any other step of the study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Pain scores were assessed by a blind observer who entered the operating room only after the analgesic intervention (IV fentanyl administration or fascia iliaca block performance) had taken place. Landmarks were drawn on all participants, and gauze was applied to the “puncture” site for all participants. Each participant was aware of his/her group allocation because we considered a placebo injection in the inguinal area not acceptable. The observer who recorded participant satisfaction was unaware of group allocation and was not involved in any other step of the study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None lost to follow‐up. One participant withdrew consent |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Low risk | Groups well balanced |
Domac 2015.
| Methods | RCT Approved by the ethics committee and informed consents obtained Setting: Turkey Funding: departmental |
|
| Participants | 40 ASA I‐III participants aged 65 to 80 years undergoing femoral fracture repair under spinal anaesthesia Excluded: patients < 65 years of age or > 80 years of age, with peripheral neurological disease, mental disorders, allergy to amide local anaesthetics, coagulation/haemostasis diseases, moderate or severe liver or kidney failure, contraindication to or refusing fascia iliaca block |
|
| Interventions |
Treatment group: fascia iliaca block with 15 mL of 0.5% bupivacaine and 15 mL of 2% lidocaine (n = 20) Control group: no block (n = 20) Spinal anaesthesia for surgery and IV patient‐controlled analgesia with morphine for postoperative analgesia for all participants |
|
| Outcomes | Pain at rest and on movement (positioning for spinal) and after surgery Opioids requirements for the first 4 and 48 hours Participant satisfaction |
|
| Notes | SD of 0.00 entered as 0.001 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "divided into two equal groups for this prospective double‐blind study", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants in control group received no block |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Study is said to be double‐blinded. No sham block reported. Unclear who was the outcome assessor for pain scores |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐out |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Low risk | Groups well balanced |
Fletcher 2003.
| Methods | RCT Approved by the ethics committee and informed consents obtained Seeting: United Kingdom Funding: unspecified |
|
| Participants | 50 participants with a hip fracture
Mean age: 78 years (range not stated)
Percentage female: 70%
Lost to follow‐up: none Excluded: confused (and therefore unable to give informed consent), bleeding diathesis or taking warfarin, local or systemic infection, previous hypersensitivity to local anaesthetics |
|
| Interventions |
Treatment group: femoral (3‐in‐1) nerve block inserted at the time of admission with 20 mL 0.5% bupivacaine, Winnie's technique and 5 minutes distal compression (n = 24) Control group: intravenous morphine alone (n = 26) Blocks performed by trained emergency physicians |
|
| Outcomes | Pneumonia from chart review at 6 months Mortality at 6 months Opioids during first 24 hours after block placement (before surgery) |
|
| Notes | Extra information supplied by trialists to confirm secure randomization and that no participants were lost to follow‐up Length of follow‐up: 6 months. Study authors re‐contacted 22 May 2015: no reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized trial: use of sealed opaque numbered envelopes with randomization generated by a random number generator |
| Allocation concealment (selection bias) | Low risk | Randomized trial: use of sealed opaque numbered envelopes with randomization generated by a random number generator |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were not blinded to group allocation because our research ethics committee considered placebo injection unacceptable. Admitting orthopaedic senior house officer was also unaware of study intervention; therefore, analgesic prescription (although standard at Rotherham) was not influenced by participant allocation. These assessments (pain scores) were made by ward nursing staff blinded to the intervention and were included in regular nursing observations undertaken at these times according to the hospital’s fractured neck of femur protocol |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "blinded assessors"; "the same blinded observer (AKF) abstracted all data" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Low risk | Groups well balanced |
Foss 2007.
| Methods | RCT Approved by the ethics committee and written informed consents obtained Setting: Denmark Funding: charity NCT00162630 |
|
| Participants | Orthopaedic hospital in Copenhagen, Denmark
48 participants with a hip fracture
Mean age: 80 years (range 69‐88)
Percentage female: 73%
Lost to follow‐up: none Excluded: refusal to participate in the study, previous surgery in the affected hip, regular prefracture opioid or glucocorticoid therapy, alcohol or substance abuse, infection at the injection site, morphine intolerance, any previous opioid administration for acute pain and non‐confirmation of hip fracture suspicion on x‐ray |
|
| Interventions |
Treatment group: fascia iliaca compartment blockade with 40 mL 1% mepivacaine and epinephrine based on landmarks to fractured limb with saline injection into the contralateral gluteal region (n = 24) Control group: saline injection into fractured side at the site of the fascia iliac block and injection of morphine (0.1 mg/kg) into the contralateral gluteal region (n = 24) After 3 hours, all participants received epidural analgesia |
|
| Outcomes | Pain scores at rest and on movement (15‐degree leg raise) 30 minutes after block Use of supplementary opiates during first 3 hours after block placement |
|
| Notes | Length of follow‐up: till 3 hours after the block No side effects attributable to the fascia iliac block were noted in any participants during their hospital stay Before block placement, pain at rest was significantly less (P = 0.05) in participants with intracapsular fractures (median 2, (interquartile range (IQR) 0–5)) vs those who had trochanteric (median 4, (IQR 2–5)) or subtrochanteric fractures (median 5, IQR 4–7), but no significant difference in movement‐associated pain between fracture types, which was median 8 (IQR 6.5–10), 9 (IQR 8–10) and 10 (IQR 8–10) for intracapsular, trochanteric and subtrochanteric fractures, respectively |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized trial: method stated as via a computer‐generated list using treatments prepared by a nurse not involved in collection of participant data |
| Allocation concealment (selection bias) | Low risk | "the medicine used for each individual patient was prepared by a nurse not otherwise involved with the collection of patient data" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study was double‐blind |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study was double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant did not have a fracture but only a severe contusion and was excluded after x‐ray; an extra participant was therefore included on a new number Two participants (1 from each group) had protocol violations because they received sufentanil as supplementation instead of morphine; both of these supplementations occurred in the post‐anaesthesia care unit more than 60 minutes after block placement |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | High risk | Groups well balanced except for higher proportion of male participants in the block group. Participants in the block group had higher pain scores on admission (P = 0.04) |
Gille 2006.
| Methods | RCT Approved by the ethics committee and informed consents obtained Setting: Germany Funding: corresponding study author had no relationship with any mentioned product nor competitors, classified as departmental resources |
|
| Participants | Orthopaedic hospital in Leipzig, Germany
100 participants with a hip fracture
Mean age: 80 years (range 35‐103)
Percentage female: 77%
Lost to follow‐up: none Excluded: < 18 years old, uncooperative, contraindications to regional anaesthesia or drugs used in the protocol, long‐term use of opioids and/or opioid dependence, history of ulcers, multiple trauma, absence of consent, anaesthetist inexperienced (fewer than 5) with the technique |
|
| Interventions |
Treatment group: femoral nerve block with catheter inserted at the time of admission (stitched in place) using 40 mL 1% prilocaine, then 30 mL 0.2% ropivacaine 6‐hourly (n = 50) Control group: no injection (n = 50) Operated 14 hours after admission. All participants had ibuprofen every 8 hours after surgery |
|
| Outcomes | Pain score on a scale of 1 to 5 (least pain level 1) at rest and passive movement (30 degrees anteflexion) 30 minutes after insertion of the block and at 24, 48 and 72 hours after surgery No severe complications related to analgesia: more specifically, no infection at insertion points of the catheters. 10 catheters were dislodged. No significant respiratory depression due to opioids, no allergic reactions |
|
| Notes | Length of follow‐up: until discharge from orthopaedic ward Extra information regarding method of randomization and length of follow‐up supplied by trialists |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized by anaesthesiologists called to the emergency room: "Sealed envelopes: information from the authors to previous reviewers" |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 20% rate of catheter dislodgement, resulting in the need for systemic analgesia Unclear whether participants with dislodged catheters were included in pain scores in their treatment group |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Low risk | Groups well balanced, including similar admission pain scores (2.50 and 2.46 at rest and 4.30 and 4.34 on movement) |
Godoy 2010.
| Methods | RCT Approved by the ethics committee and signed informed consents obtained Setting: Argentina Funding: unspecified |
|
| Participants | 154 adult participants > 65 years old who presented to the emergency department because of a previously undiagnosed and untreated hip fracture Excluded: anatomical abnormalities in the inguinal area different from fracture, known coagulation disorders, history of allergy to any of the active ingredients used during the study, refusal to participate | |
| Interventions |
Treatment group: fascia iliaca compartment block with 0.3 mL/kg 0.25% bupivacaine and 5 mL 5% dextrose (n = 92) Control group: normal saline in the fascia iliaca compartment and IV non‐steroidal anti‐inflammatory drugs (diclofenac or ketorolac) (n = 62) |
|
| Outcomes | Pain scores at rest at 15 minutes after block placement Confusion |
|
| Notes | Protocol included observing participants for 8 hours The only complications were local bruises at the site of injection |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants randomized into 2 groups (A and B) with numbers generated by the EPI‐INFO™ (Atlanta, GA: Centers for Disease Control and Prevention) programme |
| Allocation concealment (selection bias) | Low risk | Randomization list was kept by one of the study authors who did not interact with participants. He gave instructions to participants’ ED nurse about which treatment should be administered |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Nurse prepared the medication according to physician’s instructions and assigned a letter to the protocol (from a set of 10 letters: 5 for group A and 5 for group B) that designated whether the participant was receiving active medications in the fascia‐iliaca block. The physician administering medications and obtaining VAS scores did not know which medications the participant was receiving. The treating nurse was aware of the randomization group |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The physician administering the medications and obtaining the VAS scores did not know which medications the participant was receiving |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | In all, 175 participants were randomized upon presentation to the ED. A total of 21 were excluded from participation (1) because they or their legal decision‐maker declined to participate, (2) owing to systemic or laboratory abnormalities that interfered with their participation or (3) because they were subsequently found to have missing data (pain scores not recorded or incomplete vital signs on scheduled measurements) |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Low risk | Groups well balanced |
Graham 2008.
| Methods | RCT Approved by the ethics committee and informed consents obtained Setting: China Funding: unspecified |
|
| Participants | 40 adult participants (> 16 years of age) with adequate Mini Mental tests and hip fracture confirmed by x‐ray Excluded: known allergy or contraindication to morphine or bupivacaine, Mini Mental test score < 9 |
|
| Interventions |
Treatment group: femoral (3‐in‐1) nerve block with 30 mL 0.5% bupivacaine (not exceeding 3 mg/kg) (n = 18; 15 analysed) Control group : IV morphine 0.1 mg/kg (n = 22; 18 analysed) Intravenous morphine 0.1 mg/kg bolus as required 2‐ to 4‐hourly, oral dihydrocodeine 30‐60 mg 4‐hourly as required (maximum 240 mg per 24 hours), rectal diclofenac 50 mg 8‐hourly as required (maximum 150 mg per 24 hours), oral paracetamol 1 G 4‐ to 6‐hourly (maximum 4 G per 24 hours) |
|
| Outcomes | Pain scores at 30 minutes after block placement Confusion 24 hours after block placement or to surgery (whichever came first) Opioids used in 24 hours or to surgery (whichever came first) |
|
| Notes | No immediate complications in either group, defined as inadvertent vascular puncture, anaphylaxis or collapse, severe pain or inability to tolerate the procedure for the femoral block group and anaphylaxis or collapse, respiratory depression or requirement for naloxone use within 1 hour of IV morphine administration for the systemic analgesia group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were then randomized using numbered, sequential, sealed opaque envelopes" |
| Allocation concealment (selection bias) | Low risk | "Patients were then randomized using numbered, sequential, sealed opaque envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label trial |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Open‐label trial |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Seven participants had incomplete data One participant in the morphine group was unable to complete the study owing to development of an acute confusional state. Full data were unavailable for 3 other participants in the IV morphine group owing to incomplete data collection post intervention. Within the '3‐in‐1' nerve block group, 1 participant was found to have an impalpable femoral artery on the side of the hip fracture after randomization and was unable to receive a nerve block owing to lack of anatomical landmarks. Full data were unavailable for 2 other participants owing to incomplete data collection post intervention. Full results were available for 33 participants: 18 in the morphine group and 15 in the femoral nerve block group |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Unclear risk | Groups well balanced Data were not analysed in intention‐to‐treat |
Haddad 1995.
| Methods | RCT Setting: United Kingdom Funding: unspecified |
|
| Participants | Orthopaedic hospital in Stevenage, UK
50 participants with an extracapsular hip fracture
Mean age: 77 years (range 68‐89)
Percentage male: 30%
Lost to follow‐up: none Excluded: dementia, unable to rate their pain |
|
| Interventions |
Treatment group: femoral nerve block inserted at the time of admission using 0.3 mL/kg 0.25% bupivacaine (n = 25) Control group: no injection (n = 25) |
|
| Outcomes | Pneumonia Mortality Wound infection Pressure sores No local or systemic complications of femoral nerve blocks |
|
| Notes | Length of follow‐up: 24 hours for analgesia, unclear for other outcomes ("short term"; taken as in hospital) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | No placebo injections used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Admitting house surgeons and nursing staff who administered analgesia were unaware to which group participants had been allocated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None lost to follow‐up One failed block; data included |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Low risk | Groups well balanced |
Hood 1991.
| Methods | RCT Approved by the ethics committee and written informed consents obtained Setting: United Kingdom Funding: unspecified |
|
| Participants | Orthopaedic hospital in Sheffield, UK
50 participants with a hip fracture surgically treated with a pin and plate or a compression screw
Mean age: 81 years (range 62‐94)
Percentage female: 88%
Lost to follow‐up: 1 (2%) Excluded: absolute contraindication to a regional technique, allergy to local anaesthetic agents, systemic disease that indicated an alternative method of anaesthesia |
|
| Interventions | Treatment group: femoral (triple nerve block) nerve block with 35 mL 0.75% prilocaine and infiltration above the iliac crest with 8 mL 0.75% prilocaine inserted before induction of anaesthesia (n = 25) Control group: no blocks (control) (n = 25) All participants had general anaesthesia using alfentanil, etomidate, nitrous oxide, isoflurane | |
| Outcomes | Death | |
| Notes | Length of follow‐up: 24 hours No untoward sequelae were associated with nerve blocks. Venous blood for plasma prilocaine levels in the first 12 participants in group 2 was taken from an indwelling cannula at 0, 5, 10, 15, 20, 25, 30, 45 and 60 minutes after completion of the prilocaine injection. Concentrations were measured using gas chromatography. Cmax occurred no later than 25 minutes. Methemoglobin levels were not mentioned |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of 'unmarked envelopes' |
| Allocation concealment (selection bias) | Low risk | Use of 'unmarked envelopes' |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | All participants were prescribed intramuscular papaveretum 0.2 rng/kg; administration was done at the discretion of the nursing staff, who were unaware of participant groups. All participants had their skin prepared and an elastoplast placed over the possible injection site to minimize bias. Unclear for participants: blocks performed before induction of general anaesthesia |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All participants were prescribed intramuscular papaveretum 0.2 rng/kg; administration was done at the discretion of the nursing staff, who were unaware of participant groups. All participants had their skin prepared and an elastoplast placed over the possible injection site to minimize bias The operating theatre recovery sister and ward staff, who were blind to participant groups, were asked to assess the quality of analgesia after the operation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One participant lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Low risk | Groups well balanced |
Iamaroon 2010.
| Methods | RCT Approved by the ethics committee and written informed consents obtained Setting: Thailand Funding: unspecified |
|
| Participants | 64 ASA I–III participants aged 18–80 years undergoing surgery for femur fracture with body weight > 50 kg and scheduled for surgery under spinal block Excluded: multiple fractures, peripheral neuropathy, bleeding disorders, mental disorders, communication failure, allergy to local anaesthetics, use of analgesics for premedication |
|
| Interventions |
Treatment group: femoral nerve block with a nerve stimulator and 20 mL bupivacaine 0.5% and 10 mL saline 15 minutes before positioning for the spinal (n = 32) Control group: 2 doses of IV fentanyl 0.5 mcg/kg (n = 32) During positioning for the spinal (lateral position with the fracture site up), fentanyl in 0.5 mcg/kg increments was given every 5 minutes until pain scores were < or = 4 |
|
| Outcomes | Pain scores at rest and on movement (positioning for the spinal) 15 minutes after block placement No adverse systemic toxicity of bupivacaine, such as seizure, arrhythmia or cardiovascular collapse, was noted in the femoral nerve block group. Neither vascular puncture nor paraesthesia occurred. No complications, such as haematoma, infection or persistent paraesthesia, were observed within 24 hours after the operation. No participant in either group had hypoventilation (ventilatory rate < 10/min) or oxygen saturation < 95% |
|
| Notes | Although the vast majority of participants had a proximal fracture, 10 participants had a shaft (6 participants for the femoral nerve block and 1 for the control) or a distal (3 participants in the control group) fracture. The only outcomes retained in the analysis were pain scores. Pain scores at rest and on movement after block placement were finally excluded because we thought that measurement was done before the block could be effective (please see Results) An email was sent on 17 March 2016, to obtain data separately for participants with a proximal fracture. No reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were allocated by computer‐generated random numbers into 2 groups of 32 participants each |
| Allocation concealment (selection bias) | Low risk | The random allocation sequence was concealed in opaque, sealed envelopes until a group was assigned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded: "All patients were aware of their treatment group allocation" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinded: "Assessors of pain were blinded to the patients’ allocated treatment group" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None lost to follow‐up No failed block reported |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Unclear risk | Groups well balanced except that time from trauma to surgery was significantly longer in the fentanyl group than in the FNB group (P = 0.03) and most participants in the FNB had femoral neck fractures, whereas most of those in the fentanyl group had intertrochanteric fractures (P = 0.04) |
Jadon 2014.
| Methods | RCT Insitutional approval and informed consents obtained |
|
| Participants | Included: patients of both sexes, 18–70 years, weight > 50 kg, American Society of Anesthesiologists physical status I‐III, scheduled for fracture femur operation under central neuraxial block but unable to sit because of pain Excluded: could sit comfortably, any contraindication to spinal anaesthesia, FNB or local anaesthetic |
|
| Interventions |
Treatment group: femoral nerve block with 20 mL lidocaine 1.5% (15 mL lidocaine 2% and 5 mL distilled water) with epinephrine 5 mcg/mL, nerve stimulator, quadriceps response at 0.3‐0.5 mA, insulated 50 mm 22 G needle (n = 23) Control group: fentanyl 1.0 mcg/kg IV (n = 21) Positioning for spinal 5 minutes after block placement or IV fentanyl. Additional fentanyl 0.5 mcg/kg every 5 minutes in both groups allowed if VAS scores ≥ 4 until VAS scores < 4 or a maximal dose of 3 mcg/kg (whichever came first). No participant required an additional dose of fentanyl |
|
| Outcomes | Pain scores on movement (positioning for the spinal) 5 minutes after block placement (scale 0‐10) | |
| Notes | Study also includes participants with shaft fracture. We obtained results for pain scores on movement for participants with proximal fracture only from the study authors. However, we did not keep results in the analysis (see Effects of interventions) owing to the short delay between the block and the evaluation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were distributed in two groups through computer generated random numbers table" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None lost to follow‐up. For 2 participants from each group, surgery was postponed owing to infection at the surgical site and a change in the surgical plan. One participant from each group was excluded owing to refusal for spinal anaesthesia on the table after initial consent. Therefore, 6 participants were subsequently excluded, leaving 60 participants for final analysis All participants for whom a pain score could be obtained during spinal positioning had their score analysed within their assigned group |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Low risk | "Demographic data and type of surgery were comparable in both the groups" |
Jones 1985.
| Methods | RCT Informed consents obtained Setting: United Kingdom Funding: unspecified |
|
| Participants | Orthopaedic hospital in London, UK
19 participants with an extracapsular hip fracture treated with a pin and plate or a sliding hip screw
Mean age: 82 years (range 67‐93)
Percentage female: 95%
Lost to follow‐up: none Excluded: other painful lesions, signs of moderate or severe dementia, < 65 years of age, systemic disease indicating an alternative method of anaesthesia (e.g. spinal) |
|
| Interventions |
Tretament group: lateral cutaneous nerve of thigh block with 15 mL 0.5% bupivacaine and adrenaline (n = 10)
Control group: no block (control) (n = 9) All participants had general anaesthesia with fentanyl, thiopentone, suxamethonium, nitrous oxide, halothane. Blocks performed at completion of surgery |
|
| Outcomes | Death (24 hours) | |
| Notes | One participant died within 24 hours of surgery, and results for this participant were not given Length of follow‐up: not stated |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | 'Random envelopes' |
| Allocation concealment (selection bias) | Low risk | 'Random envelopes' opened at completion of surgery |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Postoperative analgesia was prescribed before allocation to respective groups, with administration performed at the discretion of the nursing staff, who were unaware of whether a block had been performed. The dose of pethidine was 25 or 50 mg intramuscularly, depending on the participant's estimated weight ‐ not on general condition |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | One lost to follow‐up Results for 1 participant who died are not included: "One patient in Group 2 who died within the 24‐hour period is not included in analysis of the results: there are thus nine patients in each group" |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Low risk | Groups well balanced |
Kullenberg 2004.
| Methods | RCT Setting: Sweden Funding: no conflict of interest declared, classified as departmental resources |
|
| Participants | Orthopaedic hospital in Sweden
80 participants with a hip fracture
Mean age: 82 years (range not stated)
Percentage female: 64%
Lost to follow‐up: not stated Excluded: inability to rate their pain |
|
| Interventions |
Treatment group: femoral nerve block inserted at the time of admission with 30 mL ropivacaine (7.5 mg/mL) (n = 40). Mean bock duration 15.8 ± 5.6 hours. Four participants had their block during transportation to the hospital. The block was repeated for 3 participants owing to a long delay before surgery (23.9 hours, 26.3 hours and 30.9 hours) Control group: no injection (n = 40) |
|
| Outcomes | Confusion (Pfeiffer test, graded according to a 4‐degree scale (0‐3: no, light, moderate and pronounced confusion) at 48 hours Time to first mobilization Pressure sores Opioids used per 24 hours |
|
| Notes | Length of follow‐up: length of acute hospital stay (mean 11 days) No complication related to the nerve block All participants indicated that they would consider a new future blockade if this would be necessary |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Use of sealed envelopes |
| Allocation concealment (selection bias) | Low risk | Use of sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None lost to follow‐up Five failed blocks; participants kept in their treatment groups |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Unclear risk | Groups well balanced except for a longer delay in arrival to surgery for the block group (15.5 hours vs 5.8 hours) |
Luger 2012.
| Methods | RCT Approved by the ethics committee and written informed consents obtained Setting: Austria Funding: departmental resources |
|
| Participants | Included: 37 very elderly participants (> 80 years) with hip fractures (of whom 3 with dementia had to be excluded) scheduled for surgery under spinal anaesthesia Excluded: score < 18 on the Mini‐Mental State Examination, surgery did not take place within 36 hours, known intolerance or allergies to drugs, planned or required general anaesthesia, refusal of consent, participation in a different study, administration of midazolam as premedication, chronic pain, contraindications and spinal anaesthesia failure, incomplete data records |
|
| Interventions |
Treatment groups: ultrasound‐guided continuous femoral (3‐in‐1) nerve block with bupivacaine (n = 10) Control group: systemic analgesia with IV/SC piritramide and IV paracetamol (n = 10) |
|
| Outcomes | Pain scores at rest and on movement at 24 hours after surgery Opioids at 24 hours after surgery Number of participants with postoperative myocardial ischaemia |
|
| Notes | Study also includes a group with epidural analgesia ‐ not retained in this review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The patients were randomized according to a computer‐generated randomization list" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drop‐out for the 2 included subgroups |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Low risk | Groups comparable |
Mossafa 2005.
| Methods | RCT Setting: Iran Funding: unspecified |
|
| Participants | 40 participants with femoral neck fracture | |
| Interventions |
Treatment group: fascia iliaca block with 20 mL 1.5% lidocaine (n = 20) Control group: IV fentanyl 1.5 mcg/kg (n = 20) Lateral decubitus position for spinal anaesthesia |
|
| Outcomes | Pain on movement 5 minutes after block placement (positioning for spinal) Participant satisfaction |
|
| Notes | Conference abstract Email sent on 26 May 2015; no reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Unclear risk | No details |
Mouzopoulos 2009.
| Methods | RCT Approved by the ethics committee and signed informed consents obtained Setting: Greece Funding: unspecified |
|
| Participants | 207 participants aged 70 years or older at intermediate or high risk of delirium scheduled for hip fracture repair Risk classification was based on the presence of 4 predictive risk factors (severity of illness, measured by acute physiology age and chronic health examination; cognitive impairment, measured by the mini‐mental state examination score; index of dehydration, measured by the ratio of blood urea nitrogen to creatinine; and visual impairment, measured by the standardized Snellen test) as described by Inouye. Intermediate risk for postoperative delirium was defined as the presence of 1 or 2 risk factors; high risk was defined as the presence of ≥ 3 risk factors Excluded: delirium at admission, metastatic hip cancer, history of bupivacaine allergy, use of cholinesterase inhibitors, severe coagulopathy, parkinsonism, epilepsy, levodopa treatment, delayed surgery more than 72 hours after admission, inability to participate in interviews (profound dementia, respiratory isolation, intubation, aphasia, coma or terminal illness) |
|
| Interventions |
Treatment group: fascia iliaca block with bupivacaine 0.3 mL/kg (0.25%?) repeated daily until delirium or surgery and at 24 hours after surgery, and daily until delirium or discharge (n = 108 randomized; n = 102 analysed) Control group: placebo medication (water for injection) identical in appearance to the active drug and administered at the same site and in the same way (n = 111 randomized; n = 105 analysed) Intravenous and intramuscular analgesics were administered as needed in both groups |
|
| Outcomes | Confusion (Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM‐IV) and Confusion Assessment Method criteria) No complications of fascia iliaca block administration report, except 3 local haematomas developed at the injection site, which resolved spontaneously. Exact number of blocks performed was not specified |
|
| Notes | Reduction was seen only in participants at intermediate risk of developing delirium ‐ not among those at high risk | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "sequentially randomly assigned" "according to a computer‐generated randomization code" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "All participants were blinded to the treatment group." "Placebo medication (water for injection) was identical in appearance to the active drug and was administered at the same site and in the same way as the fascia iliaca block was injected." However, no clear mention of blinding of personal taking care of participants |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not really clear |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | A total of 12 participants were further excluded from both groups for different reasons: 6 lost to follow‐up, 3 refused further participation and 3 died (2 pulmonary embolism and 1 stroke) between second and fourth days of admission |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Unclear risk | Groups well balanced Not in intention‐to‐treat |
Murgue 2006.
| Methods | RCT Informed consents obtained Setting: France Funding: unspecified |
|
| Participants | Orthopaedic hospital in Feurs, France
30 participants with a hip fracture
Mean age: 86 years (range 70‐96)
Percentage female: 82%
Lost to follow‐up: none Excluded: inability to rate their pain (Mini Mental score < 24), contraindication to nitrous oxide, regional anaesthesia, allergy to study drugs, renal dysfunction or prefracture opioid treatment |
|
| Interventions |
Treatment group: femoral nerve block inserted at the time of admission with 20 mL mepivacaine (n = 16) Control groups: no injection. IV morphine (n = 14) or IV paracetamol and ketoprofen (n = 15). We retained only the IV morphine group as the control group All participants received nitrous oxide for withdrawal of clothing on arrival |
|
| Outcomes | Pain score on VAS at movement (x‐ray): 20 minutes after block placement | |
| Notes | Length of follow‐up: duration of time in emergency department | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Hat drawing |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None lost to follow‐up Failed block (defined as pain scores > 4/10 at skin traction installation) 18.7%. Results included in treatment group |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Low risk | Groups well balanced |
Nie 2015.
| Methods | RCT Approved by the ethics committee and written informed obtained Setting: China Funding: governmental Open reduction and internal fixation surgery with the antirotation proximal femoral nail technique |
|
| Participants | 104 participants scheduled for open reduction of hip fracture Excluded: neuropathy involving lower extremities, bladder dysfunction, coagulopathies, known allergy to amide local anaesthetic drugs or opioids, inability to co‐operate, psychological disorders or linguistic difficulties that could interfere with pain assessment |
|
| Interventions |
Treatment group: fascia iliaca block (n = 51) Control treatment: intravenous patient‐controlled analgesia (n = 53) General anaesthesia with fentanyl, remifentanil, propofol and atracurium for surgery and flurbiprofen 40 mg at completion of surgery, plus acetaminophen and dihydrocodeine or morphine on request as rescue analgesia for all participants |
|
| Outcomes | Pain at 2, 4, 6, 12, 24 and 48 hours after surgery (taken as at rest) Acute confusional state (time point unspecified, participants screened daily; length of hospital stay 23 and 21 days for fascia iliaca and IV groups, respectively) Opioid consumption up to 48 hours Participant satisfaction (92.5% of participants receiving a fascia iliaca block were satisfied vs 94.3% of participants receiving IV analgesia) |
|
| Notes | Additional information received from study authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomly assigned according to a computer‐generated random number table" |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "Blinding could not be conducted due to differences in the analgesia procedures and infusion pumps used" |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | "Blinding could not be conducted due to differences in the analgesia procedures and infusion pumps used" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | "Patients who underwent the full protocol were included in the analysis" 2 participants withdrawn from fascia iliaca group owing to catheter failure |
| Selective reporting (reporting bias) | Unclear risk | All results mentioned in methods section given in results section, except preoperative and postoperative mini‐mental state examination. We contacted study authors who informed us that Mini Mental tests were not "collected" |
| Other bias | Unclear risk | Groups well balanced. Prophylaxis against nausea and vomiting given to IV group only Not in intention‐to‐treat |
Segado Jimenez 2009.
| Methods | RCT Approved by the ethics committee and informed consents obtained Setting: Spain Funding: unspecified |
|
| Participants | 75 participants undergoing hip fracture repair under spinal anaesthesia Excluded: general anaesthesia or intravenous administration of analgesics intraoperatively, pretreatment for chronic pain, or for ischaemic heart rhythm disorders, neurodegenerative and psychiatric diseases, lack of collaboration and/or understanding of the participant, allergy to local anaesthetics and contraindications for regional anaesthesia |
|
| Interventions |
Treatment group: femoral cutaneous and obturator nerve block (n = 25) or obturator nerve block only (n = 25). Blocks were performed after the spinal had worn off Control group: intravenous analgesia (n = 25) For all groups, investigators administered additional intravenous analgesia according to participants' demands (if VAS scores ≥ 3): metamizole 2 G or dexketoprofen trometamol 50 mg IV up to every 8 hours (depending on allergies). If pain persisted, tramadol 100 mg and metoclopramide 10 mg IIV every 8 hours was added. In addition, if needed, 0.5 mg/kg morphine chloride was used as a rescue |
|
| Outcomes | Drug expenses Time to first mobilization (sitting) Participant satisfaction (score from 1 to 5; 1 = bad, 2 = regular, 3 = good, 4 = very good and 5 = excellent) Opioids during first 48 hours after surgery Participant satisfaction (score from 1 to 5; 1 = bad, 2 = regular, 3 = good, 4 = very good and 5 = excellent) "We did not observe any complication in the realization of locoregional techniques during or subsequent to the locoregional technical" |
|
| Notes | Email sent on 26 May 2015; no reply | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly distributed", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Triple blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Triple blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No missing results |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section are given in results section |
| Other bias | Low risk | Groups well balanced |
Spansberg 1996.
| Methods | RCT Approved by the ethics committee and informed consents obtained Setting: Denmark Funding: unspecified |
|
| Participants | 20 participants with a hip fracture surgically treated Mean age: 81 years (range 58‐91) Percentage female: unclear Lost to follow‐up: none | |
| Interventions | All participants had spinal anaesthesia with 3.5 mL 0.5% bupivacaine
Postoperatively, participants received:
Treatment group: femoral nerve block with 0.4 mL/kg bolus of 0.5% bupivacaine, then infusion of 0.14 mL/kg/h 0.25% bupivacaine for 16 hours (n = 10) Control group: saline infusion for 16 hours of same volume of fluid (control) (n = 10) Regular aspirin administration and IM morphine on demand |
|
| Outcomes | Opioids used during first 18 hours after surgery No haematomas at the site of femoral catheters. Length of follow‐up unspecified |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomized by a computer after surgery |
| Allocation concealment (selection bias) | Low risk | Adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Placebo‐controlled study: participants, recovery staff and observers were blind to the solution used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Placebo‐controlled study: participants, recovery staff and observers were blind to the solution used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None lost to follow‐up Nine of 10 participants receiving bupivacaine were analgesic to pin prick in the distribution of all 3 nerves. The other participant was analgesic only in the distribution of the lateral femoral cutaneous nerve of the thigh |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Low risk | Groups well balanced |
Szucs 2012.
| Methods | RCT Approved by the ethics committee and written informed consents obtained Setting: Ireland Funding: unspecified |
|
| Participants | 24 participants presenting with fractured neck of femur, ASA I‐III and aged > 50 years Excluded: participant refusal, presence of more than 1 fracture; Mini‐Mental Score < 22; coagulation disorders; head injury; loss of consciousness; 10 mg or more morphine administration pre‐hospital; acute intercurrent heart disease; allergy to bupivacaine, morphine or paracetamol; skin lesions/infection at block site; renal dysfunction, evidence of systemic infection (clinically defined or elevated C‐reactive protein levels, leucocytosis or body temperature > 37.8°C) | |
| Interventions |
Treatment group: continuous femoral nerve block with bupivacaine 0.25% for 72 hours (n = 12) Control group: IM morphine (n = 12) All participants received paracetamol regularly and parenteral morphine up to 0.1 mg/kg IM 4‐hourly as required |
|
| Outcomes | Pain at rest and on passive movement (30 degrees flexion) at 30 minutes after block placement Participant satisfaction |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "random number sequence and sealed envelopes" |
| Allocation concealment (selection bias) | Low risk | "random number sequence and sealed envelopes" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Three participants were excluded for the following reasons: (1) elastomeric pump failure resulting in local anaesthetic administered over less than 54 hours instead of 72 hours, (2) participant confusion with subsequent pump disconnection after 12 hours, (3) late diagnosis of a complicating acetabular fracture |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Unclear risk | Groups well balanced Not in intention‐to‐treat |
Tuncer 2003.
| Methods | RCT Approved by the ethics committee and informed consents obtained Setting: Turkey Funding: unspecified |
|
| Participants | Orthopaedic hospital in Konya, Turkey
40 participants with a hip fracture undergoing surgery for a trochanteric hip fracture
Mean age: 59 years (range not stated)
Percentage male: not stated
Lost to follow‐up: none Excluded: coagulation abnormality, age < 18 or > 80 years, weight < 50 or > 100 kg, known allergy to bupivacaine or opioids, previous analgesic treatment with opioids, inability to understand pain scales or use a patient‐controlled analgesia device |
|
| Interventions | All participants had general anaesthesia with fentanyl, propofol atracurium, nitrous oxide, isoflurane. At completion of the operation, participants had: Treatment group: femoral (3‐in‐1) nerve block with 30 mL 2% lidocaine with 2% epinephrine 1:200,000, followed by continuous infusion with 0.125% bupivacaine for 48 hours (n = 20) Control group: patient‐controlled analgesia with morphine (control) (n = 20) |
|
| Outcomes | Participant satisfaction (rated as excellent, good, moderate or poor; we attributed scores from 1 to 4 to compare the data) | |
| Notes | Length of follow‐up: 48 hours Email sent to study authors on 24 May 2015, to ask for additional information; no reply |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomized at completion of surgery, method unspecified |
| Allocation concealment (selection bias) | Low risk | Randomized after inclusion |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Two missing results for participant satisfaction |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Low risk | Groups well balanced |
White 1980.
| Methods | RCT Consents obtained Setting: South Africa Funding: unspecified |
|
| Participants | Orthopaedic hospital in Cape Town, South Africa
40 participants with a hip fracture undergoing surgery
Mean age: 79 years (range not stated).
Percentage female: 81%
Lost to follow‐up: none Excluded: fracture sustained more than 8 days before admission; < 60 years old; absolute contraindication to a regional technique, such as localized sepsis, suspicion of bacteraemic process or patients receiving anticoagulant therapy; overt or suspected endocrine disorder other than diabetes mellitus |
|
| Interventions |
Treatment groups: psoas block (n = 16) or spinal (n = 20) plus 'light" general anaesthesia using althesin, nitrous oxide Control group: general anaesthesia with fentanyl, thiopentone, suxamethonium, nitrous oxide, halothane (n = 20) |
|
| Outcomes | Confusion Pneumonia Mortality |
|
| Notes | Length of follow‐up: 4 weeks Four of 20 participants allocated to receive psoas nerve block failed to achieve a satisfactory block; outcome for these participants was not given (other than for mortality) No participant showed evidence of toxicity to the local anaesthetic Trial also includes a group with spinal (n = 20) added to light general anaesthesia |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "randomly allocated", no details |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Four failed psoas compartment blocks, no other losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | All measurements mentioned in methods section given in results section |
| Other bias | Unclear risk | Groups well balanced, except for preoperative pneumonia: 1 in the psoas compartment block and 4 in the group general anaesthesia alone Not in intention‐to‐treat |
Yun 2009.
| Methods | RCT Approved by the ethics committee and written informed consents obtained Setting: Korea Funding: unspecified |
|
| Participants | 40 ASA physical status I–III participants aged 62–88 years with isolated femoral neck fracture Excluded: known allergy to amide local anaesthetics, haemorrhagic diathesis, peripheral neuropathy, mental disorders |
|
| Interventions |
Treatment group: fascia iliaca with 30 mL 0.375% ropivacaine (n = 20) Control group: IV alfentanil 10 mcg/kg followed by 0.25 mcg/kg/min starting 2 minutes before spinal (n = 20) Participants were moved to the operating suite for the spinal 20 minutes after block placement. Spinals were performed in lateral decubitus position on the side best tolerated by the participant. When a participant reported a VAS 4 during this positioning, the procedure was stopped, and 100 mg of IV alfentanil was administered in both groups |
|
| Outcomes | Pain scores on movement at 30 minutes after block placement (positioning for spinal anaesthesia) and at rest at 6 and 24 hours after surgery Participant satisfaction (yes/no: 1 = good (if necessary, I would repeat the procedure) and 2 = bad (I would never repeat the procedure again)) Opioids at 24 hours No adverse systemic toxicity of ropivacaine was noted, and neither vascular puncture nor paraesthesia was elicited in the fascia iliaca block group. No complications, such as haematoma or persistent paraesthesia, were observed in participants with a fascia iliaca block within 24 hours after the operation |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomly assigned, using an allocation sequence (which was generated by Y. H. Kim using a computer)" |
| Allocation concealment (selection bias) | Low risk | "The random allocation sequence was concealed until group was assigned (by J. W. Hwang)" |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not mentioned |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | None lost to follow‐up No failed block mentioned. 40% (8 of 20) of participants had a complete block (3 nerves) and 60% (12 of 20) had blockade of 2 nerves (lateral femoral cutaneous and femoral) |
| Selective reporting (reporting bias) | Low risk | All results mentioned in methods section given in results section |
| Other bias | Low risk | Groups well balanced |
ASA: American Society of Anesthesiologists physical status
DSM: Diagnostic and Statistical Manual of Mental Disorders
ECG or EKG: electrocardiogram
ED: emergency department
FNB: femoral nerve block
G: gram
h: hour
IM: intramuscular
IV: intravenous
IQR: interquartile range
mcg: microgram
mg: milligram
mL: millilitre
n: number
PCA: patient‐controlled analgesia
RCT: randomized controlled trial
SC: subcutaneous
VAS or VRS: = visual or verbal analogue/response scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bech 2011 | Different intervention: local anaesthetic infiltration |
| Bölükbasi 2013 | No outcome of interest. Conference abstract. Not enough details on possible outcomes of interest in the abstract. Study authors contacted on 25 May 2015. Confirmed that they were the authors of the abstracts but did not provide requested information |
| Durrani 2013 | Different population. 47 proximal fractures, 28 shaft fractures and 9 distal fractures. Mean age 42 years. Email sent 17 March 2016, to request separate data for participants with a proximal fracture. No reply |
| Foss 2005 | Different intervention: epidural analgesia |
| Fujihara 2013 | Not randomized: "The included patients were assigned to one of two groups in alternating order" |
| Ghimire 2015 | Different intervention. Comparison between fascia iliaca block and femoral nerve block for positioning for spinal anaesthesia |
| Gorodetskyi 2007 | Different intervention. This was a randomized study of 60 participants with a trochanteric hip fracture fixed with a sliding hip screw or a trochanteric external fixator. After surgery, participants were randomized to an active non‐invasive interactive neurostimulation device or to a sham device. The active device generated biphasic electrical impulses. Participants allocated to the active group had a reduced level of pain, a reduced analgesic requirement and a greater range of flexion of the injured limb. We excluded the study as it was not a study of nerve blocks |
| Hussain 2014 | Different intervention. The amount of local anaesthetic used (bupivacaine 12.5 mg/kg of body weight) exceeds recommendations |
| Hwang 2015 | No outcome of interest at our selected time points (a study may be legitimately excluded if outcomes of interest were not measured; Higgins 2011 Section 5.4.1). Here, pain intensity was measured at 2 and 3 hours after block placement only from the results of 2 conference abstracts for this study, and at 1, 4 and 24 hours after block placement in the third abstract. The third abstract also reported absence of a difference in opioid consumption "during the course of the study (no specific time point mentioned for this outcome) on preliminary results:12.0 mg of morphine equivalent (6 participants) versus 12.9 mg for the control group (8 participants); P value 0.88." This result was not retained in the analysis. Adding it would not change the conclusions for opioid consumption standardized mean difference ‐0.71 (95% confidence interval ‐0.94 to ‐0.48); I2 statistic = 44%, with this result vs standardized mean difference ‐0.77 (95% confidence interval ‐0.98 to ‐0.56); I2 statistic = 30% without. No complications were recorded in either group |
| Irwin 2012 | Retrospective study |
| Kang 2013 | Different intervention: local anaesthetic infiltration |
| Kumie 2015 | Not a RCT. Single‐institution case control study |
| Mannion 2005 | Different intervention. This was a randomized trial of 36 participants who were having hip fracture surgery. All participants had a psoas block and general anaesthesia. Participants were randomized into 3 groups. A control group received a psoas block and IV saline, another group received psoas block and IV clonidine 1 mg/kg and a third group received a psoas block and peripheral clonidine. The interval from time of completion of block to first supplementary analgesic administration was longer in the IV clonidine group. Results show no significant differences among groups regarding postoperative adverse effects. We excluded the study as investigators included no 'control' group that received no block |
| Manohara 2015 | Different intervention. Comparison between ultrasound‐guided supra‐inguinal fascia iliaca block and femoral nerve block |
| Marhofer 1998 | Different intervention. This was a randomized trial of 60 participants. 20 received a 3‐in‐1 block with ultrasound guidance with 20 mL 0.5% bupivacaine, 20 received 20 mL of 0.5% bupivacaine and 20 received 30 mL of 0.5% bupivacaine with nerve stimulator guidance. We excluded the study as investigators included no comparison with a group without nerve block |
| Matot 2003 | Different intervention: epidural analgesia |
| McRae 2015 | Different population: 6 participants with shaft fracture. Letter sent 17 March 2016, to request separate data for participants with a proximal fracture. No reply |
| Mutty 2007 | Different population. This was a randomized trial comparing femoral nerve block vs no block for 54 participants with a femoral shaft or distal femoral fracture. We excluded the study as it included no proximal femoral fractures |
| Piangatelli 2004 | Different intervention. This was a randomized study of 80 participants undergoing lower extremity surgery that compared 4 different methods. A lumbar plexus block with 30 mL 0.5% levobupivacaine or a lumbar plexus block with 30 mL 0.75% ropivacaine or a sciatic nerve block with 10 mL 0.75% ropivacaine or a sciatic nerve block with 10 mL 0.5% levobupivacaine. We excluded the study from this review, as investigators included no 'control' group without nerve block |
| Reavley 2015 | Different intervention. Comparison between fascia iliaca block and femoral (3‐in‐1) block for preoperative analgesia in the emergency department |
| Scheinin 2000 | Different intervention: epidural analgesia |
| Schiferer 2007 | Different population. This was a randomized trial of 62 participants with femoral trauma who were randomized to receive at the site of the accident a femoral nerve block or intravenous metamizole for pain. Studiy provided a variety of causes for the femoral trauma, including 20 cases of hip fracture. The nerve block was shown to reduce the degree of pain as assessed by the visual analogue scale and to reduce anxiety and heart rate. We excluded the study as it included participants with other conditions. Trialists were unable to provide separate results for hip fracture participants |
| Segado Jimenez 2010 | Study authors informed us that the trial included participants with hip fracture and participants without hip fracture undergoing elective hip arthroplasty. They could not give us data separately for participants with and without hip fracture: "I did not registered which patients were hip fractures, just the type of surgery" |
| Sia 2004 | Different population. Femoral shaft fractures |
| Turker 2003 | Different intervention. This was a randomized study of 30 participants who underwent partial hip replacement surgery. 15 received general anaesthesia plus epidural block with 15 mL of 0.5% bupivacaine, and 15 received general anaesthesia plus psoas compartment block with 30 mL of 0.5% bupivacaine. Both groups had similar pain scores, but the epidural group showed greater drops in mean arterial blood pressure from baseline and more complications. We excluded the study from this review because it did not include a control group that did not receive nerve block |
| Van Leeuwen 2000 | Different intervention. This was a randomized study of 3 different combinations of doses of local anaesthetics given to produce a 'three in one' femoral nerve block. We excluded this study from the review because it did not include a 'control' group that did not receive nerve block |
Characteristics of ongoing studies [ordered by study ID]
ACTRN12609000526279.
| Trial name or title | Ultrasound‐guided femoral nerve block using 1% ropivacaine as a method of pain control in patients who present to emergency with a fractured hip |
| Methods | Parallel RCT Open label Approved by the ethics committee |
| Participants | Inclusion criteria: 18 years or older with radiological proof of fractured neck of femur Exclusion criteria: women lactating, pregnant or of childbearing potential who are not willing to avoid becoming pregnant during the study, < 18 years old, allergy to ropivacaine, allergy to paracetamol and morphine, anticoagulated patients and those with significant coagulation abnormalities that increase their risk of bleeding. localized injection site infection. neurological deficits in the distribution of the femoral nerve noted, consent denied, documented severe hepatic disease, unable to give consent themselves, history of heart block or on amiodarone, acute cardiac event in the last 3 months |
| Interventions |
Treatment group: An ultrasound‐guided femoral nerve block will be placed with 1% ropivacaine. An ultrasound vascular probe is placed to locate structures anatomically: The main structures were the femoral nerve itself, the femoral artery and vein and the fascia iliaca. Then under real‐time ultrasound guidance via an out‐of‐plane approach, 15 mL of 1% ropivacaine is injected around the femoral nerve as visualized. A 2‐person technique is employed with the needle attached to the syringe of ropivacaine via a 90 cm minimum volume extension set. The probe and needle operator is present along with an assistant who injects the anaesthetic. Digital pressure is then placed for 30 seconds just distal to the injection site. The entire procedure takes about 15 to 20 minutes. Objective measure of the nerve block is assessed at 30 minutes by testing sensation over the anterolateral aspect of the thigh Control group: Both study and control groups will receive regular oral tablet paracetamol 1 G every 4 to 6 hours to a maximum dose of 4 G in 24 hours and parenteral (intravenous) morphine as required for pain control |
| Outcomes | Primary outcome: morphine use in patients for both groups at 24 hours Secondary outcomes: pain scores and subsequent pain scores to be looked at in each group and compared over the first 12 hours. Scores will range from 0 to 10, with 10 indicating the worst pain. Pain scores in both groups will be assessed at enrolment into the study, 30 minutes after enrolment or after nerve block has been given, then at 4 then 8 then 12 hours after enrolment |
| Starting date | 04/04/2009 |
| Contact information | Dr Edmond Park, Emergency Department, St Vincents Hospital, Victoria Street, Darlinghurst 2010 New South Wales +61 2 8382 2040, epark@stvincents.com.au |
| Notes | Recruiting |
EUCTR2006‐004001‐26‐GB.
| Trial name or title | A randomized controlled trial of fascia iliaca compartment block versus morphine for pain in fractured neck of femur in the emergency department: a pilot study ‐ fascia Iliaca compartment block versus parenteral morphine sulphate |
| Methods | RCT Open label |
| Participants | Inclusion criteria: suspected isolated hip fracture (clinical suspicion of hip fracture defined as the presence of painful, unilateral shortening and external rotation of the lower limb, preventing weight‐bearing), alert and oriented, willing to be randomized for pain relief as described, > 18 years of age Exclusion criteria: other significant injury, clinical suspicion of fracture other than to the hip, sensitivity to any study preparation, local infection at the injection site, symptoms/Injury > 8 hours, prehospital parenteral analgesia, unable to give informed consent as a result of current or pre‐existing physical or mental condition, known coagulopathy, taking warfarin or clopidogrel within 2 weeks, body mass index > 40 |
| Interventions |
Treatment group: fascia iliaca compartment block with bupivacaine 0.25% Control group: morphine |
| Outcomes | Primary outcome measure: comparison between experimental and control groups of the difference in maximum tolerable passive hip flexion from supine (verbal pain score ≤ 3), expressed in degrees measured with a goniometer at baseline (on arrival after recruitment but before randomization) vs 45 minutes post intervention Secondary objective: investigation of duration of effect, ease of use, failure rates and morphine‐sparing effect of using fascia iliaca block in this participant group |
| Starting date | |
| Contact information | Nottingham University Hospitals NHS Trust |
| Notes |
EUCTR2008‐004303‐59‐SE.
| Trial name or title | Blocking the femoral nerve in patients with suspected hip fracture ‐ does it work in clinical practice? |
| Methods | Parallel RCT Double‐blind |
| Participants | Inclusion criteria: 132 participants with suspected hip fracture, ≥ 65 years old Exclusion criteria: more fractures incurred in the context of an accident, at home for more than 12 hours after the accident, hypersensitivity to local analgesics, infection, neurovascular problems, blockade not available within a specific period, patients for whom the attending physician considers that blockade could be harmful (e.g. atrioventricular block type II or III, elderly, severe liver disease, greatly reduced renal function) |
| Interventions |
Treatment group: femoral nerve block with ropivacaine Control group: placebo, subcutaneous injection |
| Outcomes | Primary objective: pain scores Secondary outcomes: complications (decubitus ulcers), rehabilitation time, analgesic use |
| Starting date | 24/10/2008 |
| Contact information | |
| Notes |
EUCTR2010‐023871‐25‐GB.
| Trial name or title | The FINOF (Femoral Nerve‐Block Intervention in Neck Of Femur Fracture) study ‐ FINOF |
| Methods | RCT Single‐blind |
| Participants | Inclusion criteria: ≥ 70 years of age, resident in own home or in warden‐aided flat, cognitively intact (as defined by a score ≥ 7 on the Abbreviated 10 point Mental Test Score (AMTS), prior fracture New Mobility Score ≥ 3, indicating independent indoor ambulation) Exclusion criteria: pre‐fracture hospitalization, contraindications to femoral nerve block analgesia, regular pre‐fracture opioid or glucocorticoid therapy, alcohol or substance abuse, morphine intolerance, postoperative surgical restrictions for ambulation |
| Interventions |
Treatment group: femoral nerve block with ropivacaine 0.2% Control group: standard care |
| Outcomes | Primary endpoint(s): from day 1 to day 3 postoperatively: cumulative ambulation score, cumulative dynamic pain score postoperatively Secondary objective: to estimate the cost‐effectiveness of femoral nerve blockade vs usual care, to examine issues of compliance, acceptability to staff and participants |
| Starting date | 20/04/2011 |
| Contact information | Nottingham University Hospitals NHS Trust, United Kingdom; no contact provided |
| Notes |
EUCTR2015‐000078‐36‐DK.
| Trial name or title | Analgesic effect of a supplemental nerve block in patients with hip fracture |
| Methods | Parallel RCT Double‐blind |
| Participants | Inclusion criteria: clinical suspicion of hip fracture, successful sensory effect of femoral nerve block, mentally capable of comprehending and using verbal pain score and distinguishing between pain from fractured hip and pain from other location, arrival in the emergency department at times when one of the doctors who do the nerve blocks for this investigation are on call, possible visualization of necessary structures with ultrasound, verbal pain score (0‐10) > 3 at rest or > 5 with passive leg raise 30 minutes after femoral nerve block, informed consent, ≥ 18 years of age Exclusion criteria: hip fracture not confirmed by x‐ray, weight < 45 kg, previously included in this trial If participant wishes to be excluded, allergy to local anaesthetics or adrenocortical hormone, visible infection in the area of the point of needle injection |
| Interventions |
Treatment group: femoral nerve block with bupivacaine 0.25% Control group: placebo |
| Outcomes | Primary outcome: frequency of sufficient analgesia 20 minutes after a supplemental obturator nerve block vs placebo Secondary outcomes: success rate, time to perform the block, onset time |
| Starting date | 17/03/2015 |
| Contact information | Department of Anaesthesia and Intensive Care, Aarhus University Hospital, Nørrebrogade 44, 8000 Aarhus, Denmark +4528782877 thomas.dahl.nielsen@clin.au.dk |
| Notes |
ISRCTN07083722.
| Trial name or title | The effect of the use of fascia iliaca nerve blockade on patient positioning for spinal anaesthesia and the effect of continuous nerve blockade on postoperative pain and mobility outcomes in patients with hip fractures |
| Methods | RCT Double‐blind Approved by the ethics committee: Health and Social Care Research Ethics Committee (HSC REC 1) (Northern Ireland) approved on 18th of April 2008 (ref: 08/NIR01/20) Funding: governmental: Belfast Health and Social Care Trust (UK) (ref: RGHT 000559) |
| Participants | Target number of participants 100 participants ‐ 40 in first part of study and 60 in second part of study Inclusion criteria: ASA physical status class I‐IV, able to give written informed consent, requiring operative repair of fractured neck of femur, aged 18 years and over, either sex Exclusion criteria: history of dementia or difficulty in obtaining consent, history of allergy to any of the medications used in the study |
| Interventions |
Treatment group: fascia iliaca block for positioning before spinal anaesthesia and continuous infusion after surgery Part 1: Participants randomized to receive fascia iliaca compartment block with 2 mg/kg 1% lignocaine or conventional sedation with 0.2 mg/kg IV ketamine and 0.025 mg/kg IV midazolam. At completion of surgery, a fascia iliaca block with 1 mg/kg 0.25% levobupivacaine will be performed in all participants Part 2: participants randomized to receive a preoperative fascia iliaca block with 1 mg/kg of 0.25% levobupivacaine or 2 mg/kg of 1% lignocaine. After administration of fascia iliaca block, a catheter will be inserted below the fascia iliaca and secured in place. Participants will be reviewed in the postoperative period and bolus doses of 0.125% levobupivacaine will be administered through the fascia iliaca block catheter if visual analogue scale is greater than 4. The catheter will be removed no longer than 24 hours after surgery Control group: no block |
| Outcomes | Primary outcome measures: ‐ Part 1: comparison of pain score at rest and positioning for spinal anaesthesia in participants who have received a fascia iliaca compartment block or conventional sedation Part 2: comparison between postoperative pain scores among participants receiving fascia iliaca blockade with lignocaine or levobupivacaine, and effects of bolus top‐up doses of low‐dose levobupivacaine on pain scores Secondary outcome measures: length of time to first request of additional analgesia, level of assistance required for transfer from sitting to standing position, incidence and severity of motor blockade, time to mobilization with walking aid, measurement of oxygen saturations without supplemental oxygen in both groups, incidence of all complications associated with analgesic techniques in both groups, incidence of nausea and/or vomiting within first 48 hours after surgery in both groups, use of blood products in all groups |
| Starting date | 01/07/2009 |
| Contact information | Ms Rosemary Hogg Department of Anaesthetics & Intensive Care Medicine, Queen's University Belfast, 2nd Floor, Mulhouse Building, Grosvenor Road Belfast, BT12 6BJ, United Kingdom |
| Notes | Completed Protocol/serial number: RGHT000559 http://www.belfasttrust.hscni.net |
ISRCTN46653818.
| Trial name or title | Femoral nerve blockade in hip fracture patients |
| Methods | RCT Approved by the ethics committee Funding: governmental: Umeå University (Sweden) |
| Participants | Target number of participants: 250 Inclusion criteria: both males and females, aged 70 years and above, all hip fracture patients admitted to the orthopaedic department Exclusion criteria: local infection, allergy to local anaesthesia, dying patients, pathologic hip fractures |
| Interventions |
Treatment group: Participants in the intervention group will receive a femoral nerve blockade as soon as they arrive. Participants with pain scores > 4 will be given morphine IV according to the standard protocol (morphine 10 mg/mL, 1 to 5 mg when necessary) Control group: regular use of opioids Both groups will receive 1 G of paracetamol 4 times/d. Postoperative pain treatment will be given according to the standard protocol in both arms of the study. Total follow‐up for both arms will end at the time of discharge |
| Outcomes | Primary outcome measures: postoperative delirium, assessed 3 times a day; postoperative complications, such as decubital ulcers, infections, thrombosis, heart failure and pain. A cognitive test will be assessed in the ambulance pre‐hospital arrival and at 24 +/‐ 6 hours postoperatively. At days three to five, a more thorough assessment will be done including delirium, depression, cognitive status, quality of life and more Secondary outcome measures: mortality, orthopaedic recovery recorded at the time of discharge from the hospital, EQ‐5D, economics |
| Starting date | 30/03/2009 |
| Contact information | Prof Ola Winso Operationscentrum Norrlands universitetssjukhus Umea SE‐901 85 Sweden |
| Notes | Completed http://www.umu.se/umu/index_eng.html |
ISRCTN75659782.
| Trial name or title | Intra‐ and post‐operative analgesia for patients undergoing surgery for hip fracture ‐ role of fascia iliaca compartment block |
| Methods | RCT Double‐blind (participants and caregivers) Funding: governmental: Department of Health, Richmond House, 79 Whitehall, London, SW1A 2NL, United Kingdom +44 (0)20 7307 2622; dhmail@doh.gsi.org.uk, http://www.dh.gov.uk/Home/fs/en |
| Participants | 40 adult participants of ASA I‐III admitted to Selly Oak Hospital with hip fracture and scheduled for fixation will be recruited after consent is obtained inclusion criteria: scheduled for hip fracture surgery Exclusion criteria: dementia/confusion, preoperative chest infection and/or poor respiratory function, temperature ≥ 38°C, white cell count > 11,000 mm3, respiratory rate > 25 per minute, auscultation and/or chest x‐ray evidence, SpO2 < 90% on air, congestive cardiac failure, bed‐bound or use of ≥ 2 aids for mobilization pre‐fracture, malignancy, coagulopathy, known or suspected allergy to ropivacaine and/or morphine, local infection at site where the block is to be performed, refusal of permission to approach general practitioner |
| Interventions |
Treatment group: fascia iliaca compartment block (n = 20) Control group: morphine (n = 20) Each participant will have a standard preoperative assessment, standard monitoring and recovery care and will be given assessments at 1, 3, 6 and 24 hours postop. Thereafter, data will be collected on a daily basis until 30 days postop/discharge/death, whichever is earlier. Our hospital already has a framework for data collection (Integrated Care Pathway). For our study, we will be using the same data |
| Outcomes | Primary outcome measures: total dose of morphine required during first 24 hours post‐op Secondary outcome measures: time to first dose of morphine postop, pain scores in recovery room and at 13, 16 and 24 hours after surgery, time to first appropriate response to verbal commands, time to discharge from recovery room, occurrence of nausea and vomiting in recovery and number of episodes during the first 24 hours postop, total dose of cyclizine required in the first 24 hours, need for granisetron during the first 24 hours, sedation scores at 1, 3, 6 and 24 hours, mental test scores at 1, 3, 6 and 24 hours, complications, rehabilitation outcomes, mortality |
| Starting date | 04/04/2006 |
| Contact information | Dr FA Levins, Anaesthetics, Selly Oak Hospital, Birmingham, B29 6JD, United Kingdom |
| Notes | DOI 10.1186 Protocol/serial number: N0265178818 Completed |
ISRCTN92946117.
| Trial name or title | The FINOF ‐ Femoral nerve‐block Intervention in Neck Of Femur fracture study Approved by Nottingham Research Ethics Committee 2, 28th January 2011, ref: 10/H0408/113 Funding: governmental; Funder name: National Institute of Health Research (NIHR) (UK) ‐ Research for Patient Benefit (RfPB) |
| Methods | RCT |
| Participants | 150 elderly patients admitted with an acute hip fracture Inclusion criteria: aged ≥ 70 years, resident in their own home or warden‐aided flat, cognitively intact (as defined by a score ≥ 7 on the Abbreviated 10 point Mental Test Score (AMTS), and prior fracture New Mobility Score ≥ 3 (indicating independent indoor ambulation) Exclusion criteria: prefracture hospitalization, contraindications to femoral nerve block analgesia, regular pre‐fracture opioid or glucocorticoid therapy, alcohol or substance abuse, morphine intolerance, postoperative surgical restrictions for ambulation |
| Interventions |
Treatment group: femoral nerve block followed by continuous infusion Control group: standard analgesic care |
| Outcomes | Drug‐related adverse events, earlier recovery, shorter length of stay in hospital and overall, improved quality of life for patients suffering with an acute hip fracture Primary outcome measures: cumulative dynamic pain score, cumulative ambulation score measured from day 1 to day 3 postoperatively Secondary outcome measures: cumulative dynamic pain score preoperatively (at 30 minutes, 60 minutes, 12 hours after initial femoral nerve block), cumulative side effects (nausea, vomiting, constipation, delirium) (from admission to day 3 postoperatively), cumulative calorific and protein intake (from admission to day 3 postoperatively), health‐related quality of life measured on the EUROQOL EQ‐5D, informing a cost‐effectiveness analysis, hospital length of stay, rehabilitation outcome measured by New Mobility Score, participant and staff experience (qualitative study) |
| Starting date | 02/01/2012 |
| Contact information | Dr Opinder Sahota, Queens Medical Centre, Derby Road, Nottingham, NG7 2UH, United Kingdom; opinder.sahota@nuh.nhs.uk |
| Notes | Completed: DOI 10.1186/ISRCTN92946117 EudraCT number: 2010‐023871‐25 http://public.ukcrn.org.uk/Search/StudyDetail.aspx?StudyID=10929 http://www.nuh.nhs.uk/ http://www.ncbi.nlm.nih.gov/pubmed/24885267
|
NCT00749489.
| Trial name or title | Improving pain and function in hip fracture |
| Methods | RCT Single‐blind (outcome assessor) |
| Participants | Inclusion criteria: adults (≥ 60 years) presenting to the MSMC ED from 8:00 to 20:00 with a radiographically confirmed hip fracture (femoral neck, intertrochanteric or pericapsular) Exclusion criteria: history of advanced dementia, presence of multiple trauma, pathological fractures, bilateral hip fractures, previous fracture or surgery at the currently fractured site, transferred from another hospital, cirrhosis or liver failure, able to self‐report pain intensity Other exclusion criterion: < age 60 because our focus is on treatment of pain in older adults |
| Interventions |
Treatment group: femoral nerve block Control group: no intervention |
| Outcomes | Primary outcome measures: pain (11‐point numerical evaluated 3 times daily for the duration of hospital stay (average stay is 4 days)) Secondary outcome measures: delirium (confusion assessment method) evaluated 3 times daily for duration of hospital stay |
| Starting date | November 2008 |
| Contact information | R. Sean Morrison, MD, and Knox Todd, MD, Beth Israel, New York, USA 10003 |
| Notes | This study has been completed |
NCT01052974.
| Trial name or title | Perioperative analgesia by femoral perineural catheter for femoral neck fracture ‐ Study KTcol |
| Methods | Parallel RCT Double‐blind (participants, investigator) |
| Participants | Inclusion criteria: adults (≥ 18 years) with femoral neck fracture Exclusion criterion: contraindication with analgesia |
| Interventions |
Treatment group: analgesic treatment with a femoral perineural catheter (inserted from hospital admission) with continuous infusion of ropivacaine Control group: placebo |
| Outcomes | Primary outcome measures: evaluation, in patients admitted in emergencies for suspicion of femoral neck fracture, the perioperative efficiency of an analgesic treatment provided with a femoral perineural catheter from hospital admission to 24 hours after surgical operation |
| Starting date | March 2009 |
| Contact information | Guillaume Bouhours UHAngers, Angers, France |
| Notes |
NCT01219088.
| Trial name or title | Postoperative pain control among intrathecal 0.1 mg morphine, femoral nerve block or periarticular infiltration of 20 mL of 0.25% bupivacaine in patients post intramedullary hip screw |
| Methods | Parallel RCT Single‐blind (outcome assessor) |
| Participants | Inclusion criteria: 18‐90 years old, good consciousness, well co‐operated, can use patient‐controlled analgesia machine, ASA physical status I‐III, no contraindication for spinal anaesthesia, accept spinal anaesthesia, body weight > 30 kg, body mass index 20‐35 kg/m2, no history of research drug allergy Exclusion criteria: previous history of hip surgery (the same side), pathological fracture, severe infection or bone cancer |
| Interventions |
Treatment group 1: femoral nerve block, spinal anaesthesia plus femoral nerve block with 20 mL of 0.25% bupivacaine Treatment group 2: periarticular bupivacaine infiltration, spinal anaesthesia plus periarticular infiltration with 20 mL of 0.25% bupivacaine Control group 1: spinal anaesthesia with 0.5% bupivacaine alone Control group 2: spinal anaesthesia plus 0.1 mg of intrathecal morphine |
| Outcomes | Primary outcome measures: amount of morphine consumed during first 24 hours after surgery Secondary outcome measures: efficacy of pain control, patient satisfaction, incidences of adverse events (nausea, vomiting, pruritus) |
| Starting date | September 2010 |
| Contact information | Thitima Chinachoti, MD (Madihol University), Faculty of Medicine Siniraj Hospital, Bangok, Thailand, 10700 |
| Notes |
NCT01547468.
| Trial name or title | Does femoral nerve catheterization reduce the incidence of post‐operative delirium in patients presenting for hip fracture repair? |
| Methods | Parallel RCT Open label |
| Participants | 270 adults (≥ 50 years) presenting to Oschner Main Campus with a hip fracture Exclusion criteria: head trauma as reported in the medical record and/or participant response, high‐impact fracture as reported in the medical record, aphasia as reported in the medical record and/or participant response, deafness, blindness as reported in the medical record and/or participant response, true allergy (not sensitivity or side effects) to local anaesthetics or opiates, pregnant, inability to complete study activities preoperatively |
| Interventions |
Treatment group: femoral nerve catheterization Control group: intravenous opioids |
| Outcomes | Primary outcome measure: number of participants developing delirium postoperatively during first 3 days after surgery Secondary outcome measures: hospital length of stay, pain score, consumption of pain medication for breakthrough pain relief |
| Starting date | March 2012 |
| Contact information | Leslie Thomas, MD, Oschner Clinic Foundation, New Orleans, Louisianna, USA 70121 |
| Notes | Recruiting |
NCT01593319.
| Trial name or title | Is regional anaesthesia of the hip preferable over traditional analgesia in the acute stage of the management of patients with a fracture of the hip |
| Methods | Parallel RCT Double‐blind (participants, caregivers, investigator, outcomes assessors) |
| Participants | Inclusion criteria: adults (≥ 65 years) with a hip fracture Exclusion criteria: multiple fractures, delay (> 12 hours) from the time of injury until admission to the hospital, local infection, hypersensitivity to local analgesics, cognitive impairment |
| Interventions |
Treatment group: local injection (fascia iliaca block) of 150 mg ropivacaine Control group: saline injection |
| Outcomes | Primary outcome measure: pain during first 3 hours after surgery Secondary outcome measures: medical complications (number of participants who develop pressure ulcers, number of participants who develop pneumonia): under hospitalization (expected average of 10 days) |
| Starting date | January 2012 |
| Contact information | Landstinget i Värmland, Othopedikinken, Centralsjukhuset I Karlsad, Karlsad, Vamiand, Sweden, S‐65185 |
| Notes | Terminated |
NCT01638845.
| Trial name or title | Hip fracture and perineural catheter |
| Methods | Parallel RCT Open label |
| Participants | Inclusion criteria: age ≥ 60 years, written informed consent obtained, ASA physical status I‐III, undergoing surgery for hip fracture, time < 24 hours after hip fracture Exclusion criteria: contraindication to regional anaesthesia (constitutional or acquired disorder of coagulation, sepsis, local infection of puncture area, history of vascular surgery, prosthetic femoral neuropathy scalable, allergy to local anaesthetics), weight < 40 kg, receiving anticoagulant drugs or antiplatelet therapy (other than aspirin and clopidogrel), contraindication for standardized anaesthetic technique in this study, contraindication for analgesics used postoperatively (respiratory failure, severe liver failure, brain injury associated with intracranial hypertension, uncontrolled epilepsy, simultaneous treatment with monoamine oxidase inhibitors, hypersensitivity to opioids), unable to give informed consent, adults under guardianship or curator, persons not affiliated with a health insurance plan, deprived of liberty |
| Interventions |
Treatment group: continuous perineural catheter Control group: no intervention |
| Outcomes | Primary outcome measure: number of participants with cardiovascular events during the preoperative period: 3 days |
| Starting date | June 2012 |
| Contact information | Vincent Compere, University Hospital, Rouen, France |
| Notes | Recruiting |
NCT01904071.
| Trial name or title | Ultrasound‐guided femoral (three‐in‐one) nerve block versus ultrasound guided fascia iliacus compartment block versus standard treatment for pain control in patients with hip fractures in the emergency department |
| Methods | Parallel RCT Open label |
| Participants | Inclusion criteria: English‐speaking patients; ≥ 18 years of age; radiographic evidence of hip fracture; awake, alert and oriented to time, place and person; pain score ≥ 5 on 10‐point scale Exclusion criteria: cognitive deficits, allergy to amide‐type local anaesthetic or morphine, more injuries than just hip fracture |
| Interventions |
Treatment group 1 : ultrasound‐guided 3‐in‐1 femoral nerve block Treatment group 2: ultrasound‐guided fascia iliaca compartment block Control group: IV morphine |
| Outcomes | Primary outcome measure: pain score at 30 minutes Secondary outcome measures: pain score at 60, 120, 240 and 480 minutes |
| Starting date | October 2008 |
| Contact information | Eitan Dickman, MD, Maiminides Medical Center, Brooklyn, New York, USA 11219 |
| Notes | Has results |
NCT02381717.
| Trial name or title | Comparison of ultrasound guided femoral nerve blockade and standard parenteral opioid pain management alone in patients with hip fracture in the emergency department |
| Methods | Parallel RCT Open label |
| Participants | Inclusion criteria: > 18 years of age presenting with radiologically established intracapsular, extracapsular hip fracture, able to consent and participate in the study, moderate to severe pain (numerical pain score ≥ 3) at time of enrolment Exclusion criteria: previous history of hypersensitivity to local anaesthetics, signs of local infection at the site of planned needle placement |
| Interventions |
Treatment group: ultrasound‐guided femoral nerve block with 0.5% bupivacaine (2 mg/kg) Control group: IV morphine |
| Outcomes | Primary outcome measure: pain intensity reduction at 4 hours after initiation of study procedure |
| Starting date | February 2015 |
| Contact information | Elena Skomorovsky, MD, Beth Israel Deaconess Medical Center |
| Notes | This study is not yet open for participant recruitment |
NCT02406300.
| Trial name or title | Contribution of anaesthesia technique for post‐operative mortality reduction after proximal femur fractures surgical treatment ‐ a randomized clinical trial |
| Methods | Parallel RCT Double‐blind (investigator, outcome assessor) |
| Participants | Inclusion criteria: adults (≥ 60 years) admitted with a diagnosis of proximal femur fracture (ICD‐9 codes 820.0 to 820.9) and submitted to surgical internal fixation of femur or hip prosthesis (ICD‐9 codes 7935, 8151 and 8152) Exclusion criteria: multiple fractures; polytrauma, active malignancy, ASA physical status V, antiplatelet drugs (other than aspirin) in previous 5 days, known allergies to local anaesthetics, contraindication to general or regional anaesthesia |
| Interventions |
Treatment group: femoral, lateral cutaneous nerve of the thigh and anterior obturator nerve blocks with ropivacaine and inhalational general anaesthesia with sevoflurane or desflurane Control group: spinal anaesthesia with bupivacaine |
| Outcomes | Primary outcome measures: survival rate up to 1 year after surgery Secondary outcome measures: incidence of postoperative delirium (up to 1 week postoperatively and measured with the 3D‐CAM Questionnaire (Confusion Assessment Method)), quality of life recovery (measured by quality of life assessment tools (SF12v2; EQ‐5D (EuroQol) at 30 days and 1 year after surgery) |
| Starting date | April 2015 |
| Contact information | Raul Carvalho, MD, MSc, Centro Hospitalar do Porto |
| Notes | Enrolling |
NCT02433548.
| Trial name or title | Fascia iliaca block in the emergency department for analgesia after femoral neck fracture |
| Methods | Parallel RCT Double‐blind (caregiver, investigator, outcome assessor) |
| Participants | Inclusion criteria: adults (≥ 18 years) with femoral neck fracture in the emergency department Exclusion criteria: presence of dementia, body weight < 40 kg, presence of cancer or receiving chemotherapy, allergy to local anaesthetics |
| Interventions |
Treatment group: fascia iliaca block (injection of 30 mL of bupivacaine 0.5% with epinephrine 5 mcg/mL below the fascia iliaca, Carbostesin®) Control group: sham injection = subcutaneous injection of 5 mL of normal saline |
| Outcomes | Primary outcome measures: pain scores at rest: 45 minutes after injection Secondary outcome measures: pain scores at rest at 60 minutes, 4 hours, 8 hours, 12 hours and 24 hours after injection; pain scores on movement:at 60 minutes, 4 hours, 8 hours, 12 hours and 24 hours after injection; morphine consumption at 60 minutes, 4 hours, 8 hours, 12 hours and 24 hours after injection and length of stay |
| Starting date | October 2014 |
| Contact information | Eric Albrecht, Program Director, Regional Aanaesthesia, CHU Vaudois, Switzerland |
| Notes | Recruiting |
AMTS: Abbreviated 10‐point Mental Test Score
ASA: American Society of Anaesthesiologists physical status
C: Celsius
CAM Questionnaire: Confusion Assessment Method
EQ‐5D or EUROQOL: score for measurement of health‐related quality of life
G: gram
ICD‐9: list of codes for International Statistical Classification of Diseases and Related Health Problems
IV: intravenous
kg: kilogram
kg/m2: kilogram per square metre
mm: millimetre
MSMC ED: Maimonides Medical Center emergency department
n: number
NHS: Nottingham University Hospitals
OMC: orientation‐memory‐concentration
RCT: randomized controlled trial
RfPB: Research for Patient Benefit
Contributions of authors
Joanne Guay: screened abstracts, search websites, checked reference lists for new articles, selected new articles, retrieved relevant articles, graded articles for risk of bias, extracted data, analysed data, interpreted results, rated quality of evidence and drafted the update.
Martyn Parker: reviewed the update for content before submission.
Richard Griifiiths: reviewed the update for content before submission.
Sandra Kopp: screened abstracts, selected new articles, graded articles for risk of bias, extracted data, interpreted results, rated quality of evidence and drafted the update.
Sources of support
Internal sources
-
University of Sherbrooke, Canada.
University of Sherbrooke granted access to electronic databases and to major medical journals.
-
University of Quebec in Abitibi‐Temiscamingue, Canada.
Universiuty of Quebec in Abitibi‐Temiscamingue provided access to electronic databases and medical journals.
-
Cochrane Anaesthesia, Critical and Emergency Care Group, Denmark.
The review authors wish to thank Karen Hovhannisyan, who designed the search strategy for this update.
External sources
No sources of support supplied
Declarations of interest
Joanne Guay: has no direct relationship with any pharmaceutical company or equipment manufacturer in the past five years. Has not acted as a witness expert in the past five years. Not an author of any of the included or excluded studies. Does not hold any stock other than mutual funds. Editor of a multi‐author textbook on anaesthesia (including notions on general and regional anaesthesia). Receives fees as associate professor for a course on airway management from University of Quebec in Abitibi‐Temiscamingue.
Martyn Parker: has received expenses and honorarium from several commercial companies and organizations for giving lectures on different aspects of hip fracture treatment. Has received royalties from BBrawn Ltd related to design and development of an implant used for internal fixation of intracapsular hip fractures.
Richard Griffiths: chaired Association of Anaesthetists of Great Britain & Ireland guidelines on proximal femoral fracture. Member of National Institute of Health and Care Excellence 124. Chaired Association of Anaesthetists of Great Britain & Ireland guidelines on surgery in the elderly. Founder of NHS Hip Fracture Perioperative Network.
Sandra Kopp: has no conflicts of interest.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Altermatt 2013 {published and unpublished data}
- Altermatt 2015 [pers comm]. Additional information for our trial. Email to J Guay 26 May 2015.
- Altermatt RF, Fuente RF, Echevarría GC, Baeza R, Ferrada M, Fuente N, et al. Evaluation of the effect of perioperative continuous lumbar plexus block upon the incidence of Ischaemic cardiovascular events in elderly patients with hip fracture. Regional Anesthesia and Pain Medicine. 2013; Vol. 38, issue 5 (Suppl 1):E182.
Antonopoulou 2006 {published data only}
- Antonopoulo E, Papaloannou K, Konstantinou G, Papadopoulos D, Karamoulas B, Molas TH. Continous femoral block in elderly patients with hip fractures. Regional Anesthesia and Pain Medicine. 2006; Vol. 31, issue 5 Suppl 1:92.
Beaudoin 2013 {published data only}
- Beaudoin FL, Haran JP, Liebmann O. A comparison of ultrasound‐guided three‐in‐one femoral nerve block versus parenteral opioids alone for analgesia in emergency department patients with hip fractures: a randomized controlled trial. Academic Emergency Medicine 2013;20(6):584‐91. [PUBMED: 23758305] [DOI] [PubMed] [Google Scholar]
Chudinov 1999 {published data only}
- Chudinov A, Berkenstadt H, Salai M, Cahana A, Perel A. Continuous psoas compartment block for anaesthesia and perioperative analgesia in patients with hip fractures. Regional Anesthesia and Pain Medicine 1999;24(6):563‐8. [PUBMED: 10588563] [DOI] [PubMed] [Google Scholar]
- Chudinov A, Berkenstadt H, Salai M, Cahana A, Perel A. Continuous psoas compartment block for anesthesia and perioperative analgesia in patients with hip fractures [abstract]. British Journal or Anaesthesia. 1999; Vol. 82, issue Supplement 1:111. [DOI] [PubMed]
Coad 1991 {published data only}
- Coad NR. Post‐operative analgesia following femoral‐neck surgery ‐ a comparison between 3 in 1 femoral nerve block and lateral cutaneous nerve block. European Journal of Anaesthesiology 1991;8(4):287‐90. [PUBMED: 1874226] [PubMed] [Google Scholar]
Cuvillon 2007 {published data only}
- Cuvillon P, Ripart J, Debureaux S, Boisson C, Veyrat E, Mahamat A, et al. Analgesia after hip fracture repair in elderly patients: the effect of a continuous femoral nerve block: a prospective and randomised study [Analgesie postoperatoire par catheter femoral apres fracture du col du femur chez la personne agee: etude prospective randomisee]. Annales Francaises d' Anesthesie et de Reanimation 2007;26(1):2‐9. [PUBMED: 17142005] [DOI] [PubMed] [Google Scholar]
De La Tabla 2010 {published and unpublished data}
- Tabla Gonzalez SRO, Angel Martinez ND, Mercedes Echevarra MS. Influence of perioperative pain treatment in postoperative morbidity in femur fractured patients over sixty‐five years old: a randomised study. Regional Anesthesia and Pain Medicine. 2010; Vol. 35, issue 5:469.
- Ortiz de la Tabla González 2015 [pers comm]. Additional information for our trial. Email to J Guay 5 July 2015.
Diakomi 2014 {published data only}
- Diakomi M, Gkliatis E, Papaioannou M, Polymenopoulou E, Papadopoulos P, Makris A. Fascia iliaca compartment block for positioning hip fracture patients for spinal anaesthesia: a randomised trial. Regional Anesthesia and Pain Medicine. 2014; Vol. 39, issue 5 (Suppl):ESRA1‐0229. [DOI] [PubMed]
- Diakomi M, Papaioannou M, Mela A, Kouskouni E, Makris A. Preoperative fascia iliaca compartment block for positioning patients with hip fractures for central nervous blockade: a randomised trial. Regional Anesthesia and Pain Medicine 2014;39(5):394‐8. [PUBMED: 25068412] [DOI] [PubMed] [Google Scholar]
Domac 2015 {published data only}
- Domac A, Kelsaka, Sarihasan B. The effect of preoperative fascia iliaca compartment block on postoperative analgesic use in patients with femoral fracture [Femur kiriti olan hastalarda preoperatif uygulanan fasia iliaka kompartman blotunun postoperatif analjezik tuketimine etkisi]. Anestezi Dergisi 2015;23(3):144‐51. [EMBASE: AN: 2015379598 ] [Google Scholar]
Fletcher 2003 {published data only}
- Fletcher AK, Rigby AS, Heyes FL. Three‐in‐one femoral nerve block as analgesia for fractured neck of femur in the emergency department: a randomized, controlled trial. Annals of Emergency Medicine 2003;41(2):227‐33. [PUBMED: 12548273] [DOI] [PubMed] [Google Scholar]
Foss 2007 {published data only}
- Foss NB, Kristensen BB, Bundgaard M, Bak M, Heiring C, Virkelyst C, et al. Fascia iliaca compartment blockade for acute pain control in hip fracture patients: a randomized, placebo‐controlled trial. Anesthesiology 2007;106(4):773‐8. [PUBMED: 17413915] [DOI] [PubMed] [Google Scholar]
Gille 2006 {published and unpublished data}
- Gille 2006 [pers comm]. Additional information for our trial. Email to M Parker 2006.
- Gille J, Gille M, Gahr R, Wiedemann B. Acute pain management in proximal femoral fractures. Femoral nerve block (catheter technique) vs. systemic pain therapy using a clinic internal organisation model [Akutschmerztherapie bei patienten mit huftgelenknahen frakturen. N.‐femoralis‐katheter‐analgesie vs. systemische schmerztherapie unter anwendung eines klinikinternen organisationsmodells]. Der Anaesthesist 2006;55(4):414‐22. [PUBMED: 16320011] [DOI] [PubMed] [Google Scholar]
Godoy 2010 {published data only}
- Godoy Monzon D, Vazquez J, Jauregui JR, Iserson KV. Pain treatment in post‐traumatic hip fracture in the elderly: regional block vs. systemic non‐steroidal analgesics. International Journal of Emergency Medicine 2010;3(4):321‐5. [PUBMED: 21373300] [DOI] [PMC free article] [PubMed] [Google Scholar]
Graham 2008 {published data only}
- Graham CA, Baird K, McGuffie AC. A pilot randomised clinical trial of 3‐in‐1 femoral nerve block and intravenous morphine as primary analgesia for patients presenting to the emergency department with fractured hip. Hong Kong Journal of Emergency Medicine 2008;15(4):205‐11. [Google Scholar]
Haddad 1995 {published data only}
- Haddad FS, Williams RL. Femoral nerve block in extracapsular femoral neck fractures. Journal of Bone and Joint Surgery ‐ British Volume 1995;77(6):922‐3. [PUBMED: 7593107] [PubMed] [Google Scholar]
Hood 1991 {published data only}
- Hood G, Edbrooke DL, Gerrish SP. Postoperative analgesia after triple nerve block for fractured neck of femur. Anaesthesia 1991;46(2):138‐40. [PUBMED: 1872429] [DOI] [PubMed] [Google Scholar]
Iamaroon 2010 {published data only}
- Iamaroon A, Raksakietisak M, Halilamien P, Hongsawad J, Boonsararuxsapong K. Femoral nerve block versus fentanyl: analgesia for positioning patients with fractured femur. Local and Regional Anesthesia 2010;3:21‐6. [PUBMED: 22915864] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jadon 2014 {published and unpublished data}
- Jadon 2015 [pers comm]. Additional information for our trial. Email to J Guay 22 March 2016.
- Jadon A, Kedia SK, Dixit S, Chakraborty S. Comparative evaluation of femoral nerve block and intravenous fentanyl for positioning during spinal anaesthesia in surgery of femur fracture. Indian Journal of Anaesthesia 2014;58(6):705‐8. [PUBMED: 25624533] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jones 1985 {published data only}
- Jones SF, White A. Analgesia following femoral neck surgery. Lateral cutaneous nerve block as an alternative to narcotics in the elderly. Anaesthesia 1985;40(7):682‐5. [PUBMED: 3896019] [DOI] [PubMed] [Google Scholar]
Kullenberg 2004 {published data only}
- Kullenberg B, Ysberg B, Heilman M, Resch S. Femoral nerve block as pain relief in hip fracture. A good alternative in perioperative treatment proved by a prospective study [Femoralnervsblockad som smärtlindring vid höftfraktur. Bra alternativ i perioperativ behandlingsarsenal visar prospektiv studie. [Swedish]]. Lakartidningen 2004;101(24):2104‐7. [PUBMED: 15282985] [PubMed] [Google Scholar]
Luger 2012 {published data only}
- Luger TJ, Kammerlander C, Benz M, Luger MF, Garoscio I. Peridural anesthesia or ultrasound‐guided continuous 3‐in‐1 block: which is indicated for analgesia in very elderly patients with hip fracture in the emergency department?. Geriatric Orthopaedic Surgery & Rehabilitation 2012;3(3):121‐8. [PUBMED: 23569705] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mossafa 2005 {published data only}
- Mosaffa F, Esmaelijah A, Khoshnevis H. Analgesia before performing a spinal block in the lateral decubitus position in patients with a femoral neck fracture: a comparison between fascia iliaca block and IV fentanyl. Regional Anesthesia and Pain Medicine. 2005; Vol. 30, issue Suppl 1:61.
Mouzopoulos 2009 {published data only}
- Mouzopoulos G, Vasiliadis G, Lasanianos N, Nikolaras G, Morakis E, Kaminaris M. Fascia iliaca block prophylaxis for hip fracture patients at risk for delirium: a randomized placebo‐controlled study. Journal of Orthopaedics and Traumatology 2009;10(3):127‐33. [PUBMED: 19690943] [DOI] [PMC free article] [PubMed] [Google Scholar]
Murgue 2006 {published data only}
- Murgue D, Ehret B, Massacrier‐Imbert S, Durand O, Gibaud F, Maakel A, et al. Equimolar nitrous oxide/oxygen combined with femoral nerve block for emergency analgesia of femoral neck fractures. [French] [Utilisation du melange equimolaire de protoxyde d'azote/oxygene et du bloc femoral pour la prise en charge antalgique des fractures du col du femur dans un service d'urgences]. Journal Europeen Des Urgences (Jeur) 2006;19(1):9‐14. [Google Scholar]
Nie 2015 {published data only}
- Nie H. Information on our trial [personal communication]. Email to: J Guay 5 September 2016.
- Nie H, Yang YX, Wang Y, Liu Y, Zhao B, Luan B. Effects of continuous fascia iliaca compartment blocks for postoperative analgesia in hip fracture patients. Pain Research & Management 2015;20(4):210‐2. [PUBMED: 26125194] [DOI] [PMC free article] [PubMed] [Google Scholar]
Segado Jimenez 2009 {published data only}
- Segado Jimenez MI, Bayon Gago M, Arias Delgado J, Casas Garcia ML, Dominguez Hervella F, Lopez Perez A, et al. [Efficacy of obturator and femoral cutaneous nerve blocks for postoperative analgesia in hip surgery] [Eficacia del bloqueo de los nervios obturador y femorocutaneo para analgesia postoperatoria en cirugia de cadera]. Revista Espanola de Anestesiologia y Reanimacion 2009;56(10):590‐7. [PUBMED: 20151520] [DOI] [PubMed] [Google Scholar]
Spansberg 1996 {published data only}
- Spansberg NL, Anker‐Moller E, Dahl JB, Schultz P, Christensen EF. The value of continuous blockade of the lumbar plexus as an adjunct to acetylsalicyclic acid for pain relief after surgery for femoral neck fractures. European Journal of Anaesthesiology 1996;13(4):410‐2. [PUBMED: 8842667] [DOI] [PubMed] [Google Scholar]
Szucs 2012 {published data only}
- Szucs S, Iohom G. Functional recovery following operative fixation of fractured neck of femur in the elderly. Regional Anesthesia and Pain Medicine. 2010; Vol. 35, issue 5:Abstract 534.
- Szucs S, Iohom G, O'Donnell B, Sajgalik P, Ahmad I, Salah N, et al. Analgesic efficacy of continuous femoral nerve block commenced prior to operative fixation of fractured neck of femur. Perioperative Medicine (London, England) 2012;1:4. [PUBMED: 24764520] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tuncer 2003 {published data only}
- Tuncer S, Sert OA, Yosunkaya A, Mutlu M, Celik J, Okesli S. Patient‐controlled femoral nerve analgesia versus patient‐controlled intravenous analgesia for postoperative analgesia after trochanteric fracture repair. Acute Pain 2003;4(3‐4):105‐8. [Google Scholar]
White 1980 {published data only}
- White IWC, Chappell WA. Anaesthesia for surgical correction of fractured femoral neck. A comparison of three techniques. Anaesthesia 1980;35(11):1107‐10. [PUBMED: 7446914] [DOI] [PubMed] [Google Scholar]
Yun 2009 {published data only}
- Yun MJ, Kim YH, Han MK, Kim JH, Hwang JW, Do SH. Analgesia before a spinal block for femoral neck fracture: fascia iliaca compartment block. Acta Anaesthesiologica Scandinavica 2009;53(10):1282‐7. [PUBMED: 19650803] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bech 2011 {published data only}
- Bech RD, Lauritsen J, Ovesen O, Emmeluth C, Lindholm P, Overgaard S. Local anaesthetic wound infiltration after internal fixation of femoral neck fractures: a randomized, double‐blind clinical trial in 33 patients. Hip International : the Journal of Clinical and Experimental Research on Hip Pathology and Therapy 2011;21(2):251‐9. [PUBMED: 21484739] [DOI] [PubMed] [Google Scholar]
Bölükbasi 2013 {published and unpublished data}
- Bolukbasi GE, Turhan CK, Can SO, Ozcelik M, Okten F. Comparison of the effects of ultrasound‐guided fascia iliaca compartment block and remifentanil infusion on positional pain in patients undergoing hip fracture surgery under spinal anaesthesia. Regional Anesthesia and Pain Medicine 2013;38(5 Suppl 1):E182. [CENTRAL: 01061852] [Google Scholar]
- Turhan 2015 [pers comm]. Additional information for our trial. Email to J Guay 25 May 2015.
Durrani 2013 {published data only}
- Durrani HD, Butt KJ, Khosa AH, Umer A, Pervaiz M. Pain relief during positioning for spinal anaesthesia in patients with femoral fracture: a comparison between femoral nerve block and intravenous nalbuphine. Pakistan Journal of Medical and Health Sciences 2013;7(4):928‐32. [CENTRAL: 00977951] [Google Scholar]
Foss 2005 {published data only}
- Foss NB, Kristensen MT, Kristensen BB, Jensen PS, Kehlet H. Effect of postoperative epidural analgesia on rehabilitation and pain after hip fracture surgery: a randomized, double‐blind, placebo‐controlled trial. Anesthesiology 2005;102(6):1197‐204. [PUBMED: 15915033] [DOI] [PubMed] [Google Scholar]
Fujihara 2013 {published data only}
- Fujihara Y, Fukunishi S, Nishio S, Miura J, Koyanagi S, Yoshiya S. Fascia iliaca compartment block: its efficacy in pain control for patients with proximal femoral fracture. Journal of Orthopaedic Science 2013;18(5):793‐7. [PUBMED: 23744530] [DOI] [PubMed] [Google Scholar]
Ghimire 2015 {published data only}
- Ghimire A, Bhattarai B, Koirala S, Subedi A. Analgesia before performing subarachnoid block in the sitting position in patients with proximal femoral fracture: a comparison between fascia iliaca block and femoral nerve block. Kathmandu University Medical Journal (KUMJ) 2015;13(50):152‐5. [PUBMED: 26643833] [DOI] [PubMed] [Google Scholar]
Gorodetskyi 2007 {published data only}
- Gorodetskyi IG, Gorodnichenko AI, Tursin PS, Reshetnyak VK, Uskov ON. Non‐invasive interactive neurostimulation in the post‐operative recovery of patients with a trochanteric fracture of the femur. a randomised, controlled trial. Journal of Bone and Joint Surgery ‐ British Volume 2007;89(11):1488‐94. [PUBMED: 17998187] [DOI] [PubMed] [Google Scholar]
Hussain 2014 {published data only}
- Hussain R, Nazeer T, Asim A. Unilateral fascia iliaca block (FIB) for post‐operative analgesia in fracture neck of femur surgery; comparison with standard post‐operative analgesia. Pakistan Journal of Medical and Health Sciences 2014;8(4):818‐20. [Google Scholar]
Hwang 2015 {published data only}
- Dickman E, Pushkar I, Likourezos A, Todd K, Hwang U, Akhter S, Morrison S. Ultrasound‐guided nerve blocks for intracapsular and extracapsular hip fractures. Academic Emergency Medicine. 2015; Vol. 22 5 Suppl 1:S133. [CENTRAL: CN‐01080180] [DOI] [PMC free article] [PubMed]
- Dickman E, Pushkar I, Likourezos A, Todd K, Hwang U, Akhter S, Morrison S. Ultrasound‐guided nerve blocks for intracapsular and extracapsular hip fractures. The American Journal of Emergency Medicine 2016;34(3):586‐9. [PUBMED: 26809928] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson K, Akhtar S, Sandoval M, Siddiqui S, Todd K, Wirtner A. Femoral nerve block for pain management of hip fractures in the emergency department: preliminary results of a randomised,controlled trial. Annals of Emergency Medicine 2008;52(4):S164. [Google Scholar]
- Hwang U, Todd K, Dickman E, Akhtar S, Morrison S. Emergency department femoral nerve blocks for acute hip fracture pain: a randomised controlled trial. Journal of the American Geriatrics Society 2015;63(Suppl S1):S103. [Google Scholar]
- Todd K, Dickman E, Hwang U, Akhtar S, Morrison R. Emergency department femoral nerve blocks for acute hip fracture pain: a randomised controlled trial. The Journal of Pain 2015;16(4):S68. [Google Scholar]
Irwin 2012 {published data only}
- Irwin M, Mahalingam S, Mills G, Krahe L. Acute pain management for neck of femur fractures: a comparison of the fascia iliaca block versus traditional opiates. British Journal of Anaesthesia 2012;108(Suppl 2):ii422. [Google Scholar]
Kang 2013 {published data only}
- Kang H, Ha YC, Kim JY, Woo YC, Lee JS, Jang EC. Effectiveness of multimodal pain management after bipolar hemiarthroplasty for hip fracture: a randomized, controlled study. The Journal of Bone and Joint Surgery ‐ American volume 2013;95(4):291‐6. [PUBMED: 23302898] [DOI] [PubMed] [Google Scholar]
Kumie 2015 {published data only}
- Kumie FT, Gebremedhn EG, Tawuye HY. Efficacy of fascia iliaca compartment nerve block as part of multimodal analgesia after surgery for femoral bone fracture. World Journal of Emergency Medicine 2015;6(2):142‐6. [PUBMED: 26056546] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mannion 2005 {published data only}
- Mannion S, Hayes I, Loughnane F, Murphy DB, Shorten GD. Intravenous but not perineural clonidine prolongs postoperative analgesia after psoas compartment block with 0.5% levobupivacaine for hip fracture surgery. Anesthesia and Analgesia 2005;100(3):873‐8. [PUBMED: 15728081] [DOI] [PubMed] [Google Scholar]
Manohara 2015 {published data only}
- Manohara S, Lim YC. A randomized, controlled trial comparing analgesic effects of ultrasound‐guided supra‐inguinal fascia iliaca block with femoral nerve block for surgical fixation of hip fractures. Regional Anesthesia and Pain Medicine. 2015; Vol. 40 (5 Suppl 1):e93‐4. [EMBASE: AN: 72027148 ]
Marhofer 1998 {published data only}
- Marhofer P, Schrogendorfer K, Wallner T, Koinig H, Mayer N, Kapral S. Ultrasonographic guidance reduces the amount of local anesthetic for 3‐in‐1 blocks. Regional Anesthesia and Pain Medicine 1998;23(6):584‐8. [PUBMED: 9840855 ] [DOI] [PubMed] [Google Scholar]
Matot 2003 {published data only}
- Matot I, Oppenheim‐Eden A, Ratrot R, Baranova J, Davidson E, Eylon S, et al. Preoperative cardiac events in elderly patients with hip fracture randomized to epidural or conventional analgesia. Anesthesiology 2003;98(1):156‐63. [PUBMED: 12502992] [DOI] [PubMed] [Google Scholar]
McRae 2015 {published data only}
- McRae P, Bendall JC, Madigan V, Middleton P. Paramedic performed fascia iliaca compartment block (FICB) for femoral fractures: a randomised controlled trial. Australasian Journal of Prehospital Care 2013;10(3):14‐5. [Google Scholar]
- McRae PJ, Bendall JC, Madigan V, Middleton PM. Paramedic‐performed fascia iliaca compartment block for femoral fractures: a controlled trial. The Journal of Emergency Medicine 2015;48(5):581‐9. [PUBMED: 25661312] [DOI] [PubMed] [Google Scholar]
Mutty 2007 {published data only}
- Mutty CE, Jensen EJ, Manka MA Jr, Anders MJ, Bone LB. Femoral nerve block for diaphyseal and distal femoral fractures in the emergency department. Journal of Bone and Joint Surgery ‐ American Volume 2007;89(12):2599‐603. [PUBMED: 18056490] [DOI] [PubMed] [Google Scholar]
Piangatelli 2004 {published data only}
- Piangatelli C, Angelis C, Pecora L, Recanatini F, Testasecca D. Levobupivacaine versus ropivacaine in psoas compartment block and sciatic nerve block in orthopedic surgery of the lower extremity. Minerva Anestesiologica 2004;70(12):801‐7. [PUBMED: 15702061] [PubMed] [Google Scholar]
Reavley 2015 {published data only}
- Reavley P, Montgomery AA, Smith JE, Binks S, Edwards J, Elder G, et al. Randomised trial of the fascia iliaca block versus the '3‐in‐1' block for femoral neck fractures in the emergency department. Emergency Medicine Journal 2015;32(9):685‐9. [PUBMED: 25430915] [DOI] [PubMed] [Google Scholar]
Scheinin 2000 {published and unpublished data}
- Scheinin 2015 [pers comm]. Additional information for our trial. Email to: J Guay 25 May 2015.
- Scheinin H, Virtanen T, Kentala E, Uotila P, Laitio T, Hartiala J, et al. Epidural infusion of bupivacaine and fentanyl reduces perioperative myocardial ischaemia in elderly patients with hip fracture ‐ a randomized controlled trial. Acta Anaesthesiologica Scandinavica 2000;44(9):1061‐70. [PUBMED: 11028724] [DOI] [PubMed] [Google Scholar]
Schiferer 2007 {published data only}
- Schiferer A, Gore C, Gorove L, Lang T, Steinlechner B, Zimpfer M, et al. A randomized controlled trial of femoral nerve blockade administered preclinically for pain relief in femoral trauma. Anesthesia and Analgesia 2007;105(6):1852‐4. [PUBMED: 18042893] [DOI] [PubMed] [Google Scholar]
Segado Jimenez 2010 {published data only}
- Segado Jimenez I. Information on our trial [personal communication]. Email to: J Guay 24 April 2016.
- Segado Jimenez MI, Arias Delgado J, Casas Garcıa ML, Domınguez Hervella F, Lopez Perez A, Bayon Gago M, et al. Post‐surgical analgesia in hip surgery: a comparison of three techniques [Abordaje de la analgesia postoperatoria en cirugıa de cadera: comparativa de 3 tecnicas]. Revista Sociedad Espanola del Dolor 2010;17(6):259‐67. [Google Scholar]
Sia 2004 {published data only}
- Sia S, Pelusio F, Barbagli R, Rivituso C. Analgesia before performing a spinal block in the sitting position in patients with femoral shaft fracture: a comparison between femoral nerve block and intravenous fentanyl. Anesthesia and Analgesia 2004;99(4):1221‐4, table of contents. [PUBMED: 15385380] [DOI] [PubMed] [Google Scholar]
Turker 2003 {published data only}
- Turker G, Uckunkaya N, Yavascaoglu B, Yilmazlar A, Ozcelik S. Comparison of the catheter‐technique psoas compartment block and the epidural block for analgesia in partial hip replacement surgery. Acta Anaesthesiologica Scandinavica 2003;47(1):30‐6. [PUBMED: 12492794] [DOI] [PubMed] [Google Scholar]
Van Leeuwen 2000 {published data only}
- Leeuwen FL, Bronselaer K, Gilles M, Sabbe MB, Delooz HH. The 'three in one' block as locoregional analgesia in an emergency department. European Journal of Emergency Medicine 2000;7(1):35‐8. [PUBMED: 10839377] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12609000526279 {published data only}
- Ultrasound‐guided femoral nerve block using 1% ropivacaine as a method of pain control in patients who present to emergency with a fractured hip. Ongoing study 04/04/2009.
EUCTR2006‐004001‐26‐GB {published data only}
- A randomized controlled trial of fascia iliaca compartment block versus morphine for pain in fractured neck of femur in the emergency department: a pilot study ‐ fascia Iliaca compartment block versus parenteral morphine sulphate. Ongoing study Starting date of trial not provided. Contact author for more information.
EUCTR2008‐004303‐59‐SE {published data only}
- Blocking the femoral nerve in patients with suspected hip fracture ‐ does it work in clinical practice?. Ongoing study 24/10/2008.
EUCTR2010‐023871‐25‐GB {published data only}
- The FINOF (Femoral Nerve‐Block Intervention in Neck Of Femur Fracture) study ‐ FINOF. Ongoing study 20/04/2011.
EUCTR2015‐000078‐36‐DK {published data only}
- Analgesic effect of a supplemental nerve block in patients with hip fracture. Ongoing study 17/03/2015.
ISRCTN07083722 {published data only}
- The effect of the use of fascia iliaca nerve blockade on patient positioning for spinal anaesthesia and the effect of continuous nerve blockade on postoperative pain and mobility outcomes in patients with hip fractures. Ongoing study 01/07/2009.
ISRCTN46653818 {published data only}
- Femoral nerve blockade in hip fracture patients. Ongoing study 30/03/2009.
ISRCTN75659782 {published data only}
- Intra‐ and post‐operative analgesia for patients undergoing surgery for hip fracture ‐ role of fascia iliaca compartment block. Ongoing study 04/04/2006.
ISRCTN92946117 {published data only}
- Sahota O, Rowlands M, Bradley J, Walt G, Bedforth N, Armstrong S, et al. Femoral nerve block Intervention in Neck of Femur fracture (FINOF): study protocol for a randomized controlled trial. Trials 2014;15(May 24):189. [PUBMED: 24885267] [DOI] [PMC free article] [PubMed] [Google Scholar]
NCT00749489 {published data only}
- Improving pain and function in hip fracture. Ongoing study November 2008.
NCT01052974 {published data only}
- Perioperative analgesia by femoral perineural catheter for femoral neck fracture ‐ Study KTcol. Ongoing study March 2009.
NCT01219088 {published data only}
- Postoperative pain control among intrathecal 0.1 mg morphine, femoral nerve block or periarticular infiltration of 20 mL of 0.25% bupivacaine in patients post intramedullary hip screw. Ongoing study September 2010.
NCT01547468 {published data only}
- Does femoral nerve catheterization reduce the incidence of post‐operative delirium in patients presenting for hip fracture repair?. Ongoing study March 2012.
NCT01593319 {published data only}
- Is regional anaesthesia of the hip preferable over traditional analgesia in the acute stage of the management of patients with a fracture of the hip. Ongoing study January 2012.
NCT01638845 {published data only}
- Hip fracture and perineural catheter. Ongoing study June 2012.
NCT01904071 {published data only}
- Ultrasound‐guided femoral (three‐in‐one) nerve block versus ultrasound guided fascia iliacus compartment block versus standard treatment for pain control in patients with hip fractures in the emergency department. Ongoing study October 2008.
NCT02381717 {published data only}
- Comparison of ultrasound guided femoral nerve blockade and standard parenteral opioid pain management alone in patients with hip fracture in the emergency department. Ongoing study February 2015.
NCT02406300 {published data only}
- Contribution of anaesthesia technique for post‐operative mortality reduction after proximal femur fractures surgical treatment ‐ a randomized clinical trial. Ongoing study April 2015.
NCT02433548 {published data only}
- Fascia iliaca block in the emergency department for analgesia after femoral neck fracture. Ongoing study October 2014.
Additional references
Auroy 2002
- Auroy Y, Benhamou D, Bargues L, Ecoffey C, Falissard B, Mercier FJ, et al. Major complications of regional anesthesia in France: the SOS Regional Anesthesia Hotline Service. Anesthesiology 2002;97(5):1274‐80. [PUBMED: 12411815] [DOI] [PubMed] [Google Scholar]
Berde 2009
- Berde CB, Strichartz GR. Local anesthetics (Chapter 30). In: Miller RD, Eriksson LI, Fleisher LA, Wiener‐Kronish JP, Young WL editor(s). Miller's Anesthesia. 7th Edition. London, UK: Churchill Livingstone, 2009. [Google Scholar]
Brauer 2009
- Brauer CA, Coca‐Perraillon M, Cutler DM, Rosen AB. Incidence and mortality of hip fractures in the United States. JAMA 2009;302(14):1573‐9. [PUBMED: 19826027] [DOI] [PMC free article] [PubMed] [Google Scholar]
Brull 2007
- Brull R, McCartney CJ, Chan VW, El‐Beheiry H. Neurological complications after regional anesthesia: contemporary estimates of risk. Anesthesia and Analgesia 2007;104(4):965‐74. [PUBMED: 17377115] [DOI] [PubMed] [Google Scholar]
Cates 2002
- Cates CJ. Simpson's paradox and calculation of number needed to treat from meta‐analysis. BMC Medical Research Methodology 2002;2:1. [PUBMED: 11860604] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cordero 2016
- Cordero J, Maldonado A, Iborra S. Surgical delay as a risk factor for wound infection after a hip fracture. Injury 2016;47 Suppl 3:S56‐60. [PUBMED: 27692108] [DOI] [PubMed] [Google Scholar]
Cuvillon 2009
- Cuvillon P, Nouvellon E, Ripart J, Boyer JC, Dehour L, Mahamat A, et al. A comparison of the pharmacodynamics and pharmacokinetics of bupivacaine, ropivacaine (with epinephrine) and their equal volume mixtures with lidocaine used for femoral and sciatic nerve blocks: a double‐blind randomized study. Anesthesia and Analgesia 2009;108(2):641‐9. [PUBMED: 19151302] [DOI] [PubMed] [Google Scholar]
Deeks 2002
- Deeks JJ. Issues in the selection of a summary statistic for meta‐analysis of clinical trials with binary outcomes. Statistics in Medicine 2002;21(11):1575‐600. [PUBMED: 12111921] [DOI] [PubMed] [Google Scholar]
Dochez 2014
- Dochez E, Geffen GJ, Bruhn J, Hoogerwerf N, Pas H, Scheffer G. Prehospital administered fascia iliaca compartment block by emergency medical service nurses, a feasibility study. Scandinavian Journal of Trauma, Resuscitation and Emergency Medicine 2014;22:38. [PUBMED: 24957807] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gozlan 2005
- Gozlan C, Minville V, Asehnoune K, Raynal P, Zetlaoui P, Benhamou D. [Fascia iliaca block for femoral bone fractures in prehospital medicine] [Bloc iliofascial en medecine prehospitaliere pour les fractures du femur.]. Annales francaises d'anesthesie et de reanimation 2005;24(6):617‐20. [PUBMED: 15885976] [DOI] [PubMed] [Google Scholar]
Guay 2006
- Guay J. The benefits of adding epidural analgesia to general anaesthesia: a meta‐analysis. Journal of Anaesthesia 2006;20(4):335‐40. [PUBMED: 17072704] [DOI] [PubMed] [Google Scholar]
Guay 2016
- Guay J, Parker MJ, Gajendragadkar PR, Kopp S. Anaesthesia for hip fracture surgery in adults. The Cochrane Database of Systematic Reviews 2016;2:CD000521. [PUBMED: 26899415] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ. What is "quality of evidence" and why is it important to clinicians?. BMJ (Clinical research ed.) 2008;336(7651):995‐8. [PUBMED: 18456631] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction ‐ GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [PUBMED: 21195583] [DOI] [PubMed] [Google Scholar]
Guyatt 2011a
- Guyatt GH, Oxman AD, Kunz R, Woodcock J, Brozek J, Helfand M, et al. GRADE guidelines: 8. Rating the quality of evidence ‐ indirectness. Journal of Clinical Epidemiology 2011;64(12):1303‐10. [PUBMED: 21802903] [DOI] [PubMed] [Google Scholar]
Guyatt 2011b
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence ‐ imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [PUBMED: 21839614] [DOI] [PubMed] [Google Scholar]
Guyatt 2011c
- Guyatt GH, Oxman AD, Sultan S, Glasziou P, Akl EA, Alonso‐Coello P, et al. GRADE guidelines: 9. Rating up the quality of evidence. Journal of Clinical Epidemiology 2011;64(12):1311‐6. [PUBMED: 21802902] [DOI] [PubMed] [Google Scholar]
Haentjens 2010
- Haentjens P, Magaziner J, Colon‐Emeric CS, Vanderschueren D, Milisen K, Velkeniers B, et al. Meta‐analysis: excess mortality after hip fracture among older women and men. Annals of Internal Medicine 2010;152(6):380‐90. [PUBMED: 20231569] [DOI] [PMC free article] [PubMed] [Google Scholar]
Haslam 2013
- Haslam L, Lansdown A, Lee J, Vyver M. Survey of current practices: peripheral nerve block utilization by ED physicians for treatment of pain in the hip fracture patient population. Canadian Geriatrics Journal 2013;16(1):16‐21. [PUBMED: 23440013] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ (Clinical research ed.) 2003;327(7414):557‐60. [PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbookfor Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration 2011:www.cochrane‐handbook.org.
Johnson 2013
- Johnson RL, Kopp SL, Hebl JR, Erwin PJ, Mantilla CB. Falls and major orthopaedic surgery with peripheral nerve blockade: a systematic review and meta‐analysis. British Journal of Anaesthesia 2013;110(4):518‐28. [PUBMED: 23440367] [DOI] [PMC free article] [PubMed] [Google Scholar]
Johnson 2014
- Johnson RL, Duncan CM, Ahn KS, Schroeder DR, Horlocker TT, Kopp SL. Fall‐prevention strategies and patient characteristics that impact fall rates after total knee arthroplasty. Anesthesia and Analgesia 2014;119(5):1113‐8. [PUBMED: 25211392] [DOI] [PubMed] [Google Scholar]
Johnston 2016
- Johnston DF, Stafford M, McKinney M, Deyermond R, Dane K. Peripheral nerve blocks with sedation using propofol and alfentanil target‐controlled infusion for hip fracture surgery: a review of 6 years in use. Journal of Clinical Anesthesia 2016;29:33‐9. [PUBMED: 26897445] [DOI] [PubMed] [Google Scholar]
Lee 2015
- Lee LA, Caplan RA, Stephens LS, Posner KL, Terman GW, Voepel‐Lewis T, et al. Postoperative opioid‐induced respiratory depression: a closed claims analysis. Anesthesiology 2015;122(3):659‐65. [PUBMED: 25536092] [DOI] [PubMed] [Google Scholar]
Lewis 2015
- Lewis SR, Price A, Walker KJ, McGrattan K, Smith AF. Ultrasound guidance for upper and lower limb blocks. The Cochrane Database of Systematic Reviews 2015;9:CD006459. [PUBMED: 26361135] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marhofer 1997
- Marhofer P, Schrogendorfer K, Koinig H, Kapral S, Weinstabl C, Mayer N. Ultrasonographic guidance improves sensory block and onset time of three‐in‐one blocks. Anesthesia and Analgesia 1997;85(4):854‐7. [PUBMED: 9322469] [DOI] [PubMed] [Google Scholar]
McColl 1998
- McColl A, Smith H, White P, Field J. General practitioner's perceptions of the route to evidence based medicine: a questionnaire survey. BMJ (Clinical research ed.) 1998;316(7128):361‐5. [PUBMED: 9487174] [DOI] [PMC free article] [PubMed] [Google Scholar]
Memtsoudis 2014
- Memtsoudis SG, Danninger T, Rasul R, Poeran J, Gerner P, Stundner O, et al. Inpatient falls after total knee arthroplasty: the role of anesthesia type and peripheral nerve blocks. Anesthesiology 2014;120(3):551‐63. [PUBMED: 24534855] [DOI] [PubMed] [Google Scholar]
Neal 2015
- Neal JM, Barrington MJ, Brull R, Hadzic A, Hebl JR, Horlocker TT, et al. The Second ASRA Practice Advisory on Neurologic Complications Associated With Regional Anesthesia and Pain Medicine: Executive Summary 2015. Regional Anesthesia and Pain Medicine 2015;40(5):401‐30. [PUBMED: 26288034] [DOI] [PubMed] [Google Scholar]
Pace 2011
- Pace NL. Research methods for meta‐analyses. Best Practice & Research. Clinical Anaesthesiology 2011;25(4):523‐33. [PUBMED: 22099918] [DOI] [PubMed] [Google Scholar]
Parkinson 1989
- Parkinson SK, Mueller JB, Little WL, Bailey SL. Extent of blockade with various approaches to the lumbar plexus. Anesthesia and Analgesia 1989;68(3):243‐8. [PUBMED: 2919761] [PubMed] [Google Scholar]
Pogue 1998
- Pogue J, Yusuf S. Overcoming the limitations of current meta‐analysis of randomised controlled trials. Lancet 1998;351(9095):47‐52. [PUBMED: 9433436] [DOI] [PubMed] [Google Scholar]
Pompei 1994
- Pompei P, Foreman M, Rudberg MA, Inouye SK, Braund V, Cassel CK. Delirium in hospitalized older persons: outcomes and predictors. Journal of the American Geriatric Society 1994;42(8):809‐15. [PUBMED: 8046190 ] [DOI] [PubMed] [Google Scholar]
RevMan 5.3 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rucker 2011
- Rucker G, Schwarzer G, Carpenter JR, Binder H, Schumacher M. Treatment‐effect estimates adjusted for small‐study effects via a limit meta‐analysis. Biostatistics (Oxford, England) 2011;12(1):122‐42. [PUBMED: 20656692] [DOI] [PubMed] [Google Scholar]
Saunders 2010
- Saunders KW, Dunn KM, Merrill JO, Sullivan M, Weisner C, Braden JB, et al. Relationship of opioid use and dosage levels to fractures in older chronic pain patients. Journal of General Internal Medicine 2010;25(4):310‐5. [PUBMED: 20049546] [DOI] [PMC free article] [PubMed] [Google Scholar]
Singer 2015
- Singer A, Exuzides A, Spangler L, O'Malley C, Colby C, Johnston K, et al. Burden of illness for osteoporotic fractures compared with other serious diseases among postmenopausal women in the United States. Mayo Clinic Proceedings 2015;90(1):53‐62. [PUBMED: 25481833] [DOI] [PubMed] [Google Scholar]
Sites 2012
- Sites BD, Taenzer AH, Herrick MD, Gilloon C, Antonakakis J, Richins J, et al. Incidence of local anesthetic systemic toxicity and postoperative neurologic symptoms associated with 12,668 ultrasound‐guided nerve blocks: an analysis from a prospective clinical registry. Regional Anesthesia and Pain Medicine 2012;37(5):478‐82. [PUBMED: 22705953] [DOI] [PubMed] [Google Scholar]
Wallace 1987
- Wallace RB, Anderson RA. Blood lipids, lipid‐related measures, and the risk of atherosclerotic cardiovascular disease. Epidemiologic Reviews 1987;9:95‐119. [PUBMED: 3315721] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Parker 2002
- Parker MJ, Griffiths R, Appadu BN. Nerve blocks (subcostal, lateral cutaneous, femoral, triple, psoas) for hip fractures. Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI: 10.1002/14651858.CD001159] [DOI] [PubMed] [Google Scholar]


