Abstract
Background
The benefits and risks of antibiotics for acute bronchitis remain unclear despite it being one of the most common illnesses seen in primary care.
Objectives
To assess the effects of antibiotics in improving outcomes and to assess adverse effects of antibiotic therapy for people with a clinical diagnosis of acute bronchitis.
Search methods
We searched CENTRAL 2016, Issue 11 (accessed 13 January 2017), MEDLINE (1966 to January week 1, 2017), Embase (1974 to 13 January 2017), and LILACS (1982 to 13 January 2017). We searched the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) and ClinicalTrials.gov on 5 April 2017.
Selection criteria
Randomised controlled trials comparing any antibiotic therapy with placebo or no treatment in acute bronchitis or acute productive cough, in people without underlying pulmonary disease.
Data collection and analysis
At least two review authors extracted data and assessed trial quality.
Main results
We did not identify any new trials for inclusion in this 2017 update. We included 17 trials with 5099 participants in the primary analysis. The quality of trials was generally good. At follow‐up there was no difference in participants described as being clinically improved between the antibiotic and placebo groups (11 studies with 3841 participants, risk ratio (RR) 1.07, 95% confidence interval (CI) 0.99 to 1.15). Participants given antibiotics were less likely to have a cough (4 studies with 275 participants, RR 0.64, 95% CI 0.49 to 0.85; number needed to treat for an additional beneficial outcome (NNTB) 6) and a night cough (4 studies with 538 participants, RR 0.67, 95% CI 0.54 to 0.83; NNTB 7). Participants given antibiotics had a shorter mean cough duration (7 studies with 2776 participants, mean difference (MD) ‐0.46 days, 95% CI ‐0.87 to ‐0.04). The differences in presence of a productive cough at follow‐up and MD of productive cough did not reach statistical significance.
Antibiotic‐treated participants were more likely to be improved according to clinician's global assessment (6 studies with 891 participants, RR 0.61, 95% CI 0.48 to 0.79; NNTB 11) and were less likely to have an abnormal lung exam (5 studies with 613 participants, RR 0.54, 95% CI 0.41 to 0.70; NNTB 6). Antibiotic‐treated participants also had a reduction in days feeling ill (5 studies with 809 participants, MD ‐0.64 days, 95% CI ‐1.16 to ‐0.13) and days with impaired activity (6 studies with 767 participants, MD ‐0.49 days, 95% CI ‐0.94 to ‐0.04). The differences in proportions with activity limitations at follow‐up did not reach statistical significance. There was a significant trend towards an increase in adverse effects in the antibiotic group (12 studies with 3496 participants, RR 1.20, 95% CI 1.05 to 1.36; NNT for an additional harmful outcome 24).
Authors' conclusions
There is limited evidence of clinical benefit to support the use of antibiotics in acute bronchitis. Antibiotics may have a modest beneficial effect in some patients such as frail, elderly people with multimorbidity who may not have been included in trials to date. However, the magnitude of this benefit needs to be considered in the broader context of potential side effects, medicalisation for a self limiting condition, increased resistance to respiratory pathogens, and cost of antibiotic treatment.
Plain language summary
Antibiotic treatment for people with acute bronchitis
Review question
We wanted to know whether antibiotics improve outcomes for people with acute bronchitis. We also assessed potential adverse effects of antibiotic therapy.
Background
Acute bronchitis is a clinical diagnosis (based on medical signs and patient‐reported symptoms) for an acute cough, which may or may not be associated with coughing up mucus or sputum. Acute bronchitis can be caused by viruses or bacteria. Symptoms generally last for two weeks but can last for up to eight weeks. Antibiotics are commonly prescribed to treat acute bronchitis, but they can have adverse effects such as nausea and diarrhoea as well as cause more serious reactions in those who are allergic. There is no practical test to distinguish between bacterial and viral bronchitis.
Study characteristics
We included randomised controlled trials comparing any antibiotic therapy with placebo or no treatment in people with acute bronchitis or acute productive cough and no underlying chronic lung condition. We included 17 trials with 5099 participants. Co‐treatments with other medications to relieve symptoms were allowed if they were given to all participants in the study.
Key results
Our evidence is current to 13 January, 2017.
We found limited evidence of clinical benefit to support the use of antibiotics for acute bronchitis. Some people treated with antibiotics recovered a bit more quickly with reduced cough‐related outcomes. However, this difference may not be of practical importance as it amounted to a difference of half a day over an 8‐ to 10‐day period. There was a small but significant increase in adverse side effects in people treated with antibiotics. The most commonly reported side effects included nausea, vomiting, diarrhoea, headache, and rash.
This review suggests that there is limited benefit to the patient in using antibiotics for acute bronchitis in otherwise healthy individuals. More research is needed on the effects of using antibiotics for acute bronchitis in frail, elderly people with multiple chronic conditions who may not have been included in the existing trials. Antibiotic use needs to be considered in the context of the potential side effects, medicalisation for a self limiting condition, cost of antibiotic treatment, and in particular associated population‐level harms due to increasing antibiotic resistance.
Quality of the evidence
The quality of these trials was generally good, particularly for more recent studies.
Background
Description of the condition
Acute bronchitis is a common illness characterised by fever and cough that is often wheezy in nature and that may or may not be productive. The condition occurs when the bronchi become inflamed due to either viral or bacterial infection. Symptoms generally last for two weeks, but the associated cough can last for up to eight weeks (CDC 2013). Acute bronchitis is the ninth most common outpatient illness recorded by physicians in ambulatory practice in the USA (Delozier 1989), and the fifth most common outpatient illness encountered by Australian general practitioners, for whom it represents 3.5% of encounters and 2.4% of problems seen (Meza 1994). In the UK, there are 300 to 400 consultations for treatment of respiratory tract infections per 1000 registered patients each year, and while antibiotic prescribing for these conditions declined between 1995 and 2000, it has since stabilised (Gulliford 2011). Data provided by the European Centre for Disease Prevention and Control on trends in antimicrobial consumption across Europe suggests that overall antibiotic use varies across Europe, with most countries showing an increase between 1997 and 2010 (ECDC 2013).
Population‐based estimates of the incidence of acute bronchitis range from 33 to 45 cases per 1000 per year (Ayres 1986; Mainous 1996). People with bronchitis miss an average of two to three days off work per episode. The great majority of episodes of acute bronchitis in healthy individuals are presumed to be viral infections, although this has been questioned (Macfarlane 1994). Community‐based studies have isolated viruses in 8% to 23% of cases (Boldy 1990; Macfarlane 1993; Stuart‐Harris 1965). Other pathogens implicated in acute bronchitis are Mycoplasma pneumoniae, Chlamydia pneumoniae, and Bordetella pertussis, each of which has been identified in up to 25% of cases in various populations (Boldy 1990; Falck 1994; Foy 1993; Grayston 1993; Herwaldt 1991; Jonsson 1997; King 1996; Macfarlane 1993; Robertson 1987; Stuart‐Harris 1965; Thom 1994). A more recent study assessing the aetiology and outcome of acute lower respiratory tract infection in 638 adults in UK primary care showed that in 55% of cases viral or bacterial pathogens were identified (Macfarlane 2001).
Description of the intervention
The use of antibiotics in people with acute bronchial infections remains a controversial area in primary healthcare practice (Coenen 2007; Del Mar 2016; Gonzales 1995). Streptococcus pneumoniae,Haemophilus influenzae, andMoraxella catarrhalis have been isolated from sputum samples in up to 45% of people with acute bronchitis (Henry 1995; Macfarlane 1993), but their role is difficult to assess due to potential oropharyngeal colonisation in healthy individuals (Laurenzi 1961; Smith 1986). Unfortunately, there are no clinically useful criteria that accurately help distinguish bacterial from viral bronchial infections, therefore some authors have called for physicians to stop prescribing antibiotics for people with acute bronchitis (Gonzales 1995; Hueston 1997). Nevertheless, antibiotics are prescribed for 60% to 83% of people who present to physicians with the condition (Gonzales 1997; Mainous 1996; Meza 1994; Petersen 2007; Straand 1997).
How the intervention might work
Antibiotics may improve outcomes in acute bronchitis if the disease is caused by a bacterial infection. Antibiotics have no antiviral activity and are therefore not effective in viral bronchitis. In addition, antibiotics can cause harm due to their negative effect on normal bacteria colonising the intestine. The most common adverse effects of antibiotics include gastrointestinal symptoms such as nausea and diarrhoea, but they can also cause more serious reactions related to anaphylaxis in those who are allergic.
Why it is important to do this review
Some estimate of the probable effectiveness of antibiotic therapy for acute bronchitis is needed given the frequent occurrence of the condition. If found to be effective, antibiotics could shorten the course of the disease and consequently reduce the associated loss of productive work time. However, any benefit from antibiotics must be weighed against the possibility that excessive antibiotic use will lead to increases in cost and patient morbidity, as well as the development of resistant strains of common organisms, Coenen 2007; Molstad 1992, and unnecessary medicalisation of individuals with a self limiting illness (Little 2005). If antibiotics are found to be ineffective, then their use should be discontinued.
Objectives
To assess the effects of antibiotics in improving outcomes and to assess adverse effects of antibiotic therapy for people with a clinical diagnosis of acute bronchitis.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials in people with acute bronchitis assigned to treatment with an antibiotic or to a placebo or no active treatment.
Types of participants
We included trials evaluating people of either sex and any age with a clinical syndrome of cough with or without productive sputum, with a physician's diagnosis of acute bronchitis or cough with persistent cold or flu‐like illness that was not resolving. The term 'acute lower respiratory tract infection when pneumonia is not suspected' is also used to describe this clinical presentation. We excluded trials that included people with pre‐existing chronic bronchitis (i.e. acute exacerbation of chronic bronchitis).
Types of interventions
We included all randomised controlled trials comparing any antibiotic therapy versus no treatment or placebo in the management of acute bronchitis. We excluded trials comparing one antibiotic regimen with another, or trials comparing the use of other active medications (such as bronchodilators) with antibiotic therapy in this review. We included trials that allowed concurrent use of other medications such as analgesics, antitussives, antipyretics, or mucolytics if they allowed equal access to such medications to participants in both the antibiotic and the control group.
Types of outcome measures
We included the following range of cough‐related and general clinical outcomes.
Primary outcomes
-
Cough‐related outcomes including:
time to resolution of cough;
sputum production, defined as proportion of participants with or without sputum;
proportions of participants with cough, night cough, productive cough.
Global assessment of improvement by clinicians at follow‐up.
-
General clinical outcomes including:
severity of symptoms;
activity limitations;
abnormal lung examination at a designated follow‐up visit.
Secondary outcomes
Adverse effects.
Search methods for identification of studies
Electronic searches
For this updated review, we searched the Cochrane Central Register of Controlled Trials (CENTRAL) 2016, Issue 11, part of the Cochrane Library (www.cochranelibrary.com/) (accessed 13 January 2017), which includes the Cochrane Acute Respiratory Infections Group's Specialised Register, MEDLINE (1966 to January week 1, 2017), Embase (1974 to 13 January 2017), and LILACS (Latin American and Caribbean Literature in Health Sciences) (1982 to 13 January 2017). We used the search strategy described in Appendix 1 to search MEDLINE and CENTRAL. We adapted the search strategy to search Embase (Appendix 2) and LILACS (Appendix 3). Details of the 2017 update search can be found in Appendix 4.
Searching other resources
We searched the World Health Organization International Clinical Trials Registry Platform (WHO ICTRP) (www.who.int/ictrp/en/) and ClinicalTrials.gov (clinicaltrials.gov/) on 5 April 2017. We also searched the reference lists of relevant trials, and we originally searched review articles and textbook chapters to identify additional trials, including those published prior to 1966. For the original review, we included in our searches articles from the review authors’ personal collections and requested unpublished trials from trial authors. In addition, for the earlier version of this review we also contacted drug companies that manufacture antibiotics. There were no language or publication restrictions.
Data collection and analysis
Selection of studies
One review author (SS) evaluated the titles and abstracts of the identified citations and applied the inclusion criteria. We obtained the full papers of trials deemed potentially relevant for further examination. Two review authors (TF, SS) screened the full‐text papers to determine if they met the inclusion criteria. We discarded reports that were clearly irrelevant. We recorded studies that did not fulfil the inclusion criteria along with the reasons for their exclusion in the Characteristics of excluded studies table..
Data extraction and management
Two or more review authors independently extracted data using a data collection form designed for this review. Any disagreements were resolved by discussion between the review authors. We transferred data into Review Manager 5 (RevMan 2014).
Assessment of risk of bias in included studies
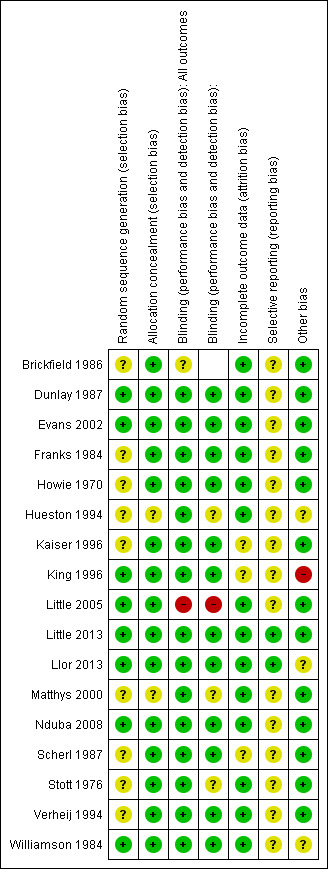
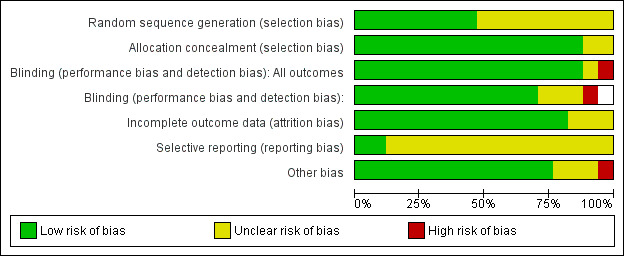
Two review authors (SS, TF) evaluated the methodological quality of each trial using Risk of Bias domains recommended in the Cochrane Handook as outlined in Figure 1 and Figure 2. Disagreements were resolved by consensus.
1.
'Risk of bias' summary: review authors' judgements about each methodological quality item for each included study.
2.
'Risk of bias' graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Measures of treatment effect
The effect measures of choice were risk ratio (RR) for categorical outcomes and mean difference (MD) for continuous data.
Unit of analysis issues
There were no cluster‐randomised trials included in this review as it involved a simple drug trial with a placebo comparator. Clinicians were generally blinded to the intervention. We identified no unit of analysis errors.
Dealing with missing data
Where data were missing we reported this in the Risk of bias in included studies section. We did not adopt any strategies to deal with missing data such as imputation. In general, missing data did not bias the review findings.
Assessment of heterogeneity
Where we considered clinical heterogeneity to be an issue, we undertook a random‐effects meta‐analysis rather than a fixed‐effect meta‐analysis. This applied in particular to the most recent analysis added to this updated version of the review (Analysis 6.1).
6.1. Analysis.
Comparison 6 Clinically improved, Outcome 1 Number of participants reporting no activity limitations or described as cured/globally improved.
Assessment of reporting biases
We examined funnel plots for each of the analyses conducted and none indicated a significant level of reporting bias.
Data synthesis
All previous versions of this review presented fixed‐effect meta‐analyses. For this update, we included a range of outcomes under the broad definition of 'clinically improved'. These were clinically heterogeneous, so we used a random‐effects meta‐analysis.
GRADE and 'Summary of findings' table
The original review and analyses were conducted prior to the use of GRADE and 'Summary of findings' tables. As we identified no new studies for inclusion in this update, we did not undertake a GRADE assessment or create a 'Summary of findings' table. We made this decision based on time and resource constraints of the author group. The update status is now 'Up to date > No new studies identified with search'.
Subgroup analysis and investigation of heterogeneity
We carried out a subgroup analysis comparing studies using a placebo control or active treatment.
Sensitivity analysis
We included only studies that limited enrolment to people with a clinical diagnosis of acute bronchitis or acute productive cough for the primary analysis. We did a sensitivity analysis that included unpublished data from subgroups of participants with a productive cough and non‐purulent tracheobronchitis from two studies that enrolled people with an influenza‐like illness or a common cold (Howie 1970; Kaiser 1996).
Results
Description of studies
Results of the search
The updated and modified CENTRAL, MEDLINE, Embase, and LILACS searches in 2017 yielded an additional 993 titles. We have identified no new studies since the 2014 update. All of the 17 trials included in the primary analysis enrolled people with a diagnosis of acute cough or acute lower respiratory tract infection. In one study participants were required to produce a sputum sample for analysis as a condition of enrolment (Franks 1984).
Included studies
We included 17 studies in this review. We identified no new studies for this update. The 2014 update added two new studies (Little 2013; Llor 2013). These were important additions, particularly the trial by Little 2013, as it is the largest trial conducted to date, involving 2061 participants recruited across 12 countries. Participants were randomised to receive either amoxicillin or placebo, and there was low risk of bias with more than 80% follow‐up of participants.
Most studies used clinical findings to exclude patients thought to have pneumonia. Four studies included chest radiographs in their protocols: two performed a chest film on all potential participants (Brickfield 1986; Nduba 2008), and in the other two, Scherl 1987 did so on people with rales or fever, and Llor 2013 did so on those with suspected pneumonia (7 of 416 participants). These four studies excluded those with radiological evidence of pneumonia or tuberculosis. One study excluded people with any abnormality noted on examination of the chest (Stott 1976). Four trials also excluded people with a clinical syndrome suggesting sinusitis (Dunlay 1987; King 1996; Verheij 1994; Williamson 1984).
In all trials the duration of illness at entry was less than 30 days. One trial limited enrolment to people who were ill for less than one week (Stott 1976), and in five trials the duration was two weeks or less (Brickfield 1986; Evans 2002; Franks 1984; King 1996; Matthys 2000).
Eight of the trials included only adults (Brickfield 1986; Dunlay 1987; Hueston 1994; Little 2013; Llor 2013; Nduba 2008; Verheij 1994; Williamson 1984). The remaining studies included both adolescents and adults (Franks 1984; Scherl 1987; Stott 1976); people aged three years or older (Little 2005); or eight years or older (King 1996).
Regarding antibiotic treatment, four trials used doxycycline (Scherl 1987; Stott 1976; Verheij 1994; Williamson 1984), four erythromycin (Brickfield 1986; Dunlay 1987; Hueston 1994; King 1996), one trimethoprim/sulfamethoxazole (Franks 1984), one azithromycin (Evans 2002), one cefuroxime (Matthys 2000), one amoxicillin or erythromycin (Little 2005), two amoxicillin (Little 2013; Nduba 2008), and one amoxicillin‐clavulanic acid (Llor 2013).
The majority of studies used a single reassessment visit to evaluate results of the intervention. The timing of this visit varied from study to study, ranging from two to 14 days after the initiation of treatment. Some investigators also asked participants to keep symptom diaries, which were used to determine the duration of symptoms or disability.
Several of the trials provided results of separate analyses of one or more subsets of patients based on characteristics such as cigarette smoking, patient age, duration of symptoms, presence of purulent sputum, or illness severity. All participants enrolled in the study by Nduba 2008 were tested for HIV and a sub‐group analysis was undertaken based on HIV status. The largest study included in the review, (Little 2013), was adequately powered for a subgroup analysis of participants aged over 60 years.
For the sensitivity analyses, we included unpublished data from two trials. In one (Howie 1970), participants began self treatment with dimethyl chlortetracycline or placebo if a cold or influenza‐like illness did not spontaneously resolve after two days. We included data from a subgroup of participants who had a productive cough prior to beginning treatment. The other study randomised participants with the common cold to amoxicillin‐clavulanic acid or placebo (Kaiser 1996). We included data from a subgroup of participants who had a concomitant diagnosis of non‐purulent tracheobronchitis, which incorporates 'acute bronchitis'. Further details on the subgroups of participants included from these studies is provided in the Characteristics of included studies table.
Excluded studies
We excluded studies for a variety of reasons based on study design and intervention criteria. Details of excluded studies are provided in the Characteristics of excluded studies table.
Risk of bias in included studies
Sixteen of the 17 included trials were randomised, double‐ or single‐blind evaluations comparing an antibiotic with a placebo. The study added in the 2011 update was the first equivalence randomised controlled trial included in the review (Nduba 2008). The earlier study by Little 2005 involved three arms comparing immediate antibiotic therapy, no active treatment, or delayed treatment; we only included the two arms comparing immediate antibiotic treatment with no treatment. The study by Llor 2013, involved three arms comparing antibiotic, placebo, and anti‐inflammatory treatment; we only included data from the antibiotic versus placebo arms. Four reports did not clearly state the randomisation method used (Brickfield 1986; Howie 1970; Kaiser 1996; Scherl 1987). Only one of the articles reported a formal evaluation of the effectiveness of the blinding procedures used (Nduba 2008). Six studies measured compliance or adherence with treatment: in five, there were no differences in the number of pills taken in the antibiotic and placebo groups (Dunlay 1987; Hueston 1994; Little 2013; Nduba 2008; Stott 1976); in the study by King 1996, 94% of the participants who returned for follow‐up took at least one‐half of their pills, and Little 2013 reported greater than 90% adherence in both groups by day five. Regarding co‐interventions with other medications, four trials asked participants to record the use of non‐prescription medications and included this as an outcome measure (Dunlay 1987; Franks 1984; Hueston 1994; King 1996); one trial restricted use to aspirin and acetaminophen, but did not have the participants record this (Scherl 1987); and one trial reported adjunctive prescriptions, but not use of over‐the‐counter medications (Verheij 1994). The majority of studies (13 out of 17) followed up more than 80% of participants (details of dropouts are provided in the Characteristics of included studies table). In some cases, no information about withdrawals was available in the paper or from the authors. However, when information was available, we included outcome data from the last point at which the participants remained in the study. To the greatest degree possible, we analysed participants on an intention‐to‐treat basis.
The overall risk of bias is presented graphically in Figure 1 and summarised in Figure 2.
Allocation
In general, there was minimal risk of allocation or selection bias: 15 out of 17 studies clearly reported adequate allocation concealment.
Blinding
In general, there was minimal risk of bias relating to lack of blinding, with 14 out of 17 studies clearly reporting adequate blinding of outcome assessors.
Incomplete outcome data
The majority of studies had adequate completion of outcome data with minimal risk of attrition bias.
Selective reporting
Most trials evaluated several different outcome measures. In some cases, the published reports included detailed data for only those outcomes found to be statistically significant. To minimise this reporting bias, we attempted to obtain additional data from the trial authors; five authors provided this information (Howie 1970; Hueston 1994; Kaiser 1996; King 1996; Williamson 1984). However, we were still unable to include data from Stott 1976 for the outcomes of cough, night cough, or activity limitations at follow‐up, which were reported in the published trial as being not significantly different between groups.
Other potential sources of bias
The main concern regarding bias was the relatively small numbers of studies that could be included in individual meta‐analyses. We have attempted to address this by adding a new, broader analysis reflecting clinical improvement. This has been further strengthened by the addition of the largest multi country trial to date (Little 2013). There were no additional concerns regarding other potential sources of bias.
Effects of interventions
All studies did not report the same outcome measures. Some studies reported the presence or absence of various symptoms and signs at a follow‐up visit; others reported the mean duration of symptoms; and still others reported only unique symptom scores. In addition, in some studies explicit data were available only for outcomes that were significantly different between the antibiotic and placebo groups. The number of studies that provided data for the outcomes in this review therefore ranged from three to 11. None of the summary outcomes in the primary analysis exhibited statistically significant heterogeneity apart from the analysis of participants 'clinically improved'. Numbers of studies and participants included in the individual meta‐analyses were generally small, although the meta‐analysis for 'clinically improved' includes 11 studies, and the meta‐analysis for adverse events includes 12 studies.
Primary outcomes
1. Cough‐related outcomes
At the follow‐up visit, participants given antibiotics were less likely to have a cough (4 studies with 275 participants, risk ratio (RR) 0.64, 95% confidence interval (CI) 0.49 to 0.85; number needed to treat for an additional beneficial outcome (NNTB) 6) (Analysis 1.1; Figure 3) or a night cough (4 studies with 538 participants, RR 0.67, 95% CI 0.54 to 0.83; NNTB 7) (Analysis 2.1). The differences in presence of a productive cough at follow‐up and days of productive cough did not reach statistical significance. Antibiotic‐treated participants only had a significant reduction in mean duration of cough when the study by Little 2005, which had a no‐treatment comparison group, was excluded (Figure 4). Llor 2013 also reported no significant difference in median days of cough between the antibiotic and placebo groups. In addition, sensitivity analysis altered the outcome of mean duration of productive cough, which was significantly reduced if the Howie 1970 study relating to upper respiratory tract infection was excluded.
1.1. Analysis.
Comparison 1 Cough at follow‐up visit, Outcome 1 Number of participants with cough.
3.
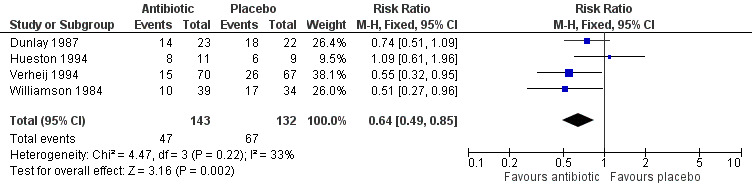
Forest plot of comparison: Cough at follow‐up visit, outcome: number of participants with cough.
2.1. Analysis.
Comparison 2 Night cough at follow‐up visit, Outcome 1 Number of participants with night cough.
4.
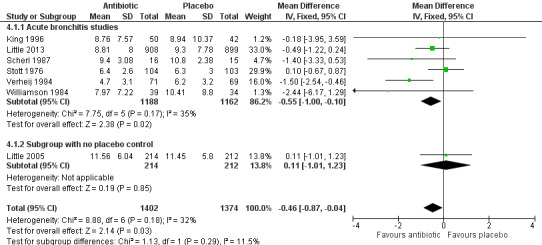
Forest plot of comparison: 8 Days of cough, outcome: mean number of days of cough.
2. Global assessment of improvement by clinicians at follow‐up: 'clinically improved'
For the 2011 update of the review, we added an analysis that included a broader outcome 'clinically improved', so that as many studies as possible could be included in a meta‐analysis. This was particularly important following the inclusion of the Nduba 2008 study in 2011, which was of high quality, included a large number of participants, and showed no benefit from antibiotic use. This outcome includes additional data from the authors of the largest included study, (Little 2013). The data from Little 2013 are based on numbers of participants no longer reporting their symptoms being "moderately bad" at one week. The published study presents mean symptom severity scores in the first few days, which indicated no significant difference between the intervention and control groups (Little 2013). This outcome reflects the proportions of participants with clinical improvement and incorporates 'cure' as measured by a greater than 75% reduction in the Acute Bronchitis Severity Score (Nduba 2008), global improvement or being well (Brickfield 1986; Llor 2013; Matthys 2000; Stott 1976; Verheij 1994; Williamson 1984), patient report of no limitations (Dunlay 1987; Evans 2002; Franks 1984), and resolution of symptoms rated as moderately bad, severe, or worsening (Little 2013). This analysis includes 11 studies involving 3841 participants and shows no statistically significant difference between groups (RR 1.07, 95% CI 0.99 to 1.15; NNTB 22) (Analysis 6.1; Figure 5). The addition of data from Little 2013 and Llor 2013 increased the heterogeneity for this analysis. A sensitivity analysis removing the studies reporting 'no limitation' made no difference to this result.
5.
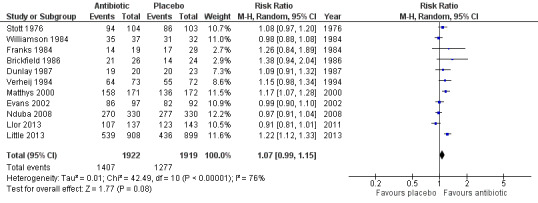
Forest plot of comparison: Clinically improved, outcome: number of participants reporting no limitations or described as cured/well/symptoms resolved or globally improved.
3. General clinical outcomes
Antibiotic‐treated participants also had a reduction in the number of days feeling ill (5 studies with 809 participants, mean difference (MD) ‐0.64, 95% CI ‐1.16 to ‐0.13) (Analysis 8.1; Figure 6) and a reduction in days with impaired activity (6 studies with 767 participants, MD ‐0.49, 95% CI ‐0.94 to ‐0.04) (Analysis 9.1). There was no significant difference in proportions of participants with activity limitations at follow‐up. Participants on antibiotics were more likely to be improved according to clinician's global assessment (6 studies with 891 participants, RR 0.61, 95% CI 0.48 to 0.79; NNTB 11) (Analysis 10.1; Figure 7) and were less likely to have an abnormal lung exam (5 studies with 613 participants, RR 0.54, 95% CI 0.41 to 0.70; NNTB 6) (Analysis 11.1). Additional clinical outcomes were reported by Little 2013, who found no significant difference in mean symptom severity scores on days two to four (intervention score 1.62 (standard deviation (SD) 0.84) versus control score 1.69 (SD 0.84), P = 0.07), and Evans 2002 found that azithromycin had no benefit in terms of health‐related quality of life at day three and day seven follow‐up. Llor 2013 also reported no difference in time to overall symptom resolution between groups.
8.1. Analysis.
Comparison 8 Days of feeling ill, Outcome 1 Mean number of days of feeling ill.
6.
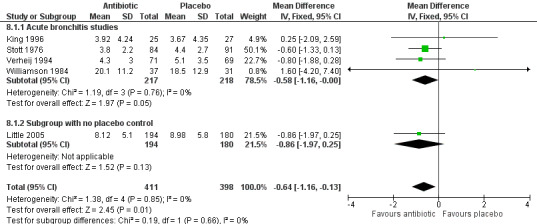
Forest plot of comparison: Days of feeling ill, outcome: mean number of days of feeling ill.
9.1. Analysis.
Comparison 9 Days of impaired activities, Outcome 1 Mean number of days of impaired activities.
10.1. Analysis.
Comparison 10 Not improved by physician's global assessment at follow‐up visit, Outcome 1 Number of participants not improved.
7.
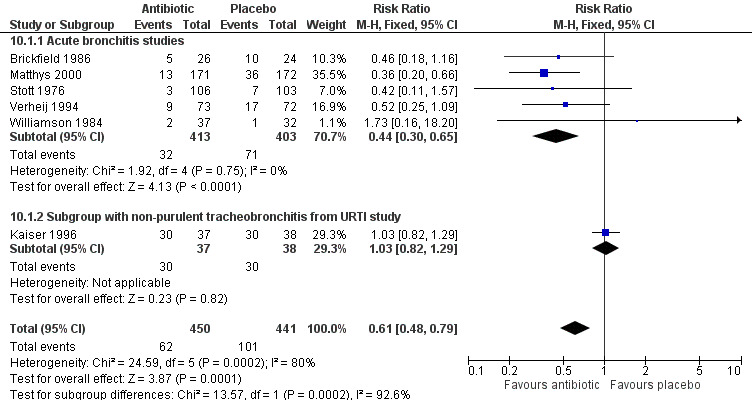
Forest plot of comparison: Not improved by physician's global assessment at follow‐up visit, outcome: number of participants not improved.
11.1. Analysis.
Comparison 11 Abnormal lung exam at follow‐up visit, Outcome 1 Number of participants with abnormal lung exams.
Secondary outcomes
1. Adverse effects
With four exceptions (Brickfield 1986; Little 2005; Matthys 2000; Nduba 2008), all of the studies found that participants in the antibiotic group reported more adverse effects than participants receiving a placebo (Figure 8). The RR of adverse effects in the antibiotic‐treated group was statistically significant at 1.20 (95% CI 1.05 to 1.36; number needed to treat for an additional harmful outcome (NNTH) 24; 12 studies with 3496 participants) (Analysis 12.1). The most commonly reported side effects included gastrointestinal symptoms such as nausea, vomiting, or diarrhoea. Headaches, skin rash, and vaginitis also occurred. Side effects seemed to be mild, as only 0% to 13% (overall 3.7%) of participants withdrew because of them, and no deaths were reported.
8.
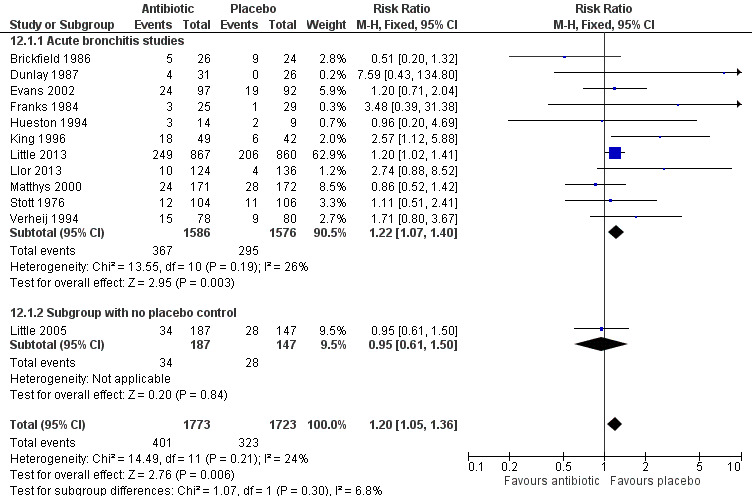
Forest plot of comparison: Number of participants with adverse effects.
12.1. Analysis.
Comparison 12 Adverse effects, Outcome 1 Number of participants with adverse effects.
Subgroups
We were not able to obtain enough explicit data from the studies for various patient subgroups, therefore we did not carry out any sensitivity analyses based on patient characteristics (such as age, duration of illness, or smoking status). Little 2013 was adequately powered to assess the effect in the subgroup of participants aged over 60 and found no significant benefit in this group. The results in the individual studies for subgroup analyses were mixed. In one trial, all of the significantly improved outcomes from antibiotics occurred in non‐smokers (Brickfield 1986). The other seven trials reported that they found no differences in antibiotic effectiveness between smokers and non‐smokers, but included no data on these comparisons in their published reports. Verheij 1994, using multiple regression, found that two subsets of patients were more likely to improve with doxycycline than placebo: participants over 55 years and those with very frequent cough who felt ill. Scherl 1987 found that only participants without coryza or sore throat had fewer days of cough or sputum with doxycycline. The only study to use Gram stains reported an earlier return to work for participants with a positive Gram stain who were treated with antibiotics (Franks 1984). Nduba 2008 also examined whether the use of amoxicillin was more effective than placebo in people who had tested positive for HIV and found no difference, though all participants had received a chest X‐ray and those with any abnormal signs were excluded.
Little 2005, which was added to the 2009 update, found no significant difference in outcomes between groups treated with immediate antibiotics compared with no antibiotic treatment. As this study did not involve a placebo control we included it in the analyses, where appropriate data were available, as a subgroup to highlight this difference. The one study added in the 2011 update was powered to detect equivalence between antibiotic and placebo and found no significant difference (Nduba 2008). In fact, the point estimates favoured placebo treatment (84% cured on placebo versus 82.4% cured on amoxicillin). The largest included study, which was added in the 2014 update, was included in the 'clinically improved' and adverse effects meta‐analyses (Little 2013).
Discussion
Summary of main results
We found mixed results across studies, with some suggesting marginal benefits for antibiotics, which are however of doubtful clinical significance. The inclusion of the largest multicentre study of the effectiveness of antibiotics in people with lower respiratory tract infections strengthens the evidence and also highlights a statistically significant increase in adverse events in the antibiotic‐treated groups. However, it is possible that older patients with multimorbidity may not have been recruited to trials, so the evidence guiding decision‐making in this group of patients is less certain.
Overall completeness and applicability of evidence
In general, the available evidence suggests we should not be using antibiotics to treat acute bronchitis or lower respiratory tract infections when pneumonia is not expected. There is a modest benefit from antibiotics for some outcomes, but these are of minimal clinical significance. Any benefit is even less apparent in the sensitivity analysis, which included data from subgroups of patients with productive cough of short duration (two to four days) in conjunction with the common cold. Of the two trials in the primary analysis that limited enrolment to people who had been ill for less than one week, one did not show any benefit from antibiotics (Stott 1976), whilst the other showed modest benefit from antibiotics (Matthys 2000).
It is possible that the overall benefit noted from antibiotics resulted from the inclusion in some trials of people who may have had pneumonia instead of acute bronchitis. There was variation between studies as to whether chest X‐rays were conducted as part of the evaluations. Only one trial obtained chest radiographs on all participants and then excluded those whose films were consistent with pneumonia (Brickfield 1986). In Little 2013, a positive chest X‐ray was not an automatic exclusion criterion, although some participants dropped out following such a finding. All of the remaining studies either excluded or obtained chest radiographs in patients with clinical findings of suspected pneumonia (which in most studies were focal findings on chest examination). Individual signs (such as crackles or fever) are not sensitive (Metlay 1997a), therefore their absence cannot be relied on to rule out pneumonia. On the other hand, since the prevalence of pneumonia in outpatients who present with cough is generally low (less than 5% in the USA) (Metlay 1997b), it is unlikely that a significant number of participants in these trials had pneumonia. In addition, this review was designed to test the effectiveness of treatment for acute bronchitis in clinical practice, and it is not standard practice to confirm the diagnosis of acute bronchitis with a chest X‐ray unless there is a clinical suspicion of underlying pneumonia. Had we only included studies with chest X‐ray confirmation of diagnosis, it would have limited the generalisability of the review findings.
Quality of the evidence
Since there is no gold standard test, the diagnosis of acute bronchitis must be made on clinical grounds. All of the trials excluded people with chronic pulmonary disease and enrolled participants with recent onset of a respiratory illness with a productive cough. The results of the studies in the primary analysis that included participants with a productive cough, without specifically stating that the participants had acute bronchitis, were similar to the studies that used this specific terminology, as one showed some benefits from antibiotics (Verheij 1994), and one did not (Stott 1976). Clinical characteristics of participants regarding the duration of illness and associated symptoms and physical findings did vary somewhat among studies, but were consistent with definitions generally used by primary care physicians (Oeffinger 1997; Verheij 1990). These results would therefore appear to be generalisable to the management of acute bronchitis in community practices.
Potential biases in the review process
This review may also be subject to bias because although we have now included 17 trials and 5099 participants, it is possible that some patient subgroups are under‐represented, as they may not have been recruited into the original trials. Little 2013 points out that while they included a large sample of older people, more severely ill older people with multimorbidities were unlikely to have been approached to participate in the trial, and in these types of patients their results should be interpreted with caution; this applies to the review results also.
Agreements and disagreements with other studies or reviews
In the current update of the review we have included a large multi country trial that shows no benefits from antibiotics even in older patients. Further analyses of the data from this study are ongoing as part of Workpackage 10 of the GRACE program (www.grace‐lrti.org). It should be noted that a recent large observational study examining symptom resolution in 2714 people with acute cough who had been prescribed amoxicillin across 13 European countries found that symptom resolution was quicker in those receiving no antibiotic (Butler 2010).
Authors' conclusions
Implications for practice.
This review confirms the impression of clinicians that antibiotics have limited, if any, beneficial effects in acute bronchitis. Where there appear to be some benefits, they are slight (such as the small improvement in mean duration of cough of less than one day) and may be of questionable clinical significance. The most recently published placebo‐controlled randomised controlled trial confirms these findings and was carried out in 12 countries, improving the generalisability of the review findings (Little 2013). Another recent randomised controlled trial included also showed no difference in cure rates between those prescribed amoxicillin and those given placebo (Nduba 2008). This trial was particularly important as it was set in a low‐income country and may increase the generalisability of the review. However, the inclusion of a range of trials in different settings does also increase heterogeneity.
While this review suggests limited if any clinical benefit from antibiotics, one could argue for prescribing antibiotics for acute bronchitis because studies of patient utilities for antibiotic treatment for respiratory infections suggest that even small benefits are seen as important by some patients (Herman 1984), and because the adverse effects associated with antibiotic treatment are minor and disappear when the medication is discontinued. On the other hand, arguments against prescribing antibiotics can be made because the modest benefits from antibiotics may not outweigh their costs, adverse effects, or negative consequences on antibiotic resistance patterns and patient expectations.
It is likely that, as with other respiratory infections (Dagnelie 1996; Kaiser 1996), antibiotics may be only effective for a subset of patients with acute bronchitis. It seems that patients who have other typical symptoms of an upper respiratory tract infection and who have been ill for less than one week may be the least likely to benefit from antibiotics. A large cohort study within the UK General Practice Research Database indicates that the risk of pneumonia as a complication of lower respiratory tract infection is substantially reduced in elderly patients when antibiotics are prescribed immediately (Petersen 2007). However, a likely confounding factor in this study was the fact that sicker patients and those more likely to suffer complications were offered immediate antibiotics, introducing potential bias (Coenen 2007). Another analysis of existing data suggests that reducing antibiotic prescribing for acute respiratory tract infections in primary care settings by 10% would be associated with one extra case of pneumonia per general practitioner every four to five years (Del Mar 2016). The trials that have been performed to date do not offer a clear method to differentiate patients with acute bronchitis who might benefit from antibiotic therapy from those who might not. In light of this uncertainty, it is especially important for clinicians to share the decision about whether to use antibiotics or not with their patients, using the expected outcomes and their magnitude from this review as a basis for their discussion.
In terms of interventions designed to reduce unnecessary antibiotic prescribing, some organisational and educational strategies have been shown to be helpful. Use of delayed or deferred antibiotic when patients consult with symptoms of acute bronchitis is of some value (Dowell 2001). In a randomised trial in 22 UK practices, 191 patients were randomised to either immediate or delayed antibiotic (prescription lodged at the family practice reception and patients were invited to collect it after one week, if required). Over half (55%) in the delayed arm did not pick up their prescriptions, though compared to the participants in the immediate arm, they were less satisfied with this strategy (Dowell 2001). In a randomised trial of a patient information leaflet in 212 patients with acute bronchitis for whom antibiotics were judged to be unnecessary by their family doctor, the leaflet reduced uptake compared to those without any information (49% versus 63%, risk ratio 0.76) (Macfarlane 2002). This review contains a subgroup of patients from a UK trial that tested the effectiveness of three prescribing strategies and an information leaflet for acute lower respiratory tract infections (Little 2005). The authors concluded that no offer or a delayed offer of antibiotics for acute uncomplicated lower respiratory tract infection is acceptable and is associated with little difference in symptom resolution. The authors argue that the strategy of delayed or no prescribing is very likely to reduce antibiotic use and beliefs in the effectiveness of antibiotics for this condition. A recent review concluded that complex interventions that included education for physicians were most likely to be effective in optimising antibiotic prescribing in primary care settings (van der Velden 2012).
Implications for research.
There is a widespread belief among clinicians and patients that antibiotics provide effective treatment for acute bronchitis. There is also widespread opinion among experts that antibiotic therapy is unwarranted in this condition. The results of this review indicate that there are, at most, limited benefits for some patients, and this must be placed in the context of the significant increase in adverse events in the antibiotic group. However, it is also possible that any apparent benefits from antibiotics are overestimated.
Ongoing research efforts should also be directed at the identification of subsets of patients who are most likely or least likely to benefit from antibiotic treatment (Coenen 2007; Little 2013). Patient age, duration and severity of illness, chest examination findings, sputum Gram stains, C‐reactive protein levels (Jonsson 1997), and cigarette smoking are variables that may be important in differentiation of these patient subsets. The ongoing GRACE programme (Genomics to Combat Resistance Against Antibiotics in Community‐Acquired Lower Respiratory Tract Infections in Europe, www.grace‐lrti.org) may provide answers to some of these questions (Coenen 2007). Given the controversy around the term 'acute bronchitis', it will also be important for researchers to be very clear on their inclusion criteria to allow comparison across studies. Finally, given the small impact, at best, of antibiotics on patient symptoms, investigators should continue the search for other effective means of relieving the most troublesome symptoms of people suffering from acute bronchitis.
Feedback
Data reported on adverse effects, 21 May 2005
Summary
We would like to draw attention to the misleading statement of authors conclusions for this review. The authors conclusion in the abstract reads: "Overall, antibiotics appear to have a modest beneficial effect in patients who are diagnosed with acute bronchitis. THE MAGNITUDE OF THIS BENEFIT, HOWEVER, IS SIMILAR TO THAT OF THE DETRIMENT FROM POTENTIAL ADVERSE EFFECTS."
The data reported on adverse effects does not seem to support this conclusion.
Graph 07 showing the number of participants with adverse effects in the antibiotics and control groups illustrates that adverse events in the antibiotics group reach significance in just one small study (King 1996), and the non‐significant pooled estimate is clearly stated in the results section: "The overall relative risk (RR) of adverse effects was 1.22 (95% CI 0.94 to 1.58)."
We would like to recommend that the authors amend their conclusions to take account of these comments. Paul Garner and Helen Smith
Submitter agrees with default conflict of interest statement: I certify that I have no affiliations with or involvement in any organisation or entity with a financial interest in the subject matter of my feedback.
Reply
We accept that our conclusions are overly pessimistic about side effects from antibiotic therapy. The pooled results from the updated review changed after inclusion of an additional RCT (Matthys 2000, see Figure 8 of the review), making adverse events less likely. We acknowledge that we did not change the tone of our conclusions to reflect this greater uncertainty concerning side effects.
We have amended the conclusion in the abstract to reflect the updated results concerning side effects to the following sentence:
"The magnitude of this benefit, however, needs to be considered in the broader context of potential side effects, medicalisation for a self‐limiting condition, increased resistance to respiratory pathogens and cost of antibiotic treatment."
Tom Fahey Lorne Becker John Smucny Rick Glazier
Correction to updated review, 1 May 2008
Summary
Dear Authors, Compared to the version published in the Cochrane Database of Systematic Reviews 2002, issue 2, the current version of the review included two more studies (Matthys, 2000; Evans, 2003). By consequence, some comparisons (02, 04‐07) were updated. Therefore, the abstract should start with "Eleven studies involving over 1250 patients" instead of "Nine trials involving over 750". But, more importantly, except for comparison 07: Adverse effects, the values of the comparisons already mentioned in the abstract still need to be adjusted, i.e. 05: Not improved by physician's global assessment at follow‐up visit (RR 0.44; 95% CI 0.30,0.65; NNT 10; 95% CI ...) instead of (RR 0.52; 95% CI 0.31,0.85; NNT 14; 95% CI 8 to 50);06: Abnormal lung exam at follow‐up visit (RR 0.54; 95% CI 0.41,0.70; NNT 6; 95% CI ...) instead of (RR 0.48; 95% CI 0.26,0.89; NNT 11; 95% CI 6 to 50); Finally, due to the inclusion of (Matthys, 2000) comparison 02: Night cough at follow‐up visit, now shows a significant difference between antibiotics and placebo(RR 0.67; 95% CI 0.54,0.83; NNT 7 ; 95% CI ...) instead of (RR 0.76; 95% CI 0.45,1.30). This comparison should thus be removed from the statement starting with "There were nog significant differences regarding the presence of night cough, productive cough, ..." and added to the previous sentence showing the benefits of antibiotic treatment. Given the difficulties in distinguishing upper from lower respiratory tract infections in daily practice and given the results of the study by Little et al. mentioned as ongoing study, the conclusions are still justified.
With kind regards Samuel Coenen
Submitter agrees with default conflict of interest statement:I certify that I have no affiliations with or involvement in any organisation or entity with a financial interest in the subject matter of my feedback.
Reply
Thank you for your comments regarding inconsistencies noted in the previous update of the review. The review has been updated again over the last nine months and these inconsistencies were noted and corrected. The Little study has since been published and is now incorporated in this new version of the review.
Contributors
Susan Smith Tom Fahey John Smucny Lorne Becker
Antibiotics for acute bronchitis, 22 June 2016
Summary
We have read your 2014 Cochrane review CD000245 "Antibiotics in Acute Bronchitis" in detail with interest and noticed two points:
1. In the published version on the Cochrane Library homepage we are not able to see Figure 1, which unfortunately seems to be missing in the document.
2. There seems to be a data‐reference disorder: In the text on page 12 concerning additional cases of adverse events it is written "NNT = 5, RR = 1.20 (1.05 to 1.36)", but in the abstract this is citated "NNT = 24, RR = 1.20 (1.05 to 1.36)"; ie the same RR‐value and 95% CI, but very different NNT.
Christian N Meyer Affiliation: Roskilde Hospital, Denmark Role: consultant, clinical
I do not have any affiliation with or involvement in any organisation with a financial interest in the subject matter of my comment
Reply
Regarding comment 1: Figure 1 has been reported to the Cochrane Library publishers and we have sought assistance to resolve this and move the Figure to the correct section.
Regarding comment 2: the NNT in the text was an error – I have corrected this.
Contributors
Susan Smith
What's new
| Date | Event | Description |
|---|---|---|
| 13 January 2017 | New citation required but conclusions have not changed | We identified no new trials for inclusion or exclusion in this update. |
| 13 January 2017 | New search has been performed | Searches updated. |
History
Protocol first published: Issue 1, 1996 Review first published: Issue 1, 1997
| Date | Event | Description |
|---|---|---|
| 14 November 2016 | Feedback has been incorporated | Feedback comment and response added to the review. |
| 6 February 2015 | Amended | Corrections: number needed to treat for an additional beneficial outcome (NNTB) for outcome 'less likely to have cough' corrected in main text to 6. NNT for 'adverse effects' corrected in Abstract and text to 24. NNTB and wording for outcome 'improved according to physcian assessment' corrected to 11 in Abstract and main text. |
| 15 February 2014 | New citation required but conclusions have not changed | Review updated and strengthens the conclusion suggesting no evidence to support use of antibiotics in people with acute bronchitis. The analysis of adverse effects has been updated by the addition of data from the largest study conducted to date and now indicates a statistically significant rate of adverse effects in the antibiotic‐treated groups. |
| 15 January 2014 | New search has been performed | Searches were updated, and one of the ongoing trials has been published and is now included in the review (Llor 2013). We identified one new included study (Little 2013). |
| 1 March 2012 | Amended | Correction made to Analysis 6.1 'Clinically improved' (Figure 5) as error noted in data entry relating to Stott 1976. This does not change the specific conclusions for this analysis or the overall conclusions of the review. |
| 6 September 2010 | New search has been performed | Searches updated and one new trial included (Nduba 2008). The conclusions remain unchanged. |
| 5 August 2010 | Amended | Contact details updated. |
| 30 April 2008 | Feedback has been incorporated | Feedback and response added. |
| 18 December 2007 | New search has been performed | Searches conducted. |
| 11 December 2007 | Amended | Converted to new review format |
| 22 May 2005 | Amended | Conclusions changed in the Abstract. |
| 21 May 2005 | Feedback has been incorporated | Feedback and response added. |
| 25 March 2004 | New search has been performed | Searches conducted. |
| 29 February 2000 | New search has been performed | Updated search Issue 4, 2000. We found no additional trials, but we were able to obtain unpublished data from three trials that were not included in the original review. The trials were not included in the original review because the participants in the trials as a whole did not meet our inclusion criteria. However, the trials each contained a subgroup of patients that did meet our inclusion criteria. We were also able to obtain additional unpublished data from some of the originally included trials. |
| 27 August 1997 | New search has been performed | Review first published Issue 4, 1997. |
Acknowledgements
We wish to thank Sarah Thorning and Justin Clark from the Cochrane Acute Respiratory Infections Group for assistance with the updated searches in 2009, 2011, 2014, and 2017; William Grant for statistical assistance on the original review; the investigators of the studies included in this review, especially William Hueston, Dana King, Harold Williamson, John Howie, and Laurent Kaiser, who provided us with unpublished data; and Mike Stephenson and Amy Schende, who also provided unpublished information. We would like to acknowledge the work of Dr Rick Glazier, who conceived and designed the original review; graded and extracted data from trials; interpreted data independently, and then as a group, and co‐wrote the 2004 update. We would also like to acknowledge Dr Warren McIsaac, who conceived and designed the original review and co‐wrote the first update. We wish to thank the following people for commenting on the 2009 updated review: Fiona Clay, Jane Nadel, Theo Verheij, Rob Ware, and Peter Morris. We wish to thank Peter Morris, Sree Nair, Theo Verheij, Amanda Young, and Teenah Handiside for comments and suggestions regarding the 2011 update. We thank Beth Stuart and Paul Little, who provided additional data for the 2013 update. Finally, we wish to thank Raghda Rashad, Theo Verheij, Conor Teljeur, and Peter Morris for commenting on the 2013 update.
Appendices
Appendix 1. MEDLINE (Ovid) and CENTRAL search strategy
1 exp Bronchitis/ 2 bronchit*.tw. 3 (bronchial adj2 infect*).tw. 4 exp Respiratory Tract Infections/ 5 or/1‐4 6 exp Anti‐Bacterial Agents/ 7 exp Lactams/ 8 exp Tetracyclines/ 9 exp Aminoglycosides/ 10 exp Glycopeptides/ 11 exp Macrolides/ 12 antibiotic*.tw. 13 (alamethicin or amdinocillin* or amikacin or amoxicillin* or ampicillin or aurodox or azithromycin or azlocillin or aztreonam or bacitracin or bacteriocin* or brefeldin* or butirosin* or candicidin or carbenicillin or carfecillin or cefaclor or cefadroxil or cefamandole or cefazolin or cefixime or cefmenoxime or cefmetazole or cefonicid or cefoperazone or cefotaxime or cefotetan or cefotiam or cefoxitin or cefsulodin or ceftazidime or ceftizoxime or ceftriaxone or cefuroxime or cephacetrile or cephalexin or cephaloglycin or cephaloridine or cephalosporin* or cephalothin or cephapirin or cephradine or chloramphenicol or chlortetracycline or citrinin or clarithromycin or clavulanic acid* or clindamycin or cloxacillin or colistin or cyclacillin or dactinomycin or daptomycin or demeclocycline or dibekacin or dicloxacillin or dihydrostreptomycin* or distamycin* or doxycycline or echinomycin or edeine or erythromycin* or floxacillin or framycetin or fusidic acid or gentamicin* or gramicidin or imipenem or lactam* or lasalocid or leucomycins or lymecycline or mepartricin or methacycline or methicillin or mezlocillin or mikamycin or minocycline or miocamycin or moxalactam or mupirocin or mycobacillin or nafcillin or nebramycin or enigericin or nisin or novobiocin or nystatin or ofloxacin or oligomycins or oxacillin or oxytetracycline or penicillanic acid or penicillic acid or penicillin* or piperacillin or pivampicillin or polymyxin* or pristinamycin* or prodigiosin or rifabutin or ristocetin or rolitetracycline or roxarsone or rutamycin or sirolimus or sisomicin or spectinomycin or streptogramin* or streptovaricin or sulbactam or sulbenicillin or talampicillin or teicoplanin or tetracycline or thiamphenicol or thiostrepton or ticarcillin or tobramycin or troleandomycin or tylosin or tyrocidine or tyrothricin or valinomycin or vancomycin or vernamycin* or viomycin* or virginiamycin* or beta‐lactam*).tw,nm. 14 or/6‐13 15 5 and 14
We combined the MEDLINE search with the Cochrane Highly Sensitive Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision); Ovid format (Lefebvre 2011).
Appendix 2. Embase.com search strategy
#2.24 #2.15 AND #2.23 #2.23 #2.18 NOT #2.22 #2.22 #2.19 NOT #2.21 #2.21 #2.19 AND #2.20 #2.20 'human'/de #2.19 'animal'/de OR 'nonhuman'/de OR 'animal experiment'/de #2.18 #2.16 OR #2.17 #2.17 random*:ab,ti OR placebo*:ab,ti OR crossover*:ab,ti OR 'cross over':ab,ti OR allocat*:ab,ti OR trial:ti OR (doubl* NEXT/1 blind*):ab,ti #2.16 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp #2.15 #2.5 AND #2.14 #2.14 #2.6 OR #2.7 OR #2.8 OR #2.9 OR #2.10 OR #2.11 OR #2.12 OR #2.13 #2.13 alamethicin:ab,ti OR amdinocillin*:ab,ti OR amikacin:ab,ti OR amoxicillin*:ab,ti OR ampicillin:ab,ti OR aurodox:ab,ti OR azithromycin:ab,ti OR azlocillin:ab,ti OR aztreonam:ab,ti OR bacitracin:ab,ti OR bacteriocin*:ab,ti OR brefeldin*:ab,ti OR butirosin*:ab,ti OR candicidin:ab,ti OR carbenicillin:ab,ti OR carfecillin:ab,ti OR cefaclor:ab,ti OR cefadroxil:ab,ti OR cefamandole:ab,ti OR cefazolin:ab,ti OR cefixime:ab,ti OR cefmenoxime:ab,ti OR cefmetazole:ab,ti OR cefonicid:ab,ti OR cefoperazone:ab,ti OR cefotaxime:ab,ti OR cefotetan:ab,ti OR cefotiam:ab,ti OR cefoxitin:ab,ti OR cefsulodin:ab,ti OR ceftazidime:ab,ti OR ceftizoxime:ab,ti OR ceftriaxone:ab,ti OR cefuroxime:ab,ti OR cephacetrile:ab,ti OR cephalexin:ab,ti OR cephaloglycin:ab,ti OR cephaloridine:ab,ti OR cephalosporin*:ab,ti OR cephalothin:ab,ti OR cephapirin:ab,ti OR cephradine:ab,ti OR chloramphenicol:ab,ti OR chlortetracycline:ab,ti OR citrinin:ab,ti OR clarithromycin:ab,ti OR 'clavulanic acid':ab,ti OR clindamycin:ab,ti OR cloxacillin:ab,ti OR colistin:ab,ti OR cyclacillin:ab,ti OR dactinomycin:ab,ti OR daptomycin:ab,ti OR demeclocycline:ab,ti OR dibekacin:ab,ti OR dicloxacillin:ab,ti OR dihydrostreptomycin*:ab,ti OR distamycin*:ab,ti OR doxycycline:ab,ti OR echinomycin:ab,ti OR edeine:ab,ti OR erythromycin*:ab,ti OR floxacillin:ab,ti OR framycetin:ab,ti OR 'fusidic acid':ab,ti OR gentamicin*:ab,ti OR gramicidin:ab,ti OR imipenem:ab,ti OR lactam*:ab,ti OR lasalocid:ab,ti OR leucomycins:ab,ti OR lymecycline:ab,ti OR mepartricin:ab,ti OR methacycline:ab,ti OR methicillin:ab,ti OR mezlocillin:ab,ti OR mikamycin:ab,ti OR minocycline:ab,ti OR miocamycin:ab,ti OR moxalactam:ab,ti OR mupirocin:ab,ti OR mycobacillin:ab,ti OR nafcillin:ab,ti OR nebramycin:ab,ti OR enigericin:ab,ti OR nisin:ab,ti OR novobiocin:ab,ti OR nystatin:ab,ti OR ofloxacin:ab,ti OR oligomycins:ab,ti OR oxacillin:ab,ti OR oxytetracycline:ab,ti OR 'penicillanic acid':ab,ti OR 'penicillic acid':ab,ti OR penicillin*:ab,ti OR piperacillin:ab,ti OR pivampicillin:ab,ti OR polymyxin*:ab,ti OR pristinamycin*:ab,ti OR prodigiosin:ab,ti OR rifabutin:ab,ti OR ristocetin:ab,ti OR rolitetracycline:ab,ti OR roxarsone:ab,ti OR rutamycin:ab,ti OR sirolimus:ab,ti OR sisomicin:ab,ti OR spectinomycin:ab,ti OR streptogramin*:ab,ti OR streptovaricin:ab,ti OR sulbactam:ab,ti OR sulbenicillin:ab,ti OR talampicillin:ab,ti OR teicoplanin:ab,ti OR tetracycline:ab,ti OR thiamphenicol:ab,ti OR thiostrepton:ab,ti OR ticarcillin:ab,ti OR tobramycin:ab,ti OR troleandomycin:ab,ti OR tylosin:ab,ti OR tyrocidine:ab,ti OR tyrothricin:ab,ti OR valinomycin:ab,ti OR vancomycin:ab,ti OR vernamycin*:ab,ti OR viomycin*:ab,ti OR virginiamycin*:ab,ti OR 'beta‐lactam':ab,ti OR 'beta‐lactams':ab,ti #2.12 antibiotic*:ab,ti #2.11 'macrolide'/exp #2.10 'glycopeptide'/de #2.9 'aminoglycoside'/de #2.8 'tetracycline derivative'/exp #2.7 'lactam'/exp #2.6 'antibiotic agent'/de #2.5 #2.1 OR #2.2 OR #2.3 OR #2.4 #2.4 'respiratory tract infection'/de OR 'lower respiratory tract infection'/de #2.3 (bronchial* NEAR/2 infect*):ab,ti #2.2 bronchit*:ab,ti #2.1 'bronchitis'/exp
Appendix 3. LILACS (BIREME) search strategy
(mh:bronchitis OR bronchit* OR bronquitis OR bronquite OR mh:c08.127.446* OR mh:c08.381.495.146* OR mh:c08.730.099* OR "bronchial infection" OR "bronchial infections" OR mh:"Respiratory Tract Infections" OR "respiratory tract infection" OR "respiratory tract infections" OR "Infecciones del Sistema Respiratorio" OR "Infecções Respiratórias") AND (mh:"Anti‐Bacterial Agents" OR antibiotic* OR antibacterianos OR mh:d27.505.954.122.085* OR mh:lactams OR lactam* OR mh:d02.065.589* OR mh:d03.383.411* OR mh:tetracyclines OR tetracyclin* OR tetraciclinas OR mh:d02.455.426.559.847.562.900* OR mh:d04.615.562.900* OR mh:aminoglycosides OR aminoglicósidos OR aminoglicosídeos OR mh:d09.408.051* OR aminoglycoside* OR mh:glycopeptides OR glycopeptide* OR glicopéptidos OR glicopeptídeos OR mh:d09.400.420* OR mh:d12.644.233* OR mh:macrolides OR macrolide* OR macrólidos OR macrolídeos OR mh:d02.540.505* OR alamethicin OR amdinocillin* OR amikacin OR amoxicillin* OR ampicillin OR aurodox OR azithromycin OR azlocillin OR aztreonam OR bacitracin OR bacteriocin* OR brefeldin* OR butirosin* OR candicidin OR carbenicillin OR carfecillin OR cefaclor OR cefadroxil OR cefamandole OR cefazolin OR cefixime OR cefmenoxime OR cefmetazole OR cefonicid OR cefoperazone OR cefotaxime OR cefotetan OR cefotiam OR cefoxitin OR cefsulodin OR ceftazidime OR ceftizoxime OR ceftriaxone OR cefuroxime OR cephacetrile OR cephalexin OR cephaloglycin OR cephaloridine OR cephalosporin* OR cephalothin OR cephapirin OR cephradine OR chloramphenicol OR chlortetracycline OR citrinin OR clarithromycin OR “clavulanic acid” OR clindamycin OR cloxacillin OR colistin OR cyclacillin OR dactinomycin OR daptomycin OR demeclocycline OR dibekacin OR dicloxacillin OR dihydrostreptomycin* OR distamycin* OR doxycycline OR echinomycin OR edeine OR erythromycin* OR floxacillin OR framycetin OR “fusidic acid” OR gentamicin* OR gramicidin OR imipenem OR lactam* OR lasalocid OR leucomycins OR lymecycline OR mepartricin OR methacycline OR methicillin OR mezlocillin OR mikamycin OR minocycline OR miocamycin OR moxalactam OR mupirocin OR mycobacillin OR nafcillin OR nebramycin OR enigericin OR nisin OR novobiocin OR nystatin OR ofloxacin OR oligomycins OR oxacillin OR oxytetracycline OR “penicillanic acid” OR “penicillic acid” OR penicillin* OR piperacillin OR pivampicillin OR polymyxin* OR pristinamycin* OR prodigiosin OR rifabutin OR ristocetin OR rolitetracycline OR roxarsone OR rutamycin OR sirolimus OR sisomicin OR spectinomycin OR streptogramin* OR streptovaricin OR sulbactam OR sulbenicillin OR talampicillin OR teicoplanin OR tetracycline OR thiamphenicol OR thiostrepton OR ticarcillin OR tobramycin OR troleandomycin OR tylosin OR tyrocidine OR tyrothricin OR valinomycin OR vancomycin OR vernamycin* OR viomycin* OR virginiamycin* OR “beta‐lactam” OR “beta‐lactams”) AND db:("LILACS") AND type_of_study:("clinical_trials")
Appendix 4. Previous search strategy
In this updated review, we searched the Cochrane Central Register of Controlled trials (CENTRAL) (The Cochrane Library 2007, issue 4), which includes the Acute Respiratory Infections (ARI) Group's Specialised Register; MEDLINE (1966 to December 2007); and EMBASE (1974 to December 2007). For details of the search strategy used, see Appendix 2.
The updated MEDLINE (OVID) search used the following search strategy:
1 RANDOMIZED CONTROLLED TRIAL.pt. (228029) 2 CONTROLLED CLINICAL TRIAL.pt. (73939) 3 RANDOMIZED CONTROLLED TRIALS.sh. (46488) 4 RANDOM ALLOCATION.sh. (56676) 5 DOUBLE BLIND METHOD.sh. (89072) 6 SINGLE‐BLIND METHOD.sh. (10505) 7 or/1‐6 (387195) 8 HUMANs.sh. (9533289) 9 ANIMALs.sh. (3970623) 10 9 not 8 (3018353) 11 7 not 10 (364156) 12 CLINICAL TRIAL.pt. (431113) 13 exp Clinical Trials/ (185629) 14 (clin$ adj25 trial$).ti,ab. (124831) 15 ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab. (88283) 16 PLACEBOS.sh. (25705) 17 placebo$.ti,ab. (99261) 18 random$.ti,ab. (357426) 19 or/12‐18 (787581) 20 19 not 10 (731504) 21 11 or 20 (748271) 22 exp BRONCHITIS/ (22484) 23 acute bronchit$.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (884) 24 exp Respiratory Tract Infections/ (215767) 25 or/22‐24 (217540) 26 Anti‐Bacterial Agents/ (157181) 27 exp Lactams/ (90537) 28 exp Tetracyclines/ (31342) 29 exp Aminoglycosides/ (97899) 30 exp Glycopeptides/ (37656) 31 exp Macrolides/ (66142) 32 antibiotic$.mp. [mp=title, original title, abstract, name of substance word, subject heading word] (166066) 33 exp alamethicin/ or exp amdinocillin/ or exp amdinocillin pivoxil/ or exp amikacin/ or exp amoxicillin/ or exp amoxicillin‐potassium clavulanate combination/ or exp ampicillin/ or exp aurodox/ or exp azithromycin/ or exp azlocillin/ or exp aztreonam/ or exp bacitracin/ or exp bacteriocins/ or exp brefeldin a/ or exp butirosin sulfate/ or exp candicidin/ or exp carbenicillin/ or exp carfecillin/ or exp cefaclor/ or exp cefadroxil/ or exp cefamandole/ or exp cefazolin/ or exp cefixime/ or exp cefmenoxime/ or exp cefmetazole/ or exp cefonicid/ or exp cefoperazone/ or exp cefotaxime/ or exp cefotetan/ or exp cefotiam/ or exp cefoxitin/ or exp cefsulodin/ or exp ceftazidime/ or exp ceftizoxime/ or exp ceftriaxone/ or exp cefuroxime/ or exp cephacetrile/ or exp cephalexin/ or exp cephaloglycin/ or exp cephaloridine/ or exp cephalosporins/ or exp cephalothin/ or exp cephapirin/ or exp cephradine/ or exp chloramphenicol/ or exp chlortetracycline/ or exp citrinin/ or exp clarithromycin/ or exp clavulanic acid/ or exp clavulanic acids/ or exp clindamycin/ or exp cloxacillin/ or exp colistin/ or exp cyclacillin/ or exp dactinomycin/ or exp daptomycin/ or exp demeclocycline/ or exp dibekacin/ or exp dicloxacillin/ or exp dihydrostreptomycin sulfate/ or exp distamycins/ or exp doxycycline/ or exp echinomycin/ or exp edeine/ or exp erythromycin/ or exp erythromycin estolate/ or exp erythromycin ethylsuccinate/ or exp floxacillin/ or exp framycetin/ or exp fusidic acid/ or exp gentamicins/ or exp gramicidin/ or exp imipenem/ or exp lactams/ or exp lasalocid/ or exp leucomycins/ or exp lymecycline/ or exp mepartricin/ or exp methacycline/ or exp methicillin/ or exp mezlocillin/ or exp mikamycin/ or exp minocycline/ or exp miocamycin/ or exp moxalactam/ or exp mupirocin/ or exp mycobacillin/ or exp nafcillin/ or exp nebramycin/ or exp nigericin/ or exp nisin/ or exp novobiocin/ or exp nystatin/ or exp ofloxacin/ or exp oligomycins/ or exp oxacillin/ or exp oxytetracycline/ or exp penicillanic acid/ or exp penicillic acid/ or exp penicillin g/ or exp penicillin g, benzathine/ or exp penicillin g, procaine/ or exp penicillin v/ or exp piperacillin/ or exp pivampicillin/ or exp polymyxin b/ or exp polymyxins/ or exp pristinamycin/ or exp prodigiosin/ or exp rifabutin/ or exp ristocetin/ or exp rolitetracycline/ or exp roxarsone/ or exp rutamycin/ or exp sirolimus/ or exp sisomicin/ or exp spectinomycin/ or exp streptogramin a/ or exp streptogramin group a/ or exp streptogramin group b/ or exp streptogramins/ or exp streptovaricin/ or exp sulbactam/ or exp sulbenicillin/ or exp talampicillin/ or exp teicoplanin/ or exp tetracycline/ or exp thiamphenicol/ or exp thiostrepton/ or exp ticarcillin/ or exp tobramycin/ or exp troleandomycin/ or exp tylosin/ or exp tyrocidine/ or exp tyrothricin/ or exp valinomycin/ or exp vancomycin/ or exp vernamycin b/ or exp viomycin/ or exp virginiamycin/ or exp beta‐lactams/ (211481) 34 or/26‐33 (499372) 35 21 and 25 and 34 (4684) 36 limit 35 to ed=20040103‐20070201 (761) 37 from 36 keep 1‐761 (761)
Data and analyses
Comparison 1. Cough at follow‐up visit.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of participants with cough | 4 | 275 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.49, 0.85] |
Comparison 2. Night cough at follow‐up visit.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of participants with night cough | 4 | 538 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.54, 0.83] |
Comparison 3. Productive cough at follow‐up visit.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of participants with productive cough | 7 | 713 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.82, 1.16] |
| 1.1 Acute bronchitis studies | 6 | 549 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.72, 1.08] |
| 1.2 Subgroup with productive cough from URTI study | 1 | 164 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.24 [0.88, 1.75] |
3.1. Analysis.
Comparison 3 Productive cough at follow‐up visit, Outcome 1 Number of participants with productive cough.
Comparison 4. Days of cough.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean number of days of cough | 7 | 2776 | Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐0.87, ‐0.04] |
| 1.1 Acute bronchitis studies | 6 | 2350 | Mean Difference (IV, Fixed, 95% CI) | ‐0.55 [1.00, ‐0.10] |
| 1.2 Subgroup with no placebo control | 1 | 426 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐1.01, 1.23] |
4.1. Analysis.
Comparison 4 Days of cough, Outcome 1 Mean number of days of cough.
Comparison 5. Days of productive cough.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean number of days of productive cough | 6 | 699 | Mean Difference (IV, Fixed, 95% CI) | ‐0.43 [‐0.93, 0.07] |
| 1.1 Acute bronchitis studies | 5 | 535 | Mean Difference (IV, Fixed, 95% CI) | ‐0.52 [‐1.03, ‐0.01] |
| 1.2 Subgroup with productive cough from URTI study | 1 | 164 | Mean Difference (IV, Fixed, 95% CI) | 1.04 [‐1.04, 3.12] |
5.1. Analysis.
Comparison 5 Days of productive cough, Outcome 1 Mean number of days of productive cough.
Comparison 6. Clinically improved.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of participants reporting no activity limitations or described as cured/globally improved | 11 | 3841 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.99, 1.15] |
Comparison 7. Limitation in work or activities at follow‐up visit.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of participants with limitations | 5 | 478 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.75 [0.46, 1.22] |
7.1. Analysis.
Comparison 7 Limitation in work or activities at follow‐up visit, Outcome 1 Number of participants with limitations.
Comparison 8. Days of feeling ill.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean number of days of feeling ill | 5 | 809 | Mean Difference (IV, Fixed, 95% CI) | ‐0.64 [‐1.16, ‐0.13] |
| 1.1 Acute bronchitis studies | 4 | 435 | Mean Difference (IV, Fixed, 95% CI) | ‐0.58 [‐1.16, ‐0.00] |
| 1.2 Subgroup with no placebo control | 1 | 374 | Mean Difference (IV, Fixed, 95% CI) | ‐0.86 [‐1.97, 0.25] |
Comparison 9. Days of impaired activities.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean number of days of impaired activities | 6 | 767 | Mean Difference (IV, Fixed, 95% CI) | ‐0.49 [‐0.94, ‐0.04] |
| 1.1 Acute bronchitis studies | 5 | 393 | Mean Difference (IV, Fixed, 95% CI) | ‐0.48 [‐0.96, 0.01] |
| 1.2 Subgroup with no placebo control | 1 | 374 | Mean Difference (IV, Fixed, 95% CI) | ‐0.57 [‐1.75, 0.61] |
Comparison 10. Not improved by physician's global assessment at follow‐up visit.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of participants not improved | 6 | 891 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.48, 0.79] |
| 1.1 Acute bronchitis studies | 5 | 816 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.30, 0.65] |
| 1.2 Subgroup with non‐purulent tracheobronchitis from URTI study | 1 | 75 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.82, 1.29] |
Comparison 11. Abnormal lung exam at follow‐up visit.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of participants with abnormal lung exams | 5 | 613 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.41, 0.70] |
Comparison 12. Adverse effects.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of participants with adverse effects | 12 | 3496 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.20 [1.05, 1.36] |
| 1.1 Acute bronchitis studies | 11 | 3162 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.22 [1.07, 1.40] |
| 1.2 Subgroup with no placebo control | 1 | 334 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.61, 1.50] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Brickfield 1986.
| Methods | Double‐blinded RCT | |
| Participants | 52 adults (aged 18 to 65), with 2 weeks or less of lower respiratory infection with sputum production and no evidence of pneumonia clinically or radiographically. Dropouts = 2/52 | |
| Interventions | Enteric‐coated erythromycin 333 mg 3 times a day for 7 days versus placebo. Volunteers kept daily logs of multiple symptoms and were re‐examined on day 8. | |
| Outcomes | Cough, sputum, fever, rhinorrhoea, chest discomfort, earache, sore throat, work disability, feeling ill, and nausea daily; and clinical impression at follow‐up | |
| Notes | 29 participants had sputum cultured (27 = normal flora, 1 = Haemophilus influenzae, 1 = Streptococcus pneumoniae), outcomes not reported; 17/23 had more than 5 white blood cells on Gram stain. Fewer than 30% of eligible patients opted to volunteer (most wanted antibiotics). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | Low risk | |
Dunlay 1987.
| Methods | Double‐blinded RCT | |
| Participants | 63 adults (age 18 years or older) with productive cough (mean duration = 7 days) and no clinical evidence of sinusitis or pneumonia. Dropouts = 15 (6 no follow‐up; 9 stopped taking pills during trial, authors state that there was no difference in results with or without the partial data from the latter 9) | |
| Interventions | Enteric‐coated erythromycin base, 333 mg 3 times a day for 10 days, versus placebo. Participants kept daily logs of 5 symptoms and had follow‐up visit at approximately day 14. | |
| Outcomes | Day cough, night cough, sputum production, congestion, sore throat, feeling poorly, activity limitation, and use of cough/cold medications daily; and cough, sputum, and abnormal lung examination at follow‐up | |
| Notes | Only 20% of eligible patients enrolled in study (but unenrolled not different clinically per chart review). 13 erythromycin participants dropped out due to gastrointestinal side effects. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Blinding (performance bias and detection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | Low risk | |
Evans 2002.
| Methods | Double‐blinded RCT | |
| Participants | 220 adults (aged 18 to 88 years) with cough (with or without sputum) of 2 to 14 days duration | |
| Interventions | Azithromycin 500 mg on day 1 and 250 mg daily on days 2 to 5 versus vitamin C 500 mg on day 1 and 250 mg daily on days 2 to 5 (total dose 1.5 g) | |
| Outcomes | Acute bronchitis health‐related quality of life on days 3 and 7, proportion of participants who had returned to usual daily activities on days 3 and 7, side effects on days 3 and 7 | |
| Notes | 88% of eligible population included. Both groups received cough suppressant (dextromethorphan) and albuterol inhaler. No difference between groups in the use of albuterol inhaler at follow‐up. 31/220 (14%) lost to follow‐up. Timing of outcome at day 3 and day 7 (day 7 taken as outcome time in this review). Study was stopped by data‐monitoring and safety committee because "outcomes were equivalent and there was sufficient precision to be confident that the likelihood of detecting a clinically meaningful difference with a larger sample was so small that continued enrolment of patients would be inappropriate". | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Blinding (performance bias and detection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | Low risk | |
Franks 1984.
| Methods | Double‐blinded RCT | |
| Participants | 67 people aged 14 years or older with fewer than 15 days of productive cough (in the absence of clinical pneumonitis). Excluded if could not produce sputum specimen for Gram stain. Dropouts = 13/67 | |
| Interventions | Trimethoprim‐sulfamethoxazole (160/800) twice daily for 7 days versus identical‐appearing placebo. Participants kept daily symptom logs. No follow‐up visit | |
| Outcomes | Cough, night cough, sputum production, general well‐being, fever, work disability, use of adjunctive medications, and side effects | |
| Notes | No mention of per cent of eligible patients who refused enrolment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Blinding (performance bias and detection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | Low risk | |
Howie 1970.
| Methods | Double‐blinded RCT | |
| Participants | 164 people with a productive cough in conjunction with a cold or influenza‐like illness that had not resolved after 2 days | |
| Interventions | Self treatment with demethyl chlortetracycline (300 mg) or placebo twice daily for 5 days. Participants kept daily symptom logs. No initial or follow‐up visits | |
| Outcomes | Duration of and presence on day 5 of cough, productive cough, and purulent sputum; and duration of time off work | |
| Notes | These were unpublished data about a subgroup of patients with a cold or influenza‐like illness; total number of people who treated themselves for a single episode of illness and returned symptom cards = 301. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Blinding (performance bias and detection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | Low risk | |
Hueston 1994.
| Methods | Double‐blinded RCT | |
| Participants | 23 adults (aged 18 to 65 years) with productive cough of fewer than 30 days duration and no clinical evidence of pneumonia. Dropouts = 0 | |
| Interventions | Erythromycin (250 mg) 4 times a day for 10 days versus identical‐looking placebo. Participants kept daily symptom log and were re‐examined on day 7 or 8. | |
| Outcomes | Cough, night cough, ability to perform normal work, and general well‐being daily and at follow‐up; overall use of over‐the‐counter medications and side effects; and abnormal lung exam at follow‐up | |
| Notes | This was part of a 2 x 2 designed study comparing erythromycin + albuterol inhaler versus erythromycin + placebo versus albuterol inhaler + placebo versus placebo + placebo. The data extracted for this review were unpublished and limited to the erythromycin + placebo group versus the placebo + placebo group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | Unclear risk | Not reported |
Kaiser 1996.
| Methods | Double‐blinded RCT | |
| Participants | 75 people (aged 16 to 64 years) with common cold and concomitant non‐purulent tracheobronchitis and no evidence of sinusitis, pharyngitis, purulent bronchitis, or pneumonia. Mean duration of illness 3 days | |
| Interventions | Amoxicillin‐clavulanic acid (375 mg 3 times a day for 5 days) versus identical‐looking placebo. Participants re‐evaluated on days 5 to 7. | |
| Outcomes | Persistent or worse symptoms versus cure at follow‐up | |
| Notes | These were unpublished data about a subgroup of patients in a study of people with common cold; total number of participants in study was 307. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Blinding (performance bias and detection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | Low risk | |
King 1996.
| Methods | Double‐blinded RCT | |
| Participants | 91 people (age 8 years or older) with cough and sputum for up to 2 weeks, and no signs of sinusitis, otitis, or pneumonia and no localised abnormal lung exam. All tested for Mycoplasma (one‐half with negative serology excluded). | |
| Interventions | Erythromycin (250 mg 4 times a day for 10 days) versus identical‐looking placebo. Participants kept daily logs and returned for follow‐up visit at days 14 to 18. | |
| Outcomes | Cough, chest congestion, use of cough medication, general well‐being, sleep, and normal activities | |
| Notes | No mention of eligible patients who refused to volunteer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Blinding (performance bias and detection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | High risk | |
Little 2005.
| Methods | RCT | |
| Participants | 426, subgroup of 807 patients with acute uncomplicated lower respiratory tract infection. Inclusion criteria: aged 3 years or older with uncomplicated LRTI for fewer than 21 days with cough as main symptom and at least 1 of sputum, chest pain, dyspnoea, and wheeze | |
| Interventions | 6‐arm RCT: (1) no leaflet or antibiotics; (2) immediate antibiotics plus leaflet; (3) immediate antibiotics and no leaflet; (4) leaflet only; (5) leaflet and delayed antibiotics; (6) no leaflet and delayed antibiotics. Only data from the no‐treatment and immediate‐antibiotic groups included in the analysis. The antibiotic used was amoxicillin 250 mg 3 times a day for 10 days (125 mg if younger than 10 years) or erythromycin 250 mg 4 times a day if penicillin allergic. | |
| Outcomes | Daily diary for 3 weeks recording antipyretic use and 6 symptoms (cough, dyspnoea, sputum production, well‐being, sleep disturbance, and activity disturbance); satisfaction questionnaire; belief in antibiotics scale; reported antibiotic use; note review for reconsultation | |
| Notes | 25% of participants lost to follow‐up in no‐treatment and immediate‐antibiotic arms | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open design |
| Blinding (performance bias and detection bias) | High risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | Low risk | |
Little 2013.
| Methods | RCT | |
| Participants | 2061 people aged 18 years or older presenting with lower respiratory tract infection with cough duration fewer than 28 days | |
| Interventions | Amoxicillin 1 g 3 times daily for 7 days | |
| Outcomes | Duration of symptoms rated as moderately bad or worsening; mean symptom severity on days 2 to 4; proportion with symptoms resolved on day 7; new or worsening symptoms presenting clinically to general practitioners; and adverse effects | |
| Notes | Adequately powered for subgroup analysis of participants aged over 60 (n = 595) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Clinicians, participants, and outcome assessors all blinded. |
| Blinding (performance bias and detection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 88% follow‐up in both intervention and control groups |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Llor 2013.
| Methods | RCT (3 arms) | |
| Participants | 420 people age 18 to 70 years presenting with respiratory tract infection of 1‐week evolution with cough as the predominant symptom. We included data from the antibiotic arm (137 participants) and the placebo arm (143 participants). | |
| Interventions | Ibuprofen or amoxicillin‐clavulanic acid (dose 500 mg/125 mg) | |
| Outcomes | Number days with frequent cough defined using a symptom diary. Secondary outcomes included clinically improved or cured, time to symptom resolution, median days with cough, and adverse effects. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised using a random number table into 3 blocks. |
| Allocation concealment (selection bias) | Low risk | Participants were unaware of allocation. Clinicians gave participants sealed containers, so they were also unaware of allocation. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants blinded and described as single‐blind study. Tablets placed in sealed containers before dispatch by an independent pharmacist. |
| Blinding (performance bias and detection bias) | Low risk | Outcomes collected in symptom diaries not seen by the investigators. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | > 90% follow‐up |
| Selective reporting (reporting bias) | Low risk | Protocol available |
| Other bias | Unclear risk | Not reported |
Matthys 2000.
| Methods | Double‐blinded RCT | |
| Participants | 294, a subgroup of 676 participants, mean age 39 (range 18 to 79) with acute bronchitis. Inclusion criteria: aged 18 years or older, symptoms of recent onset within last 5 days, nightly cough as main symptom (without at least 4 awakenings during the night) and without reduced FEV1 (more than 75% normal) | |
| Interventions | 4‐arm RCT: (1) Myrtol standardised (phytotherapeutic extract); (2) cefuroxime 500 mg twice daily; (3) ambroxol (mucolytic agent); (4) placebo capsules. Only data from cefuroxime and placebo arms were included in the analysis. | |
| Outcomes | Daytime cough, nighttime cough, type of cough, and general well‐being recorded by each participant; clinical examination at follow‐up; "overall efficacy" judged by physician and participant; bronchial hyperreactivity; change in lung function; number of participants with relapse within 4 weeks; side effects. Physician assessment at days 7 and 14; diary data on 3 follow‐up time periods: day 7, 14, 15 to 28 | |
| Notes | Secretolytics, mucolytics, and antitussives prohibited during the study. Multiple hypothesis testing for all 4 treatment groups. 3/343 (0.9%) lost to follow‐up | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | Low risk | |
Nduba 2008.
| Methods | Triple‐blind, placebo‐controlled RCT | |
| Participants | 660 participants, mean age 31; 55% female. Productive cough for < 2 weeks, no serious medical comorbidity, and no antibiotic treatment in previous 2 weeks. All participants had HIV test and chest X‐ray at baseline. Excluded if chest X‐ray showed pneumonia or tuberculosis | |
| Interventions | Amoxicillin 500 mg 3 times a day for 7 days versus identical placebo tablet | |
| Outcomes | Clinical cure at 14 days as measured by > 75% reduction in Acute Bronchitis Severity Score | |
| Notes | Reported as first study of acute bronchitis treatment that used an equivalence design. Data available for HIV‐positive patients but not included in the review. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised independently using a random number generator. |
| Allocation concealment (selection bias) | Low risk | Antibiotic or placebo tablets identical in appearance, taste, and smell were placed in identical sealed, opaque containers identifiable only with a unique study identifier. |
| Blinding (performance bias and detection bias) All outcomes | Low risk | All clinical and research staff were blinded to the allocation of participants, and the allocation schedule was kept in the office of the Chief Research Pharmacist in the host institution. |
| Blinding (performance bias and detection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | > 85% follow‐up for outcome data |
| Selective reporting (reporting bias) | Unclear risk | No access to original protocol, though selective reporting not apparent from trial description. |
| Other bias | Low risk | |
Scherl 1987.
| Methods | Double‐blinded RCT | |
| Participants | 39 people (older than 12 years) with chief complaint of cough with purulent sputum and without the following: other known bacterial infection, flu‐like syndrome, chief complaint of coryza or sore throat with minimal sputum, or chest radiograph consistent with pneumonia (not all had radiographs). Dropouts = 8/31 | |
| Interventions | Doxycycline (100 mg twice daily on day 1 and 100 mg 4 times a day on days 2 to 7) versus placebo. Kept daily symptom log and had follow‐up visit at day 14 | |
| Outcomes | Cough, sputum, feverishness, days missed from work or normal activity, chest pain, dyspnoea, side effects | |
| Notes | No mention of eligible patients who refused to volunteer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Blinding (performance bias and detection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Not reported |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | Low risk | |
Stott 1976.
| Methods | Double‐blinded RCT | |
| Participants | 212 people aged > 14 years with cough and purulent sputum for up to 1 week. Excluded if chest exam was abnormal. Dropouts = 5/212 | |
| Interventions | Participants given doxycycline or placebo (2 pills on day 1, then 1 daily for 9 days). Had follow‐up after 1 week; if "satisfied with outcome", then treatment ended; if not, then completed remaining pills and continued to record symptoms. Participants completed daily symptom logs. | |
| Outcomes | Day cough, night cough, "yellow spit", "clear spit", "off color", runny nose, sore throat, general aches, headache, vomiting, off work daily and at follow‐up; clinical impression at follow‐up; and illnesses over next 6 months | |
| Notes | No difference in average pill consumption between groups (9.3 in doxycycline group versus 9.2 in placebo group). No mention of eligible patients who refused to volunteer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Blinding (performance bias and detection bias) | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | Low risk | |
Verheij 1994.
| Methods | Double‐blinded RCT | |
| Participants | 158 adults (age 18 years or older) with cough and purulent sputum, and no clinical sinusitis or pneumonia. Dropouts = 13/158 | |
| Interventions | Doxycycline (200 mg on day 1 and 100 mg on days 2 to 10) versus placebo. Participants kept daily symptom log, and had follow‐up visit on day 11. | |
| Outcomes | Day cough, night cough, productive cough, feeling ill, impairment of activities, and side effects daily; and clinical impression and auscultatory abnormalities at follow‐up | |
| Notes | 158/209 eligible patients entered study (no difference in age, sex, or main symptoms between participants and unenrolled). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Blinding (performance bias and detection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | Low risk | |
Williamson 1984.
| Methods | Double‐blinded RCT | |
| Participants | 74 adults (age 21 to 65 years) with cough and sputum, and concurrent upper respiratory tract infection, rhonchi, or history of fever; excluded if temperature more than 39.5° C, signs or symptoms of sinus infection, or chest radiograph with consolidation (but not ordered on all). Dropouts = 5/74 | |
| Interventions | Doxycycline (100 mg twice daily on day 1, then 100 mg 4 times a day on days 2 to 7) versus identical‐looking placebo. Kept daily symptom log, returned for follow‐up visit on day 7 to 10. If not improved at follow‐up, could obtain antibiotic prescription. | |
| Outcomes | General well‐being, bother of cough, night cough, activity limitation, feverishness, sputum colour daily, doses of antitussives, and clinical impression at follow‐up | |
| Notes | No mention of eligible patients who refused to volunteer | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | |
| Allocation concealment (selection bias) | Low risk | |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Blinding (performance bias and detection bias) | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | |
| Selective reporting (reporting bias) | Unclear risk | Not reported |
| Other bias | Unclear risk | Not reported |
FEV1: forced expiratory volume in one second LRTI: lower respiratory tract infection RCT: randomised controlled trial
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Batieha 2002 | 185 participants with acute respiratory tract infection from 2 health centres in Jordan. Assignment to antibiotic (azithromycin) was by means of alternation, not randomisation. At follow‐up of 3 days, 1 week, and 2 weeks, participants administered azithromycin or placebo did similarly in terms of the proportions improved or cured and duration of illness. The authors of the study concluded that routine use of antibiotics (azithromycin) in acute respiratory tract infection is unlikely to alter the course of the illness. |
| Christ‐Crain 2004 | Randomised controlled trial concerned with application of a diagnostic test (serum calcitonin precursor, procalcitonin) that is raised in bacterial infections. 243 people admitted to hospital with suspected lower respiratory tract infections were randomly assigned to standard care (n = 119) or procalcitonin‐guided treatment (n = 124). On the basis of serum procalcitonin concentrations, use of antibiotics was more or less discouraged (< 0.1 µg/L or < 0.25 µg/L) or encouraged (≥ 0.5 µg/L or ≥ 0.25 µg/L), respectively. Re‐evaluation was possible after 6 to 24 hours in both groups. Primary endpoint was use of antibiotics. 59 (24%) participants had diagnosis of "acute bronchitis". Antibiotic use decreased in the procalcitonin group. Withholding antibiotic treatment based on procalcitonin measurement did not compromise patient outcome. |
| Dowell 2001 | Randomised controlled trial of "delayed" versus "immediate" antibiotics for acute cough. Participants randomised to "delayed" arm were asked to wait a week before collecting their prescription. 55% of participants did not pick up their prescription. More participants were satisfied and "enabled" in the immediate‐treatment arm. |
| Gordon 1974 | Participants were children with "symptoms referable to the respiratory tract", therefore likely many had upper respiratory infections (78% to 96% had runny nose, 74% to 83% had inflamed nasal mucosa). |
| Gottfarb 1994 | Post‐randomisation exclusion of 23% of the sample due to laboratory evidence of pertussis infection. Outcomes not clearly reported. |
| Stephenson 1989 | Participants were adults with upper respiratory infection. Not all had cough, and no information available on the subgroup of patients with productive cough. |
| Thomas 1978 | Explicit data from the study were not published and the data are no longer available. |
Contributions of authors
Susan Smith (SS) joined the team for the 2009, 2011, 2014, and 2017 updates and helped co‐ordinate the updates with Tom Fahey; updated the search; independently assessed potentially eligible studies; and was the lead author for the 2009, 2011, 2013, and 2017 updates.
Tom Fahey (TF) joined the team for the 2000 update and helped co‐ordinate the review with John Smucny; updated the search and independently re‐extracted data; provided unpublished data from a number of the studies; interpreted data independently, and then as a group; co‐wrote the 2004 update; helped co‐ordinate the 2009 update; independently assessed potentially eligible studies; and co‐wrote the 2009 update.
John Smucny (JS) screened the search results for acceptable trials; graded and extracted data from trials; entered and analysed data; interpreted data independently, and then as a group; updated the search and independently re‐extracted data; and co‐wrote the 2004 and 2009 updates.
Lorne Becker (LB) conceived, co‐ordinated, and designed the original review; screened the search results for acceptable trials; entered and analysed data; wrote the initial draft of the review; incorporated feedback from the other authors into the final draft; and participated in updating the review in 2004 and 2009.
Sources of support
Internal sources
Center for Evidence‐Based Practice, State University of New York, Upstate Medical University, Syracuse, New York, USA.
External sources
No sources of support supplied
Declarations of interest
Susan M Smith: None known. Tom Fahey: None known. John Smucny: None known. Lorne A Becker: None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Brickfield 1986 {published data only}
- Brickfield FX, Carter WH, Johnson RE. Erythromycin in the treatment of acute bronchitis in a community practice. Journal of Family Practice 1986;23:119‐22. [PubMed] [Google Scholar]
Dunlay 1987 {published data only}
- Dunlay J, Reinhardt R, Roi LD. A placebo‐controlled, double‐blind trial of erythromycin in adults with acute bronchitis. Journal of Family Practice 1987;25:137‐41. [PubMed] [Google Scholar]
Evans 2002 {published data only}
- Evans AT, Husain S, Durairaj L, Sadowski LS, Charles‐Damte M, Wang Y. Azithromycin for acute bronchitis: a randomised, double‐blind, controlled trial. Lancet 2002;359:1648‐54. [DOI] [PubMed] [Google Scholar]
Franks 1984 {published data only}
- Franks P, Gleiner JA. The treatment of acute bronchitis with trimethoprim and sulfamethoxazole. Journal of Family Practice 1984;19:185‐90. [PubMed] [Google Scholar]
Howie 1970 {published and unpublished data}
- Howie JGR, Clark GA. Double‐blind trial of early demethylchlortetracycline in minor respiratory illness in general practice. Lancet 1970;296(7683):1099‐102. [DOI] [PubMed] [Google Scholar]
Hueston 1994 {published and unpublished data}
- Hueston WJ. Albuterol delivered by metered‐dose inhaler to treat acute bronchitis. Journal of Family Practice 1994;39(5):437‐40. [PubMed] [Google Scholar]
Kaiser 1996 {published and unpublished data}
- Kaiser L, Lew D, Hirschel B, Auckenthaler R, Morabia A, Heald A, et al. Effects of antibiotic treatment in the subset of common‐cold patients who have bacteria in nasopharyngeal secretions. Lancet 1996;347:1507‐10. [DOI] [PubMed] [Google Scholar]
King 1996 {published and unpublished data}
- King DE, Williams WC, Bishop L, Shechter A. Effectiveness of erythromycin in the treatment of acute bronchitis. Journal of Family Practice 1996;42:601‐5. [PubMed] [Google Scholar]
Little 2005 {published and unpublished data}
- Little P, Rumsby K, Kelly J, Watson L, Moore M, Warner G, et al. Information leaflet and antibiotic prescribing strategies for acute lower respiratory tract infection. JAMA 2005;293(24):3029‐35. [DOI] [PubMed] [Google Scholar]
Little 2013 {published and unpublished data}
- Little P, Stuart B, Moore M, Coenen S, Butler CB, Godyscki‐Cwirko M, et al. Amoxicillin for acute lower‐respiratory‐tract infection when pneumonia is not suspected: a 12 country, randomised, placebo‐controlled trial. Lancet Infectious Diseases 2013;13:123‐9. [DOI] [PubMed] [Google Scholar]
Llor 2013 {published data only}
- Llor C, Moragas A, Bayona C, Morros R, Pera H, Cots JM, et al. Efficacy of anti‐inflammatory or antibiotic treatment in patients with non‐complicated acute bronchitis and discoloured sputum: randomised controlled trial. BMJ 2013;347:f5762. [DOI] [PMC free article] [PubMed] [Google Scholar]
Matthys 2000 {published data only}
- Matthys H, Mey C, Carls C, Rys A, Geib A, Wittig T. Efficacy and tolerability of myrtol standardised in acute bronchitis. Arzmeimittel Forschung 2000;50:700‐11. [DOI] [PubMed] [Google Scholar]
Nduba 2008 {published data only}
- Nduba VN, Mwachari CW, Magaret AS, Park DR, Kigo A, Hooton TM, et al. Placebo found equivalent to amoxicillin for treatment of acute bronchitis in Nairobi, Kenya: a triple blind, randomised equivalence study. Thorax 2008;63:999‐1005. [DOI] [PubMed] [Google Scholar]
Scherl 1987 {published data only}
- Scherl ER, Riegler SL, Cooper JK. Doxycycline in acute bronchitis: a randomized double‐blind trial. Journal of the Kentucky Medical Association 1987;85:539‐41. [PubMed] [Google Scholar]
Stott 1976 {published data only}
- Stott NCH, West RW. Randomised controlled trial of antibiotics in patients with cough and purulent sputum. British Medical Journal 1976;2:556‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Verheij 1994 {published data only}
- Verheij TJM, Hermans J, Mulder JD. Effects of doxycycline in patients with acute cough and purulent sputum: a double blind placebo controlled trial. British Journal of General Practice 1994;44:400‐4. [PMC free article] [PubMed] [Google Scholar]
Williamson 1984 {published and unpublished data}
- Williamson HA. A randomized controlled trial of doxycycline in the treatment of acute bronchitis. Journal of Family Practice 1984;19:481‐6. [PubMed] [Google Scholar]
References to studies excluded from this review
Batieha 2002 {published data only}
- Batieha A, Yahia G, Mahafzeh T, Omari M, Momani A, Dabbas M. No advantage of treating acute respiratory tract infections with azithromycin in a placebo‐controlled trial. Scandinavian Journal of Infectious Diseases 2002;34(4):243‐7. [DOI] [PubMed] [Google Scholar]
Christ‐Crain 2004 {published data only}
- Christ‐Crain M, Jaccard‐Stolz D, Bingisser R, Gencay MM, Huber PR, Tamm M, et al. Effect of procalcitonin‐guided treatment on antibiotic use and outcome in lower respiratory tract infections: cluster‐randomised, single‐blinded intervention trial. Lancet 2004;363(9409):600‐7. [DOI] [PubMed] [Google Scholar]
Dowell 2001 {published data only}
- Dowell J, Pitkethly M, Bain J, Martin S. A randomised controlled trial of delayed antibiotic prescribing as a strategy for managing uncomplicated respiratory tract infection in primary care. British Journal of General Practice 2001;51:200‐5. [PMC free article] [PubMed] [Google Scholar]
Gordon 1974 {published data only}
- Gordon M, Lovell S, Dugdale AE. The value of antibiotics in minor respiratory illness in children: a controlled trial. Medical Journal of Australia 1974;1:304‐6. [PubMed] [Google Scholar]
Gottfarb 1994 {published data only}
- Gottfarb P, Brauner A. Children with persistent cough ‐ outcome with treatment and role of Moraxella catarrhalis. Scandinavian Journal of Infectious Diseases 1994;26:545‐51. [DOI] [PubMed] [Google Scholar]
Stephenson 1989 {unpublished data only}
- Stephenson MJ. Antibiotics for acute bronchitis: a randomised controlled trial. Unpublished data 1989.
Thomas 1978 {published data only}
- Thomas S. Antibiotics for cough and purulent sputum. British Medical Journal 1978; Vol. 2, issue 11:1374. [DOI] [PMC free article] [PubMed]
Additional references
Ayres 1986
- Ayres JG. Seasonal pattern of acute bronchitis in general practice in the United Kingdom 1976‐83. Thorax 1986;41:106‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Boldy 1990
- Boldy DAR, Skidmore SJ, Ayres JG. Acute bronchitis in the community: clinical features, infective factors, changes in pulmonary function and bronchial reactivity to histamine. Respiratory Medicine 1990;84:377‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Butler 2010
- Butler CC, Hood K, Kelly MJ, Goossens H, Verheij T, Little P, et al. Treatment of acute cough/lower respiratory tract infection by antibiotic class and associated outcomes: a 13 European country observational study in primary care. Journal of Antimicrobial Chemotherapy 2010;65(11):2472‐8. [DOI] [PubMed] [Google Scholar]
CDC 2013
- Centers for Disease Control and Prevention. Overview of bronchitis. www.cdc.gov/getsmart/antibiotic‐use/uri/bronchitis.html 2013 (accessed 11 November 2013).
Coenen 2007
- Coenen S, Goossens H. Antibiotics for respiratory tract infections in primary care. BMJ 2007;335:946‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dagnelie 1996
- Dagnelie CF, Graf Y, DeMelker RA. Do patients with sore throat benefit from penicillin? A randomized double‐blind placebo‐controlled clinical trial with penicillin V in general practice. British Journal of General Practice 1996;46:589‐93. [PMC free article] [PubMed] [Google Scholar]
Del Mar 2016
- Mar C. Antibiotics for acute respiratory tract infections in primary care. BMJ 2016;354:i3482. [DOI] [PubMed] [Google Scholar]
Delozier 1989
ECDC 2013
- European Centre for Disease Control. Trend of antimicrobial consumption by country. www.ecdc.europa.eu/en/healthtopics/antimicrobial_resistance/esac‐net‐database/Pages/trend‐consumption‐by‐country.aspx 2013 (accessed 8 November 2013).
Falck 1994
- Falck G, Heyman L, Gnarpe J, Gnarpe H. Chlamydia pneumoniae (TWAR): a common agent in acute bronchitis. Scandinavian Journal of Infectious Diseases 1994;26:179‐87. [DOI] [PubMed] [Google Scholar]
Foy 1993
- Foy HM. Infections caused by Mycoplasma pneumoniae and possible carrier state in different populations of patients. Clinical Infectious Diseases 1993;17(Suppl 1):37‐46. [DOI] [PubMed] [Google Scholar]
Gonzales 1995
- Gonzales R, Sande M. What will it take to stop physicians from prescribing antibiotics in acute bronchitis?. Lancet 1995;345:665. [DOI] [PubMed] [Google Scholar]
Gonzales 1997
- Gonzales R, Steiner JF, Sande MA. Antibiotic prescribing for adults with colds, upper respiratory tract infections, and bronchitis by ambulatory care physicians. JAMA 1997;278:901‐4. [PubMed] [Google Scholar]
Grayston 1993
- Grayston JT, Aldous MB, Easton A, Wang SP, Kuo CC, Campbell LA, et al. Evidence that Chlamydia pneumoniae causes pneumonia and bronchitis. Journal of Infectious Diseases 1993;168:1231‐5. [DOI] [PubMed] [Google Scholar]
Gulliford 2011
- Gulliford MC, Staa T, McDermott L, Dregan A, McCann G, Ashworth M, et al. Cluster randomised trial in the General Practice Research Database: 1. Electronic decision support to reduce antibiotic prescribing in primary care (eCRT study). Trials 2001;12:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
Henry 1995
- Henry D, Ruoff GE, Rhudy J, Puopolo A, Drehobl M, Schoenberger J, et al. Effectiveness of short‐course therapy (5 days) with cefuroxime axetil in treatment of secondary bacterial infections of acute bronchitis. Antimicrobial Agents and Chemotherapeutics 1995;39:2528‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Herman 1984
- Herman JM. Patients' willingness to take risks in the management of pharyngitis. Journal of Family Practice 1984;6:767‐72. [PubMed] [Google Scholar]
Herwaldt 1991
- Herwaldt LA. Pertussis in adults: what physicians need to know. Archives of Internal Medicine 1991;151:1510‐2. [DOI] [PubMed] [Google Scholar]
Hueston 1997
- Hueston WJ. Antibiotics: neither cost effective nor 'cough' effective. Journal of Family Practice 1997;44:261‐5. [PubMed] [Google Scholar]
Jonsson 1997
- Jonsson JS, Sigurdsson JA, Kristinsson KG, Guonadottir M, Magnusson S. Acute bronchitis in adults: how close do we come to its aetiology in general practice?. Scandinavian Journal of Primary Health Care 1997;15:156‐60. [DOI] [PubMed] [Google Scholar]
Laurenzi 1961
- Laurenzi GA, Potter RT, Kass EH. Bacteriologic flora of the lower respiratory tract. New England Journal of Medicine 1961;265:1273‐8. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Macfarlane 1993
- Macfarlane JR, Colville A, Guion A, Macfarlane RM, Rose DH. Prospective study of aetiology and outcome of adult lower‐respiratory tract infections in the community. Lancet 1993;341:511‐4. [DOI] [PubMed] [Google Scholar]
Macfarlane 1994
- Macfarlane JT, Prewett J, Guion A, Gard P. Community acquired lower respiratory infection: bacterial infection not uncommon (letter). BMJ 1994;308:1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
Macfarlane 2001
- Macfarlane J, Holmes W, Gard P, Macfarlane R, Rose D, Weston V, et al. Prospective study of the incidence, aetiology and outcome of adult lower respiratory tract illness in the community. BMJ 2001;56:109‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Macfarlane 2002
- Macfarlane J, Holmes W, Gard P, Thornhill D, Macfarlane R, Hubbard R. Reducing antibiotic use for acute bronchitis in primary care: blinded, randomised controlled trial of patient information leaflet. BMJ 2002;324:91‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mainous 1996
- Mainous AG, Zoorob RJ, Hueston WJ. Current management of acute bronchitis in ambulatory care. Archives of Family Medicine 1996;5:79‐83. [DOI] [PubMed] [Google Scholar]
Metlay 1997a
- Metlay JP, Kapoor WN, Fine MJ. Does this patient have community‐acquired pneumonia? Diagnosing pneumonia by history and physical examination. JAMA 1997;278:1440‐5. [PubMed] [Google Scholar]
Metlay 1997b
- Metlay JP, Stafford RS, Singer DE. National trends in the use of antibiotics by primary care physicians for adult patients with cough. Archives of Internal Medicine 1998;158:1813‐8. [DOI] [PubMed] [Google Scholar]
Meza 1994
- Meza RA, Bridges‐Webb C, Sayer GP, Miles DA, Traynor V, Neary S. The management of acute bronchitis in general practice: results from the Australian morbidity and treatment survey, 1990‐1991. Australian Family Physician 1994;23(8):1550‐3. [PubMed] [Google Scholar]
Molstad 1992
- Molstad S, Arvidsson E, Eliasson I, Hovelius B, Kamme C, Schalén C. Production of beta‐lactamase in respiratory tract bacteria in children: relationship to antibiotic use. Scandinavian Journal of Primary Health Care 1992;10:16‐20. [DOI] [PubMed] [Google Scholar]
Oeffinger 1997
- Oeffinger KC, Snell LM, Foster BM, Panico KG, Archer RK. Diagnosis of acute bronchitis in adults: a national survey of family physicians. Journal of Family Practice 1997;45:402‐9. [PubMed] [Google Scholar]
Petersen 2007
- Petersen I, Johnson AM, Islam A, Duckworth G, Livermore DM, Hayward AC. Protective effect of antibiotics against serious complications of common respiratory tract infections: retrospective cohort study with the UK General Practice Research Database. BMJ 2007;335:982. [DOI: 10.1136/bmj.39345.405243.BE] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Robertson 1987
- Robertson PW, Goldberg H, Jarvie BH, Smith DD, Whybin LR. Bordetella pertussis infection: a cause of persistent cough in adults. Medical Journal of Australia 1987;146:522‐5. [DOI] [PubMed] [Google Scholar]
Smith 1986
- Smith JMB, Lockwood BM. Commensal or pathogen? The growing dilemma facing the general practitioner. New Zealand Medical Journal 1986;99:242‐3. [PubMed] [Google Scholar]
Straand 1997
- Straand J, Skinio Rokstad K, Sandvik H. Prescribing systemic antibiotics in general practice: a report from the More and Romsdal Prescription Study. Scandinavian Journal of Primary Health Care 1998;16:121‐7. [DOI] [PubMed] [Google Scholar]
Stuart‐Harris 1965
- Stuart‐Harris CH, Andrewes C, Andrews BE, Beale AJ, Gardner PS, Grist NR, et al. A collaborative study of the aetiology of acute respiratory infections in Britain 1961‐4. British Medical Journal 1965;29:319‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thom 1994
- Thom DH, Grayston JE, Campbell LA, Kuo CC, Diwan VK, Wang SP. Respiratory infection with Chlamydia pneumoniae in middle‐aged and older adult outpatients. European Journal of Clinical Microbiology and Infectious Diseases 1994;13:785‐92. [DOI] [PubMed] [Google Scholar]
van der Velden 2012
- Velden A, Pijpers EJ, Kuyvenhoven M, Tonkin‐Crine SKG, Little P, Verheij TJM. Effectiveness of physician‐targeted interventions to improve antibiotic use for respiratory tract infections. British Journal of General Practice 2012;62(605):e801‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Verheij 1990
- Verheij TJM, Hermans J, Kaptein AA, Mulder JD. Acute bronchitis: general practitioners' views regarding diagnosis and treatment. Family Practice 1990;7:175‐80. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Becker 1997
- Becker L, Glazier R, McIsaac W, Smucny J. Antibiotics for acute bronchitis. Cochrane Database of Systematic Reviews 1997, Issue 4. [DOI: 10.1002/14651858.CD000245.pub2] [DOI] [PubMed] [Google Scholar]
Fahey 1998
- Fahey T, Stocks N, Thomas T. Quantitative systematic review of randomised controlled trials comparing antibiotic with placebo for acute cough in adults. BMJ 1998;316:906‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
Fahey 2004
- Fahey T, Smucny J, Becker L, Glazier R. Antibiotics for acute bronchitis. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD000245.pub2] [DOI] [PubMed] [Google Scholar]
Smith 2009
- Smith SM, Fahey T, Smucny J, Becker LA. Antibiotics for acute bronchitis. Cochrane Database of Systematic Reviews 2009, Issue 1. [DOI: 10.1002/14651858.CD000245.pub2] [DOI] [Google Scholar]
Smith 2011
- Smith SM, Fahey T, Smucny J, Becker LA. Antibiotics for acute bronchitis. Cochrane Database of Systematic Reviews 2011, Issue 11. [DOI: 10.1002/14651858.CD000245.pub2] [DOI] [Google Scholar]
Smith 2014
- Smith SM, Fahey T, Smucny J, Becker L. Antibiotics for acute bronchitis. Cochrane Database of Systematic Reviews 2014, Issue 3. [DOI: 10.1002/14651858.CD000245.pub3] [DOI] [PubMed] [Google Scholar]
Smucny 1998
- Smucny JJ, Becker LA, Glazier RH, McIsaac W. Are antibiotics effective treatment for acute bronchitis? A meta‐analysis. Journal of Family Practice 1998;47:453‐60. [PubMed] [Google Scholar]
Smucny 2000
- Smucny J, Fahey T, Becker L, Glazier R, McIsaac W. Antibiotics for acute bronchitis. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI: 10.1002/14651858.CD000245.pub2] [DOI] [PubMed] [Google Scholar]


