Abstract
Background
Among subfertile women undergoing assisted reproductive technology (ART), hormone pills given before ovarian stimulation may improve outcomes.
Objectives
To determine whether pretreatment with the combined oral contraceptive pill (COCP) or with a progestogen or oestrogen alone in ovarian stimulation protocols affects outcomes in subfertile couples undergoing ART.
Search methods
We searched the following databases from inception to January 2017: Cochrane Gynaecology and Fertility Group Specialised Register, The Cochrane Central Register Studies Online, MEDLINE, Embase, CINAHL and PsycINFO. We also searched the reference lists of relevant articles and registers of ongoing trials.
Selection criteria
Randomised controlled trials (RCTs) of hormonal pretreatment in women undergoing ART.
Data collection and analysis
We used standard methodological procedures recommended by Cochrane. The primary review outcomes were live birth or ongoing pregnancy and pregnancy loss.
Main results
We included 29 RCTs (4701 women) of pretreatment with COCPs, progestogens or oestrogens versus no pretreatment or alternative pretreatments, in gonadotrophin‐releasing hormone (GnRH) agonist or antagonist cycles. Overall, evidence quality ranged from very low to moderate. The main limitations were risk of bias and imprecision. Most studies did not describe their methods in adequate detail.
Combined oral contraceptive pill versus no pretreatment
With antagonist cycles in both groups the rate of live birth or ongoing pregnancy was lower in the pretreatment group (OR 0.74, 95% CI 0.58 to 0.95; 6 RCTs; 1335 women; I2 = 0%; moderate quality evidence). There was insufficient evidence to determine whether the groups differed in rates of pregnancy loss (OR 1.36, 95% CI 0.82 to 2.26; 5 RCTs; 868 women; I2 = 0%; moderate quality evidence), multiple pregnancy (OR 2.21, 95% CI 0.53 to 9.26; 2 RCTs; 125 women; I2 = 0%; low quality evidence), ovarian hyperstimulation syndrome (OHSS; OR 0.98, 95% CI 0.28 to 3.40; 2 RCTs; 642 women; I2 = 0%, low quality evidence), or ovarian cyst formation (OR 0.47, 95% CI 0.08 to 2.75; 1 RCT; 64 women; very low quality evidence).
In COCP plus antagonist cycles versus no pretreatment in agonist cycles, there was insufficient evidence to determine whether the groups differed in rates of live birth or ongoing pregnancy (OR 0.89, 95% CI 0.64 to 1.25; 4 RCTs; 724 women; I2 = 0%; moderate quality evidence), multiple pregnancy (OR 1.36, 95% CI 0.85 to 2.19; 4 RCTs; 546 women; I2 = 0%; moderate quality evidence), or OHSS (OR 0.63, 95% CI 0.20 to 1.96; 2 RCTs; 290 women, I2 = 0%), but there were fewer pregnancy losses in the pretreatment group (OR 0.40, 95% CI 0.22 to 0.72; 5 RCTs; 780 women; I2 = 0%; moderate quality evidence). There were no data suitable for analysis on ovarian cyst formation.
One small study comparing COCP versus no pretreatment in agonist cycles showed no clear difference between the groups for any of the reported outcomes.
Progestogen versus no pretreatment
All studies used the same protocol (antagonist, agonist or gonadotrophins) in both groups. There was insufficient evidence to determine any differences in rates of live birth or ongoing pregnancy (agonist: OR 1.35, 95% CI 0.69 to 2.65; 2 RCTs; 222 women; I2 = 24%; low quality evidence; antagonist: OR 0.67, 95% CI 0.18 to 2.54; 1 RCT; 47 women; low quality evidence; gonadotrophins: OR 0.63, 95% CI 0.09 to 4.23; 1 RCT; 42 women; very low quality evidence), pregnancy loss (agonist: OR 2.26, 95% CI 0.67 to 7.55; 2 RCTs; 222 women; I2 = 0%; low quality evidence; antagonist: OR 0.36, 95% CI 0.06 to 2.09; 1 RCT; 47 women; low quality evidence; gonadotrophins: OR 1.00, 95% CI 0.06 to 17.12; 1 RCT; 42 women; very low quality evidence) or multiple pregnancy (agonist: no data available; antagonist: OR 1.05, 95% CI 0.06 to 17.76; 1 RCT; 47 women; low quality evidence; gonadotrophins: no data available). Three studies, all using agonist cycles, reported ovarian cyst formation: rates were lower in the pretreatment group (OR 0.16, 95% CI 0.08 to 0.32; 374 women; I2 = 1%; moderate quality evidence). There were no data on OHSS.
Oestrogen versus no pretreatment
In antagonist or agonist cycles, there was insufficient evidence to determine whether the groups differed in rates of live birth or ongoing pregnancy (antagonist versus antagonist: OR 0.79, 95% CI 0.53 to 1.17; 2 RCTs; 502 women; I2 = 0%; low quality evidence; antagonist versus agonist: OR 0.88, 95% CI 0.51 to 1.50; 2 RCTs; 242 women; I2 = 0%; very low quality evidence), pregnancy loss (antagonist versus antagonist: OR 0.16, 95% CI 0.02 to 1.47; 1 RCT; 49 women; very low quality evidence; antagonist versus agonist: OR 1.59, 95% CI 0.62 to 4.06; 1 RCT; 220 women; very low quality evidence), multiple pregnancy (antagonist versus antagonist: no data available; antagonist versus agonist: OR 2.24, 95% CI 0.09 to 53.59; 1 RCT; 22 women; very low quality evidence) or OHSS (antagonist versus antagonist: no data available; antagonist versus agonist: OR 1.54, 95% CI 0.25 to 9.42; 1 RCT; 220 women). Ovarian cyst formation was not reported.
Head‐to‐head comparisons
COCP was compared with progestogen (1 RCT, 44 women), and with oestrogen (2 RCTs, 146 women), and progestogen was compared with oestrogen (1 RCT, 48 women), with an antagonist cycle in both groups. COCP in an agonist cycle was compared with oestrogen in an antagonist cycle (1 RCT, 25 women). Data were scant but there was no clear evidence that any of the groups differed in rates of live birth or ongoing pregnancy, pregnancy loss or other adverse events.
Authors' conclusions
Among women undergoing ovarian stimulation in antagonist protocols, COCP pretreatment was associated with a lower rate of live birth or ongoing pregnancy than no pretreatment. There was insufficient evidence to determine whether rates of live birth or ongoing pregnancy were influenced by pretreatment with progestogens or oestrogens, or by COCP pretreatment using other stimulation protocols. Findings on adverse events were inconclusive, except that progesterone pretreatment may reduce the risk of ovarian cysts in agonist cycles, and COCP in antagonist cycles may reduce the risk of pregnancy loss compared with no pretreatment in agonist cycles.
Plain language summary
Pretreatments in in vitro fertilisation/intra‐cytoplasmic sperm injection cycles
Review question
The aim of this review was to assess whether pretreatment with a combined oral contraceptive pill (COCP) or with progestogen or oestrogen alone influences pregnancy outcomes in couples with low fertility undergoing assisted reproductive technology (ART)
Background
In vitro fertilisation (IVF; where an egg is mixed with sperm outside the body) and intra‐cytoplasmic sperm injection (ICSI; where one sperm is injected directly into the egg) are important techniques for women who have trouble getting pregnant. IVF and ICSI cycles consist of a few steps. First the woman receives hormone therapy to stimulate her ovaries in producing egg cells (called ovarian stimulation). When a few egg cells are mature enough to be fertilised, the woman receives a single hormone injection. This triggers the ovaries to release the egg cells, so they can be gathered by the clinician. The eggs are then fertilised outside the woman's body and become embryos. One or two embryos are then transferred into the womb.
Before the first step in IVF or ICSI cycles (hormone therapy), a pretreatment with a COCP can be given. A COCP contains both progestogen and oestrogen. Pretreatment with a progestogen or oestrogen alone could also be used before the hormone therapy. These pretreatments suppress the woman's own hormone production. This might improve the woman's response to the hormone therapy in IVF/ICSI cycles. In this way, side events such as cyst formation (fluid‐filled sac that develops on a woman's ovary) and the number of pregnancy losses might be reduced and pregnancy outcomes might be improved.
Study characteristics
This Cochrane Review included 30 randomized controlled trials (clinical studies where people are randomly put into one of two or more treatment groups) assessing pretreatment with COCP, progestogen or oestrogen in 5096 women undergoing ART. The evidence is current to January 2017.
Key results
Among women undergoing ovarian stimulation in antagonist protocols, COCP pretreatment was associated with a lower rate of live birth or ongoing pregnancy than no pretreatment. There was insufficient evidence to determine whether rates of live birth or ongoing pregnancy were influenced by pretreatment with progestogens or oestrogens, or by COCP pretreatment using other stimulation protocols. Findings on adverse events were inconclusive, except that progesterone pretreatment may reduce the risk of ovarian cysts in agonist cycles, and COCP in antagonist cycles may reduce the risk of pregnancy loss compared with no pretreatment in agonist cycles.
Quality of the evidence.
Overall evidence quality ranged from very low to moderate. The main problems were risk of bias and imprecision. Most studies did not describe their methods in adequate detail.
Summary of findings
Background
For definitions of terminology see our Glossary (Appendix 1).
Description of the condition
For subfertile women, assisted reproductive techniques (ART) such as in vitro fertilisation (IVF) and intra‐cytoplasmic sperm injection (ICSI) can be a way to achieve pregnancy. Pregnancy and live birth rates are higher with IVF than with expectant management (Pandian 2005).
An IVF cycle has the following stages: ovarian stimulation, oocyte retrieval, fertilisation of the egg and transfer of the embryo. Ovarian stimulation involves the administration of gonadotrophins. These hormones stimulate growth and maturation of the follicle. Gonadotrophins include follicle‐stimulating hormone (FSH) and luteinising hormone (LH). There are two different gonadotrophin preparations; human menopausal gonadotrophin (hMG) which consists of both FSH and LH, and a more recent therapy, recombinant follicle‐stimulating hormone (rFSH). There is insufficient evidence of a difference between these treatments in ongoing pregnancy or live birth rate and other aspects with relation to IVF (Van Wely 2003).
There are a number of undesirable events associated with gonadotrophin therapy that can complicate treatment and outcomes: ovarian hyperstimulation syndrome (OHSS), premature LH‐surge and multiple pregnancy (Dodson 1989). In some women undergoing IVF therapy, these problems occur because the endogenous FSH and LH production is too dominant (Awadalla 1987). Gonadotrophin‐releasing hormone analogues (GnRHa) are administered to inhibit the production of endogenous FSH and LH (Awadalla 1987; Dodson 1989). Gonadotrophin‐releasing hormone (GnRH) is a hormone that occurs naturally in the woman's body and that regulates the production of gonadotrophins. There are two different types of GnRHa: agonists or antagonists. The difference lies in their mechanism of action. GnRH agonists bind to the GnRH receptors in the pituitary gland and initially stimulate the release of gonadotrophins ('flare‐up'). Negative feedback causes a decrease in the number of GnRH receptors, which results in the release of fewer gonadotrophins. In a traditional treatment protocol, GnRH agonists are administered prior to commencing gonadotrophins, ensuring that the flare‐up will be over by the time gonadotrophins are injected. Conversely, GnRH antagonists can be started after gonadotrophin therapy has been administered because they bind competitively to the receptor, causing immediate suppression of the endogenous production of FSH and LH (Tarlatzis 2006). Therefore, GnRHa can prevent a premature LH‐surge and synchronise the follicle cohort.
The authors of one Cochrane Review comparing GnRH agonist cycles with GnRH antagonist cycles concluded that GnRH antagonists are associated with a substantial reduction in OHSS without reducing the likelihood of achieving live birth (Al‐Inany 2016).
When a few follicles reach maturity after gonadotrophin stimulation and GnRHa treatment, human chorionic gonadotrophin (hCG) is administered to trigger ovulation and 34 to 36 hours later, oocyte retrieval is undertaken and the egg is fertilised outside the body. Following fertilisation, the embryos are either transferred on day two or three (cleavage stage) or on day five or six (blastocyst stage). Luteal phase support is typically provided as a progestogen or a hCG treatment, or as a combination.
Description of the intervention
Oral contraceptive pills (OCP) are widely used by women of different ages to prevent pregnancy. They are also indicated for a range of menstrual and gynaecological conditions, such as acne vulgaris, polycystic ovary syndrome (PCOS) and menorrhagia (Arowojolu 2007; Harwood 2007; Irvine 1999). Combined oral contraceptive pills (COCP) consisting of oestrogen and progestogen reduce the women's own production of FSH and LH by way of a negative feedback (Cohen 1979; Gaspard 1984). The COCP suppresses gonadal function and, in the absence of an LH‐surge, no flare‐up or premature ovulation will occur. Only progestogen has a contraceptive effect (Erkkola 2007). Progestogen has the ability to slow GnRH pulsatility of the pituitary gland, thereby reducing gonadotrophin surges and, according to dose, inhibiting ovulation (Anderson 1990; Erkkola 2007; Le Nestour 1993; Moudgal 1985). Oestrogen is added to the COCP to regulate the bleeding patterns, though it is also capable of reducing FSH levels (De Ziegler 1998; Le Nestour 1993).
Most of progestogen‐only pills do not inhibit ovulation although higher doses of progestogen may do so (Erkkola 2007).
How the intervention might work
The COCP given prior to gonadotrophin in an IVF cycle assists synchronisation of follicular development and prevents occurrence of spontaneous LH‐surges (Gonen 1990). Huirne reported similar data as well as a reduction of the occurrence of large follicles prior to day eight (Huirne 2006a). In another study, both the COCP and progestogen had a suppressive effect on LH and FSH secretion. However, oestrogen administration (at 4 mg/day) did not suppress serum LH and FSH concentrations (Cédrin‐Durnerin 2007).
The resulting pituitary suppression of COCPs in GnRH antagonist cycles is associated with slower follicular growth and lower serum oestradiol levels than in antagonist cycles with no pretreatment in the early part of the cycle. This results in a longer duration of rFSH stimulation and a higher total rFSH consumption than in antagonist cycles without pretreatment (Cédrin‐Durnerin 2007).
COCP pretreatment in an ovarian stimulation protocol before IVF can reduce cyst formation, shorten the length of GnRHa treatment and reduce the amount of gonadotrophin needed, without negatively affecting the pregnancy rate (Biljan 1998a). Pituitary suppression seems to occur earlier with progestogen pretreatment and fewer ovarian cysts are formed, when compared with no pretreatment (Engmann 1999). COCP pretreatment can be used for scheduling oocyte retrieval on days of the working week, which is important with antagonist cycles (Barmat 2005; Gonen 1990; Huirne 2006b). Scheduling is of benefit for the clinicians and people in the laboratory, since these people usually do not work on weekends.
Why it is important to do this review
There is some debate regarding the effects of the COCP upon pregnancy rate. Higher rates of clinical pregnancy and live birth have been reported when dual suppression protocols and GnRHa were compared to a GnRHa protocol without the use of oral contraceptives in non‐randomised studies (Damario 1997; Keltz 2007). However, other non‐randomised studies have found no evidence of effect with regard to pregnancy rate (Bellver 2007; Galera 2004).
There is a lack of consensus regarding whether pretreatment with COCP in ovarian stimulation protocols improves rates of pregnancy and live birth. Furthermore, the effects of pretreatment with progestogen or oestrogen alone on IVF outcomes is unclear. The results of many small RCTs can be pooled in a systematic review and may provide a more definitive answer regarding the role of the COCP, progestogens or oestrogens in ART.
Objectives
To determine whether pretreatment with the combined oral contraceptive pill or with a progestogen or oestrogen alone in ovarian stimulation protocols affects outcomes in subfertile couples undergoing ART.
Methods
Criteria for considering studies for this review
Types of studies
We included only randomized controlled trials (RCTs) in this review. We included both published and unpublished studies and we excluded trials with quasi‐randomisation.
We excluded cross‐over trials unless pre‐crossover data were available; this type of design is inappropriate in this context.
Types of participants
Women of any age with subfertility, regardless of any cause, undergoing ART.
We only excluded two types of participants from this review. The first was women with premature ovarian failure, because these women require a different ovarian stimulation protocol. The second was women who participated in ovarian stimulation protocols as oocyte donors.
Types of interventions
Pretreatment with COCPs, progestogens or oestrogens versus no pretreatment or alternative pretreatments, in GnRH agonist or antagonist cycles. We excluded studies that compared different doses of the same pretreatment.
Types of outcome measures
Primary outcomes
-
Live birth or ongoing pregnancy (in studies not reporting live birth) per woman randomized.
Live birth defined as the delivery of a foetus with signs of life after 20 completed weeks of gestational age, counted as live birth event. When there were multiple live births (e.g. twins or triplets), we counted these as one live birth event (Griffin 2002).
Ongoing pregnancy defined as evidence of a gestational sac with foetal heart motion at 12 weeks or later, confirmed with ultrasound. When there were multiple gestational sacs in one woman, we counted these as one ongoing pregnancy (Griffin 2002).
Pregnancy loss (miscarriage) per woman randomized ‐ defined as the sum of the number of spontaneous abortions (pregnancy loss before 20 completed weeks of gestation) and the number of stillbirths (pregnancy loss after 20 completed weeks of gestation) (Griffin 2002).
Secondary outcomes
Clinical pregnancy rate per woman randomized ‐ defined as evidence of a gestational sac with foetal heart motion at six weeks or later, confirmed with ultrasound. When there were multiple gestational sacs in one woman, we counted these as one clinical pregnancy (Griffin 2002).
Multiple pregnancy rate per woman randomized.
OHSS rate per woman randomized, as defined in the included studies.
Number of oocytes retrieved per woman randomized.
Days of gonadotrophin treatment per woman randomized.
Amount of gonadotrophins administered per woman randomized.
Ovarian cyst formation rate per woman randomized ‐ defined as any intraovarian sonolucent structure with a mean diameter of 15 mm or more confirmed with ultrasound at least one week after start pituitary suppression (Biljan 1998a).
Search methods for identification of studies
We obtained all studies that described (or might have described) RCTs of pretreatment with COCP, progestogen or oestrogen therapy prior to GnRHa (agonists or antagonists) and gonadotrophins or gonadotrophins alone in women undergoing IVF, using the following search strategies.
Electronic searches
We searched the following electronic databases:
the Cochrane Gynaecology and Fertility Group (CGF) Specialised Register, searched on Procite platform from inception to 16 January 2017 (Appendix 2);
Cochrane Central Register of Studies Online (CENTRAL CRSO) searched 16 January 2017 on web platform (Appendix 3);
MEDLINE; searched on Ovid platform 1946 to 16 January 2017 (Appendix 4). We combined this search with the Cochrane highly sensitive search strategy for identifying randomized trials (Higgins 2011);
Embase; searched on Ovid platform 1980 to 16 January 2017 (Appendix 5). We combined this search with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN);
PsycINFO; searched on the Ovid platform from 1806 to 16 January 2017 (Appendix 6);
Cumulative Index to Nursing and Allied Health Literature (CINAHL Plus); searched on the Ebsco platform from 1982 to 16 January 2017 (Appendix 7). We combined this search with trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN).
We place no restrictions by language. We managed output of these searches with the reference manager, Endnote (EndNote). Through this program, duplicates can be found and removed.
Searching other resources
In addition, we searched some other resources than the electronic databases mentioned above to obtain more relevant trials. We accessed all the websites on 13 January 2017, except for OpenSIGLE.
Trial registers for ongoing and registered trials: Current Controlled Trials (www.controlled‐trials.com), ClinicalTrials.gov (clinicaltrials.gov), and the World Health Organization (WHO) International Trials Registry Platform Search Portal (www.who.int/trialsearch) (Appendix 8).
The Virtual Health Library.
Citation indexes (scientific.thomson.com/products/sci).
PubMed (www.ncbi.nlm.nih.gov/pubmed); we combined this search with random control filters for PubMed (Higgins 2011).
Conference abstracts on the ISI Web of Knowledge (isiwebofknowledge.com).
Open System for Information on Grey Literature (opensigle.inist.fr, accessed on 16 January 2017).
Reference lists of appropriate studies.
Handsearching of the abstracts of the 32nd annual meeting of the European Society of Human Reproduction and Embryology in Helsinki (Finland), 3 to 6 July 2016 (ESHRE 2016).
Data collection and analysis
Selection of studies
Two review authors (ROA, AL) independently scanned the titles and abstracts of all the studies found with the search to exclude those that did not meet the inclusion criteria. We discussed any disagreement or doubt, whether a study was eligible for inclusion or not, with a third review author (CF) to achieve consensus. We obtained the full text of those RCTs deemed eligible for inclusion where possible, and subjected them to critical appraisal of their risk of bias. Where appropriate, we included them in this systematic review.
Subsequently, we constructed a Characteristics of included studies table for those trials considered suitable for inclusion. We produced a Characteristics of excluded studies table for those that did not satisfy the inclusion criteria. In this table, we listed the reasons for exclusion.
Data extraction and management
Two review authors (ROA, AL) independently extracted the data using data extraction forms, which we designed for this particular review (Appendix 9; Appendix 10). We resolved any discrepancies by discussion and the help of a third review author (CF).
The data extraction forms included risk of bias criteria and methodological details. The information about the studies is included in the review and presented in the Characteristics of included studies table. We managed the data using Review Manager 5 software (RevMan 2014).
Assessment of risk of bias in included studies
We assessed and reported on the risk of bias of included studies in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011), which recommends the explicit reporting of the following domains.
Sequence generation
Was sequence generation adequate (e.g. use of a random number table, a computer random number generator or coin tossing) or unclear (insufficient information about the process of sequence generation)?
Allocation concealment
Was allocation concealment adequate (e.g. use of central allocation or opaque sealed envelopes), inadequate (e.g. use of an open random allocation schedule, date of birth or case record number) or unclear (insufficient information about the process of allocation concealment)?
Blinding of participants, providers and outcome assessors
Was blinding adequate (e.g. participants and researchers were all blinded and it was unlikely that blinding could have been broken, either participants or some researchers were not blinded but outcome assessment was blinded or no blinding was used but this was not likely to influence outcomes), inadequate (e.g. no blinding or incomplete blinding and outcomes were likely to be influenced by this) or unclear (insufficient information about the process of blinding)?
Incomplete outcome data
Were outcome data addressed adequately (e.g. there were no missing outcome data, reasons for missing outcome data were unlikely to be related to true outcome or missing outcome data were balanced in numbers across intervention groups), inadequate (e.g. reasons for missing outcome data were likely to be related to true outcome) or unclear (insufficient information about the process of addressing outcome data)?
Selective outcome reporting
Was the study free of selective reporting? Adequate (e.g. the study protocol was available and all prespecified outcomes were reported or the study protocol was not available but it was clear that all prespecified outcomes were reported), inadequate (e.g. not all prespecified primary outcomes were reported) or unclear (insufficient information about the process of outcome reporting).
Other sources of bias for RCTs
Was the study free of other bias? Adequate (the study seemed to be free of other bias, e.g. comparable demographic characteristics between treatment groups), inadequate (e.g. extreme baseline imbalance, a potential source of bias related to the specific study design used or early stopping) or unclear (insufficient information about other sources of bias).
By using a simple form (Appendix 9; Appendix 10), two review authors separately assessed these domains as 'low' (indicating a low risk of bias), 'unclear' (indicating an uncertain risk of bias) or 'high' (indicating a high risk of bias).
The assessments of the two review authors (ROA, AL) were compared and we resolved any discrepancies in the interpretation of the risk of bias of a study by discussion with a third review author (CF). We did not exclude any study as a result of a rating of 'Unclear' or 'High'. Where it was unclear, we contacted authors of studies about the methods used and also sought any missing data.
We presented the results of the risk of bias assessment in the Characteristics of included studies table, including commentary about each of the domains. This led to a methodological quality summary (Figure 1; Figure 2).
1.
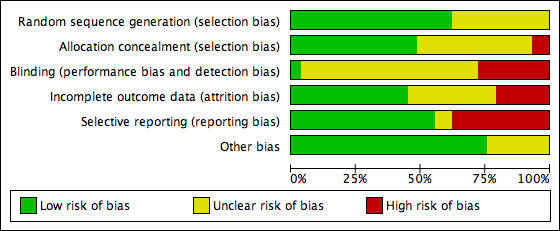
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
2.
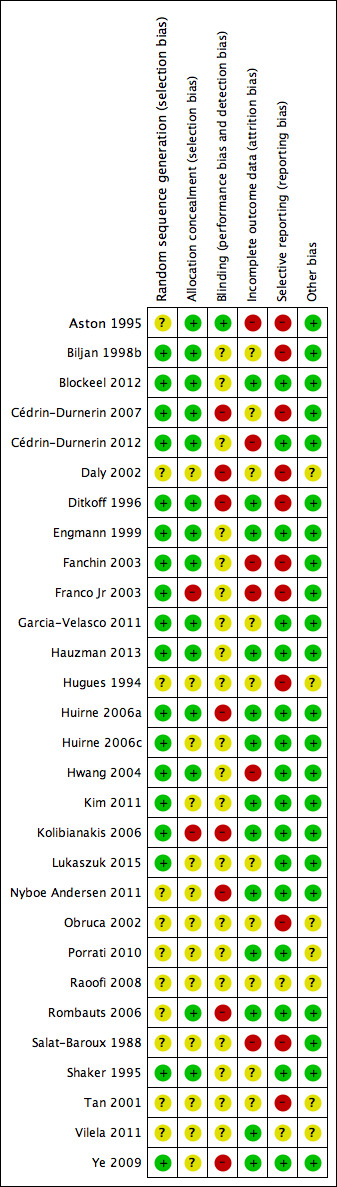
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Measures of treatment effect
For dichotomous data, we expressed results for each study as odds ratios (OR) with 95% confidence intervals (CI). For continuous variables, we reported the data as mean differences (MD) with 95% CIs.
Unit of analysis issues
To avoid analysis errors, we only pooled data that reported outcomes per woman randomized. Studies that reported 'per cycle' data were eligible for inclusion but were not included in the analyses; however, additional information was provided on such studies narratively.
Dealing with missing data
In case of missing data in the included studies, we contacted the original investigators by e‐mail or post to request relevant missing information. If we did not receive a reply, we sent a reminder to the authors two weeks later. Furthermore, we contacted the members of the CGF group to ask if they know any of the authors personally or have contact details.
We reported the data according to intention‐to‐treat (ITT) where possible. We reported live birth and ongoing pregnancy as a single outcome (i.e. live birth or ongoing pregnancy due to non‐reporting of live birth data by some studies who reported data on ongoing pregnancy). However, where a study reported data on both outcomes, we only included data for live birth in the analysis.
Assessment of heterogeneity
Before any meta‐analysis was done, we judged whether there was sufficient similarity between the eligible studies in their design and clinical characteristics to ensure that pooling was valid. We assessed statistical heterogeneity in the results of trials using the Chi2 test. A low P value (or a large Chi2 statistic relative to its degree of freedom) potentially provides evidence of heterogeneity of intervention effects and shows that results are not influenced by chance alone (Higgins 2011). We used the I2 statistic to assess the impact of the heterogeneity on the meta‐analysis. We interpreted the result of the I2 statistic as follows:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%: may represent substantial heterogeneity; and
75% to 100%: considerable heterogeneity (Higgins 2011).
If we found marked clinical or statistical heterogeneity (I2 more than 50%), we explored reasons for this heterogeneity using a sensitivity analysis.
Assessment of reporting biases
In view of the difficulty of detecting and correcting for publication bias and other reporting biases, we aimed to minimise their potential impact by ensuring a comprehensive search for eligible studies and by being alert for duplication of data. If there were 10 or more studies in an analysis, we intended to use a funnel plot to explore the possibility of small‐study effects (a tendency for estimates of the intervention effect to be more beneficial in smaller studies), but, due to the small number of studies per analysis, this was not possible.
Data synthesis
We carried out statistical analysis using Review Manager 5 (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where studies were sufficiently similar.
Comparisons were grouped separately by type of pretreatment (COCP, progesterone, oestrogen) and type of comparator (no pretreatment or alternative pretreatment) and the primary analysis was subgrouped by type of downregulation (antagonist or agonist).
Subgroup analysis and investigation of heterogeneity
Where data were available, we subgrouped the data to determine the separate evidence within the following subgroups.
-
Types of downregulating agents:
GnRH agonist in study group versus GnRH agonist in control group;
GnRH antagonist in study group versus GnRH antagonist in control group;
GnRH antagonist in study group versus GnRH agonist in control group;
GnRH agonist in study group versus GnRH antagonist in control group.
Women who were low responders (i.e. who responded poorly to controlled ovarian hyperstimulation).
Where we detected substantial heterogeneity (I2 greater than 50%), we explored possible explanations in sensitivity analyses. We took any statistical heterogeneity into consideration when interpreting the results especially if there were any variations in the direction of effect estimates.
Sensitivity analysis
We intended to conduct sensitivity analysis for the primary outcomes (live births or ongoing pregnancies and pregnancy losses) to determine whether the conclusions were robust to the choice of statistical model (fixed versus random) or summary effect measure (OR or risk ratio).
Overall quality of the body of evidence: 'Summary of findings' table
We prepared 'Summary of findings' tables using GRADEpro (GRADEpro GDT 2014) and Cochrane methods. These tables evaluated the overall quality of the body of evidence for the main review outcomes:
live birth or ongoing pregnancy;
pregnancy loss;
multiple pregnancy;
OHSS.
The tables evaluated the main review comparisons:
COCP compared to no pretreatment;
progestogen compared to no pretreatment;
oestrogen compared to no pretreatment.
We assessed the quality of the evidence using GRADE criteria: risk of bias, consistency of effect, imprecision, indirectness and publication bias). Two review authors (ROA, AL) independently made judgements about evidence quality (high, moderate, low or very low) and resolved disagreements by discussion. Judgements were justified, documented and incorporated into reporting of results for each outcome.
Results
Description of studies
Results of the search
After searching the electronic databases, we found 1792 studies: 609 studies in the GF specialized register of controlled trials, 166 studies in CENTRAL, 511 studies in MEDLINE, 463 studies in Embase, 19 studies in CINAHL and 24 studies in PsycINFO. After removing the duplicates and searching other resources, there were 1528 studies left. We retrieved 137 full‐text articles of which 29 studies (42 references) were included and 87 studies (95 references) were excluded. For further details on included/excluded studies, see Characteristics of included studies and Characteristics of excluded studies tables. The process involved in the screening and selection of eligible studies for inclusion is shown in the PRISMA flow chart (Moher 2009) (Figure 3).
3.
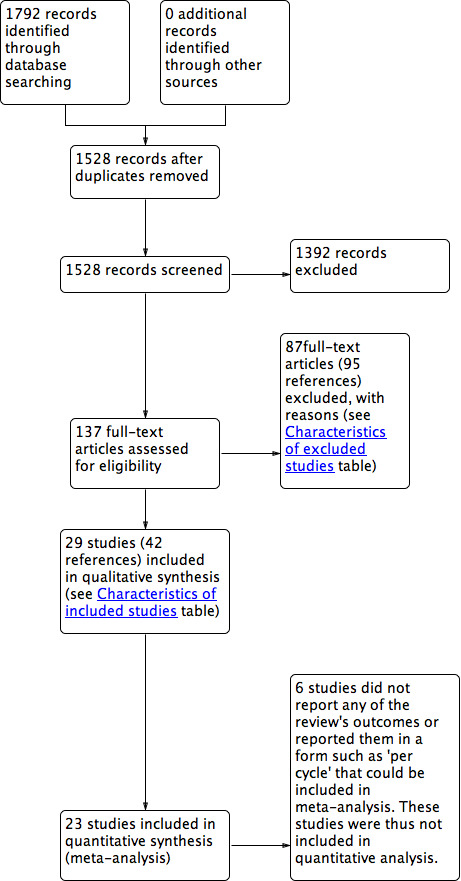
Study PRISMA flow chart
Included studies
Nine of the included studies were identified from the searches carried out in 2015 and 2016 (Blockeel 2012; Cédrin‐Durnerin 2012; Garcia‐Velasco 2011; Hauzman 2013; Kim 2011; Lukaszuk 2015; Nyboe Andersen 2011; Porrati 2010; Vilela 2011). The remaining 20 studies were identified from the previous updates of the review.
The following is a summary of the methods, participants, interventions and outcomes of the included studies. Full details of these domains (for each study separately) are in the Characteristics of included studies table.
Methods
We included 29 trials, with 4702 women randomized to treatment (Aston 1995; Biljan 1998b; Blockeel 2012; Cédrin‐Durnerin 2007; Cédrin‐Durnerin 2012; Daly 2002; Ditkoff 1996; Engmann 1999; Fanchin 2003; Franco Jr 2003; Garcia‐Velasco 2011; Hauzman 2013; Hugues 1994; Huirne 2006a; Huirne 2006c; Hwang 2004; Kim 2011; Kolibianakis 2006; Lukaszuk 2015; Nyboe Andersen 2011; Obruca 2002; Porrati 2010; Raoofi 2008; Rombauts 2006; Salat‐Baroux 1988; Shaker 1995; Tan 2001; Vilela 2011; Ye 2009). Two of the included studies were three‐arm parallel RCTs (Kim 2011; Rombauts 2006), one was a four‐arm RCT (Cédrin‐Durnerin 2007), and the remaining 26 studies were two‐arm parallel RCTs.
The largest trials were Kolibianakis 2006 (504 women), Cédrin‐Durnerin 2012 (472 women), Nyboe Andersen 2011 (442 women), Rombauts 2006 (351 women), Lukaszuk 2015 (298 women), Garcia‐Velasco 2011 (228 women), Ye 2009 (220 women) and Vilela 2011 (210 women). The remainder included fewer than 200 women. Only one study used a cross‐over design and reported pre‐cross‐over data (Daly 2002). The remaining 28 trials used a parallel design. Four studies were conducted in multiple centres (Cédrin‐Durnerin 2007; Huirne 2006a; Huirne 2006c; Rombauts 2006).
The trials took place in (or authors came from): Argentina (Porrati 2010; Vilela 2011); France (Cédrin‐Durnerin 1996; Cédrin‐Durnerin 2007; Cédrin‐Durnerin 2012; Fanchin 2003; Hugues 1994; Salat‐Baroux 1988); UK (Aston 1995; Shaker 1995); Canada (Biljan 1998b; Tan 2001); UK and Canada (Engmann 1999); USA (Daly 2002; Ditkoff 1996); USA and Europe (Nyboe Andersen 2011); Austria (Obruca 2002); Belgium (Blockeel 2012; Kolibianakis 2006); Brazil (Franco Jr 2003); China (Ye 2009); Iran (Raoofi 2008); South Korea (Kim 2011); Taiwan (Hwang 2004); Australia, Denmark, Jordan and Norway (Rombauts 2006); the Netherlands and Belgium (Huirne 2006a); Spain (Garcia‐Velasco 2011; Hauzman 2013); Poland (Lukaszuk 2015); Egypt, Iran, the Netherlands, Belgium, France and Austria (Huirne 2006c).
Of the 29 included studies, 13 performed and adhered to a power calculation (Aston 1995; Biljan 1998b; Cédrin‐Durnerin 2012; Engmann 1999; Fanchin 2003; Huirne 2006a; Huirne 2006c; Hwang 2004; Kim 2011; Kolibianakis 2006; Lukaszuk 2015; Nyboe Andersen 2011; Rombauts 2006). Seven studies did not adhere to a power calculation (Cédrin‐Durnerin 2007; Ditkoff 1996; Franco Jr 2003; Hauzman 2013; Raoofi 2008; Salat‐Baroux 1988; Shaker 1995), and in other studies this was unclear because there was only an abstract available or it was not reported.
Participants
Inclusion criteria
Of the 29 studies, 25 RCTs included women with a regular IVF/ICSI indication while four RCTs included women who had special indications for IVF: one trial included women who were low responders (i.e. women who respond poorly to controlled ovarian hyperstimulation (Kim 2011)); one included women with limited ovarian reserve (Daly 2002); one included women with PCOS (Hwang 2004); and another one included women with an ovarian cyst of over 5 mm in diameter or an endometrial thickness of over 5 mm and serum oestradiol concentration greater than 100 pmol/L after 14 days of GnRH agonist treatment (Shaker 1995).
Twenty‐one studies mentioned an age limit as an inclusion criteria. Eight studies only included women aged 38 years or less (Blockeel 2012; Cédrin‐Durnerin 2007; Cédrin‐Durnerin 2012; Franco Jr 2003; Hauzman 2013; Huirne 2006a; Salat‐Baroux 1988; Ye 2009). Nine studies only included women aged 39 years or less (Fanchin 2003; Garcia‐Velasco 2011; Huirne 2006c; Hwang 2004; Kolibianakis 2006; Nyboe Andersen 2011; Porrati 2010; Rombauts 2006; Vilela 2011). One study included women less than 40 years (Lukaszuk 2015). The other three studies used age limits above 40 years of age: one study used an upper limit of 41 years of age (Daly 2002), one study an upper limit of 42 years of age (Cédrin‐Durnerin 1996), and one study used an upper limit of 44 years of age (Engmann 1999). Six of these 21 studies defined lower limits of 18 years of age (Engmann 1999; Hauzman 2013; Huirne 2006a; Huirne 2006c; Nyboe Andersen 2011; Rombauts 2006).
Other common inclusion criteria were the presence of regular menstrual cycles (Blockeel 2012; Cédrin‐Durnerin 2007; Cédrin‐Durnerin 2012; Fanchin 2003; Garcia‐Velasco 2011; Hauzman 2013; Huirne 2006a; Nyboe Andersen 2011; Rombauts 2006; Ye 2009), and a body mass index (BMI) between 18 kg/m2 and 25 kg/m2 (Ye 2009), or less than 30 kg/m2 (Blockeel 2012; Cédrin‐Durnerin 2007; Cédrin‐Durnerin 2012; Fanchin 2003; Hauzman 2013; Huirne 2006a; Kolibianakis 2006; Rombauts 2006).
Exclusion criteria
Nine studies excluded women with an evidence of poor response. Two studies defined poor response as any previous ART cycles with less than three oocytes (Huirne 2006a; Huirne 2006c), Huirne 2006a also excluded women if they had a history of three or more consecutive ART cycles without a clinical pregnancy. One study defined poor response as development of fewer than four follicles in previous IVF or ICSI cycles (Blockeel 2012). Three studies defined poor response as fewer than five oocytes in a previous IVF attempt or fewer than five follicles in a spontaneous cycle (Cédrin‐Durnerin 2007; Cédrin‐Durnerin 2012; Hauzman 2013), and one study defined poor response as more than three unsuccessful controlled ovarian stimulation cycles or a history of low or no ovarian response during FSH/hMG (Rombauts 2006). Two studies did not mention how they defined poor response to ovarian stimulation (Garcia‐Velasco 2011; Kolibianakis 2006).
Other common exclusion criteria were: a high baseline serum FSH level (Cédrin‐Durnerin 2007; Cédrin‐Durnerin 2012; Ditkoff 1996; Engmann 1999; Huirne 2006a; Hwang 2004; Kolibianakis 2006; Nyboe Andersen 2011), evidence of ovarian cysts or endometrioma (Aston 1995; Engmann 1999; Kolibianakis 2006), and PCOS (Blockeel 2012; Garcia‐Velasco 2011; Hauzman 2013; Huirne 2006a; Kim 2011; Porrati 2010; Rombauts 2006; Vilela 2011).
Interventions
Three of the 29 studies had more than two study arms and were used in more than one comparison (four arms: Cédrin‐Durnerin 2007; three arms: Kim 2011; Rombauts 2006).
Combined oral contraceptive pill versus placebo or no pretreatment
In 17 trials (with 19 comparisons), the study group received pretreatment with a COCP, while the control group received no pretreatment. None of these studies used a placebo in the control group. Ten trials used ethinyl oestradiol as the oestrogen component in a daily dose of 30 μg (Cédrin‐Durnerin 2007; Garcia‐Velasco 2011; Huirne 2006a; Huirne 2006c; Kim 2011; Kolibianakis 2006; Nyboe Andersen 2011; Obruca 2002; Raoofi 2008; Rombauts 2006); seven trials used desogestrel 150 μg daily (Cédrin‐Durnerin 2007; Garcia‐Velasco 2011; Kolibianakis 2006; Nyboe Andersen 2011; Obruca 2002; Raoofi 2008; Rombauts 2006); three trials used levonorgestrel 150 μg daily as the progestogen component (Huirne 2006a; Huirne 2006c; Kim 2011); one trial used Diane‐35, which contained ethinyl oestradiol 35 μg and cyproterone acetate 2 mg (Hwang 2004); and two trials used a combination of ethinyl oestradiol 20 μg and levonorgestrel 100 μg (Porrati 2010; Vilela 2011). For other studies, there were not enough data available on the type of COCP used.
The starting days of pretreatment in 10 of the trials varied from cycle day one to five. Five studies started COCP pretreatment on cycle day one (Biljan 1998b; Kolibianakis 2006; Obruca 2002; Raoofi 2008; Rombauts 2006); two studies started the pretreatment on cycle day two or three (Cédrin‐Durnerin 2007; Huirne 2006a); one study started the pretreatment on a variable cycle day from one to five (Huirne 2006c); and one study started the pretreatment on cycle day five (Hwang 2004). There were not enough data available from five trials on the start day of pretreatment (Garcia‐Velasco 2011; Kim 2011; Nyboe Andersen 2011; Porrati 2010; Vilela 2011).
The duration of pretreatment in the 17 trials varied from 12 days to three consecutive cycles. Five studies used a fixed duration of 14 days of pretreatment (Biljan 1998b; Kolibianakis 2006; Raoofi 2008), or 21 days of pretreatment (Kim 2011; Porrati 2010); five studies used a variable duration of pretreatment of 12 to 16 days (Garcia‐Velasco 2011), 14 to 21 days (Nyboe Andersen 2011), 14 to 25 days (Vilela 2011), and 14 to 28 days (Huirne 2006a; Rombauts 2006). Three other studies used a variable duration of around two or three weeks minimum to around four weeks maximum (Cédrin‐Durnerin 2007, 15 to 21 days; Obruca 2002, 18 to 28 days; Huirne 2006c, 21 to 28 days). Hwang 2004 used the longest pretreatment duration of three consecutive cycles.
Two studies used agonists in both treatment groups. One study used buserelin acetate (long protocol) (Biljan 1998b) and one used a depot of triptorelin acetate (Raoofi 2008).
Ten studies used antagonists in both treatment groups. Four studies used ganirelix acetate (Blockeel 2012; Cédrin‐Durnerin 2007; Kolibianakis 2006; Rombauts 2006); three studies used cetrorelix acetate (Obruca 2002; Porrati 2010; Vilela 2011); one study used antide (Huirne 2006a); one study, a three‐arm parallel RCT, used cerotide in two of the treatment arms and a GnRH agonist in the second control arm (Kim 2011), and one study did not mention which GnRH antagonist was used (Nyboe Andersen 2011).
Four trials used an antagonist in the study group and an agonist in the control group. Two trials used cetrorelix acetate as antagonist and buserelin acetate as agonist (Huirne 2006c; Hwang 2004), and one used ganirelix acetate as antagonist and nafarelin acetate as agonist (Rombauts 2006). The other study did not mention which antagonists and agonists were used (Garcia‐Velasco 2011).
Progestogen versus placebo or no pretreatment
In seven trials, the study group was given a pretreatment with a progestogen, while the control group received placebo (Aston 1995), or no pretreatment (Cédrin‐Durnerin 2007; Ditkoff 1996; Engmann 1999; Hugues 1994; Salat‐Baroux 1988; Shaker 1995). Four studies used norethisterone 10 mg daily (Cédrin‐Durnerin 2007; Ditkoff 1996; Engmann 1999; Hugues 1994), one study used medroxyprogesterone acetate 10 mg daily (Aston 1995), and one study used ethynodiol acetate 4 mg daily (Salat‐Baroux 1988). Another study used a single injection of 100 mg, but did not mention what type of progestogen they used (Shaker 1995).
The starting days of pretreatment in all seven trials varied from cycle day one to 19. Two studies started the pretreatment with progestogen on cycle day one (Ditkoff 1996; Engmann 1999), two studies on cycle day 15 (Cédrin‐Durnerin 2007; Salat‐Baroux 1988), one study on cycle day 16 or 17 (Shaker 1995), and one study on cycle day 19 (Aston 1995). There were not enough data available from one study on the start day of pretreatment (Hugues 1994).
The duration of progestogen pretreatment varied from one to 21 days. In one study, the women received one single injection (Shaker 1995). One study used a duration of pretreatment of five days (Engmann 1999), one study used seven days (Aston 1995), and one study used eight days (Ditkoff 1996). Two trials used a variable duration of 10 to 15 days (Cédrin‐Durnerin 2007; Hugues 1994), and one trial used 11 to 17 days (Salat‐Baroux 1988).
Six trials used an agonist in both treatment groups. Three studies used buserelin acetate (Aston 1995; Engmann 1999; Shaker 1995), one study used triptorelin (Cédrin‐Durnerin 1996), one study used leuprolide acetate (Ditkoff 1996), and one study used dTRP6‐LHRH (Hugues 1994).
One trial used an antagonist (ganirelix acetate) in both treatment groups (Cédrin‐Durnerin 2007).
One trial did not use GnRH analogues for pituitary desensitisation. Women that participated in this study only received pure FSH and hMG (Salat‐Baroux 1988).
Oestrogen versus placebo or no pretreatment
In five trials, the study group was given a pretreatment with oestrogen, while the control group received no pretreatment. Three studies used 17β‐oestradiol (Cédrin‐Durnerin 2007; Cédrin‐Durnerin 2012; Fanchin 2003), and two studies used oestradiol valerate (Franco Jr 2003; Ye 2009). All these studies used 4 mg daily.
The starting days of pretreatment varied from cycle day 15 to 21. One study started the pretreatment on cycle day 20 (Fanchin 2003), and two on cycle day 21 (Franco Jr 2003; Ye 2009). One study started pretreatment seven days before the presumed onset of menses and administered up to the next Thursday after the occurrence of menstrual bleeding (Cédrin‐Durnerin 2012). One remaining study started the pretreatment 10 days before the presumed menses (Cédrin‐Durnerin 2007).
The duration of pretreatment varied from 10 to 17 days. In two studies the duration varied from 7 to 15 days (Cédrin‐Durnerin 2007; Cédrin‐Durnerin 2012). Three studies used a fixed duration of pretreatment of 10 days (Ye 2009), 11 days (Fanchin 2003), and 14 days (Franco Jr 2003).
Three trials used an antagonist in both treatment groups, one trial used ganirelix acetate (Cédrin‐Durnerin 2007), one trial used cetrorelix acetate (Fanchin 2003), and one trial did not report the name of the antagonist (Cédrin‐Durnerin 2012).
Two trials used an antagonist in the intervention group and an agonist in the control group, one trial used ganirelix acetate in the intervention group and nafarelin acetate in the control group (Franco Jr 2003), and one study used cetrotide in the intervention group and triptoreline in the control group (Ye 2009).
Combined oral contraceptive pill versus progestogen
There was only one study that compared COCP with progestogen (Cédrin‐Durnerin 2007). The women in the COCP group received ethinyl oestradiol 30 μg plus desogestrel 150 μg daily and the women in the progestogen group received norethisterone 10 mg daily. This study started the COCP pretreatment on cycle day two or three with a duration of 15 to 21 days. The progestogen pretreatment was started on cycle day 15 with a duration of 10 to 15 days. Both groups received the GnRH antagonist, ganirelix acetate.
Combined oral contraceptive pill versus oestrogen
Four trials compared a pretreatment of COCP with a pretreatment of oestrogen. One trial used ethinyl oestradiol 30 μg plus desogestrel 150 μg daily as a COCP and micronised 17β‐oestradiol 4 mg daily as oestrogen pretreatment (Cédrin‐Durnerin 2007). The COCP pretreatment started on cycle day two or three with a duration of 15 to 21 days. The oestrogen pretreatment started 10 days before the presumed menses with a duration of 10 to 15 days and both groups received the GnRH antagonist, ganirelix acetate. Another study used ethinyl oestradiol 30 μg plus levonorgestrel 150 μg daily as a COCP and oestradiol valerate 4 mg daily as oestrogen pretreatment (Hauzman 2013). The COCP pretreatment started on cycle day one or two and continued for 12 to 16 days. The oestrogen pretreatment started from day 20 of menstrual cycle for five to 12 days until the day before starting stimulation and both groups received the GnRH antagonist, ganirelix acetate. One study used ethinyl oestradiol plus desogestrel as COCP starting from day two to four of the cycle but their doses were not reported (Lukaszuk 2015). In the control group, women were pretreated with oral oestradiol 2 mg twice daily from the day 20 of the natural cycle to day one to four of the new cycle. Both groups received the GnRH agonist, triptorelin. One study did not mention which COCP was used, but used ethinyl oestradiol 2 mg as an oestrogen pretreatment (Daly 2002). This study only described that the oestrogen pretreatment was administered in the luteal phase of the preparation cycle, but did not report the exact starting days and durations of pretreatment. The COCP group received the GnRH agonist, leuprolide acetate, and the oestrogen group received the GnRH antagonist, ganirelix acetate.
Progestogen versus oestrogen
There was only one study that compared progestogen with oestrogen (Cédrin‐Durnerin 2007). The women in the progestogen group received norethisterone 10 mg daily and the women in the oestrogen group received micronised 17β‐oestradiol 4 mg daily. This study started the progestogen pretreatment on cycle day 15 with a duration of 10 to 15 days. The oestrogen pretreatment started 10 days before the presumed menses with also a duration of 10 to 15 days. Both groups received the GnRH antagonist, ganirelix acetate.
Outcomes
Primary outcome
Fifteen studies reported the number of live births or ongoing pregnancies (Cédrin‐Durnerin 2007; Cédrin‐Durnerin 2012; Daly 2002; Ditkoff 1996; Engmann 1999; Franco Jr 2003; Garcia‐Velasco 2011; Hauzman 2013; Huirne 2006a; Huirne 2006c; Kim 2011; Kolibianakis 2006; Nyboe Andersen 2011; Rombauts 2006; Ye 2009). Three studies defined ongoing pregnancy as a positive heart activity at a gestational age of 12 weeks (Huirne 2006a; Huirne 2006c; Kim 2011). One study used the same definition but did not mention when they performed the ultrasound scan (Ditkoff 1996). Two studies defined ongoing pregnancy as a pregnancy developing beyond 12 weeks (Cédrin‐Durnerin 2007; Kolibianakis 2006), and one study defined ongoing pregnancy as a pregnancy assessed by ultrasound at 12 to 16 weeks or later (Rombauts 2006). The remaining studies did not mention how they assessed ongoing pregnancy (Cédrin‐Durnerin 2012; Daly 2002; Engmann 1999; Franco Jr 2003; Garcia‐Velasco 2011; Hauzman 2013; Nyboe Andersen 2011; Ye 2009).
Ten studies reported the number of pregnancy losses. One study described this as the proportion of women with initially positive hCG in whom pregnancy failed to develop beyond 12 weeks of gestation (Kolibianakis 2006). The other nine studies did not describe a definition (Daly 2002; Engmann 1999; Franco Jr 2003; Garcia‐Velasco 2011; Hwang 2004; Kim 2011; Rombauts 2006; Salat‐Baroux 1988; Ye 2009).
Secondary outcomes
Twenty‐one studies reported the number of clinical pregnancies. Three studies defined clinical pregnancy as the presence of one or more foetal hearts confirmed with ultrasound, performed at least four weeks after embryo transfer (Fanchin 2003 or from six weeks after embryo transfer (Franco Jr 2003; Kim 2011). Two other studies used the same definition, but one of these also included the foetal sacs without heart activity (Huirne 2006c), and the other performed the ultrasound scan at seven weeks after embryo transfer (Hwang 2004). One study defined clinical pregnancy as the presence of one or more intrauterine sacs confirmed with ultrasound, at a gestational age of six weeks (Huirne 2006a). In one study, we used a positive pregnancy test with evidence of a gestational sac to define clinical pregnancy, because no clinical or ongoing pregnancy rate was available (Engmann 1999). One study defined clinical pregnancy as the evidence of a clinical gestational sac (Ditkoff 1996). In the other 12 studies, it was unclear how they defined this outcome (Aston 1995; Blockeel 2012; Cédrin‐Durnerin 2007; Cédrin‐Durnerin 2012; Daly 2002; Garcia‐Velasco 2011; Hauzman 2013; Nyboe Andersen 2011; Obruca 2002; Porrati 2010; Salat‐Baroux 1988; Ye 2009). If no clinical pregnancy rates were reported, we used the ongoing pregnancy rates (if available) for our analysis.
Ten studies reported the number of oocytes retrieved (Cédrin‐Durnerin 2007; Ditkoff 1996; Franco Jr 2003; Huirne 2006a; Huirne 2006c; Hwang 2004; Kim 2011; Obruca 2002; Rombauts 2006; Salat‐Baroux 1988). One study only mentioned the number of cumulus‐oocyte complexes (Kolibianakis 2006), and two studies mentioned the number of mature oocytes or follicles (Engmann 1999; Fanchin 2003), but we assumed that this meant the same as the number of oocytes retrieved and, therefore, we pooled the data of these studies.
Thirteen studies reported the number of days of gonadotrophin treatment (Blockeel 2012; Ditkoff 1996; Engmann 1999; Franco Jr 2003; Hauzman 2013; Huirne 2006a; Huirne 2006c; Hwang 2004; Kim 2011; Kolibianakis 2006; Porrati 2010; Rombauts 2006; Vilela 2011).
Thirteen studies reported the amount of gonadotrophins administered (Blockeel 2012; Cédrin‐Durnerin 2007; Cédrin‐Durnerin 2012; Fanchin 2003; Franco Jr 2003; Hauzman 2013; Huirne 2006a; Huirne 2006c; Kim 2011; Kolibianakis 2006; Porrati 2010; Rombauts 2006; Vilela 2011). Two studies reported the amount of gonadotrophins administered in the number of ampoules used, but we could not use these data in our analysis (Ditkoff 1996; Engmann 1999).
Other adverse outcomes
Six studies reported the number of women with ovarian cysts. In one study, we used the number of functional ovarian cysts with a diameter of 10 mm or more, measured after one week of GnRH agonist treatment (Engmann 1999). Three studies defined an ovarian cyst as an intraovarian sonolucent structure with a mean diameter of 14 mm or more, measured after seven days (Aston 1995) or eight days (Ditkoff 1996) of pituitary suppression, or on a day not specified (Franco Jr 2003). One study did not mention how they defined ovarian cyst formation and when they measured this (Huirne 2006a). One study only reported cyst formation as a reason for cycle cancellation, but it was unclear if there were more cysts formed that did not lead to cycle cancellation (Salat‐Baroux 1988). We did not use these data in our analysis.
Six studies reported the number of multiple pregnancies. One study defined multiple pregnancies as multiple clinical pregnancies (Huirne 2006c). One study described the number of ongoing or live born twin pregnancies (Hwang 2004). Four studies did not describe when the number of multiple pregnancies was measured (Cédrin‐Durnerin 2007; Franco Jr 2003; Garcia‐Velasco 2011; Kim 2011).
Five studies reported the number of women with OHSS. Two study used the WHO classification criteria to diagnose OHSS and divided the women in categories of mild (grade I), moderate (grade II) or severe (grade III) (Nyboe Andersen 2011; Rombauts 2006); the other three studies did not mention how they diagnosed OHSS (Franco Jr 2003; Hwang 2004; Ye 2009).
Six studies did not contribute data to the analyses: two of these studies reported 'per cycle' data and the numbers of cycles were not equivalent to the numbers of participants (Biljan 1998b; Shaker 1995); outcomes reported included clinical pregnancy rate, number of days of GnRH agonist and number of oocytes retrieved. One study reported outcome data in denominators other than 'per woman' such as 'per cycle', 'per embryo transfer' (Lukaszuk 2015), outcomes reported included clinical pregnancy, number of oocyte retrieved, multiple pregnancy, duration of stimulation days. Three studies had no available outcome data relevant to the review (Hugues 1994; Raoofi 2008; Tan 2001).
Excluded studies
A total of 86 studies that described pretreatments with COCPs, progestogens or oestrogens were not eligible for inclusion for various reasons. Some of these studies had multiple reasons for exclusion, but we reported the most important reason. Full details of reasons for exclusion can be found in the Characteristics of excluded studies table.
Twenty studies did not randomise their participants using standardized randomization procedures such as a computer or a random number table (Benadiva 1988; Cédrin‐rDurnerin 1995; Cohen 1987; Copperman 2003; Couzinet 1995; Ditkoff 1997; Forman 1991; Frydman 1986; Galera 2004; Godin 2003; Gonen 1990; Lindheim 1996; Neal 1993; Palomba 2008; Schoolcraft 1997; Surrey 1989; Tarlatzis 1993; Wang 2016; Weisman 1989; Yokota 2006).
Twenty‐four studies used a retrospective design (al‐Mizyen 2000; Bellver 2007; Bendikson 2006; Biljan 1998c; Chung 2006; Damario 1997; Dickey 2001; Duvan 2008; Frederick 2004; Gonzalez 1995; Keltz 2007; Kovacs 2001; Leondires 1999; Loutradis 2003; Min 2005; Mirkin 2003; Pados 1995; Pinkas 2008; Ramsewak 2005; Talebian 2004; Talebian 2007; Wei 2016; Yoshida 2005; Zhao 2008).
In five studies, participants served as their own controls in previous cycles (Branigan 1998; Fanchin 2003b; Fisch 1996; Mulangi 1997; Surrey 1998).
There was insufficient information in five studies to determine their true randomization status (Aghahosseini 2011; Bakas 2014; Davar 2014; Engels 2011; Merviel 2015).
Three studies used a cross‐over design and there were no available pre‐cross‐over data (Cédrin‐Durnerin 1996; Fanchin 2001; Wang 2008).
Eight studies used a single‐arm design (Brodt 1993; De Ziegler 1999; Gerli 1989; Hugues 1992; Meldrum 2002; Meldrum 2008; Sanghvi 2002; Tehraninejad 2010).
Nine studies compared two (or more) different dosages, timings or ways of administration of the same pretreatment (Davy 2004; Gomez 2000; Haydardedeoglu 2012; Karande 2004; Kreiner 2007; Lewin 2002; Liu 2011; Mashiach 1989; Russell 1997).
Two studies used interventions that were administered for luteal phase support and not for pretreatment (Ghanem 2015; Rashidi 2011).
In three studies, women only received ovarian stimulation, but no embryo transfer was performed as part of an ART cycle (Anderson 1990; Letterie 2000; Steinkampf 1991).
In two studies, the women were oocyte donors (Doody 2001; Martinez 2006).
In one study, the women had premature ovarian failure (Tartagni 2007).
In two studies, pretreatment was not stopped before oocyte retrieval, but continued to be used as luteal phase support (Greco 2016; Jung 2000).
In one study, the main comparison was mild versus standard stimulation (with pretreatment in one group) (Youssef 2017).
Risk of bias in included studies
A complete overview of classification of risk of bias domains can be found in the Characteristics of included studies table and in Figure 1 and Figure 2.
Allocation
Eighteen studies were at low risk of random sequence generation: 13 of these studies used computer generated random numbers to randomise the women (Biljan 1998b; Blockeel 2012; Engmann 1999; Fanchin 2003; Garcia‐Velasco 2011; Hauzman 2013; Huirne 2006a; Huirne 2006c; Hwang 2004; Kim 2011; Kolibianakis 2006; Lukaszuk 2015; Ye 2009), four studies used a table of random numbers (Cédrin‐Durnerin 2007; Cédrin‐Durnerin 2012; Franco Jr 2003; Shaker 1995), and one study accomplished the randomization by tossing a coin (Ditkoff 1996). In the remaining 11 studies, there was insufficient information on the methods used in random sequence generation; they were thus rated at unclear risk of bias with respect to random sequence generation.
If randomization is not done correctly, there might be a difference in baseline characteristics between the women in the treatment groups. This may influence the outcomes measured in the trial. Therefore, it is important that the method of randomization is reported. Due to the high number of included studies that did not report the method of randomization (15/33 studies), there might be a higher risk of bias.
Fourteen studies were at low risk of allocation concealment: eight studies used sealed envelopes to conceal the allocation (Biljan 1998b; Blockeel 2012; Cédrin‐Durnerin 2007; Cédrin‐Durnerin 2012; Garcia‐Velasco 2011; Hauzman 2013; Hwang 2004; Shaker 1995); in five studies the allocation was done by a third party (Aston 1995, hospital pharmacy and numbered bottles; Engmann 1999, clinic nurses and sealed envelopes; Fanchin 2003, independent person; Huirne 2006a, independent person from independent monitoring company; Rombauts 2006, central remote allocation); one study centralised the randomization process (Ditkoff 1996).
Two studies were classified at high risk of allocation concealment, because they reported that the sequence of allocation was not concealed (Franco Jr 2003; Kolibianakis 2006). In the remaining 14 studies, there was insufficient information to make a conclusive judgement with respect to allocation concealment and these were rated at unclear risk of bias in this domain.
Most of the outcomes of this review were objectively assessed; thus a poorly designed allocation concealment method is not likely to have a big influence on these outcomes. For example, the number of live births is not likely to be influenced by the clinician if he or she knows which treatment the woman receives. However, some outcomes, such as OHSS, are diagnosed on clinical symptoms and so there might be a more important risk of bias when the clinician was aware of the treatment assigned to each woman. Nevertheless, outcomes that are objectively assessed may be influenced indirectly if allocation is not concealed.
Blinding
One study was at low risk of blinding because it was described as double blind and placebo controlled (Aston 1995).
In 20 studies, there was insufficient information with respect to blinding of clinicians, participants and outcome assessors; these studies were assessed at unclear risk with regard to blinding (Biljan 1998b; Blockeel 2012; Cédrin‐Durnerin 2012; Engmann 1999; Fanchin 2003; Franco Jr 2003; Garcia‐Velasco 2011; Hauzman 2013; Hugues 1994; Huirne 2006c; Hwang 2004; Kim 2011; Lukaszuk 2015; Obruca 2002; Porrati 2010; Rombauts 2006; Salat‐Baroux 1988; Shaker 1995; Tan 2001; Vilela 2011).
The remaining eight studies were at high risk of bias because they were described as either open label or not blinded with personnel and participants having knowledge of the treatment allocation.
Poor blinding is less likely to influence the objective outcomes such as live birth, but it might have a bigger influence on the diagnosis of OHSS.
Incomplete outcome data
Of the 29 studies, 13 were at low risk of attrition bias (Blockeel 2012; Ditkoff 1996; Engmann 1999; Hauzman 2013; Huirne 2006a; Huirne 2006c; Kim 2011; Kolibianakis 2006; Nyboe Andersen 2011; Porrati 2010; Rombauts 2006; Vilela 2011; Ye 2009). In these trials, the proportions of, and reasons for, withdrawals were balanced between the treatment groups or data were analyzed using an ITT basis where all women randomized at baseline were included in the analysis.
In 10 studies, there were insufficient information with respect to the proportions of, and reasons for, withdrawals or losses to follow‐up and data were not analyzed on an ITT basis; these studies were rated at unclear risk of attrition bias (Biljan 1998b; Cédrin‐Durnerin 2007; Daly 2002; Garcia‐Velasco 2011; Hugues 1994; Lukaszuk 2015; Obruca 2002; Raoofi 2008; Shaker 1995; Tan 2001).
The remaining six studies were at high risk of attrition bias because the proportions of, and reasons for, withdrawals were not balanced between the treatment groups and data were not analyzed by ITT.
Incomplete outcome data can bias the results of our review, especially with regard to adverse outcomes. For example, a study might have withdrawals due to OHSS that they do not report. Also imbalances in reasons for withdrawal can occur because of differences in interventions between the study group and control group. For example, when there are more withdrawals due to OHSS in the control group, this can be in favour of the intervention used in the study group. The risk of bias might increase if authors do not report this.
Selective reporting
We were unable to retrieve the protocol of any of the included studies to examine whether all prespecified outcomes were reported. However, we examined the Methods sections of the studies to determine whether outcomes planed in the Methods section were eventually reported. In this regard, we classified 16 studies at low risk of selective reporting because these trials reported data on all the outcomes mentioned in the 'Methods' section of their publication (Blockeel 2012; Cédrin‐Durnerin 2012; Engmann 1999; Garcia‐Velasco 2011; Hauzman 2013; Huirne 2006a; Huirne 2006c; Hwang 2004; Kim 2011; Kolibianakis 2006; Lukaszuk 2015; Nyboe Andersen 2011; Porrati 2010; Rombauts 2006; Shaker 1995; Ye 2009). In two studies, there was insufficient information in the 'Methods' sections to make a conclusive judgement with respect to selective reporting of outcomes; therefore, they were rated at unclear risk of reporting bias (Raoofi 2008; Vilela 2011). In the remaining 11 studies, there was evidence of selective reporting as data were not reported on all the outcomes pre‐specified in the protocol or Methods section.
Other potential sources of bias
For other potential sources of bias, we examined the baseline demographic characteristics of participants, such as age and BMI, to determine whether there was substantial imbalance in baseline demographic characteristics between the treatment groups. In this regard, 22 studies were at low risk of bias in this domain because of the absence of substantial imbalance between in baseline demographic characteristics between the treatment groups (Aston 1995; Biljan 1998b; Blockeel 2012; Cédrin‐Durnerin 2007; Cédrin‐Durnerin 2012; Ditkoff 1996; Engmann 1999; Fanchin 2003; Franco Jr 2003; Garcia‐Velasco 2011; Hauzman 2013; Huirne 2006a; Huirne 2006c; Hwang 2004; Kim 2011; Kolibianakis 2006; Lukaszuk 2015; Nyboe Andersen 2011; Rombauts 2006; Salat‐Baroux 1988; Shaker 1995; Ye 2009).
The remaining seven studies were rated as being at unclear risk due to insufficient information to make a conclusive judgement with respect to baseline demographic characteristics in the treatment groups.
Effects of interventions
See: Table 1; Table 2; Table 3
Summary of findings for the main comparison. Combined oral contraceptive pill compared to no pretreatment for ovarian stimulation protocols for women undergoing assisted reproductive techniques.
| Combined oral contraceptive pill compared to no pretreatment for ovarian stimulation protocols for women undergoing assisted reproductive techniques | ||||||
|
Population: women undergoing ART Settings: ART clinic Intervention: COCP Comparison: no pretreatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk1 | Corresponding risk | |||||
| No pretreatment | COCP | |||||
|
Live birth or ongoing pregnancy (COCP + Ant vs Ant) |
270 per 1000 | 215 per 1000 (177 to 260) | OR 0.74 (0.58 to 0.95) | 1335 (6 studies) | ⊕⊕⊕⊝ Moderate2 | ‐ |
|
Live birth or ongoing pregnancy (COCP + Ant vs Ag) |
296 per 1000 | 273 per 1000 (212 to 345) | OR 0.89 (0.64 to 1.25) | 724 (4 studies) | ⊕⊕⊕⊝ Moderate3 | ‐ |
|
Pregnancy loss (COCP + Ant vs Ant) |
64 per 1000 | 85 per 1000 (53 to 134) | OR 1.36 (0.82 to 2.26) | 868 (5 studies) | ⊕⊕⊕⊝ Moderate3 | ‐ |
|
Pregnancy loss (COCP + Ant vs Ag) |
103 per 1000 | 44 per 1000 (25 to 76) | OR 0.40 (0.22 to 0.72) | 780 (5 studies) | ⊕⊕⊕⊝ Moderate3 | ‐ |
| Multiple pregnancy rate (COCP + Ant vs Ant) | 47 per 1000 | 98 per 1000 (25 to 313) | OR 2.21 (0.53 to 9.26) | 125 (2 studies) | ⊕⊕⊝⊝ Low4 | ‐ |
| Multiple pregnancy rate (COCP + Ant vs Ag) | 147 per 1000 | 189 per 1000 (127 to 273) | OR 1.36 (0.85 to 2.19) | 546 (4 studies) | ⊕⊕⊕⊝ Moderate3 | ‐ |
|
OHSS rate (COCP + Ant vs Ant) |
16 per 1000 |
16 per 1000 (4 to 52) |
OR 0.98 (0.28 to 3.40) |
642 (2 studies) |
⊕⊕⊝⊝ Low4 | ‐ |
|
OHSS rate (COCP + Ant vs Ag) |
55 per 1000 |
35 per 1000 (11 to 102) |
OR 0.63 (0.20 to 1.96) |
290 (2 studies) |
⊕⊕⊝⊝ Low4 | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Ag: agonist; Ant: antagonist; ART: assisted reproductive techniques; COCP: combined oral contraceptive pill; CI: confidence interval; OHSS: ovarian hyperstimulation syndrome; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Mean baseline risk of control group. 2 Downgraded one level for serious risk of bias due to poor reporting of sequence generation and allocation concealment. 3 Downgraded one level for serious imprecision: effect estimate with wide confidence intervals or low event rate (or both). 4 Downgraded two levels for very serious imprecision: small sample size or very low event rate, and effect estimate with wide confidence intervals.
Summary of findings 2. Progestogen compared to placebo or no pretreatment for ovarian stimulation protocols for women undergoing assisted reproductive techniques.
| Progestogen compared to placebo or no pretreatment for ovarian stimulation protocols for women undergoing assisted reproductive techniques | ||||||
|
Patient or population: ovarian stimulation protocols for women undergoing ART Settings: Intervention: progestogen Comparison: placebo or no pretreatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk1 | Corresponding risk | |||||
| Placebo or no pretreatment | Prog | |||||
|
Live birth or ongoing pregnancy (Prog + Ag vs Ag) |
170 per 1000 | 217 per 1000 (124 to 352) | OR 1.35 (0.69 to 2.65) | 222 (2 studies) | ⊕⊕⊝⊝ Low2 | ‐ |
| Live birth or ongoing pregnancy (Prog + Ant vs Ant) | 292 per 1000 | 217 per 1000 (69 to 512) | OR 0.67 (0.18 to 2.54) | 47 (1 study) | ⊕⊕⊝⊝ Low2 | ‐ |
|
Pregnancy loss (Prog + Ag vs Ag) |
36 per 1000 | 78 per 1000 (24 to 220) | OR 2.26 (0.67 to 7.55) | 222 (2 studies) | ⊕⊕⊝⊝ Low2 | ‐ |
|
Pregnancy loss (Prog + Ant vs Ant) |
208 per 1000 | 86 per 1000 (16 to 354) | OR 0.36 (0.06 to 2.09) | 47 (1 study) | ⊕⊕⊝⊝ Low2 | ‐ |
|
Multiple pregnancy rate (Prog + Ag vs Ag) |
No data available | ‐ | ‐ | |||
|
Multiple pregnancy rate (Prog + Ant vs Ant) |
42 per 1000 | 44 per 1000 (3 to 438) | OR 1.05 (0.06 to 17.76) | 47 (1 study) | ⊕⊕⊝⊝ Low2 | ‐ |
|
OHSS rate (Prog + Ag vs Ag) |
No data available | ‐ | ‐ | |||
|
OHSS rate (Prog + Ant vs Ant) |
No data available | ‐ | ‐ | |||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Ag: agonist; Ant: antagonist; ART: assisted reproductive techniques; CI: confidence interval; OHSS: ovarian hyperstimulation syndrome; OR: odds ratio; Prog: progestogen. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Mean baseline risk of control group. 2 Downgraded two levels for very serious imprecision: small sample size and effect estimate with wide confidence intervals.
Summary of findings 3. Oestrogen compared to no pretreatment for ovarian stimulation protocols for women undergoing assisted reproductive techniques.
| Oestrogencompared to no pretreatment for ovarian stimulation protocols for women undergoing assisted reproductive techniques | ||||||
|
Patient or population: ovarian stimulation protocols for women undergoing ART Settings: Intervention: oestrogen Comparison: no pretreatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk1 | Corresponding risk | |||||
| No pretreatment | Oestr | |||||
|
Live birth or ongoing pregnancy (Oestr + Ant vs Ant) |
299 per 1000 | 252 per 1000 (184 to 333) | OR 0.79 (0.53 to 1.17) | 502 (2 studies) | ⊕⊕⊕⊝ Moderate2 | ‐ |
|
Live birth or ongoing pregnancy (Oestr + Ant vs Ag) |
350 per 1000 | 322 per 1000 (215 to 447) | OR 0.88 (0.51 to 1.5) | 242 (2 studies) | ⊕⊝⊝⊝ Very low3,4 | ‐ |
|
Pregnancy loss (Oestr + Ant vs Ant) |
208 per 1000 | 40 per 1000 (5 to 279) | OR 0.16 (0.02 to 1.47) | 49 (1 study) | ⊕⊝⊝⊝ Very low3,4 | ‐ |
|
Pregnancy loss (Oestr + Ant vs Ag) |
72 per 1000 | 110 per 1000 (46 to 240) | OR 1.59 (0.62 to 4.06) | 220 (1 study) | ⊕⊝⊝⊝ Very low3,4 | ‐ |
|
Multiple pregnancy rate (Oestr + Ant vs Ant) |
No data available | ‐ | ‐ | |||
|
Multiple pregnancy rate (Oestr + Ant vs Ag) |
Not calculable ‐ see comment | OR 2.24 (0.09 to 53.59) | 22 (1 study) | ⊕⊝⊝⊝ Very low3,4 | Only 2 events (both in oestrogen group) | |
|
OHSS rate (Oestr + Ant vs Ant) |
No data available | ‐ | ‐ | |||
|
OHSS rate (Oestr + Ant vs Ag) |
18 per 1000 | 27 per 1000 (5 to 147) |
OR 1.54 (0.25 to 9.42) |
220 (1 study) |
⊕⊝⊝⊝ Very low3,4 | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). Ag: agonist; Ant: antagonist; ART: assisted reproductive techniques; CI: confidence interval; Oestr: oestrogen; OHSS: ovarian hyperstimulation syndrome; OR: odds ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Mean baseline risk of control group. 2 Downgraded one level for serious imprecision: effect estimate with wide confidence intervals. 3 Downgraded one level for serious risk of bias due to poor reporting on allocation concealment or high attrition (or both). 4 Downgraded two levels for very serious imprecision: small sample size and effect estimate with wide confidence intervals.
1. Combined oral contraceptive pill versus no pretreatment
Eight studies compared COCP versus no pretreatment (Cédrin‐Durnerin 2007; Garcia‐Velasco 2011; Huirne 2006c; Huirne 2006a; Kim 2011; Kolibianakis 2006; Nyboe Andersen 2011; Rombauts 2006).
Primary outcomes
1.1. Live birth or ongoing pregnancy
See Figure 4 and Analysis 1.1.
4.
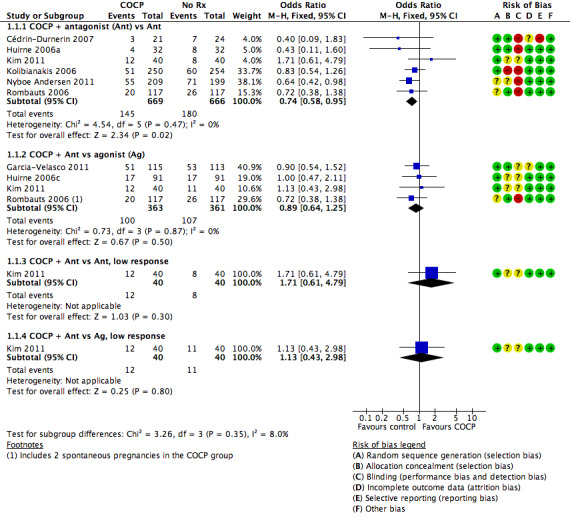
Forest plot of comparison: 1 Combined oral contraceptive pill (OCP) versus no pretreatment (Rx), outcome: 1.1 Live birth or ongoing pregnancy.
1.1. Analysis.
Comparison 1 Combined oral contraceptive pill (COCP) versus no pretreatment (Rx), Outcome 1 Live birth or ongoing pregnancy.
1.1.1. Combined oral contraceptive pill plus antagonist versus antagonist
There was a lower rate of live birth or ongoing pregnancy in women pretreated with COCP compared with no pretreatment (OR 0.74, 95% CI 0.58 to 0.95; 6 RCTs; 1335 women; I2 = 0%; moderate quality evidence). The evidence suggested that if the chance of a live birth or ongoing pregnancy following no pretreatment was assumed to be 27%, the chance following pretreatment with COCP would be between 18% and 26%.
1.1.2. Combined oral contraceptive pill plus antagonist versus agonist
There was no clear evidence of a difference between the two treatment groups in live birth or ongoing pregnancy rates (OR 0.89, 95% CI 0.64 to 1.25; 4 RCTs; 724 women; I2 = 0%; moderate quality evidence). The evidence suggested that if the chance of a live birth or ongoing pregnancy following no pretreatment was assumed to be 24%, the chance following pretreatment with COCP would be between 16% and 26%.
1.1.3. Combined oral contraceptive pill plus antagonist versus antagonist, low response
Among women with low response, there was insufficient evidence to determine whether there was a difference between the groups in live birth or ongoing pregnancy rates (OR 1.71, 95% CI 0.61 to 4.79; 1 RCT; 80 women).
1.1.4. Combined oral contraceptive pill plus antagonist versus agonist, low response
Among women with low response, there was insufficient evidence to determine whether there was a difference between the two treatment groups in live birth or ongoing pregnancy rates (OR 1.13, 95% CI 0.43 to 2.98; 1 RCT; 80 women).
1.2. Pregnancy loss
See Figure 5 and Analysis 1.2.
5.
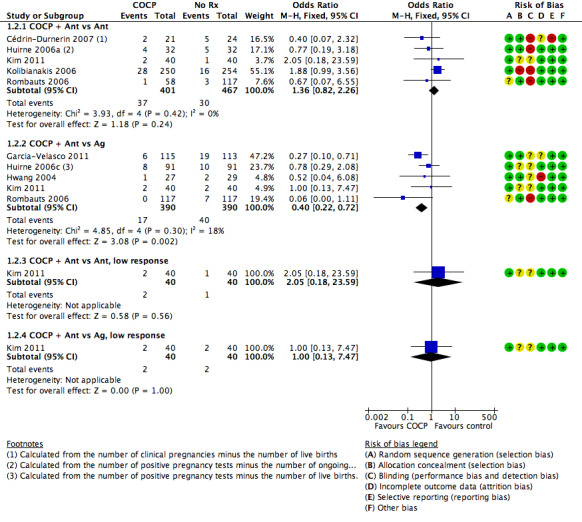
Forest plot of comparison: 1 Combined oral contraceptive pill (COCP) versus no pretreatment (Rx), outcome: 1.2 Pregnancy loss.
1.2. Analysis.
Comparison 1 Combined oral contraceptive pill (COCP) versus no pretreatment (Rx), Outcome 2 Pregnancy loss.
1.2.1. Combined oral contraceptive pill plus antagonist versus antagonist
There was no clear evidence of a difference between the groups in rates of pregnancy loss (OR 1.36, 95% CI 0.82 to 2.26; 5 RCTs; 868 women; I2 = 0%; moderate quality evidence). The evidence suggested that if the risk of a pregnancy loss following no pretreatment was assumed to be 6%, the risk following pretreatment with COCP would be between 5% and 13%.
1.2.2. Combined oral contraceptive pill plus antagonist versus agonist
There were fewer pregnancy losses recorded in women who had COCP pretreatment than in those who had no pretreatment (OR 0.40, 95% CI 0.22 to 0.72; 5 RCTs; 780 women; I2 = 18%; moderate quality evidence). The evidence suggested that if the risk of a pregnancy loss following no pretreatment was assumed to be 10%, the risk following pretreatment with COCP would be between 3% and 8%.
1.2.3. Combined oral contraceptive pill plus antagonist versus antagonist, low response
Among women with low response, there was insufficient evidence to determine whether there was a difference between the groups in rates of pregnancy loss (OR 2.05, 95% CI 0.18 to 23.59; 1 RCT; 80 women).
1.2.4. Combined oral contraceptive pill plus antagonist versus agonist, low response
Among women with low response, there was insufficient evidence to determine whether there was a difference between the groups in rates of pregnancy loss (OR 1.00, 95% CI 0.13 to 7.47; 1 RCT; 80 women).
Secondary outcomes
1.3. Clinical pregnancy rate
See Analysis 1.3.
1.3. Analysis.
Comparison 1 Combined oral contraceptive pill (COCP) versus no pretreatment (Rx), Outcome 3 Clinical pregnancy rate.
1.3.1. Combined oral contraceptive pill plus antagonist versus antagonist
There was no clear evidence of a difference between the two treatment groups in clinical pregnancy rates (OR 0.85, 95% CI 0.63 to 1.15; 5 RCTs; 740 women; I2 = 76%). The presence of substantial heterogeneity was explored in sensitivity analysis and there was no change in the evidence using a random‐effects model (OR 0.86, 95% CI 0.39 to 1.91) or risk ratio (RR) (RR 0.90, 95% CI 0.73 to 1.11).
1.3.2. Combined oral contraceptive pill plus antagonist versus agonist
There was no clear evidence of a difference between the two treatment groups in clinical pregnancy rates (OR 0.84, 95% CI 0.59 to 1.20; 4 RCTs; 546 women; I2 = 0%).
1.3.3. Combined oral contraceptive pill plus antagonist versus antagonist, low response
Among women with low response, there was insufficient evidence to determine whether there was a difference between the groups in rates of clinical pregnancy (OR 1.85, 95% CI 0.69 to 4.97; 1 RCT; 80 women).
1.3.4. Combined oral contraceptive pill plus antagonist versus agonist, low response
Among women with low response, there was insufficient evidence to determine whether there was a difference between the groups in rates of clinical pregnancy (OR 1.12, 95% CI 0.44 to 2.83); 1 RCT; 80 women).
1.3.5. Combined oral contraceptive pill plus agonist versus agonist
Two studies reported this comparison, but neither reported data suitable for analysis. One (Biljan 1998b) reported per‐cycle data and found no evidence of a difference between the groups. A second study reported a pregnancy rate of 9% in the study group and 11% in the control group, but did not report the number of women per group (Raoofi 2008).
1.4. Multiple pregnancy rate
See Analysis 1.4.
1.4. Analysis.
Comparison 1 Combined oral contraceptive pill (COCP) versus no pretreatment (Rx), Outcome 4 Multiple pregnancy rate.
1.4.1. Combined oral contraceptive pill plus antagonist versus antagonist
There was insufficient evidence to determine whether there was a difference between the groups in multiple pregnancy rates (OR 2.21, 95% CI 0.53 to 9.26; 2 RCTs; 125 women; I2 = 0%; low quality evidence). The evidence suggested that if the risk of multiple pregnancy following no pretreatment was assumed to be 5%, the risk following pretreatment with COCP would be between 3% and 31%.
1.4.2. Combined oral contraceptive pill plus antagonist versus agonist
There was insufficient evidence to determine whether there was a difference between the groups in multiple pregnancy rates (OR 1.36, 95% CI 0.85 to 2.19; 4 RCTs; 546 women; I2 = 0%; moderate quality evidence). The evidence suggested that if the risk of multiple pregnancy following no pretreatment was assumed to be 5%, the risk following pretreatment with COCP would be between 4% and 10%.
1.4.3. Combined oral contraceptive pill plus antagonist versus antagonist, low response
Among women with low response, there was insufficient evidence to determine whether there was a difference between the groups in multiple pregnancy rate (OR 2.11, 95% CI 0.36 to 12.24; 1 RCT; 80 women).
1.4.4. Combined oral contraceptive pill plus antagonist versus agonist, low response
Among women with low response, there was insufficient evidence to determine whether there was a difference between the groups in the multiple pregnancy rate (OR 1.37, 95% CI 0.29 to 6.56; 1 RCT; 80 women).
1.5. Ovarian hyperstimulation syndrome rate
See Analysis 1.5.
1.5. Analysis.
Comparison 1 Combined oral contraceptive pill (COCP) versus no pretreatment (Rx), Outcome 5 Ovarian hyperstimulation syndrome rate.
1.5.1. Combined oral contraceptive pill plus antagonist versus antagonist
There was insufficient evidence to determine whether there was a difference between the groups in OHSS rates (OR 0.98, 95% CI 0.28 to 3.40; 2 RCTs; 642 women; I2 = 0%, low quality evidence).
1.5.2. Combined oral contraceptive pill plus antagonist versus agonist
There was insufficient evidence to determine whether there was a difference between the groups in OHSS rates (OR 0.63, 95% CI 0.20 to 1.96; 2 RCTs; 290 women; I2 = 0%, low quality evidence).
1.6. Number of oocytes retrieved
See Analysis 1.6.
1.6. Analysis.
Comparison 1 Combined oral contraceptive pill (COCP) versus no pretreatment (Rx), Outcome 6 Number of oocytes retrieved.
1.6.1. Combined oral contraceptive pill plus antagonist versus antagonist
There was no clear evidence of a difference between the groups in the mean number of oocytes retrieved (MD 0.44, 95% CI ‐0.11 to 0.99; 6 RCTs; 1077 women; I2 = 59%).
1.6.2. Combined oral contraceptive pill plus antagonist versus agonist
There was no clear evidence of a difference between the two treatment groups in the mean number of oocytes retrieved (MD 0.07, 95% CI ‐0.67 to 0.81; 4 RCTs; 552 women; I2 = 0%).
1.6.3. Combined oral contraceptive pill plus antagonist versus antagonist, low response
Among women with low response, there was no clear evidence of a difference between the groups in the mean number of oocytes retrieved (MD 0.70, 95% CI ‐0.11 to 1.51; 1 RCT; 80 women).
1.6.4. Combined oral contraceptive pill plus antagonist versus agonist, low response
Among women with low response, there was no evidence of a difference between the groups in the number of oocytes retrieved (MD 0.10, 95% CI ‐0.75 to 0.95; 1 RCT; 80 women).
1.6.5. Combined oral contraceptive pill plus agonist versus agonist
Two studies looked at the number of oocytes retrieved, but the data were unsuitable for analysis. The first study reported a median of 11 oocytes retrieved (range seven to 19) in the study group (51 cycles) and a median of 10 oocytes retrieved (range seven to 15) in the control group (51 cycles) (Biljan 1998a). The second study reported a mean number of oocytes retrieved of approximately 5 (± 3) in the study group and 5 (± 6) in the control group, but did not report the number of women or cycles in each treatment group (Raoofi 2008).
1.7. Days of gonadotrophin treatment
See Analysis 1.7.
1.7. Analysis.
Comparison 1 Combined oral contraceptive pill (COCP) versus no pretreatment (Rx), Outcome 7 Days of gonadotrophin treatment.
1.7.1. Combined oral contraceptive pill plus antagonist versus antagonist
Six RCTs reported days of gonadotrophin treatment but data were unsuitable for pooling due to extreme statistical heterogeneity (I2 = 95%) with differing directions of effect.
1.7.2. Combined oral contraceptive pill plus antagonist versus agonist
Four RCTs reported days of gonadotrophin treatment but data were unsuitable for pooling due to extreme statistical heterogeneity (I2 = 95%) with differing directions of effect.
1.7.3. Combined oral contraceptive pill plus antagonist versus antagonist, low response
Among women with low response, there was no evidence of a difference between the groups in the mean days of gonadotrophin treatment (MD 0.10 days, 95% CI ‐0.47 to 0.67; 1 RCT; 80 women).
1.7.4. Combined oral contraceptive pill plus antagonist versus agonist, low response
Among women with low response, the mean number of days of gonadotrophin treatment was lower in women who had COCP treatment than in women who received no COCP pretreatment (MD ‐1.40 days, 95% CI ‐2.02 to ‐0.78; 1 RCT; n = 80).
1.7.5. Combined oral contraceptive pill plus agonist versus agonist
Only one study reported on the number of days of gonadotrophin treatment (Biljan 1998a). This study found a median of 10 days (range nine to 11) in the study group (51 cycles) and a median of 12 days (range 11 to 12) in the control group (51 cycles). The data were unsuitable for analysis.
1.8. Amount of gonadotrophins administered
See Analysis 1.8.
1.8. Analysis.
Comparison 1 Combined oral contraceptive pill (COCP) versus no pretreatment (Rx), Outcome 8 Amount of gonadotrophins administered.
1.8.1. Combined oral contraceptive pill plus antagonist versus antagonist
More gonadotrophins were administered to the group who received pretreatment with a COCP than in the group who did not (MD 190.10 IU/L, 95% CI 134.91 to 245.28; 7 RCTs; 1275 women; I2 = 88%). Sensitivity analysis using a random‐effects model did not change the statistical significance of this finding (MD 306.84 IU/L, 95% CI 112.13 to 501.56).
1.8.2. Combined oral contraceptive pill plus antagonist versus agonist
Three RCTs reported the amount of gonadotrophins but data were unsuitable for pooling due to extreme statistical heterogeneity (I2 = 94%) with differing directions of effect.
1.8.3. Combined oral contraceptive pill plus antagonist versus antagonist, low response
Among women with low response, there was no clear evidence of a difference between the two treatment groups in the mean amount of gonadotrophin administered (MD 20.00 IU/L, 95% CI ‐165.39 to 205.39; 1 RCT; 80 women).
1.8.4. Combined oral contraceptive pill plus antagonist versus agonist, low response
Among women with low response, the mean amount of gonadotrophin administered in the COCP group was lower than in the group who were not pretreated (MD ‐349.00, 95% CI ‐537.92 to ‐160.08; 1 RCT; 80 women).
1.9. Ovarian cyst formation rate
1.9.1. Combined oral contraceptive pill plus antagonist versus antagonist
There was insufficient evidence to determine whether there was a difference between the groups in the number of women with ovarian cyst formation (OR 0.47, 95% CI 0.08 to 2.75; 1 RCT; 64 women).
1.9.2. Combined oral contraceptive pill plus agonist versus agonist
Two studies reported on cyst formation, but the data were unsuitable for analysis. The first study found that there was no cyst formation in the intervention group (51 cycles) and cysts in 27 women in the control group (51 cycles) (Biljan 1998a). This result was statistically significant according to the authors (OR 0.07, 95% CI 0.03 to 0.16; P < 0.0001). Raoofi 2008 reported no women with cyst formation in either group.
2. Progestogen versus placebo or no pretreatment
Four studies compared progestogen versus placebo or no treatment (Cédrin‐Durnerin 2007; Ditkoff 1996; Engmann 1999; Salat‐Baroux 1988).
Primary outcomes
2.1. Live birth or ongoing pregnancy
2.1.1. Progestogen plus agonist versus agonist
There was insufficient evidence to determine whether there was a difference between the groups in live birth or ongoing pregnancy rates (OR 1.35, 95% CI 0.69 to 2.65; 2 RCTs; 222 women; I2 = 24%; low quality evidence). The evidence suggested that if the chance of a live birth or ongoing pregnancy following placebo or no pretreatment was assumed to be 17%, the chance following pretreatment with progestogen would be between 12% and 35%.
2.1.2. Progestogen plus antagonist versus antagonist
There was insufficient evidence to determine whether there was a difference between the groups in live birth or ongoing pregnancy rates (OR 0.67, 95% CI 0.18 to 2.54; 1 RCT; 47 women; low quality evidence). The evidence suggested that if the chance of a live birth or ongoing pregnancy following placebo or no pretreatment was assumed to be 25%, the chance following pretreatment with progestogen would be between 3% and 76%.
2.1.3. Progestogen plus gonadotrophins versus gonadotrophins
There was insufficient evidence to determine whether there was a difference between the groups in live birth or ongoing pregnancy rates (OR 0.63, 95% CI 0.09 to 4.23; 1 RCT; 42 women).
2.2. Pregnancy loss
2.2.1. Progestogen plus agonist versus agonist
There was insufficient evidence to determine whether there was a difference between the groups in rates of pregnancy loss (OR 2.26, 95% CI 0.67 to 7.55; 2 RCTs; 222 women; I2 = 0%; low quality evidence). The evidence suggested that if the risk of a pregnancy loss following placebo or no pretreatment was assumed to be 4%, the risk following pretreatment with progestogen would be between 2% and 22%.
2.2.2. Progestogen plus antagonist versus antagonist
The only study in this subgroup did not report on the number of pregnancy losses (Cédrin‐Durnerin 2007), but we calculated this number by subtracting the number of live births from the number of clinical pregnancies. There was no evidence of a difference in rates of pregnancy losses between the two treatment groups (OR 0.36, 95% CI 0.06 to 2.09; 1 RCT; 47 women; low quality evidence). The evidence suggested that if the risk of a pregnancy loss following placebo or no pretreatment was assumed to be 25%, the risk following pretreatment with progestogen would be between 1% and 67%.
2.2.3. Progestogen plus gonadotrophins versus gonadotrophins
The only trial in this subgroup found one pregnancy loss in each treatment group. Thus, there was insufficient evidence to determine whether there was a difference between the groups in rates of pregnancy loss (OR 1.00, 95% CI 0.06 to 17.12; 1 RCT; 42 women).
Secondary outcomes
2.3. Clinical pregnancy rate
2.3.1. Progestogen plus agonist versus agonist
We pooled results of three studies. In one of these studies, we used the number of positive pregnancy tests, because there were no data on clinical pregnancy rate. There was evidence of a difference in clinical pregnancy rates between the two treatment groups, with more clinical pregnancies obtained in the group pretreated with a progestogen (OR 1.99, 95% CI 1.20 to 3.28; 3 RCTs; 374 women; I2 = 0%).
2.3.2. Progestogen plus antagonist versus antagonist
There was insufficient evidence to determine whether there was a difference between the groups in clinical pregnancy rates (OR 0.52, 95% CI 0.16 to 1.71; 1 RCT; 47 women).
2.3.3. Progestogen plus gonadotrophins versus gonadotrophins
There was insufficient evidence to determine whether there was a difference between the groups in clinical pregnancy rates (OR 0.71, 95% CI 0.14 to 3.64; 1 RCT; 42 women).
2.4. Multiple pregnancy
2.4.1. Progestogen plus antagonist versus antagonist
There was insufficient evidence to determine whether there was a difference between the groups in multiple pregnancy rates (OR 1.05, 95% CI 0.06 to 17.76; 1 RCT; 47 women; low quality evidence).
2.5. Ovarian hyperstimulation syndrome rate
None of the studies reported on the number of women with OHSS.
2.6. Number of oocytes retrieved
2.6.1. Progestogen plus agonist versus agonist
There was insufficient evidence to determine whether there was a difference between the groups in the mean number of oocytes retrieved (MD ‐0.52, 95% CI ‐2.07 to 1.02; 2 RCTs; 222 women; I2 = 15%). There was one other study that reported the mean number of oocytes retrieved, but because this was analyzed per cycle (instead of per woman randomized), we were unable to include the data (Shaker 1995).
2.6.2. Progestogen plus antagonist versus antagonist
There was insufficient evidence to determine whether there was a difference between the groups in the mean number of oocytes retrieved (MD 2.70, 95% CI ‐0.98 to 6.38; 1 RCT; 47 women).
2.6.3. Progestogen plus gonadotrophins versus gonadotrophins
There was insufficient evidence to determine whether there was a difference between the groups in the mean number of oocytes retrieved (MD 0.00, 95% CI ‐0.57 to 0.57; 1 RCT; 29 women).
2.7. Days of gonadotrophin treatment
2.7.1. Progestogen plus agonist versus agonist
There was insufficient evidence to determine whether there was a difference between the groups in the mean days of gonadotrophin treatment (MD 0.11 days, 95% CI ‐0.30 to 0.52; 2 RCTs; 222 women; I2 = 88%). The presence of substantial heterogeneity was explored in sensitivity analysis using a random‐effects model and there was no substantial change in the evidence (MD 0.10, 95% CI ‐1.07 to 1.28).
2.8. Amount of gonadotrophins administered
2.8.1. Progestogen plus antagonist versus antagonist
There was insufficient evidence to determine whether there was a difference between the groups in the amount of gonadotrophin administered (MD 276.00 IU/L, 95% CI ‐75.53 to 627.53; 1 RCT; 47 women).
2.9. Ovarian cyst formation rate
2.9.1. Progestogen plus agonist versus agonist
Fewer women had ovarian cyst formation in the group pretreated with a progestogen compared with those who had no progestogen pretreatment (OR 0.16, 95% CI 0.08 to 0.32; 3 RCTs; 374 women; I2 = 1%).
3. Oestrogen versus no pretreatment
Four studies compared oestrogen versus no pretreatment (Cédrin‐Durnerin 2007; Cédrin‐Durnerin 2012; Franco Jr 2003; Ye 2009).
Primary outcomes
3.1. Live birth or ongoing pregnancy
See Figure 6 and Analysis 3.1.
6.
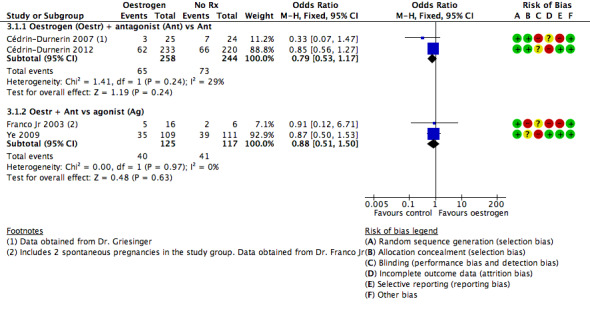
Forest plot of comparison: 3 Oestrogen versus no pretreatment (Rx), outcome: 3.1 Live birth or ongoing pregnancy.
3.1. Analysis.
Comparison 3 Oestrogen versus no pretreatment (Rx), Outcome 1 Live birth or ongoing pregnancy.
3.1.1. Oestrogen plus antagonist versus antagonist
There was insufficient evidence to determine whether there was a difference between the groups in live birth or ongoing pregnancy rates (OR 0.79, 95% CI 0.53 to 1.17; 2 RCTs; 502 women; I2 = 29%; low quality evidence). The evidence suggested that if the chance of a live birth or ongoing pregnancy following no pretreatment was assumed to be 30%, the chance following pretreatment with oestrogen would be between 19% and 35%.
3.1.2. Oestrogen plus antagonist versus agonist
There was insufficient evidence to determine whether there was a difference between the groups in live birth or ongoing pregnancy rates (OR 0.88, 95% CI 0.51 to 1.50; 2 RCTs; 242 women; I2 = 0%; very low quality evidence). The evidence suggested that if the chance of a live birth or ongoing pregnancy following no pretreatment was assumed to be 35%, the chance following pretreatment with progestogen would be between 22% and 45%.
3.2. Pregnancy loss
See Figure 7 and Analysis 3.2.
7.
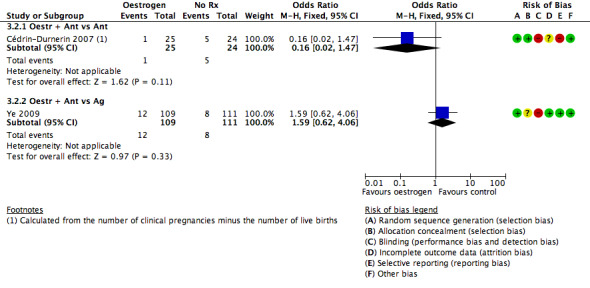
Forest plot of comparison: 3 Oestrogen versus no pretreatment (Rx), outcome: 3.2 Pregnancy loss.
3.2. Analysis.
Comparison 3 Oestrogen versus no pretreatment (Rx), Outcome 2 Pregnancy loss.
3.2.1. Oestrogen plus antagonist versus antagonist
There was insufficient evidence to determine whether there was a difference between the groups in rates of pregnancy loss (OR 0.16, 95% CI 0.02 to 1.47; 1 RCT; 49 women; very low quality evidence). The evidence suggested that if the risk of a pregnancy loss following no pretreatment was assumed to be 13%, the risk following pretreatment with oestrogen would be between 1% and 71%.
3.2.2. Oestrogen plus antagonist versus agonist
There was insufficient evidence to determine whether there was a difference between the groups in rates of pregnancy losses (OR 1.59, 95% CI 0.62 to 4.06; 1 RCT; 220 women; very low quality evidence). The evidence suggested that if the risk of a pregnancy loss following no pretreatment was assumed to be 7%, the risk following pretreatment with oestrogen would be between 5% and 24%.
Secondary outcomes
3.3. Clinical pregnancy rate
3.3.1. Oestrogen plus antagonist versus antagonist
There was insufficient evidence to determine whether there was a difference between the groups in clinical pregnancy rates (OR 0.91, 95% CI 0.66 to 1.24; 4 RCTs; 688 women; I2 = 50%).
3.3.2. Oestrogen plus antagonist versus agonist
There was insufficient evidence to determine whether there was a difference between the groups in clinical pregnancy rates (OR 0.76, 95% CI 0.45 to 1.27; 2 RCTs; 242 women; I2 = 0%).
3.4. Multiple pregnancy rate
3.4.1. Oestrogen plus antagonist versus agonist
There was insufficient evidence to determine whether there was a difference between the groups in multiple pregnancy rates (OR 2.24, 95% CI 0.09 to 53.59; 1 RCT; 22 women; very low quality evidence).
3.5. Ovarian hyperstimulation syndrome rate
3.5.1. Oestrogen plus antagonist versus agonist
There was insufficient evidence to determine whether there was a difference between the groups in OHSS rates (OR 1.54, 95% CI 0.25 to 9.42; 1 RCT; 220 women).
3.6. Number of oocytes retrieved
3.6.1. Oestrogen plus antagonist versus antagonist
More oocytes were retrieved in the group pretreated with oestrogen than in the no pretreatment group (MD 2.23, 95% CI 0.71 to 3.75; 2 RCTs; 139 women; I2 = 0%).
3.6.2. Oestrogen plus antagonist versus agonist
There was insufficient evidence to determine whether there was a difference between the groups in the number of oocytes retrieved (MD 0.40, 95% CI ‐4.47 to 5.27; 1 RCT; 22 women).
3.7. Days of gonadotrophin treatment
3.7.1. Oestrogen plus antagonist versus antagonist
Women who were pretreated with oestrogen had more days of gonadotrophin treatment compared to those who did not receive pretreatment (MD 0.83 days, 95% CI 0.58 to 1.08; 2 RCTs; 529 women; I2 = 0%).
3.7.2. Oestrogen plus antagonist versus agonist
Women pretreated with oestrogen had fewer days of gonadotrophin treatment than those who did not receive oestrogen pretreatment (MD ‐2.50 days, 95% CI ‐4.07 to ‐0.93; 1 RCT; 22 women).
3.8. Amount of gonadotrophins administered
3.8.1. Oestrogen plus antagonist versus antagonist
There was a higher total dose of gonadotrophin administered to women who were pretreated with oestrogen than to those who did not receive pretreatment (MD 168.38 IU/L, 95% CI ; 3 RCTs; 111.53 to 225.17, 668 women; I2 = 0%).
3.8.2. Oestrogen plus antagonist versus agonist
There was insufficient evidence to determine whether there was a difference between the groups in the amount of gonadotrophin administered (MD ‐16.00 IU/L, 95% CI ‐470.12 to 438.12; 1 RCT; 22 women).
3.9. Ovarian cyst formation rate
None of the studies reported ovarian cyst formation.
4. Combined oral contraceptive pill versus progestogen
One trial compared COCP versus progestogen (Cédrin‐Durnerin 2007). This trial used a GnRH antagonist in both treatment groups.
Primary outcomes
4.1. Live birth or ongoing pregnancy
4.1.1. Combined oral contraceptive pill plus antagonist versus progestogen plus antagonist
There was insufficient evidence to determine whether there was a difference between the groups in live birth or ongoing pregnancy rates (OR 0.60, 95% CI 0.12 to 2.89; 1 RCT; 44 women; very low quality evidence).
4.2. Pregnancy loss
4.2.1. Combined oral contraceptive pill plus antagonist versus progestogen plus antagonist
There was insufficient evidence to determine whether there was a difference between the groups in rates of pregnancy loss (OR 1.11, 95% CI 0.14 to 8.64; 1 RCT; 44 women)
Secondary outcomes
4.3. Clinical pregnancy rate
4.3.1. Combined oral contraceptive pill plus antagonist versus progestogen plus antagonist
There was insufficient evidence to determine whether there was a difference between the groups in clinical pregnancy rates (OR 0.71, 95% CI 0.19 to 2.73; 1 RCT; 44 women).
4.4. Multiple pregnancy rate
4.4.1. Combined oral contraceptive pill plus antagonist versus progestogen plus antagonist
There was insufficient evidence to determine whether there was a difference between the groups in multiple pregnancy rates (OR 2.32, 95% CI 0.19 to 27.59; 1 RCT; 44 women).
4.5. Ovarian hyperstimulation syndrome rate
None of the studies reported OHSS.
4.6. Number of oocytes retrieved
4.6.1. Combined oral contraceptive pill plus antagonist versus progestogen plus antagonist
There was insufficient evidence to determine whether there was a difference between the groups in the mean number of oocytes retrieved (MD 1.40, 95% CI ‐3.24 to 6.04; 1 RCT; 44 women).
4.7. Days of gonadotrophin treatment
None of the studies reported days of gonadotrophin treatment.
4.8. Amount of gonadotrophins administered
4.8.1. Combined oral contraceptive pill plus antagonist versus progestogen plus antagonist
There was insufficient evidence to determine whether there was a difference between the groups in the mean quantity of gonadotrophin administered (MD 164.00 IU/L, 95% CI ‐249.03 to 577.03; 1 RCT; 44 women).
4.9. Ovarian cyst formation rate
None of the studies reported ovarian cyst formation.
5. Combined oral contraceptive pill versus oestrogen
Three trials compared COCP versus oestrogen (Cédrin‐Durnerin 2007; Daly 2002; Hauzman 2013).
Primary outcomes
5.1. Live birth or ongoing pregnancy
5.1.1. Combined oral contraceptive pill plus antagonist versus oestrogen plus antagonist
There was insufficient evidence to determine whether there was a difference between the groups in live birth or ongoing pregnancy rates (OR 1.11, 95% CI 0.54 to 2.29; 2 RCTs; 146 women; I2 = 0%; very low quality evidence). This finding was sensitive to the choice of statistical model, and was no longer statistically significant when a sensitivity analysis was conducted to examine the effect of calculating the RR rather than OR (RR 0.15, 95% CI 0.02 to 1.08).
5.1.2. Combined oral contraceptive pill plus agonist versus oestrogen plus antagonist
There was a lower rate of live birth or ongoing pregnancy in women pretreated with COCP than in those who received oestrogen pretreatment (OR 0.08, 95% CI 0.01 to 0.79; 1 RCT; 25 women; very low quality evidence).
5.2. Pregnancy loss
5.2.1. Combined oral contraceptive pill plus agonist versus oestrogen plus antagonist
There was insufficient evidence to determine whether there was a difference between the groups in rates of pregnancy loss (OR 1.09, 95% CI 0.06 to 19.63; 1 RCT; 25 women; very low quality evidence).
Secondary outcomes
5.3. Clinical pregnancy rate
5.3.1. Combined oral contraceptive pill plus antagonist versus oestrogen plus antagonist
There was insufficient evidence to determine whether there was a difference between the groups in rates of clinical pregnancy (OR 1.19, 95% CI 0.60 to 2.37; 2 RCTs; 146 women; I2 = 0%).
5.3.2. Combined oral contraceptive pill plus agonist versus oestrogen plus antagonist
There was a higher rate of clinical pregnancy in women pretreated with oestrogen than in those who received COCP pretreatment (OR 0.13, 95% CI 0.02 to 0.82; 1 RCT; 25 women).
5.4. Multiple pregnancy rate
5.4.1. Combined oral contraceptive pill plus antagonist versus oestrogen plus antagonist
There were no data on multiple pregnancies.
5.5. Ovarian hyperstimulation syndrome rate
None of the studies reported OHSS.
5.6. Number of oocytes retrieved
5.6.1. Combined oral contraceptive pill plus antagonist versus oestrogen plus antagonist
There was insufficient evidence to determine whether there was a difference between the groups in the mean number of oocytes retrieved (MD 0.90, 95% CI ‐3.59 to 5.39; 1 RCT; 46 women).
5.7. Days of gonadotrophin treatment
5.7.1. Combined oral contraceptive pill plus antagonist versus oestrogen plus antagonist
There was no clear evidence of a difference in the number of days of gonadotrophin treatment between the two treatment groups (MD ‐0.60 days, 95% CI ‐1.23 to 0.03; 1 RCT; 100 women).
5.8. Amount of gonadotrophins administered
5.8.1. Combined oral contraceptive pill plus antagonist versus oestrogen plus antagonist
There was insufficient evidence to determine whether there was a difference between the groups in the amount of gonadotrophin administered (MD 181.56 IU/L, 95% CI ‐344.73 to 707.86; 2 RCTs; 146 women; I2 = 59%). There was no change in the evidence on sensitivity analysis using a random‐effects model (MD 113.73 IU/L, 95% CI ‐383.62 to 611.08).
5.9. Ovarian cyst formation rate
None of the studies reported ovarian cyst formation.
6. Progestogen versus oestrogen
One trial compared progestogen versus oestrogen (Cédrin‐Durnerin 2007). This trial used a GnRH antagonist in both treatment groups.
Primary outcomes
6.1. Live birth or ongoing pregnancy
6.1.1. Progestogen plus antagonist versus oestrogen plus antagonist
There was insufficient evidence to determine whether there was a difference between the groups in rates of live birth or ongoing pregnancy (OR 2.04, 95% CI 0.43 to 9.70; 1 RCT; 48 women).
6.2. Pregnancy loss
6.2.1. Progestogen plus antagonist versus oestrogen plus antagonist
There were no pregnancy losses (1 RCT; 48 women).
Secondary outcomes
6.3. Clinical pregnancy rate
6.3.1. Progestogen plus antagonist versus oestrogen plus antagonist
There was no evidence of a difference in clinical pregnancy rates between the two treatment groups (OR 2.30, 95% CI 0.57 to 9.22; 1 RCT; 48 women).
6.4. Multiple pregnancy rate
There were no multiple pregnancies (1 RCT; 48 women).
6.5. Ovarian hyperstimulation syndrome rate
The study did not report OHSS.
6.6. Number of oocytes retrieved
6.6.1. Progestogen plus antagonist versus oestrogen plus antagonist
There was insufficient evidence to determine whether there was a difference between the groups in the mean number of oocytes retrieved (MD ‐0.50, 95% CI ‐4.55 to 3.55; 1 RCT; 48 women).
6.7. Days of gonadotrophin treatment
The study did not report days of gonadotrophin treatment.
6.8. Amount of gonadotrophins administered
6.8.1. Progestogen plus antagonist versus oestrogen plus antagonist
There was insufficient evidence to determine whether there was a difference between the groups in the mean quantity of gonadotrophin administered (MD 310.00 IU/L, 95% CI ‐32.30 to 652.30; 1 RCT; 48 women).
6.9. Ovarian cyst formation rate
The study did not report ovarian cyst formation.
Discussion
Summary of main results
This is the second update of a Cochrane Review that aimed to determine whether pretreatment with a COCP, a progestogen or an oestrogen in ovarian stimulation protocols affects outcomes in subfertile couples undergoing any form of ART. The first update was conducted in 2009.
Combined oral contraceptive pill versus no pretreatment
When COCP was compared with no pretreatment in antagonist cycles, there was a lower rate of live births or ongoing pregnancies in the pretreatment group. However, there was no clear evidence of a difference between the two groups in the rates of pregnancy losses, ovarian cyst formation, multiple pregnancies and OHSS.
When COCP in antagonist cycles was compared with no pretreatment in agonist cycles, there was no evidence of a difference between the two groups in rates of live births or ongoing pregnancies. With respect to adverse effects, there was a lower rate of pregnancy loss in the pretreatment group while there was no clear evidence of a difference between the two groups in rates of ovarian cyst formation, multiple pregnancy or OHSS.
Progestogen versus no pretreatment
When progestogen was compared with no pretreatment, with the same type of cycle (antagonist, agonist or gonadotrophins) in both groups, there was no clear evidence of a difference between the groups in rates of live births or ongoing pregnancies, pregnancy losses or multiple pregnancies. However, there was a lower rate of ovarian cyst formation among the progestogen pretreatment group that used agonist cycles in both groups.
Oestrogen versus no pretreatment
When oestrogen in antagonist cycles was compared with no pretreatment in either antagonist or agonist cycles, there was no clear evidence of a difference between the groups in rates of live births or ongoing pregnancies, or in rates of pregnancy loss, multiple pregnancy or OHSS.
Head‐to‐head comparisons
We compared COCP with progestogen and oestrogen plus progestogen with oestrogen, in all cases with an antagonist cycle in both groups. We compared COCP in an agonist cycle with oestrogen in an antagonist cycle. The evidence was scant but there was no clear evidence of a difference between the groups in rates of live births or ongoing pregnancies or pregnancy losses or other adverse events except in the small study comparing COCP in an agonist cycle versus oestrogen in an antagonist cycle, which reported lower rates of live birth or ongoing pregnancy in women in the COCP group.
Overall, the evidence was insufficient to conclude whether or not pretreatment with COCPs, progestogens or oestrogens in ovarian stimulation resulted in better fertility outcomes. More studies are required for evidence‐based decision making regarding ART protocols. Besides this, there are a few other important aspects to consider when deciding if a pretreatment with a COCP, a progestogen or an oestrogen should be given. First, a pretreatment with one of these drugs may result in a longer duration and a higher amount of gonadotrophin treatment with considerable financial implications. Second, a pretreatment with one of these drugs means the need for a longer duration of the IVF/ICSI cycle and this might be a burden to the woman. In addition, if pretreatment with COCP, progestogen or oestrogen is given, this should be clearly explained to the woman, because the need for OCPs might be difficult to understand for some women trying to get pregnant.
Our finding that pretreatment with progestogen in agonist cycles has a positive effect on clinical pregnancy rates is surprising, since pretreatment with a COCP seems to yield lower clinical pregnancy rates. We also found that pretreatment with progestogen results in the formation of fewer ovarian cysts. This is important, since ovarian cysts have a negative effect on the pregnancy rate, because ART cycles have to be cancelled. However, only one study that used a COCP pretreatment reported on ovarian cyst formation and this study also found a substantial difference in favour of the COCP group. Unless more research is done on the underlying mechanism that could explain these effects, no implications for practice can be suggested.
If it is confirmed that pretreatment with progestogens results in a better IVF/ICSI outcome, this could be clinically and financially important. The administration of progestogen is easy, appears to be safe for the woman and it is less expensive than COCP pretreatment.
Overall completeness and applicability of evidence
Although we were able to include 29 studies across six comparisons, many did not report on the primary outcomes of live births or ongoing pregnancies and pregnancy loss. Using subgroups of different GnRH antagonist and agonist protocols also limited the ability to pool data. There were also limited data for many of the secondary outcomes and almost all the other adverse events.
This review included women with PCOS, but there was only one study of 56 randomized women that included a diagnosis of PCOS (Hwang 2004). Eleven other studies used PCOS or ovarian cysts as an exclusion criteria. These studies randomized 2012 women, so almost half of all the women in this review were not diagnosed with PCOS. Because of the small proportion of women with PCOS included in this review, results might be less applicable to this group of women.
With regard to poor responders, only one of the included studies used poor response to ovarian stimulation as an inclusion criterion in 120 women (Kim 2011). Therefore, the outcomes of this review might not be applicable to women with a history of poor response to controlled ovarian stimulation.
In this review, we included 29 studies with 4701 women. These studies were included in six main comparisons each comprising different subgroups. However, most of the subgroups were reported in single trials. Also, some of the review's outcome measures were not reported in some of the comparisons because of the few number of studies in each subgroup. Thus reaching a robust conclusion regarding the objective of this review was difficult.
Quality of the evidence
The overall quality of the evidence for the main outcomes ranged from very low to moderate. The main limitations of the evidence were risk of bias (associated with poor reporting of study methods) and imprecision. Most of the studies did not describe their methods in adequate detail and many were at high or unclear risk of attrition bias or selective reporting. In several cases, important clinical outcomes or usable data were not reported.
Potential biases in the review process
A strength of this review was the grouping of the studies into subgroups regarding the type of agent used for downregulation (GnRH agonist or antagonist). Nonetheless, there was still some substantial heterogeneity in a few of the statistically significant outcomes, such as the number of days of gonadotrophin administration, but this may be explained by differences in treatment protocols between studies. The combination of live birth and ongoing pregnancy as a single outcome might have given the impression of higher live birth rates than the actual results, as some ongoing pregnancies could have ended in late abortion or intrauterine foetal death. However, there was no presence of significant heterogeneity between studies involved in such combinations.
Furthermore, we were unable to construct a funnel plot, due to the small number of studies in each subgroup. Therefore, we could not examine if publication bias was present.
Agreements and disagreements with other studies or reviews
To our knowledge, there is one systematic review on COCP pretreatment available (Griesinger 2008). The review investigated the effect of COCP pretreatment in a GnRH antagonist cycle versus no pretreatment, and included four studies (Cédrin‐Durnerin 2007; Huirne 2006a; Kolibianakis 2006; Rombauts 2006). All of these studies are also included in our review, but we have included two more studies .Kim 2011; Obruca 2002). Due to a lack of data, despite contacting the author, or differences in treatment protocols, we were unable to pool their results. Because the systematic review of Dr Griesinger included the same studies and investigated almost the same outcomes, it is not surprising that we reach the same conclusions. In his review, Dr Griesinger found no significant effects on ongoing pregnancies. Also, he found a significant difference in favour of the control group with regard to the number of days and amount of gonadotrophin administration.
Authors' conclusions
Implications for practice.
Among women undergoing ovarian stimulation in antagonist protocols, COCP pretreatment was associated with a lower rate of live birth or ongoing pregnancy than no pretreatment. There was insufficient evidence to determine whether rates of live birth or ongoing pregnancy were influenced by pretreatment with progestogens or oestrogens, or by COCP pretreatment using other stimulation protocols. Findings on adverse events were inconclusive, except that progesterone pretreatment may reduce the risk of ovarian cysts in agonist cycles, and COCP in antagonist cycles may reduce the risk of pregnancy loss compared with no pretreatment in agonist cycles.
Implications for research.
More and larger trials that randomise subfertile women undergoing pretreatments with COCP, progestogen or oestrogen in gonadotrophin‐releasing hormone analogue plus gonadotrophin in in vitro fertilisation/intra‐cytoplasmic sperm injection cycles are needed. Pretreatments with COCP or progestogen should be of particular interest for further research because of their clinical importance. Research should also focus more on assessment of outcomes that are of interest to subfertile women and clinicians; these outcomes include number of live births, formation of ovarian cysts, pregnancy losses and the number of women with ovarian hyperstimulation syndrome. Furthermore, research on women with low response is necessary, because evidence in this area is scant.
Feedback
Feedback from Georg Greisinger July 2017
Summary
Main points of feedback were as follows:
For the E2 pre‐treatment, you report mean and SDs from the Fanchin study, which obviously are SEMs resulting in a skewed precision estimate, wrong weighting of the study and a potentially wrong interpretation of the clinical utility of E2 pre‐treatment to “synchronize the follicular cohort”.
What made you not even mention the large RCT on E2 pretreatment from France? https://www.ncbi.nlm.nih.gov/pubmed/20022282
Reply
Thank you for raising these points. The review authors agree that the Fanchin data incorrectly reported SEs rather than SDs and have corrected analyses 3.6.1 and 3.8.1 accordingly. This change has not substantially influenced the effect estimates for these analyses.
The review authors also agree that we should have referenced the French study (Guivarc’h‐Levêque 2009). This study was not eligible for inclusion in the review as it is quasi‐randomised and we have added it to the list of excluded studies.
Contributors
Georg Griesinger, Professor for Gynecological Endocrinology and Reproductive Medicine, University Hospital of Schleswig‐Holstein, Campus Luebeck
Review authors
What's new
| Date | Event | Description |
|---|---|---|
| 9 August 2017 | Amended | Data corrected in response to feedback. No change to conclusions. |
History
Protocol first published: Issue 3, 2006 Review first published: Issue 1, 2010
| Date | Event | Description |
|---|---|---|
| 22 May 2017 | New citation required and conclusions have changed | The addition of nine new studies has led to a change in our conclusions. |
| 22 May 2017 | New search has been performed | This review has been updated and nine new studies have been included: Blockeel 2012; Cédrin‐Durnerin 2012; Garcia‐Velasco 2011; Hauzman 2013; Kim 2011; Lukaszuk 2015; Nyboe Andersen 2011; Porrati 2010; Vilela 2011 |
| 16 August 2010 | Amended | Minor edits made no change to conclusion |
| 18 December 2008 | Amended | Title changed |
| 23 November 2008 | Amended | New authors added All aspects of original protocol revised |
| 13 April 2008 | Amended | Converted to new review format. |
| 19 May 2006 | New citation required and major changes | Substantive amendment |
Acknowledgements
For the 2017 review:
We thank Dr Julie Brown for assistance with screening, selection and data extraction during 2015. We also wish to acknowledge the contributions of Brechtje Smulders and Sanne van Oirschot to previous versions of this review.
For the 2009 review:
We would like to thank He Haojie, Bolarinde Ola, Pan LIngya and Li Shangwei, the authors of the original protocol.
We are also grateful to Marian Showell, the Information Specialist of the CGF group, for developing the search strategy and to Jane Clarke, the Review Group Co‐ordinator of the MDSG and to Vanessa Jordan, NZ Cochrane fellow, for answering our questions.
Furthermore, we would like to thank Dr G Griesinger for kindly supplying us with information on the trials that he included in his review.
Also thanks to Dr JG Franco Jr, Dr EC Ditkoff, Dr I Cédrin‐Durnerin, Dr CH Kim and M Grynberg (on behalf of Dr R Fanchin) for sending us more data on their studies.
We would like to thank all the members of the MDSG for their assistance in contacting the authors from the included trials. Special thanks to S Dias and X Zhu for their help with articles in languages other than English.
Appendices
Appendix 1. Glossary
Embryo The product of conception from the time of fertilisation to the end of the embryonic stage eight weeks after fertilisation.
Embryo transfer (ET) Procedure of which embryos are placed in the uterus or fallopian tube.
Endogenous Developed or originated inside the organism. For example, hormones produced by the pituitary gland would be an endogenous supply, but hormones produced in the laboratory and then given to the body is called an exogenous supply.
Fertilisation The penetration of the ovum by the sperm cell and fusion of genetic materials, resulting in the development of an embryo.
Follicle The sac in which an egg develops in the ovary.
Follicle cohort synchronisation In the ovaries, a few eggs are maturing at the same time. These eggs are all in a different stage of maturation. If one egg reaches a threshold at the right time in the menstrual cycle, the final maturation process will start and this egg will reach ovulation. For in vitro fertilisation/intra‐cytoplasmic sperm injection cycles it is important that more than one egg reaches this threshold at the same time, so they can be retrieved at once before spontaneous ovulation occurs. This is called synchronisation of the follicle cohort.
Follicle‐stimulating hormone (FSH) A hormone produced and released from the pituitary gland. In women it stimulates the production of oestrogen and follicles in the ovary ready for ovulation.
Gestational sac A fluid‐filled structure containing an embryo that develops early in pregnancy, usually within the uterus.
Gonadotrophin releasing hormone (GnRH) A substance produced by the hypothalamus (part of the brain) to enable the pituitary gland to secrete LH and FSH.
Gonadotrophins Pituitary hormones FSH and LH which stimulate the ovaries in women.
Human menopausal gonadotrophin (hMG) An injectable preparation that is obtained from the urine of menopausal women and has biological activity similar to that of FSH.
Luteal phase The last 14 days of the menstrual cycle.
Luteinising Hormone (LH) A hormone produced and released by the pituitary gland. In women it is responsible for ovulation and progestogen production.
Negative feedback A common regulation mechanism to stabilise the body's internal environment. An example is the temperature control of the human body. When body temperature is too high, the body will react in such a way that it cools down, by opening pores and sweating. In this way the body's temperature will not fluctuate too much. The same type of mechanism is used to keep hormone values stable. An increase in gonadotrophin values will (through negative feedback) result in fewer GnRH receptors. The binding of GnRH to a GnRH receptor in the pituitary gland will result in the release of gonadotrophins, but with fewer GnRH receptors, the releasing process will be lowered and the gonadotrophin levels in the body will drop.
Oocyte The egg from a woman's ovary.
Ova A woman's reproductive cell, also known as egg or oocyte.
Ovarian hyperstimulation syndrome (OHSS) A condition that occurs from fertility drugs when a large number of follicles in the ovary are stimulated to develop and ovulate. This stimulation causes an enlargement of the ovaries.
Ovulation The release of an egg/ova from an ovarian follicle.
Ovulation induction Medical procedure to produce ovulation.
Polycystic ovary syndrome (PCOS) When a woman has enlarged ovaries with multiple cysts and the surface of the ovary is thickened. The woman may ovulate infrequently or not at all.
Premature LH‐surge In a normal menstrual cycle an increase in LH levels (LH‐surge) is needed to start ovulation. In in vitro fertilisation (IVF)/intra‐cytoplasmic sperm injection (ISCI) cycles it is important that the ovulation does not start before the oocytes are mature enough to be retrieved. A LH‐surge that occurs too early is called premature and is an unwanted event in IVF/ICSI cycles.
Recombinant (as in recombinant FSH or rFSH) A naturally occurring hormone which has been made in the laboratory with the use of DNA technology.
Subfertility Failure to achieve pregnancy after at least one year of unprotected coitus.
Ultrasound Radiology sounds waves of a high frequency used to visualise the developing foetus in the uterus to check size, growth and the presence of abnormalities.
All these definitions (except for follicle cohort synchronisation, negative feedback and premature LH‐surge) were obtained from the glossary of the MDSG Module 2008.
Appendix 2. Cochrane Gynaecology and Fertility (CGFG) specialised register search strategy
Searched 17 January 2017
Procite platform
Keywords CONTAINS "IVF" or "in vitro fertilization" or "in‐vitro fertilisation" or "ICSI" or"intracytoplasmic sperm injection" or "Embryo" or "in‐vitro fertilization" or "ART" or "controlled ovarian " or "COH" or Title CONTAINS "IVF" or "in vitro fertilization" or "in‐vitro fertilisation" or "ICSI" or"intracytoplasmic sperm injection" or "Embryo" or "in‐vitro fertilization" or "ART" or "controlled ovarian " or "COH"
AND
Keywords CONTAINS "oral contraceptive" or "Oral Contraceptive Agent" or "oral contraceptives" or "combined oral contraceptives" or "Pretreatment" or "OCP" or "Oral Contraception" or "oral contraceptive pill" or "oral conjugated estrogen" or "OCP pretreatment" or "progestagen" or progestin"or"progestins"or"progestogen"or"progestogens"or"Progesterone"or"Norgestrel"or"Norethindrone"or "Norethisterone"or"desogestral"or"desogestrel"or"Gestagen"or"Gestodene"or"gestrinone"or"Estradiol"or"estradiol acetate" or "estradiol valerate"or"Estriol"or"oestrodiol"or"oestrogen"or"estrogen"or"Estrogens"or Title CONTAINS "oral contraceptive" or "Oral Contraceptive Agent" or "oral contraceptives" or "combined oral contraceptives" or "Pretreatment" or "OCP" or "Oral Contraception" or "oral contraceptive pill" or "oral congugated estrogen" or "OCP pretreatment" or "progestagen" or "progestin"or"progestins"or"progestogen"or"progestogens"or"Progesterone"or"Norgestrel"or"Norethindrone"or "Norethisterone" or "Estradiol" (888 hits)
Appendix 3. CENTRAL Register of Studies Online (CRSO) search strategy
Searched 17 January 2017
Web platform
#1 MESH DESCRIPTOR Reproductive Techniques, Assisted EXPLODE ALL TREES 2792
#2 (IVF or ICSI):TI,AB,KY 3537
#3 (embryo* transfer*):TI,AB,KY 2049
#4 (vitro fertili?ation ):TI,AB,KY 1954
#5 (intracytoplasmic sperm injection*):TI,AB,KY 1076
#6 COH:TI,AB,KY 212
#7 (ovar* adj2 stimulat*):TI,AB,KY 1197
#8 (assisted reproduct*):TI,AB,KY 694
#9 (ovarian hyperstimulation):TI,AB,KY 849
#10 (pituitary desensiti?ation):TI,AB,KY 82
#11 (pituitary adj2 suppression):TI,AB,KY 170
#12 (poor responder*):TI,AB,KY 391
#13 (poor ovar* response):TI,AB,KY 84
#14 (low responder*):TI,AB,KY 137
#15 #1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 6479
#16 MESH DESCRIPTOR Contraceptives, Oral EXPLODE ALL TREES 3215
#17 (oral contracepti*):TI,AB,KY 2278
#18 (pretreatment* or pre‐treatment*):TI,AB,KY 14480
#19 (gestrinone or estradiol):TI,AB,KY 7388
#20 (norgestrel or desogestrel):TI,AB,KY 893
#21 (dimethisterone or levonorgestrel):TI,AB,KY 1169
#22 (norethindrone or gestodene):TI,AB,KY 1021
#23 (norgestimate or dienogest):TI,AB,KY 233
#24 (progestogen* or progestagen*):TI,AB,KY 839
#25 (progestin* or gestagen*):TI,AB,KY 1576
#26 (Medroxyprogesterone or Diane‐35):TI,AB,KY 1851
#27 (norethisterone or ?estrogen):TI,AB,KY 9170
#28 E2:TI,AB,KY 3182
#29 #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22 OR #23 OR #24 OR #25 OR #26 OR #27 OR #28 32193
#30 #15 AND #29 1355
Appendix 4. MEDLINE search strategy
From 1946 to 17 January 2017
Ovid MEDLINE(R) Epub Ahead of Print, In Process & Other Non‐Indexed Citations, Ovid MEDLINE (R) Daily, and Ovid MEDLINE
1 reproductive techniques/ or exp reproductive techniques, assisted/ or exp embryo transfer/ or exp fertilization in vitro/ or exp sperm injections, intracytoplasmic/ or exp gamete intrafallopian transfer/ (65927) 2 (IVF or ICSI).tw. (24183) 3 embryo transfer.tw. (9681) 4 (in vitro fertilisation or in vitro fertilization).tw. (20881) 5 intracytoplasmic sperm injection$.tw. (6213) 6 COH.tw. (1533) 7 ovar$ stimulat$.tw. (5130) 8 assisted reproduct$.tw. (12517) 9 ovarian hyperstimulation.tw. (4687) 10 pituitary desensitisation.tw. (21) 11 pituitary desensitization.tw. (242) 12 pituitary suppression.tw. (327) 13 poor responder$.tw. (2039) 14 poor ovar$ response.tw. (327) 15 low responder$.tw. (2153) 16 or/1‐15 (84391) 17 oral contracepti$.tw. (26678) 18 (OC or OCP$).tw. (22318) 19 (pretreatment$ or pre‐treatment$).tw. (202425) 20 gestrinone$.tw. (199) 21 estradiol.tw. (81366) 22 desogestrel.tw. (1096) 23 dimethisterone.tw. (25) 24 levonorgestrel.tw. (4400) 25 norethindrone.tw. (1328) 26 norgestrel.tw. (1071) 27 norgestrienone.tw. (33) 28 gestodene.tw. (767) 29 norgestimate.tw. (363) 30 dienogest.tw. (376) 31 progestogen$.tw. (5441) 32 progestagen$.tw. (2202) 33 progestin$.tw. (11982) 34 gestagen$.tw. (1640) 35 Medroxyprogesterone.tw. (6404) 36 Diane‐35.tw. (87) 37 norethisterone.tw. (2044) 38 estrogen.tw. (120036) 39 E2.tw. (67681) 40 oestrogen.tw. (17720) 41 exp Contraceptives, Oral/ (47656) 42 or/17‐41 (500004) 43 randomized controlled trial.pt. (508490) 44 controlled clinical trial.pt. (98223) 45 randomized.ab. (438894) 46 randomised.ab. (87375) 47 placebo.tw. (210117) 48 clinical trials as topic.sh. (197894) 49 randomly.ab. (299032) 50 trial.ti. (202001) 51 (crossover or cross‐over or cross over).tw. (79992) 52 or/43‐51 (1282048) 53 exp animals/ not humans.sh. (4854866) 54 52 not 53 (1184243) 55 16 and 42 and 54 (977)
Appendix 5. Embase search strategy
From 1980 to 17 January 2017 Ovid platform
1 (IVF or ICSI).tw. (31186) 2 embryo transfer.tw. (11462) 3 (in vitro fertilisation or in vitro fertilization).tw. (21776) 4 intracytoplasmic sperm injection$.tw. (6743) 5 COH.tw. (1621) 6 ovar$ stimulat$.tw. (6451) 7 assisted reproduct$.tw. (14279) 8 ovarian hyperstimulation.tw. (5543) 9 pituitary desensitisation.tw. (21) 10 pituitary desensitization.tw. (263) 11 pituitary suppression.tw. (364) 12 poor responder$.tw. (2466) 13 poor ovar$ response.tw. (383) 14 low responder$.tw. (2074) 15 exp infertility therapy/ (81759) 16 exp embryo transfer/ or exp fertilization in vitro/ or exp intracytoplasmic sperm injection/ (55779) 17 or/1‐16 (97457) 18 exp oral contraceptive agent/ (53046) 19 oral contracepti$.tw. (23779) 20 (OC or OCP$).tw. (21319) 21 (pretreatment$ or pre‐treatment$).tw. (195469) 22 gestrinone$.tw. (187) 23 estradiol.tw. (74210) 24 norgestrel.tw. (706) 25 desogestrel.tw. (1122) 26 dimethisterone.tw. (11) 27 levonorgestrel.tw. (4344) 28 norethindrone.tw. (954) 29 norgestrel.tw. (706) 30 norgestrienone.tw. (15) 31 gestodene.tw. (758) 32 norgestimate.tw. (348) 33 dienogest.tw. (500) 34 progestogen$.tw. (5070) 35 progestagen$.tw. (2022) 36 progestin$.tw. (10993) 37 gestagen$.tw. (1564) 38 Medroxyprogesterone.tw. (5918) 39 Diane‐35.tw. (334) 40 norethisterone.tw. (1847) 41 estrogen.tw. (111911) 42 E2.tw. (68712) 43 oestrogen.tw. (16612) 44 or/18‐43 (485744) 45 Clinical Trial/ (845200) 46 Randomized Controlled Trial/ (372794) 47 exp randomization/ (66611) 48 Single Blind Procedure/ (20323) 49 Double Blind Procedure/ (120763) 50 Crossover Procedure/ (43072) 51 Placebo/ (257085) 52 Randomi?ed controlled trial$.tw. (117242) 53 Rct.tw. (17142) 54 random allocation.tw. (1418) 55 randomly allocated.tw. (22469) 56 allocated randomly.tw. (2023) 57 (allocated adj2 random).tw. (728) 58 Single blind$.tw. (15832) 59 Double blind$.tw. (150922) 60 ((treble or triple) adj blind$).tw. (452) 61 placebo$.tw. (214589) 62 prospective study/ (292450) 63 or/45‐62 (1465071) 64 case study/ (31878) 65 case report.tw. (282490) 66 abstract report/ or letter/ (925645) 67 or/64‐66 (1233732) 68 63 not 67 (1425818) 69 (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) (5289150) 70 68 not 69 (1369914) 71 17 and 44 and 70 (1757)
Appendix 6. PsycINFO search strategy
From 1806 to 17 January 2017 Ovid platform
1 exp Infertility/ or exp Reproductive Technology/ (3107) 2 (IVF or ICSI).tw. (512) 3 (Reproducti$ adj2 Technolog$).tw. (980) 4 embryo transfer.tw. (104) 5 (in vitro fertilisation or in vitro fertilization).tw. (648) 6 intracytoplasmic sperm injection$.tw. (49) 7 or/1‐6 (3603) 8 exp Oral Contraceptives/ (844) 9 oral contracepti$.tw. (1413) 10 (OC or OCP$).tw. (1978) 11 estradiol.tw. (5475) 12 (progestogen$ or progestin$).tw. (741) 13 (progestagen$ or gestagen$).tw. (50) 14 (norethisterone or norethindrone).tw. (41) 15 (estrogen or oestrogen).tw. (6861) 16 (pretreatment$ or pre‐treatment$).tw. (16904) 17 or/8‐16 (30114) 18 7 and 17 (58) 19 random.tw. (48701) 20 control.tw. (377497) 21 double‐blind.tw. (20370) 22 clinical trials/ (10102) 23 placebo/ (4773) 24 exp Treatment/ (671953) 25 or/19‐24 (1038320) 26 18 and 25 (23)
Appendix 7. CINAHL Plus search strategy
From 1961 to 17 January 2017 Ebsco platform
| # | Query | Results |
| S40 | S27 AND S39 | 111 |
| S39 | S28 OR S29 OR S30 OR S31 OR S32 OR S33 OR S34 OR S35 OR S36 OR S37 OR S38 | 1,105,194 |
| S38 | TX allocat* random* | 5,945 |
| S37 | (MH "Quantitative Studies") | 15,237 |
| S36 | (MH "Placebos") | 9,967 |
| S35 | TX placebo* | 43,010 |
| S34 | TX random* allocat* | 5,945 |
| S33 | (MH "Random Assignment") | 42,264 |
| S32 | TX randomi* control* trial* | 117,316 |
| S31 | TX ( (singl* n1 blind*) or (singl* n1 mask*) ) or TX ( (doubl* n1 blind*) or (doubl* n1 mask*) ) or TX ( (tripl* n1 blind*) or (tripl* n1 mask*) ) or TX ( (trebl* n1 blind*) or (trebl* n1 mask*) ) | 870,796 |
| S30 | TX clinic* n1 trial* | 197,987 |
| S29 | PT Clinical trial | 79,975 |
| S28 | (MH "Clinical Trials+") | 208,852 |
| S27 | S18 AND S26 | 364 |
| S26 | S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25 | 30,510 |
| S25 | TX Estradiol or TX oestrogen or TX estrogen or TX oestradiol | 17,624 |
| S24 | TX desogestral or TX desogestrel or TX Gestagen | 76 |
| S23 | TX Norgestrel or TX Norethindrone TX Norethisterone | 19 |
| S22 | TX progestagen* or TX progestin* or TX progestogen* | 1,722 |
| S21 | TX Pretreatment | 7,437 |
| S20 | TX oral contraceptive* | 6,329 |
| S19 | (MH "Contraceptives, Oral Combined") OR (MM "Contraceptives, Oral") | 3,014 |
| S18 | S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13 OR S14 OR S15 OR S16 OR S17 | 7,460 |
| S17 | TX (ovari* N2 induction) | 15 |
| S16 | TX COH | 119 |
| S15 | TX ovarian hyperstimulation | 403 |
| S14 | TX superovulat* | 24 |
| S13 | TX ovulation induc* | 660 |
| S12 | TX intrauterine insemination | 185 |
| S11 | TX IUI | 117 |
| S10 | TX assisted reproduct* | 1,678 |
| S9 | (MM "Reproduction Techniques+") | 4,518 |
| S8 | TX intracytoplasmic sperm injection* | 308 |
| S7 | TX embryo* N3 transfer* | 985 |
| S6 | TX ovar* N3 hyperstimulat* | 406 |
| S5 | TX ovari* N3 stimulat* | 335 |
| S4 | TX IVF or TX ICSI | 1,757 |
| S3 | (MM "Fertilization in Vitro") | 1,659 |
| S2 | TX vitro fertilization | 3,491 |
| S1 | TX vitro fertilisation | 3,491 |
Appendix 8. Virtual health library, clinicaltrials.gov and PubMed search strategies
Searched on 13 January 2017
Web platform
Clinicaltrials.gov
1. ivf and pretreatment (27 hits)
2. ivf and contraceptive* (53 hits)
3. ivf and estradiol (109 hits)
4. ivf and progestogen* (53 hits)
Virtual health library (LILACS)
1. ivf and pretreatment (1hit, limited to LILACS)
2. ivf and contraceptive* (4 hits, limited to LILACS and IBECS)
3. ivf and estradiol (4 hits, limited to LILACS and MEDCARIB)
4. ivf and progestogen* (35 hits no limit)
Pubmed
1. ivf and pretreatment (21 hits, limited from 01.01.14 to 11.06.15)
2. ivf and contraceptive* (23 hits, limited from 01.01.14 to 11.06.15)
3. ivf and estradiol (47 hits, limited from 01.12.14 to 11.06.15)
4. ivf and progestogen* (0 hits, limited from 01.01.14 to 11.06.15)
Appendix 9. Data extraction form (part 1)
| Assessment | |
| Assessor | SvO / BS |
| Date | |
| Final conclusion |
| Inclusion |
| Exclusion Reason for exclusion: |
| A. Study information | |
| 1. Title | |
| 2. First author | |
| 3. Year | |
| 4. Published | Yes / No |
| 5. Journal | |
| B. Criteria for eligibility | YES | NO | |
| Design | Described as randomised? If no, then exclude | ||
| Patients | Women with subfertility, regardless of any cause, undergoing ART | ||
| Intervention | · OCP prior to gonadotrophins · OCP prior to gonadotrophins + GnRH agonist · OCP prior to gonadotrophins + GnRH antagonist · Oestrogen prior to gonadotrophins · Oestrogen prior to gonadotrophins + GnRH agonist · Oestrogen prior to gonadotrophins + GnRH antagonist · Progestogen prior to gonadotrophins · Progestogen prior to gonadotrophins + GnRH agonist · Progestogen prior to gonadotrophins + GnRH antagonist | ||
| B. Criteria for eligibility (continued) | YES | NO | |
| Comparison | · Placebo prior to gonadotrophins · Placebo prior to gonadotrophins + GnRH agonist · Placebo prior to gonadotrophins + GnRH antagonist · No pretreatment prior to gonadotrophins · No pretreatment prior to gonadotrophins + GnRH agonist · No pretreatment prior to gonadotrophins + GnRH antagonist · OCP prior to gonadotrophins · OCP prior to gonadotrophins + GnRH agonist · OCP prior to gonadotrophins + GnRH antagonist · Oestrogen prior to gonadotrophins · Oestrogen prior to gonadotrophins + GnRH agonist · Oestrogen prior to gonadotrophins + GnRH antagonist · Progestogen prior to gonadotrophins · Progestogen prior to gonadotrophins + GnRH agonist · Progestogen prior to gonadotrophins + GnRH antagonist | ||
| Outcome | Primary: · number of live births Secondary: · no. of ongoing pregnancies · no. of clinical pregnancies · no. of oocytes retrieved · total days of gonadotrophin treatment · amount of gonadotrophin administered Adverse: · no. of pregnancy loss · no. of ovarian cyst formation · no. of multiple pregnancies · no. of ovarian hyperstimulation syndrome |
||
| Remarks: | |||
| C. Characteristics | ||||||
| C1. Trial characteristics | ||||||
| Country of investigation | ||||||
| Setting | Single Multicentre Unclear | |||||
| Academic Non‐academic Unclear | ||||||
| Duration of trial | Y = M = D = | |||||
| Design | Parallel Crossover | |||||
| Number of participants | Intervention group: Comparison group: Total: | |||||
| Remarks: | ||||||
| C2. Participants characteristics | ||||||
| Intervention group | Comparison group | |||||
| Age | Mean: SD: Not reported: | Mean: SD: Not reported: | ||||
| BMI | Mean: SD: Not reported: | Mean: SD: Not reported: | ||||
| Duration of subfertility | Mean: SD: Not reported: | Mean: SD: Not reported: | ||||
| No. of previous IVF trials | Mean: SD: Not reported: | Mean: SD: Not reported: | ||||
| Subfertility | Primary: Secondary: Not reported: | N = N = | Primary: Secondary: Not reported: | N = N = | ||
| Causes of subfertility | Tubal: Male: Endometriosis: Idiopathic: Other: Not reported: | N = N = N = N = N = | Tubal: Male: Endometriosis: Idiopathic: Other: Not reported: | N = N = N = N = N = | ||
| Poor response | YES NO Defined as: * Mature ovarian follicles: < 3 with a mean diameter ≥ 17 mm * Oocytes retrieved: < 3 * Other: |
|||||
| C2. Flowchart of participants | ||||||
| Remarks: | ||||||
| C3. Protocol characteristics | ||||||
| Pretreatment | Combined OCP Oestrogen Progestogen Name of preparation: Dosage: Start: Stop: |
|||||
| Ovarian stimulation | hMG rFSH Name of preparation: Dosage: Start: Stop: |
|||||
| Pituitary desensitization | GnRH agonist GnRH antagonist Name of preparation: Dosage: Start: Stop: Protocol: |
|||||
| Treatment schedule | ||||||
| C4. Follow‐up | |
| Duration of follow‐up | |
| Analysis of loss to follow‐up | Per protocol Intention‐to‐treat |
| Remarks: | |
| D. Risk of bias assessment | ||||
| YES | NO | Unclear | ||
| Study size | Was a power calculation performed and adhered? | |||
| Selection bias | Was the allocation sequence adequately generated? | |||
| Was the patient allocation concealment adequate? | ||||
| Detection bias | Was the length of follow‐up long enough to detect stated outcomes? | |||
| Was the investigator (performer of hormone administration) blinded? | ||||
| Was the outcome assessor blinded? | ||||
| Were the participants blinded? | ||||
| Attrition bias | Was loss to follow‐up accounted for? | |||
| Was an intention‐to‐treat analysis performed? | ||||
| Reporting bias | Where there any suggestions of selective report of outcome? | |||
| Source of funding | Is the source of funding stated? | |||
| Remarks: | ||||
Appendix 10. Data extraction form (part 2)
| D. Risk of bias assessment | ||||
| YES | NO | Unclear | ||
| Study size | Was a power calculation performed and adhered? | |||
| Selection bias | Was the allocation sequence adequately generated? | |||
| Was the patient allocation concealment adequate? | ||||
| Detection bias | Was the length of follow‐up long enough to detect stated outcomes? | |||
| Was the investigator (performer of hormone administration) blinded? | ||||
| Was the outcome assessor blinded? | ||||
| Were the participants blinded? | ||||
| Attrition bias | Was loss to follow‐up accounted for? | |||
| Was an intention‐to‐treat analysis performed? | ||||
| Reporting bias | Where there any suggestions of selective report of outcome? | |||
| Source of funding | Is the source of funding stated? | |||
| Remarks: | ||||
| E. Outcomes | ||||||||
| Comparison | a. Define treatment: b. Define control: |
|||||||
| E1. Primary outcomes | ||||||||
|
Live births Defined: YES NO |
No. of live birth | No. of no live birth | Total | |||||
| Treatment group | ||||||||
| Control group | ||||||||
| Total | ||||||||
| Remarks: | ||||||||
| E2. Secondary outcomes |
|
Ongoing pregnancy Defined: YES NO |
No. of ongoing pregnancy | No. of no ongoing pregnancy | Total | |||||
| Treatment group | ||||||||
| Control group | ||||||||
| Total | ||||||||
| Remarks: | ||||||||
|
Clinical pregnancy Defined: YES NO |
No. of clinical pregnancy | No. of no clinical pregnancy | Total | |||||
| Treatment group | ||||||||
| Control group | ||||||||
| Total | ||||||||
| Remarks: | ||||||||
|
Oocytes retrieved Defined: YES NO |
Mean no. of oocytes retrieved | SD | ||||||
| Treatment group | ||||||||
| Control group | ||||||||
| Remarks: | ||||||||
|
Days of gonadotrophins treatment Defined: YES NO |
Mean no. of days of gonadotrophins treatment | SD | ||||||
| Treatment group | ||||||||
| Control group | ||||||||
| Remarks: | ||||||||
|
Total days of treatment Defined: YES NO |
Mean no. of days of pituitary suppression | SD | ||||||
| Treatment group | ||||||||
| Control group | ||||||||
| Remarks: | ||||||||
| E3. Adverse outcomes |
|
Pregnancy loss Defined: YES NO |
No. of pregnancy loss | No. of no pregnancy loss | Total | |
| Treatment group | ||||
| Control group | ||||
| Total | ||||
| Remarks: | ||||
|
Ovarian cyst formation Defined: YES NO |
No. of ovarian cyst formation | No. of no ovarian cyst formation | Total | |
| Treatment group | ||||
| Control group | ||||
| Total | ||||
| Remarks: | ||||
|
Multiple pregnancy Defined: YES NO |
No. of multiple pregnancies | No. of no multiple pregnancies | Total | |
| Treatment group | ||||
| Control group | ||||
| Total | ||||
| Remarks: | ||||
|
Ovarian hyperstimulation
syndrome Defined: YES NO |
No. of OHS syndrome | No. of no OHS syndrome | Total | |
| Treatment group | ||||
| Control group | ||||
| Total | ||||
| Remarks: | ||||
Data and analyses
Comparison 1. Combined oral contraceptive pill (COCP) versus no pretreatment (Rx).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth or ongoing pregnancy | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 COCP + antagonist (Ant) vs Ant | 6 | 1335 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.74 [0.58, 0.95] |
| 1.2 COCP + Ant vs agonist (Ag) | 4 | 724 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.64, 1.25] |
| 1.3 COCP + Ant vs Ant, low response | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.61, 4.79] |
| 1.4 COCP + Ant vs Ag, low response | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.43, 2.98] |
| 2 Pregnancy loss | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 COCP + Ant vs Ant | 5 | 868 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.82, 2.26] |
| 2.2 COCP + Ant vs Ag | 5 | 780 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.40 [0.22, 0.72] |
| 2.3 COCP + Ant vs Ant, low response | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.05 [0.18, 23.59] |
| 2.4 COCP + Ant vs Ag, low response | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.13, 7.47] |
| 3 Clinical pregnancy rate | 8 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 COCP + Ant vs Ant | 5 | 740 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.63, 1.15] |
| 3.2 COCP + Ant vs Ag | 4 | 546 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.59, 1.20] |
| 3.3 COCP + Ant vs Ant, low response | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.85 [0.69, 4.97] |
| 3.4 COCP + Ant vs Ag, low response | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.44, 2.83] |
| 4 Multiple pregnancy rate | 5 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 COCP + Ant vs Ant | 2 | 125 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.21 [0.53, 9.26] |
| 4.2 COCP + Ant vs Ag | 4 | 546 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.36 [0.85, 2.19] |
| 4.3 COCP + Ant vs Ant, low response | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.11 [0.36, 12.24] |
| 4.4 COCP + Ant vs Ag, low response | 1 | 80 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.29, 6.56] |
| 5 Ovarian hyperstimulation syndrome rate | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 COCP + Ant vs Ant | 2 | 642 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.28, 3.40] |
| 5.2 COCP + Ant vs Ag | 2 | 290 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.20, 1.96] |
| 6 Number of oocytes retrieved | 8 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7 Days of gonadotrophin treatment | 8 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 COCP + Ant vs Ant | 6 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 COCP + Ant vs Ag | 4 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.3 COCP + Ant vs Ant, low response | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.4 COCP + Ant vs Ag, low response | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Amount of gonadotrophins administered | 8 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 COCP + Ant vs Ant | 7 | 1275 | Mean Difference (IV, Fixed, 95% CI) | 190.10 [134.91, 245.28] |
| 8.2 COCP + Ant vs Ag | 3 | 496 | Mean Difference (IV, Fixed, 95% CI) | 9.96 [‐104.09, 124.02] |
| 8.3 COCP + Ant vs Ant, low response | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | 20.0 [‐165.39, 205.39] |
| 8.4 COCP + Ant vs Ag, low response | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐349.0 [‐537.92, ‐160.08] |
| 9 Ovarian cyst formation rate | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 COCP + Ant vs Ant | 1 | 64 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.47 [0.08, 2.75] |
1.9. Analysis.
Comparison 1 Combined oral contraceptive pill (COCP) versus no pretreatment (Rx), Outcome 9 Ovarian cyst formation rate.
Comparison 2. Progestogen versus placebo/no pretreatment (Rx).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth or ongoing pregnancy | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Progestogen (Prog) + agonist (Ag) vs Ag | 2 | 222 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.69, 2.65] |
| 1.2 Prog + antagonist (Ant) vs Ant | 1 | 47 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.18, 2.54] |
| 1.3 Prog + gonadotrophin (Gon) vs Gon | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.09, 4.23] |
| 2 Pregnancy loss | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Prog + Ag vs Ag | 2 | 222 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.26 [0.67, 7.55] |
| 2.2 Prog + Ant vs Ant | 1 | 47 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.36 [0.06, 2.09] |
| 2.3 Prog + Gon vs Gon | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 17.12] |
| 3 Clinical pregnancy rate | 5 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Prog + Ag vs Ag | 3 | 374 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.99 [1.20, 3.28] |
| 3.2 Prog + Ant vs Ant | 1 | 47 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.16, 1.71] |
| 3.3 Prog + Gon vs Gon | 1 | 42 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.14, 3.64] |
| 4 Multiple pregnancy rate | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Prog + Ant vs Ant | 1 | 47 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.06, 17.76] |
| 5 Number of oocytes retrieved | 4 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Prog + Ag vs Ag | 2 | 222 | Mean Difference (IV, Fixed, 95% CI) | ‐0.52 [‐2.07, 1.02] |
| 5.2 Prog + Ant vs Ant | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | 2.70 [‐0.98, 6.38] |
| 5.3 Prog + Gon vs Gon | 1 | 29 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.57, 0.57] |
| 6 Days of gonadotrophin treatment | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Prog + Ag vs Ag | 2 | 222 | Mean Difference (IV, Fixed, 95% CI) | 0.11 [‐0.30, 0.52] |
| 7 Amount of gonadotrophins administered | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Prog + Ant vs Ant | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | 276.0 [‐75.53, 627.53] |
| 8 Ovarian cyst formation rate | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Prog + Ag vs Ag | 3 | 374 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.08, 0.32] |
2.1. Analysis.
Comparison 2 Progestogen versus placebo/no pretreatment (Rx), Outcome 1 Live birth or ongoing pregnancy.
2.2. Analysis.
Comparison 2 Progestogen versus placebo/no pretreatment (Rx), Outcome 2 Pregnancy loss.
2.3. Analysis.
Comparison 2 Progestogen versus placebo/no pretreatment (Rx), Outcome 3 Clinical pregnancy rate.
2.4. Analysis.
Comparison 2 Progestogen versus placebo/no pretreatment (Rx), Outcome 4 Multiple pregnancy rate.
2.5. Analysis.
Comparison 2 Progestogen versus placebo/no pretreatment (Rx), Outcome 5 Number of oocytes retrieved.
2.6. Analysis.
Comparison 2 Progestogen versus placebo/no pretreatment (Rx), Outcome 6 Days of gonadotrophin treatment.
2.7. Analysis.
Comparison 2 Progestogen versus placebo/no pretreatment (Rx), Outcome 7 Amount of gonadotrophins administered.
2.8. Analysis.
Comparison 2 Progestogen versus placebo/no pretreatment (Rx), Outcome 8 Ovarian cyst formation rate.
Comparison 3. Oestrogen versus no pretreatment (Rx).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth or ongoing pregnancy | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Oestrogen (Oestr) + antagonist (Ant) vs Ant | 2 | 502 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.53, 1.17] |
| 1.2 Oestr + Ant vs agonist (Ag) | 2 | 242 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.51, 1.50] |
| 2 Pregnancy loss | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Oestr + Ant vs Ant | 1 | 49 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.16 [0.02, 1.47] |
| 2.2 Oestr + Ant vs Ag | 1 | 220 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.59 [0.62, 4.06] |
| 3 Clinical pregnancy rate | 6 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 Oestr + Ant vs Ant | 4 | 688 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.66, 1.24] |
| 3.2 Oestr + Ant vs Ag | 2 | 242 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.76 [0.45, 1.27] |
| 4 Multiple pregnancies | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Oestr + Ant vs Ag | 1 | 22 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.24 [0.09, 53.59] |
| 5 Ovarian hyperstimulation syndrome rate | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Oestr + Ant vs Ag | 1 | 220 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.54 [0.25, 9.42] |
| 6 Number of oocytes retrieved | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 Oestr + Ant vs Ant | 2 | 139 | Mean Difference (IV, Fixed, 95% CI) | 2.23 [0.71, 3.75] |
| 6.2 Oestr + Ant vs Ag | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | 0.40 [‐4.47, 5.27] |
| 7 Days of gonadotrophin treatment | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 7.1 Oestr + Ant vs Ant | 2 | 529 | Mean Difference (IV, Fixed, 95% CI) | 0.83 [0.58, 1.08] |
| 7.2 Oestr + Ant vs Ag | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐2.5 [‐4.07, ‐0.93] |
| 8 Amount of gonadotrophins administered | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 8.1 Oestr + Ant vs Ant | 4 | 668 | Mean Difference (IV, Fixed, 95% CI) | 168.35 [111.53, 225.17] |
| 8.2 Oestr + Ant vs Ag | 1 | 22 | Mean Difference (IV, Fixed, 95% CI) | ‐16.0 [‐470.12, 438.12] |
3.3. Analysis.
Comparison 3 Oestrogen versus no pretreatment (Rx), Outcome 3 Clinical pregnancy rate.
3.4. Analysis.
Comparison 3 Oestrogen versus no pretreatment (Rx), Outcome 4 Multiple pregnancies.
3.5. Analysis.
Comparison 3 Oestrogen versus no pretreatment (Rx), Outcome 5 Ovarian hyperstimulation syndrome rate.
3.6. Analysis.
Comparison 3 Oestrogen versus no pretreatment (Rx), Outcome 6 Number of oocytes retrieved.
3.7. Analysis.
Comparison 3 Oestrogen versus no pretreatment (Rx), Outcome 7 Days of gonadotrophin treatment.
3.8. Analysis.
Comparison 3 Oestrogen versus no pretreatment (Rx), Outcome 8 Amount of gonadotrophins administered.
Comparison 4. Combined oral contraceptive pill (COCP) versus progestogen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth or ongoing pregnancy | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 COCP + antagonist (Ant) vs progestogen (Prog) + Ant | 1 | 44 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.6 [0.12, 2.89] |
| 2 Pregnancy loss | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 COCP + Ant vs Prog + Ant | 1 | 44 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.14, 8.64] |
| 3 Clinical pregnancy rate | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 COCP + Ant vs Prog + Ant | 1 | 44 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.19, 2.73] |
| 4 Multiple pregnancy rate | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 COCP + Ant vs Prog + Ant | 1 | 44 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.32 [0.19, 27.59] |
| 5 Number of oocytes retrieved | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 COCP + Ant vs Prog + Ant | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 1.40 [‐3.24, 6.04] |
| 6 Amount of gonadotrophins administered | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 6.1 COCP + Ant vs Prog + Ant | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | 164.0 [‐249.03, 577.03] |
4.1. Analysis.
Comparison 4 Combined oral contraceptive pill (COCP) versus progestogen, Outcome 1 Live birth or ongoing pregnancy.
4.2. Analysis.
Comparison 4 Combined oral contraceptive pill (COCP) versus progestogen, Outcome 2 Pregnancy loss.
4.3. Analysis.
Comparison 4 Combined oral contraceptive pill (COCP) versus progestogen, Outcome 3 Clinical pregnancy rate.
4.4. Analysis.
Comparison 4 Combined oral contraceptive pill (COCP) versus progestogen, Outcome 4 Multiple pregnancy rate.
4.5. Analysis.
Comparison 4 Combined oral contraceptive pill (COCP) versus progestogen, Outcome 5 Number of oocytes retrieved.
4.6. Analysis.
Comparison 4 Combined oral contraceptive pill (COCP) versus progestogen, Outcome 6 Amount of gonadotrophins administered.
Comparison 5. Combined oral contraceptive pill (COCP) versus oestrogen (Oestr).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth or ongoing pregnancy | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 COCP + antagonist (Ant) vs Oestr + Ant | 2 | 146 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.54, 2.29] |
| 1.2 COCP + agonist (Ag) vs Oestr + Ant | 1 | 25 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.08 [0.01, 0.79] |
| 2 Pregnancy loss | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 COCP + Ag vs Oestr + Ant | 1 | 25 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.06, 19.63] |
| 3 Clinical pregnancy rate | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 3.1 COCP + Ant vs Oestr + Ant | 2 | 146 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.60, 2.37] |
| 3.2 COCP + Ag vs Oestr + Ant | 1 | 25 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.13 [0.02, 0.82] |
| 4 Number of oocytes retrieved | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 COCP + Ant vs Oestr + Ant | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐3.59, 5.39] |
| 5 Days of gonadotropin treatment | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 COCP + Ant vs Oestr + Ant | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.23, 0.03] |
| 6 Amount of gonadotrophins administered | 2 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 6.1 COCP + Ant vs Oestr + Ant | 2 | 146 | Mean Difference (IV, Random, 95% CI) | 181.56 [‐344.73, 707.86] |
5.1. Analysis.
Comparison 5 Combined oral contraceptive pill (COCP) versus oestrogen (Oestr), Outcome 1 Live birth or ongoing pregnancy.
5.2. Analysis.
Comparison 5 Combined oral contraceptive pill (COCP) versus oestrogen (Oestr), Outcome 2 Pregnancy loss.
5.3. Analysis.
Comparison 5 Combined oral contraceptive pill (COCP) versus oestrogen (Oestr), Outcome 3 Clinical pregnancy rate.
5.4. Analysis.
Comparison 5 Combined oral contraceptive pill (COCP) versus oestrogen (Oestr), Outcome 4 Number of oocytes retrieved.
5.5. Analysis.
Comparison 5 Combined oral contraceptive pill (COCP) versus oestrogen (Oestr), Outcome 5 Days of gonadotropin treatment.
5.6. Analysis.
Comparison 5 Combined oral contraceptive pill (COCP) versus oestrogen (Oestr), Outcome 6 Amount of gonadotrophins administered.
Comparison 6. Progestogen (Prog) versus oestrogen (Oestr).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Live birth or ongoing pregnancy | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Prog + antagonist (Ant) vs Oestr + Ant | 1 | 48 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.04 [0.43, 9.70] |
| 2 Clinical pregnancy rate | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 Prog + Ant vs Oestr + Ant | 1 | 48 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.30 [0.57, 9.22] |
| 3 Number of oocytes retrieved | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Prog + Ant vs Oestr + Ant | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐4.55, 3.55] |
| 4 Amount of gonadotrophins administered | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4.1 Prog + Ant vs Oestr + Ant | 1 | 48 | Mean Difference (IV, Fixed, 95% CI) | 310.0 [‐32.30, 652.30] |
6.1. Analysis.
Comparison 6 Progestogen (Prog) versus oestrogen (Oestr), Outcome 1 Live birth or ongoing pregnancy.
6.2. Analysis.
Comparison 6 Progestogen (Prog) versus oestrogen (Oestr), Outcome 2 Clinical pregnancy rate.
6.3. Analysis.
Comparison 6 Progestogen (Prog) versus oestrogen (Oestr), Outcome 3 Number of oocytes retrieved.
6.4. Analysis.
Comparison 6 Progestogen (Prog) versus oestrogen (Oestr), Outcome 4 Amount of gonadotrophins administered.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aston 1995.
| Methods | Parallel group study. Number of women randomized: 152 (77 in intervention group; 75 in control group). Number of withdrawals: 8 (1 in intervention group due to endometrioma; 7 in control group: 5 due to endometrioma or cysts and 2 chose not to proceed). Number of women analyzed: 144. |
|
| Participants | Country of authors: UK. Inclusion criteria: women planning to have an IVF cycle on the Southampton (UK) IVF programme. Exclusion criteria: endometrioma or ovarian cyst seen on vaginal ultrasound scan on day 19 of the menstrual cycle (after recruitment). Mean age ± SD: intervention group: 33.8 ± 4.1 years; control group: 33.5 ± 3.5 years. |
|
| Interventions | Intervention: medroxyprogesterone acetate (10 mg/day) on cycle days 19‐25 + GnRH agonist (buserelin acetate, nasal administration 200 μg 3 times daily) from cycle day 21 + hMG 4 ampoules/day (75 IU FSH and 75 IU LH per ampoule) from day 4 of ensuing menses. Control: placebo on cycle days 19‐25 + GnRH agonist (buserelin acetate, 200 μg nasal administration, 3 times daily) from cycle day 21 + hMG 4 ampoules/day (75 IU FSH and 75 IU LH per ampoule) from day 4 of ensuing menses. Both hMG and GnRH agonist continued until hCG injection (10,000 IU, IM), administered when leading 3 follicles reached diameter of ≥ 18 mm and serum oestradiol > 300 pmol/L for every follicle > 14 mm in diameter. |
|
| Outcomes |
|
|
| Notes | Power calculation performed: yes. ITT analysis performed: no. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The hospital pharmacy randomized to contain placebo or progestogen." Method of randomization not reported. |
| Allocation concealment (selection bias) | Low risk | Quote: "The hospital pharmacy provided a series of consecutively numbered bottles." |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind. Women in control group received a placebo. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportions of withdrawals not balanced between the 2 treatment groups (1 in intervention group; 5 in control group) and data were not analyzed on ITT basis. |
| Selective reporting (reporting bias) | High risk | All planned outcomes not reported. |
| Other bias | Low risk | Quote: "No difference was seen between the study group and control group in the indication of IVF and age." |
Biljan 1998b.
| Methods | Academic centre, parallel group study. Number of women randomized: 83 women undergoing 102 cycles (51 cycles in each group; number of women per group not reported). Number of withdrawals: not reported. Number of women analyzed: only number of cycles analyzed reported (102 cycles). |
|
| Participants | Country of authors: Canada. Inclusion criteria: women who were receiving a long protocol of pituitary suppression in the early follicular phase as a part of IVF‐ET treatment. Exclusion criteria: not reported. Median age (range): intervention group: 35.2 years (32.5‐39.1); control group: 33.7 years (31.6‐38.3). |
|
| Interventions | Intervention: COCP on cycle days 1‐14 + GnRH agonist (buserelin acetate, long protocol 500 μg/day) started on cycle day 14 + hMG (75 IU FSH and 75 IU LH) or pure FSH (75 IU) started after achievement of pituitary suppression. Control: GnRH agonist (buserelin acetate, long protocol 500 μg/day) started on cycle day 2 + hMG (75 IU FSH and 75 IU LH) or pure FSH (75 IU) started after achievement of pituitary suppression. If no pituitary suppression (serum oestradiol < 40 pg/mL) achieved after 14 days of GnRH agonist administration, dosage of buserelin acetate increased to 500 μg twice daily + administration of an IM injection of progesterone 100 mg. Both hMG/FSH and GnRH agonist continued until hCG injection, administered when ≥ 3 follicles reach a mean diameter of ≥ 18 mm. |
|
| Outcomes |
|
|
| Notes | Power calculation performed: yes. ITT analysis performed: no. No per‐woman data reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "They were randomized in two groups by drawing sealed envelopes that contained randomly generated numbers." |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers and reasons for withdrawals not reported. |
| Selective reporting (reporting bias) | High risk | All planned outcomes not reported. |
| Other bias | Low risk | No significant difference in baseline characteristics between groups with regard to age, cause of infertility, number of previous attempts, and oestradiol, FSH or LH level. |
Blockeel 2012.
| Methods | Parallel group study. Number of women randomized: 86 (44 in intervention group; 42 in control group). Number of withdrawals: 14 (9 in intervention group due to cyst development, protocol violation, insufficient ovarian response, did not undergo treatment, did not receive embryo transfer; 5 in control group due to insufficient ovarian response, did not receive embryo transfer). Number of women analyzed (for pregnancy outcome): 72 (35 in intervention group; 37 in control group). |
|
| Participants | Country: Belgium. Inclusion criteria: women aged ≤ 36 years, BMI 18‐29 kg/m2, underwent a first or second treatment cycle of IVF with ICSI, serum FSH on day 3 of the menstrual cycle < 12 IU/L, normal ultrasound scan regular ovulatory menstrual cycle of 21 to 35 days. Exclusion criteria: oocyte donors, women with endometriosis ≥ grade 3, endocrine or metabolic abnormalities, PCOS or previous history of poor ovarian response (defined as development of < 4 follicles in previous IVF or ICSI cycle). Mean age ± SD: intervention group: 29.2 ± 3.0 years; control group: 30.2 ± 3.0 years. Setting: assisted reproduction programme in Belgium. |
|
| Interventions | Intervention: oestradiol valerate (2 × 2 mg/day) during 6‐10 consecutive days (from cycle day 25 onwards) prior to start of rFSH stimulation so that the first day of stimulation occurred between Friday and Sunday. Control: no pretreatment; standard GnRH antagonist protocol. Both groups received rFSH (150 IU) and on day 6 of stimulation GnRH antagonist protocol (ganirelix 0.25 mg/day). |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | Power calculation: not reported. ITT analysis: not reported. Objective of the study was to assess the ability of oestradiol to control the oocyte retrieval of GnRH antagonist cycles prior to COS. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization list. |
| Allocation concealment (selection bias) | Low risk | Consecutive sealed opaque envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for, and proportions of, withdrawals balanced between the 2 treatment groups and data were analyzed on the basis of ITT. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported, |
| Other bias | Low risk | Groups comparable at baseline, |
Cédrin‐Durnerin 2007.
| Methods | Multicentre (6 IVF centres), parallel group study. Number of women randomized: 93 (21 in COCP group; 23 in progestogen group; 25 in oestrogen group; 24 in control group). Number of withdrawals: 3 in oestrogen group (1 did not start any treatment, 1 due to an ovarian cyst and 1 due to major protocol violation). Number of women analyzed: 90. Duration of study: 10 months of recruitment. |
|
| Participants | Country of authors: France. Inclusion criteria: regular normo‐ovulatory cycles (28‐35 days), aged < 38 years, BMI 18‐30. Exclusion criteria: high levels of baseline serum FSH or oestradiol, < 5 follicles at the antral follicular count performed on day 3 of a spontaneous cycle, history of high (> 20 oocytes) or low (< 5 oocytes) ovarian response in a previous IVF attempt. Mean age ± SD: COCP group: 30.8 ± 4.6 years; progestogen group: 32.9 ± 2.5 years; oestrogen group: 31.8 ± 3.2 years; control group: 31.2 ± 4.3 years. |
|
| Interventions | COCP group: COCP (ethinyl oestradiol 30 μg + desogestrel 150 μg daily), started cycle day 2 or 3 for 15‐21 days (stopped on a Sunday) + rFSH (recombinant follitropin beta 150‐300 IU/day), started post‐treatment day 5 + GnRH antagonist (ganirelix acetate 0.25 mg/day), started when leading follicle reached 14 mm in diameter. Progestogen group: norethisterone 10 mg/day, started cycle day 15 for 10‐15 days (stopped on a Sunday) + rFSH (recombinant follitropin beta 150‐300 IU/day), started post‐treatment day 5 + GnRH antagonist (ganirelix acetate 0.25 mg/day), started when leading follicle reached 14 mm in diameter. Oestrogen group: micronised 17‐βE2 (2 mg twice daily), 10‐15 days, started 10 days before the presumed menses (stopped on a Sunday) + rFSH (recombinant follitropin beta 150‐300 IU/day), started post‐treatment day 5 + GnRH antagonist (ganirelix acetate 0.25 mg/day), started when leading follicle reached 14 mm in diameter. Control group: rFSH (recombinant follitropin beta 150‐300 IU/day), started day 3 after spontaneous menses + GnRH antagonist (ganirelix acetate 0.25 mg/day), started when leading follicle reached 14 mm in diameter. rFSH dose according to age, BMI and previous responses to stimulation; after 5 days of treatment dose adjustment according to ovarian response. Both rFSH and GnRH antagonist were continued until hCG injection (10,000 IU), administered when ≥ 3 mature (of ≥ 17 mm diameter) follicles were obtained. |
|
| Outcomes |
|
|
| Notes | Power calculation performed: no. ITT analysis performed: no (not for oestrogen group). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Random allocation sequence was generated from a table of random numbers... Randomization was stratified by centre..." |
| Allocation concealment (selection bias) | Low risk | Quote: "Random allocation sequence was concealed to each physician who enrolled and randomised patients." Sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Quote: "This study was not blind." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reasons for withdrawals and proportions of withdrawals not clearly stated. |
| Selective reporting (reporting bias) | High risk | Some planned outcomes not reported. |
| Other bias | Low risk | No significant baseline imbalance with regard to age and BMI. |
Cédrin‐Durnerin 2012.
| Methods | Parallel group study. Number of women randomized: 472 (238 in intervention group; 234 in control group). Number of withdrawals: 19 (5 in intervention group due to spontaneous pregnancy, discontinuation and no periods; 14 in control group due to spontaneous pregnancy, discontinuation, ovarian cyst and protocol change). Number of women analyzed: 453 (233 in intervention group; 220 in control group). |
|
| Participants | Country France. Inclusion criteria: regular normo‐ovulatory cycles (28‐35 days), aged < 38 years, BMI 18‐30 kg/m2 and first or second IVF/ICSI attempt. Exclusion criteria: high basal levels of serum FSH (> 12 IU/L) or oestradiol (> 80 pg/mL), < 5 follicles at the antral follicular count performed on day 3 of a spontaneous cycle or history of high (> 20 oocytes) or low (< 5 oocytes) ovarian response in earlier IVF attempt. Mean age ± SD: intervention group: 31.1 ± 3.6 years; control group: 31.2 ± 3.7 years. Setting: 10 private or university‐based centres in France. |
|
| Interventions | Intervention: oral 17 β‐oestradiol (4 mg; 2 mg x 2/day) started 7 days before the presumed onset of menses and administered up to the next Thursday after the occurrence of menstrual bleeding. Control: no pretreatment. Ovarian stimulation started on Friday in intervention group and on cycle day 2 after spontaneous menses in control group. Followed by GnRH antagonists on day 6, then hCG. |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | Power calculation for sample size (225 women per group). ITT analysis not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Table of random numbers. |
| Allocation concealment (selection bias) | Low risk | Authors stated allocation concealed by sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Reasons for withdrawals and proportions of withdrawals not balanced between groups and data not analyzed on the basis of ITT. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
| Other bias | Low risk | Groups balanced at baseline with respect to demographic characteristics. |
Daly 2002.
| Methods | Cross‐over study. Number of women randomized: 25 (13 intervention group; 12 in control group). Number of withdrawals: not reported. Number of women analyzed: unclear. |
|
| Participants | Country of authors: USA. Inclusion criteria: women, aged < 41 years, who were anticipated to have limited ovarian reserve based on transvaginal ultrasound showing limited follicles on cycle day 2‐3 or hormonal values (inhibin B, FSH, oestradiol). Exclusion criteria: not reported. Mean age: not reported. Poor response: yes ("limited ovarian reserve"). |
|
| Interventions | Intervention: COCP + GnRH agonist (leuprolide acetate, microdose) + hMG (300 IU FSH + 75 IU LH). Timing of administration of COCP, hMG and GnRH agonist not reported. Control: oestradiol (2 mg) in the luteal phase of the preparation cycle + FSH (300 IU), started cycle day 2 + GnRH antagonist (ganirelix acetate) started in late follicular phase + hMG (375 IU FSH + 150 IU LH), timing not reported. |
|
| Outcomes |
|
|
| Notes | Power calculation performed: unclear. ITT analysis performed: unclear. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized," method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Unblinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Only abstract available. Numbers and reasons for withdrawals not reported. |
| Selective reporting (reporting bias) | High risk | Data on all planned outcomes reported. Pre‐cross‐over data on primary outcome reported, but on some secondary outcomes (implantation rate, mature oocytes, good embryos) only post‐cross‐over data reported. |
| Other bias | Unclear risk | No data on baseline characteristics reported. |
Ditkoff 1996.
| Methods | Parallel group study. Number of women randomized: 105 (47 in intervention group; 58 in control group). Number of withdrawals: 0. Number of women analyzed: 105. Length of follow‐up: until end of treatment cycle. |
|
| Participants | Country: USA. Inclusion criteria: day 3 FSH values < 15 mIU/mL. Exclusion criteria: not reported. Mean age ± SD: intervention: 36.7 ± 4.8 years; control: 35.8 ± 4.57 years. |
|
| Interventions | Intervention: norethindrone acetate (10 mg/day PO) on cycle days 1‐8 + GnRH agonist (leuprolide acetate, 1 mg/day, SC), started cycle day 1 + hMG (225 IU/day IM), started when serum oestradiol level was < 30 pg/mL.
2) GnRH agonist (leuprolide acetate 1 mg/day, SC), started cycle day 1 + hMG (225 IU/day, IM), started when serum oestradiol level was < 30 pg/mL. Both hMG and GnRH agonist are continued until hCG injection (10,000 IU, IM), administered when the leading follicles reached a diameter of ≥ 18 mm. |
|
| Outcomes |
|
|
| Notes | Power calculation performed: no. ITT analysis performed: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned by tossing a coin to one of two groups." |
| Allocation concealment (selection bias) | Low risk | Centralised randomization process. |
| Blinding (performance bias and detection bias) All outcomes | High risk | No blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals. |
| Selective reporting (reporting bias) | High risk | All planned outcomes not reported. |
| Other bias | Low risk | Quote: "The various infertility diagnoses were distributed equally between the control and study groups." |
Engmann 1999.
| Methods | Parallel group study. Number of women recruited: 123. Number of women excluded: 6 (2 due to ovarian cysts ≥ 15 mm, 2 due to raised early follicular phase serum FSH, 2 did not undergo IVF). Number of women randomized: 117 (63 in intervention group; 54 in control group). Number of withdrawals: 1 (in intervention group, due to violation of the study protocol). Number of women analyzed: 116. Duration of study: 1 year of recruitment. Source of funding: Schering Health Care Limited, West Sussex, UK, supplied the norethindrone. |
|
| Participants | Country of authors: UK and Canada. Inclusion criteria: aged 18‐44 years at time of screening, duration of infertility ≥ 1 year, early follicular phase serum FSH ≤ 11.0 IU/L, good physical and mental health, suitability for the long‐term buserelin protocol for desensitisation. Exclusion criteria: endometrioma of the ovary, ovarian cysts (≥ 15 mm) in the early follicular phase, known contraindications to the use of progestogen, GnRH agonists or hMG. Mean age ± SD: intervention group: 35.3 ± 4.3 years; control group: 33.8 ± 5.5 years. |
|
| Interventions | Intervention: norethindrone (10 mg on cycle day 1 and 5 mg twice daily on cycle day 2‐5) + GnRH agonist (buserelin acetate 500 μg/day, SC, long protocol), started on cycle day 2 (dose adjustment after pituitary suppression to 200 μg/day) + hMG (Normegon, 75 IU FSH 2‐5 ampoules/day) or rFSH, started when serum oestradiol ≤ 150 pmol/L. Control: GnRH agonist (buserelin acetate 500 μg/day, SC, long protocol) started on cycle day 2 (dose adjustment after pituitary suppression to 200 μg/day) + hMG (Normegon, 75 IU FSH 2‐5 ampoules/day) or rFSH, started when serum oestradiol ≤ 150 pmol/L. Pituitary suppression achieved when there was an absence of follicular activity and endometrial thickness < 5 mm. hMG or rFSH dose according to woman's age, previous response, basal serum FSH levels and PCOS. Both hMG/rFSH and GnRH agonist continued until hCG injection (10,000 IU, IM), administered when 2 or 3 leading follicles ≥ 18 mm in diameter. |
|
| Outcomes |
|
|
| Notes | Power calculation performed: yes. ITT analysis performed: no. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Eligible patients were randomly assigned in a ratio of 1:1 by means of computer‐generated random numbers. To ensure similar distributions of age in the two groups, separate randomization schedules were drawn up for women < 40 years old and women ≥ 40 years old by use of stratified randomised blocks." |
| Allocation concealment (selection bias) | Low risk | Quote: "Selection into the groups (and of administration of the appropriate treatment protocol) was performed by the clinic nurses by using a series of consecutively numbered sealed envelopes (one for each age group)." |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "Although the patient could guess her treatment status, treatment allocation was not recorded in the clinical notes, and all clinicians were blinded to the status of study participants until the trial was over." However, no information on outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportions of withdrawals fairly balanced between the 2 groups. |
| Selective reporting (reporting bias) | Low risk | Data on all planned outcomes reported. |
| Other bias | Low risk | No significant baseline imbalance between groups with regard to age, duration of infertility, previous attempts, baseline serum FSH, polycystic ovaries and cause of infertility. |
Fanchin 2003.
| Methods | Parallel group study. Number of women randomized: 100 (number of women per group not reported). Number of withdrawals: 10 (4 due to personal reasons and 6 due to major protocol violation). Number of women analyzed: 90 (47 in intervention group; 43 in control group). Duration of study: 1 IVF‐ET cycle, from day 20 of the previous cycle to day of hCG administration (information obtained from contact person). |
|
| Participants | Country of authors: France. Inclusion criteria: aged ≤ 38 years; regular, ovulatory menstrual cycles every 25‐35 days; both ovaries present; no current or past diseases affecting ovaries or gonadotrophin or sex steroid secretion, clearance or excretion; BMI 18‐27 kg/m2; no hormone therapy during the past 6 weeks; adequate visualisation of both ovaries in transvaginal ultrasound scans. Exclusion criteria: not reported. Median age (range): intervention group: 33 (26‐38) years; control group: 33 (25‐38) years. |
|
| Interventions | Intervention: micronised 17β‐E2 (4 mg/day, PO), started cycle day 20 until day 2 of the next cycle + rFSH (225 IU/day, SC) on cycle days 3‐7 + GnRH antagonist (cetrorelix acetate 3 mg single dose, SC) when ≥ 1 follicle > 13 mm in diameter. Control: rFSH (225 IU/day SC) on cycle days 3‐7 + GnRH antagonist (cetrorelix acetate 3 mg single dose, SC) when ≥ 1 follicle > 13 mm in diameter. rFSH dose adjustments according to follicle growth determined by serum oestradiol levels and ultrasound monitoring. |
|
| Outcomes |
|
|
| Notes | Power calculation performed: yes. ITT analysis performed: no Our data analysis in this review includes 90 women with full follow‐up. We did not include all randomized women because it is unclear how many women were randomized to each group before dropouts. The study publication reports very low measures of variability which can be to be assumed SEs and which we have converted to SDs |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Women randomly received...", "...according to a computer‐generated, blocked randomization list." |
| Allocation concealment (selection bias) | Low risk | Quote: "Treatment allocation was decided by an independent person." |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No blinding, information obtained from contact person. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Unclear how many withdrew in each group and data not analyzed on the basis of ITT. |
| Selective reporting (reporting bias) | High risk | All planned outcomes not reported. |
| Other bias | Low risk | No difference in baseline characteristics with regard to age, indication for IVF‐ET, duration of infertility, rank of the current IVF‐ET attempt, menstrual cycle length, day 3 serum FSH and oestradiol. |
Franco Jr 2003.
| Methods | Parallel group study. Number of women recruited: 22. Number of women randomized: 22 (16 in intervention group; 6 in control group). Number of withdrawals: 1 (both in intervention group, due to spontaneous pregnancies). Number of women analyzed: 20. |
|
| Participants | Country of authors: Brazil. Inclusion criteria: women without specific ovulatory dysfunction, aged ≤ 37 years, who would be submitted to ovarian stimulation. Exclusion criteria: not reported. Mean age ± SD: intervention group: 32.2 ± 2.1 years; control group: 31.8 ± 1.9 years. |
|
| Interventions | Intervention: oestradiol valerate (4 mg/day) for 14 days, started cycle day 21 + rFSH (150‐300 IU) (fixed dose for 5 days), started post‐treatment day 1 + GnRH antagonist (ganirelix acetate 0.25 mg/day), started when follicular diameter ≥ 15 mm. Control: GnRH agonist (nafarelin acetate 200 μg twice daily, nasal), started cycle day 21 + rFSH (150‐300 IU) (fixed dose for 5 days), started stimulation day 14. Both rFSH and GnRH analogues continued until hCG injection (5000‐10,000 IU), administered when ≥ 2 follicles were ≥ 17 mm in diameter. |
|
| Outcomes |
|
|
| Notes | Power calculation performed: no. ITT analysis performed: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by drawing lots after constructing a table of distribution. 2:1 randomization (intervention:control). |
| Allocation concealment (selection bias) | High risk | After drawing lots, clinicians and participants could see in the table to which treatment they were assigned to. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Insufficient information to make a conclusive judgement. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There was imbalance in the proportions of withdrawals between the 2 groups and data analyzed on the basis of ITT. |
| Selective reporting (reporting bias) | High risk | All planned outcomes not reported. |
| Other bias | Low risk | No significant difference in baseline characteristics with regard to age. |
Garcia‐Velasco 2011.
| Methods | 2‐arm parallel RCT. Number of women randomized: 228 (115 in intervention; 113 in control). Withdrawals: not reported. Number of women analyzed: not reported. |
|
| Participants | Country: Spain. Inclusion criteria: regular cycle women under 39 years, < 3 previous IVF attempts. Exclusion criteria: previous low response to COH, ovarian surgery or PCOS. Mean age: intervention group: 34.1 years; control group: 33.7 years. Setting: not reported. |
|
| Interventions | Intervention: COCP (ethinyl oestradiol 30 μg + desogestrel 150 μg) for 12‐16 days and COH started on day 5 post COCP using GnRH antagonist. Control: GnRH agonist long protocol from days 20‐22 of the previous cycle. |
|
| Outcomes |
|
|
| Notes | Duration of stimulation, FSH used and number of oocyte retrieved were continuous variables. Power calculation was not reported. ITT analysis: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated list with consecutive inclusion. |
| Allocation concealment (selection bias) | Low risk | Opaque consecutively numbered envelopes with assignment under nurse control. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Proportions of withdrawals and reasons for withdrawals not reported. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
| Other bias | Low risk | Groups comparable at baseline with regard to demographic characteristics. |
Hauzman 2013.
| Methods | Parallel group study. Number of women randomized: 100 (50 in each group). Number of withdrawals: 16 (7 in intervention group; 9 in control group). Number of women analyzed: 100 (ITT analysis). |
|
| Participants | Country: Spain. Inclusion criteria: aged 18‐38 years, regular normo‐ovulatory menstrual cycles (26‐35 days), BMI < 30 kg/m2, normal cycle day 3 basal serum hormone levels (FSH < 10 IU/L and oestradiol < 60 pg/mL) and < 3 previous IVF/ICSI attempts. Exclusion criteria: previous ovarian surgery, low ovarian response (cancellation of cycle due to poor follicular development after ≥ 7 days of gonadotropin stimulation or < 5 oocytes retrieved) in previous IVF/ICSI cycle and PCOS. Mean age ± SD: intervention group: 33.9 ± 3.4 years; control group: 34.5 ± 3.1 years. Setting: single hospital clinic in Madrid, Spain. |
|
| Interventions | Intervention: COCP: (ethinyl oestradiol 30 μg + levonorgestrel 150 μg) on day 1 or 2 of cycle prior to IVF/ICSI and continued for 12‐16 days, with stimulation starting 5 days after stopping pretreatment. Control: (oestradiol valerate 4 mg/day (2 mg × 2)) from day 20 of menstrual cycle for 5‐12 days until the day before starting stimulation. In both groups, GnRH antagonist (ganirelix 0.25 mg/day) started when the leading follicle reached 13 mm in diameter and ovarian triggering performed with rhCG (250 μg) (when 2 leading follicles reached ≥ 17 mm mean diameter). |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | Power calculation for sample size: yes but not achieved because this was a single‐centre study. ITT analysis: yes. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random number list. |
| Allocation concealment (selection bias) | Low risk | Consecutively numbered opaque envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Although personnel involved in data collection and data analysis blinded it was not reported whether other personnel and participants were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for withdrawals and proportions of withdrawals were fairly balanced between groups and data were analyzed on the basis of ITT. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
| Other bias | Low risk | Groups balanced at baseline with respect to demographic characteristics. |
Hugues 1994.
| Methods | Parallel group study. Number of women randomized: 45 (20 in intervention group; 25 in control group). Number of withdrawals: not reported. Number of women analyzed: not reported. |
|
| Participants | Country of authors: France. Inclusion criteria: not reported. Exclusion criteria: not reported. Mean age: not reported. |
|
| Interventions | Intervention: norethisterone (10 mg/day) for 10‐15 days + GnRH agonist (DTRP6‐LHRH 100 μg/day). Control: GnRH agonist (DTRP6‐LHRH 100 μg/day). Timing of treatments not reported. |
|
| Outcomes |
|
|
| Notes | Power calculation performed: unclear. ITT analysis performed: unclear. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised," method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers and reasons of withdrawals not reported. |
| Selective reporting (reporting bias) | High risk | All planned outcomes not reported. No data on pregnancy rates. |
| Other bias | Unclear risk | Baseline demographic characteristics not reported. |
Huirne 2006a.
| Methods | Academic, multicentre, parallel group study. Number of women randomized: 64 (32 in each group). Number of withdrawals: 1 (in intervention group, due to unwillingness to take OCP). Number of women analyzed: 63. Source of funding: Serono Geneva supplied the antide. |
|
| Participants | Country: the Netherlands and Belgium. Inclusion criteria: regular IVF or ICSI indication (i.e. idiopathic infertility after 6 unsuccessful IUIs, infertility based on a male or tubal factor); spontaneous, regular ovulatory menstrual cycle; 2 ovaries and a normal uterine cavity; aged 18‐38 years. Exclusion criteria: FSH ≥ 12 IU/L on cycle day 2‐4; BMI > 30 kg/m2; abnormal gynaecological bleeding; extrauterine pregnancy within the last 3 months; previous ART cycles with < 3 oocytes or severe OHSS; any contraindication to receive gonadotrophins or OCP; PCOS. Mean age ± SD: intervention group: 32.3 ± 4.0 years; control group: 33.3 ± 3.8 years. |
|
| Interventions | Intervention: COCP (ethinyl oestradiol 30 μg + levonorgestrel 150 μg) for 14‐28 days, started cycle day 2 or 3 + rFSH (150‐300 IU), started post‐treatment day 2 or 3 (= stimulation day 1) + GnRH antagonist (antide 0.5 mg/mL daily), started stimulation day 6. Control: rFSH (150‐300 IU), started on cycle days 2 or 3 (= stimulation day 1) + GnRH antagonist (antide 0.5 mg/mL daily), started on stimulation day 6. rFSH dose adjustments after 5 days of stimulation (up to a maximum of 450 IU), according to number and size of oocytes and risk for OHSS. Both rFSH and GnRH antagonist were continued until hCG injection (6500 IU), administered when ≥ 1 follicle reached a diameter ≥ 18 mm + ≥ 2 follicles reached a diameter ≥ 16 mm. |
|
| Outcomes |
|
|
| Notes | Power calculation performed: yes. ITT analysis performed: no. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "64 patients were randomly allocated according to a computer‐generated, blocked randomization list. The randomization was stratified by centre." |
| Allocation concealment (selection bias) | Low risk | Quote: "Treatment allocation was decided by an independent person from an independent monitoring company..." |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportions of withdrawals were fairly balanced between groups (3% in intervention group vs 0% in control group). |
| Selective reporting (reporting bias) | Low risk | Data on all planned outcomes reported. |
| Other bias | Low risk | No significant differences in baseline characteristics with regard to age, BMI, cycle length, primary infertility, smoking habits, duration of infertility, type of infertility and antral follicle count. |
Huirne 2006c.
| Methods | Multicentre (8 IVF centres), parallel group study. Number of women recruited: 216. Number of women excluded: 34 (reasons not reported). Number of women randomized: 182 (91 in each group). Number of withdrawals: 22 (10 in intervention group: 1 due to hepatitis B, 1 due to non‐compliance, 1 due to personal reasons, 2 due to insufficient follicular response, 1 due to conversion to IUI, 1 due to absence of mature oocytes, 3 due to absence of viable embryos; 12 in control group: 2 due to spontaneous pregnancy, 3 due to failure of desensitisation, 1 due to personal reasons, 1 due to stimulation failure, 3 due to absence of 'mature' oocytes, 2 due to failure of fertilisation). Number of women analyzed: 182. |
|
| Participants | Country of authors: the Netherlands, Belgium, France and Austria. Inclusion criteria: regular IVF/ICSI indication, male partner with viable sperm in the ejaculate, aged 18‐39 years. Exclusion criteria: any previous ART cycles with < 3 oocytes or ≥ 3 consecutive ART cycles without a clinical pregnancy, any contraindication to ART, gonadotrophins or OCPs, significant systemic disease. Mean age ± SD: intervention group: 32.8 ± 3.8 years; control group: 32.2 ± 4.2 years. |
|
| Interventions | Intervention: COCP (ethinyl oestradiol 30 μg + levonorgestrel 150 μg daily), started within 5 days of onset of menses for 21‐28 days (stop on a Sunday) + r‐hFSH (150‐225 IU/day), started post‐treatment day 5 (= stimulation day 1) + GnRH antagonist (cetrorelix acetate 0.25 mg/day, SC), started stimulation day 6. Control: GnRH agonist (buserelin acetate 500 μg/day, SC), started cycle day 18‐22 (reducing dose to 200 μg/day when downregulation was achieved) + r‐hFSH (150‐225 IU/day), started when downregulation was achieved. After 5 days of r‐hFSH treatment, the dose could be adjusted by steps of 75 IU (maximal dose 450 IU/day), according to the ovarian response. Both rhFSH and GnRH analogues were continued until hCG injection, administered when the largest follicle reached a mean diameter ≥ 18 mm and ≥ 2 other follicles had a mean diameter ≥ 16 mm. |
|
| Outcomes |
|
|
| Notes | Power calculation performed: yes. ITT analysis performed: no. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "182 were randomly allocated to...", "The treatment assigned to each patient was determined according to a computer‐generated concealed randomization list. Randomization was performed by centre." |
| Allocation concealment (selection bias) | Unclear risk | "Concealed randomization list," method not reported. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All women randomized were included in final analysis. |
| Selective reporting (reporting bias) | Low risk | Data on all planned outcomes reported. |
| Other bias | Low risk | No significant difference in baseline characteristics with regard to age, race, duration of infertility, cause of infertility, smoking habits, primary infertility, number of previous ART attempts, number of follicles, endometrial thickness, FSH levels and oestradiol levels. P value of BMI was 0.04. |
Hwang 2004.
| Methods | Single‐centre, parallel group study. Number of women recruited: 60. Number of women excluded: 4 (2 refused to participate, 2 did not meet inclusion criteria). Number of women randomized: 56 (27 in intervention group; 29 in control group). Number of withdrawals: 7 (2 in intervention group: 1 due to poor ovarian response, 1 due to personal reasons; 5 in control group: 2 due to inadequate ovarian response, 3 due to risk of severe OHSS). Number of women analyzed: 49. |
|
| Participants | Country: Taiwan. Inclusion criteria: PCOS. Exclusion criteria: diagnosis of congenital adrenal hyperplasia, Cushing's syndrome, androgen‐producing tumours, hyperprolactinaemia or thyroid dysfunction; aged > 38 years; serum FSH levels > 12 mIU/mL. Mean age ± SD: intervention group: 31.4 ± 3.5 years; control group: 31.7 ± 3.7 years |
|
| Interventions | Intervention: COCP (Diane‐35, oral) on cycle days 5‐25 for 3 consecutive cycles + GnRH antagonist (cetrorelix acetate 0.25 mg single dose, SC on post‐treatment day 3; 0.125 mg/day on post‐treatment days 4‐9; and 0.25 mg/day started post‐treatment day 10 + hMG 150 IU/day), started post‐treatment day 4. Control: GnRH agonist (buserelin acetate 500 μg/day, long protocol) started day 3 of induced or spontaneous menstruation, and 250 μg/day started day of ensuing pituitary downregulation + hMG (150 IU/day) for 6 days started when pituitary downregulation was achieved. hMG dose can be adjusted according to woman's follicular response. Pituitary downregulation achieved when serum oestradiol levels < 50 pg/mL and there was an absence of ovarian cysts > 10 mm in diameter. Both GnRH analogues and hMG were continued until hCG injection (10,000 IU, IM), administered when ≥ 2 follicles reached 18 mm in diameter with adequate oestradiol response. |
|
| Outcomes |
|
|
| Notes | Power calculation performed: yes. ITT analysis performed: no |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was done by opening sealed envelopes containing computer‐generated block randomization numbers with a block size of 10." |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "The laboratory staff were blinded to the stimulation protocol." Unclear if treating physicians were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Reasons for withdrawals and proportions of withdrawals were not balanced between groups and data were not analyzed on the basis of ITT. |
| Selective reporting (reporting bias) | Low risk | Data on all planned outcomes were reported. |
| Other bias | Low risk | No significant difference in baseline characteristics with regard to age, duration of infertility, BMI and hormonal levels. |
Kim 2011.
| Methods | Parallel group study. Number of women randomized: 120. Number of withdrawals: 0. Number of women analyzed: 120. Duration of study: 1 cycle. |
|
| Participants | Country of authors: South Korea. 120 poor responders (repeated day 3 levels of FSH > 8.5 mIU/mL or antral follicle count ≤ 5, or both). 40 in each group. Inclusion criteria: not clearly stated. Exclusion criteria: PCOS (Rotterdam criteria). Mean age ± SD: group 1: 36.7 ± 3.1 years; group 2: 35.9 ± 2.8 years; group 3: 36.4 ± 3.3 years. Setting: university‐based infertility clinic, Seoul. Korea. |
|
| Interventions | Pretreatment was ethinyl oestradiol 0.03 mg and levonorgestrel 0,15 mg for 21 days in the cycle preceding COS. Group 1: GnRH antagonist multiple dose protocol after OCP pretreatment. Ovarian stimulation started 5 days after OCP discontinued using rFSH (225 IU/day, dose adjusted every 3‐4 days). Cerotide (0.25 mg) started when lead follicle was 14 mm diameter and continued until day of hCG injection. Group 2 GnRH antagonist multiple dose protocol without OCP pretreatment. Ovarian stimulation started on cycle day 3 using rFSH (225 IU/day, dose adjusted every 3‐4 days). Cerotide (0.25 mg) started when lead follicle was 14 mm diameter and continued until day of hCG injection. Group 3: GnRH agonist luteal low‐dose long protocol without OCP pretreatment. Daily injection of decapeptyl (0.1 mg) started from mid‐luteal phase and continued until menses followed by a dose reduction to 0.05 mg daily and continued until day of hCG injection. |
|
| Outcomes | Primary:
Secondary:
|
|
| Notes | Power calculation: yes. ITT analysis: yes. Earlier publications were Kim 2005 and Kim 2009. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Computer generated lists." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The sequence of allocation to the three groups was provided to the investigating physicians." |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In groups 1 and 3 there were no losses, withdrawals or cancellations. In group 2, 1 cycle was cancelled before embryo transfer; all women randomized were included in data analysis. |
| Selective reporting (reporting bias) | Low risk | All outcomes listed were reported although multiple pregnancy was reported it is not listed a priori. |
| Other bias | Low risk | Groups similar at baseline with respect to demographic characteristics. |
Kolibianakis 2006.
| Methods | Academic, single centre, parallel group study. Number of women randomized: 504 (250 in intervention group; 254 in control group). Number of withdrawals: 79 (36 in intervention group: 28 due to personal reasons, 6 due to abnormal steroid levels, 2 due to spontaneous pregnancy; 43 in control group: 31 due to personal reasons, 10 due to abnormal steroid levels, 2 due to spontaneous pregnancy). Number of women analyzed: 425. Duration of study: 3 years of recruitment. Source of funding: the Fund for Scientific Research Flanders. |
|
| Participants | Country: Belgium. Inclusion criteria: aged < 39 years, ≤ 3 previous ART attempts, BMI 18‐29 kg/m2, FSH < 10 IU/L, LH < 10 IU/L. Exclusion criteria: polycystic ovaries, endometriosis > stage II, poor response to ovarian stimulation Mean age ± SD: intervention group: 31.2 ± 0.3 years; control group: 31.5 ± 0.3 years. |
|
| Interventions | Intervention: COCP (ethinyl oestradiol 30 μg + desogestrel 150 μg) for 14 days, started cycle day 1 + rFSH (200 IU/day) (fixed dose), started post‐treatment day 5 + GnRH antagonist (ganirelix acetate). Control: rFSH (200 IU/day) (fixed dose), started cycle day 2 + GnRH antagonist (ganirelix acetate). Timing of GnRH antagonist not reported. Both rFSH and GnRH antagonist continued until hCG injection (10,000 IU), administered when ≥ 3 follicles ≥ 17 mm in diameter. |
|
| Outcomes |
|
|
| Notes | Power calculation performed: yes. ITT analysis performed: no. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomized on the basis of a computer‐generated list." |
| Allocation concealment (selection bias) | High risk | Quote: "... randomized at the outpatient clinic by the treating physician." "The sequence of allocation was not concealed and thus it was possible for the treating physician to be aware of the next treatment to be allocated." |
| Blinding (performance bias and detection bias) All outcomes | High risk | Treating physician not blinded as this was the person to allocate the participants. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for withdrawals and proportions of withdrawals fairly balanced between groups. |
| Selective reporting (reporting bias) | Low risk | Data on all planned outcomes reported. |
| Other bias | Low risk | No significant differences in baseline characteristics with regard to age, BMI, primary/secondary infertility, duration of infertility, number of previous IVF trials, indication for treatment. |
Lukaszuk 2015.
| Methods | 2‐arm parallel multicentre RCT. Number of women randomized: 298 (154 in intervention group; 144 in control group). Withdrawals: not reported. Number of women analyzed: not reported. |
|
| Participants | Country: Poland. Inclusion criteria: women aged < 40 years, AMH > 0.6 ng/mL, BMI 18‐29 kg/m2, and undergoing a first or second treatment cycle of IVF with ICSI. Exclusion criteria: presence of endometriosis and pre‐implantation diagnosis cycles. Mean age ± SD: intervention group: 32.5 ± 3.97; control group: 32.5 ± 2.96 years. BMI (mean ± SD): intervention group: 22.2 ± 1.3 kg/m2; control group: 22.4 ± 1.1 kg/m2. Setting: infertility clinics, 2 centres. |
|
| Interventions | Intervention: OCP pretreatment: pretreated with COCP (Ovulastan, Adamed, Pabianice, Poland) from day 2‐4 of the cycle. Beginning from the day 14 of cycle, pituitary was suppressed by administering triptorelin (0.1 mg) every 2 days; treatment continued for 2 weeks. Ovarian stimulation started with gonadotropin injections (150‐225 IU/day) starting from days 2‐4 of cycle and continued with a daily dose of triptorelin (0.1 mg) until hCG injection was administered 36 hours before retrieval. Control: oestradiol pretreatment: pretreated with oral oestradiol (2 mg twice daily) from day 20 of natural cycle until the day 1‐4 of the new cycle. Ovarian stimulation started with hMG injections (150‐225 IU/day) starting from days 2‐4 of cycle, 2 days after discontinuation of oestradiol administration, and continued with a daily dose of triptorelin (0.1 mg) until the hCG injection 5000 IU was administered 36 hours before retrieval. |
|
| Outcomes |
|
|
| Notes | All outcomes were measured in denominators other than "per woman randomized." Power calculation was performed. No, outcome measured in denominators other than "per woman randomized." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using "block randomization software." |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Only doctors who retrieved oocytes and embryologists were blinded to treatment groups. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information reported on reasons for withdrawals and proportion of withdrawals. |
| Selective reporting (reporting bias) | Low risk | Important outcome measures were prespecified in the methods section and data were reported. |
| Other bias | Low risk | Demographic and infertility characteristics similar between the two groups. |
Nyboe Andersen 2011.
| Methods | Parallel group multicentre study. Number of women randomized: 442 (223 in intervention group; 219 in control group). Number of withdrawals: 34 (14 in intervention group; 20 in the control group due to adverse events, withdrawal of consent, spontaneous pregnancy and "other" reasons). Number of women analyzed: 408 (209 in intervention group; 199 in control group). |
|
| Participants | Country: multicentre (USA, Europe). Inclusion criteria: aged 18‐39 years, BMI ≤ 32 kg/m2, menstrual cycle length 24‐35 days, access to ejaculatory sperm, indication for COS and IVF or ICSI or IVF plus ICSI and scheduled for first COS cycle. Exclusion criteria: history of endocrine abnormality, < 2 ovaries or any other ovarian abnormality, presence of unilateral or bilateral hydrosalpinx, clinically relevant pathology affecting the uterine cavity, fibroids ≥ 5 cm, history of recurrent miscarriage (≥ 3), with or without FSH or LH levels > 12 IU/L in the early follicular phase. Mean age ± SD: intervention group: 31.8 ± 3.7 years; control group: 31.6 ± 4.1 years. Setting: 8 centres in USA, 6 centres in Europe (Denmark, Germany, Spain and Turkey). |
|
| Interventions | Intervention: COCP pretreatment: Marvelon (ethinyl oestradiol 30 μg + desogestrel 150 μg) for 14‐21 days. Women started daily rFSH 5 days after stopping COCP pretreatment provided a withdrawal bleed occurred. Control: no pretreatment: daily rFSH on day 2 or 3 of the next menstrual cycle. In both groups, a single SC injection of rFSH (200 IU) started on stimulation day 1 and continued daily up to and including day of triggering of final oocyte maturation by urinary hCG. Maximum total duration of stimulation 19 days. Starting on day 5 of stimulation, all women received ganirelix (0.25 mg/day). |
|
| Outcomes | Primary:
Secondary:
Other:
|
|
| Notes | Power calculation for sample size: yes (200 women per group with an additional 20 to compensate for discontinuation). ITT analysis: modified: all randomized women who received at least 1 dose of rFSH. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes | High risk | Study reported as open label. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Minimal (< 10%) discontinuations and balanced between groups with similar reasons given. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
| Other bias | Low risk | Groups appeared balanced at baseline with respect to age, BMI, age at menarche, duration of infertility, cycle length, alcohol use and smoking. |
Obruca 2002.
| Methods | Parallel group study. Number of women randomized: 150 (75 in each group). Number of withdrawals: not reported. Number of women analyzed: unclear. |
|
| Participants | Country of authors: Austria. Inclusion criteria: women undergoing COS and IVF. Exclusion criteria: not reported. Mean age: not reported. |
|
| Interventions | Intervention: COCP (ethinyl oestradiol 30 μg + desogestrel 150 μg daily), started cycle day 1 for 18‐28 days (stopped on a Sunday) + rFSH (150 IU/day), started post‐treatment day 5 (= stimulation day 1) + GnRH antagonist (cetrorelix acetate 0.25 mg/day), started stimulation day 6. Control: rFSH 150 IU/day, started cycle day 3 (= stimulation day 1) + GnRH antagonist (cetrorelix acetate 0.25 mg/day), started stimulation day 6. Both rFSH and GnRH antagonist continued until final follicular maturation. |
|
| Outcomes |
|
|
| Notes | Power calculation performed: unclear. ITT analysis performed: unclear. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized," method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Number and reasons for withdrawals not reported. |
| Selective reporting (reporting bias) | High risk | All planned outcomes not reported. |
| Other bias | Unclear risk | No data on baseline characteristics reported. |
Porrati 2010.
| Methods | 2‐arm parallel group study. Number of women randomized: 150 women (75 in each group). Number of withdrawals: 4 women in intervention group; 3 in control group did not have embryo transfer. Number of women analyzed: 71 in intervention group; 72 in control group. Duration of study: 1 year of recruitment. Source of funding: Yazd IVF Centre, Yazd, Iran. |
|
| Participants | Country of authors: Argentina. Inclusion criteria: aged < 39 years, first IVF attempt, baseline FSH < 12 mlU/mL. Exclusion criteria: PCOS, prior ovarian surgery and TESE needing. Mean age: not reported. |
|
| Interventions | Intervention: COCP group: April® (ethinyl oestradiol 20 μg + levonorgestrel 100 μg) for 21 days in preceding cycle and follicular development induced using rFSH (200 IU/day) from menstrual cycle day 2‐3. Control: rFSH (200 IU/day) from menstrual cycle day 2‐3. Both groups received GnRH antagonist (cetrorelix 0.25 mg, SC) in flexible protocol starting when the leading follicle reached 14 mm continuing daily until the day of hCG administration. |
|
| Outcomes |
|
|
| Notes | Power calculation not reported. ITT: no. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportions of exclusion and reason for exclusion similar between groups. |
| Selective reporting (reporting bias) | Low risk | All reported outcomes pre‐specified in the methods section. |
| Other bias | Unclear risk | Insufficient information to make a conclusive judgement. |
Raoofi 2008.
| Methods | Academic, single‐centre, parallel group study. Number of women randomized: 54 women (number of women per group not reported). Number of withdrawals: 3 women excluded due to incomplete data. Number of women analyzed: 51. Duration of study: 1 year of recruitment. Source of funding: Yazd IVF Centre, Yazd, Iran. |
|
| Participants | Country: Iran. Inclusion criteria: women undergoing IVF and ICSI. Exclusion criteria: not reported. Mean age ± SD: intervention: 31.48 ± 5.82 years; control: 35.27 ± 4.13 years. |
|
| Interventions | Intervention: COCP (ethinyl oestradiol 30 μg + desogestrel 150 μg), on cycle days 1‐14 + GnRH agonist (triptorelin acetate depot 3.75 mg, IM) single dose on post‐treatment day 1 + hMG (FSH 75 IU + LH 75 IU), started post‐treatment day 2. Control: GnRH agonist (triptorelin acetate depot 3.75 mg, IM) single dose on cycle day 1 + hMG (FSH 75 IU + LH 75 IU), started cycle day 1. |
|
| Outcomes |
|
|
| Notes | Power calculation performed: no. ITT analysis performed: unclear. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized allocation method," method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information on reasons for withdrawals and proportions of withdrawals per treatment group. |
| Selective reporting (reporting bias) | Unclear risk | All planned outcomes not reported. |
| Other bias | Unclear risk | Number of women per group not reported. Quote: "The etiology and duration of infertility were equally distributed among the groups." No table of demographic characteristics available. |
Rombauts 2006.
| Methods | Multicentre (10 IVF centres), parallel group study. Number of women randomized: 351 (117 in each group). Number of withdrawals: 19 (5 due to spontaneous pregnancy: 2 in COCP group; 3 in GnRH antagonist group). Other reasons not reported. Number of women analyzed: 332 (111 in COCP group; 110 in GnRH antagonist group; 111 in GnRH agonist group). |
|
| Participants | Country: Australia, Denmark, Jordan and Norway.
Inclusion criteria: healthy women of infertile couples, aged 18‐39 years, BMI 18‐29 kg/m2, bodyweight ≤ 90 kg, normal menstrual cycle with a range of 24‐35 days and intra‐individual variation of ± 3 days. Exclusion criteria: contraindications for the use of gonadotrophins, endocrine abnormalities (e.g. PCOS), > 3 unsuccessful COS cycles, history of low or no ovarian response during FSH/hMG treatment, clinically relevant abnormal laboratory values (including hormones) or medical examination findings. Mean age ± SD: COCP group: 32.7 ± 3.9 years; GnRH antagonist group: 32.1 ± 3.7 years; GnRH agonist group: 32.2 ± 4.0 years. |
|
| Interventions | COCP group: COCP (ethinyl oestradiol 30 μg + desogestrel 150 μg daily), started cycle day 1 for 14‐28 days (depending on the planned started of rFSH treatment) + rFSH (follitropin beta 200 IU/day, SC), started post‐treatment day 2 (= stimulation day 1) + GnRH antagonist (ganirelix acetate 0.25 mg/day, SC), started stimulation day 5 or 6. GnRH antagonist: rFSH (follitropin beta 200 IU/day, SC), started cycle day 2 or 3 (= stimulation day 1) + GnRH antagonist (ganirelix acetate 0.25 mg/day, SC), started stimulation day 5 or 6. GnRH agonist: nafarelin acetate (0.8 mg/day, intranasal), started cycle day 21‐24 + rFSH (follitropin beta 200 IU/day, SC), started when downregulation (i.e. serum oestradiol ≤ 50 pg/mL) achieved after 2‐4 weeks of GnRH agonist treatment). After 5‐6 days of rFSH treatment, dose could be adjusted depending on the ovarian response as assessed by ultrasound. rFSH and GnRH analogues are both continued until hCG injection (10,000 IU, SC or IM), administered when ≥ 3 follicles ≥ 17 mm in diameter, or ≥ 1 follicle ≥ 20 mm in diameter. |
|
| Outcomes |
|
|
| Notes | Power calculation performed: yes. ITT analysis performed: no. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The subjects were randomly assigned..." "To improve balance, the randomization of subjects to treatment was stratified for type of infertility (primary or secondary), IVF or ICSI, centre, and age." Method not reported. |
| Allocation concealment (selection bias) | Low risk | Quote: "...by central remote allocation." |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open‐label study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportions of withdrawals fairly balanced between groups although reasons for withdrawals were not completely reported. |
| Selective reporting (reporting bias) | Low risk | Data on all planned outcomes reported. |
| Other bias | Low risk | No differences in baseline characteristics with regard to age, BMI, height and weight. |
Salat‐Baroux 1988.
| Methods | Parallel group study. 4 study arms (A1+A2 and B1+B2), of which we can only include 2 study arms (A2 and B2). Number of women randomized: 42 (21 in intervention group (A2); 21 in control group (B2)). Number of withdrawals: 13 (8 in intervention group (A2): 3 due to poorly followed treatment, 1 due to inadequate response, 2 due to spontaneous ovulation, 2 due to other reasons; 5 in control group (B2): 1 due to ovarian cyst, 4 due to inadequate response). Number of women analyzed: 29. Duration of study: 7 months of recruitment. |
|
| Participants | Country of authors: France. Inclusion criteria: infertile women scheduled for IVF treatment, aged < 38 years. Exclusion criteria: not reported. Mean age ± SD: intervention group (A1+A2): 32.8 ± 0.7 years; control group (B1+B2): 31.7 ± 0.5 years. |
|
| Interventions | Intervention (A2): progestogen (ethynodiol acetate 2 mg twice daily) for 11‐17 days, started cycle day 15 + pure FSH 4 ampoules on post‐treatment days 6‐7 and 2 ampoules on post‐treatment days 8‐9 + hMG (FSH 75 IU + LH 75 IU) 2 ampoules on post‐treatment days 10‐11. Control (B2): pure FSH 4 ampoules on cycle days 2‐3 and 2 ampoules on cycle days 4‐5 + hMG (FSH 75 IU + LH 75 IU) when needed. FSH and GnRH agonist both continued until hCG injection (10,000 IU), administration depending on follicular maturity. |
|
| Outcomes |
|
|
| Notes | Power calculation performed: no. ITT analysis performed: no. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomized," method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Proportions of, and reasons for, withdrawals not balanced between groups and data not analyzed on the basis of ITT. |
| Selective reporting (reporting bias) | High risk | All planned outcomes not reported. |
| Other bias | Low risk | Baseline demographic characteristics balanced between groups. |
Shaker 1995.
| Methods | Parallel group study. Number of women randomized: 49 (number per group not reported; 22 cycles in intervention group; 29 cycles in control group) but total number of cycles not the same as total number of women randomized. Number of withdrawals: 11 cycles (3 in intervention group: 2 due to poor response, 1 due to failure of embryo cleavage; 8 in control group: 3 due to conversion to IUI, 1 due to poor response, 2 due to failed fertilisation, 2 due to risk of OHSS). Number of women analyzed: unclear. Duration of study: 8 months of recruitment. |
|
| Participants | Country of authors: UK. Inclusion criteria: women who underwent IVF treatment cycles and had an ovarian cyst > 15 mm in diameter or an endometrial thickness > 5 mm and serum oestradiol concentration > 100 pmol/L after 14 days of GnRH agonist (buserelin acetate) treatment Exclusion criteria: relevant uterine or ovarian pathology. Mean age ± SEM: intervention group: 36.0 ± 0.86 years; control group: 35.72 ± 0.69 years. |
|
| Interventions | Intervention: GnRH agonist (buserelin acetate 500 μg/day) started cycle day 2 or 3 + progestogen (100 mg IM single dose) on cycle day 16 or 17 + hMG, started when serum oestradiol concentration ≤ 100 pmol/L. Control: GnRH agonist (buserelin acetate 500 μg/day), started cycle day 2 or 3 + hMG, started when serum oestradiol concentration ≤ 100 pmol/L. hMG start dose according to women's age, baseline serum FSH level, response to stimulation in previous treatment cycles. hMG and GnRH agonist both continued until hCG injection (10,000 IU), administered when 3 follicles ≥ 18 mm in diameter. |
|
| Outcomes |
|
|
| Notes | Power calculation performed: no. ITT analysis performed: unclear. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was done by drawing sequentially labelled sealed envelops, each containing a number obtained from a table of random numbers." |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reasons for withdrawals and proportions of participants who withdrew not reported per treatment group. |
| Selective reporting (reporting bias) | Low risk | Data on all planned outcomes reported. |
| Other bias | Low risk | No significant differences in baseline characteristics between groups with regard to age, length of infertility, number of previous IVF cycles and cause of infertility. |
Tan 2001.
| Methods | Parallel group study. Number of women randomized: 117 (number per group not reported). Number of withdrawals: not reported. Number of women analyzed: unclear. |
|
| Participants | Country of authors: Canada. Inclusion criteria: not reported. Exclusion criteria: not reported. Mean age: not reported. |
|
| Interventions | Intervention: progestogen (norethindrone) for 5 days, started cycle day 1 + GnRH agonist, start cycle day 2. Control: GnRH agonist. Timing of treatment not reported. |
|
| Outcomes |
|
|
| Notes | Power calculation performed: unclear. ITT analysis used: unclear. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No data reported. |
| Selective reporting (reporting bias) | High risk | All prespecified outcomes not reported. |
| Other bias | Unclear risk | Insufficient information to make a conclusive judgement. |
Vilela 2011.
| Methods | Parallel group study. Number of women randomized: 210 (105 to each group). Number of withdrawals: 5 (4 in intervention group; 1 in control group). Number of women analyzed: not clear; assumed 205. |
|
| Participants | Country: Argentina. Inclusion criteria: aged ≤ 39 years, first IVF attempt, basal FSH ≤ 12 mIU/mL. Exclusion criteria: PCOS, prior ovarian surgery, "TESE needing." Mean age: not reported. Setting: assume single fertility centre in Buenos Aires, Argentina. |
|
| Interventions | Intervention: COCP (ethinyl oestradiol 0.02 mg + levonorgestrel 0.1 mg) for 14/25 days in the preceding cycle. Control: no pretreatment. All women stimulated with rFSH (200 IU/day) from menstrual cycle day 2 or 3, hMG (225 UI/day) from day 4 and GnRH antagonist (cetrorelix 0.25 mg) in a flexible protocol starting with 14 mm leading follicle continuing both daily until the day of hCG. Oocyte maturation was triggered by rhCG (250 μg). Embryo transfer performed 3 days later. |
|
| Outcomes | Primary:
Other:
|
|
| Notes | Abstract with minimal data reported. Power calculation for sample size: not reported. ITT analysis: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Proportions of withdrawals fairly balanced between groups although reasons for withdrawals not reported. |
| Selective reporting (reporting bias) | Unclear risk | Outcome data not clearly reported. |
| Other bias | Unclear risk | Not clear whether groups were comparable at baseline with respect to demographic characteristics. |
Ye 2009.
| Methods | Parallel group study. Number of women randomized: 220 (109 in intervention group; 111 in control group). Number of withdrawals: none reported. Number of women analyzed: 208 cycles (103 in intervention group; 105 in control group) and numbers of cycles were equivalent to numbers of participants. |
|
| Participants | Country: China. Inclusion criteria: aged 25‐35 years; BMI 18‐25 kg/m2; number of previous IVF cycles < 3, and no previous poor response to ovarian stimulation (poor ovarian response characterised by cancellation of the cycle due to either poor follicular development or ≤ 4 cumulus‐oocyte‐complexes collected at oocyte retrieval); normal ovulatory cycles (25‐35 days); both ovaries present and normal uterus; no hormone therapy within the past 3 months; no current or past diseases affecting ovaries, gonadotrophin, sex steroid secretion, clearance or excretion. Exclusion criteria: not explicitly reported. Age range: 25‐35 years. Setting: IVF centre, China. |
|
| Interventions | Intervention: oral oestradiol valerate (4 mg/day) preceding the IVF cycle from day 21 until day 2 of next cycle before GnRH antagonist protocol. Control: standard long GnRH agonist protocol. |
|
| Outcomes |
|
|
| Notes | Outcomes measured as per 'embryo transfer cycle' but numbers of cycles transferred were equivalent to numbers of women randomized. Power calculation for sample size: not reported. ITT analysis: not reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomization allocation sequence was generated from a table of computer‐generated random numbers." |
| Allocation concealment (selection bias) | Unclear risk | Not reported. |
| Blinding (performance bias and detection bias) All outcomes | High risk | "This study was not blind." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No withdrawals reported, cycle cancellation (number of cycles were equivalent to numbers of women randomized) similar between groups. |
| Selective reporting (reporting bias) | Low risk | All prespecified outcomes reported. |
| Other bias | Low risk | Groups comparable at baseline with respect to demographic characteristics. |
17‐βE2: 17‐beta oestradiol; AMH: anti‐mullerian hormone; BMI: body mass index; COCP: combined oral contraceptive pill; COH: controlled ovarian hyperstimulation; COS: controlled ovarian stimulation; FSH: follicle‐stimulating hormone; rFSH: recombinant follicle‐stimulating hormone; GnRH: gonadotrophin‐releasing hormone; GnRHa: gonadotrophin‐releasing hormone analogue; hMG: human menopausal gonadotrophin; IM: intramuscular; ITT: intention to treat; IU: international unit; IUI: intrauterine insemination; IVF: in vitro fertilisation; IVF‐ET: in vitro fertilisation with embryo transfer; LH: luteinising hormone; OCP: oral contraceptive pill; OHSS: ovarian hyperstimulation syndrome; PCOS: polycystic ovary syndrome; PO: per os (oral); rhCG: recombinant human chorionic gonadotropin; SC: subcutaneous; SD: standard deviation; SEM: standardized mean difference; TESE: testicular epididymal sperm extraction; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aghahosseini 2011 | Randomisation status unknown. |
| al‐Mizyen 2000 | Not an RCT. Retrospective study. |
| Anderson 1990 | Not an RCT. Women only received controlled ovarian stimulation, but no embryo transfer as part of an ART cycle. |
| Bakas 2014 | Did not state that allocation randomised. |
| Bellver 2007 | Not an RCT. Retrospective study. |
| Benadiva 1988 | Not an RCT. |
| Bendikson 2006 | Not an RCT. Retrospective study. |
| Biljan 1998c | Not an RCT. Retrospective study. Each woman served as her own control. |
| Branigan 1998 | Not an RCT. Each woman served as her own control. |
| Brodt 1993 | Not an RCT. Single arm study. |
| Chung 2006 | Not an RCT. Retrospective study. Monophasic OCP vs triphasic OCP. |
| Cohen 1987 | Not an RCT. |
| Copperman 2003 | Not an RCT. |
| Couzinet 1995 | Not an RCT. Naltrexone used in treatment protocol. |
| Cédrin‐Durnerin 1995 | Not an RCT. |
| Cédrin‐Durnerin 1996 | Cross‐over design with no pre‐cross‐over data. |
| Damario 1997 | Not an RCT. Retrospective study. |
| Davar 2014 | RCT status unclear. Number of women randomised at baseline not stated. |
| Davy 2004 | Compared different durations of COCP pretreatment. |
| De Ziegler 1999 | Not an RCT. Open single‐arm study. |
| Dickey 2001 | Not an RCT. Retrospective study. |
| Ditkoff 1997 | Not an RCT. |
| Doody 2001 | Not an RCT. Women were oocyte donors. Compared different durations of COCP pretreatment. |
| Duvan 2008 | Not an RCT. Retrospective study. |
| Engels 2011 | Number of women randomised or analysed in each treatment group not reported. |
| Fanchin 2001 | Cross‐over design with no pre‐cross‐over data. |
| Fanchin 2003b | Not an RCT. Each woman served as her own control. Women only received controlled ovarian stimulation, but no embryo transfer as part of an ART cycle. |
| Feichtinger 1991 | Randomised comparison of different types of COCP. |
| Fisch 1996 | Not an RCT. Each woman served as her own control. |
| Forman 1991 | Not an RCT. |
| Frederick 2004 | Not an RCT. Retrospective study. |
| Frydman 1986 | Not an RCT. |
| Galera 2004 | Not an RCT. |
| Gerli 1989 | Not an RCT. Single‐arm study. |
| Ghanem 2015 | Intervention not relevant: luteal phase support. |
| Godin 2003 | Not an RCT. |
| Gomez 2000 | Compared 2 different ways of administration of oestrogen. |
| Gonen 1990 | Not an RCT. Clomiphene citrate used in treatment protocol. |
| Gonzalez 1995 | Not an RCT. Retrospective study. |
| Greco 2016 | Intervention not relevant: OCP used for endometrial preparation before embryo transfer and used as luteal phase support. |
| Guivarc'h‐Levêque 2009 | Quasi‐randomised |
| Haydardedeoglu 2012 | Both groups received COCP pretreatment, no control group. |
| Hugues 1992 | Not an RCT. Single‐arm study. |
| Jung 2000 | Oestrogen pretreatment not stopped before oocyte retrieval, but also used as luteal phase support. |
| Karande 2004 | Compared 2 different ways of administration of a combined contraceptive (Nuvaring vs oral Desogen). |
| Keltz 2007 | Not an RCT. Retrospective study. |
| Kovacs 2001 | Not an RCT. Retrospective study. |
| Kreiner 2007 | Interventions not relevant (OCP vs OCP). |
| Leondires 1999 | Not an RCT. Retrospective study. |
| Letterie 2000 | Women only received oestrogen plus progestogen, but no gonadotrophins or GnRH analogues as part of an ART cycle. Compared 2 different timings of administration. |
| Lewin 2002 | Compared 2 different doses of oestrogen treatment for endometrial preparation. |
| Lindheim 1996 | Not an RCT. |
| Liu 2011 | Interventions not relevant (OCP vs Nuvaring with similar contents as OCP). |
| Loutradis 2003 | Not an RCT. Retrospective study. |
| Martinez 2006 | Women were oocyte donors. |
| Mashiach 1989 | Compared different durations of COCP pretreatment. |
| Meldrum 2002 | Not an RCT. Open‐label single‐arm study. |
| Meldrum 2008 | Not an RCT. Open‐label single‐arm study. |
| Merviel 2015 | Alternate randomisation was used. |
| Min 2005 | Not an RCT. Retrospective study. |
| Mirkin 2003 | Not an RCT. Retrospective study. |
| Mulangi 1997 | Not an RCT. Each woman served as her own control. |
| Neal 1993 | Not an RCT. |
| Pados 1995 | Not an RCT. Retrospective study. Prednisolone used in treatment protocol. |
| Palomba 2008 | Not an RCT. |
| Pinkas 2008 | Not an RCT. Retrospective study. |
| Ramsewak 2005 | Not an RCT. Retrospective study. |
| Rashidi 2011 | Intervention not relevant: luteal phase support. |
| Russell 1997 | Compared different doses and timings of oestrogen pretreatments. |
| Sanghvi 2002 | Not an RCT. Retrospective study. Single‐arm study. |
| Schoolcraft 1997 | Not an RCT. |
| Steinkampf 1991 | Women only received ovulation induction, no embryo transfer as part of an ART cycle. |
| Surrey 1989 | Not an RCT. |
| Surrey 1998 | Not an RCT. Each woman served as her own control. |
| Talebian 2004 | Not an RCT. Retrospective study. |
| Talebian 2007 | Not an RCT. Retrospective study. |
| Tarlatzis 1993 | Not an RCT. |
| Tartagni 2007 | Women had premature ovarian failure. |
| Tehraninejad 2010 | Interventions not relevant (OCP vs OCP) |
| Wang 2008 | Cross‐over design with no pre‐cross‐over data. |
| Wang 2016 | Not a true RCT: randomisation according to alternate number allocation; intervention not relevant: co‐administration of medroxyprogesterone acetate with the stimulation agent. |
| Wei 2016 | Design not relevant: retrospective cohort study. |
| Weisman 1989 | Not an RCT. |
| Yokota 2006 | Not an RCT. |
| Yoshida 2005 | Not an RCT. Retrospective study. |
| Youssef 2017 | Not relevant: compared mild vs standard stimulation (with pretreatment in the mild group). |
| Zhao 2008 | Not an RCT. Retrospective study. |
ART: assisted reproductive technique; COCP: combined oral contraceptive pill; OCP: oral contraceptive pill; GnRH: gonadotrophin‐releasing hormone; RCT: randomised controlled trial.
Differences between protocol and review
The biggest change between the protocol and the review is the formation of different subgroups. In the protocol, we described that we would perform subgroup analyses on women's age; poor response; agonist long, short and ultra‐short protocol; and the duration of pretreatment. After examining the included studies, we decided it would make more sense to perform subgroup analyses on the type of GnRH analogue used in the treatment cycles. After this, we could not perform any more subgroup analysis on the planned regimens, because there were not enough studies per subgroup. Furthermore, we did not perform any sensitivity analyses due to the small number of included studies per subgroup.
Other minor changes to this review included: the exclusion of oocyte donors as participants, we rewrote the interventions to make them more understandable, we changed the outcome 'ovarian cysts per woman randomized' to 'number of women with ovarian cysts' and we removed a few items of data extraction because we thought they were less important. We were unable to perform a funnel plot because of the limited number of included studies to each subgroup.
We combined live births and ongoing pregnancies as one outcome measure in the review against two separate outcome measures in the protocol.
Contributions of authors
For the 2017 update:
ROA: selected studies, extracted and analyzed data, contacted authors of trials and drafted the results, discussion and authors' conclusions of the review.
AL: selected studies, extracted and analyzed data, and contributed to the discussion and conclusions.
CF: acted as third review author to resolve differences, acted as a clinical expert and commented on the review.
LR: acted as a clinical expert and commented on the review.
JK: acted as a clinical expert and commented on the review.
For the 2009 update:
Brechtje Smulders and Sanne van Oirschot contributed equally to the review.
Brechtje Smulders drafted the Background and Objectives of the review, and performed the search, selected the studies, extracted and analyzed the data, contacted the authors of trials and drafted the Results, Discussion and Authors' conclusions of the review together with Sanne M van Oirschot. BS also drafted half of the tables of Characteristics of included studies and drafted Table 1, Table 2 and Table 3.
Sanne M van Oirschot drafted the Methods of the review, and performed the search, selected the studies, extracted and analyzed the data, contacted the authors of trials and drafted the Results, Discussion and Authors' conclusions of the review together with Brechtje Smulders. SvO also drafted half of the tables of Characteristics of included studies and all the tables of Characteristics of excluded studies.
Cindy Farquhar helped to solve differences of opinion as a third review author, commented on the review and helped with drafting the Discussion and Authors' conclusions of the review.
Luk Rombauts acted as a clinical expert and commented on the review.
Jan Kremer acted as a clinical expert and commented on the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Stichting Nijmeegs Universiteitsfonds (SNUF), Netherlands.
Scholarship to support students of the University of Nijmegen to do a study, internship or research outside The Netherlands.
-
CVSB (Commissie Voorzieningen Studenten Budget), Netherlands.
Compensation for studying outside The Netherlands.
Declarations of interest
LR was the first author of a randomized trial about oral contraceptive pretreatment (Rombauts 2006). This study was sponsored by Organon/Schering Plough. He was not involved in selection of that study or in extraction and interpretation of data from it. He is a minority shareholder in an IVF unit that has received research grants from MSD, Merck‐Serono and Ferring. He has received educational grants from MSD, Merck‐Serono and Ferring and provided consultancy services and board membership to Ferring Australia.
ROA, AL, CF and JK have no interests to declare.
Edited (no change to conclusions)
References
References to studies included in this review
Aston 1995 {published data only}
- Aston K, Arthur I, Masson GM, Jenkins JM. Progestogen therapy and prevention of functional ovarian cysts during pituitary desensitisation with GnRH agonists. British Journal of Obstetrics and Gynaecology 1995;102:835‐7. [DOI] [PubMed] [Google Scholar]
Biljan 1998b {published data only}
- Biljan MM, Mahutte NG, Dean N, Hemmings R, Bissonnette F. Prospective randomized trial on effect of pre‐treatment with an oral contraceptive (OC) on the length of time required for pituitary suppression by gonadotropin releasing hormone agonist (GnRH‐a) and subsequent implantation and pregnancy rates. Fertility and Sterility 1998; Vol. Suppl 1, 70, issue 3:S133. [DOI] [PubMed]
- Biljan MM, Mahutte NG, Dean N, Hemmings R, Bissonnette F, Tan SL. Effects of pretreatment with an oral contraceptive on the time required to achieve pituitary suppression with gonadotropin‐releasing hormone analogues and on subsequent implantation and pregnancy rates. Fertility and Sterility 1998;70(6):1063‐9. [DOI] [PubMed] [Google Scholar]
Blockeel 2012 {published data only}
- Blockeel C, Engels S, Vos M, Haentjens P, Polyzos NP, Stoop D, et al. Oestradiol valerate pretreatment in GnRH antagonist cycles: a randomised controlled trial. Reproductive Biomedicine Online 2012;24:272‐80. [DOI] [PubMed] [Google Scholar]
Cédrin‐Durnerin 2007 {published data only}
- Cédrin‐Durnerin I, Bständig B, Parneix I, Bied‐Damon V, Avril C, Decanter C, et al. Effects of oral contraceptive, synthetic progestogen or natural estrogen pre‐treatments on the hormonal profile and the antral follicle cohort before GnRH antagonist protocol. Human Reproduction 2007;22(1):109‐16. [DOI: 10.1093/humrep/de1340] [DOI] [PubMed] [Google Scholar]
- Hugues JN, Cédrin‐Durnerin I, Bständig B, Parneix I, Bied‐Damon V, Avril C, et al. Consequences of different steroid pre‐treatments on the hormonal profile and on the antral follicular count prior to an IVF antagonist protocol. 21st Annual Meeting of the ESHRE; 2005 Jun 19‐22; Copenhagen, Denmark. 2005.
Cédrin‐Durnerin 2012 {published data only}
- Cédrin‐Durnerin I, Guivarc'h‐Leveque A, Hugues J‐N. Pretreatment with estrogen does not affect IVF‐ICSI cycle outcome compared with no pretreatment in GnRH antagonist protocol: a prospective randomised trial. Fertility and Sterility 2012;97(6):1359‐64. [DOI] [PubMed] [Google Scholar]
Daly 2002 {published data only}
- Daly DC, Daly CL, Mayo D, Jacobs A. Oocyte recruitment in IVF patients with limited ovarian reserve (LOR), maximizing quality of oocytes and embryos. Fertility and Sterility 2002; Vol. Suppl 1, 78, issue 3:S148‐9.
Ditkoff 1996 {published data only}
- Ditkoff EC, Sauer MV. A combination of norethindrone acetate and leuprolide acetate blocks the gonadotrophin‐releasing hormone agonistic response and minimizes cyst formation during ovarian stimulation. Human Reproduction 1996;11(5):1035‐7. [DOI] [PubMed] [Google Scholar]
Engmann 1999 {published data only}
- Engmann L, Maconochie N, Bekir J, Tan SL. A prospective randomized study to assess the effect of progestogen therapy during pituitary desensitization with GnRH agonist in the prevention of functional ovarian cyst formation. Abstracts from ASRM/CFAS Conjoint Annual Meeting. Toronto, Canada, 1999. Fertility and Sterility 1999;72(3, Suppl 1):S10‐1. [Google Scholar]
- Engmann L, Maconochie N, Bekir J, Tan SL. Progestogen therapy during pituitary desensitization with gonadotropin‐releasing hormone agonist prevents functional ovarian cyst formation: a prospective, randomized study. American Journal of Obstetrics and Gynecology 1999;181(3):576‐82. [DOI] [PubMed] [Google Scholar]
- Engmann L, Maconochie N, Tan SL, Bekir J. Progestogen therapy during pituitary desensitization with GnRH agonist prevents functional ovarian cyst formation ‐ a prospective randomized study. Human Fertility. 1999; Vol. 2:190‐1. [DOI] [PubMed]
Fanchin 2003 {published data only}
- Fanchin R, Salomon L, Castelo‐Branco A, Olivennes F, Frydman N, Frydman R. Luteal estradiol administration coordinates FSH‐induced follicular growth and improves the outcome of GnRH antagonist COH protocols. 19th Annual meeting of the ESHRE; 2003 Jun 29‐Jul 2; Madrid, Spain, 2003. 2003.
- Fanchin R, Salomon L, Castelo‐Branco A, Olivennes F, Frydman N, Frydman R. Luteal estradiol pre‐treatment coordinates follicular growth during controlled ovarian hyperstimulation with GnRH antagonists. Human Reproduction 2003;18(12):2698‐703. [DOI: 10.1093/humrep/deg516] [DOI] [PubMed] [Google Scholar]
Franco Jr 2003 {published data only}
- Franco JG Jr, Baruffi RLR, Petersen CG, Mauri AL, Felipe V, Contart P. Comparison of ovarian stimulation with recombinant FSH after 2nd phase protocols with GnRH analogs (I ‐ estradiol + Ganirelix versus II ‐ Nafarelin) [Comparação da estimulação ovariana com FSH recombinante após protocolo de 2a fase com análogos do GnRH (I ‐ estradiol + Ganirelix versus II ‐ Nafarelin)]. Jornal Brasileiro de Reproducao Assistida 2003;7(1):26‐32. [Google Scholar]
Garcia‐Velasco 2011 {published data only}
- Garcia‐Velasco JA, Bermejo A, Ruiz F, Martinez‐Salazar J, Requena A, Pellicer A. Cycle scheduling with oral contraceptive pills in the GnRH antagonist protocol vs the long protocol: a randomized, controlled trial. Fertility and Sterility 2011;96(3):590‐3. [DOI] [PubMed] [Google Scholar]
- Garcia‐Velasco JA, Bermejo A, Ruiz‐Flores F, Martinez‐Salazar J, Requena A, Pellicer A. Cycle scheduling with oral contraceptive pills in GnRH antagonist protocol vs long protocol: a randomized, controlled trial. Fertility and Sterility 2010;94 Suppl 1(4):S28 Abstract no. O‐93. [DOI] [PubMed] [Google Scholar]
Hauzman 2013 {published data only}
- Hauzman EE, Zapata A, Bermejo A, Iglesias C, Pellicer A, Garcia‐Velasco J. Cycle scheduling for in vitro fertilisation with oral contraceptive pills versus oral estradiol valerate: a randomised controlled trial. Reproductive Biology and Endocrinology 2013;11:96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hugues 1994 {published data only}
- Hugues JN, Cédrin‐Durnerin I, Hervé F, Huet‐Pecqueux L, Santarelli J. Incidence of preprogrammed norethisterone on the intensity of 'flare up' (flush) caused by GnRH agonist according to the IVF protocol [poster] [Incidence d'une préprogrammation par la noréthistérone sur la qualité du flare‐up induit par un agoniste du GnRH en protocole court de FIV]. Contraception, Fertilite, Sexualite 1994; Vol. 22, issue 5:331.
Huirne 2006a {published data only}
- Huirne JAF, Loenen ACD, Donnez J, Pirard C, Homburg R, Schats R, et al. Effect of an oral contraceptive pill on follicular development in IVF/ICSI patients receiving a GnRH antagonist: a randomized study. Reproductive Biomedicine Online 2006;13(2):235‐45. [DOI] [PubMed] [Google Scholar]
- Loenen ACD, Huirne JAF, Schats R, Donnez J, Lambalk CB. An open‐label multicentre, randomized, parallel, controlled phase II study to assess the feasibility of a new programming regimen using an oral contraceptive prior to the administration of recombinant FSH and a GnRH‐antagonist in patients undergoing ART (IVF‐ICSI) treatment. Human Reproduction. 2002; Vol. 17 Abstract Book 1:144‐5.
Huirne 2006c {published data only}
- Huirne JA, Hugues JN, Pirard C, Fischl F, Sage JC, Pouly JL, et al. Cetrorelix in an oral contraceptive‐pretreated stimulation cycle compared with buserelin in IVF/ICSI patients treated with r‐hFSH: a randomized, multicentre, phase IIIb study. Human Reproduction 2006;21(6):1408‐15. [DOI: 10.1093/humrep/de1030] [DOI] [PubMed] [Google Scholar]
Hwang 2004 {published data only}
- Hwang JL, Seow KM, Lin YH, Huang LW, Hsieh BC, Tsai YL, et al. Ovarian stimulation by concomitant administration of cetrorelix acetate and HMG following Diane‐35 pre‐treatment for patients with polycystic ovary syndrome: a prospective randomized study. Human Reproduction 2004;19(9):1993‐2000. [DOI: 10.1093/humrep/deh375] [DOI] [PubMed] [Google Scholar]
Kim 2011 {published data only}
- Kim C‐H, Jeon G‐H, Cheon Y‐P, Jeon I, Kim S‐H, Chae H‐D, et al. Comparison of GnRH antagonist protocol with or without oral contraceptive pill pretreatment and GnRH agonist low‐dose long protocol in low responders undergoing IVF/intracytoplasmic sperm injection. Fertility and Sterility 2009;92(5):1758‐60. [DOI] [PubMed] [Google Scholar]
- Kim C‐H, You R‐M, Kang H‐J, Ahn J‐W, Lee J‐I, Kim S‐H, et al. GnRH antagonist multiple dose protocol with oral contraceptive pill pre‐treatment in poor responders undergoing IVF/ICSI. Clinical and Experimental Reproductive Medicine 2011;38(4):228‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim CH, Lee HA, Lee JW, Lee YJ, Nah HY, Hong SH, et al. The efficacy of oral contraceptive pretreatment in controlled ovarian hyperstimulation using a GnRH antagonist for low responders [poster]. Human Reproduction. 2005; Vol. 20 Suppl 1:i105‐6.
Kolibianakis 2006 {published data only}
- Kolibianakis EM, Albano C, Tournaye H, Camus M, Steirteghem A, Devroey P. Pre‐treatment with oral contraceptive pill affects adversely implantation rate in IVF/ICSI cycles stimulated with rec‐FSH and GnRH antagonists. Fertility and Sterility 2003; Vol. 80, issue Suppl 3:S67.
- Kolibianakis EM, Papanikolaou EG, Camus M, Tournaye H, Steirteghem AC, Devroey P. Effect of oral contraceptive pill pretreatment on ongoing pregnancy rates in patients stimulated with GnRH antagonists and recombinant FSH for IVF. A randomized controlled trial. Human Reproduction 2006;21(2):352‐7. [DOI: 10.1093/humrep/dei348] [DOI] [PubMed] [Google Scholar]
Lukaszuk 2015 {published data only}
- Lukaszuk K, Liss J, Kunicki M, Kuczynski W, Pastuszek E, Jakiel G, et al. Estradiol valerate pretreatment in short protocol GNRH‐agonist cycles versus combined pretreatment with oral contraceptive pills in long protocol GNRH‐agonist cycles: a randomised controlled trial. BioMed Research International 2015;2015:1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Nyboe Andersen 2011 {published data only}
- Broekmans JF, Verweij PJM, Eijkemans MJC, Mannaerts NMJL, Witjes H. Prognostic models for high and low ovarian responses in controlled ovarian stimulation using a GnRH antagonist protocol. Human Reproduction 2014;29(8):1688‐97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyboe Andersen A, Witjes H, Gordon K, Mannaerts B. Predictive factors of ovarian response and clinical outcome after IVF/ICSI following a rFSH/GnRH antagonist protocol with or without oral contraceptive pretreatment. Human Reproduction 2011;26(12):3413‐23. [DOI] [PubMed] [Google Scholar]
- Tavmergen E, von Mauw, Witjes H, Mannaerts B. Outcome of a trial to identify predicative factors for ovarian response in a GnRH antagonist protocol with or without oral contraceptive scheduling. 25th Annual Meeting of ESHRE; 2009 28 Jun‐1 Jul; Amsterdam, the Netherlands. 2009.
Obruca 2002 {published data only}
- Obruca A, Fischl F, Huber J. Programming oocyte retrieval using oral contraceptive pretreatment before ovarian stimulation with a GnRH antagonist (Cetrotide) protocol. Human Reproduction. 2002; Vol. 17 Abstract Book 1:89.
Porrati 2010 {published data only}
- Porrati L, Vilela M, Viglierchio MI, Valcarcel A, Lombardi E, Marconi G. Oral contraceptive pretreatment achieves better pregnancy rates in IVF antagonists GnRH flexible protocols: a prospective randomized study. Human Reproduction 2010;25 Suppl 1(6):i102 Abstract no. O‐259. [Google Scholar]
Raoofi 2008 {published data only}
- Raoofi Z, Aflatoonian A. Ovarian cysts formation during depot formulation of GnRH‐a therapy and the effect of pretreatment with oral contraceptive pills on subsequent implantation and pregnancy rate in ART cycles. Iranian Journal of Pharmaceutical Research 2008;7(2):109‐13. [Google Scholar]
Rombauts 2006 {published data only}
- Rombauts L, Healy D, Norman RJ, Speirs A, Watkins B, Yovich J, et al. A comparative randomized trial to assess the impact of oral contraceptive pretreatment on follicular growth and hormone profiles in GnRH antagonist‐treated patients. Human Reproduction 2006;21(1):95‐103. [DOI: 10.1093/humrep/dei302] [DOI] [PubMed] [Google Scholar]
Salat‐Baroux 1988 {published data only}
- Salat‐Baroux J, Antoine JM, Alvarez S, Cornet D, Tibi C, Mandelbaum J, et al. Programmed ovulation induction and oocyte retrieval for in vitro fertilization. Journal of In Vitro Fertilization and Embryo Transfer 1988;5(3):153‐7. [DOI] [PubMed] [Google Scholar]
Shaker 1995 {published data only}
- Shaker AG, Pittrof R, Zaidi J, Bekir J, Kyei‐Mensah A, Tan SL. Administration of progestogens to hasten pituitary desensitization after the use of gonadotropin‐releasing hormone agonist in in vitro fertilization ‐ a prospective randomized study. Fertility and Sterility 1995;64(4):791‐5. [DOI] [PubMed] [Google Scholar]
Tan 2001 {published data only}
- Tan SL, Biljan M. Fixed scheduling of long protocol of GNRH agonists ‐ use of oral contraceptive (OC) or progestogen pre‐treatment. Gynecological Endocrinology. 2001; Vol. 15 Suppl 2:31.
Vilela 2011 {unpublished data only}
- Vilela M, Marconi M, Zappacosta MP, Porrati L, Valcarcel A, Marconi G. Oral contraceptive (OcP) pretreatment achieves better pregnancy rates in in vitro fertilisation (IVF) antagonists GnRH flexible protocols: a prospective randomised study. Fertility and Sterility 2011; Vol. 96, issue 3 (supplement):S252, P‐492.
Ye 2009 {published data only}
- Ye H, Huang G‐N, Zeng P‐H, Pei L. IVF/ICSI outcomes between cycles with luteal estradiol (E2) pre‐treatment before GnRH antagonist protocol and standard long GnRH agonist protocol: a prospective and randomized study. Journal of Assisted Reproduction and Genetics 2009;26(2‐3):105‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Aghahosseini 2011 {published data only}
- Aghahosseini M, Aleyassin A, Khodaverdi S, Esfahani F, Mohammadbeigi R, Movahedi S, et al. Estradiol supplementation during the luteal phase in poor responder patients undergoing in vitro fertilization: a randomized clinical trial. Journal of Assisted Reproduction and Genetics 2011;28:785‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
al‐Mizyen 2000 {published data only}
- al‐Mizyen E, Sabatini L, Lower AM, Wilson CMY, al‐Shawaf T, Grudzinskas JG. Does pretreatment with progestogen or oral contraceptive pills in low responders followed by the GnRHa flare protocol improve the outcome of IVF‐ET?. Journal of Assisted Reproduction and Genetics 2000;17(3):140‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Anderson 1990 {published data only}
- Anderson RE, Stein AL, Paulson RJ, Stanczyk FZ, Vijod AG, Lobo RA. Effects of norethindrone on gonadotropin and ovarian‐steroid secretion when used for cycle programming during in vitro fertilization. Fertility and Sterility 1990;54(1):96‐101. [DOI] [PubMed] [Google Scholar]
Bakas 2014 {published data only}
- Bakas P, Hassiakos D, Grigoriadis C, Vlahos NF, Liapis A, Creatsas G. Effect of a low dose combined oral contraceptive pill on the hormonal profile and cycle outcome following COS with a GnRH antagonist protocol in women over 35 years old. Gynecological Endocrinology 2014;30(11):825‐9. [DOI] [PubMed] [Google Scholar]
Bellver 2007 {published data only}
- Bellver J, Albert C, Labarta E, Pellicer A. Early pregnancy loss in women stimulated with gonadotropin‐releasing hormone antagonist protocols according to oral contraceptive pill pretreatment. Fertility and Sterility 2007;87(5):1098‐101. [DOI: 10.1016/j.fertnstert.2006.08.098] [DOI] [PubMed] [Google Scholar]
Benadiva 1988 {published data only}
- Benadiva CA, Ben‐Rafael Z, Blasco L, Tureck R, Mastroianni LJ, Flickinger GL. Ovarian response to human menopausal gonadotropin following suppression with oral contraceptives. Fertility and Sterility 1988;50(3):516‐8. [PubMed] [Google Scholar]
Bendikson 2006 {published data only}
- Bendikson K, Milki AA, Speck‐Zulak A, Westphal LM. Comparison of GnRH antagonist cycles with and without oral contraceptive pretreatment in potential poor prognosis patients. Clinical and Experimental Obstetrics and Gynecology 2006;33(3):145‐7. [PubMed] [Google Scholar]
Biljan 1998c {published data only}
- Biljan MM, Mahutte NG, Dean N, Hemmings R, Bissonnette F, Tan SL. Pretreatment with an oral contraceptive is effective in reducing the incidence of functional ovarian cyst formation during pituitary suppression by gonadotropin‐releasing hormone analogues. Journal of Assisted Reproduction and Genetics 1998;15(10):599‐604. [DOI] [PMC free article] [PubMed] [Google Scholar]
Branigan 1998 {published data only}
- Branigan EF, Estes MA. Treatment of high responders with oral contraceptive pills (OC) before ovarian hyperstimulation for IVF. Fertility and Sterility 1998; Vol. 69, issue 3:599.
Brodt 1993 {published data only}
- Brodt J, Taubert HD. Planned ovarian stimulation with preadministered contraceptives in an in‐vitro fertilization program [Programmierte ovarielle stimulation mittels ovulationshemmer‐vorbehandlung in einem in vitro fertilisations(IVF)‐programm]. Archives of Gynecology and Obstetrics 1993;254(1‐4):246‐8. [Google Scholar]
Cédrin‐Durnerin 1995 {published data only}
- Cédrin‐Durnerin I, Hervé F, Huet‐Pecqueux L, Kottler ML, Hugues JN. Progestogen pretreatment in the short‐term protocol does not affect the prognostic value of the oestradiol flare‐up in response to a GnRH agonist. Human Reproduction 1995;10(11):2904‐8. [DOI] [PubMed] [Google Scholar]
Cédrin‐Durnerin 1996 {published data only}
- Cédrin‐Durnerin I, Bulwa S, Hervé F, Martin‐Pont B, Uzan M, Hugues JN. The hormonal flare‐up following gonadotrophin‐releasing hormone agonist administration is influenced by a progestogen pretreatment. Human Reproduction 1996;11(9):1859‐63. [DOI] [PubMed] [Google Scholar]
- Cédrin‐Durnerin I, Bulwa S, Hervé F, Pecqueux L, Uzan M, Hugues JN. Influence of norethisterone pretreatment on the initial flare‐up induced by a gonadotrophin‐releasing hormone agonist in a short protocol for IVF. Human Reproduction 1995;10 Abstract Book 2:116. [Google Scholar]
Chung 2006 {published data only}
- Chung MT, Tsai YC, Chen SH, Loo TC, Tang HH, Lin LY. Influence of pituitary suppression with triphasic or monophasic oral contraceptives on the outcome of in vitro fertilization and embryo transfer. Journal of Assisted Reproduction and Genetics 2006;23:343‐6. [DOI: 10.1007/s10815-006-9056-y] [DOI] [PubMed] [Google Scholar]
Cohen 1987 {published data only}
- Cohen J, Debache C, Solal P, Serkine AM, Achard B, Boujenah A, et al. Results of planned in‐vitro fertilization programming through the pre‐administration of the oestrogen‐progesterone combined pill. Human Reproduction 1987;2(1):7‐9. [DOI] [PubMed] [Google Scholar]
Copperman 2003 {published data only}
- Copperman AB, Mukherjee T, Sandler B, Grunfeld L, Bergh PA, Scott RT. Pre‐treatment with oral contraceptive pill improves outcome in IVF cycles of poor‐responders using the GnRH antagonist. Fertility and Sterility 2003; Vol. Suppl 3, 80:S108.
Couzinet 1995 {published data only}
- Couzinet B, Young J, Brailly S, Chanson P, Schaison G. Even after priming with ovarian steroids or pulsatile gonadotropin‐releasing hormone administration, naltrexone is unable to induce ovulation in women with functional hypothalamic amenorrhoea. Journal of Clinical Endocrinology and Metabolism 1995;80(7):2102‐7. [DOI] [PubMed] [Google Scholar]
Damario 1997 {published data only}
- Damario MA, Barmat L, Liu HC, Davis OK, Rosenwaks Z. Dual suppression with oral contraceptives and gonadotrophin releasing‐hormone agonists improves in‐vitro fertilization outcome in high responder patients. Human Reproduction 1997;12(11):2359‐65. [DOI] [PubMed] [Google Scholar]
Davar 2014 {published data only}
- Davar R, Rahsepar M, Rahmani E. Outcome of a trial to identify predicative factors for ovarian response in a GnRH antagonist protocol with or without oral contraceptive scheduling. Iranian Journal of Reproductive Medicine 2014;12 Suppl 1(6):71. [Google Scholar]
Davy 2004 {published data only}
- Davy C, Guibert J, Blanchet V, Nataf E, Olivennes F. The 'wash out' period do not create asynchrony of the follicle cohort when contraceptive pill is used to program GnRH antagonist IVF cycles. Human Reproduction. 2004; Vol. 19 Suppl 1:i5‐6.
- Olivennes F Sr, Davy C, Guibert J, Nataf E, Blanchet V. Asynchrony of the follicle cohort is not induced by the “wash out” period when contraceptive pill is used to program GnRH antagonist IVF cycles. Fertility and Sterility 2004; Vol. Suppl 2, 82:S234.
De Ziegler 1999 {published data only}
- Ziegler D, Brioschi PA, Benchaa C, Campana A, Ditesheim PJ, Fanchin R, et al. Programming ovulation in the menstrual cycle by a simple innovative approach: back to the future of assisted reproduction. Fertility and Sterility 1999;72(1):77‐82. [DOI] [PubMed] [Google Scholar]
Dickey 2001 {published data only}
- Dickey RP, Sartor BM, Taylor SN, Lu PY, Rye PH, Pyrzak R. Oral contraceptives, not GnRH suppression, may be responsible for very low endogenous LH during IVF cycles. Fertility and Sterility 2001; Vol. Suppl 3, 76, issue 3:S237.
Ditkoff 1997 {published data only}
- Ditkoff EC, Prosser R, Zimmermann RC, Lindheim S, Sauer MV. The addition of norethindrone acetate to leuprolide acetate for ovarian suppression has no adverse effect on ovarian stimulation. Journal of Assisted Reproduction and Genetics 1997;14(2):92‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Doody 2001 {published data only}
- Doody KJ, Langley M, Marek D, Doody K. Oral contraceptive pre‐treatment for IVF cycles employing recombinant FSH and a GnRH antagonist. Fertility and Sterility 2001; Vol. Suppl 1, 76, issue 3:S236.
Duvan 2008 {published data only}
- Duvan CI, Berker B, Turhan NO, Satiroglu H. Oral contraceptive pretreatment does not improve outcome in microdose gonadotrophin‐releasing hormone agonist protocol among poor responder intracytoplasmic sperm injection patients. Journal of Assisted Reproduction and Genetics 2008;25:89‐93. [DOI: 10.1007/s10815-008-9203-8] [DOI] [PMC free article] [PubMed] [Google Scholar]
Engels 2011 {published data only}
- Engels S, Blockeel C, Haentjens P, Vos M, Camus M, Devroey P. How to avoid oocyte retrievals during weekend days using a GnRH antagonist protocol? A randomised controlled trial. Human Reproduction 2011;26 Suppl 1:i308 Abstract no: P‐480. [Google Scholar]
Fanchin 2001 {published data only}
- Fanchin R, Schonauer L, Cunha Filho J, Kadoch I, Cohen Bacrie P, Frydman R. Luteal E2 administration reduces size and improves homogeneity of selectable follicles on cycle‐day 3: bases for novel controlled ovarian hyperstimulation (COH) concepts. Fertility and Sterility 2001; Vol. Suppl 1, 76, issue 3:S90.
Fanchin 2003b {published data only}
- Fanchin R, Cunha Filho J, Schonauer L, Kadoch I, Cohen Bacri P, Frydman R. Co‐ordination of early antral follicles by luteal estradiol administration: basis for novel controlled ovarian hyperstimulation. Human Reproduction. 2002; Vol. 17 Abstract Book 1:88‐9.
- Fanchin R, Cunha‐Filho JS, Schonauer LM, Kadoch IJ, Cohen‐Bacri P, Frydman R. Coordination of early antral follicles by luteal estradiol administration provides a basis for alternative controlled ovarian hyperstimulation regimens. Fertility and Sterility 2003;79(2):316‐21. [DOI] [PubMed] [Google Scholar]
Feichtinger 1991 {published data only}
- Feichtinger W, Babor P, Herczeg KM, Heyda M, Hoffmann R, Plockinger B, et al. Side effects of oral contraceptives in the first treatment cycle. Weiner Medizinische Wochenschrift 1991;141(18‐19):412‐5. [PubMed] [Google Scholar]
Fisch 1996 {published data only}
- Fisch B, Royburt M, Pinkas H, Avrech OM, Goldman GA, Bar J, et al. Augmentation of low ovarian response to superovulation before in vitro fertilization following priming with contraceptive pills. Israel Journal of Medical Sciences 1996;32(12):1172‐6. [PubMed] [Google Scholar]
Forman 1991 {published data only}
- Forman RG, Demouzon J, Feinstein MC, Testart J, Frydman R. Studies on the influence of gonadotrophin‐levels in the early follicular phase on the ovarian response to stimulation. Human Reproduction 1991;6(1):113‐7. [DOI] [PubMed] [Google Scholar]
Frederick 2004 {published data only}
- Frederick J, DaCosta V, Wynter S, Reid M, Frederick C, McKenzie C. Effect of the oral contraceptive pill on patients undergoing controlled ovarian hyperstimulation. West Indian Medical Journal 2004;53(1):39‐43. [PubMed] [Google Scholar]
Frydman 1986 {published data only}
- Frydman R, Forman R, Rainhorn JD, Belaisch‐Allart J, Hazout A, Testart J. A new approach to follicular stimulation for in vitro fertilization: programmed oocyte retrieval. Fertility and Sterility 1986;46(4):657‐62. [DOI] [PubMed] [Google Scholar]
Galera 2004 {published data only}
- Galera F, Verdu V, Villafañex V, Garijo E, Rayward J, Bajo JM. Comparison of plasmatic steroid levels with and without previous oral contraceptive administration in GnRH antagonist IVF cycles. Fertility and Sterility 2004; Vol. Suppl 2, 82:S32.
Gerli 1989 {published data only}
- Gerli S, Remohi J, Partrizio P, Borrero C, Balmaceda JP, Silber SJ, et al. Programming of ovarian stimulation with norethindrone acetate in IVF/GIFT cycles. Human Reproduction 1989;4(7):746‐8. [DOI] [PubMed] [Google Scholar]
Ghanem 2015 {published data only}
- Ghanem M, Bediary MH, Helal AS, et al. Is adding estradiole (E2) to progesterone (P) luteal support in high responder long GnRH agonist ICSI cycles detrimental to outcome? Randomised controlled trial (RCT). Fertility & Sterility 2015;104 Suppl(3):e224. [Google Scholar]
Godin 2003 {published data only}
- Godin PA, Gaspard O, Jouan C, Thonon F, Hincourt N, Ravet S, et al. Importance of estradiol priming in in‐vitro maturation cycles. Human Reproduction 2003;18 Suppl 1:153. [Google Scholar]
Gomez 2000 {published data only}
- Gomez E, Ballesteros A, Landeras J, Munoz M, Martinez MC. Preparation of endometrium with oral oestradiol valerate versus transdermal oestradiol for cryopreserved embryo transfer: a prospective randomized study. Human Reproduction 2000;15:175‐6. [Google Scholar]
Gonen 1990 {published data only}
- Gonen Y, Jacobson W, Casper RF. Gonadotropin suppression with oral contraceptives before in vitro fertilization. Fertility and Sterility 1990;53(2):282‐7. [DOI] [PubMed] [Google Scholar]
Gonzalez 1995 {published data only}
- Gonzalez P, Maloul S, Ciuffardi I, Frederick JL, Balmaceda JP, Asch RH. The use of progestins for programming assisted reproductive cycles and gonadotropin‐releasing hormone agonist flare‐up protocols in older patients. Fertility and Sterility 1995;63(2):249‐51. [DOI] [PubMed] [Google Scholar]
Greco 2016 {published data only}
- Greco E, Litwicka K, Arrivi C, Varricchio MT, Caragia A, Greco A, et al. The endometrial preparation for frozen‐thawed euploidblastocyst transfer: a prospective randomized trial comparing clinical results from natural modified cycle and exogenous hormone stimulation with GnRH agonist. Journal of Assisted Reproduction and Genetics 2016;33:873‐84. [DOI: 10.1007/s10815-016-0736-y] [DOI] [PMC free article] [PubMed] [Google Scholar]
Guivarc'h‐Levêque 2009 {published data only}
- Guivarc’h‐Levêque A, Arvis P, Bouchet J‐L, Broux P‐L, Moy L, Priou G, et al. Efficiency of antagonist IVF cycle programming by estrogens [Efficacité de la programmation des cycles FIV en antagonistes par les estrogènes]. Gynécologie Obstétrique & Fertilité 2010;38:18‐22. [DOI] [PubMed] [Google Scholar]
Haydardedeoglu 2012 {published data only}
- Haydardedeoglu B, Kilicdag EB, Parlakgummus AH, Zeyneloglu HB. IVF/ICSI outcomes of the OCP plus GnRH agonist protocol versus the OCP plus GnRH antagonist protocol in women with PCOS: a randomised trial. Archives of Gynecology and Obstetrics 2012;286:763‐9. [DOI] [PubMed] [Google Scholar]
Hugues 1992 {published data only}
- Hugues JN, Attalah M, Hervé F, Martin‐Pont B, Kottler ML, Santarelli J. Effects of short‐term GnRH agonist ‐ human menopausal gonadotropin stimulation in patients pre‐treated with progestogen. Human Reproduction 1992;7(8):1079‐84. [DOI] [PubMed] [Google Scholar]
Jung 2000 {published data only}
- Jung H, Roh HK. The effects of E2 supplementation from the early proliferative phase to the late secretory phase of the endometrium in hMG‐stimulated IVF‐ET. Journal of Assisted Reproduction and Genetics 2000;17(1):28‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karande 2004 {published data only}
- Karande VC, Latash WDZ, Birkenkamp T, Cavanaugh J, Melone K, Hazlett D. Preliminary report of a prospective randomized controlled trial comparing the use of NuvaRing® to Desogen® in in vitro fertilization cycles with ganirelix acetate. Fertility and Sterility 2004; Vol. Suppl 2, 82:S31‐2.
Keltz 2007 {published data only}
- Keltz MD, Gera PS, Skorupski J, Stein DE. Comparison of FSH flare with and without pretreatment with oral contraceptive pills in poor responders undergoing in vitro fertilization. Fertility and Sterility 2007;88(2):350‐3. [DOI: 10.1016/j.fertnstert.2006.11.123] [DOI] [PubMed] [Google Scholar]
- Keltz MD, Sharma P, Stein DE. Comparison of FSH flare in poor responders undergoing in vitro fertilization (IVF) with and without prior oral contraceptive suppression. Fertility and Sterility 2003; Vol. 80:S107. [DOI] [PubMed]
Kovacs 2001 {published data only}
- Kovacs P, Barg PE, Witt BR. Hypothalamic‐pituitary suppression with oral contraceptive pills does not improve outcome in poor responder patients undergoing in vitro fertilization‐embryo transfer cycles. Journal of Assisted Reproduction and Genetics 2001;18(7):391‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kreiner 2007 {published data only}
- Kreiner D, diLandro R, Moschella J. Study of IVF success with pituitary suppression prior to follistim/HMG stimulation in ganirelix cycles using oral contraceptives and estrace. Fertility & Sterility 2007;Vol 88 Suppl 1:284, abstract no. 534. [Google Scholar]
Leondires 1999 {published data only}
- Leondires MP, Escalpes M, Segars JH, Scott RT, Miller BT. Microdose follicular phase gonadotropin‐releasing hormone agonist (GnRH‐a) compared with luteal phase GnRH‐a for ovarian stimulation at in vitro fertilization. Fertility and Sterility 1999;72(6):1018‐23. [DOI] [PubMed] [Google Scholar]
Letterie 2000 {published data only}
- Letterie GS. Inhibition of gonadotropin surge by a brief mid‐cycle regimen of ethinyl estradiol and norethindrone: possible role in in vitro fertilization. Gynecological Endocrinology 2000;14(1):1‐4. [DOI] [PubMed] [Google Scholar]
Lewin 2002 {published data only}
- Lewin A, Fatum M, Shufaro Y, Simon A, Reubinoff B, Laufer N, et al. Artificial endometrial preparation for frozen‐thawed embryo transfer using oral oestradiol and a new low‐dose vaginal progesterone preparation: Endometrin tablets. Human Reproduction 2001; Vol. Suppl 1, 16:152.
- Lewin A, Pisov G, Turgeman R, Fatum M, Shufaro Y, Simon A, et al. Simplified artificial endometrial preparation, using oral estradiol and novel vaginal progesterone tablets: a prospective randomized study. Gynecological Endocrinology 2002;16(2):131‐6. [PubMed] [Google Scholar]
Lindheim 1996 {published data only}
- Lindheim SR, Barad DH, Witt B, Ditkoff E, Sauer MV. Short‐term gonadotropin suppression with oral contraceptives benefits poor responders prior to controlled ovarian hyperstimulation. Journal of Assisted Reproduction and Genetics 1996;13(9):745‐7. [DOI] [PubMed] [Google Scholar]
Liu 2011 {published data only}
- Liu KE, Alhajri M, Greenblatt E. A randomized controlled trial of NuvaRing versus combined oral contraceptive pills for pretreatment in in vitro fertilization cycles. Fertility and Sterility 2011;96(3):605‐8. [DOI] [PubMed] [Google Scholar]
Loutradis 2003 {published data only}
- Loutradis D, Stefanidis K, Drakakis P, Kallianidis K, Sheikh A, Milingos S, et al. Does pre‐treatment with micronized progesterone affect the ovarian response to a gonadotropin releasing hormone agonist flare‐up protocol?. Gynecological Endocrinology 2003;17(2):101‐6. [PubMed] [Google Scholar]
Martinez 2006 {published data only}
- Martínez F, Boada M, Coroleu B, Clua E, Parera N, Rodríguez I, et al. A prospective trial comparing oocyte donor ovarian response and recipient pregnancy rates between suppression with gonadotrophin‐releasing hormone agonist (GnRHa) alone and dual suppression with a contraceptive vaginal ring and GnRH. Human Reproduction 2006;21(8):2121‐5. [DOI] [PubMed] [Google Scholar]
Mashiach 1989 {published data only}
- Mashiach S, Dor J, Goldenberg M, Shalev J, Blankstein J, Rudake E, et al. Protocols for induction of ovulation ‐ the concept of programmed cycles. Annals of the New York Academy of Sciences 1988;541:37‐45. [DOI] [PubMed] [Google Scholar]
- Mashiach S, Dor J, Goldenberg M, Shalev J, Levran D, Rudak E, et al. Programmed oocyte retrieval: clinical and biological effects of oral contraceptives administered before in vitro fertilization. Gynecological Endocrinology 1989;3(2):107‐15. [DOI] [PubMed] [Google Scholar]
Meldrum 2002 {published data only}
- Meldrum D, Scott R, Levy MJ, Alper M, Noyes N. A pilot study to assess oral contraceptive (OC) pretreatment in women undergoing controlled ovarian hyperstimulation (COH) in ganirelix acetate cycles. Fertility and Sterility 2002; Vol. Suppl 1, 78, issue 3:S176.
Meldrum 2008 {published data only}
- Meldrum DR, Cassidenti DL, Rosen GF, Yee B, Wisot AL. Oral contraceptive pretreatment and half dose of ganirelix does not excessively suppress LH and may be an excellent choice for scheduling IUI cycles. Journal of Assisted Reproduction and Genetics 2008;25:417‐20. [DOI: 10.1007/s10815-008-9244-z] [DOI] [PMC free article] [PubMed] [Google Scholar]
Merviel 2015 {published data only}
- Merviel P, Cabry‐Goubet R, Lourdel E, Devaux A, Belhadri‐Mansouri N, Copin H, et al. Comparative prospective study of 2 ovarian stimulation protocols in poor responders: effect on implantation rate and ongoing pregnancy. Reproductive Health 2015;12:52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheffler F, Merviel P, Lourdel E, et al. Contraceptive pill and gonadotropin‐releasing hormone (GnRH) flare‐up agonist versus GnRH antagonist protocol in poor responders: lessons to a prospective study. Human Reproduction 2015;30 Suppl:i295 Abstract no: p‐410. [Google Scholar]
Min 2005 {published data only}
- Min JK, Claman P. Oral contraceptive (OC) pre‐treatment does not influence oocyte yield in poor responders undergoing gonadotropin releasing hormone (GnRH) antagonist cycles for in vitro fertilization (IVF). Fertility and Sterility 2005; Vol. Suppl 1, 84:S45‐6.
Mirkin 2003 {published data only}
- Mirkin S, Stadtmauer LA, Gibbons WE, Oehninger S. Clinical and endocrine impact of pretreatment with oral contraceptive pills in poor responders undergoing IVF with a combination of microdose flare leuprolide acetate and high dose gonadotropins. Fertility and Sterility 2003; Vol. Suppl 3, 80:S190‐1.
Mulangi 1997 {published data only}
- Mulangi AS, Nelson‐White TM, Racowsky C, Gelety TJ. Follicular phase endocrine response to oral contraceptives (OCs) followed by gonadotropin releasing hormone agonist (GnRHa) for down‐regulation prior to controlled ovarian hyperstimulation (COH) for the purpose of IVF. Fertility and Sterility 1997; Vol. Suppl 1, 68:S6.
Neal 1993 {published data only}
- Neal GS, Sultan KM, Liu HC, Davis OK, Rosenwaks Z. A dual approach to ovarian suppression using oral contraceptive pills and leuprolide acetate in high responder patients undergoing IVF. Fertility and Sterility 1993;60(5):S111. [Google Scholar]
Pados 1995 {published data only}
- Pados G, Tarlatzis BC, Bontis J, Lagos S, Papadimas J, Spanos E, et al. Evaluation of different ovarian stimulation protocols for in vitro fertilization. Gynecological Endocrinology 1995;9:103‐12. [DOI: 10.3109/09513599509160198] [DOI] [PubMed] [Google Scholar]
Palomba 2008 {published data only}
- Palomba S, Falbo A, Orio F, Russo T, Tolino A, Zullo F. Pretreatment with oral contraceptives in infertile anovulatory patients with polycystic ovary syndrome who receive gonadotropins for controlled ovarian stimulation. Fertility and Sterility 2008;89(6):1838‐42. [DOI: 10.1016/j.fertnstert.2007.05.035] [DOI] [PubMed] [Google Scholar]
Pinkas 2008 {published data only}
- Pinkas H, Sapir O, Avrech OM, Ben‐Haroush A, Ashkenzi J, Fisch B, et al. The effect of oral contraceptive pill for cycle scheduling prior to GnRH‐antagonist protocol on IVF cycle parameters and pregnancy outcome. Journal of Assisted Reproduction and Genetics 2008;25:29‐33. [DOI: 10.1007/s10815-007-9189-7] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ramsewak 2005 {published data only}
- Ramsewak SS, Duffy S, Taylor J, Woodward B. The oral contraceptive pill effectively permits cycle batching for an intermittent in vitro fertilization programme in Trinidad and Tobago. West Indian Medical Journal 2005;54(2):127‐9. [DOI] [PubMed] [Google Scholar]
Rashidi 2011 {published data only}
- Rashidi B, Nasiri R, Rahmanpour H, Shahrokh Tehraninejad E, Deldar M. Luteal phase estradiol versus luteal phase GnRH antagonist administration: their effects on antral follicular size coordination and basal hormonal levels. Iranian Journal of Reproductive Medicine 2011;9(4):315‐8. [PMC free article] [PubMed] [Google Scholar]
Russell 1997 {published data only}
- Russell JB, Knezevich KM, Fabian KF, Dickson JA. Unstimulated immature oocyte retrieval: early versus mid follicular endometrial priming. Fertility and Sterility 1997;67(4):616‐20. [DOI] [PubMed] [Google Scholar]
Sanghvi 2002 {published data only}
- Sanghvi A, Noyes N, Krey LC. An oral contraceptive (OCP) microdose flare leuprolide acetate (LA) stimulation protocol is useful for poor prognosis patients undergoing in vitro fertilization (IVF). Fertility and Sterility 2002; Vol. Suppl 1, 78:S149.
Schoolcraft 1997 {published data only}
- Schoolcraft W, Schlenker T, Gee M, Stevens J, Wagley L. Improved controlled ovarian hyperstimulation in poor responder in vitro fertilization patients with a microdose follicle‐stimulating hormone flare, growth hormone protocol. Fertility and Sterility 1997;67(1):93‐7. [DOI] [PubMed] [Google Scholar]
Steinkampf 1991 {published data only}
- Steinkampf MP, Hammond KR, Blackwell RE. Effect of estrogen/progestin administration on the ovarian response to gonadotropins: a randomized, prospective study. Fertility and Sterility 1991;55(3):642‐3. [DOI] [PubMed] [Google Scholar]
Surrey 1989 {published data only}
- Surrey ES, Cedars MI. The effect of gonadotropin suppression on the induction of ovulation in premature ovarian failure patients. Fertility and Sterility 1989;52(1):36‐41. [DOI] [PubMed] [Google Scholar]
Surrey 1998 {published data only}
Talebian 2004 {published data only}
- Talebian S, Krey LC, Noyes N. Use of oral contraceptives with GnRH antagonists and recombinant gonadotropins in IVF cycles have no deleterious effect on pregnancy outcome. Fertility and Sterility 2004; Vol. Suppl 2, 82:S234.
Talebian 2007 {published data only}
- Talebian S, Krey LC, Reh A, Granguly N, Liu M, Noyes N. Oral contraceptive (OC) pre‐treatment in GnRH antagonist (ANT) cycles is a reasonable option to control the timing of IVF cycles. Fertility and Sterility 2007; Vol. Suppl 1, 88:S291.
Tarlatzis 1993 {published data only}
- Tarlatzis BC, Pazaitou K, Bili H, Bontis J, Papadimas J, Lagos S, et al. Growth hormone, oestradiol, progesterone and testosterone concentrations in follicular fluid after ovarian stimulation with various regimes for assisted reproduction. Human Reproduction 1993;8(10):1612‐6. [DOI] [PubMed] [Google Scholar]
Tartagni 2007 {published data only}
- Tartagni M, Cicinelli E, Pergola G, Salvia MA, Lavopa C, Loverro G. Effects of pretreatment with estrogens on ovarian stimulation with gonadotropins in women with premature ovarian failure: a randomized, placebo‐controlled trial. Fertility and Sterility 2007;87(4):858‐61. [DOI: 10.1016/j.fertnstert.2006.08.086] [DOI] [PubMed] [Google Scholar]
Tehraninejad 2010 {published data only}
- Tehraninejad ES, Nasiri R, Rashidi B, Haghollahi F, Ataie M. Comparison of GnRH antagonist with long GnRH agonist protocol after OCP pretreatment in PCOs patients. Archives of Gynecology & Obstetrics 2010;282(3):319‐25. [DOI] [PubMed] [Google Scholar]
Wang 2008 {published data only}
- Wang B, Sun HX, Hu YL, Chen H, Zhang NY. Application of GnRH‐antagonist to IVF‐ET for patients with poor ovarian response. National Journal of Andrology [Zhonghua Nan Ke Xue] 2008;14(5):423‐6. [PubMed] [Google Scholar]
Wang 2016 {published data only}
- Wang Y, Chen Q, Wang N, Chen H, Lyu Q, Kuang Y. Controlled ovarian stimulation using medroxyprogesterone acetate and hMG in patients with polycystic ovary syndrome treated for IVF a double‐blind randomized crossover clinical trial. Medicine 2016;95(9):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wei 2016 {published data only}
- Wei D, Shi Y, Li J, Wang Z, Zhang L, Sun Y, et al. Effect of pretreatment with oral contraceptives and progestins on IVF outcomes in women with polycystic ovary syndrome. Human Reproduction 2016;32(2):1‐8. [DOI: 10.1093/humrep/dew325] [DOI] [PMC free article] [PubMed] [Google Scholar]
Weisman 1989 {published data only}
- Weisman Z, Dirnfeld M, Lissak A, Sorokin Y, Abramovici H. Oral contraceptive pills and follicular fluid hormones in an in vitro fertilization program. Fertility and Sterility 1989;52(3):451‐3. [DOI] [PubMed] [Google Scholar]
Yokota 2006 {published data only}
- Yokota Y, Yokota M, Yokota H, Makita M, Sato S, Araki Y. A novel procedure for improving pregnancy rates in frequent repeated IVF failure: estrogen rebound combined with GnRH agonist flare. Human Reproduction 2006;21 Suppl 1:i130‐1. [Google Scholar]
Yoshida 2005 {published data only}
- Yoshida A, Kakinuma M, Matsuba T, Seida K, Suzuki H, Tanaka M. Effectiveness of using estradiol‐GnRH antagonist protocol with unsuccessful oral contraceptive pill‐GnRH antagonist protocol cases (full term pregnancies had not resulted) in in‐vitro fertilization (IVF) cycles. Fertility and Sterility 2005; Vol. Suppl 1, 84:S45.
Youssef 2017 {published data only}
- Youssef M, Wely M, Al‐Inany H, Madani T, Jahangiri N, Khodabakhshi S, et al. A mild ovarian stimulation in women with poor ovarian reserve undergoing IVF: a multicenter randomized non‐inferiority trial. Human Reproduction 2017;32(1):112‐8. [DOI] [PubMed] [Google Scholar]
- Youssef M, Wely M, Al‐Inany H, Madani T, Jahangiri N, Khodabakhshi S, et al. Mild versus standard ovarian stimulation in poor responder women undergoing IVF and ICSI (prima) ‐ multicenter randomised controlled study. Human Reproduction 2014;29:i50‐1. [Google Scholar]
Zhao 2008 {published data only}
- Zhao JZ, Lin XH, Huang XF, Lin JJ, Lin WQ, Ye BL. Effect of desogestrel and ethinyl estradiol pretreatment in superovulation cycles with short protocol. Chinese Journal of Obstetrics and Gynecology [Zhonghua Fu Chan Ke Za Zhi] 2008;43(2):102‐5. [PubMed] [Google Scholar]
Additional references
Al‐Inany 2016
- Al‐Inany HG, Youssef MA, Ayeleke RO, Brown J, Lam WS, Broekmans FJ. Gonadotrophin‐releasing hormone antagonists for assisted reproductive technology. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD001750.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Arowojolu 2007
- Arowojolu AO, Gallo MF, Lopez LM, Grimes DA, Garner SE. Combined oral contraceptive pills for treatment of acne. Cochrane Database of Systematic Reviews 2007, Issue 1. [DOI: 10.1002/14651858.CD004425.pub3] [DOI] [PubMed] [Google Scholar]
Awadalla 1987
- Awadalla SG, Friedman CI, Chin NW, Dodds W, Park JM, Kim MH. Follicular stimulation for in vitro fertilization using pituitary suppression and human menopausal gonadotropins. Fertility and Sterility 1987;48(5):811‐5. [DOI] [PubMed] [Google Scholar]
Barmat 2005
- Barmat LI, Chantilis SJ, Hurst BS, Dickey RP. A randomized prospective trial comparing gonadotropin‐releasing hormone (GnRH) antagonist/ recombinant follicle‐stimulating hormone (rFSH) versus GnRH‐agonist/ rFSH in women pretreated with oral contraceptives before in vitro fertilization. Fertility and Sterility 2005;83(2):321‐30. [DOI: 10.1016/j.fertnstert.2004.06.076] [DOI] [PubMed] [Google Scholar]
Biljan 1998a
- Biljan MM, Mahutte NG, Dean N, Hemmings R, Bissonnette F, Tan SL. Effects of pretreatment with an oral contraceptive on the time required to achieve pituitary suppression with gonadotropin‐releasing hormone analogues and on subsequent implantation and pregnancy rates. Fertility and Sterility 1998;70(6):1063‐9. [DOI] [PubMed] [Google Scholar]
Cohen 1979
- Cohen BL, Katz M. Pituitary and ovarian function in women receiving hormonal contraception. Contraception 1979;20(5):475‐87. [DOI] [PubMed] [Google Scholar]
De Ziegler 1998
- Ziegler D, Jaaskelainen AS, Brioschi PA, Fanchin R, Bulletti C. Synchronization of endogenous and exogenous FSH stimuli in controlled ovarian hyperstimulation (COH). Human Reproduction 1998;13(3):561‐4. [DOI] [PubMed] [Google Scholar]
Dodson 1989
- Dodson WC. Role of gonadotropin releasing hormone agonists in ovulation induction. Journal of Reproductive Medicine 1989;34(1):76‐9. [PubMed] [Google Scholar]
EndNote [Computer program]
- Thomson Reuters. EndNote. Version X1. New York: Thomson Reuters, 2008.
Erkkola 2007
- Erkkola R. Recent advances in hormonal contraception. Obstetrics and Gynecology 2007;19:547‐53. [DOI] [PubMed] [Google Scholar]
ESHRE 2016
- European Society of Human Reproduction and Embryology. 32nd annual meeting of the European Society of Human Reproduction and Embryology. Helsinki, Finland 2016. Human Reproduction 2016;31 Suppl 1:Abstract book. [Google Scholar]
Gaspard 1984
- Gaspard UJ, Dubois M, Gillain D, Franchimont P, Duvivier J. Ovarian function is effectively inhibited by a low‐dose triphasic oral contraceptive containing ethinylestradiol and levonorgestrel. Contraception 1984;29(4):305‐18. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2014 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed prior to 28 October 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Griesinger 2008
- Griesinger G, Venetis CA, Marx T, Diedrich K, Tarlatzis BC, Kolibianakis EM. Oral contraceptive pill pretreatment in ovarian stimulation with GnRH antagonists for IVF: a systematic review and meta‐analysis. Fertility and Sterility 2008;90(4):1055‐63. [DOI: 10.1016/j.fertnstert.2007.07.1354] [DOI] [PubMed] [Google Scholar]
Griffin 2002
- Griffin PD, Rowe PJ, Vayena E, editor(s). Medical, ethical and social aspects of assisted reproduction. Current practices and controversies in assisted reproduction: report of a WHO meeting. Geneva, Switzerland: World Health Organization 2002. [ISBN 92 4 159030 0]
Harwood 2007
- Harwood K, Vuguin P, DiMartino‐Nardi J. Current approaches to the diagnosis and treatment of polycystic ovarian syndrome in youth. Hormone Research 2007;68(5):209‐17. [DOI: 10.1159/000101538] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Huirne 2006b
- Huirne JA, Hugues JN, Pirard C, Fischl F, Sage JC, Pouly JL, et al. Cetrorelix in an oral contraceptive‐pretreated stimulation cycle compared with buserelin in IVF/ICSI patients treated with r‐hFSH: a randomized, multicentre, phase IIIb study. Human Reproduction 2006;21(6):1408‐15. [10.1093/humrep/del030] [DOI] [PubMed] [Google Scholar]
Irvine 1999
- Irvine GA, Cameron IT. Medical management of dysfunctional uterine bleeding. Best Practice and Research in Clinical Obstetrics and Gynaecology 1999;13(2):189‐202. [DOI] [PubMed] [Google Scholar]
Le Nestour 1993
- Nestour E, Marraoui J, Lahlou N, Roger M, Ziegler D, Bouchard P. Role of estradiol in the rise in follicle‐stimulating hormone levels during the luteal‐follicular transition. Journal of Clinical Endocrinology and Metabolism 1993;77(2):439‐42. [DOI] [PubMed] [Google Scholar]
MDSG Module 2008
- Farquhar C, Clarke J, Lethaby A, Thomas J, Proctor M, Barlow D, et al. Cochrane Menstrual Disorders and Subfertility Group, About The Cochrane Collaboration (Cochrane Review Groups (CRGs)). Available at www.thecochranelibrary.com 2008.
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA Statement. BMJ 2009;339:2535. [PMC free article] [PubMed] [Google Scholar]
Moudgal 1985
- Moudgal RN, Jagannadha Rao A, Murthy GSRC, Neelakanta R, Banavar SR, Kotagi SG, et al. Effect of intranasal administration of norethisterone and progesterone on pituitary and gonadal function in adult male and female bonnet monkeys (Macaca radiata). Fertility and Sterility 1985;44(1):120‐4. [DOI] [PubMed] [Google Scholar]
Pandian 2005
- Pandian Z, Bhattacharya S, Vale L, Templeton A. In vitro fertilisation for unexplained subfertility. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD003357.pub2] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
SIGN
- Scottish Intercollegiate Guidelines Network. Search filters. www.sign.ac.uk/methodology/filters.html#random (accessed 18 November 2008).
Tarlatzis 2006
- Tarlatzis BC, Fauser BC, Kolibianakis EM, Diedrich K, Rombauts L, Devroey P. GnRH antagonists in ovarian stimulation for IVF. Human Reproduction Update 2006;12(4):333‐40. [DOI: 10.1093/humupd/dml001] [DOI] [PubMed] [Google Scholar]
Van Wely 2003
- Wely M, Westergaard LG, Bossuyt PM, Veen F. Human menopausal gonadotropin versus recombinant follicle stimulation hormone for ovarian stimulation in assisted reproductive cycles. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD003973] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Smulders 2006
- Smulders B, Oirschot SM, Farquhar C, Rombauts L, Kremer JAM. Oral contraceptive pill, progestogen or estrogen pre‐treatment for ovarian stimulation protocols for women undergoing assisted reproductive techniques. Cochrane Database of Systematic Reviews 2006, Issue 7. [DOI: 10.1002/14651858.CD006109] [DOI] [PubMed] [Google Scholar]
Smulders 2010
- Smulders B, Oirschot SM, Farquhar C, Rombauts L, Kremer JAM. Oral contraceptive pill, progestogen or estrogen pre‐treatment for ovarian stimulation protocols for women undergoing assisted reproductive techniques. Cochrane Database of Systematic Reviews 2010, Issue 1. [DOI: 10.1002/14651858.CD006109.pub2] [DOI] [PubMed] [Google Scholar]


