Abstract
Background
Low back pain (LBP) is associated with enormous personal and societal burdens, especially when it reaches the chronic stage of the disorder (pain for a duration of more than three months). Indeed, individuals who reach the chronic stage tend to show a more persistent course, and they account for the majority of social and economic costs. As a result, there is increasing emphasis on the importance of intervening at the early stages of LBP.
According to the biopsychosocial model, LBP is a condition best understood with reference to an interaction of physical, psychological, and social influences. This has led to the development of multidisciplinary biopsychosocial rehabilitation (MBR) programs that target factors from the different domains, administered by healthcare professionals from different backgrounds.
This review is an update of a Cochrane Review on MBR for subacute LBP, which was published in 2003. It is part of a series of reviews on MBR for musculoskeletal pain published by the Cochrane Back and Neck Group and the Cochrane Musculoskeletal Group.
Objectives
To examine the effectiveness of MBR for subacute LBP (pain for a duration of six to 12 weeks) among adults, with a focus on pain, back‐specific disability, and work status.
Search methods
We searched for relevant trials in any language by a computer‐aided search of CENTRAL, MEDLINE, Embase, CINAHL, PsycINFO and two trials registers. Our search is current to 13 July 2016.
Selection criteria
We included randomised controlled trials (RCTs) of adults with subacute LBP. We included studies that investigated a MBR program compared to any type of control intervention. We defined MBR as an intervention that included a physical component (e.g. pharmacological, physical therapy) in combination with either a psychological, social, or occupational component (or any combination of these). We also required involvement of healthcare professionals from at least two different clinical backgrounds with appropriate training to deliver the component for which they were responsible.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. In particular, the data extraction and 'risk of bias' assessment were conducted by two people, independently. We used the Cochrane tool to assess risk of bias and the GRADE approach to assess the overall quality of the evidence for each outcome.
Main results
We included a total of nine RCTs (981 participants) in this review. Five studies were conducted in Europe and four in North America. Sample sizes ranged from 33 to 351. The mean age across trials ranged between 32.0 and 43.7 years.
All included studies were judged as having high risk of performance bias and high risk of detection bias due to lack of blinding, and four of the nine studies suffered from at least one additional source of possible bias.
In MBR compared to usual care for subacute LBP, individuals receiving MBR had less pain (four studies with 336 participants; SMD ‐0.46, 95% CI ‐0.70 to ‐0.21, moderate‐quality of evidence due to risk of bias) and less disability (three studies with 240 participants; SMD ‐0.44, 95% CI ‐0.87 to ‐0.01, low‐quality of evidence due to risk of bias and inconsistency), as well as increased likelihood of return‐to‐work (three studies with 170 participants; OR 3.19, 95% CI 1.46 to 6.98, very low‐quality of evidence due to serious risk of bias and imprecision) and fewer sick leave days (two studies with 210 participants; SMD ‐0.38 95% CI ‐0.66 to ‐0.10, low‐quality of evidence due to risk of bias and imprecision) at 12‐month follow‐up. The effect sizes for pain and disability were low in terms of clinical meaningfulness, whereas effects for work‐related outcomes were in the moderate range.
However, when comparing MBR to other treatments (i.e. brief intervention with features from a light mobilization program and a graded activity program, functional restoration, brief clinical intervention including education and advice on exercise, and psychological counselling), we found no differences between the groups in terms of pain (two studies with 336 participants; SMD ‐0.14, 95% CI ‐0.36 to 0.07, low‐quality evidence due to imprecision and risk of bias), functional disability (two studies with 345 participants; SMD ‐0.03, 95% CI ‐0.24 to 0.18, low‐quality evidence due to imprecision and risk of bias), and time away from work (two studies with 158 participants; SMD ‐0.25 95% CI ‐0.98 to 0.47, very low‐quality evidence due to serious imprecision, inconsistency and risk of bias). Return‐to‐work was not reported in any of the studies.
Although we looked for adverse events in both comparisons, none of the included studies reported this outcome.
Authors' conclusions
On average, people with subacute LBP who receive MBR will do better than if they receive usual care, but it is not clear whether they do better than people who receive some other type of treatment. However, the available research provides mainly low to very low‐quality evidence, thus additional high‐quality trials are needed before we can describe the value of MBP for clinical practice.
Plain language summary
Multidisciplinary treatment at the early stages of low back pain
Review Question
We reviewed the evidence about the effect of multidisciplinary treatments on pain, disability, and work status among people who had been experiencing low back pain for six to 12 weeks. We defined multidisciplinary treatments as treatments that target physical as well as psychological or social aspects of low back pain and involve a team of healthcare providers with different professional backgrounds and training. For example, a treatment that integrated exercise therapy provided by a physiotherapist with workplace adjustments provided by an ergonomist, a specialist in the design and setup of workplace equipment, would be considered to be multidisciplinary.
Background
Low back pain (LBP) is a condition that causes a great deal of pain and suffering across the world and also accounts for large costs to society due to healthcare spending and missed work. Previous research has shown that people who have back pain for more than three months are less likely to recover. As a result, there is increasing emphasis on the importance of intervening at the early stages of LBP.
The purpose of this review was to discover whether multidisciplinary treatments were better or worse than other alternatives, such a usual care (i.e. current clinical practice) or other treatments (e.g. exercise therapy alone) for people experiencing low back pain for six to 12 weeks.
Study Characteristics
The search is current to July of 2016.
Five studies were conducted in Europe and four in North America. Sample sizes ranged from 33 to 351. The mean age across trials ranged between 32.0 and 43.7 years. The majority of studies included mixed samples of male and female participants. The authors had no concerns about funding sources of any included studies.
Key Results
Overall, we found that multidisciplinary treatments may be better than usual care for people with LBP for a duration of six to 12 weeks. Individuals receiving multidisciplinary treatment had less pain, less disability, increased likelihood of return‐to‐work and fewer sick leave days at 12‐month follow‐up. However, when comparing multidisciplinary treatments to other treatments (e.g. brief clinical intervention including education and advice on exercise), we found that multidisciplinary treatments may be no better than other treatments. Although we examined adverse events as a secondary outcome, none of the included studies reported this outcome.
Quality of the Evidence
The quality of the evidence for this review was generally low to very low. This was mainly due to small sample sizes and other study limitations. Moreover, we grouped together studies with differing interventions and comparisons. For example, some of the multidisciplinary interventions were quite intense (e.g. > 30 hours of treatment), whereas others were designed to be brief (e.g. < three hours). This variability across studies makes it more challenging to interpret the findings.
In sum, there is a need for additional, large, high‐quality randomised controlled trials before we can make definitive recommendations for clinical practice.
Summary of findings
Background
Description of the condition
Low back pain (LBP) is a common condition worldwide, with a one‐month prevalence in the general population of approximately 23% (Hoy 2012). In the latest Global Burden of Disease Study, LBP was identified as the leading cause of disability globally (Vos 2015). It was estimated to be responsible for 72.3 million years lived with disability in 2013, which represents a 57% increase from 1990. In addition to the huge personal toll for individuals and their families, LBP is associated with an enormous societal burden. These costs include healthcare expenditures, as well as the indirect costs related to inability to work or reduced productivity while at work (Dagenais 2008; Luo 2004; Maetzal 2002; Stewart 2003). Research evidence indicates that the majority of people presenting to healthcare providers with LBP will recover within a few weeks, but a quarter to a third continue to report LBP after 12 months (Hayden 2010). People who reach the chronic stage of LBP (pain for a duration of more than three months) tend to show a more persistent course (Pengel 2003; Hayden 2010); over 50% of these people are not recovered one year later (Menezes Costa 2009). Indeed, it is the small proportion of people with persistent and disabling LBP that account for the majority of social and economic costs (Frymoyer 1991). As a result, there is increasing emphasis on the importance of intervening before symptoms have reached the chronic stage (Chou 2010; Chou 2011). Therefore, in the current review, we focus on subacute LBP, which is defined as back pain with a duration of six to 12 weeks.
Description of the intervention
Multidisciplinary rehabilitation programs acknowledge that although an anatomical or physiological problem can contribute to back pain, psychological factors such as fear, anxiety, mood disturbance, and a tendency to catastrophise may amplify or prolong pain (Main 2012). Similarly, social/environmental factors such as physical job demands, workplace social support, and expectations for resuming work affect long‐term disability (Shaw 2009). These insights have led to the design of interventions to address multiple factors, typically involving a combination of physical, psychological, social and/or work‐related components which are often delivered by a team of clinicians with different skills (Kamper 2014; Guzman 2006). Over time, there has been an increase in research into the multidisciplinary approach due to wider acceptance of the biopsychosocial model (Foster 2011), the ineffectiveness of monotherapies (i.e. the use of single treatments) (Artus 2010), and promising reports from clinical practice. Multidisciplinary biopsychosocial rehabilitation (MBR) may be delivered in multidisciplinary pain clinics, rehabilitation centres, or outpatient settings.
How the intervention might work
The theoretical basis for the intervention comes from the biopsychosocial model (Waddell 1987). According to this theory, chronic LBP involves impairments of physical, psychological and social functioning, and effective treatment requires intervention that specifically addresses these problems. Multidisciplinary biopsychosocial rehabilitation includes elements aimed at improving back‐related physical dysfunction as well as addressing psychological issues or targeting social or work‐related behaviours, or any combination of these.
A large Cochrane Review by Kamper and colleagues (Kamper 2014) found evidence in support of MBR for chronic LBP. They found that participants with chronic LBP receiving multidisciplinary biopsychosocial treatment generally experienced less pain and disability than those receiving usual care or a physical treatment. Although it's unclear whether these effects generalize to the subacute stage of the disorder, accumulating evidence points to the importance of early intervention. Specifically, we know that biopsychosocial risk factors play a role in the transition to chronic LBP (Chou 2011), thus interventions that target these factors in the early stages of LBP may be particularly effective and important to examine.
Why it is important to do this review
Although promising, it is notable that multidisciplinary treatments are labor‐intensive, and their availability, time demands, and costs are important barriers for healthcare providers and consumers (Deyo 2015). The most recent Cochrane review examining the effectiveness of multidisciplinary biopsychosocial treatments among individuals with subacute LBP was published over ten years ago and included only two studies (Karjalainen 2003), thus a careful review of the current state of the literature is long overdue.
Objectives
To examine the effectiveness of MBR for subacute LBP among adults, with a focus on pain, back‐specific disability status, and work status.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and quasi‐RCTs as defined in the Cochrane Handbook (Higgins 2011). We included studies when the full report was peer‐reviewed.
Types of participants
Inclusion criteria
• Adult participants with nonspecific LBP with a mean duration for the current episode greater than six weeks and less than 12 weeks. Given our interest in work status as a primary outcome, participants in the trials were required to be working age (between 18 and 65 years). In samples with mixed durations of pain, more than 75% of the study sample had to have pain that had lasted between six and 12 weeks. • Participants with or without radiating pain.
Exclusion criteria
• Studies that involved participants with LBP caused by specific pathologies (e.g. infections, neoplasms, metastases, fractures, osteoporosis, rheumatoid arthritis, radiculopathies).
• Studies that involved individuals with LBP during or immediately following pregnancy.
• Studies that recruited participants with postoperative back pain.
Types of interventions
We included studies that investigated a MBR program. This means that the intervention included a physical component (e.g. pharmacological, physical therapy) in combination with either a psychological, social, or occupational component (or any combination of these). We also required involvement of healthcare professionals from at least two different clinical backgrounds.
Physical component
The participant was assessed for physical causes of back pain by a physician, physiotherapist, or other qualified health care professionals, and the participant received pharmacological or exercise/physical therapy (including any of the following: functional restoration, back school, manual therapy, massage, ultrasound, laser therapy, and acupuncture). We excluded surgical interventions.
Psychological component
The participant received group or individual counselling targeting his or her cognitions, emotions, behaviours, beliefs, and/or motives. Cognitive‐behavioral interventions, fear‐avoidance treatment, and motivational interviewing were included here. We expected clinicians to include psychologists, counsellors, and social workers. We excluded any purely educational interventions described in terms of training, advice, skills acquisition, or education (e.g. postural re‐education, advice to stay active).
Social/occupational component
A social worker, occupational physician, case manager, ergonomist, or vocational therapist assessed the participant’s family, social and/or occupational environment and then provided an appropriate intervention.
Comparisons
We included any type of control intervention, but we evaluated the following comparisons separately:
MBR versus usual care
MBR versus other intervention
We defined usual care as care reflective of the usual management of these participants within the health care system in which the study was conducted. In contrast, we defined other interventions as interventions that were designed specifically for the RCT.
Types of outcome measures
This review focused on patient‐centred outcomes. They were categorized into three groups according to follow‐up time after randomizations.
Short‐term: up to three months
Medium‐term: > three months and less than 12 months
Long‐term: 12 months or more
We defined long term as our primary follow‐up point. Where a study reported multiple follow‐ups, the time‐points closest to three, six and 12 months were used in the meta‐analyses. Separate meta‐analyses were performed for each follow‐up period.
Primary outcomes
Pain
Back‐specific disability/functional status
Work status (return‐to‐work, sick leave)
Secondary outcomes
Generic health or quality of life (QoL)
Healthcare service utilization
Global improvement
Psychological and cognitive function (depression, anxiety, fear avoidance, coping strategies)
Adverse events
We reported the findings for the primary outcomes and adverse events in our 'Summary of Findings' tables; Table 1 and Table 2.
Summary of findings for the main comparison. Multidiscipinary rehabilitation versus usual care for subacute low back pain at long‐term follow‐up.
| Patient or population: Subacute low back pain Intervention: Multidisciplinary rehabilitation Comparison: Usual care | |||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | ||
| Risk with usual care | Risk with multidisciplinary rehabilitation | ||||||
|
Back pain long‐term Higher scores indicated more intense pain Follow‐up: median 12 months |
The baseline for the most representative study# (Karjalainen 2003) was 5.7 out of 10 (visual analogue scale). | The risk with MBR was approximately 4.67 (4.60 to 4.73) out of 10. | The mean pain in the intervention group was 0.46 standard deviations lower (0.7 lower to 0.21 lower). | 336 (4 RCTs included in meta‐analysis). | TOTAL = 532 (5 RCTs) | X X X ◯ MODERATE 1 |
This was a small to moderate effect (Cohen 1988) that is probably clinically relevant in this participant group. |
| An additional study that could not be included in meta‐analysis showed no difference between the groups. | ‐ | 196 (1 RCT included in qualitative synthesis). | |||||
|
Functional disability at the long term Higher scores indicated more disability Follow‐up: median 12 months. |
The baseline for the most representative study# (Karjalainen 2003) was 34 out of 100 (Oswestry Scale). | The risk with MBR was approximately 26.30 (25.2 to 27.4) out of 100. | The mean functional disability in the intervention group was 0.44 standard deviations lower (0.87 lower to 0.01 lower). | 240 (3 RCTs included in meta‐analysis). | TOTAL = 537 (5 RCTs) | X X ◯◯ LOW 1, 2 | This was a small to moderate effect (Cohen 1988) that is probably clinically relevant in this participant group. |
| Two additional studies could not be included in meta‐analysis. One study showed evidence in favour of MBR and the other showed no difference between the groups. | ‐ | 297 (2 RCTs included in qualitative synthesis). | |||||
|
Return‐to‐work at the long term Proportion at work Follow‐up: median 12 months. |
Study population | OR 3.19 (1.46 to 6.98) | 170 (3 RCTs included in meta‐analysis). | X ◯◯◯ VERY LOW 3, 4 | This was a moderate effect that is clinically relevant in this participant group. | ||
| 65 per 100 | 86 per 100 (95% CI from 73 to 93) | ||||||
|
Sick leave periods at long‐term Cumulative sickness absence periods over the course of the 12‐month follow‐up. |
Average sick leave in the usual care group was 997.3 hours (Bultmann 2009). | The risk with MBR was approximately 763.03 (743.3 to 782.3) sick leave hours. | The mean sick leave periods in the intervention group was 0.38 standard deviations lower (0.66 lower to 0.10 lower). | 210 (2 RCTs included in meta‐analysis). | X X ◯◯ LOW 5, 6 | This was a small to moderate effect (Cohen 1988) that is clinically relevant in this participant group. | |
| Adverse events | N/A | NO EVIDENCE | None of the included studies reported on adverse events. | ||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). #We defined the most representative sample as the study that has the largest weighting in the overall result in RevMan. CI: Confidence interval; MBR: Multidisciplinary biopsychosocial rehabilitation OR: Odds ratio | |||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | |||||||
1 Downgraded due to risk of bias, all five trials had high risk of performance bias and detection bias. One trial suffered from unclear risk of selection bias. Another trial suffered from unclear risk of attrition bias (serious bias = 1‐point downgrade).
2 Downgraded due to inconsistency, I2 statistic60% (heterogeneity = 1‐point downgrade).
3 Downgraded due to serious risk of bias, all three trials suffered from risk of performance bias and detection bias. One trial also suffered from unclear risk of selection bias and another trial suffered from unclear risk of attrition bias (very serious bias = 2‐point downgrade).
4 Downgraded due to imprecision, the total number of events was less than 300 (1‐point downgrade).
5 Downgraded due to risk of bias, both trials suffered from risk of performance bias and detection bias. One trial also suffered from unclear risk of attrition bias (serious bias = 1‐point downgrade).
6 Downgraded due to imprecision, total population size < 400 (1‐point downgrade).
Summary of findings 2. Multidisciplinary rehabilitation versus other treatment for subacute low back pain at long‐term follow‐up.
| Patient or population: Subacute low back pain Intervention: Multidisciplinary rehabilitation Comparison: Other treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with another treatment | Risk with multidisciplinary rehabilitation | |||||
|
Pain at the long term Higher scores indicated more intense pain Follow‐up: median 12 months. |
The baseline for the most representative study# (Jensen 2011) was 32.7 out of 60 (LBP Rating Scale). | The risk in the MBR group was approximately 31.02 out of 60. | The mean pain in the intervention group was 0.14 standard deviations lower (0.36 lower to 0.07 higher). | 336 (2 RCTs included in meta‐analysis). | X X ◯◯ LOW 1, 2 | This difference was not statistically or clinically relevant. |
|
Functional disability at the long term Higher scores indicated more severe functional disability. Follow‐up: median 12 months. |
The baseline for the most representative study# (Jensen 2011) was 15.6 out of 23 (Roland‐Morris). | The risk in the MBR group was approximately 15.45 out of 23. |
The mean functional disability in the intervention group was 0.03 standard deviations lower (0.24 lower to 0.18 higher). | 345 (2 RCTs included in meta‐analysis). | X X ◯◯ LOW 1, 2 | This difference was not statistically or clinically relevant. |
| Return‐to‐work at long‐term | N/A | N/A | N/A | N/A | NO EVIDENCE | None of the studies that compared MBR to another treatment assessed this outcome. |
|
Sick leave periods at long‐term Follow‐up: median 24 months. |
Average sick leave in the comparison group was 30 days (Karjalainen 2003). | The risk in the MBR group was approximately 4 (0 to 8) sick leave days. | The mean sick leave days in the intervention group was 0.25 standard deviations lower (0.98 lower to 0.47 higher). | 158 (2 RCTs included in meta‐analysis). | X ◯◯◯ VERY LOW 1, 3, 4, 5 | There was a difference between the groups but the pooled estimate was imprecise and should not be interpreted. |
| Adverse events | N/A | NO EVIDENCE | None of the included studies reported on adverse events. | |||
|
#We defined the most representative sample as the study that has the largest weighting in the overall result in RevMan. *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Downgraded due to imprecision, n < 400 (1‐point downgrade).
2Downgraded due to risk of bias, both trials suffered from high risk of performance bias and detection bias. One trial suffered from unclear risk of attrition bias (serious bias = 1‐point downgrade).
3Downgraded due to imprecision 95% confidence interval includes the no effect and the upper or lower confidence limit crosses an effect size of 0.5 (1‐point downgrade).
4Downgraded due to inconsistency, I2 statistic > 60% (1‐point downgrade).
5Downgraded due to risk of bias, the two trials suffered from high risk of performance bias and detection bias (serious bias = 1‐point downgrade).
Search methods for identification of studies
Electronic searches
We searched for relevant RCTs and quasi‐RCTs in any language in the following databases to 13 July 2016:
Cochrane Central Register of Controlled Trials (CENTRAL, which includes the Cochrane Back and Neck (CBN) group trials register; The Cochrane Library, Issue 6)
MEDLINE (Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R)) (OvidSP, 1946 to 13 July 2016)
Embase (OvidSP, 1980 to 2016 Week 28)
Cumulative Index to Nursing and Allied Health Literature (CINAHL, EBSCO, 1981 to 13 July 2016)
PsycINFO (OvidSP, 2002 to July Week 1 2016)
ClinicalTrials.gov
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP)
An information specialist from the CBN devised and ran the searches according to CBN guidelines (Furlan 2015). In 2016, MEDLINE (Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R)) was searched as it allows multiple subsets of Ovid MEDLINE to be searched at one time. The search strategies can be found in Appendix 1.
Searching other resources
We searched reference lists and contacted authors in the field for additional studies.
Data collection and analysis
The methods for this review are based on current recommendations from Cochrane (Higgins 2011) and the Cochrane Back and Neck Group (Furlan 2015). For each of the steps, review authors worked in a team of four (TJM, DVE, RC, EI) or in pairs to independently screen new studies, assess the risk of bias (RoB), and extract data. There were no language restrictions.
Selection of studies
Four reviewers (TJM, DVE, RC, EI) independently screened titles and abstracts using DistillerSR. We then assigned each selected article to a pair of reviewers who independently screened the titles and abstracts and came to consensus about retrieving the full text article. Finally, reviewers worked in pairs to assess all full‐text articles against the inclusion criteria. Moreover, we worked with translators to review all non‐English studies against the inclusion criteria. At this stage, we also reassessed the included studies from the original review against our inclusion criteria. We resolved any disagreements through discussion.
Data extraction and management
Four authors (TJM, DVE, RC, EI) worked in pairs to independently extract the data for each included study using a standardized form in DistillerSR. We then compared the data and resolved any conflicts through discussion. We extracted data on all patient‐centred outcomes.
Assessment of risk of bias in included studies
See Appendix 2 for a description of the 'risk of bias' assessment.
Measures of treatment effect
We combined the outcome measures from the individual trials through meta‐analysis where possible, taking into account clinical comparability of population, intervention and outcomes between trials. Pooled effect estimates were calculated using random‐effects models.
We analysed dichotomous outcomes by calculating the odds ratio (OR). We analysed continuous outcomes by calculating the mean difference (MD) when the same instrument was used to measure the outcomes or the standardized mean difference (SMD) when different instruments were used to measure the outcomes. We expressed the uncertainty of the effect with 95% confidence intervals (95% CI).
We examined SMD effect sizes using Cohen's 'rules of thumb', where 0.2 represented a small effect, 0.5 a moderate effect, and 0.8 a large effect (Cohen 1988).
Unit of analysis issues
All included studies randomised participants and analysed results at the individual participant level. However, studies in this review reported repeated observations on participants. To address this unit of analysis issue, we followed the guidance in section 9.3.4 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). In particular, we assessed available data from multiple follow‐up periods of the same treatment groups by conducting separate analyses based on different periods of follow‐up; short, intermediate and long term (see Types of outcome measures section above for more details).
Dealing with missing data
Where medians instead of means were reported, we planned to substitute these into the analysis. Where follow‐up standard deviations were not reported, we planned to use the standard deviation for the same measure at baseline as a substitute. Where neither the baseline nor follow‐up standard deviation was reported, we planned to calculate an estimate of the standard deviation from the same measure reported in other studies within the comparison. We attempted to contact authors of the original studies to supply data where insufficient data were reported in the article.
Data synthesis
We assessed the overall quality of the evidence for each outcome. To accomplish this, we used the GRADE approach (Atkins 2004), as recommended in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) and in the most recent method guidelines from the Cochrane Back and Neck Group (Furlan 2015). Following the GRADE guidelines, we categorized the quality of evidence as high, moderate, low, or very low. The evidence available to answer each subquestion was graded according to the following domains which are further discussed in Appendix 2 : study design, risk of bias, inconsistency, indirectness (not generalisable), and imprecision. We also planned to assess publication bias, but we were not able to assess this due to the limited number of studies identified.
'Summary of Findings' Tables
We reported the findings from our main comparisons and outcomes in 'Summary of Findings' tables.
Our main comparisons were MBR versus usual care, and MBR versus other treatments, and our main outcomes were pain, back‐specific disability, and work status, at long‐term. See Table 1 and Table 2.
Subgroup analysis and investigation of heterogeneity
We planned to conduct subgroup analyses based on baseline symptom intensity and intervention intensity. We expected that the treatment effect may vary depending on the severity of the condition in the sample, with more severe samples having the potential for greater improvement over the course of the study. Moreover, we expected that more intense interventions would be associated with greater benefits for participants.
We operationalised symptom intensity and intervention intensity as follows:
• Baseline symptom intensity. We categorized studies according to the mean score at baseline on a pain scale and a back‐specific disability measure. We categorized studies with a mean score of 60% or greater of the scale maximum for both pain and disability as 'higher baseline symptom intensity'. We categorized studies with a mean score of less than 60% of the scale maximum for both pain and disability as 'lower baseline symptom intensity'.
• Intervention intensity. We categorized interventions that involved more than 100 face‐to‐face hours and were delivered on a daily basis as having high‐intensity, and interventions that involved less that 30 hours delivered on a non‐daily basis as low‐intensity. We categorized other interventions as mid‐intensity and excluded them from these subgroup analyses (Guzman 2006; Kamper 2014).
In cases where insufficient information was reported to categorise a study, we planned to exclude the study from the subgroup analysis.
Sensitivity analysis
We planned to perform sensitivity analyses to see if the overall estimates of effectiveness changed when only studies with low risk of selection bias were considered. We categorized studies as having low risk of selection bias if they used both adequate random sequence generation and adequate allocation concealment.
Results
Description of studies
We have listed this information in the Characteristics of included studies and Characteristics of excluded studies tables.
Results of the search
Since the original review, our extensive literature search identified 17553 citations for appraisal against our inclusion and exclusion criteria. We retrieved 321 full‐text articles for further assessment and study selection. Ultimately, eight articles met our inclusion criteria. Additionally, we retained one of the two articles from the original version of this review (i.e. Loisel 1997); we excluded the other (Lindström 1992) because the multidisciplinary intervention was not carried out by clinicians from two or more disciplines. In sum, our review is based on nine studies with a total of 14 references. See Figure 1 for more details.
1.
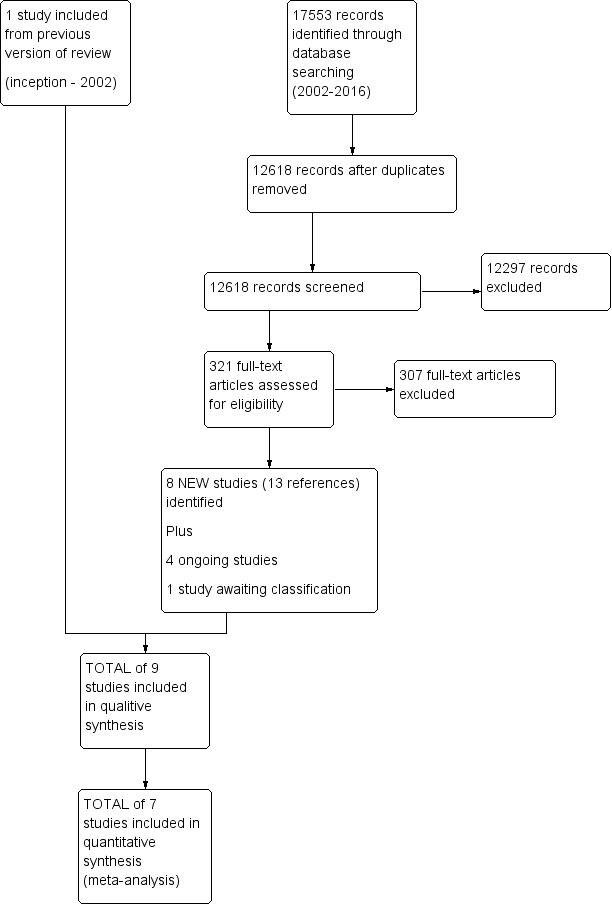
Study flow diagram.
The search for registered trials identified four ongoing studies (see Characteristics of ongoing studies) and one study awaiting classification (see Characteristics of studies awaiting classification).
Included studies
We included a total of nine RCTs in this review. Five studies were conducted in Europe and four in North America. Sample sizes ranged from 33 to 351. The mean age across trials ranged between 32.0 and 43.7 years. The majority of studies included mixed samples of male and female participants (% female ranged from approximately 40% to 60%). However, both Campello 2012 and Slater 2009 included mainly male participants (< 20% female across the groups). Eight studies reported lower baseline symptom intensity (< 60% on pain and disability scales), and there were insufficient data to categorize one study (see Characteristics of included studies).
All nine studies looked at MBR interventions with a physical component in combination with a psychological, social, and/or vocational component. Eight of the MBR interventions included an occupational component, which consisted of a worksite visit or a work rehabilitation plan or both, and two studies had a strong psychotherapy focus (Campello 2012; Schiltenwolf 2006). Seven of the MBR interventions were integrated programs, meaning that there was communication between professionals from different disciplines. The interventions ranged in duration from two to 18 weeks, with the exception of Karjalainen 2003, which included only a 1.25 hour session aimed to increase body control and exercising, as well as a 75 minute work‐site visit. None of the studies reported high‐intensity interventions (> 100 hours contact time delivered on a daily basis), three studies reported mid‐intensity interventions (> 30 and < 100 hours contact time), and four studies reported low‐intensity interventions (< 30 hours contact time delivered on a non‐daily basis). There were insufficient data to categorize two studies on intensity. See Table 3 for an overview of the MBR interventions, including the practitioners involved, methods for interdisciplinary collaboration, and the frequency/duration of the intervention.
1. Overview of MBR Interventions.
| Study | Practitioners involved | Methods for interdisciplinary collaboration | Intervention intensity |
| Anema 2007 | Ergonomist, physiotherapist | "The workplace intervention consisted of a workplace assessment and work adjustments in which all major stakeholders in the return‐to‐work process participated: i.e., the worker, the employer, the occupational physician, and the worker’s general practitioner.” | The entire program consisted of two 1‐hour sessions a week, with 26 sessions maximally (13 weeks) = low intensity |
| Bultmann 2009 | Occupational physician, occupational physiotherapist, chiropractor, psychologist, social worker | “The formulation and implementation of a coordinated, tailored and action‐oriented work rehabilitation plan collaboratively developed by an interdisciplinary team using a feedback guided approach.” | The duration of the intervention was for up to three months; insufficient information to categorize intervention intensity. |
| Campello 2012 | Physical therapist, psychologist, physician | “Backs to Work is a coordinated multidisciplinary reconditioning program” | The duration of the intervention was 3 hours per day, 3 days/week for 4 weeks = 36 hours = mid‐intensity |
| Jensen 2011 | Rehabilitation physician, specialist in clinical social medicine, physiotherapist, social worker, occupational therapist | Coordinated through case manager | The duration of the intervention was 18 weeks, average of 4 meetings with case manager = low intensity |
| Karjalainen 2003 | Physician, physiotherapist, company nurse, company physician | Physiotherapist visited patient’s workplace to involve work supervisor and company health care professionals in treatment | The duration of the mini‐intervention was 1.25‐1.5 hours and the worksite visit was approximately 75 minutes = low intensity |
| Loisel 1997 | Occupational physician, ergonomist, back pain specialist | “All described interventions were provided by a multidisciplinary medical, ergonomic, and rehabilitation staff at the Sherbrooke University Hospital back pain clinic.” | In a previous study using the same protocol (Loisel 1994), the duration of functional rehabilitation therapy ranged from 2 to 13 weeks. Insufficient information to categorize intervention intensity. |
| Schiltenwolf 2006 | Physician, physiotherapist, psychotherapist | This does not appear to be an integrated program. The physiotherapists were blind to treatment condition, indicating no communication between the physiotherapists and psychotherapists. | The duration of the intervention was 6 hours of daily treatment for 15 days in 3 weeks = mid‐intensity |
| Slater 2009 | Physician, masters‐level clinician for behavioural medicine intervention | The extent to which health care professionals communicated wasn't clear from the article text. | The duration of the intervention was 6‐10 weeks, 4 hours a week = mid‐intensity |
| Whitfill 2010 | Physical therapy and behavioral medicine sessions “provided by licensed professionals trained in their respective fields;” occupational therapist | Case management sessions and interdisciplinary team conferences held at baseline and discharge. | The duration of the intervention was from 4 to 10 weeks; 6‐9 behavioral medicine sessions; 6‐9 physical therapy sessions; Up to 6 work transitions sessions; one or more case management sessions = low intensity |
Six studies compared MBR to usual care(Anema 2007; Bultmann 2009; Campello 2012; Karjalainen 2003; Loisel 1997; Whitfill 2010). However, it should be noted that Anema 2007 differed from the other studies in that the comparison group included participants receiving usual care, as well as those who received either graded activity alone or the work intervention alone. However, their analyses statistically controlled for the effects of graded activity alone and the work intervention alone, thus the results reflected the difference between the combined intervention (i.e. MBR) and usual care.
Four studies compared MBR to other treatments (Jensen 2011; Karjalainen 2003; Schiltenwolf 2006; Slater 2009). The other treatment comparisons included (1) a 'mini' intervention with features from a light mobilization program and a graded activity program (Karjalainen 2003), (2) a brief clinical intervention including education and advice on exercise (Jensen 2011), (3) a functional restoration program of individual physiotherapy, group therapy in water, workout, and back school (Schiltenwolf 2006), and (4) usual medical care plus nondirective supportive care using a Rogerian counselling approach (Slater 2009).
Excluded studies
We excluded 307 articles (277 English and 30 nonEnglish) after full‐text screening. nonEnglish papers were reviewed by colleagues proficient in the language of the article. The most common reasons for exclusion were: study design other than RCT, inclusion of participants other than those with subacute LBP, and index interventions that did not include two or more elements of the biopsychosocial model or were not delivered by clinicians of different clinical backgrounds. See Characteristics of excluded studies for more information.
Risk of bias in included studies
We identified four studies (Anema 2007; Karjalainen 2003; Loisel 1997; Schiltenwolf 2006) as having lower risk of bias relative to the other included studies. In particular, these studies suffered from risk of bias in only two bias categories (performance bias and detection bias). Moreover, with the exception of Karjalainen 2003, these studies also included outcomes based on administrative data (e.g. return‐to‐work, sick leave data), thereby minimizing the impact of detection bias for these particular outcomes. The results of the 'risk of bias' assessment are summarized in Figure 2.
2.
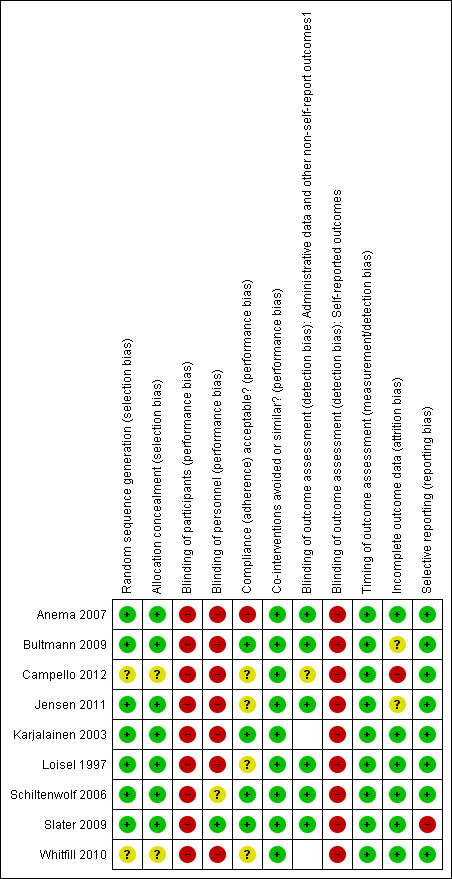
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
We classified seven of the nine included studies as having low risk of selection bias. Methods of ensuring adequate randomizations included computer‐generated random numbers and a lottery system, and treatment allocation was concealed using various methods, including sealed envelopes and conducting the allocation off site. The other two studies (Campello 2012; Whitfill 2010) were classified as having unclear risk of selection bias because they didn't specify the randomizations or allocation concealment methods used.
Blinding
Due to the nature of the MBR intervention, none of the included studies achieved blinding of participants. Indeed, it would have been very difficult to keep participants blind to an intervention that they were actively participating in. However, blinding of personnel was achieved by Slater 2009. In this study assessors and therapists were not told about the alternative treatments and hypotheses, and treatments were conducted in separate areas to prevent cross‐talk.
Measures of pain and disability rely on participant reports of their symptoms, which may be influenced by participants' knowledge of group assignment, as well as their expectations about the effectiveness of different interventions. In fact, in an MBR intervention, where there tends to be a great deal of face‐to‐face time between practitioners and participants, the experience of receiving the intervention may have an important impact on reported outcomes. However, six included studies measured more objective outcomes (e.g. sick leave data from health insurance companies). Thus, for these outcomes, detection bias was low.
Given the challenges of blinding in MBR trials, risk of performance bias and detection bias represented the main source of potential bias in this review. As a result of these study limitations, all of the evidence was downgraded by at least one point in the GRADE assessment (see Quality of the evidence section below).
Incomplete outcome data
We judged six of the nine included studies as having Iow risk of attrition bias. These studies described dropouts in detail and used an intent‐to‐treat analysis, when appropriate. Only one study was rated as having high risk of attrition bias due to over 30% loss to follow‐up in the intervention condition.
Selective reporting
We assessed all but one study in the review as having low risk of reporting bias. The one exception was the study by Slater 2009, which failed to report group differences for some study outcomes.
Other potential sources of bias
There were no additional sources of bias identified.
Effects of interventions
See the summary of findings from our main comparisons: MBR versus usual care (Table 1) and MBR versus other treatment (Table 2). We included all studies in at least one meta‐analysis, with the exception of Anema 2007 and Slater 2009. Anema 2007 reported data in a manner that did not allow for inclusion in the meta‐analysis, and Slater 2009 reported only one outcome (i.e. a dichotomous recovery outcome) that could not be combined with outcomes from other studies.
We estimated standard deviations for all data from Karjalainen 2003 by calculating an estimate of the standard deviation from the same measure reported at baseline in other studies within the comparison. However, in one case this information was not available, so we used ranges to estimate standard deviations by dividing the range by four (sick leave days; Karjalainen 2003). See Characteristics of included studies for (1) findings that could not be incorporated into meta‐analyses and (2) findings for all secondary outcomes.
Multidisciplinary biopsychosocial rehabilitation versus usual care
Pain
Pain intensity short‐term
Very low‐quality evidence from four studies with a total of 272 participants (Bultmann 2009; Campello 2012; Karjalainen 2003; Loisel 1997) showed that MBR was more effective than usual care for short‐term pain improvement (standard mean difference (SMD) ‐0.40, 95% confidence interval (CI) ‐0.74 to ‐0.06) (see Analysis 1.1, Figure 3).
1.1. Analysis.
Comparison 1 Multidisciplinary rehabilitation versus usual care, Outcome 1 Pain.
3.
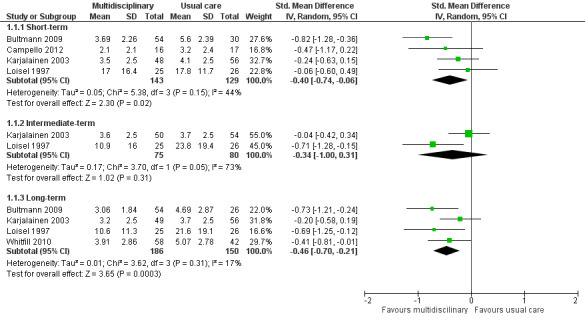
Forest plot of comparison: 1 Multidisciplinary rehabilitation versus usual care, outcome: 1.1 Pain intensity (scales varied from 0 to 10 or 0 to100).
Pain at intermediate‐term
Very low‐quality evidence from two studies with a total of 155 participants (Karjalainen 2003; Loisel 1997) showed that MBR was no better than usual care for intermediate‐term pain improvement (SMD ‐0.34, 95% CI ‐1.0 to 0.31) (see Analysis 1.1, Figure 3).
Pain at long‐term
Moderate quality evidence from five studies with a total of 532 participants reported pain intensity at long‐term. We included four studies with a total of 336 participants in the meta‐analysis (Bultmann 2009; Karjalainen 2003; Loisel 1997; Whitfill 2010) and analysed one study with 196 participants separately (Anema 2007) because results were presented as mean improvements, which could not be combined with means from other studies. Results from the meta‐analysis indicated that MBR was more effective than usual care for long‐term pain improvement (SMD ‐0.46, 95% CI ‐0.70 to ‐0.21) (see Analysis 1.1, Figure 3). Anema 2007 found no differences between the groups. Although it was unclear why the Anema 2007 findings were inconsistent with the pooled effect, it may be due to the comparison group, which included participants who received usual care, as well as those who received graded activity or the work intervention alone. These findings suggested that the combination of graded activity and the work intervention (i.e. MBR) did not have an impact over and above the independent effects of these interventions.
Disability
Disability at short‐term
Very low‐quality evidence from four studies with a total of 272 participants (Bultmann 2009; Campello 2012Karjalainen 2003; Loisel 1997) showed that MBR was more effective than usual care for disability in the short term (SMD ‐0.38, 95% CI ‐0.63 to ‐0.14) (see Analysis 1.2, Figure 4). It should be noted that Bultmann 2009 used an inverted Oswestry scale, such that lower scores indicated more severe disability. Therefore, data from this study were reverse‐coded and then entered into the meta‐analysis. This procedure applied to the long‐term disability results presented below. See Characteristics of included studies for more information about the inverted scale.
1.2. Analysis.
Comparison 1 Multidisciplinary rehabilitation versus usual care, Outcome 2 Disability.
4.
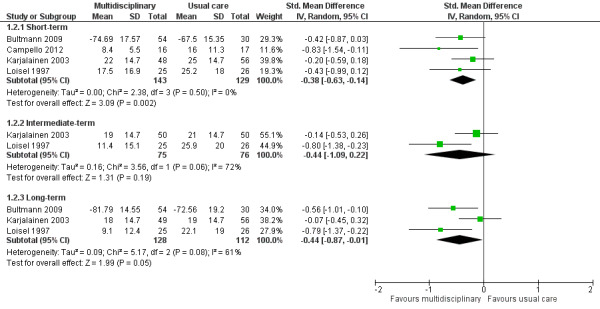
Forest plot of comparison: 1 Multidisciplinary rehabilitation versus usual care, outcome: 1.2 Disability (measured with different continuous scales)
Disability at intermediate‐term
Very low‐quality evidence from two studies with a total of 151 participants (Karjalainen 2003; Loisel 1997) showed that MBR was no better than usual care for disability in the intermediate term (SMD ‐0.44, 95% CI ‐1.09 to 0.22) (see Analysis 1.2, Figure 4).
Diability at long‐term
Low‐quality evidence from five studies with a total of 537 participants reported disability in the long‐term. We included three studies with a total of 240 participants in the meta‐analysis (Bultmann 2009; Karjalainen 2003; Loisel 1997) and reported Whitfill 2010 (101 participants) and Anema 2007 (196 participants) individually. We could not include Anema 2007 in the meta‐analysis because results were presented as mean improvements, which could not be combined with means from other studies, and Whitfill 2010 reported results of a statistical test comparing the two groups but failed to report group means. Results from the meta‐analysis showed that MBR was more effective than usual care for long‐term disability (SMD ‐0.44, 95% CI ‐0.87 to ‐0.01) (see Analysis 1.2, Figure 4). Whitfill 2010 also showed less disability in the MBR group compared to usual care; Anema 2007 found no differences between the groups. Again, it was unclear why the Anema 2007 findings deviated from those of the other studies, but it may be due to the fact that some participants in the comparison group received graded activity or the workplace intervention.
Sick Leave
Sick leave at short‐term
Very low‐quality evidence from one study with 33 participants (Campello 2012) showed that MBR was no better than usual care for short‐term work status; all subjects in both groups were back to duty by the end of the intervention period.
Sick leave at long‐term
Studies that compared MBR to usual care reported long‐term sick leave using three different outcomes: return‐to‐work, time to return‐to‐work, and sick leave periods.
Very low‐quality evidence from three studies with a total of 170 participants (Bultmann 2009; Loisel 1997; Whitfill 2010) showed that MBR was more effective than usual care for return‐to‐work at the long term (OR 3.19, 95% CI 1.46 to 6.98) (see Analysis 1.3, Figure 5).
1.3. Analysis.
Comparison 1 Multidisciplinary rehabilitation versus usual care, Outcome 3 Return‐to‐work at long‐term.
5.
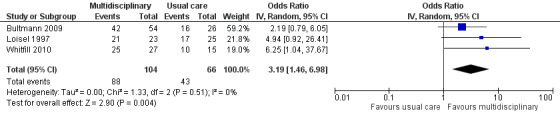
Forest plot of comparison: 1 Multidisciplinary rehabilitation versus usual care, outcome: 1.3 Return‐to‐work at long‐term.
Low‐quality evidence from one study of 196 participants (Anema 2007) showed that MBR was no better than usual care for reducing time to return‐to‐work.
Low‐quality evidence from two studies with a total of 210 participants (Bultmann 2009; Karjalainen 2003) showed that MBR was more effective than usual care in reducing sick leave periods at the long term (SMD ‐0.38, 95% CI ‐0.66 to ‐0.10) (see Analysis 1.4).
1.4. Analysis.
Comparison 1 Multidisciplinary rehabilitation versus usual care, Outcome 4 Sick leave periods at long‐term.
Secondary outcomes at short‐term
One study with 33 participants (Campello 2012) showed no differences between the groups in pain catastrophising, symptoms of depression and fear‐avoidance beliefs.
Secondary outcomes at intermediate‐term
No secondary outcomes were reported at intermediate‐term.
Secondary outcomes at long‐term
One study with 100 participants (Whitfill 2010) showed less depression in the MBR group compared to the usual care group. In terms of quality of life (measured by the SF‐36); the MBR group showed improved physical functioning compared to the usual care group, but there were no group differences in mental functioning.
One study with 105 participants (Karjalainen 2003) examined quality of life at 12 and 24 months. However, MBR was no more effective than usual care in improving quality of life at both time‐points.
One study of 51 participants (Loisel 1997) reported on generic functional status at 12 months. Results suggested that MBR was more effective in improving functional status compared to usual care.
Multidisciplinary biopsychosocial rehabilitation versus other treatment
Pain
Pain intensity short‐term
Very low‐quality evidence from two studies with a total of 165 participants (Karjalainen 2003; Schiltenwolf 2006) showed that MBR was no more effective than another treatment for short‐term pain improvement (SMD ‐0.09, 95%, CI ‐0.50 to 0.33) (see Analysis 2.1, Figure 6).
2.1. Analysis.
Comparison 2 Multidisciplinary rehabilitation versus other treatment, Outcome 1 Pain.
6.
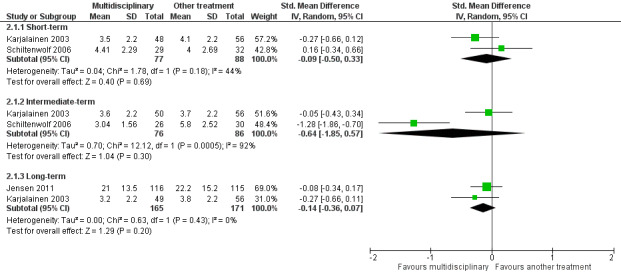
Forest plot of comparison: 2 Multidisciplinary rehabilitation versus other treatment, outcome: 2.1 Pain.
Pain at intermediate‐term
Very low‐quality evidence from two studies with a total of 162 participants (Karjalainen 2003; Schiltenwolf 2006) showed that MBR was no better than another treatment for intermediate‐term pain improvement (SMD ‐0.64, 95% CI ‐1.85 to 0.57) (see Analysis 2.1, Figure 6).
Pain at long‐term
Low‐quality evidence from two studies with a total of 336 participants (Jensen 2011; Karjalainen 2003) showed that MBR was no better than another treatment for long‐term pain improvement (SMD ‐0.14, 95% CI ‐0.36 to 0.07) (see Analysis 2.1, Figure 6).
Disability
Disability at short‐term
Low‐quality evidence from two studies with a total of 165 participants (Karjalainen 2003; Schiltenwolf 2006) showed that MBR was no more effective than another treatment for disability at the short term (SMD ‐0.00, 95%, CI ‐0.34 to 0.34) (see Analysis 2.2, Figure 7).
2.2. Analysis.
Comparison 2 Multidisciplinary rehabilitation versus other treatment, Outcome 2 Disability.
7.
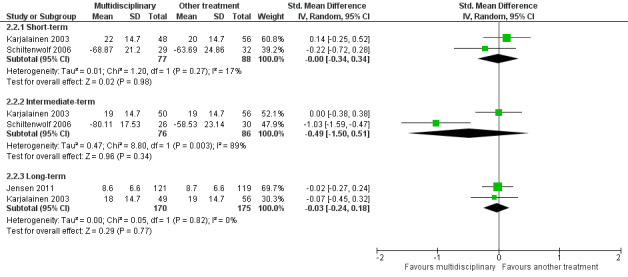
Forest plot of comparison: 2 Multidisciplinary rehabilitation versus other treatment, outcome: 2.2 Disability (Different instruments).
Disability at intermediate‐term
Very low‐quality evidence from two studies with a total of 162 participants (Karjalainen 2003; Schiltenwolf 2006) showed that MBR was no more effective than another treatment for disability at the intermediate term (SMD ‐0.49, 95% CI ‐1.50 to 0.51) (see Analysis 2.2, Figure 7).
Very low‐quality evidence from one study of 65 participants (Slater 2009) showed no difference in the proportion of participants recovered (defined in terms of pain and function) when comparing the MBR group to another treatment.
Disability at long‐term
Low‐quality evidence from two studies with a total of 345 participants (Jensen 2011; Karjalainen 2003) showed that MBR was no better than another treatment for disability at the long term (SMD ‐0.03, 95% CI ‐0.24 to 0.18) (see Analysis 2.2, Figure 7).
Sick Leave
Sick leave at long‐term
Studies that compared MBR to another treatment reported two different sick leave outcomes: time to return‐to‐work and sick leave periods.
Low‐quality evidence from one study of 351 participants (Jensen 2011) showed that MBR was no more effective than another treatment in reducing time to return‐to work.
Very low‐quality evidence from two studies with a total of 158 participants (Karjalainen 2003; Schiltenwolf 2006) showed that MBR was no more effective than another treatment in reducing sick leave periods at the long term (SMD ‐0.25, 95% CI ‐0.98 to 0.47) (see Analysis 2.3).
2.3. Analysis.
Comparison 2 Multidisciplinary rehabilitation versus other treatment, Outcome 3 Sick leave days at long‐term.
Secondary outcomes at short‐term
No secondary outcomes were reported at the short‐term.
Secondary outcomes at intermediate‐term
One study of 56 participants examined depression at six months (Schiltenwolf 2006). Results indicated that MBR effectively reduced depressive dysfunction compared to another treatment.
Secondary outcomes at long‐term
One study examined fear‐avoidance and general health (SF‐36) and mental health (SF‐36) at 12 months (numbers of participants ranged from 237 to 244) (Jensen 2011). Results indicated that MBR was no more effective in reducing fear avoidance or improving physical functioning compared to another treatment. However, MBR more effectively improved mental health compared to another treatment.
Subgroup Analyses
We planned to conduct subgroup analyses to examine the treatment effect in studies with higher versus lower baseline symptom intensity and higher versus lower intervention intensity. We were unable to conduct any subgroup analyses because we did not identify any studies that met our criteria for higher baseline symptom intensity, or studies with interventions that met our criteria for higher intervention intensity.
Sensitivity Analyses
We planned to conduct sensitivity analyses to examine the treatment effect for studies with low risk of selection bias. However, we identified too few studies to conduct these analyses, as planned.
Discussion
Summary of main results
This review included nine published RCTs, with data from close to 1000 participants. Overall, we found moderate to very low‐quality evidence in favour of MBR compared to usual care for subacute LBP. In particular, compared to individuals receiving usual care, individuals receiving MBR showed less pain and back‐specific disability, as well as increased likelihood of return‐to‐work and fewer sick leave days at 12‐month follow‐up. When comparing MBR to other treatments, we found low to very low‐quality evidence that MBR was no better than other treatments (i.e. brief intervention with features from a light mobilization program and a graded activity program, functional restoration, brief clinical intervention including education and advice on exercise, and psychological counselling) for reducing pain and disability, and reducing time away from work.
Although more evidence is needed to increase our confidence in these results, the effect estimates for pain and disability were consistent across all follow‐up points. For pain, they translated to about a 1‐point difference on a 10‐point scale, and for disability, they translated to around a 6.5‐ to 9‐point difference on a 100‐point scale. These effect sizes are at the low end of the range of estimates of clinical meaningfulness. Effects on work‐related outcomes were somewhat larger; participants receiving MBR had more than three times the odds of return‐to‐work at one year compared to participants receiving usual care, and fewer sickness absence periods (approximately 215 to 254 hours) at follow‐up.
Our findings suggest that MBR treatments are no better than other treatments for improving outcomes among people with subacute LBP. This was true across all primary outcomes, and at short‐, intermediate‐, and long‐term follow‐up points.
Findings for secondary outcomes
Studies in this review examined secondary outcomes, including symptoms of depression, pain catastrophising, fear‐avoidance beliefs, general health, generic functional status and quality of life. However, we were unable to synthesize results across studies because of a lack of common outcomes across comparisons and follow‐up periods. Results were generally inconsistent across studies ‐ some showed improvements in the MBR group when compared to usual care or another treatment, while others showed no differences between the groups. The most consistent findings related to mental health outcomes at intermediate‐ and long‐term follow‐up. Namely, two studies showed benefits of MBR for reducing symptoms of depression and one showed benefits of MBR for improving mental health more generally. This points to the potential benefits of MBR for psychological well being among participants with subacute LBP and should be considered more fully in future research. However, it should be noted that we did not assess the quality of the evidence for these findings, and it is probably too early to use them as a basis for clinical decision‐making.
Overall completeness and applicability of evidence
This review provides initial answers about the effectiveness of MBR for people with subacute LBP. However, the literature failed to cover all relevant types of participants, interventions, and outcomes. In particular, the evidence was based on a relatively homogenous group of participants. For example, all of the studies were conducted in North America and Europe, so it was unclear whether our findings generalized to people outside of these geographic areas. Moreover, all of the included studies were based on populations with relatively low baseline symptom intensity, thus we were unable to examine whether the treatment effect differed for people with high‐ versus low‐intensity symptoms. However, we categorized symptom intensity based on the cut‐off used by Kamper and colleagues in the Cochrane Review on MBR for chronic LBP (Kamper 2014) (greater than 60% of the maximum possible score on a pain and a disability measure), which may not be valid for people with subacute LBP.
We encountered the same issue when it came to classification of MBR interventions as high versus low‐intensity. In particular, although we noted MBR interventions that ranged in intensity (e.g. 'mini' intervention plus worksite visit (< three hours total)) in Karjalainen 2003 versus 36‐hour intervention in Campello 2012), none fell into the 'high‐intensity' category based on our pre‐established definition (i.e. > 100 face‐to‐face hours delivered on a daily basis) (Kamper 2014). Therefore, we were unable to examine whether intervention intensity influenced the treatment effect. Further consideration of the impact of intervention intensity on treatment outcome will be important for future updates, as it has implications for both the effectiveness and cost‐effectiveness of MBR interventions.
In regard to the outcomes reported, none of the studies in this review assessed adverse events, but given the nature of the intervention, the risk was considered low. Further, work outcomes and healthcare utilisation are key considerations for assessing the effects of MBR in this population, since they are primary determinants of the societal burden of the condition (Maetzal 2002). Many of the included studies did not report these outcomes, and when reported they were measured in different ways. For example, in our analysis of work status at long‐term follow up, we were able to statistically combine only three of the six studies that compared MBR to usual care.
Quality of the evidence
The findings from this review provided mainly low to very low‐quality evidence regarding the effectiveness of MBR in this population. We mainly downgraded studies due to lack of blinding, which increased risk of performance bias, as well as risk of detection bias (i.e. biased self‐reports of pain and disability due to knowledge of treatment group). However, effect sizes were similar for self‐report outcomes (i.e. pain and disability) and behavioral outcomes (i.e. return‐to‐work and sickness absence), which are less susceptible to bias. This consistency across the different types of outcomes suggested that our findings may not be unduly influenced by bias associated with self‐reported outcomes.
We also downgraded the evidence due to inconsistency. There was heterogeneity across participants and interventions, but we were unable to conduct subgroup analyses due to the limited number of studies identified. As a result, we lumped together some studies with a good deal of clinical heterogeneity. For example, we combined a low‐intensity MBR intervention (i.e. 'mini' clinical intervention plus worksite visit (< three hours total) (Karjalainen 2003)) together with more intense interventions, but we were unable to explore the impact of this on our treatment estimates. Despite this, our results were quite consistent across outcomes for the MBR versus usual care comparison, which increases confidence in the treatment estimates.
In regard to the MBR versus 'other treatment' findings, clinical heterogeneity among the 'other treatments' may have contributed to the inconsistent effects across studies. For example, two studies compared MBR to a brief intervention, including a physical examination and advice to stay active (Jensen 2011; Karjalainen 2003), one study compared MBR to a more extensive functional restoration program (Schiltenwolf 2006), and one study included a comparison that was mainly psychological in nature (Slater 2009). With only two studies included in each meta‐analysis, it is likely that further research would change our estimates of effectiveness. Observed inconsistencies may be due to characteristics of the MBR intervention, characteristics of the comparison intervention, or a combination of the two.
We downgraded the evidence due to imprecision for both comparisons. We were dealing with small sample sizes, especially for the analyses looking at work‐related outcomes. Therefore, the quality of the evidence and our confidence in the results will be much improved with the publication of large RCTs that consider common outcomes.
Finally, it should be noted that there is one completed study for which we were unable to find any published articles (see Characteristics of studies awaiting classification).
Potential biases in the review process
First, it is challenging to operationalise the term 'multidisciplinary', and there is no universally accepted definition of MBR (Deyo 2015). Our definition is consistent with both the biopsychosocial model and previous Cochrane Reviews on this topic (Guzman 2006; Karjalainen 2003). However, it is possible that selection of a different definition could result in inclusion of different studies and hence different effect estimates (see Kamper 2014 for a more detailed discussion).
Second, our definition of MBR was quite stringent, as we required involvement of healthcare professionals from at least two different clinical backgrounds. If future work shows that these types of MBR interventions are indeed effective for subacute LBP, a next step will be to examine whether similar effects are observed when less stringent definitions of MBR are applied.
Third, we have included in our meta‐analyses all studies irrespective of their risk of bias. Including studies with high risk of bias may have affected the point estimates, particularly in cases indicating statistical heterogeneity.
Finally, we made the decision a priori to omit non‐published and non‐peer‐reviewed studies from the review, which may have contributed to publication bias given that publication status may be associated with positive study results. Unfortunately the influence of publication bias on our results was difficult to assess due to the limited number of studies contributing to each pooled estimate. However, we have one study awaiting assessment (Rodriguez‐Blanco) with almost the same number of patients as our review (932) which is evidence of publication bias. Publication of this study could substantially change the conclusions of this review.
Agreements and disagreements with other studies or reviews
Our results were fairly consistent with those of the Cochrane Review on MBR for chronic LBP (Kamper 2014). In particular, both reviews found evidence for the effectiveness of MBR when compared to usual care. However, whereas the current review did not find any evidence that MBR was more effective than other treatments for subacute LBP, Kamper and colleagues found that MBR was more effective than other physical interventions for chronic LBP. Although it was unclear why this was the case, it may be due to varying disease trajectories among people with subacute LBP ‐ some will improve regardless of treatment, others will do well with simple monotherapies, and others will only improve with more tailored treatment approaches, such as MBR. In contrast, by the time people reach the chronic stage of LBP with its associated psychosocial stressors (e.g. increased time away from work, greater vulnerability to depression), we can expect a greater proportion of people to benefit from a multidisciplinary approach. If this is the case, it may be more effective to target subgroups of people with subacute LBP who are most likely to benefit from MBR.
We also compared our findings to a Cochrane Review on physical conditioning for workers with LBP (Schaafsma 2013), which shared three included studies with our review (Jensen 2011, Karjalainen 2003, Loisel 1997). Schaafsma and colleagues showed that physical conditioning reduced sickness absence duration compared to usual care among participants with subacute LBP, but only when the intervention took place in the workplace or it included a workplace visit. This finding is consistent with our results, which also suggested that a physical intervention may be effective when combined with a psychological or workplace intervention or both.
An important next step will be to disentangle the impact of the physical, psychological and workplace components and to identify underlying mechanisms. To this end, it would be informative to compare our results with those of other reviews examining monotherapies for subacute LBP, especially those relating to MBR, such as back schools, graded behavioral activity, workplace interventions, and psychological interventions, such as cognitive behavior therapy. However, we were unable to find up‐to‐date reviews that examined these interventions among participants with subacute LBP.
Authors' conclusions
Implications for practice.
On average, people with subacute LBP that receive MBR will do better than if they receive usual care, but it is not clear whether they do better than people who receive some other type of treatment. However, the available research provides mainly low to very low‐quality evidence, thus additional high‐quality trials are needed before we can make definitive recommendations for clinical practice.
Implications for research.
There is a need for additional large, high‐quality RCTs, which would assess the effectiveness of comprehensive MBR programs for people with subacute LBP, as well as the effectiveness of the specific components involved in rehabilitation. Moreover, given that these programs are so costly, we recommend that future RCTs include economic analyses to fully examine the costs and benefits of MBR for this population.
What's new
| Date | Event | Description |
|---|---|---|
| 1 November 2016 | New citation required and conclusions have changed | The previous version of this review (based on two included studies) (Karjalainen 2003) found evidence in favour of multidisciplinary biopsychosocial rehabilitation (MBR) for subacute low back pain. The authors concluded that MBR helped participants to return to work faster, resulted in fewer episodes of sick leave and alleviated subjective disability. In the current update (based on nine included studies), we also found evidence in support of MBR for return to work, sick leave, pain intensity, and disability, but only in comparison to usual care. When comparing MBR to other treatments, there was no benefit of MBR. Moreover, using up‐to‐date methods for grading the quality of the evidence, we determined that these findings were based on mainly low to very low‐quality evidence, indicating that additional high‐quality studies were needed before we could draw any definitive conclusions about the effectiveness of MBR for subacute low back pain. |
| 1 November 2016 | New search has been performed | For this update, we incorporated new studies and used up‐to‐date methods to assess risk of bias and the overall quality of the evidence. |
History
Protocol first published: Issue 3, 2000 Review first published: Issue 3, 2000
| Date | Event | Description |
|---|---|---|
| 19 June 2008 | Amended | Converted to new review format. |
| 31 January 2003 | New search has been performed | The literature search was last updated in November 2002 in EMBASE and MEDLINE. No new trials were identified. |
Notes
None
Acknowledgements
We are grateful to Shireen Harbin (Cochrane Back and Neck Group Managing Editor) for assisting with literature searches and the editorial process more generally, as well as Ivan Steenstra, Sara Morassaei, Sara Macdonald and Dominik Irnich for helping to translate the nonEnglish studies. Thank you to Lenny Vasanthan for assisting with the literature search and data extraction.
Appendices
Appendix 1. Search Strategies
CENTRAL
Last searched 13 July 2016. Spinal neoplasms was removed and lines 8 and 10 were truncated.
#1 MeSH descriptor: [Back Pain] explode all trees
#2 dorsalgia #3 backache
#4 MeSH descriptor: [Low Back Pain] explode all trees
#5 lumb* next pain or coccyx or coccydynia or sciatica or spondylosis
#6 MeSH descriptor: [Spine] explode all trees
#7 MeSH descriptor: [Spinal Diseases] explode all trees
#8 lumbago or discitis or disc near herniat*
#9 spinal fusion
#10 facet near joint*
#11 MeSH descriptor: [Intervertebral Disk] explode all trees
#12 postlaminectomy
#13 arachnoiditis
#14 failed near back
#15 MeSH descriptor: [Cauda Equina] explode all trees
#16 lumbar near vertebra*
#17 spinal near stenosis
#18 slipped near (disc* or disk*)
#19 degenerat* near (disc* or disk*)
#20 stenosis near (spine or root or spinal)
#21 displace* near (disc* or disk*)
#22 prolap* near (disc* or disk*)
#23 MeSH descriptor: [Sciatic Neuropathy] explode all trees
#24 sciatic*
#25 back disorder*
#26 back near pain
#27 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26
#28 MeSH descriptor: [Patient Care Team] this term only
#29 MeSH descriptor: [Patient Care Management] explode all trees
#30 MeSH descriptor: [C
omprehensive Health Care] explode all trees
#31 MeSH descriptor: [Pain Clinics] explode all trees
#32 multidisciplinary
#33 interdisciplinary
#34 multiprofessional
#35 multi‐professional
#36 multimodal
#37 multi‐modal
#38 functional restoration
#39 biopsychosocial
#40 MeSH descriptor: [Patient Education as Topic] explode all trees
#41 MeSH descriptor: [Social Support] explode all trees
#42 MeSH descriptor: [Social Environment] explode all trees
#43 (pain clinic* or pain center* or pain service* or pain relief unit* or pain centr*) #44 MeSH descriptor: [Social Work] explode all trees
#45 MeSH descriptor: [Occupational Therapy] explode all trees
#46 MeSH descriptor: [Rehabilitation, Vocational] explode all trees
#47 MeSH descriptor: [Rehabilitation Centers] explode all trees
#48 #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47
#49 #27 and #48
#50 #49 in Trials
#51 #49 Publication Year from 2015 to 2016, in Trials
2015 search. This search was revised in 2012.
#1 MeSH descriptor: [Back Pain] explode all trees
#2 dorsalgia
#3 backache
#4 MeSH descriptor: [Low Back Pain] explode all trees
#5 lumbar next pain OR coccyx OR coccydynia OR sciatica OR spondylosis
#6 MeSH descriptor: [Spine] explode all trees
#7 MeSH descriptor: [Spinal Diseases] explode all trees
#8 lumbago or discitis or disc near herniation
#9 spinal fusion
#10 spinal neoplasms
#11 facet near joints
#12 MeSH descriptor: [Intervertebral Disk] explode all trees
#13 postlaminectomy
#14 arachnoiditis
#15 failed near back
#16 MeSH descriptor: [Cauda Equina] explode all trees
#17 lumbar near vertebra*
#18 spinal near stenosis
#19 slipped near (disc* or disk*)
#20 degenerat* near (disc* or disk*)
#21 stenosis near (spine or root or spinal)
#22 displace* near (disc* or disk*)
#23 prolap* near (disc* or disk*)
#24 MeSH descriptor: [Sciatic Neuropathy] explode all trees
#25 sciatic*
#26 back disorder*
#27 back near pain
#28 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27
#29 MeSH descriptor: [Patient Care Team] this term only
#30 MeSH descriptor: [Patient Care Management] explode all trees
#31 MeSH descriptor: [Comprehensive Health Care] explode all trees
#32 MeSH descriptor: [Pain Clinics] explode all trees
#33 multidisciplinary
#34 interdisciplinary
#35 multiprofessional
#36 multi‐professional
#37 multimodal
#38 multi‐modal
#39 functional restoration
#40 biopsychosocial
#41 MeSH descriptor: [Patient Education as Topic] explode all trees
#42 MeSH descriptor: [Social Support] explode all trees
#43 MeSH descriptor: [Social Environment] explode all trees
#44 (pain clinic* or pain center* or pain service* or pain relief unit* or pain centr*)
#45 MeSH descriptor: [Social Work] explode all trees
#46 MeSH descriptor: [Occupational Therapy] explode all trees
#47 MeSH descriptor: [Rehabilitation, Vocational] explode all trees
#48 MeSH descriptor: [Rehabilitation Centers] explode all trees
#49 #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 or #44 or #45 or #46 or #47 or #48
#50 #28 and #49
#51 #50 in Trials
#52 #50 Publication Year from 2014 to 2015, in Trials
2009 search
#1 MeSH descriptor Back explode all trees
#2 MeSH descriptor Buttocks, this term only
#3 MeSH descriptor Leg, this term only
#4 MeSH descriptor Back Pain explode tree
#5 MeSH descriptor Back Injuries explode all trees
#6 MeSH descriptor Low Back Pain, this term only
#7 MeSH descriptor Sciatica, this term only
#8 (low next back next pain)
#9 (lbp)
#10 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9)
#11 MeSH descriptor Patient Care Team explode all trees
#12 MeSH descriptor Patient Care Management explode all trees
#13 MeSH descriptor Comprehensive Health Care explode all trees
#14 MeSH descriptor Pain Clinics explode all trees
#15 multidisciplinary
#16 interdisciplinary
#17 multiprofessional
#18 multi‐professional
#19 multimodal
#20 multi‐modal
#21 pain clinic
#22 functional restoration
#23 (#11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19 OR #20 OR #21 OR #22)
#24 (#10 AND #23)
#25 (#24), from 2007 to 2009
MEDLINE
Last searched 13 July 2016 in (Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R)) databases. Line 44 was edited.
randomized controlled trial.pt. (424517)
controlled clinical trial.pt. (91243)
pragmatic clinical trial.pt. (374)
randomized.ab. (363335)
placebo.ab,ti. (181201)
drug therapy.fs. (1884948)
randomly.ab,ti. (260321)
trial.ab,ti. (444976)
groups.ab,ti. (1640064)
or/1‐9 (3896754)
(animals not (humans and animals)).sh. (4244832)
10 not 11 (3364181)
multidisciplinar$.mp. (57792)
interdisciplinar$.mp. (38624)
multiprofessional$.mp. (1019)
multimodal$.mp. (37056)
exp Patient Care Team/ (59423)
exp Patient Care Management/ (631671)
exp Patient Education/ (75868)
exp Social Support/ (58144)
exp Social Environment/
exp Pain Clinics/
(pain clinic$ or pain center$ or pain service$ or pain relief unit$ or pain centr$).mp.*
exp Social Work/
exp Occupational Therapy/
exp Rehabilitation/ or exp Rehabilitation Centers/ or exp Rehabilitation, Vocational/
exp Treatment Outcome/
exp Behavior Therapy/
"Recovery of Function"/
functional restoration.mp.
*Pain/rh
or/13‐31
exp Arthritis, Rheumatoid/
exp Neoplasms/
exp Musculoskeletal Diseases/cn, su [Congenital, Surgery]
exp Central Nervous System/
exp Central Nervous System Diseases/
exp Dentistry/
exp Tooth Diseases/
or/33‐39
dorsalgia.ti,ab.
exp Back Pain/
backache.ti,ab.
((lumb$ or back) adj pain).ti,ab.
coccyx.ti,ab.
coccydynia.ti,ab.
sciatica.ti,ab.
sciatica/
spondylosis.ti,ab.
lumbago.ti,ab.
exp low back pain/
or/41‐51
52 and 12 and 32
53 not 40
limit 54 to yr=2015‐2016
limit 54 to ed=20150615‐20160713
55 or 56
*.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
2015 search. Medine In‐Process was added in 2014. Line 3 added in 2015.
randomized controlled trial.pt.
controlled clinical trial.pt.
pragmatic clinical trial.pt.
randomized.ab.
placebo.ab,ti.
drug therapy.fs.
randomly.ab,ti.
trial.ab,ti.
groups.ab,ti.
or/1‐9
(animals not (humans and animals)).sh.
10 not 11
multidisciplinar$.mp.
interdisciplinar$.mp.
multiprofessional$.mp.
multimodal$.mp.
exp Patient Care Team/
exp Patient Care Management/
exp Patient Education/
exp Social Support/
exp Social Environment/
exp Pain Clinics/
(pain clinic$ or pain center$ or pain service$ or pain relief unit$ or pain centr$).mp.*
exp Social Work/
exp Occupational Therapy/
exp Rehabilitation/ or exp Rehabilitation Centers/ or exp Rehabilitation, Vocational/
exp Treatment Outcome/
exp Behavior Therapy/
"Recovery of Function"/
functional restoration.mp.
*Pain/rh
or/13‐31
exp Arthritis, Rheumatoid/
exp Neoplasms/
exp Musculoskeletal Diseases/cn, su [Congenital, Surgery]
exp Central Nervous System/
exp Central Nervous System Diseases/
exp Dentistry/
exp Tooth Diseases/
or/33‐39
dorsalgia.ti,ab.
exp Back Pain/
backache.ti,ab.
(lumbar adj pain).ti,ab.
coccyx.ti,ab.
coccydynia.ti,ab.
sciatica.ti,ab.
sciatica/
spondylosis.ti,ab.
lumbago.ti,ab.
exp low back pain/
or/41‐51
52 and 12 and 32
53 not 40
limit 54 to yr=2014‐2015
limit 54 to ed=20140131‐20150615
55 or 56
*.mp. [mp=title, abstract, original title, name of substance word, subject heading word, keyword heading word, protocol supplementary concept word, rare disease supplementary concept word, unique identifier]
2012 search. This RCT filter was added in the 2009 search. Line 30 was added in 2012.
randomized controlled trial.pt.
controlled clinical trial.pt.
randomized.ab.
placebo.ab,ti.
drug therapy.fs.
randomly.ab,ti.
trial.ab,ti.
groups.ab,ti.
or/1‐8
(animals not (humans and animals)).sh.
9 not 10
multidisciplinar$.mp.
interdisciplinar$.mp.
multiprofessional$.mp.
multimodal$.mp.
exp Patient Care Team/
exp Patient Care Management/
exp Patient Education/
exp Social Support/
exp Social Environment/
exp Pain Clinics/
(pain clinic$ or pain center$ or pain service$ or pain relief unit$ or pain centr$).mp.*
exp Social Work/
exp Occupational Therapy/
exp Rehabilitation/ or exp Rehabilitation Centers/ or exp Rehabilitation, Vocational/
exp Treatment Outcome/
exp Behavior Therapy/
"Recovery of Function"/
functional restoration.mp.
*Pain/rh
or/12‐30
exp Arthritis, Rheumatoid/
exp Neoplasms/
exp Musculoskeletal Diseases/cn, su [Congenital, Surgery]
exp Central Nervous System/
exp Central Nervous System Diseases/
exp Dentistry/
exp Tooth Diseases/
or/32‐38
dorsalgia.ti,ab.
exp Back Pain/
backache.ti,ab.
(lumbar adj pain).ti,ab.
coccyx.ti,ab.
coccydynia.ti,ab.
sciatica.ti,ab.
sciatica/
spondylosis.ti,ab.
lumbago.ti,ab.
exp low back pain/
or/40‐50
51 and 11 and 31
52 not 39
limit 53 to yr="2009 ‐ 2012"
limit 53 to ed=20090101‐20120418
54 or 55
*.mp. [mp=title, abstract, original title, name of substance word, subject heading word, protocol supplementary concept, rare disease supplementary concept, unique identifier]
2007 search
exp "Clinical Trial [Publication Type]"/
randomized.ab,ti.
placebo.ab,ti.
dt.fs.
randomly.ab,ti.
trial.ab,ti.
groups.ab,ti.
or/1‐7
Animals/
Humans/
9 not (9 and 10)
8 not 11
multidisciplinar$.mp.
interdisciplinar$.mp.
multiprofessional$.mp.
multimodal$.mp.
exp Patient Care Team/
exp Patient Care Management/
exp Patient Education/
exp Social Support/
exp Social Environment/
exp Pain Clinics/
(pain clinic$ or pain center$ or pain service$ or pain relief unit$ or pain centr$).mp.*
exp Social Work/
exp Occupational Therapy/
exp Rehabilitation/ or exp Rehabilitation Centers/ or exp Rehabilitation, Vocational/
exp Treatment Outcome/
exp Behavior Therapy/
"Recovery of Function"/
functional restoration.mp.
or/13‐30
exp Arthritis, Rheumatoid/
exp Neoplasms/
exp Musculoskeletal Diseases/cn, su [Congenital, Surgery]
exp Central Nervous System/
exp Central Nervous System Diseases/
exp Dentistry/
exp Tooth Diseases/
or/32‐38
dorsalgia.ti,ab.
exp Back Pain/
backache.ti,ab.
(lumbar adj pain).ti,ab.
coccyx.ti,ab.
coccydynia.ti,ab.
sciatica.ti,ab.
sciatica/
spondylosis.ti,ab.
lumbago.ti,ab.
exp low back pain/
or/40‐50
12 and 31 and 51
52 not 39
limit 53 to yr="2002 ‐ 2007"
*.mp. [mp=title, original title, abstract, name of substance word, subject heading word]
EMBASE
Last searched 13 July 2016. Edited line 62 and added line 66.
Randomized Controlled Trial/
exp Controlled Clinical Trial/
Controlled Study/
Double Blind Procedure/
Single Blind Procedure/
crossover procedure/
placebo/
allocat$.mp.
assign$.mp.
blind$.mp.
((control$ or compar$ or prospectiv$ or clinical) adj25 (trial or study)).mp.
(crossover or cross‐over).mp.
factorial$.mp.
(followup or follow‐up).mp.
placebo$.mp.
random$.mp.
((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).mp.
volunteer$.mp.
or/1‐18
exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/
human/ or normal human/ or human cell/
20 and 21
20 not 22
19 not 23
multidisciplinar$.mp.
interdisciplinar$.mp.
multiprofessional$.mp.
multimodal$.mp.
patient care team.mp.
exp Patient Care/
patient care management.mp.
exp Patient Education/
exp Social Support/
exp Social Environment/
exp Pain Clinic/
(pain clinic$ or pain center$ or pain service$ or pain relief unit$ or pain centre$).mp.*
exp Occupational Therapy/
exp Social Work/
exp Vocational Rehabilitation/
exp Rehabilitation Center/
rehabilitation clinic$.mp.
exp REHABILITATION/
exp Treatment Outcome/
behavior therapy.mp. or exp Behavior Therapy/
or/25‐44
exp Rheumatoid Arthritis/
exp NEOPLASM/
exp Musculoskeletal Disease/cn, su [Congenital Disorder, Surgery]
exp Central Nervous System/
exp Central Nervous System Disease/
exp Tooth Disease/
exp Musculoskeletal System Inflammation/
exp Musculoskeletal System Malformation/
exp HEADACHE/
exp Osteoarthritis/
or/46‐55
24 and 45
57 not 56
dorsalgia.mp.
back pain.mp.
exp BACKACHE/
(lumb$ adj pain).mp.
coccyx.mp.
coccydynia.mp.
sciatica.mp.
sciatica/
exp ISCHIALGIA/
spondylosis.mp.
lumbago.mp.
exp Low Back Pain/
or/59‐70
58 and 71
limit 72 to yr="2015‐2016"
limit 72 to em=201524‐201628
73 or 74
*.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
2015 search. The study design filter was revised.
Randomized Controlled Trial/
exp Controlled Clinical Trial/
Controlled Study/
Double Blind Procedure/
Single Blind Procedure/
crossover procedure/
placebo/
allocat$.mp.
assign$.mp.
blind$.mp.
((control$ or compar$ or prospectiv$ or clinical) adj25 (trial or study)).mp.
(crossover or cross‐over).mp.
factorial$.mp.
(followup or follow‐up).mp.
placebo$.mp.
random$.mp.
((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).mp.
volunteer$.mp.
or/1‐18
exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/
human/ or normal human/ or human cell/
20 and 21
20 not 22
19 not 23
multidisciplinar$.mp.
interdisciplinar$.mp.
multiprofessional$.mp.
multimodal$.mp.
patient care team.mp.
exp Patient Care/
patient care management.mp.
exp Patient Education/
exp Social Support/
exp Social Environment/
exp Pain Clinic/
(pain clinic$ or pain center$ or pain service$ or pain relief unit$ or pain centre$).mp.*
exp Occupational Therapy/
exp Social Work/
exp Vocational Rehabilitation/
exp Rehabilitation Center/
rehabilitation clinic$.mp.
exp REHABILITATION/
exp Treatment Outcome/
behavior therapy.mp. or exp Behavior Therapy/
or/25‐44
exp Rheumatoid Arthritis/
exp NEOPLASM/
exp Musculoskeletal Disease/cn, su [Congenital Disorder, Surgery]
exp Central Nervous System/
exp Central Nervous System Disease/
exp Tooth Disease/
exp Musculoskeletal System Inflammation/
exp Musculoskeletal System Malformation/
exp HEADACHE/
exp Osteoarthritis/
or/46‐55
24 and 45
57 not 56
dorsalgia.mp.
back pain.mp.
exp BACKACHE/
(lumbar adj pain).mp.
coccyx.mp.
coccydynia.mp.
sciatica.mp.
exp ISCHIALGIA/
spondylosis.mp.
lumbago.mp.
exp Low Back Pain/
or/59‐69
58 and 70
limit 71 to yr="2014 ‐ 2015"
limit 71 to em=201404‐201524
*.mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword]
2007 search. In 2014, line 31 was revised to 14 OR 30 and the animal filter was revised to the most recent version above.
Clinical Article/
exp Clinical Study/
Clinical Trial/
Controlled Study/
Randomized Controlled Trial/
Major Clinical Study/
Double Blind Procedure/
Multicenter Study/
Single Blind Procedure/
Phase 3 Clinical Trial/
Phase 4 Clinical Trial/
crossover procedure/
placebo/
or/1‐13
allocat$.mp.
assign$.mp.
blind$.mp.
(clinic$ adj25 (study or trial)).mp.
compar$.mp.
control$.mp.
cross?over.mp.
factorial$.mp.
follow?up.mp.
placebo$.mp.
prospectiv$.mp.
random$.mp.
((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).mp.
trial.mp.
(versus or vs).mp.
or/15‐29
14 and 30
human/
Nonhuman/
exp ANIMAL/
Animal Experiment/
33 or 34 or 35
32 not 36
31 not 36
37 and 38
38 or 39
multidisciplinar$.mp.
interdisciplinar$.mp.
multiprofessional$.mp.
multimodal$.mp.
patient care team.mp.
exp Patient Care/
patient care management.mp.
exp Patient Education/
exp Social Support/
exp Social Environment/
exp Pain Clinic/
(pain clinic$ or pain center$ or pain service$ or pain relief unit$ or pain centre$).mp.*
exp Occupational Therapy/
exp Social Work/
exp Vocational Rehabilitation/
exp Rehabilitation Center/
rehabilitation clinic$.mp.
exp REHABILITATION/
exp Treatment Outcome/
behavior therapy.mp. or exp Behavior Therapy/
or/41‐60
exp Rheumatoid Arthritis/
exp NEOPLASM/
exp Musculoskeletal Disease/cn, su [Congenital Disorder, Surgery]
exp Central Nervous System/
exp Central Nervous System Disease/
exp Tooth Disease/
exp Musculoskeletal System Inflammation/
exp Musculoskeletal System Malformation/
exp HEADACHE/
exp Osteoarthritis/
or/62‐71
40 and 61
73 not 72
dorsalgia.mp.
back pain.mp.
exp BACKACHE/
(lumbar adj pain).mp.
coccyx.mp.
coccydynia.mp.
sciatica.mp.
exp ISCHIALGIA/
spondylosis.mp.
lumbago.mp.
exp Low Back Pain/
or/75‐85
74 and 86
limit 87 to yr="2002 ‐ 2007"
*.mp. [mp=title, abstract, subject headings, heading word, drug trade name, original title, device manufacturer, drug manufacturer name]
CINAHL
Last searched 13 July 2016. EBSCO has been used since 2009
S89 S87 OR S88
S88 S86 AND EM 20150615‐20160713
S87 S86 Limiters ‐ Published Date: 20150601‐20160731
S86 S85 NOT S84
S85 S49 and S76
S84 S77 or S78 or S79 or S80 or S81 or S82 or S83
S83 (MH "Tooth Diseases+")
S82 (MH "Dentistry+")
S81 (MH "Central Nervous System Diseases+")
S80 (MH "Central Nervous System+")
S79 (MH "Musculoskeletal Diseases/FG/SU")
S78 (MH "Neoplasms+")
S77 (MH "Arthritis, Rheumatoid+")
S76 S50 or S51 or S52 or S53 or S54 or S55 or S56 or S57 or S58 or S59 or S60 or S61 or S62 or S63 or S64 or S65 or S66 or S67 or S68 or S69 or S70 or S71 or S72 or S73 or S74 or S75
S75 (MH "Behavior Therapy+")
S74 (MH "Treatment Outcomes+")
S73 "rehabilitation clinic*"
S72 (MH "Rehabilitation Centers+")
S71 (MH "Rehabilitation+")
S70 (MH "Rehabilitation, Vocational+")
S69 (MH "Occupational Therapy+")
S68 (MH "Social Work+")
S67 "pain relief unit*"
S66 "pain service*"
S65 "pain centre*"
S64 "pain center*"
S63 (MH "Pain Clinics")
S62 (MH "Social Environment+")
S61 (MH "Support, Psychosocial+")
S60 (MH "Patient Education")
S59 "patient care management"
S58 (MH "Patient Centered Care")
S57 "patient care team"
S56 (MH "Combined Modality Therapy+")
S55 "multimodal"
S54 multiprofessional
S53 (MH "Collaboration")
S52 "interdisciplinary"
S51 "multidisciplinary"
S50 (MH "Multidisciplinary Care Team+")
S49 S28 and S48
S48 S35 or S43 or S47
S47 S44 or S45 or S46
S46 "lumbago"
S45 (MH "Spondylolisthesis") OR (MH "Spondylolysis")
S44 (MH "Thoracic Vertebrae")
S43 S36 or S37 or S38 or S39 or S40 or S41 or S42
S42 lumbar N2 vertebra
S41 (MH "Lumbar Vertebrae")
S40 "coccydynia" 21
S39 "coccyx" 125
S38 "sciatica" 890
S37 (MH "Sciatica") 667
S36 (MH "Coccyx") 91
S35 S29 or S30 or S31 or S32 or S33 or S34
S34 lumbar N5 pain
S33 lumbar W1 pain
S32 "backache"
S31 (MH "Low Back Pain")
S30 (MH "Back Pain+")
S29 "dorsalgia"
S28 S26 NOT S27
S27 (MH "Animals")
S26 S7 or S12 or S19 or S25
S25 S20 or S21 or S22 or S23 or S24
S24 volunteer*
S23 prospectiv*
S22 control*
S21 followup stud*
S20 follow‐up stud*
S19 S13 or S14 or S15 or S16 or S17 or S18
S18 (MH "Prospective Studies+")
S17 (MH "Evaluation Research+")
S16 (MH "Comparative Studies")
S15 latin square
S14 (MH "Study Design+")
S13 (MH "Random Sample")
S12 S8 or S9 or S10 or S11
S11 random*
S10 placebo*
S9 (MH "Placebos")
S8 (MH "Placebo Effect")
S7 S1 or S2 or S3 or S4 or S5 or S6
S6 triple‐blind
S5 single‐blind
S4 double‐blind
S3 clinical W3 trial
S2 "randomi?ed controlled trial*"
S1 (MH "Clinical Trials+")
2007 search in OvidSP
1. Randomized Controlled Trials.mp.
2. clinical trial.pt.
3. exp Clinical Trials/
4. (clin$ adj25 trial$).tw.
5. ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).tw.
6. exp PLACEBOS/
7. placebo$.tw.
8. random$.tw.
9. exp Study Design/
10. (latin adj square).tw.
11. exp Comparative Studies/
12. exp Evaluation Research/
13. Follow‐Up Studies.mp.
14. exp Prospective Studies/
15. (control$ or prospectiv$ or volunteer$).tw.
16. Animals/
17. or/1‐15
18. 17 not 16
19. neck muscles.mp. or exp Neck Muscles/
20. exp NECK/
21. exp Neck Pain/
22. exp Cervical Vertebrae/
23. exp Whiplash Injuries/
24. exp NECK INJURIES/
25. or/19‐24
26. 25 and 18
27. exp MULTIDISCIPLINARY CARE TEAM/
28. multidisciplinary.mp.
29. interdisciplinary.mp.
30. Collaboration/
31. multiprofessional.mp.
32. multimodal.mp.
33. patient care team.mp.
34. exp Patient Centered Care/ or patient care management.mp.
35. exp Patient Education/
36. exp Support, Psychosocial/
37. exp Social Environment/
38. exp Pain Clinics/
39. (pain center$ or pain centr$ or pain service$ or pain relief unit$).mp.*
40. exp Social Work/
41. exp Occupational Therapy/
42. exp Rehabilitation, Vocational/
43. exp REHABILITATION/
44. exp REHABILITATION CENTERS/
45. rehabilitation clinic$.mp.
46. exp Treatment Outcomes/
47. exp Behavior Therapy/
48. or/27‐47
49. 48 and 26
50. exp ARTHRITIS, RHEUMATOID/
51. exp Neoplasms/
52. exp Musculoskeletal Diseases/fg, su [Familial and Genetic, Surgery]
53. exp Central Nervous System/
54. exp Central Nervous System Diseases/
55. exp DENTISTRY/
56. exp Tooth Diseases/
57. or/50‐56
58. 49 not 57
59. limit 58 to yr="2002 ‐ 2007"
*.mp. [mp=title, subject heading word, abstract, instrumentation]
PsycINFO
Last searched 13 July 2016. Truncated line 4.
clinical trials/
controlled trial.mp.
RCT.mp.
random$.mp.
(clin$ adj3 trial).mp. *
(sing$ adj2 blind$).mp.
(doub$ adj2 blind$).mp.
placebo.mp. or exp Placebo/
latin square.mp.
prospective studies/
(prospective adj stud$).mp.
(comparative adj stud$).mp.
treatment effectiveness evaluation/
(evaluation adj stud$).mp.
exp Posttreatment Followup/
follow?up stud$.mp.
or/1‐16
back pain/
lumbar spinal cord/
(low adj back adj pain).mp.
(back adj pain).mp.
spinal column/
(lumbar adj2 vertebra$).mp.
coccyx.mp.
sciatica.mp.
lumbago.mp.
dorsalgia.mp.
back disorder$.mp.
"back (anatomy)"/
((disc or disk) adj degenerat$).mp.
((disc or disk) adj herniat$).mp.
((disc or disk) adj prolapse$).mp.
(failed adj back).mp.
or/18‐33
17 and 34
interdisciplinary treatment approach/
multimodal treatment approach/
multidisciplinary.mp.
patient care team.mp.
patient care management.mp.
client education/
Patient Education.mp.
social support/
Social Environments/
biopsychosocial approach/
pain clinic.mp.
pain center.mp.
pain centre.mp.
social casework/
exp case management/
occupational therapy/
rehabilitation centers/
exp vocational rehabilitation/
interdisciplinary.mp.
multiprofessional.mp.
or/36‐55
35 and 56
limit 57 to yr=2015‐2016
*.mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures]
2015 search. OvidSP has been used since 2012.
clinical trials/
controlled trial.mp.
RCT.mp.
(Random$ adj3 trial).mp.*
(clin$ adj3 trial).mp.
(sing$ adj2 blind$).mp.
(doub$ adj2 blind$).mp.
placebo.mp. or exp Placebo/
latin square.mp.
(random$ adj2 assign$).mp.
prospective studies/
(prospective adj stud$).mp.
(comparative adj stud$).mp.
treatment effectiveness evaluation/
(evaluation adj stud$).mp.
exp Posttreatment Followup/
follow?up stud$.mp.
or/1‐17
back pain/
lumbar spinal cord/
(low adj back adj pain).mp.
(back adj pain).mp.
spinal column/
(lumbar adj2 vertebra$).mp.
coccyx.mp.
sciatica.mp.
lumbago.mp.
dorsalgia.mp.
back disorder$.mp.
"back (anatomy)"/
((disc or disk) adj degenerat$).mp.
((disc or disk) adj herniat$).mp.
((disc or disk) adj prolapse$).mp.
(failed adj back).mp.
or/19‐34
18 and 35
interdisciplinary treatment approach/
multimodal treatment approach/
multidisciplinary.mp.
patient care team.mp.
patient care management.mp.
client education/
Patient Education.mp.
social support/
Social Environments/
biopsychosocial approach/
pain clinic.mp.
pain center.mp.
pain centre.mp.
social casework/
exp case management/
occupational therapy/
rehabilitation centers/
exp vocational rehabilitation/
interdisciplinary.mp.
multiprofessional.mp.
or/37‐56
36 and 57
limit 58 to yr=2014‐2015
*mp. [mp=title, abstract, heading word, table of contents, key concepts, original title, tests & measures]
2007 and 2009 search in Cambridge Scientific Abstracts (CSA)
((KW=(multidisciplinar* or interdisciplinar* or
multiprofessional*)) or (KW=(multimodal$ or (patient care team) or
(patient care management))) or (KW=((patient education) or (social
support) or (social environment))) or (KW=((pain clinic*) or (pain
center*) or (pain centre*))) or (KW=(social work)) or (DE="occupational
therapy") or (DE="rehabilitation centers") or (DE=("vocational
rehabilitation" or "rehabilitation centers"))) AND((KW=(Randomi?ed
controlled trial*) OR KW=(clinical trial*) OR KW=(clin* near trail*) OR
KW= (sing* near blind*) OR KW=(sing* near mask*) OR (doub* near blind*)
OR KW=(doubl* NEAR mask*) OR KW=(trebl* near mask*) OR KW=(trebl* near
mask*) OR KW=(tripl* near blind*) OR KW=(tripl* near mask*) OR
KW=(placebo*) OR KW=(random*) OR DE=(research design) OR KW=(Latin
square) OR KW=(comparative stud*) OR KW=(evaluation stud*) OR KW=(follow
up stud*) OR DE=(prospective stud*)OR KW=(control*) OR KW=(prospective*)
OR KW=(volunteer*)) AND(DE=(back) OR DE=(back pain) OR DE=(neck)))
ClinicalTrials.gov
Last searched 13 July 2016
((back pain OR lumbago OR dorsalgia OR sciatica OR lumbar pain OR backache OR coccydynia) AND (multidisciplinar* OR multimodal OR Multiprofessional* OR interdisciplin* OR rehab))
Studies received from 06/15/2015 to 07/13/2016
WHO ICTRP
Last searched 13 July 2016. In 2015 the intervention line was truncated as follows: multidisciplinar* OR multimodal OR Multiprofessional* OR interdisciplin* OR rehab*
Condition:back pain OR lumbago OR dorsalgia OR sciatica OR lumbar pain OR backache OR coccydynia
AND
Intervention: multidisciplinary OR multimodal OR Multiprofessional OR interdisciplinary OR rehab
Appendix 2. The GRADE Approach to Evidence Synthesis
The quality of evidence will be categorized as follows:
High (⊙⊙⊙⊙): further research is very unlikely to change the confidence in the estimate of effect.
Moderate (⊙⊙⊙○): further research is likely to have an important impact in the confidence in the estimate of effect.
Low (⊙⊙○○): further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very Low (⊙○○○): any estimate of effect is very uncertain.
We graded the evidence in the following manner:
1. Study design
For evidence from RCTs and quasi‐RCTs, we started with a rating of `high`and downgraded based on factors 2 ‐ 6 described below.
2. Risk of Bias (RoB)
We assessed RoB for included studies using the criteria recommended by the Cochrane Back and Neck (Furlan 2015), as outlined in Table 6 and Table 7 These criteria fall into five bias categories:
2. Sources of Risk of Bias.
| Bias Domain | Source of Bias | PossibleAnswers |
| Selection | (1) Was the method of randomizations adequate? | Yes/No/Unsure |
| Selection | (2) Was the treatment allocation concealed? | Yes/No/Unsure |
| Performance | (3) Was the patient blinded to the intervention? | Yes/No/Unsure |
| Performance | (4) Was the care provider blinded to the intervention? | Yes/No/Unsure |
| Detection | (5) Was the outcome assessor blinded to the intervention? | Yes/No/Unsure |
| Attrition | (6) Was the drop‐out rate described and acceptable? | Yes/No/Unsure |
| Attrition | (7) Were all randomised participants analysed in the group to which they were allocated? | Yes/No/Unsure |
| Reporting | (8) Are reports of the study free of suggestion of selective outcome reporting? | Yes/No/Unsure |
| Performance | (9) Were cointerventions avoided or similar? | Yes/No/Unsure |
| Performance | (10) Was the compliance acceptable in all groups? | Yes/No/Unsure |
| Detection | (11) Was the timing of the outcome assessment similar in all groups? | Yes/No/Unsure |
| Other | (12) Are other sources of potential bias unlikely? | Yes/No/Unsure |
3. Criteria for a Judgment of ‘‘Yes’’ for the Sources of Risk of Bias.
| 1 | A random (unpredictable) assignment sequence. Examples of adequate methods are coin toss (for studies with 2 groups), rolling a dice (for studies with 2 or more groups), drawing of balls of different colours, drawing of ballots with the study group labels from a dark bag, computer‐generated random sequence, preordered sealed envelopes, sequentially‐ordered vials, telephone call to a central office, and preordered list of treatment assignments. Examples of inadequate methods are: alternation, birth date, social insurance/security number, date in which they are invited to participate in the study, and hospital registration number. |
| 2 | Assignment generated by an independent person not responsible for determining the eligibility of the patients. This person has no information about the persons included in the trial and has no influence on the assignment sequence or on the decision about eligibility of the patient. |
| 3 | Index and control groups are indistinguishable for the patients or if the success of blinding was tested among the patients and it was successful. |
| 4 | Index and control groups are indistinguishable for the care providers or if the success of blinding was tested among the care providers and it was successful. |
| 5 | Adequacy of blinding should be assessed for each primary outcome separately. This item should be scored
''yes'' if the success of blinding was tested among the outcome assessors and it was successful or: ‐for patient‐reported outcomes in which the patient is the outcome assessor (e.g., pain, disability): the blinding procedure is adequate for outcome assessors if participant blinding is scored ''yes'' ‐for outcome criteria assessed during scheduled visit and that supposes a contact between participants and outcome assessors (e.g., clinical examination): the blinding procedure is adequate if patients are blinded, and the treatment or adverse effects of the treatment cannot be noticed during clinical examination ‐for outcome criteria that do not suppose a contact with participants (e.g., radiography, magnetic resonance imaging): the blinding procedure is adequate if the treatment or adverse effects of the treatment cannot be noticed when assessing the main outcome ‐for outcome criteria that are clinical or therapeutic events that will be determined by the interaction between patients and care providers (e.g., cointerventions, hospitalization length, treatment failure), in which the care provider is the outcome assessor: the blinding procedure is adequate for outcome assessors if item ''4'' (caregivers) is scored ''yes'' ‐for outcome criteria that are assessed from data of the medical forms: the blinding procedure is adequate if the treatment or adverse effects of the treatment cannot be noticed on the extracted data |
| 6 | The number of participants who were included in the study but did not complete the observation period or were not included in the analysis must be described and reasons given. If the percentage of withdrawals and dropouts does not exceed 20% for short‐term follow‐up and 30% for long‐term follow‐up and does not lead to substantial bias, a ''yes'' is scored. (N.B. these percentages are arbitrary, not supported by literature). |
| 7 | All randomised patients are reported/analysed in the group they were allocated to by randomizations for the most important moments of effect measurement (minus missing values) irrespective of noncompliance and cointerventions. |
| 8 | All the results from all prespecified outcomes have been adequately reported in the published report of the trial. This information is either obtained by comparing the protocol and the report, or in the absence of the protocol, assessing that the published report includes enough information to make this judgment. |
| 9 | If there were no cointerventions or they were similar between the index and control groups. |
| 10 | The reviewer determines if the compliance with the interventions is acceptable, based on the reported intensity, duration, number and frequency of sessions for both the index intervention and control intervention(s). For example, physiotherapy treatment is usually administered for several sessions; therefore, it is necessary to assess how many sessions each patient attended. For single‐session interventions (e.g., surgery), this item is irrelevant. |
| 11 | Timing of outcome assessment should be identical for all intervention groups and for all primary outcome measures. |
| 12 | Other types of biases. For example: ‐When the outcome measures were not valid. There should be evidence from a previous or present scientific study that the primary outcome can be considered valid in the context of the present. ‐Industry‐sponsored trials. The conflict of interest (COI) statement should explicitly state that the researchers have had full possession of the trial process from planning to reporting without funders with potential COI having any possibility to interfere in the process. If, for example, the statistical analyses have been done by a funder with a potential COI, usually ''unsure'' is scored. |
Selection (random sequence generation, allocation concealment, group similarities at baseline);
Performance (blinding of participants, blinding of healthcare providers, cointerventions, and compliance with intervention);
Attrition (dropouts and intention‐to‐treat (ITT) analysis);
Measurement (blinding of the outcome assessors and timing of outcome assessment);
Reporting bias (selective reporting).
To incorporate RoB into the GRADE assessment, we rated the overall extent of RoB within each bias category (e.g. performance bias) as 'bias' or 'no bias':
We did not downgrade evidence from studies judged 'no bias' for four or five categories.
We downgraded evidence (‐1 point) when three or fewer categories for each study were judged to have bias or unclear bias.
We downgraded by ‐2 points when four or more categories for each study were judged to have bias or unclear bias.
3. Inconsistency
Inconsistency refers to unexplained heterogeneity of results. Widely differing estimates of the treatment effect (such as, heterogeneity or variability in results) across trials suggest true differences in the underlying treatment effect.
Inconsistency may arise from differences among populations (e.g. multidisciplinary interventions may have larger relative effects in sicker populations), interventions (e.g. larger effects with more intense interventions), or outcomes (for example, diminishing treatment effect with time).
This item does not apply when there is only one trial. We downgraded the quality of evidence by one point when the heterogeneity or variability in results was large, as indicated by an I2 > 60% (Higgins 2011).
4. Indirectness
We assessed whether the question being addressed in this systematic review was different from the available evidence regarding the population, intervention, comparison, or outcome. We downgraded the quality of evidence by one point when there was indirectness in only one area; and by two points when there was indirectness in two or more areas.
5. Imprecision
Results are imprecise when trials include relatively few participants and few events and thus have wide CIs around the estimate of the effect.
For dichotomous outcomes, we considered imprecision for either of the following two reasons:
(1) There was only one trial. When there was more than one trial, the total number of events was < 300 (a threshold rule‐of‐thumb value) (Mueller 2007).
(2) 95% CI around the pooled or best estimate of effect included both (1) no effect and (2) appreciable benefit or appreciable harm. The threshold for 'appreciable benefit' or 'appreciable harm' is a relative risk reduction (RRR) or relative risk increase (RRI) > 25%. We downgraded the quality of the evidence by one point when there was imprecision due to (1) or (2); or by two levels when there was imprecision due to (1) and (2).
For continuous outcomes, we considered imprecision for either of the following two reasons:
(1) There was only one trial. When there was more than one trial, the total population size was < 400 (a threshold rule‐of‐thumb value; using the usual α and β, and an effect size of 0.2 SD, representing a small effect).
(2) 95% CI included no effect and the upper or lower CI crosses an effect size (standardized mean difference) of 0.5 in either direction. We downgraded the quality of the evidence by one point when there was imprecision due to (1) or (2); or by two points when there was imprecision due to (1) and (2).
6. Publication bias
Publication bias is a systematic underestimate or an overestimate of the underlying beneficial or harmful effect due to the selective publication of trials. We planned to create funnel plots for comparisons with at least 10 included studies and to downgrade the quality of the evidence by one point when the funnel plot suggested publication bias. However, we did not identify enough studies to conduct this analysis.
Data and analyses
Comparison 1. Multidisciplinary rehabilitation versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 5 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Short‐term | 4 | 272 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.74, ‐0.06] |
| 1.2 Intermediate‐term | 2 | 155 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.34 [1.00, 0.31] |
| 1.3 Long‐term | 4 | 336 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.46 [‐0.70, ‐0.21] |
| 2 Disability | 4 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Short‐term | 4 | 272 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.38 [‐0.63, ‐0.14] |
| 2.2 Intermediate‐term | 2 | 151 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐1.09, 0.22] |
| 2.3 Long‐term | 3 | 240 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.44 [‐0.87, ‐0.01] |
| 3 Return‐to‐work at long‐term | 3 | 170 | Odds Ratio (IV, Random, 95% CI) | 3.19 [1.46, 6.98] |
| 4 Sick leave periods at long‐term | 2 | 210 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.38 [‐0.66, ‐0.10] |
Comparison 2. Multidisciplinary rehabilitation versus other treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 Short‐term | 2 | 165 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.50, 0.33] |
| 1.2 Intermediate‐term | 2 | 162 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.64 [‐1.85, 0.57] |
| 1.3 Long‐term | 2 | 336 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.36, 0.07] |
| 2 Disability | 3 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 Short‐term | 2 | 165 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.00 [‐0.34, 0.34] |
| 2.2 Intermediate‐term | 2 | 162 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐1.50, 0.51] |
| 2.3 Long‐term | 2 | 345 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.24, 0.18] |
| 3 Sick leave days at long‐term | 2 | 158 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.98, 0.47] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Anema 2007.
| Methods | RCT. The study was conducted between October 2000 and October 2003. | |
| Participants | Nonspecific LBP, full or partial sick leave due to nonspecific LBP lasting 2 to 6 weeks, age between 18 and 65 years, and able to give written informed consent and to complete written questionnaires in Dutch. The trial was conducted in the Netherlands. It was designed to replicate the Canadian study by Loisel 1997 (also included). *Baseline symptom intensity for control group: Mean pain was 6.3 (1.7) on VAS (0 to 10) and mean functional status was 13.8 (4.6) on Functional Status RDQ (0 to 24); LOWER symptom intensity |
|
| Interventions |
Intervention = Usual care + workplace intervention + graded activity The workplace intervention took place directly after inclusion. Participants still sick listed at 8 weeks were randomised for graded activity. Note: Only the combination of workplace and graded activity interventions meets our criteria for multidisciplinary. Twenty‐seven participants received the combined intervention. Workplace: n = 96, mean age (SD) = 44 (8.6), 45% female. Worksite assessment and work adjustments, based on methods used in participatory ergonomics. Included an ergonomist (process leader), the injured worker, the worker's supervisor, and possible other stakeholders. Graded activity: n = 55, mean age (SD) = 41.3 (9.2), 36% female. Individual, submaximal, gradually increasing exercise program with an operant‐conditioning behavioral approach. Physiotherapist acted as a coach and supervisor, using a hands‐off approach. *The entire program consisted of two 1‐hour sessions a week, with 26 sessions maximally (13 weeks) = low intensity. Comparison = Heterogeneous group (usual care, workplace intervention only and graded activity only) After first randomizations to workplace or usual care. Usual care group 1: n = 100, age (SD) = 41.2 (10.7), 67% female. After second randomizations to graded activity or usual care. Usual care group 2: n = 57, age (SD) = 43.4 (8.3), 54% female. Usual care: The Dutch occupational guideline on LBP advises for nonspecific LBP: Education about the good prognosis and the importance of keeping up or returning to normal activities; coping with low back pain, fear of movement, and a plan for the resumption of normal activities; advice to return‐to‐work within 2 weeks in the absence of further problems; a workplace visit by an occupational therapist or ergonomist is optional; the general practitioner, or any other medical specialist, is consulted if curative treatment is considered inappropriate. |
|
| Outcomes | Return‐to‐work rate/time to return‐to‐work for workplace intervention, functional status (Roland‐Morris Disability Questionnaire, with higher scores indicating more severe disability), pain intensity. Follow‐up at 12, 26, and 52 weeks (primary at 52 weeks). Analyses compared those who received combined intervention to those who didn't receive the combined intervention (i.e. combination of workplace only, graded only, and usual care). Pain at one year: Difference in adjusted improvement over time in two groups 0.47 (‐0.42 to 1.35), NS Functional status (Roland‐Morris) at one year: Difference in adjusted improvement over time in two groups: 1.49 (‐0.33 to 3.31), NS Time to full return‐to‐work: Adjusted hazard ratio = 0.7 (95% CI, 0.3 to 1.2, P > 0.05) Adverse events: Not reported. |
|
| Notes |
Attrition: Workplace intervention = 0 lost to follow‐up. Graded activity = 0 lost to follow‐up. Usual care 1 = 0 lost to follow‐up. Usual care 2 = 0 lost to follow‐up. All analyses conducted according to ITT principles. 24 (12%), had no follow‐up data collected on secondary outcome measures (pain and function). Funding source/COIs of primary researchers: Federal funds were received in support of this work. No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript. Applicability: No concerns about generalisability of the data |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Series of random numbers (Steenstra 2003 p. 3). |
| Allocation concealment (selection bias) | Low risk | Participants were only informed after they were allocated. |
| Blinding of participants (performance bias) | High risk | Not possible due to the nature of the intervention. |
| Blinding of personnel (performance bias) | High risk | Not possible due to the nature of the intervention. |
| Compliance (adherence) acceptable? (performance bias) | High risk | For graded activity, "19 workers out of 55 were not compliant". |
| Co‐interventions avoided or similar? (performance bias) | Low risk | Cointerventions were similar across groups. |
| Blinding of outcome assessment (detection bias) Administrative data and other non‐self‐report outcomes1 | Low risk | Return‐to‐work data from automated databases. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Blinding not possible due to nature of intervention: "blinding of self‐reported outcome measurements was not possible". |
| Timing of outcome assessment (measurement/detection bias) | Low risk | Outcomes measured at standard time points. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Sick leave data collected for all participants. Follow‐up data missing on secondary outcomes (pain and function) for 12% of participants. All analyses conducted according to ITT principle (Figure 1, p. 293) |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods were addressed in results. |
Bultmann 2009.
| Methods | RCT. The study was conducted between April 2004 and April 2006 in Vejle County, Denmark. This included recruitment and one‐year follow up. | |
| Participants | Study eligibility required participants to be absent from work for 4 to 12 weeks, to have a reimbursement request indicating LBP or musculoskeletal disorder as the main cause of sick leave, and to be between 18 and 65 years of age. Understanding and speaking Danish was also required. Note that sample was mixed with respect to pain location but > 80% reported LBP in both groups. *Baseline symptom intensity for control group: Mean pain was 6.04 (2.0) on 10‐point numerical rating scale and mean functional status was 66.21 (14.7) on 0 to 100 scale, where higher scores indicated a lower level of disability; LOWER symptom intensity |
|
| Interventions |
Intervention = Work disability screening plus rehabilitation plan n = 68, mean age (SD) = 44.2 (10.8), 48.5% female. Two main components: (1) a work disability screening: a systematic, multidisciplinary assessment of disability and functioning as well as the identification of barriers for RTW; and (2) the formulation and implementation of a co‐ordinated, tailored and action‐oriented work rehabilitation plan collaboratively developed by an interdisciplinary team using a feedback guided approach. The interdisciplinary team consisted of an occupational physician, an occupational physiotherapist, a chiropractor, a psychologist, and a social worker. *The duration of the intervention was for up to three months; insufficient information to categorize intervention intensity. Comparison = Conventional case management, as provided by municipality n = 51, mean age (SD) = 42.9 (11.9), 63.8% female. |
|
| Outcomes | Registered sickness absence hours, functional disability (Oswestry Low Back Pain Disability Questionnaire, with lower scores indicating more severe disability*), initiatives and actions for RTW during the first 3 months of follow‐up, economic evaluation, work status. *Author note regarding Oswestry scale: "[We used] an inverted Oswestry with score 100 = normal functioning. The reason to do so was to focus on function and not on dysfunction (the rationale of the study and intervention) and to work with a combined function index (using all dimensions), with index 100 = normal function." Follow‐up at 3 and 12 months. The time intervals for the cumulated sickness absence hours were 0 to 3 months, 3 to 6 months, 6 to 12 months as well as 0 to 6 months and 0 to 12 months. Adverse events: Not reported. |
|
| Notes |
Attrition: 2 lost to follow‐up in intervention group and 4 lost to follow‐up in control group for primary outcome (sickness absence). For secondary outcomes (work status, pain intensity, and functional disability), 12 lost to follow‐up in intervention group and 21 lost to follow‐up in control group. Funding source/COIs of primary researchers: Kilsgaard is now the director of KIApro, an organization that develops and implements systematic programs for work rehabilitation in municipalities in Denmark. The present study was planned, designed, and performed while Kilsgaard was working at the Department of Development and Labor Market of Vejle County. Applicability: No concern about generalisability. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A randomisations protocol without stratification was computer‐generated prior to the start of the study and was undertaken by an independent information technology assistant. |
| Allocation concealment (selection bias) | Low risk | See above. |
| Blinding of participants (performance bias) | High risk | Not possible due to the nature of the intervention. |
| Blinding of personnel (performance bias) | High risk | Not possible due to the nature of the intervention. |
| Compliance (adherence) acceptable? (performance bias) | Low risk | No participants that started the intervention discontinued it. "All participants allocated to Coordinated and Tailored Work Rehabilitation underwent the multidisciplinary assessment and received a co‐ordinated, tailored, and action‐oriented RTW plan" (p. 86). |
| Co‐interventions avoided or similar? (performance bias) | Low risk | Any cointerventions were similar. |
| Blinding of outcome assessment (detection bias) Administrative data and other non‐self‐report outcomes1 | Low risk | Administration data on cumulative sickness hours used. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Participant was outcome assessor. Blinding not possible because of the nature of the intervention. |
| Timing of outcome assessment (measurement/detection bias) | Low risk | Outcomes measured at standard time points. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Administration data: all 66 participants who received the intervention (of 68 randomised) and 47 of control group (of 51 randomised to this condition) had complete data on sickness absence. For work status, pain intensity, and functional disability, intervention group 54 of 66 and control group 26 of 47 had complete data at 12 months. ITT not used. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods were presented in results. |
Campello 2012.
| Methods | RCT. Participants were recruited from May to November 2009 (Hiebert 2012). | |
| Participants | Active duty service members were eligible if they were seeking care for LBP at Sewells Point Branch Medical Clinic in Norfolk, Virginia. Must be classified as nonspecific LBP by Primary Care Manager that interfered with normal work or life for a period of between 4 and 12 weeks. *Baseline symptom intensity for control group: Mean pain was 4.5 (2.3) on a 10‐point numerical rating scale and mean functional status was 24.3 (10.5) on scale ranging from 0 to 100%; LOWER symptom intensity |
|
| Interventions |
Intervention = Physical reconditioning plus CBT with back‐to‐work focus n = 16, mean age (SD) = 33.1 (6.6), 12.5% female. Backs to Work was a co‐ordinated multidisciplinary, reconditioning program conducted by physical therapists, a psychologist, and a physician. The physical component was a graded, goal‐oriented active physical reconditioning program that included aerobic conditioning, strength training, and flexibility exercises. The psychological component included an evaluation by a psychologist to rule out psychopathology and substance abuse. CBT treatment included education about how psychosocial variables affect pain, relaxation training, modification of maladaptive beliefs and problem solving. *The duration of the intervention was 3 hours per day, 3 days/week for 4 weeks = 36 hours = mid‐intensity. Comparison = Usual Care n = 17, mean age (SD) = 32 (7.2), 5.9% female. Treatment at discretion of Primary Care Manager. Treatment conducted 2 to 3 times a week at a Sports Medicine or Chiropractic Clinic and included one or more of the following: modalities (ultrasound, heat, ice, and electrical stimulation), traction, exercises, back class, spinal manipulation. The control group did not undergo psychological examination. |
|
| Outcomes | Return to duty, pain, pain catastrophising, perceived disability (Oswestry, with higher scores indicating more severe disability), depression (CES‐D), fear of physical activity, functional performance (e.g. active trunk range of motion). Participants were followed up at 4 and 12 weeks. Means (SDs) for secondary outcomes at 12 weeks Pain catastrophising: MBR = 3.0 (3.7), usual care = 8.3 (7.9), NS. Depression: MBR = 4.4 (4.3), usual care = 8.4 (7.4), NS. Fear of physical activity (FABQ physical score): MBR = 5.7 (5.6), usual care = 10.7 (7.3), NS. Fear of physical activity (FABQ work score): MBR = 7.3 (4.9), usual care = 10.8 (9.1), NS. Adverse events: Not reported. |
|
| Notes |
Attrition Intervention group: n = 7 (3 excluded and 4 dropped out). A total of 9 completed follow‐up. Control group: n = 5 (1 dropout and 4 lost to follow‐up). A total of 12 completed follow up. Funding source and/or COIs of primary researchers: This study was sponsored by Navy & Marine Corps Public Health Centre, funded by the Assistant Secretary of the Army for Installations and Environment, and managed by Batelle. Applicability: Mainly male active duty service members. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomisations not reported. |
| Allocation concealment (selection bias) | Unclear risk | Not reported in text. |
| Blinding of participants (performance bias) | High risk | Not possible due to nature of study. |
| Blinding of personnel (performance bias) | High risk | Not possible due to nature of study. |
| Compliance (adherence) acceptable? (performance bias) | Unclear risk | Not reported and unable to ascertain. |
| Co‐interventions avoided or similar? (performance bias) | Low risk | No indication of cointerventions. |
| Blinding of outcome assessment (detection bias) Administrative data and other non‐self‐report outcomes1 | Unclear risk | Duty status was recorded by the subject's Primary Care Manager at each clinical encounter and abstracted from the subject's electronic medical record. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Participant was outcome assessor. Blinding not possible because of the nature of the intervention. |
| Timing of outcome assessment (measurement/detection bias) | Low risk | Outcomes measured at standard time points. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Loss of over 30% in intervention group. Dropouts reported, but problematic because of small sample size. ITT approach used but did not mitigate loss to follow‐up. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods were presented in results. |
Jensen 2011.
| Methods | RCT. Participants were referred to the study from November 2004 through June 2007. | |
| Participants | General practitioners in 4 municipalities with a total of 240,000 citizens received written information about the project. The general practitioners were encouraged to refer participants to the study at the Research Unit of the Spine Centre, Regional Hospital Silkeborg, Denmark, if the participants were aged 16 to 60 years and partly or fully sick‐listed from work for 4 to 12 weeks because of LBP. The first visit at the Spine Centre was not always possible within this time frame, and consequently the duration of sick leave ranged from 3 to 16 weeks at the time of inclusion. *Baseline symptom intensity for control group: Mean pain was 32.7 (12.4) on LBP rating scale 0 to 60 and mean functional status was 15.6 (5.2) on Roland‐Morris disability scale ranging from 0 to 23; LOWER symptom intensity. Exclusion: The participants were not enrolled in the study if they were unemployed, had continuing or progressive signs or symptoms of nerve root affection implicating plans for surgery, had low back surgery within the last year or specific back diseases, (e.g. tumour), were pregnant, had known dependency on drugs or alcohol, or had any primary psychiatric disease. |
|
| Interventions |
Intervention = Brief clinical intervention + multidisciplinary intervention n = 176, mean age (SD) = 42.1 (10.5), 54% female. Brief clinical intervention: A standard clinical LBP examination was carried out by the physician, relevant imaging and examinations were ordered, and treatment options were discussed. Information was given in a reassuring way and medical pain management was adjusted. The participants were advised to resume work when possible. The physiotherapy examination included a standardized, mechanical evaluation, and advice on exercise was chosen accordingly. General advice was given to increase physical activity and exercise. For all participants, a follow‐up visit at the physiotherapist was scheduled 2 weeks later, and a follow‐up visit at the physician was arranged for participants needing answers in relation to test results. Multidisciplinary intervention: In addition to the brief clinical intervention described above, participants allocated to the multidisciplinary intervention group were scheduled for an interview with a case manager within two to three workdays. This interview was standardised and included questions of work history, private life, and questions on how pain and disability were perceived. It normally lasted for 1 to 2 hours. The participant was seen one or more times by the case manager depending on need and progress. The case manager and the participant together made a tailored rehabilitation plan aiming at full or partial RTW. Each case was discussed several times by the entire multidisciplinary team including the rehabilitation physician, a specialist in clinical social medicine, a physiotherapist, a social worker, and an occupational therapist. *The duration of the intervention was 18 weeks, average of 4 meetings with case manager = low intensity. Comparison = Other intervention (brief clinical intervention alone ‐ see above) n = 175, mean age (SD) = 41.9 (10.4), 50.3% female. |
|
| Outcomes | Return‐to‐work (defined as first 4‐week period within the first year after inclusion, during which the participant received no social transfer payments), pain, disability (Roland‐Morris, with higher scores indicating more severe disability), fear avoidance, and physical functioning. All SF‐36 subscales (role‐physical, bodily pain, general health, vitality, social functioning, role‐emotional, mental health). Participants were followed up at one year. Return‐to‐work (median time until RTW) I = 18 weeks. C = 14 weeks. Unadjusted HR = 0.83 (95% CI 0.65 to 1.06), P = 0.14. Results for secondary outcomes at 12 months Fear avoidance (Orebro) (n = 237): I = 16.0 (8.5), C = 16.1 (8.1), P = 0.91. Physical functioning subscale (SF‐36, higher numbers indicated better health) (n = 244): I = 70.3 (22.0), C = 70.6 (23.2), P = 0.43. Mental health subscale (SF‐36) (n = 243): I = 75.0 (19.8), C = 70.0 (20.3), P = 0.046. Adverse events: Not reported. |
|
| Notes |
Attrition Intervention: 5 did not receive allocated intervention due to cancer diagnosis (n = 1), or unwillingness to continue after clinical examination (n = 4). Follow‐up questionnaires not answered by 47. Comparison: 2 did not receive allocated intervention due to cancer diagnosis (n = 1) or age (61 years, n = 1). Follow‐up questionnaire not answered by 53. Funding source/COIs of primary researchers: "No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript." Supported by the Danish Working Environment Research Fund. Applicability: No concerns about generalisability of the data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A secretary phoned a computer generating an automatic voice response on the basis of block randomizations designed by a data management unit at another hospital. |
| Allocation concealment (selection bias) | Low risk | Yes, it was done off site. |
| Blinding of participants (performance bias) | High risk | Participants were aware of result of randomizations. |
| Blinding of personnel (performance bias) | High risk | Personnel were aware of results of randomizations. |
| Compliance (adherence) acceptable? (performance bias) | Unclear risk | Information provided regarding frequency of contact in MBR group: "Meetings with workplace representatives were arranged with 54 participants and the case manager contacted employers directly in 33 other cases. For these 87 cases, the case manager was in contact with workplace representatives 6 times on average" (p. 1186). However, compliance with other aspects of the treatment was not reported. |
| Co‐interventions avoided or similar? (performance bias) | Low risk | There was no indication of cointerventions. |
| Blinding of outcome assessment (detection bias) Administrative data and other non‐self‐report outcomes1 | Low risk | Data on sick leave to estimate time to RTW were drawn from national registers. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Participant was outcome assessor. Blinding not possible because of the nature of the intervention. |
| Timing of outcome assessment (measurement/detection bias) | Low risk | Yes, survival analysis and standard follow‐up time. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | RTW: Dropouts were described in detail. Self‐report outcomes at one‐year follow up: Large portion failed to answer follow up questionnaire: 47 of 176 in intervention group and 53 of 175 in control ITT not used |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods were presented in results. |
Karjalainen 2003.
| Methods | RCT. Participants enrolled in study between August 1998 and May 2000. | |
| Participants | Participants were recruited from clinics in the Helsinki metropolitan area. Inclusion criteria 25 to 60‐year‐old employees with current daily low back pain (with or without sciatica), which had made working difficult for 4 weeks but less than 3 months. *Baseline symptom intensity for control group: Mean pain was 5.7 on 0 to 10 rating scale and mean functional status was 34 on Oswestry (% of maximum score of 45); LOWER symptom intensity |
|
| Interventions | A total of 164 participants with subacute low back pain were randomised to a mini‐intervention group (A), a worksite visit group (B), or a usual care group (C). Groups A (n = 56) and B (n = 51) underwent one assessment by a physician plus a physiotherapist. Group B received a worksite visit in addition. Group C served as a control group (n = 57) and was treated in municipal primary health care. All participants received a leaflet on back pain. Intervention of interest = Worksite visit group n = 51, mean age = 44 (25 to 60), 57% female. Intervention by the physicians and the physiotherapist was identical to that in the mini‐intervention group and performed without knowledge of final group assignment. The physiotherapist visited the participant's work site, along with the participant's work supervisor and company nurse, and physician. The aim of the visit, which lasted for approximately 75 minutes, was to ensure that the participant had adapted to the information and practical instructions of appropriate ways of using the back at work, to involve the supervisor and company health care professionals, and to encourage their cooperation. Comparison 1 = Mini intervention alone n = 56, mean age = 44 (25 to 60), 59% female. A physician specializing in physiatry first interviewed and examined the participants in the mini‐intervention group and encouraged them to ask anything unclear about their back pain. Working conditions were discussed and the results of the clinical examination explained to the participant and the radiograph findings and causes of pain clarified, as far as possible. The main aim of these consultations was to reduce the participants' concerns about their back pain by providing accurate information and to encourage physical activity. The physiotherapist instructed the participant no more than five exercises for improving the function of deep abdominal muscles and establishing symmetric use of the back. Other daily exercises were planned that were feasible enough for the participant to commit to and execute them. The aim of this approximately 1.5‐hour session was to increase body control and exercising in everyday life. *The duration of the mini intervention was 1.25 to 1.5 hours and the worksite visit was approximately 75 minutes = low intensity. Comparison 2 = Usual care n = 57, mean age = 43 (25 to 59), 60% female. Participants in the usual care group were not examined at FIOH but did receive a leaflet on back pain (as did all other study participants). They were treated by their GPs in primary health care in the usual manner, including specialist consultations and physiotherapy, when necessary. They were not restricted from seeking specialist treatment privately, i.e. at their own expense if they so wished. |
|
| Outcomes | Intensity of pain, daily symptoms, frequency and bothersomeness of pain, interference of pain with daily life, disability (Oswestry, with higher scores indicating more severe disability), specific and generic health‐related quality of life, satisfaction with care, days on sick leave, and use and costs of health care consumption. Participants were followed up at 3‐, 6‐, and 12‐months. MBR vs usual care between‐group differences for secondary outcomes: Quality of life, scale of 0.00 to 1.00, with higher scores indicating higher quality: 12 months: 0.00 (‐0.02 to 0.02), P = 0.834. 24 months: 0.003 (‐0.02 to 0.02), P = 0.802. Satisfaction with care: scale of 0 to 10, with higher scores indicating more satisfaction: 12 months: 2.0 (1.1 to 2.9), P < 0.00. 24 months: 2.0 (1.1 to 2.9), P = 0.00. Adverse events: Not reported. |
|
| Notes |
Attrition Mini intervention and worksite visit groups: No participants lost to follow up. Comparison group: At 3 months, 1 participant lost to follow‐up. Funding source/COIs for primary researchers: "No benefits in any form have been or will be received from a commercial party related directly or indirectly to the subject of this manuscript". Applicability: No concerns about generalisability of the data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants agreeing to participate were asked to complete baseline questionnaires at FIOH. The research nurse then randomised each participant into one of the three study groups; to ensure even distribution of participants regarding gender and age > 45 and < 45 years, four piles of sealed envelopes were used, and in each, the randomizations was done in blocks of 15. A biostatistician had prepared the order from a random number table. A secretary unconnected with the participants had numbered the envelopes sequentially to prevent their rearrangement. The research nurse and researchers were not aware of the block size and therefore could not predict the group assignments. |
| Allocation concealment (selection bias) | Low risk | See above. |
| Blinding of participants (performance bias) | High risk | Blinding not possible due to nature of design. |
| Blinding of personnel (performance bias) | High risk | Blinding not possible due to nature of design. |
| Compliance (adherence) acceptable? (performance bias) | Low risk | 49 of 51 participants received worksite visits (p. 537). |
| Co‐interventions avoided or similar? (performance bias) | Low risk | "Cointerventions, such as visits including the use of alternative medicine services, were equally distributed among the three groups". |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Participant was outcome assessor and blinding not possible because of the nature of the intervention. |
| Timing of outcome assessment (measurement/detection bias) | Low risk | Outcomes measured at standard time points |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants in each study group (except for one in the usual care group, who, without explanation, decided to withdraw from the study at the 3‐month follow‐up) were followed up by questionnaires 3, 6 and 12 months after randomizations. Participants were included in the analysis on the basis of their intervention group allocation. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods were presented in results. |
Loisel 1997.
| Methods | RCT. Participants were recruited from September 1 1991, to December 31 1993. | |
| Participants | Inclusion criteria for workplaces to participate in the study were: to have more than 175 employees and to be located within 30 km of the study site (Sherbrooke area, Quebec, Canada). Inclusion criteria for workers from these workplaces were: thoracic or lumbar back pain incurred at work that had caused an absence from work (or an assignment to light duties) for more than 4 weeks and less than 3 months, age from 18 to 65 years, and back pain accepted for compensation by the Québec Workers' Compensation Board. *Baseline symptom intensity for control group: Mean pain was 22.9 (14.2) on McGill Pain Questionnaire (0 to 78) and mean functional status was 29.8 (14.7) on Oswestry (% of maximum score of 45); LOWER symptom intensity. |
|
| Interventions |
Intervention = Occupational intervention plus graded activity n = 25, 60% female, mean age (SD) = 37.4 (8.1) Occupational The occupational intervention began after 6 weeks of absence from work and included participants' visits to an occupational physician and a participatory ergonomics evaluation conducted by an ergonomist. The occupational physician could recommend investigation or treatment or could try to set up light duties to help the participant return to usual tasks. The ergonomic intervention was a worksite evaluation that included union and employer representatives in determining the need for job modifications. Clinical intervention (graded activity) The clinical intervention included, after 8 weeks' absence from work, a visit to a back pain specialist and a school for back care education (back care school) and, after 12 weeks' absence, a multidisciplinary work rehabilitation intervention. The rehabilitation plan was a modified Mayer's intervention, including fitness development and work hardening with a cognitive‐behavioral approach. It ended with a progressive return‐to‐work, called therapeutic return‐to‐work, alternating days at the original job with progressively increased tasks and days receiving functional therapy. *In a previous study using the same protocol (Loisel 1994), the duration of functional rehabilitation therapy ranged from 2 to 13 weeks. No additional information reported on intervention intensity; insufficient information to categorize intervention intensity. Comparison = Usual care Usual care n = 26, 19.2 % female, mean age (SD) = 41.7 (10.0). Participants in the usual care group received treatment from their attending physician, who was at liberty to prescribe any test, treatment, or referral to a specialist for care. |
|
| Outcomes | Time off work, time to return‐to‐work, functional status (Oswestry, with higher scores indicating more severe disability), pain level (McGill‐Melzack questionnaire), sickness impact profile. Follow‐ups at 12, 24, 52 weeks. The means and SDs reported below were extracted from the French report. Generic functional status (Sickness impact profile, higher scores = worse health) One year: I = 3.0 (7.4) C = 9.7 (7.5) Unadjusted mean difference at one‐year was ‐6.76 (adjusted mean difference was ‐4.41, P = 0.052) Time to return to regular work Median time off regular work (days) I = 60.0 C = 120.5 Unadjusted Cox hazard ratio was 2.11 (adjusted HR = 2.23, P = 0.037) *Note that original Cochrane Review focused on Comparison 3 from Table 3 (HR = 2.41). Adverse events: Not reported. |
|
| Notes | *This study was included in the original version of the review. Attrition: Twelve workers (9%) did not respond to any follow‐up visit (nonparticipants) and were also distributed in the four groups. Hence, the comparative analyses were performed on 104 participants. The participants did not differ from the nonparticipants in gender, duration of absence from regular work, or clinical data, but the participants were older. Funding source/COIs for primary researchers: No information provided. Applicability: No concerns about generalisability of the data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The first randomizations (at workplace level) was stratified according to activity sector and according to the number of employees. Eligible workers from all workplaces were successively randomised to receive (or not) the clinical intervention. For this randomizations, 500 random numbers were generated by a computer and were given the status yes or no for clinical and rehabilitation intervention. |
| Allocation concealment (selection bias) | Low risk | Each random number was placed in order of generation into envelopes numbered from 1 to 500. Envelopes were sealed , and the first 250 were distributed in successive order to the incoming eligible workers from the workplaces not receiving the occupational intervention. |
| Blinding of participants (performance bias) | High risk | Not possible due to nature of intervention. |
| Blinding of personnel (performance bias) | High risk | Not possible due to nature of intervention. |
| Compliance (adherence) acceptable? (performance bias) | Unclear risk | No information provided and unable to ascertain. |
| Co‐interventions avoided or similar? (performance bias) | Low risk | Any cointerventions similar across groups. |
| Blinding of outcome assessment (detection bias) Administrative data and other non‐self‐report outcomes1 | Low risk | For return‐to‐work outcomes: on page 2913 it stated that the evaluation/data analysis team had no contact with study site, worksites, or participants. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Participant was outcome assessor. Blinding not possible due to nature of intervention. |
| Timing of outcome assessment (measurement/detection bias) | Low risk | Used survival analysis, measured outcomes at standard times. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts described, small sample, but distributed across groups. No mention of ITT. |
| Selective reporting (reporting bias) | Low risk | All variables reported in methods and results. |
Schiltenwolf 2006.
| Methods | RCT. The study was conducted in Germany. | |
| Participants | Participants were recruited through general practitioners and orthopaedic surgeons from 1998 to 1999. Inclusion criteria
*Baseline symptom intensity for control group: Mean pain was 5.28 (2.2) on a numeric rating scale (0 to 10) and mean functional status was 57.34 (23.7) on 0 to 100% scale, with higher scores indicating higher functioning; LOWER symptom intensity. |
|
| Interventions | The interventions were based on inpatient rehabilitation programs in both treatment arms with respect to dosage and contents. Intervention = Biopsychosocial therapy (functional restoration plus psychotherapy) n = 31 (Table 1). Note: Figure 1 suggests that there were 33 allocated to biopsychosocial therapy group (this may be a reporting error) Mean age (range) = 34.9 (19 to 50), 48% female The conventional biomedical program included a functional restoration program of individual physiotherapy, workout, and back school and aimed at stretching, strengthening, improving mobility and body control. Passive interventions (massage and physical therapy) were added. The psychological component included specifically adapted psychotherapy three times per week and relaxation therapy four times per week. A professional psychotherapist performed this part of the treatment in a group and in an individual setting. Psychotherapy contained analysis of individual psychosocial factors and conflicts contributory to persistent low back pain, enhancement of participant’s understanding of the nature and function of their pain. Psychotherapy sessions also included psychoeducation. *The duration of the intervention was 6 h of daily treatment for 15 days in 3 weeks = mid‐intensity. Comparision = Other intervention n = 33 (Table 1). Note: Figure 1 suggests that there were 31 in biomedical group. Mean age (range) = 36.7 (20 to 48), 39% female. A functional restoration program of individual physiotherapy, group therapy in water, workout, and back school and aimed at stretching, strengthening, improving mobility and body control. Passive interventions (massage and physical therapy) were added. |
|
| Outcomes | Pain intensity (numeric rating scale), functional capacity (Hannover Functional Status Questionnaire, with lower scores indicating more severe disability), depressive dysfunction (CES‐D), sick leave, clinical parameters. Participants were followed up at 3 weeks, 6 months, and 2 years (for sick leave data). Findings for secondary outcome: Depressive dysfunction (CES‐D 0 to 45) Changes since baseline Short‐term follow‐up (3 weeks): I = 2.40 (4.6), C = 3.74 (4.5). Intermediate follow‐up (6 months): I = 6.62 (7.5), C = ‐0.86 (7.8), P = 0.0034. Adverse events: Not reported. |
|
| Notes |
Attrition Treatment group: Based on Figure 1: 1 participant dropped out with cardiovascular complaints before 3 week evaluation, and 3 were lost to follow‐up after 6 months. From text: 32 completed therapy and 30 presented for follow‐up after six months. Sick leave data available for 22. 11 were lost to follow‐up after two years. Control: According to Figure 1: 2 dropped out due to cardiovascular complaints before 3 week evaluation and 5 were lost to follow‐up after 6 months. From text: 29 completed therapy and 26 presented for follow‐up at six months. Sick leave available for 20. 11 were lost to follow‐up after two years. Funding source/COIs for primary researchers: No information provided. Applicability: No concerns about the generalisability of the data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The participants were randomised in blocks of five when entering this study which was based on an inpatient treatment at the author's clinic. The physician informed an independent person working elsewhere by phone, who allocated five subsequent participants to one of the two treatment arms by using a lottery system (a piece of paper marked biomedical therapy or biopsychosocial therapy, present in equal number, was taken from a black box and returned afterwards to ensure equal binary probability). |
| Allocation concealment (selection bias) | Low risk | Allocation conducted off site. |
| Blinding of participants (performance bias) | High risk | Blinding not possible due to nature of design. |
| Blinding of personnel (performance bias) | Unclear risk | The participant's group affiliation was concealed from the physiotherapists who treated participants included in the study along with those from the rehabilitation department. Effective blinding of the physiotherapists was not confirmed. The supervising physician and the psychotherapist were not blinded to the participant's group assignment. |
| Compliance (adherence) acceptable? (performance bias) | Low risk | Authors indicated that 95% of participants completed therapy (29/31 intervention and 32/33 control). |
| Co‐interventions avoided or similar? (performance bias) | Low risk | Cointerventions such as medication, injections or chirotherapy were avoided in both groups during inpatient treatment. |
| Blinding of outcome assessment (detection bias) Administrative data and other non‐self‐report outcomes1 | Low risk | Applied to sick leave data. The observer acquiring sick leave status from health insurance companies at 2 year follow‐up (Time 3) was also blinded. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Applied to pain, function, and depression. Participant was outcome assessor. Blinding not possible because of the nature of the intervention. |
| Timing of outcome assessment (measurement/detection bias) | Low risk | Outcomes measured at standard time points. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | In regard to outcomes measured at Time 1 and Time 2. Missing outcome data balanced in numbers (3 to 5 at six months), with similar reasons for missing data across groups. For sick leave data, 30% missing data was substantial, but unlikely related to participant characteristics. Data refused by insurance company. ITT not mentioned. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods were presented in results. |
Slater 2009.
| Methods | RCT. Study dates not reported. | |
| Participants | Location = Naval medical centre in the United States. Inclusion criteria (1) age 18 to 50 years, (2) first‐onset back pain (thoracic vertebra 6 or below) present daily for at least 6 but less than 10 weeks, (3) no other major medical illness or pain disorder, and (4) not a candidate for acute surgical intervention. *Baseline symptom intensity for control group: Mean pain was 11.78 (4.1) on the 0 to 20 Descriptor Differential Scale and mean functional status was 12.73 (9.29) on 136‐item self‐report Sickness Impact Profile (reported as percentage); LOWER symptom intensity. |
|
| Interventions | Both groups received treatment consisting of 1 outpatient visit, which included (1) history, back examination, screening laboratory assessment for red flags; (2) discussion of physical findings; (3) a prescription for low‐impact aerobic exercise; (4) general health recommendations; and (5) brief education regarding the benign natural history of back pain, and a Readers Digest article, Good News for Bad Backs. Follow‐up visits occurred if requested or were indicated.
Intervention = Usual medical care (as described above) plus multi‐component chronic pain program n = 34, mean age (SD) = 28.90 (6.8), 18% female. The experimental intervention was a modification of a behavioral medicine chronic pain program revised in pilot work to fit a subacute sample. It consisted of 4 weekly, 1‐hour individual sessions, led by a masters‐level clinician trained for the study in behavioral pain management and rehabilitation methods.. *The duration of the intervention was 6 to 10 weeks, 4 hours a week = mid‐intensity. Comparison = Usual care (as described above) plus "attention control" n = 33, mean age (SD) = 32.2 (8.3), 9% female. The attention control condition delivered nonspecific therapeutic ingredients. It was delivered in 4 weekly, 1‐hour individual sessions by a master's‐level clinician with training in psychotherapy, and provided nondirective, supportive care, in contrast with the active, directive approach of the experimental treatment. |
|
| Outcomes | Proportion of participants classified as recovered, pain, disability (Sickness Impact Profile, with higher scores indicating more severe disability), health status, pain beliefs, functional work category. Participants were followed up at 6 months and 12 months. Proportion of participants recovered at six months (defined in terms of pain and function) Modified intent‐to‐treat sample (n = 65), I = 52%, C = 31%. Chi2 test = 2.75, df = 1, P = 0.09 Group differences were statistically significant when looking at (1) those completing 4 sessions (n = 50), P = 0.02, and (2) the maximum dose sample (n = 32), P = 0.002) Note: Group means for other outcomes of interest (i.e. pain and disability) were not reported. Adverse events: Not reported. |
|
| Notes |
Attrition Intervention group: 1 lost to six‐month follow‐up, 9 attended fewer than 4 sessions. Comparison group: 1 lost to six‐month follow‐up, 7 attended fewer than 4 sessions. Funding source/COIs for primary researchers: "The Chief, Bureau of Medicine and Surgery, Navy Department, Washington DC, Clinical Investigation Program, sponsored this report." "A commercial party having a direct financial interest in the results of the research supporting this article has conferred or will confer financial benefit on one of the authors. Dr. Atkinson is on the Scientific Advisory Board of Eli Lilly, which sells antidepressants, an alternative treatment method for low back pain." Applicability: Mainly male, attending Naval Medical Centre. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | After qualification and baseline assessment, participants were randomly assigned to behavioral medicine or attention control conditions. |
| Allocation concealment (selection bias) | Low risk | To guard integrity of the blind, the code for group assignment was held by a separate research unit. |
| Blinding of participants (performance bias) | High risk | Not possible due to nature of intervention. Note treatments were conducted in separate areas to prevent cross‐talk. |
| Blinding of personnel (performance bias) | Low risk | To guard integrity of the blind, the code for group assignment was held by a separate research unit. Assessors and therapists were not told about the alternative treatments and hypotheses. Treatments were conducted in separate areas to prevent cross‐talk. |
| Compliance (adherence) acceptable? (performance bias) | Low risk | Authors (Figure 1) provided information on those that completed the treatment sessions (25/34 intervention and 26/33 control). |
| Co‐interventions avoided or similar? (performance bias) | Low risk | Any cointerventions appeared to be similar across groups. |
| Blinding of outcome assessment (detection bias) Administrative data and other non‐self‐report outcomes1 | Low risk | Functional work category: "In a routine administrative action separate from the research project, each participant's physician rated physical fitness for duty". |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Participant was outcome assessor. Blinding not possible due to nature of intervention. |
| Timing of outcome assessment (measurement/detection bias) | Low risk | Outcomes measured at standard time‐points. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Given the exploratory purpose and small scale of this study, both a modified intent‐to‐treat analysis, assessing between‐group differences in proportion recovered among all enrolled participants who completed the 6‐month follow‐up assessment (n = 65), and a completer (n = 50) analysis were planned a priori. We did not include the 2 participants (1 in each group) who completed 4 treatment sessions but not the 6‐month follow‐up in these analyses because we did not have any good data from which to estimate their 6‐month recovery status. If we were to carry forward their baseline values, they would both of necessity be classified as having chronic pain based on the inclusion criteria; however, we rejected this approach, given that they chose to receive a full dose of either behavioral or attention control treatment. Noninclusion of these individuals should not have systematically biased the results in favour of one or the other condition, but could have slightly increased proportional estimates of recovery in both groups. Supplemental analyses were also conducted on participants who attended all 4 sessions and the 6‐month follow‐up (n = 50) and the maximum dose sample who attended all 4 sessions and 2 boosters (n = 32). No ITT, but not a concern due to low loss to follow‐up. |
| Selective reporting (reporting bias) | High risk | Group differences not reported for health status and work productivity/functional work category.. |
Whitfill 2010.
| Methods | RCT. Dates of study not reported. | |
| Participants | Participants involved in this investigation consisted of consecutive individuals (n = 994), referred for initial screening to The Acute Low Back Pain Program. The study was conducted in the United States. Inclusion English speakers between the ages of 18 and 65; the onset of an original case of acute LBP within 3 months of involvement in the study. *Baseline symptom intensity in control group: Mean pain was 5.95 (1.95) on VAS scale, and functional status at baseline was not reported; symptom intensity information not available. |
|
| Interventions | There were 2 treatment groups which were eventually combined because there were no differences between early intervention (EI) and EI + Work Transition (EI/WT) Intervention = Physical therapy and behavioral medicine ("Early Intervention") plus work transition for subset of participants Early Intervention (EI) n = 46, mean age (SD) = 41.8 (11.2), 38.7% female. Physical therapy sessions emphasized an active sports medicine approach involving stretching and exercise in an attempt to maintain/improve strength, endurance and range of motion. The behavioral medicine sessions lasted 45 min each, and followed a specific protocol focusing on stress management/biofeedback and other cognitive‐behavioral pain management techniques (coping skills, distraction techniques, etc.). EI + Work transition (WT) n = 43, age = not clearly reported, 55.8% female Work transition component: participants were also allowed up to 6 sessions of 45‐min each, and one or more case management sessions. The goal of the work transition sessions was to aid in the transition back to work or help address current work conditions that may have aggravated the injury. Modifications related to schedules, tasks and ergonomics were examples of areas that might benefit from adjustment. An occupational therapist specialist administered this WT component. The EI and WT treatment components were administered by licensed professionals trained in their respective fields. *The duration of the intervention was from 4 to 10 weeks; 6 to 9 behavioral medicine sessions; 6 to 9 physical therapy sessions; up to 6 work transitions sessions; one or more case management sessions = low intensity. Comparison = standard care n = 44, mean age (SD) = not clearly reported, 56.8% female. Standard care: no additional information provided. |
|
| Outcomes | Return‐to‐work (self‐report), perceived work limitations, work productivity, pain (multiple measures), depression (BDI), SF‐36 (physical and mental components), coping. Participants were followed up at 1 year. Functional disability (Million VAS) at 1 year Minimal important change classifications used. According to Chi2 test, a clinically significant reduction in MVAS was shown in the I group compared to the C group, Chi2 (1, n = 101) = 3.66, P = .04 Note: Means and SDs not reported. Mean SF‐36 at 1year (higher numbers represented higher levels of functioning) I = 40.47 (11.47), C = 39.45 (10.59). One‐way repeated measures ANOVA showed significant group differences for the physical component, F (1, 93) = 4.31, P = 0.04, but not participant's mental functioning. Means and SDs not reported for mental and physical functioning separately. Symptoms of depression at 1year I = 8.81 (9.49), C = 10.11 (10.23) One‐way repeated measures ANOVA showed that participants in the I group showed improvement in mood levels, F(1, 92) = 8.76, P < 0.01 Adverse events: Not reported. |
|
| Notes |
Attrition: Not reported. Funding source/COIs for primary researchers: The writing of this article was supported in part by grants to Dr. Gatchel from the National Institutes of Health. Applicability: Only high risk individuals randomised. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of randomizations not specified. |
| Allocation concealment (selection bias) | Unclear risk | Not mentioned in text. |
| Blinding of participants (performance bias) | High risk | Not possible due to nature of intervention. |
| Blinding of personnel (performance bias) | High risk | Not possible due to nature of intervention. |
| Compliance (adherence) acceptable? (performance bias) | Unclear risk | No information provided and unable to ascertain. |
| Co‐interventions avoided or similar? (performance bias) | Low risk | No indication of cointerventions. |
| Blinding of outcome assessment (detection bias) Self‐reported outcomes | High risk | Participant was outcome assessor. Blinding not possible because of the nature of the intervention. |
| Timing of outcome assessment (measurement/detection bias) | Low risk | Outcomes measured at standard time points. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts not described in detail, but appeared to be very few (comparing numbers randomised to baseline data and degrees of freedom in analyses). "In instance where there was missing follow‐up data for any subjects, an intent‐to‐treat analysis (using the last observation carried forward approach) was used. |
| Selective reporting (reporting bias) | Low risk | All outcomes described in methods were presented in results. |
1A blank cell for this item indicates that non‐self‐report outcomes were not used in the study.
BDI: Beck Depression Inventory
C: comparison group
CBT: cognitive behavior therapy
CES‐D: Center for Epidemiological Studies Depression Scale
COI: conflict of interest
df: degrees of freedom
EI: early intervention
FABQ: Fear‐Avoidance Belief Questionnaire
FIOH: Finnish Institute of Occupational Health
I: intervention group
ITT: intention to treat
LBP: low back pain
MVAS: Million visual analogue scale
Orebro: Orebro Musculoskeletal Pain Screening Questionnaire
RDQ: Roland‐Morris Disability Questionnaire
RTW: return‐to‐work
SD: standard deviation
SF‐36: Short Form Survey (SF‐36)
WT: work transition
VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bronfort 2000 | Rehabilitation is not multidisciplinary. |
| Bronfort 2012 | Appeared to be chronic LBP. |
| Cherkin 1996 | Rehabilitation is not multidisciplinary. |
| Cherkin 1998 | Rehabilitation is not multidisciplinary. |
| Dehlin 1981 | Rehabilitation is not multidisciplinary. |
| Ewert 2009 | Not subacute low back pain. |
| Fordyce 1986 | Non‐multidisciplinary rehabilitation for acute back pain. |
| Gohner 2006 | This study defined subacute LBP as between 7 days and 7 weeks.. |
| Hagen 2000 | Rehabilitation is not multidisciplinary. |
| Haldorsen 1998 | Rehabilitation is not multidisciplinary. |
| Hasenbring 1999 | Acute sciatica. |
| Hay 2005 | Rehabiliation is not multidisciplinary. |
| Heymans 2006 | Rehabilitation is not multidisciplinary. |
| Iles 2011 | Rehabilitation is not multidisciplinary. |
| Indahl 1995 | Rehabilitation is not multidisciplinary. |
| Indahl 1998 | Rehabilitation is not multidisciplinary. |
| Keel 1998 | Participants too chronic. |
| Lie 2008 | Rehabilitation is not multidisciplinary. |
| Lindström 1992 | This study was included in the original version of the review. However, the intervention did not meet our criteria for multidisciplinary because it was not carried out by two or more clinicians from different disciplines. |
| Linton 2000 | Rehabilitation is not multidisciplinary. |
| Moffett 1999 | Rehabilitation is not multidisciplinary. Participants were subacute and chronic low back pain patients. |
| Morrison 1988 | Fatal flaw: There was not a real control group. Participants were randomised in an index group and a control group. Both groups received rehabilitation. In the control group, baseline assessment was done before rehabilitation and in the index group after rehabilitation. Results were concluded to be unusable. |
| Pengel 2007 | Rehabilitation is not multidisciplinary. Trained physiotherapists delivered entire intervention. |
| Seferlis 1998 | Acute low back patients. Rehabilitation is not multidisciplinary. |
| Staal 2004 | Rehabilitation is not multidisciplinary. Graded activity carried out by physiotherapists. |
| Steenstra 2006 | Rehabilitation is not multidisciplinary ‐ one clinician, physiotherapist. |
| Storheim 2003 | Rehabilitation is not multidisciplinary. |
| Taimela 2000 | Rehabiliation is not multidisciplinary. |
| Whitehurst 2007 | Rehabilitation is not multidisciplinary. Trained physiotherapists delivered entire intervention. |
Characteristics of studies awaiting assessment [ordered by study ID]
Rodriguez‐Blanco.
| Methods | RCT. |
| Participants |
|
| Interventions | A multidisciplinary intervention including physical, psychological, educational, and pharmacological aspects. |
| Outcomes |
Outcomes were measured at baseline, 3 months, 6 and 12 months. |
| Notes | Trial registration: Barcelona, 01/01/2009. Study is now complete, but we were unable to find any published studies. |
LBP: low back pain SF‐12: Short Form Survey (SF‐12)
Characteristics of ongoing studies [ordered by study ID]
ISRCTN14136384.
| Trial name or title | Comparing multidisciplinary and brief intervention in sick‐listed employees with low back pain. Do job relations matter? |
| Methods | RCT. |
| Participants | Inclusion criteria:
|
| Interventions | Brief Intervention: Information about pain management + physiotherapist appointment. Multidisciplinary Intervention: Brief intervention + individual treatment plan provided by group of experts. |
| Outcomes | Return‐to‐work (RTW), which will be measured during a follow‐up period of one year. RTW is here defined as the first 4‐week period after sick‐listing, where sick leave and disability benefits are not received. Data will be retrieved from registers of public social transfer income. |
| Starting date | October 2010. |
| Contact information | |
| Notes |
NCT00908102.
| Trial name or title | Managing nonacute low back symptoms in occupational health: two trials. |
| Methods | RCT. |
| Participants | Inclusion criteria:
|
| Interventions |
|
| Outcomes | Sickness absence days (low back (LB) specific, other than LB total) (time frame: 6, 12, 24, 36, 48 months) Low back pain (VAS) (time frame: 0, 3, 6, 12, 24 months) Disability (Roland‐Morris 18) (time frame: 0, 3, 6, 12, 24 months) Quality of life (15‐Dimensional Measure of Health‐Related Quality of Life) (time frame: 0, 3, 6, 12, 24 months). |
| Starting date | September 2001. |
| Contact information | |
| Notes | Duration of LBP may exceed 3 months, in which case the study should be included in review on MBR for chronic LBP. |
NCT01690234.
| Trial name or title | Early co‐ordinated multidisciplinary intervention to prevent sickness absence and labor market exclusion in patients with low back pain. |
| Methods | RCT. |
| Participants | Inclusion criteria:
|
| Interventions | Experimental: Early co‐ordinated multidisciplinary intervention: physiotherapist, chiropractor, rheumatologist, psychologist, occupational physician, ergonomist and social worker/case manager. Active Comparator: Usual care intervention from physiotherapist, chiropractor, rheumatologist, and social worker. |
| Outcomes | Number of days off work (time frame: 12 months). |
| Starting date | September 2009. |
| Contact information | |
| Notes | Duration of LBP may exceed 3 months, in which case the study should be included in review on MBR for chronic LBP. |
NCT02609750.
| Trial name or title | Structured care with workplace interventions to improve work ability in patients with neck and/or low back pain (WorkUp). |
| Methods | Cluster RCT. |
| Participants | Inclusion criteria:
|
| Interventions | Experimental: Structured care & workplace intervention. Active Comparator: Treatment as usual. |
| Outcomes | Work ability (time frame: Changes from baseline to 3, 6, 12 months and 2 and 3 years). Work ability (defined as being at work or being eligible to the labour market during at least four weeks in a row) and time of sickness absence and return‐to‐work. Year 2 and 3 follow‐up by register data. |
| Starting date | January 2013. |
| Contact information | |
| Notes | Unclear whether study results will be reported separately for back and neck pain. |
LBP: low back pain
MBR: multidisciplinary biopsychosocial rehabilitation
OH: occupational health
RTW: return to work
VAS: visual analogue scale
Differences between protocol and review
Since the previous version of the review, we updated the methods in accordance with the Furlan 2015 method guidelines.
We only included studies when the full report was peer‐reviewed. We also worked with translators to review all non‐English studies against the inclusion criteria. In the previous version of the review, we included studies where the patients had experienced low back pain for more than four weeks but less than three months. In this update, we included patients with pain for more than six weeks but less than 12 weeks. We clarified that we included participants with or without radiating pain and excluded patients during or immediately following pregnancy.
For the intervention, we clarified that the MBR program must involve healthcare professionals from at least two different clinical backgrounds, which led to the exclusion of a previously included study, and defined the physical, psychological, and social/occupational components. We also outlined the comparisons to be included in the review. We did not include satisfaction with treatment as an outcome in this version of the review. Instead we looked at psychological and cognitive function (depression, anxiety, fear avoidance and coping satisfaction).
Contributions of authors
Rachel Couban contributed to the review by creating the search strategy and running the search, assisting with study selection, the 'risk of bias' assessment and data extraction, and reviewing final drafts of the manuscript.
Emma Irvin contributed to study selection, the 'risk of bias' assessment, date extraction, and the GRADE assessment, as well as reviewing and editing the review.
Steven Kamper contributed to data extraction and data analysis, as well as reviewing and editing the review.
Bart Koes was involved in the previous version of this review, and he reviewed and edited the present version.
Antti Malmivaara was involved in the previous version of this review, and he reviewed and edited the present version.
Teresa Marin contributed to study selection, the 'risk of bias' assessment, data extraction, the GRADE assessment, and data analysis, as well as writing the review.
Dwayne Van Eerd contributed to study selection, 'risk of bias' assessment, and data extraction, as well as reviewing and editing the review.
Maurits van Tulder was involved in the previous version of this review, and he reviewed and edited the present version.
Declarations of interest
Rachel Couban has no conflicts to declare.
Emma Irvin has no conflicts to declare.
Bart Koes has no conflicts to declare.
Antti Malmivaara has no conflicts to declare.
Steven Kamper has acted as a consultant providing methodological advice on an unrelated study to AO Spine; his salary is paid by a research fellowship from the National Health and Medical Research Council of Australia.
Teresa Marin has no conflicts to declare.
Dwayne Van Eerd has no conflicts to declare.
Maurits van Tulder has no conflicts to declare.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Anema 2007 {published data only (unpublished sought but not used)}
- Anema JR, Steenstra IA, Bongers PM, Vet HCW, Knol DL, Loisel P, et al. Multidisciplinary rehabilitation for subacute low back pain: graded activity or workplace intervention or both? A randomized controlled trial. Spine 2007;32(3):291‐8. [DOI] [PubMed] [Google Scholar]
- Steenstra IA, Anema JR, Bongers PM, Vet HC, Mechelen W. Cost effectiveness of a multi‐stage return to work program for workers on sick leave due to low back pain, design of a population based controlled trial. BMC Musculoskeletal Disorders 2003;4:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bultmann 2009 {published and unpublished data}
- Bultmann U, Sherson D, Olsen J, Hansen CL, Lund T, Kilsgaard J. Coordinated and tailored work rehabilitation: a randomized controlled trial with economic evaluation undertaken with workers on sick leave due to musculoskeletal disorders. Journal of Occupational Rehabilitation 2009;19:81‐93. [DOI] [PubMed] [Google Scholar]
Campello 2012 {published data only}
- Campello M, Ziemke G, Hiebert R, Weiser S, Brinkmeyer M, Fox B, et al. Implementation of a multidisciplinary program for active duty personnel seeking care for low back pain in a U.S. navy medical center: a feasibility study. Military Medicine 2012;177(9):1075‐80. [DOI] [PubMed] [Google Scholar]
- Hiebert R, Campello MA, Weiser S, Ziemke GW, Fox BA, Nordin M. Predictors of short‐term work‐related disability among active duty US Navy personnel: a cohort study in patients with acute and subacute low back pain. Spine Journal 2012;12:806‐16. [DOI] [PubMed] [Google Scholar]
Jensen 2011 {published data only}
- Jensen C, Jensen OK, Christiansen DH, Nielsen CV. One‐year follow‐up in employees sick‐listed because of low back pain. Spine 2011;36(15):1180‐9. [DOI] [PubMed] [Google Scholar]
- Jensen C, Nielsen CV, Jensen OK, Petersen KD. Cost‐effectiveness and cost‐benefit analyses of a multidisciplinary intervention compared with a brief intervention to facilitate return to work in sick‐listed patients with low back pain. Spine 2013;38(13):1059‐67. [DOI] [PubMed] [Google Scholar]
- Stapelfeldt CM, Christiansen DH, Jensen OK, Nielsen CV, Petersen KD, Jensen C. Subgroup analyses on return to work in sick‐listed employees with low back pain in a randomised trial comparing brief and multidisciplinary intervention. BMC Musculoskeletal Disorders 2011;12:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karjalainen 2003 {published data only (unpublished sought but not used)}
- Karjalainen K, Malmivaara A, Pohjolainen T, Hurri H, Mutanen P, Rissanen P, et al. Mini‐intervention for subacute low back pain: a randomized controlled trial. Spine 2003;28(6):533‐41. [DOI] [PubMed] [Google Scholar]
- Karjalainen K, Malmivaara A, Pohjolainen T, Mutanen P, Roine R, Hurri H, et al. Mini‐intervention for subacute low back pain: two‐year follow‐up and modifiers of effect. Spine 2004;29(10):1069‐79. [DOI] [PubMed] [Google Scholar]
Loisel 1997 {published and unpublished data}
- Loisel P, Abenhaim L, Durand P, Esdaile J, Suissa S, Gosselin L, et al. A population‐based, randomized clinical trial on back pain management. Spine 1997;22:2911‐8. [DOI] [PubMed] [Google Scholar]
Schiltenwolf 2006 {published and unpublished data}
- Schiltenwolf M, Buchner M, Heindl B, Reumont J, Muller A, Eich W. Comparison of a biopsychosocial therapy (BT) with a conventional biomedical therapy (MT) of subacute low back pain in the first episode of sick leave: a randomized controlled trial. European Spine Journal 2006;15:1083‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Slater 2009 {published data only (unpublished sought but not used)}
- Slater MA, Weickgenant AL, Greenberg MA, Wahlgren DR, Williams RA, Carter C, et al. Preventing progression to chronicity in first onset, subacute low back pain: an exploratory study. Archives of Physical Medicine and Rehabilitation 2009;90:545‐52. [DOI] [PubMed] [Google Scholar]
Whitfill 2010 {published and unpublished data}
- Whitfill T, Haggard R, Bierner SM, Pransky G, Hassett RG, Gatchel RJ. Early intervention options for acute low back pain patients: a randomized clinical trial with one‐year follow‐up outcomes. Journal of Occupational Rehabilitation 2010;20:256‐63. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bronfort 2000 {published data only}
- Bronfort G, Evans RL, Anderson AV, Schellhas KP, Garvey TA, Marks RA, et al. Nonoperative treatments for sciatica: a pilot study for a randomized clinical trial. Journal of Manipulative & Physiological Therapeutics 2000;23(8):536‐44. [DOI] [PubMed] [Google Scholar]
Bronfort 2012 {published data only}
- Bronfort G, Maiers M, Evans R, Westrom K. Individualized chiropractic and integrative care for low back pain: a randomized clinical trial. BMC Complementary and Alternative Medicine 2012;12 (Suppl 1):185 (P02.129). [Google Scholar]
Cherkin 1996 {published data only}
- Cherkin DC, Deyo RA, Street JH, Hunt M, Barlow W. Pitfalls of patient education. Limited success of a program for back pain in primary care. Spine 1996;21(3):345‐55. [DOI] [PubMed] [Google Scholar]
Cherkin 1998 {published data only}
- Cherkin DC, Deyo RA, Battie M, Street J, Barlow W. A comparison of physical therapy, chiropractic manipulation, and provision of an educational booklet for the treatment of patients with low back pain. New England Journal of Medicine 1998;339(15):1021‐29. [DOI] [PubMed] [Google Scholar]
Dehlin 1981 {published data only}
- Dehlin O, Berg S, Andersson G, Grimby G. Effect of physical training and ergonomic counselling on the psychological perception of work and on the subjective assessment of low‐back insufficency. Scandinavian Journal of Rehabilitation Medicine 1981;13:1‐9. [PubMed] [Google Scholar]
Ewert 2009 {published data only}
- Ewert T, Limm H, Wessels T, Rackwitz B, Garnier K, Freumuth R, et al. The comparative effectiveness of a multimodal program versus exercise alone for the secondary prevention of chronic low back pain and disability. Journal of Injury, Function, and Rehabilitation 2009;1(9):798‐808. [DOI] [PubMed] [Google Scholar]
Fordyce 1986 {published data only}
- Fordyce WE, Brockway JA, Bergman JA, Spengler D. Acute back pain: a control‐group comparison of behavioral vs traditional management methods. Journal of Behavioral Medicine 1986;9(2):127‐40. [DOI] [PubMed] [Google Scholar]
Gohner 2006 {published data only}
- Gohner W, Schlicht W. Preventing chronic back pain: evaluation of a theory‐based cognitive‐behavioural training programme for patients with subacute back pain. Patient Education and Counseling 2006;64:87‐95. [DOI] [PubMed] [Google Scholar]
Hagen 2000 {published data only}
- Hagen EM, Eriksen HR, Ursin H. Does early intervention with a light mobilization program reduce long‐term sick leave for low back pain?. Spine 2000;25(15):1973‐6. [DOI] [PubMed] [Google Scholar]
Haldorsen 1998 {published data only}
- Haldorsen EM, Kronholm K, Skouen JS, Ursin H. Multimodal cognitive behavioral treatment of patients sicklisted for musculoskeletal pain: a randomized controlled study. Scandinavian Journal of Rheumatology 1998;27(1):16‐25. [DOI] [PubMed] [Google Scholar]
Hasenbring 1999 {published data only}
- Hasenbring M, Ulrich HW, Hartmann M, Soyka D. The efficacy of a risk factor‐based cognitive behavioral intervention and electromyographic biofeedback in patients with acute sciatic pain. an attempt to prevent chronicity. Spine 1999;24(23):2525‐35. [DOI] [PubMed] [Google Scholar]
Hay 2005 {published data only}
- Hay EM, Mullis R, Lewis M, Vohora K, Main CJ, Watson P, et al. Comparison of physical treatments versus a brief pain‐management programme for back pain in primary care: a randomised clinical trial in physiotherapy practice. Lancet 2005;365:2024‐30. [DOI] [PubMed] [Google Scholar]
Heymans 2006 {published data only}
- Heymans MW, Vet HC, Bongers PM, Knol DLK, Koes BW, Mechelen W. The effectiveness of high‐intensity versus low‐intensity back schools in an occupational setting: a pragmatic randomized controlled trial. Spine 2006;31(10):1075‐82. [DOI] [PubMed] [Google Scholar]
Iles 2011 {published data only}
- Iles R, Taylor NF, Davidson M, O'Halloran P. Telephone coaching can increase activity levels for people with non‐chronic low back pain: a randomised trial. Journal of Physiotherapy 2011;57(4):231‐8. [DOI] [PubMed] [Google Scholar]
Indahl 1995 {published data only}
- Indahl A, Velund L, Reikeraas O. Good prognosis for low back pain when left untampered: a randomized clinical trial. Spine 1995;20:473‐7. [DOI] [PubMed] [Google Scholar]
Indahl 1998 {published data only}
- Indahl A, Haldorsen EH, Holm S, Reikerås O, Holger U. Five‐year follow‐up study of a controlled clinical trial using light mobilization and an informative approach to low back pain. Spine 1998;23(23):2625‐30. [DOI] [PubMed] [Google Scholar]
Keel 1998 {published data only}
- Keel PJ, Wittig R, Deutschmann R, Diethelm U, Knusel O, Loschmann C, et al. Effectiveness of in‐patient rehabilitation for sub‐chronic and chronic low back pain by an integrative group treatment program. Scandinavian Journal of Rehabilitation Medicine 1998;30(4):211‐9. [PubMed] [Google Scholar]
Lie 2008 {published data only}
- Lie SA, Eriksen HR, Ursin H, Hagen EM. A multi‐state model for sick‐leave data applied to a randomized control trial study of low back pain. Scandinavian Journal of Public Health 2008;36(3):279–83. [DOI] [PubMed] [Google Scholar]
Lindström 1992 {published data only}
- Lindstrom I, Ohlund C, Eek C, Wallin L, Peterson L, Fordyce W, et al. The effect of graded activity on patients with subacute low back pain: a randomized prospective clinical study with an operant‐conditioning behavioral approach. Physical Therapy 1992;72(4):279‐90. [DOI] [PubMed] [Google Scholar]
- Lindstrom I, Ohlund C, Eek C, Wallin L, Peterson L, Nachemson A. Mobility, strength, and fitness after a graded activity program for patients with subacute low back pain: a randomized prospective clinical study with a behavioral therapy approach. Spine 1992;17:641‐52. [DOI] [PubMed] [Google Scholar]
- Lindstrom I, Ohlund C, Nachemson A. Physical performance, pain, pain behavior and subjective disability in patients with subacute low back pain. Scandinavian Journal of Rehabilitation Medicine 1995;27:153‐60. [PubMed] [Google Scholar]
Linton 2000 {published data only}
- Linton SJ, Andersson T. Can chronic disability be prevented? A randomized trial of a cognitive‐behavior intervention and two forms of information for patients with spinal pain. Spine 2000;25(21):2825‐31. [DOI] [PubMed] [Google Scholar]
Moffett 1999 {published data only}
- Moffett JK, Torgerson D, Bell‐Syer S, Jackson D, Llewlyn‐Phillips H, Farrin A, et al. Randomised controlled trial of exercise for low back pain: clinical outcomes, costs, and preferences. BMJ 1999;319(7205):279‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
Morrison 1988 {published data only}
- Morrison G, Chase W, Young V, Roberts W. Back pain: treatment and prevention in a community hospital. Archives of Physical Medicine and Rehabilitation 1988;69:605‐9. [PubMed] [Google Scholar]
Pengel 2007 {published data only}
- Pengel LH, Refshauge KM, Maher CG, Nicholas MK, Herbert RD, McNair P. Physiotherapist‐directed exercise, advice, or both for subacute low back pain: a randomized trial. Annals of Internal Medicine 2007;146(11):787‐96. [DOI] [PubMed] [Google Scholar]
Seferlis 1998 {published data only}
- Seferlis T, Nemeth G, Carlsson AM, Gillstrom P. Conservative treatment in patients sick‐listed for acute low‐back pain: a prospective randomised study with 12 months' follow‐up. European Spine Journal 1998;7(6):461‐70. [DOI] [PMC free article] [PubMed] [Google Scholar]
Staal 2004 {published data only}
- Hlobil H, Uegaki K, Staal JB, Bruyne MC, Smid T, Mechelen W. Substantial sick‐leave costs savings due to a graded activity intervention for workers with non‐specific sub‐acute low back pain. European Spine Journal 2007;16(7):919‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staal JB, Hlobil H, Koke AJ, Twisk JW, Smid T, Mechelen W. Graded activity for workers with low back pain: who benefits most and how does it work?. Arthritis & Rheumatism 2008;59(5):642–9. [DOI] [PubMed] [Google Scholar]
- Staal JB, Hlobil H, Twisk JWR, Smid T, Koke AJA, Mechelen W. Graded activity for low back pain in occupational health care: a randomized, controlled trial. Annals of Internal Medicine 2004;140(2):77‐84. [DOI] [PubMed] [Google Scholar]
Steenstra 2006 {published data only}
- Steenstra IA, Anema JR, Bongers PM, Vet HCW, Knol DL, Mechelen W. The effectiveness of graded activity for low back pain in occupational healthcare. Occupational and Environmental Medicine 2006;63(11):718–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Storheim 2003 {published data only}
- Storheim K, Brox JI, Holm I, Koller AK, Bø K. Intensive group training versus cognitive intervention in sub‐acute low back pain: short‐term results of a single‐blind randomized controlled trial. Journal of Rehabilitation Medicine 2003;35(3):132‐40. [DOI] [PubMed] [Google Scholar]
Taimela 2000 {published data only}
- Taimela S, Takala EP, Asklof T, Seppala K, Parviainen S. Active treatment of chronic neck pain: a prospective randomized intervention. Spine 2000;25(8):1021‐7. [DOI] [PubMed] [Google Scholar]
Whitehurst 2007 {published data only}
- Whitehurst DG, Lewis M, Yao GL, Bryan S, Raftery JP, Mullis R, et al. A brief pain management program compared with physical therapy for low back pain: results from an economic analysis alongside a randomized clinical trial. Arthritis & Rheumatism 2007;57(3):466‐73. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Rodriguez‐Blanco {published data only}
- Rodriguez‐Blanco T, Fernández‐San‐Martin I, Balagué‐Corbella M, Berenguera A, Moix J, Montiel‐Morillo E, et al. Study protocol of effectiveness of a biopsychosocial multidisciplinary intervention in the evolution of non‐specific sub‐acute low back pain in the working population: cluster randomised trial. BMC Health Services Research 2010;10:12. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
ISRCTN14136384 {published data only}
- ISRCTN14136384. Comparing multidisciplinary and brief intervention in sicklisted employees with low back pain. Do job relations matter?. www.isrctn.com/ISRCTN14136384 (accessed 2 December 2016).
NCT00908102 {published data only}
- NCT00908102. Managing non‐acute low back symptoms in occupational health: two trials. clinicaltrials.gov/ct2/show/NCT00908102 (accessed 2 December 2016).
NCT01690234 {published data only}
- NCT01690234. Early coordinated multidisciplinary intervention to prevent sickness absence and labor market exclusion in patients with low back pain. clinicaltrials.gov/ct2/show/NCT01690234 (accessed 2 December 2016).
NCT02609750 {published data only}
- NCT02609750. WorkUp. Structured care with workplace interventions to improve work ability in patients with neck and/or low back pain. clinicaltrials.gov/ct2/show/NCT02609750 (accessed 2 December 2016).
Additional references
Artus 2010
- Artus M, Windt DA, Jordan KP, Hay EM. Low back pain symptoms show a similar pattern of improvement following a wide range of primary care treatments: a systematic review of randomized clinical trials. Rheumatology 2010;49:2346‐56. [DOI] [PubMed] [Google Scholar]
Atkins 2004
- Atkins D, Best D, Briss PA, Eccles M, Falck‐Ytter Y, GRADE Working Group. Grading quality of evidence and strength of recommendations. BMJ 2004;328:1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chou 2010
- Chou R, Shekelle P. Will this patient develop persistent disabling low back pain?. JAMA 2010;303:1295‐1302. [DOI] [PubMed] [Google Scholar]
Chou 2011
- Chou R, McCarberg B. Managing acute back pain patients to avoid the transition to chronic pain. Pain Management 2011;1(1):69‐79. [DOI] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis in the Behavioral Sciences. 2nd Edition. Hillsdale (NJ): Lawrence Erlbaum Associates, 1988. [Google Scholar]
Dagenais 2008
- Dagenais S, Caro J, Haldeman S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine Journal 2008;8(1):8‐20. [DOI: 10.1016/j.spinee.2007.10.005] [DOI] [PubMed] [Google Scholar]
Deyo 2015
- Deyo RA. Biopsychosocial care for chronic low back pain. BMJ 2015;350:h538. [DOI] [PubMed] [Google Scholar]
DistillerSR [Computer program]
- Evidence Partners. DistillerSR. Version 2. Ottawa: Evidence Partners, 2009.
Foster 2011
- Foster NE. Barriers and progress in the treatment of low back pain. BMC Medicine 2011;9:108. [DOI] [PMC free article] [PubMed] [Google Scholar]
Frymoyer 1991
- Frymoyer JW, Cats‐Baril W. An overview of the incidences and costs of low back pain. Clinical Orthopaedics and Related Research 1991;22(2):263‐71. [PubMed] [Google Scholar]
Furlan 2015
- Furlan AD, Malmivaara A, Chou R, Maher CG, Deyo R, Schoene M, et al. 2015 updated method guidelines for systematic reviews in the Cochrane Back and Neck Group. Spine 2015;40(21):1660‐73. [DOI] [PubMed] [Google Scholar]
Guzman 2006
- Guzmán J, Esmail R, Malmivaara A, Karjalainen K, Irvin E, Bombardier C. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD000963.pub2] [DOI] [Google Scholar]
Hayden 2010
- Hayden JA, Dunn KM, Windt DA, Shaw WS. What is the prognosis of back pain?. Best Practice & Research. Clinical Rheumatology 2010;24:167‐79. [DOI] [PubMed] [Google Scholar]
Hiebert 2012
- Hiebert R, Campello MA, Weiser S, Ziemke GW, Fox BA, Nordin M. Predictors of short‐term work‐related disability among active duty US Navy personnel: a cohort study in patients with acute and subacute low back pain. Spine Journal 2012;12:806‐16. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hoy 2012
- Hoy D, Bain C, Williams G, March L, Brooks P, Blyth F, et al. A systematic review of the global prevalence of low back pain. Arthritis and Rheumatism 2012;64(6):2028‐37. [DOI] [PubMed] [Google Scholar]
Kamper 2014
- Kamper SJ, Apeldoorn AT, Chiarotto A, Smeets RJ, Ostelo RWJG, Guzman J, et al. Multidisciplinary biopsychosocial rehabilitation for chronic low back pain. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD000963.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Loisel 1994
- Loisel P, Durand P, Abenhaim L, Gosselin L, Simard R, Turcotte J, et al. Management of occupational back pain: the Sherbrooke model. Results of a pilot and feasibility study. Occupational and Environmental Medicine 1994;51:597‐602. [DOI] [PMC free article] [PubMed] [Google Scholar]
Luo 2004
- Luo X, Pietrobon R, Sun SX, Liu GG, Hey L. Estimates and patterns of direct healthcare expenditures among individuals with back pain in the United States. Spine 2004;29:79‐86. [DOI] [PubMed] [Google Scholar]
Maetzal 2002
- Maetzal A, Li L. The economic burden of low back pain: a review of studies published between 1996 and 2001. Best Practice and Research Clinical Rheumatology 2002;16(1):23‐30. [DOI] [PubMed] [Google Scholar]
Main 2012
- Main CJ, Sowden G, Hill JC, Watson PJ, Hay EM. Integrating physical and psychological approaches to treatment in low back pain: the development and content of the sTarT Back trial's 'high risk' intervention. Physiotherapy 2012;98:110‐6. [DOI] [PubMed] [Google Scholar]
Menezes Costa 2009
- Menezes Costa LDC, Maher CG, McAuley JH, Hancock MJ, Herbert RD, Refshauge KM, et al. Prognosis for patients with chronic low back pain: inception cohort study. BMJ 2009;339:b3829. [DOI: 10.1136/bmj.b3829] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mueller 2007
- Mueller PS, Montori VM, Bassler D, Koenig BA, Guyatt GH. Ethical issues in stopping randomized trials early because of apparent benefit. Annals of Internal Medicine 2007;146(12):878‐81. [DOI] [PubMed] [Google Scholar]
Pengel 2003
- Pengel LH, Herbert RD, Maher CG, Refshauge KM. Acute low back pain: systematic review of its prognosis. BMJ 2003;327:323. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schaafsma 2013
- Schaafsma FG, Whelan K, Beek AJ, Es‐Lambeek LC, Ojajärvi A, Verbeek JH. Physical conditioning as part of a return to work strategy to reduce sickness absence for workers with back pain. Cochrane Database of Systematic Reviews 2013, Issue 8. [DOI: 10.1002/14651858.CD001822.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Shaw 2009
- Shaw WS, Windt DA, Main CJ, Loisel P, Linton SJ, 'Decade of the Flags' Working Group. Early patient screening and intervention to address individual‐level occupational factors ('Blue Flags') in back disability. Journal of Occupational Rehabilitation 2009;19:64‐80. [DOI] [PubMed] [Google Scholar]
Steenstra 2003
- Steenstra IA, Anema JR, Bongers PM, Vet HC, Mechelen W. Cost effectiveness of a multi‐stage return to work program for workers on sick leave due to low back pain, design of a population based controlled trial. BMC Musculoskeletal Disorders 2003;4:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stewart 2003
- Stewart WF, Ricci JA, Chee E, Morganstein D, Lipton R. Lost productive time and cost due to common pain conditions in the US workforce. JAMA 2003;290:2443‐54. [DOI] [PubMed] [Google Scholar]
Vos 2015
- Vos T, Barber RM, Bell B, Bertozzi‐Villa A, Biryukov S, Bolliger I, et al. Global, regional, and national incidence, prevalence, and years lived with disability for 301 acute and chronic diseases and injuries in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 2015;386:743‐800. [http://dx.doi.org/10.1016/ S0140‐6736(15)60692‐4 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Waddell 1987
- Waddell G. Volvo award in clinical sciences: a new clinical model for the treatment of low‐back pain. Spine 1987;12:632‐44. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Karjalainen 2003
- Karjalainen K, Malmivaara A, Tulder M, Roine R, Jauhiainen M, Hurri H, et al. Multidisciplinary biopsychosocial rehabilitation for subacute low‐back pain among working age adults. Cochrane Database of Systematic Reviews 2003, Issue 2. [DOI: 10.1002/14651858.CD002193] [DOI] [PubMed] [Google Scholar]


