Abstract
Background
Periodontal disease has been linked with a number of conditions, such as cardiovascular disease, stroke, diabetes and adverse pregnancy outcomes, all likely through systemic inflammatory pathways. It is common in women of reproductive age and gum conditions tend to worsen during pregnancy. Some evidence from observational studies suggests that periodontal intervention may reduce adverse pregnancy outcomes. There is need for a comprehensive Cochrane review of randomised trials to assess the effect of periodontal treatment on perinatal and maternal health.
Objectives
To assess the effects of treating periodontal disease in pregnant women in order to prevent or reduce perinatal and maternal morbidity and mortality.
Search methods
Cochrane Oral Health's Information Specialist searched the following databases: Cochrane Oral Health's Trials Register (to 6 October 2016), Cochrane Pregnancy and Childbirth's Trials Register (to 7 October 2016), the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 9) in the Cochrane Library, MEDLINE Ovid (1946 to 6 October 2016), Embase Ovid (1980 to 6 October 2016), and LILACS BIREME Virtual Health Library (Latin American and Caribbean Health Science Information database; 1982 to 6 October 2016). ClinicalTrials.gov and the World Health Organization International Clinical Trials Registry Platform were searched for ongoing trials on 6 October 2016. We placed no restrictions on the language or date of publication when searching the electronic databases.
Selection criteria
We included all randomised controlled trials (RCTs) investigating the effects of periodontal treatment in preventing or reducing perinatal and maternal morbidity and mortality. We excluded studies where obstetric outcomes were not reported.
Data collection and analysis
Two review authors independently screened titles and abstracts and extracted data using a prepiloted data extraction form. Missing data were obtained by contacting authors and risk of bias was assessed using Cochrane's 'Risk of bias' tool. Where appropriate, results of comparable trials were pooled and expressed as risk ratios (RR) or mean differences (MD) with 95% confidence intervals (CI) . The random‐effects model was used for pooling except where there was an insufficient number of studies. We assessed the quality of the evidence using GRADE.
Main results
There were 15 RCTs (n = 7161 participants) meeting our inclusion criteria. All the included studies were at high risk of bias mostly due to lack of blinding and imbalance in baseline characteristics of participants. The studies recruited pregnant women from prenatal care facilities who had periodontitis (14 studies) or gingivitis (1 study).The two main comparisons were: periodontal treatment versus no treatment during pregnancy and periodontal treatment versus alternative periodontal treatment. The head‐to‐head comparison between periodontal treatments assessed a more intensive treatment versus a less intensive one.
Eleven studies compared periodontal treatment with no treatment during pregnancy. The meta‐analysis shows no clear difference in preterm birth < 37 weeks (RR 0.87, 95% CI 0.70 to 1.10; 5671 participants; 11 studies; low‐quality evidence) between periodontal treatment and no treatment. There is low‐quality evidence that periodontal treatment may reduce low birth weight < 2500 g (9.70% with periodontal treatment versus 12.60% without treatment; RR 0.67, 95% CI 0.48 to 0.95; 3470 participants; 7 studies).
It is unclear whether periodontal treatment leads to a difference in preterm birth < 35 weeks (RR 1.19, 95% CI 0.81 to 1.76; 2557 participants; 2 studies; ) and < 32 weeks (RR 1.35, 95% CI 0.78 to 2.32; 2755 participants; 3 studies), low birth weight < 1500 g (RR 0.80, 95% CI 0.38 to 1.70; 2550 participants; 2 studies), perinatal mortality (including fetal and neonatal deaths up to the first 28 days after birth) (RR 0.85, 95% CI 0.51 to 1.43; 5320 participants; 7 studies; very low‐quality evidence), and pre‐eclampsia (RR 1.10, 95% CI 0.74 to 1.62; 2946 participants; 3 studies; very low‐quality evidence). There is no evidence of a difference in small for gestational age (RR 0.97, 95% CI 0.81 to 1.16; 3610 participants; 3 studies; low‐quality evidence) when periodontal treatment is compared with no treatment.
Four studies compared periodontal treatment with alternative periodontal treatment. Data pooling was not possible due to clinical heterogeneity. The outcomes reported were preterm birth < 37 weeks, preterm birth < 35 weeks, birth weight < 2500 g, birth weight < 1500 g and perinatal mortality (very low‐quality evidence). It is unclear whether there is a difference in < 37 weeks, preterm birth < 35 weeks, birth weight < 2500 g, birth weight < 1500 g and perinatal mortality when different periodontal treatments are compared because the quality of evidence is very low.
Maternal mortality and adverse effects of the intervention did not occur in any of the studies that reported on either of the outcomes.
Authors' conclusions
It is not clear if periodontal treatment during pregnancy has an impact on preterm birth (low‐quality evidence). There is low‐quality evidence that periodontal treatment may reduce low birth weight (< 2500 g), however, our confidence in the effect estimate is limited. There is insufficient evidence to determine which periodontal treatment is better in preventing adverse obstetric outcomes. Future research should aim to report periodontal outcomes alongside obstetric outcomes.
Plain language summary
Treating gum disease to prevent adverse birth outcomes in pregnant women
What is the aim of this review?
The aim of this Cochrane Review was to find out if treating gum disease can prevent adverse birth outcomes in pregnant women. Cochrane researchers collected and analysed all relevant studies to answer this question and found 15 relevant studies.
Key messages
There is no evidence that the treatment of gum disease reduces the number of babies born before 37 weeks of pregnancy, however, it may reduce the number of babies born weighing less than 2500 g. It is uncertain whether there is a difference in adverse birth outcomes when different methods of treating gum disease are compared.
What was studied in the review?
Gum health tends to worsen during pregnancy. There has been some research associating gum disease with adverse birth outcomes. The review assessed studies where pregnant women with gum disease were treated using a combination of different mechanical techniques with or without antibiotics.
What are the main results of the review?
The review authors found 15 relevant studies. Five were from North America, four from South America, three from Europe, two from Asia and one from Austalia. Eleven studies compared either scaling and root planing or scale and polish with no treatment while the other four studies compared scaling and root planing with alternative mechanical treatments.
When pregnant women with gum disease who receive periodontal treatment are compared with those who receive no treatment:
‐ there is no clear difference in the number of babies born before 37 weeks (low‐quality evidence); ‐ there may be fewer babies born weighing less than 2500 g (low‐quality evidence).
It is unclear if one periodontal treatment is better than alternative periodontal treatments in preventing adverse birth outcomes.
How up‐to‐date is this review?
The review authors searched for studies that had been published up to October 2016.
Summary of findings
Summary of findings for the main comparison. Periodontal treatment compared to no treatment for preventing adverse birth outcomes in pregnant women.
| Periodontal treatment compared to no treatment for preventing adverse birth outcomes in pregnant women | ||||||
| Patient or population: pregnant women considered to have periodontal disease after dental examination Settings: clinics and hospitals Intervention: periodontal treatment Comparison: no treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| No treatment | Periodontal treatment | |||||
| Gestational age (preterm birth < 37 weeks) | Study population | RR 0.87 (0.70 to 1.10) | 5671 (11 RCTs) | ⊕⊕⊝⊝ LOW1 | Preterm birth < 35 weeks and < 32 weeks were also reported. There was no evidence of a difference in preterm birth < 35 weeks (RR 1.19 (0.81 to 1.76), 2 studies; 2557 participants) and < 32 weeks (RR 1.35 (0.78 to 2.32), 3 studies; 2755 participants) (VERY LOW2 quality evidence) | |
| 131 per 1000 | 114 per 1000 (92 to 143) | |||||
| Birth weight (low birth weight < 2500 g) | Study population | RR 0.67 (0.48 to 0.95) | 3470 (7 RCTs) | ⊕⊕⊝⊝ LOW3 | Low birth weight < 1500 g was reported in 2 studies. There was no evidence of a difference in low birth weight < 1500 g (RR 0.80 (0.38 to 1.70); 2550 participants) (VERY LOW2 quality evidence) | |
| 126 per 1000 | 84 per 1000 (60 to 120) | |||||
| Small for gestational age | Study population | RR 0.97 (0.81 to 1.16) |
3610 (3 RCTs) | ⊕⊕⊝⊝ LOW4 | ||
| 115 per 1000 | 111 per 1000 (93 to 133) | |||||
| Perinatal mortality (including fetal and neonatal deaths up to the first 28 days after birth) | Study population | RR 0.85 (0.51 to 1.43) | 5320 (7 RCTs) | ⊕⊝⊝⊝ VERY LOW5 | ||
| 18 per 1000 | 16 per 1000 (9 to 26) | |||||
| Maternal mortality | 0% in both groups | Not estimated | 2134 (4 RCTs) | ‐ | ||
| Pre‐eclampsia | Study population | RR 1.10 (0.74 to 1.62) | 2946 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW6 | ||
| 64 per 1000 | 70 per 1000 (47 to 104) | |||||
| Adverse effects of therapy | 0% in both groups | Not estimated | 2389 (4 RCTs) | ‐ | ||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% CI) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: further research is very unlikely to change our confidence in the estimate of effect Moderate quality: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: we are very uncertain about the estimate | ||||||
1Downgraded 2 levels: serious limitation ‐ high risk of bias due to other bias (imbalance in baseline characteristics); serious inconsistency ‐ substantial heterogeneity (I2 = 66%). 2Downgraded 3 levels: serious limitation ‐ high risk of bias due to attrition; very serious imprecision ‐ low number of events and wide confidence intervals including the risk of benefit and harm. 3Downgraded 2 levels: serious limitation ‐ high risk of bias due to attrition; serious inconsistency ‐ substantial heterogeneity (I2 = 59%). 4Downgraded 2 levels: serious limitation ‐ high risk of bias due to attrition; serious inconsistency ‐ substantial heterogeneity (I2 = 54%). 5Downgraded 3 levels: very serious limitation ‐ high risk of attrition and other bias due to early termination of trial; very serious imprecision ‐ low number of events and wide confidence intervals including the risk of benefit and harm. 6Downgraded 3 levels: serious limitation ‐ high risk of attrition bias; very serious imprecision ‐ low number of events and wide confidence intervals including the risk of benefit and harm.
Background
Description of the condition
Periodontal disease is a disease of the supporting tissues of the teeth that may affect the gums, periodontal ligament membrane, cementum and bone around the tooth socket. It can present as two main types.
Gingivitis ‐ an inflammation of the gums (gingivae) around the teeth which does not cause loss of periodontal attachment (Int Workshop 1999).
Periodontitis ‐ in susceptible patients, gingivitis can progress to periodontal disease, with inflammation and destruction of the supporting tissues around the teeth.
Periodontal disease is related to low socioeconomic status (OSG 2000) and lower educational achievement (Machuca 1999). Periodontal disease has been linked to microbial infections which lead to systemic increase in proinflammatory prostaglandins and cytokines (Kim 2006). These have been hypothesised, through systemic inflammatory pathways, to lead to a number of conditions, such as cardiovascular disease, stroke, diabetes and adverse pregnancy outcomes (Papapanou 2015). While periodontal disease is common in women of reproductive age overall (e.g. 19% of Australian females 15 and over (Chrisopoulos 2012)), it is believed that gum conditions tend to worsen during pregnancy due to hormonal changes (Figuero 2013; Krejci 2002).
Observational studies in humans have shown associations between periodontal disease and adverse pregnancy outcomes including preterm birth (Ide 2013; Jeffcoat 2001; Jeffcoat 2002; Offenbacher 1996a), preterm premature rupture of the membranes (PPROM) (Offenbacher 1996b), pre‐eclampsia (Boggess 2003), pregnancy loss (Xiong 2007), and postcaesarean endometritis (Swamy 2002).
Not all observational studies, however, have found an association between preterm birth or low birth weight and periodontal disease. Davenport and colleagues in London, UK, in a case‐control study of 236 preterm infants and 507 term controls, using clinical periodontal indices measured on the labour ward, found the risk of preterm low birth weight decreased with increasing pocket depth (adjusted odds ratio (OR) 0.78, 95% confidence interval (CI) 0.64 to 0.99). The authors concluded that their results did "not support a specific drive to improve periodontal health of pregnant women as a means of improving pregnancy outcomes" (Davenport 2002). Moore and colleagues failed to find an association between preterm birth and periodontal disease in a large prospective cohort study and a smaller case‐control study (Moore 2004; Moore 2005).
Description of the intervention
Periodontal treatment may involve nonsurgical and surgical therapies, used alone or in combination. The most common periodontal therapy involves counselling on oral hygiene to educate patients on how to prevent the accumulation of dental plaque and calculus. In nonsurgical approaches, a dental hygienist or dentist removes plaque and calculus by using either hand instruments (scalers and curettes), ultrasound equipment (mechanical debridement), or polishing (Worthington 2013). When patients do not respond favourably to the initial nonsurgical treatment, surgical intervention may be required.
Antiseptic mouthwashes such as chlorhexidine can be used as a short‐term adjunct to oral hygiene measures, particularly after surgery when the patient cannot brush the area that has been operated on. Sometimes patients are given gels aimed at reducing oral bacterial load, and oral or topical antimicrobials (doxycycline, metronidazole) (Ciancio 2002). Local or systemic antibiotics may be limited to severe or aggressive periodontitis cases where symptoms persist after debridement and where good oral hygiene is evident.
How the intervention might work
The mechanism of action of periodontal treatment in preventing adverse birth outcomes is not fully understood. The general aims of treatment of periodontal disease are to resolve the inflammation by bringing the amount of plaque and calculus down to minimal levels; and to prevent or limit the tissue destruction to preserve dentition, maintain appearance and minimise discomfort (Pilot 1980; Sheiham 2002; Wennström 1990). Periodontal treatment must be followed by good oral hygiene in order for the inflammation to remain under control; resolution of this inflammation/infection may be an important outcome for preventing preterm birth. Thus instructing and motivating individuals to clean their teeth properly is an important component of periodontal treatment.
Dental care providers may be concerned that commonly used drugs such as anaesthetics, antibiotics and analgesics may harm the foetus. There may also be concern that bacteraemia caused by some dental procedures may lead to uterine infections, spontaneous abortions or preterm labour (Michalowicz 2009). Experts have recommended that dental treatment be avoided early in pregnancy during organogenesis and also late in pregnancy to avoid supine hypotension although such advice is regarded to be overly cautious by some obstetricians (Michalowicz 2008).
Why it is important to do this review
Adverse birth outcomes are traumatic and also have huge cost implications. Due to a World Health Organization (WHO) report showing that preterm birth is the second leading cause of death in children under five, addressing preterm birth has become a priority for achieving the Millennium Development Goal on infant mortality (WHO 2012). Some observational studies have stimulated interest in the treatment of women with periodontal disease in pregnancy for the possible prevention of preterm birth and other adverse birth outcomes. A systematic review of nine observational studies and three intervention studies concluded that there is some preliminary evidence to suggest that periodontal intervention may reduce adverse pregnancy outcomes (Scannapieco 2003). Many more recent reviews have since been published focusing mostly on foetal/neonatal outcomes (e.g. Fogacci 2011; Polyzos 2010). There is need for a comprehensive systematic review of randomised controlled trials assessing the effects of periodontal treatment on mothers as well as their babies.
Objectives
To assess the effects of treating periodontal disease in pregnant women in order to prevent or reduce perinatal and maternal morbidity and mortality.
Methods
Criteria for considering studies for this review
Types of studies
All published, unpublished and ongoing randomised controlled trials that compare treatments for periodontal disease during pregnancy with control treatment, or treatment with alternative interventions during pregnancy or no treatment.
Types of participants
Pregnant women considered to have periodontal disease after dental examination. The types of periodontal disease included diagnoses of gingivitis and periodontitis (Int Workshop 1999).
Types of interventions
Treatment during pregnancy for periodontal disease, performed by a dentist, dental hygienist or therapist (including mechanical debridement using scaling and root planing, polishing, or surgery), either singly or in combination with counselling on oral hygiene, antiseptic oral agents, topical or systemic antimicrobial therapies compared with either placebo (for adjunctive treatment), no treatment or alternative treatments.
Types of outcome measures
Primary outcomes
Primary outcomes were chosen to be most representative of the clinically important measures of effectiveness and safety.
Perinatal outcomes:
1. gestational age at birth (preterm birth less than 37 weeks, very preterm birth less than 34 weeks, extremely preterm birth less than 28 weeks); 2. birth weight; 3. small for gestational age (variously defined); 4. perinatal mortality.
Maternal outcomes:
5. mortality; 6. pre‐eclampsia (variously defined); 7. adverse effects of therapy.
Secondary outcomes
These include other measures of effectiveness, complications, satisfaction with care and health service use.
Maternal outcomes:
1. plaque levels measured using any appropriate scale; 2. gingival health measured using any appropriate scale; 3. changes in probing depth; 4. changes in clinical attachment levels.
We have included periodontal outcomes as secondary outcomes to establish whether or not periodontal treatment in this population results in improvements in periodontal health. This is needed in order to investigate possible sources of heterogeneity and thus to interpret findings about preterm morbidity outcomes. However, periodontal outcomes are not the main focus of the review, therefore we excluded studies not reporting any of the primary outcomes.
We collated and reported any other outcomes recorded in the studies in an appendix (Additional Table 2) to be used for developing a core outcome set in trials on pregnancy and childbirth.
1. Other obstetric outcomes.
| Outcome | Study ID |
| Birth weight ≥ 2500 g | Radnai 2009 |
| Small for gestational age (10th percentile) | Michalowicz 2006; Newnham 2009; Offenbacher 2009 |
| Preterm/low birth weight | López 2002; López 2005; Oliveira 2011; Radnai 2009; Sadatmansouri 2006; Pirie 2013 |
| Birth length | Michalowicz 2006; Newnham 2009; Offenbacher 2009; Pirie 2013 |
| Head circumference | Newnham 2009; Pirie 2013 |
| Amniotic fluid index (< 5 cm, > 25 cm) | Newnham 2009 |
| Umbilical artery S/D ratios | Newnham 2009 |
| Umbilical cord artery/vein blood (number, pH, PCO2, PO2, base excess) | Newnham 2009 |
| Meconium in amniotic fluid | Newnham 2009 |
| Decision on delivery based on electronic fetal heart rate monitoring | Newnham 2009 |
| Scalp pH measured in labour | Newnham 2009 |
| Nonreassuring fetal heart rate pattern | Newnham 2009 |
| Caesarean delivery for nonreassuring fetal heart rate | Newnham 2009 |
| Electronic fetal heart rate monitoring in labour | Newnham 2009 |
| Ventilation | Newnham 2009 |
| Continuous Positive Airway Pressure (CPAP) | Newnham 2009 |
| Oxygen | Newnham 2009 |
| Special care nursery admission | Newnham 2009 |
| 1‐min Apgar score | Pirie 2013 |
| 5‐min Apgar score (0‐3, 4‐7, 8‐10) | Offenbacher 2009; Pirie 2013 |
| Apgar score (< 7 at 1 min, < 7 at 5 min) | Michalowicz 2006; Newnham 2009; Pirie 2013 |
| Admission to neonatal intensive care unit (number admitted, length of stay > 2 days, discharged alive) | Michalowicz 2006; Offenbacher 2009 |
| Sepsis necessitating antibiotics | Newnham 2009 |
| Composite neonatal morbidity/mortality | Macones 2010; Newnham 2009; Offenbacher 2009 |
| HELLP syndrome, severe pre‐eclampsia | Herrera 2009 |
| Prenatal visits | López 2005 |
| Onset of labour (spontaneous, induced, augmented, no labour) | Newnham 2009 |
| Mode of delivery (spontaneous vaginal, assisted vaginal, elective caesarean, emergency caesarean) | Newnham 2009; Pirie 2013 |
| Fever > 37o C in labour | Newnham 2009 |
| Postpartum haemorrhage (> 1000 mL) | Newnham 2009 |
| Retained placenta | Newnham 2009 |
| Fraction of expected birth weight | Newnham 2009 |
| Urinary tract infection | Farrell 2003; López 2005 |
| Vaginosis, underweight, onset prenatal care after 20 weeks of gestation | López 2005 |
S/D = systolic/diastolic ratio.
Search methods for identification of studies
Electronic searches
Cochrane Oral Health's Information Specialist conducted systematic searches in the following databases for randomised controlled trials and controlled clinical trials. There were no language, publication year or publication status restrictions:
Cochrane Oral Health's Trials Register (searched 6 October 2016) (Appendix 1);
Cochrane Pregnancy and Childbirth's Trials Register (searched 7 October 2016) (Appendix 2);
Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 9) in the Cochrane Library (searched 6 October 2016) (Appendix 3);
MEDLINE Ovid (1946 to 6 October 2016) (Appendix 4);
Embase Ovid (1980 to 6 October 2016) (Appendix 5);
LILACS BIREME Virtual Health Library (Latin American and Caribbean Health Science Information database; 1982 to 6 October 2016) (Appendix 6).
Subject strategies were modelled on the search strategy designed for MEDLINE Ovid. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials as described in the Cochrane Handbook for Systematic Reviews of Interventions Chapter 6 (Lefebvre 2011).
Searching other resources
The following trial registries were searched for ongoing studies:
US National Institutes of Health Ongoing Trials Register ClinicalTrials.gov (clinicaltrials.gov; searched 6 October 2016) (Appendix 7);
World Health Organization International Clinical Trials Registry Platform (apps.who.int/trialsearch; searched 6 October 2016) (Appendix 7).
We searched the reference lists of included studies and relevant systematic reviews for further studies.
We did not perform a separate search for adverse effects of interventions used, we considered adverse effects described in included studies only.
Data collection and analysis
We carried out data collection and analysis according to the methods stated in the published protocol (Middleton 2015), which are based on the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011)
Selection of studies
Two review authors independently screened the titles and abstracts identified from the literature search. We discarded studies not meeting the inclusion criteria. For studies appearing to meet the inclusion criteria, or where there was insufficient information to make a clear decision, we obtained full reports and two review authors independently assessed them to establish whether the studies met the inclusion criteria. We resolved disagreements by discussion, with a third review author consulted if resolution was not possible. We entered studies rejected at this or subsequent stages in Characteristics of excluded studies tables and recorded the main reason for exclusion.
Data extraction and management
Data extraction was done independently and in duplicate into data extraction forms. We extracted relevant data from full‐text articles that met the inclusion criteria. If reported, information was collected on.
Trial setting: country and number of trial centres.
Methods: study design, total study duration and date.
Participant characteristics: age, sociodemographics, ethnicity, diagnostic criteria and total number.
Eligibility criteria: inclusion and exclusion criteria.
Intervention and comparator.
Outcomes: outcome definition, unit of measurement and time of collection.
Results: number of participants allocated to each group, missing participants, sample size.
Funding source.
We compared completed data extraction forms to check for discrepancies and made clarifications by referring to the relevant study paper. After checking data for accuracy, we entered them into the Characteristics of included studies tables.
Assessment of risk of bias in included studies
Two review authors independently assessed all studies meeting the inclusion criteria for their risk of bias using criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). The following domains were assessed.
Sequence generation (selection bias).
Allocation concealment (selection bias).
Blinding of participants and personnel (performance bias).
Blinding of outcome assessment (detection bias).
Incomplete outcome data (attrition bias).
Selective reporting (reporting bias).
Other bias.
We judged the studies to be at either low, high or unclear risk of bias for each domain assessed, based on the guidance in Higgins 2011. The different judgements on risk of bias were interpreted as follows.
Low risk of bias: plausible bias unlikely to seriously alter the results if all domains were at low risk of bias.
Unclear risk of bias: plausible bias that raises some doubt about the results if one or more domains were at unclear risk of bias.
High risk of bias: plausible bias that seriously weakens confidence in the results if one or more domains were at high risk of bias.
Measures of treatment effect
For dichotomous outcomes, we expressed the treatment effect as risk ratios (RR) with corresponding 95% confidence intervals (CI). For continuous outcomes, we expressed the treatment effect as mean difference (MD) with 95% CI. However, if the studies assessed the same continuous outcome in different ways, we planned to estimate the treatment effect using the standardised mean difference (SMD). Time to birth was expressed as hazard ratio (HR) with corresponding 95% CI.
Unit of analysis issues
The participant was the unit of analysis. For studies comparing more than two intervention groups, we made multiple pair‐wise comparisons between all possible pairs of intervention groups.
Dealing with missing data
We contacted authors where there was missing data or studies had not reported data in sufficient detail. We attempted to derive the data using relevant statistical tools and calculators. Missing standard deviations would be estimated by calculating a correlation coefficient from a study reported in considerable detail using methods outlined in the Cochrane Handbook for Systematic Reviews of Interventions Section 16.1.3.2 (Higgins 2011). Where we were unable to get missing data from authors, we presented the study results only as a narrative summary.
Assessment of heterogeneity
We tested for heterogeneity using a Chi2 test and P < 0.1 gave an indication of the presence of heterogeneity. Inconsistency was quantified and represented by the I2 statistic. The thresholds were interpreted as follows:
0% to 40%: might not be important;
30% to 60%: may represent moderate heterogeneity;
50% to 90%; may represent substantial heterogeneity;
75% to 100%: considerable heterogeneity.
Where heterogeneity was detected, we investigated possible causes and addressed them using methods described in Higgins 2011.
Assessment of reporting biases
Most reporting biases were avoided by not restricting the literature search to published literature or by language and date. We investigated publication bias for the preterm birth < 37 weeks outcome using a funnel plot. The magnitude of publication bias was to be determined by visual inspection of the asymmetry of the funnel plot. In addition, we were to test funnel plot asymmetry by performing a linear regression of intervention effect estimate against its standard error, weighted by the inverse of the variance of the intervention effect estimate (Egger 1997).
Data synthesis
We carried out a meta‐analysis where there was sufficient number of studies that assessed similar populations, interventions and outcomes. Study data were synthesised using the random‐effects model if there were more than three studies in the meta‐analysis; otherwise we used the fixed‐effect model. The random‐effects model gives wider confidence intervals for the intervention effects, resulting in a more conservative estimate of effect. We combined effect estimates of studies we considered appropriate for inclusion in the meta‐analysis. We pooled RRs for dichotomous outcomes and MDs for continuous outcomes. The primary analyses were limited to the prespecified outcomes. Studies reporting mean birth weight and gestational age were not pooled. The skewness of the data due to the rarity of the events precluded our pooling the data in a meta‐analysis. We presented study data not included in the meta‐analyses in additional tables. We presented a narrative summary of the included studies where we were unable to carry out a meta‐analysis.
Subgroup analysis and investigation of heterogeneity
We planned to undertake subgroup analyses of potential effect modifiers to investigate their influence on the effect size of the intervention if there were 10 studies or more. We identified several potential modifiers of effect: the severity of periodontal disease; who gave the treatment (periodontist, dental hygienist or general dental practitioner); the number of treatment sessions given; the gestational age at which the treatment was started; and the following prognostic factors: maternal age, smokers versus nonsmokers, and socioeconomic class. However, we were unable to undertake these analyses due to the insufficient number of studies.
Sensitivity analysis
We were to undertake a sensitivity analysis if we had a sufficient number of studies, to assess whether the findings of the review were robust to the decisions made during the review process. In particular, we planned to exclude studies at high or unclear risk of bias from analysis, as well as those with estimated standard deviations, to assess whether this affected the findings of the review.
Presentation of main results
We presented the main results in a 'Summary of findings' table. The main comparison (periodontal treatment versus no treatment) and primary outcomes were exported to GRADEprofiler software (GRADEpro GDT 2014) for quality assessment. Based on risk of bias, inconsistency, imprecision, indirectness and publication bias, we rated the quality of the evidence for each outcome as high, moderate, low or very low. These ratings have been defined as follows.
High: further research is very unlikely to change our confidence in the estimate of effect.
Moderate: further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate.
Low: further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate.
Very low: any estimate of effect is very uncertain.
Results
Description of studies
From the literature search, 786 records were retrieved after deduplication and 1 unpublished study was obtained from a study author. Titles and abstracts of these 787 records were screened by two members of the review team independently. After the screening, 752 records were discarded and we attempted to obtain 35 full‐text articles for further scrutiny. 15 studies (19 published articles) were included and nine studies (13 published articles) were excluded for different reasons. There are two trials awaiting classification and one ongoing trial. Data extraction of the 15 studies was done and all 15 studies were included in the meta‐analysis (Figure 1).
1.
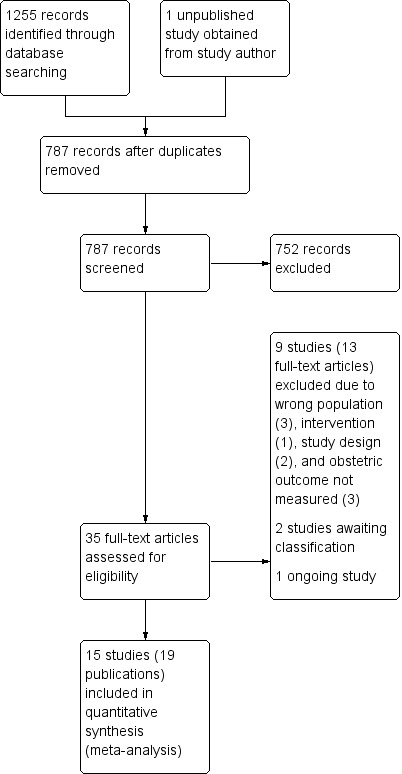
Study flow diagram.
Included studies
Fifteen studies (Farrell 2003; Herrera 2009; Jeffcoat 2003; López 2002; López 2005; Macones 2010; Michalowicz 2006; Newnham 2009; Offenbacher 2006; Offenbacher 2009; Oliveira 2011; Pirie 2013; Radnai 2009; Sadatmansouri 2006; Tarannum 2007) were included in the review. The details of these studies are reported in the Characteristics of included studies section.
Participants
The studies included 7161 pregnant women recruited from prenatal care facilities who had either periodontitis (mostly mild) or gingivitis. Participants were mostly in their first or second trimester except in two studies (Herrera 2009; Radnai 2009) where some women in the third trimester of their pregnancy were included. Mean gestational age (± standard deviation) of the participants at entry was between 14.0 ± 1.5 weeks to 39.6 ± 1.2 weeks in six studies (López 2002; Michalowicz 2006; Newnham 2009; Offenbacher 2009; Pirie 2013; Radnai 2009). Seven studies reported gestational age ranging from 9 to 34 weeks (Farrell 2003; Herrera 2009; Jeffcoat 2003; Macones 2010; Oliveira 2011; Sadatmansouri 2006; Tarannum 2007). Two studies reportedly included women at ≤ 22 weeks (López 2005) and < 22 weeks (Offenbacher 2006) gestation. Mean age of participants was reported in all but one study (Farrell 2003) and ranged between 22.2 ± 4.3 and 30.5 ± 5.5 years.
Nine studies reported baseline data on the proportion of participants with previous history of various adverse obstetric outcomes and it ranged from 3% to 55% (Jeffcoat 2003; López 2002; López 2005; Macones 2010; Michalowicz 2006; Newnham 2009; Offenbacher 2006; Offenbacher 2009; Pirie 2013). Adverse obstetric outcomes previously experienced by participants in these nine studies were preterm birth, preterm low birth weight, spontaneous abortion and stillbirth. However, there is no indication as to whether the estimates also account for participants who had experienced multiple adverse obstetric outcomes. In one study (Herrera 2009) all the participants had mild pre‐eclampsia at recruitment stage. None of the studies excluded participants on the basis of previous history of adverse obstetric outcomes except Radnai 2009. Offenbacher 2009 excluded women with "any obstetric finding that precluded enrolment in the study", however, these obstetric findings referred to were not clearly stated and some participants had a history of preterm delivery.
Severity of periodontitis ranged from moderate to severe and there was variation in definition of periodontitis across the studies. Periodontal disease was defined as four or more teeth with one or more sites with probing depth of 4 mm or more and clinical attachment level as 3 mm or more in three studies (López 2002; Oliveira 2011; Sadatmansouri 2006). In two studies (Jeffcoat 2003; Offenbacher 2009), periodontal disease was defined as three or more sites with attachment level of 3 mm or more. The other 10 studies had no common definition for periodontal disease (Additional Table 3).
2. Case definition for periodontal disease.
| Study ID | Case definition |
| Jeffcoat 2003; Offenbacher 2009 | ≥ 3 sites with CAL ≥ 3 mm |
| López 2002; Oliveira 2011; Sadatmansouri 2006 | ≥ 4 teeth with ≥ 1 sites with PD ≥ 4 mm and with CAL ≥ 3 mm |
| Farrell 2003 | ≥ 6 sites with ≥ 5 mm probing depth and ≥ 3 sites with ≥ 3 mm loss of periodontal attachment |
| Herrera 2009 | AAP criteria ‐ PPD up to 6 mm with CAL up to 4 mm |
| López 2005 | Gingival inflammation with over ≥ 25% of sites with BOP and no sites with CAL > 2 mm |
| Macones 2010 | Attachment loss ≥ 3 mm on ≥ 3 teeth |
| Michalowicz 2006 | ≥ 4 teeth with ≥ 1 sites with PD ≥ 4 mm and with CAL ≥ 2 mm |
| Newnham 2009 | PPD ≥ 4 mm at ≥ 12 probing sites in fully erupted teeth |
| Offenbacher 2006 | ≥ 2 sites measuring ≥ 5 mm probing depths plus periodontal attachment loss of 1‐2 mm at ≥ 1 sites with probing depths ≥ 5 mm |
| Pirie 2013 | ≥ 4 sites with PD ≥ 4 mm and ≥ 4 sites with CAL ≥ 4 mm |
| Radnai 2009 | Chronic: ≥ 4 mm probing depth, at least 1 site, and BOP for ≥ 50% of teeth |
| Tarannum 2007 | ≥ 2 mm attachment loss at ≥ 50% of examined sites |
AAP = American Academy of Periodontology; BOP = bleeding on probing; CAL = clinical attachment level; PD = pocket depth; PPD = periodontal pocket depth.
Study design and setting
All the included studies were published between 2003 and 2013. The studies were single‐centre (Farrell 2003; Herrera 2009; Jeffcoat 2003; López 2002; López 2005; Pirie 2013; Radnai 2009; Sadatmansouri 2006; Tarannum 2007) and multicentre (Macones 2010; Michalowicz 2006; Newnham 2009; Offenbacher 2006; Offenbacher 2009; Oliveira 2011) randomised controlled trials (RCTs) conducted in either university hospitals, public hospitals, public health clinics, antenatal clinics, maternity hospitals or a combination of university and antenatal clinics (for the multicentre trials).
Thirty‐three per cent of the studies were conducted in North America (Jeffcoat 2003; Macones 2010; Michalowicz 2006; Offenbacher 2006; Offenbacher 2009), 27% in South America (Herrera 2009; López 2002; López 2005; Oliveira 2011), 13% in Asia (Sadatmansouri 2006; Tarannum 2007), 20% in Europe (Farrell 2003; Pirie 2013; Radnai 2009), and 7% in Australia (Newnham 2009).
The source of funding was not stated in half of the studies (Farrell 2003; Herrera 2009; Jeffcoat 2003; López 2005; Macones 2010; Newnham 2009; Sadatmansouri 2006; Tarannum 2007) while the other half were funded by research institutes (Michalowicz 2006; Offenbacher 2009), the government (Pirie 2013), scientific research fund (López 2002), a university (Oliveira 2011), "institutional support" (Radnai 2009), and a manufacturer of oral healthcare products (Offenbacher 2006).
Interventions
The intervention arm in all the studies included a combination of multiple subcomponents (Additional Table 4). Apart from two studies (López 2002; Sadatmansouri 2006), none of the studies had common intervention subcomponents. The studies were split into two comparisons. Eleven studies compared periodontal treatment provided during pregnancy with no treatment and four studies did a head‐to‐head comparison of different periodontal treatments.
3. Study interventions.
| Study | Number of visits | When | Intervention | Comparator | |
| Periodontal treatment versus no treatment | |||||
| Farrell 2003 | 5 visits | 12 weeks 30 weeks Then monthly until birth | Plaque assessment Oral hygiene instruction Generalised scaling Hand instrumentation Ultrasonic instruments Irrigation with CHX before treatment Maintenance |
None | |
| Herrera 2009 | 1 session lasting 1‐2 hours | Supragingival and subgingival cleaning Oral hygiene instruction Plaque removal SRP (if necessary) with subgingival irrigation | None | ||
| López 2002 | Maintenance therapy every 2‐3 weeks till delivery CHX rinse once daily till delivery | Plaque control instruction SRP CHX rinse | None | ||
| López 2005 | Maintenance therapy every 2‐3 weeks till delivery CHX rinse once daily till delivery | Plaque control instruction Supragingival and subgingival scaling and crown polishing | None | ||
| Michalowicz 2006 | Up to 4 visits | SRP Oral hygiene instruction Tooth polishing Removal of dental plaque and calculus | None | ||
| Newnham 2009 | 3 treatments over 3 weeks | 20 weeks 21 weeks 22 weeks | Nonsurgical debridement of subgingival and supragingival plaque Removal of calculus Root planing Adjustment of overhanging restorations Oral hygiene instruction | None | |
| Offenbacher 2009 | Up to 4 sessions (mean 1.3 ± 0.4) | Supragingival and subgingival SRP Full‐mouth polishing Oral hygiene instruction | None | ||
| Oliveira 2011 | Maintenance therapy every 3 weeks till delivery | Dental prophylaxis Tooth cleaning kit + oral hygiene instruction Mechanical debridement (if necessary) | Tooth cleaning kit | ||
| Radnai 2009 | Not stated | 32 weeks | Supragingival and subgingival scaling and polishing Oral hygiene instruction | None | |
| Sadatmansouri 2006 | Not reported | 28 weeks | SRP Oral hygiene instruction CHX rinse | None | |
| Tarannum 2007 | 4‐5 sessions with a 1‐week interval between each appointment | Unclear | SRP Plaque control instruction (CHX rinse) | Plaque control instruction (toothbrushing) | |
| Periodontal treatment versus alternative periodontal treatment | |||||
| Jeffcoat 2003 | Antibiotics 3 times daily for 1 week | SRP Placebo capsule | SRP Metronidazole | Dental prophylaxis Placebo capsule | |
| Macones 2010 | Not stated | SRP | Superficial tooth cleaning | ||
| Offenbacher 2006 | 4‐6 weeks follow‐up visit | SRP Oral hygiene instruction Power toothbrush | Supragingival debridement Manual toothbrush | ||
| Pirie 2013 | Performed over 2 1‐hour sessions | Completed by end of 24 weeks | Supragingival and subgingival SRP Polishing Oral hygiene instruction | Supragingival cleaning Oral hygiene instruction | |
CHX = chlorhexidine; SRP = scaling and root planing.
Periodontal treatment (any combination of mechanical treatment) versus no treatment (Farrell 2003; Herrera 2009; López 2002; López 2005; Michalowicz 2006; Newnham 2009; Offenbacher 2009; Oliveira 2011; Radnai 2009; Sadatmansouri 2006; Tarannum 2007).
Periodontal treatment versus alternative periodontal treatment (Jeffcoat 2003; Macones 2010; Offenbacher 2006; Pirie 2013).
For the head‐to‐head comparison, the least intensive or complex intervention was regarded as the control group or 'alternative periodontal treatment'. Participants received between 1 to 5 periodontal treatment sessions. All studies except Pirie 2013 indicated that maintenance therapy was provided till delivery and this involved chlorhexidine rinse, oral hygiene instructions or dental prophylaxis. The studies rarely provided sufficient details on the number of sessions, time of treatment and maintenance and these intervention regimens varied from study to study. Periodontal treatment was administered by periodontists in three studies (Herrera 2009; Radnai 2009; Tarannum 2007) and hygienists/therapists in two studies (Macones 2010; Offenbacher 2009). Four studies referred to the professionals as 'clinicians' (Jeffcoat 2003), 'hygienists or periodontists' (Newnham 2009), 'trained personnel' (Oliveira 2011), and in one study participants were examined by periodontists, but it is not clear whether periodontists also administered the intervention (Offenbacher 2006). Six studies made no reference to the expertise of the professional who administered the intervention (Farrell 2003; López 2002; López 2005; Michalowicz 2006; Pirie 2013; Sadatmansouri 2006).
All the studies were two‐arm trials involving an intervention and a control arm except for a three‐arm trial (Jeffcoat 2003) which compared SRP (scaling and root planing) + placebo versus SRP + antimicrobial versus alternative mechanical treatment + placebo. The comparisons were divided as follows:
SRP + placebo versus alternative mechanical treatment + placebo;
SRP + antimicrobial versus alternative mechanical treatment + placebo;
SRP + antimicrobial versus SRP + placebo.
Outcomes
All the studies had to report at least one obstetric outcome to be included in the review. Ten studies (Herrera 2009; López 2002; López 2005; Michalowicz 2006; Newnham 2009; Offenbacher 2006; Offenbacher 2009; Oliveira 2011; Pirie 2013; Sadatmansouri 2006) also reported periodontal outcomes. Obstetric outcomes not specified in our protocol were collated and reported in Additional Table 3. Outcomes of interest reported in the studies are as follows.
Gestational age ‐ preterm birth < 37 weeks, < 35 weeks and < 32 weeks were reported. All 15 studies reported preterm birth < 37 weeks. Preterm birth < 35 weeks was reported in four studies (Jeffcoat 2003; Macones 2010; Michalowicz 2006; Offenbacher 2009) and preterm birth < 32 weeks was reported in three studies (Farrell 2003; Michalowicz 2006; Offenbacher 2009). Gestational age was reported as time‐to‐event data in two studies (Michalowicz 2006; Newnham 2009) and mean gestational age (weeks) was reported in six studies (López 2002; López 2005; Newnham 2009; Radnai 2009; Sadatmansouri 2006; Tarannum 2007).
Birth weight: low birth weight < 2500 g was reported in eight studies (López 2002; Macones 2010; Michalowicz 2006; Offenbacher 2009; Oliveira 2011; Radnai 2009; Sadatmansouri 2006; Tarannum 2007) and low birth weight < 1500 g was reported in three studies (Macones 2010; Michalowicz 2006; Offenbacher 2009). Mean birth weight was reported in eight studies (López 2002; López 2005; Michalowicz 2006; Newnham 2009; Offenbacher 2009; Radnai 2009; Sadatmansouri 2006; Tarannum 2007).
Small for gestational age (10th percentile) was reported in three studies (Michalowicz 2006; Newnham 2009; Offenbacher 2009).
Perinatal mortality (including fetal and neonatal deaths up to the first 28 days after birth) was reported in nine studies. Fetal deaths were reported in six studies (López 2002; López 2005; Macones 2010; Oliveira 2011; Pirie 2013; Tarannum 2007), however, the gestational age of pregnancy loss was rarely reported. Neonatal death was reported in three studies (Michalowicz 2006; Newnham 2009; Offenbacher 2009).
Maternal mortality was reported in one study (Michalowicz 2006) and data for three other studies (López 2002; López 2005; Radnai 2009) were provided through personal communication with study authors.
Pre‐eclampsia was reported in three studies (López 2002; Michalowicz 2006; Offenbacher 2009), however, one additional study (Herrera 2009) which recruited women who already had mild pre‐eclampsia reported a "progression from mild to severe pre‐eclampsia" as one of its outcomes.
Adverse effects of therapy: there were no adverse effects in López 2002; López 2005; Newnham 2009; Radnai 2009 (personal communication).
Periodontal outcomes: the periodontal outcomes probing depth, clinical attachment level, bleeding on probing, gingival index, and plaque index were reported in 10 studies (Herrera 2009; López 2002; López 2005; Michalowicz 2006; Newnham 2009; Offenbacher 2006; Offenbacher 2009; Oliveira 2011; Pirie 2013; Sadatmansouri 2006) and reported properly for inclusion in a meta‐analysis in seven of the 10 studies.
Excluded studies
We excluded nine trials and stated the reasons for exclusion (Characteristics of excluded studies table). Studies were excluded due to non‐randomisation (Gazolla 2007; Geisinger 2014), failure to report any obstetric outcome (Pack 1980; Thomson 1982), and the inclusion of participants regardless of periodontal status (Moreira 2014; Weidlich 2013). Two studies seem to have assessed the same study populations as those assessed in some included studies and lacked additional information to supplement the primary studies (Jeffcoat 2011; Penova‐Veselinovic 2015). One study assessed a single intervention which is not normally used as standalone treatment for periodontal disease (Jiang 2016).
Risk of bias in included studies
The risk of bias assessment within the included studies and across the domains is reported in the Characteristics of included studies table and summarised in Figure 2; Figure 3. All included studies were at high risk of bias.
2.
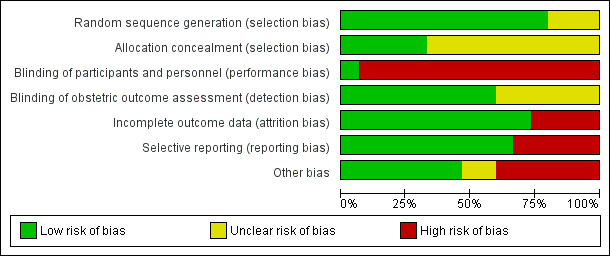
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
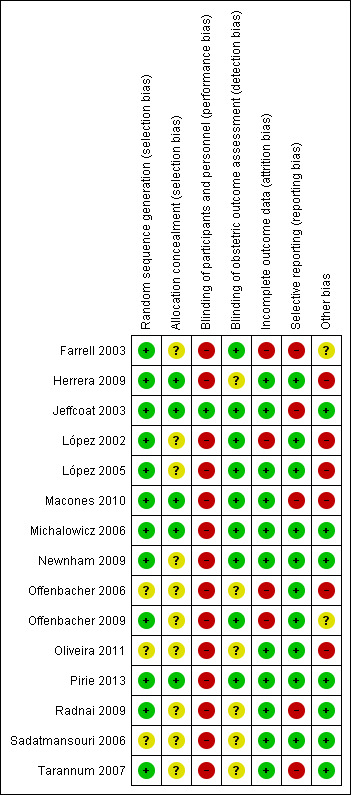
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
The studies were assessed for selection bias based on the adequacy of randomisation as well as allocation concealment. Ten studies were all at unclear risk of bias and five studies were at low risk of bias for this domain (Herrera 2009; Jeffcoat 2003; Macones 2010; Michalowicz 2006; Pirie 2013).
The studies at unclear risk of bias did not provide sufficient information on allocation concealment (Farrell 2003; López 2002; López 2005; Newnham 2009; Offenbacher 2006; Offenbacher 2009; Oliveira 2011; Radnai 2009; Sadatmansouri 2006; Tarannum 2007) or randomisation (Offenbacher 2006; Oliveira 2011; Sadatmansouri 2006) or both.
For the five studies at low risk of selection bias, randomisation code was centrally generated by a research pharmacist who also provided a double packet with coding information for each participant. Allocation was concealed in the second packet and was to be revealed only in the event of an emergency (Jeffcoat 2003). Michalowicz 2006 used permuted randomised blocks made available by telephone call to the co‐ordinating centre and for Macones 2010 permuted block randomisation was accomplished centrally. Herrera 2009 reportedly "randomised by block". This information was not sufficient but considered adequate given that allocation concealment was achieved using "...closed envelopes prepared by professionals external to the research group." One study (Pirie 2013) avoided selection bias by using computer generated allocations which were concealed by labelled opaque sealed envelopes.
Blinding
Performance bias
There was only one study (Jeffcoat 2003) at low risk of performance bias. It was placebo‐controlled and code breaking seems to have occurred only at the end of the study. All the other studies did not state whether blinding was carried out, however, they were considered to be at high risk of performance bias as the mechanical nature of the interventions made the blinding of the participants impossible.
Detection bias
Nine studies (Farrell 2003; Jeffcoat 2003; López 2002; López 2005; Macones 2010; Michalowicz 2006; Newnham 2009; Offenbacher 2009; Pirie 2013) clearly indicated that obstetric outcomes were assessed blindly or assessed independent of the caregiver. Given that obstetric outcomes are likely to have been assessed independent of the caregivers (dental professional), we had considered marking all studies 'low' for detection bias. However, there were concerns regarding whether independent assessment was sufficient to prevent detection bias, which led to marking the other six studies (Herrera 2009; Offenbacher 2006; Oliveira 2011; Radnai 2009; Sadatmansouri 2006; Tarannum 2007) with insufficient information 'unclear' .
We were not concerned as to whether periodontal outcomes were assessed blindly or not as periodontal outcomes are not the primary focus of the review.
Incomplete outcome data
Most of the studies had excluded participants who had experienced certain adverse pregnancy outcomes of relevance to this review such as eligible or indicated preterm birth (preterm births foreseen to occur due to complications), stillbirth and spontaneous abortion (pregnancy loss). These data were collected and added to the results of this review and the denominators were adjusted accordingly.
Eleven studies judged to be at low risk of bias either reported that there was no attrition (Herrera 2009; Pirie 2013; Sadatmansouri 2006) or had similarly low attrition rates across groups (Jeffcoat 2003; López 2005; Macones 2010; Michalowicz 2006; Newnham 2009; Oliveira 2011; Radnai 2009; Tarannum 2007).
Four studies were at high risk of bias due to high attrition rates of > 20% (Farrell 2003; Offenbacher 2006; Offenbacher 2009) and imbalance in attrition rates across groups (López 2002).
Selective reporting
Five studies were judged to be at high risk of reporting bias due to failure to report periodontal outcomes (Farrell 2003; Jeffcoat 2003; Macones 2010; Radnai 2009) and poor reporting of periodontal outcome follow‐up data (Tarannum 2007).
Ten studies were at low risk of reporting bias (Herrera 2009; López 2002; López 2005; Michalowicz 2006; Newnham 2009; Offenbacher 2006; Offenbacher 2009; Oliveira 2011; Pirie 2013; Sadatmansouri 2006).
Other potential sources of bias
Seven studies (Jeffcoat 2003; Michalowicz 2006; Newnham 2009; Pirie 2013; Radnai 2009; Sadatmansouri 2006; Tarannum 2007) with no other apparent source of bias were marked 'low'. Two studies reported an imbalance in the number of participants allocated to the groups (Farrell 2003), number of nulliparous women and alcohol use among participants (Offenbacher 2009) and were both marked 'unclear' as there was lack of clarity on whether these impacted on the validity of the results. Six studies (Herrera 2009; López 2002; López 2005; Macones 2010; Offenbacher 2006; Oliveira 2011) were at high risk of other bias due to an imbalance in participant characteristics across groups.
Effects of interventions
See: Table 1
Comparison 1: periodontal treatment versus no treatment
See Table 1.
Primary outcome 1: gestational age/preterm birth
Preterm birth < 37 weeks was reported in all 11 studies which compared periodontal treatment with no treatment. Three studies additionally reported on preterm birth < 35 weeks and < 32 weeks (Analysis 1.1). There is no clear difference in preterm birth < 37 weeks (risk ratio (RR) 0.87, 95% confidence interval (CI) 0.70 to 1.10; participants = 5671; studies = 11; I2 = 66%) between periodontal treatment and no treatment. This is due to low‐quality evidence downgraded for risk of bias and inconsistency. It is uncertain whether periodontal treatment leads to a difference in preterm birth < 35 weeks (RR 1.19, 95% CI 0.81 to 1.76; participants = 2557; studies = 2; I2 = 0%) and < 32 weeks (RR 1.35, 95% CI 0.78 to 2.32; participants = 2755; studies = 3; I2 = 0%) because the quality of evidence is very low. The evidence was downgraded due to high risk of bias and very serious imprecision.
1.1. Analysis.
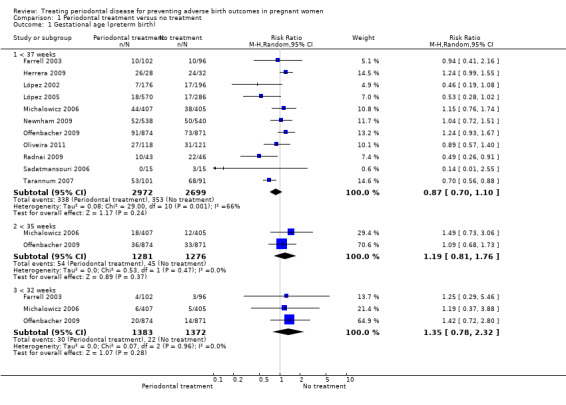
Comparison 1 Periodontal treatment versus no treatment, Outcome 1 Gestational age (preterm birth).
Some studies had excluded 'indicated preterm' births (preterm births foreseen to occur due to complications) from their analyses. We decided to include these data in the 'preterm birth' analysis of this review. Given that the studies had not specified the gestational age (in weeks) when these indicated preterm births had occurred, we included them in the < 37 weeks analyses only. Therefore the < 35 weeks and < 32 weeks preterm birth results could be underestimated.
Six studies analysing 2573 participants reported on mean gestational age in weeks and presented sufficient data for inclusion in a meta‐analysis. However, the data were not suitable for pooling in a meta‐analysis due to the skewed nature of the data. Mean difference ranged between ‐0.1 and 1.4 weeks (Additional Table 5). Two studies (Michalowicz 2006: hazard ratio 0.93, 95% CI 0.63 to 1.37; and Newnham 2009: hazard ratio 1.02, 95% CI 0.91 to 1.15) which reported gestational age as time‐to‐event data both show that there is probably no clear difference in gestational age at delivery between periodontal treatment and no treatment. The evidence was downgraded for serious imprecision due to wide confidence intervals.
4. Periodontal treatment versus no treatment ‐ mean gestational age and birth weight.
| Mean gestational age (weeks) | ||||||
| Periodontal treatment | No periodontal treatment | |||||
| Study ID | Mean | SD | Participants | Mean | SD | Participants |
| López 2002 | 39.6 | 1.2 | 163 | 39 | 2 | 188 |
| López 2005 | 39.26 | 1.5 | 560 | 38.9 | 1.7 | 283 |
| Newnham 2009 | 39.1 | 2.1 | 538 | 39.2 | 2.1 | 540 |
| Radnai 2009 | 37.5 | 1.7 | 41 | 36.1 | 2.8 | 42 |
| Sadatmansouri 2006 | 38.5 | 0.8 | 15 | 37.9 | 1.3 | 15 |
| Tarannum 2007 | 33.8 | 2.8 | 99 | 32.7 | 2.8 | 89 |
| Mean birth weight (grams) | ||||||
| Periodontal treatment | No periodontal treatment | |||||
| Study ID | Mean | SD | Participants | Mean | SD | Participants |
| López 2002 | 3501 | 429 | 163 | 3344 | 598 | 188 |
| López 2005 | 3426 | 477 | 560 | 3325 | 535 | 283 |
| Michalowicz 2006 | 3239 | 586 | 406 | 3258 | 575 | 403 |
| Newnham 2009 | 3370.6 | 613.4 | 538 | 3423.4 | 597.3 | 540 |
| Offenbacher 2009 | 3227 | 612 | 872 | 3241 | 590 | 866 |
| Radnai 2009 | 3079 | 592.3 | 41 | 2602.4 | 668.3 | 42 |
| Sadatmansouri 2006 | 3371 | 394.2 | 15 | 3059 | 389 | 15 |
| Tarannum 2007 | 2565.3 | 331.2 | 99 | 2459.6 | 380.7 | 89 |
SD = standard deviation.
Primary outcome 2: birth weight
Seven studies analysing 3470 participants reported on birth weight (low birth weight < 2500 g). Of the seven studies reporting on low birth weight < 2500 g, two studies (n = 2550) also reported on low birth weight < 1500 g. Periodontal treatment may reduce the incidence of low birth weight < 2500 g (RR 0.67, 95% CI 0.48 to 0.95; I2 = 59%; Analysis 1.2). We downgraded the evidence from high to low as a result of high risk of bias and serious inconsistency. It is uncertain whether periodontal treatment leads to a difference in low birth weight < 1500 g (RR 0.80, 95% CI 0.38 to 1.70; I2 = 45%; Analysis 1.2) compared to no treatment. The evidence was downgraded to very low as a result of high risk of bias in the studies and very serious imprecision of the results.
1.2. Analysis.
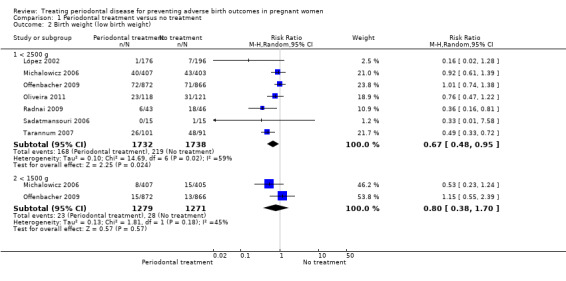
Comparison 1 Periodontal treatment versus no treatment, Outcome 2 Birth weight (low birth weight).
Eight studies analysing 5120 participants reported on birth weight (grams). However, data were not suitable for pooling in a meta‐analysis due to the skewed nature of the data. Mean difference ranged between ‐52.8 and 476.6 grams (Additional Table 5).
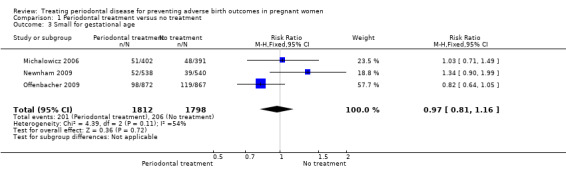
Primary outcome 3: small for gestational age
Three studies analysing 3610 participants reported outcome data on small for gestational age. Periodontal treatment may lead to no clear difference in births of babies which are small for gestational age when compared with no treatment (RR 0.97, 95% CI 0.81 to 1.16; Analysis 1.3). We used the fixed‐effect model and moderate heterogeneity was evident (Chi2 = 4.39, degrees of freedom (df) = 2 (P = 0.11); I2 = 54%). Due to risk of bias and serious inconsistency, we downgraded the evidence to low quality.
1.3. Analysis.
Comparison 1 Periodontal treatment versus no treatment, Outcome 3 Small for gestational age.
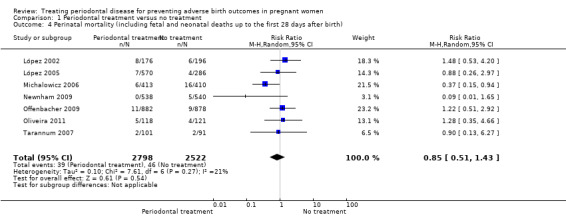
Primary outcome 4: perinatal mortality (including fetal and neonatal deaths up to the first 28 days after birth)
Fetal (spontaneous abortions and stillbirths) and neonatal deaths were reported in seven studies (n = 5320 participants); only two studies reported the exact gestational age at which mortality occurred (Michalowicz 2006; Newnham 2009). It is uncertain whether periodontal treatment increases or decreases perinatal mortality (RR 0.85, 95% CI 0.51 to 1.43; I2 = 21%; Analysis 1.4) because the quality of the evidence is very low. The evidence was downgraded for very serious limitation and very serious imprecision.
1.4. Analysis.
Comparison 1 Periodontal treatment versus no treatment, Outcome 4 Perinatal mortality (including fetal and neonatal deaths up to the first 28 days after birth).
Primary outcome 5: maternal mortality
Four studies (López 2002; López 2005; Michalowicz 2006; Radnai 2009) reported 0% maternal mortality rate.
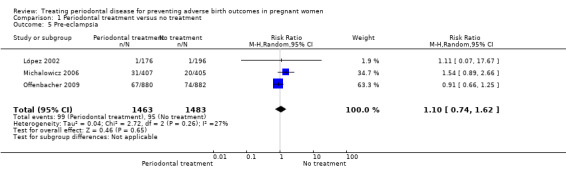
Primary outcome 6: pre‐eclampsia
Three studies analysing 2946 participants reported on pre‐eclampsia. It is uncertain whether periodontal treatment results in a difference in pre‐eclampsia when compared to no treatment (RR 1.10, 95% CI 0.74 to 1.62) because the quality of evidence is very low. We used the random‐effects model and heterogeneity was assessed as not important (Chi2 = 2.72, df = 2 (P = 0.26); I2 = 27%; Analysis 1.5). The evidence was downgraded due to high risk of bias and very serious imprecision. An additional study which evaluated 60 participants that had mild pre‐eclampsia reported on progression to severe pre‐eclampsia. Due to the quality of evidence (very low), it is uncertain whether periodontal treatment results in a difference in severe pre‐eclampsia when compared to no treatment (RR 1.21, 95% CI 0.77 to 1.92; very low quality ‐ downgraded for high risk of bias and very serious imprecision).
1.5. Analysis.
Comparison 1 Periodontal treatment versus no treatment, Outcome 5 Pre‐eclampsia.
Primary outcome 7: adverse effects of therapy
There were no adverse effects (0%) in four studies (López 2002; López 2005; Newnham 2009; Radnai 2009).
Secondary outcomes
Periodontal outcomes reported in the studies were probing depth, bleeding on probing, plaque index, and clinical attachment level. All the studies reported baseline and final scores except Offenbacher 2009 which reported mean change score. This study was included in the meta‐analysis as a subgroup. All four periodontal indices showed a reduction in favour of periodontal treatment. However due to considerable heterogeneity (91% to 100%), the results were not meta‐analysed (Analysis 1.6; Analysis 1.7; Analysis 1.8; Analysis 1.9).
1.6. Analysis.
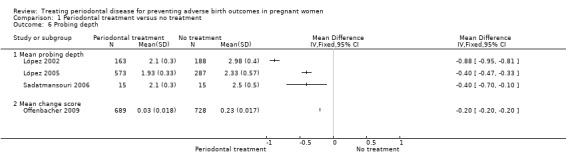
Comparison 1 Periodontal treatment versus no treatment, Outcome 6 Probing depth.
1.7. Analysis.
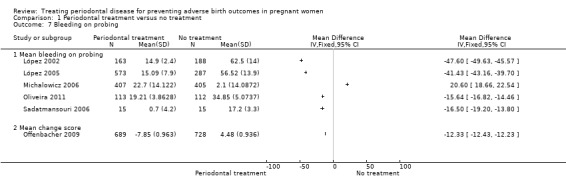
Comparison 1 Periodontal treatment versus no treatment, Outcome 7 Bleeding on probing.
1.8. Analysis.
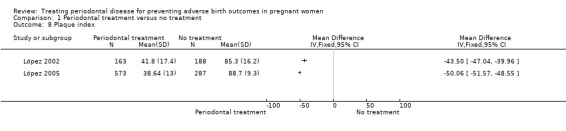
Comparison 1 Periodontal treatment versus no treatment, Outcome 8 Plaque index.
1.9. Analysis.
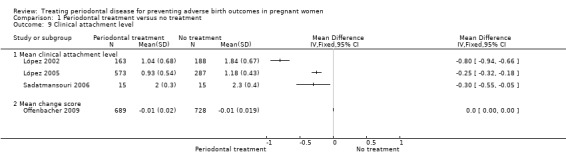
Comparison 1 Periodontal treatment versus no treatment, Outcome 9 Clinical attachment level.
Periodontal indices were also reported in seven studies (López 2002; López 2005; Michalowicz 2006; Newnham 2009; Offenbacher 2009; Oliveira 2011; Sadatmansouri 2006) in various measures that could not be incorporated into a meta‐analysis. Periodontal measures were improved in women who underwent periodontal treatment compared to no treatment in all the studies and all the outcome measures reported. These results are reported in detail in additional tables (Additional Table 6).
5. Additional periodontal outcome measures.
| Study ID | Outcome | Periodontal treatment | Number of participants | Alternative periodontal/no treatment | Number of participants | P value |
| López 2002 | % sites with PD 4‐6 mm (mean ± SD) | 2.9 ± 3.9 | 163 | 27 ± 14 | 188 | 0.001 |
| % sites with CAL ≥ 3 mm (mean ± SD) | 6.1 ± 7.8 | 163 | 25.4 ± 17.2 | 188 | 0.001 | |
| López 2005 | % sites with PD > 4 mm (mean ± SD) | 1.8 ± 2.9 | 573 | 14.5 ± 2.8 | 287 | 0.0001 |
| Michalowicz 2006 | Change PD at sites initially 4‐6 mm (mean ± SE) | 0.38 ± 0.02 | 405 | 0.88 ± 0.02 | 407 | < 0.001 |
| Change PD at sites initially ≥ 7 mm (mean ± SE) | 1.07 ± 0.14 | 405 | 1.84 ± 0.14 | 407 | < 0.001 | |
| Change % sites with CAL ≥ 2 mm (mean ± SD) | 0.84 ± 0.85 | 405 | 9.72 ± 0.87 | 407 | < 0.001 | |
| Newnham 2009 | % sites with PD > 4 mm (median (IQR)) | 3.3 (1.2‐7) | 354 | Not reported | Not reported | < 0.001 |
| % sites BOP (median (IQR)) | 28.7 (17.9‐42.5) | 354 | Not reported | Not reported | < 0.001 | |
| Offenbacher 2006 | Extent of PD ≥ 4 mm (mean ± SE) | 13.7 ± 1.5 | 25 | 10.5 ± 1.2 | 28 | < 0.0001 |
| PI ≥ 1 (mean ± SE) | 67.8 ± 5.6 | 25 | 87 ± 5.3 | 28 | 0.02 | |
| Offenbacher 2009 | Change PD at sites initially ≥ 4 mm (mean ± SD) | 1.47 ± 0.574 | 689 | 7.81 ± 0.559 | 728 | Not reported |
| Oliveira 2011 | Sites with PD ≥ 4 mm (% (95% CI)) | 1.19 (1‐1.39) | 113 | 6.36 (5.92‐6.81) | 112 | < 0.0001 |
| Sites with CAL ≥ 3 mm (% (95% CI)) | 5.72 (5.3‐6.14) | 113 | 6.58 (6.13‐7.03) | 112 | 0.0069 | |
| Pirie 2013 | Number of sites PD ≥ 4 mm (median (IQR)) | 10 (6‐22) | 45 | Not reported | 45 | Not reported |
| Number of sites PD ≥ 5 mm (median (IQR)) | 1 (0‐4) | 45 | Not reported | 45 | Not reported | |
| Number of sites AL ≥ 4 mm (median (IQR)) | 10 (5‐19) | 45 | Not reported | 45 | Not reported | |
| Number of sites AL ≥ 5 mm (median (IQR)) | 0 (0‐2) | 45 | Not reported | 45 | Not reported | |
| Number of sites plaque present (median (IQR)) | 57 (40‐82.5) | 45 | Not reported | 45 | Not reported | |
| Number of sites BOP present (median (IQR)) | 78 (63.5‐90) | 45 | Not reported | 45 | Not reported | |
| % of sites plaque present | 37 (28‐54.8) | 45 | Not reported | 45 | Not reported | |
| % of sites BOP present | 50 (42.9‐54.1) | 45 | Not reported | 45 | Not reported | |
| % of sites PD ≥ 4 mm | 78 (63.5‐90) | 45 | Not reported | 45 | Not reported | |
| Sadatmansouri 2006 | % sites with PD 4 mm (mean ± SD) | 53.31 ± 18.5 | 15 | 68.6 ± 20.2 | 15 | 0.04 |
| % sites with CAL 3 mm (mean ± SD) | 41.4 ± 18.4 | 15 | 67.1 ± 15.6 | 15 | 0.000 |
AL = attachment loss; BOP = bleeding on probing; CAL = clinical attachment level; IQR = interquartile range; PD = probing depth; PPD = periodontal pocket depth; SD = standard deviation; SE = standard error.
Comparison 2: periodontal treatment versus alternative periodontal treatment
For gestational age (preterm birth < 37 weeks and preterm birth < 35 weeks), we had three subcomparisons: SRP (scaling and root planing) versus alternative mechanical treatment; SRP + antimicrobial versus SRP + placebo; and SRP + antimicrobial versus alternative mechanical treatment + placebo. We did not pool the data due to clinical heterogeneity.
Primary outcome 1: gestational age/preterm birth
Preterm births < 37 weeks and < 35 weeks were reported in four studies. For all three subcomparisons made, it is uncertain whether there is a difference between periodontal treatment and alternative periodontal treatment in preterm birth < 37 weeks (Analysis 2.1) except for SRP + antimicrobials which may increase preterm births < 37 weeks compared to SRP + placebo (RR 3.08, 95% CI 1.15 to 8.20; participants = 243; studies = 1). With the results there is very low certainty due to very serious imprecision and high risk of bias.
2.1. Analysis.
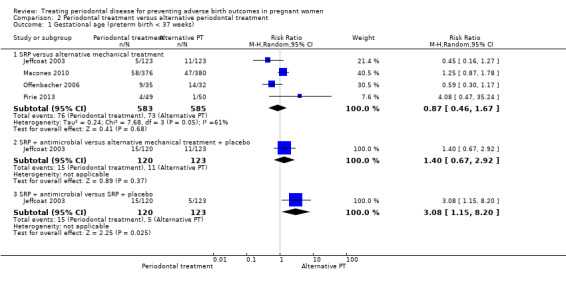
Comparison 2 Periodontal treatment versus alternative periodontal treatment, Outcome 1 Gestational age (preterm birth < 37 weeks).
SRP versus alternative mechanical treatment (RR 0.87, 95% CI 0.46 to 1.67; participants = 1168; studies = 4; I2 = 61%; very low quality).
SRP + antimicrobial versus alternative mechanical treatment + placebo (RR 1.40, 95% CI 0.67 to 2.92; participants = 243; studies = 1; very low quality).
None of the subcomparisons showed a difference in preterm births < 35 weeks (Analysis 2.2) and the quality of evidence was similarly very low in all cases.
2.2. Analysis.
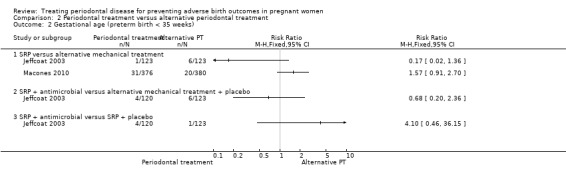
Comparison 2 Periodontal treatment versus alternative periodontal treatment, Outcome 2 Gestational age (preterm birth < 35 weeks).
SRP versus alternative mechanical treatment (not pooled due to considerable heterogeneity).
SRP + antimicrobial versus SRP + placebo.
SRP + antimicrobial versus alternative mechanical treatment + placebo.
Mean gestational age (weeks) was reported in only two studies which analysed 855 participants. Mean gestational age ranged between ‐0.6 and ‐0.2 weeks (Additional Table 7).
6. Periodontal treatment versus alternative periodontal treatment.
| Mean gestational age (weeks) | ||||||
| Periodontal treatment | Alternative periodontal treatment | |||||
| Study ID | Mean | SD | Participants | Mean | SD | Participants |
| Macones 2010 | 38.6 | 2.8 | 376 | 38.8 | 2.3 | 380 |
| Pirie 2013 | 39.4 | 2.3 | 49 | 40 | 2.5 | 50 |
| Mean birth weight (grams) | ||||||
| Macones 2010 | 3076.1 | Not reported | 376 | 3143.8 | Not reported | 380 |
| Pirie 2013 | 3510 | 650 | 49 | 3580 | 630 | 50 |
SD = standard deviation.
Primary outcome 2: birth weight
One study (Macones 2010) with 756 participants reported on low birth weight < 2500 g and < 1500 g. It is uncertain whether there is a difference in low birth weight < 2500 g (RR 1.39, 95% CI 0.92 to 2.09; participants = 756; I2 = 0%; Analysis 2.3) and low birth weight < 1500 g (RR 1.85, 95% CI 0.69 to 4.96; participants = 756; I2 = 0%; Analysis 2.3) between the periodontal treatments. In both cases we downgraded the evidence three levels to very low due to high risk of bias and very serious imprecision.
2.3. Analysis.
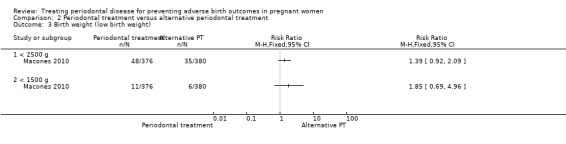
Comparison 2 Periodontal treatment versus alternative periodontal treatment, Outcome 3 Birth weight (low birth weight).
One study (Pirie 2013) analysing 99 participants reported on mean birth weight (kilograms). The study reported a mean difference in birth weight between groups of ‐0.07 kilograms. An additional study (Macones 2010) analysing 756 participants reported on mean birth weight (grams). The study reported a difference in mean birth weight of ‐67.7 grams (Additional Table 7).
Primary outcome 3: small for gestational age
Not reported.
Primary outcome 4: perinatal mortality (including fetal and neonatal deaths up to the first 28 days after birth)
This outcome was reported in two studies (n = 855 participants). It is uncertain whether there is a difference in perinatal mortality between the periodontal treatments (RR 1.06, 95% CI 0.60 to 1.85; I2 = 0%; Analysis 2.4). The evidence was downgraded to low as result of high risk of bias and very serious imprecision.
2.4. Analysis.
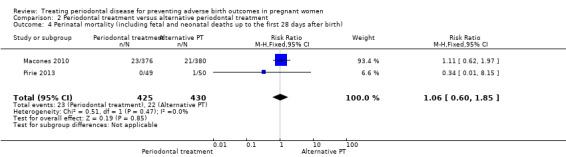
Comparison 2 Periodontal treatment versus alternative periodontal treatment, Outcome 4 Perinatal mortality (including fetal and neonatal deaths up to the first 28 days after birth).
Primary outcome 5: maternal mortality
Not reported.
Primary outcome 6: pre‐eclampsia
Not reported.
Primary outcome 7: adverse effects of therapy
Not reported.
Secondary outcomes
Periodontal indices reported were probing depth, clinical attachment level, bleeding on probing and gingival index. SRP may slightly improve probing depth, attachment loss, bleeding on probing and gingival index (Analysis 2.5; Analysis 2.6; Analysis 2.7; Analysis 2.8).
2.5. Analysis.
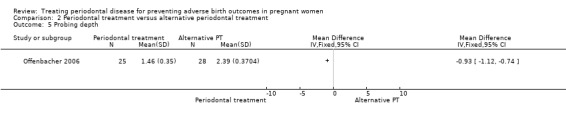
Comparison 2 Periodontal treatment versus alternative periodontal treatment, Outcome 5 Probing depth.
2.6. Analysis.
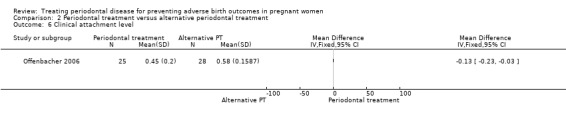
Comparison 2 Periodontal treatment versus alternative periodontal treatment, Outcome 6 Clinical attachment level.
2.7. Analysis.
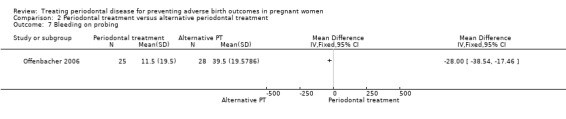
Comparison 2 Periodontal treatment versus alternative periodontal treatment, Outcome 7 Bleeding on probing.
2.8. Analysis.
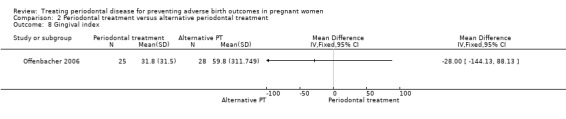
Comparison 2 Periodontal treatment versus alternative periodontal treatment, Outcome 8 Gingival index.
Probing depth: mean difference (MD) ‐0.93, 95% CI ‐1.12 to ‐0.74; participants = 53; studies = 1; I2 = 0%.
Clinical attachment level: MD ‐0.13, 95% CI ‐0.23 to ‐0.03; participants = 53; studies = 1; I2 = 0%.
Bleeding on probing: MD ‐28.00, 95% CI ‐38.54 to ‐17.46; participants = 53; studies = 1; I2 = 0%.
Gingival index: MD ‐28.00, 95% CI ‐144.13 to 88.13; participants = 53; studies = 1; I2 = 0%.
Periodontal indices were also reported in two studies (Offenbacher 2006; Pirie 2013) in various measures that could not be incorporated in the meta‐analysis. Offenbacher 2006 showed that SRP resulted in a reduction in plaque index and on the other hand an increase in extent of probing depth ≥ 4 mm (mean ± standard error) compared to the alternative periodontal treatment. Pirie 2013 made no comparison between the two groups rather it compared baseline and postintervention results in the SRP group which was not as important for this review. These results are reported in detail in additional tables (Additional Table 6).
Discussion
Summary of main results
The main results of the primary outcomes are summarised in Table 1. Fifteen randomised controlled trials provided sufficient data for inclusion in the meta‐analysis. The trials were grouped under two broad comparisons: periodontal treatment versus no treatment; periodontal treatment versus alternative periodontal treatment.
Eleven studies compared periodontal treatment and no treatment. There is no evidence of a difference in preterm birth < 37 weeks.
Periodontal treatment may reduce low birth weight (< 2500 g) (33% reduction) in pregnant women. The quality of evidence is low. The broader literature suggests that most low birth weights in high‐income countries are related to preterm births, however, this is unexpectedly not reflected in the results of this review (WHO 2012).
Periodontal treatment improves periodontal health.
For primary outcomes small for gestational age and pre‐eclampsia there is no clear difference between periodontal treatment and no treatment.
It is not clear if there is a difference in perinatal mortality outcomes (including fetal and neonatal deaths up to the first 28 days after birth) when periodontal treatment is compared with no treatment.
There were no adverse effects of the therapy or maternal mortality.
There were four studies comparing periodontal treatment with alternative periodontal treatment and it is uncertain whether there is a difference in adverse birth outcomes when periodontal treatments are compared. Periodontal data for this comparison were not pooled due to considerable heterogeneity.
We were unable to pool data on mean gestational age (weeks), mean birth weight (grams/kilograms) and periodontal data due to the skewness of the data.
Overall completeness and applicability of evidence
The studies recruited pregnant women who had different severities of periodontal disease ranging from mild to severe (mostly mild). The participants were women at various stages of pregnancy, different ages, ethnicity and socioeconomic status except two studies (Sadatmansouri 2006; Tarannum 2007) which made no reference to ethnicity. There was variation in periodontal treatment procedures across studies. This correctly reflects current disagreements in clinical practice with regards to periodontal treatment planning (John 2013). The review compares the effect of periodontal treatment versus no treatment and goes further to compare different periodontal treatments. All but one study (Jeffcoat 2003) assessed a combination of mechanical treatments compared to no treatment or in a head‐to‐head comparison. The different interventions assessed cover the range of periodontal treatments that would be given to pregnant women in practice making the results generalisable. All the primary and secondary outcomes were mostly reported. Maternal mortality and adverse effects of the therapy were rarely reported and did not occur in any of the studies that reported them (personal communication with trial authors). Both outcomes may not have been reported due to the fact that no events occurred. Five studies (Farrell 2003; Jeffcoat 2003; Macones 2010; Radnai 2009; Tarannum 2007) failed to report outcome data on periodontal health. We are aware of the fact that the efficacy of periodontal treatment on periodontal health is the basis of its theoretical effect on obstetric outcomes, therefore the absence of periodontal outcome data in the previously mentioned studies puts any potential benefits on obstetric outcomes in doubt. We also acknowledge that the Hawthorne effect (McCambridge 2014) may have resulted in an overestimation of the results by improving participant behaviour in response to their awareness of being part of the trial. This review covers a wide range of participants which would make the evidence applicable to similar population in low and middle‐income countries except that standard antenatal care in these settings may not include an oral health component.
Quality of the evidence
The quality of evidence ranged from low to very low. This was due to high risk of bias, serious imprecision and serious inconsistency. All included studies were at high risk of bias due to the lack of blinding of participants in 13 studies (93%), imbalance in baseline characteristics in seven studies (46%), failure to report periodontal data at follow‐up in four studies (26%) and attrition in four studies (26%). Imprecision was mostly due to very low number of events and for rare outcomes with insufficient sample sizes.
For the main comparison (periodontal treatment versus no treatment), effect estimates were mostly inconsistent across studies. Therefore we downgraded once for moderate to substantial heterogeneity. Heterogeneity may have resulted from variations in the average risk of the outcome events in participants. Clinical heterogeneity due to differences in severities of periodontitis, gestational age and history of preterm birth was evident. The baseline risk of adverse birth outcomes is an aggregate measure of case‐mix factors such as age, severity of periodontitis, previous history of adverse birth outcomes and participants' potential to benefit from an intervention would depend largely on their risk status. Periodontal treatment also varied greatly with studies including different combinations of interventions as part of a care package (Additional Table 4).
The evidence is relevant to the review question and was not downgraded for indirectness. We generated a funnel plot (Figure 4), however, due to the small number of studies we were unable to draw any meaningful conclusion on asymmetry. Since we had taken other measures to prevent publication bias, the evidence was not downgraded for it.
4.
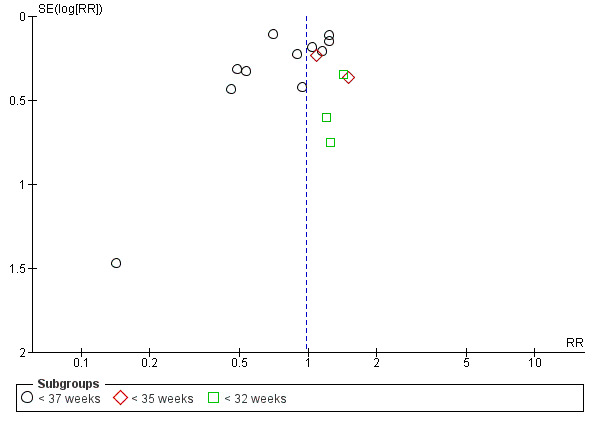
Funnel plot of Comparison 1 Periodontal treatment versus no treatment, Outcome 1.1 Gestational age (preterm birth).
Potential biases in the review process
Data extraction and risk of bias assessment had previously been carried out by a different set of review authors. To ensure consistency and avoid bias, Zipporah Iheozor‐Ejiofor and Anne‐Marie Glenny arbitrated the ratings. Studies not reporting pregnancy outcomes were excluded from the review. Studies would not normally be excluded on the basis of outcome (Higgins 2011). However, given that the primary aim of the review was to assess the efficacy of periodontal treatment in preventing adverse birth outcomes, the inclusion of studies that did not assess obstetric outcomes would have detracted from the focus of the review. Less than half of the included studies provided contact details of authors and most of the emails provided were not valid at the time we attempted to contact the authors. Only three study authors replied to our request for additional information. Therefore it was neither possible to obtain missing information nor clarify partially reported information from most of the study authors. One of the 15 included studies was not published and study data were retrieved from the author via email correspondence. The inclusion of this study meant that some study information remained unclear as we could only get partial information from one of the researchers before the research group was dissolved. This would have introduced reporting bias, however, we carried out a sensitivity analysis by removing this trial from the meta‐analyses and this did not change the direction of effect nor did the effect size change significantly. Therefore this was not considered a source of bias. Before the data synthesis, we assessed studies to assess whether there was sufficient homogeneity. Attempts to contact a study author (Macones 2010) that did not report standard deviation of birth weight were unsuccessful. This may have introduced reporting bias, however, bias was prevented by including the study results as a narrative summary. The studies were not assessed for periodontal outcome detection bias because periodontal outcomes were not the main focus of the review. Birth weight and perinatal mortality were not defined at the protocol stage. We acknowledge that the data analyses for these outcomes were mostly determined by the reporting in individual studies. We considered splitting outcome data on perinatal mortality into miscarriage, stillbirth, neonatal mortality. Given that it was not in the original protocol to split these outcome data, this would have resulted in bias (it should be noted that only two studies reported data for gestational age at which mortality occurred). Three studies (López 2002; López 2005; Tarannum 2007) involved ongoing periodontal care, plaque control, and reinforcement of oral hygiene instructions throughout pregnancy while other studies involved periodontal therapy that occurred as a one‐time (or over several appointments) event. There could be implications for reduction of inflammation that accompany ongoing therapy which may have implications for pregnancy outcomes. However, we did not assess whether duration of treatment influenced the results in a subgroup analysis. We intend to investigate this in future updates.
Agreements and disagreements with other studies or reviews
We identified some previously published reviews that had aimed to assess the effect of periodontal treatment on adverse birth outcomes. These reviews used widely similar methodology with variations in study selection, choice of outcome and data analysis. We carried out two meta‐analyses to compare periodontal treatment versus no treatment and periodontal treatment versus alternative periodontal treatment. Contrary to our approach all the published systematic reviews we identified combined all their studies in a single meta‐analysis irrespective of study comparison. The three reviews (Fogacci 2011; Polyzos 2010; Schwendicke 2015) we identified assessed the effect of periodontal treatment on preterm birth < 37 weeks of gestation, low birth weight and spontaneous abortion/stillbirths (perinatal mortality) and all concluded that periodontal treatment did not confer any advantage in pregnant women with periodontitis.
Fogacci 2011 had evaluated the effect of periodontal treatment on preterm birth and low birth weight. The results on preterm birth are in agreement with the results of our review. The authors had controlled for a number of confounding factors by carrying out separate meta‐analyses. Obtaining similar results from a review with consistent results reinforces the validity of our findings. Another review published in 2010 (Polyzos 2010) had evaluated 12 of the 14 studies included in this review. The review differed from our review in that it excluded participants whose pregnancies resulted in spontaneous abortions or stillbirths. These outcomes were considered important in our review and were extracted from the individual studies if the information was reported. For Polyzos 2010, 'high' quality trials and 'low' quality trials were analysed separately for preterm birth < 37 weeks and low birth weight. Low quality trials, unlike the high quality trials, showed statistically significant results in favour of periodontal treatment. However, there are uncertainties regarding the validity of the overall risk of bias assessment. There was only one assessment for blinding but it is not clear whether this assessment was for performance bias, detection bias or both. Again, contrary to our risk of bias assessment, Polyzos 2010 had assessed all studies including López 2002; Offenbacher 2006; Offenbacher 2009, which we had assessed as high risk, as being at low risk of attrition bias. Schwendicke 2015 stratified the results using arbitrary cut‐offs categorising studies based on high (≥ 20% for preterm birth, ≥ 20% for low birth weight, ≥ 1% for perinatal mortality) or moderate (< 20% preterm birth, < 20% low birth weight, < 1% for perinatal mortality) control group event proportions. Overall the combined results ('high' + 'low' quality in Polyzos 2010; high + moderate control group event proportions in Schwendicke 2015) of all the trials for preterm birth < 37weeks of gestation and spontaneous abortions/stillbirths reported in Polyzos 2010 and Schwendicke 2015 are in agreement with our review. Our review found that periodontal treatment may reduce low birth weight, however, this finding is in disagreement with all three systematic reviews. This variation might have resulted from the reviews combining studies that compared periodontal treatment versus no treatment together with those that did a head‐to‐head comparison between periodontal treatment and alternative periodontal treatment, thereby shifting the results towards no effect.
Authors' conclusions
Implications for practice.
The impact of periodontal treatment on preterm birth is unclear. The quality of evidence was low. There is low‐quality evidence that periodontal treatment may reduce low birth weight compared to no treatment. The GRADE meaning of 'low‐certainty evidence' is that "our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect." Additionally, "further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate." There is no evidence of effect on small for gestational age. It remains unclear whether periodontal treatment leads to a difference in perinatal mortality (including fetal and neonatal deaths up to the first 28 days after birth), and pre‐eclampsia in pregnant women with periodontal disease; the quality of evidence is very low.
There is insufficient evidence to determine which periodontal treatment during pregnancy is better in preventing adverse birth outcomes.
Implications for research.
Considering the complexity and multifactorial nature of periodontal disease, future research should identify and target interventions at specific populations based on severity, ethnicity or socioeconomic status and also aim to administer treatment no later than the first trimester to increase the likelihood of success. Studies should fully report on periodontal outcomes alongside obstetric outcomes given that the benefits of periodontal treatment on obstetric outcomes are predicated on their efficacy. There was variation in diagnosis, measurement, treatment and reporting across the trials. Periodontal status was defined based on continuous variables such as probing depth, attachment loss and bleeding on probing. These measures have been criticised for not fully reflecting periodontal status where there is a low extent of the disease (Sanz 2013). Concerns have also been raised about the lack of consensus on periodontal treatment planning (John 2013). There is need for consensus on case definition of periodontitis and more standardised reporting of periodontal and perinatal outcomes.
History
Protocol first published: Issue 2, 2005 Review first published: Issue 6, 2017
| Date | Event | Description |
|---|---|---|
| 23 December 2015 | Amended | Comprehensive re‐write of protocol as previous version was out of date |
| 5 September 2008 | Amended | Converted to new review format |
Acknowledgements
The review authors would like to thank all members of the Cochrane Oral Health editorial base for their assistance. The authors would also like to thank Natalie Thomas and Caroline Crowther for their contributions to earlier versions of the protocol for this review, and the following referees for their comments: Dr Alison Cooke, Dr Mia Geisinger, and Professor Juan Blanco Carrion.
Appendices
Appendix 1. Cochrane Oral Health's Trials Register search strategy
1 (periodont*:ti,ab) AND (INREGISTER) 2 ((scal* and polish*):ti,ab) AND (INREGISTER) 3 ((root* and plan*):ti,ab) AND (INREGISTER) 4 ((tooth and scal*) or (teeth and scal*) or (dental and scal*) :ti,ab) AND (INREGISTER) 5 (prophylaxis:ti,ab) AND (INREGISTER) 6 (("oral hygiene" or "oral health"):ti,ab) AND (INREGISTER) 7 (gingivitis:ti,ab) AND (INREGISTER) 8 (#1 or #2 or #3 or #4 or #5 or #6 or #7) AND (INREGISTER) 9 (pregnan*:ti,ab) AND (INREGISTER) 10 ((expect* and mother*):ti,ab) AND (INREGISTER) 11 (#9 or #10) AND (INREGISTER) 12 (#8 and #11) AND (INREGISTER)
Appendix 2. Cochrane Pregnancy and Childbirth's Trials Register search strategy
periodont* or (scal* and polish*) or (root* and plan*) OR (tooth and scal*) or (teeth and scal*) or (dental and scal*) or "oral hygiene" or "oral health" or gingivitis
Appendix 3. Cochrane Central Register of Controlled Trials (CENTRAL) search strategy
#1 [mh periodontics] #2 [mh "periodontal diseases"] #3 periodont* #4 [mh "dental prophylaxis"] #5 (scal* near/4 polish*) #6 (root* near/4 plan*) #7 gingivitis #8 (tooth near/6 scal*) or (teeth near/6 scal*) or (dental near/6 scal*) #9 ((oral near/3 prophylaxis) or (dental near/3 prophylaxis)) #10 [mh ^"oral hygiene"] #11 [mh ^"oral health"] #12 ("oral hygien*" or "oral health") #13 {or #1‐#12} #14 [mh Pregnancy] #15 [mh "Pregnancy complications"] #16 [mh ^"Pregnancy outcome"] #17 [mh ^"Prenatal care"] #18 pregnan* #19 (expect* near/3 mother*) #20 {or #14‐#19} #21 #13 and #20
Appendix 4. MEDLINE Ovid search strategy
1. exp Periodontics/ 2. exp Periodontal diseases/ 3. periodont$.mp. 4. Dental prophylaxis/ 5. (scal$ adj4 polish$).mp. 6. (root$ adj4 plan$).mp. 7. gingivitis.mp. 8. ((tooth adj6 scal$) or (teeth adj6 scal$) or (dental adj6 scal$)).mp. 9. ((oral adj3 prophylaxis) or (dental adj3 prophylaxis)).mp. 10. Oral hygiene/ 11. Oral health/ 12. ((oral adj hygien$) or (oral adj health)).mp. 13. or/1‐12 14. exp Pregnancy/ 15. exp Pregnancy complications/ 16. Pregnancy outcome/ 17. Prenatal care/ 18. pregnan$.mp. 19. (expect$ adj3 mother$).mp. 20. or/14‐19 21. 13 and 20
The above subject search was linked to the Cochrane Highly Sensitive Search Strategy (CHSSS) for identifying randomised trials (RCTs) in MEDLINE: sensitivity maximising version (2008 revision) as referenced in Chapter 6.4.11.1 and detailed in box 6.4.c of theCochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011) (Higgins 2011).
1. randomized controlled trial.pt. 2. controlled clinical trial.pt. 3. randomized.ab. 4. placebo.ab. 5. drug therapy.fs. 6. randomly.ab. 7. trial.ab. 8. groups.ab. 9. or/1‐8 10. exp animals/ not humans.sh. 11. 9 not 10
Appendix 5. Embase Ovid search strategy
1. exp Periodontics/ 2. exp Periodontal disease/ 3. periodont$.mp. 4. Preventive dentistry/ 5. (scal$ adj4 polish$).mp. 6. (root$ adj4 plan$).mp. 7. gingivitis.mp. 8. ((tooth adj6 scal$) or (teeth adj6 scal$) or (dental adj6 scal$)).mp. 9. ((oral adj3 prophylaxis) or (dental adj3 prophylaxis)).mp. 10. Oral hygiene/ 11. ((oral adj hygien$) or (oral adj health)).mp. 12. or/1‐11 13. exp Pregnancy/ 14. exp Pregnancy complication/ 15. Pregnancy outcome/ 16. Prenatal care/ 17. pregnan$.mp. 18. (expect$ adj3 mother$).mp. 19. or/13‐18 20. 12 and 19
This subject search was linked to an adapted version of the Cochrane Embase Project filter for identifying RCTs in Embase Ovid (see http://www.cochranelibrary.com/help/central‐creation‐details.html for information).
1. Randomized controlled trial/ 2. Controlled clinical study/ 3. Random$.ti,ab. 4. randomization/ 5. intermethod comparison/ 6. placebo.ti,ab. 7. (compare or compared or comparison).ti. 8. ((evaluated or evaluate or evaluating or assessed or assess) and (compare or compared or comparing or comparison)).ab. 9. (open adj label).ti,ab. 10. ((double or single or doubly or singly) adj (blind or blinded or blindly)).ti,ab. 11. double blind procedure/ 12. parallel group$1.ti,ab. 13. (crossover or cross over).ti,ab. 14. ((assign$ or match or matched or allocation) adj5 (alternate or group$1 or intervention$1 or patient$1 or subject$1 or participant$1)).ti,ab. 15. (assigned or allocated).ti,ab. 16. (controlled adj7 (study or design or trial)).ti,ab. 17. (volunteer or volunteers).ti,ab. 18. trial.ti. 19. or/1‐18 20. (exp animal/ or animal.hw. or nonhuman/) not (exp human/ or human cell/ or (human or humans).ti.) 21. 19 not 20
Appendix 6. LILACS BIREME Virtual Health Library (Latin American and Caribbean Health Science Information database) search strategy
(Mh Periodontal diseases or periodont$ or gingivitis or gengivite) AND (Mh Pregnancy or pregnan$ or embarazo or gravidez)
The above subject search was linked to the Brazilian Cochrane Center filter for LILACs via BIREME:
((Pt randomized controlled trial OR Pt controlled clinical trial OR Mh randomized controlled trials OR Mh random allocation OR Mh double‐blind method OR Mh single‐blind method) AND NOT (Ct animal AND NOT (Ct human and Ct animal)) OR (Pt clinical trial OR Ex E05.318.760.535$ OR (Tw clin$ AND (Tw trial$ OR Tw ensa$ OR Tw estud$ OR Tw experim$ OR Tw investiga$)) OR ((Tw singl$ OR Tw simple$ OR Tw doubl$ OR Tw doble$ OR Tw duplo$ OR Tw trebl$ OR Tw trip$) AND (Tw blind$ OR Tw cego$ OR Tw ciego$ OR Tw mask$ OR Tw mascar$)) OR Mh placebos OR Tw placebo$ OR (Tw random$ OR Tw randon$ OR Tw casual$ OR Tw acaso$ OR Tw azar OR Tw aleator$) OR Mh research design) AND NOT (Ct animal AND NOT (Ct human and Ct animal)) OR (Ct comparative study OR Ex E05.337$ OR Mh follow‐up studies OR Mh prospective studies OR Tw control$ OR Tw prospectiv$ OR Tw volunt$ OR Tw volunteer$) AND NOT (Ct animal AND NOT (Ct human and Ct animal)))and not (Ct ANIMAL AND NOT (Ct HUMAN and Ct ANIMAL)))
Appendix 7. US National Institutes of Health Ongoing Trials Register (ClinicalTrials.gov) and the WHO International Clinical Trials Registry Platform search strategy
periodontitis and pregnancy
periodontal and pregnancy
periodontitis and pregnant
periodontal and pregnant
Data and analyses
Comparison 1. Periodontal treatment versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Gestational age (preterm birth) | 11 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 < 37 weeks | 11 | 5671 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.70, 1.10] |
| 1.2 < 35 weeks | 2 | 2557 | Risk Ratio (M‐H, Random, 95% CI) | 1.19 [0.81, 1.76] |
| 1.3 < 32 weeks | 3 | 2755 | Risk Ratio (M‐H, Random, 95% CI) | 1.35 [0.78, 2.32] |
| 2 Birth weight (low birth weight) | 7 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 2.1 < 2500 g | 7 | 3470 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.48, 0.95] |
| 2.2 < 1500 g | 2 | 2550 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.38, 1.70] |
| 3 Small for gestational age | 3 | 3610 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.81, 1.16] |
| 4 Perinatal mortality (including fetal and neonatal deaths up to the first 28 days after birth) | 7 | 5320 | Risk Ratio (M‐H, Random, 95% CI) | 0.85 [0.51, 1.43] |
| 5 Pre‐eclampsia | 3 | 2946 | Risk Ratio (M‐H, Random, 95% CI) | 1.10 [0.74, 1.62] |
| 6 Probing depth | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6.1 Mean probing depth | 3 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 6.2 Mean change score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Bleeding on probing | 6 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7.1 Mean bleeding on probing | 5 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 Mean change score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Plaque index | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9 Clinical attachment level | 4 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 9.1 Mean clinical attachment level | 3 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9.2 Mean change score | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Periodontal treatment versus alternative periodontal treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Gestational age (preterm birth < 37 weeks) | 4 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 1.1 SRP versus alternative mechanical treatment | 4 | 1168 | Risk Ratio (M‐H, Random, 95% CI) | 0.87 [0.46, 1.67] |
| 1.2 SRP + antimicrobial versus alternative mechanical treatment + placebo | 1 | 243 | Risk Ratio (M‐H, Random, 95% CI) | 1.40 [0.67, 2.92] |
| 1.3 SRP + antimicrobial versus SRP + placebo | 1 | 243 | Risk Ratio (M‐H, Random, 95% CI) | 3.08 [1.15, 8.20] |
| 2 Gestational age (preterm birth < 35 weeks) | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2.1 SRP versus alternative mechanical treatment | 2 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.2 SRP + antimicrobial versus alternative mechanical treatment + placebo | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2.3 SRP + antimicrobial versus SRP + placebo | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Birth weight (low birth weight) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 < 2500 g | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 < 1500 g | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Perinatal mortality (including fetal and neonatal deaths up to the first 28 days after birth) | 2 | 855 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.60, 1.85] |
| 5 Probing depth | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Clinical attachment level | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 7 Bleeding on probing | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 8 Gingival index | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Farrell 2003.
| Methods |
Study design: RCT Location: UK Setting: Guy's and St Thomas Hospitals, UK Recruitment period: unclear |
|
| Participants |
Inclusion criteria: 12 weeks gestation, severe periodontal disease (6 or more sites with 5 mm or more probing depth and 3 or more sites with 3 mm or more loss of periodontal attachment) Exclusion criteria: not stated Age: not stated Gestational age: 12 weeks History of preterm delivery: not stated Number randomised: n = 198 Number evaluated: n = 140 (attrition n = 58 lost to follow‐up) |
|
| Interventions |
1) Antenatal periodontal treatment (n = 102): 5 visits (baseline assessment, oral hygiene instruction, scaling, hand and ultrasonic instrumentation, follow‐up at 30 weeks and maintenance every month until birth) 2) Control (n = 96): could choose to attend own dentist after birth No information on the expertise of the dental professional who administered intervention |
|
| Outcomes | Gestational age; birth weight; miscarriage/stillbirth | |
| Funding | Unclear as full study was not available | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "random allocation table" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Administrative staff allocated subjects via a random allocation table to one of the two groups, following stratification for age, ethnicity, and smoking status" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible |
| Blinding of obstetric outcome assessment (detection bias) | Low risk | Assessor was blinded to group allocation. The outcome in question was assumed to be the obstetric since the study did not report any periodontal outcome |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 40% (41/102) of the treatment group did not receive any periodontal treatment and 57% (58/102) did not attend the follow‐up visit ‐ high attrition rate |
| Selective reporting (reporting bias) | High risk | Periodontal health outcomes were not reported |
| Other bias | Unclear risk | Imbalance in numbers in the 2 groups (102 in the treatment in pregnancy group and 96 in the control group) ‐ about 6% difference |
Herrera 2009.
| Methods |
Study design: RCT Location: Colombia Setting: Hospital Universitario del Valle, Cali, Colombia Recruitment period: March 2006 and December 2007 |
|
| Participants |
Periodontal characteristics: 62% of women had chronic periodontitis (American Academy of Periodontology criteria) Inclusion criteria: pregnant women with mild pre‐eclampsia (blood pressure < 160/11 and proteinuria ≥ 300 mg/L in 24 hours urine) with gestational age between 26 and 34 weeks (no restriction on parity or mother's age); women who had not received antibiotics in the previous 3 months, or periodontal treatment in the previous 6 months before inclusion in study Exclusion criteria: history of chronic hypertension, kidney or cardiovascular disease, diabetes or past history of infections (apart from periodontal or HIV) Mean age (± standard deviation (years)): Group A = 24.7 ± 6.4, Group B = 27 ± 7.6 (P = 0.01) Mean gestational age at trial entry (weeks): Group A = 31.2, Group B = 32.4 History of preterm delivery: not reported Number randomised: n = 60 Number evaluated: n= 60 |
|
| Interventions |
A) Antenatal periodontal treatment (n = 28): between 26 and 34 weeks supragingival and subgingival cleaning with ultrasonic and manual devices (oral health education, hygiene, dental plaque removal, scaling and root planing (if necessary), subgingival irrigation without antibiotic administration in 1 single session of 1 to 2 hours) B) Postnatal periodontal treatment (n = 32): at 48 hours postpartum Periodontal treatment was performed by periodontists |
|
| Outcomes | Progression from mild to severe pre‐eclampsia; eclampsia or HELLP syndrome; number of days of clinical stability; percentile of birth weight adjusted for gestational age; preterm birth; probing depth; clinical attachment level; gingival bleeding (at probing) | |
| Funding | No funding source reported | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomised by blocks" Comment: no further details |
| Allocation concealment (selection bias) | Low risk | Quote: "Treatment intention was determined at random, in closed envelopes prepared by professionals external to the research group" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "Periodontists did not know the objectives of the research" Comment: this was not considered as adequate blinding |
| Blinding of obstetric outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | Periodontal health outcomes on the same population were reported in a linked article (Contreras A, Botero J, Jaramillo A, Soto J, Velez S, Herrera JA. Effects of periodontal treatment on the preterm delivery and low weight newborn in women with preeclampsia ‐ clinical controlled trial. Revista Odontológica Mexicana 2010;14(4):226‐30) |
| Other bias | High risk | More (57% (16/28)) of women in the treatment group had chronic mild periodontitis compared with 37% (12/32) in the control group. There were also differences in age and gestational age at entry |
Jeffcoat 2003.
| Methods |
Study design: RCT Location: USA Setting: Periodontal Clinic, University of Alabama School of Dentistry, Alabama, USA Recruitment period: not stated |
|
| Participants |
Inclusion criteria: pregnant women between 21 and 25 weeks gestational age; screened for ≥ 3 sites clinical attachment loss ≥ 3 mm; ambulatory; willingness to participate and give consent Exclusion criteria: women participating in any other treatment study; undergoing periodontal therapy; taking antibiotics during pregnancy; or using antibiotic mouthrinse; requiring treatment for bacterial vaginosis Mean age (± standard deviation (years)): Group A = 22.2 ± 4 .3, Group B = 22.8 ± 4.6, Group C = 22.4 ± 5 (P = 0.62) Gestational age at trial entry: 21 to 25 weeks (P = not reported) History of spontaneous preterm birth < 35 weeks, n (%): Group A = 6 (4.9%), Group B = 5 (4.1%), Group C = 4 (3.3%) (P = 0.83) Number randomised: n = 368 Number analysed: n = 366 (attrition n = 2 participants delivered elsewhere) |
|
| Interventions |
A) Antenatal periodontal treatment ‐ SRP + placebo capsule (n = 123): scaling and root planing was performed according to usual clinical procedures and clinicians were instructed to spend as much time and as many visits as needed B) Antenatal periodontal treatment ‐ SRP + metronidazole capsule (n = 120): metronidazole was taken 250 mg 3 times a day for 1 week. Scaling and root planing was performed according to usual clinical procedures and clinicians were instructed to spend as much time and as many visits as needed C) Antenatal periodontal treatment ‐ Dental prophylaxis + placebo capsule (n = 123): tooth cleaning and polish (supragingival scaling and rubber cup polish) + placebo capsule 3 times daily All women: received oral hygiene instructions from a dental hygienist and supplies of toothbrushes, dental floss and fluoride toothpaste Dental hygienists carried out examinations at baseline supervised by periodontists. SRP was administered by "clinicians" |
|
| Outcomes | Preterm birth rate (< 35 weeks); preterm birth rate (< 37 weeks) Intention‐to‐treat analysis was applied and the prevalence of preterm birth calculated for each of the 3 randomised treatment groups |
|
| Funding | Not stated | |
| Notes | Stratification by BMI (< 19.8 versus ≥ 19.8); presence of bacterial vaginosis as assessed by Gram stain; previous spontaneous birth prior to 35 weeks gestation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "University research pharmacist generated the randomisation code" |
| Allocation concealment (selection bias) | Low risk | Pharmacist provided a double packet with coding information for each participant ‐ the code did not need to be broken during the study |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study was placebo‐blinded and code breaking seems to have occurred only at the end of the study |
| Blinding of obstetric outcome assessment (detection bias) | Low risk | Quote: "The clinicians delivering periodontal care had no role in determining the outcome of the study... research obstetric nurses abstracted maternal records to determine the predefined age at delivery. These abstractors were completely blinded as to the periodontal status or the patients' periodontal treatment" Comment: outcome seems to have been assessed by different personnel from caregivers |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were only 2 dropouts and intention‐to‐treat analysis was applied |
| Selective reporting (reporting bias) | High risk | Periodontal health outcome was not reported |
| Other bias | Low risk | No other apparent biases |
López 2002.
| Methods |
Study design: RCT Location: Chile Setting: Consultorio Carol Urzua of Penalolen, a district of Santiago, Chile Recruitment period: recruited over a 20‐month period |
|
| Participants |
Inclusion criteria: healthy pregnant women with periodontal disease (≥ 4 teeth with ≥ 1 sites with PD ≥ 4 mm and with CAL ≥ 3 mm at the same site) randomised, aged 18 to 35 years, singleton pregnancy, between 9 and 21 weeks gestation; with fewer than 18 natural teeth Exclusion criteria: history of congenital heart disease requiring prophylactic antibiotics for invasive procedures, existing diabetes before pregnancy, current use of corticosteroids, chronic renal disease, and the intention to give birth at another hospital Mean age (± standard deviation (years)): Group A = 28 ± 4.5, Group B = 27 ± 4.3 (P = 0.04) Mean gestational age (± standard deviation (weeks)): Group A = 39.6 ± 1.2; Group B = 39 ± 2 (P = 0.002) History of preterm low birth weight (%): Group A = 4.3, Group B = 7.4 (P = 0.21) Number randomised: n = 400 Number evaluated: n = 351 (attrition n = 49: loss to follow‐up n = 10, discontinuation of treatment n = 18, spontaneous abortion n = 14, indicated preterm delivery n = 7) |
|
| Interventions |
1) Antenatal periodontal treatment (n = 200): plaque control instructions, scaling and root planing performed under local anaesthesia, each woman was instructed to rinse once a day with 0.12% chlorhexidine; periodontal therapy was completed before 28 weeks gestation and maintenance therapy was provided every 2 to 3 weeks until birth 2) Postnatal periodontal treatment (n = 200): monitoring every 4 to 6 weeks during pregnancy and treatment after birth All women: at study entry, all women received a full‐mouth periodontal examination and the following were determined: oral hygiene status, gingival inflammation, probing depth, clinical attachment level. Periodontal examination was given after 28 weeks of gestation. Carious lesions were treated and all teeth indicated for extraction were extracted from both groups No information on the expertise of the dental professional who administered intervention |
|
| Outcomes | Preterm birth < 37 weeks; low birth weight < 2500 g; preterm low birth weight; number of teeth after 28 weeks gestational age; % of sites with plaque; bleeding on probing; redness; probing depth, clinical attachment loss; after 28 weeks gestational age | |
| Funding | Supported by project grant 1981094 Fondo de Investigación Científica y Tecnológica. Dental instruments partially provided by Hu‐Friedy Co. of Chicago Illinois | |
| Notes | It was believed that 280 women in each group might detect a significant difference of preterm low birth weight between groups with a power of 80%. Data to determine the odds ratios for preterm birth, low birth weight and preterm low birth weight were analysed on an intention‐to‐treat basis. 29 women in the treatment group had severe aggressive periodontitis and were given metronidazole and amoxicillin (3 times daily) for 7 days in addition to mechanical treatment Antibiotics were always prescribed in women with severe periodontitis after they had completed at least 16 weeks of gestation |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was done equalizing periodontal disease as the relevant variable and probing depth was selected as the variable describing periodontal disease. Patients were assigned to 1 of 2 categories: those with a mean probing depth < 2.5 mm and those with a mean probing depth ≥ 2.5 mm. Patients were matched on the basis of the mean probing depth. Each patient of the matched pair was allocated to the treatment or the control group by a coin toss" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible |
| Blinding of obstetric outcome assessment (detection bias) | Low risk | Labour and delivery management decisions were made by personnel who had no knowledge that the patients were participating a research study. The obstetrician who reviewed records of patients with preterm or low birth weight was masked from the mother's periodontal data |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 24/200 (12%) of women in the intervention group were lost to follow‐up (n = 6) or withdrew (n = 18); 4/200 (2%) in the control group were lost to follow‐up There was a difference in attrition rates between groups |
| Selective reporting (reporting bias) | Low risk | No apparent evidence of selective reporting |
| Other bias | High risk | The trial was stopped early due to benefit (preterm low birth weight) ‐ 400 women recruited from target sample size of 580, statistically significant difference in maternal and gestational age between groups |
López 2005.
| Methods |
Study design: RCT Location: Chile Setting: Public Health Clinic, Santiago, Chile Recruitment period: not stated |
|
| Participants |
Inclusion criteria: healthy pregnant women with gingivitis aged 18 to 42; single gestation; ≤ 22 weeks of gestation; gingival inflammation with ≥ 25% of sites with bleeding on proving, and no sites with clinical attachment loss > 2 mm Exclusion criteria: < 18 natural teeth; indication of prophylactic antibiotics for invasive procedures; diabetes previous to pregnancy and the intention to deliver at a hospital other than that of the study Mean age (± standard deviation (years)): Group A = 25.54 ± 5.41, Group B = 24.98 ± 4.55 (P = 0.31) Gestational age: ≤ 22 weeks Previous preterm low birth weight (%): Group A = 3.44, Group B = 7.47 (P = 0.009) Number randomised: n = 870 Number evaluated: n = 834 (attrition n = 36: loss to follow‐up n = 5, withdrawal from treatment and study n = 9, spontaneous abortion n = 10, preterm delivery n = 11, stillbirth n = 1) |
|
| Interventions |
A) Antenatal periodontal treatment (n = 580): plaque control instructions (toothbrushes and mouthrinse daily), supra and subgingival scaling, and crown polishing before 28 weeks of gestation + maintenance therapy (oral hygiene instruction and supragingival plaque removal by instrumentation) every 2 to 3 weeks until delivery B) Postnatal periodontal treatment (n = 290): monitoring 2 to 3 times during pregnancy All women: repeated periodontal examinations after 30 weeks of gestation No information on the expertise of the dental professional who administered intervention |
|
| Outcomes | Preterm birth (< 37 weeks gestational age with birth weight < 2500 g following spontaneous labour and/or rupture of the membranes, regardless of route of delivery); low birth weight; gestational age; infant birth weight; plaque; bleeding on probing; probing depth; clinical attachment loss | |
| Funding | Not stated | |
| Notes | 290 women were required to detect a significant difference of preterm/low birth weight between groups with 80%. To increase statistical power a 2:1 allocation of participants to the treatment and control groups was adopted. Intention‐to‐treat principle was applied | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was done equalizing gingivitis as the relevant variable, and the percentage of bleeding on probing sites was selected as the variable describing gingivitis... One woman of each group of the three was selected by rolling a dice" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible |
| Blinding of obstetric outcome assessment (detection bias) | Low risk | Obstetrician researcher who obtained pregnancy outcome data from hospital records was masked to the periodontal characteristics of the patients. Staff involved in labour and delivery management decisions had no knowledge that the patients were participating in a research study. However, it is not clear whether periodontal outcome assessment was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates were similarly low and balanced across groups (1.7% versus 1.3%) |
| Selective reporting (reporting bias) | Low risk | Expected outcome reported |
| Other bias | High risk | More participants in the control group had a history of previous preterm/low birth weight compared to the treatment group (P = 0.009) |
Macones 2010.
| Methods |
Study design: RCT Location: USA Setting: Periodontal Infections and Prematurity Study (PIPS), a multicentre trial, was conducted in 3 antenatal clinics in metropolitan Philadelphia, USA Recruitment period: not stated |
|
| Participants |
Inclusion criteria: women between 6 and 20 weeks gestation with periodontal disease who returned for the scheduled treatment visit Exclusion criteria: periodontal treatment during pregnancy, antibiotic use within 2 weeks; use of antimicrobial mouthwash within 2 weeks, multiple gestation, and known mitral valve prolapse Mean age (± standard deviation (years)): Group A = 24.1 ± 5.2, Group B = 24.4 ± 5.7 (P = 0.41) Gestational age: 6 to 20 weeks History of preterm delivery (%): Group A = 11.7, Group B = 12.9 (P = 0.62) Periodontal characteristics: periodontal disease was defined as attachment loss ≥ 3 mm on ≥ 3 teeth. Moderate/severe ‐ Group A = 54.8, Group B = 55.3 (P = 0.9) Number randomised: n = 756 Number evaluated: n = 713 (attrition n = 43; lost to follow‐up n = 43) |
|
| Interventions |
A) Antenatal periodontal treatment ‐ Scaling and root planing (n = 376) B) Antenatal periodontal treatment ‐ Superficial tooth cleaning procedure (n = 380): superficial tooth cleaning procedure involved using the rotating cup to remove stains and plaque from the supragingival portion of the tooth using minimally abrasive polishing past. No sharp instruments were used for the subgingival removal of calculus Interventions were delivered by hygienists |
|
| Outcomes | Spontaneous preterm birth (occurring < 35 weeks of gestation because of either idiopathic preterm labour or from preterm premature rupture of the amniotic membranes); < 37 weeks of gestational age, < 35 weeks gestational age; gestational age at delivery; birth weight; neonatal adverse outcomes (respiratory distress syndrome, chronic lung disease, necrotizing enterocolitis, grade III/IV intraventricular haemorrhage (IVH), sepsis, death), stillbirth, miscarriage | |
| Funding | Not stated | |
| Notes | For a prevalence of preterm birth at < 35 weeks of gestation of 7%, it was estimated that 636 participants would be needed per treatment group and the goal was to recruit 700 subjects per treatment group. However, because of temporal restraints that were mandated by the mechanism of funding, enrolment stopped after 3 years of recruitment, which was well before the target sample size was reached | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was accomplished centrally at the University of Pennsylvania, although each clinical site had its own randomisation scheme. A permuted block randomisation procedure was used to formulate assignment lists to assure close to equal numbers of subjects in each treatment group. A uniform block size of 4 was used" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation was accomplished centrally at the University of Pennsylvania..." Comment: this suggests central allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Caregivers were unblinded |
| Blinding of obstetric outcome assessment (detection bias) | Low risk | Quote: "Members of the investigative team (including the obstetricians) who assessed our primary and secondary end points were blinded to treatment assignment" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates were similarly low and balanced across groups for all outcomes |
| Selective reporting (reporting bias) | High risk | Periodontal health outcomes were not reported |
| Other bias | High risk | There were more participants of high socioeconomic status in the control group. Enrollment stopped after 3 years of recruitment due to restraints mandated by the funding mechanism |
Michalowicz 2006.
| Methods |
Study design: RCT Location: USA Setting: the Obstetrics and Periodontal Therapy (OPT) Study, a multicentre trial, was conducted in Hennepin County Medical Centre (Minnesota), the University of Kentucky, the University of Mississippi Medical Center and Harlem Hospital (New York) Recruitment period: March 2003 to June 2005 |
|
| Participants |
Inclusion criteria: at least 16 years of age, less than 16 weeks 6 days gestation, at least 20 natural teeth, and the presence of periodontal disease (4 or more teeth with a probing depth of at least 4 mm and a clinical attachment loss of at least 2 mm, as well as bleeding on probing at 35% or more of tooth sites) Exclusion criteria: multiple pregnancy, required antibiotic prophylaxis for periodontal procedures, medical condition that precluded elective dental treatment, had extensive tooth decay, or likely to have fewer than 20 teeth after treatment of moderate to severe caries, abscesses or other non‐periodontal pathoses. Baseline assessments were conducted between 13 weeks 0 days and 16 weeks 6 days gestation Mean age (± standard deviation (years)): Group A = 26.1 ± 5.6, Group B = 25.9 ± 5.5 (P = 0.56) Mean gestational age (± standard deviation (weeks)): Group A = 15 ± 1.3, Group B = 15 ± 1.3 (P = 0.85) History of preterm delivery (%): Group A = 12.5, Group B = 16.5 (P = 0.18) Periodontal characteristics: tooth sites with probing depth ≥ 4 mm ‐ Group A = 26.5 ± 16.6, Group B = 24.8 ± 15.9 (P = 0.13). Most women were judged to have generalised early‐moderate periodontitis Number randomised: n = 823 Number evaluated: n = 823 (for gestational age); (attrition n = 11: lost to follow‐up n = 7, withdrawal n = 2, elective abortion n = 2) |
|
| Interventions |
A) Antenatal periodontal treatment; before 21 weeks gestation (n = 413): scaling and root planing until birth; removal of dental plaque and calculus from the tooth enamel and root (up to 4 treatment visits were allowed); instruction in oral hygiene, monthly tooth polishing and reinstruction in oral hygiene (actual treatment time = mean 127.7 minutes and 2.0 visits) B) Postnatal periodontal therapy (n = 410): brief oral examination at monthly follow‐ups; attended the same number of visits as the treatment in pregnancy group; periodontal therapy after birth All women: topical or systemic antimicrobials were not used; at study entry, all women were screened for periodontal disease in the obstetric clinic (assessed attachment loss, probing depth, bleeding on probing on 6 sites on each tooth, evaluation of dental plaque and calculus on selected teeth). Women were referred to a dentist for treatment of teeth that were abscessed, fractured or likely to become symptomatic during the study. Full‐mouth assessments were repeated at 21 to 24 weeks gestation and again at 29 to 32 weeks gestation Over half the women (59%) were judged to need essential dental care (239 (61%) in the treatment group and 244 (57%) in the control group) and 73% of these women (74% in the treatment group and 71% in the control group) completed the recommended treatment. Control women with progressive periodontitis at 6 or more sites were offered full‐mouth scaling and root planing. Treatment group participants with progressive disease at 6 or more tooth sites were referred to a consulting periodontist and could receive a second course of full‐mouth scaling and root planing and/or systemic antibiotics, or subgingival irrigation with antimicrobial solutions No information on the expertise of the dental professional who administered intervention |
|
| Outcomes | Periodontitis progression (increase in clinical attachment loss from baseline of at least 3 mm); birth weight; gestational age at birth; labour induced before 37 weeks (due to hypertension, diabetes or pre‐eclampsia); spontaneous abortion (loss before 20 weeks); stillbirth (loss from 20 weeks to 36 weeks and 6 days); maternal death; bacteria from subgingival plaque sampled at 29 to 32 weeks gestation; child neurodevelopment | |
| Funding | Funding from the National Institute of Dental and Craniofacial Research | |
| Notes | Calculations showed that 405 patients per group would be required to show statistical significance with a power of 90% for gestational age | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "randomization, stratified by center with the use of permuted randomized blocks of 2 and 4, was made by a telephone call to the coordinating center" |
| Allocation concealment (selection bias) | Low risk | Quote: "randomization, stratified by center with the use of permuted randomized blocks of 2 and 4, was made by a telephone call to the coordinating center" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible to blind intervention for participants and some personnel |
| Blinding of obstetric outcome assessment (detection bias) | Low risk | Quote: "(obstetrical) examiners and nurses were not aware of the study group assignments" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | The numbers evaluated varied between outcomes, however, attrition rates were similarly low and balanced across groups. 395/413 women in the treatment in pregnancy group received treatment (18 women failed to attend treatment visits or withdrew); 413 women in the treatment group and 410 women in the control group received monthly follow‐ups and 407 and 405 women respectively, were included in the gestational age analysis (99% of women overall). During pregnancy, 6 women in the treatment group withdrew (4 were lost to follow‐up, 1 withdrew consent and 1 had an elective abortion). In the control group 5 women withdrew (3 were lost to follow‐up, 1 withdrew consent and 1 had an elective abortion) |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | No indication of other sources of bias |
Newnham 2009.
| Methods |
Study design: RCT Location: Australia Setting: Smile Study was 'single‐centre' study conducted at 7 sites in public and private antenatal clinics and offices across Perth, Western Australia Recruitment period: February 2005 and December 2007 |
|
| Participants |
Inclusion criteria: > 16 years of age; absence of maternal cardiac disease that would warrant the need for antibiotics for periodontal examination or treatment; not already received periodontal treatment during the current pregnancy; ≥ 20 natural teeth; single pregnancy of > 12 and < 20 weeks gestational age; did not have any known fetal anomalies or other risk factors such as hydramnios that would place the pregnancy at imminent risk of complications; able to attend regularly for periodontal treatment if required Exclusion criteria: not stated Mean age (± standard deviation (years)): Group A = 30.5 ± 5.5, Group B = 30.5 ± 5.5 (P = 0.842) Mean gestational age (± standard deviation (weeks)): Group A = 18.1 ± 2.3, Group B = 18.2 ± 2.2 (P = 0.451) History of preterm delivery (%): Group A = 13.2, Group B = 11.1 (P = 0.412) Periodontal disease: defined as periodontal probing depth ≥ 4 mm at ≥ 12 probing sites in fully erupted teeth (excluding wisdom teeth) Number randomised: n = 1087 Number evaluated: n = 1078 (attrition n = 9: loss to follow‐up n = 1, miscarriage before treatment n = 2, multiple pregnancy n = 1, withdrew consent n = 5) |
|
| Interventions |
A) Antenatal periodontal treatment (n = 542): 3‐week protocol which included non‐surgical debridement of the subgingival and supragingival plaque, removal of local predisposing factors such as calculus, root planing, and adjustment of overhanging restorations. Oral hygiene instruction and motivation were provided at each visit. The advice included toothbrushing and flossing after mean and rinsing with chlorhexidine mouthwash. Local anaesthesia was used as required. Sessions were provided on 3 occasions at weekly intervals commencing around 20 weeks of gestation.Those women in whom the treatment had not been successful (19.6%) were offered a further 3‐week treatment regimen. In addition to the baseline and 28‐week examinations, examinations were also carried out at 32 and 36 weeks gestation B) Postnatal periodontal treatment (n = 540): periodontal care after birth commencing 6 weeks after delivery All women: examinations were carried out at baseline and 4 weeks after treatment (28 weeks gestational age) in both groups Treatments were conducted either by the hygienists or periodontists |
|
| Outcomes | Preterm birth; stillbirth; neonatal death; gestational age; onset of labour; birth weight; sepsis necessitating antibiotics; birth weight less than 10th percentile; sites with probing depth > 4 mm | |
| Funding | Not stated | |
| Notes | A sample size of 1082 women was required to detect a reduction in the preterm birth rate with 80% power. However the independent data safety monitoring committee recommended proceeding without an interim analysis after data on treatment safety and pregnancy outcomes from the trial conducted by Michalowicz 2006 were published. Primary data analysis was performed on the intention‐to‐treat principle, however, the per‐protocol analysis showed similar results as the intention‐to‐treat analysis | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was conducted using computer randomisation software specifically designed to allocate each case at random with stratification for nulliparity, history of preterm birth, and current smoking" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding of participants and caregivers not feasible |
| Blinding of obstetric outcome assessment (detection bias) | Low risk | All medical, nursing, perinatal pathology staff members as well as research midwives who extracted details of all medical, obstetric and neonatal outcomes from the medical records were also unaware of the treatment allocation of each woman |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Primary data analysis was performed on intention‐to‐treat principle, similarly low attrition rates (1.4% versus 0.2%) |
| Selective reporting (reporting bias) | Low risk | Birth weight and gestational age were reported as median and interquartile range |
| Other bias | Low risk | No apparent source of other biases |
Offenbacher 2006.
| Methods |
Study design: RCT (pilot) Location: USA Setting: 2 antenatal clinics (1 high risk) in Raleigh, NC, USA Recruitment period: January 2001 to November 2003 |
|
| Participants |
Inclusion criteria: initially pregnant women with a history of a previous preterm/low birth weight birth, but this was subsequently dropped due to very low eligibility rates. Pregnant women < 22 weeks gestation ≥ 18 years of age at time of scaling and root planing or supragingival polish, 2 or more sites measuring ≥ 5 mm probing depths plus periodontal attachment loss of 1 to 2 mm at 1 or more sites with probing depths ≥ 5 mm; ≥ 20 teeth Exclusion criteria: multiple births, a positive history of HIV, AIDS, diabetes (gestational diabetes was acceptable), any medical contraindication to periodontal probing (e.g. congenital heart disease), and use of phentermine and fenfluramine (phen‐fen) for weight loss; currently undergoing periodontal treatment, chronic regimen of aspirin or non‐steroidal anti‐inflammatory drugs, chronic use of medications that cause gingival enlargement such as phenytoin, cyclosporin‐A, or calcium channel antagonists, 5 or more teeth requiring extraction, rampant decay or any other oral condition that, in the clinician's judgement, would place the woman at unacceptable risk if treatment was delayed, prescribed or using chlorhexidine or other mouthrinses with known antiplaque or anti‐inflammatory effects Mean age (± standard deviation (years)): Group A = 26.8 ± 5.5, Group B = 25.7 ± 5.4 (P = not significant) Gestational age: < 22 weeks History of preterm delivery: Group A = 75, Group B = 88.2 (P = not stated) Number randomised: n = 109 Number evaluated: n = 67 (74 completed baseline examinations) |
|
| Interventions |
A) Antenatal periodontal treatment ‐ Scaling and root planing and polishing + oral health instructions and a sonic power toothbrush and instructions for use (n = 56 (40)) B) Antenatal periodontal treatment ‐ Supragingival debridement + manual toothbrush with no instruction (n = 53 (34)): postnatal scaling and root planing therapy was provided ˜ 6 weeks postpartum with sonic toothbrushes and instruction in their use All participants were interviewed by the dental hygienist, however, it is not clear whether they also administered intervention |
|
| Outcomes | Preterm birth; gingival index (0 = normal gingiva; 1 = mild inflammation; 2 = moderate inflammation; 3 = severe inflammation); plaque index (0 = absence of plaque on clinical crown; 3 = soft deposits covering more than 2‐thirds of the crown); probing depth (6 sites per tooth on all teeth present in the mouth); recession (6 sites per tooth on all teeth present in the mouth or isolated teeth); bleeding on probing (for each quadrant ‐ 0 = absence of bleeding; 1 = bleeding present) | |
| Funding | Study was principally supported by Philips Oral Healthcare | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "109 subjects were randomised" Comment: insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible |
| Blinding of obstetric outcome assessment (detection bias) | Unclear risk | Study was referred to as "examiner‐blinded". No further details stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | High attrition rate: 16 of the 56 women (29%) assigned to the intervention group and 19 of the 53 women assigned to the control group (36%) did not complete baseline periodontal examinations (due to moving or dropping out of the study). This includes 2 fetal deaths (not reported which group(s) these were from). A further 5 women in the intervention group and 2 in the control group did not have birth outcome data, leaving 35 women in the intervention group and 32 in the control group. Postpartum periodontal examinations were collected from 25 intervention and 28 control mothers |
| Selective reporting (reporting bias) | Low risk | Expected outcome reported |
| Other bias | High risk | "Randomization did not balance the primary exposure" of periodontal status |
Offenbacher 2009.
| Methods |
Study design: RCT Location: USA Setting: Maternal Oral Therapy to Reduce Obstetric Risk (MOTOR) Study was a multicentre trial conducted at the Duke University Medical Center (and affiliated clinic at Lincoln Health Center), The University of Alabama at Birmingham Medical Center and 2 obstetric sites of the University of Texas Health Science Center at San Antonio, USA Recruitment period: December 2003 and October 2007 |
|
| Participants |
Inclusion criteria: pregnant women presenting for obstetric care of legal age (16 years) to consent and able to complete periodontal treatment before 23 6/7 weeks gestation; with at least 20 weeks and at least 3 periodontal sites with at least 3 mm of clinical attachment: before randomisation women could receive limited dental care to reduce the likelihood of an acute infectious event during pregnancy (including extraction of hopeless teeth and restoration of pulp‐threatening caries) Exclusion criteria: women with multiple gestation; history of human immunodeficiency virus infection, acquired immunodeficiency syndrome; autoimmune disease; or diabetes (women with gestational diabetes were eligible); need for antibiotic prophylaxis for periodontal probing or periodontal treatment; any obstetric finding that precluded enrolment in the study; women with advanced caries or advanced periodontal disease requiring multiple immediate extractions Mean age (± standard deviation (years)): Group A = 25.3 ± 5.5, Group B = 25.4 ± 5.5 Mean gestational age (± standard deviation (weeks)): Group A = 19.6 ± 2.2, Group B = 19.7 ± 2.1 History of preterm delivery: Group A = 9, Group B = 10.6 (P = 0.244) Number randomised: n = 1806 Number evaluated: n = 1806 (for preterm pregnancy < 37 weeks only). Attrition ranged from 21 to 119 depending on the outcome |
|
| Interventions |
A) Antenatal periodontal treatment (n = 903 women randomised): received ≤ 4 sessions of supragingival and subgingival scaling and root planing (non‐surgical) using hand and ultrasonic instruments. Local anaesthesia was used as needed early in second trimester; plus full‐mouth polishing and oral hygiene home instructions B) Postnatal periodontal treatment (n = 903 women randomised): received periodontal care after delivery Treatment was administered by dental therapists |
|
| Outcomes | Gestational age < 37 weeks (including induced or spontaneous births, fetal demise, and miscarriage but not therapeutic abortions) [this primary outcome was originally specified as < 35 weeks]; gestational age < 35 weeks; birth weight; composite of neonatal morbidity before discharge; fetal demise after randomisation; neonatal death before discharge; respiratory distress syndrome; proven sepsis, intraventricular haemorrhage (IVH) III or IV; necrotizing enterocolitis (NEC); probing depth | |
| Funding | Supported by National Institute of Dental and Craniofacial Research (NIDCR) grant U01‐DE014577 and National Center for Research Resources (NCRR) grants RR00046 and UL1RR025747 | |
| Notes | Before randomisation, women could receive limited dental care to reduce the likelihood of an acute infectious event during pregnancy including the extraction of hopeless teeth and restoration of pulp‐threatening caries. Sample size determination used data from the University of Alabama pilot trial and estimated a preterm (gestational age < 35 weeks) birth rate of 6% in the delayed periodontal therapy group compared with 2% in the periodontal therapy group. A sample size of 900 per treatment group would provide power of 91%, however, the primary outcome was changed due to advice from the monitoring board without change of sample size | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "A permuted block randomisation scheme with a random mixture of block sizes was used, stratifying participants by clinical center" Comment: although computer generation was not mentioned, we have judged the method of sequence generation to be adequate |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible |
| Blinding of obstetric outcome assessment (detection bias) | Low risk | Quote: "Dental examiners were masked to treatment assignment of participants until after the postpartum examination, after the primary obstetric outcome was collected. Dental therapists were instructed not to divulge treatment status to study staff assigned to postnatal data collection. Participants and staff were instructed to not inform the postpartum examiner of the pregnancy outcome. The managing physicians were totally unaware of oral treatment assignments" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Attrition rates varied across outcome, ranged from 2.3% to 23% and was imbalanced for periodontal outcome, yet intention‐to‐treat analysis was only applied to the preterm pregnancy (< 37 weeks) outcome |
| Selective reporting (reporting bias) | Low risk | The authors changed the primary outcome from < 35 weeks to < 37 weeks gestational age, as recommended by the data and safety monitoring board. However, we considered this not to be a source of bias |
| Other bias | Unclear risk | Number of nulliparous pregnancies and alcohol use were higher in the treatment group compared to the control group, however, history of previous adverse pregnancy outcome was balanced between groups. Unclear whether this could be a source of bias |
Oliveira 2011.
| Methods |
Study design: RCT Location: Brazil Setting: prenatal care programmes at 2 public hospitals in Belo Horizonte, Brazil Recruitment period: not stated |
|
| Participants |
Inclusion criteria: healthy pregnant women from low socioeconomic status aged 18‐35 years, between 12‐20 weeks gestational age, current single gestation, ≥ 20 natural teeth and the presence of periodontitis Exclusion criteria: current genitourinary infection, chronic hypertension, diabetes, human immunodeficiency virus infection and/or acquired immunodeficiency syndrome, current use of tobacco (smoking), alcohol and/or illicit drug use, and any medical condition requiring antibiotic prophylaxis for dental treatment, use of any antibiotic or nonsteroidal ant‐inflammatory agents, antiseptic mouthwashes and drugs able to induce gingival overgrowth, women undergoing current periodontal treatment Mean age (± standard deviation (years)): Group A = 29.96 ± 4.38, Group B = 26.58 ± 3.96 (P = 0.5) History of preterm delivery: not stated Gestational age: 12 to 20 weeks Periodontitis was defined as: presence of 4 or more teeth with 1 or more sites with probing depth ≥ 4 mm and clinical attachment level as ≥ 3 mm Number randomised: n = 246 Number evaluated: n = 225 (attrition n = 21: spontaneous abortion n = 5, eligible preterm birth n = 8, stillbirth n = 1, abandonment n = 7) |
|
| Interventions |
A) Antenatal periodontal treatment (n = 122): informed of periodontal status and received a kit containing toothbrushes, dental floss and toothpastes, oral hygiene instructions, plaque index evaluations, dental prophylaxis, and mechanical debridement, when necessary, under local anaesthesia on all affected sites each month during the second trimester; final examination 30‐40 days later; periodontal maintenance every 3 weeks until birth. "The personnel who performed the periodontal therapy were trained", however, there was no information on the expertise of the dental professional who administered the intervention B) Postnatal periodontal treatment (n = 124): informed of their periodontal status and received a kit containing toothbrushes, dental floss and toothpastes. Examination at baseline and final periodontal examination between 30 to 32 weeks gestation; postpartum periodontal treatment offered All women: received a complete periodontal examination (probing depth, clinical attachment level, bleeding on probing at 6 sites per tooth) and were informed of their periodontal status |
|
| Outcomes | Preterm birth; low birth weight; probing depth; clinical attachment loss; and bleeding on probing | |
| Funding | Funded by Research Fund of Pontifical Catholic University of Minas Gerais, Brazil | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomly divided" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "randomly divided" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible |
| Blinding of obstetric outcome assessment (detection bias) | Unclear risk | Albeit assessed independently of the caregiver, it is not clear whether obstetric outcome assessment was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 9/122 (7%) women from the intervention group withdrew (2 spontaneous abortion, 3 'eligible preterm birth', 4 abandonment'); 12/124 (10%) women from the control group withdrew (3 spontaneous abortion, 1 stillbirth, 5 eligible preterm birth, 3 'abandonment') Attrition was similarly low and balanced across groups |
| Selective reporting (reporting bias) | Low risk | No apparent reporting bias |
| Other bias | High risk | Intervention group had worse periodontal outcomes at baseline |
Pirie 2013.
| Methods |
Study design: RCT Location: Northern Ireland Setting: Royal Jubilee Maternity Service, Belfast, Northern Ireland Recruitment period: Februrary 2005 and December 2007 |
|
| Participants |
Inclusion criteria: females >18 years old with singleton pregnancy and ≥ 20 natural teeth Exclusion criteria: multiple pregnancy, diabetes/pregnancy complications, requiring antibiotic prophylaxis before periodontal scaling, had been provided with specialist periodontal treatment in the previous 12 months or aggressive periodontitis requiring urgent intervention Mean age (± standard deviation (years)): Group A = 30.5 ± 4.5, Group B = 30.5 ± 5.5 Mean gestational age (± standard deviation (days)): Group A = 97.6 ± 10.2, Group B = 98.8 ± 10.8 Previous preterm: Group A = 0, Group B = 1 Periodontitis: it was defined as ≥ 4 mm probing depth at ≥ 4 sites and clinical attachment level ≥ 2 mm at ≥ 4 sites Number randomised: n = 99 Number evaluated: n = 99 |
|
| Interventions |
A) Antenatal periodontal treatment ‐ SRP (n = 49): oral hygiene instruction, followed by supragingival and subgingival scaling and root planing of sites with probing depths ≥ 4 mm and polishing of all the teeth. Therapy was performed over 2 1‐hour sessions under local anaesthetic (9 patients refused anaesthetic due to anxiety). Treatment was completed by 24 weeks gestational age. No information on the expertise of the dental professional who administered intervention B) Antenatal periodontal treatment ‐ Alternative mechanical treatment (n = 50): oral hygiene instruction and supragingival cleaning of all teeth at their baseline visit and the option of postpartum periodontal treatment All women: periodontal examination by calibrated examiner. Post‐treatment clinical periodontal related data were collected at 8 weeks after treatment (32 weeks gestational age) |
|
| Outcomes | Pregnancy complications such as pre‐eclampsia, type of delivery, birth weight, gestational age, probing depth, clinical attachment loss, plaque, bleeding on probing | |
| Funding | Supported by Research and Development Office, Department of Health, Northern Ireland Grant EAT/2560/03 | |
| Notes | For sample size, 50 participants in each group were to achieve a power of 80% to detect a difference of 0.6 in mean birth weight standard deviation score equating to a difference of 300 g in birth weight | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Allocations were computer generated by a third person who was not otherwise involved in the study" |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomly allocated to either the control or test arm of the study using sealed opaque envelopes labelled with a study number and containing the group allocation" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Blinding not feasible |
| Blinding of obstetric outcome assessment (detection bias) | Low risk | Birth outcomes were completed at delivery by delivery‐suite staff. These staff members were masked to the group assignments of the participants |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Intention‐to‐treat principle applied |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Low risk | Both groups were balanced for age, weight, height, BMI, alcohol consumption, smoking, periodontal condition, obstetric history except for social class with high socioeconomic status in the test group compared to the control (P = 0.02). However, the review authors did not consider this to be sufficient to bias the results |
Radnai 2009.
| Methods |
Study design: RCT Location: Hungary Setting: Department of Obstetrics and Gynaecology Szeged, Hungary Recruitment period: 2005 and 2006 |
|
| Participants |
Inclusion criteria: women with initial localised chronic periodontitis, and hospitalised due to threatening preterm birth, otherwise healthy with a singleton pregnancy Exclusion criteria: women with any systemic medical problem, multiple pregnancy, history of previous preterm birth or miscarriage, smokers, high consumption of alcohol, drug use, malnourished or women requiring antibiotics for invasive procedures Mean age (± standard deviation (years)): Group A = 29.1 ± 6.4, Group B = 28.9 ± 5.4 (P = 0.888) Mean gestational age (± standard deviation (weeks)): Group A = 31.63 ± 2.6, Group B = 31.45 ± 2.8 (P = 0.822) Previous preterm: not stated Periodontitis: it was defined as ≥ 4 mm probing depth, at least 1 site, and bleeding on probing for ≥ 50% of teeth Number randomised: n = 89 Number evaluated: n = 83 (attrition n = 6 lost to follow‐up) |
|
| Interventions |
A) Antenatal periodontal treatment (n = 43 (41)): treatment around 32 weeks gestational age (oral hygiene instruction), supra and subgingival scaling with hand instruments and/or ultrasonic scaler, teeth polishing with a fluoride paste. The women were examined and treated by a periodontist B) Postnatal periodontal treatment (n = 46 (42)): treatment was suggested postbirth |
|
| Outcomes | Preterm birth (< 37 weeks); low birth weight (< 2500 g); gestational age at birth | |
| Funding | Funded through 'institutional support' | |
| Notes | Power calculation was performed which related birth weight assessment and time of gestation. Assuming a 500 g birth weight difference at a 95% test power for a 2‐sample t‐test, n = 39 was the necessary minimum case number. To show a 2‐week difference in delivery time, at 2.5 standard deviation and 95% power, the desired minimum sample size was n = 42 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "generated a random sequence of 1's and 2's, and the treatment was allocated accordingly to the 1st or 2nd person in the blocks, leaving the other for the control group" |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible |
| Blinding of obstetric outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Attrition rates were similarly low and balanced across groups ‐ 2/43 (4.7%) women were lost to follow‐up in the intervention group and 4/46 (8.7%) were lost to follow‐up in the control group |
| Selective reporting (reporting bias) | High risk | Periodontal health outcomes were not reported |
| Other bias | Low risk | No indication of other sources of bias |
Sadatmansouri 2006.
| Methods |
Study design: RCT Location: Iran Setting: not stated Recruitment period: not stated |
|
| Participants |
Inclusion criteria: pregnant women 18‐35 years of age, with moderate or advanced periodontitis, in 13th to 20th week of pregnancy Exclusion criteria: women with a history of congenital heart disease requiring prophylactic antibiotics, diabetes, current use of corticosteroids, chronic renal disease, or with fetal congenital abnormality (evaluated by ultrasound until 20th week), obstetric disorders such as gestational diabetes, placenta previa, pre‐eclampsia eclampsia and polyhydramnios Periodontal disease: it was defined as women with at least 4 teeth, with at least 1 site of pocket depth of at least 4 mm, and clinical attachment loss of at least 3 mm Mean age (± standard deviation (years)): Group A = 29.1 ± 4.3, Group B = 28.4 ± 4.1 Gestational age: 13‐20 weeks History of preterm delivery: not stated Number randomised: n = 30 Number evaluated: n = 30 |
|
| Interventions |
A) Antenatal periodontal treatment (n = 15): first phase ‐ ultrasonic scaling and hand instrument root planing under local anaesthesia using lidocaine or mepivastesin, if needed; maintenance phase ‐ oral hygiene instructions, use of 0.2% chlorhexidine mouthrinse once a night for 1 week, and periodontal evaluation every fortnight before birth. No information on the expertise of the dental professional who administered intervention B) Postnatal periodontal treatment (n = 15) All women: repeated periodontal examination: 28th week of pregnancy for the control group and 2nd week after treatment in the intervention group (during the 30th week) |
|
| Outcomes | Probing depth; clinical attachment; bleeding on probing; preterm birth < 37 weeks; birth weight; gestational age at birth | |
| Funding | No funding source stated | |
| Notes | None of the subjects were excluded due to abortion, eclampsia, pre‐eclampsia, pregnancy diabetes, placenta previa and polyhydramnios | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "women were randomly divided into two groups" Comment: insufficient information |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible |
| Blinding of obstetric outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No postrandomisation exclusions or losses to follow‐up reported |
| Selective reporting (reporting bias) | Low risk | No apparent evidence of selective reporting bias (although perinatal mortality was not reported) |
| Other bias | Low risk | No indication of other sources of bias |
Tarannum 2007.
| Methods |
Study design: RCT Location: India Setting: The Department of Obstetrics and Gynaecology, Dr BR Ambedkar Medical College and Hospital, Bangalore, Karnataka India Recruitment period: August 2004 to August 2005 |
|
| Participants |
Inclusion criteria: healthy pregnant women aged 18 to 35 years; single gestation between 9 and 21 weeks; with ≥ 20 completely erupted teeth, excluding the third molars, and women with ≥ 2 mm attachment loss at ≥ 50% of examined sites Exclusion criteria: current use of tobacco (smoking/smokeless) or alcohol; history of congenital heart disease, current use of corticosteroids, diabetes, asthma, glomerulonephritis, or hyperthyroidism; mothers with twin pregnancy and Rh factor isoimmunity, and clinically evident systemic infection, inadequate antenatal care (< 6 visits) Mean age (± standard deviation (years)): Group A = 23 ± 3.3, Group B = 22.9 ± 3.6 (P = 0.935) Gestational age: 9 to 21 weeks History of preterm delivery: not reported Number randomised: n = 220 Number evaluated: n = 188 (attrition n = 32: loss to follow‐up n = 16, spontaneous abortions n = 4, did not receive allocated intervention n = 12) |
|
| Interventions |
A) Antenatal periodontal treatment (n = 120): plaque control instructions (rinsing twice daily with 0.2% chlorhexidine until periodontal therapy was completed) + scaling and root planing performed under local anaesthesia. Full‐mouth scaling and root planing was performed over 4 to 5 appointments, with a 1 week interval between appointments. Periondontal therapy was completed before 28 weeks gestation and maintenance therapy was provided (oral prophylaxis and reinforcement of oral hygiene instructions every 3 to 4 weeks until birth). Treatment was provided by a periodontist B) Control ‐ Plaque control (brushing) instructions only + checkups at 4 to 5‐week intervals (n = 100) All women: full‐mouth periodontal examination, including oral hygiene index (simplified); bleeding index, and clinical attachment level |
|
| Outcomes | Preterm birth (< 37 weeks); low birth weight (< 2500 g); gestational age at birth | |
| Funding | Not stated | |
| Notes | The authors claim to have undertaken intention‐to‐treat analysis involving all of the subjects regardless of whether they underwent the prescribed treatment, however, this is not reflected in the 'numbers evaluated' | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Coin flip |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not feasible |
| Blinding of obstetric outcome assessment (detection bias) | Unclear risk | Not stated |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Based on the intention‐to‐treat analysis applied to the treatment group, there was no difference in attrition between groups (16% versus 9%) |
| Selective reporting (reporting bias) | High risk | Periodontal data at follow‐up were not reported clearly |
| Other bias | Low risk | Some imbalance in numbers of women randomised to each group |
BMI = body mass index; CAL = clinical attachment loss; PD = probing depth; RCT = randomised controlled trial; SRP = scaling and root planing.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Gazolla 2007 | The control group consisted of women who refused to participate and were classed as the 'non‐treated' group. There was no randomisation |
| Geisinger 2014 | Not an RCT. The study was used in generating preliminary data for another RCT |
| Jeffcoat 2011 | Overlap with an included study (Jeffcoat 2003) and does not contain useful additional information to supplement the primary publication |
| Jiang 2016 | The study compared antimicrobial mouthrinse with toothbrushing. Antimicrobial mouthrinse alone is not considered a periodontal treatment, however, it can be used as an adjunct to a mechanical periodontal treatment |
| Moreira 2014 | Population consisted of a subsample of participants included in a study (Weidlich 2013) which was excluded for the wrong study population. There is lack of clarity on whether the concerns we had about the primary study were addressed in this study and obstetric outcomes were not reported |
| Pack 1980 | Obstetric outcome not reported |
| Penova‐Veselinovic 2015 | Participants were a subset of study population in an included study (Newnham 2009) |
| Thomson 1982 | Obstetric outcome not reported |
| Weidlich 2013 | Included pregnant women regardless of their periodontal status |
RCT = randomised controlled trial.
Characteristics of studies awaiting assessment [ordered by study ID]
NCT01533792.
| Methods | RCT |
| Participants | 34 pregnant women aged between 15 and 43 years who had at least 4 teeth with probing depth ≥ 4 mm or clinical attachment loss ≥ 3 mm, with bleeding on probing in the same place |
| Interventions | Group 1 received supra and subgingival scaling associated with oral hygiene orientation (OHO) and Group 2 received only supragingival scaling with OHO |
| Outcomes | Probing depth, clinical attachment level, hyperplasia, recession, bleeding, presence of plaque and tooth mobility on a standardized form. Quantitative parameters were evaluated at 6 sites per tooth: mesio/medium/distobuccal and mesio/medium/distolingual through millimetre periodontal probe‐type Williams. The bleeding and the presence of plaque in dichotomous variables were measured: present and absent. All patients received oral hygiene orientation |
| Notes | Reported on clinical registry as completed ‐ study not available |
NCT01549587.
| Methods | Single‐blind RCT |
| Participants | Inclusion criteria: provide written informed consent prior to participation and be given a signed copy of the informed consent form; be at least the age of legal consent; be between 8 and 24 weeks of pregnancy; have at least 20 natural teeth; have moderate‐to‐severe gingivitis during pregnancy, including at least 30 intraoral sites with evidence of marginal gingival bleeding |
| Interventions | Active comparator: regular oral hygiene. Toothpaste, toothbrush and dental floss. Interventions: drug: 0.243% sodium fluoride; device: toothbrush; device: dental floss Experimental: advanced oral hygiene + counselling. Toothpaste, toothbrush, mouthrinse and dental floss + specialized education. Interventions: drug: 0.454% stannous fluoride; device: toothbrush; drug: 0.07% cetylpyridinium chloride; device: dental floss |
| Outcomes | Gestational age (weeks); neonate birth weight (grams); preterm birth (gestational age < 37 weeks) |
| Notes | Reported on clinical registry as completed in April 2014 ‐ study not available |
RCT = randomised controlled trial.
Characteristics of ongoing studies [ordered by study ID]
CTRI/2015/02/005581.
| Trial name or title | A clinical, biochemical and interventional evaluation of possible relationship between periodontal disease and adverse pregnancy outcomes ‐ A randomized controlled trial (CTRI/2015/02/005581) |
| Methods | RCT |
| Participants | Pregnant women with periodontal disease |
| Interventions | Periodontal treatment |
| Outcomes | Unclear |
| Starting date | Unclear |
| Contact information | Vaibhavi Joshipura; vaibhavi_joshipura@yahoo.co.in |
| Notes | Author was contacted in January 2015 and confirmed that the study was not yet published |
RCT = randomised controlled trial.
Differences between protocol and review
Adverse effects of the therapy were previously considered a secondary outcome, however, due to their importance they were eventually listed as a primary outcome.
We planned to report different measures of gestational age and birth weight. Mean gestational age (weeks) and mean birth weight (grams/kilograms) were reported, however, the rarity of the outcomes and skewness of data precluded our pooling these data. We did not present these outcomes in the 'Summary of findings' tables.
We were unable to carry out any of the planned subgroup analyses due to insufficient data.
At protocol stage we stated our intention to generate 'Summary of findings' tables for each comparison. We decided to generate a 'Summary of findings' table for the main comparison alone (periodontal treatment versus no treatment).
We planned to report preterm birth < 34 weeks and < 28 weeks. Eventually we used the cut‐offs that were reported in the included studies (preterm birth < 35 and < 32 weeks).
Following editorial comments, we changed the 'Types of interventions' section to: "Treatment during pregnancy for periodontal disease, performed by a dentist, dental hygienist or therapist (including mechanical debridement using scaling and root planing, polishing, or surgery), either singly or in combination with counselling on oral hygiene, antiseptic oral agents, topical or systemic antimicrobial therapies compared with either placebo (for adjunctive treatment), no treatment or alternative treatments."
Contributions of authors
All authors contributed to all aspects of this review.
Sources of support
Internal sources
Department of Obstetrics and Gynaecology, The University of Adelaide, Australia.
Australian Department of Health and Ageing, Australia.
The Sahlgrenska Academy at Göteborg University, Sweden.
The University of Manchester, Manchester Academic Health Sciences Centre (MAHSC), NIHR Manchester Biomedical Research Centre, UK.
External sources
Swedish Medical Research Council (9495), Sweden.
-
National Institute for Health Research (NIHR), UK.
This project was supported by the NIHR, via Cochrane Infrastructure funding to Cochrane Oral Health. The views and opinions expressed herein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, the NIHR, the NHS or the Department of Health.
-
Cochrane Oral Health Global Alliance, Other.
The production of Cochrane Oral Health reviews has been supported financially by our Global Alliance since 2011 (oralhealth.cochrane.org/partnerships‐alliances). Contributors over the past year have been the American Association of Public Health Dentistry, USA; the British Association for the Study of Community Dentistry, UK; the British Society of Paediatric Dentistry, UK; the Canadian Dental Hygienists Association, Canada; the Centre for Dental Education and Research at All India Institute of Medical Sciences, India; the National Center for Dental Hygiene Research & Practice, USA; New York University College of Dentistry, USA; and NHS Education for Scotland, UK.
Declarations of interest
Zipporah Iheozor‐Ejiofor: no interests to declare. Zipporah is an Editor with Cochrane Oral Health. Philippa Middleton: no interests to declare. Marco Esposito: no interests to declare. Marco is an Editor with Cochrane Oral Health. Anne‐Marie Glenny: no interests to declare. Anne‐Marie is Deputy Co‐ordinating Editor of Cochrane Oral Health.
New
References
References to studies included in this review
Farrell 2003 {published and unpublished data}
- Farrell S. [Personal communication]. Email to: P Middleton June 2007.
- Ide M. Investigation of the effect of treatment of maternal chronic periodontitis on delivery and low birth weight. The Research Findings Register (ReFeR); 2003. Summary No.: 1031.
Herrera 2009 {published data only}
- Contreras A, Botero J, Jaramillo A, Soto J, Velez S, Herrera JA. Effects of periodontal treatment on the preterm delivery and low weight newborn in women with preeclampsia ‐ clinical controlled trial. Revista Odontológica Mexicana 2010;14(4):226‐30. [Google Scholar]
- Herrera JA, Vélez‐Medina S, Molano R, Medina V, Botero JE, Parra B, et al. Periodontal intervention effects on pregnancy outcomes in women with pre‐eclampsia. Colombia Médica 2009;40:177‐84. [Google Scholar]
- Jaramillo A, Arce R, Contreras A, Herrera JA. Effect of periodontal therapy on the subgingival microbiota in preeclamptic patients. Biomedica: Revista del Instituto Nacional de Salud 2012;32(2):233‐8. [DOI] [PubMed] [Google Scholar]
Jeffcoat 2003 {published data only}
- Jeffcoat MK, Hauth JC, Geurs NC, Reddy MS, Cliver SP, Hodgkins PM, et al. Periodontal disease and preterm birth: results of a pilot intervention study. Journal of Periodontology 2003;74(8):1214‐8. [DOI] [PubMed] [Google Scholar]
López 2002 {published data only}
- López NJ. Perinatal mortality, maternal mortality and adverse effects of therapy [personal communication]. Email to: Z Iheozor‐Ejiofor 5 October 2016.
- López NJ, Smith PC, Gutierrez J. Periodontal therapy may reduce the risk of preterm low birth weight in women with periodontal disease: a randomized controlled trial. Journal of Periodontology 2002;73(8):911‐24. [DOI] [PubMed] [Google Scholar]
López 2005 {published data only}
- López NJ. Perinatal mortality, maternal mortality and adverse effects of therapy [personal communication]. Email to: Z Iheozor‐Ejiofor 5 October 2016.
- López NJ, Silva I, Ipinza J, Gutiérrez J. Periodontal therapy reduces the rate of preterm low birth weight in women with pregnancy‐associated gingivitis. Journal of Periodontology 2005;76(11 Suppl):2144‐53. [DOI] [PubMed] [Google Scholar]
Macones 2010 {published data only}
- Jeffcoat M, Parry S, Sammel M, Clothier B, Catlin A, Macones G. Periodontal infection and preterm birth: successful periodontal therapy reduces the risk of preterm birth. BJOG 2011;118(2):250‐6. [DOI: 10.1111/j.1471-0528.2010.02713.x] [DOI] [PubMed] [Google Scholar]
- Macones GA, Parry S, Nelson DB, Strauss JF, Ludmir J, Cohen AW, et al. Treatment of localized periodontal disease in pregnancy does not reduce the occurrence of preterm birth: results from the Periodontal Infections and Prematurity Study (PIPS). American Journal of Obstetrics and Gynecology 2010;202(2):147.e1‐8. [DOI] [PubMed] [Google Scholar]
Michalowicz 2006 {published data only}
- Michalowicz BS, Hodges JS, DiAngelis AJ, Lupo VR, Novak MJ, Ferguson JE, et al. Treatment of periodontal disease and the risk of preterm birth. New England Journal of Medicine 2006;355(18):1885‐94. [DOI] [PubMed] [Google Scholar]
Newnham 2009 {published data only}
- Newnham J. Adverse effects of therapy [personal communication]. Email to: Z Iheozor‐Ejiofor 21 September 2016.
- Newnham JP, Newnham IA, Ball CM, Wright M, Pennell CE, Swain J, et al. Treatment of periodontal disease during pregnancy: a randomized controlled trial. Obstetrics and Gynecology 2009;114(6):1239‐48. [DOI] [PubMed] [Google Scholar]
Offenbacher 2006 {published data only}
- Offenbacher S, Lin D, Strauss R, McKaig R, Irving J, Barros SP, et al. Effects of periodontal therapy during pregnancy on periodontal status, biologic parameters, and pregnancy outcomes: a pilot study. Journal of Periodontology 2006;77(12):2011‐24. [DOI] [PubMed] [Google Scholar]
Offenbacher 2009 {published data only}
- Offenbacher S, Beck JD, Jared HL, Mauriello SM, Mendoza LC, Couper DJ, et al. Effects of periodontal therapy on rate of preterm delivery: a randomized controlled trial. Obstetrics and Gynecology 2009;114(3):551‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Oliveira 2011 {published data only}
- Oliveira AM, Oliveira PA, Cota LO, Magalhães CS, Moreira AN, Costa FO. Periodontal therapy and risk for adverse pregnancy outcomes. Clinical Oral Investigations 2011;15(5):609‐15. [DOI: 10.1007/s00784-010-0424-8] [DOI] [PubMed] [Google Scholar]
Pirie 2013 {published data only}
- Lopez NJ. Letter to the editor: re: intrapregnancy non‐surgical periodontal treatment and pregnancy outcome: a randomized controlled trial. Journal of Periodontology 2014;85(7):880‐1. [DOI: 10.1902/jop.2014.130626] [DOI] [PubMed] [Google Scholar]
- Pirie M, Linden G, Irwin C. Intrapregnancy non‐surgical periodontal treatment and pregnancy outcome: a randomized controlled trial. Journal of Periodontology 2013;84(10):1391‐400. [DOI: 10.1902/jop.2012.120572] [DOI] [PubMed] [Google Scholar]
Radnai 2009 {published data only}
- Novák T, Radnai M, Gorzó I, Urbán E, Orvos H, Eller J, et al. Prevention of preterm delivery with periodontal treatment. Fetal Diagnosis and Therapy 2009;25(2):230‐3. [DOI] [PubMed] [Google Scholar]
- Radnai M, Pál A, Novák T, Urbán E, Eller J, Gorzó I. Benefits of periodontal therapy when preterm birth threatens. Journal of Dental Research 2009;88(3):280‐4. [DOI] [PubMed] [Google Scholar]
Sadatmansouri 2006 {published data only}
- Sadatmansouri S, Sedighpoor N, Aghaloo M. Effects of periodontal treatment phase I on birth term and birth weight. Journal of the Indian Society of Pedodontics and Preventive Dentistry 2006;24(1):23‐6. [DOI] [PubMed] [Google Scholar]
Tarannum 2007 {published data only}
- Tarannum F, Faizuddin M. Effect of periodontal therapy on pregnancy outcome in women affected by periodontitis. Journal of Periodontology 2007;78(11):2095‐103. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Gazolla 2007 {published data only}
- Gazolla CM, Ribeiro A, Moysés MR, Oliveira LA, Pereira LJ, Sallum AW. Evaluation of the incidence of preterm low birth weight in patients undergoing periodontal therapy. Journal of Periodontology 2007;78(5):842‐8. [DOI] [PubMed] [Google Scholar]
Geisinger 2014 {published data only}
- Geisinger ML, Geurs NC, Bain JL, Kaur M, Vassilopolous PJ, Cliver SP, et al. Oral health education and therapy reduces gingivitis during pregnancy. Journal of Clinical Periodontology 2014;41(2):141‐8. [DOI: 10.1111/jcpe.12188] [DOI] [PubMed] [Google Scholar]
Jeffcoat 2011 {published data only}
- Jeffcoat M, Parry S, Sammel M, Clothier B, Catlin A, Macones G. Periodontal infection and preterm birth: successful periodontal therapy reduces the risk of preterm birth. BJOG 2011;118(2):250‐6. [DOI] [PubMed] [Google Scholar]
Jiang 2016 {published data only}
- Jiang H, Xiong X, Buekens P, Su Y, Qian X. Use of mouth rinse during pregnancy to improve birth and neonatal outcomes: a randomized controlled trial. BMC Pregnancy and Childbirth 2015;15:311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Xiong X, Su Y, Peng J, Zhu X, Wang J, et al. Use of antiseptic mouthrinse during pregnancy and pregnancy outcomes: a randomised controlled clinical trial in rural China. BJOG 2016;123 Suppl 3:39‐47. [DOI: 10.1111/1471-0528.14010] [DOI] [PubMed] [Google Scholar]
- Jiang H, Xiong X, Sue Y, Zhang Y, Wu H, Jiang Z, et al. A randomized controlled trial of pre‐conception treatment for periodontal disease to improve periodontal status during pregnancy and birth outcomes. BMC Pregnancy and Childbirth 2013;13:228. [DOI: 10.1186/1471-2393-13-228] [DOI] [PMC free article] [PubMed] [Google Scholar]
Moreira 2014 {published data only}
- Moreira CH, Weidlich P, Fiorini T, Rocha JM, Musskopf ML, Susin C, et al. Periodontal treatment outcomes during pregnancy and postpartum. Clinical Oral Investigations 2015;19(7):1635‐41. [DOI: 10.1007/s00784-014-1386-z] [DOI] [PubMed] [Google Scholar]
Pack 1980 {published data only}
- Pack AR, Thomson ME. Effects of topical and systemic folic acid supplementation on gingivitis in pregnancy. Journal of Clinical Periodontology 1980;7(5):402‐14. [DOI] [PubMed] [Google Scholar]
Penova‐Veselinovic 2015 {published data only}
- Penova‐Veselinovic B, Keelan JA, Wang CA, Newnham JP, Pennell CE. Changes in inflammatory mediators in gingival crevicular fluid following periodontal disease treatment in pregnancy: relationship to adverse pregnancy outcome. Journal of Reproductive Immunology 2015;112:1‐10. [DOI: 10.1016/j.jri.2015.05.002] [DOI] [PubMed] [Google Scholar]
Thomson 1982 {published data only}
- Thomson ME, Pack AR. Effects of extended systemic and topical folate supplementation on gingivitis of pregnancy. Journal of Clinical Periodontology 1982;9(3):275‐80. [DOI] [PubMed] [Google Scholar]
Weidlich 2013 {published data only}
- Lopez NJ. Comments about the paper “Effect of nonsurgical periodontal therapy and strict plaque control on preterm/low birth weight: a randomized controlled clinical trial”. Clinical Oral Investigations 2014;18(1):343‐4. [DOI: 10.1007/s00784-013-1145-6] [DOI] [PubMed] [Google Scholar]
- Weidlich P, Moreira CH, Fiorini T, Musskopf ML, Rocha JM, Oppermann ML, et al. Effect of nonsurgical periodontal therapy and strict plaque control on preterm/low birth weight: a randomized controlled clinical trial. Clinical Oral Investigations 2013;17(1):37‐44. [DOI: 10.1007/s00784-012-0679-3] [DOI] [PubMed] [Google Scholar]
- Weidlich P, Moreira CH, Fiorini T, Musskopf ML, Rocha JM, Oppermann ML, et al. Response to a letter to the editor addressing the publication “Effect of nonsurgical periodontal therapy and strict plaque control on preterm/low birth weight: a randomized controlled clinical trial”. Clinical Oral Investigations 2014;18(1):345‐6. [DOI: 10.1007/s00784-013-1080-6] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT01533792 {published data only}
- NCT01533792. Effect of non‐surgical periodontal treatment on pregnant women with periodontitis: a randomized clinical trial (EONSPT). clinicaltrials.gov/ct2/show/NCT01533792 (first received 28 January 2012).
NCT01549587 {published data only}
- NCT01549587. A randomized controlled clinical trial to evaluate late first to mid‐second trimester introduction of advanced daily oral hygiene on gingivitis and maternity outcomes. clinicaltrials.gov/ct2/show/NCT01549587 (first received 28 February 2012).
References to ongoing studies
CTRI/2015/02/005581 {published data only}
- CTRI/2015/02/005581. Study of relation between gum infection and pregnancy complications [A clinical, biochemical and interventional evaluation of possible relationship between periodontal disease and adverse pregnancy outcomes ‐ A randomized controlled trial]. ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=10723&EncHid=&userName=periodontal%20disease (first received 25 February 2015).
Additional references
Boggess 2003
- Boggess KA, Lieff S, Murtha AP, Moss K, Beck J, Offenbacher S. Maternal periodontal disease is associated with an increased risk for preeclampsia. Obstetrics and Gynecology 2003;101(2):227‐31. [DOI] [PubMed] [Google Scholar]
Chrisopoulos 2012
- Chrisopoulos S, Harford JE. Oral health and dental care in Australia: key facts and figures 2012. www.aihw.gov.au/publication‐detail/?id=60129543390 (accessed prior to 2 May 2017). [978‐1‐74249‐425‐8]
Ciancio 2002
- Ciancio SG. Systemic medications: clinical significance in periodontics. Journal of Clinical Periodontology 2002;29 Suppl 2:17‐21. [PubMed] [Google Scholar]
Davenport 2002
- Davenport ES, Williams CE, Sterne JA, Murad S, Sivapathasundram V, Curtis MA. Maternal periodontal disease and preterm low birthweight: case‐control study. Journal of Dental Research 2002;81(5):313‐8. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Figuero 2013
- Figuero E, Carrillo‐de‐Albornoz A, Martín C, Tobías A, Herrera D. Effect of pregnancy on gingival inflammation in systematically healthy women: a systematic review. Journal of Clinical Periodontology 2013;40(5):457‐73. [DOI] [PubMed] [Google Scholar]
Fogacci 2011
- Fogacci MF, Vettore MV, Leão AT. The effect of periodontal therapy on preterm low birth weight: a meta‐analysis. Obstetrics and Gynecology 2011;117(1):153‐65. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT 2014 [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version accessed 30 April 2017. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Ide 2013
- Ide M, Papapanou PN. Epidemiology of association between maternal periodontal disease and adverse pregnancy outcomes ‐ systematic review. Journal of Clinical Periodontology 2013;84(4 Suppl):S181‐94. [DOI] [PubMed] [Google Scholar]
Int Workshop 1999
Jeffcoat 2001
- Jeffcoat MK, Geurs NC, Reddy MS, Cliver SP, Goldenberg RL, Hauth JC. Periodontal infection and preterm birth: results of a prospective study. Journal of the American Dental Association 2001;132(7):875‐80. [DOI] [PubMed] [Google Scholar]
Jeffcoat 2002
- Jeffcoat M, Hauth J, Geurs N, Reddy M, Cliver S, Goldenberg R. Periodontal disease and preterm birth: results of an intervention study. American Journal of Obstetrics and Gynecology 2002;187(6 Suppl 1):S79 (A62). [Google Scholar]
John 2013
- John V, Lee SJ, Prakasam S, Eckert GJ, Maupome G. Consensus training: an effective tool to minimize variations in periodontal diagnosis and treatment planning among dental faculty and students. Journal of Dental Education 2013;77(8):1022‐32. [PubMed] [Google Scholar]
Kim 2006
- Kim J, Amar S. Periodontal disease and systemic conditions: a bidirectional relationship. Odontology 2006;94(1):10‐21. [DOI: 10.1007/s10266-006-0060-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
Krejci 2002
- Krejci CB, Bissada NF. Women's health issues and their relationship to periodontitis. Journal of the American Dental Association 2002;133(3):323‐9. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.handbook.cochrane.org.
Machuca 1999
- Machuca G, Khoshfeiz O, Lacalle JR, Machuca C, Bullon P. The influence of the general health and socio‐cultural variables on the periodontal condition of pregnant women. Journal of Periodontology 1999;70(7):779‐85. [DOI] [PubMed] [Google Scholar]
McCambridge 2014
- McCambridge J, Witton J, Elbourne DR. Systematic review of the Hawthorne effect: new concepts are needed to study research participation effects. Journal of Clinical Epidemiology 2014;67(3):267‐77. [DOI: 10.1016/j.jclinepi.2013.08.015] [DOI] [PMC free article] [PubMed] [Google Scholar]
Michalowicz 2008
- Michalowicz BS, DiAngelis AJ, Novak MJ, Buchanan W, Papapanou PN, Mitchell DA, et al. Examining the safety of dental treatment in pregnant women. Journal of the American Dental Association 2008;139(6):685‐95. [DOI] [PubMed] [Google Scholar]
Michalowicz 2009
- Michalowicz BS, Novak MJ, Hodges JS, DiAngelis A, Buchanan W, Papapanou PN, et al. Serum inflammatory mediators in pregnancy: changes after periodontal treatment and association with pregnancy outcomes. Journal of Periodontology 2009;87(11):1731‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moore 2004
- Moore S, Ide M, Coward PY, Randhawa M, Borkowska E, Baylis R, et al. A prospective study to investigate the relationship between periodontal disease and adverse pregnancy outcome. British Dental Journal 2004;197(5):251‐8. [DOI] [PubMed] [Google Scholar]
Moore 2005
- Moore S, Randhawa M, Ide M. A case‐control study to investigate an association between adverse pregnancy outcome and periodontal disease. Journal of Clinical Periodontology 2005;32(1):1‐5. [DOI] [PubMed] [Google Scholar]
Offenbacher 1996a
- Offenbacher S. Periodontal diseases: pathogenesis. Annals of Periodontology 1996;1(1):821‐78. [DOI] [PubMed] [Google Scholar]
Offenbacher 1996b
- Offenbacher S, Katz V, Fertik G, Collins J, Boyd D, Maynor G, et al. Periodontal infection as a possible risk factor for preterm low birth weight. Journal of Periodontology 1996;67(10 Suppl):1103‐13. [DOI] [PubMed] [Google Scholar]
OSG 2000
- US Department of Health and Human Services. Oral health in America: a report of the Surgeon General. Rockville (MD): US Department of Health and Human Services, National Institute of Dental and Craniofacial Research, National Institutes of Health; 2000.
Papapanou 2015
- Papapanou PN. Systemic effects of periodontitis: lessons learned from research on atherosclerotic vascular disease and adverse pregnancy outcomes. International Dental Journal 2015;65(6):283‐91. [DOI: 10.1111/idj.12185] [DOI] [PMC free article] [PubMed] [Google Scholar]
Pilot 1980
- Pilot T. Analysis of the overall effectiveness of treatment of periodontal disease. Efficacy of Treatment Procedures in Periodontics. Berlin: Quintessence Publishing Company, 1980:213‐30. [Google Scholar]
Polyzos 2010
- Polyzos NP, Polyzos IP, Zavos A, Valachis A, Mauri D, Papanikolaou EG, et al. Obstetric outcomes after treatment of periodontal disease during pregnancy: systematic review and meta‐analysis. BMJ 2010;341:c7017. [DOI: 10.1136/bmj.c7017] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sanz 2013
- Sanz M, Kornman K, Working Group 3 of the Joint EFP/AAP Workshop. Periodontitis and adverse pregnancy outcomes: consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. Journal of Periodontology 2013;84(4 Suppl):S164‐9. [DOI: 10.1902/jop.2013.1340016] [DOI] [PubMed] [Google Scholar]
Scannapieco 2003
- Scannapieco FA, Bush RB, Paju S. Periodontal disease as a risk factor for adverse pregnancy outcomes. A systematic review. Annals of Periodontology 2003;8(1):70‐8. [DOI] [PubMed] [Google Scholar]
Schwendicke 2015
- Schwendicke F, Karimbux N, Allareddy V, Gluud C. Periodontal treatment for preventing adverse pregnancy outcomes: a meta‐ and trial sequential analysis. PLoS One 2015;10(6):e0129060. [DOI: 10.1371/journal.pone.0129060] [DOI] [PMC free article] [PubMed] [Google Scholar]
Sheiham 2002
- Sheiham A, Netuveli GS. Periodontal diseases in Europe. Periodontology 2000 2002;29:104‐21. [DOI] [PubMed] [Google Scholar]
Swamy 2002
- Swamy G, Murtha A, Jared H, Boggess K, Lieff S, Heine P, et al. Post‐cesarean infection in women with periodontal disease. American Journal of Obstetrics and Gynecology 2002;187(6 Suppl 1):S225 (A610). [Google Scholar]
Wennström 1990
- Wennström JL, Papapanou PN, Gröndahl K. A model for decision making regarding periodontal treatment needs. Journal of Clinical Periodontology 1990;17(4):217‐22. [DOI] [PubMed] [Google Scholar]
WHO 2012
- March of Dimes, PMNCH, Save the Children, WHO. Born too soon: the global action report on preterm birth. Geneva: World Health Organization; 2012. [978 92 4 150343 3]
Worthington 2013
- Worthington HV, Clarkson JE, Bryan G, Beirne PV. Routine scale and polish for periodontal health in adults. Cochrane Database of Systematic Reviews 2013, Issue 11. [DOI: 10.1002/14651858.CD004625.pub4] [DOI] [PubMed] [Google Scholar]
Xiong 2007
- Xiong X, Buekens P, Vastardis S, Yu SM. Periodontal disease and pregnancy outcomes: state‐of‐the‐science. Obstetrical & Gynecological Survey 2007;62(9):605‐15. [PUBMED: 17705886] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Crowther 2005
- Crowther CA, Thomas N, Middleton P, Chua MC, Esposito M. Treating periodontal disease for preventing preterm birth in pregnant women. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD005297] [DOI] [Google Scholar]
Middleton 2015
- Middleton P, Esposito M, Iheozor‐Ejiofor Z. Treating periodontal disease for preventing adverse birth outcomes in pregnant women. Cochrane Database of Systematic Reviews 2015, Issue 12. [DOI: 10.1002/14651858.CD005297.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


