Abstract
Background
Acute urinary tract infection (UTI) is a common bacterial infection that affects 40% to 50% of women. Between 20% and 30% of women who have had a UTI will experience a recurrence, and around 25% will develop ongoing recurrent episodes with implications for individual well‐being and healthcare costs. Prophylactic antibiotics can prevent recurrent UTIs but there are growing concerns about microbial resistance, side effects from treatment and lack of long‐term benefit. Consequently, alternative treatments are being investigated. Chinese herbal medicine (CHM) has a recorded history of treating UTI symptoms and more recent research suggests a potential role in the management of recurrent UTIs. This review aimed to evaluate CHM for recurrent UTI.
Objectives
This review assessed the benefits and harms of CHM for the treatment of recurrent UTIs in adult women, both as a stand‐alone therapy and in conjunction with other pharmaceutical interventions.
Search methods
We searched the Cochrane Kidney and Transplant's Specialised Register to 7 May 2015 through contact with the Trials Search Co‐ordinator, using search terms relevant to this review. We also searched AMED, CINAHL and the Chinese language electronic databases Chinese BioMedical Literature Database (CBM), China Network on Knowledge Infrastructure (CNKI), VIP and Wan Fang Databases to July 2014.
Selection criteria
We included randomised controlled trials (RCTs) comparing treatments using CHM with either an inactive placebo or conventional biomedical treatment. RCTs comparing different CHM strategies and treatments were eligible for inclusion. Quasi‐randomised studies were excluded.
Data collection and analysis
Data extraction was carried out independently by two authors. Where more than one publication of one study existed, these were grouped and the publication with the most complete data was used in the analyses. Where relevant outcomes were only published in earlier versions these data were used. All meta‐analyses were performed using relative risk (RR) for dichotomous outcomes with 95% confidence intervals (CI).
Main results
We included seven RCTs that involved a total of 542 women; of these, five recruited post‐menopausal women (aged from 56 to 70 years) (422 women). We assessed all studies to be at high risk of bias. Meta‐analyses comparing the overall effectiveness of treatments during acute phases of infection and rates of recurrence were conducted. Analysis of three studies involving 282 women that looked at CHM versus antibiotics suggested that CHM had a higher rate of effectiveness for acute UTI (RR 1.21, 95% CI 1.11 to 33) and reduced recurrent UTI rates (RR 0.28, 95% CI 0.09 to 0.82). Analysis of two studies involving 120 women that compared CHM plus antibiotics versus antibiotics alone found the combined intervention had a higher rate of effectiveness for acute UTI (RR 1.24, 95% CI 1.04 to 1.47) and resulted in lower rates of recurrent infection six months after the study (RR 0.53, 95% CI 0.35 to 0.80).
One study comparing different CHM treatments found Er Xian Tang was more effective in treating acute infection in post‐menopausal women than San Jin Pian (80 women: RR 1.28, 95% CI 1.03 to 1.57). Analysis showed that active CHM treatments specifically formulated for recurrent UTI were more effective in reducing infection incidence than generic CHM treatments that were more commonly used for acute UTI (RR 0.40, 95% CI 0.21 to 0.77).
Only two studies undertook to report adverse events; neither reported the occurrence of any adverse events.
Authors' conclusions
Evidence from seven small studies suggested that CHM as an independent intervention or in conjunction with antibiotics may be beneficial for treating recurrent UTIs during the acute phase of infection and may reduce the recurrent UTI incidence for at least six months post‐treatment. CHM treatments specifically formulated for recurrent UTI may be more effective than herbal treatments designed to treat acute UTI. However, the small number and poor quality of the included studies meant that it was not possible to formulate robust conclusions on the use of CHM for recurrent UTI in women either alone or as an adjunct to antibiotics.
Keywords: Adult; Aged; Female; Humans; Middle Aged; Phytotherapy; Acute Disease; Drugs, Chinese Herbal; Drugs, Chinese Herbal/therapeutic use; Randomized Controlled Trials as Topic; Recurrence; Urinary Tract Infections; Urinary Tract Infections/drug therapy; Urinary Tract Infections/prevention & control
Plain language summary
Chinese herbal medicine for treating recurrent urinary tract infections in women
Recurrent urinary tract infections (UTIs) are a common problem that can have a serious negative impact on well‐being and healthcare costs. Although preventative antibiotics can help reduce numbers of recurrent infections, there are growing concerns about antibiotic resistance, side effects and the lack of long‐term benefits from treatment. Consequently, alternative treatments such as Chinese herbal medicine (CHM) are being considered.
We evaluated the evidence for the effectiveness and safety of CHM for treating recurrent UTIs in women. Our searches to May 2015 for Western and July 2014 for Chinese literature led to the inclusion of seven studies that met our selection criteria for this review. These involved a total of 542 women.
The studies suggested that CHM used either on its own or with antibiotic treatment may be more effective than antibiotics alone for relieving acute UTIs and preventing recurrent episodes. There were only two studies that explicitly stated that adverse events were to be reported; neither reported any adverse events.
However, studies were small and assessed as having poor methodological quality; and most study participants were post‐menopausal. Therefore, results should be interpreted cautiously and can only be considered as preliminary findings that may not be relevant to pre‐menopausal women. Further research is required to provide more rigorous evidence before CHM can be routinely recommended as a treatment option for recurrent UTIs.
Summary of findings
Summary of findings for the main comparison. CHM versus antibiotics for women with recurrent UTI.
| CHM versus antibiotics for women with recurrent UTI | ||||||
| Patient or population: women with recurrent UTI Settings: China Intervention: CHM Comparison: antibiotics | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Antibiotics | CHM | |||||
| Effectiveness | Study population | RR 1.24 (1.13 to 1.37) | 282 (3) | ⊕⊝⊝⊝ very low | ||
| 764 per 1000 | 948 per 1000 (864 to 1000) | |||||
| Moderate | ||||||
| 769 per 1000 | 954 per 1000 (869 to 1000) | |||||
| Recurrence Follow‐up: mean 5 months | Study population | RR 0.28 (0.09 to 0.82) | 282 (3) | ⊕⊝⊝⊝ very low | ||
| 550 per 1000 | 154 per 1000 (50 to 451) | |||||
| Moderate | ||||||
| 712 per 1000 | 199 per 1000 (64 to 584) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
CHM ‐ Chinese herbal medicine; UTI ‐ urinary tract infection
Summary of findings 2. CHM plus antibiotics versus antibiotics for women with recurrent UTI.
| CHM plus antibiotics versus antibiotics for women with recurrent UTI | ||||||
| Patient or population: women with recurrent UTI Settings: China Intervention: CHM plus antibiotics Comparison: antibiotics | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Antibiotics | CHM and antibiotics | |||||
| Effectiveness | Study population | RR 1.24 (1.04 to 1.47) | 120 (2) | ⊕⊝⊝⊝ very low | ||
| 733 per 1000 | 902 per 1000 (755 to 1000) | |||||
| Moderate | ||||||
| 733 per 1000 | 902 per 1000 (755 to 1000) | |||||
| Recurrence Follow‐up: mean 6 months | Study population |
RR 0.50 (0.32 to 0.77) |
120 (2) | ⊕⊝⊝⊝ very low | ||
| 567 per 1000 | 300 per 1000 (198 to 453) | |||||
| Moderate | ||||||
| 567 per 1000 | 301per 1000 (198 to 454) | |||||
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
CHM ‐ Chinese herbal medicine; UTI ‐ urinary tract infection
Summary of findings 3. CHM versus CHM for women with recurrent UTI.
| CHM versus CHM for women with recurrent UTI | ||||||
| Patient or population: women with recurrent UTI Settings: China Intervention: CHM Comparison: CHM | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Chinese herbs | Chinese herbs | |||||
| Effectiveness | Study population | RR 1.28 (1.03 to 1.57) | 80 (1) | ⊕⊝⊝⊝ very low | ||
| 725 per 1000 | 928 per 1000 (747 to 1000) | |||||
| Moderate | ||||||
| 725 per 1000 | 928 per 1000 (747 to 1000) | |||||
| Recurrence Follow‐up: mean 6 months | Study population | RR 0.4 (0.21 to 0.77) | 140 (2) | ⊕⊝⊝⊝ very low | ||
| 357 per 1000 | 143 per 1000 (75 to 275) | |||||
| Moderate | ||||||
| 367 per 1000 | 147 per 1000 (77 to 283) | |||||
| Quality of life | see comment | see comment | see comment | 80 (1) | ⊕⊝⊝⊝ very low |
Gu 2011 used SF‐36 to evaluate changes in QoL; reported that women in the active treatment arm improved the average QoL score from 96.9 to 112.1 compared with control group participants who improved the QoL score from 94.9 to 97.4 points (P < 0.05) |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
CHM ‐ Chinese herbal medicine; QoL ‐ quality of life; UTI ‐ urinary tract infection
Background
Description of the condition
Acute lower urinary tract infection (UTI), or cystitis, is a superficial bacterial infection of the bladder mucosa characterised by symptoms of burning on urination, urinary frequency including nocturia and urgency. UTIs are considered uncomplicated if the patient is not pregnant or elderly and there are no known functional or anatomical abnormalities of the genitourinary tract (Hooton 1996).
UTIs are the most common bacterial infection that women present with in primary care settings (Aydin 2014; Butler 2006; Foxman 2010; Little 2010). Up to half of all women experience one UTI during their lives, and at least 11% report UTIs annually (Kunin 1994; Silverman 2013). UTI treatment has a substantial impact on healthcare resources, and UTI accounts for 1% to 3% of all general practice consultations in the UK (Stapleton 1999), and nearly seven million office visits and one million emergency department visits, resulting in 100,000 hospitalisations, in the US (Foxman 2010). The most common pathogens causing uncomplicated UTI are Escherichia coli (E. coli) (80% to 90%), Staphylococcus saprophyticus (5% to 10%), Proteus spp. and other gram‐negative rods (Milo 2005).
Recurrent UTI is widely defined as three UTIs in the last 12 months or two episodes in the last six months (Albert 2004). Between 20% and 30% of women who have had one UTI will have a recurrence (Sanford 1975), and around 25% of these will develop subsequent recurrent episodes (Hooton 1996). Recurrent UTI can have a significant negative effect on quality of life (Flower 2014; Renard 2014) and a high impact on healthcare costs as a result of outpatient visits, diagnostic tests and prescriptions. Precise economic estimates are difficult to derive but in the US approximately 15% of all community‐prescribed antibiotics are dispensed for UTIs at an estimated annual cost of over USD 1 billion (Mazzulli 2002). The direct and indirect costs of community‐acquired UTIs in the US are estimated at around USD 2.3 billion each year (Foxman 2010).
Antibiotics are the mainstay treatment for acute and recurrent UTI. Although antibiotics can reduce the duration of severe symptoms in acute episodes (Falagas 2008; Little 2010a), antibiotic resistance is estimated at 20% for trimethoprim and cephalosporins, and 50% for amoxicillin (Christiaens 2002). Antibiotic resistance and previous UTIs have been positively associated with increased duration of severe symptoms (Little 2010). It is predicted that antibiotic resistance will continue to increase (Kumarasamy 2010).
Antibiotic prophylaxis is used to prevent recurrent UTIs. Treatment usually lasts for between six and 12 months but can be extended for up to five years (Franco 2005). A review of antibiotics for prevention of recurrent UTIs in non‐pregnant women found that given continuously for six to 12 months antibiotics were significantly more effective than placebo in preventing recurrent infection (risk ratio (RR) 0.15, 95% confidence interval (CI) 0.08 to 0.28; number needed to treat to benefit 1.85, 95% CI 1.60 to 2.20) (Albert 2004). Severe side effects such as urticaria, nausea and vomiting, and less serious but unpleasant side effects including oral and vaginal candidiasis and gastrointestinal disturbances may require treatment to be withdrawn. These side effects can cause considerable discomfort and may contribute to some women’s expressed preference to avoid using antibiotics (Leydon 2010).
Once prophylaxis is discontinued, even after extended periods of therapy, 50% to 60% of women become re‐infected within three months (Car 2003; Harding 1982); antibiotic prophylaxis does not exert a long‐term effect on the baseline infection rate.
A number of complementary therapies are used to treat recurrent UTI. Jepson 2012 found that cranberries had little effect in reducing rates of UTI recurrence. There is some evidence that CHM may be useful for treating UTI recurrence.
Description of the intervention
CHM is part of a system of Traditional Chinese Medicine (TCM). CHM involves the use of complex herbal formulae usually comprising 10 to 15 herbs delivered as decoctions (infused in water), encapsulated herbal granules, or pills. CHM formulae may be standardised or individualised according to specific needs. Although biomedical diagnoses are commonly used in CHM practice to optimise treatment effectiveness, these may be differentiated into TCM syndromes according to analysis of presenting signs and symptoms.
CHM has been used to treat UTI symptoms for over 2000 years (Maciocia 1994). Recent clinical research in China suggests that CHM may alleviate UTI symptoms (Liu 1987; Xu 1989; Zhan 2007; Zhang 2005) and reduce one year post‐treatment recurrence rates from 30% when antibiotics were used alone to 4.4% when antibiotics and CHM were combined (Zhang 2005).
How the intervention might work
The herbal products used in CHM contain highly active compounds that have been extensively researched and, in some instances, developed as pharmaceutical drugs.
The biological plausibility of CHM for recurrent UTI is supported by in vitro research suggesting that some commonly used Chinese herbs may confer significant diuretic, antibiotic, immune enhancing, antipyretic, anti‐inflammatory and pain relieving activities for treatment of recurrent UTIs (Bensky 2004; Chen 2004; Huang 1999; Zhu 1998). Individual herbs such as Huang Lian (Coptis chinensis Franch) have broad spectrum antibacterial activity but also exhibit specific action against E. coli (Yan 2008), the most common cause of recurrent UTI. CHM formulae have demonstrated a marked in vitro inhibitory activity against E. coli and other bacteria known to be responsible for UTIs including Staphylococcus aureus, Pseudomonas aeruginosa, Klebsiella pneumoniae and Proteus mirabilis (Peng 2010). Yet other CHM formulae have demonstrated a dose‐dependent ability to decrease E. coli adherence to bladder epithelial cells, to inhibit an underlying mechanism of acute and recurrent UTI (Tong 2011a). There is growing evidence that some herbal medicines can disable bacterial efflux pumps, an important mechanism underlying development of bacterial resistance to antibiotic drugs (Stavri 2007), and may serve as an important adjuvant treatment to conventional antibiotics.
Why it is important to do this review
This review evaluated the extent and quality of clinical evidence relating to CHM for treatment of recurrent UTIs. Benefits of CHM, either as stand‐alone or adjuvant treatment, may make an important contribution to managing this common and problematic condition.
Objectives
This review assessed the benefits and harms of CHM for the treatment of recurrent UTIs in adult women, both as stand‐alone therapy and in conjunction with other pharmaceutical interventions.
Methods
Criteria for considering studies for this review
Types of studies
All RCTs comparing treatment using CHM with either an inactive placebo or conventional biomedical treatment were eligible for inclusion. RCTs comparing different CHM strategies and treatments were considered. Quasi‐randomised studies were excluded because they introduced an unacceptable level of bias into the analyses.
Types of participants
Inclusion criteria
We included ambulatory women, aged 16 years and over, who had histories of three or more recurrent UTIs in the preceding 12 months. At least one episode was required to have laboratory confirmation of bacterial infection (bacterial growth of at least 102 CFU/mL in urine (Franco 2005)) in association with symptoms and signs of UTI including dysuria, frequency, urgency including nocturia, pyuria and haematuria.
Exclusion criteria
We excluded women aged up to 16 years, pregnant women, and those with complicated UTIs (such as associated with pyelonephritis, diabetes, neurological conditions or urinary tract obstruction, or in women who were catheterised) or who did not have laboratory confirmation of at least one UTI in the previous 12 months.
Types of interventions
CHM versus placebo
CHM versus biomedicine
CHM plus biomedicine versus biomedicine (with or without placebo)
CHM versus CHM.
For our purposes, 'biomedicine' referred to the practice of clinical medicine based on the current biological understanding of pathophysiological processes. All forms of oral herbal interventions (pills, herbal granules, herbal decoctions) were considered. Herbs administered as injection and CHM combined with acupuncture or another TCM therapy were excluded.
Types of outcome measures
Primary outcomes
Reduction in both symptomatic episodes, including urinary frequency, urgency, dysuria or haematuria, and bacteriologically‐confirmed episodes of UTI during the study
Rates of relapse within 12 months of completing the study.
Secondary outcomes
Reduction in severity (e.g. intensity of lower abdominal pain, urgency, frequency and burning) and duration of acute UTIs
Reduction in the use of acute and prophylactic antibiotics
Improvements in quality of life (as estimated by validated outcomes measures such as the Short Form 36 (SF‐36))
Any recorded adverse events (including liver and renal toxicity)
Health economic data relating to CHM treatment.
We did not consider changes in surrogate biochemical markers reported in studies.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Specialised Register to 7 May 2015 through contact with the Trials' Search Co‐ordinator using search terms relevant to this review. The Specialised Register contains studies identified from the following sources.
Quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Handsearching of kidney‐related journals and the proceedings of major kidney conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney journals
Searches of the World Health Organization (WHO) International Clinical Trials Register Search Portal (ICTRP) and ClinicalTrials.gov.
Studies contained in the Specialised Register were identified through search strategies for CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of these strategies as well as a list of handsearched journals, conference proceedings and current awareness alerts are available in the specialised register section of information about the Cochrane Kidney and Transplant.
See Appendix 1 for search terms used in the strategies for this review.
We searched AMED (Allied and Complementary Medicine) via OvidSP from 1995 and CINAHL (Cumulative Index to Nursing and Allied Health) via EBSCO from 1937 to November 2014. We also searched Chinese language electronic databases to July 2014 using the terms urinary tract infection, cystitis, recurrent urinary tract infection, Chinese medicine, herbal medicine, plant extract, complementary medicine.
Chinese BioMedical Literature Database (CBM) from 1978
CNKI (China Network on Knowledge Infrastructure) from 1979
VIP database from 1989
Wan Fang Database from 1990.
Searching other resources
Reference lists of review articles, relevant studies and clinical practice guidelines.
Letters seeking information about unpublished or incomplete studies sent to investigators known to be involved in previous studies.
Data collection and analysis
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies that were relevant to the review. Titles and abstracts were screened independently by two authors who discarded studies that were not applicable. However, studies and reviews that included relevant data or information on studies were retained initially. Two authors independently assessed retrieved abstracts and, if necessary, the full texts of these studies to determine which satisfied the inclusion criteria.
Data extraction and management
Data extraction was carried out independently by two authors using standard data extraction forms. Where more than one publication of one study existed, these were grouped together and the publication with the most complete data was used in the analyses. Where relevant outcomes were only published in earlier versions these data were used. Any discrepancy between published versions has been highlighted.
Assessment of risk of bias in included studies
The following items were independently assessed by two authors using the risk of bias assessment tool (Higgins 2011) (see Appendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcomes data adequately addressed (attrition bias)?
Were reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at risk of bias?
Measures of treatment effect
For dichotomous outcomes (such as a reported UTI versus no reported UTI) results were expressed as risk ratio (RR) with 95% confidence interval (CI). If continuous scales of measurement had been used to assess treatment effects (for example number of days reported with UTI symptoms), the mean difference (MD) was to be used, or the standardised mean difference (SMD) if different scales had been used. In practice, only Gu 2011 reported using a continuous scale to measure quality of life.
High levels of study heterogeneity determined our use of random‐effects models for meta‐analyses.
Because recurrent UTIs are episodic and provide outcomes that are not stable and are difficult to measure precisely, a meta‐analysis of change scores, based on a comparison of changes from baseline, was not feasible. However, we conducted meta‐analyses comparing both overall treatment effectiveness during acute phases of infection and rates of recurrence during follow‐up periods.
Unit of analysis issues
The unit of analysis was individual patients. A single measurement for each outcome from each participant was collected and analysed.
Dealing with missing data
Any further information required from the original author was requested (e.g. by e‐mailing corresponding author/s) and any relevant information obtained in this manner was included in the review. We requested further information relating to randomisation methods from the authors of seven studies and received responses from two that enabled inclusion of these studies in the review. Evaluation of important numerical data such as screened, randomised patients as well as intention‐to‐treat, as‐treated and per protocol populations was performed. Attrition rates, such as dropouts, losses to follow‐up and withdrawals, were investigated.
Assessment of heterogeneity
Heterogeneity was analysed using a Chi2 test on N‐1 degrees of freedom, with an alpha of 0.05 used for statistical significance, and with the I2 test (Higgins 2011). I2 values of 25%, 50% and 75% correspond to low, medium and high levels of heterogeneity.
Assessment of reporting biases
We included seven small studies so it was inappropriate to construct funnel plots.
Data synthesis
Data were pooled using the random‐effects model due to the highly heterogeneous nature of the included studies.
Subgroup analysis and investigation of heterogeneity
We planned to conduct subgroup analysis to explore possible sources of heterogeneity, for example the effects of different CHM formulations such as a standardised formula versus tailored formula, control group type, and the effects of treatment on diagnostic syndromes determined according to TCM theory. However, the limited number of included studies meant this was not possible.
We also planned to tabulate adverse effects and assess these using descriptive techniques to enable incorporation of a diverse range of possible measures. Risk difference was to be calculated for each adverse effect, either compared with no treatment or another agent. Because only two included studies provided limited data on adverse effects this was not possible.
Sensitivity analysis
Given the limited numbers of included studies included in the meta‐analysis and the relative parity in terms of size, duration, origin and risk of bias of the studies, it was not appropriate to conduct sensitivity analyses.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies and Characteristics of studies awaiting classification.
Results of the search
We identified 947 records, of which 231 were duplicates. We excluded 459 records that were animal studies (29); narrative reviews (36); investigated unrelated health problems (186); used non‐CHM interventions (89); were not RCTs (115); or included populations unrelated to this review (4).
Of the remaining 257 records, 214 were excluded because they: included both men and women (77); did not meet diagnostic criteria for recurrent UTIs (91); included upper and complicated UTIs (13); were not RCTs (26); did not report clinical outcomes (2); or were duplicates (5).
We assessed 43 full text studies and included seven RCTs in this review (see Characteristics of included studies; Characteristics of excluded studies; Figure 1).
1.
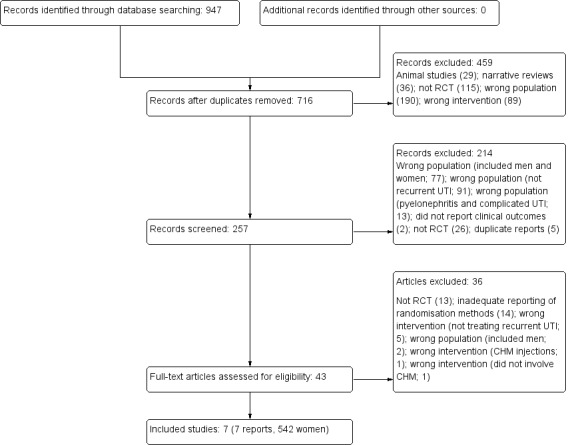
Study flow diagram
Included studies
We included seven parallel RCTs that involved 542 women. All were conducted in China and were reported in Chinese medical journals.
Comparison and control groups
Three studies (Ma 2011; Shen 2007; Zhao 2011) compared CHM with conventional antibiotic treatments. Chen 2008 and Luo 2011 compared CHM plus antibiotics versus antibiotics alone; Gu 2011 and Qin 2004 compared two different CHM regimens. The active treatment period ranged from 4 to 16 weeks.
Participants
Five studies (Chen 2008; Gu 2011; Ma 2011; Shen 2007; Zhao 2011) recruited older, post‐menopausal women whose average ages ranged from 56 to 70 years. Luo 2011 and Qin 2004 recruited younger women whose average ages were 41 years and 44 years, respectively.
Herbal treatment
Herbs were administered as decoctions in the three studies that compared CHM with antibiotics (Ma 2011; Shen 2007; Zhao 2011) and the two that compared CHM plus antibiotics versus antibiotics (Chen 2008; Luo 2011). In the studies that compared CHM with CHM, Gu 2011 compared an active decoction with a standardised pill as control; and Qin 2004 compared two CHM capsules. Details of all herbs used are presented in Characteristics of included studies.
Chen 2008 and Gu 2011 were conducted at the same hospital in China and used the same modified version of the herbal formula Er Xian Tang (Two Immortals Decoction) as active treatment. In terms of TCM, the formulae were designed to 'nourish and harmonise kidney Yin and Yang, clear empty heat, drain damp and eliminate toxins'. These descriptions refer to treatment principles aimed to alleviate well defined symptoms and signs organised into syndromes before the development of modern medicine (Maciocia 1994).
Shen 2007 combined Er Xian Tang with Bai Tou Weng Tang (Pulsatilla decoction) to target the same treatment principles but with emphasis on toxins originating from the bowel. Ma 2011 and Zhao 2011 used different formulae aimed at 'clearing liver fire, eliminating toxins and draining damp', and the formula used by Luo 2011 'eliminated toxins, drained damp and heat and nourished the kidneys and spleen'. Qin 2004 did not disclose ingredients in the active CHM capsule.
All studies except Gu 2011 and Qin 2004 reported adjusting treatment according to the precise nature of each presentation (e.g. including herbs to alleviate lower back pain or stop haematuria). Ma 2011 described two sequential formulae used during the study with initial treatment aimed at relieving acute infection followed by a nourishing treatment administered when the urine culture was negative to consolidate treatment benefits and prevent recurrence.
In Gu 2011, both groups received 14 days of antibiotic treatment to alleviate acute infection symptoms before the women being randomised to a herbal decoction or San Jin Wan (Three Golds Pill), a common proprietary herbal treatment used for UTI.
Biomedical treatment
All studies that used antibiotics as the control tested for microbial sensitivity to establish the appropriate antibiotic. Chen 2008, Ma 2011 and Zhao 2011 specified that when acute symptoms had been relieved the prescribed antibiotic dose was reduced and used prophylactically for the remainder of the study period. Luo 2011 and Shen 2007 did not specify antibiotic use.
Outcome measures
With the exception of Gu 2011, which used SF‐36 as a quality of life measure, there were no other internationally validated outcome measures used. Studies applied National Guidelines on Clinical Research of Novel Traditional Chinese Herbs for the Treatment of Urinary Tract Infection (National Guidelines 1993) to categorise the intervention effectiveness as cured, markedly effective, effective, or ineffective. These categories were commonly defined as follows.
Cured: negative urine cultures measured on two separate occasions during the study and at follow‐up. All UTI signs and symptoms such as frequency, urgency, dysuria and cloudy urine were resolved
Markedly effective: signs and symptoms improved but were not completely resolved. Negative urine culture
Effective: signs and symptoms improved but urine culture positive
Ineffective: no apparent improvement in signs and symptoms or urinalysis.
All studies combined cured, markedly effective and effective into a single overall effectiveness score expressed as a percentage, which was compared with control and subjected to statistical analysis.
All studies followed up participants to assess recurrent episodes of infection. Shen 2007 followed up participants at three months post‐treatment, all other studies at six months.
Excluded studies
We excluded 36 studies after full‐text review.
Thirteen were observational studies that lacked control groups (Flower 2012; Guo 2013; Li 2007; Liao 2005; Liu 2005; Liu 2011; Shu 2007; Tu 2002; Wang 2009; Zhang 1998; Zhang 2005b; Zhang 2013; Zhou 2007)
Hou 2011 described a case history
Seven did not provide sufficient information on randomisation (Lu 2008; Wu 2011; Xu 2009; Xu 2013; Yang 2007; Yu 2009; Zhai 2006). Attempts were made to contact authors but contact failed or authors declined to reply to our requests
Six reported inadequate or unreliable methods of randomisation including quasi‐randomisation (Huang 2007;Qin 2007), markedly unequal group sizes suggesting inadequate randomisation (Peng 2009; Yang 2012), using an unreliable method of randomisation and comparison between groups (Liu 2012a), reporting insufficient detail on randomisation, baseline equivalence or the outcomes measures used (Liu 2013)
Five reported treating urethritis or acute UTI rather than recurrent UTI (Chai 2008; Ding 2010; Li 2006b; Zhan 2007; Zhang 2005b)
Tong 2011 and Liu 2012b included both men and women; Zhan 2007 involved participants who did not meet the inclusion criteria
Albrecht 2007 did not investigate CHM
Peng 2007 investigated injection as the CHM administration route.
Risk of bias in included studies
Study design and methods were generally poorly reported leading to uncertain or high risks of bias (Figure 2; Figure 3).
2.
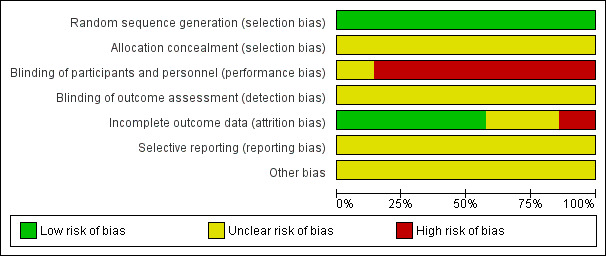
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.
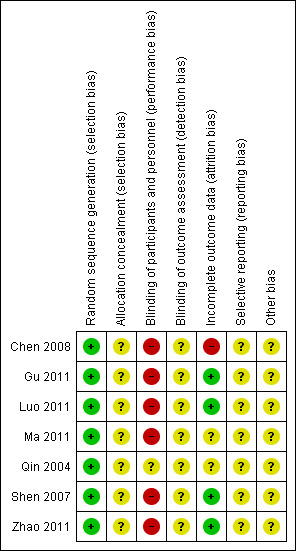
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
All included studies randomised participants into active and control treatment groups using random numbers tables. All appeared to have successfully randomised participants in terms of numbers allocated to each group, age, duration and severity of disease. Gu 2011 and Shen 2007 did not provide P values for baseline equivalence to statistically confirm successful randomisation. However, there appeared to be parity between the treatment and control groups in all studies. Overall, we assessed the included studies to be at low risk of bias for this domain. No information was provided relating to allocation concealment; this was assessed at unclear risk of bias.
Blinding
Qin 2004 compared two CHM capsules but blinding was unclear; efficacy outcomes were not reported. Six studies (Chen 2008; Gu 2011; Luo 2011; Ma 2011; Shen 2007; Zhao 2011) compared a herbal decoction with antibiotic tablets or herbal pills. The physical difference in appearance between a herbal tea (decoction) and a herbal tablet or pill meant it was not possible to blind participants to treatment; this was assessed as leading to high risk of bias. Assessor blinding was not reported.
Incomplete outcome data
Five studies (Gu 2011; Luo 2011; Qin 2004; Shen 2007; Zhao 2011) reported no dropouts during the study or any subsequent losses to follow‐up and were assessed at low risk of attrition bias. Ma 2011 reported no dropouts during the study but described a loss to follow‐up which was not taken into account in the analysis. Chen 2008 adequately reported some dropouts during the study and subsequent follow‐up but these were not accounted for in the analysis. Both Ma 2011 and Chen 2008 were assessed at high risk of attrition bias.
Selective reporting
Study protocols were not available; reporting bias was assessed as unclear.
Other potential sources of bias
None of the studies provided power calculation data to ensure adequate participant recruitment to minimise type 1 and type 2 errors. Although outcome measures conformed to Chinese national guidelines it was not clear if these were validated appropriately. None of the included studies reported funding sources.
Effects of interventions
See: Table 1; Table 2; Table 3
Overall reduction in symptoms
Chinese herbs versus antibiotic treatment
Three studies (Ma 2011; Shen 2007; Zhao 2011) assessed CHM versus antibiotics.
Effectiveness
CHM had a significantly higher rate of effectiveness than antibiotics for treating acute UTI (Analysis 1.1 (3 studies, 282 women): RR 1.21, 95% CI 1.11 to 1.33; I2 = 0%).
1.1. Analysis.
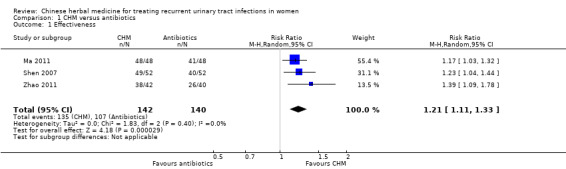
Comparison 1 CHM versus antibiotics, Outcome 1 Effectiveness.
Recurrence
CHM resulted in significantly fewer recurrent episodes of infection (Analysis 1.2 (3 studies, 282 women): RR 0.28, 95% CI 0.09 to 0.82; I2 = 80%). Shen 2007 accounted for all of the heterogeneity between the studies, but when removed there was no change in significance (RR 0.46; 95% CI 0.30 to 0.70).
1.2. Analysis.
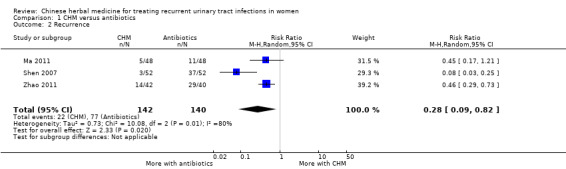
Comparison 1 CHM versus antibiotics, Outcome 2 Recurrence.
Chinese herbs plus antibiotics versus antibiotics alone
Two studies assessed Chinese herbs plus antibiotics versus antibiotics alone (Chen 2008; Luo 2011).
Effectiveness
Results from two small studies reported that CHM plus antibiotics had a higher rate of effectiveness for acute UTI than antibiotics alone (Analysis 2.1 (2 studies, 120 women): RR 1.24, 95% CI 1.04 to 1.47; I2 = 0%).
2.1. Analysis.
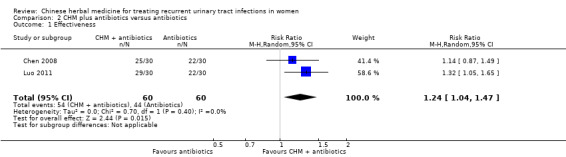
Comparison 2 CHM plus antibiotics versus antibiotics, Outcome 1 Effectiveness.
Recurrence
CHM plus antibiotics were associated with a reduced rate of recurrent infection compared with antibiotics only (Analysis 2.2 (2 studies, 120 women); RR 0.53, 95% CI 0.35 to 0.80; I2 = 0%).
2.2. Analysis.
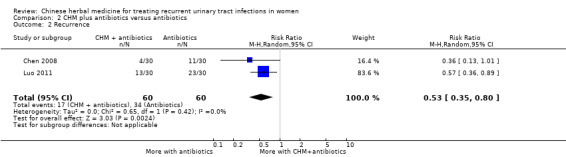
Comparison 2 CHM plus antibiotics versus antibiotics, Outcome 2 Recurrence.
Chinese herbs versus Chinese herbs
Two studies (Gu 2011; Qin 2004) compared active CHM remedies specifically formulated for recurrent UTI with a generic control CHM for acute UTIs.
Effectiveness
Gu 2011 evaluated the comparative effectiveness of a modification of the traditional formula Er Xian Tang with a patented Chinese remedy San Jin Pian. After eight weeks of treatment they reported a significant improvement in overall effectiveness scores in the Er Xian Tang group compared with women in the San Jin Pian group (Analysis 3.1 (1 study, 80 women): RR 1.28, 95% CI 1.03 to 1.57).
3.1. Analysis.
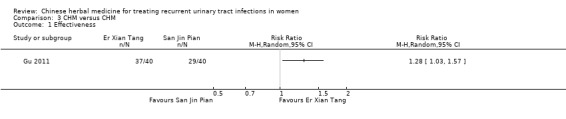
Comparison 3 CHM versus CHM, Outcome 1 Effectiveness.
Recurrence
Er Xian Tang treatment was associated with a reduced rate of recurrent infection compared with San Jin Pian at six months (Analysis 3.2 (2 studies, 140 women): RR 0.40, 95% CI 0.21 to 0.77; I2 = 0%).
3.2. Analysis.
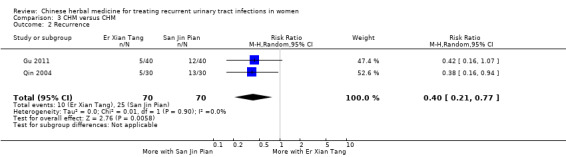
Comparison 3 CHM versus CHM, Outcome 2 Recurrence.
Quality of life
Gu 2011 used the SF‐36 to evaluate changes in quality of life. It was reported that women using Er Xian Tang improved their average quality of life scores from 96.9 to 112.1 compared with those using San Jin Pian whose scores improved from 94.9 to 97.4 points (P < 0.05).
Adverse events
Gu 2011 and Zhao 2011 reported on adverse events experienced; neither found any change in liver and kidney function in either the Er Xian Tang or San Jin Pian group. There were no other reports of serious adverse events.
Discussion
Summary of main results
We investigated CHM for the management of recurrent UTIs in women. Evidence from the included studies suggests that CHM either as an independent intervention or in conjunction with antibiotics may be beneficial for recurrent UTIs treated during the acute phase of infection and may reduce the incidence of recurrent UTI for at least six months post‐treatment. However, the reliability of this review was limited by the small size and limited number of included studies, absence of power calculations to ensure sufficient numbers of study participants, inadequate use of validated outcomes measures and overall high risk of bias.
Limited data from Ma 2011, Shen 2007 and Zhao 2011 suggest that CHM may provide more effective treatment than antibiotics alone for acute UTI episodes and when used prophylactically for long‐term management of recurrent infections in post‐menopausal women. However, these findings were generalizable to post‐menopausal women only.
Reports from Chen 2008 and Luo 2011 suggest that CHM may help to potentiate antibiotic effectiveness for acute treatment and longer‐term prophylaxis.
Analysis of data from Qin 2004 and Gu 2011, which compared two CHM remedies, showed that active CHM treatments specifically formulated for recurrent infections were more effective in preventing UTI recurrence than generic control CHM more commonly used to treat acute UTI.
Two studies that explicitly stated adverse events were to be reported did not report any episodes. There were insufficient data to establish the safety of CHM for women with UTIs.
Poor methodological quality and resultant high risk of bias meant that the results were inconclusive; more rigorous research is required to inform definitive conclusions about the role of CHM in managing recurrent UTIs for women.
Overall completeness and applicability of evidence
None of the studies used a placebo control to help identify some of the contextual effects that may be involved in CHM treatment. Whilst creating a plausible, therapeutically inert control for a herbal decoction is problematic, it is possible (Flower 2011) and desirable for CHM.
Although physical differences in treatments meant it was not possible to blind participants to herbal decoction or antibiotic pill, greater attention should have been paid to participants' expectations and treatment preferences. This could have an important influence on study participants' experiences.
Although all studies reported follow‐up periods there were considerable losses to follow‐up in Chen 2008 and Gu 2011. Follow‐up periods did not extend beyond six months, a relatively short time period for this condition, which undermines the likelihood of assessing longer‐term treatment benefits.
More details about validation of the outcome measures used need to be reported. If outcome measures are not validated this should be undertaken to ensure reliable measures of change in recurrent UTIs.
Quality of the evidence
Study reporting quality was suboptimal. None of the included studies reported on allocation concealment or assessor blinding. There was no reporting of power calculations, and it was unclear if studies recruited sufficient numbers of participants to claim statistical significance for their findings. No data were reported with confidence intervals, which further limited the generalisability of findings.
Overall, included studies were assessed at high risk of bias and the quality of the evidence was poor. The limited number of included studies and participants meant that the findings remain inconclusive, based on the current evidence. In addition there was a patient selection bias that focused on the treatment of post‐menopausal women, which limits the generalisability of these findings.
Potential biases in the review process
It is possible that there was publication bias in the studies we considered for this review as none of the included studies reported negative findings for active CHM interventions. It was also possible that some published studies may not have been included in the Chinese databases.
Agreements and disagreements with other studies or reviews
This is the first rigorous systematic review to analyse CHM for recurrent UTIs.
Authors' conclusions
Implications for practice.
We found limited evidence from seven RCTs about the possible role of CHM as a treatment for recurrent UTI, either as the sole intervention or as an adjunct to antibiotic treatment for post‐menopausal women. CHM may provide an effective treatment during the acute phase of UTI and when given prophylactically to prevent recurrence in the six months following treatment. However, the small number and poor quality of the included studies meant that it was not possible to formulate robust conclusions on the use of CHM for recurrent UTI in women, when administered alone or as an adjunct to antibiotics.
Implications for research.
Given the growing problem of microbial resistance to antibiotic treatments the results of this review should encourage new, more rigorously conducted research into the possible role of CHM for UTI recurrence.
In particular, adequate allocation concealment and assessor blinding, power calculations, use of placebo controls and properly validated outcome measures will help to clarify how CHM, particularly variations of the formula Er Xian Tang (used for recurrent UTIs in post‐menopausal women), may contribute to biomedical treatment.
What's new
| Date | Event | Description |
|---|---|---|
| 1 June 2017 | Amended | Contact details updated. |
Acknowledgements
We wish to thank the referees for their comments and feedback during the preparation of this review.
Andrew Flower is currently funded as a postdoctoral researcher by the UK National Institute of Health Research. Jianping Liu was partially funded by a grant (R24 AT001293‐10) from the National Center for Complementary and Alternative Medicine (NCCAM) of the US National Institutes of Health (www.nccam.nih.gov).
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
| AMED |
|
| CINAHL | S1 (MH "Medicine, Chinese Traditional") S2 (MH "Drugs, Chinese Herbal") S3 (MH "Medicine, Traditional") AND (MH "China") S4 (MH "Medicine, Herbal") AND (MH "China") S5 (MH "Plants, Medicinal") S6 (MH "Plant Extracts") S7 (TI traditional OR integrative) n3 (TI chinese OR medicine) S8 (AB traditional OR integrative) n3 (AB chinese OR medicine) S9 (TI "chinese medicine") OR (AB "chinese medicine") S10 (TI plant OR plants) n5 (TI chinese OR traditional OR extract* OR preparation* OR medicinal) S11 (AB plant OR plants) n5 (AB chinese OR traditional OR extract* OR preparation* OR medicinal) S12 (TI herb*) OR (AB herb*) S13 (TI decoction OR granule* OR pill OR pills OR tablet*) S14 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 S15 (MH "Urinary Tract Infections") S16 (MH "Bacteriuria") S17 (MH "Cystitis") S18 (TI urin*) n3 (TI infection*) S19 (AB urin*) n3 (AB infection*) S20 (TI uti OR utis) OR (AB uti OR utis) S21 (TI bacteriur* OR pyuri*) OR (AB bacteriur* OR pyuri*) S22 (TI cystitis) OR (AB cystitis) S23 S15 or S16 or S17 or S18 or S19 or S20 or S21 or S22 S24 S14 and S23 |
Appendix 2. Risk of bias assessment tool
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random) |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention | |
| Unclear: Insufficient information about the sequence generation process to permit judgement | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes) |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure | |
| Unclear: Randomisation stated but no information on method used is available | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon) |
| High risk of bias: Not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias |
Data and analyses
Comparison 1. CHM versus antibiotics.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Effectiveness | 3 | 282 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [1.11, 1.33] |
| 2 Recurrence | 3 | 282 | Risk Ratio (M‐H, Random, 95% CI) | 0.28 [0.09, 0.82] |
Comparison 2. CHM plus antibiotics versus antibiotics.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Effectiveness | 2 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 1.24 [1.04, 1.47] |
| 2 Recurrence | 2 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.53 [0.35, 0.80] |
Comparison 3. CHM versus CHM.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Effectiveness | 1 | Risk Ratio (M‐H, Random, 95% CI) | Totals not selected | |
| 2 Recurrence | 2 | 140 | Risk Ratio (M‐H, Random, 95% CI) | 0.40 [0.21, 0.77] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Chen 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random numbers table was used. Baseline equivalence was established between groups |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not possible to blind participants because of physical differences between a decoction and a pill |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | ITT analysis was not undertaken |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was unavailable |
| Other bias | Unclear risk | No power calculation was performed to determine the required sample size to adequately power the study to detect type 1 and type 2 errors Outcomes used were not internationally validated or available for review in the study report Funding source not reported |
Gu 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random numbers table was used. Baseline equivalence was established between groups |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not possible to blind participants because of physical differences between a decoction and a pill |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants completed the study |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was unavailable |
| Other bias | Unclear risk | No power calculation was performed to determine the required sample size to adequately power the study to detect type 1 and type 2 errors Outcomes used were not internationally validated or available for review in the study report Funding source not reported |
Luo 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer generated random numbers table was used for randomisation |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants could not be blinded because of the physical difference between decoction and antibiotic tablet |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All 60 participants completed the study and provided data at 6 months follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was unavailable |
| Other bias | Unclear risk | No power calculation was performed to determine the required sample size to adequately power the study to detect type 1 and type 2 errors Outcomes were not internationally validated or available for review in the study report Funding source not reported |
Ma 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Duration of treatment in both groups was not more than 4 weeks |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random numbers table was used. Baseline equivalence was established between groups |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not possible to blind participants because of the physical differences between decoction and pill |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No participant dropouts. However there was a disproportionate loss to follow‐up in the control group that could bias the reported results |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was unavailable |
| Other bias | Unclear risk | No power calculation was performed to determine the required sample size to adequately power the study to detect type 1 and type 2 errors Outcomes were not internationally validated or available for review in the study report Participant preference bias could exist with regard to whether herbs or antibiotics were preferred or selected. This was not assessed Funding source not reported |
Qin 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Details of the herbal components of the two capsules were not adequately reported |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A computer generated random numbers table was used |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Not reported |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No dropouts |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was unavailable |
| Other bias | Unclear risk | No power calculation was performed to determine the required sample size to adequately power the study to detect type 1 and type 2 errors Outcomes were not internationally validated or available for review in the study report Funding source not reported |
Shen 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random numbers table was used. Baseline equivalence was established between groups |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not possible to blind participants because of the physical differences between a decoction and a pill |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts or losses to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was unavailable |
| Other bias | Unclear risk | No power calculation was performed to determine the required sample size to adequately power the study to detect type 1 and type 2 errors Outcomes were not internationally validated or available for review in the study report Participant preference bias could exist with regard to whether they wanted to take herbs or antibiotics. This was not assessed Funding source not reported |
Zhao 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | A random numbers table was used. Baseline equivalence was established between groups |
| Allocation concealment (selection bias) | Unclear risk | Not reported |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | It was not possible to blind participants because of the physical differences between a decoction and a pill |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropouts or losses to follow‐up |
| Selective reporting (reporting bias) | Unclear risk | Study protocol was unavailable |
| Other bias | Unclear risk | No power calculation was performed to determine the required sample size to adequately power the study to detect type 1 and type 2 errors Outcomes were not internationally validated or available for review in the study report Participant preference bias could exist with regard to whether they wanted to take herbs or antibiotics. This was not assessed Funding source not reported |
CHM ‐ Chinese herbal medicine; TCM ‐ traditional Chinese medicine; UTI ‐ urinary tract infection
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Albrecht 2007 | Wrong intervention: not CHM |
| Chai 2008 | Wrong population: not recurrent UTI |
| Ding 2010 | Wrong population: acute UTI |
| Flower 2012 | Observational study with no control group |
| Guo 2013 | Observational study with no control group |
| Hou 2011 | Case history |
| Huang 2007 | Inadequate randomisation: allocation by odd or even number of order of admission |
| Li 2006b | Wrong population: acute UTI |
| Li 2007 | Observational study with no control group |
| Liao 2005 | Observational study with no control group |
| Liu 2005 | Observational study with no control group |
| Liu 2011 | Observational study with no control group |
| Liu 2012a | Inadequate randomisation. This study used a 2:1 randomisation for a very small population with no power calculation. Study compared 20 active versus 10 control participants and 40 active vs. 20 control participants. We do not know if these were sufficient numbers to inform meaningful comparison between groups. Adding these unequal groups together and comparing with Western medicine could create unreliable selection bias |
| Liu 2012b | Did not meet inclusion criteria ‐ it includes both men and women |
| Liu 2013 | Inadequate randomisation, baseline equivalence and outcome measures |
| Lu 2008 | Insufficient reporting of randomisation method |
| Peng 2007 | Wrong interventions: CHM administered by injection |
| Peng 2009 | Insufficient randomisation method reporting and uneven participant allocation |
| Qin 2007 | Inadequate randomisation |
| Shu 2007 | Observational study with no control group |
| Tong 2011 | Wrong population: included men and women |
| Tu 2002 | Observational study with no control group |
| Wang 2009 | An observational study with no control group |
| Wu 2011 | Insufficient reporting on randomisation method |
| Xu 2013 | Insufficient reporting on randomisation method |
| Xu 2009 | Insufficient reporting on randomisation method |
| Yang 2007 | Insufficient reporting on randomisation method |
| Yang 2012 | We found a 20% difference in group size between study arms which suggests poor randomisation, or possibly an undeclared bias or dropout rate that is not accounted for in an ITT analysis. Follow‐up period was for 6 weeks only |
| Yu 2009 | Insufficient information on randomisation method |
| Zhai 2006 | Insufficient information on randomisation method |
| Zhan 2007 | Wrong population: acute UTI |
| Zhang 1998 | Observational study with no control group |
| Zhang 2005b | Observational study with no control group |
| Zhang 2013 | Observational study with no control group |
| Zhou 2007 | Observational study with no control group |
CCHM ‐ Chinese herbal medicine; ITT ‐ intention‐to‐treat; UTI ‐ urinary tract infection
Characteristics of studies awaiting assessment [ordered by study ID]
Yin 2011.
| Methods |
|
| Participants |
|
| Interventions | Treatment group
|
| Outcomes |
|
| Notes | Authors were contacted to request further information about methods applied to measure clinical outcomes as opposed to assessing immune parameters and bacteriuria |
Differences between protocol and review
We proposed using funnel plots to assess the possible impact of small study bias or reporting bias; subgroup analysis to assess possible sources of study heterogeneity; and sensitivity analysis to assess the impact of a number of factors on study findings. We also planned to report on possible adverse effects from CHM. However, the limited number and small size of included studies meant there were insufficient data to meet these objectives.
Contributions of authors
Draft the protocol: AF, JPL, GL, PL
Study selection: QL, AF
Extract data from studies: QL, AF
Enter data into RevMan: QL, AF
Carry out the analysis: AF, QL, JPL
Interpret the analysis: AF, QL, JPL, GL, PL
Draft the final review: AF
Disagreement resolution: JPL, GL
Update the review: AF
Sources of support
Internal sources
Beijing University of Chinese Medicine (2011‐CXTD‐09), China.
External sources
National Institute of Health Research, UK.
Declarations of interest
Andrew Flower: is currently receiving a postdoctoral grant from the UK National Institute of Health Research to investigate the possible role of CHM in the treatment of recurrent UTIs. He is also a Chinese herbal practitioner who treats patients with UTIs and lectures on the subject
Li‐Qiong Wang: none known
George Lewith: none known
Jian Ping Liu: none known
Qing Li: none known
Edited (no change to conclusions)
References
References to studies included in this review
Chen 2008 {published data only}
- Chen M, Wang Y, Gu XC. Clinical observation of modified "erxian decoction" plus antibiotics in treating chronic urinary tract infection in middle‐aged and old women. Shanghai Zhongyiyao Zazhi [Shanghai Journal of Traditional Chinese Medicine] 2008;42(1):48‐9. [Google Scholar]
Gu 2011 {published data only}
- Gu XC, Xu Z, Chen M, Wang M. Study of erding erxian docoction compared with sanjin tablet in treating recurrent urinary tract infection. Zhongguo Zhongxiyi Jiehe Shenbing Zazhi [Chinese Journal of Integrated Traditional and Western Nephrology] 2011;12(7):623‐4. [Google Scholar]
Luo 2011 {published data only}
- Luo M. Clinical study of bushen tonglin decoction on female with chronic urinary tract infection. Hubei University of Chinese Medicine 2011.
Ma 2011 {published data only}
- Ma XY, Zhi Y, Zhang X, Zhao H, Gao GJ. Clinical study of Xianqing Houbu method in treating senile female recurrent urinary tract infection. Zhongguo Zhongyi Jizhen [Journal of Emergency in Traditional Chinese Medicine] 2011;20(12):1918‐9. [Google Scholar]
Qin 2004 {published data only}
- Qin SG. Clinical analysis of Ningmitai capsule on 60 cases chronic urinary tract infection. Hebei Yixue [Hebei Medicine] 2004;10(8):700‐2. [Google Scholar]
Shen 2007 {published data only}
- Shen Y, Yao Q. Clinical observation of "baitouweng decoction" and "erxian decoction" in treating lower urinary infection in 52 postmenopausal women. Shanghai Zhongyiyao Zazhi [Shanghai Journal of Traditional Chinese Medicine] 2007;41(12):37‐8. [Google Scholar]
Zhao 2011 {published data only}
- Zhao KS, Liu BL, Wei W, Ma QY, Zhao WJ, Zhang SR. Clinical study of clearing liver fire, removing dampness, strengthening spleen and tonifying kidney methods in treating middle‐aged and old woman with chronic urinary tract infection. International Journal of Traditional Chinese Medicine 2011;33(11):976‐8. [Google Scholar]
References to studies excluded from this review
Albrecht 2007 {published data only}
- Albrecht U, Goos KH, Schneider B. A randomised, double‐blind, placebo‐controlled trial of a herbal medicinal product containing Tropaeoli majoris herba (Nasturtium) and Armoraciae rusticanae radix (Horseradish) for the prophylactic treatment of patients with chronically recurrent lower urinary tract infections. Current Medical Research & Opinion 2007;23(10):2415‐22. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Chai 2008 {published data only}
- Chai D, Jiang H. Clinical effect of lomefloxacin and shenshu pill in the treatment of recurrent urethritis. Journal of Modern Clinical Medicine 2008;34(4):258‐9. [Google Scholar]
Ding 2010 {published data only}
- Ding F, Zhang Y, Chang Y. Sheshiliuhuang decoction on cytokines in patients with urinary tract infection. Zhongguo Zhong Yao Za Zhi/Zhongguo Zhongyao Zazhi [China Journal of Chinese Materia Medica] 2010;35(7):919‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Flower 2012 {published data only}
- Flower A Lewith G. A prospective case series exploring the role of Chinese herbal medicine in the treatment of recurrent urinary tract infections. European Journal of Integrative Medicine 2012;4:e421‐8. [EMBASE: 2013042266] [Google Scholar]
Guo 2013 {published data only}
- Guo HH, Yang HT. Prof Yang's experience of treating recurrent UTIs. Zhongguo Zhongxiyi Jiehe Shenbing Zazhi [Chinese Journal of Integrated Traditional and Western Nephrology] 2013;14(2):98‐9. [Google Scholar]
Hou 2011 {published data only}
- Hou YH, Wang YG. Case history of Prof Huang's application of Qing Xin Lian Zi Yin in the treatment of cystitis. Jilin Zhongyiyao [Jilin Journal of Traditional Chinese Medicine] 2011;31(11):1106‐7. [Google Scholar]
Huang 2007 {published data only}
- Huang MJ. Clinical observation of Sanling Jiedu formulae in the treatment of recurrent lower urinary tract infection in female patients. Hubei Zhongyi Zazhi [Hubei Journal of Traditional Chinese Medicine] 2007;29(6):38. [Google Scholar]
Li 2006b {published data only}
- Li Z, Fu P, Qiu H, Zhou L, Fan J, Zhang R, et al. Shenling granule for lower urinary tract infection (damp‐heat in lower‐Jiao): A randomized controlled trial. Chinese Journal of Evidence Based Medicine 2006;6(1):9‐13. [EMBASE: 2006065612] [Google Scholar]
Li 2007 {published data only}
- Li Y, Han Y. Recent developments in the treatment of recurrent UTIs in older women with Chinese medicine. Jilin Zhongyiyao [Jilin Journal of Traditional Chinese Medicine] 2007;27(3):65‐6. [Google Scholar]
Liao 2005 {published data only}
- Liao JF. Integrated traditional Chinese and Western medicine on 40 cases chronic urinary tract infection. Fujian Zhongyiyao [Fujian Journal of Traditional Chinese Medicine] 2005;36(2):29‐30. [Google Scholar]
Liu 2005 {published data only}
- Liu CJ. 42 cases of recurrent UTIs treated with Pei Yuan Tong Lin Tang (cultivate the original and free urinary obstruction decoction). Shiyong Zhongyiyao Zazhi [Journal of Practical Traditional Chinese Medicine] 2005;19(4):355. [Google Scholar]
Liu 2011 {published data only}
- Liu L. Observation study of the use of Bu Zhong Yi Qi Tang in the treatment of 35 cases of recurrent UTIs. Special Issue of the Society of Chinese Medicine 2011;19:191. [Google Scholar]
Liu 2012a {unpublished data only}
- Liu SW. Etiological analysis experimental study and clinical treatment of recurrent urinary tract infection by fuzhengqingrelishi. China Academy of Chinese Medical Sciences 2012.
Liu 2012b {unpublished data only}
- Liu HJ. Clinical effects observation of tonifying kidney clearing method in treatment of recurrent urinary. Nanjing University of Chinese Medicine 2012.
Liu 2013 {published data only}
- Liu JY. Integrated CHM and WM treatment for older women with RUTIs. Chinese medicine Modern Distance Education of China 2013;11(2):32‐3. [Google Scholar]
Lu 2008 {published data only}
- Lu Y, Zhao L, Wang D. Clinical observation of sanjin tablet in inhibit bacterial process of chronic urinary tract infection in female patients. Zhongguo Zhongyao Zazhi [China Journal of Chinese Materia Medica] 2008;33(21):2554‐5. [Google Scholar]
Peng 2007 {published data only}
- Peng GJ, Wu YS. Study of Xueshuantong injection on female palindromic urinary tract infection. Zhongguo Zhongxiyi Jiehe Zazhi [Chinese Journal of Integrated Traditional and Western Medicine] 2007;16(5):588‐9. [Google Scholar]
Peng 2009 {published data only}
- Peng GJ, Wu YS. Clinical observation of treatment of sanjin tablets in 50 cases of women with recurrent urinary tract infections. China Medical Herald 2009;6(8):74‐5. [Google Scholar]
Qin 2007 {published data only}
- Qin Y. Clinical study of warming the yang and promoting blood movement in recurrent UTIs in post‐menopausal women. ACTA Universatis Traditions Medicalis Sinensisi Pharm Shanghai 2007;21(3):46‐7. [Google Scholar]
Shu 2007 {published data only}
- Shu J, Wu ZY, Xu B. Experience of Dr Wu ZY in the treatment of recurrent UTIs. Liaoning Zhongyi Xueyuan Xuebao [Journal of Liaoning University of Traditional Chinese Medicine] 2007;9(6):96‐7. [Google Scholar]
Tong 2011 {published data only}
- Tong YN. Clinical observation of a combination of Chinese and Western medicine in the treatment of 32 older women with recurrent UTIs. Jilin Hospital Journal 2011;32(11):2156‐7. [Google Scholar]
Tu 2002 {published data only}
- Tu X, Sun JS. Clinical observation of tonify the kidneys and supplement Qi method to prevent recurrent UTIs. Journal of Zhejiang Integrated medicine 2002;12(7):441‐2. [Google Scholar]
Wang 2009 {published data only}
- Wang JW, Wang XJ. Use of San Jian Pian in the treatment of 60 cases of recurrent UTIs. Chinese Clinical Journal of Rational Drug Use 2009;2(18):51. [Google Scholar]
Wu 2011 {published data only}
- Wu QX. Clinical study of the Qingxin Lianzi Yin in the treatment of recurrent urinary tract infection in 33 cases. Xiandai Zhongyiyao [Modern Traditional Chinese Medicine] 2011;31(6):29‐30. [Google Scholar]
Xu 2009 {published data only}
- Xu WW, Chen XH. Improvement of immune state in female patients with recurrently urinary tract infection with Sanjin tablet. Chinese Journal of Difficult and Complicated Cases 2009;8(1):23‐5. [Google Scholar]
Xu 2013 {published data only}
- Xu ZY. Improvement in immune function in patients with RUTIs using San Jin Pian. Acta Universitatis Traditionis Medicalis Sinensis Pharmacologiaeque Shanghai 2013;27(1):30‐3. [Google Scholar]
Yang 2007 {published data only}
- Yang YZ. Clinical observation of Zishen Tongguan Liqi Huoxue method in the treatment of recurrent urinary tract infection. Shiyong Zhongyiyao Zazhi [Journal of Practical Traditional Chinese Medicine] 2007;23(7):434. [Google Scholar]
Yang 2012 {published data only}
- Yang ZW. Clinical observation of treatment of ziyintonglin in 36 cases of women with recurrent urinary tract infections. China Foreign Medical Treatment 2007;31(13):48,50. [Google Scholar]
Yu 2009 {published data only}
- Yu H. Clinical observation of clear heat and tonify kidneys in post menopausal women with RUTIs. Zhongyiyao Linchuang Zazhi [Clinical Journal of Traditional Chinese Medicine] 2009;21(4):306‐8. [Google Scholar]
Zhai 2006 {published data only}
- Zhai XL. Preventative and curative effect of Liu Wei Di Huang Wan in the treatment of post‐menopausal recurrent UTIs. World Science & Technology of TCM & Materia Medica 2006;8(2):99‐101. [Google Scholar]
Zhan 2007 {published data only}
- Zhan YL, Li XY, Wu SX. Clinical observation on effect of compound Shiwei Tablet in treating urinary tract infection. Zhongguo Zhongxiyi Jiehe Zazhi [Chinese Journal of Integrated Traditional and Western Medicine] 2007;27(3):249‐51. [MEDLINE: ] [PubMed] [Google Scholar]
Zhang 1998 {published data only}
- Zhang JH. The primacy of tonifying the Kidneys in the treatment of recurrent UTIs. Beijing Zhongyi [Beijing Journal of Traditional Chinese Medicine] 1998;4:25. [Google Scholar]
Zhang 2005b {published data only}
- Zhang M, Zhang D, Xu Y, Duo X, Zhang W. A clinical study on the treatment of urinary infection with Zishen Tongli Jiaonang. Journal of Traditional Chinese Medicine 2005;25(3):182‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Zhang 2013 {published data only}
- Zhang YZ, Hao YQ, Cji JM. The use of adapted Qing Xin (clear the heart) Lin Zi Yin in the treatment of 40 cases of Qi and Yin deficiency related recurrent UTIs. Heilong Journal of Traditional Chinese Medicine 2013;1:15. [Google Scholar]
Zhou 2007 {published data only}
- Zhou X, Li G. 40 cases using Xiao Chai Hu Tang and Liu Wei Di Huang Tang in the treatment of recurrent UTIs. Shiyong Zhongyi Neike Zazhi [Journal of Practical Traditional Chinese Internal Medicine] 2007;21(7):66. [Google Scholar]
References to studies awaiting assessment
Yin 2011 {published data only}
- Yin M, Zhang H, Xu X, Chen Y. Effects of sanjin tablets on T lymphocyte subsets of peripheral blood of women with recurrent urinary tract infection. Zhongguo Zhongyao Zazhi [China Journal of Chinese Materia Medica] 2011;36(16):2294‐6. [MEDLINE: ] [PubMed] [Google Scholar]
References to ongoing studies
ISRCTN95402719 {published data only}
- Flower A, Lewith G. A randomised, double‐blind, placebo controlled, feasibility study exploring the role of Chinese herbal medicine in the treatment of women with recurrent urinary tract infections. http://www.isrctn.com/ISRCTN95402719 2015.
Additional references
Albert 2004
- Albert X, Huertas I, Pereiró II, Sanfélix J, Gosalbes V, Perrota C. Antibiotics for preventing recurrent urinary tract infection in non‐pregnant women. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD001209.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Aydin 2014
- Aydin A, Ahmed K, Zaman I, Khan MS, Dasgupta PP. Recurrent urinary tract infections in women. International Urogynecology Journal 2014;4(1):1‐10. [DOI] [PubMed] [Google Scholar]
Bensky 2004
- Bensky D, Clavey S, Stoger E. Chinese Herbal Medicine: Materia Medica. 3rd Edition. Vol. 1, Eastland Press, 2004. [ISBN‐13: 978‐0939616428] [Google Scholar]
Butler 2006
- Butler CC, Hillier S, Roberts Z, Dunstan F, Howard A, Palmer S. Antibiotic‐resistant infections in primary care are symptomatic for longer and increase workload: outcomes for patients with E. coli UTIs. British Journal of General Practice 2006;56(530):686‐92. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Car 2003
- Car J, Sheikh A. Recurrent urinary tract infection in women. BMJ 2003;327(7425):1204. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chen 2004
- Chen JK, Chen TT. Chinese medical herbology and pharmacology. 1st Edition. City of Industry: Art of Medicine Press, 2004. [ISBN: 0‐9740635‐0‐9] [Google Scholar]
Christiaens 2002
- Christiaens TC, Meyere M, Verschraegen G, Peersman W, Heytens S, Maeseneer JM. Randomised controlled trial of nitrofurantoin versus placebo in the treatment of uncomplicated urinary tract infection in adult women. British Journal of General Practice 2002;52(482):729‐34. [MEDLINE: ] [PMC free article] [PubMed] [Google Scholar]
Falagas 2008
- Falagas ME, Athanasiou S, Iavazzo C, Tokas T, Antsaklis A. Urinary tract infections after pelvic floor gynecological surgery: prevalence and effect of antimicrobial prophylaxis. A systematic review. International Urogynecology Journal 2008;19(8):1165‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Flower 2011
- Flower A, Lewith G, Little P. Combining rigour with relevance: a novel methodology for testing Chinese herbal medicine. Journal of Ethnopharmacology 2011;134(2):373‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Flower 2014
- Flower A, Bishop FL, Lewith G. How women manage recurrent urinary tract infections: an analysis of postings on a popular web forum. BMC Family Practice 2014;15(1):1‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Foxman 2010
- Foxman B. The epidemiology of urinary tract infection. Nature Reviews Urology 2010;7(12):653‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Franco 2005
- Franco AV. Recurrent urinary tract infections. Best Practice & Research. Clinical Obstetrics & Gynaecology 2005;19(6):861‐73. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Harding 1982
- Harding GK, Ronald AR, Nicolle LE, Thomson MJ, Gray GJ. Long‐term antimicrobial prophylaxis for recurrent urinary tract infection in women. Reviews of Infectious Diseases 1982;4(2):438‐43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hooton 1996
- Hooton TM, Scholes D, Hughes JP, Winter C, Roberts PL, Stapleton AE, et al. A prospective study of risk factors for symptomatic urinary tract infection in young women. New England Journal of Medicine 1996;335(7):468‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Huang 1999
- Huang KC. The Pharmacology of Chinese herbs. 2nd Edition. London: CRC Press, 1999. [Print ISBN: 978‐0‐8493‐1665‐4] [Google Scholar]
Jepson 2012
- Jepson RG, Williams G, Craig JC. Cranberries for preventing urinary tract infections. Cochrane Database of Systematic Reviews 2012, Issue 10. [DOI: 10.1002/14651858.CD001321.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kumarasamy 2010
- Kumarasamy KK, Toleman MA, Walsh TR, Bagaria J, Butt F, Balakrishnan R, et al. Emergence of a new antibiotic resistance mechanism in India, Pakistan, and the UK: a molecular, biological, and epidemiological study. Lancet Infectious Diseases 2010;10(9):597‐602. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kunin 1994
- Kunin CM. Urinary tract infections in females. Clinical Infectious Diseases 1994;18(1):1‐10. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Leydon 2010
- Leydon GM, Turner S, Smith H, Little P. Women’s views about management and cause of urinary tract infection: qualitative interview study. BMJ 2010;340:c279. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Little 2010
- Little P, Merriman R, Turner S, Rumsby K, Warner G, Lowes JA, et al. Presentation, pattern, and natural course of severe symptoms, and role of antibiotics and antibiotic resistance among patients presenting with suspected uncomplicated urinary tract infection in primary care: observational study. BMJ 2010;340:b5633. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Little 2010a
- Little P, Moore MV, Turner S, Rumsby K, Warner G, Lowes JA, et al. Effectiveness of five different approaches in management of urinary tract infection: randomised controlled trial. BMJ 2010;340:c199. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 1987
- Liu P. Care of patients with acute urinary tract infection treated by Chinese herbal drugs. Chung‐Hua Hu Li Tsa Chih [Chinese Journal of Nursing] 1987;22(3):116. [MEDLINE: ] [PubMed] [Google Scholar]
Maciocia 1994
- Maciocia G. The practice of Chinese medicine. 1st Edition. London: Churchill Livingstone, 1994. [Google Scholar]
Mazzulli 2002
- Mazzulli T. Resistance trends in urinary tract pathogens and impact on management. Journal of Urology 2002;168(4 Pt 2):1720‐2. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Milo 2005
- Milo G, Katchman E, Paul M, Christiaens T, Baerheim A, Leibovici L. Duration of antibacterial treatment for uncomplicated urinary tract infection in women. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD004682.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
National Guidelines 1993
- Committee. National Guidelines on Clinical Research of Novel Traditional Chinese Herbs for urinary tract infections. In: Ministry of Health of the People's Republic of China, editor(s). National Guidelines on Clinical Research of Novel Traditional Chinese Herbs. 1. Vol. 1, Beijing: Ministry of Health of the People's Republic of China, 1993:153. [Google Scholar]
Peng 2010
- Peng MM, Fang Y, Hu W, Huang Q. The pharmacological activities of Compound Salvia Plebeia Granules on treating urinary tract infection. Journal of Ethnopharmacology 2010;129(1):59‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Renard 2014
- Renard J, Ballarini S, Mascarenhas T, Zahran M, Quimper E, Choucair J, et al. Recurrent lower urinary tract infections have a detrimental effect on patient quality of life: a prospective, observational study. Infectious Diseases & Therapy 2014;4(1):1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sanford 1975
- Sanford JP. Urinary tract symptoms and infections. Annual Review of Medicine 1975;26:485‐98. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Silverman 2013
- Silverman JA, Schreiber HL, Hooton TM, Hultgren SJ. From physiology to pharmacy: developments in the pathogenesis and treatment of recurrent urinary tract infections. Current Urology Reports 2013;14(5):448‐56. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stapleton 1999
- Stapleton A. Host factors in susceptibility to urinary tract infections. Advances in Experimental Medicine & Biology 1999;462:351‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stavri 2007
- Stavri M, Piddock LJ, Gibbons S. Bacterial efflux pump inhibitors from natural sources. Journal of Antimicrobial Chemotherapy 2007;59(6):1247‐60. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Tong 2011a
- Tong QY, Wu QC, Zhao DK, Liu YR, Cao MM, Zhang LP, et al. Effects of Chinese herbs on the hemagglutination and adhesion of Escherichia coli strain in vitro. African Journal of Traditional Complementary & Alternative Medicine 2011;8(1):82‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Xu 1989
- Xu JF, Liu BH, Cui XM, Sun SL, Yu PJ, Gao RJ, et al. Observation on therapeutic effect of clearing heat and dredging stranguria in 103 cases of urinary tract infection. Journal of Traditional Chinese Medicine 1989;9(3):163‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Yan 2008
- Yan D, Jin C, Xiao XH, Dong XP. Antimicrobial properties of berberines alkaloids in Coptis chinensis Franch by microcalorimetry. Journal of Biochemical & Biophysical Methods 2008;70(6):845–9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Zhang 2005
- Zhang M, Zhang D, Xu Y, Duo X, Zhang W. A clinical study on the treatment of urinary infection with Zishen Tongli Jiaonang. Journal of Traditional Chinese Medicine 2005;25(3):182‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Zhu 1998
- Zhu YP. Chinese Materia Medica‐Chemistry, pharmacology and applications. Amsterdam: Harwood, 1998. [ISBN: 9789057022852] [Google Scholar]
References to other published versions of this review
Flower 2013
- Flower A, Lewith G, Liu JP, Li Q. Chinese herbal medicine for treating recurrent urinary tract infections in women. Cochrane Database of Systematic Reviews 2013, Issue 3. [DOI: 10.1002/14651858.CD010446] [DOI] [PMC free article] [PubMed] [Google Scholar]


