Abstract
Background
Scalpels or electrosurgery can be used to make abdominal incisions. The potential benefits of electrosurgery may include reduced blood loss, dry and rapid separation of tissue, and reduced risk of cutting injury to surgeons. Postsurgery risks possibly associated with electrosurgery may include poor wound healing and complications such as surgical site infection.
Objectives
To assess the effects of electrosurgery compared with scalpel for major abdominal incisions.
Search methods
The first version of this review included studies published up to February 2012. In October 2016, for this first update, we searched the Cochrane Wounds Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL), Ovid MEDLINE (including In‐Process & Other Non‐Indexed Citations), Ovid Embase, EBSCO CINAHL Plus, and the registry for ongoing trials (www.clinicaltrials.gov). We did not apply date or language restrictions.
Selection criteria
Studies considered in this analysis were randomised controlled trials (RCTs) that compared electrosurgery to scalpel for creating abdominal incisions during major open abdominal surgery. Incisions could be any orientation (vertical, oblique, or transverse) and surgical setting (elective or emergency). Electrosurgical incisions were made through major layers of the abdominal wall, including subcutaneous tissue and the musculoaponeurosis (a sheet of connective tissue that attaches muscles), regardless of the technique used to incise the skin and peritoneum. Scalpel incisions were made through major layers of abdominal wall including skin, subcutaneous tissue, and musculoaponeurosis, regardless of the technique used to incise the abdominal peritoneum. Primary outcomes analysed were wound infection, time to wound healing, and wound dehiscence. Secondary outcomes were postoperative pain, wound incision time, wound‐related blood loss, and adhesion or scar formation.
Data collection and analysis
Two review authors independently carried out study selection, data extraction, and risk of bias assessment. When necessary, we contacted trial authors for missing data. We calculated risk ratios (RR) and 95% confidence intervals (CI) for dichotomous data, and mean differences (MD) and 95% CI for continuous data.
Main results
The updated search found seven additional RCTs making a total of 16 included studies (2769 participants). All studies compared electrosurgery to scalpel and were considered in one comparison. Eleven studies, analysing 2178 participants, reported on wound infection. There was no clear difference in wound infections between electrosurgery and scalpel (7.7% for electrosurgery versus 7.4% for scalpel; RR 1.07, 95% CI 0.74 to 1.54; low‐certainty evidence downgraded for risk of bias and serious imprecision). None of the included studies reported time to wound healing.
It is uncertain whether electrosurgery decreases wound dehiscence compared to scalpel (2.7% for electrosurgery versus 2.4% for scalpel; RR 1.21, 95% CI 0.58 to 2.50; 1064 participants; 6 studies; very low‐certainty evidence downgraded for risk of bias and very serious imprecision).
There was no clinically important difference in incision time between electrosurgery and scalpel (MD ‐45.74 seconds, 95% CI ‐88.41 to ‐3.07; 325 participants; 4 studies; moderate‐certainty evidence downgraded for serious imprecision). There was no clear difference in incision time per wound area between electrosurgery and scalpel (MD ‐0.58 seconds/cm2, 95% CI ‐1.26 to 0.09; 282 participants; 3 studies; low‐certainty evidence downgraded for very serious imprecision).
There was no clinically important difference in mean blood loss between electrosurgery and scalpel (MD ‐20.10 mL, 95% CI ‐28.16 to ‐12.05; 241 participants; 3 studies; moderate‐certainty evidence downgraded for serious imprecision). Two studies reported on mean wound‐related blood loss per wound area; however, we were unable to pool the studies due to considerable heterogeneity. It was uncertain whether electrosurgery decreased wound‐related blood loss per wound area. We could not reach a conclusion on the effects of the two interventions on pain and appearance of scars for various reasons such as small number of studies, insufficient data, the presence of conflicting data, and different measurement methods.
Authors' conclusions
The certainty of evidence was moderate to very low due to risk of bias and imprecise results. Low‐certainty evidence shows no clear difference in wound infection between the scalpel and electrosurgery. There is a need for more research to determine the relative effectiveness of scalpel compared with electrosurgery for major abdominal incisions.
Plain language summary
Scalpel versus electrosurgery for surgical operations on the abdomen
Review question
We reviewed the evidence about the effect of using either a scalpel (knife) or electrosurgery in surgical operations on the abdomen.
Background
During abdominal surgery, surgeons need to cut through several layers of abdominal wall tissue before reaching the target operation site. To do this, surgeons can use either a sharp‐bladed scalpel or an electrosurgical device that burns through tissue using a precise high‐frequency current (known as electrosurgery). It is thought that, compared to using a scalpel, electrosurgery may result in less blood loss, more rapid tissue separation, and a lower risk of surgeons cutting themselves. We wanted to find out about the benefits and risks of these two techniques, and to compare them in terms of safety and other measures such as risk of infection and pain.
Study characteristics
In October 2016, we searched for randomised controlled trials (RCTs; clinical studies where people are randomly put into one of two or more treatment groups) comparing scalpel‐based surgery with electrosurgery for abdominal incisions (cuts). We found seven new trials for this update, allowing us to include a total of 16 RCTs involving 2769 participants. The majority of participants were adults, although one trial included children over the age of 15 years. There were slightly more female participants than male as some trials looked exclusively at caesarean sections (an operation to deliver a baby through a cut made in the abdomen and womb) and gynaecological (female reproductive system) surgery.
Key results
There was no clear difference between scalpel and electrosurgery in the number of people whose wounds became infected. It is uncertain whether electrosurgery prevents wound breakdown (a complication that involves the breaking open of the surgical incision along the stitches/staples) following surgery, while the difference in blood loss and time required for incision between electrosurgery and scalpel was not clinically important. There was not enough information available to determine how electrosurgery compared with scalpel‐based surgery in relation to time required for wounds to heal, amount of pain during healing, and appearance of scars. More studies need to be conducted before conclusions can be drawn as to whether one method is better for pain after an operation and time to wound healing following abdominal surgery.
Quality of the evidence
We judged the certainty of evidence to be moderate to very low for all outcomes. This is because the studies were often small with a low number of events and, in many cases, were not reported in a way that meant we could be sure they had been conducted robustly. The certainty of the evidence means that we cannot make conclusive statements and better quality research is needed to form stronger conclusions.
This plain language summary is up to date as of October 2016.
Summary of findings
Summary of findings for the main comparison. Scalpel versus electrosurgery for major abdominal incisions.
| Scalpel versus electrosurgery for major abdominal incisions | ||||||
|
Participant or population: people undergoing surgery involving major abdominal incisions Setting: university hospital, general hospital, and cancer hospital Intervention: electrosurgery Comparison: scalpel | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with scalpel | Risk with electrosurgery | |||||
| Wound infection | 79 per 1000 (55 to 114) | 74 per 1000 | RR 1.07 (0.74 to 1.54) | 2178 (11 RCTs) | ⊕⊕⊝⊝ Low1 | No clear difference in risk of wound infection between groups. The 95% CI crossed the line of no effect spanning estimates consistent with possible benefit and possible harm. |
| Time to wound healing (days) | Not reported | |||||
| Wound dehiscence | 27 per 1000 (14 to 55) | 24 per 1000 | RR 1.21, 95% CI 0.58 to 2.50 | 1064 (6 RCTs) | ⊕⊝⊝⊝ Very low2 | Uncertain whether electrosurgery decreased wound dehiscence between wounds compared with scalpel. The 95% CI crossed the line of no effect spanning estimates consistent with possible benefit and possible harm. |
| Postoperative pain | ‐ | ‐ | ‐ | 949 (9 RCTs) | ‐ | Postoperative pain reported as pain score (mostly assessed using VAS) in 9 RCTs, based on the need for analgesia in 6 RCTs and PEFR in 2 RCTs. Data pooling not feasible, therefore, no further analysis carried out. Therefore, insufficient evidence to determine the effect of scalpel and electrosurgery on postoperative pain. |
| Wound incision time (seconds) | The mean wound incision time ranged from 126 seconds to 540 seconds | The mean wound incision time in the electrosurgery group was 45.74 seconds fewer (88.41 fewer to 3.07 fewer) | ‐ | 325 (4 RCTs) | ⊕⊕⊕⊝ Moderate3 | 3 RCTs (282 participants) reported wound incision time per wound area. The mean wound incision time per wound area in the electrosurgery group was 0.58 seconds/cm2 less (1.26 less to 0.09 more) and the quality of evidence was low. No clinically important difference in wound incision time between electrosurgery and scalpel. |
| Wound‐related blood loss (mL) | The mean wound‐related blood loss ranged from 23.4 mL to 105.5 mL | The mean wound‐related blood loss in the electrosurgery group was 20.10 mL less (28.16 less to 12.05 less) | ‐ | 241 (3 RCTs) | ⊕⊕⊕⊝ Moderate4 | 2 RCTs (182 participants) reported wound‐related blood loss. Due to considerable heterogeneity, we were unable to pool the studies and quality of evidence for the outcome in both studies was low. No clinically important difference in wound‐related blood loss between electrosurgery and scalpel. |
| Adhesion or scar formation | ‐ | ‐ | ‐ | 84 (1 RCT) | ‐ | 1 RCT reported scar formation; however, no further analysis was carried out. |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; PEFR: peak expiratory flow rate; RCT: randomised controlled trial; RR: risk ratio; VAS: visual analogue scale. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Downgraded two levels: serious limitation due to lack of information on randomisation and allocation concealment in three studies contributing more than 50% to the analysis; serious imprecision as 95% CIs around the estimate were wide ranging including the probability of a reduction as well as an increase in wound infection.
2 Downgraded three levels: serious limitation due to attrition in one of two studies contributing more than 50% to the analysis; very serious due to inadequate number of events and imprecision as 95% CIs around the estimate are wide ranging including the probability of a reduction as well as an increase in wound infection.
3 Downgraded one level: serious imprecision as studies were small with inadequate sample size.
4 Downgraded one level: serious imprecision as studies were few with small sample size.
Background
This review is an update of a previously published version in the Cochrane Library (2012, Issue 6) on 'Scalpel versus electrosurgery for abdominal incisions'.
Description of the condition
Millions of surgical procedures on the abdomen are conducted around the world each year. Most of these are considered 'major abdominal surgery', which require an incision to be made through abdominal wall layers including the skin, subcutaneous tissue, fascia (aponeurosis), muscle, and peritoneum to reach the abdominal cavity. Conventionally, scalpels have been used to make such surgical incisions by manually cutting through tissue using a sharp blade. However, since its introduction in the early part of the 20th century, electrosurgery has been used as an alternative tool for creating incisions (Anderson 2015). When creating a wound, key aims are to have a precise incision while minimising blood loss and the risk of surgical site infection (SSI). Based on the nature of the underlying conditions requiring surgery and degree of contamination, operative wounds are classified, with a progressive increase in risk of wound infection, into clean (class I), clean‐contaminated (class II), contaminated (class III), and dirty or infected (class IV).
Description of the intervention
Electrosurgery involves manipulation of electrons through living tissue using an alternating current density sufficient to create heat within tissue cells to destroy them (Soderstrom 2003). Two different surgical effects can be achieved with electrosurgery, namely cutting (of tissue) and coagulating. In the cutting mode, a continuous current rapidly produces extreme heat causing intracellular water to boil and cells to explode into steam (vaporisation). By moving the electrode quickly, more cells vaporise and the tissue is divided with minimal devitalised or charred tissue left along the margin of the cut surface. Thermal damage is minimal since heat evaporates as steam and is not conducted through the cut tissues, which would dry out the adjacent cells. In the coagulating mode, short bursts of electrical current are applied with a pause between each burst. As a result, the heat produced in the cells dries up the tissue but is not intense enough to evaporate intracellular water. The coagulating mode results in a greater degree of thermal damage and necrosis of adjacent tissues (Soderstrom 2003). Generally, if a surgeon chooses to make an incision using electrosurgery, it is recommended that the cutting mode is selected with a small tip or needle electrode that is activated just prior to making contact with the target tissue (Soderstrom 2003). However, many surgeons avoid using electrosurgery to divide the skin because of the concern of excessive scarring. More recently, a newer type of electrosurgery has been introduced which uses pulsed radiofrequency to produce a plasma‐mediated discharge along the exposed rim of an insulated blade. This mechanism results in effective cutting with a blade, which remains near body temperature. Studies comparing the radiofrequency approach with conventional electrosurgery and scalpels for creating incisions in animal models have reported favourably for the radiofrequency approach in terms of wound tensile strength, scar formation, and minimising blood loss and tissue damage (Loh 2009; Chang 2011).
How the intervention might work
The potential benefits of electrosurgery have been suggested to include reduced blood loss, dry and rapid separation of the tissue, and a possible decrease in the risk of accidental injury caused by the scalpel blade to operative personnel (Soderstrom 2003). However, there are concerns about the impact of electrosurgery on wound infection, wound healing, scarring, and adhesion formation, which have limited the use of electrosurgery for surgical wound creation. Data from animal studies have consistently shown that wounds created by electrosurgery (especially using the coagulation current) have more extensive tissue necrosis and inflammatory response, and a significant reduction in tensile strength during healing (Rappaport 1990). In addition, the use of electrosurgery may be associated with increased adhesion formation (i.e. scar formation between two smooth gliding surfaces) between the incision and abdominal viscera due to an out‐flowing of blood and formation of fibrin clots in the more traumatised animal tissue (Kumagai 1991; Madden 1970; Rappaport 1990). In rat and guinea pig models, necrotic tissue is an important contributory factor to the increased susceptibility of electrosurgical incisions to infection, particularly in contaminated wounds (Keenan 1984; Kumagai 1991; Madden 1970). Additionally, the use of electrosurgery has been related to an increase in the incidence of bacteraemia and death (Kumagai 1991). However, more recently, these concerns have been lowered with new‐generation electrosurgical instruments.
However, unlike animal studies which are executed under controlled conditions, there are many biological and environmental contributing factors that could affect outcomes from the use of electrosurgery in the clinical setting, although data are limited. One 10‐year prospective observational study of 62,939 surgical wounds suggested that the risk of infection for wounds resulting from clean surgery (i.e. non‐infective operative wounds with no inflammation, and none of the respiratory, alimentary, genitourinary tract, or the oropharyngeal cavity is entered) was similar for people with electrosurgery‐ and scalpel‐generated surgical incisions (Cruse 1980), although the results to support this conclusion were not presented.
Why it is important to do this review
Several prospective randomised trials have examined the effects of scalpel versus electrosurgery for wound creation on the abdomen, which have necessitated an update of the original version of this Cochrane Review which was published in 2012 (Charoenkwan 2012). We aimed to identify and assess relevant studies systematically to present a comprehensive summary of current evidence regarding the relative effectiveness of electrosurgery for making surgical incisions compared with scalpels. Importantly, this synthesis also involves assessment of evidence quality using the GRADE process (GRADE 2013). Such an evidence synthesis is important in contributing to informed decision‐making around the use of electrosurgery in practice. Current National Institute of Health and Care Excellence (NICE) guidelines for the prevention of SSI do not recommend the use of electrosurgery; however, the recommendation was based on data available approximately 10 years ago (NICE 2008).
Objectives
To assess the effects of electrosurgery compared with scalpel for major abdominal incisions.
Methods
Criteria for considering studies for this review
Types of studies
This review included randomised controlled trials (RCTs). We excluded quasi‐randomised controlled studies.
Types of participants
People undergoing major open abdominal surgery for any reason, regardless of the orientation of the incision (vertical, oblique, or transverse) and surgical setting (elective or emergency). 'Major open abdominal surgery' is defined as any abdominal surgical procedures that require an incision to be made through abdominal wall layers including the skin, subcutaneous tissue, fascia, muscle, and peritoneum to reach the abdominal cavity. For herniorrhaphy (surgical repair of a hernia; see Appendix 1 for glossary of terms), we considered wound creation to be complete if the skin, subcutaneous tissue, and external/internal oblique aponeurosis had been divided.
Types of interventions
Main intervention: wound creation using electrosurgery
The use of electrosurgery to incise major layers of abdominal wall including subcutaneous tissue and musculoaponeurosis (see Appendix 1 for glossary of terms), regardless of the techniques used to incise the abdominal skin and peritoneum.
Comparison intervention: wound creation using a scalpel
The use of a scalpel to incise all major layers of abdominal wall including skin, subcutaneous tissue, and musculoaponeurosis, regardless of the techniques used to incise the abdominal skin and peritoneum.
Types of outcome measures
Primary outcomes
Wound infection.
Time to wound healing.
Wound dehiscence.
Secondary outcomes
Postoperative pain.
Wound incision time.
Wound‐related blood loss.
Adhesion or scar formation.
Search methods for identification of studies
Electronic searches
In October 2016, we updated the searches of the following electronic databases to find reports of relevant RCTs:
Cochrane Wounds Specialised Register (searched 10 October 2016);
Cochrane Central Register of Controlled Trials (CENTRAL) (the Cochrane Library 2016, Issue 9) (searched 10 October 2016);
Ovid MEDLINE including In‐Process & Other Non‐Indexed Citations (1946 to 10 October 2016);
Ovid Embase (1974 to 10 October 2016);
EBSCO CINAHL Plus (searched 10 October 2016).
The search strategies for the Cochrane Wounds Specialised Register, CENTRAL, Ovid MEDLINE, Ovid Embase and EBSCO CINAHL Plus can be found in Appendix 2. We combined the Ovid MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision) (Lefebvre 2011). We combined the Embase search with the Ovid Embase filter developed by the UK Cochrane Centre (Lefebvre 2011). We combined the CINAHL Plus searches with the trial filters developed by the Scottish Intercollegiate Guidelines Network (SIGN 2015). Details of the search strategies used for the previous version of the review are given in Charoenkwan 2012. We also searched the following trials registries:
ClinicalTrials.gov (www.clinicaltrials.gov); a service of the US National Institutes of Health (searched 28 June 2016)
Searching other resources
We searched the citation lists of relevant publications, systematic reviews, review articles, and included studies. We contacted experts, specialists in the field, and the authors of relevant publications for information about possible unpublished studies. For publications in non‐English languages, we obtained a translation of the methods and results if the content of the abstract was thought to be relevant.
Data collection and analysis
We carried out data collection and analysis according to methods stated in the published protocol (Charoenkwan 2006), which were based on the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Selection of studies
Any two of three review authors (KC, KR, and ZIE) independently undertook study selection and screened the titles and abstracts of articles found in the search while the third review author arbitrated if there were disagreements. We discarded studies that were clearly ineligible. One review author (KC) obtained copies of the full‐text articles that appeared to be eligible. Both review authors independently assessed whether the studies met the inclusion criteria and resolved discrepancies by discussion. We contacted authors for additional information to enable decisions regarding eligibility. Excluded studies are described in the Characteristics of excluded studies table.
Data extraction and management
We extracted and summarised details of the included studies on the data extraction form. One review author (ZIE) extracted data and a second review author (KC) checked these for accuracy. We attempted to contact trial authors to obtain missing information where necessary.
We extracted the following data:
names of study authors;
year of publication;
country where study was performed;
study design;
method of randomisation;
unit of randomisation;
overall sample size and methods used to estimate statistical power;
outcome measures;
setting of operation;
participant selection criteria;
details of interventions for each study group;
number of participants in each study group;
baseline characteristics of participants in each study group;
statistical methods used for data analysis;
number of and reason for withdrawal in each study group;
outcomes
type of wound (clean, clean‐contaminated, contaminated or dirty).
Assessment of risk of bias in included studies
Methodological quality of the included trials was assessed using the Cochrane tool for assessing risk of bias (Higgins 2011). The tool addresses six specific domains of potential bias: sequence generation, allocation concealment, blinding, incomplete outcome data, selective outcome reporting, and 'other issues'. Studies were judged to be at high, low, or unclear risk of bias for each of the domains. The methodological quality assessment is presented in the 'Risk of bias' section of the Characteristics of included studies table and summarised graphically.
Measures of treatment effect
We displayed dichotomous data for each study as a risk ratio (RR) with a 95% confidence interval (CI). For continuous data, we expressed results from each study as a mean difference (MD) with a 95% CI. We planned to express time‐to‐event data (e.g. time‐to‐complete wound healing) as hazard ratios (HR) (with their 95% CI), in accordance with the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). In future updates, if such studies do not report an HR, we plan to estimate this using other reported data, such as the numbers of events, with application of available statistical methods (Parmar 1998; Tierney 2007; Wang 2013). In the absence of these measures, if there are any studies in which all wounds healed, we will consider the mean or median time to healing without survival analysis as a valid outcome (i.e. if the trial authors regarded time to healing as a continuous measure because there was no censoring). In this review, we were unable to report time to wound healing as none of the studies met these criteria.
Unit of analysis issues
Studies included in the primary analysis consisted of simple parallel‐group designs where participants were individually randomised to one of two intervention groups, and a single measurement from each participant collected and analysed. Split body studies and studies where the unit of analysis was the wound would have been analysed with parallel group studies or alone had sufficient data been reported.
Dealing with missing data
We analysed the data on an intention‐to‐treat basis. We contacted investigators from the original trials to request missing data. Where we were unable to obtain missing data from trial authors, we outlined results in additional tables and reported them as a narrative summary.
Assessment of heterogeneity
Assessment of heterogeneity comprised the initial assessment of clinical and methodological heterogeneity and the appropriateness of combining study results: that is, the degree to which the included studies varied in terms of participant, intervention, outcome, and characteristics such as length of follow‐up. This assessment of clinical and methodological heterogeneity was supplemented by information regarding statistical heterogeneity of the results. Statistical heterogeneity was assessed by visual inspection of the overlap of CIs on the forest plots alongside Chi2 tests and quantified using the I2 statistic. I2 statistic thresholds were interpreted according to Higgins 2011 as follows: 0% to 40% (may not be important), 30% to 60% (moderate heterogeneity), 50% to 90% (substantial heterogeneity), and 75% to 100% (considerable heterogeneity).
Assessment of reporting biases
Most reporting biases were avoided by conducting a broad literature search and adding search filters on year of publication or language. For studies where the protocols were published at www.clinicaltrials.gov, we examined consistency between planned and the reported outcomes. We used a funnel plot to assess publication bias.
Data synthesis
We performed statistical analyses in accordance with Cochrane guidelines using Review Manager 5 software (RevMan 2014). For dichotomous data (e.g. proportion of wounds developing infection), we expressed results for each study as RR with a 95% CI. For continuous data, we expressed results from each study as MD with 95% CI, and combined for meta‐analysis where appropriate. Since meta‐analytic methods for continuous data assume that the underlying distribution of the measurements is normal, in instances where data were clearly skewed, and the results were reported in the publication as 'median' and 'range' with non‐parametric tests of significance, we reported the results in narrative form. For a study which included participants undergoing thoracic surgery as well as those undergoing abdominal surgery, all participants were included in the analysis. For a study which included two clinically different abdominal procedures, the dichotomous data were included in meta‐analyses as a single study, while continuous data were treated and included in meta‐analyses as data from two separate studies. In terms of meta‐analytical approach, when meta‐analysis was considered viable in the presence of clinical heterogeneity (review author judgement) or there was evidence of statistical heterogeneity, or both, we used a random‐effects model. We considered a fixed‐effect approach only when clinical heterogeneity was thought to be minimal and statistical heterogeneity was estimated as non‐statistically significant for the Chi2 value and 0% for the I2 assessment (Kontopantelis 2013). This approach recognises that statistical assessments can miss potentially important between‐study heterogeneity in small samples hence the preference for the more conservative random‐effects model (Kontopantelis 2013). We decided against pooling studies with considerable heterogeneity (75% or more).
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analysis based on cutting versus coagulating modes of electrical current used in the electrosurgery group since different mechanisms of tissue separation may have impact on outcomes. However, this was not possible as the interventions were not sufficiently homogenous nor well reported to carry out this analysis. To evaluate the effect of wound contamination on the outcomes, we carried out a post hoc analysis on (class I vs. class II vs. class III vs. class IV wounds).
Sensitivity analysis
We intended to perform a sensitivity analysis to assess whether the findings of the review were robust to the decisions made during the review process. We would have excluded studies at high or unclear risk of bias from the analyses to assess whether this affected the findings of the review. However, this was not possible as none of the included studies were at low risk of bias.
'Summary of findings' tables
Two review authors (ZIE and KC) assessed the certainty of the evidence generated from the review according to GRADE methodology (GRADE 2013). We presented the main results in a 'Summary of findings' table produced using GRADEpro software. The certainty of the evidence was initially considered to be high because of the study design (RCT). We subsequently downgraded this depending on whether there were study limitations (which we judged based on the risk of bias in studies which contributed at least 50% of the weight in the analysis), whether the results were inconsistent, imprecise, the evidence was indirect or there was publication bias. The 'Summary of findings' table presents the following outcomes:
wound infection
time to wound healing
wound dehiscence
postoperative pain
wound incision time
wound‐related blood loss
adhesion or scar formation.
Results
Description of studies
Results of the search
The summary of the search results is presented in a PRISMA study flow diagram (Figure 1). At the time of the original review, we included nine RCTs that met the inclusion criteria (Argerich 2005; Dixon 1990; Groot 1994; Hemsell 1993; Hussain 1988; Johnson 1990; Kearns 2001; Pearlman 1991; Telfer 1993), and excluded seven studies (Chrysos 2005; Duxbury 2003; Franchi 2001; Ji 2006; Keel 2002; Miller 1988; Porter 1998). For this updated review, we identified 12 additional potentially eligible studies (Aird 2015; Elbohoty 2015; Eren 2010; Husnain 2006; Marsh 2015; Prakash 2015; Rongetti 2014; Shamim 2009; Shivagouda 2010; Siraj 2011; Stupart 2016; Upadhyay 2013). Seven studies met the inclusion criteria and were included in the updated analysis (Elbohoty 2015; Eren 2010; Marsh 2015; Prakash 2015; Rongetti 2014; Shivagouda 2010; Siraj 2011; Characteristics of included studies table). Three studies were excluded from the new search for failing to meet the inclusion criteria (Aird 2015; Shamim 2009; Stupart 2016; Characteristics of excluded studies table). Two studies are still awaiting further assessment (Husnain 2006; Upadhyay 2013; Characteristics of studies awaiting classification table).
1.
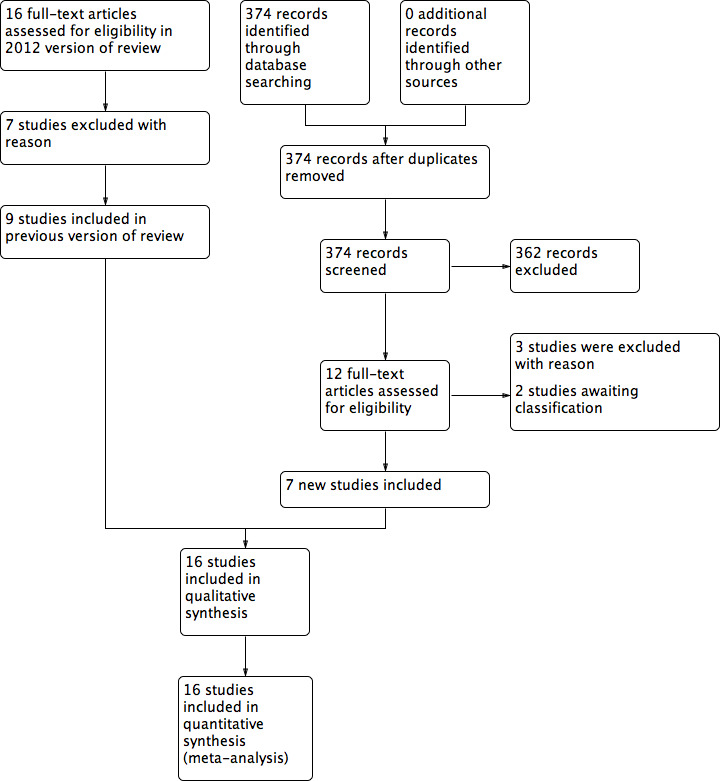
Study flow diagram of review update.
Included studies
Participants
A total of 2769 people participated in the 16 included studies. The number of participants within each study ranged from 60 (Shivagouda 2010) to 492 (Groot 1994). All studies were conducted in a single hospital setting except for Pearlman 1991, which took place at two university‐affiliated institutions. The countries represented in these studies were the UK (Dixon 1990; Johnson 1990; Marsh 2015; Telfer 1993), Brazil (Argerich 2005; Rongetti 2014), India (Prakash 2015; Shivagouda 2010), USA (Hemsell 1993; Pearlman 1991), Canada (Groot 1994), Egypt (Elbohoty 2015), Ireland (Kearns 2001), Pakistan (Siraj 2011), Saudi Arabia (Hussain 1988), and Turkey (Eren 2010).
Surgical indications and incisions varied widely among studies. Four studies were conducted in participants receiving abdominal incisions for any indication (Argerich 2005; Johnson 1990; Kearns 2001; Telfer 1993). Siraj 2011 examined participants who underwent elective midline laparotomy. Eren 2010 included participants undergoing abdominal surgery for various gastrointestinal malignancies through midline laparotomy incisions. Prakash 2015 studied participants who had a midline abdominal incision for emergency (mostly for hollow viscus perforation) and elective (most commonly for stomach cancer) abdominal surgery. One study included participants having either abdominal or thoracic operations (Groot 1994). Two studies consisted of people undergoing cholecystectomy (surgical removal of the gall bladder) (Hussain 1988; Pearlman 1991). Dixon 1990 included participants undergoing either cholecystectomy or inguinal herniorrhaphy. Shivagouda 2010 included only participants receiving inguinal herniorrhaphy. Hemsell 1993 included participants scheduled for abdominal hysterectomy for benign diseases. Rongetti 2014 examined people who underwent elective abdominal gynaecological surgery via laparotomy for diagnosis and curative or palliative cancer treatment. Elbohoty 2015 examined women with a history of one previous caesarean section who underwent planned repeat caesarean section through an existing transverse abdominal incision. Marsh 2015 considered participants who had full abdominoplasty surgery.
Four studies reported the number of participants in different levels of wound classification (class I to IV) (Argerich 2005; Groot 1994; Johnson 1990; Rongetti 2014), and three studies specified the incidences of wound infection in each level for the two study groups (Argerich 2005; Groot 1994; Johnson 1990). Studies that did not specifically report wound classification were not taken into account regardless of the nature of the procedures in order to exclude the possibility of unexpected contamination leading to misclassification.
Interventions
Electrosurgery group
Ten studies set the electrosurgical instruments in cutting mode to incise the major layers of the abdominal wall including subcutaneous tissue, muscle, and fascia (Dixon 1990; Elbohoty 2015; Hussain 1988; Johnson 1990; Kearns 2001; Pearlman 1991; Prakash 2015; Shivagouda 2010; Siraj 2011; Telfer 1993). In Hemsell 1993, although subcutaneous tissues were opened using the pure cut mode, the fascia was incised with the scalpel in all participants regardless of allocation. Four studies used coagulating mode electrosurgery instead of the cutting mode (Argerich 2005; Eren 2010; Groot 1994; Marsh 2015). In Rongetti 2014, while the subcutaneous tissue was opened to the aponeurosis with electrocoagulation, the aponeurosis and peritoneum were incised using the 'cut' mode electrosurgery.
Nine studies incised the skin by scalpel (Argerich 2005; Dixon 1990; Elbohoty 2015; Groot 1994; Hemsell 1993; Hussain 1988; Pearlman 1991; Rongetti 2014; Telfer 1993), and six studies by cutting‐mode electrosurgery (Eren 2010; Johnson 1990; Kearns 2001; Prakash 2015; Shivagouda 2010; Siraj 2011). One study did not specify the instrument used for making skin incision (Marsh 2015).
Scalpel group
For the scalpel group, a scalpel was used to create an incision through all layers in all except for two studies (Johnson 1990; Rongetti 2014). In Johnson 1990, some of the participants who required muscle cutting incision had their muscle cut using electrosurgery. In Rongetti 2014, the skin and subcutaneous tissue were divided by using scalpel to the aponeurosis. However, the aponeurosis and peritoneum were incised using the electrosurgery in 'cut' mode.
Although the technique of haemostasis and wound closure varied among studies, the same technique was used for both study groups in all trials except Elbohoty 2015 and Pearlman 1991. In those two trials, bleeding points were controlled with electrosurgery in the electrosurgery group and with ligature in the scalpel group.
Outcomes
Eleven trials reported wound infection (Argerich 2005; Elbohoty 2015; Eren 2010; Groot 1994; Hemsell 1993; Johnson 1990; Kearns 2001; Marsh 2015; Prakash 2015; Rongetti 2014; Siraj 2011). Six trials reported wound dehiscence (Dixon 1990; Elbohoty 2015; Hemsell 1993; Johnson 1990; Kearns 2001; Rongetti 2014) (see Appendix 1 for glossary of terms). The frequency of wound dehiscence reported in this review represented both total dehiscence (deep; all layers including fascia) and partial dehiscence (superficial; subcutaneous tissue only).
Seven studies reported the time taken to make the wound incision (Dixon 1990; Elbohoty 2015; Johnson 1990; Kearns 2001; Pearlman 1991; Prakash 2015; Telfer 1993). Four trials reported wound incision time as means (Dixon 1990; Kearns 2001; Pearlman 1991; Prakash 2015), and three trials as medians (Elbohoty 2015; Johnson 1990; Telfer 1993). Three trials reported mean wound incision time per wound area (seconds/cm2) (Kearns 2001; Prakash 2015; Siraj 2011).
Six studies reported wound‐related blood loss (Kearns 2001; Pearlman 1991; Prakash 2015 as means; Elbohoty 2015; Telfer 1993 as medians; Prakash 2015; Siraj 2011 as mean wound‐related blood loss per wound area). Regarding measurement of wound‐related blood loss, Elbohoty 2015, Kearns 2001, and Siraj 2011 weighed swabs used exclusively without using suction while making the incision. In Pearlman 1991, once the peritoneum was reached, the anaesthesiologist recorded incision blood loss. Telfer 1993 measured blood loss by weighing swabs using a standardised technique.
Eight studies assessed postoperative pain at different time intervals and used a variety of methods (Dixon 1990; Hussain 1988; Kearns 2001; Pearlman 1991; Prakash 2015; Shivagouda 2010; Siraj 2011; Telfer 1993). Apart from Dixon 1990, Hussain 1988, and Shivagouda 2010, all pain measurements were performed daily by using a visual analogue scale, with '0' denoting no pain and '10' denoting the most extreme pain, for the first three to five days after surgery, and were reported either as means (Kearns 2001; Siraj 2011), medians (Prakash 2015; Telfer 1993), or a three‐day sum of the scores (Pearlman 1991). Telfer 1993 also assessed peak expiratory flow rate (PEFR). Dixon 1990 assessed pain score by using a Likert scale. Hussain 1988 used a visual analogue scale to assess pain every four hours during the first 24 hours after surgery, along with the measurement of PEFR and requirement of morphine. Shivagouda 2010 employed a visual analogue scale to evaluate pain at six, 12, and 24 hours after surgery and mean dosage of analgesic requirement.
Six studies recorded analgesia requirement (Elbohoty 2015; Hussain 1988; Kearns 2001; Pearlman 1991; Shivagouda 2010; Telfer 1993).
One study reported scar formation (Dixon 1990).
None of the included studies reported time to wound healing.
Excluded studies
We excluded three studies from this updated review (Aird 2015; Shamim 2009; Stupart 2016). In Aird 2015 and Stupart 2016, the inclusion criteria with regard to comparison intervention were not met. The subcutaneous and fascial layers in all participants assigned to the scalpel group were divided using electrosurgery. Shamim 2009 was a quasi‐randomised trial. Seven studies excluded in the previous review were non‐randomised (Chrysos 2005; Franchi 2001), did not involve abdominal wound creation (Duxbury 2003; Miller 1988; Porter 1998), and compared different scalpel interventions (Ji 2006; Keel 2002).
Risk of bias in included studies
The methodological quality of the trials was assessed using the Cochrane tool and summarised in the Characteristics of included studies, the 'Risk of bias' summary (Figure 2), and the 'Risk of bias' graph (Figure 3). Incomplete reporting on various domains was evident across studies.
2.
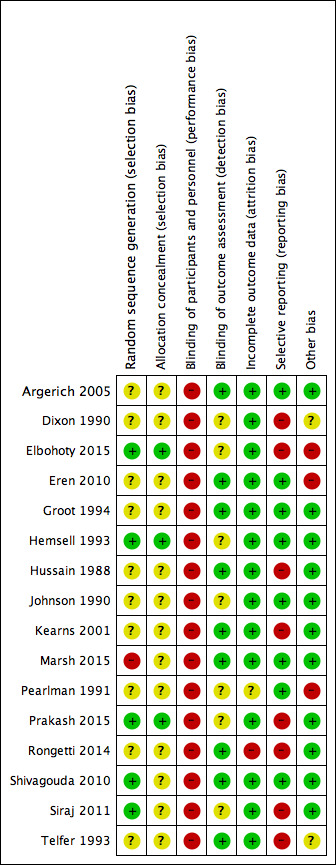
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
3.
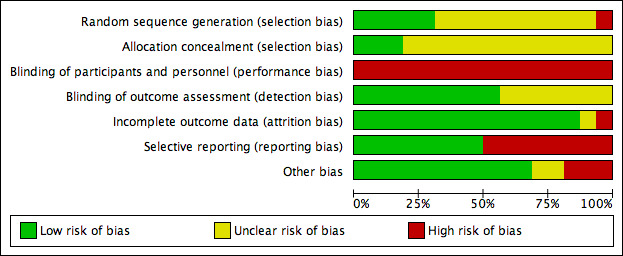
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
Allocation
Sequence generation
Five trials reported the process of sequence generation and were at low risk of bias for this domain (Elbohoty 2015; Hemsell 1993; Prakash 2015; Shivagouda 2010; Siraj 2011). The process of sequence generation in Marsh 2015 appeared at high risk. Sequence generation was unclear for the remaining 10 studies.
Allocation concealment
Allocation concealment appeared to be unclear in all except three included studies (Elbohoty 2015; Hemsell 1993; Prakash 2015). Allocation was performed by using either opaque sealed assignment envelopes (Elbohoty 2015; Prakash 2015), or central telephone assignment according to a randomly generated number list (Hemsell 1993). In 13 studies at unclear risk of bias, there was insufficient information or it was unclear whether envelopes used were opaque and sealed.
Blinding
Nine studies clearly blinded outcome assessors to assigned interventions (Argerich 2005; Eren 2010; Groot 1994; Hussain 1988; Kearns 2001; Marsh 2015; Rongetti 2014; Shivagouda 2010; Telfer 1993). Dixon 1990 reported blinding of the outcome assessors, but only for evaluating wound cosmetic appearance; therefore blinding was unclear for the other outcomes. The five remaining studies had unclear risk of blinding outcome assessors.
Due to the nature of the intervention, the review authors decided to mark all the studies high risk for performance bias since blinding of surgeons was impossible. However, blind outcome assessment is considered more important than blinding of participants and carers (Hróbjartsson 2012).
Incomplete outcome data
Across studies, participant completion was 100% or the reason for dropping out and proportion of dropouts was balanced across study groups with two exceptions (Pearlman 1991; Rongetti 2014). Pearlman 1991 excluded 10/100 participants after randomisation due to unplanned common bile duct exploration. It was at unclear risk of attrition bias as there was no information regarding which groups the excluded participants belonged to. Rongetti 2014 had high risk of attrition bias as it had 19% attrition mainly due to "digestive tract mucosa exposure." This happened more in the scalpel group compared to the electrosurgery group (15 for scalpel versus 3 for electrosurgery).
Selective reporting
Trial registrations were not available for most of the studies; however, there was no evidence to suggest selective reporting of outcomes in eight included trials. Seven studies did not report measures of variance for continuous outcomes (Dixon 1990; Elbohoty 2015; Hussain 1988; Kearns 2001; Prakash 2015; Siraj 2011; Telfer 1993). Rongetti 2014 reported means, did not report standard deviations, and did not report most outcomes according to study groups. These eight studies were at high risk of reporting bias.
Other potential sources of bias
In all studies except five (Dixon 1990; Elbohoty 2015; Eren 2010; Pearlman 1991; Telfer 1993), baseline demographic and clinical characteristics were comparable between the two study groups in each trial and no other biases were apparent. There was an imbalance in sex ratio in Telfer 1993 and baseline characteristics were not compared in Dixon 1990. Given that the method of randomisation was not clearly stated, the two studies were marked unclear as any imbalance in baseline characteristics could introduce further bias to the results. Eren 2010 reported an imbalance in number of participants assigned to the study groups and given that there was no information regarding the method of randomisation, this was considered a source of bias. The study groups varied in the way bleeding points were controlled in Elbohoty 2015 and Pearlman 1991.
Effects of interventions
See: Table 1
Sixteen studies (2769 participants) compared scalpel versus electrosurgery (Table 1).
Primary outcomes
Wound infection
Eleven studies, analysing 2178 participants, reported on wound infection events. There was no clear difference in wound infections between electrosurgery and scalpel (RR 1.07, 95% CI 0.74 to 1.54; I2 = 15%; Analysis 1.1). The evidence was of low certainty and was downgraded twice, once for serious risk of bias and once for imprecision. The results were assessed for publication bias (Figure 4). We tested for funnel plot asymmetry and by visual inspection found no evidence of publication bias. Therefore, the evidence was not downgraded for this. We used a random‐effects model due to the presence of statistical heterogeneity.
1.1. Analysis.
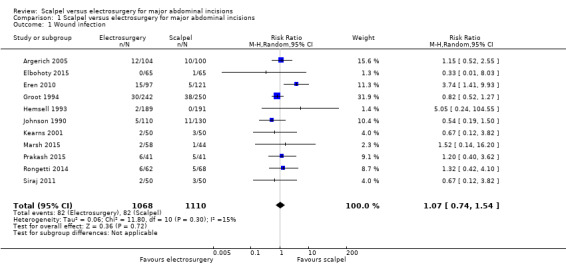
Comparison 1 Scalpel versus electrosurgery for major abdominal incisions, Outcome 1 Wound infection.
4.
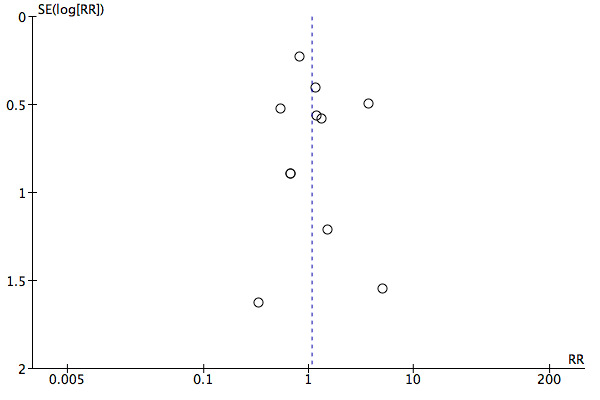
Funnel plot of comparison: 1 Scalpel versus electrosurgery for major abdominal incisions, outcome: 1.1 Wound infection.
In a post hoc subgroup analysis, there were no significant difference in risk of wound infection between the scalpel group and the electrosurgery groups in all strata of wound classification based on degree of contamination (Analysis 1.2).
1.2. Analysis.
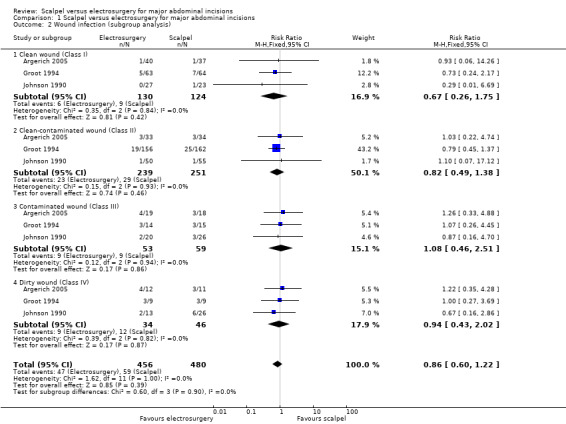
Comparison 1 Scalpel versus electrosurgery for major abdominal incisions, Outcome 2 Wound infection (subgroup analysis).
Time to wound healing
None of the included studies reported time to wound healing.
Wound dehiscence
Six studies analysing 1064 participants reported on wound dehiscence. It was uncertain whether electrosurgery decreased the incidence of wound dehiscence compared to scalpel (RR 1.21, 95% CI 0.58 to 2.50; I2 = 0%; Analysis 1.3). The certainty of the evidence was very low. It was downgraded once due to risk of bias and twice for very serious imprecision. Wound dehiscence is a relatively rare event and this meta‐analysis was underpowered to detect clinically important differences. In addition, the CIs included the possibility of both decreased and increased wound dehiscence. The fixed‐effect model was adopted as there was no statistical heterogeneity between the studies.
1.3. Analysis.
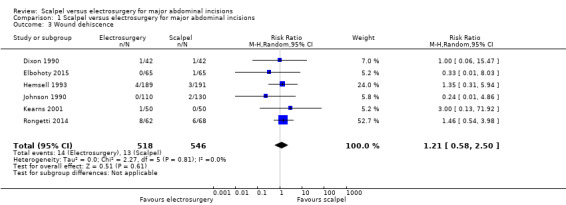
Comparison 1 Scalpel versus electrosurgery for major abdominal incisions, Outcome 3 Wound dehiscence.
Secondary outcomes
Postoperative pain
Nine studies analysing 945 participants assessed postoperative pain at different time intervals (Dixon 1990; Elbohoty 2015; Hussain 1988; Kearns 2001; Pearlman 1991; Prakash 2015; Shivagouda 2010; Siraj 2011; Telfer 1993). The studies provided data on postoperative pain score, peak expiratory flow rate (PEFR) and need for postoperative analgesia.
Given that we were unable to pool the data, we have presented the pain data in additional tables (Table 2; Table 3; Table 4; Table 5). Inconsistency in reporting, substantial heterogeneity, and failure to report measures of variance precluded the inclusion of these data in a meta‐analysis. The results were mixed with postoperative pain appearing to be reduced in the electrosurgery group in most of the studies. Pain also appeared to be similar across the study groups in some studies. As no further analyses were carried out, we are unable to draw any substantive conclusion from available data.
1. Postoperative pain: pain scores.
| Study (details) | Time postoperation | ||||||||
| 4 h | 8 h | 12 h | 16 h | 1 d | 2 d | 3 d | 4 d | 5 d | |
| Dixon 1990 (n = 60; absent = 0; mild = 1; moderate = 2; severe = 3) | |||||||||
| Scalpel | ‐ | ‐ | ‐ | ‐ | ‐ | 20 | 8 | 2 | 0 |
| Electrosurgery | ‐ | ‐ | ‐ | ‐ | ‐ | 17 | 13 | 0 | 0 |
| Hussain 1988(n = 200; mean (range)) | |||||||||
| Scalpel | 5.75 (2.7 to 8.5) | 8.85 (6 to 10) | 6.75 (1 to 8.3) | 6.75 (1 to 8.3) | 6.75 (0.7 to 8.3) | ||||
| Electrosurgery | 3.85 (1 to 6) | 5.92 (2.3 to 9.6) | 4.36 (0.7 to 7.5) | 3 (1 to 5) | 4.35 (0.7 to 7.7) | ‐ | ‐ | ‐ | ‐ |
| Kearns 2001(n = 100; mean (SEM)) | |||||||||
| Scalpel | ‐ | ‐ | ‐ | ‐ | 2.7 (0.1) | 2.4 (0.1) | 1.9 (0.1) | 1.4 (0.1) | 1.2 (0.1) |
| Electrosurgery | ‐ | ‐ | ‐ | ‐ | 2.3 (not reported) | 2 (0.1) | 1.6 (0.1) | 1.3 (0.1) | 1.1 (0.1) |
| Pearlman 1991(n = 59; objective scale; mean (SD)) | |||||||||
| Scalpel | d 1 + d 2 + d 3 = 9 (4) | ||||||||
| Electrosurgery | d 1 + d 2 + d 3 = 8 (3) | ||||||||
| Study (details) | 6 h | 8 h | 12 h | 16 h | 1 d | 2 d | 3 d | 4 d | 5 d |
| Prakash 2015 (n = 82; median (interquartile range)) | |||||||||
| Scalpel | ‐ | ‐ | ‐ | ‐ | 5 (0 to 8) | 4 (0 to 8) | 4 (0 to 6) | 3 (0 to 5) | 2 (0 to 5) |
| Electrosurgery | ‐ | ‐ | ‐ | ‐ | 5 (0 to 10) | 4 (0 to 8) | 3 (0 to 8) | 2 (0 to 7) | 2 (0 to 5) |
| Shivagouda 2010(n = 60; mean (SD)) | |||||||||
| Scalpel | 6.7 (0.53) | ‐ | 3.7 (0.64) | ‐ | 2.4 (0.51) | ‐ | ‐ | ‐ | ‐ |
| Electrosurgery | 6.6 (0.81) | ‐ | 3.8 (0.83) | ‐ | 2.5 (0.86) | ‐ | ‐ | ‐ | ‐ |
| Siraj 2011(n = 100; mean): | |||||||||
| Scalpel | ‐ | ‐ | ‐ | ‐ | 3.96 | 3 | 2.4 | 2.08 | 1.58 |
| Electrosurgery | ‐ | ‐ | ‐ | ‐ | 3.78 | 2.9 | 2.3 | 1.94 | 1.4 |
| Telfer 1993(n = 101; median (interquartile range)) | |||||||||
| Scalpel | ‐ | ‐ | ‐ | ‐ | 6 (4 to 8) | 5 (3.5 to 6) | 5 (4 to 6) | 4 (2 to 5) | ‐ |
| Electrosurgery | ‐ | ‐ | ‐ | ‐ | 6 (5 to 7) | 5 (3.75 to 7) | 5 (3 to 6) | 4 (3 to 5) | ‐ |
d: day; h: hour; n: number of participants; SD: standard deviation; SEM: standard error of the mean.
2. Postoperative pain (scalpel vs electrosurgery): visual analogue scale (reported P values).
| Study (details) | Time postoperation | ||||||||
| 4 h | 8 h | 12 h | 16 h | 1 d | 2 d | 3 d | 4 d | 5 d | |
| Dixon 1990 (n = 60) | ‐ | ‐ | ‐ | ‐ | NS | ‐ | ‐ | ‐ | ‐ |
| Hussain 1988 (n = 200) | < 0.02 | < 0.01 | < 0.02 | < 0.01 | < 0.02 | ‐ | ‐ | ‐ | ‐ |
| Kearns 2001 (n = 100) | ‐ | ‐ | ‐ | ‐ | 0.04 | 0.02 | 0.15 | 0.34 | 0.43 |
| Pearlman 1991 (n = 59) | d 1 + d 2 + d 3 = NS | ||||||||
| Study (details) | 6 h | 8 h | 12 h | 16 h | 1 d | 2 d | 3 d | 4 d | 5 d |
| Shivagouda 2010 (n = 60) | 0.475 | ‐ | 0.556 | ‐ | 0.762 | ‐ | ‐ | ‐ | ‐ |
| Siraj 2011 (n = 100) | ‐ | ‐ | ‐ | ‐ | 0.24 | 0.51 | 0.51 | 0.33 | 0.13 |
| Telfer 1993 (n = 101) | ‐ | ‐ | ‐ | ‐ | NS | ‐ | ‐ | ‐ | ‐ |
d: day; h: hour; n: number of participants; NS: not significant (as reported by study authors).
3. Postoperative pain: need for analgesia.
| Study (details) | Scalpel | Electrosurgery | P value |
| Hussain 1988(n = 200; mean (range)) | |||
| Frequency of morphine injection per 24 h | 5 (3 to 6) | 3 (2 to 5) | < 0.05 |
| Milligrams of morphine per 24 h (mg) | 52 (30 to 60) | 30 (20 to 52) | < 0.05 |
| Kearns 2001(n = 100; mean (SEM)) | |||
| Morphine (mL) | 92 (9.1) | 66 (7.6) | 0.036 |
| Days morphine required | 2.6 (0.1) | 2.2 (0.1) | 0.011 |
| Pearlman 1991(n = 59; mean (SD)) | |||
| Narcotic injections for first 3 days | 9 (4) | 8 (3) | NS |
| Elbohoty 2015 (n = 130; median (range)) | |||
| Doses of paracetamol | 4 (4 to 4) | 3 (2 to 4) | < 0.001 |
| Shivagouda 2010(n = 60; mean (SD)) | |||
| Doses of analgesic | 1.6 (0.48) | 1.8 (0.66) | 0.499 |
| Telfer 1993(n = 101; median) | |||
| Total Morphine use (mg/kg) | 1.55 | 1.49 | NS |
h: hour; n: number of participants; NS: not significant (as reported by study authors); SD: standard deviation; SEM: standard error of mean.
4. Postoperative pain: peak expiratory flow rate.
| Study (details) | 18 h | P value | 24 h | P value | ‐ |
| Hussain 1988(n = 200; mean (range)) | |||||
| Scalpel | 1.52 (1.35 to 1.75) | NS | 1.6 (1.32 to 1.88) | < 0.05 | ‐ |
| Electrosurgery | 1.64 (1.42 to 1.85) | 2.1 (1.6 to 2.2) | |||
| Study (details) | 1 d | 2 d | 3 d | 4 d | P value |
| Telfer 1993(n = 101; median (interquartile range)) | |||||
| Scalpel | 217 (180 to 265) | 220 (190 to 280) | 260 (210 to 320) | 282 (230 to 357) | NS |
| Electrosurgery | 218 (160 to 272) | 237 (182 to 272) | 272 (220 to 310) | 293 (220 to 330) | |
d: day; h: hour; n: number of participants; NS: not significant (as reported by study authors).
Wound incision time
Eight studies reported on time to create the wound (Dixon 1990; Elbohoty 2015; Johnson 1990; Kearns 2001; Pearlman 1991; Prakash 2015; Telfer 1993; Siraj 2011);
Four studies reported mean time (Dixon 1990; Kearns 2001; Pearlman 1991; Prakash 2015). There was no clinically important difference in mean incision time between electrosurgery and scalpel (MD ‐45.74 seconds, 95% CI ‐88.41 to ‐3.07; 325 participants; 4 studies; I2 = 36%; random‐effects model; Analysis 1.4), given the usual duration of the entire procedure that range from close to one hour in herniorrhaphy cases to several hours in others. The quality of the evidence was moderate. It was downgraded once for serious imprecision. In Dixon 1990 where incision time was considered separately for the two types of procedures, the MD was ‐36.00 seconds (95% CI ‐101.30 to 29.30) for herniorrhaphy cases and ‐152.00 seconds (95% CI ‐282.59 to ‐21.41) for cholecystectomy cases.
1.4. Analysis.
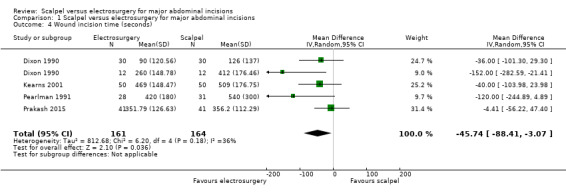
Comparison 1 Scalpel versus electrosurgery for major abdominal incisions, Outcome 4 Wound incision time (seconds).
Three studies reported on wound incision time per wound area (Kearns 2001; Prakash 2015; Siraj 2011). It was unclear whether there was a difference in wound incision time per wound area between electrosurgery and scalpel (MD ‐0.58 seconds/cm2, 95% CI ‐1.26 to 0.09; 282 participants; 3 studies; I2 = 37%; random‐effects model; Analysis 1.5). The certainty of evidence was low, downgraded twice for very serious imprecision.
1.5. Analysis.
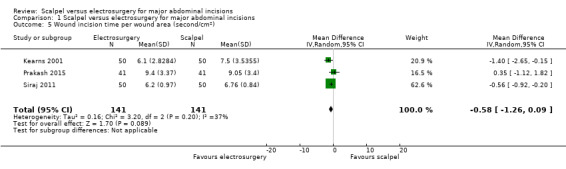
Comparison 1 Scalpel versus electrosurgery for major abdominal incisions, Outcome 5 Wound incision time per wound area (second/cm2).
Three studies reported median time (Elbohoty 2015; Johnson 1990; Telfer 1993). The wound incision time was seven minutes in the electrosurgery group versus 10 minutes in the scalpel group (P value presented, however, no further information) (Elbohoty 2015); 3.3 minutes for the electrosurgery group compared with 3.5 minutes for the scalpel group (no further information) (Johnson 1990); and six minutes for the electrosurgery group compared with five minutes for the scalpel group (P value presented, however, no further information) (Telfer 1993). No further analysis was carried out.
Wound‐related blood loss
Six studies reported wound‐related blood loss as mean, median, and mean per wound area. There was no clinically important difference in mean blood loss between electrosurgery and scalpel (MD ‐20.10 mL, 95% CI ‐28.16 to ‐12.05; 241 participants; 3 studies; I2 = 57%; random‐effects model; Analysis 1.6). The certainty of evidence was moderate due to serious imprecision resulting from the small sample size.
1.6. Analysis.
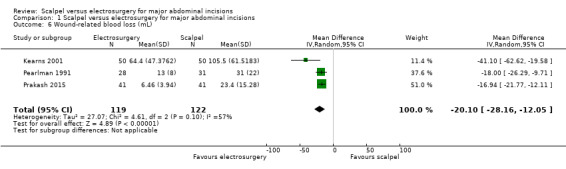
Comparison 1 Scalpel versus electrosurgery for major abdominal incisions, Outcome 6 Wound‐related blood loss (mL).
Results from two additional studies were not pooled due to insufficient data (Elbohoty 2015; Telfer 1993), therefore, it is uncertain whether electrosurgery decreases median wound‐related blood loss. Two studies reported on mean wound‐related blood loss per wound area; however, we were unable to pool the studies due to considerable heterogeneity (I2 = 95%). Therefore, it is uncertain whether electrosurgery decreases wound‐related blood loss per wound area. The evidence from the individual studies had serious imprecision (sample size ranging from 82 to 100).
Adhesion or scar formation
One study reported scar formation (Dixon 1990). Dixon 1990 rated the cosmetic appearance of scars as excellent, fair, or poor by participants, surgeon and an independent assessor. No further analysis was carried out (see Table 6).
5. Adhesion or scar formation.
| Dixon 1990 | Herniorrhaphy (60 wounds; number (%)) | Cholecystectomy (24 wounds; number) | |||||||
| Assessor | Group | Excellent | Fair | Poor | P value | Excellent | Fair | Poor | P value |
| Participant | Scalpel | 14 (47) | 13 (43) | 3 (10) | NS | 4 | 6 | 2 | NS |
| Electrosurgery | 22 (73) | 8 (27) | 0 | 8 | 3 | 1 | |||
| Surgeon | Scalpel | 6 (20) | 20 (67) | 4 (13) | < 0.001 | 3 | 8 | 1 | NS |
| Electrosurgery | 21 (70) | 9 (30) | 0 | 7 | 2 | 3 | |||
| Nurse | Scalpel | 8 (27) | 16 (53) | 6 (20) | < 0.01 | 5 | 5 | 2 | NS |
| Electrosurgery | 20 (67) | 9 (30) | 1 (3) | 6 | 3 | 3 | |||
NS: not significant (as reported by study authors).
Discussion
Summary of main results
Sixteen RCTs comparing scalpel with electrosurgery were included in this review of which nine studies were from the previous version of the review. All 16 included studies except one (Telfer 1993) were suitable for inclusion in a meta‐analysis. The studies which provided sufficient data analysed 2711 participants. The results are summarised in Table 1.
There was no clear difference in wound infection events between electrosurgery and scalpel (low‐certainty evidence).
None of the studies reported time to wound healing.
It was unclear if electrosurgery decreased wound dehiscence (very low‐certainty evidence).
There was no clinically important difference in wound incision time between electrosurgery and scalpel (moderate‐certainty evidence). There was no clear difference in incision time per wound area between electrosurgery and scalpel (low‐certainty evidence).
There was no clinically important difference in wound‐related blood loss between electrosurgery and scalpel (low‐certainty evidence).
We were unable to pool data on wound‐related blood loss per wound area, postoperative pain, and adhesion or scar formation. Therefore, it was uncertain whether electrosurgery improved these outcomes.
Overall completeness and applicability of evidence
Sixteen RCTs met the inclusion criteria of this review. The studies recruited 2769 participants aged between 18 and 100 years with different surgical indications. There was no evident exclusion of particular patient groups from the included studies, therefore, the results of this study would apply to a wide range of people undergoing surgery requiring abdominal incision. However, due to the significant discrepancies in surgical indication, underlying pathology, surgical approach, surgical technique, surgeon type, and wound biology, the overall effects demonstrated in this review should be interpreted with caution. We encourage the reader to also examine specific included studies with surgical characteristics consistent with their particular interest. In addition, there was notable variation among studies with regard to the detailed application of the main (electrosurgery) and the comparison (scalpel) intervention for dividing specific layers of abdominal wall. Frequently, the assigned intervention was not the sole tool used for dividing all layers of the abdominal wall and both electrosurgery and scalpel were employed to divide different layers of the abdominal wall in cases assigned to one intervention. Most outcomes prespecified in the protocol were reported though we were unable to meta‐analyse data on postoperative pain and scar formation. Time to wound healing was not reported. Note that post‐hoc subgroup analysis comparing wound infection between the study groups in all strata of wound classification based on degree of contamination were carried out in an attempt to evaluate the effect of wound contamination on infection. However, wound classification was not consistently reported in most of the included studies. Other clinical factors that could affect wound outcomes such as body mass index (BMI), comorbidities, performance status, methods of wound closure, and surgeon's experience were not adequately addressed in the included studies and should be considered in the future studies.
Quality of the evidence
Following a GRADE assessment, the certainty of evidence was moderate to very low across the outcomes assessed. All the included studies were at high risk of bias mostly due to performance bias and 12 of these studies were at risk of bias for additional reasons. While participant blinding was achieved in a few studies, it would not have been feasible to blind the surgeons due to the nature of intervention. Nine of the 16 included studies reported blind outcome assessment while seven studies were at unclear risk of detection bias. As stated earlier, detection bias took precedence over performance bias when we downgraded evidence for risk of bias (see: Blinding (performance bias and detection bias)). Almost all the studies (13/16: 81%) were at high or unclear risk of selection bias. Furthermore, there were many cases of selective reporting where study authors failed to report measures of variance and when contacted failed to provide details. We rarely downgraded for inconsistency as the meta‐analyses had I2 values which suggested heterogeneity that was interpreted as either moderate or not important. However, we did not pool data for the wound‐related blood loss per wound area outcome due to considerable heterogeneity (I2 = 95%). All outcome results were imprecise due to small sample size or limited number of outcome events, or both.
There was no downgrading for indirectness as the included studies were in agreement with the review question. The only outcome assessed for publication bias was the wound infection outcome. A visual inspection of the funnel plot revealed no evidence of publication bias, therefore, the evidence was not downgraded for publication bias.
Potential biases in the review process
We made a concerted effort to prevent biases during the review process by ensuring an extensive literature search and strict adherence to the published protocol. However, following the upgrade Review Manager 5 in recent years, new methods which were not previously considered were subsequently included in the review. These changes have been highlighted under the Differences between protocol and review section. These additions only serve to ensure a more robust process and methodology, we do not therefore consider them to be of concern. Husnain 2006 met our inclusion criteria; however, this was one of a few studies which were not available on the journal website (www.jsp.org.pk). We contacted the journal editor several times and were assured that efforts would be made to retrieve and send the article; however, we did not receive it. Fifty percent of the included studies reported on postoperative pain yet we were unable to pool studies in a meta‐analysis. Efforts to contact study authors to request missing data on measures of variance were unsuccessful (Kearns 2001; Siraj 2011). The protocol was not specific with regards to which measure of postoperative pain and scar formation was preferred. We avoided selective reporting by extracting all available information for outcomes specified in the review protocol.
Agreements and disagreements with other studies or reviews
The results of this review are in agreement with the NICE guidance published in 2008, which found no difference in SSI between electrosurgery and scalpel and does not recommend the use of electrosurgery for surgical incision (NICE 2008).
In Chrysos 2005, electrosurgery (65 participants) and scalpel (68 participants) incisions in tension‐free inguinal herniorrhaphy were compared for wound complications (wound strength, wound infection), the requirement for postoperative analgesia, and wound blood loss. The two groups were comparable in terms of wound strength, infectious complications (totally absent), and wound blood loss. Participants in the electrosurgery group required less parenteral analgesia on the first postoperative day. A higher proportion of participants in the scalpel group continued to need oral analgesics on the second postoperative day compared with participants in the electrosurgery group. The findings concerning wound complications and postoperative pain (need for analgesics) were in line with our review results. This was a prospective study comparing electrosurgery and scalpel incisions in tension‐free inguinal hernioplasty. Participants were allocated alternately to the intervention, so this was not true randomisation.
One cross‐sectional study compared electrosurgery in coagulation mode (433 participants) with cold scalpel (531 participants) for severe wound complications in participants undergoing midline abdominal incision for uterine malignancies (Franchi 2001). There was a higher incidence of severe wound complications in the scalpel group (8/531 versus 1/433; P < 0.05). However, there were no significant differences between groups after adjustment for confounding variables. These results support the safety of electrosurgery for making midline abdominal incisions.
Authors' conclusions
Implications for practice.
There is currently insufficient evidence on the relative effectiveness of electrosurgery compared with scalpel in abdominal surgery. While current data suggest there may be no clear difference in wound infections between the treatments, the evidence is low certainty as estimates are imprecise and span estimates consistent with both benefits and harms. Given this, decision makers are likely to follow current local and national guidelines until further evidence is available.
Implications for research.
There is a lack of high‐quality evidence on the effect of scalpel compared to electrosurgery on abdominal incisions. If this is deemed an important question to decision makers, further randomised controlled trials in the area may be warranted. Such trials should assess outcomes such as time to complete wound healing. Since incomplete outcome reporting was an important limitation in this review, future trials should follow the CONSORT statement. The reporting of outcomes such as pain should be more standardised and reported at the same time points across studies (with measures of variance) to enable data pooling. Future studies should report on wound healing as time‐to‐event data, assess clinical factors potentially affecting wound outcomes, include adequate sample sizes, and ensure complete reporting of continuous outcomes with measures of variance. Furthermore, a study designed to address the effect of coagulating‐mode electrosurgery would be worthwhile.
What's new
| Date | Event | Description |
|---|---|---|
| 6 June 2017 | New citation required and conclusions have changed | Seven additional studies included in the review and conclusions changed. |
| 1 June 2017 | New search has been performed | First update, new search. GRADE assessment of certainty of the evidence undertaken and methodology updated. ZIE and EM joined the author team. |
| 11 April 2017 | Amended | Title changed from 'Scalpel versus electrosurgery for abdominal incisions' following peer review feedback. |
History
Protocol first published: Issue 2, 2006 Review first published: Issue 6, 2012
| Date | Event | Description |
|---|---|---|
| 9 December 2008 | Amended | Converted to new review format. |
| 13 December 2007 | New citation required and conclusions have changed | Substantive amendment. |
Acknowledgements
The authors would like to acknowledge the peer referees (Jason Wong, Kurinchi Gurusamy, Jac Dinnes, Peter Moore and Jane Nadel) for their comments on the review. We would also like to acknowledge the contributions of Jenny Bellorini and Anne Lawson (copy editors) of Narain Chotirosniramit who contributed to the original version of this review.
Appendices
Appendix 1. Glossary of terms
Dehiscence: splitting open of the incision.
Haematoma: swelling due to an accumulation of blood.
Hernioplasty: when herniotomy is combined with a reinforced repair of the posterior inguinal canal wall with autogenous (person's own tissue) or heterogeneous material, such as Prolene mesh.
Herniorrhaphy: operation in which no autogenous or heterogeneous material is used for reinforcement.
Keloid: formation of unsightly scar tissue.
Musculoaponeurosis: layer of muscle and fibrous tissue that holds the abdominal viscera in place and controls movement of the trunk, located deep to the skin and subcutaneous tissue layer.
Seroma: swelling due to accumulation of serum.
Wound dehiscence: proportion of wounds breaking down.
Appendix 2. Search strategies
The Cochrane Central Register of Controlled Clinical Trials (CENTRAL)
#1 MeSH descriptor: [Electrosurgery] explode all trees #2 MeSH descriptor: [Cautery] explode all trees #3 electrosurg* or electrodissect* or electrocaut* or electrocoagul*:ti,ab,kw #4 thermocaut* or thermocoagul* #5 diathermy or diathermi:ti,ab,kw #6 "cold knife":ti,ab,kw #7 scalpel*:ti,ab,kw #8 #1 or #2 or #3 or #4 or #5 or #6 or #7 #9 MeSH descriptor: [Abdomen] explode all trees #10 abdomen near/5 (surg* or operation* or incision*):ti,ab,kw #11 abdominal near/5 (surg* or operation* or incision*):ti,ab,kw #12 MeSH descriptor: [Colorectal Surgery] explode all trees #13 MeSH descriptor: [Laparotomy] explode all trees #14 MeSH descriptor: [Hysterectomy] explode all trees #15 MeSH descriptor: [Appendectomy] explode all trees #16 MeSH descriptor: [Cholecystectomy] explode all trees #17 hysterectomy or laparotomy or cholecystectomy or appendectomy or appendicitis or "colorectal surgery":ti,ab,kw #18 MeSH descriptor: [Hernia] explode all trees #19 hernia:ti,ab,kw #20 #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 or #17 or #18 or #19 #21 #8 and #20 in Trials
Ovid MEDLINE
1 exp Electrosurgery/ 2 exp Cautery/ 3 (electrosurg$ or electrodissect$ or electrocaut$ or electrocoagul$).ti,ab. 4 (thermocaut$ or thermocoagul$).ti,ab. 5 (diathermy or diathermi$).ti,ab. 6 cold knife.ti,ab. 7 or/1‐6 8 exp Abdomen/ 9 (abdomen adj5 (surg$ or operation$ or incision$)).ti,ab. 10 (abdominal adj5 (surg$ or operation$ or incision$)).ti,ab. 11 exp Colorectal Surgery/ 12 exp Laparotomy/ 13 exp Hysterectomy/ 14 exp Appendectomy/ 15 exp Cholecystectomy/ 16 (hysterectomy or laparotomy or cholecystectomy or appendectomy or appendicitis or colorectal surgery).ti,ab. 17 exp Hernia/ 18 hernia.ti,ab. 19 or/8‐18 20 and/7,19 21 randomized controlled trial.pt. 22 controlled clinical trial.pt. 23 randomi?ed.ab. 24 placebo.ab. 25 clinical trials as topic.sh. 26 randomly.ab. 27 trial.ti. 28 or/21‐27 29 exp animals/ not humans.sh. 30 28 not 29 31 and/20,30
Ovid Embase
1 exp electrosurgery/ 2 exp Cauterization/ 3 (electrosurg$ or electrodissect$ or electrocaut$ or electrocoagul$).ti,ab. 4 (thermocaut$ or thermocoagul$).ti,ab. 5 (diathermy or diathermi$).ti,ab. 6 cold knife.ti,ab. 7 or/1‐6 8 exp Abdominal Surgery/ 9 (abdomen adj5 (surg$ or operation$ or incision$)).ti,ab. 10 (abdominal adj5 (surg$ or operation$ or incision$)).ti,ab. 11 exp Colorectal Surgery/ 12 exp Laparotomy/ 13 exp Hysterectomy/ 14 exp Appendectomy/ 15 exp Cholecystectomy/ 16 (hysterectomy or laparotomy or cholecystectomy or appendectomy or appendicitis or colorectal surgery).ti,ab. 17 exp Hernia/ 18 hernia.ti,ab. 19 or/8‐18 20 and/7,19 21 Randomized controlled trials/ 22 Single‐Blind Method/ 23 Double‐Blind Method/ 24 Crossover Procedure/ 25 (random* or factorial* or crossover* or cross over* or cross‐over* or placebo* or assign* or allocat* or volunteer*).ti,ab. 26 (doubl* adj blind*).ti,ab. 27 (singl* adj blind*).ti,ab. 28 or/21‐27 29 exp animals/ or exp invertebrate/ or animal experiment/ or animal model/ or animal tissue/ or animal cell/ or nonhuman/ 30 human/ or human cell/ 31 and/29‐30 32 29 not 31 33 28 not 32 34 and/20,33
EBSCO CINAHL Plus
S33 S19 AND S32 S32 S20 OR S21 OR S22 OR S23 OR S24 OR S25 OR S26 OR S27 OR S28 OR S29 OR S30 OR S31 S31 TI allocat* random* or AB allocat* random* S30 MH "Quantitative Studies" S29 TI placebo* or AB placebo* S28 MH "Placebos" S27 TI random* allocat* or AB random* allocat* S26 MH "Random Assignment" S25 TI randomi?ed control* trial* or AB randomi?ed control* trial* S24 AB ( singl* or doubl* or trebl* or tripl* ) and AB ( blind* or mask* ) S23 TI ( singl* or doubl* or trebl* or tripl* ) and TI ( blind* or mask* ) S22 TI clinic* N1 trial* or AB clinic* N1 trial* S21 PT Clinical trial S20 MH "Clinical Trials+" S19 S7 and S18 S18 S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 S17 TI hernia or AB hernia S16 (MH "Hernia+") S15 TI (hysterectomy or laparotomy or cholecystectomy or appendectomy or appendicitis or colorectal surgery ) or AB ( hysterectomy or laparotomy or cholecystectomy or appendectomy or appendicitis or colorectal surgery) S14 (MH "Cholecystectomy") S13 (MH "Appendectomy") S12 (MH "Hysterectomy+") S11 (MH "Laparotomy") S10 TI (abdominal N5 surg* or abdomen N5 operation* or abdomen N5 incision* ) or AB ( abdominal N5 surg* or abdomen N5 operation* or abdomen N5 incision*) S9 TI (abdomen N5 surg* or abdomen N5 operation* or abdomen N5 incision* ) or AB ( abdomen N5 surg* or abdomen N5 operation* or abdomen N5 incision*) S8 (MH "Abdomen+") S7 S1 or S2 or S3 or S4 or S5 or S6 S6 TI cold knife or AB cold knife S5 TI diatherm* or AB diatherm* S4 TI (thermocaut* or thermocoagul* ) or AB ( thermocaut* or thermocoagul*) S3 TI (electrosurg* or electrodissect* or electrocaut* or electrocoagul* ) or AB ( electrosurg* or electrodissect* or electrocaut* or electrocoagul*) S2 (MH "Cautery+") S1 (MH "Electrosurgery")
Data and analyses
Comparison 1. Scalpel versus electrosurgery for major abdominal incisions.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Wound infection | 11 | 2178 | Risk Ratio (M‐H, Random, 95% CI) | 1.07 [0.74, 1.54] |
| 2 Wound infection (subgroup analysis) | 3 | 936 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.60, 1.22] |
| 2.1 Clean wound (Class I) | 3 | 254 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.26, 1.75] |
| 2.2 Clean‐contaminated wound (Class II) | 3 | 490 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.49, 1.38] |
| 2.3 Contaminated wound (Class III) | 3 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.46, 2.51] |
| 2.4 Dirty wound (Class IV) | 3 | 80 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.43, 2.02] |
| 3 Wound dehiscence | 6 | 1064 | Risk Ratio (M‐H, Random, 95% CI) | 1.21 [0.58, 2.50] |
| 4 Wound incision time (seconds) | 4 | 325 | Mean Difference (IV, Random, 95% CI) | ‐45.74 [‐88.41, ‐3.07] |
| 5 Wound incision time per wound area (second/cm2) | 3 | 282 | Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐1.26, 0.09] |
| 6 Wound‐related blood loss (mL) | 3 | 241 | Mean Difference (IV, Random, 95% CI) | ‐20.10 [‐28.16, ‐12.05] |
| 7 Wound related blood loss per wound area (mL/cm2) | 2 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
1.7. Analysis.
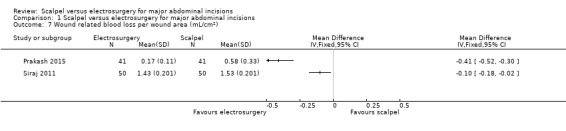
Comparison 1 Scalpel versus electrosurgery for major abdominal incisions, Outcome 7 Wound related blood loss per wound area (mL/cm2).
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Argerich 2005.
| Methods | Study design: prospective RCT in a single institution. Randomisation method: not stated. Number of participants randomised: 204. Number of participants analysed: 204 (100 in scalpel group, 104 in electrosurgery group). Analysis: full ITT. |
|
| Participants | Inclusion criteria: people undergoing abdominal surgical procedures. Location: University Hospital, Santa Maria, Brazil. Enrolment period: 15 months (August 1999 to November 2000). Exclusion criteria: people with operative wounds that did not include all layers of abdominal wall (skin, subcutaneous cellular tissue, muscle, and fascia). Age: < 40 to 100 years. Sex: not stated. Time of last evaluation: 30 days after surgery. |
|
| Interventions | Scalpel group: operative wounds created with scalpel from skin to fascia. Electrosurgery group: operative wounds created with scalpel for cutaneous incision and electrocautery, in 'coagulation' mode, for the remaining layers. |
|
| Outcomes | Surgical wound infection. Surgical time. |
|
| Notes | The 2 groups were comparable with regard to the rate of corticosteroids use, antibiotic prophylaxis, co interventions (drainage procedures), and duration of surgery. Wound classification: scalpel group vs electrosurgery group:
Funding source: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: stated to be a randomised study but no information provided for method of sequence generation. |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated; however, blinding not feasible due to nature of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: operative wounds observed and followed by professionals blinded to intervention applied using the clinical criteria. Outcome assessors should have been adequately blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: appeared that all participants completed study. |
| Selective reporting (reporting bias) | Low risk | Comment: no evidence to suggest selective reporting. |
| Other bias | Low risk | No other apparent biases. |
Dixon 1990.
| Methods | Study design: prospective RCT in a single institution. Randomisation method: sealed assignment envelopes. Number of participants randomised: 84. Number of participants analysed: 84 (42 in scalpel group, 42 in electrosurgery group). Analysis: full ITT. |
|
| Participants | Inclusion criteria: people scheduled for either inguinal herniorrhaphy (transverse incisions; 60 men) or cholecystectomy (subcostal incisions; 24 women). Location: Glenfield General Hospital, Leicester, UK. Enrolment period: 6 months. Age: not stated. Sex (M/F): 60/24. Time of last evaluation: 6 weeks. |
|
| Interventions | Scalpel group: incisions made by scalpel, with haemostasis by means of forceps coagulation using the Force 2 electrosurgical generator (Valley Laboratories Inc., Colorado, USA) producing pulsed sine waves, power 30 W. Electrosurgery group: following scoring of epidermis with a scalpel as a marker for the incision; dermal and subcutaneous layers incised with a needle electrode using the same generator to produce a pure sine‐wave current on a power setting of 70 W. Povidone iodine was sprayed throughout the subcutaneous layers in all cases, and, if the subcutaneous fat was more than 2 cm deep, interrupted plain catgut sutures were sited. Skin closure was with clips in the herniorrhaphies and a subcuticular 2/0 Prolene suture for the cholecystectomies. |
|
| Outcomes | Time taken to make incisions and achieve haemostasis. Pain scores. Wound complications (skin burn, haematoma, keloid, inadvertent incision in external oblique, inversion of wound edges) (see Appendix 1 for glossary of terms). Wound cosmetic assessment. Sepsis. Dehiscence. |
|
| Notes | Wound classification: not stated. Funding source: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Each patient was allocated at random to one of two groups." Randomisation method: no specific information regarding sequence generations provided. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated; however, blinding not feasible due to nature of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "The cosmetic appearance of the scar was graded by the patient, the surgeon and an independent assessor blind to the treatment group such as the clinic staff nurse." Comment: cosmetic outcome appeared to be blinded. However, no information provided for other outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: appeared that all participants completed study. |
| Selective reporting (reporting bias) | High risk | Comment: no evidence to suggest selective reporting, except that measures of variance were not reported for continuous outcomes. |
| Other bias | Unclear risk | Comment: baseline characteristics of study groups not specifically compared. |
Elbohoty 2015.
| Methods | Study design: prospective RCT in a single institution. Randomisation method: computerised randomisation. Number of participants randomised: 137. Number of participants analysed: 130 (65 in scalpel group, 65 in electrosurgery group). Analysis: available‐case. |
|
| Participants | Inclusion criteria: women with a history of 1 previous caesarean section at the time of their planned repeat caesarean section through a transverse abdominal incision requiring an incision to be made over the previous scar, which healed by primary intention; BMI of 18.5 to 29.9 kg/m2. Exclusion criteria: women who required midline incision, were on anticoagulant therapy, had known allergy to cephalosporin antibiotic, had pacemakers, and had chronic diseases expected to affect wound healing. Location: Ain Shams University Maternity Hospital. Enrolment period: 6 months (March to September 2013). Median age (interquartile range): scalpel group: 28.8 (21 to 34) years; electrosurgery group: 27.6 (20 to 38) years. Sex (M/F): 0/130. |
|
| Interventions | Scalpel group: incisions made by scalpel with disposable blade (No. 22) from skin to peritoneum, with proper haemostasis by application of pressure to skin blood vessels and by ligating the subcutaneous bleeders. Electrosurgery group: skin was divided by disposable scalpel blade (No. 22). A small flat blade pen electrode was used for division of subcutaneous tissue through the peritoneum. It was set on cutting mode and delivering 120‐Watt (maximum) sinusoidal current. Cutting was performed without pressure or mechanical displacement. 'Bleeders' were controlled by using diathermy, on coagulating mode and applied to a haemostat on the vessel to avoid skin necrosis and blistering. All women received IV antibiotic prophylaxis, cefradine 1 g at the time of incision and after operation paracetamol was given by IV on demand. All operations performed by the same surgeon (a lecturer of obstetrics and gynaecology). |
|
| Outcomes | Volume of blood loss from skin incision to the end of the peritoneal incision. Operative time from beginning of skin incision to completion of peritoneal incision. Wound complication. Postoperative surgical wound pain. |
|
| Notes | Wound classification: not stated. Funding source: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised randomisation using SPSS. |
| Allocation concealment (selection bias) | Low risk | Sequentially numbered sealed opaque envelopes opened in the operating room just before surgical procedure. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated; however, blinding not feasible due to nature of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar proportion of participants (3/68 in scalpel group vs 4/69 in electrosurgery group) lost to follow‐up from both study groups. |
| Selective reporting (reporting bias) | High risk | Study protocol not available and measures of variance reported for all the continuous outcomes. Authors contacted but received no reply. |
| Other bias | High risk | Comment: basic characteristics appeared comparable; however, study groups varied in way that bleeding points were controlled. |
Eren 2010.
| Methods | Study design: prospective study in a single institution. Randomisation method: not stated. Number of participants randomised: 218. Number of participants analysed: 218 (97 in scalpel group, 121 in electrosurgery group). Analysis: full ITT. |
|
| Participants | Inclusion criteria: people undergoing abdominal surgery for various gastrointestinal malignancies via sharp midline laparotomy incisions. Location: Department of General Surgery, Faculty of Medicine, Istanbul University, Istanbul, Turkey. Enrolment period: January 2002 to August 2005. Exclusion criteria: not stated. Mean age (range): 57 (17 to 93) years. Sex (M/F): 135/83. Time of last evaluation: 6 months. |
|
| Interventions | Scalpel group: incisions by scalpel from skin through fascia. Electrosurgery group: skin incised by electrosurgery in 'cut' mode and subcutaneous tissue, fascia, and muscle divided by electrocautery in the 'coagulation' mode. Bleeding points controlled by electrosurgery in 'coagulation' mode in both groups. Fascia closed with continuous semisynthetic absorbable sutures. |
|
| Outcomes | Wound infection. Incisional hernia. |
|
| Notes | Abstract stated that participants were randomly divided into 2 groups. However, no detail of randomisation described in methods section. Number of participants in the 2 groups were significantly different (97 in scalpel group vs 121 in electrosurgery group). Awaiting the author's response. Wound classification: not stated. Funding source: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were referred to as "randomly divided." Attempted to confirm details with authors and specific response not received. However, authors reported that participants in both groups had similar demographic values. |
| Allocation concealment (selection bias) | Unclear risk | Not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated; however, blinding not feasible due to nature of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Follow‐up assessments during postoperative stay and subsequent examinations blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | High risk | Imbalance in number of participants assigned to groups (45% in scalpel group vs 56% in electrosurgery group) and method of randomisation was questionable. |
Groot 1994.
| Methods | Study design: prospective RCT in a single institution. Randomisation method: sealed envelopes. Number of participants randomised: 492 (672 eligible people; 180 not randomised, of these, the randomisation assignment was forgotten in 166 and the surgeon deemed 1 method not acceptable in 14). Number of participants analysed: 492 (250 in scalpel group, 242 in electrosurgery group). Analysis: full ITT. |
|
| Participants | Inclusion criteria: people scheduled for abdominal or thoracic operations. Location: Royal University Hospital in Saskatoon, Saskatchewan, Canada. Enrolment period: 15 months (August 1989 to November 1990). Exclusion criteria: non‐full thickness operative wound; methods deemed unsuitable by the surgeon before randomisation. Surgery for morbid obesity. Age: < 40 to 100 years. Sex (M:F): scalpel group: 128/122; electrosurgery group: 119/123. Time of last evaluation: 6 weeks after hospital discharge. |
|
| Interventions | Scalpel group: incisions were made by scalpel from skin through fascia. Haemostasis was left to the preference of the surgeon. Electrosurgery group: skin incisions were created with scalpel, then subcutaneous tissue, muscle, and fascia divided with a Valleylab cautery unit (Valleylab, Boulder, Colorado) set at 6 in the 'coagulation' mode. |
|
| Outcomes | Rate of wound infection. | |
| Notes | Groups comparable with regard to independent variables of age and smoking. None of the participants had incarcerated hernia, although 2 had non‐reducible inguinal hernias. Wound classification: scalpel group vs electrosurgery group:
Funding source: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were randomly assigned to one of the two study groups before surgery." Comment: no specific information regarding sequence generation provided. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "The method to be used for each patient was indicated in a sealed envelope, opened preoperatively." Comment: there was an attempt to achieve allocation concealment in study. However, unclear whether envelopes were opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated; however, blinding not feasible due to nature of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Follow‐up assessment of the wounds was carried out by an independent observer who was unaware of the method used to create the wound." Comment: outcome assessor blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: appeared that all participants completed study. |
| Selective reporting (reporting bias) | Low risk | Comment: no evidence to suggest selective reporting. |
| Other bias | Low risk | No other apparent biases. |
Hemsell 1993.
| Methods | Study design: prospective RCT in a single institution. Randomisation method: central telephone assignment according to a randomly generated number list. Number of participants randomised: 380. Number of participants analysed: 380 (191 in scalpel group, 189 in electrosurgery group). Analysis: full ITT. |
|
| Participants | Inclusion criteria: women scheduled for elective abdominal hysterectomy for benign diseases (62% vertical incision, 38% low transverse incision). Location: Parkland Memorial Hospital, University of Texas Southwestern Medical Center, Dallas, Texas, USA. Enrolment period: 16 months (July 1987 to November 1988). Exclusion criteria: pregnancy; pelvic malignancy; allergy to cephalosporin; history of immediate hypersensitivity reaction to penicillin; requirement for other antimicrobials; history of receiving antibiotics during preceding 7 days. Mean age (± SD): scalpel: 39 ± 8.8 years; electrosurgery: 39 ± 8.3 years. Sex: women. Time of last evaluation: up to 6 weeks after hospital discharge. |
|
| Interventions | Scalpel group: incisions by scalpel from skin through fascia. Separate scalpel blades used for skin and subcutaneous tissues. Electrosurgery group: skin incisions by scalpel. Subcutaneous tissues opened by electrosurgery using the 'pure cut' mode with an SSE2 L (Valleylab, Boulder, Colorado) electrosurgical generator. Physicians selected power settings. Haemostasis of individual bleeding sites by electrosurgery or suture in either group. Pelvis irrigated with sterile saline following hysterectomy, as were subcutaneous tissues after fascial closure. Subcutaneous tissues not closed. Skin edges approximated with staples. |
|
| Outcomes | Wound complications (infection, seroma, haematoma, dehiscence) (see Appendix 1 for glossary of terms). Length of hospital stay: used to reflect the time to wound healing. However, given lack of clarity on whether women stayed in hospital until wounds healed, these data were not extracted. |
|
| Notes | Indications for surgery similar in the 2 groups (54% uterine leiomyoma, 16% chronic pelvic pain, 10% abnormal bleeding unresponsive to medical management, 8% stress urinary incontinence, 7% adnexal mass, 5% cervical intraepithelial neoplasia + abnormal uterine bleeding). Clinical and surgical variables similar in the 2 groups with the exception of race (higher black:white ratio in scalpel group (scalpel: 122:44; electrosurgery: 101/66)). Wound classification: not stated. Funding source: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "They were assigned to the scalpel or electrocautery group by a computer‐generated randomisation code." Comment: sequence generation appeared adequate. |
| Allocation concealment (selection bias) | Low risk | Comment: randomisation method was central telephone assignment according to a randomly generated number list. Allocation concealment adequate. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated; however, blinding not feasible due to nature of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: appeared that all participants completed study. |
| Selective reporting (reporting bias) | Low risk | Comment: no evidence to suggest selective reporting. |
| Other bias | Low risk | Comment: basic characteristics appeared comparable except for race ratio and given that there was clarity on method of randomisation, we did not consider this of concern. |
Hussain 1988.
| Methods | Study design: prospective RCT in a single institution. Randomisation method: sealed assignment envelopes. Number of participants randomised: 200. Number of participants analysed: 200 (100 in scalpel group, 100 in electrosurgery group). Analysis: full ITT. |
|
| Participants | Inclusion criteria: people scheduled for cholecystectomy (100% Kocher's incision). Location: King Faisal Military Hospital, College of Medicine, King Saud University, Kingdom of Saudi Arabia. Exclusion criteria: chronic obstructive airways disease, requirement for choledochostomy. Mean age (range): scalpel: 35 (28 to 56) years; electrosurgery: 37 (26 to 54) years. Sex (M/F): scalpel: 16/84; electrosurgery: 16/84. Time of last evaluation: 24 hours after surgery. |
|
| Interventions | Scalpel group: scalpel was used to create incision. Electrosurgery group: scalpel was used for cutting skin. Remaining tissues were incised with cutting diathermy. An earth‐free transistorised solid‐state diathermy generator was used. Bleeding points were controlled by coagulation diathermy in both groups. |
|
| Outcomes | Postoperative pain (VAS). PEFR as a measurement of pain. Postoperative requirement for morphine. |
|
| Notes | Chronic cholecystitis was the indication for cholecystectomy in all participants. Age comparable between groups. Wound classification: not stated. Funding source: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization was restricted and stratified for sex giving equal numbers of men and women in each group." Comment: no specific information regarding sequence generation provided. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were randomised by sealed envelopes into group A or B." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated; however, blinding not feasible due to nature of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Group identity was inevitably known to the operating surgeon but he was not involved in the postoperative assessment during the trial period." Comment: participants (assessors for pain outcome) blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: appeared that all participants completed study. |
| Selective reporting (reporting bias) | High risk | Comment: no evidence to suggest selective reporting except that measures of variance not reported for continuous outcomes. |
| Other bias | Low risk | No other apparent biases. |
Johnson 1990.
| Methods | Study design: prospective RCT in a single institution. Randomisation method: sealed assignment envelopes. Number of participants randomised: 240. Number of participants analysed: 240 (130 in scalpel group, 110 in electrosurgery group). Analysis: full ITT. |
|
| Participants | Inclusion criteria: people scheduled for abdominal surgery (scalpel group: 86 midline incision, 15 paramedian incision, 29 transverse incision; electrosurgery group: 67 midline incision, 9 paramedian incision, 34 transverse incision). Location: Kingston Hospital, Kingston‐upon‐Thames, Surrey, UK. Exclusion criteria: reoperation within 1 month of laparotomy; ruptured abdominal aortic aneurysm. Median age (IQR): scalpel: 68 (18 to 95) years; electrosurgery: 72 (26 to 92) years. Sex (M/F): scalpel: 60/70; electrosurgery: 43/67. Time of last evaluation: not stated. |
|
| Interventions | Scalpel group: scalpel used to create incision through all layers for midline incisions, but only for the skin, subcutaneous fat, and deep fascia. In muscle‐cutting incisions, diathermy was used. Electrosurgery group: cutting diathermy was used to create incision through all layers for both midline and muscle‐cutting incisions. All incisions were closed using a mass suture technique. Subcutaneous sutures and subcutaneous drains were not used. |
|
| Outcomes | Time taken to create the wound. Rate of wound inflammation, infection, and dehiscence. |
|
| Notes | Elective: emergency ratios: scalpel: 79:51; electrosurgery: 70:40. The length and depth of incisions were comparable between the groups. Wound classification: scalpel group vs electrosurgery group:
Funding source: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "patients were randomly allocated." Comment: no specific information regarding sequence generation provided. |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated; however, blinding not feasible due to nature of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: no information about blinding provided. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: appeared that all participants completed study. |
| Selective reporting (reporting bias) | Low risk | Comment: evidence to suggest selective reporting. |
| Other bias | Low risk | No other apparent biases. |
Kearns 2001.
| Methods | Study design: prospective RCT in a single institution. Randomisation method: sealed assignment envelopes. Number of participants randomised: 100. Number of participants analysed: 100 (50 in scalpel group, 50 in electrosurgery group). Analysis: full ITT. |
|
| Participants | Inclusion criteria: people scheduled for elective abdominal surgery via a midline laparotomy. Location: Beaumont Hospital, Dublin, Ireland. Exclusion criteria: diabetes, current warfarin use, previous midline laparotomy. Mean age (range): scalpel: 61 (15 to 84) years; electrosurgery: 60 (32 to 85) years. Sex ratio (M/F): scalpel: 27/23; electrosurgery: 27/23. Time of last evaluation: 1 month. |
|
| Interventions | Scalpel group: incision through all layers. Electrosurgery group: standard diathermy pen electrode, set on 'cutting' mode, and delivering a 500‐kHz sinusoidal current, used to create incision through all layers. Diathermy used for haemostasis in both groups. Wound closure achieved by mass closure using loop polyglyconate and surgical clips for skin. |
|
| Outcomes | Incision time. Wound‐related blood loss. Postoperative pain (VAS). Postoperative morphine requirement (patient‐controlled analgesia volume, duration). Wound complications (infection, dehiscence). |
|
| Notes | Malignant:benign pathology ratios similar between groups (scalpel: 33:17; electrosurgery: 32:18). Demographic and surgical characteristics comparable between groups. Wound classification: all incisions classified as clean or clean‐contaminated. Funding source: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Patients were randomised prospectively into scalpel or diathermy incision groups." Comment: no specific information regarding sequence generation provided. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Randomization was by sealed envelope upon the patient's arrival in the operating suite." Comment: unclear whether envelopes were numbered and opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated; however, blinding not feasible due to nature of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Time taken to complete the laparotomy incision was recorded by an independent observer in the operating theatre. Wound‐related pain scores and the number of days for which PCA [patient‐controlled analgesia] was required for wound‐related pain were recorded by blinded observers on the ward. Wound complications occurring at any stage after operation and at 1‐month follow‐up were recorded for all patients by independent observers blinded to the method of incision used." Comment: adequate blinding of outcome assessors achieved. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: appeared that all participants completed study. |
| Selective reporting (reporting bias) | High risk | Comment: no evidence to suggest selective reporting except that measures of variance were not reported for continuous outcomes. |
| Other bias | Low risk | No other apparent biases. |
Marsh 2015.
| Methods | Study design: RCT. Randomisation method: random number charts. Number of participants randomised: 102. Number of participants analysed: 102 (44 in scalpel group, 58 in electrosurgery group). Analysis: ITT. Power calculation: sample size based on similar studies looking at seroma formation postabdominoplasty. |
|
| Participants | Inclusion criteria: people undergoing full abdominoplasty surgery. Location: UK. Enrolment period: not stated. Exclusion criteria: reluctance to give consent, unsuitable for abdominoplasty surgery, previous abdominal surgery, smoking habit, BMI > 30 and taking antiplatelet medication. Mean age (± SD): scalpel: 45.4 ± 9.9; electrosurgery: 45.4 ± 11.2. Sex (M/F): scalpel: 5/39; electrosurgery: 3/55. |
|
| Interventions | Scalpel group: incisions made by scalpel. Used monopolar forceps to control bleeding points during flap dissection. Electrosurgery group: used handheld monopolar electrocautery dissection. |
|
| Outcomes | Seroma formation. Weight of tissue excised. Liposuction. Complications: haematoma, wound infection, delayed wound healing. |
|
| Notes | Wound classification: not stated. Funding source: authors stated there was no funding. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Random number charts with even numbers being entered into the scalpel group and odd numbers in electrosurgery group. Comment: due to possibility of predicting group assignment of participants, review authors considered method of randomisation inadequate. |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated; however, blinding not feasible due to nature of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "After the day of surgery all members of the research team were blinded as to the method of abdominal flap dissection until results analysed." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants included in analysis. |
| Selective reporting (reporting bias) | Low risk | All expected outcomes reported. |
| Other bias | Low risk | No other apparent biases. |
Pearlman 1991.
| Methods | Study design: prospective RCT in 2 university‐affiliated institutions. Randomisation method: sealed assignment envelopes. Number of participants randomised: 100. Number of participants analysed: 88 (31 in scalpel group, 28 in electrosurgery group, 29 in laser group). 12 participants excluded after randomisation because of unplanned common bile duct exploration (10 participants) and laser malfunction (2 participants). Analysis: available‐case. |
|
| Participants | Inclusion criteria: people scheduled for uncomplicated elective cholecystectomy. All but 1 participant (electrosurgery group) had a subcostal incision. Location: University of Colorado Health Sciences Center and Denver Veterans Affairs Hospital, Denver, Colorado, USA. Enrolment period: 27 months (July 1987 to October 1989). Exclusion criteria: significant comorbid conditions such as heart disease, stroke, pancreatitis, haematological disorder, or history of drug abuse; known bile common duct stones before surgery; undergoing an unplanned common bile duct exploration; laser malfunction. Mean age (± SD): scalpel: 37 ± 14 years; electrosurgery: 36 ± 13. Sex (M/F): scalpel: 4/27; electrosurgery: 3/25. Time of last evaluation: 7 days. |
|
| Interventions | 3 study groups: scalpel, electrosurgery, and laser (laser not relevant to review). Scalpel group: skin and all layers of abdominal wall to the peritoneum were incised with scalpel. Bleeding points controlled with ligature; electrosurgery not used. Electrosurgery group: skin incision to reticular dermis with a scalpel, and further incision to peritoneum with cutting currents of 25 W to 35 W. Bleeding points controlled with 35 W to 50 W cautery; ligatures not used. |
|
| Outcomes | Incision time. Incision blood loss. Subjective pain. Objective pain. Rate of wound seromas/infections. |
|
| Notes | Participants receiving electrosurgical incisions had mean weight somewhat greater than those receiving scalpel incisions, but the differences were not statistically significant. 7 participants received antibiotics after surgery because of bile spillage during gallbladder removal (5 in scalpel group, 2 in electrosurgery group). Age and weight were comparable among all groups. Wound classification: not stated. Funding source: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "they were blinded and randomised." Comment: no specific information regarding sequence generation provided. |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment probably adequate; however, insufficient information on how this was achieved. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "After surgery, patients and nursing personnel were kept blinded to incision methods used for a minimum of 3 days." Comment: blinding of surgeons not feasible due to nature of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "After surgery, patients and nursing personnel were kept blinded to incision methods used for a minimum of 3 days." Comment: unclear whether outcome assessors were sufficiently blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: appeared that 12/100 randomised participants were excluded after randomisation either because of unplanned common bile duct exploration (10 participants) or laser malfunction (2 participants). Unclear to which study groups the excluded participants belonged. |
| Selective reporting (reporting bias) | Low risk | Comment: no evidence to suggest selective reporting. |
| Other bias | High risk | Comment: basic characteristics appeared comparable; however, study groups varied in the way that bleeding points were controlled. |
Prakash 2015.
| Methods | Study design: prospective RCT in a single institution. Randomisation method: computer generated. Number of participants randomised: 90. Number of participants analysed: 82 (41 in scalpel group, 41 in electrosurgery group). Analysis: available‐case. |
|
| Participants | Inclusion criteria: people undergoing elective or emergency midline abdominal surgery. Exclusion criteria: previous midline laparotomy, concurrent anticoagulant or corticosteroid therapy, factors that could affect wound healing, and active wound infections anywhere in the body at the time of surgery. Location: Department of surgery, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), Pondicherry, India. Enrolment period: 6 months (May to December 2013). Mean age (± SD): scalpel: 47.27 ± 12.43; electrosurgery group: 47.76 ± 12.32 years. Sex (M/F): 57/25. |
|
| Interventions | Scalpel group: incision using scalpel with disposable blade of appropriate size. Electrosurgery group: incision through skin and deeper tissues with diathermy using standard diathermy pen electrode, set at 'pure‐cutting' mode and delivering 350‐kHz sinusoidal current. Diathermy was used for haemostasis of bleeding points in the subcutaneous plane in both groups. All participants were given IV ceftriaxone 1 g and metronidazole 500 mg at the time of induction and postoperatively as needed. |
|
| Outcomes | Incision time. Blood loss during incision. Postoperative incision site pain (VAS). Wound infection. |
|
| Notes | Wound classification: not stated. Funding source: not stated; however, authors reportedly had no potential or real conflict of interests. Sample size calculated to be 45 in each group considering incision time as primary variable. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers. Block randomisation into blocks of 10 carried out. |
| Allocation concealment (selection bias) | Low risk | Opaque sealed envelopes which were opened in the operating theatre after induction of the participant. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participant blinded; however, blinding of surgeon not feasible due to nature of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: blinded principal investigator noted results which were conveyed by assistants of the operating surgeons. Unclear whether these assistants were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Similar proportion of randomised participants (4/45 in scalpel group vs. 3/45 in electrosurgery group) did not complete the trial as a result of death and discharge before outcome assessment. |
| Selective reporting (reporting bias) | High risk | Trial registration available (CTRI ID: 2013/08/003870) and all prespecified outcomes reported in study; however, measures of variance not reported for pain outcome. Contacted authors but received no reply. |
| Other bias | Low risk | No other apparent biases. |
Rongetti 2014.
| Methods | Study design: RCT. Location: Barretos Cancer Hospital. Number of participants randomised: 161; scalpel group: 90; electrosurgery group: 71. Number of participants analysed: 130 (attrition 31: digestive tract mucosa exposure 18 participants, randomisation failure 6, death 2, loss to follow‐up 2, blinding disruption 1, surgery cancellation 1). Analysis: available‐case. Power calculation: taking into account an alpha error of 5%, a beta error of 20%, an expected surgical site infection of 3% to 5% and an equivalence limit of 10%, 50 to 82 participants were required per group. |
|
| Participants | Enrolment period: July 2010 to July 2012. Inclusion criteria: women > 18 years of age who agreed to participate and underwent elective abdominal gynaecological surgery via laparotomy for diagnosis and curative or palliative cancer treatment. Exclusion criteria: women who underwent surgery that involved exposure of the digestive tract mucosa, ostomies, re operations, or emergency surgeries. Mean age: scalpel: 56.2 years; electrosurgery: 54.9 years. Sex (M/F): scalpel: 0/90: electrosurgery: 0/71. |
|
| Interventions | Scalpel group: subcutaneous tissue opened to the aponeurosis with conventional scalpel. Electrosurgery group: subcutaneous tissue opened to the aponeurosis with electrosurgery in 'cut' mode. Conventional scalpel used to open the epidermis for all participants. Bleeding vessels in the subcutaneous tissue were spot coagulated using electrocautery. Follow‐up visits at 14 and 30 days following the operation. |
|
| Outcomes | Surgical time (day 14 postsurgery). Surgical complications: surgical site infection, discharge, dehiscence and epidermolysis (day 30 postsurgery). |
|
| Notes | Wound classification: scalpel group vs electrosurgery group:
Funding: supported by the Teaching and Research Instituted of the Barretos Cancer Hospital and no grant from a funding agency. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...Stratified randomisation based on BMI... Immediately before surgery, the attending surgeon randomly selected the type of scalpel to be used to open the skin and subcutaneous tissue." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "...non‐transparent envelopes." Comment: no mention of whether the envelopes were sealed and numbered. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated; however, blinding not feasible due to nature of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Wound assessments were performed by two nurses who specialised in standardised post‐operative care but had no prior knowledge as to the type of scalpel used in the surgery." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 19% attrition. Single main reason for attrition was 'digestive tract mucosa exposure' which happened more in the scalpel group (15 participants) compared to the electrosurgery group (3 participants). |
| Selective reporting (reporting bias) | High risk | Where means were reported, SDs were not reported and most outcomes were not reported according to study groups. |
| Other bias | Low risk | No other apparent bias. |
Shivagouda 2010.
| Methods | Study design: prospective RCT in a single institution. Randomisation method: not stated. Number of participants randomised: 60. Number of participants analysed: 60 (30 in scalpel group, 30 in electrosurgery group). Analysis: full ITT. |
|
| Participants | Inclusion criteria: people undergoing inguinal hernia repair. Location: KLES Dr. Prabhakar Kore Hospital, Belgaum, India. Enrolment period: 12 months. Exclusion criteria: complicated inguinal hernia; preoperative use of analgesics for > 3 days per week for > 3 months; people aged < 12 years and > 50 years; people with chronic pain > 3 months; history of drug or alcohol abuse; severe hepatic, renal, and cardiovascular dysfunction; diabetes mellitus; immunocompromised status. Mean age (± SD): scalpel: 47.7 ± 13.95 years; electrosurgery: 47.8 ± 16.21 years. Sex (M/F): not stated. Time of last evaluation: 7 days. |
|
| Interventions | Scalpel group: skin incisions with scalpel. Bleeding controlled by forceps coagulation using pulse sine wave on 30 W power supply. Electrosurgery group: incisions with electrocautery needle using pulse sine wave current and power setting of 70 W. Haemostasis achieved with forceps coagulation. Abdomen closed using continuous proline for external aponeurosis, intermittent plane catgut for subcutaneous tissue, and mattress suture with 2‐0 silk for skin. |
|
| Outcomes | Postoperative pain at 6, 12, and 24 hours (VAS). Postoperative wound complications (seroma, haematoma, purulent discharge). Postoperative analgesic requirement. |
|
| Notes | Age comparable between groups. Wound classification: not stated. Funding source: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization done according to computerized randomisation table with block length of 10x6." Comment: random sequence generation probably acceptable. |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not stated. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated; however, blinding not feasible due to nature of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The observer is blinded to the type of incision used and gave his observation based on the predefined criteria." Comment: adequate blinding of outcome assessors achieved. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: appeared that all participants completed study. |
| Selective reporting (reporting bias) | Low risk | Comment: no evidence to suggest selective reporting. |
| Other bias | Low risk | No other apparent biases. |
Siraj 2011.
| Methods | Study design: prospective RCT in a single institution. Randomisation method: coin tossing. Number of participants randomised: 100. Number of participants analysed: 100 (50 in scalpel group, 50 in electrosurgery group). Analysis: full ITT. |
|
| Participants | Inclusion criteria: people undergoing elective midline laparotomy. Only clean and clean contaminated cases were included. Location: Department of Surgery, PNS Shifa, Karachi, Pakistan. Enrolment period: 16 months (March 2007 to June 2008). Exclusion criteria: previous midline laparotomy and concurrent anticoagulant or corticosteroid therapy. Mean age (± SD): 46.85 ± 5.38 (range 18 to 87) years. Sex (M/F): 56/44; 28:22 in both groups. Time of last evaluation: 5 days. |
|
| Interventions | Scalpel group: incision with disposable scalpel blade from skin to peritoneum. Electrosurgery group: electrosurgical unit, set at 'pure cutting' mode and delivering 417‐kHz sinusoidal current, used to incise skin and all deeper layers. Diathermy was used in 'coagulation' mode for haemostasis in both groups. |
|
| Outcomes | Wound‐related pain (VAS). Wound infection. |
|
| Notes | Age comparable between groups. Wound classification: all incisions classified as clean or clean‐contaminated. Funding source: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "They were randomised by coin toss method into two equal groups." Comment: random sequence generation appeared adequate. |
| Allocation concealment (selection bias) | Unclear risk | Comment: concealment method not described in sufficient detail. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated; however, blinding not feasible due to nature of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Comment: blinding process not described. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: appeared that all participants completed study. |
| Selective reporting (reporting bias) | High risk | Comment: no evidence to suggest selective reporting except that measures of variance were not reported for continuous outcomes. |
| Other bias | Low risk | No other apparent biases. |
Telfer 1993.
| Methods | Study setting: prospective RCT in a single institution. Randomisation method: sealed assignment envelopes. Number of participants randomised: 101. Number of participants analysed: 101 (50 in scalpel group, 51 in electrosurgery group). Analysis: full ITT. |
|
| Participants | Inclusion criteria: people scheduled for abdominal surgery, for any reason, via a midline incision. Location: Gartnavel General Hospital, Glasgow, UK. Enrolment period: 18 months. Exclusion criteria: difficulty co‐operating with the measurement techniques to be used in the postoperative period or unable to give informed consent. Median age (IQR): scalpel: 58 (38 to 66) years; electrosurgery: 64 (54 to 71) years. Sex (M/F): scalpel: 15/35; electrosurgery: 26/25 (difference in sex ratio significant). Time of last evaluation: 96 hours after surgery. |
|
| Interventions | Scalpel group: scalpel incision through all layers. Electrosurgery group: scalpel was used to score the dermis, the remainder of the incision was made using an electrosurgical technique. An Eschmann TD 411‐S (Eschmann and Walsh, Lansing, UK) electrosurgical unit was used, and was set to supply a pure cutting current. Coagulation diathermy with a pinpoint coagulation current was used in both groups to control bleeding points. Wound closure consisted of a mass closure technique using a monofilament non‐absorbable suture for the abdominal wall and a monofilament absorbable suture placed in the subcuticular layer for the skin. |
|
| Outcomes | Incision time. Postoperative pain (VAS). Postoperative PEFR. Postoperative requirements for analgesia. |
|
| Notes | Age and weight were comparable between groups. Majority (72%) of participants underwent operation for colorectal cancer; remainder had procedures to treat inflammatory bowel disease and a variety of other conditions. The groups were almost identical with regard to diagnosis and type of surgical procedures. Wound classification: not stated. Funding source: not stated. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no specific information regarding sequence generation provided. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were randomised by drawing an unmarked envelope in the operating room." Comment: unclear whether envelopes were sealed and opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not stated; however, blinding not feasible due to nature of intervention. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All postoperative assessment was undertaken by an investigator unaware of the incision method used." Comment: outcome assessor adequately blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: appeared that all participants completed study. |
| Selective reporting (reporting bias) | High risk | Comment: no evidence to suggest selective reporting except that measures of variance were not reported for continuous outcomes. |
| Other bias | Unclear risk | Comment: basic characteristics appeared comparable except for sex ratio. |
BMI: body mass index; F: female; ITT: intention to treat; IV: intravenous; M: male; PEFR: peak expiratory flow rate; RCT: randomised controlled trial; SD: standard deviation; VAS: visual analogue scale.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aird 2015 | Inclusion criteria with regard to comparison intervention not met. Subcutaneous and fascial layers in all participants assigned to the scalpel group were divided by using electrosurgery. |
| Chrysos 2005 | Prospective study comparing electrosurgery and scalpel incisions in tension‐free inguinal hernioplasty. Allocated alternately to intervention, not a true randomisation. |
| Duxbury 2003 | Randomised trial of scalpel or diathermy in excision of pilonidal disease, not abdominal wound creation. |
| Franchi 2001 | Large cross‐sectional study of scalpel vs electrocautery for midline abdominal incision, not a randomised trial. |
| Ji 2006 | Randomised trial comparing 3 different approaches for abdominal incision on wound healing. high‐frequency electric surgical knives (not electrosurgery) for cutting tissues and electric coagulation for haemostasis vs scalpel for cutting tissues and electric coagulation for haemostasis vs scalpel for cutting tissues and silk thread suturing for haemostasis. |
| Keel 2002 | Randomised trial of diamond laser scalpel vs steel scalpel for fusiform cutaneous excision, not scalpel vs electrosurgery. |
| Miller 1988 | Randomised trial of scalpel vs electrocautery in modified radical mastectomy, not abdominal wound creation. |
| Porter 1998 | Randomised trial of scalpel vs electrocautery in dissection of the mastectomy flaps, not abdominal wound creation. |
| Shamim 2009 | Blocked randomisation used for allocation into 2 groups (A and B). Participants divided in blocks of 2, and within each block the first participant was allocated to group A and the second participant to group B. Considered quasi‐randomised trial. |
| Stupart 2016 | Inclusion criteria with regard to comparison intervention not met. In both groups, tissues deep to the skin were incised using diathermy. |
Characteristics of studies awaiting assessment [ordered by study ID]
Husnain 2006.
| Methods | Unclear. |
| Participants | People undergoing elective cholecystectomy. |
| Interventions | Scalpel vs electrosurgery. |
| Outcomes | Unclear. |
| Notes | Awaiting full text: study not available on the journal website. The editor was contacted on the 23 February 2016 and we were informed that some old issues including Husnain 2006 were missing and contact details of authors were unavailable. |
Upadhyay 2013.
| Methods | Unclear. |
| Participants | People undergoing inguinal hernioplasty. |
| Interventions | Electrosurgery vs scalpel. |
| Outcomes | Unclear. |
| Notes | Awaiting full text. |
Differences between protocol and review
The title has been changed from 'Scalpel versus electrosurgery for abdominal incisions' to 'Scalpel versus electrosurgery for major abdominal incisions' in response to peer review feedback.
We have populated sections of the 'Methods' and 'Discussion' which were previously not available in the older version of Review Manager as follows: Unit of analysis issues; Dealing with missing data; Assessment of reporting biases; Table 1; Overall completeness and applicability of evidence; Potential biases in the review process.
We intended to use 'length of hospital stay' to reflect 'time to wound healing'. However, 'time‐to‐complete wound healing' is usually expressed as hazard ratios (HR) (with their 95% confidence interval), in accordance with the methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Deeks 2011). In future updates, if studies reporting time‐to‐event data (e.g. time to wound healing) do not report a HR, we plan to estimate this using other reported data, such as the numbers of events, with application of available statistical methods (Parmar 1998; Tierney 2007; Wang 2013). In the absence of these measures, if there are any studies in which all wounds healed, we will consider the mean or median time to healing without survival analysis as a valid outcome (i.e. if the trial authors regarded time to healing as a continuous measure because there was no censoring).
Following peer review feedback, we carried out a post hoc subgroup analysis on wound contamination.
Differences between previous review and this update
The previous review had deviated from the protocol by reporting outcomes which had not been prespecified. We corrected this by reporting both primary and secondary outcomes as stated in the protocol.
Contributions of authors
Kittipat Charoenkwan: conceived, designed and coordinated the review update; extracted data; checked the quality of data extraction; analysed and interpreted data; undertook quality assessment; performed statistical analysis; produced the first draft of the review update; contributed to writing and editing the review update; performed previous work that was the foundation of the current review update; approved the final review update prior to submission and is a guarantor of the review update.
Zipporah Iheozor‐Ejiofor: designed and coordinated the review update; extracted data; checked the quality of data extraction; analysed and interpreted data; undertook quality assessment; performed statistical analysis; produced the first draft of the review update; contributed to writing and editing the review update; wrote to study author/experts/companies and approved the final review update prior to submission.
Kittipan Rerkasem: coordinated the review update; checked quality assessment; checked the quality of the statistical analysis; contributed to writing and editing the review update; advised on the review update; performed previous work that was the foundation of the current review update and approved the final review update prior to submission.
Elizabeth Matovinovic: coordinated the review update; checked quality assessment; checked the quality of the statistical analysis; contributed to writing and editing the review update; advised on the review update and approved the final review update prior to submission.
Contributions of editorial base:
Nicky Cullum (Editor): edited the previous version of the review, advised on methodology, interpretation and review content. Approved the previous version of the review prior to submission.
Gill Norman (Editor): edited this review update, advised on methodology, interpretation and content. Approved the updated review prior to submission.
Sally Bell‐Syer (Managing Editor): co‐ordinated the editorial process for the previous version of the review. Advised on methodology, interpretation, and content. Edited and copy edited the previous version of the review.
Gill Rizzello (Managing Editor): co‐ordinated the editorial process for this update. Advised on interpretation, and content. Edited and copy edited the updated review.
Ruth Foxlee (Information Specialist): designed the search strategy, ran the searches, and edited the search methods section for the previous version of this review.
Naomi Shaw (Information Specialist): updated the search strategy, ran the searches, and edited the search methods section for this review update.
Ursula Gonthier (Editorial Assistant): edited the plain language summary and reference sections for this review update.
Sources of support
Internal sources
Faculty of Medicine, Chang Mai University, Thailand.
Division of Nursing, Midwifery and Social Work, School of Health Sciences, Faculty of Biology, Medicine and Health, University of Manchester, UK.
External sources
-
National Institute of Health Research (NIHR), UK.
This project was supported by the National Institute of Health Research (NIHR) via Cochrane Infrastructure funding to Cochrane Wounds. The views expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or Department of Health.
Declarations of interest
Kittipat Charoenkwan: none known.
Zipporah Ejiofor‐Iheozor: none known.
Kittipat Rerkasem: is funded by the Faculty of Medicine, Chiang Mai University.
Elizabeth Matovinovic: none known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Argerich 2005 {published data only}
- Argerich AL, Santos JC, Lima MA, Mikalauscas RR, Hassan E. Electrocautery effects in infection rates of operative abdominal wounds [Efeitos do eletrocauterio nas taxas de infeccao de feridas operatorias abdominais]. Jornal Brasileiro de Medicina 2005;89(4):12‐6. [Google Scholar]
Dixon 1990 {published data only}
- Dixon AR, Watkin DF. Electrosurgical skin incision versus conventional scalpel: a prospective trial. Journal of the Royal College of Surgeons of Edinburgh 1990;35(5):299‐301. [PubMed] [Google Scholar]
Elbohoty 2015 {published data only}
- Elbohoty AE, Gomaa MF, Abdelaleim M, Abd‐El‐Gawad M, Elmarakby M. Diathermy versus scalpel in transverse abdominal incision in women undergoing repeated cesarean section: a randomized controlled trial. Journal of Obstetrics and Gynaecology Research 2015;41:1541‐6. [DOI] [PubMed] [Google Scholar]
Eren 2010 {published data only}
- Eren T, Balik E, Ziyade S, Yamaner S, Akyuz A, Bugra D. Do different abdominal incision techniques play a role in wound complications in patients operated on for gastrointestinal malignancies? "Scalpel vs. electrocautery". Acta Chirurgica Belgica 2010;110(4):451‐6. [DOI] [PubMed] [Google Scholar]
Groot 1994 {published data only}
- Groot G, Chappell EW. Electrocautery used to create incisions does not increase wound infection rates. American Journal of Surgery 1994;167(6):601‐3. [DOI] [PubMed] [Google Scholar]
Hemsell 1993 {published data only}
- Hemsell DL, Hemsell PG, Nobles B, Johnson ER, Little BB, Heard M. Abdominal wound problems after hysterectomy with electrocautery vs. scalpel subcutaneous incision. Infectious Diseases in Obstetrics and Gynecology 1993;1(1):27‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hussain 1988 {published data only}
- Hussain SA, Hussain S. Incisions with knife or diathermy and postoperative pain. British Journal of Surgery 1988;75(12):1179‐80. [DOI] [PubMed] [Google Scholar]
Johnson 1990 {published data only}
- Johnson CD, Serpell JW. Wound infection after abdominal incision with scalpel or diathermy. British Journal of Surgery 1990;77(6):626‐7. [DOI] [PubMed] [Google Scholar]
Kearns 2001 {published data only}
- Kearns SR, Connolly EM, McNally S, McNamara DA, Deasy J. Randomized clinical trial of diathermy versus scalpel incision in elective midline laparotomy. British Journal of Surgery 2001;88(1):41‐4. [DOI] [PubMed] [Google Scholar]
Marsh 2015 {published data only}
- Marsh DJ, Fox A, Grobbelaar AO, Chana JS. Abdominoplasty and seroma: a prospective randomised study comparing scalpel and handheld electrocautery dissection. Journal of Plastic, Reconstructive and Aesthetic Surgery 2015;68(2):192‐6. [DOI] [PubMed] [Google Scholar]
Pearlman 1991 {published data only}
- Pearlman NW, Stiegmann GV, Vance V, Norton LW, Bell RC, Staerkel R, et al. A prospective study of incisional time, blood loss, pain, and healing with carbon dioxide laser, scalpel, and electrosurgery. Archives of Surgery 1991;126(8):1018‐20. [DOI] [PubMed] [Google Scholar]
Prakash 2015 {published data only}
- Prakash LD, Balaji N, Kumar SS, Kate V. Comparison of electrocautery incision with scalpel incision in midline abdominal surgery: a double blind randomized controlled trial. International Journal of Surgery 2015;19:78‐82. [DOI] [PubMed] [Google Scholar]
Rongetti 2014 {published data only}
- Rongetti RL, Oliveira e Castro PD, Vieira RA, Serrano SV, Mengatto MF, Fregnani JH. Surgical site infection: an observer‐blind, randomized trial comparing electrocautery and conventional scalpel. International Journal of Surgery 2014;12(7):681‐7. [DOI] [PubMed] [Google Scholar]
Shivagouda 2010 {published data only}
- Shivagouda P, Gogeri BV, Godhi AS, Metgud SC. Prospective randomized control trial comparing the efficacy of diathermy incision versus scalpel incision over skin in patients undergoing inguinal hernia repair. Recent Research in Science and Technology 2010;2(8):44‐7. [Google Scholar]
Siraj 2011 {published data only}
- Siraj A, Gilani AA, Dar MF, Raziq S. Elective midline laparotomy: comparison of diathermy and scalpel incisions. Professional Medical Journal 2011;18:106‐11. [Google Scholar]
Telfer 1993 {published data only}
- Telfer JR, Canning G, Galloway DJ. Comparative study of abdominal incision techniques. British Journal of Surgery 1993;80(2):233‐5. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aird 2015 {published data only}
- Aird LN, Bristol SG, Phang PT, Raval MJ, Brown CJ. Randomized double‐blind trial comparing the cosmetic outcome of cutting diathermy versus scalpel for skin incisions. British Journal of Surgery 2015;102(5):489‐94. [DOI] [PubMed] [Google Scholar]
Chrysos 2005 {published data only}
- Chrysos E, Athanasakis E, Antonakakis S, Xynos E, Zoras O. A prospective study comparing diathermy and scalpel incisions in tension‐free inguinal hernioplasty. American Surgeon 2005;71(4):326‐9. [PMID: 15943407] [DOI] [PubMed] [Google Scholar]
Duxbury 2003 {published data only}
- Duxbury MS, Blake SM, Dashfield A, Lambert AW. A randomised trial of knife versus diathermy in pilonidal disease. Annals of the Royal College of Surgeons of England 2003;85(6):405‐7. [DOI: 10.1308/003588403322520799] [DOI] [PMC free article] [PubMed] [Google Scholar]
Franchi 2001 {published data only}
- Franchi M, Ghezzi F, Benedetti‐Panici PL, Melpignano M, Fallo L, Tateo S, et al. A multicentre collaborative study on the use of cold scalpel and electrocautery for midline abdominal incision. American Journal of Surgery 2001;181(2):128‐32. [PMID: 11425052] [DOI] [PubMed] [Google Scholar]
Ji 2006 {published data only}
- Ji GW, Wu YZ, Wang X, Pan HX, Li P, Du WY, et al. Experimental and clinical study of influence of high‐frequency electric surgical knives on healing of abdominal incision. World Journal of Gastroenterology 2006;12(25):4082‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Keel 2002 {published data only}
- Keel DM, Goldman MP, Fitzpatrick RE, Butterwick KJ. Diamond laser scalpel vs steel scalpel: a side by side comparison of cutaneous wound healing. Lasers in Surgery and Medicine 2002;31(1):41‐4. [DOI: 10.1002/lsm.10072] [DOI] [PubMed] [Google Scholar]
Miller 1988 {published data only}
- Miller E, Paull DE, Morrissey K, Cortese A, Nowak E. Scalpel versus electrocautery in modified radical mastectomy. American Surgeon 1988;54(5):284‐6. [PMID: 3364865] [PubMed] [Google Scholar]
Porter 1998 {published data only}
- Porter KA, O'Connor S, Rimm E, Lopez M. Electrocautery as a factor in seroma formation following mastectomy. American Journal of Surgery 1998;176(1):8‐11. [PMID: 9683123] [DOI] [PubMed] [Google Scholar]
Shamim 2009 {published data only}
- Shamim M. Diathermy vs. scalpel skin incisions in general surgery: double‐blind, randomized, clinical trial. World Journal of Surgery 2009;33:1594‐9. [DOI] [PubMed] [Google Scholar]
Stupart 2016 {published data only}
- Stupart DA, Sim FW, Chan ZH, Guest GD, Watters DA. Cautery versus scalpel for abdominal skin incisions: a double blind, randomized crossover trial of scar cosmesis. Australian and New Zealand Journal of Surgery 2016;86:303‐6. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Husnain 2006 {published data only}
- Husnain SS, Chaudhry AK, Tariq GR, Sheikh IA, Munir I. Comparison of frequency of postoperative wound infection with the use of scalpel/diathermy during elective cholecystectomy. Journal of Surgery Pakistan 2006;11(3):116‐8. [Google Scholar]
Upadhyay 2013 {published data only}
- Upadhyay S, Bansal N. Electrocautery versus scalpel incision in inguinal hernioplasty. Research Journal of Pharmaceutical, Biological and Chemical Sciences 2013;4(4):499‐503. [Google Scholar]
Additional references
Anderson 2015
- Anderson TL, Thomassee MS. Principles of electrosurgery and laser energy applied to gynecologic surgery. In: Jones HW III, Rock JA editor(s). Te Linde's Operative Gynecology. 11th Edition. Philadelphia: Wolters Kluwer, 2015:249‐64. [Google Scholar]
Chang 2011
- Chang EI, Carlson GA, Vose JG, Huang EJ, Yang GP. Comparative healing of rat fascia following incision with three surgical instruments. Journal of Surgical Research 2011;167(1):e47‐54. [DOI] [PubMed] [Google Scholar]
Cruse 1980
- Cruse PJ, Foord R. The epidemiology of wound infection: a 10‐year prospective study of 62,939 wounds. Surgical Clinics of North America 1980;60:27‐40. [DOI] [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JP, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
GRADE 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editor(s), GRADE working group. GRADE Handbook. gdt.guidelinedevelopment.org/central_prod/_design/client/handbook/handbook.html (accessed 5 February 2016).
GRADEpro [Computer program]
- GRADE Working Group, McMaster University. GRADEpro. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Higgins 2011
- Higgins JP, Altman DG, Sterne JA, editor(s). Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hróbjartsson 2012
- Hróbjartsson A, Thomsen AS, Emanuelsson F, Tendal B, Hilden J, Boutron I, et al. Observer bias in randomised clinical trials with binary outcomes: systematic review of trials with both blinded and non‐blinded outcome assessors. BMJ 2012;344:e1119. [DOI: 10.1136/bmj.e1119] [DOI] [PubMed] [Google Scholar]
Keenan 1984
- Keenan KM, Rodeheaver GT, Kenney JG, Edlich RF. Surgical cautery revisited. American Journal of Surgery 1984;147:818‐21. [DOI] [PubMed] [Google Scholar]
Kontopantelis 2013
- Kontopantelis E, Springate DA, Reeves D. A re‐analysis of the Cochrane Library data: the dangers of unobserved heterogeneity in meta‐analyses. PloS One 2013;26:e69930. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kumagai 1991
- Kumagai SG, Rosales RF, Hunter GC, Rappaport WD, Witzke DB, Chvapil TA, et al. Effects of electrocautery on midline laparotomy wound infection. American Journal of Surgery 1991;162:620‐3. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Loh 2009
- Loh SA, Carlson GA, Chang EI, Huang E, Palanker D, Gurtner GC. Comparative healing of surgical incisions created by the PEAK PlasmaBlade, conventional electrosurgery, and a scalpel. Plastic and Reconstructive Surgery 2009;124(6):1849‐59. [DOI] [PubMed] [Google Scholar]
Madden 1970
- Madden JE, Edlich RF, Custer JR, Panek PH, Thul J, Wangensteen OH. Studies in the management of the contaminated wound. American Journal of Surgery 1970;119:222‐4. [DOI] [PubMed] [Google Scholar]
NICE 2008
- National Institute of Health and Care Excellence (NICE). Surgical site infection: prevention and treatment of surgical site infection. NICE Clinical guideline CG74, 2008. www.nice.org.uk/guidance/cg74 (accessed 2 May 2017).
Parmar 1998
- Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta‐analyses of the published literature for survival endpoints. Statistics in Medicine 1998;17(30):2815‐34. [DOI] [PubMed] [Google Scholar]
Rappaport 1990
- Rappaport WD, Hunter GC, Allen R, Lick S, Halldorsson A, Chvapil T, et al. Effect of electrocautery on wound healing in midline laparotomy incisions. American Journal of Surgery 1990;160:618‐20. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
SIGN 2015
- Scottish Intercollegiate Guidelines Network (SIGN). Search filters. www.sign.ac.uk/methodology/filters.html#random (accessed 10 March 2017).
Soderstrom 2003
- Soderstrom R. Principles of electrosurgery as applied to gynecology. In: Rock JA, Jones HW III editor(s). Te Linde's Operative Gynecology. 9th Edition. Philadelphia (PA): Lippincott Williams & Wilkins, 2003:291‐308. [Google Scholar]
Tierney 2007
- Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2007;8:16. [DOI: 10.1186/1745-6215-8-16] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wang 2013
- Wang Y, Zeng T. Response to: practical methods for incorporating summary time‐to‐event data into meta‐analysis. Trials 2013;14:391. [DOI: 10.1186/1745-6215-14-391] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Charoenkwan 2006
- Charoenkwan K, Chotirosniramit N, Rerkasem K. Scalpel versus electrosurgery for abdominal incisions. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD005987] [DOI] [PubMed] [Google Scholar]
Charoenkwan 2012
- Charoenkwan K, Chotirosniramit N, Rerkasem K. Scalpel versus electrosurgery for abdominal incisions. Cochrane Database of Systematic Reviews 2012, Issue 6. [DOI: 10.1002/14651858.CD005987.pub2] [DOI] [PubMed] [Google Scholar]


