Abstract
Background
Dementia is a clinical syndrome with a number of different causes which is characterised by deterioration in cognitive, behavioural, social and emotional functions. Pharmacological interventions are available but have limited effect to treat many of the syndrome's features. Less research has been directed towards non‐pharmacological treatments. In this review, we examined the evidence for effects of music‐based interventions as a treatment.
Objectives
To assess the effects of music‐based therapeutic interventions for people with dementia on emotional well‐being including quality of life, mood disturbance or negative affect, behavioural problems, social behaviour, and cognition at the end of therapy and four or more weeks after the end of treatment.
Search methods
We searched ALOIS, the Specialized Register of the Cochrane Dementia and Cognitive Improvement Group (CDCIG) on 14 April 2010 using the terms: music therapy, music, singing, sing, auditory stimulation. Additional searches were also carried out on 3 July 2015 in the major healthcare databases MEDLINE, Embase, psycINFO, CINAHL and LILACS; and in trial registers and grey literature sources. On 12 April 2016, we searched the major databases for new studies for future evaluation.
Selection criteria
We included randomized controlled trials of music‐based therapeutic interventions (at least five sessions) for people with dementia that measured any of our outcomes of interest. Control groups either received usual care or other activities.
Data collection and analysis
Two reviewers worked independently to screen the retrieved studies against the inclusion criteria and then to extract data and assess methodological quality of the included studies. If necessary, we contacted trial authors to ask for additional data, including relevant subscales, or for other missing information. We pooled data using random‐effects models.
Main results
We included 17 studies. Sixteen studies with a total of 620 participants contributed data to meta‐analyses. Participants in the studies had dementia of varying degrees of severity, but all were resident in institutions. Five studies delivered an individual music intervention; in the others, the intervention was delivered to groups of participants. Most interventions involved both active and receptive musical elements. The methodological quality of the studies varied. All were at high risk of performance bias and some were at high risk of detection or other bias. At the end of treatment, we found low‐quality evidence that music‐based therapeutic interventions may have little or no effect on emotional well‐being and quality of life (standardized mean difference, SMD 0.32, 95% CI −0.08 to 0.71; 6 studies, 181 participants), overall behaviour problems (SMD −0.20, 95% CI −0.56 to 0.17; 6 studies, 209 participants) and cognition (SMD 0.21, 95% CI −0.04 to 0.45; 6 studies, 257 participants). We found moderate‐quality evidence that they reduce depressive symptoms (SMD −0.28, 95% CI −0.48 to −0.07; 9 studies, 376 participants), but do not decrease agitation or aggression (SMD −0.08, 95% CI −0.29 to 0.14; 12 studies, 515 participants). The quality of the evidence on anxiety and social behaviour was very low, so effects were very uncertain. The evidence for all long‐term outcomes was also of very low quality.
Authors' conclusions
Providing people with dementia with at least five sessions of a music‐based therapeutic intervention probably reduces depressive symptoms but has little or no effect on agitation or aggression. There may also be little or no effect on emotional well‐being or quality of life, overall behavioural problems and cognition. We are uncertain about effects on anxiety or social behaviour, and about any long‐term effects. Future studies should employ larger sample sizes, and include all important outcomes, in particular 'positive' outcomes such as emotional well‐being and social outcomes. Future studies should also examine the duration of effects in relation to the overall duration of treatment and the number of sessions.
Keywords: Aged, Humans, Music Therapy, Dementia, Dementia/rehabilitation, Dementia/therapy, Mental Disorders, Mental Disorders/therapy, Randomized Controlled Trials as Topic
Music‐based therapeutic interventions for people with dementia
Background
People with dementia gradually develop difficulties with memory, thinking, language and daily activities. Dementia is often associated with emotional and behavioural problems and may lead to a reduction in a person's quality of life. In the later stages of dementia it may be difficult for people to communicate with words, but even when they can no longer speak they may still be able to hum or play along with music. Therapy involving music may therefore be especially suitable for people with dementia. Music therapists are specially qualified to work with individuals or groups of people, using music to try to help meet their physical, psychological and social needs. Other professionals may also be trained to provide similar treatments.
Purpose of this review
We wanted to see if we could find evidence that treatments based on music improve the emotional well‐being and quality of life of people with dementia. We were also interested in evidence about their effects on emotional, behavioural, social or cognitive (e.g. thinking and remembering) problems in people with dementia.
What we did
We searched for trials in which people with dementia were randomly allocated to a music‐based treatment or to a comparison group, and in which any of the outcomes we were interested in were measured. The comparison groups might have had no special treatment, or might have been offered a different activity. The trials had to have offered at least five sessions of treatment because we thought fewer sessions than this were unlikely to have much effect. If we judged that the trials were similar enough, then we combined their results in order to estimate the effect of the treatment as accurately as possible.
What we found
We found seventeen trials to include in the review and we were able to combine results for at least some outcomes from 620 people. All of the people in the trials were living in care homes. People with all severities of dementia were included. Some trials compared music‐based treatments with usual care, and some compared it with other activities, such as cooking or painting. The quality of the trials and how well they were reported varied, and this affected our confidence in the results. First, we looked at outcomes immediately after a course of therapy ended. From our results, we could be moderately confident that music‐based treatments improve symptoms of depression, but do not help with agitated or aggressive behaviour. We were less confident in our results on emotional well‐being including quality of life, overall behavioural problems, and cognition, but music‐based treatments may have little or no effect on these outcomes. We had very little confidence in our results on anxiety and social interaction. Some studies also looked to see whether there were any lasting effects four weeks or more after treatment ended. However, there were few data and we were very uncertain about the results. Further trials are likely to have a significant impact on what we know about the effects of music‐based treatments for people with dementia, and so continuing research is important.
Summary of findings
Summary of findings for the main comparison.
Music‐based therapeutic interventions compared to usual care or other activities for people with dementia: end of treatment effects
| Music‐based therapeutic interventions compared to usual care or other activities for people with dementia: end of treatment effects | |||
| Patient or population: people with dementia (all resided in institutional settings) Intervention: music‐based therapeutic interventions Comparison: usual care or other activities | |||
|
Outcomes (end of treatment) measured with a variety of scales except for social behaviour |
Anticipated absolute effects* (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) |
|
Score with music therapy compared with usual care or other activities | |||
| Emotional well‐being including quality of life | The score in the intervention group was 0.32 SDs higher (0.08 lower to 0.71 higher) | 181 (6 RCTs) | ⊕⊕⊝⊝ LOW 1 2 |
| Mood disturbance or negative affect: depression | The score in the intervention group was 0.28 SDs lower (0.48 lower to 0.07 lower) | 376 (9 RCTs) | ⊕⊕⊕⊝ MODERATE 1 |
| Mood disturbance or negative affect: anxiety | The score in the intervention group was 0.50 SDs lower (0.84 lower to 0.16 lower) | 365 (11 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 3 5 |
| Behavioural problems: agitation or aggression | The score in the intervention group was 0.08 SDs lower (0.29 lower to 0.14 higher) | 515 (12 RCTs) | ⊕⊕⊕⊝ MODERATE 1 |
| Behavioural problems: overall | The score in the intervention group was 0.20 SDs lower (0.56 lower to 0.17 higher) | 209 (6 RCTs) | ⊕⊕⊝⊝ LOW 1 2 |
| Social behaviour: music vs other activities | The score in the intervention group was 0.54 SDs higher (0.06 higher to 1.02 higher) | 70 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 4 |
| Cognition | The score in the intervention group was 0.21 SDs higher (0.04 lower to 0.45 higher) | 257 (6 RCTs) | ⊕⊕⊝⊝ LOW 1 2 |
| *Interpretation of SMD: a difference of < 0.40 standard deviations can be regarded as a small effect, 0.40 to 0.70 a moderate effect, and > 0.70 a large effect. CI: Confidence interval; SMD: standardised mean difference; SD: standard deviation | |||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect. | |||
1 Risk of bias: no blinding of therapists and patients (not possible), and often no blinding of outcome assessment
2 Imprecision: small number of participants and rather broad confidence interval
3 Inconsistency: more non‐overlapping confidence intervals
4 Imprecision: very small number of participants and broad confidence interval
5 Publication bias: funnel plot is based on a limited number of studies but suggests there may be publication bias
Summary of findings 2.
Music‐based therapeutic interventions compared to usual care or other activities for people with dementia: long‐term effects (scores 4 weeks or more after treatment ended)
|
Music‐based therapeutic interventions compared to usual care or other activities for people with dementia: long‐term effects (scores 4 weeks or more after treatment ended) Interpretation of SMD: a difference of < 0.40 standard deviations can be regarded as a small effect, 0.40 to 0.70 a moderate effect, and > 0.70 a large effect. | |||
| Patient or population: people with dementia (all resided in institutional settings) Intervention: music‐based therapeutic interventions Comparison: usual care or other activities | |||
|
Outcomes (long‐term) measured with a variety of scales except for social behaviour |
Anticipated absolute effects* (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) |
|
Score with music therapy compared with usual care or other activities | |||
| Emotional well‐being including quality of life | The score in the intervention group was 0.47 SDs higher (0.10 lower to 1.05 higher) | 48 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 |
| Mood disturbance or negative affect: depression | The score in the intervention group was 0.01 SDs lower (0.27 lower to 0.24 higher) | 234 (5 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 3 |
| Mood disturbance or negative affect: anxiety | The score in the intervention group was 0.23 SDs lower (0.86 lower to 0.41 higher) | 160 (5 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 4 |
| Behavioural problems: agitation or aggression | The score in the intervention group was 0.02 SDs lower (0.36 lower to 0.33 higher) | 225 (4 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 3 4 |
| Behavioural problems: overall | The score in the intervention group was 0.05 SDs higher (0.30 lower to 0.41 higher) | 125 (3 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 3 |
| Social behaviour ‐ music vs other activities | The score in the intervention group was 0.53 SDs higher (0.53 lower to 1.6 higher) | 48 (2 RCTs) | ⊕⊝⊝⊝ VERY LOW 1 2 |
| Cognition ‐ music vs usual care | The score in the intervention group was 0.13 SDs higher (0.26 lower to 0.52 higher) | 100 (1 RCT) | ⊕⊝⊝⊝ VERY LOW 1 2 |
| *Interpretation of SMD: a difference of < 0.40 standard deviations can be regarded as a small effect, 0.40 to 0.70 a moderate effect, and > 0.70 a large effect. CI: Confidence interval; SMD: standardised mean difference; SD: standard deviation | |||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect | |||
1 Risk of bias: no blinding of therapists and patients (not possible), and no blinding of outcome assessment
2 Imprecision: very small number of participants and broad confidence interval includes both benefit and harm
3 Imprecision: small number of participants and rather broad confidence interval
4 Inconsistency: multiple non‐overlapping confidence intervals
Background
Description of the condition
Dementia is a clinical syndrome characterised by progressive decline in cognitive functions. Dementia of the Alzheimer's type is the most common form of dementia, followed by vascular dementia, Lewy body dementia and frontotemporal dementia (ADI 2015).
Dementia is a collective name for progressive degenerative brain syndromes which affect memory, thinking, behaviour and emotion (ADI 2015). Symptoms may include:
loss of memory;
difficulty in finding the right words or understanding what people are saying;
difficulty in performing previously routine tasks;
personality and mood changes.
Alzheimer’s Disease International estimates that worldwide currently 46.8 million people are suffering from dementia; and that this figure will increase to 74.7 million by 2030 and to 131.5 million people by 2050 (ADI 2015).
Research is pursuing a variety of promising findings related to describing the causes of dementia and for the treatment of dementia. Pharmacological interventions are available but have limited ability to treat many of the syndrome's features. Little research has been directed towards non‐pharmacological treatments.
As dementia is due to damage to the brain, one approach is to limit the extent and rate of progression of the pathological processes producing this damage. At present the scope of this approach is limited and an equally important approach is to help people with dementia and their caregivers to cope with the syndrome's social and psychological manifestations. As well as trying to slow cognitive deterioration, care should aim to stimulate abilities, improve quality of life, and reduce problematic behaviours associated with dementia. The therapeutic use of music might achieve these aims.
Description of the intervention
Many treatments of dementia depend on the client's ability to communicate verbally. When the ability to speak or understand language has been lost, music might offer alternative opportunities for communication. People who cannot speak anymore may still be able to hum or play along with music.
Music therapy is defined by the World Federation of Music Therapy (WFMT) as "the professional use of music and its elements as an intervention in medical, educational, and everyday environments with individuals, groups, families, or communities who seek to optimize their quality of life and improve their physical, social, communicative, emotional, intellectual, and spiritual health and wellbeing". Research, practice, education, and clinical training in music therapy are based on professional standards according to cultural, social, and political contexts (WFMT, 2011). The American Music Therapy Association (AMTA) defines music therapy as "the clinical and evidence‐based use of music interventions to accomplish individualized goals within a therapeutic relationship by a credentialed professional who has completed an approved music therapy program" (AMTA). It describes assessment of the client, interventions ("including creating, singing, moving to, and/or listening to music"), benefits and research, and explains that music therapy is used "within a therapeutic relationship to address physical, emotional, cognitive, and social needs of individuals". We reviewed music‐based interventions, which may share these therapeutic goals even if not provided by an accredited music therapist.
Two main types of music therapy can be distinguished — receptive (or passive) and active music therapy — and these are often combined (Guetin 2013). Receptive music therapy consists of listening to music by the therapist who sings, plays or selects recorded music for the recipients. In active music therapy, recipients are actively involved in the music‐making, by playing on small instruments for instance. The participants may be encouraged to participate in musical improvisation with instruments or voice, with dance, movement activities or singing.
Music may also be used in ways which are less obviously therapy or therapeutic, for example playing music during other activities such as meals or baths, or during physiotherapy or movement, or as part of an arts programme or other psychosocial interventions. 'Music as therapy' includes more narrowly defined music therapy provided by "a formally credentialed music major with a therapeutic emphasis" (Ing‐Randolph 2015). In order to benefit people with dementia, those providing music‐based interventions with a therapeutic goal may need to draw on the skills of both musicians and therapists to select and apply musical parameters adequately, tailored to a recipient's individual needs and goals.
How the intervention might work
Music‐based therapeutic interventions, including interventions provided by a certified music therapist, mostly consist of singing, listening, improvising or playing along on musical instruments. Music and singing may stimulate hemispheric specialization. Clinical observations indicate that singing critically depends upon right‐hemisphere structures. By contrast, people suffering from aphasia due to left‐hemisphere lesions often show strikingly preserved vocal music capabilities. Singing may be exploited to facilitate speech reconstruction when suffering from aphasia (Riecker 2000). Singing can further help the development of articulation, rhythm, and breath control. Singing in a group setting can improve social skills and foster a greater awareness of others. For those with dementia, singing may encourage reminiscence and discussions of the past, while reducing anxiety and fear. For individuals with compromised breathing, singing can improve oxygen saturation rates. For individuals who have difficulty speaking following a stroke, music may stimulate the language centres in the brain promoting the ability to sing. In sum, singing may improve a range of physical and psychosocial parameters (Clift 2016). Playing instruments may improve gross and fine motor coordination in individuals with motor impairments or neurological trauma related to a stroke, head injury or a disease process (Magee 2017; WFMT, 2010).
Whereas cognitive functions decline during disease progression, receptivity to music may remain until the late phases of dementia (Adridge 1996; Baird 2009; Cowles 2003 ). Even in the latest stage of the disease, they may remain responsive to music where other stimuli may no longer evoke a reaction (Norberg 1986).This may be related to musical memory regions in the brain being relatively spared in Alzheimer's disease (Jacobsen 2015). Possibly, the fundamentals of language are musical, and precede lexical functions in language development (Adridge 1996). Listening to music itself may decrease stress hormones such as cortisol; and helps patients to cope with, for instance, pre‐operative stress (Spintge 2000). Music therapy can bring relaxation and has a positive effect on enhancing communication and emotional well‐being (Brotons 2000). Music therapy enables the recall of life experiences and the experience of emotions. Many important life events are accompanied by music; most of the time these 'musical memories' are stored for a longer time than the ones from the same period that were not accompanied by music (Broersen 1995; Baird 2009). If words are not recognized any longer, familiar music may provide a sense of safety and well‐being, which in turn may decrease anxiety.Musical rhythm may help people with Alzheimer's disease to organize time and space. Patients are able to experience group contact through musical communication with other participants, without having to speak. Owing to its non‐verbal qualities, music‐based interventions might help people with dementia at all levels of severity to cope with the effects of their illness.
Why it is important to do this review
In this review we examine current research literature to assess whether music‐based therapeutic interventions, including music therapy, are an efficacious approach to the treatment of emotional, behavioural, social, and cognitive problems in people with dementia. We also investigate whether, in the absence of specific problems, these interventions have an effect on emotional well‐being, including quality of life, or social behaviour in people with dementia. Quality of life is often an appropriate goal of care for people with dementia (ADI 2016), and it is important to assess evidence as to whether music‐based therapeutic intervention can contribute to quality of life or related outcomes.
Objectives
To assess the effects of music‐based therapeutic interventions for people with dementia on emotional well‐being including quality of life, mood disturbance or negative affect, behavioural problems, social behaviour, and cognition at the end of therapy and four or more weeks after the end of treatment
Methods
Criteria for considering studies for this review
Types of studies
We included both parallel and cross‐over randomized controlled trials (RCTs).
Types of participants
We included people who were formally diagnosed as having any type of dementia according to DSM‐IV or DSM‐5, ICD‐10 or other accepted diagnostic criteria. In order to be relevant to clinical practice, we also accepted a physician's diagnosis of dementia if no data on formal criteria such as DSM‐IV, DSM‐5 or comparable instruments were available. We included people living in diverse settings including in the community, hospitals or nursing homes and all severities of dementia. We did not use a criterion for age so as not to exclude studies in which some participants were below age 65.
Types of interventions
We included any music‐based interventions, either active or receptive, delivered to individuals or groups. We required a minimum of five sessions in order to ensure that a therapeutic intervention could have taken place. We defined therapeutic music‐based interventions as: therapy provided by a qualified music therapist, or interventions based on a therapeutic relationship and meeting at least two of the following criteria/indicators: (a) therapeutic objective which may include communication, relationships, learning, expression, mobilisation and other relevant therapeutic objectives; (b) music matches individual preferences; (c) active participation of the people with dementia using musical instruments or singing; (d) participants had a clinical indication for the intervention or were referred for the intervention by a clinician. We also required music to be a main element of the intervention (e.g. not merely moving with use of music). Simple participation in a choir would not meet our definition of a therapeutic intervention.
The music‐based interventions could be compared with any other type of therapy or no therapy. Control groups could not receive any music‐based therapeutic intervention (even if fewer sessions than the intervention group).
Types of outcome measures
Emotional well‐being, including quality of life and positive affect. Facial expressions (in the absence of interaction with the observer) may also indicate emotional well‐being.
Mood disturbance or negative affect: depression (depressive symptoms) or anxiety.
Behavioural problems: agitation and/or aggression, overall behavioural problems or neuropsychiatric symptoms. (We combined agitation and aggression outcomes consistent with the International Psychogeriatric Association consensus definition of agitation requiring presence of one of "excessive motor activity, verbal aggression, or physical aggression" (Cummings 2015)).
Social behaviour, such as (verbal) interaction.
Cognition.
Any other adverse effects.
For these outcomes, we accepted all assessment tools used in the primary studies. Outcomes were assessed at the end of treatment (a minimum of five sessions), irrespective of the duration and number of sessions in excess of four. If there was evidence of no different effect over time, then reported outcomes could include earlier assessments. We also looked for outcomes a minimum of four weeks after the treatment ended in order to assess long‐term effects.
Primary outcomes
The protocol did not prioritise outcomes. We prioritised the outcomes related to emotions (emotional well‐being including quality of life, and mood disturbance or negative affect) as being of critical importance because these outcomes (e.g. depression) are closely related to quality of life of people with dementia (Banerjee 2009; Beerens 2014). Depression and anxiety are also prevalent and rather persistent during the course of the dementia (van der Linde 2016; Zhao 2016). We further prioritised behavioural problems because these affect relationships and caregiver burden (e.g. van der Linde 2012); and some may also be indicators of distress.
Secondary outcomes
Social behaviour and cognition were important but secondary outcomes, as the benefit for the participants themselves was not as obvious as for outcomes more closely related to their quality of life.
Search methods for identification of studies
We searched ALOIS, the Cochrane Dementia and Cognitive Improvement Group’s Specialized Register. The search terms used were: music therapy, music, singing, sing, auditory stimulation.
ALOIS is maintained by the Trials Search Co‐ordinator for CDCIG and contains studies in the areas of dementia prevention, dementia treatment and cognitive enhancement in the healthy. Details of the search strategies used for the retrieval of reports of trials from the healthcare databases, the Cochrane Central Register of Controlled Trials (CENTRAL) and conference proceedings can be viewed in the ‘Methods used in reviews’ section within the editorial information about the Dementia and Cognitive Improvement Group. To view a list of all sources searched for ALOIS see About ALOIS on the ALOIS web site.
We performed additional searches in each of the sources listed above to cover the timeframe from the last searches performed for ALOIS to 3 July 2015. The search strategies for the above described databases are presented in Appendix 1.
In addition, we searched Geronlit/Dimdi, Research Index, Carl Uncover/Ingenta, Musica and Cairss in January 2006 and June 2010, with the following search terms: music therapy, music, singing, dance, dementia, alzheimer. We also searched on these dates specific music therapy databases, as made available by the University of Witten‐Herdecke on www.musictherapyworld.de, based in Germany. We checked the reference lists of all relevant articles and a clinical librarian conducted a forward search from key articles using SciSearch. In addition, conference proceedings of European and World Music Therapy conferences on www.musictherapyworld.de and European music therapy journals, such as the Nordic Journal of Music Therapy, the British Journal of Music Therapy the Musiktherapeutische Umschau and the Dutch Tijdschrift voor Vaktherapie were hand searched to find music therapy studies (RCTs) for people with dementia, in January 2006, June 2010, and July 2015. A new database search was performed on 12 April 2016 to identify new studies published after 3 July 2015. Potentially eligible new studies (based on abstract review with two independent reviewers) were included under Characteristics of studies awaiting classification.
Data collection and analysis
Selection of studies
Two review authors independently assessed publications for eligibility by checking the title and, if available, the abstract. If any doubt existed as to an article's relevance they retrieved and assessed the full article.
Data extraction and management
Two reviewers extracted and cross‐checked outcome data independently of each other. They discussed any discrepancies or difficulties with a third reviewer. We reviewed articles in English, French, German and Dutch and searched for Cochrane collaborators to assess articles in other languages. We emailed authors for additional information when unclear (for example, about the type of control group or setting); and for additional data if that would help inclusion of the study data in meta‐analyses (for example, if estimates from graphical presentation were imprecise, SDs were lacking, or item‐level data if items of global tools represented relevant outcomes).
We first extracted data on the design (RCT), population (dementia diagnosis), the criteria for music therapy, outcomes and timing of outcome assessment, to evaluate eligibility of the study, Of the eligible studies, we subsequently recorded the following characteristics.
Data collection period.
Setting: nursing home, residential home, hospital, ambulatory care, other.
Participant characteristics: age, sex, severity and type of the dementia.
Number of participants included, randomized and lost to follow up.
Type, frequency and duration of active interventions and control interventions.
Description of activities in the control group if not usual care.
Outcomes: type of outcome measures about emotional well‐being, emotional problems (mood disturbance or negative affect), problematic or challenging behaviours (in general; and more specifically, agitation or aggression), social behaviours and cognition. Whether outcomes were being referred to as primary or secondary outcomes.
Timing of outcome measurement including the long term, after treatment ended.
Research hypotheses if specified, and a description of the results.
Any methodological problems and comments.
Funding sources.
A 'Risk of bias' assessment (below).
For each study, we extracted relevant outcome data, i.e. means, standard deviations and number of participants in each group for continuous data and numbers with each outcome in each group for dichotomous data. If needed or helpful, we contacted authors for clarification; or for data, such as from relevant subscales.
Assessment of risk of bias in included studies
Two reviewers (neither of whom was an author on any of the studies that they assessed) independently of each other assessed included studies for risk of bias according to the guidelines in the Cochrane Handbook for Systematic Reviews of Interventions, and using the 'Risk of bias' assessment tool (Higgins 2011). They looked at the following elements of study quality: selection bias (random sequence generation, allocation concealment); performance bias (blinding of participants and personnel); detection bias (blinding of outcome assessment); attrition bias (incomplete outcome data); reporting bias (selective reporting); and other potential threats to validity. They assessed performance, detection and attrition bias for each outcome.
Measures of treatment effect
We used the risk ratio to summarize any effects on dichotomous outcome variables and the mean difference (or if different instruments or scales were used, the standardised mean difference) for continuous variables.
Unit of analysis issues
Only participant‐level outcomes were considered, and all were continuous measures. For cross‐over trials, we extracted data for the first period only because of the likelihood of carry‐over.
Dealing with missing data
We considered if there were missing outcome data, with reasons reported, for example due to participants who moved or died, and how these were dealt with (exclusion of cases for analyses or were dealt with otherwise).
Assessment of heterogeneity
We interpreted I² according to criteria in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011: chapter 9.5.2). Further, a low P value for the Chi² statistic indicated heterogeneity of intervention effects, which we evaluated against the combined 'usual care' and 'other activities' control groups. Because of small numbers of participants and studies for most outcomes, a non‐significant P value was not decisive in the evaluation of consistency, and we also considered overlap of confidence intervals in the forest plots.
Assessment of reporting biases
Selective outcome reporting is covered by the risk of bias assessment, and for this we searched the articles about included studies and related articles for references to study protocols and trial registrations. If available, we compared with outcomes and prioritisation of outcomes in the article. If no research protocol was available, risk of reporting bias was set to either unclear, or high when appropriate. To detect possible publication bias, we examined funnel plots for outcomes with at least 10 studies available.
Data synthesis
We included studies about all eligible interventions in similar groups of people in different stages of dementia, and we pooled the results of studies that examined effects on the same seven outcomes. We discriminated between effects at the end of treatment, and long‐term effects (a minimum of four weeks after treatment ended). In case of clinically homogeneous studies, results would have been combined using a fixed‐effect model. In case of statistical heterogeneity (assessed by visual inspection of the forest plots) and the availability of at least five studies, a random‐effects model was used.
We were interested in both usual care and other activity‐control interventions because usual practice with regard to activities offered is variable, and the question as to whether music‐based therapeutic interventions should be introduced and the question as to whether they are superior to other activities are both relevant in practice. We presented data by type of control intervention: usual care or other activities. A control group with other activities controls for increased social contact and stimulation. However, it is unclear whether this increases or decreases contrast with the music‐based intervention group for different outcomes (e.g. agitation, anxiety). We therefore analysed effects against all control groups as planned in the protocol, but for purposes of possible hypothesis generation we present forest plots by subgroup.
With probable selective outcome reporting, we ran the analyses for the reported outcomes while omitting the particular study, to evaluate change and direction of change of the estimate.
Sensitivity analysis
Post hoc, we performed a series of sensitivity analyses because there are different possible criteria as to what constitutes music therapy, and because funding related to music therapy potentially involves an intellectual conflict of interest. We first reran all analyses on end‐of‐treatment effects with studies in which the intervention was probably or definitely (when mentioned explicitly) delivered by a professional music therapist only. Second, we restricted these analyses to studies definitely delivered by a music therapist. Third, we restricted the analyses to studies definitely delivered by a music therapist and with no potential conflict of interest related to funding or no reported funding source.
Presentation of results and 'Summary of findings' table
We used GRADE methods to rate the quality of evidence (high, moderate or low) behind each effect estimate in the review (Guyatt 2011). This rating refers to our level of confidence that the estimate reflects the true effect, taking account of risk of bias in the included studies, inconsistency between studies, imprecision in the effect estimate, indirectness in addressing our review question and the risk of publication bias. We produced 'Summary of findings' tables for end‐of‐treatment and long‐term outcome comparisons to show the effect estimate and the quantity and quality of the supporting evidence for outcomes for which more studies were available. The summary of findings was generated with Review Manager 5 (RevMan 5) data imported into the GradePro Guideline Development Tool (2015).
Results
Description of studies
Results of the search
The total number of included studies was 17. For the first version of this review (Vink 2003), we identified 354 references related to music‐based interventions and dementia (Figure 1). Of those, 254 were discarded as they did not refer to a research study or were identified as anecdotal or reports of case studies on the basis of their abstracts. Hard copies were obtained for the remaining 100 studies. We then discarded a further 74 studies as they involved participant series or case studies. A total of 26 studies remained in 2003 of which five met the criteria for inclusion at that time (Groene 1993; Lord 1993; Clark 1998; Brotons 2000; Gerdner 2000). In 2008 an additional eighteen studies were reviewed, of which three studies met the criteria at that time (Sung 2006; Svansdottir 2006; Raglio 2008). For the update of 2010 we retrieved a total of 188 references of possible relevance. After a first assessment 16 references remained which were further assessed, of which two studies met the criteria of this review (Guétin 2009; Raglio 2010b). In total, 10 studies were included in the previous update. In 2015, due to clarified criteria for eligibility of interventions, randomization, and more stringent application of criteria for analyses of outcomes after a minimum number of sessions, we excluded five of the 10 previously included studies (Groene 1993; Brotons 2000; Gerdner 2000; Sung 2006; Raglio 2008; Characteristics of excluded studies). However, we included 12 new studies after evaluating 121 references including 25 full‐text evaluations, which resulted in the total of 17 included studies. A new search on 12 April 2016 identified eight potentially eligible additional studies which still warrant review against inclusion criteria for the next update of this review (Hsu 2015; Raglio 2015; Curto Prieto 2015; Hsiung 2015; Rouch 2017; Thornley 2016; 신보영, 황은영. 2015; 채경숙 2015), in addition to a study for which we are waiting for clarification from the authors about the results (Hong 2011). These are listed under Characteristics of studies awaiting classification and Characteristics of ongoing studies.
Figure 1.
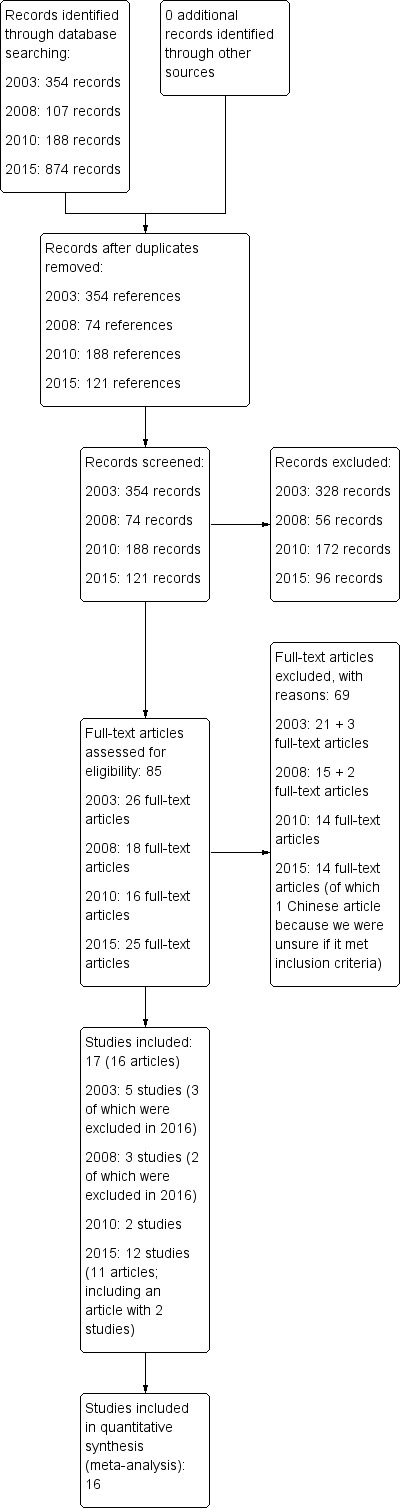
Study flow diagram.
Included studies
Details of the included studies are presented in the Characteristics of included studies table. One article (Narme and colleagues 2012: Narme 2012‐study 1 and Narme 2012‐study 1a) reported on two studies with rather similar designs indicated with study 1 and study 2 in the article (note that study 2 is indicated with 1a in our analyses). More articles with additional results or background of the study were available for five studies (Cooke 2010;Raglio 2010b;Lin 2011;Vink 2013; Narme 2014).
Fourteen studies had a parallel groups design (Lord 1993; Svansdottir 2006; Guétin 2009; Raglio 2010a; Raglio 2010b; Lin 2011; Ceccato 2012; Narme 2012‐study 1 and Narme 2012‐study 1a (also referred to as study 2); Sung 2012; Sakamoto 2013; Vink 2013; Narme 2014; Liesk 2015); and three used a crossover design with first‐period data available for all (Clark 1998; Cooke 2010; Ridder 2013).
The seventeen studies were performed in 11 countries. Whereas the two oldest studies were from the USA (Lord 1993; Clark 1998), the studies published after 1998 were from a variety of other regions and countries: 11 studies conducted in seven countries in Europe (Italy, France, Germany, the Netherlands, and Iceland, including also one study performed in two countries, Denmark and Norway; Ridder 2013), three studies from two countries in Asia (Taiwan and Japan), and one study from Australia. The studies were all performed in institutional settings of nursing homes, residential homes and geriatric hospital wards. Dementia severity varied.
The interventions were active (Cooke 2010; Raglio 2010a; Raglio 2010b; Sung 2012; Liesk 2015); receptive (Clark 1998; Guétin 2009); or a mixture of the two forms (the other 10 studies). Appendix 2 describes the music‐based therapeutic intervention and other activities of all studies. Music included live or recorded music that met preferences of the group or individual. The active forms often combined playing of instruments and singing activities, and some also combined with movement such as clapping hands and dance. Sessions varied in duration between half an hour and two hours. The total number of sessions ranged from six (Narme 2012‐study 1) to 156 (Lord 1993), with a median total number of 12 sessions until the end of treatment assessment. The frequency ranged between one session per week (Guétin 2009; Sakamoto 2013) and six sessions per week (Lord 1993), with a median and more typical number (mode) of two sessions per week (two per week was employed in 10 studies). These figures probably reflect number of sessions offered, as the number of attended session may be lower. There are few reports about implementation fidelity including adherence and dose received, but Ridder 2013 reports that a minimum of 12 sessions were offered, but the participants received 10 sessions on average.
In seven of the studies, we could be sure from the report that the interventions had been delivered by an accredited music therapist (Svansdottir 2006; Raglio 2010a; Raglio 2010b; Lin 2011; Ceccato 2012; Ridder 2013; Vink 2013). In four studies, it was unclear whether a music therapist was involved (no profession reported in the older studies, Lord 1993 and Clark 1998; probably delivered by trained music therapists but it was not stated explicitly in Guétin 2009; and delivered by musicians trained in the delivery of sessions and in working with older people with dementia but unclear if these were formally trained music therapists in Cooke 2010). In the other six studies, the intervention was not delivered by a music therapist (psychologist and other supervisor(s) with no training in music therapy, Narme 2012‐study 1; Narme 2012‐study 1a; Narme 2014; trained research assistants, Sung 2012; music facilitator, Sakamoto 2013; music teacher specialised in teaching older people, Liesk 2015).
Seven of the 17 studies compared the music intervention with an active control intervention, all with the same number of sessions and frequency as the music group. Two‐armed studies compared with the following interventions: reading (Cooke 2010; Guétin 2009), a cognitive stimulation intervention (Liesk 2015), painting (Narme 2012‐study 1), cooking (Narme 2012‐study 1 and Narme 2012‐study 1a — also referred to as study 2; Narme 2014), or variable recreational activities which included handwork, playing shuffleboard, and also cooking and puzzle games (Vink 2013). Two studies had three arms with the active control group working on jigsaw puzzles (Lord 1993); or receiving a passive group music intervention which did not meet our inclusion criteria for a therapeutic music‐based intervention (Sakamoto 2013).
The outcomes 'emotional well‐being' including quality of life, mood disturbance or negative affect (also as part of behavioural scales), and 'behavioural problems' (agitation or aggression, and behaviour overall) and 'cognition' were often assessed. Social behaviour was less commonly assessed (Lord 1993; Narme 2012‐study 1; Narme 2012‐study 1a; Narme 2014); and the meta‐analyses of end‐of‐treatment scores included only the three studies from Narme and colleagues. The Cohen‐Mansfield Agitation Inventory (CMAI, for agitation; Cohen‐Mansfield 1986), Mini‐Mental State Examination (MMSE, for cognition; Folstein 1975), and the Neuropsychiatric Inventory (NPI, for behaviour; Cummings 1994) in particular were frequently used. Item‐level NPI outcome data were reported in the article or the author additionally provided data about depression, anxiety, and agitation outcomes.
Excluded studies
We screened a total of 737 records and we excluded 652 (Figure 1). We excluded 69 of 85 records examined in full text (see Characteristics of excluded studies for a selection of excluded studies which were close but did not qualify upon careful consideration). They were often excluded because the participants did not have dementia, or because of a design other than an RCT. Further, and often less obvious, we critically reviewed whether the intervention met the inclusion criteria for a music‐based therapeutic intervention, and whether the reported outcomes included any assessments after fewer than five sessions. There are a number of studies on group music interventions such as group music in addition to movement interventions (e.g. Sung 2006): these were excluded because music was not the main or only therapeutic element, or was not provided with individual therapeutic intent. Further, some studies assessed outcomes during the treatment sessions only, combining immediate effects, for example on behaviour during the first session, with effects after multiple sessions (e.g. Gerdner 2000). Studies awaiting classification included conference abstracts, articles about studies in Asia which we could not retrieve or evaluate in time, and new studies published after the search (Characteristics of studies awaiting classification).
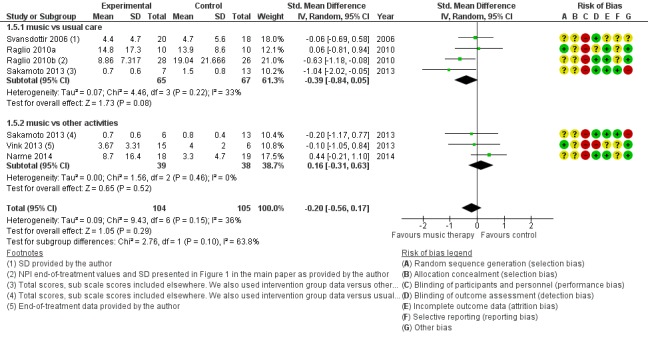
Risk of bias in included studies
The results of the assessment of risk of bias are presented in the Risk of bias in included studies tables, in Figure 2 and Figure 3, and in funnel plots (Figure 4 and Figure 5).
Figure 2.
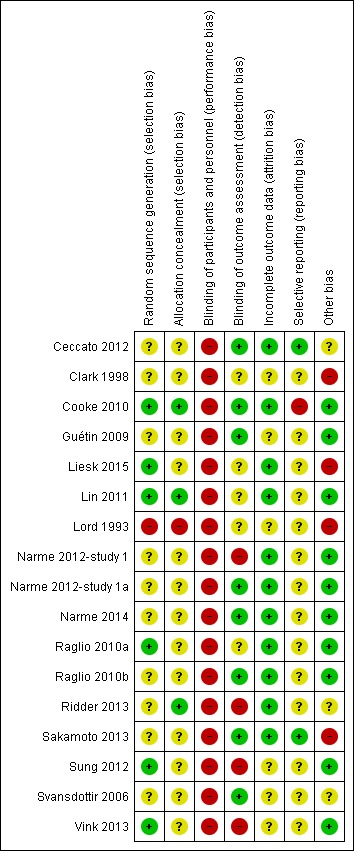
Methodological quality summary: review authors' judgements about each methodological quality item for each included study.
Figure 3.
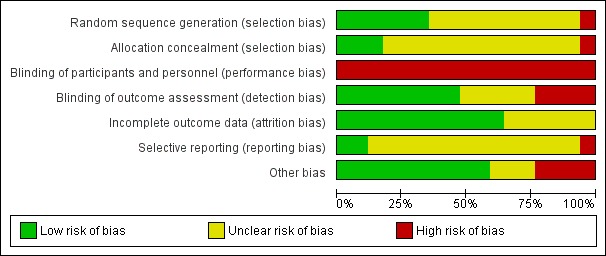
Methodological quality graph: review authors' judgements about each methodological quality item presented as percentages across all included studies.
Figure 4.
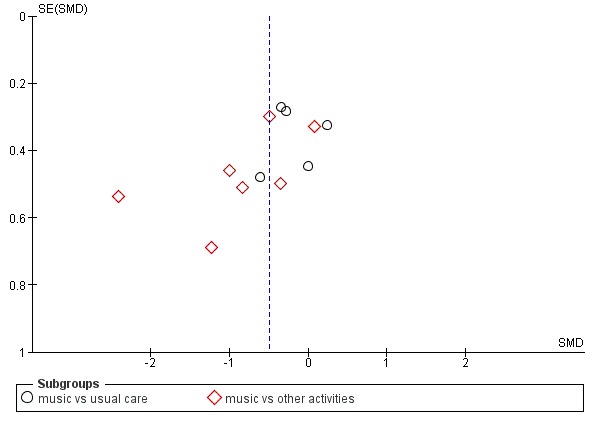
Funnel plot of comparison: 1 Music therapy versus usual care or versus other activities: end of treatment, outcome: 1.3 Negative affect or mood disturbances: anxiety (11 studies, 12 dots because 1 study used 2 control groups, one with usual care and one with other activities)
Figure 5.
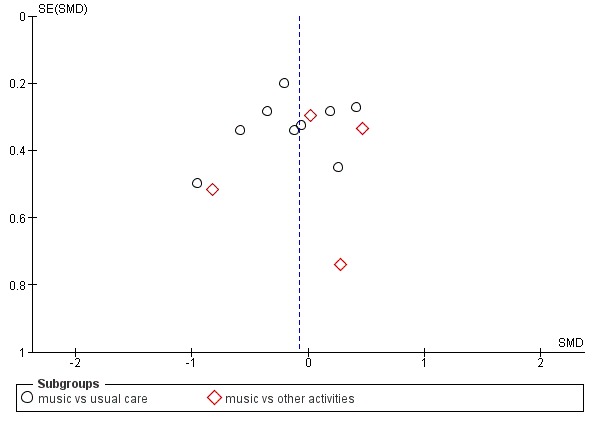
Funnel plot of comparison: 1 Music therapy versus usual care or versus other activities: end of treatment, outcome: 1.4 Problematic behaviour: agitation or aggression (12 studies, 13 dots because 1 study used 2 control groups, one with usual care and one with other activities)
There were a number of possible biases and often we could not assess the risk of bias due to poor reporting. Only risk of attrition bias was either low or unclear, as often no or only few participants were lost to follow‐up and there were few missing outcome assessments. Risk of performance bias was high for all studies because participants and staff could not be blinded to the intervention. Regarding the other items, in more recent studies risk of bias was lower and the reporting in terms of interventions, rationale, chosen procedures, design and results was generally better. Still, we are unsure about the methodological quality of a number of studies because several items were rated as unclear.
Allocation
All included studies were RCTs. However, the randomization procedure was not always described in detail (Figure 2). Moreover, allocation concealment was described and adequate in detail in only three studies, all of which were published in 2010 or later (Cooke 2010; Lin 2011; Ridder 2013). One older study stated that participants were "non‐systematically separated" into groups without further detail, which we considered posed a high risk of selection bias (Lord 1993).
Blinding
Blinding of therapists and participants to the intervention is not possible. Therefore, the studies are at high risk of performance bias even though therapists do not generally assess outcomes and participants may not be aware or have no specific expectations or are unable to self‐report. The outcomes were assessed unblinded, by the research team or unblinded nurses, in at least four studies (Figure 2). For example, Narme and colleagues describe two studies differing in detection bias (Narme 2012‐study 1; Narme 2012‐study 1a). The first study involved a high risk of detection bias because the outcomes 'anxiety' (measured with the State‐Trait Anxiety Inventory for adults, STAI‐A) and, as assessed from the first two minutes of filmed interviews, 'emotions' (from facial expressions) and 'social behaviour' (discourse content), were assessed by nurses who were not blinded for the interventions (music intervention or painting) (Narme 2012‐study 1). By contrast, in the second study (Narme 2012‐study 1a), risk of detection bias was low because the outcomes were assessed by five independent observers who were blinded for the type of intervention (music intervention or cooking). Risk of detection bias resulted in downgrading of the quality of the evidence for all end‐of‐treatment outcomes (to 'serious'; Table 1); and for all long‐term outcomes (to 'very serious' — all outcome assessment was unblinded; Table 2).
Incomplete outcome data
Self‐reported outcomes were rarely employed. Incomplete outcome data were not identified as problematic in any of the studies. Occasionally death, hospitalisation, acute illness, or no interest in the therapy occurred across the different study arms; and cases with no outcome data were not included in the analyses. Therefore, attrition bias was probably not highly prevalent, and probably did not affect the pooled estimates. Newer studies often visualized cases lost to follow‐up and missing outcome assessment in detail using flow diagrams. Nevertheless, in addition to the two oldest studies, some newer studies also only reported the number of cases randomized and analysed and did not explicitly report reasons for missing outcome data by study arm, or how these were handled.
Selective reporting
Most studies did not refer to initial plans, a study protocol or trial registration. Therefore, it is unclear to what extent bias due to selective outcome reporting is pertinent. We found some indication of inconsistent reporting of primary and secondary outcomes which, however, did not seem to affect the pooled estimate (Cooke 2010). Only one study clearly referred to a change in initial plans (Ceccato 2012); and one study referred to a trial registration, and outcome reporting was consistent with the registration (Sakamoto 2013). We did not downgrade the quality of the evidence because of unclear risk of selective reporting.
Regarding publication bias: funnel plots for outcomes with sufficient studies (anxiety, 11 studies of which one with both a 'usual care' and 'other activity' control group, Figure 4; and agitation or aggression, 12 studies, also one with two types of control groups, Figure 5) indicate possible publication bias for the anxiety outcome. For anxiety, the largest effects were found in studies with the largest standard error, and publications about studies with small effects and large standard error might be missing.
Other potential sources of bias
We found some potential other sources of bias. Outcome assessment may be either imprecise or biased by the use of non‐validated outcome measures with suboptimal distributions (such as skewed distributions, e.g. number of times yelling was observed; Clark 1998) and different procedures for the baseline and outcome assessment (Sakamoto 2013). Further, we found problems with the reporting of outcomes or we suspected errors (Lord 1993; and for this reason, Hong 2011 was moved to Studies awaiting classification). Implementation fidelity, including non‐adherence, was infrequently described, but Liesk 2015, the only study with null findings, reported on this in detail. Finally, there may be bias through a financial or intellectual conflict of interest when funding was provided by a source with a potential interest in the effectiveness of music therapy. This may apply to two studies (Ceccato 2012; Ridder 2013), but it should be noted that no source of funding was reported for more studies (Lord 1993; Clark 1998; Raglio 2010a; Raglio 2010b; Lin 2011; Liesk 2015). Only two studies were both definitely delivered by a music therapist and funded by a source unrelated to music or music therapy (no potential financial conflict of interest, but the music therapists (co)authored the article; Svansdottir 2006; Vink 2013). More studies did not report any funding source.
Effects of interventions
Results at the end of treatment are summarised in Table 1 and longer term effects in Table 2.
Of the 17 included studies, 16 studies with a total of 620 participants contributed to meta‐analyses of effects. One study reported data on emotional well‐being, social behaviour and cognition, but not in enough detail for us to include it in meta‐analyses (Lord 1993). Several authors provided additional data such as SDs or item‐level outcome data of scales for general behavioural assessments. We pooled data for all end‐of‐treatment outcomes, and for all but one long‐term outcome — cognition — because there was only one study. Of note: of the 17 studies, all but one study — Liesk 2015 — reported some significant improvement in outcomes of the music intervention versus control (all outcomes, including also, e.g. physiological outcomes that we did not evaluate). The methodological quality in terms of risk of bias, but also other quality considerations, varied substantially across the studies and the particular outcomes.
Emotional well‐being including quality of life
We included six studies with 181 participants in the analysis of end‐of‐treatment scores for the critically important outcome of emotional well‐being and quality of life. In half of the studies, a validated quality‐of‐life measure was used (the Dementia Quality of Life, DQOL (Cooke 2010), a German translation of the Dementia Quality of Life Instrument, DEMQOL (Liesk 2015), and a Danish translation of the Alzheimer’s Disease‐Related Quality of Life, ADRQL (Ridder 2013). In the three studies conducted by Narme and colleagues (Narme 2012‐study 1; Narme 2012‐study 1a; Narme 2014) emotional well‐being referred to counts of positive and negative facial expressions as assessed from the first two minutes of filmed interviews. There was no clear evidence of an effect at the end of treatment (Table 1; standardized mean difference, SMD 0.32, 95% CI −0.08 to 0.71; Analysis 1.1 and Figure 6). Heterogeneity was only low to moderate (I² = 38%; Chi² P = 0.15). There was no blinding of outcome assessment in two of the six studies. The overall quality for effects of music‐based interventions on emotional well‐being and quality of life at end of treatment was low, downgraded for serious risk of bias and imprecision (wide confidence interval). The quality was very low for long‐term outcomes for which there were only two very small studies and very serious imprecision (Narme 2012‐study 1a; Narme 2014). There was no clear evidence of an effect (SMD 0.47, 95% CI −0.10 to 1.05; Analysis 2.1; Table 2), but because of the very low quality, this is a very uncertain result.
Analysis 1.1.
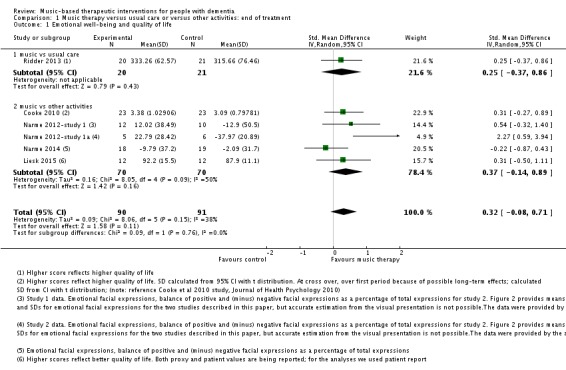
Comparison 1 Music therapy versus usual care or versus other activities: end of treatment, Outcome 1 Emotional well‐being and quality of life.
Figure 6.
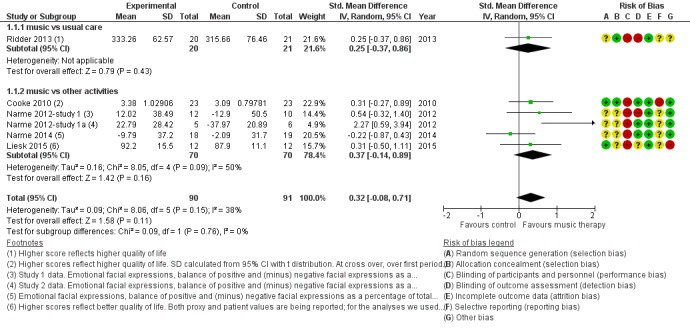
Forest plot of comparison: 1 Music therapy versus usual care or versus other activities: end of treatment, outcome: 1.1 Emotional well‐being and quality of life .
Analysis 2.1.
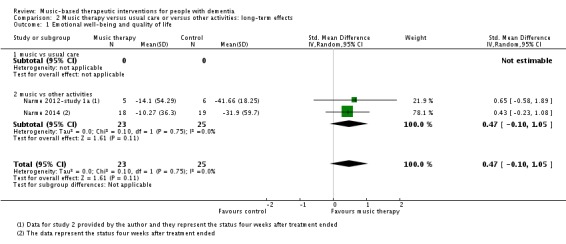
Comparison 2 Music therapy versus usual care or versus other activities: long‐term effects, Outcome 1 Emotional well‐being and quality of life.
Mood disturbance or negative affect: depression
Nine studies contributed 376 participants to the analysis on end‐of‐treatment effect (Figure 7) and five studies contributed 234 participants to the analysis on long‐term effects. Depression or depressive symptoms were measured with (translated versions of) the Geriatric Depression Scale (GDS) or with a subscale of the Behavioural Pathology in Alzheimer's Disease (BEHAVE‐AD) or the Neuropsychiatric Inventory (NPI). Heterogeneity was not important (I² = 0%) for either end‐of‐treatment or long‐term outcomes. We downgraded both outcomes for risk of bias, due to lack of blinding in many studies. Imprecision was more of an issue for long‐term outcomes. The overall quality of the evidence was moderate for end‐of‐treatment effects and very low for long‐term outcomes. We found that music‐based therapeutic interventions probably reduced depressive symptoms at the end of treatment (SMD −0.28, 95% CI −0.48 to −0.07; Table 1; Analysis 1.2 and Figure 7). There was no evidence of a reduction in the longer term, with a smaller estimate and a confidence interval including no effect (SMD −0.01, 95% CI −0.27 to 0.24; Table 2; Analysis 2.2) although again the very low quality of the evidence made this result very uncertain.
Figure 7.
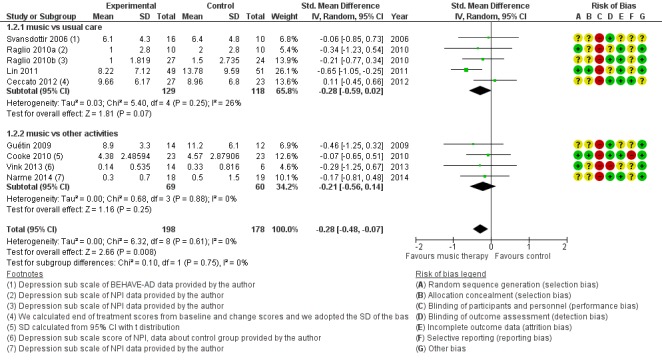
Forest plot of comparison: 1 Music therapy versus usual care or versus other activities: end of treatment, outcome: 1.2 Negative affect or mood disturbances: depression.
Analysis 1.2.
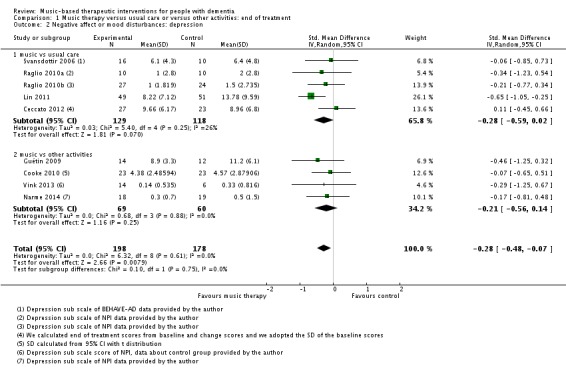
Comparison 1 Music therapy versus usual care or versus other activities: end of treatment, Outcome 2 Negative affect or mood disturbances: depression.
Analysis 2.2.
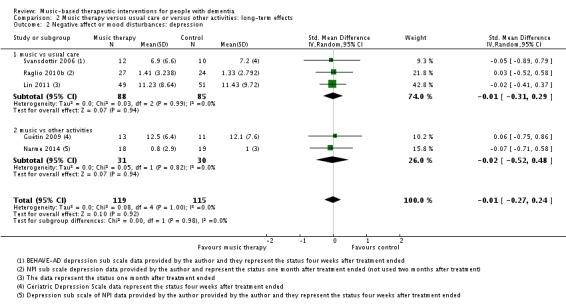
Comparison 2 Music therapy versus usual care or versus other activities: long‐term effects, Outcome 2 Negative affect or mood disturbances: depression.
Mood disturbance or negative affect: anxiety
The other mood item we considered was anxiety. For this outcome, at the end of treatment, we included 11 studies with 365 participants. A variety of (translated) outcome measures were used; Rating Anxiety in Dementia Scale (RAID), State‐Trait Anxiety Inventory for Adults (STAI‐A), Hamilton Anxiety Scale, and subscale scores of the BEHAVE‐AD and NPI. Heterogeneity was substantial for end‐of‐treatment effects (I² = 56%; Chi² P = 0.009) and longer‐term effects (I² = 72%; Chi² P = 0.006). In addition to serious inconsistency, there was serious risk of bias, imprecision and — for the end of treatment outcome — possible publication bias (Figure 4). Hence we judged the quality of the evidence at both time points to be very low. We can therefore have very little confidence in the results. Anxiety was lower in the music intervention group at the end of treatment (SMD −0.50, 95% CI −0.84 to −0.16; 11 studies with 365 participants; Table 1; Analysis 1.3 and Figure 8), but not in the longer term (SMD −0.23, 95% CI −0.86 to 0.41; 5 studies with 160 participants; Table 2; Analysis 2.3).
Analysis 1.3.
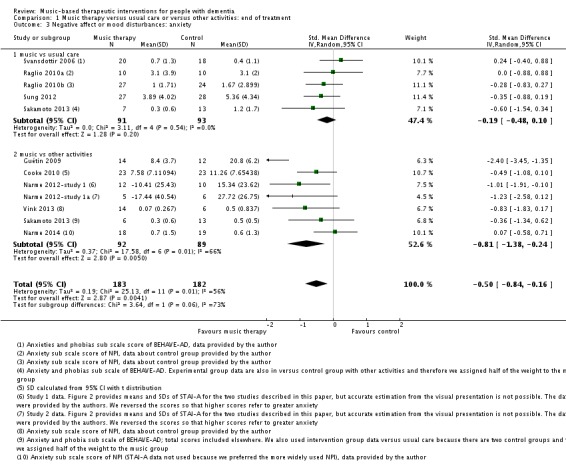
Comparison 1 Music therapy versus usual care or versus other activities: end of treatment, Outcome 3 Negative affect or mood disturbances: anxiety.
Figure 8.
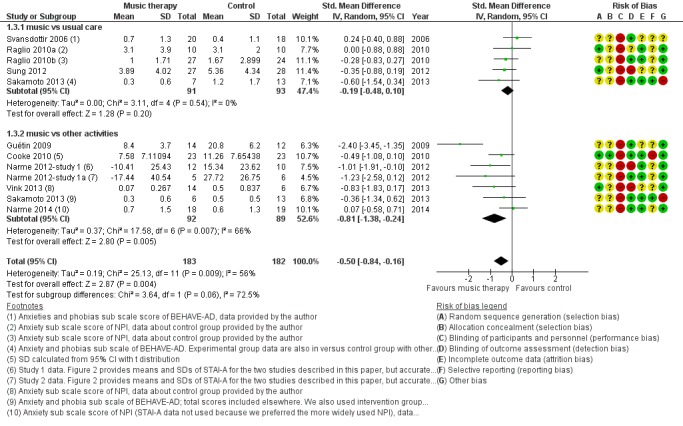
Forest plot of comparison: 1 Music therapy versus usual care or versus other activities: end of treatment, outcome: 1.3 Negative affect or mood disturbances: anxiety.
Analysis 2.3.
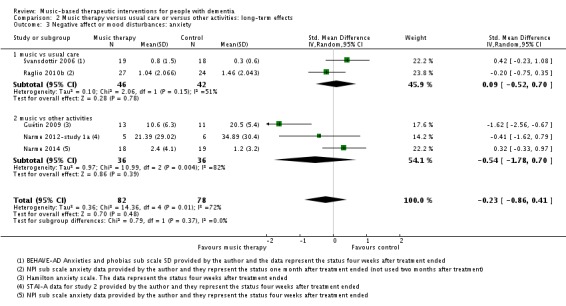
Comparison 2 Music therapy versus usual care or versus other activities: long‐term effects, Outcome 3 Negative affect or mood disturbances: anxiety.
Behavioural problems: agitation or aggression
Twelve studies with 515 participants contributed to the end‐of‐treatment effect analysis, and four studies with 225 participants contributed to the long‐term effect analysis. Outcome measures used for agitation were (translated versions of) the Cohen‐Mansfield Agitation Inventory (CMAI) and the agitation subscale of the NPI; and for aggression, the aggressiveness subscale of the BEHAVE‐AD and counts of observed aggressive behaviour. Heterogeneity was low to moderate at end of treatment and longer term (I² = 27%, Chi² P = 0.18, and I² = 37%, Chi² P = 0.19, respectively). Inconsistency and imprecision were not serious for the end‐of‐treatment outcome, but inconsistency was serious for the long‐term outcome, as was imprecision. Both outcomes were downgraded for risk of bias. There was no evidence of publication bias (regarding end‐of‐treatment effect; Figure 5). We rated the quality of the evidence as moderate for the end‐of‐treatment outcome but very low for the long‐term outcome. We found no evidence of an effect on agitation or aggression at the end of treatment (SMD −0.08, 95% CI −0.29 to 0.14; Table 1; Analysis 1.4 and Figure 9) nor in the long term (SMD −0.02, 95% CI −0.36 to 0.33; ; Table 2; Analysis 2.4).
Analysis 1.4.
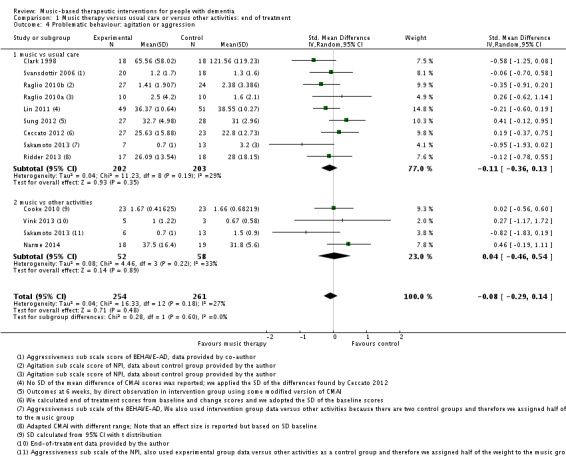
Comparison 1 Music therapy versus usual care or versus other activities: end of treatment, Outcome 4 Problematic behaviour: agitation or aggression.
Figure 9.
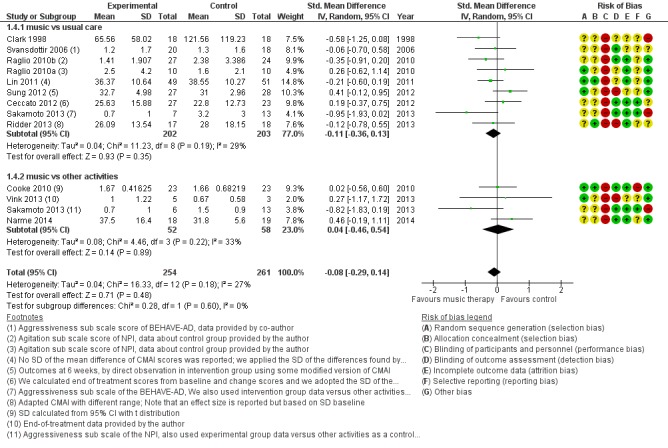
Forest plot of comparison: 1 Music therapy versus usual care or versus other activities: end of treatment, outcome: 1.4 Problematic behaviour: agitation or aggression.
Analysis 2.4.
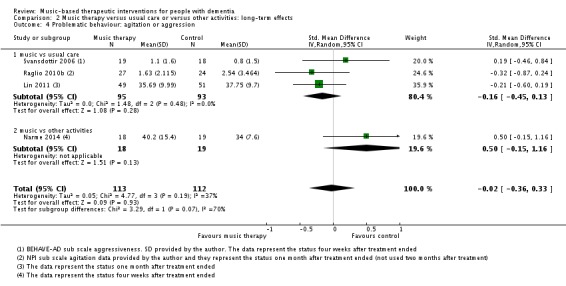
Comparison 2 Music therapy versus usual care or versus other activities: long‐term effects, Outcome 4 Problematic behaviour: agitation or aggression.
Behavioural problems overall
Six studies with 209 participants contributed to the end‐of‐treatment effect analysis, and three studies with 125 participants contributed to the analysis of longer term effects. Outcome measures were (translated versions of) the BEHAVE‐AD and NPI. Heterogeneity was low to moderate for the end of treatment effect (I² = 36%, Chi² P = 0.15). The quality of the evidence was low due to serious risk of bias and imprecision. We found no evidence of an effect of music‐based therapeutic interventions on problematic behaviour overall at the end of treatment (SMD −0.20, 95% CI −0.56 to 0.17; Table 1; Analysis 1.5 and Figure 10). There was no evidence of a long‐term effect either (SMD 0.05, 95% CI −0.30 to 0.41; I² = 0%, Chi² P = 0.38, heterogeneity was not important; Table 2; Analysis 2.5) although we considered this very low quality evidence.
Analysis 1.5.
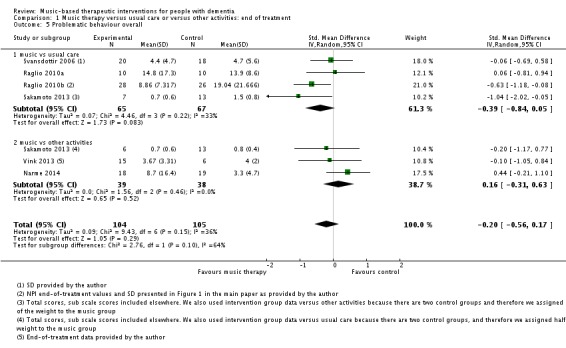
Comparison 1 Music therapy versus usual care or versus other activities: end of treatment, Outcome 5 Problematic behaviour overall.
Figure 10.
Forest plot of comparison: 1 Music therapy versus usual care or versus other activities: end of treatment, outcome: 1.5 Problematic behaviour overall.
Analysis 2.5.
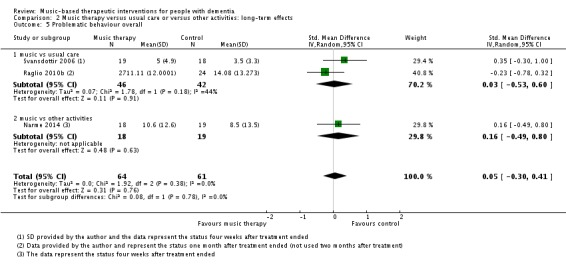
Comparison 2 Music therapy versus usual care or versus other activities: long‐term effects, Outcome 5 Problematic behaviour overall.
Social behaviour
The three studies of Narme and colleagues (Narme 2012‐study 1; Narme 2012‐study 1a; Narme 2014) contributed 70 participants to the end‐of‐treatment effect analysis and two of them contributed 48 participants to the analyses of longer term effects. For all, the outcome was the contents of conversation (positive versus negative expressions when interviewed about current feelings and personal history). Lord 1993 reported on effects on their self‐made questionnaire on social interaction, mood and recall (combined outcome) but there were no separate figures for social interaction and therefore we could not use the data for the meta‐analysis. We downgraded the evidence at both time points due to serious or very serious risk of bias and very serious imprecision. There was also moderate to substantial heterogeneity (I² = 54%, Chi² P = 0.14) in the long‐term analysis. We considered the quality of the evidence to be very low for both outcomes and were therefore very uncertain about the result of more positive expressions in the music‐based interventions group at the end of treatment (SMD 0.54, 95% CI 0.06 to 1.02; 3 studies; I² = 0%, Chi² P = 0.70; Table 1, Analysis 1.6 and Figure 11). There was a similar SMD but an even wider confidence interval in the analysis of long‐term effects (SMD 0.53, 95% CI −0.53 to 1.60; Analysis 2.6; Table 2).
Analysis 1.6.
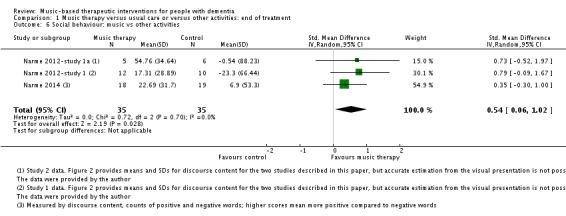
Comparison 1 Music therapy versus usual care or versus other activities: end of treatment, Outcome 6 Social behaviour: music vs other activities.
Figure 11.
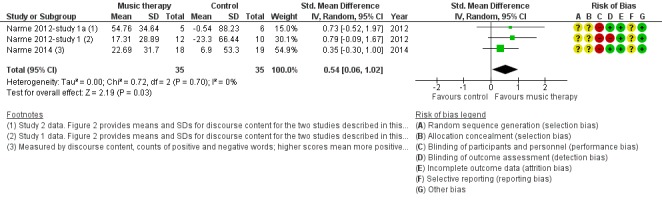
Forest plot of comparison: 1 Music therapy versus usual care or versus other activities: end of treatment, outcome: 1.6 Social behaviour: music vs other activities.
Analysis 2.6.
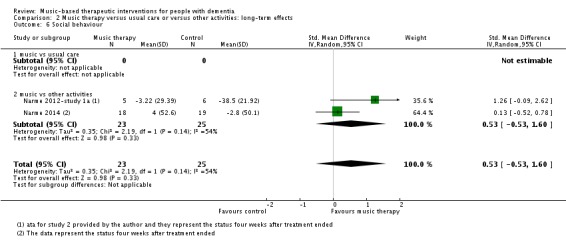
Comparison 2 Music therapy versus usual care or versus other activities: long‐term effects, Outcome 6 Social behaviour.
Cognition
Six studies contributed 257 participants to the end‐of‐treatment effect analysis and there was only one study, with 100 participants, that assessed long‐term effects. Outcome measures used in the analyses were (translated versions of) the Mini‐Mental State Examination (MMSE) and the Severe Impairment Battery (SIB). We used the MMSE data if these were available in addition to other cognition measures such as Prose Memory tests, the FAS‐Test (Controlled‐Oral‐Word‐Association Test), or the Alzheimer's Disease Assessment Scale cognitive subscale (ADAS‐cog). The end‐of‐treatment results were imprecise but not inconsistent. There was no important heterogeneity (I² = 0%; Chi² P = 0.84). There was serious risk of bias. The overall quality of the evidence was low and suggested that music‐based interventions may not affect cognition at the end of treatment (SMD 0.21, 95% CI −0.04 to 0.45; Table 1; Analysis 1.7 and Figure 12). The only study that assessed long‐term effects found a SMD of 0.13 (95% CI −0.26 to 0.52); we considered this very low quality evidence.
Analysis 1.7.
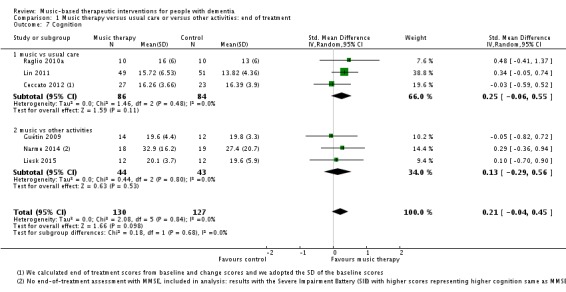
Comparison 1 Music therapy versus usual care or versus other activities: end of treatment, Outcome 7 Cognition.
Figure 12.
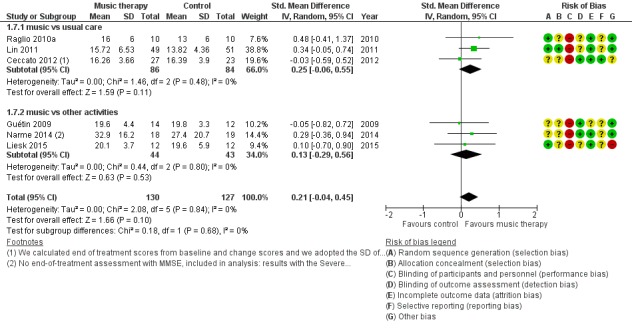
Forest plot of comparison: 1 Music therapy versus usual care or versus other activities: end of treatment, outcome: 1.7 Cognition.
Any other adverse effects
These were not reported.
Effects of interventions delivered by a music therapist and in studies with a potential financial conflict of interest
The sensitivity analyses with analyses restricted to studies where the intervention was definitely or possibly delivered by a qualified music therapist resulted in similar end‐of‐treatment effect estimates (there was no sensitivity analysis for the social behaviour outcome because no study remained). When restricting to studies that were definitely delivered by a music therapist, most effects were similar, but there was a smaller effect on anxiety. In the four of 11 studies in which the intervention was definitely delivered by a music therapist, the estimate for anxiety was −0.15 (SMD −0.15, 95% CI −0.54 to 0.24; with less heterogeneity; I² = 16%, Chi² P = 0.31; 129 participants).
When we restricted analyses further to studies definitely delivered by a music therapist and having no potential financial conflict of interest, or no funding source reported, we found somewhat different estimates, but there were very small numbers of studies and participants in these analyses.
Discussion
Summary of main results
The aim of this review was to evaluate the effect of music‐based therapeutic interventions on a range of outcomes relevant for people with dementia. The specific focus was to assess whether they can improve emotional well‐being including quality of life, mood disturbance or negative affect, behavioural problems, social behaviour, and cognition.
Seventeen studies have been included in this review, and we were able to perform meta‐analyses on effects at the end of treatment and longer term (mostly four weeks after treatment ended). We found moderate‐quality evidence that at the end of treatment music‐based therapeutic interventions improved depressive symptoms and did not improve agitation or aggression, and low‐quality evidence that it had no effect on emotional well‐being including quality of life, overall behavioural problems and cognition. There was very low quality evidence of benefit on anxiety and social behaviour. Sensitivity analyses suggested that the effects were not larger in studies in which the intervention was delivered by a qualified music therapist. There was no evidence of effects four weeks or more after the end of treatment, but the quality of this evidence for all outcomes was also very low.
Overall completeness and applicability of evidence
Only three studies used social behaviour as an outcome, and these were from a single group of researchers in France (Narme 2012‐study 1; Narme 2012‐study 1a; Narme 2014). The evidence in this review applies to therapeutic effects of music‐based therapeutic interventions after at least five sessions. It excludes some group interventions which involved music, but where music was not the main or only therapeutic element. It excludes direct effects during sessions.
Quality of the evidence
The quality of the evidence was moderate for depression and for agitation or aggression at the end of treatment. For all other outcomes it was low or very low. All end‐of‐treatment outcomes were downgraded for risk of bias; all except depression and agitation or aggression were downgraded for imprecision; and some outcomes were also downgraded for inconsistency.
Many studies used validated outcome measures for behaviour (e.g. the NPI (Cummings 1994) or BEHAVE‐AD (Reisberg 1987)), two widely used measures which are recommended because of favourable psychometric properties (Jeon 2011), and for cognition (e.g. the MMSE (Folstein 1975)). We included subscales of the behavioural scales as outcome measures. However, there is less evidence for validity of subscales compared to total scores (Lai 2014). We combined agitation and aggression in meta‐analyses because this is consistent with the definition given by the International Psychogeriatric Association (Cummings 2015); and these items are also combined in the widely used CMAI (Cohen‐Mansfield 1986). Some have raised conceptual issues such as overlap of a broad definition of agitation with resistance to care (Volicer 2007).
The quality of the reporting was sometimes poor which resulted in uncertainty about the exact methodological quality of the included studies and the evidence for effects. Overall, the studies had small sample sizes. Few studies reported on fidelity of the implementation of the music intervention and other activities, or on other aspects of a process evaluation. Implementation fidelity is often defined as the degree to which an intervention or programme is delivered as intended (Carroll 2007); and in music therapy trial specifically, treatment fidelity refers to "methodological strategies used to monitor the delivery of the music therapy intervention as described in the treatment manual" (Bradt 2012). Treatment fidelity includes adherence to an intervention, exposure or dose, quality of delivery, participant responsiveness, and programme differentiation to identify essential components of the intervention (Carroll 2007), and therefore includes, but is not limited to, participant (or staff) adherence and responsiveness.
Some of the included studies selected people with agitated behaviour before the intervention, or people who were more likely to be interested in music‐based interventions. On the other hand, there were studies in which people with musical knowledge were excluded (Raglio 2010a), or without such selection criteria. Dropout was mostly due to health‐related conditions such as hospitalisation, illness or mortality. Dropout due to lack of interest was reported for a control activity (cognitive stimulation programme) and dropout due to "problems in the group" in a music intervention group (Liesk 2015), but none of the other studies reported any unfavourable effects of the music‐based interventions. We do not know if there were any unreported adverse effects such as a sore throat after singing. We also do not know if, without selectively including people based on subjective judgement of whether they will probably accept the intervention, some individuals with dementia might experience disadvantages of the intervention. Possibly, effects in these studies depend on participants having problems at baseline (being selected as in need of treatment for specific problems) and hence to there being substantial room for improvement. Specific subgroups might benefit from music‐based therapeutic interventions more than others.
There may be publication bias through selective reporting of studies and selective outcome reporting in the relevant literature. Although few protocols were registered, we found inconsistencies in the reporting of outcome measures in one study that reported on the study in multiple papers (Cooke 2010). Moreover, despite most of the meta‐analyses we ran not resulting in significant pooled effects, 16 of the 17 studies (all, except for Liesk 2015) reported at least one significant effect. For some studies this included outcomes beyond the scope of this review, such as heart rate, but it could indicate selective reporting of significant findings or analytic methods that resulted in significant findings. Further, the funnel plot showing end‐of‐treatment effects on anxiety suggested possible publication bias. There may also be a financial conflict of interest if the study is funded by a source interested in the outcomes, or an intellectual conflict of interest in case the study is performed by the music therapist who authors the article, but there were insufficient data to examine possible effects of conflicts of interest.
Potential biases in the review process
Although we have done an extensive literature search in the most commonly used databases and also thoroughly handsearched music therapy journals, it may however be that not all conducted RCTs were retrieved.
Agreements and disagreements with other studies or reviews
A recent review on effects of music therapy on behavioural and psychological symptoms of dementia found larger SMDs for behavioural problems overall (−0.49, 95% CI −0.82 to −0.17) and for anxiety (−0.64, 95% CI −1.05 to −0.24) compared with our findings (Ueda 2013). However, that review included non‐randomized trials and cohort studies and studies which we excluded because they did not meet our criteria for therapeutic interventions. They found an even larger effect for studies that lasted three months or longer (−0.93, 95% CI −1.72 to −0.13), a subgroup we did not analyse separately.
The review by Chang 2015 included 10 studies, including Raglio 2008 which we excluded in the updated version of our review because after re‐evaluation, we judged this to be a quasi‐randomized study; Sung 2006 which after re‐evaluation did not meet our criteria for a music‐based therapeutic intervention (it was music with movement); and Janata 2012 which we excluded because streaming music also did not meet our criteria for a therapeutic intervention. Chang 2015 included studies that compared with usual care, excluding other activities except for reading sessions as the comparator (Guétin 2009; Cooke 2010; perhaps also including a study on ICU patients with no dementia). Our review had a longer search period than 2000 to 2014 and we included articles in French and German. Both we and Chang 2015 found substantial heterogeneity in our analyses of anxiety, but we also found that the funnel plot indicated possible publication bias and that the quality of the evidence for an effect on anxiety was very low. Effect sizes for cognition were smaller than for mood in both reviews. However, Chang 2015 found a significant effect on "disruptive behaviours" whereas we did not find evidence of an effect on behavioural problems (agitation/aggression), and we found an effect on depression which they did not, despite a larger effect size than in our review (−0.39 and −0.28, respectively).
A recent review by Zhang 2017 included non‐randomized studies and studies that we excluded because of insufficient therapeutic‐based goals and their methods and findings differed in a number of other ways. Their subgroup analyses for effect on "disruptive behaviour" (overall behavioural scales and agitation) suggested a higher SMD for non‐randomized studies (−1.02 versus −0.65 (text) or −0.52 (Table) for parallel RCTs). They found a larger SMD for disruptive behaviour (−0.42, 95% CI −0.74 to −0.11, compared to −0.20 in our work). Compared to our review, they found a similar SMD for cognition (SMD 0.20, 95% CI −0.09 to 0.49), and smaller SMDs for anxiety (−0.20, 95% CI −0.37 to −0.02), depression (SMD −0.16, 95% CI −0.41 to 0.08) and quality of life (SMD −0.12, −0.36 to 0.12; negative SMDs however favoured music therapy). Zhang 2017 performed different analyses, probably comparing scores before and after the intervention to calculate a SMD with a general check of whether there were baseline differences. This may explain different SMDs also for individual studies, and the quality assessments of the same included studies rarely corresponded with ours. For example, Svansdottir 2006 was an outlier for effect on behaviour in Zhang 2017 (SMD −3.88), compared with an SMD of −0.06 for end‐of‐treatment scores in our work. Also, in this case, Zhang 2017 assigned points for quality because of blinding of the therapist whereas we rated high risk for performance bias for all studies (in view of standardized methods to allow for comparison of very different interventions and situations) and in this case, Svansdottir 2006 also disclosed that the first author "conducted the music therapy." Zhang 2017 judged all studies to be of acceptable quality, even those with a total score of 3 (Supplemental table) or higher than 4 (text) on a 0 to 10 scale where one of the items was the random allocation. Finally, their secondary outcomes (depression, anxiety and quality of life) were prioritised in our review because of the evident importance for the person with dementia him/herself.
Multiple other recent reviews have summarized effects and concluded, without meta‐analyses, that the intervention is beneficial. Some focused on specific outcomes such as behavioural and psychological symptoms of dementia (e.g. Raglio 2012); or covered different types of outcomes such as physiological outcomes (e.g. McDermott 2013. who also noted a lack of evidence on long‐term effects). Petrovsky 2015 focused on effects on anxiety and depression in people with mild dementia, but included studies with participants who had varying severity of dementia as long as it was not limited to severe dementia. They found that the evidence was inconclusive based on 10 studies, including some with a pre‐post test design. We were able to include more RCTs because authors provided data about mood items in overall behavioural scales. Ing‐Randolph 2015 reviewed effects of group music interventions, including music therapy, on anxiety. They found that music interventions reduced anxiety in seven of eight included studies.
The clinical importance of the effect of music‐based interventions on depression is somewhat uncertain because of the variety of scales used, although there was no heterogeneity in effects across the studies. The SMD for depression (−0.28) and anxiety (−0.50; but very uncertain due to serious risk of bias, imprecision, inconsistency and probable publication bias) was within the range of, or larger than, pooled estimates of effects of medication on depression in people with dementia (antidepressants, 6 trials, SMD favouring medication 0.29, 95% CI 0.02 to 0.60, Nelson 2011; selective serotonin reuptake inhibitors, 12 trials, effect sizes favouring medication 0.06 to 0.10, Sepehry 2012). There may be fewer side effects of music‐based therapeutic interventions compared with medication.
Authors' conclusions
Music‐based therapeutic interventions may be used for people with dementia residing in institutional settings, to treat depressive symptoms. Depression is very common in people with dementia irrespective of the stage of dementia (Verkaik 2007); and it is related to low quality of life (Banerjee 2009; Beerens 2014). It is not clear whether effects will persist beyond the intervention period and music‐based interventions may need to be continued for prolonged periods for a sustained effect.
Guidelines for the design and implementation of RCTs of music therapy are available (Bradt 2012). For dementia, more well‐conducted studies are needed to establish more precisely the effects of music therapy and related interventions in the treatment of people with dementia, including effects on positive outcomes such as emotional well‐being, quality of life and social behaviour. Outcomes may also cover behaviour that may not be disturbing to others but compromises quality of life, such as apathy, which is highly prevalent and often highly persistent over the course of dementia (dementia or cognitive impairment, van der Linde 2016; Alzheimer's disease, Zhao 2016). Outcomes such as pain and discomfort have been used for testing effects of music therapy at the end of life, mostly among people with cancer (McConnell 2016); these are also important outcomes for people with dementia. Overall behavioural scales (which include items on hallucinations, euphoria, etc.) might be rather too broad for use as outcome scales for effects of music therapy. Future studies should follow the CONSORT guidelines for reporting of randomized trials, use adequate methods of randomization with adequate concealment of allocation of the participants to (parallel) treatment groups, blind the outcome assessors to treatment allocation (and report this) and be of sufficient duration to assess persistence of effects after the end of treatment. Blinding of participants is difficult but not impossible, especially with active control groups, when the participants are unaware of the hypothesis of the study and which intervention is considered the active intervention (Bradt 2012). We discourage the use of cross‐over designs because possible long‐term effects of music‐based interventions risk carry‐over effects when crossing over. Study protocols should be registered and primary and secondary outcomes should be reported accordingly. Reporting of effects should preferably include mean differences and standard deviation of differences between baseline and follow‐up, or effect sizes, which only a few studies (namely Ceccato 2012 and Ridder 2013) have reported so far. Funding sources should be reported and any potential conflict of interest through possible interest in the outcomes should be considered and disclosed, such as an interest in finding favourable effects of the therapy. This also includes cases where the therapist delivering the intervention (co)authors the article.
More research is needed to differentiate between various therapeutic approaches using music: to examine, for example, whether there is a difference between receptive and active approaches, or how response relates to duration of individual sessions (noting that any dose‐response relationships may not be linear, due to participants' difficulties with sustaining concentration or the risk of overstimulation with longer sessions). It is important to establish whether pre‐existing problematic or challenging behaviour moderates the effects. Further research is also required to compare music‐based therapeutic activities in which music is the main or only therapeutic element, to other group activities involving music. If more data were available, it would be helpful for future analyses to distinguish between usual care and other musical or non‐musical activities in the control group. In the existing literature, the professional background of the therapist was sometimes unclear, or there was no information about the training of the music therapists or their experience of delivering music‐based therapeutic interventions specifically to people with dementia. It is important to provide detail on who delivers the intervention in order to facilitate classification of interventions as music therapy delivered by a qualified, trained and experienced music therapist, other music‐based therapeutic interventions, or other interventions involving music, and to allow corresponding subgroup analyses. However, targeted studies may be more appropriate to evaluate effects of training because subgroup analyses risk confounding if, for example, qualified therapists see people with more complex problems. Further studies may also focus on effects in special groups such as young‐onset dementia, or on different settings, including community settings with more people with early dementia.
Acknowledgements
We gratefully acknowledge the contributions of the following.
The Cochrane Dementia and Cognitive Improvement Group and their peer reviewers.
Ingrid Riphagen, clinical librarian of the University of Groningen for her kind assistance.
Prof. Dr. Joris Slaets (University of Groningen, the Netherlands) for methodological and geriatric clinical advice.
The music therapy students from the Conservatory Enschede, ArtEZ School of music, the Netherlands for their help in retrieving possible studies.
Our lay reviewer Mr. Joost de Haas.
Jacqueline Birks for her valuable contribution to the first two versions of this review.
The authors who provided additional data and answered our questions about their studies.
Appendices
Appendix 1. Sources searched and search strategies used
| Source searched | Search strategy | Hits |
| MEDLINE In‐process and other non‐indexed citations and MEDLINE 1950 to present | 1. exp Dementia/ 2. Delirium/ 3. Wernicke Encephalopathy/ 4. Delirium, Dementia, Amnestic, Cognitive Disorders/ 5. dement*.mp. 6. alzheimer*.mp. 7. (lewy* adj2 bod*).mp. 8. deliri*.mp. 9. (chronic adj2 cerebrovascular).mp. 10. ("organic brain disease" or "organic brain syndrome").mp. 11. ("normal pressure hydrocephalus" and "shunt*").mp. 12. "benign senescent forgetfulness".mp. 13. (cerebr* adj2 deteriorat*).mp. 14. (cerebral* adj2 insufficient*).mp. 15. (pick* adj2 disease).mp. 16. (creutzfeldt or jcd or cjd).mp. 17. huntington*.mp. 18. binswanger*.mp. 19. korsako*.mp. 20. or/1‐19 21. music*.mp. 22. exp Music Therapy/ 23. singing.mp. 24. sing.mp. 25. "auditory stimul*".mp. 26. piano.mp. 27. or/21‐26 28. 27 and 20 29. randomized controlled trial.pt. 30. controlled clinical trial.pt. 31. random*.ab. 32. placebo.ab. 33. trial.ab. 34. groups.ab. 35. or/29‐34 36. (animals not (humans and animals)).sh. 37. 35 not 36 38. 28 and 37 39. (2008* or 2009* or 2010*).ed. 40. 38 and 39 |
15 |
| Embase 1980 to 2010 week 14 |
1. exp dementia/ 2. Lewy body/ 3. delirium/ 4. Wernicke encephalopathy/ 5. cognitive defect/ 6. dement*.mp. 7. alzheimer*.mp. 8. (lewy* adj2 bod*).mp. 9. deliri*.mp. 10. (chronic adj2 cerebrovascular).mp. 11. ("organic brain disease" or "organic brain syndrome").mp. 12. "supranuclear palsy".mp. 13. ("normal pressure hydrocephalus" and "shunt*").mp. 14. "benign senescent forgetfulness".mp. 15. (cerebr* adj2 deteriorat*).mp. 16. (cerebral* adj2 insufficient*).mp. 17. (pick* adj2 disease).mp. 18. (creutzfeldt or jcd or cjd).mp. 19. huntington*.mp. 20. binswanger*.mp. 21. korsako*.mp. 22. CADASIL.mp. 23. or/1‐22 24. music*.mp. 25. exp music therapy/ 26. singing.mp. 27. sing.mp. 28. exp singing/ 29. "auditory stimul*".mp. 30. exp auditory stimulation/ 31. piano.mp. 32. or/24‐31 33. 23 and 32 34. randomized controlled trial/ 35. exp controlled clinical trial/ 36. random*.ab. 37. placebo.ab. 38. trial.ab. 39. groups.ab. 40. or/34‐39 41. 33 and 40 42. (2008* or 2009* or 2010*).em. 43. 41 and 42 |
28 |
| PsycINFO 1806 to April week 1 2010 |
1. exp Dementia/ 2. exp Delirium/ 3. exp Huntingtons Disease/ 4. exp Kluver Bucy Syndrome/ 5. exp Wernickes Syndrome/ 6. exp Cognitive Impairment/ 7. dement*.mp. 8. alzheimer*.mp. 9. (lewy* adj2 bod*).mp. 10. deliri*.mp. 11. (chronic adj2 cerebrovascular).mp. 12. ("organic brain disease" or "organic brain syndrome").mp. 13. "supranuclear palsy".mp. 14. ("normal pressure hydrocephalus" and "shunt*").mp. 15. "benign senescent forgetfulness".mp. 16. (cerebr* adj2 deteriorat*).mp. 17. (cerebral* adj2 insufficient*).mp. 18. (pick* adj2 disease).mp. 19. (creutzfeldt or jcd or cjd).mp. 20. huntington*.mp. 21. binswanger*.mp. 22. korsako*.mp. 23. ("parkinson* disease dementia" or PDD or "parkinson* dementia").mp. 24. or/1‐23 25. music*.mp. 26. exp Music Therapy/ 27. sing.mp. 28. singing.mp. 29. exp Singing/ 30. "auditory stimul*".mp. 31. *Auditory Stimulation/ 32. piano.mp. 33. or/25‐32 34. 24 and 33 35. exp Clinical Trials/ 36. random*.ti,ab. 37. trial.ti,ab. 38. group.ab. 39. placebo.ab. 40. or/35‐39 41. 34 and 40 42. (2008* or 2009* or 2010*).up. 43. 41 and 42 |
26 |
| CINAHL | S1 (MH "Dementia+") S2 (MH "Delirium") or (MH "Delirium, Dementia, Amnestic, Cognitive Disorders") S3 (MH "Wernicke's Encephalopathy") S4 TX dement* S5 TX alzheimer* S6 TX lewy* N2 bod* S7 TX deliri* S8 TX chronic N2 cerebrovascular S9 TX "organic brain disease" or "organic brain syndrome" S10 TX "normal pressure hydrocephalus" and "shunt*" S11 TX "benign senescent forgetfulness" S12 TX cerebr* N2 deteriorat* S13 TX cerebral* N2 insufficient* S14 TX pick* N2 disease S15 TX creutzfeldt or jcd or cjd S16 TX huntington* S17 TX binswanger* S18 TX korsako* S19 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 or S18 S20 TX music* S21 (MH "Music Therapy") or (MH "Music Therapy (Iowa NIC)") S22 TX sing S23 TX singing S24 (MM "Singing") S25 TX "auditory stimul*" S26 (MM "Acoustic Stimulation") S27 S20 or S21 or S22 or S23 or S24 or S25 or S26 S28 S19 and S27 S29 (MH "Clinical Trials+") S30 AB random* S31 AB trial S32 AB placebo S33 AB group* S34 S29 or S30 or S31 or S32 or S33 S35 S28 and S34 S36 EM 2008 S37 EM 2009 S38 EM 2010 S39 S36 or S37 or S38 S40 S35 and S39 |
18 |
| Web of Science with Conference Proceedings (1945 to present) | Topic=(music* OR singing OR sing OR "auditory stimul*") AND Topic=(dement* OR alzheimer* OR "lew* bod*" OR huntington*) AND Topic=(random* OR trial OR placebo OR "double blind*" OR "single blind*" OR groups) Timespan=2008‐2010. Databases=SCI‐EXPANDED, A&HCI, SSCI, CPCI‐S |
33 |
| LILACS | demen$ [Words] and music OR singing [Words] | 7 |
| ALOIS | Advanced search: [study aim: Treatment Dementia] AND [study design: RCT OR CCT] AND [intervention (contains any): music OR singing OR audirory) | 29 |
| UMIN (Clinical Trial Register of Japan) | Free Keyword: music OR singing OR auditory | 0 |
| CENTRAL | #1 MeSH descriptor Dementia explode all trees #2 MeSH descriptor Delirium, this term only #3 MeSH descriptor Wernicke Encephalopathy, this term only #4 MeSH descriptor Delirium, Dementia, Amnestic, Cognitive Disorders, this term only #5 dement* #6 alzheimer* #7 "lewy* bod*" #8 deliri* #9 "chronic cerebrovascular" #10 "organic brain disease" or "organic brain syndrome" #11 "normal pressure hydrocephalus" and "shunt*" #12 "benign senescent forgetfulness" #13 "cerebr* deteriorat*" #14 "cerebral* insufficient*" #15 "pick* disease" #16 creutzfeldt or jcd or cjd #17 huntington* #18 binswanger* #19 korsako* #20 (#1 OR #2 OR #3 OR #4 OR #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 OR #13 OR #14 OR #15 OR #16 OR #17 OR #18 OR #19) #21 MeSH descriptor Music Therapy explode all trees #22 music* #23 singing #24 sing #25 "auditory stimul*" #26 (#21 OR #22 OR #23 OR #24 OR #25) #27 (#20 AND #26), from 2008 to 2010 |
10 |
| ClincalTrials.gov | dementia OR alzheimer OR alzheimers OR alzheimer's | music OR sing OR singing OR auditory | received from 01/01/2008 to 04/14/2010 | 2 |
| ICTRP Search Portal (WHO portal) | Advanced search: [condition: Dementia OR alzheimer OR alzheimers] AND [Intervention: music OR singing OR sing OR auditory] AND [date registration: 01/01/08 to 14/04/10] | 20 |
Appendix 2. Description of the interventions
Ceccato 2012
Music‐based therapeutic intervention: Sound Training for Attention and Memory in Dementia (STAM‐Dem) (versus a control group of usual care)
A 45‐minute mixed (active and receptive) group intervention delivered by “professionally trained music therapists trained to administer the STAM‐Dem protocol.” Highly structured, progressive series music sessions, with a minimum of four and a maximum of five participants per group. The music therapists were instructed to “pay attention to the relational atmosphere” and “maintain the level of motivation as high as possible.”
The intervention included “step‐by‐step exercises aimed at stimulating and checking both attention and memory.” Participants were asked to perform specific movements, count, clap hands, alternate clapping hands and tapping the table, repeat sequences of previously recorded sounds (not stated how) after listening to recorded and live played music. It is a mixed intervention because the active component was combined with listening to music.
The STAM‐Dem protocol comprises four phases, one for each specific cognitive function that is trained (selective attention, sustained attention, alternate attention and working memory). The phases involve: 1) stimulus‐movement association, 2) reaction to acoustic stimuli, 3) shifting attention with two exercises, and 4) orderly and inverted repetition. It is not clear from the text if the phases each last four sessions, and are progressive, but as described in other sources (not cited in the article) they are (STAM protocol). Each phase then lasts four sessions and is followed by the next. However, the intervention phase lasted 12 weeks, in which 24 sessions were held.
Clark 1998
Music‐based therapeutic intervention: preferred, recorded music during bathing episodes with aggressive behaviour (versus a control group with no music during bathing)
A receptive individual intervention with music, listening through speakers, delivered by nursing staff. Duration followed established nursing routines and varied from 11 to 18 minutes.
Preferred music was recorded and selections played via an audiotape recorder during the bathing episode. Background information on participants’ music experiences and preferences was obtained by interviews with the family member or responsible agent. “Bathing times were scheduled for either morning or afternoon” “following established nursing routines.” Participants received either a partial bath which was given in the participant’s room, or a full bath, which was given in the shower on the nursing unit.
Nursing staff delivered the bathing session. It is not clear from the text whether nursing staff were responsible for turning on the music, but it is highly probable that this was done by the observer: “Initially, consideration was given to having nursing staff be responsible for turning on the audiotape recorder...However, during pilot testing of the procedures, this proved too cumbersome for already overburdened nursing staff.” The sessions were given 10 times over two weeks.
Cooke 2010
Music‐based therapeutic intervention: Active group music sessions with live and recorded music (versus a reading group as the control condition)
An active, structured 40‐minute group music session delivered by two musicians. The session consisted of singing and playing on instruments accompanied by live familiar songs and recorded instrumental music. The group had a maximum of 16 participants.
The session covered 30 minutes of musician‐led familiar song‐singing with guitar accompaniment, and 10 minutes of pre‐recorded instrumental music. A set repertoire was established for each of three sessions and this was repeated for eight weeks.
“Residents were encouraged to participate actively through singing/humming, playing instruments and… movement.” Choice of the instruments is not described. The repertoire selection was based primarily on participants’ musical preferences, musicians’ repertoire knowledge, and the findings from a practice session (conducted in an alternative aged care setting). The 10 minutes of listening to pre‐recorded music allowed the musicians and participants to have a short rest from performance and singing and to cater for participants who had a preference for more instrumental music. The sessions were delivered three mornings a week (Monday, Wednesday and Friday) for eight weeks, with a total of 24 sessions.
Control intervention: reading group
An interactive reading session included a range of reading and social activities, such as reading local news stories, short stories, telling jokes and undertaking quiz activities. The sessions were led by one trained Research Assistant. A maximum number of attendees is not clear from the text. The control sessions took 40 minutes, and were delivered three times a week (Monday, Wednesday and Friday) for eight weeks, totaling a number of 24 sessions.
Guétin 2009
Music‐based therapeutic intervention: Individual receptive therapy with the ‘U‘ sequence method (versus a reading group as the control condition)
An individual receptive music therapy method, the ‘U‐sequence’ method involved listening to music sequences, selected from a limited number of musical styles delivered through headphones, in the patient’s room. The musical style was chosen based on the patients’ personal tastes following an interview or questionnaire. From the suggested different musical styles, a musical sequence was selected. This usual musical sequence, lasting 20 minutes, was broken down into several phases, according to the ‘U sequence’ method and making use of a computer program especially designed for this method. Musical rhythm, orchestral formation, frequency and volume were reduced. After a phase of sustained reduced musical rhythm, orchestral formation, frequency and volume, a re‐enlivening phase followed in which musical rhythm, orchestral formation, frequency and volume increased again, and ended at a moderate level in comparison to the beginning phase. The style of music varied from one session to another for a given patient.
“Patients were either in a supine position or seated in a comfortable armchair and were offered a mask so as to avoid visual stimuli.” Details on the ‘U sequence’ method are retrievable through this external link (not included in the paper): http://www.music‐care.com/en/page/treatment
Sessions were extended by a period of time spent listening to the patient. This period of time served “to create a ‘psychotherapist’‐type of therapeutic relationship and …reinforced the effect triggered by listening to music.” Duration of this ‘listening’ intervention with a therapist was not reported.
Personnel delivering the music and the listening intervention is not clear from the text. Sessions were delivered once a week, lasted 20 minutes (plus time spent listening to patients' responses – duration of which is not stated), and 16 sessions were delivered.
Control intervention: reading group
“Rest and reading under the same conditions and at the same intervals. “
Liesk 2015
Music‐based therapeutic intervention: A ‘Musikgeragogik’ group music programme (versus a cognitive stimulation intervention as the control condition)
A 90‐minute structured active group music intervention based on the principles of ‘Musikgeragogik’ by T Hartogh (2005) which was designated as “music education for elders.” Sessions consisted of singing folk songs, rounds and playing on instruments (woodblocks, bells, tambourine and maracas). Participants were stimulated to improvise in a structured way according to cues in the song lyrics, alternated with spontaneous expression of individual impressions provoked by the songs that were played or sung. It is probable that the music used was live as the music intervention was “created as an active therapy form,” but this is not explicitly mentioned in the text.
A music recreational therapist (‘Musikgeragogin’) delivered the intervention. Duration of sessions was 90 minutes and frequency was twice a week, during six weeks, totaling 12 sessions.
Control intervention
A cognitive stimulation program in which cognitive function is trained through quiz questions of differing complexity and theme‐focused conversations, a Cognitive training program of NEUROvitalis from a group in Cologne (Baller and colleagues, 2010), adapted for people with dementia. A gerontologist delivered the intervention. The sessions lasted 90 minutes, twice a week over six weeks, totaling 12 sessions.
Lin 2011
Music‐based therapeutic intervention: Group music therapy (versus a control group with usual care that “continued to perform their usual daily activities”)
This was a 30‐minute structured mixed group music therapy intervention, based on the protocol developed by Clair and Bernstein (1990). The size of the group is not clear from the text.
The intervention consisted of rhythmic music and slow‐tempo instrumental activities (choice of instruments not specified), therapeutic singing, listening to specially selected music, glockenspiel playing and musical activities and traditional holiday and ‘music creator’ activities. “…before the therapy sessions a subject’s fondness for music was evaluated through an interview, and the musical activities in the group sessions were arranged according to the interview findings.”
The person delivering the intervention was a researcher schooled in two university music therapy courses. The sessions lasted 30 minutes and were conducted twice a week for six consecutive weeks. The total number of sessions was 12.
Lord 1993
Music‐based therapeutic intervention: A group music programme (versus two control groups, Jigsaw puzzle activities, and no special treatment)
A 30‐minute mixed group music intervention, during which music of the “Big Bands” of the 1920s and 1930s were played. It is not clear if the music used was repeated every session or varied from session to session. The group had a size of 20 participants. Active music making (on triangles and tambourines) and singing was possible. It is not clear to what degree active music‐making was stimulated by personnel or depended on participants’ initiative only.
Personnel delivering the session was an “activities specialist” and two nurses. Sessions were delivered six times per week and continued for six months, therefore totaling 156 sessions.
Control intervention 1
This group was given several puzzle‐play activities (cardboard jigsaw cutouts and pegboard puzzles), new puzzles were introduced periodically.
Control intervention 2
Another group received the usual recreational activities of drawing, painting, and watching television.
Narme 2012
Music‐based therapeutic interventions: Group music programme (versus the control condition of art therapy in study 1, and versus cooking in study 2)
Study 1
A 2‐hour structured mixed group intervention, with a maximum of 12 participants. Music selections were chosen independent of participants’ preference and were played through a loudspeaker. The selections varied from classical music to songs from the 1950s and included instrumental and vocal music, and varied from ‘calming’ to ‘dynamic’ music. Calming music was used at the start and end of each session. The order of the musical selections was the same for every session, and pieces were played twice if participants expressed the wish to hear a song again. Participants were encouraged to play along (on percussion instruments, maracas or bell chains), sing and improvise. Participants were stimulated to express their feeling and memories evoked by the activity.
Control intervention study 1
The control intervention in study 1 was another pleasant art therapy intervention. Painting session offered participants the use of wax crayons, colouring pencils, felt pens and gouache painting. They were stimulated to create simple drawings, to make circular movements with different materials and to make drawings based on their imagination. Participants were also encouraged to express their feeling and memories evoked by the activity.
Personnel delivering the two interventions were two psychologists. All sessions lasted two hours and were delivered twice a week during three weeks, totaling 12 hours during six sessions.
Study 2
The same 2‐hour structured mixed group intervention was delivered by two psychologists, and the sessions were delivered twice a week, but during four weeks, and therefore totaling 16 hours during eight sessions.
Control intervention study 2
The control intervention in study 2 was cooking, because it was a pleasant activity that stimulates a number of senses. There was more interaction compared to the painting control condition. Further, more similar with the music therapy intervention, the cooking intervention also involved alternating productive (prepare a recipe) and receptive phases (taste a dessert). The sessions included preparing a different recipe collectively, with roles distributed according to the participants' abilities. Participants were encouraged to taste ingredients, and verbalize remembrances.
Narme 2014
Music‐based therapeutic intervention: A group music programme (versus cooking as the control condition)
A 60‐minute structured mixed group intervention, with a maximum of eight participants. Music selections were chosen independent of the participants’ preferences, and were played on a CD player (loudspeaker). The selections varied from classical music to songs from the 1950 to 1980s, included minor and major keys) and were ‘calming’ with slow to moderate tempo and ‘arousing’ music with a higher tempo. Calming music was used at the start and end of the session. The same playlist was used in the same order for each music session, but pieces were played twice if participants expressed the wish to hear a song again. Participants were asked to listen or to play along (on percussion instruments: clapping or playing hand drums) and sing along. Receptive and active phases were alternated. Participants were encouraged to express their feelings and autobiographical memories evoked by the activity.
The sessions were delivered twice a week, for a period of four weeks, totaling eight one‐hour sessions. Personnel delivering the intervention were “two supervisors,” including one psychologist, with no prior education in music therapy.
Control intervention
A cooking intervention, in which participants were asked to make a different recipe for each session (e.g. chocolate cake; French pancakes). Each session commenced with a game about ingredients where participants were asked to collectively prepare a given recipe. Roles were distributed according to patients’ abilities (e.g. cutting, peeling, measuring quantities, mixing or cooking). Receptive (tasting) and productive phases were alternated. Participants were encouraged to express their feelings and autobiographical memories evoked by the activity.
The sessions had a duration of one hour and were delivered twice a week, for a period of four weeks, totaling eight one‐hour sessions. Personnel delivering the intervention were “two supervisors,” including one psychologist, with no prior education in music therapy.
Raglio 2010a
Music‐based therapeutic intervention: Individual music therapy based on relationship (versus a control group with usual care)
A 30‐minute active non‐verbal individual music therapy intervention, in which free musical improvisation is used to build a relationship between participant and music therapist. During the session the participant and the music therapist have a non‐verbal dialogue and express their feelings and emotions through non‐verbal behaviours (possibly by using voice and tapping, not specified in the text) and by playing musical instruments. Choice of instruments included rhythmic‐melodic instruments, percussions, glockenspiels, xylophones, etc. Sharing emotions, raising awareness and the possibility of introducing new ways of expression and communication are a focus of the session and may lead to empathetic processes and mutual calibration.
A music therapist delivered the sessions, which were twice a week for 15 weeks, with a total of 30 sessions.
Raglio2010b
Music‐based therapeutic intervention: Group music therapy based on relationship (versus a control group with usual care)
A 30‐minute active non‐verbal group music therapy intervention, in which free musical improvisation is used to build a relationship between participant and music therapist. Groups had a size of three participants. The intervention focuses on favouring the moments of attunement that help organise and regulate the participants’ behaviours and emotions. Participants and music therapist interact and express their feelings and emotions through non‐verbal behaviours and using musical instruments. Note that this approach is inspired by the intersubjective psychology (Fogel 1993; Stern 1985, 2004; Trevarthen & Aitkin 2001; Tronick 1998; references provided in the article).
A music therapist delivered the sessions. The sessions were delivered in three non‐continuous treatment cycles consisting of four weeks of three sessions per week followed by one month of no treatment (washout; however, not in the context of a cross‐over design). The total number of sessions was 36, within a period of six months.
Ridder 2013
Music‐based therapeutic intervention: Individual mixed music therapy (versus a control group with usual care)
An individual mixed music therapy intervention, not pre‐structured, delivered by music therapists with an average duration of almost 34 minutes (33.8, SD 9.91). The aim of the music therapy was phrased in a more positive way than a goal of reducing, e.g. challenging behaviour ("to facilitate initiative, engagement, self‐expression and mutual understanding"). The authors refer to Tom Kitwood for the theoretical basis of a relation‐based and person‐centred approach in music therapy.
Vocal or instrumental improvisation, singing, dancing/moving, listening and talking/going for a walk could be part of the session. The music accompanying the activities was pre‐recorded or live music, and consisted of ‘free’ improvisation or based on songs/melodies. The overall aim of the music therapy was to facilitate initiative, engagement, self‐expression and mutual understanding. Clinicians were instructed to be aware of at least three different ways of applying music in therapy: catching attention and creating a safe setting, regulating arousal level to a point where self‐regulation is possible and engaging in social communication to fulfil psychosocial needs. The session was not especially focused on decreasing agitation.
Music therapists with university‐level training delivered the intervention which were twice a week for a period of six weeks, with 12 sessions offered in total. The average number of sessions received was 10 (SD 2.82, range 0 to 13).
Sakamoto 2013
Music‐based therapeutic intervention: An individual mixed music (therapy) intervention (versus 2 control groups)
A 30‐minute individual mixed music therapy intervention. The selection of music was based on determination of a period of the participant’s life that was recalled most frequently, interviews with participants and their family, and links to special memories. Music was selected for probable evoking of positive emotions such as pleasure or joy.
The selected music was played via a CD player (loudspeaker). The participants also participated in activities guided by a music facilitator, including clapping, singing and dancing. The sessions took place in a familiar room.
During the session an eye was kept on participants to confirm that “the music was suitable in terms of engaging the participants and eliciting a joyful emotional state.” Participants’ attention was directed to the music, and “an interactive approach that responded to the participants’ emotional reactions to the music” was used.
The sessions were delivered by music therapists, occupational therapists and nurses, each trained for ten days in delivering the intervention. The sessions took place weekly for a period of 10 weeks (10 sessions in total), and were scheduled between 10 a.m. and 11 a.m.
Control group 1: Passive individual music intervention (the music intervention did not meet our criteria for music‐based therapeutic interventions)
A 30‐minute individual music intervention. The selection of music was made based on determination of a period of participants’ life that was recalled most frequently, interviews with participants and their family, and links to special memories. Music was selected for probable evoking of positive emotions such as pleasure or joy.
The selected music was played via a CD player (loudspeaker). Personnel delivering the intervention was a caregiver and a music provider, but no interaction took place between personnel and participants during the intervention. The session took place in a familiar room.
The sessions took place weekly for a period of 10 weeks (10 sessions in total), and were scheduled between 10 a.m. and 11 a.m.
Control group 2
Spending 30 minutes in their own room as usual in a silent environment, with a caregiver observing from a distance and no interaction between caregiver and participant. The sessions took place weekly for a period of 10 weeks (10 sessions in total), and were scheduled between 10 a.m. and 11 a.m.
Sung 2012
Music‐based therapeutic intervention: Active group music intervention (versus a control group with usual care)
A 30‐minute active group music therapy intervention with movement. The sessions included five minutes of warm‐up and five minutes of cooling down (stretching major muscle groups and breathing exercise with music). During the main part of the session, participants were guided in the use of percussion instruments (hand bell, tambourine, maracas, guiro tone block, flapper and loop bell) while listening to music and songs familiar to the participants. Participants’ music preferences were assessed through interviewing the participants, caregivers, families or nursing staff. The preferred music was Taiwanese and Chinese songs from the 1950 to 1970s with moderate rhythm and tempo.
Sessions were delivered by a nursing researcher and two research assistants trained in providing the music intervention, twice a week for six weeks, with a total of 12 sessions.
Svansdottir 2006
Music‐based therapeutic intervention: Mixed group music therapy (versus a control group with usual care)
A 30‐minute mixed music therapy intervention, with three or four participants per group. The sessions were accompanied by guitar playing and consisted of (listening to) singing with the help of songbooks, playing along on various kind of instruments (choice of instruments not specified), instrumental improvisation, and moving/dancing, if “patients had an urge to move and dance.” The music therapist selected a collection of songs that were familiar to the residents.
A music therapist delivered the sessions which were three times a week for six weeks, totaling 18 sessions.
Vink 2014
Music‐based therapeutic intervention: Mixed group music therapy (versus a control condition with general recreational activities)
A 40‐minute mixed group music therapy intervention which consisted of a welcome song, listening to music selected, sung or played by the therapist (Dutch familiar songs, classical and folk music), and singing, dancing or playing along (on simple rhythm instruments). Within the group session the therapist adjusted the level of each intervention to individual capacities. The music accompanying the session was played live on e.g., piano or guitar and was selected with the goal of inciting pleasant memories and reducing agitation. For this musical parameters were used “such as slow tempo and little instrumentation.”
Music therapists delivered the intervention, in rooms away from the nursing home ward. The sessions were delivered twice a week for four months, with a total of up to 34 sessions.
Control intervention
General recreational activities, such as handwork, playing shuffleboard, making flower bouquets and playing games. The sessions lasted 40 minutes and were also held in rooms away from the nursing home ward.
Data and analyses
Comparison 1.
Music therapy versus usual care or versus other activities: end of treatment
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Emotional well‐being and quality of life | 6 | 181 | Std. Mean Difference (IV, Random, 95% CI) | 0.32 [‐0.08, 0.71] |
| 1.1 music vs usual care | 1 | 41 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.37, 0.86] |
| 1.2 music vs other activities | 5 | 140 | Std. Mean Difference (IV, Random, 95% CI) | 0.37 [‐0.14, 0.89] |
| 2 Negative affect or mood disturbances: depression | 9 | 376 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.48, ‐0.07] |
| 2.1 music vs usual care | 5 | 247 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.28 [‐0.59, 0.02] |
| 2.2 music vs other activities | 4 | 129 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.56, 0.14] |
| 3 Negative affect or mood disturbances: anxiety | 11 | 365 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐0.84, ‐0.16] |
| 3.1 music vs usual care | 5 | 184 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.48, 0.10] |
| 3.2 music vs other activities | 7 | 181 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.81 [‐1.38, ‐0.24] |
| 4 Problematic behaviour: agitation or aggression | 12 | 515 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.29, 0.14] |
| 4.1 music vs usual care | 9 | 405 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.36, 0.13] |
| 4.2 music vs other activities | 4 | 110 | Std. Mean Difference (IV, Random, 95% CI) | 0.04 [‐0.46, 0.54] |
| 5 Problematic behaviour overall | 6 | 209 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.20 [‐0.56, 0.17] |
| 5.1 music vs usual care | 4 | 132 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.39 [‐0.84, 0.05] |
| 5.2 music vs other activities | 3 | 77 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.31, 0.63] |
| 6 Social behaviour: music vs other activities | 3 | 70 | Std. Mean Difference (IV, Random, 95% CI) | 0.54 [0.06, 1.02] |
| 7 Cognition | 6 | 257 | Std. Mean Difference (IV, Random, 95% CI) | 0.21 [‐0.04, 0.45] |
| 7.1 music vs usual care | 3 | 170 | Std. Mean Difference (IV, Random, 95% CI) | 0.25 [‐0.06, 0.55] |
| 7.2 music vs other activities | 3 | 87 | Std. Mean Difference (IV, Random, 95% CI) | 0.13 [‐0.29, 0.56] |
Comparison 2.
Music therapy versus usual care or versus other activities: long‐term effects
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Emotional well‐being and quality of life | 2 | 48 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [‐0.10, 1.05] |
| 1.1 music vs usual care | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 music vs other activities | 2 | 48 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [‐0.10, 1.05] |
| 2 Negative affect or mood disturbances: depression | 5 | 234 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.27, 0.24] |
| 2.1 music vs usual care | 3 | 173 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.31, 0.29] |
| 2.2 music vs other activities | 2 | 61 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.52, 0.48] |
| 3 Negative affect or mood disturbances: anxiety | 5 | 160 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.23 [‐0.86, 0.41] |
| 3.1 music vs usual care | 2 | 88 | Std. Mean Difference (IV, Random, 95% CI) | 0.09 [‐0.52, 0.70] |
| 3.2 music vs other activities | 3 | 72 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.54 [‐1.78, 0.70] |
| 4 Problematic behaviour: agitation or aggression | 4 | 225 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.36, 0.33] |
| 4.1 music vs usual care | 3 | 188 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.16 [‐0.45, 0.13] |
| 4.2 music vs other activities | 1 | 37 | Std. Mean Difference (IV, Random, 95% CI) | 0.50 [‐0.15, 1.16] |
| 5 Problematic behaviour overall | 3 | 125 | Std. Mean Difference (IV, Random, 95% CI) | 0.05 [‐0.30, 0.41] |
| 5.1 music vs usual care | 2 | 88 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.53, 0.60] |
| 5.2 music vs other activities | 1 | 37 | Std. Mean Difference (IV, Random, 95% CI) | 0.16 [‐0.49, 0.80] |
| 6 Social behaviour | 2 | 48 | Std. Mean Difference (IV, Random, 95% CI) | 0.53 [‐0.53, 1.60] |
| 6.1 music vs usual care | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 music vs other activities | 2 | 48 | Std. Mean Difference (IV, Random, 95% CI) | 0.53 [‐0.53, 1.60] |
| 7 Cognition | 1 | Std. Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1 music vs usual care | 1 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7.2 music vs other activities | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Analysis 2.7.
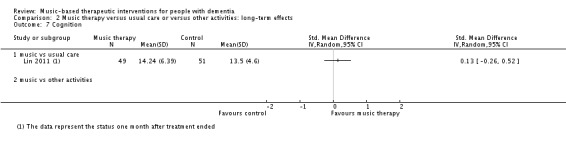
Comparison 2 Music therapy versus usual care or versus other activities: long‐term effects, Outcome 7 Cognition.
What's new
Last assessed as up‐to‐date: 12 April 2016.
| Date | Event | Description |
|---|---|---|
| 11 April 2017 | New citation required and conclusions have changed | New studies included. Conclusions changed. New author. |
| 12 April 2016 | New search has been performed | Updated search and potentially eligible studies included under studies awaiting classification |
History
Protocol first published: Issue 1, 2002 Review first published: Issue 3, 2004
| Date | Event | Description |
|---|---|---|
| 14 April 2010 | New search has been performed | An update search was performed for this review on 14 April 2010. New studies were retrieved for possible inclusion or exclusion within the review. Two new studies have been included in this update |
| 26 November 2008 | New search has been performed | A new update search was performed on 20 March 2008. New studies were retrieved for possible inclusion or exclusion in the review. Three new studies have been included in this update, and 15 new studies have been excluded Risk of Bias tables have been completed for all included studies |
| 23 January 2006 | New search has been performed | January 2006: The update searches of 5 December 2005 yielded 4 new trials which were not suitable for inclusion. The results and conclusions of this review remain unchanged |
Differences between protocol and review
We adapted terminology for relevant outcomes. The protocol formulated the objective in terms of problems only while emotions and (social) behaviour is broader than that (protocol: “To assess the effects of music therapy in the treatment of behavioural, social, cognitive and emotional problems in older people with dementia.”). In the review, we now consistently refer to: (1) emotional well‐being including quality of life; mood disturbance or negative affect, which includes (2) depression and (3) anxiety; behavioural problems which includes (4) agitation or aggression, and (5) behaviour overall; (6) social behaviour; and (7) cognition. We also searched for any (other) possible adverse effects. We adapted the objectives in the abstract to cover both the original aims and how we broadened it to include more positive outcomes as well. Also, the protocol referred to effects in "older people" but there has not been an exclusion criterion based on age. We therefore dropped reference to "older" people.
Publications were assessed by two, and not three reviewers independently. Data were extracted by two reviewers and if needed, in consultation with other reviewers as per protocol. We included RCTs only because unlike at the time the protocol was written, we expected more RCTs to be available. We accepted a physician's diagnosis of dementia if no data on formal criteria such as DSM‐IV, DSM‐5 or comparable instruments were available for reason of relevance to clinical practice and known underreporting. We did not analyse by length of treatment (months, length in three groups as in the protocol), but we analysed end‐of‐treatment data accepting variable durations and number of sessions as long as the outcomes were assessed after a minimum of five sessions. Rather, we aimed at assessing long‐term effects, analysing data about assessments at a minimum of four weeks after the end of treatment (with at least five studies available).
We used more stringent criteria with respect to: (1) assessing whether an article reported about a music intervention with an individual therapeutic intent, including — but not limited to — interventions provided by qualified music therapists, (2) analyses referring to outcome assessments after a minimum of five sessions or analyses that include earlier assessments if there is evidence of no different effect over time, (3) control group, and (4) risk of bias.
Regarding (4): if no research protocol was available, risk of reporting bias was set to either unclear or, for specific reasons, as high (also if rated as low in previous versions of the review). With regard to (1), we defined music‐based therapeutic interventions or music therapy as: therapy provided by a qualified music therapist, or an intervention meeting at least two of the following criteria: (a) therapeutic objective which may include communication, relationships, learning, mobilisation, expression, mobilisation and other relevant therapeutic objectives; (b) music matches individual preferences; (c) active participation of the people with dementia using music instruments; (d) participants had a clinical indication for the interventions or were referred to the intervention by a clinician. We also required music to be a main element of the intervention (e.g. not moving with use of music). We therefore focused on therapeutic aspects and elements that are more complex and required special skills while also targeted to the individual compared with, for example, playing recorded music for a group activity. We did not require a certified music therapist to provide the intervention, because the profession, exact qualification, training and experience was often unclear, and training programmes may vary between countries. Moreover, the importance of requiring a qualification is unclear in relation to the importance of having experience with the specific needs of people with dementia (for example, a trained music therapist with no experience in comparison with a musician with years of experience in providing therapy to people with dementia). Further (point 3), we required control groups to not receive any music‐based therapeutic intervention (even if fewer sessions than the active intervention group).We re‐assessed previously included studies by the new criteria and when in doubt, we consulted the lead author of the earlier versions.
Finally, we conducted a series of post hoc sensitivity analyses to explore possible effects of using more stringent criteria with respect to a requirement of a music therapist to deliver the intervention, and funding by parties with a possible interest in effectiveness of music therapy.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | RCT (parallel) No information on data collection period reported |
|
| Participants | Country: Italy. 5 support centres. N = 51 people with dementia and 50 of them were included in analyses (1 had only pre‐test data); 28 participants in experimental group (27 in analyses) and 23 participants in control group (21 female in experimental group and 19 female in control group). Mean age: 85.5 years (SD 5.9) in experimental group and 87.2 years (SD 7.1) in control group. Dementia diagnosis: formally diagnosed with the DSM‐IV. Inclusion criterion was Mini‐Mental State Examination (MMSE) score from mild (MMSE 18 to 24) to moderate (MMSE 12 to 18). People with acute medical illness were excluded, and a number of additional inclusion criteria applied, including being "sensitive to sound/musical stimuli"; "the desire and capacity to remain in the setting"; "presence of sufficient (also residual) hearing and perceptive‐communicative and relational skills". |
|
| Interventions | 1) Sound Training for Attention and Memory in Dementia (STAM‐Dem). Mixed active‐receptive group intervention with 24 sessions of 45 minutes in 12 weeks. STAM‐Dem includes 4 phases: 1) stimulus‐movement association, 2) reaction to acoustic stimuli, 3) shifting attention, and 4) orderly and inverted repetition. The intervention combines listening to music, clapping hands, tapping the table, and repeating sounds. The professional music therapists were trained to administer the STAM‐Dem protocol. Supervision was provided throughout the course of the intervention by the protocol's author. 2). Those in the control group continued with the normal ʺstandard careʺ provided. |
|
| Outcomes | Primary outcome
Secondary outcomes
|
|
| Notes | Randomization was done separately for each centre (6 randomizations in total). This is also the reason why there were more people in the experimental group (28 versus 23). Funding: F.S. Zerbato Centre at Tregnago (president, director and manager) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "They were divided up using an online randomization program by personnel not involved in the study, thereby ensuring totally ʺblindʺ conditions." However, there were 6 randomizations with small numbers. |
| Allocation concealment (selection bias) | Unclear risk | Unclear how blinded. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind the convener and participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "Pre‐ and postintervention testing was also administered by professionals who had no other role in the project; blind conditions were thus obtained for assignment treatment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant dropped out and 1 participant had no post‐test data. Unclear if this was the same participant as the number allocated to the intervention group was incorrect in the Figure. |
| Selective reporting (reporting bias) | Low risk | They admit that they did not follow the plans here: no follow‐up was conducted after the intervention because of a lack of funding. |
| Other bias | Unclear risk | Funding sources might have an interest in the study outcomes. |
| Methods | RCT (crossover 2 weeks + 2 weeks). No information on data collection period reported. |
|
| Participants | Country: USA. N = 18 (14 female, 4 male). Mean age: 82 (range 55 to 95), residents in a nursing home with Alzheimer‐type dementia. Inclusion criteria presence of dementia and a history of aggressive behaviour exhibited during care giving routines. Presence of dementia was assessed with the MMSE (Mean = 10, range 0 to 22); most residents had severe dementia. Exclusion criteria:
|
|
| Interventions | (1) Favourite music during bathing (receptive intervention). (2) No music during bathing. Following a 2‐week (10‐sessions) observation period, conditions were reversed. A total of 20 sessions (bathing episodes; 10 control, 10 experimental) were observed over a period of approximately 4 weeks. Probably the intervention was provided for all bathing episodes and all were observed. |
|
| Outcomes | Behaviour: frequency of aggressive behaviours (no specific measure was used, but counts and mean counts across specific behaviours). | |
| Notes | No information about funding available. Note that the study also included younger people with dementia. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "After being enrolled in the study, participants were randomly scheduled for observation during bath time under either a control (no music) condition or an experimental condition." No further information is provided on randomization. |
| Allocation concealment (selection bias) | Unclear risk | No information provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind the convener and participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information provided. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available. |
| Other bias | High risk | Questionable outcome measure and distribution. The authors report in the article on the effects of the extreme intra‐subject and inter‐subject variability characteristic of this population in this study. Quote: "For example, one subject was responsible for 408 and 84 occurrences of yelling behaviour in the no music and music conditions, respectively". Therefore, highly skewed distributions (the observation hardly occurred) causing imprecision. |
| Methods | RCT (crossover). Data collection from October 2008 to March 2009. |
|
| Participants | Country: Australia. 2 mixed‐gender long‐term care facilities, which provided low (assisted living) and high (nursing home) care. N = 47 (33 female and 14 male). Age: 3 people between 65 and 74, 13 between 75 and 84, 28 between 85 and 94 and 3 people aged 95+. Dementia diagnosis: a confirmed diagnosis of early‐ to mid‐stage dementia, OR probable dementia (i.e. a cognitive impairment level of 12 to 24 on MMSE) OR Alzheimer’s dementia according to DSM‐IV criteria. At baseline, the mean MMSE score was 16.51, representing middle‐stage dementia (SD = 6.737). Parcipants had "a documented behavioural history of agitation/aggression on nursing/medical records within the last month." |
|
| Interventions | 1) Active live group music programme (30 minutes per session) and listening to pre‐recorded instrumental music (10 minutes per session) led by 2 musicians. 2) Reading group chosen as the control group activity so as to provide a comparable activity. The facilitator of the 40‐minute sessions was trained research assistant. Both the active group music programme and the control activities ran 3 mornings a week (Monday, Wednesday and Friday) for 8 weeks, and the facilitators were trained in the delivery of the sessions and in working with older people with dementia. |
|
| Outcomes | Primary outcome
Secondary outcome
|
|
| Notes | Funding: funded by the National Health & Medical Research Council, Australia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation process was conducted by the study’s biostatistician, who was blinded to the identity of potential participants, using a computer‐generated programme". |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomisation process was conducted by the study’s biostatistician, who was blinded to the identity of potential participants, using a computer‐generated programme". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind the convener and participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote about CMAI‐SF: "Aged care staff who provided most care to the participant, but blinded to treatment groups, were asked to rate the ..." Quote about RAID: "Research assistants (RAs) blinded to the treatment groups asked participants to rate, on a scale from ‘1 = absent’ to ‘3 = severe’, how often he/she had experienced each symptom in the previous two weeks". Research assistant completed DQOL and GDS (Figure 1). Quote: "Both measures were conducted by trained RAs blinded to the treatment groups at a time most convenient for the participant (i.e. any day of the week from 9am–5pm). The RAs took the role as interviewer, taking the participants through the measures by asking them questions to elicit their response". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Prior to all sessions, participants were asked if they wished to attend. This resulted in some refusals and differences in attendance levels amongst participants. Following a missing values analysis, which indicated data to be missing at random, an ITT analysis, in which all randomized participants were included (n = 47), was undertaken. Missing values in the outcome measures were imputed with multiple imputation methods. |
| Selective reporting (reporting bias) | High risk | Inconsistencies compared with the trial registration which was retrospectively registered in 2012. Number of registration therefore not in article. Registration points to anxiety as a secondary outcome, not a primary outcome. Moreover, quality of life and depression were not reported as secondary outcomes. |
| Other bias | Low risk | |
| Methods | RCT parallel group trial; total duration 18 months, with a follow‐up period of 6 months. Participants resided in the nursing home between September 2007 and April 2008. |
|
| Participants | Country: France. N = 30 (22 female, 8 male), 1 centre. Mean age: experimental group 85.2 (range 75 to 93 years); control group 86.9 (range 74 to 95 years) Diagnosis of dementia: mild to moderate stages of AD; Inclusion criteria
Exclusion criteria
|
|
| Interventions | 1) Individual receptive music therapy method, the ‘U‐sequence method’, which involved listening to music sequences, selected from preferred musical styles delivered through headphones, in the participant’s room. 2) Reading sessions Weekly sessions during 16 weeks (total of 16 sessions). |
|
| Outcomes | 1) Level of anxiety (Hamilton Scale; total score ranging from 0 to 56) 2) Level of depression (Geriatric Depression Scale; maximum score is 30) 3) Mini‐Mental State Examination Score (MMSE). Outcomes assessed at day 0, week 4, week 8, week 16 and week 24 by an independent neuropsychologist assessor. |
|
| Notes | Funding: this research could be carried out thanks to support from Centres Mémoire de Ressources et de Recherches, Les Violettes nursing home, Université René Descartes – Paris V, Institut Alzheimer, the Rotary Club and La Fondation Médéric Alzheimer. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Probably yes, but no details provided. “The study design corresponded to a randomised, controlled, comparative, single‐centre study, with the results evaluated under blind conditions.” “The patients were allocated to the different groups by randomisation at the end of the inclusion visit.” |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind the convener and participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and caregivers not blinded, outcome assessor blinded: “The results obtained at D0, W4, W8, W16 and W24 were collected by an independent neuropsychologist assessor (D.L.), not belonging to the care team and unaware of the type of intervention.” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear whether dropouts caused bias. "Two patients were prematurely withdrawn from the study in the intervention group: 1 between W8 and W16 owing to an intercurrent event not related to the study (life‐threatening situation, hospitalisation), and the second died between W16 and W24. Four patients were withdrawn from the study in the control group: 1 between W4 and W8 due to dropping out, 1 between W4 and W8 owing to an intercurrent event not related to the study (hospitalisation), 1 patient died between W4 and W8, and the last patient dropped out between W16 and W24”. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol available. |
| Other bias | Low risk | Baseline imbalances do not appear to have caused bias. |
| Methods | RCT (parallel). No information on data collection period reported. |
|
| Participants | Country: Germany. 5 nursing homes. 26 participants with dementia were randomized. 2 had no complete baseline data, and 24 (12 in each group) were included in analyses. Mean age: 83.6 years in music group (SD 5.1; range 72 to 89); and 84.3 in cognitive stimulation group (SD 5.4, range 70 to 90). Diagnosis of dementia: partly formally diagnosed with ICD 10 and partly not formally diagnosed. People with mild to moderate dementia were included. People with vision or hearing impairment or life‐threatening illness were excluded. |
|
| Interventions | 1) Active group music intervention ‘Musikgeragogik’ which included singing folk songs and canons and instrumental performance, 12 sessions of 90 minutes in 6 weeks. 2) Cognitive stimulation intervention: adapted Cognitive training program from NEUROvitalis, 12 sessions of 90 minutes in 6 weeks. |
|
| Outcomes | Cognition was measured with the Mini‐Mental‐Status‐Test (MMST), DEmTect (and subscales), MTF/ROF (Modified Taylor Figure/Rey‐Osseterrieth Figure), Mac‐Q (Selbteinschatzung‐Gedachtnis), Trail Making Test A, FAS‐Test (Controlled‐Oral‐Word‐Association Test), BTA (brief test of attention). Quality of life was measured with a quality of life instrument (DEMQOL) and DEMQOL‐Proxy (no full name, developed by Smith and colleauges; Smith 2005). ADL was measured with the Barthel‐index, IADL and ADL (Aktivitaten des taglichen Lebens). Also the NOSGER (nurses' observation scale for geriatric patients) was measured, but it is unclear for which outcome. Outcomes were measured at baseline (before randomization) and 1 or 2 days after the last session. |
|
| Notes | No explanation about the instruments that were used. The instruments are only mentioned in the Table with results. Unknown for which outcome the NSOGER‐enschatzungen is used. Low fidelity in music intervention group (see 'Other bias' quote below). Bottom effect cognitive measure and more problems described (also in Discussion section of the article) which was part of the goal of the article. No information about funding reported. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Die randomisierte Zuteilung der Programme auf die Einrichtungen fand computergestutzt statt”. (Randomized computer‐assisted allocation of the programs [at the level of individuals with dementia] was performed at the facilities) |
| Allocation concealment (selection bias) | Unclear risk | No description about allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind the convener and participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear who administered the instruments and whether these persons were blinded for the intervention type. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few participants missed outcome data and this was clearly reported. |
| Selective reporting (reporting bias) | Unclear risk | No research protocol available. |
| Other bias | High risk | Participants in the control group frequently developed an acute illness resulting in missing sessions. Quote: "Während keiner der 12 Teilnehmer des MP akut erkrankte, fielen 5 der 12 Teilnehmer des KS zwischen zwei und vier Sitzungen aus." (While none of the 12 participants in the music intervention group became acutely ill, 5 of the 12 participants in the cognitive stimulation group missed between two and four sessions). People who attended fewer than 8 of 12 sessions were excluded from the analyses, so these people still contributed to outcome data. Therefore, adherence or fidelity may be a problem even though they already preselected people who were probably interested in music therapy. |
| Methods | RCT (parallel). Data collection between August 2008 and January 2009. |
|
| Participants | Country: Taiwan. 3 nursing home facilities. Of 104 included people with dementia (52 per group), 100 participants (49 participants in experimental group and 51 in control group) were included in analyses (53% female in total group; 53.06% female in experimental group and 52.94% female in control group). Mean age: 82 in total group (range 65 to 97, SD 6.80), 81.46 years in experimental group and 82.15 years in control group. Diagnosis of dementia: participants had been diagnosed by a physician as having dementia, using the Diagnostic and Statistical Manual of Mental Disorders, 4th edition Text Revision (DSM‐IV‐TR). |
|
| Interventions | 1) Experimental group: mixed active‐receptive music group intervention modified of the protocol developed by Clair and Berstein, 12 sessions of 30 minutes in 6 weeks; provided by a music therapist. 2) Control group: continued to engage in their normal daily activities. |
|
| Outcomes | Physically non‐aggressive behaviours, physically aggressive behaviours, verbally non‐aggressive behaviours, and verbally aggressive behaviours were measured with Chinese Version of the Cohen‐Mansfield Agitation Inventory (C‐CMAI). The instrument rates a subject’s agitated behaviour and its frequency over the previous 2 weeks. The C‐CMAI includes 29 items, each rated on a 7‐point scale (1 to 7) ranging from never (1 point) to several times an hour (7 points), with a total score of 29 (minimum) to 203 (maximum). CMAI frequency referred to the previous 2 weeks. Depression was measured with the Chinese Version of the Cornell Scale for Depression in Dementia (C‐CSDD). Cognition was measured with the Chinese Version of the Mini‐Mental State Examination (C‐MMSE). These outcomes were measured by another member of the research team in experimental and control group at baseline (1 week before start intervention), immediately after 6th and 12th sessions, and at 1 month after cessation of the intervention. Cortisol levels were used as a biomarker for depression and was measured at baseline, immediately after 6th and 12th sessions. |
|
| Notes | Funding: no information provided. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "subjects consisted of a total of 104 elderly persons who were randomly assigned to the experimental (n = 52) and control group (n = 52) by permuted block randomization.” (p 671, Lin 2011) and “permuted block randomisation computer‐based program” (p 672, Lin 2011). Quote: "Using permuted‐block randomisation, a separate researcher randomized participants into the experimental or usual‐care control group within each nursing home. We determined blocked randomization with a block size of 26 using the Research Randomizer computer program, which generates a list of random numbers to be used for allocating participants to the two groups. We generated the allocation sequence with the Research Randomizer program prior to the recruitment of participants and …" (Chu 2014). |
| Allocation concealment (selection bias) | Low risk | Quote: "participants and ……(continued) concealed the results in sequentially numbered and sealed opaque envelopes, which we opened when participant were ready for allocation. After four randomization series, we assigned the 104 participants to the experimental or control condition in a blinded manner" (Chu 2014). |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind the convener and participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Unclear who reported the C‐CMAI However, Chu 2014 described that the C‐CSDD and MSSE were reported by an other member of the research team. Quote: "Another member of the research team administered the study instruments 1 week before the start of the intervention (Time 1), immediately following the 6th (Time 2) and 12th (Time 3) sessions of the intervention, and 1 month after the final intervention session (Time 4) and collected salivary cortisol samples at Times 1–3. The same person administered the instruments each time" (Chu 2014). |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Few cases lost to follow up, and only 1 in experimental group was not interested. |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | |
| Methods | RCT (parallel), total duration of 6 months. No information provided about begin and end dates of the study. |
|
| Participants | Country: USA. N = 60 (42 female, 18 male) residents in a privately funded home for older people. Age range : 72 to 103 years. Diagnosis of dementia: all clinically diagnosed with dementia of the Alzheimer's type (method not specified). The 60 participants were "randomly selected from approximately 200 patients clinically diagnosed as having Alzheimer disease". |
|
| Interventions | (1) Mixed active‐receptive group intervention with music listening and playing along (30‐minute sessions delivered 6 times per week for a period of 6 months). (2) Jigsaw puzzle activities (30‐minute sessions 6 times per week for a period of 6 months). (3) No special treatment, but involved in usual recreational activities of drawing, painting, and watching television. | |
| Outcomes | Cognition, social skills (interaction) and emotional well‐being as assessed with a self‐made questionnaire: general impressions (assessed before and after intervention period) + participants' disposition and social coaction (assessed with a focused 30‐sec, observation on 1 subject for 3 periods during each activity session for the first 2 weeks and final 2 weeks of the study (resulting in 36 observations for each participant in the first 2 weeks and 36 observations in the last 2 weeks). | |
| Notes | No information reported about funding. Randomization was stratified by gender. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "The patients were non‐systematically separated into three groups of equal size". |
| Allocation concealment (selection bias) | High risk | Quote: "To assure equal representation by gender, the random division was implemented first with the female and then with the male patients". No further information provided on the method to conceal the allocation sequence. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind the convener and participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | No information was provided on blinding of the outcome assessors. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided. |
| Selective reporting (reporting bias) | Unclear risk | Not enough detail was reported about the outcome measures. No study protocol available. |
| Other bias | High risk | We were unable to reproduce the results. No statistical tests were reported for the between‐group comparisons, only for the within‐group. The article reports that the number of correct answers for each of the 3 groups was summed for baseline and post treatment, and then a 1‐way analysis of variance conducted. No information on how the data were analysed, whether the baseline was used as a covariate. Table 1 analysis of variance, although showing significant differences between the 3 therapies, does not seem valid. For example, the degrees of freedom within groups are not correct. To interpret this table far more information is required. Even if the results in table 2 are accepted, all that can be deduced is that the treatments were different. They may be different in the level of participation in the therapies, but that does not explain whether the therapy itself brought any benefit. |
| Methods | RCT (parallel). Study 1 lasted 6 weeks. Start and end dates are not reported. |
|
| Participants | Country: France. The first study enrolled 22 participants who resided on a geriatric unit, which is part of Valenciennes hospital. In study 1, 10 of 22 were female (6 of 11 in the music intervention group, and 4 of 11 in a painting group). MMSE 3 to 18 for study 1, age is not described. No diagnostic criteria for dementia are mentioned. |
|
| Interventions | Study 1 1) Mixed active‐receptive group music therapy, 6 × 2‐hour sessions, 2 per week (over 3 weeks). 2) Art therapy involving painting sessions with a variety of materials, 6 × 2‐hour sessions, 2 per week. Both interventions were delivered by 2 psychologists. |
|
| Outcomes | Outcomes were hypothesized to be more favourable for music therapy compared with the other activity.
|
|
| Notes | Funding: l’Agence nationale pour la recherche du ministère français de l’enseignement supérieur et de la recherche (ANR‐09‐BLAN‐0310‐02) et de l’Institut Universitaire de France à Séverine Samson | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No explanation how random sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind the convener and participants. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Study 1: high risk of bias because outcomes were assessed by nurses who were not blinded for the interventions. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only few were lost to follow‐up. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol was available. |
| Other bias | Low risk | |
| Methods | Study 1a is indicated as "Study 2" in the article. RCT (parallel). Study 2 lasted 9 weeks. Start and end dates are not reported. |
|
| Participants | Country: France. Study 2 enrolled 14 participants, of whom 11 were included in the analyses. Participants resided on a geriatric unit, which is part of Valenciennes hospital. Gender and age are not described. Participants had moderate to severe Alzheimer's dementia (MMSE < 12 for study 2, no diagnostic criteria mentioned). |
|
| Interventions | Study 2 1) Mixed active‐receptive group music therapy, 8 × 2‐hour sessions, 2 per week (over 4 weeks). 2) Cooking sessions, 8 × 2‐hour sessions, 2 per week that included preparing a different recipe collectively, with roles distributed according to the participants' abilities. Participants were encouraged to taste ingredients, and verbalize remembrances. Both interventions were delivered by 2 psychologists. |
|
| Outcomes | Outcomes for which stronger and more sustainable effects were hypothesized for music therapy compared with the other activity (measured 2 and 4 weeks after the last intervention).
|
|
| Notes | Funding: l’Agence nationale pour la recherche du ministère français de l’enseignement supérieur et de la recherche (ANR‐09‐BLAN‐0310‐02) et de l’Institut Universitaire de France à Séverine Samson | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No explanation how random sequence was generated. |
| Allocation concealment (selection bias) | Unclear risk | No information about allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind the convener and participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcomes of study 2 were assessed by 5 independent and blinded observers. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only a few were lost to follow up. |
| Selective reporting (reporting bias) | Unclear risk | No study protocol was available. |
| Other bias | Low risk | |
| Methods | RCT (parallel). The study lasted 10 weeks. Start and end dates are not reported. |
|
| Participants | Country: France. 48 participants living in a residential care home which is part of Reims University Hospital. At baseline, 37 were included in the analyses of which 32 were female participants (15 in the music therapy group, and 17 in a cooking group). Mean age in the music intervention group was 86.7 years (SD 6.4); in the cooking group, it was 87.5 years (SD 6, no decimal provided). Participants had Alzheimer's or mixed dementia according to DSM‐IV criteria. Inclusion criterion: Mini‐Mental State Examination (MMSE) 20 or lower. Mean MMSE in the music therapy group was 9.6 (SD 5.3), and in the cooking group, it was 10.8 (SD 8.4). "Only native French speakers were recruited in order to ensure familiarity with the songs selected for music sessions." Medication use was stable. |
|
| Interventions | 1) Mixed active‐receptive group music therapy, alternating listening and playing and singing along; 8 × 1‐hour sessions, twice a week (during 4 weeks). 2) Cooking sessions as another pleasant activity in a group setting, which included preparing a different recipe during 8 sessions, twice a week, collectively, with roles distributed according to the participants' abilities. |
|
| Outcomes | 1) Main outcomes (outcomes for which improvement was hypothesized) were as follows.
2) Another outcome (for which an effect "to a lesser extent" was hypothesized) was improved cognition measured with the Severe Impairment Battery (SIB). |
|
| Notes | Also, an effect "to a lesser extent" was hypothesized as improved professional caregiver's distress measured with an adapted version of the NPI, a distress scale. Funding: “Agence Nationale pour la Recherche” of the French Ministry of Research (contract n° ANR‐09‐BLAN‐0310‐02). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | There was no explanation as to how the participants were randomly assigned to each of the groups. |
| Allocation concealment (selection bias) | Unclear risk | There was also no information about allocation concealment. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind the convener and participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | All observers were blind to the group to which the participant was allocated, although only one was blind to the pre‐ or post‐test treatment phase. Further, only the first 3 minutes of interviews were analysed, which we feel decreases chances that raters can infer the group from the interviews. Regarding other outcomes, these were assessed by blinded caregivers and psychologist. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Probably about the same number was missing in each of the groups and health problems (n = 6) and death (n = 2) is not likely related to the intervention. Refusal (n = 3) may be more of a problem, but this was the case in only 3 of 48 randomized (although unknown in which group). |
| Selective reporting (reporting bias) | Unclear risk | No protocol available. |
| Other bias | Low risk | |
| Methods | RCT (parallel). Study duration or start and end dates not reported. |
|
| Participants | Country: Italy. N = 20 residents of a nursing home, of whom 15 were female (8/10 in the experimental group and 7/10 in the control group). Mean age was 84 (SD 6) for the experimental group, and 87 (SD 6) in the control group. The participants had Alzheimer's disease according to NINCDS‐ADRDA criteria or vascular dementia according to NINDS‐AIREN criteria. Clinical Dementia Rating (CDR) scale means were 1.9 (SD 0.9) in the music therapy group, and 2.2 (SD 0.7) in control group. Mean Mini‐Mental State Examination (MMSE) scores at baseline were 17 ± 6, and 13 ± 4, respectively. "Patients with musical competence or knowledge about music therapy were excluded". |
|
| Interventions | 1) Active, individual music therapy intervention in which free musical improvisation is used to build a relationship between participant and music therapist; 30 sessions of 30 minutes, twice a week (during 15 weeks). 2) Control group with no music exposure but educational and occupational activities such as personal care, lunch, bath, cognitive stimulation reading a newspaper etc. Frequency or duration is not reported, and these activities are referred to as "standard care". |
|
| Outcomes | 1) Main outcome (in line with study aims): behavioural and psychological symptoms of dementia measured with the Neuropsychiatric Inventory (NPI; no timeframe reported but reference provided), including depression subscore 2) Other outcomes were cognition, measured with the MMSE and the Alzheimer's Disease Assessment Scale cognitive sub scale (ADAS‐cog), and depression measured with the NPI. Heart rate (variability) and (I)ADL were outcomes as well. |
|
| Notes | Funding source not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Software mentioned: “patients were randomised to music therapy treatment or standard care by using the randomisation program QuickCalcs”. |
| Allocation concealment (selection bias) | Unclear risk | Not described. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind the convener and participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not clear who assessed the outcomes. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No dropout. |
| Selective reporting (reporting bias) | Unclear risk | No protocol of the (pilot) study available. |
| Other bias | Low risk | |
| Methods | RCT (parallel). This study took place from March to November 2007 in 3 cycles of 12 sessions. |
|
| Participants | Country: Italy. N = 60 (55 female, 5 male); residents from 5 nursing homes. Mean age (age range) experimental group: 85.4 (74 to 99). Mean age (age range) control group: 84.6 (69 to 96). Inclusion criteria
|
|
| Interventions | All participants in the experimental and control groups received standard care (i.e. educational and entertainment activities such as reading a newspaper, performing physical activities, etc.). In addition, the experimental group received 3 cycles of 12 active music therapy sessions (total of 36 sessions) each, 3 times a week. Each session included a group of 3 people and lasted 30 minutes. Each cycle of treatment was followed by 1 month of washout period (in the context of a parallel design) while the standard care activities continued over time. The total duration was 6 months. |
|
| Outcomes | Neuropsychiatric Inventory (NPI). | |
| Notes | No information about funding reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Probably yes, but no details provided “Sixty patients from 5 nursing homes [...] were eligible and were randomly assigned to experimental or control group”. |
| Allocation concealment (selection bias) | Unclear risk | No details provided. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind the convener and participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome assessor was blinded: “The assessments were made by NH healthcare assistants who were blinded to the aim of the study”. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts did not appear to cause bias. “During the study 7 patients dropped out, 3 in the experimental and 4 in the control group. The drops‐out were due to death (n = 5), transfer to acute hospital because of hip fracture (n = 1) and transfer to another NH (n = 1)” |
| Selective reporting (reporting bias) | Unclear risk | Changes in Barthel Index scores and MMSE were not presented. In addition, “The patients’ communicative and relational skills did not improve from baseline to the end of the treatment in the experimental group (data not shown).” No study protocol available. |
| Other bias | Low risk | Baseline imbalances do not appear to have caused bias. |
| Methods | RCT, crossover with 2 periods of 6 weeks for the different conditions. "Data were collected in three 15‐week periods during fall 2010, spring 2011 and fall 2011". |
|
| Participants | Countries: Denmark and Norway. 42 people participated from 14 nursing homes (4 in Denmark and 10 in Norway); most were from Norway (76% of the participants). Most (69%) were female and mean age was 81 years (ranging from 66 to 96 years) for the 26% of participants for whom this information was available. The participants had a diagnosis of dementia ("stated in medical journal," no criteria mentioned); 40% had Alzheimer's dementia, for 38% the type was not specified, 22% had other types of dementia such as vascular, Lewy body, frontotemporal or mixed dementia. Eligible people had moderate to severe dementia. Participants who received music therapy first had a baseline mean Mini‐Mental State Examination (MMSE) score of 9.84 (SD 5.97). For the control condition first, mean MMSE was 5.25 (SD 4.83), Global Deterioration Scale (GDS) means were 5.54 (SD 0.69), and 5.80 (SD 0.62), respectively. Included participants had symptoms of agitation. |
|
| Interventions | 1) Individual mixed active‐receptive music therapy, a minimum of 12 sessions were offered, but the participants received an average of 10 sessions (SD 2.82, range 0 to 13). Frequency: twice a week (over 6 weeks). Average duration: 33.80 minutes (SD 9.91). 2) The control group (period) received usual care which for some participants meant participating in group sing‐along sessions. |
|
| Outcomes | Primary outcome: agitation as measured with the Cohen‐Mansfield Agitation Inventory (CMAI). Timeframe adapted from 2, to 1 week (previous week). In addition to the 7‐point frequency scale, a later version of CMAI was used with a 5‐point disruptiveness scale. The frequency scale, CMAI‐fr, ranged from 1 (never) to 7 (several times per hour), and the disruptiveness scale, CMAI‐di, from 1 (not at all) to 5 (extremely). The CMAI‐fr 1 to 7 point scale was transformed to scores 0 to 6, leading to a maximum total score of 66 and the 1 to 5 point CMAI‐di scale was transformed to scores 0 to 4, leading to a maximum total score of 44. Secondary outcome: quality of life measured with the Alzheimer’s Disease‐Related Quality of Life (ADRQL). Timeframe adapted from 2, to 1 week (previous week). |
|
| Notes | Psychotropic medication use was measured and considered as an outcome as well. Funding: GC Rieber Foundation in Bergen and Aalborg University |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants were randomly allocated to 1 of 2 groups (music therapy or standard care first) but it is not described how. |
| Allocation concealment (selection bias) | Low risk | "[A] concealed sequence procedure" was used, witnessed and signed by someone who was not involved in the study. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind the convener and participants. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Interviewers and proxy respondents were not blinded to the treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Only a few values were missing; and sensitivity analyses were performed with last observation carried forward. |
| Selective reporting (reporting bias) | Unclear risk | "The researchers designed the study protocol in collaboration with a group of clinicians from Denmark and Norway" but there is no reference to compare with. |
| Other bias | Unclear risk | Funding source might have an interest in the study outcomes. |
| Methods | RCT (parallel). Study duration, start and end dates not reported. |
|
| Participants | Country: Japan. N = 39 people residing in 4 group homes or a special dementia hospital, 32 of whom were female; mean age of the females was 81 years; of the males, slightly lower. Participants had Alzheimer's dementia according to DSM‐IV criteria. Inclusion criterion: Clinical Dementia Rating scale 3 (severe dementia). The mean Mini‐Mental State Examination (MMSE) score at baseline was 4.6 ± 3.5 for the music therapy group, 4.7 ± 4.8 for the passive music intervention group, and 4.7 ± 3.9 for the control group that received usual care. Participants had no relevant hearing disorders and no experience of playing musical instruments. |
|
| Interventions | 1) Interactive mixed active‐receptive music therapy intervention with 10 × 30‐minute sessions once a week (over 10 weeks). 2) Passive individual music intervention (not therapy) with 10 × 30‐minute sessions once a week. 3) Control group: "Each control group participant spent time with one caregiver in their own room as usual, without any music intervention (silent environment)". |
|
| Outcomes | Behavioural and psychological symptoms of dementia as measured with the Behavioural Pathology in Alzheimer's Disease (BEHAVE‐AD) rating scale. Timeframe: last 2 weeks, but any changes were by direct observation. Another outcome was stress levels which were also measured with the Faces Scale but only on the short term. |
|
| Notes | Funding: MEXT KAKENHI grant numbers 19592567, 22592586 (2007–2009, 2010–2013). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Stratified randomisation" at the level of gender and MMSE, but it was not described how exactly this was performed. |
| Allocation concealment (selection bias) | Unclear risk | "Participants were randomly and blindly assigned to either control, passive, or interactive group" but there is no description of the blinding process. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind the convener and participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The primary experimenters were not involved in the intervention or evaluation, and the evaluators did not act as music facilitators." Further, occupational therapists and nurses who did not work in the study institution completed the BEHAVE‐AD. "The short‐ and long‐term effects of intervention were evaluated by two trained occupational therapists and four trained nurses in a blinded fashion". |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There was no drop out. |
| Selective reporting (reporting bias) | Low risk | The study protocol is available and all pre‐specified outcomes are reported in the article. |
| Other bias | High risk | Outcomes (changes in behaviour) were observed by blinded professional caregivers, probably over the last 2 weeks, while baseline assessments seemed to refer to direct observation before the therapy by the therapist. |
| Methods | RCT (parallel). Total study duration or begin and end dates are not reported. |
|
| Participants | Country: Taiwan. N = 60 recruited from a residential care facility, of which 55 participated. Most (65.8%) were female. Mean age (SD) experimental group, 81.37 (9.14); for the control group, 79.5 (8.76). Diagnosis of dementia is not described. Inclusion criterion was "ability to engage in a simple activity and follow simple directions." The participants had mild to moderate cognitive impairment according to the Short Portable Mental Status Questionnaire (mean 6.56, SD 2.86 for the music therapy group, and 4.43, SD 3.17 for the control group.) The participants had the "ability to engage in a simple activity and follow simple directions, ability to understand Taiwanese or Chinese, no severe hearing impairment, presence of behavioural and psychological symptoms reported by nursing staff and no obvious symptoms of acute pain or infection". |
|
| Interventions | 1) Active group music intervention using percussion instruments, familiar music, and also movement. A nursing researcher and 2 trained research assistants delivered 12 sessions of 30 minutes, twice a week (over 6 weeks). 2) No intervention; usual care. |
|
| Outcomes | 1) Agitation assessed with a modified Cohen‐Mansfield Agitation Inventory (CMAI). Timeframe unclear with observations during music therapy session ("The behaviours of the participants during each music session were assessed by the observer assistants using modified CMAI"), and also “frequency of occurrence over 2 weeks.” Unclear how the CMAI was modified. 2) Anxiety assessed with the Rating of Anxiety in Dementia scale (RAID) assessed over previous 2 weeks |
|
| Notes | Most people (76.2%) had not received any formal education. Included residents had behavioural and psychological symptoms as reported by nursing staff. Funding: Taiwan National Science Council [NSC 96‐2314‐B‐277‐003‐MY2]. Unclear if agitation effects includes an immediate effect through observations during the music therapy sessions. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Participants were randomly assigned to either the experimental or the control group using simple random sampling method with a computer‐generated list". |
| Allocation concealment (selection bias) | Unclear risk | Unclear who handled the allocation schedule. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind the convener and participants. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Detection bias (blinding of outcome assessment): observer assistants completed the CMAI and RAID over the last 2 weeks. Unclear if these were other people than the trained research assistants who gave the music therapy (probably, these were people who knew the person but they were also aware of the intervention because the assessment was during the intervention). |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Handling of missing data is not reported; 60 were randomized and 55 were analysed. |
| Selective reporting (reporting bias) | Unclear risk | No published study protocol available. |
| Other bias | Low risk | |
| Methods | RCT (parallel). 6‐weeks intervention and 4‐weeks follow‐up. No information reported about start and end dates of data collection. |
|
| Participants | Country: Iceland. N = 38 (? female, ? male); residents in 2 nursing homes and 2 psychogeriatric wards. Age range: 71 to 87 (recruited sample, N = 48). Diagnosis of dementia: all diagnosed with Alzheimers disease (ICD‐10); Global Deterioration Scale (GDS) score of 5 to 7 (moderate to severe dementia). |
|
| Interventions | 1) Group music therapy (three or four participants per session), mixed active (playing instruments) and receptive (listening), 3 times a week for 6 weeks (total of 18 sessions), 30 minutes per session. 2) Standard care as usual. |
|
| Outcomes | Behavioural and psychological symptoms of dementia (BPSD) assessed with the Behaviour Pathology in Alzheimer's disease Rating Scale (BEHAVE‐AD). | |
| Notes | No clear baseline characteristics presented. Funded by the Research Fund for Alzheimer’s Disease and Related Disorders, Landspitali University Hospital. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | No information provided. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "...The 46 remaining patients were then randomised to a music therapy group or a control group, with 23 individuals in each group". |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind the convener and participants. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcome assessors were blinded. Quote: "Two nurses were trained in using the BEHAVE‐AD scale and they were blinded to the therapy used. The nurses were not part of the staff of the wards".. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided. |
| Selective reporting (reporting bias) | Unclear risk | No data. |
| Other bias | Unclear risk | No clear baseline characteristics presented. First author (HBS) provided the music therapy. Quote: "Throughout the study the same qualified music therapist (H.B.S.) conducted the music therapy". |
| Methods | RCT (parallel). Exact duration of total study or start and end dates are not reported, but therapy was provided over a period of 4 months. |
|
| Participants | Country: the Netherlands. N = 94 residents of 6 nursing homes of which 77 were included in the analyses. 70% (n = 54) were female; mean age was 82.16 (SD 6.87) Participants had any type of dementia according to DSM‐IV criteria, Cohen‐Mansfield Agitation Inventory (CMAI) score of > 44. |
|
| Interventions | 1) Mixed active‐receptive group music therapy, which involved listening to live music, interacting with the therapist and playing simple instruments. A maximum of 34 sessions of 40 minutes each were held, twice weekly, over a period of 4 months. 2) General recreational activities such as handwork, playing shuffleboard, cooking, and puzzle games. Sessions lasted 40 minutes, similarly twice weekly over 4 months. |
|
| Outcomes | 1) Agitation assessed with the CMAI modified through dichotomising of items resulting in a total score range of 0 to 29. Presence and absence of behaviour was presumably measured by direct observation or with very short time frames (because it was assessed 1 hour before the session, 1 hour after the session, 2 hours after the session and 4 hours after the session). 2) Neuropsychiatric symptoms (behaviour overall, NPI). |
|
| Notes | Funding: ZonMW (the Netherlands Organisation for Health Research and Development), the Dutch Alzheimer Foundation (Alzheimer Nederland) and the Triodos Foundation. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “To ensure randomised allocation, sealed envelopes were used, with at least two persons present to ensure appropriate randomisation". |
| Allocation concealment (selection bias) | Unclear risk | Only sealing is described; it remains unclear whether envelopes were sequentially numbered and opaque. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not possible to blind the convener and participants. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Quote: "Some of the nurse caregivers who rated the modified CMAI scores were at occasion responsible for taking the residents to either the activity or music therapy room. Complete blinding for some of the nurse caregivers could therefore not be guaranteed.” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The explanation of missing data was not clear. There were 7 missing cases in the baseline data in the general activities group, and 4 of the participants died out of 47 allocated. It is not clear if baseline data were missing because participants died before the baseline assessment. |
| Selective reporting (reporting bias) | Unclear risk | The study protocol is not available. |
| Other bias | Low risk | |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Arroyo‐Anlló 2013 | Not clear whether it is a RCT and the outcome is self‐consciousness. |
| Ballard 2009 | RCT, no music‐based therapeutic intervention. A small proportion of the study sample followed individualised music as an intervention (n = 35). A non‐significant improvement was found on the total CMAI score. |
| Brotons 2000 | Only 4 therapy sessions. |
| Bruer 2007 | RCT, crossover, 8 weeks, comparison of group music therapy to video presentation on cognition (MMSE score). Participants were involved in fewer than 5 sessions. |
| Bugos 2005 | RCT, people with dementia were excluded in this study, focus on healthy older adults (effects of individualized piano instruction on executive functioning and working memory). |
| Clair 1996 | Not clear if participants were randomized; and they participated in fewer than 5 sessions. |
| Cohen‐Mansfield 2010 | Not an RCT, no control group included. |
| Davidson 2011 | Not an RCT, no control group included. |
| Garland 2007 | RCT, crossover, comparing audiotapes with simulated family presence to audiotapes with preferred music and a neutral placebo tape to reduce agitation. Fewer than 5 sessions in each group, in which participants listened to preferred music. |
| Gerdner 2000 | The analyses covered directly observed agitation, probably over the combined sessions (so inclusive of the first 4 sessions). |
| Groene 1993 | The control group received music therapy as well. |
| Hanser 1994 | RCT, participants did not have dementia but depression. |
| Hicks‐Moore 2008 | RCT, comparison of favourite music and hand massage, fewer than 5 sessions. |
| Hokkanen 2008 | RCT, no music therapy, the study involved dance and movement therapeutic methods |
| Holmes 2006 | RCT, comparison of live interactive music, passive pre‐recorded music or silence for 30 minutes in a single session. Fewer than 5 sessions. |
| Janata 2012 | The intervention did not meet our criteria for a therapeutic‐based intervention in which contact with a therapist or facilitator is essential. The intervention created "a musical atmosphere" with music programs streamed to the rooms of individuals assigned to a music group for several hours per day |
| Noice 2009 | RCt, no music therapy: a theatrically based intervention was given to 122 older adults who took lessons twice a week for 4 weeks. |
| Otto 1999 | RCT, participants did not have dementia. |
| Pomeroy 1993 | RCT, music was part of physiotherapy. |
| Raglio 2008 | Quasi‐randomized study. |
| Riegler 1980 | RCT, not clear whether participants were diagnosed with dementia. |
| Satoh 2014 | No music‐based therapeutic intervention, but physical exercise combined with music. |
| Sung 2006 | No music‐based therapeutic intervention, but music with movement intervention. |
| Särkämö 2014 | No music‐based therapeutic intervention, but singing coaching for family caregivers and nurses, and listening to music. |
| Thompson 2005 | RCT, single test moment, music as cue to facilitate performance on a category fluency task. No therapeutic intervention. |
| Van de Winckel 2004 | RCT, no music‐based therapeutic intervention, but music based exercises. |
| Vanderark 1983 | RCT, not clear whether participants were diagnosed with dementia. |
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | RCT (parallel) |
| Participants | 35 persons with Alzheimer’s disease living in “an institution for the dependent elderly” in France, with Mini‐Mental State Examination (MMSE) score between 5 and 20 |
| Interventions | Intervention group: receptive intervention using “’U’ sequence: the musical sequence lasts 20 minutes and is made up of several phases that progressively induce a relaxed state in the patient. The phase of maximum relaxation is followed by a stimulating phase.” Control group: “Interview with an occupational activity (such as discussion of personal pictures or news) with the caregiver in charge of music therapy sessions with the same period” |
| Outcomes | Quality of life, agitation, and overall behavioural problems were secondary outcomes (in addition to outcomes other than the seven outcomes of interest for the Cochrane review) |
| Notes | ClinicalTrials.gov: The study has been completed June 2015; the study has been terminated. No study results are posted (accessed 16 April 2017). If a report on possible results should become available, eligibility should be reviewed, in particular if the intervention meets our criteria for music‐based therapeutic interventions |
| Methods | |
| Participants | |
| Interventions | |
| Outcomes | |
| Notes | No publication was found up to 2017 |
| Methods | Either RCT or quasi‐experimental design |
| Participants | “Institutionalized” people with dementia (24), “in phases 5 and 6” (moderate to advanced dementia) |
| Interventions | Intervention group: a form of group music therapy Control group: reminiscence‐recreation group |
| Outcomes | Mood and cognition, perhaps also (social) behaviour |
| Notes | Conference abstract. When a full report becomes available, the design needs careful evaluation (a ”quasi‐experimental study“ with a ”pre‐post test design with a control group“ wherein groups were ”randomly assigned to a music therapy group or a reminiscence group“) |
| Methods | RCT (parallel) |
| Participants | Thirty people with mild to moderate Alzheimer’s disease |
| Interventions | Intervention group: ”individual, receptive music therapy. The musical style was chosen by the patient. The validated ‘U’ technique was employed” Control group: reading sessions |
| Outcomes | Anxiety (primary) and depression (secondary) |
| Notes | Conference abstract. When methods and results (means and SDs) are being reported in more detail, eligibility should be reviewed, in particular if the intervention meets our criteria for music‐based therapeutic interventions |
| Methods | RCT (parallel) |
| Participants | Nursing home residents (30) in the Republic of Korea |
| Interventions | 1) Songwriting; music therapy programme employing song‐writing activities. 3 stages: a) preparing songwriting, b) songwriting; c) reinforcing songwriting. A therapist administered the active individual intervention. Session of 60 minutes were given for 16 weeks (once per week). 2) Control group: free time was given to the control group instead |
| Outcomes | Cognition (assessed with the MMSE‐K) |
| Notes | Presentation of results (Figure 2a,b) is incorrect. The intervention and control group are reversed. There is little variability in MMSE‐K scores with either no change or change in one direction only |
| Methods | Pilot RCT (cross‐over) |
| Participants | Ten persons with Alzheimer’s disease, Mini‐Mental State Examination (MMSE) score range 6‐28 |
| Interventions | Intervention condition: music therapy by a trained music therapist; no detail on type of intervention is reported Control condition: not reported |
| Outcomes | Overall behavioural problems was a primary outcome; secondary outcomes included quality of life, depression and cognition (additionally there were outcomes other than the seven outcomes of interest for the Cochrane review) |
| Notes | Conference abstract. If a full report becomes available, the type of intervention should be reviewed against our criteria for music‐based therapeutic interventions |
| Methods | RCT (cross‐over) |
| Participants | Persons (27) with moderate Alzheimer’s disease |
| Interventions | Intervention condition: “music therapy by an accredited music therapist following a standardized structured protocol (Clair & Bernstein 1990).” Control condition: “waiting” (probably usual care) |
| Outcomes | Overall behavioural problems was a primary outcome; secondary outcomes included quality of life, depression, agitation and cognition (additionally there were outcomes other than the seven outcomes of interest for the Cochrane review) |
| Notes | Conference abstract. If a full report becomes available, the exact type of intervention should be reviewed against our criteria for music‐based therapeutic interventions |
| Methods | Mixed quantitative‐qualitative feasibility study which included a cluster‐randomized trial (randomized at nursing home unit level) |
| Participants | Nursing home residents with dementia (17) but also 10 staff from two nursing homes in the UK (see Notes) |
| Interventions | Intervention group: individual active music therapy by music therapist and training of care staff Control group: “standard care” |
| Outcomes | Well‐being and overall behavioural problems (in addition to outcomes other than the seven outcomes of interest for the Cochrane review) |
| Notes | Needs a detailed evaluation against the inclusion criteria for possible inclusion in the next update of the review. In particular, we will need to decide on comparability with individually randomized trials and whether the intervention meets our criteria for music‐based therapeutic interventions. Further, music therapists delivered the intervention but the intervention also included video clips of the sessions which were used to train care staff to improve caregiving. We therefore need to decide if the training of care staff provided in parallel to music therapy is sufficiently comparable with music therapy provided as a single intervention in other studies |
| Methods | “Case control study” but “The participants (…) were assigned randomly to a music therapy group and a control group.” |
| Participants | Persons with moderate Alzheimer’s disease residing in one of four participating long‐term care centres were randomized (probably 120 were randomized and 82 participated). |
| Interventions | Intervention group: music therapy with active elements provided by music therapists Control group: “standard care” |
| Outcomes | Behavioural problems overall measured with the BEHAVE‐AD; however, aims and results are about agitation disruptiveness (additionally there were outcomes other than the seven outcomes of interest for the Cochrane review) |
| Notes | Conference abstract. If a full report becomes available, the design needs careful consideration as to whether it qualifies as an RCT |
| Methods | RCT (parallel) |
| Participants | Persons with mild dementia (93, Clinical Dementia Rating (CDR) score of 0.5 or 1.0) staying in a geriatric hospital in China |
| Interventions | Intervention group: group music therapy that included singing lyrics provided by a music therapist. Two other groups: “lyrics control group” where the same lyrics were read without music, supervised by the music therapist and “blank control group” which represented usual care |
| Outcomes | Probably overall behavioural problems, and cognition (memory and language; additionally there were outcomes other than the seven outcomes of interest for the Cochrane review) |
| Notes | We could not timely evaluate the article in Chinese in detail. The study may meet our inclusion criteria but we do not know yet if the analyses were limited to assessments after at least 5 therapy sessions |
| Methods | RCT (parallel) |
| Participants | Persons with moderate to severe dementia (120) residing in nine institutions (geriatric department, geriatric center, or nursing home) in Italy. |
| Interventions | Intervention group: active music therapy delivered by a music therapist Two other groups: individualized listening which did “not involve any kind of direct relationship with a therapist,” and usual care |
| Outcomes | Quality of life, overall behavioural problems, depression (and observed social behaviour in participants of the active music therapy group only) |
| Notes | This study meets our inclusion criteria and will be included in the future update of the Cochrane review |
| Methods | RCT (parallel) |
| Participants | Persons with mild Alzheimer's disease or mild cognitive impairment (59; but "Patient with a different etiology of cognitive disorder that of Alzheimer's disease" are excluded), in France |
| Interventions | Intervention group: singing sessions Control group: painting sessions |
| Outcomes | Primary outcome: "Physical and moral pain" or "pain intensity" rated at "a simplified visual scale;" secondary outcome: other pain intensity scale (Brief Pain Inventory) |
| Notes | Study completed in June 2016. When study results become available, needs an assessment as to whether people with no dementia have been included, whether we accept pain as an outcome for the review, and whether analyses included outcomes assessed after fewer than 5 sessions |
| Methods | Pilot RCT (parallel) |
| Participants | Persons with dementia (16) and moderate to severe cognitive impairment admitted to an inpatient psychiatric unit within a large academic hospital in Canada |
| Interventions | Intervention group: individual, active music therapy provided by an accredited music therapist Control group: active engagement and attention intervention provided by a social worker |
| Outcomes | Agitation, overall behavioural problems, and some individual item scores of the outcome instruments were reported as well |
| Notes | “A number of the patients enrolled in this study were hospitalised for two to three weeks” only, and some may therefore not have had the minimum of 5 therapy sessions. Moreover, end‐of‐treatment scores were reported for only part of the outcomes |
| Methods | RCT (parallel) |
| Participants | Estimated 30 people with “a mild dementia diagnosis” (or “mild to moderate”) dementia in Taiwan |
| Interventions | Intervention group: mixed active‐receptive music therapy Control group: “no intervention” (usual care) |
| Outcomes | Quality of life, depression, and agitation were secondary outcomes; additionally there were outcomes other than the seven outcomes of interest for the Cochrane review |
| Notes | Estimated trial completion date: September 2014. However, ClinicalTrial.gov reports (status 17 April 2017): "Study has passed its completion date and status has not been verified in more than two years" |
| Methods | RCT (parallel) |
| Participants | Estimated 30 people with mild to moderate dementia in Taiwan |
| Interventions | Intervention group: “Musical Dual Task Training protocol is structured with musical content and patients are required to do musical tasks including singing and playing instruments contingent on visual or auditory cues while walking” delivered by a “qualified music therapist” Control group: “walking and talking:” “read a newspaper article prior to a walk and have a conversation with the music therapist based on the content of the news while walking.” |
| Outcomes | Cognition (primary outcome); agitation (secondary outcome and outcomes other than the seven outcomes of interest for the Cochrane review) |
| Notes | Estimated primary completion date October 2013. However, ClinicalTrial.gov reports (status 17 April 2017): "Study has passed its completion date and status has not been verified in more than two years" |
| Methods | “Pretest‐posttest control group design” and “people were randomly assigned to the experimental and control groups” |
| Participants | Persons with dementia (34) attending a day care center in South Korea |
| Interventions | Intervention group: music therapy Control group: usual care or other not reported in the abstract |
| Outcomes | Cognition |
| Notes | We could not retrieve the full text. First, we would like to evaluate if this was an RCT |
| Methods | RCT (parallel) |
| Participants | Persons with mild dementia (20) “who reside in G Welfare Foundation in D city” (Korea) |
| Interventions | Intervention group: group music therapy Control group: usual care or other not reported in the abstract |
| Outcomes | Quality of life and depression |
| Notes | We could not retrieve the full text. Type of analyses not clear from the abstract. We would need to review if analyses were limited to effects after at least 5 sessions |
| Methods | Unclear (“17 of them were assigned to experimental group and the other 17 people were assigned to control group. The musical activities with visual supportive strategies were carried out both experimental group and control group for 10 sessions”) |
| Participants | Persons with dementia (34) attending a day care center in South Korea |
| Interventions | Intervention group: musical activities with visual supportive strategies Control group: unclear |
| Outcomes | Cognition |
| Notes | Unclear if this was an RCT and how effectiveness could be derived if the control group received the same intervention (“According to this results, it was shown that the musical activities with visual supportive strategies were effective intervention for the cognitive rehabilitation of elderly people with dementia”). It is also unclear if this is music therapy or a combination of more types of therapy. We still need to retrieve the full text to evaluate eligibility |
| Methods | Not reported in the abstract |
| Participants | Nursing home residents (59) with dementia |
| Interventions | Intervention group: music therapy Two other groups: “laughing therapy” and “control group” |
| Outcomes | Depression (in addition to outcomes other than the seven outcomes of interest for the Cochrane review) |
| Notes | We still need to evaluate the article in Korean, in particular whether this is an RCT, and if so, what the music therapy intervention entails and whether the analyses were limited to results after at least 5 therapy sessions |
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Personalized Music Therapy and Agitation in Dementia |
| Methods | Unclear (Intervention Model: Single Group Assignment?). |
| Participants | Inclusion Criteria:
Exclusion Criteria:
|
| Interventions | Listening to personalized and either non‐personalized or no music during daily hygiene care (grooming). |
| Outcomes | Changes in agitation. |
| Starting date | May 2014. |
| Contact information | Dr C Tartaglia, University Health Network, Toronto, Canada. |
| Notes | Registered trial. Estimated completion in June 2017 (final data collection date for primary outcome measure). |
Contributions of authors
Jenny van der Steen, Mirjam van Soest, Annemiek Vink, Rob Scholten and Hans (Johannes) van der Wouden contributed to all aspects of the review.
Manon Bruinsma assisted with data‐extraction and commenting on drafts.
Consumer editor: Joost de Haas. Contact editor: Leon Flicker.
The review has been peer reviewed anonymously.
Sources of support
Internal sources
Rijksuniversiteit Groningen, Netherlands.
ArtEZ School of Music, Enschede, Netherlands.
External sources
The Netherlands Organisation for Scientific Research (NWO); Innovational Research Incentives Scheme: Vidi grant number 917.11.339, Netherlands.
-
NIHR, UK.
This update was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to the Cochrane Dementia and Cognitive Improvement group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health
Declarations of interest
Annemiek Vink and Manon Bruinsma are involved in music therapy research and dementia. We included a study of Annemiek Vink, which was, however, evaluated by two other authors. The lead author and the co‐authors, who are Cochrane experts, made the final decisions about analyses, presentation and interpretation of the data and they do not have a conflict of interest related to finding effects of music therapy.
Notes
2016: this new citation version was written with three additional authors. Inclusion of studies until the 2011 update were reconsidered according to the new and more stringent criteria. A further update is planned for the end of 2017 to incorprate studies awaiting classification.
May 2004: this is a completely new review of music‐based interventions for people with dementia written by a new and different team of reviewers (Vink and colleagues) from the previous, now permanently withdrawn review of music therapy (Koger and colleagues).
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
- Ceccato E, Vigato G, Bonetto C, Bevilacqua A, Pizziolo P, Crociani S, et al. STAM protocol in dementia: a multicenter, single‐blind, randomized, and controlled trial. American Journal of Alzheimer's Disease and Other Dementias 2012;27(5):301‐10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark ME, Lipe AW, Bilbrey M. Use of music to decrease aggressive behaviors in people with dementia. Journal of Gerontological Nursing 1998;24(7):10‐7. [DOI] [PubMed] [Google Scholar]
- Cooke M, Moyle W, Shum D, Harrison S, Murfield J. A randomized controlled trial exploring the effect of music on quality of life and depression in older people with dementia. Journal of Health Psychology 2010;15(5):765‐76. [DOI: 10.1177/1359105310368188] [DOI] [PubMed] [Google Scholar]; Cooke ML, Moyle W, Shum DH, Harrison SD, Murfield JE. A randomized controlled trial exploring the effect of music on agitated behaviours and anxiety in older people with dementia. Aging & Mental Health 2010;14(8):905‐16. [DOI: 10.1080/13607861003713190] [DOI] [PubMed] [Google Scholar]; Murfield J, Cooke M, Moyle W, Shum D, Harrison S. Conducting randomized controlled trials with older people with dementia in long‐term care: Challenges and lessons learnt. International Journal of Nursing Practice 2011;17(1):52‐9. [DOI: 10.1111/j.1440-172X.2010.01906.x] [DOI] [PubMed] [Google Scholar]
- Guétin S, Portet F, Picot MC, Pommie C, Messaoudi M, Djabelkir L, et al. Effect of music therapy on anxiety and depression in patients with Alzheimer's type dementia: Randomised, controlled study. Dementia and Geriatric Cognitive Disorders 2009;28(1):36‐46. [DOI: 10.1159/000229024] [DOI] [PubMed] [Google Scholar]
- Liesk J, Hartogh T, Kalbe E. Cognitive stimulation and music intervention for people with dementia in nursing homes: A pilot study, problems and perspectives [Kognitive Stimulation und Musikintervention bei stationär versorgten Menschen mit Demenz Eine Pilotstudie, Probleme und Perspektiven]. Zeitschrift für Gerontologie und Geriatrie 2015;48(3):275‐81. [DOI] [PubMed] [Google Scholar]
- Chu H, Yang CY, Lin Y, Ou KL, Lee TY, O'Brien AP, et al. The impact of group music therapy on depression and cognition in elderly persons with dementia: a randomized controlled study. Biological Research for Nursing 2014;16(2):209‐17. [DOI: 10.1177/1099800413485410] [DOI] [PubMed] [Google Scholar]; Lin Y, Chu H, Yang CY, Chen CH, Chen SG, Chang HJ, et al. Effectiveness of group music intervention against agitated behavior in elderly persons with dementia. International Journal of Geriatric Psychiatry 2011;26(7):670‐8. [DOI: 10.1002/gps.2580] [DOI] [PubMed] [Google Scholar]
- Lord TR, Garner JE. Effects of music on Alzheimer patients. Perceptual and Motor Skills 1993;76(2):451‐5. [DOI] [PubMed] [Google Scholar]
- Narme P, Tonini A, Khatir F, Schiaratura L, Clément S, Samson S. Non pharmacological treatment for Alzheimer's disease: comparison between musical and non‐musical interventions [Thérapies non médicamenteuses dans la maladie d’Alzheimer: comparaison d’ateliers musicaux et non musicaux]. Geriatrie et Psychologie Neuropsychiatrie du Vieillissement 2012;10(2):215‐24. [DOI] [PubMed] [Google Scholar]
- Narme P, Tonini A, Khatir F, Schiaratura L, Clément S, Samson S. Non pharmacological treatment for Alzheimer's disease: comparison between musical and non‐musical interventions [Thérapies non médicamenteuses dans la maladie d’Alzheimer: comparaison d’ateliers musicaux et non musicaux]. Geriatrie et Psychologie Neuropsychiatrie du Vieillissement 2012;10(2):215‐24. [In the article, this is referred to as study 2] [DOI] [PubMed] [Google Scholar]
- Narme P, Clément S, Ehrlé N, Schiaratura L, Vachez S, Courtaigne B, et al. Efficacy of musical interventions in dementia: evidence from a randomized controlled trial. Journal of Alzheimer's Disease 2014;38(2):359‐69. [DOI: 10.3233/JAD-130893] [DOI] [PubMed] [Google Scholar]; Samson S, Clément S, Narme P, Schiaratura L, Ehrlé N. Efficacy of musical interventions in dementia: methodological requirements of nonpharmacological trials. Annals of the New York Academy of Sciences 2015;1337(Mar):249‐55. [DOI: 10.1111/nyas.12621] [DOI] [PubMed] [Google Scholar]
- Raglio A, Oasi O, Gianotti M, Manzoni V, Bolis S, Ubezio MC, et al. Effects of music therapy on psychological symptoms and heart rate variability in patients with dementia: A pilot study. Current Aging Science 2010;3(3):242‐6. [DOI] [PubMed] [Google Scholar]
- Raglio A, Bellelli G, Traficante D, Gianotti M, Ubezio MC, Gentile S, et al. Addendum to 'Efficacy of music therapy treatment based on cycles of sessions: a randomised controlled trial' (Raglio et al., 2010). Aging & Mental Health 2012;16(2):265‐7. [DOI: 10.1080/13607863.2011.630376] [DOI] [PubMed] [Google Scholar]; Raglio A, Bellelli G, Traficante D, Gianotti M, Ubezio MC, Gentile S, et al. Efficacy of music therapy treatment based on cycles of sessions: A randomised controlled trial. Aging & Mental Health 2010;14(8):900‐4. [DOI: 10.1080/13607861003713158] [DOI] [PubMed] [Google Scholar]
- Ridder HM, Stige B, Qvale LG, Gold C. Individual music therapy for agitation in dementia: an exploratory randomized controlled trial. Aging & Mental Health 2013;17(6):667‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakamoto M, Ando H, Tsutou A. Comparing the effects of different individualized music interventions for elderly individuals with severe dementia. International Psychogeriatrics / IPA 2013;25(5):775‐84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung HC, Lee WL, Li TL, Watson R. A group music intervention using percussion instruments with familiar music to reduce anxiety and agitation of institutionalized older adults with dementia. International Journal of Geriatric Psychiatry 2012;27(6):621‐7. [DOI] [PubMed] [Google Scholar]
- Svansdottir HB, Snaedal J. Music therapy in moderate and severe dementia of Alzheimer's type: A case‐control study. International Psychogeriatrics / IPA 2006;18(4):613‐21. [DOI] [PubMed] [Google Scholar]
- Vink AC, Zuidersma M, Boersma F, Jonge P, Zuidema SU, Slaets JP. Effect of music therapy versus recreational activities on neuropsychiatric symptoms in elderly adults with dementia: an exploratory randomized controlled trial. Journal of the American Geriatrics Society 2014;62(2):392‐3. [DOI: 10.1111/jgs.12682] [DOI] [PubMed] [Google Scholar]; Vink AC, Zuidersma M, Boersma F, Jonge P, Zuidema SU, Slaets JP. The effect of music therapy compared with general recreational activities in reducing agitation in people with dementia: A randomised controlled trial. International Journal of Geriatric Psychiatry 2013;28(10):1031‐8. [DOI: 10.1002/gps.3924] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Arroyo‐Anlló EM, Díaz JP, Gil R. Familiar music as an enhancer of self‐consciousness in patients with Alzheimer's disease. BioMed Research International 2013;2013:752965. [DOI: 10.1155/2013/752965] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballard C, Brown R, Fossey J, Douglas S, Bradley P, Hancock J, et al. Brief psychosocial therapy for the treatment of agitation in Alzheimer disease (the CALM‐AD trial). American Journal of Geriatric Psychiatry 2009;17(9):726‐33. [DOI] [PubMed] [Google Scholar]
- Brotons M, Koger SM. The impact of music therapy on language functioning in dementia. Journal of Music Therapy 2000;XXXVII(3):183‐95. [DOI] [PubMed] [Google Scholar]
- Bruer RA, Spitznagel E, Cloninger CR. The temporal limits of cognitive change from music therapy in elderly persons with dementia or dementia‐like cognitive impairment: A randomized controlled trial. Journal of Music Therapy 2007;44(4):308‐28. [DOI] [PubMed] [Google Scholar]
- Bugos JA. The effects of individualized piano instruction on executive functions in older adults (ages 60‐85). Dissertation Abstracts International Section A: Humanities and Social Sciences. 2005;66(1‐A):18. [Google Scholar]
- Clair AA. The effect of singing on alert responses in persons with late stage dementia. Journal of Music Therapy 1996;XXXIII(4):234‐47. [Google Scholar]
- Cohen‐Mansfield J, Marx MS, Dakheel‐Ali M, Regier NG, Thein K, Freedman L. Can agitated behavior of nursing home residents with dementia be prevented with the use of standardized stimuli?. Journal of the American Geriatrics Society 2010;58(8):1459‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson JW, Fedele J. Investigating group singing activity with people with dementia and their caregivers: Problems and positive prospects. Musicae Scientiae 2011;15(3):402‐22. [Google Scholar]
- Garland K, Beer E, Eppingstall B, O'Connor DW. A comparison of two treatments of agitated behavior in nursing home residents with dementia: Simulated family presence and preferred music. American Journal of Geriatric Psychiatry 2007;15(6):514‐21. [DOI] [PubMed] [Google Scholar]
- Gerdner LA. Effects of individualized versus classical "relaxation" music on the frequency of agitation in elderly persons with Alzheimer's disease and related disorders. International Psychogeriatrics / IPA 2000;12(1):49‐65. [DOI] [PubMed] [Google Scholar]
- Groene RW. Effectiveness of music therapy 1:1 intervention with individuals having senile dementia of the Alzheimer's type. Journal of Music Therapy 1993;30(3):138‐57. [Google Scholar]
- Hanser SB, Thompson LW. Effects of a music therapy strategy on depressed older adults. Journal of Gerontology 1994;49(6):P265‐P269. [DOI] [PubMed] [Google Scholar]
- Hicks‐Moore SL, Robinson BA. Favorite music and hand massage: Two interventions to decrease agitation in residents with dementia. Dementia 2008;7(1):95‐108. [Google Scholar]
- Hokkanen L, Rantala L, Remes AM, Härkönen B, Viramo P, Winblad I. Dance and movement therapeutic methods in management of dementia: A randomized, controlled study. Journal of the American Geriatrics Society 2008;56(4):771‐2. [DOI] [PubMed] [Google Scholar]
- Holmes C, Knights A, Dean C, Hodkinson S, Hopkins V. Keep music live: music and the alleviation of apathy in dementia subjects. International Psychogeriatrics / IPA 2006;18(4):623‐30. [DOI] [PubMed] [Google Scholar]
- Janata P. Effects of widespread and frequent personalized music programming on agitation and depression in assisted living facility residents with Alzheimer‐type dementia. Music and Medicine 2012;4(1):8‐15. [Google Scholar]
- Noice H, Noice T. An arts intervention for older adults living in subsidized retirement homes. Aging, Neuropsychology, and Cognition 2009;16(1):56‐79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto D, Cochran VV, Johnson G, Clair AA. The influence of background music on task engagement in frail, older persons in residential care. Journal of Music Therapy 1999;36(3):182‐95. [DOI] [PubMed] [Google Scholar]
- Pomeroy VM. The effect of physiotherapy input on mobility skills of elderly people with severe dementing illness. Clinical Rehabilitation 1993;7(2):163‐70. [Google Scholar]
- Raglio A, Bellelli G, Traficante D, Gianotti M, Ubezio MC, Villani D, et al. Efficacy of music therapy in the treatment of behavioral and psychiatric symptoms of dementia. Alzheimer Disease and Associated Disorders 2008;22(2):158‐62. [DOI: 10.1097/WAD.0b013e3181630b6f] [DOI] [PubMed] [Google Scholar]
- Riegler J. Comparison of a reality orientation program for geriatric patients with and without music. Journal of Music Therapy 1980;17(1):26‐33. [DOI] [PubMed] [Google Scholar]
- Särkämö T, Tervaniemi M, Laitinen S, Numminen A, Kurki M, Johnson JK, et al. Cognitive, emotional, and social benefits of regular musical activities in early dementia: randomized controlled study. Gerontologist 2014;54(4):634‐50. [DOI] [PubMed] [Google Scholar]
- Satoh M, Ogawa J, Tokita T, Nakaguchi N, Nakao K, Kida H, et al. The effects of physical exercise with music on cognitive function of elderly people: Mihama‐Kiho project. PLos One 2014;9(4):e95230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung HC, Chang SM, Lee WL, Lee MS. The effects of group music with movement intervention on agitated behaviours of institutionalized elders with dementia in Taiwan. Complementary Therapies in Medicine 2006;14(2):113‐9. [DOI] [PubMed] [Google Scholar]
- Thompson RG, Moulin CJ, Hayre S, Jones RW. Music enhances category fluency in healthy older adults and Alzheimer's disease patients. Experimental Aging Research 2005;31(1):91‐9. [DOI] [PubMed] [Google Scholar]
- Vanderark S, Newman I, Bell S, Akron T. The effects of music participation on quality of life of the elderly. Music Therapy 1983;3(1):71‐81. [Google Scholar]
- Winckel A, Feys H, Weerdt W, Dom R. Cognitive and behavioural effects of music‐based exercises in patients with dementia. Clinical Rehabilitation 2004;18(3):253‐60. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
- Music therapy in Alzheimer's Disease. ClinicalTrials.gov: NCT02020356. The study has been completed June 2015.
- Asmussen S, Shah V, Goldstein A, Tomaino C, Scheiby B, Ramsey D. Research abstract: The effect of a music therapy intervention on the levels of depression, anxiety/agitation, and quality of life experienced by individuals diagnosed with early and middle stage dementia. A controlled study. Beth Abraham Health Services, Center for research and education. 1997. [Google Scholar]
- Curto Prieto D, Sole Serrano C, Castro M, Mercadal Brotons M, Asensio FM. Effects of music therapy in institutionalized elderly with dementia. European Geriatric Medicine 2015;11th International Congress of the European UnionGeriatric Medicine Society, EUGMS, 2015:S48. [Google Scholar]
- Guetin S, Portet F, Touchon J. Effects of music therapy on anxiety and depression in patients with Alzheimer's disease: A randomised controlled trial. Neuro‐degenerative diseases Conference: 10th International Conference AD/PD ‐ Alzheimer's and Parkinson's Diseases: Advances, Concepts and New Challenges Barcelona Spain. 2011. [Google Scholar]
- Hong IS, Choi MJ. Songwriting oriented activities improve the cognitive functions of the aged with dementia. The Arts in Psychotherapy 2011;38:221‐228. [Google Scholar]
- Hsiung G‐Y, Kirkland K, Hswen Y, Slack PJ, Summers S, Boyd L, Jacova C. A pilot, randomized study of music therapy for people with Alzheimer's disease. Alzheimer's & Dementia 2013 Conference: Alzheimer's Association International Conference 2013 Boston, MA United States. 2013. [Google Scholar]
- Hsiung G‐YR, Kirkland K, Summers SG, Beattie BL, Jacova C. A randomized controlled trial of music therapy in managing behavioral symptoms in Alzheimer disease. Alzheimer's & Dementia 2015;Alzheimer's Association International conference 2015:749. [Google Scholar]
- Hsu MH, Flowerdew R, Parker M, Fachner J, Odell‐Miller H. Individual music therapy for managing neuropsychiatric symptoms for people with dementia and their carers: A cluster randomised controlled feasibility study. BMC Geriatrics 2015;15(July 18):84. [DOI: 10.1186/s12877-015-0082-4] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak K, Bae N, Jang WY. Music therapy with moderate Alzheimer's disease in a long‐term care center. Alzheimer's & dementia 2013 Conference: Alzheimer's Association International Conference 2013 Boston, MA United States. 2012. [Google Scholar]; Kwak K‐P. Music therapy with moderate Alzheimer's disease in long‐term care center. International Psychogeriatrics 2013 Conference: 16th International Congress of the International Psychogeriatric Association, IPA 2013 Seoul South Korea. 2013. [Google Scholar]
- Lyu J, Gao T, Li M, Xie L, Li W, Jin W, Hao Z, Mu H. The effect of music therapy on memory, language and psychological symptoms of patients with mild Alzheimer's disease. Chinese Journal of Neurology 2014;47(12):831‐835. [Google Scholar]
- Raglio A, Bellandi D, Baiardi P, Gianotti M, Ubezio MC, Zanacchi E, Granieri E, Imbriani M, Stramba‐Badiale M. Effect of active music therapy and individualized listening to music on dementia: A multicenter randomized controlled trial. Journal of the American Geriatrics Society 2015;63(8):1534‐1539. [DOI] [PubMed] [Google Scholar]
- Rouch I, Labruyere C. Support by singing sessions on physical and moral pain: assessment of Its effectiveness in Alzheimer’s Disease: Multicenter study LACME. ClinicalTrials.gov 21 April 2017. [NCT02670993] [Google Scholar]
- Thornley J, Hirjee H, Vasudev A. Music therapy in patients with dementia and behavioral disturbance on an inpatient psychiatry unit: results from a pilot randomized controlled study. International Psychogeriatrics 2016;28(5):869‐871. [DOI] [PubMed] [Google Scholar]
- Unknown. Musical dual task training to improve attention control for dementia. ClinicalTrials.gov Estimated primary completion date October 2013. However, ClinicalTrial.gov reports (status 16 April 2016): "The recruitment status of this study is unknown because the information has not been verified recently".
- Unknown. Music therapy to promote emotion and cognition for dementia. ClinicalTrials.gov Estimated trial completion date: September 2014. However, ClinicalTrials.gov reports (status 16 April 2016): "The recruitment status of this study is unknown because the information has not been verified recently".
- 권서령, 강경선. The Effect of Music Therapy Using Korean Folk Song and Rhythm on Improving the Cognitive Function of Elderly People with Dementia. Journal of Arts Psychotherapy 2013;2:1‐17. [Google Scholar]
- 김현정, 정재원. A study on the effect of Korean traditional music therapy with on depression and quality of life of the elderly with mild dementia. Journal of Arts Psychotherapy 2013;3:19‐38. [Google Scholar]
- 신보영, 황은영. A research on the effects of music activities adopting visual supportive strategies on cognitive rehabilitation of elderly dementia patients. Journal of Arts Psychotherapy 2015;2:41‐62. [Google Scholar]
- 채경숙. Effects of laughing and music therapy on depression and activities of the autonomic nervous system in the elderly with dementia. Journal of Korean Biological Nursing Science 2015;3:245‐252. [Google Scholar]
References to ongoing studies
- Personalized Music Therapy and Agitation in Dementia. ClinicalTrials.gov: NCT02147652. Estimated primary completion date: May 2016.
Additional references
- Prince M, Wilmo A, Guerchet M, Ali G‐C, Wu Y‐T, Prina M. World Alzheimer Report 2015: The Global Economic Impact of Dementia: An analysis of prevalence, incidence, cost and trends. www.alz.co.uk/research/WorldAlzheimerReport2015.pdf.
- Alzheimer's Disease International (ADI). World Alzheimer Report 2016: Improving healthcare for people living with dementia ‐ coverage, quality and costs now and in the future. https://www.alz.co.uk/research/WorldAlzheimerReport2016.pdf 2016. [Google Scholar]
- Aldridge D. Music Therapy Research and Practice in Medicine: From out of the Silence. London: Jessica Kingsley Publishers, 1996. [Google Scholar]
- Baird A, Samson S. Memory for music in Alzheimer's disease: unforgettable?. Neuropsychology Review 2009;19:85‐101. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Samsi K, Petrie CD, Alvir J, Treglia M, Schwam EM, et al. What do we know about quality of life in dementia? A review of the emerging evidence on the predictive and explanatory value of disease specific measures of health related quality of life in people with dementia. International Journal of Geriatric Psychiatry 2009;24(1):15‐24. [DOI: 10.1002/gps.2090] [DOI] [PubMed] [Google Scholar]
- Beerens HC, Sutcliffe C, Renom‐Guiteras A, Soto ME, Suhonen R, Zabalegui A, et al. RightTimePlaceCare Consortium. Quality of life and quality of care for people with dementia receiving long term institutional care or professional home care: the European RightTimePlaceCare study. Journal of the American Medical Directors Association 2014;15(1):54‐61. [DOI: 10.1016/j.jamda.2013.09.010] [DOI] [PubMed] [Google Scholar]
- Bradt J. Randomized controlled trials in music therapy: Guidelines for design and implementation. Journal of Music Therapy 2012;49(2):120–49. [DOI] [PubMed] [Google Scholar]
- Broersen M, Groot R, Jonker C. Music Therapy for Alzheimer Patients [Muziektherapie bij Alzheimer Patienten. Enkele richtlijnen op basis van de literatuur]. Tijdschrift voor Kreatieve Therapie 1995;14(1):9‐14. [Google Scholar]
- Carroll C, Patterson M, Wood S, Booth A, Rick J, Balain S. A conceptual framework for implementation fidelity. Implementation Science 2007;30(2):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang YS, Chu H, Yang CY, Tsai JC, Chung MH, Liao YM, et al. The efficacy of music therapy for people with dementia: A meta‐analysis of randomised controlled trials. Journal of Clinical Nursing 2015;24(23‐24):3425‐40. [DOI] [PubMed] [Google Scholar]
- Clift C, Gilbert R, Vella‐Burrows T. A review of research on the value of singing for older people. A Choir in Every Care Home working paper 6. achoirineverycarehome.files.wordpress.com/2016/04/wp6‐research‐review‐v2‐1.pdf.
- Cohen‐Mansfield J, Billig N. Agitated behaviours in the elderly I: A conceptual review. Journal of the American Geriatrics Society 1986;34:711‐21. [DOI] [PubMed] [Google Scholar]
- Cowles A, Beatty WW, Nixon SJ, Lutz LJ, Paulk J, Paulk K. Musical skill in dementia: a violinist presumed to have Alzheimer’s disease learns to play a new song. Neurocase 2003;9(6):493‐503. [DOI] [PubMed] [Google Scholar]
- Cummings JL, Mega M, Gray K. The Neuropsychiatric Inventory: Comprehensive assessment of psychopathology in dementia. Neurology 1994;44(12):2308‐14. [DOI] [PubMed] [Google Scholar]
- Cummings J, Mintzer J, Brodaty H, Sano M, Banerjee S, Devanand DP, et al. International Psychogeriatric Association. Agitation in cognitive disorders: International Psychogeriatric Association provisional consensus clinical and research definition. International Psychogeriatrics / IPA 2015;27(1):7‐17. [DOI: 10.1017/S1041601214001963] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. "Mini‐mental state": A practical method for grading the cognitive state of patients for the clinician. Journal of Psychiatric Research 1975;12(3):189‐98. [DOI] [PubMed] [Google Scholar]
- Gerdner LA. Effects of individualized versus classical "relaxation" music on the frequency of agitation in elderly persons with Alzheimer's disease and related disorders. International Psychogeriatrics / IPA 2000;12(1):49‐65. [DOI] [PubMed] [Google Scholar]
- Guetin S, Charras K, Berard A, Arbus C, Berthelon P, Blanc F, et al. An overview of the use of music therapy in the context of Alzheimer's disease: a report of a French expert group. Dementia (London, England) 2013;12(5):619‐34. [DOI: 10.1177/1471301212438290] [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE guidelines: a new series of articles in the Journal of Clinical Epidemiology. Journal of Clinical Epidemiology 2011;64(4):380‐82. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- Ing‐Randolph AR, Phillips LR, Williams AB. Group music interventions for dementia‐associated anxiety: A systematic review. International Journal of Nursing Studies 2015;52(11):1775‐84. [DOI: 10.1016/j.ijnurstu.2015.06.014] [DOI] [PubMed] [Google Scholar]
- Jacobsen JH, Stelzer J, Fritz TH, Chételat G, Joie R, Turner R. Why musical memory can be preserved in advanced Alzheimer's disease. Brain 2015;138((Pt 8)):2438‐50. [DOI: 10.1093/brain/awv135] [DOI] [PubMed] [Google Scholar]
- Jeon YH, Sansoni J, Low LF, Chenoweth L, Zapart S, Sansoni E, et al. Recommended measures for the assessment of behavioral disturbances associated with dementia. American Journal of Geriatric Psychiatry 2011;19(5):403‐15. [DOI] [PubMed] [Google Scholar]
- Lai CK. The merits and problems of Neuropsychiatric Inventory as an assessment tool in people with dementia and other neurological disorders. Clinical Interventions in Aging 2014;9:1051‐61. [DOI: 10.2147/CIA.S63504] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magee WL, Clark I, Tamplin J, Bradt J. Music interventions for acquired brain injury. Cochrane Database Syst Rev 2017;Jan 20;1:CD006787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McConnell T, Scott D, Porter S. Music therapy for end‐of‐life care: An updated systematic review. Palliative Medicine 2016;30(9):877‐83. [DOI] [PubMed] [Google Scholar]
- McDermott O, Crellin N, Ridder HM, Orrell M. Music therapy in dementia: a narrative synthesis systematic review. International Journal of Geriatric Psychiatry 2013;28(8):781‐94. [DOI: 10.1002/gps.3895] [DOI] [PubMed] [Google Scholar]
- Nelson JC, Devanand DP. A systematic review and meta‐analysis of placebo‐controlled antidepressant studies in people with depression and dementia. Journal of the American Geriatrics Society 2011;59(4):577‐85. [DOI: 10.1111/j.1532-5415.2011.03355.x] [DOI] [PubMed] [Google Scholar]
- Norberg A, Melin E. Reactions to music, touch and object presentation in the final stage of dementia: an exploratory study. International Journal of Nursing Studies 1986;40(5):473‐9. [DOI] [PubMed] [Google Scholar]
- Petrovsky D, Cacchione PZ, George M. Review of the effect of music interventions on symptoms of anxiety and depression in older adults with mild dementia. International Psychogeriatrics / IPA 2015;27(10):1661‐70. [DOI: 10.1017/S1041610215000393] [DOI] [PubMed] [Google Scholar]
- Raglio A, Bellelli G, Mazzola P, Bellandi D, Giovagnoli AR, Farina E, et al. Music, music therapy and dementia: a review of literature and the recommendations of the Italian Psychogeriatric Association. Maturitas 2012;72(4):305‐10. [DOI: 10.1016/maturitas.2012.05.016] [DOI] [PubMed] [Google Scholar]
- Reisberg B, Borenstein J, Salob SP, Ferris SH, Franssen E, Georgotas, A. Behavioral symptoms in Alzheimer's disease: Phenomenology and treatment. Journal of Clinical Psychiatry 1987;48(Suppl.):9‐15. [PubMed] [Google Scholar]
- Riecker A, Ackermann H, Wildgruber D, Dogil G, Grodd W. Opposite hemispheric lateralization effects during speaking and singing. Neuroreport 2000;11(9):1997‐2000. [DOI] [PubMed] [Google Scholar]
- Sepehry AA, Lee PE, Hsiung GY, Beattie BL, Jacova C. Effect of selective serotonin reuptake inhibitors in Alzheimer's disease with comorbid depression: a meta‐analysis of depression and cognitive outcomes. Drugs & Aging 2012;29(10):793‐806. [DOI] [PubMed] [Google Scholar]
- Smith SC, Lamping DL, Banerjee S, Harwood R, Foley B, Smith P, et al. Measurement of health‐related quality of life for people with dementia: development of a new instrument (DEMQOL) and an evaluation of current methodology. Health Technology Assessment 2005;9(10):1‐93, iii‐iv. [DOI] [PubMed] [Google Scholar]
- Spintge R. [Musik in Anästhesie und Schmertztherapie]. Anästhesiol Intensivmed Notfallmed Schmerzther 2000;35:254‐61. [DOI] [PubMed] [Google Scholar]
- Ueda T, Suzukamo Y, Sato M, Izumi S. Effects of music therapy on behavioral and psychological symptoms of dementia: a systematic review and meta‐analysis. Ageing Research Reviews 2013;12(2):628‐41. [DOI: 10.1016/j.arr.2013.02.003] [DOI] [PubMed] [Google Scholar]
- Linde RM, Stephan BC, Savva GM, Dening T, Brayne C. Systematic reviews on behavioural and psychological symptoms in the older or demented population. Alzheimer's Research & Therapy 2012;4(4):28. [DOI: 10.1186/alzrt131] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linde RM, Dening T, Stephan BC, Prina AM, Evans E, Brayne C. Longitudinal course of behavioural and psychological symptoms of dementia: systematic review. The British Journal of Psychiatry 2016;209(5):366‐377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkaik R, Nuyen J, Schellevis F, Francke A. The relationship between severity of Alzheimer's disease and prevalence of comorbid depressive symptoms and depression: a systematic review. International Journal of Geriatric Psychiatry 2007;22(11):1063‐86. [DOI] [PubMed] [Google Scholar]
- Volicer L, Bass EA, Luther SL. Agitation and resistiveness to care are two separate behavioral syndromes of dementia. Journal of the American Medical Directors Association 2007;8(8):527‐32. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Cai J, An L, Hui F, Ren T, Ma H, Zhao Q. Does music therapy enhance behavioral and cognitive function in elderly dementia patients? A systematic review and meta‐analysis. Ageing Res Rev 2017;35:1‐11. [DOI] [PubMed] [Google Scholar]
- Zhao QF, Tan L, Wang HF, Jiang T, Tan MS, Tan L, et al. The prevalence of neuropsychiatric symptoms in Alzheimer's disease: Systematic review and meta‐analysis. Journal of Affective Disorders 2016;190:264‐71. [DOI: 10.1016/j.jad.2015.09.069] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
- Vink AC, Birks JS, Bruinsma MS, Scholten RJPM. Music therapy for people with dementia. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD003477.pub2] [DOI] [PubMed] [Google Scholar]
- Vink AC, Bruinsma MS, Scholten RJPM. Music therapy for people with dementia. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD003477.pub2] [DOI] [PubMed] [Google Scholar]


