Abstract
Background
Cervical cerclage is a well‐known surgical procedure carried out during pregnancy. It involves positioning of a suture (stitch) around the neck of the womb (cervix), aiming to give mechanical support to the cervix and thereby reduce risk of preterm birth. The effectiveness and safety of this procedure remains controversial. This is an update of a review last published in 2012.
Objectives
To assess whether the use of cervical stitch in singleton pregnancy at high risk of pregnancy loss based on woman's history and/or ultrasound finding of 'short cervix' and/or physical exam improves subsequent obstetric care and fetal outcome.
Search methods
We searched Cochrane Pregnancy and Childbirth's Trials Register (30 June 2016) and reference lists of identified studies.
Selection criteria
We included all randomised trials of cervical suturing in singleton pregnancies. Cervical stitch was carried out when the pregnancy was considered to be of sufficiently high risk due to a woman's history, a finding of short cervix on ultrasound or other indication determined by physical exam. We included any study that compared cerclage with either no treatment or any alternative intervention. We planned to include cluster‐randomised studies but not cross‐over trials. We excluded quasi‐randomised studies. We included studies reported in abstract form only.
Data collection and analysis
Three review authors independently assessed trials for inclusion. Two review authors independently assessed risk of bias and extracted data. We resolved discrepancies by discussion. Data were checked for accuracy. The quality of the evidence was assessed using the GRADE approach.
Main results
This updated review includes a total of 15 trials (3490 women); three trials were added for this update (152 women).
Cerclage versus no cerclage
Overall, cerclage probably leads to a reduced risk of perinatal death when compared with no cerclage, although the confidence interval (CI) crosses the line of no effect (RR 0.82, 95% CI 0.65 to 1.04; 10 studies, 2927 women; moderate quality evidence). Considering stillbirths and neonatal deaths separately reduced the numbers of events and sample size. Although the relative effect of cerclage is similar, estimates were less reliable with fewer data and assessed as of low quality (stillbirths RR 0.89, 95% CI 0.45 to 1.75; 5 studies, 1803 women; low quality evidence; neonatal deaths before discharge RR 0.85, 95% CI 0.53 to 1.39; 6 studies, 1714 women; low quality evidence). Serious neonatal morbidity was similar with and without cerclage (RR 0.80, 95% CI 0.55 to 1.18; 6 studies, 883 women; low‐quality evidence). Pregnant women with and without cerclage were equally likely to have a baby discharged home healthy (RR 1.02, 95% CI 0.97 to 1.06; 4 studies, 657 women; moderate quality evidence).
Pregnant women with cerclage were less likely to have preterm births compared to controls before 37, 34 (average RR 0.77, 95% CI 0.66 to 0.89; 9 studies, 2415 women; high quality evidence) and 28 completed weeks of gestation.
Five subgroups based on clinical indication provided data for analysis (history‐indicated; short cervix based on one‐off ultrasound in high risk women; short cervix found by serial scans in high risk women; physical exam‐indicated; and short cervix found on scan in low risk or mixed populations). There were too few trials in these clinical subgroups to make meaningful conclusions and no evidence of differential effects.
Cerclage versus progesterone
Two trials (129 women) compared cerclage to prevention with vaginal progesterone in high risk women with short cervix on ultrasound; these trials were too small to detect reliable, clinically important differences for any review outcome. One included trial compared cerclage with intramuscular progesterone (75 women) which lacked power to detect group differences.
History indicated cerclage versus ultrasound indicated cerclage
Evidence from two trials (344 women) was too limited to establish differences for clinically important outcomes.
Authors' conclusions
Cervical cerclage reduces the risk of preterm birth in women at high‐risk of preterm birth and probably reduces risk of perinatal deaths. There was no evidence of any differential effect of cerclage based on previous obstetric history or short cervix indications, but data were limited for all clinical groups. The question of whether cerclage is more or less effective than other preventative treatments, particularly vaginal progesterone, remains unanswered.
Plain language summary
Can inserting a cervical stitch prevent early births of single babies?
What is the issue?
Cervical cerclage is a surgical procedure performed during pregnancy to place a stitch around the neck of the womb (cervix). The stitch is aimed to support the cervix and reduce risk of an early birth.
Why is this important?
The cervix stays tightly closed until towards the end of normal pregnancies, before starting to shorten and gradually soften to prepare for labour and delivery. However, sometimes the cervix starts to shorten and widen too early, causing either late miscarriage or an early birth. Inserting a cervical stitch may reduce the chance of late miscarriage or early birth.
What evidence did we find?
We searched for evidence up to 30 June 2016. This review includes 15 studies involving 3490 women (3 studies involving 152 women were added for this update).
Women with a stitch are less likely to have a baby who is born too early. Babies whose mothers had a stitch are also less likely to die during the first week of life. It is not clear whether a cervical stitch can prevent stillbirth or improve the baby's health once born.
What does this mean?
Inserting a stitch helps pregnant women who are at high risk avoid early births compared to no stitch. Inserting a stitch may also improve a baby's chance for survival. We found too few clinical trials to understand whether cervical stitch is more effective than other treatments for preventing early births, such as progesterone (a hormone drug used to prevent early birth). We found too few data to understand if it is better to have a stitch inserted early in pregnancy (based on the mother's previous history) or to wait to perform an ultrasound scan later in pregnancy to see if the cervix has become shortened.
Summary of findings
Summary of findings for the main comparison. Cerclage versus no cerclage.
| Cerclage versus no cerclage | ||||||
| Patient or population: preventing preterm birth in women with singleton pregnancy Setting: Belgium, Brazil, Canada, Chile, France, Greece, Hungary, Iceland, Ireland, Italy, Netherlands, Norway, South Africa, Slovenia, UK, USA, Zimbabwe Intervention: cerclage Comparison: no cerclage | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no cerclage (SoF outcomes) | Risk with cerclage | |||||
| All perinatal losses | Study population | RR 0.82 (0.65 to 1.04) | 2927 (10 RCTs) | ⊕⊕⊕⊝ MODERATE1 | ||
| 92 per 1000 | 75 per 1000 (60 to 96) | |||||
| Serious neonatal morbidity | Study population | RR 0.80 (0.55 to 1.18) | 883 (6 RCTs) | ⊕⊕⊝⊝ LOW 2 | ||
| 116 per 1000 | 93 per 1000 (64 to 136) | |||||
| Baby discharged home healthy | Study population | RR 1.02 (0.97 to 1.06) | 657 (4 RCTs) | ⊕⊕⊕⊝ MODERATE3 | ||
| 912 per 1000 | 930 per 1000 (885 to 967) | |||||
| Stillbirths | Study population | RR 0.89 (0.45 to 1.75) | 1803 (5 RCTs) | ⊕⊕⊝⊝ LOW 2 | ||
| 19 per 1000 | 17 per 1000 (9 to 33) | |||||
| Neonatal deaths before discharge | Study population | RR 0.85 (0.53 to 1.39) | 1714 (6 RCTs) | ⊕⊕⊝⊝ LOW 2 | ||
| 35 per 1000 | 30 per 1000 (19 to 49) | |||||
| Preterm birth before 34 completed weeks | Study population | average RR 0.77 (0.66 to 0.89) | 2415 (9 RCTs) | ⊕⊕⊕⊕ HIGH4 | ||
| 238 per 1000 | 183 per 1000 (157 to 212) | |||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Wide confidence interval crossing the line of no effect (‐1).
2 Wide confidence interval crossing the line of no effect and small sample size (‐2)
3 Estimate based on small sample size (‐1).
4 Random effects model retained from primary analysis; there is no substantive difference in the risk estimate or the confidence intervals with fixed or random effects.
Background
Description of the condition
During normal pregnancy the neck of the womb (cervix) stays tightly closed, allowing the pregnancy to reach full term. Towards the end of pregnancy, the cervix starts to shorten and progressively becomes softer (more favourable) ‐ these changes are physiological preparations for normal labour and delivery.
Sometimes, the cervix starts to shorten and dilates too early, causing either late miscarriage or preterm birth. In the absence of uterine contractions, the cause of this pathological condition is considered to be cervical insufficiency (sometimes also called incompetence). The condition has been described as early as the 17th century (Riverius 1658). It has been suggested that cervical insufficiency complicates about 1% of an obstetric population (McDonald 1980) and 8% of a recurrent miscarriage population who have experienced mid‐trimester pregnancy losses (Drakeley 1998). There is however, no consistent definition of cervical insufficiency (Berry 1995) which hampers any attempt to establish the true incidence.
Some researchers have defined cervical insufficiency as "the history of painless dilatation of the cervix resulting in second or early third trimester delivery and the passage, without resistance, of size nine Hegar dilator (an instrument which is used to measure the size of cervical dilatation in millimetres)" (Berry 1995). Other descriptions include: recurrent second trimester or early third trimester loss of pregnancy caused by the inability of the uterine cervix to retain a pregnancy until term (Althuisius 2001) and a physical defect in the strength of the cervical tissue that is either congenital (inherited) or acquired, i.e. caused by previous damage (Rust 2000).
Description of the intervention
Cervical cerclage is one of the best known surgical procedures in obstetrics. It involves the positioning of a suture (stitch) around the neck of the womb (cervix), aimed to provide mechanical support to the cervix and keep the cervix closed during the pregnancy.
There are a number of proposed surgical methods designed to keep the cervix closed until the expected time of birth. All interventions require at least regional anaesthesia in the form of a spinal or epidural block. Shirodkar 1955 reported the insertion of a cervical stitch (suture) at around 14 weeks of pregnancy. The anterior vaginal wall is cut and the bladder reflected (pushed) back and upwards allowing an access close to the level of the internal cervical os by the vaginal route. A stitch, usually silk, tape, or other non‐absorbable material, is inserted around the cervix, enclosing it. McDonald 1957 described a simpler purse string stitch technique, whereby the stitch is inserted around the body of the cervix visible in the vagina in three or four bites. Athough the internal os is often not reached, the procedure is easier to perform with less bleeding. These techniques were described as elective (planned) procedures.
Total cervical occlusion is another proposed variation where, in addition to the standard cerclage, the external cervical os is closed with continuous nylon (Saling 1984; Secher 2007). The rationale for this technique is based on the observation that the mucous plug has a double role in preventing preterm labour. The plug is a mechanical barrier between the vagina and uterus, but its intrinsic richness in immune components also makes it a very important element in defending the fetal compartment from ascending infections. Intuitively, protective nylon could keep the plug in situ, thereby increasing the innate defence of the cervical canal.
There has been some suggestion recently that suture material may have an important influence on the outcome of pregnancy. However, the surgical methods for cerclage, including the choice of material, are beyond the scope of this review.
Stitches are normally inserted via the vaginal route, but transabdominal cerclage has also been proposed. This approach is used for women when vaginal stitches have failed, or when a woman has a short, scarred cervix making vaginal stitch insertion technically difficult (Anthony 1997; Gibb 1995). Initally, cerclage procedures have been carried out in early pregnancy around 12 weeks of gestation, but are increasingly being scheduled before pregnancy. Either way, during laparotomy, the bladder is reflected downwards away from the uterus and the cervical stitch is placed at the level of the internal cervical os. Vaginally inserted cervical stitches are either taken out at 37 weeks' gestation, or when the woman presents in labour, usually without an anaesthetic. Abdominal cervical stitches are left in place and the baby is delivered by caesarean section.
Cervical cerclage, by whichever technique employed, carries risks for the pregnancy. Surgical manipulation of the cervix can cause uterine contractions, bleeding or infection which may lead to miscarriage or preterm labour. These risks must be carefully balanced against the benefit from mechanical support of the cervix.
Cervical cerclage can either be inserted as a planned procedure based on previous history (history‐indicated), because of a short cervical length detected on transvaginal ultrasound (ultrasound‐indicated), or as an emergency procedure when women with threatened miscarriage present at the hospital (physical exam‐indicated) (Chanrachakul 1998; Wong 1993). Ultrasound‐ and physical exam‐indicated cerclages tend to be performed later in pregnancy; history‐indicated procedures are usually planned around 14 weeks.
How the intervention might work
Intuitively, in the presence of a short cervix at ultrasound, or history of recurrent spontaneous mid‐trimester losses, reinforcing the cervix by positioning a mechanical support should prolong pregnancy and reduce the risk of preterm birth and its sequelae.
Why it is important to do this review
Controversies concerning cervical cerclage include effectiveness, safety and risk/benefit to both mother and unborn baby. The avoidance of surgical trauma to the cervix may be as effective as intervention. Grant 1989 reviewed the evidence for the benefits and hazards of treatment by cervical cerclage to prolong pregnancy and suggested that cervical cerclage in women with a previous mid‐trimester loss (or preterm delivery) may help to prevent one delivery before 33 weeks for every 20 stitches inserted (Grant 1989). Since 1989 there have been a number of randomised and non‐randomised studies published, however, the issues surrounding effectiveness in preventing neonatal sequelae of prematurity, timing of cerclage and optimal techniques have not been addressed adequately. The evidence on which to base practice for physical exam‐indicated cerclage is even less robust. A meta‐analysis estimated the effectiveness of physical examination‐indicated cerclage versus expectant management in the setting of second‐trimester cervical dilatation (14 to 27 gestational weeks) (Ehsanipoor 2015). The physical examination‐indicated cerclage was associated with a significant increase in neonatal survival and prolongation of pregnancy. However, as well as including randomised controlled trials, Ehsanipoor 2015 also included retrospective and prospective cohort studies in the meta‐analysis. A previous Cochrane Review on this topic did not find clear benefit, although heterogeneity was high for some important obstetric outcomes. In their meta‐analysis of individual patient data, Berghella 2005 concluded that cerclage could be beneficial in women with singleton pregnancies, short cervix and experience of prior preterm birth. In a similar meta‐analysis, no statistical significance was found for singleton pregnancies (Jorgensen 2007). Both meta‐analyses showed no benefit for multiple gestation pregnancies. In an indirect comparison meta‐analysis of randomised controlled trials, Conde‐Agudelo 2013 et al found that either cerclage or vaginal progesterone are equally efficacious in the prevention of preterm birth in women with sonographic short cervix in the mid trimester, singleton gestation and previous preterm birth.
A Cochrane Review investigating cervical cerclage for preventing preterm birth in multiple gestation pregnancies has been published (Rafael 2014).
Objectives
To assess whether the use of cervical stitch in singleton pregnancy at high risk of pregnancy loss based on woman's history and/or ultrasound finding of 'short cervix' and/or physical exam improves subsequent obstetric care and fetal outcome.
Methods
Criteria for considering studies for this review
Types of studies
All randomised trials comparing cervical stitch in singleton pregnancies of women considered to be at high risk of pregnancy loss. We planned to include cluster‐randomised studies but not cross‐over trials. We excluded quasi‐randomised studies. We included studies reported in abstract form only.
Types of participants
Women with singleton pregnancies considered to be at high risk for pregnancy loss based any of the following: woman's history (e.g. previous preterm birth); prior cervical surgery (loop excision, cone biopsy, surgical termination of pregnancy); short cervix on ultrasound scanning; or physical exam‐detected cervical changes (including emergency or rescue cerclage). Cervical cerclage for multiple pregnancies was investigated in another Cochrane Review (Rafael 2014).
Types of interventions
Cervical stitch in singleton pregnancies considered for women to be at high risk for pregnancy loss.
Comparisons
Cervical stitch (cerclage) versus no stitch according to clinical subgroups (history‐ versus ultrasound‐ versus physical exam‐indicated cerclage).
Cervical stitch (cerclage) versus any alternative preventative treatment (e.g. progesterone or pessary).
Any comparison of different cerclage protocols (history‐ versus ultrasound‐ versus physical exam‐indicated cerclage).
Types of outcome measures
We selected outcome domains based on consensus work undertaken to define core outcome measures for clinical research and evidence synthesis for pregnancy and childbirth generally (Devane 2007) and for preterm birth prevention specifically (van 't Hooft 2016).
Primary outcomes
Perinatal loss: all losses including miscarriages, stillbirth and neonatal deaths.
Serious neonatal morbidity (as defined by trialists).
Baby discharged home healthy (without obvious pathology ‐ as defined by trialists).
It may seem unusual to not include preterm birth rates as the primary outcome. In the context of this review, preterm births should be regarded as a surrogate for mortality and morbidity. More importantly, there is a real possibility that prolongation of pregnancy may be misinterpreted as benefit, when in fact, an early birth in a setting with adequate neonatal care resources may be better for the infant.
Secondary outcomes
Neonatal
Stillbirth: intra‐uterine death at 24 weeks or more weeks; or greater than 500 g fetal weight or reaching viability as defined by trialist.
Neonatal death before discharge.
Miscarriages: perinatal loss before 24 weeks.
Preterm birth (birth before 28, 34 and 37 completed weeks of pregnancy).
Serious intracranial pathology, e.g. intraventricular haemorrhage or periventricular leukomalacia (as defined by trialists).
Serious respiratory morbidity, e.g. respiratory distress syndrome or oxygen dependency after 28 days of life.
Necrotising enterocolitis requiring surgery.
Retinopathy of prematurity.
Apgar less than seven at five minutes.
Maternal
Caesarean section (elective and emergency).
Maternal infection requiring intervention, e.g. antibiotics or delivery.
Maternal side effects (vaginal discharge, bleeding, pyrexia not requiring antibiotics).
We also planned to report non‐prespecified outcomes if they were reported by more than one included trial.
Not prespecified outcomes
Any intravenous, oral or combined tocolysis.
Preterm premature rupture of the membranes (PPROM).
Chorioamnionitis.
Search methods for identification of studies
The following methods section of this review is based on a standard template used by Cochrane Pregnancy and Childbirth.
Electronic searches
We searched Cochrane Pregnancy and Childbirth’s Trials Register by contacting their Information Specialist (30 June 2016).
The Register is a database containing over 22,000 reports of controlled trials in the field of pregnancy and childbirth. For full search methods used to populate Pregnancy and Childbirth’s Trials Register including the detailed search strategies for CENTRAL, MEDLINE, Embase and CINAHL; the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service, please follow this link to the editorial information about the Cochrane Pregnancy and Childbirth in the Cochrane Library and select the ‘Specialized Register’ section from the options on the left side of the screen.
Briefly, Cochrane Pregnancy and Childbirth’s Trials Register is maintained by their Information Specialist and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE (Ovid);
weekly searches of Embase (Ovid);
monthly searches of CINAHL (EBSCO);
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Search results are screened by two people and the full text of all relevant trial reports identified through the searching activities described above is reviewed. Based on the intervention described, each trial report is assigned a number that corresponds to a specific Pregnancy and Childbirth review topic (or topics), and is then added to the Register. The Information Specialist searches the Register for each review using this topic number rather than keywords. This results in a more specific search set which has been fully accounted for in the relevant review sections (Included studies; Excluded studies; Studies awaiting classification; Ongoing studies).
Searching other resources
We searched the reference lists of the studies identified. We did not apply any language or date restrictions.
Data collection and analysis
Methods used in the previous version of this review are presented in Alfirevic 2012. The following methods were used for this update to assess records identified as a result of the 2016 search.
Selection of studies
Two review authors independently assessed all potential studies identified as a result of the search for inclusion. We resolved any disagreement through discussion or, if required, we consulted the third review author.
Data extraction and management
We designed a data extraction form. Two review authors extracted data from eligible studies using the form. We resolved discrepancies through discussion or, if required, we consulted the third review author. Data were entered into Review Manager software (RevMan 2014) and checked for accuracy.
When information was unclear, we planned to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). Any disagreement was resolved by discussion or by involving a third assessor.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3.1) Blinding of participants and personnel (checking for possible performance bias)
We described for each included study the methods used, if any, to blind study participants and personnel from knowledge of which intervention a participant received. We considered that studies were at low risk of bias if they were blinded, or if we judged that the lack of blinding unlikely to affect results. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
(3.2) Blinding of outcome assessment (checking for possible detection bias)
We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or could be supplied by the trial authors, we planned to re‐include missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s pre‐specified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we had about other possible sources of bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Handbook (Higgins 2011). With reference to (1) to (6) above, we planned to assess the likely magnitude and direction of the bias and whether we considered it is likely to impact on the findings. In future updates, we will explore the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Assessment of the quality of the evidence using the GRADE approach
For this update, we assessed evidence quality using the GRADE approach as outlined in the GRADE handbook relating to the following outcomes:
perinatal loss: all losses including miscarriages, stillbirth and neonatal deaths;
serious neonatal morbidity (as defined by trialists);
baby discharged home healthy (without obvious morbidity, as defined by trialists);
Stillbirth: intra‐uterine death at 24 or more weeks or more than 500 g fetal weight or reaching viability as defined by trialists;
neonatal death before discharge; and
preterm birth before 34 completed weeks of pregnancy.
GRADEpro GDT was used to import data from Review Manager 5.3 (RevMan 2014) to create ’Summary of findings’ tables. A summary of the intervention effect and a measure of quality for each of the above outcomes was produced using the GRADE approach. The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from 'high quality' by one level for serious (or by two levels for very serious) limitations, depending on assessments for risk of bias, indirectness of evidence, serious inconsistency, imprecision of effect estimates or potential publication bias.
Measures of treatment effect
Dichotomous data
We presented results as summary risk ratio with 95% confidence intervals for dichotomous data.
Continuous data
No continuous data were analysed in this review. In future updates, if applicable, we will use the mean difference if outcomes are measured in the same way between trials. We will use the standardised mean difference to combine trials that measure the same outcome, but use different methods.
Unit of analysis issues
Cluster‐randomised trials
For this update, we did not include any cluster‐randomised trials. If in future updates of the review we find cluster‐randomised trials, we will include these trials in the analyses along with individually randomised trials. We will adjust their sample sizes or standard errors using the methods described in the Handbook (Section 16.3.4 or 16.3.6) (Higgins 2011) using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial, or from a study of a similar population. If we use ICCs from other sources, we will report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we identify both cluster‐randomised trials and individually‐randomised trials, we plan to synthesise the relevant information. We will consider it reasonable to combine the results from both if there is little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit is considered to be unlikely.
We will also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Cross‐over trials
Cross‐over trials are not feasible for the population of interest or for interventions relevant to this systematic review.
Other unit of analysis issues
Multiple pregnancy was not eligible for inclusion in this review. Where trials reported both singleton and multiple pregnancy, we used data for women with singleton pregnancies.
Dealing with missing data
Levels of attrition were noted for included studies. In future updates, if more studies are included, the impact of including studies with high levels of missing data in the overall assessment of treatment effect will be explored in sensitivity analyses.
Analyses for all outcomes were carried out, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the Tau², I² and Chi² statistics. We regarded heterogeneity as substantial if I² was greater than 30% and either Tau² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity. If we identified substantial heterogeneity (above 30%), we explained in the text possibly sources of clinical heterogeneity between trials. See also Data synthesis.
Assessment of reporting biases
In future updates, if there are 10 or more studies in the meta‐analysis, we will investigate reporting biases (such as publication bias) using funnel plots. We will assess funnel plot asymmetry visually. If asymmetry is suggested by a visual assessment, we will perform exploratory analyses to investigate.
Data synthesis
We carried out statistical analysis using Review Manager software (RevMan 2014). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar.
If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differed between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average range of possible treatment effects. We also discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials. If we used random‐effects analyses, the results were presented as the average treatment effect with 95% confidence intervals, and the estimates of Tau² and I².
Within each comparison, analyses for all outcomes are displayed according to clinical groups (history‐indicated, physical‐exam indicated, etc). Subgroup analysis was conducted only for comparison of cerclage versus no cerclage.
Subgroup analysis and investigation of heterogeneity
If we found substantial heterogeneity (I² > 30%) for our primary outcomes, and had adequate numbers of included trials in each relevant subgroup, we planned to investigate sources using subgroup analyses to consider whether an overall summary was meaningful, and if so, to use random‐effects analysis to investigate.
We planned to carry out the following subgroup analyses for the main comparison (cerclage versus no cerclage). Five potential subgroups were examined: history‐indicated cerclage; one‐off ultrasound‐indicated cerclage in high‐risk women, serial ultrasound‐indicated cerclage, physical exam‐indicated cerclage (rescue cerclage) and one‐off ultrasound‐indicated cerclage in low or unspecified risk women. There were too few trials in each subgroup to make meaningful conclusions regarding differences in effect in subgroups. Forest plots show trials within the appropriate subgroup for display only.
If in future updates, if we have adequate numbers of trials, we will assess subgroup differences by interaction tests available within RevMan (RevMan 2014). If evidence of subgroup differences are identified, we plan to report the results of subgroup analyses quoting the Chi² statistic and P value, and the interaction test I² value.
Sensitivity analysis
For primary outcomes only, we carried out sensitivity analyses to explore the impact of trial quality, assessed as high quality if the trial reported adequate methods for sequence generation and allocation concealment and had no other clear markers of poor trial quality (unacceptable attrition, for example). We reported whether or not the exclusion of studies with substantial risks of bias changed the overall effect estimate or its interpretation.
Results
Description of studies
Results of the search
An updated search (June 2016) identified 22 new reports. We also re‐assessed Althuisius 2001, and included Althuisius 2003, which had previously been listed as a report of this study. We also included two new studies (five reports) from the 2016 search (Chandiramani 2010; Ionescu 2012), added five additional reports of two already included studies (MRC/RCOG 1993 (1 report); Owen 2009 (4 reports)). We also identified and excluded another report of a previously excluded study (Secher 2007). We excluded six new studies (Hui 2013;Israfil‐Bayli 2014 (two reports); Ismail 2014; Üçyiğit 2013 (two reports); Zakhera 2015; Zolghadri 2014). There are two ongoing studies (Hezelgrave 2015; Koulalli 2014) and one study (Ragab 2015) awaiting classification. See Figure 1.
1.
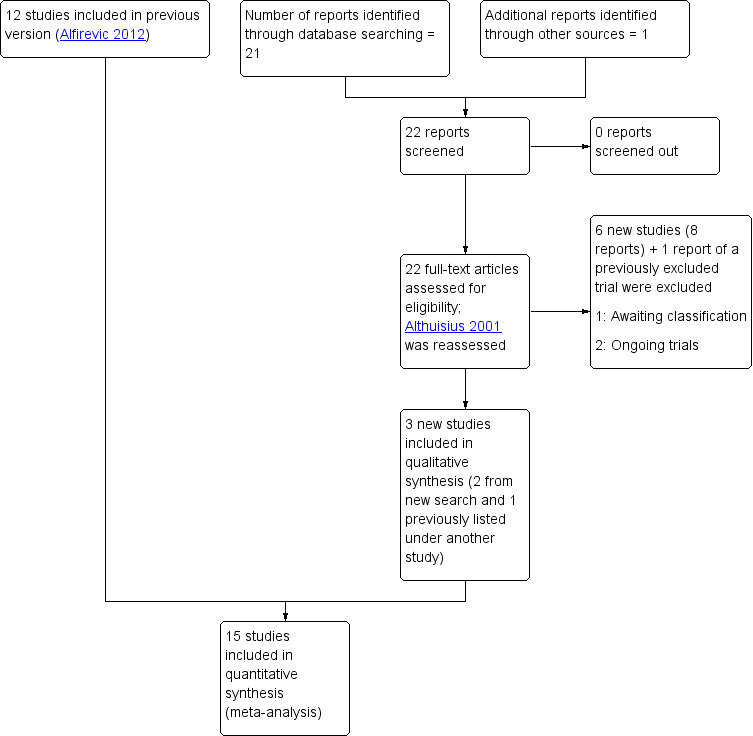
Study flow diagram
Included studies
Interventions
Most included studies (n = 10) compared cerclage versus no cerclage (Althuisius 2001; Althuisius 2003; Berghella 2004; Ezechi 2004; Lazar 1984; MRC/RCOG 1993; Owen 2009; Rush 1984; Rust 2000; To 2004). Of these, two studies required women in both the intervention (cerclage) and control (no cerclage) groups to undertake bed rest (Althuisius 2001; Berghella 2004). Three studies incorporated a rescue arm for women randomised to the control group based on physical exam (Owen 2009) or ultrasound‐detected changes of the cervix (Althuisius 2001; Rust 2000).
Two studies compared cerclage versus progesterone for pregnant women with a history of preterm birth undergoing serial ultrasound who developed short cervix (< 25 mm) (Chandiramani 2010; Ionescu 2012). One study compared cervical cerclage versus weekly intramuscular injections of 17 OHP‐C (Keeler 2009).
Two studies compared different management protocols for cervical cerclage: elective cerclage based on previous obstetrical history versus cerclage based on cervical changes on serial transvaginal ultrasound scans (Beigi 2005; Simcox 2009).
Setting
Studies took place in many countries including: USA (4), UK (2), France (2), Netherlands (3), South Africa (2), Brazil, Slovenia, Greece, Chile, Iran, Nigeria, Romania, Hungary, Norway, Italy, Belgium, Zimbabwe, Iceland, Ireland, Belgium and Canada. Two trials took place in multiple countries (MRC/RCOG 1993; To 2004).
Population
Only women at high risk of preterm labour were included in 11 studies. Risk of preterm labour was assessed based on previous obstetrical history (n = 5; Beigi 2005; Ezechi 2004; MRC/RCOG 1993; Rush 1984; Simcox 2009) and serial ultrasound scans (Owen 2009). Lazar 1984 used a mixed scoring system based on obstetrical history, serial ultrasound scans of the cervix and physical exam. Althuisius 2001 assessed risk of preterm labour based on previous obstetrical history in half the population and serial ultrasound scans of the cervix in the other half. Althuisius 2003 assessed women with ultrasound and physical exam. Ionescu 2012 and Chandiramani 2010 included pregnant women with both history of preterm birth and short cervix < 25 mm on serial ultrasound.
To 2004 included an unselected general obstetric population with the need for cerclage assessed using a one‐off ultrasound scan. Three studies included a mixed population, with indication for cerclage based either on serial ultrasound scans of the cervix in women at high risk of preterm birth, or a one‐off ultrasound scan in women at low risk (Berghella 2004; Keeler 2009; Rust 2000).
Nine studies involved singleton pregnancies only (Althuisius 2001; Beigi 2005; Chandiramani 2010; Keeler 2009; Lazar 1984; Owen 2009; Rush 1984; Simcox 2009; To 2004) and four assessed both singleton and multiple pregnancies (Althuisius 2003; Berghella 2004; MRC/RCOG 1993; Rust 2000). Two trials did not state if only singleton pregnancies were included (Ezechi 2004; Ionescu 2012); however, Ezechi 2004 reported individual patient data for singletons only.
We classified trials according to clinical groups for display purposes only: pregnant women with a history of preterm birth (Beigi 2005; Ezechi 2004; Lazar 1984; MRC/RCOG 1993; Rush 1984; Simcox 2009); pregnant women with one‐off ultrasound (To 2004); serial ultrasound (Althuisius 2001; Owen 2009) or using both ultrasound protocols (Berghella 2004; Rust 2000). We included Althuisius 2003 in the physical exam‐indicated subgroup. Three trials compared cerclage with natural progesterone (Chandiramani 2010; Ionescu 2012) or 17 OHP‐C (Keeler 2009).
Excluded studies
We excluded a total of 17 studies; of these, six were excluded based on assessments for the 2016 search. Three studies included only twin pregnancies (Dor 1982; Nicolaides 2001; Rust 2001); six compared different types of cervical cerclage (Broumand 2011; Caspi 1990; Secher 2007; Tsai 2009; Üçyiğit 2013; Zolghadri 2014). We excluded two studies that did not use adequate randomisation procedures (Kassanos 2001; Von Forster 1986). Blair 2002 compared outpatient cerclage with inpatient cerclage. Hui 2013 compared Arabin pessary with no treatment for women with sort cervix at 20 to 24 weeks' gestation. Three trials compared suture materials (Israfil‐Bayli 2014; Ismail 2014). Zakhera 2015 included women for cerclage on the basis of recurrent early bleeding in pregnancy; women did not have a short cervix or history of preterm birth. Varma 1986 is a study protocol, and we doubt that this trial was carried out.
Risk of bias in included studies
The overall quality of most studies was good, with adequate reporting of sequence generation, allocation concealment and outcome data. However, several trials had insufficient information in published reports to inform assessment of these key domains. It is not feasible to blind cerclage treatment, and therefore, all trials were assessed at high risk of performance bias due to lack of blinding. We feel that the impact of lack of blinding in trials will vary by outcomes, and we took this into consideration for our GRADE assessments (Characteristics of included studies; Figure 2).
2.
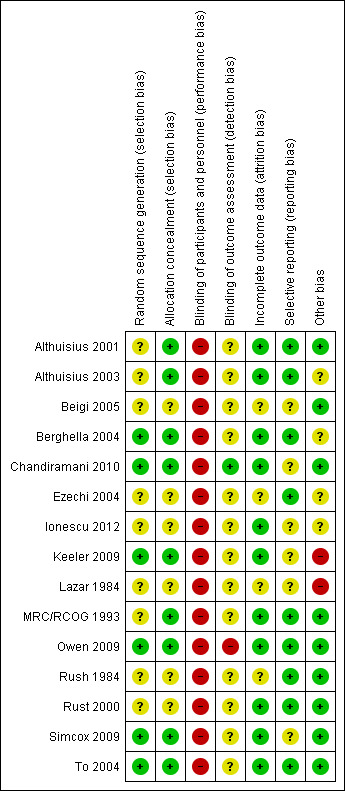
Risk of bias summary: review authors' judgements about each risk of bias item for each included study
Allocation
Six studies reported adequate methods for random sequence generation and concealment allocation (Berghella 2004; Chandiramani 2010; Keeler 2009; Owen 2009; Simcox 2009; To 2004). Allocation concealment was judged as low risk of bias, but sequence generation was unclear in three studies (Althuisius 2001; Althuisius 2003; MRC/RCOG 1993). Six studies had both unclear sequence generation and concealment allocation (Beigi 2005; Ezechi 2004; Ionescu 2012; Lazar 1984; Rush 1984; Rust 2000).
Blinding
Blinding of participants and personnel was not feasible due to the nature of the intervention. Nevertheless, information on attempts to protect against biased assessment of the outcomes (detection bias) was available in one study (Owen 2009). Chandiramani 2010 had adequate blinding for laboratory staff assessing the primary aim of the study (cytokine concentrations).
Incomplete outcome data
Eleven studies adequately addressed the issue of incomplete outcome data assessment (attrition bias) (Althuisius 2001; Althuisius 2003; Berghella 2004; Chandiramani 2010; Ionescu 2012; Keeler 2009; MRC/RCOG 1993; Owen 2009; Rust 2000; Simcox 2009; To 2004). In four studies, the quality of outcome data assessment was judged as unclear (Beigi 2005; Ezechi 2004; Lazar 1984; Rush 1984). Only a few studies provided information on the number of women approached to take part in the study, the number eligible for inclusion, and the overall refusal rate. Although not sources of bias, high exclusion and refusal rates may affect the generalisability of findings and interpretation of results.
Selective reporting
With one exception(To 2004), trial protocols were not available to inform assessment of prespecified primary and secondary outcomes. Despite this, we judged nine studies to be free of selective reporting on the basis that prespecified data extraction forms were provided by the authors (Althuisius 2001; Althuisius 2003; Berghella 2004; Ezechi 2004; MRC/RCOG 1993; Owen 2009; Rush 1984; Rust 2000; To 2004). Selective reporting was judged as unclear in the remaining included studies (Beigi 2005; Chandiramani 2010; Ionescu 2012; Keeler 2009; Lazar 1984; Simcox 2009).
Other potential sources of bias
We assessed 10 studies to be free of other sources of bias (Althuisius 2001; Althuisius 2003; Beigi 2005; Chandiramani 2010; MRC/RCOG 1993; Owen 2009; Rush 1984; Rust 2000; Simcox 2009; To 2004); three studies were judged as unclear (Berghella 2004; Ezechi 2004; Ionescu 2012). Two studies were stopped early and considered to be of high risk of bias (Keeler 2009; Lazar 1984).
Sensitivity analyses
To determine which studies to exclude in sensitivity analyses based on their quality, we referred to both adequate (low risk of bias) labelled sequence generation and adequate (low risk of bias) allocation concealment as essential criteria for adequate quality. If there were obvious additional sources of risk of bias, such as unacceptable attrition or the was trial stopped early, we also considered these factors. We assessed five studies (Berghella 2004; Chandiramani 2010; Owen 2009; Simcox 2009; To 2004) to be at overall low risk of bias (Figure 2).
Effects of interventions
See: Table 1
Some trial data included in the analyses for all perinatal losses and baby discharged home healthy outcomes were based on individual patient data meta‐analyses published in Jorgensen 2007. Data for some trials may not match the published reports because we obtained data sets from trial authors (see Characteristics of included studies).
The denominator used for the outcomes of neonatal death, baby discharged home healthy and Apgar less than seven at five minutes, was as far as possible, live births (where reported, we subtracted the number of stillbirths and miscarriages from the total number randomised to calculate live births). The denominator for all other outcomes was the total number of participants randomised. The all perinatal losses outcome includes miscarriage, stillbirth and neonatal death events.
Trial effect estimates are reported according to clinical groups based on indication for cerclage (history‐ or physical‐exam indicated) and trial protocol (one‐off or serial ultrasound) for Comparison 1. We pooled effect estimates for all analyses where heterogeneity was not substantial and did not formally discuss subgroup interaction tests. The small number of trials in clinical groups means these interaction tests are not valid. Plausible explanations for sources of substantial heterogeneity are provided.
GRADEpro GDT software is unable to analyse data split into clinical groups. Therefore, we collapsed the clinical groups for summary of findings outcomes from Comparison 1 and assessed these in Comparison 5 (Cerclage versus no cerclage (Summary of findings outcomes)).
Comparison 1. Cerclage versus no cerclage
Several trials in this comparison were split according to clinical groups as shown in the forest plots.
Primary outcomes
1.1 All perinatal losses
Cerclage may lead to reduced risk of perinatal death when compared with no cerclage, although the confidence interval (CI) just crosses the line of no effect (RR 0.82, 95% CI 0.65 to 1.04; 10 studies, 2927 participants; moderate‐quality evidence; Analysis 1.1).
1.1. Analysis.
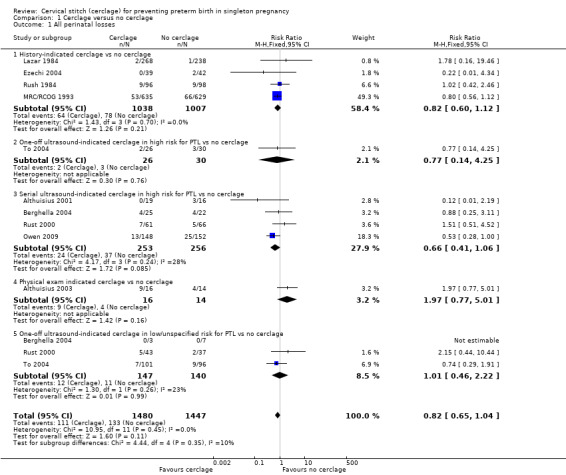
Comparison 1 Cerclage versus no cerclage, Outcome 1 All perinatal losses.
1.2 Serious neonatal morbidity
Treatment groups had similar rates of serious neonatal morbidity (RR 0.80, 95% CI 0.55 to 1.18; 6 studies, 883 participants; low‐quality evidence; Analysis 1.2).
1.2. Analysis.
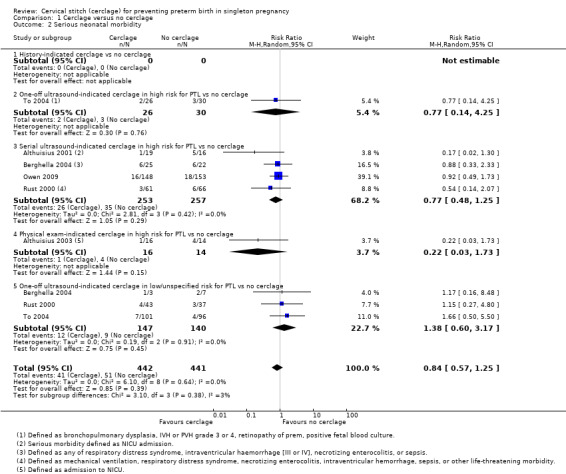
Comparison 1 Cerclage versus no cerclage, Outcome 2 Serious neonatal morbidity.
1.3 Baby discharged home healthy
In four trials similar numbers of women with and without cerclage had healthy babies discharged home (RR 1.02, 95% CI 0.97 to 1.06; 4 studies, 657 participants; moderate‐quality evidence; Analysis 1.3).
1.3. Analysis.
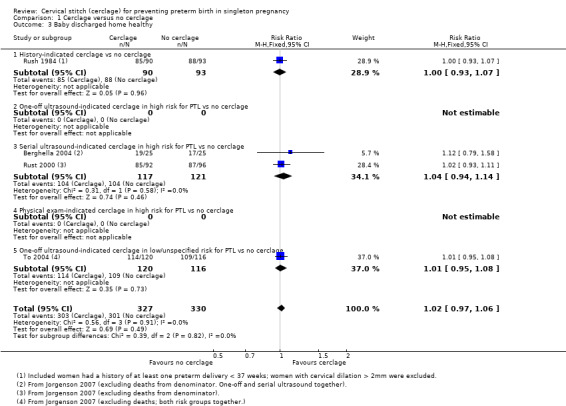
Comparison 1 Cerclage versus no cerclage, Outcome 3 Baby discharged home healthy.
Secondary outcomes
1.4 Stillbirth and 1.6 Miscarriage
There was no evidence that cerclage had an impact on rates of stillbirth (RR 0.89, 95% CI 0.45 to 1.75; 5 studies, 1803 participants; low‐quality evidence; Analysis 1.4) or miscarriage (RR 0.84, 95% CI 0.58 to 1.22; 7 studies, 2091 participants; Analysis 1.6).
1.4. Analysis.
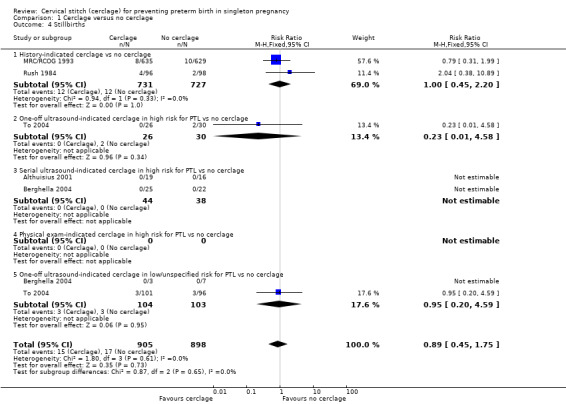
Comparison 1 Cerclage versus no cerclage, Outcome 4 Stillbirths.
1.6. Analysis.
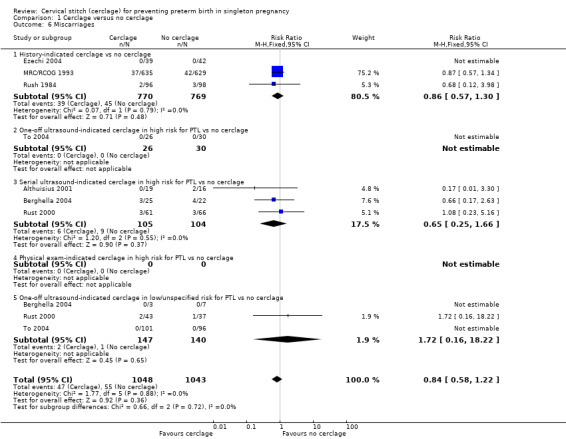
Comparison 1 Cerclage versus no cerclage, Outcome 6 Miscarriages.
1.5 Neonatal deaths before discharge
There was no clear evidence that cerclage prevented neonatal deaths before discharge (RR 0.85, 95% CI 0.53 to 1.39; 6 studies, 1714 participants; low‐quality evidence; Analysis 1.5).
1.5. Analysis.
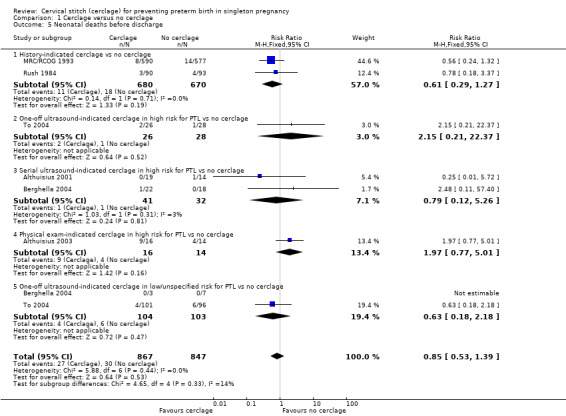
Comparison 1 Cerclage versus no cerclage, Outcome 5 Neonatal deaths before discharge.
1.7 Preterm birth < 37 weeks, 1.8 Preterm birth < 34 weeks, 1.9 Preterm birth < 28 weeks
Cerclage was associated with reduced risk of preterm births before 37 weeks, with some heterogeneity noted (average RR 0.80, 95% CI 0.69 to 0.95; 9 studies, 2898 participants; I² = 39%; Analysis 1.7). Pregnant women who underwent cerclage were also less likely to give birth before 34 weeks' gestation (average RR 0.77, 95% CI 0.66 to 0.89; 9 studies, 2415 participants; high‐quality evidence; Analysis 1.8) and also probably less likely to give birth before 28 weeks, although this result was marginal, with the CI meeting the line of no effect (RR 0.80, 95% CI 0.64 to 1.00; 8 studies, 2392 participants; Analysis 1.9).
1.7. Analysis.
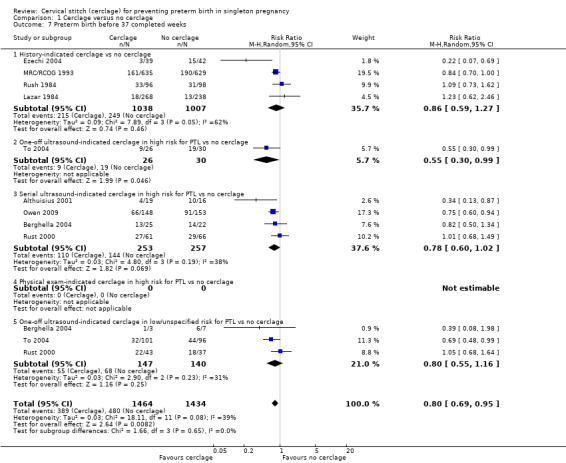
Comparison 1 Cerclage versus no cerclage, Outcome 7 Preterm birth before 37 completed weeks.
1.8. Analysis.
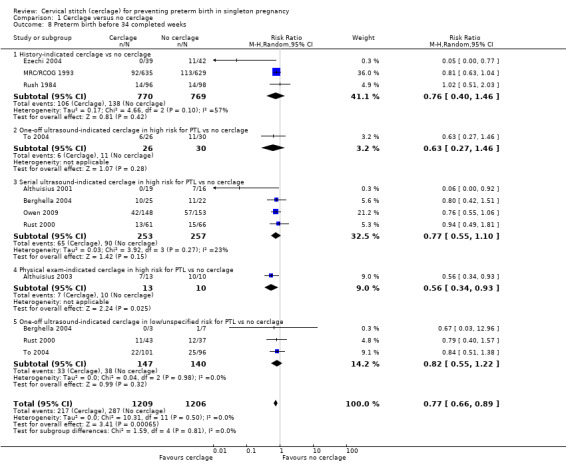
Comparison 1 Cerclage versus no cerclage, Outcome 8 Preterm birth before 34 completed weeks.
1.9. Analysis.
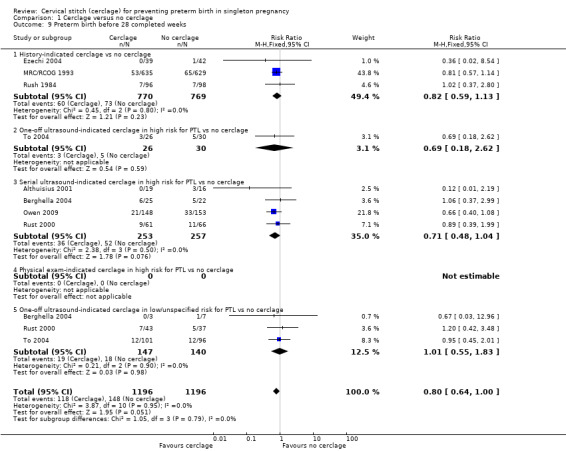
Comparison 1 Cerclage versus no cerclage, Outcome 9 Preterm birth before 28 completed weeks.
Reporting of various aspects of neonatal morbidity was inconsistent and meta‐analyses showed no clear evidence of an effect from cerclage. There was marginally more respiratory morbidity in the cerclage group (Analysis 1.11), but less intracranial pathology (Analysis 1.10), less necrotising enterocolitis (Analysis 1.12) and less retinopathy of prematurity (Analysis 1.13) with cerclage. None of these differences reached statistical significance.
1.11. Analysis.
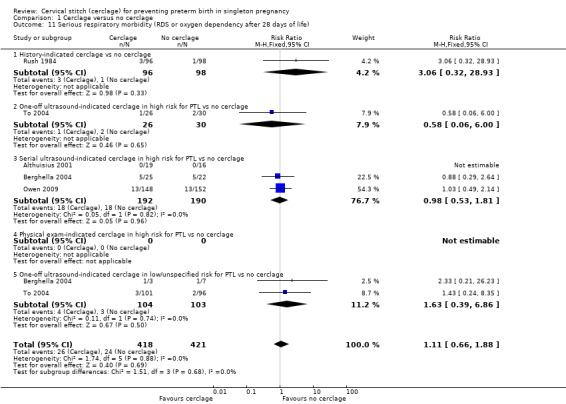
Comparison 1 Cerclage versus no cerclage, Outcome 11 Serious respiratory morbidity (RDS or oxygen dependency after 28 days of life).
1.10. Analysis.
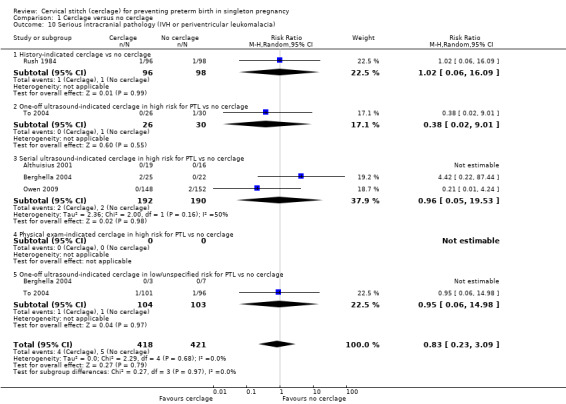
Comparison 1 Cerclage versus no cerclage, Outcome 10 Serious intracranial pathology (IVH or periventricular leukomalacia).
1.12. Analysis.
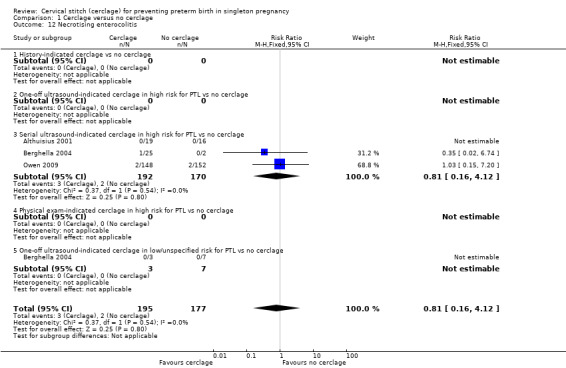
Comparison 1 Cerclage versus no cerclage, Outcome 12 Necrotising enterocolitis.
1.13. Analysis.
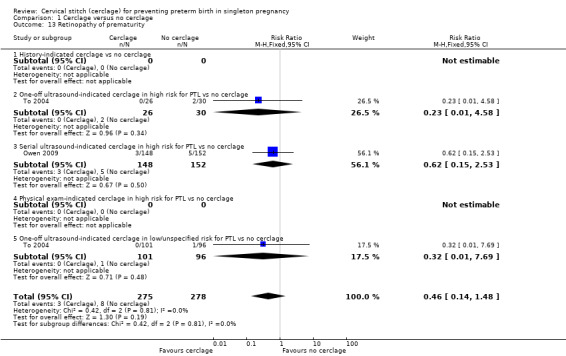
Comparison 1 Cerclage versus no cerclage, Outcome 13 Retinopathy of prematurity.
One small trial reported similar numbers of babies with Apgar score less than seven at five minutes in both treatment arms (RR 0.68, 95% CI 0.40 to 1.15; 301 participants; Analysis 1.14).
1.14. Analysis.
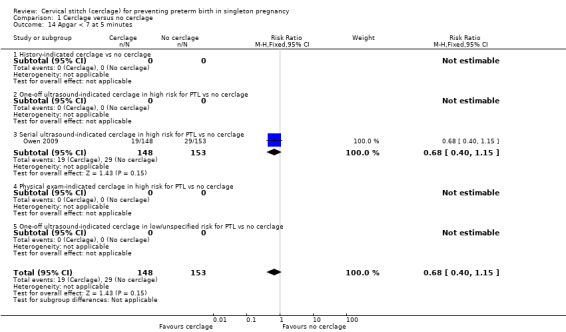
Comparison 1 Cerclage versus no cerclage, Outcome 14 Apgar < 7 at 5 minutes.
1.15 Caesarean section (emergency and elective)
Women with cerclage were more likely to have caesarean sections, although the CI for this result was marginal (RR 1.19, 95% CI 1.01 to 1.40; 8 studies, 2817 participants; Analysis 1.15).
1.15. Analysis.
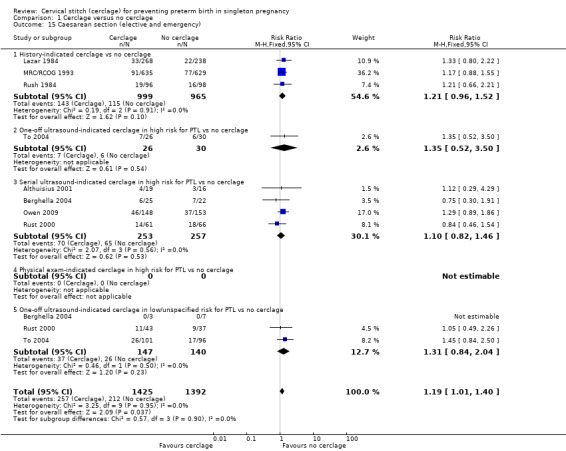
Comparison 1 Cerclage versus no cerclage, Outcome 15 Caesarean section (elective and emergency).
1.16 Maternal side effects
Cervical cerclage was associated with a higher rate of maternal side effects (vaginal discharge and bleeding and pyrexia) although this result did not reach statistical significance and had substantial heterogeneity (average RR 2.25, 95% CI 0.89 to 5.69; 3 studies, 953 participants; I² = 66%; Analysis 1.16). An increased risk of pyrexia appears to be a particular problem, with three trials reporting significantly higher rates in cerclage groups (6% versus 2.4%) (RR 2.39, 95% CI 1.35 to 4.23; 1245 participants; Analysis 1.17).
1.16. Analysis.
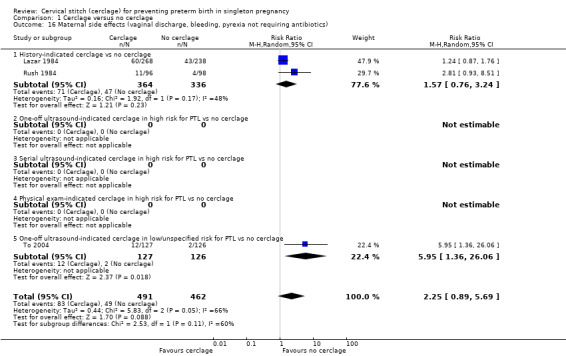
Comparison 1 Cerclage versus no cerclage, Outcome 16 Maternal side effects (vaginal discharge, bleeding, pyrexia not requiring antibiotics).
1.17. Analysis.
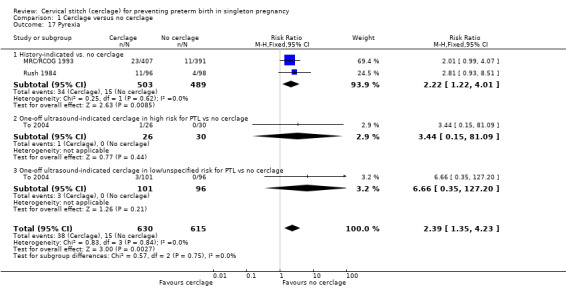
Comparison 1 Cerclage versus no cerclage, Outcome 17 Pyrexia.
Two small trials reported similar numbers of women receiving any intravenous, oral or combined tocolysis in both arms (RR 1.28, 95% CI 0.80 to 2.05; 2 studies, 217 participants; Analysis 1.18).
1.18. Analysis.
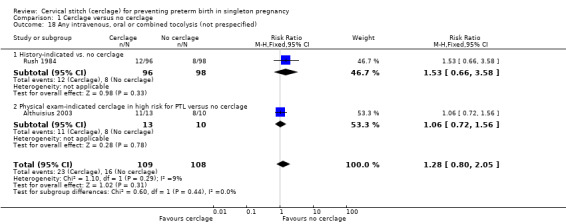
Comparison 1 Cerclage versus no cerclage, Outcome 18 Any intravenous, oral or combined tocolysis (not prespecified).
1.19 Preterm premature rupture of membranes (PPROM) (not prespecified)
There was no evidence of a difference in the rates of PPROM, although this analysis had substantial heterogeneity (average RR 0.96, 95% CI 0.62 to 1.48; 6 studies, 2010 participants; I² = 33%; Analysis 1.19).
1.19. Analysis.
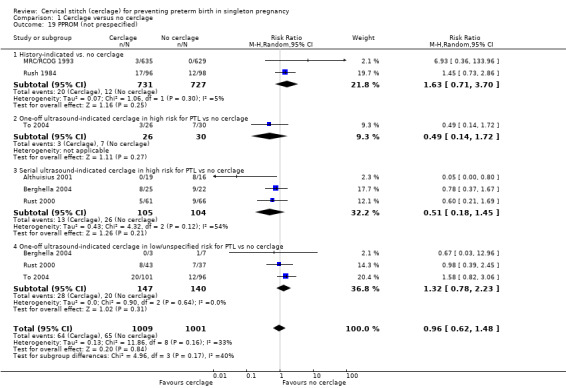
Comparison 1 Cerclage versus no cerclage, Outcome 19 PPROM (not prespecified).
1.20 Chorioamnionitis (not prespecified)
There were similar group rates of chorioamnionitis showing no evidence of benefit of cerclage, with the exception of Althuisius 2001. However, Althuisius 2001 contributed to substantial heterogeneity in the analysis (average RR 0.84, 95% CI 0.26 to 2.72; 3 studies, 1506 participants; I² = 58%; Analysis 1.20).
1.20. Analysis.
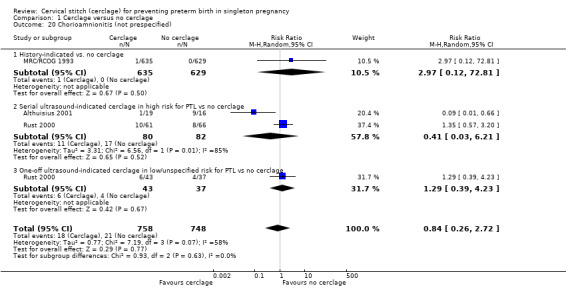
Comparison 1 Cerclage versus no cerclage, Outcome 20 Chorioamnionitis (not prespecified).
Subgroup analysis
Where possible, five potential subgroups were examined: history‐indicated cerclage; one‐off ultrasound‐indicated cerclage in high risk women, serial ultrasound‐indicated cerclage, physical exam‐indicated cerclage (rescue cerclage) and one‐off ultrasound‐indicated cerclage in low or unspecified risk women. There were too few trials in each subgroup to make meaningful conclusions.
Sensitivity analysis
Three studies were assessed as high quality (Berghella 2004; Owen 2009; To 2004) based on adequate reported methods of sequence generation and allocation concealment. Confidence intervals overlapped for estimates of primary outcomes, and conclusions regarding effect estimates for our primary outcomes did not change when trials of worse quality were removed from analyses (data not shown).
Comparison 2. Cerclage versus vaginal progesterone
Chandiramani 2010 compared cerclage and natural progesterone (Cyclogest) in a small randomised study nested in a larger prospective observational study. All pregnant women underwent serial ultrasound, but only those with a history of preterm birth who developed a short cervix (< 25 mm) at less than 24 weeks' gestation were randomised to receive treatment. Ionescu 2012 randomised pregnant women with short cervix (< 25 mm) at 19 to 24 weeks' gestation; this trial was reported as an abstract only, but received additional information and unpublished data through correspondence with the author. Few data per outcome limit the conclusions that can be made for this comparison.
There was considerable heterogeneity for several outcomes in this comparison. Differences in relative effects may be due to the different trial objectives (the primary outcome in Chandiramani 2010 was cervical cytokines); the dose of progesterone also differed (400 mg/day Chandiramani 2010 and 200 mg/day Ionescu 2012).
There were no group differences detected for any review outcome, apart from greater incidence of PPROM in the cerclage arm in a single small trial (N = 92)(Ionescu 2012).
Primary outcomes
2.1 All perinatal losses
Cerclage and progesterone had similar efficacy to prevent perinatal deaths (RR 0.94, 95% CI 0.36 to 2.48; 2 studies, 108 participants; Analysis 2.1).
2.1. Analysis.
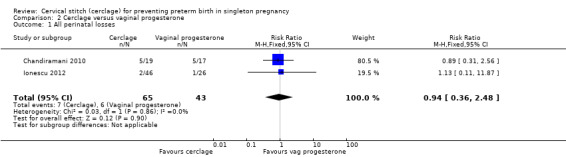
Comparison 2 Cerclage versus vaginal progesterone, Outcome 1 All perinatal losses.
2.2 Serious neonatal morbidity
Two small trials reached different conclusions regarding the relative effect of progesterone on serious morbidity (average RR 0.49, 95% CI 0.05 to 4.52; 2 studies, 120 participants; I² = 84%; Analysis 2.2).
2.2. Analysis.
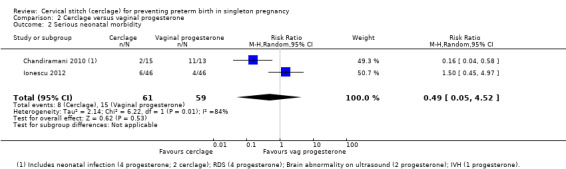
Comparison 2 Cerclage versus vaginal progesterone, Outcome 2 Serious neonatal morbidity.
2.3 Baby discharged home healthy
There were no clear differences in the number of babies who went home healthy (RR 0.97, 95% CI 0.88 to 1.07; 2 studies, 119 participants; Analysis 2.3).
2.3. Analysis.
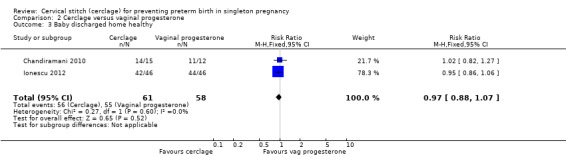
Comparison 2 Cerclage versus vaginal progesterone, Outcome 3 Baby discharged home healthy.
Secondary outcomes
2.4 Stillbirth
There were no treatment group differences detected in rates of stillbirth (RR 2.70, 95% CI 0.12 to 62.17; 2 studies, 128 participants; Analysis 2.4).
2.4. Analysis.
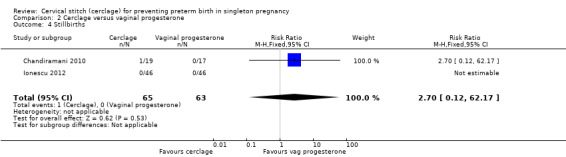
Comparison 2 Cerclage versus vaginal progesterone, Outcome 4 Stillbirths.
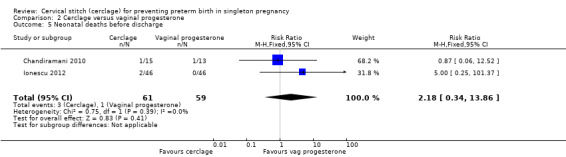
2.5 Neonatal deaths before discharge
There were no treatment group differences detected for rates of neonatal death (RR 2.18, 95% CI 0.34 to 13.86; 2 studies, 120 participants; Analysis 2.5).
2.5. Analysis.
Comparison 2 Cerclage versus vaginal progesterone, Outcome 5 Neonatal deaths before discharge.
2.6 Miscarriages
Similar numbers of pregnant women miscarried in each treatment group (RR 0.58, 95% CI 0.17 to 2.01; 2 studies, 128 participants; Analysis 2.6).
2.6. Analysis.
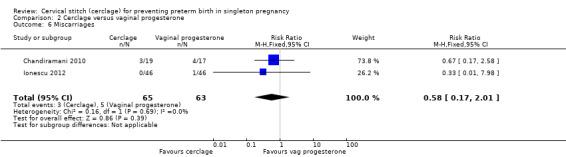
Comparison 2 Cerclage versus vaginal progesterone, Outcome 6 Miscarriages.
2.7 Preterm birth < 37 weeks, 2.8 Preterm birth < 34 weeks, 2.9 Preterm birth < 28 weeks
Data were sparse, and results for preterm birth at all time points showed no evidence of a difference between treatments: preterm birth < 37 weeks (RR 1.16, 95% CI 0.64 to 2.08; 2 studies, 128 participants; Analysis 2.7); preterm birth < 34 weeks (RR 1.01, 95% CI 0.51 to 2.01; 2 studies, 128 participants; Analysis 2.8); preterm birth < 28 weeks (RR 0.92, 95% CI 0.37 to 2.27; 2 studies, 128 participants; Analysis 2.9).
2.7. Analysis.
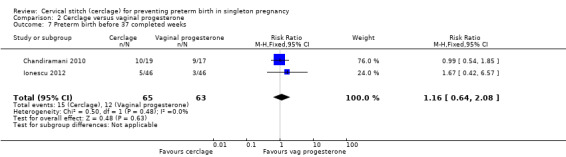
Comparison 2 Cerclage versus vaginal progesterone, Outcome 7 Preterm birth before 37 completed weeks.
2.8. Analysis.
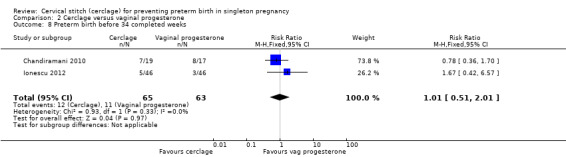
Comparison 2 Cerclage versus vaginal progesterone, Outcome 8 Preterm birth before 34 completed weeks.
2.9. Analysis.
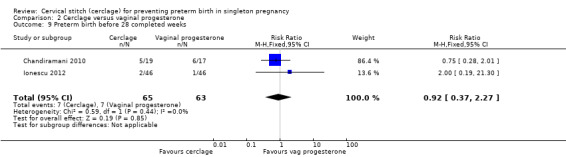
Comparison 2 Cerclage versus vaginal progesterone, Outcome 9 Preterm birth before 28 completed weeks.
There was no evidence of group differences for the following review outcomes: serious intracranial pathology (intraventricular haemorrhage or periventricular leukomalacia: RR 0.96, 95% CI 0.17 to 5.28; 2 studies, 128 participants; Analysis 2.10); serious respiratory morbidity (respiratory distress syndrome or oxygen dependency after 28 days of life (average RR 0.48, 95% CI 0.04 to 6.41; 2 studies, 128 participants; I² = 64%; Analysis 2.11); Apgar less than seven at five minutes (RR 1.90, 95% CI 0.37 to 9.80; 2 studies, 120 participants; Analysis 2.14); caesarean section (average RR 0.67, 95% CI 0.18 to 2.47; 2 studies, 128 participants; I² = 70%; Analysis 2.15); and chorioamnionitis (RR 1.53, 95% CI 0.10 to 23.61; 2 studies, 128 participants; I² = 54%; Analysis 2.21).
2.10. Analysis.
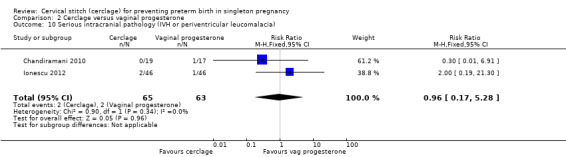
Comparison 2 Cerclage versus vaginal progesterone, Outcome 10 Serious intracranial pathology (IVH or periventricular leucomalacia).
2.11. Analysis.
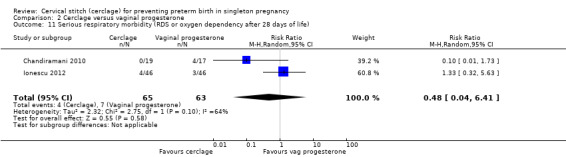
Comparison 2 Cerclage versus vaginal progesterone, Outcome 11 Serious respiratory morbidity (RDS or oxygen dependency after 28 days of life).
2.14. Analysis.
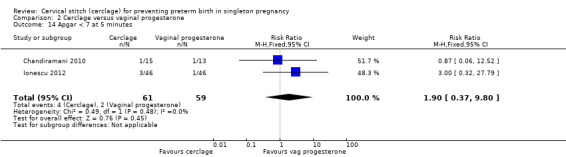
Comparison 2 Cerclage versus vaginal progesterone, Outcome 14 Apgar < 7 at 5 minutes.
2.15. Analysis.
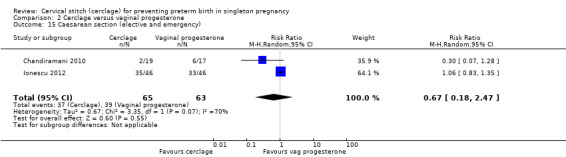
Comparison 2 Cerclage versus vaginal progesterone, Outcome 15 Caesarean section (elective and emergency).
2.21. Analysis.
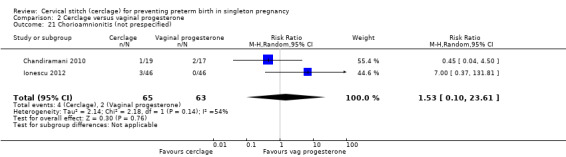
Comparison 2 Cerclage versus vaginal progesterone, Outcome 21 Chorioamnionitis (not prespecified).
Ionescu 2012 reported very few events and no group differences for necrotising enterocolitis (RR 3.00, 95% CI 0.13 to 71.78; 92 participants; Analysis 2.12) and retinopathy of prematurity (RR 1.00, 95% CI 0.06 to 15.51; 92 participants; Analysis 2.13).
2.12. Analysis.
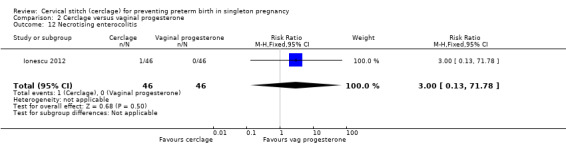
Comparison 2 Cerclage versus vaginal progesterone, Outcome 12 Necrotising enterocolitis.
2.13. Analysis.
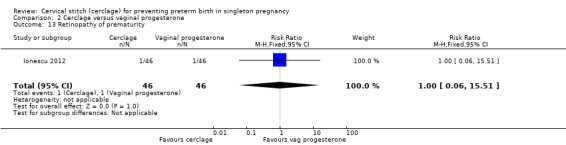
Comparison 2 Cerclage versus vaginal progesterone, Outcome 13 Retinopathy of prematurity.
Ionescu 2012 reported very few maternal side effects (vaginal discharge, bleeding or pyrexia not requiring antibiotics) (RR 3.00, 95% CI 0.32 to 27.79; 92 participants; Analysis 2.17) and no instances of maternal pyrexia in either treatment arm (RR not calculated due to zero events in both arms; 92 participants).
2.17. Analysis.
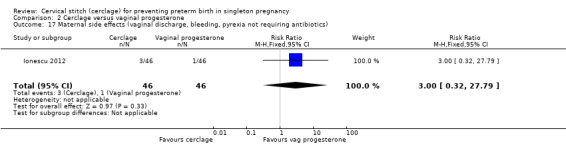
Comparison 2 Cerclage versus vaginal progesterone, Outcome 17 Maternal side effects (vaginal discharge, bleeding, pyrexia not requiring antibiotics).
No trials reported maternal infection requiring intervention (antibiotics or delivery).
Progesterone led to fewer women with preterm premature rupture of membranes, although this result was based on a single trial (Ionescu 2012) with few events and small sample size (RR 8.00, 95% CI 1.04 to 61.42; 92 participants; Analysis 2.20).
2.20. Analysis.
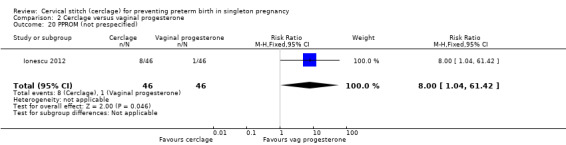
Comparison 2 Cerclage versus vaginal progesterone, Outcome 20 PPROM (not prespecified).
Sensitivity analysis
There were too few studies in this comparison to conduct sensitivity analysis.
Comparison 3. Cerclage versus intramuscular progesterone
Keeler 2009 (79 participants) compared cerclage with weekly intramuscular injections of 17 α‐hydroxyprogesterone caproate in women with a short cervix detected by transvaginal ultrasound scan. The study was interrupted after three years of recruitment because interim analysis did not reveal any obvious differences in obstetric and neonatal outcomes. Therefore the results of this trial must be interpreted with caution (Keeler 2009).
Primary outcomes
3.1 All perinatal losses
There was no evidence of a difference in prevention of perinatal death (RR 1.12, 95% CI 0.58 to 2.16; Analysis 3.1).
3.1. Analysis.
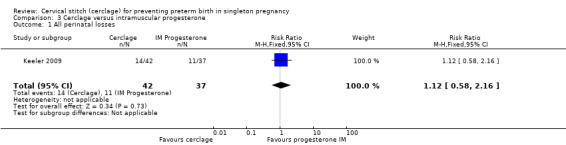
Comparison 3 Cerclage versus intramuscular progesterone, Outcome 1 All perinatal losses.
3.2 Serious neonatal morbidity
There were similar rates of neonatal morbidity in treatment groups (RR 1.13, 95% CI 0.47 to 2.74; Analysis 3.2).
3.2. Analysis.
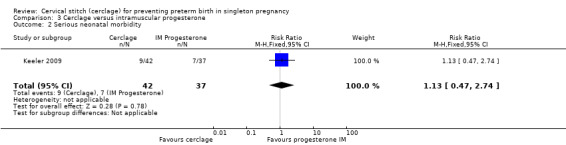
Comparison 3 Cerclage versus intramuscular progesterone, Outcome 2 Serious neonatal morbidity.
3.3 Baby discharged home healthy
Similar numbers of healthy infants were reported in both treatment arms (RR 1.17, 95% CI 0.82 to 1.67; Analysis 3.3).
3.3. Analysis.
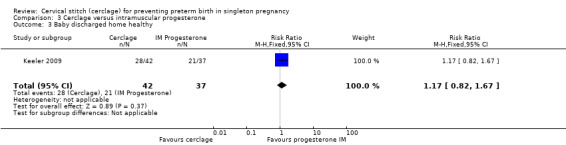
Comparison 3 Cerclage versus intramuscular progesterone, Outcome 3 Baby discharged home healthy.
Secondary outcomes
No trials reported the following secondary outcomes: stillbirth, neonatal death before discharge, preterm birth less than 34 weeks, serious intracranial pathology, serious respiratory morbidity, necrotising enterocolitis, retinopathy of prematurity, Apgar less than seven at five minutes, caesarean section, maternal infection, maternal side effects or maternal pyrexia. Keeler 2009 (79 participants) provided data for the following analyses.
3.6 Miscarriages
There was no clear evidence of an impact on the risk of miscarriage (RR 1.47, 95% CI 0.38 to 5.73; Analysis 3.6).
3.6. Analysis.
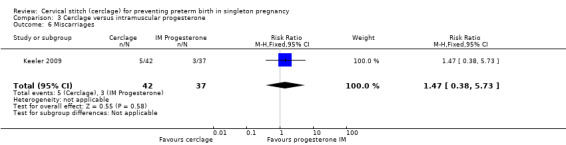
Comparison 3 Cerclage versus intramuscular progesterone, Outcome 6 Miscarriages.
Data were sparse, and results for preterm birth at all time points showed no evidence of a difference between treatments.
3.7 Preterm birth < 37 weeks
Cerclage and intramuscular progesterone were associated with similar risks of preterm birth (RR 0.88, 95% CI 0.60 to 1.30; Analysis 3.7).
3.7. Analysis.
Comparison 3 Cerclage versus intramuscular progesterone, Outcome 7 Preterm birth before 37 completed weeks.
3.9 Preterm birth < 28 weeks
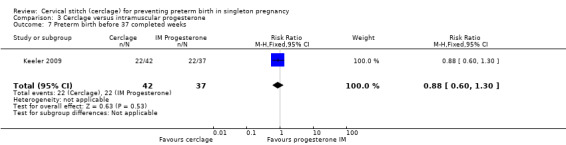
There was no clear evidence of group differences for preterm birth less than 28 weeks, although data were few (RR 1.26, 95% CI 0.53 to 2.97; Analysis 3.9).
3.9. Analysis.
Comparison 3 Cerclage versus intramuscular progesterone, Outcome 9 Preterm birth before 28 completed weeks.
3.19 Preterm premature rupture of membranes
Pregnant women with cerclage and intramuscular progesterone experienced similar rates of preterm premature rupture of membranes (RR 0.88, 95% CI 0.47 to 1.65; Analysis 3.19).
3.19. Analysis.
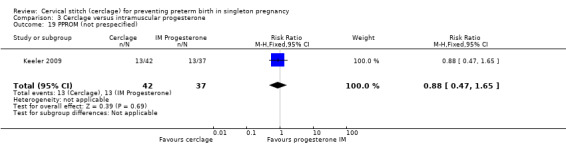
Comparison 3 Cerclage versus intramuscular progesterone, Outcome 19 PPROM (not prespecified).
3.20 Chorioamnionitis
Pregnant women in both treatment groups had similar rates of chorioamnionitis (RR 1.32, 95% CI 0.61 to 2.88; Analysis 3.20).
3.20. Analysis.
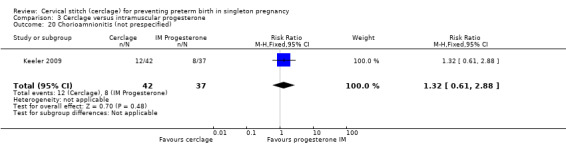
Comparison 3 Cerclage versus intramuscular progesterone, Outcome 20 Chorioamnionitis (not prespecified).
Sensitivity analysis
There were too few studies in this comparison to conduct sensitivity analysis.
Comparison 4. Cerclage versus pessary
There were no included trials eligible for this comparison and therefore no data for any review outcome.
Comparison 5. Comparisons of different cerclage protocols
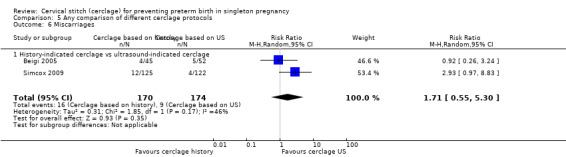
Simcox 2009 and Beigi 2005 compared the benefits of two cerclage protocols in women at high risk of preterm birth. In one group, the indication to perform cerclage was based on previous history, in the other women had cerclage only if the cervix was found to be short on transvaginal ultrasound (≤ 20 mm). The trials were not entirely comparable because only 20% of high risk women in Simcox 2009 received cerclage when assigned to elective management (80% were left untreated). Beigi 2005 treated all women; one arm were treated with elective cerclage and the other arm with serial transvaginal sonography followed by ultrasound‐indicated cerclage. Of the women randomised to this second arm, 54% received cerclage.
There was no significant difference in any of the primary and secondary outcomes in either of these trials. Miscarriage rate was the only prespecified outcome reported by both trials (Analysis 5.6).
5.6. Analysis.
Comparison 5 Any comparison of different cerclage protocols, Outcome 6 Miscarriages.
Sensitivity analysis
Simcox 2009 was assessed as a high‐quality study, but with only two studies included in this comparison, formal sensitivity analysis based on quality was not appropriate.
Comparison 6. Summary of findings outcomes
We include GRADE assessments in our reporting of Comparison 1; the outcomes reported under this comparison are identical to those above in Comparison 1.
Discussion
Summary of main results
The evidence from 15 included randomised trials demonstrated that, compared with expectant management, the placement of cervical cerclage in women at risk of preterm birth reduced risk of preterm birth.
The key issue is whether such prolongation of pregnancy improves the outcome for the baby; there is a distinct possibility that a baby may be better off after an early birth in a setting with adequate neonatal care resources. The difference in all perinatal losses was not established because the upper limit for the 95% confidence interval (CI) for the pooled effect estimate crossed the line of no effect (RR 0.82, 95% CI 0.65 to 1.04; 10 studies, 2927 participants). Women with cerclage and expectant management had a similar rate of serious neonatal morbidity and a similar chance of having a healthy baby at discharge.
The key question regarding long‐term development in terms of neurological and respiratory outcomes was not addressed; most trials did not follow‐up mother and baby after discharge from hospital. Data for short‐term neonatal morbidity are also sparse because of inconsistencies between trials in terms of how this outcome was defined and reported.
In terms of safety, it is clear that cerclage is associated with a higher rate of maternal side effects, especially pyrexia. However, side effects tend to be self‐limiting (vaginal discharge and bleeding) or treatable (pyrexia) and do not appear to put maternal health at risk. The higher rates of caesarean section after cervical cerclage have not been reported previously. This is unsurprising given few participants in primary studies and relatively modest increase in absolute terms (3% absolute risk increase; 95% CI 0.06% to 5.5% increase). The exact mechanism is difficult to establish, but we were mindful that none of the trials was double‐blind. The decision to perform caesarean section is very subjective, and therefore, the knowledge of allocated treatment may have been a significant source of bias. It is possible that cervical cerclage causes damage to the cervix that increases the need for caesarean section. However, we also speculate that increased caesarean section is due to biased (more frequent) diagnosis of failed induction or failure to progress in labour when clinicians know that a woman had cervical cerclage earlier in pregnancy.
We prespecified three clinical scenarios based on the indications for cervical cerclage in current clinical practice:
history‐indicated cerclage ‐ usually because of previous preterm births and sometimes referred to as elective cerclage;
cerclage performed because a short cervix is found on transvaginal sonography (one‐off ultrasound indicated cerclage and serial ultrasound‐indicated cerclage); and
physical exam‐indicated cerclage, also called emergency or rescue cerclage, when symptomatic women are found to have either significant cervical shortening or cervical dilatation detected on vaginal examination (performed digitally or with speculum).
We found four trials of history‐indicated cerclage, five trials of ultrasound‐indicated cerclage and one small trial of physical exam‐indicated cerclage.
Women with previous preterm birth are often extremely anxious in subsequent pregnancies and there are an increasing number of specialist clinics for these women. The issue of prevention is clearly a hot topic, particularly when a cervix is found to be short on transvaginal sonography. Treatment options include daily vaginal pessaries of natural progesterone (Fonseca 2007; Hassan 2011), weekly intramuscular injections of 17 α‐hydroxyprogesterone (Meis 2003), or Arabin pessary (Arabin 2003).
No robust conclusions could be made about cerclage versus alternative interventions such as vaginal and intramuscular progesterone or pessary. Two studies compared cerclage to vaginal progesterone (Chandiramani 2010; Ionescu 2012). These two trials had different objectives (the primary outcome of the Chandiramani 2010 trial was cervical cytokines) and used different dose of progesterone ‐ differences which likely contributed to the significant heterogeneity noted in meta‐analyses.
Only Keeler 2009 attempted to compare ultrasound‐indicated cerclage with 17 α‐hydroxyprogesterone, but this trial was halted prematurely and was too small for any meaningful conclusions to be made. No included trials assessed cerclage versus pessary. These findings underline the necessity of high quality data.
There is also the question of whether it is better to perform a prophylactic procedure electively in early pregnancy, or wait and see if the cervix gets shorter before performing cerclage. Simcox 2009 and Beigi 2005 attempted to answer this question but both studies were quite small and important clinical outcomes were reported inconsistently, precluding meaningful comparisons and conclusions from pooled data. Interestingly, in the Simcox 2009 study only 20% of the women managed without ultrasound scans had cerclage, despite being identified as of high risk. An improved design may have been for women to be randomised only if clinicians were in equipoise whether to perform prophylactic cerclage or wait for ultrasound shortening of the cervix, as was the case in Beigi 2005.
Overall completeness and applicability of evidence
The consistency in the size and direction of effects across all clinical scenarios is reassuring. However, the lack of robust neonatal morbidity data and lack of long‐term follow‐up studies, in particular, are considerable weaknesses. As the data are emerging that natural vaginal progesterone has a more pronounced protective effect for women with a short cervix (Fonseca 2007; Hassan 2011), the role of cervical cerclage in the prevention of preterm birth remains unclear.
There is often a lot of pressure to perform cervical cerclage in early pregnancy as a prophylaxis for women who have experienced late miscarriage in a previous pregnancy. Unfortunately, the results from Simcox 2009 and Beigi 2005 are inconclusive and further similar studies are urgently needed with strict inclusion criteria and firm management protocols.
We were unable to provide what would be considered as definitive evidence regarding benefits, or harms, associated with rescue cerclage, i.e. cerclage performed when women are found to have a dilated cervix in the second trimester of pregnancy. Published observational data are likely to be biased (Pereira 2007), but consenting and randomising this group of patients is very difficult.
Quality of the evidence
Overall, most included trials were at low risk of bias. Selective reporting of the results is always a concern when trial protocols are unavailable for review. We significantly minimised this risk by asking study authors to provide outcome data for prespecified outcomes, including individual patient data if available. It was particularly gratifying that the response was excellent and additional information was provided by Althuisius 2001; Chandiramani 2010; MRC/RCOG 1993; Owen 2009; Rush 1984; Rust 2000 and To 2004.
Performance bias (blinding of personnel and participants) will always be an issue in cerclage trials; it is not practical to blind participants to the type of treatment. However, several key outcomes (perinatal mortality, serious neonatal morbidity) and gestational age at birth are objective and therefore, unlikely to be influenced by lack of blinding.
For the comparison of cerclage versus no cerclage we assessed six primary and secondary outcomes using GRADE methods. Perinatal deaths evidence was assessed as moderate quality (good quality trials and adequate sample size); we downgraded the evidence one level because the confidence interval just crossed 1. We assessed evidence for preterm birth before 34 weeks' gestation to be of high quality. Evidence for baby discharged home healthy was assessed as moderate quality, downgraded one level due to small sample size. Serious neonatal morbidity, neonatal death and stillbirth were all assessed as low quality due to small sample size and wide confidence intervals crossing the line of no effect.
Potential biases in the review process
We followed the proscribed Cochrane methods for reducing bias in the process of writing a systematic review. We conducted a comprehensive search of the literature and have no reason to believe any relevant trials were left out. We completed study selection, appraisal and data extraction in duplicate.
Agreements and disagreements with other studies or reviews
Systematic reviews
An indirect meta‐analysis compared progesterone and cerclage for women with ultrasound‐detected short cervix (< 25 mm), singleton pregnancy and history of preterm birth. Treatments were estimated to be of similar efficacy for preventing preterm birth. Compared with placebo or no cerclage, both interventions reduced the risk of preterm birth before 32 weeks and composite perinatal morbidity and mortality (Conde‐Agudelo 2013).
A recent network meta‐analysis compared use of cerclage, progesterone and pessary. The review included 40 trials and 11,637 women and found pessary ranked best for preterm birth before 37 weeks, followed by progesterone with cerclage not more effective than control. For births before 34 weeks, no single treatment (cerclage, pessary or progesterone) was significantly better than control (Jarde 2016).
An individual patient data meta‐analysis comparing cerclage versus no cerclage in patients at high risk of preterm labour did not demonstrate a statistically significant reduction of perinatal loss in the cerclage group (Jorgensen 2007). Furthermore, the main indication for cerclage (obstetric history versus short cervical length) did not influence the effect estimate for pregnancy loss.
A meta‐analysis by Berghella 2011a compared cerclage versus no cerclage in a subgroup of women with short cervix and previous preterm delivery. Berghella 2011a reported a significant decrease in preterm births in the cerclage group, together with a significant decrease in composite perinatal mortality and morbidity. When considered individually, perinatal mortality and composite morbidity decreased in the cerclage group (perinatal mortality 8.8% versus 13.8% and composite neonatal morbidity 8.2% versus 14.3% respectively), although statistical significance was not achieved. These data were broadly in accordance with our results.
Berghella and colleagues published a separate meta‐analysis comparing history‐indicated cerclage with ultrasound‐indicated cerclage in women at high risk for preterm labour (Berghella 2011b). Berghella 2011b did not identify any differences in terms of preterm birth or perinatal outcomes between management strategies and concluded that women with prior preterm birth may be monitored safely with ultrasound‐indicated cerclage. Berghella 2011b suggested that history‐indicated cerclage should be reserved for women with three prior early preterm births or second‐trimester losses.
Our analysis did not find significant differences in key primary and secondary outcomes; however, we urge caution in interpreting data. Unlike Berghella 2011b, we excluded Kassanos 2001 from our analysis, because this was likely to be a quasi‐randomised study. Data from Althuisius 2001 were not included because primary randomisation was to prophylactic cerclage or no treatment. Two included studies comparing history‐indicated with ultrasound‐indicated cerclage (Beigi 2005; Simcox 2009) are not entirely comparable because in Simcox 2009 only 20% of women randomised to the elective cerclage group received the intervention. For this reason, we feel that it is too premature to conclude that both management strategies are equally safe.
Emergency cerclage
A recent meta‐analysis pooled data on the use of emergency cerclage in pregnant women with singleton pregnancy and cervical dilation of at least 0.5 cm. Evidence comparing cerclage with no cerclage from 10 studies (1 randomised controlled trial and 9 cohort studies) and 757 women showed an association between emergency cerclage and improved neonatal survival as well as prolongation of pregnancy for approximately one month (Ehsanipoor 2015).
A retrospective study of 158 pregnant women receiving emergency cerclage for cervical dilation and bulging membranes (mean gestation 21.45 weeks; SD 2.23) reported that cerclage placement led to live birth for 130/158 women. The study authors compared women with dilation > 3 cm and women with dilation < 3 cm; survival, birthweight and suture‐to‐delivery interval were all greater for women with cervical dilation < 3 cm (Zhu 2015).
Observational evidence
A retrospective, multicentre cohort study examined a specific subset of pregnant women with singleton pregnancy. All included women had a preterm birth before 37 weeks for their first pregnancy. All women had ultrasound‐indicated cerclage for short cervix (< 25 mm) during their second pregnancy. At the third singleton pregnancy, women received either history‐indicated cerclage or transvaginal ultrasound screening. The cohort study compared outcomes from the third pregnancy; 38 women received cervical length screening and 64 women underwent cerclage. Pregnancy outcomes were similar for women managed with either cerclage or ultrasound, but just under half of women receiving ultrasound screening developed short cervix < 25 mm and required cerclage (Suhag 2015).
Khalifeh 2016 argued for a cervical length screening programme for all pregnant women, with cut‐off of < 25 mm as standard; the study authors proposed that such a test is acceptable to women, effective in preventing the prevalent condition of preterm birth, and cost‐effective.
Authors' conclusions
Implications for practice.
Cervical cerclage prevents preterm births, but so does the natural progesterone given vaginally to women with a short cervix, without an increased risk of caesarean section (Romero 2012). However, transvaginal sonography and prolonged treatment with progesterone may not be affordable for all. Also, the progesterone option may be unacceptable to women who have already had a successful pregnancy with cervical cerclage. Therefore, the decision on how best to minimise the risk of recurrent preterm birth in women at risk, either because of poor history or a short or dilated cervix, has to be personalised and based on the clinical circumstances, the skill and expertise of the clinical team and, most importantly, the woman's informed choice.
Implications for research.
Women with a short cervix on transvaginal sonography should be randomised to either cervical cerclage, natural progesterone, neither, or both. It would be important to report separately results for women who had routine transvaginal sonography screening (low risk) and for those who had serial ultrasound scans because of previous preterm birth or other risk factors.
Further randomised data that includes women with a dilated cervix found on physical examination (digital/speculum) would be welcome.
We need definitive studies to ascertain whether it is better for women at particularly high risk of preterm birth to have cervical cerclage early (as prophylaxis), or to have serial transvaginal scanning.
All future studies should have neonatal morbidity as the primary outcome on which sample size calculations should be based. Such studies will have more than adequate power to address the impact on preterm births and most safety aspects. Studies that use gestational age as the primary outcome do so primarily to justify the smaller (more feasible) sample size. It is unlikely that these will have adequate power to answer the key question of whether there is a benefit for mother and baby.
What's new
| Date | Event | Description |
|---|---|---|
| 30 June 2016 | New search has been performed | Search updated and three trials added data to the review (Chandiramani 2010; Ionescu 2012; Althuisius 2003). We added a 'Summary of findings' table with GRADE assessments. |
| 30 June 2016 | New citation required but conclusions have not changed | Conclusions have not changed. There is still a lack of evidence comparing cervical cerclage with cervical pessary or vaginal progesterone. |
Acknowledgements
We are particularly grateful to Dr Sietske Althuisius, Dr Vincenzo Berghella, Dr Orion Rust, Dr RW Rush, Prof Kypros Nicolaides, Dr John Owen and Dr Jeff Szychowski and National Perinatal Epidemiology Unit in Oxford (Dr Sarah Ayers) for providing additional information including unpublished data and access to individual patient databases. We also thank Rachel Tribe and Paul Seed for providing an individual patient data file for Chandiramani 2010 study.
We thank Andrea Jorgensen and Devender Roberts for contributions to previous versions of this review.
Nancy Medley's contribution to this project is supported by the University of Liverpool's Harris‐Wellbeing of Women Preterm Birth Centre research award.
This project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pregnancy and Childbirth. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Data and analyses
Comparison 1. Cerclage versus no cerclage.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All perinatal losses | 10 | 2927 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.65, 1.04] |
| 1.1 History‐indicated cerclage vs no cerclage | 4 | 2045 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.60, 1.12] |
| 1.2 One‐off ultrasound‐indicated cerclage in high risk for PTL vs no cerclage | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.77 [0.14, 4.25] |
| 1.3 Serial ultrasound‐indicated cerclage in high risk for PTL vs no cerclage | 4 | 509 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.66 [0.41, 1.06] |
| 1.4 Physical exam indicated cerclage vs no cerclage | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [0.77, 5.01] |
| 1.5 One‐off ultrasound‐indicated cerclage in low/unspecified risk for PTL vs no cerclage | 3 | 287 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.46, 2.22] |
| 2 Serious neonatal morbidity | 6 | 883 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.57, 1.25] |
| 2.1 History‐indicated cerclage vs no cerclage | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 One‐off ultrasound‐indicated cerclage in high risk for PTL vs no cerclage | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.14, 4.25] |
| 2.3 Serial ultrasound‐indicated cerclage in high risk for PTL vs no cerclage | 4 | 510 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.48, 1.25] |
| 2.4 Physical exam‐indicated cerclage in high risk for PTL vs no cerclage | 1 | 30 | Risk Ratio (M‐H, Random, 95% CI) | 0.22 [0.03, 1.73] |
| 2.5 One‐off ultrasound‐indicated cerclage in low/unspecified risk for PTL vs no cerclage | 3 | 287 | Risk Ratio (M‐H, Random, 95% CI) | 1.38 [0.60, 3.17] |
| 3 Baby discharged home healthy | 4 | 657 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.97, 1.06] |
| 3.1 History‐indicated cerclage vs no cerclage | 1 | 183 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.93, 1.07] |
| 3.2 One‐off ultrasound‐indicated cerclage in high risk for PTL vs no cerclage | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.3 Serial ultrasound‐indicated cerclage in high risk for PTL vs no cerclage | 2 | 238 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.94, 1.14] |
| 3.4 Physical exam‐indicated cerclage in high risk for PTL vs no cerclage | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.5 One‐off ultrasound‐indicated cerclage in low/unspecified risk for PTL vs no cerclage | 1 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.95, 1.08] |
| 4 Stillbirths | 5 | 1803 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.45, 1.75] |
| 4.1 History‐indicated cerclage vs no cerclage | 2 | 1458 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.45, 2.20] |
| 4.2 One‐off ultrasound‐indicated cerclage in high risk for PTL vs no cerclage | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.01, 4.58] |
| 4.3 Serial ultrasound‐indicated cerclage in high risk for PTL vs no cerclage | 2 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 Physical exam‐indicated cerclage in high risk for PTL vs no cerclage | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.5 One‐off ultrasound‐indicated cerclage in low/unspecified risk for PTL vs no cerclage | 2 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.20, 4.59] |
| 5 Neonatal deaths before discharge | 6 | 1714 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.53, 1.39] |
| 5.1 History‐indicated cerclage vs no cerclage | 2 | 1350 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.29, 1.27] |
| 5.2 One‐off ultrasound‐indicated cerclage in high risk for PTL vs no cerclage | 1 | 54 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.15 [0.21, 22.37] |
| 5.3 Serial ultrasound‐indicated cerclage in high risk for PTL vs no cerclage | 2 | 73 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.12, 5.26] |
| 5.4 Physical exam‐indicated cerclage in high risk for PTL vs no cerclage | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.97 [0.77, 5.01] |
| 5.5 One‐off ultrasound‐indicated cerclage in low/unspecified risk for PTL vs no cerclage | 2 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.63 [0.18, 2.18] |
| 6 Miscarriages | 7 | 2091 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.58, 1.22] |
| 6.1 History‐indicated cerclage vs no cerclage | 3 | 1539 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.57, 1.30] |
| 6.2 One‐off ultrasound‐indicated cerclage in high risk for PTL vs no cerclage | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.3 Serial ultrasound‐indicated cerclage in high risk for PTL vs no cerclage | 3 | 209 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.65 [0.25, 1.66] |
| 6.4 Physical exam‐indicated cerclage in high risk for PTL vs no cerclage | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.5 One‐off ultrasound‐indicated cerclage in low/unspecified risk for PTL vs no cerclage | 3 | 287 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.72 [0.16, 18.22] |
| 7 Preterm birth before 37 completed weeks | 9 | 2898 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.69, 0.95] |
| 7.1 History‐indicated cerclage vs no cerclage | 4 | 2045 | Risk Ratio (M‐H, Random, 95% CI) | 0.86 [0.59, 1.27] |
| 7.2 One‐off ultrasound‐indicated cerclage in high risk for PTL vs no cerclage | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.55 [0.30, 0.99] |
| 7.3 Serial ultrasound‐indicated cerclage in high risk for PTL vs no cerclage | 4 | 510 | Risk Ratio (M‐H, Random, 95% CI) | 0.78 [0.60, 1.02] |
| 7.4 Physical exam‐indicated cerclage in high risk for PTL vs no cerclage | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7.5 One‐off ultrasound‐indicated cerclage in low/unspecified risk for PTL vs no cerclage | 3 | 287 | Risk Ratio (M‐H, Random, 95% CI) | 0.80 [0.55, 1.16] |
| 8 Preterm birth before 34 completed weeks | 9 | 2415 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.66, 0.89] |
| 8.1 History‐indicated cerclage vs no cerclage | 3 | 1539 | Risk Ratio (M‐H, Random, 95% CI) | 0.76 [0.40, 1.46] |
| 8.2 One‐off ultrasound‐indicated cerclage in high risk for PTL vs no cerclage | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.63 [0.27, 1.46] |
| 8.3 Serial ultrasound‐indicated cerclage in high risk for PTL vs no cerclage | 4 | 510 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.55, 1.10] |
| 8.4 Physical exam‐indicated cerclage in high risk for PTL vs no cerclage | 1 | 23 | Risk Ratio (M‐H, Random, 95% CI) | 0.56 [0.34, 0.93] |
| 8.5 One‐off ultrasound‐indicated cerclage in low/unspecified risk for PTL vs no cerclage | 3 | 287 | Risk Ratio (M‐H, Random, 95% CI) | 0.82 [0.55, 1.22] |
| 9 Preterm birth before 28 completed weeks | 8 | 2392 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.64, 1.00] |
| 9.1 History‐indicated cerclage vs no cerclage | 3 | 1539 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.59, 1.13] |
| 9.2 One‐off ultrasound‐indicated cerclage in high risk for PTL vs no cerclage | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.18, 2.62] |
| 9.3 Serial ultrasound‐indicated cerclage in high risk for PTL vs no cerclage | 4 | 510 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.48, 1.04] |
| 9.4 Physical exam‐indicated cerclage in high risk for PTL vs no cerclage | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.5 One‐off ultrasound‐indicated cerclage in low/unspecified risk for PTL vs no cerclage | 3 | 287 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.55, 1.83] |
| 10 Serious intracranial pathology (IVH or periventricular leukomalacia) | 5 | 839 | Risk Ratio (M‐H, Random, 95% CI) | 0.83 [0.23, 3.09] |
| 10.1 History‐indicated cerclage vs no cerclage | 1 | 194 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.06, 16.09] |
| 10.2 One‐off ultrasound‐indicated cerclage in high risk for PTL vs no cerclage | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.38 [0.02, 9.01] |
| 10.3 Serial ultrasound‐indicated cerclage in high risk for PTL vs no cerclage | 3 | 382 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.05, 19.53] |
| 10.4 Physical exam‐indicated cerclage in high risk for PTL vs no cerclage | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 10.5 One‐off ultrasound‐indicated cerclage in low/unspecified risk for PTL vs no cerclage | 2 | 207 | Risk Ratio (M‐H, Random, 95% CI) | 0.95 [0.06, 14.98] |
| 11 Serious respiratory morbidity (RDS or oxygen dependency after 28 days of life) | 5 | 839 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.11 [0.66, 1.88] |
| 11.1 History‐indicated cerclage vs no cerclage | 1 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.06 [0.32, 28.93] |
| 11.2 One‐off ultrasound‐indicated cerclage in high risk for PTL vs no cerclage | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.06, 6.00] |
| 11.3 Serial ultrasound‐indicated cerclage in high risk for PTL vs no cerclage | 3 | 382 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.53, 1.81] |
| 11.4 Physical exam‐indicated cerclage in high risk for PTL vs no cerclage | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11.5 One‐off ultrasound‐indicated cerclage in low/unspecified risk for PTL vs no cerclage | 2 | 207 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.63 [0.39, 6.86] |
| 12 Necrotising enterocolitis | 3 | 372 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.16, 4.12] |
| 12.1 History‐indicated cerclage vs no cerclage | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 One‐off ultrasound‐indicated cerclage in high risk for PTL vs no cerclage | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.3 Serial ultrasound‐indicated cerclage in high risk for PTL vs no cerclage | 3 | 362 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.16, 4.12] |
| 12.4 Physical exam‐indicated cerclage in high risk for PTL vs no cerclage | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.5 One‐off ultrasound‐indicated cerclage in low/unspecified risk for PTL vs no cerclage | 1 | 10 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Retinopathy of prematurity | 2 | 553 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.46 [0.14, 1.48] |
| 13.1 History‐indicated cerclage vs no cerclage | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 One‐off ultrasound‐indicated cerclage in high risk for PTL vs no cerclage | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.01, 4.58] |
| 13.3 Serial ultrasound‐indicated cerclage in high risk for PTL vs no cerclage | 1 | 300 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.62 [0.15, 2.53] |
| 13.4 Physical exam‐indicated cerclage in high risk for PTL vs no cerclage | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.5 One‐off ultrasound‐indicated cerclage in low/unspecified risk for PTL vs no cerclage | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.32 [0.01, 7.69] |
| 14 Apgar < 7 at 5 minutes | 1 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.40, 1.15] |
| 14.1 History‐indicated cerclage vs no cerclage | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 One‐off ultrasound‐indicated cerclage in high risk for PTL vs no cerclage | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.3 Serial ultrasound‐indicated cerclage in high risk for PTL vs no cerclage | 1 | 301 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.40, 1.15] |
| 14.4 Physical exam‐indicated cerclage in high risk for PTL vs no cerclage | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.5 One‐off ultrasound‐indicated cerclage in low/unspecified risk for PTL vs no cerclage | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Caesarean section (elective and emergency) | 8 | 2817 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.19 [1.01, 1.40] |
| 15.1 History‐indicated cerclage vs no cerclage | 3 | 1964 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.21 [0.96, 1.52] |
| 15.2 One‐off ultrasound‐indicated cerclage in high risk for PTL vs no cerclage | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.35 [0.52, 3.50] |
| 15.3 Serial ultrasound‐indicated cerclage in high risk for PTL vs no cerclage | 4 | 510 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.10 [0.82, 1.46] |
| 15.4 Physical exam‐indicated cerclage in high risk for PTL vs no cerclage | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.5 One‐off ultrasound‐indicated cerclage in low/unspecified risk for PTL vs no cerclage | 3 | 287 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.84, 2.04] |
| 16 Maternal side effects (vaginal discharge, bleeding, pyrexia not requiring antibiotics) | 3 | 953 | Risk Ratio (M‐H, Random, 95% CI) | 2.25 [0.89, 5.69] |
| 16.1 History‐indicated cerclage vs no cerclage | 2 | 700 | Risk Ratio (M‐H, Random, 95% CI) | 1.57 [0.76, 3.24] |
| 16.2 One‐off ultrasound‐indicated cerclage in high risk for PTL vs no cerclage | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.3 Serial ultrasound‐indicated cerclage in high risk for PTL vs no cerclage | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.4 Physical exam‐indicated cerclage in high risk for PTL vs no cerclage | 0 | 0 | Risk Ratio (M‐H, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 16.5 One‐off ultrasound‐indicated cerclage in low/unspecified risk for PTL vs no cerclage | 1 | 253 | Risk Ratio (M‐H, Random, 95% CI) | 5.95 [1.36, 26.06] |
| 17 Pyrexia | 3 | 1245 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.39 [1.35, 4.23] |
| 17.1 History‐indicated vs. no cerclage | 2 | 992 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.22 [1.22, 4.01] |
| 17.2 One‐off ultrasound‐indicated cerclage in high risk for PTL vs no cerclage | 1 | 56 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.44 [0.15, 81.09] |
| 17.3 One‐off ultrasound‐indicated cerclage in low/unspecified risk for PTL vs no cerclage | 1 | 197 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.66 [0.35, 127.20] |
| 18 Any intravenous, oral or combined tocolysis (not prespecified) | 2 | 217 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.80, 2.05] |
| 18.1 History‐indicated vs. no cerclage | 1 | 194 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.66, 3.58] |
| 18.2 Physical exam‐indicated cerclage in high risk for PTL versus no cerclage | 1 | 23 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.72, 1.56] |
| 19 PPROM (not prespecified) | 6 | 2010 | Risk Ratio (M‐H, Random, 95% CI) | 0.96 [0.62, 1.48] |
| 19.1 History‐indicated vs. no cerclage | 2 | 1458 | Risk Ratio (M‐H, Random, 95% CI) | 1.63 [0.71, 3.70] |
| 19.2 One‐off ultrasound‐indicated cerclage in high risk for PTL vs no cerclage | 1 | 56 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.14, 1.72] |
| 19.3 Serial ultrasound‐indicated cerclage in high risk for PTL vs no cerclage | 3 | 209 | Risk Ratio (M‐H, Random, 95% CI) | 0.51 [0.18, 1.45] |
| 19.4 One‐off ultrasound‐indicated cerclage in low/unspecified risk for PTL vs no cerclage | 3 | 287 | Risk Ratio (M‐H, Random, 95% CI) | 1.32 [0.78, 2.23] |
| 20 Chorioamnionitis (not prespecified) | 3 | 1506 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.26, 2.72] |
| 20.1 History‐indicated vs. no cerclage | 1 | 1264 | Risk Ratio (M‐H, Random, 95% CI) | 2.97 [0.12, 72.81] |
| 20.2 Serial ultrasound‐indicated cerclage in high risk for PTL vs no cerclage | 2 | 162 | Risk Ratio (M‐H, Random, 95% CI) | 0.41 [0.03, 6.21] |
| 20.3 One‐off ultrasound‐indicated cerclage in low/unspecified risk for PTL vs no cerclage | 1 | 80 | Risk Ratio (M‐H, Random, 95% CI) | 1.29 [0.39, 4.23] |
Comparison 2. Cerclage versus vaginal progesterone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All perinatal losses | 2 | 108 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.36, 2.48] |
| 2 Serious neonatal morbidity | 2 | 120 | Risk Ratio (M‐H, Random, 95% CI) | 0.49 [0.05, 4.52] |
| 3 Baby discharged home healthy | 2 | 119 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.97 [0.88, 1.07] |
| 4 Stillbirths | 2 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.7 [0.12, 62.17] |
| 5 Neonatal deaths before discharge | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.18 [0.34, 13.86] |
| 6 Miscarriages | 2 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.58 [0.17, 2.01] |
| 7 Preterm birth before 37 completed weeks | 2 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.64, 2.08] |
| 8 Preterm birth before 34 completed weeks | 2 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.01 [0.51, 2.01] |
| 9 Preterm birth before 28 completed weeks | 2 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.37, 2.27] |
| 10 Serious intracranial pathology (IVH or periventricular leucomalacia) | 2 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.17, 5.28] |
| 11 Serious respiratory morbidity (RDS or oxygen dependency after 28 days of life) | 2 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 0.48 [0.04, 6.41] |
| 12 Necrotising enterocolitis | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 71.78] |
| 13 Retinopathy of prematurity | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.06, 15.51] |
| 14 Apgar < 7 at 5 minutes | 2 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.90 [0.37, 9.80] |
| 15 Caesarean section (elective and emergency) | 2 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 0.67 [0.18, 2.47] |
| 16 Maternal infection requiring intervention(antibiotics or delivery) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Maternal side effects (vaginal discharge, bleeding, pyrexia not requiring antibiotics) | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.32, 27.79] |
| 18 Pyrexia | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 Any intravenous, oral or combined tocolysis (not prespecified) | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.75 [1.93, 7.29] |
| 20 PPROM (not prespecified) | 1 | 92 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.0 [1.04, 61.42] |
| 21 Chorioamnionitis (not prespecified) | 2 | 128 | Risk Ratio (M‐H, Random, 95% CI) | 1.53 [0.10, 23.61] |
2.18. Analysis.
Comparison 2 Cerclage versus vaginal progesterone, Outcome 18 Pyrexia.
2.19. Analysis.
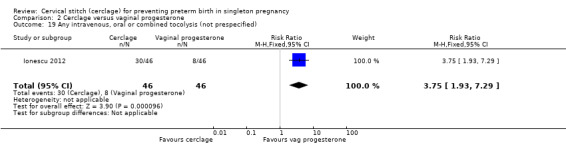
Comparison 2 Cerclage versus vaginal progesterone, Outcome 19 Any intravenous, oral or combined tocolysis (not prespecified).
Comparison 3. Cerclage versus intramuscular progesterone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All perinatal losses | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.58, 2.16] |
| 2 Serious neonatal morbidity | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.13 [0.47, 2.74] |
| 3 Baby discharged home healthy | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.82, 1.67] |
| 4 Stillbirths | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Neonatal deaths before discharge | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Miscarriages | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.47 [0.38, 5.73] |
| 7 Preterm birth before 37 completed weeks | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.60, 1.30] |
| 8 Preterm birth before 34 completed weeks | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Preterm birth before 28 completed weeks | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.26 [0.53, 2.97] |
| 10 Serious intracranial pathology (IVH or periventricular leucomalacia) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Serious respiratory morbidity (RDS or oxygen dependency after 28 days of life) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Necrotising enterocolitis | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Retinopathy of prematurity | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Apgar < 7 at 5 minutes | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Caesarean section (elective and emergency) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Maternal infection requiring intervention(antibiotics or delivery) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Maternal side effects (vaginal discharge, bleeding, pyrexia not requiring antibiotics) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Pyrexia | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 PPROM (not prespecified) | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.88 [0.47, 1.65] |
| 20 Chorioamnionitis (not prespecified) | 1 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.32 [0.61, 2.88] |
Comparison 4. Cerclage versus pessary.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All perinatal losses | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Serious neonatal morbidity | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Baby discharged home healthy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Stillbirths | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5 Neonatal deaths before discharge | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Miscarriages | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7 Preterm birth before 37 completed weeks | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8 Preterm birth before 34 completed weeks | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9 Preterm birth before 28 completed weeks | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10 Serious intracranial pathology (IVH or periventricular leucomalacia) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Serious respiratory morbidity (RDS or oxygen dependency after 28 days of life) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Necrotising enterocolitis | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Retinopathy of prematurity | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Apgar < 7 at 5 minutes | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Caesarean section (elective and emergency) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Maternal infection requiring intervention(antibiotics or delivery) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Maternal side effects (vaginal discharge, bleeding, pyrexia not requiring antibiotics) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Pyrexia | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 19 PPROM (not prespecified) | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 20 Chorioamnionitis | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only |
Comparison 5. Any comparison of different cerclage protocols.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All perinatal losses | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 History‐indicated cerclage vs ultrasound‐indicated cerclage | 1 | 247 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.37 [0.63, 2.96] |
| 2 Serious neonatal morbidity | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 2.1 History‐indicated cerclage vs ultrasound‐indicated cerclage | 1 | 247 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.51, 5.69] |
| 3 Baby discharged home healthy | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.1 History‐indicated cerclage vs ultrasound‐indicated cerclage | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 History‐indicated cerclage vs physical exam‐indicated cerclage | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Stillbirths | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 History‐indicated cerclage vs ultrasound‐indicated cerclage | 1 | 247 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.49 [0.04, 5.31] |
| 5 Neonatal deaths before discharge | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 History‐indicated cerclage vs ultrasound‐indicated cerclage | 1 | 247 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.24 [0.03, 2.15] |
| 5.2 History‐indicated cerclage vs physical exam‐indicated cerclage | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Miscarriages | 2 | 344 | Risk Ratio (M‐H, Random, 95% CI) | 1.71 [0.55, 5.30] |
| 6.1 History‐indicated cerclage vs ultrasound‐indicated cerclage | 2 | 344 | Risk Ratio (M‐H, Random, 95% CI) | 1.71 [0.55, 5.30] |
| 7 Preterm birth before 37 completed weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 7.1 History‐indicated cerclage vs ultrasound‐indicated cerclage | 1 | 97 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.72 [0.25, 2.05] |
| 8 Preterm birth before 34 completed weeks | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 History‐indicated cerclage vs ultrasound‐indicated cerclage | 1 | 247 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.57, 1.87] |
| 9 Preterm birth before 28 completed weeks | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.1 History‐indicated cerclage vs ultrasound‐indicated cerclage | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 9.2 History‐indicated cerclage vs physical exam‐indicated cerclage | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Serious intracranial pathology (IVH or periventricular leucomalacia) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 10.1 History‐indicated cerclage vs ultrasound‐indicated cerclage | 1 | 247 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.95 [0.36, 10.46] |
| 10.2 History‐indicated cerclage vs physical exam‐indicated cerclage | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Serious respiratory morbidity (RDS or oxygen dependency after 28 days of life) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 11.1 History‐indicated cerclage vs ultrasound‐indicated cerclage | 1 | 247 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.46 [0.25, 8.61] |
| 11.2 History‐indicated cerclage vs physical exam‐indicated cerclage | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12 Necrotising enterocolitis | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.1 History‐indicated cerclage vs ultrasound‐indicated cerclage | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 12.2 History‐indicated cerclage vs physical exam‐indicated cerclage | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13 Retinopathy of prematurity | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.1 History‐indicated cerclage vs ultrasound‐indicated cerclage | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 13.2 History‐indicated cerclage vs physical exam‐indicated cerclage | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14 Apgar < 7 at 5 minutes | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.1 History‐indicated cerclage vs ultrasound‐indicated cerclage | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 14.2 History‐indicated cerclage vs physical exam‐indicated cerclage | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15 Caesarean section (elective and emergency) | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.1 History‐indicated cerclage vs ultrasound‐indicated cerclage | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 15.2 History‐indicated cerclage vs physical exam‐indicated cerclage | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 16 Maternal infection requiring intervention(antibiotics or delivery) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 16.1 History‐indicated cerclage vs ultrasound‐indicated cerclage | 1 | 247 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.91] |
| 16.2 History‐indicated cerclage vs physical exam‐indicated cerclage | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 17 Maternal side effects (vaginal discharge, bleeding, pyrexia not requiring antibiotics) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 17.1 History‐indicated cerclage vs ultrasound‐indicated cerclage | 1 | 243 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.54 [0.21, 1.42] |
| 17.2 History‐indicated cerclage vs physical exam‐indicated cerclage | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Tocolysis (not prespecified) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 18.1 History‐indicated cerclage vs ultrasound‐indicated cerclage | 1 | 247 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.44 [0.16, 1.24] |
5.1. Analysis.
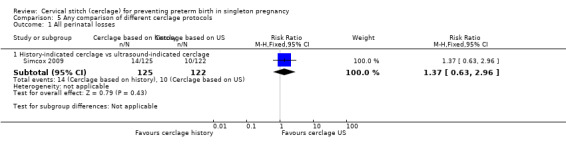
Comparison 5 Any comparison of different cerclage protocols, Outcome 1 All perinatal losses.
5.2. Analysis.
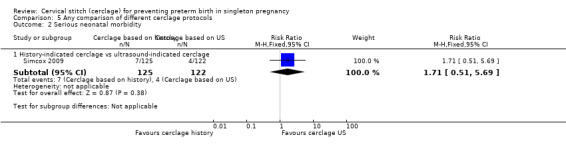
Comparison 5 Any comparison of different cerclage protocols, Outcome 2 Serious neonatal morbidity.
5.4. Analysis.
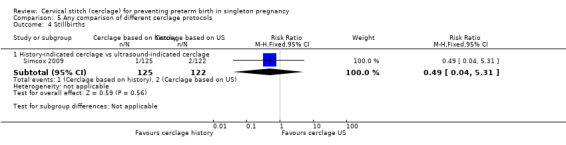
Comparison 5 Any comparison of different cerclage protocols, Outcome 4 Stillbirths.
5.5. Analysis.
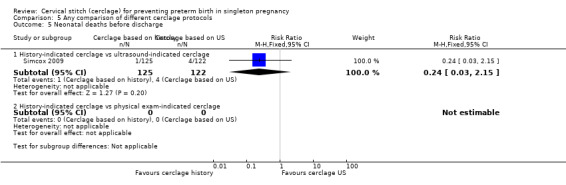
Comparison 5 Any comparison of different cerclage protocols, Outcome 5 Neonatal deaths before discharge.
5.7. Analysis.
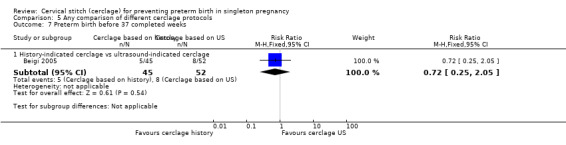
Comparison 5 Any comparison of different cerclage protocols, Outcome 7 Preterm birth before 37 completed weeks.
5.8. Analysis.
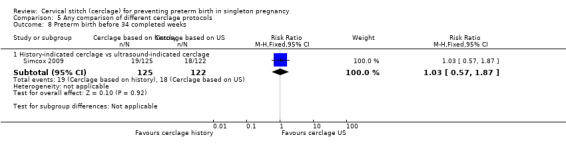
Comparison 5 Any comparison of different cerclage protocols, Outcome 8 Preterm birth before 34 completed weeks.
5.10. Analysis.
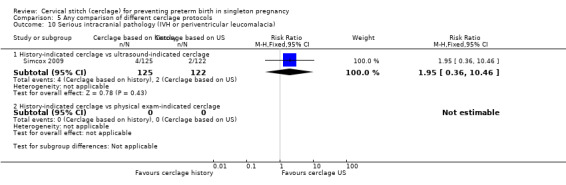
Comparison 5 Any comparison of different cerclage protocols, Outcome 10 Serious intracranial pathology (IVH or periventricular leucomalacia).
5.11. Analysis.
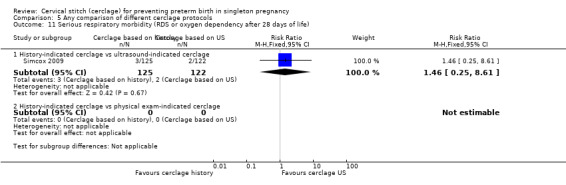
Comparison 5 Any comparison of different cerclage protocols, Outcome 11 Serious respiratory morbidity (RDS or oxygen dependency after 28 days of life).
5.16. Analysis.
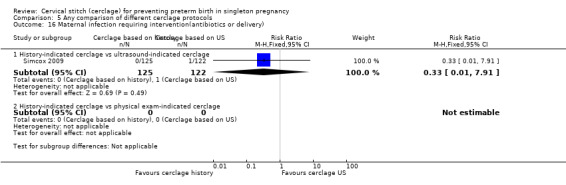
Comparison 5 Any comparison of different cerclage protocols, Outcome 16 Maternal infection requiring intervention(antibiotics or delivery).
5.17. Analysis.
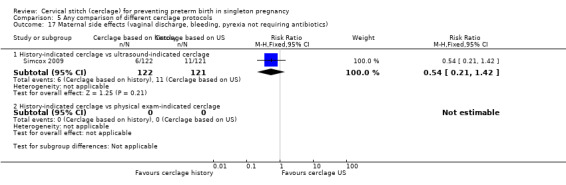
Comparison 5 Any comparison of different cerclage protocols, Outcome 17 Maternal side effects (vaginal discharge, bleeding, pyrexia not requiring antibiotics).
5.18. Analysis.
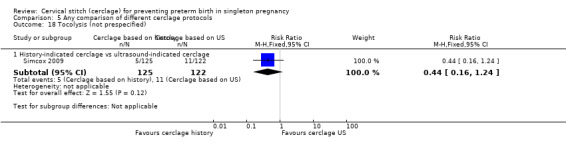
Comparison 5 Any comparison of different cerclage protocols, Outcome 18 Tocolysis (not prespecified).
Comparison 6. Cerclage versus no cerclage (Summary of findings outcomes).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All perinatal losses | 10 | 2927 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.65, 1.04] |
| 2 Serious neonatal morbidity | 6 | 883 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.80 [0.55, 1.18] |
| 3 Baby discharged home healthy | 4 | 657 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.97, 1.06] |
| 4 Stillbirths | 5 | 1803 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.45, 1.75] |
| 5 Neonatal deaths before discharge | 6 | 1714 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.53, 1.39] |
| 6 Preterm birth before 34 completed weeks | 9 | 2415 | Risk Ratio (M‐H, Random, 95% CI) | 0.77 [0.66, 0.89] |
6.1. Analysis.
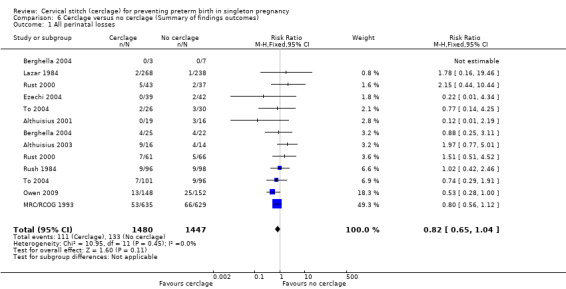
Comparison 6 Cerclage versus no cerclage (Summary of findings outcomes), Outcome 1 All perinatal losses.
6.2. Analysis.
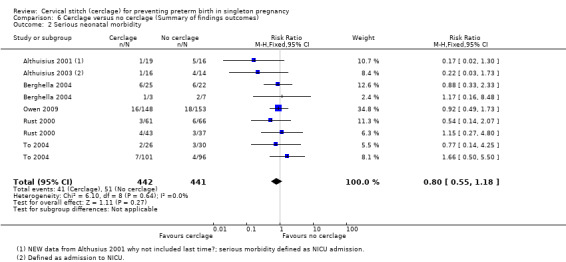
Comparison 6 Cerclage versus no cerclage (Summary of findings outcomes), Outcome 2 Serious neonatal morbidity.
6.3. Analysis.
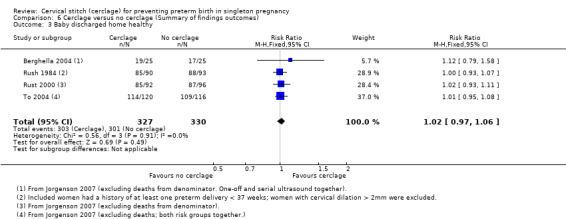
Comparison 6 Cerclage versus no cerclage (Summary of findings outcomes), Outcome 3 Baby discharged home healthy.
6.4. Analysis.
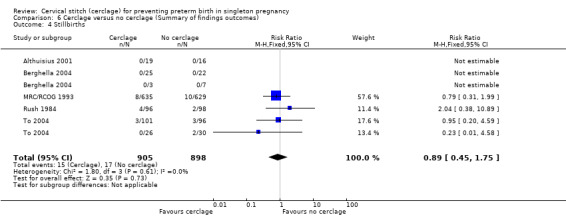
Comparison 6 Cerclage versus no cerclage (Summary of findings outcomes), Outcome 4 Stillbirths.
6.5. Analysis.
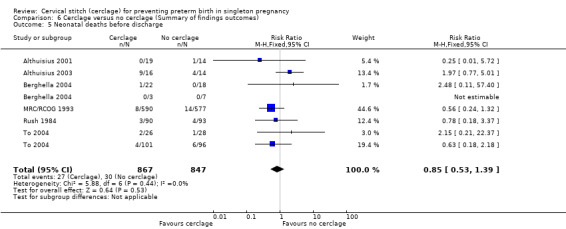
Comparison 6 Cerclage versus no cerclage (Summary of findings outcomes), Outcome 5 Neonatal deaths before discharge.
6.6. Analysis.
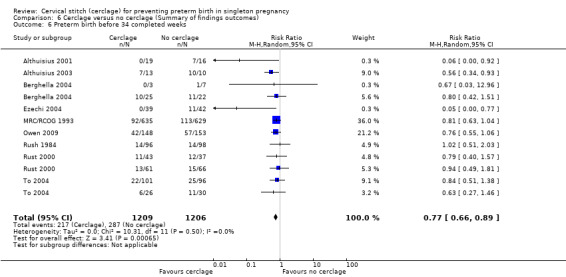
Comparison 6 Cerclage versus no cerclage (Summary of findings outcomes), Outcome 6 Preterm birth before 34 completed weeks.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Althuisius 2001.
| Methods | RCT ‐ block randomisation. July 1995 to July 2000. University Hospital Vrije Universiteit and Olze Lieve Vrouwe Gastus, Amsterdam |
|
| Participants | Eligible participants from 3 populations:
Women randomised and included in this review came from groups I ( N = 18), II (N = 8) and III (N = I0) Inclusion criteria: “high risk of PTL as diagnosed by cervical length of < 25 mm before gestational age of 27 weeks.” “…cervical length was measured by TV US in women with risk factors or symptoms of cervical incompetence” “only patients with singleton pregnancies were included”. Exclusion criteria: women with pregnancies complicated by fetal congenital/chromosomal anomalies, PROM, membranes bulging into the vagina, or intrauterine infection in the current pregnancy were not eligible for trial entry |
|
| Interventions | Therapeutic cerclage (N = 20) with bed rest compared to bed rest only (N = 16). One woman was excluded due to bulging membranes, leaving 19 women in the cerclage group | |
| Outcomes |
Primary: PTL < 34 weeks, neonatal morbidity defined as admission to NICU and/or neonatal death and neonatal survival. Secondary: not stated |
|
| Notes | Additional information and the database for cross‐checking of the published results provided by the first author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Random allocation was stratified for the different inclusion criteria and the 2 participating hospitals and organised in balanced blocks. It is not stated how was the random sequence generated |
| Allocation concealment (selection bias) | Low risk | Telephone randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind for participants and clinicians |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated if outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Any loss of participants to follow‐up at each data collection point:
Any exclusion of participants after randomisation:
Intention‐to‐treat analysis |
| Selective reporting (reporting bias) | Low risk | Full study protocol not available, but prespecified data extraction form provided by authors. Secondary outcome not prespecified in the article |
| Other bias | Low risk | Study was not stopped early. No baseline imbalance |
Althuisius 2003.
| Methods | RCT ‐ block randomisation. July 1995 to July 2000. University Hospital Vrije Universiteit and Olze Lieve Vrouwe Gastus, Amsterdam. This trial recruited women alongside Althuisius 2001 and reported identical methodology |
|
| Participants | Women were recruited at the same time as for Althuisius 2001. For Althuisius 2003, all women were < 27 weeks' gestation and had imminent preterm birth due to cervical incompetence with membranes bulging at or beyond the cervical os. Women were evaluated for trial entry with transvaginal ultrasound and an additional speculum examination when cervical length < 25 mm. Exclusion criteria: signs of infection including fever, uterine tenderness, fetal tachycardia, leukocytosis, and/or elevated C‐reactive protein |
|
| Interventions |
Emergency cerclage (N = 13, 10 singleton and 3 twins): Emergency cerclage (MacDonald) and indomethacin 100 mg suppository 2 hours before and 6 hours after the operation Bed rest (N = 10, 6 singleton and 4 twins) Women in both arms received amoxicillin/clavulanic acid 1 g intravenously every 6 hours and metronidazole 500 mg intravenously every 8 hours for 1 week. All women remained hospitalised and on bed rest until 30 weeks' gestation. Cerclage removed on indication or at 37 weeks' gestation. One woman had membranes rupture during cerclage placement and the intervention was abandoned |
|
| Outcomes | Preterm delivery at < 34 weeks of gestation, compound neonatal morbidity (defined as admission to the neonatal intensive care unit and/or neonatal death), and neonatal survival. We did not include deaths in the review outcome of 'neonatal morbidity' |
|
| Notes | Data from this trial were not included in previous versions of this review. We included women reported here in the 'physical‐exam indicated' subgroup | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Sequence generation not described |
| Allocation concealment (selection bias) | Low risk | Telephone allocation |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated if outcomes assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ITT analysis. No losses to follow‐up |
| Selective reporting (reporting bias) | Low risk | Stated outcomes are reported |
| Other bias | Unclear risk | Demographics at baseline comparable |
Beigi 2005.
| Methods | RCT. January 2001 to September 2003. Arash Maternity Hospital, Tehran University of Medical Sciences |
|
| Participants |
N = 97 Inclusion criteria: “singleton pregnancies with an obstetric history of spontaneous midtrimester loss or early preterm delivery (between 15 and 32 weeks) accompanied by painless and progressive dilatation of cervix and/or PROM without preceding contractions, in the absence of other possible causes of midtrimester loss or early PTD were included”. Exclusion criteria: multiple pregnancies, major fetal defect and intra‐uterine fetal death |
|
| Interventions | Elective cerclage ‐ cerclage placement at 12 to 15 weeks' gestation versus serial transvaginal sonography of the cervix and cerclage only if indicated by cervical changes. Serial TV sonography of the cervix performed every 2 weeks, beginning at 14 weeks' gestation, and were offered an emergency cerclage placement only if the endocervical canal length shortened to 20 mm or less | |
| Outcomes |
Primary: GA at delivery. Secondary: not stated |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Random assignment was performed immediately after inclusion in the trial and women were allocated to receive either an elective cerclage or serial transvaginal sonography of the cervix.” |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind to participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated if outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Any loss of participants to follow‐up at each data collection point:
Any exclusion of participants after randomisation:
Was the analysis intention‐to‐treat?
|
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available. Secondary outcome not prespecified in the article |
| Other bias | Low risk | Study was not stopped early There seemed to be no baseline imbalance |
Berghella 2004.
| Methods | RCT Thomas Jefferson University Hospital from February 1998 until June 2003 and University of Pennsylvania Hospital from February 2002 until June 2003 |
|
| Participants |
Participants (N = 61)
Inclusion criteria
Exclusion criteria
|
|
| Interventions | Cerclage with bed rest | |
| Outcomes |
Primary:
Secondary:
|
|
| Notes | Additional information and the data base for cross‐checking published results provided by the first author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomization included allocation that was accomplished by computer‐generated numbers in permuted blocks of 6.” |
| Allocation concealment (selection bias) | Low risk | “These were concealed in sequentially numbered, opaque, sealed envelopes.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind to participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated if the outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No losses of participants. Describe any exclusion of participants after randomisation:
Although not stated, from numbers, study seems to be intention‐to‐treat |
| Selective reporting (reporting bias) | Low risk | Full study protocol not available, but prespecified data extraction form provided by authors |
| Other bias | Unclear risk | Study was not stopped early.
|
Chandiramani 2010.
| Methods |
|
|
| Participants | 1223 women assessed for eligibility; 112 women enrolled for study. 101 allocated to treatment arms. Inclusion criteria: pregnant women (14 to 24 weeks' gestation) with at least 1 previous preterm delivery and short cervix (< 25 mm) at < 24 weeks' gestation were randomised. Women who did not develop a short cervix served as an additional third arm of controls. We have only included data for randomised women in this review. Exclusion criteria: multiple pregnancy, previous iatrogenic preterm birth, unable to consent |
|
| Interventions | Cerclage versus progesterone; N = 37
|
|
| Outcomes | Cytokine concentrations in the cervico‐vaginal fluid prior to cervical shortening, and before and after the treatment; many obstetric/delivery outcomes were also recorded and not reported in published reports. We obtained data directly from study authors | |
| Notes | Authors: "The study was not designed or powered to directly compare the two treatment groups (e.g. for cytokine concentrations, cervical length or preterm birth), although some exploratory comparisons have been included”. Funding: Action Medical Research and Tommy’s Charity. Results from this study formed the rationale for the NIHR funded SUPPORT trial comparing progesterone, cerclage and pessary. 4 women in the progesterone group received cerclage for bulging membranes. We obtained unpublished individual patient data from the authors for this review from Rachel Tribe, MD. Where events are discrepant between reports, we used data from the data set |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated sequence |
| Allocation concealment (selection bias) | Low risk | Personal communication from authors: allocation concealed in password‐protected database. Investigator performing allocations blind to assignment |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind these interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Laboratory staff were blind to allocation for the principal aims of the study (cytokine concentrations). It is unclear if those collecting delivery data were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 woman excluded from cerclage arm due to incomplete sample collection |
| Selective reporting (reporting bias) | Unclear risk | We obtained unpublished outcome data relevant to this review directly from authors |
| Other bias | Low risk | Personal communication from authors clarified methods and data in published reports |
Ezechi 2004.
| Methods | July 2000 to June 2002. Havana Specialist Hospital Lagos, Nigeria |
|
| Participants |
|
|
| Interventions | Cerclage at 14 weeks of gestation versus no cerclage | |
| Outcomes | GA at delivery, birthweight, neonatal admission and outcome, hospital stay and cumulative hospital bill | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "The women were randomised into cerclage (cases) and non cerclage (controls) after their consent had been obtained." |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind to participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated if outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No description available |
| Selective reporting (reporting bias) | Low risk | Full study protocol not available, but prespecified data extraction form provided by authors. Primary outcome only described in the article |
| Other bias | Unclear risk | No description available |
Ionescu 2012.
| Methods | RCT (abstract only); unpublished data and additional information obtained from authors. Tertiary care obstetrics and gynaecology department at a University Hospital, Romania |
|
| Participants | Women were recruited between 19 and 24 weeks' gestation. Pregnant women had a history of 1 or more previous preterm birth (N = 92 randomised); all women also had short cervix detected with serial TVU (< 25 cm) at 19 to 24 weeks' gestation and were randomised to treatment with cerclage or vaginal progesterone. Singleton pregnancy only. Exclusion criteria: not stated |
|
| Interventions | All women: cerclage (N = 46) and progesterone (N = 46). All women with short cervix: Cerclage (N = 46) treatment Shrodikar cerclage. Progesterone (N = 46) 200 mg/day intravaginal capsule of progesterone |
|
| Outcomes | Mean GA at delivery; preterm birth < 34 weeks; several other unpublished data obtained directly from author | |
| Notes | Study reported in abstract form only. Data reported as percentages only. Mean GA reported without standard deviations in published abstract. All data used in meta‐analyses for this review came directly from trial author. This trial followed 92 women with serial TVU. Of these 92, 90% had 1 previous preterm birth; the remaining had more than 1 previous preterm birth |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Study described as randomised; no further details |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Not described but not possible to blind these interventions |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No loss to follow up described. Unpublished data for all 92 women randomised |
| Selective reporting (reporting bias) | Unclear risk | Not apparent, but study reported in abstract form only. We have obtained unpublished outcome data from authors |
| Other bias | Unclear risk | Baseline characteristics not described; regimen and dose of progesterone not described in abstract but obtained from authors |
Keeler 2009.
| Methods | RCT. November 2003 to December 2006. Lehigh Valley Hospital Perinatal Testing Center. Pennsylvania, USA. |
|
| Participants | Participants (N = 79)
Exclusion criteria
|
|
| Interventions | McDonald cerclage versus weekly intramuscular injections of 17 OHP‐C | |
| Outcomes |
Primary: spontaneous preterm birth prior to 35 weeks' gestation. Secondary: obstetrical complications and neonatal morbidity and mortality.
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Randomisation was accomplished by computer generated assignment…” “The randomisation sequence was secured by administrative stuff until enrolment was terminated.” |
| Allocation concealment (selection bias) | Low risk | “Assignments were concealed in sequentially numbered opaque envelopes by a coordinator not involved in screening, enrolment, or randomisation.” “Randomisation was accomplished by handing out the sequentially numbered opaque envelopes.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | “Due to the intrinsic nature of the study design, there was no masking in this trial.” |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated if outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
|
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Other bias | High risk | Study was stopped early: “We anticipated randomising 160 patients to allow for attrition during the study. However, the trial was stopped early by the authors because 3 years of recruitment, an interim analysis showed no difference in outcome between treatment groups”. No known baseline imbalance |
Lazar 1984.
| Methods | RCT Dates of data collection: not stated. Setting:
|
|
| Participants | N = 506 (268 cerclage, 238 no cerclage) Inclusion criteria “The eligibility of the rest was assessed using a scoring system." The scores were established by points given to two kinds of risk factors: ”permanent” (factors present before the index pregnancy) and “evolving” (factors that appeared or changed during the pregnancy).” “Patients with score ≥ 20 points at the first visit were deemed to be ineligible for the trial. Similarly, low risk patients with scores < 9 at the first or subsequent visits were also deemed to be ineligible. Women became eligible for the entry into the trial as soon as a score of ≥ 9 had been reached, and they remained in the trial whether or not the score subsequently rose to ≥ 20. The target trial population were pregnant women who had a risk of cervical incompetence that was lower than the pregnant women excluded for the following" Exclusion criteria
|
|
| Interventions | Cerclage versus no cerclage | |
| Outcomes | Not specified | |
| Notes | 242/268 women in cerclage arm received cerclage; 26 women in no cerclage arm had cerclage | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “…eligible patients were randomly allocated (using prepared envelopes) into…” |
| Allocation concealment (selection bias) | Unclear risk | “…eligible patients were randomly allocated (using prepared envelopes) into…” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind to participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated if the outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Losses to follow up not reported. Exclusion of participants after randomisation not reported. Analysis appears to be intention‐to‐treat “Of the women entered into the trial, 90% received the management to which they had been allocated.” |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available |
| Other bias | High risk | Study stopped early: “It was decided to conduct a first analysis of the data after about 500 patients had been recruited, and to decide in the light of the results whether or not to pursue the trial. The results reported here are those of the first analysis.” Baseline imbalance: “Women allocated to the cerclage policy, however, were more likely to have had previous abortions. This difference is largely a reflection of a difference between the experimental and control groups in one of the four centres. Although selection bias may have been operating in this centre we have included data derived from cases and controls managed there because analyses conducted after excluding these patients did not make any difference to the conclusions we have reached after analysing data derived from all four centres.” |
MRC/RCOG 1993.
| Methods | RCT ‐ block randomisation. 1981 to 1988. Multicentre – the trial involved more than 200 obstetricians in the UK and 11 other countries: UK, France, Hungary, Norway, Italy, Belgium, Zimbabwe, South Africa, Iceland, Ireland, Netherlands, Canada |
|
| Participants | Participants (N = 1292): twins and singletons. Inclusion criteria: “Women whose obstetricians were uncertain whether to recommend cervical cerclage, most of whom had a history of early delivery or cervical surgery”. Exclusion criteria: not specified |
|
| Interventions | Recommendation to insert suture as soon as possible versus recommendation to avoid the suture | |
| Outcomes |
Primary: length of pregnancy (deliveries < 33 and < 37 weeks); vital status of the baby at the time of completion of the form. Secondary: postpartum pyrexia; causes of fetal/neonatal death; indications for CS; usual technique of cervical cerclage |
|
| Notes | Additional information and the data base for cross‐checking of the published results provided by the authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “Most obstetricians used the randomisation service provided by the Clinical Trial Service Unit in Oxford, but other randomisation centres were established in Hungary, Italy and Zimbabwe.” “Randomisation was organized in balanced blocks, but no prognostic stratification was used.” |
| Allocation concealment (selection bias) | Low risk | “Most women were entered and assigned a random allocation by telephone; a few were registered by post.” “Once basic identifying and descriptive data had been given over the telephone, a random allocation was made to one of two clinical policies.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated if the outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2% participants lost to follow‐up. Not stated if participants were excluded of after randomisation. Analysis intention‐to‐treat analysis: 598/647 in cerclage group received cerclage; 49/645 in no cerclage group received cerclage |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but the authors provided individual data for independent data extraction |
| Other bias | Low risk | Study was not stopped early. No baseline imbalance |
Owen 2009.
| Methods | RCT January 2003 to November 2007. 15 ultrasound clinical centres |
|
| Participants | Participants (N = 302) “Healthy multiparous women carrying a singleton gestation who enrolled for prenatal care were screened to identify those with at least 1 prior spontaneous preterm birth between 17 + 0 and 33 + 6 weeks' gestation.” Inclusion criteria
Exclusion criteria
|
|
| Interventions | Cerclage versus “Women in the no‐cerclage group could receive a physical examination indicated cerclage for acute cervical insufficiency diagnosed on clinical examination”. | |
| Outcomes |
Primary
Secondary
|
|
| Notes | Additional information and data provided by the first author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “Centralized random assignment.” “Randomization in predetermined blocks was stratified by each centre and qualifying cervical length less than 20 mm vs 20‐24 mm.” Stratified randomisation sequence was generated by SAS, permuted in blocks of size 2, 4, and 6. There was a 1:1 cerclage to no‐cerclage allocation ratio throughout. Early in the study the intent to use progesterone stratification was added |
| Allocation concealment (selection bias) | Low risk | Centralised randomisation ‐ via the cerclage web site |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | “Because the cerclage intervention was not masked, managing physicians might infer that the cervical length was less than 25 mm, but they were otherwise masked to the results of the sonographic evaluations except in cases of complete placenta previa, oligohydramnios, or fetal death." Impossible to blind |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | At delivery, randomisation assignment may or may not have been known. There was no attempt to blind at delivery |
| Incomplete outcome data (attrition bias) All outcomes | Low risk |
Analysis was intention‐to‐treat |
| Selective reporting (reporting bias) | Low risk | Study protocol not available, but Cochrane data extraction sheet completed by the authors, so any selective reporting unlikely |
| Other bias | Low risk | Study was not stopped early. Baseline imbalance: 691 participants declined participation; 1044 met initial criteria and consented |
Rush 1984.
| Methods | RCT. 20 January 1979 to 19 April 1982. Reproductive failure clinic at the Groote Schuur Maternity Centre, Peninsula Maternity and Neonatal Service, Capetown, South Africa. Women entered the study at 15 to 21 weeks' gestation. |
|
| Participants | Participants (N = 194): high‐risk women for PTL or late abortion. Inclusion criteria:
Exclusion criteria: age > 35 years; smoking > 5 cigarettes/day; medical disorders (cardiac disease, hypertension, diabetes, thyroid disease); obstetric/gynaecological conditions (recurrent 1st trimester abortions, multiple gestation in present pregnancy, congenital uterine abnormality, uterine fibromyomata, previous cervical surgery – cone biopsy, trachelorrhaphy, cervical cerclage); cervix < 2.0 cm long or dilated at entry |
|
| Interventions | Cervical suture versus no suture | |
| Outcomes | Not stated | |
| Notes | Additional information and the data base for cross‐checking of the published results provided by the first author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “…patients were allocated at random either to have a cervical suture (96 patients) or to be managed without a suture (98 patients) by reference to a series of sealed envelopes.” |
| Allocation concealment (selection bias) | Unclear risk | “…reference to a series of sealed envelopes.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind to participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated if outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Loss of participants not stated. Exclusion of participants after randomisation not stated. Intention‐to‐treat analysis: “All but two of 194 women entered into the trial received the management to which they were allocated.” |
| Selective reporting (reporting bias) | Low risk | Study protocol not available. The full database was provided by the authors, so any selective reporting unlikely |
| Other bias | Low risk | Study was not stopped early. Baseline imbalance: “Although the frequency of two or more previous second trimester abortions or preterm deliveries was somewhat greater in women allocated to cerclage, this difference was not statistically significant.” |
Rust 2000.
| Methods | RCT. May 1998 to August 2000. Lehigh Valley Hospital Outpatient Perinatal Testing Center. USA |
|
| Participants |
Participants (N = 61): “Any patients between the gestational ages of 16 and 24 weeks with transvaginal ultrasound demonstration of (1) dilatation of the internal os, (2) prolapse of the membranes into the endocervical canal but not beyond the external os, (3) a shortened distal cervical length, and (4) exacerbation of these 3 findings associated with transfundal pressure was considered a candidate for enrolment”. Inclusion criteria: “Inclusion criteria consisted of demonstrable dilatation of the internal os and either prolapse of membranes at least 25% of the total cervical length or a distal cervical length of < 2.5 cm”. “Those patients, who met the inclusion criteria and provided informed consent, underwent an amniocentesis to rule out infection.” “A rescue arm of the study was designed for each group. Any patient at < 24 weeks. gestation who had prolapsed membranes beyond the level of the cerclage or to the external os (without cerclage) was offered a revision, or rescue cerclage procedure”. Exclusion criteria: “Exclusion criteria included membrane prolapse beyond the external os, any fetal lethal congenital or chromosomal anomaly, clinical evidence of abruption placenta, unexplained vaginal bleeding, chorioamnionitis (diagnosed by clinical or amniocentesis criteria and confirmed by histopathologic features), persistent uterine activity accompanied by cervical change (consistent with the diagnose of preterm labour), or any other contraindication for a cerclage procedure.” |
|
| Interventions | McDonald cerclage (N = 31) versus no cerclage (N = 30) All prospective participants had indomethacin and clindamycin before randomisation. Women in the cerclage group had indomethacin and clindamycin for 24 h after the cerclage procedure, while women in the no cerclage group had indomethacin and clindamycin stopped at 24 h after randomisation. Women were send home after 24 h and monitored weekly by ultrasound |
|
| Outcomes | Perinatal death, neonatal morbidity according to 4 categories: none (routine neonatal care), minimal (intensive care admission with no mechanical ventilation or serious morbidity), serious (mechanical ventilation, respiratory distress syndrome, necrotizing enterocolitis, intraventricular haemorrhage, sepsis, or other life‐threatening morbidity), and perinatal death (stillborn fetus or death during the first 28 days after birth) | |
| Notes | Additional information and the data base for cross‐checking of the published results provided by the first author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | “If the patients continued to meet inclusion criteria, they were randomly assigned to receive a McDonald cerclage under regional anaesthesia or not cerclage therapy.” |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind to participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated if the outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss of participants:
Exclusion of participants after randomisation:
Intention‐to‐treat analysis: “A rescue arm of the study was designed for each group. Any patient at < 24 weeks' gestation who had prolapsed membranes beyond the level of the cerclage or to the external os (without cerclage) was offered a revision or rescue cerclage procedure. Data were analysed on the basis of intention to treat”. |
| Selective reporting (reporting bias) | Low risk | Study protocol not available. The full database was provided by the authors, so any selective reporting unlikely |
| Other bias | Low risk | Study was not stopped early. No apparent baseline imbalance |
Simcox 2009.
| Methods | RCT. November 2003 to March 2006. 9 UK hospitals |
|
| Participants | Participants (N = 248): pregnant women < 24 weeks of gestation. Inclusion criteria: singleton pregnancy with at least 1 previous spontaneous delivery between 16 + 0 and 34 + 0 weeks. Exclusion criteria: unable to give informed consent |
|
| Interventions | Cerclage based on history “For those women allocated to the history‐indicated arm of the trial, a history‐indicated suture was offered if the treating clinicians considered that the obstetric history justified a cerclage. There were no prescribed minimum criteria for history‐indicated suture insertion. The decision to insert a cerclage or not, based on history, was made in every case before randomisation by the attending clinician, and then carried out if the patient was randomised to history arm” versus Cerclage based on serial US scanning “Women allocated to the scanning arm of the trial underwent cervical length assessment by transvaginal US every 2 weeks from entry into the trial until 24 + 0 weeks of gestation. If the cervix shortened to ≤ 20 mm, a cervical cerclage was inserted.” |
|
| Outcomes |
Primary: PTD before 34 weeks. Secondary: frequency of suture insertion, incidence of histological chorioamnionitis, incidence of maternal pyrexia, hospital admissions, bed rest, use of steroids, tocolysis and progesterone. Neonatal outcomes: need for oxygen therapy at 28 days and US evidence of brain abnormality |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “The randomisation sequence was computer generated in balanced block multiples. Stratification was performed to control for gestation of last delivery before 24 weeks.” |
| Allocation concealment (selection bias) | Low risk | "Allocation was made by telephone to the central trials office in London, UK.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Impossible to blind to participants and personnel |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated if the outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss of participants to follow‐up: “primary outcome data were available on 247/248 women (99.6%)”. Exclusion of participants after randomisation: 5 women were excluded “three were subsequently identified as not fitting eligibility criteria and a further two were excluded from analysis as they elected to terminate the pregnancy after a diagnosis of fetal anomaly”. Intention‐to‐treat analysis: “There were 9 patients who did not receive the randomisation intervention. Eight women in the history arm were scanned” “All analysis was conducted according to the original allocation, following the intention to treat principle." |
| Selective reporting (reporting bias) | Unclear risk | Study protocol not available. All outcomes prespecified in the article were reported |
| Other bias | Low risk | Study was not stopped early Baseline imbalance: “One women in each arm declined a suture.” |
To 2004.
| Methods | RCT ‐ block randomisation. January 1998 to May 2002. “Women with singleton pregnancies undergoing routine antenatal care in 12 hospitals in UK, Brazil, South Africa, Slovenia, Greece and Chile." |
|
| Participants |
N = 253 “Women with singleton pregnancies”, “women attending for the 22‐24 week scan were offered a transvaginal scan to measure cervical length, as a screening test for spontaneous preterm delivery.” Inclusion criteria: “women with a cervical length of 15 mm or less were invited to participate in the randomised study of cervical cerclage”. Exclusion criteria: “women with major fetal abnormalities, painful regular uterine contractions, or history of ruptured membranes and cervical cerclage in situ were excluded from screening, and women with dilatated cervix during screening were excluded from the randomised study.” |
|
| Interventions | Shirodkar cerclage (N = 127) versus no cerclage (N = 126). | |
| Outcomes |
Primary: delivery before 33 completed weeks (231 days) of gestation. Secondary: centile‐adjusted birthweight, stillbirth, and neonatal death or major adverse outcome before discharge from hospital (bronchopulmonary dysplasia, intraventricular or periventricular haemorrhage grade 3 or 4, retinopathy of prematurity, or positive fetal blood culture), maternal morbidity during antenatal hospital stay (fever of 38°C or more on 2 occasions), or symptomatic vaginal discharge." |
|
| Notes | Additional information and the data base for cross‐checking of the published results provided by the first author | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | “the randomisation sequence was computer generated for individual centres in balanced block multiples of ten. These codes were held at a central trials office in London, UK.” |
| Allocation concealment (selection bias) | Low risk | “…allocation was made by telephone.” |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | “Because of the invasive nature of the cervical cerclage, masking of treatment allocation to participants and investigators was not practical in this study." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Not stated if the outcome assessors were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Loss of participants to follow‐up: 1 neonate lost to follow‐up in cerclage group. Exclusion of participants after randomisation: 2 in cerclage group ruptured the membranes. Intention‐to‐treat analysis: 4/127 in cerclage group did not have cerclage; 2/126 in no cerclage group had cerclage |
| Selective reporting (reporting bias) | Low risk | Study protocol available, but primary outcome only specified. The full database was provided by the authors, so any selective reporting unlikely |
| Other bias | Low risk | Baseline imbalance: 470 eligible patients, 217 (54%) declined participation. Women who declined participation did not differ from the study group in their main demographic characteristics and preterm delivery rate (data not shown) |
CS: caesarean section GA: gestational age h: hour NEC: necrotising enterocolitis NICU: neonatal intensive care unit PROM: premature rupture of membranes PPROM: preterm premature rupture of membranes PTB: preterm birth PTD: preterm delivery PTL: preterm labour RCT: randomised controlled trial TA: transabdominal TVU: transvaginal ultrasound US: ultrasound
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Blair 2002 | Outpatient cerclage versus inpatient cerclage |
| Broumand 2011 | Double cerclage versus traditional cerclage |
| Caspi 1990 | Cervical internal os cerclage versus Shirodkar cerclage |
| Dor 1982 | Twin pregnancies |
| Hui 2013 | This trial compared use of Arabin pessary with no treatment for pregnant women with short cervix < 25 mm at 20 to 24 weeks' gestation |
| Ismail 2014 | This protocol described a trial to compare suture types for cervical cerclage |
| Israfil‐Bayli 2014 | This was a feasibility RCT to compare 2 types of suture materials for cervical cerclage |
| Kassanos 2001 | Likely to be quasi‐randomised study: “the patients were randomised to be treated either by elective cerclage or by weekly serial vaginal US (every second patient) with the possibility of an emergency cerclage and were divided into 2 groups.” |
| Nicolaides 2001 | Twin pregnancies |
| Rust 2001 | Multiple gestation |
| Secher 2007 | Protocol for a randomised study comparing double cerclage compared with a single cerclage |
| Tsai 2009 | Double cervical cerclage versus traditional single cervical cerclage |
| Varma 1986 | We have been unable to find any published report to suggest that this proposed study of cerclage was ever carried out. Therefore, we have moved this report from awaiting assessment to excluded studies |
| Von Forster 1986 | Quasi‐randomised study: “patients were divided into 3 groups on the basis of initial letter of their surname.” |
| Zakhera 2015 | The inclusion criteria for this trial was recurrent bleeding in early pregnancy. Women did not have short cervix on US or physical exam or previous history of preterm birth |
| Zolghadri 2014 | This report describes and RCT to compare McDonald cerclage vs a double cerclage method |
| Üçyiğit 2013 | RCT, Compared low vaginal, high vaginal and abdominal cerclage |
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Ragab 2015.
| Methods |
|
| Participants |
|
| Interventions |
Cerclage + progesterone versus progesterone alone (N = 100)
|
| Outcomes | Primary outcome: duration of prolongation of pregnancy, live birth, neonatal morbidity and mortality |
| Notes | Authors contacted to clarify preterm birth outcome data (reported only in the discussion of the paper) and the high number of neonatal deaths in published report (emailed May 2016). We are still awaiting the response ‐ it should be noted that these data, as published, significantly change the result of meta‐analysis for the outcome of neonatal death |
h: hour
Characteristics of ongoing studies [ordered by study ID]
Hezelgrave 2015.
| Trial name or title | SuPPoRT: Stitch, Progesterone or Pessary: a randomised controlled trial The prevention of pre‐term birth in women who develop a short cervix. A multi‐centre randomised controlled trial to compare 3 treatments; cervical cerclage, cervical pessary and vaginal progesterone |
| Methods | 3‐arm randomised controlled trial. Main objective of the trial: for asymptomatic women at risk of preterm birth who develop a short cervix on transvaginal ultrasound scan, which is the optimal preventative strategy; cervical cerclage, arabin pessary or vaginal progesterone? Secondary objectives of the trial: does the success of the intervention depend on early pregnancy biomarker expression? |
| Participants |
Planned number of subjects: 540 Principal inclusion criteria: women with singleton pregnancies who are found to have cervical length < 25 mm on transvaginal ultrasound between 14 + 0 weeks’ gestation (dated by ultrasound or LMP and adjusted for ultrasound estimated date of delivery once ultrasound performed if no miscarriage prior to dating ultrasound) until 23 + 6 weeks’ gestation and 1 or more of the following risk factors.
Principal exclusion criteria:
Any contra‐indications or cautions to the investigational medicinal product including:
|
| Interventions | Cervical cerclage versus progesterone (Cyclogest 200 mg) versus arabin pessary |
| Outcomes |
Primary end point: delivery < 37 completed weeks’ gestation (powered). Timepoint of evaluation of this end point: date of delivery. Secondary end point(s):
|
| Starting date | Ethical approval May 2015 |
| Contact information | Dr Natahsa Hezelgrave, natasha.hezelgrave@gstt.nhs.uk |
| Notes | EudraCT Number: 2015‐000456‐15 Funding: NIHR (UK), Tommy's Charity, Guys and St Thomas' NHS Foundation Trust |
Koulalli 2014.
| Trial name or title | PC‐study. Pessary or Cerclage to Prevent Preterm birth in women with short cervical length and a history preterm birth |
| Methods | Parallel randomised controlled trial |
| Participants |
Target number of participants: 440 Inclusion criteria:
Exclusion criteria:
|
| Interventions | Pessary versus cervical cerclage |
| Outcomes | Primary outcome: preterm birth < 32 weeks' gestation. Secondary outcomes: preterm rate birth before 24, 28, 34 and 37 weeks, time from intervention to delivery, (early) premature rupture of membranes, maternal infection, maternal side effects and composite bad neonatal outcome including both morbidity and mortality rate of children as well as costs |
| Starting date | 2014. End date 2018 |
| Contact information | Dr B Koullali, pc@studies‐obsgyn.nl and Dr E Pajkrt, d.pajkrt@amc.uva.nl |
| Notes |
NTR4415 Sponsor: Academic Medical Center, Amsterdam Funding: ZON‐MW, The Netherlands Organization for Health Research and Development |
Differences between protocol and review
For the 2012 update we replaced 'any preventable perinatal loss' with 'all perinatal losses'. We also added the non‐prespecified outcomes:
Any intravenous, oral or combined tocolysis.
Preterm premature rupture of membranes (PPROM).
Chorioamnionitis.
For the 2017 update, we removed the primary outcome of composite perinatal deaths and serious neonatal morbidity. We were concerned about the possible double counting of babies with serious morbidity who also died. A clearer indicator of efficacy and safety together is whether or not babies go home without serious morbidity. Therefore, we moved the outcome of baby discharged home healthy to primary outcomes. Methods have been updated to current Cochrane Pregnancy and Childbirth standards and a 'Summary of findings' table was added for this update.
Contributions of authors
Nancy Medley assessed reports for inclusion, extracted and entered data, created the SoF table and contributed to writing the text of the review.
Tamara Stampilija assessed reports for inclusion, extracted and entered data, created the SoF table and contributed to writing the text of the review.
Zarko Alfirevic assessed reports for inclusion and contributed to writing the text of the review.
Sources of support
Internal sources
University of Liverpool, UK.
External sources
Harris‐Wellbeing of Women Preterm Birth Centre, UK.
Declarations of interest
Zarko Alfirevic: My employer (University of Liverpool) has received grants from UK National Institute of Health Research, Wellbeing of Women charity and Perkin Elmer to support my research group's work related to preterm birth prevention and my Cochrane editorial work.
Tamara Stampalija: none known.
Nancy Medley: Nancy Medley's work was financially supported by the University of Liverpool's Harris‐Wellbeing of Women Preterm Birth Centre research award.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Althuisius 2001 {published data only}
- Althuisius S, Dekker G, Hummel P, Bedekam D, Kuik D, Geijn H. Cervical incompetence prevention randomized cerclage trial (CIPRACT): effect of therapeutic cerclage with bed rest vs. bed rest only on cervical length. Ultrasound in Obstetrics and Gynecology 2002;20(2):163‐7. [DOI] [PubMed] [Google Scholar]
- Althuisius S, Dekker G, Hummel P, Bekedam D, Geijn H. CIPRACT (cervical incompetence prevention randomized cerclage trial): final results [abstract]. American Journal of Obstetrics and Gynecology 2001;184(1):S2. [DOI] [PubMed] [Google Scholar]
- Althuisius SM, Dekker GA, Hummel P, Bekedam DJ, Geijn HP. Final results of the cervical incompetence prevention randomized cerclage trial (CIPRACT): therapeutic cerclage versus bedrest alone. American Journal of Obstetrics and Gynecology 2001;185(5):1106‐12. [DOI] [PubMed] [Google Scholar]
- Althuisius SM, Dekker GA, Geijn HP, Bekedam DJ, Hummel P. Cervical incompetence prevention randomized cerclage trial, preliminary results. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):S20. [DOI] [PubMed] [Google Scholar]
- Althuisius SM, Dekker GA, Geijn HP, Bekedam DJ, Hummel P. Cervical incompetence prevention randomized cerclage trial (CIPRACT): study design and preliminary results. American Journal of Obstetrics and Gynecology 2000;183(4):823‐9. [DOI] [PubMed] [Google Scholar]
- Bowes WA. Cervical incompetence prevention randomized cerclage trial (CIPRACT): effect of therapeutic cerclage with bed rest vs. bed rest only on cervical length. Obstetrical & Gynecological Survey 2003;58(2):88‐9. [Google Scholar]
Althuisius 2003 {published data only}
- Althuisius S, Dekker G, Hummel P, Geijn H. Cervical incompetence prevention randomized cerclage trial (CIPRACT): emergency cerclage with bed rest versus bed rest alone. American Journal of Obstetrics and Gynecology 2002;187(6 Pt 2):S86. [DOI] [PubMed] [Google Scholar]
- Althuisius SM, Dekker GA, Hummel P, Geijn HPV. Cervical incompetence prevention randomized cerclage trial: emergency cerclage with bed rest versus bed rest alone. American Journal of Obstetrics and Gynecology 2003;189(4):907‐10. [DOI] [PubMed] [Google Scholar]
Beigi 2005 {published data only}
- Beigi A, Zarrinkoub F. Elective versus ultrasound‐indicated cervical cerclage in women at risk for cervical incompetence. Medical Journal of the Islamic Republic of Iran 2005;19(2):103‐7. [Google Scholar]
Berghella 2004 {published data only}
- Berghella V, Odibo A, Tolosa J. Cerclage for prevention of preterm birth with a short cervix on transvaginal ultrasound: a randomized trial [abstract]. American Journal of Obstetrics and Gynecology 2003;189(6 Suppl 1):S167. [DOI] [PubMed] [Google Scholar]
- Berghella V, Odibo AO, Tolosa JE. Cerclage for prevention of preterm birth in women with a short cervix found on transvaginal ultrasound examination: a randomized trial. American Journal of Obstetrics and Gynecology 2004;191(4):1311‐7. [DOI] [PubMed] [Google Scholar]
Chandiramani 2010 {published data only}
- Abbott D, Chin‐Smith E, Seed P, Chandiramani M, Shennan A, Tribe R. Relationship between cervical‐vaginal fluid elafin concentrations and subsequent cervical shortening in women at high risk of spontaneous preterm birth. Reproductive Sciences 2012;19(3 Suppl):73A. [Google Scholar]
- Chandiramani M, Seed P, Shennan AH, Tribe R. Association between cervicovaginal cytokines and fetal fibronectin in women at risk of spontaneous preterm labour. Archives of Disease in Childhood: Fetal & Neonatal Edition 2011;96(Suppl 1):Fa123‐4. [Google Scholar]
- Chandiramani M, Seed PT, Bennett PR, Shennan AH, Tribe RM. Serum progesterone concentrations in women with a previous preterm birth treated with vaginal progesterone supplementation. Reproductive Sciences 2012;19(3 Suppl):189A. [Google Scholar]
- Chandiramani M, Seed PT, Ekbote UV, Orsi NM, Bennett PR, Shennan AH, et al. CLIC: a longitudinal study of inflammatory markers and cervical change in women at risk of spontaneous preterm labour. Archives of Disease in Childhood: Fetal & Neonatal Edition 2010;95(Suppl 1):Fa10. [Google Scholar]
- Chandiramani M, Seed PT, Orsi NM, Ekbote UV, Bennett PR, Shennan AH, et al. Limited relationship between cervico‐vaginal fluid cytokine profiles and cervical shortening in women at high risk of spontaneous preterm birth. PLoS ONE 2012;7(12):e52412. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ezechi 2004 {published data only}
- Ezechi OC, Kalu BKE, Nwokoro CA. Prophylactic cerclage for the prevention of preterm delivery. International Journal of Gynaecology and Obstetrics 2004;85(3):283‐4. [DOI] [PubMed] [Google Scholar]
Ionescu 2012 {published data only}
- Ionescu AC, Gheorghiu D, Pacu I, Davitoiu B, Dimitriu M, Haradja H. Randomized trial of cerclage and progesterone to prevent spontaneous preterm birth in high‐risk women with a short cervix. Journal of Perinatal Medicine 2012;39(Suppl):Abstract 008. [Google Scholar]
Keeler 2009 {published data only}
- Keeler SM, Kiefer D, Rochon M, Quinones JN, Novetsky AP, Rust O. A randomized trial of cerclage vs. 17 alpha‐hydroxyprogesterone caproate for treatment of short cervix. Journal of Perinatal Medicine 2009;37(5):473‐9. [DOI] [PubMed] [Google Scholar]
Lazar 1984 {published data only}
- Lazar P, Gueguen S, Dreyfus J, Renaud R, Pontonnier G, Papiernik E. Multicentred controlled trial of cervical cerclage in women at moderate risk of preterm delivery. British Journal of Obstetrics and Gynaecology 1984;91(8):731‐5. [DOI] [PubMed] [Google Scholar]
MRC/RCOG 1993 {published data only}
- Anonymous. MRC/RCOG randomised trial of cervical cerclage. 23rd British Congress of Obstetrics and Gynaecology; 1983 July 12‐15; Birmingham, UK. 1983:187.
- Anonymous. MRC/RCOG randomised trial of cervical cerclage. 24th British Congress of Obstetrics and Gynaecology;1986 April 15‐18; Cardiff, UK. 1986:268.
- Knight KM, Hackney DN. Re‐evaluation of the subgroup analysis from the Royal College of Obstetricians and Gynaecologists randomized controlled trial of cervical cerclage. Journal of Maternal‐Fetal & Neonatal Medicine 2012;25(6):864‐5. [DOI] [PubMed] [Google Scholar]
- Lampe L [pers comm]. Effectiveness of cervical cerclage before pregnancy [personal communication]. Letter to; National Perinatal Epidemiology Unit, Oxford, UK 16 March 1988.
- MRC/RCOG working party on cervical cerclage. Final report of the Medical Research Council/Royal College of Obstetricians and Gynaecologists multicentre randomised trial of cervical cerclage. British Journal of Obstetrics and Gynaecology 1993;100(6):516‐23. [DOI] [PubMed] [Google Scholar]
- MRC/RCOG working party on cervical cerclage. Interim report of the Medical Research Council/Royal College of Obstetricians and Gynaecologists multicentre randomized trial of cervical cerclage. British Journal of Obstetrics and Gynaecology 1988;95(5):437‐45. [DOI] [PubMed] [Google Scholar]
- Szeverenyi M, Chalmers I, Grant AM, Nagy T, Nagy J, Balogh I, et al. Operative treatment of the cervical incompetency during the pregnancy: judgement of the competence of cerclage operation. Orvosi Hetilap 1992;133:1823‐6. [PubMed] [Google Scholar]
Owen 2009 {published data only}
- Berghella V, Figueroa D, Szychowski J. 17‐Alpha‐hydroxyprogesterone caproate for the prevention of preterm birth in women with prior preterm birth and a short cervical length. American Journal of Obstetrics and Gynecology 2009;201(6 Suppl 1):S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghella V, Figueroa D, Szychowski JM, Owen J, Hankins GD, Iams JD, et al. 17‐alpha‐hydroxyprogesterone caproate for the prevention of preterm birth in women with prior preterm birth and a short cervical length. American Journal of Obstetrics and Gynecology 2010;202(4):351.e1‐351.e6. [DOI: 10.1016/j.ajog.2010.02.019] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berghella V, Szychowski JM, Owen J, Hankins G, Iams JD, Sheffield JS, et al. Suture type and ultrasound‐indicated cerclage efficacy. Journal of Maternal‐Fetal and Neonatal Medicine 2012;25(11):2287‐90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farinelli C, Wing D, Owen J, Szychowski J. Association between body mass index (BMI) and pregnancy outcome in a randomized trial of cerclage for short cervix. Reproductive Sciences 2011;18(3 Suppl 1):305A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farinelli CK, Wing DA, Szychowski JM, Owen J, Hankins G, Iams JD, et al. Association between body mass index and pregnancy outcome in a randomized trial of cerclage for short cervix. Ultrasound in Obstetrics & Gynecology 2012;40(6):669‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa D, Mancuso M, Maddox Paden M, Szychowski J, Owen J. Does mid‐trimester Nugent score or vaginal pH predict gestational age at delivery in women at risk for preterm birth. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S215. [DOI] [PubMed] [Google Scholar]
- Mancuso M, Szychowski J, Owen J. Cervical funnelling: effect on gestational length and ultra‐sound indicated cerclage in high‐risk women. American Journal of Obstetrics and Gynecology 2009;201(6 Suppl 1):S23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso M, Szychowski J, Owen J. Dynamic cervical shortening in high‐risk women: effect on gestational length and ultrasound‐indicated cerclage. American Journal of Obstetrics and Gynecology 2009;203(6 Suppl 1):S130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso MS, Figueroa D, Szychowski JM, Paden MM, Owen J. Midtrimester bacterial vaginosis and cervical length in women at risk for preterm birth. American Journal of Obstetrics and Gynecology 2011;204(4):342.e1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancuso MS, Szychowski JM, Owen J, Hankins G, Iams JD, Sheffield JS, et al. Cervical funneling: effect on gestational length and ultrasound‐indicated cerclage in high‐risk women. American Journal of Obstetrics and Gynecology 2010;203(3):259.e1‐295.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NCT00059683. Vaginal ultrasound cerclage trial. clinicaltrials.gov/ct2/show/NCT00059683 (first received 1 May 2003).
- Owen J. Multicenter randomized trial of cerclage for preterm birth prevention in high‐risk women with shortened mid‐trimester cervical length. American Journal of Obstetrics and Gynecology 2008;199(6 Suppl 1):S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen J, Hankins G, Iams JD, Berghella V, Sheffield JS, Perez‐Delboy A, et al. Multicenter randomized trial of cerclage for preterm birth prevention in high‐risk women with shortened midtrimester cervical length. American Journal of Obstetrics and Gynecology 2009;201(4):375.e1‐375.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen J, Szychowski J. Association between post‐randomization sonographic cervical length and birth gestational age in a multicenter trial of ultrasound‐indicated cerclage. American Journal of Obstetrics and Gynecology 2009;201(6 Suppl 1):S197. [Google Scholar]
- Owen J, Szychowski J. Can the optimal cervical length for placing ultrasound‐indicated cerclage be identified?. American Journal of Obstetrics and Gynecology 2011;204(1 Suppl):S198‐S199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen J, Szychowski J. Does mid‐trimester cervical length 25 mm predict preterm birth in high risk women?. American Journal of Obstetrics and Gynecology 2009;201(6 Suppl 1):S9‐S10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen J, Szychowski J. Neonatal morbidities in a multicenter randomized trial of ultrasound‐indicated cerclage for shortened cervical length (CL). American Journal of Obstetrics and Gynecology 2011;204(1 Suppl 1):S17‐S18. [Google Scholar]
- Owen J, Szychowski J. Scheduled timing of and patient compliance with longitudinal sonographic cervical length (CL) measurement in a multicenter randomized trial of ultrasound‐indicated cerclage. American Journal of Obstetrics and Gynecology 2011;204(1 Suppl):S98. [Google Scholar]
- Silver R. Vaginal ultrasound cerclage trial (ongoing). Evanston Northwestern Healthcare (www.enh.org) (accessed 14 June 2005).
- Szychowski JM, Berghella V, Owen J, Hankins G, Iams JD, Sheffield JS, et al. Cerclage for the prevention of preterm birth in high risk women receiving intramuscular 17‐alpha‐hydroxyprogesterone caproate. Journal of Maternal‐Fetal and Neonatal Medicine 2012;25(12):2686‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szychowski JM, Owen J, Hankins G, Iams J, Sheffield J, Perez‐Delboy A, et al. Timing of mid‐trimester cervical length shortening in high‐risk women. Ultrasound in Obstetrics & Gynecology 2009;33(1):70‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing D, Szychowski J. Influence of earliest gestational age of prior spontaneous birth on subsequent mid‐trimester cervical length, pregnancy duration and cerclage efficacy. American Journal of Obstetrics and Gynecology 2009;201(6 Suppl 1):S190. [Google Scholar]
Rush 1984 {published data only}
- Rush RW, Isaacs S. Prophylactic cervical cerclage and gestational age at delivery. 2nd Conference on Priorities in Perinatal Care; 1983; South Africa. 1983:132‐7.
- Rush RW, Isaacs S, McPherson K, Jones L, Chalmers I, Grant AM. A randomized controlled trial of cervical cerclage in women at high risk of spontaneous preterm delivery. British Journal of Obstetrics and Gynaecology 1984;91(8):724‐30. [DOI] [PubMed] [Google Scholar]
Rust 2000 {published data only}
- Rust O, Atlas R, Jones K, Benham B, Balducci J. A randomized trial of cerclage vs no cerclage in patients with sonographically detected 2nd trimester premature dilation of the internal os. American Journal of Obstetrics and Gynecology 2000;182(1 Pt 2):Ss13. [DOI] [PubMed] [Google Scholar]
- Rust O, Atlas R, Reed J, Gaalen J, Balducci J. Regression analysis of perinatal morbidity for second‐trimester sonographic evidence of internal os dilation and shortening of the distal cervix. American Journal of Obstetrics and Gynecology 2001;184(1):S26. [Google Scholar]
- Rust O, Atlas R, Wells M, Kimmel S. Does cerclage location influence perinatal outcome. American Journal of Obstetrics and Gynecology 2001;185(6 Suppl):S111. [DOI] [PubMed] [Google Scholar]
- Rust O, Larkin R, Roberts W, Quinones J, Rochon M, Reed J, et al. A randomized trial of cerclage versus 17‐hydroxyprogesterone (17p) for the treatment of short cervix. American Journal of Obstetrics and Gynecology 2006;195(6 Suppl 1):S112. [Google Scholar]
- Rust OA, Atlas RO, Jones KJ, Benham BN, Balducci J. A randomized trial of cerclage versus no cerclage among patients with ultrasonographically detected second‐trimester preterm dilatation of the internal os. American Journal of Obstetrics and Gynecology 2000;183(4):830‐5. [DOI] [PubMed] [Google Scholar]
- Rust OA, Atlas RO, Reed J, Gaalen J, Balducci J. Revisiting the short cervix detected by transvaginal ultrasound in the second trimester: why cerclage therapy may not help. American Journal of Obstetrics and Gynecology 2001;185(5):1098‐105. [DOI] [PubMed] [Google Scholar]
- Rust OA, Atlas RO, Wells M, Kimmel S. Second trimester dilatation of the internal os and a history of prior preterm birth [abstract]. Obstetrics and Gynecology 2002;99(4 Suppl):14S. [Google Scholar]
Simcox 2009 {published data only}
- Shennan A, Maternal and Fetal Research Unit (MFRU). CIRCLE study: cerclage in relation to cervical length. www.mfru.org.uk/CIRCLE.htm (accessed 25 February 2004).
- Simcox R, Bennett F, Teoh TG, Shennan AH. A randomised controlled trial of cervical scanning vs history to determine cerclage in high risk women (circle trial). Journal of Obstetrics and Gynaecology 2007;27(Suppl 1):S18. [DOI] [PubMed] [Google Scholar]
- Simcox R, Seed PT, Bennett P, Teoh TG, Poston L, Shennan AH. A randomized controlled trial of cervical scanning vs history to determine cerclage in women at high risk of preterm birth (CIRCLE trial). American Journal of Obstetrics and Gynecology 2009;200(6):623.e1‐623.e6. [DOI] [PubMed] [Google Scholar]
To 2004 {published data only}
- ISRCTN61066532. Randomised controlled trial of cervical cerclage in women with a short cervix identified by routine sonography at 23 weeks of pregnancy. www.isrctn.com/ISRCTN61066532 (accessed prior to 29 May 2017).
- To MS, Alfirevic Z, Heath VCF, Cicero S, Cacho AM, Williamson PR, et al. Cervical cerclage for prevention of preterm delivery in women with short cervix: randomised controlled trial. Lancet 2004;363(9424):1849‐53. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Blair 2002 {published data only}
- Blair O, Fletcher H, Kulkarni S. A randomised controlled trial of outpatient versus inpatient cervical cerclage. Journal of Obstetrics and Gynaecology 2002;22(5):493‐7. [DOI] [PubMed] [Google Scholar]
Broumand 2011 {published data only}
- Broumand F, Bahadori F, Behrouzilak T, Yekta Z, Ashrafi F. Viable extreme preterm birth and some neonatal outcomes in double cerclage versus traditional cerclage: a randomized clinical trial. ScientificWorldJournal 2011;11:1660‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Caspi 1990 {published data only}
- Caspi E, Schneider DF, Mor Z, Langer R, Weinraub Z, Bukovsky I. Cervical internal os cerclage: description of a new technique and comparison with Shirodkar operation. American Journal of Perinatology 1990;7(4):347‐9. [DOI] [PubMed] [Google Scholar]
Dor 1982 {published data only}
- Dor J, Shalev J, Mashiach S, Blankstein J, Serr DM. Elective cervical suture of twin pregnancies diagnosed ultrasonically in the first trimester following induced ovulation. Gynecologic and Obstetric Investigation 1982;13(1):55‐60. [DOI] [PubMed] [Google Scholar]
Hui 2013 {published data only}
- Hui SY, Chor CM, Lau TK, Lao TT, Leung TY. Cerclage pessary for preventing preterm birth in women with a singleton pregnancy and a short cervix at 20 to 24 weeks: a randomized controlled trial. American Journal of Perinatology 2013;30(4):283‐8. [DOI] [PubMed] [Google Scholar]
Ismail 2014 {published data only}
- ISRCTN15373349. Cerclage suture type for an insufficient cervix and its effect on health outcomes (C‐STICH). isrctn.com/ISRCTN15373349 Date first received: 3 December 2014.
Israfil‐Bayli 2014 {published data only}
- ISRCTN17866773. Cerclage outcome by the type of suture material (COTS) study. isrctn.com/ISRCTN17866773 (first received 4 February 2013).
- Israfil‐Bayli F, Toozs‐Hobson P, Lees C, Slack M, Ismail K. Cerclage outcome by the type of suture material (COTS): study protocol for a pilot and feasibility randomised controlled trial. Trials 2014;15(1):415. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kassanos 2001 {published data only}
- Kassanos D, Salamalekis E, Vitoratos N, Panayotopoulos N, Loghis C, Creatsas C. The value of transvaginal ultrasonography in diagnosis and management of cervical incompetence. Clinical and Experimental Obstetrics & Gynecology 2001;28(4):266‐8. [PubMed] [Google Scholar]
Nicolaides 2001 {published data only}
- Nicolaides K. Randomised controlled trial of cervical cerclage in women with twin pregnancies found to have an asymptomatic short cervix. Current Controlled Trials (www.controlled‐trials.com/mrct) (accessed 26 July 2001).
Rust 2001 {published data only}
- Rust O, Atlas R, Wells M, Rawlinson K. Cerclage in multiple gestation with midtrimester dilatation of the internal os [abstract]. American Journal of Obstetrics and Gynecology 2001;185(6 Suppl):S111. [Google Scholar]
Secher 2007 {published data only}
- Brix N, Secher N, McCormack C, Helmig R, Hein M, Weber T, et al. Randomised trial of cervical cerclage, with and without occlusion, for the prevention of preterm birth in women suspected for cervical insufficiency. BJOG: an international journal of obstetrics and gynaecology 2013;120(5):613‐20. [DOI] [PubMed] [Google Scholar]
- Secher NJ, McCormack CD, Weber T, Hein M, Helmig RB. Cervical occlusion in women with cervical insufficiency: protocol for a randomised, controlled trial with cerclage, with and without cervical occlusion. BJOG: an international journal of obstetrics and gynaecology 2007;114(5):649‐e6. [DOI] [PubMed] [Google Scholar]
Tsai 2009 {published data only}
- Tsai YL, Lin YH, Chong KM, Huang LW, Hwang JL, Seow KM. Effectiveness of double cervical cerclage in women with at least one previous pregnancy loss in the second trimester: a randomized controlled trial. Journal of Obstetrics and Gynaecology Research 2009;35(4):666‐71. [DOI] [PubMed] [Google Scholar]
Üçyiğit 2013 {published data only}
- Vousden N, Carter J, Üçyiğit A, Shennan A. Risk of infertility following pre‐conception abdominal cerclage: evidence from a randomised control. BJOG: an international journal of obstetrics and gynaecology 2015;122(Suppl S2):17. [Google Scholar]
- Üçyiğit A, Hezelgrave N, Shennan A. Risk of infertility and abdominal cerclage: Conception following placement in a randomised controlled trial. BJOG: an international journal of obstetrics and gynaecology 2013;120:189. [Google Scholar]
Varma 1986 {published data only}
- Varma TR [pers comm]. To assess further the value of cervical cerclage in pregnancy [personal communication]. Letter to; National Perinatal Epidemiology Unit, Oxford, UK 13 September 1989.
Von Forster 1986 {published data only}
- Forster F, During R, Schwarzlos G. Treatment of cervix incompetence ‐ cerclage versus pessary?. Zentralblatt für Gynäkologie 1986;108:230‐7. [PubMed] [Google Scholar]
Zakhera 2015 {published data only}
- Zakhera M. Cervico‐isthmic cerclage for the treatment of recurrent bleeding in early pregnancy: a randomized clinical trial. International Journal of Gynecology and Obstetrics 2015;131(Suppl 5):E538. [Google Scholar]
Zolghadri 2014 {published data only}
- Zolghadri J, Younesi M, Asadi N, Khosravi D, Behdin S, Tavana Z, et al. Double versus single cervical cerclage for patients with recurrent pregnancy loss: a randomized clinical trial. Journal of Obstetrics and Gynaecology Research 2014;40(2):375‐80. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Ragab 2015 {published data only}
- Ragab A, Mesbah Y. To do or not to do emergency cervical cerclage (a rescue stitch) at 24‐28 weeks gestation in addition to progesterone for patients coming early in labor? A prospective randomized trial for efficacy and safety. Archives of Gynecology and Obstetrics 2015;292(6):1255‐60. [PUBMED: 26041325] [DOI] [PubMed] [Google Scholar]
References to ongoing studies
Hezelgrave 2015 {published data only}
- 2015‐000456‐15. The prevention of pre‐term birth in women who develop a short cervix. A multi‐centre randomised controlled trial to compare three treatments; cervical cerclage, cervical pessary and vaginal progesterone. clinicaltrialsregister.eu/2015‐000456‐15 (first received 11 March 2015).
Koulalli 2014 {published data only}
- NTR4415. Pessary or cerclage to prevent preterm birth in women with short cervical length and a history preterm birth. trialregister.nl/trialreg/admin/rctview.asp?TC=4415 (first received 29 January 2014).
Additional references
Anthony 1997
- Anthony GS, Walker RG, Cameron AD, Price JL, Walker JJ, Calder AA, et al. Trans‐abdominal cervico‐isthmic cerclage in the management of cervical incompetence. European Journal of Obstetrics & Gynecology and Reproductive Biology 1997;72:127‐30. [DOI] [PubMed] [Google Scholar]
Arabin 2003
- Arabin B, Halbesma JR, Vork F, Hubener M, Eyck J. Is treatment with vaginal pessaries an option in patients with a sonographically detected short cervix. Journal of Perinatal Medicine 2003;31:122‐33. [DOI] [PubMed] [Google Scholar]
Berghella 2005
- Berghella V, Odibo AO, To MS, Rust OA, Althuisius SM. Cerclage for short cervix on ultrasonography: meta‐analysis of trials using individual patients level data. Obstetrics & Gynecology 2005;106:181‐9. [DOI] [PubMed] [Google Scholar]
Berghella 2011a
- Berghella V, Rafael TJ, Szychowski JM, Rust OA, Owen J. Cerclage for short cervix on ultrasonography in women with singleton gestation and previous preterm birth. Obstetrics and Gynecology 2011;117(3):663‐71. [DOI] [PubMed] [Google Scholar]
Berghella 2011b
- Berghella V, Mackeen AD. Cervical length screening with ultrasound‐indicated cerclage compared with history indicated cerclage for prevention of preterm birth. Obstetrics & Gynecology 2011;118(1):148‐55. [DOI] [PubMed] [Google Scholar]
Berry 1995
- Berry CW, Brambati B, Eskes TKAB, Exalto N, Fox H, Geraedts JPM, et al. The Euro‐Team Early Pregnancy (ETEP) protocol for recurrent miscarriage. Human Reproduction 1995;1(11):1516‐20. [DOI] [PubMed] [Google Scholar]
Chanrachakul 1998
- Chanrachakul B, Herabutya Y. Emergency cervical cerclage: Ramathibodi Hospital Experience. Journal of the Medical Association of Thailand 1998;1(11):858‐61. [PubMed] [Google Scholar]
Conde‐Agudelo 2013
- Conde‐Agudelo A, Romero R, Nicolaides K, Chaiworapongsa T, O'Brien JM, Cetingoz E, et al. Vaginal progesterone vs cervical cerclage for the prevention of preterm birth in women with a sonographic short cervix, previous preterm birth, and singleton gestation: a systematic review and indirect comparison meta‐analysis. American Journal of Obstetrics and Gynecology 2013;208:42.e1‐18. [DOI: 10.1016/j.ajog.2012.10.877] [DOI] [PMC free article] [PubMed] [Google Scholar]
Devane 2007
- Devane D, Begley CM, Clarke M, Horey D, OBoyle C. Evaluating maternity care: a core set of outcome measures. Birth (Berkeley, Calif.) 2007;34(2):164‐72. [PUBMED: 17542821] [DOI] [PubMed] [Google Scholar]
Drakeley 1998
- Drakeley AJ, Quenby S, Farquharson RG. Mid‐trimester loss‐‐appraisal of a screening protocol. Human Reproduction 1998;13(7):1975‐80. [DOI] [PubMed] [Google Scholar]
Ehsanipoor 2015
- Ehsanipoor RM, Seligman NS, Saccone G, Szymanski LM, Wissinger C, Werner EF, et al. Physical examination‐indicated cerclage: a systematic review and meta‐analysis. Obstetrics & Gynecology 2015;126:125‐35. [DOI: 10.1097/AOG.0000000000000850] [DOI] [PubMed] [Google Scholar]
Fonseca 2007
- Fonseca EB, Celik E, Parra M, Singh M, Nicolaides KH, Fetal Medicine Foundation Second Trimester Screening Group. Progesterone and the risk of preterm birth among women with a short cervix. New England Journal of Medicine 2007;357(5):462‐9. [DOI] [PubMed] [Google Scholar]
Gibb 1995
- Gibb DMF, Salaria DA. Transabdominal cervicoisthmic cerclage in the management of recurrent second trimester miscarriage and pre‐term delivery. British Journal of Obstetrics and Gynaecology 1995;102:802‐6. [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- GRADE Working Group, McMaster University. GRADEpro GDT. Version (accessed prior to 30 May 2017). Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Grant 1989
- Grant A. Cervical cerclage to prolong pregnancy. In: Chalmers I, Enkin M, Keirse MJNC editor(s). Effective Care in Pregnancy and Childbirth. Oxford: Oxford University Press, 1989:633‐46. [Google Scholar]
Hassan 2011
- Hassan SS, Romero R, Vidyadhari D, Fusey S, Baxter JK, Khandelwal M, et al. Vaginal progesterone reduces the rate of preterm birth in women with a sonographic short cervix: a multicenter, randomized, double‐blind, placebo‐controlled trial. Ultrasound in Obstetrics and Gynecology 2011;38(1):18‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Jarde 2016
- Jarde A, Lutsiv O, Park C, Gulmezoglu M, Shah P, Biringer A, et al. Progesterone, cervical cerclage and cervical pessary for primary prevention of preterm birth in high risk singleton pregnancies: a systematic review and network meta‐analysis. American Journal of Obstetrics and Gynecology. 2016; Vol. Suppl:S249. [http://www.ajog.org/article/S0002‐9378(15)01798‐6/pdf]
Jorgensen 2007
- Jorgensen AL, Alfirevic Z, Tudur Smith C, Williamson PR. Cervical stitch (cerclage) for preventing pregnancy loss: individual patient data meta‐analysis. BJOG: an international journal of obstetrics and gynaecology 2007;114(12):1460‐76. [DOI] [PubMed] [Google Scholar]
Khalifeh 2016
- Khalifeh A, Berghella V. Universal cervical length screening in singleton gestations without a previous preterm birth: ten reasons why it should be implemented. American Journal of Obstetrics and Gynecology 2016;214(5):603.e1‐603.e5. [DOI] [PubMed] [Google Scholar]
McDonald 1957
- McDonald IA. Suture of the cervix for inevitable miscarriage. Journal of Obstetrics and Gynaecology of the British Commonwealth 1957;64(3):346‐50. [DOI] [PubMed] [Google Scholar]
McDonald 1980
- McDonald IA. Cervical cerclage. Clinical Obstetrics and Gynecology 1980;7(3):461‐79. [PubMed] [Google Scholar]
Meis 2003
- Meis PJ, Klebanoff M, Thom E, Dombrowski MP, Sibai B, Moawad AH, et al. Prevention of recurrent preterm delivery by 17 alfa‐hydroxyprogesterone caproate. New England Journal of Medicine 2003;348:2379‐85. [DOI] [PubMed] [Google Scholar]
Pereira 2007
- Pereira L, Cotter A, Gómez R, Berghella V, Prasertcharoensuk W, Rasanen J, et al. Expectant management compared with physical examination‐indicated cerclage (EM‐PEC) in selected women with a dilated cervix at 14 (0/7) ‐ 25 (6/7) weeks: results from the EM‐PEC international cohort study. American Journal of Obstetrics and Gynecology 2007;197(5):483.e1‐483.e8. [DOI] [PubMed] [Google Scholar]
Rafael 2014
- Rafael TJ, Berghella V, Alfirevic Z. Cervical stitch (cerclage) for preventing preterm birth in multiple pregnancy. Cochrane Database of Systematic Reviews 2014, Issue 9. [DOI: 10.1002/14651858.CD009166.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Riverius 1658
- Riverius L, Culpeper N, Cole A. On barrenness. The Practice of Physic. London: Peter Cole, 1658. [Google Scholar]
Romero 2012
- Romero R, Nicolaides K, Conde‐Agudelo A, Tabor A, O'Brien JM, Cetingoz E, et al. Vaginal progesterone in women with an asymptomatic sonographic short cervix in the midtrimester decreases preterm delivery and neonatal morbidity: a systematic review and meta analysis of individual patient data. American Journal of Obstetrics and Gynecology 2012;206(2):124.e1‐124.e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
Saling 1984
- Saling E. Prevention of habitual abortion and prematurity by early total occlusion of the external os uteri. European Journal of Obstetrics, Gynecology, and Reproductive Biology 1984;17(2‐3):165‐70. [DOI] [PubMed] [Google Scholar]
Shirodkar 1955
- Shirodkar VN. A new method of operative treatment for habitual abortions in the second trimester of pregnancy. Antiseptic 1955;52:299‐300. [Google Scholar]
Suhag 2015
- Suhag A, Reina J, Sanapo L, Martinelli P, Saccone G, Simonazzi G, et al. Prior ultrasound‐indicated cerclage: comparison of cervical length screening or history‐indicated cerclage in the next pregnancy. Obstetrics and Gynecology 2015;126(5):962‐8. [DOI] [PubMed] [Google Scholar]
van 't Hooft 2016
- ʼt Hooft J, Duffy JM, Daly M, Williamson PR, Meher S, Thom E, et al. Global Obstetrics Network. A core outcome set for evaluation of interventions to prevent preterm birth. Obstetrics and Gynecology 2016;127(1):49‐58. [DOI: 10.1097/AOG.0000000000001195] [DOI] [PMC free article] [PubMed] [Google Scholar]
Wong 1993
- Wong GP, Farquharson DF, Dansereau J. Emergency cerclage: a retrospective review of 51 cases. American Journal of Perinatology 1993;10(5):341‐7. [DOI] [PubMed] [Google Scholar]
Zhu 2015
- Zhu LQ, Chen H, Chen LB, Liu YL, Tian JP, Wang YH, et al. Effects of emergency cervical cerclage on pregnancy outcome: a retrospective study of 158 cases. Medical Science Monitor 2015;21:1395‐401. [DOI: 10.12659/MSM.893244] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Alfirevic 2012
- Alfirevic Z, Stampalija T, Roberts D, Jorgensen AL. Cervical stitch (cerclage) for preventing preterm birth in singleton pregnancy. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD008991.pub2] [DOI] [PubMed] [Google Scholar]
Drakeley 2003
- Drakeley AJ, Roberts D, Alfirevic Z. Cervical stitch (cerclage) for preventing pregnancy loss in women. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD003253] [DOI] [PMC free article] [PubMed] [Google Scholar]


