Abstract
Background
Although routine administration of pharmacologic sedation or analgesia during mechanical ventilation in preterm neonates is not recommended, its use in clinical practice remains common. Alpha‐2 agonists, mainly clonidine and dexmedetomidine, are used as adjunctive (or alternative) sedative agents alongside opioids and benzodiazepines. Clonidine has not been systematically assessed for use in neonatal sedation during ventilation.
Objectives
To assess whether clonidine administered to term and preterm newborn infants receiving mechanical ventilation reduces morbidity and mortality rates. To compare the intervention versus placebo, no treatment, and dexmedetomidine; and to assess the safety of clonidine infusion for potential harms.
To perform subgroup analyses according to gestational age; birth weight; administration method (infusion or bolus therapy); dose, duration, and route of clonidine administration; and pharmacologic sedation as a co‐intervention.
Search methods
We used the standard search strategy of the Cochrane Neonatal Review Group to search the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 12) in the Cochrane Library, MEDLINE via PubMed (1966 to January 10, 2017), Embase (1980 to January 10, 2017), and the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to January 10, 2017). We also searched clinical trials databases, conference proceedings, and the reference lists of retrieved articles for randomized controlled trials and quasi‐randomized trials.
Selection criteria
We searched for randomized controlled trials, quasi‐randomized controlled trials, and cluster trials comparing clonidine versus placebo, no treatment, or dexmedetomidine administered to term and preterm newborns receiving mechanical ventilation via an endotracheal tube.
Data collection and analysis
For the included trial, two review authors independently extracted data (e.g. number of participants, birth weight, gestational age, all‐cause death during initial hospitalization, duration of respiratory support, sedation scale, duration of hospital stay) and assessed risk of bias (e.g. adequacy of randomization, blinding, completeness of follow‐up). This review considered primary outcomes of all‐cause neonatal death, all‐cause death during initial hospitalization, and duration of mechanical ventilation in days.
Main results
One trial, which included 112 infants, met the inclusion criteria for this review. Term newborn infants on mechanical ventilation with the need for continuous analgesia and sedation with fentanyl and midazolam were eligible for enrollment during the first 96 hours of ventilation. Study authors administered clonidine 1 μg/kg/h or placebo on day 4 after intubation.
We found no differences between the two groups in all‐cause death during hospitalization (risk ratio [RR] 0.69, 95% confidence interval [CI] 0.12 to 3.98). The quality of the evidence supporting these findings is low owing to imprecision of the estimates (one study; few events). The median (interquartile range) duration of mechanical ventilation was 7.1 days (5.7 to 9.1 days) in the clonidine group and 5.8 days (4.9 to 7.9 days) in the placebo group, respectively (P = 0.070). Among secondary outcomes, we found no differences in terms of duration of stay in the intensive care unit. Sedation scale values (COMFORT) and analgesia scores (Hartwig) during the first 72 hours of infusion of study medication were lower in the clonidine group than in the placebo group.
Authors' conclusions
At present, evidence is insufficient to show the efficacy and safety of clonidine for sedation and analgesia in term and preterm newborn infants receiving mechanical ventilation.
Keywords: Humans; Infant, Newborn; Clonidine; Clonidine/administration & dosage; Clonidine/adverse effects; Dexmedetomidine; Dexmedetomidine/administration & dosage; Dexmedetomidine/adverse effects; Hypnotics and Sedatives; Hypnotics and Sedatives/administration & dosage; Hypnotics and Sedatives/adverse effects; Respiration, Artificial; Cause of Death; Hospital Mortality; Intensive Care Units, Neonatal; Intensive Care Units, Neonatal/statistics & numerical data; Length of Stay; Length of Stay/statistics & numerical data; Randomized Controlled Trials as Topic
Plain language summary
Clonidine for neonates receiving mechanical ventilation
Review question: Does clonidine reduce mortality and the duration of mechanical ventilation in term and preterm newborn infants?
Background: Although routine pharmacologic sedation or analgesia during mechanical ventilation in preterm neonates is not recommended, its use in clinical practice remains common. Clonidine may be used as an adjunctive (or alternative) sedative agent alongside other opioids and benzodiazepines. This review reported and critically analyzed available evidence on the effectiveness of clonidine in term and preterm newborn infants on a ventilator.
Study characteristics: In medical literature searches completed to January 2017, we identified and included one trial with 112 newborns comparing clonidine with placebo.
Study funding resources: We did not identify funding by industry for the included trial.
Results: Clonidine did not reduce death, duration of mechanical ventilation, or duration of stay in the intensive care unit. Sedation and pain scale values were lower among newborns receiving clonidine.
Conclusions: Owing to the small number of newborns included in the single included trial, we are uncertain as to whether clonidine is effective or safe in providing analgesia and sedation for mechanically ventilated neonates.
Summary of findings
Summary of findings for the main comparison. Clonidine versus placebo for neonates receiving mechanical ventilation.
| Clonidine versus placebo for neonates receiving mechanical ventilation | ||||||
| Patient or population: neonates receiving mechanical ventilation Settings: neonatal intensive care unit Intervention: clonidine vs placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Control | Clonidine vs placebo | |||||
| All‐cause mortality during initial hospitalization | Study population | RR 0.69 (0.12 to 3.98) | 112 (1 study) | ⊕⊕⊝⊝ lowa | Downgraded for imprecision: 1 study identified with few events | |
| *The basis for the assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
aOne study and few events
Background
Description of the condition
Neonatal pain was poorly understood and often unrecognized until the 1980s, when research describing the developmental physiology of nociception and adverse responses of neonates to noxious stimuli emerged (Anand 1987a; Anand 1987b). Despite early maturation of ascending neural pathways responsible for nociception, descending inhibitory pathways, which localize and mitigate pain, do not form until later in maturation (Fitzgerald 1986). Moreover, normal brain development is abruptly interrupted by preterm birth, which results in unique susceptibility to neurologic remodeling after repetitive noxious stimuli (Taddio 2009). Despite growing knowledge about long‐term consequences of neonatal pain and discomfort, consensus has not been reached regarding a safe and effective strategy for controlling these complications in many routine clinical situations.
Mechanical ventilation is a common stressful experience among preterm neonates (Hall 2007). Non‐pharmacologic therapies, including non‐nutritive sucking and swaddling, form the foundation for relief of neonatal pain and agitation, but in many cases, pharmacologic support is needed to provide comfort during invasive ventilation (Golianu 2007). Although routine administration of pharmacologic sedation or analgesia during mechanical ventilation in preterm neonates is not recommended, use of benzodiazepines and opiates in clinical practice remains common because alternative therapies are lacking (Clark 2006; Kumar 2008). Benzodiazepines have no analgesic effect, and data from two randomized controlled trials (RCTs) show that midazolam may increase the incidence of brain injury (Anand 1999; Jacqz‐Aigrain 1994). Furthermore, the Cochrane review titled "Intravenous midazolam infusion for sedation of infants in the neonatal intensive care unit" reported controversial data on the neurologic effects of midazolam, raising questions regarding the safety of this drug (Ng 2017). Additionally, studies in rodent models have shown widespread neuroapoptosis and suppressed neurogenesis elicited by early benzodiazepine exposure (Stefovska 2008; Young 2005).
Morphine and fentanyl are the opiates most commonly utilized in neonates (Clark 2006; Kumar 2008). Three large RCTs examined the impact of morphine on acute brain injury in mechanically ventilated preterm neonates (Anand 1999; Anand 2004; Simons 2003). The first ‐ the Neonatal Outcome and Prolonged Analgesia in Neonates (NOPAIN) trial ‐ demonstrated that the incidence of the composite outcome of severe intraventricular hemorrhage (IVH), periventricular leukomalacia (PVL), or death was decreased in the morphine group (4%) compared with the midazolam (32%) and placebo (24%) groups (Anand 1999). However, in the two RCTs that followed, investigators detected no difference in the composite outcome of severe IVH, PVL, or death (Anand 2004; Simons 2003). In addition, they noted no impact of fentanyl on the incidence of the composite outcome of severe IVH, PVL, or death (Lago 1998). A later Cochrane review, in which review authors described that infants receiving morphine needed a longer time to achieve a full enteral feeding (Bellù 2008), confirmed this finding.
Research has shown that early opiate exposure in rodent models can diminish neuronal density and dendritic length, and can increase apoptosis (Hammer 1989; Ricalde 1990; Seatriz 1993). Further, rodents exposed to postnatal morphine have exhibited reduced brain growth (Zagon 1977) and persistently decreased motor activity and impaired learning ability (Handelmann 1985; Ma 2007; McPherson 2007). Conflicting results have been reported for human neonates with regard to the long‐term neurodevelopmental impact of early morphine exposure. It has been shown that morphine‐treated children had smaller head circumference, impaired short‐term memory, and greater social problems when compared with placebo‐treated children (Ferguson 2012).
Data on the impact of morphine therapy on the intelligence quotient are controversial (de Graaf 2011; Ferguson 2012). Children treated with morphine displayed a lower overall intelligence quotient than was seen in children given placebo (de Graaf 2011). This difference disappeared after correction for the treatment condition, open‐label morphine consumption over the first 28 days, and determination of a propensity score for clinically relevant co‐variables in multiple regression analyses. Of note, scores on one intelligence quotient (IQ) subtest, "visual analysis," were significantly negatively related to receipt of morphine and open‐label morphine consumption in the first 28 days. In a small pilot follow‐up study (NEOPAIN population), children treated with morphine completed 27% less of the short‐term memory task than children in the placebo group, although overall IQ did not differ between groups (Ferguson 2012).
Description of the intervention
Thus, investigators have tested alternative sedation strategies. Alpha‐2 agonists, mainly clonidine and dexmedetomidine, may be used as adjunctive (or alternative) sedative agents alongside opioids and benzodiazepines. They have a wide range of effects, including sedation, analgesia, and relief of anxiety (Mantz 2011; Pichot 2012). Alpha‐2‐adrenergic receptor subtype agonism within the locus ceruleus is known to mediate these effects. Both clonidine and dexmedetomidine reduce the activity of neurons in the locus ceruleus without affecting the respiratory drive (Hoy 2011). Moreover, it has been suggested that alpha‐2 agonists might show neuroprotective and anti‐inflammatory actions (Mantz 2011). Both drugs preserve neutrophil function and inhibit the cytokine response in animal models of endotoxic shock (Nishina 1999; Taniguchi 2004; Taniguchi 2008). Researchers have confirmed the impact of dexmedetomidine on cytokine levels in septic adult humans (Tasdogan 2009). Both alpha‐2 agonists reduced the number of damaged neurons in vitro and reduced the size of the lesions in vivo (Laudenbach 2002; Paris 2006). Adverse events of alpha‐2 agonists, such as bradycardia and hypotension, are mediated via alpha‐2 adenoreceptors in the medullary dorsal motor nucleus and motor complex, and thus are independent of sedative effect (Gregoretti 2009; Pichot 2012). Traditionally, investigators have used clonidine to treat attention deficit hyperactivity disorder (ADHD) (Hazell 2003) and opioid withdrawal (Gold 1978), and as an anesthetic adjuvant (Gregoretti 2009; Lambert 2014). Its use for sedation remains "off label" in many countries. However, in the critically ill pediatric population, clonidine is frequently used as a sedative agent, particularly as an adjunctive agent when response to opioids and benzodiazepines is inadequate, or to facilitate weaning from mechanical ventilation (Duffett 2012). Dexmedetomidine has a higher alpha‐ 2/alpha‐1 selectivity ratio (dexmedetomidine 1620:1, clonidine 220:1) (Virtanen 1988). The US Food and Drug Admnistration approved dexmedetomidine in 1999 for short‐term sedation in adults. Currently, dexmedetomidine is not approved for pediatric use but is widely used in critically ill children and infants (Mason 2011). The first case report regarding the use of dexmedetomidine in an extremely preterm newborn was published in 2009 (O'Mara 2009); this was followed by a retrospective description of the efficacy and safety of dexmedetomidine infusion for mechanically ventilated preterm neonates (O'Mara 2012).
How the intervention might work
Clonidine is a centrally acting alpha‐2 selective adrenergic agonist. It has been postulated that alpha‐2 agonists exert their sedative effects via stimulation of the pre‐synaptic alpha‐2 adrenoceptors of the locus ceruleus, decreasing norepinephrine release (Jamadarkhana 2010). Clonidine also has shown action in cholinergic, purinergic, and serotonergic pathways, resulting in analgesia (Jamadarkhana 2010). Mechanically ventilated preterm neonates treated with dexmedetomidine infusion required less adjunctive sedation when compared with historical controls treated with fentanyl infusion (O'Mara 2012). These data support the findings of RCTs that included adult participants (Riker 2009; Ruokonen 2009). Moreover, clonidine may exert neuroprotective effects by preventing apoptosis induced by anesthesia (Pontén 2012).
Why it is important to do this review
Publiahed Cochrane reviews have examined pharmacologic treatment of newborns receiving mechanical ventilation (Bellù 2008; Ng 2017). Important issues raised by review authors include lack of data on safety and on long‐term neurodevelopmental effects of midazolam and opioid treatment; however, extremely preterm infants, who constitute the largest population requiring mechanical ventilation in neonatal intensive care units, are under‐represented in these clinical trials.
Clonidine has not been systematically assessed for neonatal sedation during ventilation; the Cochrane review titled "Alpha‐2 agonists for long‐term sedation during mechanical ventilation in critically ill patients" excluded neonates (Chen 2015). One systematic review, which focused only on pediatric patients in the intensive care unit, found that adjunctive clonidine use decreased the requirement for other sedative agents, decreased withdrawal symptoms during weaning off of benzodiazepines or opiates, and was associated with minimal clinically significant adverse effects (Duffett 2012). A Cochrane review titled "Dexmedetomidine for analgesia and sedation in newborn infants receiving mechanical ventilation" is in preparation (Ibrahim 2016); however, despite the theoretical advantages of clonidine, safety and efficacy for both short‐term and long‐term use remain unclear. A comprehensive synthesis is needed to assess whether clonidine is safe, and whether it confers advantages over traditional sedatives for long‐term sedation.
Objectives
To assess whether clonidine administered to term and preterm newborn infants receiving mechanical ventilation reduces morbidity and mortality rates. To compare the intervention versus placebo, no treatment, and dexmedetomidine; and to assess the safety of clonidine infusion for potential harms.
To perform subgroup analyses according to gestational age; birth weight; administration method (infusion or bolus therapy); dose, duration, and route of clonidine administration; and pharmacologic sedation as a co‐intervention.
Methods
Criteria for considering studies for this review
Types of studies
We included RCTs, quasi‐randomized controlled trials, and cluster trials. We excluded cross‐over trials.
Types of participants
We included full‐term and preterm newborns receiving mechanical ventilation via an endotracheal tube.
Types of interventions
Clonidine versus placebo
Clonidine versus no intervention
Clonidine versus dexmedetomidine
We included any route of administration, dose, frequency, timing of initiation, and duration for clonidine, dexmedetomidine, and co‐interventions.
We planned to assess in subgroup analyses inclusion of pharmacologic co‐interventions within sedation and pain management (e.g. morphine, fentanyl, midazolam).
Types of outcome measures
Primary outcomes
All‐cause neonatal death (death within 28 days of birth)
All‐cause death during initial hospitalization
Duration of mechanical ventilation (days)
Secondary outcomes
Sedation assessed with tools or scales such as COMFORT (Ista 2005). We planned to report mean values from sedation scales assessed at 30 minutes and at 3 hours post administration of the drug in question
Analgesia assessed via validated pain scales with age‐appropriate behavioral measures and physiologic parameters such as COMFORTneo (van Dijk 2009), Échelle Douleur Inconfort Nouveau‐Né (neonatal pain and discomfort scale; EDIN) (Debillon 2001), Astrid Lindgren and Lund Children’s Hospitals Pain and Stress Assessment Scale for Preterm and Sick Newborn Infants (ALPS‐Neo) (Lundqvist 2014), the Neonatal Infant Pain Scale (NIPS) (Lawrence 1993), and the Pain Assessment Tool (PAT) (Hodgkinson 1994). See Appendix 1 for a more detailed list. We planned to report mean values from analgesia scales assessed at 30 minutes and at 3 hours post administration of the drug in question
Duration of any co‐interventions (e.g. morphine, fentanyl, midazolam) in days. We planned not to report this outcome if the study protocol mandated sedation with a co‐intervention
Any intraventricular hemorrhage: any IVH, grade 1 to 4 (according to Papile classification (Papile 1978)); severe IVH (grades 3 and 4)
Cerebellar hemorrhage on brain ultrasound in the first month of life (yes/no; Graça 2013)
Cystic periventricular leukomalacia at brain ultrasound in the first month of life (yes/no)
Brain magnetic resonance imaging (MRI) abnormalities at term equivalent age (yes/no), defined as white matter lesions (i.e. cavitations (Rutherford 2010)) and punctate lesions (Cornette 2002); germinal matrix (GM)‐IVH (Parodi 2015); and cerebellar hemorrhage (Limperopoulos 2007)
Retinopathy of prematurity (ICROP 1984): any; requiring laser therapy
Pneumothorax (on chest x‐ray)
Duration of respiratory support (intermittent positive‐pressure ventilation [IPPV] or continuous positive airway pressure in days)
Duration of oxygen therapy in days
Duration of hospital stay in days
-
Bronchopulmonary dysplasia (BPD)/chronic lung disease (CLD), defined as:
Respiratory support or oxygen, or both, at 28 days of life (NIH 1979);
Treatment with oxygen greater than 21% for at least 28 days, with grade of severity scored at 36 weeks of postmenstrual age (PMA) (Jobe 2001); or
Physiologic definition (measured at 36 weeks' post menstrual age) (Walsh 2004)
Necrotizing enterocolitis (any grade; requiring surgery)
Need for treatment (medical; surgical) for persistent ductus arteriosus (PDA)
Time to full enteral feeding in days
Episodes of bradycardia, defined as a fall in heart rate greater than 30% below baseline or less than 100 beats per minute for 10 seconds or longer, during exposure to the intervention
Major neurodevelopmental disability: cerebral palsy, developmental delay (Bayley Mental Developmental Index (Bayley 1993; Bayley 2006) or Griffiths Mental Development Scale (Griffiths 1954) assessment more than two standard deviations [SDs] below the mean), intellectual impairment (IQ > 2 SD below the mean), blindness (vision < 6/60 in both eyes), or sensorineural deafness requiring amplification (Jacobs 2013). We plan to evaluate each of these components as a separate outcome and to extract data on this long‐term outcome from studies that evaluated children after 18 months of chronological age. Data on children aged 18 to 24 months and those aged three to five years are to be assessed separately
Search methods for identification of studies
Electronic searches
We used the criteria and standard methods of Cochrane and the Cochrane Neonatal Review Group (see the Cochrane Neonatal search strategy for specialized register).
We conducted a comprehensive search that included the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 12) in the Cochrane Library; MEDLINE via PubMed (1966 to January 10, 2017); Embase (1980 to January 10, 2017); and the Cumulative Index to Nursing and Allied Health Literature (CINAHL; 1982 to January 10, 2017) using the following search terms: (clonidine OR alpha‐2 agonists), plus database‐specific limiters for RCTs and neonates (see Appendix 2 for full search strategies for each database). We applied no language restrictions.
Searching other resources
We searched clinical trials registries for ongoing or recently completed trials (clinicaltrials.gov; the World Health Organization International Trials Registry and Platform [www.whoint/ictrp/search/en/], and the ISRCTN Registry).
Data collection and analysis
We used the standard methods of the Cochrane Neonatal Review Group as described below.
Selection of studies
Two review authors (OR, MB) independently searched and identified eligible trials that met the inclusion criteria. We screened titles and abstracts to identify potentially relevant citations, and we retrieved the full texts of all potentially relevant articles and independently assessed study eligibility by filling out eligibility forms designed in accordance with the specified inclusion criteria. We reviewed studies for relevance on the basis of study design and types of participants, interventions, and outcome measures. We resolved disagreements by discussion and, when necessary, by consultation with a third review author (MGC). We planned to provide details of studies excluded from the review in the Characteristics of excluded studies table, along with reasons for exclusion. We contacted trial authors when details of primary trials were not clear.
Data extraction and management
Two review authors (OR, MB) independently extracted data using a data extraction form developed ad hoc and integrated with a modified version of the Cochrane Effective Practice and Organisation of Care Group data collection checklist (Cochrane EPOC 2013).
We extracted the following characteristics from each included study.
Administrative details: author(s); published or unpublished; year of publication; year in which study was conducted; details of other relevant papers cited.
Details of the study: study design; type, duration, and completeness of follow‐up (i.e. > 80%); country and location of study; informed consent; ethics approval.
Details of participants: sex, birth weight, gestational age, number of participants.
Details of intervention: initiation, dose, and duration of the intervention (clonidine) and of co‐interventions, if any.
Details of outcomes as mentioned above under Types of outcome measures.
We resolved disagreements by discussion. We planned to describe ongoing studies identified by our search, when available, detailing the primary author, research question(s), methods, and outcome measures, together with an estimate of the reporting date.
When queries arose, or when additional data were required, we contacted the original study investigators/authors for clarification. Two review authors (MGC, MB) used Cochrane statistical software (Revman 2014) for data entry.
Assessment of risk of bias in included studies
Two review authors (OR, MGC) independently assessed risk of bias in all included studies using the Cochrane tool for assessing risk of bias (Higgins 2011).
We assessed the following items.
-
Selection bias: random sequence generation and selection bias, that is:
random sequence generation (biased allocation to interventions) due to inadequate generation of a randomized sequence; and
allocation concealment (selection bias [biased allocation to interventions] due to inadequate concealment of allocations before assignment.
Blinding of participants and personnel: performance bias due to knowledge of allocated interventions by participants and personnel during the study.
Blinding of outcome assessment: detection bias due to knowledge of allocated interventions by outcome assessors.
Incomplete outcome data: attrition bias due to quantity, nature, or handling of incomplete outcome data.
Selective reporting: reporting bias due to selective outcome reporting.
Other bias: bias due to problems not covered elsewhere in the table.
We used a "Risk of bias" graph to illustrate risk across studies. We resolved disagreements by consensus and, if necessary, by consultation with a third review author (MB).
Random sequence generation (selection bias)
For each included study, we categorized risk of bias regarding random sequence generation as follows.
Low risk: Investigators describe a random component in the sequence generation process such as referral to a random number table, use of a computer random number generator, coin tossing, shuffling of cards or envelopes, throwing of dice, drawing of lots, minimization.
High risk: Investigators describe a non‐random component in the sequence generation process (sequence generated by odd or even date of birth, sequence generated by some rule based on date or day of admission, sequence generated by some rule based on hospital or clinic record number, allocation by judgment of the clinician, allocation by preference of the participant, allocation based on results of a laboratory test or series of tests, allocation by availability of the intervention).
Unclear risk: no or unclear information provided.
Allocation concealment (selection bias)
For each included study, we categorized risk of bias regarding allocation concealment as follows.
Low risk: Participants and investigators enrolling participants could not foresee assignment because they used one of the following, or an equivalent method, to conceal allocation: central allocation (including telephone, web‐based, and pharmacy‐controlled randomization), sequentially numbered drug containers of identical appearance, sequentially numbered sealed opaque envelopes.
High risk: Participants and investigators enrolling participants could possibly foresee assignments and thus introduce selection bias, such as allocation based on open random allocation schedule (e.g. a list of random numbers), unsealed or non‐opaque envelopes, alternation or rotation, date of birth, or case record number.
Unclear risk: no or unclear information provided.
Blinding of study participants and personnel (performance bias)
For each included study, we categorized methods used to blind study participants and personnel from knowledge of which intervention a participant received as follows.
Criteria for a judgment of "low risk": no blinding or incomplete blinding, but review authors judge that the outcome is not likely to be influenced by lack of blinding; or blinding of participants and key study personnel ensured and unlikely that blinding could have been broken.
Criteria for a judgment of "high risk": no blinding or incomplete blinding and the outcome is likely to be influenced by lack of blinding; or blinding of key study participants and personnel attempted but likely that blinding could have been broken and the outcome is likely to be influenced by lack of blinding.
Unclear risk: no or unclear information provided.
Blinding of outcome assessors (detection bias)
For each included study, we categorized methods used to blind outcome assessors from knowledge of which intervention a participant received as follows.
Criteria for a judgment of "low risk": no blinding or incomplete blinding but review authors judge that the outcome is not likely to be influenced by lack of blinding; or blinding of participants and key study personnel ensured and unlikely that blinding could have been broken.
Criteria for a judgment of "high risk": no blinding of outcome assessment and the outcome is likely to be influenced by lack of blinding; or blinding of outcome assessment but likely that blinding could have been broken and outcome measurement is likely to be influenced by lack of blinding.
Unclear risk: no or unclear information provided.
Incomplete outcome data (attrition bias)
For each included study and for each outcome, we described completeness of data including attrition and exclusions from analysis as follows.
-
Criteria for a judgment of "low risk".
No missing outcome data.
Reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias).
Missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups.
For dichotomous outcome data, proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardized differences in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size.
Missing data imputed through appropriate methods.
-
Criteria for a judgment of "high risk".
Reasons for missing outcome data likely to be related to true outcome, with imbalance in numbers or reasons for missing data across intervention groups.
For dichotomous outcome data, proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate.
For continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size.
"As‐treated" analysis done with substantial departure of the intervention received from that assigned at randomization.
Potentially inappropriate application of simple imputation.
Unclear risk: no or unclear information provided.
Selective reporting (reporting bias)
For each included study, we described how we investigated the risk of selective outcome reporting bias and what we found. We planned to attempt to access all protocols of the included studies through clinical trials registries (e.g. ClinicalTrials.gov (ClinicalTrials.gov), the International Standard Randomised Controlled Trial Number (ISRCTN) registry (www.controlled‐trials.com)) and through direct contact with study authors.
We assessed methods as follows.
Low risk: Study protocol is available and all of the study's prespecified (primary and secondary) outcomes of interest in the review have been reported in the prespecified way; or study protocol is not available but it is clear that published reports include all expected outcomes, including those that were prespecified (convincing text of this nature may be uncommon).
High risk: Not all of the study’s prespecified primary outcomes have been reported; or one or more primary outcomes have been reported using measurements or analysis methods or subsets of data (e.g. subscales) that were not prespecified; or one or more reported primary outcomes were not prespecified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); or one or more outcomes of interest in the review have been reported incompletely so cannot be entered into a meta‐analysis; or the study report fails to include results for a key outcome that would be expected to have been reported for such a study.
Unclear risk: no or unclear information provided (study protocol was not available).
Other potential sources of bias (other bias)
For each included study, we described any important concerns we had about other possible sources of bias (e.g. whether a potential source of bias was related to the specific study design used).
We assessed whether each study was free of other problems that could put it at risk of bias as follows.
Low risk: appears to be free of other sources of bias.
High risk: has at least one important risk of bias (e.g. study has a potential source of bias related to the specific study design used or has been claimed to have been fraudulent or has some other problem).
Unclear risk: possible risk of bias, but insufficient information to assess whether an important risk of bias exists; or insufficient rationale or evidence that an identified problem will introduce bias.
Measures of treatment effect
We followed standard methods of the Cochrane Neonatal Review Group for data synthesis. We extracted categorical data for each intervention group and calculated risk ratios (RRs) and absolute risk differences (RDs). We obtained means and standard deviations for continuous data and performed analyses using mean differences (MDs). For each measure of effect, we also calculated corresponding 95% confidence intervals (CIs). We planned to present the number needed to treat for an additional beneficial outcome (NNTB) and the number needed to treat for an additional harmful outcome (NNTH) when we found that RDs were statistically significant (P < 0.05).
Unit of analysis issues
The unit of randomization was the intended unit of analysis (individual neonate). If we identified any cluster‐randomized trials for inclusion, we planned to adjust their sample sizes using methods described in the Cochrane Handbook for Systematic Reviews of Interventions (Section 16.3.4 or 16.3.6) using an estimate of the intracluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial, or from a study of a similar population (Higgins 2011). If we used ICCs from other sources, we planned to report this and to conduct sensitivity analyses to investigate effects of variation in the ICC.
Dealing with missing data
When data were missing, we contacted the original study investigators to request the missing data. We planned to obtain a drop‐out rate for each study. If we found a significant drop‐out rate (> 20%), we planned to contact study authors to request additional data. We planned to perform a sensitivity analysis to evaluate overall results with and without inclusion of studies with a significant drop‐out rate. If a study reported outcomes only for participants who completed the trial or only for participants who followed the protocol, we planned to contact study authors to ask them to provide additional information to facilitate an intention‐to‐treat analysis; when this was not possible, we planned to perform a complete case analysis.
Assessment of heterogeneity
We planned to assess clinical heterogeneity by comparing the distribution of important participant factors between trials and trial factors (randomization concealment, blinding of outcome assessment, loss to follow‐up, treatment type, co‐interventions). We planned to assess statistical heterogeneity by examining the I² statistic (Higgins 2011) ‐ a quantity that describes the proportion of variation in point estimates that is due to variability across studies rather than to sampling error.
We planned to interpret the I² statistic as described by Higgins 2003.
< 25%: no heterogeneity.
25% to 49%: low heterogeneity.
50% to 74%: moderate heterogeneity.
≥ 75%: high heterogeneity.
We planned to consider statistical heterogeneity as substantial when I² was greater than 50%. In addition, we planned to employ the Chi² test of homogeneity to determine the strength of evidence that heterogeneity is genuine. We planned to explore clinical variation across studies by comparing the distribution of important participant factors among trials and trial factors (randomization concealment, blinding of outcome assessment, loss to follow‐up, treatment type, and co‐interventions). We planned to consider a threshold P value of less than 0.1 as indicating whether heterogeneity (genuine variation in effect sizes) was present.
Assessment of reporting biases
We planned to investigate publications by preparing funnel plots if we included at least 10 clinical trials (Egger 1997; Higgins 2011).
Data synthesis
We summarized all eligible studies in Review Manager 5 (Revman 2014) and utilized standard methods for meta‐analysis as described in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We used the fixed‐effect model and presented all results with 95% CIs. We planned to calculate RR, RD, and NNTB or NNTH if RD was significant, each with 95% CI, for categorical outcomes; and MD with 95% CI for continuous outcomes. For outcomes for which included studies were not sufficiently homogeneous, or for which insufficient data were available for meta‐analysis, we planned to present a narrative synthesis.
Quality of evidence
We used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes: all‐cause neonatal death, all‐cause death during initial hospitalization, and duration of mechanical ventilation in days; important outcomes were intraventricular hemorrhage; duration of hospital stay; and major neurodevelopmental disability.
Two review authors independently assessed the quality of the evidence for each of the outcomes above. We considered evidence from RCTs as high quality but downgraded the evidence one level for serious (or two levels for very serious) limitations on the basis of the following: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates, and presence of publication bias. We used the GRADEpro GDT Guideline Development Tool to create a "Summary of findings" table to report the quality of the evidence.
The GRADE approach yields an assessment of the quality of a body of evidence according to four grades.
High: We are very confident that the true effect lies close to the estimate of effect.
Moderate: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect but may be substantially different.
Low: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect.
Very low: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
We planned to present data from the following subgroups.
Gestational age: preterm (< 37 weeks' gestational age) versus term infants (≥ 37 weeks); extreme preterm (< 28 weeks) versus preterm infants (≥ 28 but < 37 weeks).
Birth weight: less than 1500 grams versus greater than or equal to 1500 grams.
Parenteral versus enteral administration of the intervention.
Dose of clonidine (low: < 0.3 mcg/kg/h; standard: 0.3 to 1 mcg/kg/h; high: > 1 mcg/kg/h).
Duration of treatment (< 24 hours; 1 to 5 days; ≥ 5 days).
With versus without pharmacologic sedation and pain management as co‐interventions.
Within studies that included co‐interventions: studies in which the protocol allowed co‐interventions for sedation and pain management for one or both of the intervention groups versus studies in which the protocol mandated sedation with co‐interventions.
Sensitivity analysis
We planned to conduct sensitivity analyses to explore the effect of the methodological quality of trials, while checking to ascertain whether studies at high risk of bias overestimated effects of treatment.
Results
Description of studies
See the Characteristics of included studies table.
Results of the search
The literature search run in January 2017 identified 628 references (Figure 1). After screening, we included only one RCT (Hünseler 2014).
1.
Study flow diagram.
We found no relevant studies on the clinical trials registries for ongoing or recently completed trials.
Included studies
One trial recruiting 112 term infants met the inclusion criteria (Hünseler 2014; see the Characteristics of included studies table).
Hünseler 2014 is a prospective, double‐blind, randomized, placebo‐controlled, multicenter trial conducted at 28 level 3 German pediatric/neonatal intensive care units. Investigators computed a randomization scheme in blocks, stratified according to study center and age (stratum I: 1 to 28 days; stratum II: 29 to 120 days; stratum III: 121 days to 2 years); we considered in this review only data from the first stratum. A designated pharmacist at each study center received the lists of treatment group assignments. Upon inclusion, researchers assigned the study participant to the appropriate study medication in neutral, blinded ampoules forwarded to the local investigator by the local pharmacy. Term newborn infants (gestational age ≥ 37.0 weeks) with a duration of mechanical ventilation greater than three days and of an expected six days with the need for continuous analgesia and sedation with fentanyl and midazolam were eligible for enrollment during the first 96 hours of ventilation. Infants in postoperative care or with respiratory failure for other reasons were included. Criteria for exclusion were states precluding pain assessment (encephalopathy, encephalitis, severe head injury, cerebral edema, and neuromuscular blockade) and maternal drug abuse during pregnancy. Participants received clonidine 1 μg/kg/h or placebo on day 4 after intubation. Fentanyl and midazolam were adjusted to achieve a defined level of analgesia and sedation according to Hartwig score. Primary outcome measures were consumption of fentanyl and midazolam and use of thiopentone during the 72 hours following study medication. Secondary outcome measures were number of protocol violations (administration of analgesics or sedatives not provided by study protocol), blood pressure, heart rate, catecholamines and volume replacement, diuresis, "therapeutic intervention scoring system," Hartwig and Comfort scores during the first 72 hours of infusion of study medication, withdrawal symptoms (Finnegan score), therapy for withdrawal symptoms, length of intensive care unit stay, mortality, and serum concentration of clonidine in steady state. There was no statistically significant difference in baseline demographic characteristics among the two groups of newborns at randomization. Infusion with clonidine was associated with significantly lower mean fentanyl and midazolam consumption compared with placebo; investigators reported values as dose per unit of time ‐ not as duration of treatment as specified in this review. Frequencies of administration of at least one thiopentone dose were similar between clonidine and placebo groups. Hartwig score values were significantly lower in the clonidine group during the first 72 hours of infusion of study medication (11.1 ± 2.0 vs 12.5 ± 2.4; P < 0.001).
Excluded studies
None of the other identified studies were eligible.
Risk of bias in included studies
Figure 2 summarizes risk of bias for the trial included in this review.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Investigators carried out randomization (in blocks and stratified according to study center) using a computer‐generated randomization sequence. A designated pharmacist at each study center received the lists of treatment group assignments. Upon inclusion, researchers assigned the study participant to the appropriate age stratum and treatment arm, and study medication in neutral, blinded ampoules was forwarded to local investigator by the local pharmacy.
Blinding
Parents and investigators remained blinded to medications administered throughout the study period.
Incomplete outcome data
Study authors reported outcomes for all randomized infants (no drop‐outs).
Selective reporting
The study protocol was available (ISRCTN77772144, Controlled.Trials.com.gov).
Other potential sources of bias
The trial appeared free of other biases.
Effects of interventions
See: Table 1
Clonidine versus placebo
We identified only one trial, which included a total of 112 newborns (Hünseler 2014). Tests for heterogeneity were not applicable for any of the analyses, as we identified only one study.
Primary outcomes
All‐cause neonatal death (death within 28 days of birth)
The study did not report all‐cause death during initial hospitalization.
All‐cause death during initial hospitalization
Two infants in the clonidine group and three in the placebo group died (typical RR 0.69, 95% CI 0.12 to 3.98; typical RD ‐0.02, 95% CI ‐0.09 to 0.06; one study, 112 infants; Analysis 1.1; Figure 3). These five events occurred during stay in the intensive care unit (we obtained data for this outcome directly from trial authors). The test for heterogeneity was not applicable. The quality of the evidence supporting these findings is low owing to imprecision of the estimates (one study; few events).
1.1. Analysis.
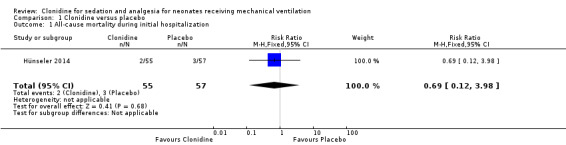
Comparison 1 Clonidine versus placebo, Outcome 1 All‐cause mortality during initial hospitalization.
3.
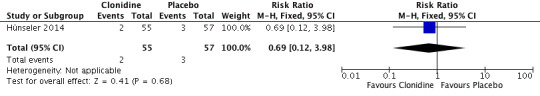
Forest plot of comparison: 1 Clonidine versus placebo, outcome: 1.1 All‐cause mortality during initial hospitalization.
Duration of mechanical ventilation
We reported this outcome as medians (interquartile ranges) ‐ not as means or SDs. Medians for duration of mechanical ventilation were 7.1 days (5.7 to 9.1 days) in the clonidine group and 5.8 days (4.9 to 7.9 days) in the placebo group, respectively (P = 0.070).
Secondary outcomes
Sedation assessed with COMFORT
Sedation scale values refer to the first 72 hours of infusion of study medication ‐ not to mean values assessed at 30 minutes and 3 hours post administration, as prespecified in our protocol. Analgesia assessed with COMFORT was significantly lower in the clonidine group than in the placebo group (13.4 ± 2.2 vs 14.8 ± 2.4; P < 0.004).
Analgesia assessed with Hartwig
Although not explicitly listed in our protocol, we report here the Hartwig score, as this score has been validated in newborns (Hünseler 2011). Analgesia scale values refer to the first 72 hours of infusion of study medication ‐ not to mean values assessed at 30 minutes and 3 hours post administration, as prespecified in our protocol. Analgesia assessed with Hartwig during the first 72 hours of infusion of study medication was significantly lower in the clonidine group than in the placebo group (11.1 ± 2.0 vs 12.5 ± 2.4; P < 0.001).
Duration of hospital stay
The included study reported this outcome as duration of stay in the intensive care unit ‐ not as total hospital stay, as prespecified in our protocol. Medians for duration of intensive care unit stay were 16.9 days (10.7 to 25.8 days) in the clonidine group and 16.8 days (13.0 to 21.2 days) in the placebo group, respectively.
Outcomes with no available data
Duration of co‐interventions
Intraventricular hemorrhage (any; grades 3 and 4)
Cerebellar hemorrhage on brain ultrasound
Cystic periventricular leukomalacia on brain ultrasound
Brain MRI abnormalities at term equivalent age
Retinopathy of prematurity (any; requiring laser therapy)
Pneumothorax
Duration of respiratory support
Duration of oxygen therapy
Bronchopulmonary dysplasia/chronic lung disease
Necrotizing enterocolitis (any grade; requiring surgery)
Need for treatment (medical; surgical) for persistent ductus arteriosus
Time to full enteral feeding
Episodes of bradycardia during exposure to the intervention
Major neurodevelopmental disability
In addition, we found no data on all‐cause neonatal death.
Subgroup analysis
We were unable to conduct any of the planned subgroup analyses, as we included only one trial.
Discussion
Summary of main results
We evaluated the efficacy and safety of clonidine in neonates receiving mechanical ventilation. Only one trial including 112 term newborns with duration of mechanical ventilation greater than three days and of expectedly six days with the need for continuous analgesia and sedation with fentanyl and midazolam met the inclusion criteria (Hünseler 2014). Participants received clonidine 1 μg/kg/h or placebo on day 4 after intubation. Study authors reported no differences in the primary outcomes of this review (i.e. mortality and duration of mechanical ventilation). Sedation scale values (COMFORT) and analgesia scores (Hartwig) during the first 72 hours of infusion of study medication were lower in the clonidine group than in the placebo group. The quality of the evidence was low owing to imprecision of the estimates.
Overall completeness and applicability of evidence
The only identified study included few newborns (112) and was underpowered to assess the outcomes specified in this review. While taking this important limitation into account, study authors reported no adverse effects in the 55 newborns treated with clonidine. They admininstered no loading dose: It has been argued that in this case, because of the long half‐life of clonidine, steady state would have been achieved in newborns only toward the end of the 72‐hour study period (Sheng 2015). The included trial compared clonidine versus placebo; we identified no trials comparing clonidine versus other alpha‐2 agonists (e.g. dexmedetomidine). We could not perform a priori subgroup analyses (gestational age; birth weight; dose, duration, and route of clonidine administration; and presence of pharmacologic sedation as a co‐intervention) to detect differential effects, as we included only one randomized controlled trial (RCT).
Quality of the evidence
The included trial had low risk of bias and no relevant limitations in trial design. However, the overall quality of the evidence was affected by imprecision owing to the small number of infants (112) and few events in the included study (see Table 1).
Potential biases in the review process
It is unlikely that the literature search applied to this review may have missed relevant trials; thus, we are confident that this systematic review summarizes all presently available evidence from RCTs on clonidine for neonates receiving mechanical ventilation. We did not exclude any potentially relevant trial. We designed the methods of the review to minimize the introduction of additional bias. Two review authors independently completed data screening, data extraction, and "risk of bias" ratings (OR, MB). We contacted the authors of the included trial (Hünseler 2014) but could not obtain additional information on the outcomes specified in this review. Deviations from the protocol (see Differences between protocol and review) had no effect on the conclusions of this review.
Agreements and disagreements with other studies or reviews
A systematic review on the pediatric use of alpha‐2 agonists for the indication of sedation included three RCTs on dexmedetomidine and three RCTs on clonidine (Hayden 2016). Investigators compared clonidine with either placebo (two trials) or midazolam (one trial). However, Hünseler 2014 was the only included trial on clonidine in newborns that was included in Hayden 2016 as well as in our review. Moreover, we agree with the judgment of risk of bias provided by Hayden and collaborators in Hünseler 2014. Another systematic review on clonidine for sedation is under preparation, although the population of interest excludes studies exclusively enrolling neonates (Jing Wang 2015).
Authors' conclusions
Implications for practice.
Evidence is of low quality and is insufficient to establish the efficacy and safety of clonidine for treatment of the ventilated newborn. The only included trial, which reported a small sample size, showed no differences in any clinically relevant outcomes. Given the imprecision of our estimates, this systematic review found no benefit and no detrimental effect of clonidine and cannot provide a definitive answer to the review question.
Implications for research.
As dosage regimen is undefined, dose‐finding studies are needed to guide the design of future trials in which clonidine might be administered as the primary sedation agent or adjunctively with other interventions and might be compared with either placebo or dexmedetomidine. Trials in extremely preterm newborn infants would require additional caution as regards pharmacokinetics and effects on long‐term neurodevelopmental outcomes.
Acknowledgements
We thank Roger Soll for his advice, and Colleen Ovelman and Yolanda Brosseau for their kind and efficient support. We thank Mohan Pammi for revising this review.
Appendices
Appendix 1. Neonatal pain scores
Comfort‐Neo (van Dijk 2009)
Échelle Douleur Inconfort Nouveau‐Né (neonatal pain and discomfort scale; EDIN) (Debillon 2001)
Astrid Lindgren and Lund Children’s Hospitals Pain and Stress Assessment Scale for Preterm and Sick Newborn Infants (ALPS‐Neo) (Lundqvist 2014)
Neonatal Infant Pain Scale (NIPS) (Lawrence 1993)
Pain Assessment Tool (PAT) (Hodgkinson 1994)
Premature Infant Pain Profile (PIPP) (Stevens 1996)
APN: evaluation behavioral scale of acute pain in newborn infants (Carbajal 1997)
Neonatal Facial Coding System (NFCS) (Grunau 1986; Peters 2003)
DAN (Douleur Aiguë du Nouveau‐né) (Carbajal 2005)
ABC Pain Scale (Bellieni 2005)
Neonatal Pain, Agitation, and Sedation Scale (N‐PASS) (Hummel 2010)
'Faceless' Acute Neonatal Pain Scale (FANS) (Milesi 2010)
Premature Infant Pain Profile ‐ Revised (PIPP‐R) (Gibbins 2014)
Appendix 2. Standard search methods
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR randomized [tiab] OR placebo [tiab] OR drug therapy [sh] OR randomly [tiab] OR trial [tiab] OR groups [tiab]) NOT (animals [mh] NOT humans [mh]))
Embase: (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial)
CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
Cochrane Library: (infant or newborn or neonate or neonatal or premature or preterm or very low birth weight or low birth weight or VLBW or LBW)
Data and analyses
Comparison 1. Clonidine versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 All‐cause mortality during initial hospitalization | 1 | 112 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.12, 3.98] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Hünseler 2014.
| Methods | Prospective, double‐blind, randomized, controlled multicenter (three 28‐level German pediatric/neonatal intensive care units) clinical trial Randomization in blocks stratified according to study center and age; only data from the first stratum (1 to 28 days) have been included in this review The study participant was assigned to the appropriate study medication forwarded in neutral, blinded ampoules to the local investigator by the local pharmacy |
|
| Participants | Inclusion criteria: Term (≥ 37 weeks) infants with a duration of mechanical ventilation > 3 days and of expectedly 6 days with the need for continuous analgesia and sedation with fentanyl and midazolam were eligible for enrollment during the first 96 hours of ventilation; infants in postoperative care or with respiratory failure for other reasons were included Exclusion criteria: states precluding pain assessment (encephalopathy, encephalitis, severe head injury, cerebral edema, and neuromuscular blockade) and maternal drug abuse during pregnancy |
|
| Interventions | Clonidine 1 μg/kg/h or placebo on day 4 after intubation Fentanyl and midazolam were adjusted to achieve a defined level of analgesia and sedation according to Hartwig score |
|
| Outcomes | Primary outcomes: consumption of fentanyl (µg/kg/h) and midazolam (µg/kg/h) and use of thiopentone during the 72 hours following study medication Secondary outcomes: number of protocol violations (administration of analgesics or sedatives not provided by study protocol), blood pressure, heart rate, catecholamines and volume replacement, diuresis, "therapeutic intervention scoring system," Hartwig and COMFORT scores, withdrawal symptoms (Finnegan score), therapy for withdrawal symptoms, length of stay in the intensive care unit, mortality, and serum concentration of clonidine in steady state |
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomization sequence, in blocks, stratified according to study center and age |
| Allocation concealment (selection bias) | Low risk | A designated pharmacist at each study center received the lists of treatment group assignments. Upon inclusion, the study participant was assigned to the appropriate age stratum and treatment arm, and study medication was forwarded in neutral, blinded ampoules to the local investigator by the local pharmacy |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Study was blinded |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Study was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No outcome data were missing |
| Selective reporting (reporting bias) | Low risk | Study protocol was available (ISRCTN77772144, Controlled.Trials.com.gov) |
| Other bias | Low risk | Study appears to be free of other sources of bias |
Differences between protocol and review
Although not explicitly listed in our protocol, we report analgesia assessment based on Hartwig score, as this score has been validated in newborns (Hünseler 2011).
We modified the definition of the dose of clonidine as follows: low: < 0.3 mcg/kg/h; standard: 0.3 to 1 mcg/kg/h; high: > 1 mcg/kg/h.
We changed the title on the basis of editorial input: from "Clonidine for neonates receiving mechanical ventilation" to "Clonidine for sedation and analgesia for neonates receiving mechanical ventilation".
Changes included bolus administration (in the protocol, we specified only infusion).
Contributions of authors
OR and MB reviewed the literature and wrote the review.
MGC assisted in the review of literature and in writing of the review.
EN commented on and reviewed the review.
Sources of support
Internal sources
-
Institute for Clinical Sciences, Lund University, Lund, Sweden.
to OR, EN and MB
-
Istituto Giannina Gaslini, Genoa, Italy.
to MGC
External sources
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA.
Editorial support for the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201600005C
Declarations of interest
OR, MGC, EN, and MB declare no known conflicts of interest.
New
References
References to studies included in this review
Hünseler 2014 {published data only}
- Hünseler C, Balling G, Röhlig C, Blickheuser R, Trieschmann U, Lieser U, et al. Clonidine Study Group. Continuous infusion of clonidine in ventilated newborns and infants: a randomized controlled trial. Pediatric Critical Care Medicine 2014;15(6):511‐22. [DOI: 10.1097/PCC.0000000000000151; PUBMED: 24751788 ] [DOI] [PubMed] [Google Scholar]
Additional references
Anand 1987a
- Anand KJ, Hickey PR. Pain and its effects in the human neonate and fetus. New England Journal of Medicine 1987;371(21):1321‐9. [DOI: 10.1056/NEJM198711193172105; PUBMED: 3317037 ] [DOI] [PubMed] [Google Scholar]
Anand 1987b
- Anand KJ, Sippell WG, Aynsley‐Green A. Randomised trial of fentanyl anaesthesia in preterm babies undergoing surgery: effects on the stress response. Lancet 1987;1(8524):62‐6. [PUBMED: 2879174 ] [DOI] [PubMed] [Google Scholar]
Anand 1999
- Anand KJ, Barton BA, McIntosh N, Lagercrantz H, Pelausa E, Young TE, et al. Analgesia and sedation in preterm neonates who require ventilatory support: results from the NOPAIN trial. Neonatal Outcome and Prolonged Analgesia in Neonates. Archives of Pediatrics and Adolescent Medicine 1999;153(4):331‐8. [PUBMED: 10201714 ] [DOI] [PubMed] [Google Scholar]
Anand 2004
- Anand KJ, Hall RW, Desai N, Shephard B, Bergqvist LL, Young TE, et al. NEOPAIN Trial Investigators Group. Effects of morphine analgesia in ventilated preterm neonates: primary outcomes from the NEOPAIN randomised trial. Lancet 2004;363(9422):1673‐82. [DOI: 10.1016/S0140-6736(04)16251-X; PUBMED: 15158628 ] [DOI] [PubMed] [Google Scholar]
Bayley 1993
- Bayley N. Bayley Scales of Infant Development. 2nd Edition. San Antonio, TX: Psychological Corporation, 1993. [Google Scholar]
Bayley 2006
- Bayley N. Bayley Scales of Infant and Toddler Development. 3rd Edition. San Antonio, TX: Harcourt Assessment, 2006. [Google Scholar]
Bellieni 2005
- Bellieni CV, Bagnoli F, Sisto R, Neri L, Cordelli D, Buonocore G. Development and validation of the ABC pain scale for healthy full‐term babies. Acta Paediatrica 2005;94(10):1432‐6. [PUBMED: 16299876 ] [DOI] [PubMed] [Google Scholar]
Bellù 2008
- Bellù R, Waal KA, Zanini R. Opioids for neonates receiving mechanical ventilation. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD004212.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Carbajal 1997
- Carbajal R, Paupe A, Hoenn E, Lenclen R, Olivier‐Martin M. APN: evaluation behavioral scale of acute pain in newborn infants. Archives de Pediatrie 1997;4(7):623‐8. [PUBMED: 9295899 ] [DOI] [PubMed] [Google Scholar]
Carbajal 2005
- Carbajal R, Lenclen R, Jugie M, Paupe A, Barton BA, Anand KJ. Morphine does not provide adequate analgesia for acute procedural pain among preterm neonates. Pediatrics 2005;115(6):1494‐500. [DOI: 10.1542/peds.2004-1425; PUBMED: 15930209 ] [DOI] [PubMed] [Google Scholar]
Chen 2015
- Chen K, Lu Z, Xin YC, Cai Y, Chen Y, Pan SM. Alpha‐2 agonists for long‐term sedation during mechanical ventilation in critically ill patients. Cochrane Database of Systematic Reviews 2015, Issue 1. [DOI: 10.1002/14651858.CD010269.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Clark 2006
- Clark RH, Bloom BT, Spitzer AR, Gerstmann DR. Reported medication use in the neonatal intensive care unit: data from a large national data set. Pediatrics 2006;117(6):1979‐87. [DOI: 10.1542/peds.2005-1707; PUBMED: 16740839 ] [DOI] [PubMed] [Google Scholar]
Cochrane EPOC 2013
- Effective Practice, Organisation of Care (EPOC). Data extraction and management. EPOC resources for review authors. Oslo: Norwegian Knowledge Centre for the Health Services, 2013. epoc.cochrane.org/epoc‐specific‐resources‐review‐authors.
Cornette 2002
- Cornette LG, Tanner SF, Ramenghi LA, Miall LS, Childs AM, Arthur RJ, et al. Magnetic resonance imaging of the infant brain: anatomical characteristics and clinical significance of punctate lesions. Archives of Disease in Childhood. Fetal and Neonatal Edition 2002;86(3):F171‐7. [PUBMED: 11978747] [DOI] [PMC free article] [PubMed] [Google Scholar]
de Graaf 2011
- Graaf J, Lingen RA, Simons SH, Anand KJ, Duivenvoorden HJ, Weisglas‐Kuperus N, et al. Long‐term effects of routine morphine infusion in mechanically ventilated neonates on children’s functioning: five‐year follow‐up of a randomized controlled trial. Pain 2011;152(6):1391‐7. [DOI: 10.1016/j.pain.2011.02.017; PUBMED: 21402444 ] [DOI] [PubMed] [Google Scholar]
Debillon 2001
- Debillon T, Zupan V, Ravault N, Magny JF, Dehan M. Development and initial validation of the EDIN scale, a new tool for assessing prolonged pain in preterm infants. Archives of Disease in Childhood. Fetal and Neonatal Edition 2001;85(1):F36‐41. [PUBMED: 11420320 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Duffett 2012
- Duffett M, Koop A, Menon K, Meade MO, Cook DJ. Clonidine for the sedation of critically ill children: a systematic review. Journal of Pediatric Intensive Care 2012;1(1):5‐15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. British Medical Journal (Clinical Research Ed.) 1997;315(7109):629‐34. [PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferguson 2012
- Ferguson SA, Ward WL, Paule MG, Hall RW, Anand KJ. A pilot study of preemptive morphine analgesia in preterm neonates: effects on head circumference, social behavior, and response latencies in early childhood. Neurotoxicology and Teratology 2012;34(1):47‐55. [DOI: 10.1016/j.ntt.2011.10.008; PUBMED: 22094261 ] [DOI] [PubMed] [Google Scholar]
Fitzgerald 1986
- Fitzgerald M, Koltzenburg M. The functional development of descending inhibitory pathways in the dorsolateral funiculus of the newborn rat spinal cord. Brain Research 1986;389(1‐2):261‐70. [PUBMED: 3948011 ] [DOI] [PubMed] [Google Scholar]
Gibbins 2014
- Gibbins S, Stevens BJ, Yamada J, Dionne K, Campbell‐Yeo M, Lee G, et al. Validation of the Premature Infant Pain Profile‐Revised (PIPP‐R). Early Human Development 2014;90(4):189‐93. [DOI: 10.1016/j.earlhumdev.2014.01.005; PUBMED: 24491511 ] [DOI] [PubMed] [Google Scholar]
Gold 1978
- Gold M, Redmond DE, Kleber H. Clonidine blocks acute opiate‐withdrawal symptoms. Lancet 1978;2(8090):599‐602. [PUBMED: 80526 ] [DOI] [PubMed] [Google Scholar]
Golianu 2007
- Golianu B, Krane E, Seybold J, Almgren C, Anand KJ. Non‐pharmacological techniques for pain management in neonates. Seminars in Perinatology 2007;31(5):318‐22. [DOI: 10.1053/j.semperi.2007.07.007; PUBMED: 17905187 ] [DOI] [PubMed] [Google Scholar]
GRADEpro GDT [Computer program]
- Grade Working Group, McMaster University. GRADEpro [www.gradepro.org]. Version Version 14 September 2014. Hamilton (ON): Grade Working Group, McMaster University, 2014.
Graça 2013
- Graça AM, Geraldo AF, Cardoso K, Cowan FM. Preterm cerebellum at term age: ultrasound measurements are not different from infants born at term. Pediatric Research 2013;74(6):698‐704. [DOI: 10.1038/pr.2013.154; PUBMED: 24002327 ] [DOI] [PubMed] [Google Scholar]
Gregoretti 2009
- Gregoretti C, Moglia B, Pelosi P, Navalesi P. Clonidine in perioperative medicine and intensive care unit: more than an anti‐hypertensive drug. Current Drug Targets 2009;10(8):799‐814. [PUBMED: 19702526 ] [DOI] [PubMed] [Google Scholar]
Griffiths 1954
- Griffiths R. The Abilities of Babies: A Study in Mental Measurement. New York, NY: McGraw‐Hill Book Co. Inc., 1954. [Google Scholar]
Grunau 1986
- Grunau RE, Oberlander T, Holsti L, Whitfield MF. Bedside application of the Neonatal Facial Coding System in pain assessment of premature neonates. Pain 1986;76(3):277‐86. [PUBMED: 9718246 ] [DOI] [PubMed] [Google Scholar]
Hall 2007
- Hall RW, Boyle E, Young T. Do ventilated neonates require pain management?. Seminars in Perinatology 2007;31(5):289‐97. [DOI: 10.1053/j.semperi.2007.07.002; PUBMED: 17905183 ] [DOI] [PubMed] [Google Scholar]
Hammer 1989
- Hammer RP Jr, Ricalde AA, Seatriz JV. Effects of opiates on brain development. Neurotoxicology 1989;10(3):475‐83. [PUBMED: 2696899] [PubMed] [Google Scholar]
Handelmann 1985
- Handelmann GE, Dow‐Edwards D. Modulation of brain development by morphine: effects on central motor systems and behavior. Peptides 1985;6(Suppl 2):29‐34. [PUBMED: 4080616] [DOI] [PubMed] [Google Scholar]
Hayden 2016
- Hayden JC, Breatnach C, Doherty DR, Healy M, Howlett MM, Gallagher PJ, et al. Efficacy of α2‐agonists for sedation in pediatric critical care: a systematic review. Pediatric Critical Care Medicine 2016;17(2):e66‐75. [DOI: 10.1097/PCC.0000000000000599; PUBMED: 26704469] [DOI] [PubMed] [Google Scholar]
Hazell 2003
- Hazell PL, Stuart JE. A randomized controlled trial of clonidine added to psychostimulant medication for hyperactive and aggressive children. Journal of the American Academy of Child and Adolescent Psychiatry 2003;42(8):886‐94. [DOI: 10.1097/01.CHI.0000046908.27264.00; PUBMED: 12874489] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. British Medical Journal (Clinical Research Ed.) 2003;327(7414):557‐60. [DOI: 10.1136/bmj.327.7414.557; PUBMED: 12958120] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. handbook.cochrane.org.
Hodgkinson 1994
- Hodgkinson K, Bear M, Thorn J, Blaricum S. Measuring pain in neonates: evaluating an instrument and developing a common language. Australian Journal of Advanced Nursing 1994;12(1):17‐22. [PUBMED: 7786451] [PubMed] [Google Scholar]
Hoy 2011
- Hoy SM, Keating GM. Dexmedetomidine: a review of its use for sedation in mechanically ventilated patients in an intensive care setting and for procedural sedation. Drugs 2011;71(11):1481‐501. [DOI: 10.2165/11207190-000000000-00000; PUBMED: 21812509] [DOI] [PubMed] [Google Scholar]
Hummel 2010
- Hummel P, Lawlor‐Klean P, Weiss MG. Validity and reliability of the N‐PASS assessment tool with acute pain. Journal of Perinatology 2010;30(7):474‐8. [DOI: 10.1038/jp.2009.185; PUBMED: 19924132] [DOI] [PubMed] [Google Scholar]
Hünseler 2011
- Hünseler C, Merkt V, Gerloff M, Eifinger F, Kribs A, Roth B. Assessing pain in ventilated newborns and infants: validation of the Hartwig score. European Journal of Pediatrics 2011;170(7):837‐43. [DOI: 10.1007/s00431-010-1354-9; PUBMED: 21120525] [DOI] [PubMed] [Google Scholar]
Ibrahim 2016
- Ibrahim M, Jones LJ, Lai NM, Tan K. Dexmedetomidine for analgesia and sedation in newborn infants receiving mechanical ventilation. Cochrane Database of Systematic Reviews 2016, Issue 9. [DOI: 10.1002/14651858.CD012361] [DOI] [PMC free article] [PubMed] [Google Scholar]
ICROP 1984
- Committee for the Classification of Retinopathy of Prematurity. An international classification of retinopathy of prematurity. Pediatrics 1984;74(1):127‐33. [PUBMED: 6547526] [PubMed] [Google Scholar]
Ista 2005
- Ista E, Dijk M, Tibboel D, Hoog M. Assessment of sedation levels in pediatric intensive care patients can be improved by using the COMFORT "behavior" scale. Pediatric Critical Care Medicine 2005;6(1):58‐63. [DOI: 10.1097/01.PCC.0000149318.40279.1A; PUBMED: 15636661 ] [DOI] [PubMed] [Google Scholar]
Jacobs 2013
- Jacobs SE, Berg M, Hunt R, Tarnow‐Mordi WO, Inder TE, Davis PG. Cooling for newborns with hypoxic ischaemic encephalopathy. Cochrane Database of Systematic Reviews 2013, Issue 1. [DOI: 10.1002/14651858.CD003311.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jacqz‐Aigrain 1994
- Jacqz‐Aigrain E, Daoud P, Burtin P, Desplanques L, Beaufils F. Placebo‐controlled trial of midazolam sedation in mechanically ventilated newborn babies. Lancet 1994;344(8923):646‐50. [PUBMED: 7915348] [DOI] [PubMed] [Google Scholar]
Jamadarkhana 2010
- Jamadarkhana S, Gopal S. Clonidine in adults as a sedative agent in the intensive care unit. Journal of Anaesthesiology, Clinical Pharmacology 2010;26(4):439‐45. [PUBMED: 21547166] [PMC free article] [PubMed] [Google Scholar]
Jing Wang 2015
- Jing Wang G, Belley‐Coté E, Burry L, Duffett M, Karachi T, Perri D, et al. Clonidine for sedation in the critically ill: a systematic review and meta‐analysis (protocol). Systematic Reviews 2015;6(4):154. [DOI: 10.1186/s13643-015-0139-7; PUBMED: 26542363] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jobe 2001
- Jobe AH, Bancalari E. Bronchopulmonary dysplasia. American Journal of Respiratory and Critical Care Medicine 2001;163(7):1723‐9. [DOI: 10.1164/ajrccm.163.7.2011060; PUBMED: 11401896] [DOI] [PubMed] [Google Scholar]
Kumar 2008
- Kumar P, Walker JK, Hurt KM, Bennett KM, Grosshans N, Fotis MA. Medication use in the neonatal intensive care unit: current patterns and off‐label use of parenteral medications. Journal of Pediatrics 2008;152(3):412‐5. [DOI: 10.1016/j.jpeds.2007.07.050; PUBMED: 18280851] [DOI] [PubMed] [Google Scholar]
Lago 1998
- Lago P, Benini F, Agosto C, Zacchello F. Randomised controlled trial of low dose fentanyl infusion in preterm infants with hyaline membrane disease. Archives of Disease in Childhood. Fetal and Neonatal Edition 1998;79(3):F194–7. [PUBMED: 10194990] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lambert 2014
- Lambert P, Cyna AM, Knight N, Middleton P. Clonidine premedication for postoperative analgesia in children. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD009633.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Laudenbach 2002
- Laudenbach V, Mantz J, Lagercrantz H, Desmonts JM, Evrard P, Gressens P. Effects of alpha(2)‐adrenoceptor agonists on perinatal excitotoxic brain injury: comparison of clonidine and dexmedetomidine. Anesthesiology 2002;96(1):134‐41. [PUBMED: 11753013] [DOI] [PubMed] [Google Scholar]
Lawrence 1993
- Lawrence J, Alcock D, McGrath P, Kay J, MacMurray SB, Dulberg C. The development of a tool to assess neonatal pain. Neonatal Network 1993;12(6):59‐66. [PUBMED: 8413140] [PubMed] [Google Scholar]
Limperopoulos 2007
- Limperopoulos C, Bassan H, Gauvreau K, Robertson RL Jr, Sullivan NR, Benson CB, et al. Does cerebellar injury in premature infants contribute to the high prevalence of long‐term cognitive, learning, and behavioral disability in survivors?. Pediatrics 2007;120(3):584‐93. [DOI: 10.1542/peds.2007-1041; PUBMED: 17766532] [DOI] [PubMed] [Google Scholar]
Lundqvist 2014
- Lundqvist P, Kleberg A, Edberg AK, Larsson BA, Hellström‐Westas L, Norman E. Development and psychometric properties of the Swedish ALPS‐Neo pain and stress assessment scale for newborn infants. Acta Paediatrica 2014;103(8):833‐9. [DOI: 10.1111/apa.12672; PUBMED: 24813238] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ma 2007
- Ma MX, Chen YM, He J, Zeng T, Wang JH. Effects of morphine and its withdrawal on Y‐maze spatial recognition memory in mice. Neuroscience 2007;147(4):1059‐65. [DOI: 10.1016/j.neuroscience.2007.05.020; PUBMED: 17601672] [DOI] [PubMed] [Google Scholar]
Mantz 2011
- Mantz J, Josserand J, Hamada S. Dexmedetomidine: new insights. European Journal of Anaesthesiology 2011;28(1):3‐6. [DOI: 10.1097/EJA.0b013e32833e266d; PUBMED: 20881501] [DOI] [PubMed] [Google Scholar]
Mason 2011
- Mason KP, Lerman J. Review article: dexmedetomidine in children: current knowledge and future applications. Anesthesia and Analgesia 2011;113(5):1129‐42. [DOI: 10.1213/ANE.0b013e31822b8629; PUBMED: 21821507] [DOI] [PubMed] [Google Scholar]
McPherson 2007
- McPherson RJ, Gleason C, Mascher‐Denen M, Chan M, Kellert B, Juul SE. A new model of neonatal stress which produces lasting neurobehavioral effects in adult rats. Neonatology 2007;92(1):33‐41. [DOI: 10.1159/000100084; PUBMED: 17596735] [DOI] [PubMed] [Google Scholar]
Milesi 2010
- Milesi C, Cambonie G, Jacquot A, Barbotte E, Mesnage R, Masson F, et al. Validation of a neonatal pain scale adapted to the new practices in caring for preterm newborns. Archives of Disease in Childhood. Fetal and Neonatal Edition 2010;95(4):F263‐6. [DOI: 10.1136/adc.2008.144758; PUBMED: 19221401] [DOI] [PubMed] [Google Scholar]
Ng 2017
- Ng E, Taddio A, Ohlsson A. Intravenous midazolam infusion for sedation of infants in the neonatal intensive care unit. Cochrane Database of Systematic Reviews 2017, Issue 1. [DOI: 10.1002/14651858.CD002052.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
NIH 1979
- National Institutes of Health. Report of Workshop on Bronchopulmonary Dysplasia; December 4‐6, 1978. Bethesda, MD: National Institutes of Health, National Heart, Lung, and Blood Institute; Division of Lung Diseases, 1979. Report No.: 80‐1660.
Nishina 1999
- Nishina K, Akamatsu H, Mikawa K, Shiga M, Maekawa N, Obara H, et al. The effects of clonidine and dexmedetomidine on human neutrophil functions. Anesthesia and Analgesia 1999;88(2):424‐8. [PUBMED: 9972773 ] [DOI] [PubMed] [Google Scholar]
O'Mara 2009
- O'Mara K, Gal P, Ransom JL, Wimmer JE Jr, Carlos RQ, Dimaguila MA, et al. Successful use of dexmedetomidine for sedation in a 24‐week gestational age neonate. Annals of Pharmacotherapy 2009;43(10):1707–13. [DOI: 10.1345/aph.1M245; PUBMED: 19755621] [DOI] [PubMed] [Google Scholar]
O'Mara 2012
- O'Mara K, Gal P, Wimmer J, Ransom JL, Carlos RQ, Dimaguila MA, et al. Dexmedetomidine versus standard therapy with fentanyl for sedation in mechanically ventilated premature neonates. Journal of Pediatric Pharmacology and Therapeutics 2012;17(3):252‐62. [DOI: 10.5863/1551-6776-17.3.252; PUBMED: 23258968] [DOI] [PMC free article] [PubMed] [Google Scholar]
Papile 1978
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. Journal of Pediatrics 1978;92(4):529‐34. [PUBMED: 305471] [DOI] [PubMed] [Google Scholar]
Paris 2006
- Paris A, Mantz J, Tonner PH, Hein L, Brede M, Gressens P. The effects of dexmedetomidine on perinatal excitotoxic brain injury are mediated by the alpha2A‐adrenoceptor subtype. Anesthesia and Analgesia 2006;102(2):456‐61. [DOI: 10.1213/01.ane.0000194301.79118.e9; PUBMED: 16428542] [DOI] [PubMed] [Google Scholar]
Parodi 2015
- Parodi A, Morana G, Severino MS, Malova M, Natalizia AR, Sannia A, et al. Low‐grade intraventricular hemorrhage: is ultrasound good enough?. Journal of Maternal‐Fetal & Neonatal Medicine 2015;28(Suppl 1):2261‐4. [DOI: 10.3109/14767058.2013.796162; PUBMED: 23968243] [DOI] [PubMed] [Google Scholar]
Peters 2003
- Peters JW, Koot HM, Grunau RE, Boer J, Druenen MJ, Tibboel D, et al. Neonatal Facial Coding System for assessing postoperative pain in infants: item reduction is valid and feasible. Clinical Journal of Pain 2003;19(6):353‐63. [PUBMED: 14600535] [DOI] [PubMed] [Google Scholar]
Pichot 2012
- Pichot C, Ghignone M, Quintin L. Dexmedetomidine and clonidine: from second‐to‐first‐line sedative agents in the critical care setting?. Journal of Intensive Care Medicine 2012;27(4):219‐37. [DOI: 10.1177/0885066610396815; PUBMED: 21525113] [DOI] [PubMed] [Google Scholar]
Pontén 2012
- Pontén E, Viberg H, Gordh T, Eriksson P, Fredriksson A. Clonidine abolishes the adverse effects on apoptosis and behaviour after neonatal ketamine exposure in mice. Acta Anaesthesiologica Scandinavica 2012;56(8):1058‐65. [DOI: 10.1111/j.1399-6576.2012.02722.x; PUBMED: 22694670] [DOI] [PubMed] [Google Scholar]
Revman 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Ricalde 1990
- Ricalde AA, Hammer RP Jr. Perinatal opiate treatment delays growth of cortical dendrites. Neuroscience Letters 1990;115(2‐3):137‐43. [PUBMED: 2172870] [DOI] [PubMed] [Google Scholar]
Riker 2009
- Riker RR, Shehabi Y, Bokesch PM, Ceraso D, Wisemandle W, Koura F, et al. SEDCOM (Safety and Efficacy of Dexmedetomidine Compared With Midazolam) Study Group. Dexmedetomidine vs midazolam for sedation of critically ill patients: a randomized trial. JAMA 2009;301(5):489‐99. [DOI: 10.1001/jama.2009.56; DOI: ] [DOI] [PubMed] [Google Scholar]
Ruokonen 2009
- Ruokonen E, Parviainen I, Jakob SM, Nunes S, Kaukonen M, Shepherd ST, et al. "Dexmedetomidine for Continuous Sedation" Investigators. Dexmedetomidine versus propofol/midazolam for long‐term sedation during mechanical ventilation. Intensive Care Medicine 2009;35(2):282‐90. [DOI: 10.1007/s00134-008-1296-0; PUBMED: 18795253] [DOI] [PubMed] [Google Scholar]
Rutherford 2010
- Rutherford MA, Supramaniam V, Ederies A, Chew A, Bassi L, Groppo M, et al. Magnetic resonance imaging of white matter diseases of prematurity. Neuroradiology 2010;52(6):505‐21. [DOI: 10.1007/s00234-010-0700-y; PUBMED: 20422407] [DOI] [PubMed] [Google Scholar]
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. GRADE Working Group. GRADE Handbook for Grading Quality of Evidence and Strength of Recommendations. https://gdt.gradepro.org/app/handbook/handbook.html. Updated October 2013.
Seatriz 1993
- Seatriz JV, Hammer RP Jr. Effects of opiates on neuronal development in the rat cerebral cortex. Brain Research Bulletin 1993;30(5‐6):523‐7. [PUBMED: 8384517 ] [DOI] [PubMed] [Google Scholar]
Sheng 2015
- Sheng Y, Standing JF. Pharmacokinetic reason for negative results of clonidine sedation in long‐term‐ventilated neonates and infants. Pediatric Critical Care Medicine 2015;16(1):92‐3. [DOI: 10.1097/PCC.0000000000000267; PUBMED: 25560290] [DOI] [PubMed] [Google Scholar]
Simons 2003
- Simons SH, Dijk M, Lingen RA, Roofthooft D, Duivenvoorden HJ, Jongeneel N, et al. Routine morphine infusion in preterm newborns who received ventilatory support: a randomized controlled trial. JAMA 2003;290(18):2419‐27. [DOI: 10.1001/jama.290.18.2419; PUBMED: 14612478] [DOI] [PubMed] [Google Scholar]
Stefovska 2008
- Stefovska VG, Uckermann O, Czuczwar M, Smitka M, Czuczwar P, Kis J, et al. Sedative and anticonvulsant drugs suppress postnatal neurogenesis. Annals of Neurology 2008;64(4):434‐45. [DOI: 10.1002/ana.21463; PUBMED: 18991352] [DOI] [PubMed] [Google Scholar]
Stevens 1996
- Stevens B, Johnston C, Petryshen P, Taddio A. Premature Infant Pain Profile: development and initial validation. Clinical Journal of Pain 1996;12(1):13‐22. [PUBMED: 8722730] [DOI] [PubMed] [Google Scholar]
Taddio 2009
- Taddio A, Shah V, Atenafu E, Katz J. Influence of repeated painful procedures and sucrose analgesia on the development of hyperalgesia in newborn infants. Pain 2009;144(1‐2):43–8. [DOI: 10.1016/j.pain.2009.02.012; PUBMED: 19329255] [DOI] [PubMed] [Google Scholar]
Taniguchi 2004
- Taniguchi T, Kidani Y, Kanakura H, Takemoto Y, Yamamoto K. Effects of dexmedetomidine on mortality rate and inflammatory responses to endotoxin‐induced shock in rats. Critical Care Medicine 2004;32(6):1322‐6. [PUBMED: 15187514] [DOI] [PubMed] [Google Scholar]
Taniguchi 2008
- Taniguchi T, Kurita A, Kobayashi K, Yamamoto K, Inaba H. Dose‐ and time‐related effects of dexmedetomidine on mortality and inflammatory responses to endotoxin‐induced shock in rats. Journal of Anesthesia 2008;22(3):221‐8. [DOI: 10.1007/s00540-008-0611-9; PUBMED: 18685927] [DOI] [PubMed] [Google Scholar]
Tasdogan 2009
- Tasdogan M, Memis D, Sut N, Yuksel M. Results of a pilot study on the effects of propofol and dexmedetomidine on inflammatory responses and intraabdominal pressure in severe sepsis. Journal of Clinical Anesthesia 2009;21(6):394‐400. [PUBMED: 10.1016/j.jclinane.2008.10.010; PUBMED: 19833271] [DOI] [PubMed] [Google Scholar]
van Dijk 2009
- Dijk M, Roofthooft DW, Anand KJ, Guldemond F, Graaf J, Simons S, et al. Taking up the challenge of measuring prolonged pain in (premature) neonates: the COMFORTneo scale seems promising. The Clinical Journal of Pain 2009;25(7):607‐16. [DOI: 10.1097/AJP.0b013e3181a5b52a; PUBMED: 19692803] [DOI] [PubMed] [Google Scholar]
Virtanen 1988
- Virtanen R, Savola JM, Saano V, Nyman L. Characterization of the selectivity, specificity and potency of medetomidine as an alpha 2‐adrenoceptor agonist. European Journal of Pharmacology 1988;150(1‐2):9‐14. [PUBMED: 2900154] [DOI] [PubMed] [Google Scholar]
Walsh 2004
- Walsh MC, Yao Q, Gettner P, Hale E, Collins M, Hensman A, et al. National Institute of Child Health and Human Development Neonatal Research Network. Impact of a physiologic definition on bronchopulmonary dysplasia rates. Pediatrics 2004;114(5):1305‐11. [DOI: 10.1542/peds.2004-0204; PUBMED: 15520112] [DOI] [PubMed] [Google Scholar]
Young 2005
- Young C, Jevtovic‐Todorovic V, Qin YQ, Tenkova T, Wang H, Labruyere J, et al. Potential of ketamine and midazolam, individually or in combination, to induce apoptotic neurodegeneration in the infant mouse brain. British Journal of Pharmacology 2005;146(2):189‐97. [DOI: 10.1038/sj.bjp.0706301; PUBMED: 15997239] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zagon 1977
- Zagon IS, McLaughlin PJ. Morphine and brain growth retardation in the rat. Pharmacology 1977;15(3):276‐82. [PUBMED: 866403] [DOI] [PubMed] [Google Scholar]


