Abstract
Background
Despite substantial improvements in myocardial preservation strategies, coronary artery bypass grafting (CABG) is still associated with severe complications. It has been reported that remote ischaemic preconditioning (RIPC) reduces reperfusion injury in people undergoing cardiac surgery and improves clinical outcome. However, there is a lack of synthesised information and a need to review the current evidence from randomised controlled trials (RCTs).
Objectives
To assess the benefits and harms of remote ischaemic preconditioning in people undergoing coronary artery bypass grafting, with or without valve surgery.
Search methods
In May 2016 we searched CENTRAL, MEDLINE, Embase and Web of Science. We also conducted a search of ClinicalTrials.gov and the International Clinical Trials Registry Platform (ICTRP). We also checked reference lists of included studies. We did not apply any language restrictions.
Selection criteria
We included RCTs in which people scheduled for CABG (with or without valve surgery) were randomly assigned to receive RIPC or sham intervention before surgery.
Data collection and analysis
Two review authors independently assessed trials for inclusion, extracted data and checked them for accuracy. We calculated mean differences (MDs), standardised mean differences (SMDs) and risk ratios (RR) using a random‐effects model. We assessed quality of the trial evidence for all primary outcomes using the GRADE methodology. We completed a ’Risk of bias’ assessment for all studies and performed sensitivity analysis by excluding studies judged at high or unclear risk of bias for sequence generation, allocation concealment and incomplete outcome data. We contacted authors for missing data. Our primary endpoints were 1) composite endpoint (including all‐cause mortality, non‐fatal myocardial infarction or any new stroke, or both) assessed at 30 days after surgery, 2) cardiac troponin T (cTnT, ng/L) at 48 hours and 72 hours, and as area under the curve (AUC) 72 hours (µg/L) after surgery, and 3) cardiac troponin I (cTnI, ng/L) at 48 hours, 72 hours, and as area under the curve (AUC) 72 hours (µg/L) after surgery.
Main results
We included 29 studies involving 5392 participants (mean age = 64 years, age range 23 to 86 years, 82% male). However, few studies contributed data to meta‐analyses due to inconsistency in outcome definition and reporting. In general, risk of bias varied from low to high risk of bias across included studies, and insufficient detail was provided to inform judgement in several cases. The quality of the evidence of key outcomes ranged from moderate to low quality due to the presence of moderate or high statistical heterogeneity, imprecision of results or due to limitations in the design of individual studies.
Compared with no RIPC, we found that RIPC has no treatment effect on the rate of the composite endpoint with RR 0.99 (95% confidence interval (CI) 0.78 to 1.25); 2 studies; 2463 participants; moderate‐quality evidence. Participants randomised to RIPC showed an equivalent or better effect regarding the amount of cTnT release measured at 72 hours after surgery with SMD ‐0.32 (95% CI ‐0.65 to 0.00); 3 studies; 1120 participants; moderate‐quality evidence; and expressed as AUC 72 hours with SMD ‐0.49 (95% CI ‐0.96 to ‐0.02); 3 studies; 830 participants; moderate‐quality evidence. We found the same result in favour of RIPC for the cTnI release measured at 48 hours with SMD ‐0.21 (95% CI ‐0.40 to ‐0.02); 5 studies; 745 participants; moderate‐quality evidence; and measured at 72 hours after surgery with SMD ‐0.37 (95% CI ‐0.59 to ‐0.15); 2 studies; 459 participants; moderate‐quality evidence. All other primary outcomes showed no differences between groups (cTnT release measured at 48 hours with SMD ‐0.14, 95% CI ‐0.33 to 0.06; 4 studies; 1792 participants; low‐quality evidence and cTnI release measured as AUC 72 hours with SMD ‐0.17, 95% CI ‐0.48 to 0.14; 2 studies; 159 participants; moderate‐quality evidence).
We also found no differences between groups for all‐cause mortality after 30 days, non‐fatal myocardial infarction after 30 days, any new stroke after 30 days, acute renal failure after 30 days, length of stay on the intensive care unit (days), any complications and adverse effects related to ischaemic preconditioning. We did not assess many patient‐centred/salutogenic‐focused outcomes.
Authors' conclusions
We found no evidence that RIPC has a treatment effect on clinical outcomes (measured as a composite endpoint including all‐cause mortality, non‐fatal myocardial infarction or any new stroke, or both, assessed at 30 days after surgery). There is moderate‐quality evidence that RIPC has no treatment effect on the rate of the composite endpoint including all‐cause mortality, non‐fatal myocardial infarction or any new stroke assessed at 30 days after surgery, or both. We found moderate‐quality evidence that RIPC reduces the cTnT release measured at 72 hours after surgery and expressed as AUC (72 hours). There is moderate‐quality evidence that RIPC reduces the amount of cTnI release measured at 48 hours, and measured 72 hours after surgery. Adequately‐designed studies, especially focusing on influencing factors, e.g. with regard to anaesthetic management, are encouraged and should systematically analyse the commonly used medications of people with cardiovascular diseases.
Keywords: Adult; Aged; Aged, 80 and over; Female; Humans; Male; Middle Aged; Coronary Artery Bypass; Area Under Curve; Cause of Death; Heart Valves; Heart Valves/surgery; Ischemic Preconditioning; Ischemic Preconditioning/adverse effects; Ischemic Preconditioning/methods; Ischemic Preconditioning/mortality; Myocardial Infarction; Myocardial Infarction/epidemiology; Randomized Controlled Trials as Topic; Stroke; Stroke/epidemiology; Troponin I; Troponin I/metabolism; Troponin T; Troponin T/metabolism
Plain language summary
Effects of remote ischaemic preconditioning in patients undergoing coronary artery bypass graft surgery (with or without valve surgery)
Review question
We reviewed the evidence about the effect of remote ischaemic preconditioning (RIPC, the temporary blockage of arterial blood flow to one arm or one leg before surgery after induction of anaesthesia) in people undergoing coronary artery bypass graft surgery with or without additional valve surgery.
Background
Coronary artery disease (CAD) results from progressive blockage of the coronary arteries. If coronary arteries are partly or fully blocked, they cannot supply the heart with enough oxygen. Symptoms of CAD include shortness of breath, pain in the upper body (e.g. arms, left shoulder, back, etc). CAD can be treated with medical therapy, percutaneous coronary intervention (PCI) or coronary artery bypass grafting (CABG). Despite substantial improvements in surgical strategies, cardiac surgery is associated with severe complications. Several approaches have been implemented to reduce the risk during surgery (hypothermia, cardioplegic solutions, and the limitation of procedure times). These strategies have led to a pronounced reduction in mortality and morbidity, however, biomarkers of ischaemia indicate persisting postoperative myocardial damage. RIPC has been reported to reduce these biomarkers of ischaemia in people who undergo cardiac surgery. The aim of this systematic review was to assess whether this practice improves clinical outcomes.
Study characteristics
We searched scientific databases for randomised trials in which people scheduled for CABG (with or without valve surgery) were randomly assigned to receive RIPC or sham intervention before surgery. The evidence is current to May 2016. We did not identify any source of bias related to the funding of included studies.
Key results
We identified 29 studies involving 5392 participants (mean age = 64 years, age range 23 to 86 years, 82% male). RIPC does not improve clinical outcome in people undergoing CABG with or without valve surgery (measured as a composite endpoint including all‐cause mortality, non‐fatal myocardial infarction or any new stroke, or both, assessed at 30 days after surgery, moderate‐quality evidence). There is moderate‐quality evidence that RIPC reduces the amount of cardiac troponin T release measured at 72 hours and measured as AUC (72 hours). There is moderate‐quality evidence that cardiac troponin I release measured at 48 hours and 72 hours after surgery is lower in the RIPC group than in the control group. Regarding troponin T measured at 48 hours and troponin I measured as AUC 72 hours after surgery there was no difference between groups (low‐ and moderate‐quality evidence). However, this effect on biomarkers does not result in improved clinical outcome.
Quality of the evidence
We used reliable methods to assess the quality of the trial evidence. The quality of the evidence of key outcomes ranged from moderate to low quality due to the presence of moderate or high statistical heterogeneity, imprecision of results or due to limitations in the design of individual studies.
Summary of findings
Summary of findings for the main comparison. RIPC versus no RIPC in people undergoing CABG (with or without valve surgery).
| RIPC versus no RIPC in people undergoing CABG (with or without valve surgery) | ||||||
|
Patient or population: people undergoing CABG (with or without valve surgery)
Settings: hospital
Intervention: RIPC Comparison: no RIPC | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk no RIPC | Corresponding risk RIPC | |||||
| Composite endpoint (including all‐cause mortality, non‐fatal myocardial infarction and/or any new stroke assessed at 30 days after surgery) | 98 per 1000 | 97 per 1000 (77 to 123) | RR 0.99 (0.78 to 1.25) | 2463 (2) | ⊕⊕⊕⊖ moderate1 | |
| Cardiac troponin T (cTnT) 48 h after surgery (ng/L) | The mean cTnT 48 h after surgery (ng/L) ranged across control groups from 2.39 to 2.61 | The mean cTnT 48 h after surgery (ng/L) in the intervention groups was 0.04lower (0.1 lower to 0.01 higher) | SMD ‐0.14 (‐0.33 to 0.06) | 1792 (4) | ⊕⊕⊖⊖ low2 | A lower troponin value indicates improvement |
| cTnT 72 h after surgery (ng/L) | The mean cTnT 72 h after surgery (ng/L) ranged across control groups from 2.457 to 2.563 | The mean cTnT 72 h after surgery (ng/L) in the intervention groups was 0.1lower (0.21 to 0 lower) | SMD ‐0.32 (‐0.65 to ‐0.00) | 1120 (3) | ⊕⊕⊕⊖ moderate3 | A lower troponin value indicates improvement |
| cTnT AUC 72 h (ng/L) | The mean cTnT AUC 72 h after surgery (ng/L) ranged across control groups from 1.399 to 1.562 | The mean cTnT AUC 72 h (µg/L) in the intervention groups was 0.12lower (0.23 to 0.02 lower) | SMD ‐0.49 (‐0.96 to ‐0.02) | 830 (3) | ⊕⊕⊖⊖ moderate3 | A lower troponin value indicates improvement |
| Cardiac troponin I (cTnI) 48 h after surgery (ng/L) | The mean cTnI 48 h after surgery (ng/L) ranged across control groups from ‐0.663 to 0.41 | The mean cTnI 48 h after surgery (µg/L) in the intervention groups was 0.11 lower (0.17 lower to 0.05 lower) | SMD ‐0.21 (‐0.40 to ‐0.02) | 745 (5) | ⊕⊕⊕⊖ moderate3 | A lower troponin value indicates improvement |
| cTnI 72 h after surgery (ng/L) | The mean cTnI 72 h after surgery (ng/L) ranged across control groups from ‐0.631 to 0.141 | The mean cTnI 72 h after surgery (µg/L) in the intervention groups was 0.18 lower (0.29 lower to 0.06 lower) | SMD ‐0.37 (‐0.59, ‐0.15) | 459 (2) | ⊕⊕⊕⊖ moderate4 | A lower troponin value indicates improvement |
| cTnI AUC 72 h (ng/L) | The mean cTnI AUC 72 h after surgery (ng/L) ranged across control groups from 1.583 to 2.394 | The mean cTnI AUC 72 h (µg/L) in the intervention groups was 0.07lower (0.19 lower to 0.06 higher) | SMD ‐0.17 (‐0.48 to 0.14) | 159 (2) | ⊕⊕⊕⊖ moderate1 | A lower troponin value indicates improvement |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). AUC: area under the curve; CABG: coronary artery bypass grafting; CI: confidence interval; RIPC: remote ischaemic preconditioning; RR: risk ratio; SMD: standardised mean difference (we used the SMD to combine data of troponin values as these biomarkers were most likely measured by using different laboratory assays.) | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Downgraded one step due to imprecision of results as shown in wide confidence intervals crossing line of no effect 2Downgraded two steps due to presence of moderate or substantial heterogeneity and imprecision of results as shown in wide confidence intervals crossing line of no effect 3Downgraded one step due to presence of moderate or substantial heterogeneity 4Downgraded one step due limitations in the design and implementation of the two available studies suggesting high likelihood of bias (one study was planned with 80 participants, during the course of the study it was decided to include another 50 participants "to increase power"; general small sample size n = 459)
Background
Cardiovascular disease (CVD) is the major contributor to the burden of disease and the number one cause of death worldwide. In 2008, 30% of all global deaths (17.3 million) were attributed to CVD (World Health Organization 2015). Of these deaths, an estimated 7.3 million were due to coronary artery disease (World Health Organization 2011).
Despite substantial improvements in myocardial preservation strategies, cardiac surgery is still associated with severe complications. This includes atrial arrhythmias, a very common and most often benign complication, with little influence on the postoperative course or long‐term outcome (Bojar 2011). However, less common complications (such as stroke, mediastinitis, tamponade, renal failure, or an acute abdomen) may be catastrophic and lead to death or prolonged hospitalisation with multisystem organ failure (Bojar 2011). The incidence of complications will further increase in the future as cardiac surgery is increasingly being performed on an aging population with increased numbers of comorbid conditions and complex coronary lesions. Analyses of large patient databases indicate that major complications including death, myocardial infarction, cardiac arrest and failure, renal failure, stroke, gastrointestinal complications and respiratory failure occur in up to 16% of all people during the initial hospital stay (Ghosh 2004).
Description of the condition
Worldwide, an estimated 800,000 to 1,000,000 CABG procedures are performed annually, with about 400,000 in the USA alone (Centers for Disease Control and Prevention 2015). Most commonly, the procedure is performed through a median sternotomy with the use of cardiopulmonary bypass (CPB) and cardioplegic arrest. Revascularisation during CABG is obtained by creating new routes for the blood (bypasses) around narrowed or blocked coronary arteries. Autologous venous or arterial graft material is harvested from the patients' internal thoracal wall or from the patients' extremities and re‐implanted in aortocoronary position. In addition to the open surgical procedure under circulatory arrest, both minimally‐invasive and beating‐heart strategies exist.
Several approaches have been implemented to reduce the perioperative risk of myocardial ischaemia. Among the most commonly applied are hypothermia, cardioplegic solutions and the general limitation of procedure times. These strategies have led to a pronounced reduction in procedural mortality and morbidity (Estafanous 2001). Nevertheless, postoperative elevated creatine kinase or troponin levels indicate persisting myocardial damage due to intraoperative ischaemia reperfusion (I/R) injury. Methods of pre‐ and post conditioning have been proven to reduce I/R damage in vitro, however the translation into a clinically relevant protective strategy is still challenging.
Description of the intervention
Ischaemic preconditioning is an experimental method to increase the body's resistance to a projected reduced oxygen supply. In the heart, ischaemic preconditioning is an intrinsic process whereby repeated short episodes of ischaemia protect the myocardium against successive ischaemic insults by decreasing the infarct size.
Since the mid‐1980s, the existence of preconditioning as a protective mechanism has been known from animal models (Murry 1986). A brief stimulus of sub‐lethal ischaemia was able to induce at least two time windows in which the myocardium is protected from otherwise deleterious noxa. In recent decades, several alternative stimuli (e.g. opioids, volatile anaesthetics, noble gases) have been shown to induce at least a partially similar effect. Amazingly, the effect can also be induced by a remotely applied temporary ischaemia. The term remote ischaemic preconditioning was first coined by Przylenk in 1993 (Przyklenk 1993). The remote ischaemic preconditioning intervention is performed by temporary inflation of a blood pressure cuff above the systolic arterial pressure on one chosen extremity. The blood flow from and to this extremity is blocked and local ischaemia occurs. Reperfusion washes released mediators from the isolated tissue into the circulation aiming to enhance the circulating level of cardioprotective substances.
Within the past decade, remote ischaemic preconditioning has been rapidly translated from experimental studies to promising proof‐of‐principle clinical trials. Various studies have demonstrated that RIPC reduces myocardial injury in various surgical settings (Ali 2007; Cheung 2006; Hausenloy 2007; Hausenloy 2010; Hausenloy 2012; Heusch 2010; Thielmann 2010). In contrast, other reports have not confirmed the positive effects of RIPC (Karuppasamy 2011; Young 2012). The underlying reasons might be the RIPC protocol itself or the use of volatile anaesthetics in several of these studies, which in itself is known to protect the heart against ischaemia/reperfusion injury (Karuppasamy 2011; Kottenberg 2012).
Currently, adverse events associated with RIPC are not known. The repeated pumping up of the blood pressure cuff is considered to be safe, and since the intervention occurs after the induction of anaesthesia, additional pain or feelings of stress through the actual intervention are unlikely. The potential risk of thrombosis, plaque rupture or embolisation in people with pre‐existing atherosclerosis in the upper extremities is also regarded as low.
How the intervention might work
In remote ischaemic preconditioning, temporal ischaemia of a distant compartment positively affects the human heart. The humoral factors involved are unclear, but it is largely agreed upon that the effect is mediated via the blood stream. Suspected mediators are nitrites (Corti 2014; Rassaf 2014), microRNA (Li 2014; Slagsvold 2014), and other chemokines. Within the myocardium, the effect is supposedly mediated via the reperfusion injury salvage kinase (RISK) and survivor activating factor enhancement (SAFE) pathways (Hausenloy 2011), and preserves mitochondrial function (Slagsvold 2014) and myocardial performance (Illes 1998; Li 1999; Lu 1997) in ischaemia/reperfusion.
Why it is important to do this review
Several randomised trials comparing RIPC versus no RIPC in people undergoing CABG have been conducted, but the results have varied and most trials were too underpowered to reach a conclusion on clinically relevant outcome measures. Following the conduct of several small trials, two major trials on remote ischaemic preconditioning have now completed recruitment and provided data on 3012 people undergoing CABG/cardiac surgery (Hausenloy 2015; Meybohm 2015). Final consensus is needed on the effectiveness of RIPC for cardiac patients scheduled for CABG.
Objectives
To assess the benefits and harms of remote ischaemic preconditioning in people undergoing coronary artery bypass grafting, with or without valve surgery.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs), studies reported in full text, those published as an abstract only and unpublished data.
Types of participants
We included adults (people aged 18 years or more) scheduled for CABG (with or without valve surgery).
Types of interventions
We included trials comparing remote ischaemic preconditioning before CABG (with or without valve surgery) with no remote ischaemic preconditioning before CABG (with or without valve surgery).
Types of outcome measures
As no core outcome set for clinical studies investigating CABG is available, we have chosen the list of outcomes based on outcome measures from possible matching studies.
Primary outcomes
Composite endpoint (including all‐cause mortality, non‐fatal myocardial infarction or any new stroke, or both) assessed at 30 days after surgery
Cardiac troponin T (cTnT, ng/L) at 48 hours, 72 hours, and as area under the curve (AUC) 72 hours (µg/L) after surgery
Cardiac troponin I (cTnI, ng/L) at 48 hours, 72 hours, and as area under the curve (AUC) 72 hours (µg/L) after surgery
Secondary outcomes
All‐cause mortality after 30 days
Non‐fatal myocardial infarction after 30 days
Any new stroke after 30 days
Acute renal failure after 30 days
Length of stay on the intensive care unit (days)
Any complications and adverse effects related to ischaemic preconditioning, as reported by trial authors
Any patient‐centred/salutogenic‐focused outcome, as reported in included study
Cardiac troponin T (cTnT, ng/L) at 6 hours, 12 hours, and 24 hours after surgery
Cardiac troponin I (cTnI, ng/L) at 6 hours, 12 hours, and 24 hours after surgery
Search methods for identification of studies
Electronic searches
We identified trials through systematic searches of the following bibliographic databases on 4 May 2016:
Cochrane Central Register of Controlled Trials (CENTRAL, 2016, Issue 4) in the Cochrane Library;
MEDLINE (Ovid, 1946 to April week 3 2016);
Embase (Ovid, 1980 to 2016 week 18);
Web of Science Core Collection (Thomson Reuters, 1900 to 3 May 2016).
We used the search strategies developed by Cochrane Heart for the database searches. The preliminary search strategy for MEDLINE (Ovid) was adapted for use in the other databases (Appendix 1). We applied the Cochrane sensitivity‐maximising RCT filter (Lefebvre 2011) to MEDLINE (Ovid) and adaptations of it to the other databases, except CENTRAL.
We also conducted a search of ClinicalTrials.gov (www.ClinicalTrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) search portal (apps.who.int/trialsearch/) in May 2016, for possible matching studies using the search terms "remote" and "preconditioning OR pre‐conditioning".
We searched all databases from their inception to the present, and we imposed no restriction on language of publication.
Searching other resources
We checked the reference lists of all primary studies and review articles for additional references. We contacted study authors for missing data. We also contacted principal investigators of identified studies to ascertain if they were aware of any other relevant published or unpublished matching clinical studies.
Data collection and analysis
The methods used in this review are in accordance with the recommendations provided in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a).
Selection of studies
We imported citations from each database into the reference management software Papers (Version 3.4.0) and removed duplicates. Two authors (CB, CS) independently screened the titles and abstracts of all the potential studies we identified as a result of the search and coded them as 'retrieve' (eligible or potentially eligible/unclear) or 'do not retrieve'. In two cases of disagreements, we asked a third author to arbitrate (AG).
We retrieved the full‐text study reports/publication and two authors (CB, AG) independently screened the full text and identified studies for inclusion. We identified and recorded reasons for the exclusion of ineligible studies (see 'Characteristics of excluded studies' tables). We resolved any disagreement through discussion. We identified and excluded duplicates and collated multiple reports of the same study so that each study rather than each report was the unit of interest in the review. We recorded the selection process in sufficient detail to complete a PRISMA flow diagram (Figure 1) (Moher 2009) and Characteristics of excluded studies tables.
1.

Study flow diagram
Data extraction and management
We used a purposely pre‐developed data collection form for study characteristics and outcome data, which we piloted on one study in the review. Two review authors (CB, JN) extracted the following study characteristics from the included studies (also compare Table 2).
1. Overview characteristics of included studies.
| Type of publication | Type of data included in this review | Number of participants randomised (total n = 5409) | Type of surgery | Off or on‐pump procedure | Anaesthestetic gas used | Location of RIPC and number of cycles of RIPC | RIPC intervention | Control/comparison intervention | |
| Ahmad 2014 | Journal article | Published data only | 67 | CABG | On‐pump | Sevoflurane/ isoflurane anaesthesia | Right upper arm, 3 cycles | 200 mmHg for 5 min | Deflated cuff |
| Ali 2010 | Journal article | Published data only | 100 | CABG | Not reported | Not reported | Right or left upper arm, 3 cycles | 200 mmHg for 5 min | Deflated cuff (30 min) |
| Candilio 2015 | Journal article | Published and unpublished data | 180 | CABG and/or valve surgery | On‐pump | Isoflurane, sevoflurane, propofol anaesthesia | Upper arm + upper thigh, 2 cycles | 200 mmHg or 15 mmHg above systolic blood pressure for 5 min | Deflated cuff (20 min) |
| Gallagher 2015 | Journal article | Published data only | 86 | CABG with or without aortic valve replacement | On‐pump | 85% isoflurane anaesthesia, rest unknown | Forearm, 3 cycles | Not reported | Deflated cuff (30 min) |
| Gegouskov 2009 | abstract | Published data only | 40 | CABG | Not reported | Not reported | Upper arm, 3 cycles | 200 mmHg for 5 min | Not reported |
| Günaydin 2000 | Journal article | Published data only | 8 | CABG | On‐pump | Fentanyl anaesthesia | Right upper arm, 2 cycles | 300 mmHg for 3 min | Deflated cuff |
| Hausenloy 2007 | Journal article | Published data only | 57 | CABG | On‐pump | Propofol anaesthesia | Right upper arm, 3 cycles | 200 mmHg for 5 min | Deflated cuff (30 min) |
| Hausenloy 2015 | Journal article | Published and unpublished data | 1612 | CABG with or without valve surgery | On‐pump | Propofol and volatile anaesthesia | Upper arm, 4 cycles | 200 mmHg for 5 min | Simulated RIPC |
| Hong 2010 | Journal article | Published data only | 130 | CABG | Off‐pump | Sevoflurane anaesthesia | Upper arm, 4 cycles | 200 mmHg for 5 min | Simulated RIPC |
| Joung 2013 | Journal article | Published data only | 98 | CABG | Off‐pump | Propofol anaesthesia | Upper arm, 4 cycles | 200 mmHg for 5 min | Deflated cuff |
| Karuppasamy 2011 | Journal article | Published data only | 54 | CABG | On‐pump | Propofol anaesthesia | Left upper arm, 3 cycles | 200 mmHg for 5 min | Deflated cuff (30 min) |
| Kottenberg 2014 | Journal article | Published data only | 24 | CABG | On‐pump | Propofol anaesthesia | Left upper arm, 3 cycles | 200 mmHg for 5 min | Deflated cuff |
| Krawczyk 2010 | abstract | Published data only | 14 | CABG | Off‐pump | Not reported | Right upper arm, 3 cycles | 200 mmHg for 5 min | Not reported |
| Krawczyk 2011 | abstract | Published data only | 19 | CABG | Off‐pump | Not reported | Right upper arm, 3 cycles | 200 mmHg for 5 min | Not reported |
| Krawczyk 2012 | abstract | Published data only | 30 | CABG | Off‐pump | Not reported | Right upper arm, 3 cycles | 200 mmHg for 5 min | Not reported |
| Krogstad 2015 | Journal article | Published data only | 92 | CABG | On‐pump | Propofol and isoflurane anaesthesia | Upper arm, 3 cycles | 200 mmHg for 5 min | Deflated cuff |
| Lomivorotov 2012 | Journal article | Published and unpublished data | 80 | CABG | On‐pump | isoflurane anaesthesia | Upper arm, 3 cycles | 200 mmHg for 5 min | Deflated cuff (30 min) |
| Lucchinetti 2012 | Journal article | Published data only | 55 | CABG | On‐pump | Isoflurane anaesthesia | Lower limb, 4 cycles | 300 mmHg for 5 min | Not reported |
| Meybohm 2013 | Journal article | Published and unpublished data | 180 | Cardiac surgery | On‐pump | Propofol anaesthesia | Upper arm, 4 cycles | 200 mmHg or 15 mmHg above systolic blood pressure for 5 min | Deflated cuff |
| Meybohm 2015 | Journal article | Published and unpublished data | 1385 | Cardiac surgery | On‐pump | Propofol anaesthesia | Upper arm, 4 cycles | 200 mmHg or 15 mmHg above systolic blood pressure for 5 min | Dummy arm used for similar cycles of inflation and deflation |
| Rahman 2010 | Journal article | Published data only | 162 | CABG | On‐pump | Propofol anaesthesia | Upper arm, 3 cycles | 200 mmHg for 5 min | Dummy arm used for similar cycles of inflation and deflation |
| Saxena 2013 | Journal article | Published data only | 30 | CABG | On‐pump | Not reported | Upper arm, 3 cycles | 20 mmHg above systolic blood pressure for 5 min | Deflated cuff |
| Shmyrev 2011 | abstract | Published data only | 31 | CABG | On‐pump | Not reported | Upper arm, 3 cycles | Not reported | Not reported |
| Slagsvold 2014 | Journal article | Published data only | 60 | CABG | On‐pump | Isoflurane anaesthesia | Upper arm, 3 cycles | 200 mmHg for 5 min | Deflated cuff |
| Sosorburam 2014 | abstract | Published data only | 268 | CABG | On‐pump | Not reported | 3 cycles | Not reported | Deflated cuff |
| Thielmann 2013 | Journal article | Published and unpublished data | 329 | CABG | On‐pump | Isoflurane or propofol anaesthesia | Upper arm, 3 cycles | 200 mmHg for 5 min | Deflated cuff |
| Venugopal 2009 | Journal article | Published data only | 45 | CABG with or without aortic valve replacement | On‐pump | Isoflurane/sevoflurane or propofol anaesthesia | Right upper arm, 3 cycles | 200 mmHg for 5 min | Deflated cuff (30 min) |
| Yildirim 2016 | Journal article | Published data only | 60 | CABG | On‐pump | Fentanyl anaesthesia | Left lower limb, 3 cycles | 200 mmHg for 5 min | Deflated cuff (25 min) |
| Young 2012 | Journal article | Published and unpublished data | 96 | CABG and/or valve surgery | On‐pump | Volatile anaesthesia | Upper arm, 3 cycles | 200 mmHg for 5 min | Dummy arm used for similar cycles of inflation and deflation |
CABG: coronary artery bypass grafting; RIPC: remote ischaemic preconditioning
Methods: study design, total duration of study, details of any 'run‐in' period, number of study centres and location, study setting, withdrawals and date of study
Participants: number (N), mean age, age range, gender, severity of condition (e.g. number of affected vessels, left ventricular ejection fraction), inclusion and exclusion criteria, reported differences between intervention and comparison groups
Interventions: intervention, comparison, concomitant medications and excluded medications, types of anaesthesia
Outcomes: primary and secondary outcomes specified and collected, and time points reported
Notes: funding for trial and notable conflicts of interest of trial authors
Two authors (CB, JN) independently extracted outcome data from the included studies. We resolved disagreements by consensus. One author (CB) transferred data into the Cochrane statistical software Review Manager 5 (RevMan 5) (RevMan 2014). We double‐checked that data were entered correctly (CB, AG, JN) by comparing the data presented in the systematic review with the study reports. A second author (JN) checked the study characteristics for accuracy against the trial report.
Assessment of risk of bias in included studies
Two authors (CB, JN) independently assessed risk of bias for each study using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We resolved any disagreements by discussion or by involving another author (AG, CS). We summarised the results of the 'Risk of bias' assessment in both a 'Risk of bias' graph (Figure 2) and a 'Risk of bias' summary (Figure 3). Seven 'Risk of bias' domains (random sequence generation (checking for possible selection bias); blinding of participants and personnel (checking for possible performance bias); blinding of outcome assessment (checking for possible detection bias); allocation concealment (checking for possible selection bias); incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations); selective reporting (checking for reporting bias); and other bias (checking for other biases)) have been identified and we outline in Appendix 2 how we assessed the risk in relation to each of these domains.
2.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study
3.
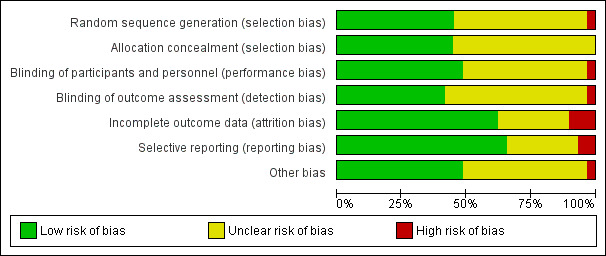
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
We summarised the risk of bias judgements across different studies for each of the domains listed (see Figure 2 and Figure 3). Where information on risk of bias relates to unpublished data or correspondence with a trialist, we noted this in the 'Risk of bias' table. We interpreted the results of the systematic review and meta‐analyses in light of the findings with respect to risk of bias. When considering treatment effects, we took into account the risk of bias for the studies that contributed to that outcome.
Assessment of bias in conducting the systematic review
We conducted the review according to the published protocol of the review (Benstoem 2015a) and report any deviations from it in the Differences between protocol and review section of this review.
Assessment of the quality of the evidence
For this review we assessed the quality of the evidence using the GRADE approach (Schunemann 2009). The GRADE approach considers five areas (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The evidence can be downgraded from ’high quality’ by one level for serious (or by two levels for very serious) limitations. We assessed the quality of the body of evidence for our primary outcomes for the comparison RIPC versus no RIPC to create a Table 1.
Measures of treatment effect
We analysed dichotomous data as risk ratios (RR) with 95% confidence intervals (CI). For continuous data, we used the mean difference (MD) with 95% CI for outcomes measured in the same way between trials. We used the standardised mean difference (SMD) with 95% CI to combine data of troponin values, as these biomarkers are typically measured by using different laboratory assays. As the distribution of troponin values is heavily skewed, approximate normal distribution is only existent on a logarithmic (log) scale. Following statistical advice, we therefore asked all included trials to provide data (means and standard deviation) on a log scale. In the case where this data could not be provided, we used the existing mean and standard deviation on a linear scale to approximately estimate the log scaled mean and standard deviation. eWe have described the formula for this in the Data synthesis section.
Unit of analysis issues
As we only included RCTs with a parallel design, unit of analysis issues did not occur.
Dealing with missing data
We contacted investigators (see Characteristics of included studies/Characteristics of excluded studies tables) to verify key study characteristics and to obtain missing numerical outcome data where possible (e.g. when a study was identified as an abstract only). Where this was not possible, and the missing data was thought to introduce serious bias, we explored the impact of including such studies in the overall assessment of results by a sensitivity analysis. We carried out analyses on an intention‐to‐treat basis for all outcomes, as far as possible.
Assessment of heterogeneity
Where we pooled data using meta‐analysis, we assessed the presence heterogeneity by visual inspection of forest plots and by examining the Chi² test for heterogeneity (Deeks 2009). We also assessed statistical heterogeneity in each meta‐analysis using the Tau², I² (Higgins 2003) and Chi² statistics. We regarded heterogeneity as substantial if:
the I² value was high (exceeding 30%); and either
there was inconsistency between trials in the direction or magnitude of effects (judged visually), or there was a low P value (< 0.10) in the Chi² test for heterogeneity; or
the estimate of between‐study heterogeneity (Tau²) was above zero.
Assessment of reporting biases
As we were not able to pool more than 10 trials, we did not create a funnel plot to explore possible small study biases for the primary outcomes by assessing funnel plot asymmetry visually and by using formal tests for funnel plot asymmetry (Egger 1997; Harbord 2006).
Data synthesis
We carried out statistical analysis using RevMan 5 (RevMan 2014). We undertook meta‐analyses only where this was meaningful, that is, the treatments, participants and the underlying clinical questions were similar enough for pooling to make sense.
As previously mentioned, in the case of missing log scale data, we transformed the linear scale mean m and variance v using the following formula: m(log) = log(m) ‐ v(log)/2 and v(log) = log(exp(log(v) ‐ 2log(m)) + 1). We then transformed data to a base of 10 for better readability of data. As all troponin data are presented on a log scale, a value of 1 therefore represents a raw value of 10, a value of 2 represents a raw value of 100 and a value of 0 represents a raw value of 1. Negative values represent raw values between 0 and 1. For transparency, we included the extracted untransformed data from the original studies in additional tables (Table 3; Table 4; Table 5; Table 6; Table 7).
2. 1st Comparison, untransformed data for troponins.
| RIPC versus no RIPC in people undergoing CABG (with or without valve surgery) | ||||||
| RIPC | No RIPC | |||||
| 1.5 cTnT 6 h after surgery (ng/L) | Mean | SD | Total | Mean | SD | Total |
| Candilio 2015 | 614 | 306 | 66 | 780 | 491 | 64 |
| Hausenloy 2007 | 310 | 290 | 27 | 590 | 450 | 30 |
| Hausenloy 2015 | 960.49 | 862 | 580 | 984.54 | 838 | 606 |
| Meybohm 2015 | 870 | 848 | 276 | 879 | 734 | 263 |
| Young 2012 | 1533 | 1784 | 48 | 837 | 547 | 46 |
| 1.6 cTnT 12 h after surgery (ng/L) | ||||||
| Candilio 2015 | 543 | 344 | 66 | 694 | 428 | 64 |
| Hausenloy 2007 | 370 | 190 | 27 | 690 | 480 | 30 |
| Hausenloy 2015 | 859 | 911 | 579 | 887.39 | 748 | 592 |
| Meybohm 2015 | 714 | 820 | 271 | 729 | 761 | 255 |
| Young 2012 | 1608 | 2361 | 48 | 725 | 589 | 46 |
| 1.7 cTnT 24 h after surgery (ng/L) | ||||||
| Meybohm 2015 | 560 | 1146 | 280 | 510 | 588 | 275 |
| Hausenloy 2015 | 695.56 | 949 | 604 | 689 | 657 | 605 |
| Hausenloy 2007 | 300 | 140 | 27 | 520 | 330 | 30 |
| Candilio 2015 | 370 | 213 | 66 | 494 | 304 | 64 |
| 1.8 cTnT 48 h after surgery (ng/L) | ||||||
| Candilio 2015 | 272 | 144 | 65 | 379 | 278 | 63 |
| Hausenloy 2007 | 300 | 170 | 27 | 520 | 490 | 30 |
| Hausenloy 2015 | 588 | 1,566 | 546 | 557 | 592 | 536 |
| Meybohm 2015 | 374 | 520 | 264 | 339 | 317 | 261 |
| 1.9 cTnT 72 h after surgery (ng/L) | ||||||
| Candilio 2015 | 232 | 161 | 63 | 378 | 325 | 63 |
| Hausenloy 2007 | 250 | 160 | 27 | 480 | 640 | 30 |
| Hausenloy 2015 | 440 | 419 | 459 | 501 | 502 | 478 |
| 1.10 cTnT AUC 72 h (µg/L) | ||||||
| Hausenloy 2007 | 20.58 | 9.58 | 27 | 36.12 | 26.08 | 30 |
| Hausenloy 2015 | 32.7 | 0 | 801 | 36.4 | 0 | 811 |
| Venugopal 2009 | 18.16 | 6.67 | 23 | 31.53 | 24.04 | 22 |
| 1.11 cTnI 1 h after surgery (µg/L) | ||||||
| Hong 2010 | 0.79 | 0.83 | 65 | 1.19 | 2.06 | 65 |
| Thielmann 2013 | 5.33 | 4 | 162 | 7.24 | 10 | 167 |
| 1.12 cTnI 6 h after surgery (µg/L) | ||||||
| Hong 2010 | 1 | 1.68 | 65 | 2.25 | 4.1 | 65 |
| Krawczyk 2011 | 0.62 | 0.43 | 9 | 3.73 | 2.25 | 10 |
| Krawczyk 2012 | 0.78 | 0.55 | 15 | 3.56 | 1.89 | 15 |
| Lomivorotov 2012 | 2.13 | 1.38 | 40 | 2.37 | 2.19 | 40 |
| Meybohm 2015 | 6 | 6 | 104 | 8 | 12 | 108 |
| Saxena 2013 | 10.14 | 7 | 15 | 14.17 | 8 | 15 |
| Thielmann 2013 | 10.78 | 78 | 162 | 12.45 | 17 | 167 |
| 1.13 cTnI 12 h after surgery (µg/L) | ||||||
| Hong 2010 | 1.14 | 1.66 | 65 | 1.54 | 2.13 | 65 |
| Krawczyk 2011 | 0.56 | 0.34 | 9 | 4.03 | 2.68 | 10 |
| Krawczyk 2012 | 0.66 | 0.44 | 15 | 3.38 | 2.39 | 15 |
| Meybohm 2015 | 4 | 4 | 103 | 6 | 11 | 112 |
| Saxena 2013 | 4.79 | 2.69 | 15 | 6.55 | 4 | 15 |
| Thielmann 2013 | 8.07 | 5 | 162 | 11.18 | 14 | 167 |
| 1.14 cTnI 24 h after surgery (µg/L) | ||||||
| Hong 2010 | 0.76 | 1.2 | 65 | 1.05 | 1.54 | 65 |
| Krawczyk 2011 | 0.42 | 0.23 | 9 | 2.46 | 2.14 | 10 |
| Krawczyk 2012 | 0.42 | 0.23 | 15 | 2.33 | 1.77 | 15 |
| Lomivorotov 2012 | 0.93 | 2.67 | 40 | 0.87 | 1.9 | 40 |
| Meybohm 2015 | 3 | 5 | 114 | 5 | 16 | 108 |
| Saxena 2013 | 2.28 | 2 | 15 | 2.94 | 1 | 15 |
| Thielmann 2013 | 5.41 | 5 | 162 | 7.71 | 9.95 | 167 |
| 1.15 cTnI 48 h after surgery (µg/L) | ||||||
| Hong 2010 | 0.61 | 0.92 | 65 | 0.76 | 1.1 | 65 |
| Lomivorotov 2012 | 0.76 | 2.37 | 40 | 0.54 | 1.23 | 40 |
| Meybohm 2015 | 2 | 3.7 | 84 | 2 | 7 | 92 |
| Saxena 2013 | 1.18 | 0.773 | 15 | 1.75 | 0.811 | 15 |
| Thielmann 2013 | 2.94 | 3 | 162 | 4.6 | 7 | 167 |
| 1.16 cTnI 72 h after surgery (µg/L) | ||||||
| Hong 2010 | 0.35 | 0.56 | 65 | 0.39 | 0.52 | 65 |
| Thielmann 2013 | 1.55 | 2 | 162 | 2.53 | 4 | 167 |
| 1.17 cTnI AUC 72 h (µg/L) | ||||||
| Hong 2010 | 53.2 | 72.9 | 65 | 67.4 | 97.7 | 65 |
| Kottenberg 2014 | 285 | 201 | 15 | 330 | 290 | 14 |
AUC: area under the curve; CABG: coronary artery bypass grafting; cTnI: cardiac troponin I; cTnT: cardiac troponin T; RIPC: remote ischaemic preconditioning
3. 2nd Comparison, untransformed data for troponins.
| RIPC versus no RIPC (subgroup analysis: isolated CABG without additional surgery) | ||||||
| RIPC | No RIPC | |||||
| Mean | SD | Total | Mean | SD | Total | |
| 2.2 cTnT 48 h after surgery (ng/L) | ||||||
| Hausenloy 2007 | 300 | 170 | 27 | 520 | 490 | 30 |
| Hausenloy 2015 | 449.017 | 537.042 | 290 | 439.049 | 539.901 | 265 |
| Meybohm 2015 | 308 | 517 | 188 | 295 | 309 | 189 |
| 2.3 cTnT 72 h after surgery (ng/L) | ||||||
| Hausenloy 2007 | 250 | 160 | 27 | 480 | 640 | 30 |
| Hausenloy 2015 | 393.952 | 480.83 | 251 | 391.335 | 431.575 | 233 |
| 2.4 cTnT AUC 72 h (µg/L) | ||||||
| Hausenloy 2007 | 20.58 | 9.58 | 27 | 36.12 | 26.08 | 30 |
| 2.5 cTnI 48 h after surgery (µg/L) | ||||||
| Hong 2010 | 0.61 | 0.92 | 65 | 0.76 | 1.1 | 65 |
| Lomivorotov 2012 | 0.76 | 2.37 | 40 | 0.54 | 1.23 | 40 |
| Meybohm 2015 | 1.037 | 1.277 | 64 | 2.308 | 8.167 | 75 |
| Saxena 2013 | 1.18 | 0.773 | 15 | 1.75 | 0.811 | 15 |
| Thielmann 2013 | 2.94 | 3.436 | 162 | 4.6 | 6.849 | 167 |
| 2.6 cTnI 72 h after surgery (µg/L) | ||||||
| Hong 2010 | 0.35 | 0.56 | 65 | 0.39 | 0.52 | 65 |
| Thielmann 2013 | 1.55 | 2.418 | 162 | 2.53 | 3.876 | 167 |
| 2.7 cTnI AUC 72 h (µg/L) | ||||||
| Hong 2010 | 53.2 | 72.9 | 65 | 67.4 | 97.7 | 65 |
| Kottenberg 2014 | 285 | 201 | 15 | 330 | 290 | 14 |
AUC: area under the curve; CABG: coronary artery bypass grafting; cTnI: cardiac troponin I; cTnT: cardiac troponin T; RIPC: remote ischaemic preconditioning
4. 3rd Comparison, untransformed data for troponins.
| RIPC versus no RIPC (subgroup analysis in people undergoing CABG, with or without valve surgery and a high preoperative risk status, EuroSCORE ≥ 6) | ||||||
| RIPC | No RIPC | |||||
| Mean | SD | Total | Mean | SD | Total | |
| 3.2 cTnT 48 h after surgery (ng/L) | ||||||
| Hausenloy 2015 | 625.659 | 1822.498 | 393 | 592.255 | 596.452 | 376 |
| Meybohm 2015 | 543 | 536 | 61 | 433 | 365 | 64 |
| 3.3 cTnT 72 h after surgery (ng/L) | ||||||
| Hausenloy 2015 | 441.336 | 421.671 | 333 | 538.079 | 54.254 | 328 |
| 3.4 cTnI 48 h after surgery (µg/L) | ||||||
| Meybohm 2015 | 3.409 | 7.224 | 19 | 2.429 | 2.423 | 19 |
AUC: area under the curve; CABG: coronary artery bypass grafting; cTnI: cardiac troponin I; cTnT: cardiac troponin T; RIPC: remote ischaemic preconditioning
5. 4th Comparison, untransformed data for troponins.
| RIPC versus no RIPC (subgroup analysis: isoflurane anaesthesia or similar volatile agents versus propofol anaesthesia in people undergoing CABG, with or without valve surgery) | ||||||
| RIPC | No RIPC | |||||
| Mean | SD | Total | Mean | SD | Total | |
| 4.2 cTnT 48 h after surgery (ng/L) | ||||||
| 4.2.1 Isoflurane anaesthesia or similar volatile agents | ||||||
| Hausenloy 2015 | 448.4 | 309.462 | 10 | 304.167 | 127.589 | 6 |
| 4.2.2 Propofol anaesthesia | ||||||
| Hausenloy 2007 | 300 | 170 | 27 | 520 | 490 | 30 |
| Hausenloy 2015 | 578.383 | 652.856 | 256 | 582.749 | 628.337 | 235 |
| Meybohm 2015 | 375 | 525 | 259 | 337 | 318 | 258 |
| 4.3 cTnT 72 h after surgery (ng/L) | ||||||
| 4.3.1 Isoflurane anaesthesia or similar volatile agents | ||||||
| Hausenloy 2015 | 411.778 | 283.678 | 9 | 235.333 | 119.652 | 6 |
| 4.3.2 Propofol anaesthesia | ||||||
| Hausenloy 2007 | 250 | 160 | 27 | 480 | 640 | 30 |
| Hausenloy 2015 | 480.421 | 494.477 | 216 | 533.91 | 576.106 | 212 |
| 4.4 cTnT AUC 72 h (µg/L) | ||||||
| 4.4.1 Isoflurane anaesthesia or similar volatile agents | ||||||
| Venugopal 2009 | 18.16 | 6.67 | 23 | 31.53 | 24.04 | 22 |
| 4.4.2 Propofol anaesthesia | ||||||
| Hausenloy 2007 | 20.58 | 9.58 | 27 | 36.12 | 26.08 | 30 |
| 4.5 cTnI 48 h after surgery (µg/L) | ||||||
| 4.5.1 Isoflurane anaesthesia or similar volatile agents | ||||||
| Hong 2010 | 0.61 | 0.92 | 65 | 0.76 | 1.1 | 65 |
| Lomivorotov 2012 | 0.76 | 0.37 | 40 | 0.54 | 1.23 | 40 |
| 4.5.2 Propofol anaesthesia | ||||||
| Meybohm 2015 | 1.715 | 3.7 | 84 | 2.478 | 7.524 | 90 |
| 4.6 cTnI 72 h after surgery (µg/L) | ||||||
| 4.6.1 Isoflurane anaesthesia or similar volatile agents | ||||||
| Hong 2010 | 0.35 | 0.56 | 65 | 0.39 | 0.52 | 65 |
| 4.6.2 Propofol anaesthesia | ||||||
| ‐ | ||||||
| 4.7 cTnI AUC 72 h (µg/L) | ||||||
| 4.7.1 Isoflurane anaesthesia or similar volatile agents | ||||||
| Hong 2010 | 53.2 | 72.9 | 65 | 67.4 | 97.7 | 65 |
| 4.7.2 Propofol anaesthesia | ||||||
| Kottenberg 2014 | 285 | 201 | 15 | 330 | 290 | 14 |
AUC: area under the curve; CABG: coronary artery bypass grafting; cTnI: cardiac troponin I; cTnT: cardiac troponin T; RIPC: remote ischaemic preconditioning
6. 5th Comparison, untransformed data for troponins.
| RIPC versus no RIPC (sensitivity analysis by sequence generation, allocation concealment and incomplete outcome data in patients undergoing CABG, with or without valve surgery) | ||||||
| RIPC | No RIPC | |||||
| Mean | SD | Total | Mean | SD | Total | |
| 5.2 cTnT 48 h after surgery (ng/L) | ||||||
| Hausenloy 2015 | 587.624 | 1566.324 | 546 | 556.907 | 591.826 | 536 |
| Meybohm 2015 | 374 | 520 | 264 | 339 | 317 | 261 |
| 5.3 cTnT 72 h after surgery (ng/L) | ||||||
| Hausenloy 2015 | 440.166 | 418.592 | 459 | 500.646 | 502.244 | 478 |
| 5.4 cTnI 48 h after surgery (µg/L) | ||||||
| Hong 2010 | 0.61 | 0.92 | 65 | 0.76 | 1.1 | 65 |
| Meybohm 2015 | 1.715 | 3.7 | 84 | 2.432 | 7.447 | 92 |
| Thielmann 2013 | 2.94 | 3.436 | 162 | 4.6 | 6.849 | 167 |
| 5.5 cTnI 72 h after surgery (µg/L) | ||||||
| Hong 2010 | 0.35 | 0.56 | 65 | 0.39 | 0.52 | 65 |
| Thielmann 2013 | 1.55 | 2.418 | 162 | 2.53 | 3.876 | 167 |
| 5.6 cTnI AUC 72 h (µg/L) | ||||||
| Hong 2010 | 53.2 | 72.9 | 65 | 67.4 | 97.7 | 65 |
AUC: area under the curve; CABG: coronary artery bypass grafting; cTnI: cardiac troponin I; cTnT: cardiac troponin T; RIPC: remote ischaemic preconditioning
Given the clinical heterogeneity in the modus operandi of RIPC, the heart surgery performed (CABG with or without valve surgery) and during the postoperative course, we used random‐effects meta‐analysis to produce an overall summary of average treatment effect across trials. We treated the random‐effects summary as the average range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine trials. We present results as the average treatment effect with its 95% confidence interval, and the estimates of Tau² and I².
Subgroup analysis and investigation of heterogeneity
We identified potential sources of heterogeneity a priori in relation to:
differences in concomitant or no concomitant cardiovascular procedure to CABG (with or without valve surgery);
differences in the number of RIPC cycles or their length;
differences in the localisation of RIPC (upper or lower limb);
differences in surgical techniques (off‐pump versus on‐pump CABG).
However, during the course of this systematic review, no noteworthy differences became apparent between included studies with regard to 1) differences in the number of RIPC cycles or their length; 2) differences in the localisation of RIPC (upper or lower limb); or 3) differences in surgical techniques (off‐pump versus on‐pump CABG) (for a detailed description see Characteristics of included studies and Table 2). Therefore, to explain heterogeneity among study results, we performed three subgroup analyses:
RIPC versus no RIPC (subgroup analysis: isolated CABG without additional surgery);
RIPC versus no RIPC in participants with a high preoperative risk status (EuroSCORE 6 or more) versus no RIPC (CABG with or without valve surgery versus isolated CABG) in participants with a high preoperative risk status (EuroSCORE 6 or more); and
RIPC versus no RIPC (in participants undergoing CABG, with or without valve surgery, subgroup analysis: isoflurane anaesthesia or similar volatile agents versus propofol anaesthesia).
We used random‐effects analysis to produce it and restricted them to the primary outcomes. We used the formal test for subgroup interactions in RevMan 5 (RevMan 2014) and reported the results of subgroup analyses quoting the Chi² test and P value, and the I² value of the interaction test.
Sensitivity analysis
We performed sensitivity analysis by limiting analyses to studies at low risk of bias. This was done by excluding studies judged at high or unclear risk of bias for sequence generation, allocation concealment and incomplete outcome data. We give the criteria for these judgements in the Assessment of risk of bias in included studies section for each included study. We limited sensitivity analyses to primary outcomes (see Types of outcome measures).
Reaching conclusions
We based our conclusions only on findings from the quantitative or narrative synthesis of included studies for this review. We avoided making recommendations for practice and our implications for research suggest priorities for future research and outline what the remaining uncertainties are in the area.
Results
Description of studies
See Characteristics of included studies and Characteristics of excluded studies as well as overview of baseline characteristics of included studies (Additional tables).
Results of the search
We performed the database searches in May 2016 and identified 700 citations with potential for inclusion. We had knowledge of two additional, recently published possibly matching studies (Gallagher 2015; Zarbock 2015) on RIPC that were not included in the initial search results and a further nine were identified through other sources. After removal of duplicates, we excluded 321 citations during the initial screening of titles and abstracts. Nine studies were ongoing at the time of this review, and results were not yet published; we contacted the study authors. Those that responded wished to withhold results until after publication (see Characteristics of ongoing studies).
Overall, we assessed 76 records on the basis of a full‐text review. We found seven studies (Candilio 2015; Meybohm 2015; Walsh 2016; Young 2012; Zarbock 2015; Zimmerman 2011; Zitta 2014 (Meybohm 2013) that randomised people scheduled CABG with or without valve surgery and valve surgery alone, which does not correspond with our inclusion criteria. However, we contacted the study authors to ask if they could provide data on CABG with or without valve surgery only. Of these, three studies (Candilio 2015; Meybohm 2015; Young 2012) provided data sets excluding valve‐only participants from the analysis and were therefore included in our review. The authors of Zitta 2014 (Meybohm 2013) informed us that the study is a subgroup analysis as part of the pilot study Meybohm 2013. The authors of the remaining three studies (Walsh 2016; Zarbock 2015; Zimmerman 2011) did not respond. In total 22 studies failed to meet the inclusion criteria (see Characteristics of excluded studies) and one study is awaiting classification (Gasparovic 2014a). Thus, 29 studies (reported in 50 publications) are included in this review (please refer to the PRISMA chart for overview of the selection process, Figure 1).
Included studies
A total of 29 RCTs are included in this review (Ahmad 2014; Ali 2010; Candilio 2015; Gallagher 2015; Gegouskov 2009; Günaydin 2000; Hausenloy 2007; Hausenloy 2015; Hong 2010; Joung 2013; Karuppasamy 2011; Kottenberg 2014; Krawczyk 2010; Krawczyk 2011; Krawczyk 2012; Krogstad 2015; Lomivorotov 2012; Lucchinetti 2012; Meybohm 2013; Meybohm 2015; Rahman 2010; Saxena 2013; Shmyrev 2011; Slagsvold 2014; Sosorburam 2014; Thielmann 2013; Venugopal 2009; Yildirim 2016; Young 2012). We provided detailed descriptions of these individual studies in the Characteristics of included studies tables. These studies involved 5392 participants (mean age = 64 years, age range 23 to 86 years, 82% male) randomly assigned to either receive remote ischaemic preconditioning or sham remote ischaemic preconditioning before cardiac surgery. All trials that met the inclusion criteria used a standard parallel‐group design. For a detailed account of the criteria required for inclusion, see Criteria for considering studies for this review. Six citations (Gegouskov 2009; Krawczyk 2010; Krawczyk 2011; Krawczyk 2012; Shmyrev 2011; Sosorburam 2014) referred only to an abstract. We contacted the study authors (if contact details were obtainable) to get further information on the studies, but they did not respond. We also contacted the authors of three studies (Lomivorotov 2012; Thielmann 2013; Young 2012), who provided us with additional information, as in their final reports data were only presented as a graph, and absolute figures (e.g. on troponin values) were missing.
The majority of studies were single‐centre studies. Only two studies were performed as multicentre trials (Hausenloy 2015, Meybohm 2015). Most studies were performed in Europe (seven studies in the UK (Candilio 2015; Gallagher 2015; Hausenloy 2007; Hausenloy 2015; Karuppasamy 2011; Rahman 2010; Venugopal 2009), five studies in Germany (Gegouskov 2009; Kottenberg 2014; Meybohm 2013; Meybohm 2015; Thielmann 2013), three studies in Poland (Krawczyk 2010; Krawczyk 2011; Krawczyk 2012), two studies in Turkey (Günaydin 2000; Yildirim 2016), two studies in Norway (Krogstad 2015; Slagsvold 2014)). The other studies were performed in North America, Australia and in Asia (one study each in Canada (Lucchinetti 2012), Australia (Saxena 2013) and New Zealand (Young 2012); two studies each in Pakistan (Ahmad 2014; Ali 2010), Korea (Hong 2010; Joung 2013), and Russia (Lomivorotov 2012; Shmyrev 2011)). Only half of the studies provided details on funding (e.g. institutional funding or funding by an independent Health Department or Research Foundation). No study reported details that would raise concern for bias with regard to funding.
The sample size in the included studies ranged from eight participants (Günaydin 2000) to 1612 participants (Hausenloy 2015). Most studies did not perform a power analysis. We noted a large gender imbalance across all studies in favour of male participants, ranking only from 0% (Günaydin 2000) to 31% (Hong 2010) of participants being female. The majority of studies included people scheduled for isolated CABG (Hong 2010; Joung 2013; Karuppasamy 2011; Kottenberg 2014; Krawczyk 2010; Krawczyk 2011; Krawczyk 2012; Krogstad 2015; Lomivorotov 2012; Lucchinetti 2012; Rahman 2010; Saxena 2013; Shmyrev 2011; Slagsvold 2014; Sosorburam 2014; Thielmann 2013; Yildirim 2016), seven studies (Candilio 2015; Gallagher 2015; Hausenloy 2015; Meybohm 2013; Meybohm 2015; Venugopal 2009; Young 2012) also included participants with combined procedures (CABG with or without valve surgery). Only a minority was performed as off‐pump procedure (Hong 2010; Joung 2013; Krawczyk 2010; Krawczyk 2011; Krawczyk 2012).
RIPC was performed almost exclusively on the right or left upper arm; only one included study (Candilio 2015) chose a mixed approach and performed RIPC on the upper arm and the upper thigh. The number of cycles of RIPC carried out ranked from two to four cycles, with two studies (Candilio 2015; Günaydin 2000) performing only two cycles of RIPC and six studies (Hausenloy 2015; Hong 2010; Joung 2013; Lucchinetti 2012; Meybohm 2013; Meybohm 2015) performing four cycles of RIPC. All other studies executed three cycles of RIPC. The majority of studies applied 200 mmHg pressure during one cycle of RIPC, two studies applied 300 mmHg (Günaydin 2000; Lucchinetti 2012) and one study specified that they would apply 20 mmHg above systolic blood pressure (Saxena 2013). The control intervention was in most cases a deflated cuff around the forearm, two studies (Hausenloy 2015; Hong 2010) simulated RIPC by inflating the cuff with the valve open, three studies used a dummy arm (Meybohm 2015; Rahman 2010; Young 2012). Eight studies (Ali 2010; Gegouskov 2009; Krawczyk 2010; Krawczyk 2011; Krawczyk 2012; Saxena 2013; Shmyrev 2011; Sosorburam 2014) did not report which anaesthetic gas was used during surgery. In the remaining studies, propofol anaesthesia and isoflurane anaesthesia was used equally often or a mixed approach was chosen.
Only three of the included studies considered the skew distribution of troponin values (Hausenloy 2015, Thielmann 2013, Young 2012). Due to the variation of outcomes assessed in cardiac trials and inconsistency in outcome definition and reporting (Benstoem 2015b) none of the included studies contributed data to all of the outcomes assessed in this systematic review. Two studies (Hausenloy 2015; Meybohm 2015) performed a secondary analysis tailored to the needs of this systematic review. If we needed to convert data in order to include them in one of the meta‐analyses, we described this in detail in the Characteristics of included studies tables.
Excluded studies
Overall, we excluded 22 studies during the full‐text screening process. Ten studies were systematic reviews, five studies were laboratory tissue studies, four studies assessed a different study population, and three studies used a different intervention. The Characteristics of excluded studies provides full details of the excluded studies.
Risk of bias in included studies
Risk of bias varied across included studies, and insufficient detail was provided to inform judgement in several included studies (see Figure 2, 'Risk of bias' summary table, and Figure 3, 'Risk of bias' graph, for an overview).
Allocation
We judged 13 included studies (Candilio 2015; Hausenloy 2015; Hong 2010; Joung 2013; Kottenberg 2014; Krogstad 2015; Lucchinetti 2012; Meybohm 2015; Rahman 2010; Slagsvold 2014; Thielmann 2013; Venugopal 2009; Young 2012) as having low risk of bias in random sequence generation. Information was insufficient to permit a decision in with regard to 15 trials. We rated one study as having high risk of bias (Ali 2010).
With regard to allocation concealment, we judged 13 studies (Ali 2010; Candilio 2015; Hausenloy 2015; Hong 2010; Kottenberg 2014; Krogstad 2015; Lucchinetti 2012; Meybohm 2013; Meybohm 2015; Rahman 2010; Slagsvold 2014; Thielmann 2013; Young 2012) as having low risk of bias. The remaining 16 studies provided insufficient information on which to base judgements.
Blinding
With regard to performance bias, we judged 14 studies (Candilio 2015; Gallagher 2015; Hausenloy 2007; Hausenloy 2015; Karuppasamy 2011; Krogstad 2015; Lucchinetti 2012; Meybohm 2013; Meybohm 2015; Rahman 2010; Saxena 2013; Thielmann 2013; Yildirim 2016; Young 2012) as having low risk of bias and one study as having high risk of bias (Ali 2010). The the remaining 14 studies provided insufficient information on which to base judgements.
As a result of the nature of the objective outcomes assessed in included studies (e.g. not likely to be influenced by lack of blinding e.g. cardiac troponin T (cTnT), cardiac troponin I (cTnI), etc.) we do not expect detection bias to have greater impact on study results, especially as we did not assess many possibly subjective outcomes that were likely to be influenced by lack of blinding (e.g. “Any patient‐centred/salutogenic‐focused outcome, as reported in included studies”), combining results in meta‐analyses was not meaningful and therefore not done. However, we only rated 12 studies (Ali 2010; Candilio 2015; Hausenloy 2015; Hong 2010; Kottenberg 2014; Krogstad 2015; Lucchinetti 2012; Meybohm 2015; Slagsvold 2014; Thielmann 2013; Yildirim 2016, Young 2012) as low risk of bias, as the level of reporting of whether outcome assessment was blinded was relatively poor across studies. We judged one study as having high risk of bias (Gallagher 2015) and the remaining 16 studies provided insufficient information on which to base judgements.
Incomplete outcome data
We judged 18 studies (Ahmad 2014; Ali 2010; Gallagher 2015; Günaydin 2000; Hausenloy 2007; Hausenloy 2015; Hong 2010; Karuppasamy 2011; Krogstad 2015; Lomivorotov 2012; Lucchinetti 2012; Meybohm 2015; Rahman 2010; Saxena 2013; Slagsvold 2014; Thielmann 2013; Venugopal 2009; Yildirim 2016) as having low risk of attrition bias and three studies as having high risk: 29% of participants dropped out of Joung 2013; 21% of participants dropped out of Kottenberg 2014; and 27% of participants dropped out of Meybohm 2013. Information was insufficient on which to base judgements in the remaining eight studies.
Selective reporting
We found no trial registration protocol for most studies to confirm whether all prespecified outcomes were reported in the publication. For two included studies (Hausenloy 2015, Meybohm 2015) a study protocol was available. However, outcomes listed in the methods section of the included studies were reported in the results section, with the exception of two studies that we judged to have high risk of reporting bias (i.e. Günaydin 2000 who did not report lactate, PO2 and pH values; and Slagsvold 2014 who did not report troponin T values for all prespecified time points). As we were not able to pool more than 10 trials, we did not include funnel plots in this review.
Other potential sources of bias
For most studies (Ahmad 2014; Ali 2010; Gegouskov 2009; Günaydin 2000; Joung 2013; Kottenberg 2014; Krawczyk 2010; Krawczyk 2011; Krawczyk 2012; Lucchinetti 2012; Shmyrev 2011; Sosorburam 2014; Thielmann 2013; Young 2012), information was insufficient on which to base judgements for low risk of bias. However, we rated one study (Hong 2010) as high risk of bias for other potential sources of bias, as the authors stated that, although the study was planned with 80 participants, during the course of the study it was decided to include another 50 participants "to increase power", which might have had impact on the study results.
Effects of interventions
See: Table 1
See Table 1 for the comparison RIPC before CABG (with or without valve surgery) versus no RIPC before CABG (with or without valve surgery).
RIPC versus no RIPC (in participants undergoing CABG, with or without valve surgery)
For this comparison, we included all participants scheduled for CABG with or without valve surgery who were randomly assigned in the included studies and received either RIPC or no RIPC. We did not include study data on participants scheduled for isolated valve surgery (see Characteristics of included studies tables for details). We also undertook three subgroup analyses as discussed in the Subgroup analysis and investigation of heterogeneity section.
Primary outcomes
Composite endpoint (including all‐cause mortality, non‐fatal myocardial infarction and/or any new stroke) assessed at 30 days after surgery
Among the 29 trials that met the inclusion criteria of the meta‐analysis, only two (Hausenloy 2015; Meybohm 2015) made an attempt to measure the overall treatment effect by means of a composite endpoint. However, in their initial study reports the composite endpoint was not defined uniformly (ERICCA trial: death from cardiovascular causes, nonfatal myocardial infarction, coronary revascularisation, or stroke, assessed 12 months after randomisation (Hausenloy 2015); and RipHEART study: death, myocardial infarction, stroke, or acute renal failure up to the time of hospital discharge (Meybohm 2015)) and a secondary analysis (unpublished, exclusively performed for this systematic review) provided the data for this meta‐analysis. Participants randomised to receive RIPC had, on average, no difference in the rate of the composite endpoint when compared to participants allocated to the control group with RR 0.99 (95% CI 0.78 to 1.25); 2 studies; 2463 participants; I2 = 0%; moderate‐quality evidence, Analysis 1.1. A detailed description of grading the evidence is presented in the Table 1). For this outcome, we did not observe statistical heterogeneity.
1.1. Analysis.

Comparison 1 RIPC versus no RIPC in people undergoing CABG (with or without valve surgery), Outcome 1 Composite endpoint (including all‐cause mortality, non‐fatal myocardial infarction and/or any new stroke assessed at 30 days after surgery).
cTnT after CABG (ng/L) at 48 hours, 72 hours, and as area under the curve (AUC) 72 hours (µg/L) after surgery
A total of four trials measured cTnT 48 hours after CABG, with or without valve surgery. We found that, on average, the amount of cTnT released at 48 hours after surgery was not reduced among participants allocated to RIPC compared with those allocated to no RIPC with SMD ‐0.14 (95% CI ‐0.33 to 0.06); 4 studies; 1792 participants; I2 = 64%; low‐quality evidence, Analysis 1.8. However, measured at 72 hours after CABG, we found that participants randomised to receive RIPC showed, on average, an equivalent or better effect regarding the amount of cTnT release with SMD ‐0.32 (95% CI ‐0.65 to 0.00); 3 studies; 1120 participants; I2 = 68%; moderate‐quality evidence, Analysis 1.9. Also cTnT release measured as the AUC (72 hours) showed, on average, the same result in favour of RIPC with SMD ‐0.49 (95% CI ‐0.96 to ‐0.02); 3 studies; 830 participants; I2 = 73%; moderate‐quality evidence, Analysis 1.10. Heterogeneity identified was substantial for Analysis 1.8, Analysis 1.9 and Analysis 1.10, as Tau² was greater than zero, and in all cases, I² was greater than 30% and the P value for the Chi² test was less than 0.10. We undertook subgroup and sensitivity analyses to try to explore heterogeneity; although findings are presented later, they did not explain the high level of heterogeneity.
1.8. Analysis.
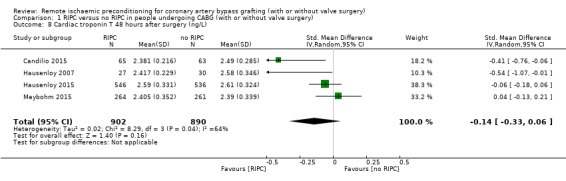
Comparison 1 RIPC versus no RIPC in people undergoing CABG (with or without valve surgery), Outcome 8 Cardiac troponin T 48 hours after surgery (ng/L).
1.9. Analysis.
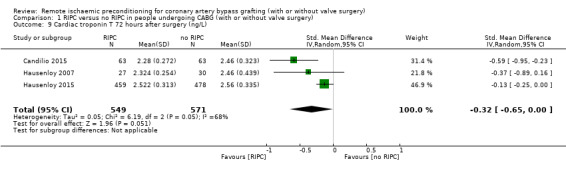
Comparison 1 RIPC versus no RIPC in people undergoing CABG (with or without valve surgery), Outcome 9 Cardiac troponin T 72 hours after surgery (ng/L).
1.10. Analysis.
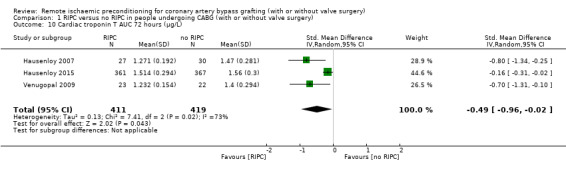
Comparison 1 RIPC versus no RIPC in people undergoing CABG (with or without valve surgery), Outcome 10 Cardiac troponin T AUC 72 hours (µg/L).
cTnI after CABG (ng/L) at 48 hours, 72 hours, and as AUC 72 hours (µg/L) after surgery
A total of five trials measured cTnI 48 hours after CABG, with or without valve surgery. We found that participants randomised to receive RIPC showed, on average, a benefit regarding the amount of cTnI release after 48 hours with SMD ‐0.21 (95% CI ‐0.40 to ‐0.02); 5 studies; 745 participants; I2 = 31%; moderate‐quality evidence, Analysis 1.15. The analysis showed that cTnI measured at 72 hours with SMD ‐0.37 (95% CI ‐0.59 to ‐0.15); 2 studies; 459 participants; I2 = 24%; moderate‐quality evidence Analysis 1.16, on average, was also reduced in favour of RIPC. In contrast, measured as AUC (72 hours) after surgery with SMD ‐0.17 (95% CI ‐0.48 to 0.14); 2 studies; 159 of participants; I2 = 0%; moderate‐quality evidence, Analysis 1.17, the analysis showed, on average, no difference among participants. The troponin I analyses measured at 48 hours and as AUC (72 hours) showed moderate presence of heterogeneity in the results obtained.
1.15. Analysis.

Comparison 1 RIPC versus no RIPC in people undergoing CABG (with or without valve surgery), Outcome 15 Cardiac troponin I 48 hours after surgery (µg/L).
1.16. Analysis.
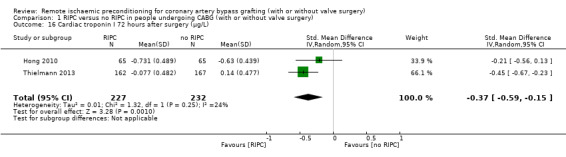
Comparison 1 RIPC versus no RIPC in people undergoing CABG (with or without valve surgery), Outcome 16 Cardiac troponin I 72 hours after surgery (µg/L).
1.17. Analysis.
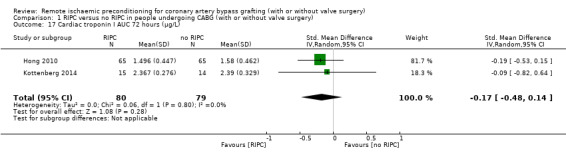
Comparison 1 RIPC versus no RIPC in people undergoing CABG (with or without valve surgery), Outcome 17 Cardiac troponin I AUC 72 hours (µg/L).
Secondary outcomes
All‐cause mortality after 30 days
A total of eight studies measured all‐cause mortality 30 days after surgery. On average, no difference with regard to mortality after 30 days was reported among participants randomised to receive RIPC compared with those randomised to no RIPC with RR 1.05 (95% CI 0.66 to 1.68); 8 studies; 3288 participants; I2 = 0%, Analysis 1.2.
1.2. Analysis.

Comparison 1 RIPC versus no RIPC in people undergoing CABG (with or without valve surgery), Outcome 2 All‐cause mortality after 30 days.
Non‐fatal myocardial infarction after 30 days
Two studies reported on the number of non‐fatal myocardial infarction after 30 days. On average, there was no difference regarding this secondary outcome between both groups with RR 0.84 (95% CI 0.62 to 1.15); 2 studies; 2463 participants; I2 = 0%; Analysis 1.3.
1.3. Analysis.

Comparison 1 RIPC versus no RIPC in people undergoing CABG (with or without valve surgery), Outcome 3 Non‐fatal myocardial infarction after 30 days.
Any new stroke after 30 days
Similar to non‐fatal myocardial infarction after 30 days, we found, on average, no difference in the number of new strokes after 30 days among participants allocated to the intervention or the comparison group with RR 1.12 (95% CI 0.60 to 2.07); 2 studies; 2463 participants; I2 = 0%; Analysis 1.4.
1.4. Analysis.

Comparison 1 RIPC versus no RIPC in people undergoing CABG (with or without valve surgery), Outcome 4 Any new stroke after 30 days.
Acute renal failure after 30 days
Only one study reported on acute renal failure after 30 days. We observed no difference between the two study groups with RR 1.37 (95% CI 0.78 to 2.40); 1 study; 851 participants; I2 not applicable; Analysis 1.18.
1.18. Analysis.

Comparison 1 RIPC versus no RIPC in people undergoing CABG (with or without valve surgery), Outcome 18 Acute renal failure after 30 days.
Length of stay on the intensive care unit (days)
Eight studies reported on the duration of ICU stay after CABG, with or without valve surgery. On average, no difference with regard to ICU duration was reported among participants allocated to RIPC compared with those allocated to no RIPC with MD ‐0.01 (95% CI ‐0.15 to 0.12); 8 studies; 3102 participants; I2 = 25%; Analysis 1.19.
1.19. Analysis.
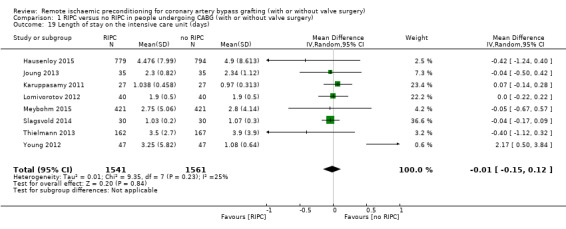
Comparison 1 RIPC versus no RIPC in people undergoing CABG (with or without valve surgery), Outcome 19 Length of stay on the intensive care unit (days).
Any complications and adverse effects related to ischaemic preconditioning, as reported by trial authors
Overall, only three studies reported adverse effects related to RIPC. Two studies observed no adverse effects, neither in the intervention nor in the control group. One study observed significantly more events (skin petechiae exclusively) in the intervention group than in the control group with RR 18.59 (95% CI 4.49 to 76.92); 3 studies; 2414 participants; I2 not applicable; Analysis 1.20.
1.20. Analysis.
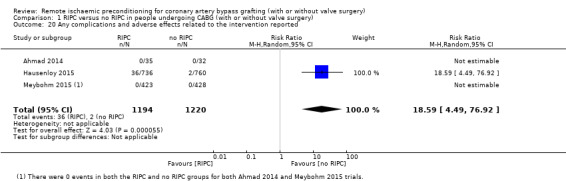
Comparison 1 RIPC versus no RIPC in people undergoing CABG (with or without valve surgery), Outcome 20 Any complications and adverse effects related to the intervention reported.
Any patient‐centred/salutogenic‐focused outcome, as reported in included studies
Included studies did hardly assess patient‐centred/salutogenic‐focused outcomes. Only one study assessed health‐related quality of life as measured by the European Quality of Life–5 Dimensions score at baseline, 6 weeks, and 3 months, 6 months, 9 months, and 12 months (Hausenloy 2015). They found no difference between groups with regard to quality of life. No other patient‐centred or salutogenic‐focused outcome was evaluated. Therefore, we were not able to pool data by means of meta‐analysis.
cTnT after CABG (ng/L) at 6 hours, 12 hours, and 24 hours after surgery
A total of five studies measured cTnT at 6 hours after surgery, with SMD ‐0.13 (95% CI ‐0.38 to 0.12); 5 studies; 2006 participants; I2 = 80%, Analysis 1.5; and at 12 hours after surgery, with SMD ‐0.14 (95% CI ‐0.40 to 0.13); 5 studies; 1978 participants; I2 = 82%, Analysis 1.6; and a total of four studies at 24 hours after surgery, with SMD ‐0.25 (95% CI ‐0.50 to 0.01); 4 studies; 1951 participants; I2 = 80%, Analysis 1.7, but did not find, on average, a benefit in the amount of cTnT released. In all three meta‐analyses, we identified substantial statistical heterogeneity.
1.5. Analysis.
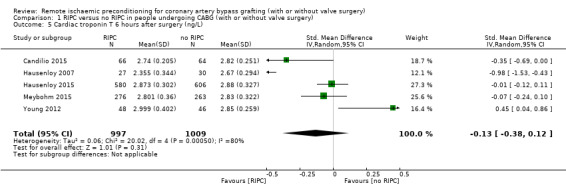
Comparison 1 RIPC versus no RIPC in people undergoing CABG (with or without valve surgery), Outcome 5 Cardiac troponin T 6 hours after surgery (ng/L).
1.6. Analysis.

Comparison 1 RIPC versus no RIPC in people undergoing CABG (with or without valve surgery), Outcome 6 Cardiac troponin T 12 hours after surgery (ng/L).
1.7. Analysis.

Comparison 1 RIPC versus no RIPC in people undergoing CABG (with or without valve surgery), Outcome 7 Cardiac troponin T 24 hours after surgery (ng/L).
cTnI after CABG (ng/L) at 6 hours, 12 hours, and 24 hours after surgery
A total of seven studies measured cTnI at 6 hours after surgery, with SMD ‐0.85 (95% CI ‐1.39 to ‐0.30); 7 studies; 830 participants; I2 = 91%, Analysis 1.12; six studies at 12 hours after surgery, with SMD ‐0.89 (95% CI ‐1.42 to ‐0.36); 6 studies; 753 participants; I2 = 89%, Analysis 1.13; and seven studies at 24 hours after surgery, with SMD ‐0.61 (95% CI ‐1.01 to ‐0.21); 7 studies; 840 participants; I2 = 83%, Analysis 1.14, and did find, on average, a difference in the amount of cTnI released. In all three meta‐analyses, we identified substantial statistical heterogeneity.
1.12. Analysis.
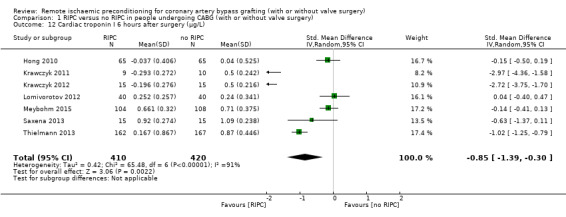
Comparison 1 RIPC versus no RIPC in people undergoing CABG (with or without valve surgery), Outcome 12 Cardiac troponin I 6 hours after surgery (µg/L).
1.13. Analysis.

Comparison 1 RIPC versus no RIPC in people undergoing CABG (with or without valve surgery), Outcome 13 Cardiac troponin I 12 hours after surgery (µg/L).
1.14. Analysis.
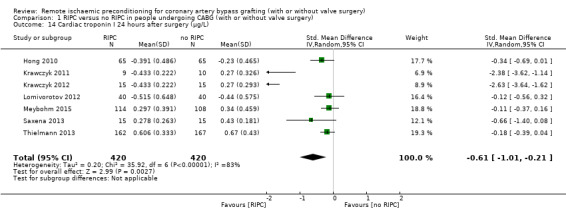
Comparison 1 RIPC versus no RIPC in people undergoing CABG (with or without valve surgery), Outcome 14 Cardiac troponin I 24 hours after surgery (µg/L).
Subgroup and sensitivity analyses
RIPC versus no RIPC (subgroup analysis: isolated CABG without additional surgery)
A total of 22 studies focused on isolated CABG without additional surgery, of these, 10 studies (Candilio 2015; Hausenloy 2007; Hausenloy 2015; Hong 2010; Kottenberg 2014; Lomivorotov 2012; Meybohm 2015; Saxena 2013; Thielmann 2013; Venugopal 2009) contributed data to this subgroup analysis, but not all studies to all outcomes. With regard to the composite endpoint, the evidence suggested no difference in treatment effect for this subgroup with RR 1.03 (95% CI 0.73 to 1.45); 2 studies; 1447 participants; I2 = 0%, Analysis 2.1. With regard to cTnT release measured as AUC 72 hours after surgery, (SMD ‐0.80 (95% CI ‐1.34 to ‐0.25); 1 study; 57 participants; I2 not applicable; Analysis 2.4), cTnI at 48 hours after surgery, (SMD ‐0.19 (95% CI ‐0.41 to ‐0.02); 5 studies; 708 participants; I2 = 42%, Analysis 2.5), cTnI at 72 hours after surgery (SMD ‐0.37 (95% CI ‐0.59 to ‐0.15); 2 studies; 459 participants; I2 = 24%, Analysis 2.6) and cTnI measured as AUC 72 hours after surgery, (SMD ‐0.17 (95% CI ‐0.48 to 0.14); 2 studies; 159 participants; I2 = 0%, Analysis 2.7), the evidence suggested a benefit of RIPC for participants undergoing isolated CABG. No difference was reported for cTnT 48 hours after surgery between both groups with SMD ‐0.18 (95% CI ‐0.46 to 0.11); 3 studies; 989 participants; I2 = 74%, Analysis 2.2).
2.1. Analysis.
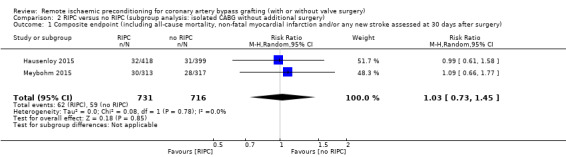
Comparison 2 RIPC versus no RIPC (subgroup analysis: isolated CABG without additional surgery), Outcome 1 Composite endpoint (including all‐cause mortality, non‐fatal myocardial infarction and/or any new stroke assessed at 30 days after surgery).
2.4. Analysis.

Comparison 2 RIPC versus no RIPC (subgroup analysis: isolated CABG without additional surgery), Outcome 4 Cardiac troponin T AUC 72 hours (µg/L).
2.5. Analysis.
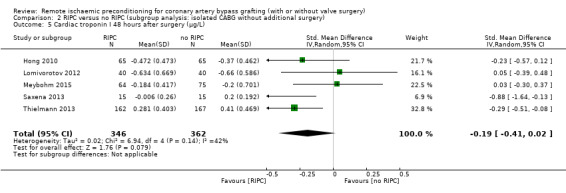
Comparison 2 RIPC versus no RIPC (subgroup analysis: isolated CABG without additional surgery), Outcome 5 Cardiac troponin I 48 hours after surgery (µg/L).
2.6. Analysis.
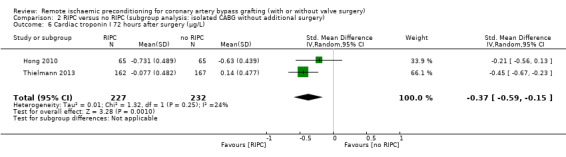
Comparison 2 RIPC versus no RIPC (subgroup analysis: isolated CABG without additional surgery), Outcome 6 Cardiac troponin I 72 hours after surgery (µg/L).
2.7. Analysis.
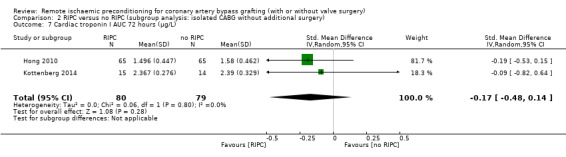
Comparison 2 RIPC versus no RIPC (subgroup analysis: isolated CABG without additional surgery), Outcome 7 Cardiac troponin I AUC 72 hours (µg/L).
2.2. Analysis.
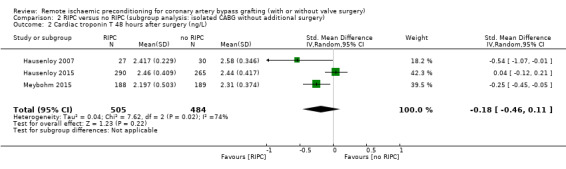
Comparison 2 RIPC versus no RIPC (subgroup analysis: isolated CABG without additional surgery), Outcome 2 Cardiac troponin T 48 hours after surgery (ng/L).
RIPC versus no RIPC (in people undergoing CABG, with or without valve surgery and a high preoperative risk status, EuroSCORE 6 or more)
Only two studies (Hausenloy 2015; Meybohm 2015) were included in this subgroup analysis and were able to contribute data on participants based on their individual preoperative risk status. Cardiac troponin T 72 hours after surgery was reduced in the RIPC group with SMD ‐0.90 (95% CI ‐1.06 to ‐0.74); 1 study; 661 participants; assessment of heterogeneity not applicable, Analysis 3.3; showing that participants with a high preoperative risk status (EuroSCORE 6 or more) also had a reduced troponin T release 72 hours after surgery. We found no evidence that there is a treatment effect for this subgroup with regard to the composite endpoint (RR 1.02 (95% CI 0.74 to 1.38); 2 studies; 1317 participants; I2 = 0%, Analysis 3.1), cTnT measured 48 hours after surgery (SMD ‐0.21 (95% CI ‐0.98 to 0.56); 2 studies; 894 participants; I2 = 94%, Analysis 3.2) or cTnI measured 48 hours after surgery (SMD ‐0.15 (95% CI ‐0.79 to 0.49); 1 study; 38 participants; assessment of heterogeneity not applicable, Analysis 3.4).
3.3. Analysis.
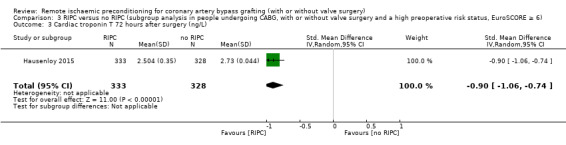
Comparison 3 RIPC versus no RIPC (subgroup analysis in people undergoing CABG, with or without valve surgery and a high preoperative risk status, EuroSCORE ≥ 6), Outcome 3 Cardiac troponin T 72 hours after surgery (ng/L).
3.1. Analysis.

Comparison 3 RIPC versus no RIPC (subgroup analysis in people undergoing CABG, with or without valve surgery and a high preoperative risk status, EuroSCORE ≥ 6), Outcome 1 Composite endpoint (including all‐cause mortality, non‐fatal myocardial infarction and/or any new stroke assessed at 30 days after surgery).
3.2. Analysis.
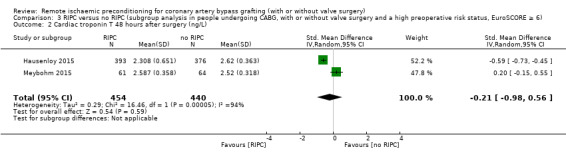
Comparison 3 RIPC versus no RIPC (subgroup analysis in people undergoing CABG, with or without valve surgery and a high preoperative risk status, EuroSCORE ≥ 6), Outcome 2 Cardiac troponin T 48 hours after surgery (ng/L).
3.4. Analysis.
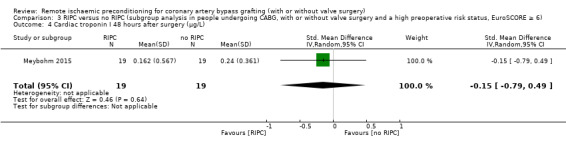
Comparison 3 RIPC versus no RIPC (subgroup analysis in people undergoing CABG, with or without valve surgery and a high preoperative risk status, EuroSCORE ≥ 6), Outcome 4 Cardiac troponin I 48 hours after surgery (µg/L).
RIPC versus no RIPC (subgroup analysis: isoflurane anaesthesia or similar volatile agents versus propofol anaesthesia in people undergoing CABG, with or without valve surgery)
A total of seven studies (Hausenloy 2007; Hausenloy 2015; Hong 2010; Kottenberg 2014; Lomivorotov 2012; Meybohm 2015; Venugopal 2009) contributed data to the subgroup analysis investigating any possible influence of type of anaesthetic gas used on study results. We found no evidence of a treatment effect between subgroups (Analysis 4.1; Analysis 4.2; Analysis 4.3; Analysis 4.4; Analysis 4.5; Analysis 4.6; Analysis 4.7).
4.1. Analysis.
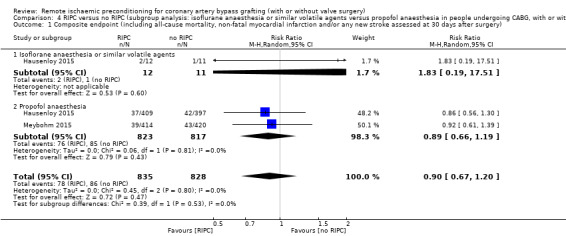
Comparison 4 RIPC versus no RIPC (subgroup analysis: isoflurane anaesthesia or similar volatile agents versus propofol anaesthesia in people undergoing CABG, with or without valve surgery), Outcome 1 Composite endpoint (including all‐cause mortality, non‐fatal myocardial infarction and/or any new stroke assessed at 30 days after surgery).
4.2. Analysis.

Comparison 4 RIPC versus no RIPC (subgroup analysis: isoflurane anaesthesia or similar volatile agents versus propofol anaesthesia in people undergoing CABG, with or without valve surgery), Outcome 2 Cardiac troponin T 48 hours after surgery (ng/L).
4.3. Analysis.
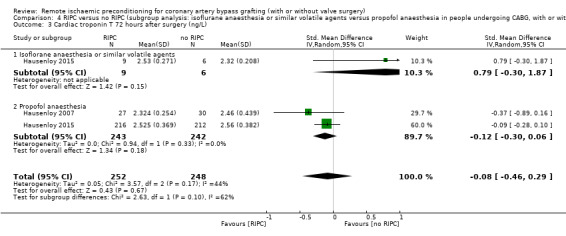
Comparison 4 RIPC versus no RIPC (subgroup analysis: isoflurane anaesthesia or similar volatile agents versus propofol anaesthesia in people undergoing CABG, with or without valve surgery), Outcome 3 Cardiac troponin T 72 hours after surgery (ng/L).
4.4. Analysis.
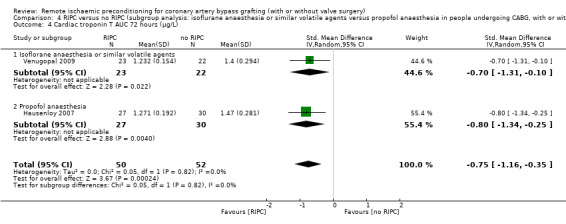
Comparison 4 RIPC versus no RIPC (subgroup analysis: isoflurane anaesthesia or similar volatile agents versus propofol anaesthesia in people undergoing CABG, with or without valve surgery), Outcome 4 Cardiac troponin T AUC 72 hours (µg/L).
4.5. Analysis.
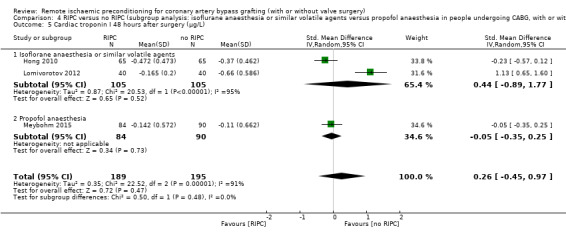
Comparison 4 RIPC versus no RIPC (subgroup analysis: isoflurane anaesthesia or similar volatile agents versus propofol anaesthesia in people undergoing CABG, with or without valve surgery), Outcome 5 Cardiac troponin I 48 hours after surgery (µg/L).
4.6. Analysis.

Comparison 4 RIPC versus no RIPC (subgroup analysis: isoflurane anaesthesia or similar volatile agents versus propofol anaesthesia in people undergoing CABG, with or without valve surgery), Outcome 6 Cardiac troponin I 72 hours after surgery (µg/L).
4.7. Analysis.
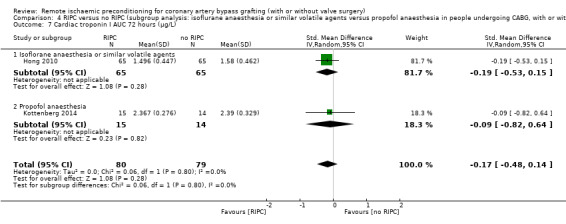
Comparison 4 RIPC versus no RIPC (subgroup analysis: isoflurane anaesthesia or similar volatile agents versus propofol anaesthesia in people undergoing CABG, with or without valve surgery), Outcome 7 Cardiac troponin I AUC 72 hours (µg/L).
RIPC versus no RIPC (sensitivity analysis by random sequence generation, allocation concealment and incomplete outcome data in people undergoing CABG, with or without valve surgery)
A sensitivity analysis included only studies of high quality (studies for which both allocation concealment and incomplete outcome data were rated as low risk) (see 'Risk of bias' summary, Figure 2). Eight studies met the criteria for high quality (Hausenloy 2015; Hong 2010; Krogstad 2015; Lucchinetti 2012; Meybohm 2015; Rahman 2010; Slagsvold 2014; Thielmann 2013). Effect estimates were consistent with overall summary effect estimates for all primary outcomes when contributing data were restricted to high‐quality studies (Analysis 5.1; Analysis 5.2; Analysis 5.3; Analysis 5.4; Analysis 5.5; Analysis 5.6).
5.1. Analysis.
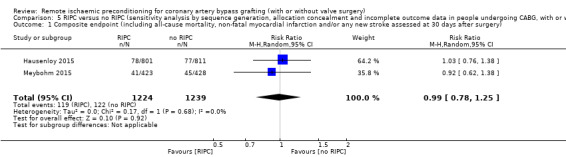
Comparison 5 RIPC versus no RIPC (sensitivity analysis by sequence generation, allocation concealment and incomplete outcome data in people undergoing CABG, with or without valve surgery), Outcome 1 Composite endpoint (including all‐cause mortality, non‐fatal myocardial infarction and/or any new stroke assessed at 30 days after surgery).
5.2. Analysis.

Comparison 5 RIPC versus no RIPC (sensitivity analysis by sequence generation, allocation concealment and incomplete outcome data in people undergoing CABG, with or without valve surgery), Outcome 2 Cardiac troponin T 48 hours after surgery (ng/L).
5.3. Analysis.

Comparison 5 RIPC versus no RIPC (sensitivity analysis by sequence generation, allocation concealment and incomplete outcome data in people undergoing CABG, with or without valve surgery), Outcome 3 Cardiac troponin T 72 hours after surgery (ng/L).
5.4. Analysis.
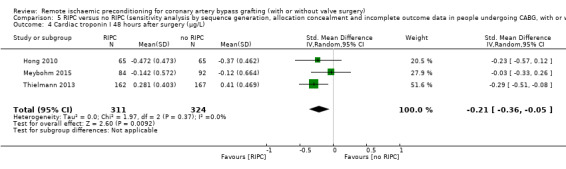
Comparison 5 RIPC versus no RIPC (sensitivity analysis by sequence generation, allocation concealment and incomplete outcome data in people undergoing CABG, with or without valve surgery), Outcome 4 Cardiac troponin I 48 hours after surgery (µg/L).
5.5. Analysis.

Comparison 5 RIPC versus no RIPC (sensitivity analysis by sequence generation, allocation concealment and incomplete outcome data in people undergoing CABG, with or without valve surgery), Outcome 5 Cardiac troponin I 72 hours after surgery (µg/L).
5.6. Analysis.
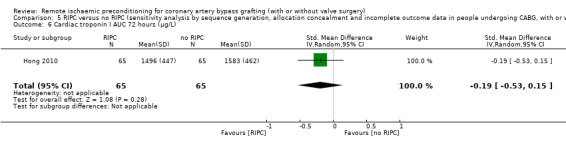
Comparison 5 RIPC versus no RIPC (sensitivity analysis by sequence generation, allocation concealment and incomplete outcome data in people undergoing CABG, with or without valve surgery), Outcome 6 Cardiac troponin I AUC 72 hours (µg/L).
Discussion
Summary of main results
This review summarised 29 studies involving 5392 participants randomly assigned to either receive RIPC or sham RIPC before CABG (with or without additional surgery). This is the first version of this review. The majority of studies included participants scheduled for isolated CABG, some studies included participants with combined procedures (CABG with valve surgery). Only a minority was performed as off‐pump procedure. The sample size in included studies ranged from eight participants to 1612 participants; only a few studies provided data on power analysis. We noted a large gender imbalance across all studies in favour of male participants. RIPC was performed almost exclusively on the right or left upper arm. The number of cycles of RIPC carried out ranked from two to four cycles and the majority of studies applied 200 mmHg pressure during one cycle of RIPC; the control intervention was in most cases a deflated cuff around the forearm.
We found moderate‐quality evidence that participants randomised to receive RIPC showed, on average, a benefit regarding the amount of cTnT release measured at 72 hours after surgery and expressed as AUC 72 hours after surgery. In addition, we found moderate‐quality evidence that cTnI measured at 48 hours and 72 hours after surgery showed, on average, a benefit in favour of RIPC. All other primary outcomes showed no benefit of RIPC. However, the results of our meta‐analyses demonstrated that the effect of RIPC on a surrogate parameter of myocardial damage, showed minor clinical relevance and did not affect the overall incidence of all‐cause mortality, myocardial infarction, stroke, renal failure or length of stay on the intensive care unit. Also when measured as a composite endpoint (all‐cause mortality, non‐fatal myocardial infarction or any new stroke, or both, assessed at 30 days after surgery) RIPC showed no benefit in favour of the intervention. Hausenloy 2015 observed significantly more adverse events (skin petechiae) in the intervention group. Skin petechiae are a small (1–2 mm) red spot on the skin, caused by a minor bleed from broken capillary blood vessels, in the context of RIPC skin petechiae are not hurtful and do not require any action or therapy. Also the three subgroup analyses performed did not indicate that RIPC might yield a benefit in any relevant subgroup. Yet, the mid‐ to long‐term effects of a reduced troponin release were not adequately addressed in any of the cited studies. As both myocardial damage and organ injury determine a patient's outcome after cardiac surgery, further investigations are encouraged to either combine cardio‐ and organ‐protective strategies.
Two other aspects of our systematic review warrant comment. A great number of studies investigated a possible benefit of RIPC in cardiac patients and more studies are currently recruiting that matched the inclusion criteria of this systematic review. However, due to inconsistency in outcome definition and reporting in cardiac trials (Benstoem 2015b), available evidence is limited. Secondly, it is important to highlight that patient‐centred outcomes focusing on individual health‐related quality of life were rarely assessed; pooling of data was not possible.
Overall completeness and applicability of evidence
During the last decades an impressive body of evidence has demonstrated the promising effects of RIPC in various experimental and small clinical trials, which urged the need for large‐scale, multicentre trials in order to confirm its clinical relevance. Beside its significance in people with myocardial infarction, cardiac surgery patients were thought to represent a population that may benefit from this promising cardioprotective strategy, as people increasingly present with severe co‐morbidities in old age. Very recently, both the RIPHeart (Meybohm 2015) and ERICCA study (Hausenloy 2015) have been published, which demonstrated neutral results with respect to the occurrence of death from any cause, non‐fatal myocardial infarction, new stroke, acute kidney injury (RIPHeart only) and coronary revascularisation (ERICCA only). These results stimulated the need for the present meta‐analysis, in order to draw conclusions about the current evidence on the effect of RIPC in people undergoing cardiac surgery.
Although the number of trials meeting our inclusion criteria was relatively large, none of the included studies reported all the assessed outcomes. Only two studies (Hausenloy 2015; Meybohm 2015) reported a composite endpoint. However, both of these studies were of high quality (see Figure 2, 'Risk of bias' summary) and adequately powered. We found a sufficient number of studies reporting the two additional primary and our secondary outcomes. Regarding the perioperative release of troponin T (measured at 6 hours, 12 hours, 24 hours, and 48 hours after surgery), we could not detect, on average, a difference in treatment effect at each single time point, which is in contrast to earlier clinical studies in people with CABG procedures. Notably, we found a reduced troponin T release measured at 72 hours and overall troponin T release (AUC 72 hours) during the postoperative course.
The reasons for these heterogeneous results and failure to confirm beneficial results from animal studies remains unclear. One reason may be due to the fact that RIPC is known to provide cardioprotective effects, whereas knowledge about its organ‐protective effects remain sparse. This is of particular relevance because the postoperative dysfunctions of other organs are well‐known determinants of the patients' postoperative outcome. Therefore, future research should focus on RIPC's potential organ‐protective properties or encourage the combination of cardio‐ and organ‐protective strategies in order to improve the outcome of cardiac surgical patients. Besides the postulated, but not confirmed effect of propofol, one potential explanation may be an improvement in surgical techniques and anaesthetic management, which reduces a periprocedural myocardial injury and therefore the resulting overall morbidity and mortality (Siregar 2014).
The recently published RIPHeart study (Meybohm 2015) and ERICCA study (Hausenloy 2015) have raised the question of whether the use of concomitant medications may inhibit the cardioprotective effects of RIPC in cardiac surgery patients. However, in both studies, no effect was detected for any commonly used long‐term medication. It still is debated if the use of propofol anaesthesia may, at least in part, have interfered with the cardioprotective effects of RIPC in cardiac surgery patients (Kottenberg 2012; Kottenberg 2014). In the past, numerous experimental studies (Li 2012; Lim 2005) and one small clinical study in cardiac surgery (Sayin 2002) demonstrated that propofol may provide cardioprotective effects, which were mediated by its antioxidant properties and prevention of mPTP opening. In the present meta‐analyses, we could not detect any difference in treatment effect between the use propofol and volatile anaesthetics. However, given the limited validity of the results of this subgroup analysis received from very heterogeneous studies, we acknowledge the need to systematically address this question in both experimental, and following adequately powered and designed clinical studies. So far the mechanism for this interaction remains unclear and needs to be investigated (Heusch 2016). Furthermore, the application of nitric oxide donors, is supposed to interfere with the RIPC‐mediated effects and is currently investigated in the ERIC‐GTN trial (Hamarneh 2015). In our systematic review, due to insufficient data, we were unable to address this question, but it needs to be carefully considered as an interfering factor. Therefore, we conclude that the various mediations as part of the anaesthetic management may inhibit the conditioning effects although they may either be cardioprotective or not.
Surgical procedures on the heart are primarily undertaken with the intention to reduce mortality or to relieve symptoms, or both, resulting in improved quality of life. Today, the protective strategies mainly focus on reducing the perioperative inflammatory response. Considering the major goal of RIPC is to reduce myocardial injury in order to improve the overall outcome for cardiac surgical patients, it is doubtful if this alone can adequately capture patients’ postoperative complications and patients' perspective after discharge from hospital. Cardioprotective interventions should not exclusively attempt to reduce mortality, but should also aim to improve postsurgical morbidity and quality of life. These endpoints need to be considered in cardiac trials (Benstoem 2015b; Stoppe 2016).
Given the clinical heterogeneity in the modus operandi of RIPC, the heart surgery performed (CABG with or without valve surgery) and during the postoperative course, we used random‐effects meta‐analysis to produce an overall summary of average treatment effect across trials. We treated the random‐effects summary as the average range of possible treatment effects. However, we would also like to highlight important aspects on the applicability of the evidence with regard to troponins. We included troponin values T and I measured at 48 hours, 72 hours, and as area under the curve (AUC) 72 hours after surgery as primary outcomes, and troponin values T and I measured at 6 hours, 12 hours, and 24 hours after surgery as secondary outcomes. By doing so, we highlighted outcomes that are clinically most meaningful as primary outcomes. In general, troponin measures might be biased if there are many missing data. For example, if sicker patients do not get their bloods measured, the mean troponin may be lower. One study (Hausenloy 2015) evaluated this possible effect of missing data by using multiple imputation to replace any missing values for troponin T assays. In their complete‐case analysis (n = 728 participants) with full data for troponin T (45.2% of all 1612 participants), they found a 10% lower AUC for the troponin T level in participants undergoing RIPC as compared with those undergoing the sham procedure (geometric mean 32.7 versus 36.4 ng/ml; 95% CI, 0.81 to 0.99). However, this effect largely disappeared when multiple imputation analysis was undertaken (geometric mean, 34.2 vs. 34.8 ng/ml; 95% CI, 0.91 to 1.06). This finding was also supported by examination of the data for the 1282 participants (79.5%) who had at least one perioperative high‐sensitivity troponin T assay result, which also showed little difference between both groups. Therefore, with regard to troponins, the findings of this systematic review have to be interpreted with caution. The Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2011b) advises against the common approach to deal with missing data by imputing outcomes and to treat them as if they are real measurements, however, sometimes circumstances warrant the use of multiple imputation methods. Whether or not multiple imputation is appropriate or not will depend on some assumptions being reasonable and rather than having a pre‐specified policy on missing data imputation. One might argue that not doing multiple imputation and using only complete‐case analysis also relies on an assumption: that this subset of the data can provide an unbiased estimation of the real effect. In order to calculate the AUC for troponin for a participant, one needs all need all five readings of troponin T for that participant at 6 hours, 12 hours, 24 hours, 48 hours and 72 hours. If one of those readings is missing then, technically speaking, the AUC cannot be calculated and the whole participant would be missing from the analysis. In the ERICCA study (Hausenloy 2015) about half of the participants had at least one of these measurement missing. Not doing any imputation would have meant throwing away half of the data. It seems not entirely unreasonable to input only part of the outcome for an individual (some of these missing observations) to avoid losing all the recorded measures of that participant. The statistical findings of this systematic review have to be interpreted in light of the above.
In addition, different assays of troponin analyses are available and in most cases trial authors did not specify which type of laboratory analysis was performed, possibly limiting the ability to combine results, as the modest difference seen between groups could be due to a Type I error simply resulting from the diversity of the assay methods for this biomarker. Therefore, we used the standardised mean difference with 95% CI to combine data of troponin values. On the other hand, assuming that the differences in enzyme release reflect a real benefit of RIPC, the relevance of this finding cannot be underscored because of the well‐documented effects of early postoperative troponin release on long‐term outcomes (Domanski 2011). As not all studies that contributed data to the meta‐analyses of cTnT and cTnI were large trials, the results obtained must be interpreted with caution due to the skewed nature of the data (Deeks 2009). This problem is encountered by performing meta‐analyses on the scale of the log‐transformed data, which we implemented in this review.
Quality of the evidence
We assessed the quality of trial evidence for the following outcomes using the criteria outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011) and by employing the GRADE approach to assess the quality of result findings. GRADEpro GDT (gradepro.org) allowed us to import data from RevMan 5 to create a 'Summary of findings' table for our primary outcomes.
Composite endpoint (including all‐cause mortality, non‐fatal myocardial infarction and/or any new stroke) assessed at 30 days after surgery
cTnT after CABG (ng/L) at 48 hours, 72 hours, and as area under the curve (AUC) 72 hours (µg/L) after surgery
cTnI after CABG (ng/L) at 48 hours, 72 hours, and as area under the curve (AUC) 72 hours (µg/L) after surgery
We graded the quality of evidence for the composite endpoint as of moderate quality due to imprecision of results as shown in wide confidence intervals crossing the line of no effect. We graded cTnT measured at 48 hours as of low quality, due to the presence of substantial heterogeneity and imprecision of results, as shown in wide confidence intervals crossing the line of no effect. We graded cTnT measured at 72 hours and as AUC (72 hours) as of moderate quality due to the presence of substantial heterogeneity. We graded cardiac troponin I measured at 48 hours, at 72 hours or as AUC (72 hours) hours as of moderate quality, due to the presence of moderate heterogeneity, imprecision of results as shown in wide confidence intervals crossing line of no effect or due to limitations in the design and implementation of available studies, suggesting high likelihood of bias. Details are outlined in the Table 1.
Potential biases in the review process
We undertook this systematic review in accordance with the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011a). We carried out a comprehensive search across major databases and scanned reference lists of included studies, and we have described the process of study selection comprehensively and in full detail (see Figure 1, Study flow diagram). In addition, we screened reference lists of systematic reviews and contacted study authors for additional data when needed. We did not apply any language or date restrictions. Two review authors performed all steps of the selection process independently and analyses were conducted by one reviewer and checked by a colleague. Review authors who participated in included studies were not involved in the selection of these studies. We gave reasons why a study was not included in this systematic review. We described each included study in full detail and made explicit judgements about whether studies were at low or high risk of bias. Two secondary analyses (unpublished) tailored to the needs of this systematic review (Hausenloy 2015; Meybohm 2015) contributed data to this systematic review. However, both analyses were performed by independent statisticians and not by the principal investigators (DH, PM) and entered into RevMan 5 by a third person (CB, AG). We carried out sensitivity analyses to explore the effect of trial quality assessed by sequence generation, allocation concealment and incomplete outcome data with poor quality studies being excluded from the analyses to assess whether this made any difference to the overall result. Again, review authors who had participated in included studies were not involved in the quality assessment of these studies. We identified no other potential sources of bias in the review process.
Agreements and disagreements with other studies or reviews
The results of our systematic review are consistent with those of the most recent meta analysis by the Remote Preconditioning Trialists’ Group (Healy 2014), which included 23 trials of RIPC involving a total of 2200 participants undergoing cardiac surgery. Healy and colleagues concluded that RIPC has no effect on clinically relevant outcomes, including death, myocardial infarction, acute renal failure, stroke, mesenteric ischaemia, and hospital or ICU length of stay.
Authors' conclusions
Implications for practice.
We found no evidence that remote ischaemic preconditioning (RIPC) has a treatment effect on clinical outcomes (all‐cause mortality, non‐fatal myocardial infarction, stroke, acute renal failure or length of stay on ICU). There is moderate‐quality evidence that RIPC has no treatment effect on the rate of the composite endpoint including all‐cause mortality, non‐fatal myocardial infarction or any new stroke, or both, assessed at 30 days after surgery. We found low‐quality evidence that RIPC reduces the cardiac troponin T release measured at 72 hours after surgery and expressed as AUC (72 hours). There is low‐ and moderate‐quality evidence that RIPC reduces the amount of cardiac troponin I release measured at 48 hours and 72 hours after surgery.
Implications for research.
The results above show the need for further, adequately‐designed studies, especially focusing on influencing factors, e.g. anaesthetic management. Mechanistic studies on the protective effects of RIPCs should systematically analyse the commonly‐used medications of people with cardiovascular diseases.
Acknowledgements
We acknowledge the authors of the primary studies included in the meta‐analyses, who have kindly provided additional data and information regarding their studies. Our special thanks go to Prof. Derek Hausenloy, Prof. Tim Clayton and Prof. Derek Yellon for sharing their valuable expertise on RIPC with us. We would also like to thank Nicole Martin from Cochrane Heart for her support and for providing assistance during the editorial process. The methods section of this review is based on a standard template used by Cochrane Heart.
Appendices
Appendix 1. Search strategies
Search strategy for CENTRAL
#1 MeSH descriptor: (Ischemic Preconditioning) explode all trees
#2 (isch?em* near/2 (precondit* or pre‐condit* or "pre condit*")):ti,ab,kw (Word variations have been searched)
#3 IPC:ti,ab,kw (Word variations have been searched)
#4 #1 or #2 or #3
#5 MeSH descriptor: (Coronary Disease) explode all trees
#6 coronary heart disease:ti,ab,kw (Word variations have been searched)
#7 MeSH descriptor: (Coronary Artery Bypass) explode all trees
#8 aortocoronary bypass:ti,ab,kw (Word variations have been searched)
#9 cabg:ti,ab,kw (Word variations have been searched)
#10 (coronary near/5 bypass):ti,ab,kw (Word variations have been searched)
#11 #5 or #6 or #7 or #8 or #9 or #10
#12 #4 and #11
Search strategy for MEDLINE OVID
1 exp Ischemic Preconditioning/
2 (isch?em* adj2 (precondit* or pre‐condit* or "pre condit*")).tw.
3 IPC.tw.
4 or/1‐3
5 exp Coronary Disease/
6 coronary heart disease.tw.
7 exp Coronary Artery Bypass/
8 aortocoronary bypass.tw.
9 cabg.tw.
10 (coronary adj5 bypass).tw.
11 or/5‐10
12 4 and 11
13 randomized controlled trial.pt.
14 controlled clinical trial.pt.
15 randomized.ab.
16 placebo.ab.
17 drug therapy.fs.
18 randomly.ab.
19 trial.ab.
20 groups.ab.
21 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20
22 exp animals/ not humans.sh.
23 21 not 22
24 12 and 23
Search strategy for Embase OVID
1 exp Ischemic Preconditioning/
2 (isch?em* adj2 (precondit* or pre‐condit* or "pre condit*")).tw.
3 IPC.tw.
4 or/1‐3
5 exp Coronary Artery Disease/
6 coronary heart disease.tw.
7 exp Coronary Artery Bypass Graft/
8 aortocoronary bypass.tw.
9 cabg.tw.
10 (coronary adj5 bypass).tw.
11 or/5‐10
12 4 and 11
13 random$.tw.
14 factorial$.tw.
15 crossover$.tw.
16 cross over$.tw.
17 cross‐over$.tw.
18 placebo$.tw.
19 (doubl$ adj blind$).tw.
20 (singl$ adj blind$).tw.
21 assign$.tw.
22 allocat$.tw.
23 volunteer$.tw.
24 crossover procedure/
25 double blind procedure/
26 randomized controlled trial/
27 single blind procedure/
28 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 or 21 or 22 or 23 or 24 or 25 or 26 or 27
29 (animal/ or nonhuman/) not human/
30 28 not 29
31 12 and 30
Search strategy for WEB OF SCIENCE
# 10 #9 AND #6 AND #1
Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years
# 9 #8 OR #7
Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years
# 8 TOPIC: ((isch?em* near/2 (precondit* or pre‐condit* or "pre condit*")))
Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years
# 7 TOPIC: (IPC)
Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years
# 6 #5 OR #4 OR #3 OR #2
Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years
# 5 TOPIC: ("coronary heart disease")
Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years
# 4 TOPIC: ("aortocoronary bypass")
Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years
Select to delete this set.
# 3 TOPIC: (cabg)
Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years
# 2 TOPIC: ((coronary near/5 bypass))
Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years
# 1 TS=(random* or blind* or allocat* or assign* or trial* or placebo* or crossover* or cross‐over*)
Indexes=SCI‐EXPANDED, CPCI‐S Timespan=All years
Appendix 2. Assessment of risk of bias in included studies
Random sequence generation (checking for possible selection bias)
For each included study, we described the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups. The method was assessed as follows:
low risk (any truly random process, e.g. random number table; computer random number generator);
high risk (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk (insufficient information to permit judgement).
Blinding of participants and personnel (checking for possible performance bias)
We described whether participants and personnel were blind to the allocation to the intervention or control groups in our 'Risk of bias' assessment. We assessed the methods as:
low, high or unclear risk of bias for participants;
low, high or unclear risk of bias for personnel.
Blinding of outcome assessment (checking for possible detection bias)
For each included study, we described the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We considered studies to be at low risk of bias if they were blinded or if we judged that the lack of blinding could not have affected the results. We assessed the method as follows:
low risk (no blinding of outcome assessment but the authors judged that the outcome was not likely to be influenced by this);
high risk (no blinding of outcome assessment and the outcome measurement was likely to have been influenced by this);
unclear risk (insufficient information to permit judgement; the study did not address this).
Allocation concealment (checking for possible selection bias)
For each included study, we described the method used to conceal the allocation sequence and determine whether the intervention allocation could have been foreseen in advance of or during recruitment, or changed after assignment. We assessed the method as follows:
low risk (e.g. telephone or central randomisation; consecutively numbered, sealed, opaque envelopes);
high risk (open random allocation; unsealed or non‐opaque envelopes; alternation; date of birth);
unclear risk (insufficient information to permit judgement).
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
For each included study and for each outcome or class of outcomes, we described the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total number of randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. We assessed the method as follows:
low risk (20% or less missing data);
high risk (more than 20% missing data);
unclear risk (insufficient reporting to permit judgement; the study did not address this).
Selective reporting (checking for reporting bias)
We investigated the possibility of selective outcome reporting bias by identifying the outcomes in the study protocol (if available) and in the methods section of the publication, and by cross‐checking to see if these outcomes are reported in the results section of the trial publication(s). We assessed the method as follows:
low risk (where it was clear that all of the study's prespecified outcomes as identified in the study protocol (where available) and in the methods section were reported on; that all expected outcomes of interest to the review were reported on);
high risk (where it was clear that not all of the study's prespecified outcomes as identified in the study protocol (where available) and in the methods section were reported on; failure to include a key outcome that would have been expected to have been included);
unclear risk (insufficient information to permit judgement).
Other bias (checking for other biases)
For each included study, we described any important concerns we had about other possible sources of bias, for example sources of research funding. We assessed whether each study was free of other problems that could put it at risk of bias as follows:
low risk (study appeared to be free of bias);
high risk (had at least one important risk of bias, for example related to study design);
unclear risk (insufficient information to permit judgement).
Data and analyses
Comparison 1. RIPC versus no RIPC in people undergoing CABG (with or without valve surgery).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Composite endpoint (including all‐cause mortality, non‐fatal myocardial infarction and/or any new stroke assessed at 30 days after surgery) | 2 | 2463 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.78, 1.25] |
| 2 All‐cause mortality after 30 days | 8 | 3288 | Risk Ratio (M‐H, Random, 95% CI) | 1.05 [0.66, 1.68] |
| 3 Non‐fatal myocardial infarction after 30 days | 2 | 2463 | Risk Ratio (M‐H, Random, 95% CI) | 0.84 [0.62, 1.15] |
| 4 Any new stroke after 30 days | 2 | 2463 | Risk Ratio (M‐H, Random, 95% CI) | 1.12 [0.60, 2.07] |
| 5 Cardiac troponin T 6 hours after surgery (ng/L) | 5 | 2006 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.38, 0.12] |
| 6 Cardiac troponin T 12 hours after surgery (ng/L) | 5 | 1978 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.40, 0.13] |
| 7 Cardiac troponin T 24 hours after surgery (ng/L) | 4 | 1951 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.50, 0.01] |
| 8 Cardiac troponin T 48 hours after surgery (ng/L) | 4 | 1792 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.14 [‐0.33, 0.06] |
| 9 Cardiac troponin T 72 hours after surgery (ng/L) | 3 | 1120 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.32 [‐0.65, 0.00] |
| 10 Cardiac troponin T AUC 72 hours (µg/L) | 3 | 830 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.49 [‐0.96, ‐0.02] |
| 11 Cardiac troponin I 1 hour after surgery (µg/L) | 2 | 459 | Std. Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.16, 0.21] |
| 12 Cardiac troponin I 6 hours after surgery (µg/L) | 7 | 830 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.85 [‐1.39, ‐0.30] |
| 13 Cardiac troponin I 12 hours after surgery (µg/L) | 6 | 753 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.89 [‐1.42, ‐0.36] |
| 14 Cardiac troponin I 24 hours after surgery (µg/L) | 7 | 840 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.61 [‐1.01, ‐0.21] |
| 15 Cardiac troponin I 48 hours after surgery (µg/L) | 5 | 745 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.40, ‐0.02] |
| 16 Cardiac troponin I 72 hours after surgery (µg/L) | 2 | 459 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.59, ‐0.15] |
| 17 Cardiac troponin I AUC 72 hours (µg/L) | 2 | 159 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.48, 0.14] |
| 18 Acute renal failure after 30 days | 1 | 851 | Risk Ratio (M‐H, Random, 95% CI) | 1.37 [0.78, 2.40] |
| 19 Length of stay on the intensive care unit (days) | 8 | 3102 | Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.15, 0.12] |
| 20 Any complications and adverse effects related to the intervention reported | 3 | 2414 | Risk Ratio (M‐H, Random, 95% CI) | 18.59 [4.49, 76.92] |
1.11. Analysis.

Comparison 1 RIPC versus no RIPC in people undergoing CABG (with or without valve surgery), Outcome 11 Cardiac troponin I 1 hour after surgery (µg/L).
Comparison 2. RIPC versus no RIPC (subgroup analysis: isolated CABG without additional surgery).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Composite endpoint (including all‐cause mortality, non‐fatal myocardial infarction and/or any new stroke assessed at 30 days after surgery) | 2 | 1447 | Risk Ratio (M‐H, Random, 95% CI) | 1.03 [0.73, 1.45] |
| 2 Cardiac troponin T 48 hours after surgery (ng/L) | 3 | 989 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐0.46, 0.11] |
| 3 Cardiac troponin T 72 hours after surgery (ng/L) | 2 | 541 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.34, 0.12] |
| 4 Cardiac troponin T AUC 72 hours (µg/L) | 1 | 57 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐1.34, ‐0.25] |
| 5 Cardiac troponin I 48 hours after surgery (µg/L) | 5 | 708 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.41, 0.02] |
| 6 Cardiac troponin I 72 hours after surgery (µg/L) | 2 | 459 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.59, ‐0.15] |
| 7 Cardiac troponin I AUC 72 hours (µg/L) | 2 | 159 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.48, 0.14] |
2.3. Analysis.
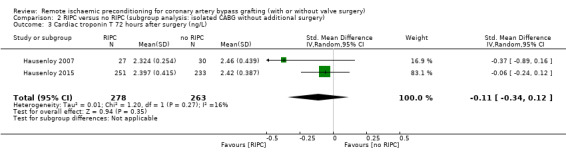
Comparison 2 RIPC versus no RIPC (subgroup analysis: isolated CABG without additional surgery), Outcome 3 Cardiac troponin T 72 hours after surgery (ng/L).
Comparison 3. RIPC versus no RIPC (subgroup analysis in people undergoing CABG, with or without valve surgery and a high preoperative risk status, EuroSCORE ≥ 6).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Composite endpoint (including all‐cause mortality, non‐fatal myocardial infarction and/or any new stroke assessed at 30 days after surgery) | 2 | 1317 | Risk Ratio (M‐H, Random, 95% CI) | 1.02 [0.74, 1.38] |
| 2 Cardiac troponin T 48 hours after surgery (ng/L) | 2 | 894 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.98, 0.56] |
| 3 Cardiac troponin T 72 hours after surgery (ng/L) | 1 | 661 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.90 [‐1.06, ‐0.74] |
| 4 Cardiac troponin I 48 hours after surgery (µg/L) | 1 | 38 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.15 [‐0.79, 0.49] |
Comparison 4. RIPC versus no RIPC (subgroup analysis: isoflurane anaesthesia or similar volatile agents versus propofol anaesthesia in people undergoing CABG, with or without valve surgery).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Composite endpoint (including all‐cause mortality, non‐fatal myocardial infarction and/or any new stroke assessed at 30 days after surgery) | 2 | 1663 | Risk Ratio (M‐H, Random, 95% CI) | 0.90 [0.67, 1.20] |
| 1.1 Isoflorane anaesthesia or similar volatile agents | 1 | 23 | Risk Ratio (M‐H, Random, 95% CI) | 1.83 [0.19, 17.51] |
| 1.2 Propofol anaesthesia | 2 | 1640 | Risk Ratio (M‐H, Random, 95% CI) | 0.89 [0.66, 1.19] |
| 2 Cardiac troponin T 48 hours after surgery (ng/L) | 3 | 1081 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.28, 0.06] |
| 2.1 Isoflorane anaesthesia or similar volatile agents | 1 | 16 | Std. Mean Difference (IV, Random, 95% CI) | 0.47 [‐0.56, 1.50] |
| 2.2 Propofol anaesthesia | 3 | 1065 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.29, 0.04] |
| 3 Cardiac troponin T 72 hours after surgery (ng/L) | 2 | 500 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.08 [‐0.46, 0.29] |
| 3.1 Isoflorane anaesthesia or similar volatile agents | 1 | 15 | Std. Mean Difference (IV, Random, 95% CI) | 0.79 [‐0.30, 1.87] |
| 3.2 Propofol anaesthesia | 2 | 485 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.12 [‐0.30, 0.06] |
| 4 Cardiac troponin T AUC 72 hours (µg/L) | 2 | 102 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.75 [‐1.16, ‐0.35] |
| 4.1 Isoflorane anaesthesia or similar volatile agents | 1 | 45 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.70 [‐1.31, ‐0.10] |
| 4.2 Propofol anaesthesia | 1 | 57 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.80 [‐1.34, ‐0.25] |
| 5 Cardiac troponin I 48 hours after surgery (µg/L) | 3 | 384 | Std. Mean Difference (IV, Random, 95% CI) | 0.26 [‐0.45, 0.97] |
| 5.1 Isoflorane anaesthesia or similar volatile agents | 2 | 210 | Std. Mean Difference (IV, Random, 95% CI) | 0.44 [‐0.89, 1.77] |
| 5.2 Propofol anaesthesia | 1 | 174 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.05 [‐0.35, 0.25] |
| 6 Cardiac troponin I 72 hours after surgery (µg/L) | 1 | 130 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.56, 0.13] |
| 6.1 Isoflorane anaesthesia or similar volatile agents | 1 | 130 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.56, 0.13] |
| 6.2 Propofol anaesthesia | 0 | 0 | Std. Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Cardiac troponin I AUC 72 hours (µg/L) | 2 | 159 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.48, 0.14] |
| 7.1 Isoflorane anaesthesia or similar volatile agents | 1 | 130 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.53, 0.15] |
| 7.2 Propofol anaesthesia | 1 | 29 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.09 [‐0.82, 0.64] |
Comparison 5. RIPC versus no RIPC (sensitivity analysis by sequence generation, allocation concealment and incomplete outcome data in people undergoing CABG, with or without valve surgery).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Composite endpoint (including all‐cause mortality, non‐fatal myocardial infarction and/or any new stroke assessed at 30 days after surgery) | 2 | 2463 | Risk Ratio (M‐H, Random, 95% CI) | 0.99 [0.78, 1.25] |
| 2 Cardiac troponin T 48 hours after surgery (ng/L) | 2 | 1607 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.33 [‐0.70, 0.04] |
| 3 Cardiac troponin T 72 hours after surgery (ng/L) | 1 | 937 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.13 [‐0.26, ‐0.00] |
| 4 Cardiac troponin I 48 hours after surgery (µg/L) | 3 | 635 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.21 [‐0.36, ‐0.05] |
| 5 Cardiac troponin I 72 hours after surgery (µg/L) | 2 | 459 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.59, ‐0.15] |
| 6 Cardiac troponin I AUC 72 hours (µg/L) | 1 | 130 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.53, 0.15] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ahmad 2014.
| Methods |
Study design: RCT Total duration of study: 16 months, March 2012‐June 2013 Setting: single‐centre study, Department of Cardiac Surgery, Chaudhry Pervaiz Elahi Institute of Cardiology, Multan, Pakistan (tertiary care hospital) Withdrawals: not reported |
|
| Participants |
N = 67 (control = 32, intervention = 35) participants randomised Mean age = 55 years (± 11 years) Gender: 78% male, 22% female Severity of condition: participants diagnosed with triple vessel coronary disease, EF = 51%, history of diabetes = 52%, history of MI = 24% Inclusion criteria: class III Angina, triple vessel coronary artery disease, ACC and AHA Class I indications for CABG, informed consent given Exclusion criteria: major surgical event, i.e. major surgical injury, revision of graft, aortic cross clamp time > 100 min Reported differences between intervention and comparison groups: none Anaesthestetic gas used: sevoflurane/isoflurane anaesthesia |
|
| Interventions |
Intervention: 3 cycles of RIPC (occluding pressure in cuff: 200 mmHg) for 5 min in the right arm, alternated with in‐between rest periods of 5 min after induction of anaesthesia Comparison: blood pressure cuff applied without any occluding pressure Concomitant medications: not reported Excluded medications: not reported |
|
| Outcomes |
Outcomes and time points measured in the study: Defibrillation on weaning from cardiopulmonary bypass Intra‐aortic balloon pump placement Low cardiac output state demanding high inotropic support (> 0.1 μ/kg/min of adrenaline, dobutamine, or noradrenaline) Use of antiarrhythmics CK‐MB levels at 1 h, 6 h, 12 h and 24 h postoperatively Serum creatinine level on the first postoperative day Ejection fraction on third postoperative day Low CO state In‐hospital mortality One‐year mortality Limb ischaemia Neurapraxia of right limb |
|
| Notes |
Funding for trial: not reported Notable conflicts of interest of authors: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to form judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to form judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to form judgement however, the objective outcomes such as CK‐MB levels, serum creatinine level, ejection fraction and mortality are not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to form judgement however, the objective outcomes such as CK‐MB levels, serum creatinine level, ejection fraction and mortality are not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methods section were adequately reported or explained in results |
| Other bias | Unclear risk | Insufficient information to form judgement |
Ali 2010.
| Methods |
Study design: RCT Total duration of study: 6 months, January‐June 2008 Setting: single‐centre study, Surgical Department of Armed Forces Institute of Cardiology/National Institute of Heart Disease, Rawalpindi, Pakistan Withdrawals: not reported |
|
| Participants |
N = 100, (control = 50, intervention = 50) participants randomised Mean age = 54 years (± 9 years) Gender: 89% male 11% female Severity of condition: double and triple vessel disease, EF > 55% = 50, EF 30‐55% = 42, EF < 30% = 8, history of diabetes = 19%, history of recent MI = 19%, NYHA I = 51%, NYHA II = 44%, NYHA III = 5% Inclusion criteria: people with double and triple vessel disease undergoing elective CABG and given written informed consent Exclusion criteria: significant renal or hepatic disease, which could influence the enzyme kinetics in blood, haemodynamic instability ECG changes and/or raised cardiac enzymes suggesting ongoing ischaemia and infarction, acute myocardial infarction during last 4 weeks, and significant peripheral vascular disease Reported differences between intervention and comparison groups: no statistical difference between groups with regard to baseline characteristics Anaesthestetic gas used: not reported |
|
| Interventions |
Intervention: 3 cycles of RIPC (occluding pressure in cuff: 200 mmHg) for 5 min in the right or left arm, alternated with in‐between rest periods of 5 min after induction of anaesthesia Comparison: deflated cuff on upper arm for 30 min Concomitant medications: aspirin: 8%, ß‐blocker = 88%, ACE inhibitor = 42%, long acting nitrates = 86% Excluded medications: not reported |
|
| Outcomes |
Outcomes and time points measured in the study: Postoperative CKMB levels measured at 8 h, 16 h, 24 h and 48 h after surgery Cross clamp time Bypass time BMI |
|
| Notes |
Funding for trial: not reported Notable conflicts of interest of authors: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | "Random allocation of patients to either rIPC group or controlled group was done by lottery method." |
| Allocation concealment (selection bias) | Low risk | "Sealed non‐transparent envelops allocated patients to either of the study groups." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | "The operating surgeon or anaesthetist in charge opened the randomisation envelope and was thus aware of the allocated group/procedure." Since the RIPC protocol was implemented after the patient was anaesthetised, blinding of the participant is likely. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "All data were finally collected by a research investigator blinded to the treatment allocation." In addition, the objective outcomes such as CK‐MB levels, cross clamp and bypass time and BMI are not likely to be influenced by lack of blinding of the participants and personnel. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methods section were adequately reported or explained in results. |
| Other bias | Unclear risk | Insufficient information to form judgement |
Candilio 2015.
| Methods |
Study design: RCT Total duration of study: 19 months, December 2010 ‐ July 2012 Setting: single‐centre study, Cardiovascular Institute, University College London, UK Withdrawals: 2 study subjects excluded from data analysis, no reason given |
|
| Participants |
N = 180, (control = 90, intervention = 90) participants randomised Mean age = 66 years (± 10 years) Gender: 78% male; 22% female Severity of condition: CABG = 73%, valve surgery = 23%; single to quadruple vessel disease, EF > 50% = 77%, EF 30‐50% = 18.5%, EF < 30% = 4.5%, history of diabetes = 29.5%, recent MI = 29%, NYHA 0 = 9%, NYHA I = 31%, NYHA II = 45%, NYHA III = 14%, NYHA IV = 1% Inclusion criteria: adults (> 18 years of age) undergoing on‐pump CABG and/or valve surgery Exclusion criteria: cardiogenic shock or cardiac arrest in the current hospital admission, positive baseline serum hsTnT, pregnancy, significant peripheral arterial disease affecting upper and/or lower limbs, significant hepatic (INR > 2.0), pulmonary (forced expiratory volume‐1 < 40% predicted) or renal disease (estimated glomerular filtration rate < 30 mL/min/1.73 m2), and concomitant therapy with glibenclamide or nicorandil Reported differences between intervention and comparison groups: no statistical difference between groups with regard to baseline characteristics. With regards to treatments during surgery, participants in the control group received intravenous glyceryl trinitrate more often than those from the intervention group (76% vs 60%) Anaesthestetic gas used: isoflurane, sevoflurane, propofol |
|
| Interventions |
Intervention: RIPC was delivered with 2 standard blood pressure cuffs, 1 placed on the upper arm and another placed on the upper thigh. Two cycles of RIPC (occluding pressure in cuff: 200 mmHg or 15 mmHg above systolic blood pressure) for 5 min, alternated with in‐between rest periods of 5 min after induction of anaesthesia Comparison: 2 cuffs were placed on the upper arm and upper thigh and left uninflated for 20 min Concomitant medications: aspirin = 80%, clopidogrel = 29.5%, warfarin = 8.5%, ß‐blocker = 64.5%, ca‐blocker = 31.5%, statin = 83.5%, ACE inhibitor = 68%, long‐acting nitrates = 15%, diuretics = 33.5% Excluded medications: glibenclamide or nicorandil |
|
| Outcomes |
Outcomes and time points measured in the study: Perioperative MI, assessed by measuring the total 72‐h AUC hsTnT Blood samples for hsTnT preoperatively and 6 h, 12 h, 24 h, 48 h and 72 h postoperatively AKI score (serum creatinine and urine output measured preoperatively and 24 h, 48 h and 72 h postoperatively) Inotrope requirements (measured every 24 h over the 72‐h postoperative period as dosages mg/kg/min) Length of ICU and hospital stay (days) Incidence of postoperative atrial fibrillation within 72 h postoperatively Major adverse cardiovascular events at 6 weeks postoperatively (death, non‐fatal MI, coronary artery revascularisation and stroke) |
|
| Notes |
Funding for trial: study was funded by the British Heart Foundation (grant numbers RG/03/007 and FS/10/039/28270), the Rosetrees Trust and supported by the National Institute for Health Research University College London Hospitals Biomedical Research Centre Notable conflicts of interest of authors: none to declare |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated list of randomised numbers." |
| Allocation concealment (selection bias) | Low risk | "Sequentially numbered opaque sealed envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Randomisation, treatment allocation and delivery of RIPC or control protocols were performed by an unblinded investigator not involved in data collection or analysis. The investigator collecting and analysing the data, patients, cardiac surgeons and anaesthetists, operating theatre staff and staff on intensive care unit (ICU) and cardiac wards were all blinded to treatment allocation." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The investigator collecting and analysing the data, patients, cardiac surgeons and anaesthetists, operating theatre staff and staff on intensive care unit (ICU) and cardiac wards were all blinded to treatment allocation." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 180 participants were enrolled in the study. No lost‐to‐follow‐up or discontinued intervention described in the study flow chart. However, only 178 participants were included in the final analysis, the reason for excluding two participants missing |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methods section were adequately reported or explained in results. |
| Other bias | Low risk | No other bias identified |
Gallagher 2015.
| Methods |
Study design: RCT Total duration of study: February 2011‐April 2012 Setting: Single‐centre study, Department of Cardiology, Barts Health NHS Trust, London, UK Withdrawals: none |
|
| Participants |
N = 86, (control = 43, intervention = 43) participants randomised Mean age = 71 years (± 9.7 years) Gender: 85% male; 15% female Severity of condition: triple vessel disease = 74%, LVEF = 52%, NYHA class > 3 = 34%, recent MI = 53%, diabetes = 66% Inclusion criteria: people were eligible for the study if they were aged 18‐85 years, had established CHD, and were scheduled to undergo non emergent CABG with or without aortic valve replacement Exclusion criteria: MI within 7 days of surgery, off‐pump surgery, ‘re‐do’ surgery, end‐stage renal failure or the presence of a renal transplant, coronary angiography within 7 days of surgery, and presurgical AKI, defined as an increase in serum creatinine concentration 40.3 mg/dL between the two preoperative measurements Reported differences between intervention and comparison groups: no statistical difference between groups with regard to baseline characteristics Anaesthestetic gas used: 87% isoflurane anaesthesia |
|
| Interventions |
Intervention: Three 5‐min cycles of forearm ischaemia followed by reperfusion after induction of anaesthesia, induced by inflating a 9 cm blood pressure cuff placed around the upper arm to a pressure of 50 mmHg greater than the participant’s systolic blood pressure. Each cycle of ischaemia was separated by a 5‐min period of reperfusion, during which the blood pressure cuff was deflated. Comparison: 9 cm blood pressure cuff placed on the upper arm (but not inflated) for 30 min Concomitant medications: aspirin = 97%, Clopidogrel = 22%, ß‐blocker = 81%, calcium‐n‐blocker = 37%, lipid‐lowering therapy = 92%, ACE antagonist = 79%, long‐acting nitrate = 40%, insulin = 28%, metformin = 34%, sulphonylurea = 21% Excluded medications: not reported |
|
| Outcomes |
Outcomes and time points measured in the study: Development of AKI defined as an increase in serum creatinine concentration over 0.3 mg/dL within 48 h of surgery Serum biomarkers of renal injury: 1) (NGAL), 2) (IL‐18), and 3) (CyC); and urinary biomarkers of renal injury 1) (NGAL), 2) (IL‐18), and 3) (KIM‐1) all measured 6 h, 12 h, and 24 h after surgery 72‐h serum (TnT) AUC (ng/L) Inotrope use Extubation time ICU length of stay (h) Hospital length of stay (days) 30‐day mortality |
|
| Notes |
Funding for trial: this study was funded by a project grant from Barts and the London Charity Notable conflicts of interest of authors: none to declare |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to form judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to form judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, anaesthetists, surgeons, and critical care teams were blinded to study group allocation, although investigators were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Investigators were not blinded. However, the objective outcomes such as 7‐h serum TnT, ICU and hospital length of stay and mortality are not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methods section were adequately reported or explained in results |
| Other bias | Low risk | No other bias identified |
Gegouskov 2009.
| Methods |
Study design: RCT Total duration of study: not reported Setting: single‐centre study, Department of Cardiac Surgery, University of Heidelberg, Germany Withdrawals: not reported |
|
| Participants |
N = 40 participants randomised Mean age = not reported Gender: not reported Severity of condition: not reported Inclusion criteria: people undergoing elective CABG Exclusion criteria: not reported Reported differences between intervention and comparison groups: not reported Anaesthestetic gas used: not reported |
|
| Interventions |
Intervention: three 5‐min cycles of upper limb ischaemia inflating a blood pressure cuff up to 200 mmHg Comparison: not reported Concomitant medications: not reported Excluded medications: not reported |
|
| Outcomes |
Outcomes and time points measured in the study: Serum concentrations of cardiac enzymes (CK, CK‐MB, troponin‐T) Interleukins ‐1, ‐8, ‐10 TNF‐alpha, macrophage migration inhibitory factor (MIF) High mobility group box 1 (HMGB1) NFkappaB (nuclear‐factor 'kappa‐light‐chain‐enhancer' of B‐cells) CD‐40 protein S‐100 protein Human leukocyte antigen complex (HLA‐DR) Insulin‐like growth factor (IGF) Blood samples were collected before surgery and at 6 h, 12 h, 24 h and 48 h after surgery |
|
| Notes |
Funding for trial: not reported Notable conflicts of interest of authors: not reported This study was only reported as an abstract, we contacted study authors for more details but we did not receive a response. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to form judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to form judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to form judgement. However, the measured biochemical markers in blood samples are objective outcomes and thus not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to form judgement. However, the measured biochemical markers in blood samples are objective outcomes and thus not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to form judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to form judgement |
| Other bias | Unclear risk | Insufficient information to form judgement |
Günaydin 2000.
| Methods |
Study design: RCT Total duration of study: not reported Setting: single‐centre study, Department of Cardiovascular Surgery, Gazi University, Turkey Withdrawals: not reported |
|
| Participants |
N = 8 (control = 4, intervention = 4) participants randomised Mean age = 61 years (± 10 years) Gender: 100% male Severity of condition: 37.5% single‐vessel disease, 25%; two‐vessel 37.5%; three‐vessel, EF > 40% Inclusion criteria: men with NYHA class II or III , scheduled to undergo coronary artery bypass surgery Exclusion criteria: suffering from unstable angina pectoris, diabetes mellitus type I or II, angiographically diagnosed left ventricular aneurysm or having an ejection fraction < 40% Reported differences between intervention and comparison groups: no statistical difference between groups with regard to baseline characteristics Anaesthestetic gas used: fentanyl anaesthesia |
|
| Interventions |
Intervention: a double cuffed tourniquet, which is especially used for intravenous regional anaesthesia for extremity operations, was wrapped around the right upper extremity of the participant close to the shoulder. Following CPB, the tourniquet was inflated to ap‐ proximately 300 mmHg for 3 min ischaemia and it was deflated for 2 min reperfusion. The cycle was repeated twice. Comparison: cuff left uninflated Concomitant medications: not reported Excluded medications: not reported |
|
| Outcomes |
Outcomes and time points measured in the study: CPK CPK‐MB LDH Lactate pH and PO2 from the coronary sinus blood before CPB, before clamping aorta and 5 min after declamping the aorta |
|
| Notes |
Funding for trial: not reported Notable conflicts of interest of authors: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to form judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to form judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to form judgement. However, the measured biochemical markers in blood samples are objective outcomes and thus not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to form judgement. However, the measured biochemical markers in blood samples are objective outcomes and thus not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported |
| Selective reporting (reporting bias) | High risk | Data of lactate, PO2 and pH values are not reported |
| Other bias | Unclear risk | Insufficient information to form judgement |
Hausenloy 2007.
| Methods |
Study design: RCT Total duration of study: 12 months, February 2006‐February 2007 Setting: single‐centre study, Cardiovascular Institute, University College London Hospital, London, UK Withdrawals: not reported |
|
| Participants |
N = 57 (control = 30, intervention = 27) participants randomised Mean age = 67 years (± 12 years) Gender: 79% male, 21% female Severity of condition: Single‐vessel disease = 3,5%, two‐vessel disease = 19,5%, three‐vessel disease = 66,5%, four‐vessel disease = 10,5%, EF > 55% = 67%, EF 35%‐50% = 22,5%, EF<35% = 3,5%, EF not known = 7%, diabetes 42%, previous MI 31,5%. Inclusion criteria: people with coronary artery disease referred for elective coronary artery bypass graft surgery Exclusion criteria: people > 80 years, unstable angina, left main stem disease, hepatical/renal/pulmonal disease and those with peripheral vascular disease affecting the upper limbs, participants taking antidiabetic sulphonylurea, glibenclamide Reported differences between intervention and comparison groups: no statistical difference between groups with regard to baseline characteristics Anaesthestetic gas used: propofol |
|
| Interventions |
Intervention: RIPC consisted of three 5‐min cycles of right upper arm ischaemia, which was induced by an automated cuff‐inflator placed on the right upper arm and inflated to 200 mm Hg, with an intervening 5 min of reperfusion during which the cuff was deflated Comparison: deflated cuff placed on the right upper arm for 30 min Concomitant medications: aspirin = 96.5%, ß‐blocker = 63%, statins = 95%, ACE inhibitor = 61.5%, long‐acting nitrates 15.5% Excluded medications: antidiabetic sulphonylurea, glibenclamide |
|
| Outcomes |
Outcomes and time points measured in the study: Serum troponin‐T concentration (measured before surgery and at 6 h, 12 h, 24 h, 48 h, and 72 h after surgery) |
|
| Notes |
Funding for trial: Department of Health’s NIHR Biomedical Research Centres Notable conflicts of interest of authors: none to declare |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to form judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to form judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Patients and the cardiac surgeon were blinded to treatment allocation." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to form judgement. However, the objective outcome troponin T is not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methods section were adequately reported or explained in results. |
| Other bias | Low risk | No other bias identified |
Hausenloy 2015.
| Methods |
Study design: RCT Total duration of study: 36 months, April 2011 ‐ March 2014 Setting: multicentre study, 30 cardiac surgery centres in the UK Withdrawals: 28 (3.5%) in the control group and 19 (2.4%) in the intervention group |
|
| Participants |
N = 1612 (control = 811, intervention = 801) participants randomised Mean age = 76 years (± 6.5 years) Gender: 71% male, 29% female Severity of condition: diabetes 25.9%, previous MI 39.9%, no details on number of vessels, EF or NYHA class reported Inclusion criteria: adults (≥ 18 years of age) with an additive EuroSCORE of ≥ 5 (with higher scores indicating a greater risk of death; 0 indicates minimum risk and ≥ 6 indicates high risk) who were undergoing on‐pump CABG (with or without valve surgery) with myocardial protection provided by blood cardioplegia Exclusion criteria: cardiogenic shock or cardiac arrest during the current admission, pregnancy, clinically significant peripheral arterial disease affecting the arms, hepatic dysfunction (bilirubin level of > 20 μmol per litre (1.2 mg per decilitre) or international normalised ratio of > 2.0), pulmonary disease (forced expiratory volume in 1 second of < 40% of the predicted value) or renal failure (estimated glomerular filtration rate of < 30 mL per minute per 1.73 m2 of body‐surface area), and concomitant therapy with glibenclamide or nicorandil Reported differences between intervention and comparison groups: no statistical difference between groups with regard to baseline characteristics Anaesthestetic gas used: propofol and volatile anaesthesia |
|
| Interventions |
Intervention: four 5‐min inflations to 200 mmHg and deflations of a standard blood‐pressure cuff on the upper arm after induction of anaesthesia and before surgical incision Comparison: four 5‐min cycles of simulated remote ischaemic preconditioning, simulated by inflating the cuff with the valve open Concomitant medications: sulphonylurea = 5.5% Excluded medications: glibenclamide and nicorandil |
|
| Outcomes |
Outcomes and time points measured in the study: The combined primary end point was death from cardiovascular causes, nonfatal MI, coronary revascularisation, or stroke, assessed 12 months after randomisation. Secondary endpoints Death from cardiovascular causes, non‐fatal MI, coronary revascularisation, or stroke assessed at 30 days and 12 months Death from any cause, assessed at 12 months AUC troponin T 72 h (obtained preoperatively and 6 h, 12 h, 24 h, 48 h, and 72 h) Grade 1, 2, or 3 AKI within 72 h after surgery 24‐h AUC for plasma neutrophil gelatinase– associated lipocalin Maximum inotrope score in the 72‐h postoperative period Length of ICU stay and hospital stay 6‐min walk test at baseline, 6 weeks, and 12 months Health‐related quality of life as assessed by the European Quality of Life–5 Dimensions score at baseline, 6 weeks, and 3, 6, 9, and 12 months |
|
| Notes |
Funding for trial: funded by the Efficacy and Mechanism Evaluation Program (a Medical Research Council and National Institute of Health Research partnership) and the British Heart Foundation Notable conflicts of interest of authors: none to declare |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Randomisation was conducted by means of a secure website (Sealed Envelope)" |
| Allocation concealment (selection bias) | Low risk | "Randomisation was conducted by means of a secure website (Sealed Envelope)" |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The patients, anaesthesiologists, cardiac surgeons, intensive care unit (ICU) and ward staff, and study investigators collecting and analysing the data were all unaware of the treatment assignments" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The patients, anaesthesiologists, cardiac surgeons, intensive care unit (ICU) and ward staff, and study investigators collecting and analysing the data were all unaware of the treatment assignments" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Less than 20% lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the study protocols and in the methods section were adequately reported or explained in results. |
| Other bias | Low risk | No other bias identified |
Hong 2010.
| Methods |
Study design: RCT Total duration of study: 10 months, December 2008‐May 2009, November 2009‐February 2010 Setting: single‐centre study, Seoul National University Hospital, Seoul, Korea Withdrawals: not reported |
|
| Participants |
N = 130 (control = 65, intervention = 65) participants randomised Mean age = 65 years (± 9 years) Gender: 70% male, 31% female Severity of condition: three‐vessel disease, EF = 55.5%, diabetes = 35%, previous MI = 6.5% Inclusion criteria: people undergoing elective off‐pump CABG Exclusion criteria: age > 80 years, unstable angina, preoperative use of inotropic agents or mechanical‐assist devices, left ventricular EF < 30%, major combined operation such as vascular surgery, severe liver/renal or pulmonary disease, MI within last 7 days, preoperative use of nicorandil, peripheral vascular disease, or amputation upper limb Reported differences between intervention and comparison groups: no statistical difference between groups with regard to baseline characteristics Anaesthestetic gas used: sevoflurane anaesthesia |
|
| Interventions |
Intervention: RIPC induction by using a pneumatic blood pressure cuff on the upper arm, inflated to 200 mmHg by an automated cuff inflator. There were 4 cycles of 5‐min ischaemia and 5‐min reperfusion. RIPC protocol was done after anaesthesia induction Comparison: all procedures were the same in the control group, except for the fact that the 3‐way stop clock between the pneumatic cuff and the cuff inflator was opened and therefore the cuff pressure did not increase. Concomitant medications: aspirin = 94%, ß‐blocker = 53%, ca‐blocker = 40%, ACE inhibitor = 51%, statin = 63%, long‐acting nitrates = 64%, insulin = 8%, sulphonylurea = 20% Excluded medications: antiplatelet therapies other than aspirin were stopped one week before operation and started again on the first postop day, oral hypoglycaemic agents such as sulphonylureas were stopped 3 days before operation, also preoperative use of nicorandil, inotropic agents |
|
| Outcomes |
Outcomes and time points measured in the study: Serum troponin I (preoperatively and 1 h, 6 h, 12 h, 24 h, 48 h and 72 h postoperatively) |
|
| Notes |
Funding for trial: not reported Notable conflicts of interest of authors: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomly allocated to the RIPC or control group by a computer‐generated random code." |
| Allocation concealment (selection bias) | Low risk | "An anaesthesia nurse who was not involved in patient treatment performed the patient allocation and RIPC procedure." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "The surgeons, anaesthesiologists, including researchers were blinded to the group assignment." As RIPC protocol was applied after induction of anaesthesia, it is likely that participants were blinded to group allocation, but no details provided. However, the objective outcome troponin T is not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "The surgeons, anaesthesiologists, including researchers were blinded to the group assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Detailed description of lost‐to‐follow‐up in the study flow chart (< 20%) |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methods section were adequately reported or explained in results. |
| Other bias | High risk | Study was planned with 80 participants, during the course of the study it was decided to include another 50 participants "to increase power". |
Joung 2013.
| Methods |
Study design: RCT Total duration of study: 24 months, October 2009‐October 2011 Setting: single‐centre study, Department of Anesthesiology and Pain Medicine, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea Withdrawals: 11 intervention group, 13 control group |
|
| Participants |
N = 98 (control = 49, intervention = 49) participants randomised Mean age = 60 years (± 7.8 years) Gender: 81% male, 19% female Severity of condition: 3‐vessel disease, average EF = 57.5%, diabetes = 38% Inclusion criteria: all patients who underwent off‐pump coronary artery bypass graft surgery within the study duration Exclusion criteria: emergency surgery, age < 40 and > 80, supported mechanical assist device, preoperative inotropic use, EF < 30%, history of neuropsychiatric disease, people who did not want to participate Reported differences between intervention and comparison groups: no statistical difference between groups with regard to baseline characteristics Anaesthestetic gas used: propofol anaesthesia |
|
| Interventions |
Intervention: Four, 5‐min cycles of ischaemia with 5‐min of reperfusion on the upper limb using a blood pressure cuff inflated to 200 mmHg before coronary artery anastomosis Comparison: blood pressure cuff placed on the upper limb, without inflation Concomitant medications: ACE‐inhibitor = 23%, Ca‐ blocker = 47%, ß‐blocker = 51%, lipid‐lowering agents = 37% Excluded medications: not reported |
|
| Outcomes |
Outcomes and time points measured in the study: Development cognitive dysfunction 7 days postoperatively Extubation time Max SOFA score Duration of ICU stay |
|
| Notes |
Funding for trial: not reported Notable conflicts of interest of authors: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The enrolled patients were divided into control and rIPC groups using a computer‐generated randomisation table." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to form judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to form judgement however, the subjective outcome cognitive dysfunction is likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to form judgement however, the subjective outcome cognitive dysfunction is likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 29% of data is missing due to "loss to follow‐up, or using CPB system". |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methods section were adequately reported or explained in results. |
| Other bias | Unclear risk | Insufficient information to form judgement |
Karuppasamy 2011.
| Methods |
Study design: RCT Total duration of study: 11 months, October 2008‐September 2009 Setting: single‐centre study, Department of Cardiothoracic Surgery, King's College Hospital NHS Foundation Trust, London, UK Withdrawals: not reported |
|
| Participants |
N = 54 (control = 27, intervention = 27) participants randomised Mean age = 67 years (± 11 years) Gender: 83% male, 17% female Severity of condition: three‐vessel disease = 63%, NYHA class = 2.2, previous MI = 35%, diabetes = 21%, average EF = 54.2% Inclusion criteria: adults referred for elective CABG surgery without concomitant valve or aortic surgery Exclusion criteria: age > than 85 years, with unstable angina, significant hepatic, pulmonary or renal disease, peripheral vascular disease in the upper limbs, taking sulphonylureas or other drugs that may activate or inhibit preconditioning Reported differences between intervention and comparison groups: no statistical difference between groups with regard to baseline characteristics Anaesthestetic gas used: propofol anaesthesia |
|
| Interventions |
Intervention: Three 5‐min cycles of left upper arm ischaemia, which was induced by a blood pressure cuff inflated to 200 mmHg with 5‐min cycles of deflation Comparison: deflated cuff placed on the left upper arm for 30 min Concomitant medications: aspirin = 70.5%, ß‐blocker = 78%, cholesterol‐lowering drug = 100%, ACE‐inhibitor = 70.5%, insulin = 13% Excluded medications: sulphonylureas or other drugs that may activate or inhibit preconditioning |
|
| Outcomes |
Outcomes and time points measured in the study: Troponin I (cTnI) measured preoperatively and after 6 h, 12 h, 24 h and 48 h postoperatively Brain natriuretic peptide (BNP) CK‐MB Panel of cytokines and growth factors measured perioperatively (interferon‐c (INF‐c), interleukins (IL‐2, IL‐4, IL‐6, IL‐8, IL‐10, IL‐1a, IL‐1b), TNF‐a, and monocyte chemoattractant, protein 1 (MCP‐1) |
|
| Notes |
Funding for trial: not reported Notable conflicts of interest of authors: none to declare |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to form judgement |
| Allocation concealment (selection bias) | Unclear risk | "Patients were randomised by sealed envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Patients and surgeons were blinded to treatment allocation." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to base judgement. However, the measured biochemical markers in blood samples are objective outcomes and thus not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methods section were adequately reported or explained in results. |
| Other bias | Low risk | No other bias identified |
Kottenberg 2014.
| Methods |
Study design: RCT Total duration of study: 19 months, June 2010‐December 2011 Setting: single‐centre study, Department of Cardiothoracic Surgery, Westgerman Heart Centre Essen, University Hospital Essen, Germany Withdrawals: 5 participants due to unclear biopsy assignment |
|
| Participants |
N = 24 (control = 12, intervention = 12) participants randomised Mean age = 65 years (± 9 years) Gender: 88% male, 12% female Severity of condition: 3‐vessel coronary artery disease Inclusion criteria: all > 18 years of age who were scheduled for elective isolated first‐time CABG surgery for 3‐vessel coronary artery disease Exclusion criteria: any type of diabetes mellitus (controlled by diet, oral drugs, or insulin), renal insufficiency (serum creatinine > 2 mg/dL), peripheral vascular disease affecting the upper limbs, acute coronary syndrome, acute or recent MI, preoperative inotropic support before induction of anaesthesia, any kind of mechanical assist device, those with any condition potentially increasing preoperative troponin I concentration (cTnI), e.g. coronary interventions within the previous 6 weeks, or those having received any type of emergency surgery, combined CABG/valve surgery, or those with any previous cardiac operations Reported differences between intervention and comparison groups: no statistical difference between groups with regard to baseline characteristics Anaesthestetic gas used: propofol anaesthesia |
|
| Interventions |
Intervention: repetitive left upper arm a (three cycles of 5‐min cuff occlusion/ 5‐ min reperfusion each, cuff pressure 200 mmHg Comparison: un‐inflated cuff placed around the left upper arm Concomitant medications: not reported Excluded medications: diabetes medication |
|
| Outcomes |
Outcomes and time points measured in the study: Serum cTnI (measured preoperatively and 1 h, 6 h, 12 h, 24 h, 48 h, and 72 h postoperatively, AUC) Several cardioprotective cytokines (measured preoperatively and during reperfusion) |
|
| Notes |
Funding for trial: not reported Notable conflicts of interest of authors: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer‐generated randomisation schedules were generated and placed in sequentially numbered sealed envelopes." |
| Allocation concealment (selection bias) | Low risk | "Computer‐generated randomisation schedules were generated and placed in sequentially numbered sealed envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Lab personal, patients, surgeons, critical care teams were blinded to treatment and type of anaesthesia. The resident anaesthetists, who applied the protocol by inflating or not inflating the cuff could not be blinded to the group assignment but had no part in the data sampling or analysis." However, the measured biochemical markers in blood samples are objective outcomes and thus not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Lab personal, patients, surgeons, critical care teams were blinded to treatment and type of anaesthesia. The resident anaesthetists, who applied the protocol by inflating or not inflating the cuff could not be blinded to the group assignment but had no part in the data sampling or analysis." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 21% of data is missing due to "loss to follow‐up" (due to unclear biopsy assignment) |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methods section were adequately reported or explained in results. |
| Other bias | Unclear risk | Insufficient information to form judgement |
Krawczyk 2010.
| Methods |
Study design: RCT Total duration of study: not reported (completed by December 2009) Setting: Wojskowy Instytut Medyczny, Warsaw, Poland Withdrawals: not reported |
|
| Participants |
N = 14 (control = 6, intervention = 8) patients randomised Mean age = not reported Gender: not reported Severity of condition: not reported Inclusion criteria: adults undergoing elective off‐pump CABG Exclusion criteria: not reported Reported differences between intervention and comparison groups: not reported Anaesthestetic gas used: not reported |
|
| Interventions |
Intervention: Three 5‐min cycles of right upper limb ischaemia, induced by a manual cuff‐inflator placed on the right upper arm and inflated to 200 mmHg, with intervening reperfusion periods of 3 min when the cuff was deflated after induction Comparison: not reported Concomitant medications: not reported Excluded medications: not reported |
|
| Outcomes |
Outcomes and time points measured in the study: Serum troponin I (cTnI) CKMB Heart‐type fatty acid‐binding proteins (h‐FAB) (measured preoperatively and at 6 h, 12 h, 24 h postoperatively) |
|
| Notes |
Funding for trial: not reported Notable conflicts of interest of authors: not reported This study was only reported as an abstract, we were not able to find any contact details of the authors, it remains unclear if the three studies Krawczyk 2010, 2011, 2012 report independent results. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to form judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to form judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to form judgement. However, the measured biochemical markers in blood samples are objective outcomes and thus not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to form judgement. However, the measured biochemical markers in blood samples are objective outcomes and thus not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to form judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to form judgement |
| Other bias | Unclear risk | Insufficient information to form judgement |
Krawczyk 2011.
| Methods |
Study design: RCT Total duration of study: not reported Setting: single‐centre study, Department of Cardiac Surgery, Military Institute of Medicine, Warsaw, Poland Withdrawals: not reported |
|
| Participants |
N = 19 (control = 9, intervention = 10) participants randomised Mean age = not reported Gender: not reported Severity of condition: not reported Inclusion criteria: adults undergoing elective off‐pump CABG Exclusion criteria: not reported Reported differences between intervention and comparison groups: not reported Anaesthestetic gas used: not reported |
|
| Interventions |
Intervention: three 5‐min cycles of right upper limb ischaemia, induced by a manual cuff‐inflator placed on the right upper arm and inflated to 200 mmHg, with intervening reperfusion periods of 3 min when the cuff was deflated after induction Comparison: not reported Concomitant medications: not reported Excluded medications: not reported |
|
| Outcomes |
Outcomes and time points measured in the study: Serum troponin I (cTnI) Creatine kinase MB (CKMB) Measured preoperatively and 6 h, 12 h, 24 h postoperatively |
|
| Notes |
Funding for trial: not reported Notable conflicts of interest of authors: not reported This study was only reported as an abstract, we were not able to find any contact details of the authors, it remains unclear if the three studies Krawczyk 2010, 2011, 2012 report independent results. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to form judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to form judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to form judgement. However, the measured biochemical markers in blood samples are objective outcomes and thus not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to form judgement. However, the measured biochemical markers in blood samples are objective outcomes and thus not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to form judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to form judgement |
| Other bias | Unclear risk | Insufficient information to form judgement |
Krawczyk 2012.
| Methods |
Study design: RCT Total duration of study: not reported Setting: Wojskowy Instytut Medyczny, Warsaw, Poland Withdrawals: not reported |
|
| Participants |
N = 30 (control = 15, intervention = 15) participants randomised Mean age = not reported Gender: not reported Severity of condition: not reported Inclusion criteria: adults undergoing elective off‐pump CABG Exclusion criteria: not reported Reported differences between intervention and comparison groups: not reported Anaesthestetic gas used: not reported |
|
| Interventions |
Intervention: three 5‐min cycles of right upper limb ischaemia, induced by a manual cuff‐inflator placed on the right upper arm and inflated to 200 mmHg, with intervening reperfusion periods of 3 min when the cuff was deflated after induction Comparison: not reported Concomitant medications: not reported Excluded medications: not reported |
|
| Outcomes |
Outcomes and time points measured in the study: Serum troponin I (cTnI) Creatine kinase MB (CKMB) measured preoperatively and 6 h, 12 h, 24 h postoperatively |
|
| Notes |
Funding for trial: not reported Notable conflicts of interest of authors: not reported This study was only reported as an abstract, we were not able to find any contact details of the authors, it remains unclear if the three studies Krawczyk 2010, 2011, 2012 report independent results. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to form judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to form judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to form judgement. However, the measured biochemical markers in blood samples are objective outcomes and thus not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to form judgement. However, the measured biochemical markers in blood samples are objective outcomes and thus not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to form judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to form judgement |
| Other bias | Unclear risk | Insufficient information to form judgement |
Krogstad 2015.
| Methods |
Study design: RCT Total duration of study: not reported Setting: Department of Cardiothoracic Surgery, St. Olav’s University Hospital, Trondheim, Norway Withdrawals: 4 due to administrative reasons |
|
| Participants |
N = 92 (control = 47, intervention = 45) participants randomised Mean age = 64 years (± 8 years) Gender: 84% male, 16% female Severity of condition: EF = 53%, diabetes = 23%, previous MI = 41% Inclusion criteria: > 18 years of age, scheduled to undergo elective, isolated, primary CABG surgery with cardiopulmonary bypass Exclusion criteria: severe pulmonary disease, renal failure (GFR 30 mL/min/1.73 m2) liver failure, peripheral vascular disease affecting the upper limbs or atrial fibrillation in their case history, > 80 years of age and used sulphonylurea derivatives Reported differences between intervention and comparison groups: none Anaesthestetic gas used: isoflurane and propofol anaesthesia |
|
| Interventions |
Intervention: 3 cycles of 5‐min right upper arm ischaemia after induction of anaesthesia, which was induced by inflating a blood pressure cuff to 200 mmHg, with an intervening 5 min of reperfusion during which the cuff was deflated Comparison: Deflated cuff placed on the right upper arm throughout the same period Concomitant medications: not reported Excluded medications: sulphonylurea derivatives |
|
| Outcomes |
Outcomes and time points measured in the study: Incidence of postoperative atrial fibrillation Circulating microRNA in blood plasma |
|
| Notes |
Funding for trial: the study was supported by Skipsreder Tom Wilhelmsens Stiftelse Notable conflicts of interest of authors: none to declare |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Internet‐based randomisation application created by the Unit of Applied Clinical Research at the Norwegian University of Science and Technology (NTNU) |
| Allocation concealment (selection bias) | Low risk | Internet‐based randomisation application created by the Unit of Applied Clinical Research at the Norwegian University of Science and Technology (NTNU) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, cardiac surgeons, the investigator and the staff at both the ward and intensive care unit were all blinded to treatment allocation. Theatre nurses and anaesthetists were present during the RIPC or control procedure and were consequently not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants, cardiac surgeons, the investigator and the staff at both the ward and intensive care unit were all blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methods section were adequately reported or explained in results |
| Other bias | Low risk | No other bias identified |
Lomivorotov 2012.
| Methods |
Study design: RCT Total duration of study: 9 months, June 2010‐March 2011 Setting: single‐centre study, Department of Anaesthesiology, Academician EN Meshalkin Novosibirsk State Research Institute of Circulation Pathology, Novosibirsk, Russia Withdrawals: not reported |
|
| Participants |
N = 80 (control = 40, intervention = 40) participants randomised Mean age = 57 years (± 9 years) Gender: 91% male, 9% female Severity of condition: NYHA class II = 27.5%, NYHA class III = 72.5%, average EF = 59% Inclusion criteria: 80 adults with stable CHD referred for CABG under cardiopulmonary bypass were recruited Exclusion criteria: reduced left ventricular EF (< 50%), renal failure, hepatic or pulmonary disease, diabetes and MI within the past 4 weeks Reported differences between intervention and comparison groups: no statistical difference between groups with regard to baseline characteristics Anaesthestetic gas used: isoflurane anaesthesia |
|
| Interventions |
Intervention: Three 5‐min cycles of right upper limb ischaemia, induced by a blood pressure cuff placed on the right upper arm and inflated to 200 mm Hg, with an intervening 5 min of reperfusion during which the cuff was deflated Comparison: control participants had a deflated cuff placed on the right upper arm for 30 min Concomitant medications: not reported Excluded medications: not reported |
|
| Outcomes |
Outcomes and time points measured in the study: Cardiac index Stroke index Central venous pressure Pulmonary capillary wedge pressure Mean arterial pressure and heart rate before RIPC, after RIPC and 5 min, 30 min, 2 h, 4 h, 6 h and 24 h after discontinuing CPB troponin I (cTnI) preoperatively and 6 h, 24 h and 48 h after CPB CK‐MB preoperatively and 6 h, 24 h and 48 h after CPB Duration of ventilation postoperatively ICU stay Complications Blood loss Mortality |
|
| Notes |
Funding for trial: institutional funding Notable conflicts of interest of authors: none to declare |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to form judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to form judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to form judgement. However, the measured biochemical markers in blood samples and haemodynamic intraoperative factors are objective outcomes and thus not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to form judgement. However, the measured biochemical markers in blood samples and haemodynamic intraoperative factors are objective outcomes and thus not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methods section were adequately reported or explained in results. |
| Other bias | Low risk | No other bias identified |
Lucchinetti 2012.
| Methods |
Study design: RCT Total duration of study: 23 months, September 2008‐July 2010 Setting: single‐centre study, University Hospital of Alberta, Edmonton, Alberta, Canada Withdrawals: 2 (logistic reasons) |
|
| Participants |
N = 55 (control = 28, intervention = 27) patients randomised Mean age = 61 years (± 10 years) Gender: 91% male, 9% female Severity of condition: three‐vessel disease, average EF = 51%, previous MI = 42%, Inclusion criteria: scheduled for elective on‐pump CABG surgery, aged 50–85 years Exclusion criteria: emergency surgery, MI within 48 h before surgery as defined by increased plasma concentrations for cardiac enzymes, diabetes mellitus, BMI > 35, concomitant non‐cardiac surgery, or severe peripheral vascular disease Reported differences between intervention and comparison groups: no statistical difference between groups with regard to baseline characteristics Anaesthestetic gas used: isoflurane anaesthesia |
|
| Interventions |
Intervention: Four 5‐min cycles of lower limb ischaemia‐reperfusion induced by a tourniquet inflated to 300 mmHg Comparison: no treatment Concomitant medications: ß‐blockers = 91%, ACE‐inhibitor = 51%, angiotensin antagonists = 24%, Ca‐ blockers = 14,5%, statins = 96%, Aspirin = 94,5%, clopidogrel = 11%, diuretics = 20% Excluded medications: not reported |
|
| Outcomes |
Outcomes and time points measured in the study: High‐sensitivity cardiac troponin T (hscTnT) (peak hscTnT values and AUC) at pre induction, pre bypass, immediately post bypass, 60 min post cross‐clamp release, 24 h, 48 h, and 72 h after surgery Plasma levels of N‐terminal pro‐brain natriuretic peptide (NTproBNP) High‐sensitivity C‐reactive protein (hsCRP) S100 Perioperative composite endpoint: new arrhythmias and new MIs Late (6 month) adverse events:
|
|
| Notes |
Funding for trial: not reported Notable conflicts of interest of authors: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "A 1:1 block randomisation with no further stratification was generated by an independent person using a computer random number generator." |
| Allocation concealment (selection bias) | Low risk | "The results were stored in numbered, sealed, opaque envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "RIPC procedure was executed by an operating room technician, who was not otherwise involved in the study." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Collection and analysis of all clinical and laboratory data were performed by study personnel blinded for group assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported |
| Selective reporting (reporting bias) | Unclear risk | hscTnT and cThT values were measured at several time points (preoperatively, pre bypass, post bypass, 1 h post clamp, postoperative day 1, 2, 3) but are not given by numbers and only shown as a figure. Only the AUC is given, however, it remains unclear to which of the two biomarkers the AUC relates. |
| Other bias | Unclear risk | Insufficient information to form judgement |
Meybohm 2013.
| Methods |
Study design: RCT Total duration of study: 23 months, January 2009‐November 2010 Setting: single‐centre study, Department of Cardiovascular Surgery, University Hospital Schleswig‐Holstein, Cammpus Kiel, Germany Withdrawals: 48 drop outs |
|
| Participants |
N = 180 (control = 90, intervention = 90) participants randomised Mean age = 69 (age range = 23‐86) Gender: 81% male, 19% female Severity of condition: 59% CABG (three‐vessel disease), valve surgery = 18%, combined surgery procedure = 23%; pre op EF = 67.5%, diabetes = 15%, previous MI = 24% Inclusion criteria: written informed consent, > 18 years, scheduled to undergo differing types of cardiac surgery in which a cardiopulmonary bypass would be used Exclusion criteria: surgery‐related exclusions (off‐pump heart surgery, concomitant carotid surgery, minimally invasive surgery with lateral thoracotomy, selective antegrade cerebral perfusion, previous heart surgery, aorta descendens surgery, normothermic cardiopulmonary bypass), cardiac‐related exclusions (previous MI within the last 7 days; EF 30%; previous atrial fibrillation within the last 6 months; receiving therapy with amiodarone, digitalis, and other antiarrhythmic agents; having an implanted pacemaker or defibrillator; and emergency cases), previous stroke within the last 2 months, renal failure (defined as a plasma creatinine level of > 2.0 mg/dL), liver failure, severe alcohol abuse, severe chronic obstructive pulmonary disease, receiving therapy with sulphonamide and nicorandil (which are preconditioning blocking and preconditioning mimetic medications, respectively), previous serious psychiatric disorders (e.g. schizophrenia or dementia), previous serious neurologic illnesses (e.g. Parkinson’s disease), and a Mini‐Mental State Examination (MMSE) score < 24 points Reported differences between intervention and comparison groups: significantly more MIs in the last 7 days before surgery in the RIPC group (30 vs 14) Anaesthestetic gas used: propofol anaesthesia |
|
| Interventions |
Intervention: 4 cycles of 5‐min upper limb ischaemia via blood pressure cuff inflation to 200 mmHg (a cuff‐pressure at least 15 mm Hg higher than the systolic arterial pressure measured via the arterial line) and 5‐min cuff deflation Comparison: 4 cycles of 5‐min blood pressure cuff inflation to 20 mmHg and 5‐min cuff deflation without any limb ischaemia Concomitant medications: ß‐blockers = 65%, ACE antagonist = 41%, long‐acting nitrates = 3%, insulin/metformin = 12%, statins = 52% Excluded medications: amiodarone, digitalis, and other antiarrhythmic agents, therapy with sulphonamide and nicorandil |
|
| Outcomes |
Outcomes and time points measured in the study: Post‐op neurocognitive dysfunction 5‐7 days after surgery Ventilatory support Incidence of re‐intubation Total length of hospital stay AKI assessed by serum creatinine (the day before, 24 h after, and 48 h after surgery) according to AKIN criteria Myocardial injury assessed by troponin T (the day before surgery; after ICU admission; and 12 h, 24 h, and 48 h following surgery) New onset of atrial fibrillation within 4 days after surgery |
|
| Notes |
Funding for trial: the study is funded by a junior research funding award of the University of Kiel (PM), a Clinical Scholar Research Award of the International Anesthesia Research Society (PM), and a Clinical Research Grant of the German Society of Anesthesiology and Intensive Care Medicine (PM). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript Notable conflicts of interest of authors: the study authors have declared that no competing interests exist |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to form judgement |
| Allocation concealment (selection bias) | Low risk | "Sealed envelopes were used for randomisation." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Blinding concerned (1) the individual patient, (2) the staff involved in intraoperative (anaesthesia and cardiac surgery team) and perioperative (intensive care unit (ICU) staff) care, and (3) investigators obtaining data, performing the neurocognitive assessment, visiting patients during follow‐up, and documenting the study." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | "Blinding concerned (1) the individual patient, (2) the staff involved in intraoperative (anaesthesia and cardiac surgery team) and perioperative (intensive care unit (ICU) staff) care, and (3) investigators obtaining data, performing the neurocognitive assessment, visiting patients during follow‐up, and documenting the study." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 27% lost to follow‐up (reasons: death, leaving city, refused assessment, others) |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methods section were adequately reported or explained in results |
| Other bias | Low risk | No other bias identified |
Meybohm 2015.
| Methods |
Study design: RCT Total duration of study: 41 months, January 2011‐May 2014 Setting: Multicentre study in 14 German university hospitals:
Withdrawals: Of 1403 patients who underwent randomisation, 42 did not receive the assigned intervention, owing mainly to logistic reasons. A total of 18 participants (10 in the RIPC group and 8 in the sham‐RIPC group) were not included in the full analysis set, because no information on the primary end point was available. A total of 218 participants had protocol violations, resulting in 1167 (83.2%) participants in the per‐protocol set. |
|
| Participants |
N = 1385 (control = 693, intervention = 692) participants randomised Mean age = 66 years (± 10.4) Gender: 74% male, 26% female Severity of condition: Ischaemic heart disease = 75.3%, isolated CABG = 45.5%, previous MI = 29%, diabetes = 25%, EF > 55% = 75.5%, EF 30%‐55% = 24.4%, EF < 30% = 0.1%, EUROscore = 4.2 ± 2.6. Inclusion criteria: ≥ 18 years scheduled for elective cardiovascular surgery requiring cardiopulmonary bypass and who provided written informed consent Exclusion criteria: key exclusion criteria were related to specific surgical procedures (e.g. off‐pump heart surgery or urgent surgery) and severe organ dysfunction (e.g. EF < 30% or severe renal failure)
Reported differences between intervention and comparison groups: none Anaesthestetic gas used: propofol anaesthesia |
|
| Interventions |
Intervention: 4 cycles of 5‐min upper limb ischaemia via blood pressure cuff inflation to 200 mmHg (a cuff‐pressure at least 15 mm Hg higher than the systolic arterial pressure measured via the arterial line) and 5‐min cuff deflation Comparison: a dummy arm was used for similar cycles of inflation and deflation Concomitant medications: ß‐blocker = 63%, ACE‐i = 52%, cholesterol‐lowering drug = 65.5% Excluded medications: drug therapy with sulphonylureas and nicorandil |
|
| Outcomes |
Outcomes and time points measured in the study: Composite endpoint (including all‐cause mortality, non‐fatal MI, any new stroke, and/or acute renal failure) until hospital discharge (within a maximum of 14 days when length of hospital stay was longer) Secondary endpoints: Any individual component of the composite at 30 and 90 days after surgery Duration of mechanical ventilation, length of stay on the ICU and in hospital. Troponin T/I (preoperative, 6, 12, 24, and 48 h after surgery) Creatinine (preoperative, 24 h and 48 h postoperative as well as maximum creatinine). Modified RIFLE criteria were Used to specify acute kidney injury Vasopressor and inotropic support New onset of atrial fibrillation (within 4 days after surgery) Incidence of postoperative delirium was assessed with the CAM‐ICU score (preoperative, 24 h, 48 h, 72 h, and 96 h after surgery) |
|
| Notes |
Funding for trial: supported by a grant (ME 3559/1‐1) from the German Research Foundation Notable conflicts of interest of authors: Dr Bein reports receiving fees for serving on advisory boards from Pulsion Medical Systems, 3M, and Merck Sharp & Dohme; acting as medical advisor to the Medicines Company; receiving consulting and lecture fees from Edwards Lifesciences, CSL Behring, Orion Pharma, AbbVie, and GE Healthcare; receiving devices for research purposes from 3M; and receiving grant support from AbbVie and GE Healthcare. Dr Böning reports receiving honoraria for presentations from Maquet, Bayer, AstraZeneca, and Orion Pharma. Dr Stehr reports receiving honoraria and travel support from Teva/Ratiopharm. Dr Heringlake reports receiving consulting and lecture fees from Orion Pharma. Dr Sander reports receiving grant support from Ratiopharm, Pulsion Medical Systems, Edwards Lifesciences, the Medicines Company, and Fresenius Medical Care. No other potential conflict of interest relevant to this article was reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The trial statistician prepared the allocation algorithm, which was implemented at the Clinical Trial Centre. After written informed consent the Data Management (Clinical Trial Centre Leipzig) was contacted via an internet‐based randomisation tool." |
| Allocation concealment (selection bias) | Low risk | "Internet‐based randomisation tool." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "To ensure double blinding, the intervention was performed on patients who were already anaesthestized, on the arm with no arterial line, and surgical drapes were used to cover the blood‐pressure cuffs on both the patient’ arm and the dummy arm. The individual patient, the staff involved in intraoperative care (anaesthesia and cardiac‐surgery team) and postoperative care, the investigators who obtained and documented data and performed follow‐up assessments, and the clinical end‐point committee, whose members assessed all available electrocardiograms for reference analysis, were unaware of the study‐group assignments." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "To ensure double blinding, the intervention was performed on patients who were already anaesthestized, on the arm with no arterial line, and surgical drapes were used to cover the blood‐pressure cuffs on both the patient’ arm and the dummy arm. The individual patient, the staff involved in intraoperative care (anaesthesia and cardiac‐surgery team) and postoperative care, the investigators who obtained and documented data and performed follow‐up assessments, and the clinical end‐point committee, whose members assessed all available electrocardiograms for reference analysis, were unaware of the study‐group assignments." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported. Loss to follow‐up < 20% |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the study protocols and in the methods section were adequately reported or explained in results. |
| Other bias | Low risk | No other bias identified |
Rahman 2010.
| Methods |
Study design: RCT Total duration of study: 27 months, January 2007‐March 2009 Setting: single‐centre study, Department of Cardiothoracic Surgery, University Hospital Birmingham NHS trust, Birmingham, UK Withdrawals: 8 pre‐randomisation for logistical reasons |
|
| Participants |
N =162 (control = 82, intervention = 80) participants randomised Mean age = 64 years (age range = 57‐73) Gender: 88% male, 12% female Severity of condition: NYHA class II, elective surgery 49%, urgent surgery 51%. Preop EF 60.5%, Euroscore 3 Inclusion criteria: first time multi‐vessel CABG on CPB. Both elective and urgent adult patients were included Exclusion criteria: ST elevation MI within 30 days, any angina pain within 48 h of procedure, diabetes mellitus, pregnancy, preoperative dialysis, intended additional non‐CABG surgery, or radial artery usage Reported differences between intervention and comparison groups: none Anaesthestetic gas used: 79.5% enflurane, 18.5% sevoflurane, 2% propofol |
|
| Interventions |
Intervention: 3 cycles of upper limb ischaemia via cuff inflation (200 mmHg)/deflation for 5‐min Comparison: the placebo stimulus was an identical cuff inflation applied on a wooden cylinder acting as a dummy arm Concomitant medications: ß‐blocker = 81%, Ca2+‐blockers = 35.5%, statins = 91%, diuretics = 16.5%, oral nitrates = 30%, aspirin = 88%, ACE‐i = 65% Excluded medications: none |
|
| Outcomes |
Outcomes and time points measured in the study: The primary end point: 48‐h AUC troponin T (cTnT) Secondary cardiac endpoints: 6‐h and peak cTnT Incidence of myocardial injury on 12‐lead ECG Serial cardiac and left ventricular stroke work indices Low cardiac output episode incidence Inotrope usage and dosage Reperfusion ventricular fibrillation Perioperative ventricular tachyarrhythmia incidence and functional echocardiographic change ICU and hospital length of stay Postoperative creatinine on days 0 and 4. Urinary albumin‐creatinine ratios at 0 h, 12 h, and 24 h, and ‐1 microglobulin at 0 and 24 h Arterial pO2 (mm Hg): FiO2 ratios at 6 h and 12 h after surgery |
|
| Notes |
Funding for trial: this study was funded by the British Heart Foundation (project grant number: PG/05/125/19869) Notable conflicts of interest of authors: disclosures: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer generated 1:1 randomisation, stratified by surgeon." |
| Allocation concealment (selection bias) | Low risk | "Randomisation schedules were placed in sequentially numbered sealed envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Patients, investigators, anaesthetists, surgeons, and critical care teams were blinded to group allocation." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to form judgement however, measured biochemical markers (such as troponin T and creatine), perioperative haemodynamic factors (such as inotrope usage) and postoperative development including ICU stay are not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 18% loss to follow‐up due to incomplete data sets, however, only 6% of the cTnT measurements were reported incompletely. |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methods section were adequately reported or explained in results. |
| Other bias | Low risk | No other bias identified |
Saxena 2013.
| Methods |
Study design: RCT Total duration of study: 11 months, February‐December 2010 Setting: single‐centre study; Sir Charles Gairdner Hospital, Perth, Australia Withdrawals: not reported |
|
| Participants |
N = 30 (control = 15, intervention =15) participants randomised Mean age = 66.9 years (± 9.15) Gender: 93% male, 7% female Severity of condition: LVEF = 56%, diabetes = 27%, number grafts = 4.3 (± 0.8) Inclusion criteria: elective CABG with CPB at Sir Charles Gairdner Hospital Exclusion criteria: evolving MI, those taking oral sulphonyl urea medication or insulin for diabetes, those with a LVEF < 35% and those undergoing perioperative haemodialysis Reported differences between intervention and comparison groups: none Anaesthestetic gas used: not reported |
|
| Interventions |
Intervention: Three 5‐min cycles of ischaemia and reperfusion by inflation of a standard blood pressure cuff, placed on the upper arm, to a pressure exceeding systolic pressure by 20 mmHg Comparison: a blood pressure cuff was placed around the upper arm but was not inflated Concomitant medications: not reported Excluded medications: oral sulphonyl urea or insulin |
|
| Outcomes |
Outcomes and time points measured in the study: Expression of kinin receptors Plasma concentrations of IL‐6, IL‐8, IL‐10, TNF‐α and neutrophil elastase at baseline (before RIPC/sham), before surgery (after RIPC/sham) and 30 min and 24 h after surgery Plasma bradykinin levels before and after RIPC/sham, and at 30 min, 6 h, 12 h and 24 h after surgery Serum creatine kinase (CK), troponin I, CRP and lactate levels immediately prior to surgery and 30 min, 6 h, 12 h, 24 h and 48 h after surgery |
|
| Notes |
Funding for trial: National Health and Medical Research Council of Australia Notable conflicts of interest of authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to form judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to form judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "Double‐blind trial." |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to form judgement however, biochemical markers measured in blood samples are objective subjects and thus not likely to be influenced by lack of blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methods section were adequately reported or explained in results. |
| Other bias | Low risk | No other bias identified |
Shmyrev 2011.
| Methods |
Study design: RCT abstract Total duration of study: not reported Setting: single‐centre study, academician EN Meshalkin Research Institute of Circulation Pathology, Novosibirsk, Russia Withdrawals: not reported |
|
| Participants |
N =31 participants randomised Mean age = not reported (age range = not reported) Gender: not reported Severity of condition: not reported Inclusion criteria: stable CABG patients operated under cardiopulmonary bypass Exclusion criteria: not reported Reported differences between intervention and comparison groups: not reported Anaesthestetic gas used: not reported |
|
| Interventions |
Intervention: three 5‐min cycles of upper limb ischaemia and reperfusion using a blood pressure cuff Comparison: not reported Concomitant medications: not reported Excluded medications: not reported |
|
| Outcomes |
Outcomes and time points measured in the study: Haemodynamics Levels of troponin I Creatine kinase MB (mass) Heat shock protein 70 (Hsp70) Interleukin‐6 (IL‐6), interleukin‐8 (IL‐8), interleukin‐10 (IL‐10) were assessed perioperatively |
|
| Notes |
Funding for trial: not reported Notable conflicts of interest of authors: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to form judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to form judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to base judgement however, biochemical markers measured in blood samples are objective subjects and thus not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to base judgement however, biochemical markers measured in blood samples are objective subjects and thus not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to form judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to form judgement |
| Other bias | Unclear risk | Insufficient information to form judgement |
Slagsvold 2014.
| Methods |
Study design: RCT Total duration of study: not reported Setting: single‐centre study, Department of Cardiothoracic Surgery, St. Olav's university hospital, Trondheim, Norway Withdrawals: none |
|
| Participants |
N = 60 (control =30, intervention = 30) participants randomised Mean age = 66 years (± 8.6) Gender: 83% male, 17% female Severity of condition: coronary graft anastomose = 3.37, EF > 50% = 78%, EF 31%‐50% = 20%, EF 21%‐30% = 1.5% EF < 20% = 0%, previous MI = 57%, diabetes = 21%, Euroscore = 1.33 Inclusion criteria: undergoing CABG surgery at St Olavs Hospital Exclusion criteria: severe hepatic, renal or pulmonary disease, and peripheral vascular disease affecting the upper limbs Reported differences between intervention and comparison groups: significantly more calcium channel blocker in control group Anaesthestetic gas used: isoflurane |
|
| Interventions |
Intervention: 3 cycles of 5‐min limb ischaemia by inflating the blood pressure cuff to 200 mm Hg, interrupted by 5‐min reperfusion intervals Comparison: the blood pressure cuff remained deflated Concomitant medications: statin = 86.5%, ß‐ blocker = 81.5%, Ca antagonist = 17%, ACE inhibitor = 42%, diuretics = 18%, aspirin = 95%, clopidogrel = 40%, insulin/metformin = 17%, sulphonylurea = 3%, glibenclamide = 1.5%, organic nitrates =15%, warfarin = 5% Excluded medications: none |
|
| Outcomes |
Outcomes and time points measured in the study: Mitochondrial respiration Postop atrial fibrillation Cardiac troponin T (cTnT) CK‐MB, or N‐terminal probrain natriuretic peptide ‐ preoperative (T1), 3 h (T2) and 6 h after removal of arterial cross clamp (T3), and on the first postoperative day (T4) |
|
| Notes |
Funding for trial: this study was supported by Liaison Committee between the Central Norway Regional Health Authority and the Norwegian University of Science and Technology (NTNU), Unimed Innovation’s Research Fund, and Department of Circulation and Medical Imaging, Faculty of Medicine, NTNU Notable conflicts of interest of authors: disclosure: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Patients were randomised with an Internet‐based randomisation database through the Unit for Applied Clinical Research of St. Olav’s Hospital." |
| Allocation concealment (selection bias) | Low risk | "Patients were randomised with an Internet‐based randomisation database through the Unit for Applied Clinical Research of St. Olav’s Hospital." |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | "Patients, surgeons, personnel in postoperative intensive care and laboratory personnel were blinded to which group the patients were randomised." In addition, biochemical markers measured in blood samples are objective subjects and thus not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Group allocations were revealed after data collection, and analyses were completed." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported |
| Selective reporting (reporting bias) | High risk | Troponin T values are not reported for every time point. Only pre‐ and postoperative values are given. |
| Other bias | Low risk | No other bias identified |
Sosorburam 2014.
| Methods |
Study design: RCT abstract Total duration of study: 11 months, March 2012‐February 2013 Setting: single‐centre medical college in China Withdrawals: not reported |
|
| Participants |
N =268 participants randomised Mean age = not reported (age range = not reported) Gender: not reported Severity of condition: not reported Inclusion criteria: having CABG surgery with cardiopulmonary bypass machine Exclusion criteria: not reported Reported differences between intervention and comparison groups: none Anaesthestetic gas used: not reported |
|
| Interventions |
Intervention: 3 cycles of 5‐min RIPC is applied to limbs before surgery Comparison: Placebo Concomitant medications: not reported Excluded medications: not reported |
|
| Outcomes |
Outcomes and time points measured in the study: TnT CK‐MB 4 h, 12 h, 24 h intervals after CPB ICU stay Mechanical ventilation |
|
| Notes |
Funding for trial: not reported Notable conflicts of interest of authors: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to form judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to form judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to base judgement however, biochemical markers measured in blood samples are objective subjects and thus not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to base judgement however, biochemical markers measured in blood samples are objective subjects and thus not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to form judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to form judgement |
| Other bias | Unclear risk | Insufficient information to form judgement |
Thielmann 2013.
| Methods |
Study design: RCT Total duration of study: 55 months, April 2008‐October 2012 Setting: single‐centre study, West‐ German Heart Centre, Essen, Germany Withdrawals: not reported |
|
| Participants |
N =329 (control 167, intervention 162) participants randomised Mean age = 68.7 years (± 9.8) Gender: 81.5% male, 18.5% female Severity of condition: bypass grafts = 2.7; Diabetes = 8.5%, LVEF = 51.3%, previous MI = 14.5%, EuroSOCORE II = 0.5% Inclusion criteria: adults with three‐vessel coronary artery disease, who were scheduled to undergo primary, isolated, elective CABG surgery under cardiopulmonary bypass at the West‐German Heart Centre, Essen, Germany, between April 2008, and October 2012 Exclusion criteria: preoperative renal insufficiency (serum creatinine > 200 μmol/L), peripheral vascular disease affecting the upper limbs, acute coronary syndrome within the previous 4 weeks, inotropic or mechanical circulatory support before induction of anaesthesia, any disorder that could potentially increase preoperative cTnI concentrations (e.g. percutaneous coronary intervention within the previous 6 weeks), coronary surgery without cardiopulmonary bypass, and emergency, repeat, or concomitant surgery Reported differences between intervention and comparison groups: none Anaesthestetic gas used: isoflurane or propofol anaesthesia, however after becoming aware of the RIPC interference with propofol, propofol anaesthesia was discontinued |
|
| Interventions |
Intervention: Three cycles of 5‐min ischaemia, achieved by inflation of a blood pressure cuff to 200 mm Hg, followed by 5‐min reperfusion while the cuff was deflated were applied to the upper left arm Comparison: cuff was placed, around the arm but not inflated Concomitant medications: aspirin = 86%, clopidogrel = 30%, ß‐blockers = 69%, statins = 78%, ACE inhibitor = 46.5% Excluded medications: not reported |
|
| Outcomes |
Outcomes and time points measured in the study: Serum AUC for cTnI before and 1 h, 6 h, 12 h, 24 h, 48 h, and 72 h after surgery Secondary endpoints were assessed at 30 days, 1 year, and at completion of follow‐up: Death from any cause Major adverse cardiac events (MI) Cerebrovascular events (including cerebrovascular accident or stroke) Need for repeat revascularization |
|
| Notes |
Funding for trial: German Research Foundation Notable conflicts of interest of authors: the sponsor of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Codes were computer generated and kept in sealed envelopes at a central location." |
| Allocation concealment (selection bias) | Low risk | "Codes were computer generated and kept in sealed envelopes at a central location." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "A junior staff anaesthetist not involved in the study or analysis opened the envelope in the preparation room where anaesthesia was induced. Patients, cardiac surgeons and intensive care physicians were unaware of treatment assignment." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Data of outcomes were obtained from medical records and reviewed by two consultant cardiologists who did not participate in the study." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methods section were adequately reported or explained in results. |
| Other bias | Unclear risk | Unclear influence of study programme extension (although the original study protocol excluded participants with diabetes and those undergoing combined surgical procedures, the authors extended the study in 2012 and included these participants in the intention‐to‐treat analysis (n = 329). In the separate pre‐protocol analysis (n = 258) these participants were excluded) |
Venugopal 2009.
| Methods |
Study design: RCT Total duration of study: 12 months, February 2007‐February 2008 Setting: single‐centre study; Heart hospital, University College London Hospitals NHS Trust, London UK Withdrawals: none |
|
| Participants |
N = 45 participants randomised Mean age = 63 (± 9.4) Gender: male: 84.5%, female 15.5% Severity of condition: previous MI ?= 22.5%, NYHA = 1.55, EF > 55% = 91%, EF 35%‐55% = 7%, EF<35% = 2%, concomitant aortic valve replacement = 18% Inclusion criteria: adults with coronary artery disease consecutively referred for elective CABG surgery with or without concomitant aortic valve replacement between February 2007 and February 2008 Exclusion criteria: > 80 years of age, with unstable angina, diabetes mellitus, hepatic, renal or pulmonary disease, were excluded, as were people with peripheral vascular disease affecting the upper limbs Reported differences between intervention and comparison groups: none Anaesthestetic gas used: isoflurane/sevoflurane or propofol anaesthesia |
|
| Interventions |
Intervention: three 5‐min cycles of right forearm ischaemia, induced by inflating a blood pressure cuff on the upper arm to 200 mm Hg, with an intervening 5‐min reperfusion Comparison: deflated cuff placed on the upper arm for 30 min Concomitant medications: aspirin = 49%, ß‐blocker = 73.5%, cholesterol‐lowering agent = 82.5%, ACE inhibitor = 51%, nitrate = 13.5% Excluded medications: not reported |
|
| Outcomes |
Outcomes and time points measured in the study: Serum troponin T preoperatively and at 6 h, 12 h, 24 h, 48 h and 72 h after surgery and AUC at 72 h |
|
| Notes |
Funding for trial: this work was undertaken at UCLH/UCL who received a proportion of funding from the Department of Health’s NIHR Biomedical Research Centres funding scheme Notable conflicts of interest of authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "Computer randomised table with random numbers." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to form judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants and the cardiac surgeons were blinded to treatment allocation, investigators and anaesthetists were not blinded. In addition, biochemical markers measured in blood samples are objective subjects and thus not likely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to form judgement However, biochemical markers measured in blood samples are objective subjects and thus not likely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methods section were adequately reported or explained in results. |
| Other bias | Low risk | No other bias identified |
Yildirim 2016.
| Methods |
Study design: RCT Total duration of study: 26 months, January 2012‐February 2014 Setting: single‐centre study, Department of Cardiovascular Surgery, Celal Bayar University, School of Medicine, Manisa, Turkey Withdrawals: none |
|
| Participants |
N = 60 (control 30, intervention 30) participants randomised Mean age = 63 years (± 2) Gender: 81.7% male, 18.3% female Severity of condition: bypass grafts = 2 ± 0.2; diabetes = 42.5%, EUROScore II = 4 ± 0.4 Inclusion criteria: adults scheduled to undergo nonemergency, non repeat isolated CABG using CPB Exclusion criteria: severe left ventricular dysfunction (< 30% or end‐diastolic pressure > 16 mmHg), MI within the last 4 weeks. pulmonary disease, renal or hepatic dysfunction, diabetes treated with glibenclamide, age > 75 years, weight > 100 kg, BMI > 35 kg/m2 (large enough blood pressure cuff was not available), severe peripheral vascular disease, white blood cell count > 10.000 mm3, infection during the week before surgery, smoking during the month before surgery; or the preoperative use of antibiotics, beta‐blockers, corticosteroids, aspirin, nonsteroidal anti‐inflammatory drugs, allopurinol, calcium dobesilate, trimetazidine, pentoxifylline, or nicorandil Reported differences between intervention and comparison groups: None Anaesthestetic gas used: isoflurane or propofol anaesthesia, however after becoming aware of the RIPC interference with propofol, propofol anaesthesia was discontinued |
|
| Interventions |
Intervention: three cycles of 5‐min ischaemia, achieved by inflation of a blood pressure cuff to 200 mm Hg, followed by 5‐min reperfusion while the cuff was deflated were applied to the left lower tight Comparison: cuff was placed around the left upper tight for 25 min but not inflated Concomitant medications: not reported Excluded medications: antibiotics, beta‐blockers, corticosteroids, aspirin, nonsteroidal anti‐inflammatory drugs, allopurinol, calcium dobesilate, trimetazidine, pentoxifylline, or nicorandil |
|
| Outcomes |
Outcomes and time points measured in the study: Measures of SOD, GPx and MDA before induction of anaesthesia, 1 min and 15 min after release of cross‐clamp Measures of hs‐cTnT levels before induction of anaesthesia, 1 h, 6 h after skin closure, 24 h postoperatively |
|
| Notes |
Funding for trial: not reported Notable conflicts of interest of authors: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to form judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to form judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | "The patients, the ICU staff, and biochemical analysts were blinded with respect to the study group of patients." Investigators and anaesthetists were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | "Biochemical analysts were blinded with respect to the study group of patients." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All data reported |
| Selective reporting (reporting bias) | Low risk | All outcomes stated in the methods section were adequately reported or explained in results. |
| Other bias | Low risk | No other bias identified |
Young 2012.
| Methods |
Study design: RCT Total duration of study: 12 months, 18 May 2010‐23 June 2011 Setting: single‐centre study, Department of Cardiac Surgery, Wellington Hospital, Wellington, New Zealand Withdrawals: 4 due to missing data |
|
| Participants |
N = 96 participants randomised Mean age = 65 (± 13.5) Gender: 62.5% male, 37.5% female Inclusion criteria: eligible for inclusion if having one of the following ‘high‐risk’ cardiac surgical procedures with cardiopulmonary bypass: double‐valve or triple‐valve surgery, mitral valve surgery, CABG plus valve(s), CABG with documented pre‐operative EF of < 50%, or any ‘redo’ operation Exclusion criteria: < 18 years of age, had known peripheral vascular disease affecting the upper limbs, required deep hypothermic circulatory arrest, or were being considered for radial artery conduit harvesting Reported differences between intervention and comparison groups: not reported Anaesthestetic gas used: volatile anaesthesia |
|
| Interventions |
Intervention: 3 cycles of 5 min of upper‐limb ischaemia induced by inflating a blood pressure cuff to 200 mmHg with 5 min of reperfusion. This process began with the first surgical incision and was repeated three times. Comparison: in the control group, the same cycles were performed using a tourniquet wrapped around a towel lying next to the participant on the operating table. Concomitant medications: not reported Excluded medications: not reported |
|
| Outcomes |
Outcomes and time points measured in the study: Four main end points: Post‐operative plasma high‐sensitivity troponin T (hsTNT) levels measured at 6 h following aortic cross‐clamp removal hsTNT measured 12 h following aortic cross‐clamp removal Duration of noradrenaline use amongst ICU survivors Worst postoperative renal injury determined by RIFLE criteria. Second end points: Duration of mechanical ventilation amongst ICU survivors Haemodynamic parameters of cardiac index Mixed venous oxygen saturation Mean arterial pressure recorded at 3 h, 6 h, and 12 h from the time of ICU admission |
|
| Notes |
Funding for trial: this study was funded by unrestricted grants from the New Zealand Lotteries Commission and the National Heart Foundation of New Zealand Notable conflicts of interest of authors: none |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised in a 1:1 fashion to either RIPC or control. The random allocation sequence was generated by a third party using an online randomisation sequence generator using block randomisation with a block size of eight |
| Allocation concealment (selection bias) | Low risk | The allocation to RIPC or control was concealed in sequentially‐numbered opaque envelopes until the intervention was applied by an anaesthetic technician without loss of masking |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Participants, all theatre staff (with the exception of a technician), ICU staff and those assessing outcomes were masked as to treatment allocation. Concealment of allocation was maintained until data collection was completed for all participants |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants, all theatre staff (with the exception of a technician), ICU staff and those assessing outcomes were masked as to treatment allocation. Concealment of allocation was maintained until data collection was completed for all participants |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to form judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to form judgement |
| Other bias | Unclear risk | Insufficient information to form judgement |
ACC: American College of Cardiology; ACE inhibitor: angiotensin‐converting‐enzyme inhibitor; AHA: American Heart Association; AKI: acute kidney injury; AUC: area under the curve, BMI: Body mass index; BNP: Brain natriuretic peptide; CABG: coronary artery bypass graft; Ca‐ blocker: calcium channel blocker; CHD: coronary heart disease; CK: Creatine kinase; CK‐MB: cardiac marker, bound combination of two variants (isoenzymes CKM and CKB) of the enzyme phosphocreatine kinase, CO: cardiac output, CPB: cardiopulmonary bypass, CPK: Creatinin phosphokinase, Creatinin phosphokinase muscle brain (CPK‐MB); ECG: echocardiogram; EF: ejection fraction; EuroSCORE: European System for Cardiac Operative Risk Evaluation Score; h: hour; HLA‐DR Human leukocyte antigen complex; HMGB: high mobility group box; hsTnT: high sensitive troponin T; GFR: glomerular filtration rate; IABP: intra‐aortic balloon pump; ICU: intensive care unit; IGF: Insulin‐like growth factor; INR: international normalised ratio; LDH: Lactate dehydrogenase; LVEF: left ventricular ejection fraction; MI: myocardial infarction; MIF: migration inhibitory factor; min: minute, MMSE: Mini‐Mental State Examination; NYHA: New York Heart Association (NYHA) Functional Classification for Heart Failure; RIPC: remote ischaemic preconditioning; SOFA: Sepsis‐related organ failure assessment score; TNF: tumour necrosis factor; TnT: troponin T.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alreja 2012 | Systematic review, no additional reference to any other matching study |
| Brevoord 2011 | Study protocol, authors were contacted but could not provide any further details as the study is still awaiting publication. The study randomised people scheduled for elective CABG to one of the following arms:
This approach does not correspond to the inclusion criteria of this review, we therefore classified this study as an excluded study |
| Cain 1998 | Laboratory tissue study |
| Contractor 2009a | Laboratory tissue study (abstract) |
| Contractor 2009b | Laboratory tissue study (same abstract as Contractor 2009a) |
| D'Ascenzo 2012 | Systematic review, no additional reference to any other matching study |
| Deng 2015 | Systematic review, no additional reference to any other matching study |
| Healy 2014 | Systematic review, no additional reference to any other matching study |
| Hong 2014 | Intervention performed included pre‐ and post‐conditioning on people undergoing cardiac surgery |
| King 2016 | Conditioning techniques and ischaemic reperfusion injury in relation to on‐pump cardiac surgery |
| Marczak 2012 | Systematic review, no additional reference to any other matching study |
| Pei 2014 | Systematic review, no additional reference to any other matching study |
| Pilcher 2012 | Systematic review, no additional reference to any other matching study |
| Sabbagh 2013 | Systematic review, no additional reference to any other matching study |
| Sirohi 2012 | Laboratory tissue study (abstract) |
| Sivaraman 2010 | Laboratory tissue study |
| Wagner 2010 | Intervention RIPC was performed on the day before surgery |
| Walsh 2016 | Study randomised patients scheduled CABG with or without valve surgery and valve surgery alone. Authors were contacted if they could provide data on CABG with or without valve surgery only but did not respond. |
| Yang 2014 | Systematic review, no additional reference to any other matching study |
| Zarbock 2015 | Study randomised patients scheduled CABG with or without valve surgery and valve surgery alone. Authors were contacted if they could provide data on CABG with or without valve surgery only but did not respond. |
| Zhao 2014 | Systematic review, no additional reference to any other matching study |
| Zimmerman 2011 | Study randomised patients scheduled CABG with or without valve surgery and valve surgery alone. Authors were contacted if they could provide data on CABG with or without valve surgery only. Authors responded kindly, but did not assess the outcomes of this review in their study. |
CABG: coronary artery bypass grafting; RIPC: remote ischaemic preconditioning
Characteristics of studies awaiting assessment [ordered by study ID]
Gasparovic 2014a.
| Methods | RCT |
| Participants | Patients scheduled for elective coronary artery bypass grafting with the use of cardiopulmonary bypass |
| Interventions | Three cycles of RIPC (200 mmHg for 5 min) followed by 5‐min reperfusion period |
| Outcomes | New ischaemic lesions on brain MRI Postprocedural impairment in brain connectivity on resting‐state functional MRI (rs‐fMRI) Significant new declines in neurocognitive performance Individual components of the primary endpoint measures, expressed as continuous variables, troponin T release on postoperative day 1 and the incidence of major adverse cardiovascular events at 3 months postoperatively. Major adverse cardiovascular events, including accumulating cardiovascular mortality, stroke, nonfatal MI, and rehospitalisation for ischaemia, will form a composite endpoint measure |
| Notes | Study is awaiting classification as it has not been published yet, but completed recruiting. |
MI: myocardial infarction; MRI: magnetic resonance imaging; RIPC: remote ischaemic preconditioning; RCT: randomised controlled trial; rs‐fMRI: resting state functional MRI
Characteristics of ongoing studies [ordered by study ID]
Akhyari 2012.
| Trial name or title | Effects of Remote Ischemic PreConditioning in Off‐pump versus On‐pump coronary artery bypass grafting (RIPCON) |
| Methods | RCT (4 groups) Double‐blind (subject, investigator, outcomes assessor) Follow‐up: 72 h, 30 d, 3 months, 1 year |
| Participants | n = 100 Inclusion criteria
Exclusion Criteria
|
| Interventions | RIPC: upper arm ischaemia by inflation cuff to 200 mmHg for 3 x 5 min, interspersed with 5 min of no inflation and thus reperfusion after induction of anaesthesia but before surgery
|
| Outcomes | Primary outcome
Secondary outcomes
|
| Starting date | May 2012 |
| Contact information | Payam Akhyari, MD; +492118118331, payam.akhyari@med.uni‐duesseldorf.de |
| Notes | The recruitment status of this study is unknown because the information has not been verified recently. Results not yet published |
Choi 2009.
| Trial name or title | Effect of remote ischemic preconditioning on cognitive function after off‐pump coronary artery bypass graft |
| Methods | RCT (2 groups) Double‐blind (subject, investigator, outcomes assessor) Follow‐up: 7 d, 6 months |
| Participants | n = 270 Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes | Primary outcome
|
| Starting date | October 2009 |
| Contact information | In‐Cheol Choi, Professor; +82‐2‐3010‐3862; icchoi@amc.seoul.kr |
| Notes | The recruitment status of this study is unknown because the information has not been verified recently. Results not yet published |
Dong 2009.
| Trial name or title | The neuroprotection of remote ischemic preconditioning (RIPC) on cardiac surgery in multicenter |
| Methods | RCT (2 groups) Double‐blind (subject, outcomes assessor) Follow‐up: 6h, 24h, 48h, and 72h, 6 months |
| Participants | n = 150 Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes | Primary outcome
Secondary outcomes
|
| Starting date | June 2009 |
| Contact information | Hailong Dong, MD,PhD; 86‐2984775337; hldong6@hotmail.com |
| Notes | The recruitment status of this study is unknown because the information has not been verified recently. Results not yet published |
Gasparovic 2014b.
| Trial name or title | Impact of remote ischemic preconditioning preceding coronary artery bypass grafting on inducing neuroprotection |
| Methods | RCT (2 groups) Double‐blind (subject, investigator, outcomes assessor) Follow‐up: 1d, 7d, 3 months |
| Participants | n = 70 Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes | Primary outcomes:
Secondary outcomes:
|
| Starting date | March 2014 |
| Contact information | Hrvoje Gašparović, MD, PhD; +38512367517; hgasparovic@gmail.com |
| Notes | The study is currently recruiting participants. Results not yet published |
Kamler 2013.
| Trial name or title | Transfer of cardioprotection during RIPC |
| Methods | RCT (2 groups) Double‐blind (subject, investigator, outcomes assessor) Primary purpose: basic science Follow‐up: post‐op, 72 h, 30 d, 1year |
| Participants | n = 332 Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes | Primary outcome
Secondary outcomes:
|
| Starting date | September 2013 |
| Contact information | Markus Kamler, MD; +49‐201‐2802211; markus.kamler@uk‐essen.de |
| Notes | The study is currently recruiting participants. Results not yet published |
Lofti 2010.
| Trial name or title | Effect of remote ischemic preconditioning on incidence of atrial fibrillation in patients undergoing coronary artery bypass graft surgery |
| Methods | RCT (2 groups) Single‐blind (subject) Follow‐up: 7 d |
| Participants | n = 410 Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes | Primary outcome
Secondary outcome:
|
| Starting date | December 2010 |
| Contact information | Amir Lotfi, MD, 413 7934 4490, amir.lotfi@bhs.org |
| Notes | The recruitment status of this study is unknown because the information has not been verified recently. Results not yet published |
Petrucci 2013.
| Trial name or title | Evaluation of remote preconditioning on heart resistance to ischemia and reperfusion injury |
| Methods | RCT (2 groups) Double‐blind (subject, caregiver) Follow‐up: 2 d, 10 d, 30 d, 180 d |
| Participants | n = 120 Inclusion criteria
Exclusion criteria
|
| Interventions |
|
| Outcomes | Primary outcome
Secondary outcome:
|
| Starting date | August 2013 |
| Contact information | Orlando Petrucci, MD, PhD; +55 19 97253335; petrucci@cardiol.br |
| Notes | The recruitment status of this study is unknown because the information has not been verified recently. Results not yet published |
Preckel 2010.
| Trial name or title | A clinical study on the effect of remote ischemic conditioning on atrial fibrillation and outcome after coronary artery bypass grafting |
| Methods | RCT (4 groups) Double‐blind (subject, investigator, outcomes assessor) Follow‐up: 72 h, 3 months, 6 months, 1 year |
| Participants | n = 191 Inclusion criteria
Exclusion criteria
|
| Interventions | RIPC: fore arm ischaemia by inflation cuff to 200 mmHg for 3 x 5 min, interspersed with 5 min of no‐inflation and thus reperfusion
|
| Outcomes | Primary outcome
Secondary outcomes:
|
| Starting date | April 2010 |
| Contact information | B Preckel, Academisch Medisch Centrum ‐ Universiteit van Amsterdam |
| Notes | Results not yet published |
Yellon 2010.
| Trial name or title | Effect of remote ischemic preconditioning in patient undergoing cardiac bypass surgery |
| Methods | RCT (2 groups) Single‐blind (subject) Follow‐up: 3 d |
| Participants | n = 200 Inclusion criteria
Exclusion Criteria:
|
| Interventions |
|
| Outcomes | Primary outcome:
|
| Starting date | December 2010 |
| Contact information | Contact: Derek M Yellon, PhD DSc; +44 203 447 9888; d.yellon@ucl.ac.uk |
| Notes | The study is currently recruiting participants. Results not yet published |
AUC: area under the curve; CABG: coronary artery bypass grafting; CPB: cardiopulmonary bypass; d: days; EF: ejection fraction; h: hour(s); ICU: intensive care unit; LVEF: left ventricular ejection fraction; MACCE: major adverse cardiac and cerebrovascular events; MI: myocardial infarction; MMSE: mini‐mental state examination; MRI: magnetic resonance imaging; OPCAB: off‐pump coronary artery bypass; RCT: randomised controlled trial; RIPC: remote ischaemic preconditioning; rs‐fMRI: resting state functional MRI
Differences between protocol and review
We phrased the title of the review more precisely and added "with or without valve surgery".
As we were able to assess a composite endpoint as primary endpoint, we changed the order of the primary outcomes.
When the protocol was composed, we did not know what time points trialists would have reported; therefore we did not specify any time points for troponins in the protocol. We included troponin values T and I measured at 48 hours, 72 hours, and as area under the curve (AUC) 72 hours after surgery as primary outcomes, and troponin values T and I measured at 6 hours, 12 hours, and 24 hours after surgery as secondary outcomes. By doing so we highlighted outcomes that are clinically most meaningful as primary outcomes.
We performed different subgroup analyses than initially planned in the protocol.
We assessed the secondary outcome "Length of stay on the intensive care unit" in days and not in hours.
We did not include "Assessment of the quality of the evidence, GRADE assessment and 'Summary of Findings' tables" in the protocol but in the review.
We analysed the highly skewed troponin values on the log scale.
During the course of this systematic review it became apparent that RCTs that assessed RIPC in the setting of cardiac surgery did not include “with a diagnosis of acute coronary heart disease” as an explicit inclusion criteria but the need for CABG with or without valve surgery. Accordingly, for the studies that assessed the effects of RIPC on cardiac surgical patients, we were not able to confirm whether or not patients were indeed diagnosed with 'acute coronary heart disease'. As we regarded the need for cardiac surgery more specific to investigate RIPC performed during cardiac surgery than the wording we chose within the study protocol, we amended the definition of types of participants accordingly.
Contributions of authors
CB selected trials, extracted data, assessed the methodological quality of trials, was responsible for handling data in RevMan, checked entered data in RevMan, designed the meta‐analyses, interpreted the results, drafted the review, revised the manuscript and approved the final version. CB is the guarantor of this review. CS selected trials, assessed the methodological quality of trials, designed the meta‐analyses, interpreted the results, contributed important content to the drafting of this review, revised the manuscript and approved the final version. OL provided support and guidance throughout the review, contributed important content to the drafting of this review, and approved the final version. JN extracted data, assessed the methodological quality of trials, checked entered data in RevMan, contributed important content to the drafting of this review, and approved the final version. DH designed the meta‐analyses, contributed important content to the drafting of this review, and approved the final version. PM provided support and guidance throughout the review, contributed important content to the drafting of this review, and approved the final version. AG selected trials, assessed the methodological quality of trials, was responsible for handling data in RevMan, checked entered data in RevMan, designed the meta‐analysis, contributed important content to the drafting of this review, revised the manuscript and approved the final version.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Cochrane Heart, USA.
The Cochrane Heart US Satellite is supported by intramural support from the Northwestern University Feinberg School of Medicine and the Northwestern University Clinical and Translational Science (NUCATS) Institute (UL1TR000150), USA
-
National Institute for Health Research, UK.
This project was supported by the National Institute for Health Research via Cochrane Infrastructure funding to Cochrane Heart. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health, UK.
Declarations of interest
CS and PM are investigators of the RIPHeart Study, which investigates the effects of remote ischaemic preconditioning in cardiac surgery patients. The RIPHeart trial contributed published and unpublished data to this review. We independently judged eligibility and risk of bias (performed by CB, AG and JN). PM received research grants from the German Research Foundation, German Society of Anesthesiology and Intensive Care Medicine and International Anesthesia Research Society for his research focusing on remote ischaemic preconditioning. He also received grants from Fresenius Kabi, B. Braun Melsungen, Vifor Pharma, CSL Behring, Pfizer. CB no conflict of interest. AG no conflict of interest. JN no conflict of interest. DH no conflict of interest. OJL no conflict of interest.
New
References
References to studies included in this review
Ahmad 2014 {published data only}
- Ahmad AM, Ali GS, Tariq W. Remote ischemic preconditioning is a safe adjuvant technique to myocardial protection but adds no clinical benefit afer on‐pump coronary artery bypass grafting. Heart Surgery Forum 2014;17(4):220‐3. [DOI] [PubMed] [Google Scholar]
Ali 2010 {published data only}
- Ali N, Rizwi F, Iqbal A, Rashid A. Induced remote ischemic pre‐conditioning on ischemia‐reperfusion injury in patients undergoing coronary artery bypass. Journal of the College of Physicians and Surgeons Pakistan 2010;20(7):427‐31. [PubMed] [Google Scholar]
Candilio 2015 {published and unpublished data}
- Candilio L, Malik A, Ariti C, Barnard M, Salvo C, Lawrence D, et al. Effect of remote ischaemic preconditioning on clinical outcomes in patients undergoing cardiac bypass surgery: a randomised controlled clinical trial. Heart 2015;101(3):185‐92. [DOI] [PubMed] [Google Scholar]
- Candilio L, Malik A, Ariti C, Barnard M, Wright S, Smith A, et al. The effects of multi‐limb remote ischaemic preconditioning in patients undergoing cardiac bypass surgery. Heart 2013;99(2):74. [DOI] [PubMed] [Google Scholar]
Gallagher 2015 {published data only}
- Gallagher SM, Jones DA, Kapur A, Wragg A, Harwood SM, Mathur R, et al. Remote ischemic preconditioning has a neutral effect on the incidence of kidney injury after coronary artery bypass graft surgery. Kidney International 2015;87(2):473‐81. [DOI] [PubMed] [Google Scholar]
Gegouskov 2009 {published data only}
- Gedik N, Thielmann M, Kottenberg E, Peters J, Jakob H, Heusch G, et al. No evidence for activated autophagy in left ventricular myocardium at early reperfusion with protection by remote ischemic preconditioning in patients undergoing coronary artery bypass grafting. PLOS One 2014;9(5):e96567. [DOI] [PMC free article] [PubMed] [Google Scholar]
Günaydin 2000 {published data only}
- Günaydin B, Cakici I, Soncul H, Kalaycioglu S, Cevik C, Sancak B, et al. Does remote organ ischaemia trigger cardiac preconditioning during coronary artery surgery?. Pharmacological Research 2000;41(4):493‐6. [DOI] [PubMed] [Google Scholar]
Hausenloy 2007 {published data only}
- Hausenloy DJ, Mwamure PK, Venugopal V, Harris J, Barnard M, Grundy E, et al. Effect of remote ischaemic preconditioning on myocardial injury in patients undergoing coronary artery bypass graft surgery: a randomised controlled trial. Lancet 2007;370(9587):575‐9. [DOI] [PubMed] [Google Scholar]
Hausenloy 2015 {published and unpublished data}
- Hausenloy DJ, Candilio L, Evans R, Ariti C, Jenkins DP, Kolvekar S, et al. Remote ischemic preconditioning and outcomes of cardiac surgery. New England Journal of Medicine 2015;373(15):1408‐17. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Candilio L, Laing C, Kunst G, Pepper J, Kolvekar S, et al. Effect of remote ischemic preconditioning on clinical outcomes in patients undergoing coronary artery bypass graft surgery (ERICCA): rationale and study design of a multi‐centre randomized double‐blinded controlled clinical trial. Clinical Research in Cardiology 2012;101(5):339‐48. [DOI] [PubMed] [Google Scholar]
Hong 2010 {published data only}
- Hong DM, Mint JJ, Kim JH, Sohn IS, Lim TW, Lim YJ, et al. The effect of remote ischaemic preconditioning on myocardial injury in patients undergoing off‐pump coronary artery bypass graft surgery. Anaesthesia and Intensive Care 2010;38(5):924‐9. [DOI] [PubMed] [Google Scholar]
Joung 2013 {published data only}
- Joung KW, Rhim JH, Chin JH, Kim WJ, Choi DK, Lee EH, et al. Effect of remote ischemic preconditioning on cognitive function after off‐pump coronary artery bypass graft: a pilot study. Korean Journal of Anesthesiology 2013;65(5):418‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
Karuppasamy 2011 {published data only}
- Karuppasamy P, Chaubey S, Desai J. Simultaneous anaesthetic and remote preconditioning during cardiac surgery ‐ a clinical strategy?. Cardiovascular Research 2010;1:S33. [Google Scholar]
- Karuppasamy P, Chaubey S, Dew T. Does remote ischaemia modulate cytokines and growth factors? A clinical study in patients undergoing coronary artery bypass graft surgery. Cardiovascular Research 2010;87:78. [Google Scholar]
- Karuppasamy P, Chaubey S, Dew T, Musto R, Sherwood R, Desai J, et al. Remote intermittent ischemia before coronary artery bypass graft surgery: a strategy to reduce injury and inflammation?. Basic Research in Cardiology 2011;106(4):511‐9. [DOI] [PubMed] [Google Scholar]
Kottenberg 2014 {published data only}
- Kottenberg E, Musiolik J, Thielmann M, Jakob H, Peters J, Heusch G. Interference of propofol with signal transducer and activator of transcription 5 activation and cardioprotection by remote ischemic preconditioning during coronary artery bypass grafting. Journal of Thoracic and Cardiovascular Surgery 2014;147(1):376‐82. [DOI] [PubMed] [Google Scholar]
Krawczyk 2010 {published data only}
- Krawczyk K, Rybicki. Remote ischaemic preconditioning in patients undergoing off‐pump coronary bypass grafting: prospective study and preliminary results. Cardiac Track Program. 2019:25.
Krawczyk 2011 {published data only}
- Krawczyk K, Burnos Z, Gierak E, Golowicz J, Gryszko L, Suwalski G, et al. Remote ischemic preconditioning is cardioprotective in patients undergoing off‐pump coronary bypass grafting a prospective randomised controlled study. Cardiac Track Program 2011;6(3):146. [Google Scholar]
Krawczyk 2012 {published data only}
- Krawczyk K, Grzegorz S, Gryszko L, Gołowicz J, Burnos Z, Szałański P, et al. Routine remote ischemic preconditioning in patients undergoing off‐pump coronary artery bypass grafting improves results in controlled randomized prospective study. Innovations: Technology & Techniques in Cardiothoracic & Vascular Surgery 2012;7(2):137. [Google Scholar]
Krogstad 2015 {published data only}
- Krogstad LE, Slagsvold KH, Wahba A. Remote ischemic preconditioning and incidence of postoperative atrial fibrillation. Scandinavian Cardiovascular Journal 2015;49(3):117‐22. [DOI] [PubMed] [Google Scholar]
Lomivorotov 2012 {published data only}
- Lomivorotov VV, Shmyrev VA, Nepomnyaschih VA, Ponomarev DN, Knyazkova LG, Lomivorotov VN, et al. Remote ischaemic preconditioning does not protect the heart in patients undergoing coronary artery bypass grafting. Interactive Cardiovascular and Thoracic Surgery 2012;15(1):18‐22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomivorotov VV, Shmyrev VA, Ponomarev DN, Efremov SM, Shilova AN, Postnov VG. Influence of remote ischemic preconditioning on brain injury markers dynamics during cardiopulmonary bypass. Anesteziologiia i Reanimatologiia 2015;60(1):33‐38. [PubMed] [Google Scholar]
Lucchinetti 2012 {published data only}
- Lucchinetti E, Bestmann L, Feng J, Freidank H, Clanachan AS, Finegan BA, et al. Remote ischemic preconditioning applied during isoflurane inhalation provides no benefit to the myocardium of patients undergoing on‐pump coronary artery bypass graft surgery: lack of synergy or evidence of antagonism in cardioprotection?. Anesthesiology 2012;116(2):296‐310. [DOI] [PubMed] [Google Scholar]
Meybohm 2013 {published data only}
- Meybohm P, Renner J, Broch O, Caliebe D, Albrecht M, Cremer J, et al. Postoperative neurocognitive dysfunction in patients undergoing cardiac surgery after remote ischemic preconditioning: a double‐blind randomized controlled pilot study. PLOS One 2013;8(5):e64743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zitta K, Meybohm P, Bein B, Gruenewald M, Lauer F, Steinfath M, et al. Activities of cardiac tissue matrix metalloproteinases 2 and 9 are reduced by remote ischemic preconditioning in cardiosurgical patients with cardiopulmonary bypass. Journal of Translational Medicine 2014;12:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
Meybohm 2015 {published and unpublished data}
- Meybohm P, Bein B, Brosteanu O, Cremer J, Gruenewald M, Stoppe C, et al. A multicenter trial of remote ischemic preconditioning for heart surgery. New England Journal of Medicine 2015;373(15):1397‐401. [DOI] [PubMed] [Google Scholar]
- Meybohm P, Zacharowski K, Cremer J, Roesner J, Kletzin F, Schaelte G, et al. Remote ischaemic preconditioning for heart surgery. The study design for a multi‐center randomized double‐blinded controlled clinical trial‐‐the RIPHeart‐Study. European Heart Journal 2012;33(12):1423‐6. [PubMed] [Google Scholar]
Rahman 2010 {published data only}
- Rahman IA, Mascaro J, Green D. Does remote ischaemic preconditioning during on‐pump coronary artery bypass grafting enhance lung preservation?. Interactive Cardiovascular and Thoracic Surgery 2010;10(Suppl 1):C16‐2. [Google Scholar]
- Rahman IA, Mascaro JG, Steeds RP, Frenneaux MP, Nightingale P, Gosling P, et al. Remote ischemic preconditioning in human coronary artery bypass surgery: from promise to disappointment?. Circulation 2010;122(11):53‐9. [DOI] [PubMed] [Google Scholar]
Saxena 2013 {published data only}
- Saxena P, Aggarwal S, Misso NL, Passage J, Newman MA, Thompson PJ, et al. Remote ischaemic preconditioning down‐regulates kinin receptor expression in neutrophils of patients undergoing heart surgery. Interactive Cardiovascular and Thoracic Surgery 2013;17(4):653‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shmyrev 2011 {published data only}
- Lomivorotov V, Shmyrev V, Ponomarev D. Effects of remote ischaemic preconditioning on oxidative stress in cardiac surgery: a singleblind randomised study. EACTA 2013: 28th Annual Meeting of the European Association of Cardiothoracic Anaesthesiologists 2013;17:155‐6. [Google Scholar]
- Shmyrev V, Lomivorotov V, Ponomarev D, Knyazkova L. Remote ischaemic preconditioning is not beneficial in patients operated during cardiopulmonary bypass. Journal of Cardiothoracic and Vascular Anesthesia 2011;25(3):26. [Google Scholar]
- Shmyrev V, Ponomarev D, Lomivorotov V. Effects of remote ischaemic preconditioning on cognitive function and neurologic injury in cardiac surgery. 29th Annual Meeting of the European Association of Cardiothoracic Anaesthesiologists (EACTA) and 14th International Congress on Cardiovascular Anesthesia (ICCVA) 2014;20:59. [Google Scholar]
Slagsvold 2014 {published data only}
- Slagsvold KH, Rognmo O, Høydal M, Wisløff U, Wahba A. Remote ischemic preconditioning preserves mitochondrial function and influences myocardial microRNA expression in atrial myocardium during coronary bypass surgery. Circulation Research 2014;114(5):851‐9. [DOI] [PubMed] [Google Scholar]
Sosorburam 2014 {published data only}
- Sosorburam T, Xing Ping W. Ischemic preconditioning before cardiopulmonary bypass reduces ischemic‐reperfusion injury in patients undergoing GABG surgery. Cardiovascular Research 2014;103(1):121. [Google Scholar]
Thielmann 2013 {published and unpublished data}
- Gedik N, Thielmann M, Kottenberg E, Peters J, Jakob H, Heusch G, et al. No evidence for activated autophagy in left ventricular myocardium at early reperfusion with protection by remote ischemic preconditioning in patients undergoing coronary artery bypass grafting. PLOS One 2014;103:77‐78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gedik N, Thielmann M, Kottenberg E, Peters J, Jakob H, Heusch G, et al. No evidence for activated autophagy in left ventricular myocardium at early reperfusion with protection by remote ischemic preconditioning in patients undergoing coronary artery bypass grafting. PLOS One 2014;9(5):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heusch G, Musiolik J, Kottenberg E, Peters J, Jakob H, Thielmann M. STAT5 activation and cardioprotection by remote ischemic preconditioning in humans: short communication. Circulation Research 2012;110(1):111‐5. [DOI] [PubMed] [Google Scholar]
- Kleinbongard P, Thielmann M, Jakob H, Peters J, Heusch G, Kottenberg E. Nitroglycerin does not interfere with protection by remote ischemic preconditioning in patients with surgical coronary revascularization under isoflurane anesthesia. Cardiovascular Drugs and Therapy 2013;27(4):359‐61. [DOI] [PubMed] [Google Scholar]
- Kottenberg E, Thielmann M, Bergmann L, Heine T, Jakob H, Heusch G, et al. Protection by remote ischemic preconditioning during coronary artery bypass graft surgery with isoflurane but not propofol ‐ a clinical trial. Acta Anaesthesiologica Scandinavica 2012;56(1):30‐8. [DOI] [PubMed] [Google Scholar]
- Thielmann M, Kottenberg E, Boengler K, Raffelsieper C, Neuhaeuser M, Peters J, et al. Remote ischemic preconditioning reduces myocardial injury after coronary artery bypass surgery with crystalloid cardioplegic arrest. Basic Research in Cardiology 2010;105(5):657‐64. [DOI] [PubMed] [Google Scholar]
- Thielmann M, Kottenberg E, Kleinbongard P, Wendt D, Gedik N, Pasa S, et al. Cardioprotective and prognostic effects of remote ischaemic preconditioning in patients undergoing coronary artery bypass surgery: a single‐centre randomised, double‐blind, controlled trial. Lancet 2013;382(9892):597‐604. [DOI] [PubMed] [Google Scholar]
- Thielmann M, Kottenberg E, Kleinbongard P, Wendt D, Gedik N, Pasa S, et al. Cardioprotective and prognostic effects of remote ischaemic preconditioning in patients undergoing coronary artery bypass surgery: a single‐centre randomised, double‐blind, controlled trial. Lancet 2013;382(9896):940. [DOI] [PubMed] [Google Scholar]
- Thielmann M, Kottenberg E, Musiolik J, Wendt D, Gedik N, Schlúter M, et al. Prognostic benefit from remote ischemic preconditioning in 300 patients undergoing coronary artery bypass surgery: a randomized controlled trial. Circulation 2012;126:A14034. [Google Scholar]
Venugopal 2009 {published data only}
- Venugopal V, Hausenloy DJ, Ludman A, Salvo C, Kolvekar S, Yap J, et al. Remote ischaemic preconditioning reduces myocardial injury in patients undergoing cardiac surgery with cold‐blood cardioplegia: a randomised controlled trial. Heart 2009;95(19):1567‐71. [DOI] [PubMed] [Google Scholar]
- Venugopal V, Hausenloy DJ, Ludman A, Liang C. Remote ischaemic preconditioning reduces renal injury in patients undergoing coronary artery bypass graft surgery. Cardiovascular Surgery 2009;30:597. [Google Scholar]
- Venugopal V, Laing CM, Ludman A, Yellon DM, Hausenloy D. Effect of remote ischemic preconditioning on acute kidney injury in nondiabetic patients undergoing coronary artery bypass graft surgery: a secondary analysis of 2 small randomized trials. American Journal of Kidney Diseases: The Official Journal of the National Kidney Foundation 2010;56(6):1043‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yildirim 2016 {published data only}
- Yildirim F, Iskesen I, Kurdal AT, Ozturk T, Taneli F, Gozukara C, et al. Is "Attenuation of Oxidative Stress" helpful to understand the mechanism of remote ischemic preconditioning in cardiac surgery?. Journal of Cardiothoracic and Vascular Anesthesia 2016;30(1):134‐40. [DOI] [PubMed] [Google Scholar]
Young 2012 {published and unpublished data}
- Williams JM, Young P, Pilcher J, Weatherall M, Miller JH, Beasley R, et al. Remote ischaemic preconditioning does not alter perioperative cytokine production in high‐risk cardiac surgery. Heart Asia 2012;4:97‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young PJ, Dalley P, Garden A, Horrocks C, Flamme A, Mahon B, et al. A pilot study investigating the effects of remote ischemic preconditioning in high‐risk cardiac surgery using a randomised controlled double‐blind protocol. Basic Research in Cardiology 2012;107(3):256. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Alreja 2012 {published data only}
- Alreja G, Bugano D, Lotfi A. Effect of remote ischemic preconditioning on myocardial and renal injury: meta‐analysis of randomized controlled trials. Journal of Invasive Cardiology 2012;24(2):42‐8. [PubMed] [Google Scholar]
Brevoord 2011 {published data only}
- Brevoord D, Hollmann MW, Hert SG, Dongen EH, Heijnen BG, Bruin A, et al. Effect of remote ischemic conditioning on atrial fibrillation and outcome after coronary artery bypass grafting (RICO‐trial). BMC Anesthesiology 2011;11(11):1‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cain 1998 {published data only}
- Cain BS, Meldrum DR, Meng X, Pulido EJ, Banerjee A, Harken AH. Therapeutic antidysrhythmic and functional protection in human atria. Journal of Surgical Research 1998;76(2):143‐8. [DOI] [PubMed] [Google Scholar]
Contractor 2009a {published data only}
- Contractor H, Ashrafian H, Rahman I, Juul‐Dam K, Redington A, Mascaro CJ. Beta‐catenin pathway expression in human myocardium is increased by a remote ischaemic preconditioning stimulus. Research Gate 2009;54(5):1173–74. [Google Scholar]
Contractor 2009b {published data only}
- Contractor H, Ashrafian H, Rahman I, Redington A, Frenneaux M, Mascaro CJ, et al. Increased beta‐catenin pathway expression by a remote ischaemic preconditioning stimulus: first evidence in humans. Journal of the American College of Cardiology 2009;53(10):A309. [Google Scholar]
D'Ascenzo 2012 {published data only}
- D'Ascenzo F, Cavallero E, Moretti C, Omedè P, Sciuto F, Rahman IA, et al. Remote ischaemic preconditioning in coronary artery bypass surgery: a meta‐analysis. Heart 2012;98(17):1267‐71. [DOI] [PubMed] [Google Scholar]
Deng 2015 {published data only}
- Deng QW, Xia ZQ, Qiu YX, Wu Y, Liu JX, Li C, et al. Clinical benefits of aortic cross‐clamping versus limb remote ischemic preconditioning in coronary artery bypass grafting with cardiopulmonary bypass: a meta‐analysis of randomized controlled trials. Journal of Surgical Research 2015;193(1):52‐68. [DOI] [PubMed] [Google Scholar]
Healy 2014 {published data only}
- Healy DA, Khan WA, Wong CS, Moloney MC, Grace PA, Coffey JC, et al. Remote preconditioning and major clinical complications following adult cardiovascular surgery: systematic review and meta‐analysis. International Journal of Cardiology 2014;176(120‐31):20‐31. [DOI] [PubMed] [Google Scholar]
Hong 2014 {published data only}
- Hong DM, Lee EH, Kim HJ, Min JJ, Chin JH, Choi DK, et al. Does remote ischaemic preconditioning with postconditioning improve clinical outcomes of patients undergoing cardiac surgery? Remote Ischaemic Preconditioning with Postconditioning Outcome Trial. European Heart Journal 2014;35(3):176‐83. [DOI] [PubMed] [Google Scholar]
King 2016 {published data only}
- King N, Dieberg G, Smart NA. Remote ischaemic pre‐conditioning does not affect clinical outcomes following coronary artery bypass grafting. A systematic review and meta‐analysis. Clinical Trials and Regulatory Science in Cardiology 2016;17:1‐8. [Google Scholar]
Marczak 2012 {published data only}
- Marczak ED, Marzec J, Zeldin DC, Kleeberger SR, Brown NJ, Pretorius M, et al. Is remote ischaemic preconditioning of benefit to patients undergoing cardiac surgery?. Pharmacogenetics and Genomics 2012;22(8):620‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pei 2014 {published data only}
- Pei H, Wu Y, Wei Y, Yang Y, Teng S, Zhang H. Remote ischemic preconditioning reduces perioperative cardiac and renal events in patients undergoing elective coronary intervention: a meta‐analysis of 11 randomized trials. PLOS One 2014;9(12):e115500. [DOI] [PMC free article] [PubMed] [Google Scholar]
Pilcher 2012 {published data only}
- Pilcher JM, Young P, Weatherall M, Rahman I, Bonser RS, Beasley RW. A systematic review and meta‐analysis of the cardioprotective effects of remote ischaemic preconditioning in open cardiac surgery. Journal of the Royal Society of Medicine 2012;105(10):436‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sabbagh 2013 {published data only}
- Sabbagh S, Henry Salzman MM, Kloner RA, Simkhovich BZ, Rezkalla SH. Remote ischemic preconditioning for coronary artery bypass graft operations. The Annals of Thoracic Surgery 2013;96(2):727‐36. [DOI] [PubMed] [Google Scholar]
Sirohi 2012 {published data only}
- Sirohi R, Candilio L, Babu G, Roberts N, Lawrence D, Sheik A, et al. Remote ischaemic preconditioning and human atrial trabeculae in the diabetic heart. Heart 2012;98(1):A61. [Google Scholar]
Sivaraman 2010 {published data only}
- Sivaraman V, Hausenloy DJ, Wynne AM, Yellon DM. Preconditioning the diabetic human myocardium. Journal of Cellular and Molecular Medicine 2010;14(6B):1740‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wagner 2010 {published data only}
- Wagner R, Piler P, Bedanova H, Adamek P, Grodecka L, Freiberger T. Myocardial injury is decreased by late remote ischaemic preconditioning and aggravated by tramadol in patients undergoing cardiac surgery: a randomised controlled trial. Interactive Cardiovascular and Thoracic Surgery 2010;11(6):758‐62. [DOI] [PubMed] [Google Scholar]
Walsh 2016 {published data only}
- Walsh M, Whitlock R, Garg AX, Légaré JF, Duncan AE, Zimmerman R, et al. Effects of remote ischemic preconditioning in high‐risk patients undergoing cardiac surgery (Remote IMPACT): a randomized controlled trial. Canadian Medical Association Journal 2016;188(5):329‐36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2014 {published data only}
- Yang L, Wang G, Du Y, Ji B, Zheng Z. Remote ischemic preconditioning reduces cardiac troponin I release in cardiac surgery: a meta‐analysis. Journal of Cardiothoracic and Vascular Anesthesia 2014;28(3):682‐9. [DOI] [PubMed] [Google Scholar]
Zarbock 2015 {published data only}
- Zarbock A, Schmidt C, Aken H, Wempe C, Martens S, Zahn PK, et al. Effect of remote ischemic preconditioning on kidney injury among high‐risk patients undergoing cardiac surgery a randomized clinical trial. JAMA 2015;213(21):2133‐41. [DOI] [PubMed] [Google Scholar]
Zhao 2014 {published data only}
- Zhang B, Zhou J, Li H, Zhou M, Chen A, Zhao Q. Remote ischemic preconditioning does not improve the clinical outcomes in patients undergoing coronary artery bypass grafting: a meta‐analysis of randomized controlled trials. International Journal of Cardiology 2014;172(1):e36‐8. [DOI] [PubMed] [Google Scholar]
Zimmerman 2011 {published data only}
- Zimmerman RF, Ezeanuna PU, Kane JC, Cleland CD, Kempananjappa TJ, Lucas FL, et al. Ischemic preconditioning at a remote site prevents acute kidney injury in patients following cardiac surgery. Kidney International 2011;80(8):861‐7. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Gasparovic 2014a {published data only}
- Gasparovic H, Kopjar T, Rados M, Anticevic A, Rados M, Malojcic B, et al. Impact of remote ischemic preconditioning preceding coronary artery bypass grafting on inducing neuroprotection (RIPCAGE): study protocol for a randomized controlled trial. Trials 2014;15(414):1‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Akhyari 2012 {unpublished data only}
- NCT01608984. Effects of Remote Ischemic PreConditioning in Off‐pump Versus On‐pump Coronary Artery Bypass Grafting(RIPCON) (RIPCON) [Effects of Remote Ischemic PreConditioning in Off‐pump Versus On‐pump Coronary]. https://clinicaltrials.gov/ct2/show/NCT01608984 (first received 29 May 2012).
Choi 2009 {unpublished data only}
- NCT00953368. Effect of Remote Ischemic Preconditioning on Cognitive Function After Off‐Pump Coronary Artery Bypass Graft. https://clinicaltrials.gov/ct2/show/NCT00953368 (first received 5 August 2009). [DOI] [PMC free article] [PubMed]
Dong 2009 {unpublished data only}
- NCT01231789. The Neuroprotection of Remote Ischemic Preconditioning (RIPC) on Cardiac Surgery in Multicenter. https://clinicaltrials.gov/ct2/show/NCT01231789 (first received 15 September 2010).
Gasparovic 2014b {unpublished data only}
- NCT02177981. Impact of Remote Ischemic Preconditioning Preceding Coronary Artery Bypass Grafting on Inducing nEuroprotection (RIPCAGE). https://clinicaltrials.gov/ct2/show/NCT02177981 (first received 7 April 2014).
Kamler 2013 {unpublished data only}
- NCT01956708. Transfer of Cardioprotection During RIPC. https://clinicaltrials.gov/ct2/show/NCT01956708 (first received 27 September 2013).
Lofti 2010 {unpublished data only}
- NCT01500369. Effect of Remote Ischemic Preconditioning on Incidence of Atrial Fibrillation in Patients Undergoing Coronary Artery Bypass Graft Surgery. https://clinicaltrials.gov/ct2/show/NCT01500369 (first received 22 December 2011).
Petrucci 2013 {unpublished data only}
- NCT01906918. Application of Remote Ischemic Preconditioning in Patients Undergoing Open Heart Surgeries [Evaluation of Remote Preconditioning on Heart Resistance to Ischemia and Reperfusion Injury]. https://clinicaltrials.gov/ct2/show/NCT01906918 (first received 21 July 2013).
Preckel 2010 {unpublished data only}
- NCT01107184. Remote Ischemic Conditioning and Atrial Fibrillation After Coronary Artery Bypass Grafting (CABG) (RICO) [A Clinical Study on the Effect of Remote Ischemic Conditioning on Atrial Fibrillation and Outcome After Coronary Artery Bypass Grafting]. https://clinicaltrials.gov/ct2/show/NCT01107184 (first received 19 April 2010).
Yellon 2010 {unpublished data only}
- NCT00397163. Effect of Remote Ischemic Preconditioning in Patient Undergoing Cardiac Bypass Surgery. https://clinicaltrials.gov/ct2/show/NCT00397163 (first received 6 November 2006).
Additional references
Ali 2007
- Ali ZA, Callaghan CJ, Lim E, Ali AA, Nouraei SA, Akthar AM, et al. Remote ischemic preconditioning reduces myocardial and renal injury after elective abdominal aortic aneurysm repair: a randomized controlled trial. Circulation 2007;116:I98–I105. [DOI] [PubMed] [Google Scholar]
Benstoem 2015b
- Benstoem C, Moza A, Autschbach R, Stoppe C, Goetzenich A. Evaluating outcomes used in cardiothoracic surgery interventional research: a systematic review of reviews to develop a core outcome set. PLOS One 2015;10(4):1‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bojar 2011
- Bojar RM. Manual of Perioperative Care in Adult Cardiac Surgery. Fifth. Chichester, UK: Wiley‐Blackwell, 2011. [Google Scholar]
Centers for Disease Control and Prevention 2015
- Centers for Disease Control and Prevention. Inpatient Surgery, Data for the U.S. (online). Available from: www.cdc.gov/nchs/fastats/inpatient‐surgery.htm (accessed 4 April 2015).
Cheung 2006
- Cheung MM, Kharbanda RK, Konstantinov IE, Shimizu M, Frndova H, Li J, et al. Randomized controlled trial of the effects of remote ischemic preconditioning on children under‐going cardiac surgery: first clinical application in humans. Journal of the American College of Cardiology 2006;47:2277–82. [DOI] [PubMed] [Google Scholar]
Corti 2014
- Corti P, Gladwin MT. Is nitrite the circulating endocrine effector of remote ischemic preconditioning?. Circulation Research 2014;114(10):1554‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Deeks 2009
- Deeks JJ, Higgins JPT, Altman DG (editors). Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Domanski 2011
- Domanski MJ, Mahaffey K, Hasselblad V, Brener SJ, Smith PK, Hillis G, et al. Association of myocardial enzyme elevation and survival following coronary artery bypass graft surgery. JAMA 2011;305:585‐91. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Estafanous 2001
- Estafanous FG, Barash PG, Reves JG. Cardiac Anesthesia: Principles and Practice. 1. Philadelphia, USA: Lippincott Williams & Wilkins, 2001. [Google Scholar]
Ghosh 2004
- Ghosh P, Schistek R, Unger F. Coronary revascularization in DACH: 1991‐2002. Journal of Thoracic and Cardiovascular Surgery 2004;52(6):356–64. [DOI] [PubMed] [Google Scholar]
Hamarneh 2015
- Hamarneh A, Sivaraman V, Bulluck H, Shanahan H, Kyle B, Ramlall M, et al. The effect of remote ischemic conditioning and glyceryl trinitrate on perioperative myocardial injury in cardiac bypass surgery patients: rationale and design of the ERIC‐GTN study. Clinical Cardiology 2015;38:641‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harbord 2006
- Harbord RM, Egger M, Sterne JA. A modified test for small‐study effects in meta‐analyses of controlled trials with binary endpoints. Statistics in Medicine 2006;25(20):3443–57. [DOI] [PubMed] [Google Scholar]
Hausenloy 2010
- Hausenloy DJ, Yellon DM. The therapeutic potential of ischemic conditioning: an update. Nature Reviews Cardiology 2010;8:619–29. [DOI] [PubMed] [Google Scholar]
Hausenloy 2011
- Hausenloy DJ, Lecour S, Yellon DM. Reperfusion injury salvage kinase and survivor activating factor enhancement prosurvival signaling pathways in ischemic postconditioning: two sides of the same coin. Antioxidants & Redox Signaling 2011;14(5):893‐907. [DOI] [PubMed] [Google Scholar]
Hausenloy 2012
- Hausenloy DJ, Boston‐Griffiths E, Yellon DM. Cardioprotectionduring cardiac surgery. Cardiovascular Research 2012;94:253‐65. [DOI] [PMC free article] [PubMed] [Google Scholar]
Heusch 2010
- Heusch G. Cardioprotection: chances and challenges of itstranslation to the clinic. Lancet 2012;381(9861):166–75. [DOI] [PubMed] [Google Scholar]
Heusch 2016
- Heusch G, Gersh BJ. ERICCA and RIPHeart: two nails in the coffin for cardioprotection by remote ischemic conditioning? Probably not!. European Heart Journal 2016;37(2):200‐2. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011a
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Higgins 2011b
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Illes 1998
- Illes RW, Swoyer KD. Prospective, randomized clinical study of ischemic preconditioning as an adjunct to intermittent cold blood cardioplegia. Annals of Thoracic Surgery 1998;65(3):748‐52. [DOI] [PubMed] [Google Scholar]
Kottenberg 2012
- Kottenberg E, Thielmann M, Bergmann L, Heine T, JakobH, Heusch G, et al. Protection by remote ischemic preconditioningduring coronary artery bypass graft surgerywith isoflurane but not propofol ‐ a clinical trial. Acta Anaesthesiologica Scandinavica 2012;56:30‐8. [DOI] [PubMed] [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Li 1999
- Li G, Chen S, Lu E, Li Y. Ischemic preconditioning improves preservation with cold blood cardioplegia in valve replacement patients. European Journal of Cardiothoracic Surgery 1999;15(5):653‐7. [DOI] [PubMed] [Google Scholar]
Li 2012
- Li W, Zhang Y, Liu Y, Yue F, Lu Y, Qiu H, et al. In vitro kinetic evaluation of the free radical scavenging ability of propofol. Anesthesiology 2012;116:1258–66. [DOI] [PubMed] [Google Scholar]
Li 2014
- Li J, Rohailla S, Gelber N, Rutka J, Sabah N, Gladstone RA, et al. MicroRNA‐144 is a circulating effector of remote ischemic preconditioning. Basic Research in Cardiology 2014;109(5):423. [DOI] [PubMed] [Google Scholar]
Lim 2005
- Lim KH, Halestrap AP, Angelini GD, Suleiman MS. Propofol is cardioprotective in a clinically relevant model of normothermic blood cardioplegic arrest and cardiopulmonary bypass. Experimental Biology and Medicine Journal 2005;230(6):413‐20. [DOI] [PubMed] [Google Scholar]
Lu 1997
- Lu EX, Chen SX, Yuan MD, Hu TH, Zhou HC, Luo WJ, et al. Preconditioning improves myocardial preservation in patients undergoing open heart operations. Annals of Thoracic Surgery 1997;64(5):1320‐4. [DOI] [PubMed] [Google Scholar]
Moher 2009
- Moher D, Liberati A, Tetzlaff J, Altman DG, The PRISMA Group. Preferred reporting items for systematic reviews and meta‐analyses: The PRISMA Statement. PLoS Medicine 2009;6(7):e1000097. [DOI: 10.1371/journal.pmed1000097] [DOI] [PMC free article] [PubMed] [Google Scholar]
Murry 1986
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation 1986;74(5):1124‐36. [DOI] [PubMed] [Google Scholar]
Przyklenk 1993
- Przyklenk K, Bauer B, Ovize M, Kloner RA, Whittaker P. Regional ischemic 'preconditioning' protects remote virgin myocardium from subsequent sustained coronary occlusion. Circulation 1993;87(3):893‐9. [DOI] [PubMed] [Google Scholar]
Rassaf 2014
- Rassaf T, Totzeck M, Hendgen‐Cotta UB, Shiva S, Heusch G, Kelm M. Circulating nitrite contributes to cardioprotection by remote ischemic preconditioning. Circulation Research 2014;114(10):1601‐10. [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sayin 2002
- Sayin MM, Ozatamer O, Taşöz R, Kilinç K, Unal N. Propofol attenuates myocardial lipid peroxidation during coronary artery bypass grafting surgery. British Journal of Anaesthesia 2002;89(2):242‐6. [DOI] [PubMed] [Google Scholar]
Schunemann 2009
- Schunemann HJ. GRADE: from grading the evidence to developing recommendations. A description of the system and a proposal regarding the transferability of the results of clinical research to clinical practice [GRADE: von der evidenz zur empfehlung. beschreibung des systems und losungsbeitrag zur ubertragbarkeit von studienergebnissen]. Zeitschrift fur Evidenz, Fortbildung und Qualitat im Gesundheitswesen 2009;103(6):391‐400. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Siregar 2014
- Siregar S, Heer F, Groenwold RH, Versteegh MI, Bekkers JA, Brinkman ES, et al. Trends and outcomes of valve surgery: 16‐year results of Netherlands Cardiac Surgery National Database. European Journal of Cardiothoracic Surgery 2014;46:386‐97. [DOI] [PubMed] [Google Scholar]
Stoppe 2016
- Stoppe C, McDonald B, Benstoem C, Elke G, Meybohm P, Whitlock R, et al. Evaluation of persistent organ dysfunction plus death as a novel composite outcome in cardiac surgical patients. Journal of Cardiothoracic and Vascular Anesthesia 2016;30(1):30‐8. [DOI] [PubMed] [Google Scholar]
Thielmann 2010
- Thielmann M, Kottenberg E, Boengler K, RaffelsieperC, Neuhaeuser M, Peters J, et al. Remote ischemic preconditioning reduces myocardial injury after coronaryartery bypass surgery with crystalloid cardioplegic arrest. Basic Research in Cardiology 2010;105:657‐64. [DOI] [PubMed] [Google Scholar]
World Health Organization 2011
- World Health Organization. Global Status Report on Noncommunicable Diseases 2010. Geneva: World Health Organization, 2011. [Google Scholar]
World Health Organization 2015
- World Health Organization. Cardiovascular diseases (CVD), Fact sheet N°317 (online). Available from: www.who.int/mediacentre/factsheets/fs317/en/ (accessed 4 April 2015).
References to other published versions of this review
Benstoem 2015a
- Benstoem C, Stoppe C, Liakopoulos OJ, Meybohm P, Clayton TC, Yellon DM, et al. Remote ischaemic preconditioning for coronary artery bypass grafting. Cochrane Database of Systematic Reviews 2015, Issue 11. [DOI: 10.1002/14651858.CD011719.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


