Abstract
Background
The long‐acting bronchodilator tiotropium and single‐inhaler combination therapy of inhaled corticosteroids and long‐acting beta2‐agonists (ICS/LABA) are commonly used for maintenance treatment of patients with chronic obstructive pulmonary disease (COPD). Combining these treatments, which have different mechanisms of action, may be more effective than administering the individual components.
Objectives
To assess relative effects of the following treatments on markers of exacerbations, symptoms, quality of life and lung function in patients with COPD.
• Tiotropium plus LABA/ICS versus tiotropium.
• Tiotropium plus LABA/ICS versus LABA/ICS.
Search methods
We searched the Cochrane Airways Group Specialised Register of Trials (April 2015), ClinicalTrials.gov (www.ClinicalTrials.gov), the World Health Organization (WHO) trials portal and reference lists of relevant articles.
Selection criteria
We included parallel, randomised controlled trials (RCTs) lasting three months or longer conducted to compare ICS and LABA combination therapy in addition to inhaled tiotropium versus tiotropium alone or combination therapy alone.
Data collection and analysis
We independently assessed trials for inclusion, then extracted data on trial quality and outcome results. We contacted study authors to ask for additional information. We collected trial information on adverse effects.
Main results
Tiotropium plus LABA/ICS versus tiotropium
We included six studies (1902 participants) with low risk of bias that compared tiotropium in addition to inhaled corticosteroid and long‐acting beta2‐agonist combination therapy versus tiotropium alone. We found no statistically significant differences in mortality between treatments (odds ratio (OR) 1.80, 95% confidence interval (CI) 0.55 to 5.91; two studies; 961 participants) as well as in the all‐cause hospitalisations (OR 0.84, 95% CI 0.53 to 1.33; two studies; 961 participants). The effect on exacerbations was heterogeneous among trials and was not meta‐analysed. Health‐related quality of life measured by St. George’s Respiratory Questionnaire (SGRQ) showed a statistically significant improvement in total scores with use of tiotropium + LABA/ICS compared with tiotropium alone (mean difference (MD) ‐3.46, 95% CI ‐5.05 to ‐1.87; four studies; 1446 participants). Lung function was significantly different in the combined therapy (tiotropium + LABA/ICS) group, although average benefit with this therapy was small. None of the included studies included exercise tolerance as an outcome.
A pooled estimate of these studies did not show a statistically significant difference in adverse events (OR 1.16, 95% CI 0.92 to 1.47; four studies; 1363 participants), serious adverse events (OR 0.86, 95% CI 0.57 to 1.30; four studies; 1758 participants) and pneumonia (Peto OR 1.62, 95% CI 0.54 to 4.82; four studies; 1758 participants).
Tiotropium plus LABA/ICS versus LABA/ICS
One of the six studies (60 participants) also compared combined therapy (tiotropium + LABA/ICS) versus LABA/ICS therapy alone. This study was affected by lack of power; therefore results did not allow us to draw conclusions for this comparison.
Authors' conclusions
This review update includes three additional studies and provides new low quality evidence supporting the finding that tiotropium + LABA/ICS‐based therapy improves the disease‐specific quality of life. The current evidence is insufficient to support the benefit of tiotropium + LABA/ICS‐based therapy for mortality, hospital admission or exacerbations (moderate and low quality evidence). Compared with use of tiotropium alone, tiotropium + LABA/ICS‐based therapy does not seem to increase undesirable effects nor serious non‐fatal adverse events.
Plain language summary
Are tiotropium plus combination inhalers better than tiotropium or combination inhalers alone for the treatment of COPD?
Background
Chronic obstructive pulmonary disease (COPD) is a lung disease that includes the conditions chronic bronchitis and/or emphysema. COPD is characterised by narrowing of the airways and lung tissue destruction. Symptoms include breathlessness and long‐term cough. Symptoms of COPD are treatable, but the condition cannot be reversed or cured. It is usually brought on by airway irritants, such as smoking or inhaled dust.
Inhalers with bronchodilators (which allow the airways in the lungs to relax and expand) and/or anti‐inflammatory agents are commonly used to ease symptoms and minimise the long‐term decline in health caused by COPD. Examples of these treatments are tiotropium, which is a bronchodilator, and combination inhalers, which contain another type of bronchodilator (long‐acting beta‐agonists) together with anti‐inflammatory agents (steroids). These treatments work in different ways and therefore might be more beneficial if used together.
Study characteristics
This review found six studies, involving 1902 participants, comparing the long‐term efficacy and side effects of tiotropium combined with combination inhalers for treatment of patients with COPD. Not all of the people included in these studies had COPD that was severe enough to be recommended for combined therapy according to current guidelines.
Key results
Current evidence shows potential benefits of treatment with tiotropium in addition to inhaled corticosteroid and long‐acting beta2‐agonist combination therapy through increased health‐related quality of life and a small improvement i n lung function in patients receiving this combined therapy. However, this evidence does not allow us to draw conclusions about the effects of these treatments on mortality, hospitalisation for all causes and exacerbations. The frequency of serious and non‐serious adverse events was not increased in either of the two groups.
Quality of the evidence
Overall, we assessed the evidence presented in this review to be of moderate or low quality, which means we are reasonably confident in some of the findings, but less confident in others.
Summary of findings
Summary of findings for the main comparison. Tiotropium + LABA/ICS combination compared with tiotropium for chronic obstructive pulmonary disease.
| Tiotropium + LABA/ICS combination compared with tiotropium for chronic obstructive pulmonary disease | ||||||
|
Patient or population: patients with chronic obstructive pulmonary disease Settings: ambulatory clinics Intervention:tiotropium + LABA/ICS combination Comparison: tiotropium | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | Number of participants (studies) | Quality of the evidence (GRADE) | Comments | |
|
Assumed risk Tiotropium |
Corresponding risk Tiotropium + LABA/ICS combination |
|||||
| Mortality (all‐cause) | 8 per 1000 | 15 per 1000 (5 to 47) | OR 1.80 (0.55 to 5.91) | 961 (2 studies) | ⊕⊕⊕⊝ Moderatea,b | |
| Hospital admission (all causes) | 156 per 1000 | 101 per 1000 (69 to 145) | OR 0.84 (0.53 to 1.33) | 961 (2 studies) | ⊕⊕⊝ ⊝ Lowa,b | |
| Exacerbation ‐ at 12‐month follow‐up | 628 per 1000 | 601 per 1000 (486 to 704) | OR 0.89 (0.56 to 1.41) | 301 (1 study) | ⊕⊕⊝⊝ Lowa,b | |
| Serious adverse events (non‐fatal) | 60 per 1000 | 52 per 1000 (35 to 76) | OR 0.86 (0.57 to 1.30) | 1758 (4 studies) | ⊕⊕⊝⊝ a,c Low | |
| Quality of life up to 6 months (SGRQ) | Mean SGRQ up to 6 months in the intervention groups was 3.46 lower (5.05 to 1.87 lower) | ‐ | (4 studies) | ⊕⊕⊝⊝ d Low | A lower score indicates better quality of life | |
| FEV1 pre‐dose ‐ FEV1 3‐6 months mean difference | Mean FEV1 pre‐dose ‐ FEV1 3‐6 months mean difference in the intervention groups was 0.06 (0.04 to 0.08 ) | ‐ | (4 studies) | ⊕⊕⊕⊝ Moderatee | ||
| FEV1 pre‐dose ‐ FEV1 1 year | Mean FEV1 pre‐dose ‐ FEV1 1 year mean difference in the intervention groups was 0.06 (0 to 0.12 ) | ‐ | (1 study) | ⊕⊕⊕⊝ Moderatea,b | ||
| *The basis for assumed risk (e.g. median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; OR: odds ratio | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate Very low quality: We are very uncertain about the estimate | ||||||
aDowngraded one level because of imprecision (95% confidence interval includes both no effect and appreciable harm)
bDowngraded one level because of study limitations (incomplete outcome assessment in Aaron 2007)
cDowngraded once because of study limitations (incomplete outcome assessment in Aaron 2007 and Hanania 2011; unclear risk of selection bias in Hanania 2011; possible detection bias in Jung 2012)
dDowngraded two levels because of study limitations (unclear risk of selection bias and detection bias and incomplete outcome assessment in Hoshino 2011; unclear risk of detection bias in Jung 2012; incomplete outcome assessment in Aaron 2007)
eDowngraded one level because of study limitations (unclear risk of selection and detection bias in Cazzola 2007; unclear risk of detection bias in Jung 2012; incomplete outcome assessment in Aaron 2007)
Background
Description of the condition
Chronic obstructive pulmonary disease (COPD) is a general term that refers to chronic bronchitis or emphysema, or both. COPD occurs when airflow to the lungs is restricted by narrowing of the airways. Symptoms include cough, breathlessness and reduced exercise capacity. The Global Initiative for Chronic Obstructive Lung Disease (GOLD 2015) guidelines describe COPD as a preventable and treatable condition that is not fully reversible. Worldwide, the main cause of COPD is tobacco smoking, but air pollution, burning of biomass and occupational exposure are also risk factors (GOLD 2015). The prevalence, morbidity and mortality of the disease vary across populations, and the disorder causes a substantial economic and social burden.
Various pharmacological treatments are commonly used in COPD management to relieve symptoms, improve exercise tolerance and quality of life, reduce mortality and prevent and treat exacerbations. Exacerbations of COPD impair patients' quality of life, and a large part of the economic burden of COPD is attributed to the cost of managing exacerbations, particularly those resulting in the use of acute care services or hospitalisations (Hutchinson 2010). Appropriate pharmacological management of the disease is therefore important to reduce and prevent exacerbations. Management of COPD tends to begin with one treatment, and additional therapies are introduced as necessary to control symptoms (GOLD 2015). Self‐management, education, vaccination and rehabilitation can accompany these pharmacological interventions (Effing 2007; Lacasse 2006; Sehatzadeh 2012).
Description of the intervention
The first pharmacological step in treating patients with COPD consists of the use of short‐acting bronchodilators for symptom control when needed. These include short‐acting beta2‐agonists (SABA) and the short‐acting anticholinergic agent ipratropium. To manage persistent COPD symptoms, long‐acting bronchodilators can be introduced (GOLD 2015). Regular treatment with long‐acting bronchodilators is more efficient and convenient than treatment with regular short‐acting bronchodilators (Beeh 2010). Long‐acting bronchodilators include long‐acting beta2‐agonists (LABA) and the long‐acting anticholinergic agent tiotropium. Tiotropium bromide has gained widespread acceptance as once daily maintenance therapy in COPD (Barr 2005; GOLD 2015). Tiotropium reduces COPD exacerbations and related hospitalisations compared with ipratropium (Barr 2005). Most LABA are taken twice daily. They improve lung function compared with ipratropium, but little difference is shown in improving COPD symptoms and exercise tolerance (Appleton 2006). For symptomatic patients with severe or very severe COPD (forced expiratory volume in one second (FEV1) < 50% predicted) and with repeated exacerbations, GOLD 2015 recommends the addition of inhaled corticosteroids (ICS) to bronchodilator treatment. Inhaled corticosteroids are licensed as combination inhalers with LABA. The most common combinations of ICS and LABA in combination inhalers are fluticasone and salmeterol; budesonide and formoterol; and mometasone and formoterol. Combination therapy reduces exacerbation rates and mortality compared with ICS alone (Nannini 2013). Also compared with LABA alone, combination therapy is more effective in reducing exacerbation rates, but with no significant difference in mortality (Nannini 2007b). For patients who continue to have symptoms and are at high risk of experiencing exacerbations, triple therapy with LABA, long‐acting muscarinic antagonists (LAMA, e.g. tiotropium) and ICS is recommended. Such patients are referred to as 'Group D' in the GOLD guidelines; this group typically includes patients classified as GOLD 3 and 4, i.e. FEV1 < 50% of predicted value (GOLD 2015). Benefits of combination inhalers should be viewed against the possible increased risk of pneumonia (Nannini 2007b; Nannini 2013). Potential risks and benefits of treatment with combination inhaler compared with tiotropium are uncertain (Welsh 2010), as are risks and benefits of treatment with combination inhaler in addition to tiotropium, which will be explored in this review.
How the intervention might work
Tiotropium
Tiotropium (TIO) is a long‐acting anticholinergic agent that targets bronchospasm in COPD by relaxing the smooth muscle of the airways. Tiotropium is structurally related to ipratropium, a short‐acting anticholinergic agent that binds to M1, M2 and M3 muscarinic receptors, which in turn open the bronchi (Barr 2005). Although tiotropium binds to the same receptors as ipratropium, it has different kinetic selectivity. Tiotropium dissociates slowly from M1 and M3 receptors, giving a bronchodilator effect lasting over 24 hours, but rapidly from M2 receptors. It appears that M2 receptors are feedback inhibitory receptors, and blocking them (as is the case for ipratropium) releases acetylcholine rather than reducing it as desired (Barr 2005). Benefits of tiotropium, in comparison with placebo, include reduced COPD exacerbations and exacerbation‐related hospitalisations, and improved health‐related quality of life and symptom scores among patients with moderate and severe disease (Barr 2005). Anticholinergic side effects can occur with tiotropium and include dry mouth, constipation and tachycardia.
Inhaled beta2‐agonist plus inhaled corticosteroids
Inhaled beta2‐agonists activate beta2‐receptors in the smooth muscle of the airways, releasing adenylate cyclase and increasing intracellular cyclic adenosine monophosphate (cAMP), which leads to a cascade of reactions resulting in bronchodilation. Beta2‐agonists may act through other mechanisms such as respiratory muscle function or mucociliary clearance; patients have shown improvement in symptoms whilst showing no improvement in lung function tests. Beta2‐agonists are particularly useful because they reverse bronchoconstriction regardless of its initial cause. Side effects include muscle tremors, nervousness and occasional insomnia, but, as with all inhaled medications, systemic side effects are minimised by a comparatively low dose administered directly to the lungs. Inhaled corticosteroids are anti‐inflammatory drugs that have been associated with reduced risk of exacerbation in patients with COPD and with better quality of life outcomes when compared with placebo, with no effect on overall mortality or long‐term FEV1 (GOLD 2015; Yang 2012). Combination inhalers including ICS and LABA reduce exacerbation rates and all‐cause mortality and improve lung function and quality of life compared with placebo (Nannini 2007a). These effects are thought to be greater for combination inhalers than for the component preparations (GOLD 2015). Use of inhaled corticosteroids, alone or in combination with beta2‐agonists, potentially increases the risk of pneumonia (GOLD 2015; Yang 2012).
The combination inhalers currently available are fluticasone/salmeterol (FSC); budesonide/formoterol (BUD/F); and beclomethasone/formoterol (DPB/F).
Combination therapy
The nature of the interaction between the two systems is not yet fully understood, but combining beta2‐adrenergic receptor agonists and muscarinic acetylcholine receptor antagonists is pharmacologically reasonable, given that airway tone is regulated by the parasympathetic and sympathetic nervous systems. The synergistic effect of these therapies can be explained in several ways. One explanation is that the addition of a beta2‐adrenergic receptor agonist decreases release of acetylcholine (ACh) and amplifies bronchial smooth muscle relaxation; another is that the addition of a muscarinic acetylcholine receptor antagonist can reduce bronchoconstrictor effects of ACh and amplify bronchodilation through direct stimulation of the smooth muscle beta2‐adrenergic receptor (Cazzola 2010). An animal model showed activation of calcium‐activated potassium (KCa) channels thought to hyperpolarise the cell membrane, causing reductions in the concentration of intracellular calcium (Ca) and ACh release in prejunctional cholinergic nerves (Brichetto 2003).
Why it is important to do this review
The previous version of this review showed a significant effect of combination therapy tiotropium + LABA/ICS on FEV1 in participants with stable COPD, in comparison with tiotropium therapy alone. However, sparse evidence was found to support similar beneficial effects on other important outcomes, such as all‐cause hospitalisations, exacerbations and mortality. New published trials have been conducted with the aim of comparing these therapies; therefore it is necessary to include their results as part of this review to obtain more precise estimations of treatment effects on outcomes for which combination therapy effects remain unclear.
Objectives
To assess relative effects of the following treatments on markers of exacerbations, symptoms, quality of life and lung function in patients with COPD.
Tiotropium plus LABA/ICS versus tiotropium.
Tiotropium plus LABA/ICS versus LABA/ICS.
Methods
Criteria for considering studies for this review
Types of studies
For effectiveness and safety objectives, we included randomised controlled trials (RCTs) of parallel design conducted in patients with stable COPD who received the trial treatment for at least 12 weeks.
For economic objectives, we included economic evaluation studies such as cost‐effectiveness analyses and cost‐utility analyses addressing the same interventions in the population of interest for this review. We considered for inclusion the economic evaluation conducted alongside the RCT or economic evaluation modelling studies based on a comprehensive systematic review of the literature. We excluded partial economic evaluation studies reporting cost analysis or cost‐outcome descriptions.
Types of participants
Populations with a diagnosis of COPD. We included only studies that used an external set of criteria to screen participants for this condition (e.g. ATS; BTS; GOLD 2015; TSANZ).
Types of interventions
Inhaled combination corticosteroid and long‐acting beta2‐agonist (such as fluticasone/salmeterol, budesonide/formoterol, beclomethasone/formoterol) and tiotropium bromide versus:
inhaled tiotropium bromide alone; or
inhaled corticosteroid and long‐acting beta2‐agonist combination.
Types of outcome measures
Primary outcomes
Mortality (all‐cause).
Exercise tolerance.
Hospital admissions: all‐cause and due to exacerbations.
Exacerbations: all‐cause, requiring short burst oral corticosteroids or antibiotics as defined by agreed criteria.
Health‐related quality of life (measured with a validated scale for COPD, e.g. St George's Respiratory Questionnaire (SGRQ), Chronic Respiratory Disease Questionnaire (CRQ)).
Serious adverse events non‐fatal.
Pneumonia.
Secondary outcomes
Symptoms.
Forced expiratory volume in one second (FEV1).
Adverse events.
Side effects.
Cost‐effectiveness of interventions.
Search methods for identification of studies
Electronic searches
The previously published version of this review (Karner 2011) included searches up to July 2010. The search period for this update is July 2010 to April 2015.
For this update, we identified trials from the Cochrane Airways Group Specialised Register (CAGR), which is maintained by the Information Specialist for the Group. The Register contains trial reports identified through systematic searches of bibliographic databases including the Cochrane Central Register of Controlled Trials (CENTRAL), MEDLINE, EMBASE, the Cumulative Index to Nursing and Allied Health Literature (CINAHL), the Allied and Complementary Medicine Database (AMED) and PsycINFO, and by handsearching of respiratory journals and meeting abstracts (please see Appendix 1 for further details). We searched all records in the CAGR using the search strategy provided in Appendix 2.
We also conducted a search of ClinicalTrials.gov (www.ClinicalTrials.gov) and the World Health Organization (WHO) trials portal (www.who.int/ictrp/en/). We searched all databases from their inception to the present, with no restriction on the language of publication. We conducted the latest search in April 2015.
Searching other resources
We reviewed reference lists of all primary studies and review articles for additional references. We contacted authors of identified trials and asked them to identify other published or unpublished studies.
Data collection and analysis
Selection of studies
Two review authors (OMG and RJD) screened the titles and abstracts of citations retrieved through literature searches and obtained those deemed to be potentially relevant. We assigned each reference to a study identifier and assessed all references against the inclusion criteria of the protocol.
Two review authors (OMG and RJD) independently examined titles and abstracts for the selection of health economics studies to be included in the critical review of economic data. We removed records that did not report on cost‐effectiveness or cost‐utility analysis. Two review authors (MXR and RJD) independently examined full‐text reports to determine which studies met the eligibility criteria of this review. We resolved disagreements by discussion between review authors. We included only full economic evaluations of high methodological and reporting quality.
Data extraction and management
We extracted the following characteristic information from each study.
Design (design, total duration of study and run‐in, number of study centres and locations, withdrawals, date of study).
Participants (N, mean age, age range, gender, COPD severity, diagnostic criteria, baseline lung function, smoking history, inclusion criteria, exclusion criteria).
Interventions (run‐in, intervention treatment and inhaler type, control treatment and inhaler type).
Outcomes (primary and secondary outcomes specified and collected, time points reported).
Two review authors (MXR and OMG) extracted data from the studies onto data collection forms. Review authors discussed discrepancies in the data and resolved them and transferred data from data collection forms into RevMan (RevMan 2014).
Data obtained by authors from the previous version of this review regarding all cause hospital admissions that were supplied by Aaron 2007 and by AstraZeneca (for Welte 2009) on request, were kept for this update without changes.
Assessment of risk of bias in included studies
We assessed all included studies for risk of bias according to the recommendations outlined in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011) for the following items.
Allocation sequence generation.
Concealment of allocation.
Blinding of participants and investigators.
Incomplete outcome data.
Selective outcome reporting.
We noted other sources of bias and graded each potential source of bias as having high, low or unclear risk.
We assessed the methodological quality of economic evaluations by using the Drummond checklist (Drummond 1996), which addresses the following methodological and reporting aspects.
Was a well‐defined question posed?
Was a comprehensive description of competing alternatives given?
Does the paper provide evidence that the programme would be effective (i.e. would the programme do more harm than good)?
Were all important and relevant resource uses (costs) for each alternative identified?
Were all important and relevant health outcome consequences for each alternative identified?
Were costs measured accurately in appropriate units before evaluation and valued credibly?
Were health outcome consequences measured credibly?
Were costs and health outcome consequences adjusted for the different times at which they occurred (i.e. was discounting applied)?
Was an incremental analysis of the consequences and costs of alternatives performed?
Was an adequate sensitivity analysis performed?
Quality of the body of evidence for each outcome
We assessed the quality of evidence for the main comparison at the outcome level using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach (Guyatt 2011). This methodological approach considers RCTs as providing high‐quality evidence that may be rated down by limitations in any of five areas: design (risk of bias), consistency across studies, directness of the evidence, precision of estimates and presence of publication bias (Guyatt 2011). The GRADE approach results in an assessment of the quality of a body of evidence in one of four grades: (1) high: We are very confident that the true effect lies close to that of the estimate of effect; (2) moderate: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of effect but may be substantially different; (3) low: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of effect; (4) very low: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect (GRADE 2013).
Two review authors (OMG and MXR) independently assessed the quality of the body of evidence found for each of the outcomes identified as critical or important for clinical decision making: mortality, hospital admission (all causes), exacerbation at 12 months, improvement in FEV1, serious adverse events and quality of life. In the case that the study authors did not take measures to ensure concealment of allocation, randomised assignment, completion to follow‐up or blinded outcome assessment, we downgraded the quality of evidence because of design limitations (GRADE 2013). We evaluated consistency by similarity of point estimates, extent of overlap of confidence intervals (CIs) and application of statistical criteria including testing for heterogeneity (I2). We planned to downgrade the quality of evidence if we detected substantial unexplained heterogeneity across study results (i.e. some studies suggest important benefit and others no effect or harm without a clinical explanation) (GRADE 2013). We assessed precision according to the 95% CI around the pooled estimate (GRADE 2013). When studies were conducted in populations other than the target population, the GRADE framework suggests that the quality of evidence should be downgraded because of indirectness (GRADE 2013).
We entered data (i.e. pooled estimates of effects and corresponding 95% CIs) and explicit judgements that were made for each of the above aspects into the GRADEprofiler (GDT), the software used to create Summary of findings (SoF) tables. We explained in the SoF table footnotes all judgements involved in assessment of the aspects of the evidence described above.
Measures of treatment effect
We performed all statistical analyses using RevMan software (RevMan 2014). We analysed dichotomous data (such as mortality, hospital admission, number of participants with one or more exacerbations) using the Mantel‐Haenszel odds ratio (OR) and risk difference (RD), unless events were rare, in which case we employed the Peto OR (as this does not require a continuity correction for zero cells). For statistically significant results of categorical variables, we reported the number needed to treat for an additional beneficial outcome (NNTB).
We analysed continuous outcome data (such as quality of life (score) and FEV1) using the mean difference (MD). We reported the 95% CI on all estimates as fixed‐effect mean differences with 95% CI. When treatment effects were reported as a mean difference with 95% CI, we entered the MD and standard errors calculated from the 95% CI and analysed data using the generic inverse variance (GIV) tool.
Unit of analysis issues
We analysed dichotomous data by using participants as the unit of analysis (rather than events) to avoid counting the same participant more than once.
Dealing with missing data
We contacted investigators and study sponsors to verify key study characteristics and to obtain missing numerical outcome data.
Assessment of heterogeneity
We assessed the amount of statistical variation between study results by using the I2 measurement.
Assessment of reporting biases
We minimised reporting bias from non‐publication of studies or selective outcome reporting by using a broad search strategy, by contacting study authors directly and by checking references of included studies. We planned to assess reporting bias by visual inspection of funnel plots.
Data synthesis
We combined dichotomous data using the Mantel‐Haenszel OR with 95% CIs by using a fixed‐effect model. We combined rate ratios and hazard ratios using GIV in a fixed‐effect model and compared them with the random‐effects model. We planned to calculate the NNTB outcome from the pooled OR and its CI, and to apply appropriate levels of baseline risk. We have presented the findings of our primary outcomes in Table 1, which we generated by using GradePro software.
We did not perform pooled calculations of economic data. Rather, we presented the characteristics and results of included economic studies in a descriptive way in the additional tables (Table 2; Table 3), including the final incremental cost‐effectiveness ratios (ICERs) reported by study authors in Euros (EUR). We did not adjust the values of ICERs provided by study authors because most identified studies were conducted in similar settings and during a similar time period (2009 to 2010) using the same information resource, as all are based on the same clinical trial data.
1. Characteristics of included economic evaluations.
| Study ID | Country | Study design |
Population (N participants, severity indicators, smoking history |
Economic outcomes | Interventiona (doses) | Perspective | Price year | Time horizon | ICER reported and adjusted to Euros 2014 |
| Najafzadeh 2008 | Canada | Cost utility | Based on the Aaron 2007 study: N = 449, patients with ≥ 1 exacerbation within 12 months, moderate and severe obstruction defined as FEV1 < 65% post‐bronchodilator, ≥ 10 pack‐years |
Incremental cost per exacerbation avoided and incremental cost per QALY with tiotropium + LABA/ICS relative to tiotropium | • Tiotropium 18 mcg once daily + placebo twice daily • Tiotropium 18 mcg once daily + FS 250/25 mg/puff, 2 puffs twice daily |
Healthcare system perspective |
2006 | 1 year | Per exacerbation avoid CAN$6510 Per QALY CAN$243180 |
| Mittmann 2011 | Australia, Canada and Sweden | CEA | Based on the Welte 2009 study: N = 659, aged ≥ 40 years, symptoms for ≥ 2 years, ≥ 1 exacerbation within 12 months requiring systemic steroids and/or antibiotics, FEV1 ≤ 50% of predicted normal, FEV1 /FVC < 70% pre‐dose, ≥ 10 pack‐years |
Incremental cost‐effectiveness ratio for exacerbation avoided with tiotropium + LABA/ICS relative to tiotropium | • Tiotropium (Handihaler) 18 mcg once daily + budesonide/formoterol (Symbicort Turbuhaler) 320/9 mcg one inhalation twice daily • Tiotropium 18 mcg once daily + placebo (identical Turbuhaler) twice daily |
Healthcare system payer perspective | 2009 | 3 months | Per avoiding severe exacerbation: Australia: tiotropium + LABA/ICS dominant Canadian: tiotropium + LABA/ICS dominant Sweden: 244,36 EUR |
| Nielsen 2013 | Denmark, Finland, Norway and Sweden | CEA | Based on the Welte 2009 study: N = 659, aged ≥ 40 years, clinical diagnosis of COPD and symptoms ≥ 2 years, ≥ 1 exacerbation in the previous 12 months requiring systemic steroids and/or antibiotics, FEV1 ≤ 50% predicted normal value, FEV1 /FVC < 70% pre‐dose, ≥10 pack‐years |
Incremental cost‐effectiveness ratio for exacerbation avoided with tiotropium + LABA/ICS relative to tiotropium | • Tiotropium (Handihaler) 18 mcg once daily + budesonide/formoterol (Symbicort Turbuhaler) 320/9 mcg one inhalation twice daily • Tiotropium 18 mcg once daily + placebo (identical Turbuhaler) twice daily |
Healthcare system payer perspective | 2010 | 3 months | ICER excluding antibiotics Denmark: 212 EUR Finland: 307 EUR Norway: tiotropium + LABA/ICS dominant Sweden: 165 EUR |
aTiotropium was compared with tiotropium + budesonide/formoterol in all included economic evaluations.
CEA: cost‐effectiveness analysis
FEV1: forced expiratory volume in one second
FVC: forced vital capacity
ICER: incremental cost‐effectiveness ratio
ICS: inhaled corticosteroids
LABA: long‐acting beta‐agonists
QALY: quality‐adjusted life‐year
2. Quality assessment of included economic evaluations (Drummond checklist).
| Study ID | Well‐defined question? | Competing alternatives described? | Effectiveness established? | Relevant costs and consequences identified? | Costs and consequences measured accurately? | Costs and consequences valued credibly? | Discounting performed? | Incremental analysis of costs and consequences performed? | Sensitivity analysis performed? |
| Mittmann 2011 | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Najafzadeh 2008 | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes |
| Nielsen 2013 | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
Subgroup analysis and investigation of heterogeneity
We planned to perform subgroup analyses for effectiveness and safety data on the basis of types of combination therapy and differences in baseline risk (severity of disease at baseline), provided at least three studies per subgroup were included in a specific comparison. However, included studies did not provide data for these subgroup analyses. Included studies reported outcomes at different follow‐up periods, and different follow‐periods may be associated with different treatment effects; therefore, we decided to include three subgroup analyses: at three‐month follow‐up; at six‐month follow‐up; and at 12‐month follow‐up.
Sensitivity analysis
The sensitivity analysis takes into account biases that could significantly impact the outcomes of included studies. We planned to perform a sensitivity analysis to assess how results of the meta‐analysis would be affected by excluding studies determined to be at a high risk of bias. Two studies (Hoshino 2011; Jung 2012) were open‐label studies; therefore we performed a sensitivity analysis for outcomes of quality of life (QoL) and for all severe adverse events (non‐fatal).
Results
Description of studies
Results of the search
The initial search carried out in July 2010 yielded 101 references, from which only three studies (Aaron 2007; Cazzola 2007; Welte 2009) were included and one RCT was classified as awaiting assessment (Fang 2008). Details of the search results from the previous review are described in Appendix 3. We updated these searches in April 2015 and identified 250 new references from July 2010. Of these, 13 references were selected as potentially relevant and underwent full‐text review. Three new studies (Hanania 2011; Hoshino 2011; Jung 2012) and three economic analyses (Mittmann 2011; Najafzadeh 2008; Nielsen 2013) met the criteria for inclusion, two RCTs were classified as ongoing studies (Betsuyaku 2013; Cohuet 2013) and one RCT was classified as awaiting assessment (Lee 2014) because only results from the abstract presented at a scientific meeting were available. We excluded four studies (see the Excluded studies section). We did not find published results of the Fang 2008 study for this review; therefore, it is still awaiting assessment.
In summary, for the clinical effectiveness objective we included a total of six studies (Aaron 2007; Cazzola 2007; Hanania 2011; Hoshino 2011; Jung 2012; Welte 2009), randomising 1902 participants to comparisons of interest for the review. We included three studies (Mittmann 2011; Najafzadeh 2008; Nielsen 2013) for the economic evaluation objective related to cost‐effectiveness (Figure 1).
1.
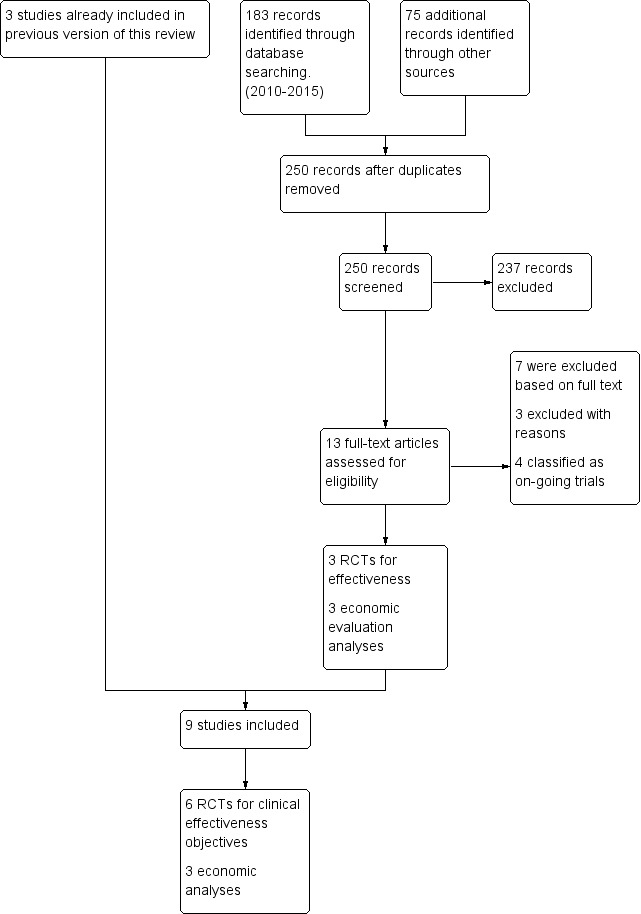
Study flow diagram.
Included studies
Randomised controlled trials (RCTs)
We included a total of six RCTs in this review. All included studies (Aaron 2007; Cazzola 2007; Hanania 2011; Hoshino 2011; Jung 2012; Welte 2009) assessed the effectiveness of tiotropium plus LABA/ICS in comparison with tiotropium, and just one study (Cazzola 2007) also compared the effectiveness of tiotropium plus LABA/ICS versus LABA/ICS (see the Characteristics of included studies table and Table 4). Not all of the participants enrolled in the included studies were eligible for triple therapy according to the current guidance (GOLD 2015).
3. Characteristics of included studies: baseline COPD severity and exacerbation status.
| Study ID | Baseline COPD severity | Baseline FEV1% predicted | Exacerbation status preceding enrolment |
| Aaron 2007 | Moderate or severe | Inclusion criteria < 65% predicted Mean FEV1% predicted tiotropium + LABA/ICS group 42.2% and in tiotropium group 42.1% |
Required to have had ≥ 1 exacerbation in previous year |
| Cazzola 2007 | Severe and very severe | Inclusion criteria ≤ 50% predicted Mean FEV1% predicted LABA/ICS group = 36.9%, tiotropium group = 38.5%, tiotropium + LABA/ICS group = 39% |
No information on exacerbation status before enrolment |
| Hanania 2011 | Moderate: tiotropium + LABA/ICS = 63% and tiotropium = 72% Severe: tiotropium + LABA/ICS = 37% and tiotropium = 28% |
Inclusion criteria ≥ 40 to ≤ 80% predicted Mean FEV1% predicted tiotropium + LABA/ICS group = 56% and tiotropium group = 57.4% |
Exacerbations in past 12 months requiring antibiotics/corticosteroid: tiotropium + LABA/ICS: 1 exacerbation = 37%, ≥ 2 exacerbations = 6% Tiotropium: 1 exacerbation = 27%, ≥ 2 exacerbations = 6% |
| Hoshino 2011 | Mild to very severe Overall: mild = 10%, moderate = 33%, severe = 36.6%, very severe = 20%. |
Mean FEV1% predicted tiotropium + LABA/ICS group = 64.6% and tiotropium group = 57.1% | Participants were excluded if they had experienced an exacerbation in the previous 12 weeks before commencement of the study. No other information on exacerbation status |
| Jung 2012 | Moderate: tiotropium + LABA/ICS = 56.6% and tiotropium = 60.3% Severe: tiotropium + LABA/ICS = 40.8% and tiotropium = 35.5% Very severe: tiotropium + LABA/ICS = 2.7% and tiotropium = 3.5% |
Inclusion criteria < 65% predicted Mean FEV1% predicted tiotropium + LABA/ICS group = 47.4% and tiotropium group = 47.5% |
No information on exacerbation before enrolment |
| Welte 2009 | Severe and very severe | Inclusion criteria ≤ 50% predicted Mean FEV1% predicted tiotropium + LABA/ICS group = 38.1% and tiotropium group = 37.7% |
Required to have had ≥ 1 exacerbation in previous year Mean exacerbations last year: 1.4 for both groups, range 1‐7 |
COPD: chronic obstructive pulmonary disease
FEV1: forced expiratory volume in one second
LABA: long‐acting beta‐agonists
ICS: inhaled corticosteroids
Aaron 2007 is a randomised, double‐blind, placebo‐controlled study that was conducted in Canada with the aim of comparing the safety and effectiveness of tiotropium plus placebo versus tiotropium plus fluticasone plus salmeterol as one‐year maintenance therapy in participants with moderate or severe COPD. This study included 449 participants (301 in a comparison of interest in this review) older than 35 years of age, with a forced expiratory volume in one second (FEV1)–to‐forced vital capacity (FVC) ratio of less than 0.70 and a post‐bronchodilator FEV1 of less than 65% of the predicted value. Participants had to have at least one exacerbation of COPD that required treatment with systemic steroids or antibiotics within the 12 months before randomisation. Participants were assigned to receive tiotropium plus placebo or tiotropium plus fluticasone‐salmeterol. Measures of efficacy included the proportion of participants who experienced an exacerbation of COPD that required treatment with systemic steroids or antibiotics, lung function, disease‐specific quality of life, number of hospitalisations for COPD exacerbations and all‐cause hospitalisations. It is likely that most participants in this study would be eligible for triple therapy according to current guidance (GOLD 2015).
Cazzola 2007 is a randomised, double‐blind, double‐dummy, parallel‐group study that was conducted in Italy to compare the efficacy and safety of three treatments for 12 weeks: (1) fluticasone/salmeterol (FSC) 500/50 mg Diskus, one inhalation twice daily + placebo Handihaler, one inhalation once daily; (2) tiotropium 18 mg Handihaler, one inhalation once daily + placebo Diskus, one inhalation twice daily; (3) FSC 500/50 mg Diskus, one inhalation twice daily + tiotropium 18 mg Handihaler, one inhalation once daily. This study included 90 participants 50 years of age or older with well‐controlled severe or very severe COPD (FEV1% predicted ≤ 50%) who were current or former smokers (20 or more pack‐years) and were randomised to receive FSC, tiotropium or their combination. Study authors provided no information on the exacerbation status of participants during the year before enrolment. The primary efficacy measure was the mean change from baseline in pre‐dose FEV1 after three‐months of treatment. Secondary efficacy measures included change from baseline in the validity assessment score (VAS) assessing dyspnoea and supplemental salbutamol. It is likely that most participants in this study would be eligible for triple therapy according to current guidance (GOLD 2015).
Hanania 2011 is a randomised, double‐blind, parallel‐group, multi‐centre study of 24 weeks, conducted at 33 centres in the USA to compare the efficacy and safety of FSC (250/50 mcg twice daily) when added to tiotropium (18 mcg once daily) in participants with symptomatic moderate to severe COPD. The study included 342 participants who were 40 years of age or older with a cigarette smoking history ≥ 10 pack‐years and with a diagnosis of COPD and post‐bronchodilator FEV1 ≥ 40% to ≤ 80% of predicted normal and FEV1/FVC of 0.70. In the year before enrolment, 43% of participants in the tiotropium plus FSC group had experienced at least one exacerbation, and 33% in the tiotropium alone group. Participants were randomised in a 1:1 double‐blind fashion to open‐label tiotropium 18 mcg once daily plus FSC 250/50 mcg twice daily or open‐label tiotropium 18 mcg once daily plus placebo twice daily. Measures of efficacy included evaluation of lung function (pre‐dose FEV1, post‐dose FEV1, pre‐dose FVC and post‐dose FVC), use of rescue medication, healthcare utilisation for COPD exacerbations, health status evaluated with domain scores on the Chronic Respiratory Disease Questionnaire‐Self Administered Standardised (CRQ‐SAS) and safety. It is likely that most participants in this study would not be recommended triple therapy according to current guidance (GOLD 2015).
Hoshino 2011 is a randomised, open‐label, parallel‐group study conducted in Japan with the aim of comparing the efficacy and tolerability of salmeterol/fluticasone propionate added to tiotropium for 12 weeks. This study included 30 participants with an FEV1/FVC ratio less than 0.70, a smoking history > 10 pack‐years and no history of asthma or atopy. Eligible participants had mild to very severe COPD and were newly diagnosed or had not been treated previously with LAMA, LABA or ICS. Investigators provided no information on the exacerbation status of participants in the year before enrolment. They were randomised to receive inhaled tiotropium once daily or inhaled SFC twice daily, in combination with tiotropium once daily, for 12 weeks. Measures of efficacy included changes in airway dimensions on computed tomography (CT), pulmonary function testing and assessments of health‐related quality of life using the SGRQ. It is unclear what proportion of participants in this study would be eligible for triple therapy according to current guidance (GOLD 2015).
Jung 2012 is a randomised, open‐label, multi‐centre two‐arm parallel‐group study conducted in 30 academic hospital‐based pulmonary clinics in Korea with the aim of comparing the efficacy of tiotropium (18 mg once daily) plus FSC (250/50 mg twice daily) versus tiotropium monotherapy. This study included 479 participants diagnosed with moderate to very severe COPD, who had a post‐bronchodilator FEV1/FVC ratio less than 0.70 and FEV1 less than 65% of predicted value; eligible participants were 40 to 80 years of age and had a smoking history of at least 10 pack‐years. Investigators provided no information on the exacerbation status of participants in the year before enrolment. Participants were randomised to one of two treatment groups for 24‐week treatment: tiotropium 18 mg once daily; or tiotropium 18 mg once daily plus FSC, 250/50 mg/puff, one puff twice daily. Measures of efficacy included evaluation of lung function (change in pre‐bronchodilator FEV1 (L); changes in pre‐bronchodilator inspiratory capacity (IC); FVC and percent predicted (% pred) values for FEV1); mean changes in health‐related quality of life; frequency of COPD exacerbations; exacerbations requiring hospitalisation, emergency room visits or outpatient clinic visits; and hospitalisation rates for all causes. It is unclear what proportion of participants in this study would be recommended triple therapy according to current guidance (GOLD 2015), but likely it would be less than half.
Welte 2009 is a randomised, double‐blind, parallel‐group, multi‐centre study conducted to compare the efficacy and tolerability of budesonide/formoterol added to tiotropium for 12 weeks. This study included 660 participants with severe or very severe COPD, with a pre‐bronchodilator FEV1 not exceeding 50% of predicted normal value and a history of at least one exacerbation requiring systemic steroids and/or antibiotics in the previous year. Participants were randomised to receive tiotropium 18 mg once daily plus budesonide/formoterol 320/9 mg one inhalation twice daily or placebo twice daily. Measures of efficacy included clinic assessment of lung function and health status (change in pre‐dose FEV1, pre‐dose and post‐dose spirometry measurements and SGRQ for COPD), morning lung function assessments, COPD symptoms and morning activities, use of reliever medication, exacerbations and tolerability. It is likely that most of the participants in this study would be eligible for triple therapy according to current guidance (GOLD 2015).
Economic evaluation analysis
Of the three economic analyses included, two (Mittmann 2011; Nielsen 2013) reported on the economic evaluation conducted alongside the Welte 2009 clinical trial (the CLIMB trial) in six of the nine participant countries; Nielsen 2013 conducted the economic evaluation in four Nordic countries (Sweden, Denmark, Finland, Norway) and Mittmann 2011 in three countries (Canada, Australia, Sweden); both study authors reported on the incremental cost‐effectiveness ratio for exacerbation avoided with tiotropium + LABA/ICS relative to tiotropium from the healthcare system perspective. Najafzadeh 2008 conducted the economic evaluation from the Canadian healthcare system perspective alongside the Aaron 2007 study (OPTIMAL trial) and reported on the incremental cost‐effectiveness ratio per exacerbation avoided and the incremental cost‐effectiveness ratio per quality‐adjusted life‐year (QALY). Investigators calculated the utilities used for the cost‐utility analysis from the results of SGRQ as applied to trial participants.
We have presented detailed characteristics of these economic evaluations in Table 2.
Excluded studies
The initial search carried out in July 2010 revealed eight studies that failed to meet eligibility criteria for the review (see the Characteristics of excluded studies table). Four of these compared tiotropium alone with combination therapy (Ando 2008; Bateman 2008; Golabi 2006; Hara 2007), and one study compared tiotropium with LABA alone (Petroianni 2008). The remaining three studies were shorter than three months in duration (Biscione 2009; Perng 2006), and one used a cross‐over design (Singh 2008).
Searches updated to April 2015 identified seven studies that failed to meet eligibility criteria for the review (see the Characteristics of excluded studies table). Two of these evaluated tiotropium versus placebo (Tashkin 2008; Troosters 2008), and one study compared tiotropium alone versus the LABA/ICS combination (Sarac 2013). One study (Maltais 2013) was shorter than three months, and four were added to Studies awaiting classification and Ongoing studies.
Risk of bias in included studies
We have presented the assessment of risk of bias in the Characteristics of included studies table, and an overview of the findings in Figure 2.
2.
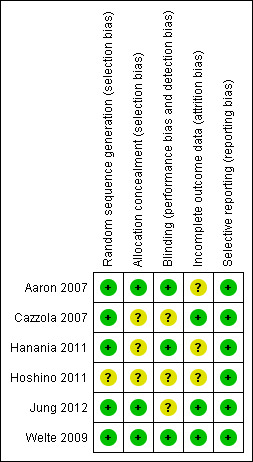
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
We have summarised results of the quality assessment of economic evaluation studies in Table 3. We assessed all included economic evaluations according to their full‐text publications. In general, the economic evaluations included met the methodological and reporting aspects evaluated by the Drummond checklist, and their results can be considered valid (Drummond 1996). In the economic evaluations conducted by Mittmann 2011, Nielsen 2013 and Najafzadeh 2008, discounting was not applied to costs and consequences. However, this was considered to be methodologically correct because the time horizon used in these analyses was three months and one year, respectively, making discounting unnecessary.
Allocation
Aaron 2007, Welte 2009 and Jung 2012 reported adequate sequence generation and allocation concealment. Details for Welte 2009 were supplied on request. For these three studies, randomisation was computer‐generated through central allocation, and both research staff and participants were blinded to the treatment assignment until the end of the study. For Cazzola 2007, Hanania 2011 and Hoshino 2011, sequence generation and/or allocation concealment is unclear because study authors did not report full details and did not respond to personal communication.
Blinding
Aaron 2007, Hanania 2011 and Welte 2009 performed a blinded outcome assessment. In the trial arms of Aaron 2007, inhalers containing placebo and fluticasone/salmeterol were identical in taste and appearance, and they were enclosed in identical tamper‐proof blinding devices. Medication canisters within the blinding devices were stripped of identifying labelling. Clinical data for suspected exacerbations were reviewed by a blinded committee to judge whether data met the study definition of COPD exacerbation. Blinding of participants was not broken for participants who prematurely discontinued treatment with study medications, and the statistician who performed the analysis was initially blinded to participant group assignments. In Hanania 2011, the DISKUS inhalers containing placebo and fluticasone‐salmeterol were identical in taste and appearance. In Welte 2009, treatment assignment was concealed, as active and placebo inhalers were of identical appearance and both clinicians and participants were blinded to treatment until completion of the study. Hoshino 2011 was an open study; however, for evaluation of airway dimensions, a single observer, who was blind to all participant data, measured the outcome. Cazzola 2007 and Jung 2012 did not report details of the outcome assessment; therefore blinding is unclear for these studies.
Incomplete outcome data
Cazzola 2007, Jung 2012 and Welte 2009 reported comparable attrition rates (< 14%) for both intervention and control groups; reasons for attrition were provided in all cases, making the risk of bias low. In Aaron 2007, withdrawal rates were different between intervention groups (74 participants (47%) withdrew from the tiotropium + placebo group, and 37 participants (26%) from the tiotropium + LABA/ICS group); however, mortality data were obtained for all participants, apart from six (2/145 on tiotropium + LABA/ICS and 4/156 on tiotropium + placebo) who withdrew and declined to be involved further in the study; therefore, we rated risk of bias as unclear. Hanania 2011 had high withdrawal rates in both groups (21% in fluticasone/salmeterol (FSC) + tiotropium group and 25% in tiotropium + placebo group); reasons for attrition were provided and were similar among groups; therefore, we rated this study as having an unclear risk of bias. In Hoshino 2011, a total of 36 participants were enrolled in the study, but only 30 were included in the analysis (16 participants on FSC + tiotropium and 14 on tiotropium + placebo); therefore the withdrawal rate was 20% because of loss to follow‐up, making risk of attrition bias unclear.
Selective reporting
All six studies adequately reported outcome data for primary and secondary outcomes that were pre‐specified in the study record.
Effects of interventions
See: Table 1
Because of the small number of eligible studies for the two comparisons (tiotropium + LABA/ICS vs tiotropium alone and vs LABA/ICS alone), no subgroup analysis by disease severity or by type of combination therapy was possible.
Comparison 1. Tiotropium plus LABA/ICS versus tiotropium
We identified six RCTs addressing the comparison of tiotropium + LABA/ICS versus tiotropium + placebo (Aaron 2007; Cazzola 2007; Hanania 2011; Hoshino 2011; Jung 2012; Welte 2009).
Primary outcomes
All‐cause mortality
Two studies (Aaron 2007; Welte 2009) reported mortality at three months and 12 months of follow‐up, respectively. Both studies recruited participants who, on average, were likely to have fulfilled current GOLD criteria for triple therapy (GOLD 2015). These two studies did not find a significant effect on mortality with the use of tiotropium + LABA/ICS compared with tiotropium + placebo. Meta‐analysis of these studies showed a non‐statistically significant trend towards reduced risk of mortality with the use of tiotropium + LABA/ICS (two studies; 961 participants; OR 1.80, 95% CI 0.55 to 5.91; I2 = 0%). The quality of evidence for this outcome is moderate because of imprecision in estimates of effect (Table 1).
Hospital admission (all causes)
The same two studies (Aaron 2007; Welte 2009) reported on all causes of hospital admission at three months and 12 months of follow‐up, respectively. Welte 2009 did not find a significant difference in hospital admissions at three months of follow‐up. Aaron 2007 found a statistically significant reduction in hospital admission at 12 months of follow‐up with the use of tiotropium + LABA/ICS. Meta‐analysis of these studies (Figure 3) showed a non statistically significant trend towards to decreased risk of hospital admission associated with the use of tiotropium + LABA/ICS (two studies; 961 participants; OR 0.84, 95% CI 0.53 to 1.33; I2 = 0%); the quality of evidence for this outcome is low because of the risk of bias in included studies and imprecision of the estimate (Table 1).
3.
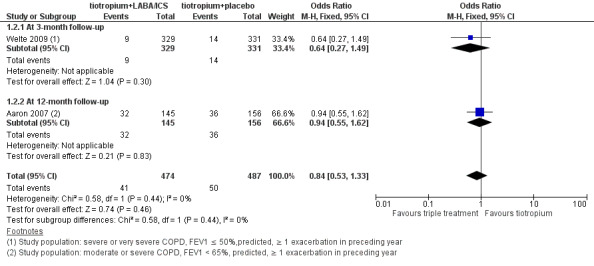
Forest plot of comparison: 1 Tiotropium + LABA/ICS combination versus tiotropium + placebo, outcome: 1.2 Hospital admission (all causes).
Exacerbations
Three studies (Aaron 2007; Jung 2012; Welte 2009) reported on exacerbations at three, six and 12 months of follow‐up, respectively. Welte 2009 found a significant difference in exacerbations at three months of follow‐up with the use of combined therapy tiotropium + LABA/ICS (one study; 660 participants; OR 0.36, 95% CI 0.22 to 0.60). Jung 2012 did not find a significant difference in exacerbations at six months of follow‐up (one study; 479 participants; OR 0.83, 95%CI 0.52 to 1.34). Aaron 2007 did not find a statistically significant reduction in exacerbations at 12 months of follow‐up with the use of combined therapy tiotropium + LABA/ICS (one study; 301 participants; OR 0.89, 95% CI 0.56 to 1.41). We did not pool study results because statistical heterogeneity across studies was considerable. Of note, Jung 2012 may have recruited a population of participants with less severe COPD, not all of whom would be recommended triple therapy according to current guidelines. The quality of evidence for this outcome is low because of the risk of bias and imprecision in estimates of effect (Table 1).
Quality of life
Four studies (Aaron 2007; Hoshino 2011; Jung 2012; Welte 2009) reported on quality of life using the SGRQ (Meguro 2007) at six months. The meta‐analysis of these studies showed a statistically significant difference in quality of life (SGRQ total score) in favour of combined therapy of tiotropium + LABA/ICS compared with tiotropium + placebo (Figure 4) (four studies; 1446 participants; MD ‐3.46, 95% CI ‐5.05 to ‐1.87; I2 = 16%). Only one study reported on the percentage of participants who were responders to treatment. Welte 2009 reported the percentage of participants with improvement in SGRQ score greater than four units, which was significantly higher in the tiotropium + LABA/ICS group (49.5%) than in the tiotropium + placebo group (40.0%) (P value = 0.016). The percentage of participants who showed deterioration in SGRQ score greater than four units was similar in the two groups (tiotropium + LABA/ICS 27.6%, tiotropium + placebo 29.7%).
4.
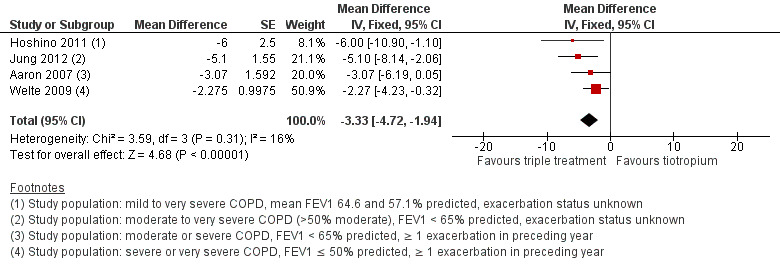
Forest plot of comparison: 1 Tiotropium + LABA/ICS combination versus tiotropium + placebo, outcome: 1.4 Quality of life up to 6 months (SGRQ).
The sensitivity analysis excluding two open‐label studies, which also included participants with less severe COPD (Hoshino 2011; Jung 2012), revealed no changes in the direction of treatment effect (two studies; 961 participants; MD ‐2.5, 95% CI ‐4.16 to ‐0.84; I2 = 0%).
The quality of evidence for this outcome is low because of very serious risk of bias in the trial design (Table 1).
Exercise tolerance
None of the included studies reported exercise tolerance as an outcome.
Serious adverse events non‐fatal (all reported)
Four studies (Aaron 2007; Hanania 2011; Jung 2012; Welte 2009) reported on serious adverse events (non‐fatal). Aaron 2007 reported no differences in serious adverse events between intervention groups; a total of 19 serious adverse events not related to COPD (respiratory failure, cancer and myocardial infarction or acute arrhythmia) were reported in both intervention groups, and one case of pneumonia in the combined therapy group. Jung 2012 reported no differences in serious adverse event rates between trial arms; the event most commonly reported in the combined therapy group was productive cough, whereas dyspnoea was the most common event in the tiotropium group. Two cases of pneumonia were reported in each intervention group. Hanania 2011 reported no statistically significant differences in serious adverse event rates between therapy groups; two cases of pneumonia were reported in the combined therapy group, and nobody in the tiotropium presented with pneumonia. In Welte 2009, six cases of pneumonia were reported in each trial arm as serious adverse events, representing < 1% of the total adverse events reported by the trial.
Meta‐analysis for all non‐fatal serious adverse events reported in these studies showed no statistically significant differences (four studies; 1758 participants; OR 0.86, 95% CI 0.57 to 1.30; I2 = 9%) (Figure 5). The sensitivity analysis excluding the open‐label study (Jung 2012) revealed no differences in the treatment effect estimation (three studies; 1303 participants; OR 0.67, 95% CI 0.4 to 1.13; I2 = 0%). Exclusion of both Hanania 2011 and Jung 2012 from the meta‐analysis on the basis of the less severe population recruited widened the CIs but had little impact on size and direction of the effect.
5.
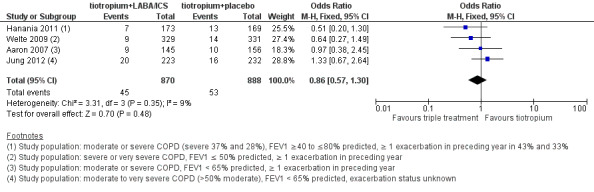
Forest plot of comparison: 1 Tiotropium + LABA/ICS combination versus tiotropium + placebo, outcome: 1.7 Serious adverse events all reported (non‐fatal).
The quality of evidence for this outcome is low because of risk of bias and imprecision in estimates of effect (Table 1).
Independent meta‐analysis for pneumonia including four studies (Aaron 2007; Hanania 2011; Jung 2012; Welte 2009) revealed no statistically significant differences in effects on pneumonia between treatments (four studies; 1758 participants; Peto OR 1.62, 95% CI 0.54 to 4.82; I2 = 0%).
Secondary outcomes
Symptoms
Welte 2009 was the only included study that reported changes in COPD symptom scores for breathlessness (MD ‐0.142, 95% CI ‐0.214 to ‐0.069), night awakening (MD ‐0.157, 95% CI ‐0.222 to ‐0.092), chest tightness (MD ‐0.142, 95% CI ‐0.212 to ‐0.072) and cough (MD ‐0.161, 95% CI ‐0.238 to ‐0.084) among 660 participants. Scores for all symptoms favoured the tiotropium + LABA/ICS group compared with the tiotropium + placebo group.
Forced expiratory volume in one second (FEV1)
Six studies (Aaron 2007; Cazzola 2007; Hanania 2011; Hoshino 2011; Jung 2012; Welte 2009) reported mean change in FEV1 at three to six months. These studies found statistically significant changes in FEV1 with the use of tiotropium + LABA/ICS compared with tiotropium + placebo (four studies; 1678 participants; MD 0.06, 95% CI 0.04 to 0.08; I2 = 0%; Figure 6); however, these changes are not clinically significant. The quality of evidence for this outcome is moderate as a result of the risk of bias.
6.
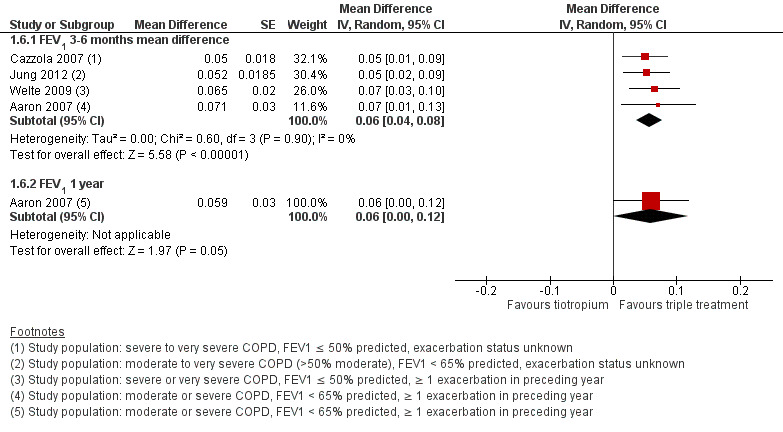
Forest plot of comparison: 1 Tiotropium + LABA/ICS combination versus tiotropium + placebo, outcome: 1.4 FEV1 pre‐dose.
Hoshino 2011 was excluded from the analysis because all data were not available. Hanania 2011 was not included in the pooled estimates of effect because this study generated significant heterogeneity (I2 > 90%). We considered the statistical heterogeneity to result from differences in baseline risk, as participants in Hanania 2011 had on average a greater degree of dyspnoea (modified Medical Research Council scale (MRCm) > 2), which may have resulted in a greater response to pharmacological management.
Exclusion of Jung 2012 due to concerns about the relevance of the recruited population to current guidelines on triple therapy had a minimal impact on size and direction of the effect estimate (GOLD 2015).
Adverse events (not serious)
Four studies (Aaron 2007; Cazzola 2007; Hanania 2011; Welte 2009) reported adverse events. These studies did not find statistically significant differences with the use of tiotropium + LABA/ICS compared to tiotropium + placebo. Meta‐analysis of these studies did not show a statistically significant difference (four studies; 1363 participants OR 1.16, 95% CI 0.92 to 1.47, I2 = 0%). Removing Hanania 2011, a study in which the participants may not, on average, have fulfilled current GOLD criteria for receiving triple therapy, had little impact on the outcome (GOLD 2015).
Cost‐effectiveness of interventions
In the economic evaluation conducted by Mittmann 2011; Nielsen 2013 Tiotropium+ LABA/ICS was the dominant strategy (less costly and more effective) in three of the countries where it was assessed, in comparison with Tiotropium (Canada, Australia and Norway) (Table 2). In all other countries including Sweden, Finland, Denmark and Norway the ICER per exacerbation avoided was under the established willingness to pay threshold (between 600 to 1000 EUR); therefore the Tiotropium + LABA/ICS therapy resulted in a cost‐effective alternative. Sensitivity analyses in both studies indicated that the variables with the largest effect on the ICER were hospitalisation costs, the incidence of exacerbations and hospital admission‐related costs.
In the study conducted by Najafzadeh 2008 Tiotropium + LABA/ICS showed significantly better quality of life and less hospital admissions than Tiotropium alone, but this improvement in health outcomes was associated with increased costs. The Tiotropium therapy showed the highest probability of being cost‐effective when the "willingness to pay" is CAN $6.000°°; when QALY was used as the effectiveness outcome, treatment with Tiotropium had the highest probability of being the best option compared to Tiotropium + LABA/ICS.
Comparison 2. Tiotropium plus LABA/ICS versus LABA/ICS
Cazzola 2007 was the only eligible study identified that compared tiotropium + LABA/ICS versus LABA/ICS + placebo (60 participants) and recruited those with severe or very severe COPD at baseline, most of whom were likely to have met current GOLD criteria for triple therapy (GOLD 2015). This study reported results for the following outcomes of interest for this review.
Primary outcome
Mortality (all‐cause)
Cazzola 2007 reported zero serious adverse events; therefore, we assumed that no deaths occurred during the study.
Secondary outcomes
Forced expiratory volume in one second (FEV1)
Tiotropium in combination with LABA/ICS improves FEV1 significantly compared with LABA/ICS + placebo (MD 0.05, 95% CI 0.00 to 0.09), but MD and CI were below the minimally clinically important difference of 100 to 140 mL.
Serious adverse events (non‐fatal)
No serious adverse events were reported in either intervention group.
Adverse events
More adverse events were reported in the tiotropium + LABA/ICS group (15/30) than in the tiotropium + placebo group (8/30), but the CI was wide because of the small numbers of participants (OR 2.75, 95% CI 0.93 to 8.10).
Withdrawal
Fewer withdrawals were reported in the tiotropium + LABA/ICS group (1/30) than in the tiotropium + placebo group (4/30), but the number of events was small and was not statistically significant (OR 0.22, 95% CI 0.02 to 2.14).
Discussion
Summary of main results
This systematic review set out to investigate the long‐term (≥ three months) effects of tiotropium in combination with long‐acting beta‐agonists/inhaled corticosteroids (LABA/ICS) compared with either LABA/ICS alone or tiotropium alone for the treatment of chronic obstructive pulmonary disease (COPD). We identified six randomised controlled trials (RCTs). All six studies looked at the effects of combination therapy (tiotropium + LABA/ICS) compared with tiotropium alone, whereas only one of these studies (Cazzola 2007) compared triple therapy versus LABA/ICS alone. Additionally, we included three cost‐effectiveness analyses based on data from two of these studies (Aaron 2007; Welte 2009) for evaluation of economic outcomes.
Tiotropium + LABA/ICS versus tiotropium
Since the first version of this systematic review was published, three clinical trials comparing tiotropium + LABA/ICS versus tiotropium alone have been published (Hanania 2011; Hoshino 2011; Jung 2012). These three trials reported on quality of life; exacerbations, FEV1 and non serious adverse events but do not report on hospital admission and mortality; therefore the evidence for the last two outcomes remain the same that failed to show a statistically significant difference in mortality and hospital admission between tiotropium + LABA/ICS versus tiotropium alone (moderate and low quality of evidence). Participants recruited to the two studies included in the analyses of hospitalisations and mortality (Aaron 2007; Welte 2009) are likely to have been candidates for tiotropium + LABA/ICS therapy according to current guidance GOLD 2015 (i.e. forced expiratory volume in one second (FEV1) < 50% predicted and frequent exacerbations ), suggesting that these findings are clinically applicable.
Even though investigators found statistically significant differences between treatment arms for other important outcomes such as FEV1 and quality of life, these results must be interpreted with caution, as the differences found may not be clinically significant. According to Jones 2005, the minimal clinically important difference (MCID) for FEV1 may vary but is accepted to be within the range of 100 to 140 mL (American Thoracic Society/European Respiratory Society Task Force). In this review, the difference in treatment effect on FEV1 was 60 mL; this difference did not reach the MCID needed to have a beneficial impact on participants' quality of life (Jones 2005). Similarly, the MCID in quality of life scores evaluated with St. George's Respiratory Questionnaire (SGRQ) has been proposed to be four units of improvement; the meta‐analysis for this outcome showed a difference smaller than a four‐unit change that could be reached just as part of a Hawthorne effect. Westwood et al described that in participants with COPD, a Hawthorne effect influences SGRQ scores in COPD trials; typically, this results in improvement of two to three points on the SGRQ with placebo (Westwood 2011). Welte 2009 reported a percentage of participants with improvement in SGRQ score greater than four units, which was significantly higher in the tiotropium + LABA/ICS group (49.5%) than in the tiotropium + placebo group (40.0%) (P value = 0.016). The percentage of participants with a decrease in SGRQ score greater than four units was similar in the two groups (tiotropium + LABA/ICS 27.6%, tiotropium + placebo 29.7%).
Of note, Hoshino 2011 and Jung 2012 contributed data to the quality of life analysis, and Jung 2012 to the FEV1 analysis, but on average, participants included in these studies may not have been candidates for tiotropium + LABA/ICS therapy according to current guidelines (GOLD 2015). However, removing these studies from the analyses had little impact on the size or direction of the effect estimate.
We did not pool data on exacerbations reported in these studies, as we considered that several sources of variation among the studies resulted in important heterogeneity (I2 > 80%) that could not be removed by subgroup analyses based on length of follow‐up (three, six and 12 months) (Aaron 2007; Jung 2012; Welte 2009) nor by the definition of exacerbation used (Aaron 2007 defined exacerbation as worsening of COPD leading to treatment with systemic steroids and/or antibiotics; Jung 2012 cited the definition of Rodriguez‐Roisin: sustained worsening of the patient's condition from the stable state and beyond normal day‐to‐day variations that is acute in onset and necessitates a regular change in medication in a patient with underlying COPD; and Welte 2009 defined an exacerbation as worsening of COPD leading to treatment with systemic steroids and/or hospitalisation/emergency room visits). All these individual studies failed to show significant differences in exacerbations between the two treatment arms at six and 12 months of follow‐up. The study by Welte 2009 showed a significant reduction in the risk of exacerbation at three‐month follow‐up associated with triple therapy in comparison with tiotropium alone. However, this finding may be considered clinically irrelevant because the follow‐up period needed to define the real effect that any COPD treatment could have on exacerbation is 52 months (Cazzola 2008; Miravitlles 2004).
The effect of tiotropium + LABA/ICS combination treatment on mortality remains uncertain because of the small number of events. The difference in serious adverse event rates between intervention groups was not statistically significant. For pneumonia, the number of cases in each study was small compared with the number of withdrawals and the number of participants lost to follow‐up. Withdrawals did not seem to be linked to adverse events but rather to the efficacy of treatment. Even though use of inhaled corticosteroids has been associated with pneumonia, our findings suggest no safety concerns related to use of the tiotropium + LABA/ICS combination in the treatment of patients with COPD when compared with tiotropium alone.
Economic evaluation results show a high probability that tiotropium + LABA/ICS combination treatment could be a cost‐effective alternative in various settings, as it was associated with fewer hospital admissions and better quality of life, which may drive most of the long‐term costs associated with this condition. The two economic evaluations conducted in Canada (Mittmann 2011; Najafzadeh 2008) differ in the final conclusions presented regarding cost‐effectiveness of combined therapy (tiotropium + LABA/ICS); these differences may be explained by differences in willingness to pay thresholds used in the sensitivity analyses, making comparison of the conclusions of these two studies impossible.
LABA/ICS + tiotropium versus LABA/ICS + placebo
The one pilot study (Cazzola 2007; no publications after 2007) that looked at the effect of LABA/ICS + tiotropium versus LABA/ICS + placebo showed significantly greater improvement in FEV1 with tiotropium + LABA/ICS compared with LABA/ICS; however, the mean difference in FEV1 was not clinically significant. All other outcomes of interest were not studied, revealed no events or did not achieve a statistically significant difference.
Overall completeness and applicability of evidence
For the comparison of benefits and risks of treatment with tiotropium + LABA/ICS versus LABA/ICS, we identified just one small eligible study (Cazzola 2007), which did not look at, or report on, any of the primary outcomes specified in this review, except for mortality. Therefore, this review found little applicable evidence for this comparison.
Current international guidance suggests that triple therapy with long‐acting muscarinic agonists (LAMA), LABA and ICS should be reserved for patients who continue to have symptoms despite receiving dual therapy with either LABA/ICS or LABA + LAMA, have an FEV1 < 50% predicted and are at a high risk of experiencing exacerbations (i.e. ≥ two exacerbations in the preceding year) (GOLD 2015). Lack of detailed reporting of baseline characteristics has somewhat limited our ability to assess to what extent the studies included in this review recruited participants who would meet these criteria. This could potentially limit the generalisability of our findings to a clinical setting. Table 4 summarises the available information, and, when relevant, we performed a sensitivity analysis that excluded the study or studies in which investigators raised concerns about the relevance of the recruited population. This did not have a substantial impact on any of the effect estimates. We have also included in the individual analyses footnotes that detail the baseline characteristics of participants included in the analysis.
Quality of the evidence
Methods used for randomisation and outcome assessment in some of the included studies were not clearly described and in some cases explanations were missing, thus presenting a source of potential bias.
The quality of the evidence, according to the GRADE (Grades of Recommendation, Assessment, Development and Evaluation Working Group) framework, was moderate for mortality but low for hospital admissions and other important outcomes such as exacerbations, adverse events and quality of life (GRADE 2013).
Potential biases in the review process
The issue of large and/or uneven numbers of withdrawals, as mentioned above (Quality of the evidence), will, even if addressed, have a high likelihood of introducing selection bias, as no consensus has been reached on how best to handle participants for whom no data are available. The high drop‐off rates observed in these types of studies may have been a consequence of the long‐term follow‐up required to measure effectiveness outcomes.
We analysed available data as specified in the protocol. However, we expanded the review question from the protocol to include the comparison of tiotropium + LABA/ICS versus LABA/ICS + placebo. We also highlighted the percentage of participants with a clinically significant change in health‐related quality of life as reported by study authors, although this was not specified in Measures of treatment effect.
Agreements and disagreements with other studies or reviews
Liu 2014 and Rodrigo 2012 published reviews that evaluated the long‐term efficacy and adverse effects of tiotropium + LABA/ICS treatment compared with tiotropium. Both reviews revealed benefits for lung function, quality of life and exacerbation risk. Our review validates these findings. However, with respect to this last outcome, we believe it is not advisable to combine the results, given that length of follow‐up differed among the studies, that the only study that showed length of follow‐up to 52 weeks is Aaron 2007 and that results should be presented independently, as has been done in the present review. The present review also describes a beneficial impact on hospitalisation risk, another clinically relevant outcome that was not previously considered. Additionally, the current review presents results reflecting the GRADE method, which allows the reader to consider the quality of the evidence for each outcome ‐ a critical piece of information on which to base clinical decisions.
A systematic review looking at LABA/ICS combination treatment compared with placebo has shown that combination treatment significantly reduces mortality and exacerbation rates and improves lung function (Nannini 2013). LABA/ICS also increases the risk of pneumonia compared with placebo. A systematic review comparing tiotropium versus placebo showed that tiotropium treatment was associated with a significant improvement in participants' quality of life and reduced the risk of exacerbations, with a number needed to treat for an additional beneficial outcome (NNTB) of 16 to prevent one exacerbation. Tiotropium also reduced exacerbations leading to hospitalisation when compared with placebo (Karner 2014).
Authors' conclusions
Implications for practice.
This review update includes three additional studies and provides new low quality evidence supporting the finding that tiotropium + LABA/ICS‐based therapy improves the disease‐specific quality of life but is insufficient to support the benefit of tiotropium + LABA/ICS‐based therapy for mortality, hospital admission or exacerbations (moderate and low quality evidence). Compared with use of tiotropium alone, tiotropium + LABA/ICS‐based therapy does not seem to increase undesirable effects nor serious non‐fatal adverse events.
Implications for research.
Randomised controlled trials with complete follow‐up of 12 months are required to reduce uncertainty about the impact that tiotropium in combination with LABA/ICS might have on mortality and exacerbations when used as treatment for patients with COPD.
Feedback
Errors in data entry introduced by update, 8 April 2017
Summary
In this 2016 review, I was surprised to read the authors' conclusions read "we found new moderate‐quality evidence that combined tiotropium + LABA/ICS therapy compared with tiotropium plus placebo decreases hospital admission." The forest plot (Figure 3) shows that two RCTs (Welte 2009 and Aaron 2007) contribute to this analysis: hospital admission (all cause): OR 0.61 [95%CI 0.40, 0.92]. These are the same two RCTs used in the 2011 version of the same review which did not find a reduction in hospital admission (all cause): OR 0.84 [95%CI 0.53, 1.33]. The discrepancy seems to originate from the data input for Aaron 2007 in the 2016 review. The 2016 review reports: 41 events for triple therapy compared to 62 events for tiotropium. The 2011 review reports: 32 events for triple therapy compared to 36 events tiotropium. The 2011 review reports that the data for Aaron 2007 were supplied by the authors, that is, the numbers of people with one or more hospitalization (all cause) was not reported in the Annals of Internal Medicine publication of the trial. Therefore the numbers contributing to the analysis in the 2016 review are total numbers of events, not numbers of people with one or more events.The 2016 review reads "We analysed dichotomous data by using participants as the unit of analysis (rather than events) to avoid counting the same participant more than once". The use of events for this outcome would not be consistent with that analysis plan. I do hope the discrepancies can be clearly addressed.
Reply
We thank Dr O'Sullivan for their feedback and interest in our review.
The data used in our 2016 review update for the analysis of the “Hospital admissions” outcome was that reported by Aaron 2007 in the Annals of Internal Medicine paper. As correctly pointed out, the data used in the 2011 review were unpublished data supplied directly by Aaron to the prior review authors. We have since obtained this data and have corrected the meta‐analysis for “hospital admissions” and the corresponding sections (“Effects of interventions”, SoF table and conclusions) in accordance with these new findings.
Contributors
Cait O'Sullivan (PharmD)Island Health, British Columbia, Canada
I certify that I have no affiliations with or involvement in any organisation or entity with a direct financial interest in the subject matter of my criticisms.
What's new
| Date | Event | Description |
|---|---|---|
| 24 May 2017 | Amended | Feedback and reply added. Data in analys es 1.2 (hospital admisssion) reverted to that shown in the original review. Clarifications made in the text. |
| 24 May 2017 | Feedback has been incorporated | New feedback received |
History
Protocol first published: Issue 6, 2010 Review first published: Issue 3, 2011
| Date | Event | Description |
|---|---|---|
| 10 April 2015 | New citation required and conclusions have changed | Three new studies were added (Hanania 2011; Hoshino 2011; Jung 2012), increasing the number of participants contributing data to the review from 1021 to 1902. In this update, we found that combined therapy tiotropium + LABA/ICS compared with tiotropium plus placebo In this update, we also included a synthesis of economic evidence addressing the same question of interest for this review |
| 10 April 2015 | New search has been performed | This review was updated following a new literature search up to April 2015 |
| 11 April 2013 | Amended | NIHR acknowledgement added |
Acknowledgements
The review authors thanks Liz Stovold, Information Specialist from the Cochrane Airways Group, for running the new searches used to update this review. We also thank Rebecca Normansell, Deputy Co‐ordinating Editor, for her invaluable help.
John White was the Editor for this review and commented critically on the review.
Professors Olga Milena García Morales, María Ximena Rojas‐Reyes and Rodolfo J. Dennis thank Melissa Giraldo Duque, Research Assistant at the Clinical Epidemiology and Biostatistics Department of the Pontificia Universidad Javeriana, who contributed to the risk of bias assessment of included studies and assisted review authors in extracting data from new studies.
The Faculty of Medicine of the Pontificia Universidad Javeriana, Bogotá, Colombia, supported the time dedicated by authors Olga Milena García Morales, María Ximena Rojas‐Reyes and Rodolfo J. Dennis to update this review as part of a collaboration agreement with the Iberoamerican Cochrane Center.
This project was supported by the National Institute for Health Research (NIHR), via Cochrane Infrastructure funding to the Cochrane Airways Group. The views and opinions expressed therein are those of the review authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, the National Health Service (NHS) or the Department of Health.
Appendices
Appendix 1. Sources and search methods for the Cochrane Airways Group Specialised Register (CAGR)
Electronic searches: core databases
| Database | Frequency of search |
| CENTRAL (The Cochrane Library) | Monthly |
| MEDLINE (Ovid) | Weekly |
| EMBASE (Ovid) | Weekly |
| PsycINFO (Ovid) | Monthly |
| CINAHL (EBSCO) | Monthly |
| AMED (EBSCO) | Monthly |
Handsearches: core respiratory conference abstracts
| Conference | Years searched |
| American Academy of Allergy, Asthma and Immunology (AAAAI) | 2001 onwards |
| American Thoracic Society (ATS) | 2001 onwards |
| Asia Pacific Society of Respirology (APSR) | 2004 onwards |
| British Thoracic Society Winter Meeting (BTS) | 2000 onwards |
| Chest Meeting | 2003 onwards |
| European Respiratory Society (ERS) | 1992, 1994, 2000 onwards |
| International Primary Care Respiratory Group Congress (IPCRG) | 2002 onwards |
| Thoracic Society of Australia and New Zealand (TSANZ) | 1999 onwards |
MEDLINE search strategy used to identify trials for the CAGR
COPD search
1. Lung Diseases, Obstructive/
2. exp Pulmonary Disease, Chronic Obstructive/
3. emphysema$.mp.
4. (chronic$ adj3 bronchiti$).mp.
5. (obstruct$ adj3 (pulmonary or lung$ or airway$ or airflow$ or bronch$ or respirat$)).mp.
6. COPD.mp.
7. COAD.mp.
8. COBD.mp.
9. AECB.mp.
10. or/1‐9
Filter to identify RCTs
1. exp "clinical trial [publication type]"/
2. (randomized or randomised).ab,ti.
3. placebo.ab,ti.
4. dt.fs.
5. randomly.ab,ti.
6. trial.ab,ti.
7. groups.ab,ti.
8. or/1‐7
9. Animals/
10. Humans/
11. 9 not (9 and 10)
12. 8 not 11
The MEDLINE strategy and RCT filter are adapted to identify trials in other electronic databases.
Appendix 2. Search strategy to identify relevant trials from the CAGR
#1 MeSH DESCRIPTOR Pulmonary Disease, Chronic Obstructive Explode All
#2 MeSH DESCRIPTOR Bronchitis, Chronic
#3 (obstruct*) near3 (pulmonary or lung* or airway* or airflow* or bronch* or respirat*)
#4 COPD:MISC1
#5 (COPD OR COAD OR COBD):TI,AB,KW
#6 #1 OR #2 OR #3 OR #4 OR #5
#7 tiotropium*
#8 Spiriva
#9 glycopyrronium*
#10 glicopirronio*
#11 Seebri
#12 #7 or #8 or #9 or #10 or #11
#13 budesonide
#14 fluticasone
#15 beclomethasone
#16 mometasone
#17 ciclesonide
#18 steroid* or corticosteroid*
#19 #13 or #14 or #15 or #16 or #17 or #18
#20 *formoterol
#21 salmeterol
#22 indacaterol
#23 olodaterol
#24 beta* NEAR agonist*
#25 #20 or #21 or #22 or #23 or #24
#26 #19 and #25
#27 symbicort
#28 viani
#29 seretide
#30 advair
#31 foster
#32 fostair
#33 inuvair
#34 fostex
#35 kantos
#36 combination*
#37 #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36
#38 #6 and #12 and (#26 or #37)
[In search line #4, MISC1 denotes the field in the record where the reference has been coded for condition, in this case, COPD]
Appendix 3. Results of the search 2010
The initial search identified 101 references. Of these, we identified 24 as potentially relevant, and we obtained full‐text versions for further assessment. Fourteen of these were eligible for inclusion and belonged to three studies (Aaron 2007; Cazzola 2007; Welte 2009) (see Characteristics of included studies table). Peer review identified one further potentially eligible study; this is noted in the Characteristics of studies awaiting classification table (Fang 2008).
Data and analyses
Comparison 1. Tiotropium + LABA/ICS combination versus tiotropium + placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality (all‐cause) | 2 | 961 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.80 [0.55, 5.91] |
| 1.1 At 3‐month follow‐up | 1 | 660 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.03 [0.12, 74.59] |
| 1.2 At 12‐month follow‐up | 1 | 301 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.64 [0.45, 5.93] |
| 2 Hospital admission (all causes) | 2 | 961 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.53, 1.33] |
| 2.1 At 3‐month follow‐up | 1 | 660 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.27, 1.49] |
| 2.2 At 12‐month follow‐up | 1 | 301 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.55, 1.62] |
| 3 Exacerbation | 3 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 At 3‐month follow‐up | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 At 6‐month follow‐up | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 At 12‐month follow‐up | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Quality of life up to 6 months (SGRQ) | 4 | Mean Difference (Fixed, 95% CI) | ‐3.33 [‐4.72, ‐1.94] | |
| 5 Sensitivity analysis ‐ QoL up to 6 months (SGRQ) | 2 | Mean Difference (Random, 95% CI) | ‐2.50 [‐4.16, ‐0.84] | |
| 6 FEV1 pre‐dose | 4 | Mean Difference (Random, 95% CI) | Subtotals only | |
| 6.1 FEV1 3‐6 months mean difference | 4 | Mean Difference (Random, 95% CI) | 0.06 [0.04, 0.08] | |
| 6.2 FEV1 1 year | 1 | Mean Difference (Random, 95% CI) | 0.06 [0.00, 0.12] | |
| 7 Serious adverse events all reported (non‐fatal) | 4 | 1758 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.57, 1.30] |
| 8 Pneumonia | 4 | 1758 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.62 [0.54, 4.82] |
| 9 Sensitivity analysis ‐ SAE all reported (non‐fatal) | 3 | 1303 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.67 [0.40, 1.13] |
| 10 Adverse event | 4 | 1363 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.16 [0.92, 1.47] |
1.1. Analysis.
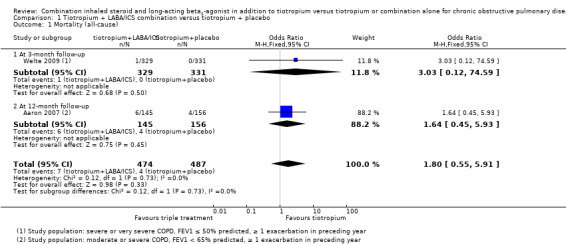
Comparison 1 Tiotropium + LABA/ICS combination versus tiotropium + placebo, Outcome 1 Mortality (all‐cause).
1.2. Analysis.
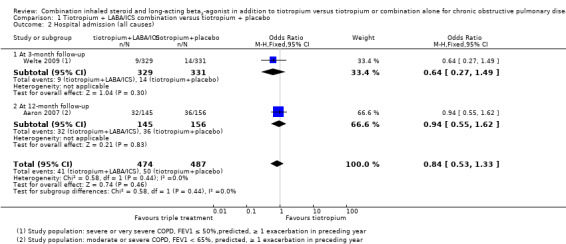
Comparison 1 Tiotropium + LABA/ICS combination versus tiotropium + placebo, Outcome 2 Hospital admission (all causes).
1.3. Analysis.
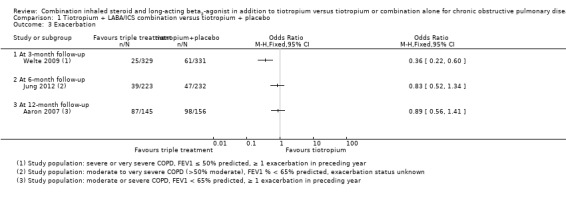
Comparison 1 Tiotropium + LABA/ICS combination versus tiotropium + placebo, Outcome 3 Exacerbation.
1.4. Analysis.
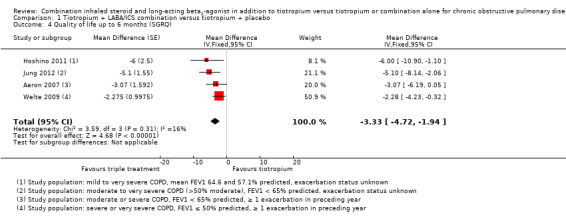
Comparison 1 Tiotropium + LABA/ICS combination versus tiotropium + placebo, Outcome 4 Quality of life up to 6 months (SGRQ).
1.5. Analysis.
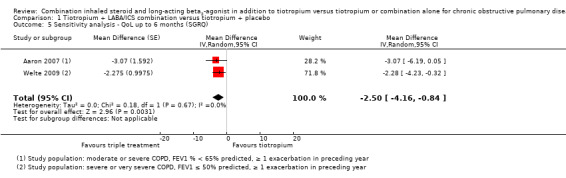
Comparison 1 Tiotropium + LABA/ICS combination versus tiotropium + placebo, Outcome 5 Sensitivity analysis ‐ QoL up to 6 months (SGRQ).
1.6. Analysis.
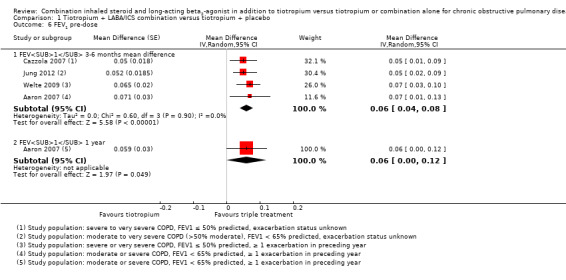
Comparison 1 Tiotropium + LABA/ICS combination versus tiotropium + placebo, Outcome 6 FEV1 pre‐dose.
1.7. Analysis.
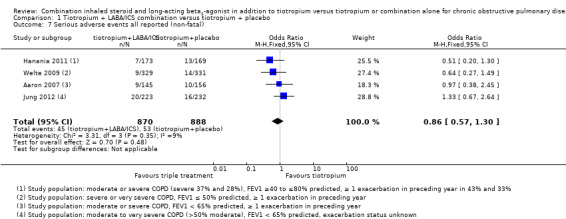
Comparison 1 Tiotropium + LABA/ICS combination versus tiotropium + placebo, Outcome 7 Serious adverse events all reported (non‐fatal).
1.8. Analysis.
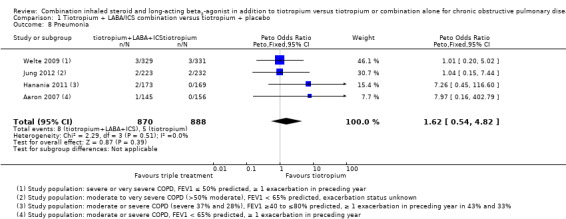
Comparison 1 Tiotropium + LABA/ICS combination versus tiotropium + placebo, Outcome 8 Pneumonia.
1.9. Analysis.
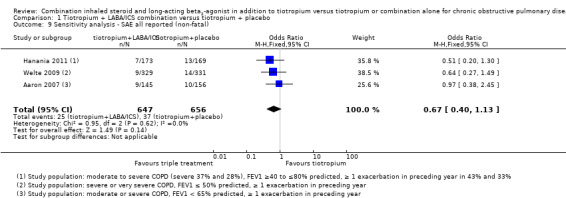
Comparison 1 Tiotropium + LABA/ICS combination versus tiotropium + placebo, Outcome 9 Sensitivity analysis ‐ SAE all reported (non‐fatal).
1.10. Analysis.
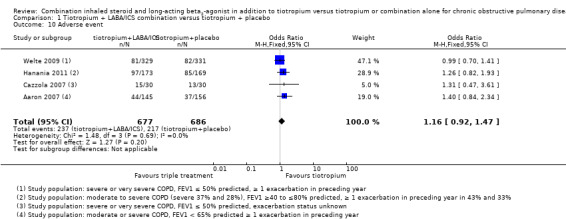
Comparison 1 Tiotropium + LABA/ICS combination versus tiotropium + placebo, Outcome 10 Adverse event.
Comparison 2. Tiotropium + LABA/ICS combination vs LABA/ICS combination + placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 FEV1 GIV | 1 | Mean Difference (Fixed, 95% CI) | Totals not selected | |
| 2 Adverse event | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 FEV1 | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
2.1. Analysis.

Comparison 2 Tiotropium + LABA/ICS combination vs LABA/ICS combination + placebo, Outcome 1 FEV1 GIV.
2.2. Analysis.
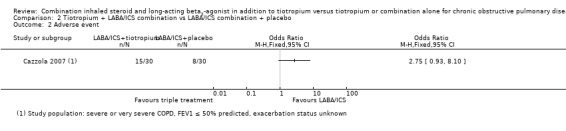
Comparison 2 Tiotropium + LABA/ICS combination vs LABA/ICS combination + placebo, Outcome 2 Adverse event.
2.3. Analysis.
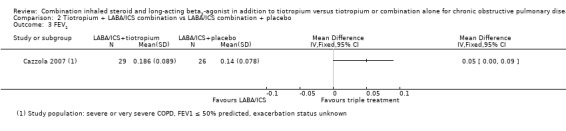
Comparison 2 Tiotropium + LABA/ICS combination vs LABA/ICS combination + placebo, Outcome 3 FEV1.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aaron 2007.
| Methods | Design: a randomised, double‐blind, placebo‐controlled, parallel‐group trial from October 2003 to January 2006. The trial included 27 Canadian medical centres; 20 centres were academic hospital‐based pulmonary clinics, 5 were community‐based pulmonary clinics and 2 were community‐based primary care clinics | |
| Participants |
Population: 449 adults with a clinical history of moderate or severe COPD as defined by ATS and GOLD guidelines Baseline characteristics: mean age 68 years. COPD severity moderate to severe with mean FEV1 predicted of 39%. 44% women Inclusion criteria: at least 1 exacerbation of COPD that required treatment with systemic steroids or antibiotics within the 12 months before randomisation; age older than 35 years; history of 10 or more pack‐years of cigarette smoking; documented chronic airflow obstruction, with an FEV1/FVC ratio < 0.70 and a post‐bronchodilator FEV1 < 65% of predicted value Exclusion criteria: history of physician‐diagnosed asthma before 40 years of age; history of physician‐diagnosed chronic congestive heart failure with known persistent severe left ventricular dysfunction; those receiving oral prednisone; those with a known hypersensitivity or intolerance to tiotropium, salmeterol or fluticasone‐salmeterol; history of severe glaucoma or severe urinary tract obstruction, previous lung transplantation or lung volume reduction surgery or diffuse bilateral bronchiectasis; those who were pregnant or breastfeeding |
|
| Interventions | • Tiotropium + salmeterol + fluticasone: tiotropium (Spiriva, Handihaler (Boehringer Ingelheim Pharma, Ingelheim, Germany)), 18 mcg once daily, plus fluticasone‐salmeterol (Advair (GlaxoSmithKline, Research Triangle Park, North Carolina, USA)), 250/25 mcg/puff, 2 puffs twice daily • Tiotropium + salmeterol: tiotropium, 18 mcg once daily, plus salmeterol (Serevent (GlaxoSmithKline)), 25 mcg/puff, 2 puffs twice daily • Tiotropium + placebo: tiotropium, 18 mcg once daily, plus placebo inhaler, 2 puffs twice daily |
|
| Outcomes |
Primary: proportion of participants with ≥ 1 exacerbation of COPD Secondary: mean number of COPD exacerbations per patient‐year; total number of exacerbations that resulted in urgent visits to a healthcare provider or emergency department; number of hospitalisations for COPD; total number of hospitalisations for all causes; changes in health‐related quality of life, dyspnoea or lung function |
|
| Notes |
Co‐medication: All study participants were provided with inhaled albuterol and were instructed to use it when necessary to relieve symptoms. Any treatment with ICS, LABA and anticholinergics that the patient may have been using before entry was discontinued on entry into the study. Therapy with other respiratory medications, such as oxygen, antileukotrienes and methylxanthines, was continued in all patient groups Funding source: The Canadian Institutes of Health Research and The Ontario Thoracic Society provided peer‐reviewed funding for this study |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done through central allocation of a randomisation schedule that was prepared from a computer‐generated random listing of the 3 treatment allocations in variable blocks of 9 or 12 and stratified by site |
| Allocation concealment (selection bias) | Low risk | Neither research staff nor participants were aware of the treatment assignment before or after randomisation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Metered‐dose inhalers containing placebo, salmeterol and fluticasone‐salmeterol were identical in taste and appearance and were enclosed in identical tamper‐proof blinding devices. Medication canisters within the blinding devices were stripped of all identifying labelling |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The number of people who stopped drug therapy was high, with large variations between groups (74 (47%) tiotropium + placebo and 37 (26%) tiotropium + LABA/ICS comb). However, the number of people who did not complete the trial was smaller, although large variations between groups were evident (30 (19%) tiotropium + placebo and 15 (10%) tiotropium + LABA/ICS comb). The issue of incomplete data was addressed by sensitivity analyses of the data comprising alternative assumptions for participants who prematurely withdrew from treatment |
| Selective reporting (reporting bias) | Low risk | Results for all listed primary and secondary outcomes were reported |
Cazzola 2007.
| Methods | Design: a randomised, double‐blind, double‐dummy, parallel‐group trial over 12‐weeks | |
| Participants |
Population: 90 participants with well‐controlled COPD Baseline characteristics: mean age 66 years. Severe to very severe COPD with mean FEV1 predicted of 38%. 11% women Inclusion criteria: baseline FEV1 < 50% predicted and post‐bronchodilator FEV1/FVC < 70% following salbutamol 400 mcg according to the GOLD criteria of severity Exclusion criteria: current evidence of asthma as primary diagnosis; unstable respiratory disease requiring oral/parenteral corticosteroids within 4 weeks before the beginning of the study; upper or lower respiratory tract infection within 4 weeks of the screening visit; unstable angina or unstable arrhythmias; concurrent use of medications that affected COPD; evidence of alcohol abuse |
|
| Interventions | • LABA/ICS comb + placebo: FSC 500/50 mcg Diskus, 1 inhalation twice daily + placebo Handihaler 1 inhalation once daily • Tiotropium + placebo: tiotropium 18 mcg Handihaler, 1 inhalation once daily + placebo Diskus, 1 inhalation twice daily • Tiotropium + LABA/ICS comb: FSC 500/50 mcg Diskus, 1 inhalation twice daily + tiotropium 18 mcg Handihaler, 1 inhalation once daily |
|
| Outcomes | Mean change from baseline in pre‐dose FEV1 after 3‐month treatment, change from baseline in VAS score assessing dyspnoea and in supplemental salbutamol | |
| Notes | Run‐in: Participants entered a 2‐week run‐in period during which their regular treatment for COPD (all were receiving regular treatment with a LABA and an ICS, many (81 out of 90) with theophylline also) was stopped, with the exception of stable regimens of theophylline (no change in dose for 1 month before screening), and they received salbutamol for relief of breakthrough symptoms. Use of all other inhaled or oral bronchodilators, systemic corticosteroids, ipratropium bromide, oxitropium bromide or leukotriene modifiers was prohibited | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Participants were randomised to receive FSC, tiotropium or their combination by a computer‐generated list. Randomisation was performed in blocks of 9 |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropout rate 10% |
| Selective reporting (reporting bias) | Low risk | Results for all listed outcomes were reported |
Hanania 2011.
| Methods | Design: a randomised, double‐blind, parallel‐group, multi‐centre study over 24‐weeks. The trial included 33 centres in the USA | |
| Participants |
Population: 342 adults with a clinical history of moderate to severe COPD as defined by ATS and ERS guidelines Baseline characteristics: mean age 61 years. Moderate to severe COPD with mean FEV1 predicted of 56% Inclusion criteria: age ≥ 40 years; diagnosis of COPD according to ATS‐ERS criteria; history of 10 or more pack‐years of cigarette smoking; post‐albuterol FEV1 > 40 to < 80% of predicted normal and post‐albuterol FEV1/FVC ratio < 0.70 according to NHANES III reference values Exclusion criteria: clinical diagnosis of respiratory disorder other than COPD; long‐term oxygen; BMI > 40 kg/m2; clinically significant and uncontrolled medical disorder; lung resection surgery within the past year; inability to give informed consent |
|
| Interventions | • Tiotropium 18 mcg once daily via HandiHaler + fluticasone/salmeterol 250/50 mcg via DISKUS (FSC; Advair, Seretide, GlaxoSmithKline, Research Triangle Park, North Carolina, USA) twice daily • Tiotropium 18 mcg once daily + placebo DISKUS twice daily |
|
| Outcomes |
Primary: AM pre‐dose FEV1 Secondary: 2hours post‐dose FEV1; AM pre‐dose FVC; 2 hours post‐dose FVC; AM pre‐dose IC; domain scores on the CRQ‐SAS; rescue albuterol use and healthcare utilisation for COPD exacerbations |
|
| Notes |
Co‐medication: All study participants were provided with inhaled albuterol and were instructed to use it when necessary to relieve symptoms. Use of concurrent inhaled long‐acting bronchodilators (beta2‐agonist and anticholinergic), ipratropium/albuterol combination products, oral beta2‐agonists, ICS and OCS and theophylline preparations was not allowed during the treatment period Funding source: Hanania has received research grant support and honoraria for serving as a consultant and on the speaker bureau of GlaxoSmithKline; Niewoehner has received advisory or consulting fees from Boehringer Ingelheim, AstraZeneca, GlaxoSmithKline, Forest Research, Novartis, Merck, Nycomed, Sanofi Aventis, Sepracor and Bayer Schering |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | After 4‐week treatment with open‐label tiotropium 18 mcg once daily, participants were randomised in a double‐blind fashion to either the addition of FSC 250/50 DISKUS twice daily or matching placebo |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) All outcomes | Low risk | The DISKUS inhalers containing placebo and fluticasone‐salmeterol were identical in taste and appearance |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Drop‐out rate 23%. FSC + tiotropium 137/173 (79%) and tiotropium + placebo 127/169 (75%) |
| Selective reporting (reporting bias) | Low risk | Results for all listed primary and secondary outcomes were reported |
Hoshino 2011.
| Methods | Design: a randomised, open‐label, parallel‐group study over 12 weeks | |
| Participants |
Population: 30 adults with COPD with post‐bronchodilator FEV1/FVC < 0.7 Baseline characteristics: mean age 73 years; the proportions of participants at each disease stage, according to the GOLD criteria, were as follows: stage I, 10%; stage II, 33.3%; stage III, 36.6%; stage IV, 20%. Proportions of men and women were 14/0 (Tiotropium) and 14/2 (SFC + tiotropium) Inclusion criteria: participants with COPD confirmed on the basis of spirometry (post‐bronchodilator FEV1/FVC < 0.7), smoking history of > 10 pack‐years and no history of asthma or atopy as defined by a positive skin prick test to one or more common allergens. Participants were newly diagnosed with COPD or had not previously used tiotropium, OCS or ICS or LABA Exclusion criteria: use of supplemental oxygen and respiratory infection or COPD exacerbation in the 12 weeks before commencement of the study |
|
| Interventions | • Tiotropium 18 mcg (Boehringer Ingelheim Pharma, Ingelheim, Germany) once daily • SFC 50/250 mcg (GlaxoSmithKline, London, UK) twice daily, in combination with Tio, 18 mcg once daily |
|
| Outcomes |
Primary: analysis of airway dimensions Secondary: mean change in FVC , FEV1, IC, FCR, RV/TLC and DLCO/VA after 3‐months of treatment; change on the SGRQ |
|
| Notes | Participants entered a 2‐week washout period before the start of the study, during which all current COPD medications were discontinued. Use of additional bronchodilators was not permitted throughout the study period, except for SABA as required | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was done in a 1:1 ratio. No details about the method were provided |
| Allocation concealment (selection bias) | Unclear risk | No details |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Open, but assessor(s) were blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Drop‐out rate 20%. A total of 36 participants were enrolled in the study, but 6 participants were withdrawn because of lack of follow‐up |
| Selective reporting (reporting bias) | Low risk | Results for all listed primary and secondary outcomes were reported |
Jung 2012.
| Methods | Design: a randomised, open‐label, multi‐centre, 2‐arm, parallel study from April 2009 to March 2010. The trial was conducted at 30 academic hospital‐based pulmonary clinics in Korea | |
| Participants |
Population: 479 participants with COPD Baseline characteristics: mean age 67 years. Moderate to very severe COPD with mean FEV1 predicted of 50.8%. 98% men Inclusion criteria: participants diagnosed with COPD who had a post‐bronchodilator FEV1/FVC ratio < 0.70 and FEV1 < 65% of predicted value in the past 1 year or at screening. Eligible participants were 40 to 80 years of age and had a smoking history of 10 or more pack‐years Exclusion criteria: a history of physician‐diagnosed asthma or a chronic respiratory disorder other than COPD that was clinically significant; any uncontrollable or serious disease that might affect participation in the study; use of systemic corticosteroids or immunosuppressants within 4 weeks before study entry; any malignant disease; a history of severe glaucoma, urinary tract obstruction or previous lung volume reduction surgery; women who were pregnant or lactating; known hypersensitivity or intolerance to tiotropium or FSC |
|
| Interventions | • Tiotropium (Spiriva HandiHaler (Boehringer Ingelheim Pharma, Ingelheim, Germany)), 18 mcg once daily • Tiotropium 18 mcg once daily + FSC (Seretide Diskus (GlaxoSmithKline, Brentford, UK)), 250/50 mcg/puff, 1 puff twice daily |
|
| Outcomes |
Primary: change in pre‐bronchodilator FEV1 (L) from baseline to week 24 Secondary: mean changes in pre‐bronchodilator FEV1 (L) from baseline to weeks 4, 8 and 16; mean changes in pre‐bronchodilator inspiratory capacity (IC); FVC and percent predicted (% pred) values for FEV1; mean changes in HRQoL; frequency of COPD exacerbations; exacerbations requiring hospitalisations, emergency room visits or outpatient clinic visits; hospitalisation rates for all causes |
|
| Notes |
Co‐medication: All participants were provided with a salbutamol inhalation aerosol and were instructed to use it when necessary to relieve symptoms. Before the run‐in period, participants stopped their usage of ICA and long‐acting bronchodilators, but therapy with other regular medications such as oxygen, mucolytics and methylxanthines was allowed throughout the study for all participants This study was supported by a grant from the Korea Healthcare Technology R&D Project, Ministry for Health and Welfare, Republic of Korea (A102065), and from GlaxoSmithKline Korea |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done in a 1:1 ratio through a computerised random‐number generator |
| Allocation concealment (selection bias) | Low risk | Neither research staff nor participants were aware of treatment assignment until randomised |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | No details |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐out rates were 14% in the tiotropium + placebo group and 13% in the tiotropium + LABA/ICS comb group |
| Selective reporting (reporting bias) | Low risk | Results were reported for all listed primary and secondary outcomes |
Welte 2009.
| Methods | Design: a randomised, double‐blind, parallel‐group, multi‐centre trial from May 2007 to June 2008. The trial included 102 centres in 9 countries: Australia (10 centres), Canada (16), France (12), Germany (12), Hungary (13), Poland (10), Slovakia (13), Spain (6) and Sweden (10) | |
| Participants |
Population: 660 participants with COPD eligible for LABA/ICS combination therapy, with pre‐bronchodilator FEV1 not exceeding 50% of predicted normal value and a history of exacerbations requiring systemic steroids and/or antibiotics Baseline characteristics: mean age 62 years. Moderate, severe or very severe COPD with mean FEV1 predicted of 38%. 25% women Inclusion criteria: participants with COPD eligible for LABA/ICS combination therapy ≥ 40 years of age, with a clinical diagnosis of COPD and symptoms for ≥ 2 years; ≥ 1 COPD exacerbation in the previous 12 months requiring systemic steroids and/or antibiotics; current or previous smokers with a smoking history of ≥ 10 pack‐years; forced expiratory volume in 1 second (FEV1) ≤ 50% of predicted normal value and FEV1 /FVC < 70% pre‐dose Exclusion criteria: worsening of COPD during run‐in or within 4‐weeks before visit 2 requiring hospitalisation; a course of OCS and/or ICS and/or antibiotics; use of ICS within 2 weeks before visit 2; use of oral/parenteral glucocorticosteroids within 4 weeks before visit 2; a history of asthma or any significant disease/disorder that, in the opinion of the investigator, may put the patient at risk or might influence results |
|
| Interventions | • Tiotropium + LABA/ICS comb: tiotropium (Handihaler) 18 mcg once daily + budesonide/formoterol (Symbicort Turbuhaler; AstraZeneca, Lund, Sweden) 320/9 mcg 1 inhalation twice daily • Tiotropium + placebo: tiotropium 18 mcg once daily + placebo (identical Turbuhaler) twice daily |
|
| Outcomes |
Primary: change in pre‐dose FEV1 from randomisation (week 0) to full treatment period (mean FEV1 at 1, 6 and 12 weeks of treatment) Secondary: pre‐dose and post‐dose spirometry measurements (pre‐dose FVC and inspiratory capacity and post‐treatment FEV1 (5 and 60 min), FVC (5 and 60 min) and inspiratory capacity (60 min)) and SGRQ |
|
| Notes |
Run‐in: Before entering the study, participants stopped their LABA and ICS medications (4 weeks and 2 weeks before run‐in, respectively). During the 2‐week run‐in period, all participants used tiotropium (Spiriva HandiHaler, Boehringer Ingelheim Pharma, Ingelheim, Germany) 18 mcg once daily. Terbutaline 0.5 mg/inhalation (Bricanyl Turbuhaler, AstraZeneca, Lund, Sweden) was used as needed for symptom relief during the run‐in period Co‐medication: Terbutaline 0.5 mg/inhalation (Bricanyl Turbuhaler, AstraZeneca, Lund, Sweden) was used as needed for symptom relief during the treatment period in both treatment arms |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation codes were sequentially assigned to participants from a computer‐generated list at AstraZeneca R&D, Lund, Sweden, as they became eligible |
| Allocation concealment (selection bias) | Low risk | Investigators were provided with a blinded randomisation code for each participant. Both clinicians and participants were blinded to treatment until completion of the study |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Treatment assignment was concealed, as active and placebo Turbuhalers were of identical appearance |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Drop‐out rates were 9% in the tiotropium + placebo group and 8% in the tiotropium + LABA/ICS comb group |
| Selective reporting (reporting bias) | Low risk | All collected data were reported |
ATS: American Thoracic Society
BMI: body mass index
CRQ‐SAS: Chronic Respiratory Disease Questionnaire‐Self‐Administered Standardized
COPD: chronic obstructive pulmonary disease
DLCO/VA: diffusing capacity of the lungs for carbon monoxide/alveolar volume
ERS: European Respiratory Society
FCR: functional residual capacity
FEV1: forced expiratory volume in one second
FSC: fluticasone/salmeterol
FVC: forced vital capacity
GOLD: Global Initiative for Chronic Obstructive Lung Disease
HRQoL: health‐related quality of life
IC: inspiratory capacity
ICS: inhaled corticosteroids
LABA: long‐acting beta2‐agonists
NHANES: National Health and Nutrition Examination Survey
OCS: oral corticosteroids
RV/TCL: residual volume/total lung capacity
SABA: short‐acting beta2‐agonists
SGRQ: St. George's Respiratory Questionnaire
Tio: tiotropium
VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Ando 2008 | Evaluated the effects of tiotropium alone vs LABA/ICS combination |
| Bateman 2008 | Evaluated the effects of tiotropium alone vs LABA/ICS combination |
| Biscione 2009 | Treatment period: 4 weeks |
| Golabi 2006 | Evaluated the effects of tiotropium alone vs LABA/ICS combination |
| Hara 2007 | Evaluated the effects of tiotropium alone vs LABA/ICS combination |
| Maltais 2013 | Time of follow up was less than 8 weeks; treatment period for triple therapy was just 4 weeks |
| Perng 2006 | Treatment period: 4 weeks |
| Petroianni 2008 | Evaluated effects of tiotropium alone vs formoterol alone |
| Sarac 2013 | Assessed for effects of tiotropium alone vs LABA/ICS combination |
| Singh 2008 | 14 days of treatment and of cross‐over design |
| Tashkin 2008 | Evaluated tiotropium vs placebo; co‐treatment allowed |
| Troosters 2008 | Evaluated tiotropium vs placebo; co‐treatment allowed |
ICS: inhaled corticosteroids
LABA: long‐acting beta‐agonists
Characteristics of studies awaiting assessment [ordered by study ID]
Fang 2008.
| Methods | Design: randomised, parallel‐group, 12 months of treatment |
| Participants | 126 participants (M/F: 92/34) with COPD |
| Interventions | Salmeterol/fluticasone (50/250 mcg) twice daily and tiotropium 18 mcg once daily (n = 33, M/F: 23/10) Salmeterol/fluticasone (50/250 mcg) twice daily (n = 32, M/F: 24/8) Tiotropium 18 mcg once daily (n = 32, M/F: 23/9) Blank control group (n = 29, M/F: 22/7): Participants in this group did not receive inhaled anticholinergic drugs, LABA or glucocorticoid therapy |
| Outcomes | Symptoms, health status, use of rescue medication, frequency of exacerbations, FEV1 |
| Notes |
Lee 2014.
| Methods | Design: multi‐centre, randomised, parallel‐group, open‐label study |
| Participants | 578 participants with COPD. Mean age: 67 years, 96% male |
| Interventions | Following a 14‐day run‐in period during which participants received tiotropium 18 mcg once daily, participants were randomised to BUD/FORM 160/4.5 mcg 2 inhalations twice daily + tiotropium 18 mcg once daily (BUD/FORM+T), or tiotropium alone (18 mcg once daily), for 12 weeks |
| Outcomes |
Primary endpoint: ratio of treatment period mean to baseline in pre‐dose FEV1 Secondary outcomes: post‐dose FEV1, pre‐dose FVC, post‐dose IC, pre‐dose PEF, use of reliever medication, change in COPD symptoms, COPD exacerbations |
| Notes |
BUD: budesonide
COPD: chronic obstructive pulmonary disease
FEV1: forced expiratory volume in one second
FORM: formoterol
FVC: forced vital capacity
IC: inspiratory capacity
LABA: long‐acting beta‐agonists
PEF: peak expiratory flow
T: tiotropium
Characteristics of ongoing studies [ordered by study ID]
Betsuyaku 2013.
| Trial name or title | Evaluating the Control of COPD Symptoms in Patients Treated With Tiotropium Bromide 18 mcg Once Daily Alone, ADOAIR 50/250 mcg Twice Daily Alone or ADOAIR 50/250 mcg Plus Tiotropium Bromide 18 mcg |
| Methods | Design: multi‐centre, randomised, parallel‐ group study; 24 weeks of treatment |
| Participants | Participants will be 40–80 years of age with an established clinical history of COPD as defined by the GOLD guidelines, with a current or former smoking history of > 10 pack‐years, post‐bronchodilator FEV1 > 30% to < 80% of predicted normal value, post‐bronchodilator FEV1/FVC ratio < 70% and grade ≥ 1 on the MRCm scale |
| Interventions | • Salmeterol/fluticasone propionate (SFC) 50/250 mcg twice daily delivered via the Diskus • Tiotropium bromide 18 mcg delivered once daily via the Handihaler inhalation device • Salmeterol/fluticasone propionate (SFC) 50/250 mcg twice daily delivered via the Diskus + tiotropium bromide 18 mcg delivered once daily via the Handihaler inhalation device |
| Outcomes |
Primary: proportion of participants able to remain on the randomised therapy Secondary: • Proportion of participants who switched to triple therapy • Proportion of participants controlled by triple therapy • Proportion of participants controlled by randomised therapy + triple therapy • Time to switch to triple therapy • Time to first exacerbation • Proportion of exacerbations confirmed by EXACT • Proportion of exacerbations detected by EXACT not diagnosed • CAT score change • Change in FEV1 |
| Starting date | February 2013 |
| Contact information | GSKClinicalSupportHD@gsk.com |
| Notes | ClinicalTrials.gov register NCT01762800 |
Cohuet 2013.
| Trial name or title | A Study to Compare the Effect of Inhaled Treatments: The Combination of 3 Components (Beclometasone/Formoterol/Glycopyrrolate) to a Known Single Treatment (Tiotropium) or the Double Combination of Tiotropium (Spiriva) and Beclometasone plus Formoterol in Participants With COPD Treated for One Year |
| Methods | Design: randomised, double‐blind, double‐dummy, parallel‐group study; 52‐weeks of treatment |
| Participants | Participants > 40 years of age with a diagnosis of COPD (according to GOLD guidelines, updated February 2013) ≥ 12 months before the screening visit. Current smokers or ex‐smokers who quit smoking ≥ 6 months before screening visit, with a smoking history of ≥ 10 pack‐years (pack‐years = (number of cigarettes per day × number of years)/20) Post‐bronchodilator FEV1 < 50% of predicted normal value and post‐bronchodilator FEV1/FVC ratio < 0.7 within 30 min after 4 puffs (4 × 100 mcg) of salbutamol pMDI |
| Interventions | • Beclometasone dipropionate + formoterol fumarate + glycopyrrolate bromide administered via pMDI • Tiotropium bromide • Beclometasone dipropionate + formoterol fumarate administered via pMDI and tiotropium bromide |
| Outcomes |
Primary: moderate and severe COPD exacerbation rate Secondary: Change from baseline in pre‐dose morning FEV1 COPD exacerbation (moderate or severe, rate and time to first) FEV1 response (change from baseline in pre‐dose morning FEV1 ≥ 100 mL) SGRQ score (change from baseline in total/domain scores) Use of rescue medication PK analysis |
| Starting date | November 2013 |
| Contact information | g.cohuet@chiesi.com |
| Notes | EudraCT number: 2013‐000063‐91 |
COPD: chronic obstructive pulmonary disease
EXACT: Emboshield and Xact Post Approval Carotid Stent Trial
FEV1: forced expiratory volume in one second
FVC: forced vital capacity
GOLD: Global Initiative for Chronic Obstructive Lung Disease
mMRC: modified Medical Research Council
pMDI: pressurised metered‐dose inhaler
SFC: salmeterol/fluticaso ne
SGRQ: St. George’s Respiratory Questionnaire
Differences between protocol and review
We included the comparison of treatment with tiotropium + LABA/ICS versus LABA/ICS. We narratively reported the percentage of participants with a clinically significant change in health‐related quality of life as reported by study authors, although this was not specified in Measures of treatment effect.
In this update, we included a synthesis of economic evidence addressing the same question and performed sensitivity analyses that excluded studies that may have recruited large numbers of participants who would not be candidates for tiotropium + LABA/ICS according to current guidance.
Contributions of authors
Charlotta Karner and Chris Cates wrote the first version of this review. Olga Milena García Morales, María Ximena Rojas‐Reyes and Rodolfo J. Dennis drafted the update protocol and conducted the updating process. Olga Milena García Morales and Rodolfo J. Dennis conducted the screening search and data extraction; María Ximena Rojas‐Reyes and Rodolfo J. Dennis conducted the screening search and extracted data from economic articles. The three review authors interpreted findings and wrote the review conclusions.
Charlotta Karner reviewed the document of the updated review, submitted it for editorial approval and accepted the final publication.
Sources of support
Internal sources
-
The Department of Clinical Epidemiology and Biostatistics of the Faculty of Medicine of the Pontificia Universidad Javeriana, Bogotá, Colombia.
Supported the time dedicated by the review authors, Olga Milena García, María Ximena Rojas and Rodolfo Dennis, to update this review at the Cochrane Collaboration Center
External sources
-
NIHR, UK.
National Institute for Health Research supported this work through funding for both authors who worked on the previous version of this review
Declarations of interest
Dr. OM Garcia has received financial support to attend scientific meetings from pharmaceutical companies which manufacture tiotropium preparations. The remaining three authors were not aware of any conflict of interest that should be declared covering the past three years.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Aaron 2007 {published data only}
- Aaron SD, Vandemheen K, Ferguson D, FitzGerald M, Maltais F, Boureau J, et al. The Canadian optimal therapy of COPD trial: design, organization and patient recruitment. Canadian Respiratory Journal 2004;11(8):581‐5. [DOI] [PubMed] [Google Scholar]
- Aaron SD, Vandemheen KL, Fergusson D, Maltais F, Bourbeau J, Goldstein R, et al. Tiotropium in combination with placebo, salmeterol, or fluticasone‐salmeterol for treatment of chronic obstructive pulmonary disease: a randomized trial. Annals of Internal Medicine 2007;146(8):545‐55. [DOI] [PubMed] [Google Scholar]
- Kaplan A. Effects of tiotropium combined with either salmeterol or salmeterol/fluticasone in moderate to severe COPD. Primary Care Respiratory Journal 2007;16(4):258‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Najafzadeh M, Marra CA, Sadatsafavi M, Aaron SD, Sullivan SD, Vandemheen KL, et al. Cost effectiveness of therapy with combinations of long acting bronchodilators and inhaled steroids for treatment of COPD. Thorax 2008;63(11):962‐7. [DOI] [PubMed] [Google Scholar]
- Roisman G. Tiotropium in combination with placebo, salmeterol, or fluticasone‐salmeterol for treatment of chronic obstructive pulmonary disease. A randomized trial. Revue de Pneumologie Clinique 2007;63(6):390‐1. [DOI] [PubMed] [Google Scholar]
Cazzola 2007 {published data only}
- Cazzola M, Ando F, Santus P, Ruggeri P, Marco F, Sanduzzi A, et al. A pilot study to assess the effects of combining fluticasone propionate/salmeterol and tiotropium on the airflow obstruction of patients with severe‐to‐very severe COPD. Pulmonary Pharmacology and Therapeutics 2007;20(5):556‐61. [DOI] [PubMed] [Google Scholar]
- D'Amato M, Ando F, Santus P, Ruggeri P, Marco F, Cazzola M. Clinical effects of adding fluticasone propionate/salmeterol (FSC) and tiotropium (TIO) in severe‐to‐very severe COPD [Abstract]. European Respiratory Journal 2005;26(Suppl 49):218. [Google Scholar]
Hanania 2011 {published data only}
- Hanania NA, Crater GD, Morris AN, Emmett AH, O'Dell DM, Niewoehner DE. Benefits of adding fluticasone propionate/salmeterol to tiotropium in moderate to severe COPD. Respiratory Medicine 2012;106(1):91‐101. [PUBMED: PMID: 22040533.] [DOI] [PubMed] [Google Scholar]
Hoshino 2011 {published data only}
- Hoshino M, Ohtawa J. Effects of adding salmeterol/fluticasone propionate to tiotropium on airway dimensions in patients with chronic obstructive pulmonary disease. Respirology 2011;16(1):95–101. [DOI: 10.1111/j.1440-1843.2010.01869.x] [DOI] [PubMed] [Google Scholar]
Jung 2012 {published data only}
- Jung KS, Park HY, Park SY, Kim SK, Kim YK, Shim JJ, et al. Comparison of tiotropium plus fluticasone propionate/salmeterol with tiotropium in COPD: a randomized controlled study. Respiratory Medicine 2012;106(3):382‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Welte 2009 {published data only}
- Mittmann N, Hernandez P. Cost effectiveness of budesonide/formoterol added to tiotropium bromide versus placebo added to tiotropium bromide in patients with chronic obstructive pulmonary disease: Australian, Canadian and Swedish Healthcare Perspectives. Pharmacoeconomics 2011;29(5):403‐14. [PUBMED: 21504240] [DOI] [PubMed] [Google Scholar]
- Nielsen R, Kankaanranta H, Bjermer L. Cost effectiveness of adding budesonide/formoterol to tiotropium in COPD in four Nordic countries. Respiratory Medicine 2013;107(11):1709‐21. [DOI: ] [DOI] [PubMed] [Google Scholar]
- Welte T, Hartman L, Polanowski T, Hernandez P. Budesonide/formoterol added to tiotropium is well tolerated and reduces risk of severe exacerbations in COPD patients [Abstract]. American Thoracic Society International Conference; 2009 May 15‐20 San Diego. 2009:A6188 [Poster #215].
- Welte T, Miravitlles M, Hernandez P, Eriksson G, Peterson S, Polanowski T, et al. Addition of budesonide/formoterol to tiotropium reduces the number of exacerbation days compared with tiotropium alone. Chest 2009;136(4):26S‐f. [Google Scholar]
- Welte T, Miravitlles M, Hernandez P, Eriksson G, Peterson S, Polanowski T, et al. Budesonide/formoterol added to tiotropium provides rapid improvements in lung function and ability to undertake morning activities. Chest 2009;136(4):24S‐g. [Google Scholar]
- Welte T, Miravitlles M, Hernandez P, Eriksson G, Peterson S, Polanowski T, et al. Efficacy and tolerability of budesonide/formoterol added to tiotropium in patients with chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine 2009;180(8):741‐50. [DOI] [PubMed] [Google Scholar]
- Welte T, Miravitlles M, Hernandez P, Hartman L, Polanowski T, Kessler R. Budesonide/formoterol added to tiotropium improves lung function, health status, symptoms & morning activities in COPD patients [Abstract]. European Respiratory Society Annual Congress; Sep 12‐16; Vienna. 2009:[P2005].
- Welte T, Miravitlles M, Hernandez P, Peterson S, Polanowski T, Kessler R. Budesonide/formoterol added to tiotropium improves exacerbations and exacerbation‐related antibiotic use in patients with COPD [Abstract]. European Respiratory Society Annual Congress; September 12‐16; Vienna. 2009:[P2012].
- Welte T, Miravitlles M, Peterson S, Polanowski T, Kessler R. Budesonide/formoterol added to tiotropium improves the management of COPD patients [Abstract]. American Thoracic Society International Conference; 2009 May 15‐20; San Diego. 2009:A6192 [Poster #216].
References to studies excluded from this review
Ando 2008 {published data only}
- Ando F, Ruggeri P, Girbino G, Cazzola M. Tiotropium and salmeterol/fluticasone combination do not cause oxygen desaturation in COPD. Respiratory Medicine 2008;102(6):815‐8. [DOI] [PubMed] [Google Scholar]
Bateman 2008 {published data only}
- Bateman E, Van‐Dyk M, Chang A. A pilot study comparing tiotropium to salmeterol plus fluticasone in moderate COPD [Abstract]. European Respiratory Journal 2005;26(Suppl 49):Abstract No. 851. [Google Scholar]
- Bateman ED, Dyk M, Sagriotis A. Comparable spirometric efficacy of tiotropium compared with salmeterol plus fluticasone in patients with COPD: a pilot study. Pulmonary Pharmacology and Therapeutics 2008; Vol. 21, issue 1:20‐5. [DOI] [PubMed]
Biscione 2009 {published data only}
- Biscione G, Crigna G, Auciello L, Pasqua F, Cazzola M. Addition of tiotropium (T) to a regular treatment with long‐acting beta‐agonist + inhaled corticosteroid (LABA + ICS) in patients with severe to very‐severe COPD under in‐patient pulmonary rehabilitation program (PRP) [Abstract]. European Respiratory Society Annual Congress; 2009 Sep 12‐16; Vienna. 2009:[P526].
Golabi 2006 {published data only}
- Golabi P, Topaloglu N, Karakurt S, Celikel T. Effects of tiotropium and salmeterol/fluticasone combination on lung hyperinflation dyspnea and exercise tolerance in COPD [Abstract]. European Respiratory Journal 2006; Vol. 28, issue Suppl 50:33s [E304].
Hara 2007 {published data only}
- Hara K, Kurashima K, Tokunaga D, Ueno M, Aoyagi K, Isobe Z, et al. Single blind comparison of tiotropium and salmeterol plus fluticasone propionate of treatment in patients with chronic obstructive pulmonary disease (COPD) [Abstract]. American Thoracic Society International Conference; 2007 May 18‐23; San Francisco. 2007:Poster #A1.
Maltais 2013 {published data only}
- Maltais F, Mahler D. Effect of fluticasone propionate/salmeterol plus tiotropium versus tiotropium on walking endurance in COPD. European Respiratory Journal 2013;42:539–41. [DOI: 10.1183/09031936.00074113] [DOI] [PubMed] [Google Scholar]
Perng 2006 {published data only}
- Perng DW, Wu CC, Su KC, Lee YC, Perng RP, Tao CW. Additive benefits of tiotropium in COPD patients treated with long‐acting beta2 agonists and corticosteroids. Respirology 2006;11(5):598‐602. [DOI] [PubMed] [Google Scholar]
Petroianni 2008 {published data only}
- Petroianni A, Ceccarelli D, Conti V, Graziani E, Terzano C. Evening administration of tiotropium during combination therapy reduces night symptoms in COPD patients [Abstract]. European Respiratory Society 18th Annual Congress; 2008 Oct 3‐7; Berlin 2008:[E4282].
Sarac 2013 {published data only}
- Sarac P. Comparison of the efficacy and safety of long‐acting anticholinergic and a combination of inhaled steroids and long‐acting beta‐2 agonist in moderate chronic obstructive pulmonary disease. European Respiratory Journal 2013;42(Suppl 57):P4143. [Google Scholar]
Singh 2008 {published data only}
- Singh D, Brooks J, Hagan G, Cahn A, O'Connor BJ. Superiority of "triple" therapy with salmeterol/fluticasone propionate and tiotropium bromide versus individual components in moderate to severe COPD. Thorax 2008;63(7):592‐8. [DOI] [PubMed] [Google Scholar]
- Singh D, Hagan G, Cahn A, Leonard TB, Riley JH, O'Connor BJ. Individual and combined responses to salmeterol/fluticasone propionate combination (SFC) and tiotropium (Tio) shown in a COPD clinical trial [Abstract]. American Thoracic Society International Conference; 2008 May 16‐21; Toronto. 2008:A648[#F10].
Tashkin 2008 {published data only}
- Tashkin D. Impact of tiotropium on the course of moderate‐to‐very severe chronic obstructive pulmonary disease: the UPLIFT® trial. Expert Review of Respiratory Medicine 2010;4(3):279–89. [DOI] [PubMed] [Google Scholar]
Troosters 2008 {published data only}
- Troosters T, Celli B, Kesten S. Effectiveness of combination therapy with tiotropium in COPD. A secondary analysis of the UPLIFT trial. Primary Care Respiratory Journal 2010;19(2):A13. [Google Scholar]
References to studies awaiting assessment
Fang 2008 {published data only}
- Fang L, Liang X, Zhang F, Liu L, Fu W, Zhao Z, et al. Combination of inhaled salmeterol/fluticasone and tiotropium in the treatment of chronic obstructive pulmonary disease: a randomised controlled trial. Zhonghua Jiehe He Huxi Zazhi [Chinese Journal of Tuberculosis and Respiratory Diseases] 2008;31(11):811‐4. [PubMed] [Google Scholar]
Lee 2014 {published and unpublished data}
References to ongoing studies
Betsuyaku 2013 {published data only}
- Betsuyaku T, Kato M. A study to assess COPD symptom‐based management and to optimise treatment strategy in Japan (COSMOS‐J) based on GOLD 2011. International Journal of Chronic Obstructive Pulmonary Disease 2013;8:453‐9. [DOI: 10.2147/COPD.S48298] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohuet 2013 {published data only}
- Cohuet Geraldine. A study to compare the effect of inhaled treatments: the combination of 3 components (beclometasone/formoterol/glycopyrrolate) to a known single treatment (tiotropium) or the double combination of tiotropium (Spiriva) and beclometasone plus formoterol in patients with chronic obstructive pulmonary disease treated for one year.. The European Union Clinical Trials Register 2013‐10‐11. [EudraCT Number:2013‐000063‐91]
Additional references
Appleton 2006
- Appleton S, Jones T, Poole P, Pilotto L, Adams R, Lasserson TJ, et al. Ipratropium bromide versus long‐acting beta‐2 agonists for stable chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD006101] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barr 2005
- Barr RG, Bourbeau J, Camargo CA. Tiotropium for stable chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD002876.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Beeh 2010
- Beeh KM, Beier J. The short, the long and the "ultra‐long": why duration of bronchodilator action matters in chronic obstructive pulmonary disease. Advances in Therapy 2010;27(3):150‐9. [DOI] [PubMed] [Google Scholar]
Brichetto 2003
- Brichetto L, Song P, Crimi E. Modulation of cholinergic responsiveness through the [beta]‐adrenoceptor signal transmission pathway in bovine trachealis. Journal of Applied Physiology 2003;95(2):735‐41. [DOI] [PubMed] [Google Scholar]
Cazzola 2008
- Cazzola M, MacNee W, Martinez FJ, Rabe KF, Franciosi LG, Barnes PJ, et al. American Thoracic Society; European Respiratory Society Task Force on outcomes of COPD. Outcomes for COPD pharmacological trials: from lung function to biomarkers. European Respiratory Journal 2008;31(2):416–69. [DOI: 10.1183/09031936.00099306] [DOI] [PubMed] [Google Scholar]
Cazzola 2010
- Cazzola M, Molimard M. The scientific rationale for combining long‐acting b2‐agonists and muscarinic antagonists in COPD. Pulmonary Pharmacology & Therapeutics 2010;23(4):257‐67. [DOI: 10.1016/j.pupt.2010.03.003] [DOI] [PubMed] [Google Scholar]
Drummond 1996
- Drummond MF, Jefferson TO. Guidelines for authors and peer reviewers of economic submissions to the BMJ. BMJ 1996;313:275‐83. [DOI: 10.1136/bmj.313.7052.275] [DOI] [PMC free article] [PubMed] [Google Scholar]
Effing 2007
- Effing T, Monninkhof EEM, Valk PP, Zielhuis GGA, Walters EH, Palen JJ, et al. Self‐management education for patients with chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD002990.pub2] [DOI] [PubMed] [Google Scholar]
GOLD 2015
- From the Global Strategy for the Diagnosis, Management and Prevention of COPD, Global Initiative for Chronic Obstructive Lung Disease (GOLD). http://www.goldcopd.org/ (accessed 11 June 2015).
GRADE 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A. Handbook for grading the quality of evidence and the strength of recommendations using the GRADE approach. http://www.guidelinedevelopment.org/handbook/#h.buaodtl66dyx (accessed 11 June 2015).
Guyatt 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction—GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383–94. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. www.cochrane‐handbook.org (accessed 11 June 2015).
Hutchinson 2010
- Hutchinson A, Brand C, Irving L, Roberts C, Thompson P, Campbell D. Acute care costs of patients admitted for management of chronic obstructive pulmonary disease exacerbations: contribution of disease severity, infection and chronic heart failure. Internal Medical Journal 2010;40(5):364‐71. [1445‐5994: (Electronic)] [DOI] [PubMed] [Google Scholar]
Jones 2005
- Jones PW. St. George’s Respiratory Questionnaire: MCID. COPD 2005;2:75‐9. [DOI] [PubMed] [Google Scholar]
Karner 2014
- Karner C, Chong J, Poole P. Tiotropium versus placebo for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2014, Issue 7. [DOI: 10.1002/14651858.CD009285.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lacasse 2006
- Lacasse Y, Goldstein R, Lasserson TJ, Martin S. Pulmonary rehabilitation for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2006, Issue 4. [DOI: 10.1002/14651858.CD003793.pub2] [DOI] [PubMed] [Google Scholar]
Liu 2014
- Liu Y, Shi H, Sun X. Benefits of adding fluticasone propionate/salmeterol to tiotropium in COPD: a meta‐analysis. European Journal of Internal Medicine 2014;25(5):491‐5. [DOI: 10.1016/j.ejim.2014.04.007] [DOI] [PubMed] [Google Scholar]
Meguro 2007
Miravitlles 2004
- Miravitlles M, Ferrer M, Pont A, Zalacain R, Alvarez‐Sala JL, Masa F. Effect of exacerbations on quality of life in patients with chronic obstructive pulmonary disease: a 2 year follow up study. Thorax 2004;59(5):387–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mittmann 2011
- Mittmann N, Hernandez P. Cost effectiveness of budesonide/formoterol added to tiotropium bromide versus placebo added to tiotropium bromide in patients with chronic obstructive pulmonary disease: Australian, Canadian and Swedish Healthcare Perspectives. Pharmacoeconomics 2011;29(5):403‐14. [PUBMED: 21504240] [DOI] [PubMed] [Google Scholar]
Najafzadeh 2008
- Najafzadeh M, Marra CA, Sadatsafavi M, Aaron SD, Sullivan SD, Vandemheen KL, et al. Cost effectiveness of therapy with combinations of long acting bronchodilators and inhaled steroids for treatment of COPD. Thorax 2008;63(11):962‐7. [DOI] [PubMed] [Google Scholar]
Nannini 2007a
- Nannini L, Cates CJ, Lasserson TJ, Poole P. Combined corticosteroid and long‐acting beta‐agonist in one inhaler versus placebo for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD003794.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nannini 2007b
- Nannini LJ, Cates CJ, Lasserson TJ, Poole P. Combined corticosteroid and long‐acting beta‐agonist in one inhaler versus long‐acting beta‐agonists for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD003794.pub2] [DOI] [PubMed] [Google Scholar]
Nannini 2013
- Nannini LJ, Poole P, Milan SJ, Holmes R, Normansell R. Combined corticosteroid and long‐acting beta2‐agonist in one inhaler versus placebo for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2013, Issue 11. [DOI: 10.1002/14651858.CD003794.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Nielsen 2013
- Nielsen R, Kankaanranta H, Bjermer L. Cost effectiveness of adding budesonide/formoterol to tiotropium in COPD in four Nordic countries. Respiratory Medicine 2013;107(11):1709‐21. [DOI: ] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Copenhagen, The Nordic Cochrane Centre: The Cochrane Collaboration. Review Manager (RevMan). Version 5.3.5. Copenhagen, The Nordic Cochrane Centre: The Cochrane Collaboration, 2014.
Rodrigo 2012
- Rodrigo GJ, Plaza V, Castro‐Rodríguez JA. Comparison of three combined pharmacological approaches with tiotropium monotherapy in stable moderate to severe COPD: a systematic review. Pulmonary Pharmacology & Therapeutics 2012;25(1):40‐7. [DOI: 10.1016/j.pupt.2011.10.006] [DOI] [PubMed] [Google Scholar]
Sehatzadeh 2012
- Sehatzadeh S. Influenza and pneumococcal vaccinations for patients with chronic obstructive pulmonary disease (COPD): an evidence‐based analysis. Ontario Health Technology Assessment Series 2012;12(3):1‐64. [PUBMED: 3384373] [PMC free article] [PubMed] [Google Scholar]
Welsh 2010
- Welsh EJ, Cates CJ, Poole P. Combination inhaled steroid and long‐acting beta2‐agonist versus tiotropium for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2010, Issue 5. [DOI: 10.1002/14651858.CD007891.pub2] [DOI] [PubMed] [Google Scholar]
Westwood 2011
- Westwood M, Bourbeau J, Jones PW, Cerulli A, Capkun‐Niggli G, Worthy G. Relationship between FEV1 change and patient‐reported outcomes in randomised trials of inhaled bronchodilators for stable COPD: a systematic review. Respiratory Research 2011;12:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
Yang 2012
- Yang IA, Clarke MS, Sim EHA, Fong KM. Inhaled corticosteroids for stable chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2012, Issue 7. [DOI: 10.1002/14651858.CD002991.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Karner 2011
- Karner C, Cates CJ. Combination inhaled steroid and long‐acting beta2‐agonist in addition to tiotropium versus tiotropium or combination alone for chronic obstructive pulmonary disease. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD008532.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]


