Abstract
Importance
Human genetic studies have indicated that plasma lipoprotein(a) (Lp[a]) is causally associated with the risk of coronary heart disease (CHD), but randomized trials of several therapies that reduce Lp(a) levels by 25% to 35% have not provided any evidence that lowering Lp(a) level reduces CHD risk.
Objective
To estimate the magnitude of the change in plasma Lp(a) levels needed to have the same evidence of an association with CHD risk as a 38.67-mg/dL (ie, 1-mmol/L) change in low-density lipoprotein cholesterol (LDL-C) level, a change that has been shown to produce a clinically meaningful reduction in the risk of CHD.
Design, Setting, and Participants
A mendelian randomization analysis was conducted using individual participant data from 5 studies and with external validation using summarized data from 48 studies. Population-based prospective cohort and case-control studies featured 20 793 individuals with CHD and 27 540 controls with individual participant data, whereas summarized data included 62 240 patients with CHD and 127 299 controls. Data were analyzed from November 2016 to March 2018.
Exposures
Genetic LPA score and plasma Lp(a) mass concentration.
Main Outcomes and Measures
Coronary heart disease.
Results
Of the included study participants, 53% were men, all were of white European ancestry, and the mean age was 57.5 years. The association of genetically predicted Lp(a) with CHD risk was linearly proportional to the absolute change in Lp(a) concentration. A 10-mg/dL lower genetically predicted Lp(a) concentration was associated with a 5.8% lower CHD risk (odds ratio [OR], 0.942; 95% CI, 0.933-0.951; P = 3 × 10−37), whereas a 10-mg/dL lower genetically predicted LDL-C level estimated using an LDL-C genetic score was associated with a 14.5% lower CHD risk (OR, 0.855; 95% CI, 0.818-0.893; P = 2 × 10−12). Thus, a 101.5-mg/dL change (95% CI, 71.0-137.0) in Lp(a) concentration had the same association with CHD risk as a 38.67-mg/dL change in LDL-C level. The association of genetically predicted Lp(a) concentration with CHD risk appeared to be independent of changes in LDL-C level owing to genetic variants that mimic the relationship of statins, PCSK9 inhibitors, and ezetimibe with CHD risk.
Conclusions and Relevance
The clinical benefit of lowering Lp(a) is likely to be proportional to the absolute reduction in Lp(a) concentration. Large absolute reductions in Lp(a) of approximately 100 mg/dL may be required to produce a clinically meaningful reduction in the risk of CHD similar in magnitude to what can be achieved by lowering LDL-C level by 38.67 mg/dL (ie, 1 mmol/L).
Apolipoprotein(a), which is encoded by the LPA gene, co-valently binds to a cholesterol-rich low-density lipo-protein (LDL) particle to form lipoprotein(a) (Lp[a]).1 Meta-analyses of prospective observational studies have reported that higher plasma Lp(a) concentration is associated with dose-dependent higher risk of coronary heart disease (CHD).2 Furthermore, mendelian randomization analyses have provided strong evidence that the association between Lp(a) and risk of CHD is likely to be causal.3–5 However, several large randomized trials evaluating therapies that lower Lp(a) concentration by between 20% and 35% (including niacin, cholesterol ester transfer protein inhibitors, and PCSK9 inhibitors) have not provided clear evidence that lowering plasma Lp(a) concentration reduces the risk of cardiovascular events beyond that which would be expected from the observed LDL-lowering effect of these therapies.6–11 Although these trials were not specifically designed to assess the Lp(a)-lowering effect of these agents, these trials raise the question of how much Lp(a) concentration must be lowered to produce a clinically meaningful reduction in cardiovascular events. Therapies that more specifically and potently lower Lp(a) concentrations by up to 90% by inhibiting apolipoprotein(a) synthesis are in development.12 Whether lowering Lp(a) concentrations with these new therapies will reduce the risk of cardiovascular events is unknown.
Owing to the skewed distribution of plasma Lp(a) concentration, prior studies have reported the association between log-transformed concentrations of Lp(a) and CHD risk.2,13–15 Changes in log-transformed Lp(a) concentrations represent proportional changes in Lp(a) concentrations. However, proportional reduction is not a useful metric for assessing the potential clinical benefit of lowering Lp(a) level because concentrations can vary by as much as 1000-fold among members of the same population, and therefore, the same proportional change in Lp(a) concentration can result in markedly different absolute changes, depending on the initial Lp(a) concentration.16
Importantly, statins and other therapies that reduce LDL particle concentrations are associated with a dose-dependent reduction in the risk of cardiovascular events that is determined by the absolute (rather than the proportional) change in LDL cholesterol (LDL-C) level.17–19 Because Lp(a) contains an LDL particle, we hypothesized that there would be evidence to support a clinical association of Lp(a) with the risk of CHD that may also be proportional to the absolute change in circulating Lp(a) mass concentration. To test this hypothesis, we created a genetic score to estimate the magnitude and shape of the relationship of Lp(a) with the risk of CHD. We then estimated the absolute change in plasma Lp(a) concentration required to achieve the equivalent change in CHD risk as a 38.67-mg/dL (ie, 1-mmol/L) change in LDL-C level (to convert to millimoles per liter, multiply by 0.0259), a change in LDL-C that has been demonstrated to produce a clinically meaningful 20% to 25% reduction in the risk of cardiovascular events in short-term trials.17–19 Our objective was to make inferences about how much Lp(a) concentration must be reduced pharmacologically to produce a clinically meaningful reduction in CHD risk and thereby determine who is most likely to benefit from treatment with Lp(a)-lowering therapy to inform clinical guidelines and the design of randomized trials evaluating Lp(a)-lowering therapies.
Methods
Study Population and Outcomes
We studied 48 333 participants of European descent (including 20 793 with CHD) from 5 studies for whom individual participant–level data were available as part of the CHD Exome+ Consortium. Descriptions of the included studies are provided in eMethods 1 of the Supplement. The primary outcome was CHD, defined as CHD death, nonfatal myocardial infarction, or (for 3 of the studies) other coronary events with International Statistical Classification of Diseases and Related Health Problems, Tenth Revision codes I20-25. Participants provided written informed consent for genetic studies. As this was an analysis of anonymized data that had already been collected, ethical approval was not sought for this particular investigation.
LPA Genetic Score
All CHD Exome+ Consortium participants were genotyped using a customized version of the Illumina Exome Beadchip array, which included ultrafine mapping of the LPA gene region involving 2426 variants genotyped within a 660-kb window (eFigure 1 in the Supplement). To select variants for inclusion in the genetic score, we identified variants in the LPA gene region that were conditionally associated with Lp(a) concentrations at a genome-wide level of significance (P < 5 × 10−8) using forward stepwise regression among participants free from CHD at baseline in each study. We adjusted for study, age, sex, and 5 principal components of ancestry. Genetic variants correlated with a selected variant at r2 greater than 0.4 were excluded from further steps of the procedure (eFigure 2 in the Supplement). For each participant, we calculated a weighted genetic score by summing the number of Lp(a)-raising alleles inherited at each variant included in the score, weighted by each variant’s association with absolute change in Lp(a) mass concentration (measured in milligrams per deciliter). In sensitivity analyses, we repeated the primary analyses using different choices of variants in the genetic scores, as described in eMethods 2 in the Supplement.
Study Design
To assess the dose-response shape of the association between genetically predicted Lp(a) and CHD risk, we divided participants into deciles of the genetic score and measured the association between each decile of genetically predicted Lp(a) concentration and the risk of CHD using the first decile as the reference group. Informed by the shape of the association, we estimated the association between the LPA score and the risk of CHD for absolute changes in Lp(a) concentration.
To estimate the absolute reduction in Lp(a) concentration required to have the same change in CHD risk as a 38.67-mg/dL decrease in LDL-C level, we used the following protocol (eFigure 3 in the Supplement). First, we measured the association between the LPA score and the risk of CHD per 10-mg/dL decrease in genetically predicted Lp(a) concentration. Next, we measured the association between a genetic score consisting of variants in or near genes that encode the targets of currently available LDL-C–lowering therapies and CHD risk per 10-mg/dL decrease in genetically predicted LDL-C (eTable 1 in the Supplement).20 We then calculated the ratio between these 2 estimates to obtain the change in Lp(a) concentration that has an equivalent association with CHD risk as a 1-mg/dL change in LDL-C level. To estimate the amount Lp(a) concentration must be reduced to have the same association with CHD risk as a 38.67-mg/dL reduction in LDL-C level, we multiplied this ratio by 38.67. Finally, we estimated the predicted short-term change associated with different magnitudes of pharma-cological lowering of Lp(a) concentration by converting the change in Lp(a) concentration into a change in LDL-C level having an equivalent predicted effect on CHD risk and using the estimated change associated with statin treatment per 38.67-mg/dL reduction in LDL-C level, as reported by the Cholesterol Treatment Trialists’ Collaboration.17
Statistical Analyses
We estimated the association of each variant with Lp(a) or LDL-C concentration using linear regression and with CHD risk using logistic regression. All regression analyses were performed separately in each of the studies, adjusting for age, sex, and the first 5 principal components of ancestry; these estimates were combined across studies in a fixed-effects inverse variance-weighted meta-analysis. Heterogeneity was assessed using the I2 statistic. Mendelian randomization estimates were then obtained from these variant-specific estimates using a previously reported method that accounts for correlation between variants.21 Nonlinearity in the mendelian randomization estimates of the shape of the association of Lp(a) change with the risk of CHD was assessed using fractional polynomials, as described elsewhere.22 For external replication in an independent sample, we performed the same analyses using summarized genetic associations with CHD risk from the Coronary Artery Disease Genome Wide Replication and Meta-analysis (CARDIOGRAM) plus The Coronary Artery Disease (C4D) Genetics (CARDIOGRAMplusC4D) consortium in up to 62 240 patients and 127 299 controls.23
All analyses were performed using the statistical software platform R version 3.4.1 (R Programming). A detailed description of the methods is provided in eMethods 2 of the Supplement.
Results
Participant Characteristics
The baseline characteristics of participants are presented in Table 1. Across the 5 studies contributing to the initial sample, the median Lp(a) concentration varied from 13.6 mg/dL to 43.3 mg/dL (eTable 2 and eFigure 4 in the Supplement).
Table 1. Baseline Characteristics of Participants.
| Source | No | Patients With CHD No | Lp(a) Measured No.a | Age, Mean (SD), y | Men, No | Lp(a) Concentration, mg/dL | |
|---|---|---|---|---|---|---|---|
| Mean (SD) | Median | ||||||
| CCHS | 7808 | 1943 | 7396 | 58 (15) | 3463 | 29.3 (33.6) | 16.9 |
| CGPS-CIHDS | 17 120 | 7740 | 9964 | 59 (13) | 9635 | 25.0 (28.1) | 13.6 |
| EPIC-CVD | 20 780 | 9810 | 15 899 | 55 (10) | 9792 | 52.6 (37.1) | 43.3 |
| PROSPER | 1279 | 641 | 0 | 76 (4) | 708 | NA | NA |
| WOSCOPS | 1346 | 659 | 1017 | 56 (6) | 1346 | 36.0 (39.3) | 19.0 |
Abbreviations: CCHS, Copenhagen City Heart study; CGPS, Copenhagen General Population study; CHD, coronary heart disease; CIHDS, Copenhagen Ischemic Heart Disease study; EPIC-CVD, European Prospective Investigation Into Cancer and Nutrition-Cardiovascular Disease study; Lp(a), lipoprotein (a); PROSPER, Prospective Study of Pravastatin in the Elderly at Risk study; WOSCOPS, West of Scotland Coronary Prevention study.
Values of Lp(a) concentration were winsorized at 130 mg/dL.
LPA Genetic Score
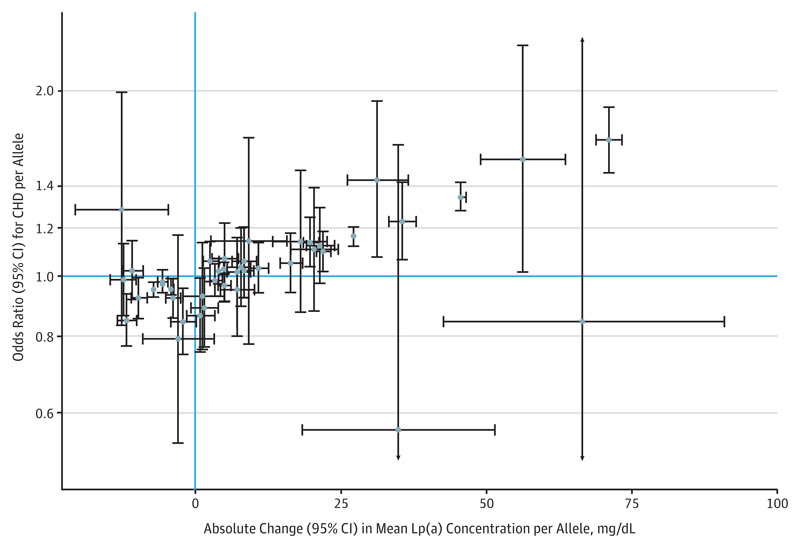
The stepwise selection procedure identified 43 genetic variants conditionally associated with Lp(a) (eTable 3 in the Supplement). The genetic score comprising these variants explained 51% to 63% of the variance in Lp(a) concentration in each study (eFigure 5 in the Supplement). This explanatory ability is lower than observed previously24 because our genetic score was constructed conservatively to minimize bias owing to overfitting. Associations of each variant with Lp(a) concentration and CHD risk are displayed in Figure 1.
Figure 1. Association of LPA Variants With Lipoprotein(a) (Lp[a]) Concentration and Coronary Heart Disease (CHD) Risk.
Marginal genetic associations with Lp(a) and CHD risk (error bars indicate 95% confidence intervals) obtained in the CHD Exome+ consortium for 43 variants included in the LPA genetic score. Associations are orientated to the minor allele.
Association of LPA Genetic Score With CHD
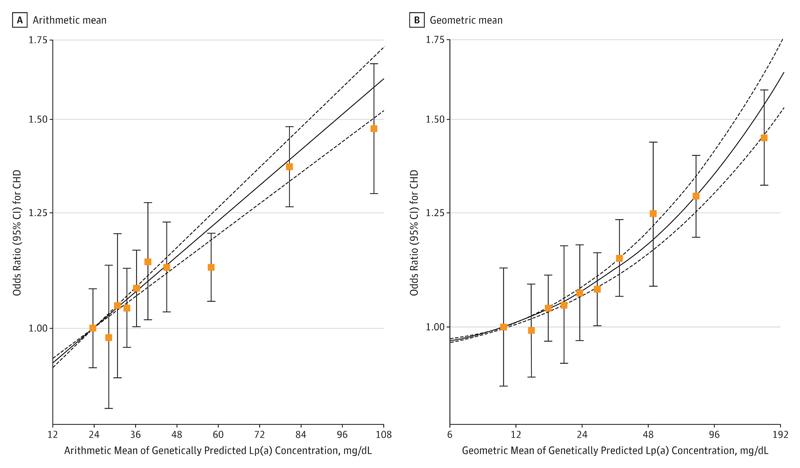
In analyses dividing the population into deciles of genetically predicted absolute Lp(a) mass concentration, the exposure–outcome association for log-transformed CHD risk was approximately linear, ie, fixed changes in absolute Lp(a) concentrations led to equal odds ratios (ORs) for CHD regardless of the starting Lp(a) concentration (Figure 2A). By contrast, the exposure–outcome association for deciles of log-transformed Lp(a) concentration was curvilinear (Figure 2B), with fixed proportional changes in Lp(a) concentrations leading to greater log-ORs for individuals with higher baseline Lp(a) concentrations (and hence, increasingly greater absolute changes in Lp[a] concentrations). These findings are consistent and support the hypothesis that the risk of CHD is log-linearly proportional to absolute changes in Lp(a) concentration.
Figure 2. Shape of Association Between Genetically Predicted Lipoprotein(a) (Lp[a]) and Coronary Heart Disease (CHD) Risk.
A, Arithmetic mean of Lp(a) in each decile (untransformed, linear scale).
B, Geometric mean of Lp(a) in each decile (log-transformed, log-scale). Points on the curve indicate mendelian randomization estimates in each decile of genetically predicted Lp(a) (error bars indicate 95% confidence intervals; first decile is reference group). The solid line indicates the best-fitting fractional polynomial (left, linear term only; right, square root and cubic terms) to model the dose-dependent relationship; the dotted lines indicate the 95% confidence intervals for the relationship.
Overall, each 10-mg/dL lower genetically predicted Lp(a) level was associated with a 5.8% lower risk of CHD (OR, 0.942; 95% CI, 0.933-0.951; P = 3 × 10−37). There was no evidence of heterogeneity with similar genetic association estimates obtained across all studies independent of the type of Lp(a) assay used (eFigures 6 and 7 in the Supplement). Estimates were also similar in sensitivity analyses that varied the number of genetic variants included in the LPA score (eTable 4 in the Supplement). In external replication analyses involving participants from CARDIOGRAMplusC4D, a 10-mg/dL lower genetically predicted Lp(a) level was associated with a 5.2% lower risk of CHD (OR, 0.948; 95% CI, 0.941-0.955; P = 1 × 10−47).
Expected Clinical Benefit of Lowering Lp(a) Concentration
Using the LDL-C genetic score, a 10-mg/dL genetically predicted lower LDL-C level was associated with a 14.5% lower risk of CHD (OR, 0.855; 95% CI, 0.818-0.893; P = 2 × 10−12) (eFigure 8 in the Supplement). This finding suggests that a 1-mg/dL difference in LDL-C level has the same association with CHD risk as a 2.63-mg/dL difference in Lp(a) concentration (ie, log[0.855] / log[0.942] = 2.63), and therefore, a 38.67-mg/dL difference in LDL-C level has the same association as a 101.5-mg/dL (95% CI, 71.0-137.0) difference in Lp(a) concentration. In external replication analyses using data from CARDIOGRAMplusC4D, a 10-mg/dL lower LDL-C level was associated with a 14.0% lower CHD risk (OR, 0.860; 95% CI, 0.841-0.879; P = 3 × 10−40), suggesting that a 109.1-mg/dL (95% CI, 89.0-133.1) difference in Lp(a) concentration has the same association with CHD risk as a 38.67-mg/dL difference in LDL-C level.
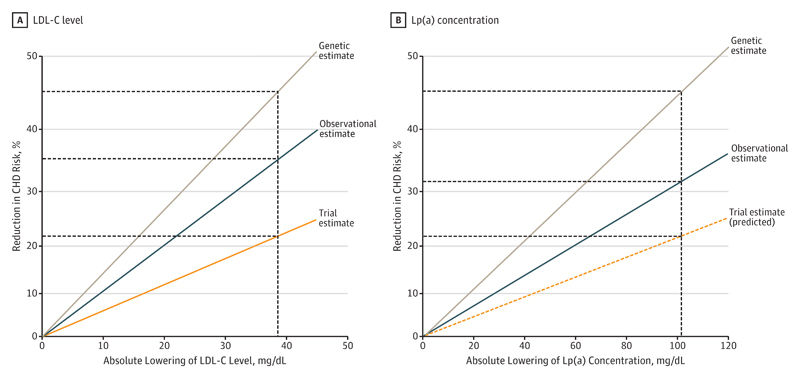
Changes in genetically predicted Lp(a) and LDL-C concentrations represent lifelong exposure to these lipoproteins. Hence, to estimate the effect of lowering Lp(a) concentration in a short-term trial, we assumed that if lifelong exposure to 101.5-mg/dL lower Lp(a) concentration has the same association with CHD risk as lifelong exposure to 38.67-mg/dL lower LDL-C level, then short-term exposure to 101.5-mg/dL lower Lp(a) concentration should have the same association with CHD risk as short-term exposure to 38.67-mg/dL lower LDL-C levels observed in randomized trials. This assumption is valid only if changes in Lp(a) concentration and LDL-C level have similar cumulative associations with CHD over time. It is further supported by the observation that the ratio of the association of lifelong exposure to Lp(a) with CHD risk estimated from mendelian randomization to the association of intermediate-term exposure to Lp(a), estimated from observational studies in the Emerging Risk Factors Consortium,2 is very similar to the ratio of the association of lifelong exposure to LDL-C with CHD risk estimated from mendelian randomization to the association of intermediate-term exposure to LDL-C in the Emerging Risk Factors Consortium (Figure 3; eFigure 9 in the Supplement).25 Therefore, Lp(a) and LDL-C appear to have similar cumulative associations with the risk of CHD over time.
Figure 3. Estimates of Coronary Heart Disease (CHD) Risk Reduction With Lowering of Low-Density Lipoprotein Cholesterol (LDL-C) Level and Lipoprotein(a) (Lp[a]) Concentration.
Genetic estimates of lifelong lowering from mendelian randomization (brown line), observational estimates from prospective cohort studies (blue line), and (A) trial estimate from short-term statin trials (for LDL-C) or (B) predicted trial estimate (for Lp[a]) (orange line). The vertical line is at 38.67 mg/dL (ie, 1 mmol/L) for LDL-C level and at 101.5 mg/dL for Lp(a) concentration, the estimated equivalent lowering in Lp(a) for the same reduction in CHD risk. To convert LDL-C to millimoles per liter, multiply by 0.0259.
Table 2 shows the expected clinical benefit in CHD risk from both lifelong and short-term exposure to absolute differences in Lp(a) concentration. Lifelong estimates are conventional mendelian randomization estimates, while short-term estimates are calculated using the difference in Lp(a) concentration needed to achieve the same change for a given reduction in LDL-C level over a median of 5 years of treatment with a statin, as reported by the Cholesterol Treatment Trialists’ Collaboration.18
Table 2. Expected Clinical Benefit of Lowering Lp(a).
| Reduction in Lp(a) Concentration, mg/dL | Reduction in LDL-C Level for Equivalent CHD Risk Reduction, mg/dL (95% CI)a | Estimated Lifelong Proportional Risk Reduction Owing to Genetically Decreased Exposure, % (95% CI)b | Estimated Short-term Proportional Risk Reduction in Randomized Trial, % (95% CI)c |
|---|---|---|---|
| 120 | 45.7 (34.1-65.4) | 51.1 (45.5-56.2) | 27.7 (20.9-37.5) |
| 100 | 38.1 (28.4-54.5) | 44.9 (39.7-49.8) | 23.7 (17.8-32.4) |
| 80 | 30.5 (22.7-43.6) | 38.0 (33.2-42.3) | 19.4 (14.5-26.9) |
| 50 | 19.0 (14.2-27.3) | 25.8 (22.3-29.1) | 12.6 (9.3-17.8) |
| 30 | 11.4 (8.5-16.4) | 16.4 (14.1-18.7) | 7.8 (5.7-11.1) |
| 20 | 7.6 (5.7-10.9) | 11.3 (9.6-12.9) | 5.3 (3.8-7.5) |
| 10 | 3.8 (2.8-5.5) | 5.8 (4.9-6.7) | 2.7 (1.9-3.9) |
| 5 | 1.9 (1.4-2.7) | 2.9 (2.5-3.4) | 1.3 (1.0-1.9) |
Abbreviations: CHD, coronary heart disease; LDL-C, low-density lipoprotein cholesterol; Lp(a), lipoprotein (a).
SI conversion factor: To convert LDL-C to millimoles per liter, multiply by 0.0259.
Each mg/dL lower Lp(a) has an association with CHD risk that is equivalent to a 0.38-mg/dL reduction in LDL-C based on the ratio of the associations of the genetic scores with CHD risk.
Effect size (95% confidence interval) for Lp(a) reduction obtained from mendelian randomization approach.
Effect size (95% confidence interval) for Lp(a) reduction obtained by considering equivalent lowering of LDL-C and in comparison with estimate from randomized trials of statins on major coronary events (risk ratio, 0.76; 95% CI, 0.73-0.78).
Independent Association of Lp(a) and LDL-C–Lowering Therapies
To assess whether the association of lowering Lp(a) concentration with the risk of CHD is likely to be independent of lowering LDL-C level with statins, we divided the population into 3 groups based on the number of LDL-C–lowering alleles each participant inherited at a common variant (rs12916) in the 3-hydroxy-3-methyl-glutaryl-CoA reductase (HMGCR) gene, which encodes the target of statins.26,27 The LPA score had nearly identical associations per 10-mg/dL lower Lp(a) concentration in each of these 3 groups (CC genotype group: OR, 0.945; 95% CI, 0.927-0.964; CT genotype group: OR, 0.939; 95% CI, 0.927-0.952; TT genotype group: OR, 0.945; 95% CI, 0.932-0.957; P = .79 for difference) (eFigure 10 in the Supplement), suggesting that the relative risk reduction of lowering Lp(a) concentration is likely to be independent of lowering LDL-C level with statins. Similar findings were observed for genetic variants in the PCSK9 and NPC1L1 gene regions that mimic the changes associated with PCSK9 inhibitors and ezetimibe, respectively.
Discussion
We found that the association of genetically predicted plasma Lp(a) with the risk of CHD was linearly proportional to the absolute difference in Lp(a) concentration. Absolute differences in Lp(a) concentration of approximately 100 mg/dL had an equivalent association with CHD risk as a 38.67-mg/dL difference in LDL-C level. The results of this study may have important implications for informing clinical practice guidelines on the use of Lp(a)-lowering therapies, for designing randomized trials to evaluate Lp(a)-lowering therapies currently in development, and for designing screening programs to reduce the global burden of CHD.
Because a 100-mg/dL difference in Lp(a) concentration had the same association with CHD risk as a 38.67-mg/dL difference in LDL-C level, the results of this study suggest that pharmacologically lowering Lp(a) concentration by approximately 100 mg/dL should reduce the risk of CHD (CHD death or nonfatal myocardial infarction) by approximately 22% to 25% in a 3- to 5-year randomized trial, similar to the association that has been observed for a 38.67-mg/dL reduction in LDL-C level during treatment with a statin.17–19 Therefore, it follows that lowering Lp(a) concentration by 80 mg/dL might be expected to reduce the risk of CHD events by approximately 18% to 20%, while lowering Lp(a) concentration by 50 mg/dL might reduce CHD events by 10% to 12% (Table 2), assuming that there are no unrecognized competing risks associated with lowering Lp(a) concentration. Therefore, only persons with very high Lp(a) concentrations are likely to benefit substantially from therapies that reduce Lp(a) concentration.
This finding likely explains why therapies that reduce Lp(a) concentration by 20% to 35% have failed to provide clear evidence that lowering Lp(a) concentration reduces the risk of cardiovascular events in previous randomized trials even though Lp(a) is a genetically supported target. The median Lp(a) concentration among participants enrolled in these trials was approximately 12 to 20 mg/dL.7–11 Therefore, a 30% reduction in Lp(a) concentration would translate into only a 3- to 6-mg/dL absolute reduction in circulating plasma Lp(a) concentration, a small absolute reduction that was likely far too modest to reduce the risk of cardiovascular events appreciably in a short-term randomized trial.
The results of this study suggest that randomized trials evaluating new, more potent Lp(a)-lowering therapies in development should be designed to enroll individuals with very high baseline Lp(a) concentrations of 90 to 100 mg/dL or more. Reducing Lp(a) concentration by 80% to 90% in such individuals should translate into large absolute reductions in Lp(a) concentrations of 70 to 90 mg/dL, which should in turn translate into approximately a 15% to 20% proportional reduction in the risk of CHD events. Enrolling patients at high risk of CHD owing to markedly elevated Lp(a) concentration in the initial proof-of-concept clinical trials is similar to the strategy used by the Scandinavian Simvastatin Survival Study trial,28 which enrolled high-risk patients with markedly elevated LDL-C concentrations and was the first trial to demonstrate that treatment with statins led to large, clinically meaningful reductions in the risk of cardiovascular events.
The magnitude of the pharmacologic reduction in Lp(a) mass that is likely needed to produce clinically meaningful reductions in CHD risk estimated in this study is larger than estimated in a 2018 study evaluating changes in Lp(a) during treatment with niacin.29 However, whereas that previous study involved informal estimates of the reversible CHD risk by lowering Lp(a) concentrations in a short-term trial,29 we used a more systematic approach. In particular, our study estimated the differences in genetically predicted Lp(a) and LDL-C concentrations needed to have the same change in lifetime CHD risk and incorporated an assessment of the differential cumulative associations of Lp(a) and LDL-C with CHD risk over time to estimate how much Lp(a) concentration must be lowered pharmacologically to produce the same change as lowering LDL-C level by 38.67 mg/dL (ie, 1 mmol/L) with a statin. This approach has been successfully used to accurately anticipate the results of several recent trials.26,27,30 Similar analyses to those used in the current study are needed before it would be possible to accurately anticipate the potential effect of pharmacologically lowering Lp(a) on the risk of stroke, peripheral vascular disease, aortic stenosis, or composite end points that include these outcomes.31
Finally, it should be noted that plasma Lp(a) concentration is largely heritable. Therefore, if the linear relationship with CHD risk continues at very high absolute Lp(a) concentrations (as occurs for LDL-C), then Lp(a) concentrations in excess of 200 mg/dL may be associated with a 3- to 4-fold increased lifetime risk of CHD (OR, 3.30; 95% CI, 2.75-3.96) and thus may represent an inherited lipoprotein disorder that is associated with a similar lifetime risk of CHD as heterozygous familial hypercholesterolemia but with a prevalence that may be 2-fold higher than that of heterozygous familial hypercholesterolemia.32,33 Therefore, screening for individuals with extremely elevated Lp(a) concentrations and treating them with one of the new Lp(a)-lowering therapies in development could potentially have the same effect on reducing the global burden of CHD as current screening programs designed to detect and treat individuals with familial hypercholesterolemia.
Limitations
Our study has limitations. Multiple different assays were used to measure Lp(a) concentrations in the included studies. However, we focused only on absolute differences in Lp(a) associated with genetic variants, which were very similar across all included studies, regardless of assay used or baseline Lp(a) concentrations. In addition, our estimate of the effect of lowering Lp(a) is agnostic to the mechanism of action, and hence our use of plasma Lp(a) mass concentration to estimate the dose-response relationship does not imply that our estimates are solely via changes in plasma Lp(a) mass concentration. If pharmacologic Lp(a) lowering has associations not adequately captured by the genetic variants (eg, antithrombotic associations), then smaller absolute reductions in Lp(a) than estimated here may produce clinically meaningful reductions in CHD risk.34
Conclusions
The association of genetically predicted Lp(a) with CHD risk was linearly proportional to the absolute change in Lp(a) mass concentration. Large absolute reductions in Lp(a) concentration of approximately100mg/dLare likely necessary to achieve clinically meaningful reductions in the risk of CHD similar in magnitude to what can be achieved by lowering LDL-C level by 38.67mg/dL (ie, 1 mmol/L) with a statin.
Supplementary Material
Key Points.
Question How much does plasma lipoprotein(a) need to be lowered to produce a clinically meaningful reduction in the risk of coronary heart disease?
Findings In a mendelian randomization analysis involving more than 80 000 patients and more than 150 000 controls, coronary heart disease risk was proportionally associated with the absolute change in plasma lipoprotein(a) mass concentration; a 101.5-mg/dL change in lipoprotein(a) concentration was associated with the same coronary heart disease risk as a 38.67-mg/dL (ie, 1-mmol/L) change in low-density lipoprotein cholesterol level.
Meaning Lipoprotein(a) concentration must be lowered by approximately 100 mg/dL to achieve the same reduction in coronary heart disease risk as can be achieved by lowering low-density lipoprotein cholesterol level by 38.67 mg/dL.
Funding/Support
The study’s coordinating centre has been underpinned by grants G0800270 and MR/L003120/1 from the UK Medical Research Council, grants SP/09/002, RG/08/014, and RG13/13/30194 from the British Heart Foundation, grants from the National Institute for Health Research through the Cambridge Biomedical Research Centre, grant HEALTH-F2-2012-279233 from the European Commission Framework 7 through the EPIC-CVD award, and grants from the European Research Council through an Advanced Investigator Award 268834 to Dr Danesh. Dr Burgess is supported by a Sir Henry Dale Fellowship jointly funded by the Wellcome Trust and the Royal Society (grant number 204623/Z/16/Z). Dr Danesh holds a BHF Professorship and NIHR Senior Investigator Award. Aspects of the analysis were supported by the Cambridge Substantive Site of Health Data Research UK.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Author Affiliations:
Medical Research Council Biostatistics Unit, University of Cambridge, Cambridge, United Kingdom (Burgess); MRC/BHF Cardiovascular Epidemiology Unit, Department of Public Health and Primary Care, University of Cambridge, Cambridge, United Kingdom (Burgess, Ference, Staley, Freitag, Mason, Willeit, Young, Surendran, Karthikeyan, Bolton, Peters, Di Angelantonio, Howson, Butterworth, Danesh); School of Medicine, Wayne State University, Detroit, Michigan (Ference); Institute for Advanced Studies, University of Bristol, Bristol, United Kingdom (Ference); MRC Integrative Epidemiology Unit, Bristol Medical School, University of Bristol, Bristol, United Kingdom (Staley); Department of Clinical Biochemistry, Herlev and Gentofte Hospital, Copenhagen University Hospital, Copenhagen, Denmark (Nielsen, Kamstrup, Langsted, Vedel-Krogh, Kobylecki, Nordestgaard); The Copenhagen General Population Study, Herlev and Gentofte Hospital, Copenhagen University Hospital, Copenhagen, Denmark (Nielsen, Kamstrup, Tybjærg-Hansen, Benn, Langsted, Vedel-Krogh, Kobylecki, Nordestgaard); Faculty of Health and Medical Sciences, University of Copenhagen, Copenhagen, Denmark (Nielsen, Tybjærg-Hansen, Benn, Vedel-Krogh, Kobylecki, Nordestgaard); Department of Neurology, Medizinische Universität Innsbruck, Innsbruck, Austria (Willeit); Institute of Health and Wellbeing, University of Glasgow, Glasgow, United Kingdom (Young, Ford); Department of Clinical Biochemistry, Rigshospitalet, Copenhagen University Hospital, Copenhagen, Denmark (Tybjærg-Hansen, Benn); Copenhagen City Heart Study, Frederiksberg Hospital, Copenhagen University Hospital, Copenhagen, Denmark (Tybjærg-Hansen, Schnohr, Nordestgaard); Institute of Cardiovascular and Medical Sciences, University of Glasgow, United Kingdom (Packard, Sattar); Department of Cardiology, Leiden University Medical Centre, Leiden, the Netherlands (Trompet, Jukema); Department of Gerontology and Geriatrics, Leiden University Medical Center, Leiden, the Netherlands (Trompet); Netherlands Heart Institute, Utrecht, the Netherlands (Jukema); National Institute for Health Research Blood and Transplant Research Unit in Donor Health and Genomics, University of Cambridge, Cambridge, United Kingdom (Di Angelantonio, Butterworth, Danesh); Department of Biostatistics and Epidemiology, Perelman School of Medicine at the University of Pennsylvania, Philadelphia (Saleheen); Centre for Non-Communicable Diseases, Karachi, Pakistan (Saleheen); Wellcome Trust Sanger Institute, Hinxton, United Kingdom (Danesh).
The EPIC-CVD Consortium:
Principal investigators of the EPIC-CVD Consortium include the following: Kim Overvad, MD, PhD (Department of Public Health, Section for Epidemiology, Aarhus University, Aarhus, Denmark); Anne Tjønneland, MD, PhD, DMSc (Diet, Genes and Environment, Danish Cancer Society Research Center, Copenhagen, Denmark); Francoise Clavel-Chapelon, PhD (INSERM, Centre for Research in Epidemiology and Population Health, U1018, Nutrition, Hormones, and Women’s Health Team, Institut Gustave Roussy, Villejuif, France); Rudolf Kaaks, PhD (Division of Cancer Genetic Epidemiology, German Cancer Research Centre, Heidelberg, Germany); Heiner Boeing, PhD (Department of Epidemiology, German Institute of Human Nutrition, Potsdam-Rehbrücke, Germany); Antonia Trichopoulou, MD, PhD (WHO Collaborating Center for Nutrition and Health, Unit of Nutritional Epidemiology and Nutrition in Public Health, Department of Hygiene, Epidemiology and Medical Statistics, University of Athens Medical School, Athens, Greece); Pietro Ferrari, PhD (International Agency for Research on Cancer, Lyon, France); Domenico Palli, MD (Molecular and Nutritional Epidemiology Unit, Centro per lo Studio e la Prevenzione Oncologica-Scientific Institute of Tuscany, Florence, Italy); Vittorio Krogh, MD (Epidemiology and Prevention Unit, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy); Salvatore Panico, MD, MS (Dipartimento di Medicina Clinica e Chirurgia, Federico II University, Naples, Italy); Rosario Tumino, MD (Cancer Registry and Histopathology Unit, Civic-M. P. Arezzo Hospital, ASP Ragusa, Italy); Giuseppe Matullo, PhD (Human Genetics Foundation, Turin, Italy); Jolanda Boer, PhD (Centre for Nutrition, Prevention and Health Services, National Institute for Public Health and the Environment, Bilthoven, the Netherlands); Yvonne van der Schouw, PhD (Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands); Elisabete Weiderpass, MD, PhD (Department of Community Medicine, Faculty of Health Sciences, University of Tromsø, The Arctic University of Norway, Tromsø, Norway); J. Ramon Quiros, MD (Public Health Directorate, Asturias, Spain); María-José Sánchez, MD, PhD (Escuela Andaluza de Salud Pública, Instituto de Investigación Biosanitaria ibs GRANADA Hospitales Universitarios de Granada/Universidad de Granada, Granada, Spain); Carmen Navarro, MD, PhD (Epidemiology Department, Murcia Health Authority, Murcia, Spain); Conchi Moreno-Iribas, MD (Public Health Institute of Navarra, Pamplona, Spain); Larraitz Arriola, MD (Public Health Division of Gipuzkoa, Instituto Bio-Donostia, Basque Government, CIBERESP, Spain); Olle Melander, MD, PhD (Department of Clinical Sciences, Hypertension and Cardiovascular Disease, Clinical Research Centre, Malmö University Hospital, Malmö, Sweden); Patrik Wennberg, MD, PhD (Department of Public Health and Clinical Medicine, Family Medicine, Umeå University, Umeå, Sweden); Nicholas J. Wareham, MBBS, PhD (Medical Research Council Epidemiology Unit, University of Cambridge, Cambridge, United Kingdom); Timothy J. Key, PhD (Cancer Epidemiology Unit, Nuffield Department of Population Health, University of Oxford, United Kingdom); Elio Riboli, MD, PhD (School of Public Health, Imperial College London, United Kingdom); Adam S. Butterworth, PhD (MRC/BHF Cardiovascular Epidemiology Unit, Department of Public Health and Primary Care, University of Cambridge, United Kingdom); and John Danesh, DPhil, FMedSci (MRC/BHF Cardiovascular Epidemiology Unit, Department of Public Health and Primary Care, University of Cambridge, United Kingdom).
Corresponding Author:
Brian A Ference, MD, MPhil, MSc, MRC/BHF Cardiovascular Epidemiology Unit, Department of Public Health and Primary Care, University of Cambridge, Cambridge, CB1 8RN, United Kingdom (baf29@medschl.cam.ac.uk); Stephen Burgess, PhD, Medical Research Council Biostatistics Unit, University of Cambridge, Cambridge CB2 0SR, United Kingdom (sb452@medschl.cam.ac.uk).
Footnotes
Author Contributions: Dr Burgess had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Drs Burgess and Ference contributed equally.
Study concept and design: Burgess, Ference, Benn, Packard, Jukema, Di Angelantonio, Butterworth, Danesh.
Acquisition, analysis, or interpretation of data: Burgess, Ference, Staley, Freitag, Mason, Nielsen, Willeit, Young, Surendran, Kamstrup, Tybjærg-Hansen, Benn, Langsted, Vedel-Krogh, Kobylecki, Ford, Packard, Trompet, Jukema, Di Angelantonio, Nordestgaard, Butterworth, Danesh.
Drafting of the manuscript: Burgess, Ference, Surendran, Packard, Butterworth, Danesh.
Critical revision of the manuscript for important intellectual content: Ference, Staley, Freitag, Mason, Nielsen, Willeit, Young, Kamstrup, Tybjærg-Hansen, Benn, Langsted, Vedel-Krogh, Kobylecki, Ford, Packard, Trompet, Jukema, Di Angelantonio, Nordestgaard, Butterworth.
Statistical analysis: Burgess, Ference, Staley, Freitag, Willeit, Surendran, Trompet.
Obtained funding: Di Angelantonio, Nordestgaard, Butterworth, Danesh.
Administrative, technical, or material support: Ference, Nielsen, Young, Benn, Ford, Packard, Nordestgaard.
Study supervision: Ference, Freitag, Tybjærg-Hansen, Vedel-Krogh, Kobylecki, Jukema, Nordestgaard, Butterworth.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Ference has received grants from Merck & Co, Amgen, Esperion Therapeutics, and Novartis as well as personal fees from Merck & Co, Amgen, Ionis Pharmaceuticals, Krka, d. d., Novo mesto, Medicines Company, and Sanofi Regeneron. Dr Freitag has been a full-time employee of Bayer AG since October 2015. Dr Peters has received travel and accommodation expenses to speak at Olink-sponsored academic meetings. Dr Packard has received grants from Merck Sharp & Dohme as well as personal fees from Pfizer, Amgen, Sanofi Regeneron, and Daiichi Sankyo. Dr Sattar has received personal fees from Amgen and Sanofi. Dr Nordestgaard has received personal fees for consultation or speaking from AstraZeneca, Sanofi Regeneron, Ionis Pharmaceuticals, Aegerion Pharmaceuticals, Dezima Pharma, and Amgen. Dr Butterworth has received grants from Pfizer, Novartis, Merck, Biogen, and AstraZeneca as well as personal fees from Novartis. Dr Danesh has received grants from the UK Medical Research Council, the British Heart Foundation, the UK National Institute of Health Research, and the European Commission during the conduct of the study; grants from the European Research Council, Merck & Co, NHS Blood and Transplant, Novartis, Pfizer, Wellcome Trust, and AstraZeneca; personal fees and nonfinancial support from Merck Sharp & Dohme UK Atherosclerosis; and has served on the Novartis cardiovascular and metabolic advisory board and the Pfizer population research advisory panel. No other disclosures were reported.
Additional Contributions: We thank staff from the EPIC-CVD and EPIC-InterAct Coordinating Centres for carrying out sample preparation and data-handling work, particularly Sarah Spackman, MMath (MRC/BHF Cardiovascular Epidemiology Unit, Department of Public Health and Primary Care, University of Cambridge, Cambridge, United Kingdom), for her work as the EPIC-CVD data manager. She is a paid employee of the University of Cambridge but was otherwise not compensated for her work.
References
- 1.Marcovina SM, Koschinsky ML. Lipoprotein(a) as a risk factor for coronary artery disease. Am J Cardiol. 1998;82(12A):57U–66U. doi: 10.1016/s0002-9149(98)00954-0. [DOI] [PubMed] [Google Scholar]
- 2.Erqou S, Kaptoge S, Perry PL, et al. Emerging Risk Factors Collaboration Lipoprotein(a) concentration and the risk of coronary heart disease, stroke, and nonvascular mortality. JAMA. 2009;302(4):412–423. doi: 10.1001/jama.2009.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kamstrup PR, Tybjaerg-Hansen A, Steffensen R, Nordestgaard BG. Genetically elevated lipoprotein(a) and increased risk of myocardial infarction. JAMA. 2009;301(22):2331–2339. doi: 10.1001/jama.2009.801. [DOI] [PubMed] [Google Scholar]
- 4.Clarke R, Peden JF, Hopewell JC, et al. PROCARDIS Consortium Genetic variants associated with Lp(a) lipoprotein level and coronary disease. N Engl J Med. 2009;361(26):2518–2528. doi: 10.1056/NEJMoa0902604. [DOI] [PubMed] [Google Scholar]
- 5.Nordestgaard BG, Langsted A. Lipoprotein (a) as a cause of cardiovascular disease: insights from epidemiology, genetics, and biology. J Lipid Res. 2016;57(11):1953–1975. doi: 10.1194/jlr.R071233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boden WE, Probstfield JL, Anderson T, et al. AIM-HIGH Investigators Niacin in patients with low HDL cholesterol levels receiving intensive statin therapy. N Engl J Med. 2011;365(24):2255–2267. doi: 10.1056/NEJMoa1107579. [DOI] [PubMed] [Google Scholar]
- 7.Landray MJ, Haynes R, Hopewell JC, et al. HPS2-THRIVE Collaborative Group Effects of extended-release niacin with laropiprant in high-risk patients. N Engl J Med. 2014;371(3):203–212. doi: 10.1056/NEJMoa1300955. [DOI] [PubMed] [Google Scholar]
- 8.Barter PJ, Caulfield M, Eriksson M, et al. ILLUMINATE Investigators Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357(21):2109–2122. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 9.Lincoff AM, Nicholls SJ, Riesmeyer JS, et al. ACCELERATE Investigators Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N Engl J Med. 2017;376(20):1933–1942. doi: 10.1056/NEJMoa1609581. [DOI] [PubMed] [Google Scholar]
- 10.Bowman L, Hopewell JC, Chen F, et al. HPS3/TIMI55–REVEAL Collaborative Group Effects of anacetrapib in patients with atherosclerotic vascular disease. N Engl J Med. 2017;377(13):1217–1227. doi: 10.1056/NEJMoa1706444. [DOI] [PubMed] [Google Scholar]
- 11.Sabatine MS, Giugliano RP, Keech AC, et al. FOURIER Steering Committee and Investigators Evolocumab and clinical outcomes in patients with cardiovascular disease. N Engl J Med. 2017;376(18):1713–1722. doi: 10.1056/NEJMoa1615664. [DOI] [PubMed] [Google Scholar]
- 12.Viney NJ, van Capelleveen JC, Geary RS, et al. Antisense oligonucleotides targeting apolipoprotein(a) in people with raised lipoprotein(a): two randomised, double-blind, placebo-controlled, dose-ranging trials. Lancet. 2016;388(10057):2239–2253. doi: 10.1016/S0140-6736(16)31009-1. [DOI] [PubMed] [Google Scholar]
- 13.Nordestgaard BG, Chapman MJ, Ray K, et al. European Atherosclerosis Society Consensus Panel Lipoprotein(a) as a cardiovascular risk factor: current status. Eur Heart J. 2010;31(23):2844–2853. doi: 10.1093/eurheartj/ehq386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bennet A, Di Angelantonio E, Erqou S, et al. Lipoprotein(a) levels and risk of future coronary heart disease: large-scale prospective data. Arch Intern Med. 2008;168(6):598–608. doi: 10.1001/archinte.168.6.598. [DOI] [PubMed] [Google Scholar]
- 15.Emdin CA, Khera AV, Natarajan P, et al. CHARGE–Heart Failure Consortium; CARDIoGRAM Exome Consortium Phenotypic characterization of genetically lowered human lipoprotein(a) levels. J Am Coll Cardiol. 2016;68(25):2761–2772. doi: 10.1016/j.jacc.2016.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamstrup PR, Benn M, Tybjaerg-Hansen A, Nordestgaard BG. Extreme lipoprotein(a) levels and risk of myocardial infarction in the general population: the Copenhagen City Heart Study. Circulation. 2008;117(2):176–184. doi: 10.1161/CIRCULATIONAHA.107.715698. [DOI] [PubMed] [Google Scholar]
- 17.Baigent C, Blackwell L, Emberson J, et al. Cholesterol Treatment Trialists’ (CTT) Collaboration Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomised trials. Lancet. 2010;376(9753):1670–1681. doi: 10.1016/S0140-6736(10)61350-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mihaylova B, Emberson J, Blackwell L, et al. Cholesterol Treatment Trialists’ (CTT) Collaborators The effects of lowering LDL cholesterol with statin therapy in people at low risk of vascular disease: meta-analysis of individual data from 27 randomised trials. Lancet. 2012;380(9841):581–590. doi: 10.1016/S0140-6736(12)60367-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silverman MG, Ference BA, Im K, et al. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;316(12):1289–1297. doi: 10.1001/jama.2016.13985. [DOI] [PubMed] [Google Scholar]
- 20.Teslovich TM, Musunuru K, Smith AV, et al. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466(7307):707–713. doi: 10.1038/nature09270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Burgess S, Dudbridge F, Thompson SG. Combining information on multiple instrumental variables in mendelian randomization: comparison of allele score and summarized data methods. Stat Med. 2016;35(11):1880–1906. doi: 10.1002/sim.6835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Staley JR, Burgess S. Semiparametric methods for estimation of a nonlinear exposure-outcome relationship using instrumental variables with application to mendelian randomization. Genet Epidemiol. 2017;41(4):341–352. doi: 10.1002/gepi.22041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nikpay M, Goel A, Won HH, et al. A comprehensive 1,000 genomes-based genome-wide association meta-analysis of coronary artery disease. Nat Genet. 2015;47(10):1121–1130. doi: 10.1038/ng.3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boerwinkle E, Leffert CC, Lin J, Lackner C, Chiesa G, Hobbs HH. Apolipoprotein(a) gene accounts for greater than 90% of the variation in plasma lipoprotein(a) concentrations. J Clin Invest. 1992;90(1):52–60. doi: 10.1172/JCI115855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Angelantonio E, Sarwar N, Perry P, et al. Emerging Risk Factors Collaboration Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302(18):1993–2000. doi: 10.1001/jama.2009.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ference BA, Majeed F, Penumetcha R, Flack JM, Brook RD. Effect of naturally random allocation to lower low-density lipoprotein cholesterol on the risk of coronary heart disease mediated by polymorphisms in NPC1L1, HMGCR, or both: a 2 × 2 factorial mendelian randomization study. J Am Coll Cardiol. 2015;65(15):1552–1561. doi: 10.1016/j.jacc.2015.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ference BA, Robinson JG, Brook RD, et al. Variation in PCSK9 and HMGCR and risk of cardiovascular disease and diabetes. N Engl J Med. 2016;375(22):2144–2153. doi: 10.1056/NEJMoa1604304. [DOI] [PubMed] [Google Scholar]
- 28.Pedersen TR, Kjekshus J, Berg K, et al. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344(8934):1383–1389. [PubMed] [Google Scholar]
- 29.Parish S, Hopewell JC, Hill MR, et al. HPS2-THRIVE Collaborative Group Impact of apolipoprotein(a) isoform size on lipoprotein(a) lowering in the HPS2-THRIVE study. Circ Genom Precis Med. 2018;11(2):e001696. doi: 10.1161/CIRCGEN.117.001696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ference BA, Kastelein JJP, Ginsberg HN, et al. Association of genetic variants related to CETP inhibitors and statins with lipoprotein levels and cardiovascular risk. JAMA. 2017;318(10):947–956. doi: 10.1001/jama.2017.11467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ference BA. How to use mendelian randomization to anticipate the results of randomized trials. Eur Heart J. 2018;39(5):360–362. doi: 10.1093/eurheartj/ehx462. [DOI] [PubMed] [Google Scholar]
- 32.Varvel S, McConnell JP, Tsimikas S. Prevalence of elevated Lp(a) mass levels and patient thresholds in 532 359 patients in the United States. Arterioscler Thromb Vasc Biol. 2016;36(11):2239–2245. doi: 10.1161/ATVBAHA.116.308011. [DOI] [PubMed] [Google Scholar]
- 33.Langsted A, Kamstrup PR, Benn M, Tybjærg-Hansen A, Nordestgaard BG. High lipoprotein(a) as a possible cause of clinical familial hypercholesterolaemia: a prospective cohort study. Lancet Diabetes Endocrinol. 2016;4(7):577–587. doi: 10.1016/S2213-8587(16)30042-0. [DOI] [PubMed] [Google Scholar]
- 34.Langsted A, Kamstrup PR, Nordestgaard BG. High lipoprotein(a) and low risk of major bleeding in brain and airways in the general population: a mendelian randomization study. Clin Chem. 2017;63(11):1714–1723. doi: 10.1373/clinchem.2017.276931. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.