Abstract
Recent evidence suggested a weak relationship between alcohol consumption and pancreatic cancer (PC) risk. In this study, the association between lifetime and baseline alcohol intakes and the risk of PC was evaluated, including the type of alcoholic beverages and potential interaction with smoking. Within the European Prospective Investigation into Cancer and Nutrition (EPIC) study, 1,283 incident PC (57% women) were diagnosed from 476,106 cancer-free participants, followed up for 14 years. Amounts of lifetime and baseline alcohol were estimated through lifestyle and dietary questionnaires, respectively. Cox proportional hazard models with age as primary time variable were used to estimate PC hazard ratios (HR) and their 95% confidence interval (CI). Alcohol intake was positively associated with PC risk in men. Associations were mainly driven by extreme alcohol levels, with HRs comparing heavy drinkers (>60 g/day) to the reference category (0.1-4.9 g/day) equal to 1.77 (95% CI: 1.06, 2.95) and 1.63 (95% CI: 1.16, 2.29) for lifetime and baseline alcohol, respectively. Baseline alcohol intakes from beer (>40 g/day) and spirits/liquors (>10 g/day) showed HRs equal to 1.58 (95% CI: 1.07, 2.34) and 1.41 (95% CI: 1.03, 1.94), respectively, compared to the reference category (0.1-2.9 g/day). In women, HR estimates did not reach statistically significance. The alcohol and PC risk association was not modified by smoking status. Findings from a large prospective study suggest that baseline and lifetime alcohol intakes were positively associated with PC risk, with more apparent risk estimates for beer and spirits/liquors than wine intake.
Introduction
Pancreatic cancer (PC) is a major public health concern. It is one of the most fatal cancers worldwide, accounting for a mortality-incidence ratio close to 1, and a 7% survival beyond five years after diagnosis.1,2 The total number of deaths due to PC is expected to rise in the coming years among the American and European populations and is set to surpass breast, prostate, and colorectal cancers to become the second leading cause of cancer-related death after lung cancers.3,4 This evidence highlights the importance of understanding risk factors of PC to enhance its primary prevention.
The majority of PC cases currently occurs in high-income countries, such as the United States and Western European countries, where incidence rates are nearly three times higher than in middle- and low-income countries.5 This incidence pattern suggests that PC occurrence is related to lifestyle factors specifically prevalent in the Western world. The etiology of PC has been extensively researched, leading to the identification of tobacco smoking, obesity, type-II diabetes mellitus and chronic pancreatitis as well as inherited genetic disorders as major risk factors.6–9
In 2012, international expert panels reviewed the association between alcohol and cancer and considered the epidemiologic evidence for PC inconsistent, highlighting the possibility of residual confounding by smoking and the lack of knowledge on whether results differ by type of alcoholic beverages.6,10 The most recent prospective studies suggested that alcohol consumption may increase PC risk but with an excess risk limited to high levels of consumption.11–14 The majority of these investigations primarily focused on baseline alcohol intake, whereas two early analysis from the European Prospective Investigation on Cancer and Nutrition (EPIC) study indicated that neither baseline nor cumulative lifetime alcohol intake were related to PC risk.15,16 Recent meta-analyses have shown that alcohol intake increased the risk of PC by at least 15% in heavy drinkers consuming more than 25 g/day when compared to light drinkers.17,18 Although the association was also investigated among never smokers, as well as the interaction with tobacco smoking,11,12,14 it has been more often explored in case-control studies in comparison to prospective studies19 due to the small number of cases being both heavy drinker and never smoker.
In the light of these findings, relationship between alcohol intake and PC risk was comprehensively examined in the EPIC study involving a larger number of incident PC cases than earlier evaluations,15,16 and presenting risk estimates according to lifetime and baseline intakes, as well as according to the type of alcoholic beverages and smoking habits.
Material and Methods
EPIC is an ongoing multicenter prospective study aiming to investigate prospectively the etiology of cancer in relation to diet, lifestyle and environmental factors, and for which the study design has been previously describe in detail.20 From 1992 to 2000, a total of 521,324 participants were recruited across 10 European countries, mostly from the general population, of which 70% are women, aged from 35 to 70 years. Exceptions were the French cohort (members of a health insurance for school and university employees), some of the Spanish and Italian centers (blood donors), Utrecht and Florence sub-cohorts (only breast cancer screening participants), and Oxford sub-cohort (vegetarians and ‘health conscious’ participants). The cohorts of France and Norway and the national sub-cohorts of Utrecht and Naples consist of women only. Approval for this study was obtained from the relevant ethical review boards of the participating institutions and study participants provided informed consent before they completed diet, lifestyle and medical questionnaires at baseline.
Assessment of alcohol intake and covariates
Diet was assessed at recruitment by validated center-/country- specific dietary questionnaires20 designed to capture local-dietary habits with high compliance.21 Data on weight and height (self-reported in France, Norway and the UK Oxford center), occupational and physical activities, previous illness, smoking status and lifetime alcohol intake were collected through lifestyle questionnaires.
Baseline alcohol intake was computed from the number of glasses of beer and/or cider, wine, sweet liquors and/or distilled spirits, and fortified wines drunk per day or week during the 12 months preceding recruitment. For each country, an average daily alcohol intake expressed in grams per day was calculated based on the standard glass volume and ethanol content for each type of alcoholic beverage using information collected through 24-hour dietary recalls from a subgroup of the cohort.22–24
Lifetime alcohol consumption was measured through the number of glasses from the different types of beverages consumed per week at 20, 30, 40, and 50 years of age, including the intake at recruitment. The average lifetime alcohol intake was calculated as a weighted average of intakes at different ages with weights equal to the time of exposure to alcohol at different ages. Information was available for 76.3% of the study participants, as data on lifetime alcohol exposure were not collected in Naples (Italy), Bilthoven (The Netherlands), Sweden, and Norway.
Ascertainment of disease outcome and vital status
The identification of cancer cases during follow-up was based on population cancer registries in 7 of the participating countries (Denmark, Italy, Netherlands, Norway, Spain, Sweden, and the United Kingdom), and on a combination of methods, including health insurance records, contacts with cancer and pathology registries, and active follow-up of EPIC participants and their next of kin (France, Germany, and Greece). Mortality data were collected from, either the cancer, or mortality registries at the regional or national level. Currently, the vital status is known for 98.4% of all EPIC participants, as well as the proportion of participants who had emigrated to another country, withdrew or had unknown vital status (1.6%).
For the present study, we used information on the most recent vital status and cancer diagnosis update. The follow-up period ended as follows: December 2009 (Varese, Murcia), December 2010 (Florence, Ragusa, Turin, Asturias, Bilthoven, and Utrecht), December 2011 (Granada, Navarra, San Sebastian, and Cambridge), December 2012 (Oxford, Umeå, Denmark, and Norway), and December 2013 (Malmö). For France, Germany, Greece and Naples, the end of follow-up was considered to be the last known contact with study participants: June 2008 for France, December 2009 for Heidelberg and Potsdam, December 2010 for Naples and December 2012 for Greece. Cases of PC defined in this study were primary incident exocrine tumor of the pancreas. They were coded according to International Classification of Diseases-Oncology (3rd edition), including all invasive pancreatic cancers coded as C25 (C25.0–C25.3, C25.7–C25.9). As they represent around 95% of PC cases, this study focused only on exocrine PC, while endocrine tumors of the pancreas were not considered (C25.4). Microscopically confirmed PC represented 67% of the cases (n=854) based on histology, cytology or hematology reports. Other cases were obtained from clinical or surgical observations (n=344), medical imaging technics (n=57), death certificates (n=17) and laboratory techniques (n=11).
Statistical analysis
EPIC participants without lifestyle or dietary information (n= 6,902), participants with ratio of estimated energy intake over energy requirement in the top or bottom 1% (n=10,241),25 prevalent cancer cases (n=21,401), PC cases with missing date of diagnosis (n=18), participants with missing follow-up information (n=18) and PC cases having a neuroendocrine or endocrine tumor (n=54) were excluded. For lifetime alcohol analysis, participants without information on past alcohol use were excluded (n=112,841).
The association between alcohol intake and PC incidence was evaluated using multivariable Cox proportional hazard models. Age was the primary time variable, and Breslow’s method was adopted for handling ties.26 The time at entry was the age at recruitment, whereas the exit time was the age at cancer diagnosis, death, loss, or end of follow-up, whichever came first.
All models were stratified by study center to control for different effects in questionnaires, follow-up procedures and other center-specific features.25 To further control for the effect of age as possible confounding, models were also stratified by age at recruitment in 1-year categories. Separate models were run by gender to account for the behavioral differences of alcohol uses between men and women. Baseline and lifetime alcohol intake were first modeled by categories, as non-consumers, 0.1-4.9 g/day (reference category), 5-14.9 g/day, 15-29.9 g/day, 30-59.9 g/day and >60 g/day. In women, the last two categories were collapsed into a ≥ 30 g/day group. In analyses on lifetime alcohol intake, former drinkers at baseline were separated out from never consumers. Overall tests for significance of HRs related to alcohol in categories were determined by p-values comparing Wald test statistics to a χ2 distribution with degree of freedom equal to the number of alcohol categories minus one. Analyses were also carried out in continuous, expressing HRs per 12 g/day increase of alcohol intake as 12 grams of alcohol corresponds to about one standard glass of either wine, beer or spirits/liquors. Tests for trend were computed accordingly.
The following confounding variables were consistently included in all analyses: smoking intensity (never; current, 1-15 cig/day; current, 16-25 cig/day; current +26 cig/day; former, quit<10 years; former, quit 11-20 years; former, quit +20 years, current, pipe/cigar occasionally, unknown (n=7,921)), education level (no degree, primary school, secondary school, technical or professional school, university degree, unknown (n=10,706)), physical activity index (inactive, moderately inactive, moderately active, active, unknown (n=8,823))27, type 1 and type 2 diabetes mellitus status combined (no, yes, unknown (n=2,324)), body mass index (BMI) in kg.m-2 (continuous), height in cm (continuous). The inclusion of energy intake from non-alcohol sources to perform iso-caloric comparisons and partially control for errors in alcohol estimation did not alter the magnitude or risk estimates, and was not pursued. Models evaluating lifetime alcohol consumption were further adjusted on the duration of alcohol drinking (in years), time since quitting (in years), and an indicator variable for drinkers. Associations between alcohol subtypes, namely beer, wine and spirits/liquors and PC were assessed in adjusted models for energy intake from alcohol sources other than the one under evaluation using the following categories: never, 0.1–2.9 g/day (reference), 3–9.9, 10–19.9, 20–39.9 and ≥ 40 g/day. For women, the two last categories were merged into a ≥ 20 g/day group. All models were compatible with the proportional hazards assumption, assessed through analyses of Schoenfeld residuals.28
Dose-response analyses were performed for baseline and lifetime alcohol intake in men. Potential departures from linearity in the association between alcohol intakes and PC were examined by fitting restricted cubic spline models29 with alcohol category-specific knots placed at 0.1, 5, 30, 60 and 100. Non-linearity was evaluated by comparing the difference in log-likelihood of models with linear term and fractional polynomials to a χ2 distribution.
Effect modification in the relationship between alcohol and PC risk by, in turn, smoking status (never, current smokers), sex and country was evaluated through comparisons of models with and without interaction terms. The differences in log-likelihood were compared to a χ2 distribution, with degrees of freedom equal to the total number of interaction terms minus one. For analysis by smoking status, parameter estimates were not altered by the inclusion in the models of smoking duration and age at smoking initiation (data not shown). Sensitivity analyses were performed to assess the robustness of the findings. First, as reverse causation may bias the association between alcohol and PC, cases occurring during the first 2 years of follow-up were further excluded. Second, models on baseline alcohol intake in women were further adjusted for baseline information for menopausal status, ever use of hormone therapy, and number of full-term pregnancies. Finally, in the absence of information on chronic pancreatitis in EPIC, a sensitivity analysis was carried out to account for the potential confounding role of chronic pancreatitis (Z) between baseline alcohol intake (X) and risk of pancreatic cancer (D) using external information.30 A PC HR for baseline heavy drinkers (>60g/day) vs. moderate drinkers (0.1-4.9g/day) not adjusted for chronic pancreatitis in EPIC was estimated as large as 1.64 (95% CI: 1.22, 2.21), for men and women combined. Assuming values from the literature for relative risk estimates of chronic pancreatitis associated with alcohol intake greater than 25 g/day compared to the never drinkers ranging from 2 to 6,31,32 pancreatitis prevalence among moderate drinkers ranging from 0.005 to 0.0232 and relative risk estimates of PC associated with chronic pancreatitis ranging from 1.5 to 15,33–35 PC HR for heavy drinkers vs. moderate drinkers adjusted for chronic pancreatitis were estimated.
Two-sided p-values were provided with nominal level of statistical significance set to 5%. Analyses were performed using Stata.36
Results
EPIC population characteristics
This study was based on a population of 476,106 participants, 70% women, with an overall median age at recruitment of 52 years. Within a mean follow-up time of 14 years, and a total of 6,640,000 person-years, 1,283 incident pancreatic cancers were diagnosed (727 women) as reported in Table 1, with a median age at diagnosis of 67 years and age standardized incidence rate equal to 5.4 per 100,000 person-years.
Table 1. Country- and sex-specific frequencies of PC cases and other characteristics of the study population.
| Participants | PC cases | Follow-up (years)1 | PY2 | Age (years)3 | Baseline alcohol (g/day)4 | BNC2 | Baseline beer (g/day)4 | Baseline wine (g/day)4 | Baseline spirits/liquors (g/day)4 | Lifetime alcohol (g/day) 4 | % LNC2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Men | |||||||||||||||||
| Italy | 14,023 | 35 | 14 | 195,883 | 50 | 24 (1-54) | 4 | 2 (0-3) | 20 (0-47) | 2 (0-5) | 24 (2-49) | 3 | |||||
| Spain | 15,138 | 55 | 16 | 239,109 | 50 | 28 (0-67) | 15 | 3 (0-9) | 22 (0-56) | 3 (0-11) | 43 (4-91) | 4 | |||||
| United Kingdoms | 22,848 | 69 | 14 | 329,209 | 53 | 13 (0-34) | 6 | 5 (0-22) | 5 (0-12) | 2 (0-3) | 15 (1-34) | 2 | |||||
| The Netherlands | 9,627 | 20 | 15 | 140,422 | 44 | 18 (0-44) | 9 | 10 (0-29) | 4 (0-10) | 4 (0-11) | -6 | -6 | |||||
| Greece | 10,814 | 25 | 10 | 112,787 | 52 | 18 (0-44) | 10 | 4 (0-9) | 9 (0-26) | 5 (0-15) | 30 (0-74) | 6 | |||||
| Germany | 21,177 | 72 | 10 | 219,536 | 53 | 24 (2-53) | 4 | 15 (0-40) | 8 (0-22) | 2 (0-7) | 27 (5-56) | 1 | |||||
| Sweden | 22,304 | 93 | 16 | 358,863 | 51 | 9 (0-23) | 10 | 4 (0-8) | 2 (0-8) | 2 (0-7) | -6 | -6 | |||||
| Denmark | 26,287 | 187 | 15 | 382,609 | 56 | 28 (4-62) | 2 | 14 (1-36) | 10 (0-30) | 3 (0-8) | 21 (5-41) | 1 | |||||
| All Men | 142,218 | 556 | 14 | 1,978,417 | 53 | 20 (0-50) | 7 | 7 (0-22) | 10 (0-30) | 3 (0-7) | 26 (3-55) | 2 | |||||
| Women | |||||||||||||||||
| France | 67,395 | 56 | 13 | 869,319 | 52 | 11 (0-29) | 14 | 1 (0-2) | 9 (0-24) | 0 (0-1) | 7 (0-17) | 15 | |||||
| Italy | 30,511 | 69 | 14 | 434,978 | 51 | 9 (0-24) | 23 | 1 (0-1) | 7 (0-24) | 0 (0-1) | 6 (0-17) | 15 | |||||
| Spain | 24,848 | 51 | 16 | 398,811 | 48 | 4 (0-14) | 53 | 1 (0-2) | 3 (0-12) | 0 (0-0) | 4 (0-14) | 39 | |||||
| United Kingdoms | 52,56 | 119 | 15 | 793,462 | 48 | 7 (0-17) | 6 | 1 (0-4) | 5 (0-12) | 1 (0-3) | 9 (0-21) | 4 | |||||
| The Netherlands | 26,906 | 73 | 14 | 384,205 | 53 | 8 (0-24) | 18 | 1 (0-2) | 4 (0-11) | 1 (0-3) | 7 (0-18) | 7 | |||||
| Greece | 15,233 | 19 | 11 | 168,491 | 54 | 3 (0-9) | 35 | 1 (0-1) | 2 (0-6) | 1 (0-2) | 3 (0-10) | 33 | |||||
| Germany | 27,379 | 44 | 10 | 284,937 | 48 | 9 (0-23) | 5 | 2 (0-7) | 6 (0-17) | 1 (0-1) | 7 (1-15) | 2 | |||||
| Sweden | 26,368 | 111 | 17 | 442,242 | 51 | 5 (0-13) | 18 | 1 (0-3) | 2 (0-7) | 0 (0-1) | -6 | -6 | |||||
| Denmark | 28,719 | 138 | 15 | 432,414 | 56 | 14 (1-34) | 3 | 3 (0-7) | 8 (0-30) | 2 (0-5) | 9 (1-19) | 18 | |||||
| Norway | 33,969 | 47 | 13 | 452,121 | 48 | 3 (0-7) | 21 | 1 (0-2) | 2 (0-5) | -5 | -6 | -6 | |||||
| All women | 333,888 | 727 | 14 | 4,660,980 | 51 | 8 (0-22) | 17 | 1 (0-4) | 5 (0-15) | 1 (0-2) | 7 (0-17) | 11 | |||||
| All participants | 476,106 | 1283 | 14 | 6,639,397 | 52 | 12 (0-32) | 14 | 3 (0-8) | 7 (0-21) | 1 (0-3) | 13 (0-31) | 8 | |||||
Means;
PY: Person-years; BNC: Percentage of baseline non-consumers (<0.1 g/day); LNC: Percentage of lifetime never consumers (<0.1 g/day);
Medians;
Means (10th-90th percentiles);
Information on alcohol coming from spirits/liquors not available in Norway;
Information on lifetime alcohol not collected in Naples (Italy), Bilthoven (The Netherlands), Sweden, and Norway;
Lifetime and baseline alcohol consumptions were 2- and 4-fold higher in men than in women, respectively. On average, beer and wine represented, respectively, 35% and 50% of total alcohol intake in men, and 12.5% and 63% in women. These patterns of consumption were consistent across countries in women, while consumptions were more heterogeneous in men. The proportion of non-drinkers was higher in women than in men. Men and women non-drinkers (< 0.1 g/day) differed by their educational attainment, physical activity level and diabetes mellitus status when they were compared to alcohol consumers. Percentage of smokers at recruitment was higher among alcohol drinkers than among alcohol non-drinkers. Characteristics by categories of baseline alcohol intake are shown into the Table 2.
Table 2. Baseline characteristics of the EPIC participants by categories of baseline alcohol intake1.
| Baseline alcohol intake categories (g/day) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Men | Total | < 0.1 | 0.1 - 4.9 | 5 - 14.9 | 15 - 29.9 | 30 - 59.9 | > 60 | |
| Number of participants | (n) | 142,219 | 9,709 | 30,494 | 37,890 | 29,450 | 25,272 | 9,404 |
| Number of PC cases | - | 556 | 40 | 95 | 134 | 120 | 104 | 63 |
| Person-Years | - | 1,978,418 | 131,552 | 433,917 | 534,503 | 405,407 | 347,434 | 125,605 |
| Age at recruitment | (years) | 52 ± 10 | 54 ± 11 | 51 ± 12 | 52 ± 11 | 52 ± 9 | 53 ± 9 | 53 ± 8 |
| Height | (cm) | 175 ± 7 | 172 ± 8 | 175 ± 7 | 175 ± 7 | 175 ± 7 | 175 ± 7 | 174 ± 7 |
| Weight | (kg) | 81 ± 12 | 80 ± 13 | 80 ± 12 | 81 ± 12 | 81 ± 11 | 82 ± 12 | 83 ± 13 |
| BMI | (kg/m2) | 27 ± 4 | 27 ± 4 | 26 ± 4 | 26 ± 4 | 26 ± 3 | 27 ± 4 | 27 ± 4 |
| Diabetes mellitus | (%) | 4 | 7 | 4 | 3 | 3 | 3 | 4 |
| Smokers at recruitment | (%) | 29 | 29 | 23 | 25 | 29 | 36 | 49 |
| Participants moderately active | (%) | 24 | 21 | 23 | 24 | 25 | 25 | 25 |
| Participants with at least a university degree | (%) | 27 | 15 | 23 | 29 | 31 | 29 | 22 |
| Energy from non-alcohol drinking | (kcal/day) | 2,268 ± 637 | 2,282 ± 686 | 2,222 ± 659 | 2,260 ± 626 | 2,297 ± 620 | 2,277 ± 620 | 2,318 ± 637 |
| Lifetime alcohol intakes | (g/day) | 26 ± 29 | 23 ± 47 | 10 ± 20 | 15 ± 15 | 24 ± 18 | 38 ± 23 | 65 ± 41 |
| Baseline alcohol intakes | (g/day) | 20 ± 23 | 0 ± 0 | 2 ± 1 | 9 ± 3 | 21 ± 4 | 42 ± 8 | 82 ± 25 |
| Women | Units | < 0.1 | 0.1 - 4.9 | 5 - 14.9 | 15 - 29.9 | > 30 | ||
| Number of participants | (n) | 333,887 | 56,754 | 132,159 | 89,804 | 35,695 | 19,475 | - |
| Number of PC cases | - | 727 | 127 | 278 | 187 | 81 | 54 | - |
| Person-Years | - | 4,660,979 | 799,607 | 1,841,850 | 1,261,568 | 490,471 | 267,483 | - |
| Age at recruitment | (years) | 51 ± 10 | 52 ± 9 | 50 ± 10 | 51 ± 10 | 51 ± 9 | 52 ± 8 | - |
| Height | (cm) | 162 ± 7 | 160 ± 7 | 163 ± 7 | 163 ± 6 | 163 ± 6 | 163 ± 6 | - |
| Weight | (kg) | 66 ± 12 | 68 ± 13 | 66 ± 12 | 65 ± 11 | 64 ± 10 | 65 ± 11 | - |
| BMI | (kg/m2) | 25 ± 4 | 26 ± 5 | 25 ± 5 | 24 ± 4 | 24 ± 4 | 24 ± 4 | - |
| Diabetes melitus | (%) | 2 | 4 | 2 | 1 | 1 | 2 | - |
| Smokers at recruitment | (%) | 19 | 17 | 18 | 18 | 22 | 31 | - |
| Participants moderately active | (%) | 27 | 22 | 29 | 28 | 27 | 26 | - |
| Participants with at least a university degree | (%) | 23 | 13 | 20 | 28 | 30 | 32 | - |
| Energy from non-alcohol drinking | (kcal/day) | 1,877 ± 529 | 1,831 ± 534 | 1,845 ± 521 | 1,910 ± 520 | 1,963 ± 540 | 1,915 ± 558 | - |
| Lifetime alcohol intakes | (g/day) | 7 ± 9 | 1 ± 6 | 3 ± 5 | 8 ± 7 | 14 ± 9 | 23 ± 14 | - |
| Baseline alcohol intakes | (g/day) | 8 ± 12 | 0 ± 0 | 2 ± 1 | 9 ± 3 | 21 ± 4 | 43 ± 15 | - |
Means ± SD are presented for continuous variables, frequencies for categorical variables.
Baseline alcohol intake
In men, baseline alcohol intake was statistically significantly associated with PC risk, with HR comparing alcohol intake greater than 60 g/day to the reference category (0.1-4.9g/day) equal to 1.63 (95%CI: 1.16, 2.29; pWald=0.03), as reported in Table 3. The association remained statistically significant when baseline alcohol intake was modelled as a continuous variable (HR for every increment of 12g/day: 1.05; 95% CI: 1.01, 1.09; ptrend=0.02). For women, no statistically significant association between baseline alcohol intake and PC risk was observed, either as a categorical (pWald=0.68) or as a continuous (HR for every increment of 12g/day: 1.04; 95% CI: 0.97, 1.12; ptrend=0.28) exposure.
Table 3. Hazard Ratio (HR) estimates (95% CI) for baseline and lifetime alcohol intakes and PC.
| Men | Baseline alcohol | Lifetime alcohol | |||||||
| cases | PY | HR1 | 95%CI | cases | PY | HR2 | 95%CI | ||
| Continuous (12 g/day)3 | |||||||||
| 556 | 1,978,417 | 1.05 | (1.01, 1.09) | 429 | 1,460,432 | 1.06 | (1.02, 1.10) | ||
| ptrend | 0.02 | <0.01 | |||||||
| Categories (g/day) | |||||||||
| Ex-consumers | - | - | - | - | 24 | 61,485 | 1.78 | (0.75, 4.22) | |
| Non consumers | 40 | 131,552 | 1.23 | (0.84, 1.79) | 4 | 33,366 | 0.53 | (0.16, 1.74) | |
| 0.1 - 4.94 | 101 | 439,915 | 1.00 | (Ref) | 41 | 176,469 | 1.00 | (Ref) | |
| 5 - 14.9 | 132 | 532,427 | 0.99 | (0.76, 1.29) | 119 | 400,402 | 1.22 | (0.82, 1.81) | |
| 15 - 29.9 | 116 | 403,985 | 1.11 | (0.83, 1.47) | 116 | 389,206 | 1.26 | (0.84, 1.90) | |
| 30 - 59.9 | 104 | 345,443 | 1.10 | (0.82, 1.47) | 88 | 287,583 | 1.42 | (0.93, 2.17) | |
| ≥60 | 63 | 125,095 | 1.63 | (1.16, 2.29) | 37 | 111,921 | 1.77 | (1.06, 2.95) | |
| pWald5 | 0.03 | 0.23 | |||||||
| Women | Baseline alcohol | Lifetime alcohol | |||||||
| cases | PY | HR1 | 95%CI | cases | PY | HR2 | 95%CI | ||
| Continuous (12 g/day)3 | |||||||||
| 727 | 4,660,980 | 1.04 | (0.97, 1.12) | 537 | 3,486,009 | 1.01 | (0.88, 1.14) | ||
| ptrend | 0.28 | 0.90 | |||||||
| Categories (g/day) | |||||||||
| Ex-consumers | - | - | - | - | 31 | 176,499 | 1.07 | (0.54, 2.11) | |
| Non consumers | 127 | 799,607 | 0.98 | (0.78, 1.23) | 63 | 495,243 | 0.72 | (0.47, 1.10) | |
| 0.1 - 4.94 | 280 | 1,852,494 | 1.00 | (Ref) | 210 | 1,296,401 | 1.00 | (Ref) | |
| 5 - 14.9 | 187 | 1,257,465 | 1.00 | (0.82, 1.21) | 165 | 1,052,229 | 1.06 | (0.85, 1.34) | |
| 15 - 29.9 | 80 | 487,565 | 1.11 | (0.86, 1.44) | 59 | 382,037 | 1.16 | (0.85, 1.59) | |
| ≥30 | 53 | 263,849 | 1.16 | (0.85, 1.59) | 9 | 83,601 | 0.93 | (0.47, 1.85) | |
| pWald5 | 0.68 | 0.79 | |||||||
Models for baseline alcohol intake were stratified by center and age at recruitment. Systematic adjustment was undertaken for smoking intensity, physical activity level, educational attainment, diabetes status, BMI, height;
Models for lifetime alcohol intake were stratified by center and age at recruitment. Systematic adjustment was undertaken for smoking intensity, physical activity level, educational attainment, diabetes status, BMI, height, duration of alcohol drinking, time since quitting, and an indicator variable for drinkers;
12g of alcohol correspond to about one standard glass of either wine, beer or spirits;
The category 0.1 to 4.9 g/days was used as the reference category;
Wald test for overall significance, according to the χ2 distribution with degrees of freedom equal to the number of categories minus one.
Lifetime alcohol intake
Compared to the reference category, HR for men heavy drinkers (>60 g/day) was 1.77 (95% CI: 1.06, 2.95) without overall statistical significance among categories (pWald=0.23), as reported in Table 3. Analyses in continuous showed HR for a 12 g/day increase equal to 1.06 (95% CI: 1.02, 1.10; ptrend<0.01). No statistically significant associations were observed in women.
Type of alcoholic beverages
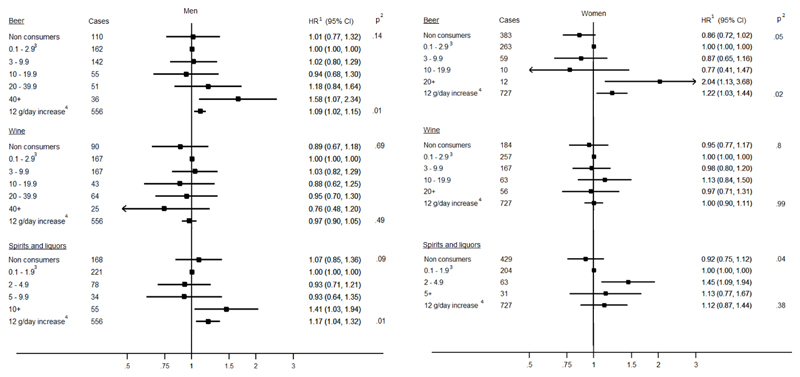
Mutually adjusted HR estimates for baseline alcoholic beverages are shown in Figure 1. Beer consumption was positively associated with PC risk with a 9% (95% CI: 1.02, 1.15; ptrend=0.01) and a 22% (95% CI: 1.03, 1.44; ptrend=0.02) risk increase for 12 g/day in men and women, respectively. The highest levels of beer consumption (>40 g/day in men and >20 g/day in women) were statistically significantly associated with PC risk compared to the reference category (0.1-2.9 g/day) with HR equal to 1.58 (95% CI: 1.10, 2.40) and 2.04 (95% CI: 1.13, 3.68) for men and women, respectively. Spirits/liquors in men were associated with a 17% higher risk (95% CI: 1.04, 1.32; ptrend=0.01) for a 12 g/day increase, while no relationships were observed in women. Wine intake was not associated with PC risk, consistently in men and women. Similar results were observed for lifetime alcohol intake from the different beverages and PC risk (Supplementary Figure 1).
Figure 1. Baseline intake of beer, wine and spirits/liquors (g/day) and Hazard Ratio (HR) of pancreatic cancer in men and women.
1 Models for baseline alcohol intake by subtypes were stratified by center and age at recruitment. Systematic adjustment was undertaken for smoking intensity, physical activity level, educational attainment, diabetes status, BMI, height, and baseline energy intake from other alcohol subtypes;
2 pWald for overall significance across categories were performed according to the χ2 distribution with degrees of freedom equal to the number of categories minus one. Trend tests were performed for continuous variable;
3 The category of light drinkers was used as the reference category (0.1-2.9 g/day for beer and wine, and 0.1-1.9 g/day for spirits/liquors);
4 12g of alcohol correspond to about one standard glass of either wine, beer or spirits/liquors.
Dose-response relationship
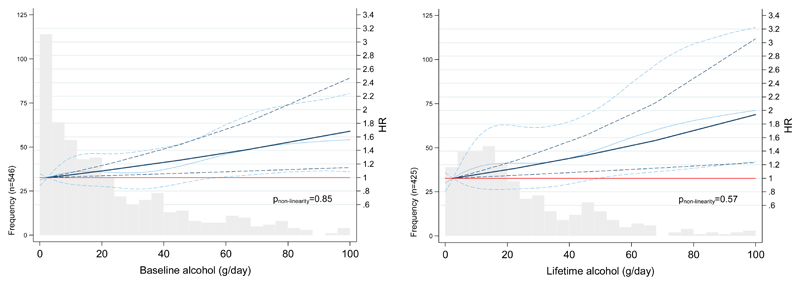
Figure 2 illustrates the dose-response relationship of the baseline and lifetime alcohol intake and PC risk in men, using restricted cubic splines. The trend for baseline and lifetime alcohol intake suggests a linear-shaped association, without evidence for departure from linearity either for baseline (pnon-linearity=0.83) or lifetime alcohol (pnon-linearity=0.57).
Figure 2. Hazard Ratio (HR) functions and corresponding 95% confidence intervals (95% CI) describing the linear (dark blue) and the curvilinear (light blue) dose–response relationship between baseline and lifetime alcohol intake (g/day) and PC risk, according to pancreatic cancer frequencies in men.
Evaluating heterogeneity
Heterogeneity tests by sex and country for baseline alcohol intake were not statistically significant, with p-values equal to 0.63 (data not shown) and 0.33 (Supplementary Figure 2), respectively. Alcohol intake was not associated with PC risk among never smokers with HRs per 12g/day increase equal to 1.06 (95%CI: 0.98, 1.15; ptrend=0.13), unlike current smokers with HR equal to 1.05 (95%CI: 1.00, 1.11; ptrend=0.04). However, the overall interaction test for heterogeneity between alcohol and smoking status was not statistically significant (pheterog=0.84) (Table 4). Thus, the association between baseline alcohol and PC risk was not different across smoking status.
Table 4. Hazard Ratio1 (95% CI) for overall pancreatic cancer risk by categories of baseline alcohol use (g/day) and smoking status (never and current smokers at baseline).
| All participants | Never smokers | Current smokers | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Cases | PY | HR3 | (95%CI) | Cases | PY | HR3 | (95%CI) | pheterogeneity4 | |
| Continuous (12 g/day)2 | |||||||||
| 494 | 3,286,210 | 1.06 | (0.98, 1.15) | 422 | 1,469,414 | 1.05 | (1.00, 1.11) | ||
| ptrend | 0.13 | 0.04 | 0.84 | ||||||
| Categories (g/day) | |||||||||
| Non consumers | 86 | 578,541 | 0.94 | (0.71, 1.23) | 51 | 176026.5 | 1.26 | (0.89, 1.77) | |
| 0.1 - 4.9 | 182 | 1,261,449 | 1.00 | (Ref) | 105 | 439200.4 | 1.00 | (Ref) | |
| 5 - 14.9 | 126 | 879,891 | 0.99 | (0.79, 1.25) | 88 | 360975.2 | 0.91 | (0.68, 1.22) | |
| 15 - 29.9 | 60 | 359,073 | 1.08 | (0.82, 1.50) | 73 | 227177.8 | 1.19 | (0.87, 1.63) | |
| 30 - 59.9 | 28 | 176,457 | 0.93 | (0.65, 1.48) | 71 | 193786.8 | 1.25 | (0.91, 1.73) | |
| ≥ 60 | 12 | 30,799 | 2.17 | (1.18, 3.99) | 34 | 72247.58 | 1.50 | (1.00, 2.28) | |
| pWald5 | 0.14 | 0.10 | 0.41 | ||||||
Models were stratified by centre, age at recruitment and sex. Systematic adjustment was undertaken for smoking status, physical activity level, educational attainment, diabetes status, BMI, height, and an indicator variable for drinkers;
12g of alcohol correspond to about one standard glass of either wine, beer or spirits/liquors;
Models included interaction terms between baseline alcohol use and a smoking indicator (0=never smokers; 1=current smokers), keeping as reference category the group of light alcohol users (0.1–4.9 g/day) among never smokers, whereas former smokers and participants without information on their smoking status were excluded;
Differences in HRs were assessed comparing the log-likelihood of models with and without interaction terms between alcohol and smoking status to one degree of freedom χ2 distribution for analyses in continuous, and to five degrees of freedom χ2 distribution for analyses in categories;
P-value was determined using a Wald test for contrasts according to a χ2 distribution with five degrees of freedom.
Sensitivity analyses
After exclusion of the first two years of follow-up no substantial differences in results was observed in the association with baseline alcohol intake (data not shown). Among women, adjustment for menopausal status, ever use of hormone therapy, and number of full-term pregnancies in women did not alter estimates appreciably. The sensitivity analysis for external adjustment by history of chronic pancreatitis indicated that unadjusted HR estimate comparing baseline heavy drinkers (>60 g/day) vs. moderate drinkers (0.1-4.9 g/day) was marginally attenuated for estimates of relative risk between alcohol and chronic pancreatitis as large as 4 and estimates of the PC relative risk associated with chronic pancreatitis not exceeding 5. Larger attenuations of HR estimates were observed for more extreme scenarios, as displayed in Supplementary Table 1.
Discussion
In this study, alcohol was positively associated with PC risk in men, the relation being particularly apparent among heavy drinkers compared to light drinkers, consistently for baseline and lifetime alcohol intakes, controlling for a comprehensive list of confounding factors. There was no statistically significant association between alcohol consumption and PC in women. Analyses by alcoholic subtypes showed positive relationships for beer and spirits/liquors but not for wine. These results were virtually unaltered after sensitivity analyses.
These findings support observations from other prospective studies.11,12,14,37,38 Our results showed that each 12 g/day of alcohol in men was linearly associated with a 5% increase in PC risk for baseline intakes, with a stronger association with the largest amounts of alcohol greater than 60 g/day, consistently with results from the most recent meta-analyses.13,17,18. While alcohol drinking has been related to PC risk in men, fewer studies found an association in women.14,37 Women drink generally less than men,39 as it was notably the case in the EPIC study, the chance to observe a significant association with PC risk is weaker in women, particularly if such association is apparent at high level of alcohol intake. However, no evidence for heterogeneity across genders between alcohol and PC risk emerged in our study (pheterog=0.63), suggesting that an association with PC risk in women would have been observed if they were showing exposure to alcohol as high as levels observed in men.
Our study used information on lifetime alcohol intake, less often investigated in relation to PC risk. It revealed a statistically significant positive relationship with total lifetime alcohol consumption in men, whether it was modelled as continuous variable with a 6% increase risk for 12g/day or as categories, with men with the highest level of lifetime consumption (>60g/day) having a 77% higher risk when compared to the light drinkers category. Although, one case control study from California showed a more than three-fold significantly increased OR for those with a history of binge drinking,40 this association has not been shown in previous prospective analyses.15,16,40
Specific analyses on alcohol subtypes in this study showed that PC risk was statistically significantly associated with spirits/liquors and beer in men, consistently using baseline and lifetime intake. In women, results were more heterogeneous, showing associations with beer intake at baseline, but not with lifetime intake. These findings are in line with previous studies showing spirits/liquors consumption frequently associated with PC risk.12,14,16,18,37,38 However, the association between beer consumption and PC risk was not reported in recent prospective studies, especially in women. Our results also showed no association with wine intakes, consistent observations with the other prospective studies.12,14,16,18,37 Moreover, country-specific associations showed HR homogeneous estimates despite the variability of drinking patterns across EPIC countries.
The consumption of alcoholic beverages leads to the production of acetaldehyde, the most important metabolite derived from ethanol which increases the production of reactive oxygen species and DNA-adducts.41 Acetaldehyde was classified as carcinogenic in 2012 by the IARC Monograph program.10 Although oxidative stress produced by ethanol may induce damage in pancreatic tissues through lipid peroxidation,42,43 associations observed in this study varied depending on alcoholic subtypes. In vitro models investigating non-alcoholic compounds of alcoholic beverages have shown that beer, unlike pure ethanol or wine, may dose-dependently increase amylase secretion of rat’s acinar cells, and potentially disturb exocrine activity of the pancreas through alteration of cells’ functions.44 In parallel, the absence of association between wine and PC risk could be partially explained by the fact that wine contains molecules with anti-oxidative properties like polyphenols that may counteract ethanol.45 Resveratrol, a well-known polyphenolic compound of wine, has been reported to suppress cell transformation, to induce apoptosis through a p53-dependent pathway and to have chemo-preventive effects.46 More recently, in vitro and ex-vivo models have shown resveratrol suppressive action on pancreatic cells through inhibition of leukotriene A4 hydrolase, an enzyme involved into pancreatic cancer cells growth.47
It has been suggested that cigarette smoking in combination to ethanol may be associated with pancreatic stellate cells activation in cells culture, which are the cells responsible for pancreas fibrosis - a pre-cancerous lesion of PC.48 Despite some evidence for interaction between smoking and alcohol consumption on PC risk in case-control studies,19 this finding has not been replicated in prospective studies,11,12,14 possibly due to the lack of sufficient statistical power. In this study, no interaction between alcohol and smoking was observed, consistently with one large American prospective study.14 This evidence lends further support to the hypothesis that the relationship between alcohol and PC risk does not depend on smoking.
Our study has several strengths and limitations. We took advantage of the large number of PC cases accrued in the EPIC study over a median of 14 years follow up, larger than previous evaluations within EPIC,15,16 where no association was observed between alcohol intake and PC. However, as EPIC participants are volunteers, they may be healthier and not representative of the general population. Thus, the variability of alcohol intake could be lower than in the general population. Moreover, self-reported assessments of alcohol intake are prone to measurement errors, and could have biased the estimates of the association between alcohol and PC risk. However, a previous calibration study in EPIC showed an absence of impact in the assessment of the diet/disease association.25
Study subjects with heavy alcohol consumption are susceptible to develop chronic pancreatitis,49 a known risk factor for PC.50 Accounting for chronic pancreatitis may provide useful information on the mechanism of the relationship between alcohol and PC risk. To address this, a sensitivity analysis was performed. For this analysis to be informative, a priori assumptions were set using evidence from the literature, i.e. the relative risk estimates of chronic pancreatitis associated with PC risk,35 the prevalence of chronic pancreatitis among moderate drinkers32 and the relative risk estimates of chronic pancreatitis comparing extreme to light alcohol drinkers.31 The sensitivity analysis suggests that PC HR estimate in relation to alcohol intake was not substantially altered when information on chronic pancreatitis was accounted for, thus suggesting that alcohol intake exerts its carcinogenic role only partially through chronic pancreatitis.
Conclusion
In summary, our study has shown a moderate but statistically significant increase in PC risk with high alcohol intake, either baseline or lifetime, and particularly with beer and spirits/liquors. These findings provide epidemiologic evidence for the role of alcohol consumption as a potential carcinogen of the pancreas.
Supplementary Material
Novelty and Impact.
Pancreatic cancer (PC) has been inconsistently associated with alcohol consumption owing to the challenge of investigating a rare disease in prospective studies, providing a limited number of incident events. Through a comprehensive evaluation that included 1,283 incident cases, our study indicated that baseline and lifetime alcohol intakes were positively related to PC, with stronger risks estimated for beer and spirits/liquors than wine intake. Associations were not modulated by smoking habits.
Financial disclosure
This work was supported by the Direction Générale de la Santé (French Ministry of Health) (Grant GR-IARC-2003-09-12-01), by the European Commission (Directorate General for Health and Consumer Affairs) and the International Agency for Research on Cancer. The national cohorts are supported by the Danish Cancer Society (Denmark); the Ligue Contre le Cancer, the Institut Gustave Roussy, the Mutuelle Générale de l’Education Nationale and the Institut National de la Santé et de la Recherche Médicale (France); the Deutsche Krebshilfe, the Deutsches Krebsforschungszentrum, and the Federal Ministry of Education and Research (Germany); the Hellenic Health Foundation, the Stavros Niarchos Foundation and the Hellenic Ministry of Health and Social Solidarity (Greece); the Italian Association for Research on Cancer and the National Research Council (Italy); the Dutch Ministry of Public Health, Welfare and Sports, the Netherlands Cancer Registry, LK Research Funds, Dutch Prevention Funds, the Dutch Zorg Onderzoek Nederland, the World Cancer Research Fund and Statistics Netherlands (the Netherlands); the Health Research Fund, Regional Governments of Andalucýa, Asturias, Basque Country, Murcia (project 6236) and Navarra, Instituto de Salud Carlos III, Redes de Investigacion Cooperativa (RD06/0020) (Spain); the Swedish Cancer Society, the Swedish Scientific Council and the Regional Government of Skåne (Sweden); Cancer Research UK (14136 to EPIC-Norfolk; C570/A16491 and C8221/A19170 to EPIC-Oxford), Medical Research Council (1000143 to EPIC-Norfolk, MR/M012190/1 to EPIC-Oxford) (United Kingdom), the Stroke Association, the British Heart Foundation, the Department of Health, the Food Standards Agency, and the Wellcome Trust (UK). This work was part of Sabine Naudin’s PhD at Claude Bernard Lyon 1 University (France), funded by the Communautée de Recherche Academique de la Région Auvergne Rhône-Alpes (France).
Abbreviations
- EPIC
European prospective investigation into cancer and nutrition
- HR
hazard ratio
- CI
confidence interval
- IARC
International Agency for Research on Cancer
- WCRF/AICR
World cancer research fund / American institute of cancer research
Footnotes
Conflict of interest
None to declare.
Data sharing statement
For information on how to submit an application for gaining access to EPIC data and/or biospecimens, please follow the instructions at http://epic.iarc.fr/access/index.ph
References
- 1.Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–386. doi: 10.1002/ijc.29210. [DOI] [PubMed] [Google Scholar]
- 2.Lepage C, Capocaccia R, Hackl M, Lemmens V, Molina E, Pierannunzio D, Sant M, Trama A, Faivre J. Survival in patients with primary liver cancer, gallbladder and extrahepatic biliary tract cancer and pancreatic cancer in Europe 1999–2007: Results of EUROCARE-5. European Journal of Cancer. 2015;51:2169–78. doi: 10.1016/j.ejca.2015.07.034. [DOI] [PubMed] [Google Scholar]
- 3.Rahib L, Smith BD, Aizenberg R, Rosenzweig AB, Fleshman JM, Matrisian LM. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 2014;74:2913–21. doi: 10.1158/0008-5472.CAN-14-0155. [DOI] [PubMed] [Google Scholar]
- 4.Malvezzi M, Bertuccio P, Levi F, La Vecchia C, Negri E. European cancer mortality predictions for the year 2014. Ann Oncol. 2014;25:1650–6. doi: 10.1093/annonc/mdu138. [DOI] [PubMed] [Google Scholar]
- 5.Bray F, Ren J-S, Masuyer E, Ferlay J. Global estimates of cancer prevalence for 27 sites in the adult population in 2008. Int J Cancer. 2013;132:1133–45. doi: 10.1002/ijc.27711. [DOI] [PubMed] [Google Scholar]
- 6.American Institute for World Cancer Research Fund International. Pancreatic cancer | Continuous Update Project Report. Food, Nutrition, Physical Activity, and the Prevention of Pancreatic Cancer. 2012 [Internet] Available from: http://www.dietandcancerreport.org. [Google Scholar]
- 7.Maisonneuve P, Lowenfels AB. Risk factors for pancreatic cancer: a summary review of meta-analytical studies. Int J Epidemiol. 2015;44:186–98. doi: 10.1093/ije/dyu240. [DOI] [PubMed] [Google Scholar]
- 8.Barone E, Corrado A, Gemignani F, Landi S. Environmental risk factors for pancreatic cancer: an update. Arch Toxicol. 2016;90:2617–42. doi: 10.1007/s00204-016-1821-9. [DOI] [PubMed] [Google Scholar]
- 9.Ilic M, Ilic I. Epidemiology of pancreatic cancer. World J Gastroenterol. 2016;22:9694–705. doi: 10.3748/wjg.v22.i44.9694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Personal habits and indoor combustions. Volume 100 E. A review of human carcinogens. IARC Monogr Eval Carcinog Risks Hum. 2012;100:1–538. [PMC free article] [PubMed] [Google Scholar]
- 11.Heinen MM, Verhage BAJ, Ambergen TAW, Goldbohm RA, van den Brandt PA. Alcohol consumption and risk of pancreatic cancer in the Netherlands cohort study. Am J Epidemiol. 2009;169:1233–42. doi: 10.1093/aje/kwp028. [DOI] [PubMed] [Google Scholar]
- 12.Jiao L, Silverman DT, Schairer C, Thiébaut ACM, Hollenbeck AR, Leitzmann MF, Schatzkin A, Stolzenberg-Solomon RZ. Alcohol use and risk of pancreatic cancer: the NIH-AARP Diet and Health Study. Am J Epidemiol. 2009;169:1043–51. doi: 10.1093/aje/kwp034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tramacere I, Scotti L, Jenab M, Bagnardi V, Bellocco R, Rota M, Corrao G, Bravi F, Boffetta P, La Vecchia C. Alcohol drinking and pancreatic cancer risk: a meta-analysis of the dose-risk relation. Int J Cancer. 2010;126:1474–86. doi: 10.1002/ijc.24936. [DOI] [PubMed] [Google Scholar]
- 14.Gapstur SM, Jacobs EJ, Deka A, McCullough ML, Patel AV, Thun MJ. Association of alcohol intake with pancreatic cancer mortality in never smokers. Arch Intern Med. 2011;171:444–51. doi: 10.1001/archinternmed.2010.536. [DOI] [PubMed] [Google Scholar]
- 15.Bueno de Mesquita HB, Maisonneuve P, Moerman CJ, Runia S, Boyle P. Lifetime consumption of alcoholic beverages, tea and coffee and exocrine carcinoma of the pancreas: a population-based case-control study in The Netherlands. Int J Cancer. 1992;50:514–22. doi: 10.1002/ijc.2910500403. [DOI] [PubMed] [Google Scholar]
- 16.Rohrmann S, Linseisen J, Vrieling A, Boffetta P, Stolzenberg-Solomon RZ, Lowenfels AB, Jensen MK, Overvad K, Olsen A, Tjonneland A, Boutron-Ruault M-C, et al. Ethanol intake and the risk of pancreatic cancer in the European Prospective Investigation into Cancer and Nutrition (EPIC) Cancer Causes Control. 2009;20:785–94. doi: 10.1007/s10552-008-9293-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bagnardi V, Rota M, Botteri E, Tramacere I, Islami F, Fedirko V, Scotti L, Jenab M, Turati F, Pasquali E, Pelucchi C, et al. Alcohol consumption and site-specific cancer risk: a comprehensive dose-response meta-analysis. Br J Cancer. 2015;112:580–93. doi: 10.1038/bjc.2014.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y-T, Gou Y-W, Jin W-W, Xiao M, Fang H-Y. Association between alcohol intake and the risk of pancreatic cancer: a dose-response meta-analysis of cohort studies. BMC Cancer. 2016;16:212. doi: 10.1186/s12885-016-2241-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.La Torre G, Sferrazza A, Gualano MR, de Waure C, Clemente G, De Rose AM, Nicolotti N, Nuzzo G, Siliquini R, Boccia A, Ricciardi W. Investigating the Synergistic Interaction of Diabetes, Tobacco Smoking, Alcohol Consumption, and Hypercholesterolemia on the Risk of Pancreatic Cancer: A Case-Control Study in Italy. Biomed Res Int. 2014;2014 doi: 10.1155/2014/481019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Riboli E, Hunt KJ, Slimani N, Ferrari P, Norat T, Fahey M, Charrondière UR, Hémon B, Casagrande C, Vignat J, Overvad K, et al. European Prospective Investigation into Cancer and Nutrition (EPIC): study populations and data collection. Public Health Nutr. 2002;5:1113–24. doi: 10.1079/PHN2002394. [DOI] [PubMed] [Google Scholar]
- 21.Kaaks R, Slimani N, Riboli E. Pilot phase studies on the accuracy of dietary intake measurements in the EPIC project: overall evaluation of results. European Prospective Investigation into Cancer and Nutrition. Int J Epidemiol. 1997;26(Suppl 1):S26–36. doi: 10.1093/ije/26.suppl_1.s26. [DOI] [PubMed] [Google Scholar]
- 22.Slimani N, Ferrari P, Ocké M, Welch A, Boeing H, Liere M, Pala V, Amiano P, Lagiou A, Mattisson I, Stripp C, et al. Standardization of the 24-hour diet recall calibration method used in the european prospective investigation into cancer and nutrition (EPIC): general concepts and preliminary results. Eur J Clin Nutr. 2000;54:900–17. doi: 10.1038/sj.ejcn.1601107. [DOI] [PubMed] [Google Scholar]
- 23.Sieri S, Agudo A, Kesse E, Klipstein-Grobusch K, San-José B, Welch AA, Krogh V, Luben R, Allen N, Overvad K, Tjønneland A, et al. Patterns of alcohol consumption in 10 European countries participating in the European Prospective Investigation into Cancer and Nutrition (EPIC) project. Public Health Nutr. 2002;5:1287–96. doi: 10.1079/PHN2002405. [DOI] [PubMed] [Google Scholar]
- 24.Slimani N, Deharveng G, Unwin I, Southgate DaT, Vignat J, Skeie G, Salvini S, Parpinel M, Møller A, Ireland J, Becker W, et al. The EPIC nutrient database project (ENDB): a first attempt to standardize nutrient databases across the 10 European countries participating in the EPIC study. Eur J Clin Nutr. 2007;61:1037–56. doi: 10.1038/sj.ejcn.1602679. [DOI] [PubMed] [Google Scholar]
- 25.Ferrari P, Day NE, Boshuizen HC, Roddam A, Hoffmann K, Thiébaut A, Pera G, Overvad K, Lund E, Trichopoulou A, Tumino R, et al. The evaluation of the diet/disease relation in the EPIC study: considerations for the calibration and the disease models. Int J Epidemiol. 2008;37:368–78. doi: 10.1093/ije/dym242. [DOI] [PubMed] [Google Scholar]
- 26.Thiébaut ACM, Bénichou J. Choice of time-scale in Cox’s model analysis of epidemiologic cohort data: a simulation study. Stat Med. 2004;23:3803–20. doi: 10.1002/sim.2098. [DOI] [PubMed] [Google Scholar]
- 27.Cust AE, Smith BJ, Chau J, van der Ploeg HP, Friedenreich CM, Armstrong BK, Bauman A. Validity and repeatability of the EPIC physical activity questionnaire: a validation study using accelerometers as an objective measure. International Journal of Behavioral Nutrition and Physical Activity. 2008;5:33. doi: 10.1186/1479-5868-5-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schoenfeld D. Partial Residuals for The Proportional Hazards Regression Model. Biometrika. 1982;69:239–41. [Google Scholar]
- 29.Durrleman S, Simon R. Flexible regression models with cubic splines. Stat Med. 1989;8:551–61. doi: 10.1002/sim.4780080504. [DOI] [PubMed] [Google Scholar]
- 30.Greenland S. Basic Methods for Sensitivity Analysis of Biases. Int J Epidemiol. 1996;25:1107–16. [PubMed] [Google Scholar]
- 31.Samokhvalov AV, Rehm J, Roerecke M. Alcohol Consumption as a Risk Factor for Acute and Chronic Pancreatitis: A Systematic Review and a Series of Meta-analyses. EBioMedicine. 2015;2:1996–2002. doi: 10.1016/j.ebiom.2015.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Setiawan VW, Pandol SJ, Porcel J, Wilkens LR, Le Marchand L, Pike MC, Monroe KR. Prospective Study of Alcohol Drinking, Smoking, and Pancreatitis: The Multiethnic Cohort. Pancreas. 2016;45:819–25. doi: 10.1097/MPA.0000000000000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Raimondi S, Lowenfels AB, Morselli-Labate AM, Maisonneuve P, Pezzilli R. Pancreatic cancer in chronic pancreatitis; aetiology, incidence, and early detection. Best Pract Res Clin Gastroenterol. 2010;24:349–58. doi: 10.1016/j.bpg.2010.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Duell EJ, Lucenteforte E, Olson SH, Bracci PM, Li D, Risch HA, Silverman DT, Ji BT, Gallinger S, Holly EA, Fontham EH, et al. Pancreatitis and pancreatic cancer risk: a pooled analysis in the International Pancreatic Cancer Case-Control Consortium (PanC4) Ann Oncol. 2012 doi: 10.1093/annonc/mds140. mds140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dhar P, Kalghatgi S, Saraf V. Pancreatic Cancer in Chronic Pancreatitis. Indian J Surg Oncol. 2015;6:57–62. doi: 10.1007/s13193-014-0373-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.StataCorp LP. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP; 2015. www.stata.com. [Google Scholar]
- 37.Genkinger JM, Spiegelman D, Anderson KE, Bergkvist L, Bernstein L, van den Brandt PA, English DR, Freudenheim JL, Fuchs CS, Giles GG, Giovannucci E, et al. Alcohol intake and pancreatic cancer risk: a pooled analysis of fourteen cohort studies. Cancer Epidemiol Biomarkers Prev. 2009;18:765–76. doi: 10.1158/1055-9965.EPI-08-0880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michaud DS, Vrieling A, Jiao L, Mendelsohn JB, Steplowski E, Lynch SM, Wactawski-Wende J, Arslan AA, Bas Bueno-de-Mesquita H, Fuchs CS, Gross M, et al. Alcohol intake and pancreatic cancer: a pooled analysis from the pancreatic cancer cohort consortium (PanScan) Cancer Causes Control. 2010;21:1213–25. doi: 10.1007/s10552-010-9548-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wilsnack RW, Wilsnack SC, Kristjanson AF, Vogeltanz-Holm ND, Gmel G. Gender and alcohol consumption: patterns from the multinational GENACIS project. Addiction. 2009;104:1487–500. doi: 10.1111/j.1360-0443.2009.02696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gupta S, Wang F, Holly EA, Bracci PM. Risk of pancreatic cancer by alcohol dose, duration, and pattern of consumption, including binge drinking: a population-based study. Cancer Causes Control. 2010;21:1047–59. doi: 10.1007/s10552-010-9533-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Seitz HK, Stickel F. Molecular mechanisms of alcohol-mediated carcinogenesis. Nat Rev Cancer. 2007;7:599–612. doi: 10.1038/nrc2191. [DOI] [PubMed] [Google Scholar]
- 42.Criddle DN, Raraty MGT, Neoptolemos JP, Tepikin AV, Petersen OH, Sutton R. Ethanol toxicity in pancreatic acinar cells: mediation by nonoxidative fatty acid metabolites. Proc Natl Acad Sci USA. 2004;101:10738–43. doi: 10.1073/pnas.0403431101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palmieri VO, Grattagliano I, Palasciano G. Ethanol induces secretion of oxidized proteins by pancreatic acinar cells. Cell Biol Toxicol. 2007;23:459–64. doi: 10.1007/s10565-007-9007-0. [DOI] [PubMed] [Google Scholar]
- 44.Gerloff A, Singer MV, Feick P. Beer and its Non-Alcoholic Compounds: Role in Pancreatic Exocrine Secretion, Alcoholic Pancreatitis and Pancreatic Carcinoma. Int J Environ Res Public Health. 2010;7:1093–104. doi: 10.3390/ijerph7031093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Howard A, Chopra M, Thurnham DI, Strain JJ, Fuhrman B, Aviram M. Red wine consumption and inhibition of LDL oxidation: what are the important components? Medical Hypotheses. 2002;59:101–4. doi: 10.1016/s0306-9877(02)00144-5. [DOI] [PubMed] [Google Scholar]
- 46.Jang M, Cai L, Udeani GO, Slowing KV, Thomas CF, Beecher CW, Fong HH, Farnsworth NR, Kinghorn AD, Mehta RG, Moon RC, et al. Cancer chemopreventive activity of resveratrol, a natural product derived from grapes. Science. 1997;275:218–20. doi: 10.1126/science.275.5297.218. [DOI] [PubMed] [Google Scholar]
- 47.Oi N, Jeong C-H, Nadas J, Cho Y-Y, Pugliese A, Bode AM, Dong Z. Resveratrol, a Red Wine Polyphenol, Suppresses Pancreatic Cancer by Inhibiting Leukotriene A4 Hydrolase. Cancer Res. 2010;70:9755–64. doi: 10.1158/0008-5472.CAN-10-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lee ATK, Xu Z, Pothula SP, Patel MB, Pirola RC, Wilson JS, Apte MV. Alcohol and cigarette smoke components activate human pancreatic stellate cells: implications for the progression of chronic pancreatitis. Alcohol Clin Exp Res. 2015;39:2123–33. doi: 10.1111/acer.12882. [DOI] [PubMed] [Google Scholar]
- 49.Yadav D, Lowenfels AB. The Epidemiology of Pancreatitis and Pancreatic Cancer. Gastroenterology. 2013;144:1252–61. doi: 10.1053/j.gastro.2013.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Herreros-Villanueva M, Hijona E, Bañales JM, Cosme A, Bujanda L. Alcohol consumption on pancreatic diseases. World J Gastroenterol. 2013;19:638–47. doi: 10.3748/wjg.v19.i5.638. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.