Abstract
Background
Poor interprofessional collaboration (IPC) can adversely affect the delivery of health services and patient care. Interventions that address IPC problems have the potential to improve professional practice and healthcare outcomes.
Objectives
To assess the impact of practice‐based interventions designed to improve interprofessional collaboration (IPC) amongst health and social care professionals, compared to usual care or to an alternative intervention, on at least one of the following primary outcomes: patient health outcomes, clinical process or efficiency outcomes or secondary outcomes (collaborative behaviour).
Search methods
We searched CENTRAL (2015, issue 11), MEDLINE, CINAHL, ClinicalTrials.gov and WHO International Clinical Trials Registry Platform to November 2015. We handsearched relevant interprofessional journals to November 2015, and reviewed the reference lists of the included studies.
Selection criteria
We included randomised trials of practice‐based IPC interventions involving health and social care professionals compared to usual care or to an alternative intervention.
Data collection and analysis
Two review authors independently assessed the eligibility of each potentially relevant study. We extracted data from the included studies and assessed the risk of bias of each study. We were unable to perform a meta‐analysis of study outcomes, given the small number of included studies and their heterogeneity in clinical settings, interventions and outcomes. Consequently, we summarised the study data and presented the results in a narrative format to report study methods, outcomes, impact and certainty of the evidence.
Main results
We included nine studies in total (6540 participants); six cluster‐randomised trials and three individual randomised trials (1 study randomised clinicians, 1 randomised patients, and 1 randomised clinicians and patients). All studies were conducted in high‐income countries (Australia, Belgium, Sweden, UK and USA) across primary, secondary, tertiary and community care settings and had a follow‐up of up to 12 months. Eight studies compared an IPC intervention with usual care and evaluated the effects of different practice‐based IPC interventions: externally facilitated interprofessional activities (e.g. team action planning; 4 studies), interprofessional rounds (2 studies), interprofessional meetings (1 study), and interprofessional checklists (1 study). One study compared one type of interprofessional meeting with another type of interprofessional meeting. We assessed four studies to be at high risk of attrition bias and an equal number of studies to be at high risk of detection bias.
For studies comparing an IPC intervention with usual care, functional status in stroke patients may be slightly improved by externally facilitated interprofessional activities (1 study, 464 participants, low‐certainty evidence). We are uncertain whether patient‐assessed quality of care (1 study, 1185 participants), continuity of care (1 study, 464 participants) or collaborative working (4 studies, 1936 participants) are improved by externally facilitated interprofessional activities, as we graded the evidence as very low‐certainty for these outcomes. Healthcare professionals' adherence to recommended practices may be slightly improved with externally facilitated interprofessional activities or interprofessional meetings (3 studies, 2576 participants, low certainty evidence). The use of healthcare resources may be slightly improved by externally facilitated interprofessional activities, interprofessional checklists and rounds (4 studies, 1679 participants, low‐certainty evidence). None of the included studies reported on patient mortality, morbidity or complication rates.
Compared to multidisciplinary audio conferencing, multidisciplinary video conferencing may reduce the average length of treatment and may reduce the number of multidisciplinary conferences needed per patient and the patient length of stay. There was little or no difference between these interventions in the number of communications between health professionals (1 study, 100 participants; low‐certainty evidence).
Authors' conclusions
Given that the certainty of evidence from the included studies was judged to be low to very low, there is not sufficient evidence to draw clear conclusions on the effects of IPC interventions. Neverthess, due to the difficulties health professionals encounter when collaborating in clinical practice, it is encouraging that research on the number of interventions to improve IPC has increased since this review was last updated. While this field is developing, further rigorous, mixed‐method studies are required. Future studies should focus on longer acclimatisation periods before evaluating newly implemented IPC interventions, and use longer follow‐up to generate a more informed understanding of the effects of IPC on clinical practice.
Keywords: Female, Humans, Cooperative Behavior, Health Personnel, Interprofessional Relations, Professional Practice, Allied Health Occupations, Checklist, Delivery of Health Care, Nurses, Pharmacists, Physicians, Quality of Health Care, Randomized Controlled Trials as Topic, Social Workers, Telecommunications
Plain language summary
How effective are strategies to improve the way health and social care professional groups work together?
What is the aim of this review?
The aim of this Cochrane Review was to find out whether strategies to improve interprofessional collaboration (the process by which different health and social care professional groups work together), can positively impact the delivery of care to patients. Cochrane researchers collected and analysed all relevant studies to answer this question, and found nine studies with 5540 participants.
Key messages
Strategies to improve interprofessional collaboration between health and social care professionals may slightly improve patient functional status, professionals' adherence to recommended practices, and the use of healthcare resources. Due to the lack of clear evidence, we are uncertain whether the strategies improved patient‐assessed quality of care, continuity of care, or collaborative working.
What was studied in this review?
The extent to which different health and social care professionals work well together affects the quality of the care that they provide. If there are problems in how these professionals communicate and interact with each other, this can lead to problems in patient care. Interprofessional collaboration practice‐based interventions are strategies that are put into place in healthcare settings to improve interactions and work processes between two or more types of healthcare professionals. This review studied different interprofessional collaboration interventions, compared to usual care or an alternative intervention, to see if they improved patient care or collaboration.
What are the main results of the review?
The review authors found nine relevant studies across primary, secondary, tertiary and community care settings. All studies were conducted in high‐income countries (Australia, Belgium, Sweden, UK and USA) and lasted for up to 12 months. Most of the studies were well conducted, although some studies reported that many participants dropped out. The studies evaluated different methods of interprofessional collaboration, namely externally facilitated interprofessional activities (e.g. collaborative planning/reflection activities led by an individual who is not part of the group/team), interprofessional rounds, interprofessional meetings, and interprofessional checklists.
Externally facilitated interprofessional activities may slightly improve patient functional status and health care professionals' adherence to recommended practices, and may slightly improve use of healthcare resources. We are uncertain whether externally facilitated interprofessional activities improve patient‐assessed quality of care, continuity of care, or collaborative working behaviours. The use of interprofessional rounds and interprofessional checklists may slightly improve the use of healthcare resources. Interprofessional meetings may slightly improve adherence to recommended practices, and may slightly improve use of healthcare resources.
Further research is needed, including studies testing the interventions at scale to develop a better understanding of the range of possible interventions and their effectiveness, how they affect interprofessional collaboration and lead to changes in care and patient health outcomes, and in what circumstances such interventions may be most useful.
How up to date is this review?
The review authors searched for studies that had been published to November 2015.
Summary of findings
Summary of findings for the main comparison. Effects of practice‐based interprofessional collaboration (IPC) interventions on professional practice and healthcare outcomes compared to usual care.
| Effects of practice‐based interprofessional collaboration (IPC) interventions on professional practice and healthcare outcomes compared to usual care | |||
| Patient or population: health and social care professionals involved in the delivery of health services and patient care Settings: primary, secondary, tertiary and community care settings, primarily in the USA and the UK Intervention: practice‐based interprofessional collaboration (IPC) interventions with an explicit objective of improving collaboration between two or more health or social care professionals Comparison: usual care | |||
| Outcomes | Impacts | No. of studies (participants) | Certainty of the evidence (GRADE) |
| Patient health outcomes | |||
| Patient functional status | Externally facilitated interprofessional activities may slightly improve stroke patients' functional status (Strasser 2008). | 1 (464) |
⊕⊕⊖⊖ Lowa |
| Patient‐assessed quality of care | It is uncertain if externally facilitated interprofessional activities increases patient‐assessed quality of care because the certainty of this evidence is very low (Black 2013). | 1 (1185) |
⊕⊖⊖⊖ Very lowb |
| Patient mortality, morbidity or complication rates | None of the included studies reported patient mortality, morbidity or complication rates. | ‐‐ | ‐‐ |
| Clinical process or efficiency outcomes | |||
| Adherence to recommended practices | The use of interprofessional activities with an external facilitator or interprofessional meetings may slightly improve adherence to recommended practices and prescription of drugs (Cheater 2005; Deneckere 2013; Schmidt 1998). | 3 (2576) |
⊕⊕⊖⊖ Lowc |
| Continuity of care | It is uncertain if externally facilitated interprofessional activities improves continuity of care because the certainty of this evidence is very low (Strasser 2008). | 1 (464) |
⊕⊖⊖⊖ Very lowd |
| dUse of healthcare resources | Interprofessional checklists (Calland 2011), interprofessional rounds (Curley 1998; Wild 2004) or externally facilitated interprofessional activities (Strasser 2008), may slightly improve overall use of resources, length of hospital stay, or costs. | 4 (1679) |
⊕⊕⊖⊖ Lowe |
| Collaborative behaviour outcomes | |||
| Collaborative working; team communication; team co‐ordination | It is uncertain whether externally facilitated interprofessional activities (Black 2013; Calland 2011; Cheater 2005; Deneckere 2013) improve collaborative working, team communication, and co‐ordination because the certainty of this evidence is very low. | 4 (1936) |
⊕⊖⊖⊖ Very lowf |
| GRADE Working Group grades of evidence High‐certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate‐certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very‐certainty: We are very uncertain about the estimate. | |||
a We assessed the certainty of the evidence as low because of high risk of bias (no blinding of outcome assessment).
b We assessed the certainty of the evidence as very low because of the risk of bias (high risk of attrition and detection bias; details about allocation sequence generation and concealment were not reported).
c We assessed the certainty of the evidence as low due to potential indirectness (both studies were conducted in one country and the outcomes may not be transferable to other settings), and risk of bias (high risk of attrition, unclear selection and reporting risk).
d We assessed the certainty of the evidence as very low because of risk of bias (high risk of attrition and detection bias, and unclear risk of selection bias).
e We assessed the certainty of evidence as low because of high risk of bias (attrition and detection), and unclear risk of bias (selection, reporting, and contamination).
f We assessed the certainty of the evidence as very low due to high risk of bias (selection, attrition, and detection) or unclear risk of bias (reporting and contamination).
Summary of findings 2. Effects of practice‐based interprofessional collaboration (IPC) interventions on professional practice and healthcare outcomes compared with alternative IPC intervention.
| Effects of practice‐based interprofessional collaboration (IPC) interventions on professional practice and healthcare outcomes compared with alternative IPC intervention | |||
|
Patient or population: health and social care professionals involved in the delivery of health services and patient care Settings: two hospitals in Australia Intervention: multidisciplinary video conferencing Comparison: multidisciplinary audio conferencing | |||
| Outcomes | Impacts | No. of studies (participants) | Certainty of the evidence (GRADE) |
| Patient health outcomes | The study did not report patient health outcomes. | ‐ | ‐ |
| Clinical process or efficiency outcomes | Video conferencing may reduce the average length of treatment, compared to audio conferencing and may improve process/efficiency outcomes by reducing the number of multidisciplinary conferences needed per patient and patient length of stay. | 1 (100) | ⊕⊕⊖⊖ Lowa |
| Collaborative behaviour outcomes | There was little or no difference between the interventions in the number of communications between health professionals. | 1 (100) | ⊕⊕⊖⊖ Lowa |
| GRADE Working Group grades of evidence High‐certainty: Further research is very unlikely to change our confidence in the estimate of effect. Moderate‐certainty: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low‐certainty: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low‐certainty: We are very uncertain about the estimate. | |||
a We assessed the certainty of evidence as low because of high risk of bias (attrition and detection) and unclear risk of bias (selection, reporting, and contamination).
Background
Description of the condition
Interprofessional collaboration (IPC) is the process by which different health and social care professional groups work together to positively impact care. IPC involves regular negotiation and interaction between professionals, which values the expertise and contributions that various healthcare professionals bring to patient care. However, IPC can be affected by problems linked to imbalances of authority, limited understanding of others’ roles and responsibilities, and professional boundary friction when delivering patient care (Baker 2011; Reeves 2010).
Research has repeatedly documented the impact of collaboration problems on work processes and patient safety (Lillebo 2015; Van Leijen‐Zeelenberg 2015). For example, failures of collaboration were found to be at the centre of a number of care failures across the globe (Francis 2013; The Joint Commission 2016). Therefore, professionals must ensure that they collaborate in an effective manner to deliver safe, high‐quality patient care.
Different health policy makers across the globe have repeatedly called for the use of IPC as a key approach to improve the quality and safety of patient care (Department of Health 1997; Health Canada 2003; Institute of Medicine 2000; Institute of Medicine 2013; WHO 1976; WHO 2010). During the past 10 years in particular, IPC has been at the forefront of much curricular, research, policy, and regulatory activity at national and international levels. The promotion of IPC stems from the complexity and multifaceted nature of patients' health and care needs and the health system, and research that suggests that improved collaboration between multiple professionals may be essential for the provision of effective and comprehensive care.
Research in the area of IPC is complicated by the use of varied terms (interdisciplinary collaboration, multidisciplinary co‐ordination, transprofessional teamwork), which has resulted in conceptual confusion within the field (Reeves 2010). As a result, one must take care when evaluating such studies, to ensure one understands the nature and key activities of the intervention, whatever it may be named.
Description of the intervention
An IPC intervention involves members of more than one health or social care profession interacting together with the explicit purpose of improving IPC. A scoping review examining the nature of interventions used in the interprofessional field found three main types: education‐based interventions, practice‐based interventions, and organisationally‐based interventions (Reeves 2011).
This review focuses on interprofessional practice‐based interventions, also called practice‐based IPC interventions. An interprofessional practice‐based intervention involves the deployment in the workplace of a tool or routine to improve IPC; examples include communication tools, interprofessional meetings, and checklists.
A review focusing solely on interprofessional education (an education‐based intervention) was recently updated (Reeves 2013). In this review, an interprofessional education intervention was defined as 'members of more than one health or social care profession learning interactively together, for the explicit purpose of improving interprofessional collaboration, the health and well‐being of patients, or both.' Interactive learning requires active learner participation and active exchange between learners from different professions.
An interprofessional organisationally‐based intervention involves a change at an organisational level to improve interprofessional collaboration; examples include policy and staffing changes (Reeves 2010). A review of the effects of this type of intervention still needs to be undertaken, to generate a more holistic understanding of the nature of these different, but complementary, interventions.
How the intervention might work
A practice‐based IPC intervention might work by incorporating a tool, routine, or activity to improve interprofessional interaction (e.g. communication, co‐ordination) into clinical practice. In turn, this may improve how healthcare professionals work together and deliver health care, leading to improved health outcomes.
Why it is important to do this review
Research identifying various problems with IPC, and the delivery of care and patient outcomes, continues to accumulate (Körner 2016; Van Leijen‐Zeelenberg 2015). Therefore, It is important to understand the effectiveness of interventions aimed at improving IPC on health and social care. Governments around the globe continue to institute major changes and invest significant resources to improve IPC. Ideally, these policy decisions should be based on evidence of the effectiveness of these approaches. The aim of this review is to update a previous review, and synthesise evidence from randomised trials of practice‐based IPC interventions, to inform such decision‐making (Zwarenstein 2009).
Objectives
To assess the impact of practice‐based interventions designed to improve IPC amongst health and social care professionals, compared to usual care or to an alternative intervention, on at least one of the following primary outcomes: patient health outcomes, clinical process or efficiency outcomes or secondary outcomes (collaborative behaviour).
Methods
Criteria for considering studies for this review
Types of studies
We only considered individual or cluster‐randomised, which provide the most reliable evidence for the effects of practice‐based interprofessional collaboration (IPC).
Types of participants
We included interventions that targeted any type of health and social care professional (e.g. chiropodists or podiatrists, complementary therapists, dentists, dietitians, doctors or physicians, hygienists, midwives, nurses, occupational therapists, pharmacists, physiotherapists, psychologists, psychotherapists, radiographers, social workers, or speech therapists).
Types of interventions
We included any practice‐based intervention with an explicit objective of improving collaboration between two or more health or social care professionals. We used the following criterion to include interventions.
Evaluations of a practice‐based IPC intervention, where the study explicitly noted an objective to improve collaboration amongst two or more types of health or social care professionals. Other terms besides IPC could have been used, and were accepted as equivalent to IPC, such as communication, co‐ordination, and teamwork.
The comparator was usual care or an alternative intervention, We placed no restrictions on interventions or settings (e.g. hospitals, primary care, community‐based care).
Types of outcome measures
Primary outcomes
-
Patient health outcomes (objectively measured or self‐reported, using a validated instrument)
mortality
morbidity
disease incidence
disease duration
cure rates
quality of life measures
functional status
complication rate
patient‐assessed quality of care
-
Clinical process or efficiency outcomes
readmission rates
adherence to recommended practices (by healthcare providers)
continuity of care
use of healthcare resources (i.e. cost‐benefit analysis)
participant satisfaction
Secondary outcomes
Collaborative behaviour (objective or self‐reported outcomes, using a validated instrument)
We excluded interprofessional learning (interprofessional education) as a secondary outcome. Whilst interprofessional education can support IPC in the workplace, these are distinct activities and our focus was the latter.
Search methods for identification of studies
Electronic searches
We searched the following sources.
Cochrane Central Register of Controlled Trials (CENTRAL; 2015, issue 11) in the Cochrane Library (searched on 24 November 2015; full strategy available in Appendix 1).
MEDLINE Ovid: 2007 to 2015 (searched on 10 November 2015; full strategy available in Appendix 2).
CINAHL EBSCO: 2007 to 2015 (searched on 10 November 2015; full strategy available in Appendix 3).
ClinicalTrials.gov and WHO International Clinical Trials Registry Platform (ICTRP): 2007 to 2015 (searched on 24 November 2015; full strategy available in Appendix 4).
We placed no language restrictions on the search strategy. We did not search Embase, as a review of the studies included previously showed that none were indexed in this database. We included all the trials identified by the previous version of the review (Zwarenstein 2009).
Searching other resources
We handsearched the Journal of Interprofessional Care (2007 to November 2015), and reviewed the reference lists of included studies.
Data collection and analysis
Selection of studies
At least two review authors (SR, FP) independently reviewed each of the titles and abstracts retrieved in the searches, to identify those that met the review's inclusion criteria.
We obtained the full‐text of all potentially relevant articles. At least two review authors (SR, FP) independently assessed each full‐text article to determine if it met all of the criteria. We resolved disagreements by consultation with another review author (MZ). As a further quality check, this additional review author reviewed all included articles.
Data extraction and management
Two review authors (SR, FP) independently extracted the following information from included studies.
Study setting (country, healthcare setting).
Types of study participants.
Description of IPC intervention.
Description of any other interventions.
Outcomes (primary and secondary).
Data for the main outcomes.
Assessment of risk of bias in included studies
We used the suggested criteria recommended by Cochrane Effective Practice and Organisation of Care (EPOC) to assess risk of bias in all studies included in the review (EPOC 2016), an approach that assessed the key areas of:
selection bias;
performance bias;
detection bias;
attrition bias;
reporting bias; and
any other potential sources of bias.
For each criterion, we described the relevant information provided by the trial authors, and judged each item as being at: 1) high risk of bias (plausible bias that seriously weakens confidence in the results); 2) low risk of bias (plausible bias unlikely to seriously alter the results); or 3) unclear risk of bias (lack of information or uncertainty over the potential for bias).
We reported all included studies in the Risk of bias in included studies section below. We did not exclude studies on the basis of their risk of bias. We made an overall assessment of the risk of bias for each outcome (across criteria) within and across studies, using the approach suggested by EPOC 2016.
Measures of treatment effect
We had initially planned to conduct a meta‐analysis, however, this was not possible due to differences in populations and interventions (see Results). Therefore, we presented a narrative summary of the results.
Unit of analysis issues
We critically examined the methods of analysis of all study types. We identified cluster‐randomised trials, and where appropriate, commented on unit of analysis errors in the results and discussion.
Dealing with missing data
It was not possible to undertake a meta‐analysis of the included studies due to heterogeneity and therefore the issue of missing data in statistical analysis did not arise.
Assessment of heterogeneity
We did not assess heterogeneity statistically. Our narrative of randomised trials compared the characteristics of the study populations, interventions, and outcomes (see Data synthesis).
Assessment of reporting biases
We attempted to reduce the risk of reporting bias by undertaking comprehensive searches of multiple databases and trial registers. We found too few studies reporting the primary outcomes to allow any assessment of reporting bias.
Data synthesis
We could not complete a meta‐analysis of study outcomes for this review due to the small number of included studies, and the differences in IPC subtypes, settings, participants, and outcomes across the studies. Consequently, we presented the results in a narrative format. In producing this narrative, we grouped (or categorised) the following types of practice‐based IPC interventions as: externally facilitated interprofessional activities, interprofessional rounds, interprofessional meetings, and interprofessional checklists.
Two review authors (SR, MZ) independently assessed the certainty of the evidence (high, moderate, low, and very low) as it related to the main outcomes, using the five GRADE considerations (risk of bias, consistency of effect, imprecision, indirectness, and publication bias) Guyatt 2008.
We developed two 'Summary of findings' tables for the comparisons: (1) practice‐based IPC compared with usual care and; (2) practice‐based IPC compared with an alternative practice‐based IPC. We included the following outcomes.
Patient health outcomes: patient functional status; patient‐assessed quality of care; patient mortality, morbidity or complication rates.
Clinical process or efficiency outcomes: adherence to recommended practices; continuity of care; use of healthcare resources.
Collaborative behaviour outcomes: collaborative working; team communication or co‐ordination.
We applied the methods and recommendations described in Section 8.5 and Chapter 12 of the Cochrane Handbook of Systematic Reviews of Interventions (Higgins 2011), and the EPOC worksheets (EPOC 2013). We justified all decisions to downgrade or upgrade the certainty of the evidence using footnotes, and made comments to aid readers' understanding of the review where necessary.
Subgroup analysis and investigation of heterogeneity
As we did not undertake a meta‐analysis, we could not conduct a subgroup analysis.
Sensitivity analysis
We had planned to perform a sensitivity analysis for pooled results based on the risk of bias information recorded from the included studies. However, a meta‐analysis was not possible, due to a variation in the intervention and study methods used in the included studies.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
Our searches generated a total of 2493 abstracts. Once duplicate abstracts were removed (N = 1083), the total number of abstracts reviewed was 1410 (see Figure 1). The handsearch did not produce any additional articles. Following assessment of each of the abstracts, we identified 34 studies that potentially met our inclusion criteria (1 from EPOC, 1 from CENTRAL, 29 from MEDLINE, and 3 from CINAHL).
1.
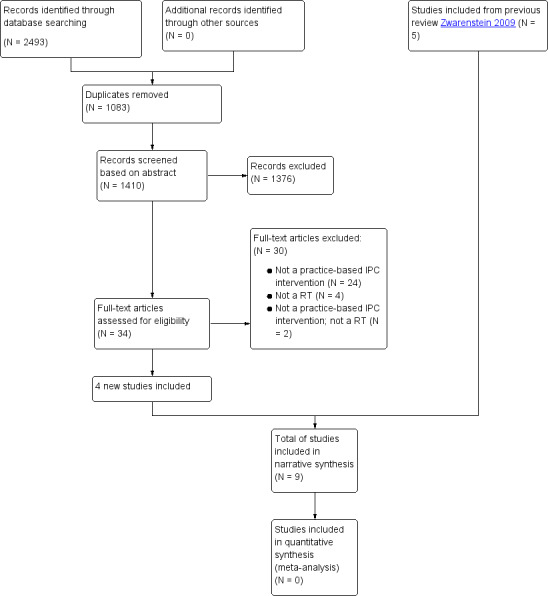
Flow diagram of study selection
We included nine studies (N = 6540), all conducted in high‐income countries (Australia, Belgium, Sweden, UK, and USA). We identified four studies for this update (Black 2013; Calland 2011; Deneckere 2013; Strasser 2008).
Eight studies compared an interprofessional collaboration (IPC) intervention to a control group, which received usual care (Black 2013; Calland 2011; Cheater 2005; Curley 1998; Deneckere 2013; Schmidt 1998; Strasser 2008; Wild 2004); one study compared two different IPC interventions (Wilson 2004).
All of the studies reported an objectively measured, or self‐reported (using a validated instrument), patient, clinical process or efficiency outcome. The secondary outcome, collaborative behaviour, was only evaluated in four of the studies (see below).
Included studies
Externally facilitated interprofessional activities
Black 2013 evaluated the effectiveness of an externally facilitated IPC intervention, based in primary care practices, to support nurses, administrative staff, practice managers, and receptionists to work collaboratively with general practitioners (GPs) to enhance the delivery of patient care when implementing practice systems that supported chronic disease care. Sixty primary care practices, involving 1185 participants based across Australia, took part in the study. The intervention included the following activities: structured appointment systems; patient disease registers; patient recall systems with reminders; patient education; planned care; definition of roles, responsibilities and job descriptions for each professional or staff member; communication and meetings; practice billing; record keeping; and quality improvement. Before the intervention, facilitators conducted workshops with primary care staff, followed by practice visits. Staff were provided with resource manuals and workbooks for each of the different elements of the intervention. Facilitators routinely followed up practices by telephone and email. All control teams received the IPC intervention after the 12‐month follow‐up data collection in each intervention practice.
Cheater 2005 evaluated an externally facilitated programme aimed at improving IPC by the use of a multidisciplinary audit in a secondary care setting. Twenty‐two multidisciplinary teams from five acute care hospitals in the UK participated. Each team consisted of nurses and physicians, and a representative from one or more of other health professional groups (e.g. pharmacist, social worker, physiotherapist), service support staff (e.g. ward clerk, care assistant), and managers (N = 141). A range of specialties were represented. After participating in a two‐day skills workshop, external facilitators facilitated five meetings over a period of six months, for each of the multidisciplinary teams randomised to the intervention group. Intervention teams were required to undertake a collaborative audit (the specific focus of these audits was not identified) and submit an audit report. Control teams provided usual care at their institution .
Deneckere 2013 evaluated an interprofessional intervention for 30 teams caring for patients with chronic obstructive pulmonary disease and proximal femur fracture. Seventeen intervention teams and 13 control teams based in Belgian hospitals were involved in the study (N = 581), the aim of which was to examine effects of the use of care pathways on interprofessional teamwork, in an acute care setting. The intervention involved three facilitated components. To promote IPC the intervention included: feedback on the team's performance before the implementation of care pathways; receipt of evidence‐based key indicators for implementing care pathways in practice; and review and training in the development and implementation of care pathways. The teams randomised to the control group provided usual care (i.e. did not implement care pathways). The intervention teams were given nine months to implement the intervention. After this time period, a summative evaluation was performed, in which the performance on team indicators was compared between the intervention and control groups.
Strasser 2008 evaluated the effects of an externally facilitated intervention aimed at improving collaboration to support better care delivered to patients following a stroke. Thirty‐one teams from different Veteran Affairs' rehabilitation units based in the USA participated in the study. Participants included physicians, nurses, occupational therapists, speech‐language pathologists, physical therapists, case managers, or social workers (N = 464). Intervention teams received the following activities, delivered in three phases: phase 1) an off‐site facilitated workshop emphasising team dynamics, problem‐solving, the use of performance feedback data, and the development of action plans (specific team performance profiles with recommendations) for process improvement; phase 2) development of written action plans to address team process problems, based on discussions at the earlier workshop; and phase 3) telephone and video conference consultation on advice for implementation of action plans, and facilitation of team process skills. Control teams continued with usual care (i.e. specific team performance profile information).
For further details on these studies: see Characteristics of included studies
Interprofessional rounds
Curley 1998 examined the effects of daily interdisciplinary rounds in inpatient medical wards at an acute care hospital in the USA. The intervention group consisted of three ward services that implemented interdisciplinary work rounds; the control group consisted of three other ward services that continued usual care (i.e. traditional work rounds) (N = 1102). Team members included: medical interns and residents, staff nurses, nursing supervisors, respirologists, pharmacists, nutritionists, and social workers. To reduce baseline variability the authors used a process of random allocation of patient and clinical staff to either intervention wards or control wards.
Wild 2004 studied the effects of daily interdisciplinary rounds in a telemetry unit of a community hospital in the USA. In this study, patients were randomised to a medical team that performed daily interdisciplinary rounds, and patients were randomised to a medical team that provided usual care (N = 84). During the interdisciplinary rounds, the resident physicians, nurses, a case manager, pharmacist, dietitian, and physical therapist spent two to five minutes discussing each patient, and identifying and addressing possible discharge problems. No information on the duration of the intervention was provided.
For further details on these studies: see Characteristics of included studies.
Interprofessional meetings
Schmidt 1998 evaluated the impact of multidisciplinary team meetings on the quality and quantity of psychotropic drug prescribing in Swedish nursing homes. Thirty‐six nursing homes were randomised to either receive the intervention or were randomised to the control group (N = 1854). In the experimental nursing homes, the pharmacist in the homes helped organise team meetings that occurred approximately once a month, over a period of 12 months. The nursing home pharmacists attended two training sessions prior to, and three sessions during, the programme. The team meeting participants in the nursing homes included the pharmacist, a physician, and selected nurses and nursing assistants. All participants were encouraged to take part in the meeting discussions about the drug use of individual residents. Usual care continued to be used in the control homes. Nursing home residents' prescriptions were recorded one month before, and one month after, the 12‐month intervention.
Wilson 2004 compared two forms of multidisciplinary virtual team conferencing: usual care (audio conferencing) with video conferencing (including audio). Participating team members consisted of medical staff specialists, medical registrars, nurses, a speech pathologist, occupational therapists, a social worker, and medical students. Patients were randomly assigned to the audio conferencing or video conferencing (N = 100). At each conference session, the audio conferences were conducted before the video conferences, with the same multidisciplinary team.
For further details on these studies: see Characteristics of included studies.
Interprofessional checklists
Calland 2011 studied the effects of a procedure checklist for interprofessional surgical teams during laparoscopic cholecystectomies. Ten USA‐based general surgery teams were randomly assigned to an intervention or a control group (N = 29). The intervention consisted of preoperative steps: a briefing with introductions from all operating team members (surgeons, anaesthetists and nurses), a review of the patient's history, laboratory and radiographic studies, and a discussion of any unusual care circumstances. Surgeons in the intervention group received instructions on using the checklist and reminders before each surgery. In addition, a checklist copy was posted on the anaesthesia monitor in the operating room during cases, and team members were encouraged to use a call‐and‐repeat method to ensure that key steps of the checklist were neither omitted nor performed in a suboptimal manner. Control teams performed the laparoscopic cholecystectomy procedure in their normal fashion, without the use of a checklist.
For further details on this study: see Characteristics of included studies.
Excluded studies
We excluded 30 studies. The main reason for exclusion was that the intervention was not practice‐based interprofessional collaboration (IPC). See: Characteristics of excluded studies.
Risk of bias in included studies
The risk of bias in the nine included studies varied. A brief summary is presented below. Further information for each included study is presented in the Characteristics of included studies tables; Figure 2 and Figure 3 provide further overviews.
2.
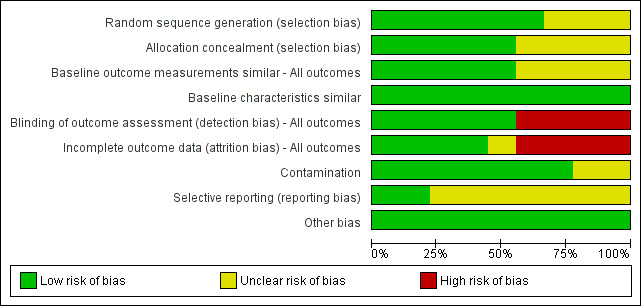
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies, based on EPOC methods.
3.
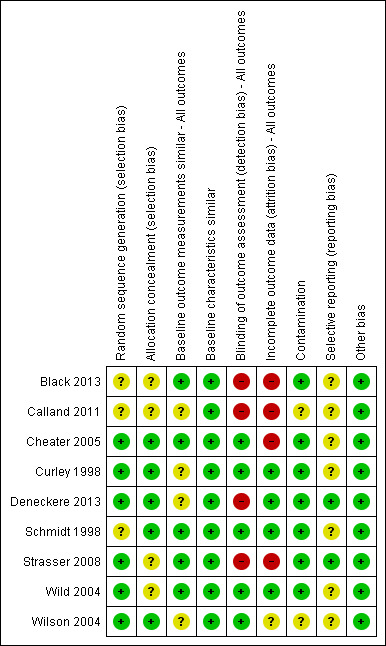
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study, based on EPOC methods.
Allocation
We classified the sequence generation for six studies as 'low risk'. In Cheater 2005 and Strasser 2008, randomisation was determined by computer; in Wild 2004, randomisation was performed with random numerical assignments in pre‐sealed envelopes; Curley 1998 randomised with a firm system; Wilson 2004 used a table of random numbers, while Deneckere 2013 used stratified randomisation to render intervention and control interprofessional teams comparable. We assessed the sequence generation for three studies as 'unclear risk', as there was insufficient information reported about the sequence generation process (Black 2013; Calland 2011; Schmidt 1998).
For allocation concealment, we classified five of the studies as 'low risk' (Cheater 2005; Curley 1998; Deneckere 2013; Schmidt 1998; Wilson 2004). In these studies, participating professionals or investigators enrolled participants. They could not foresee assignment because allocation took place in a location separate from recruitment; a sequentially numbered, blind or pre‐sealed envelope was used for allocation concealment, or both. We classified four studies at 'unclear risk', since there was insufficient information provided to assess this criteria (Black 2013; Calland 2011; Schmidt 1998; Wild 2004) .
Blinding
We considered four studies to represent 'high risk' for blinding of outcome assessment (Black 2013; Calland 2011; Deneckere 2013; Strasser 2008). These studies did not prevent knowledge of the allocated interventions, as no blinding was performed, and the outcome measurement was likely to be affected by lack of blinding. We classed detection bias as 'low risk' in five studies (Cheater 2005; Curley 1998; Schmidt 1998; Wild 2004; Wilson 2004).
Incomplete outcome data
We assessed four studies as being at 'low risk' of attrition bias (Curley 1998; Deneckere 2013; Schmidt 1998; Wild 2004). These studies provided an adequate description of participant flow through the study, with missing outcome data relatively balanced between groups, and judged to be unlikely to be related to the outcomes of interest. We assessed four studies as having 'high risk' of attrition bias for the following reasons: they acknowledged that sites dropped out but intention‐to‐treat analysis was not mentioned in the text; there were differences in characteristics related to study outcomes between completers and non‐completers (Black 2013; Calland 2011; Cheater 2005; Strasser 2008). We judged attrition bias at 'unclear risk' in one study, as the study authors did not report sufficient information about attrition and missing data (Wilson 2004).
Selective reporting
Two of the more recently completed studies published research protocols prior to study commencement (Deneckere 2013; Strasser 2008). We judged seven studies at 'unclear risk' for this bias, due to the difficulty in establishing what outcomes may have been planned in the protocol and collected, but not reported in the final published study report.
Other potential sources of bias
We assessed two of the studies as being at 'unclear risk' for contamination bias (Calland 2011; Wilson 2004). In both trials, some measures were taken to prevent contamination, but some of the subjects crossed over from one arm of the study to the other (and were involved in both interventions), thereby, potentially contaminating the initial randomisation process. We recorded studies at 'unclear risk' for baseline outcome measurements and baseline characteristics due to lack of information presented in the published paper.
Effects of interventions
This section reports on primary and secondary outcomes from the nine included studies. Data presented below are taken directly from the published articles of the included studies. We report point estimates and confidence intervals whenever reported by the study authors. For further detailed information see Table 1 and Table 2.
Practice‐based interprofessional collaboration (IPC) interventions compared with usual care
Eight studies compared IPC interventions with usual care.
Externally facilitated interprofessional activities
Patient health outcomes
Patient functional status
Externally facilitated interprofessional activities may slightly improve stroke patients' motor function (low‐certainty evidence, 1 study, N = 464). Strasser 2008 reported a difference between the intervention and control groups in patient health, with changes from admission to discharge in the motor skills component of the Functional Independence Measure (FIM) score. For the patients with stroke, 13.6% more of those in the intervention group gained in excess of the median gain, 23 points, when compared to the control group (P = 0.032).
Patient‐assessed quality of care
It is uncertain if externally facilitated interprofessional activities increase patient‐assessed quality of care at 12‐month follow‐up (very low‐certainty evidence, 1 study, N = 1185) (Black 2013).
Patient mortality, morbidity or complication rates
None of the included studies reported patient mortality, morbidity or complication rates.
Clinical process or efficiency outcomes
Adherence to recommended practices
The use of interprofessional activities with an external facilitator may slightly improve adherence to recommended practices and prescription of drugs (low‐certainty evidence, 2 studies, N = 722).
Cheater 2005 reported an increase in collaborative audit activity, with six of the 11 intervention teams completing the full audit cycle. Only three control teams undertook any audit (first data collection).
Deneckere 2013 reported improvements in the following outcomes: conflict management (slope of difference between intervention and control group (β) 0.30, 95% confidence interval (CI) 0.08 to 0.53); team climate for innovation (β 0.29, 95% CI 0.09 to 0.49); and level of organised care (β 5.56, 95% CI 1.35 to 9.76). Deneckere 2013 also reported that the intervention group scored lower in emotional exhaustion (β 0.57, 95% CI 0.14 to 1.00) and higher in level of competence (β 0.39, 95% CI 0.15 to 0.64).
Continuity of care
It is uncertain if externally facilitated interprofessional activities improve continuity of care (low‐certainty evidence, 1 study, N = 464).
Use of healthcare resources
While Strasser 2008 reported that externally facilitated interprofessional activities may improve use of resources (low‐certainty evidence, 1 study, N = 464), Strasser 2008 also reported that there was little or no difference between the intervention and control groups for length of stay or discharge disposition.
Collaborative behaviour outcomes
Collaborative working, team communication and team co‐ordination
It is uncertain whether externally facilitated interprofessional activities improve collaborative working, team communication, and co‐ordination (very low‐certainty evidence, 3 studies, N = 1907).
Black 2013 reported differences between the intervention and control groups in the mean change from baseline to follow‐up in staff role scores assessed using the Chronic Care Team Profile (CCTP). These differences were in the non‐GP clinical staff function (P = 0.023), the administrative staff function (P < 0.001), and the total score (P = 0.03). These changes included, for example, the creation of a diabetes care co‐ordinator to perform tasks such as managing the recall and reminder system for patients with diabetes, and organising staff meetings to improve communication and practice systems.
Cheater 2005 report that Collaborative Practice Scale (CPS) scores on co‐operation went from 83.5 at baseline to 88.5 after the intervention in the intervention group, compared to 84.5 at baseline to 84.5 after the intervention in the control group. These differences are presented with no measures of variability and the change appears small in relation to the value at baseline.
Deneckere 2013 found little effect of the intervention on relational co‐ordination, which assessed the process of communication and relationship between team members in order to complete the task.
Interprofessional rounds
Patient health outcomes
None of the included studies reported patient health outcomes.
Clinical process or efficiency outcomes
Use of healthcare resources
Interprofessional rounds may improve use of healthcare resources (low‐certainty evidence, 2 studies, N = 1186).
Curley 1998 found differences in length of stay and costs for patients in the interdisciplinary group compared to the traditional care group. The mean length of stay for patients in the interdisciplinary rounds group was 5.46 days, compared with 6.06 days for traditional care (P = 0.006). The mean total charges were USD 6681 and USD 8090 for the two groups, respectively (P = 0.002). For respiratory therapy, 91.7% of the nursing and pharmacy orders in the interdisciplinary rounds group were appropriate, compared with 73.6% for the traditional rounds group (P = 0.075).
Wild 2004 found little or no change in the length of hospital stay, between the experimental group (3.2 ± 2.7 days), which participated in interdisciplinary rounds, and the control group (3.2 ± 3.2 days (P = 0.90)).
Collaborative behaviour outcomes
None of the included studies reported collaborative behaviour outcomes.
Interprofessional meetings
Patient health outcomes
The included study did not report patient health outcomes.
Clinical process or efficiency outcomes
Adherence to recommended practices
Interprofessional meetings may slightly improve adherence to recommended practices and prescription of drugs (low‐certainty evidence, 1 study, N = 1854).
Schmidt 1998 found that the change from baseline to end of study in the proportion of patients receiving drugs was the same in experimental and control homes (1.3% intervention and 1.3% control). The mean number of psychotropic drugs increased from 2.07 to 2.08 (1%) in the intervention group and from 2.06 to 2.20 (7%) in the control group. The use of non‐recommended hypnotics declined by 37% in the experimental homes versus a decrease of only 3% in the control homes. There was little or no change in the prescribing of non‐recommended anxiolytics in the experimental homes, but there was an increase of 7% in the control homes. Non‐recommended antidepressant drugs decreased by 59% in experimental homes but by only 34% in control homes.
Collaborative behaviour outcomes
The included study did not report collaborative behaviour outcomes.
Interprofessional checklists
Patient health outcomes
None of the included studies reported patient health outcomes.
Clinical process or efficiency outcomes
Use of healthcare resources
Interprofessional checklists may improve use of healthcare resources (low‐certainty evidence, 1 study, N = 29).
In Calland 2011, there was little or no difference between surgeons and team members in the intervention group (who received basic team training and used a pre‐procedural checklist), and the control group (who performed standard laparoscopic cholecystectomies), in patient outcomes, length of operation, discharge status, readmission rates, and technical proficiency. Overall, situational awareness did not differ between the two groups. Surgeons and team members in the intervention group consistently rated their cases as involving less satisfactory levels of comfort, team efficiency, and communication compared to the control group.
Collaborative behaviour outcomes
Collaborative working, team communication and team co‐ordination
Calland 2011 reported that participants in the intervention (checklist) group were more likely to introduce team members, assign team roles, give case presentations, devise contingency plans, and complete post‐case performance reviews. The intervention may make little or no difference to collaborative behaviour.
Practice‐based IPC intervention compared with alternative IPC intervention
Wilson 2004 compared one type of IPC intervention (video conferencing) with a second IPC intervention (audio conferencing).
Interprofessional meetings
Patient health outcomes
The included study did not report patient health outcomes.
Clinical process or efficiency outcomes
Use of healthcare resources
Interprofessional meetings may improve use of healthcare resources (low‐certainty evidence, 1 study, N = 100). Wilson 2004 reported that the mean number of audio conferences held per patient (mean 3.3; standard deviation (SD) 4.4) was greater than the mean number of video conferences held (mean 1.9; SD 1.3; P = 0.04). Video conferencing may reduce the average length of treatment (mean 6.0; SD 4.5 days), compared to audio conferencing (mean 10.2; SD 12.3 days; P = 0.03). The use of video conferencing may improve process/efficiency outcomes by reducing the number of multidisciplinary conferences needed per patient and patient length of stay.
Collaborative behaviour outcomes
Collaborative working, team communication and team co‐ordination
Wilson 2004 reported little or no difference between the groups in the number of communications between health professionals, as recorded in the notes (low‐certainty evidence, 1 study, N = 100).
Discussion
Summary of main results
Eight studies compared an interprofessional collaboration (IPC) intervention with usual care (Black 2013; Calland 2011; Cheater 2005; Curley 1998; Deneckere 2013; Schmidt 1998; Strasser 2008; Wild 2004). One study compared one IPC intervention (video/audio conferencing) with another (audio conferencing) (Wilson 2004). See Table 1 and Table 2.
IPC interventions compared with usual care
Each of the eight included studies in this comparison is discussed below in relation to the specific IPC intervention they employed. None of the included studies reported on patient mortality, morbidity or complication rates.
Externally facilitated interprofessional activities
Four studies reported the effects of this intervention. Externally facilitated interprofessional activities may slightly improve patient functional status (1 study, N = 464) and adherence to recommended practices (2 studies, N = 722), and may improve use of resources (1 study, N = 464). There was low‐certainty evidence for all outcomes. It is uncertain whether externally facilitated interprofessional activities improve patient‐assessed quality of care (1 study, N = 1185), continuity of care (1 study, N = 464), or collaborative working (3 studies, N = 1907), as we graded the evidence as very low‐certainty.
The included studies reported varied activities. Strasser 2008 implemented a multiphase, IPC programme, delivered over six months, that aimed to enhance the effectiveness of interdisciplinary stroke rehabilitation teams. Cheater 2005 reported an IPC intervention where an external facilitator used strategies to encourage collaborative working. Deneckere 2013 developed a care pathway for patients hospitalised in an acute hospital setting with either a proximal femur fracture or an exacerbation of chronic obstructive pulmonary disease. Black 2013 used a multimodal intervention involving educational sessions, practice visits, and resource manuals and workbooks.
Interprofessional rounds
Two studies reported the effects of this intervention. Overall, interprofessional rounds may improve use of resources (low‐certainty evidence, 2 studies, N = 1186 participants). The studies implemented the intervention in an acute care hospital and in a community hospital telemetry ward (Curley 1998; Wild 2004). Wild 2004 suggested that their finding of little or no change in clinical process outcomes could be because, for many admissions, there was already a clinical pathway with standardised care for their diagnoses, the patients were more stable, at a lower risk for complications, and possibly healthier overall, and so the interdisciplinary rounds provided no additional advantage.
Interprofessional meetings
One study reported the effects of this intervention. Interprofessional meetings may slightly improve adherence to recommended practices (low‐certainty evidence, 1 study, N = 1854) and may improve use of resources (low‐certainty evidence, 1 study). Schmidt 1998 implemented a collaborative team meeting in nursing homes.
Interprofessional checklists
One study reported the effects of this intervention. Interprofessional checklists may improve the use of resources (low‐certainty evidence, 1 study, N = 29). Calland 2011 used a pre‐procedural checklist with surgical teams.
IPC intervention compared with alternative IPC intervention
Wilson 2004 assessed the impacts of interprofessional meetings facilitated using two different technologies, and found that video/audio conferencing may be more efficient than audio conferencing alone.
Overall completeness and applicability of evidence
The included studies covered four types of practice‐based IPC interventions: externally facilitated interprofessional activities, interprofessional rounds, interprofessional meetings, and interprofessional checklists. We did not find any studies that used other types of practice‐based IPC interventions, such as debriefing. Given the range of practice‐based interventions aimed at promoting IPC, and the different types of participants, settings, and clinical areas addressed in these interventions, further studies are required to provide better insight into the effectiveness of these interventions alone or in combination, in a variety of target groups and clinical areas.
Other issues affected the completeness of the evidence. For example, Schmidt 1998 acknowledged that their study could not provide robust evidence about the participating teams' decision‐making processes, or the strategies used by pharmacists in their role as team facilitators.
Secondary outcomes, focused on examining interprofessional collaboration processes, were not well examined in most of the studies, with only four studies reporting on this type of outcome (Black 2013; Cheater 2005; Deneckere 2013; Wilson 2004).
Six of the nine included studies were cluster‐randomised trials; this was appropriate, given the complex nature of interventions and their inherently clustered nature, the difficulty of blinding, and the consequential threat of contamination.
Whilst we identified four new studies for this review, the number of practice‐based IPC studies remains small. Some of the studies offered some evidence that IPC interventions may be effective in improving clinical processes/efficiency outcomes, but the small number of studies and the methodological limitations precluded definitive conclusions. Therefore, we still know little about the processes of collaboration, and how they contribute to changes in clinical process/efficiency and patient outcomes.
Certainty of the evidence
The review included nine randomised trials; the findings for the two comparisons are summarised in Table 1 and Table 2. Based on our GRADE assessment, we found the certainty of the evidence from the included studies to be low or very low due to risk of bias (attrition, detection, selection, reporting, and contamination bias), and potential indirectness (as outcomes generated in one country or clinical setting may not be transferable to other settings).
A number of other study limitations may also have contributed to the risk of bias: Curley 1998 used a non‐validated survey to examine interdisciplinary communication on the ward. Wild 2004 used a questionnaire to ask about communication, but this was only administered to the experimental group. Similarly, Cheater 2005 used a modified Collaborative Practice Scale (CPS), which was also only completed by the experimental group. Wilson 2004 used the number of communications between health professionals, recorded in the notes, to measure communication, which is a limited measurement of collaboration. Deneckere 2013 used a post‐test only; as a result, there was no baseline assessment measured before the intervention. Also, the results were primarily based on self‐reported outcomes, which caused some limitations, such as possible social desirability bias.
Potential biases in the review process
The searches were sensitive, but some literature may be under‐represented. We did not contact authors of the included studies for this review, which may have introduced some bias. In the next review, Scopus should be added to the databases to be searched, and corresponding authors of included studies should be contacted to clarify published information, and to seek unpublished data. The limited number of studies reporting data on both primary and secondary outcomes limited exploration of publication bias and sensitivity analyses.
Agreements and disagreements with other studies or reviews
There were no comparable reviews in this area.
Authors' conclusions
Implications for practice.
The findings from the nine studies included in this review suggested that interventions aimed at improving interprofessional collaboration (IPC) through practice changes may slightly improve clinical process/efficiency and patient health outcomes compared to usual care or an alternative intervention. Nine randomised trials of four different IPC interventions, in nine different clinical settings and health conditions provided mixed results. We judged the certainty of evidence from these randomised trials to be low to very low. Based on these included studies we do not have sufficient evidence to draw clear conclusions on the effects of IPC interventions.
Implications for research.
Given the problems that health professionals encounter with IPC in their clinical practice (e.g. Körner 2016; Van Leijen‐Zeelenberg 2015), it is encouraging that research on the effects of IPC interventions has increased since the previous Cochrane Review of this intervention (Zwarenstein 2009). While this research field is developing, further rigorous, mixed‐method studies are required. It is recommended that future randomised trials have a clear and explicit focus on IPC, longer acclimatisation periods before evaluating newly implemented teamwork interventions, and longer follow‐up.
Future research should also focus on the conceptualisation and measurement of collaboration. While there are some scales that measure collaboration (e.g. Kenaszchuk 2010), there are limitations with their validity, reliability, the extent to which they could be used with different professional groups, and how well they examine issues of collaborative practice.
The studies included in this updated Cochrane Review used a variety of terms to describe their interventions (e.g. interdisciplinary, multidisciplinary), which contributes to an on‐going confusion of terminology. The absence of a consistent approach to terminology of these interventions undermines our ability to synthesise them in order to develop a more informed understanding of their effects. Further work is needed to clarify the conceptualisation of IPC, interprofessional education, and interprofessional organisationally‐based interventions to support consistency in the use and understanding of these terms and their related interventions. While we have published an initial classification (Reeves 2010; Reeves 2011), future empirical work could test these conceptualisations to generate more detailed knowledge related to their implementation. Finally, quantitative and qualitative methods should be used in single studies to improve our understanding of how the intervention addresses collaboration, the nature of changes that occur in relation to collaboration, and how they in turn lead to the outcomes achieved.
What's new
| Date | Event | Description |
|---|---|---|
| 26 July 2018 | Amended | Contact person/author Scott Reeves deceased May 2018. Contact person role reassigned to Merrick Zwarenstein. |
History
Protocol first published: Issue 3, 1996 Review first published: Issue 2, 1997
| Date | Event | Description |
|---|---|---|
| 13 November 2015 | New citation required but conclusions have not changed | This update found four new studies. As a result, the review now includes nine studies. While the number of studies has increased slightly, the main conclusions from the previous update remain unchanged (Zwarenstein 2009). There have been changes to the author team, with the inclusion of two new authors. |
| 10 November 2015 | New search has been performed | New searches performed to 10 November 2015. Four new studies identified. |
| 13 May 2009 | New citation required and conclusions have changed | Conclusions changed, based on additional studies. Criteria for included study designs, included participants and specification of the intervention changed from the 1997 review. This first review included randomised trials, controlled before‐after studies and interrupted time series designs, whereas this update included only randomised trials. The types of participants included in the first review were physicians and nurses, whereas this update included all types of healthcare professionals. The first review included studies in which the interventions may not have specified their intent to change interprofessional collaboration, whereas this update only included studies with an explicit focus on collaboration. These changes were intended to increase the validity of the conclusions, and to widen their applicability to professions other than nursing and medicine. |
| 13 May 2009 | New search has been performed | New search and four additional studies identified and included in the review. |
| 20 August 2008 | New search has been performed | Converted to new review format. |
| 11 January 2000 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
The authors would like to acknowledge their respective academic institutions for the support in undertaking this updated Cochrane Review. The authors would also like to thank the following Effective Practice and Organisation of Care (EPOC) editors and staff for their advice and feedback on the review: Pierre Durieux, Daniela Gonçalves Bradley, Simon Lewin, Paul Miller, and Julia Worswick. We would like to thank Vicki Pennick for copy‐editing the review. Finally, the authors would like to thank the peer reviewers Kunal D Patel and Andreas Xyrichis who generously gave up their time to provide comments on the review, which helped improve its quality.
The National Institute for Health Research (NIHR), via Cochrane Infrastructure, funds the Effective Practice and Organisation of Care (EPOC) Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, National Health Service (NHS), or the Department of Health.
Appendices
Appendix 1. CENTRAL search strategy
#1[mh "Interprofessional Relations"] and (collaborat* or team*)
#2 [mh "Patient Care Team"] and (collaborat* or team*)
#3 ((interprofession* or inter‐profession*) next (collaborat* or team*))
#4 ((interdisciplin* or inter‐disciplin*) next (collaborat* or team*))
#5 ((interoccupation* or inter‐occupation*) next (collaborat* or team*))
#6 ((multiprofession* or multi‐profession*) next (collaborat* or team*))
#7 ((multidisciplin* or multi‐disciplin*) next (collaborat* or team*))
#8((multioccupation* or multi‐occupation*) next (collaborat* or team*))
#9((transdisciplin* or trans‐disciplin*) next (collaborat* or team*))
#10(team* next collaborat*)
#11{or #1‐#10}
Appendix 2. MEDLINE search strategy
1 exp Interprofessional Relations/ and (collaborat$ or team$).tw. (8220)
2 exp Patient Care Team/ and (collaborat$ or team$).tw. (13439)
3 ((interprofession$ or inter‐profession$) adj (collaborat$ or team$)).tw. (853)
4 ((interdisciplin$ or inter‐disciplin$) adj (collaborat$ or team$)).tw. (2660)
5 ((interoccupation$ or inter‐occupation$) adj (collaborat$ or team$)).tw. (0)
6 ((multiprofession$ or multi‐profession$) adj (collaborat$ or team$)).tw. (355)
7 ((multidisciplin$ or multi‐disciplin$) adj (collaborat$ or team$)).tw. (7856)
8 ((multioccupation$ or multi‐occupation$) adj (collaborat$ or team$)).tw. (0)
9 ((transdisciplin$ or trans‐disciplin$) adj (collaborat$ or team$)).tw. (105)
10 (team$ adj collaborat$).tw. (158)
11 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 (26183)
12 randomized controlled trial.pt. (276233)
13 controlled clinical trial.pt. (42446)
14 randomized controlled trials/ (83918)
15 random allocation/ (45887)
16 double blind method/ (80591)
17 single blind method/ (16519)
18 12 or 13 or 14 or 15 or 16 or 17 (452549)
19 animals/ not humans/ (1793680)
20 18 not 19 (410246)
21 11 and 20 (954)
22 limit 21 to yr="2007 ‐Current" (595)
Appendix 3. CINAHL search strategy
1 (MH "Interprofessional Relations+") AND TX ((collaborat* or team*))
2 (MH " Multidisciplinary Care Team+") AND TX ((collaborat* or team*))
3 TX ((interprofession* or inter‐profession*) N1 (collaborat* or team*))
4 TX ((interdisciplin* or inter‐disciplin*)) N1 (collaborat* or team*))
5 TX ((interoccupation* or inter‐occupation*) N1 (collaborat* or team*))
6 TX ((multiprofession* or multi‐profession*) N1 (collaborat* or team*))
7 TX ((multidisciplin* or multi‐disciplin*) N1 (collaborat* or team*))
8 TX ((multioccupation* or multi‐occupation*) N1 (collaborat* or team*))
9 TX ((multioccupation* or multi‐occupation*) N1 (collaborat* or team*))
10 TX ((transdisciplin* or trans‐disciplin*) N1 (collaborat* or team*))
11 TX team* N1 collaborat*
12 S1 OR S2 OR S3 OR S4 OR S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11
13 (MH "Clinical Trials+")
14 PT Clinical trial
15 TX ( (trebl* n1 blind*) or (trebl* n1 mask*) )
16 TX ( (tripl* n1 blind*) or (tripl* n1 mask*) )
17 TX ( (doubl* n1 blind*) or (doubl* n1 mask*) )
18 TX ( (singl* n1 blind*) or (singl* n1 mask*) )
19 TX randomi* control* trial*
20 (MH "Random Assignment")
21 TX random* allocat*
22 TX placebo*
23 (MH "Placebos")
24 (MH "Quantitative Studies")
25 TX allocat* random*
26 S13 OR S14 OR S15 OR S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23 OR S24 OR S25
27 S12 AND S26
28 S12 AND S26. Limiters ‐ Publication Year: 2007‐2014; Clinical Trial
Appendix 4. ClinicalTrials.gov and ICTRP search strategies
ClinicalTrials.gov search strategy
(collaboration OR team) AND (interdisciplinary OR interprofessional OR multidisciplinary OR multiprofessional)
ICTRP search strategy
#1 collaboration AND interdisciplinary
#2 collaboration AND interprofessional
#3 collaboration AND multidisciplinary
#4 collaboration AND multiprofessional
#5 team AND interdisciplinary
#6 team AND interprofessional
#7 multidisciplinary team
#8 team AND multiprofessional
#9 OR/1‐8
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Black 2013.
| Methods | Cluster‐randomised trial to test the effectiveness of an intervention involving non GP‐staff in GP practices, on the quality of care for patients with diabetes or cardiovascular disease. | |
| Participants | Country: Australia General practitioners, nurses, practice managers, receptionists, and other administrative staff. 60 general practices were randomised to receive a 6‐month teamwork intervention immediately (intervention, n = 637) or after 12 months (control, n = 548). |
|
| Interventions | To assist non‐GP staff (e.g. nurses, administrative staff (practice managers, receptionists)) to work as a team with GPs, the intervention included a number of activities including: the use of structured appointment systems, recall and reminders, planned care, the use of roles, responsibilities, and job descriptions, as well as communication and meetings. | |
| Outcomes | Quality of care (12‐month follow‐up) | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation is mentioned: “…Following baseline‐data collection, practices were stratified according to size (solo, 2 to 4 GPs or 5+ GPs) and randomised to receive the 6‐month teamwork intervention immediately, or after 12 months…”, but method not specified. |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation method. |
| Baseline outcome measurements similar ‐ All outcomes | Low risk | At baseline, the quality of care PACIC outcomes in the intervention group (3.01, SD 0.30) and control group (2.87, SD 0.34) were similar. |
| Baseline characteristics similar | Low risk | Intervention and control teams look reasonably similar. Quote: "Control practices were more likely to be in an urban location compared with the intervention practices, have a lower full‐time equivalent level of practice nurses and were also more likely to have a higher score on the CCTP with more administrative functions for chronic disease managed by non‐GP staff. There were no key differences between the control and intervention practices for total levels of non‐GP staffing." |
| Blinding of outcome assessment (detection bias) ‐ All outcomes | High risk | It did not appear that there was any blinding. |
| Incomplete outcome data (attrition bias) ‐ All outcomes | High risk | Acknowledged sites dropped out, but ITT is not mentioned in the text. Practice level: Quote: "Of these, 69% (60/87) finally participated in the study, and three of these (3/60) withdrew at follow up…Reasons for withdrawal of three practices included concern about the extent of data collection and other reasons not pertaining to the study." Patient level: There were 3349 patients invited to participate in the study, with 2642 (79%) providing informed consent. Of these, 2552 (96.6%) returned the PACIC questionnaire at baseline, with 2135 (73.7%) completing all 20 items. To be included in the factor analysis, at least 17 questions needed to be completed, and 2438 participants met this criterion. The multilevel regression included data for which all relevant variables were available, resulting in a final sample size of 1853 patients. |
| Contamination | Low risk | Allocation was by practice, and it is unlikely that the control practices received the intervention. |
| Selective reporting (reporting bias) | Unclear risk | All relevant outcomes in the method section (p B) were reported in the results section (p D‐E). A study protocol was not available and there was insufficient information to permit judgement of high or low risk of bias. |
| Other bias | Low risk | Cluster‐randomised trial with appropriate statistical analysis. |
Calland 2011.
| Methods | A RT of an IPC intervention aimed to determine the effectiveness of procedural checklists for surgical teams during 47 laparoscopic cholecystectomies. General surgeons were randomly assigned to an intervention (i.e. the use of the checklist) or a control group. | |
| Participants | Country: USA Ten general surgeon teams consisting of surgeons, anaesthetists and nurses. Twenty‐three patients in the control group and 24 in the intervention group. Eighteen patients dropped out between the randomisation and the analysis. |
|
| Interventions | An intraoperative procedural checklist including preoperative, intraoperative, and postoperative items. | |
| Outcomes | Clinical process or efficiency outcomes: length of operation, discharge status, readmission rates and technical proficiency. Collaborative behavioural outcomes: team behaviours (e.g. team communication and co‐ordination). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation was mentioned: "a total of 65 cases were randomized (by attending surgeon) to…", but method not specified. |
| Allocation concealment (selection bias) | Unclear risk | No description of allocation method. |
| Baseline outcome measurements similar ‐ All outcomes | Unclear risk | Not reported. |
| Baseline characteristics similar | Low risk | Quote: "Length of operation, discharge status, and readmission rates as indication of case outcome showed nonstatistical differences between groups." |
| Blinding of outcome assessment (detection bias) ‐ All outcomes | High risk | It did not appear that there was any blinding. |
| Incomplete outcome data (attrition bias) ‐ All outcomes | High risk | Acknowledged sites dropped out but ITT was not mentioned in the text. Patient level: Quote: "A total of 65 cases were randomized..." Quote: "Eighteen subjects/cases dropped out between randomization and analysis: two in the checklist group declined to use the checklist or requested that their cases be withdrawn after videotaping, three cases were excluded due to the conversion from laparoscopic to open procedure, procedure cancellations occurred in four cases, and scheduling difficulties or mechanical problems precluded participation for the nine remaining dropouts." |
| Contamination | Unclear risk | Randomised at the level of surgeon, but as noted by the authors "there exists the possibility that residents and other staff participated in both control and intervention cases and this contaminated our results" (p 1137). |
| Selective reporting (reporting bias) | Unclear risk | All relevant outcomes in the method section (p 1132‐3) were reported in the results section (p 1133‐6). A study protocol was not available and there was insufficient information to permit judgement of high or low risk of bias. |
| Other bias | Low risk | None detected. |
Cheater 2005.
| Methods | A RT where 22 multidisciplinary teams from five acute care hospitals were randomised to an intervention group that participated in a facilitated programme on multidisciplinary audit or a control group. | |
| Participants | Country: UK Nurses, physicians and other professionals (e.g. pharmacist, social worker, physiotherapist), service support staff (e.g. ward clerk, care assistant), and managers. A range of specialties (e.g. surgery, medicine, and nephrology) were included. There were 11 teams with a total of 77 participants in the intervention group and 11 teams with a total of 64 participants in the control group. |
|
| Interventions | Five facilitated meetings over 6 months with activities designed to support multidisciplinary teams to undertake an audit. | |
| Outcomes | Collaborative audit activity. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Teams within the same hospital were stratified on mean self‐reported KSA scores, perceived level of team collaboration and medical or surgical specialty before randomisation. The project secretary under the supervision of [a researcher] randomised 22 teams to intervention or control groups, using a computer random number generator. |
| Allocation concealment (selection bias) | Low risk | Quote: "With the exception of two accident and emergency teams in different hospitals, teams from the same organisation were randomised in pairs. Other researchers were blind to allocation." |
| Baseline outcome measurements similar ‐ All outcomes | Low risk | At baseline, both groups were equivalent for baseline variables in relation to KSA scores, and on the scores for the Collaborative Practice Scale. |
| Baseline characteristics similar | Low risk | Quote: "At baseline, both groups were equivalent for all outcome variables except two. In comparison to the intervention group, the control arm reported higher levels of audit knowledge (median score 32.5 vs 25.0, z = ‐3.001, P = 0.003) and skills (median score 32.5 vs. 24.6, z = ‐ 2.990, P = 0.003). Baseline differences were adjusted for in the analysis. Baseline differences were not found for WWTs." |
| Blinding of outcome assessment (detection bias) ‐ All outcomes | Low risk | Quote: "Two members of the research team (RB and HH) independently assessed the quality of the reports (blind to group allocation) and the percentage inter‐rater agreement did not fall below 82%." |
| Incomplete outcome data (attrition bias) ‐ All outcomes | High risk | Practice level: Quote: "Participation in the intervention programme was associated with increased audit activity, with 9 of the 11 teams reporting improvements to care and seven teams completing the full audit cycle. In contrast, the majority of teams in the control group had made no progress with undertaking an audit and only two teams had undertaken a first data collection and implemented changes." Patient level: Results were provided about the quality of the audits in relation to their compliance with the 55 quality criteria, but no further information was provided in relation to any patient level outcomes. |
| Contamination | Low risk | Only intervention teams participated in the facilitation programme. |
| Selective reporting (reporting bias) | Unclear risk | All relevant outcomes in the method section (p 781‐2) were reported in the results section (p 785‐7). A study protocol was not available and there was insufficient information to permit judgement of high or low risk of bias. |
| Other bias | Low risk | None detected. |
Curley 1998.
| Methods | Randomised trial ‐ Firm trial: patients and staff from inpatient medical wards at an acute care hospital were randomised to one of six medical wards. Three wards were allocated to the intervention group that implemented daily interdisciplinary work rounds, and three wards were allocated to the control group that continued traditional work rounds. | |
| Participants | Country: USA Interns and residents in medicine, staff nurses, nursing supervisors, respirologists, pharmacists, nutritionists, and social workers. There were 567 patients in the intervention group and 535 patients in the control group. |
|
| Interventions | Daily interdisciplinary work rounds. | |
| Outcomes | Length of stay, total charges, orders for administration of aerosols. | |
| Notes | Unit of analysis error ‐ allocated intervention to wards but analysed patients without correction for clustering. However, this correction may not substantially change the conclusion because randomisation of staff and patients limits variation between clusters. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The firm system randomization procedures and their validation have been reviewed extensively in the literature. Each inpatient firm has two physician teams or ward services. For this trial the six ward services were divided so that three ward services continued traditional work rounds as usual and the three ward services implemented the CQI designed interdisciplinary work rounds." |
| Allocation concealment (selection bias) | Low risk | Quote: "The firm system randomization procedures and their validation have been reviewed extensively in the literature. Each inpatient firm has two physician teams or ward services. For this trial the six ward services were divided so that three ward services continued traditional work rounds as usual and the three ward services implemented the CQI designed interdisciplinary work rounds." |
| Baseline outcome measurements similar ‐ All outcomes | Unclear risk | Not reported. |
| Baseline characteristics similar | Low risk | Quote: "After controlling for baseline differences in case‐mix using a multivariate propensity score, the length of stay and total charges for the hospital stay for the patients included in the trial were evaluated." |
| Blinding of outcome assessment (detection bias) ‐ All outcomes | Low risk | Quote: "Patient data were retrieved from the hospital’s administrative and billing system. Thus, patient specific cost and efficiency outcomes were limited to resource utilization in the form of hospital length of stay and total charges." "...the Respiratory Therapy (RT) Department conducted a study of aerosol use appropriateness, as determined by criteria previously devised and tested by the RT Department." |
| Incomplete outcome data (attrition bias) ‐ All outcomes | Low risk | Practice level: Quote: "The outcome measures reported in this review were at the patient level. The study does report results from satisfaction surveys completed by 19 providers of the traditional rounds group and 21 providers of the interdisciplinary rounds group but provides no information about the total number of providers in each group." Patient level: Quote: "Study patients included all patients admitted to the medical inpatient units between November 8, 1993, and May 31, 1994, who spent at least 50% of their hospital stay on that unit and were discharged from that unit. If patients were readmitted during the trial, each admission was considered separately.” “Patient data were retrieved from the hospital’s administrative and billing system." |
| Contamination | Low risk | Quote: "Patients were excluded from analysis if their hospital stay was not on their assigned medical firm because they had been ’de‐firmed’ because of excess admissions to one service or if they were ’boarding’ on a floor that was not the ward team’s home floor. Patients were excluded from the trial if they were transferred from medicine to another service (e.g. surgery) or if less than 50% of their stay occurred on the medical floor..." |
| Selective reporting (reporting bias) | Unclear risk | All relevant outcomes in the method section (AS6) were reported in the results section (AS7‐9). There was no published protocol so we cannot be sure all planned analyses were conducted. |
| Other bias | Low risk | None detected. |
Deneckere 2013.
| Methods | A post‐test‐only cluster‐RT of 30 teams caring for patients with COPD and PFF. 17 intervention teams and 13 control teams examined how the use of CPs improved teamwork in an acute hospital setting. | |
| Participants | Country: Belgium Doctors (i.e. orthopaedic surgeons or pneumologists), head nurses, nurses, and allied health professionals (i.e. physiotherapists and social workers). 581 participants: 346 in the intervention teams (N = 17) and 235 in the control teams (N = 13). |
|
| Interventions | The intervention involved the development and implementation of CPs including 3 components: 1) feedback on team's performance before CP implementation; 2) receipt of evidence‐based key‐indicators for implementing CPs in practice to review; 3) training in CP development. Control teams: usual care. | |
| Outcomes | Conflict management, team climate for innovation, level of organised care, emotional exhaustion, level of competence, relational co‐ordination. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Stratified randomisation was used to assign the teams to an intervention group (using care pathways) and a control group (usual care). Interprofessional teams were randomised. COPD/PFF was used as blocking factor." |
| Allocation concealment (selection bias) | Low risk | Quote: "Before the start of the randomisation process, random numbers were assigned to each cluster by a researcher not involved in the study, using the online available tool 'Research Randomizer' www.randomizer.org). Next, the researcher randomly allocated the coded clusters to the intervention or control group using the same online tool." |
| Baseline outcome measurements similar ‐ All outcomes | Unclear risk | Not reported. |
| Baseline characteristics similar | Low risk | Intervention and control teams were reasonably similar. Quote: "No significant differences in organizational or team member characteristics were found, except for the number of years of experience, which was significantly higher in the control group" (Table 2). |
| Blinding of outcome assessment (detection bias) ‐ All outcomes | High risk | It did not appear that there was any blinding. |
| Incomplete outcome data (attrition bias) ‐ All outcomes | Low risk | Practice level ITT was not mentioned. Authors acknowledged that sites dropped out. Quote: "A potential weakness of the study is the dropout of 7 teams and its possible impact on the results." |
| Contamination | Low risk | Only intervention teams participated in the development and implementation of CP. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes in the method section (p 100‐1) were reported in the results section (p 102‐4). There was also a published protocol and all planned analyses were conducted. |
| Other bias | Low risk | Cluster‐RT with appropriate statistical analysis. |
Schmidt 1998.
| Methods | A RT of 33 nursing homes, 15 experimental homes and 18 control homes, to examine the effects of monthly facilitated multidisciplinary rounds on the quality and quantity of psychotropic drug prescribing. | |
| Participants | Country: Sweden Physician, pharmacists, selected nurses, and nursing assistants. 1854 long‐term residents: 626 in experimental homes and 1228 in control homes. |
|
| Interventions | Pharmacist led team meetings once a month over a period of 12 months. | |
| Outcomes | Proportion of patients receiving drugs, number of psychotropic drugs, use of non‐recommended hypnotics, use of non‐recommended anxiolytics, use of non‐recommended antidepressant drugs. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not reported. |
| Allocation concealment (selection bias) | Low risk | Quote: "Thirty‐six nursing homes, representing 5% of all nursing homes in Sweden, participated in the study. The sampling process consisted of three steps. At the time of the study, the National Corporation of Swedish Pharmacies was organized into 36 regions, 18 of which were randomly selected for this study. Each regional pharmacy director then selected two facilities in his or her region using several criteria....Researchers randomly assigned one home in each pair to receive the intervention." |
| Baseline outcome measurements similar ‐ All outcomes | Low risk | Quote: "At baseline, we found no significant differences in the proportion of residents with scheduled psychotropics (64% vs 65%), number of drugs among residents with psychotropics (2.07 vs 2.06)." |
| Baseline characteristics similar | Low risk | Quote: "There were no significant differences in the demographic, functional, or psychiatric characteristics of residents in experimental and control homes at baseline." Quote: "The overall level of prescribing was similar in experimental and control homes before the intervention (Table 2). At baseline, we found no significant differences in the proportion of residents with scheduled psychotropics (64% vs 65%), number of drugs among residents with psychotropics (2.07 vs 2.06), or proportion of residents with polymedicine (46% vs 47%). Baseline rates of therapeutic duplication were also comparable in the experimental and control homes." |
| Blinding of outcome assessment (detection bias) ‐ All outcomes | Low risk | Quote: "Lists of each resident’s prescriptions were collected 1 month before and 1 month after the 12‐month intervention in both experimental homes and control homes. Trained coders, supervised by pharmacists, classified and coded all scheduled and PRN (pro re nata) orders." |
| Incomplete outcome data (attrition bias) ‐ All outcomes | Low risk | 3 intervention homes out of 18 became ineligible. |
| Contamination | Low risk | Quote: "Pharmacists assigned to experimental homes had no contact with control nursing homes. In the control homes, no efforts were made beyond normal routine to influence drug prescribing." |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement of high or low risk of bias. There was no published protocol so we cannot be sure all planned analyses were conducted. |
| Other bias | Low risk | None detected. |
Strasser 2008.
| Methods | RT, in which patients with a stroke were treated by 31 teams from 31 Veteran Affair rehabilitation units before and after a multifaceted intervention, aimed at improving interprofessional collaboration. | |
| Participants | Country: USA Medical doctors, nurses, occupational therapists, speech‐language pathologists, physical therapists, and case managers or social workers. 464 participants: 227 in the intervention teams (N = 15) and 237 in the control teams (N = 16). Patients with a stroke were randomly assigned to each group. |
|
| Interventions | Intervention teams: received the following multifaceted intervention: 1) an off‐site workshop emphasising team dynamics, problem‐solving, and the use of performance feedback data; 2) action plans (specific team performance profiles with recommendations) for process improvement; 3) telephone and video conference consultations to sustain improvement in collaboration. Control teams only received specific team performance profile Information. |
|
| Outcomes | Functional improvement (as measured by the change in motor items of the FIM instrument), length of stay (LOS), rates of community discharge | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “... we randomized sites to either intervention or control group using a computer; each stratum was force randomized to have 4 sites in 1 arm.” |
| Allocation concealment (selection bias) | Unclear risk | Method of concealment was not described. |
| Baseline outcome measurements similar ‐ All outcomes | Low risk | The mean FIM scores at baseline were similar for the intervention group (52.2 ± 3.9) and for the control group (52.4 ± 3.8). |
| Baseline characteristics similar | Low risk | Quote: “…There were no differences between study conditions in demographic characteristics (table 2). Control sites admitted stroke patients with lower initial (admission) motor FIM scores during the pre‐intervention periods (P.002); thus, we adjusted all analyses using FRGs … a classification based on initial motor FIM and age.” |
| Blinding of outcome assessment (detection bias) ‐ All outcomes | High risk | It did not appear that there was any blinding. |
| Incomplete outcome data (attrition bias) ‐ All outcomes | High risk | Acknowledged sites dropped out but ITT was not mentioned in the text Practice level Quote: “Of 33 eligible sites, a total of 31 sites agreed to participate, initiated the IRB approval, and were randomized. One control site was unable to complete the IRB process and withdrew, and 1 intervention site did not report data to the FSOD, leaving 15 sites in the control group and 14 in the intervention group. |
| Contamination | Low risk | No reason to think contamination had occurred. |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes in the methods section (p 11) were reported in the results section (p 14). There was a published protocol and all planned analyses were conducted. |
| Other bias | Low risk | None detected. |
Wild 2004.
| Methods | Randomised trial in which patients in inpatient telemetry ward in a community hospital were randomised to the intervention medical team, which conducted interdisciplinary rounds or to the control team, which provided standard care. | |
| Participants | Country: USA Resident physicians, nurses, a case manager, pharmacist, dietician, and physical therapist. Eighty‐four patients were enrolled: 42 in intervention and 42 in standard care. |
|
| Interventions | Intervention: daily interdisciplinary rounds. Control group: standard care. |
|
| Outcomes | Length of hospital stay | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed using random numerical assignments in pre‐sealed envelopes." |
| Allocation concealment (selection bias) | Unclear risk | Envelope randomisation. |
| Baseline outcome measurements similar ‐ All outcomes | Low risk | Mean length of stay (days) was similar in the intervention group (3.04 ± 1.8) compared with the control group (2.7 ± 1.8). |
| Baseline characteristics similar | Low risk | Quote: "There were no significant differences between groups for admission diagnosis; number of co‐morbidities; number of abnormal laboratory data; ability to perform activities of daily living; presence of dementia or diabetes, or whether there was a home health aide. In spite of randomization, the gender composition between groups was somewhat different...and the number of readmissions in the IR Team was higher than in the non‐IR Team (P = 0.003)." |
| Blinding of outcome assessment (detection bias) ‐ All outcomes | Low risk | Quote: "Charts were surveyed to determine patient characteristics and LOS. LOS was measured as the difference between discharge and admission date." |
| Incomplete outcome data (attrition bias) ‐ All outcomes | Low risk | Practice level: Quote: "Questionnaire return was 80%", but these results were not reported in this review because they did not meet outcome criteria. Patient level: All participants were accounted for and none were lost to follow‐up. |
| Contamination | Low risk | Quote: "Patients were randomly assigned to two medical teams: the intervention group received IRs and the control subjects received standard care." |
| Selective reporting (reporting bias) | Unclear risk | All relevant outcomes in the method section (p 64) were reported in the results section (p 67). There was no published protocol so we cannot be sure all planned analyses were conducted. |
| Other bias | Low risk | None detected. |
Wilson 2004.
| Methods | RT comparing multidisciplinary audio conferencing and multidisciplinary video conferencing with a team that worked at two hospitals. | |
| Participants | Country: Australia Medical staff specialists, medical registrars, nurses, speech pathologist, occupational therapists, social worker, medical students. Fifty patients were randomly assigned to each group. |
|
| Interventions | Multidisciplinary audio conferences and video conferences. At each conference session, the audio conferences were conducted before the video conferences, with the same multidisciplinary team. | |
| Outcomes | Number of audio conferences held per patient, number of video conferences held, length of treatment. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The random allocation was done by an independent administrative assistant, using a table of random numbers." |
| Allocation concealment (selection bias) | Low risk | Quote: "The random allocation was done by an independent administrative assistant, using a table of random numbers." |
| Baseline outcome measurements similar ‐ All outcomes | Unclear risk | None reported. |
| Baseline characteristics similar | Low risk | Quote: "The two groups were similar in terms of age, sex and diagnosis (Table 1)." |
| Blinding of outcome assessment (detection bias) ‐ All outcomes | Low risk | Quote: "Conference times were recorded by an independent observer and files were reviewed by an independent medical practitioner blinded to the randomization." |
| Incomplete outcome data (attrition bias) ‐ All outcomes | Unclear risk | Practice level: Quote: "Only 14 of 29 (including 6 medical students) completed a staff satisfaction survey. These results are not reported in this review because they did not meet outcome criteria." Patient level" Quote: "There were no deaths, and all patients recruited completed the trial." |
| Contamination | Unclear risk | Quote: "Within each meeting of the multidisciplinary team, the audioconferences were conducted before the videoconferences, to ensure that there was no visual contact between the two locations until the latter part of the session." "The team remained consistent at either site for both the audio‐ and videoconferences held on each individual day of the conference, but the team members rotated between sites over the study period." While measures were taken to prevent contamination, the same team members were involved in both types of conferencing. |
| Selective reporting (reporting bias) | Unclear risk | All relevant outcomes in the method section (p 353‐4) were reported in the results section (p 354). Insufficient information was provided to permit judgement of high or low risk of bias. There was no published protocol so we cannot be sure all planned analyses were conducted. |
| Other bias | Low risk | None detected. |
CCTP = Chronic care team profile COPD = chronic obstructive pulmonary disease CP = care pathway CQI = Continuous quality improvement FIM = Functional independence measure FRG = Functional‐related groups FSOD = Functional status outcomes database GP = General practitioner IPC = Interprofessional collaboration IRs = interdisciplinary rounds IRB = Institutional Research Board ITT = Intention‐to‐treat KSA = Knowledge, skills, attitudes LOS = length of stay PACIC = Patient assessment of chronic illness care PFF = proximal femur fracture RT = randomised trial WWT = Wider ward teams
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bekelman 2015 | Not a practice‐based IPC intervention |
| Boet 2013 | Not a practice‐based IPC intervention |
| Boone 2008 | Not a practice‐based IPC intervention |
| Chen 2013 | Not a practice‐based IPC intervention |
| Cheng 2013 | Not a practice‐based IPC intervention |
| Curtis 2012 | Not a RT |
| Dhalla 2014 | Not a practice‐based IPC intervention |
| Döpp 2013 | Not a practice‐based IPC intervention |
| Fransen 2012 | Not a practice‐based IPC intervention |
| Goud 2009 | Not a practice‐based IPC intervention |
| Hallin 2011 | Not a practice‐based IPC intervention |
| Hobgood 2010 | Not a practice‐based IPC intervention |
| Hoffmann 2014 | Not a practice‐based IPC intervention |
| Jankouskas 2011 | Not a practice‐based IPC intervention |
| Jenkins 2013 | Not a practice‐based IPC intervention |
| Katakam 2012 | Not a practice‐based IPC intervention |
| Keller 2012 | Not a practice‐based IPC intervention |
| Kemper 2011 | Not a RT |
| Koerner 2014 | Not a practice‐based IPC intervention |
| Kunkler 2007 | Not a practice‐based IPC intervention |
| Körner 2012 | Not a practice‐based IPC intervention |
| Lee 2013 | Not a practice‐based IPC intervention |
| Marsteller 2013 | Not a practice‐based IPC intervention |
| Mohaupt 2012 | Not a practice‐based IPC intervention |
| Musick 2011 | Not a practice‐based IPC intervention |
| O'Leary 2010 | Not a RT |
| Rörtgen 2013 | Not a practice‐based IPC intervention |
| Van de Ven 2010 | Not a RT |
| Weller 2014 | Not a practice‐based IPC intervention |
| Wittenberg‐Lyles 2013 | Not a practice‐based IPC intervention |
IPC: interprofessional collaboration RT: randomised trial
Differences between protocol and review
Review authorship changed between protocol and review updates. The title has changed from the protocol: 'The effects on patient care of interventions to change collaboration between nurses and doctors' (Zwarenstein 1996). Changes were also made to criteria for included study designs, included participants, and specification of the intervention between the published protocol and this update. The protocol planned to include randomised trials, controlled before‐after studies, and interrupted time series designs, whereas the last update (Zwarenstein 2009) and this update included only randomised trials. The protocol planned to include only physicians and nurses, whereas this update included all types of health and social care professionals. The protocol also planned to include studies in which the interventions may not have specified their intent to change interprofessional collaboration, whereas this update included only studies with an explicit focus on collaboration. These changes were made to increase the validity of the conclusions, and to widen their applicability to professions beyond nursing and medicine.
Contributions of authors
SR and FP undertook the searches and both independently reviewed each of the titles and abstracts located to ensure they met the inclusion criteria. SR and FP independently assessed each full‐text article to ensure they met the criteria. MZ resolved any disagreements during the screening processes. SR and MZ independently assessed the certainty of evidence for the included studies. All authors (SR, FP, RH, JG, MZ) analysed and interpreted the data and contributed to writing the review. SR is guarantor for the review.
Scott Reeves (SR) died in May 2018 following the publication of this review The contributions as stated above were provided before the author died.
Sources of support
Internal sources
Faculty of Health, Social Care and Education, Kingston University and St George's, University of London, UK.
Continuing Education and Professional Development, Faculty of Medicine, University of Toronto, Canada.
External sources
Canadian Institutes of Health Research, Canada.
Declarations of interest
Scott Reeves: (deceased May 2018), none known. This declaration of interest was provided before the author died. Ferruccio Pelone: none known Reema Harrison: none known Joanne Goldman: none known Merrick Zwarenstein: none known.
The authors have no personal or professional interests as to whether this review shows benefits of practice‐based interventions on interprofessional collaboration. Author deceased; [declarations of interest if provided before the author died
Deceased
Edited (no change to conclusions)
References
References to studies included in this review
Black 2013 {published data only}
- Black DA, Taggart J, Jayasinghe UW, Proudfoot J, Crookes P, Beilby J, et al. Teamwork Research Team. The Teamwork Study: enhancing the role of non‐GP staff in chronic disease management in general practice. Australian Journal of Primary Health 2013;19(3):184‐9. [DOI] [PubMed] [Google Scholar]
Calland 2011 {published data only}
- Calland JF, Turrentine FE, Guerlain S, Bovbjerg V, Poole GR, Lebeau K, et al. The surgical safety checklist: lessons learned during implementation. The American Surgeon 2011;77(9):1131‐7. [PubMed] [Google Scholar]
Cheater 2005 {published data only}
- Cheater FM, Hearnshaw H, Baker R, Keane M. Can a facilitated programme promote effective multidisciplinary audit in secondary care teams? An exploratory trial. International Journal of Nursing Studies 2005;42:779‐91. [DOI] [PubMed] [Google Scholar]
Curley 1998 {published data only}
- Curley C, McEachern JE, Speroff T. A firm trial of interdisciplinary rounds on the inpatient medical wards. Medical Care 1998;36(8 Suppl):AS4‐12. [DOI] [PubMed] [Google Scholar]
Deneckere 2013 {published data only}
- Deneckere S, Euwema M, Lodewijckx C, Panella M, Mutsvari T, Sermeus W, et al. Better interprofessional teamwork, higher level of organized care, and lower risk of burnout in acute health care teams using care pathways: a cluster randomized controlled trial. Medical Care 2013;51(1):99‐107. [DOI] [PubMed] [Google Scholar]
Schmidt 1998 {published data only}
- Schmidt I, Claesson CB, Westerholm B, Nilsson LG, Svarstad BL. The impact of regular multidisciplinary team interventions on psychotropic prescribing in Swedish nursing homes. Journal of the American Geriatrics Society 1998;46:77‐82. [DOI] [PubMed] [Google Scholar]
Strasser 2008 {published data only}
- Strasser DC, Falconer JA, Stevens AB, Uomoto JM, Herrin J, Bowen SE, et al. Team training and stroke rehabilitation outcomes: a cluster randomized trial. Archives of Physical Medicine and Rehabilitation 2008;89(1):10‐5. [DOI] [PubMed] [Google Scholar]
Wild 2004 {published data only}
- Wild D, Nawaz H, Chan W, Katz DL. Effects of interdisciplinary rounds on length of stay in a telemetry unit. Journal of Public Health Management and Practice 2004;10:63‐9. [DOI] [PubMed] [Google Scholar]
Wilson 2004 {published data only}
- Wilson SF, Marks R, Collins N, Warner B, Frick L. Benefits of multidisciplinary case conferencing using audiovisual compared with telephone communication: a randomized controlled trial. Journal of Telemedicine and Telecare 2004;10:351‐4. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bekelman 2015 {published data only}
- Bekelman DB, Plomondon ME, Carey EP, Sullivan MD, Nelson KM, Hattler B, et al. Primary results of the patient‐centered disease management (PCDM) for heart failure study: a randomized clinical trial. JAMA Internal Medicine 2015;175(5):725‐32. [DOI] [PubMed] [Google Scholar]
Boet 2013 {published data only}
- Boet S, Bould MD, Sharma B, Reeves S, Naik VN, Triby E, et al. Within‐team debriefing versus instructor‐led debriefing for simulation‐based education: a randomized controlled trial. Annals of Surgery 2013;258(1):53‐8. [DOI] [PubMed] [Google Scholar]
Boone 2008 {published data only}
- Boone BN, King ML, Gresham LS, Wahl P, Suh E. Conflict management training and nurse‐physician collaborative behaviors. Journal for Nurses in Staff Development 2008;24(4):168‐75. [DOI] [PubMed] [Google Scholar]
Chen 2013 {published data only}
- Chen Z, Ernst ME, Ardery G, Xu Y, Carter BL. Physician‐pharmacist co‐management and 24‐hour blood pressure control. Journal of Clinical Hypertension 2013;15(5):337‐443. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cheng 2013 {published data only}
- Cheng A, Hunt EA, Donoghue A, Nelson‐McMillan K, Nishisaki A, Leflore J, et al. EXPRESS Investigators. Examining pediatric resuscitation education using simulation and scripted debriefing: a multicenter randomized trial. JAMA Pediatrics 2013;167(6):528‐36. [DOI] [PubMed] [Google Scholar]
Curtis 2012 {published data only}
- Curtis JR, Ciechanowski PS, Downey L, Gold J, Nielsen EL, Shannon SE, et al. Development and evaluation of an interprofessional communication intervention to improve family outcomes in the ICU. Contemporary Clinical Trials 2012;33(6):1245‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dhalla 2014 {published data only}
- Dhalla IA, O'Brien T, Morra D, Thorpe KE, Wong BM, Mehta R, et al. Effect of a post‐discharge virtual ward on readmission or death for high‐risk patients: a randomized clinical trial. JAMA 2014;312(13):1305‐12. [DOI] [PubMed] [Google Scholar]
Döpp 2013 {published data only}
- Döpp CM, Graff MJ, Teerenstra S, Nijhuis‐van der Sanden MW, Olde Rikkert MG, Vernooij‐Dassen MJ. Effectiveness of a multifaceted implementation strategy on physicians' referral behavior to an evidence‐based psychosocial intervention in dementia: a cluster randomized controlled trial. BMC Family Practice 2013;14:70. [DOI: 10.1186/1471-2296-14-70] [DOI] [PMC free article] [PubMed] [Google Scholar]
Fransen 2012 {published data only}
- Fransen AF, Ven J, Merién AE, Wit‐Zuurendonk LD, Houterman S, Mol BW, et al. Effect of obstetric team training on team performance and medical technical skills: a randomised controlled trial. BJOG 2012;119(11):1387‐93. [DOI] [PubMed] [Google Scholar]
Goud 2009 {published data only}
- Goud R, Keizer NF, ter Riet G, Wyatt JC, Hasman A, Hellemans IM, et al. Effect of guideline based computerised decision support on decision making of multidisciplinary teams: cluster randomised trial in cardiac rehabilitation. BMJ 2009;338:b1440. [DOI: 10.1136/bmj.b1440] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hallin 2011 {published data only}
- Hallin K, Henriksson P, Dalén N, Kiessling A. Effects of interprofessional education on patient perceived quality of care. Medical Teacher 2011;33(1):e22‐6. [DOI] [PubMed] [Google Scholar]
Hobgood 2010 {published data only}
- Hobgood C, Sherwood G, Frush K, Hollar D, Maynard L, Foster B, et al. Interprofessional Patient Safety Education Collaborative. Teamwork training with nursing and medical students: does the method matter? Results of an interinstitutional, interdisciplinary collaboration. Quality & Safety in Health Care 2010;19(6):e25. [DOI] [PubMed] [Google Scholar]
Hoffmann 2014 {published data only}
- Hoffmann B, Müller V, Rochon J, Gondan M, Müller B, Albay Z, et al. Effects of a team‐based assessment and intervention on patient safety culture in general practice: an open randomised controlled trial. BMJ Quality & Safety 2014;23(1):35‐46. [DOI] [PubMed] [Google Scholar]
Jankouskas 2011 {published data only}
- Jankouskas TS, Haidet KK, Hupcey JE, Kolanowski A, Murray WB. Targeted crisis resource management training improves performance among randomized nursing and medical students. Simulation in Healthcare 2011;6(6):316‐26. [DOI] [PubMed] [Google Scholar]
Jenkins 2013 {published data only}
- Jenkins VA, Farewell D, Farewell V, Batt L, Wagstaff J, Langridge C, et al. Teams Talking Trials Steering Committee. Teams Talking Trials: results of an RCT to improve the communication of cancer teams about treatment trials. Contemporary Clinical Trials 2013;35(1):43‐51. [DOI] [PubMed] [Google Scholar]
Katakam 2012 {published data only}
- Katakam LI, Trickey AW, Thomas EJ. Speaking up and sharing information improves trainee neonatal resuscitations. Journal of Patient Safety 2012;8(4):202‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Keller 2012 {published data only}
- Keller C, Records K, Coe K, Ainsworth B, Vega López S, Nagle‐Williams A, et al. Promotoras' roles in integrative validity and treatment fidelity efforts in randomized controlled trials. Family & Community Health 2012;35(2):120‐9. [DOI] [PubMed] [Google Scholar]
Kemper 2011 {published data only}
- Kemper PF, Bruijne M, Dyck C, Wagner C. Effectiveness of classroom based crew resource management training in the intensive care unit: study design of a controlled trial. BMC Health Services Research 2011;11:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koerner 2014 {published data only}
- Koerner M, Wirtz M, Michaelis M, Ehrhardt H, Steger AK, Zerpies E, et al. A multicentre cluster‐randomized controlled study to evaluate a train‐the‐trainer programme for implementing internal and external participation in medical rehabilitation. Clinical Rehabilitation 2014;28(1):20‐35. [DOI] [PubMed] [Google Scholar]
Körner 2012 {published data only}
- Körner M, Ehrhardt H, Steger AK, Bengel J. Interprofessional SDM train‐the‐trainer program "Fit for SDM": provider satisfaction and impact on participation. Patient Education and Counseling 2012;89(1):122‐8. [DOI] [PubMed] [Google Scholar]
Kunkler 2007 {published data only}
- Kunkler IH, Prescott RJ, Lee RJ, Brebner JA, Cairns JA, Fielding RG, et al. TELEMAM: a cluster randomised trial to assess the use of telemedicine in multi‐disciplinary breast cancer decision making. European Journal of Cancer 2007;43(17):2506‐14. [DOI] [PubMed] [Google Scholar]
Lee 2013 {published data only}
- Lee GA, Murray A, Bushnell R, Niggemeyer LE. Challenges developing evidence‐based algorithms for the trauma reception and resuscitation project. International Emergency Nursing 2013;21(2):129‐35. [DOI] [PubMed] [Google Scholar]
Marsteller 2013 {published data only}
- Marsteller JA, Hsu YJ, Wen M, Wolff J, Frick K, Reider L, et al. Effects of Guided Care on providers' satisfaction with care: a three‐year matched‐pair cluster‐randomized trial. Population Health Management 2013;16(5):317‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohaupt 2012 {published data only}
- Mohaupt J, Soeren M, Andrusyszyn MA, Macmillan K, Devlin‐Cop S, Reeves S. Understanding interprofessional relationships by the use of contact theory. Journal of Interprofessional Care 2012;26(5):370‐5. [DOI] [PubMed] [Google Scholar]
Musick 2011 {published data only}
- Musick BS, Robb SL, Burns DS, Stegenga K, Yan M, McCorkle KJ, et al. Development and use of a web‐based data management system for a randomized clinical trial of adolescents and young adults. Computers, Informatics, Nursing: CIN 2011;29(6):337‐43. [DOI] [PMC free article] [PubMed] [Google Scholar]
O'Leary 2010 {published data only}
- O'Leary KJ, Wayne DB, Haviley C, Slade ME, Lee J, Williams MV. Improving teamwork: impact of structured interdisciplinary rounds on a medical teaching unit. Journal of General Internal Medicine 2010;25(8):826‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rörtgen 2013 {published data only}
- Rörtgen D, Bergrath S, Rossaint R, Beckers SK, Fischermann H, Na IS, et al. Comparison of physician staffed emergency teams with paramedic teams assisted by telemedicine – a randomized, controlled simulation study. Resuscitation 2013;84(1):85‐92. [DOI] [PubMed] [Google Scholar]
Van de Ven 2010 {published data only}
- Ven J, Houterman S, Steinweg RA, Scherpbier AJ, Wijers W, Mol BW, et al. TOSTI‐Trial Group. Reducing errors in health care: cost‐effectiveness of multidisciplinary team training in obstetric emergencies (TOSTI study); a randomised controlled trial. BMC Pregnancy Childbirth 2010;10:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Weller 2014 {published data only}
- Weller JM, Torrie J, Boyd M, Frengley R, Garden A, Ng WL, et al. Improving team information sharing with a structured call‐out in anaesthetic emergencies: a randomized controlled trial. British Journal of Anaesthesia 2014;112(6):1042‐9. [DOI] [PubMed] [Google Scholar]
Wittenberg‐Lyles 2013 {published data only}
- Wittenberg‐Lyles E, Oliver DP, Kruse RL, Demiris G, Gage LA, Wagner K. Family caregiver participation in hospice interdisciplinary team meetings: how does it affect the nature and content of communication?. Health Communication 2013;28(2):110‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Baker 2011
- Baker L, Egan‐Lee E, Martimianakis M, Reeves S. Relationships of power: implications for interprofessional education and practice. Journal of Interprofessional Care 2011;25:98‐104. [DOI] [PubMed] [Google Scholar]
Department of Health 1997
- UK Department of Health. The New NHS: Modern and Dependable. Available from www.gov.uk/government/publications/the‐new‐nhs.
EPOC 2013
- Cochrane Effective Practice, Organisation of Care (EPOC). EPOC worksheets for preparing a 'Summary of findings' table using GRADE. EPOC resources for review authors, 2013. Available from epoc.cochrane.org/epoc‐specific‐resources‐review‐authors.
EPOC 2016
- Cochrane Effective Practice, Organisation of Care (EPOC). Suggested risk of bias criteria for EPOC reviews. EPOC resources for review authors, 2016. Available from epoc.cochrane.org/epoc‐specific‐resources‐review‐authors.
Francis 2013
- Francis R. Report of the Mid Staffordshire NHS Foundation Trust public inquiry. Available from www.gov.uk/government/uploads/system/uploads/attachment_data/file/279124/0947.pdf.
Guyatt 2008
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schünemann HJ, GRADE Working Group. What is "quality of evidence" and why is it important to clinicians?. BMJ 2008;336(7651):995‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Health Canada 2003
- Health Canada. 2003 First Ministers' Accord on Health Care Renewal. Available from www.scics.gc.ca/CMFiles/800039004_e1GTC‐352011‐6102.pdf.
Higgins 2011
- Higgins JP, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Institute of Medicine 2000
- Institute of Medicine (US) Committee on Quality of Health Care in America, Kohn LT, Corrigan JM, Donaldson MS, editor(s). To Err Is Human: Building a Safer Health System. Washington (DC): National Academies Press (US), 2000. [PubMed] [Google Scholar]
Institute of Medicine 2013
- Global Forum on Innovation in Health Professional Education, Board on Global Health, Institute of Medicine. Interprofessional Education for Collaboration: Learning how to improve health from interprofessional models across the continuum of education to practice: Workshop summary. Washington (DC): The National Academies Press (US), 2013. [PubMed] [Google Scholar]
Kenaszchuk 2010
- Kenaszchuk C, Reeves S, Nicholas D, Zwarenstein M. Validity and reliability of a multiple‐group measurement scale for interprofessional collaboration. BMC Health Services Research 2010;10:83. [DOI: 10.1186/1472-6963-10-83] [DOI] [PMC free article] [PubMed] [Google Scholar]
Körner 2016
- Körner M, Bütof S, Müller C, Zimmermann L, Becker S, Bengel J. Interprofessional teamwork and team interventions in chronic care: A systematic review. Journal of Interprofessional Care 2016;30(1):15‐28. [DOI] [PubMed] [Google Scholar]
Lillebo 2015
- Lillebo B, Faxvaag A. Continuous interprofessional coordination in perioperative work: an exploratory study. Journal of Interprofessional Care 2015;29(2):125‐30. [DOI] [PubMed] [Google Scholar]
Reeves 2010
- Reeves S, Lewin S, Espin S, Zwarenstein M. Interprofessional Teamwork for Health and Social Care. London: Blackwell‐Wiley, 2010. [Google Scholar]
Reeves 2011
- Reeves S, Goldman J, Gilbert J, Tepper J, Silver I, Suter E, et al. A scoping review to improve conceptual clarity of interprofessional interventions. Journal of Interprofessional Care 2011;25:167‐74. [DOI] [PubMed] [Google Scholar]
Reeves 2013
- Reeves S, Perrier L, Goldman J, Freeth D, Zwarenstein M. Interprofessional education: effects on professional practice and healthcare outcomes (update). Cochrane Database of Systematic Reviews 2013, Issue 3. [DOI: 10.1002/14651858.CD002213.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
The Joint Commission 2016
- The Joint Commission (USA). Sentinel Event Data ‐ Root Causes by Event Type. www.jointcommission.org/se_data_event_type_by_year_/.
Van Leijen‐Zeelenberg 2015
- Leijen‐Zeelenberg J, Raak A, Duimel‐Peeters I, Kroese M, Brink P, Vrijhoef H. Interprofessional communication failures in acute care chains: How can we identify the causes?. Journal of Interprofessional Care 2015;29(4):320‐30. [DOI] [PubMed] [Google Scholar]
WHO 1976
- World Health Organization. Continuing Education of Health Personnel, 1976. www.who.int/genomics/professionals/education/en/.
WHO 2010
- World Health Organization. Framework for action on interprofessional education and collaborative practice, 2010. www.who.int/hrh/resources/framework_action/en/.
References to other published versions of this review
Zwarenstein 1996
- Zwarenstein M, Bryant W, Baillie R. The effects on patient care of interventions to change collaboration between nurses and doctors. Cochrane Database of Systematic Reviews 1996, Issue 3. [DOI: 10.1002/14651858.CD000072] [DOI] [PubMed] [Google Scholar]
Zwarenstein 1997
- Zwarenstein M, Bryant W, Baillie R, Sibthorpe B. Interventions to change collaboration between nurses and doctors. Cochrane Database of Systematic Reviews 1997, Issue 2. [DOI: 10.1002/14651858.CD000072.pub2] [DOI] [PubMed] [Google Scholar]
Zwarenstein 2009
- Zwarenstein M, Goldman J, Reeves S. Interprofessional collaboration: effects of practice‐based interventions on professional practice and healthcare outcomes. Cochrane Database of Systematic Reviews 2009, Issue 8. [DOI: 10.1002/14651858.CD000072.pub2] [DOI] [PubMed] [Google Scholar]


