Abstract
Background
Nausea and vomiting are common symptoms in patients with terminal, incurable illnesses. Both nausea and vomiting can be distressing. Haloperidol is commonly prescribed to relieve these symptoms. This is an updated version of the original Cochrane review published in Issue 2, 2009, of Haloperidol for the treatment of nausea and vomiting in palliative care patients.
Objectives
To evaluate the efficacy and adverse events associated with the use of haloperidol for the treatment of nausea and vomiting in palliative care patients.
Search methods
For this updated review, we performed updated searches of CENTRAL, EMBASE and MEDLINE in November 2013 and in November 2014. We searched controlled trials registers in March 2015 to identify any ongoing or unpublished trials. We imposed no language restrictions. For the original review, we performed database searching in August 2007, including CENTRAL, MEDLINE, EMBASE, CINAHL and AMED, using relevant search terms and synonyms. Handsearching complemented the electronic searches (using reference lists of included studies, relevant chapters and review articles) for the original review.
Selection criteria
We considered randomised controlled trials (RCTs) of haloperidol for the treatment of nausea or vomiting, or both, in any setting, for inclusion. The studies had to be conducted with adults receiving palliative care or suffering from an incurable progressive medical condition. We excluded studies where nausea or vomiting, or both, were thought to be secondary to pregnancy or surgery.
Data collection and analysis
We imported records from each of the electronic databases into a bibliographic package and merged them into a core database where we inspected titles, keywords and abstracts for relevance. If it was not possible to accept or reject an abstract with certainty, we obtained the full text of the article for further evaluation. The two review authors independently assessed studies in accordance with the inclusion criteria. There were no differences in opinion between the authors with regard to the assessment of studies.
Main results
We considered 27 studies from the 2007 search. In this update we considered a further 38 studies from the 2013 search, and two in the 2014 search. We identified one RCT of moderate quality with low risk of bias overall which met the inclusion criteria for this update, comparing ABH (Ativan®, Benadryl®, Haldol®) gel, applied to the wrist, with placebo for the relief of nausea in 22 participants. ABH gel includes haloperidol as well as diphenhydramine and lorazepam. The gel was not significantly better than placebo in this small study; however haloperidol is reported not to be absorbed significantly when applied topically, therefore the trial does not address the issue of whether haloperidol is effective or well‐tolerated when administered by other routes (e.g. by mouth, subcutaneously or intravenously). We identified one ongoing trial of haloperidol for the management of nausea and vomiting in patients with cancer, with initial results published in a conference abstract suggesting that haloperidol is effective for 65% of patients. The trial had not been fully published at the time of our review. A further trial has opened, comparing oral haloperidol with oral methotrimeprazine (levomepromazine) for patients with cancer and nausea unrelated to their treatment, which we aim to include in the next review update.
Authors' conclusions
Since the last version of this review, we found one new study for inclusion but the conclusion remains unchanged. There is incomplete evidence from published RCTs to determine the effectiveness of haloperidol for nausea and vomiting in palliative care. Other than the trial of ABH gel vs placebo, we did not identify any fully published RCTs exploring the effectiveness of haloperidol for nausea and vomiting in palliative care patients for this update, but two trials are underway.
Plain language summary
Haloperidol for the treatment of nausea and vomiting in palliative care patients
Haloperidol is often used to help control nausea (feeling sick) or vomiting (being sick), both of which are common problems for patients with serious life‐threatening illnesses. Haloperidol can be given by mouth or by injection. There has been some research looking at how this drug works in nausea and vomiting caused by surgery and when trying to prevent nausea and vomiting caused by anti‐cancer treatments.
This is an update of the original review published in 2009 for which no studies met the inclusion criteria. For this update, in a search of the published literature in November 2014 we found one moderate quality randomised controlled trial which compared ABH (Ativan®, Benadryl®, Haldol®) gel, containing haloperidol and two other medications, to placebo.
The trial showed no difference between ABH gel and placebo. However it has previously been shown that haloperidol is not absorbed after applying ABH gel, so this result is not surprising. We identified a trial of haloperidol for nausea and vomiting in patients with cancer, with initial results presented at a conference. This suggests that haloperidol is effective in 65% of patients, but the results were not fully published at the time of our review. A further trial has opened in Australia, comparing haloperidol with another medication used for nausea, methotrimeprazine (levomepromazine).
Background
This is an updated version of the original Cochrane review published in Issue 2, 2009, of Haloperidol for the treatment of nausea and vomiting in palliative care patients.
All patients with terminal illness should have access to palliative care, independent of their diagnosis, and we wanted to reflect this in our review. Defining this population has been identified as a problem in previous reviews. We used the same definition as Hirst 2001 to give some consistency with other Cochrane reviews: "adult patients in any setting, receiving palliative care or suffering an incurable progressive medical condition".
Description of the condition
Nausea and vomiting are common symptoms for patients with terminal, incurable illnesses. Both symptoms can be distressing. Between 6% and 68% of patients with advanced cancer are troubled by nausea (Solano 2006), and nausea and vomiting are also common in other conditions, for example long‐term lung conditions and heart failure (Edmonds 2001; Klinkenberg 2004; Solano 2006). There are many potential causes in patients with terminal illness, including biochemical abnormalities (for example, kidney failure, high calcium salts in the blood), drugs (for example, iron supplements or morphine), or the underlying illness (for example, cancer deposits in the liver or brain). Anxiety can also be associated with nausea. Medications used to improve nausea and vomiting are called antiemetics. Antiemetics can help control symptoms while actions are taken to try and treat the underlying cause (Twycross 1998; Bentley 2001; Mannix 2004; Reuben 1986).
Description of the intervention
Haloperidol is in the butyrophenone class of drugs and acts as an antagonist on dopamine receptors. Haloperidol is used alone or in combination with other antiemetics orally, subcutaneously, intravenously or intramuscularly and can also be used intranasally (Miller 2008). It is also available in a compound gel including lorazepam and diphenhydramine, although it is reported not to be absorbed by this route (Smith 2012a).
Possible side effects of haloperidol include sedation, movement disturbance and arrhythmias (Twycross 2014). Neuroleptic malignant syndrome is a more serious but less common adverse event. This has numerous features including fever, altered consciousness and muscle rigidity, and can be fatal (Susman 2001).
How the intervention might work
Dopamine is an important neurotransmitter in the vomiting centre in the brain. Haloperidol acts as an antagonist at dopamine receptors in the brain (Smith 2012b; Twycross 2014).
Why it is important to do this review
There is little evidence from published randomised trials for many of the drugs used for these symptoms in this patient group (for example, cyclizine, haloperidol or levomepromazine) (Glare 2004). Haloperidol is commonly used in this setting to treat nausea and vomiting (Critchley 2001; Smith 2012b; To 2014). A small uncontrolled study (42 participants with cancer and nausea or vomiting unrelated to cancer treatment) suggests some evidence for effectiveness in the setting: 61% of evaluable participants had partial or complete control of nausea at day two (47% of all participants) and 74% at day five (40% of all participants) (Hardy 2010). We felt it important to update the previous systematic review to establish the current evidence base from randomised trials (Perkins 2009).
Objectives
To evaluate the efficacy and adverse events associated with the use of haloperidol for the treatment of nausea and vomiting in palliative care patients.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs) of haloperidol for the treatment of nausea or vomiting, or both, in any setting.
Types of participants
Inclusion criteria
Adults receiving palliative care or suffering from an incurable progressive medical condition. Adults suffering from nausea or vomiting, or both.
Exclusion criteria
Nausea or vomiting, or both, thought to be secondary to pregnancy or surgery.
Types of interventions
Studies where haloperidol was used as an antiemetic (alone or in addition to other agents) including any dose of haloperidol, via any route, over any duration of follow‐up.
Acceptable comparators
Placebo.
Other drug.
Non‐pharmacological intervention.
Types of outcome measures
Primary outcomes
Patient‐reported nausea severity.
Patient‐reported vomiting severity.
As there is a wide variety of instruments to measure these symptoms, we accepted any measure.
Secondary outcomes
Quality of life measurement.
Acceptability of treatment.
Need for rescue antiemetic medication.
Adverse events.
Withdrawal from study because of side effects.
Ideally, valid outcome measures would have been used but we would not have excluded studies on the basis of their outcome measures.
Search methods for identification of studies
For the original review we searched the electronic databases including CENTRAL, MEDLINE (1950 to August 2007), EMBASE (1980 to August 2007), CINAHL (1981 to August 2007) and AMED (1985 to August 2007), using relevant search terms and synonyms. The basic search strategy was ("haloperidol" OR "butyrophenone") AND ("nausea" OR "vomiting"), modified for each database. For the original review, handsearching complemented the electronic searches (using reference lists of included studies, relevant chapters and review articles). We did not impose a language restriction on studies. See Appendix 1 for the MEDLINE search strategy. We performed database searching in August 2007. For this update, we performed updated electronic searches of CENTRAL, MEDLINE and EMBASE in September 2012 and again in November 2013 and in November 2014.
We also searched clinical trials registers, the WHO International Clinical Trials Registry Platform (http://www.who.int/ictrp/en/), ClinicalTrials.gov, Current Controlled Trials (www.controlled-trials.com/) and the EU Clinical Trials Register (clinicaltrialsregister.eu) for this update on 06 March 2015, using the search term "haloperidol".
Electronic searches
We searched the following databases without language restrictions.
The Cochrane Central Register of Controlled Trials (CENTRAL) (via The Cochrane Library, 2007) for the previous version, and (Issue 10 of 12, 2014) for this update.
MEDLINE (via Ovid) 1946 to August 2007 for the previous version, and November 2014 for this update.
EMBASE (via Ovid) 1974 to August 2007 for the previous version, and November 2014 for this update.
CINAHL (via EBSCO) 1981 to August 2007 for the previous version.
AMED (via Ovid) 1985 to August 2007 for the previous version.
We used medical subject headings (MeSH) or equivalent and text word terms. There were no language or date restrictions. The search strategies for CENTRAL, MEDLINE, EMBASE, CINAHL and AMED are in Appendix 1.
Searching other resources
We searched the metaRegister of controlled trials (mRCT) (www.controlled-trials.com/mrct), clinicaltrials.gov (www.clinicaltrials.gov) and the World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (http://apps.who.int/trialsearch/) on 06 March 2015 to identify additional completed or ongoing studies.
Data collection and analysis
For the original review, records were imported from each of the above electronic databases into the bibliographic package EndNote 9 and merged into a core database where titles, keywords and abstracts were inspected for relevance. If it was not possible to accept or reject an abstract with certainty, the full text of the article was obtained for further evaluation. We reviewed the updated searches from 2013 and 2014 in a Word document including title, keywords and abstracts.
Selection of studies
Two review authors independently assessed abstracts and possible studies for inclusion in accordance with the above inclusion criteria (PP and SD for the review published in 2009 (Perkins 2009) and FM‐B and SD for this updated review). There were no differences in opinion between review authors with regards to assessment of studies.
Data extraction and management
Data were entered into RevMan 5.3 (RevMan 2014).
We planned to assess studies for treatment effect (see Types of outcome measures above), specifying numbers needed to treat for an additional beneficial outcome (NNTB) and numbers needed to treat for an additional harmful outcome (NNTH).
Assessment of risk of bias in included studies
We graded studies that met the inclusion criteria in the Risk of bias table below (see Characteristics of included studies).
Measures of treatment effect
Treatment effect was given as the mean difference in the change in nausea scores from baseline (Fletcher 2014). Other estimations of treatment effect would also have been considered.
Unit of analysis issues
We accepted randomisation of the individual patient.
Dealing with missing data
Had missing data been potentially relevant to the findings of the review, we would have contacted the authors to include the missing data if available; if unavailable, we would have used imputation (e.g. last outcome value carried forward).
Assessment of heterogeneity
Palliative care populations can be diverse, including a range of diagnoses as well as demographic characteristics. We did not exclude studies on the basis of scales used, raising the possibility of several different outcome measures being used. If the heterogeneity of studies allowed we would have performed a meta‐analysis. If the I2 statistic value was > 50% we would have used a random‐effects model (Higgins 2003).
Assessment of reporting biases
We planned to assess heterogeneity using L’Abbé plots (L’Abbé 1987), a visual method for assessing differences in results of individual studies.
Data synthesis
Quantiative meta‐analysis would only be used if studies were sufficiently similar to do so on the basis of I2 statistical test to assess heterogeneity (Higgins 2003). In the qualitative synthesis, risk of bias is noted.
Subgroup analysis and investigation of heterogeneity
If there had been sufficient data we had planned to perform subgroup analyses using the following subgroups identified a priori:
Population subgroups
Diagnoses of patients.
Likely mechanism of nausea/vomiting.
Prognoses of patients.
Age of patients.
Gender of patients.
Intervention subgroups
Route of administration.
Drug dose (< 2 mg/24 hours; 2 mg to 5 mg/24 hours; > 5 mg/24 hours),
Outcome subgroups
Nausea.
Vomiting.
Sensitivity analysis
Sensitivity analysis was not undertaken as there were insufficient studies identified to warrant it.
Results
Description of studies
Results of the search
We obtained 27 full studies (one in German) as potentially fitting the inclusion criteria for the 2009 review; we obtained a further 38 studies in 2013 as potentially fitting the inclusion criteria. None of these studies met the criteria for inclusion (see Figure 1). A further two studies were obtained from the search in November 2014 including one randomised study (Fletcher 2014) and one published as a conference abstract (ACTRN12610000481077).
1.
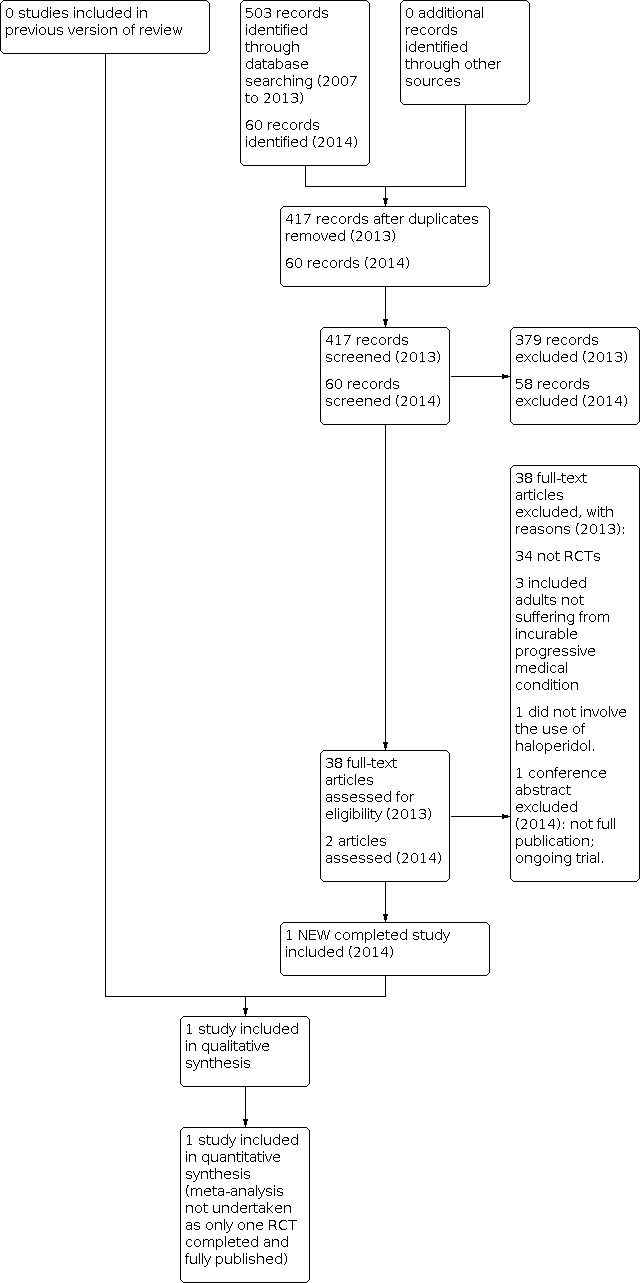
Study flow diagram.
We also identified the two ongoing trials and one uncompleted trial below in trials registers, from 190 records (163 trials) in the WHO International Clinical Trials Registry Platform, 137 records in ClinicalTrials.gov, 18 records in Current Controlled Trials and 33 records in the EU Clinical Trials Register (date of searches 06 March 2015):
Trials in progress
A two‐stage trial of response to antiemetic therapy in patients with cancer and nausea not related to anticancer therapy. Study 1 is a randomised, open‐label study of guideline‐driven, targeted antiemetic therapy versus single‐agent antiemetic therapy (ACTRN12610000481077). Haloperidol is the treatment in arm 2 of this study (escalated in a three‐step schedule from 1 mg/24 hours to 3 mg/24hours orally or subcutaneously). It is also used in combination with dexamethasone for a subset of arm 1 (participants with mechanical bowel obstruction). Preliminary results have been published as a conference abstract (ACTRN12610000481077). The authors conclude that haloperidol is effective for 65% of patients, with no significant difference between haloperidol or targeted antiemetic therapy, however the full results were not published at the time of our review.
A study for participants with cancer who experience ongoing nausea, not related to their treatment, despite taking standard and usual medications, that studies the effectiveness of oral methotrimeprazine (levomepromazine) versus oral haloperidol. This study was registered 23 February 2015, with a target sample size of 126. Patients will be randomised to receive blinded encapsulated oral methotrimeprazine (6.25mg) or oral haloperidol (1.5mg) given once daily for three days, with a potential to increase to twice daily if there is no response at 24 hours or 48 hours. Responses will be assessed using a 0 to 10 numeric rating scale of nausea (ACTRN12615000177550).
Trial stopped early
A study of olanzapine versus haloperidol for the relief of nausea and vomiting in patients with advanced cancer (registered 7 June 2005 but stopped early due to poor recruitment on 30 June 2008; Pereira 2012).
Included studies
Fletcher 2014 compared topical "ABH Gel" (Ativan® (lorazepam), Benadryl® (diphenhydramine) and Haldol® (haloperidol)) with placebo gel in 22 patients with nausea. Participants had cancer, or had a consultation with the palliative care team; patients receiving chemotherapy were excluded. The study sample size was reported to be large enough to detect a significant change in nausea (two points on a ten point scale), with a power of 80%. Patients were randomised to a sequence of treatments: one group that applied the ABH gel initially and then the placebo; the other group applied the placebo first followed by ABH gel. Twenty patients completed both treatments. It is unclear which arm was completed by the two participants who dropped out, ABH gel or placebo. The study is of moderate quality overall (GRADE criteria, Schünemann 2011).
Excluded studies
In total 27 studies were excluded in the initial review (Perkins 2009) with a further 37 studies excluded in this update (Excluded studies, with reasons shown below in Characteristics of excluded studies).
Risk of bias in included studies
The comparison of ABH gel with placebo (Fletcher 2014) recruited 22 participants of whom 20 completed the trial. The study sample size was calculated using a paired t‐test to be adequate to show that placebo was not inferior to ABH gel (power 80%, common standard deviation 1.5 at a one‐sided significance level of 5%). Risk of bias is summarised in Figure 2 and Figure 3.
2.

Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.

Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
In the study of ABH gel (Fletcher 2014), participants were allocated a sequence of treatments randomly, using a randomisation list generated by the study statistician.
Blinding
The study of ABH gel (Fletcher 2014) is described as double‐blind. Participants received ABH gel or placebo gel in randomised order. Participants and investigators were not aware of which treatment was being used.
Incomplete outcome data
Two of the 22 participants did not complete the study (Fletcher 2014). It is not clear which arm of the study they were in. This is unlikely to affect the overall results of the study.
Selective reporting
Placebo is reported to be non‐inferior to ABH gel (Fletcher 2014). Reporting bias appears unlikely in this instance.
Other potential sources of bias
There is some risk of recruitment bias in Fletcher 2014: all participants were reported to have an active cancer diagnosis, and some were recruited from a bone marrow transplant clinic. The study population may not therefore fully reflect the heterogeneity of patients seen by palliative care services. Also, some potential participants with more severe nausea may have chosen not to take part in the study because they preferred a subcutaneous or intravenous injection of medication for nausea, with a likely more rapid onset of action.
Effects of interventions
Haloperidol in the form of ABH topical gel did not reduce nausea or vomiting any more than placebo.
The primary study outcome was nausea which was self‐assessed using a 0–10 scale, with zero being no nausea and 10 being the worst possible nausea. The number of episodes of vomiting over time was recorded and participants completed the Memorial Symptom Assessment Scale‐Condensed which was used to determine the secondary outcomes and side effects. Participants were also asked at the end of each treatment period, "Did you feel the treatment was effective?,” and “Did you have any side effects from the drug?” On completion of both arms of the study, participants were asked, “Which drug helped you more?,” “Which drug did you think was the real ABH gel?,” and “Which was the placebo?” (Fletcher 2014)
Primary review outcomes
Patient‐reported nausea: no significant difference was found between placebo and ABH gel rubbed into the wrists, with both treatments showing a small reduction in nausea scores at 60 minutes (1.7+/‐ 2.05 for ABH gel, 0.9 +/‐ 2.45 for placebo; not statistically significant P value = 0.42).
Patient‐reported vomiting: observed episodes of vomiting were recorded rather than patient‐reported. ABH gel did not reduce vomiting more than placebo (not statistically significant P value = 0.34), but most patients did not have episodes of vomiting in any case.
Secondary review outcomes
Quality of life: not measured (note short term study).
Acceptability of treatment: of the 21 participants answering the question, seven thought the ABH gel was effective and 14 thought it was not. One reported a side effect of drowsiness.
Need for rescue antiemetic medication: not reported.
Adverse events: of 21 participants answering the question "Did you have any side effects from the drug?", 20 said they had no side effects from the drug and one said they did (drowsiness). One participant did not answer the question.
Withdrawal from study because of side effects: none (two participants did not complete both arms of the study, said to be because they did not want to wait to complete the study; they denied side effects) (Fletcher 2014).
Discussion
This is an updated version of the original Cochrane review published in Issue 2, 2009, on Haloperidol for the treatment of nausea and vomiting in palliative care patients.
For this update, we identified one small RCT of moderate quality which compared ABH gel, containing haloperidol, diphenhydramine and lorazepam, with placebo (Fletcher 2014). However haloperidol is not absorbed significantly from application of ABH gel (Smith 2012a) so this study does not add to the evidence base for the effectiveness of haloperidol as an antiemetic. Other than this (Fletcher 2014) there are no fully published randomised controlled trials of haloperidol for nausea or vomiting in a palliative care population, although it is frequently used by palliative care physicians (Prommer 2012; Smith 2012b; To 2014).
A previous systematic review of haloperidol in this context retrieved case reports and case series only (Critchley 2001). In a systematic review of antiemetics in the treatment of nausea in far‐advanced cancer only case series were found to support the use of haloperidol (Glare 2004). A review of the management of nausea and vomiting in people with cancer and other chronic diseases described haloperidol as "likely to be beneficial" based on consensus (Keeley 2007). Indeed haloperidol is thought by some to be one of four essential drugs for the management of symptoms at the end of life (Lindqvist 2013).
However, initial results from a trial comparing haloperidol with a targeted antiemetic approach suggests that either approach is effective for 65% of patients (ACTRN12610000481077); the full results were not published at the time of our review. This is similar to the findings from a previous uncontrolled study which suggested a response rate of 61% at day two (47% on intention to treat analysis (Hardy 2010). It is encouraging that despite the difficulties encountered by some researchers trying to conduct RCTs of the effectiveness of haloperidol, two randomised studies are currently underway (ACTRN12610000481077; ACTRN12615000177550).
There are RCTs of haloperidol in post‐operative nausea and vomiting (Barton 1975), gastrointestinal disorders (Christman 1974; Robbins 1975), prophylaxis against nausea and vomiting associated with radiotherapy (Cole 1974) and chemotherapy (Neidhart 1981). A meta‐analysis calculated that the number needed to treat for an additional beneficial outcome (NNTB) (with 2 mg) to prevent postoperative nausea or vomiting compared with placebo was four (Buttner 2004). It is not clear to what extent these studies are applicable to palliative care populations, although they may help to inform practice (McLean 2013). The causes and mechanisms of nausea and vomiting may be somewhat different in palliative care. This may have an impact on the effectiveness of interventions.
Summary of main results
For this update, we identified one RCT which compared ABH gel, containing haloperidol, diphenhydramine and lorazepam, with placebo (Fletcher 2014). However haloperidol is not absorbed significantly from topical application of ABH gel (Smith 2012a) so this study does not add to the evidence base for the effectiveness of haloperidol as an antiemetic. Other than Fletcher 2014 there are no fully published randomised controlled trials of haloperidol for nausea or vomiting in a palliative care population, although two randomised studies are currently underway (ACTRN12610000481077; ACTRN12615000177550).
Overall completeness and applicability of evidence
There is incomplete evidence from published RCTs to determine the effectiveness of haloperidol for nausea and vomiting in palliative care. The study of ABH gel (Fletcher 2014) does not refute the apparent anti‐emetic effect of haloperidol in clinical practice and studies in other settings (Christman 1974; Cole 1974; Barton 1975; Robbins 1975; Neidhart 1981; Buttner 2004; McLean 2013) since haloperidol is not absorbed by this route (Smith 2012a).
Haloperidol remains frequently prescribed as an antiemetic by palliative care physicians (Prommer 2012; Smith 2012b; To 2014).
Quality of the evidence
Only one published RCT of moderate quality has been identified in this updated review (Fletcher 2014). This is a small study of ABH gel (applied topically) compared with placebo. Since haloperidol is not absorbed via this route (Smith 2012a), this study neither supports nor refutes the role of haloperidol as an antiemetic.
Potential biases in the review process
The review methods sought to minimise bias by conducting a thorough search of the published literature to identify relevant studies, using predefined criteria to select studies for inclusion, and independent review by two authors. The review authors sometimes prescribe haloperidol as an antiemetic in palliative care settings.
Agreements and disagreements with other studies or reviews
A previous systematic review of haloperidol in this context retrieved case reports and case series only (Critchley 2001). In a systematic review of antiemetics in the treatment of nausea in far‐advanced cancer only case series were found to support the use of haloperidol (Glare 2004). A review of the management of nausea and vomiting in people with cancer and other chronic diseases described haloperidol as "likely to be beneficial" based on consensus (Keeley 2007). Indeed haloperidol is thought by some to be one of four essential drugs for the management of symptoms at the end of life (Lindqvist 2013).
Although we did not systematically review the evidence in other contexts, we found RCTs of haloperidol in post‐operative nausea and vomiting (Barton 1975), gastrointestinal disorders (Christman 1974; Robbins 1975), prophylaxis against nausea and vomiting associated with radiotherapy (Cole 1974) and chemotherapy (Neidhart 1981). A meta‐analysis calculated that the number needed to treat for a beneficial outcome (NNTB) (with 2 mg) to prevent postoperative nausea or vomiting compared with placebo was four (Buttner 2004). It is not clear to what extent these studies are applicable to palliative care populations, although they may help to inform practice (McLean 2013). The causes and mechanisms of nausea and vomiting may be somewhat different in palliative care. This may have an impact on the effectiveness of interventions.
Authors' conclusions
Implications for practice.
Since the last version of this review, we found one new study for inclusion (Fletcher 2014) but the conclusions remain unchanged.
There is a lack of published evidence for the use of haloperidol for nausea and vomiting in palliative care. A study comparing ABH gel (containing haloperidol, diphenhydramine and lorazepam) to placebo showed no significant difference (Fletcher 2014). However haloperidol is not absorbed significantly following application of the gel (Smith 2012a). No other RCTs of haloperidol in this setting have been fully published but two trials are underway (ACTRN12610000481077; ACTRN12615000177550).
Implications for people with nausea or vomiting in the context of advanced disease
Haloperidol is often used to help control nausea (feeling sick) or vomiting (being sick). The evidence to support this is largely from other settings ‐ for example, haloperidol to control sickness after surgical operations, chemotherapy or radiotherapy. There are no published randomised trials of haloperidol to control nausea and vomiting in people with advanced disease, other than a small study of a gel containing haloperidol (ABH gel) compared with placebo. No significant difference was found between ABH gel and placebo, which is not surprising as haloperidol is not absorbed by this route. Two randomised studies are underway to compare haloperidol with other medications for nausea and vomiting.
Implications for clinicians
Haloperidol may still be used as an antiemetic, though there is no evidence from RCTs in the palliative care context to quantify its effectiveness used orally or by injection. A small study of a gel containing haloperidol (ABH gel) compared with placebo found no significant difference (Fletcher 2014), which is not surprising as haloperidol is not absorbed by this route (Smith 2012a). Initial results from a trial which is underway suggest that it may be effective in 65% of patients (ACTRN12610000481077). Further results from this ACTRN12610000481077 and another ongoing study, ACTRN12615000177550, will help to inform practice which so far has been guided by clinical experience, consensus (Keeley 2007), an uncontrolled study (Hardy 2010) and research in other settings (McLean 2013).
Implications for policy makers
Haloperidol is frequently prescribed as an antiemetic (Prommer 2012; Smith 2012b; To 2014), although there is no evidence from RCTs in the palliative care context to quantify its effectiveness used orally or by injection. A small study of a topically applied gel containing haloperidol (ABH gel) compared with placebo found no significant difference (Fletcher 2014), which is not surprising as haloperidol is not absorbed by this route (Smith 2012a). Initial results from a trial which is underway suggest that it may be effective in 65% of patients (ACTRN12610000481077). Further results from ACTRN12610000481077 and another ongoing study, ACTRN12615000177550, will help to inform practice which so far has been guided by clinical experience, consensus (Keeley 2007) and research in other settings (McLean 2013).
Implications for funders
There is limited evidence for the use of haloperidol as an antiemetic in palliative care although it is commonly prescribed. Current clinical practice is guided by clinical experience, consensus and research in other settings. Some research to compare haloperidol with other antiemetics is underway, however this is a relatively under‐researched area. Nausea and vomiting are common, distressing symptoms with a profound impact on quality of life. Further studies are needed to determine the most effective strategies for managing these symptoms.
Implications for research.
Much has been written about methodological challenges when conducting research in palliative medicine (Jordhoy 1999; Ewing 2004). Nevertheless, two trials of haloperidol compared with other antiemetics are underway (ACTRN12610000481077; ACTRN12615000177550).
There is scope to build on this evidence further, using variations in intervention (e.g. dose, route of administration) and variations in trial design.
For instance, a randomised controlled trial could be conducted with participants with advanced disease who have nausea or vomiting, not associated with surgery, chemotherapy, radiotherapy or pregnancy, using an intervention of haloperidol, compared with another antiemetic, for example levomepromazine. Oral or subcutaneous routes of administration could be appropriate as these are often used in palliative care settings. Suggested outcomes include relief from nausea and from vomiting, anxiety, sedation, and extrapyramidal side effects. The duration of the study would be likely to have an impact on recruitment and attrition rates, but some side effects may only be apparent after weeks or months of use.
The pros and cons of placebo‐controlled studies have been debated (Hardy 1997; Kirkham 1997; Sanderson 2013). Placebo effects are likely to be significant, particularly in the context of a therapeutic relationship and environment (Sanderson 2013). Kris et al recommended that further trials of antiemetics in the context of chemotherapy should be conducted using active agents rather than placebo as comparator (Kris 1996). However a recent study of antiemetics in the emergency department compared metoclopramide, ondansetron and placebo and found no significant difference; most participants were satisfied with the treatment of their nausea (Egerton‐Warburton 2014). The feasibility and ethical acceptability of a placebo arm may in part depend on the participants' perception of the severity of their symptoms and the urgency of the need for treatment.
What's new
| Date | Event | Description |
|---|---|---|
| 27 October 2020 | Review declared as stable | See Published notes. |
History
Protocol first published: Issue 4, 2006 Review first published: Issue 2, 2009
| Date | Event | Description |
|---|---|---|
| 4 May 2017 | Review declared as stable | See Published notes. |
| 6 March 2015 | New search has been performed | This review has been updated; a risk of bias table has been added and a PRISMA flowchart have been included for this update. Two ongoing studies have been identified in trials registers. |
| 15 October 2014 | New citation required but conclusions have not changed | One new study has been identified for inclusion, but the conclusions remain unchanged. |
| 8 June 2014 | Amended | Edited by authors |
| 24 March 2014 | New search has been performed | Review updated |
| 24 March 2014 | Amended | Change of author |
| 9 November 2009 | Amended | Contact details updated. |
| 30 June 2008 | Amended | Protocol converted to new review format. |
Notes
Assessed for updating in 2017
A restricted search in May 2017 did not identify any potentially relevant studies likely to change the conclusions. Therefore, this review has now been stabilised following discussion with the authors and editors. We are aware of one relevant ongoing study, which we will assess when available. We will update the review if new evidence likely to change the conclusions is published, or if standards change substantially which necessitate major revisions.
Assessed for updating in 2019/20
We updated the searches in full in June 2019 with the intention of completing a full update; unfortunately the author team is no longer available. The current version of this review has been permanently stabilised. We are currently undertaking a priority setting process for palliative care titles. If this topic is prioritised, a new protocol will be required for this title.
Acknowledgements
With thanks to Paul Perkins for his significant contributions to the original systematic review, Yvonne Roy and colleagues in the Cochrane Pain, Palliative & Supportive Care Review Group, Sylvia Bickley for help with refining the search strategy and two review authors for their comments on an earlier draft of the protocol. Thanks also to Karen Rigby (Hinchingbrooke Hospital NHS Foundation Trust) who helped with some literature searching, Beccy Day who helped input some references and Jo Abbott, Caroline Struthers and Jane Hayes for helping us bring the literature search up to date.
Cochrane Review Group funding acknowledgement: The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane PaPaS Group. Disclaimer: The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, National Health Service (NHS) or the Department of Health.
Appendices
Appendix 1. Search strategies
CENTRAL
#1 MeSH descriptor: [Vomiting] explode all trees
#2 MeSH descriptor: [Nausea] explode all trees
#3 vomit*:ti,ab,kw (Word variations have been searched)
#4 nause*:ti,ab,kw (Word variations have been searched)
#5 (emesis or emet*):ti,ab,kw (Word variations have been searched)
#6 (antiemet* or anti‐emet*):ti,ab,kw (Word variations have been searched)
#7 #1 or #2 or #3 or #4 or #5 or #6
#8 MeSH descriptor: [Haloperidol] this term only
#9 (haloperidol or enabran* or halopidol* or halozen* or limerix* or neupram* or zetoridal* or haldol* or serenace* or haloper* or loperidol* or "uni haloper*" or novo‐peridol* or peridol* or alternus* or serenase* or buteridol* or duraperidol* or elaubat* or haloneural* or sigaperidol* or aloperidin* or sevium$.mp. or cizoren* or pericate* or peridor* or bioperidol* or kepsidol* or pulsit* or serenelfi*):ti,ab,kw (Word variations have been searched)
#10 (senorm* or cereen* or h‐tab* or halo‐p or halomed* or halopol* or haricon* or haridol* or perida* or polyhadon* or schizopol* or tensidol* or dozic* or fortunan* or halperon*):ti,ab,kw (Word variations have been searched)
#11 butyrophenone*:ti,ab,kw (Word variations have been searched)
#12 MeSH descriptor: [Butyrophenones] explode all trees
#13 #8 or #9 or #10 or #11 or #12
MEDLINE
1. RANDOMIZED CONTROLLED TRIAL.pt. 2. CONTROLLED CLINICAL TRIAL.pt. 3. RANDOMIZED CONTROLLED TRIALS.sh. 4. RANDOM ALLOCATION.sh. 5. DOUBLE BLIND METHOD.sh. 6. SINGLE BLIND METHOD.sh. 7. 1 or 2 or 3 or 4 or 5 or 6 8. (ANIMALS not HUMAN).sh. 9. 7 not 8 10. CLINICAL TRIAL.pt. 11. exp CLINICAL TRIALS/ 12. (clin$ adj25 trial$).ti,ab. 13. ((singl$ or doubl$ or trebl$ or tripl$) adj25 (blind$ or mask$)).ti,ab. 14. PLACEBOS.sh. 15. placebo$.ti,ab. 16. random$.ti,ab. 17. RESEARCH DESIGN.sh. 18. 10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 19. 18 not 8 20. 19 not 9 21. COMPARATIVE STUDY.sh. 22. exp EVALUATION STUDIES/ 23. FOLLOW UP STUDIES.sh. 24. PROSPECTIVE STUDIES.sh. 25. (control$ or prospectiv$ or volunteer$).ti,ab. 26. 21 or 22 or 23 or 24 or 25 27. 26 not 8 28. 27 not (9 or 20) 29. 9 or 20 or 28 30. nause$.mp. 31. vomit$.mp. 32. emesis.mp. 33. emet$.mp. 34. anti‐eme$.mp. 35. antieme$.mp. 36. antiemetics.sh. 37. nausea.sh. 38. vomiting.sh. 39. 30 or 31 or 32 or 33 or 34 or 35 or 36 or 37 or 38 40. haloperidol.mp. 41. enabran$.mp. 42. halopidol$.mp. 43. halozen$.mp. 44. limerix$.mp. 45. neupram$.mp. 46. zetoridal$.mp. 47. haldol$.mp. 48. serenace$.mp. 49. haloper$.mp. 50. loperidol$.mp. 51. "uni haloper$".mp. 52. novo‐peridol$.mp. 53. peridol$.mp. 54. alternus$.mp. 55. serenase$.mp. 56. buteridol$.mp. 57. duraperidol$.mp. 58. elaubat$.mp. 59. haloneural$.mp. 60. sigaperidol$.mp. 61. aloperidin$.mp. 62. sevium$.mp. 63. cizoren$.mp. 64. pericate$.mp. 65. peridor$.mp. 66. bioperidol$.mp. 67. avant$.mp. 68. kepsidol$.mp. 69. pulsit$.mp. 70. serenelfi$.mp. 71. senorm$.mp. 72. cereen$.mp. 73. h‐tab$.mp. 74. halo‐p.mp. 75. halomed$.mp. 76. halopol$.mp. 77. haricon$.mp. 78. haridol$.mp. 79. perida$.mp. 80. polyhadon$.mp. 81. schizopol$.mp. 82. tensidol$.mp. 83. dozic$.mp. 84. fortunan$.mp. 85. halperon$.mp. 86. 40 or 41 or 42 or 43 or 44 or 45 or 46 or 47 or 48 or 49 or 50 or 51 or 52 or 53 or 54 or 55 or 56 or 57 or 58 or 59 or 60 or 61 or 62 or 63 or 64 or 65 or 66 or 67 or 68 or 69 or 70 or 71 or 72 or 73 or 74 or 75 or 76 or 77 or 78 or 79 or 80 or 81 or 82 or 83 or 84 or 85 87. butyrophenone$.mp. 88. exp butyrophenones/ 89. 87 or 88 90. 86 or 89 91. 29 and 39 and 90
EMBASE
1 exp Vomiting/ (131250) 2 exp Nausea/ (152267) 3 vomit*.tw. (73323) 4 nause*.tw. (66297) 5 (emesis or emet*).tw. (12303) 6 (antiemet* or anti‐emet*).tw. (9283) 7 or/1‐6 (248098) 8 exp Butyrophenones/ (66670) 9 butyrophenone*.tw. (961) 10 (senorm* or cereen* or h‐tab* or halo‐p or halomed* or halopol* or haricon* or haridol* or perida* or polyhadon* or schizopol* or tensidol* or dozic* or fortunan* or halperon*).tw. (178) 11 (haloperidol or enabran* or halopidol* or halozen* or limerix* or neupram* or zetoridal* or haldol* or serenace* or haloper* or loperidol* or "uni haloper*" or novo‐peridol* or peridol* or alternus* or serenase* or buteridol* or duraperidol* or elaubat* or haloneural* or sigaperidol* or aloperidin* or sevium* or cizoren* or pericate* or peridor* or bioperidol* or kepsidol* or pulsit* or serenelfi*).tw. (22865) 12 or/8‐11 (68762) 13 random$.tw. (927492) 14 factorial$.tw. (24183) 15 crossover$.tw. (50980) 16 cross over$.tw. (22914) 17 cross‐over$.tw. (22914) 18 placebo$.tw. (209888) 19 (doubl$ adj blind$).tw. (151391) 20 (singl$ adj blind$).tw. (15179) 21 assign$.tw. (249822) 22 allocat$.tw. (88369) 23 volunteer$.tw. (186012) 24 Crossover Procedure/ (40652) 25 double‐blind procedure.tw. (222) 26 Randomized Controlled Trial/ (355913) 27 Single Blind Procedure/ (19061) 28 or/13‐27 (1477437) 29 (animal/ or nonhuman/) not human/ (4687675) 30 28 not 29 (1306909) 31 7 and 12 and 30 (1217) 32 (201311* or 201312* or 2014*).dd. (1574688) 33 31 and 32 (34)
CINAHL
S13 S7 AND S12
S12 S8 OR S9 OR S10 OR S11
S11 (haloperidol or enabran* or halopidol* or halozen* or limerix* or neupram* or zetoridal* or haldol* or serenace* or haloper* or loperidol* or "uni haloper*" or novo‐peridol* or peridol* or alternus* or serenase* or buteridol* or duraperidol* or elaubat* or haloneural* or sigaperidol* or aloperidin* or sevium* or cizoren* or pericate* or peridor* or bioperidol* or kepsidol* or pulsit* or serenelfi*)
S10 (senorm* or cereen* or h‐tab* or halo‐p or halomed* OR halopol* OR haricon* or haridol* or perida* or polyhadon* or schizopol* or tensidol* or dozic* or fortunan* or halperon*)
S9 butyrophenone*
S8 (MH "Antipsychotic Agents, Butyrophenone+")
S7 S1 OR S2 OR S3 OR S4 OR S5 OR S6
S6 (antiemet* or anti‐emet*)
S5 (emesis or emet*)
S4 nause*
S3 vomit*
S2 (MH "Nausea")
S1 (MH "Vomiting+")
AMED
1. exp Vomiting/
2. exp Nausea/
3. vomit*.tw.
4. nause*.tw.
5. (emesis or emet*).tw.
6. (antiemet* or anti‐emet*).tw.
7. or/1‐6
8. butyrophenone*.tw.
9. (senorm* or cereen* or h‐tab* or halo‐p or halomed* or halopol* or haricon* or haridol* or perida* or polyhadon* or schizopol* or tensidol* or dozic* or fortunan* or halperon*).tw.
10. (haloperidol or enabran* or halopidol* or halozen* or limerix* or neupram* or zetoridal* or haldol* or serenace* or haloper* or loperidol* or "uni haloper*" or novo‐peridol* or peridol* or alternus* or serenase* or buteridol* or duraperidol* or elaubat* or haloneural* or sigaperidol* or aloperidin* or sevium* or cizoren* or pericate* or peridor* or bioperidol* or kepsidol* or pulsit* or serenelfi*).tw.
11. or/8‐10
12. 7 and 11
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Fletcher 2014.
| Study characteristics | ||
| Methods | Randomised, double‐blind, placebo‐controlled non‐inferiority crossover trial. | |
| Participants | Adults with active cancer and self‐reported nausea at least 4 out of 10 (n=22 enrolled, 20 of whom completed the study). Many participants (unspecified) were attending the bone marrow transplant clinic, some were enrolled from the palliative care clinic or palliative care in‐patient setting. For full inclusion and exclusion criteria see Fletcher 2014. | |
| Interventions | ABH gel (contains lorazepam, diphenhydramine and haloperidol) or placebo gel rubbed on the wrist. ABH gel contains lorazepam 20mg, diphenhydramine 250mg, haloperidol 20mg, lecithin organogel 2mL, ethoxydiglycol 0.83mL, water 0.2mL, pluronic gel 20% (sufficient to make 10mL). These 10mL were divided into ten doses of 1mL. Placebo was 1mL pluronic lecithin organogel alone. If no effect (up to 1 point on 0 to 10 scale) was seen after one hour, the participant was offered other medication for nausea and was considered to have completed the trial. Otherwise the participant crossed over to the other treatment after four hours. |
|
| Outcomes | Primary outcome: difference in nausea score from baseline to 60 minutes after intervention. A change of 2 points on a 0 to 10 scale was thought to be significant. Secondary outcomes: difference in vomiting episodes and side effects determined by the Memorial Symptom Assessment Scale ‐ Condensed. Assessments were made at baseline (before administration of gel), 30, 60, 90, 120, 180, 240 minutes. Participants were also asked questions about the perceived effectiveness of each gel. |
|
| Notes | There was no significant difference in the change in nausea scores for each group (1.7 +/‐ 2.05 for the ABH gel group and 0.9 +/‐ 2.45 for the placebo group on a 0 to10 scale; P = 0.42). Authors concluded that placebo was non‐inferior to ABH gel. A previous study had shown that there was no significant absorption of haloperidol via this route (Smith 2012a). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The study biostatistician generated the randomisation list." |
| Allocation concealment (selection bias) | Low risk | "Randomisation was done in the investigational drug pharmacy with allocation concealed by the use of sealed opaque envelopes not accessible to investigators." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Described as double‐blind. Participants received ABH gel or placebo gel in randomised order. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants and investigators not aware of which treatment was being used. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Two of the 22 participants did not complete both arms of the study, because they "did not feel like waiting the length of the study but denied side effects from the medications". It is not clear which treatments were completed for the two who did not complete the study. |
| Selective reporting (reporting bias) | Low risk | Placebo is reported to be non‐inferior to ABH gel in this study. |
| Other bias | Unclear risk | This is a relatively small study. Participants were enrolled from the bone marrow transplant clinic, inpatient palliative care unit or seen as an inpatient by the palliative care team. The study authors state that, "Many patients were bone marrow transplant patients" but it is not clear what proportion. The study participants may not be fully representative of patients receiving palliative care in other settings. However, this is unlikely to have been a significant cause of bias for the study findings. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdelsayed 2007 | Review article ‐ not a RCT |
| Bleicher 2008 | Pilot retrospective study ‐ not a RCT; unclear whether population is palliative; unable to isolate effectiveness of haloperidol as given with other agents |
| Bregni 1991 | Not a RCT; prophylaxis not treatment; bone marrow transplantation rather than palliative context |
| Buttner 2004 | Review article ‐ not a RCT |
| Casey 2011 | Review article ‐ not a RCT; unclear whether population is palliative |
| Cerchietti 2000 | RCT but not of haloperidol |
| Cheung 2011 | Review article ‐ not a RCT |
| Chiu 2007 | Review article ‐ not a RCT; focus on antiemetics other than haloperidol |
| Chow 2010 | Review article ‐ not a RCT; not a palliative population (acute gastroenteritis); no mention of haloperidol |
| Christman 1974 | Not a palliative population |
| Christo 2003 | Review article ‐ not a RCT |
| Clary 2009 | Review article ‐ not a RCT |
| Cole 1974 | Unclear whether patient group restricted to palliative care patients |
| Cole 1994 | Case report |
| Davis 2010 | Systematic review, but no RCTs including haloperidol described |
| De Vries 1969 | Not a palliative population (mixed ‐ with diagnosis of "functional gastrointestinal disorders" with "an evident psychosomatic component") |
| DiVall 2007 | Review article ‐ not a RCT |
| Ettinger 2007 | Review article ‐ not a RCT |
| Ettinger 2009 | Review article ‐ not a RCT |
| Feyer 2011 | Review article ‐ not a RCT; no mention of haloperidol |
| Findlay 1993 | Unable to isolate effectiveness of haloperidol as given with other agents; not a palliative care population |
| Fischberg 2007 | RCT but not of haloperidol (droperidol), and not restricted to palliative care patients |
| Getto 2011 | Review article ‐ not a RCT; not a palliative population |
| Glare 2004 | Review article ‐ not a RCT |
| Glare 2011 | Review article ‐ not a RCT |
| Gonzales 2011 | Review article ‐ not a RCT |
| Grunberg 1984 | Randomised controlled trial but not restricted to palliative care patients |
| Grunberg 2010 | Review article ‐ not a RCT; no mention of haloperidol |
| Hardy 2010 | Prospective, open‐label, uncontrolled study ‐ not a RCT |
| Herndon 2002 | Review article ‐ not a RCT |
| Herrstedt 2008 | Review article ‐ not a RCT |
| Jordan 2007 | Review article ‐ not a RCT |
| Kohara 2005 | Retrospective notes review |
| Ladabaum 1999 | Review ‐ no mention of haloperidol |
| Liem‐Moolenar 2010 | Not a palliative population ‐ healthy male volunteers |
| Lohr 2008 | Review article ‐ not a RCT |
| McHugh 2011 | Review article ‐ not a RCT |
| McNicol 2003 | Review article ‐ not a RCT |
| Mercadante 2007 | Systematic review but no mention of haloperidol |
| Naeim 2008 | Review article ‐ not a RCT; no mention of haloperidol |
| Navari 2007 | Review article ‐ not a RCT |
| Neidhart 1981 | Prophylaxis rather than treatment of emesis; unclear whether population is palliative |
| O'Connor 2011 | Review article ‐ not a RCT |
| Owens 1984 | Prophylaxis rather than treatment of emesis; unclear whether population is palliative |
| Perkins 2009 | Cochrane systematic review ‐ not a RCT |
| Pleuvry 2009 | Review article ‐ not a RCT |
| Porreca 2009 | Review article ‐ not a RCT; not a palliative population (patients with chronic pain) |
| Ripamonti 2001 | Review article ‐ not a RCT |
| Robbins 1975 | Population not necessarily palliative; treatment of symptoms of gastritis/gastroenteritis |
| Roeland 2010 | Review article ‐ not a RCT; stem cell transplant rather than palliative context |
| Saller 1985 | Prophylaxis not treatment of chemotherapy‐associated nausea and vomiting |
| Siden 2008 | Case report ‐ not a RCT; child |
| Silvey 1988 | Prophylaxis rather than treatment of emesis; unclear whether population is palliative |
| Smith 2011 | Population not palliative (volunteers) |
| Smith 2012a | Population not palliative (volunteers) |
| Sperry 2007 | Review article ‐ not a RCT |
| Stapleton 2009 | Review article ‐ not a RCT; not a palliative population (patients with gastroparesis from any cause) |
| Tatum 2009 | Review article ‐ not a RCT |
| Tornetta 1971 | Postoperative nausea and vomiting |
| Tornetta 1972 | Prophylaxis of postoperative nausea and vomiting |
| Trigg 2008 | Review article ‐ not a RCT; stem cell transplant rather than palliative context |
| Trigg 2010 | Review article ‐ not a RCT |
| Weschules 2005 | Not a RCT ‐ retrospective notes review; haloperidol given with other agents |
| White 2006 | Case study ‐ not a RCT |
RCT ‐ randomised controlled trial
Characteristics of ongoing studies [ordered by study ID]
ACTRN12610000481077.
| Study name | The effectiveness of guideline‐driven antiemetic therapy versus single agent antiemetic therapy in patients with cancer and nausea not related to cancer therapy. A two‐stage trial of response to antiemetic therapy in patients with cancer and nausea not related to anticancer therapy. Study 1: a randomised open label study of guideline‐driven targeted antiemetic therapy versus single agent antiemetic therapy. |
| Methods | Randomised Controlled Trial Multi‐centre, open‐label randomised parallel arm trial. Participants are randomised to active treatment arms 1 or 2. |
| Participants | Patients with cancer and nausea not related to anticancer therapy. |
| Interventions | Arm 1: targeted guideline‐driven antiemetic therapy dependent on presumed cause of nausea. Arm 2: haloperidol in a three‐step dose escalation schedule from 1mg over 24 hours to 3mg over 24 hours given orally or parenterally (subcutaneously). |
| Outcomes | Primary outcome: response to treatment at 72 hours. Response defined as at least a two‐point improvement in average nausea score from baseline and final score less than 3 on an 11‐point numeric rating scale. |
| Starting date | First enrolment 15 June 2010. |
| Contact information | Professor Patsy Yates Head of School, Faculty of Health, School ‐ Nursing Queensland University of Technology, Brisbane QLD 4001 Australia |
| Notes | Preliminary results of this study were published as a conference abstract in 2014 (see Yates 2010 secondary reference). |
ACTRN12615000177550.
| Study name | A randomised, controlled, double blind study of oral methotrimeprazine versus oral haloperidol in patients with cancer and nausea not related to anticancer therapy (Nausea study 3), to compare the effectiveness of oral methotrimeprazine versus oral haloperidol in improving the management of nausea in patients with cancer and nausea not related to anticancer therapy. |
| Methods | Randomised controlled trial. |
| Participants | Patients with cancer and nausea not related to anticancer therapy, with nausea severity of at least 3 on an 11 point numeric rating scale (0 no nausea to 10 worst possible nausea). |
| Interventions | Blinded encapsulated oral methotrimeprazine (6.25mg) or haloperidol (1.5mg) given once daily for three intervention days. If no response at 24 or 48 hours, the dose of the study drug can be increased to twice daily. Rescue medication of metoclopramide or domperidone will be available. |
| Outcomes | Primary outcome: response to treatment at 72 hours from first study drug administration. Response defined as more than or equal to a two point improvement from baseline for average nausea over the preceding 24 hours on an 11 point numeric rating scale. Secondary outcomes: best nausea score over the preceding 24 hours; complete response (defined as at least two‐point improvement from baseline and a score of less than 3 for average nausea over preceding 24 hours, on 0 to 10 numeric rating scale; response; number of episodes of vomiting, not including retching; number of rescue antiemetic doses; toxicity. |
| Starting date | First enrolment 02 March 2015 |
| Contact information | Prof Janet R Hardy, Director of Palliative Care Mater Health Services, Raymond Terrace, South Brisbane QLD 4101 Australia +617 31632775 janet.hardy@mater.org.au |
| Notes | http://www.anzctr.org.au/ACTRN12615000177550.aspx |
Differences between protocol and review
We had planned to use the Jadad score (Jadad 1996) to assess risk of bias but used the Cochrane risk of bias table instead, as recommended by the Cochrane Collaboration.
Contributions of authors
Paul Perkins and SD designed the original systematic review and wrote the protocol. SD developed the search strategy with comments from PP and advice from Sylvia Bickley. Both authors independently reviewed all titles and abstracts yielded by the search strategy for inclusion or exclusion and achieved a consensus by discussion (PP and SD for the original review, FM‐B and SD for the updated review). FM‐B and SD updated the text of the full review, which was originally written by PP and SD. SD is responsible for further updates.
Declarations of interest
FMB has no known conflicts of interest to declare that are relevant to the development of this review.
SD has no known conflicts of interest to declare that are relevant to the development of this review.
Stable (no update expected for reasons given in 'What's new')
References
References to studies included in this review
Fletcher 2014 {published data only}
- Fletcher DS, Coyne PJ, Dodson PW, Parker GG, Wan W, Smith TJ. A randomized trial of the effectiveness of eopical "ABH Gel" (Ativan®, Benadryl®, Haldol®) vs. placebo in cancer patients with nausea. Journal of Pain and Symptom Management 2014;48(5):797-803. [DOI: 10.1016/j.jpainsymman.2014.02.010] [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abdelsayed 2007 {published data only}
- Abdelsayed G. Management of radiation-induced nausea and vomiting. Experimental Haematology 2007;35(4 Suppl):34-6. [DOI] [PubMed] [Google Scholar]
Bleicher 2008 {published data only}
- Bleicher J, Bhaskara A, Huyck T, Constantino S, Bardia A, Loprinzi CL, et al. Lorazepam, diphenhydramine, and haloperidol transdermal gel for rescue from chemotherapy-induced nausea and vomiting: Results of two pilot trials. Journal of Supportive Oncology 2008;6(1):27-32. [PubMed] [Google Scholar]
Bregni 1991 {published data only}
- Bregni M, Siena S, Di Nicola M, Bonadonna G, Gianni AM. Tropisetron plus haloperidol to ameliorate nausea and vomiting associated with high-dose alkylating agent cancer chemotherapy. European Journal of Cancer 1991;27(5):561-5. [DOI] [PubMed] [Google Scholar]
Buttner 2004 {published data only}
- Buttner M, Walder B, Elm E, Tramer MR. Is low-dose haloperidol a useful antiemetic? A meta-analysis of published and unpublished randomized trials. Anaesthesiology 2004;101:1454-63. [DOI] [PubMed] [Google Scholar]
Casey 2011 {published data only}
- Casey C, Chen LM, Rabow MW. Symptom management in gynaecologic malignancies. Expert Review of Anticancer Therapy 2011;11(7):1077-89. [DOI] [PubMed] [Google Scholar]
Cerchietti 2000 {published data only}
- Cerchietti L, Navigante A, Sauri A, Palazzo F. Hypodermoclysis for control of dehydration in terminal-stage cancer. International Journal of Palliative Nursing 2000;6(8):370-4. [DOI] [PubMed] [Google Scholar]
Cheung 2011 {published data only}
- Cheung W, Zimmerman C. Pharmacologic management of cancer-related pain, dyspnoea and nausea. Seminars in Oncology 2011;38(3):450-9. [DOI] [PubMed] [Google Scholar]
Chiu 2007 {published data only}
- Chiu W. Update on antiemetics of chemotherapy. Journal of Internal Medicine of Taiwan 2007;18(6):342-9. [Google Scholar]
Chow 2010 {published data only}
- Chow C, Leung AK, Hon KL. Acute gastroenteritis: from guidelines to real life. Clinical and Experimental Gastroenterology 2010;3(1):97-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Christman 1974 {published data only}
- Christman RS, Weinstein RA, Larose JB. Low-dose haloperidol as antiemetic treatment in gastrointestinal disorders: a double-blind study. Current Therapeutic Research 1974;16(11):1171-5. [PubMed] [Google Scholar]
Christo 2003 {published data only}
- Christo PJ. Opioid effectiveness and side effects in chronic pain. Anesthesiology Clinics of North America 2003;21:699-713. [DOI] [PubMed] [Google Scholar]
Clary 2009 {published data only}
- Clary P, Lawson P. Pharmacologic pearls for end of life care. American Family Physician 2009;79(12):1059-65. [PubMed] [Google Scholar]
Cole 1974 {published data only}
- Cole DR, Duffy DF. Haloperidol for radiation sickness: control of associated nausea, vomiting, and anorexia. New York State Journal of Medicine 1974;74(9):1558-662. [PubMed] [Google Scholar]
Cole 1994 {published data only}
- Cole RM, Robinson F, Harvey L, Trethowan K, Murdoch B. Successful control of intractable nausea and vomiting requiring combined ondansetron and haloperidol in a patient with advanced cancer. Journal of Pain and Symptom Management 1994;9:48-50. [DOI] [PubMed] [Google Scholar]
Davis 2010 {published data only}
- Davis M, Hallerberg G. A systematic review of the treatment of nausea and/or vomiting in cancer unrelated to chemotherapy of radiation. Journal of Pain and Symptom Management 2010;39(4):756-67. [DOI] [PubMed] [Google Scholar]
De Vries 1969 {published data only}
- De Vries PM. A double-blind cross-over clinical valuation of metoclopramide and a combination of haloperidol and isopropamide iodide in gastroenterology. Arzneimittel-Forschung 1969;19:1766-7. [PubMed] [Google Scholar]
DiVall 2007 {published data only}
- DiVall M, Cervosimo R. Prevention and treatment of chemotherapy-induced nausea and vomiting. Formulary 2007;42(6):378-88. [Google Scholar]
Ettinger 2007 {published data only}
- Ettinger DS, Bierman PJ, Bradbury B, Comish CC, Ellis G, Ignoffo RJ, et al. Antiemesis: clinical practice guidelines in oncology. Journal of the National Comprehensive Cancer Network 2007;5:12-33. [DOI] [PubMed] [Google Scholar]
Ettinger 2009 {published data only}
- Ettinger D, Armstrong DK, Barbour S, Berger MJ, Bierman PJ, Bradbury B, et al. Antiemesis. Journal of the National Comprehensive Cancer Network 2009;7(5):572-95. [DOI] [PubMed] [Google Scholar]
Feyer 2011 {published data only}
- Feyer P, Jordan K. Update and new trends in antiemetic therapy: the continuing need for novel therapies. Annals of Oncology 2011;22(1):30-8. [DOI] [PubMed] [Google Scholar]
Findlay 1993 {published data only}
- Findlay M, Simes RJ, Cox K, Carmichael K, Chey T, McNeil E, et al. A randomised cross-over trial of antiemetic therapy for platinum-based chemotherapy. Improved control with an intensive multiagent regimen. European Journal of Cancer 1993;29A(3):309-15. [DOI] [PubMed] [Google Scholar]
Fischberg 2007 {published data only}
- Fischberg D. Randomised controlled trial of three antiemetics in the emergency department. Journal of Pain and Palliative Care Pharmacotherapy 2007;21(1):73-4. [Google Scholar]
Getto 2011 {published data only}
- Getto L, Zeserson E, Breyer M. Vomiting, diarrhoea, constipation and gastroenteritis. Emergency Medicine Clinics of North America 2011;29(2):211-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
Glare 2004 {published data only}
- Glare P, Pereira G, Kristjanson LJ, Stockler M, Tattersall M. Systematic review of the efficacy of antiemetics in the treatment of nausea in patients with far-advanced cancer. Supportive Care Cancer 2004;12:432-40. [DOI] [PubMed] [Google Scholar]
Glare 2011 {published data only}
- Glare P, Miller J, Nikolova T, Tickoo R. Treating nausea and vomiting in palliative care: a review. Clinical Interventions in Ageing 2011;6(1):243-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gonzales 2011 {published data only}
- Gonzales M, Widera E. Nausea and other nonpain symptoms in long-term care. Clinics in Geriatric Medicine 2011;27(2):213-28. [DOI] [PubMed] [Google Scholar]
Grunberg 1984 {published data only}
- Grunberg SM, Gala KV, Lampenfeld M, Jamin D, Johnson K, Cariffe P, et al. Comparison of the antiemetic effect of high-dose intravenous metoclopramide and high-dose intravenous haloperidol in a randomized double-blind crossover study. Journal of Clinical Oncology 1984;2(7):782-7. [DOI] [PubMed] [Google Scholar]
Grunberg 2010 {published data only}
- Grunberg S, Clark-Snow RA, Koeller J. Chemotherapy-induced nausea and vomiting: contemporary approaches to optimal management. Supportive Care in Cancer 2010;18 Suppl 1:S1-10. [DOI] [PubMed] [Google Scholar]
Hardy 2010 {published data only}
- Hardy J, O'Shea A, White C, Gilshenan K, Welch L, Douglas C. The efficacy of haloperidol in the management of nausea and vomiting in patients with cancer. Journal of Pain and Symptom Management 2010;40(1):111-6. [DOI] [PubMed] [Google Scholar]
Herndon 2002 {published data only}
- Herndon CM, Jackson IK, Hallin PA. Management of opioid-induced gastrointestinal effects in patients receiving palliative care. Pharmacotherapy 2002;22:240-50. [DOI] [PubMed] [Google Scholar]
Herrstedt 2008 {published data only}
- Herrstedt J. Antiemetics: an update and the MASCC guidelines applied in clinical practice. Nature Clinical Practice Oncology 2008;5(1):32-43. [DOI] [PubMed] [Google Scholar]
Jordan 2007 {published data only}
- Jordan K, Schmoll HJ, Aapro MS. Comparative activity of anti-emetic drugs. Critical Reviews in Oncology/Hematology 2007;61(2):162-75. [DOI] [PubMed] [Google Scholar]
Kohara 2005 {published data only}
- Kohara H, Ueoka H, Takeyama H, Murakami T, Morita T. Sedation for terminally ill patients with cancer with uncontrollable physical distress. Journal of Palliative Medicine 2005;8(1):20-5. [DOI] [PubMed] [Google Scholar]
Ladabaum 1999 {published data only}
- Ladabaum U, Hasler WL. Novel approaches to the treatment of nausea and vomiting. Digestive Diseases 1999;17:125-32. [DOI] [PubMed] [Google Scholar]
Liem‐Moolenar 2010 {published data only}
- Liem-Moolenaar M, te Beek ET, Kam ML, Franson KL, Kahn RS, Hijman R, et al. Central nervous system effects of haloperidol on THC in healthy male volunteers. Journal of Psychopharmacology 2010;24(11):1697-708. [DOI] [PubMed] [Google Scholar]
Lohr 2008 {published data only}
- Lohr L. Chemotherapy-induced nausea and vomiting. Cancer Journal 2008;14(2):85-93. [DOI] [PubMed] [Google Scholar]
McHugh 2011 {published data only}
- McHugh M, Miller-Saultz D. Assessment of gastrointestinal symptoms in advanced illness. Primary Care - Clinics in Office Practice 2011;38(2):225-46. [DOI] [PubMed] [Google Scholar]
McNicol 2003 {published data only}
- McNicol E, Horowicz-Mehler N, Fisk RA, Bennett K, Gialeli-Goudas M, Chew PW, et al. Management of opioid side effects in cancer-related and chronic noncancer pain: a systematic review. Journal of Pain 2003;4(5):231-56. [DOI] [PubMed] [Google Scholar]
Mercadante 2007 {published data only}
- Mercadante S, Casuccio A, Mangione S. Medical treatment for inoperable malignant bowel obstruction: a qualitative systematic review. Journal of Pain and Symptom Management 2007;33(2):217-23. [DOI] [PubMed] [Google Scholar]
Naeim 2008 {published data only}
- Naeim A. Evidence-based recommendations for cancer nausea and vomiting. Journal of Clinical Oncology 2008;26(23):3903-10. [DOI] [PubMed] [Google Scholar]
Navari 2007 {published data only}
- Navari M. Overview of the updated antiemetic guidelines for chemotherapy-induced nausea and vomiting. Community Oncology 2007;4 Suppl 1:3-11. [Google Scholar]
Neidhart 1981 {published data only}
- Neidhart JA, Gagen M, Young DM, Wilson HE. Specific antiemetics for specific cancer chemotherapeutic agents: haloperidol versus benzquinamide. Cancer 1981;47:1439-43. [DOI] [PubMed] [Google Scholar]
O'Connor 2011 {published data only}
- O'Connor B, Creedon B. Pharmacological treatment of bowel obstruction in cancer patients. Expert Opinion on Pharmacotherapy 2011;12(14):2205-14. [DOI] [PubMed] [Google Scholar]
Owens 1984 {published data only}
- Owens NJ, Schauer AR, Nightingale CH, Golub GR, Martin RS, Williams HM, et al. Antiemetic efficacy of prochlorperazine, haloperidol, and droperidol in cisplatin-induced emesis. Clinical Pharmacy 1984;3:167-70. [PubMed] [Google Scholar]
Perkins 2009 {published data only}
- Perkins P, Dorman S. Haloperidol for the treatment of nausea and vomiting in palliative care patients. Cochrane Database of Systematic Reviews 2009, Issue 2. Art. No: CD006271. [DOI: 10.1002/14651858.CD006271.pub2] [DOI] [PubMed] [Google Scholar]
Pleuvry 2009 {published data only}
- Pleuvry B. Physiology and pharmacology of nausea and vomiting. Anaesthesia and Intensive Care Medicine 2009;10(12):597-601. [Google Scholar]
Porreca 2009 {published data only}
- Porreca F, Ossipov M. Nausea and vomiting side effects with opioid analgesics during treatment of chronic pain: Mechanisms, implications and management options. Pain Medicine 2009;10(4):654-62. [DOI] [PubMed] [Google Scholar]
Ripamonti 2001 {published data only}
- Ripamonti C, Twycross R, Baines M, Bozzetti F, Capri S, De Conno F, et al. Clinical-practice recommendations for the management of bowel obstruction in patients with end-stage cancer. Supportive Care in Cancer 2001;9:223-33. [DOI] [PubMed] [Google Scholar]
Robbins 1975 {published data only}
- Robbins EL, Nagel JD. Haloperidol parenterally for treatment of vomiting and nausea from gastrointestinal disorders in a group of geriatric patients: double-blind, placebo-controlled study. Journal of the American Geriatrics Society 1975;23:38-41. [DOI] [PubMed] [Google Scholar]
Roeland 2010 {published data only}
- Roeland E, Mitchell W, Elia G, Thornberry K, Herman H, Cain J, et al. Symptom control in stem cell transplantation: a multidisciplinary palliative care team approach. Part 1: Physical symptoms. Journal of Supportive Oncology 2010;8(3):100-16. [PubMed] [Google Scholar]
Saller 1985 {published data only}
- Saller R, Hellenbrecht D. The benefit and risk of high dose metoclopramide in comparison to high dose haloperidol or trifluorpromazine in cisplatin-induced emesis. Klinische Wochenschrift 1985;63:428-32. [DOI] [PubMed] [Google Scholar]
Siden 2008 {published data only}
- Siden H. Haloperidol as a palliative anti-emetic in a toddler: an evidence-base challenge. Journal of Pain and Symptom Management 2008;35(3):235-8. [DOI] [PubMed] [Google Scholar]
Silvey 1988 {published data only}
- Silvey L, Carpenter JT, Wheeler RH, Lee J, Conolley C. A randomized comparison of haloperidol plus dexamethasone versus prochlorperazine plus dexamethasone in preventing nausea and vomiting in patients receiving chemotherapy for breast cancer. Journal of Clinical Oncology 1988;6(9):1397-400. [DOI] [PubMed] [Google Scholar]
Smith 2011 {published data only}
- Smith T, Ritter JK, Coyne PJ, Parker GL, Dodson P, Fletcher DS. Testing the cutaneous absorption of lorazepam, diphenhydramine and haloperidol gel (ABH gel) used for cancer-related nausea. Journal of Clinical Oncology 2011;29:Abstract 9021. [Google Scholar]
Smith 2012a {published data only}
- Smith T, Fletcher D, Coyne P, Dodson P, Parker G. ABH gel is not absorbed from the skin of normal volunteer so cannot be effective against nausea. In: Journal of Pain and Symptom Management. Conference: Annual Assembly of the American Academy of Hospice and Palliative Medicine and the Hospice and Palliative Nurses Association. Denver, US, 2012.
Sperry 2007 {published data only}
- Sperry M. A review of the 2006 ASCO antiemetic guidelines update. US Pharmacist 2007;32(1):22-8. [Google Scholar]
Stapleton 2009 {published data only}
- Stapleton J, Wo J. Current treatment of nausea and vomiting associated with gastroparesis: antiemetics, prokinetics, tricyclics. Gastrointestinal Endoscopy Clinics of North America 2009;19(1):57-72. [DOI] [PubMed] [Google Scholar]
Tatum 2009 {published data only}
- Tatum P. Haloperidol for nausea and vomiting. Journal of Pain and Palliative Care Pharmacotherapy 2009;23(4):426-7. [Google Scholar]
Tornetta 1971 {published data only}
- Tornetta FJ. Haloperidol as an antiemetic - guest discussion. Anesthesia and Analgesia - Current Researches 1971;50(6):1024-6. [PubMed] [Google Scholar]
Tornetta 1972 {published data only}
- Tornetta FJ. Double-blind evaluation of haloperidol for antiemetic activity. Anesthesia and analgesia. Current Researches 1972;51(6):964-7. [PubMed] [Google Scholar]
Trigg 2008 {published data only}
- Trigg M, Inverso D. Nausea and vomiting with high-dose chemotherapy and stem cell rescue therapy: a review of antiemetic regimens. Bone Marrow Transplantation 2008;42(8):501-6. [DOI] [PubMed] [Google Scholar]
Trigg 2010 {published data only}
- Trigg M. Chemotherapy-induced nausea and vomiting: antiemetic trials that impacted clinical practice. Journal of Oncology Pharmacy Practice 2010;16(4):233-44. [DOI] [PubMed] [Google Scholar]
Weschules 2005 {published data only}
- Weschules DJ. Tolerability of the compound ABHR in hospice patients. Journal of Palliative Medicine 2005;8(6):1135-43. [DOI] [PubMed] [Google Scholar]
White 2006 {published data only}
- White C, McPherson A, McCann MA, Sadler A, Fyvie J. Prolonged extra-pyramidal side effects after discontinuation of haloperidol as an antiemetic. Palliative Medicine 2006;20:215-6. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ACTRN12610000481077 {published data only}
- Hardy J, Yates P, Philip J, Martin P, Glare P, Currow D. A targeted versus empiric approach to antiemetic use in advanced cancer. In: In: MASCC/ISOO International Symposium on Supportive Care in Cancer. 26 June 2014. [http:/mascc2014.meetingxpert.net/MASCC_595/poster_96766/program.aspx/anchor96766]
- Yates P. The effectiveness of guideline driven antiemetic therapy versus single agent antiemetic therapy in patients with cancer and nausea not related to cancer therapy. Australian New Zealand Clinical Trials Registry 2010. [ACTRN12610000481077] [https://www.anzctr.org.au/Trial/Registration/TrialReview.aspx?ACTRN=12610000481077]
ACTRN12615000177550 {published data only}
- Hardy JR. A randomised, controlled, double blind study of oral methotrimeprazine versus oral haloperidol in patients with cancer and nausea not related to anticancer therapy (Nausea study 3), to compare the effectiveness of oral methotrimeprazine versus oral haloperidol in improving the management of nausea in patients with cancer and nausea not related to anticancer therapy. World Health Organization International Clinical Trials Registry Platform 23 February 2015. [ACTRN12615000177550] [http://www.anzctr.org.au/ACTRN1261500077550.aspx]
Additional references
Barton 1975
- Barton MD, Libonati M, Cohen PJ. The use of haloperidol for treatment of postoperative nausea and vomiting--a double-blind placebo-controlled trial. Anesthesiology 1975;42(4):508-12. [DOI] [PubMed] [Google Scholar]
Bentley 2001
- Bentley A, Boyd K. Use of clinical pictures in the management of nausea and vomiting. Palliative Medicine 2001;15:247-53. [DOI] [PubMed] [Google Scholar]
Critchley 2001
- Critchley P, Plach N, Grantham M, Marshall D, Taniguchi A, Latimer E, et al. Efficacy of haloperidol in the treatment of nausea and vomiting in the palliative patient: a systematic review. Journal of Pain and Symptom Management 2001;22:631-4. [DOI] [PubMed] [Google Scholar]
Edmonds 2001
- Edmonds P, Karlsen S, Khan S, Addington-Hall J. A comparison of the palliative care needs of patients dying from chronic respiratory diseases and lung cancer. Palliative Medicine 2001;15:287-95. [DOI] [PubMed] [Google Scholar]
Egerton‐Warburton 2014
- Egerton-Warburton D, Meek R, Mee MJ, Braitberg G. Antiemetic use for nausea and vomiting in adult emergency department patients: randomized controlled trial comparing ondansetron, metoclopramide, and placebo. Annals of Emergency Medicine 2014;64(5):526-32. [DOI] [PubMed] [Google Scholar]
Ewing 2004
- Ewing G, Rogers M, Barclays S, McCabe J, Martin A, Todd C. Recruiting patients into a primary care based study of palliative care: why is it so difficult? Palliative Medicine 2004;18:452-9. [DOI] [PubMed] [Google Scholar]
Hardy 1997
- Hardy JR. Placebo-controlled trials in palliative care: the argument for. Palliative Medicine 1997;11(5):415-8. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003;327(7414):557-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hirst 2001
- Hirst A, Sloan R. Benzodiazepines and related drugs for insomnia in palliative care. Cochrane Database of Systematic Reviews 2001, Issue 4. Art. No: CD003346. [DOI: 10.1002/14651858.CD003346] [DOI] [PubMed] [Google Scholar]
Jadad 1996
- Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the quality of randomized clinical trials: is blinding necessary? Controlled Clinical Trials 1996;17:1-12. [DOI] [PubMed] [Google Scholar]
Jordhoy 1999
- Jordhoy M, Kaasa S, Fayers P, Ovreness T, Underland G, Ahlner-Elmqvist M. Challenges in palliative care research; recruitment, attrition and compliance: experience from a randomized controlled trial. Palliative Medicine 1999;13:299-310. [DOI] [PubMed] [Google Scholar]
Keeley 2007
- Keeley P. Nausea and vomiting in people with cancer and other chronic diseases. BMJ Clinical Evidence 2007;06:2406. [PMC free article] [PubMed] [Google Scholar]
Kirkham 1997
- Kirkham SR, Abel J. Placebo-controlled trials in palliative care: the argument against. Palliative Medicine 1997;11(6):489-92. [DOI] [PubMed] [Google Scholar]
Klinkenberg 2004
- Klinkenberg M, Willems DL, Wal G, Deeg DJH. Symptom burden in the last week of life. Journal of Pain and Symptom Management 2004;27:5-13. [DOI] [PubMed] [Google Scholar]
Kris 1996
- Kris MG, Cubeddu LX, Gralla RJ, Cupissol D, Tyson LB, Venkatraman E, Homesley HD. Are more antiemetic trials with a placebo necessary? Report of patient data from randomized trials of placebo antiemetics with cisplatin. Cancer 1996;78(10):2193-8. [DOI] [PubMed] [Google Scholar]
Lindqvist 2013
- Lindqvist O, Lundquist G, Dickman A, Bükki J, Lunder U, Hagelin CL, et al. Four essential drugs needed for quality care of the dying: a Delphi-study based international expert consensus opinion. Journal of Palliative Medicine 2013;16(1):38-43. [DOI] [PubMed] [Google Scholar]
L’Abbé 1987
- L’Abbé KA, Detsky AS, O’Rourke K. Meta-analysis in clinical research. Annals of Internal Medicine 1987;107:224-32. [DOI] [PubMed] [Google Scholar]
Mannix 2004
- Mannix KA. Palliation of nausea and vomiting. In: Oxford Textbook of Palliative Medicine. Oxford University Press, 2004. [Google Scholar]
McLean 2013
- McLean SL, Blenkinsopp A, Bennett MI. Using haloperidol as an antiemetic in palliative care: informing practice through evidence from cancer treatment and postoperative contexts. Journal of Pain and Palliative Care Pharmacotherapy 2013;27(2):132-5. [DOI] [PubMed] [Google Scholar]
Miller 2008
- Miller JL, Ashford JW, Archer SM, Rudy AC, Wermeling DP. Comparison of intranasal administration of haloperidol with intravenous and intramuscular administration. Pharmacotherapy 2008;28(7):875-82. [DOI] [PubMed] [Google Scholar]
Pereira 2012
- Pereira J. Study comparing olanzapine with haloperidol for the relief of nausea and vomiting in patients with advanced cancer. clinicaltrials.gov. [ISRCTN58624349] [NCT00124930] [https://clinicaltrials.gov/ct2/show/NCT00124930?term=Haloperidol&rank=59]
Prommer 2012
- Prommer E. Role of haloperidol in palliative medicine: an update. American Journal of Hospice and Palliative Medicine 2012;29(4):295-301. [DOI] [PubMed] [Google Scholar]
Reuben 1986
- Reuben DB, Mor V. Nausea and vomiting in terminal cancer patients. Archives of Internal Medicine 1986;146:2021-3. [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Sanderson 2013
- Sanderson C, Hardy J, Spruyt O, Currow DC. Placebo and nocebo effects in randomized controlled trials: the implications for research and practice. Journal of Pain and Symptom Management 2013;46(5):722-30. [DOI] [PubMed] [Google Scholar]
Schünemann 2011
- Schünemann HJ, Oxman AD, Higgins JPT, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and ‘Summary of findings' tables. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.
Smith 2012b
- Smith HR, Cox LR, Smith BR. Dopamine receptor antagonists. Annals of Palliative Medicine 2012;1(2):137-42. [DOI] [PubMed] [Google Scholar]
Solano 2006
- Solano JP, Gomes B, Higginson IJ. A comparison of symptom prevalence in far advanced cancer, AIDS, heart disease, chronic obstructive pulmonary disease and renal disease. Journal of Pain and Symptom Management 2006;31(1):58-69. [DOI] [PubMed] [Google Scholar]
Susman 2001
- Susman VL. Clinical management of neuroleptic malignant syndrome. Psychiatric Quarterly 2001;72:325-36. [DOI] [PubMed] [Google Scholar]
To 2014
- To TH, Agar M, Yates P, Currow DC. Prescribing for nausea in palliative care: a cross-sectional national survey of Australian palliative medicine doctors. Journal of Palliative Medicine 2014;17(9):1032-6. [DOI] [PubMed] [Google Scholar]
Twycross 1998
- Twycross R, Back I. Nausea and vomiting in advanced cancer. European Journal of Palliative Care 1998;5:39-45. [Google Scholar]
Twycross 2014
- Twycross R, Wilcock A, Howard P. Palliative Care Formulary. 5th edition. palliativedrugs.com, 2014. [Google Scholar]


