Abstract
Background
Chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) is a disease that causes progressive or relapsing and remitting weakness and numbness. It is probably caused by an autoimmune process. Immunosuppressive or immunomodulatory drugs would be expected to be beneficial. This review was first published in 2003 and has been updated most recently in 2016.
Objectives
To assess the effects of immunomodulatory and immunosuppressive agents other than corticosteroids, immunoglobulin, and plasma exchange in CIDP.
Search methods
On 24 May 2016, we searched the Cochrane Neuromuscular Specialised Register, the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 4) in the Cochrane Library, MEDLINE, Embase, CINAHL, and LILACS for completed trials, and clinical trial registers for ongoing trials. We contacted the authors of the trials identified and other disease experts seeking other published and unpublished trials.
Selection criteria
We sought randomised and quasi‐randomised trials of all immunosuppressive agents, such as azathioprine, cyclophosphamide, methotrexate, ciclosporin, mycophenolate mofetil, and rituximab, and all immunomodulatory agents, such as interferon (IFN) alfa and IFN beta, in participants fulfilling standard diagnostic criteria for CIDP. We included all comparisons of these agents with placebo, another treatment, or no treatment.
Data collection and analysis
We used standard methodological procedures expected by Cochrane. We wanted to measure the change in disability after one year as our primary outcome. Our secondary outcomes were change in disability after four or more weeks (from randomisation); change in impairment after at least one year; change in maximum motor nerve conduction velocity and compound muscle action potential amplitude after one year; and for participants who were receiving corticosteroids or intravenous immunoglobulin (IVIg), the amount of this medication given during at least one year after randomisation. Participants with one or more serious adverse events during the first year was also a secondary outcome.
Main results
Four trials fulfilled the selection criteria: one of azathioprine (27 participants), two of IFN beta‐1a (77 participants in total) and one of methotrexate (60 participants). The risk of bias was considered low in the trials of IFN beta‐1a and methotrexate but high in the trial of azathioprine. None of the trials showed significant benefit in any of the outcomes selected by their authors. The results of the outcomes which approximated most closely to the primary outcome for this review were as follows.
In the azathioprine trial there was a median improvement in the Neuropathy Impairment Scale (scale range 0 to 280) after nine months of 29 points (range 49 points worse to 84 points better) in the azathioprine and prednisone treated participants compared with 30 points worse (range 20 points worse to 104 points better) in the prednisone alone group. There were no reports of adverse events.
In a cross‐over trial of IFN beta‐1a with 20 participants, the treatment periods were 12 weeks. The median improvement in the Guy's Neurological Disability Scale (range 1 to 10) was 0.5 grades (interquartile range (IQR) 1.8 grades better to zero grade change) in the IFN beta‐1a treatment period and 0.5 grades (IQR 1.8 grades better to 1.0 grade worse) in the placebo treatment period. There were no serious adverse events in either treatment period.
In a parallel group trial of IFN beta‐1a with 67 participants, none of the outcomes for this review was available. The trial design involved withdrawal from ongoing IVIg treatment. The primary outcome used by the trial authors was total IVIg dose administered from week 16 to week 32 in the placebo group compared with the IFN beta‐1a groups. This was slightly but not significantly lower in the combined IFN beta‐1a groups (1.20 g/kg) compared with the placebo group (1.34 g/kg, P = 0.75). There were four participants in the IFN beta‐1a group and none in the placebo group with one or more serious adverse events, risk ratio (RR) 4.50 (95% confidence interval (CI) 0.25 to 80.05).
The methotrexate trial had a similar design involving withdrawal from ongoing corticosteroid or IVIg treatment. At the end of the trial (approximately 40 weeks) there was no significant difference in the change in the Overall Neuropathy Limitations Scale, a disability scale (scale range 0 to 12), the median change being 0 (IQR −1 to 0) in the methotrexate group and 0 (IQR −0.75 to 0) in the placebo group. These changes in disability might have been confounded by the reduction in corticosteroid or IVIg dose required by the protocol. There were three participants in the methotrexate group and one in the placebo with one or more serious adverse events, RR 3.56 (95% CI 0.39 to 32.23).
Authors' conclusions
Low‐quality evidence from randomised trials does not show significant benefit from azathioprine or interferon beta‐1a and moderate‐quality evidence from one randomised trial does not show significant benefit from a relatively low dose of methotrexate for the treatment of CIDP. None of the trials was large enough to rule out small or moderate benefit. The evidence from observational studies is insufficient to avoid the need for randomised controlled trials to discover whether these drugs are beneficial. Future trials should have improved designs, more sensitive outcome measures relevant to people with CIDP, and longer treatment durations.
Plain language summary
Immune system treatments other than corticosteroids, immunoglobulin and plasma exchange for CIDP
Review question
We reviewed the evidence for giving people with chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) treatment that regulates the immune system other than corticosteroids, immunoglobulin and plasma exchange.
Background
CIDP is an uncommon disease that causes weakness and numbness of the arms and legs. The condition may progress steadily or have periods of worsening and improvement. The cause is inflammation that damages the insulating layer (myelin) around individual nerve fibres. In severe cases, the disease affects the actual nerve fibres themselves. According to Cochrane systematic reviews, three immune system treatments are known to help. These are corticosteroids ('steroids'), plasma exchange (which removes and replaces blood plasma), and intravenous immunoglobulin (infusions into a vein of human antibodies). However, benefit from these is often inadequate or does not last long. We wanted to discover whether other drugs that suppress or change the activity of the immune system are of benefit.
Study characteristics
We found four trials. A trial with 27 participants compared the effects of azathioprine together with steroids to steroids alone for nine months. Azathioprine is a drug that is often used to treat autoimmune diseases because it suppresses the harmful immune cells. This trial had a parallel‐group design, which means that the participants were divided into groups that each received only one of the treatments.
A cross‐over design trial with 10 participants compared the immune‐regulating drug interferon (IFN) beta‐1a with placebo (dummy treatment) for 12 weeks. The cross‐over design means that all participants received both treatments in random order. A parallel‐group trial with 67 participants also compared interferon beta‐1a with placebo, but for 32 weeks. Another parallel‐group trial with 60 participants compared the cytotoxic drug methotrexate with placebo for 40 weeks. The IFN beta‐1a trials, but not the azathioprine or methotrexate trials, received pharmaceutical company support or sponsorship.
Key results
None of these trials showed significant benefit or harm from the drugs. We selected disability scores as our primary measure of the effect of treatment. All the trials were too small to detect or rule out anything but major benefit or harm. We rated the quality of the evidence as moderate or low for IFN beta‐1a and methotrexate because of problems with trial design, and because the small number of participants made the result imprecise. We rated the quality of the evidence for azathioprine as low because of lack of blinding and imprecision. Observational studies of these and other drugs, including the cytotoxic drugs cyclophosphamide, ciclosporin, mycophenolate, rituximab, alemtuzumab and natalizumab, peripheral blood stem cell transplantation, and the immune regulating drug interferon (IFN) alfa, exist but are of insufficient quality to determine whether any of these treatments are beneficial.
The evidence is up to date to May 2016.
Summary of findings
Background
Description of the condition
Chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) is a disease causing progressive or relapsing and remitting weakness and numbness. Its minimum prevalence is at least one or two cases per 100,000 of the population and may be as high as 8.9 per 100,000 (Lunn 1999; Mygland 2001; Chiò 2007; Laughlin 2009; Mahdi‐Rogers 2014). A prevalence study in the South East Thames region of England in 2008 found that 76.2% of 84 people with CIDP had needed treatment at some time and 31.7% of 41 people could not walk independently on the prevalence date (Mahdi‐Rogers 2008; Mahdi‐Rogers 2014).
CIDP began to emerge as a syndrome separate from Guillain‐Barré syndrome (GBS, an acute paralysing disorder usually due to acute inflammatory demyelinating polyradiculoneuropathy) with the description of recurrent steroid‐responsive neuropathy by Austin in 1958 (Austin 1958). The description of larger series of people with CIDP led to a clearer clinical picture (Dyck 1975; Prineas 1976; McCombe 1987; Barohn 1989), and eventually an arbitrary definition (Ad Hoc 1991). This definition has been widely used and was the basis of the diagnostic criteria in the first version of this review although it has been criticised as too restrictive, excluding people who are considered by the authors of case series to have a clinical condition identical to CIDP (Saperstein 2001; Koski 2009). Other criteria have been published, including less restrictive criteria agreed by a consensus committee (EFNS/PNS 2010), which we have accepted in updates of this review.
A number of conditions that are forms of, or resemble, CIDP require special consideration. The duration of the onset phase in CIDP is more than eight weeks, while that of GBS is less than four weeks. Some cases of CIDP have an acute onset like GBS, or are subacute (Hughes 1992; Oh 2003; Ruts 2005; Ruts 2010). GBS itself may recur on one or sometimes several occasions (Kuitwaard 2009). While some relapses are acute and easily recognisable as GBS, others may be subacute or chronic and these cases are difficult to distinguish from CIDP. For this reason we would have included people with recurrent GBS in this review, but noted in which studies they were included; we are not aware of any such participants. A form of asymmetrical sensory and motor neuropathy with persistent conduction block, the Lewis‐Sumner syndrome (Lewis 1982; Saperstein 1999; Viala 2004), has been recognised as a distinct subgroup of CIDP that differs from other cases in the persistence of the conduction block at individual sites over long periods. We would have included such cases in this review if trials had included them. However, the syndrome of multifocal motor neuropathy (MMN) with conduction block is a pure motor syndrome that differs from CIDP in its response to steroids and might differ in its response to other agents (EFNS/PNS 2010). Consequently, we have excluded MMN from this review; a separate review has been published (Umapathi 2015). By contrast, in the sensory form of CIDP there is electrophysiological evidence of motor involvement (Oh 1992). We therefore regarded this as a form of CIDP and would have included such people. We would also have included variants with cranial nerve or upper‐limb onset or involvement (Thomas 1996), distal or proximal and distal involvement (Katz 2000; Saperstein 2001), and subclinical (magnetic resonance imaging) evidence of central nervous system involvement (Ormerod 1990; Waddy 1989).
There has been debate as to whether people with the clinical features of an acquired demyelinating neuropathy and a systemic disease, such as cancer, diabetes mellitus, systemic lupus erythematosus, and other connective tissue diseases should be categorised as having CIDP (Ad Hoc 1991; EFNS/PNS 2006; EFNS/PNS 2010). To confine this review to as homogeneous a group as possible, we would have excluded such cases where the results were available separately from the whole trial. About 10% of people with an acquired demyelinating neuropathy have a serum paraprotein (Kelly 1981). About half such people have an IgM paraprotein in which the paraprotein is an autoantibody directed against carbohydrate epitopes on myelin‐associated glycoprotein, other myelin proteins, and glycolipids. This condition and some of the other paraprotein‐associated demyelinating neuropathies have different underlying pathology and presumably pathogenesis. Consequently, we have left treatments for all paraprotein‐associated demyelinating neuropathies for other reviews to consider. A Cochrane Review of immunotherapy for IgM anti‐myelin‐associated glycoprotein paraprotein‐associated peripheral neuropathies (Lunn 2016) and another for IgG and IgA paraprotein‐associated demyelinating neuropathy have been published (Stork 2015).
Description of the intervention
Although not proven, CIDP is generally considered to be an autoimmune disease caused by either humoral or cell‐mediated immunity directed against myelin or Schwann cell antigens which have not been identified (Köller 2005). Consequently, various forms of immunotherapy have been tried in its treatment. A Cochrane systematic review of corticosteroid treatment concluded that the one randomised controlled trial (RCT) identified gave only weak support for the conventional view that such drugs are beneficial (Hughes 2015). In a RCT of high‐dose monthly oral dexamethasone versus standard prednisolone treatment for CIDP, 10 of 24 people in the dexamethasone group and six of 16 in the prednisolone group (odds ratio 1.2, 95% CI 0.3 to 4.4) went into remission after 12 months (van Schaik 2010). Two RCTs have reported that plasma exchange is more effective than sham exchange (Dyck 1986; Hahn 1996a). A Cochrane systematic review concluded that plasma exchange does produce benefit but this is short term and may be followed by rebound worsening (Mehndiratta 2015). A Cochrane systematic review of five trials including a total of 235 participants (Vermeulen 1993; Hahn 1996b; Thompson 1996; Mendell 2000; Hughes 2008), concluded that a single course of intravenous immunoglobulin (IVIg) significantly reduces disability and weakness (Eftimov 2013).
Immunoglobulin (Ig) is beginning to be given in large subcutaneous doses. One trial in which 30 participants were withdrawn from intravenous Ig (IVIg) and randomised to the same dose of subcutaneous Ig (SCIg) or placebo for 12 weeks supports this practice. There was an increase in isometric muscle strength with SCIg and a decline with placebo, the difference being significant (Markvardsen 2013).
One RCT showed that three‐weekly treatment with IVIg has a sustained effect for at least 24 weeks (Hughes 2008). Another showed that plasma exchange was not significantly different in efficacy from IVIg (Dyck 1994). A trial comparing oral prednisolone with IVIg showed slightly but not significantly more benefit from IVIg (Hughes 2001). A trial comparing IVIg and intravenous methylprednisolone (IVMP) showed IVIg was both more effective and better tolerated in more participants compared to IVMP, but those who responded to IVMP relapsed less frequently after stopping treatment (Nobile‐Orazio 2012). An open‐label trial comparing IVIg with oral prednisone, only published in abstract, reported that more participants improved with IVIg, but the result was not statistically significant (Camdessanché 2013). Corticosteroids have serious long‐term side effects, such as hypertension, osteoporosis, diabetes mellitus, and obesity. Both plasma exchange and IVIg are expensive, require hospitalisation, and have only short‐term benefits. Therefore, there is a need for more effective, longer‐lasting treatment regimens.
Why it is important to do this review
In response to the need for better treatments many neurologists have tried treating CIDP with immunosuppressive and immunomodulatory agents that have been found useful in multiple sclerosis, rheumatoid arthritis, other connective tissue diseases, and renal transplant rejection. These agents include azathioprine, interferon beta (IFNb), cyclophosphamide, ciclosporin, methotrexate, etanercept, alemtuzumab, rituximab, natalizumab, fingolimod, and autologous peripheral blood stem cell transplantation (PBSCT). When this review was first published in 2003, the only published trials were one small RCT of azathioprine combined with prednisone compared with prednisone alone (Dyck 1985) and another of interferon beta‐1a (Hadden 1999). Since then only one further trial, of methotrexate, has been completed (RMC 2009). The topic of immunosuppressive and immunomodulatory agents for CIDP has regularly been the subject of non‐systematic reviews (Hahn 2005; Köller 2005; Léger 2005; Nobile‐Orazio 2005; van Doorn 2005; Saperstein 2008; Stübgen 2013), but we know of no other systematic review. This review aims to provide the best evidence about such immunomodulatory treatment for CIDP. It is now updated until 2016 to make sure that it includes the latest evidence.
Objectives
To assess the effects of immunomodulatory and immunosuppressive agents other than corticosteroids, immunoglobulin, and plasma exchange in CIDP.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomised and quasi‐randomised trials of immunosuppressive and immunomodulatory agents for chronic inflammatory demyelinating polyradiculoneuropathy (CIDP), except corticosteroids, intravenous immunoglobulin (IVIg) and plasma exchange. Quasi‐randomised trials are those trials in which treatment allocation was intended to be random but might have been biased (for example alternate allocation or allocation according to the day of the week).
Types of participants
We included results from participants of all ages who fulfilled criteria which approximated to the definition of probable CIDP by an American Academy of Neurology ad hoc committee (Ad Hoc 1991) or the Joint Task Force of the European Federation of Neurological Societies and the Peripheral Nerve Society (EFNS/PNS 2005), which were updated in 2010 (EFNS/PNS 2010). Briefly, these criteria require progressive weakness with an onset phase lasting more than eight weeks due to polyradiculoneuropathy, fulfilment of strict criteria for a demyelinating neuropathy, and the absence of significant central nervous system involvement or alternative causes. For the reasons described in the background, we included people with cranial nerve or upper limb onset, proximal and distal or distally predominant weakness, symmetrical or asymmetrical involvement, and sensory predominant deficit, but not multifocal motor neuropathy. We did not include people with cancer, connective tissue diseases, or immunoglobulin M (IgM) paraprotein with antibodies to myelin‐associated glycoprotein.
Types of interventions
We included all immunosuppressive agents such as azathioprine, cyclophosphamide, methotrexate, ciclosporin, mycophenolate mofetil, etanercept, rituximab and alemtuzumab, immunomodulatory agents such as interferon alfa (IFN alfa) and interferon beta (IFN beta), and autologous peripheral blood stem cell transplantation (PBSCT). We did not include corticosteroids, plasma exchange or intravenous immunoglobulin (IVIg). We also included studies of combinations of these interventions or of these interventions with corticosteroids, plasma exchange or IVIg where the objective of the study was to evaluate the effectiveness of cytotoxic drugs or interferons.
Types of outcome measures
Primary outcomes
Change in disability at 26 or more weeks after randomisation measured by a disability scale designed and validated for CIDP treatment trials such as the Inflammatory Neuropathy Cause and Treatment (INCAT) scale (Hughes 2001), the Overall Disability Status Score (ODSS) (Merkies 2002), Overall Neuropathy Limitations Scale (ONLS) (Graham 2006) (Appendix 1), or Rasch‐built Overall Disability Scale (R‐ODS) scale (van Nes 2011). Failing that, we would have accepted a validated generic disability scale such as the Modified Rankin Scale (van Swieten 1988) (Appendix 2).
We selected a disability outcome as the primary outcome on the grounds that a patient‐centred outcome is more relevant to the needs of people with CIDP and healthcare purchasers than a measure of impairment or neurophysiology. We originally chose one year as the period for measurement of outcome on the grounds that the risk, expense and inconvenience of cytotoxic agents and interferons would not be worthwhile unless improvements lasted for at least 12 months. However, none of the trials of immunomodulatory drugs in CIDP have lasted as long as one year so we have reported change in disability at 26 or more weeks as the primary outcome since the 2010 update of the review.
Secondary outcomes
Change in disability four or more weeks after randomisation, measured by the Modified Rankin Scale, ONLS or a similar disability scale.
Change in impairment at least one year after randomisation, measured by an impairment scale similar to the Mayo Neuropathy Impairment Scale (NIS) (Dyck 2005).
Change in maximum motor nerve conduction velocity (MNCV) and compound muscle action potential (CMAP) amplitude one year after randomisation. Where more than one nerve was studied, the average of all the nerves studied was to be used.
For participants who were receiving corticosteroids or IVIg, the amount of this medication given during at least one year after randomisation.
Participants with one or more serious adverse events during the first year after randomisation. A serious adverse event is one that is life‐threatening, requires or prolongs hospitalisation, is severely or permanently disabling, or is a new malignancy.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Neuromuscular Specialised Register (24 May 2016), the Cochrane Central Register of Controlled Trials (CENTRAL; 2016, Issue 4) in the Cochrane Library (searched 24 May 2016), MEDLINE (January 1966 to 24 May 2016), Embase (January 1980 to 24 May 2016), LILACS (January 1982 to 24 May 2016) and CINAHL Plus (January 1982 to 24 May 2016). We additionally searched MEDLINE and Embase for observational studies for inclusion in the discussion.
Electronic strategies
The detailed search strategies are in the appendices: Cochrane Neuromuscular Specialised Register (Appendix 3), CENTRAL (Appendix 4), MEDLINE (Appendix 5), Embase (Appendix 6), LILACS (Appendix 7), CINAHL (Appendix 8) and trial registries (Appendix 9). The additional searches for non‐randomised studies are in Appendix 10 for MEDLINE and Appendix 11 for Embase.
Searching other resources
We contacted the authors of the trials identified and other disease experts seeking other published and unpublished trials. We also searched ClinicalTrials.gov and www.who.int/ictrp/en/ (World Health Organization International Clinical Trials Registry Platform) for any ongoing trials. We applied no language limitations.
Data collection and analysis
Selection of studies
Two review authors (MM‐R and RACH) checked the titles and abstracts of the articles identified by the search and decided upon inclusion independently. If there had been disagreement, we would have reached agreement by discussion and adjudication by a third review author.
Data extraction and management
Two review authors (MM‐R and RACH) extracted data using a specially designed form. If there had been disagreement, we would have reached agreement by discussion and adjudication by a third author.
Assessment of risk of bias in included studies
Two review authors assessed the risk of bias of the included trials using the methods described in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If there had been disagreement, we would have reached agreement by discussion and adjudication by a third author.
Measures of treatment effect
Although there were two trials of IFNb‐1a, the efficacy outcomes were different. If there had been similar outcomes we would have calculated a treatment effect across trials using the Cochrane statistical package Review Manager 5 (RevMan) (RevMan 2014). We would have expressed results as risk ratios (RRs) with 95% confidence intervals (CIs) for dichotomous outcome measures and differences between means with 95% CIs for continuous outcomes. In the event that our preferred outcomes had not been available but others were available for more than one trial we would have used whatever outcomes were available to calculate a treatment effect across trials.
Unit of analysis issues
In the one cross‐over trial included we reported the results for both treatment periods as if they were separate groups of participants regardless of the order of treatment.
Dealing with missing data
If data were missing from the original reports we reported the omission and did not make any assumptions.
Assessment of heterogeneity
We would have tested the results for heterogeneity across trials if necessary.
Assessment of reporting biases
There were too few trials to warrant the use of a funnel plot to explore small study biases.
Data synthesis
We reported the results for each comparison separately.
We would have used a fixed‐effect model for data synthesis, with a sensitivity analysis using the random‐effects model if we had found heterogeneity.
'Summary of findings' tables
We included 'Summary of findings' tables to present the quality of the body of evidence for methotrexate and IFN beta‐1a for each available main outcome. We assessed the evidence using the five GRADE considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias). We chose the following outcomes: change in disability after 26 or more weeks, and participants able to reduce corticosteroid or IVIg dose by more than 20% for methotrexate; and participants who completed the study and did not restart IVIg therapy after IVIg withdrawal for IFN beta‐1a. We chose serious adverse events for both.
Subgroup analysis and investigation of heterogeneity
If the data had allowed we would also have tested the effect of interventions in the subgroups mentioned in Types of participants.
Sensitivity analysis
If heterogeneity had been found, we would have undertaken sensitivity analyses by repeating the calculation after omitting the trials which we judged at a high risk of bias on individual risk of bias items.
Results
Description of studies
Results of the search
The searches for updates on 21 October 2014 and 24 May 2016 found 21 papers in the Cochrane Neuromuscular Specialised Register, 23 papers in CENTRAL, 127 papers in MEDLINE, 40 in Embase, 53 in CINAHL, 0 in LILACS, but no new trials. Previous searches had identified four trials (Dyck 1985; Hadden 1999; RMC 2009; Hughes 2010). See Figure 1 for a flow chart of the study selection process. The searches for randomised trials also identified nearly all the non‐randomised studies included in the Discussion. Additional searches for non‐randomised studies on 19 July 2016 found 94 references in MEDLINE and 52 in Embase, total 146. This total reduced to 128 after deduplication and removal of references already attached to the review. Only two references were suitable for inclusion in the Discussion (Ryan 2000; Ware 2014).
1.
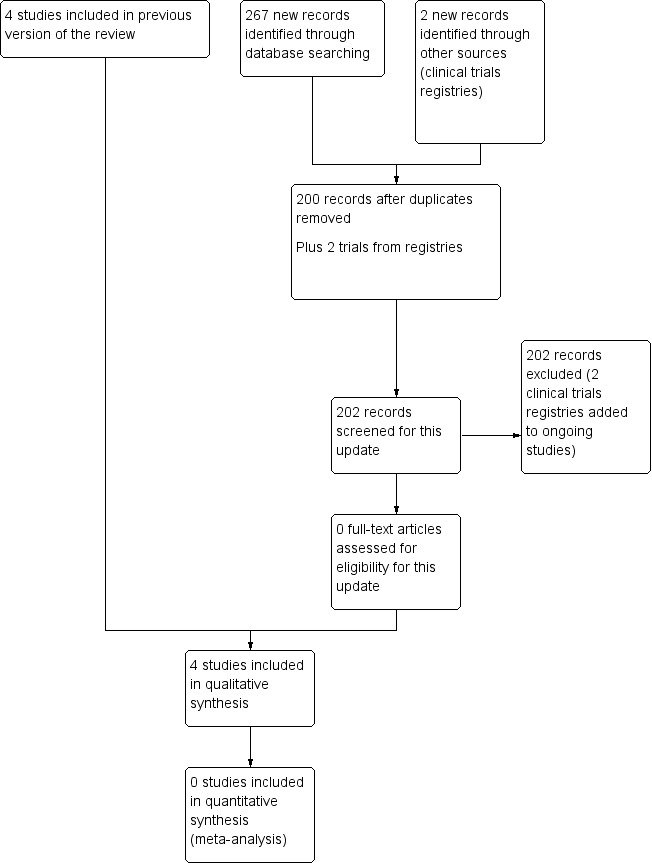
Study flow diagram.
Three randomised trials (of fingolimod, FORCIDP trial; cyclosporin, Japanese cyclosporin trial; and mycophenolate, MYCOPID trial) and one open non‐randomised study (Haematopoietic stem cell transplantation trial) are ongoing (12 July 2016) (see Characteristics of ongoing studies). One study awaits classification (Hu 2009) (see Characteristics of studies awaiting classification). No further details of this study are available in 2016.
We have included relevant observational studies in the Discussion.
Included studies
We included four trials, one of azathioprine, one of methotrexate, and two of the immunomodulatory drug interferon beta (IFN beta). We will describe these in turn, but more details are available in Characteristics of included studies.
Dyck 1985 compared 14 people with CIDP randomised to azathioprine 2 mg/kg and prednisone with 13 people with CIDP randomised to prednisone alone. The trial had a parallel‐group design and treatment lasted nine months. The observers and participants were not blinded to the intervention. There were multiple explicit outcome criteria but none were stipulated as primary. Follow‐up data were not available for one azathioprine and prednisone participant and three prednisone‐alone participants. There were no significant differences after four or nine months in any of 16 variables, including the Neuropathy Impairment Scale (NIS), other measures of impairment, motor nerve conduction parameters, and cerebrospinal fluid protein concentration.
Hadden 1999 compared interferon beta‐1a (IFN beta‐1a) with placebo in a trial with a cross‐over design. Ten adults with treatment‐resistant CIDP were randomised to receive IFNb‐1a (Rebif, Serono) 11 μg subcutaneously thrice weekly for two weeks and then 22 μg thrice weekly for 10 weeks, or a similar‐appearing placebo. After a four‐week washout period participants started on the opposite treatment to that which they received during the first treatment period. The primary outcome measure was a clinically significant improvement in at least three of the following measurements:
five disability scales:
one grade in the Ambulation Index;
one grade in the upper limb subscale of the Guy's Neurological Disability Scale;
> 4 units in the expanded Medical Research Council (MRC) sum score;
at least 2 points on the Hammersmith Motor Ability Scale;
at least 10 points on the Functional Independence Measure;
two impairment scales:
> 20% from baseline on the time taken to remove and replace the pegs from a board in the nine‐hole peg test;
> 20% from baseline value (and > 2 seconds) in the time taken to walk 10 metres;
and one quality of life scale:
at least 33 points on the EuroQoL visual analogue scale.
If the participant worsened on any of these measures, then improvement had to be observed on two more measures than the number that had worsened. Only one participant fulfilled the criteria proposed for the primary outcome measure in the IFNb‐1a treatment period and two in the placebo treatment period. The secondary outcome measures were the changes in the same measurements and in parameters of motor nerve conduction in the median nerves at 12 weeks. The only statistically significant difference was a trivial one‐point improvement in the median of the Functional Independence Measure in the placebo treatment period compared with no change in the IFNb‐1a treatment period.
Hughes 2010 compared the effect of intramuscular IFNb‐1a with placebo in a double‐blind, placebo‐controlled, parallel‐group, dose‐ranging study. Sixty‐seven intravenous immunoglobulin (IVIg)‐dependent participants were randomised: 45 to different doses of intramuscular IFNb‐1a and 22 to placebo. Participants received two intramuscular injections of IFNb‐1a or placebo weekly for 32 weeks. There were five treatment groups: IFNb‐1a 30 μg weekly and placebo, IFNb‐1a 60 μg weekly and placebo, IFNb‐1a 30 μg twice weekly, IFNb‐1a 60 μg twice weekly, and placebo twice weekly. At the end of week 16 of the trial, IVIg was stopped. IVIg was restarted if the participant had a clinical relapse, which was defined as a two‐point deterioration on the Medical Research Council (MRC) sum score and one functional disability grade on Overall Disability Sum Score, or study termination at 32 weeks. The primary outcome was the total IVIg dose (g/kg) administered in the 16 weeks after the 16‐week visit.
RMC 2009 was a randomised, double‐blind, parallel‐group, placebo‐controlled trial of methotrexate in people with CIDP who had previously responded to and were still receiving corticosteroids, IVIg, or both. Sixty participants were randomised to receive oral methotrexate or placebo (starting at 7.5 mg weekly, increasing to 10 mg weekly after four weeks, and 15 mg weekly after eight weeks) both with folic acid (5 mg twice weekly for 40 weeks). After 16 weeks (15 weeks if on three or five times weekly IVIg and 14 weeks if on seven times weekly IVIg), corticosteroids or IVIg were reduced, subject to satisfactory progress, at a rate of 20% of the baseline dose every four weeks. The primary outcome was the percentage change in mean weekly dose of corticosteroids or IVIg during the last four weeks, usually from week 37 to week 40, compared with the mean during weeks one to four. Secondary disability outcomes were changes in disability measured in two ways and at two times: the Amsterdam Linear Disability Score (ALDS), ranging from 0, which is death, to 100, indicating fully able (Holman 2004), and the Overall Neuropathy Limitations Scale (ONLS) (Graham 2006), both measured from baseline to week 16 and to week 40. The secondary impairment measures were the changes in the expanded (to include the first dorsal interosseous and extensor hallucis longus muscles) MRC sum score from baseline to week 16 and to week 40.
Risk of bias in included studies
See Figure 2 for a summary of the review authors' 'Risk of bias' assessments of included studies. Three trials were at low risk of bias (Hadden 1999; RMC 2009; Hughes 2010). The fourth trial was at high risk of bias because of lack of allocation concealment, lack of blinding and unexplained dropouts (Dyck 1985).
2.
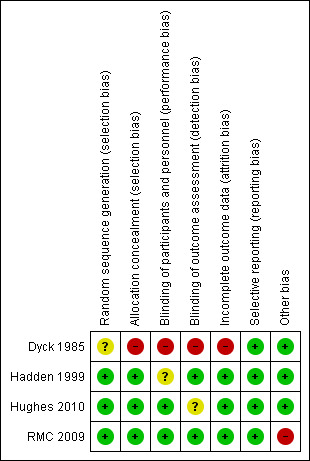
'Risk of bias' summary: review authors' judgements about each 'Risk of bias' item for each included study. Red (‐) = high risk of bias; yellow (?) = unclear risk of bias; green (+) = low risk of bias.
Effects of interventions
Summary of findings for the main comparison. Methotrexate compared to placebo for CIDP.
| Methotrexate compared to placebo for CIDP | ||||||
| Patient or population: people with CIDP Settings: hospital Intervention: methotrexate Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Methotrexate | |||||
| Participants able to reduce corticosteroid or IVIg dose by more than 20% Follow‐up: mean 40 weeks. | 44 per 100 | 53 per 100 (18 to 100) | RR 1.21 (0.4 to 3.7) | 59 (1 study) | ⊕⊕⊕⊝ moderate1,3 | This was the primary outcome in the trial although not specified as a primary outcome in the review. |
| Change in disability after 26 or more weeks Overall Neuropathy Limitation Scale. Scale from: 0 to 12. Follow‐up: median 40 weeks. | The median change in disability after 26 or more weeks in the control groups was 0 points | The median change in disability after 26 or more weeks in the intervention groups was 0 points | 59 (1 study) | ⊕⊕⊝⊝ low2 | No significant difference. | |
| Change in disability after 26 or more weeks Amsterdam Linear Disability Scale. Scale from: 0 to 100. Follow‐up: mean 40 weeks. | The mean change in disability after 26 or more weeks in the control groups was −0.48 | The mean change in disability after 26 or more weeks in the intervention groups was 0.47 lower (3.62 lower to 1.87 higher) | 59 (1 study) | ⊕⊕⊝⊝ low2 | No significant difference. | |
| Serious adverse events | 3 per 100 | 11 per 100 (1 to 100) | RR 3.56 (0.39 to 32.23) | 59 (1 study) | ⊕⊕⊕⊝ moderate3 | No significant difference. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; CIDP: chronic inflammatory demyelinating polyradiculoneuropathy | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 The high rate of responders in the placebo group reduced the power of the trial to detect an effect of methotrexate. 2 Participants in the two groups received different dosages of IVIg or corticosteroids which risked confounding this comparison. 3 Wide 95% CI.
Summary of findings 2. Interferon beta‐1a compared to placebo for CIDP.
| Interferon beta‐1a compared to placebo for CIDP | ||||||
| Patient or population: people with CIDP Settings: hospital Intervention: interferon beta‐1a Comparison: placebo | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Placebo | Interferon beta‐1a | |||||
| Change in disability after 26 or more weeks | ‐ | ‐ | ‐ | ‐ | ‐ | Not measured |
| Participants who completed the study and did not restart IVIg therapy after IVIg withdrawal Follow‐up: median 32 weeks. | 47 per 100 | 47 per 100 (25 to 89) | RR 1 (0.54 to 1.88) | 49 (1 study) | ⊕⊕⊕⊝ moderate1 | This was the primary outcome in the trial although not specified as a primary outcome in the review. |
| Serious adverse events | See comment2 | See comment | RR 4.5 (0.25 to 80.05) | 67 (1 study) | ⊕⊕⊕⊝ low3 | 0 of 22 in the control and 4 of 45 with IFN beta‐1a had serious adverse events. |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio; CIDP: chronic inflammatory demyelinating polyradiculoneuropathy; IVIg: intravenous immunoglobulin; IFN: interferon | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 There was a high rate of responders in the placebo group which reduced the power to detect an effect of IFN beta‐1a. 2 There were no serious adverse events in the control group but RevMan software automatically makes a correction to allow calculation of a RR. 3 Wide 95% CI.
Azathioprine and prednisone versus prednisone alone
Dyck 1985 (N = 27) studied this comparison but did not provide data for any of the outcomes specified for this review, since there was no measure of disability and the trial only lasted nine months. However, two trial outcomes approximated to two of the review authors' specified secondary outcomes.
Secondary outcomes
Change in impairment at least one year after randomisation measured by an impairment scale similar to the NIS
After nine months there was a median improvement of 29 points (range 49 points worse to 84 points better) in the 13 evaluable participants who received azathioprine and prednisone compared with a worsening of 30 points (range 20 points worse to 104 points better) in the 10 who received prednisone alone.
Change in maximum MNCV and CMAP amplitude one year after randomisation
After nine months the median maximum motor nerve conduction velocity (MNCV) and compound muscle action potential (CMAP) amplitude of the ulnar, median and peroneal nerves showed small changes after nine months in both groups of participants. The changes were not clinically significant. The mean changes of all the nerves tested could not be computed from the published data.
As stated above, there were no significant differences after four or nine months in any of the other measures of impairment reported by the trial authors (See Characteristics of included studies). Adverse events were not reported.
Methotrexate versus placebo
RMC 2009 (N = 60, 59 with follow‐up data) studied this comparison and provided data on only the following review outcomes.
Primary outcome
Change in disability 26 or more weeks after randomisation measured by the ONLS or a similar disability scale
All analyses were adjusted for age, baseline score and baseline corticosteroid or IVIg dose. At the end of the trial (approximately 40 weeks) there was no significant change in activity limitation (disability) measured with the ONLS (scale 0 to 12, higher scores indicating more disability) or ALDS (scale 0 to 100 where 100 is fully able). The median change of ONLS was 0 (interquartile range −1 to 0) in the methotrexate group and 0 (interquartile range −0.75 to 0) in the placebo group. The mean change from baseline ALDS score of the methotrexate group was −0.66 (standard deviation (SD) 4.25) and placebo −0.48 (SD 2.40). The mean change from the baseline ALDS score of the methotrexate group was −0.47 (95% confidence interval (CI) −3.62 to 1.87) points less than that of the placebo group, adjusting for ALDS score at baseline, baseline IVIg or corticosteroid dose per week per kg and age. These changes in disability might have been confounded by the reduction in the corticosteroid or IVIg doses required by the protocol.
Secondary outcomes
Change in disability four or more weeks after randomisation measured by the ONLS or a similar disability scale
The ONLS and ALDS were also measured at the mid‐trial visit (approximately 16 weeks). There was no significant change in limitations (disability) measured with the ONLS, the median change being 0 (interquartile range −1 to 0) in both the methotrexate and placebo groups. The mean change from baseline ALDS score of the methotrexate group was 1.51 (SD 2.71) and placebo −0.42 (SD 2.51). The mean improvement from the baseline ALDS score of the methotrexate group was 1.79 (95% CI 0.12 to 3.05) points more than in the placebo group adjusting for ALDS score at baseline, baseline IVIg or corticosteroid dose per week per kg and age. Since this scale ranges from 0 which is death to 100 indicating fully able, such a small change is unlikely to be clinically significant.
Participants with one or more serious adverse events during the first year after randomisation
There were three participants in the methotrexate group and one in the placebo with one or more serious adverse events, risk ratio (RR) 3.56 (95% CI 0.39 to 32.23) (Analysis 1.1).
1.1. Analysis.
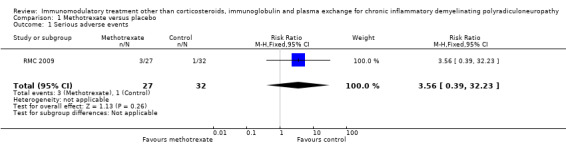
Comparison 1 Methotrexate versus placebo, Outcome 1 Serious adverse events.
More results are given in Table 1 and Discussion.
Interferon beta‐1a versus placebo
Hadden 1999 (N = 10), a cross‐over trial with treatment periods of only 12 weeks, and Hughes 2010 (N = 67) studied this comparison. One participant deteriorated after four weeks during the first treatment period (IFNb) and was immediately switched to the alternative treatment (placebo). One participant showed clinically significant improvement during the IFNb treatment and two during placebo. The only trial outcomes also stipulated for this review were as follows.
Secondary outcomes
Change in disability four or more weeks after randomisation measured by the Modified Rankin Scale or a similar disability scale
One trial, Hadden 1999, contributed data for this outcome.
The median improvement in the combined upper and lower limb components of the Guy's Neurological Disability Scale (range worst (48) to best (0)) was 0.5 grades (interquartile range 1.8 grades better to zero grade change) in the IFNb‐1a treatment period and 0.5 grades (interquartile range 1.8 grades better to 1.0 grade worse) in the placebo treatment period.
Participants with one or more serious adverse events during the first year after randomisation
There were no serious adverse events during either treatment period in Hadden 1999.
In Hughes 2010, four of the 45 participants in the IFNb‐1a group and none of 22 participants in the placebo group had one or more serious adverse events, RR 4.50 (95% CI 0.25 to 80.05) (Analysis 2.1).
2.1. Analysis.
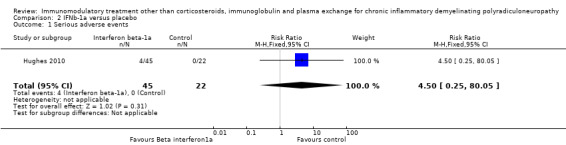
Comparison 2 IFNb‐1a versus placebo, Outcome 1 Serious adverse events.
More results are given in Table 2 and the Discussion.
Discussion
Summary of main results
This review only found four small trials of immunomodulatory agents in CIDP. One trial of azathioprine, two of IFN beta‐1a and one of methotrexate showed no significant benefit. However the quality of the evidence was low or moderate and none of the trials was large enough to rule out small or moderate benefit.
In this discussion we also review the observational studies concerning each of these drugs in turn and also non‐randomised studies of other immunomodulatory drugs.
Azathioprine
Azathioprine is a broad‐spectrum immunosuppressive agent and is probably the one most commonly used in chronic inflammatory demyelinating polyradiculoneuropathy (CIDP). The trial by Dyck and colleagues described above is the only controlled trial and did not detect a significant effect on any of the 16 outcomes tested by the authors or on the outcomes that we had selected for this review (Dyck 1985). However, the trial was small and lacked power to detect or exclude any but very large treatment effects. Furthermore, it only tested a dose of 2 mg/kg of azathioprine whereas a dose of 2.5 mg/kg or sometimes 3.0 mg/kg has been used in other conditions such as multiple sclerosis and Crohn's disease (BDMSATG 1988). The trial also only continued treatment for nine months, whereas in a similar trial in myasthenia gravis a treatment effect did not become evident until after 12 months (Palace 1998). Consequently, it would be premature to draw conclusions about the efficacy of azathioprine from this trial alone (Dyck 1985).
Dyck 1985 did not mention the occurrence of side effects from azathioprine, but it may cause nausea, vomiting, diarrhoea and allergic reactions including rash, which prevent its continuation in about 10% of participants. It also causes leucopenia, altered liver function, increased susceptibility to infection, and a theoretical risk of neoplasia (Confavreux 1996; Kissel 1986). A large retrospective cohort study of immunosuppressive agents in autoimmune ocular disease did not show an increased incidence of neoplasia in people with CIDP treated with antimetabolites such as azathioprine, methotrexate and mycophenolate mofetil after 17,316 person years (Kempen 2009).
Azathioprine is less expensive than most immunosuppressive drugs. The annual cost of giving 150 mg daily for a year is about GBP 101 (EUR 125) (BNF 2014). To this cost must be added the costs of monitoring haematological and liver function, which is required for as long as treatment is continued, and the costs of side effects caused by the drug. However, if the drug is effective it might result in savings because of reduced healthcare costs arising from the disability that CIDP causes, from the reduced usage of intravenous immunoglobulin (IVIg), which is one of the preferred treatments for CIDP (Eftimov 2013), and from the frequent potentially disabling and expensive side effects that can be associated with the use of corticosteroids.
Uncontrolled observations from case reports and case series in the literature provide little information about the value of azathioprine in CIDP. In 1981, Dalakas and colleagues described azathioprine taken as a 3 mg/kg single daily dose as their immunosuppressive drug of choice (see Table Summary of observational studies of azathioprine) (Dalakas 1981). They considered that a clinical effect could be detected in between one and 12 weeks. They reported improvement in three of four people with steroid‐resistant CIDP to almost 90% to 95% of normal. They also considered that azathioprine has a steroid‐sparing action in those who responded to steroids. Seven of a series of 92 people with CIDP were treated with azathioprine and four improved by at least one point on a six‐point disability scale (McCombe 1987). In a series of 59 treated people, Barohn 1989 reported that 56 (95%) responded to immunosuppressive treatment, which started with prednisone and then included azathioprine in the event of a relapse or poor response; the number who received azathioprine was not stated. Simmons 1995 followed up 69 people with CIDP, of whom 50 were being treated and eight were receiving azathioprine: the authors did not state how effective they thought azathioprine to be. Ryan and colleagues reported no benefit in two people with CIDP, one of whom experienced deranged liver function on both occasions they received azathioprine (Ryan 2000). Monaco and colleagues state their own experience that low doses of azathioprine (1 mg/kg) and prednisolone (0.25 to 0.5 mg/kg) prevent relapses, in particular in people with CIDP who have responded poorly to IVIg, but do not give figures to support this impression (Monaco 2004). In a disease with a relapsing–remitting course and a drug that has a slow onset of action, conclusions are particularly difficult to draw from anecdotal observations. A retrospective Italian multicentre study of 158 people with CIDP treated with immunosuppressive drugs included 77 people treated with azathioprine, of whom 21 were reported to improve (Cocito 2011).
Summary of observational studies of azathioprine
| Series | Dose | Duration | Total | Improved | Notes |
| Dalakas 1981 | 3 mg/kg daily | Not stated | 4 | 3 | Corticosteroid‐resistant patients |
| McCombe 1987 | Not stated | Not stated | 7 | 4 | |
| Ryan 2000 | Not stated in 1 3 mg/kg in 1 |
19 days in 1 8 months twice in 1 |
2 | 0 | Deranged liver function twice in 1 |
| Cocito 2011 | 100 mg to 200 mg daily | ≥ 12 months | 77 | 21 | Italian retrospective study |
| All studies | 90 | 28 |
Cyclophosphamide
Cyclophosphamide is an alkylating agent that can be given orally or by intravenous injection. Four people with CIDP who were worsening despite treatment with corticosteroids had sustained improvement when given oral cyclophosphamide 50 to 150 mg daily for two to nine months (Prineas 1976). Dalakas and colleagues reported that oral cyclophosphamide 2 mg/kg was beneficial in the one person with CIDP in whom they tried it (Dalakas 1981). McCombe and colleagues described benefit in four of five people with CIDP but did not mention the dose or route (McCombe 1987). Bouchard and colleagues described the treatment of 36 people with CIDP using in turn, depending on response, corticosteroids, IVIg, plasma exchange and finally oral cyclophosphamide 2 mg/kg daily for six to 12 months (Bouchard 1999). Ryan and colleagues treated one child with 4 mg/kg for 9 months with benefit evident within one month and sustained after stopping the drug nine months later (Ryan 2000). Three people with CIDP who had not responded to previous agents also failed to respond to this cyclophosphamide regimen. Brannagan and colleagues gave high‐dose cyclophosphamide to four people with CIDP that had responded inadequately to other treatments (Brannagan 2002). The dose was 200 mg/kg over four days accompanied by a forced diuresis and the drug mesna to prevent haemorrhagic cystitis. All four people improved in functional status and had their other treatment stopped. Complications included alopecia in four, infections, transient amenorrhoea, transient renal failure, heart failure, mucositis and diarrhoea. In a follow‐up study of the same four and one additional person after a median 2.9 years, four of five showed improvements in the Modified Rankin score, four of five improved more than two points on the Medical Research Council (MRC) sum score, and three of five had increases in summed compound muscle action potential (CMAP) amplitudes of more than 1.0 mV (Gladstone 2005). Jasmin 2012 reported the successful treatment of one person with oral cyclophosphamide and corticosteroids, but this person has not been included in the summary table because he also had systemic lupus erythematosus. The largest series is that of Good 1998, who treated 15 people with CIDP with intravenous pulses of 1 g/m² monthly for a maximum of six months with careful precautions to avoid dehydration and premedication to reduce nausea. Twelve people showed marked improvement, 11 improving to normal. Three did not improve, of whom one worsened. Six had minor side effects and two alopecia. None developed haematuria, prolonged bone marrow depression or neoplasia, all of which are feared side effects of high‐dose cyclophosphamide. Additional side effects are increased susceptibility to infection and ovarian failure. The results in these case series strongly suggest but do not prove treatment benefit. The risk of serious side effects puts many people with CIDP and their neurologists off using this drug.
Summary of observational studies of cyclophosphamide
| Series | Dose | Duration | No. of people with CIDP | No. improved | Notes |
| Prineas 1976 | 50 mg to 150 mg daily | 2 to 9 months | 4 | 4 | |
| Dalakas 1981 | 2 mg/kg daily | Not stated | 1 | 1 | |
| McCombe 1987 | Not stated | Not stated | 5 | 4 | |
| Ryan 2000 | 4 mg/kg | 9 months | 1 | 1 | |
| Bouchard 1999 | 2 mg/kg daily | 6 to 12 months | 3 | 0 | Refractory to other treatments |
| Brannagan 2002 | 200 mg/kg daily | 4 days | 4 | 4 | |
| Gladstone 2005 | Not stated | Median 2.9 years | 5 | 4 | Follow‐up of Brannagan 2002: included the 4 people in Brannagan and one additional person |
| Good 1998 | 1 g/m² monthly | Maximum 6 months | 15 | 12 | 11 improving to normal |
| Cocito 2011 | 1 g/m² iv monthly, or 2 mg/kg oral daily | ≥ 12 months | 13 | 5 | Italian retrospective study |
| All studies | 51 | 35 |
Ciclosporin
Another drug which has been used quite frequently in CIDP is ciclosporin, which particularly inhibits the proliferation of T cells. One person with CIDP improved on ciclosporin but developed irreversible renal failure as a side effect (Kolkin 1987). Another improved when treated with the combination of plasma exchange and ciclosporin, but treatment had to be discontinued because of a rise in serum creatinine (Hefter 1990). The largest series comes from Sydney (Barnett 1998; Hodgkinson 1990), where 19 people with CIDP were treated but five had a paraprotein which excluded them from consideration in this review. At the beginning of their series they used a high dose, 10 mg/kg daily, reduced to 8 mg/kg daily after one month and 5 mg/kg daily after three months, but later they reduced the starting dose to 3 to 7 mg/kg and the maintenance dose to 2 mg/kg to 3 mg/kg. All 14 without a paraprotein improved either with a reduction in disability by at least one grade or by a reduction in the annual relapse rate. Of the 19 people treated, 11 had side effects, nephrotoxicity in four, hypertension in four, nausea in three, oedema in three and hirsutism in four. The side effects were less with lower doses. In a North American series, three of eight people with CIDP improved (Mahattanakul 1996). In a Japanese series, all of seven people with CIDP inadequately controlled by other agents including corticosteroids and IVIg improved following treatment with oral ciclosporin 5 mg/kg daily to keep the trough plasma concentration between 100 and 150 ng/ml. All seven people had improvements in disability measured with the Modified Rankin scale and increases in grip strength within three months. None had side effects attributed to ciclosporin (Matsuda 2004). One child treated with 5 mg/kg daily for 3 months did not improve (Ryan 2000). Five people were treated with ciclosporin 3 mg/kg daily adjusted to keep the trough plasma concentration between 100 and 150 ng/ml: four improved, including one who had failed to respond to intravenous cyclophosphamide (Odaka 2005). Three of 12 people treated with ciclosporin improved in a retrospective study (Cocito 2011). In a report of two people treated with oral ciclosporin, one had a significant improvement in grip strength and went into remission and the other showed an improvement in the modified Rankin score and muscle strength. The authors made the point that in addition to monitoring trough levels, it was important to measure the area under the concentration time curve (AUC) from 0 to 4 hours. They aimed for a trough level of 150 ng/ml and an AUC of 2500 ng/ml per hour (Takeuchi 2012). These series suggest but do not prove that ciclosporin is also beneficial in CIDP; there is no doubt that it causes potentially serious side effects, especially renal failure.
Summary of observational studies of ciclosporin
| Series | Dose | Duration | No. of people with CIDP | No. improved | Notes |
| Hefter 1990 | Not stated | Not stated | 1 | 1 | Combination of ciclosporin and plasma exchange |
| Kolkin 1987 | Not stated | Not stated | 1 | 1 | |
| Barnett 1998; Hodgkinson 1990 | 10 mg/kg to 8 mg/kg daily after 1 month and 5 mg/kg daily after 3 months but later 3 mg/kg to 7 mg/kg and then maintained at 2 mg/kg to 3 mg/kg | Not stated | 19 | 14 | 11 had side effects |
| Mahattanakul 1996 | 3 mg/kg to 5 mg/kg daily | Not stated | 8 | 3 | |
| Matsuda 2004 | 5 mg/kg daily | Not stated | 7 | 7 | Trough plasma concentration maintained at 100 to 150 ng/ml |
| Odaka 2005 | 3 mg/kg daily | Not stated | 5 | 4 | Trough plasma concentration maintained at 100 to 150 ng/ml |
| Ryan 2000 | 5 mg/kg daily | 3 months | 1 | 0 | |
| Cocito 2011 | 100 mg to 300 mg daily | ≥ 12 months | 12 | 3 | Italian multicentre retrospective study |
| Takeuchi 2012 | 150 mg to 250 mg daily in combination with corticosteroids and IVIg | 2 | 2 | Trough plasma concentration of 150 ng/ml and AUC of 2500 ng/ml per hour for first 4 hours | |
| All studies | 56 | 35 |
Fingolimod
Fingolimod, an oral immunosuppressant drug approved for use in multiple sclerosis, has been tested in a randomised trial in CIDP but the trial was stopped prematurely after an interim analysis showed that continuing the trial would be futile (FORCIDP trial). There is a report of benefit from the drug in a person who had both CIDP and central nervous system demyelination (Erdener 2014).
Fludarabine
Fludarabine is a nucleoside analogue used in the treatment of B cell malignancies. Leitch 2015 reported improvement in three people with CIDP resistant to first‐line treatments after being given a combination of cyclophosphamide and fludarabine.
Tacrolimus
Tacrolimus (FK506), which is related to ciclosporin, has only been used in a single published report of one person with CIDP, in whom it appeared beneficial (Ahlmén 1998).
Sirolimus
We have not found any reports of the use of sirolimus (rapamycin) in CIDP.
Methotrexate
Methotrexate is one of the favoured disease‐modifying agents in treatment of rheumatoid arthritis, but until recently there were no reports of its use in CIDP. It is a folate‐inhibiting drug, which is well tolerated for long periods and is reasonably safe when used in low, weekly oral doses of about 15 mg/kg to 20 mg/kg. One child improved following treatment with oral methotrexate 10 mg weekly (Ryan 2000). A consecutive series of 10 people with inadequate responses to other agents was treated with oral methotrexate 10 mg to 15 mg weekly for at least 32 weeks. Five people reported symptomatic benefit and seven had increased MRC sum scores indicating increased strength. However, none showed improvements in the Overall Disability Status Score (ODSS), indicating that improvements were modest (Fialho 2006). The results of this study, the excellent tolerability of methotrexate, and its efficacy in rheumatoid arthritis motivated the RMC trial (RMC 2009).
The RMC trial was a double‐blind, randomised, parallel‐group trial of methotrexate versus placebo (RMC 2009). Some of the results have been reported in the Description of studies above. In the trial authors' own analysis of its primary outcome, the results were dichotomised into non‐responders (reduction of corticosteroid or IVIg dose by 20% or less) and responders (reduction of corticosteroid or IVIg dose by more than 20%). There were 14 responders out of 27 (52%) in the methotrexate group and 14 of 32 (44%) in the placebo group; the RR of being a responder in the methotrexate group was 1.18 (95% CI 0.69 to 2.02) more than in the placebo group, which is not statistically significant. The average change from baseline in the expanded MRC sum score (graded out of 80) for people in the methotrexate group at the mid‐trial visit was an improvement of 2.05 (95% CI −0.21 to 4.32) points more in the methotrexate group than the placebo group. Although the CI included the possibility of no effect, problems inherent in the trial mean that we cannot exclude mild or moderate benefit. These problems included especially the high proportion of responders in the placebo group, the complexity of the trial design, and the subjective component to the decision about dose adjustment on which the primary outcome depended.
In the Italian retrospective study, only two of 12 people with CIDP treated with 7.5 mg to 15 mg of methotrexate weekly responded (Cocito 2011). There has been one case report of the use of a higher dose (20 mg weekly) of methotrexate in a person with CIDP refractory to conventional treatment. In this report, there was a 14‐point improvement in MRC sum score and three times weekly IVIg was stopped after eight months on methotrexate 20 mg weekly. The improvement was sustained and prednisolone dose was reduced from 50 mg on alternate days to 10 mg on alternate days (Díaz‐Manera 2009).
Mycophenolate mofetil
Mycophenolate mofetil is becoming popular as an alternative to azathioprine and ciclosporin in prevention of rejection of renal transplants and has been used in eight published series of people with CIDP. Chaudhry and colleagues treated three people with CIDP with 1000 mg twice daily and one improved (Chaudhry 2001). Mowzoon and colleagues reported two people, both of whom improved (Mowzoon 2001). According to an abstract report, three of six people with CIDP improved with mycophenolate (Radziwill 2006). In two people with CIDP, it was possible to reduce the amount of IVIg being used by 50% without any deterioration in their condition (Benedetti 2004). By contrast there was no improvement in any of four people in another consecutive series (Umapathi 2002). Gorson and colleagues treated 12 people with CIDP and in the group as a whole there was no significant improvement in average impairment or disability compared with baseline. However, three people did improve significantly (Gorson 2004). In another series, eight people with CIDP received mycophenolate mofetil (mean dose 2 g/day; median duration 15.2 months): all eight improved and their average (SD) Neuropathy Impairment Score (NIS) improved from a baseline of 72.3 (35) to 37.8 (37) (P < 0.001) after treatment. Six of these eight people either stopped corticosteroids or IVIg or reduced their doses and frequency by 50% or more (Bedi 2010). Three of 12 people in the Cocito 2011 series improved with mycophenolate. There is an on‐going RCT of mycophenolate in CIDP. Its aim is to assess the effectiveness of mycophenolate by determining the proportion of people with CIDP on placebo or mycophenolate who relapse during the tapering of IVIg and after IVIg is withdrawn (MYCOPID trial).
Summary of observational studies of mycophenolate
| Series | Dose | Duration | No. of people with CIDP | No. improved | Notes |
| Chaudhry 2001 | 1000 mg twice daily | Not stated | 3 | 1 | |
| Mowzoon 2001 | Not stated | Not stated | 2 | 2 | |
| Radziwill 2006 | Not stated | Not stated | 6 | 3 | |
| Benedetti 2004 | Not stated | Not stated | 2 | 2 | It was possible to reduce the amount of IVIg being used by 50% without any deterioration in condition |
| Umapathi 2002 | Not stated | Not stated | 4 | 0 | |
| Gorson 2004 | Not stated | Not stated | 12 | 3 | In the group as a whole there was no significant improvement in average impairment or disability compared with baseline |
| Bedi 2010 | 2 g daily | Median 15.2 months | 8 | 8 | All 8 people had improved impairment scores and 6 either stopped corticosteroids or IVIg or reduced their doses and frequency by ≥ 50% |
| Cocito 2011 | 1 to 2 g daily | ≥ 12 months | 12 | 3 | Italian retrospective study |
| All studies | 49 | 22 |
Etanercept
The tumour necrosis factor‐α antagonist etanercept is beneficial in rheumatoid arthritis. Three of 10 people with treatment‐resistant CIDP were considered to gain significant benefit from it (Chin 2003). However, there are also reports of Guillain‐Barré syndrome, multifocal motor neuropathy and CIDP developing in people being treated with tumour necrosis factor inhibitors for other conditions (Hamon 2007; Lozeron 2009; Alshekhlee 2010; Ahmed 2011).
Rituximab
Rituximab, a chimeric (mouse/human) monoclonal antibody against CD20+ B lymphocytes, beneficial in lymphoma and rheumatoid arthritis, has been used in small series of people with paraproteinaemic demyelinating neuropathy with modest benefit in some. Two randomised controlled trials of its use in IgM anti‐myelin‐associated glycoprotein demyelinating neuropathy showed benefit in some participants (Dalakas 2009; Léger 2013; Lunn 2016). In CIDP, it has been reported as beneficial in 17 of 22 people with CIDP in published case reports and small series (see Table of Summary of observational studies of rituximab). An email survey of international experts reported benefit from rituximab in 12 of 20 people with CIDP (Lunn 2009). An Italian retrospective survey reported improvement in six of 18 people with CIDP treated with rituximab (Cocito 2011). The reports of Lunn 2009 and Cocito 2011 have been omitted from our Summary of observational studies, since there may have been overlap with case reports and series already included in the table. The people in whom rituximab was reported to be beneficial often had other autoimmune disease or haematological disease. The table does not include people who had simultaneous treatment with other agents, such as one with associated non‐Hodgkin lymphoma co‐treated with cyclophosphamide, doxorubicin and prednisone and then etoposide (Kasamon 2002), those with IgM paraproteinaemia, such as four reported by (Kilidireas 2006), or a participant who also had systemic lupus erythematosus (Sanz 2012).
Summary of observational studies of rituximab
| Series | Dose | Duration | No. of participants | No. improved | Notes |
| Bodley‐Scott 2005 | 700 mg every 3 weeks | 7 courses | 1 | 1 | Self‐report |
| Briani 2004; Benedetti 2008; Benedetti 2011 | 375 mg/m² weekly | 4 weeks | 10 | 6 | 3 people with IgM paraprotein in these series were excluded |
| D'Amico 2012 | 375 mg /m² weekly | not stated | 1 | 1 | |
| Gorson 2007 | 375 mg/m² weekly | 4 weeks | 2 | 1 | |
| Knecht 2004 | 375 mg/m² weekly | 7 months | 1 | 1 | With associated Evans syndrome |
| Münch 2007 | 375 mg/m² weekly | 4 weeks | 1 | 1 | With type 2 diabetes |
| Rose 2010 | 900 mg weekly | 4 weeks | 1 | 1 | |
| Sadnicka 2011 | 1 g every 2 weeks | 2 doses | 1 | 1 | With Morvan's syndrome and myasthenia gravis |
| Velardo 2014 | 1 g every 2 weeks | 2 doses | 2 | 2 | Both of those treated had been refractory to other immunomodulatory treatment including cyclophosphamide |
| Ware 2014 | not stated | Not stated | 2 | 2 | children: marked improvement in strength in 2 months |
| Total | 22 | 17 |
Interferons
Interferon beta
Interferon beta (IFN beta) is a naturally occurring cytokine, which downregulates inflammatory responses and has been shown to reduce relapse frequency and blood‐brain barrier leakage in multiple sclerosis. IFN beta‐1a is a recombinant protein manufactured in mammalian cells that exactly replicates human IFNb. An apparently beneficial effect was reported in one person with treatment‐resistant CIDP (Choudhary 1995). In a prospective open study, four people with moderately severe CIDP received a six‐month course of IFN beta‐1a 22 μg thrice weekly for three weeks and then 44 μg thrice weekly for 8.5 to 10.3 months (Kuntzer 1999). There was no statistically significant benefit: two people showed moderate improvement and one relapsed on treatment with IFN‐b1a alone. When the treatment was combined with IVIg improvement did occur but this might have been due to the known beneficial effect of IVIg. Martina and colleagues reported benefit in an open study from IFN beta‐1a 22 μg thrice weekly in one person with pure motor CIDP, as well as three with the related condition of multifocal motor neuropathy (Martina 1999). The encouraging anecdotes led to the first randomised trial described in this review (Hadden 1999). Its negative result has to be considered in the context of the facts that the participants were resistant to other treatments, only received treatment for 12 weeks, and received a low dose (maximum 22 μg subcutaneously three times a week). In subsequent trials in multiple sclerosis, 44 μg three times weekly has been more effective on most parameters than 22 μg three times weekly (PRISMS 1998; PRISMS 2001). Radziwill and colleagues reported in an abstract that four of five people with CIDP improved on IFN beta‐1a 22 μg three times weekly or on alternate days (Radziwill 2001). In a non‐randomised open study of intramuscular IFN beta‐1a 30 μg weekly, seven (35%) of 20 participants treated showed clinical improvement, 10 (50%) remained stable and three (15%) worsened (Vallat 2003).
These results led to the second randomised trial reported in this review which also failed to confirm benefit in the intention‐to‐treat analysis (Hughes 2010). In this trial, treatment lasted 32 weeks and the dose given ranged from 30 μg weekly up to 60 μg intramuscularly twice weekly. The primary outcome used by the trial authors was total IVIg dose (g/kg) administered from week 16 to week 32 in the placebo group compared with the IFN beta‐1a group. This was slightly lower in the combined IFN beta‐1a groups (1.20 g/kg; P = 0.75) compared with the placebo group (1.34 g/kg) but the difference was not statistically significant. However, in an exploratory analysis, participants who were more severely disabled or on higher doses of IVIg had a statistically significant response to IFN beta‐1a.
The evidence from the observational studies does not provide much support for the use of IFN beta‐1a and, while differences in design and outcomes prevented meta‐analysis, neither of the randomised trials identified in this review showed significant benefit. The observation in an exploratory analysis of significant reduction of IVIg in the participants receiving high doses might be worth pursuing with further trials. However, the occurrence of worsening of CIDP following treatment of co‐existent multiple sclerosis with IFN beta‐1a in one person urges caution in using the drug outside a clinical trial (Matsuse 2005). This conclusion is supported by the occurrence of side effects and especially the expense of IFN beta. IFN beta‐1a often causes minor alterations of liver function and white cell counts and, with subcutaneous preparations, skin reactions but serious side effects are rare. In the UK, the cost of being on 30 μg of IFN beta‐1a once a week for one year is about GBP 8502 (EUR 10,481) (BNF 2014).
Summary of observational studies of interferon beta‐1a
| Series | Dose | Duration | No. of participants | No. improved | Notes |
| Choudhary 1995 | Six month course of IFNb‐1a 22 μg 3 times weekly for 3 weeks and then 44 μg thrice weekly for 8.5 to 10.3 months | Not stated | 1 | 1 | |
| Kuntzer 1999 | Not stated | Not stated | 4 | 2 | There was no statistically significant benefit. 2 participants showed moderate improvement and 1 relapsed on treatment with IFN beta‐1a alone |
| Martina 1999 | Not stated | Not stated | 1 | 1 | Pure motor (3 other participants with MMN also improved) |
| Radziwill 2001 | 22 μg 3 times weekly or on alternate days | Not stated | 5 | 4 | |
| Vallat 2003 | 30 μg weekly | Not stated | 20 | 7 | 10 (50%) remained stable and 3 (15%) worsened) |
| Cocito 2011 | 6 million units (30 μg) weekly | ≥ 12 months | 3 | 0 | |
| All studies | 34 | 15 |
Interferon alfa
Interferon alfa (IFN alfa) is another naturally occurring cytokine which has complex, incompletely understood immunoregulatory actions. It is used to enhance immune reactions to combat hepatitis C. It upregulates immune responses and has been reported to cause autoimmune diseases including CIDP (Marzo 1998; Meriggioli 2000). Despite this, IFN alfa has been used to treat CIDP. The largest study treated 16 participants (of whom two had a paraprotein) with IFN alfa‐2a 3 million international units (MIU) subcutaneously three times a week for six weeks (Gorson 1997). Of the 14 participants without a paraprotein, nine responded to IFN alfa‐2a: five had a sustained improvement and one improved, received plasma exchange and then had sustained improvement, and three relapsed. Minor side effects consisting of fatigue, fever, malaise, and myalgia and arthralgia were common. Pavesi and colleagues reported dramatic improvement with IFN alfa in a single person with treatment‐resistant CIDP. This person had severe symmetric distal and proximal muscle weakness and made complete functional recovery six months after she was started on IFN alfa 3 MIU subcutaneously twice a week (Pavesi 2002). The Italian retrospective study reported improvement in four of 11 people with CIDP treated with 800,000 units to 3 million units every one to three weeks for at least a year (Cocito 2011). IFN alfa is expensive. We share the view of Saperstein and colleagues that randomised trials would be needed to establish whether IFN alfa is beneficial (Saperstein 2001).
Autologous and allogeneic peripheral blood stem cell transplantation
Autologous peripheral blood stem cell transplantation (PBSCT) is an extreme form of immunosuppression in which the person is treated with high‐dose cyclophosphamide and then granulocyte stimulating factor to allow harvesting of bone marrow stem cells from their own blood. The person is then treated with very high dose cyclophosphamide, anti‐T cell antibodies and, in some regimens, whole body irradiation to ablate their immune system. Finally, the immune system is reconstituted with the stored stem cells. There are seven reports of people with CIDP who underwent PBSCT (See table Summary of studies of autologous and allogeneic PBSCT). Vermeulen and colleagues reported success in one person with CIDP who had been dependent on frequent IVIg: the person improved and became able to stop all immunotherapy afterwards (Vermeulen 2002), but relapsed after five years (Vermeulen 2007). Barreira and colleagues reported a 24‐year‐old man with a 12‐year history of CIDP who underwent PBSCT with only a transient response that lasted for a month (Barreira 2007). Oyama and colleagues reported a 32‐year‐old woman who had had an inadequate response to standard treatments. She had clinical and electrophysiological improvement after her transplantation. Her Rankin function score improved from 4 to 1 and she came off corticosteroids, IVIg and plasma exchange (Oyama 2007). A case series followed up three people with CIDP, two of whom were from an earlier publication (Mahdi‐Rogers 2007). Of the three people, two improved: one had a response which was transient (18 months) and the other was in remission after six months (Mahdi‐Rogers 2009). An open‐label, non‐randomised study of PBSCT included 42 participants with probable or definite CIDP who had not responded to at least two first‐line treatments. Twenty‐four of 32 participants had a drug‐free remission after one year. None developed neutropenic sepsis but two died of diseases unrelated to PBSCT during the follow‐up (see table for more details) (Allen 2013). In another series, 11 participants who had been refractory to other immunotherapies (including one included in a previous report (Axelson 2008)) were treated with PBSCT. After a median follow‐up of 28 months, eight had entered remission without the need for any immunomodulatory treatment (Press 2014).
The use of allogeneic peripheral blood stem cell transplantation, which requires even more aggressive immunosuppression, induced complete remission in one person with CIDP; no relapse had occurred 6.5 years after transplantation (Remenyi 2007).
Summary of studies of autologous and allogeneic peripheral blood stem cell transplantation
| Series | No. of participants | No. improved | Notes (autologous transplant unless noted) |
| Vermeulen 2002 | 1 | 1 | Relapse after 5 years |
| Barreira 2007 | 1 | 1 | Improvement lasted for 1 month |
| Oyama 2007 | 1 | 1 | No disease exacerbation 22 months after transplant. By 6 months after transplant the person's Rankin function score had improved from 4 to 1 and corticosteroids, IVIg, and plasma exchange were withdrawn |
| Mahdi‐Rogers 2009 | 3 | 2 | 1 of the 2 who benefited relapsed after 18 months |
| Remenyi 2007 | 1 | 1 | Allogeneic transplant. No relapse after 6.5 years |
| Allen 2013 | 32 at 1 year |
24 at 1 year |
Follow up: 6 months (n = 38), 1 year (n = 32), 2 years (n = 20), 3 years (n = 14), 4 years (n = 9), and 5 years (n = 5). Drug‐free remission was observed in 68% of 28 at 6 months, 75% of 32 at 1 year, 75% of 20 at 2 years, 78% of 14 at 3 years, 55% of 9 at 4 years, and 40% of 5 at 5 years. and Short Form 36 Health Survey questionnaire significantly improved at last follow‐up (P < 0.001) |
| Press 2014 | 11 | 8 | The median follow‐up period was 28 months. 8 of 11 of those treated went into drug‐free remission |
| Total | 50 | 38 |
Alemtuzumab
Alemtuzumab is a monoclonal antibody against CD52 antigen. Hirst 2006 reported remission for 16 months after administration of five daily infusions of alemtuzumab 30 mg/day in a person with CIDP with 11 relapses in 18 months after disease onset. A subsequent report included this person and six others (Llewelyn 2009; Marsh 2010). All had been treated with oral prednisolone as well as conventional immunosuppressants without significant benefit. Each received nine courses of alemtuzumab (dose range 60 to 150 mg). After treatment, mean monthly IVIg dose reduced from 202 g to 149 g (26%) and frequency of IVIg infusion from 22 to 136 days. Two people had prolonged remission, another two had a partial response and three had no clear benefit. Responders had a younger age at disease onset and a shorter disease duration than non‐responders. Three of the seven people treated developed an autoimmune condition, including Graves’ disease and autoimmune haemolytic anaemia (Marsh 2010). There is an ongoing open‐label multicentre trial of alemtuzumab in the treatment of CIDP (Alemtuzumab trial).
Natalizumab
Natalizumab, a humanised monoclonal antibody against the T‐cell adhesion molecule α4 integrin, has been approved for treatment of multiple sclerosis. A person with CIDP who had failed to respond to corticosteroids, IVIg, plasma exchange, azathioprine, cyclophosphamide, and mycophenolate mofetil was treated with a single intravenous infusion of 300 mg of natalizumab and worsened (Wolf 2010). An open‐label study of natalizumab evaluated three people with CIDP with a sensory variant of CIDP (Vallat 2015). They received natalizumab after they had become less responsive or refractory to IVIg and corticosteroids. One improved markedly and was well after three years of treatment, another improved significantly after eight months and the third remained stable during 12 months of treatment. Natalizumab was stopped in all three because of the detection of JC virus in their serum.
Future trials
New trials are needed to investigate the efficacy of immunosuppressive and immunomodulatory drugs in CIDP. Of those reported in this review, azathioprine, a higher dose of methotrexate, ciclosporin, and rituximab are all candidates. Fingolimod is being tested in a commercially sponsored trial (FORCIDP trial), which closed early for futility. There is also an ongoing multicentre RCT of mycophenolate for CIDP (MYCOPID). Many other agents such as dimethyl fumarate, teriflunomide, and laquinimod are used or being tested in multiple sclerosis and other inflammatory diseases and would be worth consideration in CIDP (Melzer 2014).
Our ignorance of the detailed underlying pathogenesis of CIDP complicates the choice of agents to try. The occurrence of new onset CIDP in people being treated with tumour necrosis factor inhibitors has already been mentioned. To this we must add the occurrence of CIDP following solid organ transplantation. Echaniz‐Laguna 2012 found that 10 of 1557 (0.6%) people developed a monophasic CIDP‐like illness in the first 12 months after a transplant for which they had received immunosuppression (Echaniz‐Laguna 2012).
Future trials should preferably last longer than those undertaken so far. The 12‐month duration recommended by a consensus group (ENMC 2005), which we selected for this review as the time for measuring the primary outcome, seems appropriate.
Measuring disability is preferred to impairment or nerve conduction parameters as the primary outcome because it has greater relevance to the individual. A disability scale developed for, and validated in, CIDP, such as the Overall Neuropathy Limitations Scale (ONLS) (Graham 2006), would be preferable to a generic scale. Hitherto such scales have been ordinal scales. The Rasch‐built Overall Disability Scale (R‐ODS) scale is a numerical scale that captures a wider range of disability than the ONLS and should now be preferred since it has been shown to be more responsive (van Nes 2011; Draak 2014). A RCT of long‐term use of IVIg for CIDP found the measurement of grip strength using a vigorimeter to be a sensitive tool in showing clinically significant change in participants (Vanhoutte 2013). It is likely to be used as an outcome measure in future trials and we will include it as a secondary outcome measure in future updates of this review.
On the other hand, measurement of neurophysiological parameters is fraught with technical difficulties, especially in the multicentre and even multinational trials which are needed in CIDP, and is not being done in current trials. We will remove neurophysiological parameters from secondary outcome measures in future updates of this review.
An alternative outcome measure in people who are receiving IVIg or corticosteroids is to introduce the agent being tested, stop the IVIg or corticosteroids and watch carefully for worsening. This was done by measuring the percentage IVIg dose reduction in the Hughes 2010 trial of IFN beta, or the percentage of participants who could reduce the dose of IVIg or corticosteroids in the RMC 2009 trial. An alternative way of testing the same thing is to compare the times to relapse between the two groups, as was done in the trial of fingolimod (FORCIDP trial).
A high proportion of participants in the placebo groups, 14 of 32 in the RMC trial (RMC 2009), and 8 of 17 in the trial by IFN beta‐1a (Hughes 2010), were able to reduce their dose of IVIg or corticosteroids without deteriorating. This suggested that some participants had been on a higher dose at baseline than they required. This high proportion of 'responders' in the placebo groups reduced the sensitivity of these trials to detect a significant difference in the reduction of IVIg or corticosteroids in withdrawal trials, which made the assessment of the efficacy of methotrexate and IFNb‐1a difficult. Future trials should allow for this in power calculations or include a run‐in period during which the IVIg or corticosteroid dosage is reduced, after which only participants who had been observed to worsen and require the reintroduction of IVIg or corticosteroids would be randomised. The observations in these trials have led guidelines to encourage drug holidays to discover whether IVIg or corticosteroids are still needed (Van den Bergh 2010).
Overall completeness and applicability of evidence
Our search for randomised trials has been exhaustive. Enquiry from members of the international Peripheral Nerve Society, the relevant medical professional organisation, in June 2016 did not reveal any other completed or ongoing trials (www.pnsociety.com). No robust method exists for systematically identifying and reviewing non‐randomised studies, but we included all relevant articles known to us, discovered during our search for randomised trials or identified in our additional search for non‐randomised studies. We referenced and briefly described case reports and series that gave the number of people treated with individual immunosuppressive agents and the number whom the study authors deemed to have improved. We do not claim to have discovered all such reports or series and the criteria for diagnosis, improvement and completeness of follow‐up have often not been fully reported even in those to which we have referred. Any conclusions from these non‐randomised data require extreme caution in interpretation.
Quality of the evidence
The evidence from trials of azathioprine and IFN beta‐1a was low and that of methotrexate moderate. The evidence from non‐randomised studies is so low as to be uninterpretable beyond stating that none of the treatments tested is universally effective.
Potential biases in the review process
Three of the authors of this review were investigators in one or more of the trials considered in this review and have received funding for research or consultancies for firms which produce human immunoglobulin which is a recognised treatment for CIDP (see Declarations of interest). The absence of any claim for efficacy of any of the drugs reviewed here reduces any potential bias from this source.
Agreements and disagreements with other studies or reviews
We know of no other systematic reviews of immunosuppressive immunomodulatory drugs for CIDP. We include the same trials and studies as in recent narrative reviews (Kleyman 2015).The conclusions of this review are in line with the international guideline on management of CIDP which states that there is only class IV evidence on which to base practice (Van den Bergh 2010).
Authors' conclusions
Implications for practice.
One randomised controlled trial of azathioprine, two of interferon beta‐1a (IFN beta‐1a) and one of methotrexate have been performed in chronic inflammatory demyelinating polyradiculoneuropathy (CIDP). None showed a significant effect but none were adequate to support or refute mild or moderate benefit or harm. Small numbers of case reports and case series described the use of other immunosuppressant drugs, especially cyclophosphamide, ciclosporin and rituximab, but none consistently produced sufficient improvement to recommend their use without further research.
Implications for research.
More research is needed to determine whether immunosuppressive drugs or IFNs are beneficial in CIDP. Future trials should measure outcomes including disability after at least one year. It is necessary to use scales for measuring disability and impairment that are more responsive, biometrically sound and relevant to people with CIDP. If trials use change in dosage of corticosteroids or intravenous immunoglobulin (IVIg), or the need for rescue treatment when IVIg is stopped as an outcome measure, their design should allow for the fact that these drugs may no longer be needed in a proportion of participants.
What's new
| Date | Event | Description |
|---|---|---|
| 8 October 2019 | Amended | Clarification message added to Declarations of interest about the review's compliance with the Cochrane Commercial Sponsorship Policy. |
History
Protocol first published: Issue 4, 2001 Review first published: Issue 1, 2003
| Date | Event | Description |
|---|---|---|
| 6 August 2016 | New search has been performed | New search. No new trials found. |
| 6 August 2016 | New citation required but conclusions have not changed | Background and Discussion updated. Conclusions unchanged. Two additional authors joined the review team. |
| 14 June 2013 | Amended | Correction to author affiliation, acknowledgements and sources of support |
| 6 February 2013 | New citation required but conclusions have not changed | Results of new searches fully incorporated |
| 9 July 2012 | New search has been performed | New search to 9 July 2012. No new completed trials. Three ongoing trials added. Text updated. Search strategies updated with intervention terms. |
| 30 July 2008 | New citation required and conclusions have changed | Change of first author Two new trials added Change of title. This Cochrane review was previously published as Cytotoxic drugs and interferons for chronic inflammatory demyelinating polyradiculoneuropathy. |
| 27 April 2008 | Amended | Converted to new review format. |
| 21 March 2007 | New search has been performed | The searches were updated in March 2007. No new trials were identified. |
| 1 January 2006 | New search has been performed | We updated the searches of the NMD Group Trials Register, MEDLINE and EMBASE (to September 2005). No new randomised trials were found. We included additional non‐randomised studies in the Discussion section of the review. |
| 1 July 2004 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
We thank the GBS/CIDP Foundation International for funding for MM‐R.
We thank Dr Tony Swan, statistician, who co‐authored earlier versions of this review.
The Assistant Managing Editor for Cochrane Neuromuscular, Dr Katherine Jones, entered additional characteristics of ongoing studies.
This project was supported by the National Institute for Health Research via Cochrane Infrastructure funding to Cochrane Neuromuscular. The views and opinions expressed herein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health. Cochrane Neuromuscular is also supported by the MRC Centre for Neuromuscular Disease.
Appendices
Appendix 1. Overall Neuropathy Limitations Scale (ONLS) (Graham 2006)
Arm grade
0 = Normal
1= Minor symptoms in one or both arms but not affecting any of the functions:
washing and brushing their hair;
turning a key in a lock;
using a knife and fork together (or spoon, if knife and fork not used);
doing or undoing buttons or zips;
dressing the upper part of their body excluding buttons or zips
2= Disability in one or both arms affecting but not preventing any of the above functions
3= Disability in one or both arms preventing at least one but not all the above functions
4= Disability in both arms preventing all the above functions but purposeful movement still possible
5= Disability in both arms preventing all purposeful movements
Leg grade
0= Walking/climbing stairs/running not affected
1= Walking/climbing stairs/running is affected, but gait does not look abnormal
2= Walks independently but gait looks abnormal
3= Requires unilateral support to walk 10 metres (stick, single crutch, one arm)
4= Requires bilateral support to walk 10 metres (sticks, crutches, crutch and arm, frame)
5= Requires wheelchair to travel 10 metres but able to stand and walk 1 metre with the help of one person
6= Restricted to wheelchair, unable to stand and walk 1 metre with the help of one person, but able to make
some purposeful leg movements
7= Restricted to wheelchair or bed most of the day, unable to make any purposeful movements of the legs
Overall grade = arm grade + leg grade (range: 0 (no disability) to 12 (maximum disability))
Appendix 2. Modified Rankin Scale
0: asymptomatic
1: non‐disabling symptoms not interfering with lifestyle
2: minor disability symptoms leading to some restriction of lifestyle but not interfering with the patient's capacity to look after themselves
3: moderate disability symptoms significantly interfering with lifestyle or preventing fully independent existence
4: moderately severe disability symptoms preventing independent existence although patients do not need constant attention day and night
5: totally dependent, requiring constant attention day and night
Appendix 3. Cochrane Neuromuscular Specialised Register (CRS) search strategy
#1 chronic NEAR inflammatory NEAR demyelinating NEAR (neuropath* or polyneuropath* or polyradiculoneuropath* or polyradiculoneuritis or polyneuritis) [REFERENCE] [STANDARD] #2 cidp [REFERENCE] [STANDARD] #3 #1 or #2 [REFERENCE] [STANDARD] #4 MeSH DESCRIPTOR Immunologic Factors Explode All [REFERENCE] [STANDARD] #5 MeSH DESCRIPTOR Cyclophosphamide Explode All [REFERENCE] [STANDARD] #6 immunologic or immunosuppress* or immunomodulat* or alemtuzumab or azathioprine or cyclosporin* or etanercept or cyclophosphamide or methotrexate or "mycophenolate mofetil" or Nataluzimab or rituximab or Sirolimus or Tacrolimus [REFERENCE] [STANDARD] #7 MeSH DESCRIPTOR Peripheral Blood Stem Cell Transplantation [REFERENCE] [STANDARD] #8 "autologous peripheral blood stem cell transplantation" [REFERENCE] [STANDARD] #9 interferon* and (alfa or alpha or beta) [REFERENCE] [STANDARD] #10 #4 or #5 or #6 or #7 or #8 or #9 [REFERENCE] [STANDARD] #11 #3 and #10 [REFERENCE] [STANDARD] #12 (#3 and #10) AND (INREGISTER) [REFERENCE] [STANDARD]
Appendix 4. CENTRAL (CRSO) search strategy
Search run on Tue May 24 2016 #1 (polyneuritis or polyradiculoneuritis or polyradiculoneuropath* or polyneuropathy*):TI,AB,KY625 #2 (inflammatory demyelinating):TI,AB,KY140 #3 chronic:TI,AB,KY69174 #4 #1 AND #2 AND #3110 #5 (chronic inflammatory demyelinating polyradiculoneuropathy):TI,AB,KY61 #6 MESH DESCRIPTOR Polyradiculoneuropathy, Chronic Inflammatory Demyelinating EXPLODE ALL TREES25 #7 #4 OR #5 OR #6110 #8 MESH DESCRIPTOR Immunologic Factors EXPLODE ALL TREES34332 #9 MESH DESCRIPTOR Adjuvants, Immunologic EXPLODE ALL TREES4893 #10 MESH DESCRIPTOR Immunosuppressive Agents EXPLODE ALL TREES15973 #11 MESH DESCRIPTOR Peripheral Blood Stem Cell Transplantation171 #12 (immunologic or immunosuppress* or immunomodulat* or mycophenolate mofetil or etanercept or rituximab or alemtuzumab or autologous PBSCT or autologous peripheral blood stem cell transplantation):TI,AB,KY18024 #13 #8 OR #9 OR #10 OR #11 OR #1243184 #14 #7 AND #1333
Appendix 5. MEDLINE (OvidSP) search strategy
Database: Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) <1946 to Present> May Week 2 2016 Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 randomized controlled trial.pt. (416993) 2 controlled clinical trial.pt. (90735) 3 randomized.ab. (353953) 4 placebo.ab. (172264) 5 drug therapy.fs. (1858912) 6 randomly.ab. (253758) 7 trial.ti,ab. (432115) 8 groups.ab. (1583319) 9 or/1‐8 (3803835) 10 exp animals/ not humans.sh. (4244988) 11 9 not 10 (3279838) 12 inflammatory demyelinating.tw. (3910) 13 (polyradiculoneuropath$3 or polyneuropath$3).tw. (12899) 14 Polyneuropathies/ or Polyradiculoneuropathy/ (8193) 15 (polyneuritis or polyradiculoneuritis).tw. (1949) 16 13 or 14 or 15 (19006) 17 12 and 16 and chronic.mp. (1986) 18 Polyradiculoneuropathy, Chronic Inflammatory Demyelinating/ (1095) 19 (chronic inflammatory demyelinating polyradiculoneuropathy or cidp).mp. (1557) 20 or/17‐19 (2324) 21 exp immunologic factors/ (1219643) 22 exp adjuvants, immunologic/ (149440) 23 exp Immunosuppressive Agents/ (275831) 24 Peripheral Blood Stem Cell Transplantation/ (3146) 25 (immunologic or immunosuppress* or immunomodulat* or mycophenolate mofetil or etanercept or rituximab or alemtuzumab or autologous PBSCT or autologous peripheral blood stem cell transplantation).mp. (439433) 26 exp Cyclophosphamide/ (49523) 27 exp Methotrexate/ (33959) 28 exp cyclosporins/ (37374) 29 (interferon adj1 (alfa or alpha or beta)).mp. (48037) 30 (Cyclosporine or Azathioprine or Tacrolimus or Sirolimus or Nataluzimab).mp. (85088) 31 or/21‐30 (1451586) 32 11 and 20 and 31 (479) 33 remove duplicates from 32 (472)
Observational studies strategy
Database: Epub Ahead of Print, In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid MEDLINE(R) <1946 to Present> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 randomized controlled trial.pt. (424764) 2 controlled clinical trial.pt. (91261) 3 randomized.ab. (363400) 4 placebo.ab. (176413) 5 drug therapy.fs. (1885888) 6 randomly.ab. (259624) 7 trial.ti,ab. (445060) 8 groups.ab. (1616474) 9 or/1‐8 (3872481) 10 exp animals/ not humans.sh. (4278331) 11 9 not 10 (3342037) 12 inflammatory demyelinating.tw. (3966) 13 (polyradiculoneuropath$3 or polyneuropath$3).tw. (13053) 14 Polyneuropathies/ or Polyradiculoneuropathy/ (8245) 15 (polyneuritis or polyradiculoneuritis).tw. (1966) 16 13 or 14 or 15 (19195) 17 12 and 16 and chronic.mp. (2016) 18 Polyradiculoneuropathy, Chronic Inflammatory Demyelinating/ (1125) 19 (chronic inflammatory demyelinating polyradiculoneuropathy or cidp).mp. (1585) 20 or/17‐19 (2359) 21 exp immunologic factors/ (1230953) 22 exp adjuvants, immunologic/ (150762) 23 exp Immunosuppressive Agents/ (279014) 24 Peripheral Blood Stem Cell Transplantation/ (3184) 25 (immunologic or immunosuppress* or immunomodulat* or mycophenolate mofetil or etanercept or rituximab or alemtuzumab or autologous PBSCT or autologous peripheral blood stem cell transplantation).mp. (445205) 26 exp Cyclophosphamide/ (50025) 27 exp Methotrexate/ (34376) 28 exp cyclosporins/ (37605) 29 (interferon adj1 (alfa or alpha or beta)).mp. (48615) 30 (Cyclosporine or Azathioprine or Tacrolimus or Sirolimus or Nataluzimab).mp. (86177) 31 or/21‐30 (1466442) 32 11 and 20 and 31 (484) 33 remove duplicates from 32 (474) 34 20 and 31 (1086) 35 limit 34 to (clinical trial or consensus development conference or consensus development conference, nih or controlled clinical trial or guideline or meta analysis or multicenter study or practice guideline or randomized controlled trial) (102) 36 exp cohort studies/ or (cohort$ or case‐control$ or case control$).mp. (1928112) 37 34 and 36 (163) 38 35 or 37 (234) 39 exp animals/ not humans.sh. (4278331) 40 38 not 39 (234) 41 40 not 32 (96) 42 remove duplicates from 41 (94) 43 limit 42 to yr="1976 ‐Current" (94)
Appendix 6. Embase (OvidSP) search strategy
Database: Embase <1980 to 2016 Week 21> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 crossover‐procedure.sh. (47056) 2 double‐blind procedure.sh. (128360) 3 single‐blind procedure.sh. (22094) 4 randomized controlled trial.sh. (401464) 5 (random$ or crossover$ or cross over$ or placebo$ or (doubl$ adj blind$) or allocat$).tw,ot. (1251337) 6 trial.ti. (199070) 7 or/1‐6 (1400710) 8 (animal/ or nonhuman/ or animal experiment/) and human/ (1481547) 9 animal/ or nonanimal/ or animal experiment/ (3568731) 10 9 not 8 (2953303) 11 7 not 10 (1289354) 12 limit 11 to embase (1063514) 13 demyelination/ or demyelinating neuropathy/ or demyelinating disease/ (28726) 14 inflammatory demyelinating.tw. (5739) 15 13 or 14 (31905) 16 polyneuropathy/ or peripheral neuropathy/ or peripheral nervous system/ or polyradiculoneuropathy/ (104562) 17 (polyradiculoneur$ or polyneur$).mp. (27333) 18 16 or 17 (114576) 19 15 and 18 and chronic.mp. (3557) 20 (chronic inflammatory demyelinating neuropath$ or cidp).mp. (2341) 21 19 or 20 (4016) 22 exp immunomodulating agent/ (677888) 23 exp immunological adjuvant/ (26926) 24 (immunologic or immunosuppress$ or anti?inflammatory).mp. (503535) 25 exp interferon/ (404450) 26 exp antiinflammatory agent/ (1332044) 27 exp immunosuppressive agent/ (607925) 28 (azanthioprine or cyclophosphamide or methotrexate or ciclosporin or cyclosporin or mycophenolate mofetil or etanercept or rituximab or alemtuzumab or autologous PBSCT or autologous peripheral blood stem cell transplantation).mp. (422780) 29 (alemtuzumab or azanthioprine or ciclosporin or cyclosporin or cyclophosphamide or etanercept or methotrexate or mycophenolate mofetil or nataluzimab or rapamycin or rituximab or sirolimus or tacrolimus).mp. (494213) 30 (interferon adj1 (alfa or alpha or beta)).mp. (85007) 31 (autologous PBSCT or autologous peripheral blood stem cell transplantation).mp. (2366) 32 or/22‐31 (2192064) 33 12 and 21 and 32 (158) 34 remove duplicates from 33 (156)
Observational studies strategy
Database: Embase <1980 to 2016 Week 29> Search Strategy: ‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐‐ 1 crossover‐procedure.sh. (47903) 2 double‐blind procedure.sh. (129905) 3 single‐blind procedure.sh. (22486) 4 randomized controlled trial.sh. (410130) 5 (random$ or crossover$ or cross over$ or placebo$ or (doubl$ adj blind$) or allocat$).tw,ot. (1276259) 6 trial.ti. (203468) 7 or/1‐6 (1427201) 8 (animal/ or nonhuman/ or animal experiment/) and human/ (1500351) 9 animal/ or nonanimal/ or animal experiment/ (3596641) 10 9 not 8 (2973342) 11 7 not 10 (1314424) 12 limit 11 to embase (1084796) 13 demyelination/ or demyelinating neuropathy/ or demyelinating disease/ (29128) 14 inflammatory demyelinating.tw. (5869) 15 13 or 14 (32404) 16 polyneuropathy/ or peripheral neuropathy/ or peripheral nervous system/ or polyradiculoneuropathy/ (105315) 17 (polyradiculoneur$ or polyneur$).mp. (27679) 18 16 or 17 (115447) 19 15 and 18 and chronic.mp. (3640) 20 (chronic inflammatory demyelinating neuropath$ or cidp).mp. (2415) 21 19 or 20 (4115) 22 exp immunomodulating agent/ (685472) 23 exp immunological adjuvant/ (27163) 24 (immunologic or immunosuppress$ or anti?inflammatory).mp. (510036) 25 exp interferon/ (410357) 26 exp antiinflammatory agent/ (1348019) 27 exp immunosuppressive agent/ (614578) 28 (azanthioprine or cyclophosphamide or methotrexate or ciclosporin or cyclosporin or mycophenolate mofetil or etanercept or rituximab or alemtuzumab or autologous PBSCT or autologous peripheral blood stem cell transplantation).mp. (427096) 29 (alemtuzumab or azanthioprine or ciclosporin or cyclosporin or cyclophosphamide or etanercept or methotrexate or mycophenolate mofetil or nataluzimab or rapamycin or rituximab or sirolimus or tacrolimus).mp. (499976) 30 (interferon adj1 (alfa or alpha or beta)).mp. (85706) 31 (autologous PBSCT or autologous peripheral blood stem cell transplantation).mp. (2376) 32 or/22‐31 (2219446) 33 12 and 21 and 32 (160) 34 21 and 32 (1565) 35 exp case control study/ or exp cohort analysis/ (358714) 36 (cohort$ or case‐control$ or case control$).mp. (767448) 37 35 or 36 (767448) 38 34 and 37 (58) 39 38 not 33 (54) 40 39 not 10 (54) 41 limit 40 to embase (52) 42 limit 41 to yr="1976 ‐Current" (52)
Appendix 7. LILACS (IAHx) search strategy
(MH:"Polyradiculoneuropathy, Chronic Inflammatory Demyelinating" or cidp or "chronic inflammatory demyelinating polyneuropathy" or (chronic and demyelinating and (polyradiculoneuropathy or polyneuropathy or polyneuritis))) and (MH:D27.505.696.477$ or "factores inmunologicos" or "factores imunologicos" or alemtuzumab or azathioprine or cyclophosphamide or ciclosporin or cyclosporin or etanercept or interferon or methotrexate or nataluzimab or rapamycin or rituximab or sirolimus or tacrolimus or "analogous PBSCT" or "peripheral blood stem cell transplantation") and ((PT:"Randomized Controlled Trial" or "Randomized Controlled trial" or "Ensayo Clínico Controlado Aleatorio" or "Ensaio Clínico Controlado Aleatório" or PT:"Controlled Clinical Trial" or "Ensayo Clínico Controlado" or "Ensaio Clínico Controlado" or "Random allocation" or "Distribución Aleatoria" or "Distribuição Aleatória" or randon$ or Randomized or randomly or "double blind" or "duplo‐cego" or "duplo‐cego" or "single blind" or "simples‐cego" or "simples cego" or placebo$ or trial or groups) AND NOT (B01.050$ AND NOT (humans or humanos or humanos)))
Appendix 8. CINAHL (EBSCOhost) search strategy
Tuesday, May 24, 2016 7:33:41 AM S39 S32 and S38 60 S38 S33 or S34 or S35 or S36 or S37 134,634 S37 autologous PBSCT or autologous peripheral blood stem cell transplantation 74 S36 etanercept or rituximab or alemtuzumab 4,759 S35 immunologic or immunosuppress* or azathioprine or cyclophosphamide or methotrexate or ciclosporin or cyclosporin or mycophenolate mofetil 31,118 S34 (MH "Immunologic Factors+") 110,799 S33 (MH "Immunosuppressive Agents+") 14,101 S32 S18 and S31 111 S31 S28 or S29 or S30 398 S30 chronic n3 inflammatory n3 demyelinating n3 polyradiculoneuropathy 130 S29 cidp 226 S28 S22 and S26 and S27 355 S27 chronic 180,521 S26 S23 or S24 or S25 4,949 S25 polyradiculoneuropath* or polyneuropath* or polyneuritis 1,924 S24 (MH "Polyneuritis+") 332 S23 (MH "Polyradiculoneuritis+") or (MH "Polyradiculopathy") 3,399 S22 S21 or (S19 and S20) 639 S21 inflammatory n3 demyelinating 551 S20 TI inflammatory or AB inflammatory 41,871 S19 (MH "Demyelinating Diseases") 1,197 S18 S1 or S2 or S3 or S4 or S5 or S6 or S7 or S8 or S9 or S10 or S11 or S12 or S13 or S14 or S15 or S16 or S17 833,262 S17 ABAB design* 91 S16 TI random* or AB random* 172,898 S15 ( TI (cross?over or placebo* or control* or factorial or sham? or dummy) ) or ( AB (cross?over or placebo* or control* or factorial or sham? or dummy) ) 343,315 S14 ( TI (clin* or intervention* or compar* or experiment* or preventive or therapeutic) or AB (clin* or intervention* or compar* or experiment* or preventive or therapeutic) ) and ( TI (trial*) or AB (trial*) ) 124,235 S13 ( TI (meta?analys* or systematic review*) ) or ( AB (meta?analys* or systematic review*) ) 46,136 S12 ( TI (single* or doubl* or tripl* or trebl*) or AB (single* or doubl* or tripl* or trebl*) ) and ( TI (blind* or mask*) or AB (blind* or mask*) ) 26,647 S11 PT ("clinical trial" or "systematic review") 131,987 S10 (MH "Factorial Design") 972 S9 (MH "Concurrent Prospective Studies") or (MH "Prospective Studies") 282,226 S8 (MH "Meta Analysis") 24,580 S7 (MH "Solomon Four‐Group Design") or (MH "Static Group Comparison") 49 S6 (MH "Quasi‐Experimental Studies") 7,849 S5 (MH "Placebos") 9,720 S4 (MH "Double‐Blind Studies") or (MH "Triple‐Blind Studies") 33,445 S3 (MH "Clinical Trials+") 198,457 S2 (MH "Crossover Design") 13,749 S1 (MH "Random Assignment") or (MH "Random Sample") or (MH "Simple Random Sample") or (MH "Stratified Random Sample") or (MH "Systematic Random Sample") 72,638
Appendix 9. Clinical trials registries search strategies
Chronic inflammatory demyelinating polyradiculoneuropathy or CIDP
Appendix 10. Additional MEDLINE search strategy for non‐randomised studies
32 11 and 20 and 31 (484) Total for RCT search
33 remove duplicates from 32 (474)
34 20 and 31 (1086)
35 limit 34 to (clinical trial or consensus development conference or consensus development conference, nih or controlled clinical trial or guideline or meta analysis or multicenter study or practice guideline or randomized controlled trial) (102)
36 exp cohort studies/ or (cohort$ or case‐control$ or case control$).mp. (1928112)
37 34 and 36 (163)
38 35 or 37 (234)
39 exp animals/ not humans.sh. (4278331)
40 38 not 39 (234)
41 40 not 32 (96)
42 remove duplicates from 41 (94)
43 limit 42 to yr="1976 ‐Current" (94)
Appendix 11. Additional Embase search strategy for non‐randomised studies
33 12 and 21 and 32 (160) Total for RCT search
34 21 and 32 (1565)
35 exp case control study/ or exp cohort analysis/ (358714)
36 (cohort$ or case‐control$ or case control$).mp. (767448)
37 35 or 36 (767448)
38 34 and 37 (58)
39 38 not 33 (54)
40 39 not 10 (54)
41 limit 40 to embase (52)
42 limit 41 to yr="1976 ‐Current" (52)
Data and analyses
Comparison 1. Methotrexate versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serious adverse events | 1 | 59 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.56 [0.39, 32.23] |
Comparison 2. IFNb‐1a versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Serious adverse events | 1 | 67 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.5 [0.25, 80.05] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Dyck 1985.
| Methods | Open, parallel‐group, randomised trial | |
| Participants | 30 adults with CIDP defined by criteria which approximate to the Ad Hoc 1991 criteria published later. 15 were randomised to azathioprine and prednisone but 1 was removed because of a changed diagnosis. 15 were randomised to prednisone only but 2 were removed because of a changed diagnosis. The median time from CIDP diagnosis to enrolment was 29 months (range 8 to 168) for those who received prednisone and 21 months (range 6 to 84) for those who received azathioprine and placebo. "Slowly progressive and relapsing cases were included... none had symptoms for less than 6 months; all were static or worsening, had not been treated with prednisone or immunotherapy for at least 3 months, had no associated disease that might cause neuropathy,had a neurologic disability score (NDS) greater than 50 points, were willing to be assigned randomly to either schedule of therapy, could return for evaluations at 4 and 9 months, and signed a consent form." No exclusion criteria specified. |
|
| Interventions | Azathioprine 2 mg/kg for 9 months: prednisone was given to both groups starting at 120 mg every other day with subsequent tapering over 9 months. | |
| Outcomes | Neuropathy impairment score, maximal inspiratory pressure, maximal expiratory pressure, hand grip, finger pinch, and motor nerve conduction in 1 ulnar, median and peroneal nerve. | |
| Additional outcomes not specified in review | ‐ | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Restricted randomisation" according to age, sex and disease severity. |
| Allocation concealment (selection bias) | High risk | Not attempted. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants were not blinded. |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Assessors were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Follow‐up data were available on 13/14 azathioprine and prednisone and 10/13 prednisone only participants. The report provided no reasons for dropouts. |
| Selective reporting (reporting bias) | Low risk | The trial reports all the pre‐specified clinical and electrophysiological outcome measurements. |
| Other bias | Low risk | None identified. |
Hadden 1999.
| Methods | Double blind, cross‐over trial | |
| Participants | 10 adults with treatment‐resistant probable CIDP according to the Ad Hoc 1991 criteria. The median time from CIDP diagnosis to trial enrolment was 11 years (range 3 to 23). "Patients had not received corticosteroids, immunosuppressants, immunoglobulin, or plasma exchange during the previous 12 weeks, or had received them only in an unchanging regimen." Exclusion criteria: "Patients were excluded if they had a paraprotein on serum electrophoresis, previous unrelated neurologic deficit, or a severe concurrent medical condition that might cause neuropathy or interfere with treatment, or were pregnant or younger than 16 years of age." |
|
| Interventions | IFNb‐1a 11 μg thrice weekly for 2 weeks then 22 μg thrice weekly for 10 weeks or placebo, a 4‐week washout period then the opposite regime for 12 weeks. | |
| Outcomes | Primary: number with clinically important improvement in at least 3 measurements of disability, impairment and quality of life (see Description of studies) Secondary: changes in the same clinical measures and in median nerve compound muscle action potential amplitudes, distal motor latencies and motor conduction velocities after 12 weeks | |
| Additional outcomes not specified in review | In the secondary outcome measures the only statistically significant abnormality was a trivial improvement in the functional independence measure in favour of placebo treatment. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Cross‐over design. Participants received either IFNb or placebo in the first arm and the opposite in the second arm. IFNb‐1a and placebo injections were supplied in coded packages according to a sequence of random numbers. |
| Allocation concealment (selection bias) | Low risk | IFNb and placebo injections were supplied in coded packages according to a sequence of random numbers. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Participants were assessed independently by treating neurologists masked to the intervention, at entry and at 2, 4, 8 and 12 weeks during each arm of the trial. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Participants were assessed independently by treating neurologists masked to the intervention, at entry and at 2, 4, 8 and 12 weeks during each arm of the trial. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 participant was on the first arm (IFNb‐1a) for only 4 weeks because he deteriorated. The participant had no washout and was transferred to the second arm. |
| Selective reporting (reporting bias) | Low risk | Detailed report of all clinical and electrophysiological outcomes. |
| Other bias | Low risk | None identified. |
Hughes 2010.
| Methods | Double‐blind, placebo‐controlled, parallel‐group, dose‐ranging. | |
| Participants | 67 adults, men and women between 18 and 75 years of age with a diagnosis of CIDP for at least 6 months. The median time from CIDP diagnosis to trial enrolment was 2.9 years (range 0.6 to 29) for those who received placebo and 3.6 (range 0.4 to 23) for those who received IFNb‐1a. Participants met the following criteria. 1. Fulfilled the modified Inflammatory Neuropathy Cause and Treatment (INCAT) neurophysiological criteria for CIDP, or had CIDP with a motor deficit responsive to IVIg and alternative electrophysiologic data justifying inclusion, or had supportive pathologic or laboratory data. 2. Prior to screening had documented evidence of loss of muscle strength associated with CIDP, such that the MRC sum score was less than or equal to 58. 3. Were negative for IgM monoclonal gammopathy or positive for IgM monoclonal gammopathy but MAG‐antibody negative and proven to be IVIg responsive. 4. Were clinically stable while on a constant regimen of IVIg in the 3 months prior to screening. Exclusion criteria Associated systemic disorder that might cause neuropathy. Diabetes mellitus, unless well‐controlled, with no retinopathy or nephropathy, and with a normal sensory nerve action potential (SNAP) amplitude recorded in the sural nerve on at least one side of the body. History of a seizure disorder. Pure motor syndrome fulfilling criteria for multifocal motor neuropathy with conduction block or pure sensory CIDP, or any other variant of CIDP without motor involvement. History of (or abnormal laboratory values indicative of) any significant major organ system disease, or known drug hypersensitivity that precluded IM IFNb‐1a administration. Serious local or systemic infection within the 6 months prior to screening. History of suicidal ideation or severe depression within 3 months of baseline. Use of IFNb at any time, or use of plasma exchange, plasmapheresis, or any immunosuppressant within 6 months prior to screening. |
|
| Interventions | Intramuscular IFNb‐1a 30 μg once weekly, 60 μg once weekly, 30 μg twice weekly, or 60 μg twice weekly for 32 weeks, or placebo. | |
| Outcomes | Total IVIg dose (g/kg) administered in the 16 weeks after 16‐week visit (from weeks 16 to 32). | |
| Additional outcomes not specified in review | Total IVIg dose (g/kg) administered from week 16 to week 32 was slightly lower in the combined IFNb‐1a groups (1.20 g/kg) compared with the placebo group (1.34 g/kg, P = 0.75). | |
| Notes | Full trial data made available by the authors This trial is that referred to in ClinicalTrials.gov as NCT00099489 |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation took place across all investigational sites using a centralised interactive voice response system (IVRS) at randomisation. |
| Allocation concealment (selection bias) | Low risk | At randomisation the IVRS assigned a unique 6 digit identification number to each participant. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | To maintain the double blind status of the study, all participants received 2 injections per week. Consequently, participants allocated to a once weekly active treatment also received placebo once weekly. All drugs were packaged identically. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | To maintain the double blind status of the study, all participants received 2 injections per week. Consequently, participants allocated to a once weekly active treatment also received placebo once weekly. All drugs were packaged identically. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 34/45 (76%) IFN beta‐1a and 19/22 (86%) placebo participants completed the study. In the combined intramuscular IFN beta‐1a groups, 31% of participants discontinued the study drug but completed study evaluations vs 14% in the placebo group. The main reasons for study drug discontinuation were adverse events (11%) and voluntary withdrawal (11%) in the combined IFN beta‐1a groups, and adverse events (5%), voluntary withdrawal (5%), and worsening of disease (5%) in the placebo group. The main reasons for study withdrawal were adverse events (9%) and voluntary withdrawal (7%) in the combined IFNb‐1a groups, and adverse events (5%), voluntary withdrawal (5%), and worsening of disease (5%) in the placebo group. 80% of participants completed at least 28 weeks of the study and 71% received at least 87.5% of their expected dose (56 of 64 injections of study drug). 4 non‐compliant participants (2 on IFN beta‐1a, 2on placebo) were excluded from efficacy analysis but included in the safety analysis. |
| Selective reporting (reporting bias) | Low risk | The trial reported all pre‐planned primary and secondary outcomes. |
| Other bias | Low risk | None identified. |
RMC 2009.
| Methods | Randomised, double blind, placebo controlled. | |
| Participants | 60 adults with CIDP (diagnosed by a consultant neurologist) with or without paraprotein (but not anti‐myelin‐associated‐glycoprotein antibodies), who were receiving corticosteroids or IVIg or both. "Patients had to have met neurophysiological criteria for definite or probably CIDP (EFNS/PNS 2005; Hughes 2001) within the previous 3 years." "Patients must have responded to and still be receiving intravenous immunoglobulin (equivalent to at least 0.4 g/kg every 4 weeks and given at least every 8 weeks) or corticosteroids (equivalent to at least 15 mg daily prednisolone). The dose must have been stable (within 25%) for at least 12 weeks and not changed for 4 weeks. Patients had to have at least moderate limitations in arms or legs (overall neuropathy limitations scale [ONLS] ≥2) and Medical Research Council (MRC) grade 4 or less weakness in at least one muscle at baseline or after reduction of corticosteroid or intravenous immunoglobulin dose at sometime in the previous 12 months." Exclusion criteria "'neurogenic sphincter disturbance; pregnancy; planning for pregnancy; breastfeeding, or unwillingness to practise contraception; severe concurrent medical disorders that would prevent treatment or assessment, including significant haematological, renal, or liver‐function disorders (e.g. serum liver enzyme concentrations more than twice the upper limit of normal) or abnormalities on chest radiography, vasculitis, and haematological and non‐haematological malignant disease; and alternative causes of peripheral neuropathy, such as drug or toxin induced or hereditary neuropathy, HIV infection, Lyme disease, chronic active hepatitis, systemic lupus erythematosus and IgM paraprotein with antibodies to myelin associated glycoprotein; and multifocal motor neuropathy fulfilling European Federation of Neurological Sciences/Peripheral Nerve Society criteria (EFNS/PNS 2006). Patients with diabetes mellitus or IgG, IgA, and IgM paraproteins without antibodies to myelin‐associated glycoprotein were not excluded. Additional exclusion criteria were immunomodulatory treatment other than intravenous immunoglobulin or corticosteroids during the previous 12 weeks, treatment with methotrexate at any time, and participation in a controlled trial of any investigational drug within the previous 12 weeks. Patients with atypical CIDP with pure sensory or persistent unifocal impairment or significant CNS involvement were also excluded." |
|
| Interventions | Methotrexate 7.5 mg weekly for 4 weeks, then 10 mg weekly for 4 weeks, then 15 mg weekly for 32 weeks, or placebo. Both groups received folic acid 5 mg twice weekly. | |
| Outcomes | Primary: percentage reduction in mean dose of corticosteroids or IVIg in weeks 37 to 40 compared with weeks 1 to 4. Secondary: change in expanded MRC sum score, Overall Neuropathy Limitation Score and Amsterdam Linear Disability Score from baseline to week 16 and to week 40. |
|
| Additional outcomes not specified in review | In the analysis of the primary outcome measure, the results were dichotomised into non‐responders (reduction of corticosteroid or IVIg dose by 20% or less) and responders (reduction of corticosteroid or IVIg dose by more than 20%). There were 14 of 27 (52%) responders in the methotrexate group and 14 of 32 in the placebo group (44%), adjusted odds ratio 1.21 (95% CI 0.40 to 3.70). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The trial statistician prepared a computer‐generated list of random assignments stratified by centre and in blocks in order to allocate participants to methotrexate or placebo in a balanced way within centres. |
| Allocation concealment (selection bias) | Low risk | Each trial pharmacy dispensed treatment packs according to the randomisation allocation sequence. Treatment packs were supplied in separate packs for each 4‐week period in the trial labelled with a unique code for that participant. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | The active medication and placebo were presented as tablets of identical colour and external markings. Treatment packs were supplied in separate packs for each 4‐week period in the trial labelled with a unique code for that participant. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Disability scales and impairment measures were collected by a blinded neurologist or other trained health professional at scheduled outpatient visits. This individual did not have access to the laboratory records and did not ask questions about possible side effects. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no follow‐up measurements for one participant. |
| Selective reporting (reporting bias) | Low risk | All the primary and secondary outcomes pre‐specified in the study protocol were reported. |
| Other bias | High risk | The protocol specified a gradual reduction of concomitant IVIg and/or corticosteroid treatment after about 16 weeks which may have biased interpretation of changes in disability or impairment after that time. |
IFN beta: interferon beta; i.m.: intramuscular
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Alemtuzumab trial | Study failed to recruit any participants and was discontinued. Information from chief investigator Dr David Cornblath on 12 July 2016 |
| Lipoic acid trial | Study failed to recruit participants and was discontinued. No results available. Information from chief investigator Dr Lou Jau‐Shin jaushin.lou@med.und.edu on 11 July 2016 |
Characteristics of studies awaiting assessment [ordered by study ID]
Hu 2009.
| Methods | Randomised study |
| Participants | 60 |
| Interventions | Gullong tongluo (Chinese herbal remedy) |
| Outcomes | Changes before and after 3 months of treatment in terms of muscle force, functional and sensory disturbance of extremities, as well as scoring by Activity of Daily Living Scale (ADL) and electromyogram (EMG) for nerve conduction velocity |
| Note | ‐ |
| Notes | ‐ |
Characteristics of ongoing studies [ordered by study ID]
FORCIDP trial.
| Trial name or title | Evaluate efficacy and safety of fingolimod 0.5 mg orally once daily versus placebo in chronic inflammatory demyelinating polyradiculoneuropathy patients. |
| Methods | Double‐blind, randomised, multicentre, placebo‐controlled, parallel‐group study. |
| Participants | "Confirmed diagnosis of chronic inflammatory demyelinating polyradiculoneuropathy and treated with IVIg, corticosteroids, or both therapies prior to study entry" |
| Interventions | Oral fingolimod (0.5 mg/day) or matching placebo in a ratio of 1:1. The IVIg or corticosteroids are stopped or tailed off when the participant is randomised. |
| Outcomes | The primary outcome measure is time to first confirmed worsening on the adjusted INCAT Disability Scale by 1 point or more from the value at baseline, in people being treated with IVIg and/or corticosteroids prior to the study start. |
| Starting date | November 2012. |
| Contact information | Novartis Pharmaceuticals +1(862)778‐8300 |
| Notes | Clinicaltrials.gov NCT01625182 |
Haematopoietic stem cell transplantation trial.
| Trial name or title | Hematopoetic stem cell transplantation in chronic inflammatory demyelinating polyneuropathy. |
| Methods | Non‐randomised study |
| Participants | 50 |
| Interventions | Non‐myeloablative autologous hematopoetic stem cell transplant. |
| Outcomes | Time to worsening as the primary outcome. |
| Starting date | January 1996. |
| Contact information | Dr Spahovic: d‐spahovic@northwestern.edu |
| Notes | Clinicaltrials.gov NCT00278629 |
Japanese cyclosporin trial.
| Trial name or title | Cyclosporin A (Neoral) for chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) requiring repetitive administration of high‐dose intravenous immunoglobulin. |
| Methods | Parallel group, open, randomised. |
| Participants | "Definite or probable CIDP according to classification criteria of the EFNS/PNS CIDP guideline" |
| Interventions | "Cyclosporin A is given orally at an initial dose of 3 mg/kg/day. The dosage of cyclosporine A is adjusted to keep a serum trough concentration between 100 and 150 ng/ml. Administration of high‐dose intravenous immunoglobulin (0.4g/kg) is continued same as observation period" |
| Outcomes | Primary outcomes: neurological symptom; frequency of high‐dose intravenous immunoglobulin; toxicity. |
| Starting date | 1 August 2006 |
| Contact information | Shu‐ichi Ikeda 0263‐37‐2671; hmorita@hsp.md.shinshu‐u.ac.jp |
| Notes | ICTRP Search Portal JPRN‐UMIN000000674 |
MYCOPID trial.
| Trial name or title | Does the mycophenolate improve the ability of weaning patients off the treatment in chronic inflammatory demyelinating polyradiculopathy (CIDP) |
| Methods | Randomised, multicentre, double‐blind, placebo‐controlled, parallel‐assignment, efficacy study. |
| Participants | Definite or probable CIDP according to EFNS/PNS criteria, or atypical CIDP. Participants need to meet clinical EFNS/PNS criteria and at least 2 criteria among the EFNS/PNS supplementary criteria. |
| Interventions | Oral mycophenolate mofetil 2 g/day or placebo. The dose of IVIg is tapered until complete withdrawal. |
| Outcomes | Primary outcome: occurrence of a relapse during the tapering‐off period (up to 18 months after baseline) or after withdrawal during the monitoring period.
Secondary outcomes (with time frame): Proportion of withdrawn participants (6 months after the withdrawal). Proportion of withdrawn participants at the end of the study (24 months). Sparing treatment (composite criteria) (24 months): extension of the mean interval between IVIg courses at month 12 and month 24 compared to baseline, reduction of the total cumulative dose of IVIg at month 12 and month 24 in the mycophenolate group. Time to reach withdrawal (24 months). Visual analog scale (VAS) pain score (12 and 24 months). Overall Neuropathy Limitations Scale (ONLS) 12 and 24 months). Rasch‐built Overall Disability Scale (R‐ODS) (12 and 24 months). Medical Research Council (MRC) scale (12 and 24 months). Inflammatory Neuropathy Cause and Treatment (INCAT) sensory test (12 and 24 months). 10 meters test (12 and 24 months). Short‐Form 36 Health Survey (SF‐36) quality of life scale (12 and 24 months). Nottingham quality of life scale (12 and 24 months). Global cost (24 months): comparison of the global cost in each group. Occurrence of a relapse during the tapering‐off period (up to 18 months after baseline) or after the withdrawal during the monitoring period (the withdrawal is defined by the ability to reach the last day of IVIG treatment). |
| Starting date | November 2013 |
| Contact information | Karine Viala Karine.viala@psl.aphp.fr |
| Notes | NCT02494505 |
EFNS/PNS: European Federation of Neurological Societies/Peripheral Nerve Society; IVIg: intravenous immunoglobulin; INCAT: Inflammatory Neuropathy Cause And Treatment; IVIg: intravenous immunoglobulin
Differences between protocol and review
In the 2010 update, we accepted the EFNS/PNS 2005 criteria as adequate for diagnosis, the Overall Neuropathy Limitations Scale as the preferred disability scale (Graham 2006), and 24‐week outcomes in the absence of the 12‐month outcomes which we would have preferred. We added 'Risk of bias' and 'Summary of findings' tables. MM‐R joined the review team for the 2010 update of the review.
In the 2014 update we updated the Methods section to comply with current Cochrane reporting standards.
Dr Tony Swan, an author of the protocol, withdrew from authorship of the review. In the 2016 update RB and AAG joined the review team and we added an extra search for non‐randomised studies.
Contributions of authors
MM‐R wrote the first draft of the 2010, 2013 and 2014 updates of this review. PAvD commented on all versions of this review. RACH wrote the first draft of previous versions and edited the subsequent updates. The Managing Editor for Cochrane Neuromuscular (RB) assisted with revising the text to meet current methodological and reporting requirements. The Information Specialist for Cochrane Neuromuscular (AAG) conducted database searches. All authors approved the final version of each update.
Sources of support
Internal sources
King's College London School of Medicine, UK.
University Hospital Rotterdam/Erasmus University Medical Center Rotterdam, Netherlands.
-
GBS/CIDP Foundation International, USA.
Funding for MM‐R
External sources
Donation from the late Mr Chris Lazari and Mrs Lazari, UK.
Declarations of interest
MM‐R was an investigator and trial coordinator for RMC 2009 and has received a travel grant from Grifols.
AAG is the Information Specialist of the Cochrane Neuromuscular. She has no commercial conflicts of interest.
RB is the Managing Editor of Cochrane Neuromuscular. She has no commercial conflicts of interest. She played no role in the later stages of the editorial process in accordance with Cochrane policy (Cochrane 2016).
PAvD participated in RMC 2009. He and his institution are in receipt of funding from Talecris and CSL Behring in relation to serving on the scientific board of the ICE trial in chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) and scientific board on IVIg in chronic polyneuropathy. His institution is in receipt of a grant from Baxter to conduct a randomised controlled trial (RCT) comparing intravenous immunoglobulin (IVIg) with IVIg and steroids in Guillain‐Barré Syndrome (GBS), a grant from Sanquin to conduct a RCT investigating the effect of a second course of IVIg (SID‐trial) in people with GBS with a poor prognosis and a grant from Talecris to conduct a prospective international study on the effect of a second course of IVIg in people with GBS with a poor prognosis (I‐SID study).
RACH was the principal investigator of an investigator‐led trial of IFNb‐1a in CIDP (Hadden 1999) which was funded by Serono, a Biogen sponsored trial of IFNb‐1a for CIDP (Hughes 2010), and an investigator‐led trial of methotrexate for CIDP (RMC 2009), all of which are included in this review. He is chair of the steering committee for the ongoing fingolimod trial (FORCIDP trial). RACH has consulted or is consulting for Baxter, CSL Behring, Grifols, LFB and Octapharma which all manufacture human immunoglobulin, an alternative treatment for CIDP not considered in this review. RACH has consulted for Biogen and Serono which manufacture BIFN‐1a, which is considered in this review with the conclusion that it is not effective. RACH is also consulting for Novartis, which is conducting a trial of fingolimod in CIDP (FORCIDP trial). He will demit authorship of this review when fingolimod is considered. RACH is an honorary member of the Board of GBS CIDP Foundation International and Medical Patron of GAIN, the British charity which cares for CIDP. RACH is a member of the Cochrane Neuromuscular Editorial Board. He did not participate in the editorial process for this review.
The review is not compliant with the Cochrane Commerical Sponsorship policy. The update willl have a majority of authors and lead author free of conflicts.
Edited (no change to conclusions)
References
References to studies included in this review
Dyck 1985 {published data only}
- Dyck PJ, O'Brien P, Swanson C, Low P, Daube J. Combined azathioprine and prednisone in chronic inflammatory demyelinating polyneuropathy. Neurology 1985;35(8):1173‐6. [PUBMED: 4022350] [DOI] [PubMed] [Google Scholar]
Hadden 1999 {published data only}
- Hadden RD, Sharrack B, Bensa S, Soudain SE, Hughes RA. Randomized trial of interferon beta‐1a in chronic inflammatory demyelinating polyradiculoneuropathy. Neurology 1999;53(1):57‐61. [PUBMED: 10408537] [DOI] [PubMed] [Google Scholar]
Hughes 2010 {published and unpublished data}
- Gorson K, Hughes R, Cros D, Pollard J, Vallat J, Riester K, et al. Efficacy of interferon beta‐1a in patients chronic inflammatory demyelinating polyradiculoneuropathy (CIDP). Neurology 2008;70(11 Suppl 1):A369. [DOI] [PubMed] [Google Scholar]
- Gorson KC, Hughes RAC, Cros DP, Pollard JD, Vallat J‐M, Riester K, et al. Efficacy of interferon beta1A in patients with chronic inflammatory demyelinating polyneuropathy (CIDP). Journal of the Peripheral Nervous System 2008;13(2):170. [Google Scholar]
- Hughes RAC, Gorson KC, Cros D, Griffin J, Pollard J, Vallat JM, et al. Avonex CIDP Study Group. Intramuscular interferon beta‐1a in chronic inflammatory demyelinating polyradiculoneuropathy. Neurology 2010;74(8):651‐7. [PUBMED: 20177118] [DOI] [PubMed] [Google Scholar]
RMC 2009 {published data only}
- EUCTR2005‐003382‐16‐GB. Pilot randomised controlled trial of methotrexate for chronic inflammatory demyelinating polyradiculoneuropathy (RMC trial). ‐ RMC Trial. apps.who.int/trialsearch/Trial2.aspx?TrialID=EUCTR2005‐003382‐16‐GB (accessed 4 December 2015).
- Mahdi‐Rogers M, for the Randomised Methotrexate Chronic Inflammatory Demyelinating Polyradiculoneuropathy (RMC) Trial Group. A pilot randomised controlled trial of methotrexate for CIDP: Lessons for future trials. Journal of the Peripheral Nervous System : JPNS 2008;13(2):176. [Google Scholar]
- Pace AA, Hughes RAC, Schaik IN, Hobart JC, for the RMC Trial Group. Randomised trial of methotrexate in CIDP (RMC) study: Did the measurement of strength prove to be a weakness?. Journal of the Peripheral Nervous System : JPNS 2009;14(Suppl s2):116‐7. [Google Scholar]
- RMC trial Group. Randomised controlled trial of methotrexate for chronic inflammatory demyelinating polyradiculoneuropathy (RMC trial): a pilot, multicentre study. Lancet Neurology 2009; Vol. 8, issue 2:158‐64. [PUBMED: 19136303] [DOI] [PubMed]
References to studies excluded from this review
Alemtuzumab trial {published data only}
- NCT01757574. Alemtuzumab in chronic inflammatory demyelinating polyradiculoneuropathy (CIDP). clinicaltrials.gov/show/NCT01757574 (accessed 10 January 2013; December 2015).
Lipoic acid trial {published data only}
- NCT00962429. Lipoic acid to treat chronic inflammatory demyelinating polyneuropathy—a randomised, double‐blind, placebo controlled pilot study. clinicaltrials.gov/show/NCT00962429 (accessed 10 July 2016).
References to studies awaiting assessment
Hu 2009 {published data only}
- Hu JY, Chen JL, Zhang ZH. Clinical observation on treatment of 10 patients with chronic inflammatory demyelinating polyneuropathy by gullong tongluo capsule combined with prednisone. Zhongguo Zhong Xi Yi Jie He Za Zhi (Chinese) July 2009;29(7):649‐51. [PubMed] [Google Scholar]
References to ongoing studies
FORCIDP trial {published data only}
- Hartung H, Sobue G, Dalakas M, Latov N, Leger JM, Merkies I, et al. Fingolimid (FTY720) oral for the treatment of chronic inflammatory demyelinating polyradiculoneuropathy (CIDP): study design of the phase 3 FORCIDP trial. Neurology 2014;82(Suppl):Poster no: P6.103. [Google Scholar]
- Hughes R, Dalakas M, Latov N, Léger J‐M, Merkies I, Nobile‐Orazio E, et al. Fingolimod (FTY720) oral for the treatment of chronic inflammatory demyelinating polyradiculoneuropathy (CIDP): Study design of the phase 3 FORCIDP trial. Journal of the Neurological Sciences 2013;333(Suppl 1):e361. [EMBASE: 71188889] [Google Scholar]
- Hughes RAC, Dalakas M, Latov N, Leger J‐M, Merkies ISJ, Nobile‐Orazio E, et al. Oral fingolimod (FTY720) for the treatment of chronic inflammatory demyelinating polyradiculoneuropathy (CIDP): Study design of the phase 3 FORCIDP trial. Journal of the Peripheral Nervous System : JPNS 2013;18(Suppl s2):S48‐9. [EMBASE: 71124949] [Google Scholar]
- NCT01625182. Evaluate efficacy and safety of fingolimod 0.5 mg orally once daily versus placebo in chronic inflammatory demyelinating polyradiculoneuropathy patients. clinicaltrials.gov/show/NCT01625182 (accessed 10 January 2013; December 2015).
Haematopoietic stem cell transplantation trial {published data only}
- NCT00278629. Hematopoetic stem cell transplantation in chronic inflammatory demyelinating polyneuropathy. clinicaltrials.gov/show/NCT00278629 (accessed 10 January 2013; December 2015).
Japanese cyclosporin trial {published data only}
- JPRN‐UMIN000000674. Cyclosporin A (Neoral) for chronic inflammatory demyelinating polyradiculoneuropathy (CIDP) requiring repetitive administration of high‐dose intravenous immunoglobulin. apps.who.int/trialsearch/Trial2.aspx?TrialID=JPRN‐UMIN000000674 (accessed 4 December 2015).
MYCOPID trial {published data only}
- NCT012494505. Interest of Mycophenolate for CIDP Weaning (MYCOPID). clinicaltrials.gov/ct2/show/NCT02494505 (accessed 16 June 2016).
Additional references
Ad Hoc 1991
- Ad Hoc Subcommittee of the American Academy of Neurology AIDS Task Force. Research criteria for the diagnosis of chronic inflammatory demyelinating polyradiculoneuropathy (CIDP). Neurology 1991;41(5):617‐8. [PUBMED: 2027473] [PubMed] [Google Scholar]
Ahlmén 1998
- Ahlmén J, Andersen O, Hallgren G, Peilot B. Positive effect of tacrolimus (FK 506) in a case of CIDP. 3rd International Conference on New Trends in Clinical and Experimental Immunosuppression 1998:110.
Ahmed 2011
- Ahmed Z, Powell R, Llewelyn G, Anstey A. Chronic inflammatory demyelinating polyradiculoneuropathy complicating anti TNF α therapy for chronic plaque psoriasis. BMJ Case Reports 2011, issue pii: bcr0820114674. [DOI: 10.1136/bcr.08.2011.4674; PUBMED: 22674940] [DOI] [PMC free article] [PubMed]
Allen 2013
- Allen JA, Sufit R, Ajroud‐Driss S, Burt R. Nonmyeloablative autologous hematopoetic stem cell transplant for the treatment of CIDP: An interim report. Journal of the Peripheral Nervous System : JPNS 2013;18(Suppl):S5. [Google Scholar]
Alshekhlee 2010
- Alshekhlee A, Basiri K, Miles JD, Ahmad SA, Katirji B. Chronic inflammatory demyelinating polyneuropathy associated with tumor necrosis factor‐alpha antagonists. Muscle & Nerve 2010;41(5):723‐7. [PUBMED: 20405504] [DOI] [PubMed] [Google Scholar]
Austin 1958
- Austin JH. Recurrent polyneuropathies and their corticosteroid treatment; with five‐year observations of a placebo‐controlled case treated with corticotrophin, cortisone, and prednisone. Brain 1958;81(2):157‐92. [PUBMED: 13572689] [DOI] [PubMed] [Google Scholar]
Axelson 2008
- Axelson HW, Oberg G, Askmark H. Successful repeated treatment with high dose cyclophosphamide and autologous blood stem cell transplantation in CIDP. Journal of Neurology, Neurosurgery, and Psychiatry 2008;79(5):612‐4. [PUBMED: 18408093] [DOI] [PubMed] [Google Scholar]
Barnett 1998
- Barnett MH, Pollard JD, Davies L, McLeod JG. Cyclosporin A in resistant chronic inflammatory demyelinating polyradiculoneuropathy. Muscle & Nerve 1998;21(4):454‐60. [PUBMED: 9533779] [DOI] [PubMed] [Google Scholar]
Barohn 1989
- Barohn RJ, Kissel JT, Warmolts JR, Mendell JR. Chronic inflammatory demyelinating polyradiculoneuropathy. Clinical characteristics, course, and recommendations for diagnostic criteria. Archives of Neurology 1989;46(8):878‐84. [PUBMED: 2757528] [DOI] [PubMed] [Google Scholar]
Barreira 2007
- Barreira AA, Marques W Jr, Rezende C, Souza DGB, Voltarelli J. Autologous stem cell transplantation of a patient with chronic demyelinating neuropathy and persistent conduction block. Journal of the Peripheral Nervous System : JPNS 2007;12(s1):7. [Google Scholar]
BDMSATG 1988
- British and Dutch Multiple Sclerosis Azathioprine Trial Group. Double‐masked trial of azathioprine in multiple sclerosis. Lancet 1988;2(8604):179‐83. [PUBMED: 2899660] [PubMed] [Google Scholar]
Bedi 2010
- Bedi G, Brown A, Tong T, Sharma KR. Chronic inflammatory demyelinating polyneuropathy responsive to mycophenolate mofetil therapy. Journal of Neurology, Neurosurgery, and Psychiatry 2010;81(6):634‐6. [PUBMED: 20176598] [DOI] [PubMed] [Google Scholar]
Benedetti 2004
- Benedetti L, Grandis M, Nobbio L, Beronio A, Ghiglione E, Manzino M, et al. Mycophenolate mofetil in dysimmune neuropathies: a preliminary study. Muscle & Nerve 2004;29(5):748‐9. [PUBMED: 15116381] [DOI] [PubMed] [Google Scholar]
Benedetti 2008
Benedetti 2011
- Benedetti L, Briani C, Franciotta D, Fazio R, Paolasso I, Comi C, et al. Rituximab in patients with chronic inflammatory demyelinating polyradiculoneuropathy: a report of 13 cases and review of the literature. Journal of Neurology, Neurosurgery, and Psychiatry 2011;82(3):306‐8. [PUBMED: 20639381] [DOI] [PubMed] [Google Scholar]
BNF 2014
- Joint Formulary Committee. British National Formulary. 8th Edition. London: BMJ Group and Pharmaceutical Press, September 2014. [Google Scholar]
Bodley‐Scott 2005
- Bodley‐Scott DD. Chronic inflammatory demyelinating polyradiculoneuropathy responding to rituximab. Practical Neurology 2005;5(4):242‐5. [DOI: 10.1111/j.1474-7766.2005.00328.x; EMBASE: 2005372287 ] [DOI] [Google Scholar]
Bouchard 1999
- Bouchard C, Lacroix C, Planté V, Adams D, Chedru F, Guglielmi JM, et al. Clinicopathologic findings and prognosis of chronic inflammatory demyelinating polyneuropathy. Neurology 1999;52(3):498‐503. [PUBMED: 10025777] [DOI] [PubMed] [Google Scholar]
Brannagan 2002
- Brannagan TH, Pradhan A, Heiman‐Patterson T, Winkelman AC, Styler MJ, Topolsky DL, et al. High‐dose cyclophosphamide without stem‐cell rescue for refractory CIDP. Neurology 2002;58(12):1856‐8. [PUBMED: 12084892] [DOI] [PubMed] [Google Scholar]
Briani 2004
- Briani C, Zara G, Zambello R, Trentin L, Rana M, Zaja F. Rituximab‐responsive CIDP. European Journal of Neurology 2004;11(11):788. [PUBMED: 15525302] [DOI] [PubMed] [Google Scholar]
Camdessanché 2013
- Camdessanché J‐P, Ferraud K, Lagrange E, Viala K, Chan V, Guy N, et al. Multicentre, randomised, open‐label trial to compare efficacy and tolerance of corticosteroids and IVIg in patients with CIDP on a one‐year follow‐up. Journal of the Peripheral Nervous System : JPNS 2013;18(Suppl):S17‐8. [Google Scholar]
Chaudhry 2001
- Chaudhry V, Cornblath DR, Griffin JW, O'Brien R, Drachman DB. Mycophenolate mofetil: a safe and promising immunosuppressant in neuromuscular diseases. Neurology 2001;56(1):94‐6. [PUBMED: 11148242] [DOI] [PubMed] [Google Scholar]
Chin 2003
- Chin RL, Sherman WH, Sander HW, Hays AP, Latov N. Etanercept (Enbrel) therapy for chronic inflammatory demyelinating polyneuropathy. Journal of the Neurological Sciences 2003;210(1‐2):19‐21. [PUBMED: 12736082] [DOI] [PubMed] [Google Scholar]
Chiò 2007
- Chiò A, Cocito D, Bottacchi E, Buffa C, Leone M, Plano F, et al. Idiopathic chronic inflammatory demyelinating polyneuropathy: an epidemiological study in Italy. Journal of Neurology, Neurosurgery, and Psychiatry 2007; Vol. 78, issue 12:1349‐53. [PUBMED: 17494979] [DOI] [PMC free article] [PubMed]
Choudhary 1995
- Choudhary PP, Thompson N, Hughes RAC. Improvement following interferon beta in chronic inflammatory demyelinating polyradiculoneuropathy. Neurology 1995;242(4):252‐3. [PUBMED: 7798127] [DOI] [PubMed] [Google Scholar]
Cochrane 2016
- Cochrane Editorial Unit. Cochrane Editorial and Publishing Resource (updated May 2015). (community.cochrane.org/editorial‐and‐publishing‐policy‐resource) (accessed 31 March 2017).
Cocito 2011
- Cocito D, Grimaldi S, Paolasso I, Falcone Y, Antonini G, Benedetti L, et al. Italian Network for CIDP Register. Immunosuppressive treatment in refractory chronic inflammatory demyelinating polyradiculoneuropathy. A nationwide retrospective analysis. European Journal of Neurology 2011;18(12):1417‐21. [PUBMED: 21819489] [DOI] [PubMed] [Google Scholar]
Confavreux 1996
- Confavreux C, Saddier P, Grimaud J, Moreau T, Adeleine P, Aimard G. Risk of cancer from azathioprine therapy in multiple sclerosis: a case‐control study. Neurology 1996;46(6):1607‐12. [PUBMED: 8649558] [DOI] [PubMed] [Google Scholar]
D'Amico 2012
- D'Amico A, Catteruccia M, Benedetti F, Vivarelli M, Colucci M, Cascioli S, et al. Rituximab in a childhood‐onset idiopathic refractory chronic inflammatory demyelinating polyneuropathy. European Journal of Paediatric Neurology: EJPN 2012;16(3):301‐3. [PUBMED: 21903431] [DOI] [PubMed] [Google Scholar]
Dalakas 1981
- Dalakas MC, Engel WK. Chronic relapsing (dysimmune) polyneuropathy: pathogenesis and treatment. Annals of Neurology 1981;9(Suppl):134‐45. [PUBMED: 7224612] [DOI] [PubMed] [Google Scholar]
Dalakas 2009
Draak 2014
- Draak TH, Vanhoutte EK, Nes SI, Gorson KC, Pol WL, Notermans NC, et al. Changing outcome in inflammatory neuropathies: Rasch‐comparative responsiveness. Neurology 2014;83(23):2124‐32. [PUBMED: 25378677 ] [DOI] [PubMed] [Google Scholar]
Dyck 1975
- Dyck PJ, Lais AC, Ohta M, Bastron JA, Okazaki H, Groover RV. Chronic inflammatory polyradiculoneuropathy. Mayo Clinic Proceedings 1975;50(11):621‐37. [PUBMED: 1186294] [PubMed] [Google Scholar]
Dyck 1986
- Dyck PJ, Daube J, O'Brien P, Pineda A, Low PA, Windebank AJ, et al. Plasma exchange in chronic inflammatory demyelinating polyradiculoneuropathy. New England Journal of Medicine 1986;314(8):461‐5. [PUBMED: 3511382] [DOI] [PubMed] [Google Scholar]
Dyck 1994
- Dyck PJ, Litchy WJ, Kratz KM, Suarez GA, Low PA, Pineda AA, et al. A plasma exchange versus immune globulin infusion trial in chronic inflammatory demyelinating polyradiculoneuropathy. Annals of Neurology 1994;36(6):838‐45. [PUBMED: 7998769] [DOI] [PubMed] [Google Scholar]
Dyck 2005
- Dyck PJ, Hughes RAC, O'Brien PC. Quantitating overall neuropathic symptoms, impairments, and outcomes. In: Dyck PJ, Thomas PK editor(s). Peripheral Neuropathy. 4th Edition. Philadelphia: Elsevier Saunders, 2005:1031‐52. [Google Scholar]
Díaz‐Manera 2009
- Díaz‐Manera J, Rojas‐García R, Gallardo E, Illa I. Response to methotrexate in a chronic inflammatory demyelinating polyradiculoneuropathy patient. Muscle & Nerve 2009;39(3):386‐8. [PUBMED: 19208395] [DOI] [PubMed] [Google Scholar]
Echaniz‐Laguna 2012
- Echaniz‐Laguna A, Séze J, Chanson JB. Chronic inflammatory demyelinating polyradiculoneuropathy in solid organ transplant recipients: a prospective study. Journal of Neurology, Neurosurgery, and Psychiatry 2012;83(7):699‐705. [PUBMED: 22577230 ] [DOI] [PubMed] [Google Scholar]
EFNS/PNS 2005
- Joint Task Force of the European Federation of Neurological Societies and the Peripheral Nerve Society. European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Journal of the Peripheral Nervous System : JPNS 2005;10(3):220‐8. [PUBMED: 16221283] [DOI] [PubMed] [Google Scholar]
EFNS/PNS 2006
- European Federation of Neurological Societies/Peripheral Nerve Society Guideline on management of multifocal motor neuropathy. Report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society. Journal of the Peripheral Nervous System : JPNS 2006; Vol. 11, issue 1:1‐8. [PUBMED: 16519777] [DOI] [PubMed]
EFNS/PNS 2010
- Bergh PY, Hadden RD, Bouche P, Cornblath DR, Hahn A, Illa I, et al. European Federation of Neurological Societies, Peripheral Nerve Society. European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society ‐ first revision. Journal of the Peripheral Nervous System : JPNS 2010; Vol. 17, issue 3:356‐63. [PUBMED: 20456730] 20456730
Eftimov 2013
- Eftimov F, Winer JB, Vermeulen M, Haan R, Schaik IN. Intravenous immunoglobulin for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database of Systematic Reviews 2013, Issue 12. [DOI: 10.1002/14651858.CD001797.pub3] [DOI] [PubMed] [Google Scholar]
ENMC 2005
- Hughes RAC on behalf of the CIDP and MMN Trial Group. 129th ENMC International Workshop: Clinical Trials for Chronic Inflammatory Demyelinating Polyradiculoneuropathy and Multifocal Motor Neuropathy, 27th October 2004, Schiphol airport, The Netherlands. Neuromuscular Disorders : NMD 2005;15(4):321‐5. [PUBMED: 15792873] [DOI] [PubMed] [Google Scholar]
Erdener 2014
- Erdener SE, Nurlu G, Gocmen R, Erdem‐Ozdamar S, Kurne A. Remission with fingolimod in a case of demyelinating polyneuropathy. Muscle & Nerve 2014;50(4):615‐7. [PUBMED: 24909140] [DOI] [PubMed] [Google Scholar]
Fialho 2006
- Fialho D, Chan YC, Allen DC, Reilly MM, Hughes RA. Treatment of chronic inflammatory demyelinating polyradiculoneuropathy with methotrexate. Journal of Neurology, Neurosurgery, and Psychiatry 2006; Vol. 77, issue 4:544‐7. [PUBMED: 16543541 ] [DOI] [PMC free article] [PubMed]
Gladstone 2005
- Gladstone DE, Prestrud AA, Brannagan TH 3rd. High‐dose cyclophosphamide results in long‐term disease remission with restoration of a normal quality of life in patients with severe refractory chronic inflammatory demyelinating polyneuropathy. Journal of the Peripheral Nervous System : JPNS 2005;10(1):11‐6. [PUBMED: 15703014 ] [DOI] [PubMed] [Google Scholar]
Good 1998
- Good JL, Chehrenama M, Mayer RF, Koski CL. Pulse cyclophosphamide therapy in chronic inflammatory demyelinating polyneuropathy. Neurology 1998;51(6):1735‐8. [PUBMED: 9855536] [DOI] [PubMed] [Google Scholar]
Gorson 1997
- Gorson KC, Allam G, Simovic D, Ropper AH. Improvement following interferon‐alpha 2A in chronic inflammatory demyelinating polyneuropathy. Neurology 1997;48(3):777‐80. [PUBMED: 9065566] [DOI] [PubMed] [Google Scholar]
Gorson 2004
- Gorson KC, Amato AA, Ropper AH. Efficacy of mycophenolate mofetil in patients with chronic immune demyelinating polyneuropathy. Neurology 2004;63(4):715‐7. [PUBMED: 15326250] [DOI] [PubMed] [Google Scholar]
Gorson 2007
- Gorson KC, Natarajan N, Ropper AH, Weinstein R. Rituximab treatment in patients with IVIg‐dependent immune polyneuropathy: a prospective pilot trial. Muscle & Nerve 2007;35(1):66‐9. [PUBMED: 16967492] [DOI] [PubMed] [Google Scholar]
Graham 2006
- Graham RC, Hughes RA. A modified peripheral neuropathy scale: the Overall Neuropathy Limitations Scale. Journal of Neurology, Neurosurgery, and Psychiatry 2006;77(8):973‐6. [PUBMED: 16574730] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hahn 1996a
- Hahn AF, Bolton CF, Pillay N, Chalk C, Benstead T, Bril V, et al. Plasma exchange therapy in chronic inflammatory demyelinating polyneuropathy. A double‐blind, sham‐controlled, cross‐over study. Brain 1996;119(Pt 4):1055‐66. [PUBMED: 8813270] [DOI] [PubMed] [Google Scholar]
Hahn 1996b
- Hahn AF, Bolton CF, Zochodne D, Feasby TE. Intravenous immunoglobulin treatment in chronic inflammatory demyelinating polyneuropathy. A double‐blind placebo‐controlled cross‐over study. Brain 1996;119(Pt 4):1067‐77. [PUBMED: 8813271] [DOI] [PubMed] [Google Scholar]
Hahn 2005
- Hahn AF, Hartung H‐P, Dyck PJ. Chronic Inflammatory Demyelinating Polyradiculoneuropathy. In: Dyck PJ, Thomas PK editor(s). Peripheral Neuropathy. Fourth. Vol. 2, Philadelphia: Elsevier, 2005:2221‐53. [Google Scholar]
Hamon 2007
- Hamon MA, Nicolas G, Deviere F, Letournel F, Dubas F. Demyelinating neuropathy during anti‐TNF alpha treatment with a review of the literature [Neuropathie démyélinisante au cours d'un traitement par anti‐TNF alpha et revue de la litérature]. Revue Neurologique 2007;163(12):1232‐5. [PUBMED: 18355471] [DOI] [PubMed] [Google Scholar]
Hefter 1990
- Hefter H, Sprenger KB, Arendt G, Hafner D. Treatment of chronic relapsing inflammatory demyelinating polyneuropathy by cyclosporin A and plasma exchange. A case report. Journal of Neurology 1990;237(5):320‐3. [PUBMED: 2230850] [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hirst 2006
- Hirst C, Raasch S, Llewelyn G, Robertson N. Remission of chronic inflammatory demyelinating polyneuropathy after alemtuzumab (Campath 1H). Journal of Neurology, Neurosurgery, and Psychiatry 2006;77(6):800‐2. [PUBMED: 16705208] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hodgkinson 1990
- Hodgkinson SJ, Pollard JD, McLeod JG. Cyclosporin A in the treatment of chronic demyelinating polyradiculoneuropathy. Journal of Neurology, Neurosurgery, and Psychiatry 1990;53(4):327‐30. [PUBMED: 2341846 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Holman 2004
- Holman R, Lindeboom R, Vermeulen M, Haan RJ. The AMC Linear Disability Score project in a population requiring residential care: psychometric properties. Health and Quality of Life Outcomes 2004; Vol. 2:42. [PUBMED: 15291958] [DOI] [PMC free article] [PubMed]
Hughes 1992
- Hughes R, Sanders E, Hall S, Atkinson P, Colchester A, Payan P. Subacute idiopathic demyelinating polyradiculoneuropathy. Archives of Neurology 1992;49(6):612‐6. [PUBMED: 1317700] [DOI] [PubMed] [Google Scholar]
Hughes 2001
- Hughes R, Bensa S, Willison H, Bergh P, Comi G, Illa I, et al. Randomized controlled trial of intravenous immunoglobulin versus oral prednisolone in chronic inflammatory demyelinating polyradiculoneuropathy. Annals of Neurology 2001;50(2):195‐201. [PUBMED: 11506402] [DOI] [PubMed] [Google Scholar]
Hughes 2008
- Hughes RA, Donofrio P, Bril V, Dalakas MC, Deng C, Hanna K, et al. ICE Study Group. Intravenous immune globulin (10% caprylate‐chromatography purified) for the treatment of chronic inflammatory demyelinating polyradiculoneuropathy (ICE Study): a randomised placebo‐controlled trial. Lancet Neurology 2008; Vol. 7, issue 2:136‐44. [PUBMED: 18178525] [DOI] [PubMed]
Hughes 2015
- Hughes RA, Mehndiratta MM. Corticosteroids for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database of Systematic Reviews 2015, Issue 1. [DOI: 10.1002/14651858.CD002062.pub3] [DOI] [PubMed] [Google Scholar]
Jasmin 2012
- Jasmin R, Sockalingam S, Shahrizaila N, Cheah TE, Zain AA, Goh KJ. Successful treatment of chronic inflammatory demyelinating polyneuropathy (CIDP) in systemic lupus erythematosus (SLE) with oral cyclophosphamide. [Review]. Lupus 2012;21(10):1119‐23. [PUBMED: 22433918] [DOI] [PubMed] [Google Scholar]
Kasamon 2002
- Kasamon YL, Nguyen TN, Chan JA, Nascimento AF. EBV‐associated lymphoma and chronic inflammatory demyelinating polyneuropathy in an adult without overt immunodeficiency. American Journal of Hematology 2002;69(4):289‐93. [PUBMED: 11921025] [DOI] [PubMed] [Google Scholar]
Katz 2000
- Katz JS, Saperstein DS, Gronseth G, Amato AA, Barohn RJ. Distal acquired demyelinating symmetric neuropathy. Neurology 2000;54(3):615‐20. [PUBMED: 10680792] [DOI] [PubMed] [Google Scholar]
Kelly 1981
- Kelly JJ, Kyle RA, O'Brien PC, Dyck PJ. The prevalence of monoclonal protein in peripheral neuropathy. Neurology 1981;31(11):1480‐3. [PUBMED: 6273767] [DOI] [PubMed] [Google Scholar]
Kempen 2009
- Kempen JH, Daniel E, Dunn JP, Foster CS, Gangaputra S, Hanish A, et al. Overall and cancer related mortality among patients with ocular inflammation treated with immunosuppressive drugs: retrospective cohort study. BMJ 2009; Vol. 339:b2480. [PUBMED: 2714688] [DOI] [PMC free article] [PubMed]
Kilidireas 2006
- Kilidireas C, Anagnostopoulos A, Karandreas N, Mouselimi L, Dimopoulos MA. Rituximab therapy in monoclonal IgM‐related neuropathies. Leukemia and Lymphoma 2006;47(5):859‐64. [PUBMED: 16753870] [DOI] [PubMed] [Google Scholar]
Kissel 1986
- Kissel JT, Levy RJ, Mendell JR, Griggs RC. Azathioprine toxicity in neuromuscular disease. Neurology 1986;36(1):35‐9. [PUBMED: 3941781] [DOI] [PubMed] [Google Scholar]
Kleyman 2015
- Kleyman I, Brannagan TH 3rd. Treatment of chronic inflammatory demyelinating polyneuropathy. Current Neurology and Neuroscience Reports 2015;15(7):47. [PUBMED: 26008811] [DOI] [PubMed] [Google Scholar]
Knecht 2004
- Knecht H, Baumberger M, Tobòn A, Steck A. Sustained remission of CIDP associated with Evans syndrome. Neurology 2004;63(4):730‐2. [PUBMED: 15326255] [DOI] [PubMed] [Google Scholar]
Kolkin 1987
- Kolkin S, Nahman NS Jr, Mendell JR. Chronic nephrotoxicity complicating cyclosporin treatment of chronic inflammatory demyelinating polyradiculoneuropathy. Neurology 1987;37(1):147‐9. [PUBMED: 3796829] [DOI] [PubMed] [Google Scholar]
Koski 2009
- Koski CL, Baumgarten M, Magder LS, Barohn RJ, Goldstein J, Graves M, et al. Derivation and validation of diagnostic criteria for chronic inflammatory demyelinating polyneuropathy. Journal of the Neurological Sciences 2009;277(1‐2):1‐8. [PUBMED: 19091330] [DOI] [PubMed] [Google Scholar]
Kuitwaard 2009
Kuntzer 1999
- Kuntzer T, Radziwill AJ, Lettry‐Trouillat R, Naegeli C, Ochsner F, Erne B, et al. Interferon‐beta 1a in chronic inflammatory demyelinating polyneuropathy. Neurology 1999;53(6):1364‐5. [PUBMED: 10522905] [DOI] [PubMed] [Google Scholar]
Köller 2005
- Köller H, Kieseier BC, Jander S, Hartung HP. Chronic inflammatory demyelinating polyneuropathy. New England Journal of Medicine 2005;352(13):1343‐56. [PUBMED: 15800230] [DOI] [PubMed] [Google Scholar]
Laughlin 2009
- Laughlin RS, Dyck PJ, Melton LJ 3rd, Leibson C, Ransom J, Dyck PJ. Incidence and prevalence of CIDP and the association of diabetes mellitus. Neurology 2009; Vol. 73, issue 1:39‐45. [PUBMED: 19564582] [DOI] [PMC free article] [PubMed]
Leitch 2015
- Leitch MM, Sherman WH, Brannagan TH 3rd. Fludarabine in the treatment of refractory chronic inflammatory demyelinating neuropathies. Journal of Clinical Neuromuscular Disease 2015;17(1):1‐5. [PUBMED: 26301372 ] [DOI] [PubMed] [Google Scholar]
Lewis 1982
- Lewis RA, Sumner AJ, Brown MJ, Asbury AK. Multifocal demyelinating neuropathy with persistent conduction block. Neurology 1982;32(9):958‐64. [PUBMED: 7202168] [DOI] [PubMed] [Google Scholar]
Llewelyn 2009
Lozeron 2009
- Lozeron P, Denier C, Lacroix C, Adams D. Long‐term course of demyelinating neuropathies occurring during tumor necrosis factor‐blocker therapy. Archives of Neurology 2009;66(4):490‐7. [PUBMED: 19364934] [DOI] [PubMed] [Google Scholar]
Lunn 1999
- Lunn MPT, Manji H, Choudhary PP, Hughes RAC, Thomas PK. Chronic inflammatory demyelinating polyradiculoneuropathy: a prevalence study in south east England. Journal of Neurology, Neurosurgery, and Psychiatry 1999;66(5):677‐80. [PUBMED: 10209187] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lunn 2009
- Lunn MPT. Rituximab usage in CIDP: A retrospective e‐mail based data collection. Journal of the Peripheral Nervous System : JPNS 2009; Vol. 14, issue s2:92. [DOI: 10.1111/j.1529-8027.2009.00212.x] [DOI]
Lunn 2016
- Lunn M, Nobile‐Orazio E. Immunotherapy for IgM anti‐myelin‐associated glycoprotein paraprotein‐associated peripheral neuropathies. Cochrane Database of Systematic Reviews 2016, Issue 10. [DOI: 10.1002/14651858.CD002827.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Léger 2005
- Léger J‐M. A review of the medical management of chronic inflammatory demyelinating polyradiculoneuropathy. Expert Opinion on Pharmacotherapy 2005;6(4):569‐82. [PUBMED: 15934883] [DOI] [PubMed] [Google Scholar]
Léger 2013
- Léger JM, Viala K, Nicolas G, Créange A, Vallat JM, Pouget J, et al. RIMAG Study Group (France and Switzerland). Placebo‐controlled trial of rituximab in IgM anti‐myelin‐associated glycoprotein neuropathy. Neurology 2013;80(24):2217‐25. [PUBMED: 23667063] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mahattanakul 1996
- Mahattanakul W, Crawford TO, Griffin JW, Goldstein JM, Cornblath DR. Treatment of chronic inflammatory demyelinating polyneuropathy with cyclosporin‐A. Journal of Neurology, Neurosurgery, and Psychiatry 1996;60(2):185‐7. [PUBMED: 8708650] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mahdi‐Rogers 2007
- Mahdi‐Rogers M, Hughes RAC, Kazmi M, Ferner R. Autologous peripheral blood progenitor cell transplantation for Chronic inflammatory demyelinating polyradiculoneuropathy. Journal of the Peripheral Nervous System : JPNS 2007;12(s1):55. [DOI: 10.1111/j.1529-8027.2007.00123.x] [DOI] [Google Scholar]
Mahdi‐Rogers 2008
- Mahdi‐Rogers, M, Al‐Chalabi A, Hughes RAC. Prevalence and morbidity of chronic inflammatory neuropathies in South East England. Journal of the Peripheral Nervous System : JPNS 2008; Vol. 13, issue 2:176‐7. [DOI: 10.1111/j.1529-8027.2008.00174.x] [DOI]
Mahdi‐Rogers 2009
Mahdi‐Rogers 2014
- Mahdi‐Rogers M, Hughes RA. Epidemiology of chronic inflammatory neuropathies in southeast England. European Journal of Neurology 2014;21(1):28‐33. [PUBMED: 23679015] [DOI] [PubMed] [Google Scholar]
Markvardsen 2013
- Markvardsen LH, Debost JC, Harbo T, Sindrup SH, Andersen H, Christiansen I, et al. Subcutaneous immunoglobulin in responders to intravenous therapy with chronic inflammatory demyelinating polyradiculoneuropathy. European Journal of Neurology 2013;20(5):836‐42. [PUBMED: 23294032] [DOI] [PubMed] [Google Scholar]
Marsh 2010
- Marsh EA, Hirst CL, Llewelyn JG, Cossburn MD, Reilly MM, Krishnan A, et al. Alemtuzumab in the treatment of IVIG‐dependent chronic inflammatory demyelinating polyneuropathy. Journal of Neurology 2010;257(6):913‐9. [PUBMED: 20049473] [DOI] [PubMed] [Google Scholar]
Martina 1999
- Martina IS, Doorn PA, Schmitz PI, Meulstee J, Meché FG. Chronic motor neuropathies: response to interferon‐beta 1a after failure of conventional therapies. Journal of Neurology, Neurosurgery, and Psychiatry 1999;66(2):197‐201. [PUBMED: 10071099] [DOI] [PMC free article] [PubMed] [Google Scholar]
Marzo 1998
- Marzo ME, Tintoré M, Fabregues O, Montalbán X, Codina A. Chronic inflammatory demyelinating polyradiculoneuropathy during treatment with interferon‐alpha. Journal of Neurology, Neurosurgery, and Psychiatry 1998;65(4):604. [PUBMED: 9771799] [DOI] [PMC free article] [PubMed] [Google Scholar]
Matsuda 2004
- Matsuda M, Hoshi K, Gono T, Morita H, Ikeda S. Cyclosporin A in treatment of refractory patients with chronic inflammatory demyelinating polyradiculoneuropathy. Journal of the Neurological Sciences 2004;224(1‐2):29‐35. [PUBMED: 15450768] [DOI] [PubMed] [Google Scholar]
Matsuse 2005
- Matsuse D, Ochi H, Tashiro K, Nomura T, Murai H, Taniwaki T, et al. Exacerbation of chronic inflammatory demyelinating polyradiculoneuropathy during interferon beta‐1b therapy in a patient with childhood‐onset multiple sclerosis. Internal Medicine (Tokyo, Japan) 2005;44(1):68‐72. [PUBMED: 15704667] [DOI] [PubMed] [Google Scholar]
McCombe 1987
- McCombe PA, Pollard JD, McLeod JG. Chronic inflammatory demyelinating polyradiculoneuropathy. A clinical and electrophysiological study of 92 cases. Brain 1987;110(Pt 6):1617‐30. [PUBMED: 3427403] [DOI] [PubMed] [Google Scholar]
Mehndiratta 2015
- Mehndiratta MM, Hughes RA, Pritchard J. Plasma exchange for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database of Systematic Reviews 2015, Issue 8. [DOI: 10.1002/14651858.CD003906.pub4] [DOI] [PMC free article] [PubMed] [Google Scholar]
Melzer 2014
- Melzer N, Meuth SG. Disease‐modifying therapy in multiple sclerosis and chronic inflammatory demyelinating polyradiculoneuropathy: common and divergent current and future strategies. [Review]. Clinical and Experimental Immunology 2014;175(3):359‐72. [PUBMED: 24032475 ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Mendell 2000
- Mendell JR, Barohn RJ, Freimer ML, Kissel JT, King W, Nagaraja HN, et al. Randomized controlled trial of IVIg in untreated chronic inflammatory demyelinating polyradiculoneuropathy. Neurology 2001;56(4):445‐9. [PUBMED: 11222785] [DOI] [PubMed] [Google Scholar]
Meriggioli 2000
- Meriggioli MN, Rowin J. Chronic inflammatory demyelinating polyneuropathy after treatment with interferon‐alpha. Muscle & Nerve 2000;23(3):433‐5. [PUBMED: 10679722] [DOI] [PubMed] [Google Scholar]
Merkies 2002
- Merkies IS, Schmitz PI, Meché FG, Samijn JP, Doorn PA, Inflammatory Neuropathy Cause and Treatment (INCAT) group. Clinimetric evaluation of a new overall disability scale in immune mediated polyneuropathies. Journal of Neurology, Neurosurgery, and Psychiatry 2002;72(5):596‐601. [PUBMED: 11971045] [DOI] [PMC free article] [PubMed] [Google Scholar]
Monaco 2004
- Monaco S, Turri E, Zanusso G, Maistrello B. Treatment of inflammatory and paraproteinemic neuropathies. Current Drug Targets. Immune, Endocrine and Metabolic Disorders 2004;4(2):141‐8. [PUBMED: 15180454] [DOI] [PubMed] [Google Scholar]
Mowzoon 2001
- Mowzoon N, Sussman A, Bradley WG. Mycophenolate (CellCept) treatment of myasthenia gravis, chronic inflammatory polyneuropathy and inclusion body myositis. Journal of the Neurological Sciences 2001;185(2):119‐22. [PUBMED: 11311292] [DOI] [PubMed] [Google Scholar]
Mygland 2001
Münch 2007
- Münch C, Anagnostou P, Meyer R, Haas J. Rituximab in chronic inflammatory demyelinating polyneuropathy associated with diabetes mellitus. Journal of the Neurological Sciences 2007;256(1‐2):100‐2. [PUBMED: 17382963] [DOI] [PubMed] [Google Scholar]
Nobile‐Orazio 2005
- Nobile‐Orazio E. Treatment of dys‐immune neuropathies. Journal of Neurology 2005;252(4):385‐95. [PUBMED: 15834625] [DOI] [PubMed] [Google Scholar]
Nobile‐Orazio 2012
- Nobile‐Orazio E, Cocito D, Jann S, Uncini A, Beghi E, Messina P, et al. Intravenous immunoglobulin versus intravenous methylprednisolone for chronic inflammatory demyelinating polyradiculoneuropathy: a randomised controlled trial. Lancet Neurology 2012;11(6):493‐502. [PUBMED: 2012287474] [DOI] [PubMed] [Google Scholar]
Odaka 2005
- Odaka M, Tatsumoto M, Susuki K, Hirata K, Yuki N. Intractable chronic inflammatory demyelinating polyneuropathy treated successfully with ciclosporin. Journal of Neurology, Neurosurgery, and Psychiatry 2005;76(8):1115‐20. [PUBMED: 16024890] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oh 1992
- Oh SJ, Joy JL, Kuruoglu R. 'Chronic sensory demyelinating neuropathy': chronic inflammatory demyelinating polyneuropathy presenting as a pure sensory neuropathy. Journal of Neurology, Neurosurgery, and Psychiatry 1992;55(8):677‐80. [PUBMED: 1326601] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oh 2003
- Oh SJ, Kurokawa K, Almeida DF, Ryan HF Jr, Claussen GC. Subacute inflammatory demyelinating polyneuropathy. Neurology 2003;61(11):1507‐12. [PUBMED: 14663033] [DOI] [PubMed] [Google Scholar]
Ormerod 1990
- Ormerod IE, Waddy HM, Kermode AG, Murray NM, Thomas PK. Involvement of the central nervous system in chronic inflammatory demyelinating polyneuropathy: a clinical, electrophysiological and magnetic resonance imaging study. Journal of Neurology, Neurosurgery, and Psychiatry 1990;53(9):789‐93. [PUBMED: 2174078] [DOI] [PMC free article] [PubMed] [Google Scholar]
Oyama 2007
- Oyama Y, Sufit R, Loh Y, Statkute L, Yaung K, Quigley K, et al. Nonmyeloablative autologous hematopoietic stem cell transplantation for refractory CIDP. Neurology 2007;69(18):1802‐3. [PUBMED: 17967996] [DOI] [PubMed] [Google Scholar]
Palace 1998
- Palace J, Newsom‐Davis J, Lecky B. A randomized double‐blind trial of prednisolone alone or with azathioprine in myasthenia gravis. Myasthenia Gravis Study Group. Neurology 1998;50(6):1778‐83. [PUBMED: 9633727] [DOI] [PubMed] [Google Scholar]
Pavesi 2002
- Pavesi G, Cattaneo L, Marbini A, Gemignani F, Mancia D. Long‐term efficacy of interferon‐alpha in chronic inflammatory demyelinating polyneuropathy. Journal of Neurology 2002;249(6):777‐9. [PUBMED: 12173577] [DOI] [PubMed] [Google Scholar]
Press 2014
- Press R, Askmark H, Svenningsson A, Andersen O, Axelson HW, Strömberg U, et al. Autologous haematopoietic stem cell transplantation: A viable treatment option for CIDP. Journal of Neurology, Neurosurgery, and Psychiatry 2014;85(6):618‐24. [PUBMED: 24262917] [DOI] [PubMed] [Google Scholar]
Prineas 1976
- Prineas JW, McLeod JG. Chronic relapsing polyneuritis. Journal of the Neurological Sciences 1976;27(4):427‐58. [PUBMED: 1262904] [DOI] [PubMed] [Google Scholar]
PRISMS 1998
- PRISMS (Prevention of Relapses and Disability by Interferon beta‐1a Subcutaneously in Multiple Sclerosis) Study Group. Randomised, double‐blind, placebo‐controlled study of interferon‐beta 1a in relapsing‐remitting multiple sclerosis. PRISMS (Prevention of Relapses and Disability by Interferon beta‐1a Subcutaneously in Multiple Sclerosis) Study Group. Lancet 1998;352(9139):1498‐504. [PUBMED: 9820297] [PubMed] [Google Scholar]
PRISMS 2001
- PRISMS Study Group and the University of British Columbia MS/MRI Analysis Group. PRISMS‐4: Long‐term efficacy of interferon‐beta‐1a in relapsing MS. Neurology 2001;56(12):1628‐36. [PUBMED: 11425926] [DOI] [PubMed] [Google Scholar]
Radziwill 2001
- Radziwill AJ, Kuntzer T, Fuhr P, Steck AJ. Long term follow up in chronic inflammatory demyelinating polyneuropathy (CIDP) under interferon beta‐1a (IFN‐b1a) and intravenous immunoglobulin (IVIg). Journal of Neurology, Neurosurgery, and Psychiatry 2001;70:273. [Google Scholar]
Radziwill 2006
Remenyi 2007
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Reference Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rose 2010
- Rose WN, Dwyre DM, Erickson YO, Klutts JS. Chronic inflammatory demyelinating polyradiculoneuropathy refractory to multiple treatment modalities responds to rituximab. American Journal of Clinical Pathology 2010;134:510‐1. [Google Scholar]
Ruts 2005
- Ruts L, Koningsveld R, Doorn PA, Dutch GBS Study Group. Distinguishing acute‐onset CIDP from Guillain–Barré syndrome with treatment related fluctuations. Neurology 2005;65(1):138‐40. [PUBMED: 16009902] [DOI] [PubMed] [Google Scholar]
Ruts 2010
- Ruts L, Drenthen J, Jacobs BC, Doorn PA, Dutch GBS Study Group. Distinguishing acute‐onset CIDP from fluctuating Guillain‐Barre syndrome: a prospective study. Neurology 2010;74(21):1680‐6. [PUBMED: 20427754] [DOI] [PubMed] [Google Scholar]
Ryan 2000
- Ryan MM, Grattan‐Smith PJ, Procopis PG, Morgan G, Ouvrier RA. Childhood chronic inflammatory demyelinating polyneuropathy: clinical course and long‐term outcome. Neuromuscular Disorders 2000;10(6):398‐406. [DOI] [PubMed] [Google Scholar]
Sadnicka 2011
- Sadnicka A, Reilly MM, Mummery C, Brandner S, Hirsch N, Lunn MP. Rituximab in the treatment of three coexistent neurological autoimmune diseases: chronic inflammatory demyelinating polyradiculoneuropathy, Morvan syndrome and myasthenia gravis. Journal of Neurology, Neurosurgery, and Psychiatry 2011;82(2):230‐2. [PUBMED: 20462915] [DOI] [PubMed] [Google Scholar]
Sanz 2012
- Sanz PG, García Méndez CV, Cueto AL, Silva VB, Walther JC, Diez RA, et al. Chronic inflammatory demyelinating polyradiculoneuropathy in a patient with systemic lupus erythematosus and good outcome with rituximab treatment. Rheumatology International 2012;32(12):4061‐3. [PUBMED: 21922339 ] [DOI] [PubMed] [Google Scholar]
Saperstein 1999
- Saperstein DS, Amato AA, Wolfe GI, Katz JS, Nations SP, Jackson CE, et al. Multifocal acquired demyelinating sensory and motor neuropathy: the Lewis‐Sumner syndrome. Muscle & Nerve 1999;22(5):560‐6. [PUBMED: 10331353] [DOI] [PubMed] [Google Scholar]
Saperstein 2001
- Saperstein DS, Katz JS, Amato AA, Barohn RJ. Clinical spectrum of chronic acquired demyelinating polyneuropathies. Muscle & Nerve 2001;24(3):311‐24. [PUBMED: 11353415] [DOI] [PubMed] [Google Scholar]
Saperstein 2008
- Saperstein DS. Chronic acquired demyelinating polyneuropathies. Seminars in Neurology 2008;28(2):168‐84. [PUBMED: 18351519] [DOI] [PubMed] [Google Scholar]
Simmons 1995
- Simmons Z, Albers JW, Bromberg MB, Feldman EL. Long‐term follow‐up of patient with chronic inflammatory demyelinating polyradiculoneuropathy, without and with monoclonal gammopathy. Brain 1995;118(Pt 2):359‐68. [PUBMED: 7735878] [DOI] [PubMed] [Google Scholar]
Stork 2015
- Stork ACJ, Lunn MPT, Nobile‐Orazio E, Notermans NC. Treatment for IgG and IgA paraproteinaemic neuropathy. Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD005376.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Stübgen 2013
- Stübgen JP. A review of the use of biological agents for chronic inflammatory demyelinating polyradiculoneuropathy. Journal of the Neurological Sciences 2013;326(1‐2):1‐9. [PUBMED: 23337197] [DOI] [PubMed] [Google Scholar]
Takeuchi 2012
- Takeuchi A, Shirai S, Horiuchi K, Takahashi I, Matsushima M, Hirotani M, et al. [Successful cyclosporine treatment in 2 patients with refractory CIDP, involving monitoring of both AUC(0‐4h) and trough levels]. [Japanese]. Rinsho Shinkeigaku [Clinical Neurology] 2012;52(3):172‐7. [PUBMED: 22453042 ] [DOI] [PubMed] [Google Scholar]
Thomas 1996
- Thomas PK, Claus D, Jaspert A, Workman JM, King RH, Larner AJ, et al. Focal upper limb demyelinating neuropathy. Brain 1996;119(Pt 3):765‐74. [PUBMED: 8673489] [DOI] [PubMed] [Google Scholar]
Thompson 1996
Umapathi 2002
- Umapathi T, Hughes R. Mycophenolate in treatment‐resistant inflammatory neuropathies. European Journal of Neurology 2002;9(6):683‐5. [PUBMED: 12453086] [DOI] [PubMed] [Google Scholar]
Umapathi 2015
- Umapathi T, Hughes RAC, Nobile‐Orazio E, Léger JM. Immunosuppressant and immunomodulatory treatments for multifocal motor neuropathy. Cochrane Database of Systematic Reviews 2015, Issue 3. [DOI: 10.1002/14651858.CD003217.pub5] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vallat 2003
- Vallat JM, Hahn AF, Léger JM, Cros DP, Magy L, Tabaraud F, et al. Interferon beta‐1a as an investigational treatment for CIDP. Neurology 2003;60(8 Suppl 3):S23‐8. [PUBMED: 12707419 ] [DOI] [PubMed] [Google Scholar]
Vallat 2015
- Vallat JM, Mathis S, Ghorab K, Milor MA, Richard L, Magy L. Natalizumab as a disease‐modifying therapy in chronic inflammatory demyelinating polyneuropathy ‐ a report of three cases. European Neurology 2015;73(5‐6):294‐302. [DOI: 10.1159/000381767; PUBMED: 25925430] [DOI] [PubMed] [Google Scholar]
Van den Bergh 2010
- Bergh PY, Hadden RD, Bouche P, Cornblath DR, Hahn A, Illa I, et al. European Federation of Neurological Societies, Peripheral Nerve Society. European Federation of Neurological Societies/Peripheral Nerve Society guideline on management of chronic inflammatory demyelinating polyradiculoneuropathy: report of a joint task force of the European Federation of Neurological Societies and the Peripheral Nerve Society ‐ first revision. European Journal of Neurology 2010;17(3):356‐63. [PUBMED: 20456730] [DOI] [PubMed] [Google Scholar]
van Doorn 2005
- Doorn PA. Treatment of Guillain‐Barré syndrome and CIDP. Journal of the Peripheral Nervous System : JPNS 2005;10(2):113‐27. [PUBMED: 15958124] [DOI] [PubMed] [Google Scholar]
van Nes 2011
- Nes SI, Vanhoutte EK, Doorn PA, Hermans M, Bakkers M, Kuitwaard K, et al. Rasch‐built Overall Disability Scale (R‐ODS) for immune‐mediated peripheral neuropathies. Neurology 2011;76(4):337‐45. [PUBMED: 21263135] [DOI] [PubMed] [Google Scholar]
van Schaik 2010
- Schaik IN, Eftimov F, Doorn PA, Brusse E, Berg LH, Pol WL, et al. Pulsed high‐dose dexamethasone versus standard prednisolone treatment for chronic inflammatory demyelinating polyradiculoneuropathy (PREDICT study): a double‐blind, randomised, controlled trial. Lancet Neurology 2010;9(3):245–53. [PUBMED: 20133204] [DOI] [PubMed] [Google Scholar]
van Swieten 1988
- Swieten JC, Koudstaal PJ, Visser MC, Schouten HJ, Gijn J. Interobserver agreement for the assessment of handicap in stroke patients. Stroke 1988;19(5):604‐7. [PUBMED: 3363593] [DOI] [PubMed] [Google Scholar]
Vanhoutte 2013
- Vanhoutte EK, Latov N, Deng C, Hanna K, Hughes RAC, Bril V, et al. Vigorimeter grip strength in CIDP: a responsive tool that rapidly measures the effect of IVIG ‐ the ICE study. European Journal of Neurology 2013;20(5):748‐55. [PUBMED: 22891893] [DOI] [PubMed] [Google Scholar]
Velardo 2014
- Velardo D, Bianchi F, Mannina D, Mauri E, Comi G, Fazio R. Rituximab in two cases of chronic inflammatory demyelinating polyradiculoneuropathy not responsive to other treatments. Journal of the Peripheral Nervous System : JPNS 2014;19(s1):S34‐5. [Google Scholar]
Vermeulen 1993
- Vermeulen M, Doorn PA, Brand A, Strengers PF, Jennekens FG, Busch HF. Intravenous immunoglobulin treatment in patients with chronic inflammatory demyelinating polyneuropathy: A double blind, placebo controlled study. Journal of Neurology, Neurosurgery, and Psychiatry 1993;56(1):36‐9. [PUBMED: 8429321] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vermeulen 2002
- Vermeulen M, Oers MH. Successful autologous stem cell transplantation in a patient with chronic inflammatory demyelinating polyneuropathy. Journal of Neurology, Neurosurgery, and Psychiatry 2002;72(1):127‐8. [PUBMED: 11784845] [DOI] [PMC free article] [PubMed] [Google Scholar]
Vermeulen 2007
- Vermeulen M, Oers MH. Relapse of chronic inflammatory demyelinating polyneuropathy 5 years after autologous stem cell transplantation. Journal of Neurology, Neurosurgery, and Psychiatry 2007;78(10):1154. [PUBMED: 17878198] [DOI] [PMC free article] [PubMed] [Google Scholar]
Viala 2004
Waddy 1989
- Waddy HM, Misra VP, King RH, Thomas PK, Middleton L, Ormerod IE. Focal cranial nerve involvement in chronic inflammatory demyelinating polyneuropathy: clinical and MRI evidence of peripheral and central lesions. Journal of Neurology 1989;236(7):400‐5. [PUBMED: 2809641] [DOI] [PubMed] [Google Scholar]
Ware 2014
- Ware TL, Kornberg AJ, Rodriguez‐Casero MV, Ryan MM. Childhood chronic inflammatory demyelinating polyneuropathy: an overview of 10 cases in the modern era. Journal of Child Neurology 2014;29(1):43‐8. [DOI] [PubMed] [Google Scholar]
Wolf 2010
- Wolf C, Menge T, Stenner MP, Meyer zu Hörste G, Saleh A, Hartung HP, et al. Natalizumab treatment in a patient with chronic inflammatory demyelinating polyneuropathy. Archives of Neurology 2010;67(7):881‐3. [PUBMED: 20625098] [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Hughes 2002
- Hughes RAC. Systematic reviews of treatment for inflammatory demyelinating neuropathy. Journal of Anatomy 2002;200(4):331‐9. [PUBMED: 12090400] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hughes 2003
- Hughes RAC, Swan AV, Doorn PA. Cytotoxic drugs and interferons for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD003280] [DOI] [PubMed] [Google Scholar]
Hughes 2004
- Hughes RAC, Swan AV, Doorn PA. Cytotoxic drugs and interferons for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD003280.pub2] [DOI] [PubMed] [Google Scholar]
Mahdi‐Rogers 2010
- Mahdi‐Rogers M, Swan AV, Doorn PA, Hughes RAC. Immunomodulatory treatment other than corticosteroids, immunoglobulin and plasma exchange for chronic inflammatory demyelinating polyradiculoneuropathy. Cochrane Database of Systematic Reviews 2010, Issue 11. [DOI: 10.1002/14651858.CD003280.pub3] [DOI] [PubMed] [Google Scholar]


