Abstract
Background
Cognitive impairment in people with traumatic brain injury (TBI) could affect multiple facets of their daily functioning. Cognitive rehabilitation brings about clinically significant improvement in certain cognitive skills. However, it is uncertain if these improved cognitive skills lead to betterments in other key aspects of daily living. We evaluated whether cognitive rehabilitation for people with TBI improves return to work, independence in daily activities, community integration and quality of life.
Objectives
To evaluate the effects of cognitive rehabilitation on return to work, independence in daily activities, community integration (occupational outcomes) and quality of life in people with traumatic brain injury, and to determine which cognitive rehabilitation strategy better achieves these outcomes.
Search methods
We searched CENTRAL (the Cochrane Library; 2017, Issue 3), MEDLINE (OvidSP), Embase (OvidSP), PsycINFO (OvidSP), and clinical trials registries up to 30 March 2017.
Selection criteria
We identified all available randomized controlled trials of cognitive rehabilitation compared with any other non‐pharmacological intervention for people with TBI. We included studies that reported at least one outcome related to : return to work, independence in activities of daily living (ADL), community integration and quality of life.
Data collection and analysis
Two review authors independently selected trials. We used standard methodological procedures expected by Cochrane. We evaluated heterogeneity among the included studies and performed meta‐analysis only when we could include more than one study in a comparison. We used the online computer programme GRADEpro to assess the quality of evidence, and generate 'Summary of findings' tables.
Main results
We included nine studies with 790 participants. Three trials (160 participants) compared cognitive rehabilitation versus no treatment, four trials (144 participants) compared cognitive rehabilitation versus conventional treatment, one trial (120 participants) compared hospital‐based cognitive rehabilitation versus home programme and one trial (366 participants) compared one cognitive strategy versus another. Among the included studies, we judged three to be of low risk of bias.
There was no difference between cognitive rehabilitation and no intervention in return to work (risk ratio (RR) 1.80, 95% confidence interval (CI) 0.74 to 4.39, 1 study; very low‐quality evidence). There was no difference between biweekly cognitive rehabilitation for eight weeks and no treatment in community integration (Sydney Psychosocial Reintegration Scale): mean difference (MD) ‐2.90, 95% CI ‐12.57 to 6.77, 1 study; low‐quality evidence). There was no difference in quality of life between cognitive rehabilitation and no intervention immediately following the 12‐week intervention(MD 0.30, 95% CI ‐0.18 to 0.78, 1 study; low‐quality evidence). No study reported effects on independence in ADL.
There was no difference between cognitive rehabilitation and conventional treatment in return to work status at six months' follow‐up in one study (RR 1.43, 95% CI 0.87 to 2.33; low‐quality evidence); independence in ADL at three to four weeks' follow‐up in two studies (standardized mean difference (SMD) ‐0.01, 95% CI ‐0.62 to 0.61; very low‐quality evidence); community integration at three weeks' to six months' follow‐up in three studies (Community Integration Questionnaire: MD 0.05, 95% CI ‐1.51 to 1.62; low‐quality evidence) and quality of life at six months' follow‐up in one study (Perceived Quality of Life scale: MD 6.50, 95% CI ‐2.57 to 15.57; moderate‐quality evidence).
For active duty military personnel with moderate‐to‐severe closed head injury, there was no difference between eight weeks of cognitive rehabilitation administered as a home programme and hospital‐based cognitive rehabilitation in achieving return to work at one year' follow‐up in one study (RR 0.95, 95% CI 0.85 to 1.05; moderate‐quality evidence). The study did not report effects on independence in ADL, community integration or quality of life.
There was no difference between one cognitive rehabilitation strategy (cognitive didactic) and another (functional experiential) for adult veterans or active duty military service personnel with moderate‐to‐severe TBI (one study with 366 participants and one year' follow‐up) on return to work (RR 1.10, 95% CI 0.83 to 1.46; moderate‐quality evidence), or on independence in ADL (RR 0.90, 95% CI 0.75 to 1.08; low‐quality evidence). The study did not report effects on community integration or quality of life.
None of the studies reported adverse effects of cognitive rehabilitation.
Authors' conclusions
There is insufficient good‐quality evidence to support the role of cognitive rehabilitation when compared to no intervention or conventional rehabilitation in improving return to work, independence in ADL, community integration or quality of life in adults with TBI. There is moderate‐quality evidence that cognitive rehabilitation provided as a home programme is similar to hospital‐based cognitive rehabilitation in improving return to work status among active duty military personnel with moderate‐to‐severe TBI. Moderate‐quality evidence suggests that one cognitive rehabilitation strategy (cognitive didactic) is no better than another (functional experiential) in achieving return to work in veterans or military personnel with TBI.
Plain language summary
Cognitive rehabilitation for people with brain injury due to trauma to help them return to work
Background
Traumatic brain injuries (head injuries) are becoming increasingly common, and their impact on people's lives can be devastating. Depending on which part of the brain is injured and to what extent, impairments could be in physical functions such as walking, and use of hands and legs, or in mental functions (also known as 'cognitive functions'). Problems with mental functions can be related to memory, understanding language, using appropriate words to express oneself, analyzing options in a situation and making appropriate decisions . Problems with mental functions could lead to difficulty in 'occupational activities', a term that refers to employment, pursuing education and managing daily routines. Limitations in these activities could lead to a poor quality of life and withdrawal from social life.
'Cognitive rehabilitation' is the term used to refer to the training given to people with brain injury to address and improve the specific mental abilities that are impaired. This is usually done to improve return to work, independence in managing daily routines, and quality of life.
Review question
Does cognitive rehabilitation for people with traumatic brain injury improve their return to work, independence in daily activities, community integration and quality of life?
Study characteristics
We included nine studies with 790 participants. Seven of the studies were conducted in the US, and one each in Australia and China. Follow‐up (monitoring) duration in the studies ranged between two weeks and two years.
Key findings
Cognitive rehabilitation compared to no treatment
There was insufficient evidence to conclude that cognitive rehabilitation, as compared to no other treatment, led to better return to work, community integration or quality of life in adults with traumatic brain injury. We judged the quality of this evidence as low or very low because of poor reporting of both the methods used and the results.
Cognitive rehabilitation compared to other conventional rehabilitation
There was inadequate evidence to conclude that adults with traumatic brain injury who received cognitive rehabilitation had better return to work, independence in daily living, community integration or quality of life when compared to adults who received conventional rehabilitation. We judged the quality of evidence for these outcomes to vary between moderate and very‐low because of poor reporting of the methods used, different types of 'conventional' treatment and imprecise results.
Home‐based cognitive rehabilitation training compared to hospital‐based training
In one study on active military personnel, those who received a home programme for cognitive rehabilitation training had similar return to work when compared to those who received cognitive rehabilitation training in a hospital. We judged this evidence to be of moderate quality due to imprecise results.
Different types of cognitive rehabilitation compared against each other
One study compared trial‐and‐error type cognitive rehabilitation (cognitive didactic) to another type of cognitive rehabilitation that provided cues to avoid errors (functional‐experiential) for veterans or active military personnel with traumatic brain injury. The study found no evidence to suggest one type of cognitive rehabilitation was better than the other in improving return to work or the ability to live independently. We judged the quality of evidence to be of moderate (return to work) and low quality (ability to live independently) because of imprecise results.
None of the studies reported information about harms from cognitive rehabilitation.
Summary of findings
Background
Description of the condition
Traumatic brain injury (TBI) is defined as an alteration in brain function, or other evidence of brain pathology, caused by an external force (Menon 2010). TBI has become one of the leading causes of death and disability worldwide (Gean 2010). The incidence is highest in people aged 16 to 60 years (Chesnut 1998). Consequences of TBI range from physical disabilities to long‐term cognitive, social and behavioural deficits, resulting in family disruption, restriction in community participation, loss of earning potential, considerable expense over a lifetime and poor quality of life (Khan 2003).
Description of the intervention
Cognition is the process of knowing. Cognition includes the selection, acquisition, understanding and retention of information, and the application of the knowledge thus acquired in appropriate situations (Cicerone 2000). Cognitive dysfunction (or cognitive impairment) can be defined as functioning below expected normative levels or loss of ability in any area of cognitive functioning. Cognitive impairments include difficulties in arousal, attention, memory, problem solving, decision making and insight. These impairments impede a person's ability to perform their occupations in everyday life (Toglia 1991). As defined by the American Occupational Therapy Association's practice framework, and as referenced in other published literature, the term 'occupation' refers not just to paid employment, but also purposeful activities that people perform in their daily life such as work, self‐care (activities of daily living (ADL)), leisure activities or social participation (AOTA 2014; Ibrahim 2015).
The term cognitive rehabilitation has been widely discussed and used in a variety of contexts. However, there is no singular, consensus‐based definition. Cognitive rehabilitation refers to the methods to restore cognitive functions and to the techniques to compensate for the decline of cognitive functions (Sohlberg 1989). Various names have been used to describe cognitive rehabilitation strategies, including remedial, compensatory (Sarajuuri 2006), functional experiential, cognitive didactic (Vanderploeg 2008), errorless learning (Middleton 2012), multi‐context treatment (Toglia 1991), and intensive cognitive rehabilitation programme (Cicerone 2008). Most of these intervention strategies overlap, making it difficult to compare one strategy with another.
How the intervention might work
Cognitive rehabilitation refers to the therapeutic process of increasing or improving a person's capacity to process and use information to allow increased functioning in everyday life. This includes methods to restore cognitive functions, as well as techniques for compensating for the decline of cognitive functions. This could be achieved by various approaches, including 1. reinforcing, strengthening, or re‐establishing previously learned patterns of behaviour; 2. establishing new patterns through internal compensatory mechanisms; 3. establishing new patterns of activity through external compensatory mechanisms such as environmental structuring and support and 4. enabling people to adapt to their cognitive disability without establishing any new patterns of activity but with the existing patterns. Review articles published since the 2000s have suggested beneficial effects of cognitive rehabilitation strategies on specific cognitive aspects such as memory, visuospatial abilities, apraxia and aphasia in people with acquired brain injury (Cicerone 2000; Cicerone 2005; Cicerone 2011). Exact mechanisms of how each cognitive rehabilitation intervention works have not been elucidated. It is likely that a combination of the above factors might influence clinical improvements in cognitive functions.
Although focused interventions to improve specific cognitive aspects are commonplace, these programmes are geared towards bringing about an improvement in the overall performance of people with brain injury in their daily lives. This would include the ability to return to a vocation, to be independent in daily activities, to be able to live independently and to engage in interactions with the community. Neuropsychological tests for cognitive functions could correlate with functional outcome measures in people with TBI (Barman 2016). Considerable improvements in these aspects of daily functioning are likely to lead to better satisfaction with quality of life among people with brain injury (Juengst 2015).
Why it is important to do this review
Available systematic reviews on effectiveness of cognitive rehabilitation have looked at intermediate outcomes of cognitive performance and not definite endpoints such as return to work status. Previous reviews have also included studies on non‐traumatic brain injuries (Cicerone 2000; Cicerone 2005; Cicerone 2011). Moreover, the authors did not do meta‐analyses. In a related review, while doing a meta‐analysis on pre‐existing reviews, the authors reported limitations including reliance on a predominant number of single group pre‐post studies, differing control groups, heterogeneity and confounders such as different aetiologies, age and recovery levels (Rohling 2009). Several Cochrane Reviews on the effectiveness of cognitive rehabilitation in people with acquired brain injury caused by aetiologies such as stroke were unable to obtain conclusive evidence supporting or refuting the usefulness of such interventions in the short or long term (Bowen 2013; Chung 2013; Loetscher 2013). Given such conflicting conclusions from related literature, it is imperative that we assess the effectiveness of cognitive rehabilitation interventions on practically relevant occupational outcomes of return to work, independence in daily activities, ability to live independently, community integration and quality of life in people with TBI.
Objectives
To evaluate the effects of cognitive rehabilitation on return to work, independence in daily activities, community integration (occupational outcomes) and quality of life in people with traumatic brain injury, and to determine which cognitive rehabilitation strategy better achieves these outcomes.
Methods
Criteria for considering studies for this review
Types of studies
We included all randomized controlled trials (RCT; including parallel, factorial, wait‐list/cross‐over trials) of cognitive rehabilitation following TBI.
Types of participants
We included studies conducted with adults (aged 16 years and above) who had sustained a TBI of any clinical severity. We excluded studies if participants with non‐traumatic aetiology were also recruited.
Types of interventions
We included studies with any type of non‐pharmacological rehabilitation intervention aimed at improving cognitive functions. We included studies with non‐intervention controls or alternative interventions as a control group, categorized into four comparisons:
cognitive rehabilitation versus no treatment;
cognitive rehabilitation versus conventional treatment (conventional treatment included those rehabilitation interventions that did not have a specific cognitive strategy);
hospital‐based cognitive rehabilitation versus home programme;
one cognitive strategy versus another cognitive strategy.
Types of outcome measures
We included studies that reported at least one of the primary or secondary outcome measures.
We categorized outcomes into short term (less than three months), medium term (three to 12 months) and long term (more than one year).
Primary outcomes
Return to work.
Independence in ADL measured using standard tools (e.g. Functional Independence Measure (FIM)) or the status of independent living (or both).
Community integration measured using standard tools (e.g. Community Integration Questionnaire).
Secondary outcomes
Quality of life measured using standard tools (e.g. Perceived Quality of Life (PQOL) scale).
Search methods for identification of studies
The Cochrane Injuries Group trials search co‐ordinators conducted the following electronic searches.
Electronic searches
CENTRAL (the Cochrane Library; March 2017, Issue 3).
Ovid MEDLINE(R), Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid OLDMEDLINE(R) 1946 to March 2017
Embase Classic + Embase (OvidSP) 1947 to March 2017
PsycINFO (OvidSP) 1806 to March 2017
Clinical trial register (www.clinicaltrials.gov).
Controlled Trials metaRegister (www.controlled‐trials.com).
Search strategies are listed in Appendix 1; Appendix 2; Appendix 3 and Appendix 4.
Data collection and analysis
Selection of studies
Two sets of review authors (KSK) and (SS and AV worked in pair) independently undertook a preliminary screen of titles and abstracts, applying the inclusion and exclusion criteria. We resolved disagreements by mutual consent. We obtained the full‐text of these potentially relevant articles for further assessment. After the secondary screening, we have two studies awaiting cassification and we included nine studies in this review.
Data extraction and management
Three review authors (KSK independently; SS and AV worked in pair) extracted data on methods, participant characteristics, intervention characteristics and outcome measures of each trial.
Assessment of risk of bias in included studies
Three review authors (KSK independently; SS and AV worked in pair) assessed the risk of bias in the included trials as per the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). If there was any disagreement, we discussed this, and where necessary the fourth review author (AM) resolved the disagreement. For each study, we judged the following items as having a high, low or unclear risk of bias: sequence generation; allocation concealment; blinding of participants, personnel and outcome assessors; incomplete outcome data; selective outcome reporting and 'other' identified potential sources of bias like rehabilitation provider's and assessor's competency, their qualification and credentials, etc. We did not prespecify in our protocol the criteria to judge the overall risk of bias of each study (K SK 2009). Since our primary outcome, return to work, was an objective measure, we decided to classify individual studies as having high risk of bias if one or more of the domains of random sequence generation, allocation concealment and blinding of outcome assessment were at high risk of bias. We supported our judgements with observations and with direct quotes from the articles where possible.
Measures of treatment effect
We calculated the treatment effects by using data tables in Review Manager 5 (RevMan 2014). We used risk ratios (RRs) for dichotomous outcomes, and mean differences (MDs) or standardized mean differences (SMDs) for continuous outcomes and reported their 95% confidence intervals (CI).
Dealing with missing data
We contacted authors of included studies when necessary to clarify study methodology and obtain missing numerical data.
Assessment of heterogeneity
We considered similarity of participants, intervention, control and outcomes of the included studies to assess homogeneity of the results. We considered participants as homogeneous when they were people with TBI. We considered interventions and controls as homogeneous when they fitted the descriptions explained in the Types of interventions section. We considered outcomes as homogeneous when they fitted in the descriptions explained in the Types of outcome measures section.
In analyses that included data from more than one trial, we used the I² statistic to measure heterogeneity among the trials for each analysis. We considered I² values more than 50% as substantial heterogeneity.
Data synthesis
We pooled RRs for dichotomous outcomes and MDs for continuous outcomes. When studies reported a continuous outcome using different tools, we calculated SMDs. When we had more than one study contributing data for an outcome, and if we regarded them to be sufficiently homogeneous, we performed a meta‐analysis. All statistical analyses were performed using Review Manager 5 (RevMan 2014). When heterogeneity was indicated by an I² statistic less than 50%, we used a fixed‐effect model. We decided to use a random‐effects model when the I² statistic was greater than 50%, and to not perform a meta‐analysis if the I² statistic was greater than 80%. We did not prespecify these I² statistic cutoffs in our protocol (K SK 2009).
We used the online computer programme GRADEpro GDT to assess the quality of evidence across studies and to generate 'Summary of findings' tables for the comparisons (GRADEpro 2014). We assessed the domains of limitations in study design, consistency of results, directness, precision and publication bias to determine the quality of study as per the guidelines to use GRADEpro. We reported our justifications for judgement in each of these domains as footnotes in the 'Summary of findings' tables. We judged the study design to have limitations when the studies contributing data to the outcome in a comparison had unclear or high risk of bias for randomization, unclear allocation concealment or blinding of outcome assessment.
Subgroup analysis and investigation of heterogeneity
We did not identify enough studies that could be included in the analysis to warrant subgroup analysis at this time.
Sensitivity analysis
We performed sensitivity analyses to assess the robustness of our conclusions from analyses by including only studies that we judged to have a low risk of bias.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies tables.
Results of the search
We identified 3369 records from our search. Of the 3369 records retrieved, we identified 50 potentially relevant records after discarding reports that were duplicates and that were not relevant to this review. We scrutinized the full texts of the 50 studies. Of these 50 studies, we excluded 39 studies. Seven studies were non‐randomized/quasi‐randomized studies, nine did not meet the inclusion criteria, five had an intervention that was not appropriate for this review, and 18 studies did not report the outcomes of interest for this review. There were 11 studies left for inclusion. Of this 11, two studies are awaiting classification, nine RCTs met the eligibility criteria and so we included them. We describe the process of selecting the included trials in Figure 1.
1.
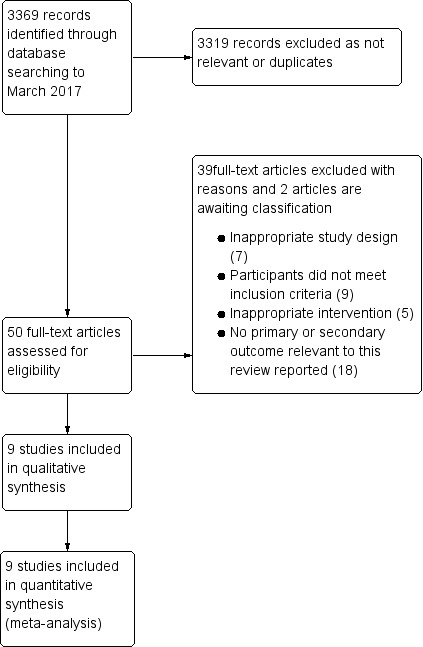
PRISMA study flow diagram.
Included studies
We describe the nine included RCTs in detail in the Characteristics of included studies table. The nine included trials randomized 790 participants.
Study designs
Nine of the included studies were RCTs. Seven trials had parallel arm controls. Two studies that employed a wait‐list control strategy, in which participants were randomly allocated to an immediate‐intervention arm or to a control group that was placed on a wait‐list before they received the intervention, analysed data only for the outcomes that were assessed immediately on completion of the wait‐list period (Bornhofen 2008a; Cantor 2014).
Country and time period
One of the included studies was conducted before the year 2000, while the remainder were performed between 2000 and 2012. Seven studies had been carried out in the US, and one each in Australia and Hong Kong (China).
Type of settings and participants
Eight studies were conducted by rehabilitation centres, three of which were US army centres. Four studies recruited inpatients, while five used outpatient settings. Among the seven studies that administered individual therapies, three had additional group therapy components.
Five studies recruited people with moderate‐to‐severe brain injury, one severe brain injury, one moderate brain injury, one mild‐to‐moderate brain injury and one at least mild brain injury.
Sample sizes
The number of participants was fewer than 25 in three studies, more than 25 but fewer than 75 in three studies, more than 75 but fewer than 300 in two studies and more than 300 in one study.
Interventions
Ten study arms in nine included studies examined cognitive rehabilitation interventions. One study arm assessed interventions for emotional perception (Bornhofen 2008a). One study arm assessed the effect of a Short Term Executive Plus (STEP) programme (Cantor 2014). One study arm assessed Cognitive Symptom Management and Rehabilitation Therapy (cogSMART) (Twamley 2014). Two study arms examined interventions for self‐awareness (Cheng 2006; Goverover 2007). One study arm evaluated a categorization programme (Constantinidou 2008). Four study arms in three studies assessed methods of comprehensive cognitive rehabilitation strategies (Cicerone 2008; Salazar 2000; Vanderploeg 2008).
Type of control group
Two studies used a wait‐list control group (Bornhofen 2008a, Cantor 2014). Four studies compared an active cognitive rehabilitation programme to a standard/conventional rehabilitation programme (Cheng 2006; Cicerone 2008; Constantinidou 2008; Goverover 2007). One study compared an inpatient programme to a limited home programme (Salazar 2000). One study compared a combination of cognitive rehabilitation and supported employment against a control group that received supported employment only (Twamley 2014). One study compared two active interventions (Vanderploeg 2008).
Outcomes
Four studies reported return to work (Cicerone 2008; Salazar 2000; Twamley 2014; Vanderploeg 2008).
One study reported functional independence defined as the ability to live independently with less than three hours of assistance in one week (Vanderploeg 2008). One study reported independence in ADL using FIM (Cheng 2006), and one study used Assessment of Motor and Process Skills (AMPS) scale (Goverover 2007).
Three studies reported community integration as assessed by Community Integration Questionnaire (Cicerone 2008; Constantinidou 2008; Goverover 2007), and one study reported using the Sydney Psychosocial Reintegration Scale (SPRS) (Bornhofen 2008a).
Two studies reported quality of life assessment using the PQOL scale (Cantor 2014; Cicerone 2008).
Follow‐up
Short‐term
There were five studies in which the last outcome measurement was at the end of the intervention (Bornhofen 2008a; Cantor 2014; Cheng 2006; Constantinidou 2008; Goverover 2007). In one study, the last outcome measurement was within two weeks of completion of the intervention (Twamley 2014).
Medium‐term
In two studies, last follow‐up measurement was six months to one year after intervention (Cicerone 2008; Vanderploeg 2008).
Long‐term
There was one study in which the last follow‐up measurement was more than one year after the intervention (Salazar 2000).
Excluded studies
We excluded 39 studies. See Characteristics of excluded studies table for details.
Study design: seven studies were not RCTs (Braverman 1999; Culley 2010; Dawson 2013; Fish 2007; Man 2006a; Man 2006b; Tam 2004).
Participants: nine studies had recruited participants with non‐traumatic aetiology of brain injury such as stroke (Bertens 2015; Bjorkdahl 2013; Bovend’Eerdt 2010; Hallock 2016; Park 2015; Spikman 2010; Tlustos 2016; Tornas 2016; Yip 2013).
Intervention: five studies did not involve interventions that could be categorized as cognitive rehabilitation (Bell 2005; Lannin 2014; Niemann 1990; Tiersky 2005; Trexler 2016).
Outcomes: 18 studies did not report any of the primary or secondary outcomes relevant for this review (Bornhofen 2008b; Bourgeois 2007; Couillet 2010; Dahlberg 2007; Dirette 1999; Dou 2006; Hewitt 2006; Hildebrandt 2006; Kaschel 2002; Kurowski 2013; Neistadt 1992; Neumann 2015; Niemann 1990; Rath 2003; Richter 2015; Ryan 1988; Shum 2011; Thickpenny‐Davis 2007).
Risk of bias in included studies
Our judgements about overall risk of bias across all included studies are summarized in Figure 2. Our judgements about each risk of bias item for each included study are depicted in Figure 3. Details about each individual study are provided in the 'Risk of bias' sections accompanying the Characteristics of included studies table.
2.
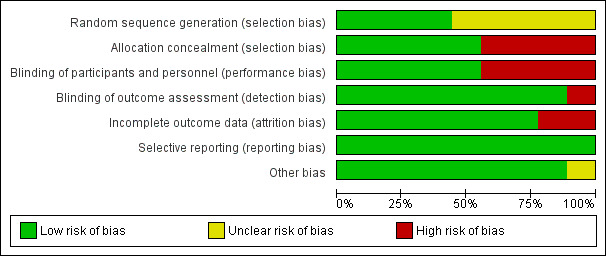
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
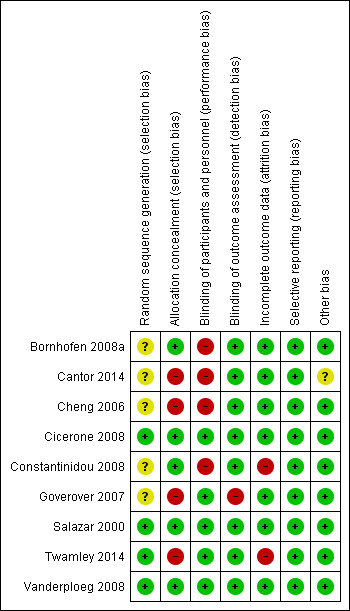
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
We judged four studies that explained the method of sequence generation to have low risk of bias (Cicerone 2008; Salazar 2000; Twamley 2014; Vanderploeg 2008). We judged the five studies that did not adequately describe the method of random sequence generation as having unclear risk of bias (Bornhofen 2008a; Cantor 2014; Cheng 2006; Constantinidou 2008; Goverover 2007).
Allocation concealment
Five studies reported methods to ensure concealment of allocation, and we judged these as having low risk of bias for this item (Bornhofen 2008a; Cicerone 2008; Constantinidou 2008; Salazar 2000; Vanderploeg 2008). We regarded the methodology used in four studies as inadequate to ensure allocation concealment, and judged them to have a high risk of bias (Cantor 2014; Cheng 2006; Goverover 2007; Twamley 2014).
Blinding
It is not possible to implement blinding of participants and personnel in wait‐list controlled trials by design. Three studies described adequate methods for blinding of participants and outcome assessors (Cicerone 2008; Salazar 2000; Vanderploeg 2008). Though Goverover 2007 and Twamley 2014 did not adequately describe measures to ensure blinding of participants and personnel, we judged them as having low risk of bias for this item since the key objective outcomes were unlikely to be influenced by blinding or the lack of it. We regarded four studies to have a high risk of performance bias since self‐reported outcomes are likely to be influenced by the knowledge of the intervention arm to which the trial participants belong (Bornhofen 2008a; Cantor 2014; Cheng 2006; Constantinidou 2008).
We judged blinding of outcome assessors as adequate and of low risk of bias in all but one (Goverover 2007) studies.
Incomplete outcome data
Two studies reported a high dropout rate of more than 30%, and we judged these as having a high risk of attrition bias (Constantinidou 2008; Twamley 2014). We judged all the other included studies to have a low risk of bias with respect to incomplete outcome data because they reported dropout rates less than 20% of those recruited (Bornhofen 2008a; Cantor 2014; Cheng 2006; Cicerone 2008; Goverover 2007; Salazar 2000; Vanderploeg 2008). Details including the reasons participants dropped out were also described adequately.
Selective reporting
We were able to locate prospectively registered protocols of two studies (Cantor 2014; Twamley 2014). We judged all the included studies to have a low risk of bias with respect to selective reporting, if either the studies reported all key intended outcomes mentioned in the protocol, or in our judgement that all outcomes that would be expected of such a study were reported.
Other potential sources of bias
We did not identify any other significant potential sources of bias in the included studies.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4
Summary of findings for the main comparison. Cognitive rehabilitation compared to no treatment for occupational outcomes after traumatic brain injury.
| Cognitive rehabilitation compared to no treatment for occupational outcomes after traumatic brain injury | ||||||
| Patient or population: traumatic brain injury ‐ mild, moderate or severe Setting: outpatient centres in US and Australia Intervention: cognitive rehabilitation Comparison: no treatment | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with no treatment | Risk with cognitive rehabilitation | |||||
|
Return to work Assessed by attainment of work within 14 weeks (medium‐term) of initiating intervention |
Study population | RR 1.80 (0.74 to 4.39) | 50 (1 RCT) | ⊕⊝⊝⊝ Very low1,2 | ‐ | |
| 278 per 1000 | 500 per 1000 (206 to 1000) | |||||
|
Community integration
Assessed with Sydney Psychosocial Reintegration Scale (self‐reported) Scores range from 0 to 72, higher scores indicate better reintegration. Follow‐up: 1 month (short‐term) |
The mean community integration was 54.5 | MD 2.90 lower (12.57 lower to 6.77 higher) | ‐ | 12 (1 RCT) | ⊕⊕⊝⊝ Low1,3 | ‐ |
|
Quality of life Assessed with Life‐3. Follow‐up: none |
The mean quality of life was 4.0 | MD 0.30 higher (0.18 lower to 0.78 higher) | ‐ | 98 (1 RCT) | ⊕⊕⊝⊝ Low1,3 | ‐ |
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RCT: randomized controlled trial; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | ||||||
1 Downgraded by 1 level because the study was at high risk of bias.
2 Downgraded by 2 levels because of imprecision. Confidence interval overlapped with both 0.75 and 1.25.
3 Downgraded by 1 level because of imprecision. Total population was size fewer than 400.
Summary of findings 2. Cognitive rehabilitation compared to conventional treatment for people with traumatic brain Injury.
| Cognitive rehabilitation compared to conventional treatment for people with traumatic brain injury | ||||||
| Patient or population: people with traumatic brain injury Settings: inpatient and outpatient rehabilitation units in Hong Kong and the US Intervention: cognitive rehabilitation Comparison: conventional treatment | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Conventional treatment | Cognitive rehabilitation | |||||
| Return to work Return to work status Follow‐up: 6 months (medium‐term) | 412 per 1000 | 589 per 1000 (358 to 959) | RR 1.43 (0.87 to 2.33) | 68 (1 study) | ⊕⊕⊝⊝ L ow1 | ‐ |
|
Independence in ADL
FIM, with 18 items in basic and psychosocial functional activities. Score ranges from 0 to 126; higher scores indicate higher functional independence. Assessment of motor and process skills, score ranges from 4 to 144; higher scores indicate better independence in ADL. Follow‐up: 3‐4 weeks (short‐term) |
Mean FIM score in the control group of the trial reporting this scale was 100 | The mean FIM score in the intervention group at 4 weeks was 0.16 lower (10.35 lower to 10.18 higher) | SMD ‐0.01 (‐0.62 to 0.61) | 41 (2 studies) | ⊕⊝⊝⊝ Very low2,3 | Analysis conducted on a standardized scale with data from studies that used different assessor‐rated scales of independence in daily living (FIM and Assessment of Motor and Process Skills (AMPS)). The effect size of the meta‐analysis has been back transformed to the FIM scale by using the mean standard deviation of the control group of the study that used FIM scale to report this outcome. |
|
Community integration
Community Integration Questionnaire. Score ranges from 0 to 29, higher scores indicate better community integration. Follow‐up: mean 6 months (medium‐term) |
The mean community integration ranged across control groups from 12.9 to 17.59 points4 | The mean community integration in the intervention groups was 0.05 higher (1.51 lower to 1.62 higher) | ‐ | 123 (3 studies) | ⊕⊕⊝⊝ Low3,5 | ‐ |
|
Quality of life
Perceived Quality of Life scale. Scores range from 10 to 100, higher scores indicate better quality of life. Follow‐up: 6 months (medium‐term) |
The mean quality of life in the control groups was 59.6 points6 | The mean quality of life in the intervention groups was 6.5 higher (2.57 lower to 15.57 higher) | ‐ | 68 (1 study) | ⊕⊕⊕⊝ Moderate3 | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). ADL: activities of daily living; CI: confidence interval; FIM: Functional Independence Measure; RR: risk ratio; SMD: standardized mean difference. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded by 2 levels because of imprecision. Confidence intervals overlapped 1 and 1.25. Number of events was fewer than 300. 2 Downgraded by 2 levels because of very serious risk of bias due to unclear random sequence generation, allocation concealment and blinding in the two studies. 3 Downgraded by 1 level because of imprecision. Total population size was fewer than 400. 4 Final scores using Community Integration Questionnaire. 5 Downgraded by 1 level because of serious risk of bias in two of the three studies. 6 Final scores on Perceived Quality of Life scale.
Summary of findings 3. Hospital‐based cognitive rehabilitation compared to home programme for people with traumatic brain injury.
| Hospital‐based cognitive rehabilitation compared to home programme for people with traumatic brain injury | ||||||
| Patient or population: active duty military personnel within 3 months of moderate‐to‐severe traumatic brain injury Settings: army medical centre, US Intervention: hospital‐based cognitive rehabilitation Comparison: home programme | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Home programme | Hospital‐based cognitive rehabilitation | |||||
| Return to work Return to work status Follow‐up: 24 months (long‐term) | 943 per 1000 | 896 per 1000 (802 to 991) | RR 0.95 (0.85 to 1.05) | 120 (1 study) | ⊕⊕⊕⊝ Moderate1 | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded by 1 level because of imprecision. The number of events was fewer than 300.
Summary of findings 4. Cognitive didactic therapy compared to functional experiential therapy for people with traumatic brain injury.
| Cognitive didactic therapy compared to functional experiential therapy for people with traumatic brain injury | ||||||
| Patient or population: adult veterans or active duty military service personnel with moderate‐to‐severe traumatic brain injury Settings: acute inpatient rehabilitation brain injury programmes at 4 Veterans Administration medical centres, US Intervention: cognitive didactic therapy Comparison: functional experiential therapy | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Functional experiential therapy | Cognitive didactic therapy | |||||
| Return to work Return to work status Follow‐up: 1 year (medium‐term) | 354 per 1000 | 389 per 1000 (294 to 516) | RR 1.10 (0.83 to 1.46) | 366 (1 study) | ⊕⊕⊕⊝ Moderate1 | ‐ |
| Independence in ADL Structured interview Follow‐up: 1 year (medium‐term) | 616 per 1000 | 554 per 1000 (462 to 665) | RR 0.90 (0.75 to 1.08) | 366 (1 study) | ⊕⊕⊝⊝ Low2 | ‐ |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). A DL: activities of daily living; CI: confidence interval; RR: risk ratio. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1 Downgraded by 1 level because of imprecision. Confidence interval overlapped with both 1 and 1.25. The total number of events was fewer than 300. 2 Downgraded by 2 levels because of imprecision. Confidence interval overlapped with both 0.75 and 1.25. The total number of events was fewer than 300.
We included data from nine studies and we present these within four main comparisons:
cognitive rehabilitation versus no treatment (three studies, 160 participants);
cognitive rehabilitation versus conventional treatment (four studies, 144 participants);
hospital‐based cognitive rehabilitation versus home programme (one study, 120 participants);
one cognitive strategy (cognitive didactic) versus another cognitive strategy (functional experiential) (one study, 366 participants).
1. Cognitive rehabilitation versus no treatment
We found three studies comparing cognitive rehabilitation versus no treatment (Bornhofen 2008a; Cantor 2014; Twamley 2014; 160 participants; Table 1).
1.1. Return to work
Twamley 2014 found no difference in return to work in 14 weeks (medium‐term) between cognitive rehabilitation and no intervention (RR 1.80, 95% CI 0.74 to 4.39; Analysis 1.1).
1.1. Analysis.
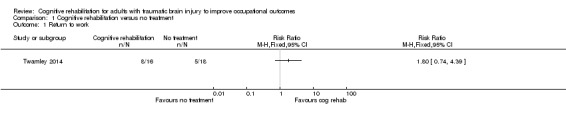
Comparison 1 Cognitive rehabilitation versus no treatment, Outcome 1 Return to work.
1.2. Independence in activities of daily living
We found no studies reporting independence in ADL.
1.3. Community integration
Bornhofen 2008a found no difference between cognitive rehabilitation and no treatment in community integration at one month follow‐up (short‐term) measured using the SPRS (MD ‐2.90, 95% CI ‐12.57 to 6.77; Analysis 1.2).
1.2. Analysis.
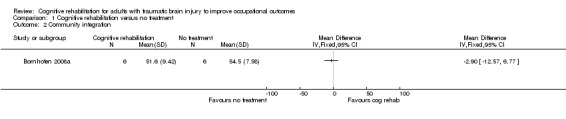
Comparison 1 Cognitive rehabilitation versus no treatment, Outcome 2 Community integration.
1.4. Quality of life
Cantor 2014 reported no difference in quality of life assessed with Life‐3 between cognitive rehabilitation and no intervention on completion of 12 weeks of intervention without any follow‐up (MD 0.30, 95% CI ‐0.18 to 0.78; Analysis 1.3).
1.3. Analysis.
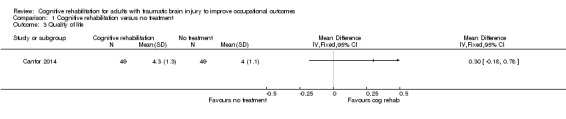
Comparison 1 Cognitive rehabilitation versus no treatment, Outcome 3 Quality of life.
2. Cognitive rehabilitation versus conventional treatment
We found four studies comparing cognitive rehabilitation versus conventional treatment (Cheng 2006; Cicerone 2008; Constantinidou 2008; Goverover 2007; 144 participants; Table 2).
2.1. Return to work
Cicerone 2008 found no difference in return to work at six months (medium‐term) between cognitive rehabilitation and conventional treatment (RR 1.43, 95% CI 0.87 to 2.33; 68 participants; Analysis 2.1).
2.1. Analysis.
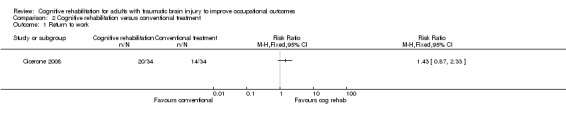
Comparison 2 Cognitive rehabilitation versus conventional treatment, Outcome 1 Return to work.
2.2. Independence in activities of daily living
Cheng 2006 and Goverover 2007 found no difference between cognitive rehabilitation and conventional treatment in improving independence in ADL by four weeks (short‐term), measured using the FIM and AMPS (SMD ‐0.01, 95% CI ‐0.62 to 0.61; 41 participants; Analysis 2.2).
2.2. Analysis.
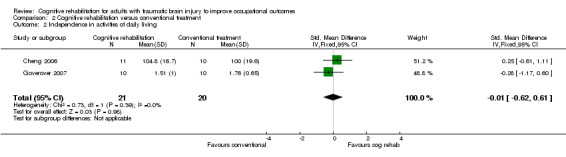
Comparison 2 Cognitive rehabilitation versus conventional treatment, Outcome 2 Independence in activities of daily living.
2.3. Community integration
Cicerone 2008, Constantinidou 2008 and Goverover 2007 found no statistically significant effect of cognitive rehabilitation compared with conventional treatment on community integration measured by six months (medium‐term) with the Community Integration Questionnaire (MD 0.05, 95% CI ‐1.51 to 1.62; 123 participants; Analysis 2.3).
2.3. Analysis.
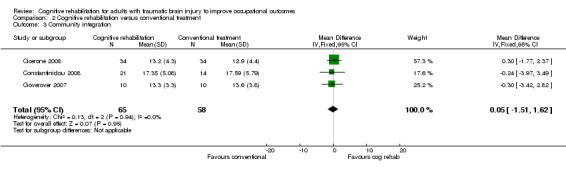
Comparison 2 Cognitive rehabilitation versus conventional treatment, Outcome 3 Community integration.
Sensitivity analysis: risk of bias
Removing the studies we judged as having an unclear or high risk of bias for random sequence generation or allocation concealment left only one study (Cicerone 2008; 68 participants), demonstrating a similar direction of effect (MD 0.30, 95% CI ‐1.77 to 2.37).
2.4. Quality of life
Cicerone 2008 found no difference between cognitive rehabilitation and conventional treatment in terms of quality of life measured by six months (medium‐term) using the PQOL scale (MD 6.50, 95% CI ‐2.57 to 15.57; 68 participants; Analysis 2.4).
2.4. Analysis.
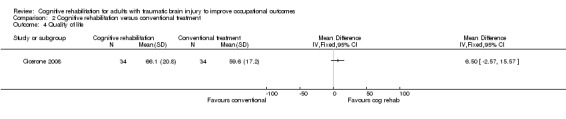
Comparison 2 Cognitive rehabilitation versus conventional treatment, Outcome 4 Quality of life.
3. Hospital‐based cognitive rehabilitation versus home programme
We found one study comparing hospital‐based cognitive rehabilitation versus home programme (Salazar 2000; 120 participants; Table 3).
3.1. Return to work
Salazar 2000 found no difference in rates of return to work between hospital‐based cognitive rehabilitation and home cognitive programme in follow‐up assessment at two years (long‐term) (RR 0.95, 95% CI 0.85 to 1.05; 120 participants; Analysis 3.1).
3.1. Analysis.
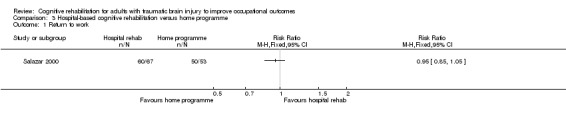
Comparison 3 Hospital‐based cognitive rehabilitation versus home programme, Outcome 1 Return to work.
3.2. Independence in activities of daily living
We found no studies reporting independence in activities of daily living.
3.3. Community integration
We found no studies reporting community integration.
3.4. Quality of life
We found no studies reporting quality of life.
4. One cognitive strategy (cognitive didactic) versus another cognitive strategy (functional experiential)
We found one study comparing one cognitive strategy (cognitive didactic) versus another cognitive strategy (functional experiential (Vanderploeg 2008; 366 participants; Table 4).
4.1. Return to work
Vanderploeg 2008 showed no difference between one cognitive strategy (cognitive didactic) and another cognitive strategy (functional experiential) in terms of return to work in one year (medium‐term) (RR 1.10, 95% CI 0.83 to 1.46; 366 participants; Analysis 4.1).
4.1. Analysis.
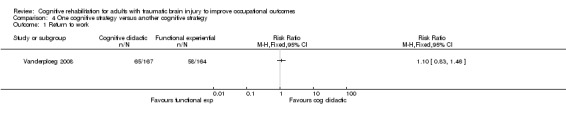
Comparison 4 One cognitive strategy versus another cognitive strategy, Outcome 1 Return to work.
4.2. Independence in activities of daily living
Vanderploeg 2008 found no difference in independent living status in one year (medium‐term) when one cognitive strategy (cognitive didactic) was compared with another cognitive strategy (functional experiential) (RR 0.90, 95% CI 0.75 to 1.08; 366 participants; Analysis 4.2).
4.2. Analysis.
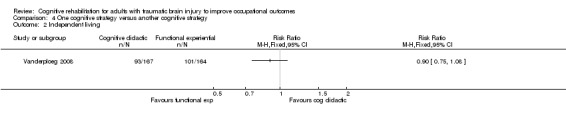
Comparison 4 One cognitive strategy versus another cognitive strategy, Outcome 2 Independent living.
4.3. Community integration
We found no studies reporting community integration.
4.4. Quality of life
We found no studies reporting quality of life.
GRADE assessment
For all comparisons, we assessed the quality of the evidence using GRADE. We judged studies contributing data to the first and second comparisons to have high risk of bias due to unclear random sequence generation, inadequate allocation concealment and blinding, and we downgraded the quality of evidence by one level. In all the comparisons, when there were fewer than 400 participants or if the meta‐analysis results had wide CIs that introduced uncertainty about appreciable clinical benefit or harm, we downgraded for imprecision. Overall, the quality of the evidence for outcomes across all comparisons was moderate to very low. The arguments on which we based our GRADE assessment decisions for all the comparisons that reported the outcome of return to work are given in Table 5. We report our assessment of the level of evidence provided by all key outcomes in Table 1; Table 2; Table 3; and Table 4.
1. GRADE assessment for return to work.
| Comparison | Risk of bias | Inconsistency | Indirectness | Imprecision | Publication bias | Level of evidence |
| Cognitive rehabilitation vs no treatment | 1 study, downgraded by 1 level | N/A | No | 50 participants. CI overlapped with RR 0.75 and RR 1.25: downgraded by 2 levels | N/A | Very low quality |
| Cognitive rehabilitation vs conventional treatment 6 months' follow‐up |
1 study, not downgraded | N/A | No | 68 participants. CI overlapped with RR 1 and RR 1.25: downgraded 2 levels | N/A | Low quality |
| Hospital‐based cognitive rehabilitation vs home programme 24 months' follow‐up |
1 study, not downgraded | N/A | No | 120 participants, downgraded by 1 level | N/A | Moderate quality |
| Cognitive didactic therapy vs functional experiential 1 year' follow‐up |
1 study, not downgraded | N/A | No | 366 participants. CI overlapped with RR 1 and RR 1.25: downgraded by 1 level | N/A | Moderate quality |
CI: confidence interval; N/A: not available; RR: risk ratio.
Discussion
Summary of main results
Cognitive rehabilitation when compared to no intervention did not lead to better return to work. Evidence for this was of very low quality. Cognitive rehabilitation did not result in better community integration or quality of life, as supported by low‐quality evidence.
There was no difference between cognitive rehabilitation and a conventional rehabilitation programme for return to work (low‐quality evidence), independence in ADL (very low‐quality evidence) and community integration (low‐quality evidence). There was no difference in quality of life between cognitive rehabilitation and conventional rehabilitation. Evidence for this was of moderate quality.
For active duty military personnel with moderate‐to‐severe closed head injury, there was no difference between eight weeks of cognitive rehabilitation provided as a home programme and hospital‐based cognitive rehabilitation in achieving return to work at one year. This was supported by moderate‐quality evidence.
There was no difference between one intervention strategy (cognitive didactic) and another (functional experiential) for adult veterans or active duty military service personnel with moderate‐to‐severe TBI in return to work (moderate‐quality evidence) or in independent living (low‐quality evidence).
Overall completeness and applicability of evidence
Due to the absence of accepted standardizations for many cognitive intervention strategies, the included studies used various terminologies to describe the type of interventions, such as awareness training, categorization programme and holistic neuropsychological rehabilitation programme. Similarly, components of 'conventional treatment' varied between different trials. The term 'conventional treatment' could not be generalized, since each rehabilitation centre would have its own 'convention'.
There was no consistent rationale reported for a few aspects of interventions in the included studies, such as individual therapy versus group therapy; daily therapy versus intermittent therapy; varying length of interventions (ranging from a few weeks to a few months) and home‐based versus hospital‐based cognitive rehabilitation.
The outcomes assessed in the included studies varied too, ranging from assessment of one specific domain of cognition such as 'attention span', to categorical endpoints such as 'return to work'. There was reasonable uniformity in the scales used to report functional independence and community integration.
Seven of the included studies were performed in the US, and one each in Australia and China (Hong Kong). Consequently, there is an absence of data from low‐ and middle‐income regions of the world.
There was no uniformity of inclusion criteria throughout, with different screening tools used including Glasgow Coma Score (GCS), Rancho Los Amigos (RLA) and post‐traumatic amnesia. Three studies recruited participants based on RLA stages ranging from 5 to 7. One study included high functioning people (Cicerone 2008); one study included people with GCS 15/15 (Cheng 2006); and one study recruited people with severe chronic brain injury with apparent disregard or lack of awareness of social cues (Bornhofen 2008a).
There was a considerable difference among the studies in terms of chronicity of brain injury at the time of recruitment. Only one study specifically included those within three months of injury (Salazar 2000).
Quality of the evidence
Quality of evidence for most of the outcomes was low to very low, overall. Many studies did not adequately report the methodology used. Random sequence generation and allocation concealment were commonly not reported. Imprecision of the results and risk of bias were the most common causes for downgrading the level of evidence. Assessment of precision for continuous outcomes that were measured by scores was challenging due to the lack of proven or cursory estimates of minimally important clinical benefits or harms.
Description of rationale for choice of interventions, intensity and duration was generally lacking. Sample size determination was not explained in most studies.
Fewer than half of the included studies had reported return to work. Many outcomes that we assessed were reported by single studies only, thus precluding meta‐analysis.
Potential biases in the review process
Though the search strategy included various terms used to mean 'cognitive rehabilitation', it is possible that some studies might have been missed since there is no globally accepted definition for what constitutes cognitive rehabilitation. Also, there are other existing Cochrane Reviews that focus on specific subdomains of cognition such as memory and executive functions. It is likely that our use of the wider terminology of 'cognitive rehabilitation' might not have covered all studies that have evaluated these subdomains.
Publication bias could not be studied with funnel plot asymmetry since we could only include very few studies in each comparison. However, such bias is unlikely because none of the interventions had evidence of significant effects (Dwan 2013).
Agreements and disagreements with other studies or reviews
One narrative systematic review of cognitive rehabilitation interventions in brain injury and stroke assessed various components of cognitive functions, but did not include occupational outcomes (Cicerone 2011). Moreover, the review included non‐randomized studies, and the authors reported that biases of included studies were not analysed. A meta‐analysis of the data from an earlier version of the review also did not report occupational outcomes (Rohling 2009). Though these two reviews indicated a possible beneficial effect of cognitive rehabilitation strategies in improving specific aspects of cognition, there is a complete lack of reporting of objective outcomes such as return to work.
It is possible that focused cognitive rehabilitation strategies bring about beneficial effects in one or more individual cognitive functions. These are probably not translated into significant, appreciable changes in return to work status or daily activities and other occupational outcomes that are reported in this review. If such a lack of causal effect could be confirmed, it might have significant implications for the goal setting process, and shared decision making in rehabilitation of people with TBI.
Authors' conclusions
Implications for practice.
There is low‐ to very low‐quality evidence that cognitive rehabilitation does not result in better return to work, community integration or quality of life in short‐ to medium‐term follow‐up when compared to no treatment for people with traumatic brain injury.
There is moderate‐ to very low‐quality evidence that cognitive rehabilitation when compared to conventional rehabilitation treatment does not result in better return to work, independence in activities of daily living, community integration or quality of life in short‐ to medium‐term follow‐up for people with traumatic brain injury.
There is moderate‐quality evidence that hospital‐based cognitive rehabilitation is similar to home‐based rehabilitation in improving return to work among active duty military personnel with moderate‐to‐severe traumatic brain injury at long‐term follow‐up.
There is moderate‐ to low‐quality evidence that one cognitive strategy (cognitive‐didactic) is no different from another (functional experiential) in improving return to work and independent living at medium‐term follow‐up.
Implications for research.
The current evidence does not conclusively support or refute the effectiveness of any particular form of cognitive rehabilitation strategy. Further trials are therefore warranted to arrive at conclusive evidence. We suggest the following factors be considered in future trials to improve the evidence base.
Recruitment: recruiting participants who have similar characteristics of severity and duration of brain injury, or factoring the baseline differences by stratification at the time of recruitment, is likely to improve the robustness of the results. Considering return to work as the primary outcome, if the control group return to work rate with just the conventional rehabilitation treatment is 35%, to be able to detect an increased return to work rate of least 55% with cognitive rehabilitation intervention, assuming α = 0.05 and β = 0.80, a sample size of 212 would be needed.
Outcomes: participant‐reported outcome measures and outcomes that are practically relevant occupational endpoints should be given priority over surrogate or intermediate measures while assessing outcomes of rehabilitation programmes. Longer‐term outcomes measured in follow‐up durations of more than one year are needed.
Setting: trials need to validate evidence for potential advantages of home‐ and community‐based cognitive rehabilitation interventions as against hospital‐based cognitive rehabilitation. Effects of such interventions in resource‐constrained settings (that include high‐, low‐ and middle‐income country settings) should also be studied.
Reporting: interventions should be clearly defined and reported using the TIDieR checklist (Hoffmann 2014) so that homogeneity of similar trials can be assessed. The population sampled, content of interventions and outcome measures should be detailed systematically to enable replication and comparison of outcomes across studies.
Acknowledgements
We would like to acknowledge Mr Kalidas for the help with the screening process during the search update. We would like to thank Ian Roberts, Emma Sydenham, Deirdre Beecher and Karen Blackhall of the Cochrane injuries group for the help with literature search. We thank Cristina Bornhofen, Fofi Constantinidou and Yael Goverover for providing further information about their studies. The principal author Suresh Kumar was a recipient of the 'Capacity strengthening strategic award' by the Wellcome Trust and the Public Health Foundation of India for his PhD programme. This facilitated receiving technical and scientific support from the Cochrane Injuries Group at the London School of Hygiene and Tropical Medicine, especially with the initial run of the searches.
We thank Jani Ruotsalainen, Managing Editor, and Jos Verbeek, Coordinating Editor from Cochrane Work Review Group for their help in all stages of the current review. We also thank Editors Karen Nieuwenhuijsen and external peer referee Mitchell Batavia for their comments and Anne Lawson for copy editing the text.
Appendices
Appendix 1. CENTRAL search strategy
CENTRAL (the Cochrane Library) to May 2016 #1MeSH descriptor: [Craniocerebral Trauma] explode all trees #2MeSH descriptor: [Brain Edema] explode all trees #3MeSH descriptor: [Glasgow Coma Scale] explode all trees #4MeSH descriptor: [Glasgow Outcome Scale] explode all trees #5MeSH descriptor: [Unconsciousness] explode all trees #6MeSH descriptor: [Cerebrovascular Trauma] explode all trees #7((head or crani* or cerebr* or capitis or brain* or forebrain* or skull* or hemispher* or intra?cran* or inter?cran* or intracran* or intercran*) near/3 (injur* or trauma* or damag* or lesion* or wound* or destruction* or oedema* or edema* or contusion* or concus* or fracture*)) (Word variations have been searched) #8((head or crani* or cerebr* or brain* or intra?cran* or inter?cran* or intracran* or intercran*) near/3 (haematoma* or hematoma* or haemorrhag* or hemorrhag* or bleed* or pressur*)) (Word variations have been searched) #9(Glasgow next (coma or outcome) next (scale* or score*)) (Word variations have been searched) #10"rancho los amigos scale" (Word variations have been searched) #11("diffuse axonal injury" or "diffuse axonal injuries") (Word variations have been searched) #12((brain or cerebral or intracranial) near/3 (oedema or edema or swell*)) (Word variations have been searched) #13((unconscious* or coma* or concuss* or 'persistent vegetative state') near/3 (injur* or trauma* or damag* or wound* or fracture* or contusion* or haematoma* or hematoma* or haemorrhag* or hemorrhag* or pressur*)) (Word variations have been searched) #14(#1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13) #15MeSH descriptor: [Activities of Daily Living] explode all trees #16MeSH descriptor: [Rehabilitation, Vocational] explode all trees #17MeSH descriptor: [Occupational Therapy] explode all trees #18MeSH descriptor: [Cognition Disorders] explode all trees #19MeSH descriptor: [Memory Disorders] explode all trees #20MeSH descriptor: [Attention] explode all trees #21MeSH descriptor: [Perceptual Disorders] explode all trees #22MeSH descriptor: [Quality of Life] explode all trees #23MeSH descriptor: [Karnofsky Performance Status] explode all trees #24cognitive rehabilitation:ti,ab,kw (Word variations have been searched) #25"Quality of Life":ti,ab,kw (Word variations have been searched) #26community integration:ti,ab,kw (Word variations have been searched) #27assessment near/3 (cognitive or cognition):ti,ab,kw (Word variations have been searched) #2828return* near/3 work:ti,ab,kw (Word variations have been searched) #29"Karnofsky Performance Status":ti,ab,kw (Word variations have been searched) #30(living or social) near/3 skill*:ti,ab,kw (Word variations have been searched) #31"living skills":ti,ab,kw (Word variations have been searched) #32"sickness impact profile":ti,ab,kw (Word variations have been searched) #33limitation* near/3 activit*:ti,ab,kw (Word variations have been searched) #34rehabilitat* near/3 disabilit*:ti,ab,kw (Word variations have been searched) #35occupational near/3 outcome*:ti,ab,kw (Word variations have been searched) #36(head or crani* or cerebr* or capitis or brain* or forebrain* or skull* or hemispher* or intra‐cran* or inter‐cran*) near/3 (injur* or trauma* or damag* or wound* or fracture* or contusion*) near/15 (memory or attention or concentration or percept* or memori* or learn* or retention or recall or recogni*):ti,ab,kw (Word variations have been searched) #37executive near/3 abilit*:ti,ab,kw (Word variations have been searched) #38(#15 or #16 or #17 or #18 or #19 or #20 or #21 or #22 or #23 or #24 or #25 or #26 or #27 or #28 or #29 or #30 or #31 or #32 or #33 or #34 or #35 or #36 or #37) #39(#14 and #38)
Appendix 2. MEDLINE search strategy
Ovid MEDLINE(R), Ovid MEDLINE(R) In‐Process & Other Non‐Indexed Citations, Ovid MEDLINE(R) Daily and Ovid OLDMEDLINE(R) to May 2016
1.exp rehabilitation, vocational/ 2.exp occupational therapy/ 3.exp Cognition Disorders/ 4.exp Memory Disorders/ 5.exp Attention/ 6.exp Perceptual Disorders/ 7."cognitive rehabilitation".ab,ti. 8."community integration".ab,ti. 9.(assessment adj3 (cognitive or cognition)).ab,ti. 10.(return$ adj3 work$).ab,ti. 11."living skills".ab,ti. 12.((living or social) adj3 skill$).mp. 13.(limitation$ adj3 activit$).ab,ti. 14.(rehabilitat$ adj3 disabilit$).ab,ti. 15.(occupational adj3 outcome$).ab,ti. 16.((head or crani$ or cerebr$ or capitis or brain$ or forebrain$ or skull$ or hemispher$ or intracran$ or intercran$) adj3 (injur$ or trauma$ or damag$ or wound$ or fracture$ or contusion$) adj15 (memory or attention or concentration or percept$ or memori$ or learn$ or retention or recall or recogni$)).ab,ti. 17.(executive adj3 abilit$).ab,ti. 18.exp Craniocerebral Trauma/ 19.exp Glasgow Coma Scale/ 20.exp Glasgow Outcome Scale/ 21.exp Cerebral hemorrhage, traumatic/ 22.((head or crani* or cerebr* or capitis or brain* or forebrain* or skull* or hemispher* or intra?cran* or inter?cran* or intracran* or intercran*) adj3 (injur* or trauma* or damag* or lesion* or wound* or destruction* or oedema* or edema* or contusion* or concus* or fracture*)).ab,ti.
23.((head or crani* or cerebr* or brain* or intra?cran* or inter?cran* or intracran* or intercran*) adj3 (haematoma* or hematoma* or haemorrhag* or hemorrhag* or bleed* or pressur*)).ti,ab. 24.(Glasgow adj (coma or outcome) adj (scale* or score*)).ab,ti. 25."rancho los amigos scale".ti,ab. 26.("diffuse axonal injury" or "diffuse axonal injuries").ti,ab. 27.((brain or cerebral or intracranial) adj3 (oedema or edema or swell*)).ab,ti. 28. ((unconscious* or coma* or concuss* or 'persistent vegetative state') adj3 (injur* or trauma* or damag* or wound* or fracture* or contusion* or haematoma* or hematoma* or haemorrhag* or hemorrhag* or pressur*)).ti,ab. 29.randomi?ed.ab,ti. 30.randomized controlled trial.pt. 31.controlled clinical trial.pt. 32.clinical trials as topic.sh. 33.trial.ti. 34.(animals not (humans and animals)).sh. 35.or/1828 36.or/2933 37.36 not 34 38.or/117 39.35 and 38 40.39 and 37
Appendix 3. Embase search strategy
Embase Classic + Embase (OvidSP) to May 2016
1."cognitive rehabilitation".ab,ti. 2."community integration".ab,ti. 3.(assessment adj3 (cognitive or cognition)).ab,ti. 4."living skills".ab,ti. 5. ((living or social) adj3 skill$).mp. [mp=title, abstract, heading word, drug trade name, original title, device manufacturer, drug manufacturer, device trade name, keyword] 6.(limitation$ adj3 activit$).ab,ti. 7.(rehabilitat$ adj3 disabilit$).ab,ti. 8.(occupational adj3 outcome$).ab,ti. 9.((head or crani$ or cerebr$ or capitis or brain$ or forebrain$ or skull$ or hemispher$ or intracran$ or intercran$) adj5 (injur$ or trauma$ or damag$ or wound$ or fracture$ or contusion$) adj15 (memory or attention or concentration or percept$ or memori$ or learn$ or retention or recall or recogni$)).ab,ti. 10.exp daily life activity/ 11.exp vocational rehabilitation/ 12.exp cognitive defect/ 13.exp occupational therapy/ 14.exp memory disorder/ 15.exp attention disturbance/ 16.exp perception disorder/ 17.*"quality of life"/ 18.or/117 19.exp Brain Injury/ 20.exp Glasgow Coma Scale/ 21.exp Glasgow Outcome Scale/ 22.exp Rancho Los Amigos Scale/ 23.exp Unconsciousness/ 24.((head or crani$ or cerebr$ or capitis or brain$ or forebrain$ or skull$ or hemispher$ or intracran$ or intercran$) adj5 (injur$ or trauma$ or damag$ or wound$ or fracture$ or contusion$)).ab,ti. 25.(Glasgow adj (coma or outcome) adj (scale$ or score$)).ab,ti. 26.Rancho Los Amigos Scale.ab,ti. 27.((unconscious$ or coma$ or concuss$ or 'persistent vegetative state') adj3 (injur$ or trauma$ or damag$ or wound$ or fracture$)).ti,ab. 28.Diffuse axonal injur$.ab,ti. 29.or/1928 30.18 and 29 31.exp Randomized Controlled Trial/ 32.exp controlled clinical trial/ 33.randomi?ed.ab,ti. 34.*Clinical Trial/ 35.randomly.ab. 36.trial.ti. 37.or/3136 38.exp animal/ not (exp human/ and exp animal/) 39.37 not 38 40.30 and 39 41.limit 40 to exclude medline journals
Appendix 4. PsychINFO search strategy
PsycINFO (OvidSP) to May 2016 1.head injuries.sh. 2.BrainDamage.sh. 3.TraumaticBrainInjury.sh. 4.BrainConcussion.sh. 5.((head or crani* or cerebr* or capitis or brain* or forebrain* or skull* or hemispher* or intracran* or intercran*) adj5 (injur* or trauma* or damag* or wound* or fracture* or contusion*)).ab,ti. 6.1 or 2 or 3 or 4 or 5 7.exp "Activities of Daily Living"/ 8.exp Vocational Rehabilitation/ 9.exp Occupational Therapy/ 10.exp Cognitive Impairment/ 11.exp Cognitive Ability/ 12.exp Memory Disorders/ 13.exp Perceptual Disturbances/ or exp Perceptual Distortion/ 14."cognitive rehabilitation".ab,ti. 15."community integration".ab,ti. 16.(assessment adj3 (cognitive or cognition)).ab,ti. 17.(return$ adj3 work$).ab,ti. 18."living skills".ab,ti. 19.((living or social) adj3 skill*).mp. 20.(limitation* adj3 activit*).ab,ti. 21.(rehabilitat* adj3 disabilit*).ab,ti. 22.(occupational adj3 outcome*).ab,ti. 23.or/722 24.6 and 23 25.exp clinical trials/
26.exp experimental design/ 27.clinical trial*.ab,ti. 28.controlled clinical trial.ab,ti. 29.randomi?ed controlled trial.ab,ti. 30.randomi?ed.ab,ti. 31.randomly.ab. 32.trial.ti. 33.or/2532 34.exp animals/ 35.exp human females/ 36.exp human males/ 37.35 or 36 38.34 not (34 and 37) 39.33 not 38 40.24 and 39
Data and analyses
Comparison 1. Cognitive rehabilitation versus no treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Return to work | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Community integration | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 3 Quality of life | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 2. Cognitive rehabilitation versus conventional treatment.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Return to work | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Independence in activities of daily living | 2 | 41 | Std. Mean Difference (IV, Fixed, 95% CI) | ‐0.01 [‐0.62, 0.61] |
| 3 Community integration | 3 | 123 | Mean Difference (IV, Fixed, 95% CI) | 0.05 [‐1.51, 1.62] |
| 4 Quality of life | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Comparison 3. Hospital‐based cognitive rehabilitation versus home programme.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Return to work | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 4. One cognitive strategy versus another cognitive strategy.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Return to work | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Independent living | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bornhofen 2008a.
| Methods |
Design: randomized, 2‐arm, wait‐list control trial. Duration of study: December 2003 to May 2004. |
|
| Participants |
Number randomized: 12. 6 in each arm (outpatient volunteers with severe, chronic TBI). Gender: 11 men, 1 woman. Age range: 20‐57 years. Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention: remedial cognitive programme. Designed to address emotion perception with 2 techniques Errorless Learning and Self Instruction Training. Emphasis was on graduated practice of increasingly complex, guided tasks relevant to perception of static and dynamic emotion cues. Greater independence was promoted as ability improved. Task requirements included group activities, notebook maintenance and home practice tasks. Duration: 1.5‐hour sessions, biweekly, for 8 weeks. Control: wait‐list. 1 week after the completion of 8 weeks of treatment for intervention group, the wait‐list group received the same treatment. |
|
| Outcomes | Generalization measures: SPRS (self‐reported). Identification of Static Emotions: 2 facial expression tasks (labelling and matching emotions from Ekman and Friesen's photographs). Labelling of dynamic audio‐visual emotional displays: TASIT, Part 1. Identification of social inferences based on emotional demeanour: TASIT Parts 2 and 3. |
|
| Notes |
Setting: outpatient services, Liverpool Hospital Brain Injury Rehabilitation Unit, Sydney. Country: Australia. Duration of follow‐up: 1 month following treatment. Dropouts: 1 dropout from intervention group before completing post‐test assessment. 1 further dropout in the wait‐list group after completing assessment at the post‐treatment phase for the treatment group but prior to completing wait‐list treatment. Funding: project grant from National Medical and Research Council of Australia. Comments: at baseline, SPRS scores were significantly different between the groups, hence, results to be interpreted with caution. Long term maintenance of treatment effects cannot be observed/compared due to wait‐list control design. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: unclear method of random sequence generation. |
| Allocation concealment (selection bias) | Low risk | Quote: "random allocation to treatment or wait‐list group was completed off‐site by an independent person unfamiliar with the individuals." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: no details provided in the report regarding blinding of participants and personnel. Self‐reported outcome (SPRS) likely to be influenced by lack of blinding of participants. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Comment: no details provided in the report regarding blinding of outcome assessors. Since the primary outcome was a self‐reported scale, lack of blinding of outcome assessment was unlikely to influence the outcome the study. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 1 dropout in each arm. No reason for dropout provided. No significant differences in the pretest scores of the dropouts except in TASIT Part 1 scores where they performed poorer when compared with those who completed the treatments. |
| Selective reporting (reporting bias) | Low risk | Comment: all stated outcomes were reported. |
| Other bias | Low risk | No other bias detected. |
Cantor 2014.
| Methods |
Design: randomised, wait‐list controlled trial with minimization and blinded outcome assessment. Duration of study: January 2008 to June 2012. |
|
| Participants |
Number randomized: 80 participants randomized and 18 participants directly grouped for study convenience, resulting in 49 people in each group. Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention: Short Term Executive Plus (STEP) programme 2 × 45‐minute group sessions (emotional regulation and problem solving) and 1 × 60‐minute individual session (attention training and advising) per day, 3 days per week, for 12 weeks, for a total of 108 sessions. Rolling admissions was used with a monthly start date for new group members. Group size was generally 4‐6 people. Control: wait‐list. Duration: 2 × 45‐minute group sessions, 1 × 60‐minute individual session per day, 3 days per week for 12 weeks. |
|
| Outcomes | Quality of life: Life‐3. Participation: Participation Objective Participation Subjective (POPS). Executive function: composite score. Problem Solving Inventory. Self‐efficacy questionnaire. |
|
| Notes |
Setting: community dwelling participants, institutional intervention. Country: US. Duration of follow‐up: 12 weeks of intervention followed by assessment. Dropouts: 9. In the treatment arm, 8 withdrew prior to completion of 12 weeks, and 1 did not start treatment. Funding: Supported by the Centers for Disease Control and Prevention (grant no. 1R49CE001171‐01). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Although the study used random assignment with minimization and some participants were assigned to groups based on group size, we have used the term randomization throughout because this was the principal mode of group allocation." "We allocated 18 participants without randomization when this was necessary to keep the size of the treatment group between 3 and 8; these participants were allocated in strict order of qualification." Comment: method of random sequence generation not specified. Unclear whether minimization method of allocating 18 participants had introduced bias in random allocation. |
| Allocation concealment (selection bias) | High risk | Quote: "We entered scores into the Minim program to determine treatment allocation." Comment: authors using the software was likely to have unblinded the allocation sequence. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: though the wait‐list control design made it impossible to blind the participants, we rated this at high risk of bias since the self‐reported outcomes were likely to be influenced by the knowledge of allocation to active intervention group or the wait‐list group. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "Assessors were blind to allocation at all assessments conducted after randomization." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 9 dropouts from intervention arm were not due to treatment‐related reasons. |
| Selective reporting (reporting bias) | Low risk | Comment: all key outcomes mentioned in the protocol published in the clinical trials registry were reported. |
| Other bias | Unclear risk | Quote: "Another limitation is the reliance on self‐report measures of function." "narrative reports from STEP participants to the treatment team suggested the presence of benefits of treatment that we did not measure." Comment: unclear whether reliance on self‐report measures for functional outcomes instead of using objective real‐life measures would impact the internal and external validity of the interpretations from this trial. |
Cheng 2006.
| Methods |
Design: randomized, parallel‐group control (pretest‐post‐test control group design). Duration of study: September 2004 to March 2005. |
|
| Participants |
Number randomized: 21. 11 allocated to intervention group, 10 to control group. Inclusion criteria:
Exclusion criteria: None reported. |
|
| Interventions |
Intervention: Awareness Intervention Programme (AIP). Individual therapy. Content of AIP included:
Duration: 2 sessions per day, 5 days per week for 4 weeks Control: conventional rehabilitation programme. Group therapy. 2 or 3 sessions every day including physical, functional and cognitive aspects of occupational therapy, for 4 weeks. |
|
| Outcomes | FIM. Lawton IADL score. Self‐Awareness of Deficits Interview (SADI). |
|
| Notes |
Setting: inpatients at MacLehose Medical Rehabilitation Center, Hong Kong. Country: China. Duration of follow‐up: none. Dropouts: none. Funding: none declared. Comment: return to work status and community integration not reported. Long‐term maintenance of treatment effects could not be studied as there was no follow‐up evaluation. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Ten of the participants were randomly assigned to a control group and 11 were allocated to the experimental group according to their admission sequence." Comment: in view of the potential non‐random component (admission sequence) in the sequence generation process, we judged this to be of unclear risk of bias. |
| Allocation concealment (selection bias) | High risk | Quote: "Allocation according to admission sequence." Comment: allocation by admission sequence is likely to have unblinded the allocation. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Quote: "limitation is that this was not a blinded study." Comment: self‐reported outcomes are likely to be influenced by the knowledge of allocation. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "Scoring was primarily conducted by a therapist who was not involved in the programme implementation." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no dropouts reported. |
| Selective reporting (reporting bias) | Low risk | All 3 rating scales listed in methods were reported. |
| Other bias | Low risk | Comment: no other sources of bias detected. |
Cicerone 2008.
| Methods |
Design: prospective, randomized clinical trial. Randomization: 2‐arm, block randomization, with stratification for referral source as either clinical or community. Duration of study: January 2003 to December 2006. |
|
| Participants |
Number randomized: 68 participants, 34 received the intervention and 34 received control. Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention: Intensive Cognitive Rehabilitation Programme Individual and group therapy. Intervention based on principles of comprehensive holistic neuropsychiatric rehabilitation emphasizing the integration of interventions for cognitive deficits, emotional difficulties, interpersonal behaviours and functional skills within the context of a therapeutic environment. Duration: 16 weeks, with 15 hours of therapy 3 days per week, that included 11 hours of group therapy, 3 hours of individual therapy and 1 hour of individual neuropsychological treatment. Control: standard neurorehabilitation. Predominantly individual therapy. Comprehensive interdisciplinary day treatment programme, consisted of physical occupational and speech therapies, along with neuropsychological treatment. Duration: 16 weeks. Amount and combination of specific treatments for each participant in the standard neurorehabilitation programme condition varied based on person's needs and routine clinical decision making, but group treatments were limited to no more than 3 hours per week. |
|
| Outcomes | Community Integration Questionnaire (CIQ) and Perceived Quality of Life (PQOL) scale. Vocational and educational outcomes measured by Vocational Integration Scale, ratings of which were collapsed into a dichotomous variable to classify participants as either engaged in community‐based employment (Vocational Integration Scale levels 3‐5) or unemployed (Vocational Integration Scale levels 1‐2). Other secondary outcome measures were neuropsychological functioning and perceived self‐efficacy. |
|
| Notes |
Setting: Department of Cognitive Rehabilitation and Department of Physical Medicine and Rehabilitation, JFK‐Johnson Rehabilitation Institute, Edison, New Jersey. Country: US. Duration of follow‐up: 6 months. Dropouts: of the 34 allocated to each arm, 2 from the intervention group and 4 from the control group did not complete the protocol. On completion of the protocols, 2 from each arm did not respond to requests for 6‐month follow‐up evaluation. Funding: National Institute on Disability and Rehabilitation Research. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was conducted through the web‐based interactive statistical calculation pages," "randomisation occurred in unequal blocked multiples of 4." |
| Allocation concealment (selection bias) | Low risk | Quote: "The allocation of participants to treatment condition was concealed by placing the individual randomized assignments in sequentially numbered, opaque, sealed envelopes." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "participants and therapists had knowledge that both treatments were clinically established programs that were expected to be beneficial, with no assumption regarding differential benefits and no further information about the specific intent of the study." |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "Data entry and scoring for these measures were conducted by a research assistant who was blind to treatment condition." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Dropouts reported, 2 in each arm, and included in the final analysis. |
| Selective reporting (reporting bias) | Low risk | Published report contained all expected outcomes including subgroup analysis of certain outcome measures. |
| Other bias | Low risk | None identified. |
Constantinidou 2008.
| Methods |
Design: prospective randomized controlled trial. Randomization: 2‐arm, parallel group, multi‐centre trial. Duration of study: 2004‐2008. |
|
| Participants |
Number randomized: 49 people undergoing rehabilitation following TBI. 29 assigned to intervention group, 20 to control group. Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention: categorization programme Intervention consisted of 2 types of tasks:
Duration: mean of 13 weeks to complete categorization programme. Participants received approximately 57 hours of individual cognitive treatment, averaging 2‐3 hours per week on the categorization programme‐related tasks, for a total of 27 hours of categorization programme treatment and about 4.5 hours of total individual therapy per week. Control: standard rehabilitation programme at each rehabilitation centre.
Duration: mean 80 hours of individual cognitive treatment over an 18‐week period, averaging 4.5 hours of individual therapy per week. |
|
| Outcomes | Community Integration Questionnaire (CIQ) along with the following cognitive assessment tools: Wechsler Abbreviated Scale of Intelligence, Scales of Cognitive Ability for Traumatic Brain Injury, Rey Complex Figure Test, Trail Making Tests, Wechsler Memory Scale III, California Verbal Learning Test II, Wisconsin Card Sorting Test, The Booklet Category Test, Symbol Digits Modalities Test, Control Oral Word Association, subsets from Woodcock‐Johnson III, Mayo‐Portland Adaptability Inventory III (MPAI‐3). |
|
| Notes |
Setting: 5 residential brain injury rehabilitation centres. Country: US. Duration of follow‐up: none. Dropouts: intervention group: 2 discontinued rehabilitation, 2 developed complications, 5 discharged due to insurance‐related issues. Control group: 6 discharged due to insurance‐related issues. Funding: grants from the National Institute of Child Health and Human Development, National Institutes of Health, and the Center for NeuroSkills, Bakersfield, CA. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomly assigned by project investigators who were off location and did not have direct contact with participants." Comment: method of random sequence generation not reported. Author could not provide specific details to clarify this. |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomly assigned by project investigators who were off location and did not have direct contact with participants." Comment: allocation concealment was adequate since it was performed off‐location. |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Comment: not blinded, self‐reported outcomes are likely to be influenced by the knowledge of allocation. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "The functional outcome measures in most cases were conducted by the case management staff who was not involved in patient training and, therefore, was not informed of the participant's group assignment." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Data from patients unable to complete the assigned treatment regimen were included in the analyses to the fullest extent possible. If partial data were useful for certain analyses, then those data were analysed. Therefore, the intention‐to‐treat principle was followed." Comment: we rated this as high risk of bias because there were 15 dropouts (31%). |
| Selective reporting (reporting bias) | Low risk | Comment: published report contains all expected outcomes. |
| Other bias | Low risk | Comment: no additional biases detected. |
Goverover 2007.
| Methods |
Design: single‐blind (participants) randomizedd clinical trial. Randomization: 2‐arm parallel group. Duration of study: not reported. |
|
| Participants |
Number randomized: 20 participants living in community with moderate‐to‐severe acquired brain injury, aged 18‐55 years. Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention: self‐awareness training. Performance of instrumented activities of daily living:
Participants were asked to predict the performance before completing each task and then asked to assess their performance immediately following the completion of each task. If a participant identified a specific problem, he/she was asked to think of a strategy for better and easier task performance. Duration: 6 individualized treatment sessions over 3 weeks, 1 session per day on 2 or 3 days every week. Each session consisted of a maximum of 45 minutes. Control: same ADL task as the treatment group, but participants were not given specific self‐awareness intervention by the therapist. They were given conventional practice of corrective feedback from the therapist. Duration: same as intervention group. |
|
| Outcomes | Community Integration Questionnaire (CIQ); Assessment of Motor and Process Skills (AMPS) to assess ADL and IADL. Awareness Questionnaire, Assessment of Awareness of Disability, Self‐Regulation Skills Inventory, Satisfaction with quality of care. |
|
| Notes |
Setting: Cognitive Remediation Program at Kessler Institute for Rehabilitation, New Jersey. Country: US. Duration of follow‐up: none. Dropouts: none. Funding: National Institute on Disability and Rehabilitation Research; Mary E. Switzer Research Fellowship Program (Grant Award Number: H133F0400180). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Participants were then randomly assigned to either the control or experimental group by the second author of this paper." Comment: insufficient information about the method of randomization. The author could not provide further details. |
| Allocation concealment (selection bias) | High risk | Quote: "Participants were then randomly assigned to either the control or experimental group by the second author of this paper." Comment: insufficient allocation concealment since 1 of the authors was involved in the allocation process, and method of allocation concealment could not be verified. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Participants remained blind to the group membership." Comment: blinding of participants was adequate. Blinding of personnel not reported. |
| Blinding of outcome assessment (detection bias) Objective outcomes | High risk | Comment: blinding of outcome assessment not reported. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no dropouts. |
| Selective reporting (reporting bias) | Low risk | Comment: none identified. |
| Other bias | Low risk | Comment: no additional biases were detected. |
Salazar 2000.
| Methods |
Design: single‐centre, parallel group, randomized trial (not blinded). Randomization: 2‐arm parallel group. Duration of study: January 1992 to February 1997. |
|
| Participants |
Number randomized: 120 participants randomized. 67 assigned to intervention and 53 to control using blocked randomization by an independent study statistician. Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention: in‐hospital rehabilitation. Physical fitness training and group and individual cognitive, speech, occupational and coping skills therapies. Specific group therapies were planning and organization, cognitive skills, pragmatic speech, milieu, psychotherapy and community re‐entry. Duration: 8 weeks of standardized, protocol‐defined structured daily routine. Control: home rehabilitation. TBI education and individual counselling from a psychiatric nurse. Education materials were given and strategies recommended for enhancing cognitive and organizational skills. They were trained in various number and card game exercises, were encouraged to watch news programmes and read magazines and books. Duration: 8 weeks. Weekly 30‐minutes telephone call from the psychiatric nurse inquiring about the week's events and offering support and advice in addressing problems. Daily physical exercises at own pace. |
|
| Outcomes | Return to work and fitness for military duty at 1‐year post‐treatment as determined by interview, military records or both. 'Work' defined as either full time (≥ 35 hours per week) or part time (≤ 35 hours per week) gainful military or civilian employment. 'Fitness for duty' included all people who were still on active military duty or had received a normal discharge from service but excluded people who had a medical discharge or whose discharge was pending. |
|
| Notes |
Setting: Walter Reed Army Medical Center (WRAMC), Washington, DC. Country: US. Duration of follow‐up: 24 months. Dropout: 7 withdrew from hospital rehabilitation (2 medical reasons, 5 voluntary non‐medical); 6 from home rehabilitation group received supplemental therapy and were excluded. Funding: Defense and Veterans Head Injury Program and Medical Research Service of the Department of Veterans Affairs. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Blocked randomisation was done by an independent study statistician using variable‐sized blocks to prevent investigators from guessing the code." |
| Allocation concealment (selection bias) | Low risk | Quote: "Blocked randomisation was done by an independent study statistician using variable‐sized blocks to prevent investigators from guessing the code." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Programs were implemented by separate teams of therapists who generally functioned independently of each other and of the outcome evaluation personnel, although complete blinding was not possible." Comment: no blinding but study outcomes unlikely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "Programs were implemented by separate teams of therapists who generally functioned independently of each other and of the outcome evaluation personnel, although complete blinding was not possible." Comment: no blinding but study outcomes unlikely to be influenced by lack of blinding. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "Seven patients failed to complete the full hospital program, 2 for medical reasons and 5 who voluntarily withdrew an average of 3 weeks into the program. Likewise, 6 patients in the home treatment group required supplemental therapy because of persistent behavioural or mood problems, 4 of them after completing the home program. All these randomized patients were included in the principal intent‐to‐treat analysis. However, excluding them from repeat analysis did not change the results substantially." |
| Selective reporting (reporting bias) | Low risk | Quote: "Forty‐seven eligible patients who refused to participation were similar to the 120 study participants in demographics, injury severity, and clinical status at study entry. Data were analysed using the intent‐to‐treat analysis that included all randomized patients." |
| Other bias | Low risk | Comment: no additional biases detected. |
Twamley 2014.
| Methods |
Design: randomized controlled, trial comparing 2 alternative TBI treatment approaches. Randomization: computerized randomization in 1 block, 2‐arm, parallel group. Duration of study: September 2008 to February 2012. |
|
| Participants |
Number randomized: 50 adult veterans with mild‐to‐moderate TBI. Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention: supported employment + cognitive Symptom Management and Rehabilitation Therapy (cogSMART). Portable and practical intervention designed to be implemented without extensive training. 12‐week, multi‐modal compensatory cognitive training intervention emphasizing habit learning and compensatory strategies in prospective memory, attention, learning, memory and executive functioning. The treatment manual was informed by consultation with the acquired brain injury programme at Mesa College in San Diego, CA, and other cognitive remediation experts. Control: enhanced supported employment without cogSMART. Duration: 12 weeks. 1 employment specialist delivered CogSMART for 1 hour per week in addition to 1 hour of standard supported employment, to make it 2 visits per week. For the control group, another employment specialist delivered enhanced supported employment, making it 2 visits per week. |
|
| Outcomes | Return to work: data on attainment of competitive work by 14 weeks collected on a weekly basis. QUality Of Life Interview ‐ Brief version (QUOLI‐Brief). Wide Range Achievement Test 3rd edition (WRAT‐3) Reading test. Prospective memory ‐ Memory for Intentions Screening Test (MIST). Wechsler Adult Intelligence Scale ‐ 3rd edition. California Verbal Learning Test ‐ 2nd edition. Delis‐Kaplan Executive Function System (D‐KEFS). Wisconsin Card Sorting Test. Postconcussive symptoms ‐ Neurobehavioral Symptom Inventory (NSI). Clinician Administered PTSD (post‐traumatic stress disorder) scale (CAPS). Hamilton Depression rating scale (HAM‐D). |
|
| Notes |
Setting: hospital rehabilitation centre. Country: US. Duration of follow‐up: up to 2 weeks after completion of 12 weeks' intervention. Dropouts: of the 50 randomized, 8 (4 from each arm) reported to have dropped out. Post‐intervention data available only for 34 participants, 16 in the intervention arm and 18 in the control arm. Funding: project was "based on work supported by the Department of Defense (award W81XWH‐08‐2‐0193)." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was carried out by the principal investigator using a randomisation scheme generated by Randomization.com, with 50 participants in one block." |
| Allocation concealment (selection bias) | High risk | Comment: concealment of allocation could not have been plausible since the principal investigator carried out randomization using an online generator. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Comment: though the participants and personnel were not blinded, this is unlikely to introduce bias in the objective outcome of return to work. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "Outcome assessment was not blinded; however, most of our outcome measures were either objective (neuropsychological test performance, attainment of competitive work) or reported by the participant." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Fifty Veterans receiving healthcare at the VA San Diego Healthcare System enrolled in the study". "Eight participants dropped out, four from each group (two decided not to pursue work, one moved, and five were lost to follow‐up). Posttreatment data were available for 34 participants at 3 mo [months]." Comment: of the 16 dropouts (32% of the participants initially randomized), only 8 were accounted for. |
| Selective reporting (reporting bias) | Low risk | Comment: though no details were available regarding prospective registration of the trial protocol, the outcomes reported were adequate from a trial of this nature. |
| Other bias | Low risk | Comment: no other bias detected. |
Vanderploeg 2008.
| Methods |
Design: randomized, controlled, intention‐to‐treat trial comparing 2 alternative TBI treatment approaches. Single blind (outcome assessors). Randomization: 2‐arm, parallel group, stratified by centre, blocked in randomly ordered block sizes. Duration of study: not reported. |
|
| Participants | 366 adult veterans or active duty military service personnel with moderate‐to‐severe TBI. 184 in the cognitive didactic rehabilitation arm and 182 in functional experiential rehabilitation arm. Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention 1: cognitive‐didactic. 4 cognitive domains targeted: attention, memory, executive functions and pragmatic communication. Paper and pencil, or computerized cognitive tasks in 1 to 1 cognitive therapy sessions given. Trial‐and‐error learning approach used. Therapists frequently asked questions calling attention to participant's self‐awareness. Intervention 2: functional‐experiential rehabilitation therapy. Real‐life performance situations and common tasks were used to remediate or compensate for functional deficits after brain injury. Functional protocol treatment interventions occurred in group setting and natural environments. Treatment focused on learning and doing functional daily activities using an errorless treatment strategy. Therapists emphasized instructional cues and attempted to anticipate and minimize participant errors by providing structure or directions. Duration: 1.5‐2.5 hours' daily of protocol‐specific therapy in addition to 2‐2.5 hours daily of occupational therapy and physiotherapy to both groups. Duration of protocol treatment days varied from 20 to 60 days depending on the clinical needs and progress of each participant. |
|
| Outcomes | Functional independence (ability to live independently with < 3 hours of assistance per week). Return to work or school (current status of paid employment or school enrolment either full or part time, not sheltered workshop). These were determined by structured interview questions. Secondary outcomes were FIM, Disability Rating Scale score and items from the Present State Exam, Apathy Evaluation Scale and Neurobehavioral Rating Scale. |
|
| Notes |
Setting: acute inpatient rehabilitation brain injury programmes at 4 participating Veterans Administration Medical Centres in Minneapolis, Palo Alto, Richmond and Tampa. Country: US. Duration of follow‐up: 1 year. Dropouts: cognitive didactic group, 3 rescinded consent before protocol treatment began, 13 lost to follow‐up. Functional experiential group, 2 rescinded consent before protocol treatment began, 16 lost to follow‐up. 1‐year analysis on 360 participants. Funding: Defense and Veterans Brain Injury Center, Uniformed Services University of the Health Sciences, Bethesda, MD, the Department of Veterans Affairs, Veterans Health Administration, and a Department of Defense award administered through the Henry Jackson Foundation (grant no. MDA 905‐03‐2‐0003). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were randomized to the comparative treatments by an independent study statistician using random number tables. Randomization was stratified by centre and blocked in randomly ordered block sizes. This method provides approximately even group assignments across centres and is recommended for multicenter clinical trials." |
| Allocation concealment (selection bias) | Low risk | Quote: "Participants were randomized to the comparative treatments by an independent study statistician using random number tables. Randomization was stratified by centre and blocked in randomly ordered block sizes. This method provides approximately even group assignments across centres and is recommended for multicenter clinical trials." |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The interactive nature of the experimental conditions precluded subject blinding. Independent teams of therapists functioned at each site to deliver the separate treatments, and by necessity were not blinded to treatment." Comment: no blinding but study outcomes unlikely to be influenced by lack of blinding. |
| Blinding of outcome assessment (detection bias) Objective outcomes | Low risk | Quote: "Given the interactive nature of the interventions, patients and treating clinicians could not remain blinded. However, independent evaluators collected the outcome data and were blinded to treatment arm assignment." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "366 subjects consented and were randomized. Five subjects rescinded consent before study procedures began, and 1 withdrew consent later, leaving 360 subjects, 180 in each treatment arm. Data were analysed using an intent‐to‐treat analysis including all randomized patients." |
| Selective reporting (reporting bias) | Low risk | Quote: "All preplanned and exploratory analyses are reported." |
| Other bias | Low risk | None identified. |
ADL: activities of daily living; FIM: Functional Independence Measure; GCS: Glasgow Coma Score; IADL: Instrumental Activities of Daily Living; SPRS: Psychosocial Reintegration Scale; TASIT: The Awareness of Social Inferences Test; TBI: traumatic brain injury.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bell 2005 | No specific cognitive rehabilitation component in the telephonic intervention. |
| Bertens 2015 | Population included non‐TBI. |
| Bjorkdahl 2013 | Population included non‐TBI. |
| Bornhofen 2008b | No occupational outcome measured. |
| Bourgeois 2007 | No occupational outcome measured. |
| Bovend’Eerdt 2010 | Population included non‐TBI. |
| Braverman 1999 | No control group ‐ intervention arm of another randomized controlled trial described in this paper. |
| Couillet 2010 | No occupational outcome measured. |
| Culley 2010 | Non‐randomized study design. |
| Dahlberg 2007 | Participants with impairment in communication skills due to TBI. Intervention was targeted at improving communication skills. |
| Dawson 2013 | > 50% of participants were not allocated randomly. |
| Dirette 1999 | No occupational outcome measured (only computer tasks). |
| Dou 2006 | No occupational outcome measured. |
| Fish 2007 | Non‐randomized study design. |
| Hallock 2016 | Systematic review of randomized and non‐randomized studies. |
| Hewitt 2006 | No occupational outcome measured. |
| Hildebrandt 2006 | No occupational outcome measured. |
| Kaschel 2002 | No occupational outcome measured. |
| Kurowski 2013 | No occupational outcome measured. |
| Lannin 2014 | Intervention did not include a component of cognitive rehabilitation. |
| Man 2006a | Quasi‐experimental design. |
| Man 2006b | Quasi‐experimental design. |
| Neistadt 1992 | No occupational outcome measured. |
| Neumann 2015 | No occupational outcome measured. |
| Niemann 1990 | No occupational outcome measured. |
| Niemeier 2010 | No specific cognitive rehabilitation component in the vocational intervention. |
| Park 2015 | Population included non‐TBI. |
| Rath 2003 | No occupational outcome measured. |
| Richter 2015 | No occupational outcome measured. |
| Ryan 1988 | No occupational outcome measured. |
| Shum 2011 | No primary occupational outcome measured. |
| Spikman 2010 | Population included non‐TBI. |
| Tam 2004 | Quasi‐experimental design. |
| Thickpenny‐Davis 2007 | No occupational outcome measured. |
| Tiersky 2005 | No specific cognitive rehabilitation component in the (combined CBT and Cognitive rehabilitation) intervention. |
| Tlustos 2016 | Participants were adolescents. |
| Tornas 2016 | Population included non‐TBI. |
| Trexler 2016 | Intervention did not include any component of cognitive rehabilitation. |
| Yip 2013 | Population not specified as traumatic aetiology for brain injury. |
TBI: traumatic brain injury. ABI: acquired brain injury
Characteristics of studies awaiting assessment [ordered by study ID]
Twamley 2015.
| Methods |
Design: randomized controlled, trial comparing 2 alternative TBI treatment approaches. Randomization: computerized randomization in 1 block, 2‐arm, parallel group. Duration of study: 12 month trial |
| Participants |
Number randomized: 50 adult veterans with mild‐to‐moderate TBI. Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention: supported employment + cognitive Symptom Management and Rehabilitation Therapy (cogSMART). Portable and practical intervention designed to be implemented without extensive training. 12‐week, multi‐modal compensatory cognitive training intervention emphasizing habit learning and compensatory strategies in prospective memory, attention, learning, memory and executive functioning. The treatment manual was informed by consultation with the acquired brain injury programme at Mesa College in San Diego, CA, and other cognitive remediation experts. Control: enhanced supported employment without cogSMART. Duration: 12 weeks. 1 employment specialist delivered CogSMART for 1 hour per week in addition to 1 hour of standard supported employment, to make it 2 visits per week. For the control group, another employment specialist delivered enhanced supported employment, making it 2 visits per week. |
| Outcomes | Return to work: data on attainment of competitive work by 14 weeks collected on a weekly basis. Quality Of Life Interview ‐ Brief version (QOLI‐Brief). Prospective memory ‐ Memory for Intentions Screening Test (MIST). Wechsler Adult Intelligence Scale ‐ 3rd edition. California Verbal Learning Test ‐ 2nd edition. Delis‐Kaplan Executive Function System (D‐KEFS). Wisconsin Card Sorting Test. Postconcussive symptoms ‐ Neurobehavioral Symptom Inventory (NSI). UCSD Performance‐Based Skills Assessment (UPSA). |
| Notes | Corresponding author is contacted to provide more details related to the following: 1. Is this the same study published in 2014 or a different study? 2. ARe the participants different? 3. Is this an extended follow‐up of the same participant? |
Vas 2011.
| Methods |
Design: single blinded randomized control trial Randomization: 2‐arm parallel group. Duration of study: not mentioned |
| Participants |
Number randomized: 28 participants with Chronic TBI Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention 1: strategy‐based strategic memory and reasoning training (SMART program) Intervention 2: information‐based Brain Health Workshop (BHW). Duration: Participants in both groups received a minimum of 15 hours of training over 8 weeks. Both SMART and BHW programs offered a total of 18 hours of training during 12 group sessions (1.5 hours each session) conducted over 8 weeks. The first 15 hours of training over 10 sessions were conducted in the first 5 weeks (ie, 2 sessions per week). The final 3 hours of training, over 2 booster sessions, took place at spaced intervals over the next 3 weeks (ie, session 11 during week‐6 and session 12 in the eighth‐week). Two trained clinicians (a speech pathologist and an occupational therapist) who had experience in TBI rehabilitation led each group. Each group consisted of 4 to 5 participants |
| Outcomes | Test of strategic learning (TOSL) Wechler adult intelligence scale (WAIS III) Delis‐Kaplan Executive Function System (DKEFS) Glasgow outcome scale ‐ extended (GOS‐E), Functional status examination (FSE) Community Integration Questionnaire (CIQ) |
| Notes | Corresponding author is contacted to provide more details related to the following: 1. majority of the participants sustained their injury in their preteen, teen, or early adulthood years. 2. reliable documentation of acute severity of TBI amonmg participants not available. Documenting initial injury severity is critical to accurately establish the relation between initial injury severity, later recovery level, and response to cognitive treatment protocol 3. the study examined functional gains on self‐rated questionnaires that may represent one's perception of gains made post training. This could be even more complex if its TBI participants with cognitive dysfunctions. |
Differences between protocol and review
Author
We added Anand Viswanathan onto the author team following the publication of our protocol (K SK 2009).
Objectives
We included the word 'Adult' in the title and objectives of the review to be specific about the age group we looked at.
In the objectives, we have now specified the following as occupational outcomes (AOTA 2014): return to work, independence in daily living and community integration. In the protocol, we just mentioned "occupations refers to all the things that people do in their everyday life, not just paid employment."
We dropped the following secondary objective that was mentioned in the protocol: to evaluate the effectiveness of cognitive rehabilitation interventions aimed at improving cognitive functions for people with traumatic brain injury. We did this since we realized that cognitive functions are intermediate measures, whereas the primarily focus of this review is on practically relevant occupational outcomes. We have specified community integration as a primary outcome measure, because social participation is within the domain of 'occupation' (K SK 2009).
Search
We did not search the following databases as intended in our protocol due to limitations in accessing them at the review stage (K SK 2009).
CINAHL;
ISI Web of Science: Science Citation Index Expanded (SCI‐EXPANDED);
ISI Web of Science: Social Science Citation Index Expanded (SCI‐EXPANDED);
ISI Web of Science: Conference Proceedings Citation Index‐Science (CPCI‐S);
ZETOC.
Interventions
We had not defined in the protocol what the control groups and the comparisons would be. Hence, to categorize the screened studies objectively, we specified the four comparisons that we agreed would be clinically relevant.
In studies that employed a wait‐list control design, we analysed outcomes after the initial wait‐list period only, and not at the end of the entire follow‐up duration. We had not specified this in the protocol and all authors agreed on this decision to analyse the differences in outcomes between the intervention arm and the non‐intervention control arm.
We have used the term 'conventional treatment' in the review, instead of the term 'standard care' described in the protocol to refer to the interventions in the control arm that did not have a specific cognitive strategy. We made this change since we realized that 'standard' norms would vary between different institutions and health systems, and that any existing standard of care in a system could be better described as 'conventional'.
We decided to label individual studies as having high risk of bias if one or more of the domains random sequence generation, allocation concealment and blinding of outcome assessment were judged to have a high risk of bias. We had not prespecified this in the protocol (K SK 2009).
Results
We used RR instead of OR for dichotomous results. We did not compare trials that used an ITT analysis with those that did not use an ITT analysis due to lack of data (K SK 2009).
Contributions of authors
K Suresh Kumar led the review. He developed the idea and analysed the rationale for the review, drafted the protocol, screened records, extracted trial details and data, read and edited the final drafts of the review.
Selvaraj Samuelkamaleshkumar acted as a second review author, screened records, extracted trial data, carried out data analysis and wrote the final drafts of the review.
AV acted as a second review author, screened records, extracted trial data, carried out data analysis and wrote the final drafts of the review.
AM provided methodological expertise, and read and commented on the drafts.
Sources of support
Internal sources
-
Christian Medical College, Vellore, India.
Salary for Suresh Kumar, Selvaraj Samuelkamaleshkumar, Anand Viswanathan, Ashish Macaden
-
Public Health Foundation of India ‐ Indian Institute of Public Health, Hyderabad, India.
Salary for Suresh Kumar
-
Cochrane South Asia, India.
Capacity building in research synthesis by way of training workshops on protocol development and systematic review completion.
External sources
-
Cochrane Injuries Group, UK.
Logistic support in the initial stages ‐ publication of protocol and searches
Declarations of interest
K Suresh Kumar: none known.
Selvaraj SSamuelkamaleshkumar: none known.
Anand Viswanathan: none known.
Ashish Macaden: none known.
New
References
References to studies included in this review
Bornhofen 2008a {published data only}
- Bornhofen C, McDonald S. Treating deficits in emotion perception following traumatic brain injury. Neuropsychological Rehabilitation 2008;18(1):22‐44. [DOI] [PubMed] [Google Scholar]
Cantor 2014 {published data only}
- Cantor J, Ashman T, Dams‐O'Connor K, Dijkers MP, Gordon W, Spielman L, et al. Evaluation of the Short‐Term Executive Plus intervention for executive dysfunction after traumatic brain injury: a randomized controlled trial with minimization. Archives of Physical Medicine and Rehabilitation 2014;95(1):1‐9.e3. [DOI] [PubMed] [Google Scholar]
Cheng 2006 {published data only}
- Cheng SKW, Man DWK. Management of impaired self‐awareness in persons with traumatic brain injury. Brain Injury 2006;20(6):621‐8. [DOI] [PubMed] [Google Scholar]
Cicerone 2008 {published data only}
- Cicerone KD, Mott T, Azulay J, Sharlow‐Galella MA, Ellmo WJ, Paradise S, et al. A randomized controlled trial of holistic neuropsychologic rehabilitation after traumatic brain injury. Archives of Physical Medicine and Rehabilitation 2008;89(12):2239‐49. [DOI] [PubMed] [Google Scholar]
Constantinidou 2008 {published data only}
- Constantinidou F, Thomas RD, Robinson L. Benefits of categorization training in patients with traumatic brain injury during post acute rehabilitation: additional evidence from a randomized control trial. Journal of Head Trauma Rehabilitation 2008;23(5):312‐28. [DOI] [PubMed] [Google Scholar]
Goverover 2007 {published data only}
- Goverover Y, Johnston MV, Toglia J, Deluca J. Treatment to improve self‐awareness in persons with acquired brain injury. Brain Injury 2007;21(9):913‐23. [DOI] [PubMed] [Google Scholar]
Salazar 2000 {published data only}
- Salazar AM, Warden DL, Schwab K, Spector J, Braverman S, Walter J, et al. Cognitive rehabilitation for traumatic brain injury: A randomized trial. JAMA 2000;283(23):3075‐81. [DOI] [PubMed] [Google Scholar]
Twamley 2014 {published data only}
- Twamley EW, Jak AJ, Delis DC, Bondi MW, Lohr JB. Cognitive Symptom Management and Rehabilitation Therapy (CogSMART) for veterans with traumatic brain injury: pilot randomized controlled trial. Journal of Rehabilitation Research and Development 2014;51(1):59‐70. [DOI] [PubMed] [Google Scholar]
Vanderploeg 2008 {published data only}
- Vanderploeg RD, Schwab K, Walker WC, Fraser JA, Signford BJ, Date ES, et al. Rehabilitation of traumatic brain injury in active duty military personnel and veterans: defense and veterans brain injury center randomized controlled trail of two rehabilitation approaches. Archives of Physical Medicine and Rehabilitation 2008;89(12):2227‐38. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Bell 2005 {published data only}
- Bell KR, Temkin NR, Esselman PC, Doctor JN, Bombardier CH, Fraser RT, et al. The effect of a scheduled telephone intervention on outcome after moderate to severe traumatic brain injury: a randomized trial. Archives of Physical Medicine and Rehabilitation 2005;86(5):851‐6. [DOI] [PubMed] [Google Scholar]
Bertens 2015 {published data only}
- Bertens D, Kessels RPC, Fiorenzato E, Boelen DHE, Fasotti L. Do old errors always lead to new truths? A randomized controlled trial of errorless goal management training in brain‐injured patients. Journal of the International Neuropsychological Society: JINS 2015;21(8):639‐49. [DOI] [PubMed] [Google Scholar]
Bjorkdahl 2013 {published data only}
- Bjorkdahl A, Akerlund E, Svensson S, Esbjornsson E. A randomized study of computerized working memory training and effects on functioning in everyday life for patients with brain injury. Brain Injury 2013;27(13‐14):1658‐65. [DOI] [PubMed] [Google Scholar]
Bornhofen 2008b {published data only}
- Bornhofen C, McDonald S. Comparing strategies for treating emotion perception deficits in traumatic brain injury. Journal of Head Trauma Rehabilitation 2008;23(2):103‐15. [DOI] [PubMed] [Google Scholar]
Bourgeois 2007 {published data only}
- Bourgeois MS, Lenius K, Turkstra L, Camp C. The effects of cognitive teletherapy on reported everyday memory behaviours of persons with chronic traumatic brain injury. Brain Injury 2007;21(12):1245‐57. [DOI] [PubMed] [Google Scholar]
Bovend’Eerdt 2010 {published data only}
- Bovend’Eerdt TJ, Dawes H, Sackley C, Izadi H, Wade DT. An integrated motor imagery program to improvefunctional task performance in neurorehabilitation: a single‐blind randomized controlled trial. Archives of Physical Medicine and Rehabilitation 2010;91:939‐46. [DOI] [PubMed] [Google Scholar]
Braverman 1999 {published data only}
- Braverman SE, Spector J, Warden DL, Wilson BC, Ellis TE, Bamdad MJ, et al. A multidisciplinary TBI inpatient rehabilitation programme for active duty service members as part of a randomized clinical trial. Brain Injury 1999;13(6):405‐15. [DOI] [PubMed] [Google Scholar]
Couillet 2010 {published data only}
- Couillet J, Soury S, Lebornec G, Asloun S, Joseph PA, Mazaux JM, et al. Rehabilitation of divided attention after severe traumatic brain injury: a randomised trial. Neuropsychological Rehabilitation 2010;20(3):321‐39. [DOI] [PubMed] [Google Scholar]
Culley 2010 {published data only}
- Culley C, Evans JJ. SMS text messaging as a means of increasing recall of therapy goals in brain injury rehabilitation: a single‐blind within‐subjects trial. Neuropsychological Rehabilitation 2010;20(1):103‐19. [DOI] [PubMed] [Google Scholar]
Dahlberg 2007 {published data only}
- Dahlberg CA, Cusick CP, Hawley LA, Newman JK, Morey CE, Harrison‐Felix CL, et al. Treatment efficacy of social communication skills training after traumatic brain injury: a randomized treatment and deferred treatment controlled trial. Archives of Physical Medicine and Rehabilitation 2007;88(12):1561‐73. [DOI] [PubMed] [Google Scholar]
Dawson 2013 {published data only}
- Dawson DR, Binns MA, Hunt A, Lemsky C, Polatajko HJ. Occupation‐based strategy training for adults with traumatic brain injury: a pilot study. Archives of Physical Medicine and Rehabilitation 2013;94(10):1959‐63. [DOI] [PubMed] [Google Scholar]
Dirette 1999 {published data only}
- Dirette DK, Hinojosa J, Carnevale GJ. Comparison of remedial and compensatory interventions for adults with acquired brain injuries. Journal of Head Trauma Rehabilitation 1999;14(6):595‐601. [DOI] [PubMed] [Google Scholar]
Dou 2006 {published data only}
- Dou ZL, Man DWK, Ou HN, Zheng JL, Tam SF. Computerized errorless learning‐based memory rehabilitation for Chinese patients with brain injury: a preliminary quasi‐experimental clinical design study. Brain Injury 2006;20(3):219‐25. [DOI] [PubMed] [Google Scholar]
Fish 2007 {published data only}
- Fish J, Evans JJ, Nimmob M, Martin E, Kersel D, Bateman A, et al. Rehabilitation of executive dysfunction following brain injury: "content‐free" cueing improves everyday prospective memory performance. Neuropsychologia 2007;45(6):1318‐30. [DOI] [PubMed] [Google Scholar]
Hallock 2016 {published data only}
- Hallock H, Collins D, Lampit A, Deol K, Fleming J, Valenzuela M. Cognitive training for post‐acute traumatic brain injury: A systematic review and meta‐analysis. Frontiers in Human Neuroscience 2016;10:537. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hewitt 2006 {published data only}
- Hewitt J, Evans JJ, Dritschel B. Theory driven rehabilitation of executive functioning: improving planning skills in people with traumatic brain injury through the use of an autobiographical episodic memory cueing procedure. Neuropsychologia 2006;44(8):1468‐74. [DOI] [PubMed] [Google Scholar]
Hildebrandt 2006 {published data only}
- Hildebrandt H, Bussmann‐MorkGünter B, Schwendemann G. Group therapy for memory impaired patients: a partial remediation is possible. Journal of Neurology 2006;253(4):512‐9. [DOI] [PubMed] [Google Scholar]
Kaschel 2002 {published data only}
- Kaschel R, Della Sala S, Cantagallo A, Fahlbock A, Laaksonen R, Kazen M. Imagery mnemonics for the rehabilitation of memory: a randomised group controlled trial. Neuropsychological Rehabilitation 2002;12(2):127‐53. [Google Scholar]
Kurowski 2013 {published data only}
- Kurowski BG, Wade SL, Kirkwood MW, Brown TM, Stancin T, Taylor HG. Online problem‐solving therapy for executive dysfunction after child traumatic brain injury. Pediatrics 2013;132(1):e158‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lannin 2014 {published data only}
- Lannin N, Carr B, Allaous J, Mackenzie B, Falcon A, Tate R. A randomized controlled trial of the effectiveness of handheld computers for improving everyday memory functioning in patients with memory impairments after acquired brain injury. Clinical Rehabilitation 2014;28(5):470‐81. [DOI] [PubMed] [Google Scholar]
Man 2006a {published data only}
- Man DWK, Soong WYL, Tam SF, Hui‐Chan CWY. Self‐efficacy outcomes of people with brain injury in cognitive skill training using different types of trainer‐trainee interaction. Brain Injury 2006;29(9):959‐70. [DOI] [PubMed] [Google Scholar]
Man 2006b {published data only}
- Man DWK, Soong WYL, Tam SF, Hui‐Chan CWY. A randomized clinical trial study on the effectiveness of a tele‐analogy‐based problem‐solving programme for people with acquired brain injury (ABI). NeuroRehabilitation 2006;21(3):205‐17. [PubMed] [Google Scholar]
Neistadt 1992 {published data only}
- Neistadt ME. Occupational therapy treatments for constructional deficits. American Journal of Occupational Therapy 1992;46(2):141‐8. [DOI] [PubMed] [Google Scholar]
Neumann 2015 {published data only}
- Neumann D, Babbage DR, Zupan B, Willer B. A randomized controlled trial of emotion recognition training after traumatic brain injury. Journal of Head Trauma Rehabilitation 2015;30(3):E12‐23. [DOI] [PubMed] [Google Scholar]
Niemann 1990 {published data only}
- Niemann H, Ruff RM, Baser CA. Computer‐assisted attention retraining in head‐injured individuals: a controlled efficacy study of an outpatient program. Journal of Consulting and Clinical Psychology 1990;58(6):811‐7. [DOI] [PubMed] [Google Scholar]
Niemeier 2010 {published data only}
- Niemeier JP, DeGracea SM, Farrara LF, Ketchuma JS, Berman AJ, Young JA. Effectiveness of a comprehensive, manualized intervention for improving productivity and employability following brain injury. Journal of Vocational Rehabilitation 2010;33(3):167‐79. [Google Scholar]
Park 2015 {published data only}
- Park, Hae Y, Maitra, Kinsuk M, Kristina M. The Effect of Occupation‐based Cognitive Rehabilitation for Traumatic Brain Injury: A Meta‐analysis of Randomized Controlled Trials. Occupational therapy international 2015;22(2):104‐16. [DOI] [PubMed] [Google Scholar]
Rath 2003 {published data only}
- Rath JF, Simon D, Langenbahn DM, Sherr RLynn, Diller L. Group treatment of problem‐solving deficits in outpatients with traumatic brain injury: a randomised outcome study. Neuropsychological Rehabilitation 2003;13(4):461‐88. [Google Scholar]
Richter 2015 {published data only}
- Richter KM, Modden C, Eling P, Hildebrandt H. Working memory training and semantic structuring improves remembering future events, not past events. Neurorehabilitation and Neural Repair 2015;29(1):33‐40. [DOI] [PubMed] [Google Scholar]
Ryan 1988 {published data only}
- Ryan TV, Ruff RM. The efficacy of structured memory retraining in a group comparison of head trauma patients. Archives of Clinical Neuropsychology 1988;3(2):165‐79. [PubMed] [Google Scholar]
Shum 2011 {published data only}
- Shum D, Fleming J, Gill H, Gullo MJ, Strong J. A randomized controlled trial of prospective memory rehabilitation in adults with traumatic brain injury. Journal of Rehabilitation Medicine 2011;43(3):216‐23. [DOI] [PubMed] [Google Scholar]
Spikman 2010 {published data only}
- Spikman JM, Boelen DHE, Lamberts KF, Brouwer WH, Fasotti L. Effects of a multifaceted treatment programfor executive dysfunction after acquired brain injury on indications of executive functioning in daily life. Journal of the International Neuropsychological Society 2010;16:118‐129. [DOI] [PubMed] [Google Scholar]
Tam 2004 {published data only}
- Tam SF, Man WK. Evaluating computer‐assisted memory retraining programmes for people with post‐head injury amnesia. Brain Injury 2004;18(5):461‐70. [DOI] [PubMed] [Google Scholar]
Thickpenny‐Davis 2007 {published data only}
- Thickpenny‐Davis KL, Barker‐Collo SL. Evaluation of a structured group format memory rehabilitation program for adults following brain injury. Journal of Head Trauma Rehabilitation 2007;22(5):303‐13. [DOI] [PubMed] [Google Scholar]
Tiersky 2005 {published data only}
- Tiersky LA, Anselmi V, Johnston MV, Kurtyka J, Roosen E, Schwartz T, DeLuca J. A trial of neuropsychologicrehabilitation in mild‐spectrum traumatic brain injury. Arch Phys Med Rehabil 2005;86:1565‐74. [DOI] [PubMed] [Google Scholar]
Tlustos 2016 {published data only}
- Tlustos SJ, Kirkwood, Michael W, Taylor H. Gerry ST, Brown TM, Wade SL. A randomized problem‐solving trial for adolescent brain injury: Changes in social competence. Rehabilitation Psychology 61;4:347‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Tornas 2016 {published data only}
- Tornas S, Løvstad M, Solbakk AK, Evans J, Endestad T, Hol Per K, et al. Rehabilitation of executive functions in patients with chronic acquired brain injury with goal management training, external cuing, and emotional regulation: a randomized controlled trial. Journal of the International Neuropsychological Society: JINS 2016;22(4):436‐52. [DOI] [PubMed] [Google Scholar]
Trexler 2016 {published data only}
- Trexler LE, Parrott DR, Malec JF. Replication of a prospective randomized controlled trial of resource facilitation to improve return to work and school after brain injury. Archives of Physical Medicine and Rehabilitation 2016;97(2):204‐10. [DOI] [PubMed] [Google Scholar]
Yip 2013 {published data only}
- Yip BCB, Man DWK. Virtual reality‐based prospective memory training program for people with acquired brain injury. NeuroRehabilitation 2013;32(1):103‐15. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Twamley 2015 {published data only}
- Twamley EW, Thomas KR, Gregory AM, Jak J, Bondi MW, Delis DC, Lohr JB. CogSMART Compensatory Cognitive Training for Traumatic Brain Injury: Effects Over 1 Year. The Journal of head trauma rehabilitation 2015;30(6):391‐401. [DOI] [PubMed] [Google Scholar]
Vas 2011 {published data only}
- Vas AK, Chapman SB, Cook LG, Elliott AC, Keebler M. Higher‐Order Reasoning Training Years AfterTraumatic Brain Injury in Adults. The Journal of Head Trauma Rehabilitation 2011;26(3):224‐39. [DOI] [PubMed] [Google Scholar]
Additional references
AOTA 2014
- American Occupational Therapy Association. Occupational therapy practice framework: domain and process (3rd edition). American Journal of Occupational Therapy 2014;68(Suppl 1):S1‐S48. [Google Scholar]
Barman 2016
- Barman A, Chatterjee A, Bhide R. Cognitive impairment and rehabilitation strategies after traumatic brain injury. Indian Journal of Psychological Medicine 2016;38(3):172‐81. [PUBMED: 27335510] [DOI] [PMC free article] [PubMed] [Google Scholar]
Bowen 2013
- Bowen A, Hazelton C, Pollock A, Lincoln NB. Cognitive rehabilitation for spatial neglect following stroke. Cochrane Database of Systematic Reviews 2013, Issue 7. [DOI: 10.1002/14651858.CD003586.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chesnut 1998
- Chesnut RM, Carney N, Maynard H, Patterson P, Mann NC, Helfand M. Rehabilitation for Traumatic Brain Injury: Summary. AHRQ Evidence Report Summaries. Vol. 2, Rockville (MD): Agency for Healthcare Research and Quality, 1998:1‐176. [PMC free article] [PubMed] [Google Scholar]
Chung 2013
- Chung CSY, Pollock A, Campbell T, Durward BR, Hagen S. Cognitive rehabilitation for executive dysfunction in adults with stroke or other adult non‐progressive acquired brain damage. Cochrane Database of Systematic Reviews 2013, Issue 4. [DOI: 10.1002/14651858.CD008391.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cicerone 2011
- Cicerone KD, Langenbahn DM, Braden C, Malec JF, Kalmar K, Fraas M, et al. Evidence‐based cognitive rehabilitation: Updated review of the literature from 2003 through 2008. Archives of Physical Medicine and Rehabilitation 2011;92(4):519‐30. [DOI] [PubMed] [Google Scholar]
Cicerone 2000
- Cicerone KD, Dahlberg C, Kalmar K, Langenbahn DM, Malec JF, Bergquist TF, et al. Evidence‐based cognitive rehabilitation: recommendations for clinical practice. Archives of Physical Medicine and Rehabilitation 2000;81(12):1596‐615. [DOI] [PubMed] [Google Scholar]
Cicerone 2005
- Cicerone KD, Dahlberg C, Malec JF, Langenbahn DM, Felicetti T, Kneipp S, et al. Evidence‐based cognitive rehabilitation: updated review of the literature from 1998 through 2002. Archives of Physical Medicine and Rehabilitation 2005;86(8):1681‐92. [DOI] [PubMed] [Google Scholar]
Dwan 2013
- Dwan K, Gamble C, Williamson PR, Reporting Bias Group. Systematic review of the empirical evidence of study publication bias and outcome reporting bias ‐ an updated review. PLoS One 2013;8(7):e66844. [DOI: 10.1371/journal.pone.0066844] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gean 2010
- Gean AD, Fischbein NJ. Head trauma. Neuroimaging Clinics of North America 2010;20(4):527‐56. [DOI] [PubMed] [Google Scholar]
GRADEpro 2014 [Computer program]
- Brozek J, Oxman A, Schünemann H. GRADEpro GDT. Version accessed 7 September 2016. Hamilton (ON): GRADE Working Group, McMaster University, 2014.
Higgins 2011
- Higgins JPT, Green S, editor(s). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
Hoffmann 2014
- Hoffmann TC, Glasziou PP, Boutron I, Milne R, Perera R, Moher D, et al. Better reporting of interventions: template for intervention description and replication (TIDieR) checklist and guide. BMJ 2014;Mar 7:348. [DOI: 10.1136/bmj.g1687] [DOI] [PubMed] [Google Scholar]
Ibrahim 2015
- Ibrahim SAS, Dahlan A. Engagement in occupational activities and purpose in life amongst older people in the community and institutions. Procedia ‐ Social and Behavioral Sciences 2015;202:263‐72. [Google Scholar]
Juengst 2015
- Juengst SB, Adams LM, Bogner JA, Arenth PM, O'Neil‐Pirozzi TM, Dreer LE, et al. Trajectories of life satisfaction after traumatic brain injury: influence of life roles, age, cognitive disability, and depressive symptoms. Rehabilitation Psychology 2015;60(4):353‐64. [PUBMED: 26618215] [DOI] [PMC free article] [PubMed] [Google Scholar]
Khan 2003
- Khan F, Baguley IJ, Cameron ID. Rehabilitation after traumatic brain injury. Medical journal of Australia 2003;178(6):290‐5. [DOI] [PubMed] [Google Scholar]
Loetscher 2013
- Loetscher T, Lincoln NB. Cognitive rehabilitation for attention deficits following stroke. Cochrane Database of Systematic Reviews 2013, Issue 5. [DOI: 10.1002/14651858.CD002842.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Menon 2010
- Menon DK, Schwab K, Wright DW, Maas AI. Position statement: definition of traumatic brain injury. Archives of Physical Medicine and Rehabilitation 2010;91(11):1637‐40. [DOI] [PubMed] [Google Scholar]
Middleton 2012
- Middleton EL, Schwartz MF. Errorless learning in cognitive rehabilitation: a critical review. Neuropsychological Rehabilitation 2012;22(2):138‐68. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Rohling 2009
- Rohling ML, Faust ME, Beverly B, Demakis G. Effectiveness of cognitive rehabilitation following acquired brain injury: a meta‐analytic re‐examination of Cicerone et al.'s (2000, 2005) systematic reviews. Neuropsychology 2009;23(1):20‐39. [DOI] [PubMed] [Google Scholar]
Sarajuuri 2006
- Sarajuuri JM, Koskinen SK. Holistic neuropsychological rehabilitation in Finland: the INSURE program ‐ a transcultural outgrowth of perspectives from Israel to Europe via the USA. International Journal of Psychology 2006;41(5):362‐70. [Google Scholar]
Sohlberg 1989
- Sohlberg MM, Mateer CA. Cognitive Rehabilitation: an Integrated Neuropsychological Approach. 2nd Edition. Vol. 16, New York: Guilford Press, 2001. [Google Scholar]
Toglia 1991
- Toglia JP. Generalization of treatment: a multicontext approach to cognitive perceptual impairment in adults with brain injury. American Journal of Occupational Therapy 1991;45(6):505‐16. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
K SK 2009
- K SK, Kumar SK, Macaden AS. Cognitive rehabilitation for occupational outcomes after traumatic brain injury (Protocol). Cochrane Database of Systematic Reviews 2009, Issue 3. [DOI: 10.1002/14651858.CD007935] [DOI] [PMC free article] [PubMed] [Google Scholar]


