Abstract
The three extended synaptotagmins (E-Syts) are endoplasmic reticulum (ER)-localized membrane proteins that mediate tethering of the ER to the plasma membrane (PM) via C2 domain-dependent interactions regulated by Ca2+ and/or PI(4,5)P2. The E-Syts also contains a Synaptotagmin-like Mitochondrial lipid-binding Protein (SMP) domain, a lipid-harboring module through which they mediate lipid transport between the two adjacent membranes. Here, we describe in vitro liposome-based methods to study the membrane tethering and lipid transport functions of E-Syt1. Its membrane tethering activity is monitored through a turbidity-based assay, and its lipid transport property is analyzed via fluorescence resonance energy transfer (FRET)-based assay. These in vitro methods have enabled us to gain insight into the mechanism of action and regulation of E-Syt1, such as the role of Ca2+ in releasing E-Syt1 from an autoinhibitory conformation. The same methods could be adapted to the study of other lipid transport proteins that function at membrane contact sites.
Keywords: C2 domain, SMP domain, Ca2 +, Membrane contact sites, Diacylglycerol
1. Introduction
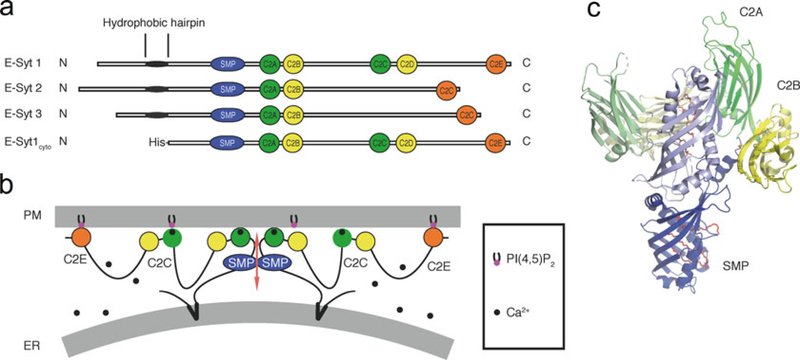
Areas of close apposition between the ER and the PM occur in all eukaryotes [1–4]. These appositions involve proteins that tether the two membranes and have a variety of functions. One such class of proteins are the three Extended synaptotagmins (E-Syt1, E-Syt2, and E-Syt3, Fig. 1a), which are homologous to the yeast tricalbins [6–9]. The E-Syts are anchored to the ER through an N-terminal hydrophobic hairpin (HRs) and mediate tethering to the PM via C-terminal C2 domains (Fig. 1b) [6, 10]. All three E-Syts also comprise a Synaptotagmin-like Mitochondrial lipid-binding Protein (SMP) domain (Fig. 1), which is a lipid-binding module of the TULIP domain superfamily [11–14]. Recruitment of all E-Syts to contacts of the ER with the PM requires PI(4,5)P2 in the PM, and also elevation of cytosolic Ca2+ in the case of E-Syt1 [6, 15–17]. The SMP domain, which is not required for ER-PM tethering [6], is typically found in proteins that mediate lipid transport at contacts between intracellular membranes [6, 7, 9, 18–21]. Crystallographic and biochemical studies of a fragment of E-Syt2 revealed that the SMP domain dimerizes to form a 90-Å-long cylinder with a hydrophobic groove that is along the cylinder and contains glycerophospholipids (Fig. 1c) [14].
Fig. 1.
Architecture and structure of the E-Syts. (a) Domain organization of the E-Syts and of the E-Syt1 construct used for the membrane tethering and lipid transfer assays. (b) Schematic representation of the putative arrangement of E-Syt1 at ER-PM contacts in the presence of elevated cytosolic Ca2+ (see also [5]). The protein dimerizes via its SMP domain. The C2A and the C2C domains have Ca2+-binding sites. (c) Crystal structure of an E-Syt2 fragment comprising its SMP domain and C2A-C2B domains (PDB code: 4P42). One monomer is shown in full colors and the other in pale colors. The SMP domain is in blue, the C2A domain is in green, and the C2B domain is in yellow. Lipid molecules are represented as sticks in red
To prove a lipid transfer function of the E-Syts, and gain insight into the regulation of such function as well as into their tethering properties, we used in vitro liposome-based assays and purified fragments of the cytosolic portion of E-Syt1 [5, 10, 22]. Such fragments had a Histidine tag at their N-terminus so that they could be anchored to liposomes containing a nickel-chelating lipid [23].
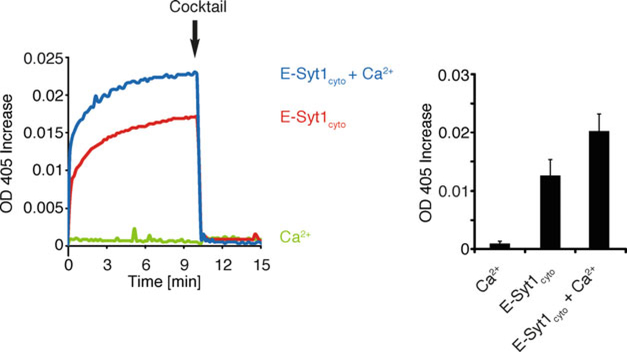
We initially focused on the membrane tethering activity of E-Syt1. In this assay, the entire purified N-terminal His-tagged cytosolic fragment of human E-Syt1 (the fragment missing the N-terminus including the hydrophobic hairpin (E-Syt1cyto), Fig. 1a), was incubated with ER-like liposomes and PM-like liposomes. ER-like liposomes comprised phosphatidylcholine (PC), NBD-phosphatidylethanolamine (PE), rhodamine-PE (Rhod-PE), and a nickel-chelating lipid [DGS-NTA(Ni)]. PM-like liposomes comprised PC and the acidic phospholipids phosphatidylserine (PS) and PI(4,5)P2. Turbidity, which reflects the size of clustered liposomes, was measured as optical density at 405 nm. Presence of E-Syt1cyto in the liposome mixture increased the turbidity observed in the absence of the protein and this occurred even in the absence of Ca2+ (Fig. 2). However, the turbidity increase was strongly stimulated by Ca2+ (Fig. 2). These findings were consistent with the presence of a fraction of E-Syt1 at ER-PM contacts at resting Ca2+ level [6, 16], and with the striking recruitment to the PM upon cytosolic Ca2+ elevation [6, 15–17]. Addition of a “cocktail” of EGTA (to chelate the Ca2+ in the solution), imidazole (to disrupt nickel His-tag interactions), and proteinase K (to digest E-Syt1cyto) at the end of the reaction completely reversed the increase in turbidity, thus ruling out fusion of liposomes as an explanation for the increase of turbidity (Fig. 2).
Fig. 2.
Tethering of donor and acceptor liposomes in the absence or presence of E-Syt1cyto (protein: lipid ratio 1:2000) and 100 μM Ca2+, as assessed by increase in turbidity (OD at 405 nm). A cocktail of EGTA, imidazole, and Proteinase K (Cocktail) was added after 10 min. Time-courses are at left and bar graphs showing quantification of OD405 increases at the time of addition of the cocktail (arrows in the left panel) are at right. Mean and SD of three independent experiments
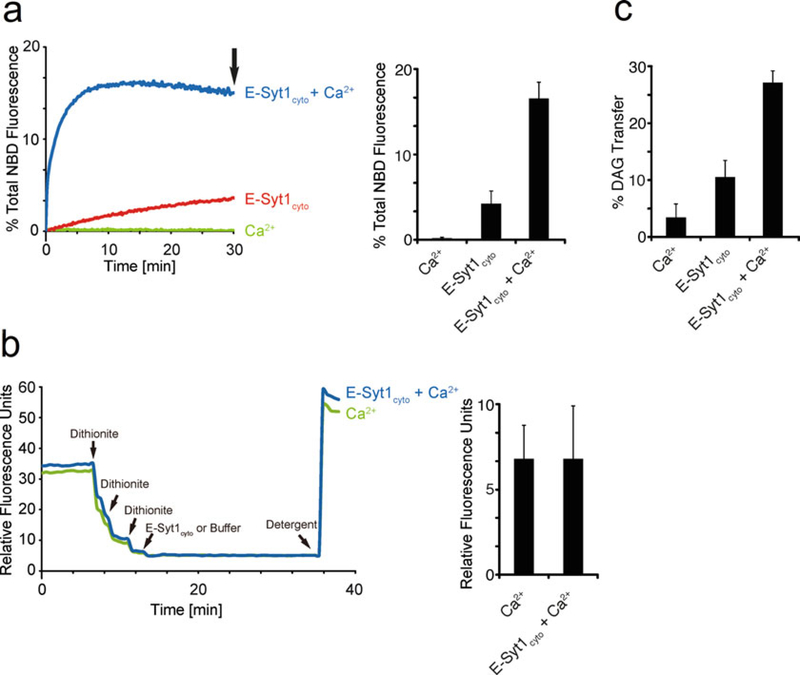
We next examined the lipid transfer activity of E-Syt1 constructs using the same liposome-based system in a fluorescence resonance energy transfer (FRET) assay. In this assay, transfer of a fluorescently labeled lipid (NBD-PE) from ER-like liposomes (hence referred to as donor liposomes) to PM-like liposomes (hence referred to as acceptor liposomes) was measured by monitoring the increase of the NBD fluorescence, which is self-quenched in the donor liposomes, as NBD-PE was diluted by transfer to acceptor liposomes. In ongoing follow-up experiments we used a modification of this assay in which NBD-PE fluorescence in donor liposomes is quenched by the additional presence of Rhod-PE in these liposomes, as in this case the quenching efficiency is greater [24]. We found that Ca2+ in the micromolar range was required for efficient lipid transfer (Fig. 3a) and that the effect of Ca2+ was due to the release of E-Syt1 from an autoinhibitory conformation affecting both its membrane tethering properties and accessibility of lipid to its lipid-binding pocket [5]. Dequenching could also be the result of fusion, but this was excluded by control experiments in which sodium dithionite, a poorly membrane permeable compound, was added to liposomes before protein addition to quench NBD fluorescence in their outer leaflet [25]. As NBD-PE is present on both leaflets of the donor liposome bilayer, if fusion of donor and acceptor liposomes occurred, the residual fluorescence after dithionite addition should undergo dequenching, but this was not the case (Fig. 3b).
Fig. 3.
E-Syt1 is a Ca2+-dependent lipid transfer protein. (a) On the left: time-course of normalized NBD fluorescence signals of liposomes mixtures in the absence or presence of 100 μM Ca2+ at room temperature. E-Syt1cyto was added at time 0 (protein: lipid ratio 1:1000). On the right: quantification of the increase in NBD fluorescence at the end of the incubation (indicated by an arrow in the left graph). (b) As in (a), but with 1 mM sodium dithionite added three times before addition of E-Syt1cyto. Raw fluorescence values, rather than percent fluorescence changes are shown in this figure. Quantifications of NBD fluorescence before addition of detergent are at right. (c) Bar graphs show the quantification of the percentage of 3H radioactivity in the supernatant (light liposomes) over the sum of the total radioactivity in the supernatant and pellet (light plus heavy liposomes). Mean and SD of three independent experiments
Finally, we used a liposome-based system to assess transfer of a natural and physiological relevant 3H-labeled lipid devoid of fluorescent tags. We examined transfer of 3H-diacylglycerol (DAG), as studies of the E-Syts showed that cells lacking all the three E-Syts have a delayed clearance of DAG at the PM following PLC activation [10]. In this assay, one population of liposomes was loaded with sucrose (heavy liposomes comprising PC, PS, PI(4,5)P2, DAG, and 3H-DAG) to allow their separation by centrifugation from the other liposome population [light liposomes comprising PC and DGS-NTA(Ni)] at the end of the reaction. Liposomes were incubated with or without purified E-Syt1cyto. Subsequently, E-Syt1cyto-tethered liposomes were separated by the addition of the “cocktail” (as describe above but with no EGTA) followed by centrifugation. Analysis of radioactivity in the two populations of liposomes indicated robust SMP-domain-dependent and Ca2+-stimulated 3H-DAG accumulation in light liposomes, proving the ability of E-Syt1 to transport DAG (Fig. 3c).
Work based on these assays validated the hypothesis that E-Syt1 has both membrane tethering and lipid transfer activities and provided insight into the regulation of its activities [5, 10, 22]. These assays can be adapted to explore and/or characterize the membrane tethering and lipid transfer properties of other proteins.
2. Materials
2.1. Expression and Purification of E-Syt1cyto
Plasmids: pCMV6-AN-His vector (e.g., OriGene) containing the region coding for residues 93–1104 of human E-Syt1 (see [5, 10] for detailed information for making this construct).
Expi293F cells (ThermoFisher).
Expi293 expression medium (ThermoFisher).
ExpiFectamine 293 Transfection Kit containing the ExpiFectamine 293 Reagent, the ExpiFectamine 293 Transfection Enhancer 1 and the ExpiFectamine 293 Transfection Enhancer 2 (ThermoFisher).
Nalgene Single-Use PETG Erlenmeyer Flasks with Plain Bottom: Sterile (ThermoFisher).
His60 Ni Superflow Resin (TaKaRa).
Econo-Pac Chromatography Column (Bio-rad).
PBS buffer: 1.8 mM KH2PO4, 10 mM Na2HPO4, 2.7 mM KCl, 137 mM NaCl, pH 7.4.
Lysis buffer: 25 mM Tris–HCl, pH 8, 300 mM NaCl, 0.5 mM TCEP ((tris(2-carboxyethyl)phosphine), 10 mM imidazole supplemented with EDTA-free Protease Inhibitor Cocktail (Roche).
Wash buffer: 25 mM Tris–HCl, pH 8, 300 mM NaCl, 0.5 mM TCEP, 20 mM imidazole.
Elution buffer: 25 mM Tris–HCl, pH 8, 300 mM NaCl, 0.5 mM TCEP, 400 mM imidazole.
Gel filtration buffer: 25 mM Tris–HCl, pH 8, 100 mM NaCl, 0.5 mM TCEP.
Superspeed centrifuge, highspeed microcentrifuge, and polycarbonate centrifuge tubes.
Amicon Ultra-15 Centrifugal filters, regenerated cellulose 100,000 NMWL (Millipore).
Superdex 200 Increase 10/300 GL (GE Healthcare).
NanoDrop 2000c Spectrophotometer (ThermoFisher).
ÄKTA pure Chromatography System (GE Healthcare).
2.2. Liposome Preparation
Non-radiolabeled lipids (Avanti Polar Lipids): 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), 1-palmitoyl-2-oleoyl-sn-glycero-3-phospho-L-serine (POPS), 1,2-dioleoyl-sn-glycero-3-[(N-(5-amino-1-carboxypentyl)iminodiacetic acid)succinyl] (nickel salt) (DGS-NTA(Ni)), L-α-phosphatidylinositol-4,5-bisphosphate (PI(4,5)P2), 1,2-dipalmitoyl-sn-glycero-3-phosphoethanolamine-N-(7-nitro-2–1,3-benzoxadiazol-4-yl) (NBD-PE), 1,2-dioleoyl-sn-glycero-3-phosphoethanolamine-N-(lissamine rhodamine B sulfonyl) (Rhod-PE), 1–2-dioleoyl-sn-glycerol (DAG).
1,2-dioleoyl [9,10-3H] rac-glycerol (3H-DAG, ART).
Chloroform.
Glass vials.
Gastight syringes with a 250–500 μL volume (Hamilton).
N2 gas.
LiposoFast Liposome Factory Basic (Sigma-Aldrich).
Nuclepore Track-Etched Polycarbonate Membrane Filter, diameter: 1.9 cm, pore size: 0.05 μm (Whatman).
Gel filtration buffer: see details in Subheading 2.1.
2.3. Membrane Tethering and Lipid Transfer Assays
Gel filtration buffer: see details in Subheading 2.1.
100 mM sodium dithionite stock solution in gel filtration buffer. Prepare freshly.
2.5% (w/v) n-dodecyl-ß-D-maltoside (DDM) solution.
Cocktail solution: 1.8 M imidazole, 15 mM EGTA, and 1.15 mg/mL proteinase K (Sigma-Aldrich) in gel filtration buffer. Prepare freshly.
EGTA-free cocktail solution: 1.8 M imidazole and 1.15 mg/mL proteinase K in gel filtration buffer. Prepare freshly.
1 M CaCl2 stock solution.
96-well flat bottom plate and 96-well black, flat bottom plate (Corning).
Plate Reader for turbidity and fluorescence analysis (e.g., Molecular Devices, SpectraMax M5 Microplate Reader).
Liquid Scintillation counter for radioactivity analysis (e.g., Wallac 1409DSA Liquid Scintillation Counter).
EcoScint scintillation liquid (National Diagnostics).
3. Methods
3.1. Expression and Purification of E-Syt1cyto
Culture Expi293F cells in 30 mL of Expi293 expression medium in an orbital shaker (125 rpm) at 37 °C in 8% CO2. Cells are considered ready for transfection when at three million viable cells/mL.
Pellet and resuspend the cells in 25.5 mL of fresh Expi293 expression medium, pre-warmed to 37 °C in a water bath.
Dilute 30 μg of plasmid encoding E-Syt1cyto in 1.5 mL of Opti-MEM, and 81 μL of ExpiFectamine 293 Reagent in 1.5 mL of Opti-MEM. Incubate for 5 min at room temperature.
Add 1.5 mL of diluted ExpiFectamine 293 Reagent into 1.5 mL of the DNA mixture, mix gently, and incubate for 20 min at room temperature.
Slowly pipette the mixture to Expi293F cells.
After 18 h, enhance protein expression with 150 μL of ExpiFectamine 293 Transfection Enhancer 1 and 1.5 mL of ExpiFectamine 293 Transfection Enhancer 2.
Harvest cells 72 h post transfection by centrifugation at 500 × g for 10 min at room temperature.
Resuspend 1 volume of cell pellet with an equal volume of lysis buffer.
Lyse cells by 3 cycles of freezing with liquid nitrogen and thawing in a 37 °C water bath.
Clarify the lysates by centrifugation at 17,000 × g for 30 min at 4 °C.
Equilibrate 500 μL of His60 Ni Superflow Resin with lysis buffer (see Note 1).
Transfer clarified lysate in a 50 mL falcon tube and add equilibrated His60 Ni Superflow Resin. Incubate at 4 °C for 1 h with gentle agitation.
Transfer the mixture to an Econo-Pac Chromatography Column, and wash the resin with 50 mL of washing buffer.
Add 15 mL of elution buffer to elute the protein (see Note 2).
Concentrate the protein to 500 μL using 100 K Amicon Ultra-15 Centrifugal filters.
Further purify the protein by size exclusion chromatography using Superdex 200 Increase 10/300 GL connected to an ÄKTA pure Chromatography System in gel filtration buffer at a flow rate of 0.5 mL/min (see Note 3). Collect all the fractions of 1 mL each.
Analyze all the fractions by SDS–PAGE (10% of acrylamide), stain the gel with Coomassie blue, and destain with distilled water. Fractions containing His-tagged E-Syt1cyto are pooled and stored at 4 °C (see Note 4). The molar concentration of the protein is calculated by dividing the absorbance of the sample at 280 nm with a spectrophotometer by the molar extinction coefficient of the protein.
3.2. Preparation of Liposomes
Warm up the lipid stocks to room temperature.
Add individual lipids to a glass vial using a glass syringe. The total lipids amount is 2.5 μmoL (see Note 5). For tethering and FRET-based lipid transfer assay (see Subheadings 3.3 and 3.4), prepare donor liposomes (mol%) with 82% DOPC, 15% DGS-NTA(Ni), 1.5% NBD-PE, and 1.5% Rhod-PE and acceptor liposomes with 85% DOPC, 10% POPS, and 5% PI(4,5)P2. For the radioactivity-based, centrifugation-based lipid transfer assay (see Subheading 3.5), prepare heavy donor liposomes with 84% DOPC, 10% POPS, 5% PI(4,5)P2, 0.9% DAG, and 0.1% 3H-DAG and light acceptor liposomes with 85% DOPC and 15% DGS-NTA(Ni).
Dry the lipid mixture with a stream of N2 gas. Incubate the dried lipids at 37 °C for 10 min (see Note 6).
Further dry the lipids completely in vacuum for 2 h.
Hydrate the lipid film with 500 μL of gel filtration buffer and incubate at 37 °C for 30 min. In the case of the heavy liposomes, gel filtration buffer should also contain 0.75 M sucrose.
Transfer the lipid mixture into 1.5 mL conical microcentrifuge tubes and generate liposomes by 10 cycles of freezing with liquid nitrogen and thawing in a 37 °C water bath.
For all liposomes mixtures (with the exception of heavy liposomes), extrude them 11 times through polycarbonate membrane filters (pore size: 0.05 μm) in a LiposoFast Liposome factory. For heavy liposomes, dilute them in 1 mL of gel filtration buffer, pellet them at 17,000 × g for 10 min at room temperature, and resuspend them with 500 μL of gel filtration buffer.
Transfer the liposomes to a conical microcentrifuge tube and store at 4 °C (see Note 7).
3.3. Membrane Tethering Assay
Prepare a 96-well flat bottom plate. Add 35 μL of gel filtration buffer with or without 100 μM CaCl2 to the well, followed by 2.5 μL of donor liposomes and 2.5 μL of acceptor liposomes. Incubate the reaction mixture at room temperature for 10 min (see Note 8).
Dilute His-tagged E-Syt1cyto in gel filtration buffer to 1.25 μM.
Add 10 μL of His-tagged E-Syt1cyto to the reaction mix, and immediately start reading the absorbance of the solution at 405 nm (OD405) every 10 s for 10 min using a microplate reader (see Note 9) to monitor turbidity changes.
After 10 min, add 10 μL of cocktail solution to reverse liposome aggregation. Read the absorbance of the solution at 405 nm again for another 5 min.
3.4. FRET-Based Lipid Transfer Assay
Prepare a 96-well black, flat bottom plate. Add 35 μL of gel filtration buffer with or without 100 μM CaCl2 to each well, followed by 2.5 μL of donor liposomes and 2.5 μL of acceptor liposomes. Incubate the reaction mix at room temperature for 10 min (see Note 10).
For the dithionite-quenching assay, add 0.5 μL of 100 mM dithionite stock solution to the reaction mixture multiple times, until the fluorescence intensity of NBD with an excitation wavelength of 460 nm and emission wavelength of 538 nm using a fluorescence microplate reader does not change (see Note 11).
Dilute His-tagged E-Syt1cyto in gel filtration buffer to 2.5 μM.
Add 10 μL of His-tagged E-Syt1cyto to the reaction mix prepared as in step 1 for the lipid transfer assays or as in step 2 for the control experiments, and immediately start reading the fluorescence intensity of the solution with an excitation wavelength of 460 nm and emission wavelength of 538 nm every 10 s for 30 min using the microplate reader.
After 30 min, add 10 μL of 2.5% DDM solution to the reaction mixture (to solubilize liposomes) and read the total NBD fluorescence.
The data were expressed as a percentage of the total NBD fluorescence, after subtracting the baseline values obtained at 0 min.
3.5. Radioactivity-Based Lipid Transfer Assay
Add 70 μL of gel filtration buffer with or without 100 μM CaCl2 into a 1.5 mL conical microcentrifuge tube, followed by 3.3 μL of heavy liposomes and 6.7 μL of light liposomes.
Dilute His-tagged E-Syt1cyto in gel filtration buffer to 2.5 μM.
Add 10 μL of His-tagged E-Syt1cyto to the reaction mixture and incubate this material at room temperature for 30 min.
Terminate the reaction by addition of 20 μL of EGTA-free cocktail solution (see Note 12) to reverse liposome aggregation.
Separate the heavy and light liposomes by centrifugation at16,000 × g for 15 min at room temperature.
Resuspend the pellets with 100 μL of gel filtration buffer.
Mix supernatants and resuspended pellets with scintillation liquid and measure radioactivity by a liquid scintillation counter at room temperature.
Acknowledgments
We thank Frederic Pincet, Joshua Lees, Frank Wilson, Heather Wheeler, Louise Lucast, and Yiying Cai for technical assistance. P. D.C. was supported in part by NIH grants NS036251 and DA018343. X.B. was supported by a Human Frontier Science Program long-term fellowship.
Footnotes
Do not use EGTA and DTT in the buffer used for Ni affinity chromatography. EGTA chelates Ni and DTT reduces Ni, thus decreasing the binding efficiency of the protein.
The Ni resin can be eluted with a smaller volume of buffer that can be run multiple times through the resin to achieve a greater concentration of the eluted protein.
Proteins are unstable in buffers containing high concentrations of imidazole. Remove the imidazole by gel filtration as soon as possible after the elution from the Ni resin.
We have found that E-Syt1cyto is stable and functional at 4 °C for at least 1 week. Freeze the protein in liquid nitrogen and store it at −80 °C for longer storage.
Always use glass syringes and glass vials to transfer and store lipids in chloroform.
The solubility of PI(4,5)P2 will decrease during the evaporation of chloroform, due to the cooling resulting from this evaporation. It is recommended to keep the vial in a 37 °C bath during the drying process.
The stability of liposomes when stored at 4 °C varies with different lipids. Liposomes used here can be stored at 4 °C for 1 week. It is not recommended to freeze liposomes.
Lipid transfer mediated by E-Syt1cyto increases with temperature. We typically perform our reactions at room temperature.
Excessive aggregation of liposomes will cause fluctuation of the OD405 curve. Decreasing the concentration of liposomes or of the protein will solve this problem.
Increase the ratio of acceptor to donor liposomes will raise the maximum percentage of NBD fluorescence increase.
Dithionite is very unstable in water. Always use freshly solubilized dithionite.
Do not add EGTA to this cocktail solution as it will cause a slight aggregation of light liposomes.
References
- 1.Friedman JR, Voeltz GK (2011) The ER in 3D: a multifunctional dynamic membrane network. Trends Cell Biol 21:709–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gallo A, Vannier C, Galli T (2016) Endoplasmic reticulum-plasma membrane associations: structures and functions. Annu Rev Cell Dev Biol 32:279–301 [DOI] [PubMed] [Google Scholar]
- 3.Saheki Y, De Camilli P (2017) Endoplasmic reticulum-plasma membrane contact sites. Annu Rev Biochem 86:659–684 [DOI] [PubMed] [Google Scholar]
- 4.Stefan CJ, Manford AG, Emr SD (2013) ER-PM connections: sites of information transfer and inter-organelle communication. Curr Opin Cell Biol 25:434–442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bian X, Saheki Y, De Camilli P (2018) Ca(2+) releases E-Syt1 autoinhibition to couple ER-plasma membrane tethering with lipid transport. EMBO J 37:219–234 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giordano F, Saheki Y, Idevall-Hagren O et al. (2013) PI(4,5)P(2)-dependent and Ca(2+)-regulated ER-PM interactions mediated by the extended synaptotagmins. Cell 153:1494–1509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Manford AG, Stefan CJ, Yuan HL et al. (2012) ER-to-plasma membrane tethering proteins regulate cell signaling and ER morphology. Dev Cell 23:1129–1140 [DOI] [PubMed] [Google Scholar]
- 8.Min SW, Chang WP, Sudhof TC (2007) E-Syts, a family of membranous Ca2+–sensor proteins with multiple C2 domains. Proc Natl Acad Sci U S A 104:3823–3828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toulmay A, Prinz WA (2012) A conserved membrane-binding domain targets proteins to organelle contact sites. J Cell Sci 125:49–58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saheki Y, Bian X, Schauder CM et al. (2016) Control of plasma membrane lipid homeostasis by the extended synaptotagmins. Nat Cell Biol 18:504–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kopec KO, Alva V, Lupas AN (2010) Homology of SMP domains to the TULIP superfamily of lipid-binding proteins provides a structural basis for lipid exchange between ER and mitochondria. Bioinformatics 26:1927–1931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kopec KO, Alva V, Lupas AN (2011) Bioinformatics of the TULIP domain superfamily. Biochem Soc Trans 39:1033–1038 [DOI] [PubMed] [Google Scholar]
- 13.Lee I, Hong W (2006) Diverse membrane-associated proteins contain a novel SMP domain. FASEB J 20:202–206 [DOI] [PubMed] [Google Scholar]
- 14.Schauder CM, Wu X, Saheki Y et al. (2014) Structure of a lipid-bound extended synaptotagmin indicates a role in lipid transfer. Nature 510:552–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chang CL, Hsieh TS, Yang TT et al. (2013) Feedback regulation of receptor-induced Ca2+ signaling mediated by E-Syt1 and Nir2 at endoplasmic reticulum-plasma membrane junctions. Cell Rep 5:813–825 [DOI] [PubMed] [Google Scholar]
- 16.Fernandez-Busnadiego R, Saheki Y, De Camilli P (2015) Three-dimensional architecture of extended synaptotagmin-mediated endoplasmic reticulum-plasma membrane contact sites. Proc Natl Acad Sci U S A 112:E2004–E2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Idevall-Hagren O, Lu A, Xie B et al. (2015) Triggered Ca2+ influx is required for extended synaptotagmin 1-induced ER-plasma membrane tethering. EMBO J 34:2291–2305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kornmann B, Currie E, Collins SR et al. (2009) An ER-mitochondria tethering complex revealed by a synthetic biology screen. Science 325:477–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lees JA, Messa M, Sun EW et al. (2017) Lipid transport by TMEM24 at ER-plasma membrane contacts regulates pulsatile insulin secretion. Science 355:709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu LK, Choudhary V, Toulmay A et al. (2017) An inducible ER-Golgi tether facilitates ceramide transport to alleviate lipotoxicity. J Cell Biol 216:131–147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reinisch KM, De Camilli P (2016) SMP-domain proteins at membrane contact sites: structure and function. Biochim Biophys Acta 1861:924–927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yu H, Liu Y, Gulbranson DR et al. (2016) Extended synaptotagmins are Ca2+–dependent lipid transfer proteins at membrane contact sites. Proc Natl Acad Sci U S A 113:4362–4367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bornhorst JA, Falke JJ (2000) Purification of proteins using polyhistidine affinity tags. Methods Enzymol 326:245–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nichols JW, Pagano RE (1983) Resonance energy transfer assay of protein-mediated lipid transfer between vesicles. J Biol Chem 258:5368–5371 [PubMed] [Google Scholar]
- 25.Angeletti C, Nichols JW (1998) Dithionite quenching rate measurement of the inside-outside membrane bilayer distribution of 7-nitrobenz-2-oxa-1,3-diazol-4-yl-labeled phospholipids. Biochemistry 37:15114–15119 [DOI] [PubMed] [Google Scholar]