This systematic review and meta-analysis of 20 observational studies and 1 randomized clinical trial, including a total of 3534 patients, evaluates outcomes of cutaneous squamous cell carcinoma in the head and neck region with regional lymph node metastasis.
Key Points
Question
What are the risk factors most significantly associated with overall survival, locoregional recurrence, locoregional control, and disease-specific survival in cutaneous squamous cell carcinoma in the head and neck region with regional lymph node metastasis (McSCCHN)?
Findings
This systematic review and meta-analysis of 3534 patients with McSCCHN in 20 observational studies and 1 randomized clinical trial showed that immunosuppression, extracapsular spread, and adjuvant radiotherapy were the most significant risk factors associated with overall survival. Immunosuppression and adjuvant radiotherapy were also significantly associated with disease-specific survival.
Meaning
These data can be used for refinements of staging systems, to help inform future clinical trials, and to better counsel patients with McSCCHN about their prognosis.
Abstract
Importance
There is a need to summarize the available evidence and provide quantitative data of the most important prognostic factors for patients with metastatic cutaneous squamous cell carcinoma of the head and neck region with regional lymph node metastasis (McSCCHN).
Objective
To undertake a PRISMA-compliant systematic review and meta-analysis of all published studies on the risk factors for overall survival (OS), locoregional control (LRC), locoregional recurrence (LRR), and disease-specific survival (DSS) for patients with McSCCHN.
Data Sources
PubMed, CINAHL, and Embase were searched from 1946 to August 2018 for English-language articles.
Study Selection
Inclusion criteria were randomized clinical trials or observational studies reporting on at least 10 patients with McSCCHN; studies analyzing 1 defined risk factor; reporting OS, LRC, LRR, or DSS; and clinical follow-up of 1 year of more. For the final analysis we included risk factors that were analyzed for the same outcome in at least 3 studies. Of the 2923 articles screened, 21 articles met the inclusion criteria.
Data Extraction and Synthesis
PRISMA guidelines were used for abstracting the data. Two reviewers independently abstracted the data. Risk of bias was estimated with the Newcastle-Ottawa Scale. Meta-analysis was performed using the random-effects model. All analysis took place between January and October 2018.
Main Outcomes and Measures
The primary end point was OS. Secondary end points included LRC, LRR, and DSS.
Results
A total of 20 observational studies and 1 randomized clinical trial were identified, representing 3534 patients (some reviewed articles reported no demographic characteristics), and were included in the analysis. Significant risk factors associated with OS were immunosuppression (hazard ratio [HR] of death, 2.66; 95% CI, 2.26-3.13), extracapsular spread (HR, 1.90; 95% CI, 1.12-3.23), adjuvant radiotherapy (HR, 0.45; 95% CI, 0.27-0.78), lymph node ratio (HR, 1.91; 95% CI, 1.09-3.35), and advanced age (HR, 1.03; 95% CI, 1.00-1.07). Immunosuppression (HR, 3.82; 95% CI, 2.47-5.92) and adjuvant radiotherapy (HR, 0.52; 95% CI, 0.33-0.84) were also significant risk factors for DSS.
Conclusions and Relevance
Immunosuppressed patients and those with extracapsular extension have poor prognosis. Adjuvant radiotherapy is associated with an improvement in OS. These risk factors will assist with better risk stratification and may also help to inform future clinical trials.
Introduction
Cutaneous squamous cell carcinoma (cSCC) rates among the most common cancers worldwide. The majority of cases arise in the head and neck region, and it frequently occurs in geographic areas with a predominantly white population and high solar UV exposure, with an incidence that is likely to continue to rise.1 In the United States, approximately 200 000 to 400 000 new cases of cSCC are diagnosed each year, leading to more than 3000 deaths.2,3,4
Approximately 5% of patients with cSCC present with nodal metastasis,5 with higher rates in immunosuppressed patients and those with recurrent and higher-stage tumors. Survival of patients with nodal metastasis has been reported between 50% and 70%6,7 at 5 years after appropriate surgery and adjuvant treatment. Although the metastatic risk is low compared with that of other malignant tumors, the high prevalence of cSCC portends that the absolute number of patients harboring nodal metastasis and dying from this disease is substantial.
There is extensive dermatologic literature focused on nonmetastatic cSCC, with multicenter prospective trials and systematic reviews addressing the implications of different risk factors on outcomes of this entity.5,6,8,9 In contrast, most of our knowledge of metastatic cSCC is mainly based on retrospective single-center experiences, resulting in a limited ability to risk stratify and prognosticate outcomes. The objective of this study was to undertake the first systematic review and meta-analysis of the published literature of regionally metastatic cSCC in the head and neck region (McSCCHN) to our knowledge so as to identify risk factors that can be used for prognostication, risk-stratification, and patient selection for future trials.
Methods
Methods of the analysis and inclusion criteria were defined in advance and documented in a prespecified protocol (PROSPERO ID, CRD42017062506). PRISMA guidelines for abstracting data were used. Research ethics board approval was waived for this study.
Eligibility Criteria
Inclusion criteria were randomized clinical trials or observational studies of at least 10 patients with McSCCHN that reported comparative data associating at least 1 defined risk factor (pertaining to the patient, disease, or treatment) and an outcome of interest. Exclusion criteria were studies of noncutaneous SCC; articles referring to nonmetastatic non–head and neck cSCC; cSCC data that could not be extracted from data on other cutaneous cancers (ie, basal cell carcinoma and melanoma); articles with mixed metastatic and nonmetastatic cases with indistinguishable data between the 2 groups; and patients with distant metastasis.
Information Sources and Literature Search
A comprehensive and systematic search was conducted, using the following databases: Ovid MEDLINE In-Process & Other Non-Indexed Citations, MEDLINE, Embase, Cochrane Central Register of Controlled Trials, Cochrane Database of Systematic Reviews, ClinicalTrials.gov, CINAHL, and Scopus. The article search was performed from each database’s earliest records up to and including August 2018 by an experienced librarian with the input of 2 of us (A.S.) and (S.D.M.). Only studies published in or translated into English were included, and bibliographies of selected articles were reviewed for additional relevant studies. Gray literature and conference abstracts were also tracked. Details of the full electronic search are provided in eMethods in the Supplement.
Study Selection and Data Collection Process
Two of us (A.S and D.H.Y.) independently selected studies on the basis of the inclusion and exclusion criteria. Disparities in selection were resolved through discussion and ultimately by the lead investigator (S.D.M.) Articles were initially reviewed on the basis of title and abstract, and those deemed relevant were further reviewed. In cases of duplicate or overlapping data, the more recent version was included.
Data extraction was conducted with a template and compiled in a spreadsheet once studies had been selected and consensus was reached between each reviewer. The following information was obtained from each study: (1) study characteristics (years, location, number of patients); (2) patient risk factors, including age, sex, and immune status; presence of nodal disease (number of nodes, number of positive neck and parotid lymph nodes, node location, size, lymph node positive margins, extracapsular spread [ECS], lymph node ratio [LNR]); pathologic characteristics (lymphovascular and perineural invasion [PNI], tumor dimension, margin status, and tumor differentiation); and treatment (adjuvant radiotherapy [AR] and chemoradiotherapy); and (3) outcomes (overall survival [OS], locoregional control [LRC], locoregional recurrence [LRR], and disease-specific survival [DSS]).
Risk of Bias Assessment
The Newcastle-Ottawa Scale10 was used to assess the quality of the studies. Each reviewer independently generated a Newcastle-Ottawa Scale score, and this value was reviewed for agreement. For studies that had high risk of bias (score <6) both reviewers would deliberate on whether such studies should be excluded.
Statistical Analysis
Effect estimates presented as either a hazard ratio (HR) or odds ratio (OR), and associated 95% confidence intervals (CIs) for each risk factor and outcome of interest were extracted. When available, the effect estimates from multivariable Cox or logistic regression models were used for this study analysis. Meta-analysis was performed using STATA, version 14 (StataCorp LP, 2015) and Review Manager, version 5.3 (The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). Random-effects models were adopted for all analysis. For each model, risk factors were only included within the meta-analysis if relevant data from a minimum of 3 studies were provided. When studies reported both continuous and categorical effect estimates for the same risk factor (eg, age), categorical data were then converted to continuous; this methodology was only implemented if the following 2 qualifications were met: (1) linearity between predictor and outcome, and (2) all events within the continuous predictor occurred within its mid-range (see eMethods in the Supplement for a sample conversion). In cases where these qualifications did not apply, only the first level of the categorical predictor was included within the meta-analysis. An a priori sensitivity analysis was then conducted to evaluate the presence of this predictor on the overall meta-analytical effect estimate. Furthermore, in situations where effect estimates of categorical estimates were inversed (ie, female vs male as opposed to male vs female), the corresponding effect estimates and 95% CIs were inversed.
To assess the degree of heterogeneity, the I2 statistic11 was also calculated, and a value exceeding 50% implied substantial heterogeneity. Forest plots were constructed for all associations of risk factors and outcomes.
Results
Study Selection
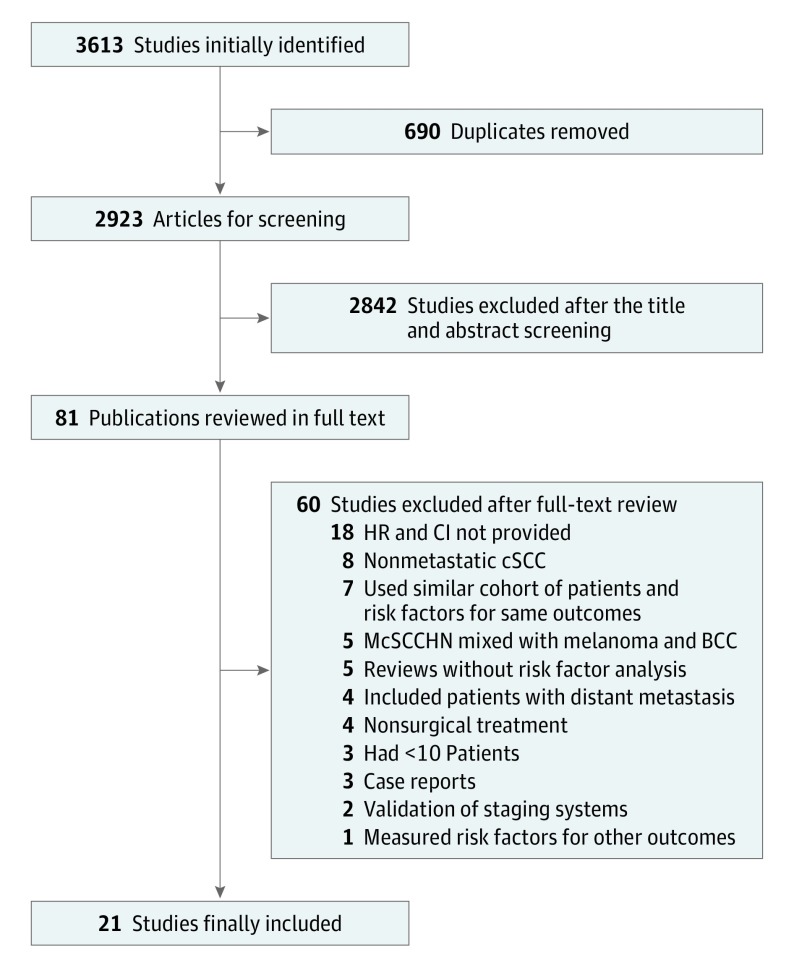
There were 3613 records initially identified. After 690 duplicates were removed, a total of 2923 records remained for title and abstract analysis, and 81 studies were reviewed in full text. Twenty-one articles were finally included. The study inclusion flow diagram can be seen in Figure 1.
Figure 1. Flow Diagram of Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) for the Systematic Literature Search.
BCC indicates basal cell carcinoma; CI, confidence interval; cSCC, cutaneous squamous cell carcinoma; HR, hazard ratio; McSCCHN, cSCC in the head and neck region with regional lymph node metastasis.
Overlapping Cohorts in the Final Random-Effects Model
The full-text review found 28 studies that fulfilled inclusion criteria. We found reports from the same institutions involving coincident time periods, risk factors, patient populations, and analyzed outcomes. In an effort to prevent overlapping cohorts between reports including analogous patient populations, in the cases where the same risk factors for the same outcomes were analyzed between studies with similar patient cohorts, we selected the studies that combined the largest data set of patients and the greater number or risk factors. eTable 1 in the Supplement details the studies with overlapping cohorts. In cases in which the studies with overlapping cohorts for the same outcome measured other outcomes that could be combined, they were included (eg, the study by Ebrahimi et al12 had an overlapping cohort with that of Brunner et al13 for OS and with that of Ch’ng et al14 for DSS, but the study by Ebrahimi et al12 was still included to analyze age for locoregional control).
Study Characteristics and Meta-analysis
A total of 21 studies were included, representing 3534 patients included in the analysis. Twenty studies were observational, and 1 was a randomized clinical trial. The Table summarizes the characteristics of these 21 studies.7,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31
Table. Characteristics of the Included Studies.
| Source | Study Years | Location | No. of Patients | Measured Risk Factorsa | Analyzed Outcomes | Median Follow-up, mo |
|---|---|---|---|---|---|---|
| Amoils et al,15 2017 | 2009-2014 | United States (California) | 80 | Age, sex, IS, AR, primary lesion (>2 cm, depth >2mm), tumor features (recurrent presentation, PNI, lymphovascular invasion, ECS, poor differentiation) | OS | 36 |
| Bobin et al,16 2018 | 2005-2015 | Nantes (France) | 35 | Age, IS, FN involvement, invaded neck nodes, positive laryngeal margins | OS, DSS | 11 |
| Brunner et al, 201513 | 1980-2010 | Australia (NSW, SHNCI/RPAH) | 672 | Age, sex, IS, AR, nodal classification, ECS | OS | 36 |
| Ch’ng et al,14 2013 | 1978-2010 | Australia (NSW, Westmead Hospital) | 239 | IS, AR, lymph node–positive margins, tumor features (well differentiated vs others) | DSS | 37.2 |
| Ebrahimi et al,12 2013 | 1980-2010 | Australia (NSW, Westmead Hospital) | 229 | Age, sex, IS, AR, lymph node positive margins, primary lesion (>2 vs 2 cm; thickness, >5 vs 5 mm; involved margin, PNI, LVI, recurrent primary tumor), tumor features (poor or undifferentiated) | LRC, DSS | 45.6 |
| Givi et al,17 2011 | 1993-2007 | United States (Oregon) | 61 | Age, AR, IS, node location (parotid only vs cervical lymph nodes), tumor features (recurrent presentation, well/moderate/poor differentiation, lymphovascular invasion, PNI, ECS) | OS | 15 |
| Hirshoren et al,18 2017 | 2001-2014 | Peter MacCallum Center (Australia, Victoria) | 149 | Age, IS, AR, nodal classification, nodal size, No. of involved nodes, LNR, lymph node positive margins, primary lesion (increasing diameter of primary lesion), ECS, T-N interval | OS, LRC | 37.7 |
| Lam et al,19 2018 | 1986-2016 | Australia (NSW, Westmead Hospital) | 69 | IS | OS, LRR | 70 |
| Makki et al,20 2013 | 2000-2010 | Canada (Halifax) | 54 | Age, nodal classification,b primary lesion (size), tumor features (tumor differentiation, PNI, lymphovascular invasion) | LRR | 24 |
| Manyam et al,21 2015 | 2000-2011 | United States (Cleveland) | 40 | ECS | LRR | 24 |
| McDowell et al,22 2016 | 2000-2014 | Peter MacCallum Center (Australia, Victoria) | 132 | IS, largest nodal size, lymphovascular space invasion | DSS | 60 |
| Mizrachi et al,23 2013 | 1990-2008 | Israel (Tel Aviv) | 71 | Age, sex, AR, nodal classification, LNR, tumor features (moderate vs well differentiated/ poorly vs well differentiated) | OS | 49.2 |
| Pramana et al,24 2012 | 1994-2008 | Australia (NSW, St George Cancer Care Centre) | 75 | Nodal size, surgical margins | DSS | 60 |
| Porceddu et al,7 2018 | 2005-2014 | Multi-institutional (New Zealand and Australia, TROG) | 310 | Chemoradiation | OS, DFS, FFLR | 60 |
| Shao et al,25 2014 | 1989-2010 | New Zealand (Auckland) | 160 | IS | OS, DSS | 60 |
| Smith et al,26 2018 | 1987-2015 | Australia (SHNCI) | 442 | Age, AR, ECS, multiple nodes vs single node/none | DSS, DFS | 21 |
| Southwell et al,27 2006c | 1992-2002 | New Zealand (Auckland) | 49 | Age, IS, AR, previous surgery, tumor features (poor differentiation), surgical margins, VII nerve sacrifice, ECS | LRR | 20 |
| Tanvetyanon et al,28 2015 | 1998-2011 | United States (Florida) | 61 | Chemoradiation, nodal classification, tumor features (poor differentiation, PNI) | OS | 35.5 |
| Thom et al,29 2014 | 1994-2010 | United States (Minnesota) | 42 | Nodes location (deep lobe of parotid) | OS | 36.4 |
| Tseros et al,30 2016 | 1995-2016 | Australia (NSW, Westmead Hospital) | 238 | Age, sex, LNR | OS | 55 |
| Vasan et al,31 2018 | 1988-2015 | Australia (SHNCI) | 326 | LNR | OS, DFS | 40 |
Abbreviations: AR, adjuvant radiotherapy; DFS, disease-free survival; DSS, disease-specific survival; ECS, extracapsular invasion; FFLR, freedom from locoregional relapse; FN, fibronectin; IS, immunosuppression; LNR, lymph node ratio; LRC, locoregional control; LRR, locoregional recurrence; LVI, lymphovascular invasion; NSW, New South Wales; OS, overall survival; PNI, perineural invasion; RPAH, Royal Prince Alfred Hospital; SHNCI, Sydney Head and Neck Cancer Institute; TROG, Trans Tasman Radiation Oncology Group.
Compared risk factors are in bold type.
O’Brien classification.
This article was the only which calculated the odds ratio, therefore their outcomes and risk factors could not be combined.
Age, sex, AR, ECS, immunosuppression (IS), LNR, nodal classification, and PNI were the risk factors analyzed for the same outcome in at least 3 studies and were therefore included in the final analysis.
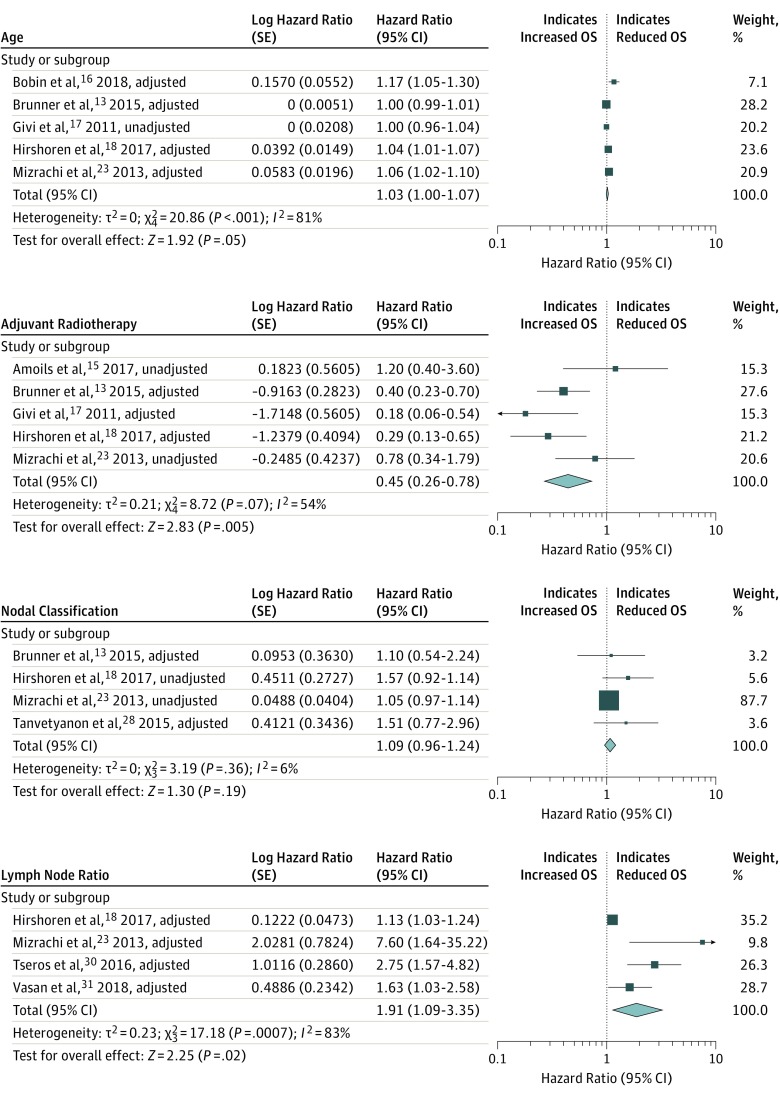
Significant risk factors for OS were IS (HR, 2.66; 95% CI, 2.26-3.13), ECS (HR, 1.90; 95% CI, 1.12-3.22), AR (HR, 0.45; 95% CI, 0.26-0.78), LNR (HR, 1.91; 95% CI, 1.09-3.35), and age (HR, 1.03; 95% CI, 1.00-1.07). The forest plots are depicted in Figure 2 and Figure 3.13,15,16,17,18,19,23,25,28,30,31 Significant heterogeneity was observed for the presence of ECS (I2 = 68%), AR (I2 = 53%), LNR (I2 = 83%), and age (I2 = 81%). After excluding the studies with unadjusted variables, ECS and AR HRs were 2.89 and 0.33, respectively, and both I2 scores were nonsignificant (I2 = 0%) (not shown). Patient sex, nodal classification and PNI were not significant risk factors for OS (Figure 2 and Figure 3).
Figure 2. Forest Plots for Risk Factor Associations With the Outcome of Overall Survival (OS).
Figure 3. Forest Plots for Risk Factor Associations With the Outcome of Overall Survival (OS).
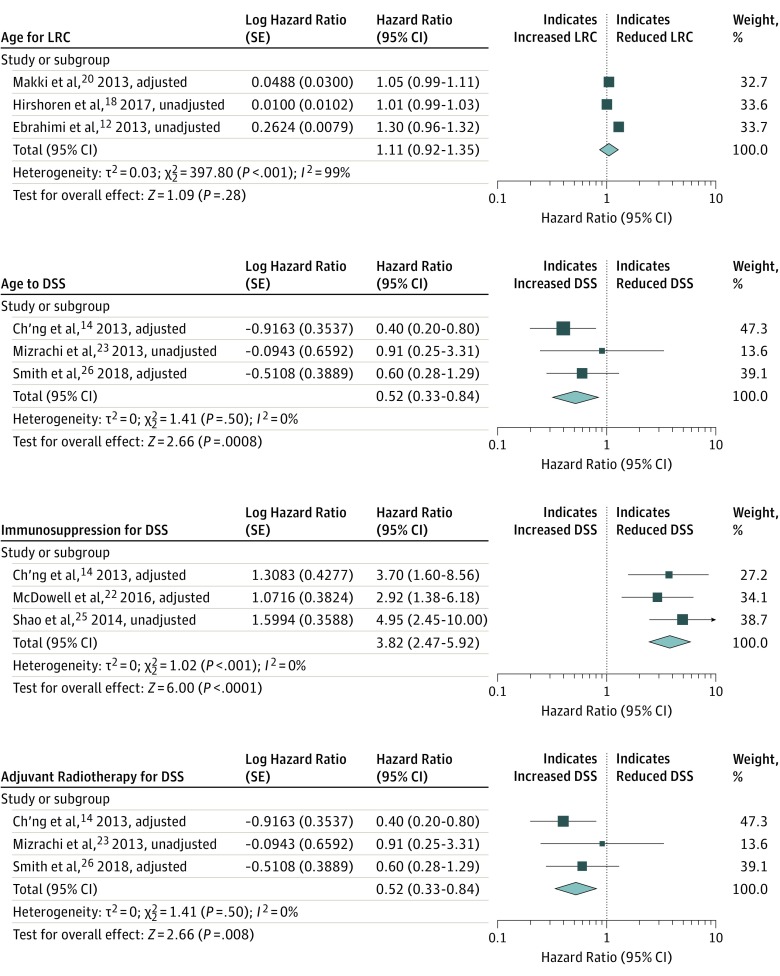
Regarding LRC and DSS, age, IS, and AR were the risk factors that were included in at least 3 studies. A significant association was found between IS (HR, 3.82; 95% CI, 2.47-5.92) and a reduced DSS, whereas AR (HR, 0.52; 95% CI, 0.33-0.84) was associated with increased DSS. Age was not a significant risk factor for LRC, and the I2 score was 99%. Figure 4 summarizes these findings.12,14,18,20,22,23,25,26
Figure 4. Forest Plots for Risk Factor Associations With the Outcomes of Locoregional Control (LRC) and Disease-Specific Survival (DSS).
There were many risk factors for different outcomes among the selected studies that could not be pooled because they were variably reported. eTable 2 in the Supplement details these risk factors for each outcome studied.
Quality of the Studies
eTable 3 in the Supplement details the scores of the Newcastle-Ottawa Scale for selection, comparability, and outcome of the studies. All of the studies had values greater than 6. Most studies were poor in the comparability aspect of the scale because the adjustment of variables varied among the studies and risk factors; this is detailed in the Table.
Discussion
Our analysis determined that IS, ECS, AR, LNR, and age were significant risk factors for OS. Immunosuppression and AR were also associated with reduced DSS. This meta-analysis provides robust evidence of a deleterious effect of IS and ECS and also suggests that AR improves outcomes in patients with McSCCHN.
There are some notable implications of this analysis. Immunosuppression had the highest HR for decreased OS and also was significantly associated with decreased DSS. This risk factor has been considered in the recent AJCC Cancer Staging Manual, 8th Edition,32 as an additional factor recommended for clinical care of patients with cSCC in the head and neck region. Discontinuing immunosuppressive therapy in patients with McSCCHN, especially in patients with regional metastasis, may represent an option whenever possible, and risks should be weighed against the benefits, as such a strong association between IS and decreased OS and DSS.33,34 Moreover, there was only 1 article in our analysis that characterized IS.18 Further studies specifying the nature of the IS are needed.
Extracapsular spread had the second highest HR associated with OS in our meta-analysis. As with IS, the latest AJCC staging system32 also included this risk factor, dividing the N category in N3a and N3b, attributing the highest N classification to patients having clinical or pathological ECS. This brings up the question of whether systemic treatment should be offered to patients with ECS in keeping with the evidence for offering systemic treatment to patients with mucosal squamous cancers.35,36 Escalating treatment by offering adjuvant chemoradiotherapy to patients with McSCCHN was evaluated in 2 studies, and 1 of them was included in the final analysis.28 The other is a recent multicenter randomized clinical trial7 that could not be included in the final random-effects model. No benefit in OS was found in these studies. Authors of the trial reported that the study was not powered to detect a smaller benefit from chemotherapy and that carboplatin was used rather than cisplatin. Thus, it is important to include anti–epidermal growth factor receptor agents and immunotherapies in future studies, and to recruit larger patient populations to elucidate if CRT is advantageous in patients with ECS.
The use of AR was associated with increased OS and DSS in our analysis. The 2018 NCCN Guidelines37 highlight the variability of the published results using AR in the treatment of cSCC with regional metastasis, stating that the diversity of study populations makes direct comparison of treatment outcomes difficult, but they declare it should be recommended for these patients. After homogenizing the analyzed studies and accounting for the characteristics of the included patients, as well as excluding overlapping cohorts, our meta-analysis showed a beneficial association between AR treatment and McSCCHN. Nonetheless, a more exact analysis controlling the N stage of the patients with the use of AR could contribute to identify whether there is a subset of patients with regional metastasis who would benefit from AR more than others. It should be noted that the risk of bias in our reviewed retrospective nonrandomized studies is high because the patients with worse prognosis and comorbidities might have not received AR, thus selecting the ones with better outcomes.
Evaluating LNR (defined as the ratio of number of metastatic lymph nodes to the number examined lymph nodes)23 represents a novel concept introduced as an independent risk factor in McSCCHN. This rationale emerged from characterization of other cancers (pancreatic, breast, and melanoma) for which LNR is an independent risk factor for survival as well.38,39,40,41 The Association of LNR with certain outcomes reached statistical significance in our review, but heterogeneity between the studies was substantial because the articles were discordant in their reporting of LNR, setting thresholds of more than 10 lymph nodes or a greater than 6% ratio. Age was also significantly associated with reduced OS, although the clinical implications were not as meaningful, and heterogeneity among studies was 81%.
Sex, nodal classification, and PNI were not significantly associated with OS. We expected to find a significant association between nodal classification and OS. We could not include the studies cited by the AJCC Cancer Staging Manual, 8th Edition,32 and the NCCN37 guidelines42,43,44,45,46 to stratify patients according to their nodal stage because there were overlapping cohorts, or the studies did not report HRs or CIs. One possible explanation for not finding significance with nodal classification is that some of the studies reported this factor variably as N-positive only,23 “per each N increase,”28 or N2a vs N1.13 Further and more detailed analyses discerning among the different N stages could help to elucidate the importance of this risk factor more accurately. While in the NCCN guidelines37 the presence of PNI increases T stage in patients, we were unable to find a significant association between PNI and OS.
Strengths and Limitations
Among the strengths of our study is our previously reported protocol in PROSPERO, defining in advance the criteria of our analysis, and we strictly followed the PRISMA guidelines. Also, we performed a comprehensive literature search performed by an experienced librarian. In addition, we perused all the available literature in an effort to exclude studies with overlapping cohorts and obtained results from studies with large patient populations.
The limitations of this study are mainly represented by the retrospective, single-cohort nature of the included articles with inherent biases. Moreover, many studies performed analyses without adjusting for confounding factors, which influenced the quality of included studies and also the heterogeneity of the studies. Finally, there was a notable heterogeneity in reporting risk factors and outcomes throughout the studies that hindered the inclusion in the final random-effects models.
Conclusions
To our knowledge, this is the first systematic review and meta-analysis of McSCCHN providing a higher level of evidence and demonstrating important risk factors for survival in patients with McSCCHN. The present findings can help physicians in their daily practice to better stratify and counsel their patients in that we have provided data on the weight of different risk factors. Our findings can be helpful for further refinements in staging systems for cSCC and may be used to direct future research about the subject. Subsequent research should report on well-defined survival outcomes, using consistent risk factor reporting and adjusted Cox proportional outcomes analyses.
eMethods.
eTable 1. Studies with overlapping cohorts
eTable 2. General summary of outcomes
eTable 3. Newcastle-Ottawa Scale for assessing the quality of nonrandomised studies in meta-analyses
eReferences.
References
- 1.Alam M, Ratner D. Cutaneous squamous-cell carcinoma. N Engl J Med. 2001;344(13):975-983. doi: 10.1056/NEJM200103293441306 [DOI] [PubMed] [Google Scholar]
- 2.Kim JYS, Kozlow JH, Mittal B, Moyer J, Olenecki T, Rodgers P; Work Group; Invited Reviewers . Guidelines of care for the management of cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2018;78(3):560-578. doi: 10.1016/j.jaad.2017.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karia PS, Han J, Schmults CD. Cutaneous squamous cell carcinoma: estimated incidence of disease, nodal metastasis, and deaths from disease in the United States, 2012. J Am Acad Dermatol. 2013;68(6):957-966. doi: 10.1016/j.jaad.2012.11.037 [DOI] [PubMed] [Google Scholar]
- 4.Demers AA, Nugent Z, Mihalcioiu C, Wiseman MCKE, Kliewer EV. Trends of nonmelanoma skin cancer from 1960 through 2000 in a Canadian population. J Am Acad Dermatol. 2005;53(2):320-328. doi: 10.1016/j.jaad.2005.03.043 [DOI] [PubMed] [Google Scholar]
- 5.Brantsch KD, Meisner C, Schönfisch B, et al. . Analysis of risk factors determining prognosis of cutaneous squamous-cell carcinoma: a prospective study. Lancet Oncol. 2008;9(8):713-720. doi: 10.1016/S1470-2045(08)70178-5 [DOI] [PubMed] [Google Scholar]
- 6.Thompson AK, Kelley BF, Prokop LJ, Murad MH, Baum CL. Risk factors for cutaneous squamous cell carcinoma recurrence, metastasis, and disease-specific death: a systematic review and meta-analysis. JAMA Dermatol. 2016;152(4):419-428. doi: 10.1001/jamadermatol.2015.4994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Porceddu SV, Bressel M, Poulsen MG, et al. . Postoperative concurrent chemoradiotherapy versus postoperative radiotherapy in high-risk cutaneous squamous cell carcinoma of the head and neck: the randomized phase III TROG 05.01 trial. J Clin Oncol. 2018;36(13):1275-1283. doi: 10.1200/JCO.2017.77.0941 [DOI] [PubMed] [Google Scholar]
- 8.Patel GK, Goodwin R, Chawla M, et al. . Imiquimod 5% cream monotherapy for cutaneous squamous cell carcinoma in situ (Bowen’s disease): a randomized, double-blind, placebo-controlled trial. J Am Acad Dermatol. 2006;54(6):1025-1032. doi: 10.1016/j.jaad.2006.01.055 [DOI] [PubMed] [Google Scholar]
- 9.Campoli M, Brodland DG, Zitelli J. A prospective evaluation of the clinical, histologic, and therapeutic variables associated with incidental perineural invasion in cutaneous squamous cell carcinoma. J Am Acad Dermatol. 2014;70(4):630-636. doi: 10.1016/j.jaad.2013.11.034 [DOI] [PubMed] [Google Scholar]
- 10.Wells GA, Shea B, O’Connell DPJ, Welch V, Losos MTP The Newcastle–Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. The Ottawa Hospital Research Institute; 2014. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed January 22, 2019.
- 11.Higgins JP, Thompson SG, Deeks JJAD, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557-560. doi: 10.1136/bmj.327.7414.557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ebrahimi A, Clark JR, Ahmadi N, Palme CE, Morgan GJ, Veness MJ. Prognostic significance of disease-free interval in head and neck cutaneous squamous cell carcinoma with nodal metastases. Head Neck. 2013;35(8):1138-1143. doi: 10.1002/hed.23096 [DOI] [PubMed] [Google Scholar]
- 13.Brunner M, Ng BC, Veness MJ, Clark JR. Assessment of the new nodal classification for cutaneous squamous cell carcinoma and its effect on patient stratification. Head Neck. 2015;37(3):336-339. doi: 10.1002/hed.23602 [DOI] [PubMed] [Google Scholar]
- 14.Ch’ng S, Clark JR, Brunner M, Palme CE, Morgan GJ, Veness MJ. Relevance of the primary lesion in the prognosis of metastatic cutaneous squamous cell carcinoma. Head Neck. 2013;35(2):190-194. doi: 10.1002/hed.22941 [DOI] [PubMed] [Google Scholar]
- 15.Amoils M, Lee CS, Sunwoo J, et al. . Node-positive cutaneous squamous cell carcinoma of the head and neck: survival, high-risk features, and adjuvant chemoradiotherapy outcomes. Head Neck. 2017;39(5):881-885. doi: 10.1002/hed.24692 [DOI] [PubMed] [Google Scholar]
- 16.Bobin C, Ingrand P, Dréno B, Rio E, Malard O, Espitalier F. Prognostic factors for parotid metastasis of cutaneous squamous cell carcinoma of the head and neck. Eur Ann Otorhinolaryngol Head Neck Dis. 2018;135(2):99-103. doi: 10.1016/j.anorl.2017.09.006 [DOI] [PubMed] [Google Scholar]
- 17.Givi B, Andersen PE, Diggs BS, Wax MK, Gross ND. Outcome of patients treated surgically for lymph node metastases from cutaneous squamous cell carcinoma of the head and neck. Head Neck. 2011;33(7):999-1004. doi: 10.1002/hed.21574 [DOI] [PubMed] [Google Scholar]
- 18.Hirshoren N, Danne J, Dixon BJ, et al. . Prognostic markers in metastatic cutaneous squamous cell carcinoma of the head and neck. Head Neck. 2017;39(4):772-778. doi: 10.1002/hed.24683 [DOI] [PubMed] [Google Scholar]
- 19.Lam JKS, Sundaresan P, Gebski V, Veness MJ. Immunocompromised patients with metastatic cutaneous nodal squamous cell carcinoma of the head and neck: Poor outcome unrelated to the index lesion. Head Neck. 2018;40(5):985-992. doi: 10.1002/hed.25069 [DOI] [PubMed] [Google Scholar]
- 20.Makki FM, Mendez AI, Taylor SM, et al. . Prognostic factors for metastatic cutaneous squamous cell carcinoma of the parotid. J Otolaryngol Head Neck Surg. 2013;42(1):14. doi: 10.1186/1916-0216-42-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Manyam BV, Gastman B, Zhang AY, et al. . Inferior outcomes in immunosuppressed patients with high-risk cutaneous squamous cell carcinoma of the head and neck treated with surgery and radiation therapy. J Am Acad Dermatol. 2015;73(2):221-227. doi: 10.1016/j.jaad.2015.04.037 [DOI] [PubMed] [Google Scholar]
- 22.McDowell LJ, Tan T-J, Bressel M, et al. . Outcomes of cutaneous squamous cell carcinoma of the head and neck with parotid metastases. J Med Imaging Radiat Oncol. 2016;60(5):668-676. doi: 10.1111/1754-9485.12484 [DOI] [PubMed] [Google Scholar]
- 23.Mizrachi A, Hadar T, Rabinovics N, et al. . Prognostic significance of nodal ratio in cutaneous squamous cell carcinoma of the head and neck. Eur Arch Otorhinolaryngol. 2013;270(2):647-653. doi: 10.1007/s00405-012-2050-3 [DOI] [PubMed] [Google Scholar]
- 24.Pramana A, Browne L, Graham PH. Metastatic cutaneous squamous cell carcinoma to parotid nodes: the role of bolus with adjuvant radiotherapy. J Med Imaging Radiat Oncol. 2012;56(1):100-108. doi: 10.1111/j.1754-9485.2011.02326.x [DOI] [PubMed] [Google Scholar]
- 25.Shao A, Wong DKC, McIvor NP, et al. . Parotid metastatic disease from cutaneous squamous cell carcinoma: prognostic role of facial nerve sacrifice, lateral temporal bone resection, immune status and P-stage. Head Neck. 2014;36(4):545-550. doi: 10.1002/hed.23323 [DOI] [PubMed] [Google Scholar]
- 26.Smith JA, Virk S, Palme CE, et al. . Age is not a predictor of prognosis in metastatic cutaneous squamous cell carcinoma of the head and neck. ANZ J Surg. 2018;88(4):E273-E277. doi: 10.1111/ans.13757 [DOI] [PubMed] [Google Scholar]
- 27.Southwell KE, Chaplin JM, Eisenberg RL, McIvor NP, Morton RP. Effect of immunocompromise on metastatic cutaneous squamous cell carcinoma in the parotid and neck. Head Neck. 2006;28(3):244-248. doi: 10.1002/hed.20321 [DOI] [PubMed] [Google Scholar]
- 28.Tanvetyanon T, Padhya T, McCaffrey J, et al. . Postoperative concurrent chemotherapy and radiotherapy for high-risk cutaneous squamous cell carcinoma of the head and neck. Head Neck. 2015;37(6):840-845. doi: 10.1002/hed.23684 [DOI] [PubMed] [Google Scholar]
- 29.Thom JJ, Moore EJ, Price DL, Kasperbauer JL, Starkman SJ, Olsen KD. The role of total parotidectomy for metastatic cutaneous squamous cell carcinoma and malignant melanoma. JAMA Otolaryngol Head Neck Surg. 2014;140(6):548-554. doi: 10.1001/jamaoto.2014.352 [DOI] [PubMed] [Google Scholar]
- 30.Tseros EA, Gebski V, Morgan GJ, Veness MJ. Prognostic significance of lymph node ratio in metastatic cutaneous squamous cell carcinoma of the head and neck. Ann Surg Oncol. 2016;23(5):1693-1698. doi: 10.1245/s10434-015-5070-6 [DOI] [PubMed] [Google Scholar]
- 31.Vasan K, Low T-HH, Gupta R, et al. . Lymph node ratio as a prognostic factor in metastatic cutaneous head and neck squamous cell carcinoma. Head Neck. 2018;40(5):993-999. doi: 10.1002/hed.25066 [DOI] [PubMed] [Google Scholar]
- 32.Amin MB, Edge S, Greene F, et al. , eds. AJCC Cancer Staging Manual. 8th ed New York, NY: Springer; 2017. doi: 10.1007/978-3-319-40618-3 [DOI] [Google Scholar]
- 33.Otley CC, Griffin MD, Charlton MR, Edwards BS, Neuburg M, Stasko T; Reduction of Immunosuppression Task Force of the International Transplant Skin Cancer Collaborative . Reduction of immunosuppression for transplant-associated skin cancer: thresholds and risks. Br J Dermatol. 2007;157(6):1183-1188. doi: 10.1111/j.1365-2133.2007.08203.x [DOI] [PubMed] [Google Scholar]
- 34.Otley CC, Berg D, Ulrich C, et al. ; Reduction of Immunosuppression Task Force of the International Transplant Skin Cancer Collaborative and the Skin Care in Organ Transplant Patients Europe . Reduction of immunosuppression for transplant-associated skin cancer: expert consensus survey. Br J Dermatol. 2006;154(3):395-400. doi: 10.1111/j.1365-2133.2005.07087.x [DOI] [PubMed] [Google Scholar]
- 35.Forastiere AA, Goepfert H, Maor M, et al. . Concurrent chemotherapy and radiotherapy for organ preservation in advanced laryngeal cancer. N Engl J Med. 2003;349(22):2091-2098. doi: 10.1056/NEJMoa031317 [DOI] [PubMed] [Google Scholar]
- 36.Bernier J, Domenge C, Ozsahin M, et al. ; European Organization for Research and Treatment of Cancer Trial 22931 . Postoperative irradiation with or without concomitant chemotherapy for locally advanced head and neck cancer. N Engl J Med. 2004;350(19):1945-1952. doi: 10.1056/NEJMoa032641 [DOI] [PubMed] [Google Scholar]
- 37.National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines). https://www.nccn.org/professionals/physician_gls/default.aspx#site. Accessed January 28, 2018.
- 38.Rossi CR, Mocellin S, Pasquali S, Pilati P, Nitti D. N-ratio: a novel independent prognostic factor for patients with stage-III cutaneous melanoma. Ann Surg Oncol. 2008;15(1):310-315. doi: 10.1245/s10434-007-9641-z [DOI] [PubMed] [Google Scholar]
- 39.Woodward WA, Vinh-Hung V, Ueno NT, et al. . Prognostic value of nodal ratios in node-positive breast cancer. J Clin Oncol. 2006;24(18):2910-2916. doi: 10.1200/JCO.2005.03.1526 [DOI] [PubMed] [Google Scholar]
- 40.Kuru B. Prognostic significance of total number of nodes removed, negative nodes removed, and ratio of positive nodes to removed nodes in node positive breast carcinoma. Eur J Surg Oncol. 2006;32(10):1082-1088. doi: 10.1016/j.ejso.2006.06.005 [DOI] [PubMed] [Google Scholar]
- 41.Berger AC, Watson JC, Ross EA, Hoffman JP. The metastatic/examined lymph node ratio is an important prognostic factor after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am Surg. 2004;70(3):235-240. [PubMed] [Google Scholar]
- 42.Forest V-I, Clark JJ, Veness MJ, Milross C. N1S3: a revised staging system for head and neck cutaneous squamous cell carcinoma with lymph node metastases: results of 2 Australian Cancer Centers. Cancer. 2010;116(5):1298-1304. doi: 10.1002/cncr.24855 [DOI] [PubMed] [Google Scholar]
- 43.Palme CE, O’Brien CJ, Veness MJ, McNeil EB, Bron LP, Morgan GJ. Extent of parotid disease influences outcome in patients with metastatic cutaneous squamous cell carcinoma. Arch Otolaryngol Head Neck Surg. 2003;129(7):750-753. doi: 10.1001/archotol.129.7.750 [DOI] [PubMed] [Google Scholar]
- 44.Andruchow JL, Veness MJ, Morgan GJ, et al. . Implications for clinical staging of metastatic cutaneous squamous carcinoma of the head and neck based on a multicenter study of treatment outcomes. Cancer. 2006;106(5):1078-1083. doi: 10.1002/cncr.21698 [DOI] [PubMed] [Google Scholar]
- 45.Audet N, Palme CE, Gullane PJ, et al. . Cutaneous metastatic squamous cell carcinoma to the parotid gland: analysis and outcome. Head Neck. 2004;26(8):727-732. doi: 10.1002/hed.20048 [DOI] [PubMed] [Google Scholar]
- 46.Ch’ng S, Maitra A, Allison RS, et al. . Parotid and cervical nodal status predict prognosis for patients with head and neck metastatic cutaneous squamous cell carcinoma. J Surg Oncol. 2008;98(2):101-105. doi: 10.1002/jso.21092 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eMethods.
eTable 1. Studies with overlapping cohorts
eTable 2. General summary of outcomes
eTable 3. Newcastle-Ottawa Scale for assessing the quality of nonrandomised studies in meta-analyses
eReferences.