Key Points
Question
What is the real-world risk of fracture for patients treated with denosumab compared with those treated with alendronate?
Findings
In this Danish cohort study including 4624 individuals treated with denosumab and 87 731 individuals treated with alendronate, 3-year cumulative incidence of hip fracture was 3.7% and 3.1% among the denosumab and alendronate cohorts, respectively. The 3-year cumulative incidence of any fracture was 9.0% for both cohorts.
Meaning
In routine clinical practice, initiation of denosumab and alendronate treatments were associated with similar risks of hip and any fracture over a 3-year period.
This cohort study of individually linked data from Danish health registries compares the risk of hip and any fracture in adult patients treated with denosumab vs alendronate in routine practice settings.
Abstract
Importance
Head-to-head randomized clinical trials showed greater efficacy of denosumab vs alendronate in improving bone mineral density. Although there is an association of changes in bone mineral density with reductions in fracture risk, the magnitude of the association is not well established.
Objective
To compare the risk of hip and any fracture in patients treated with denosumab and alendronate in routine practice settings.
Design, Setting, and Participants
This Danish nationwide, population-based, historical cohort study of a population with universal access to health care used prospectively collected, individually linked data from Danish health registries with complete follow-up. Cohorts consisted of 92 355 individuals 50 years or older who were new users of denosumab (n = 4624) or alendronate (n = 87 731) from May 2010 to December 2017 after at least 1 year without an antiosteoporosis medication dispensing.
Exposures
Initiation of denosumab or alendronate.
Main Outcomes and Measures
The primary outcome was hospitalization for hip fracture, and the secondary outcome was hospitalization for any fracture. Inverse probability of treatment weights and the intention-to-treat approach were used to calculate cumulative incidences and adjusted hazard ratios (aHRs) with 95% CIs.
Results
Of the 92 355 included patients, 75 046 (81.3%) were women, and the mean (SD) age was 71 (10) years. The denosumab cohort had a lower proportion of men than the alendronate cohort (12.7% [589] vs 19.0% [16 700]), while age distributions were similar in the 2 cohorts. Within 3 years of follow-up, initiation of denosumab or alendronate was associated with cumulative incidences of 3.7% and 3.1%, respectively, for hip fracture and 9.0% and 9.0%, respectively, for any fracture. Overall, the aHRs for denosumab vs alendronate were 1.08 (95% CI, 0.92-1.28) for hip fracture and 0.92 (95% CI, 0.83-1.02) for any fracture. The aHR of denosumab vs alendronate for hip fracture was 1.07 (95% CI, 0.85-1.34) among patients with a history of any fracture and 1.05 (95% CI, 0.83-1.32) among patients without history of fracture. The aHR for any fracture for denosumab vs alendronate was 0.84 (95% CI, 0.71-0.98) among patients with a history of any fracture and 0.77 (95% CI, 0.64-0.93) among patients with no history of fracture.
Conclusions and Relevance
Treatment with denosumab and alendronate was associated with similar risks of hip or any fracture over a 3-year period, regardless of fracture history.
Introduction
Osteoporosis is characterized by progressive deterioration of bone structure and decreased bone mineral density (BMD).1 Fractures are a common manifestation of osteoporosis,2,3 with hip fracture being the most serious.4,5 Worldwide, osteoporosis is the most common metabolic skeletal disease,6 affecting 200 million persons and causing nearly 9 million fractures annually.2
Bisphosphonates are a mainstay of prevention and treatment of postmenopausal osteoporosis, with alendronate being the first-line choice, given its effectiveness and low price.7,8 Denosumab was approved in the European Union, including Denmark, in May 2010 for the treatment of osteoporosis in postmenopausal women and in men at high fracture risk.9 Both denosumab and alendronate are antiresorptive drugs and inhibit osteoclasts, albeit via different mechanisms; while denosumab binds the cytokine receptor activator of nuclear factor–κB ligand and thereby blocks osteoclasts’ formation, maturation, activation, and survival, alendronate binds to bone mineral, where it is absorbed by mature osteoclasts, inducing osteoclast apoptosis and suppressing resorption.10
Some adverse effects reported among bisphosphonates users, such as gastrointestinal tract effects or acute kidney injury, are rarely seen with denosumab, owing to differences in administration and clearance between the 2 agents.11 In contrast to weekly oral alendronate treatment, denosumab treatment, administered subcutaneously every 6 months, removes the need for patient adherence for that period.12 On the other hand, denosumab is promptly cleared after 6 months, while bisphosphonates linger in bone for many years, potentially partially compensating for suboptimal adherence. Denosumab is a cost-effective treatment for osteoporosis with €50 000 to €60 000 (US$56 232-$67 478) per quality-adjusted life-year gained13 and is a potential alternative to alendronate in patients 75 years or older, those with renal impairment, and those with low expected adherence.14
In randomized clinical trials (RCTs), denosumab was more efficacious than bisphosphonates in increasing bone mass among postmenopausal women.15,16 Although there is an association of change in BMD with reduction in fracture risk,17,18 the magnitude of the association is not well established. The RCTs examined the risk of any fracture as a secondary outcome, finding no difference in the fracture risk between denosumab and alendronate within 1 year of treatment initiation.15,16 However, these studies were based on a low number of fractures, and the extent of fracture reduction with denosumab compared with alendronate in routine clinical practice is unclear from the data. Using routinely collected data from population-based health registries in Denmark, we conducted a nationwide cohort study to compare risk of hip and any fracture in patients treated with denosumab and alendronate.
Methods
Setting and Data Sources
Danish residents have access to universal health care, including subsidized prescription costs. Furthermore, numerous population-based linkable registries enable nearly complete capture of important life and health events. To construct the analysis data set for this study, we linked data from the Danish Civil Registration System, which assigns a unique personal identifier to all Danish residents and tracks vital status and migration19; the Danish National Health Services Prescription Database, which has recorded reimbursed medication dispensings from all community pharmacies since 200420; and the Danish National Patient Registry, which contains discharge dates and diagnoses from all hospitalizations since 1977 and from all outpatient clinic and emergency department contacts since 1995.21 Although dispensings are imperfect representations of true treatment status, they are considered a better measure of medication intake compared with most alternatives.22 Completeness and positive predictive values of the fracture diagnoses in the Danish National Patient Register exceed 90%,23,24 and positive predictive value of the comorbidities is 90%.25
As this cohort study did not involve contact with patients or an intervention, it was not necessary to obtain permission from the Danish Scientific Ethical Committee. The study was approved by the Danish Data Protection Agency. Danish law does not require informed consent or ethical approval for studies based solely on registry data. This report follows the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline for cohort studies.
Patients
We used an active-comparator, new-user approach26 to include patients taking denosumab (the denosumab cohort) and patients taking alendronate (the alendronate cohort), defined as persons with the first dispensing of denosumab or alendronate between May 26, 2010, and December 31, 2017. The date of the first dispensing of denosumab or alendronate during the study period was the index date in each respective cohort. We excluded patients with a recorded dispensing of any osteoporosis medication (eg, denosumab, alendronate, raloxifene, ipriflavone, strontium ranelate, other bisphosphonates, teriparatide, calcitonin) in the 12 months preceding the index date. We furthermore excluded patients younger than 50 years on the index date and those with a history of cancer or Paget disease in the 10 years before index date (both of which are alternative indications for denosumab and alendronate).
Outcome
The primary outcome was hip fracture, identified by primary or secondary diagnosis during hospitalization. The secondary outcome was any fracture, including primary or secondary diagnosis during hospitalization for hip or vertebral fractures and primary or secondary diagnosis for nonvertebral nonhip fractures recorded during any hospital encounter (inpatient, outpatient, or emergency).
Covariates
We measured the following covariates at the index date: age, sex, and comorbidities, including history of fracture, alcohol-related disorders, obesity, and diseases from the Charlson Comorbidity Index (CCI). We summarized diseases from the CCI using the Romano modification,27 ie, excluding chronic obstructive pulmonary disease (a marker of smoking) and chronic renal impairment (a predictor of denosumab treatment), which were treated as separate covariates. We classified the CCI scores as no comorbidity (CCI score, 0), moderate comorbidity (CCI score, 1-2), or severe comorbidity (CCI score, ≥3). We also included data on dispensings of comedications affecting bone metabolism or fracture risk since 2004. Included comedications were oral corticosteroids, anticoagulants and antithrombotics, hormone replacement therapy, anxiolytics and sedatives, antipsychotics, antidepressants, statins, nonsteroid anti-inflammatory drugs, antihypertensive drugs, drugs for treatment of chronic obstructive pulmonary disease, opioids, and antithyroid drugs. History of hip and any fracture was defined using the same codes as those used to define hip and any fracture outcome.
Statistical Analysis
We tabulated the characteristics of the denosumab cohort and the alendronate cohort, then computed crude rates of fracture outcomes per 1000 person-years. Analogous to the intention-to-treat approach used in RCTs,26 a patient was considered treated with the agent initiated on the index date. We included all patient data from the index date to the date of a fracture outcome, death, emigration, or December 31, 2017, whichever occurred first. Occurrence of a fracture at a site other than the hip did not censor follow-up for the hip fracture end point. We plotted cohort-specific cumulative incidence curves for hip fracture and any fracture, considering death as a competing risk and weighting all observations by stabilized inverse probability of treatment weights (IPTWs) to balance the measured covariates.28 Inverse probability of treatment weights were derived from propensity scores, which were computed using logistic regression as predicted probability of initiating denosumab or alendronate treatment as a function of the covariates and interaction terms of sex with comedication and of calendar year with all other covariates. Weights were stabilized by multiplying the weights in the denosumab cohort by the proportion of denosumab users in the total cohort and by multiplying the weights in the alendronate cohort by the proportion of alendronate users in the total cohort.28 We used standardized mean differences to assess the balance of covariates in the 2 cohorts, with the aim of achieving a standardized mean difference of less than 0.1 for all covariates.
Furthermore, we computed risk differences (based on cumulative incidences) with 95% CIs. We used Cox proportional hazards regression to compute crude and adjusted hazard ratios (aHRs) with 95% CIs for each outcome, overall and stratified by age group, sex, and fracture history. In the stratified analyses, new stabilized IPTWs were computed within each subgroup. The proportionality of hazards assumptions was found not to be violated, according to log-log plots.
We tested the robustness of the results in a series of sensitivity analyses. First, we repeated the analyses while excluding patients with a recent fracture at the same site as a given outcome, varying the definition of recent from 30 to 730 days before the index date. Second, we used a stricter definition of treatment initiation by extending the requirement of absence of pre–index date osteoporosis therapy from 1 year to 5 years. Third, we excluded atypical femoral fractures (approximated, in the absence of specific diagnostic codes for atypical fractures or access to results of radiography, by diagnostic codes for fractures of the femoral shaft or subtrochanteric region) from the hip fracture definition and computed new aHRs for hip fracture. Fourth, we repeated the analyses while censoring follow-up at discontinuation of or switch from the initial treatment (analogous to the per-protocol approach in RCTs). Discontinuation was defined as a gap of 90 days or more between the expiration of the days supplied in a given dispensing for the denosumab cohort and as a gap of 180 days or more for the alendronate cohort, thereby accounting for differences in the pharmacokinetic properties of the 2 agents (ie, faster clearance of denosumab).10 We tested different combinations of discontinuation-defining intervals for denosumab/alendronate: 90/90 days, 60/150 days, 120/210 days, and 120/180 days. A switch was defined as a dispensing of a different antiosteoporosis agent before discontinuation of the initial agent. Analyses were performed with SAS version 9.4 (SAS Institute Inc).
Results
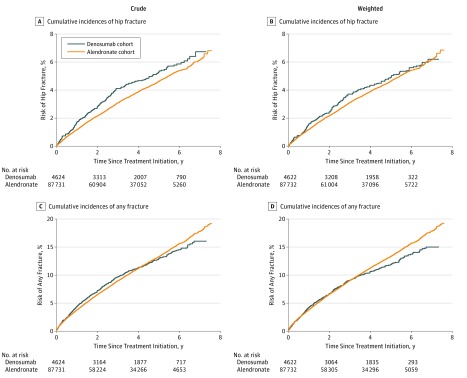
We identified 4624 initiators of denosumab and 87 731 initiators of alendronate. Of the 92 355 included patients, 75 046 (81.3%) were women, and the mean (SD) age was 71 (10) years. Table 1 shows patient characteristics in the 2 cohorts before applying IPTWs. There was a sharp increase in the number of users of alendronate in 2012. This increased prescription rate was because alendronate’s status changed from individual to general reimbursement. The denosumab cohort had a lower proportion of men than the alendronate cohort (12.7% [589] vs 19.0% [16 700]), while age distributions were similar in the 2 cohorts. In the denosumab cohort, 604 patients (13.1%) were aged 50 to 59 years, 1382 (29.9%) were 60 to 69 years, 1496 (32.4%) were 70 to 79 years, and 1142 (24.7%) were 80 years and older. In the alendronate cohorts, 12 344 of patients (14.1%) were aged 50 to 59 years, 26 923 (30.7%) were 60 to 69 years, 28 320 (32.3%) were 70 to 79 years, and 20 144 (23.0%) were 80 years and older. The denosumab cohort had higher prevalences of comorbidity and comedications than the alendronate cohort, including higher prevalence of renal impairment (3.5% [161] vs 1.3% [1131]). While standardized mean differences before applying IPTW were as high as 0.63 for some covariates, in the IPTW-stabilized population all standardized mean differences were less than 0.02 (Table 1). Median (interquartile range) follow-up time was 3.3 (1.5-5.3) years in the denosumab cohort and 3.1 (1.4-5.0) years in the alendronate cohort. The Figure shows cumulative incidences of hip fracture and any fracture during the follow-up time in the denosumab cohort and the alendronate cohort in the unweighted and IPTW populations.
Table 1. Baseline Characteristics of the Denosumab and Alendronate Cohorts in Denmark, 2010 to 2017.
| Characteristic | No. (%) | Standardized Mean Difference | |||
|---|---|---|---|---|---|
| Denosumab (n = 4624) | Alendronate (n = 87 731) | Total (N = 92 355) | Before Weighting | After Stabilized IPTW | |
| Year | |||||
| 2010 | 168 (3.6) | 2856 (3.3) | 3024 (3.3) | 0.03 | 0 |
| 2011 | 940 (20.3) | 4973 (5.7) | 5913 (6.4) | 0.63 | 0 |
| 2012 | 845 (18.3) | 24 876 (28.4) | 25 721 (27.9) | 0.34 | 0.01 |
| 2013 | 583 (12.6) | 13 641 (15.5) | 14 224 (15.4) | 0.12 | 0.01 |
| 2014 | 617 (13.3) | 10 926 (12.5) | 11 543 (12.5) | 0.04 | 0.01 |
| 2015 | 582 (12.6) | 10 495 (12.0) | 11 077 (12.0) | 0.03 | 0 |
| 2016 | 472 (10.2) | 9489 (10.8) | 9961 (10.8) | 0.03 | 0 |
| 2017 | 417 (9.0) | 10 475 (11.9) | 10 892 (11.8) | 0.14 | 0 |
| Men | 589 (12.7) | 16 700 (19.0) | 17 289 (18.7) | 0.24 | 0 |
| Age group, y | |||||
| 50-59 | 604 (13.1) | 12 344 (14.1) | 12 948 (14.0) | 0.04 | 0.01 |
| 60-69 | 1382 (29.9) | 26 923 (30.7) | 28 305 (30.6) | 0.02 | 0.01 |
| 70-79 | 1496 (32.4) | 28 320 (32.3) | 29 816 (32.3) | 0.00 | 0 |
| ≥80 | 1142 (24.7) | 20 144 (23.0) | 21 286 (23.0) | 0.06 | 0 |
| Charlson Comorbidity Index score | |||||
| 0 (No comorbidity) | 2741 (59.3) | 53 787 (61.3) | 56 528 (61.2) | 0.06 | 0.01 |
| 1-2 (Moderate) | 1519 (32.9) | 28 671 (32.7) | 30 190 (32.7) | 0.01 | 0.01 |
| ≥3 (High) | 364 (7.9) | 5273 (6.0) | 5637 (6.1) | 0.10 | 0.01 |
| Specific comorbidities | |||||
| History of fracture | 1508 (32.6) | 26 190 (29.9) | 27 698 (30.0) | 0.08 | 0 |
| Chronic renal impairment | 161 (3.5) | 1131 (1.3) | 1292 (1.4) | 0.20 | 0 |
| COPD | 827 (17.9) | 14 257 (16.3) | 15 084 (16.3) | 0.06 | 0 |
| Alcohol-related disorders | 118 (2.6) | 2014 (2.3) | 2132 (2.3) | 0.02 | 0.02 |
| Obesity | 141 (3.0) | 2613 (3.0) | 2754 (3.0) | 0.01 | 0.01 |
| Comedication | |||||
| Corticosteroids | 1699 (36.7) | 36 153 (41.2) | 37 852 (41.0) | 0.13 | 0 |
| Anticoagulants | 1943 (42.0) | 34 496 (39.3) | 36 439 (39.5) | 0.08 | 0 |
| Hormone replacement therapy | 1796 (38.8) | 28 854 (32.9) | 30 650 (33.2) | 0.18 | 0.01 |
| Antidepressants, antipsychotics, and sedatives | 1799 (38.9) | 28 820 (32.9) | 30 619 (33.2) | 0.18 | 0.01 |
| Statins | 1854 (40.1) | 34 274 (39.1) | 36 128 (39.1) | 0.03 | 0.01 |
| NSAIDs | 3576 (77.3) | 67 209 (76.6) | 70 785 (76.6) | 0.02 | 0.01 |
| Antidiabetics | 320 (6.9) | 6985 (8.0) | 7305 (7.9) | 0.06 | 0.01 |
| Antihypertensives | 2513 (54.3) | 46 095 (52.5) | 48 608 (52.6) | 0.05 | 0 |
| Drugs for treatment of COPD | 1501 (32.5) | 25 978 (29.6) | 27 479 (29.8) | 0.09 | 0 |
| Opioids | 3124 (67.6) | 53 044 (60.5) | 56 168 (60.8) | 0.21 | 0.01 |
| Antithyroid drugs | 170 (3.7) | 2972 (3.4) | 3142 (3.4) | 0.02 | 0 |
Abbreviations: COPD, chronic obstructive pulmonary disease; IPTW, inverse probability of treatment weight; NSAIDs, nonsteroidal anti-inflammatory drugs.
Figure. Crude and Weighted Cumulative Incidences of Hip Fracture and Any Fracture Following Initiation of Denosumab and Alendronate.
Hip Fracture
Within 3 years of follow-up, initiation of denosumab vs alendronate was associated with a weighted cumulative incidence for hip fracture of 3.7% vs 3.1%, respectively, corresponding to absolute risk differences of 0.6% (95% CI, −0.3% to 1.5%). The aHR for denosumab vs alendronate was 1.08 (95% CI, 0.92 to 1.28) for hip fracture (Table 2).
Table 2. Crude Rates and Crude and Adjusted Hazard Ratios of Hip Fracture in Denosumab and Alendronate Cohorts.
| Hip Fracture Outcome | Cohort | Patients, No. | Cases, No. | Person-Years | Crude Rate per 1000 Person-Years (95% CI) | Hazard Ratio (95% CI) | |
|---|---|---|---|---|---|---|---|
| Crude | IPTW | ||||||
| Overall | Denosumab | 4624 | 208 | 16 510.4 | 12.6 (10.9-14.4) | 1.18 (1.03-1.36) | 1.08 (0.92-1.28) |
| Alendronate | 87 731 | 3160 | 294 584.3 | 10.7 (10.4-11.1) | 1 [Reference] | 1 [Reference] | |
| Age, y | |||||||
| <80 y | Denosumab | 3482 | 95 | 13 210.8 | 7.2 (5.8-8.8) | 1.04 (0.84-1.28) | 1.00 (0.78-1.28) |
| Alendronate | 67 587 | 1641 | 236 634.2 | 6.9 (6.6-7.3) | 1 [Reference] | 1 [Reference] | |
| ≥80 y | Denosumab | 1142 | 113 | 3 299.6 | 34.2 (28.2-41.2) | 1.30 (1.08-1.58) | 1.21 (0.97-1.51) |
| Alendronate | 20 144 | 1519 | 57 950.1 | 26.2 (24.9-27.6) | 1[Reference] | 1 [Reference] | |
| Sex | |||||||
| Male | Denosumab | 589 | 25 | 1745.3 | 14.3 (9.3-21.1) | 1.38 (0.93-2.07) | 1.24 (0.79-1.95) |
| Alendronate | 16 700 | 525 | 50 782.9 | 10.3 (9.5-11.3) | 1 [Reference] | 1 [Reference] | |
| Female | Denosumab | 4035 | 183 | 14 765.0 | 12.4 (10.7-14.3) | 1.15 (0.99-1.34) | 1.03 (0.87-1.22) |
| Alendronate | 71 031 | 2635 | 243 801.4 | 10.8 (10.4-11.2) | 1 [Reference] | 1 [Reference] | |
| History of any fracture | |||||||
| No | Denosumab | 3116 | 106 | 11 477.1 | 9.2 (7.6-11.2) | 1.17 (0.96-1.42) | 1.05 (0.83-1.32) |
| Alendronate | 61 541 | 1673 | 212 601.9 | 7.9 (7.5-8.3) | 1 [Reference] | 1 [Reference] | |
| Yes | Denosumab | 1508 | 102 | 5033.2 | 20.3 (16.5-24.6) | 1.13 (0.93-1.38) | 1.07 (0.85-1.34) |
| Alendronate | 26 190 | 1487 | 81 982.4 | 18.1 (17.2-19.1) | 1 [Reference] | 1 [Reference] | |
| History of hip fracture | |||||||
| No | Denosumab | 4139 | 161 | 15 122.8 | 10.6 (9.1-12.4) | 1.15 (0.98-1.35) | 1.04 (0.86-1.26) |
| Alendronate | 78 644 | 2496 | 268 782.3 | 9.3 (8.9-9.7) | 1 [Reference] | 1 [Reference] | |
| Yes | Denosumab | 485 | 47 | 1 387.5 | 33.9 (24.9-45.0) | 1.33 (0.99-1.79) | 1.25 (0.89-1.76) |
| Alendronate | 9087 | 664 | 25 802.0 | 25.7 (23.8-27.8) | 1 [Reference] | 1 [Reference] | |
Abbreviation: IPTW, inverse probability of treatment weight.
Hazard ratios for hip fracture were similar for denosumab vs alendronate, regardless of sex or age (Table 2). The aHR of denosumab vs alendronate for hip fracture was 1.07 (95% CI, 0.85 to 1.34) among patients with a history of any fracture and 1.05 (95% CI, 0.83 to 1.32) among patients without history of any fracture (Table 2). Weighted cumulative incidences for hip fracture within 3 years among patients with a history of any fracture were 6.1% and 5.1% in the denosumab cohort and the alendronate cohort, respectively, with an absolute risk difference of 1.0% (95% CI, −0.7 to 2.7). Weighted cumulative incidences of hip fracture within 3 years among patients without history of any fracture were 2.9% and 2.3% in the denosumab cohort and the alendronate cohort, respectively, with an absolute risk difference of 0.6% (95% CI, −0.3 to 1.5).
Any Fracture
Within 3 years of follow-up, the initiation of denosumab and alendronate were both associated with a cumulative incidence of 9.0% for any fracture. The overall aHR for denosumab vs alendronate was 0.92 (95% CI, 0.83-1.02) for any fracture (Table 3). For denosumab vs alendronate, the aHR for any fracture was 0.84 (95% CI, 0.71-0.98) among patients with a history of any fracture and 0.77 (95% CI, 0.64-0.93) among patients without a history of any fracture. Results of stratified analyses for any fracture are presented in Table 3 and are very similar across strata.
Table 3. Crude Rates and Crude and Adjusted Hazard Ratios of Any Fracture in Denosumab and Alendronate Cohorts.
| Any Fracture Outcome | Cohort | Patients, No. | Cases, No. | Person-Years | Crude Rate per 1000 Person-Years (95% CI) | Hazard Ratio (95% CI) | |
|---|---|---|---|---|---|---|---|
| Crude | IPTW | ||||||
| Overall | Denosumab | 4624 | 511 | 15 749.0 | 32.4 (29.7-35.4) | 0.99 (0.91-1.08) | 0.92 (0.83-1.02) |
| Alendronate | 87 731 | 9213 | 280 839.0 | 32.8 (32.1-33.5) | 1 [Reference] | 1 [Reference] | |
| Age | |||||||
| <80 y | Denosumab | 3482 | 313 | 12 655.1 | 24.7 (22.1-27.6) | 0.93 (0.83-1.04) | 0.85 (0.75-0.97) |
| Alendronate | 67 587 | 6052 | 226 136.1 | 26.8 (26.1-27.4) | 1 [Reference] | 1 [Reference] | |
| ≥80 y | Denosumab | 1142 | 198 | 3093.9 | 64.0 (55.4-73.6) | 1.10 (0.95-1.27) | 1.06 (0.89-1.26) |
| Alendronate | 20 144 | 3161 | 54 702.9 | 57.8 (55.8-59.8) | 1 [Reference] | 1 [Reference] | |
| Sex | |||||||
| Male | Denosumab | 589 | 46 | 1707.6 | 26.9 (19.7-35.9) | 1.17 (0.87-1.57) | 0.96 (0.68-1.36) |
| Alendronate | 16 700 | 1137 | 49 511.2 | 23.0 (21.6-24.3) | 1[Reference] | 1 [Reference] | |
| Female | Denosumab | 4035 | 465 | 14 041.4 | 33.1 (30.2-36.3) | 0.95 (0.86-1.04) | 0.89 (0.80-0.99) |
| Alendronate | 71 031 | 8076 | 231 327.8 | 34.9 (34.2-35.7) | [1 Reference] | 1 [Reference] | |
| History of any fracture | |||||||
| No | Denosumab | 1508 | 149 | 3357.7 | 44.4 (37.5-52.1) | 0.82 (0.70-0.97) | 0.77 (0.64-0.93) |
| Alendronate | 26 190 | 2556 | 45 211.4 | 56.5 (54.4-58.8) | 1 [Reference] | 1 [Reference] | |
| Yes | Denosumab | 1508 | 223 | 4722.2 | 47.2 (41.2-53.8) | 0.89 (0.78-1.02) | 0.84 (0.71-0.98) |
| Alendronate | 26 190 | 4054 | 76 222.9 | 53.2 (51.6-54.8) | 1 [Reference] | 1 [Reference] | |
| History of hip fracture | |||||||
| No | Denosumab | 4139 | 428 | 14 452.6 | 29.6 (26.9-32.6) | 0.98 (0.89-1.08) | 0.89 (0.79-0.99) |
| Alendronate | 78 644 | 7778 | 256 616.5 | 30.3 (29.6-31.0) | 1 [Reference] | 1 [Reference] | |
| Yes | Denosumab | 485 | 83 | 1296.4 | 64.0 (51.0-79.4) | 1.07 (0.86-1.34) | 1.11 (0.85-1.44) |
| Alendronate | 9087 | 1435 | 24 222.5 | 59.2 (56.2-62.4) | 1 [Reference] | 1 [Reference] | |
Abbreviation: IPTW, inverse probability of treatment weight.
Sensitivity Analyses
The results did not materially change after excluding patients with a recent fracture at the same site as a given outcome or after excluding atypical femoral fractures from the hip fracture outcome definition. Extending the required therapy-free period to 5 years to define new use yielded an aHR for hip fracture of 1.07 (95% CI, 0.88-1.30) and for any fracture of 0.93 (95% CI, 0.82-1.05) for denosumab compared with alendronate (eTable 1 in the Supplement).
Patients switching between treatments were rare. Discontinuation was more frequent; thus, we lost approximately 30% of person-years in the denosumab group and approximately 44% for the alendronate cohort (these figures were similar for hip and any fracture). Nevertheless, the on-treatment analyses were consistent with the findings for hip fracture in the primary analysis (eTable 2 in the Supplement).
Discussion
This large nationwide historical cohort study was conducted using routinely and prospectively collected data originating from a health care system with universal coverage. Initiation of denosumab and initiation of alendronate were associated with similar risks of hip fracture or any fracture during a median follow-up of approximately 3 years, regardless of sex, age, or previous fracture.
Randomized clinical trials evaluating head-to-head efficacy of denosumab vs alendronate used BMD as the primary outcome and clinical fractures as an adverse effect or complication.15,16 These RCTs, conducted in postmenopausal women, regardless of prior treatment for osteoporosis, both found denosumab to be more efficacious than alendronate in increasing BMD, possibly the best proxy outcome for subsequent fracture risk. Overall, 6 of 8 RCTs29,30,31,32,33,34 included in 2 meta-analyses15,16 reported results on risk of any fracture. Our finding of no clinically relevant differences between denosumab and alendronate in the risk of hip and any fracture is in line with findings of the RCTs.15,16 In addition, our absolute risk estimates are similar to those from the RCTs; however, the absolute number of fractures in our study is considerably higher than the total number of fractures from RCTs included in the 2 meta-analyses.15,16 Kendler et al30 reported the risk of any fracture during 12 months of treatment as 3.2% (8 of 253 patients) in denosumab initiators and 1.6% (4 of 249 patients) in alendronate initiators. McClung et al32 reported 3.8% (12 of 314 patients) vs 2.2% (1 of 46 patients) risk of any fracture among individuals taking denosumab vs alendronate, respectively, and Brown et al29 reported 4.0% (24 of 593 patients) vs 3.2% (19 of 586 patients) risk of any fracture after 1 year among individuals taking denosumab vs alendronate. Two additional studies reported the risk of any fracture being 4.4% vs 3.5%34 and 3.6% vs 3.2%33 for denosumab users vs alendronate users, respectively, after 1 year of treatment, whereas a third study reported the risk of any fracture within 2 years of treatment as 6.7% vs 4.3%.31 To our knowledge, no head-to-head RCTs were powered to examine the risk of specific fractures, such as hip fractures, between denosumab and alendronate users. A 1996 meta-analysis based on women has suggested that the risk of hip fracture changes 2.6-fold for each standard deviation in BMD at the hip.18 The gradient of risk is lower (1.4 vs 1.6) in predicting any other osteoporotic fractures, regardless of the site measured.
It is well known that BMD can identify individuals who are at increased risk of developing a fracture, but it cannot with certainty identify individuals who will eventually develop fractures.18 Therefore, it is important to use data from clinical practice to examine treatment effectiveness in terms of fracture risk (clinically relevant outcome). Randomized clinical trials have not provided a clear answer on that question, especially as applied to long-term outcomes. In this study, it was not feasible to directly assess the full treatment-BMD-fracture pathway. However, BMD increase while receiving treatment accounts for only a small proportion of actual observed fracture risk reduction. A number of other factors, such as lifestyle factors, medication, comorbidities, or socioeconomic status, can influence the association of BMD with fracture risk and could explain why higher efficacy of denosumab on BMD does not translate into higher effectiveness in reducing hip fracture risk. In addition, we observed no difference in secondary hip fracture reduction between denosumab and alendronate treatment, which does not support current recommendations for prescribing denosumab to high-risk patients. The cost-effectiveness of denosumab treatment compared with alendronate is an argument for prescribing denosumab rather than alendronate to prevent hip fractures.
Limitations
Our study had limitations. The most important limitation when comparing a new-in-class agent with an established treatment is the possibility of residual confounding. For example, renal impairment was more prevalent in the denosumab cohort than in the alendronate cohort but is incompletely measured by hospital diagnoses.35 We lacked measures of frailty, socioeconomic status, and lifestyle, which could affect our results. Furthermore, no data on BMD were available, which is likely the main driver of treatment choice. Most guidelines, including National Institute for Health and Care Excellence technology appraisal guideline 204 from 2010,36 recommend the use of denosumab only in patients with lower BMD or in secondary fracture prevention, if first-line alendronate is not tolerated or contraindicated. Thus, confounding by indication is another limitation of our study, despite comprehensive availability of confounders and use of stabilized IPTWs in statistical analyses. Also, despite the large sample size, it was not possible to divide patients into more than 2 age groups.
Conclusions
In this nationwide cohort study based on routinely collected data in Denmark, treatment with denosumab and alendronate were associated with similar risks of hip and any fracture over a 3-year period. Sex, age, and fracture history were not associated with patients’ risk.
eTable 1. Rates and hazard ratios of the fracture outcomes in initiators of denosumab and alendronate, defining initiation by requiring 5 years’ absence of osteoporosis treatment before the index date (sensitivity analysis)
eTable 2. Rates and hazard ratios of the fracture outcomes for denosumab and alendronate, with follow-up censored at discontinuation or switch
References
- 1.Kanis JA, Melton LJ III, Christiansen C, Johnston CC, Khaltaev N. The diagnosis of osteoporosis. J Bone Miner Res. 1994;9(8):-. doi: 10.1002/jbmr.5650090802 [DOI] [PubMed] [Google Scholar]
- 2.Hernlund E, Svedbom A, Ivergård M, et al. . Osteoporosis in the European Union: medical management, epidemiology and economic burden: a report prepared in collaboration with the International Osteoporosis Foundation (IOF) and the European Federation of Pharmaceutical Industry Associations (EFPIA). Arch Osteoporos. 2013;8(1-2):136. doi: 10.1007/s11657-013-0136-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lane NE. Clinical practice: osteoarthritis of the hip. N Engl J Med. 2007;357(14):1413-1421. doi: 10.1056/NEJMcp071112 [DOI] [PubMed] [Google Scholar]
- 4.Keene GS, Parker MJ, Pryor GA. Mortality and morbidity after hip fractures. BMJ. 1993;307(6914):1248-1250. doi: 10.1136/bmj.307.6914.1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Omsland TK, Emaus N, Tell GS, et al. . Mortality following the first hip fracture in Norwegian women and men (1999-2008): a NOREPOS study. Bone. 2014;63:81-86. doi: 10.1016/j.bone.2014.02.016 [DOI] [PubMed] [Google Scholar]
- 6.Lin JT, Lane JM. Osteoporosis: a review. Clin Orthop Relat Res. 2004;(425):126-134. doi: 10.1097/01.blo.0000132404.30139.f2 [DOI] [PubMed] [Google Scholar]
- 7.Maraka S, Kennel KA. Bisphosphonates for the prevention and treatment of osteoporosis. BMJ. 2015;351:h3783. doi: 10.1136/bmj.h3783 [DOI] [PubMed] [Google Scholar]
- 8.Kanis JA, Burlet N, Cooper C, et al. ; European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO) . European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int. 2008;19(4):399-428. doi: 10.1007/s00198-008-0560-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.European Medicines Agency. Prolia (denosumab). https://www.ema.europa.eu/en/medicines/human/EPAR/prolia. Accessed March 6, 2019.
- 10.Baron R, Ferrari S, Russell RG. Denosumab and bisphosphonates: different mechanisms of action and effects. Bone. 2011;48(4):677-692. doi: 10.1016/j.bone.2010.11.020 [DOI] [PubMed] [Google Scholar]
- 11.Cummings SR, San Martin J, McClung MR, et al. ; FREEDOM Trial . Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med. 2009;361(8):756-765. doi: 10.1056/NEJMoa0809493 [DOI] [PubMed] [Google Scholar]
- 12.Freemantle N, Satram-Hoang S, Tang ET, et al. ; DAPS Investigators . Final results of the DAPS (Denosumab Adherence Preference Satisfaction) study: a 24-month, randomized, crossover comparison with alendronate in postmenopausal women. Osteoporos Int. 2012;23(1):317-326. doi: 10.1007/s00198-011-1780-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jönsson B, Ström O, Eisman JA, et al. . Cost-effectiveness of denosumab for the treatment of postmenopausal osteoporosis. Osteoporos Int. 2011;22(3):967-982. doi: 10.1007/s00198-010-1424-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mori T, Crandall CJ, Ganz DA. Cost-effectiveness of denosumab versus oral alendronate for elderly osteoporotic women in Japan. Osteoporos Int. 2017;28(5):1733-1744. doi: 10.1007/s00198-017-3940-4 [DOI] [PubMed] [Google Scholar]
- 15.Benjamin B, Benjamin MA, Swe M, Sugathan S. Review on the comparison of effectiveness between denosumab and bisphosphonates in post-menopausal osteoporosis. Osteoporos Sarcopenia. 2016;2(2):77-81. doi: 10.1016/j.afos.2016.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin T, Wang C, Cai XZ, et al. . Comparison of clinical efficacy and safety between denosumab and alendronate in postmenopausal women with osteoporosis: a meta-analysis. Int J Clin Pract. 2012;66(4):399-408. doi: 10.1111/j.1742-1241.2011.02806.x [DOI] [PubMed] [Google Scholar]
- 17.Cummings SR, Black DM, Nevitt MC, et al. ; The Study of Osteoporotic Fractures Research Group . Bone density at various sites for prediction of hip fractures. Lancet. 1993;341(8837):72-75. doi: 10.1016/0140-6736(93)92555-8 [DOI] [PubMed] [Google Scholar]
- 18.Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ. 1996;312(7041):1254-1259. doi: 10.1136/bmj.312.7041.1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmidt M, Pedersen L, Sørensen HT. The Danish Civil Registration System as a tool in epidemiology. Eur J Epidemiol. 2014;29(8):541-549. doi: 10.1007/s10654-014-9930-3 [DOI] [PubMed] [Google Scholar]
- 20.Johannesdottir SA, Horváth-Puhó E, Ehrenstein V, Schmidt M, Pedersen L, Sørensen HT. Existing data sources for clinical epidemiology: the Danish National Database of Reimbursed Prescriptions. Clin Epidemiol. 2012;4:303-313. doi: 10.2147/CLEP.S37587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sørensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449-490. doi: 10.2147/CLEP.S91125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneeweiss S, Avorn J. A review of uses of health care utilization databases for epidemiologic research on therapeutics. J Clin Epidemiol. 2005;58(4):323-337. doi: 10.1016/j.jclinepi.2004.10.012 [DOI] [PubMed] [Google Scholar]
- 23.Hudson M, Avina-Zubieta A, Lacaille D, Bernatsky S, Lix L, Jean S. The validity of administrative data to identify hip fractures is high: a systematic review. J Clin Epidemiol. 2013;66(3):278-285. doi: 10.1016/j.jclinepi.2012.10.004 [DOI] [PubMed] [Google Scholar]
- 24.Sing CW, Woo YC, Lee ACH, et al. . Validity of major osteoporotic fracture diagnosis codes in the Clinical Data Analysis and Reporting System in Hong Kong. Pharmacoepidemiol Drug Saf. 2017;26(8):973-976. doi: 10.1002/pds.4208 [DOI] [PubMed] [Google Scholar]
- 25.Thygesen SK, Christiansen CF, Christensen S, Lash TL, Sørensen HT. The predictive value of ICD-10 diagnostic coding used to assess Charlson Comorbidity Index conditions in the population-based Danish National Registry of Patients. BMC Med Res Methodol. 2011;11:83. doi: 10.1186/1471-2288-11-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lund JL, Richardson DB, Stürmer T. The active comparator, new user study design in pharmacoepidemiology: historical foundations and contemporary application. Curr Epidemiol Rep. 2015;2(4):221-228. doi: 10.1007/s40471-015-0053-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Romano PS, Roos LL, Jollis JG. Adapting a clinical comorbidity index for use with ICD-9-CM administrative data: differing perspectives. J Clin Epidemiol. 1993;46(10):1075-1079. doi: 10.1016/0895-4356(93)90103-8 [DOI] [PubMed] [Google Scholar]
- 28.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661-3679. doi: 10.1002/sim.6607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brown JP, Prince RL, Deal C, et al. . Comparison of the effect of denosumab and alendronate on BMD and biochemical markers of bone turnover in postmenopausal women with low bone mass: a randomized, blinded, phase 3 trial. J Bone Miner Res. 2009;24(1):153-161. doi: 10.1359/jbmr.0809010 [DOI] [PubMed] [Google Scholar]
- 30.Kendler DL, Roux C, Benhamou CL, et al. . Effects of denosumab on bone mineral density and bone turnover in postmenopausal women transitioning from alendronate therapy. J Bone Miner Res. 2010;25(1):72-81. doi: 10.1359/jbmr.090716 [DOI] [PubMed] [Google Scholar]
- 31.Lewiecki EM, Miller PD, McClung MR, et al. ; AMG 162 Bone Loss Study Group . Two-year treatment with denosumab (AMG 162) in a randomized phase 2 study of postmenopausal women with low BMD. J Bone Miner Res. 2007;22(12):1832-1841. doi: 10.1359/jbmr.070809 [DOI] [PubMed] [Google Scholar]
- 32.McClung MR, Lewiecki EM, Cohen SB, et al. ; AMG 162 Bone Loss Study Group . Denosumab in postmenopausal women with low bone mineral density. N Engl J Med. 2006;354(8):821-831. doi: 10.1056/NEJMoa044459 [DOI] [PubMed] [Google Scholar]
- 33.Recknor C, Czerwinski E, Bone HG, et al. . Denosumab compared with ibandronate in postmenopausal women previously treated with bisphosphonate therapy: a randomized open-label trial. Obstet Gynecol. 2013;121(6):1291-1299. doi: 10.1097/AOG.0b013e318291718c [DOI] [PubMed] [Google Scholar]
- 34.Roux C, Hofbauer LC, Ho PR, et al. . Denosumab compared with risedronate in postmenopausal women suboptimally adherent to alendronate therapy: efficacy and safety results from a randomized open-label study. Bone. 2014;58:48-54. doi: 10.1016/j.bone.2013.10.006 [DOI] [PubMed] [Google Scholar]
- 35.Kim SM, Long J, Montez-Rath M, Leonard M, Chertow GM. Hip fracture in patients with non-dialysis-requiring chronic kidney disease. J Bone Miner Res. 2016;31(10):1803-1809. doi: 10.1002/jbmr.2862 [DOI] [PubMed] [Google Scholar]
- 36.National Institute for Health and Care Excellence Denosumab for the prevention of osteoporotic fractures in postmenopausal women. https://nice.org.uk/guidance/ta204. Accessed November 5, 2018.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eTable 1. Rates and hazard ratios of the fracture outcomes in initiators of denosumab and alendronate, defining initiation by requiring 5 years’ absence of osteoporosis treatment before the index date (sensitivity analysis)
eTable 2. Rates and hazard ratios of the fracture outcomes for denosumab and alendronate, with follow-up censored at discontinuation or switch